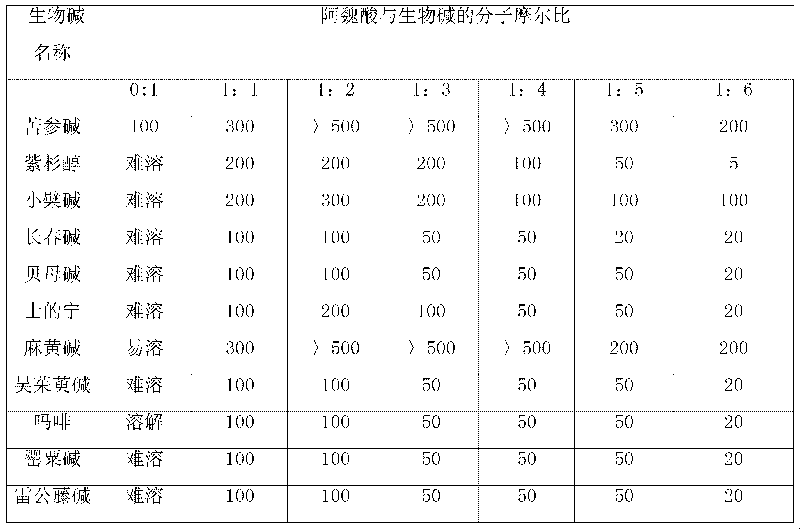
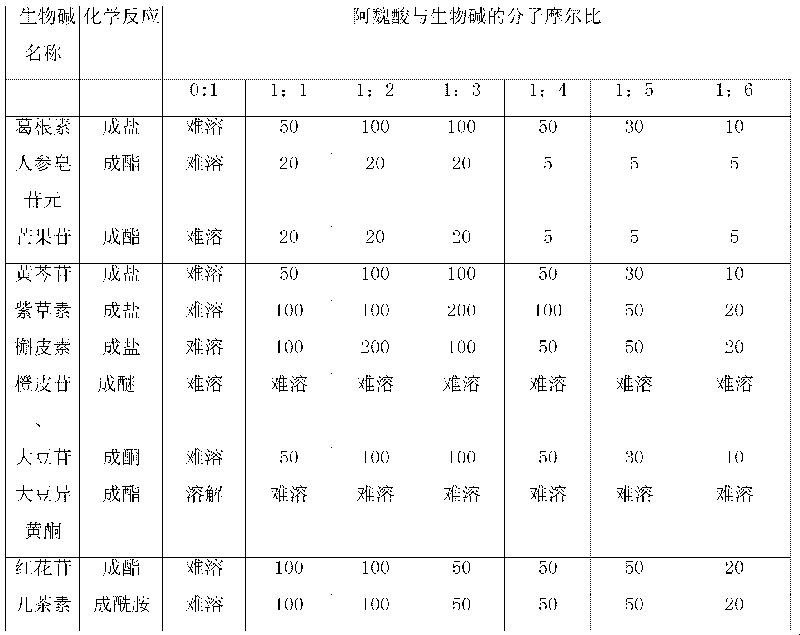
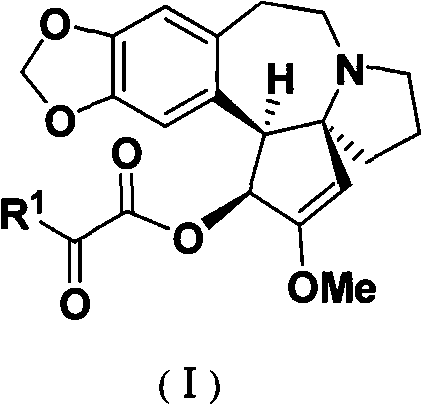
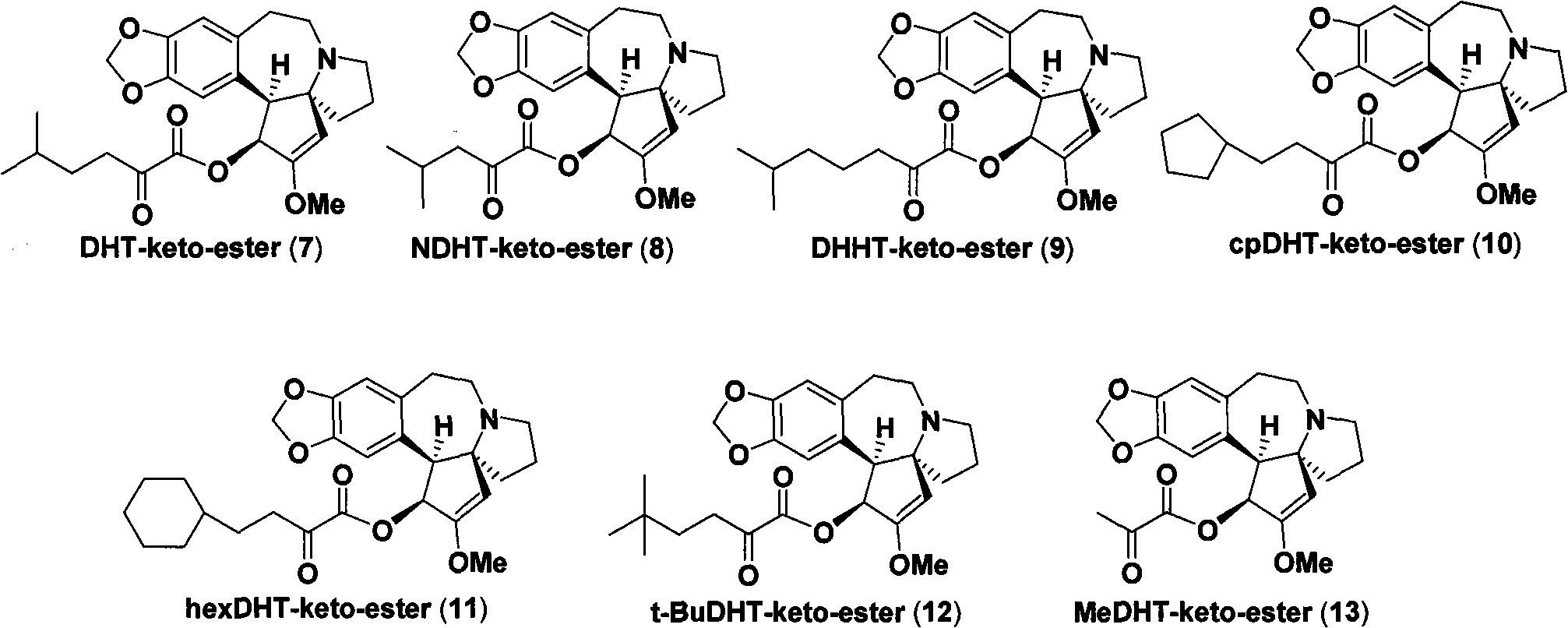
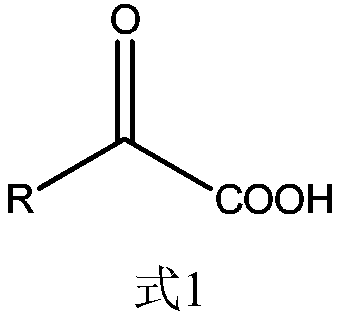
Patents
Literature
156 results about "Ketonic acids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
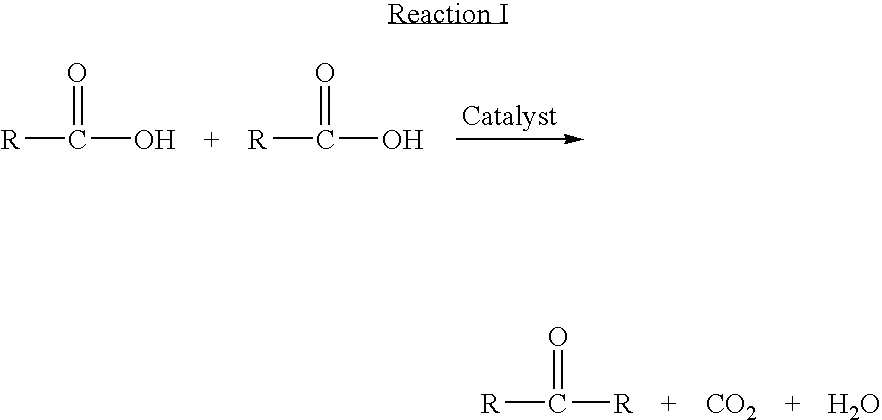
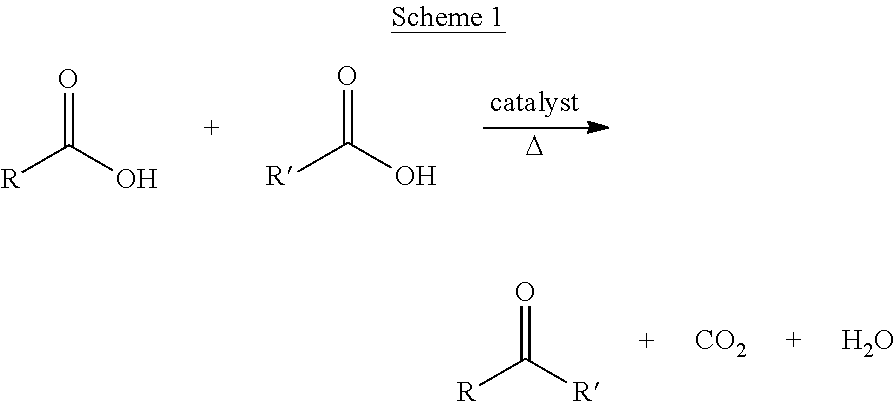
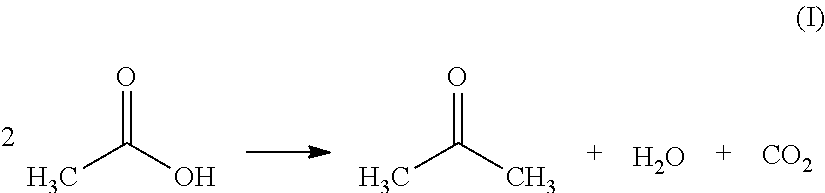
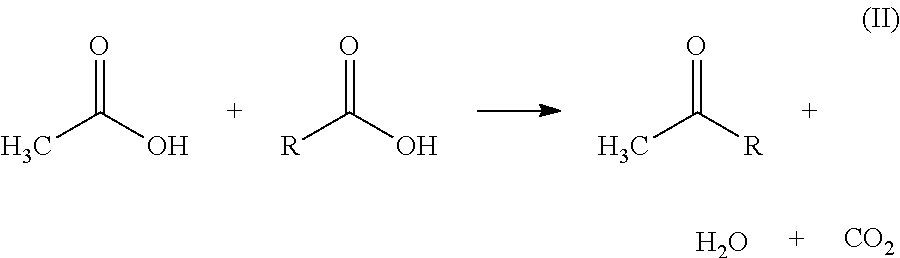
Ketonic decarboxylation (also known as ketonization) is a type of organic reaction and a decarboxylation converting two equivalents of a carboxylic acid to a symmetric ketone by the application of heat with expulsion of one equivalent of water and one equivalent of carbon dioxide. Bases promote this reaction.
Production of gasoline from fermentable feedstocks
ActiveUS20100137647A1High speedHigh oil contentOrganic compound preparationBiofuelsBiodieselKetonic acids
Methods are disclosed for forming heptan-4-one, and, optionally, heptan-4-ol, from fermentable sugars. The sugars are fermented using a bacteria or yeast that predominantly forms butyric acid. The butyric acid is subjected to catalytic ketonization conditions to form heptan-4-one, with concomitant loss of water and carbon dioxide. The heptan-4-one can be subjected to catalytic hydrogenation to form heptan-4-ol, an either of these can be included in gasoline compositions. In one aspect, the fermentable sugars are derived from lignocellulosic materials such as wood products, switchgrass, or agricultural wastes, which are delignified to form lignin, cellulose and hemicellulose. The cellulose and hemicellulose can be depolymerized to form glycose and xylose, either or both of which can be fermented by the bacteria. Thus, the methods described herein can convert biomass to a fuel composition or fuel additive, which can be used in a conventional gasoline engine, unlike traditional fuels such as ethanol or biodiesel.
