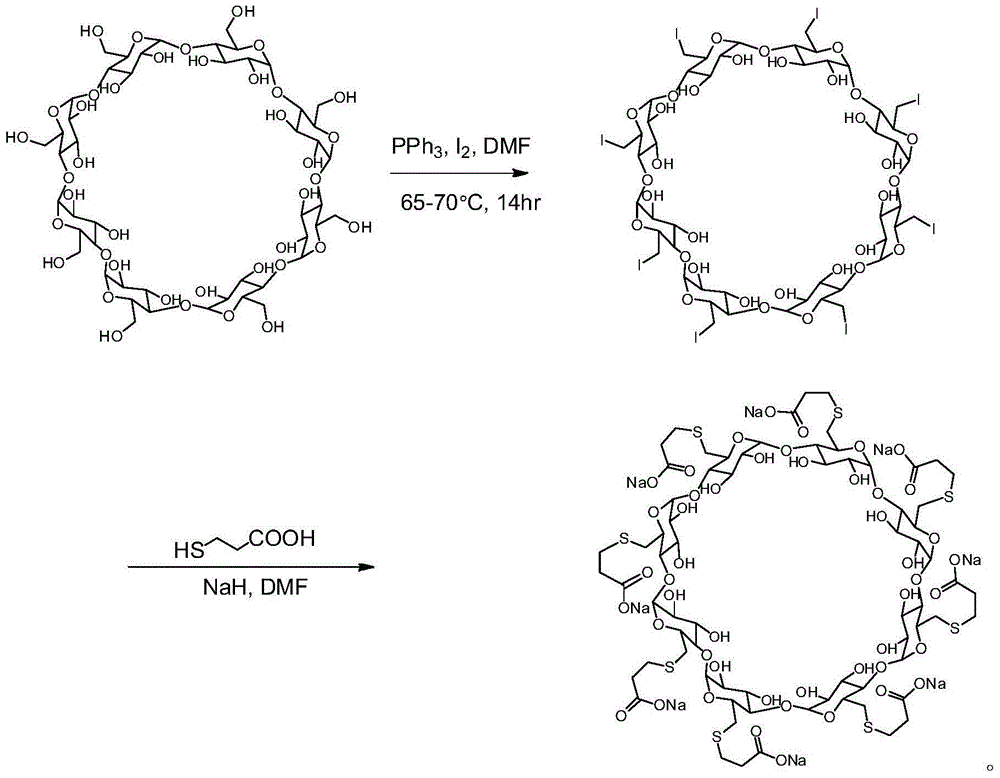
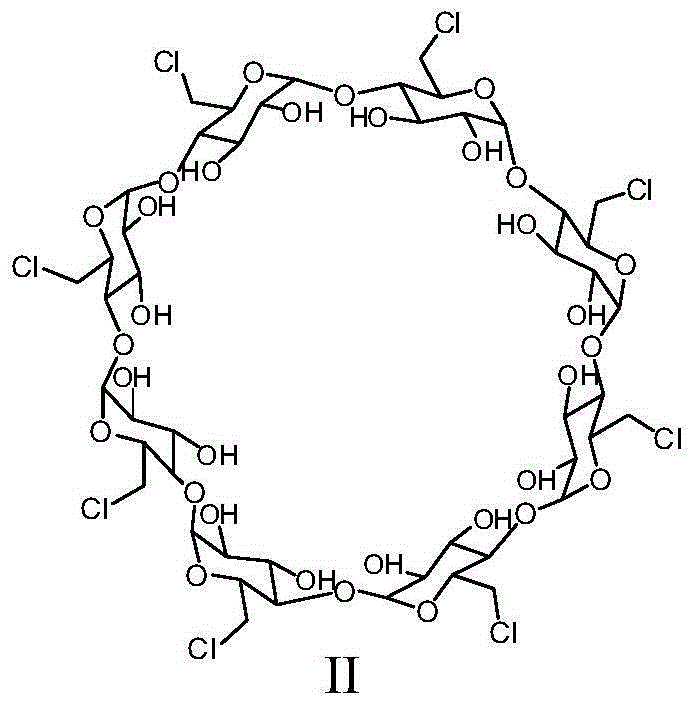
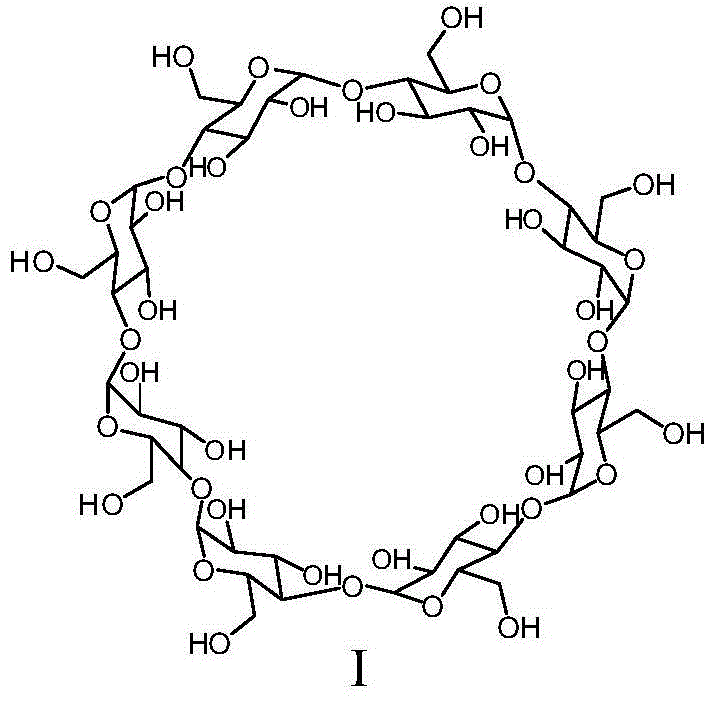
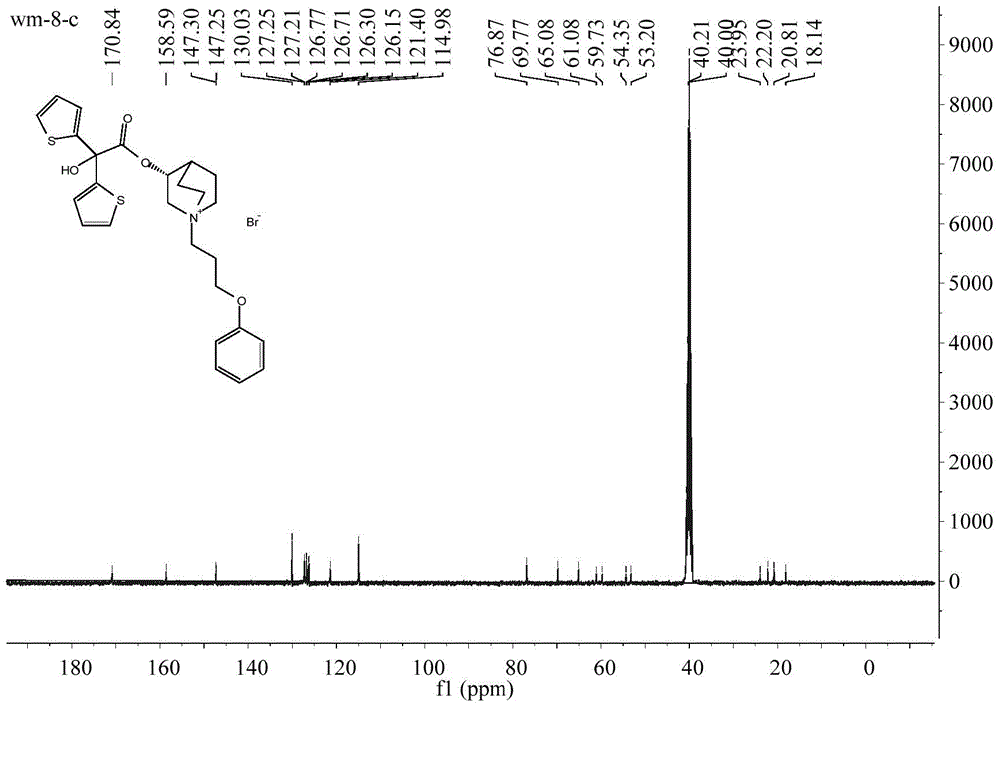
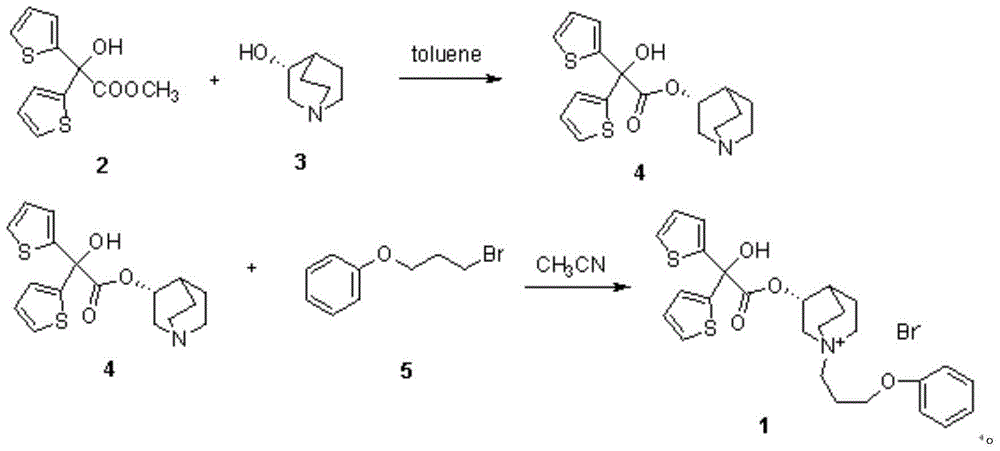
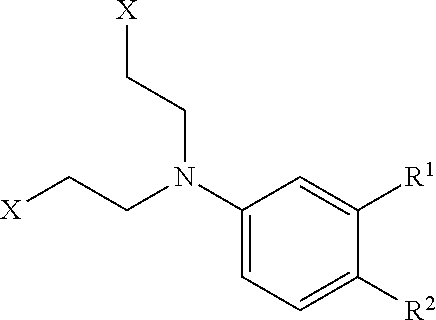
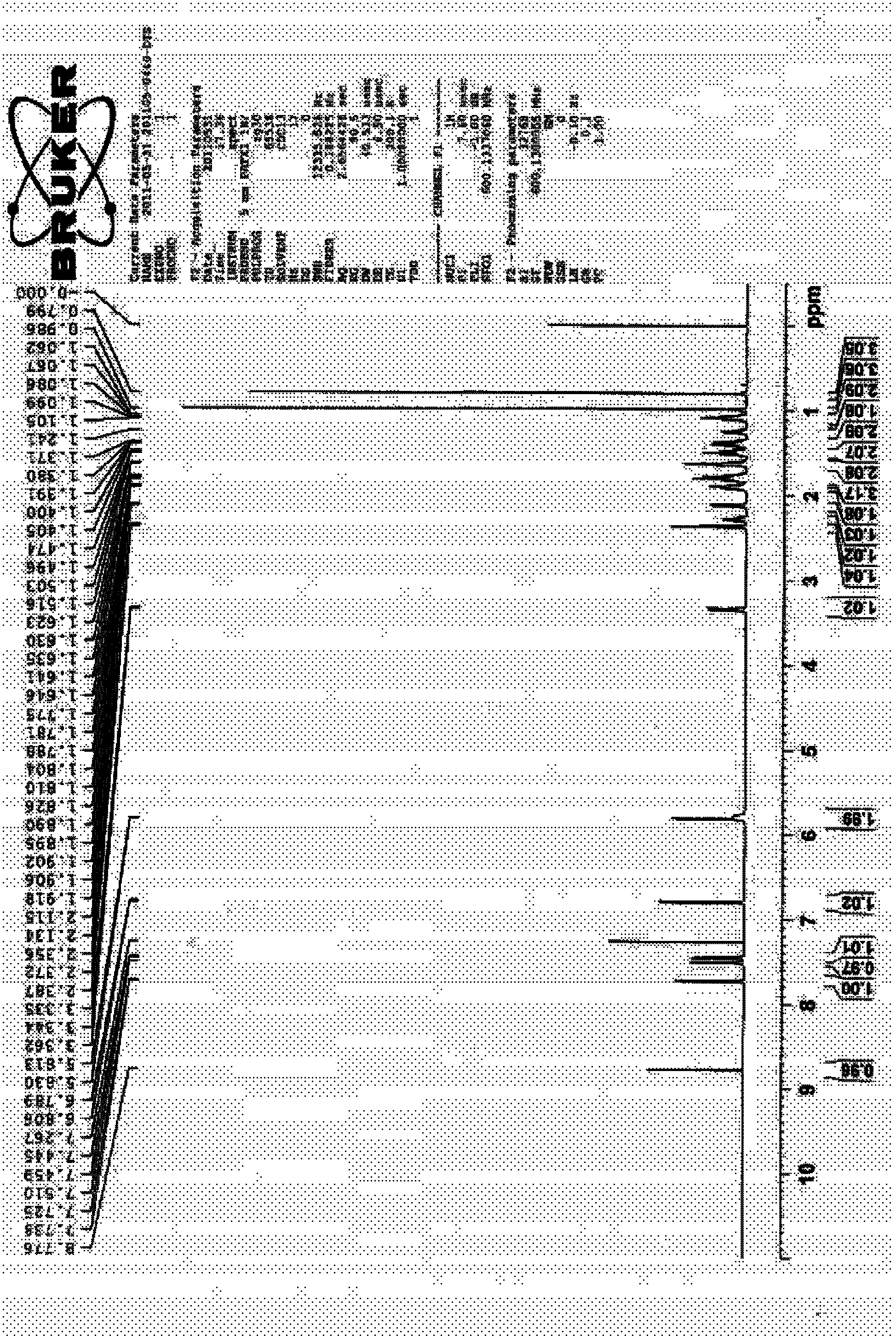
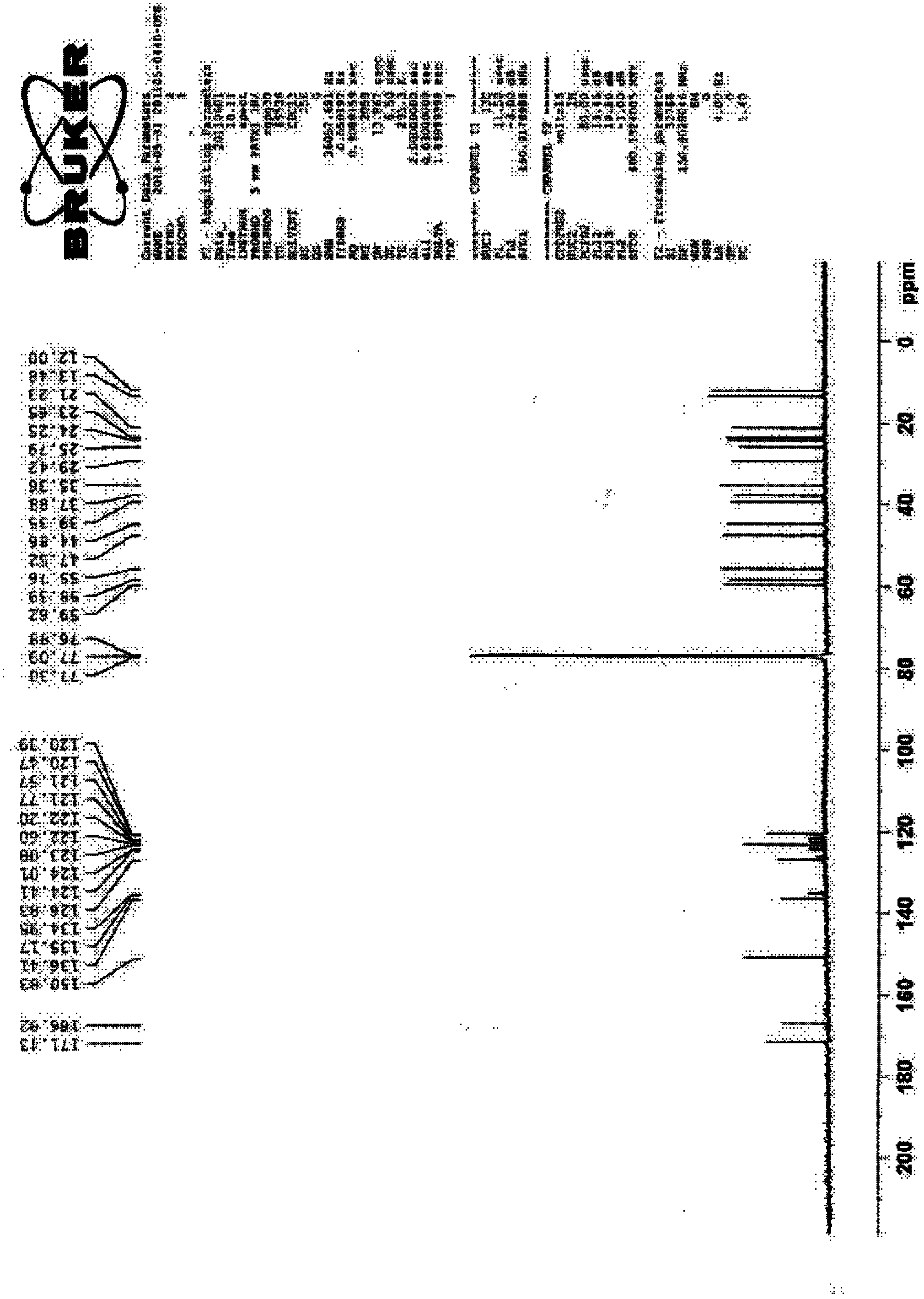
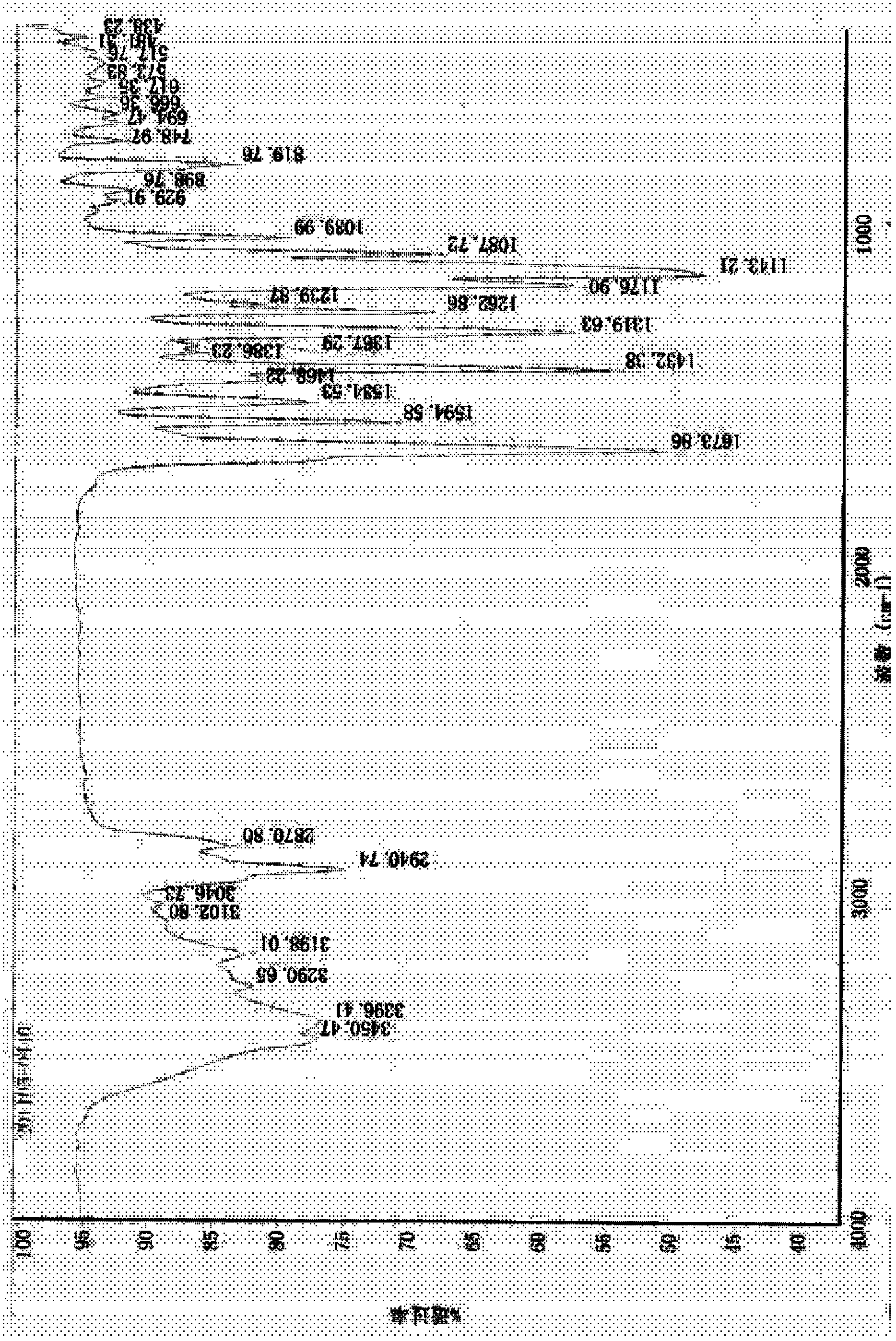
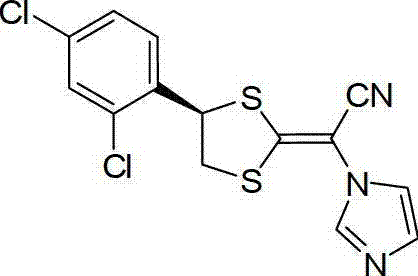
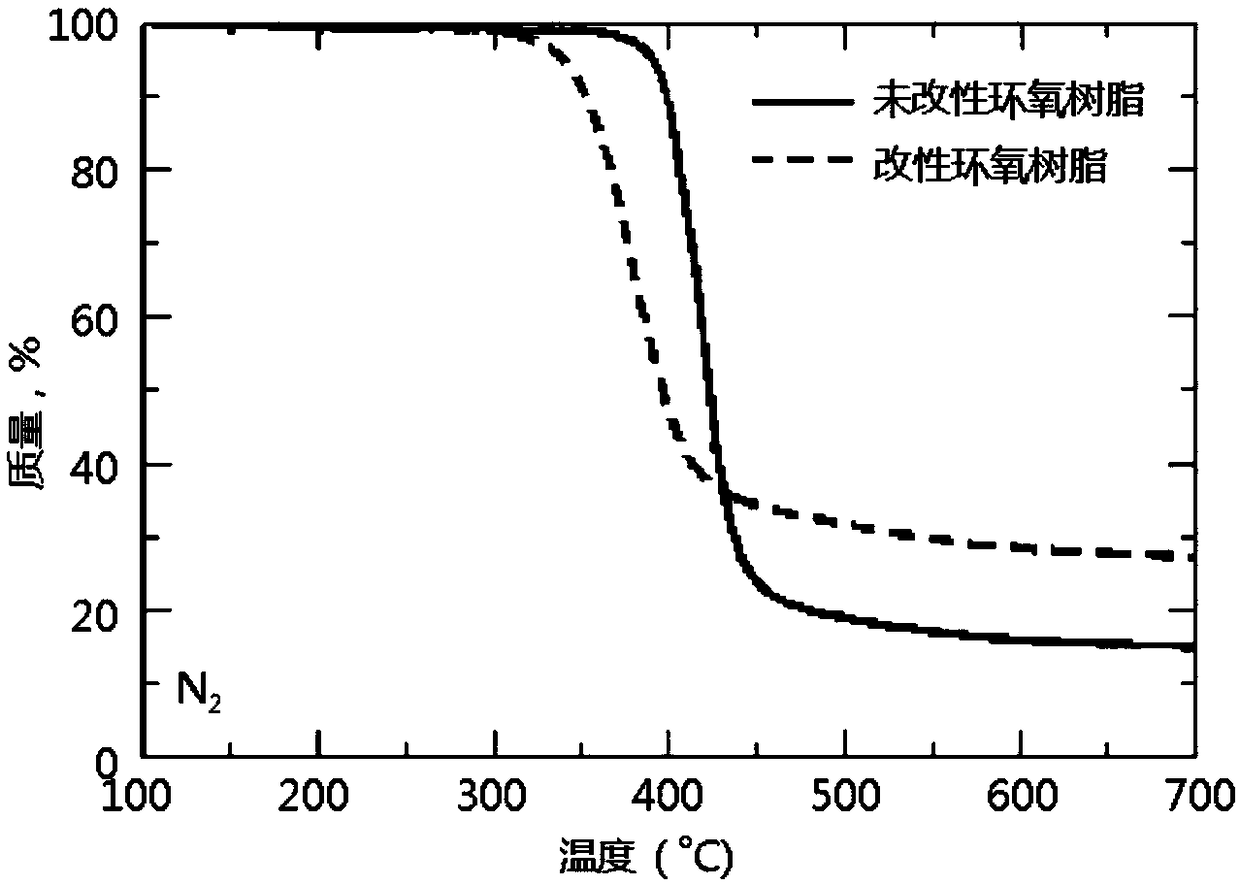
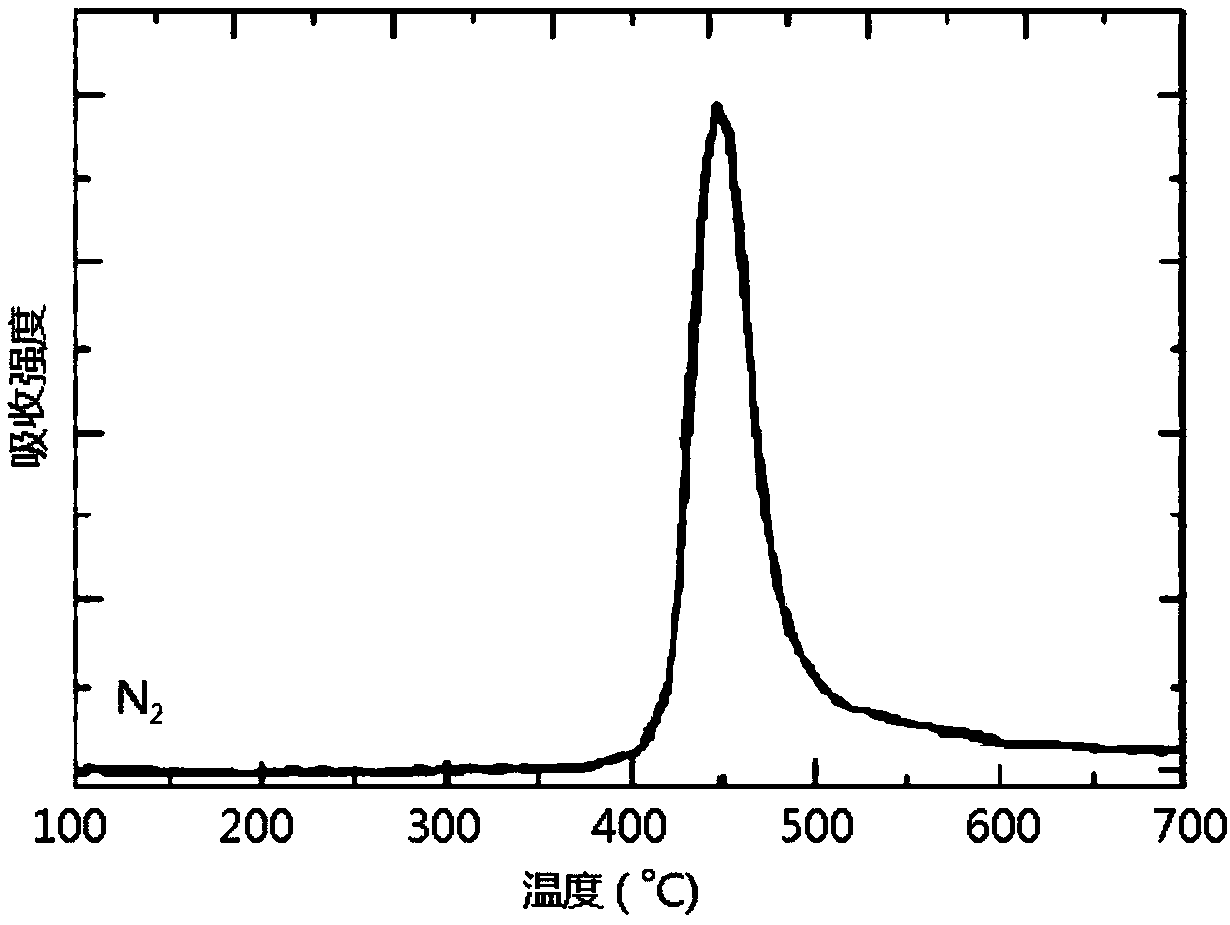
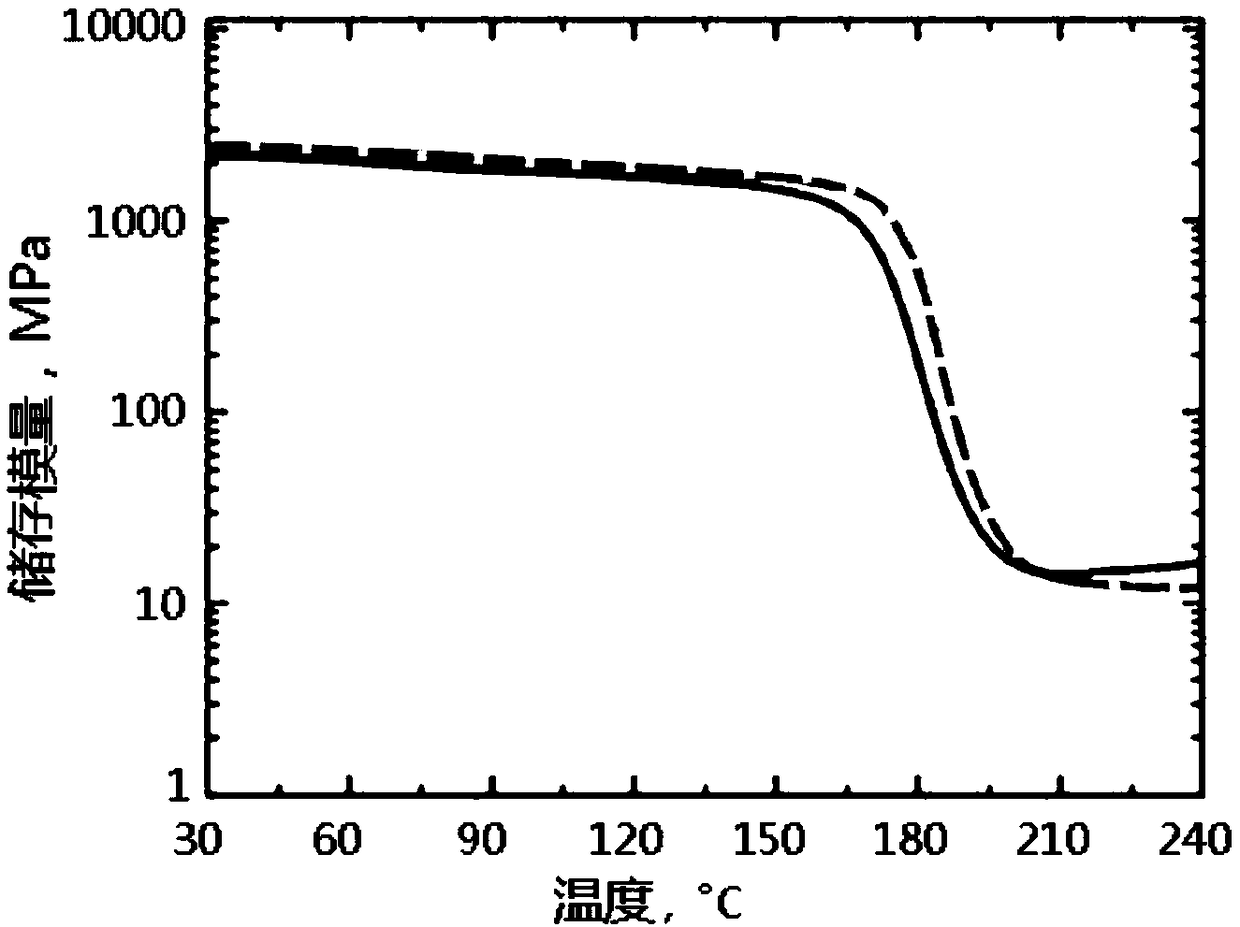
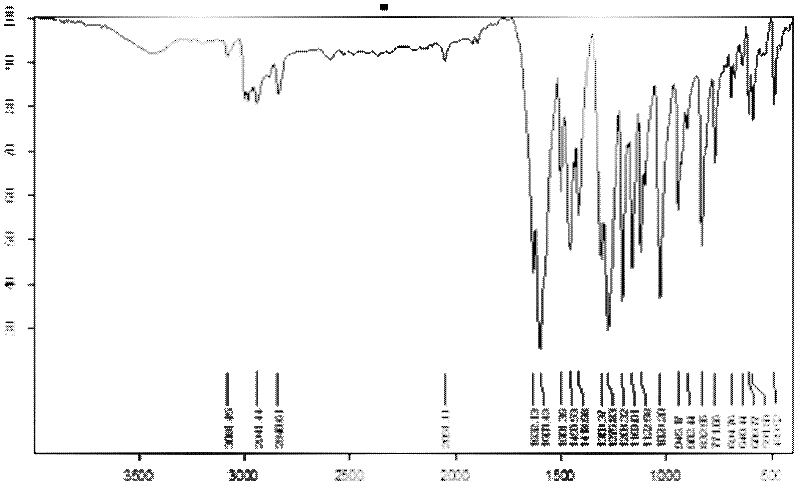
