Application of physalis pubescens lactone B in preparing anti-cancer drugs
An anti-cancer drug, physalis technology, applied in the direction of drug combination, anti-tumor drugs, pharmaceutical formulations, etc., can solve the problem of synergistic effect that has not been reported in detail, and achieve the goal of being conducive to market promotion, improving anti-cancer activity, The effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
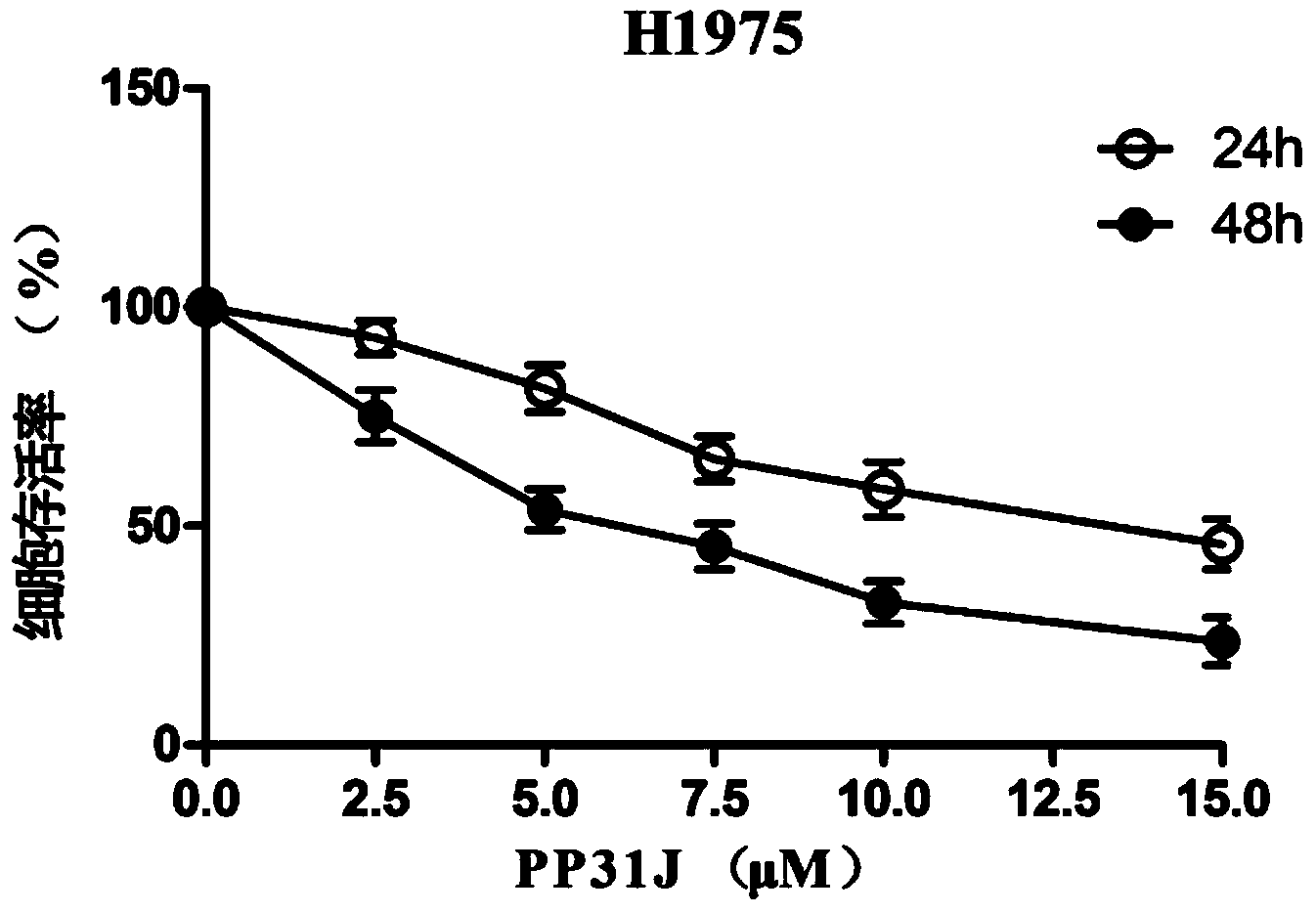
[0038] Example 1: physapubescin B has an inhibitory effect on the proliferation of human non-small cell lung cancer cell NCI-H1975.
