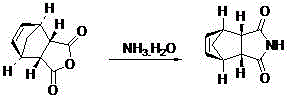Patents
Literature
313 results about "Piperazidine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
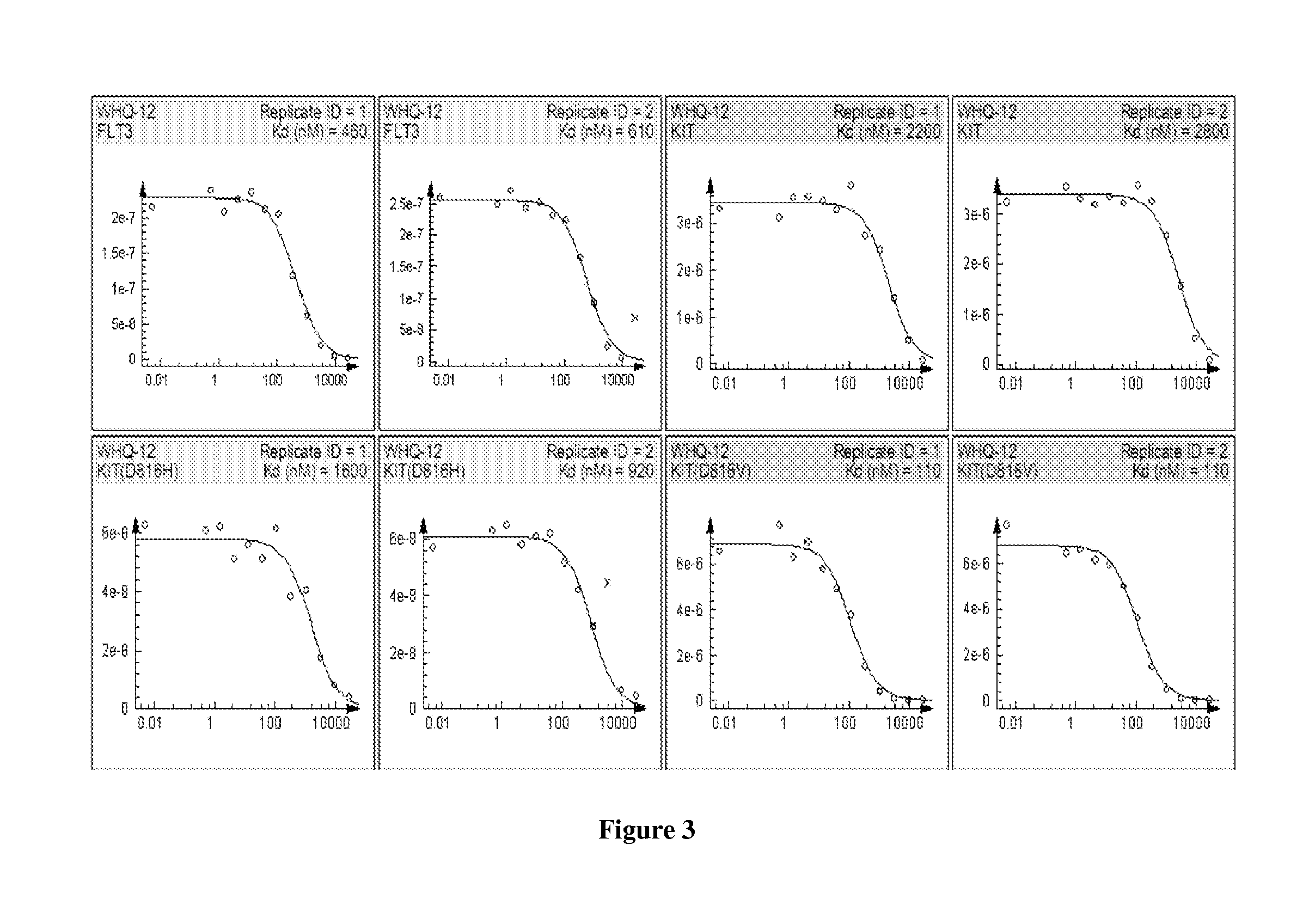
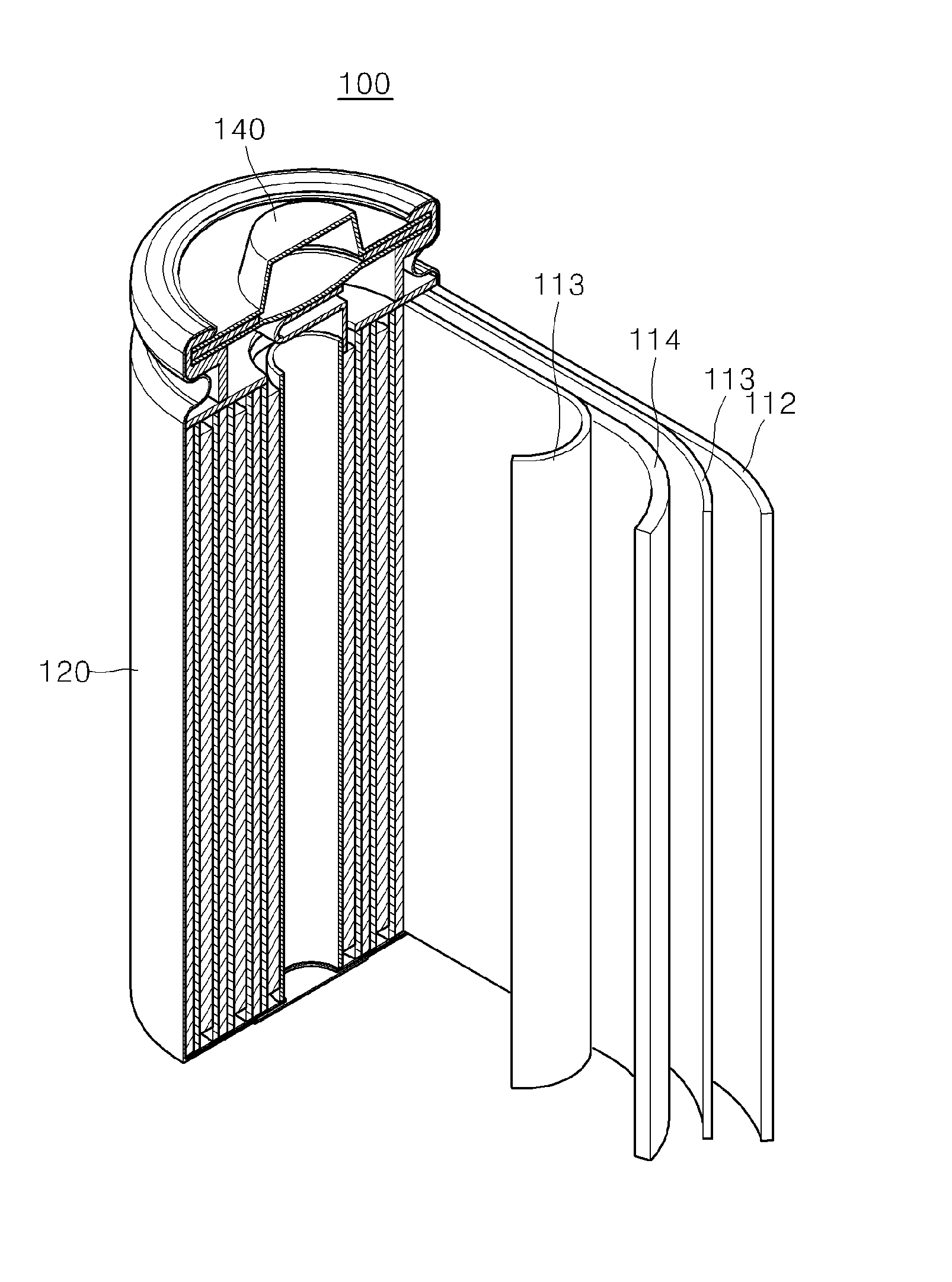
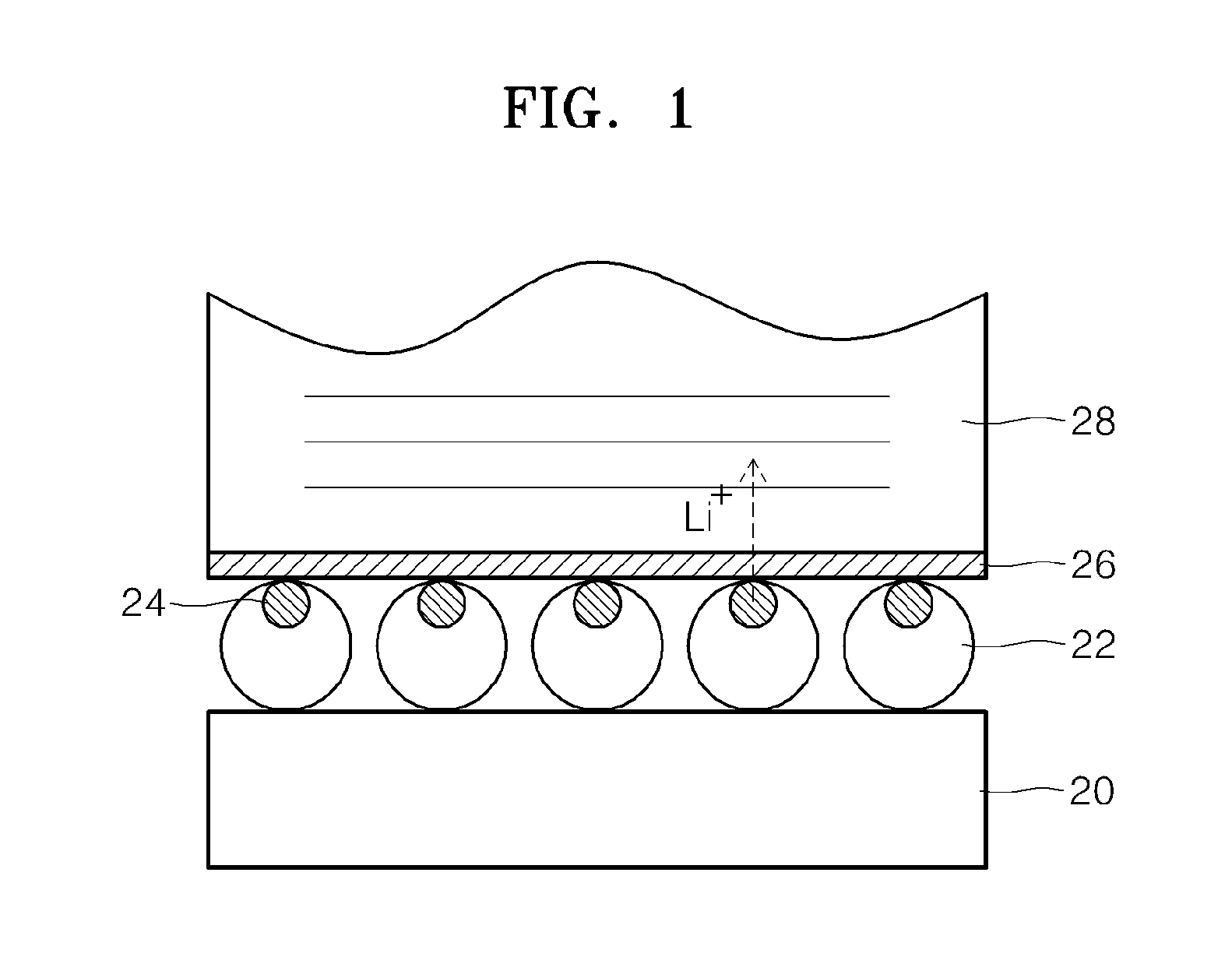
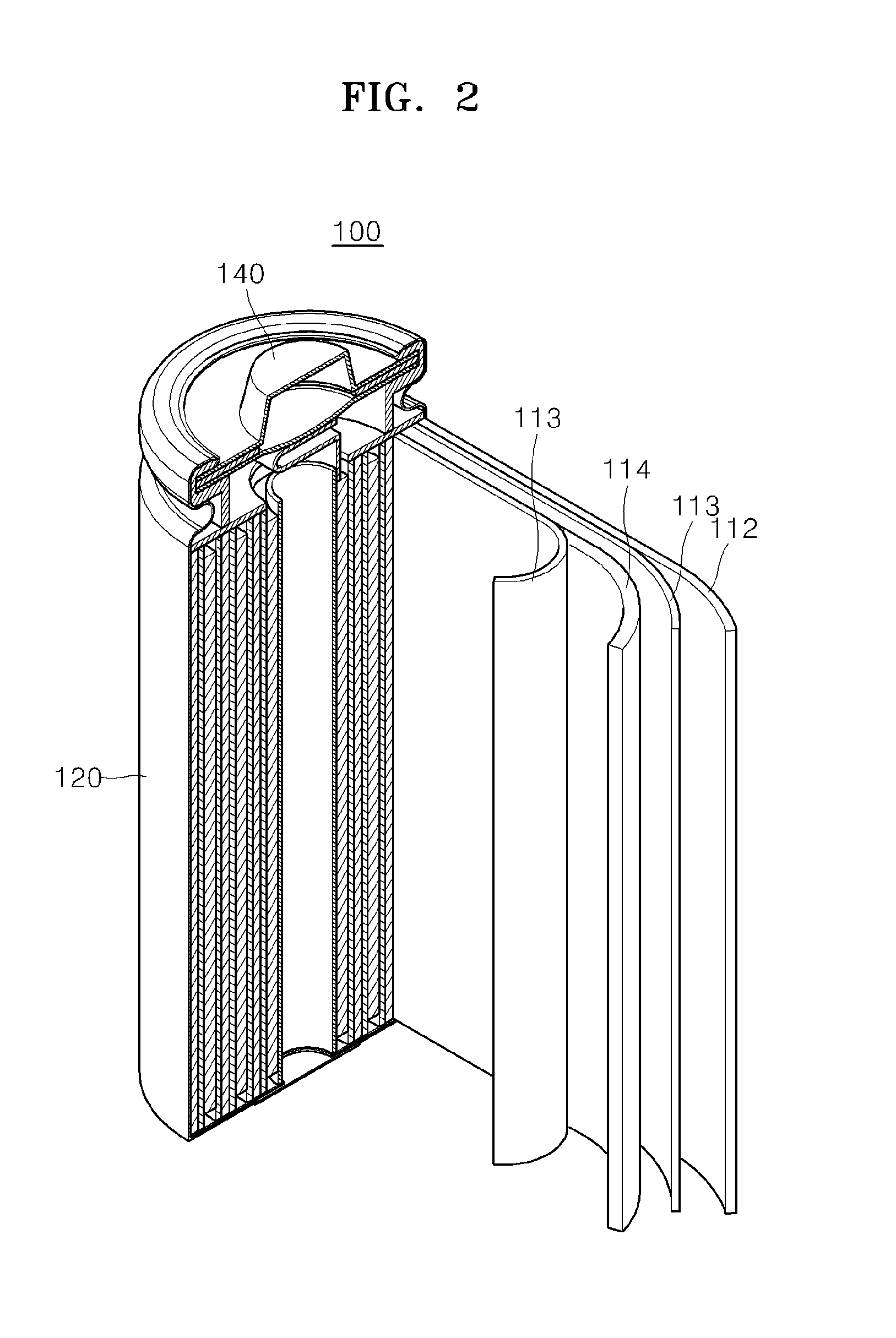
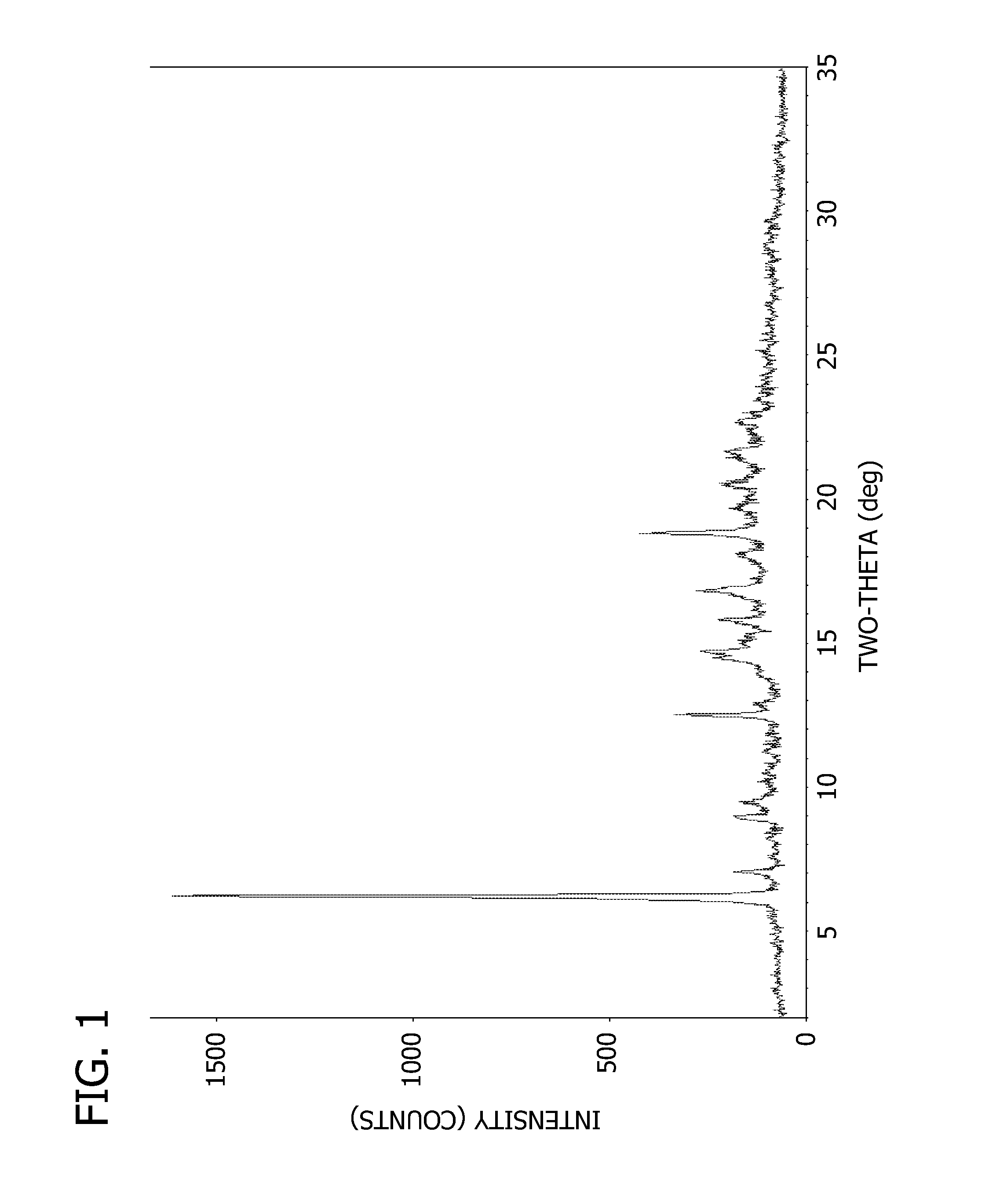
Electrolyte for lithium secondary battery and lithium secondary battery using the same
ActiveUS20150064578A1Excellent characteristicsImprove life characteristicsCell electrodesOrganic electrolyte cellsOrganic solventElectrical battery
An electrolyte for a lithium secondary battery, the electrolyte including: a lithium salt; a non-aqueous organic solvent; and a piperazine derivative represented by Formula 1 having an oxidation potential lower than an oxidation potential of the non-aqueous organic solvent by about 2 V to about 4 V:wherein, in Formula 1, X, Y, and R1 to R4 are defined in the specification.
Owner:SAMSUNG ELECTRONICS CO LTD
Salts and crystalline forms of an apoptosis-inducing agent
Salts and crystalline forms of 4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)-N-({3-nitro-4-[(tetrahydro-2H-pyran-4-ylmethyl)amino]phenyl}-sulfonyl)-2-(1H-pyrrolo[2,3-b]pyridin-5-yloxy)benzamide are suitable active pharmaceutical ingredients for pharmaceutical compositions useful in treatment of a disease characterized by overexpression of one or more anti-apoptotic Bcl-2 family proteins, for example cancer.
Owner:ABBVIE INC
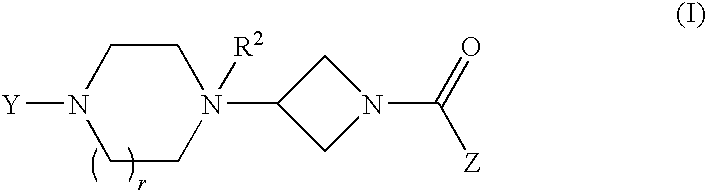
Piperazine derivative, preparation thereof and use thereof in medicine