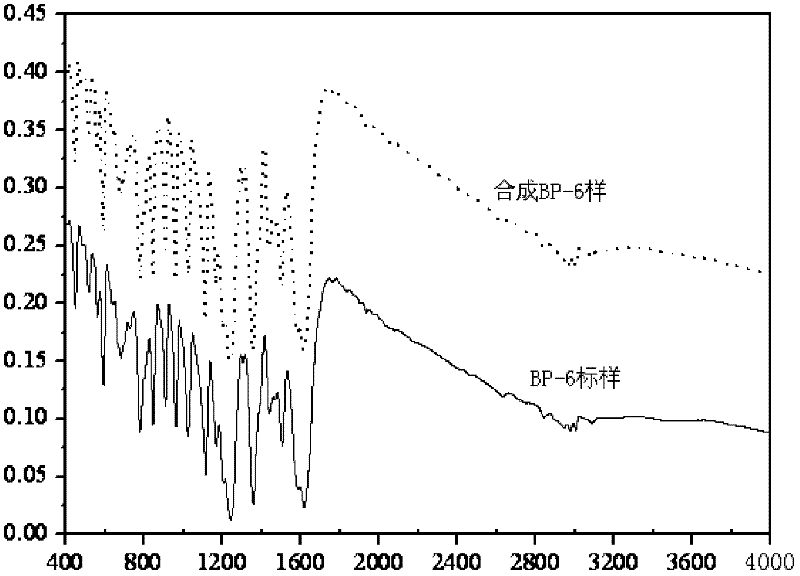
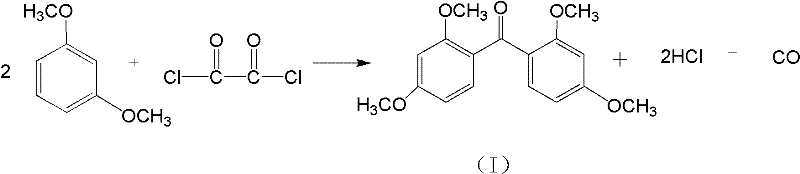
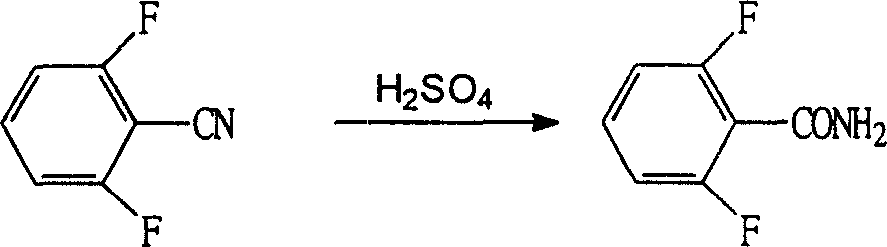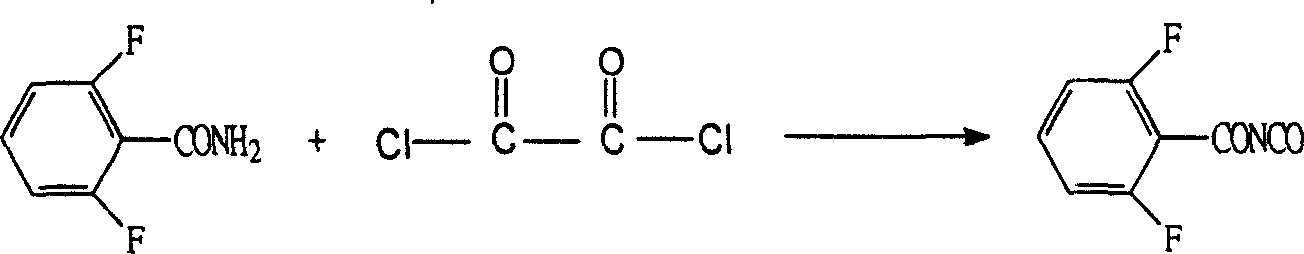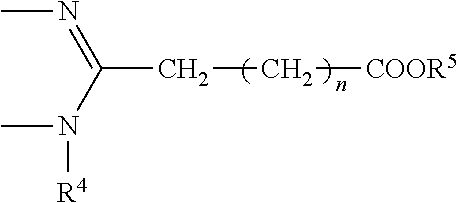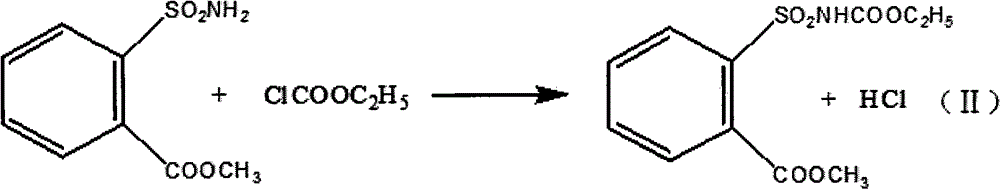Patents
Literature
392 results about "Oxalyl chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oxalyl chloride is a chemical compound with the formula (COCl)₂. This colourless, sharp-smelling liquid, the diacyl chloride of oxalic acid, is a useful reagent in organic synthesis. It can be prepared by treating oxalic acid with phosphorus pentachloride.
