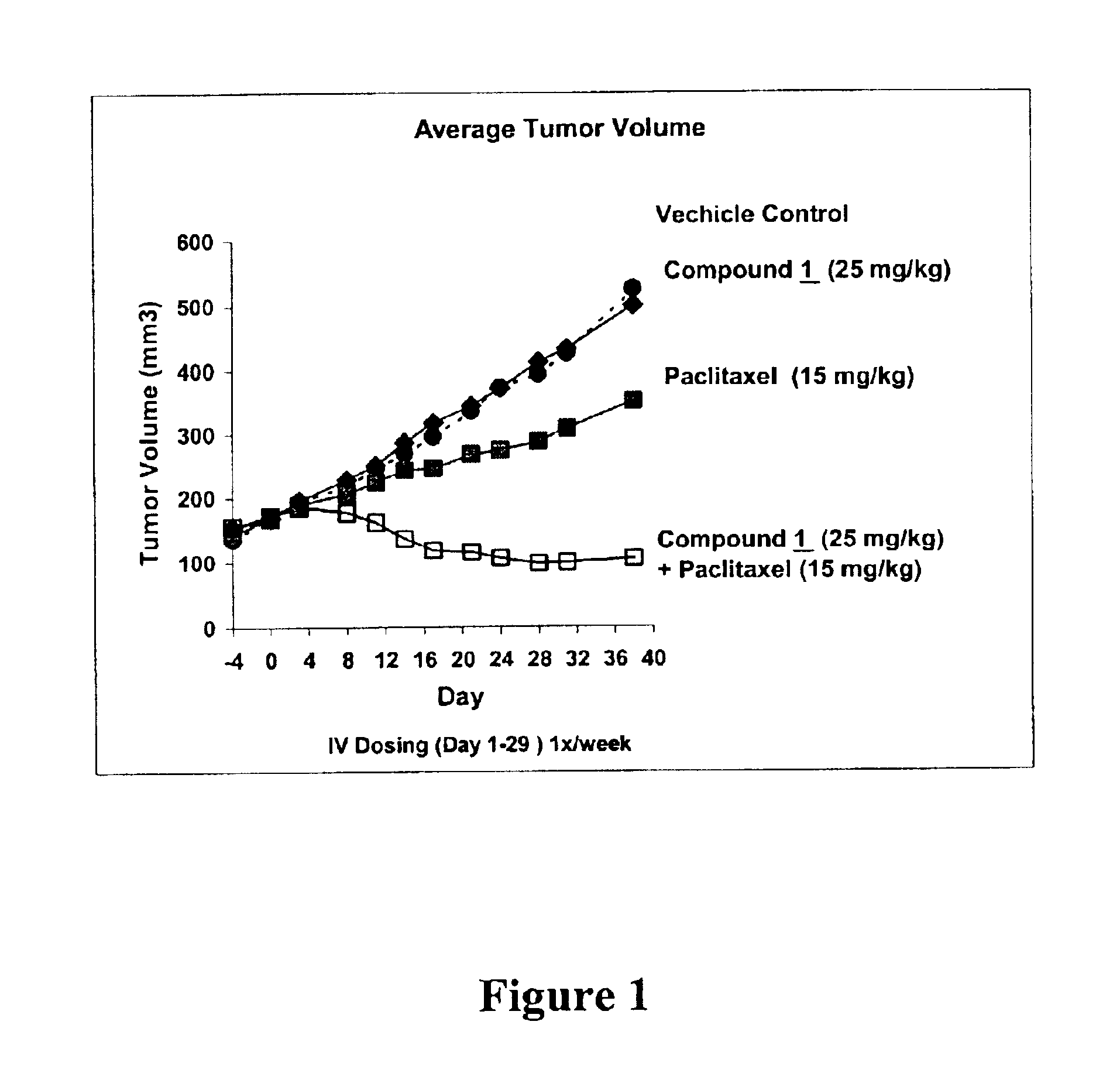
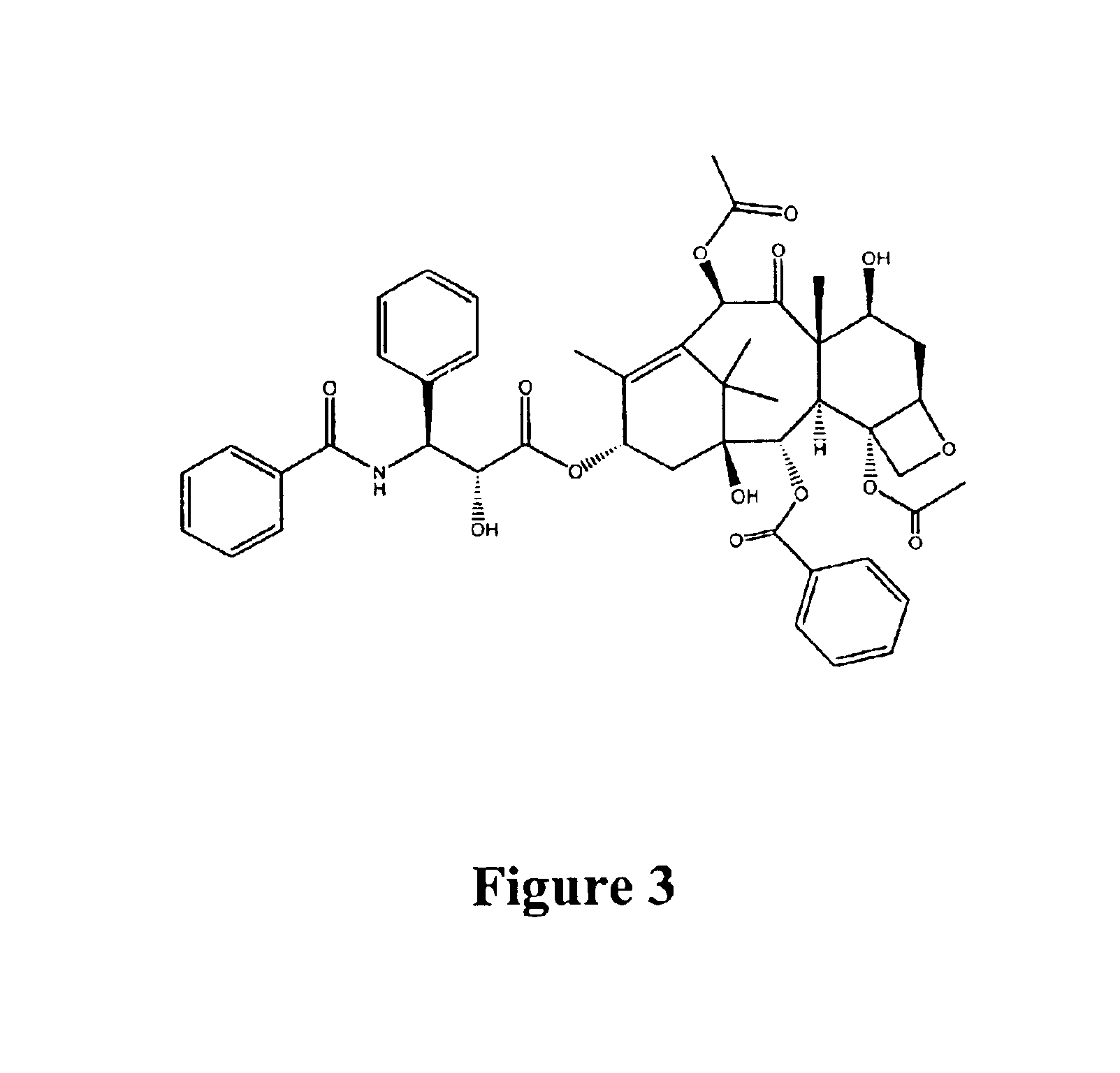
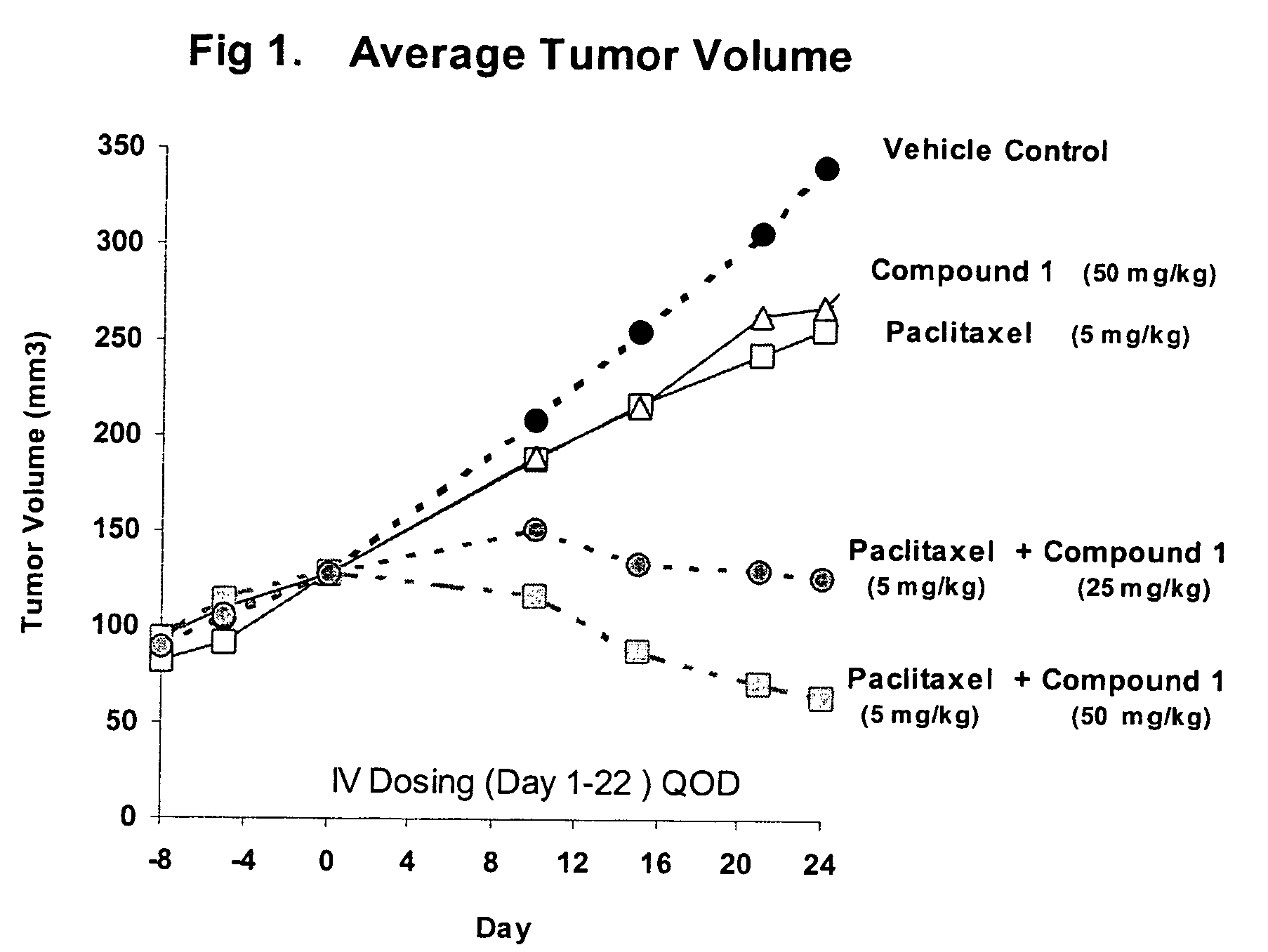
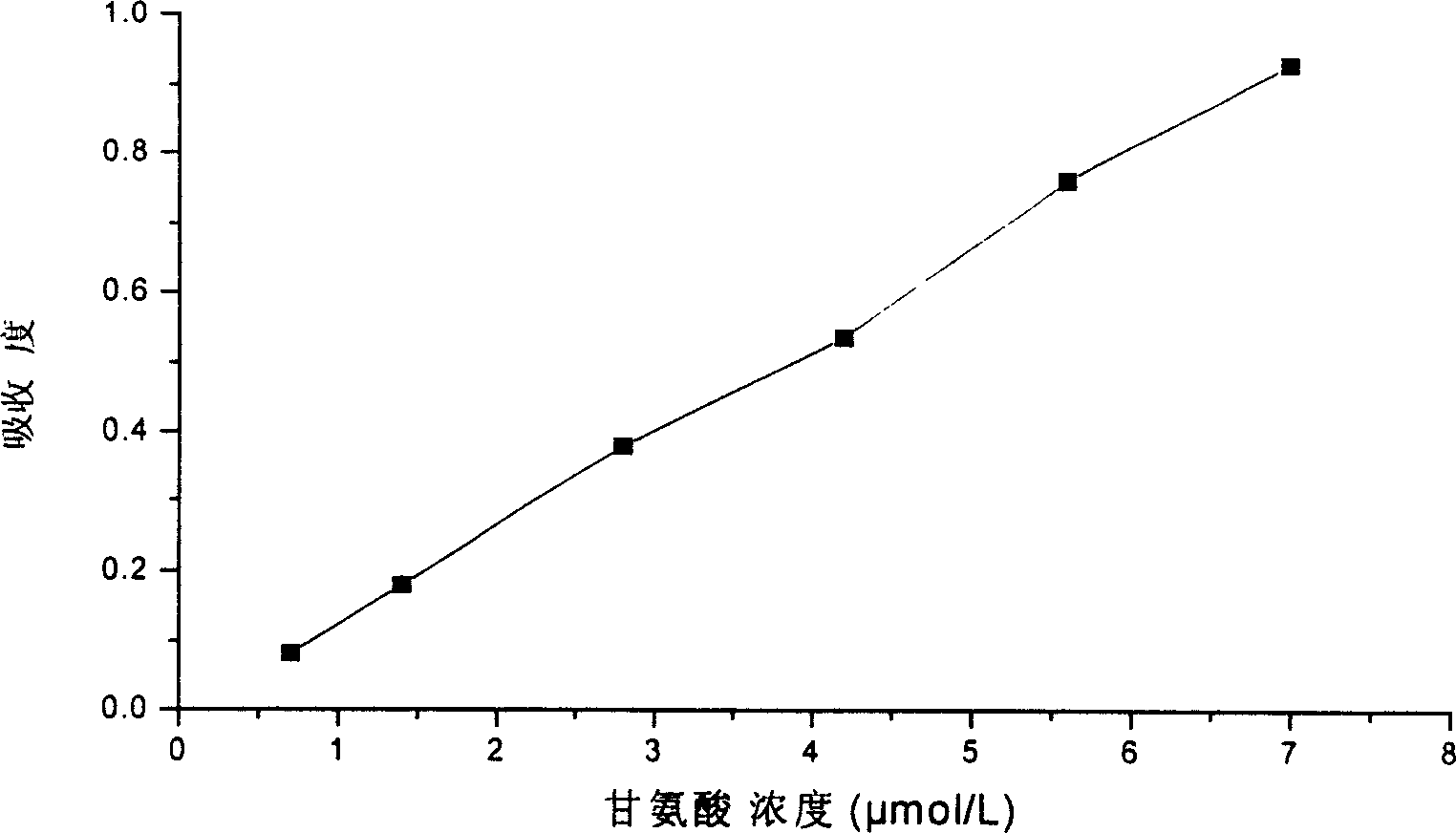
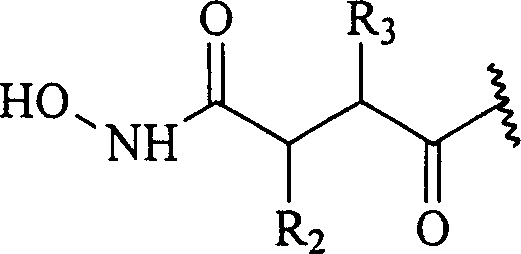
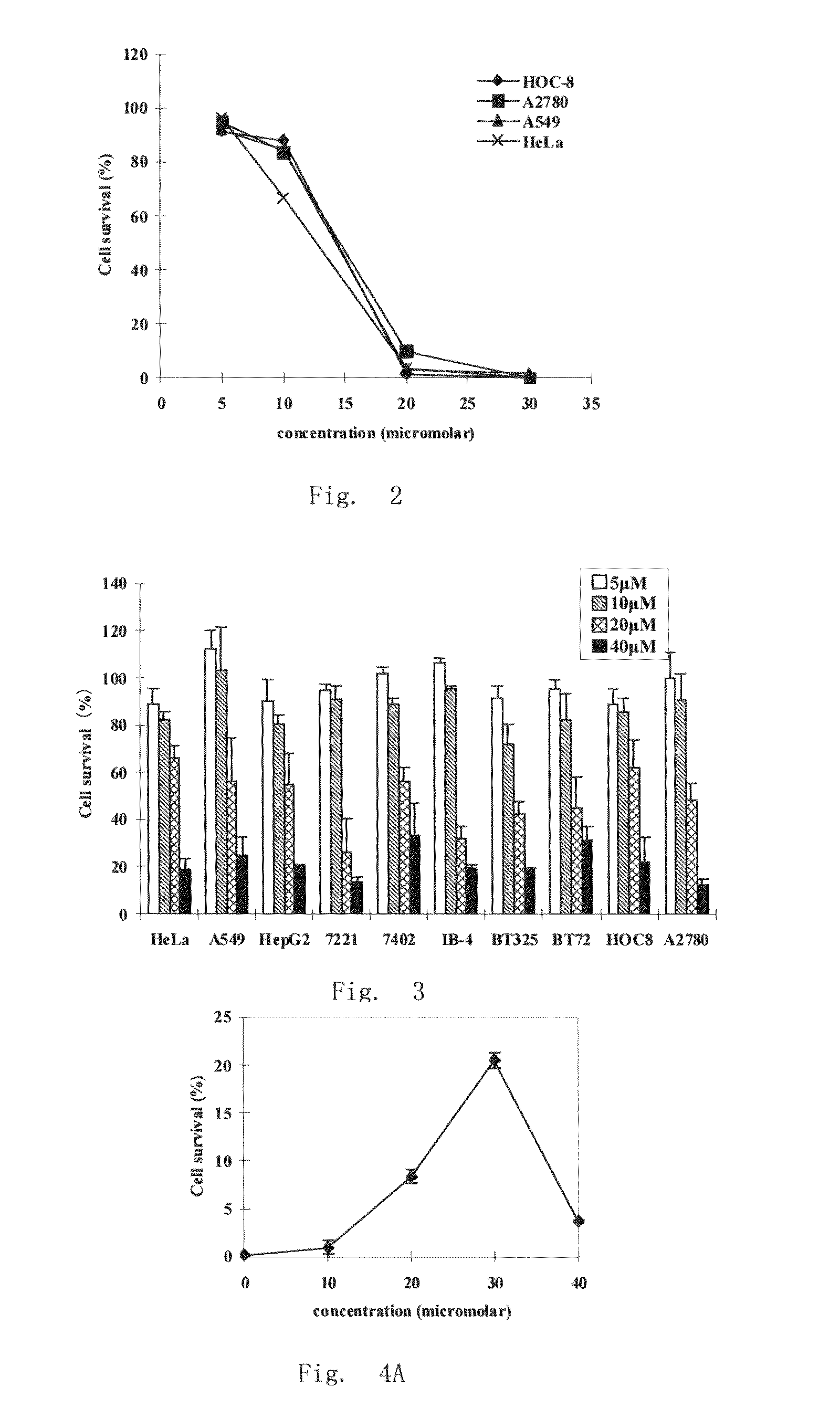
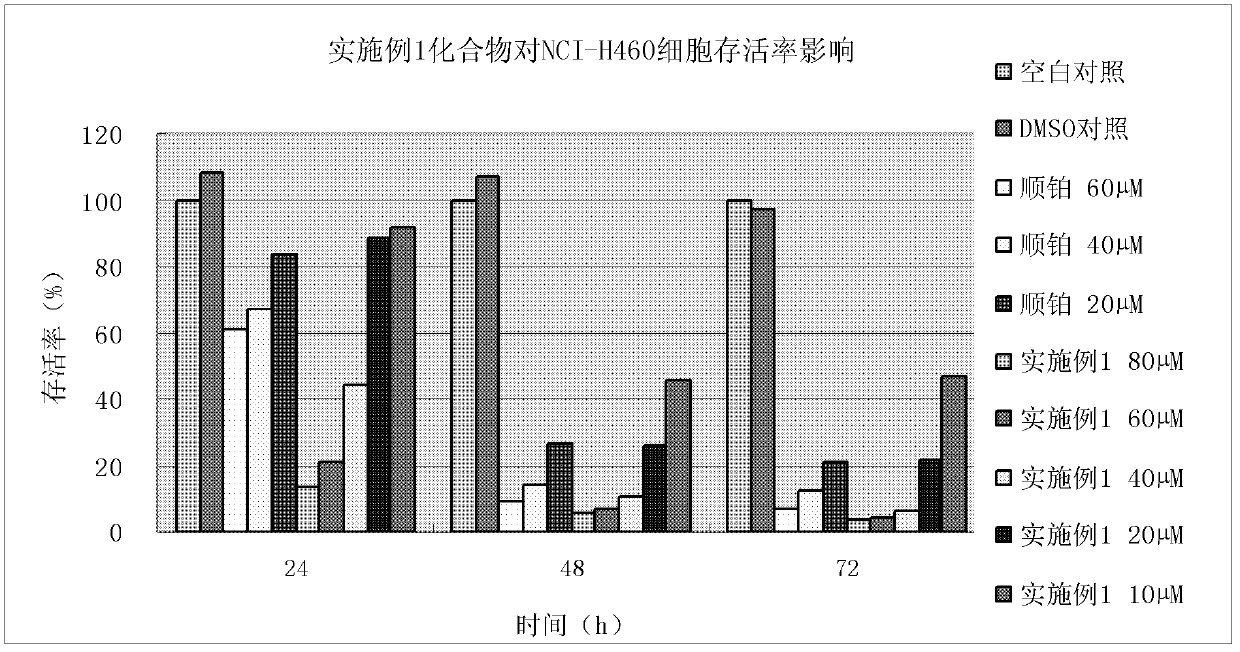
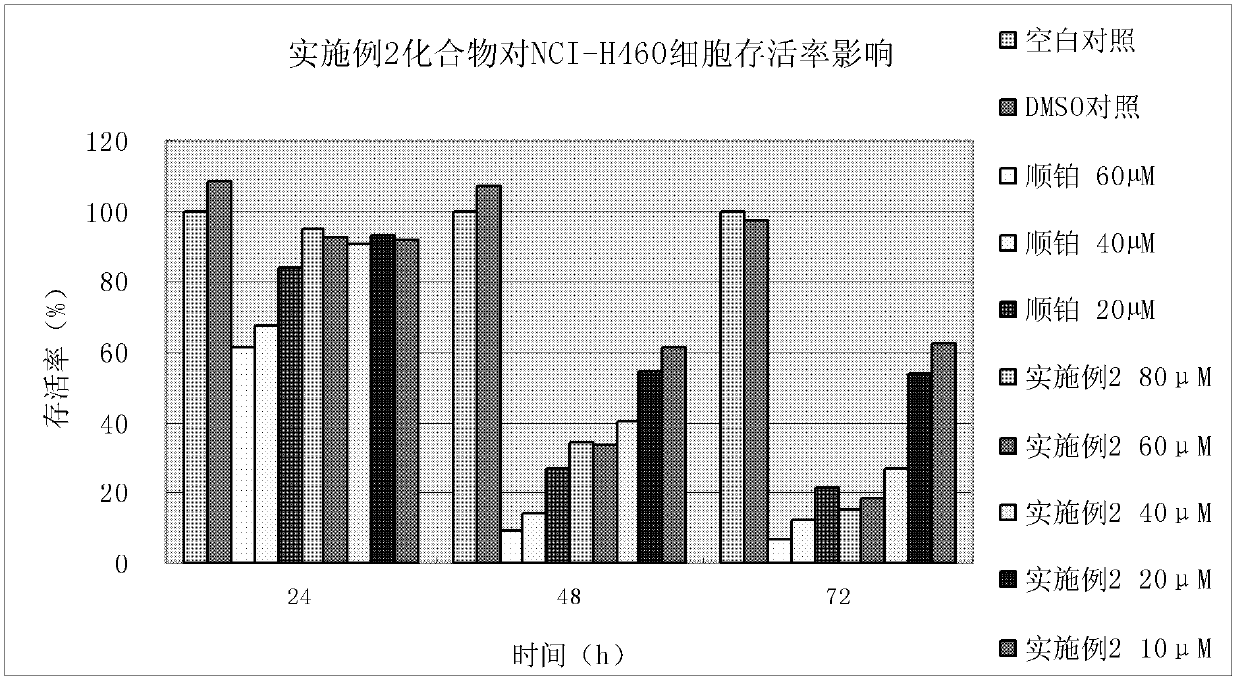
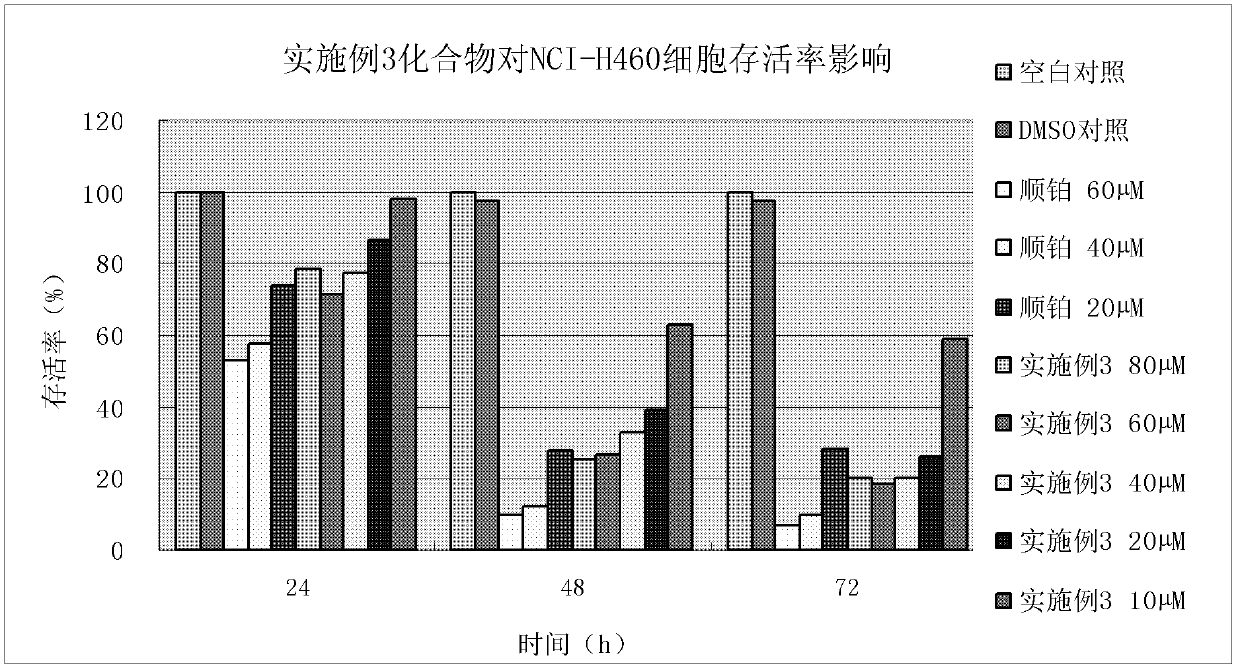
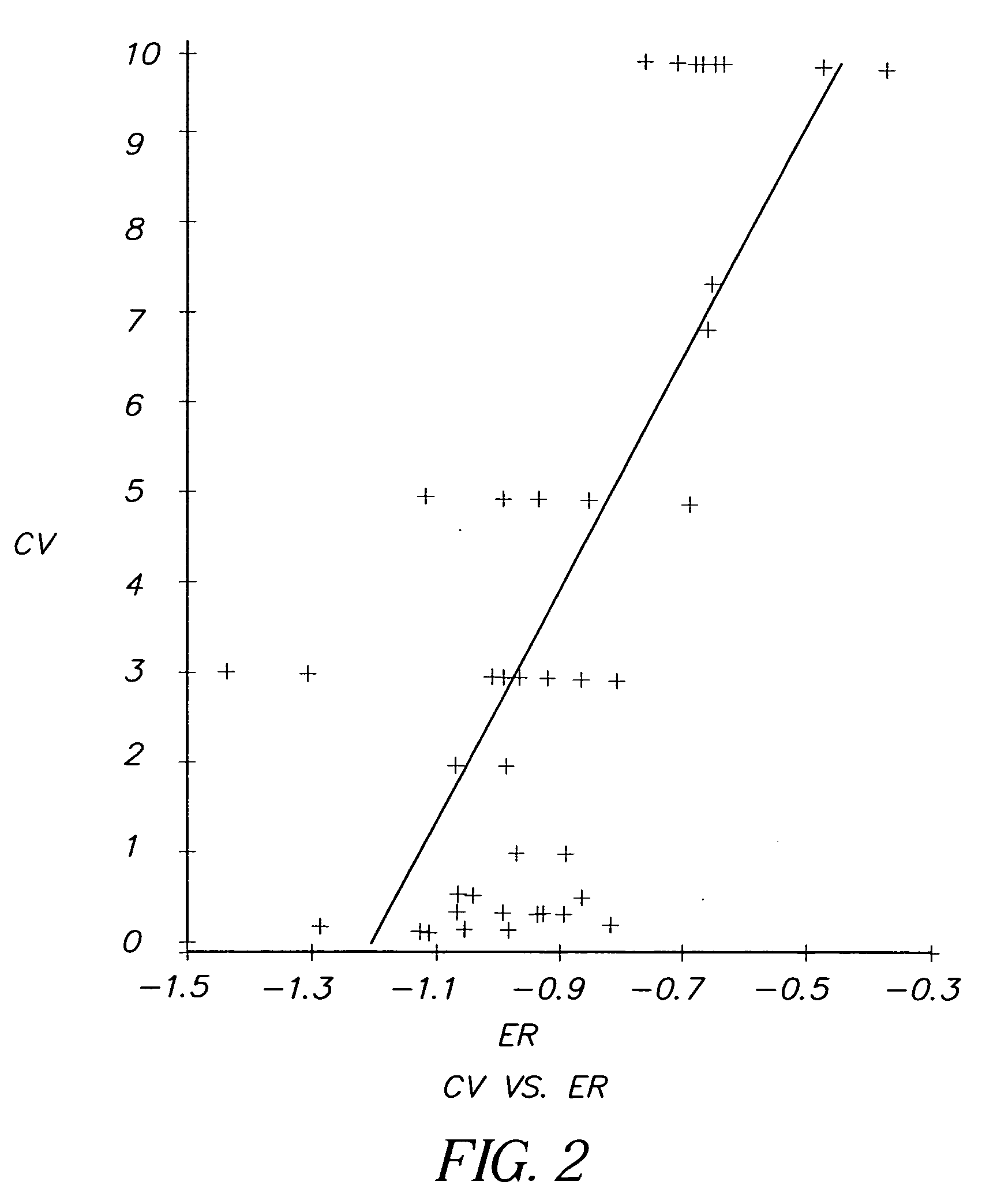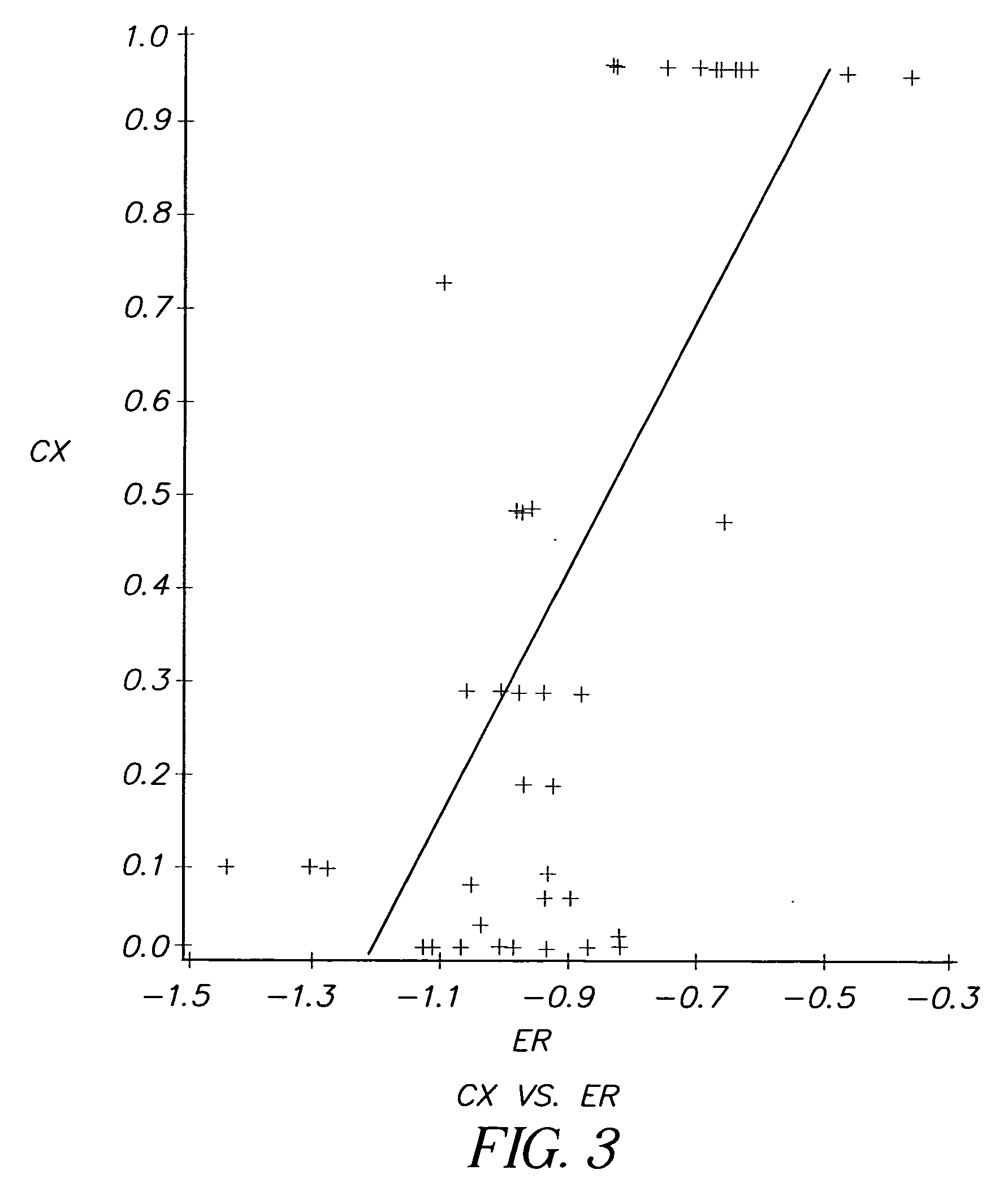Patents
Literature
900results about How to "High anticancer activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
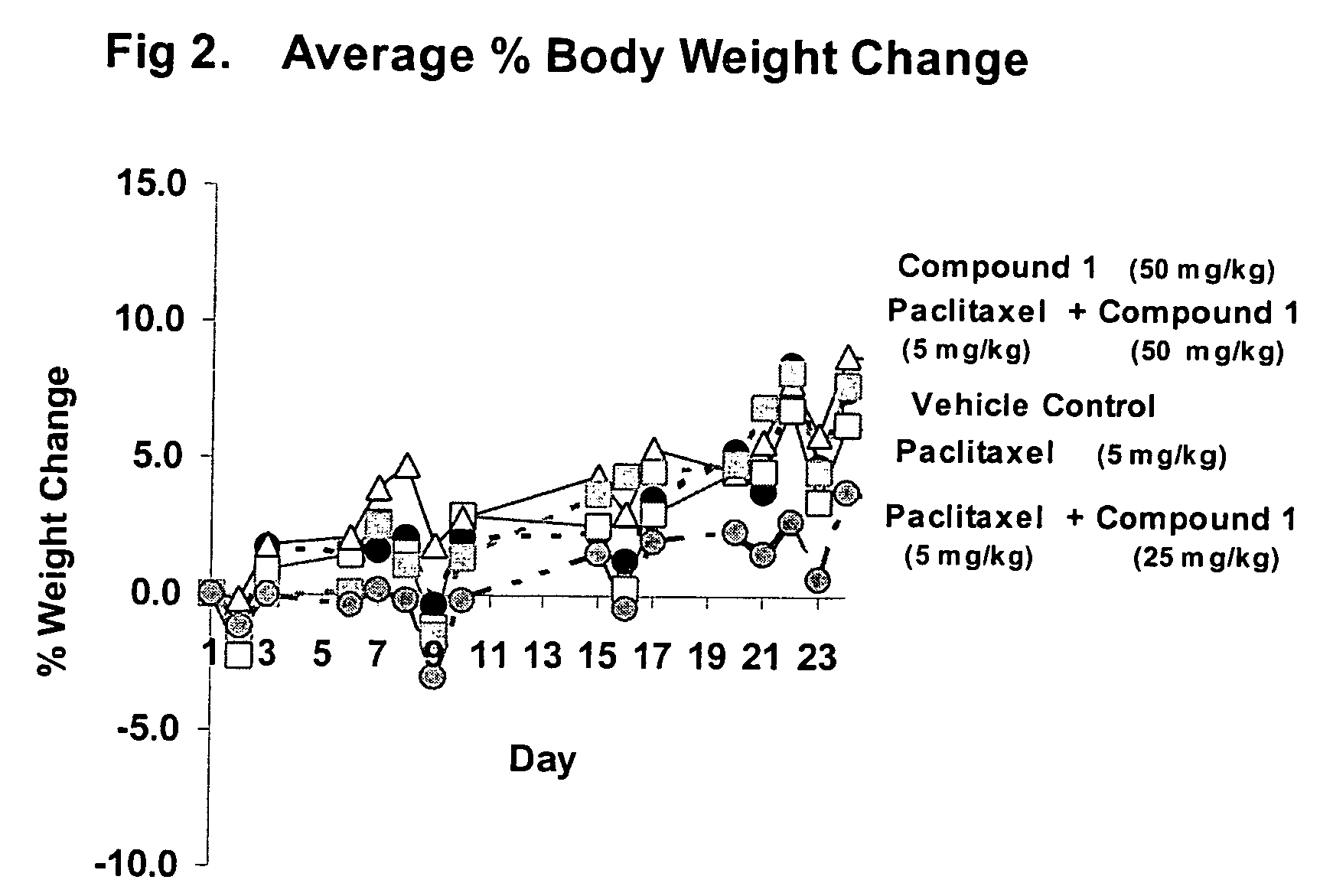
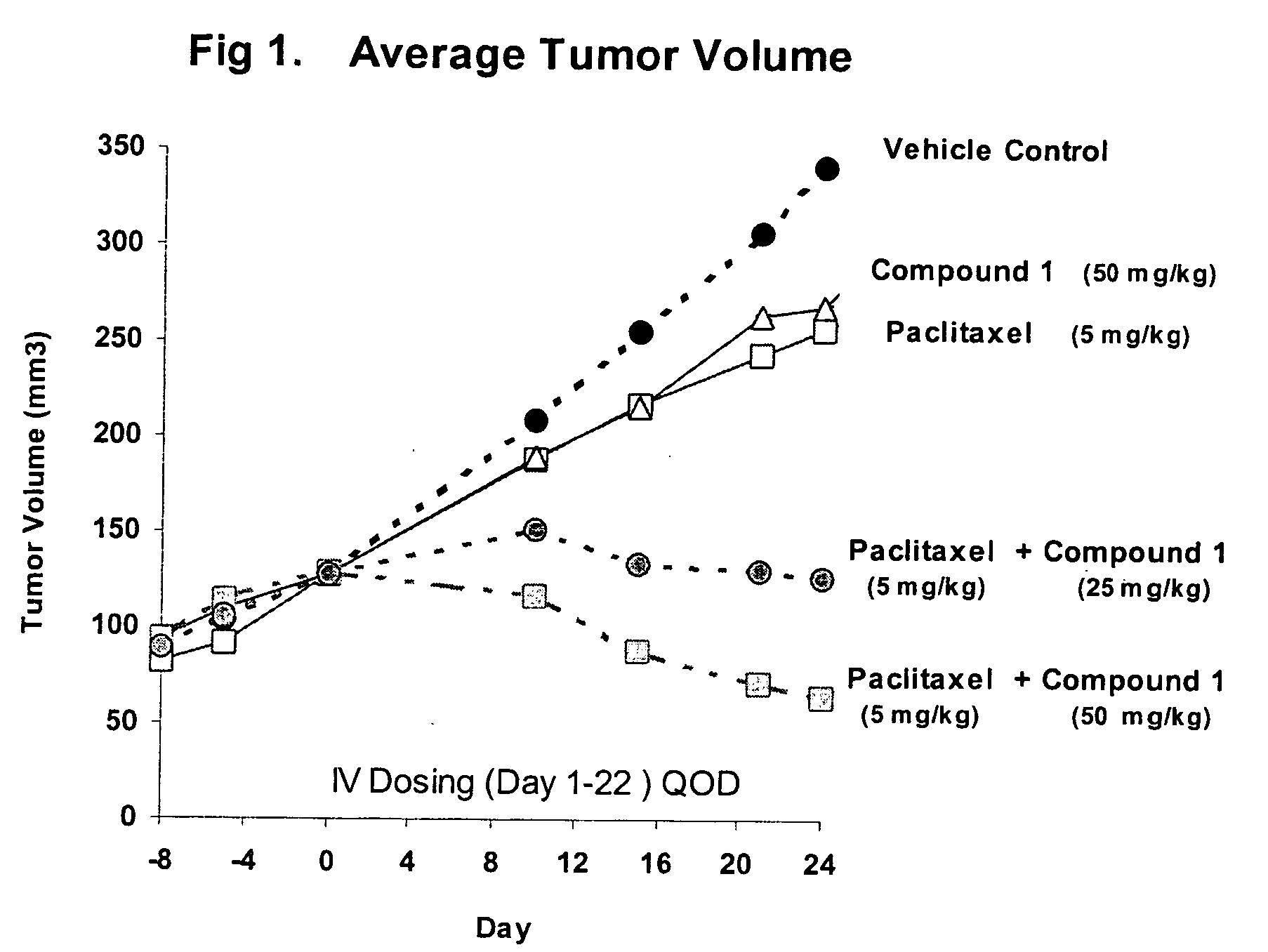
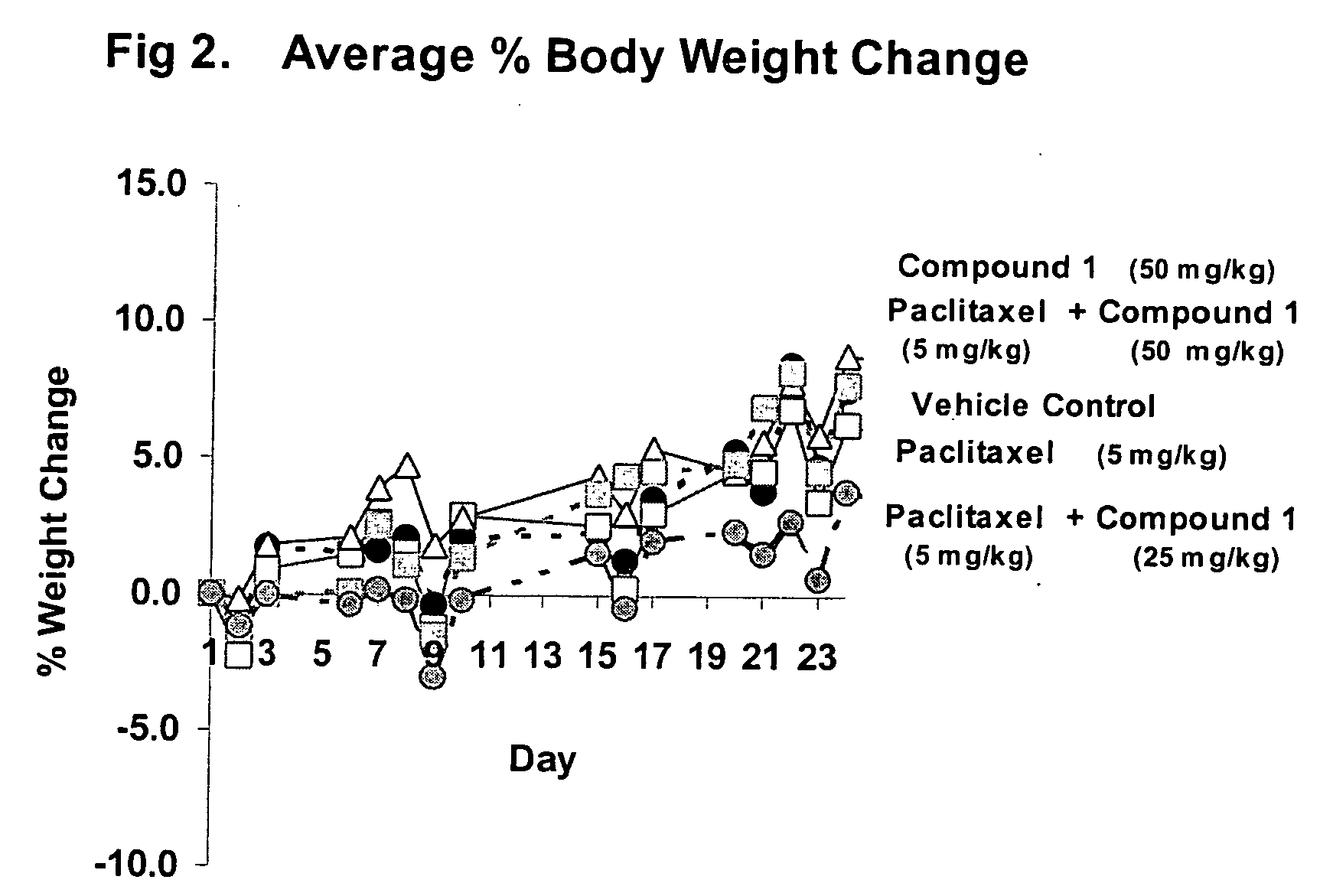
Taxol enhancer compounds
InactiveUS6924312B2High anticancer activityImprove efficiencyBiocideOrganic chemistryArylStructural formula
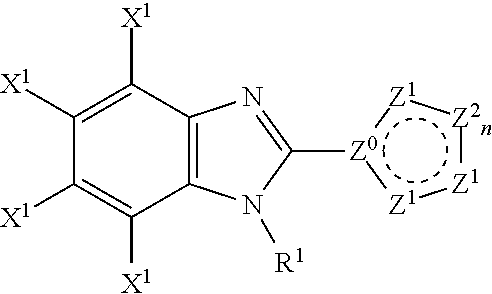
Disclosed is a compound represented by the Structural Formula (I): Y is a covalent bond, a phenylene group or a substituted or unsubstituted straight chained hydrocarbyl group. In addition, Y, taken together with both >C═Z groups to which it is bonded, is a substituted or unsubstituted aromatic group. Preferably, Y is a covalent bond or —C(R7R8)—.R1 and R2 are independently an aryl group or a substituted aryl group, R3 and R4 are independently —H, an aliphatic group, a substituted aliphatic group, an aryl group or a substituted aryl group.R5-R6 are independently —H, an aliphatic group, a substituted aliphatic group, an aryl group or a substituted aryl group.R7 and R8 are each independently —H, an aliphatic or substituted aliphatic group, or R7 is —H and R8 is a substituted or unsubstituted aryl group, or, R7 and R8, taken together, are a C2-C6 substituted or unsubstituted alkylene group.Z is ═O or ═S.Also disclosed are pharmaceutical compositions comprising the compound of the present invention and a pharmaceutically acceptable carrier or diluent. Also disclosed is a method of treating a subject with cancer by administering to the subject a compound of Structural Formula (I) in combination with taxol or an analog of taxol.
