Patents
Literature
68results about "Magnesium halides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for producing nitride semiconductor, crystal growth rate increasing agent, single crystal nitride, wafer and device
InactiveUS20100104495A1Improve performanceIncrease probabilityPolycrystalline material growthFrom normal temperature solutionsNitrogenCrystal structure
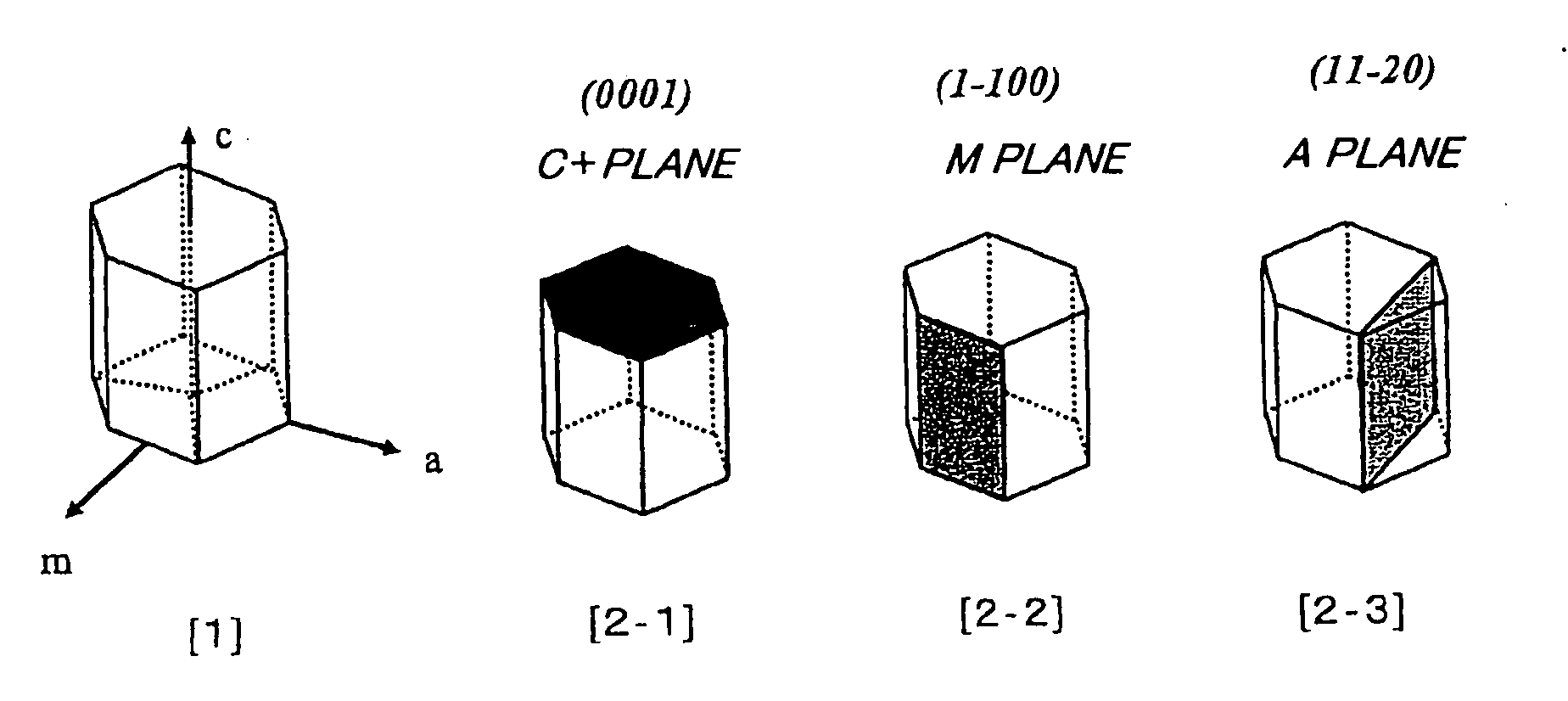
A method for producing a nitride semiconductor, comprising controlling temperature and pressure in a autoclave containing a seed having a hexagonal crystal structure, a nitrogen element-containing solvent, a raw material substance containing a metal element of Group 13 of the Periodic Table, and a mineralizer so as to put said solvent into a supercritical state and / or a subcritical state and thereby ammonothermally grow a nitride semiconductor crystal on the surface of said seed, wherein the crystal growth rate in the m-axis direction on said seed is 1.5 times or more the crystal growth rate in the c-axis direction on said seed. By the method, a nitride semiconductor having a large-diameter C plane or a nitride semiconductor thick in the m-axis direction can be efficiently and simply produced.
Owner:MITSUBISHI CHEM CORP +1
Processes for preparing highly pure lithium carbonate and other highly pure lithium containing compounds
ActiveUS20110200508A1Electrolysis componentsLithium organic compoundsLithium carbonateLithium hydroxide
Owner:TERRALITHIUM LLC
Method for separating magnesium and concentrating lithium from brine in salt lake
ActiveCN1626443AHighly selective extractionEfficient separationMagnesium halidesLithium halidesLithiumSalt lake
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Use of an ammonia storage device in production of energy
An electric power generating unit comprising (i) an ammonia storage device in the form of a container comprising an ammonia absorbing and releasing salt of the general formula: Ma(NH3)nXz, wherein M is one or more cations selected from alkali metals, alkaline earth metals, and transition metals such as Li, K, Mg, Ca, V, Cr, Mn, Fe, Co, Ni, Cu or Zn, X is one or more anions selected from fluoride, chloride, bromide, iodide, nitrate, thiocyanate, sulphate, molybdate, phosphate, and chlorate ions, a is the number of cations per salt molecule, Z is the number of anions per salt molecule, and n is the coordination number of 2 to 12. (ii) means for heating said container and ammonia absorbing and releasing salt for releasing ammonia gas and (iiia) a fuel cell for converting ammonia directly into electric power; or (iiib1) a reactor for dissociating ammonia into hydrogen and nitrogen and (iiib2) a fuel cell for converting hydrogen into electric power.
Owner:AMMINEX
Positive electrode active material, positive electrode, battery, battery pack, electronic device, electric vehicle, power storage device, and power system
ActiveUS20170207444A1Large capacityExcellent cycle characteristicsMagnesium halidesCell electrodesFluorideCrystallinity
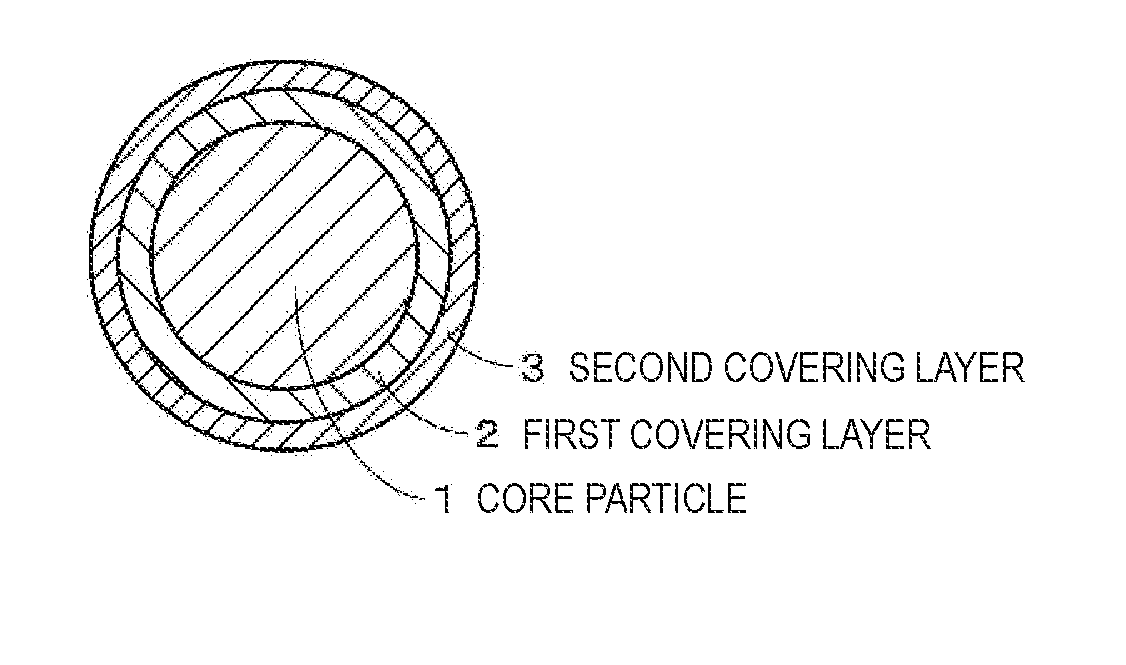
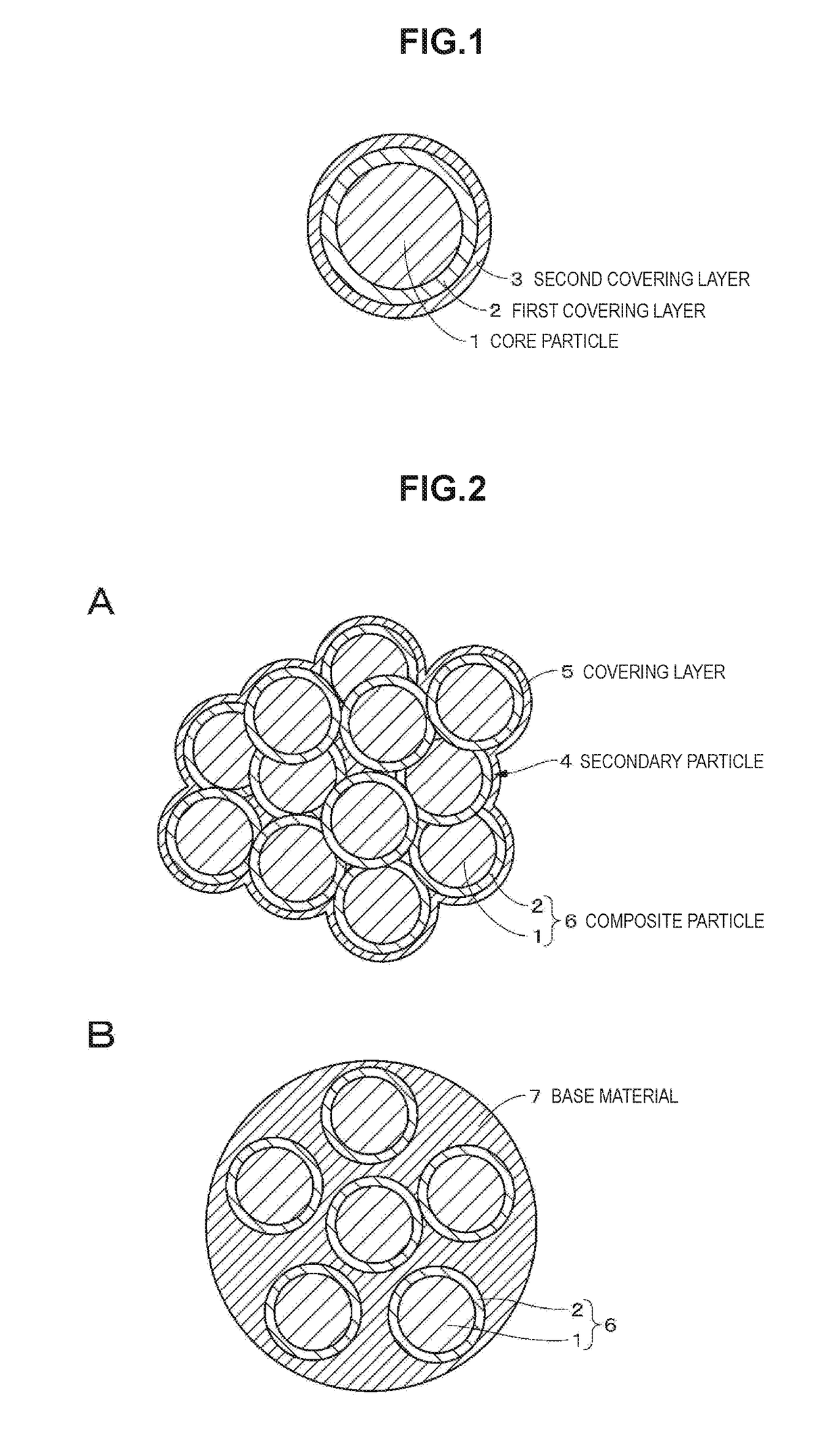
A positive electrode active material includes: a particle including a lithium composite oxide; a first layer that is provided on a surface of the particle and includes a lithium composite oxide; and a second layer that is provided on a surface of the first layer. The lithium composite oxide included in the particle and the lithium composite oxide included in the first layer have the same composition or almost the same composition, the second layer includes an oxide or a fluoride, and the lithium composite oxide included in the first layer has lower crystallinity than the lithium composite oxide included in the particle.
Owner:MURATA MFG CO LTD
Use Of An Ammonia Storage Device In Production Of Energy
InactiveUS20070207351A1Reactant parameters controlMagnesium halidesAlkaline earth metalAmmonia storage
An electric power generating unit comprising (i) an ammonia storage device in the form of a container comprising an ammonia absorbing and releasing salt of the general formula: Ma(NH3)nXz, wherein M is one or more cations selected from alkali metals, alkaline earth metals, and transition metals such as Li, K, Mg, Ca, V, Cr, Mn, Fe, Co, Ni, Cu or Zn, X is one or more anions selected from fluoride, chloride, bromide, iodide, nitrate, thiocyanate, sulphate, molybdate, phosphate, and chlorate ions, a is the number of cations per salt molecule, Z is the number of anions per salt molecule, and n is the coordination number of 2 to 12. (ii) means for heating said container and ammonia absorbing and releasing salt for releasing ammonia gas and (iiia) a fuel cell for converting ammonia directly into electric power; or (iiib1) a reactor for dissociating ammonia into hydrogen and nitrogen and (iiib2) a fuel cell for converting hydrogen into electric power is useful for large stationary energy producing facilities, but also for use for is useful for large stationary energy producing facilities, but also for use for small rechargeable and / or replaceable power supply units for micro-fabricated or miniaturized ammonia decomposition reactors for use in mobile units and portable devices may be used for large energy producing facilities, and by use of small rechargeable and / or replaceable ammonia storage decomposition reactors, it is also possible to provide energy for mobile units and portable devices.
Owner:AMMINEX
Process for producing lithium-containing composite oxide for positive electrode for lithium secondary battery
ActiveUS20060154146A1Improve featuresSolve the small densityMagnesium halidesCell electrodesAlkaline earth metalNiobium
It is to provide a positive electrode active material for a lithium secondary battery, which has a large volume capacity density and high safety, is excellent in uniform coating properties and is excellent in the charge and discharge cyclic durability and low temperature characteristics even at a high charge voltage. A process for producing a lithium-containing composite oxide represented by the formula LipQqNxMyOzFa (wherein Q is at least one element selected from the group consisting of titanium, zirconium, niobium and tantalum, N is at least one element selected from the group consisting of Co, Mn and Ni, M is at least one element selected from the group consisting of Al, alkaline earth metal elements and transition metal elements other than the Q element and the N element, 0.9≦p≦1.1, 0<q≦0.03, 0.97≦x<1.00, 0≦y<0.03, 1.9≦z≦2.1, q+x+y=1 and 0≦a≦0.02) from a lithium source, an Q element source and an N element source, and if necessary, at least one source selected from the group consisting of an M element source and a fluorine source, characterized by using as the Q element source an Q element compound aqueous solution having a pH of from 0.5 to 11.
Owner:SUMITOMO CHEM CO LTD
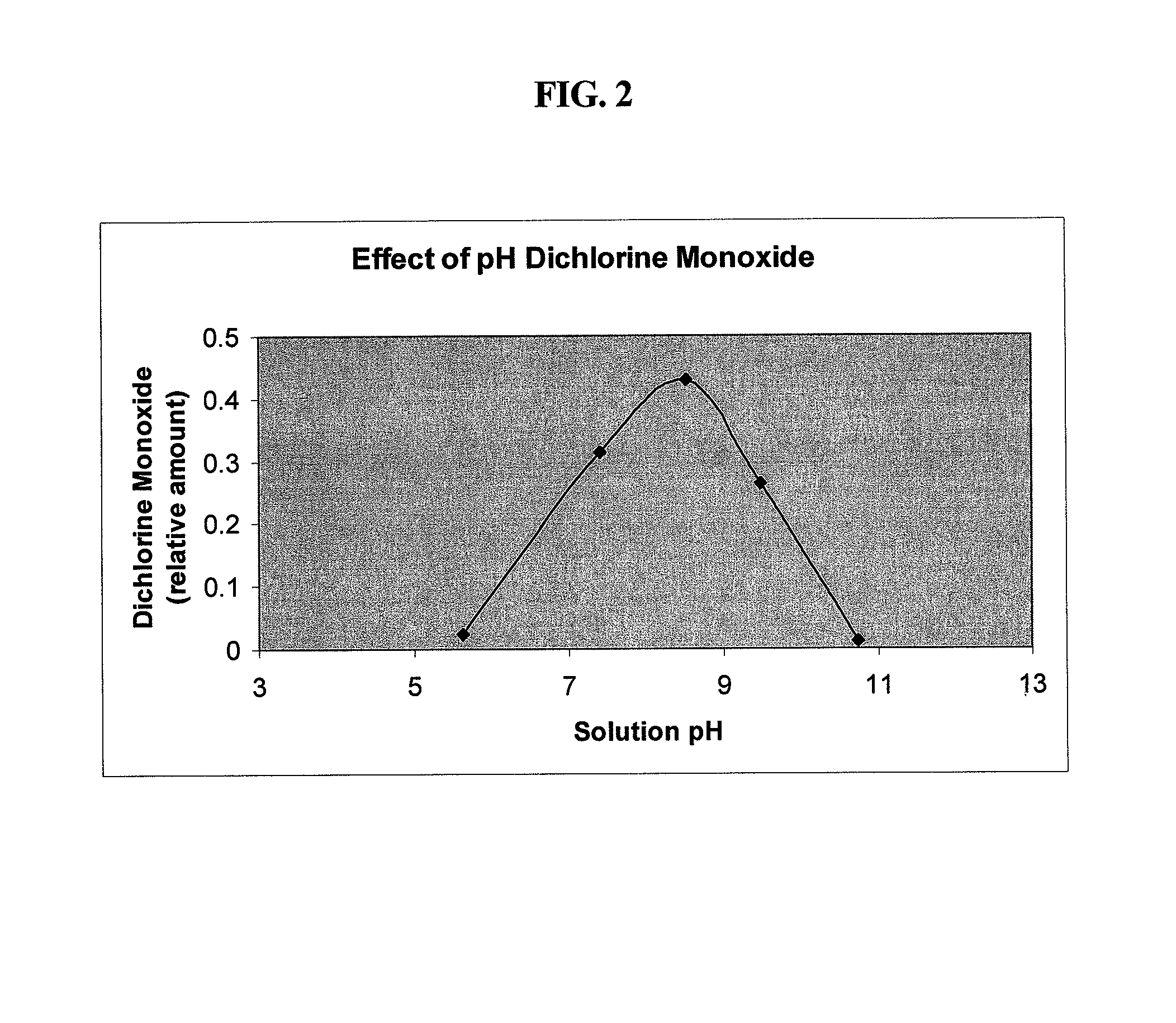
Antimicrobial solutions containing dichlorine monoxide and methods of making and using the same
Methods and products are provided for treating a wound or infection in a mammal or disinfecting a surface with a hypochlorous acid solution that has been activated by a catalyst. Additionally provided is a process for preparing an antimicrobial product that produces an activated hypochlorous acid solution for use as an antimicrobial.