Preparation method for sugammadex and intermediates thereof
The invention provides a preparation method for sugammadex and intermediates thereof. The method comprises a step that a compound in the formula (I) and a chloride agent are subjected to a chlorination reaction, wherein the chloride agent is selected from thionyl chloride and oxalyl chloride. The formula (I) is shown in the specification. The method is advantaged by simple technology process, high yield, high purity and no pollution, and is suitable for industrial production.
Owner:SICHUAN HAISCO PHARMA CO LTD
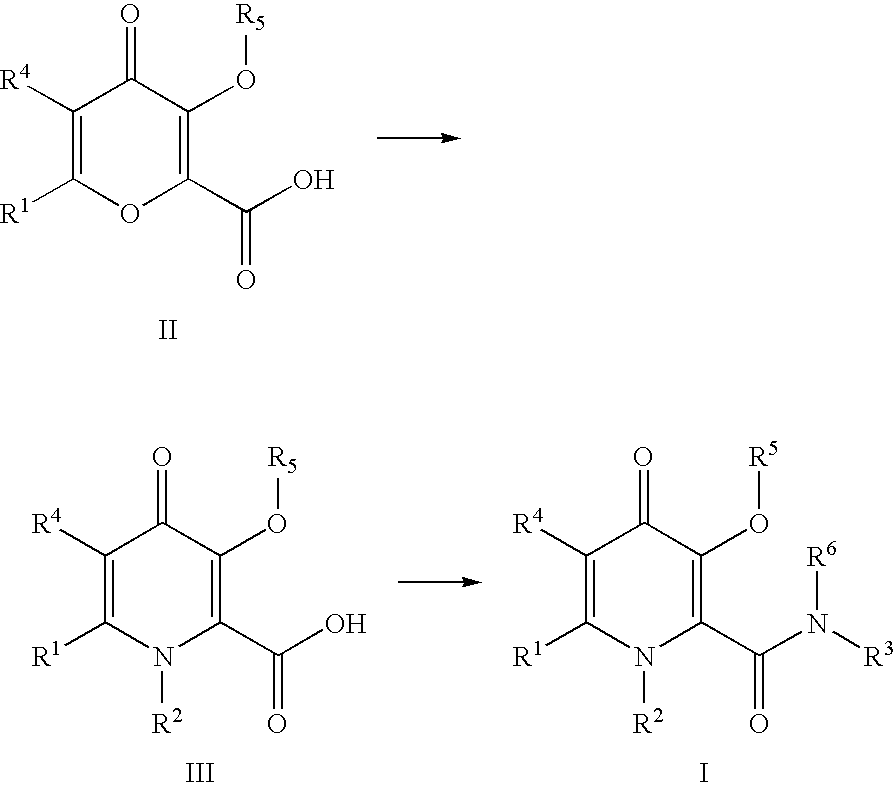
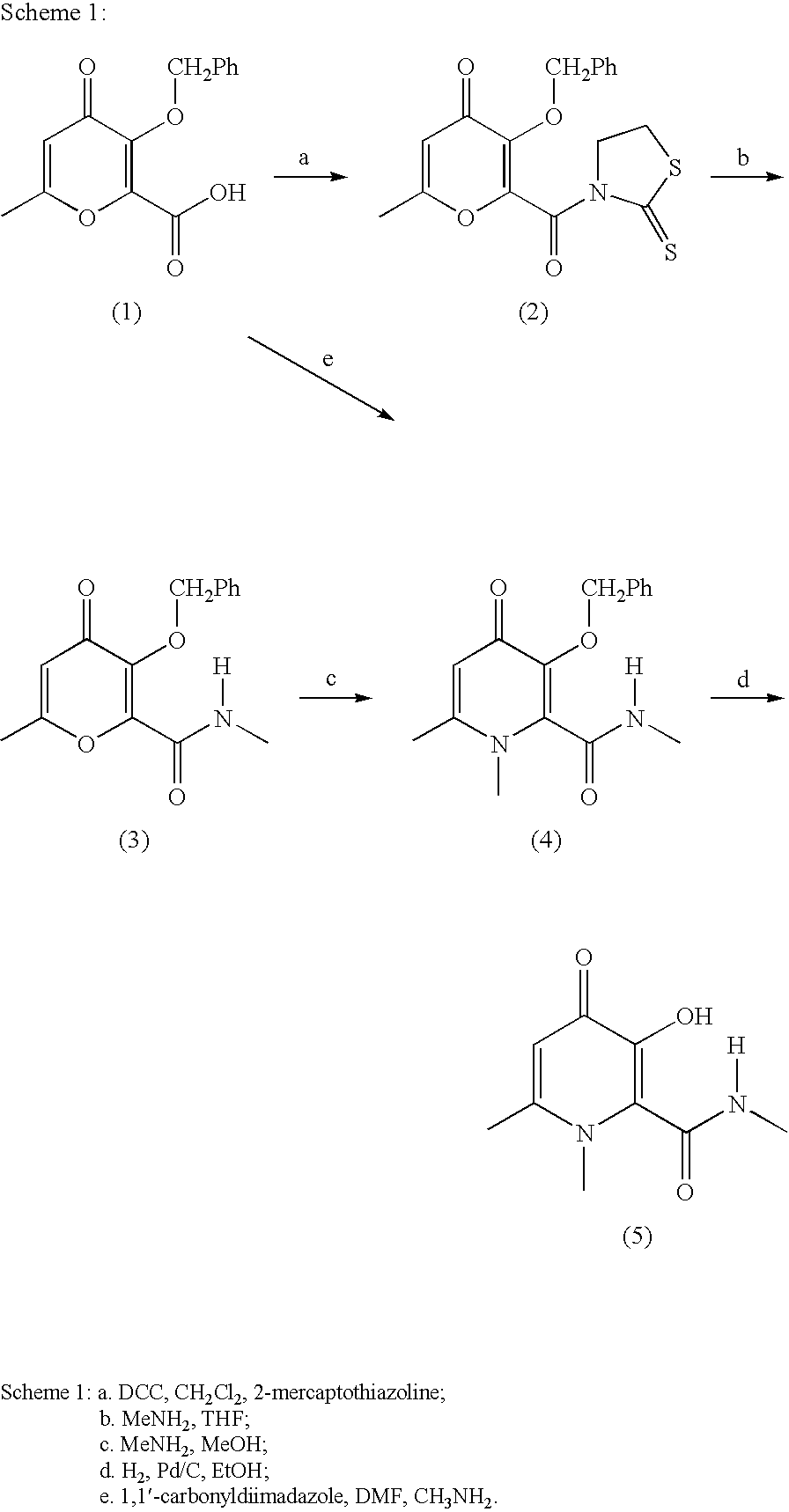
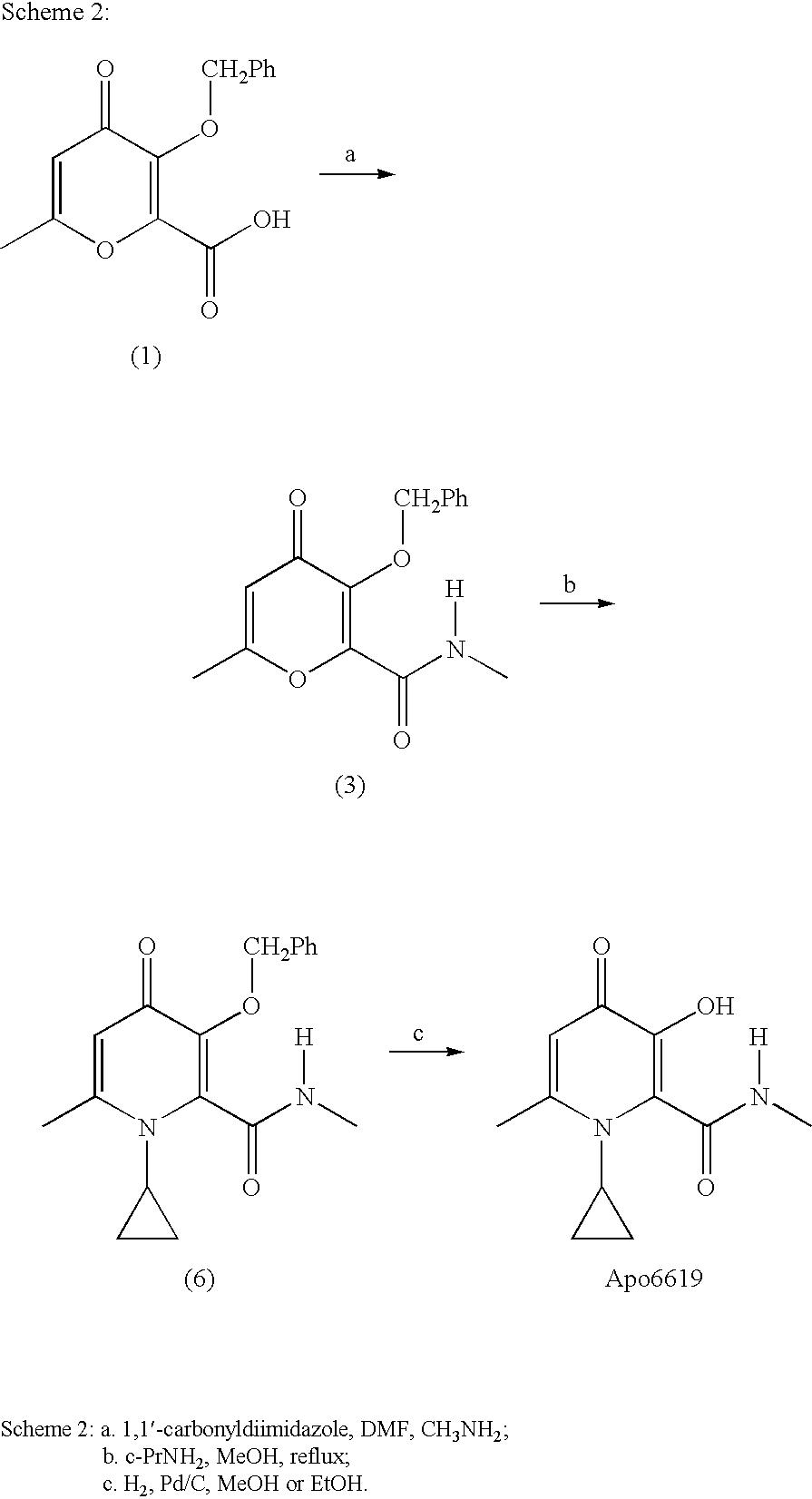
Process For The Manufacture Of 3-Hydroxy-N-Alkyl-1-Cycloalkyl-6-Alkyl-4-Oxo-1,4-Dihydropyridine-2-Carboxamide And Its Related Analogues
InactiveUS20080096886A1Removes costly aspectReduce the amount requiredBiocideOrganic chemistryOxalyl chlorideAmmonium chloride mixture
The present invention relates to a novel process for the preparation of 1-alkyl or 1-cycloalkyl derivatives of 3-hydroxy-4-oxo-1,4-dihydropyridine-2-carboxamide of formula I. The process includes reacting an amine R2NH2 with a compound of formula II in a solution of metal hydroxide in water to give a compound of formula III. Subsequent reaction of the compound of formula III with an acid chloride formation reagent in an inert solvent gives compounds of formula I. The acid chloride formation reagent is selected from oxalyl chloride and dimethylformamide, dimethylchloromethylene-ammonium chloride and thionyl chloride and dimethylformamide. If desired, a compound of formula I where R5 is hydrogen may be formed when an intermediate substituent is used wherein R5 is an alcohol protective group removable by catalytic hydrogenation.
Owner:APOTEX INC
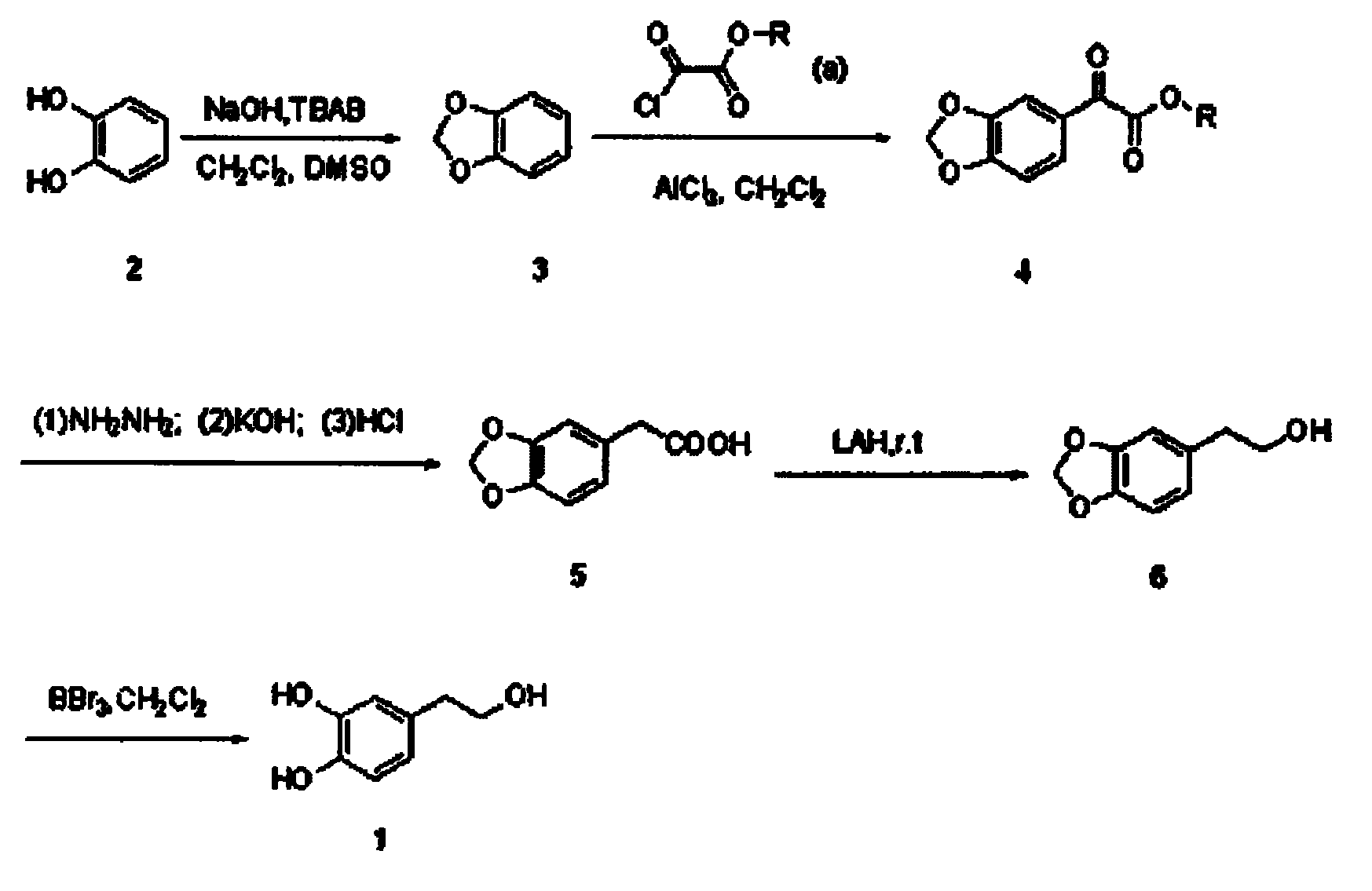
Synthetic method of hydroxytyrosol
InactiveCN103664536AMild reaction conditionsLower reaction costOrganic chemistryOrganic compound preparationChemical synthesisHydroxytyrosol
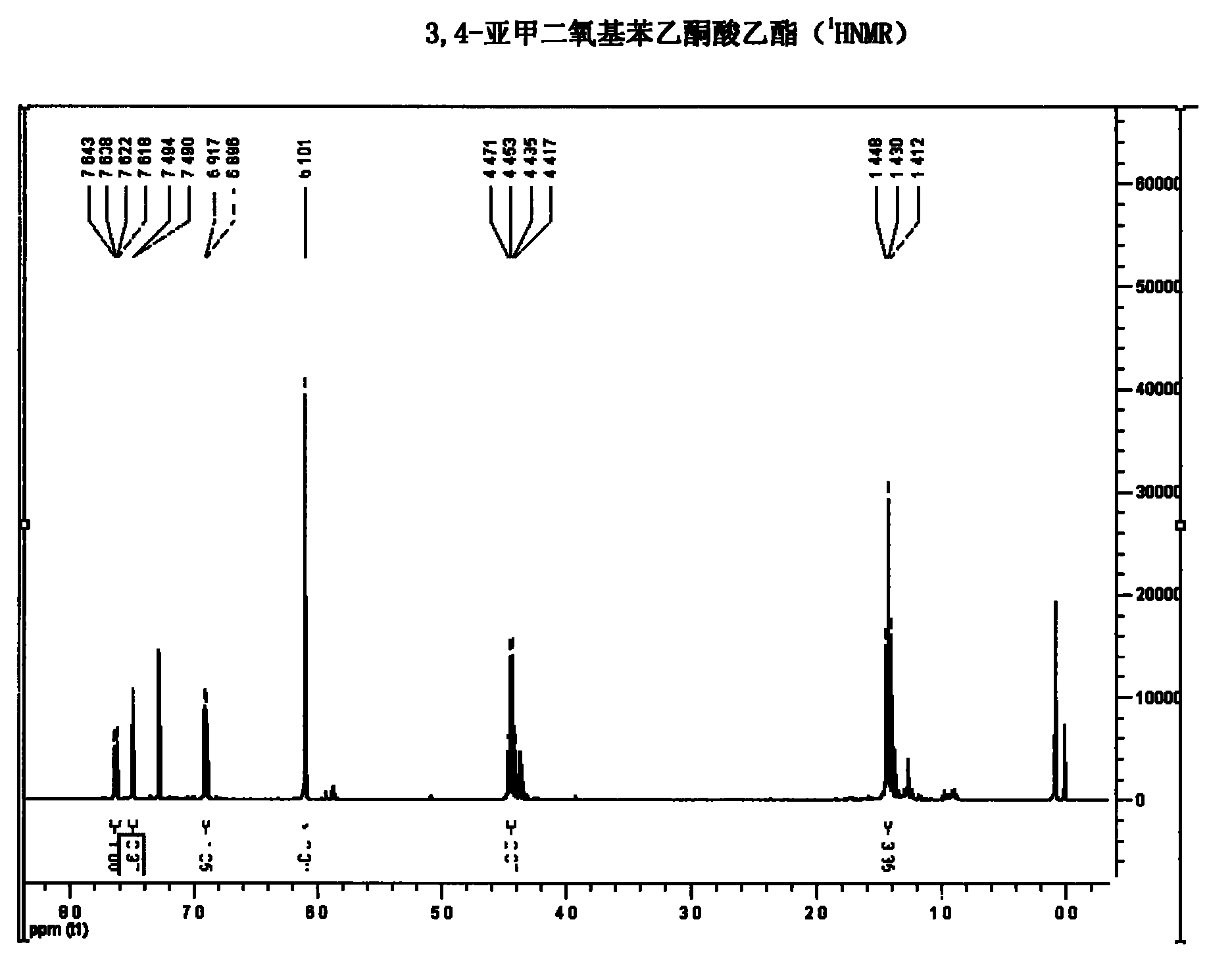
The invention belongs to the technical field of medicament synthesis, and in particular relates to a chemical synthetic method of hydroxytyrosol. The chemical synthetic method comprises the steps of (1) protecting two phenolic hydroxyl groups of catechol by using dichloromethane, and enabling catechol to react with dichloromethane to prepare 1,2-methylenedioxybenzene; (2) enabling 1,2-methylenedioxybenzene to react with various monoesters of oxalyl chloride to prepare 3,4-methylenedioxy phenylglyoxylic acid ester; (3) preparing 3,4-methylenedioxy phenylacetic acid by using 3,4-methylenedioxy phenylglyoxylic acid ester through a Wollff-kishner-Huang Minglong reduction reaction; and (4) reducing the 3,4-methylenedioxy phenylacetic acid by using lithium aluminum hydride, lithium borohydride or sodium borohydride to prepare 3,4-methylenedioxy phenethyl alcohol, and then removing methylene protection of the 3,4-methylenedioxy phenethyl alcohol by using boron tribromide or palladium on activated carbon to prepare hydroxytyrosol. A reactive reagent used in the synthetic method disclosed by the invention is easy to obtain and low in price, the reaction condition is mild, and the final yield of the whole reaction is 23%.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Preparation method of LCZ696 intermediate
ActiveCN105884656AImprove quality indicatorsEasy to get materialsCarbamic acid derivatives preparationOrganic compound preparationPhenylboronic acidEnvironmental resistance
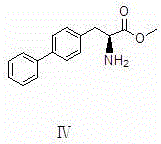
The invention discloses a preparation method of LCZ696 intermediate and relates to the technical field of preparation of an aromatic nucleus compound containing 2 benzene rings and 1 chiral center. The preparation method includes the steps of S1, allowing benzyl magnesium bromide to react with methyl oxalyl chloride to obtain a compound as shown in formula I; S2, allowing the compound as shown in formula I to have a bromination reaction with a bromination reagent to generate a compound as shown in formula II; S3, coupling the compound as shown in formula II with phenylboronic acid to obtain a compound as shown in formula III; S4, performing reductive ammoniation on the compound as shown in formula III to obtain a compound as shown in formula IV; S5, applying Boc to the compound as shown in formula IV to obtain a compound as shown in formula V; S6, performing ester group reduction on the compound as shown in formula V to obtain a compound as shown in formula VI. The preparation method has the advantages that overall raw material consumption is lowered, and product productivity and market competiveness are increased; by the overall process optimization, the reaction of each step can be performed and controlled easily, the use of heavy metal catalysts is reduced and avoided, and accordingly the quality index of the final product is increased, and an economic and environment-friendly process route is developed.