Owner:SYNTA PHARMA CORP
Taxol enhancer compounds
Disclosed is a compound represented by the Structural Formula (I): Y is a covalent bond, a phenylene group or a substituted or unsubstituted straight chained hydrocarbyl group. In addition, Y, taken together with both >C═Z groups to which it is bonded, is a substituted or unsubstituted aromatic group. Preferably, Y is a covalent bond or —C(R7R8)—.R1 and R2 are independently an aryl group or a substituted aryl group, R3 and R4 are independently —H, an aliphatic group, a substituted aliphatic group, an aryl group or a substituted aryl group.R5–R6 are independently —H, an aliphatic group, a substituted aliphatic group, an aryl group or a substituted aryl group.R7 and R8 are each independently —H, an aliphatic or substituted aliphatic group, or R7 is —H and R8 is a substituted or unsubstituted aryl group, or, R7 and R8, taken together, are a C2–C6 substituted or unsubstituted alkylene group.Z is ═0 or ═S.Also disclosed are pharmaceutical compositions comprising the compound of the present invention and a pharmaceutically acceptable carrier or diluent.Also disclosed is a method of treating a subject with cancer by administering to the subject a compound of Structural Formula (I) in combination with taxol or an analog of taxol.
Owner:SYNTA PHARMA CORP
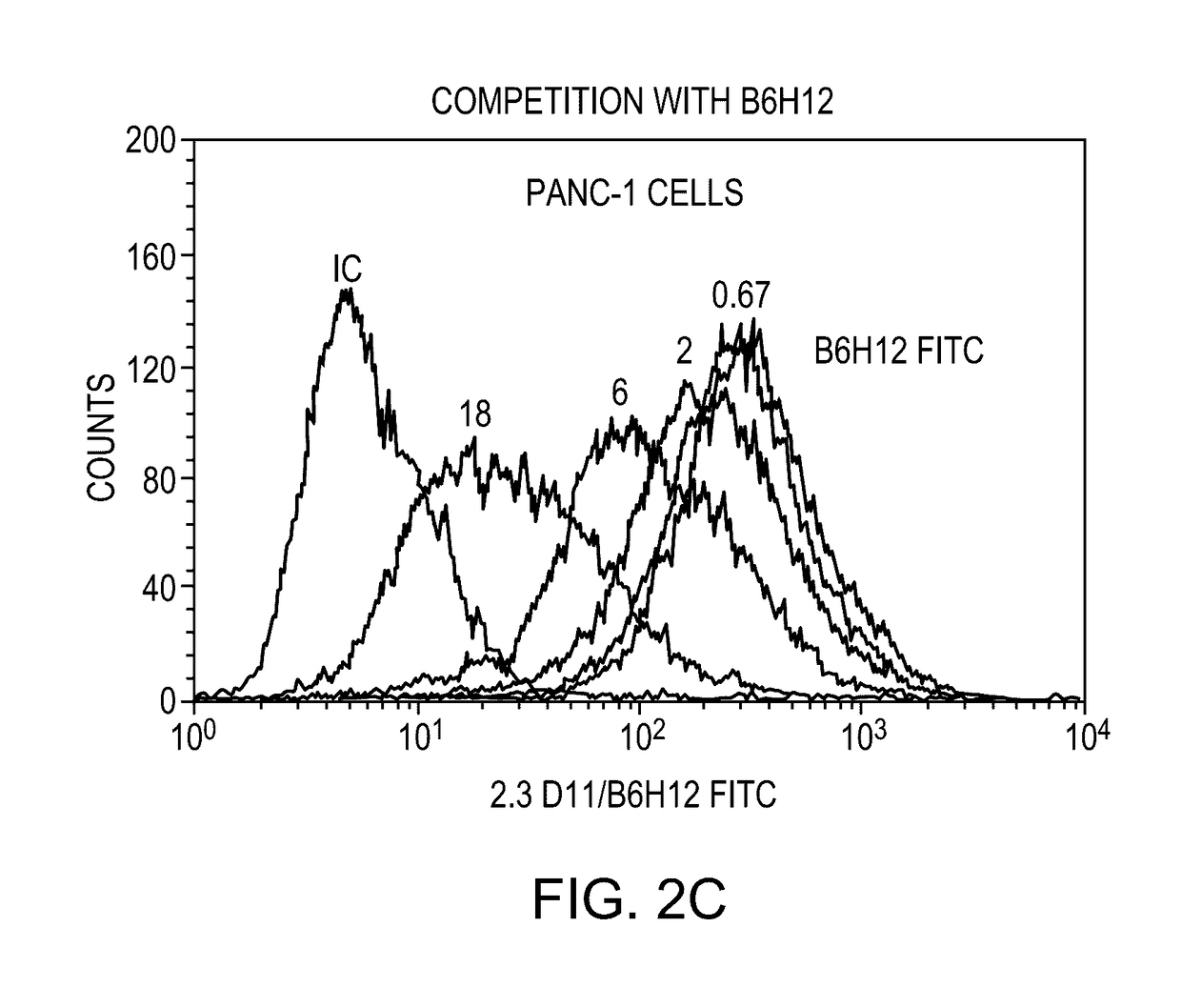
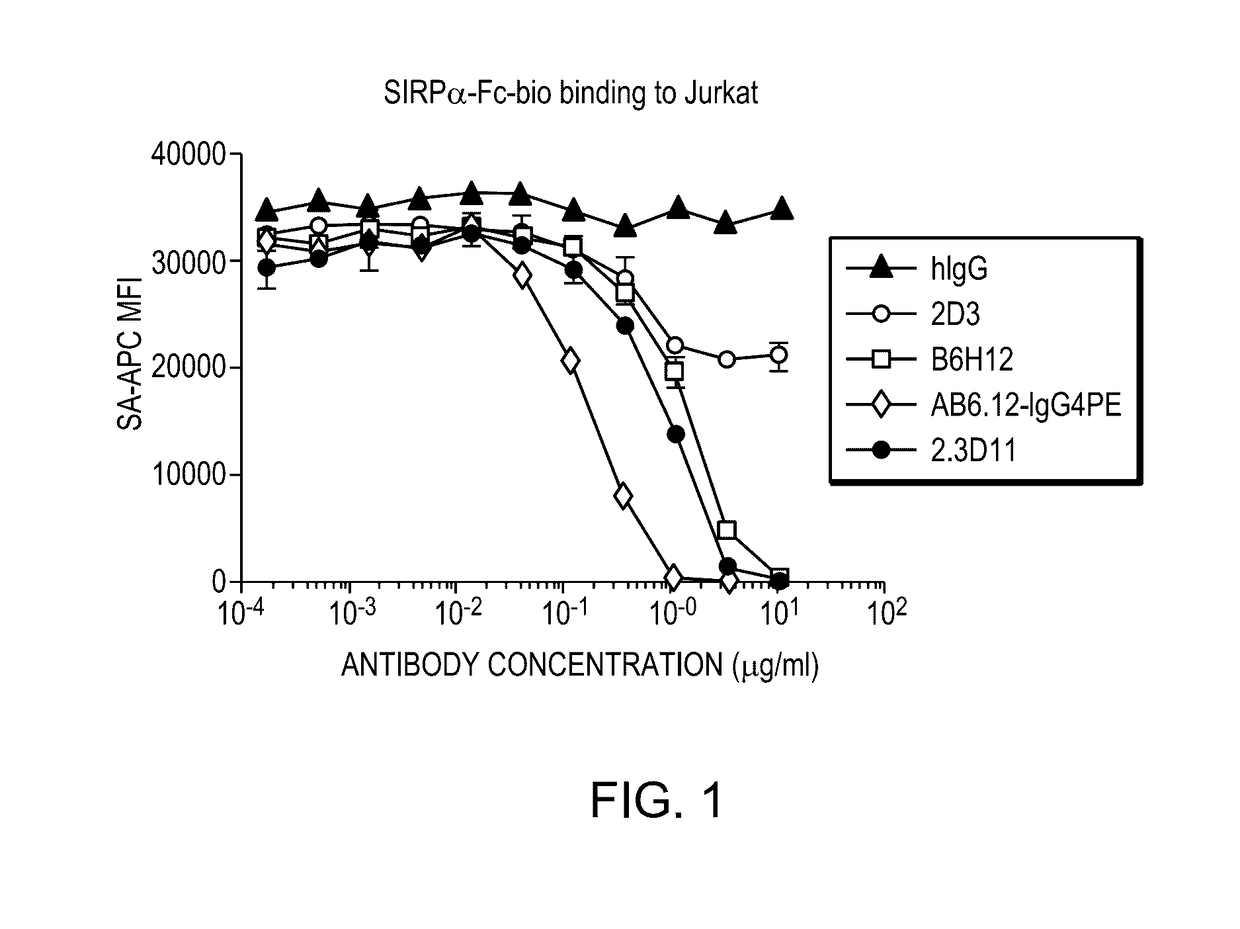
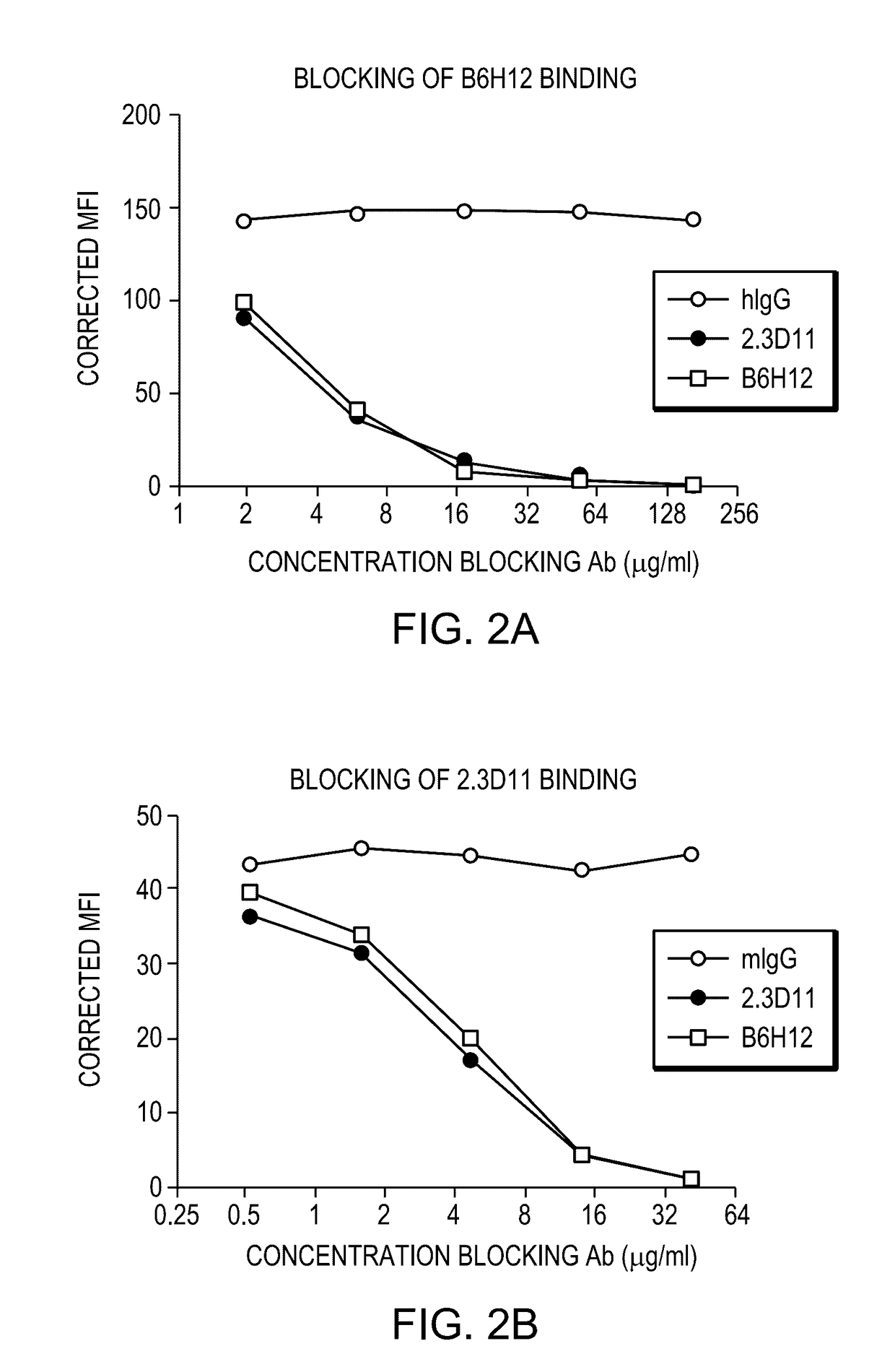
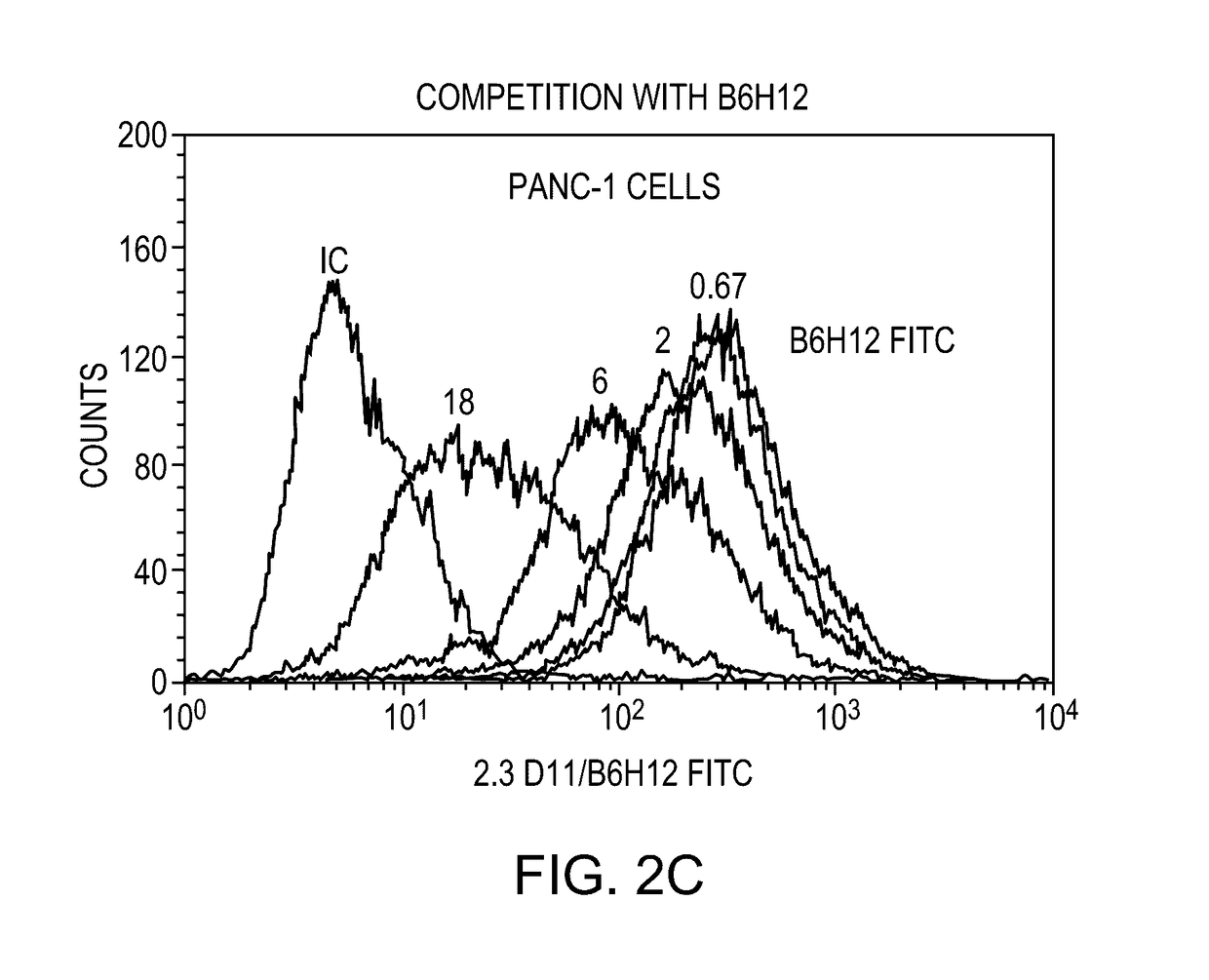
Anti-cd47 antibodies and methods of use
ActiveUS20170081407A1Little and no hemagglutinationProgression moreImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsSolid tumorCD47
