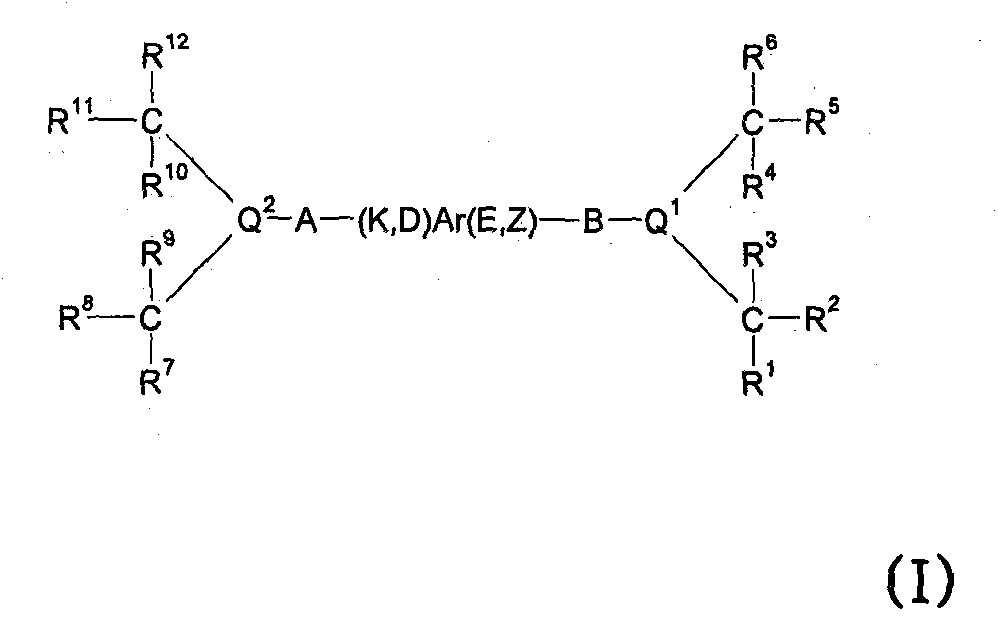
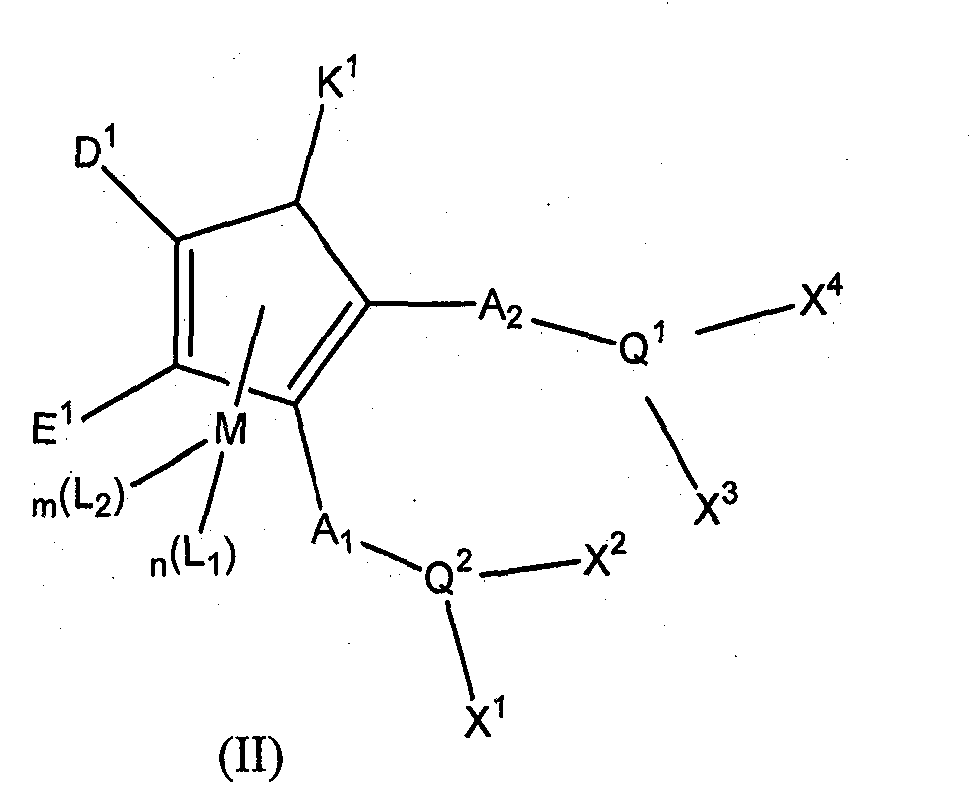
Patents
Literature
35results about "Platinum group organic compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
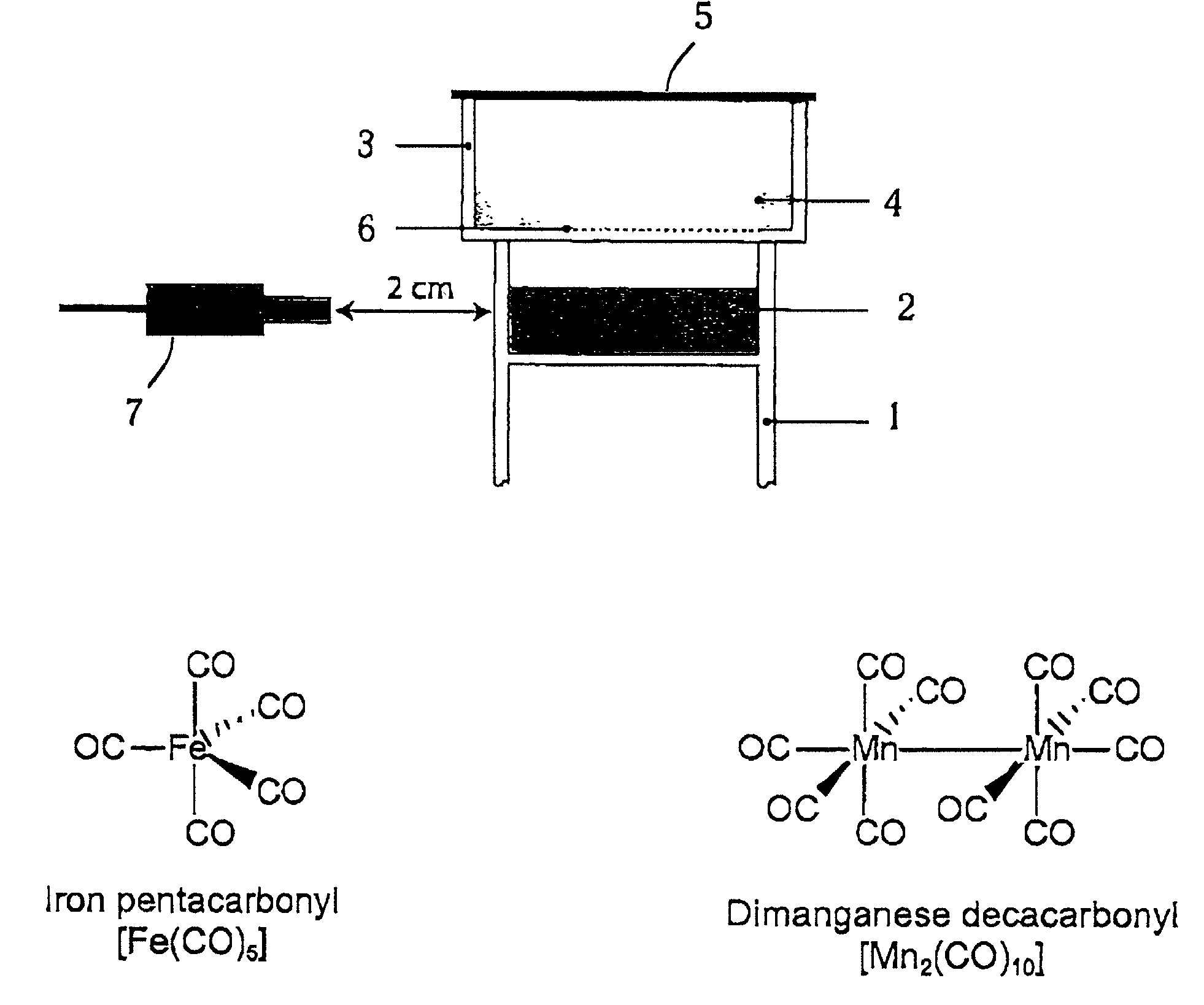
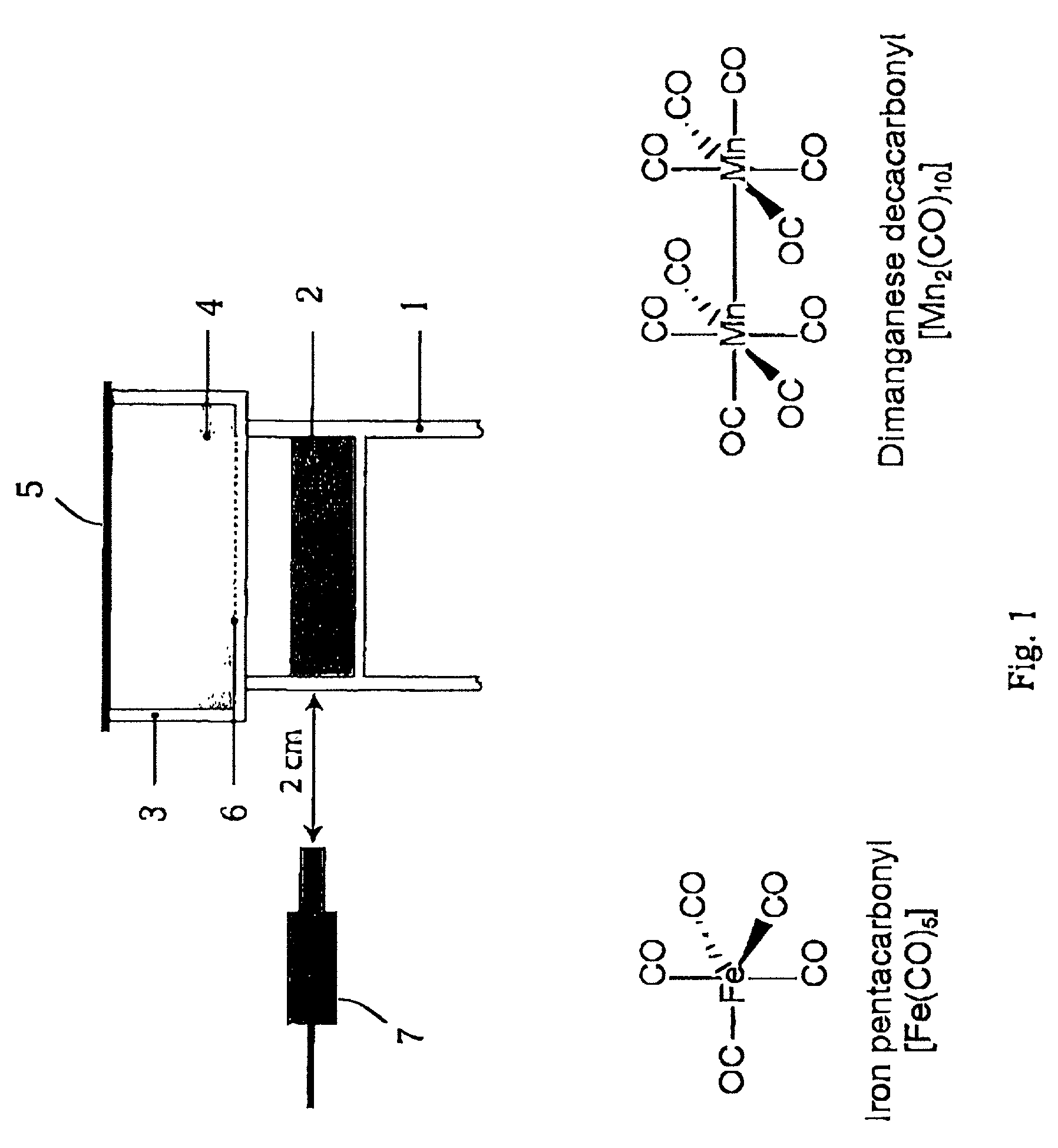
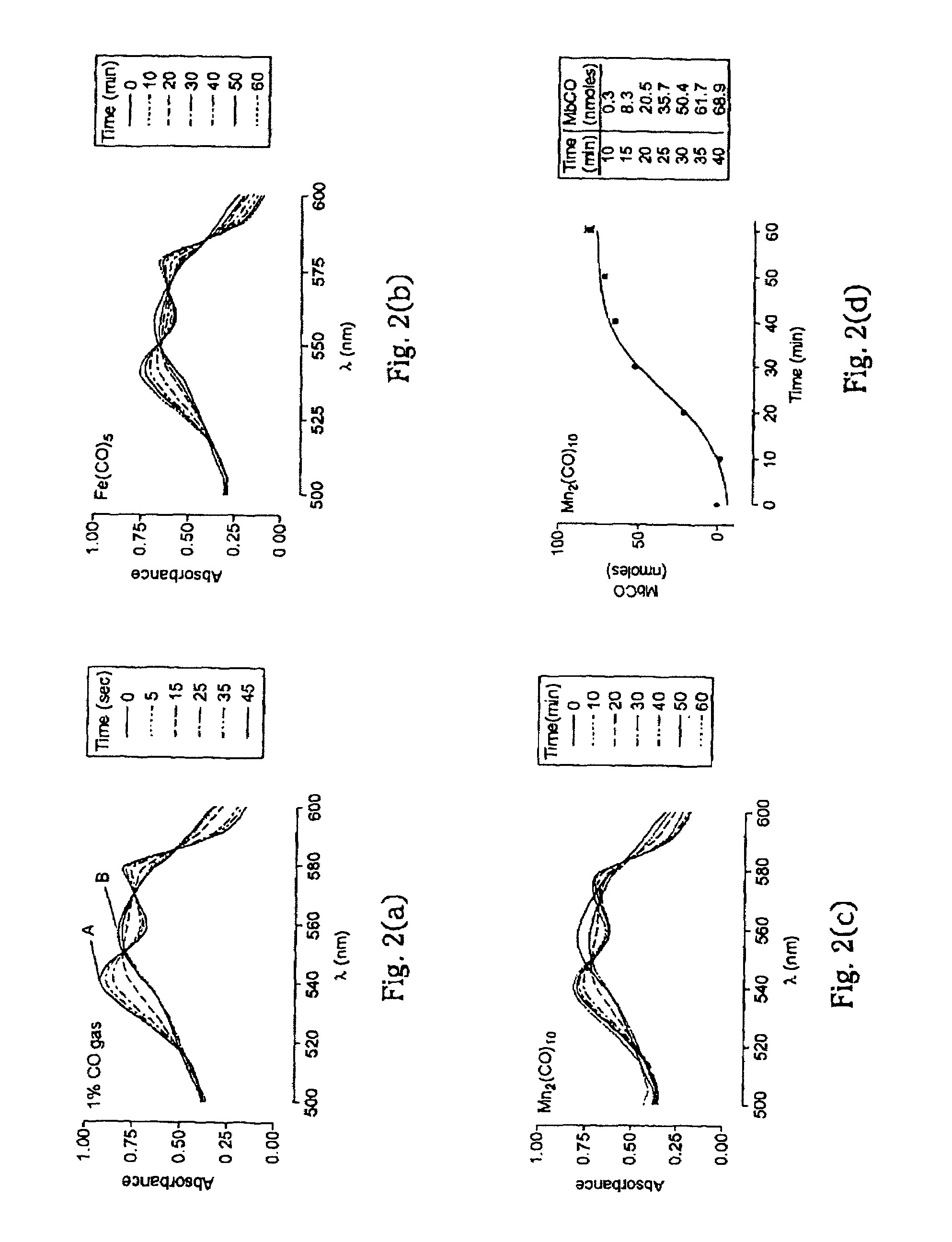
Therapeutic delivery of carbon monoxide
InactiveUS7045140B2Increase stimulationRegulate expressionOrganic active ingredientsPlatinum group organic compoundsSolubilityTransplant rejection
Metal carbonyls are used to deliver CO having biological activity, for example vasodilatation and inhibition of transplant rejection. The metal of the carbonyl is typically of groups 7 to 10, e.g. Fe and Ru. The carbonyl preferably has one or more ligands other than CO, such as amino acids, to modulate the CO release property and solubility.