Owner:MICROSAFE GRP DMCC
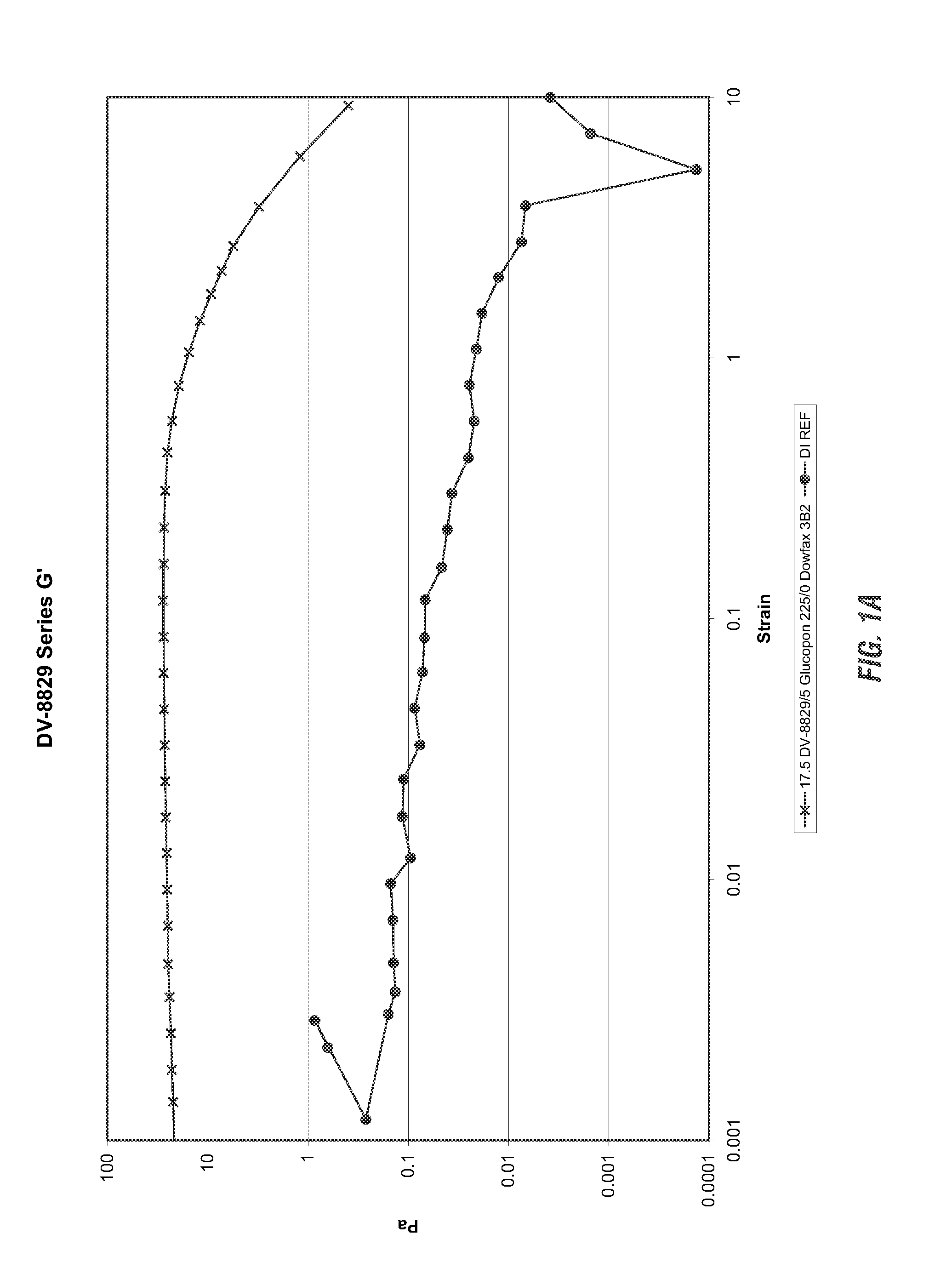
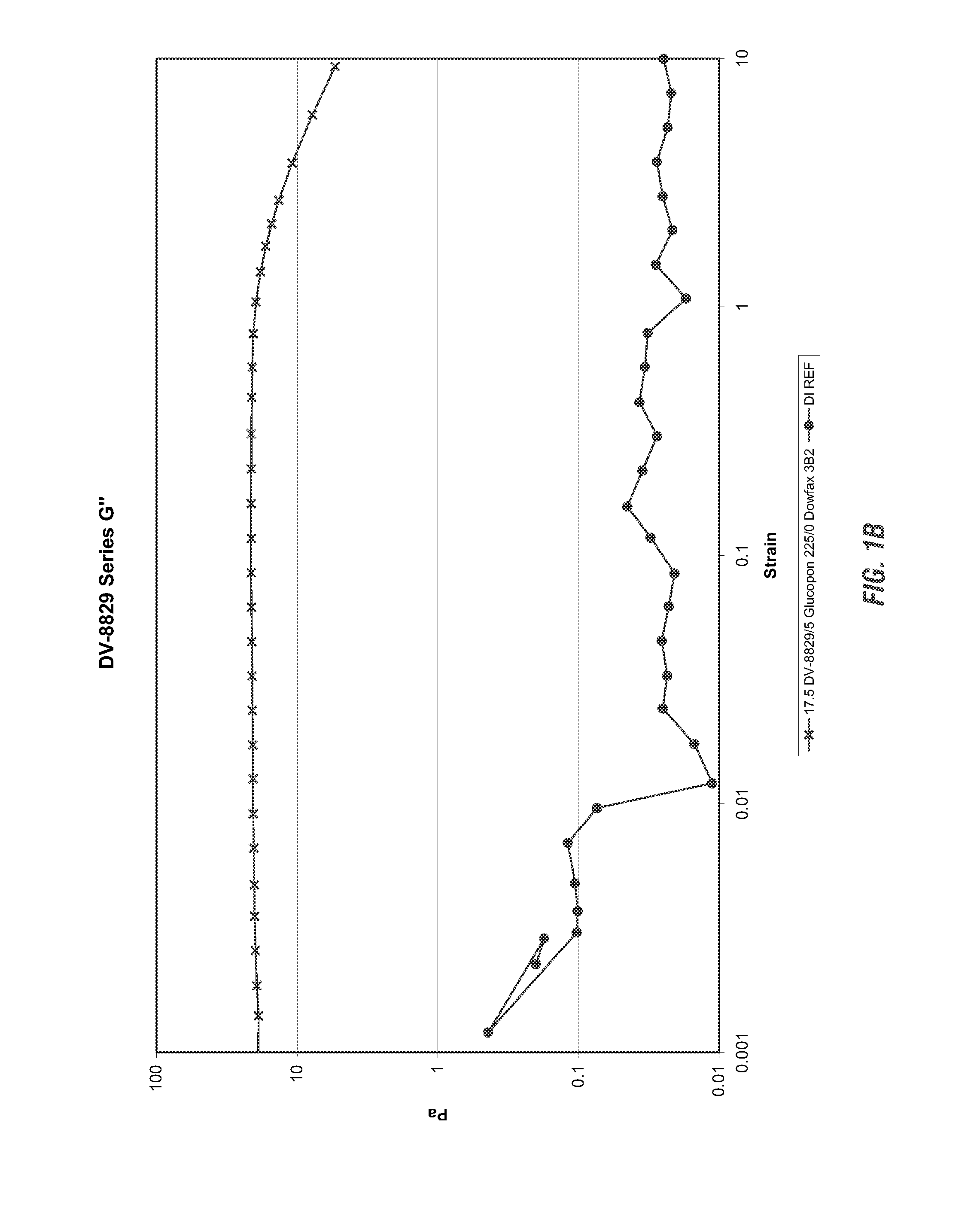
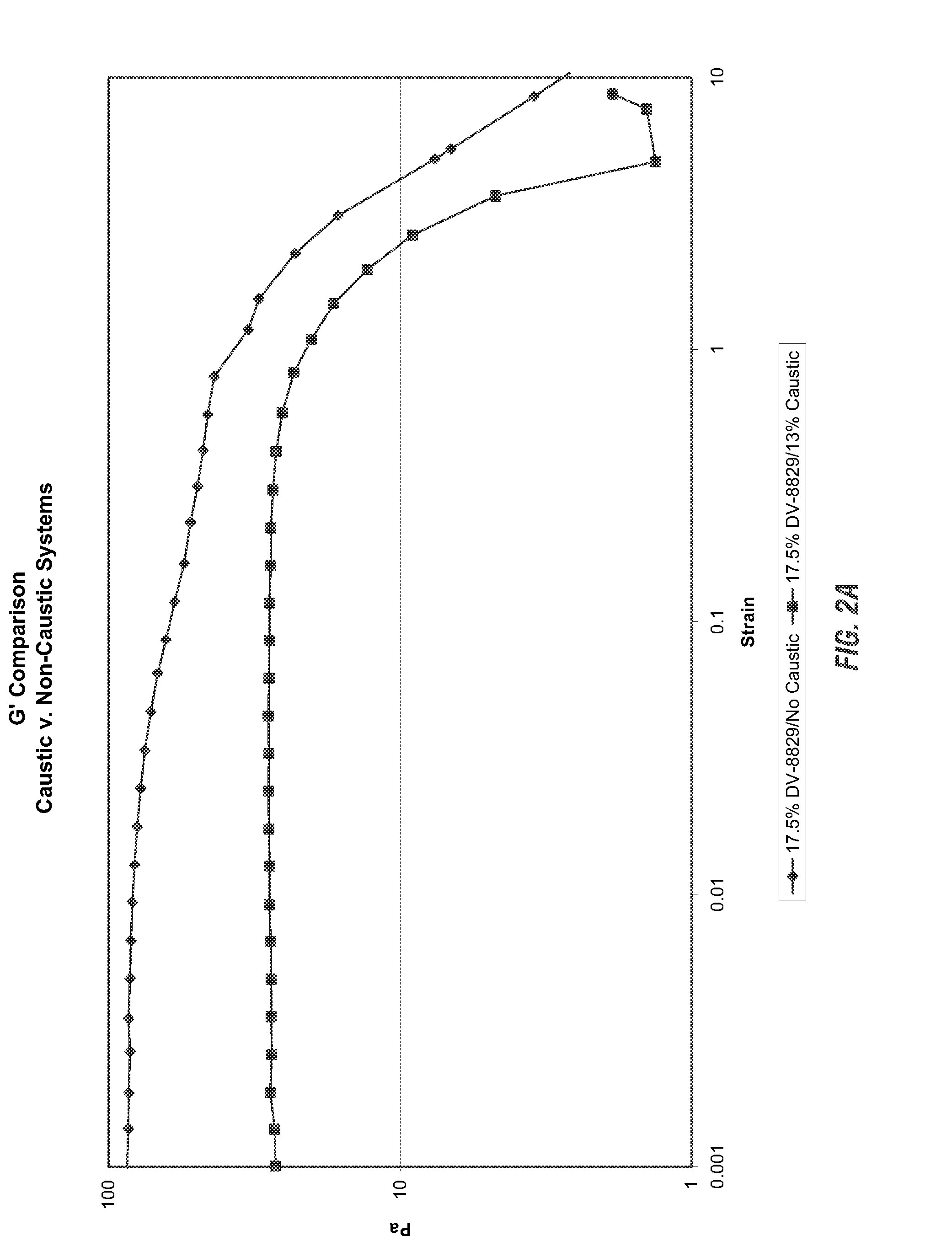
Viscoelastic surfactant based cleaning compositions
ActiveUS20140148371A1Improve performanceAdditional componentInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsViscoelasticitySURFACTANT BLEND
Alkaline or neutral viscoelastic cleaning compositions are disclosed which use non polymer thickening agents. According to the invention, cleaning compositions have been developed using viscoelastic surfactants in a neutral, acidic or alkaline cleaning formulations. These provide the dual benefit of thickening as well as an additional cleaning, thereby improving performance. Applicants have also identified several pseudo linking agents which when, used with viscoelastic surfactants provide viscoelasticity in alkaline cleaning compositions.
Owner:ECOLAB USA INC
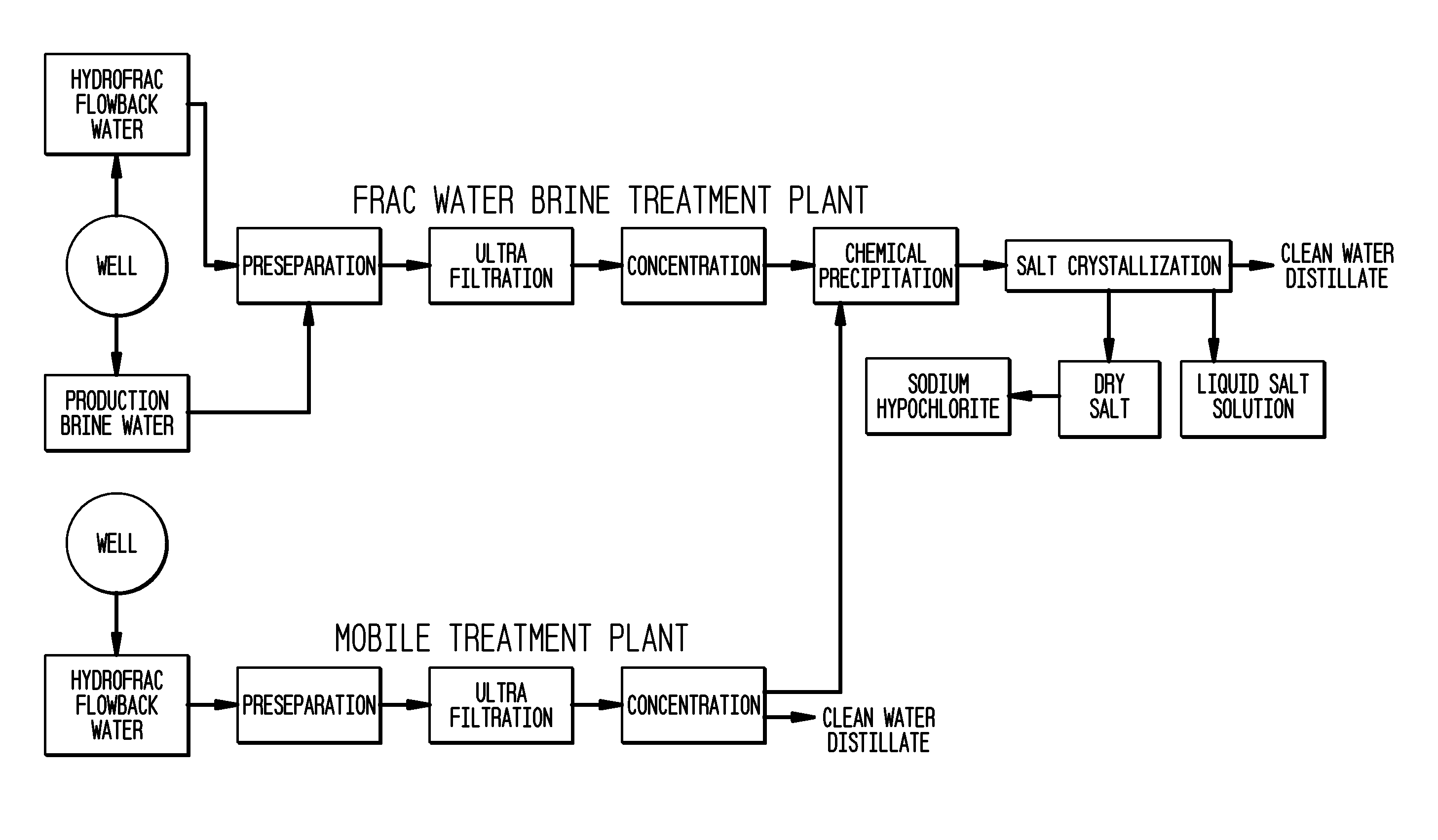
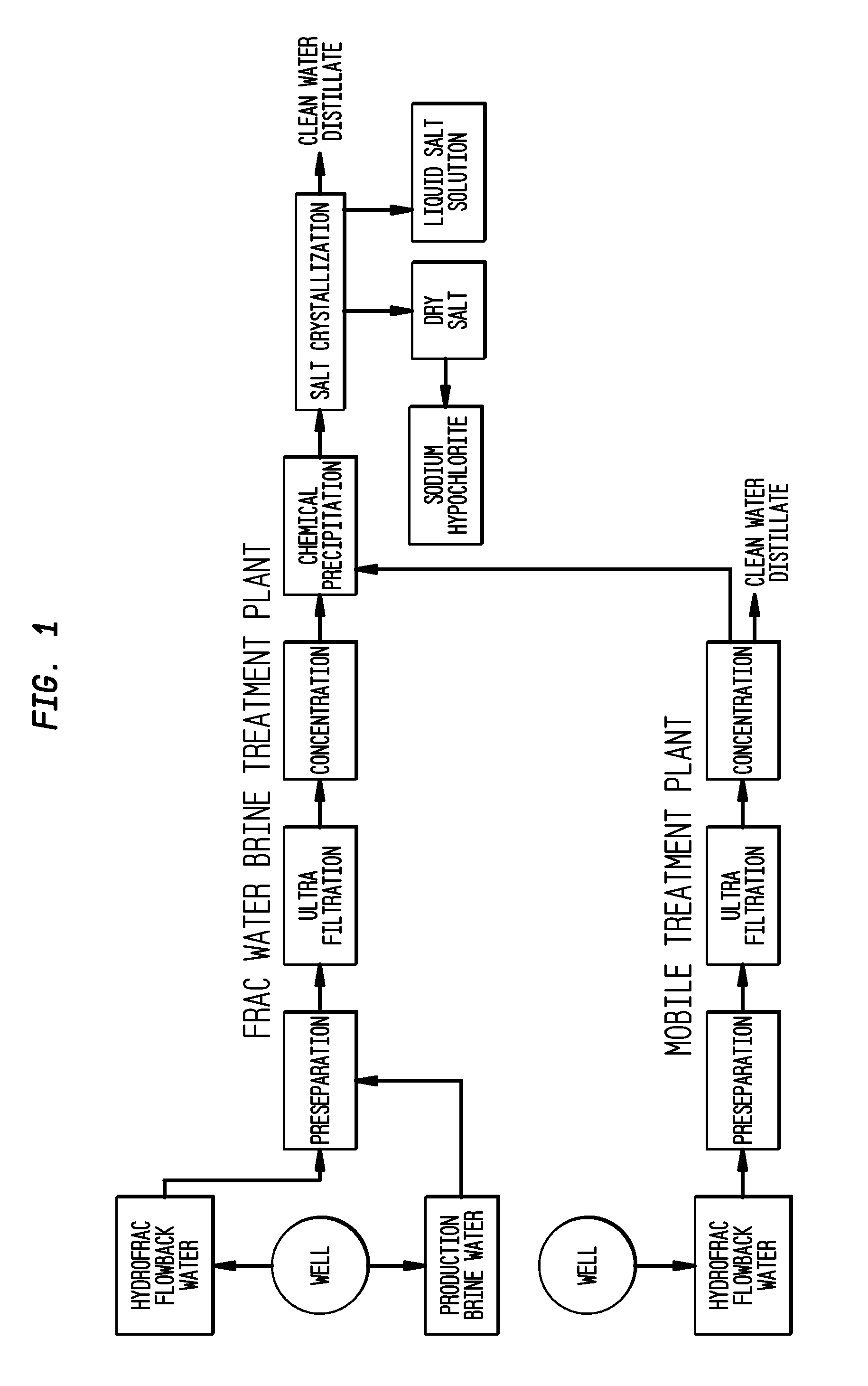
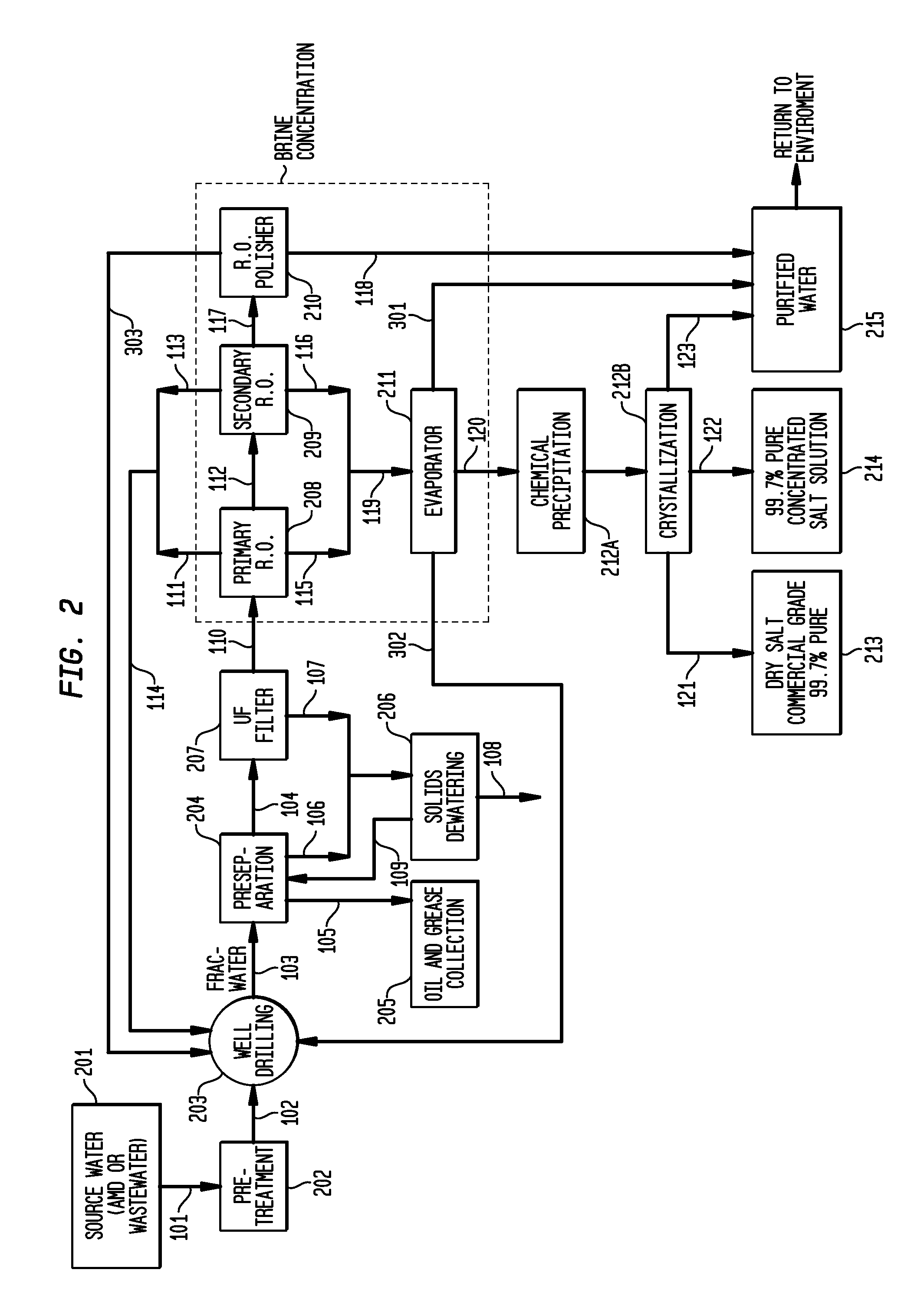
Method of making pure salt from FRAC-water/wastewater
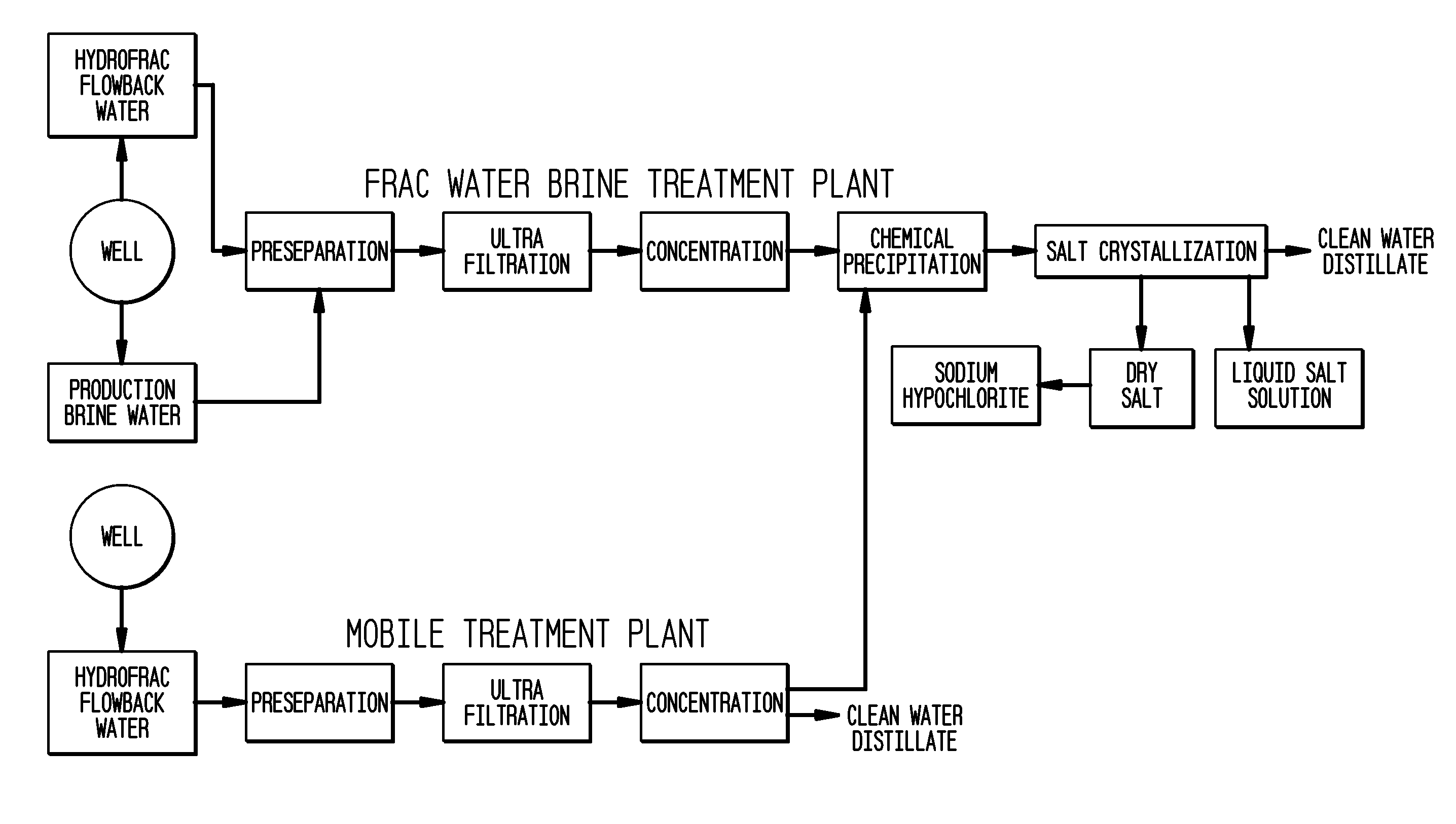
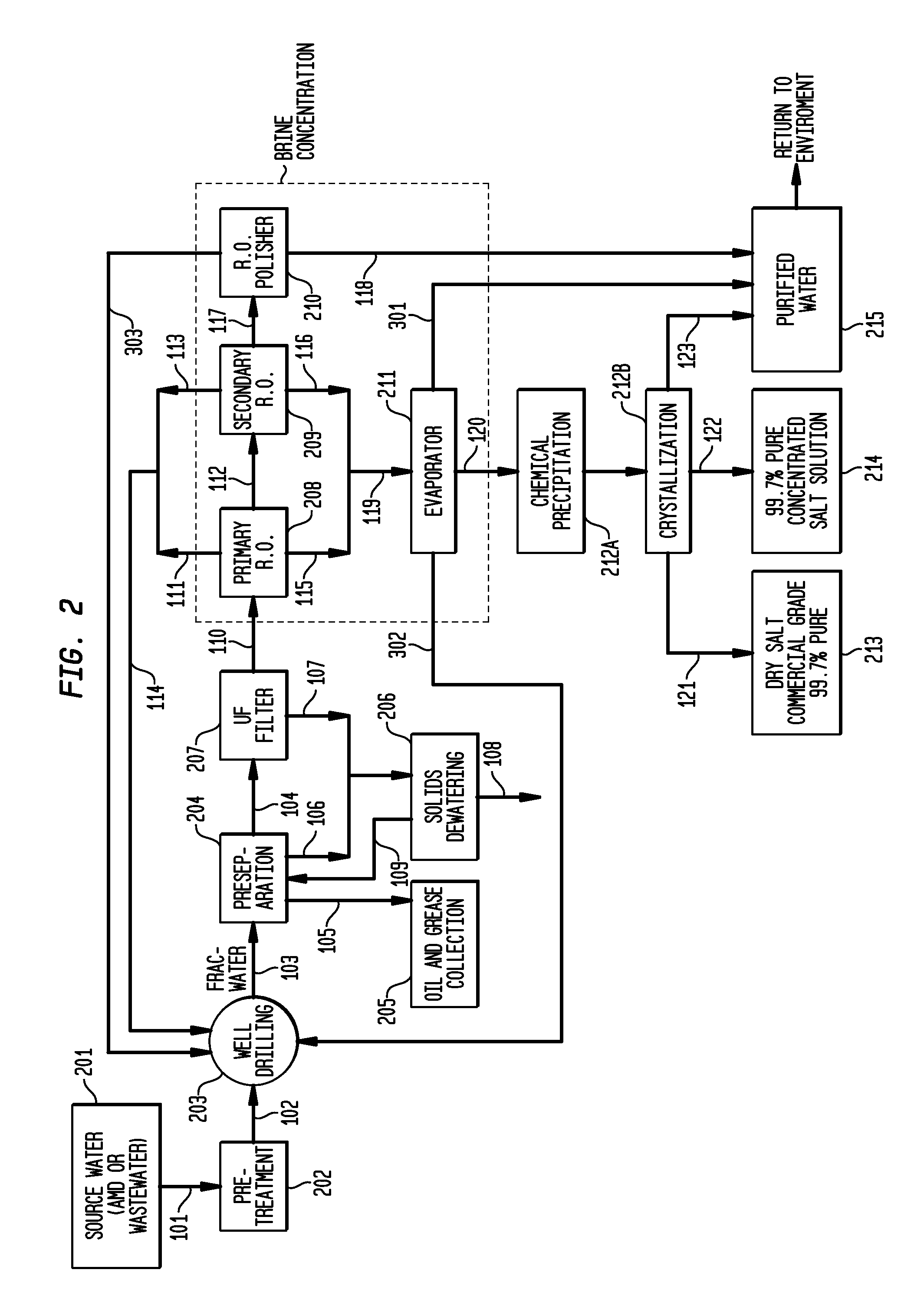
ActiveUS8158097B2Yield maximizationQuality improvementCalcium/strontium/barium carbonatesCalcium/strontium/barium chloridesWater useParticulates
The present invention relates to a method for making pure salt comprises recapturing post-drilling flowback water from hydro-fracturing; removing oil from the flowback water; filtering the flowback water using an ultra filter with a pore size of about 0.1 microns or less to remove solid particulates and large organic molecules, such as benzene, ethylbenzene, toluene, and xylene, from the water; concentrating the flowback water to produce a brine that contains from about 15 wt % to about 40 wt % of salt relative to the total weight of the flowback brine; performing one or more chemical precipitation process using an effective amount of reagents to precipitate out the desired high quality commercial products, such as, barium sulfate, strontium carbonate, calcium carbonate; and crystallizing the chemically treated and concentrated flowback brine to produce greater than 99.5% pure salt products, such as sodium and calcium chloride.