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
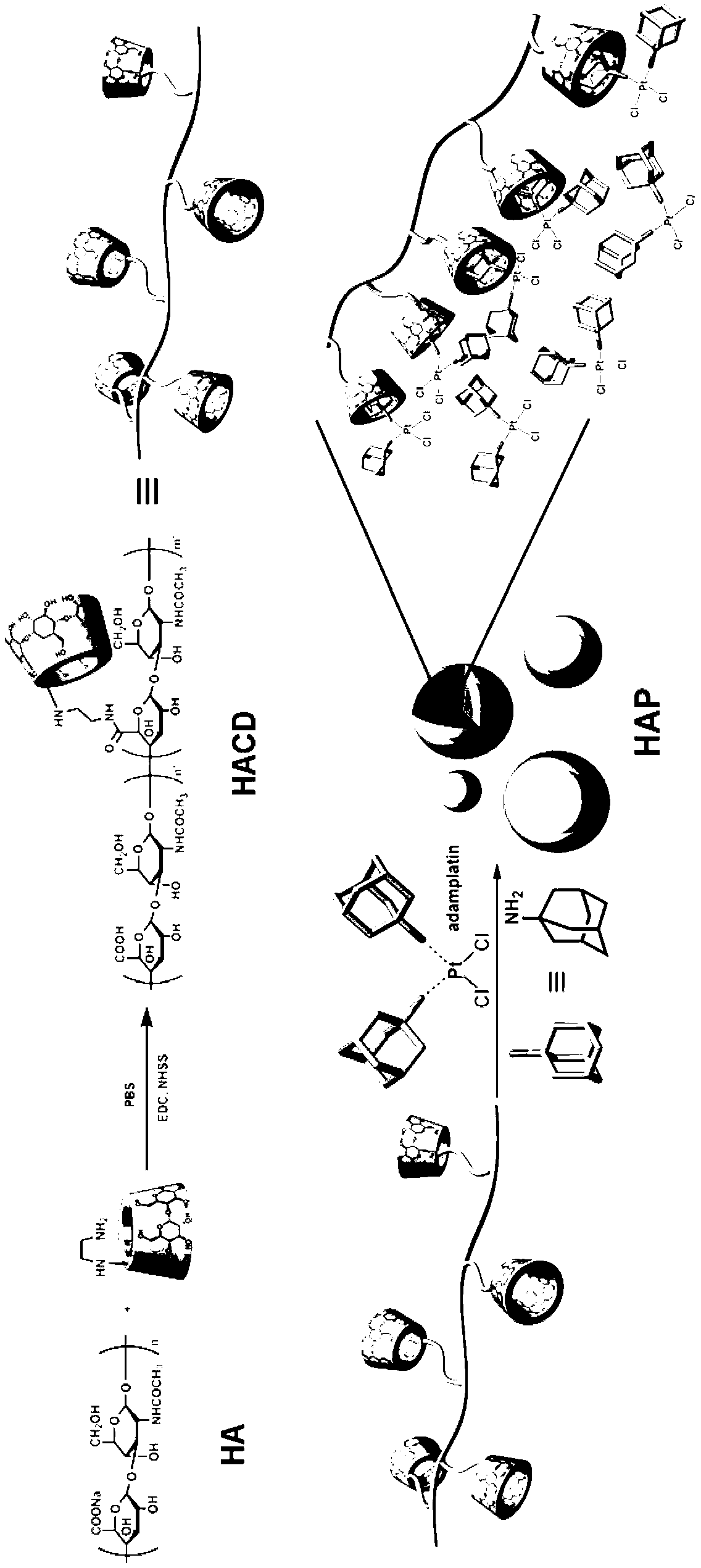
Supramolecule assembly of targeting-delivery anticancer adamplatin and preparation of supramolecule assembly
InactiveCN102698286AAchieve selective killingSmall toxicityHeavy metal active ingredientsPharmaceutical non-active ingredientsSide effectCancer cell
The invention discloses a supramolecule assembly of targeting-delivery anticancer adamplatin. The supramolecule assembly is a binary supramolecule assembly which is synthesized on the basis of cyclodextrin-decorated hyaluronic acid and adamplatin. A preparation method of the supramolecule assembly is characterized in that the cyclodextrin-decorated hyaluronic acid and the adamplatin are respectively synthesized, and through the strong non-covalent interaction of cyclodextrin and adamantine and the amphiphilic action of molecules, a supermolecule nano particle which takes the hydrophilic hyaluronic acid as a shell and the adamplatin as a core is formed. The supramolecule assembly disclosed by the invention has the advantages that the supramolecule assembly of the targeting-delivery anticancer adamplatin has a simple synthetic route, is low in preparation cost and high in productivity, and is suitable for amplification synthesis and practical production application; and through endocytosis in which a malignant cell surface hyaluronic acid receptor serves as a medium, the supramolecule assembly (HAP) is brought in cancer cells in a target manner, so that the protection of normal cells and the targeting selective killing of cancer cells are realized, the anti-cancer activity is obviously improved, and toxic and side effects are obviously reduced.
Owner:NANKAI UNIV
Beta-element nitrogenous derivative, and its preparing method and use
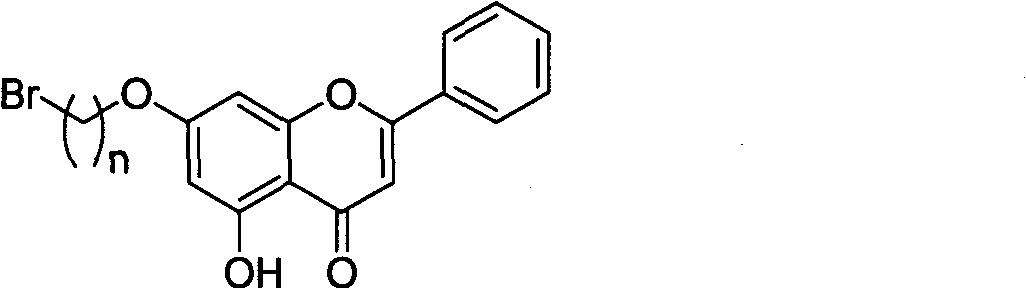
InactiveCN1850779AHigh anticancer activityGood water solubilityOrganic active ingredientsOrganic chemistrySolubilityFatty amine