[0039] The specific implementation method is as follows:
[0040] Human non-small cell lung cancer cells NCI-H1975 were cultured in RPMI1640 medium containing 10% (wt) fetal bovine serum (FBS) (37°C, 5% CO 2 ), digested and counted by conventional methods, and the cells in the logarithmic growth phase were divided into 4×10 4Inoculate 200 μl at a concentration of / ml in a 96-well culture plate, set a blank control group, a drug group of physapubescin B (2.5 μM, 5 μM, 7.5 μM, 10 μM, 15 μM) and a solvent dimethyl sulfoxide (DMSO) blank control group, Each group made 3 replicate holes. When the cell growth density reaches 60%-70%, add different concentrations of physapubescin B respectively, after continuing to cultivate for 24h and 48h, add 5mg / ml tetramethylazozolium salt (MTT) 20μl solution, after continuing to cultivate for 4 hours, Terminat...
Embodiment 2
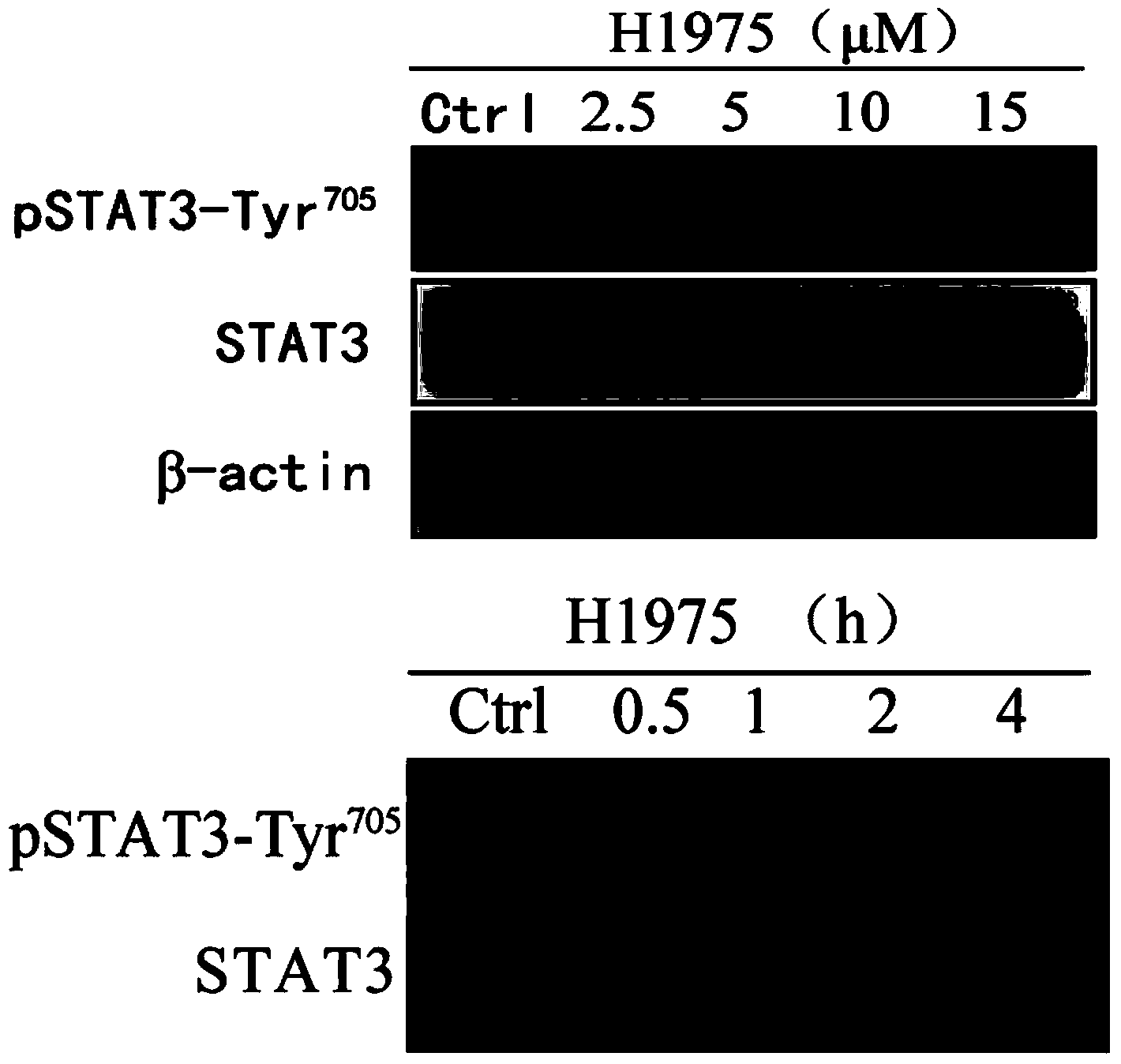
[0042] Embodiment 2: physapubescin B inhibits STAT3 kinase 705 tyrosine (Tyr 705 ) phosphorylation level.