Owner:HEMOCORM
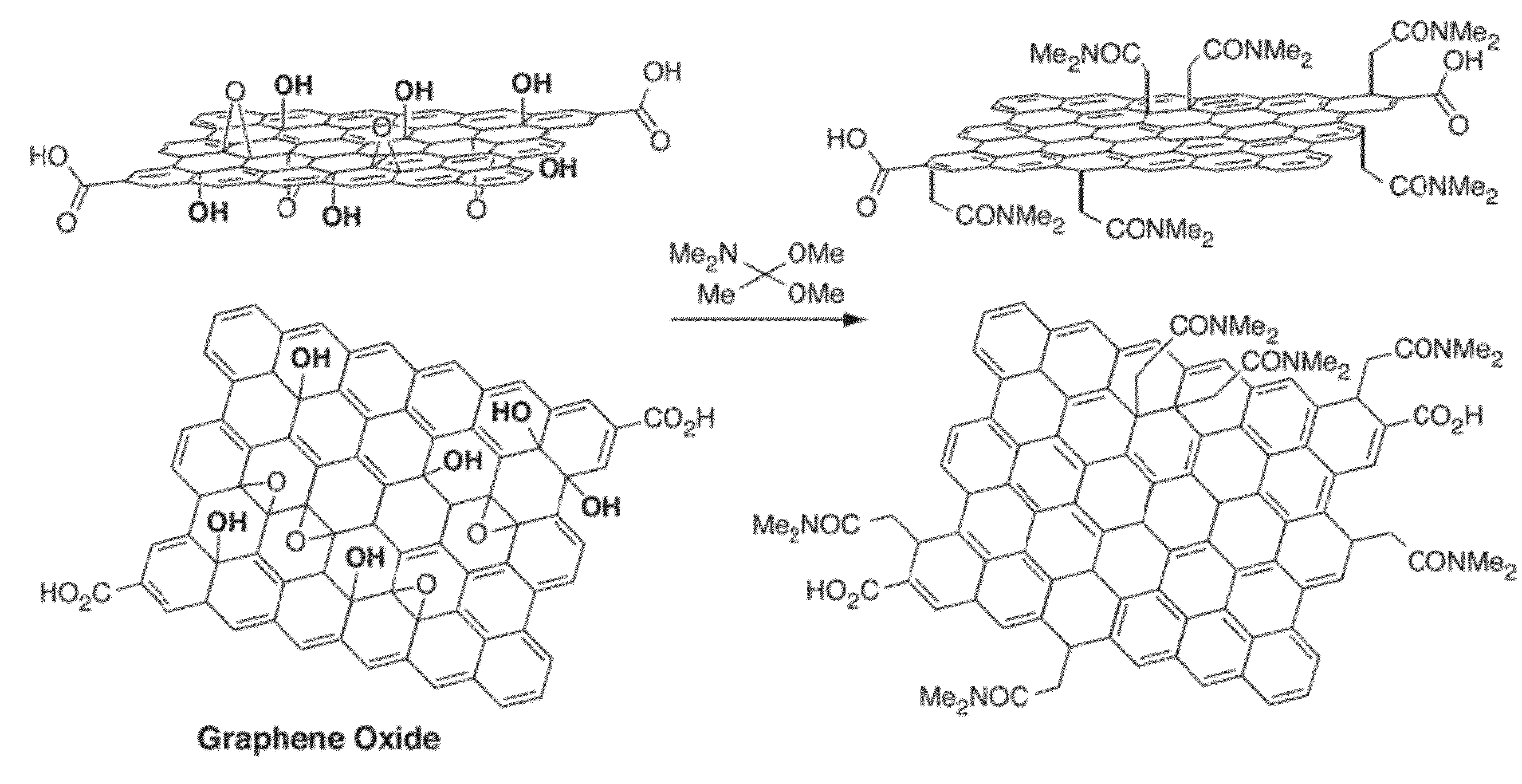
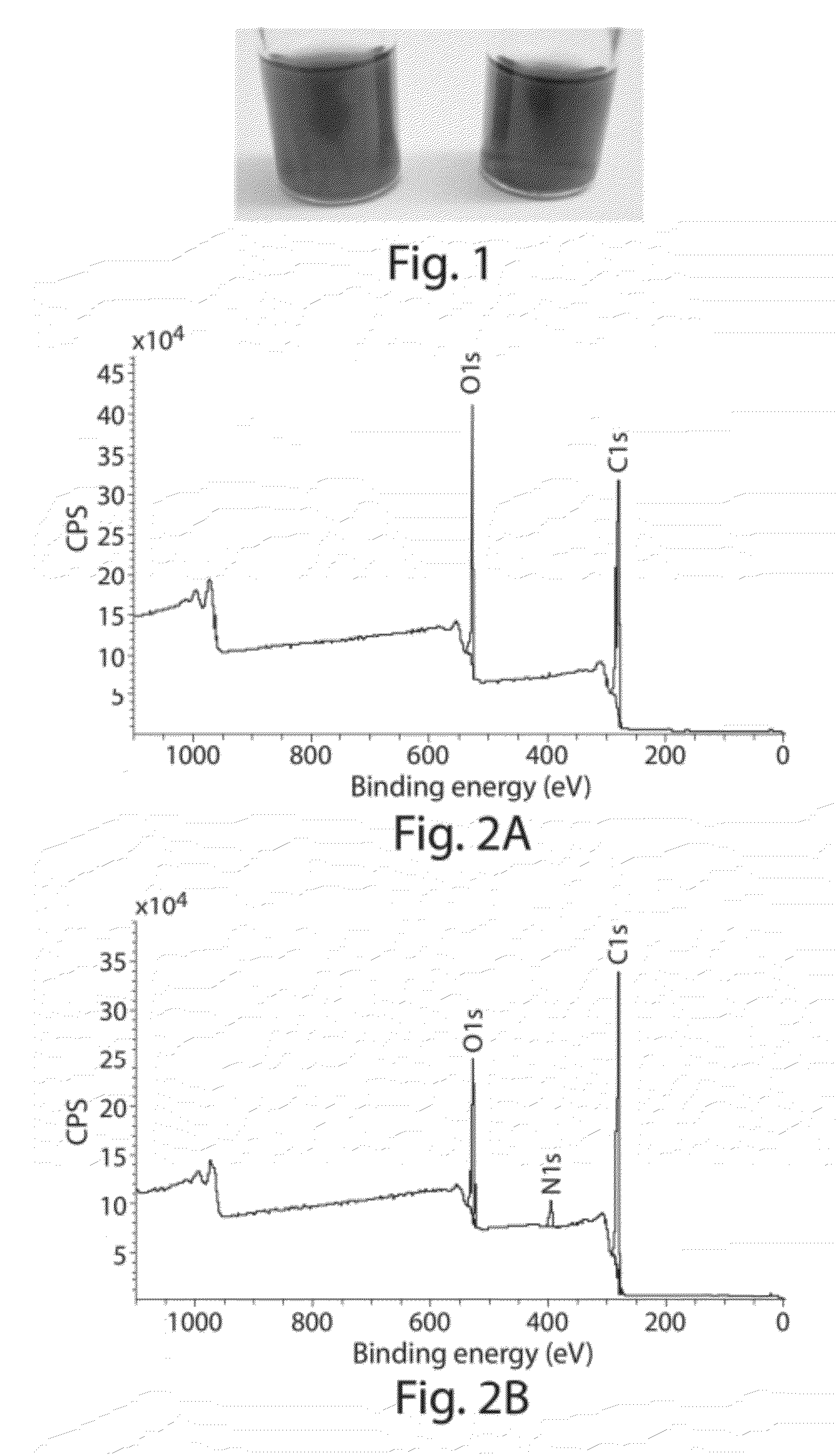
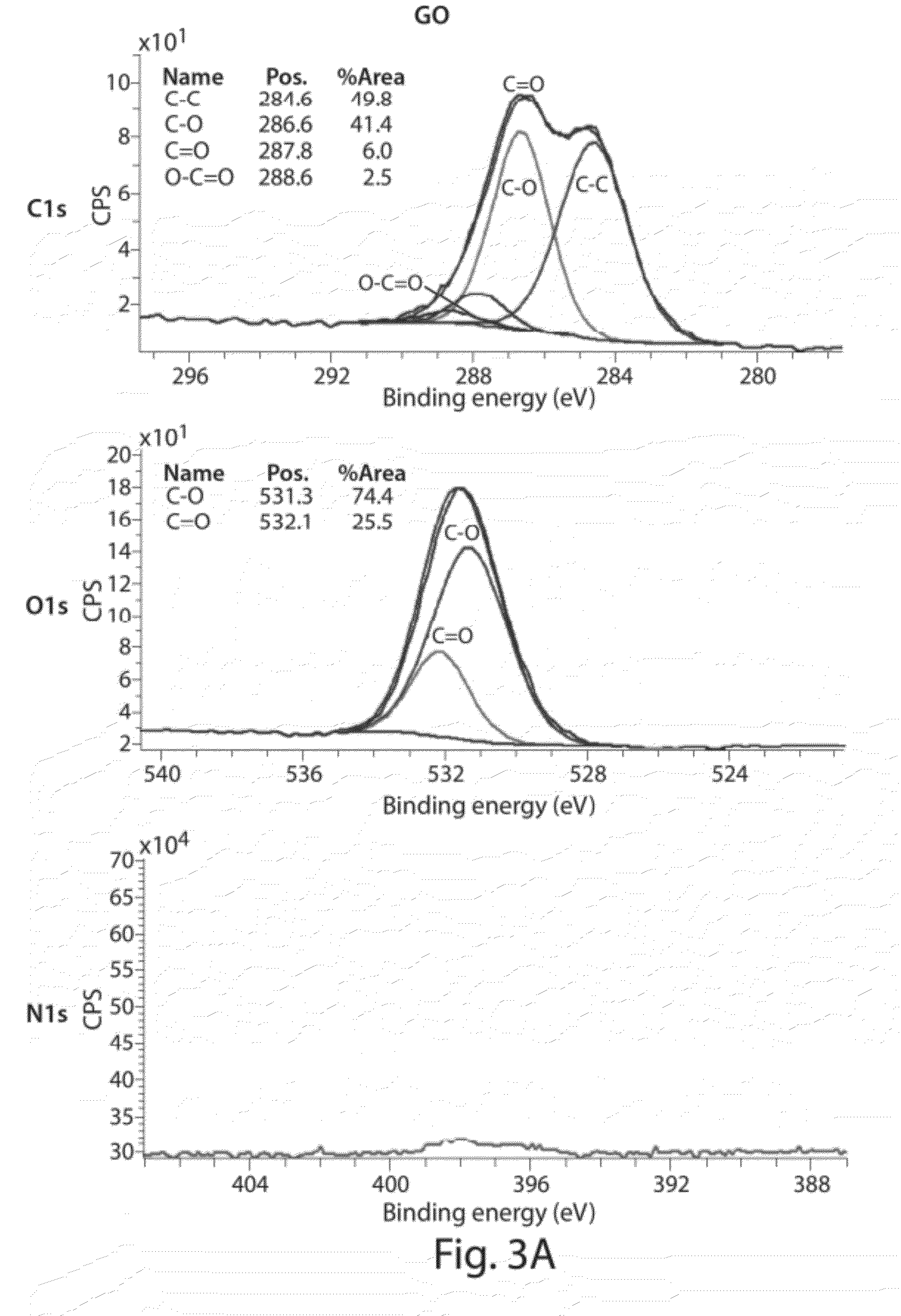
Compositions comprising functionalized carbon-based nanostructures and related methods
InactiveUS20120171093A1Reduce the amount requiredReduce concentrationPlatinum group organic compoundsNitrogen compoundsCrystallographyNanostructure
Owner:MASSACHUSETTS INST OF TECH
Preparation of metal alkoxides
InactiveUS6355821B1Heat dissipationReduce thermal decompositionPlatinum group organic compoundsCopper oxides/halidesSolventEthanol
Methods of forming metal alkoxides and methods of forming precursor solutions of metal alkoxides suitable for the coating of glass in the manufacture of electrochromic devices are disclosed. The method of forming metal alkoxides involves dissolving the metal halide in an anhydrous solvent and reacting it with an alcohol and (together with the addition of the alcohol or subsequently) adding an epoxide, and then evaporating-off the volatile components of the reaction product to leave a solid metal alkoxide that is substantially free of halide. The alkoxide may then be dissolved in a solvent including an alcohol (preferably ethanol) containing a small proportion of water to produce a precursor solution suitable for coating glass, the coating then being hydrolyzed to form a sol-gel and then baked to remove volatile components and to yield a thin layer of metal oxide.
Owner:DYESOL PTY LTD
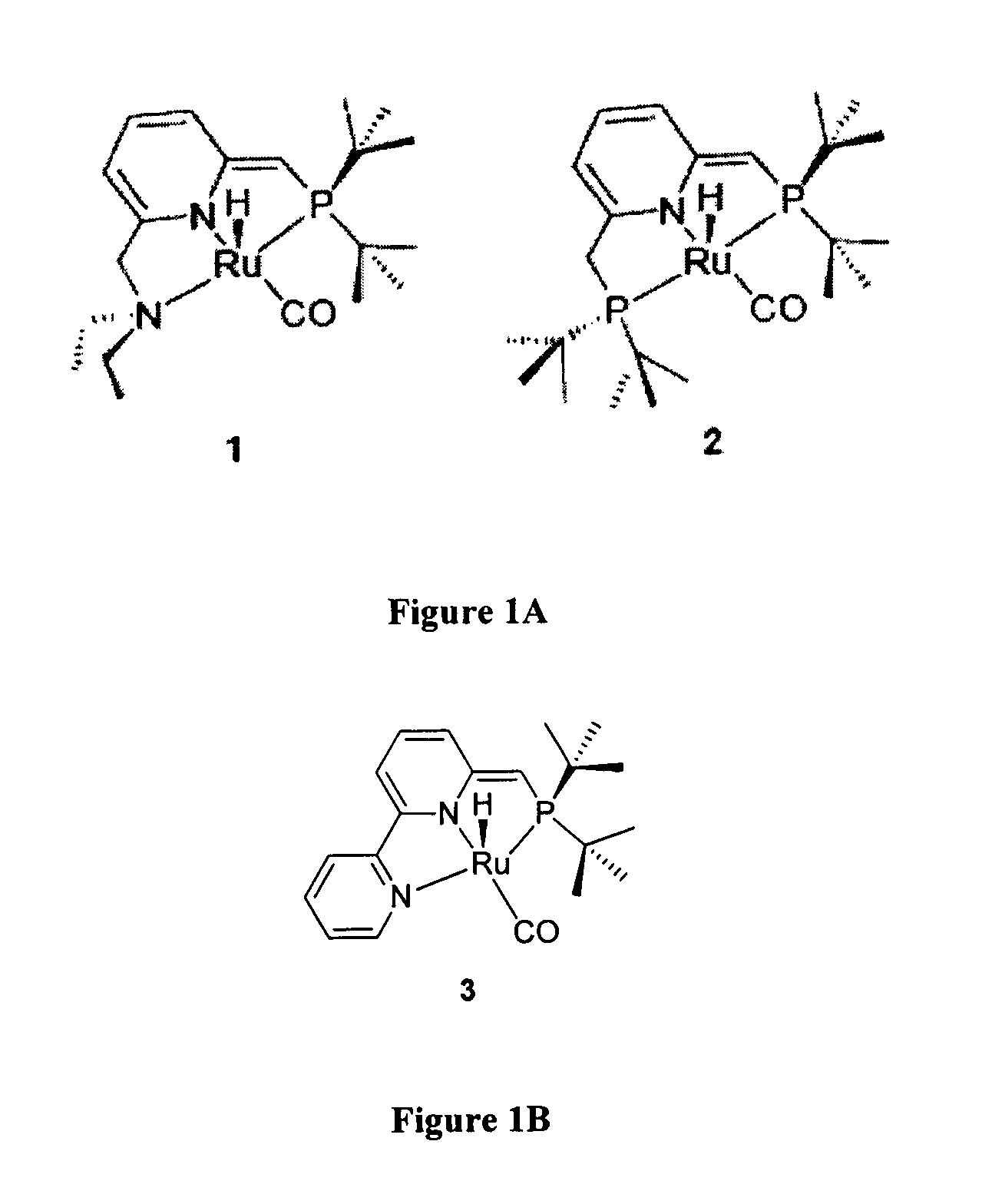
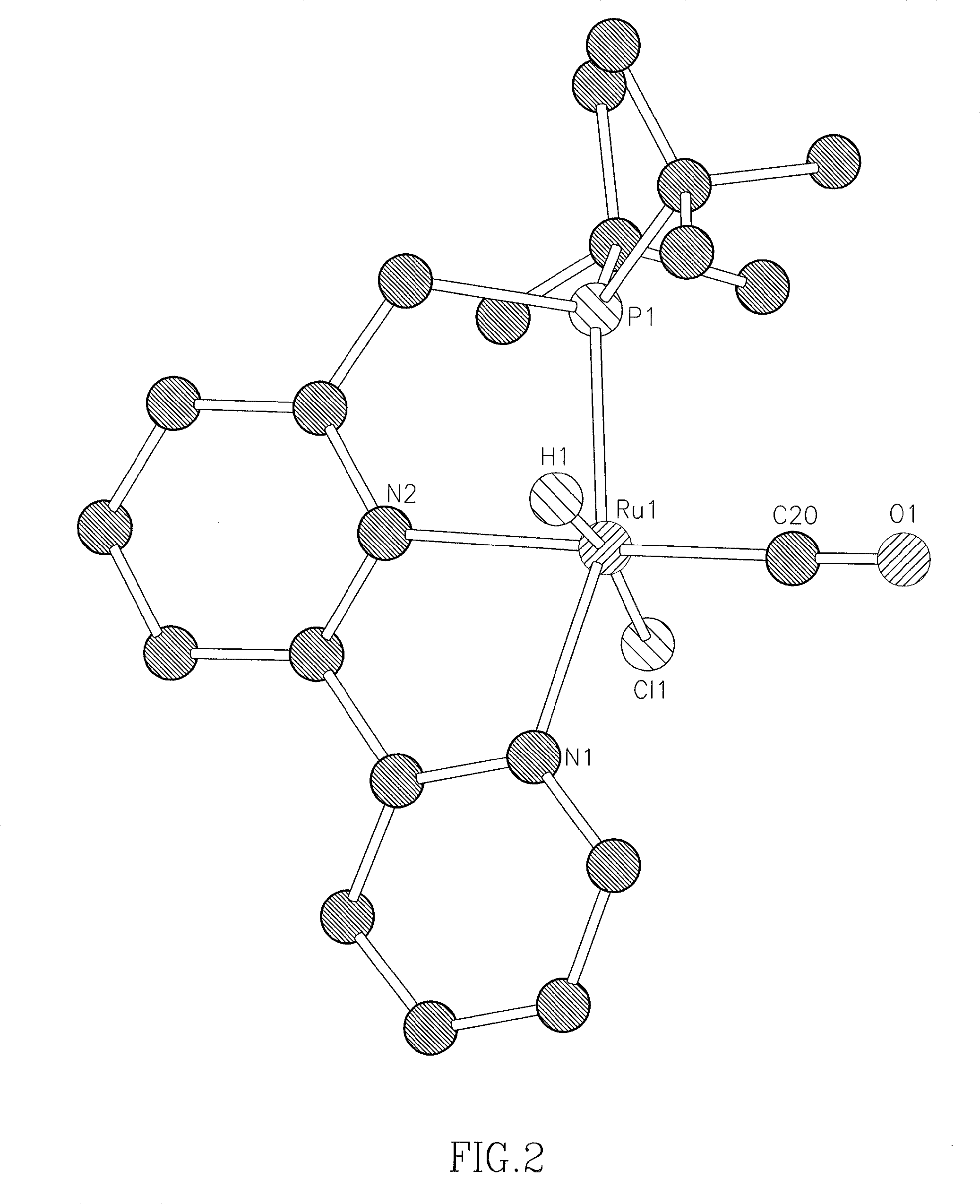
Novel ruthenium complexes and their uses in processes for formation and/or hydrogenation of esters, amides and derivatives thereof
ActiveUS20130281664A1High yieldImprove turnover ratePreparation from carboxylic acid saltsPlatinum group organic compoundsUrea derivativesPolyester
The present invention relates to novel Ruthenium catalysts and related borohydride complexes, and the use of such catalysts, inter alia, for (1) hydrogenation of amides (including polyamides) to alcohols and amines; (2) preparing amides from alcohols with amines (including the preparation of polyamides (e.g., polypeptides) by reacting dialcohols and diamines and / or by polymerization of amino alcohols); (3) hydrogenation of esters to alcohols (including hydrogenation of cyclic esters (lactones) or cyclic di-esters (di-lactones) or polyesters); (4) hydrogenation of organic carbonates (including polycarbonates) to alcohols and hydrogenation of carbamates (including polycarbamates) or urea derivatives to alcohols and amines; (5) dehydrogenative coupling of alcohols to esters; (6) hydrogenation of secondary alcohols to ketones; (7) amidation of esters (i.e., synthesis of amides from esters and amines); (8) acylation of alcohols using esters; (9) coupling of alcohols with water to form carboxylic acids; and (10) dehydrogenation of beta-amino alcohols to form pyrazines. The present invention further relates to the novel uses of certain pyridine Ruthenium catalysts.
