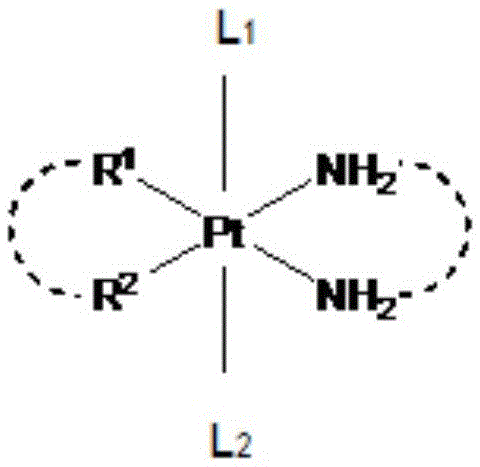
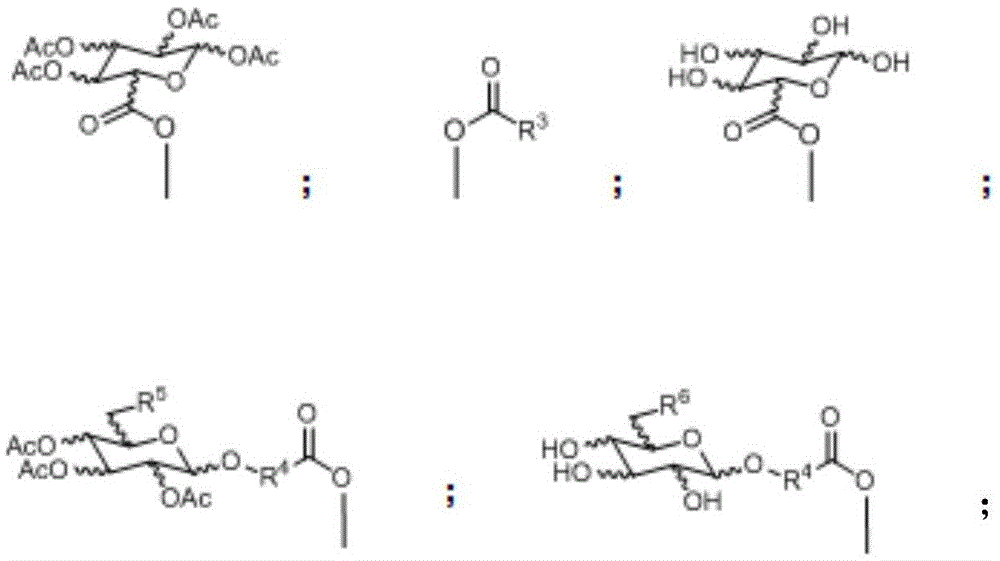
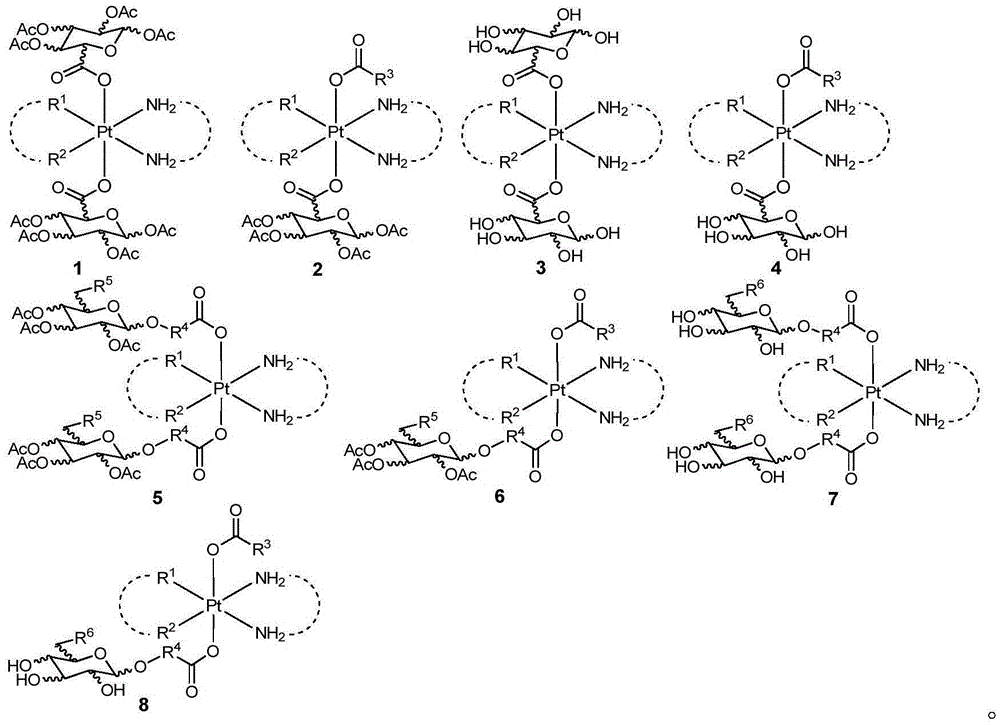
Glycosylated quadrivalent platinum compounds with anticancer activity, and preparation method and application thereof
A technology for anticancer activity, compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Example 1: Preparation of glycosyl-modified tetravalent platinum compound represented by general formula 1
[0096] 1. Synthesis of Hydroxycisplatin (IV) 10-1
[0097]
[0098] Add 1.0 g of cisplatin and 30 mL of distilled water into a 250 mL round bottom flask, stir to disperse them, slowly add 50 mL of 30% hydrogen peroxide dropwise into the reaction system, raise the temperature to 60°C and stir for 4 hours. Stop the reaction, place it at -4°C for crystallization for 12 hours, separate by filtration to obtain a yellow solid, add appropriate amount of distilled water, heat to 80°C to dissolve it, place it at 4°C for crystallization for 12 hours, and filter to obtain yellow crystals of compound 10-1 (0.82g ,74%).
[0099] 2. Synthesis of hydroxyoxaliplatin (IV) 10-2
[0100]
[0101] Add 1.0 g of oxaliplatin and 30 mL of distilled water to a 250 mL round bottom flask, stir to disperse them, slowly add 50 mL of 30% hydrogen peroxide dropwise to the reaction system...
Embodiment 2
[0112] Example 2: Preparation of asymmetric naked sugar modified tetravalent platinum represented by general formula 4
[0113] 1. Synthesis of tetravalent platinum compound 12-1
[0114]