InactiveCN101468988AOrganic active ingredientsOrganic chemistryDipeptidyl peptidasePerylene derivatives
The invention relates to piperazidine derivatives shown in a general formula (I), a preparation method thereof, a medicine composition containing the derivatives, and use of the medicine as a therapeutic agent, in particular dipeptidyl peptidase-4 inhibitor, wherein the definitions of various substitutional groups in the general formula (I) are the same as those in the description.
Owner:SHANGHAI HENGRUI PHARM CO LTD
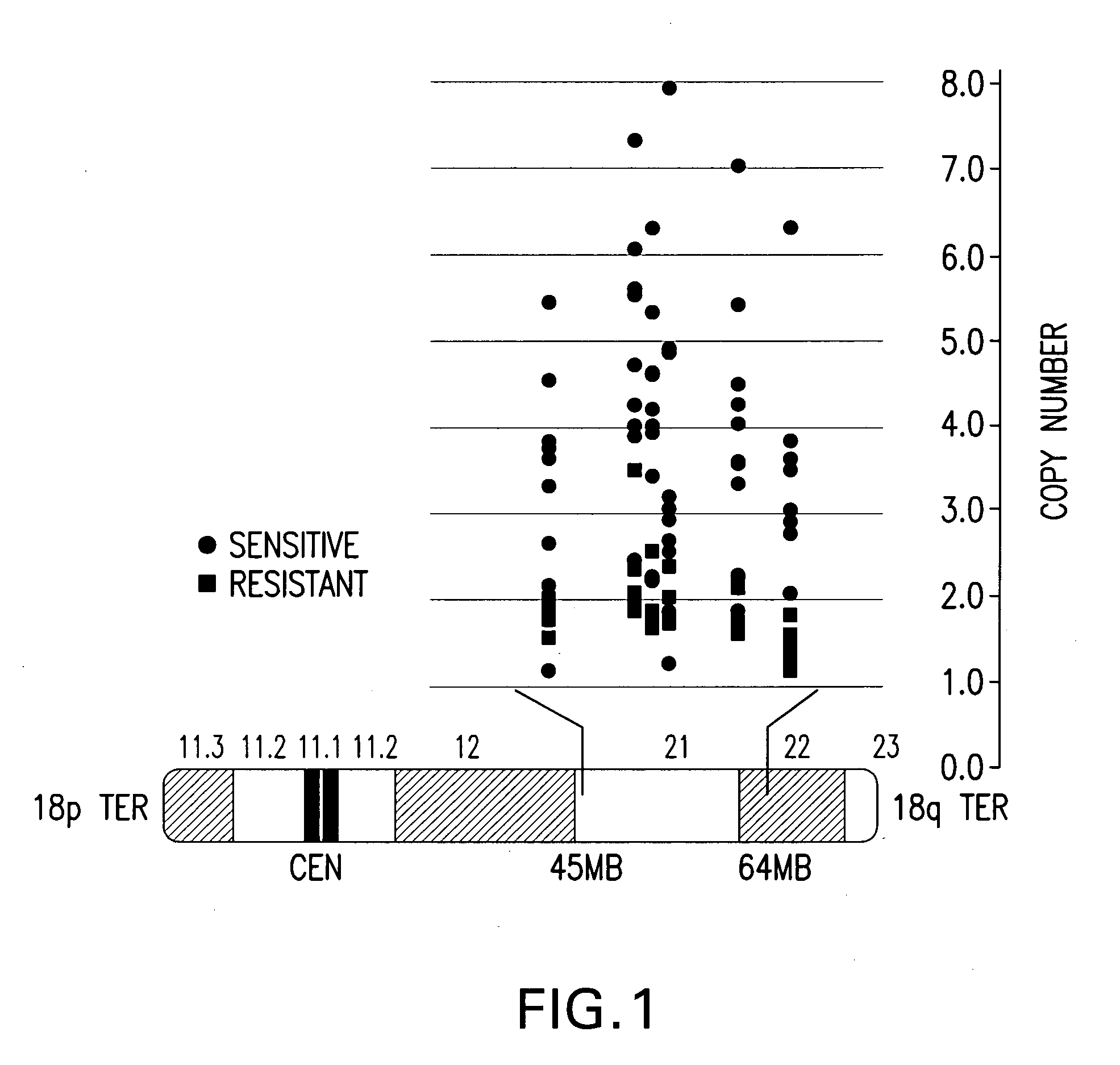
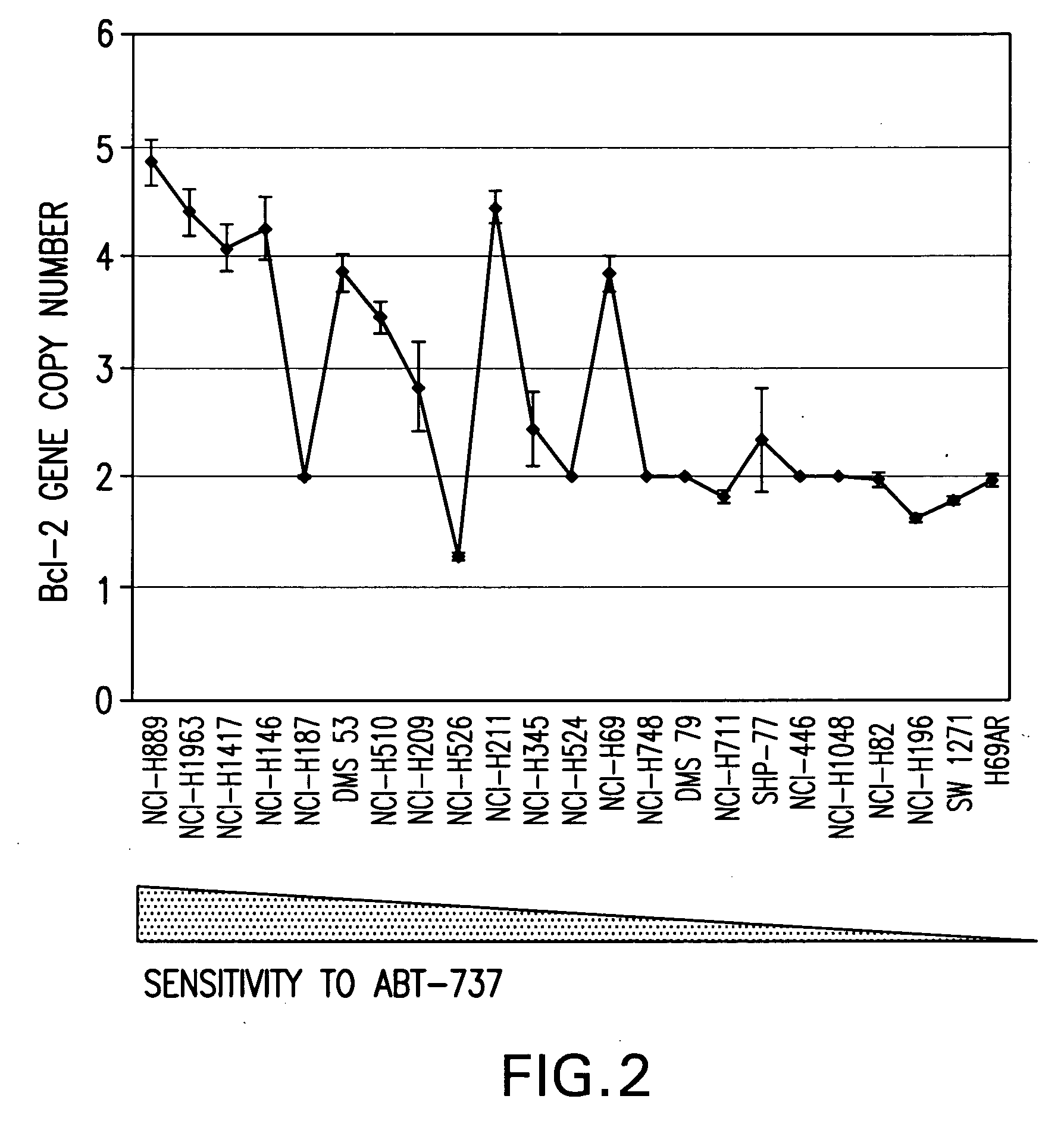
Companion diagnostic assays for cancer therapy
InactiveUS20080193943A1Promote stratificationParticular utilitySugar derivativesMicrobiological testing/measurementPhenacylOncology
A method for classifying cancer patients as eligible to receive cancer therapy with a small molecule inhibitor of Bcl-2 comprising determination of the presence or absence in a patient tissue sample of chromosomal copy number status at the chromosomal locus 13q14 comprising the microRNA's miR-15a and miR-16-1 or at the chromosomal locus 11q23.1 comprising the microRNA miR-34c. The classification of cancer patients based upon the presence or absence of 13q14 loss or gain allows better selection of patients to receive chemotherapy with a small molecule Bcl-2 inhibitor such as N-(4-(4-((2-(4-chlorophenyl)-5,5-dimethyl-1-cyclohex-1-en-1-yl) methyl)piperazin-1-yl)benzoyl)-4-(((1R)-3-(morpholin-4-yl)-1-((phenylsulfanyl) methyl)propyl)amino)-3-((trifluoromethyl)sulfonyl)benzenesulfonamide, and for monitoring patient response to this therapy.