Owner:FRACPURE HLDG
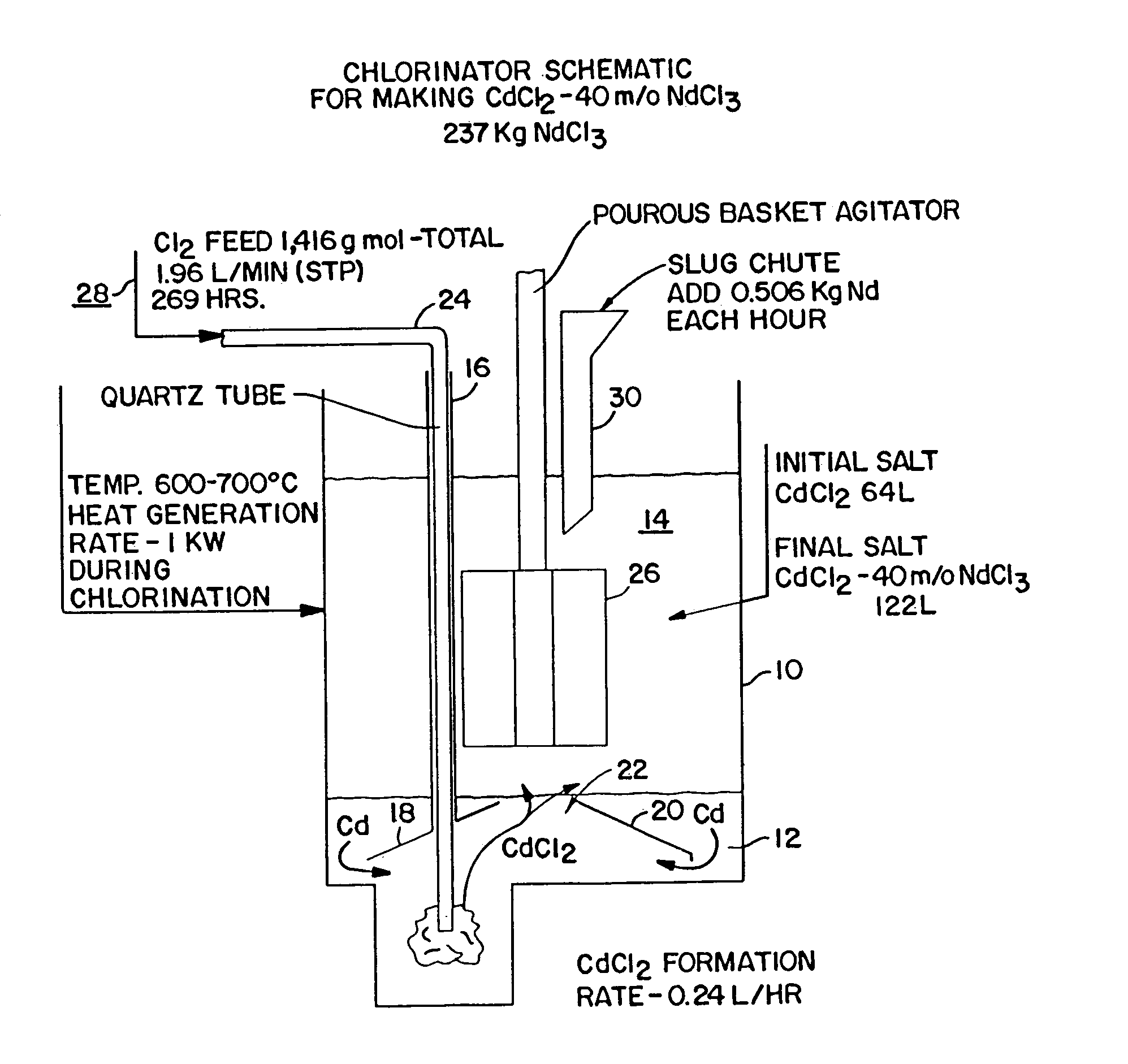
Apparatus and method for making metal chloride salt product
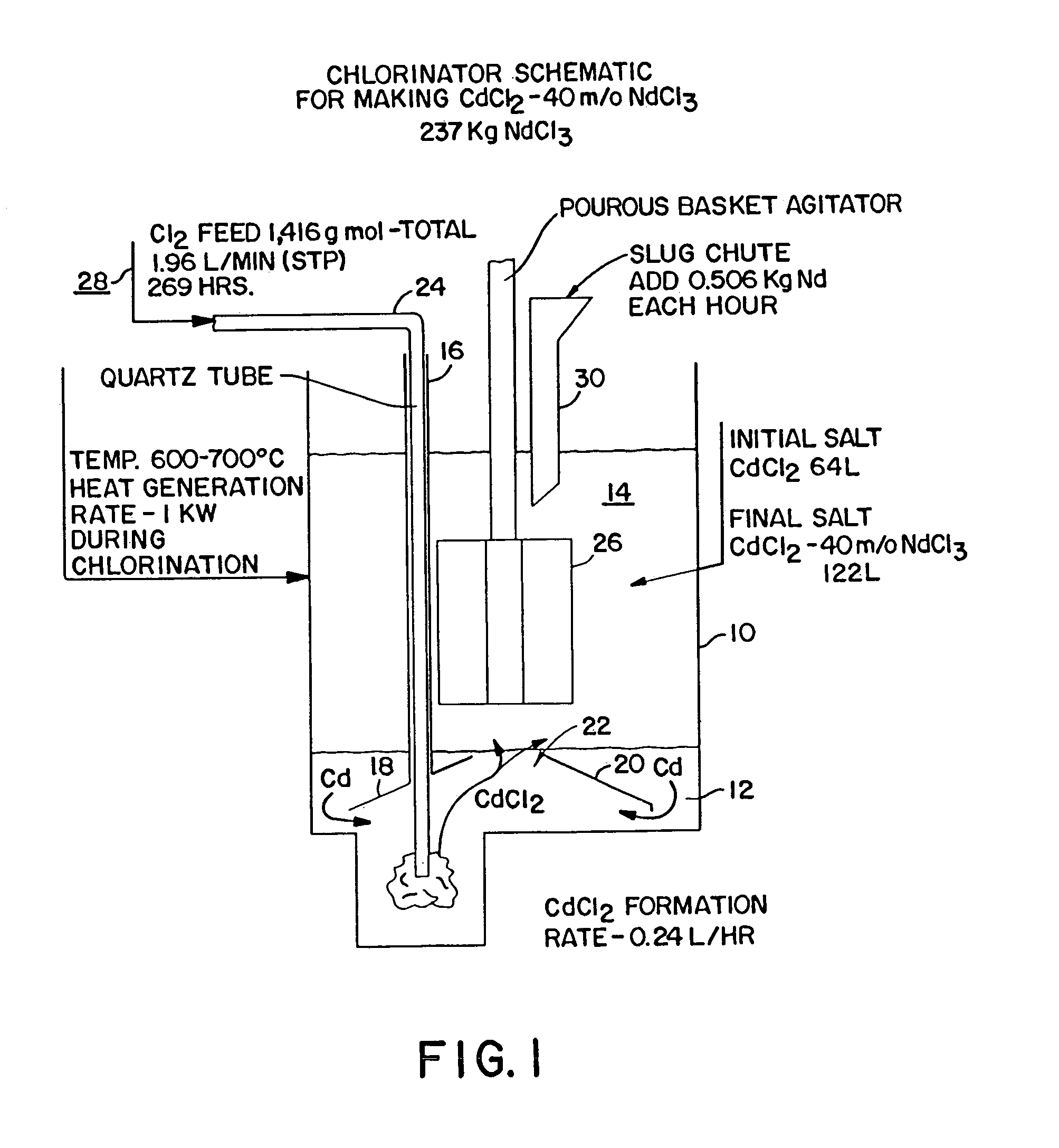
InactiveUS7217402B1Low densityRare earth metal chloridesChloride preparationMetal chlorideDistillation
A method of producing metal chlorides is disclosed in which chlorine gas is introduced into liquid Cd. CdCl2 salt is floating on the liquid Cd and as more liquid CdCl2 is formed it separates from the liquid Cd metal and dissolves in the salt. The salt with the CdCl2 dissolved therein contacts a metal which reacts with CdCl2 to form a metal chloride, forming a mixture of metal chloride and CdCl2. After separation of bulk Cd from the salt, by gravitational means, the metal chloride is obtained by distillation which removes CdCl2 and any Cd dissolved in the metal chloride.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
Method of making pure salt from frac-water/wastewater
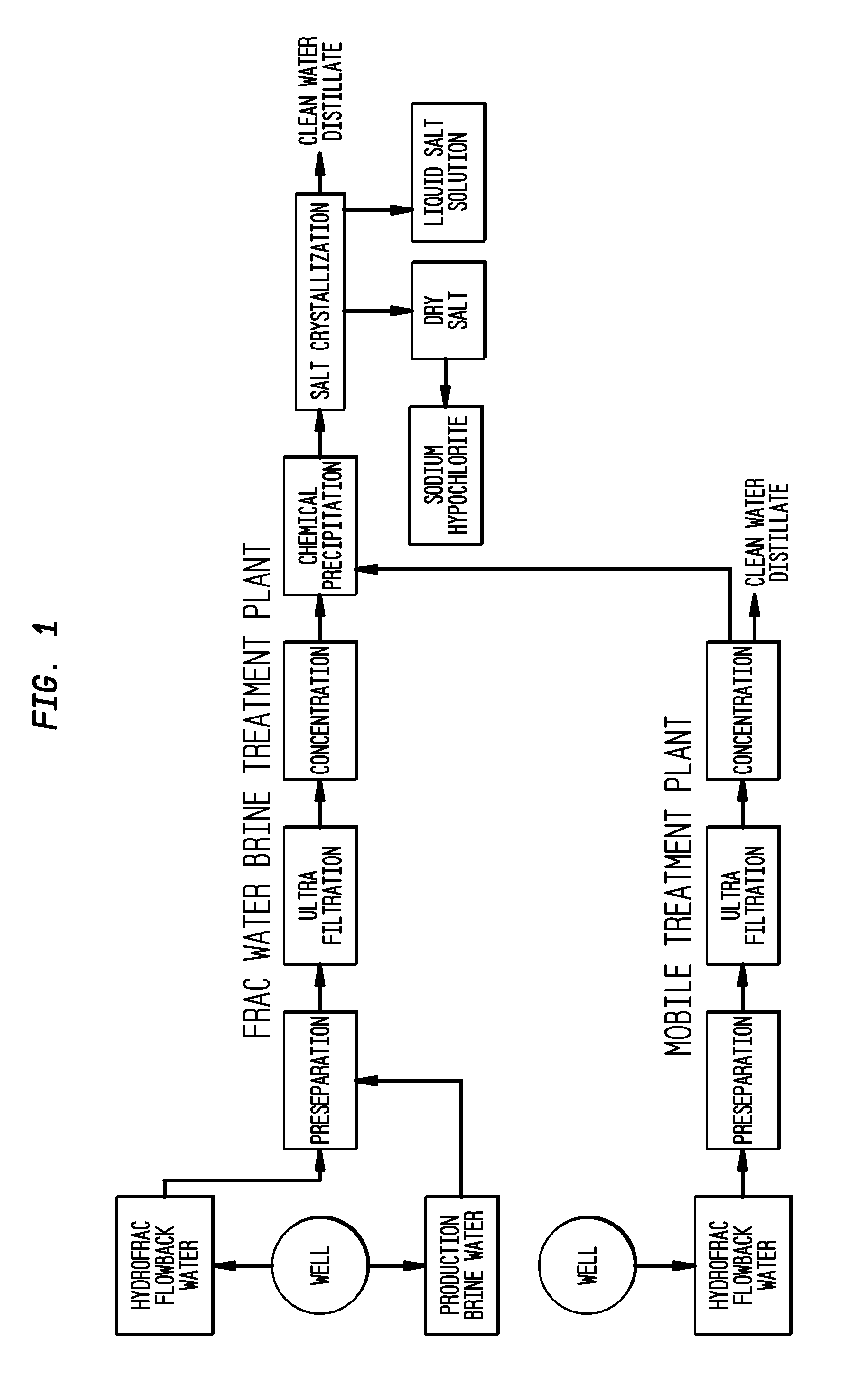
ActiveUS20110104038A1Easy to produceQuality improvementCalcium/strontium/barium carbonatesCalcium/strontium/barium chloridesParticulatesWater use
The present invention relates to a method for making pure salt comprises recapturing post-drilling flowback water from hydro-fracturing; removing oil from the flowback water; filtering the flowback water using an ultra filter with a pore size of about 0.1 microns or less to remove solid particulates and large organic molecules, such as benzene, ethylbenzene, toluene, and xylene, from the water; concentrating the flowback water to produce a brine that contains from about 15 wt % to about 40 wt % of salt relative to the total weight of the flowback brine; performing one or more chemical precipitation process using an effective amount of reagents to precipitate out the desired high quality commercial products, such as, barium sulfate, strontium carbonate, calcium carbonate; and crystallizing the chemically treated and concentrated flowback brine to produce greater than 99.5% pure salt products, such as sodium and calcium chloride.