Owner:CPS BIOFUELS INC
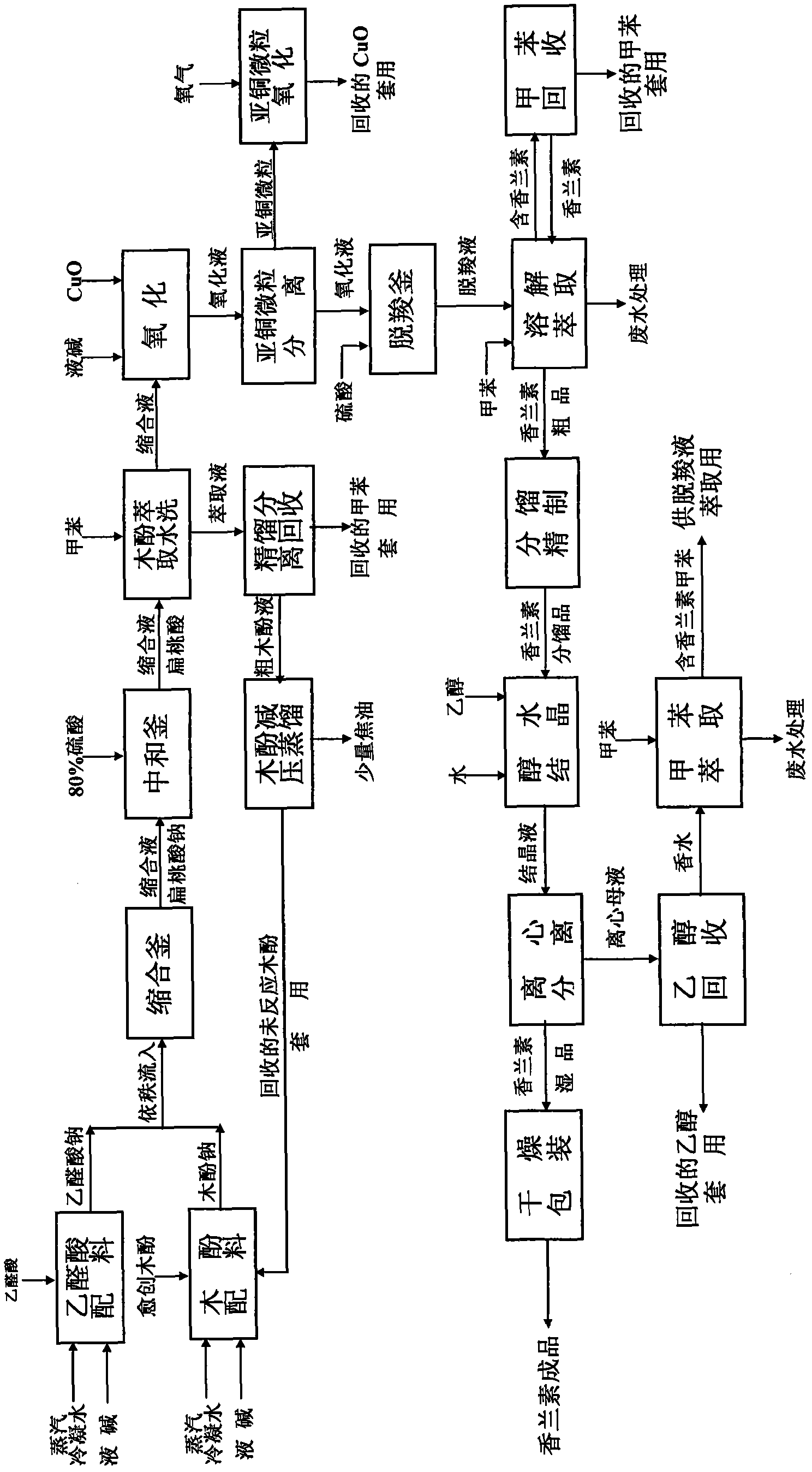
Productive technology of vanlillin by glyoxylic acid method
ActiveCN102010310AReduce organic contentReduce pollution sourcesOrganic compound preparationCarbonyl compound separation/purificationKetonic acidsFractionation
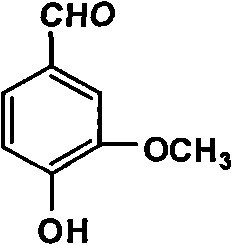
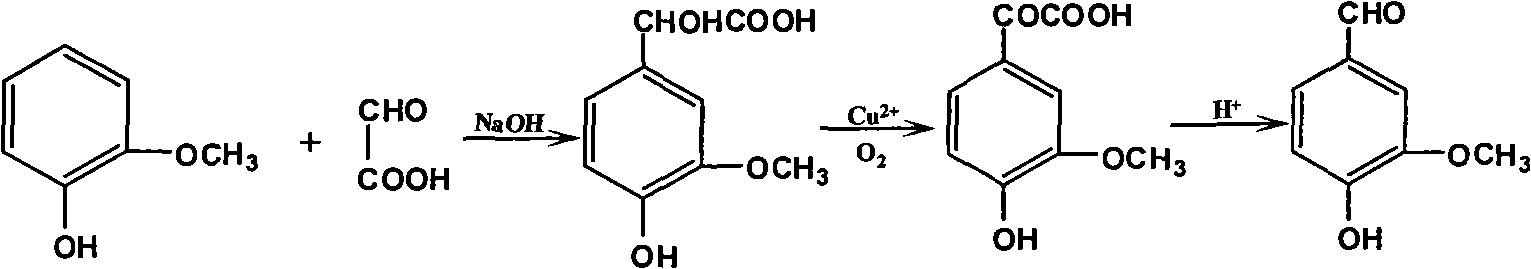
The invention discloses a productive technology of vanlillin by a glyoxylic acid method. The productive technology comprises a synthesis process, a fractionation process and a purification process, wherein the synthesis process comprises condensation treatment, oxidation treatment and decarboxylation treatment of methyl catechol and glyoxylic acid. The productive technology particularly comprisesthe following steps: respectively converting the methyl catechol and the glyoxylic acid into guaiacol sodium and sodium glyoxylate in a sodium hydroxide system; carrying out condensation treatment onthe guaiacol sodium and the sodium glyoxylate; after recovering the unreacted methyl catechol in a condensation liquid, carrying out oxidation treatment, namely carrying out catalytic oxidation on anethanol group in 4-hydroxy-3-methoxybenzene sodium glycolate by using copper oxide in the sodium hydroxide system to form a ketone group, thereby generating a corresponding ketonic acid compound; after separating red copper oxide particles from an oxidation liquid, carrying out decarboxylation treatment, namely using sulfuric acid to acidize the oxidation liquid, and simultaneously converting an acid group in the ketonic acid compound into carbon dioxide so as to generate 4-hydroxy-3- methoxybenzaldehyde; and carrying out the fractionation process and the purification process to obtain the vanlillin.
Owner:喜孚狮王龙香料(宁波)有限公司
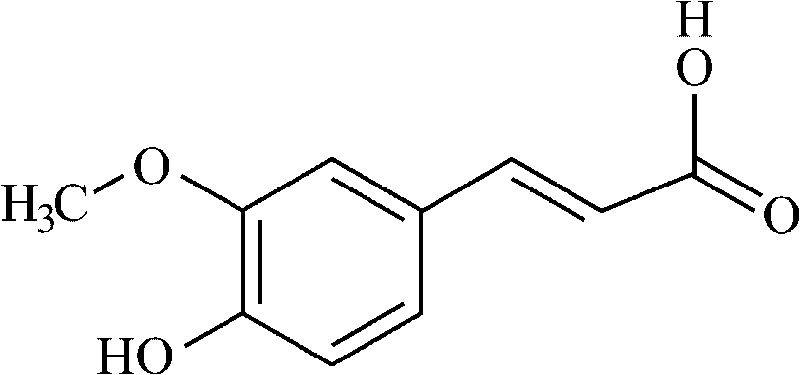
Action of ferulic acid on enhancing drug effect of some medicaments and purpose thereof
InactiveCN101721400AOrganic active ingredientsPharmaceutical non-active ingredientsCatharanthineChemical reaction
The invention relates to an action of ferulic acid on enhancing the drug effect of some medicaments and a purpose thereof, wherein the medicaments mainly comprise alkaloid medicaments including matrine, kushenin, sophocarpine, hyoscyamine, narceine, hanfangchin A, jamaicin, morphine, codeine, evodiamine, strychnine, catharanthine, vincristine, taxol, verticine, peimine, peiminine, ephedrine, pseudoephedrine, wilfordine, triptolide, tripdiolide and the like, and flavonoid medicaments including puerarin, ginsenoside, ginsengenin, mangiferin, scutelloside, alkannin, meletin, rutin, hesperidin, daidzin, soybean isoflavone, daidzein, carthamin, catechin and the like. The ferulic acid and the medicaments can form a compound or a medicament compound, or the ferulic acid and the medicaments can generate a chemical reaction (including salification, esterification, amidation, ketonization, etherification and the like), and / or the ferulic acid and the medicaments can generate a synergistic effect and an additive effect.