Owner:ABBOTT LAB INC
Dioxygen piperazidine compounds and preparation method and usage thereof
The invention relates to preparation methods and uses of two dioxypiperazine compounds. The invention adopts aspergillus effuses H1-1 from root mud of Fujian mangrove forest to product novel structural dioxypiperazine compounds. Tests prove that: the compounds can be used as cellular breeding depressant or anti-tumor agent.
Owner:OCEAN UNIV OF CHINA +1
Non-halogen flame-retardant synthetic resin composition
ActiveUS20110092622A1Improve flame retardant performanceMaintain good propertiesDyeing processHalogenHydrogen atom
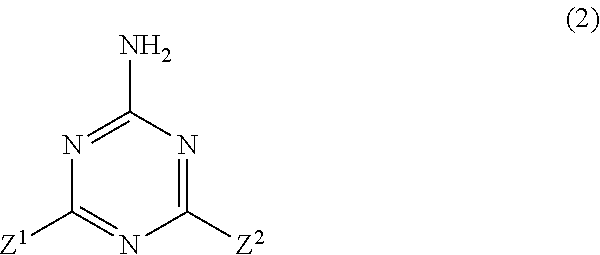
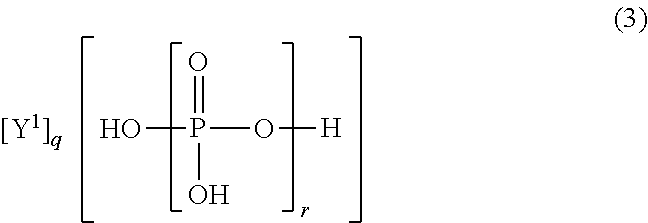
The present invention is the non-halogen flame-retardant synthetic resin composition characterized in that the said synthetic resin contains the (poly)phosphate compounds represented by the general formulae (1) and (3), described in Claim 1, and layered silicate;n in the general formula (1) represents a number of 1˜100, X1 is ammonia, or a triazine derivative represented by the general formula (2) described in Claim 1, p is a number satisfying the relational expression of 0<p≦n+2;Z1 and Z2 in the general formula (2) are groups selected independently from groups consisting of a —NR5R6 group, a hydroxyl group or the like, and the above R5 and R6 are a hydrogen atom, an alkyl group having 1-6 carbon atoms, or a methylol group independently.r in the general formula (3) represents a number of 1-100, Y1 is a diamine containing a [R1R2N(CH2)mNR3R4], piperazine or a diamine containing a piperazine ring, R2˜R4 are a hydrogen atom, an alkyl group having 1-5 carbon atoms independently. m is an integer of 1-10, q is a number satisfying the relational expression of 0<q≦r+2.
Owner:ADEKA CORP
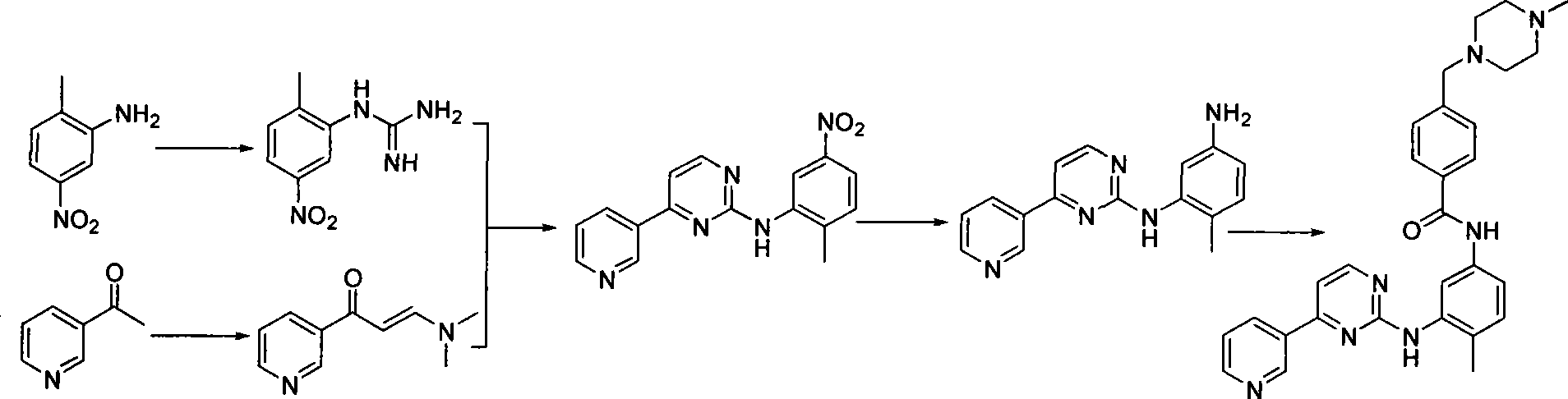
Process for synthesizing imatinib
The invention discloses a method for synthesizing imatinib. The method comprises that: N-(4-methyl-3-3-aminophenyl)-4-(4-methyl-piperazinyl-1-methyl)-benzamide is used as a raw material, and reacts with 4-methyl-(3-pyridyl)-2-pyrimidone under actions of a polypeptide condensation agent and an organic alkali so as to generate the imatinib. The method has the advantages of mild reaction conditions, easy operation, high reaction yield and suitability for industrialized production.
Owner:FUJIAN SOUTH PHARMA CO LTD
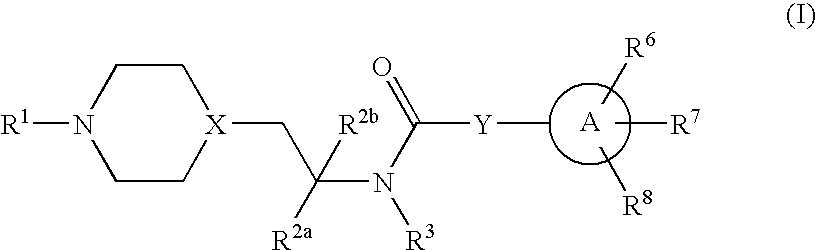
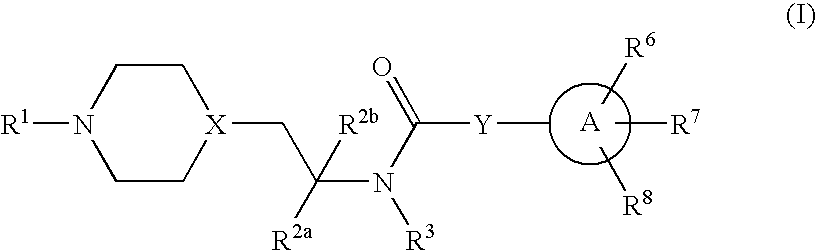
Inhibitors of P2X3
Compounds of formula 1 are modulators of P2X3 useful for the treatment of pain and genitourinary, gastrointestinal, and respiratory disorders:whereinR1 is —C(═S)CH3, pyridyl, pyrimidinyl, pyrazinyl, thiazolyl, furyl, furylcarbonyl, acetyl, or carbamoyl; R2a and R2b are independently H, methyl, or ethyl; R3 is H or methyl; Y is a bond, —(CR4R5)n— or —CR4═CR5—; wherein R4 and R5 are each independently H or methyl and n is 1 or 2; X is N or CH; A is phenyl, 5-membered heterocyclyl, or 6-membered heterocyclyl; R6, R7 and R8 are each independently H, halo, lower alkyl, cycloalkyl, alkylthio, alkylthio-lower alkyl, alkylsulfonyl-lower alkyl, di(lower alkyl)amino-lower alkyl, morpholinyl-lower alkyl, 4-methyl-piperazinyl-methyl, trifluoromethyl, pyridyl, tetrazolyl, thiophenyl, phenyl, biphenyl, or benzyl (where thiophenyl, phenyl and benzyl are substituted with 0-3 lower alkyl, halo, sulfonamido, trifluoromethyl, lower alkoxy or lower alkylthio) or R6 and R7 together form a 5-membered or 6-membered carbocyclic or heterocyclic ring substituted with 0-3 substituents selected from the group consisting of lower alkyl, lower alkoxy, oxo, halo, thiophenyl-lower alkyl, phenyl, benzyl (where phenyl and benzyl are substituted with 0-3 lower alkyl, halo, sulfonamido, trifluoro-methyl, lower alkoxy, lower alkylthio, amino-lower alkyl, lower alkylamino-lower alkyl, or di(lower alkyl)amino-lower alkyl); and pharmaceutically acceptable salts thereof; wherein when R1 is pyrimidin-2-yl, X is N, Y is a bond and A is oxazol-5-yl the carbon atom at position 4 in said oxazol-5-yl is not substituted by propyl when the carbon atom at position 2 in said oxazol-5-yl is substituted by substituted phenyl and the carbon atom at position 4 in said oxazol-5-yl is not substituted by phenyl when the carbon atom at position 2 is substituted by unsubstituted or substituted phenyl.
