Method for preparing unsymmetrical rhodamine
An asymmetric, ketoacid technology, applied in the field of preparation of organic fluorescent dyes, can solve the problem of not finding naphthalenediol, etc., and achieve the effects of simple equipment, high fluorescence intensity and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
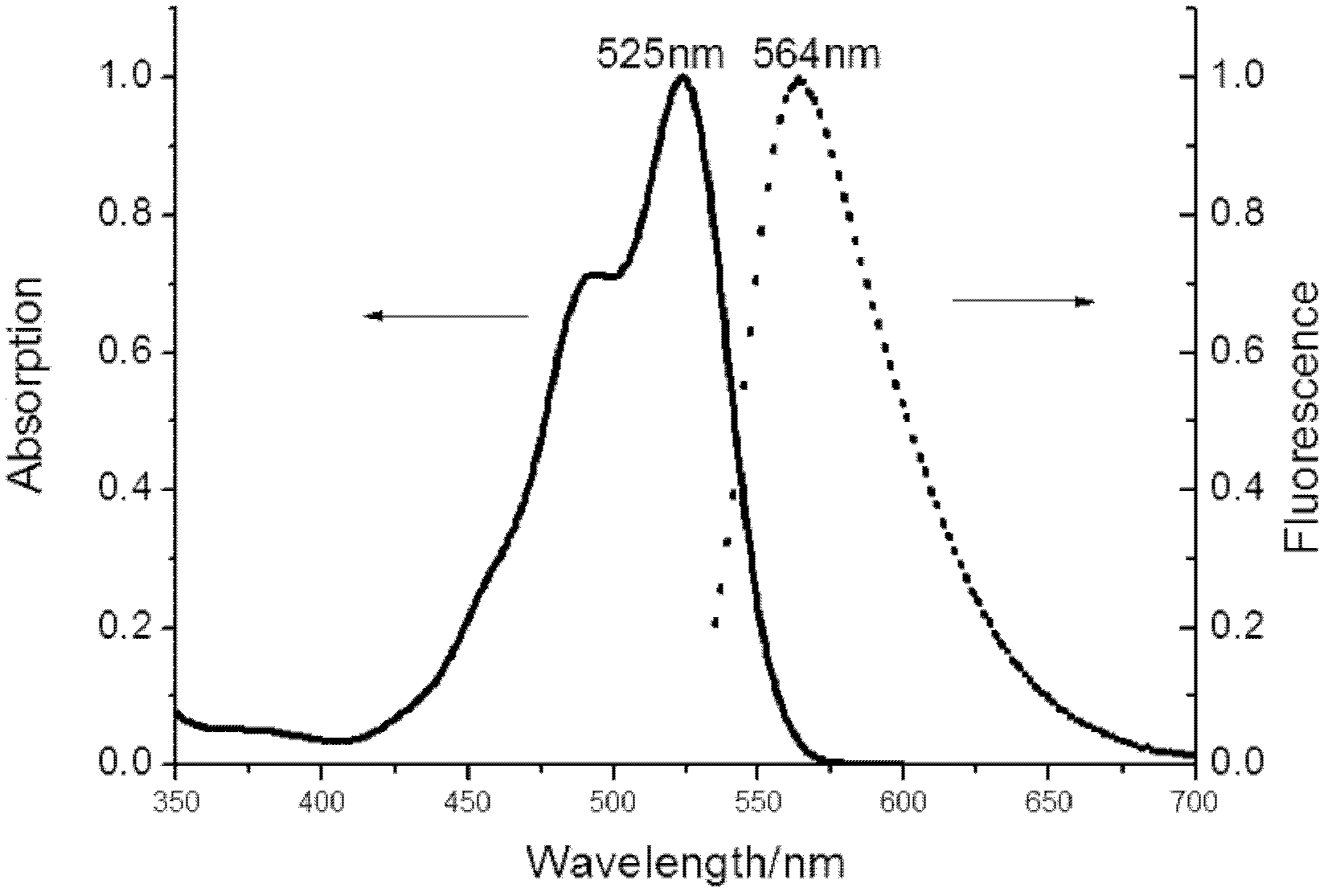
[0031] Preparation and purification of asymmetric rhodamine I:
[0032] Add 9.9g of N,N-diethylm-aminophenol and 9.0g of phthalic anhydride in a 250ml three-neck flask equipped with magnetic stirring, dissolve with 150ml of toluene, N 2 Protect, stir, stop the reaction after heating to reflux for 8 hours, suction filter after cooling to room temperature, and recrystallize the filter cake with water to obtain 11.7 g of light yellow columnar crystals, namely keto acid, with a yield of 62.5%.
[0033] Add 3.1 g of keto acid, 4.6 g of ethyl naphthalene diol formate, and 1.3 g of zinc chloride into a 100 mL round bottom flask equipped with magnetic stirring. Heated and stirred at 120°C for 6h. The obtained mixture was put into 200ml of water to precipitate a precipitate, filtered with suction, and the filter cake was dried to obtain a crude product.