Owner:YEDA RES & DEV CO LTD
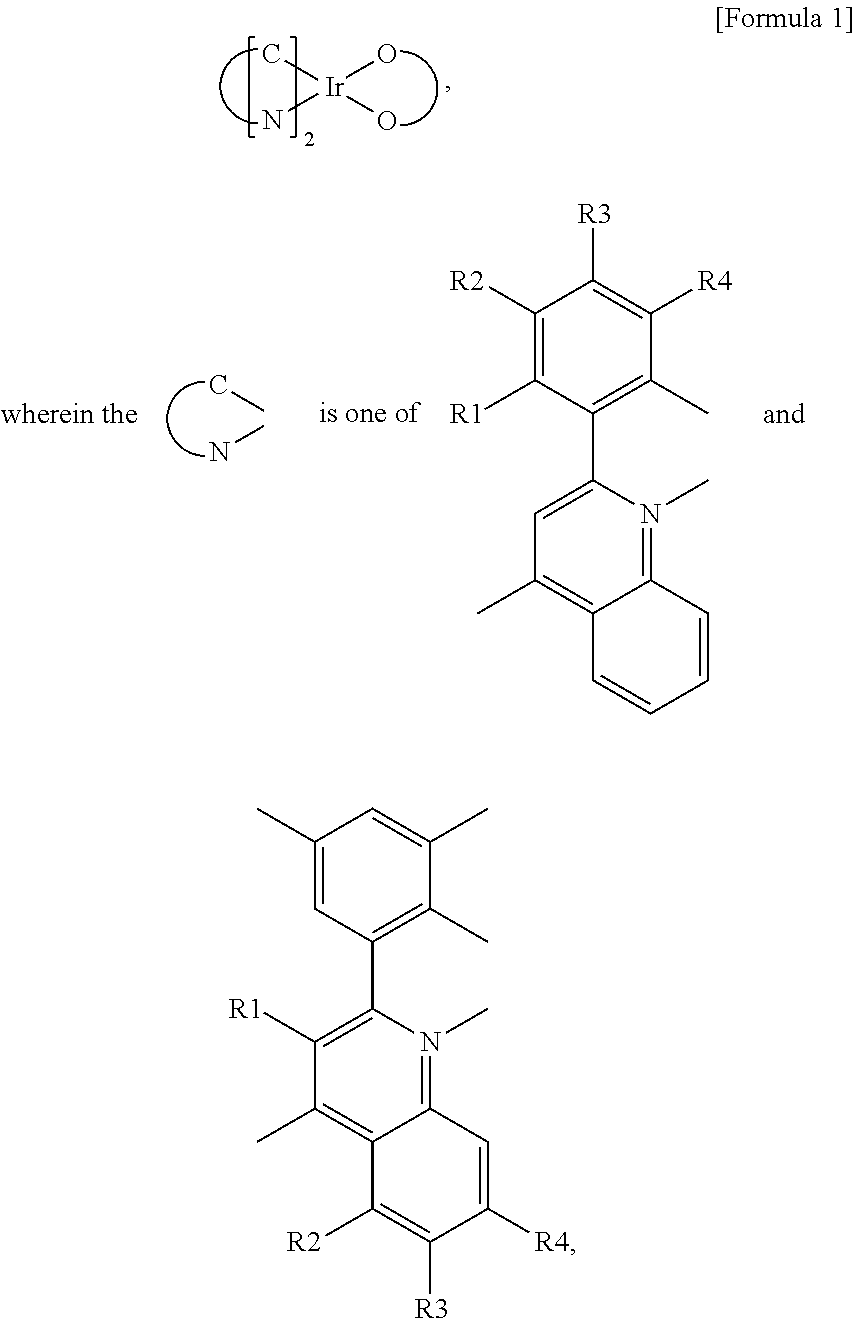
Metal coordination compound and organic luminescence device
InactiveUS6783873B2Platinum group organic compoundsGroup 5/15 element organic compoundsOrganic light emitting deviceTetradentate ligand
Owner:CANON KK
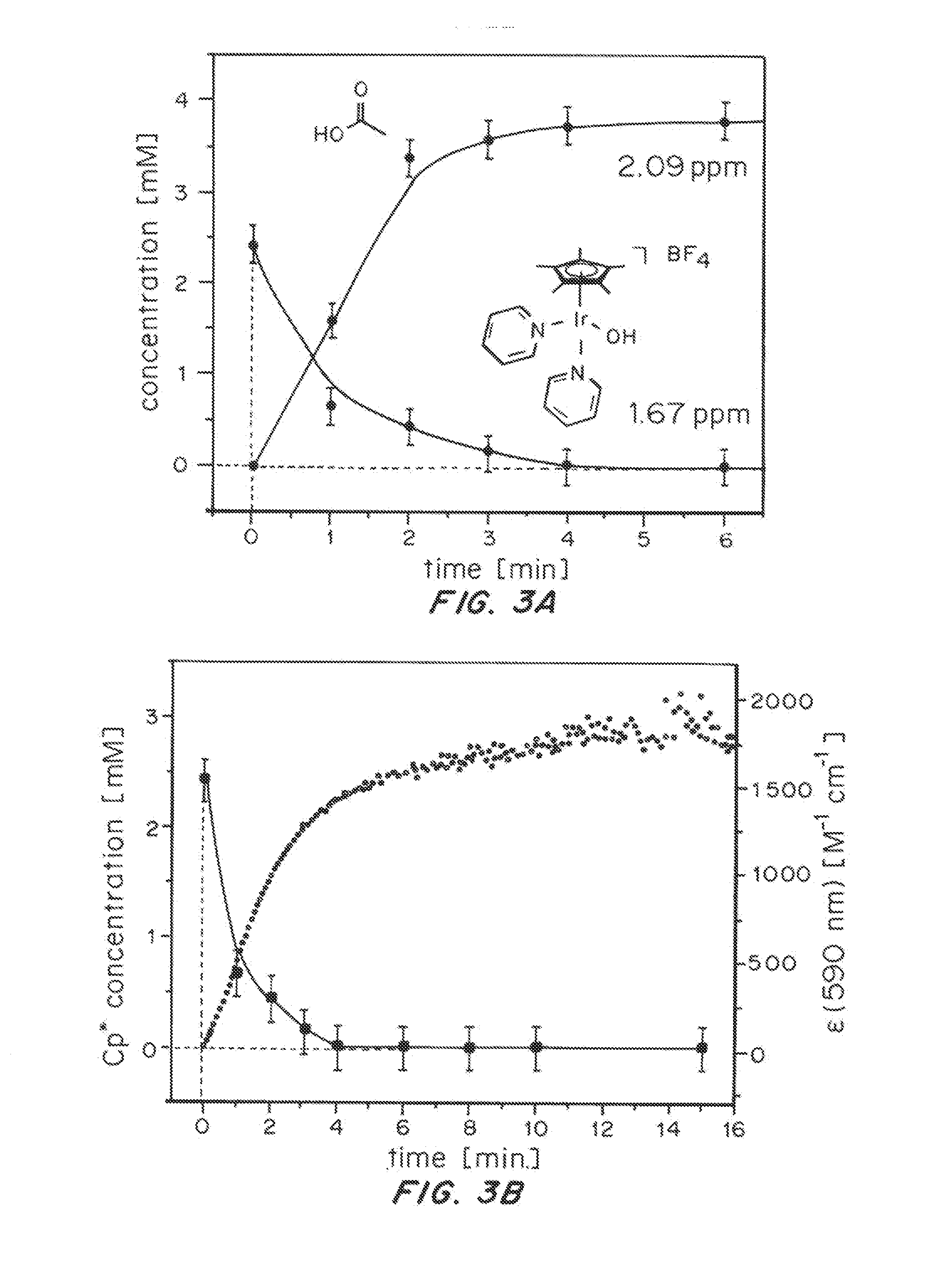
Iridium complexes for electrocatalysis
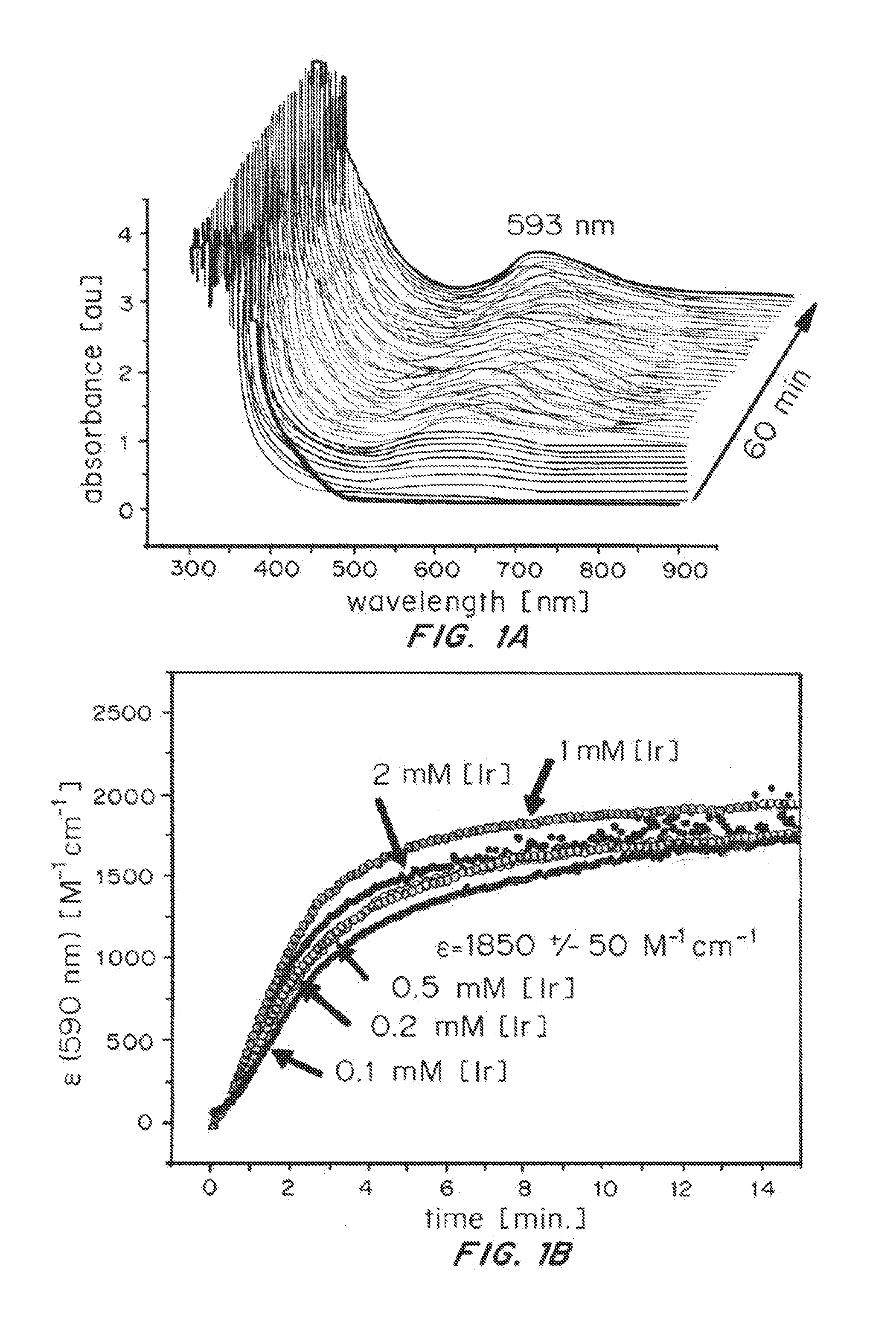
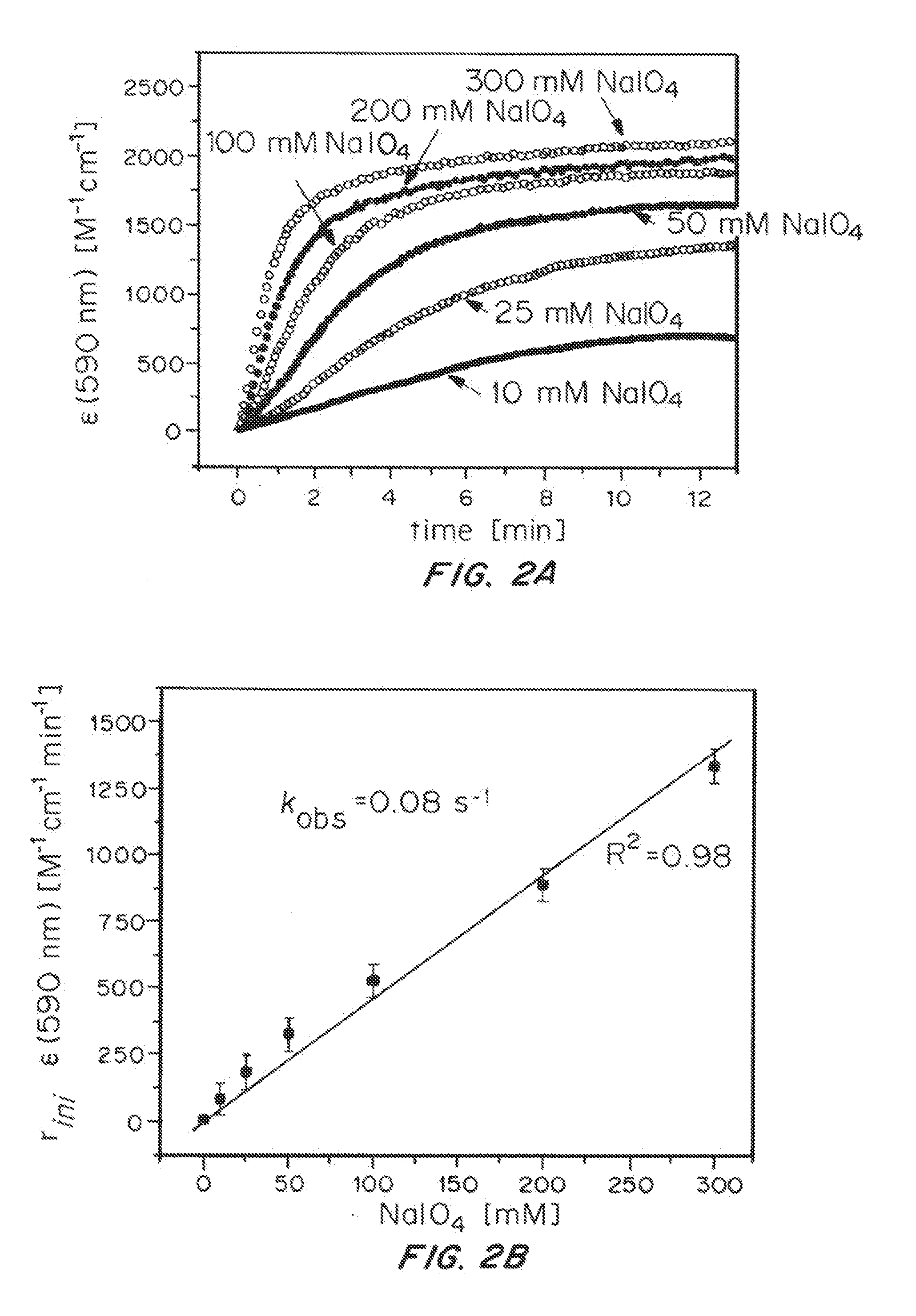
ActiveUS20150021194A1Improve stabilityConvenient bindingPlatinum group organic compoundsFrom normal temperature solutionsIridiumPentamethylcyclopentadiene
Solution-phase (e.g., homogeneous) or surface-immobilized (e.g., heterogeneous) electrode-driven oxidation catalysts based on iridium coordination compounds which self-assemble upon chemical or electrochemical oxidation of suitable precursors and methods of making and using thereof are. Iridium species such as {[Ir(LX)x(H2O)y(μ-O)]zm+}n wherein x, y, m are integers from 0-4, z and n from 1-4 and LX is an oxidation-resistant chelate ligand or ligands, such as such as 2(2-pyridyl)-2-propanolate, form upon oxidation of various molecular iridium complexes, for instance [Cp*Ir(LX)OH] or [(cod)Ir(LX)] (Cp*=pentamethylcyclopentadienyl, cod=cis-cis,1,5-cyclooctadiene) when exposed to oxidative conditions, such as sodium periodate (NaIO4) in aqueous solution at ambient conditions.
