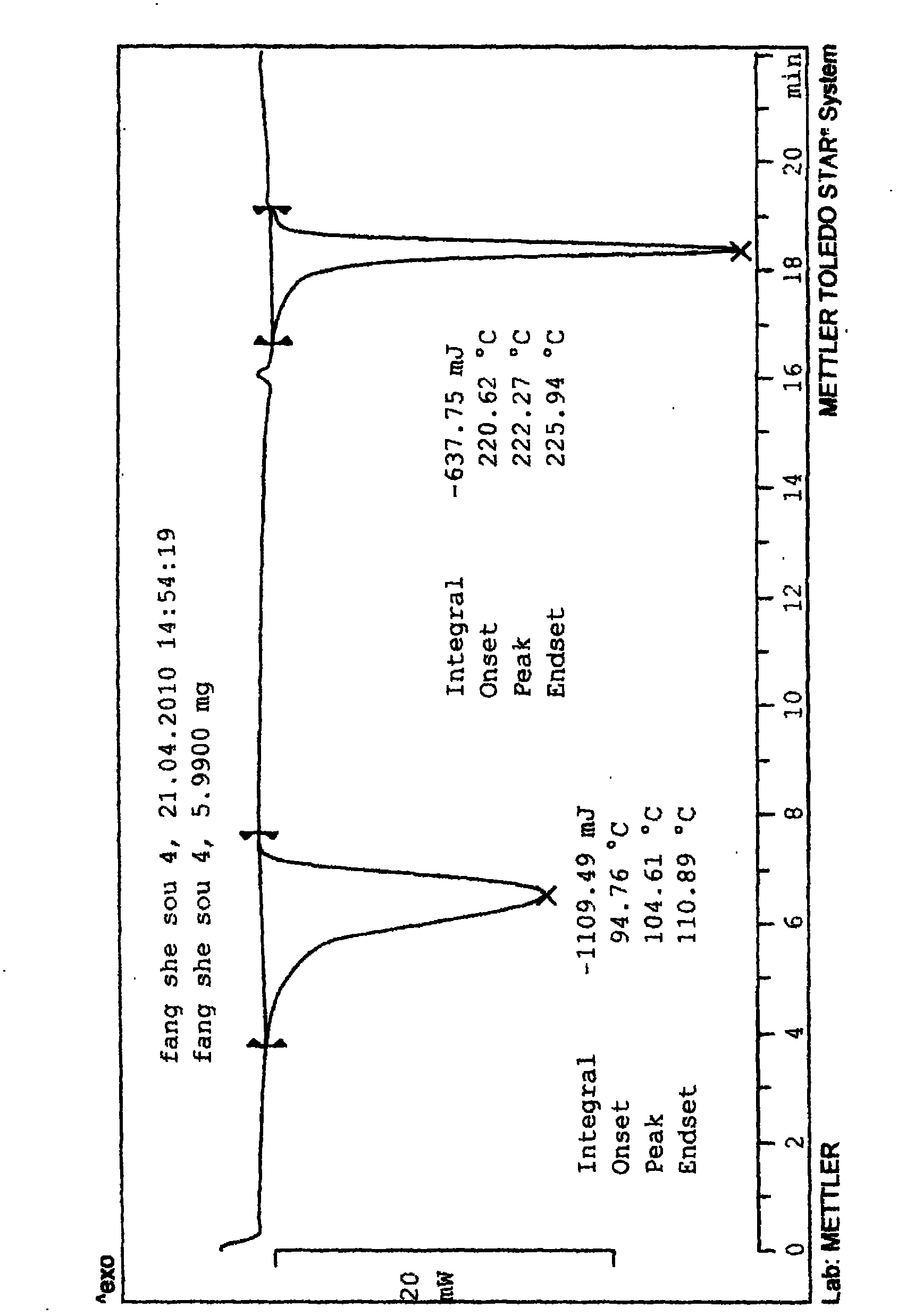
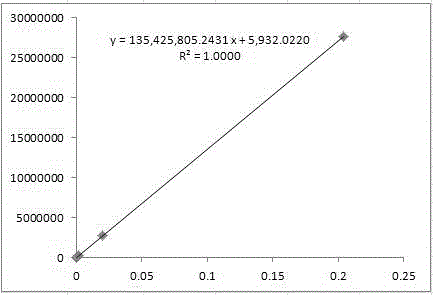
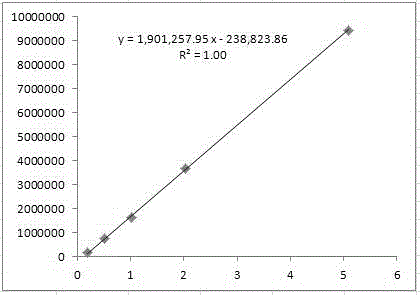
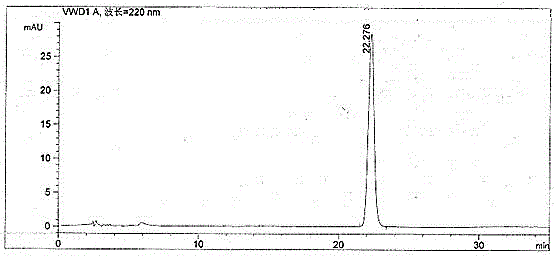
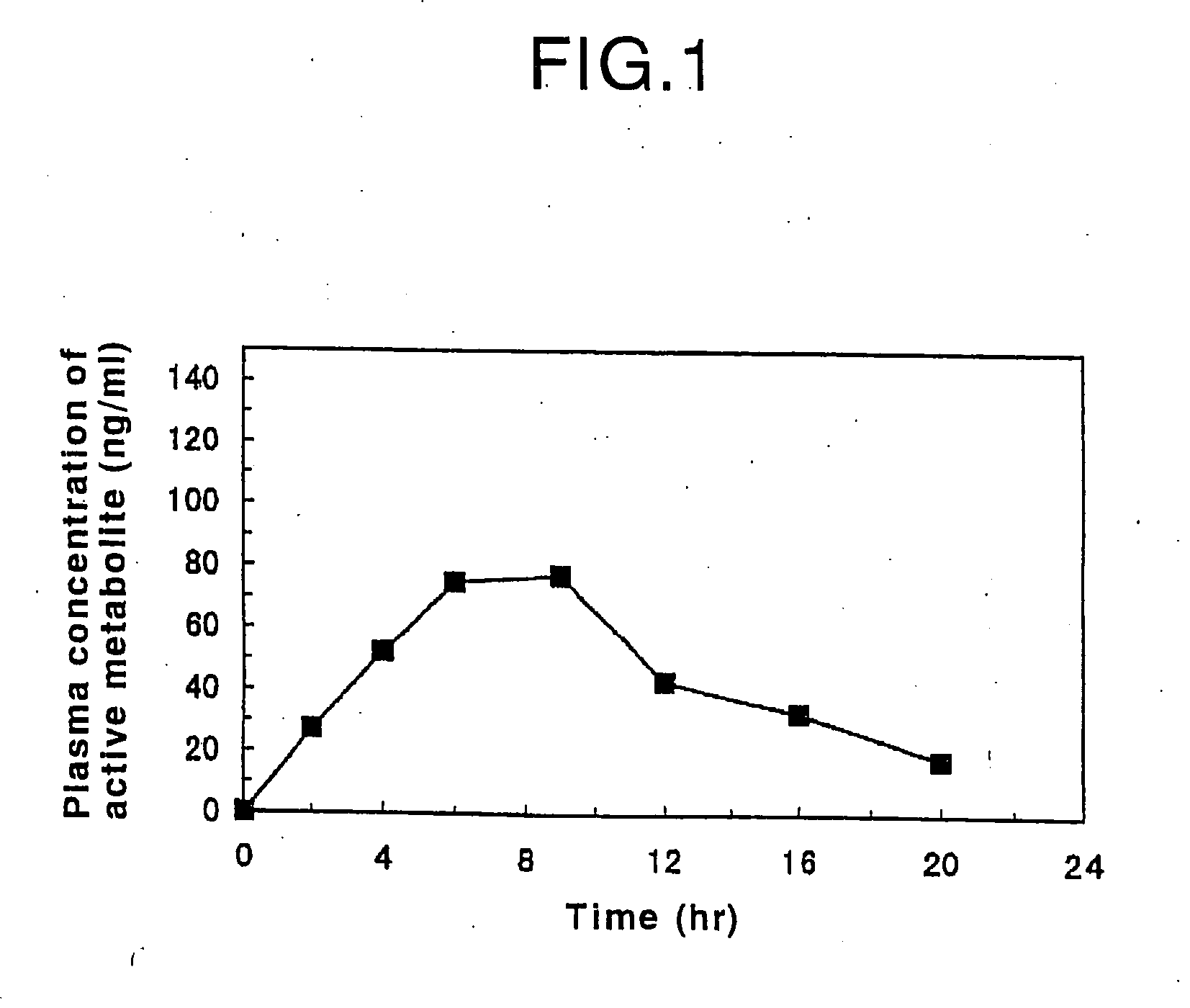
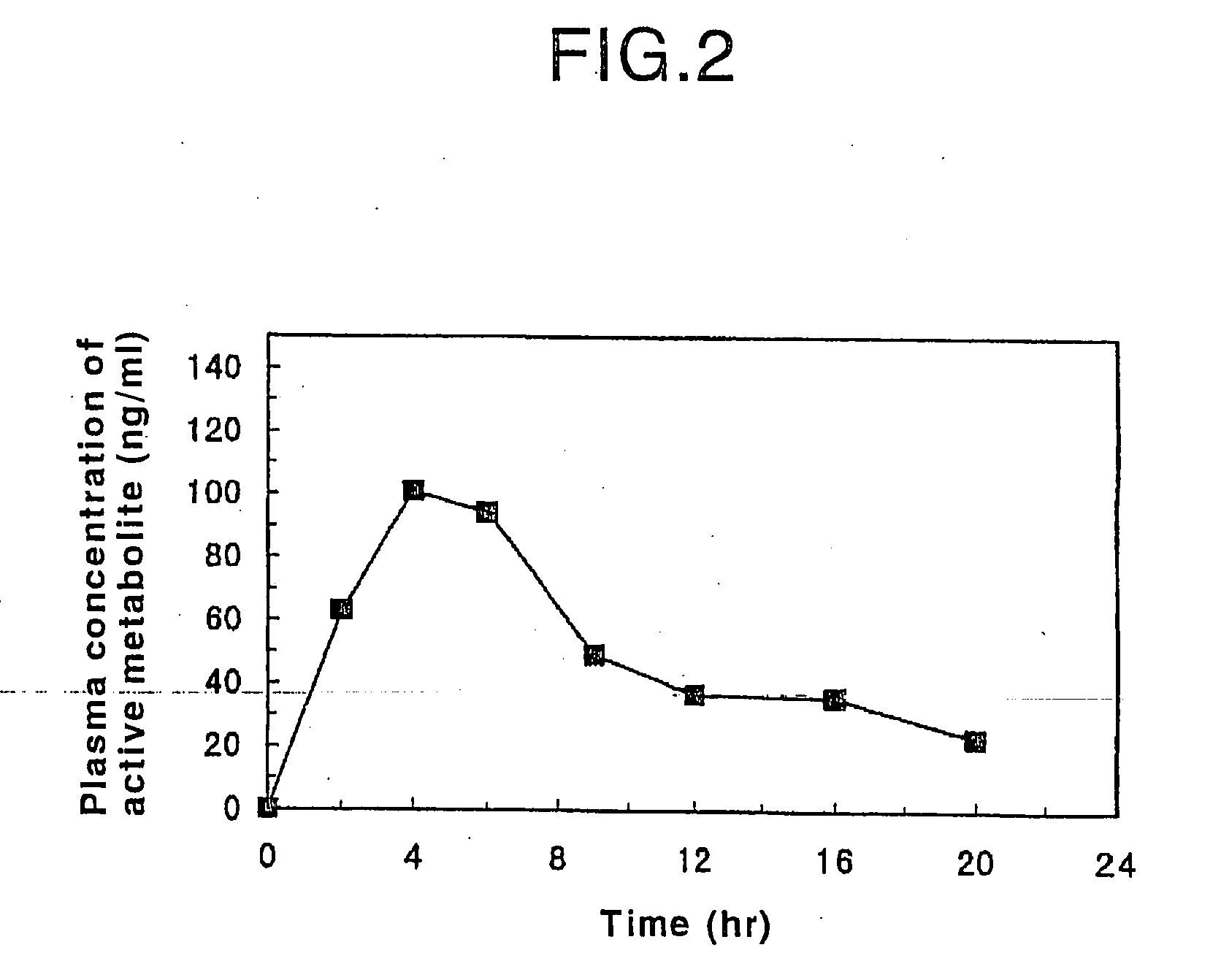
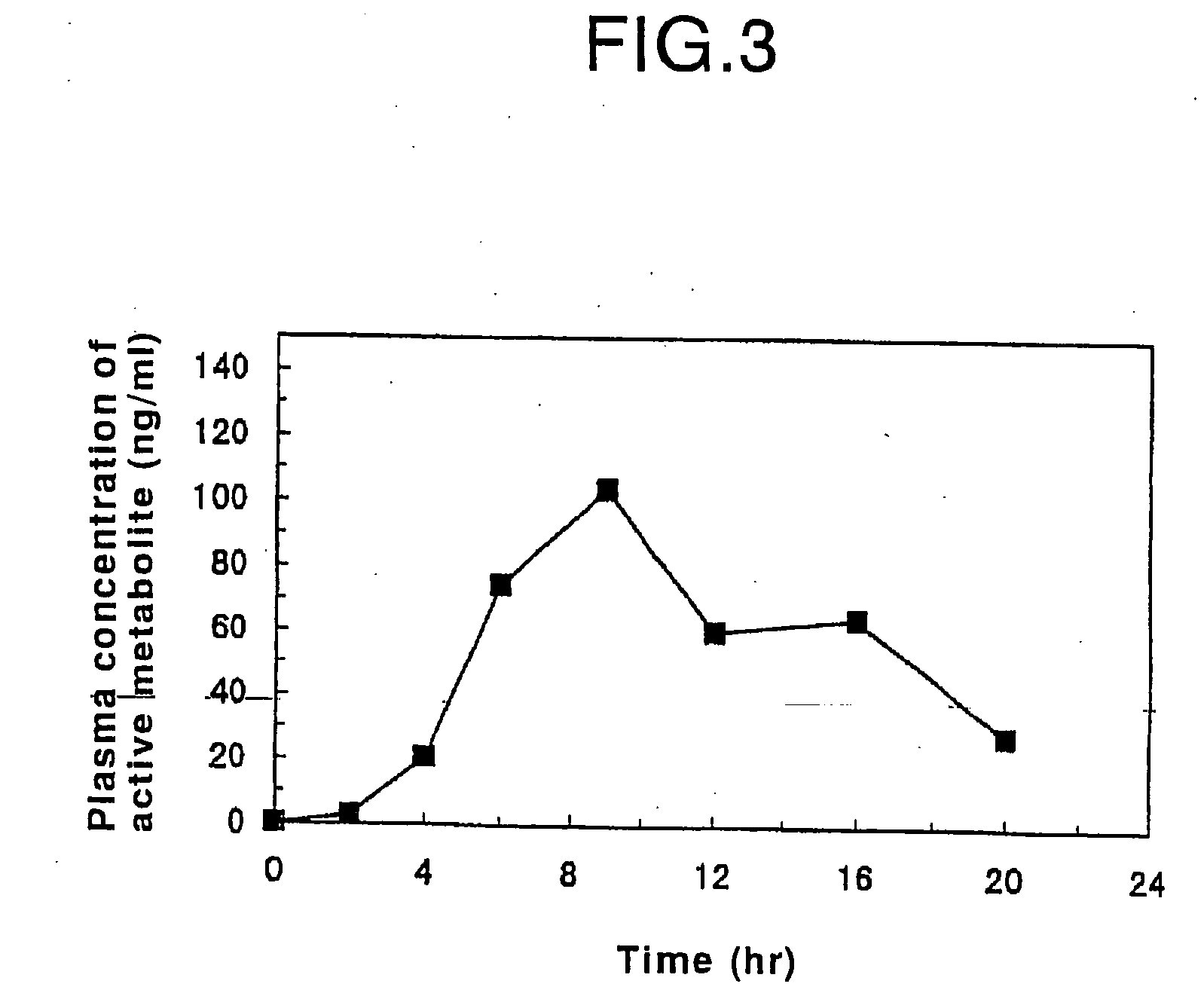
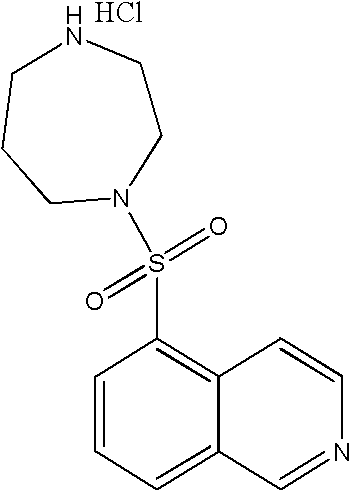
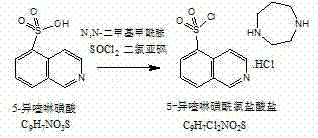Patents