Owner:ROCHE PALO ALTO LLC
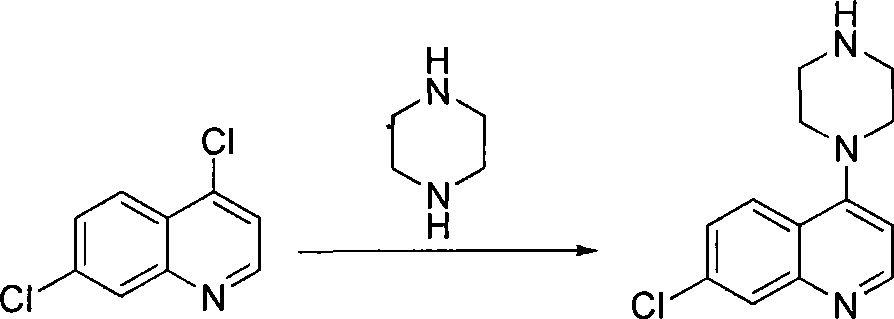
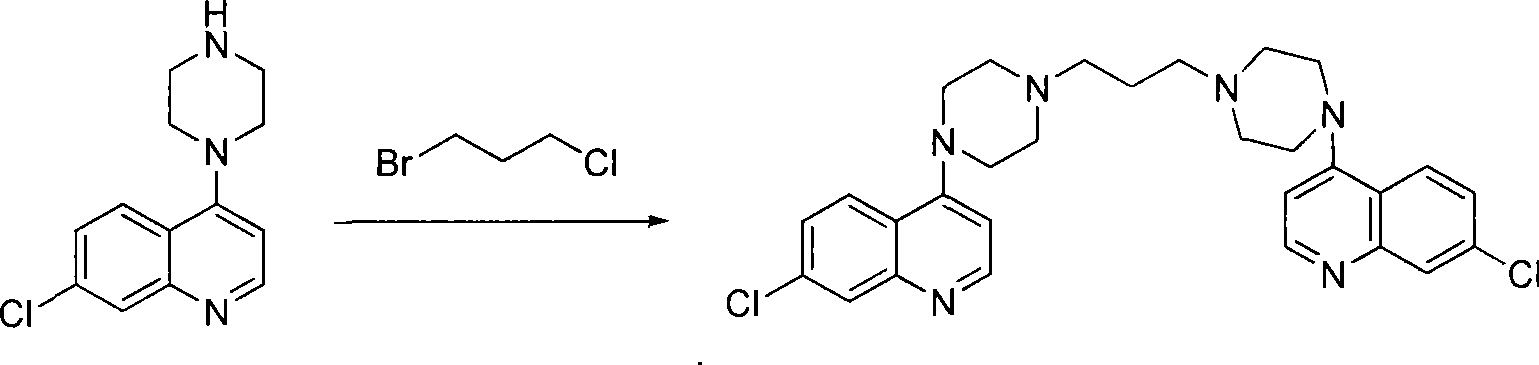
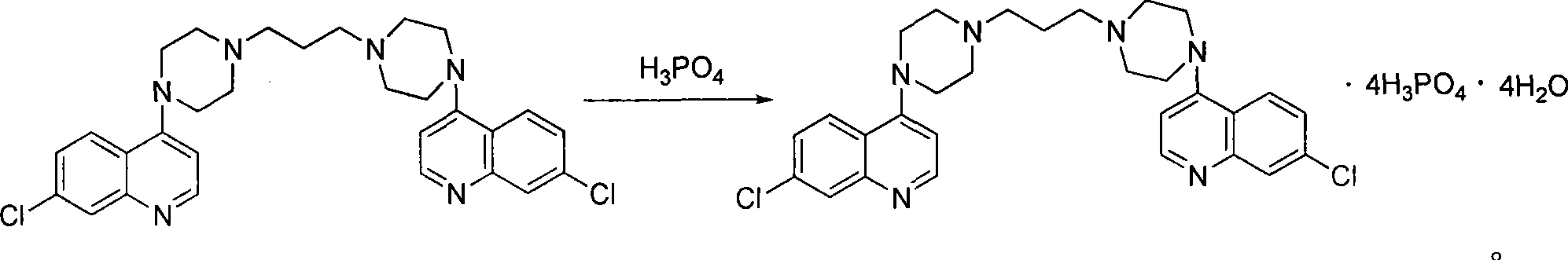
Preparation of piperaquini phosphatis
The invention discloses a method for preparing piperaquine phosphate. 4,7-dichloroquinoline is taken as an initial raw material and is subjected to condensation reaction with anhydrous piperazidine first to obtain 7-chloro-4-(1-piperazinyl)quinoline, then the 7-chloro-4-(1-piperazinyl)quinoline is subjected to condensation reaction with 1, 3-bromochloropropane to obtain piperaquine, and finally the piperaquine is salified with phosphorous acid to obtain the piperaquine phosphate. The method has a simple process, is easy to purify intermediate products and a final product, has high product yield and good quality, avoids the use of toxic reagents, has small pollution to the environment and low production cost, and is suitable for industrialized production.
Owner:CHONGQING KANGLE PHARMA
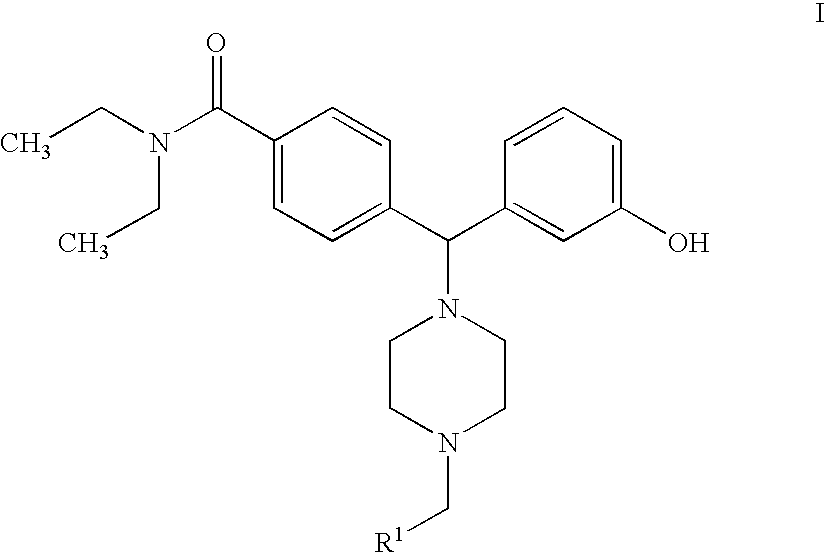
Hydroxyphenyl-piperazinyl-methyl-benzamide derivatives for the treatment of pain
The present application describes compounds of general formula Iwhere R<1 >is selected from any one of pyridinyl, thienyl, furanyl, imidazolyl, and triazolyl;and where each R<1 >heteroaromatic ring may optionally and independently be further substituted by 1, 2 or 3 substituents selected from straight and branched C1-C6 alkyl, NO2, CF3, C1-C6 alkoxy, chloro, fluoro, bromo, and iodo. The substitutions on the heteroaromatic ring may take place in any position on the ring system. The invention also includes enantiomers, salts and pharmaceutical compositions containing the compounds. The compounds may be used in treating patients for pain.
Owner:ASTRAZENECA AB
Pharmaceutical compositions for the treatment of rhinitis
InactiveUS6489329B2Increasing possible adverse effects of the treatmentImproved efficiency over each pharmaceutical substanceBiocideOrganic active ingredientsAcetic acidPseudoephedrine
Owner:UCB PHARMA SA
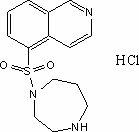
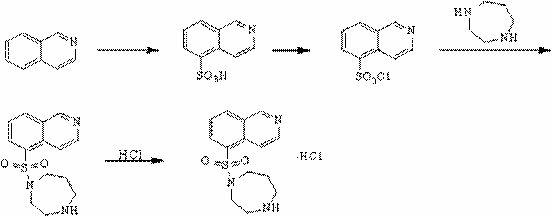
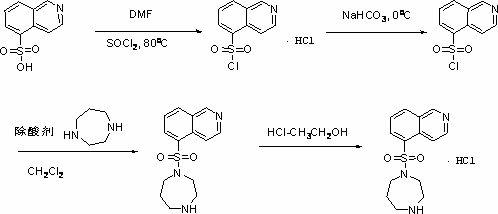
Synthesis and preparation method of fasudil hydrochloride
InactiveCN102603715AReduce usageReduce manufacturing costOrganic chemistrySulfonyl chlorideIce water
The invention discloses a synthesis and preparation method of fasudil hydrochloride, which comprises the steps of taking 5-isoquinoline sulfoacid as raw material, refluxing in thionyl chloride to obtain 5-isoquinoline sulfonyl chloride hydrochloride, adding dichloromethane after dissolving with ice water, adjusting pH value of the solution to be neutral and performing liquid eparation; washing organic phase with water and edible salt, drying and filtering to obtain dichloromethane solution of 5-isoquinoline sulfonyl chloride; performing reaction of the solution with high purity piperazine in the presence of other alkaline reagents, washing the reaction solution with hydrochloric acid aqueous solution and sodium hydroxide aqueous solution after reaction, and extracting the washing solution respectively with dichloromethane; combining organic phases, washing with water, drying and filtering, and dropwise adding saturated hydrogen chloride ethanol solution to separate out crude product of fasudil hydrochloride; and recrystalizing the crude product with ethanol aqueous solution to obtain fasudil hydrochloride. The method provided by the invention greatly reduces the usage amount of high purity piperazine that is expensive by using deacidifying agents that are low in cost so as to reduce the preparation cost of fasudil hydrochloride effectively.
Owner:NOWA PHARMA
Heteroaromatic and aromatic piperazinyl azetidinyl amides as monoacylglycerol lipase inhibitors
Owner:JANSSEN PHARMA NV
Piperazidine or homopiperazine oxalyl hydrazine class compound, preparation and use thereof
ActiveCN101565409AHigh anticancer activityGrowth inhibitionOrganic chemistryAntineoplastic agentsHydrazine compoundPiperazine
The invention relates to a novel piperazidine or homopiperazine oxalyl hydrazine class compound as represented by formula I, wherein each symbol has meanings defined in the description. The invention further relates to the preparation of the piperazidine or homopiperazine oxalyl hydrazine class compound, use of the piperazidine or homopiperazine oxalyl hydrazine class compound in preparing medicament for treating and / or preventing tumor and / or cancer, medicament composition containing the piperazidine or homopiperazine oxalyl hydrazine class compound. The piperzzidine or homopiperazine oxalyl hydrazine class compound has effective anti-tumor activity.
Owner:DONGGUAN ZHENGXING BEITE MEDICINE TECH CO LTD
Piperazinone-substituted tetrahydro-carboline MCH-1 antagonists, methods of making, and uses thereof
InactiveUS8697700B2High affinityHigh indexNervous disorderOrganic chemistryMelanin-concentrating hormoneCombinatorial chemistry
The present invention relates to piperazinone-substituted tetrahydrocarboline derivatives of formula (I):having the substituents as described herein which are melanin-concentrating hormone (MCH-1) receptor antagonists. The present invention also relates to pharmaceutical compositions including these compounds, and methods of preparation and use thereof.
Owner:ALBANY MOLECULAR RESEARCH INC
Aralkyl piperidine (piperazidine) derivate and use thereof in mental disease treatment
InactiveCN101302214AHigh affinityHigh antagonistic activityOrganic active ingredientsNervous disorderDiseaseSchizophrenia
The invention discloses an aralkyl piperidine (oxazine) derivative and an application of the derivative in treating neurological and mental diseases. Pharmacological tests show that the derivative has a good effect of resisting schizophrenia and little toxicity. The derivative is free alkali or salt of a compound having the following structure general formula.
Owner:JIANGSU HENGYI PHARMA +1
Catalyst for producing piperazidine and triethylenediamine and preparation method thereof
InactiveCN103240116AOvercome the defect of easy poisoning and inactivationWeak acid strengthOrganic chemistryMolecular sieve catalystsPeristaltic pumpWater vapor
The invention relates to a catalyst for producing piperazidine and triethylenediamine and a preparation method thereof, and belongs to the technical field of a zeolite molecular sieve catalyst. The preparation method comprises the following steps of: sequentially carrying out water vapor treatment, NaOH solution soaking, 600-800 DEG C roasting on H-ZSM-5; then adding to a straight type condensation tube; sealing both ends of a molecular sieve by respectively using filter papers; plugging rubber plugs with stainless steel tubes on both ends of the condensation tube; circularly accessing a metal nitrate solution into the condensation tube through a peristaltic pump; carrying out 60-110 DEG C oil bath on the shell of the condensation tube; taking out the molecular sieve, carrying out suction filtration, placing a filter cake into a quartz glass tube, placing into liquid nitrogen in a sealing way, and taking out the filter cake for drying; and placing into muffle furnace for roasting. The catalyst disclosed by the invention keeps high selectivity on the triethylenediamine, keeps the activity still good with the change of time and has long life.
Owner:BEIJING UNIV OF CHEM TECH
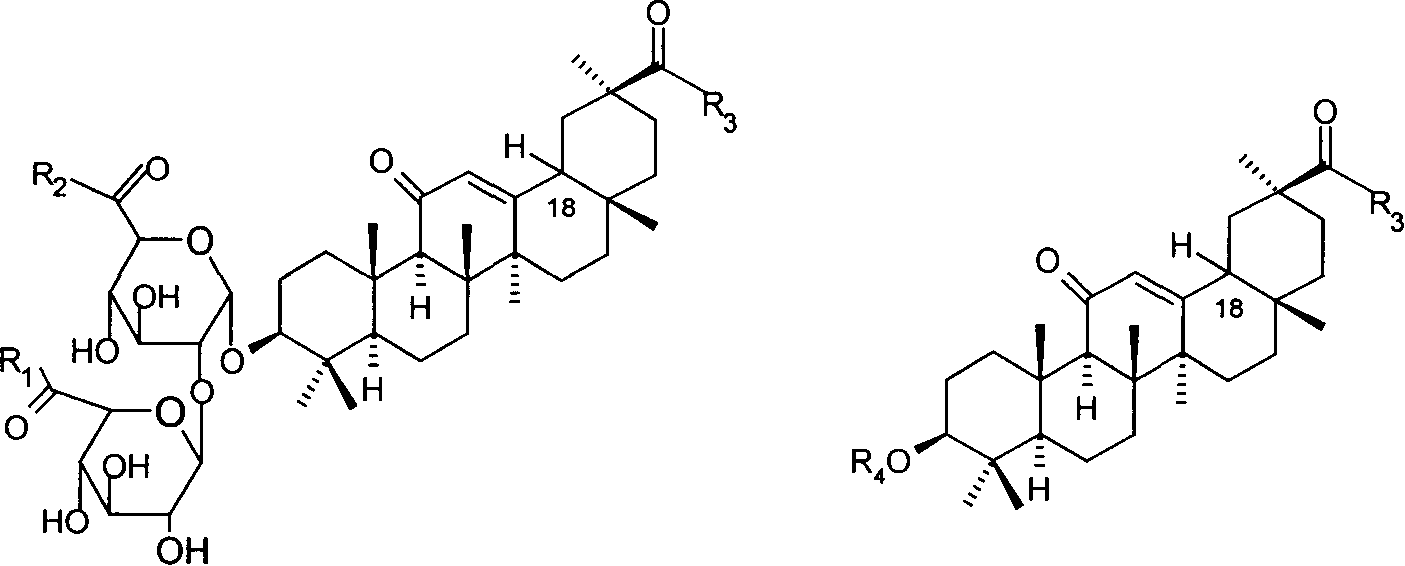
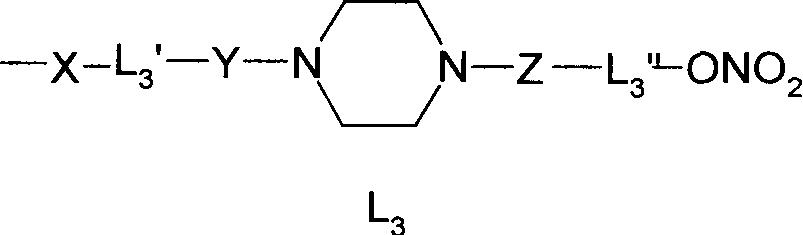
Nitrate derivatives of glycyrrhetic acid and glycyrrhetinic acid and pharmaceutical use thereof
Disclosed are nitrate derivant of glycyrrhizic acid or glycor represented by formula Ia and Ib and its nontoxic salt which can be accepted in the sphere of pharmacy, their precoss for preparing, and components and usage of the drugs which contain these compounds.Thereinto, R1, R2 and R3 stand for hydroxyl or -X-L-oxo-oxonitryl (X stands for oxygen,imino; L stands for alkyl with 2-6 of canbon atoms, substituted alkyl or cycloalkyl, hydroxyl amino acid residue or N,Ní»-disubstituted piperazine); R3 stands for hydrogen, oxonitryl or carbonyl -Lí»oxo-oxonitryl (Lí»stands for alkyl with 1-6 of canbon atoms, substituted alkyl or cycloalkyl, aralkyl, N,Ní»-disubstituted piperazine); in formula Ib, R1, R2 and R3 may be the same or different, but in which at least one of them contains -X-L-oxo-oxonitryl; in formula Ib, at least one of R3 or R4 must contain oxonitryl. In formula Ia and Ib, hydrogen of 18-carbon can be alpha-isomer or beta-isomer.