Owner:YALE UNIV
Therapeutic delivery of carbon monoxide
InactiveUS20060115542A1Increase stimulationRegulate expressionOrganic active ingredientsNervous disorderSolubilityTransplant rejection
Metal carbonyls are used to deliver CO having biological activity, for example vasodilatation and inhibition of transplant rejection. The metal of the carbonyl is typically of groups 7 to 10, e.g. Fe and Ru. The carbonyl preferably has one or more ligands other than CO, such as amino acids, to modulate the CO release property and solubility.
Owner:HEMOCORM
Metal oxide-organic hybrid materials for heterogeneous catalysis and methods of making and using thereof
ActiveUS20150065339A1Improve robustnessPreparation by oxidation reactionsPlatinum group organic compoundsElectrolysisCobalt
Catalysts prepared from abundant, cost effective metals, such as cobalt, nickel, chromium, manganese, iron, and copper, and containing one or more neutrally charged ligands (e.g., monodentate, bidentate, and / or polydentate ligands) and methods of making and using thereof are described herein. Exemplary ligands include, but are not limited to, phosphine ligands, nitrogen-based ligands, sulfur-based ligands, and / or arsenic-based ligands. In some embodiments, the catalyst is a cobalt-based catalyst or a nickel-based catalyst. The catalysts described herein are stable and active at neutral pH and in a wide range of buffers that are both weak and strong proton acceptors. While its activity is slightly lower than state of the art cobalt-based water oxidation catalysts under some conditions, it is capable of sustaining electrolysis at high applied potentials without a significant degradation in catalytic current. This enhanced robustness gives it an advantage in industrial and large-scale water electrolysis schemes.
Owner:YALE UNIV
High-performance organic light-emitting diode
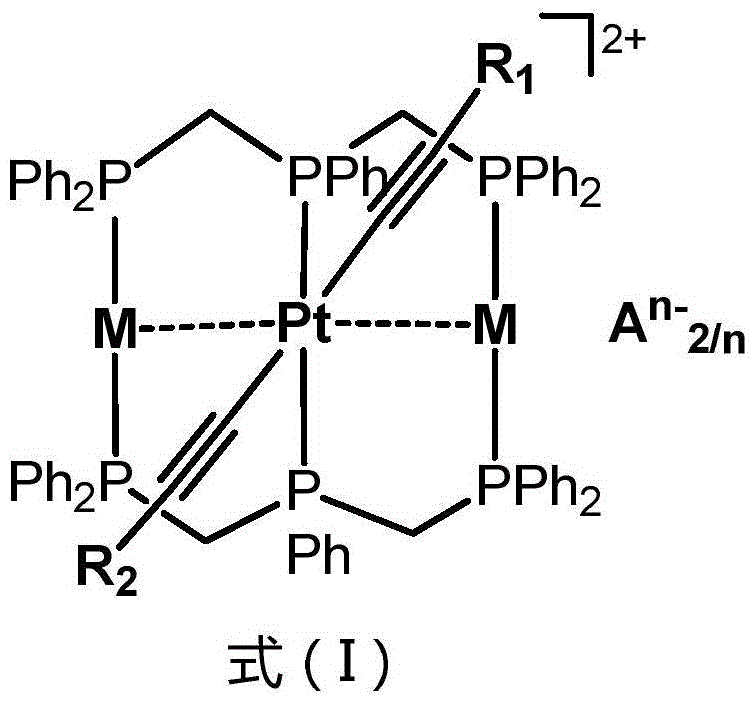
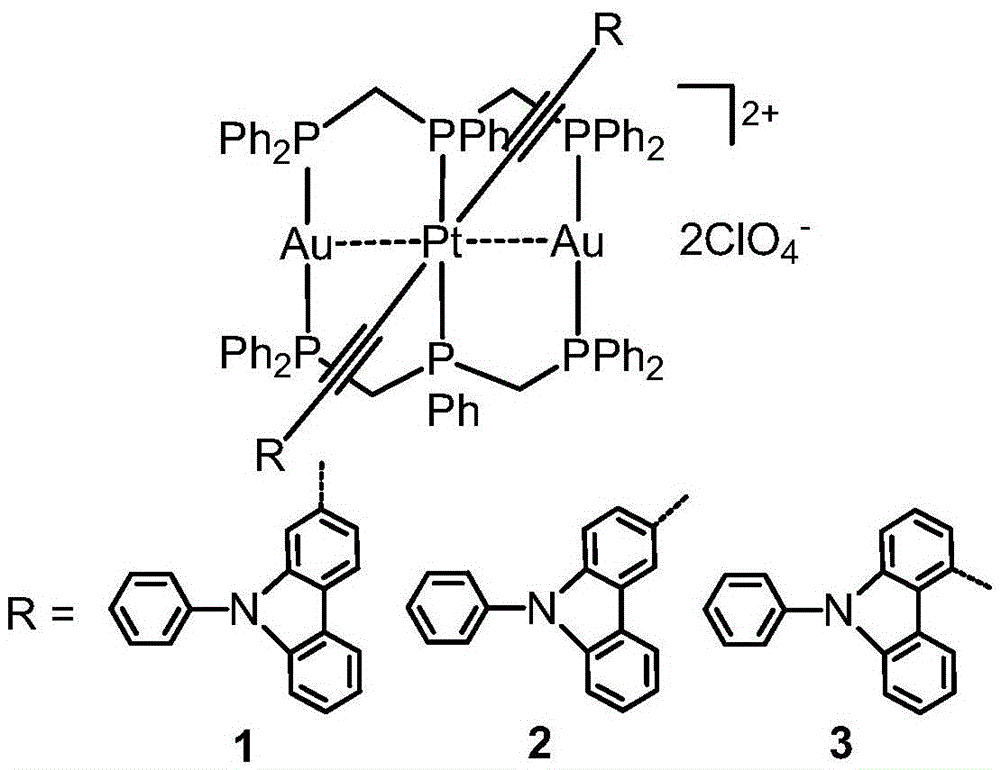
ActiveCN105481910AStrong phosphorescent emissionImprove performancePlatinum group organic compoundsSolid-state devicesQuantum efficiencyAryl
The invention relates to an ionic phosphorescent metal complex, and a preparation method and application thereof. The structure of the complex is as shown in a general formula (I) which is described in the specification. In the general formula (I), R1 and R2 are same or different and are independently selected from a group consisting of aryl-substituted heteroaryl groups; M is any one selected from the group consisting of Ag(I) and Au(I); A<n-> is ClO4<-->, Pf6<-->, SbF6<-->, BF4<--> and SiF6<2-->; and n is 1 or 2. The invention also relate to an organic light-emitting diode and a preparation method and application thereof. The organic light-emitting diode is prepared with the phosphorescent metal complex as a doping body of a luminescent layer, has high-performance organic electroluminescence and maximum external quantum efficiency of up to 20% and can be applied to panel display.
Owner:GUANGDONG JUHUA PRINTING DISPLAY TECH CO LTD
Red phoshorescent compound and organic electroluminescent device using the same
ActiveUS20100133524A1High luminous efficiencyPlatinum group organic compoundsElectroluminescent light sourcesHydrogen atomAlkoxy group
A red phosphorescent compound includes a host material being capable of transporting an electron or a hole; and a dopant material represented by following Formula 1:and each of R1 to R4 is one of the group consisting of hydrogen atom (H), C1 to C6 substituted or non-substituted alkyl group, C1 to C6 substituted or non-substituted alkoxy group, and halogen atom.
Owner:LG DISPLAY CO LTD
Cobalt Containing Hydrosilylation Catalysts and Compositions Containing the Catalysts
InactiveUS20140231702A1Group 1/11 organic compounds without C-metal linkagesPlatinum group organic compoundsSilanesOrganic group
A composition contains (A) a hydrosilylation reaction catalyst and (B) an aliphatically unsaturated compound having an average, per molecule, of one or more aliphatically unsaturated organic groups capable of undergoing hydrosilylation reaction. The composition is capable of reacting via hydrosilylation reaction to form a reaction product, such as a silane, a gum, a gel, a rubber, or a resin. Ingredient (A) contains a metal-ligand complex that can be prepared by a method including reacting a metal precursor and a ligand.
Owner:DOW CORNING CORP
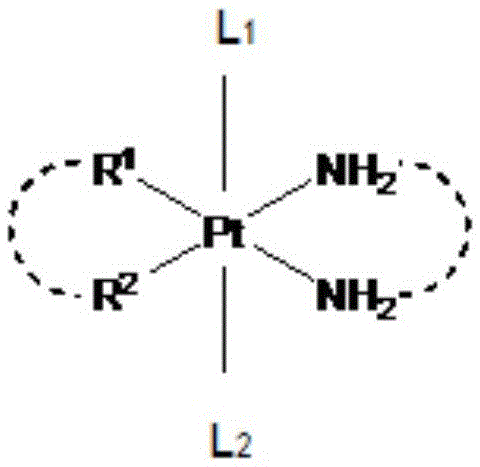
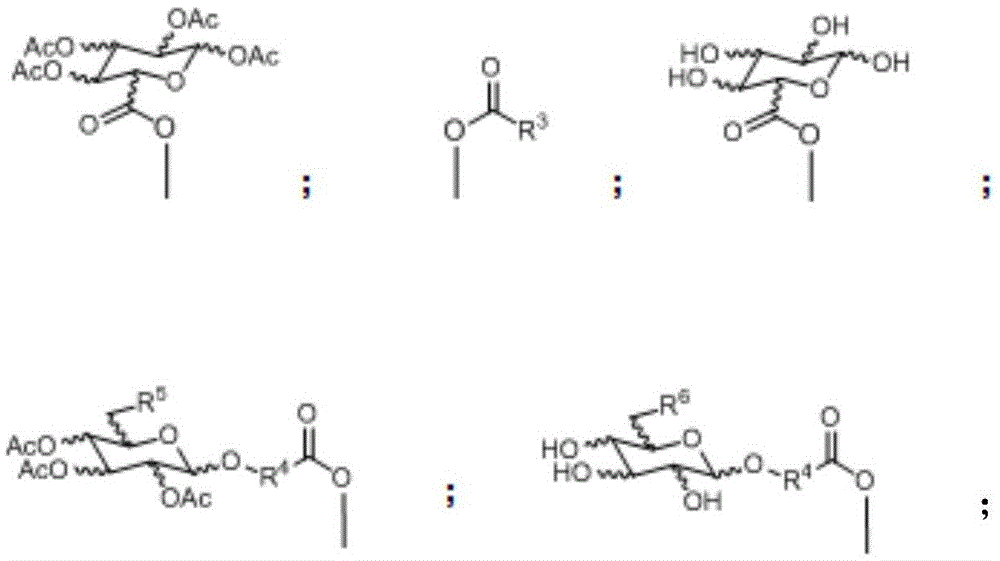
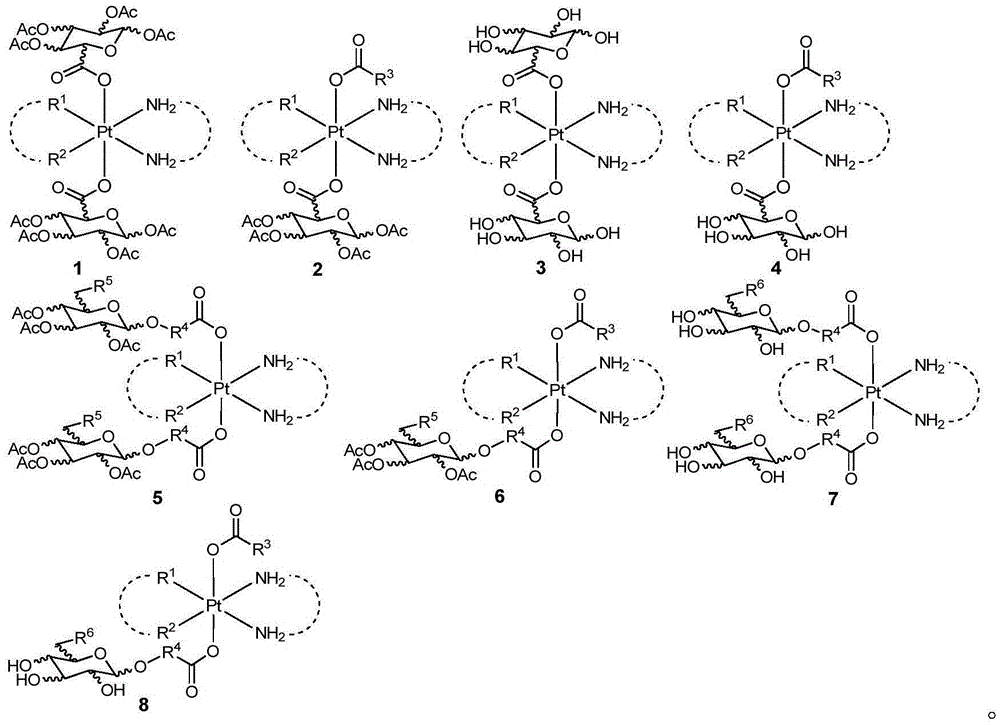
Glycosylated quadrivalent platinum compounds with anticancer activity, and preparation method and application thereof
InactiveCN105622673AHas anticancer activityEsterified saccharide compoundsOrganic active ingredientsPlatinum CompoundGlycosyl
The invention relates to glycosylated quadrivalent platinum compounds with anticancer activity, and a preparation method and application thereof. The glycosyl group is introduced into the quadrivalent platinum parent nucleus for the first time to design and synthesize a series of novel glycosylation-modified quadrivalent platinum compounds of which the anticancer and antitumor capacities are inspected. A series of original creative researches are hopeful to obtain multiple precursor molecules which are effective for tumors, thereby providing new candidate drug molecules for the existence of the traditional bivalent platinum drugs, and also providing a new pathway for modification of the quadrivalent platinum compounds. The basically creative drug researches have important theoretical value and practical meanings for national economical and social development, health of people and the like.
Owner:NANKAI UNIV
Metal oxide-organic hybrid materials for heterogeneous catalysis and methods of making and using thereof
ActiveUS20160152648A1Improve robustnessPreparation by oxidation reactionsPlatinum group organic compoundsElectrolysisCobalt
Owner:YALE UNIV
Control of composite covalent organic framework by varying functional groups inside the pore
An ordered functional nanoporous material (OFMN) composition includes a pore defined by a sidewall, the sidewall comprising N—C—N linkages therein. A process for synthesis of a reagent includes the reaction of a 6,7-diaminoquinoxaline having R groups with hexaketocyclohexane (HKH) octahydrate, where R is independently in each occurrence H, Cl, Br, I, C4H4S (thiophenyl), SO3−, CO2−, C≡CH, CH═CH2, NH2, OH, C≡N, C1-C4 alkyl, (CH2)xCH═CH2, or (CH2)yCH═CH(CH2)z where x or (y+z) is an integer of 0 to 4 inclusive, (CH2)jCH≡CH, or (CH2)kCH≡C(CH2)r where j or (k+r) 0 to 4 inclusive. A process of degasification that includes extracting a gas from a mixture by exposing the mixture to an OFNM to selectively pass the gas therethrough. A process of dehydrogenation includes exposing an aliphatically unsaturated feedstock to platinum modified OFNM under conditions to form hydrogen and selectively passing the hydrogen through the platinum modified OFNM.
Owner:UNIVERSITY OF WYOMING
Arylamine tetradentate cyclometalated platinum complex near-infrared electroluminescent materials as well as preparation and application thereof
ActiveCN105481906AImprove carrier transport performanceIncrease the degree of π conjugationPlatinum group organic compoundsSolid-state devicesPlatinum complexCarbazole
The invention discloses arylamine tetradentate cyclometalated platinum complex near-infrared electroluminescent materials as well as preparation and application thereof. The cyclometalated platinum complexes contain ligands comprising dual donor units (triphenylamine fluorene or carbazole) and dual C^N tetradentate coordination structures; the donor units in cyclometalated complex molecules can further enlarge a conjugated system of the complexes, red shift of the emission spectra of the complexes is realized, and the complexes have a near-infrared emission characteristic. Efficient near-infrared emission can be realized when the cyclometalated platinum complexes are applied to preparation of polymer electroluminescent devices.