Owner:QINGDAO QIYUAN BIO TECH CO LTD
Method for detecting organic acid/amino acid metabolic product by filter paper shect gas chromatography-mass spectrum analysis
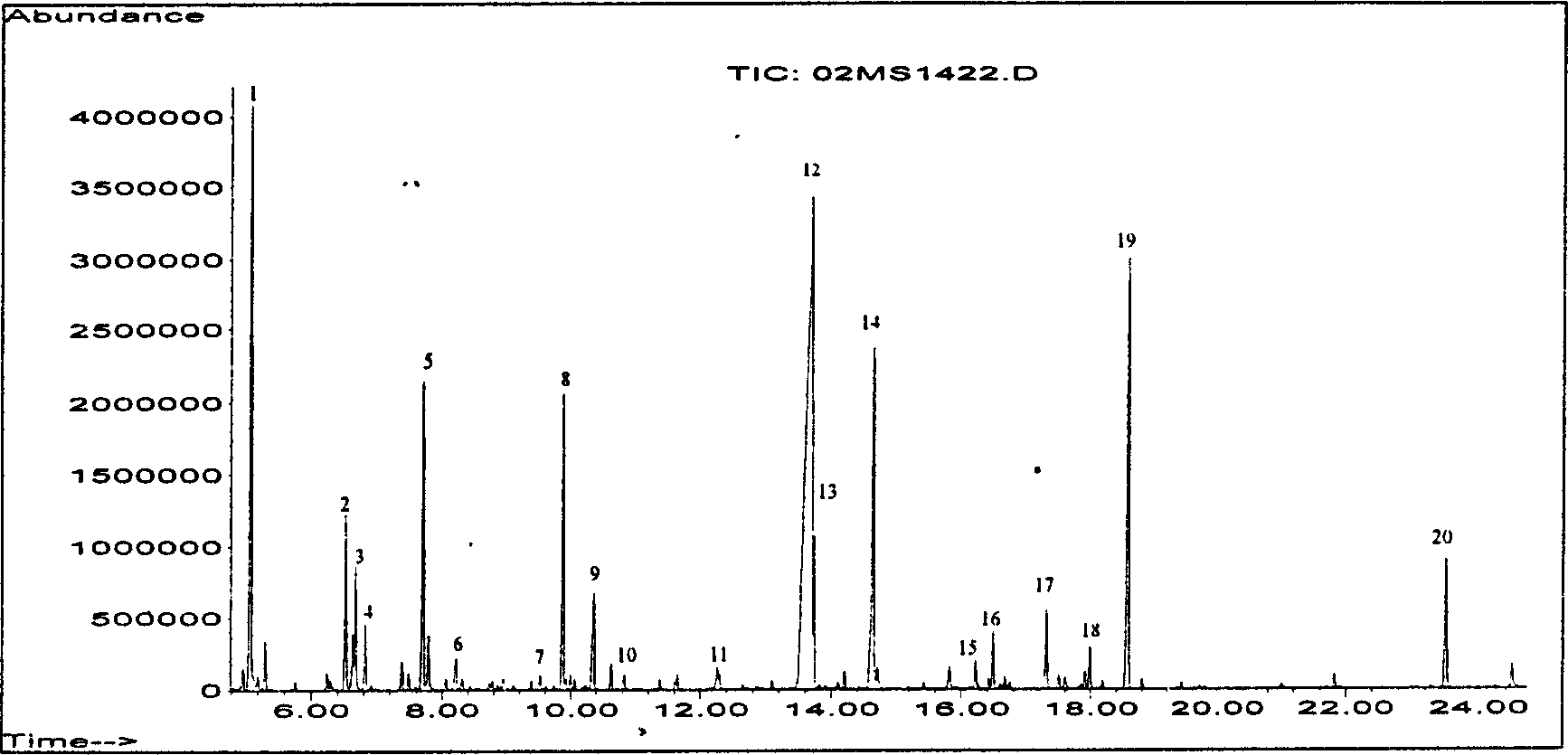
The present invention discloses filter paper sheet gas chromatography-mass spectrum analysis process of organic acid / amino acid metabolic product in urine. The process includes elution of urine collected with filter paper, oximation reaction, extraction, methyl silane derivation, and united detection with chromatographic instrument and mass spectrograph. The process of the present invention is superior in that the filter paper collection of urine favors remote analysis of genetic metabolic diseases; the oximation reaction processing favors alpha-ketonic acid detection for diagnosis of branched chain ketonic acid metabolic disorder; the extraction has high recovery rate of organic acid / amino acid metabolic product, high repeatability, less chromatographic impurity peaks and no pollution to chromatographic capillary column and mass spectrographic ion source; and the united detection is suitable for screening analysis of great amount of samples.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Catalyst and process for the preparation of unsymmetrical ketones
InactiveUS20070100166A1Return to normal activitiesOrganic compound preparationCarbonyl compound preparation by condensationKetonic acidsCarboxylic acid
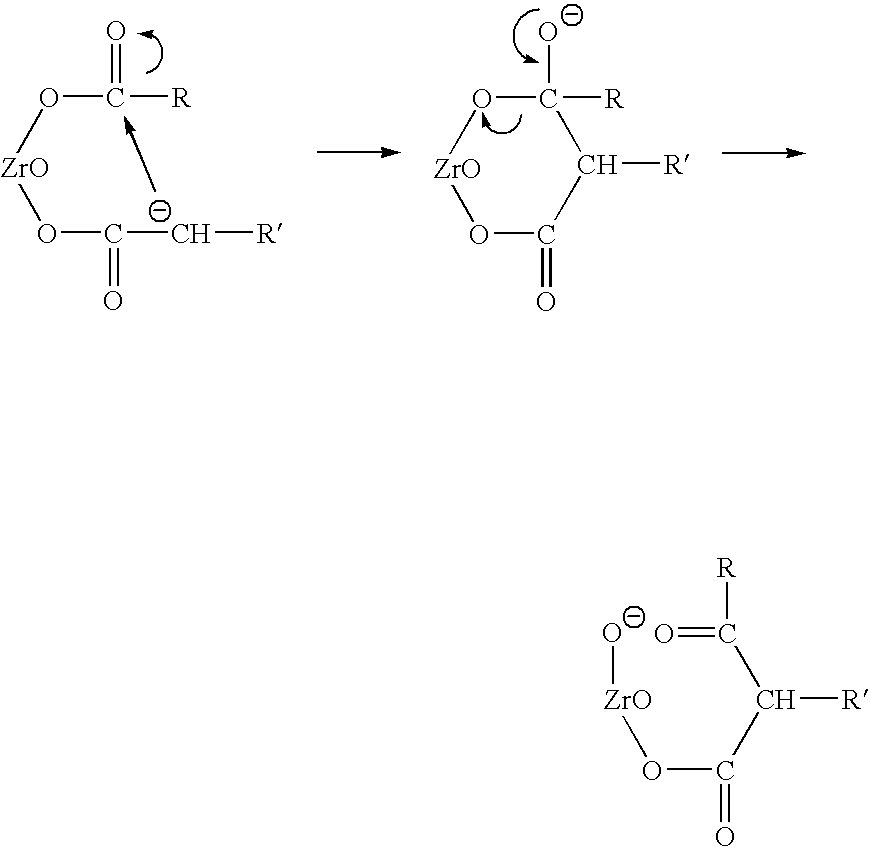
Carboxylic acid mixtures form unsymmetrical ketones in yields approaching statistical using zirconia catalysts promoted with Group IA and IIA elements. Active catalysts exist in their monoclinic or tetragonal but not cubic form. And the level of promoter loading is generally less than ten percent. The advantages of this catalyst over other ketonization catalysts include its high selectivity to ketones, its low formation of dehydrogenated byproducts, and its stability. The catalyst stability permits its regeneration to remove carbon accumulations by air oxidation. This regeneration restores full catalytic activity.
Owner:EASTMAN CHEM CO
Method for synthesizing coumarin and derivatives thereof under catalysis of choline ionic liquids
InactiveCN103613573AEasy to separateNot generatedOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsProtonationKetonic acids
The invention discloses a method for synthesizing coumarin and derivatives thereof under catalysis of choline ionic liquids. According to the technical scheme, the method is characterized in that the coumarin and the derivatives thereof are prepared from a phenolic compound and beta-ketonic acid or keto ester under the catalytic action of choline ionic liquids and the choline ionic liquids include an ionic liquid of melted binary acid and choline chloride, a functionalized acidic choline ionic liquid and a protonized choline ionic liquid. According to the method disclosed by the invention, the synthesized choline ionic liquid is used for Pechmann condensation reaction; the negative and positive ions of the ionic liquids are adjusted according to the catalytic effect based on different acid-base properties of the ionic liquids, so that better catalytic effect can be achieved; the choline ionic liquids take the place of dangerous concentrated sulfuric acid when the choline ionic liquids are used for catalyzing the Pechmann reaction; the reaction conditions are simple, the product is easy to separate and no by-product is generated; more importantly, the catalyst can be reused.
Owner:HENAN NORMAL UNIV
Compositions for the treatment and prevention of plant pathogens
An antimicrobial composition is disclosed which is particularly useful in the protection of plants against microbial attack. The composition may also be used to treat plants that are infected with microorganisms. The composition comprises in colloidal form, an antimicrobially effective amount of a product formed by the reaction in water of a water soluble cupric tetra amine salt with an acid or a salt thereof selected from the group comprising hydroxy carboxylic acids, dicarboxylic acids, hydroxy di- and tri-carboxylic acids, polyhydric dicarboxylic acids, ketonic acids and mixtures and isomers thereof; the molar ratio of the water soluble Cupric tetramine salt (as copper) to the acid or salt thereof (as carboxylate groups) being from 4:1 to 1:4; and, a pectin in an amount of 0.05 to 2.00.% w / v and having a degree of esterification of 2-20 or derivatives or mixtures thereof. Optionally, a wetting agent to promote the wetting of the plant or parts thereof by the composition, a spreading agent to promote the distribution of the composition onto the plant or parts thereof, and an adherent to promote the retention of the composition onto the plant or parts thereof may be included.
Owner:BIOACUMEN
Compound alpha-ketoacid chewing tablet and preparation method thereof
InactiveCN101416947AExpand the range of dosage formsExpand the range of dosage forms, with a dispersed stateUrinary disorderPill deliveryDiseaseAdditive ingredient
The invention relates to a drug preparation and a preparation method thereof, in particular to a compound Alpha-ketonic acid chewable tablet used for curing chronic renal failure, and a preparation method thereof. The compound Alpha-ketonic acid chewable tablet comprises the following components: Alpha-ketophenylalanine calcium, Alpha-hydroxymethionine calcium, Alpha-ketoleucine calcium, Alpha-ketoisoleucine calcium, Alpha-ketovaline calcium, tryptophan, histidine, tyrosine, threonine, lysine, acetate, a filling agent, a bonding agent, a taste-masking agent, a lubricant and a coating agent. The compound Alpha-ketonic acid chewable tablet not only widens the dosage form range of the compound Alpha-ketonic acid chewable tablets, but also has the advantages of having good dispersing state, short disintegration time, fast drug dissolution, rapid absorption, high biological availability and convenient taking, being capable of being swallowed, chewed and sucked and being especially suitable for old people, stroke patients, patients with special diseases and patients having swallowing difficulty.
Owner:无锡曙辉药业有限公司
Engineering bacteria and application thereof
ActiveCN107586752APoor substrate specificityHigh activityBacteriaMicroorganism based processesEscherichia coliEnzyme Gene
The invention discloses escherichia coli gene engineering bacteria for four enzyme co-expression. The engineering bacteria is characterized by introducing an L-amino acid oxidase gene, an alpha-ketonic acid decarboxylase gene, an alcohol dehydrogenase gene, and an enzyme gene capable of reducing NAD(P) to NAD(P)H. The invention further discloses a construction method and application of recombinantescherichia coli. The engineering bacteria is applied to biological synthesis of phenylethyl alcohol compounds, has the characteristics of simple operation, low cost, high product synthetic efficiency, and high optical purity, and has bright industrial prospects.
Owner:HONGTAOSIM RES INST OF ANALYCAL SCI & TECH LTD CO
Method for asymmetrically synthesizing chiral beta-acetenyl ketone from beta-ketonic acid
InactiveCN104513146AHigh reactivityHigh stereoselectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsKetonic acidsKetone
The invention relates to a method for synthesizing chiral beta-acetenyl ketone by catalytic intermolecular decarboxylation from beta-ketonic acid and a propargyl compound. A chiral copper catalyst adopted in the invention is synthesized in stiu from a copper salt and a chiral P,N,N-tridentate ligand in various polar solvents and nonpolar solvents. According to the invention, various chiral beta-acetenyl ketone compounds with substituent groups can be synthesized conveniently, and can obtain a percent enantiomeric excess as high as 95%. The method in the invention is advantaged by operational simplicity, available raw materials, wide substrate application range, high enantioselectivity, and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing alpha-calcium picrolonate
InactiveCN101759553AEasy to separateEasy to purifyCarboxylic acid salt preparationCalcium bicarbonateKetonic acids
The invention relates to a method for preparing alpha-calcium picrolonate, which comprises the following steps of: adding alpha-ketonic acid into a proper amount of water; adjusting a PH value to 3-6 by using calcium carbonate or calcium bicarbonate; controlling the temperature to be 0-40 DEG C; concentrating, crystallizing and obtaining the product alpha-calcium picrolonate. The process is simple, is easy for operation, can finish reaction in one step and has high product yield and high quality without producing other by-products and can be used for the industrialized production of the alpha-calcium picrolonate.
Owner:YANGTZE RIVER PHARMA GRP BEIJING HAIYAN PHARMA
Production of gasoline from fermentable feedstocks
ActiveUS8148579B2Easy to separateHigh energy per unit volumeOrganic compound preparationBiofuelsBiodieselKetonic acids
Methods are disclosed for forming heptan-4-one, and, optionally, heptan-4-ol, from fermentable sugars. The sugars are fermented using a bacteria or yeast that predominantly forms butyric acid. The butyric acid is subjected to catalytic ketonization conditions to form heptan-4-one, with concomitant loss of water and carbon dioxide. The heptan-4-one can be subjected to catalytic hydrogenation to form heptan-4-ol, an either of these can be included in gasoline compositions. In one aspect, the fermentable sugars are derived from lignocellulosic materials such as wood products, switchgrass, or agricultural wastes, which are delignified to form lignin, cellulose and hemicellulose. The cellulose and hemicellulose can be depolymerized to form glycose and xylose, either or both of which can be fermented by the bacteria. Thus, the methods described herein can convert biomass to a fuel composition or fuel additive, which can be used in a conventional gasoline engine, unlike traditional fuels such as ethanol or biodiesel.