Literature
85 results about "Fasudil" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
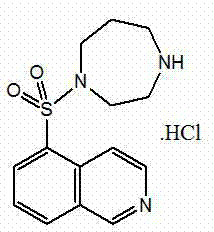
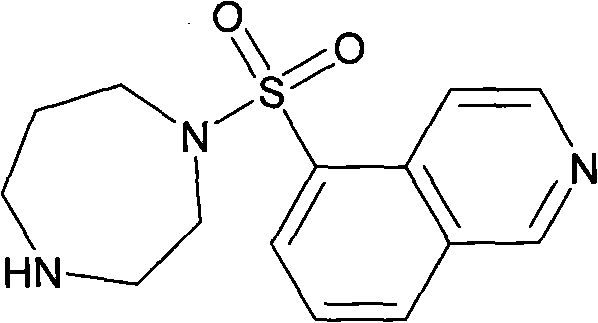
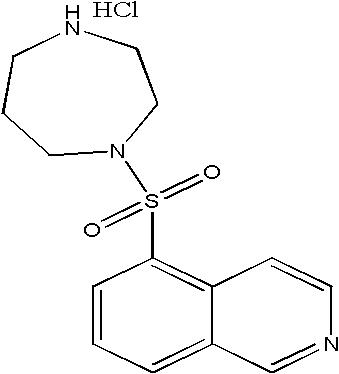
Fasudil (INN) is a potent Rho-kinase inhibitor and vasodilator. Since it was discovered, it has been used for the treatment of cerebral vasospasm, which is often due to subarachnoid hemorrhage, as well as to improve the cognitive decline seen in stroke patients. It has been found to be effective for the treatment of pulmonary hypertension. It was demonstrated in February 2009 that fasudil could improve memory in normal mice, identifying the drug as a possible treatment for age-related or neurodegenerative memory loss.