Owner:FRACPURE HLDG
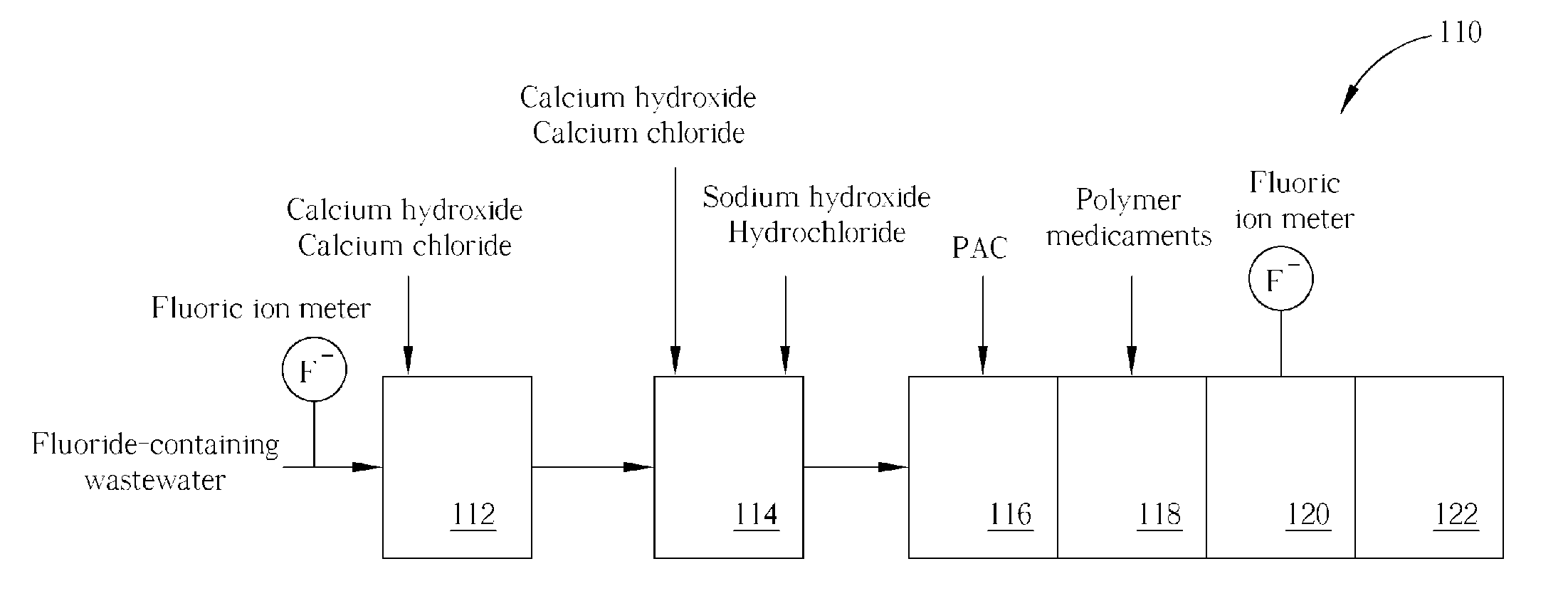
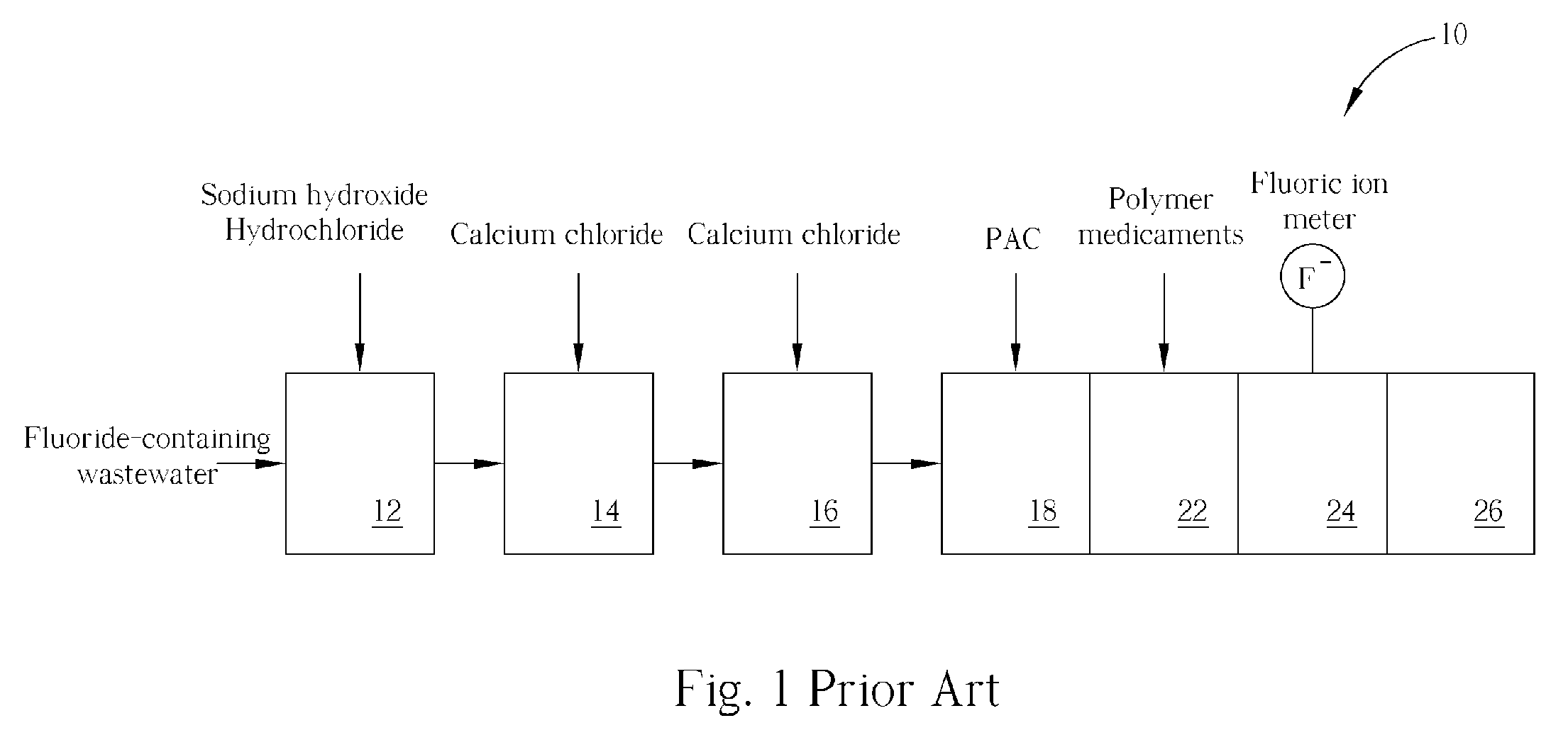
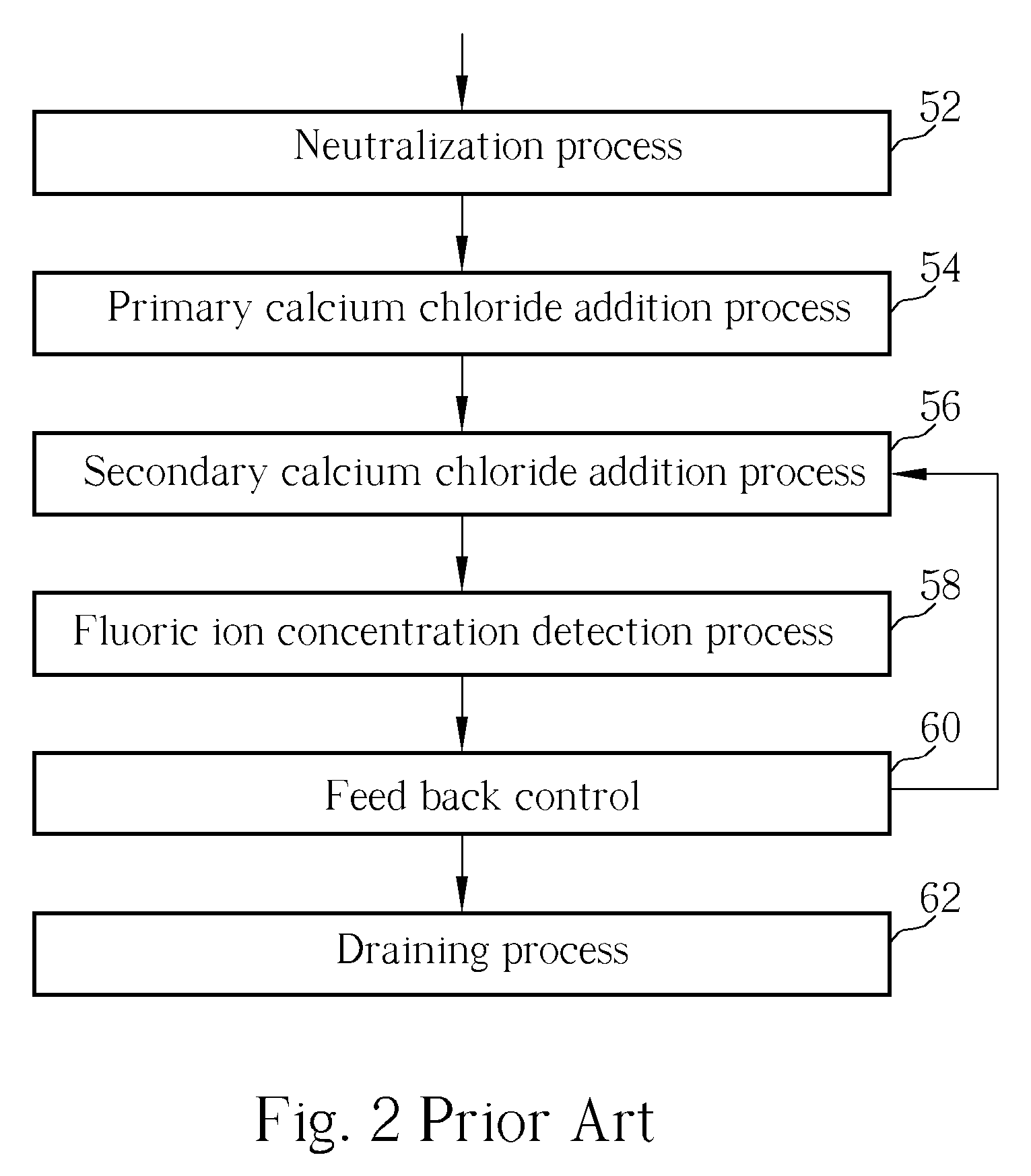
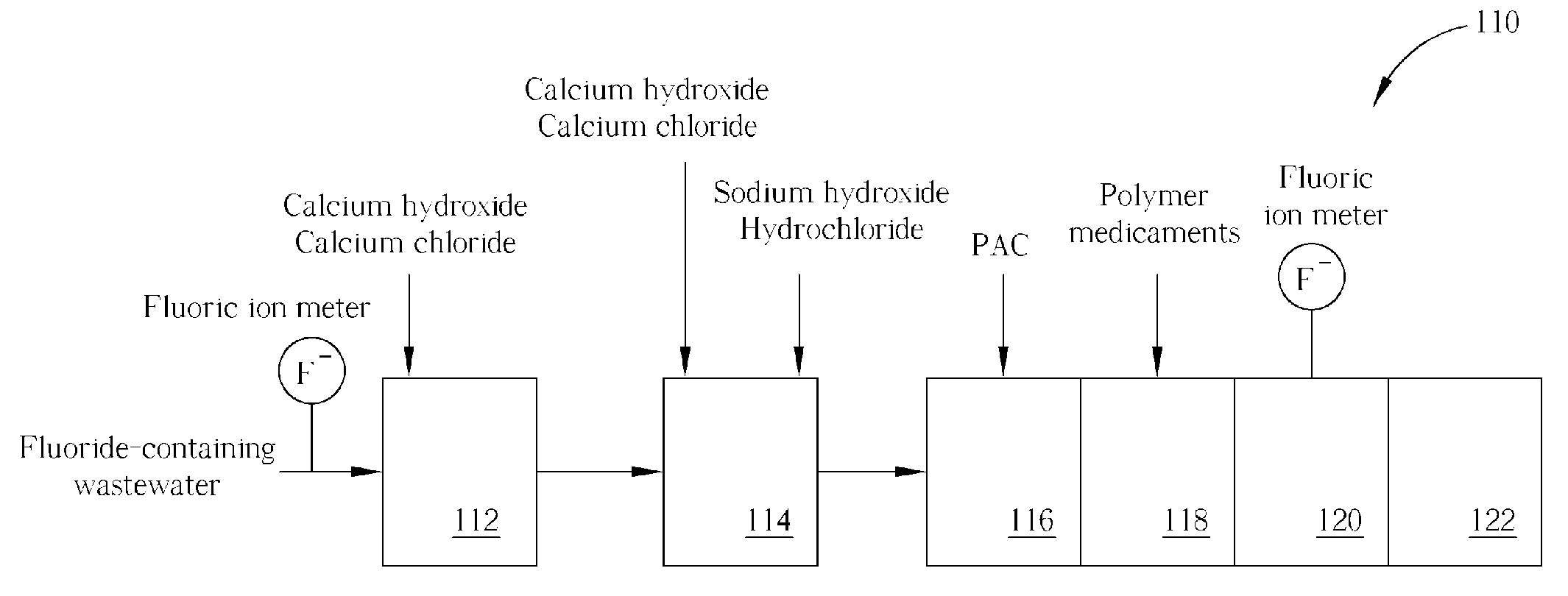
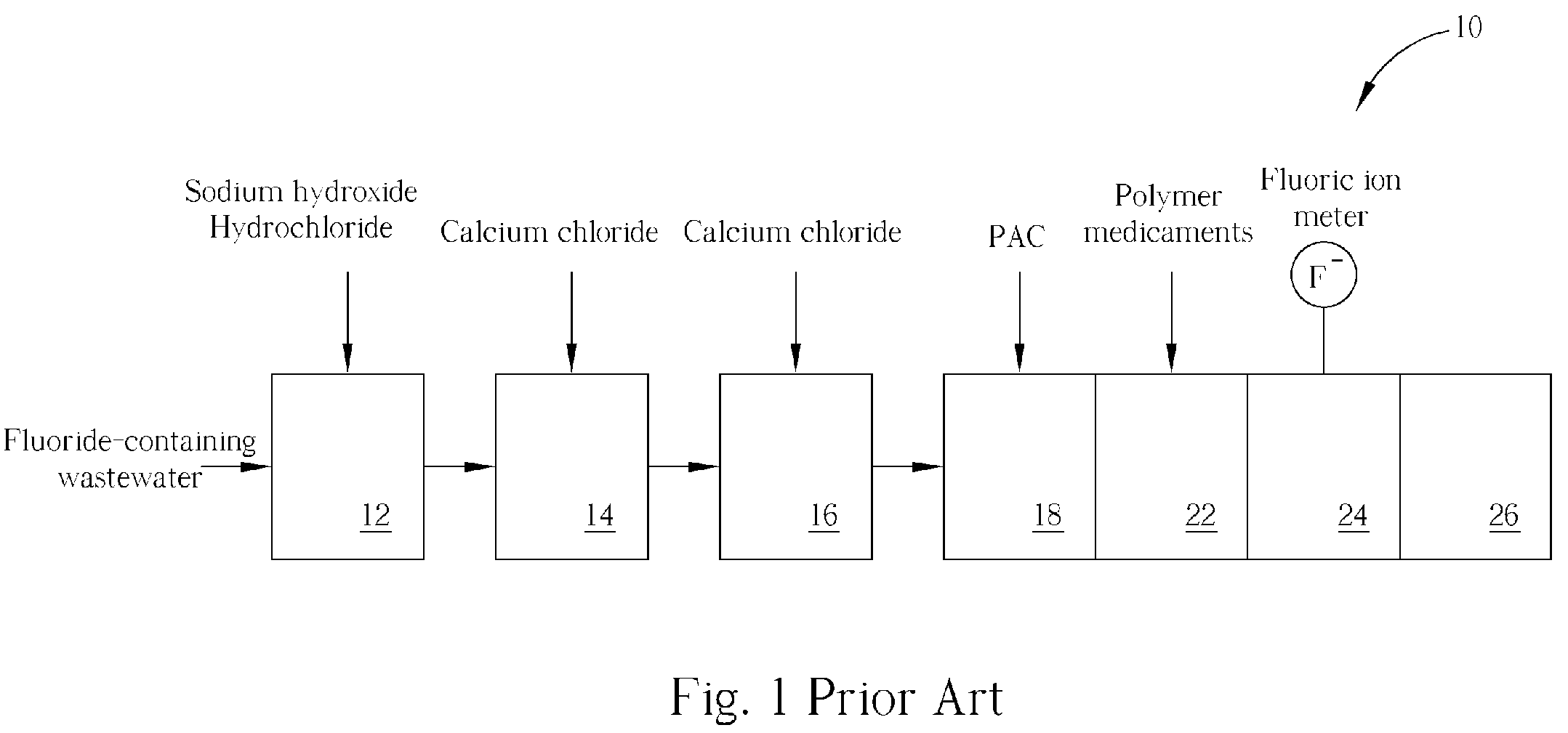
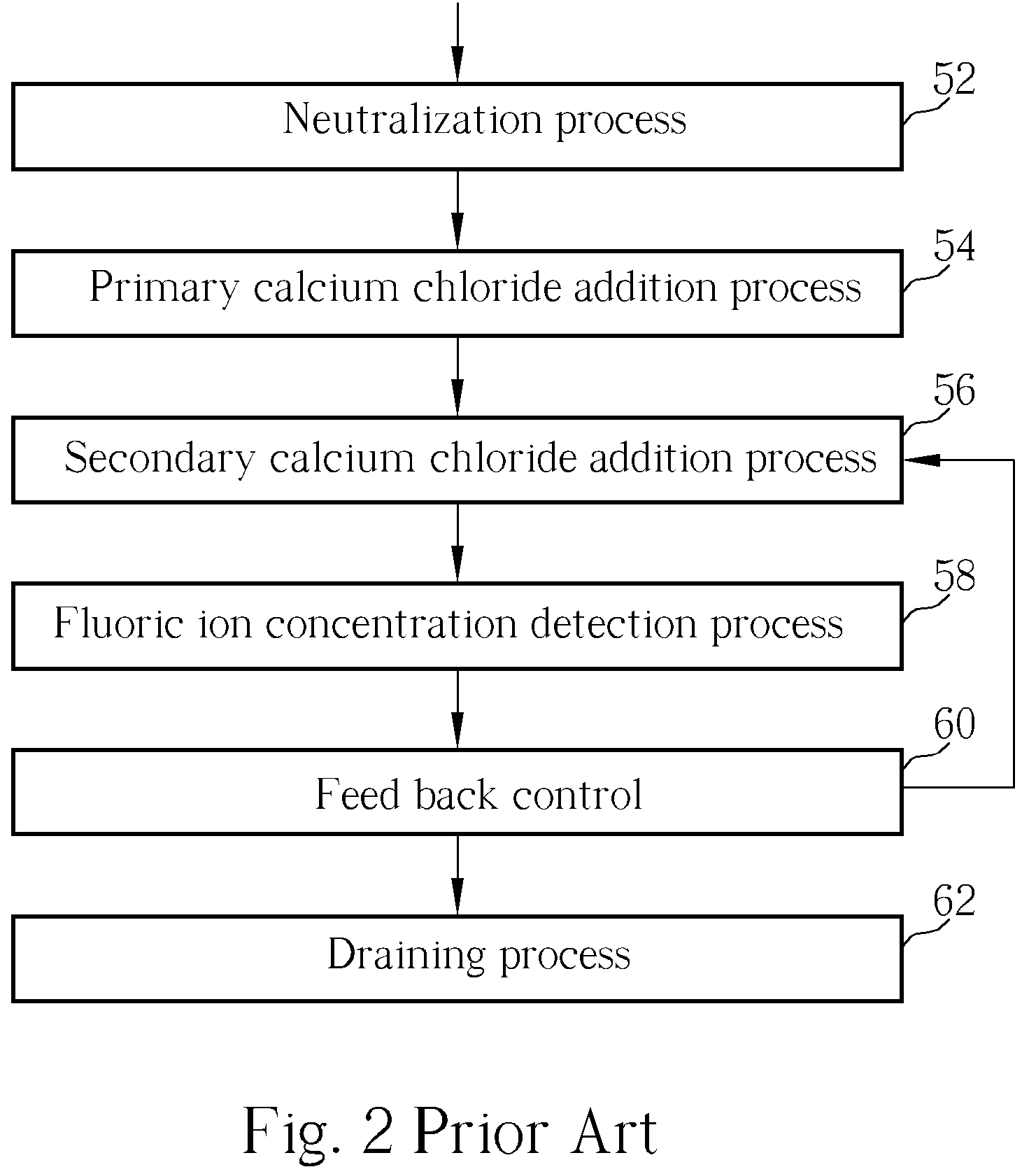
Method of fluoride-containing wastewater treatment
First, a primary fluoric ion concentration detection process is performed, and a primary calcium salt addition process is performed to add calcium salt in a first reaction tank, wherein the dosage of the calcium salt in the primary calcium salt addition process is determined according to the detected fluoric ion concentration. Thereupon, a secondary calcium salt addition process is performed to add calcium salt into the second reaction tank. Following that, a solid-liquid separation process is performed to separate calcium fluoride from the wastewater, and a secondary fluoric ion concentration detection process is performed upon the wastewater after the calcium fluoride is separated. Finally, the dosage of the calcium salt in the secondary calcium salt addition process is determined in a feed back control manner according to the detected fluoric ion concentration.
Owner:POWERCHIP SEMICON MFG CORP
Method for producing a halide brine
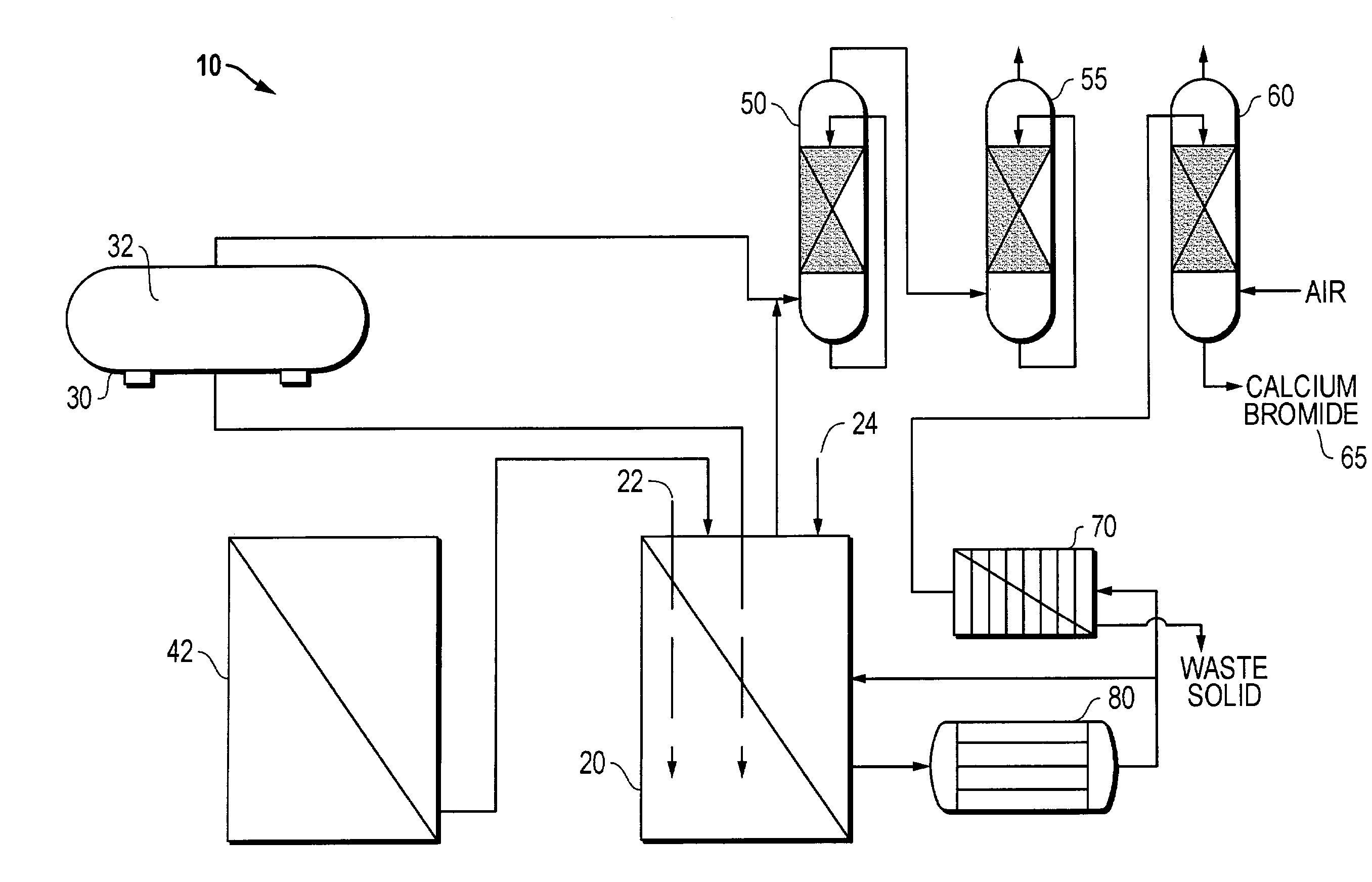
ActiveUS7087209B2Simple and inexpensive methodLow costMagnesium halidesGallium/indium/thallium compoundsHalogenSalt water
A method for producing halide brine wherein an alkali and a reducing agent are added to an aqueous fluid having a density greater than 8.30 lb / gal., (0.996 kg / L) water, waste water or sea water for example. The resulting fluid is then contacted with a halogen to form a halide brine. The reaction occurs in a conventional reactor such as a mixing tank.