[0043] The specific implementation method is as follows:
[0044] NCI-H1975 was cultured with RPMI1640 medium containing 10% (wt) FBS (GIBCO). When the cells grew to 70%-80%, different concentrations (0, 2.5 μM, 5 μM, 10 μM, 15 μM) of the drug physapubescin B were added. After 24 hours, the cells were collected into a 15ml Eppendorf tube, centrifuged at 800rpm for 5min. After the supernatant was discarded, the cells were transferred to a 1.5ml Eppendorf tube, centrifuged at 8000rpm for 5min. Discard the supernatant, add RIPA lysate containing 1% (wt) phosphorylase inhibitor, pipette evenly, lyse the cells on ice for 30 min, and shake once every 10 min. After the cells are fully lysed, centrifuge at 13,000rpm for 10min, take the supernatant, measure the protein concentration with a BCA protein quantification kit, add an equal volume of 2×SDS loading buffer, denature in...
Embodiment 3
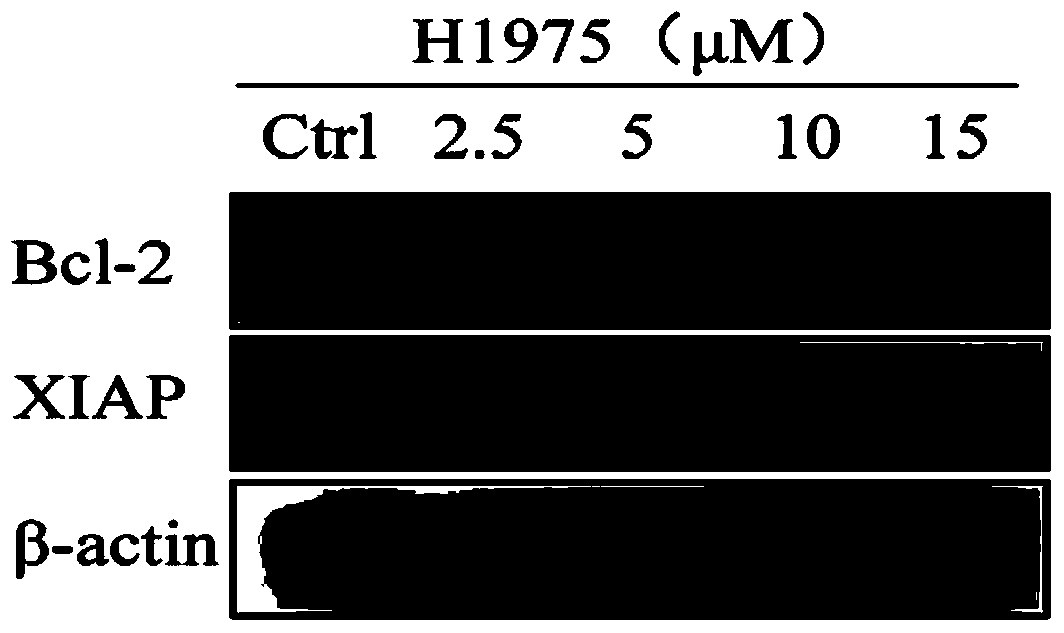
[0046] Example 3: Physapubescin B down-regulates the protein expression levels of the downstream genes Bcl-2 and XIAP in human non-small cell lung cancer cells NCI-H1975STAT3.
[0047] The specific implementation method is as follows:
[0048] NCI-H1975 was cultured with RPMI1640 medium containing 10% (wt) FBS (GIBCO). When the cells grew to 70%-80%, drugs of different concentrations (0, 2.5 μM, 5 μM, 10 μM, 15 μM) were added. After 24 hours, the cells were collected into 15 ml Eppendorf tubes, centrifuged at 800 rpm for 5 min. After the supernatant was discarded, the cells were transferred to a 1.5ml Eppendorf tube, centrifuged at 8000rpm for 5min. Discard the supernatant, add RIPA lysate containing 1% (wt) phosphorylase inhibitor, pipette evenly, lyse the cells on ice for 30 min, and shake once every 10 min. After the cells are fully lysed, centrifuge at 13,000rpm for 10min, take the supernatant, measure the protein concentration with a BCA protein quantification kit, add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com