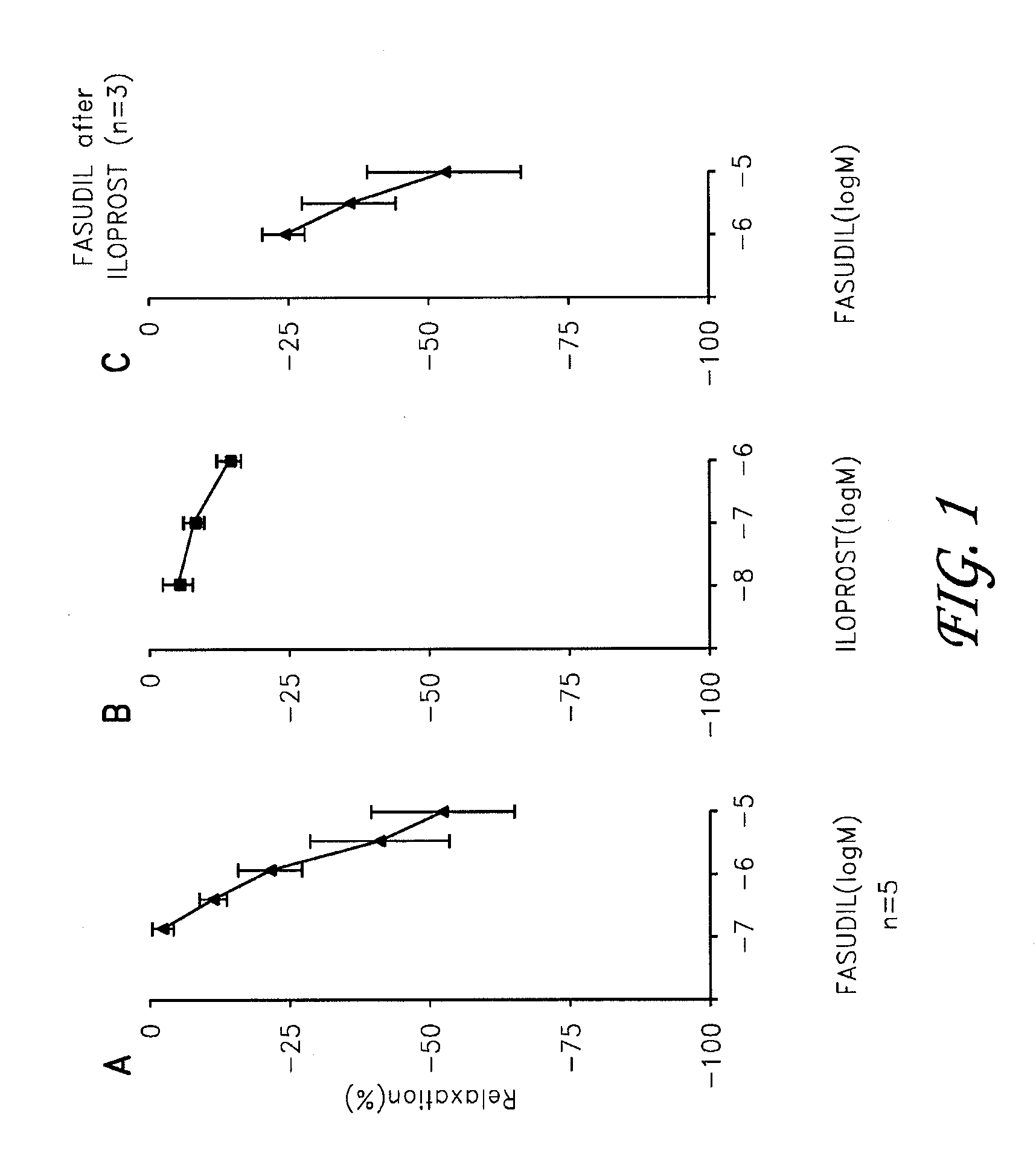
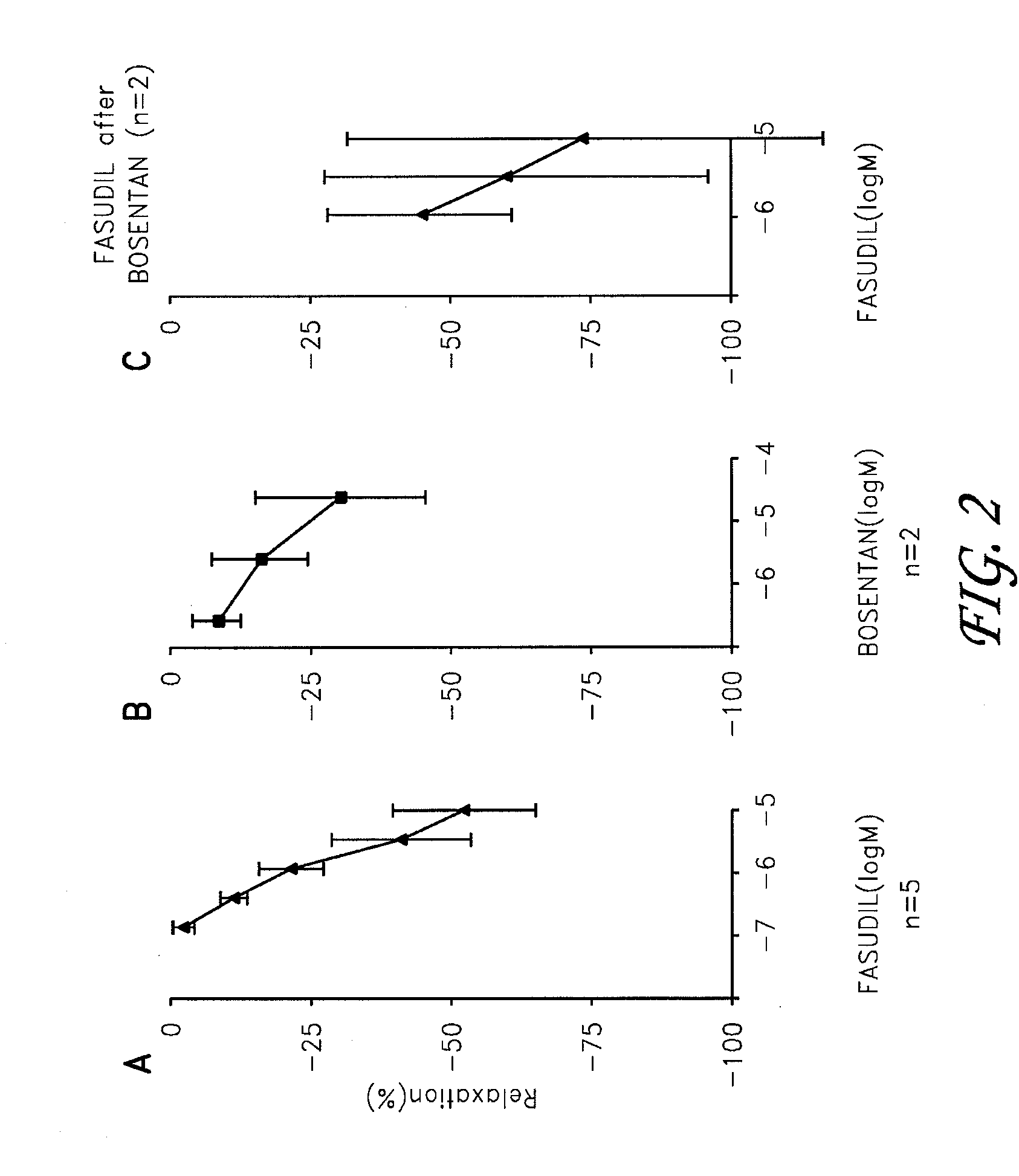
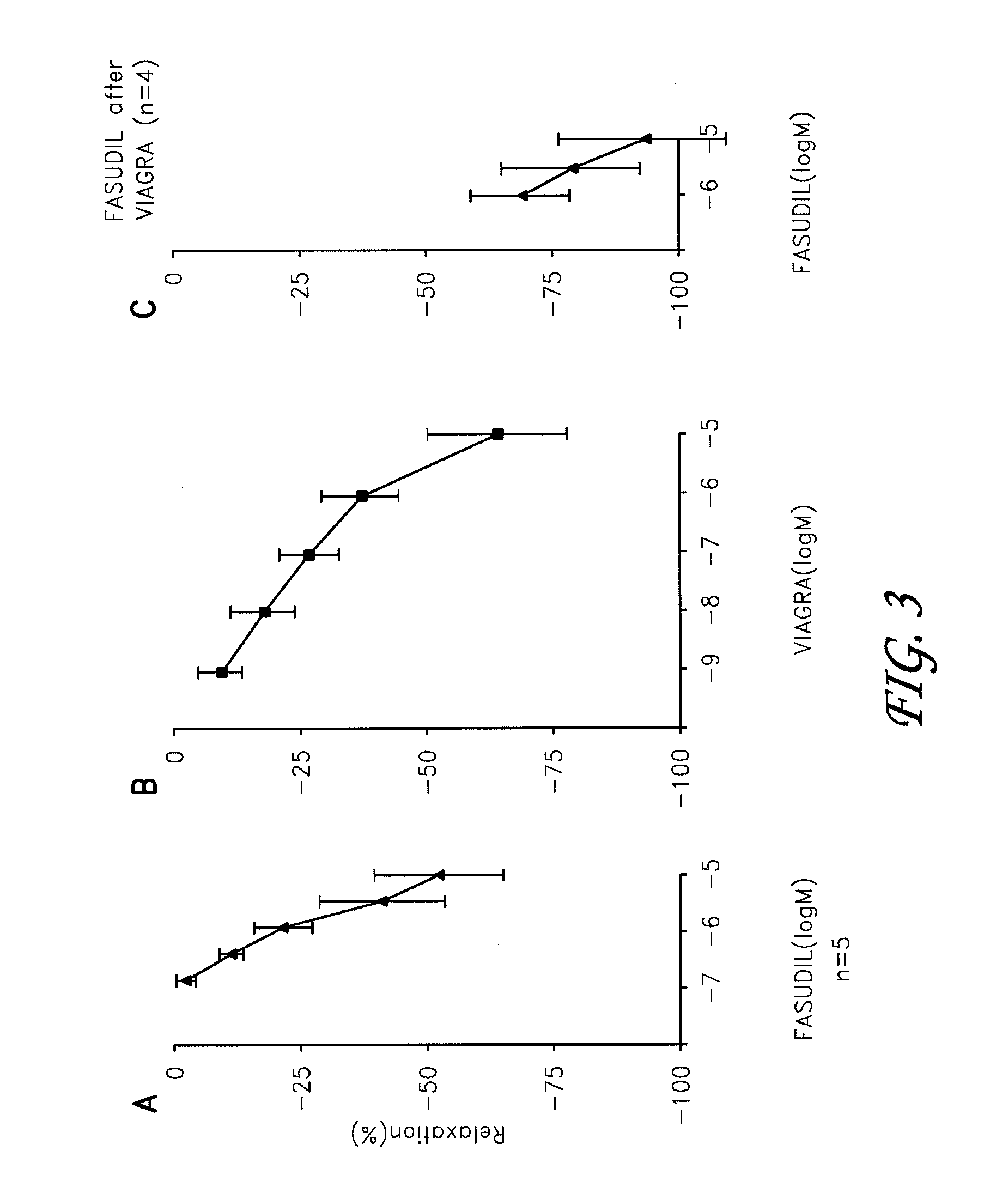
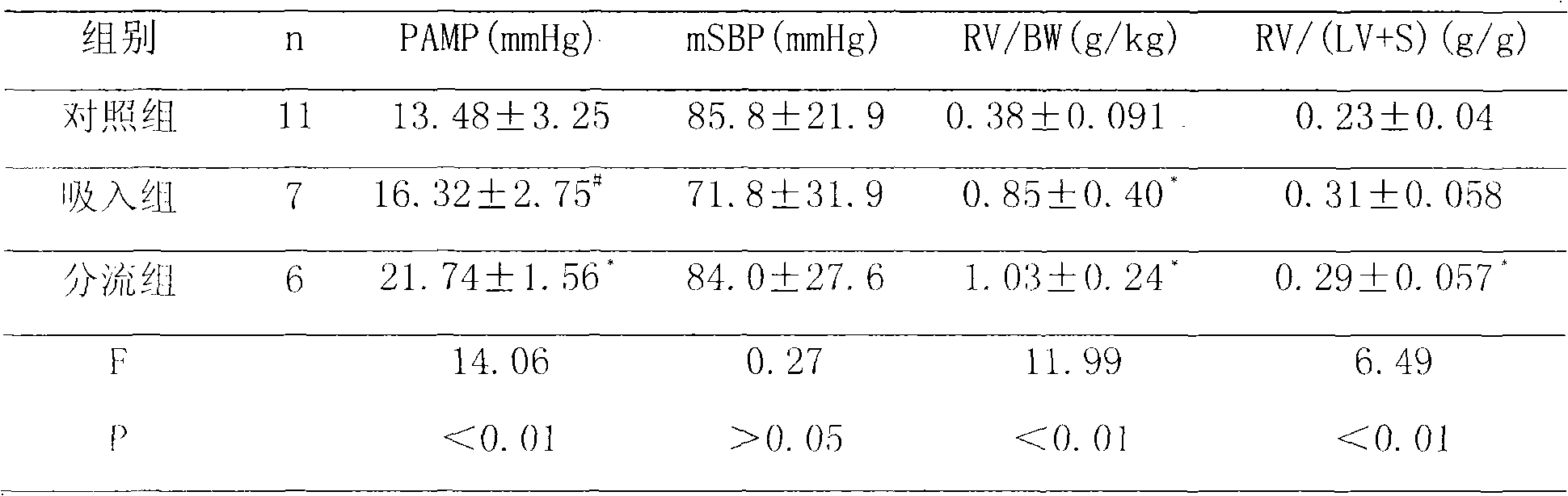
Fasudil in combination therapies for the treatment of pulmonary arterial hypertension
Preferred embodiments of the present invention are related to novel therapeutic drug combinations and methods for treating and / or preventing pulmonary arterial hypertension and / or stable angina. More particularly, aspects of the present invention are related to therapeutic combinations comprising a Rho-kinase inhibitor, such as fasudil, and one or more additional compounds selected from the group consisting of prostacyclins, such as iloprost, endothelin receptor antagonists, PDE inhibitors, calcium channel blockers, 5-HT2A antagonists, such as sarpogrelate, selective serotonin reuptake inhibitors, such as fluoxetine, statins, and vascular remodeling modulators, such as Gleevec.