Owner:XIANGTAN UNIV
Methods for solubilizing and recovering fluorinate compounds
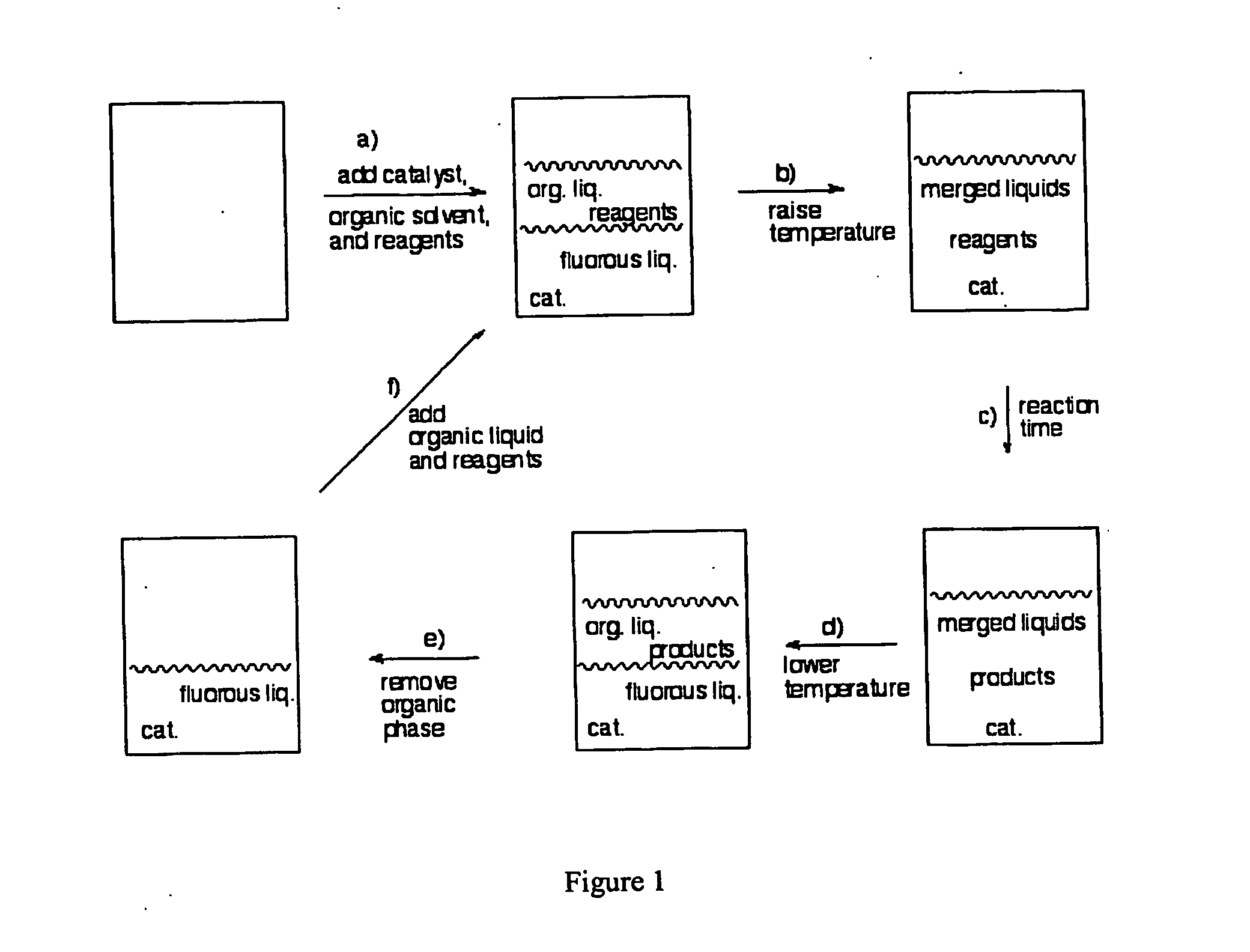
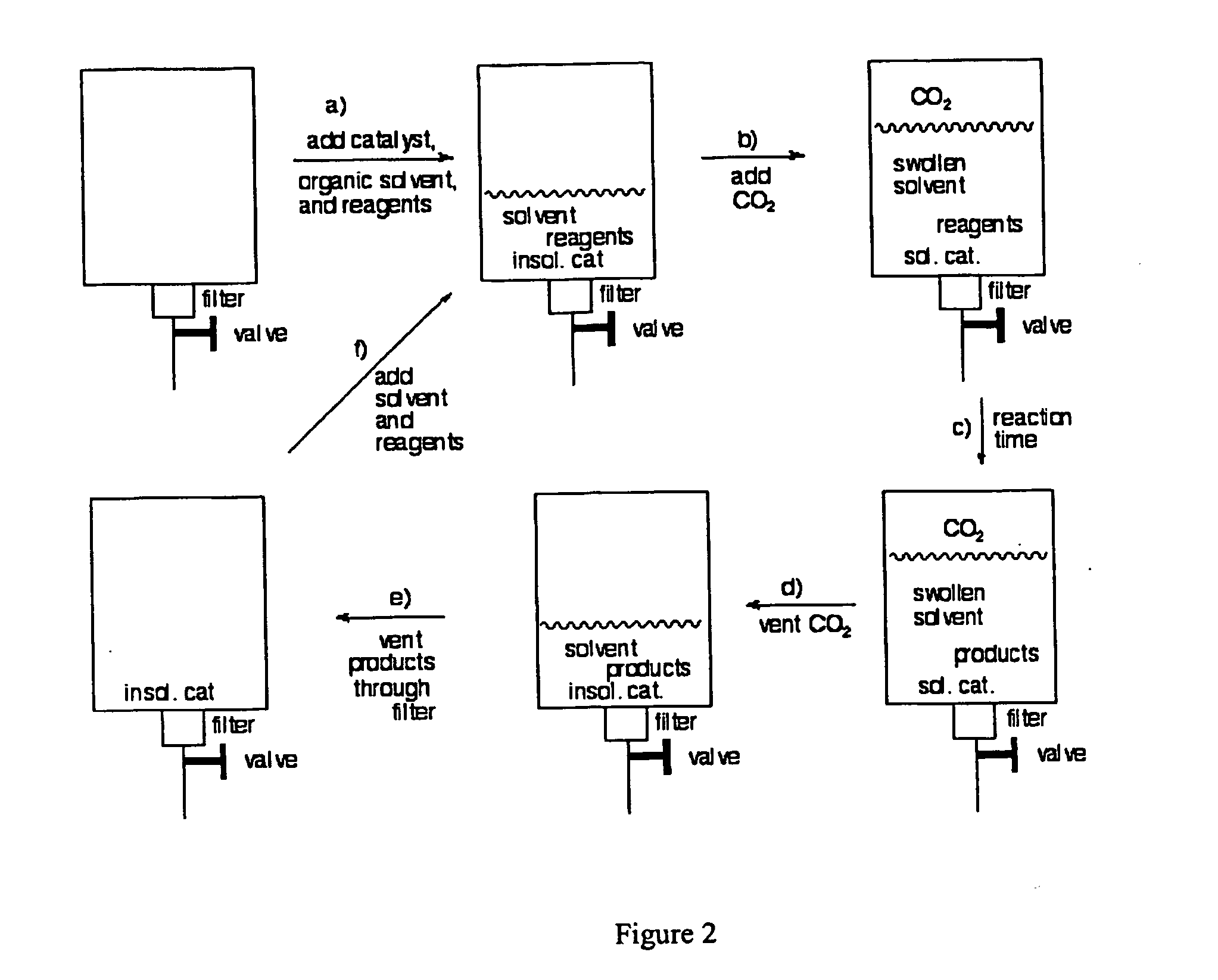
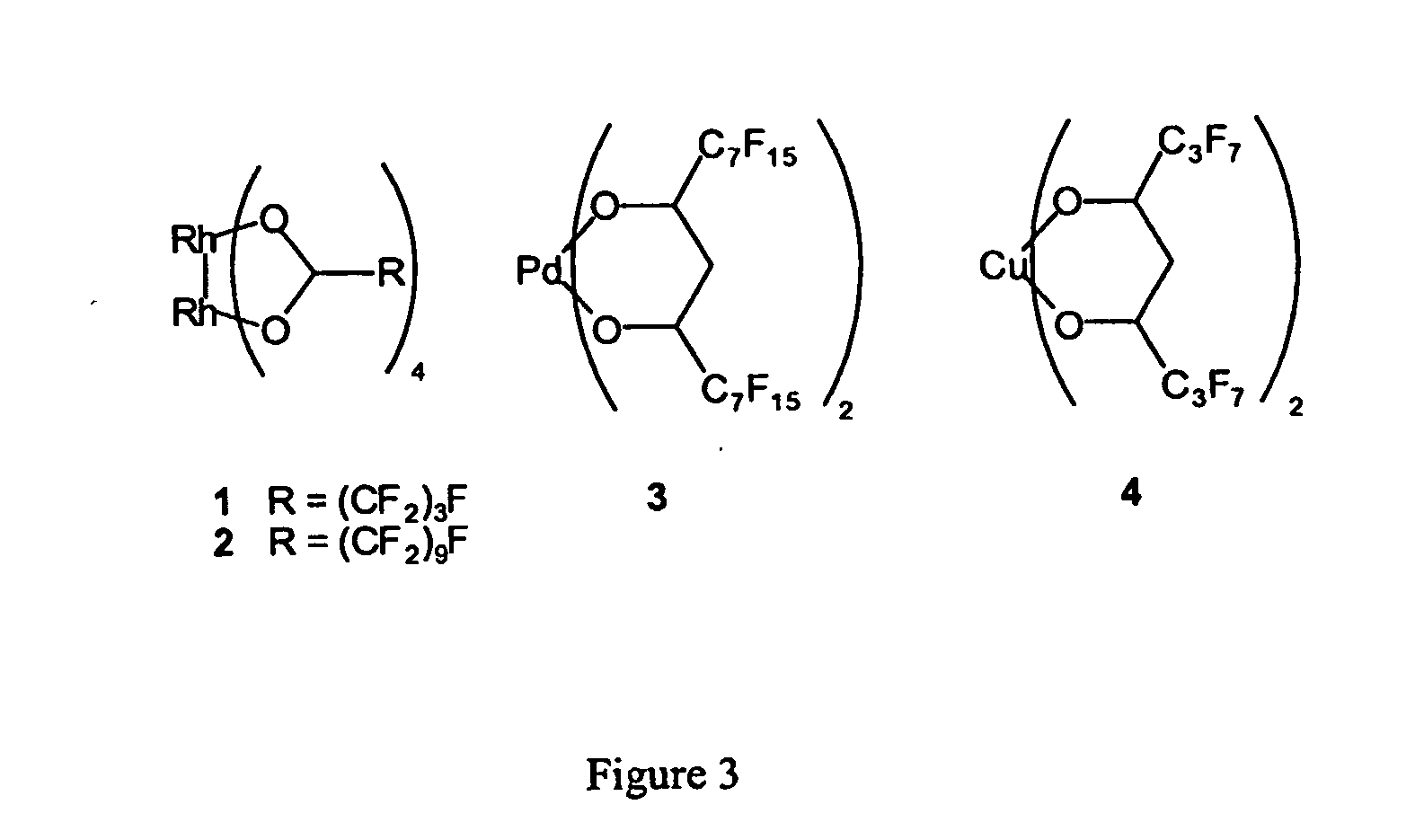
InactiveUS20050015936A1Reducing carbon dioxide gaseous pressureEffective recoveryPlatinum group organic compoundsMagnesium halidesSolubilityOrganic solvent
Methods of enhancing the solubility of a fluorinated compound in an organic solvent are provided. In one embodiment, carbon dioxide gas pressure is applied to the solvent at a pressure effective to enhance the solubility of the fluorinated compound. The method may further include recrystallizing the fluorinated compound by reducing the pressure of the carbon dioxide gas. Also provided are methods of conducting a reaction using a fluorinated compound in an organic solvent In one embodiment, the method comprises applying carbon dioxide pressure to an organic solvent comprising at least one substrate and a fluorinated catalyst, in an effective amount to solubilize the catalyst; and permitting the fluorinated catalyst to catalyze the reaction of the substrate to form a product. The catalyst is optionally separated from the reaction product and solvent after the reaction by the release of the pressure.
Owner:GEORGIA TECH RES CORP +1
Hydrosilylation Catalysts Made With Terdentate Nitrogen Ligands And Compositions Containing The Catalysts
InactiveUS20150224490A1Group 1/11 organic compounds without C-metal linkagesPlatinum group organic compoundsOrganic groupNitrogen
A composition contains (A) a hydrosilylation reaction catalyst and (B) an aliphatically unsaturated compound having an average, per molecule, of one or more aliphatically unsaturated organic groups capable of undergoing hydrosilylation reaction. The composition is capable of reacting via hydrosilylation reaction to form a reaction product, such as a silane, a gum, a gel, a rubber, or a resin. Ingredient (A) contains a metal-ligand complex that can be prepared by a method including reacting a metal precursor and a ligand.
Owner:DOW CORNING CORP
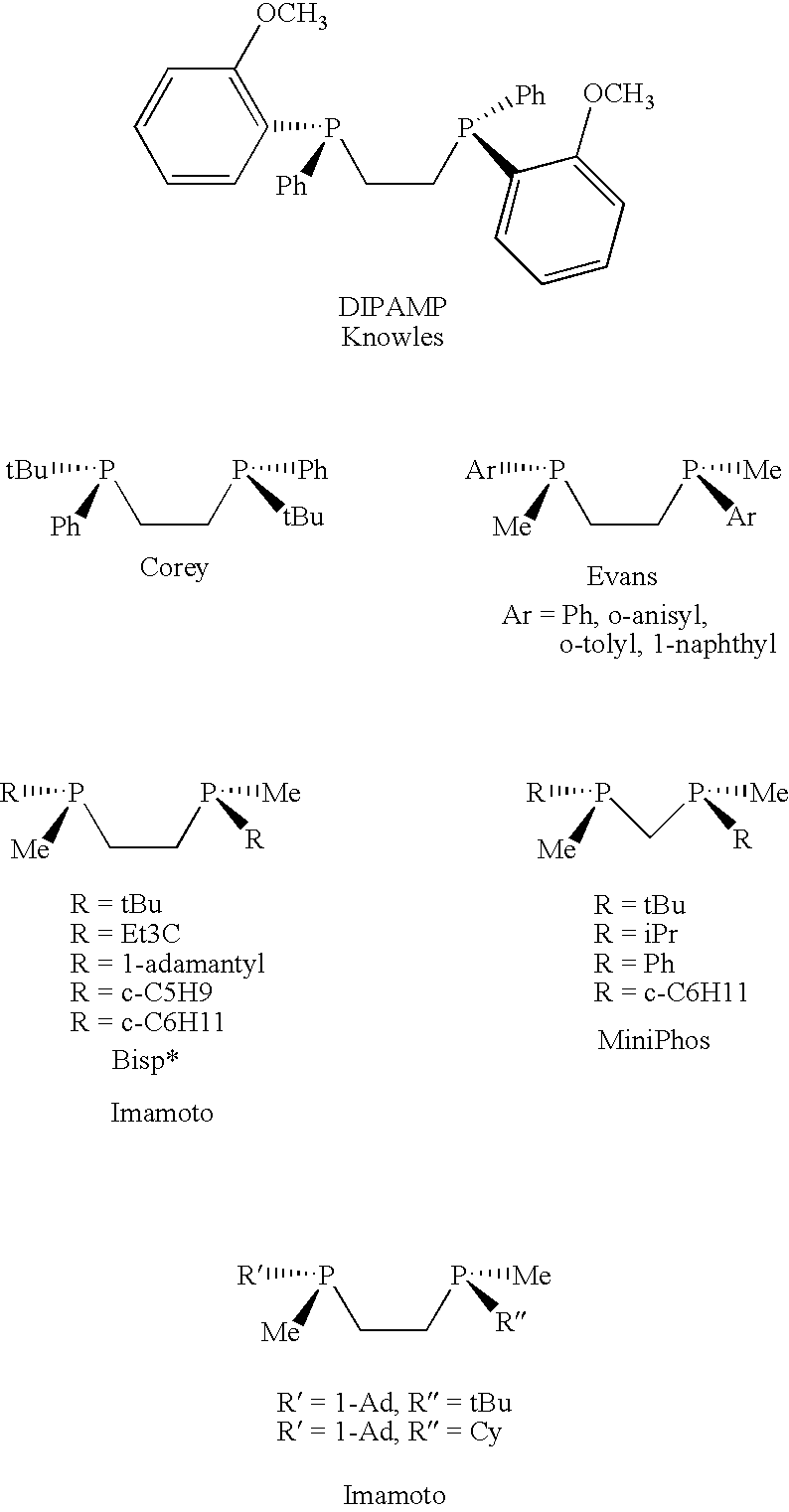
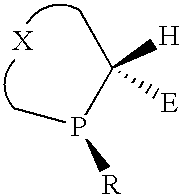
P-chiral phospholanes and phosphocyclic compounds and their use in asymmetric catalytic reactions
InactiveUS7153809B2SelectiveOrganic active ingredientsGroup 1/11 organic compounds without C-metal linkagesIsomerizationCycloaddition
Chiral ligands and metal complexes based on such chiral ligands useful in asymmetric catalysis are disclosed. The metal complexes according to the present invention are useful as catalysts in asymmetric reactions, such as, hydrogenation, hydride transfer, allylic alkylation, hydrosilylation, hydroboration, hydrovinylation, hydroformylation, olefin metathesis, hydrocarboxylation, isomerization, cyclopropanation, Diels-Alder reaction, Heck reaction, isomerization, Aldol reaction, Michael addition; epoxidation, kinetic resolution and [m+n] cycloaddition. Processes for the preparation of the ligands are also described.