Owner:CANGZHOU SENARY CHEM SCI TEC
Hexa alkyl guanidine salt ion liquid and preparing process
The present invention relates to hexa-alkyl guanidine salt ion liquid and its preparation process. The materials 1 ,3-dimethyl-2-imidazolinone and tetra-alkyl urea are produced into intermediate Wilsman salt under the action of phosphorus oxychlooride, oxalyl chloride, thiophosgene, phosgene or dichluoro sulfoxide; the intermediate is then reacted with C10 below aliphatic amine to produce penta-alkyl guanidine; and penta-alkyl guanidine is finally reacted with alkyl bromide or alkyl iodide and methyl or ethyl ester to produce hexa-alkyl guanidine salt ion liquid. The bromine salt and iodine salt may further reacted with various inorganic salt to produce hexa-alkyl guanidine salt ion liquid containing various negative ions. The present invention lays down foundation for the research and development of ionic liquid and provides systemic technology for the industrial production of hexa-alkyl guanidine salt ion liquid..
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Method for preparing trifloxystrobin
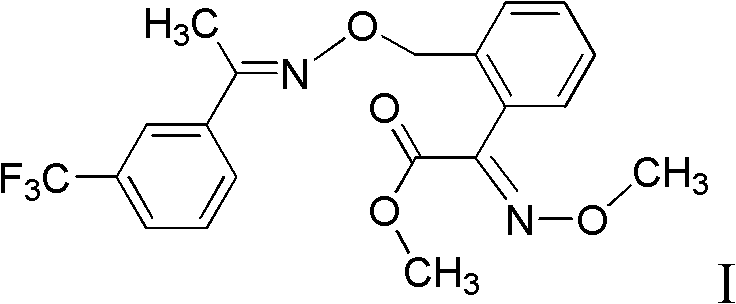
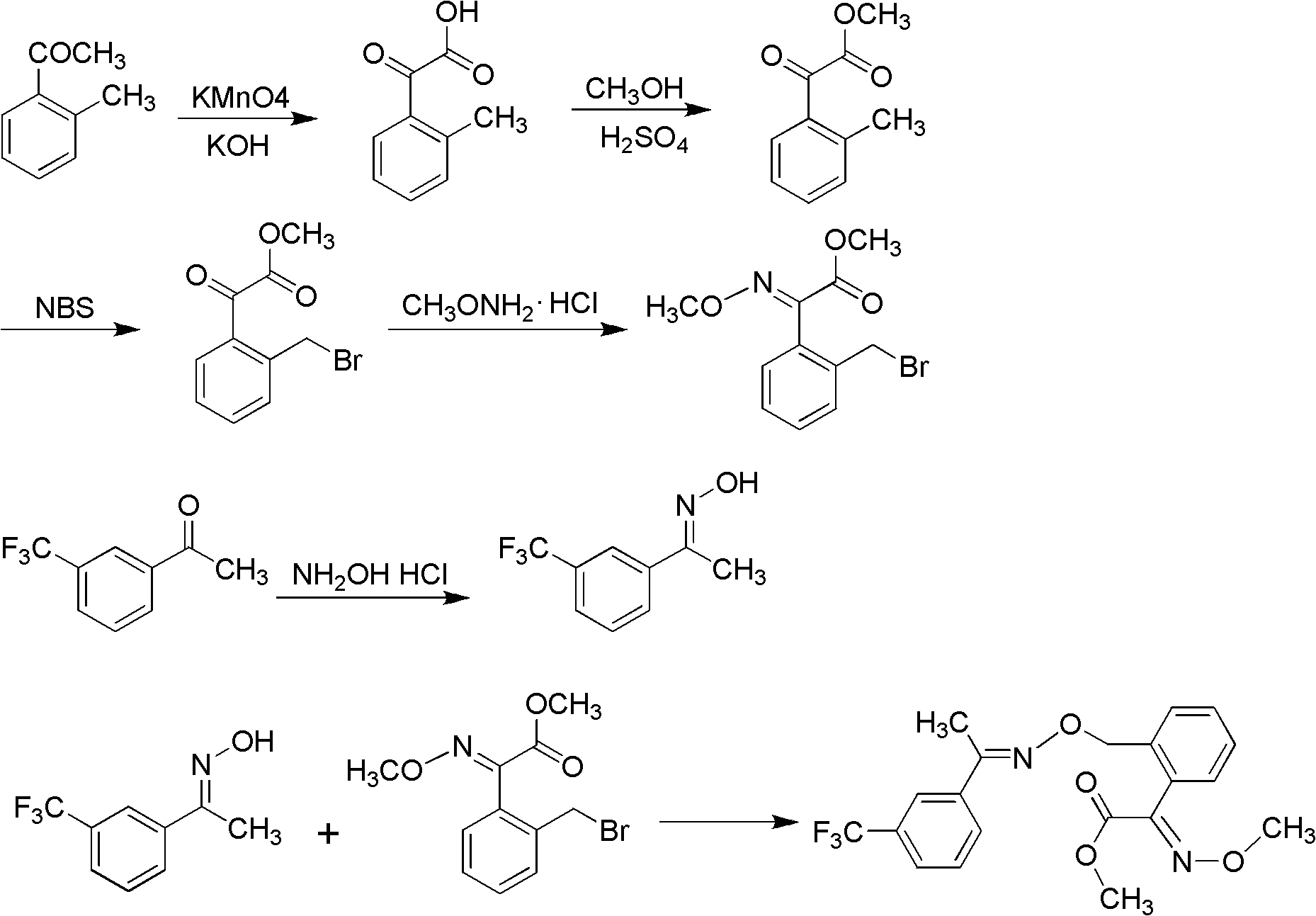
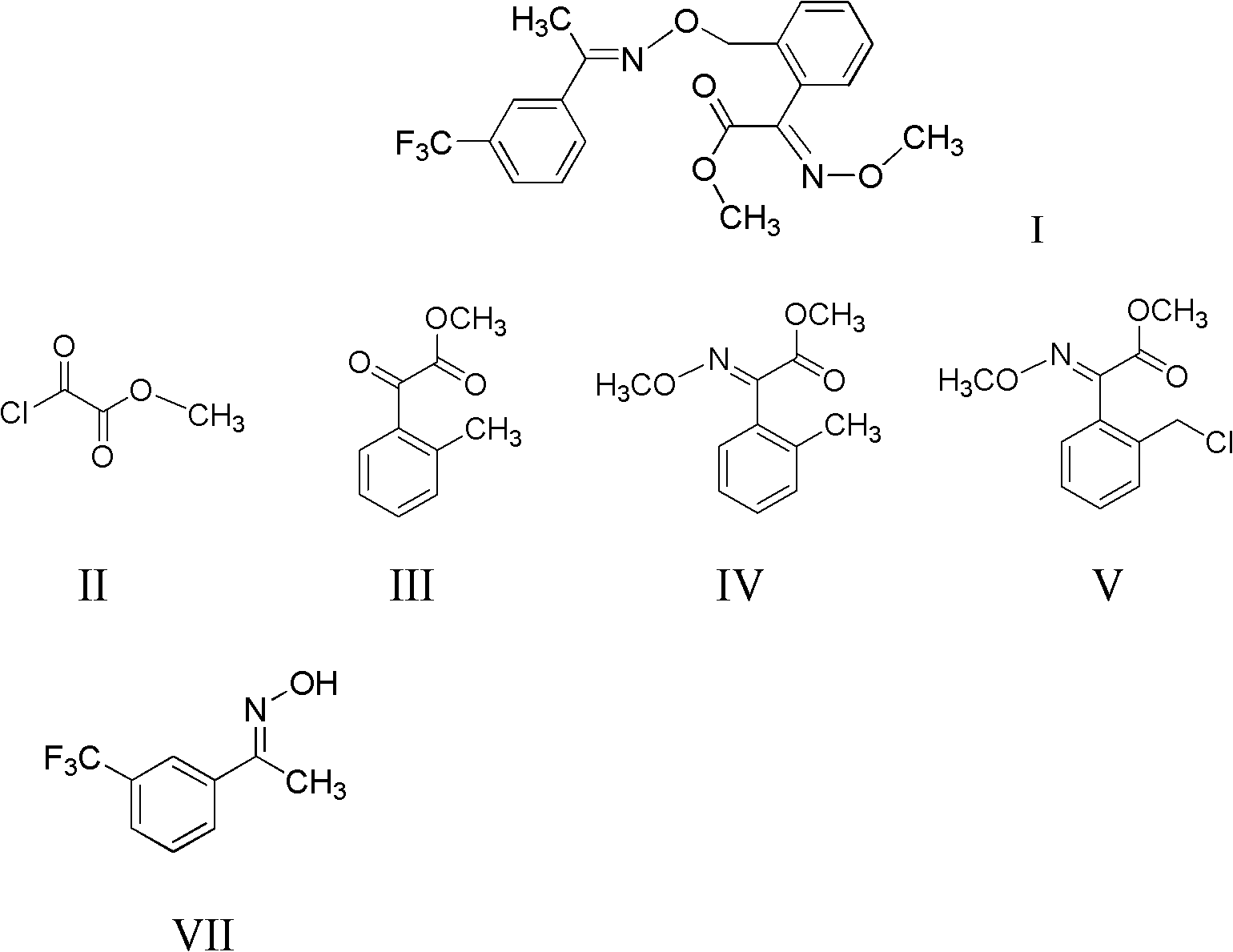
InactiveCN101941921ARaw materials are cheap and easy to getHigh reaction yieldOximes preparationReaction stepOxalyl chloride
The invention discloses a method for preparing (E,E)-2-[1'-(3'-trifluoromethyl phenyl)-ethyl-imine-oxyl-tolyl]-2-carbonyl methyl acetate-O-ketoxime. The method comprises the following steps of: performing acylation reaction on toluene and methyl oxalyl chloride in the presence of anhydrous aluminum chloride to prepare 2-(2'-methyl phenyl)-2-carbonyl methyl acetate; reacting the 2-(2'-methyl phenyl)-2-carbonyl methyl acetate with methoxy amine hydrochloride to prepare (E)-2-(2'-methyl phenyl)-2-carbonyl methyl acetate-O-methyl ketoxime; and finally performing condensation reaction on the (E)-2-(2'-methyl phenyl)-2-carbonyl methyl acetate-O-methyl ketoxime and m-trifluoromethyl phenyl ethyl ketoxime under the action of alkaline substance to prepare the trifloxystrobin. The method has the advantages of a few reaction steps, simple synthesis process, readily available raw materials, mild reaction conditions, great industrial value and great social and economic benefit.
Owner:YUEYANG DIPU CHEM TECH
Substituted benzoyl urea insect growth regulator synthesizing method
InactiveCN1580042AApplicable to industrial production scaleHigh yieldBiocideOrganic chemistryPotassium fluorideBenzonitrile
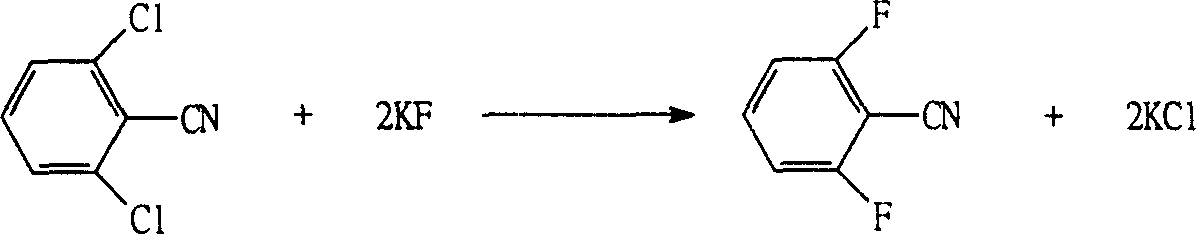
The invention refers to a synthetic method of insect growth regulator of substituted benzoyl urea and to be specific involves the synthetic method of fluorin bell urea, fluorin insect urea and diflubenzuron. It chooses the 2, 6-difluoro benzonitrile as the starting material and gets the compound of the substituted benzoyl urea after fluorination, hydrolysis, esterification and addition. The method adopts the potasium fluoride with high activity and quaternary ammonium salt as the catalyzer during the fluorination, which shortens the time of the fluorination reaction greatly, chooses the base catalysis during the hydrolysis, which reduces the special requirement to the equipment, uses the phosgene instead of the oxalyl chloride during the esterification to decrease the reaction cost and employs the catalyzer during the addition to increase the yield of the reaction. The invention is featured by the high yield, simple steps and cheap raw material and is more adaptable to the scale of the industrial production. Through the method, the yield of the diflubenzuron is 97%, which of the fluorin bell urea 93% and that of the fluorin insect urea 86%.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
Ferrocene-DOPO biradical polyester type flame retarding and smoke suppressing agent, preparation method of ferrocene-DOPO biradical polyester type flame retarding and smoke suppressing agent, and application of ferrocene-DOPO biradical polyester type flame retarding and smoke suppressing agent to flame retarding polymer
The invention discloses a ferrocene-DOPO biradical polyester type flame retarding and smoke suppressing agent and a preparation method of the ferrocene-DOPO biradical polyester type flame retarding and smoke suppressing agent. The preparation method of the polymer is as follows: putting 10-(2,5-dyhydroxyl phenyl)-9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide into a reactor, adding a solvent,stirring for 5-10 minutes, adding an acid-binding agent; taking R1,R2'-ferrocene oxalyl chloride, adding the solvent, mixing, adding into the reactor dropwise, thermally insulating, stirring and reacting for 1-15 hours at the temperature of 0-50 DEG C under the protection of nitrogen after finishing adding dropwise; pouring a liquid obtained after the reaction into a precipitator while stirring, wherein the volume of the precipitator is 5-10 times that of the liquid, precipitating a yellow solid, carrying out suction filtration, washing a filter cake by using the precipitator, and drying to obtain the ferrocene-DOPO biradical polyester type flame retarding and smoke suppressing agent. The ferrocene-DOPO biradical polyester type flame retarding and smoke suppressing agent can be used to retard flame and suppress smoke of high polymer materials like polyolefins and epoxy resins.