Owner:TETRA TECH INC
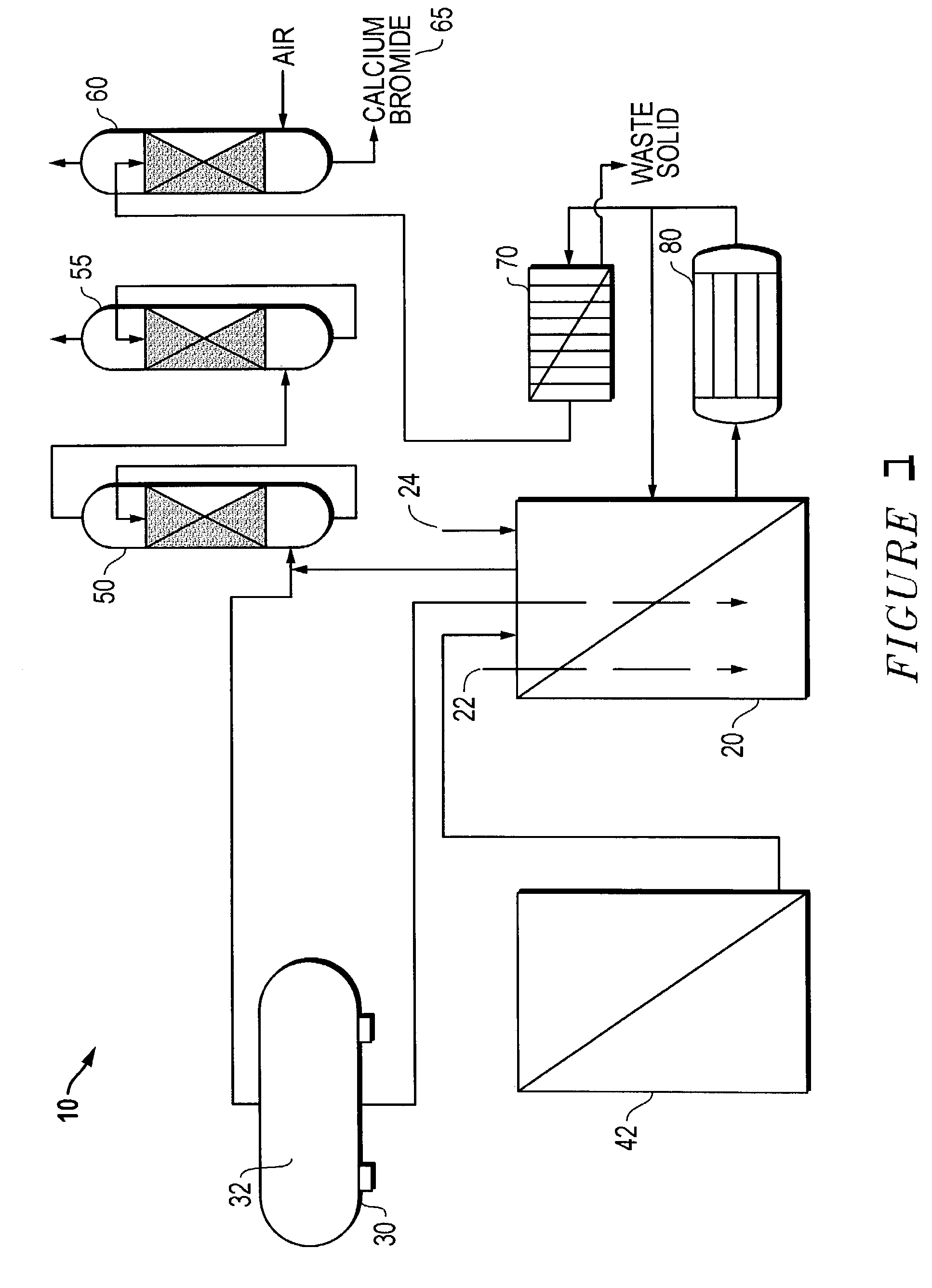
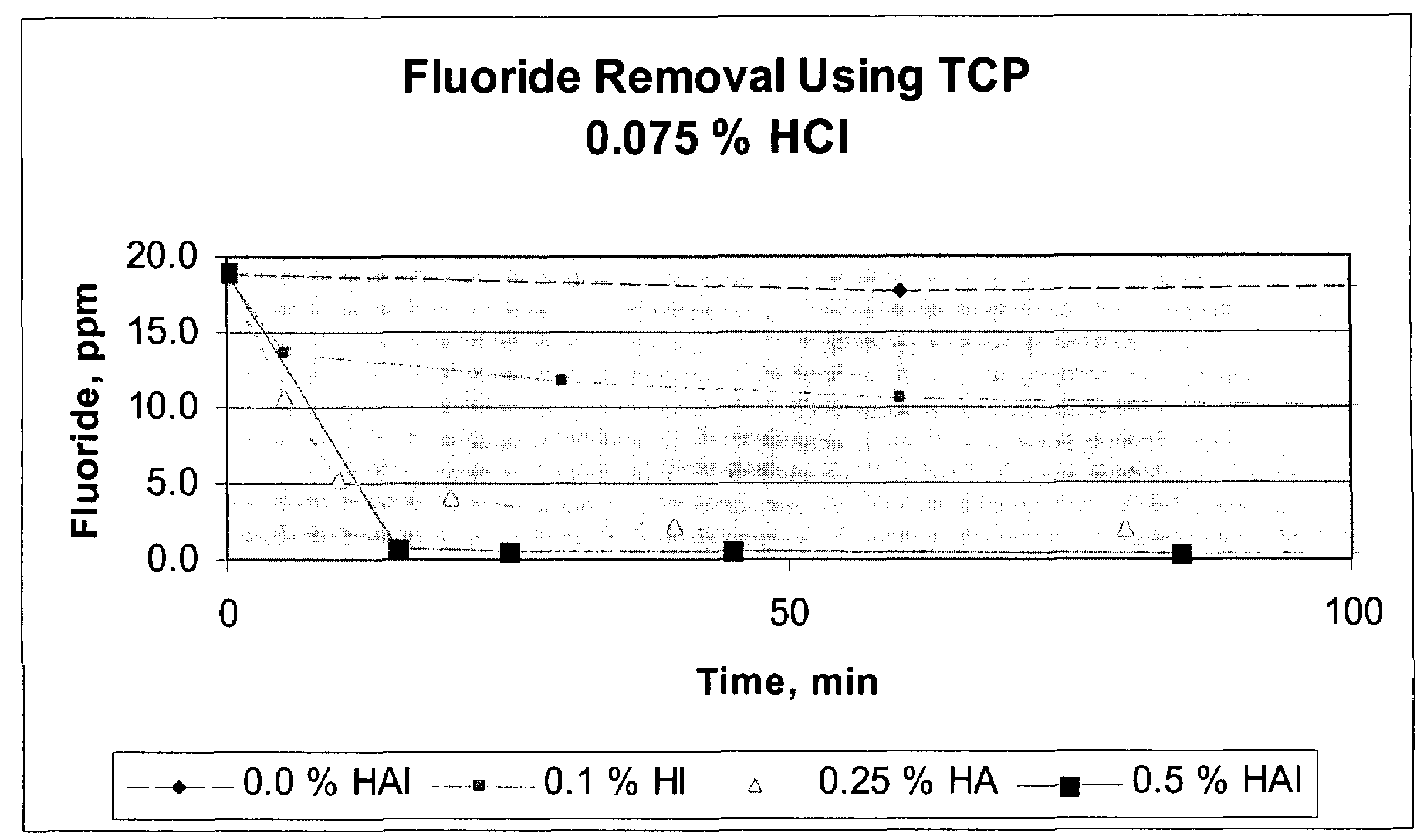
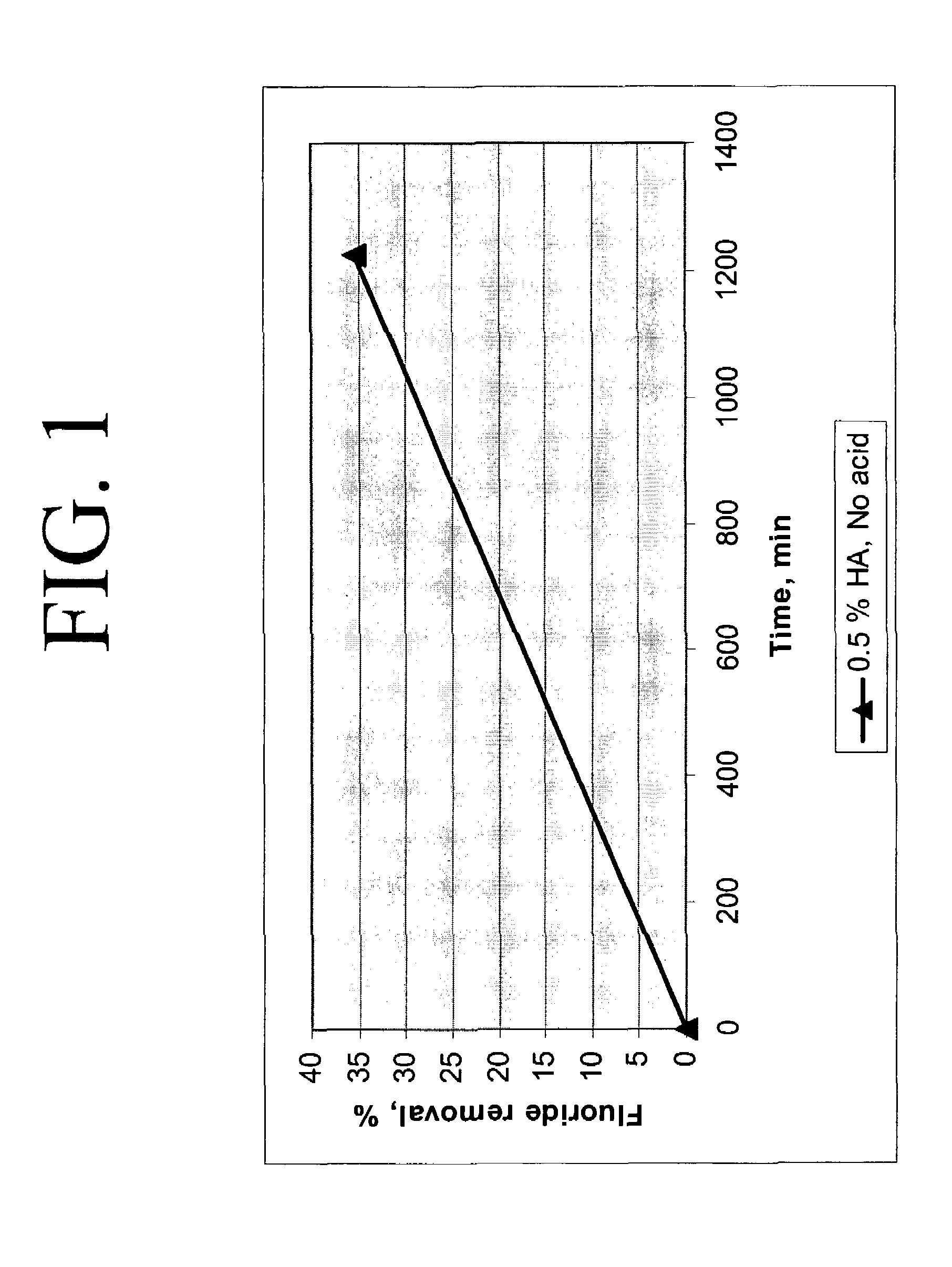
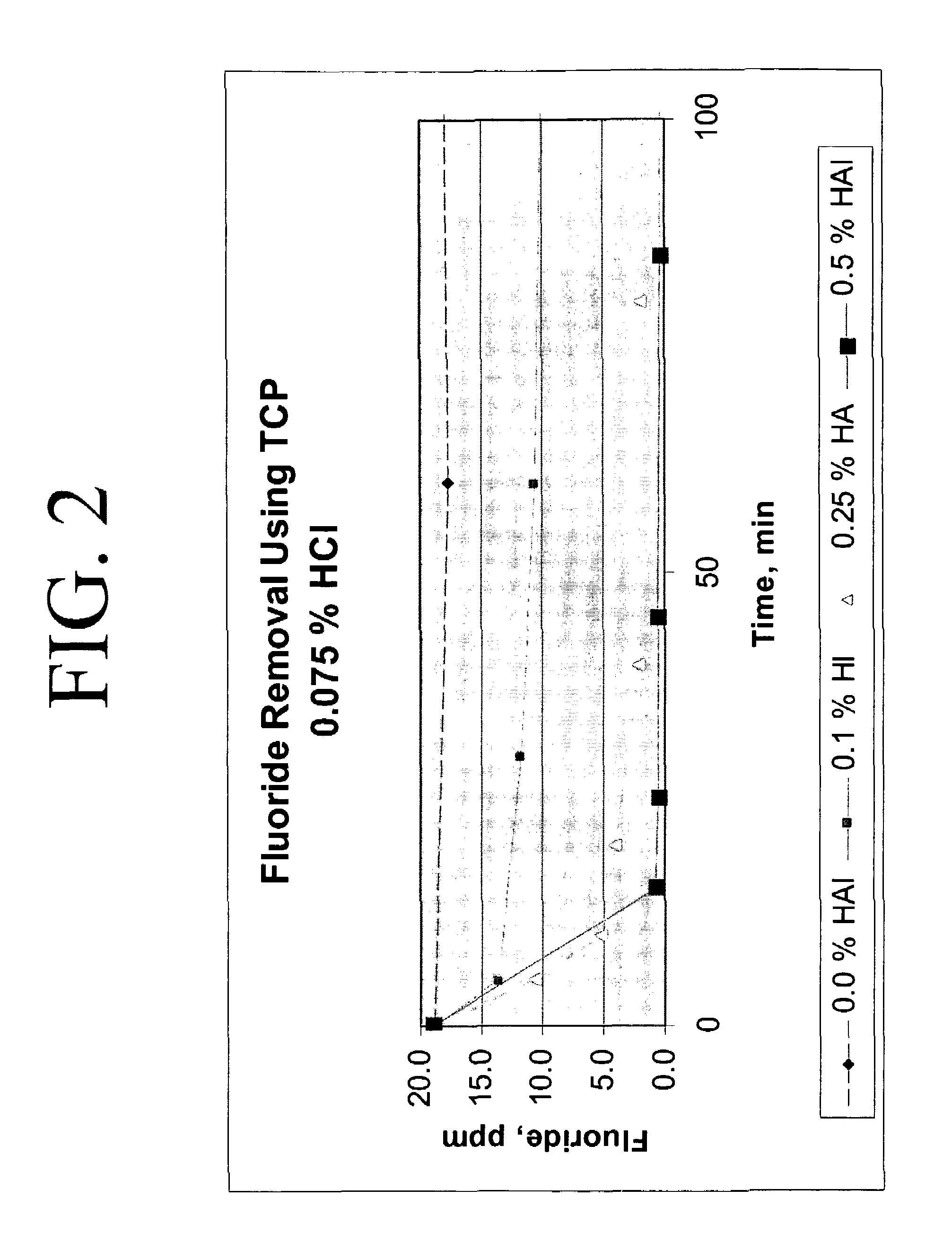
Calcium chloride purification
Significant amounts of soluble fluoride, known to create problems in processes requiring high quality grade calcium chloride, are removed from calcium chloride solution using hydroxyapatite as a removal mechanism. Under acidic conditions, calcium chloride solution is purified to about less than 10 ppm fluoride, significantly, to less than 1 ppm fluoride. At least 0.1 weight percent hydroxyapatite and concentrated hydrochloric acid are added to calcium chloride solution and slurried to remove fluoride and create a highly purified calcium chloride solution, substantially free of fluoride.
Owner:HONEYWELL INT INC
Treatment of solid containing material derived from effluent
InactiveUS6425973B1Reducing and eliminating cost and environmental impactSimpler and cheapCalcium/strontium/barium carbonatesMagnesium halidesParticulatesSludge
A method of treating solid containing material derived from effluent or sludge from a plant for de-inking paper, the material containing calcium in the form of one or more insoluble calcium compounds, the method including the steps of treating the material with an acid to cause dissolution of the calcium thereby forming a calcium ion-containing solution in which insoluble solids are suspended, separating the solution from the insoluble solids and incinerating the separated solids. The solution containing calcium ions may be treated by adding one or more reagents to form a calcium compound precipitate, eg calcium carbonate. The particulate solids produced following the incineration step and following the precipitate formation may be employed as pigments or fillers in paper making or paper coating.
Owner:IMERYS MINERALS
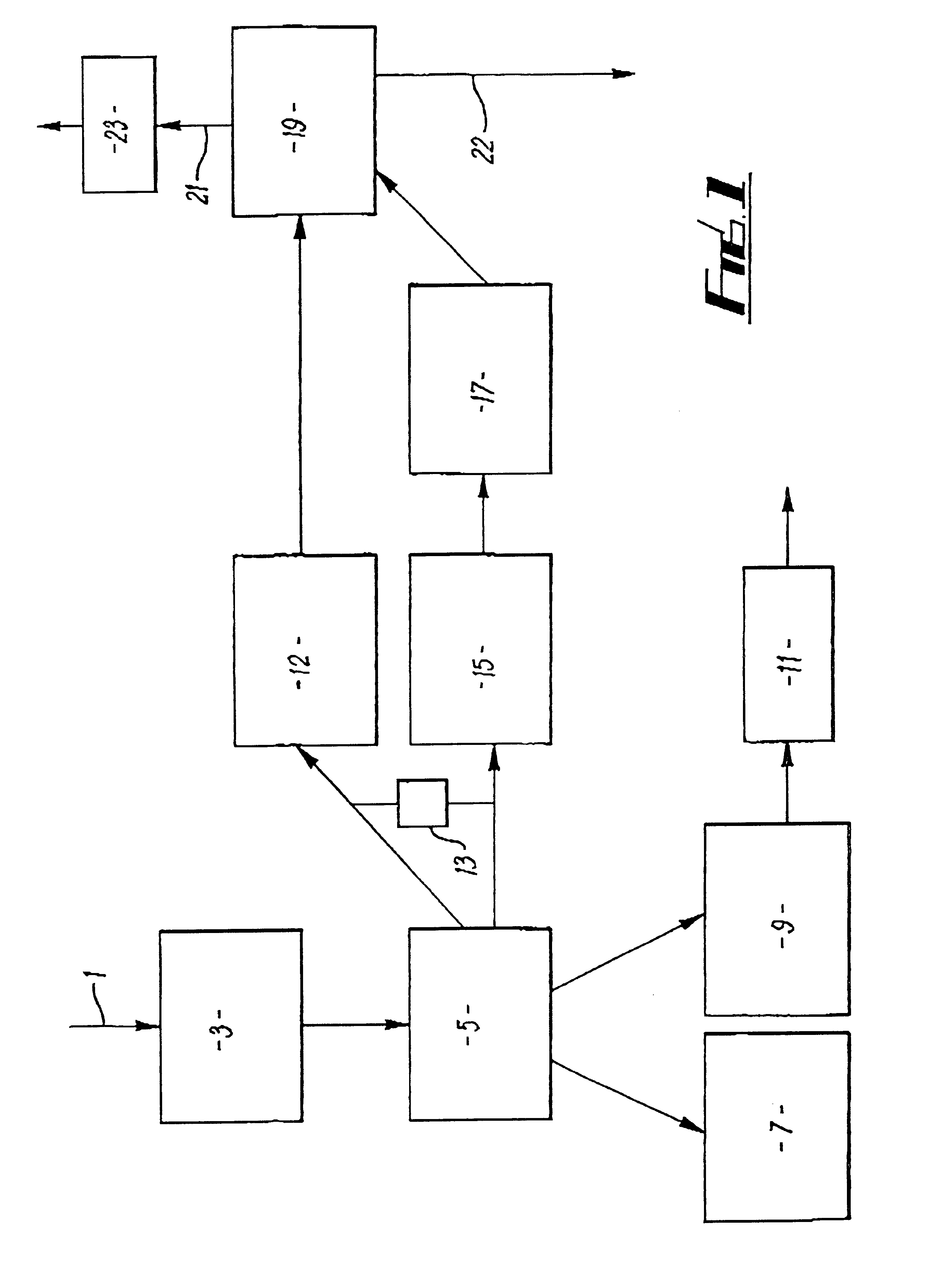
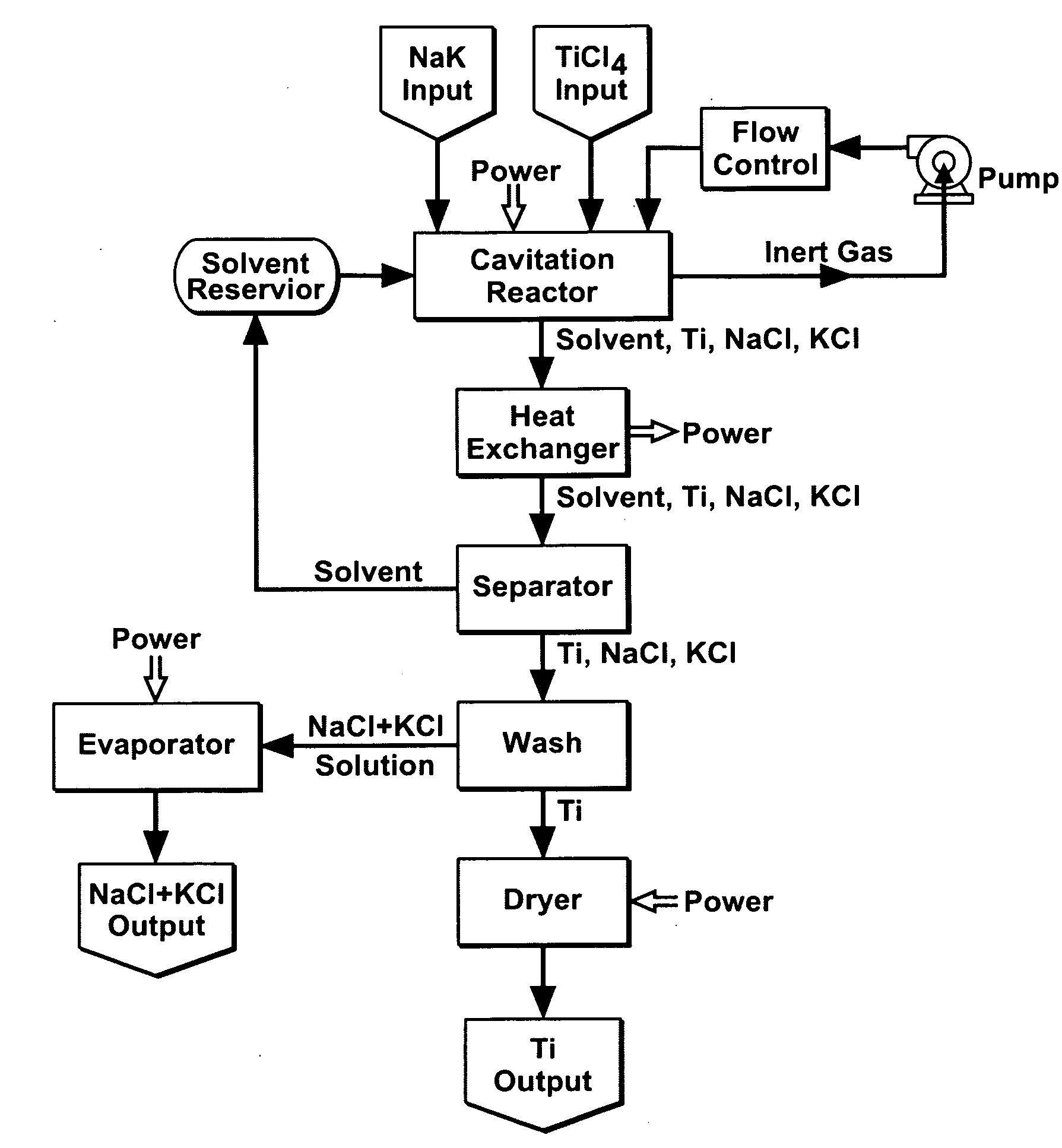
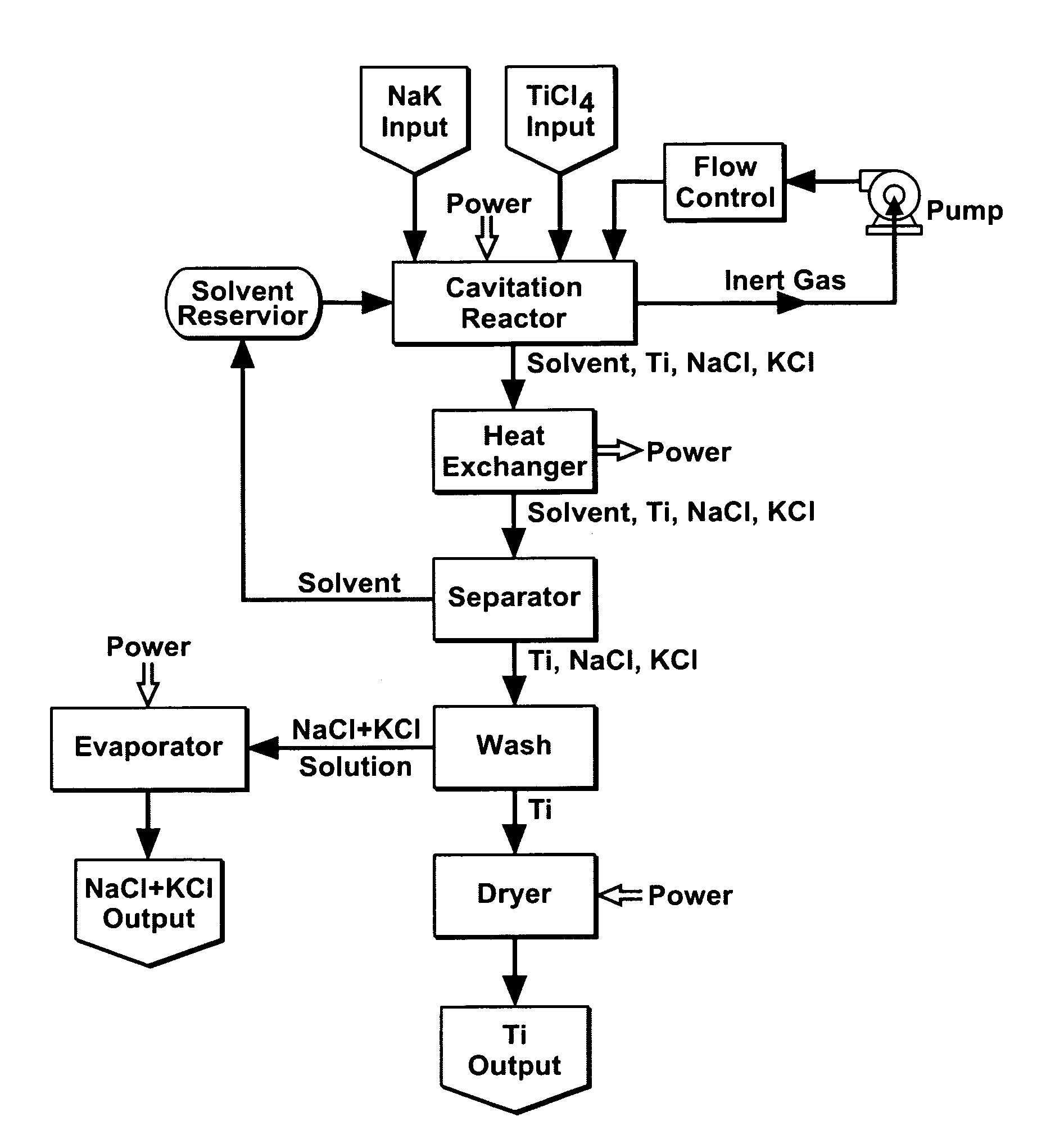
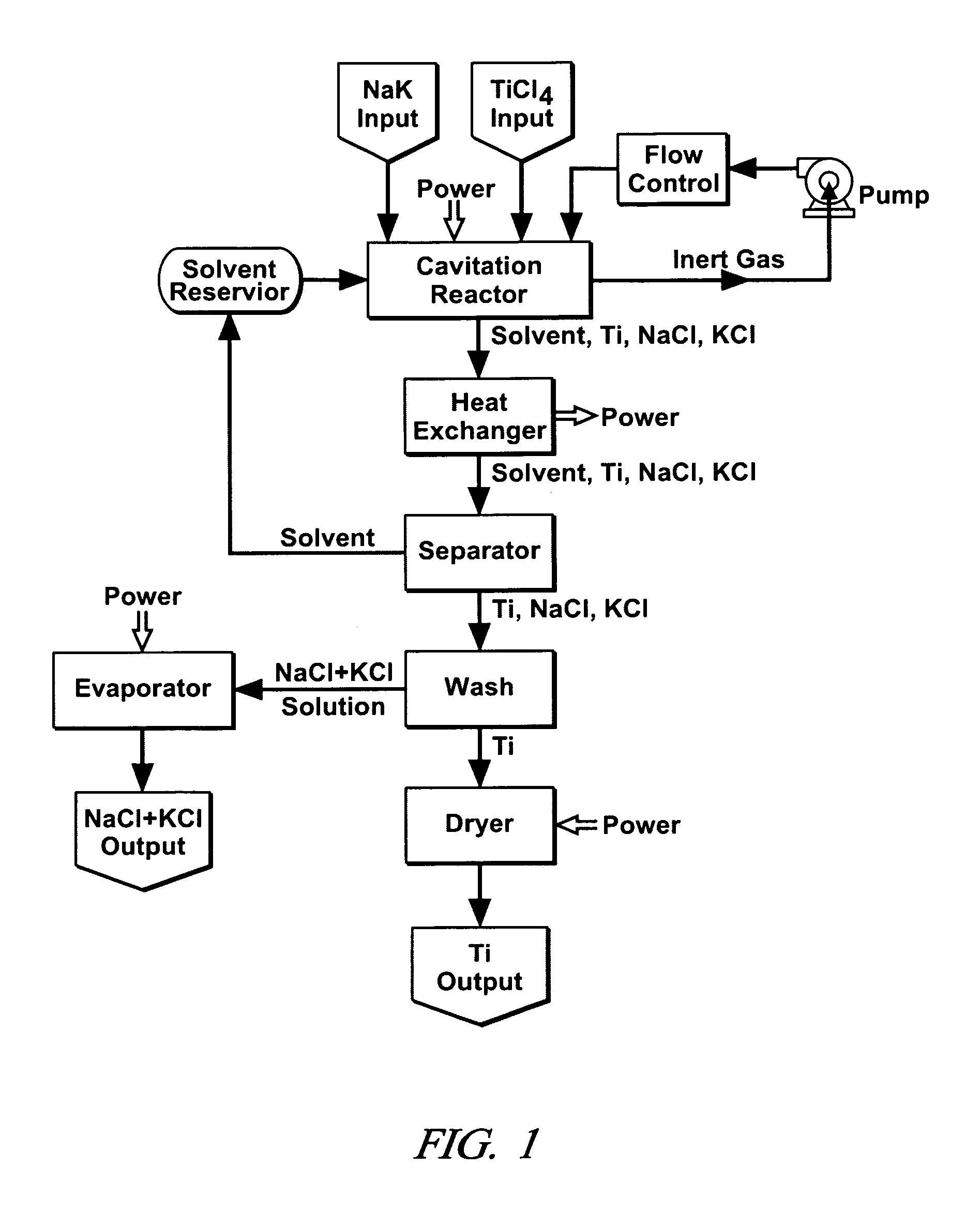
Cavitation process for products from precursor halides
ActiveUS20080295645A1Promote lowerLow vapor pressureMagnesium halidesZirconium compoundsAlkaline earth metalLiquid medium
A precursor halide compound is reduced to a predetermined product at substantially ambient conditions. The halide is added to an anhydrous liquid reaction medium containing one or more alkali metals or alkaline earth metals as reductants. The metal reductants are dispersed as very small globules in the liquid by cavitation of the liquid, such as by application of high intensity ultrasonic vibrations or high-shear mixing to the reaction vessel. Continued cavitation of the liquid medium affects low temperature reduction of the precursor halide(s) to produce a metal, metal alloy, metal compound, ceramic material, metal matrix-ceramic composite material, or the like. The practice may be applied, for example, to titanium tetrachloride, alone or with other chlorides, to produce titanium metal, titanium alloys (for example Ti-6Al-4V), and titanium compounds (TiSi2).