Owner:BEIJING MEIBEITA DRUG RES
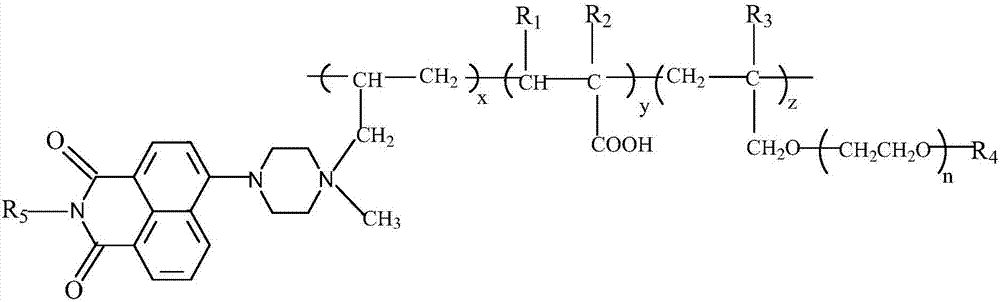
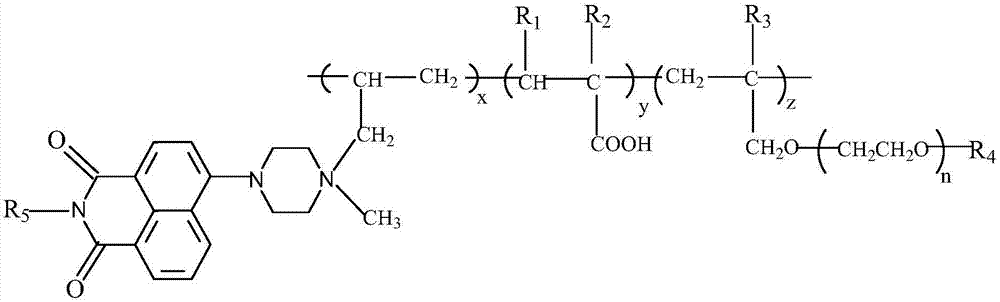
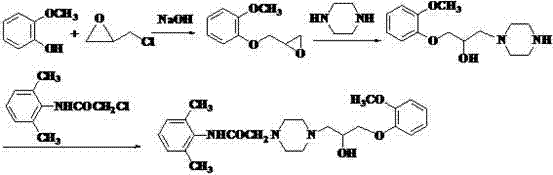
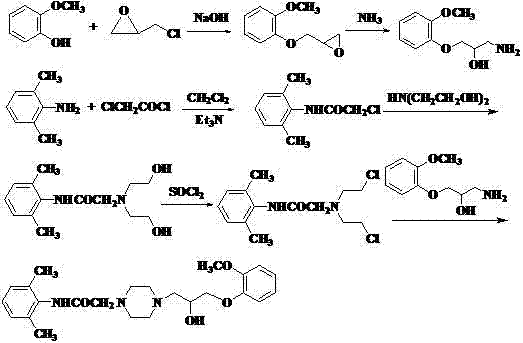
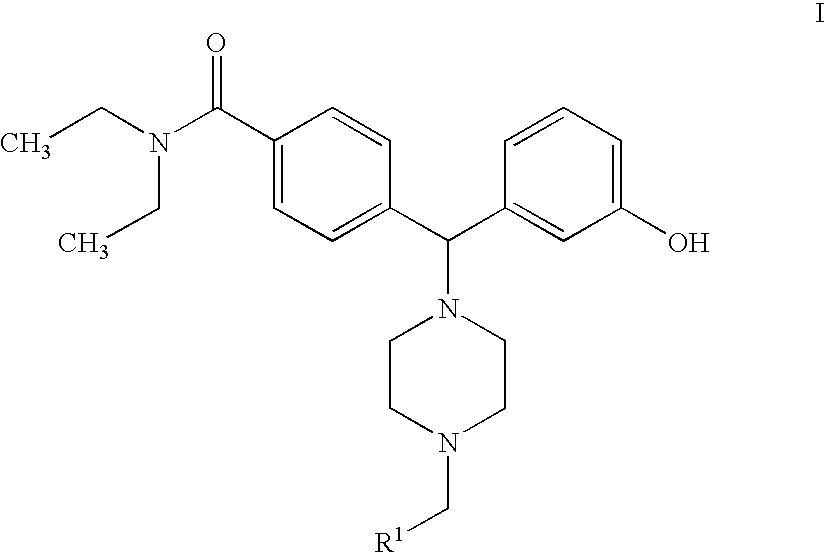
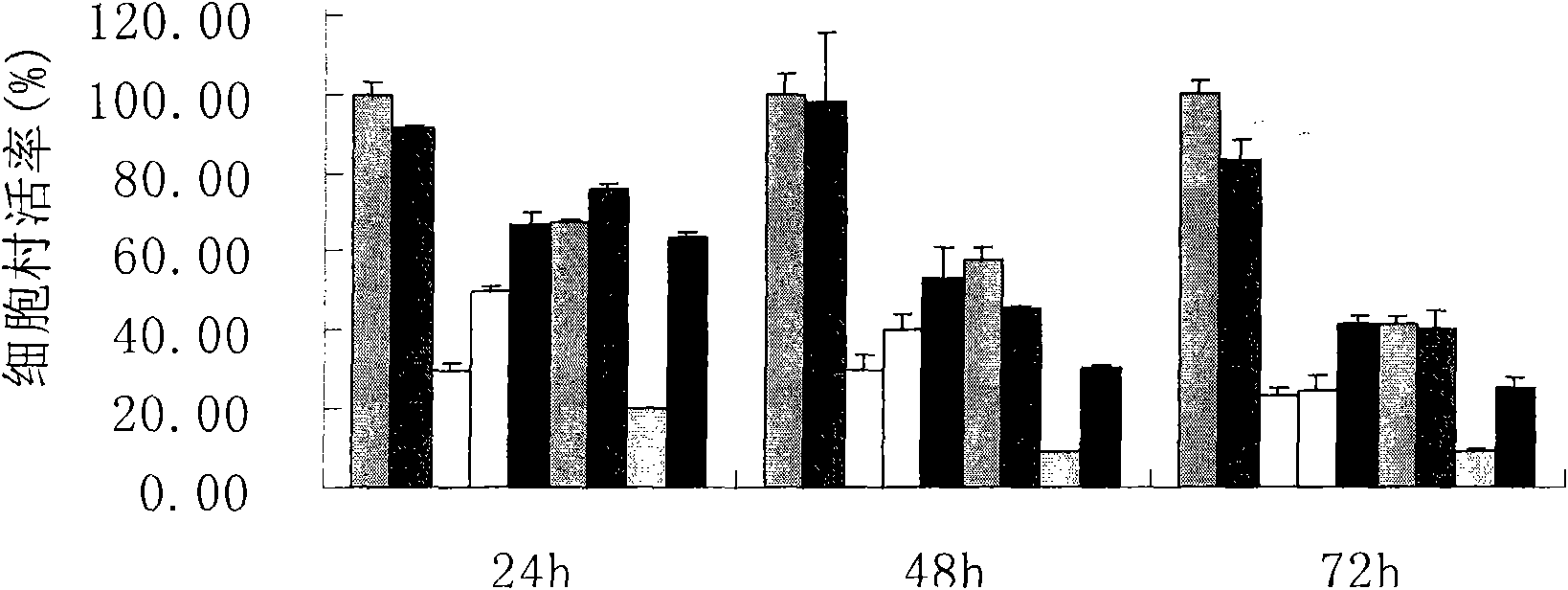
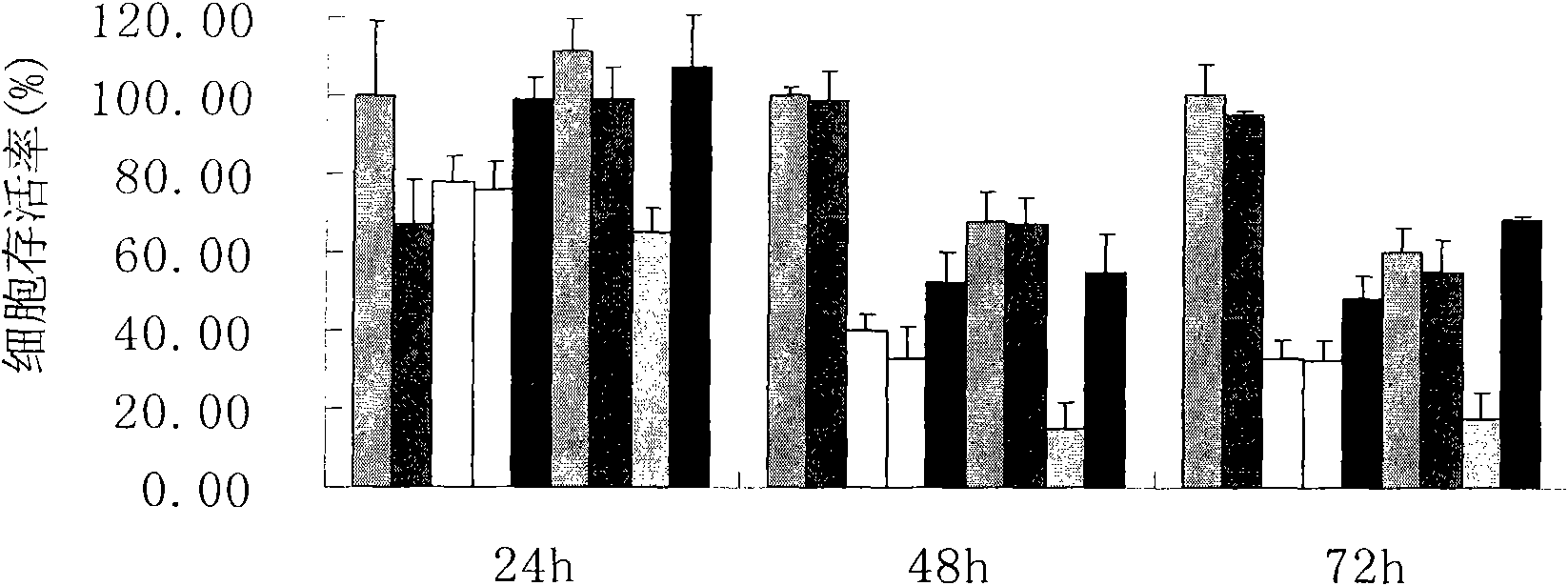
4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative and preparation method thereof
ActiveCN103073524AHigh affinityEffective treatmentNervous disorderOrganic chemistryReaction intermediateStructural formula
The invention discloses a phenyl piperazidine heterocyclic medicinal compound. The compound has high affinity to a dopamine D3 receptor, so that the compound can be used for treating addiction to and dependence on medicines such as cocaine, and a central nervous system disorder relevant to the addiction and the dependence. The compound is a 4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative with a structural formula as Formula (1) as shown in the specification. A synthetic method of the derivate comprises the steps that substituted aniline and 2-(beta-chloroethyl) amine hydrochloride reacts in a solvent by taking inorganic base as an acid-binding agent to form corresponding substituted phenyl piperazidine hydrochloride 1; substituted phenyl piperazidine hydrochloride 1 and N-(delta-bromobutyl) phthalimide react in acetonitrile by taking K2CO3 as an acid-binding agent and under catalysis of KI to form a reaction intermediate 2; the intermediate 2 is subjected to hydrazinolysis to form an intermediate 3; and the intermediate 3 and an intermediate 5 are condensed by taking triethylamine as an acid-binding agent and a catalyst to form a target product I. The intermediate 5 is obtained in a manner that triphosgene and substituted aromatic phenol conduct partial condensation reaction in methylene chloride.