Owner:ASAHI KASEI PHARMA
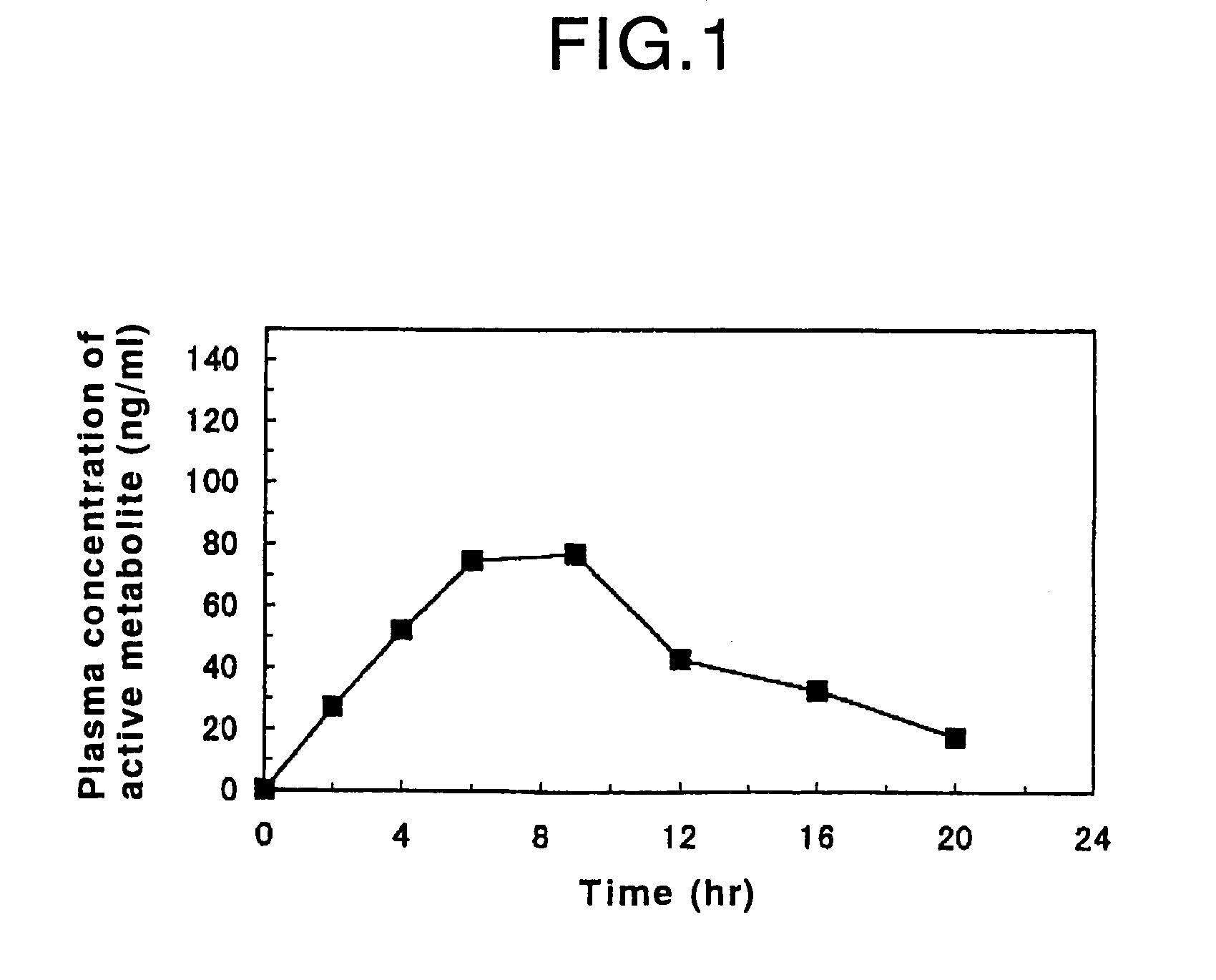
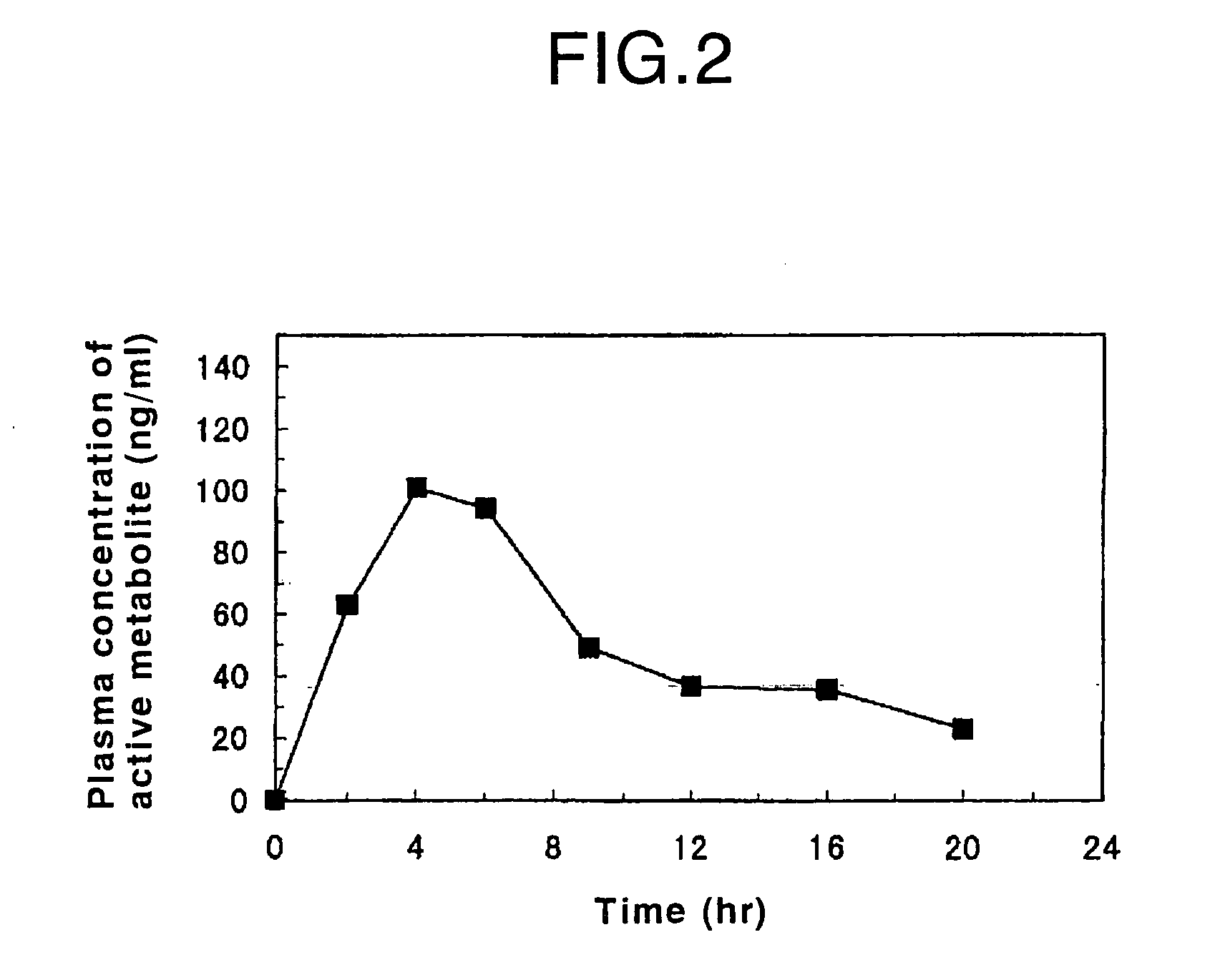
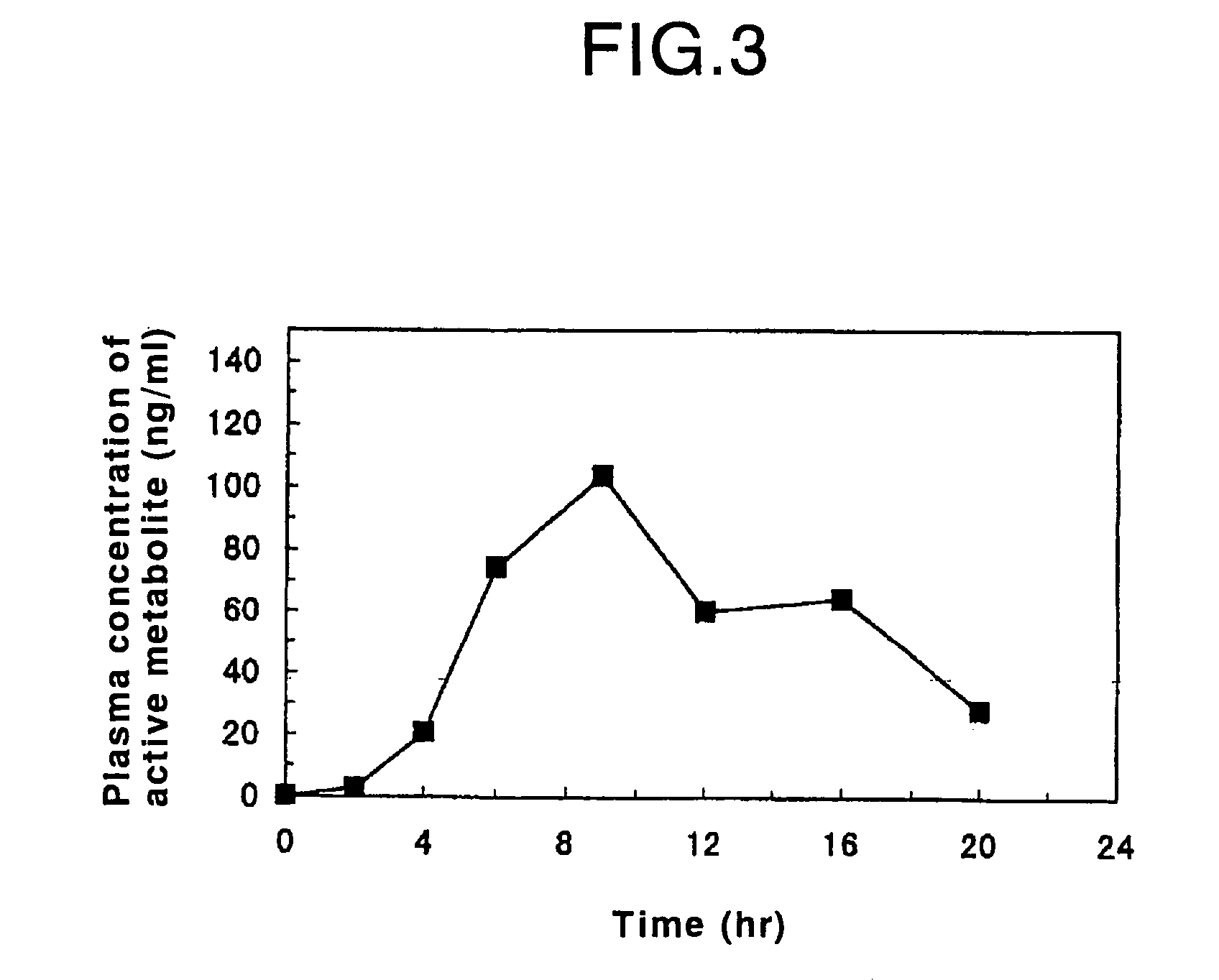
Process for producing an oral sustained-release preparation of fasudil hydrochloride
InactiveUS7125567B2Good effectReduce the burden onBiocidePowder deliveryDissolutionBULK ACTIVE INGREDIENT
Disclosed is an oral sustained-release preparation which contains at least one active ingredient selected from the group consisting of fasudil hydrochloride and a hydrate thereof, the preparation comprising at least one sustained-release coated particle comprising a core having a surface and a coating formed on the surface of the core, wherein the core contains the active ingredient and the coating comprises a coating base material and a specific insoluble auxiliary material, and wherein the preparation exhibits, with respect to the active ingredient, a specific dissolution rate, as measured by the dissolution test. By using the oral sustained-release preparation of the present invention, it becomes possible to surely control the release of fasudil hydrochloride from the preparation, so that the effect of the active ingredient is maintained for a long period of time. Therefore, the burden of the patient who has to take the preparation can be decreased and the compliance with respect to the administration of the preparation can be improved. Also disclosed is a method for evaluating an oral sustained-release preparation containing the active ingredient, wherein the evaluation is conducted with respect to the sustained-release ability of the active ingredient.