Owner:GM GLOBAL TECH OPERATIONS LLC
Method of reclaiming and using high-pollution low-concentration waste acid
InactiveCN101058408AReduce processing costsSolve industry problemsChlorine/hydrogen-chlorideMagnesium halidesInorganic saltsSulfate
The invention discloses a high-pollution low-density recycling and utilizing method of waste acid, which is characterized by the following: adding the waste water with sulphuric acid into inorganic salt MX to react with sulphuric acid after multi-effective concentration and filtering to remove impurity; generating with gas and acid composite salt; utilizing the acid composite salt or refining and separating through neutralizing, removing impurity and decoloring. The X in the halogenated salt is Cl-or Br-and M is cation to form soluble sulfate with sulfuric radical. The invention modifies and improves the disposing method to recycle and utilize low-density waste acid, which saves waste water disposing cost to provide a new resource integrated concept.
Owner:ZHEJIANG RUNTU
Persistent and fast acting antiseptics and disinfectants based on calcium fluoride
Antiseptic compounds that act as persistent and fast acting antiseptics and disinfectants. The base of these antiseptic actions is CaF2 as the persistent part, preventing the colonization of tissue and nonliving surfaces with microorganisms through the targeted on-demand release of fluorine ions. For fighting heavy contamination and invasion of transient microbes through new application of the solution, fast acting alcohols and toxic solutions have been added in small percentage. They act fast and evaporate fast, leaving the natural protection of skin undamaged and coated with a persistent antiseptic.
Owner:VOEGELI +2
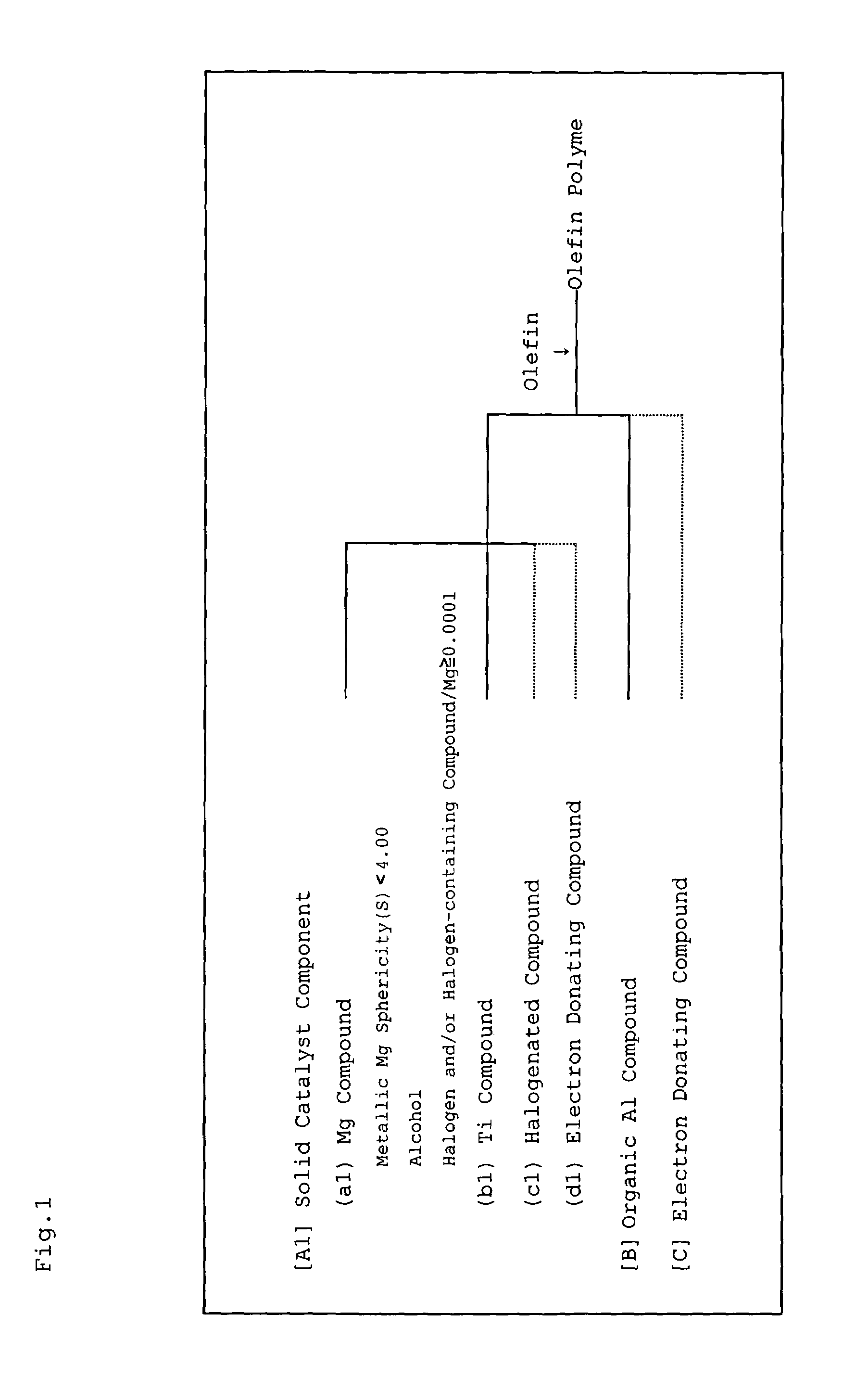
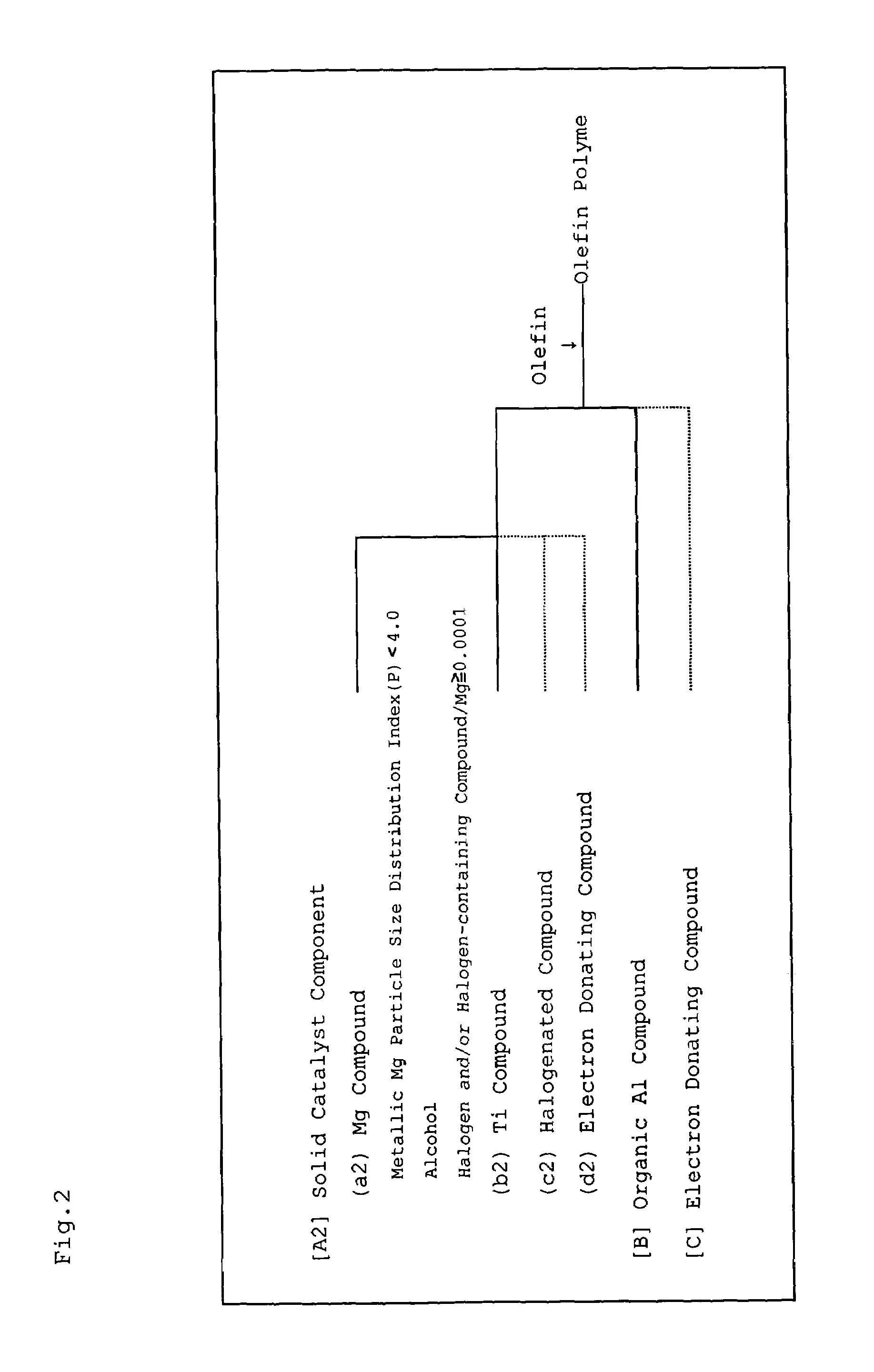
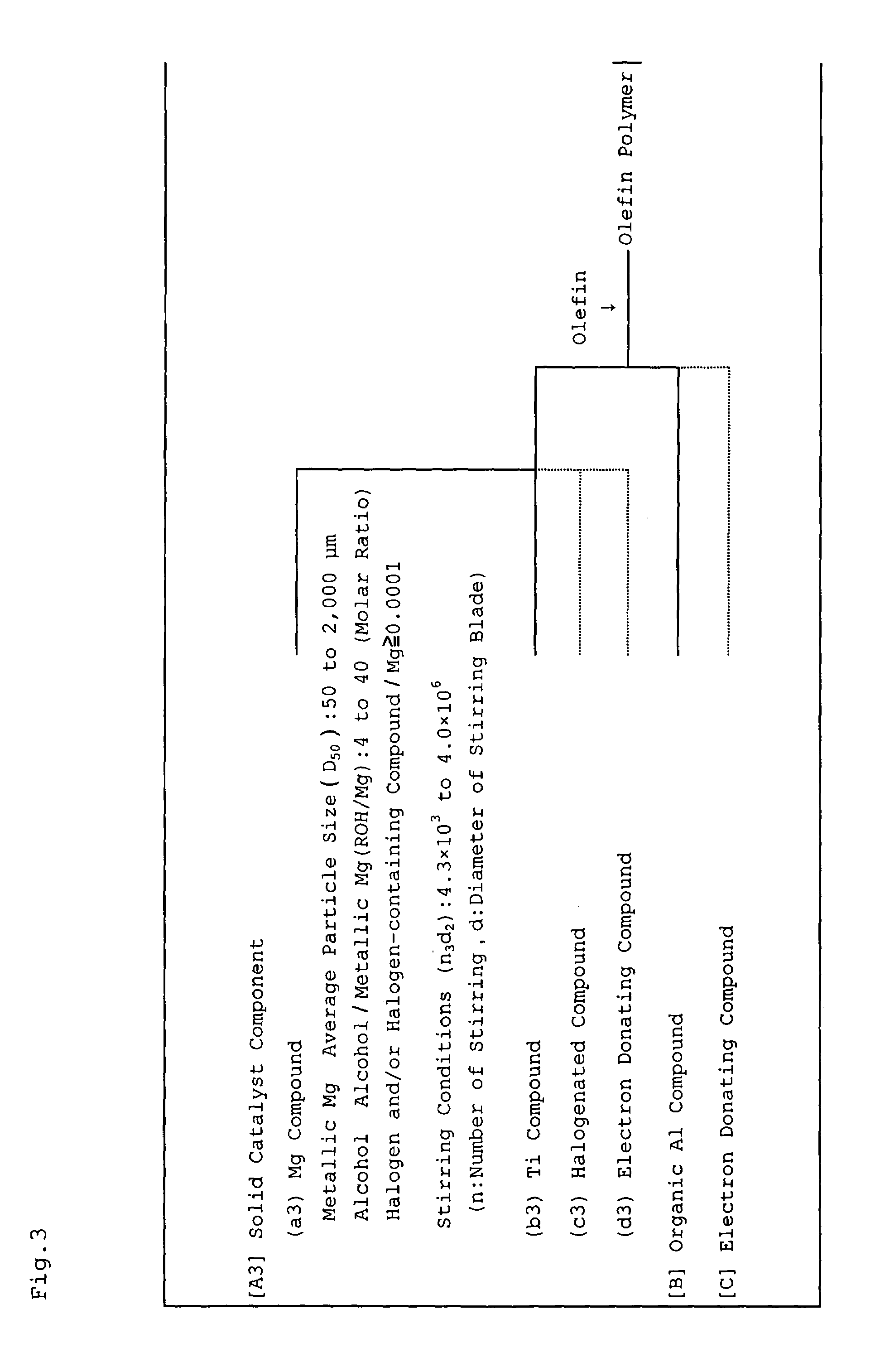
Magnesium compound, solid catalyst component for olefin polymerization, catalyst for olefin polymerization and method for producing polyolefin
InactiveUS7387979B2Magnesium halidesOrganic-compounds/hydrides/coordination-complexes catalystsPolyolefinMaximum diameter
A magnesium compound obtained by reacting metallic magnesium having a sphericity (S) of less than 4.00, the sphericity (S) being represented by the following formula (I), an alcohol, and a halogen and / or a halogen-containing compound containing halogen atoms in an amount of 0.0001 gram atom or more relative to one gram atom of the metallic magnesium,S=(L1 / L2)3 (I)wherein L1 represents the maximum diameter of projection views of metallic magnesium determined by photographing with a scanning electron microscope and thereafter an image processing, and L2 represents a diameter of a circle having an area equal to the area of the projection view of metallic magnesium. A solid catalyst component is obtained from the magnesium compound and a titanium compound, and a catalyst for olefin polymerization is obtained using the solid catalyst component.
Owner:IDEMITSU KOSAN CO LTD
Process for producing inorganic fine grains, inorganic fine grains, rare earth element-activated barium fluorohalide fluorescent substance, and radiation image conversion panel
InactiveUS20030005552A1Improve image qualityX-ray/infra-red processesMagnesium halidesRare-earth elementFluorescence
A process for producing inorganic fine grains in a definite form having a small grain size, the inorganic fine grains obtained by this process, a rare earth element-activated barium fluorohalide fluorescent substance made using the grains, and a radiation image conversion panel with a layer of the fluorescent substance. The process features adding, to a solution containing an inorganic compound, a solid matter substantially insoluble in the solution, promoting crystallization or precipitation in the solution to form crystal or precipitate, and separating out the resulting crystal or precipitate. The inorganic fine grains produced by this process are represented by the formula BaFI:xLn (Ln represents at least one of Ce, Pr, Sm, Eu, Tb, Dy, Ho, Nd, Er, Tm and Yb, and 0<x.ltoreq.0.2), have a cubic form and have a volume-average grain size of 1 to 10 .mu.m.
Owner:FUJIFILM HLDG CORP +1
Method of fluoride-containing wastewater treatment
First, a primary fluoric ion concentration detection process is performed, and a primary calcium salt addition process is performed to add calcium salt in a first reaction tank, wherein the dosage of the calcium salt in the primary calcium salt addition process is determined according to the detected fluoric ion concentration. Thereupon, a secondary calcium salt addition process is performed to add calcium salt into the second reaction tank. Following that, a solid-liquid separation process is performed to separate calcium fluoride from the wastewater, and a secondary fluoric ion concentration detection process is performed upon the wastewater after the calcium fluoride is separated. Finally, the dosage of the calcium salt in the secondary calcium salt addition process is determined in a feed back control manner according to the detected fluoric ion concentration.
Owner:POWERCHIP SEMICON MFG CORP
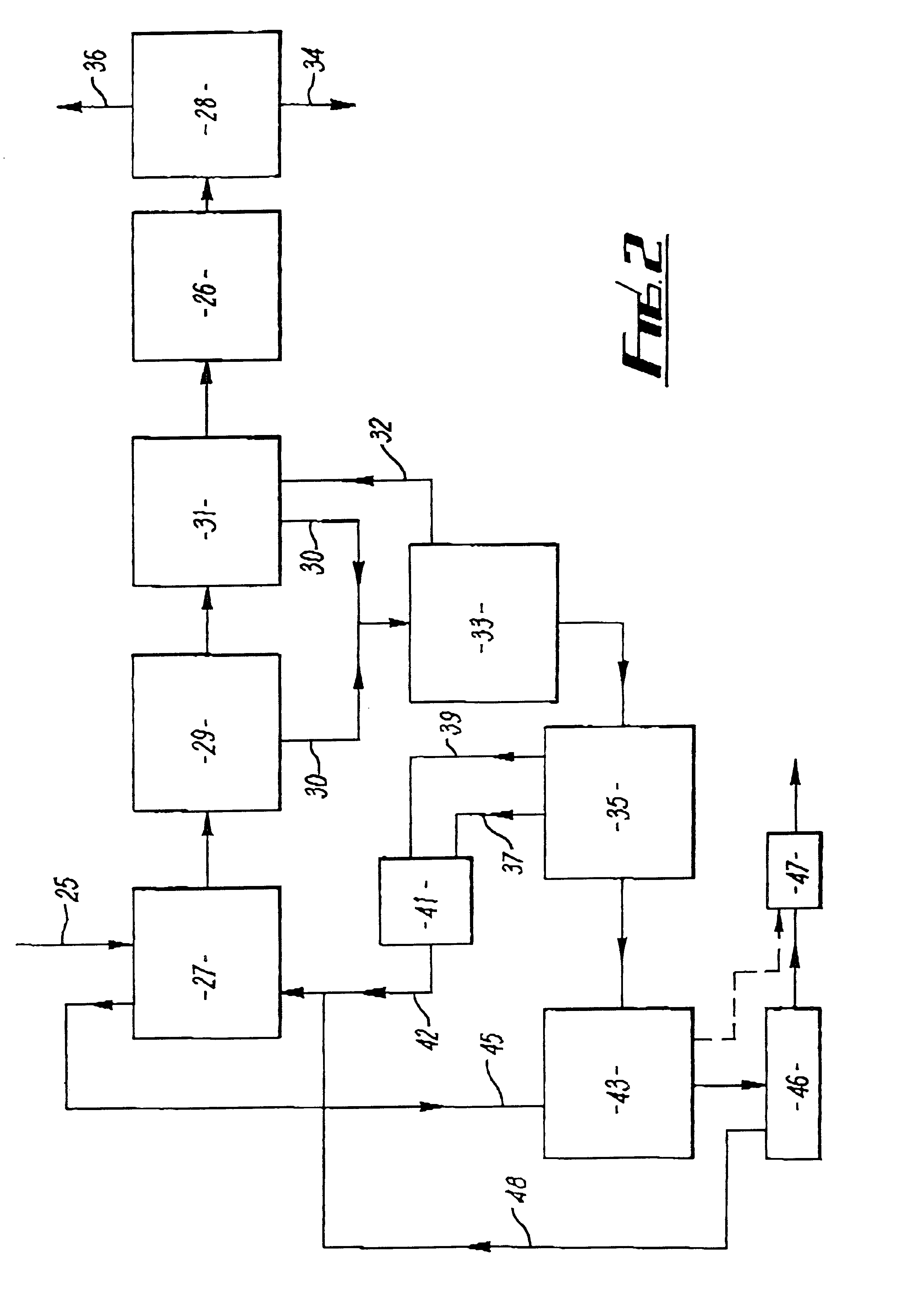
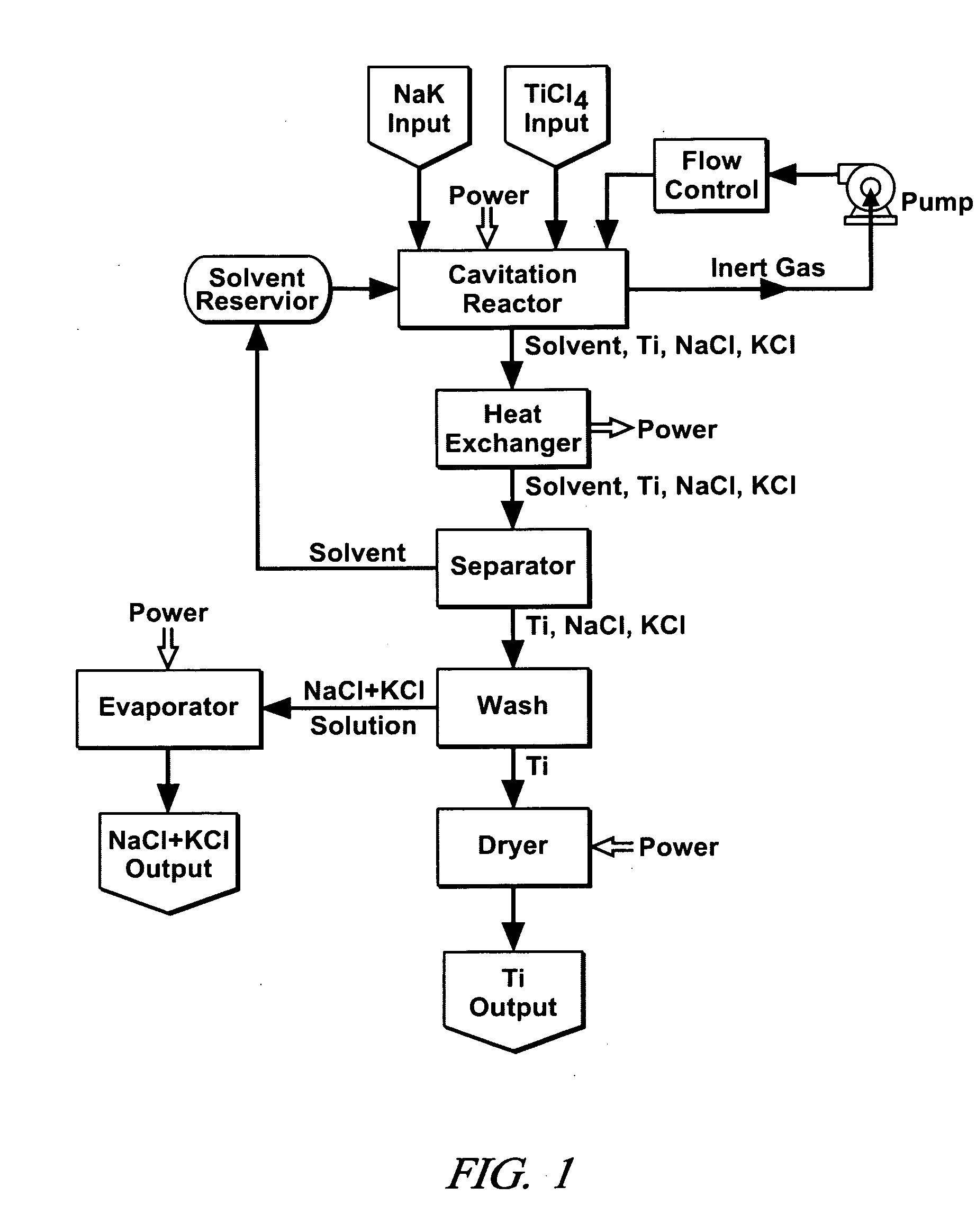
Cavitation process for products from precursor halides
ActiveUS7465333B1Promote lowerLow vapor pressureMagnesium halidesUsing liquid separation agentLiquid mediumCavitation
A precursor halide compound is reduced to a predetermined product at substantially ambient conditions. The halide is added to an anhydrous liquid reaction medium containing one or more alkali metals or alkaline earth metals as reductants. The metal reductants are dispersed as very small globules in the liquid by cavitation of the liquid, such as by application of high intensity ultrasonic vibrations or high-shear mixing to the reaction vessel. Continued cavitation of the liquid medium affects low temperature reduction of the precursor halide(s) to produce a metal, metal alloy, metal compound, ceramic material, metal matrix-ceramic composite material, or the like. The practice may be applied, for example, to titanium tetrachloride, alone or with other chlorides, to produce titanium metal, titanium alloys (for example Ti-6Al-4V), and titanium compounds (TiSi2).