Owner:SOUTHWEAT UNIV OF SCI & TECH
Synthesis method for N-Boc-3-piperidone
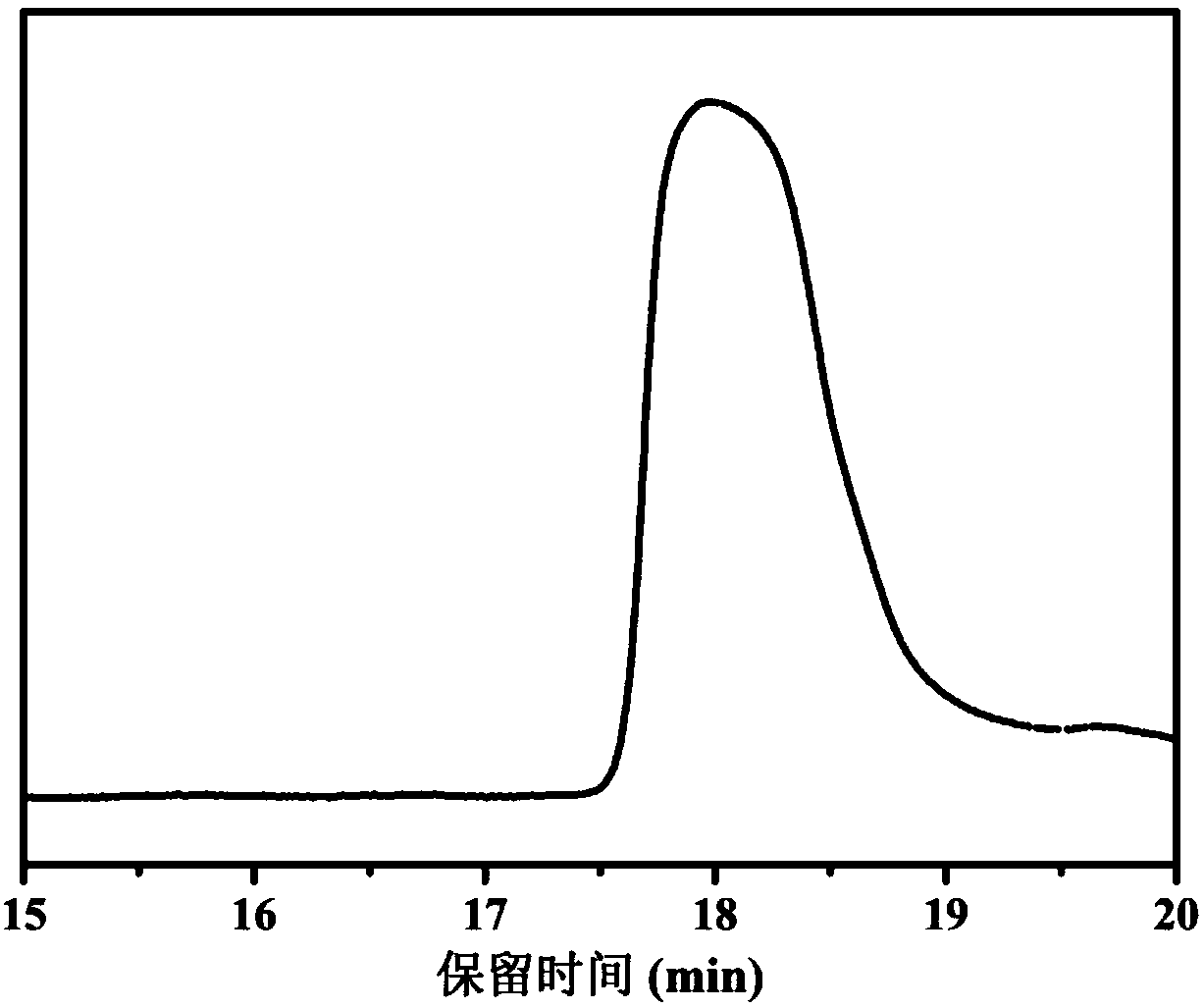
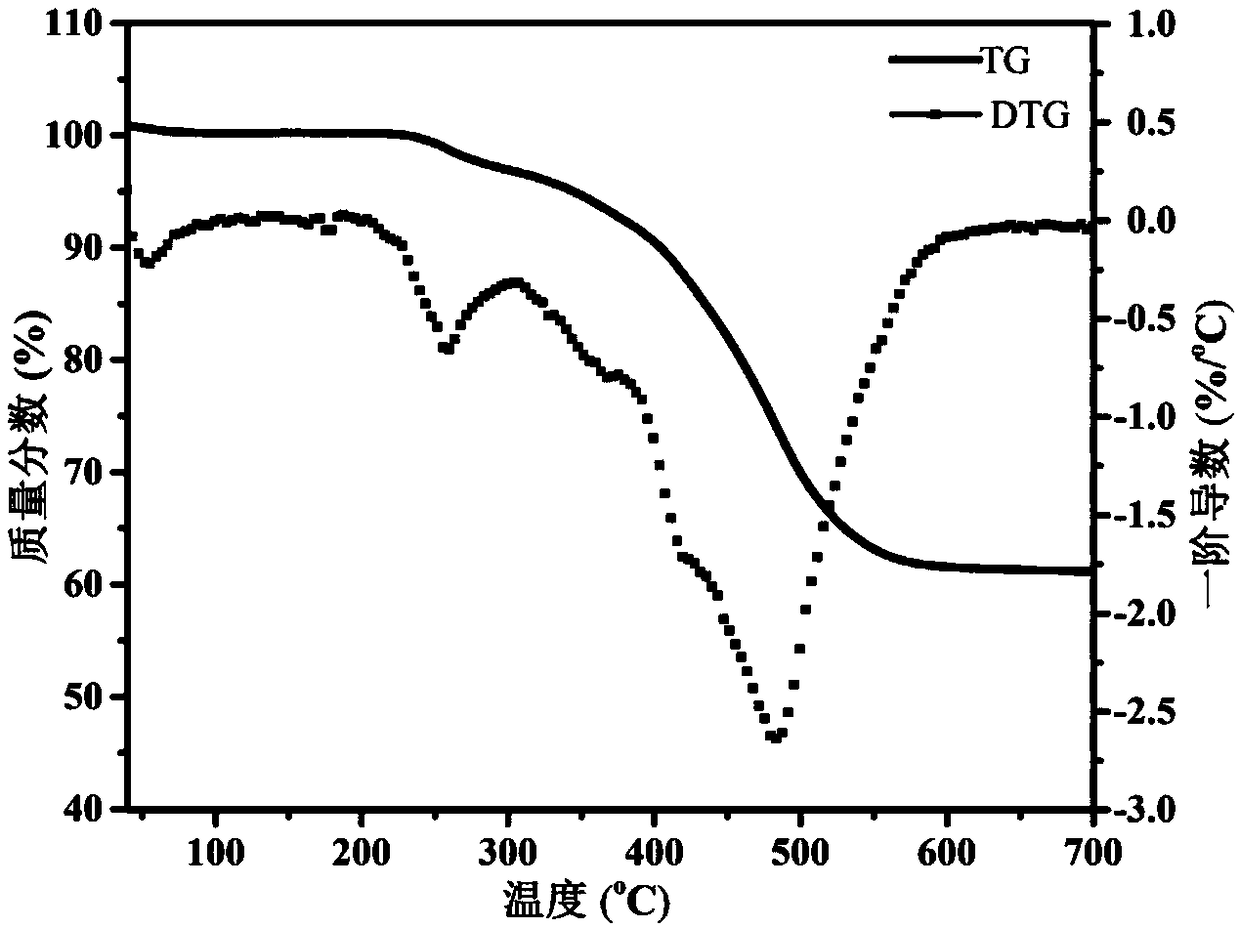
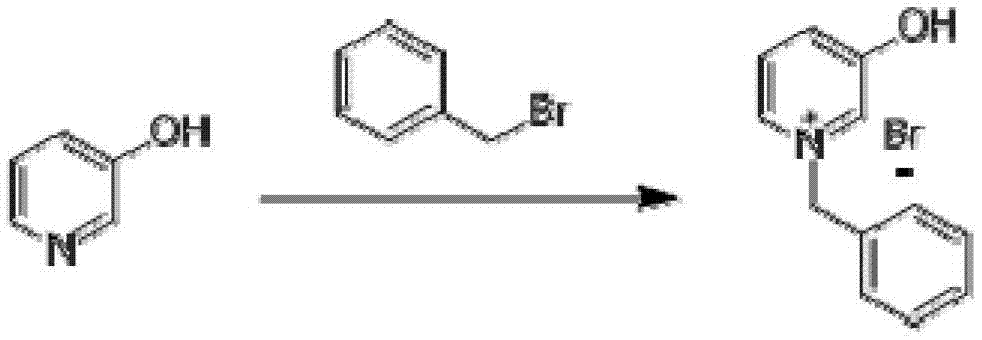
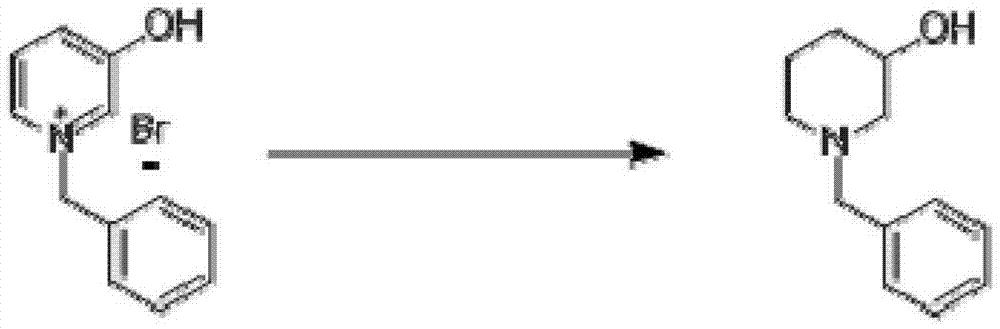
InactiveCN103204801AEasy to separate and purifyShort synthetic routeOrganic chemistryPalladium on carbonBenzoyl bromide
The invention discloses a synthesis method for N-Boc-3-piperidone. The synthesis method comprises the following steps of: reacting 3-hydroxyl pyridine with benzyl bromide in an organic solvent to obtain an N-benzyl-3-hydroxyl pyridine quaternary ammonium salt; reducing the N-benzyl-3-hydroxyl pyridine quaternary ammonium salt by sodium borohydride to obtain N-benzyl-3-hydroxyl piperidine; reacting N-benzyl-3-hydroxyl piperidine with di-tert-butyl dicarbonate ester to obtain N-Boc-3-hydroxyl piperidine under hydrogen protection and the catalysis of a palladium-carbon catalyst; and reacting N-Boc-3-hydroxyl piperidine with the mixed oxidant of dimethyl sulfoxide and oxalyl chloride to obtain N-Boc-3-piperidone under the action of an organic base. Compared with the existing synthesis method, the synthesis method disclosed by the invention is shorter in synthesis route, and easier for separation and purification of reactants, thus reducing the production cost, the energy consumption and the pollution; and the total productivity of N-Boc-3-piperidone can achieve more than 42%, and the purity thereof is greater than 98%.
Owner:甘肃天骄商贸有限公司
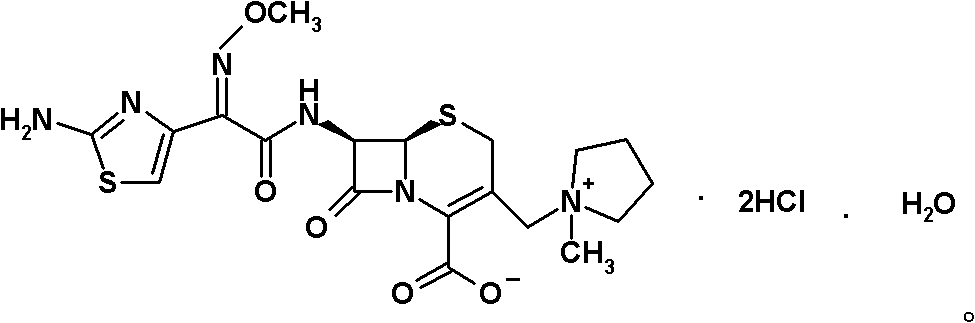
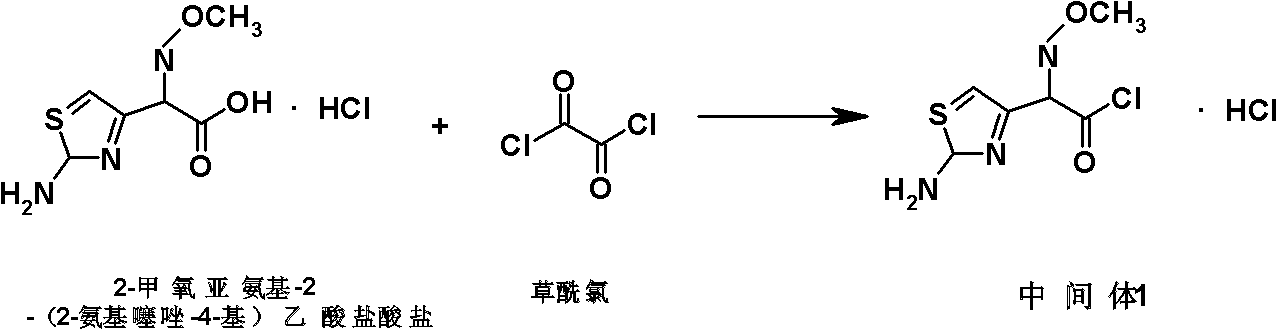
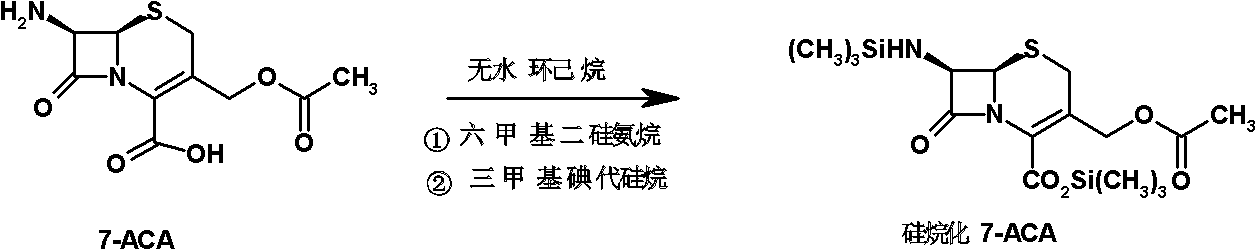
Preparation method of cefepime hydrochloride
ActiveCN101935325ASimple processAvoid the phenomenon of inhomogeneous crystal form and poor fluidityOrganic chemistryCefepime hydrochlorideBetaine
The invention discloses a preparation method of cefepime hydrochloride, comprising the following steps of: reacting oxalyl chloride with 2-methoxyimino-2-(2-aminothiazole-4-yl) acetic acid hydrochloride to obtain a midbody I, i.e. 2-methoxyimino-2-(2-aminothiazole-4-yl) acetyl chloride hydrochloride; mixing silanized 7-aminoce-phalosporanic acid and silanized N-methylpyrrolidine, and reacting to obtain a midbody II, i.e. hydriodic acidification (6R, 7R)-7-amino-3-[(1-methyl-1-tetrahydro pyrrolidine) methyl]-3-cephem-4-formic betaine, in the presence of trimethyl idodine silicon hydride, isopropanol and an aqueous solution of hydrogen iodide; dissolving the midbody II into dichloromethane, sequentially adding trimethylchlorosilane and hexamethyldisilazane for reaction, and then adding the midbody I and triethylamine to react to prepare the cefepime hydrochloride. The cefepime hydrochloride prepared by the method has the advantages of uniform crystal form, good flowability and simple process and is suitable for industrialized production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
STAT3 small molecular selective inhibitor and preparation method and application thereof
InactiveCN102417479ARich varietyEasy to prepareOrganic chemistryAntineoplastic agentsCancer cellQuinoline
The invention discloses an STAT3 small molecular selective inhibitor and a preparation method and applications thereof, and the STAT3 small molecular selective inhibitor comprises four structure general formulae as shown in formula I, formula II, formula III and formula IV. The preparation comprises the following steps: allowing 2-phenyl substituted quinoline-4-carboxylic acid thionyl chloride or oxalyl chloride to react to generate substituted acyl chloride, reacting with substituted arylamine to generate substituted quinoline-4-amide derivatives. The applications comprise an application in the preparation of medicaments for treating cancers related to abnormally-activated STAT3 pathway, and an application in the preparation of antitumor medicaments where the STAT3 small molecular selective inhibitor is used as an inhibitor of the STAT3 signal pathway. The STAT3 inhibitor of the invention is a small molecular selective inhibitor; based on results obtained by detecting its effect on cancer cells and evaluating its activity, the small molecular STAT3 selective inhibitor of the invention is applicable to the development of related cancer-treatment medicaments, has quite wide applications, and has very good medicine curative effect. The small molecular STAT3 selective inhibitor of the invention has various types, easily available raw materials, a simple preparation method, high product purity, high yield, and strong practicality.
