Preparation method of muscarinic receptor antagonist glycopyrronium bromide
A technology of muscarinic receptors and glycopyrronium bromide, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
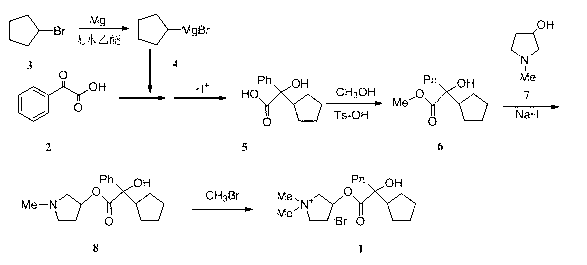
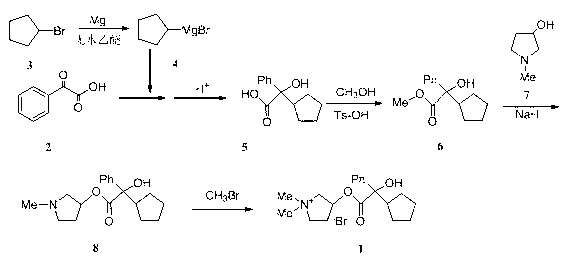
[0013] Alpha-cyclopentylmandelic acid (5)
[0014] Magnesium chips (1.5g, 62mmol), anhydrous ether (20mL) and a small amount of iodine were added to a 250mL three-necked flask. Heated to 30°C, and a small amount of bromocyclopentane ( 3 ) diethyl ether solution, heating to initiate the reaction, when the iodine color disappears, add dropwise under stirring 3 (8.94g, 60mmol) in diethyl ether (15mL), heated to 37°C and refluxed for 20min after dropping, then cooled to 0~5°C. Join in batches 2 (3g, 20mmol), after the addition, add 40mL of anhydrous diethyl ether, raise the temperature to 30°C, stir for 10h, add hydrochloric acid (30mL, 4 mol / L) and stir until the solid is completely dissolved, separate the diethyl ether layer, and wash the water layer with diethyl ether (20mL , 20mL, 10mL) extracted three times. Combine the ether layers, extract twice with 10% sodium carbonate aqueous solution (60mL, 40mL), combine the water layers, adjust the pH to 1~2 with concentrated hy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com