Owner:GM GLOBAL TECH OPERATIONS LLC
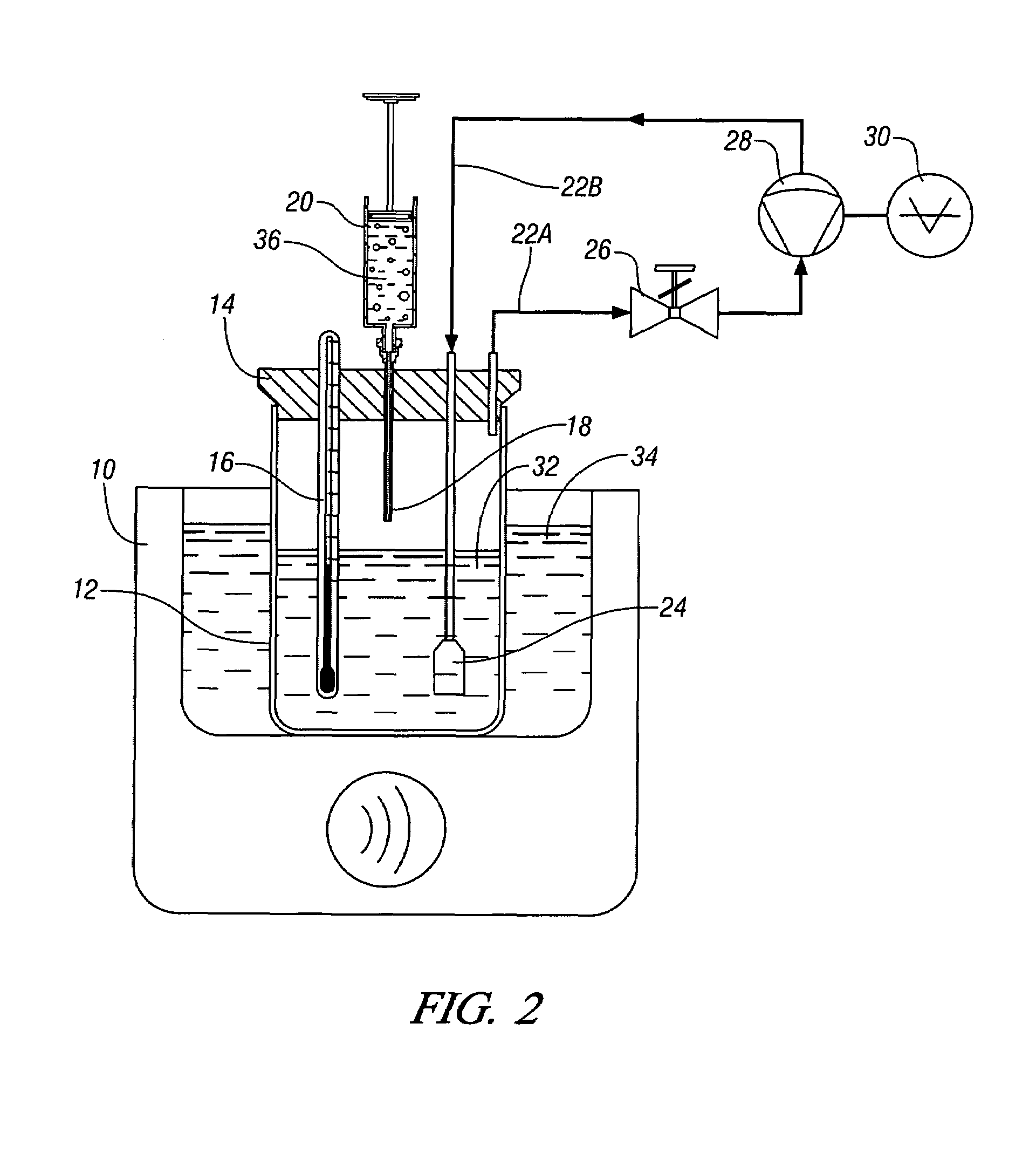
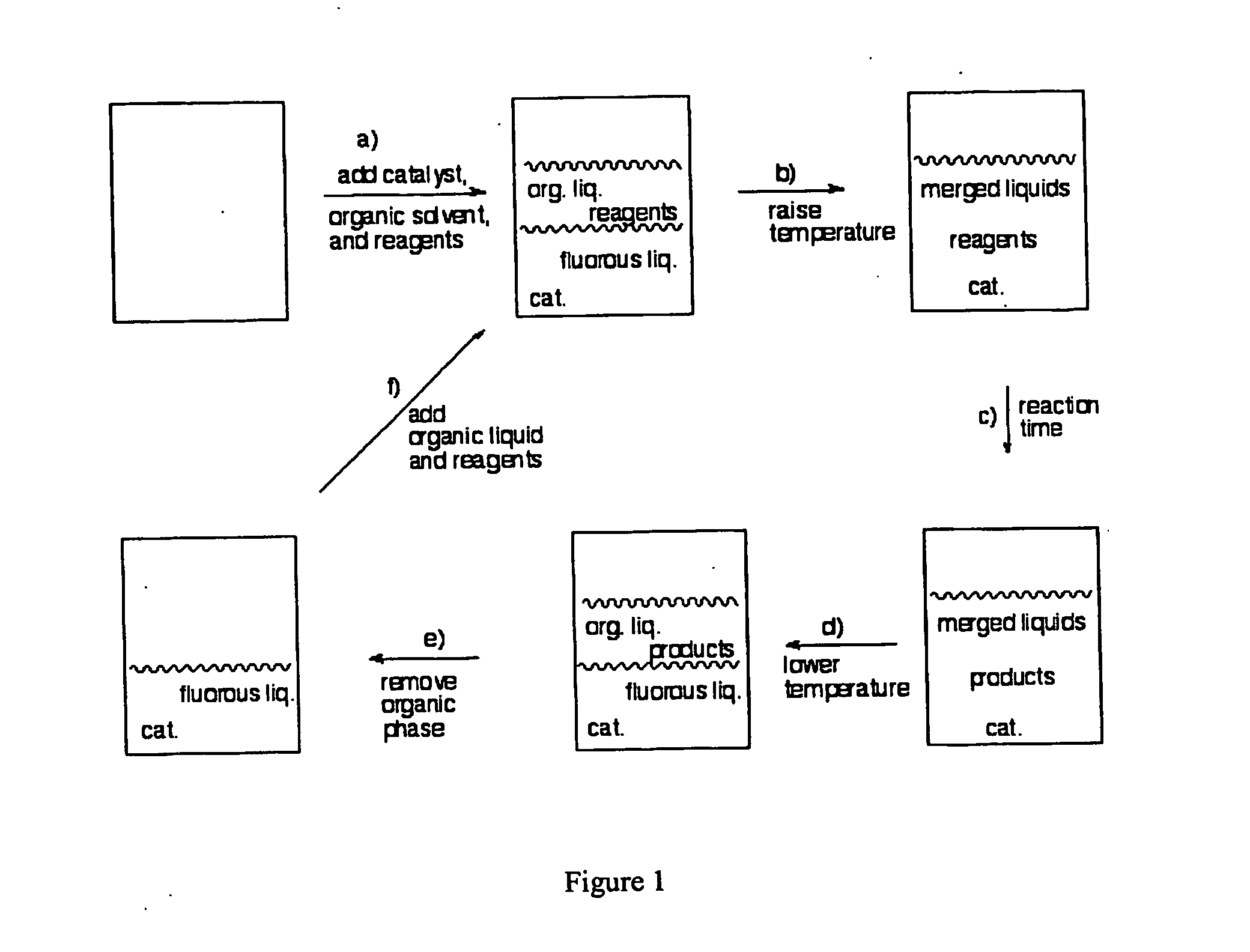
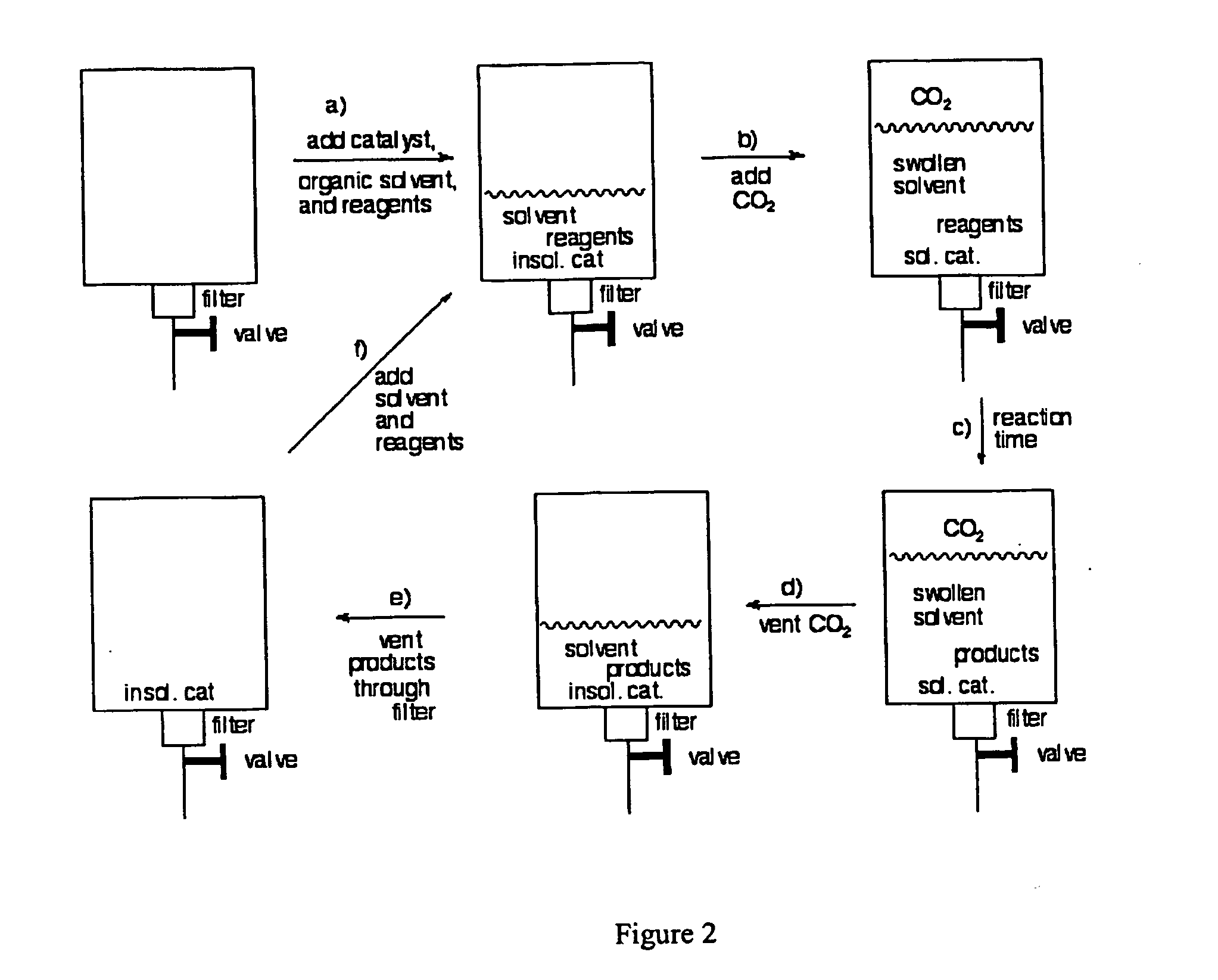
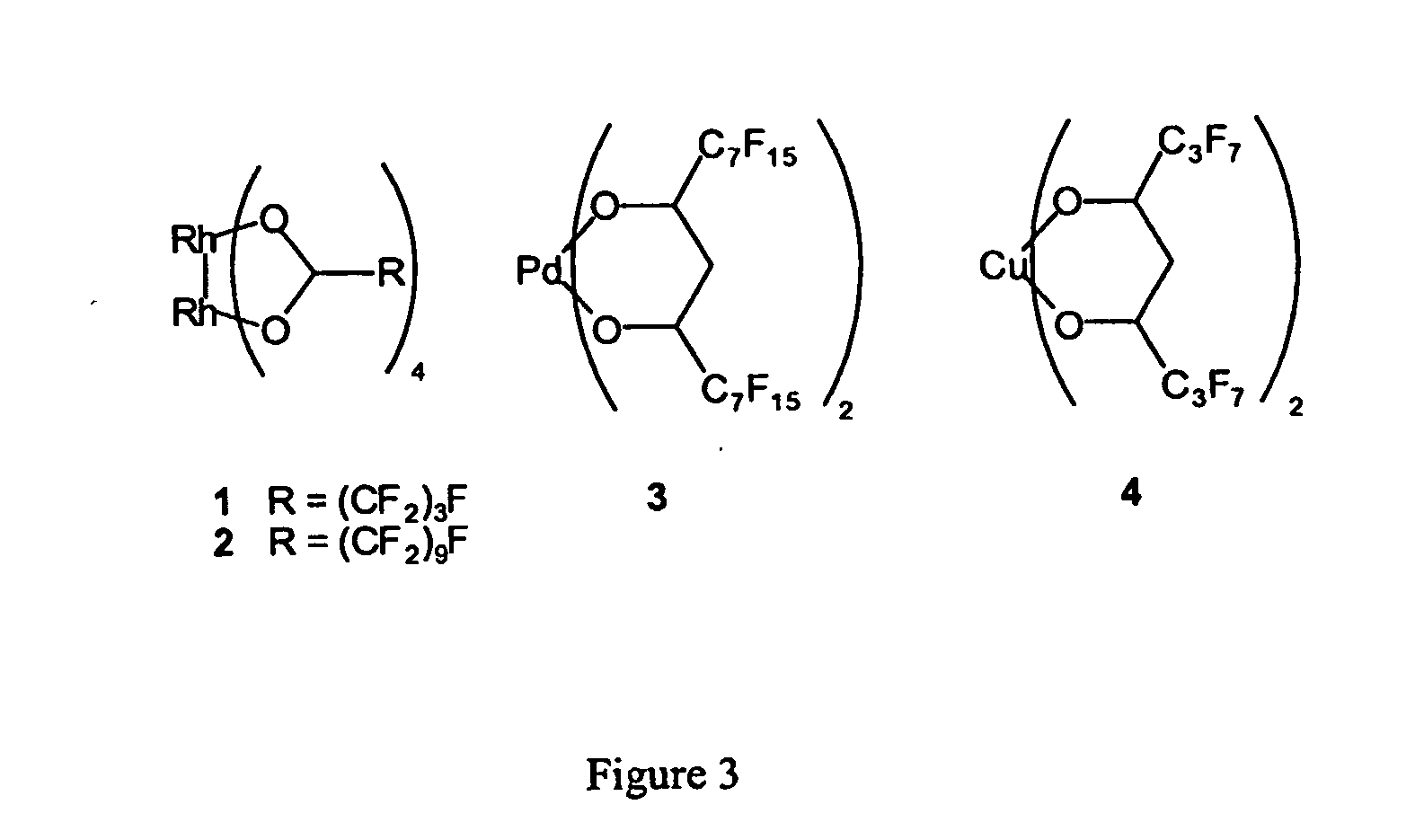
Methods for solubilizing and recovering fluorinate compounds
InactiveUS20050015936A1Reducing carbon dioxide gaseous pressureEffective recoveryPlatinum group organic compoundsMagnesium halidesSolubilityOrganic solvent
Methods of enhancing the solubility of a fluorinated compound in an organic solvent are provided. In one embodiment, carbon dioxide gas pressure is applied to the solvent at a pressure effective to enhance the solubility of the fluorinated compound. The method may further include recrystallizing the fluorinated compound by reducing the pressure of the carbon dioxide gas. Also provided are methods of conducting a reaction using a fluorinated compound in an organic solvent In one embodiment, the method comprises applying carbon dioxide pressure to an organic solvent comprising at least one substrate and a fluorinated catalyst, in an effective amount to solubilize the catalyst; and permitting the fluorinated catalyst to catalyze the reaction of the substrate to form a product. The catalyst is optionally separated from the reaction product and solvent after the reaction by the release of the pressure.
Owner:GEORGIA TECH RES CORP +1
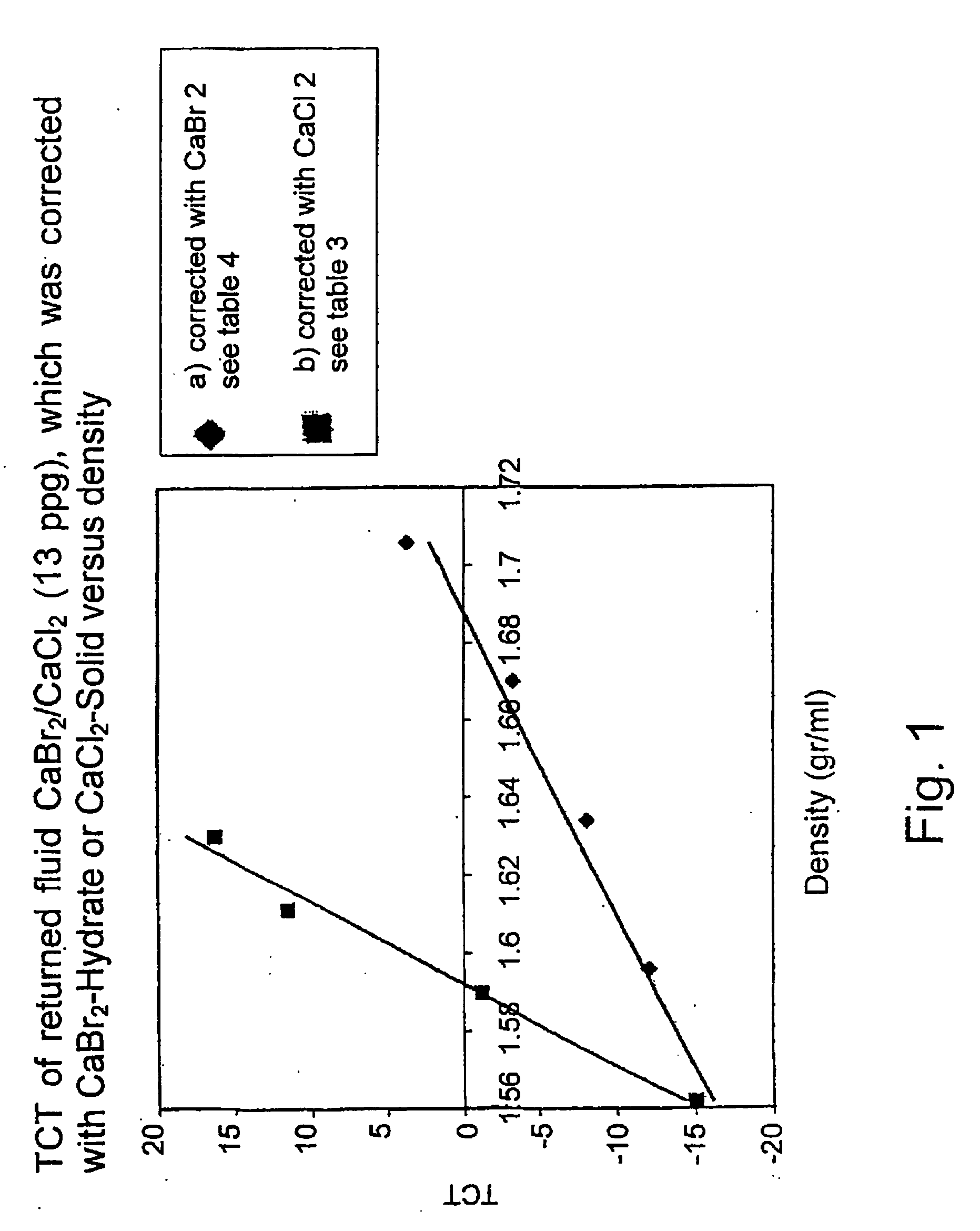
Process for the preparation of cabr2 hydrates and uses thereof
InactiveUS20060127301A1Magnesium halidesContaminated soil reclamationSoil remediationVolumetric Mass Density
A process for the preparation of Solid Calcium Bromide Hydrates comprises preparing a highly concentrated Calcium Bromide solution from an initial solution having a lower concentration, bringing it into contact with a cold surface, whereby solid Calcium Bromide hydrates form on said surface, and detaching said solid hydrates from said surface. The concentration of the initial solution is not higher than 60 wt %, typically 52 wt %, and is brought to from 65 to 78 wt %. The said Calcium Hydrates are mainly tetrahydrate Calcium Bromide. The Solid Calcium Hydrates have many uses in the processing of oil wells, for instance preparation of completion fluids, work-over fluids or drill-in fluids in the processing of wells, restoring already used and / or depleted such fluids to the desired, original density. They also have other uses, particularly for soil remediation.
Owner:ELITZUR RAPHAEL +2
Selective Oxidation Agent of Hydrocarbons to Synthesis Gas Based on Separate Particles of O-Carrier and Hydrocarbon Activator
A solid material is presented for the partial oxidation of natural gas. The solid material includes a solid oxygen carrying agent and a hydrocarbon activation agent. The material precludes the need for gaseous oxygen for the partial oxidation and provides better control over the reaction.