Owner:CPS BIOFUELS INC
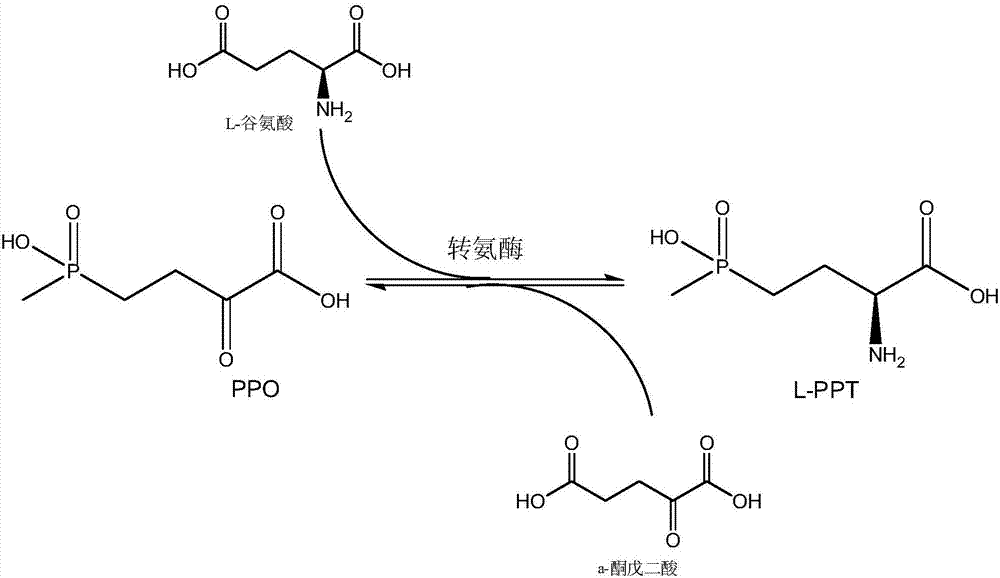
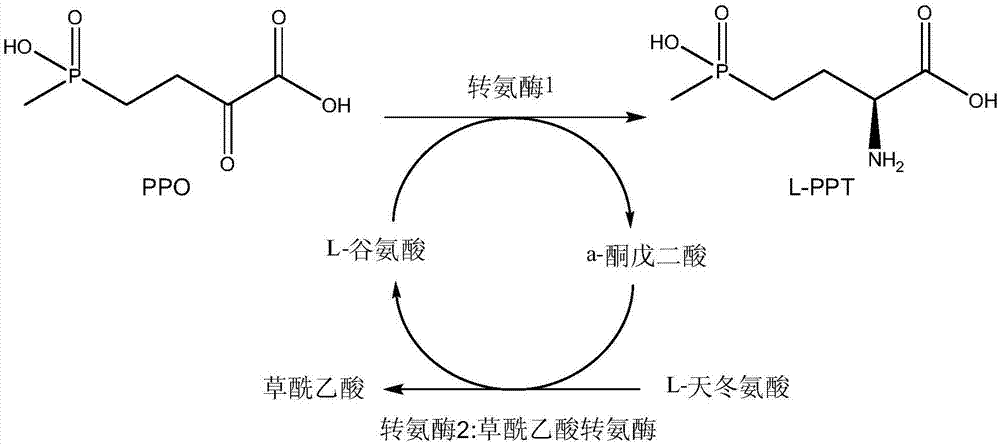
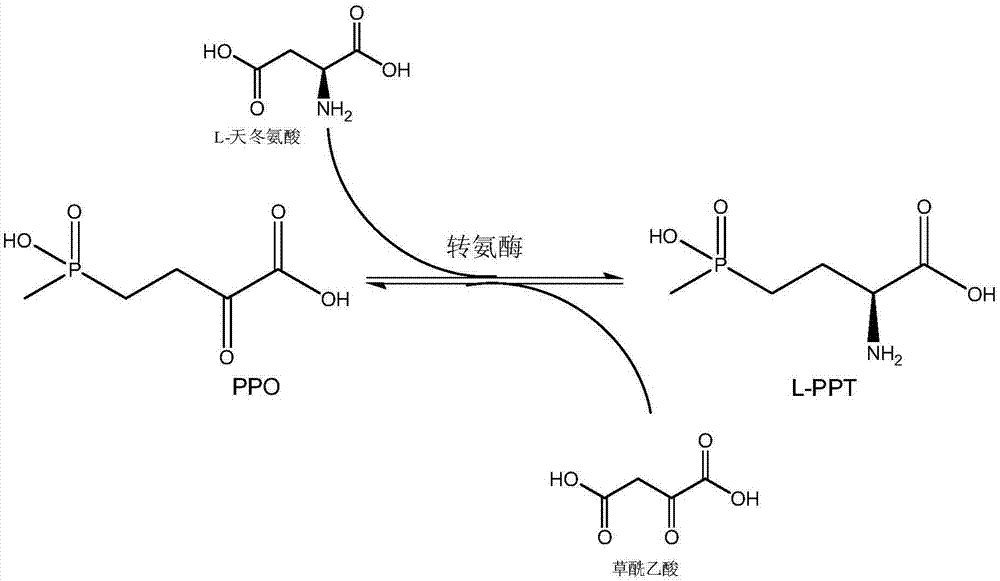
Method for producing L-glufosinate-ammonium by using transaminase and ethylene-forming enzyme
The invention discloses a method for producing L-glufosinate-ammonium by using transaminase and an ethylene-forming enzyme. According to the method, 2-carbonyl-4-(hydroxyl methyl phosphoryl) butyric acid or salt thereof is taken as a raw material, and the L-glufosinate-ammonium is obtained through a catalytic reaction of the transaminase and the ethylene-forming enzyme under the condition of taking glutamic acid or salt thereof as an amidogen donor. According to the method, the 2-carbonyl-4-(hydroxyl methyl phosphoryl) butyric acid or the salt thereof is taken as a substrate, and the L-glufosinate-ammonium is obtained through the co-catalysis of the transaminase and the ethylene-forming enzyme under the condition of taking the glutamic acid or the salt thereof as the amidogen donor, so that by-product alpha-dibasic ketonic acid after a transamination reaction is completely converted into carbon dioxide and ethylene through catalysis of the ethylene-forming enzyme, under the condition of guaranteeing few using amount of the raw materials, the conversion rate of the raw materials is obviously improved, the cost of the raw materials is reduced, the subsequent purification technology is simplified, and the product total recovery is improved.
Owner:ZHEJIANG UNIV
Method for producing 2-phenylethanol under biological catalysis
ActiveCN106957878AImprove conversion rateIncrease productionMicroorganism based processesFermentationPhenylalanine dehydrogenaseKetonic acids
The invention relates to the field of bioengineering and biotechnologies, and discloses a method for producing 2-phenylethanol under biological catalysis. The method comprises the following steps: adding wet cells of E.coli / Pdh, E.coli / Kdc and E.coli / ADH undergoing induced expression into a biological catalysis system taking L-phenylalanine as a substrate for performing a catalytic reaction; centrifuging after finishing the reaction; extracting supernatant to obtain the 2-phenylethanol. In the method, the L-phenylalanine is taken as the substrate, and recombinant escherichia coli transformed with a phenylalanine dehydrogenase gene, a 2-keto acid decarboxylase gene and an alcohol dehydrogenase gene is added into a reaction system for performing a biological deamination, decarboxylation and reduction three-step catalytic reaction in order to generate a product, namely, phenylethanol; a coenzyme, namely, NAD (Nicotinamide Adenine Dinucleotide) is added once and can be recycled, excess ketonic acid and hydrogen sources do not need to be added, no unnecessary side products are generated, a high substrate transforming rate is achieved, and the yield of the phenylethanol is increased remarkably.
Owner:BOTON SHANGHAI BIOLOGICAL TECH CO LTD
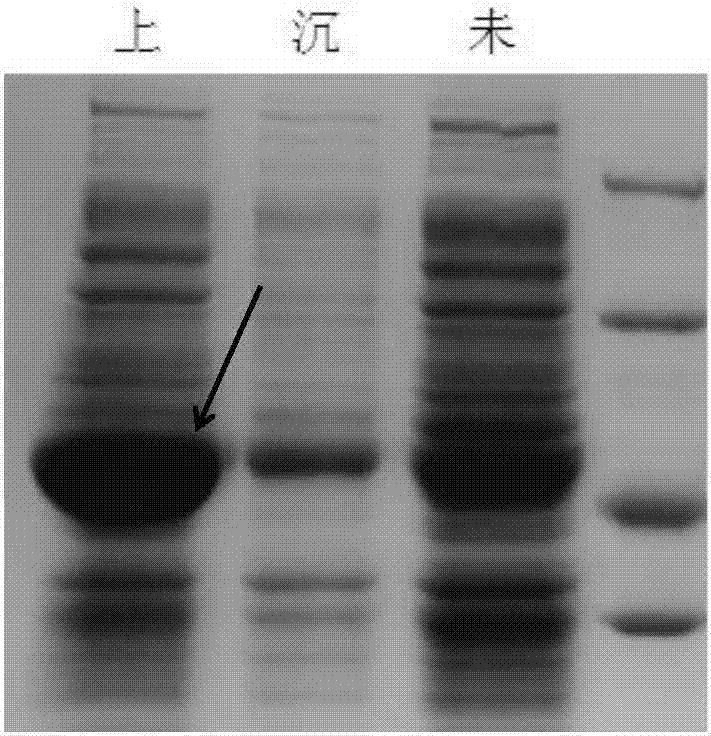
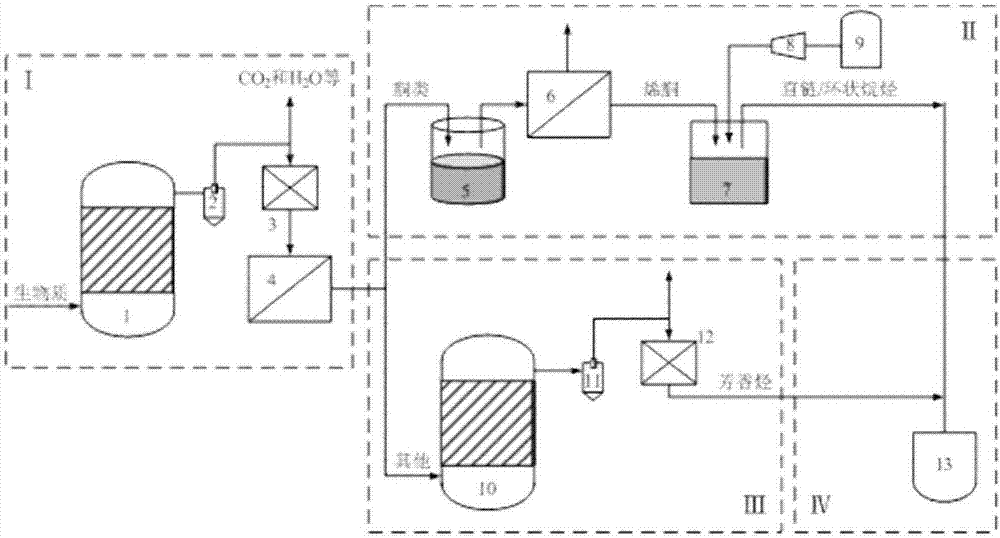
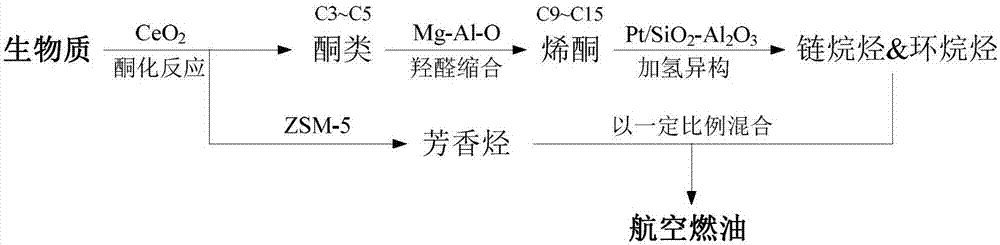
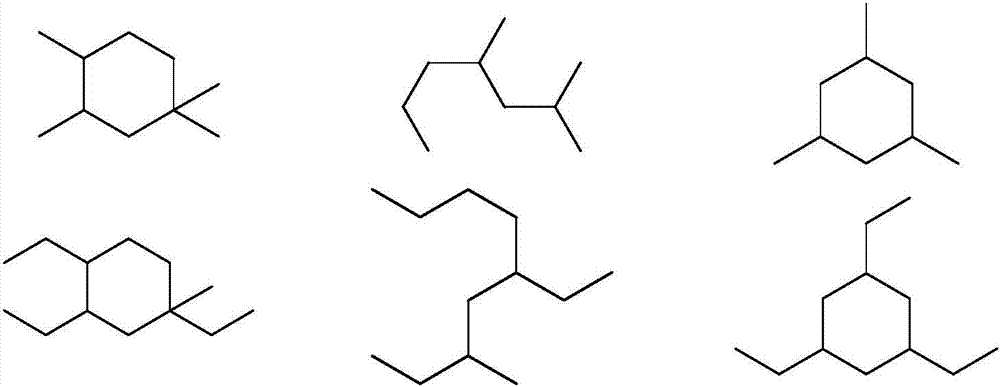
Device and method for preparing bioaviation fuel based on ketone platform compounds
ActiveCN107022369ARealize comprehensive utilizationReduce yieldLiquid hydrocarbon mixture productionHydrocarbon oils treatmentAlkaneKetonic acids
The invention provides a device and a method for preparing bioaviation fuel. The device comprises a biomass ketonization reaction system, a carbon chain elongation reaction system, a zeolite catalytic reaction system and an alkane and arene mixing system, wherein the biomass ketonization reaction system is used for catalytically converting biomass into ketones and other monofunctional compounds in an orientated manner, and separating the ketones from the other monofunctional compounds; the carbon chain elongation reaction system is used for converting the ketone platform compounds produced by the biomass ketonization reaction system into alkanes and cycloalkanes through aldol condensation, hydrogenation isomerism and other reactions; the zeolite catalytic reaction system is used for converting the other monofunctional compounds produced by the biomass ketonization reaction system into arenes through zeolite catalytic conversion; the alkane and arene mixing system is used for mixing the alkanes, the cycloalkanes and the arenes according to a certain ratio to obtain the final aviation fuel. With the method, full-component utilization of the biomass can be achieved, the aviation fuel containing the straight-chain alkanes, the cycloalkanes, the arenes and many other components can be prepared, and the ratio of the components is controllable; a novel way of converting the biomass into the aviation fuel is provided.