This invention discloses beta-elemene nitrogen contained derivant. Nitrogen contain hetero atomic ring is brought in beta-elemene nitrogen structure to synthesize new structure beta-elemene nitrogen contained derivant. Its molecular structure formula is (I), R represents C1-C20 fatty amine group and aromatic amine group, C1-C20 contains hetero atomic ring amido group, cyclohexane backbone has three chirality centers. The derivent has some biological activities like alkaloid, it can increase polar at the same time, and make the synthesized derivent display certain alkalinity, so the goal of improve water-solubility by it with mineral acid or organic acid salt.
Owner:SHENYANG PHARMA UNIVERSITY
Naphthoyl amine derivative, and preparation method and application thereof
InactiveCN103288728ALower reaction costHigh yieldOrganic chemistryMetabolism disorderPTK InhibitorsEther
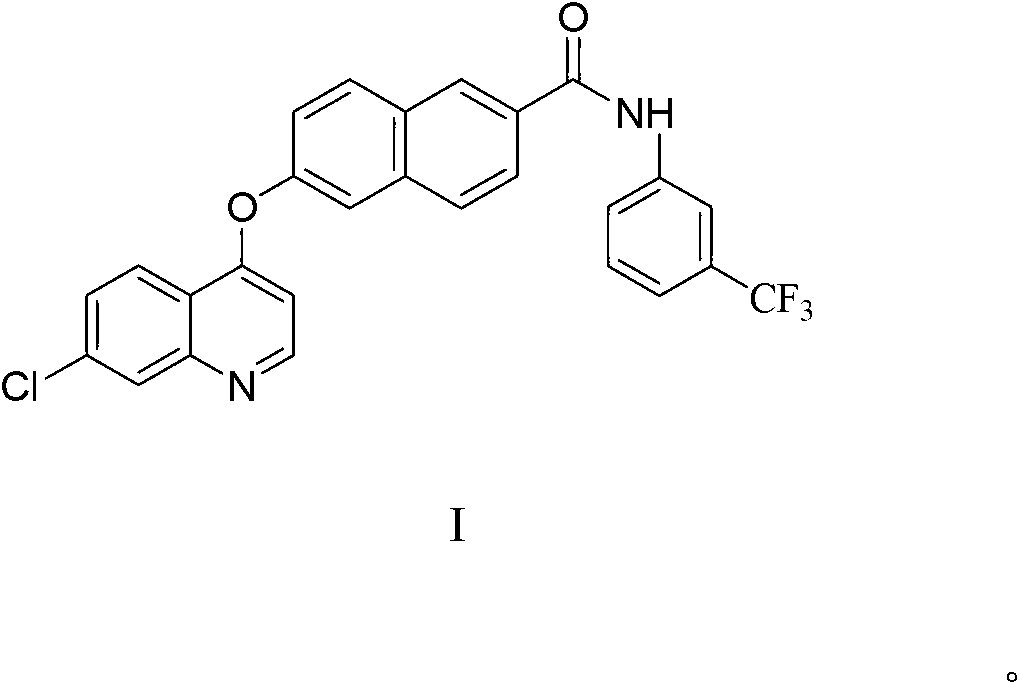
The invention provides a naphthoyl amine derivative, and a preparation method and an application of the same. The naphthoyl amine derivative is N-(3- trifluoromethyl phenyl)-6-[(7- chloroquinoline-4-oxo) phenolic ether]-2- naphthoyl amine; the N-(3- trifluoromethyl phenyl)-6-[(7- chloroquinoline-4-oxo) phenolic ether]-2- naphthoyl amine can be used as protein kinase inhibitor and histone deacetylase inhibitor, and can be used for treating diseases caused by protein kinase dysregulation. The naphthoyl amine derivative can effectively treat the diseases caused by protein kinase dysregulation, and has the advantages of high bioavailability, obvious antitumor activity and low toxicity; moreover, the naphthoyl amine derivative is low in preparation reaction cost and higher in yield, the reaction process is simple and easy to control, so that the naphthoyl amine derivative is suitable for industrial production.