Owner:宁波市微循环与莨菪类药研究所 +1
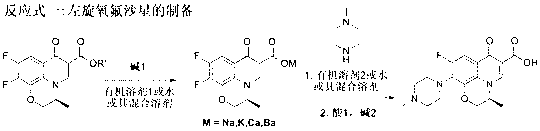
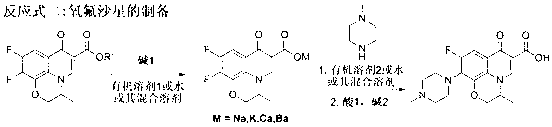
One-step synthesizing method of levofloxacin and ofloxacin
The invention provides a one-step synthesizing method of levofloxacin and ofloxacin. According to the invention, S-9,10-difluoro-2,3-dihydro-3-methyl-7-oxygen-7H-pyrido[1,2,3-delta]-[1,4]-benzoxazine-6-carboxylic acid ester or 9,10-difluoro-2,3-dihydro-3-methyl-7-oxo-7H-pyrido[1,2,3-delta]-[1,4]-benzoxazine-6-carboxylic acid ester is adopted as a raw material; the raw material is subjected to a reaction with alkali in an organic solvent or water or a mixed solvent of an organic solvent and water, such that a corresponding carboxylic acid salt is formed; the solvent is directly removed by evaporation after a hydrolysis process; The product is directly added into N-methylpiperazine in a form of a carboxylic acid salt; and a piperazine concentration reaction is carried out, such that levofloxacin or ofloxacin is obtained. The method provided by the invention is simple to operate. With the method, a hydrolysis and then acid adjusting process is not needed, reaction cost is reduced, production period is short, pollution is low, raw material utilization rate is high, the method is economical and simple, and the yield and purity of obtained levofloxacin and ofloxacin are high.
Owner:ZHEJIANG UNIV +1
High piperazine acetydrazide derivatives, and preparation and use thereof
ActiveCN101503394AHigh anticancer activityGrowth inhibitionOrganic active ingredientsOrganic chemistryAntitumor activityPiperazine
The invention relates to a novel high piperazidine acethydrazide-type derivative indicated by formula I or II. In the formula, the definitions of various symbols are the same as those in the Description. The invention also relates to a method for preparing the high piperazidine acethydrazide-type derivative, the usage of the high piperazidine acethydrazide-type derivative in preparing medicines for curing and / or preventing tumors and / or cancers, and a pharmaceutical composition containing the high piperazidine acethydrazide-type derivative. The high piperazidine acethydrazide-type derivative has effective antitumor activity.
Owner:DONGGUAN ZHENGXING BEITE MEDICINE TECH CO LTD
Piperazinylpyrimidine analogues as protein kinase inhibitors
The invention provides novel compounds based on piperazinylpyrimidine derivatives to be used as protein kinase inhibitors. The compounds may be useful in treating or preventing different cellular proliferation disorders, such as cancer. The present invention also provides methods of preparing these compounds, and methods of using the same.
Owner:UNIVERSITY OF THE PACIFIC
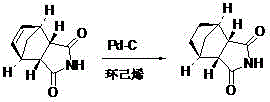
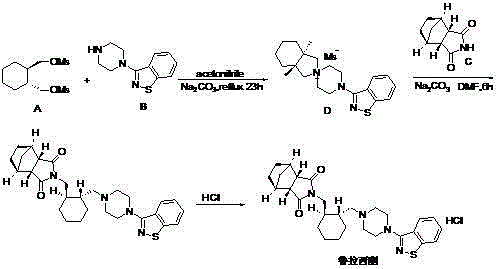
Preparation method of lurasidone
The invention provides a preparation method of lurasidone. On the basis of the existing preparation method of lurasidone, a one-pot method is adopted to replace the method including multiple steps and obtain a target product once. The preparation method comprises the following steps: adding 3-(1-piperazinyl)-1,2-benzisothiazole in toluene, stirring to dissolve; adding (1R,2R)-1,2-bis(methanesulfonyloxymethyl)cyclohexane and an inorganic alkali, heating and carrying out reflux reaction for 12-36 hours; adding (3alpha R,4S,7R,7alpha S)4,7-methano-1H-isoindole-1,3(2H)-dione; heating and refluxing; recycling toluene at reduced pressure; adding ethyl acetate in the residue, stirring to dissolve, washing for 2-3 times with 5% hydrochloric acid, separating out the organic layers, drying for 20-120 minutes, filtering to remove the drying agent, concentrating the obtained ethyl acetate solution, dropwise adding concentrated hydrochloric acid, precipitating the solid, and performing suction filtration to obtain crude lurasidone; and refining crude lurasidone to obtain pure lurasidone. By adopting the preparation method of lurasidone, the solvent can be recycled conveniently and the method is simple in operation.
Owner:SHANDONG BESTCOMM PHARMA CO LTD
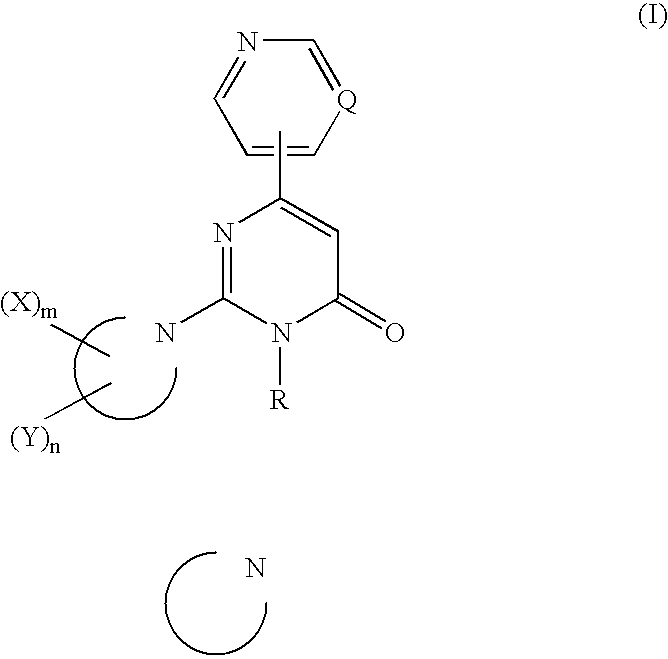
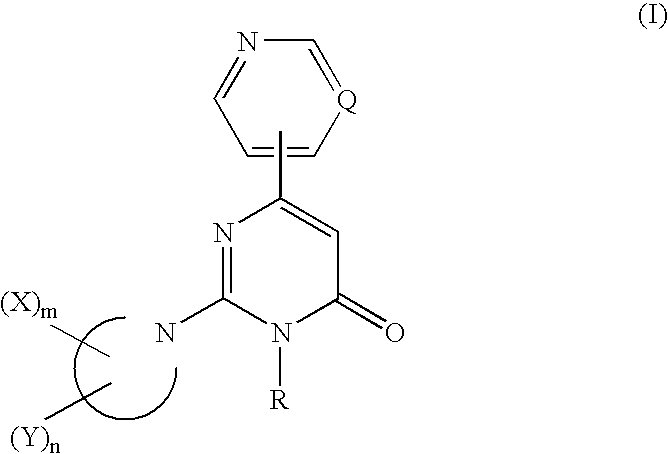
2,3,6-Trisubstituted-4-pyrimidone derivatives
InactiveUS7504411B2Inhibition of neurotoxic effectsAvoid deathOrganic active ingredientsBiocideArylHalogen
A pyrimidone derivative having tau protein kinase 1 inhibitory activity which is represented by formula (I) or a salt thereof, or a solvate thereof or a hydrate thereof; useful for prventive and / or therapeutic treatment of diseass such as neurodegenerative diseases (e.g. Alzheimer disease); wherein Q represents CH or nitrogen atom; R represents a C1-C12 alkyl group; the ring of Formula (I): represents piperazine ring or piperidine ring; each X independently represents a C1-C8 alkyl group, an optionally partially hydrogenated C6-C10 aryl ring, an indan ring or the like; m represents an integer of 1 to 3; each independently represents a halogen atom, a hydroxy group, a cyano group, a C1-C6 alkyl group or the like; n represents an integer of 0 to 8; when X and Y or two Y groups are attached on the same carbon atom, they may combine to each other to form a C2-C6 alkylene group.
Owner:SANOFI SA +1
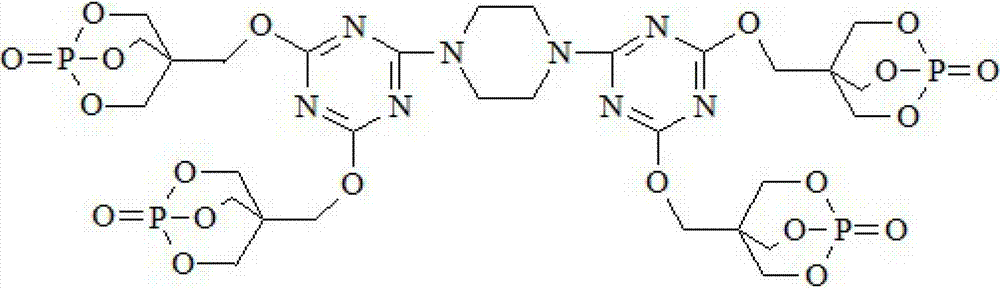
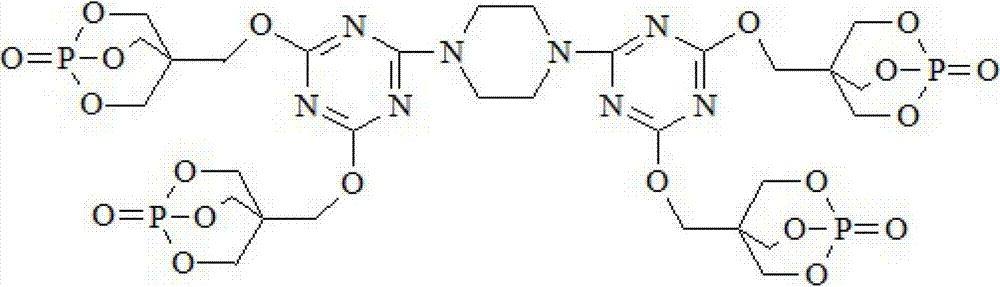
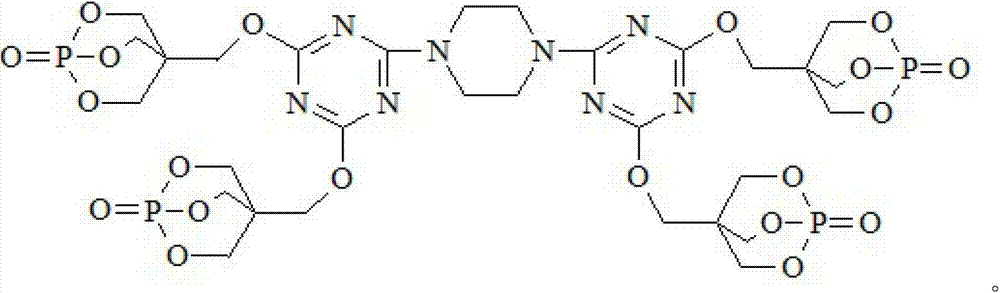
Triazine ring structure containing caged organic phosphate and preparation method thereof
ActiveCN103113409AImprove thermal stabilityImprove charcoal abilityGroup 5/15 element organic compoundsPhosphateFire retardant
The invention discloses a triazine ring structure containing caged organic phosphate and a preparation method of the caged organic phosphate. Cyanuric chloride, piperazidine and 1-oxo-4-hydroxymethyl-2,6,7-trioxa-1-phosphabicyclo[2,2,2]octane (PEPA) are used as main raw materials to prepare the triazine ring structure containing caged organic phosphate, and the preparation method has the characteristics of accessible raw materials, simple, convenient and easy process, safe production and operation, and the like. According to the triazine ring structure containing caged organic phosphate disclosed by the invention, three components, namely, a carbon source, an acid source and a gas source are integrated in one molecular structure, phosphorus-nitrogen synergy and compatibility charring effect in the same molecule is exerted, charring performance and flame retardant performance of a flame retardant system are greatly improved, and in addition, the triazine ring structure and a caged structure both exist in the molecular structure so that the caged organic phosphate has extremely good thermal stability, can be used as a flame retardant in most polymer materials, and has good application prospect.
Owner:DONGHUA UNIV
Triethylene diamine synthesis method
InactiveCN107163054AImprove universalityRaise the ratioOrganic chemistryMolecular sieve catalystsAlkaline earth metalSynthesis methods
The invention discloses a triethylene diamine synthesis method, which comprises the following steps of performing preheating gasification on a water solution of raw materials; then, at 180 to 400 DEG C, performing contact reaction with modified molecular sieve catalysts to obtain triethylene diamine, wherein the modified molecular sieve catalysts are obtained by soaking HZSM type molecular sieves into a water solution of alkaline earth metal compounds and then performing drying and roasting; the raw materials are at least one kind of materials from piperazidine, piperazidine derivatives, straight chain alcohol amine and amines. The molecular sieve catalysts are modified through the alkaline earth metal, so that the acidity and the acid size of Brphinsted acid on the surfaces of the molecular sieve catalysts are obviously reduced; the proportion of the Lewis acid sites is improved; the reaction temperature is greatly lowered; the universality of the catalysts is good; the product yield is improved. Compared with a conventional triethylene diamine synthesis method, the triethylene diamine synthesis method achieves economic effects, green effects and high efficiency.