[0034] 3 g of the obtained crude product was dissolved in 100 ml of absolute ethanol, and 5 ml of concentrated sulfuric acid was a...
Embodiment 2
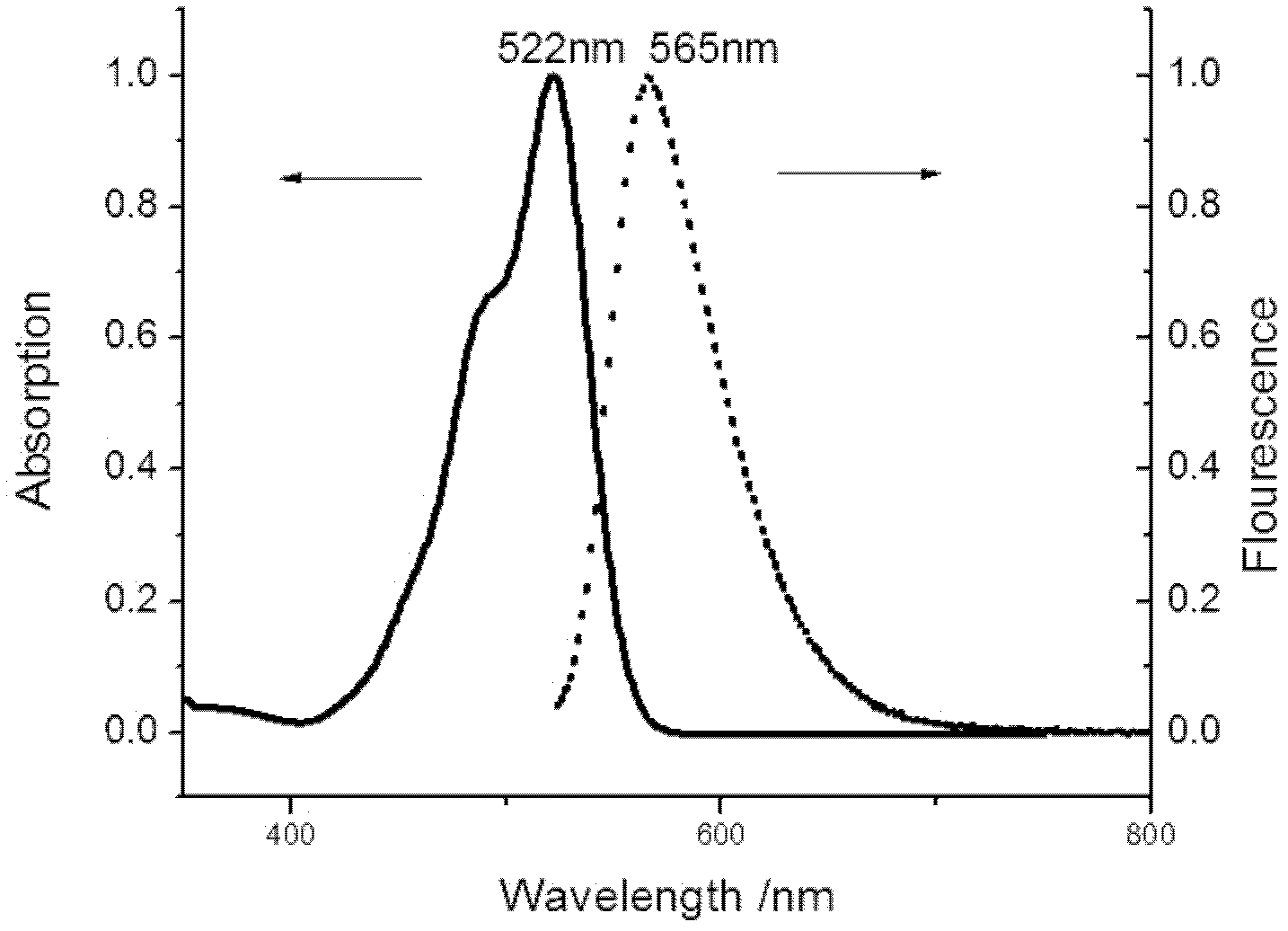
[0039] Preparation and purification of asymmetric rhodamine II:
[0040] Add 9.9g of N,N-diethylm-aminophenol and 9.0g of phthalic anhydride in a 250ml three-neck flask equipped with magnetic stirring, dissolve with 150ml of toluene, N 2 Protect, stir, stop the reaction after heating to reflux for 8 hours, suction filter after cooling to room temperature, and recrystallize the filter cake with water to obtain 11.7 g of light yellow columnar crystals, namely keto acid, with a yield of 62.5%.
[0041] Add 3.1 g of keto acid, 4.6 g of ethyl naphthalene diol formate, and 10 ml of methanesulfonic acid into a 100 mL round bottom flask equipped with magnetic stirring. Heated and stirred at 100°C for 10h. The obtained mixture was put into 200ml of water to precipitate a precipitate, filtered with suction, and the filter cake was dried to obtain a crude product.