Owner:PENN STATE RES FOUND
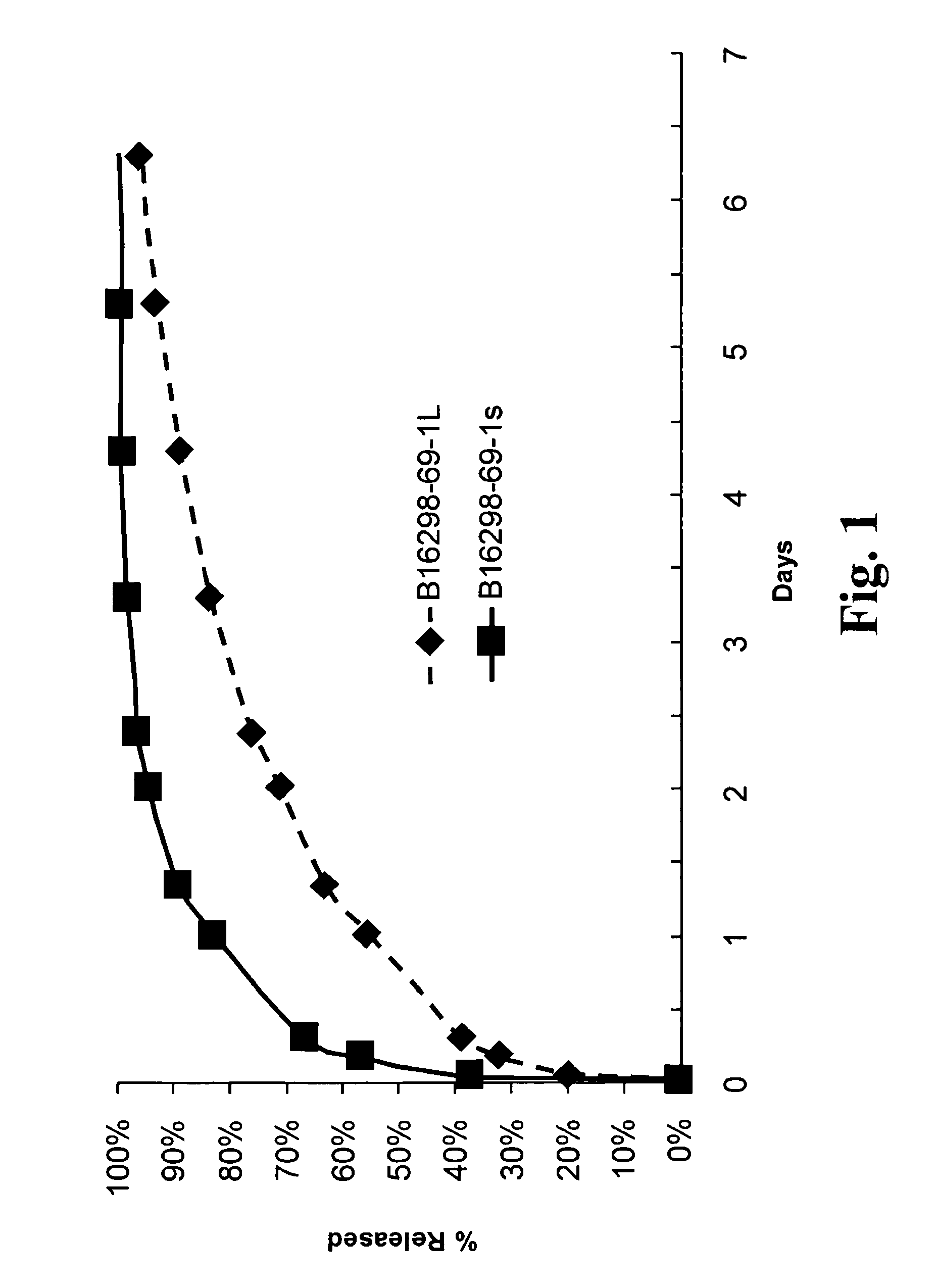
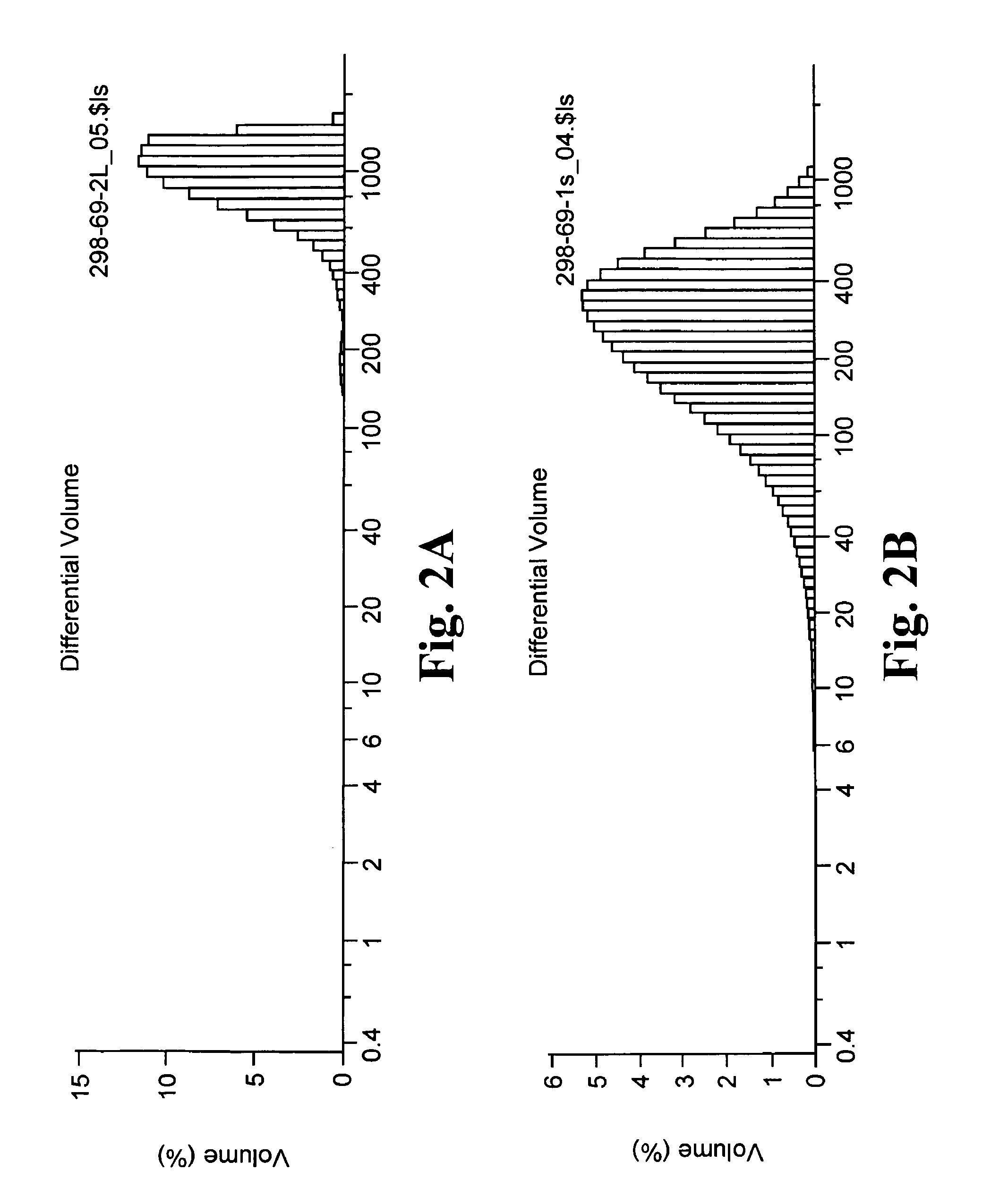
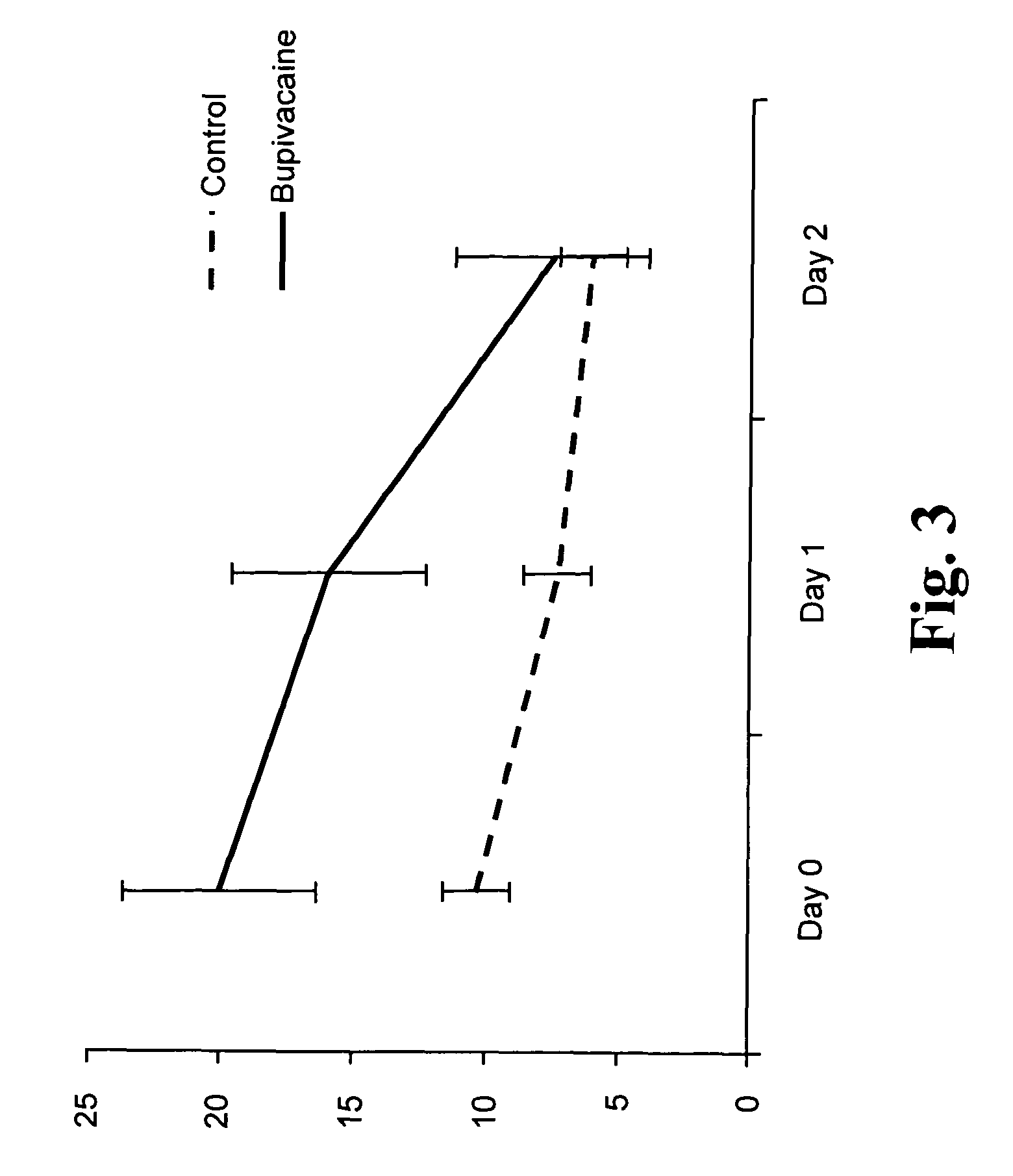
Slow release of organic salts of local anesthetics for pain relief
ActiveUS8920843B2Improve performanceExtended releasePowder deliveryOrganic active ingredientsAnesthetic AgentOrganic acid
Particles of an organic acid salt of an amino acid amide or ester local anesthetic are employed as agents for the improved alleviation of pain. Particularly, the particles find use with surgically created wounds, where the particles may be administered directly into the bed of the wound or topically for transdermal transport.
Owner:SVIP5
Molybdenum Containing Hydrosilylation Reaction Catalysts and Compositions Containing the Catalysts
InactiveUS20140231703A1Group 1/11 organic compounds without C-metal linkagesPlatinum group organic compoundsSilanesOrganic group
A composition contains (A) a hydrosilylation reaction catalyst and (B) an aliphatically unsaturated compound having an average, per molecule, of one or more aliphatically unsaturated organic groups capable of undergoing hydrosilylation reaction. The composition ′ capable of reacting via hydrosilylation reaction to form a reaction product, such as a silane, a gum, a gel, a rubber, or a resin. Ingredient (A) contains a metal-ligand complex that can be prepared by a method including reacting a metal precursor and a ligand.
Owner:DOW CORNING CORP
Metal complexes for use in the carbonylation of ethylenically unsaturated compounds
InactiveCN101448773AExtended Catalyst SystemPlatinum group organic compoundsPreparation by carbon monoxide or formate reactionProtein carbonylCarbonylation
The invention concerns metal complexes and their preparation, in particular a metal complex MLnXm, where M is a metal of group 8, 9 or 10 and X is a halide, HCO3-, NO3-, CO32- or carboxylate. n is a number equal to or less than the coordination number of the metal and m is 1 or 2 and is equal to the oxidation state of the metal. The ligand L may be a bidentate phosphine of formula (I), (II), (III) or (IV) as set out herein. The process of production comprises reacting an ammine compound of metal M with a complexing compound, which is preferably a phosphine.