Owner:HUAQIAO UNIVERSITY
Compound, a process for its preparation, a pharmaceutical composition, use of a compound, a method for modulating or regulating serine/threonine kinases and a serine/threonine kinases modulating agent
InactiveUS20120184535A1Improve effectivenessInhibitory activityBiocideNervous disorderDiseaseTyrosine
A compound, a process for its preparation, a pharmaceutical composition, use of a compound, a method for modulating or regulating serine / threonine and tyrosine kinases and a serine / threonine and tyrosine kinases modulating agent. Novel small-molecule compounds with kinase inhibitory activity, having superior properties as pharmaceutical agents, production method thereof and uses thereof. In particular, new derivatives of tetrahalogenated benzimidazole with serine / threonine and tyrosine kinases inhibitory properties, preferably selected from the group of PIM, HIPK, DYRK, CLK, CDK, FLT, PKG, Haspin, MER, TAO, MNK, TRK kinases which exhibit superior pharmacological actions, and can be useful for the treatment of disease conditions, especially cancers depending on serine / threonine and tyrosine kinases, such as but not limited to leukemias and solid tumors.
Owner:SELVITA SA
Composite edible fungi polysaccharides and preparation method thereof
ActiveCN102093598AHigh anticancer activityImprove immunityMetabolism disorderDigestive systemVacuum dryingGanoderma lucidum
The invention relates to composite edible fungi polysaccharides and a preparation method thereof. The preparation method comprises the following steps: selecting fruiting bodies of Lentinus edodes, Auricularia aurlcula, Ganoderma lucidum and Grifola frondosa as raw materials; weighing at a ratio of (1:2):(2:1), mixing, pulverizing, adding distilled water, homogeneously mixing, and sequentially performing microwave extraction and condensation reflux extraction; separating residues and liquid; adding residues into distilled water, and sequentially performing microwave extraction and condensation reflux extraction for 1-2 times; mixing extractive solutions, carrying out vacuum concentrating until the volume of the extractive solution is 1 / 3-1 / 4 of the original volume; and precipitating with alcohols, vacuum drying, and pulverizing to obtain composite edible fungi polysaccharides. The composite edible fungi polysaccharides provided by the invention have good physiological activity and complete efficacy, and has the functions of resisting viruses, reducing blood lipid, regulating immune system, resisting platelet aggregation, resisting tumor, protecting liver, resisting gene mutation and resisting acquired immunodeficiency syndrome (AIDS) and the like. The preparation method of the composite edible fungi polysaccharides has the advantages of high yield of polysaccharides, reasonable process, and high operability.
Owner:山东海普盾生物科技有限公司
Treatment for cancers
InactiveUS7763658B2Cause side effectSolve low usageBiocideAmide active ingredientsArylMulti drug resistant
One embodiment of the present invention is a method of treating a subject with a multi-drug resistant cancer. The method comprises administering to the subject an effective amount of a compound represented by Structural Formula (I):Y is a covalent bond or a substituted or unsubstituted straight chained hydrocarbyl group, or, Y, taken together with both >C═Z groups to which it is bonded, is a substituted or unsubstituted aromatic group.R1-R4 are independently —H, an aliphatic group, a substituted aliphatic group, an aryl group or a substituted aryl group, or R1 and R3 taken together with the carbon and nitrogen atoms to which they are bonded, and / or R2 and R4 taken together with the carbon and nitrogen atoms to which they are bonded, form a non-aromatic heterocyclic ring optionally fused to an aromatic ring. Preferably R1 and R2 are the same and R3 and R4 are the same.R5-R6 are independently —H, an aliphatic group, a substituted aliphatic group, an aryl group or a substituted aryl group.Z is ═O or ═S.
Owner:SYNTA PHARMA CORP
Novel formulations of pharmacological agents, methods for the preparation thereof and methods for the use thereof
InactiveUS20070117863A1Eliminate side effectsHigh anticancer activityPowder deliveryBiocideSolventActive agent
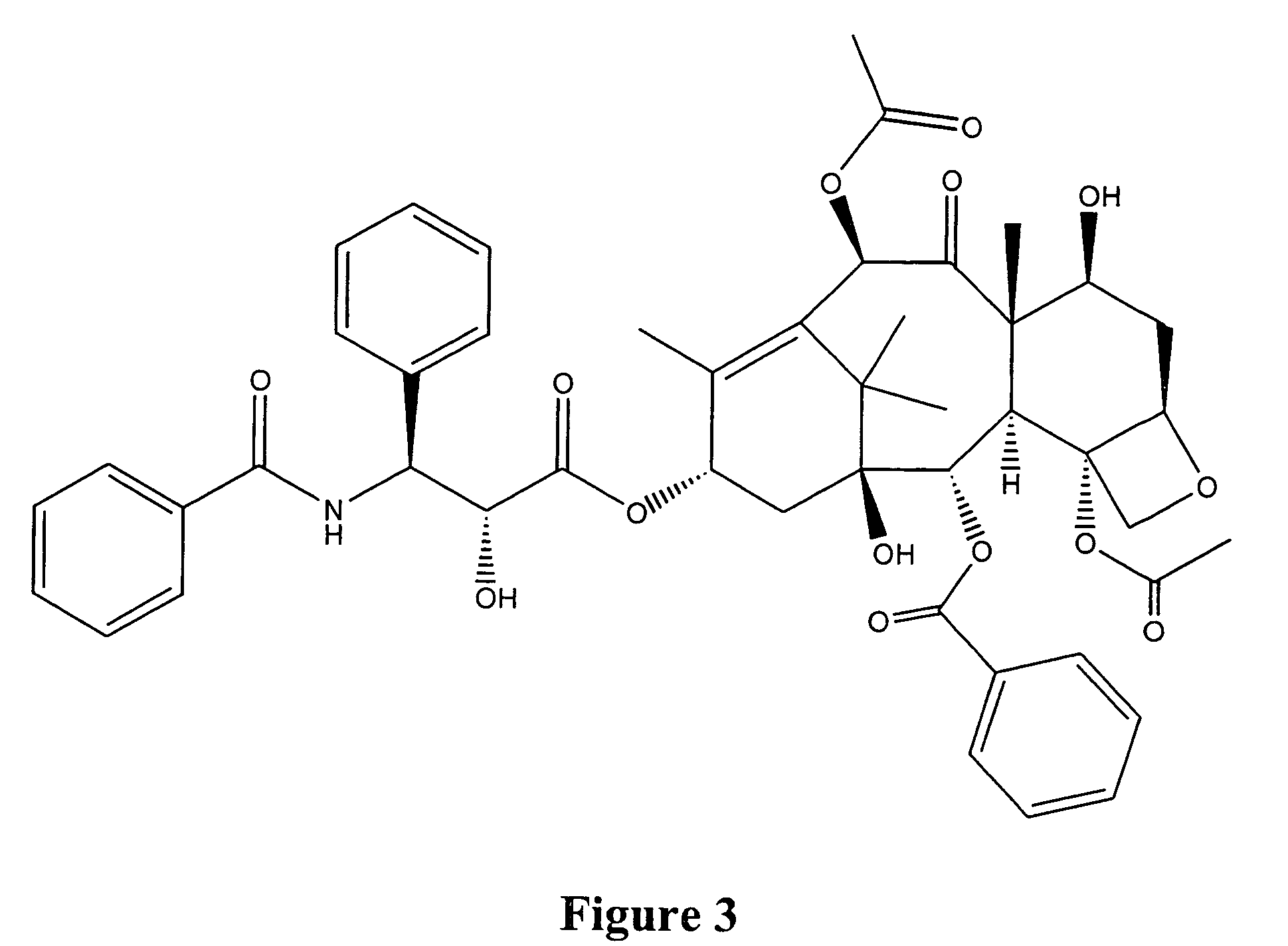
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of substantially water insoluble pharmacologically active agents (such as the anticancer drug paclitaxel) in which the pharmacologically active agent is delivered in the form of suspended particles coated with protein (which acts as a stabilizing agent). In particular, protein and pharmacologically active agent in a biocompatible dispersing medium are subjected to high shear, in the absence of any conventional surfactants, and also in the absence of any polymeric core material for the particles. The procedure yields particles with a diameter of less than about 1 micron. The use of specific composition and preparation conditions (e.g., addition of a polar solvent to the organic phase), and careful selection of the proper organic phase and phase fraction, enables the reproducible production of unusually small nanoparticles of less than 200 nm diameter, which can be sterile-filtered. The particulate system produced according to the invention can be converted into a redispersible dry powder comprising nanoparticles of water-insoluble drug coated with a protein, and free protein to which molecules of the pharmacological agent are bound. This results in a unique delivery system, in which part of the pharmacologically active agent is readily bioavailable (in the form of molecules bound to the protein), and part of the agent is present within particles without any polymeric matrix therein.
Owner:ABRAXIS BIOSCI LLC
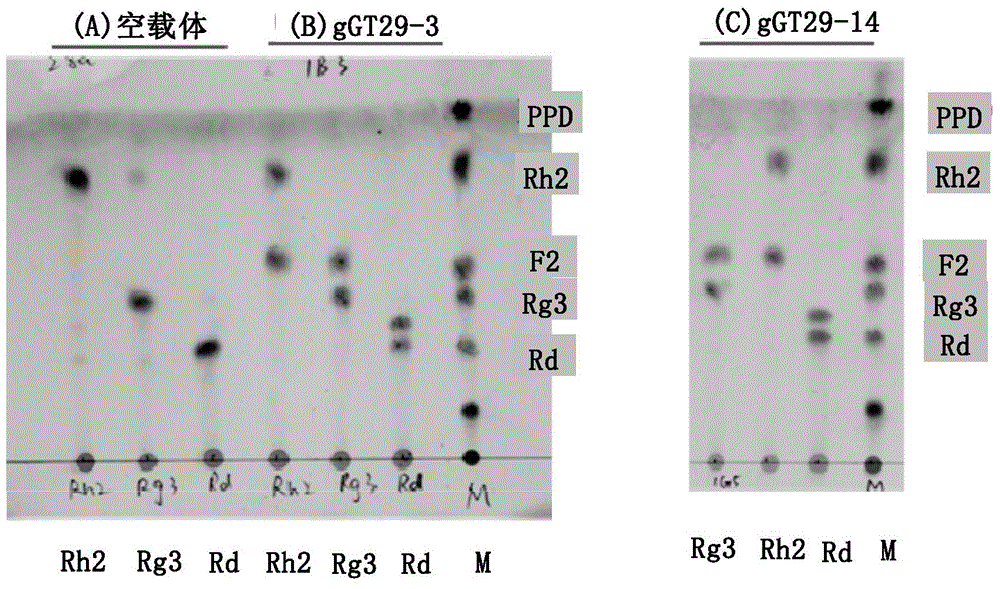
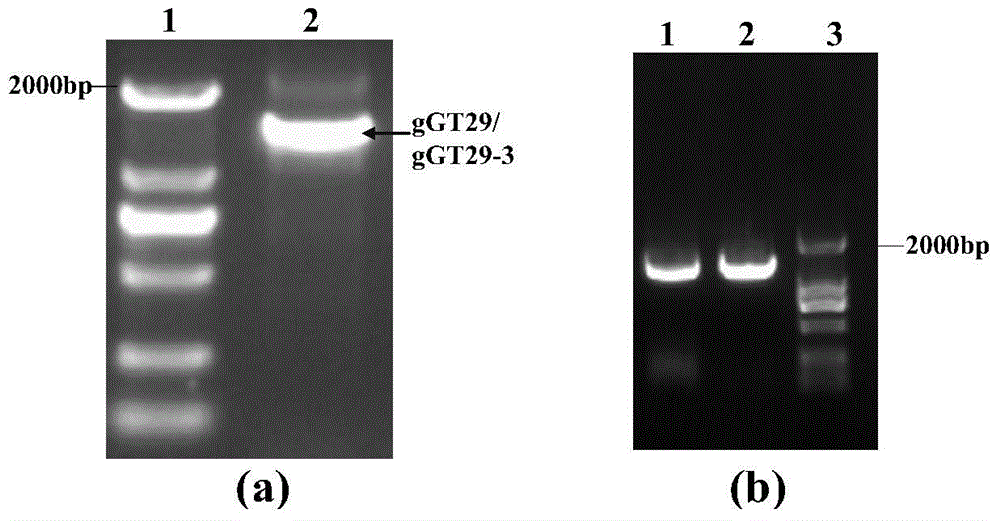
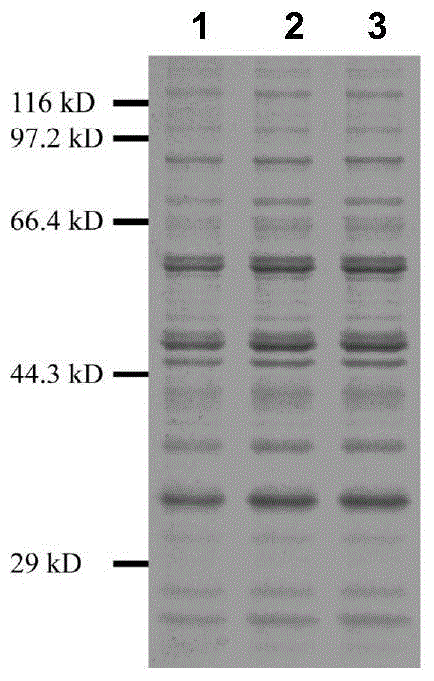
A group of glycosyl transferase, and applications thereof
InactiveCN105177100AHigh anticancer activityResolve source issuesTransferasesPlant peptidesTriterpeneTriterpenoid
The present invention relates to a group of glycosyl transferase and applications thereof, and specifically provides applications of the glycosyl transferase gGT29-7 and a derived polypeptide thereof in terpene compound glycosylation catalysis and new saponin synthesis, wherein the glycosyl transferase can specifically and efficiently transfer the glycosyl from a glycosyl donor to the first glycosyl on C-3 site and / or C-6 site of a tetracyclic triterpene compound so as to extend the sugar chain. The glycosyl transferase of the present invention can further be used for constructing artificial ly-synthesized rare ginsenosides and a variety of new ginsenosides and derivatives thereof.
Owner:周志华
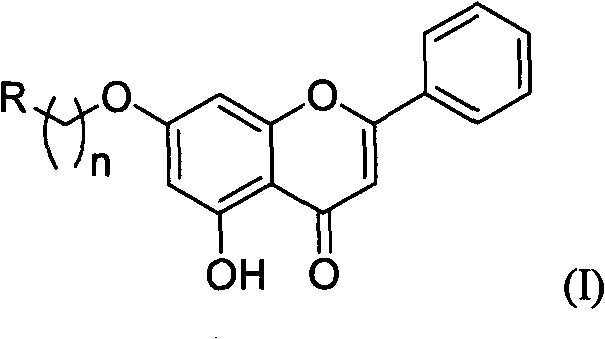
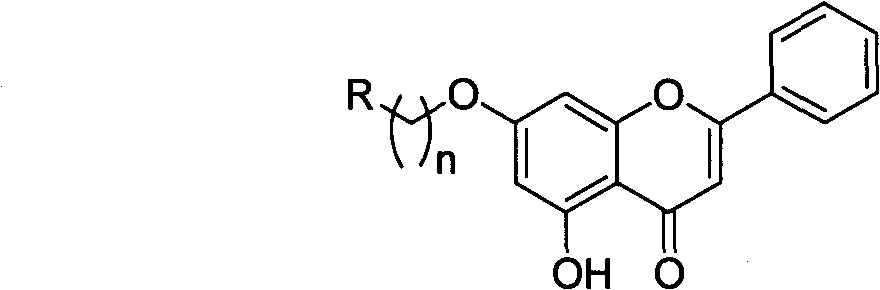
Chrysin nitrogen-containing derivative as well as preparation method and purpose thereof
InactiveCN101774993AGood water solubilityHigh anticancer activityOrganic active ingredientsOrganic chemistrySolubilityCarbon chain
The invention discloses a chrysin nitrogen-containing derivative. Nitrogen-containing groups are introduced to a chrysin structure to synthesize the chrysin nitrogen-containing derivative with a novel structure. The molecular structure of the chrysin nitrogen-containing derivative is shown as (I), wherein R represents fatty amidogens of C1-C20, aromatic amidogens of C6-C20, heterocycle-containing amidogens of C1-C20 and alcohol amidogens or benzyl amidogens of C1-C20, and n represents the carbon chains of C2-C20. The derivative can have the biological activities of some similar alkaloids and simultaneously enlarge polarities, and the synthesized derivative presents a certain alkalinity, which is convenient to achieve the purpose of improving the water solubility with inorganic acid or organic acid finished salts.
Owner:JIANGSU POLYTECHNIC UNIVERSITY
Beta-elemene derivatives containing nitrogen and their preparation method and use
InactiveCN1844105AHigh anticancer activityGood water solubilityOrganic chemistryAmine active ingredientsOrganic acidSolubility
The invention discloses an A-elemene nitrogenous derivant, it providing the preparation and usage of A-elemene nitrogenous derivant, the structural formula is as picture shows, wherein R represent aliphatic amido of C1-C20, aromatic amido; amido of C1-C20 containing heterocycle; wherein cyclohexane skeleton has three chirality center. This derivant may has some physiologically active like alkaloid; meanwhile, it can increase polarity and making the synthesized derivant has certain alkalinity and it can reach the purpose of improving water-solubility salt-forming with inorganic acid or organic acid.