Owner:NANJING UNIV
Fluorescence molecular probe compound as well as preparation method and application thereof
InactiveCN104059005AElimination of heavy atom effectsCrossover inhibitionOrganic chemistryFluorescence/phosphorescenceGrignard reagentFluorescence
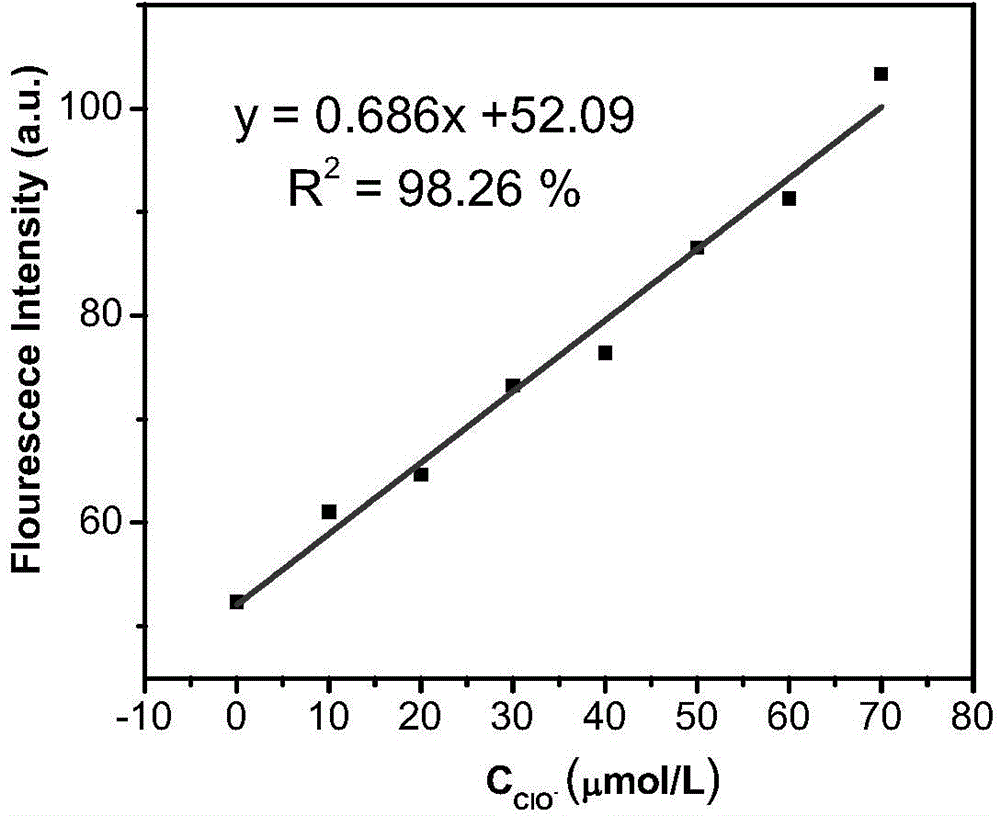
The invention discloses a fluorescence molecular probe compound as well as a preparation method and an application thereof. The preparation method of the fluorescence molecular probe compound comprises the following steps: allowing a benzophenone derivative containing a halogen substituent group to react under the catalysis of Zn / TiCl4; allowing the reaction products to react with magnesium to generate a Grignard reagent, subsequently allowing the Grignard reagent to react with CO2 and acidifying; acylating the acidification products by use of oxalyl chloride and then amidating the acylated acidification products with secondary amine; and further reacting with an Lawesson's reagent to obtain the fluorescence molecular probe compound which is high in stability and good in oxidation resistance. The preparation method is simple to operate, the raw materials are easy to get and the reaction condition is moderate; the prepared fluorescence molecular probe compound can be used for detecting hypochlorite ions in a water solution, has the characteristics of being high in sensitivity, high in capability of identifying pypocholoride, rapid in response, wide in range and low in limit of detection, and can be widely applied to the fields including water detection, environmental monitoring and the like.
Owner:CENT SOUTH UNIV
Optically pure alpha-ketoacyl harringtonine and preparing and purifying method thereof
The present invention relates to an optically pure alpha- ketoacyl harringtonine and a preparing and purifying method thereof. In the temperature of -80 DEG C to 50 DEG C, the alpha-ketoacyl chlorine which is prepared through reacting alpha-ketonic acid and oxalyl chloride reacts with the cephalotaxine in an inert organic solvent while the organic base is used as an acid-binding agent for obtaining the oily product represented by the formula (I). The purifying steps are as follows: dissolving the oily product with the inert organic solvent, adding the saturated NaHSO3 solution, mixing and separating the liquid; after washing the water phase with the organic solvent, adjusting the pH of the water phase with saturated NaHSO3 solution to 7-8, extracting with the organic solvent; washing the organic phase with the buffering solution with pH of 6.8 and the saturated saline solution, drying and filtering the organic phase, removing the solvent for obtaining the pale-yellow solid; and then recrystallizing with the organic dissolvent for obtaining the white solid or colorless crystal. The optically pure alpha- ketoacyl harringtonine is a key intermediate for synthesizing the medicine of harringtonine alkaloid, which is widely applied for anti-tumor (malignant tumor and benign tumor), antiparasitic, antifungal and antibacterial chemotherapy. The synthesizing method is suitable for purifying and preparing the large amount of optically pure compound represented by the structural formula of (I).
Owner:NANKAI UNIV
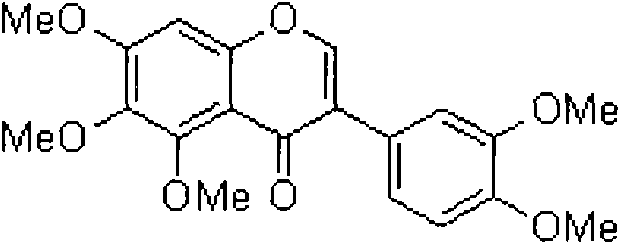
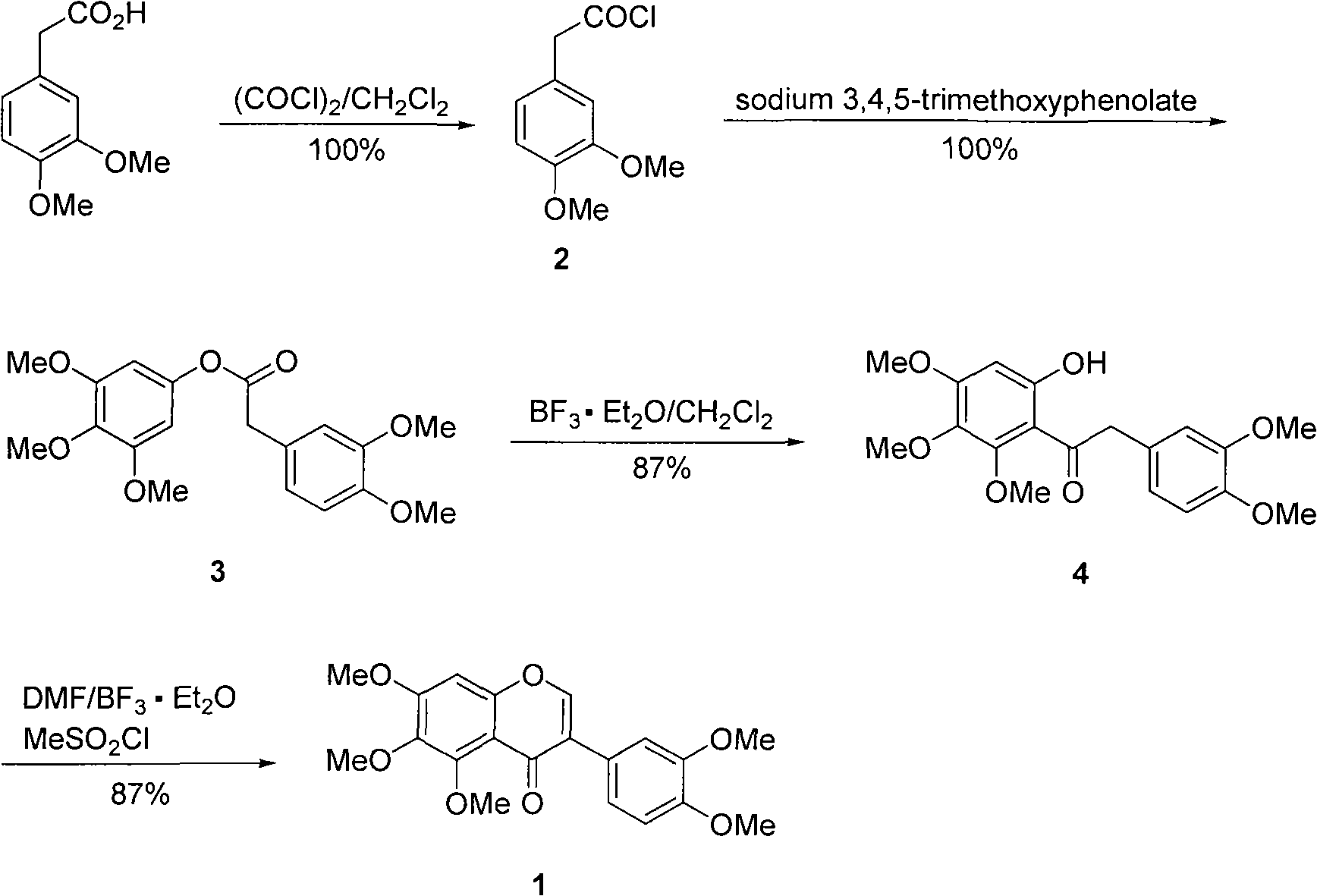
Preparation method of COMT inhibitor 5, 6, 7, 3', 4'-pentamethoxyl isoflavone
InactiveCN101643465AEasy to operateShort reaction timeOrganic active ingredientsOrganic chemistrySulfonyl chlorideFormylation reaction
The invention relates to a preparation method for COMT inhibitor 5, 6, 7, 3', 4'-pentamethoxyl isoflavone, comprising the following steps: mixing 3, 4-dimethoxyphenylacetic acid and oxalyl chloride indichloromethane solution to carry out reflux reaction for 2 to 3 h, and cooling to room temperature; adding 3, 4, 5-trimethoxy sodium phenolate to carry out esterification reaction to carry out Friesrearrangement reaction under the catalysis of Lewis acid boron trifluoride ether; and finally carrying out reaction with DMF with the existence of the Lewis acid boron trifluoride ether; and adding methane sulfonyl chloride to promote Vilsmeier-Haack formylation reaction and subsequent cyclization reaction to obtain light yellow crystals 5, 6, 7, 3', 4'-pentamethoxyl isoflavone. The preparation method has simple and easily available raw materials, simple operation, short reaction time, mild reaction condition, environment protection and high yield of 76 %, and is suitable for industrial production.
Owner:DONGHUA UNIV
Method for preparing amide
ActiveCN106045870AEasy to synthesizeImprove conversion rateOrganic compound preparationCarboxylic acid amides preparationOrganic acidOrganic solvent
The invention provides a method for preparing amide. The method includes weighing triphenyl phosphine oxide, oxalyl chloride, organic acid and organic amine according to a molar ratio of 1-5:1-5:1:0.3-4; adding the triphenyl phosphine oxide, the oxalyl chloride, the organic acid and the organic amine into a reaction vessel, stirring the triphenyl phosphine oxide, the oxalyl chloride, the organic acid and the organic amine in N2 environments and carrying out reaction on the organic acid and the organic amine in the presence of organic solvents at the reaction temperature of 10-40 DEG C for the reaction time of 0.5-5 hours to generate the corresponding amide. The method has the advantages that the triphenyl phosphine oxide is used as a novel carboxylic acid activating agent to promote synthesis of the amide, the method is low in reaction temperature and short in reaction time, and the triphenyl phosphine oxide which is the carboxylic acid activating agent is inexpensive, is easily available and can be recycled; only reaction byproducts CO and CO2 can be generated, and accordingly the method is high in atom utilization rate.
Owner:SHANGHAI INST OF TECH
Low dielectric constant polyamide aerogel thermal insulation material and preparation method thereof
The invention discloses a low dielectric constant polyamide aerogel thermal insulation material and a preparation method thereof. According to the low dielectric constant polyamide aerogel thermal insulation material, aromatic oxalyl chloride and aromatic diamine are taken as raw materials, polyamine or polybasic acyl chloride are taken as a cross-linking agent, a sol-to-gel process is adopted to prepare and obtain polyamide gel with a three-dimensional net structure, and the low dielectric constant polyamide aerogel thermal insulation material is obtained by carrying out CO2 supercritical drying on the polyamide gel. The aromatic oxalyl chloride is TPC or IPC or a mixture of the TPC and the IPC; the aromatic diamine is ODA or pPDA; the polyamine is TAB or OAPS; the polybasic acyl chloride is BTC. The thermal insulation material provided by the invention is relatively low in dielectric constant, low in density, low in heat conductivity and good in heat-insulating property; the preparation method provided by the invention has the advantages that a preparation process is simple, the raw materials are easy to obtain, the cost is relatively low, the requirement on environment is relatively low, a whole technological process is short in consumed time, and the material is suitable for industrial production.
Owner:NAT UNIV OF DEFENSE TECH
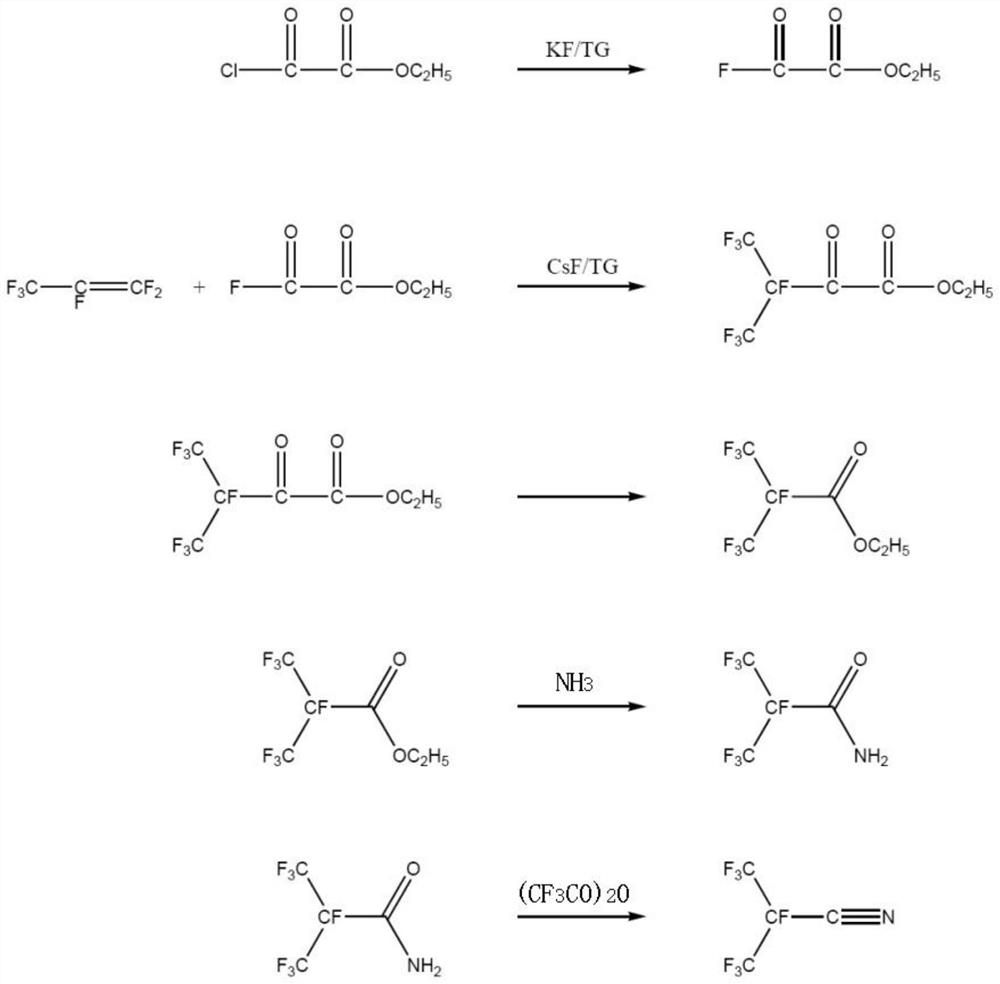
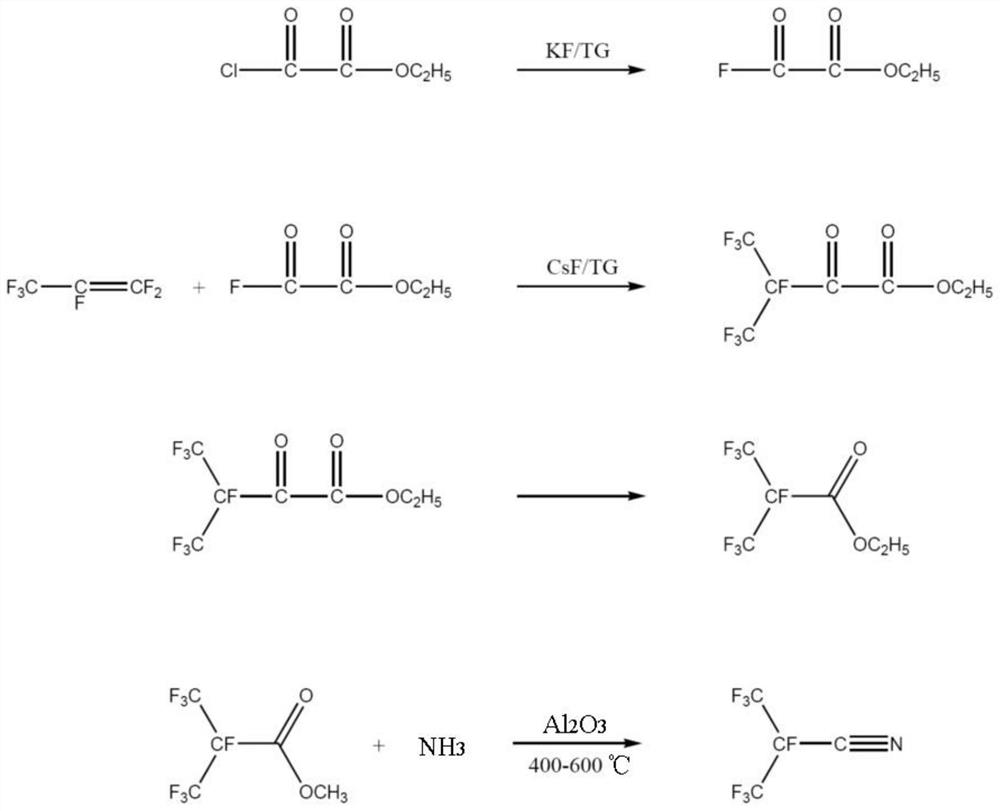
Synthetic method of perfluoroisobutyronitrile
PendingCN111825568ASynthetic process safetyEfficient synthesis processOrganic compound preparationCarboxylic acid esters preparationOxalyl fluoridePtru catalyst
Owner:福建省漳平市九鼎氟化工有限公司
Choline M receptor antagonist aclidinium bromide and preparation method thereof
InactiveCN104478871AShort reaction timeQuick costOrganic active ingredientsOrganic chemistryGrignard reagentStructural formula
The invention relates to choline M receptor antagonist aclidinium bromide and a preparation method thereof. The chemical structural formula of the choline M receptor antagonist aclidinium bromide is as shown in the specification. The preparation method comprises the following steps: mixing R-quinine-3 alcohol, alkali and an organic solvent, dripping methyl oxalyl chloride, after the methyl oxalyl chloride is completely dripped, heating, performing heating reflux for 1-20 hours, and separating and purifying, thereby obtaining a substance A; adding iodine into a tetrahydrofuran solution of 2-bromothiophene and magnesium powder to initiate reaction for 1-5 hours, thereby preparing a Grignard reagent of 2-bromothiophene, adding the substance A, stirring to react for 10-30 minutes at the room temperature, performing heating reflux for 4-6 hours, separating and purifying, thereby preparing 2,2-dithienyl-2-glycolic acid-R-quinine-3-base ester, and performing quaterisation reaction with 3-phenoxypropyl bromine, thereby obtaining aclidinium bromide. The aclidinium bromide provided by the invention is simple in reaction operation, high in yield, low in price, short in reaction route, small in waste generation and easy in industrialization production.