Owner:LUCITE INT UK LTD
Ruthenium complexes and their uses in processes for formation and/or hydrogenation of esters, amides and derivatives thereof
ActiveUS9045381B2Good atom economyHigh yieldPreparation from carboxylic acid saltsPlatinum group organic compoundsUrea derivativesPolyester
Owner:YEDA RES & DEV CO LTD
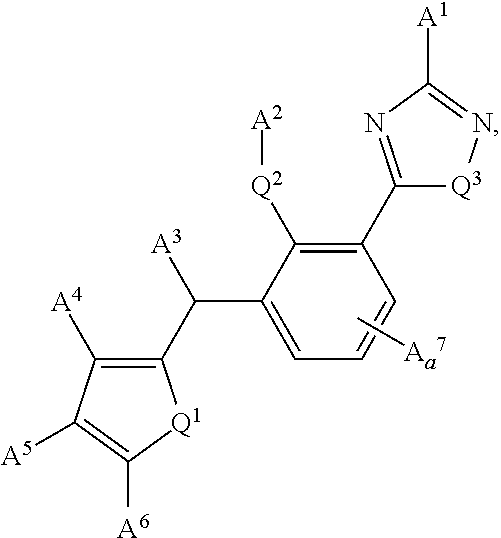
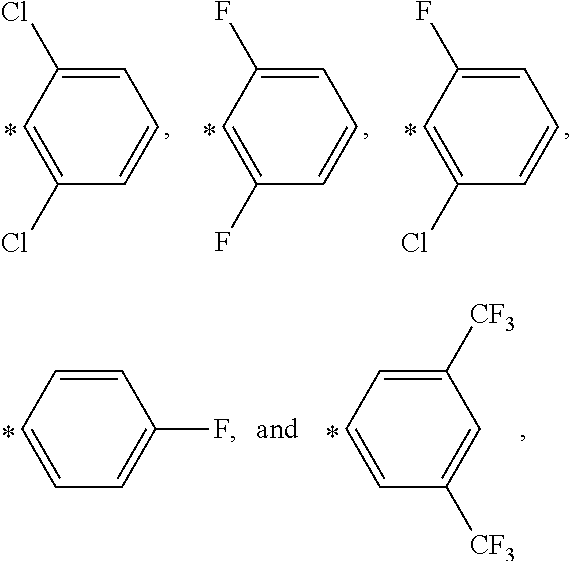
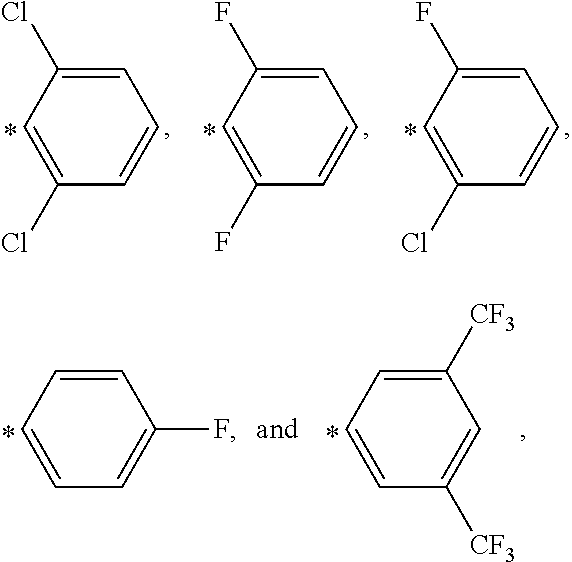
Substituted dibenzo[FG,OP]tetracenes and formulations or electronic devices containing the same
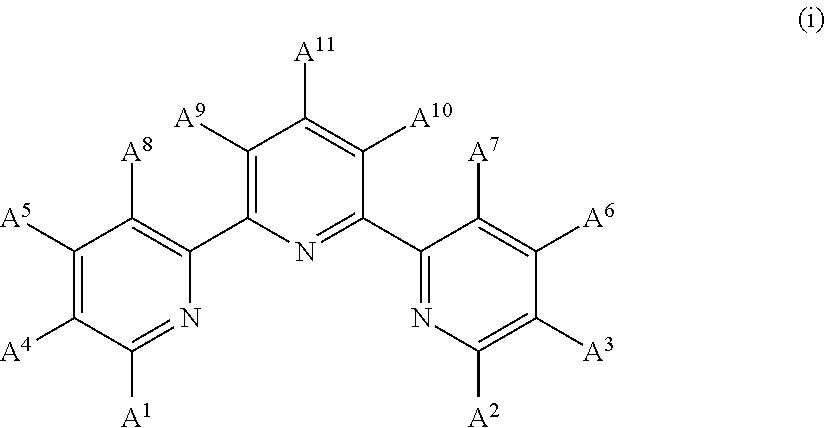
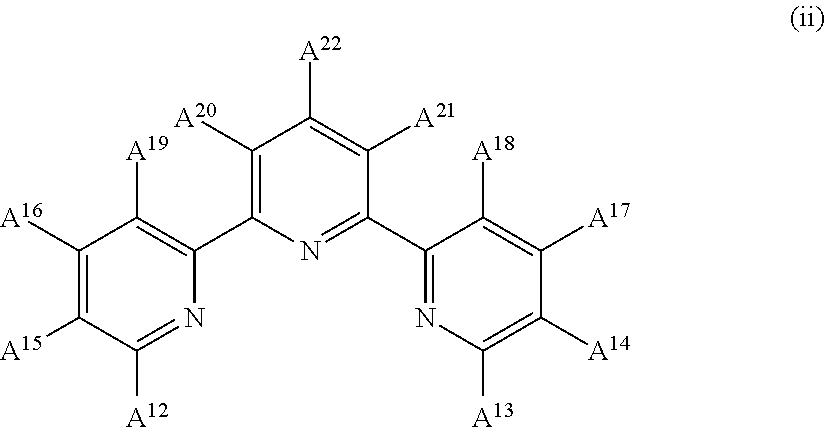
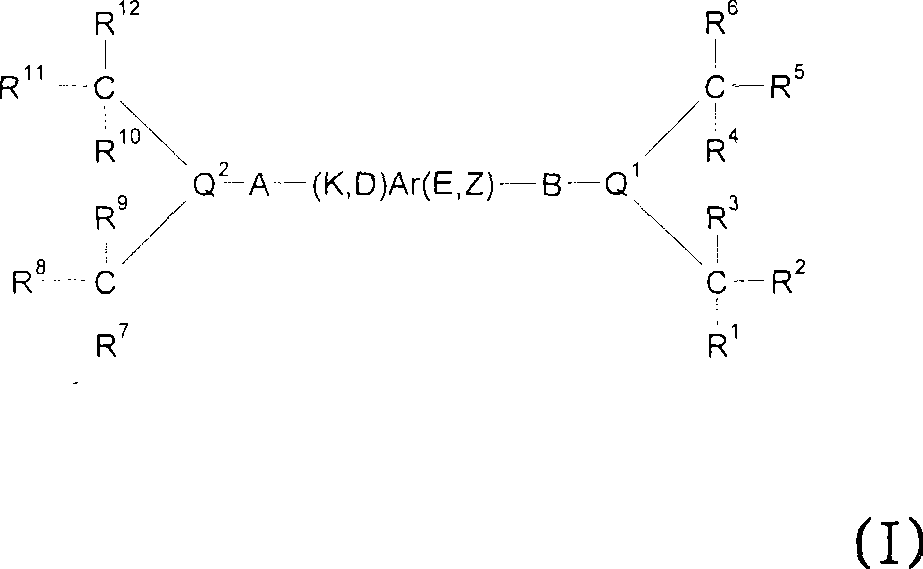
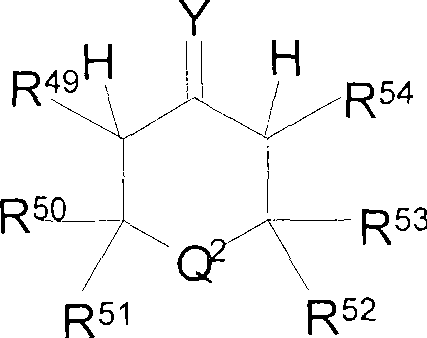
InactiveUS9029425B2Improve solubilityOrganic active ingredientsSenses disorderEngineeringCombinatorial chemistry
Owner:MERCK PATENT GMBH
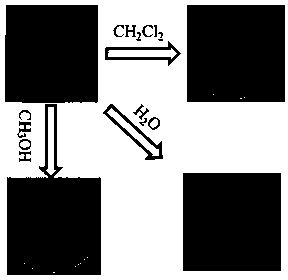
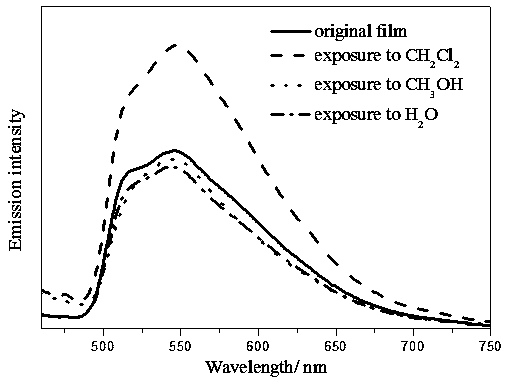
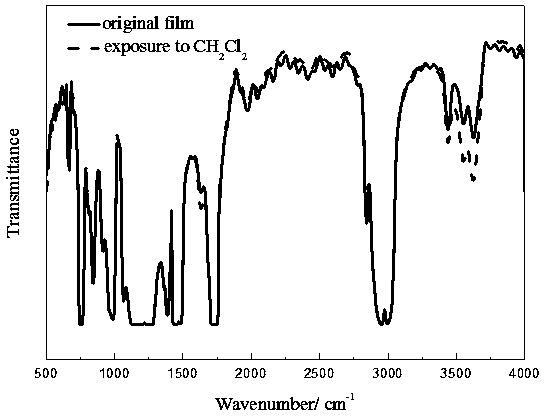
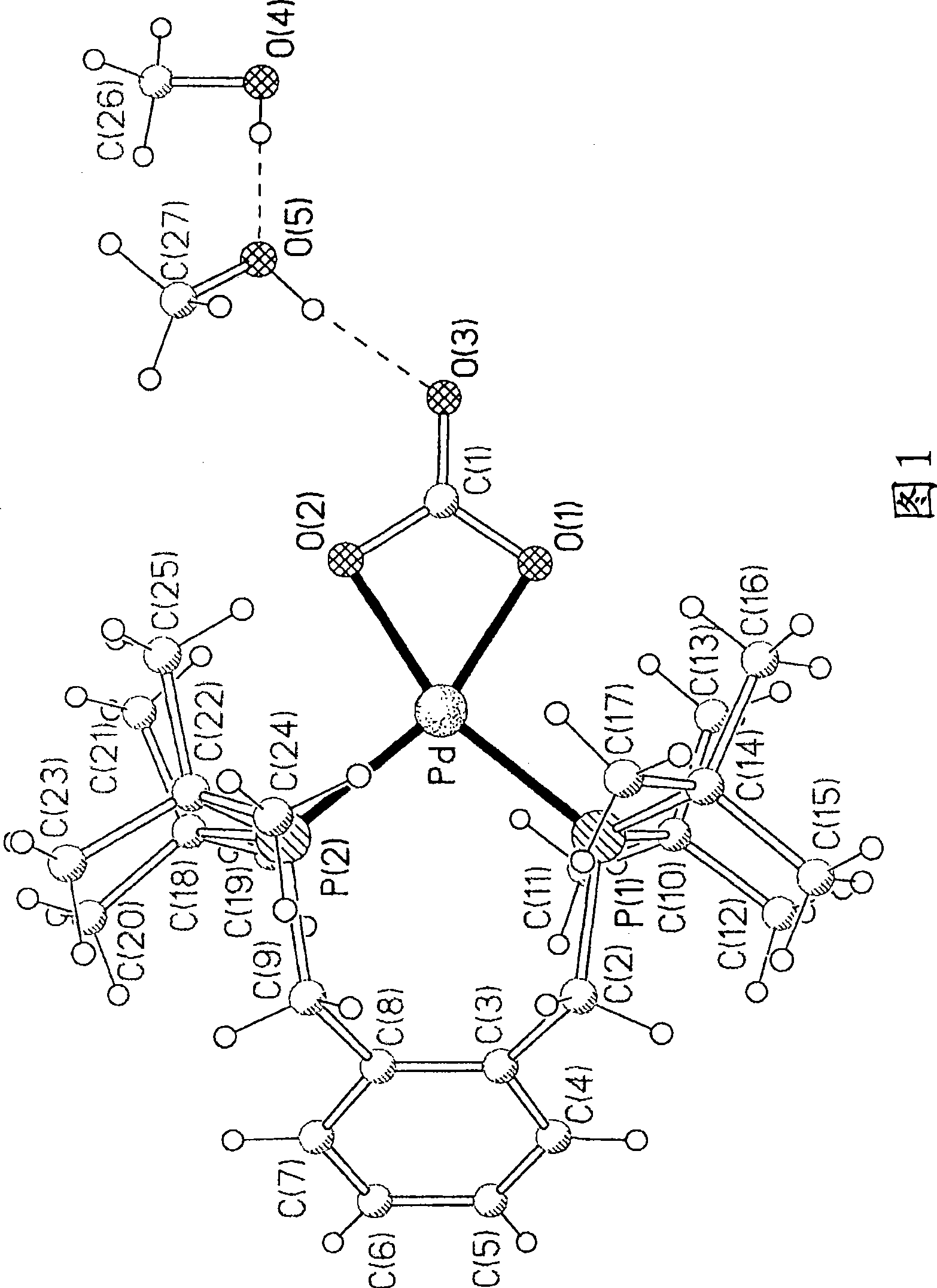
Preparation method and application of multi-response platinum complex luminescent thin film
PendingCN110591129AHigh emission intensityReduce quenchingPlatinum group organic compoundsColor/spectral properties measurementsPlatinum complexPolymer solution
The invention relates to the field of luminescent thin film materials, in particular to a preparation method and an application of a multi-response platinum complex luminescent thin film. The preparation method of the multi-response platinum complex luminescent thin film comprises the following steps: (1), a platinum complex precursor and 2,6-dimethylphenyl isocyanide are subjected to a reaction,the platinum complex is prepared and is recrystallized in a chloroform solution, and the platinum complex is prepared; (2), the platinum complex is dissolved in a good solvent S1, and a platinum complex solution is prepared; (3), high-molecular polymers are dissolved in a good solvent S2, and a high-molecular polymer solution is prepared; (4), the platinum complex solution and the high-molecular polymer solution are mixed, and a compound solution is prepared; and (5), the compound solution is dropwise added to an evaporation dish and subjected to tape-casting to be flat, and the platinum complex luminescent thin film is prepared. The raw materials are easily available, the preparation method is simple and has a good effect, and the platinum complex luminescent thin film can be widely applied to the fields of volatile organic compound detection, thermosensitive sensing and the like.
Owner:HAINAN NORMAL UNIV
Metal complexes for use in the carbonylation of ethylenically unsaturated compounds
InactiveCN101448773BExtended Catalyst SystemPlatinum group organic compoundsPreparation by carbon monoxide or formate reactionOxidation stateCarbonylation
The invention concerns metal complexes and their preparation, in particular a metal complex MLnXm, where M is a metal of group 8, 9 or 10 and X is a halide, HCO3-, NO3-, CO32- or carboxylate. n is a number equal to or less than the coordination number of the metal and m is 1 or 2 and is equal to the oxidation state of the metal. The ligand L may be a bidentate phosphine of formula (I), (II), (III) or (IV) as set out herein. The process of production comprises reacting an ammine compound of metal M with a complexing compound, which is preferably a phosphine.