Owner:SHENYANG PHARMA UNIVERSITY
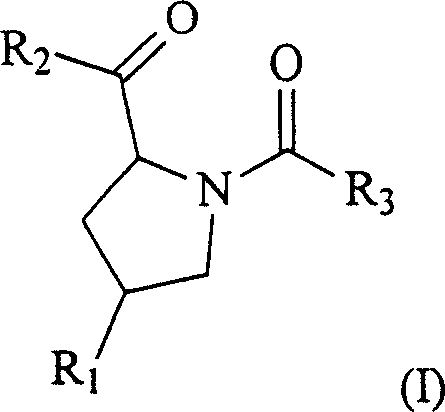
Pyrrolidine matrix metall oprotease inhibitor and preparing method thereof
InactiveCN1528745ANo cytotoxic activityHigh anticancer activityOrganic chemistryActive componentPyrrolidine
The invention is a pyrrol alkyl matrix metalloproteinase inhibitor and preparing method, relating to the corresponding compound preparation, active test of inhibiting matrix metalloproteinase and the drug combination in which it acts as active component.
Owner:SHANDONG UNIV
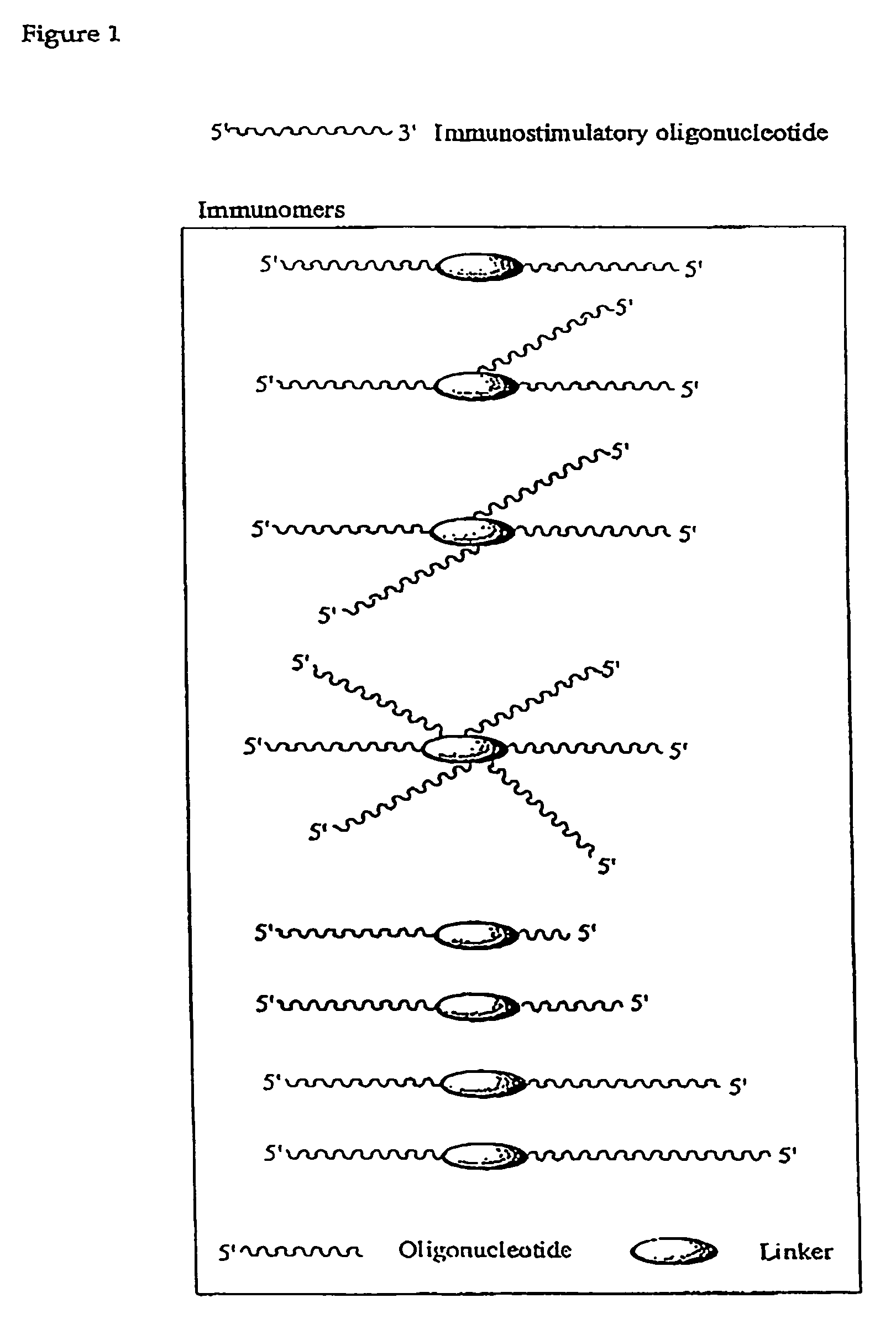
Synergistic treatment of cancer using immunomers in conjunction with therapeutic agents
ActiveUS7569554B2Enhancing the anti-cancer activity of immunostimulatoryHigh anticancer activityOrganic active ingredientsBiocideTherapeutic effectOligonucleotide
The invention relates to the therapeutic use of immunostimulatory oligonucleotides and / or immunomers in combination with chemotherapeutic agents to provide a synergistic therapeutic effect.
Owner:IDERA PHARMA INC
Method for extracting and preparing medlar leaf flavone
ActiveCN102000208AIncrease contentImprove biological activityAntinoxious agentsAntineoplastic agentsDiseaseColloid
The invention relates to a method for extracting and preparing medlar leaf flavone. The method comprises the following steps of: (1) after picking and removing impurities manually, drying medlar leaves indoor to obtain a medlar leaf dry sample; (2) crushing the medlar leaf dry sample and screening the crushed medlar leaf dry sample to obtain medlar powder; (3) leaching the medlar powder by using ethanol to obtain leaching liquor; (4) grinding the leaching liquor by using a colloid grinder to obtain colloidal medlar leaf leaching solution; (5) homogenizing and separating the colloidal medlar leaf leaching solution to obtain liquid supernatant and filter residue; (6) removing impurities from the liquid supernatant by using a ceramic membrane to obtain filtrate; (7) adsorbing the filtrate by using weak-polarity macroporous adsorption resin and eluting the filtrate by using ethanol solution to obtain medlar leaf flavone-containing eluent; and (8) after