Owner:DONGHUA UNIV
Process for the production of bendamustine alkyl ester, bendamustine, and derivatives thereof
ActiveUS8481751B2Amino compound purification/separationOrganic compound preparationHalogenBendamustine
Methods are provided for the production of bendamustine alkyl ester, bendamustine, as well as derivatives thereof. With the methods the production of these compounds is possible in reproducibly high yields. To this end, hydroxyl-group-containing esters are used as the starting material, whose hydroxyl groups are substituted in a simple way by halogen groups. This substitution is possible in the presence of (i) oxalyl chloride and (ii) dialkylformamide, dialkyl acetamide or dimethyl sulfoxide. In a subsequent reaction, the resulting esters can be hydrolyzed to form the acid.
Owner:HERAEUS PRECIOUS METALS GMBH & CO KG
Methods of Synthesizing a Prostacyclin Analog
InactiveUS20150315114A1Increase productionToxic reductionGroup 4/14 element organic compoundsEther separation/purificationProstacyclinChemical compound
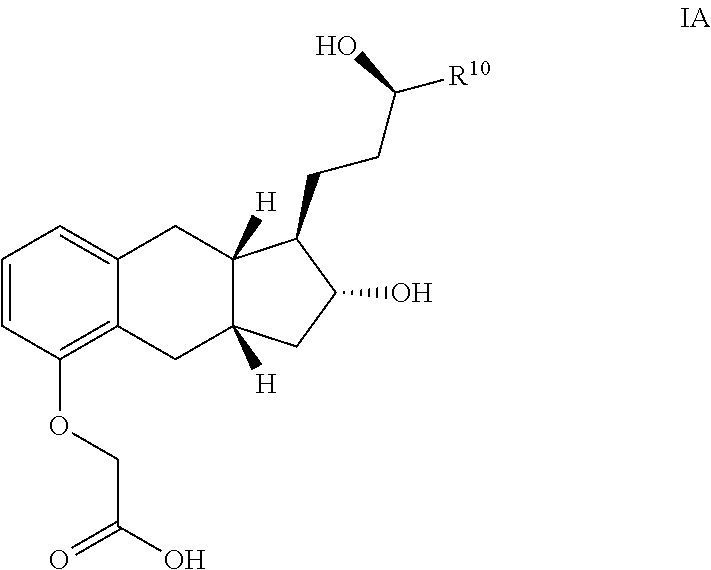
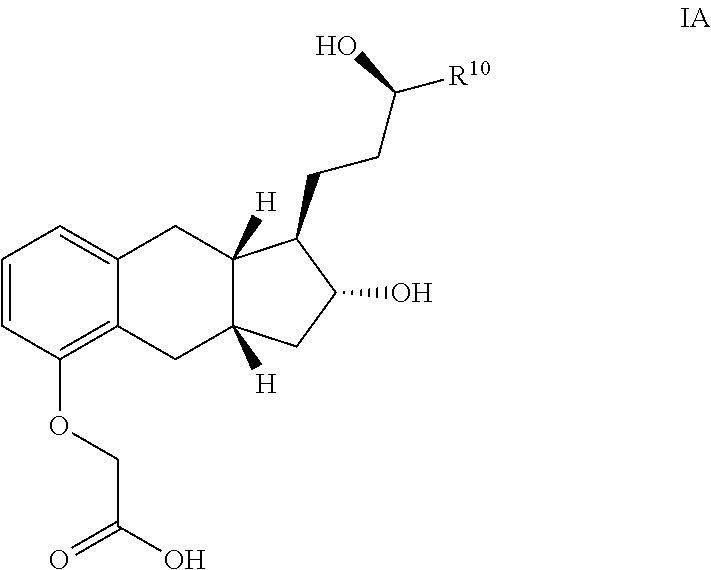
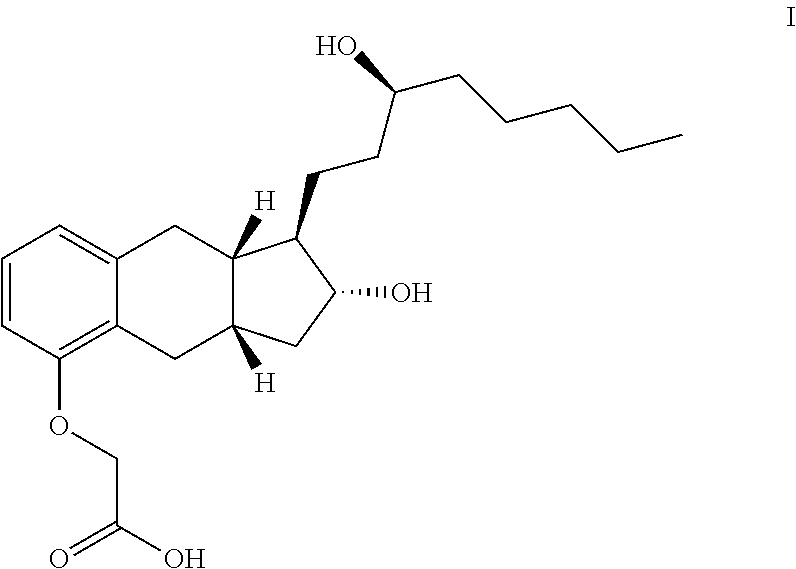
The present invention provides processes for preparing a prostacyclin analogue of Formula (I) or a pharmaceutically acceptable salt thereof, wherein R10 is a linear or branched C1-6 alkyl. The processes of the present invention comprise steps that generate improved yields and fewer byproducts than traditional methods. The processes of the present invention employ reagents (e.g., the oxidizing reagent) that are less toxic that those used in the traditional methods (e.g., oxalyl chloride). Many of the processes of the present invention generate intermediates with improved e.e. and chemical purity; thereby eliminating the need of additional chromatography steps. And, the processes of the present invention are scalable to generate commercial quantities of the final compound.
Owner:CAYMAN CHEMICAL COMPANY
Synthesis technology of dutasteride
The invention discloses a synthesis technology of dutasteride, comprising the following steps of: using the acidity of DT4 carboxyl to directly react with ammonia to generate DT4-ammonium salt; dehydrating the obtained ammonium salt into DT4-amide, and performing an amino exchange reaction between amide and BTFMA in the presence of a catalyst so as to prepare dutasteride. With DT4 as a starting material, dutasteride is synthesized by three reactions of salt formation, dehydration and amino exchange. During the reactions, thionyl chloride, oxalyl chloride, pivaloyl chloride or methylsulfonyl chloride which is sensitive to the environment is avoided, special expensive catalysts such as DBU, copper powder and the like are not needed, and harmful and poisonous chemical materials are avoided. The product has good product yield and high purity, and is easy to refine. The synthesis technology has advantages of low cost and few ''three wastes'', is easy and simple to operate, conforms to green chemical synthesis requirements, and lays a good industrial foundation for realizing large-scale green clean production of high-yield and high-purity dutasteride.
Owner:HUBEI TIANSHENG PHARMA
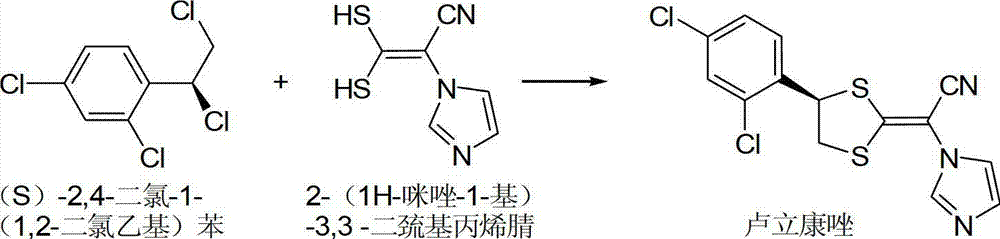
Method for synthesizing luliconazole intermediate-(S)-2,4-dichloro-1-(1,2-dichloroethyl) benzene
InactiveCN103044192AReduce dosageEasy to recycleOrganic compound preparationHydroxy compound preparationBenzeneChemical synthesis
The invention belongs to the field of chemical synthesis processes, particularly relates to a method for synthesizing a luliconazole intermediate-(S)-2,4-dichloro-1-(1,2-dichloroethyl) benzene, and solves the technical problems of high cost, lower yield and low possibility of industrialization in the conventional synthesis method. The invention adopts the following technical scheme: the method comprises the steps as follows: a, preparing a chiral catalyst; b, adding a surfactant or a phase transfer catalyst and omega-chloro-2,4-dichloroacetophenone as well as a hydrogen source into the chiral catalyst for reaction; c, extracting and re-crystallizing a reaction solution and drying a solid to obtain (R)-2-chloro-1-(2,4-dichlorophenyl) ethanol; and d, reacting (R)-2-chloro-1-(2,4-dichlorophenyl) ethanol with oxalyl chloride, and extracting, purifying and drying the reaction solution to obtain (S)-2,4-dichloro-1-(1,2-dichloroethyl) benzene as a product. The invention provides a novel method for preparing (S)-2,4-dichloro-1-(1,2-dichloroethyl) benzene with high yield and low cost.
Owner:ASTATECH CHENGDU PHARMA
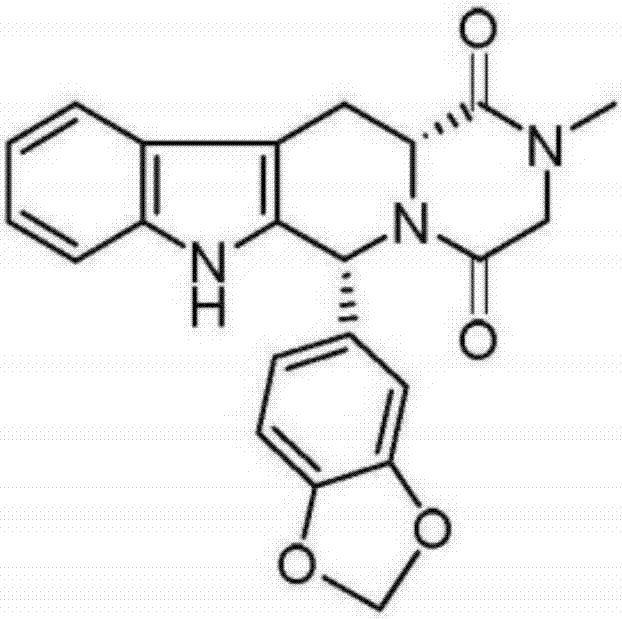
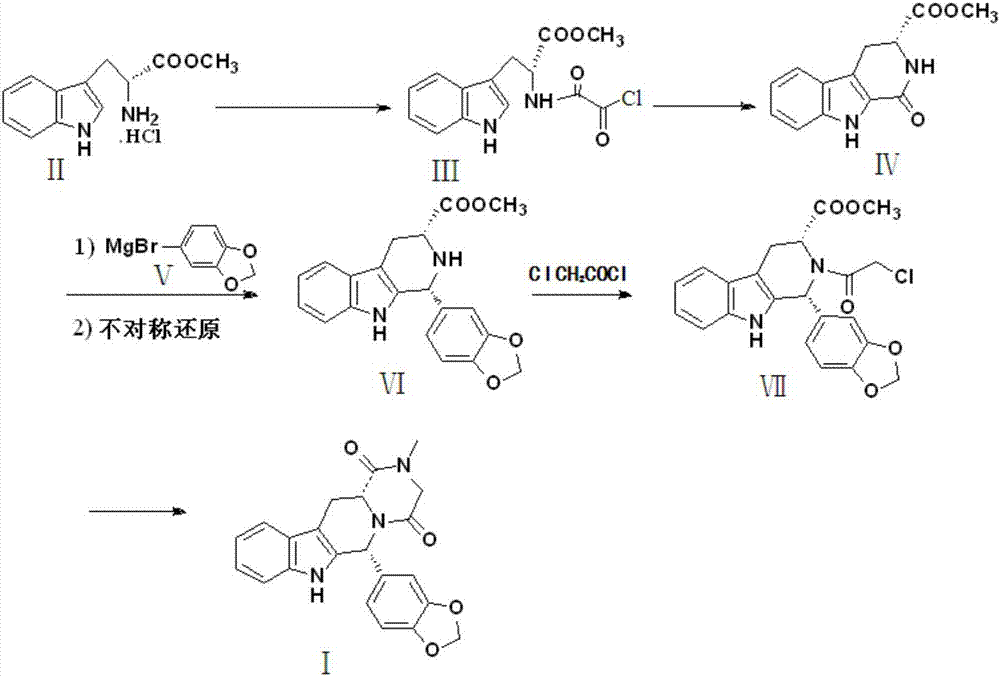
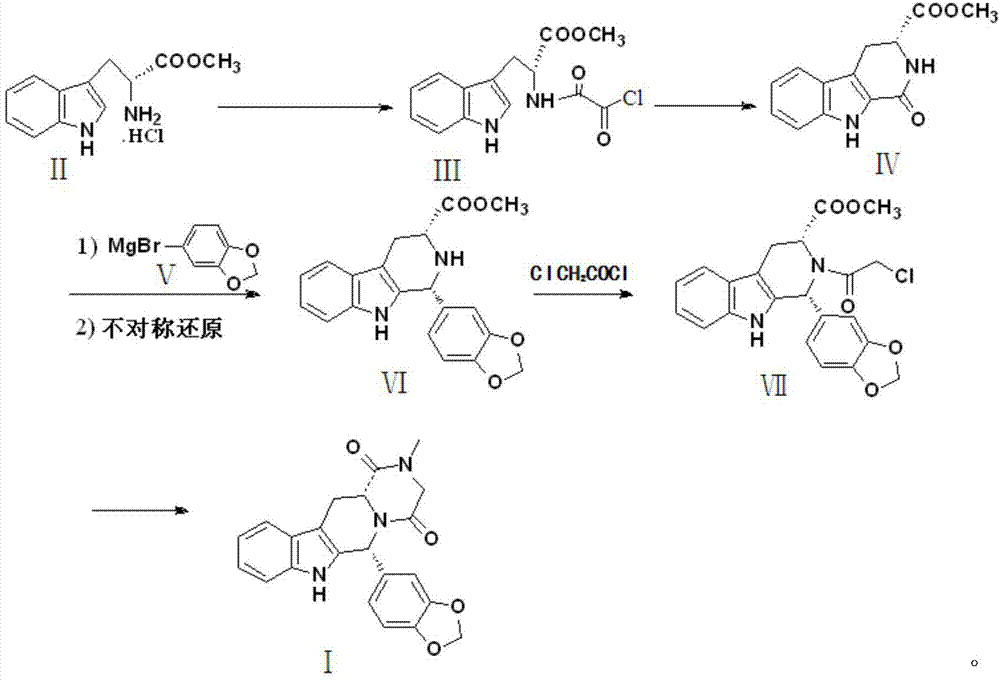
Preparation method of tadalafil
The invention discloses a preparation method of tadalafil, starting material D-Tryptophan methyl ester hydrochloride is reacted with oxalyl chloride to obtain an intermediate III, and the final product tadalafil (I) is obtained through cyclization, Grignard reaction, asymmetric reduction, substitution and condensation reaction. The use of national control chemical piperonal is avoided, an intermediate VI can be highly-selectively obtained by the asymmetric reduction, and the method has the advantages of simple postprocessing, short synthesis steps and high product total yield, and is suitable for industrialized production.
Owner:SHANDONG YUXIN PHARMA CO LTD
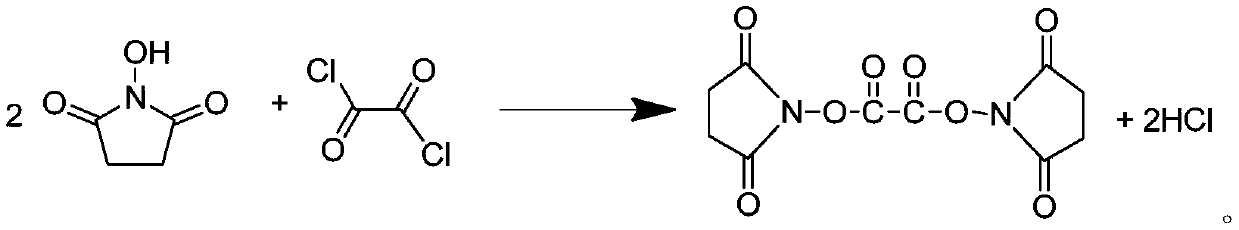
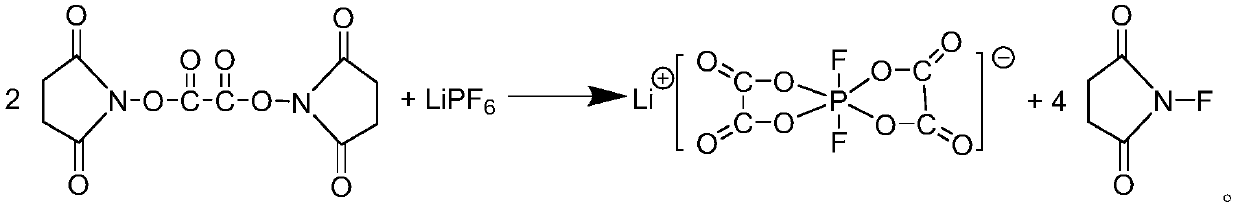
Preparation method of lithium difluorobisoxalate phosphate solution
ActiveCN110204576AMild reaction conditionsHigh yieldGroup 5/15 element organic compoundsSecondary cellsOxalatePhosphate
The invention provides a preparation method of a lithium difluorobisoxalate phosphate solution, and the method comprises the following steps: 1) reacting N-hydroxysuccinimide and oxalyl chloride in anon-aqueous solvent to obtain a di (N-succinimido) oxalic acid solution; and 2) adding lithium hexafluorophosphate into the di (N-succinimido) oxalic acid solution, and reacting to obtain the lithiumdifluorobisoxalate phosphate solution. According to the preparation method, the obtained solution can be directly used for lithium salt electrolyte materials of lithium ion batteries without further purification, and the method has the advantages of cheap and easily available reaction raw materials, simple operation, environmental friendliness of byproducts, and suitability for industrial production.