Owner:SHAOXING XINGXIN CHEM
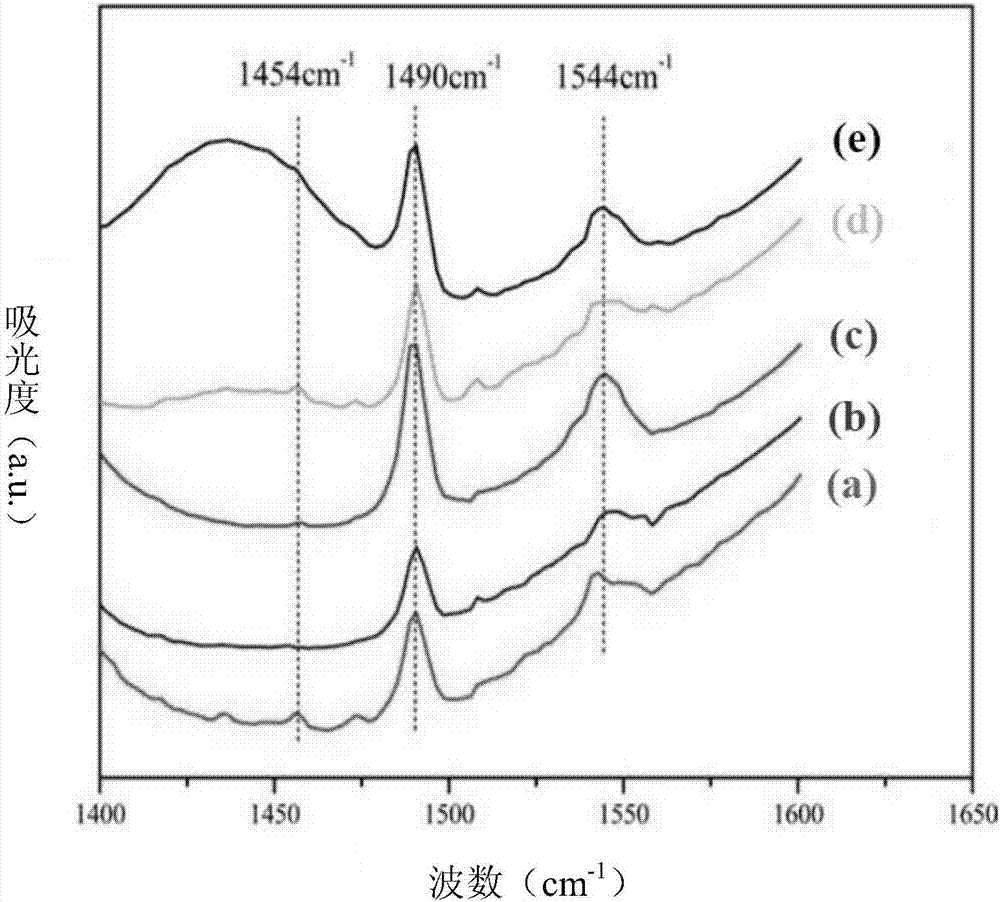
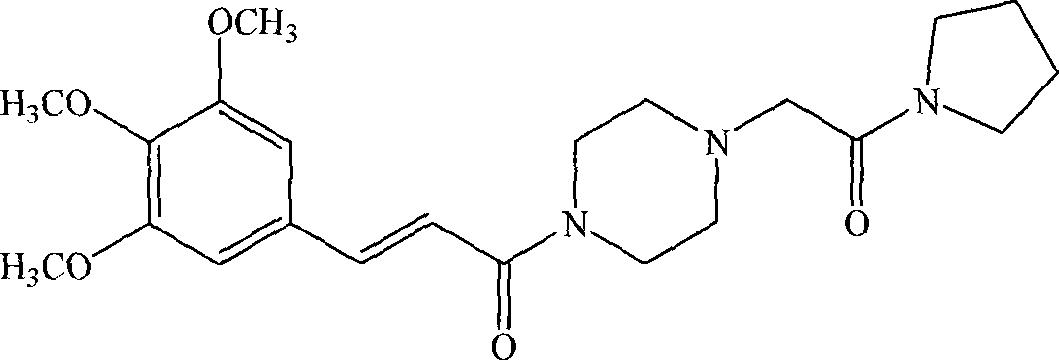
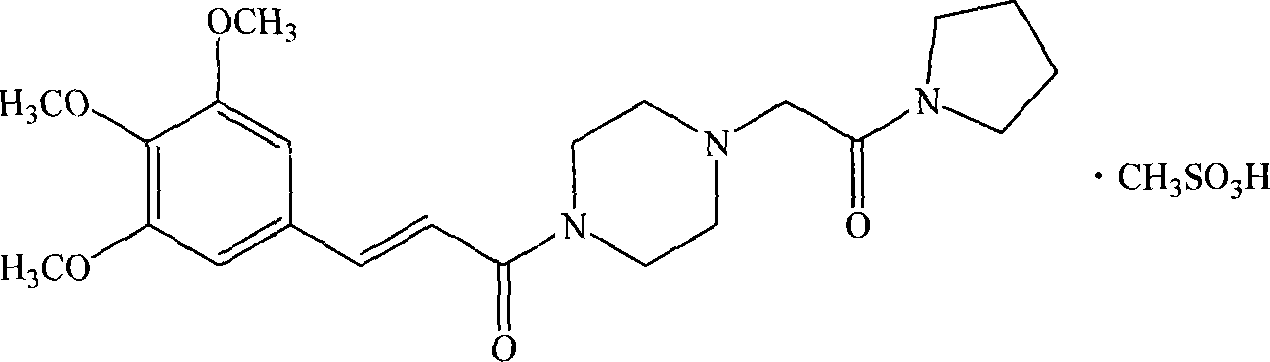
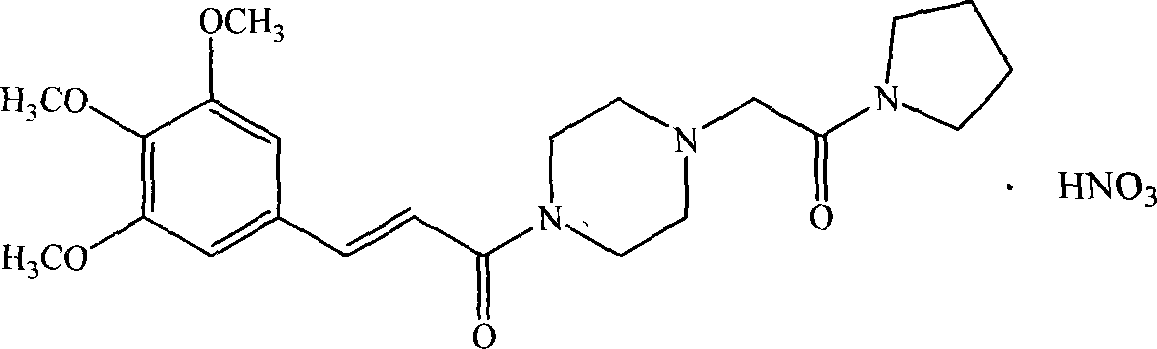
Novel medicinal salt for cinepazide and preparation method thereof
InactiveCN101058568AGood choiceGood water solubilityOrganic active ingredientsOrganic chemistryDiseaseSolubility
The invention discloses a new medicinal salt of (E)-1-{4-[(3',4',5'-trimethoxy cinnamon acyl]-1-piperazidine} acetopyrrole as cinprazole, which comprises the following parts: mesilate, phosphate, nitrate, sulfate, hydrobromide, p-toluenesulfonic acid salt, tartrate, fumarate, citrate, succinate, malonate, optimizing the mesilate, nitrate, phosphate and sulfate, for mesilate at best. The drug can be used to treat and / or prevent cardiovascular and cerebrovascular disease and peripheral vessel disease. The drug possesses good stability and superior solubility, which can be made into acceptable pharmacy agent.
Owner:车冯升
Ultraviolet and fluorescent dual-tracing concrete admixture and preparation method thereof
InactiveCN107383284ASolve the shortcomings of untraceable detectionAvoid joiningPolymer scienceEther
The invention discloses an ultraviolet and fluorescent dual-tracing concrete admixture. The ultraviolet and fluorescent dual-tracing concrete admixture is obtained by performing a free radical polymerization reaction on an allyloxy polyvinyl ether monomer or a blocked allyloxy polyvinyl ether monomer, an unsaturated carboxylic acid monomer or an unsaturated carboxylic acid anhydride monomer and an unsaturated double bond-containing water-soluble fluorescent monomer, wherein the unsaturated double bond-containing water-soluble fluorescent monomer is 4-(N'-methyl-1-piperazinyl)-N-hydroxyethyl-1,8-naphthal allyl ammonium chloride or 4-(N'-hydroxyethyl-piperazinyl)-N-hydroxyethyl-1,8-naphthal allyl ammonium chloride. Raw materials for synthesizing a polymer are non-toxic and harmless, so that the characteristic of clean production is met; when the polymer is used as a water-reducing agent, a polymer product can be directly used without being separated; the polymer product has a relatively small addition amount as well as excellent dispersion with relatively high mobility, and the advantage of the relatively small solid addition amount can greatly reduce the cost and increase economic benefits, so that the polymer product is suitable for market promotion.
Owner:JIANGSU OPEN UNIV
Improved method for synthesizing ranolazine
ActiveCN102558097AReflux reaction time shortenedShort reaction timeOrganic chemistryDimethylaniline N-oxideDimethylphenylpiperazinium
The invention discloses an improved method for synthesizing an antianginal medicine ranolazine and belongs to the field of pharmaceutical chemistry. The method comprises the following steps of: amidating 2,6-dimethylaniline which is taken as a raw material, and alkylating to obtain N-(2,6-dimethylphenyl)-2-(1-piperazino)-acetamide; and reacting the N-(2,6-dimethylphenyl)-2-(1-piperazino)-acetamide and 2-(2-methylphenoxymethyl)ethylene oxide which is obtained by reaction of 2-methoxyphenol and epichlorohydrin to obtain crude ranolazine, and recrystallizing to obtain refined ranolazine. A reaction solvent, the molar ratio of raw materials, a phase transfer catalyst, a recrystallization solvent and the like are optimized; and the improved method is convenient to operate and is suitable for industrialized production, the production cost is reduced, and the yield is improved.
Owner:FUREN PHARMA GROUP +1
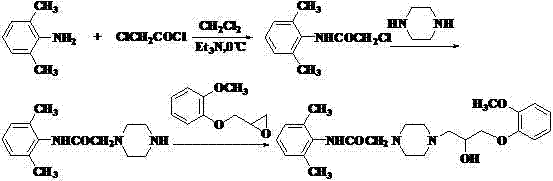
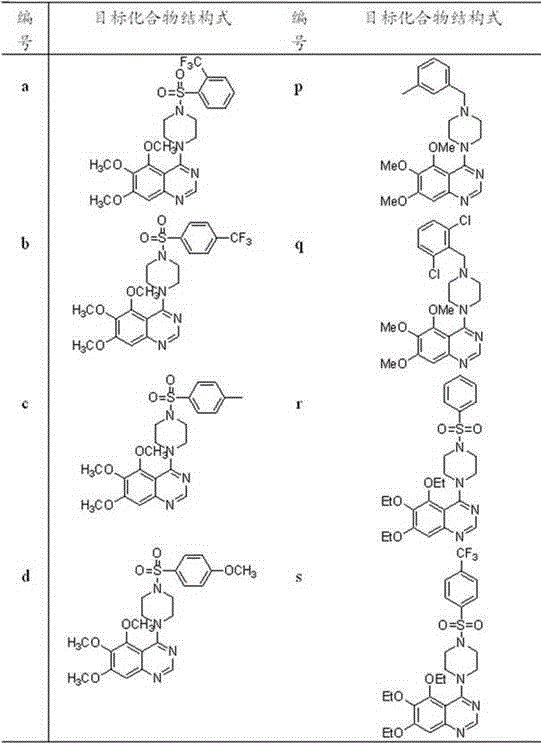
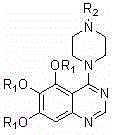
4-(4-substituted piperazine)-5,6,7-trialkoxy quinazoline type compound as well as preparation method and application of 4-(4-substituted piperazine)-5,6,7-trialkoxy quinazoline type compound
The invention discloses a 4-(4-substituted piperazine)-5,6,7-trialkoxy quinazoline type compound as well as a preparation method and application of the 4-(4-substituted piperazine)-5,6,7-trialkoxy quinazoline type compound, wherein the compound structure is shown as the following general formula (I). A series of novel 4-(4-substituted piperazine)-5,6,7-trialkoxy quinazoline type compounds are synthesized by using 2,3,4-trihydroxy benzoic acid, dimethyl sulfate, diethyl sulfate, methanol, sulfuric acid, nitric acid, hydrogen gas, formamide, phosphorus oxychloride, N-Boc piperazine, hydrochloric acid, aryl sulfonyl chloride and 4-aromatic (benzyl, pyridine and morpholine propyl) substituted piperazine as raw materials through multiple steps. The compound has a better anticancer effect and a plant fungus inhibition effect and can be used for preparing anticancer medicine and plant fungus resistance pesticide. (I) is shown as the accompanying drawing.