concentrating and drying the medlar leaf flavone-containing eluent under a reduced pressure in a vacuum, obtaining the medlar leaf flavone powder. The method has low cost and high extraction rate; and the obtained medlar leaf flavone has stable properties, high content, and high biological activity of heart head blood-vessel disease resistance, cancer resistance, oxidation resistance and the like.
Owner:CHINA ACAD OF SCI NORTHWEST HIGHLAND BIOLOGY INST
Taxol enhancer compounds
InactiveUS20060116374A1High anticancer activityImprove efficiencyBiocideOrganic chemistryArylDiluent
Owner:SYNTA PHARMA CORP
Angiogenesis-inhibiting chimeric protein and the use
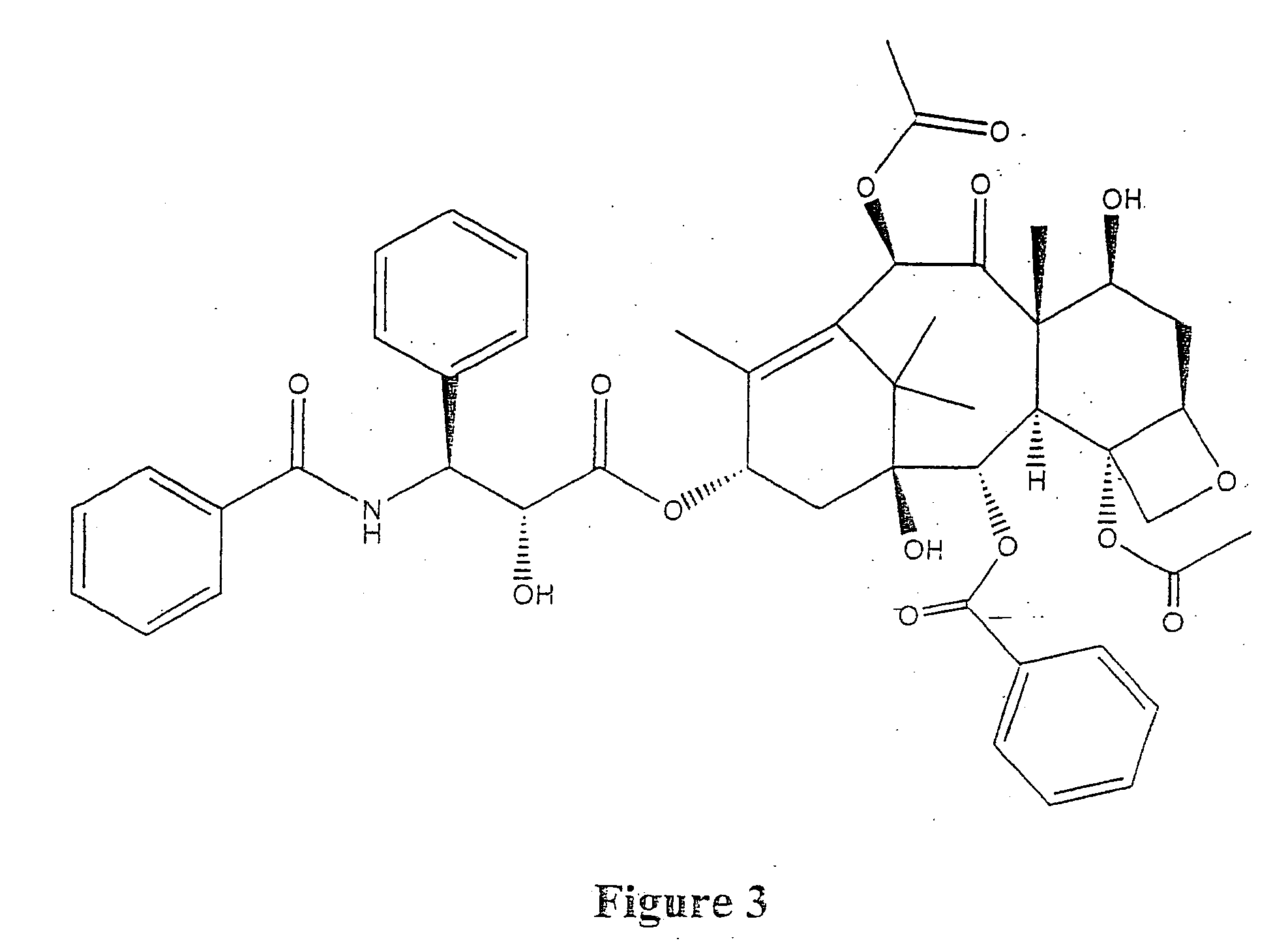
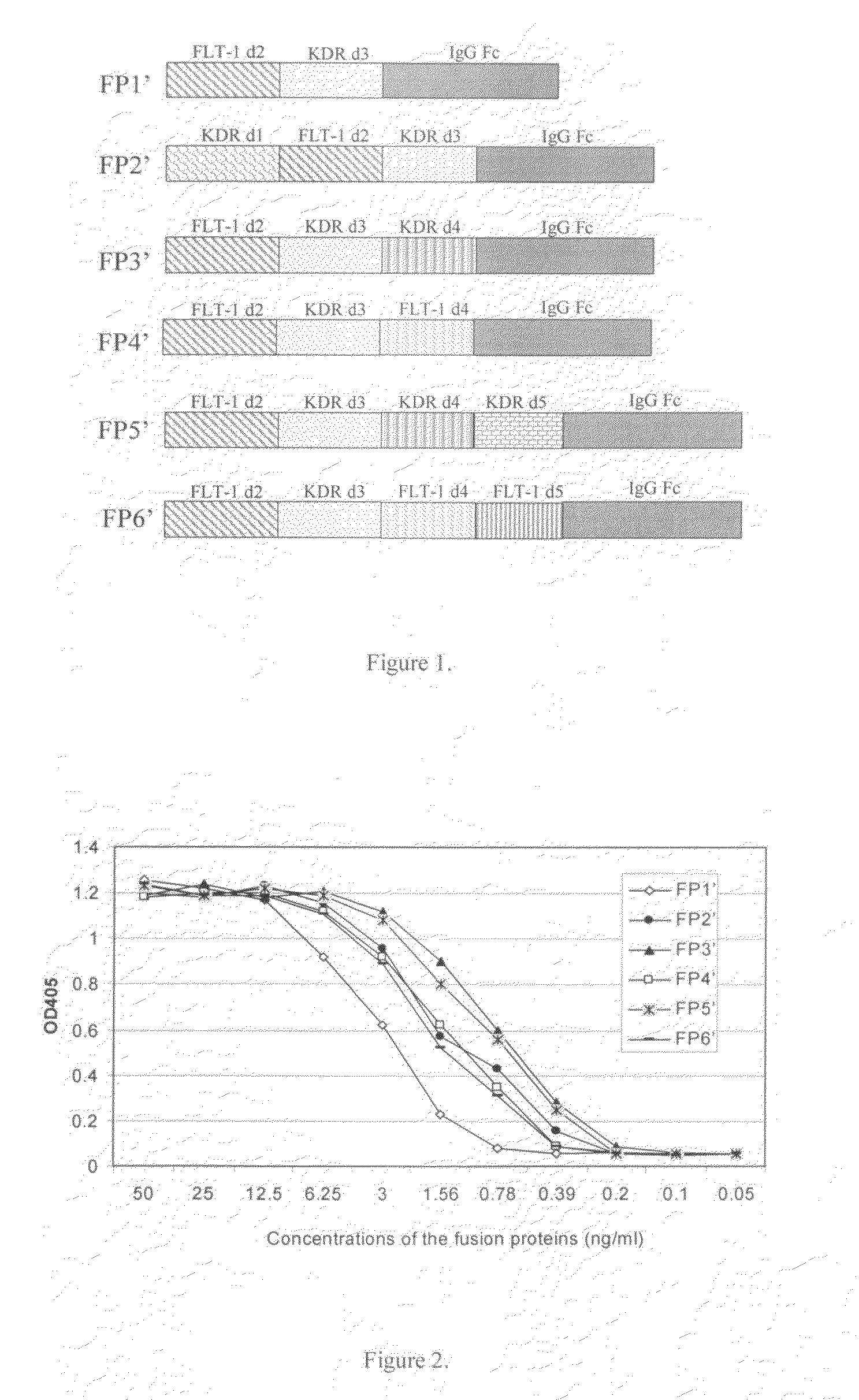
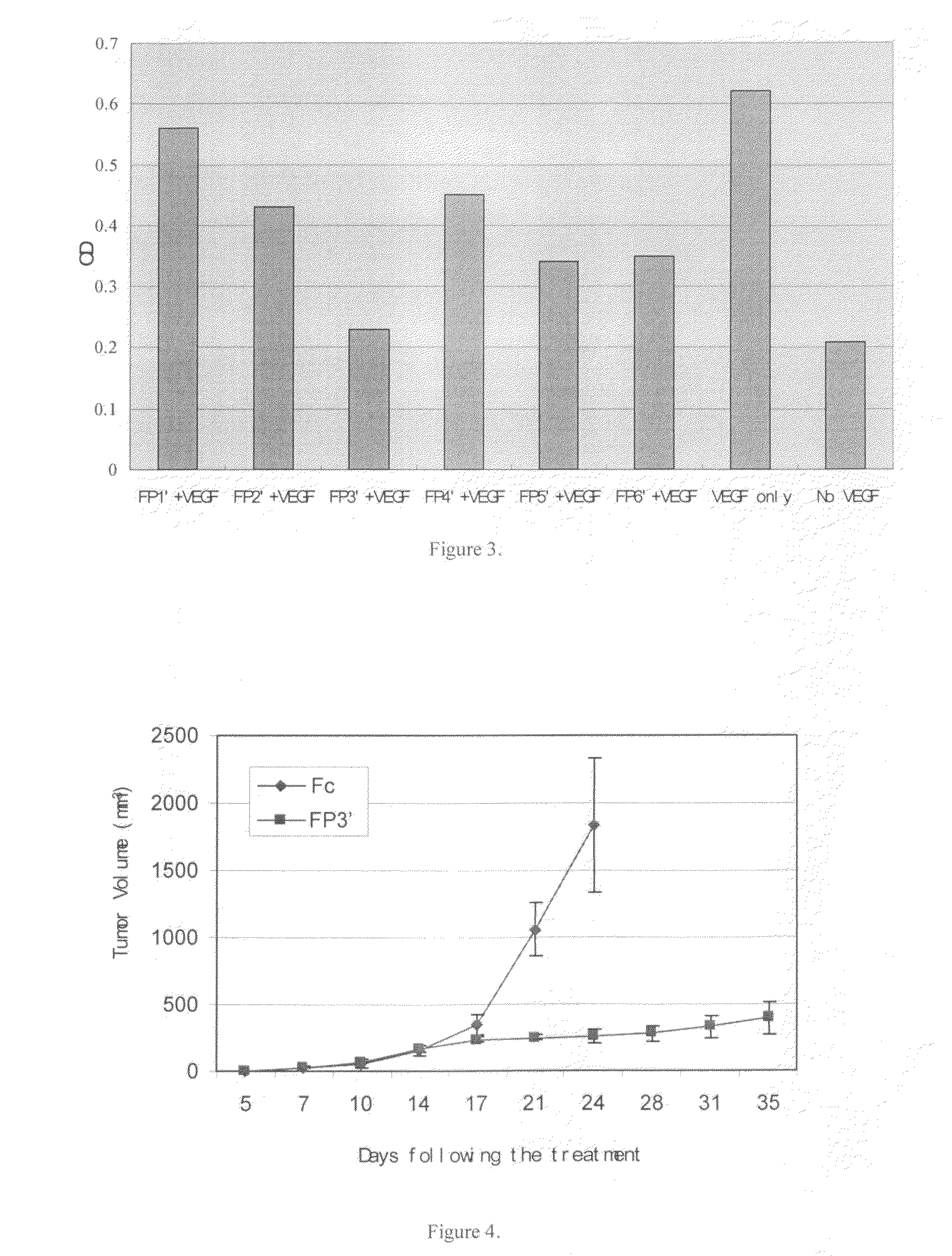
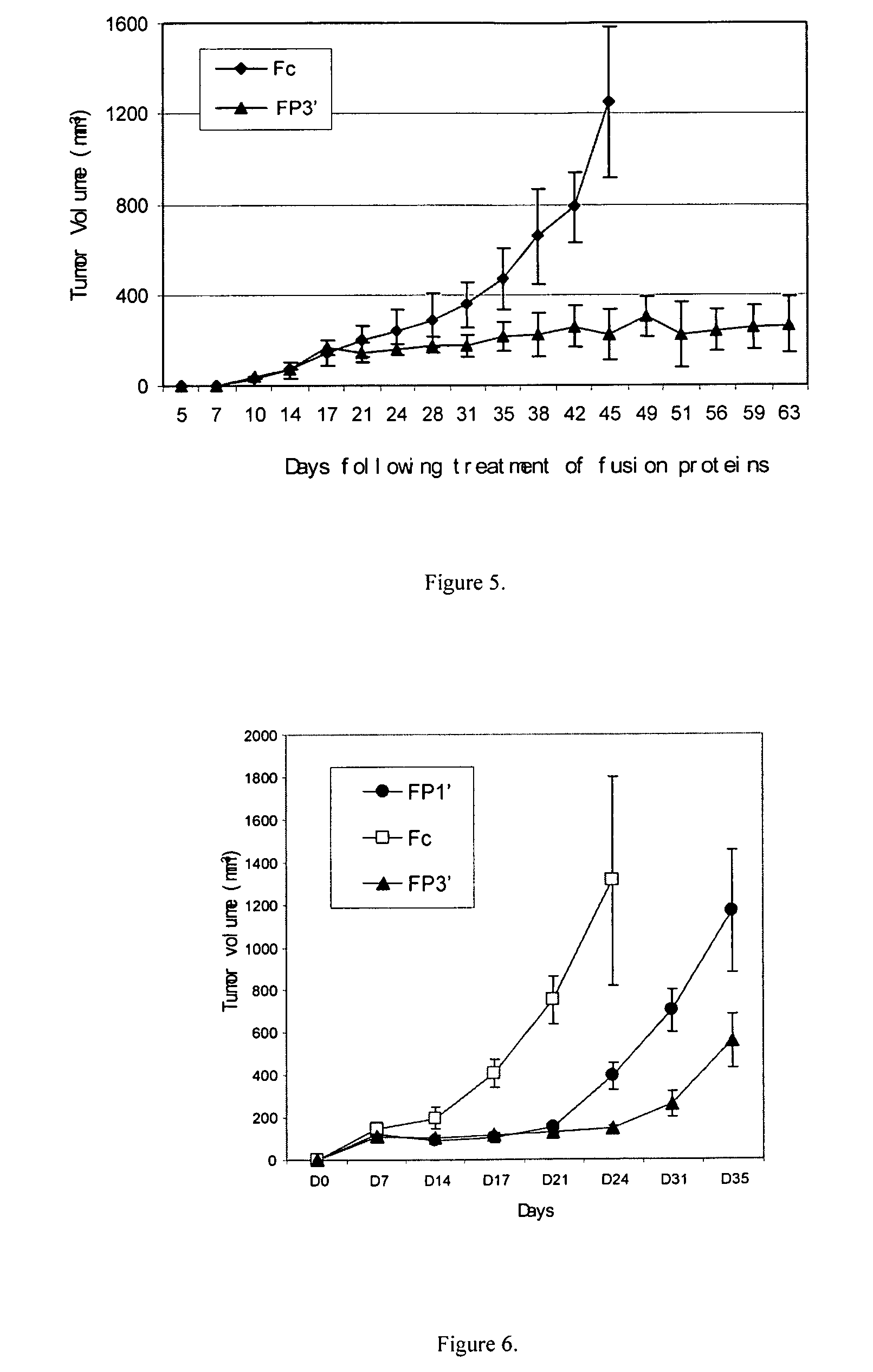
ActiveUS7750138B2Increase binding siteHigh affinitySenses disorderPeptide/protein ingredientsAngiogenesis growth factorChimera Protein
Owner:CHENGDU KANGHONG BIOTECH
Anti-cancer pharmaceutical composition
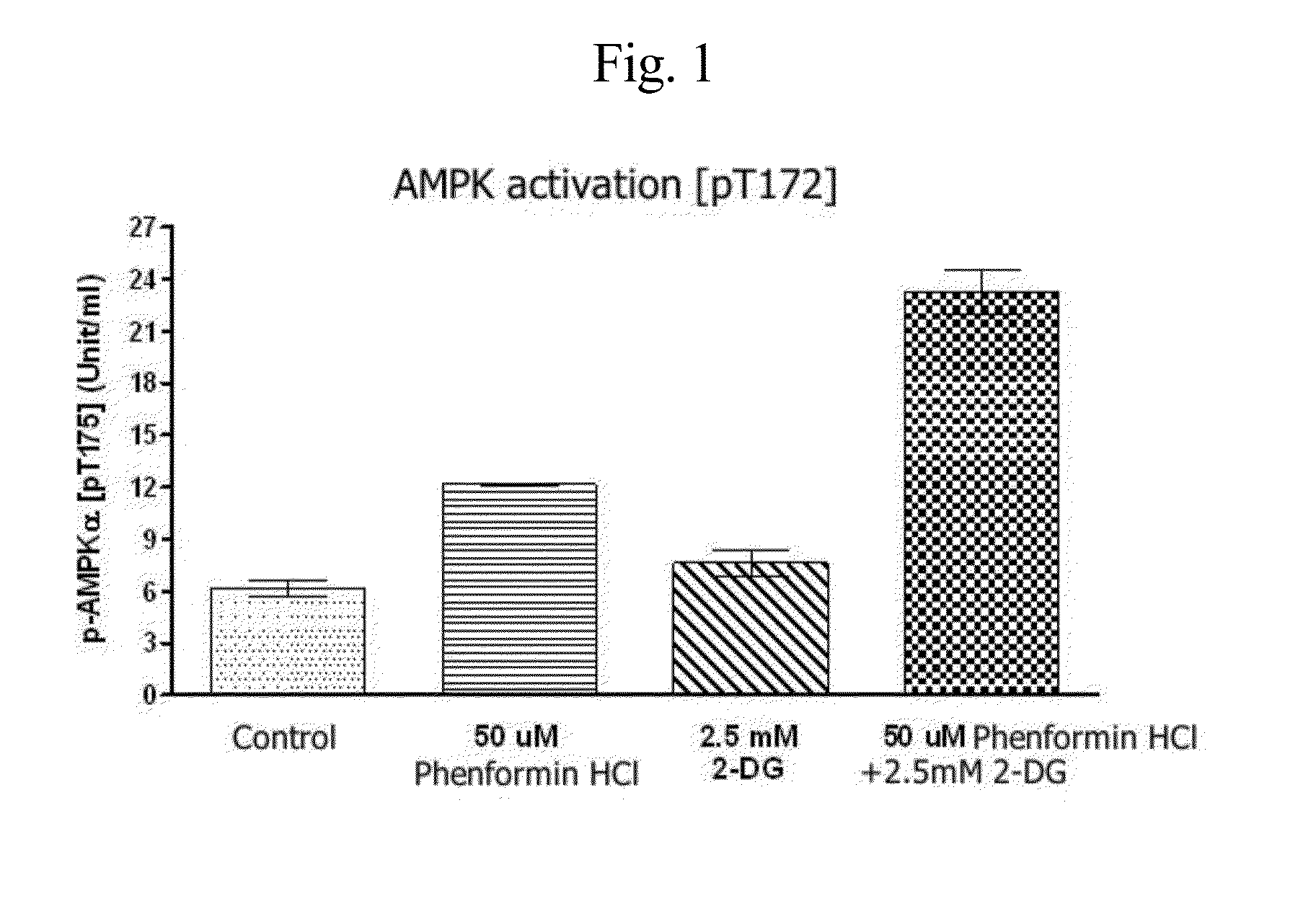
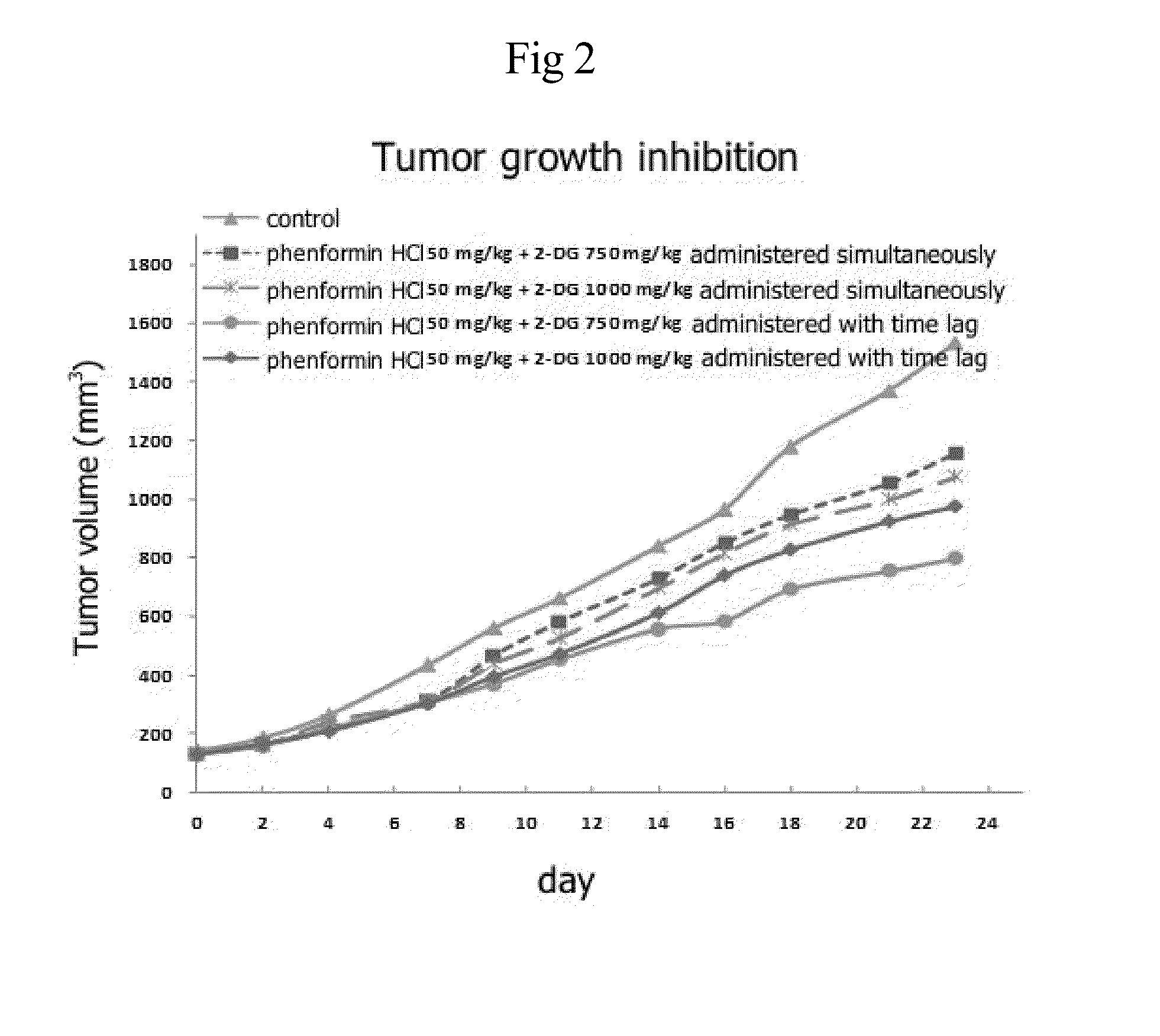
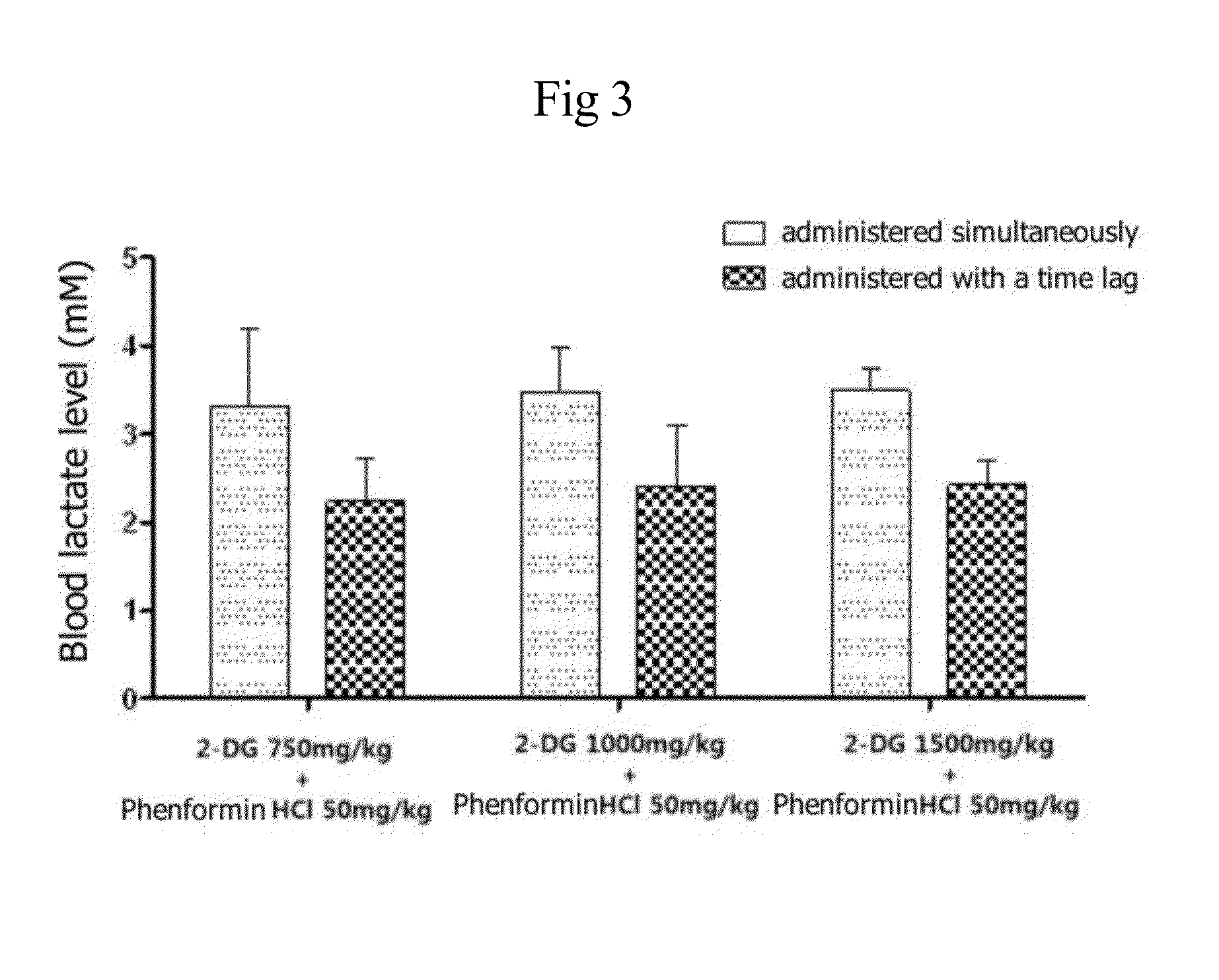
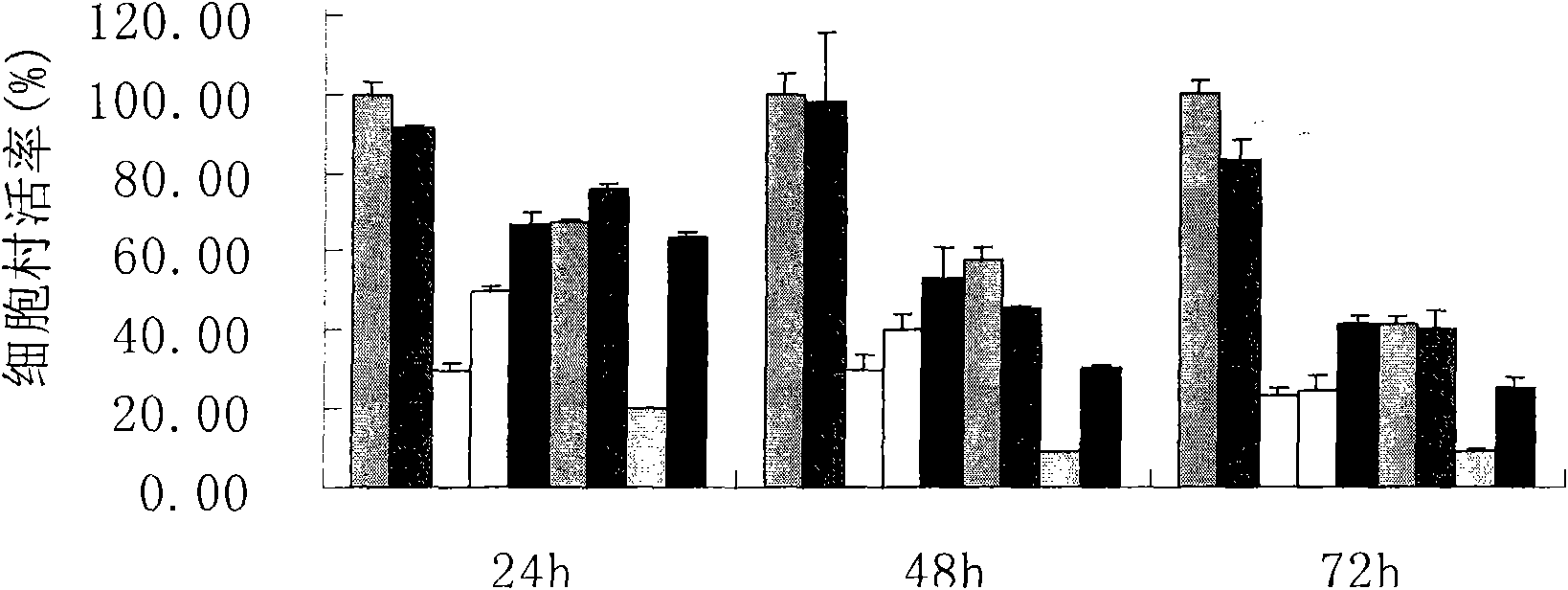
InactiveUS20140235559A1Inhibitory activityDecrease blood lactic acid levelBiocideMetabolism disorderTime lagCancer cell
Disclosed is an anticancer pharmaceutical composition comprising phenformin or a pharmaceutically acceptable salt thereof, and 2-deoxy-D-glucose as active ingredients. These ingredients act in synergy with each other, thus exhibiting more potent inhibitory activity against the growth of cancer cells, compared to individual ingredients. Also, the synergistic anticancer activity allows the individual drugs to be used in lower amounts, which leads to a reduction in the occurrence of adverse effects. In addition, the time-lag release or administration of the ingredients decreases blood lactic acid levels to significantly mitigate the adverse effect of lactic acidosis, as well as exerting high anticancer effects. Particularly, the pharmaceutical composition can be formulated to dosage forms effective for therapy, increasing the drug compliance of the subject.