[0042] 3 g of the obtained crude product was dissolved in 100 ml of absolute ethanol, and 5 ml of concentrated sulfuric a...
Embodiment 3
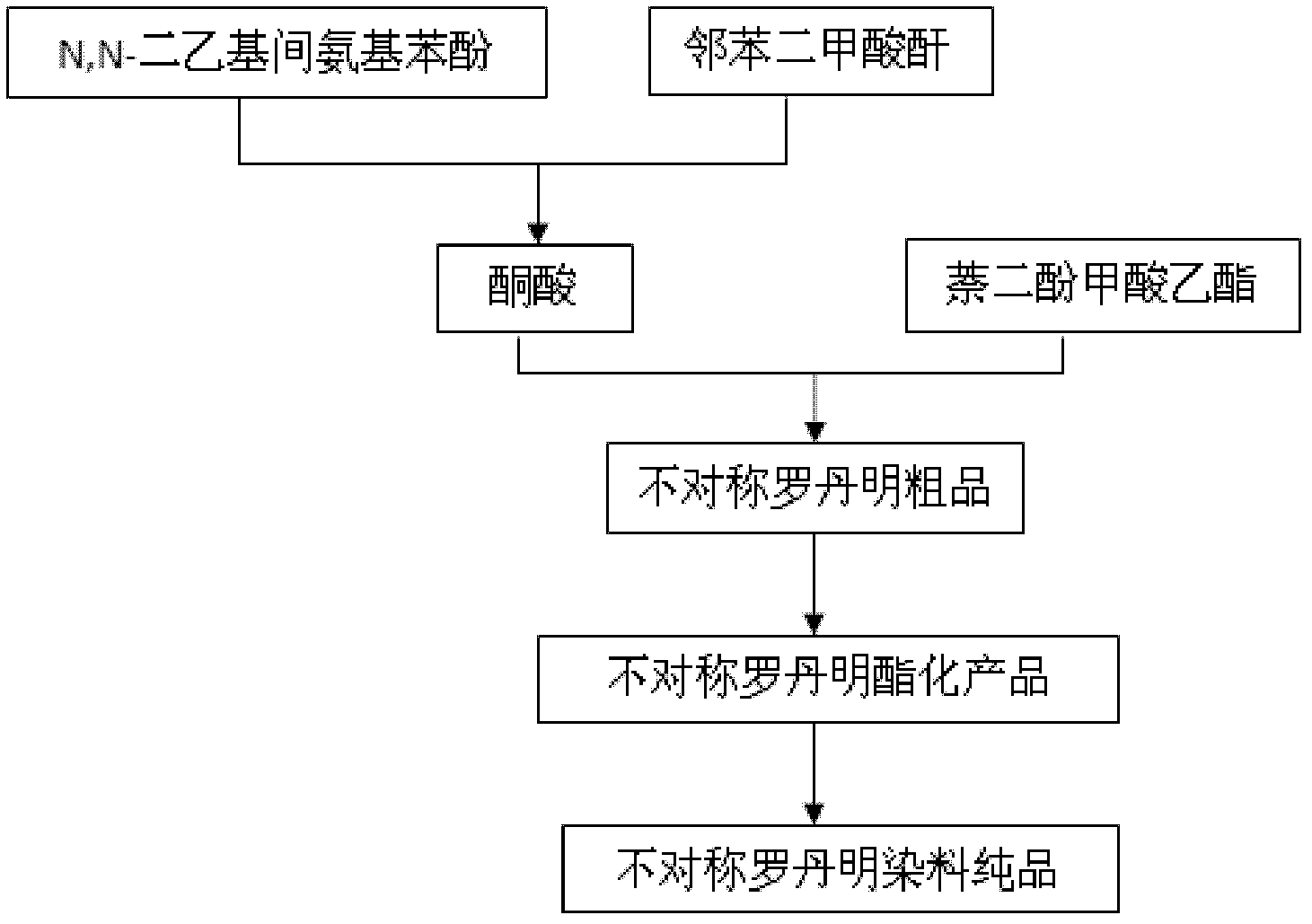
[0047] A method for preparing asymmetric rhodamine, its technological process is as follows image 3 As shown, the method includes the following steps:
[0048] (1) Preparation of intermediate keto acid: take N, N-diethyl m-aminophenol and its equimolar phthalic anhydride as raw materials, dissolve with 10 times the mass of toluene, stir under nitrogen protection conditions, and heat Reflux for 5 hours, and suction filter after cooling to obtain the intermediate ketoacid;
[0049] (2) The preparation of asymmetric rhodamine: get the intermediate ketoacid prepared by step (1), the naphthalenediol ethyl formate and catalyst zinc chloride of equal molar weight, the weight of zinc chloride and intermediate ketoacid The ratio is 1:4, heated to 100°C and reacted for 10 hours, the obtained mixture is put into 40-80 times the mass of water to precipitate a precipitate, filtered by suction, and dried to obtain a crude product;
[0050] (3) Esterification of asymmetric rhodamine crude...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com