[0115] Add compound 10-1 (0.6g, 1.8mmol) and palmitic anhydride (0.94g, 1.9mmol) into a 250mL round bottom flask, add 30mL of anhydrous DMSO under nitrogen protection, stir and react at 30°C for 7 days, reduce DMSO was removed by pressure evaporation, acetone was added, and a large amount of white solid powder was precipitated, which was filtered by suction and washed 3 times with acetone to obtain compound 12-1 (0.99 g, 96%).
[0116] 2. Synthesis of tetravalent platinum compound 12-2
[0117]
[0118] Add compound 10-2 (0.8g, 1.8mmol) and palmitic anhydride (0.94g, 1.9mmol) to a 100mL round bottom flask, add 40mL of anhydrous DMSO under nitrogen protection, stir and react at 30°C for 7 days, reduce DMSO was removed by pressure evaporation, and acetone was added to precipitate a wh...
Embodiment 3
[0129] Example 3: Preparation of bridged peracetylglycosylated tetravalent platinum compounds with general formula 5
[0130]
[0131] Add tetravalent platinum 10 and 1-glycosidic bond-modified acid chloride compound 16 into a 50 mL round bottom flask, replace the air in the system with nitrogen, add anhydrous acetone, stir at room temperature for 12-36 hours, and stop the reaction. Acetone was removed by concentration, and the target product 5 was obtained by column chromatography.
[0132]
[0133] 5-1: yellow solid; 1 HNMR (400MHz, CDCl 3 )δ6.05(br,6H),5.21(s,2H),5.05(d,J=11.9Hz,4H),4.63(s,2H),4.28(d,J=49.9Hz,8H),3.76( s,2H),2.35–1.74(m,24H). 13 CNMR (101MHz, CDCl 3 )δ178.22, 171.32, 170.83, 170.10, 169.51, 100.21, 72.36, 72.15, 71.07, 68.20, 65.84, 61.63, 21.12, 21.02, 20.61. HRMS: Calcd.forC 32 h 48 Cl 2 N 2 o 24 Pt(M+NH 4 ) + :1128.1952,found:1128.1893.
[0134] 5-2: yellow solid; 1 HNMR (400MHz, CDCl 3 )δ6.33–5.83(br,6H),5.41(s,2H),5.32–5.12(m,2H),5....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com