Method for synthesizing coumarin and derivatives thereof under catalysis of choline ionic liquids
A technology of ionic liquids and phenolic compounds, which is applied in the field of synthesis of coumarin and its derivatives, can solve the problems of expensive catalysts, cumbersome experimental treatment process, and environmental hazards, and achieve easy separation, good catalytic effect, and reaction conditions simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
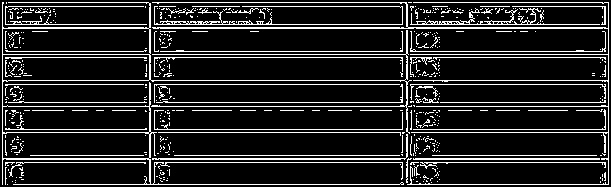
[0019] Effect of Different Catalysts on Pechmann Reaction
[0020] Based on the reaction of resorcinol and ethyl acetoacetate, different choline ionic liquids are used to catalyze the reaction. The ratio of the amount of choline ionic liquid to the amount of resorcinol is 1:1 , the temperature of the oil bath was controlled at 90°C, no solvent was added, and the progress of the reaction was followed by TLC to optimize a suitable ionic liquid. The experimental results are shown in Table 1. It can be seen from Table 1 that [DMEA][HSO in entry1 4 ] has the best catalytic effect, and the yield can reach 87% after 1.5h of reaction.
[0021] Table 1 Catalytic effects of different choline-based ionic liquids on the Pechmann reaction
[0022]
Embodiment 2
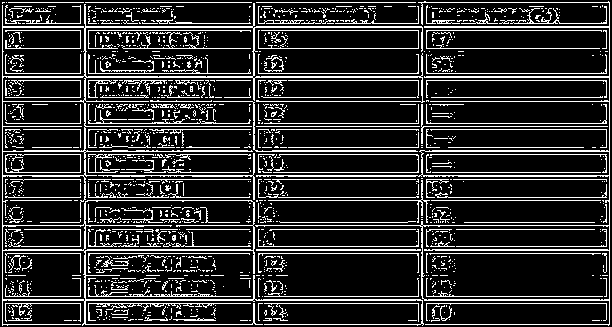
[0024] Effects of Catalyst Consumption and Reaction Temperature on Pechmann Reaction
[0025] With resorcinol and ethyl acetoacetate as the reaction raw materials, the choline-based ionic liquid [DMEA][HSO 4 ] The conditions such as the dosage and temperature are optimized, and the results are shown in Table 2. First, we optimized the amount of choline ionic liquid in the range of 0.02-1.5 mmol, and found that when the amount of catalyst was in the range of 0.05-1.5 mmol, with the increase of the amount of choline ionic liquid, the yield of the target product was reversed. Decrease, continue to reduce the amount of choline-based ionic liquid to 0.02mmol (entry8), the yield dropped to 90%, which shows that the most appropriate when the amount of choline-based ionic liquid is 0.05mmol. The reaction was carried out at 25°C, 60°C, 90°C, and 120°C. At 25°C, no product was produced for 4 hours; at 60°C, for 5.5 hours, the yield was only 75%; at 120°C, Solids were quickly formed in...
Embodiment 3
[0029]Expansion of reaction substrates
[0030] According to the above optimization results, we choose to synthesize coumarin and its derivatives by Pechmann reaction under the optimal conditions. As can be seen from Table 3, in the choline-based ionic liquid [DMEA] [HSO 4 ] Under the condition of catalyst, monohydric phenol, dihydric phenol and trihydric phenol can all undergo Pechmann condensation reaction with ethyl acetoacetate, among which resorcinol has the highest activity. Due to the low activity of monohydric phenols, in [DMEA][HSO 4 ] almost no reaction when used as a catalyst, so the reaction (entry1-3) was carried out with substrates containing amino, methoxy and other strong electron-donating substituents, and the yields were 50% and 70% respectively. In addition, the activity of phenols is related to the position of substituents, such as p-aminophenol, p-hydroxyanisole (entry4, 5) does not react with ethyl acetoacetate; the electron-withdrawing substituents con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com