Owner:JIANGSU UNIV
Process for producing ketones from fattyacids
ActiveUS20130324449A1Organic compound preparationPreparation by hydrogenolysisKetonic acidsGas phase
The invention relates to a process for producing ketones or hydrocarbon base oil from fatty acids preferably derived from a biological origin or other renewable source. The process is directed at making an aliphatic ketone or a mixture of aliphatic ketones having 14 to 52 carbon atoms, comprising a ketonization reaction of a fatty acid in a vapor phase with a decarboxylation-coupling catalyst to provide ketones, which can be deoxygenated to give saturated hydrocarbons, unsaturated hydrocarbons, and mixtures thereof. Base oils and transportation fuels may be produced from the process herein.
Owner:CHEVROU USA INC
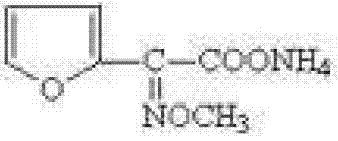
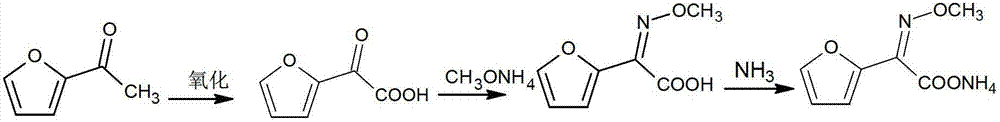
Preparation method of 2-methoxyiminofurylacetic acid amonium salt
The invention relates to a preparation method of 2-methoxyiminofurylacetic acid amonium salt, and belongs to the technical field of medical intermediate preparation. The preparation method of the 2-methoxyiminofurylacetic acid amonium salt comprises the steps as follows: reacting acetylfuran and sodium nitrite to obtain furan ketonic acid under the catalyzing of metal salt; and then mixing the furan ketonic acid and methoxy-ammonium salt to obtain the 2-methoxyiminofurylacetic acid amonium salt. According to the preparation method provided by the invention, the transformation rate of the furan ketonic acid can be improved under the catalysis of the added metal salt, the side reaction is reduced, therefore, the yield of the 2-methoxyiminofurylacetic acid amonium salt is improved; the preparation method is simple and convenient in operation, low in cost, high in safety, and high in yield; and the quality of the prepared product meets the requirement of national standard.
Owner:SHANDONG JINCHENG PHARMACEUTICAL GROUP CO LTD +1
Optically pure alpha-ketoacyl harringtonine and preparing and purifying method thereof
The present invention relates to an optically pure alpha- ketoacyl harringtonine and a preparing and purifying method thereof. In the temperature of -80 DEG C to 50 DEG C, the alpha-ketoacyl chlorine which is prepared through reacting alpha-ketonic acid and oxalyl chloride reacts with the cephalotaxine in an inert organic solvent while the organic base is used as an acid-binding agent for obtaining the oily product represented by the formula (I). The purifying steps are as follows: dissolving the oily product with the inert organic solvent, adding the saturated NaHSO3 solution, mixing and separating the liquid; after washing the water phase with the organic solvent, adjusting the pH of the water phase with saturated NaHSO3 solution to 7-8, extracting with the organic solvent; washing the organic phase with the buffering solution with pH of 6.8 and the saturated saline solution, drying and filtering the organic phase, removing the solvent for obtaining the pale-yellow solid; and then recrystallizing with the organic dissolvent for obtaining the white solid or colorless crystal. The optically pure alpha- ketoacyl harringtonine is a key intermediate for synthesizing the medicine of harringtonine alkaloid, which is widely applied for anti-tumor (malignant tumor and benign tumor), antiparasitic, antifungal and antibacterial chemotherapy. The synthesizing method is suitable for purifying and preparing the large amount of optically pure compound represented by the structural formula of (I).
Owner:NANKAI UNIV
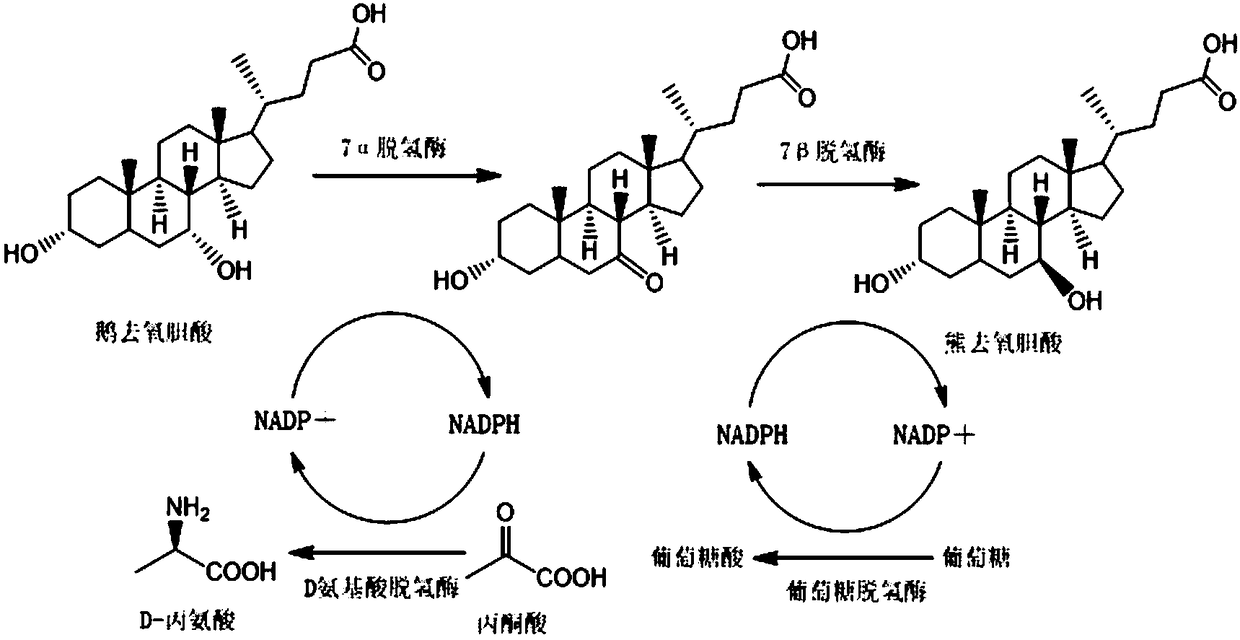
Method for synthesizing ursodeoxycholic acid and high-chiral-purity D-amino acid based on enzyme-method coupling technology
The invention discloses a method for synthesizing ursodeoxycholic acid (UDCA) and high-chiral-purity D-amino acid based on an enzyme-method coupling technology. The method comprises the following steps: putting chenodeoxycholic acid and alpha-ketonic acid into a solution system containing 7alpha-HSDH (Homoserine Dehydrogenase), DAADH and NADP (Nicotinamide Adenine Dinucleotide Phosphate) and carrying out enzyme catalysis reaction; separating a reaction solution by adopting an ultra-filtration membrane to obtain a concentrated mixed enzyme solution; regulating the pH (Potential of Hydrogen) ofa dialysis solution and crystallizing; filtering and separating to obtain 7-KLCA wet powder and filtrate; carrying out chromatographic treatment on the filtrate to obtain the D-amino acid; putting the7-KLCA wet powder into a solution system containing glucose, the NADP, the 7alpha-HSDH and GDH (Glutamate Dehydrogenase) and carrying out enzyme catalysis reaction; separating the reaction solution by adopting the ultra-filtration membrane to obtain the concentrated mixed enzyme solution; crystallizing, filtering and separating the dialysis solution, so as to obtain ursodeoxycholic acid. By adopting the method provided by the invention, UDCA and the high-chiral-purity D-amino acid can be obtained at the same time, the enzyme utilization rate is high, synthesis steps are simple and the cost isreduced; meanwhile, a metal reduction reagent and an organic solvent do not need to be added in a reaction process and conditions are mild; the method is environmentally friendly and is suitable forindustrial production.
Owner:HUNAN BAOLISHI BIOTECH
Preparation method of muscarinic receptor antagonist glycopyrronium bromide
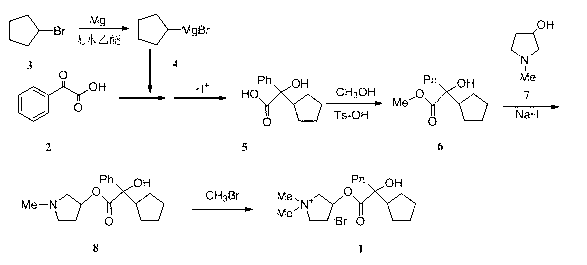
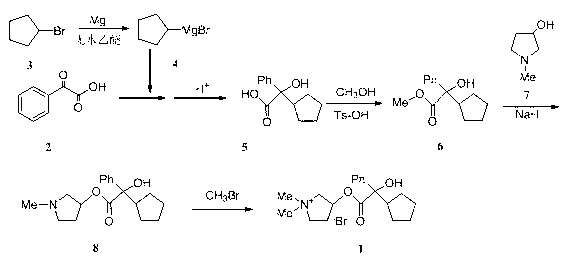
The invention belongs to the technical field of medicine and discloses a preparation method of muscarinic receptor antagonist glycopyrronium bromide. The preparation method of the muscarinic receptor antagonist glycopyrronium bromide is characterized in that Alpha-cyclopentyl mandelic acid methyl ester is obtained by aceptophenone ketonic acid Grignard reaction and esterification reaction, and then glycopyrronium bromide is obtained by N-methyl pyrrolidine-3-alcohol ester exchange and quaterisation.
Owner:SHENYANG PHARMA UNIVERSITY +1
Electrochemical catalyzed synthesis method for acyl substituted electron-deficient nitrogen-containing heterocyclic compound
ActiveCN107460497ALower internal resistanceReduce decomposition voltageElectrolysis componentsElectrolytic organic productionSynthesis methodsKetonic acids
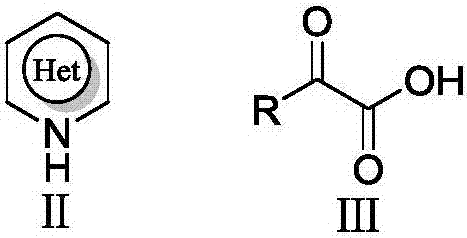
The invention relates to an electrochemical catalyzed synthesis method for an acyl substituted electron-deficient nitrogen-containing heterocyclic compound, and belongs to the technical field of acyls nitrogen-containing aromatic heterocyclic compounds. The method comprises the following steps: taking alpha-ketonic acid and an electron-deficient nitrogen-containing heterocyclic compound as raw materials in an electrolytic cell; taking halide ions as an electrocatalyst in electrolyte; carrying out electrolysis in the presence of additives, wherein the reaction temperature is 25-70 DEG C, and the current density is 1-5 mA / cm<2>; and consuming 2.0-3.5 F / mol of quantity of electric charge to obtain an acyls nitrogen-containing aromatic heterocyclic compound. According to the method, the acyls nitrogen-containing aromatic heterocyclic compound is synthesized by an electrochemical catalyzed indirect electrolyzing method which is simple to operate for the first time, use of expensive metal compounds such as silver nitrate and peroxide such as stoichiometric (NH4)2S2O8 is avoided, therefore, the atom economy can be realized, the cost is greatly reduced, operation is simplified, and the acyls nitrogen-containing aromatic heterocyclic compound is suitable for being produced industrially.