Owner:LUCITE INT UK LTD
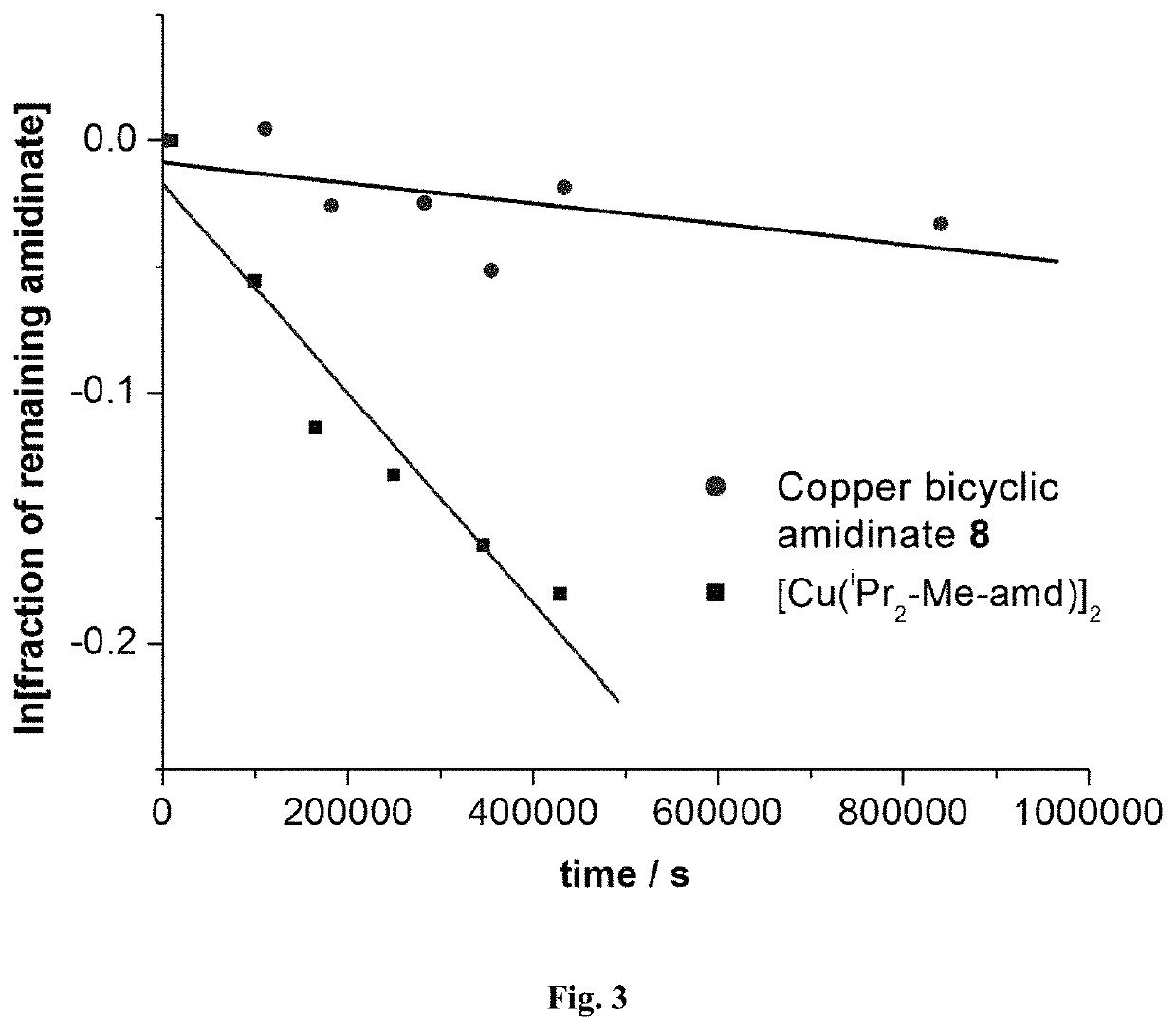
Metal bicyclic amidinates
ActiveUS11161857B2Reduce in quantityImprove thermal stabilityPlatinum group organic compoundsCopper organic compoundsGermanideHafnium
Compounds are synthesized with bicyclic amidinate ligands attached to one or more metal atoms. These compounds are useful for the synthesis of materials containing metals. Examples include pure metals, metal alloys, metal oxides, metal nitrides, metal phosphides, metal sulfides, metal selenides, metal tellurides, metal borides, metal carbides, metal silicides and metal germanides. Techniques for materials synthesis include vapor deposition (chemical vapor deposition and atomic layer deposition), liquid solution methods (sol-gel and precipitation) and solid-state pyrolysis. Copper metal films are formed on heated substrates by the reaction of copper(I) bicyclic amidinate vapor and hydrogen gas, whereas reaction with water vapor produces copper oxide. Silver and gold films were deposited on surfaces by reaction of their respective bicyclic amidinate vapors with hydrogen gas. Reaction of cobalt(II) bis(bicyclic amidinate) vapor, ammonia gas and hydrogen gas deposits cobalt metal films on heated substrates, while reaction with ammonia produces cobalt nitride and reaction with water vapor produces cobalt oxide. Ruthenium metal films are deposited by reaction of ruthenium(II) bis(bicyclic amidinate) or ruthenium(III) tris(bicyclic amidinate) at a heated surface either with or without a co-reactant such as hydrogen gas or ammonia or oxygen. Suitable applications include electrical interconnects in microelectronics and magnetoresistant layers in magnetic information storage devices. Hafnium oxide films are deposited by reaction of hafnium(IV) tetrakis(bicyclic amidinate) with oxygen sources such as water, hydrogen peroxide or ozone. The HfO2 films have high dielectric constant and low leakage current, suitable for applications as an insulator in microelectronics. The films have very uniform thickness and complete step coverage in narrow holes.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
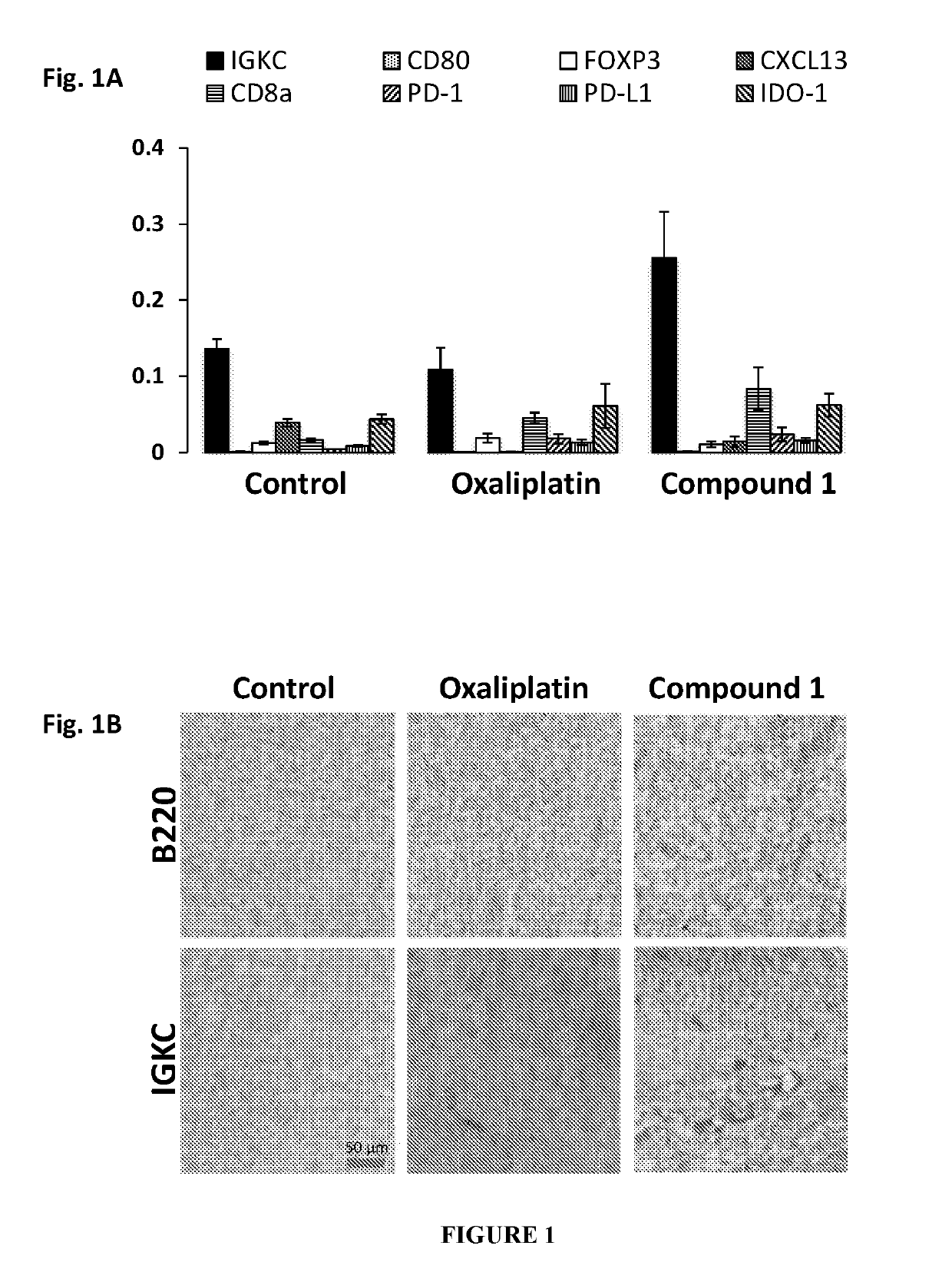
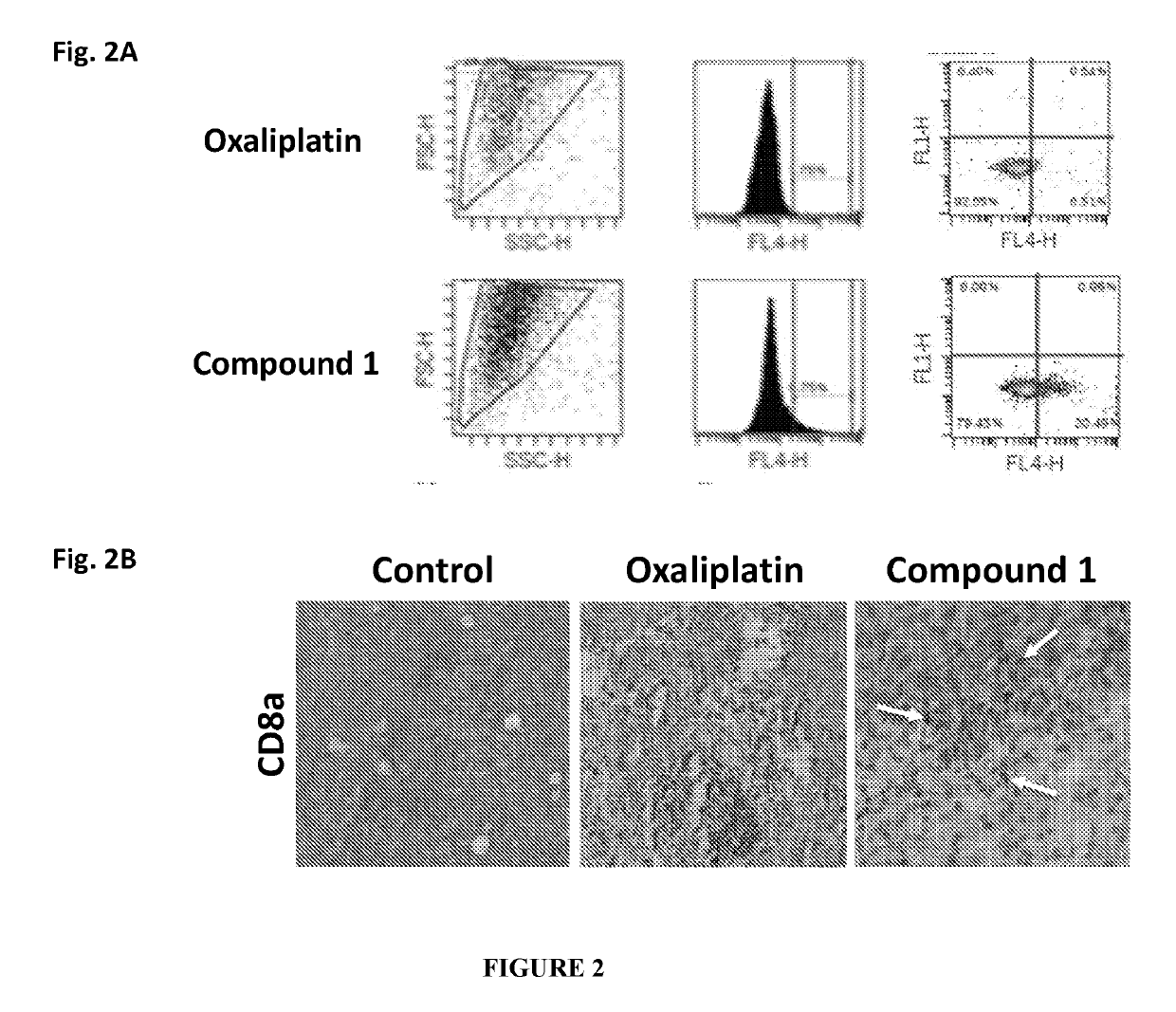
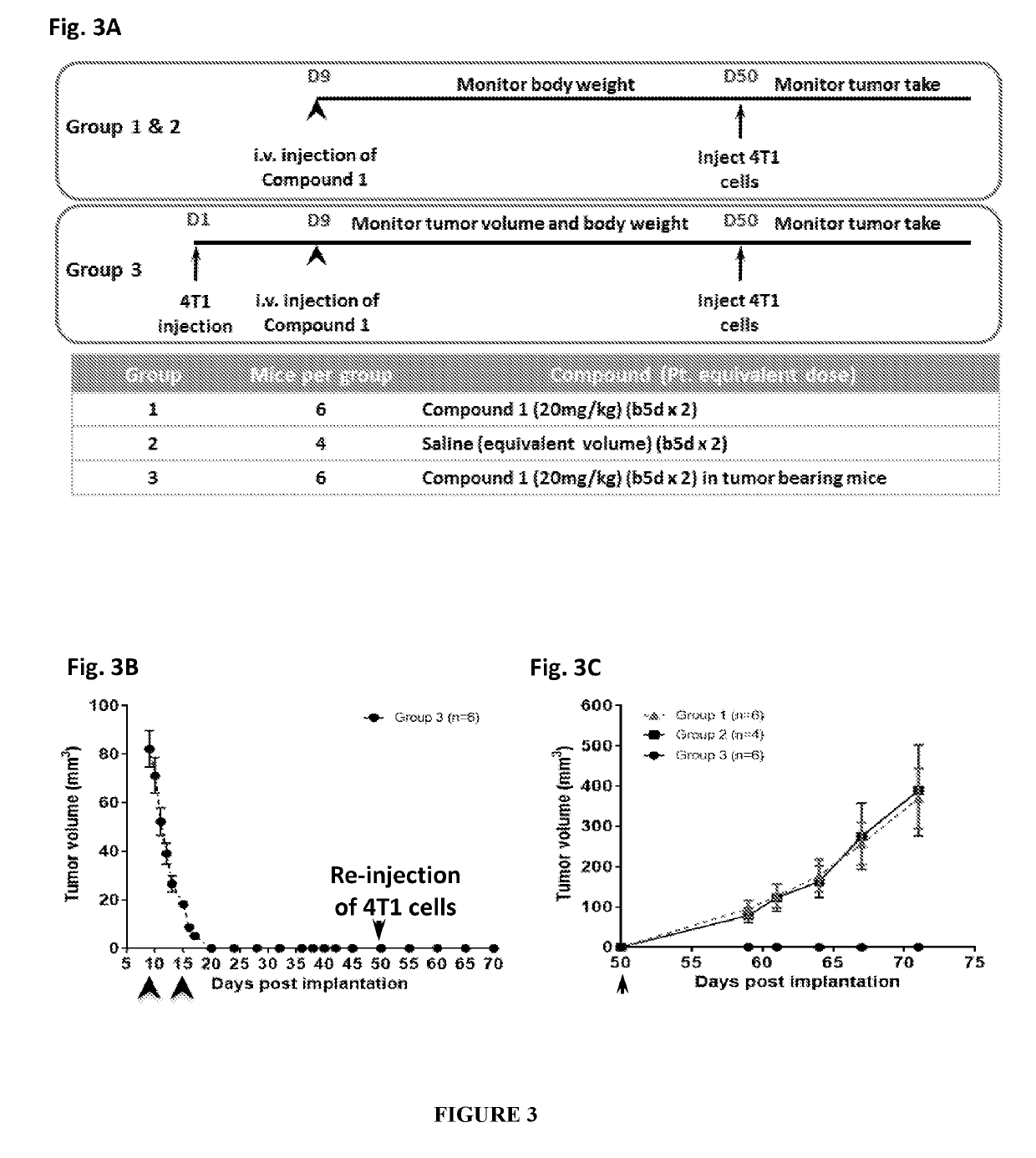
Immune memory induction by platinum based compounds
PendingUS20190209513A1High expressionPrevents metastasis and relapsePlatinum group organic compoundsSugar derivativesPlatinumLymphatic Spread
The present invention pertains to a method of treating cancer or its relapse in mammals by employing platinum based compounds. More particularly, the present invention provides to enhance immunity in a mammal, using a compound of Formula I and / or Formula II, preferably Compound 1 or its derivative, salt, tautomeric form, isomer, polymorph, solvate, or intermediates thereof. The method of inducing an immune response in a mammal is mediated through immune memory. The present invention also provides for such platinum based compounds and their use in treating cancer, metastasis or cancer relapse.
Owner:AKAMARA THERAPEUTICS INC
Novel ruthenium complexes and their uses in processes for formation and/or hydrogenation of esters, amides and derivatives thereof
InactiveUS20150284417A1Good atom economyHigh yieldPreparation from carboxylic acid saltsPlatinum group organic compoundsUrea derivativesCarbamate
The present invention relates to novel Ruthenium complexes and related borohydride complexes, and their use for (1) hydrogenation of amides (including polyamides) to alcohols and amines; (2) preparing amides from alcohols with amines (including preparing polyamides (e.g., polypeptides) by reacting dialcohols and diamines or by polymerization of amino alcohols); (3) hydrogenation of esters to alcohols (including hydrogenation of cyclic esters (lactones), cyclic di-esters (di-lactones) or polyesters); (4) hydrogenation of organic carbonates (including polycarbonates) to alcohols and of carbamates (including polycarbamates) or urea derivatives to alcohols and amines; (5) dehydrogenative coupling of alcohols to esters; (6) hydrogenation of secondary alcohols to ketones; (7) amidation of esters (synthesis of amides from esters and amines); (8) acylation of alcohols using esters; (9) coupling of alcohols with water to form carboxylic acids; and (10) dehydrogenation of beta-amino alcohols to form pyrazines. The present invention further relates to novel uses of certain pyridine Ruthenium complexes.
Owner:YEDA RES & DEV CO LTD
Water-soluble anionic bacteriochlorophyll derivatives and their uses
ActiveUS20110117029A1Antibacterial agentsOrganic active ingredientsChlorophyll derivativesPhotodynamic therapy
The invention provides anionic water-soluble tetracyclic and pentacyclic bacteriochlorophyll derivatives (Bchls) containing at least one, preferably two or three, negatively charged groups and / or acidic groups that are converted to negatively charged groups at the physiological pH, preferably Bchls having a group COO<−>, COS<−>, SO3<−>, PO3<2−>, COOH, COSH, SO3H, and / or PO3H2 bound through an ester or amide bond to one or more of the positions 17<3>, 13<3>, and 3<2> of the tetracyclic or pentacyclic Bchl molecule, for photodynamic therapy and diagnosis.
Owner:YEDA RES & DEV CO LTD
Red phosphorescent composition and organic electroluminescent device using the same
ActiveUS8986853B2High luminous efficiencyPlatinum group organic compoundsDischarge tube luminescnet screensHydrogen atomAlkoxy group
A red phosphorescent compound includes a host material being capable of transporting an electron or a hole; and a dopant material represented by following Formula 1:and each of R1 to R4 is one of the group consisting of hydrogen atom (H), C1 to C6 substituted or non-substituted alkyl group, C1 to C6 substituted or non-substituted alkoxy group, and halogen atom.
Owner:LG DISPLAY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
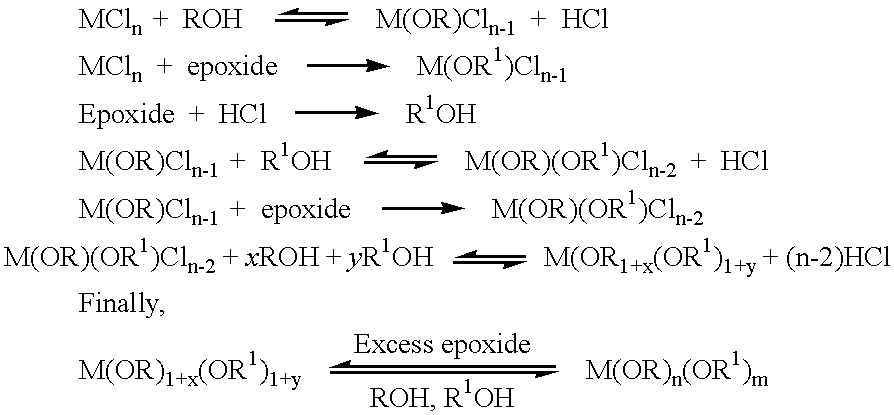
![Substituted dibenzo[FG,OP]tetracenes and formulations or electronic devices containing the same Substituted dibenzo[FG,OP]tetracenes and formulations or electronic devices containing the same](https://images-eureka.patsnap.com/patent_img/45511ced-6485-40a5-b46e-caafa19666a7/US09029425-20150512-C00001.PNG)
![Substituted dibenzo[FG,OP]tetracenes and formulations or electronic devices containing the same Substituted dibenzo[FG,OP]tetracenes and formulations or electronic devices containing the same](https://images-eureka.patsnap.com/patent_img/45511ced-6485-40a5-b46e-caafa19666a7/US09029425-20150512-C00002.PNG)
![Substituted dibenzo[FG,OP]tetracenes and formulations or electronic devices containing the same Substituted dibenzo[FG,OP]tetracenes and formulations or electronic devices containing the same](https://images-eureka.patsnap.com/patent_img/45511ced-6485-40a5-b46e-caafa19666a7/US09029425-20150512-C00003.PNG)