Owner:DONGGUAN DONGYANG SOLAR SCI RES & DEV CO LTD
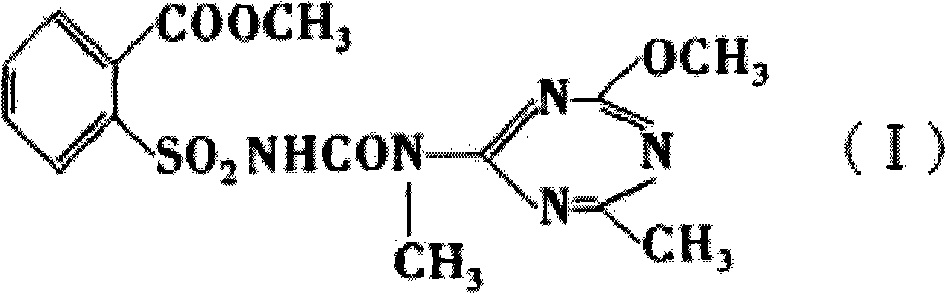
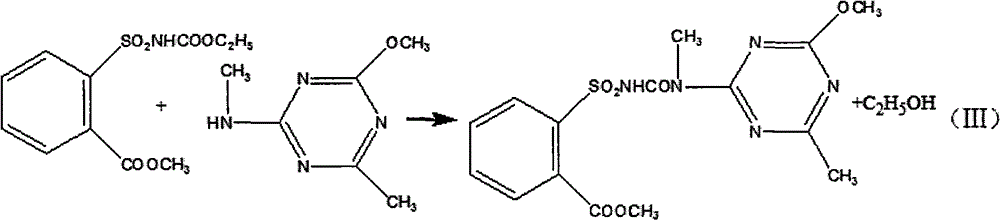
Method for preparing tribenuron-methyl
InactiveCN102718723AEmission reductionEasy to operateOrganic chemistryEthyl chloroformateReaction temperature
The invention discloses a method for preparing tribenuron-methyl, which comprises the following steps: ortho carbomethoxyl group benzene sulfonamide and ethyl chloroformate are reacted in a solvent, filtered and dried to obtain an ortho carbomethoxyl group benzene sulfonamide ethyl formate solid, the ortho carbomethoxyl group benzene sulfonamide ethyl formate solid is dissolved in the solvent, a 2-methylamino-4-methoxy-6-methyl-s-triazine solution is added drop by drop, stirred and heated, and reacted to obtain the tribenuron-methyl, wherein the purity is greater than or equal to 95%. According to the invention, ethyl chloroformate is used as an amide agent, the reaction temperature can be controlled by ice bath, and the speed for adding ethyl chloroformate drop by drop can be controlled, thereby the ethyl chloroformate is reacted with ortho carbomethoxyl group benzene sulfonamide in the solvent to obtain the ortho carbomethoxyl group benzene sulfonamide ethyl formate, and then reacted with s-triazine to obtain tribenuron-methyl. The present invention adopts ethyl chloroformate as a raw material, compared with phosgene, triphosgene and oxalyl chloride, and the method for preparing the tribenuron-methyl has the advantages of simple operation, high production security, low cost and less generation amount of three wastes.
Owner:HEFEI JIUYI AGRI DEV
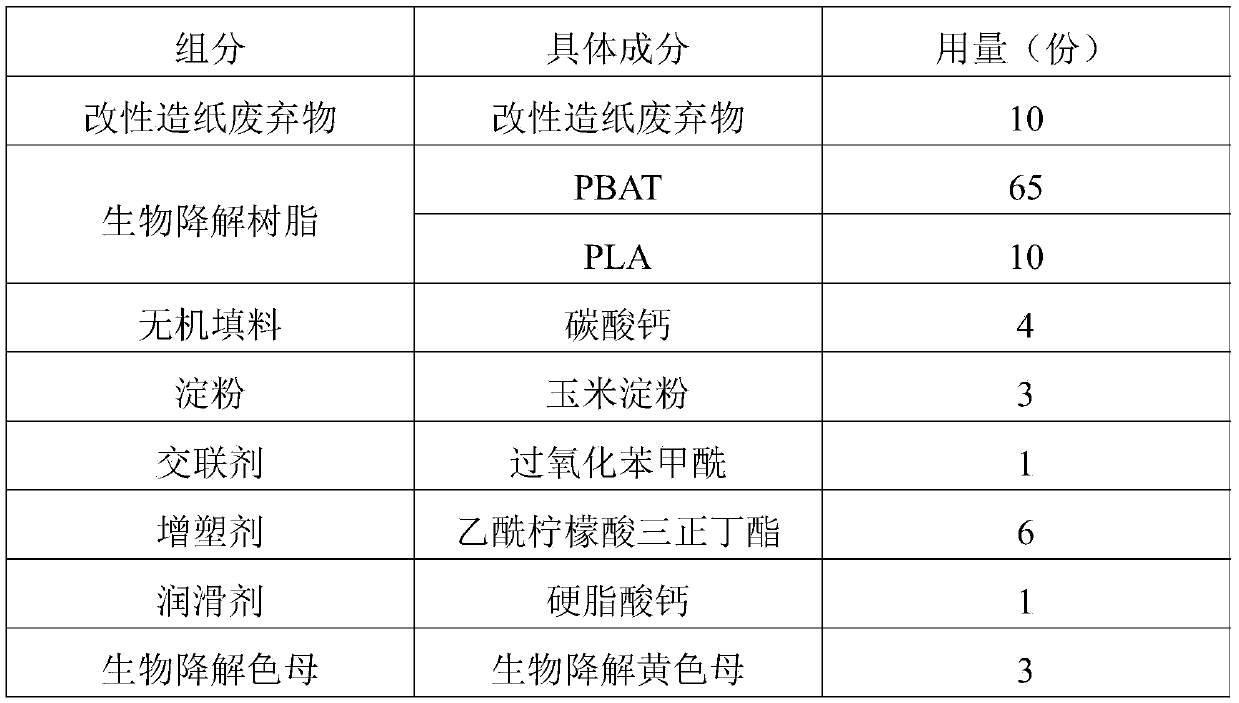
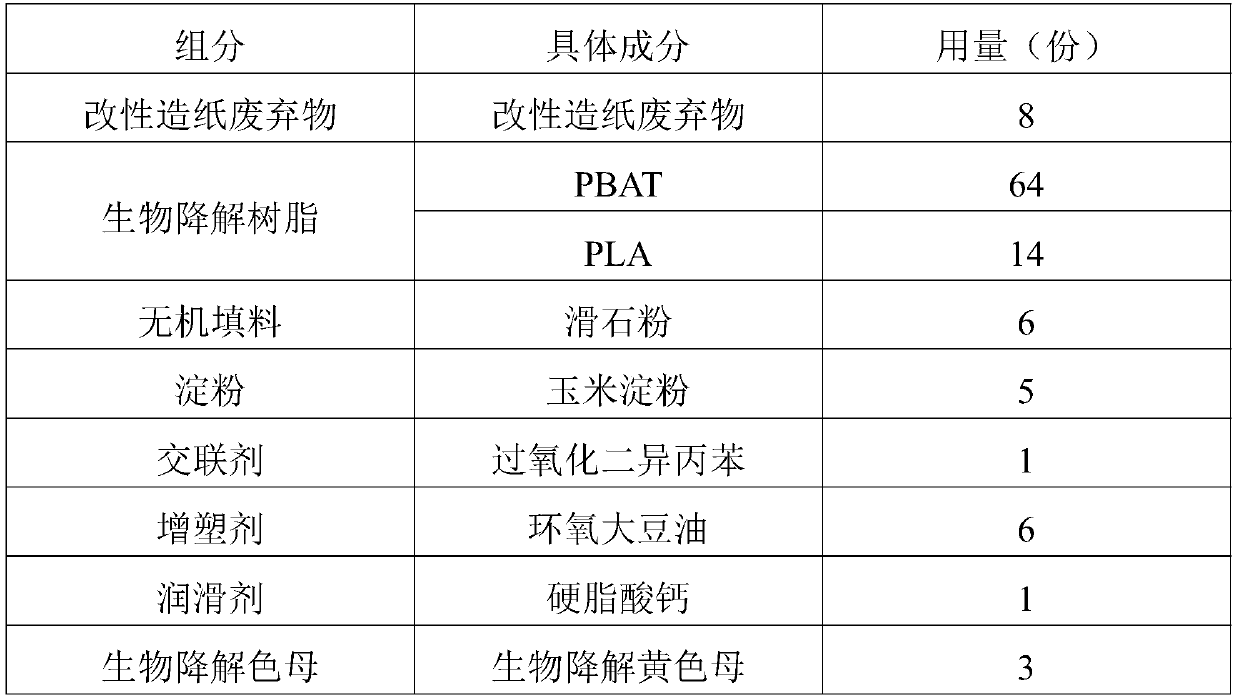
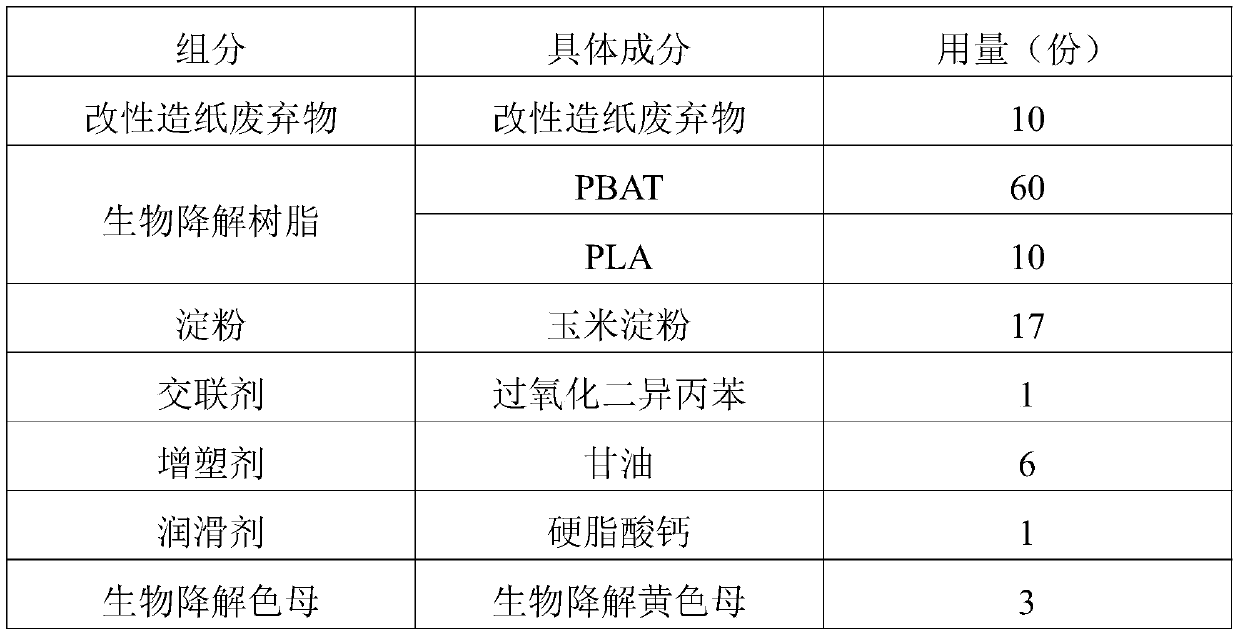
Application of raw material composition in preparation of biodegradable insect trapping plate
InactiveCN111234477AHigh tensile strengthHigh elongation at breakInsect catchers and killersBiotechnologyChloroacetyl chloride
The invention discloses an application of a raw material composition in preparation of a biodegradable insect trapping plate. The raw material composition comprises the following components in parts by weight: 5-20 parts of modified papermaking waste, 50-90 parts of a biodegradable resin and 1-10 parts of auxiliaries, wherein the modified papermaking waste comprises 100 parts of papermaking waste,1-50 parts of a modifier and 0.1-5 parts of a phase transfer agent, and the modifier is one or more of stearoyl chloride, acetyl chloride, benzoyl chloride, oxalyl chloride, chloroacetyl chloride andtrichloroacetyl chloride. The master batch of the biodegradable insect trapping plate and the biodegradable insect trapping plate can be completely degraded by microorganisms in the nature, so that the environment is not polluted, waste utilization can be achieved, and the cost is reduced. The modified papermaking waste is adopted, so that the compatibility of the modified papermaking waste and biodegradable resin can be effectively improved, and the tensile strength and elongation at break of the biodegradable insect trapping plate are improved. The insect trapping amount of the biodegradable insect trapping plate is obviously superior to that of a traditional PP insect trapping plate and that of a traditional PVC insect trapping plate.
Owner:SHANGHAI CHANGFA NEW MATERIAL CO LTD
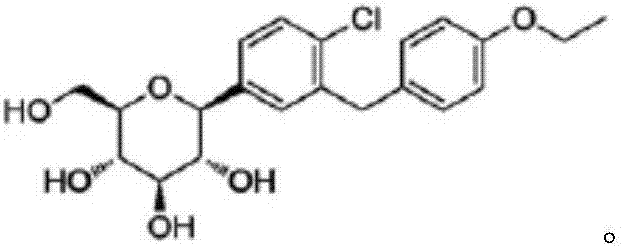
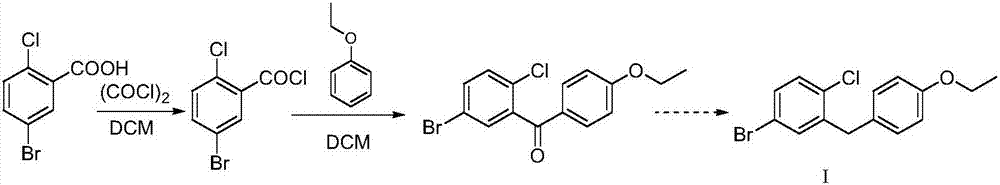
Preparation method of Dapagliflozin intermediate used for treating II-type diabetes
InactiveCN107200683AHigh yieldGood reserve supportOrganic compound preparationCarbonyl compound preparation by condensationTert-butyldimethylsilyl chloride2-Chlorobenzoic acid
The invention discloses a preparation method of a Dapagliflozin intermediate used for treating II-type diabetes. The preparation method comprises the following steps: 1) performing a reaction on 5-bromine-2-chlorobenzoic acid and oxalyl chloride in anhydrous dichloromethane under the catalysis of DMF (dimethyl formamide), so as to obtain 5-bromine-2-chloro-benzoyl chloride; 2) under the condition that tert-Butyldimethylsilyl chloride exists, performing a reaction on 5-bromine-2-chloro-benzoyl chloride obtained in step 1) and phenetole under the catalysis of ferric trichloride, so as to obtain 5-bromine-2-chloro-4'-ethyoxyl benzophenone. According to the preparation method provided by the invention, no ortho-by-product is generated, and the yield of a target product is high, so that the good support of storage of raw materials is provided for Dapagliflozin. Additionally, the preparation method is mild in conditions and short in reaction time, therefore, the preparation method is suitable for industrial production and promotion.
Owner:QINGDAO CHENDA BIOLOGICAL SCI & TECH
Phenyl phosphine diamide derivative as well as synthesis method and application thereof
InactiveCN108659040AFair priceLow boiling pointGroup 5/15 element organic compoundsEpoxySynthesis methods
The invention provides a phenyl phosphine diamide derivative as well as a synthesis method and application thereof. The synthesis method comprises the following steps: by taking phenyl phosphine oxalyl chloride and primary amine as reaction raw materials, tetrahydrofuran or diethyl ether as reaction solvents, and triethylamine as a reaction catalyst, dissolving the primary amine into ethyl ether or tetrahydrofuran, and by taking triethylamine as an equal mass as a substrate, slowly dropping a phenyl phosphine oxalyl chloride containing ethyl ether or tetrahydrofuran solution while stirring ata uniform speed, wherein the dropping time is longer than 2 hours; controlling a reaction temperature to be below 5 DEG C, and continuously stirring for more than 5 hours at the room temperature afterdropping is completed; filtering a product to remove triethylamine hydrochloride, carrying out vacuum distillation, and washing an obtained product with deionized water, so as to obtain a crude product. The product can be applied to flame-retardant modification on an epoxy resin. The product provided by the invention can be used as a flame-retardant modifier, meanwhile is low in synthesis raw material price, simple and gentle in synthesis process and low in solvent melting point in a reaction process and can be recycled and repeatedly used, and energy consumption is correspondingly reduced.
Owner:FUJIAN CONSTR ENG GRP BUILDING MATERIAL SCI & TECH DEV
Synthesis method of 2, 2'-dihydroxy-4, 4'-dimethoxybenzophenone
InactiveCN102329207AEasy to operateMild reaction conditionsOrganic compound preparationCarbonyl compound preparationChlorobenzeneBenzoyl peroxide
The invention relates to a synthesis method of 2, 2'-dihydroxy-4, 4'-dimethoxybenzophenone. The method comprises the following steps: 1) enabling m-dimethoxybenzene and oxalyl chloride to react in the presence of a catalyst at the temperature of 70-80 DEG C for getting an intermediate product 2, 2', 4, 4'-tetramethoxy benzophenone, wherein the weight ratio of the m-dimethoxybenzene to the oxalyl chloride is 1:1-20, the used catalyst is azo isobutyronitrile or benzoyl peroxide, and the using quantity of the catalyst is 0.5%-2% of the weight of the m-dimethoxybenzene; and 2) enabling the obtained intermediate product and Lewis acid to react under the condition of taking an organic reagent as a solvent, wherein the reaction temperature is 50 DEG C and the reaction time is 2-3 hours; and stopping the reaction, adding water for hydrolysis, skimming, performing rotary evaporation and recrystallizing to get the 2, 2'-dihydroxy-4, 4'-dimethoxybenzophenone. The Lewis acid is one of AlCl3, ZnCl2, BF3 and polyphosphoric acid; and the organic reagent is one of dichloroethane, toluene, xylene, nitrobenzene and chlorobenzene. According to the method, the reaction temperature is appropriate, the catalyst is simple and easy to get, the using quantity of the catalyst is low, the environmental pollution is small, and the method is closest to the requirements of green chemistry.
Owner:HUBEI POLYTECHNIC UNIV
Phosphate siloxane high-temperature proton exchange membrane with hydrolytic stability and preparation method thereof
InactiveCN105037733AImprove proton conductivityPromote formationFinal product manufactureSolid electrolyte fuel cellsCross-linkAminosilochrome
The invention belongs to the technical field of fuel cells and particularly relates to a phosphate siloxane high-temperature proton exchange membrane with hydrolytic stability and a preparation method thereof. The preparation method of the proton exchange membrane comprises the following steps: firstly, carrying out acylation reaction between phosphonoacetic acid and oxalyl chloride to obtain phosphoryl chloride; then, carrying out amidation reaction between phosphoryl chloride and amino siloxane to obtain phosphate siloxane; and finally, hydrolyzing and cross-linking phosphate siloxane and tetraethoxysilane together, and preparing a membrane through a sol-gel process. According to the invention, reaction conditions are relatively mild, the preparation process is simple, phosphoric acid in the prepared proton exchange membrane exists in the membrane in a form of C-P bonds, and the hydrolytic stability is better.
Owner:WUHAN UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com