Owner:BEIJING UNIV OF TECH
Catalyst for C12-C12 fat carboxylic acid ketonization and its application
ActiveCN1765490AInhibit high temperature cokingAvoid carbonizationPreparation by hydrogenolysisCatalyst activation/preparationKetonic acidsLanthanide
The invention provides a C2-C12 aliphatic carboxylic acid ketone activator. The invention uses ª†-aluminum oxide as carrier and the single, double elements, and ternary elements of lanthanum, cerium, praseodymium and neodymium of light lanthanide series rare earth as active components. While the activating carrier method comprises (1) the heat treatment process while the treatment temperature is 480-800 Deg. C; (2) azotic acid aqueous solution method while the concentration of azotic acid aqueous solution is 5-45%; (3) evacuation method. In addition, the activator is prepared via the immersion method while it can be used to C2-C12 aliphatic carboxylic acid ketone.
Owner:PETROCHINA CO LTD
Method for synthetizing chiral cyclic alkyl amino acid by amino transferase
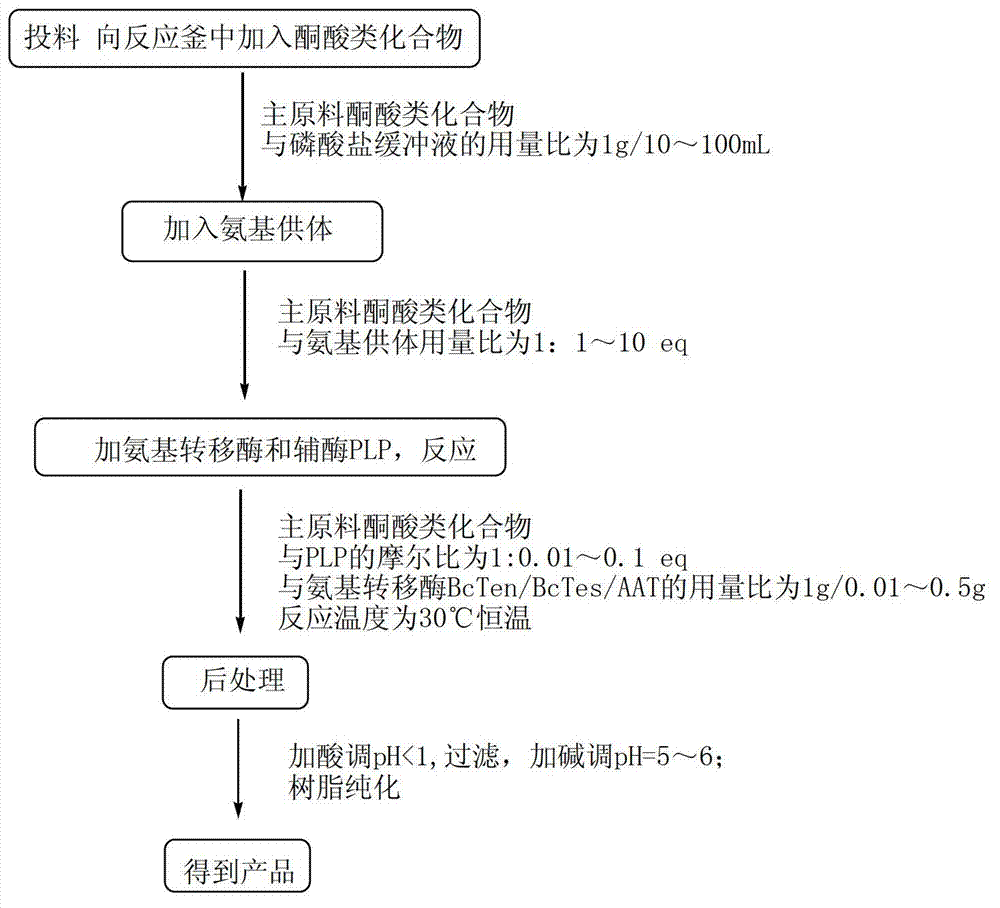
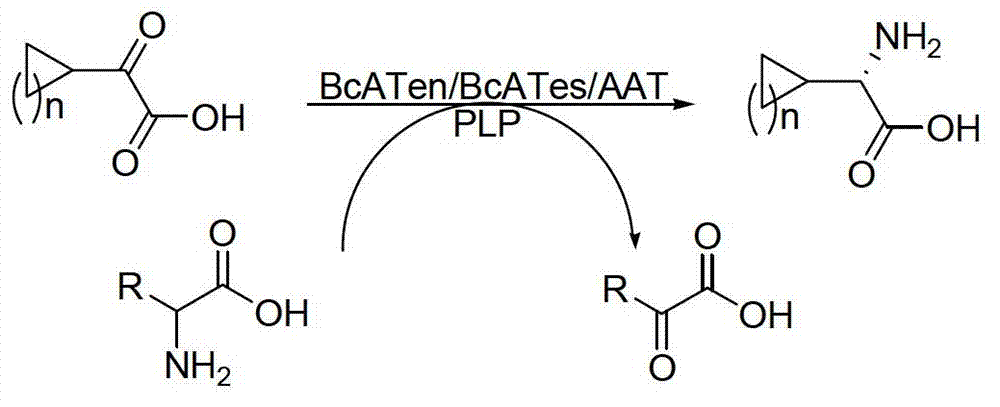
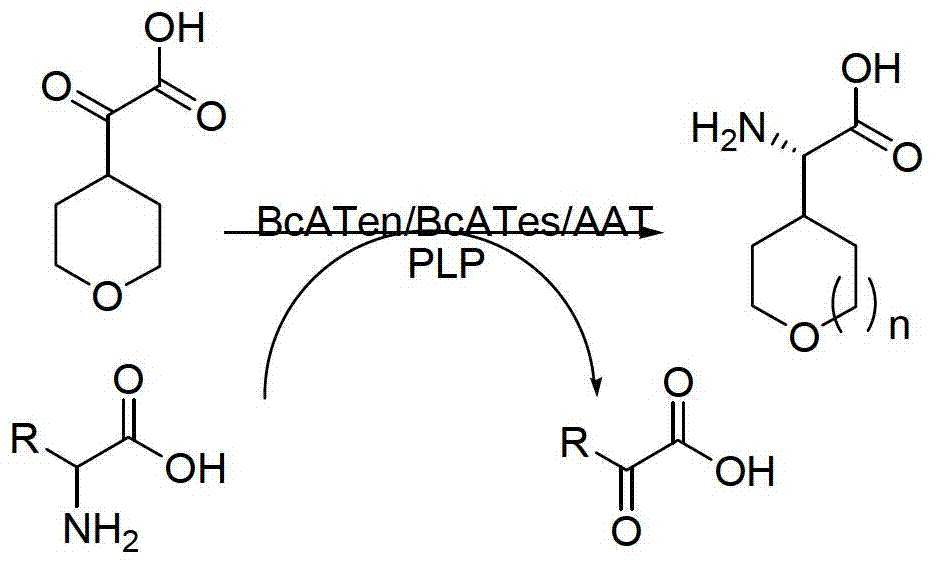
The invention discloses a method for synthetizing chiral cyclic alkyl amino acid by amino transferase. The commercialized material ketonic acid or corresponding soluble ketonic acid salt compound in the market is selected as an initial material; the initial material is dissolved into phosphate buffer, and added to an amino supply body; pyridoxal phosphate (PLP) and amino transferase main enzyme are added to a system containing the amino supply body and main material ketonic acid or corresponding soluble ketonic acid salt compound to react under constant temperature, and obtaining a product with a high ee value, wherein n is equal to 1, 2, 3, 4, 5, or obtaining a product wherein n is equal to 0 and 1. The method is stable in technological condition, simple to operate, high in yield, low in cost, and suitable for large-scale production, and beneficial for environmental protection; and a novel train of thought and a method are provided for the preparation of chiral cyclic alkyl amino acid compound.
Owner:ASYMCHEM LAB TIANJIN +5
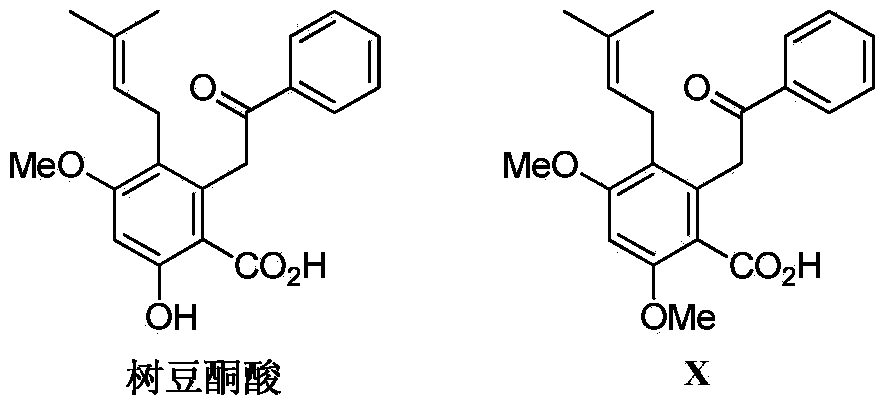
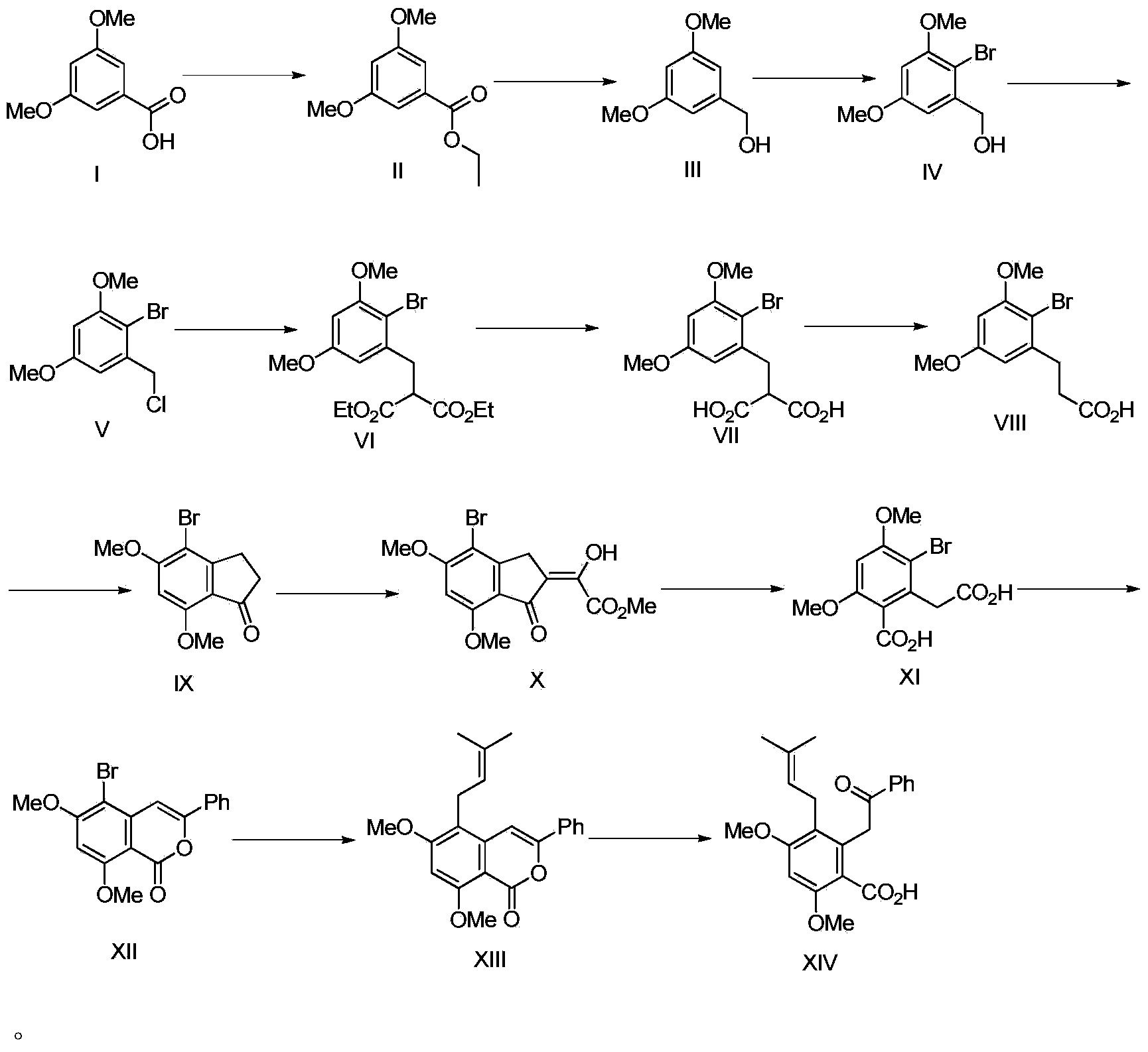
Synthesis method of diethylstilbestrol compound methyl pigeon pea ketonic acid A
ActiveCN103772189AGet efficientlyMass synthesisOrganic compound preparationCarboxylic acid esters preparationAlkyl transferKetonic acids
The invention discloses a synthesis method of diethylstilbestrol compound methyl pigeon pea ketonic acid A. The synthesis method comprises the steps: esterifying a compound with a formula (I) to generate a compound with a formula (II); reducing the compound with the formula (II) to generate a compound with a formula (III); carrying out substitution on the compound with the formula (III) to generate a compound with a formula (IV); carrying out substitution on the compound with the formula (IV) to generate a compound with a formula (V); carrying out alkylation on the compound with the formula (V) to generate a compound with a formula (VI); carrying out esterolysis on the compound with the formula (VI) to generate a compound with a formula (VII); carrying out decarboxylation on the compound with the formula (VII) to generate a compound with a formula (VIII); carrying out dehydration cyclization on the compound with the formula (VIII) to generate a compound with a formula (IX); carrying out condensation on the compound with the formula (IX) to generate a compound with a formula (X); carrying out five-membered ring breakage on the compound with the formula (X) to generate a compound with a formula (XI); carrying out condensation on the compound with the formula (XI) to generate a compound with a formula (XII); carrying out coupling on the compound with the formula (XII) to generate a compound with a formula (XIII); carrying out esterolysis on the compound with the formula (XIII) to generate a compound with a formula (XIV), namely methyl pigeon pea ketonic acid A.
