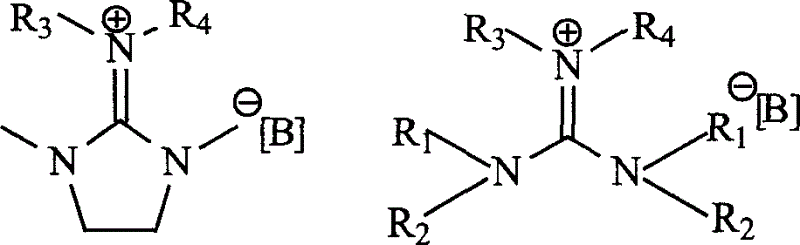
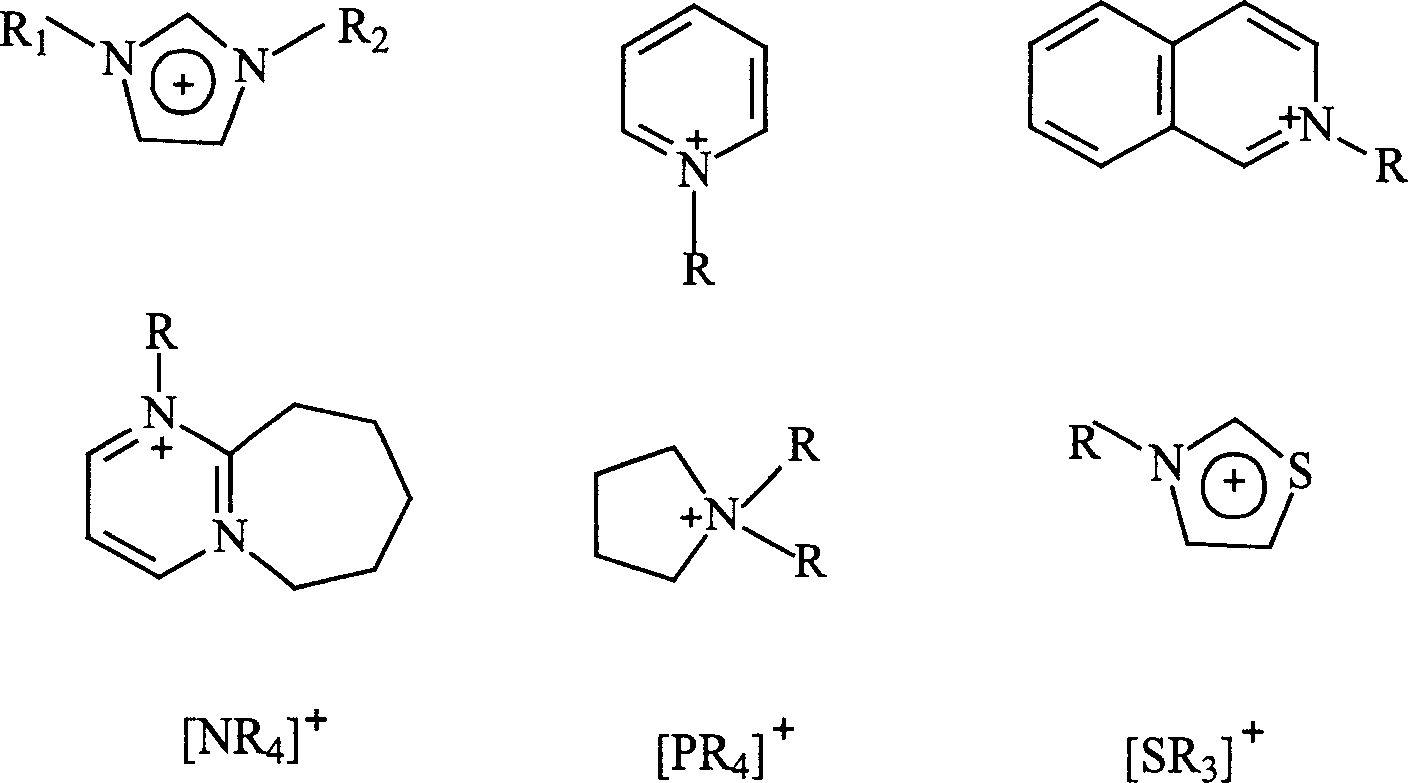
Hexa alkyl guanidine salt ion liquid and preparing process
A kind of technology of hexaalkylguanidine and ionic liquid, applied in the field of hexaalkylguanidine salt ionic liquid and preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: 1, the synthesis of 3-dimethyl-2-butyl-2-methyl-1,3-ethyl cyclic guanidinium iodide ionic liquid ([BMCG] + I - ):
[0032] Dissolve 0.3 mol of 1,3-dimethyl-2-imidazolidinone in 30 ml of refined toluene, slowly add 0.30 mol of phosphorus oxychloride dropwise, react at 65 degrees Celsius for 12 hours, add 50 ml of dichloromethane, slowly drop Add 0.60mol of n-butylamine, reflux for 12 hours, drop in 35% NaOH aqueous solution to make the system alkaline, extract the reaction mixture with dichloromethane, combine the organic phases, and wash with anhydrous NaOH 2 SO 4 After drying, remove the solvent, distill under reduced pressure to obtain pentaalkylguanidine, take 0.3mol of pentaalkylguanidine and dissolve it in 50ml of refined acetocyanide, add 0.3mol of methyl iodide under nitrogen protection, react for 12 hours and remove the solvent under reduced pressure to obtain the target product.
Embodiment 2
[0033] Example 2: [BMCG] + PF 6 - Synthesis of ionic liquids:
[0034] Take [BMCG] + I - Dissolve 0.3mol in 50ml distilled water, add 0.3mol KPF dropwise 6 The aqueous solution was reacted for 1 hour, and the oil layer was separated, and the oil layer was dissolved in 50ml of dichloromethane, washed with water, dried, and the solvent was removed under reduced pressure to obtain the target product.
Embodiment 3
[0035]Example 3: [BMCG] + BF 4 - Synthesis of ionic liquids:
[0036] Take [BMCG] + I - Dissolve 0.3mol in 200ml distilled water, add 0.32mol NaBF dropwise 4 Aqueous solution, reacted for 5 hours, removed water under reduced pressure, added 100ml of chloroform, and filtered. The organic phase is dried. The solvent was removed under reduced pressure to obtain the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com