Owner:HANALL PHARMA CO LTD
Piperazidine or homopiperazine oxalyl hydrazine class compound, preparation and use thereof
ActiveCN101565409AHigh anticancer activityGrowth inhibitionOrganic chemistryAntineoplastic agentsHydrazine compoundPiperazine
The invention relates to a novel piperazidine or homopiperazine oxalyl hydrazine class compound as represented by formula I, wherein each symbol has meanings defined in the description. The invention further relates to the preparation of the piperazidine or homopiperazine oxalyl hydrazine class compound, use of the piperazidine or homopiperazine oxalyl hydrazine class compound in preparing medicament for treating and / or preventing tumor and / or cancer, medicament composition containing the piperazidine or homopiperazine oxalyl hydrazine class compound. The piperzzidine or homopiperazine oxalyl hydrazine class compound has effective anti-tumor activity.
Owner:DONGGUAN ZHENGXING BEITE MEDICINE TECH CO LTD
Application of 2-bromide-isovanillin for the manufacture of a medicament for anti-cancer or/and radiation/chemotherapy sensitization
ActiveUS20080221221A1Induce apoptosisHigh sensitivityBiocideAldehyde active ingredientsSide effectTherapeutic effect
Use of 2-bromo-isovanillin in the preparation of an anticancer medicament and / or radio- and chemotherapy sensitizing medicament is disclosed. The medicament for the treatment of cancers and / or for radio- and chemotherapy sensitization comprising 2-bromo-isovanillin as active ingredient provided herein has the following features: (1) low toxicity, without evident adverse effects; (2) significant therapeutic effect, with remarkable proliferation inhibiting and pro-apoptotic effects in tumor cells; (3) a broad-spectrum anticancer activity; (4) suitable to be used in combination with antimetabolites, to enhance the effects and meanwhile lower the toxicity, and also to reduce multi-drug resistance; (5) convenient and safe administration, the main route being oral.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Tobacco additive for increasing smoke concentration and preparation method and application thereof
InactiveCN101810367AIncrease concentrationIncrease sweetnessTobacco treatmentWater bathsMurraya kwangsiensis
The invention relates to a tobacco additive for increasing smoke concentration and a preparation method and application thereof. Shortleaf kyllinga herb, lysimachia foenum-graecum and murraya kwangsiensis are used as main raw materials. The preparation method comprises the following steps: soaking a raw material mixture in an organic solvent or performing reflux extraction for 1 to 360 hours; leaching the mixture by using qualitative filter paper and filtering off residues to obtain natural tobacco additive extracting solution; performing concentration on the extracting solution under reduced pressure in a water bath at the temperature of between 50 and 80 DEG C; recycling a solvent of the extracting solution to obtain a natural tobacco additive; dissolving the tobacco additive in a certain amount of single or mixed solution consisting of water, ethanol and propylene glycol; and spraying the mixture accounting for 0.005 to 2.5 percent of the weight of cigarette cut tobacco, cut stems or thin tobacco slices onto sample cut tobacco according to the conventional flavoring process. The tobacco additive can remarkably enrich tobacco flavor, effectively increase the smoke concentration of the cigarette, enhance sweet feeling in an oral cavity and achieve fine and soft smoke. The additive has the advantages of simple preparation process, low cost and wide market prospect.
Owner:CHINA TOBACCO GUANGXI IND
Benzenesulfonyl piperazine compounds or benzoyl piperazine compounds, preparation methods and uses thereof
ActiveCN102838567AHigh anticancer activityReduce neurotoxicityOrganic active ingredientsOrganic chemistryEffective treatmentPiperazine
The present invention relates to benzenesulfonyl piperazine compounds represented by a formula I or benzoyl piperazine compounds represented by a formula II, or pharmaceutically acceptable salts, solvates, stereoisomers or precursor drugs thereof, wherein various symbols are described in an instruction. The present invention further relates to preparation methods for the compounds, uses of the compounds in preparation of drugs for treatment and / or prevention of tumors and / or cancers, methods for treatment and / or prevention of tumors and / or cancers by using the compounds, and drug compositions containing the compounds having an effective treatment and / or prevention amount. The compounds of the present invention have characteristics of effective anticancer activity and low toxicity.
Owner:DONGGUAN ZHENGXING BEITE MEDICINE TECH CO LTD
Preparation of demethylated manganese cantharidate
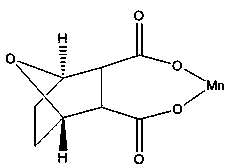
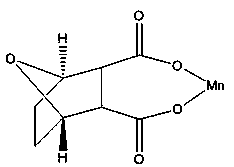
InactiveCN103910744AHigh purityGood water solubilityOrganic chemistryAntineoplastic agentsPhysical chemistryManganese
The invention discloses a demethylated manganese cantharidate of which the structural formula (I) is disclosed in the specification. The preparation method comprises the following steps: heating a certain volume of deionized water to 70-80 DEG C, and adding a certain amount of demethylated cantharidin until the end concentration is 12.5 mmol / L; adding a certain amount of manganous hydroxide until the mole ratio of the demethylated cantharidin to the manganous hydroxide is 1:2-1:5; heating while magnetically stirring to react for 30-60 minutes, centrifuging at 10000g for 30 minutes, and discarding the precipitate to reserve the supernatant; and drying the supernatant at 60 DEG C under reduced pressure to obtain the colorless random crystal. The demethylated manganese cantharidate has the characteristics of higher anticancer activity, simple technique, favorable safety, stable finished product performance and high purity, and the preparation method is easy to operate.
Owner:ZUNYI MEDICAL UNIVERSITY
Pectin manufacture technology
ActiveCN103275242AHigh anticancer activityHighlight substantive featuresPectinaseManufacturing technology
The invention provides a pectin manufacture technology. The pectin manufacture technology comprises the following steps: firstly, cleaning and washing peel pomace; secondly, performing hydrolysis; thirdly, performing separation and concentration; fourthly, performing enzymolysis on pectin: adjusting the pH value of the concentrate C to be 3.0-5.0, adding pectolase of 0.2-0.5 permillage, insulating at 30-45 DEG C for 4-6 hours, wherein during the insulation process, the pectolase can act on the specific position of the pectin molecular chain, a D-galacturonic acid Alpha-1,4-glucosidic bond locates and decomposes the original pectin into small molecules, and the molecular weight of the pectin is 10,000-20,000 g / mol; and fifthly, performing vacuum drying, adopting vacuum low-temperature drying with the vacuity of -0.70 to -0.80 MPa and the temperature of 60-70 DEG C to obtain the end product. According to the invention, an enzymic method degradation bio-engineering technology is adopted to reduce the molecule weight of the pectin to 10,000-20,000 g / mol; and the pectin after selective modification of the molecule chain has an obvious antitumor activity, and functions of removing in vivo heavy metal and adjusting blood sugar and blood fat.
Owner:YANTAI ANDRE PECTIN
Correlation of anti-cancer activity of dyes with redox potentials
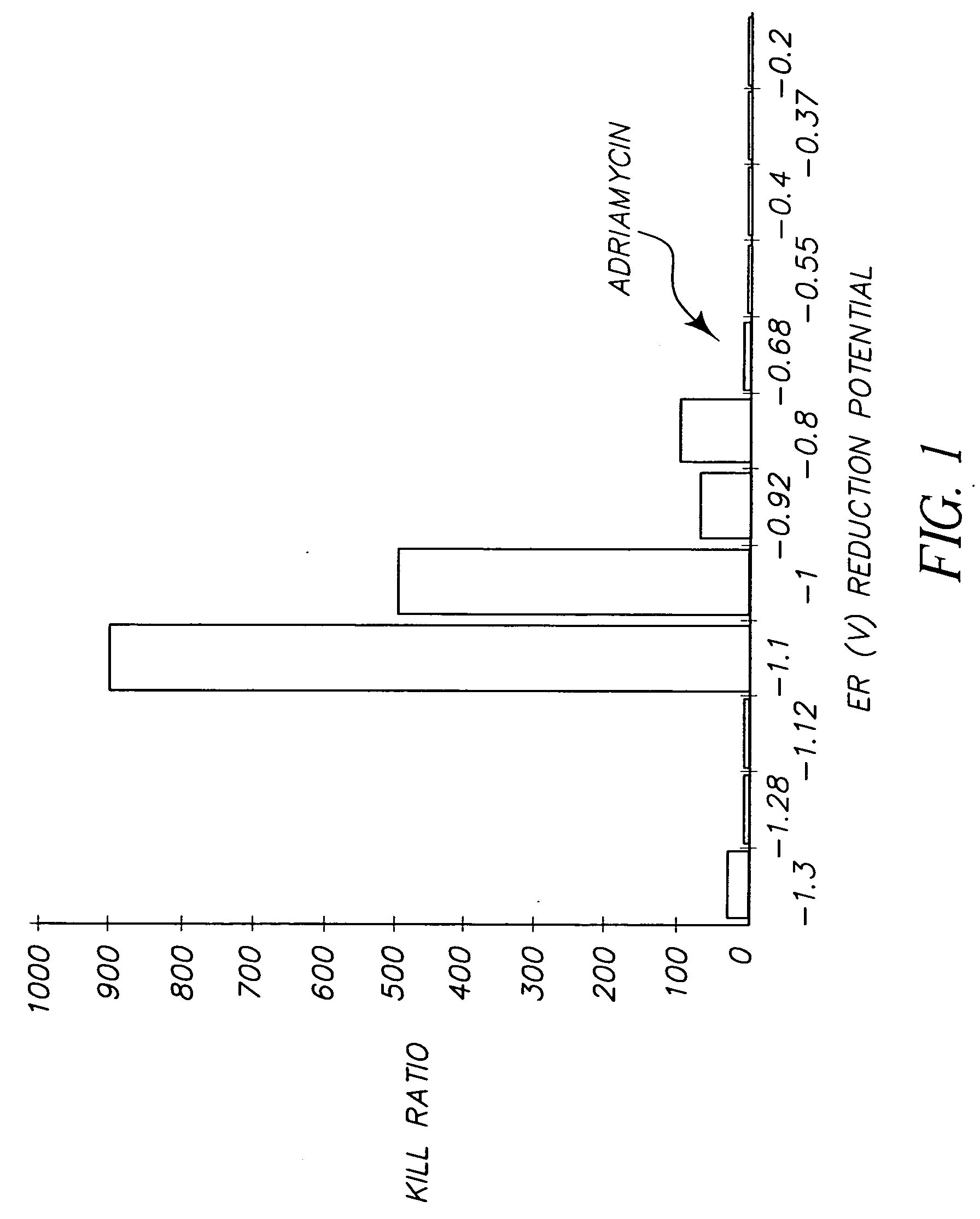
InactiveUS20060099712A1High anticancer activityReduce the amount requiredMethine/polymethine dyesMaterial analysis by electric/magnetic meansCancer cellCyanine
The present invention relates to a method for selecting pharmacological compounds for selective inhibition of cancer cells comprising identifying a compound, determining the reduction potential (ER) of the compound, and selecting the compound which has a reduction potential from −1.1 to −0.8 volts. The invention also relates to a pharmacological compound comprising at least one cyanine dye or merocyanine dye, wherein the dye has at least one cationic substituent, wherein the dye has a reduction potential of from—1.1 to 0.8 volts, and wherein the pharmacological compound demonstrates selective inhibition of cancer cells.
Owner:CARESTREAM HEALTH INC
Anti-CD47 antibodies and methods of use
ActiveUS9650441B2Little and no hemagglutinationProgression moreImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsSolid tumorCD47
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Method for extracting and refining anthocyanin in black rice
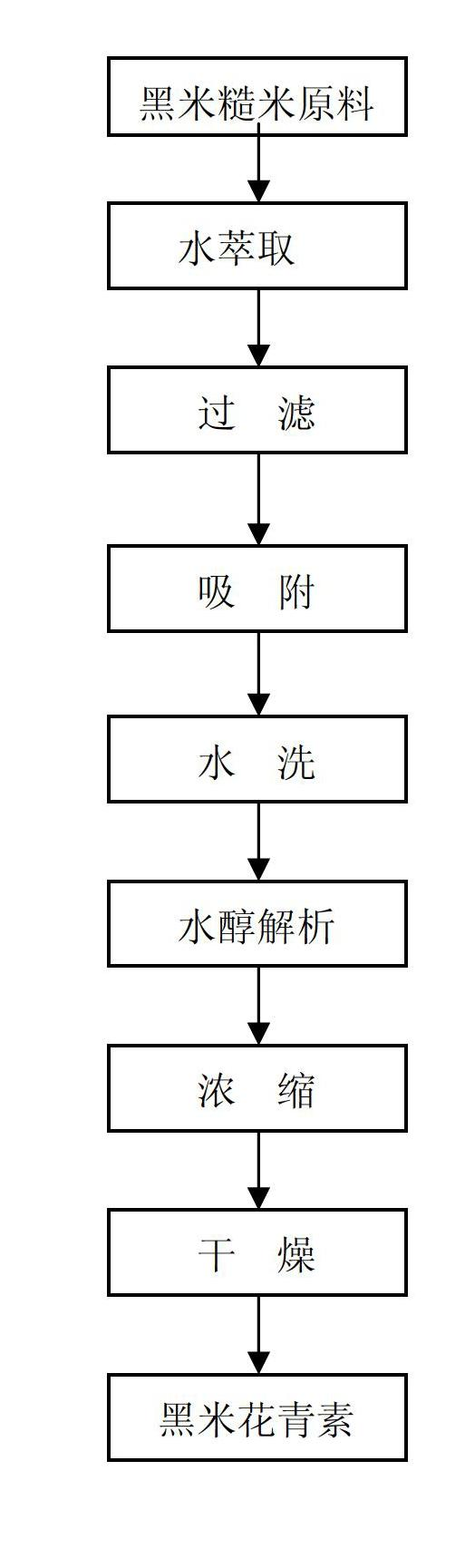
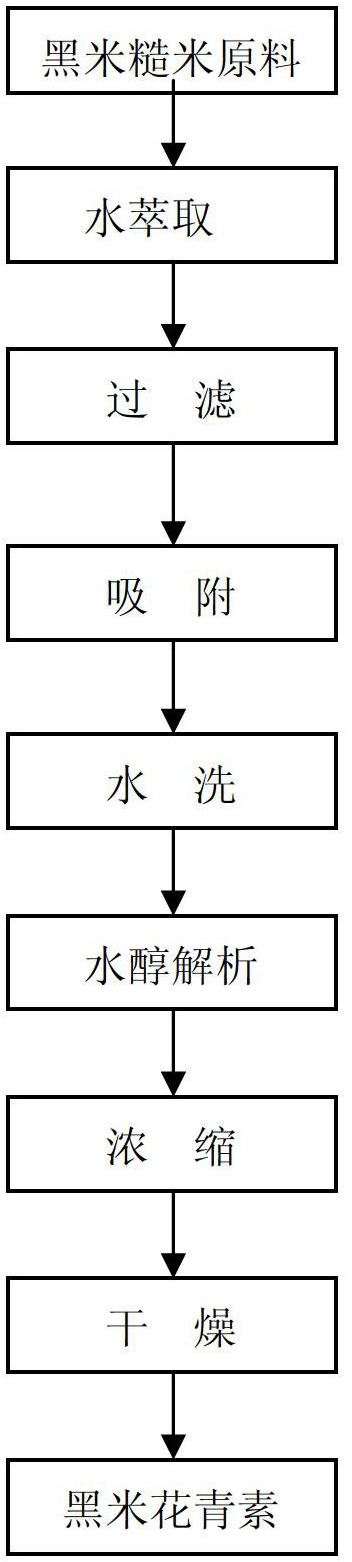
InactiveCN102659871AHigh purityImprove efficiencySenses disorderSugar derivativesAlcoholAqueous solution
The invention relates to a process for extracting and refining anthocyanin in coarse rice, rice bran of black rice. According to the technical scheme, the process comprises the following steps of: extracting raw materials by using an acidic aqueous solution under the condition of keeping temperature, filtering, mixing extracting solutions, adsorbing by macroporous adsorbent resin, washing the resin by using water, desorbing by using an alcohol aqueous solution with appropriate concentration, concentrating a desorbed solution until the relative density is 1.1 to 1.2, and performing spray drying or vacuum drying to obtain an anthocyanin extract, wherein the content of the anthocyanin is more than 25 percent.
Owner:魏有良
Methods and formulations for delivery of pharmacologically active agents
InactiveUS20060257326A1Eliminate side effectsHigh anticancer activityPowder deliveryBiocideActive agentIn vivo
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of a pharmaceutically active agent, wherein the agent is associated with a polymeric biocompatible material.
Owner:ABRAXIS BIOSCI LLC
Taxol enhancer compounds
InactiveUS20050009920A1High anticancer activityMinimal toxic side-effectsBiocideOrganic chemistryArylDiluent
Disclosed is a compound represented by the Structural Formula (I): Y is a covalent bond, a phenylene group or a substituted or unsubstituted straight chained hydrocarbyl group. In addition, Y, taken together with both >C═Z groups to which it is bonded, is a substituted or unsubstituted aromatic group. Preferably, Y is a covalent bond or —C(R7R8)—. R1 and R2 are independently an aryl group or a substituted aryl group, R3 and R4 are independently —H, an aliphatic group, a substituted aliphatic group, an aryl group or a substituted aryl group. R5-R6 are independently —H, an aliphatic group, a substituted aliphatic group, an aryl group or a substituted aryl group. R7 and R8 are each independently —H, an aliphatic or substituted aliphatic group, or R7 is —H and R8 is a substituted or unsubstituted aryl group, or, R7 and R8, taken together, are a C2-C6 substituted or unsubstituted alkylene group. Z is ═0 or ═S. Also disclosed are pharmaceutical compositions comprising the compound of the present invention and a pharmaceutically acceptable carrier or diluent. Also disclosed is a method of treating a subject with cancer by administering to the subject a compound of Structural Formula (I) in combination with taxol or an analog of taxol.
Owner:SYNTA PHARMA CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com