Owner:山东龙辰药业有限公司
Catalyst for preparing acetone through gas phase catalytic ketonization of acetic acid and preparation method and application of catalyst
ActiveCN104174397AMild reaction conditionsHigh selectivityOrganic compound preparationCarbonyl compound preparationActivated carbonAcetic acid
The invention discloses a catalyst for preparing acetone through gas phase catalytic ketonization of acetic acid. The catalyst is characterized by consisting of the following components in percentage by weight: 5-40 percent of activated carbon, 55-85 percent of oxide carrier and 3-25 percent of active component oxide. The catalyst disclosed by the invention has the advantages of simple preparation method, low cost, high activity and high selectivity.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI +1
Method for preparing unsymmetrical rhodamine
InactiveCN102321063AHigh fluorescence intensityImprove stabilityOrganic chemistryAzo dyesM-aminophenolKetonic acids
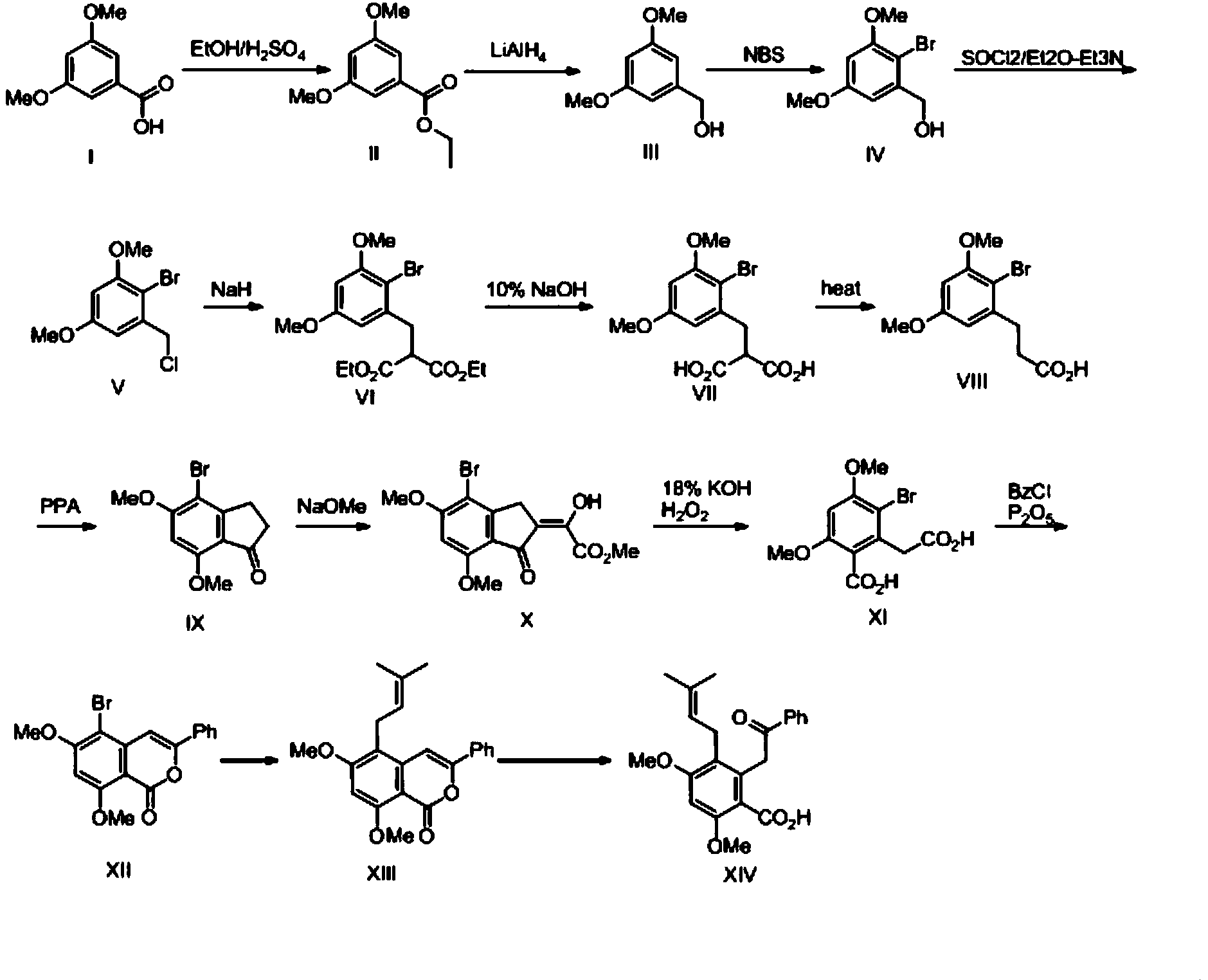
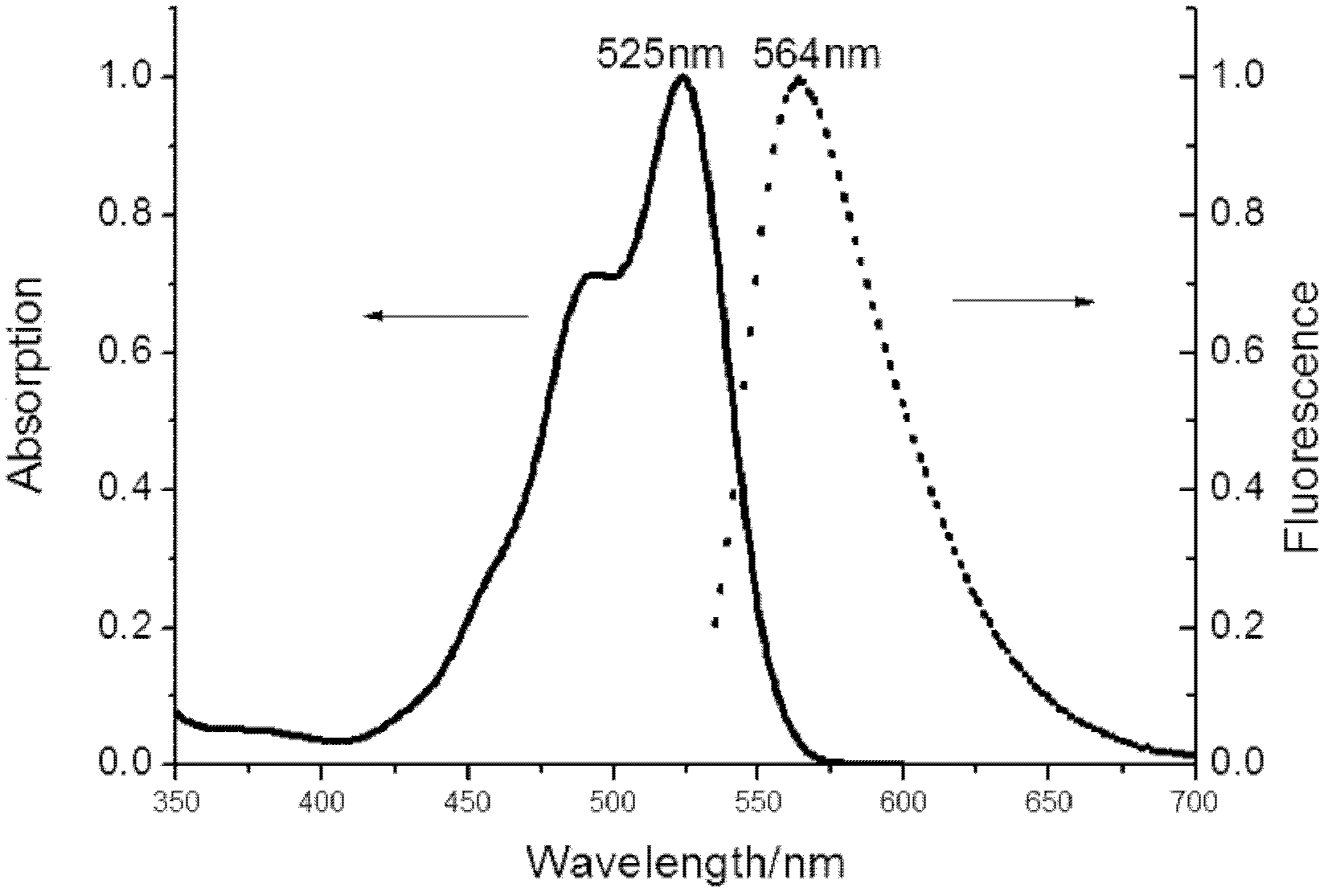
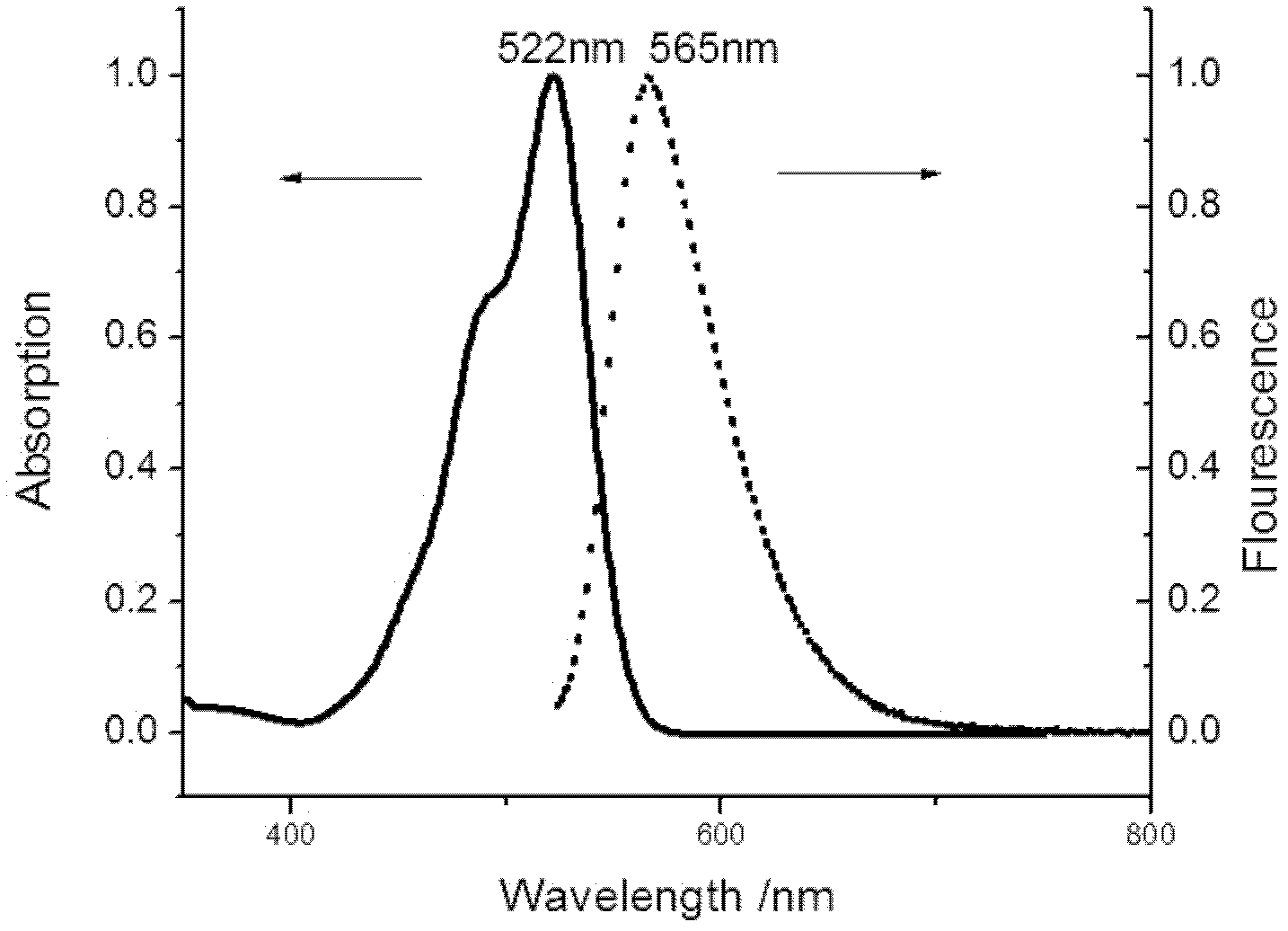
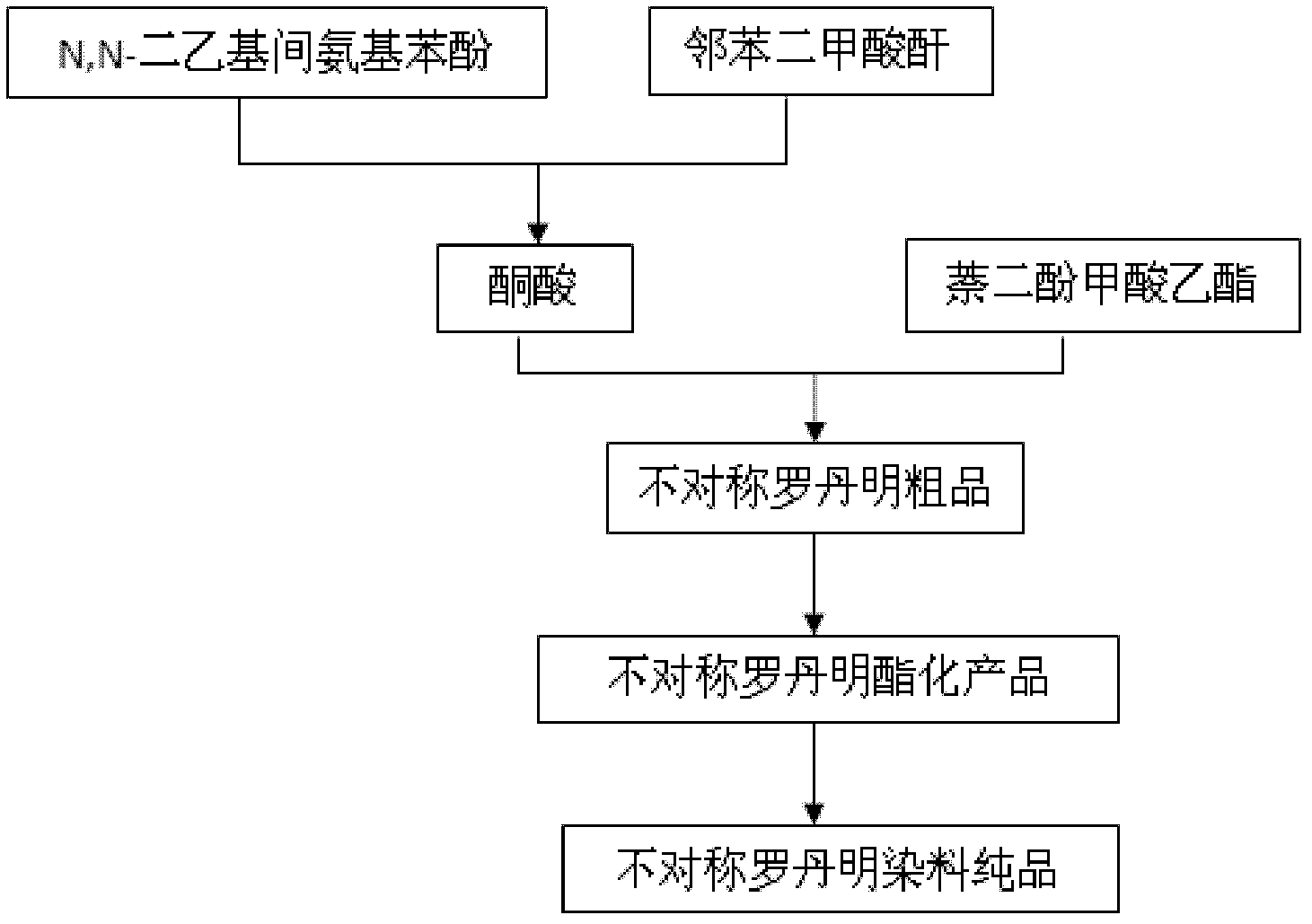
The invention relates to a method for preparing unsymmetrical rhodamine. The method comprises the following steps of: reacting N,N-diethyl m-aminophenol with phthalic anhydride to prepare an intermediate ketonic acid; reacting the ketonic acid with the corresponding binaphthol structure under the action of a catalyst to generate the unsymmetrical rhodamine; directly performing ethyl esterification on the crude products and performing column chromatography; and recrystallizing to obtain unsymmetrical rhodamine dye which meets the requirements and has different structures. Compared with the prior art, the method has the advantages of low requirement on equipment, low production cost and high production efficiency and is easy to operate. The obtained novel rhodamine has good fluorescence property and high stability and can be widely applied.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Application of pigeon pea ketonic acid A in terms of preparation of medicines for accompanied diseases of diabetes mellitus and hyperlipidaemia
ActiveCN102670576ALower cholesterol levelsNormal blood sugarOrganic active ingredientsMetabolism disorderSerum triglyceride levelsKetonic acids
The invention discloses application of pigeon pea ketonic acid A in terms of preparation of medicines for accompanied diseases of diabetes mellitus or hyperlipidaemia. Animal experiment data show that the pigeon pea ketonic acid A can remarkably reduce the serum triglyceride level, the total cholesterol level and the low-density lipoprotein cholesterol level of SD (Sprague-Dawley) rats with type-2 diabetes mellitus, and can treat or improve the hyperlipidaemia as an accompanied disease of the diabetes mellitus; and pathological research results prove that the pigeon pea ketonic acid A can reduce spotty necrosis of hepatocytes, can treat or improve liver injury as an accompanied disease of the diabetes mellitus, reverses injury to tissues of pancreas inlets of the rats due to glucoses, and has protection and repair effects on pancreas. The pigeon pea ketonic acid A can remarkably reduce the serum triglyceride level and the total cholesterol level of Zucker rats with congenital obesity, and is proved to be capable of treating or improving the hyperlipidaemia.