Owner:UOP LLC
Method and apparatus for treating exhaust gas
ActiveUS8420034B2Reduce the amount requiredCurb energy consumptionGas treatmentMagnesium halidesAmmoniaMetal
Owner:MITSUBISHI HEAVY IND LTD
Processes for preparing highly pure lithium carbonate and other highly pure lithium containing compounds
ActiveUS20120100056A1Electrolysis componentsLithium organic compoundsLithium carbonateLithium hydroxide
Owner:TERRALITHIUM LLC
Method of cutting single crystals
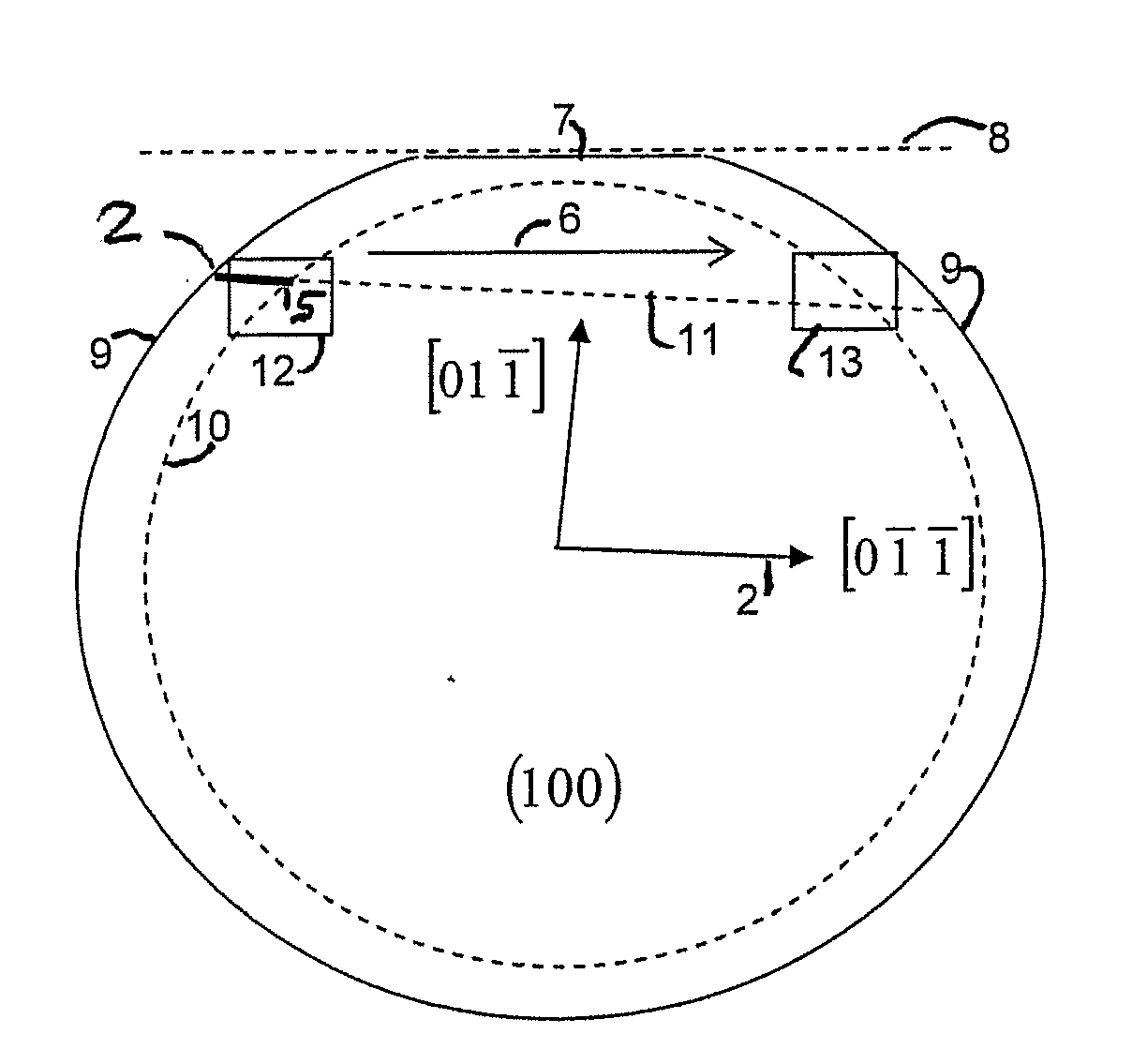
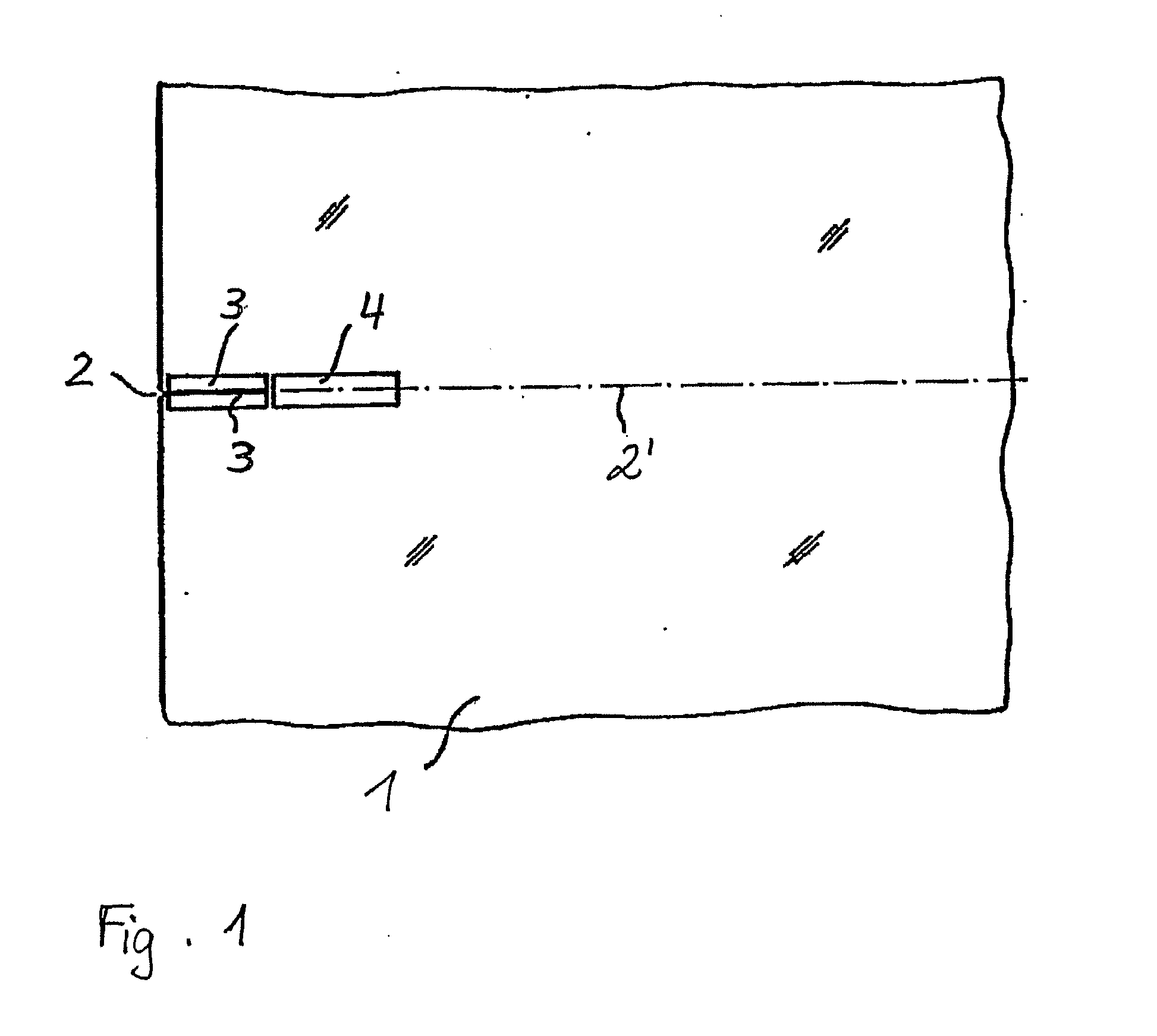
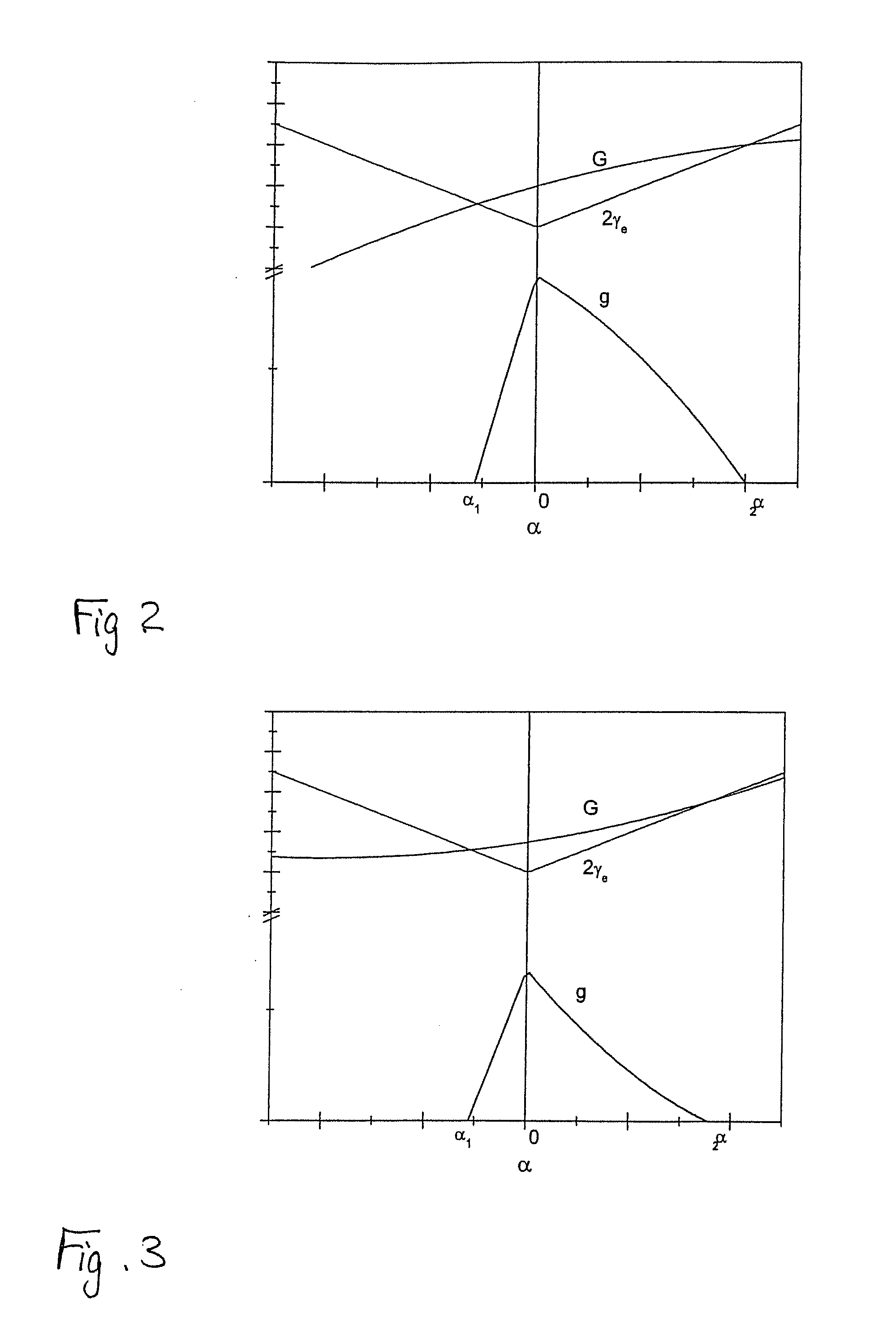
ActiveUS20090283761A1Quality improvementImprove accuracyGrinding machine componentsNitrogen compoundsClassical mechanicsSingle crystal
A method of dividing single crystals, particularly of plates of parts thereof, is proposed, which can comprise: pre-adjusting the crystallographic cleavage plane (2′) relative to the cleavage device, setting a tensional intensity (K) by means of tensional fields (3′, 4′), determining an energy release rate G(α) in dependence from a possible deflection angle (α) from the cleavage plane (2′) upon crack propagation, controlling the tensional fields (3′, 4′) such that the crack further propagates in the single crystal, wherein G(0)≧2γe(0) and simultaneously at least one of the following conditions is satisfied:∂G∂αα=0≤2βehif∂2G∂α2≤0or(2.1)∂G∂α≤2βeh∀α:α1<α<α2,(2.2)
Owner:FREIBERGER COMPOUND MATERIALS
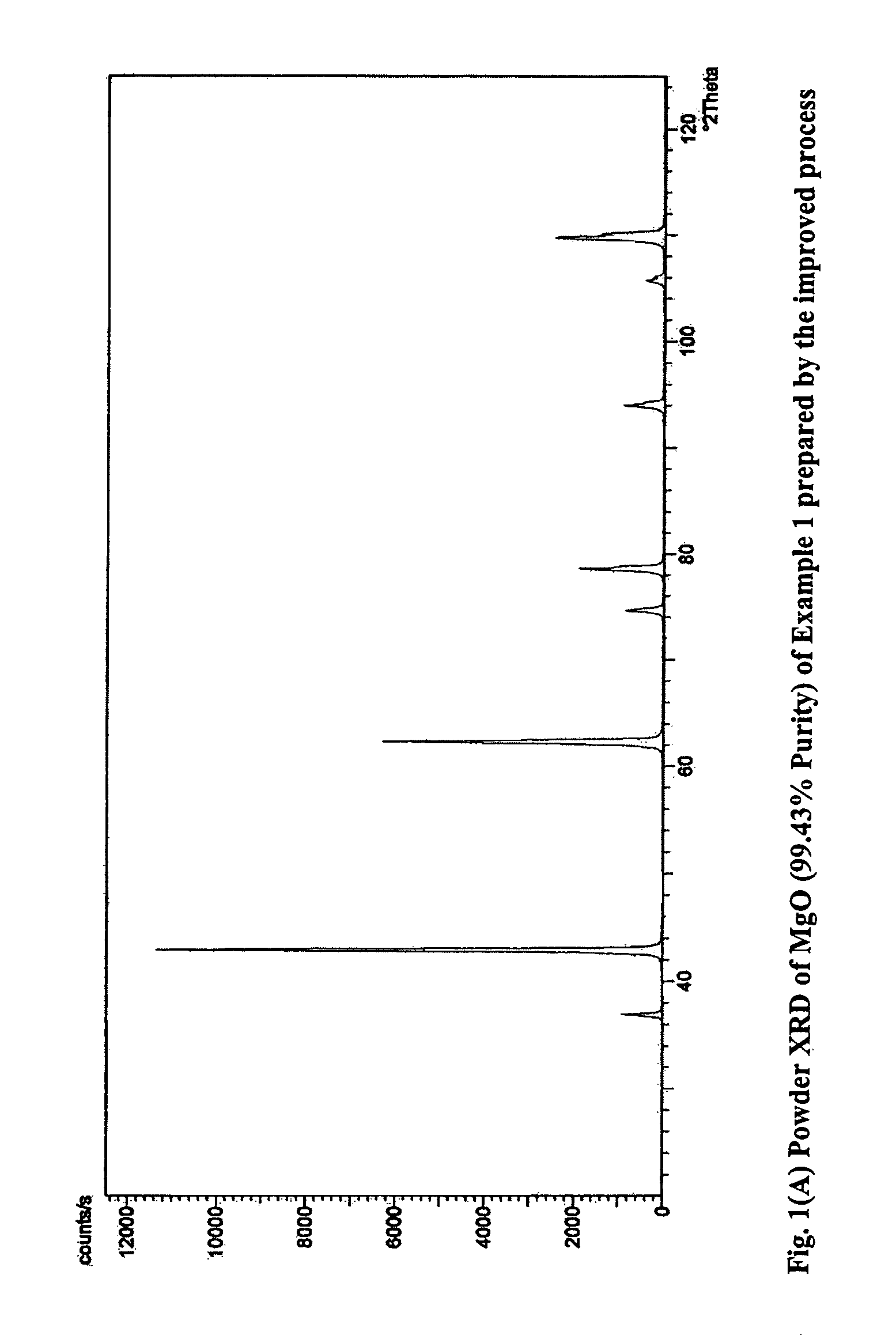
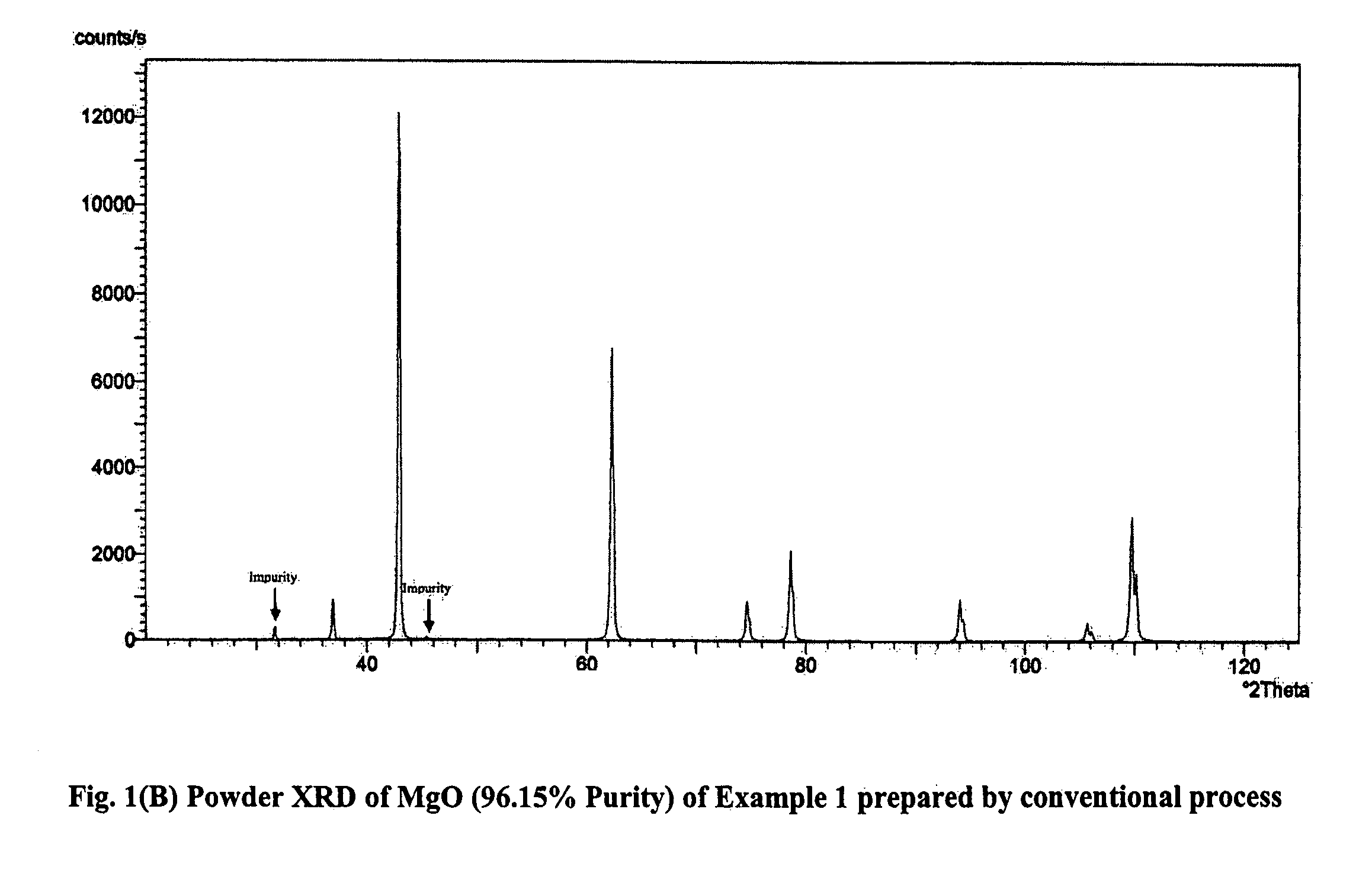
Process for the preparation of magnesia (MgO) from crude Mg (OH)2
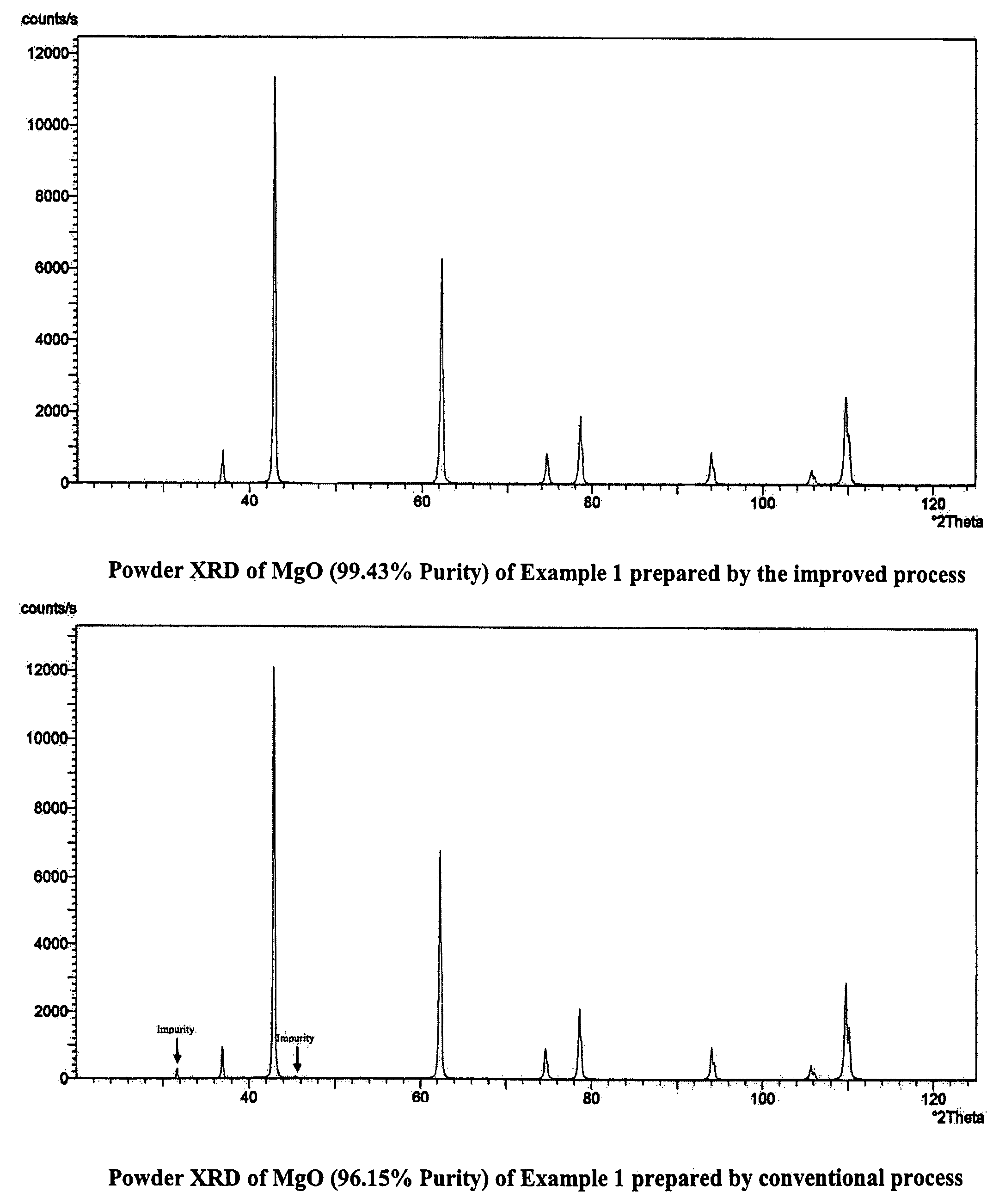
InactiveUS7771682B2Easily washed off with waterReduce boron impurityOxide/hydroxide preparationMolecular sieve catalystsMagnesium saltPhysical chemistry
The process provides for the preparation of MgO from the reaction of magnesium salt and alkali / lime. The crude Mg(OH)2 is directly calcined and then treated with water to disintegrate the mass spontaneously to yield a slurry and dissolve away the soluble salts. This slurry is much easier to filter and wash than the original Mg(OH)2 slurry, which helps to speed up the purification operation and also conserve fresh water. Another important advantage of the present method is that even pasty or dough like reaction products that are processed using dough mixers and similar equipment can be worked up with ease. There is no compromise in the quality of MgO achieved in this manner.
Owner:COUNCIL OF SCI & IND RES
Popular searches
Nitrogen-metal/silicon/boron binary compounds Semiconductor/solid-state device manufacturing From melt solutions From frozen solutions Using seed in melt Eutectic material solidification Single crystal growth details Calcium/strontium/barium halides Electrolytic organic production Group 3/13 element organic compounds
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com