Owner:GUIZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
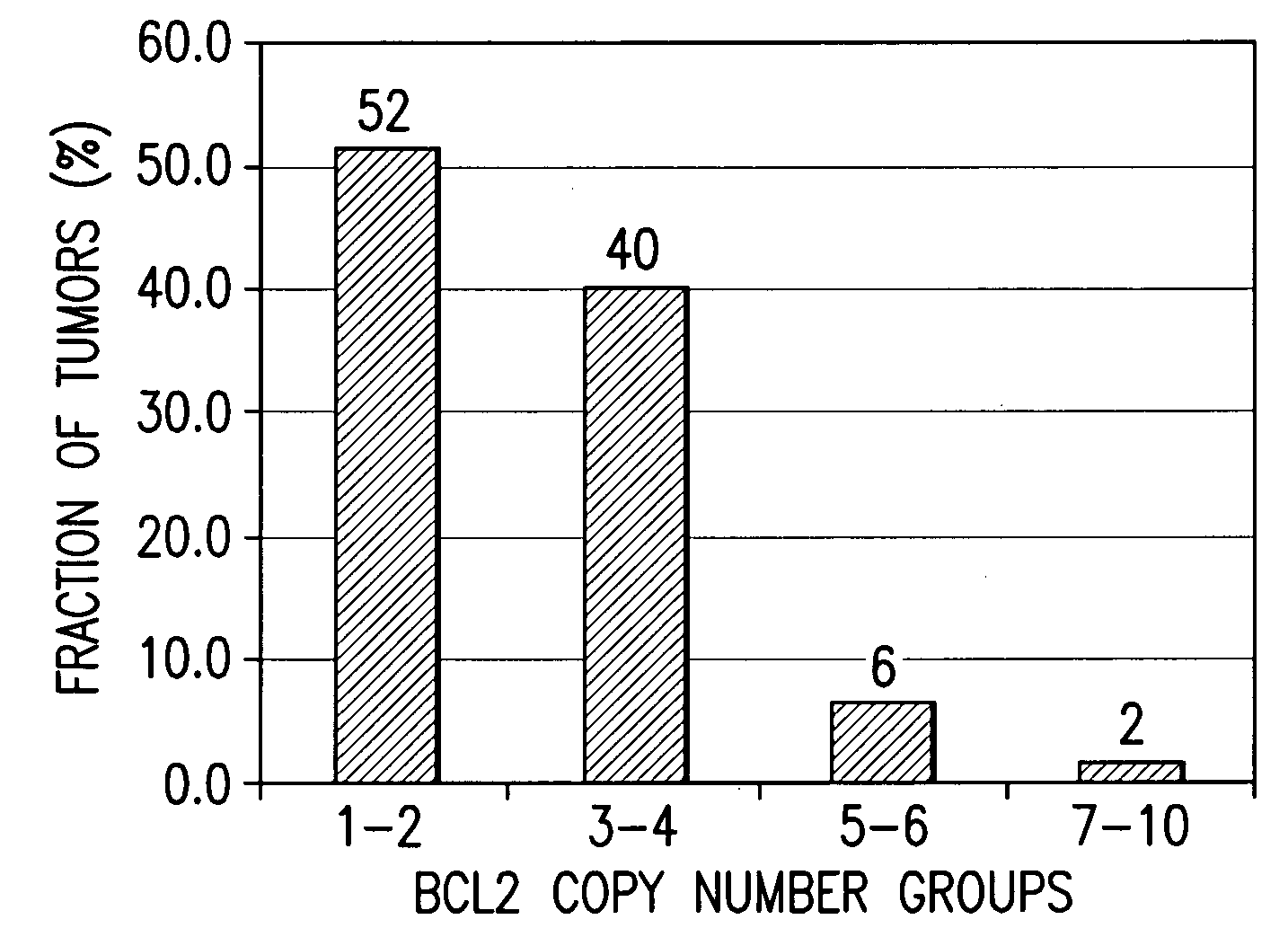
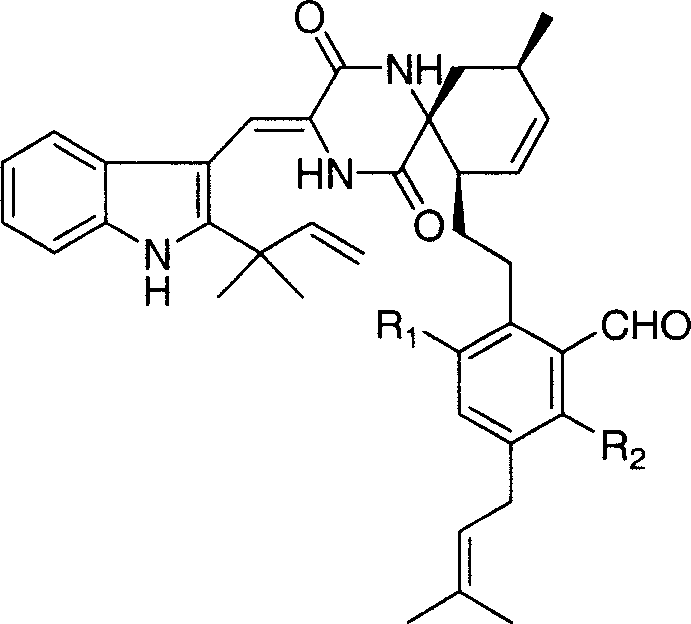
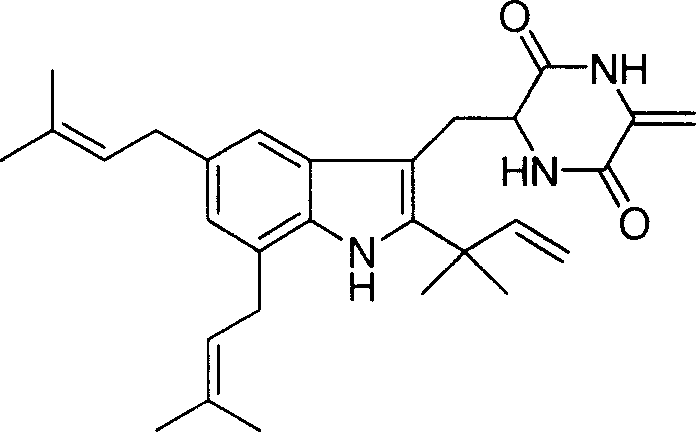
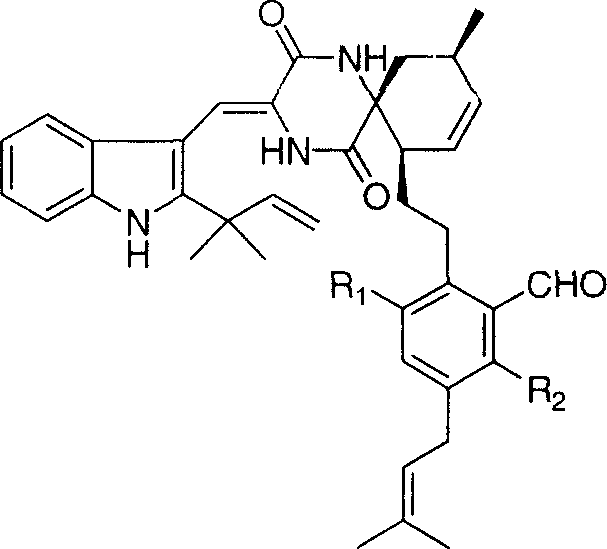
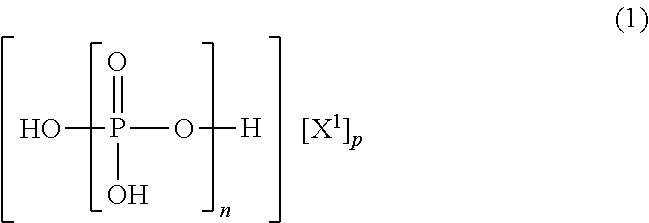
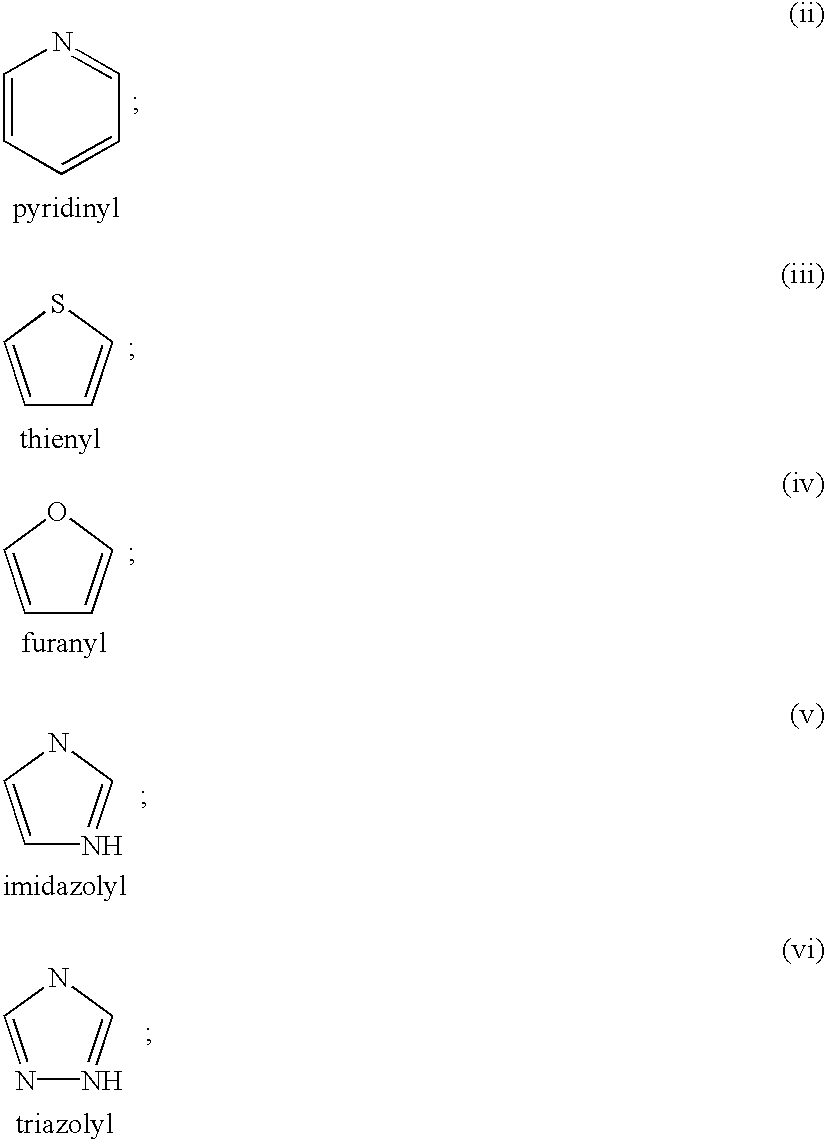
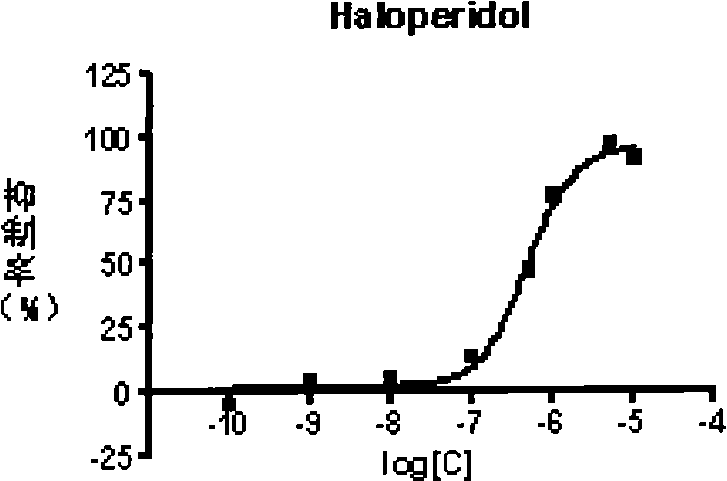
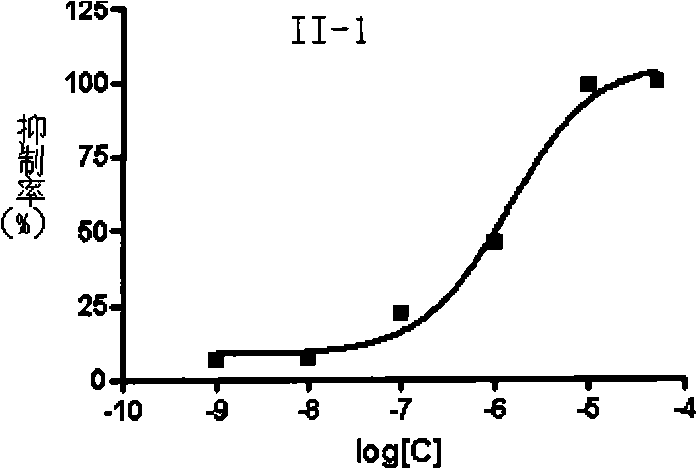
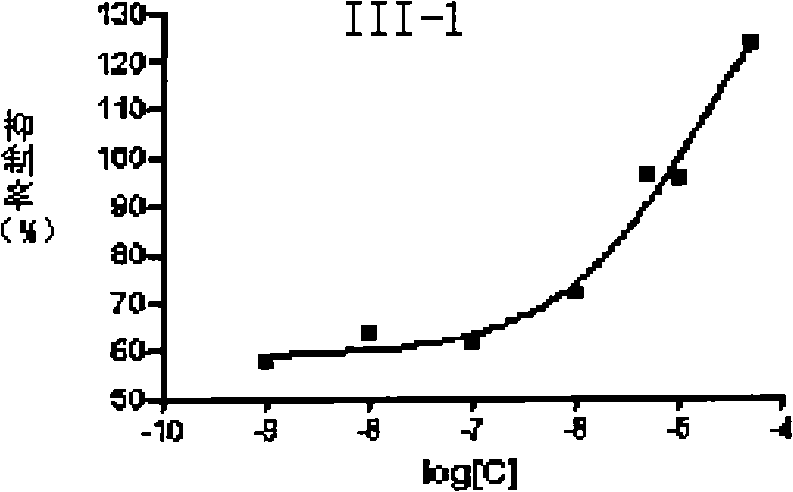
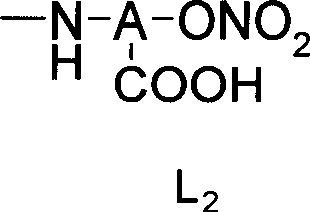
![4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative and preparation method thereof 4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative and preparation method thereof](https://images-eureka.patsnap.com/patent_img/ccda6d25-26d1-4761-b15f-5f57af727bcc/BDA00002781366000011.PNG)
![4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative and preparation method thereof 4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative and preparation method thereof](https://images-eureka.patsnap.com/patent_img/ccda6d25-26d1-4761-b15f-5f57af727bcc/BDA00002781366000021.PNG)
![4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative and preparation method thereof 4-[4-(substituted phenyl) piperazine piperazinyl-1]-butylcarbamic acid substituted aromatic ester derivative and preparation method thereof](https://images-eureka.patsnap.com/patent_img/ccda6d25-26d1-4761-b15f-5f57af727bcc/BDA00002781366000041.PNG)