Owner:GUANGZHOU YUNZHONG BIOTECH
High-density potassium salt-resistant cementing slurry and preparation method for same
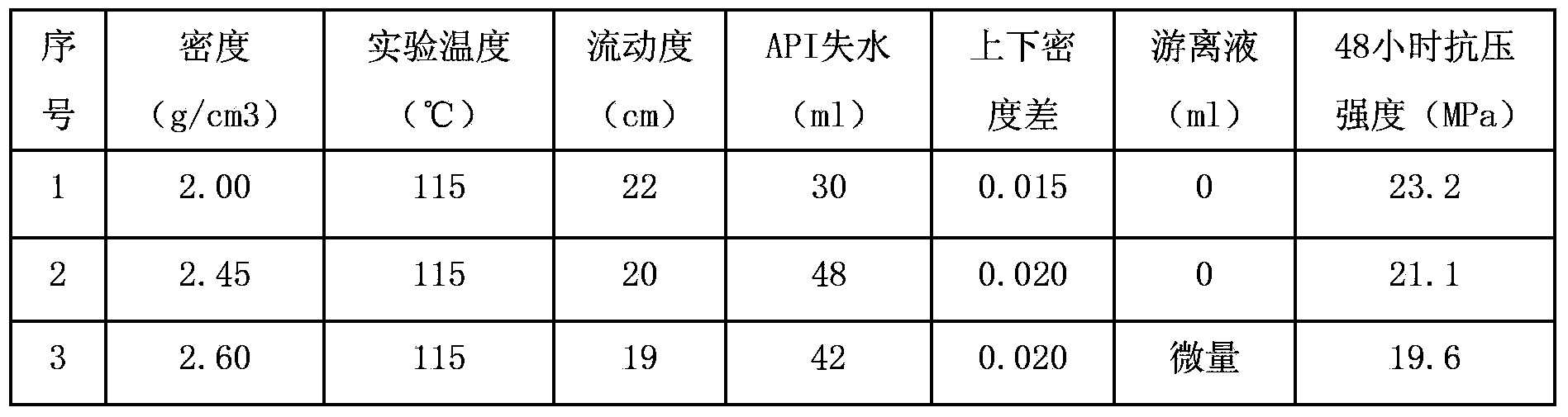
ActiveCN103450862ASimple on-site operationImprove liquidityDrilling compositionMicro nanoKetonic acids
The invention provides a high-density potassium salt-resistant cementing slurry applied to high-pressure oil-gas wells and high-pressure potassium salt well cementing construction. The high-density potassium salt-resistant cementing slurry is prepared from the raw materials in percentage by mass: 29-58% of Jiahua grade-G cement, 12-56% of a weighting agent, 1.8-3.5% of active micro-nano silicon, 10-20% of 80-mesh quartz sand, 1-2% of a salt-resistant dispersing agent, 1.8-4% of a salt-resistant fluid loss agent, and 0.3-1.0% of salt-resistant retarder, wherein the weighting agent is a mixture of 250-mesh hematite and 1200-mesh hematite, the salt-resistant dispersing agent is a formaldehyde / acetone copolymer, the salt-resistant fluid loss agent is the polymer of N,N-dimethacrylamide and maleic anhydride, and the retarder is the compound of ketonic acid and organic phosphate; the density of the cementing slurry is 2.0-2.6 g / cm<3>, the fluidity of the cementing slurry is 19-21 cm, the API (American Petroleum Institute) fluid loss of the cementing slurry is not greater than 50 ml, the rheological property n of the cementing slurry is not less than 0.5, and K is not greater than 2.
Owner:中石化江汉石油工程有限公司钻井一公司
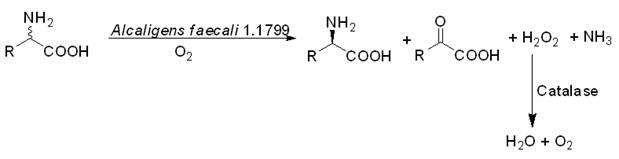
Biological catalysis method for preparing D-amino acid through deracemizing DL-amino acid
InactiveCN102586384AHigh optical purityHigh chemical purityMicroorganism based processesFermentationPtru catalystKetonic acids
The invention provides a biological catalysis method for preparing D-amino acid through deracemizing DL-amino acid. According to the method, manure production base coli cells subjected to permeability are used as a biological catalyst, L-amino acid oxidase in the biological catalyst is utilized to oxidize and denitrify the specificity of L-antipode into corresponding ketonic acid under the existence of air, and D-antipode is retained; and meanwhile, hydrogen peroxide generated in a decomposition reaction process of co-expressed catalase is utilized. The method avoids derivation and derivation removal steps of a traditional split method, has the characteristics of simple process, low cost, high product optical purity and good environmental protection and is suitable for the industrial production of the D-amino acid.
Owner:CHONGQING CHIRAL BIOCATALYSIS TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com