Owner:ASAHI KASEI PHARMA
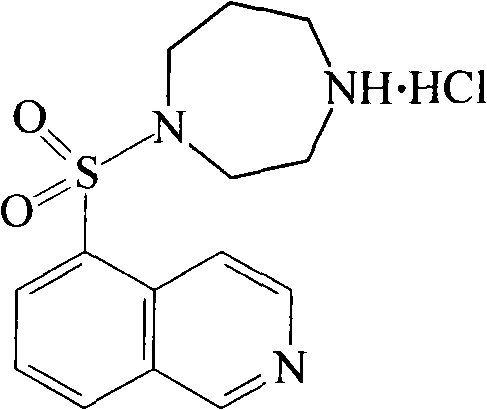
High purity fasudil hydrochloride compound
The invention relates to high purity fasudil hydrochloride compound; the method can obtain high purity fasudil hydrochloride finished goods and belongs to the technical field of medicine. The purity of the fasudil hydrochloride is greatly increased by acid-base reaction, silicagel column elution and activated carbon adsorption. The method has simple technique, low cost and high yield and is suitable for industrialization production.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
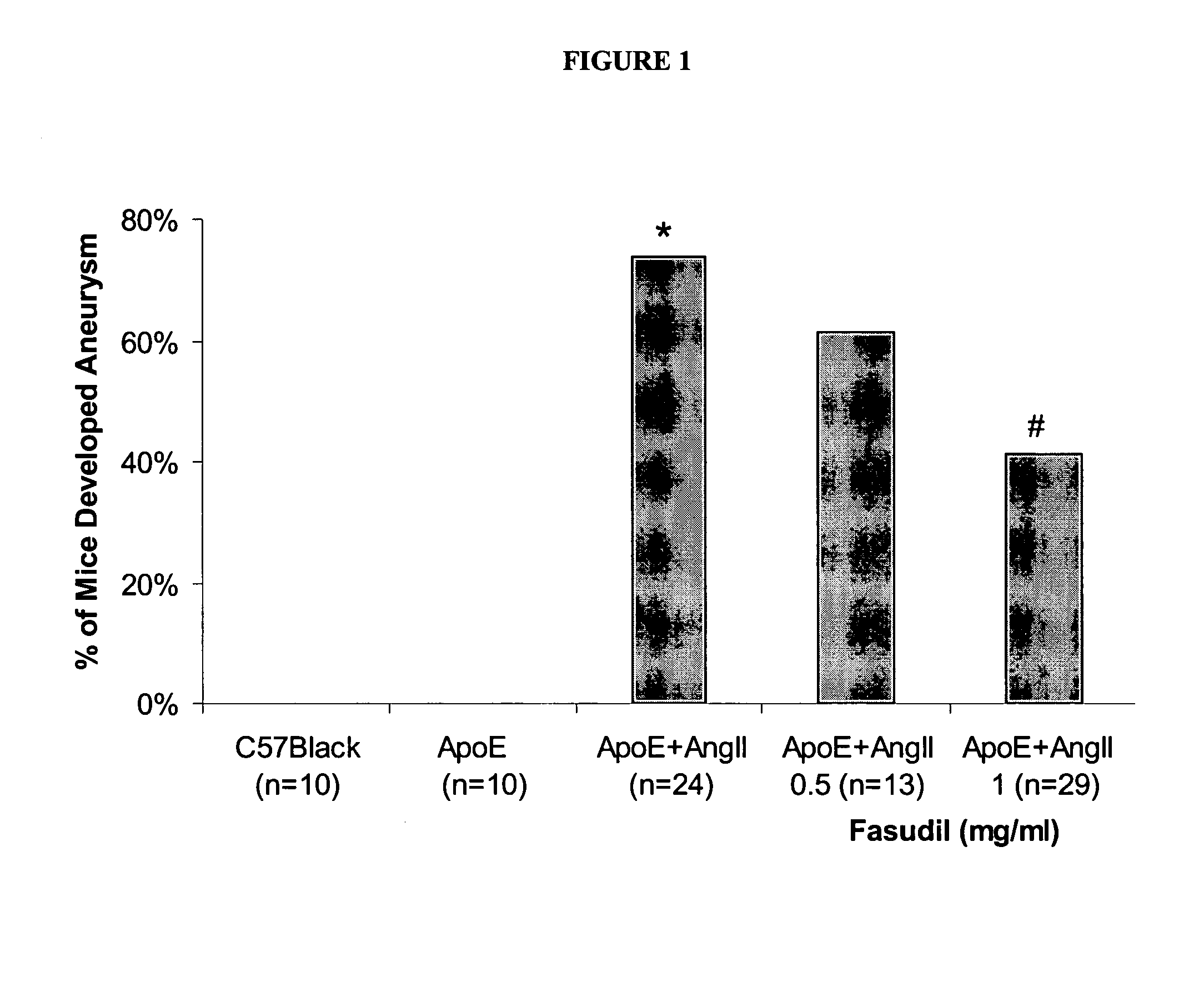
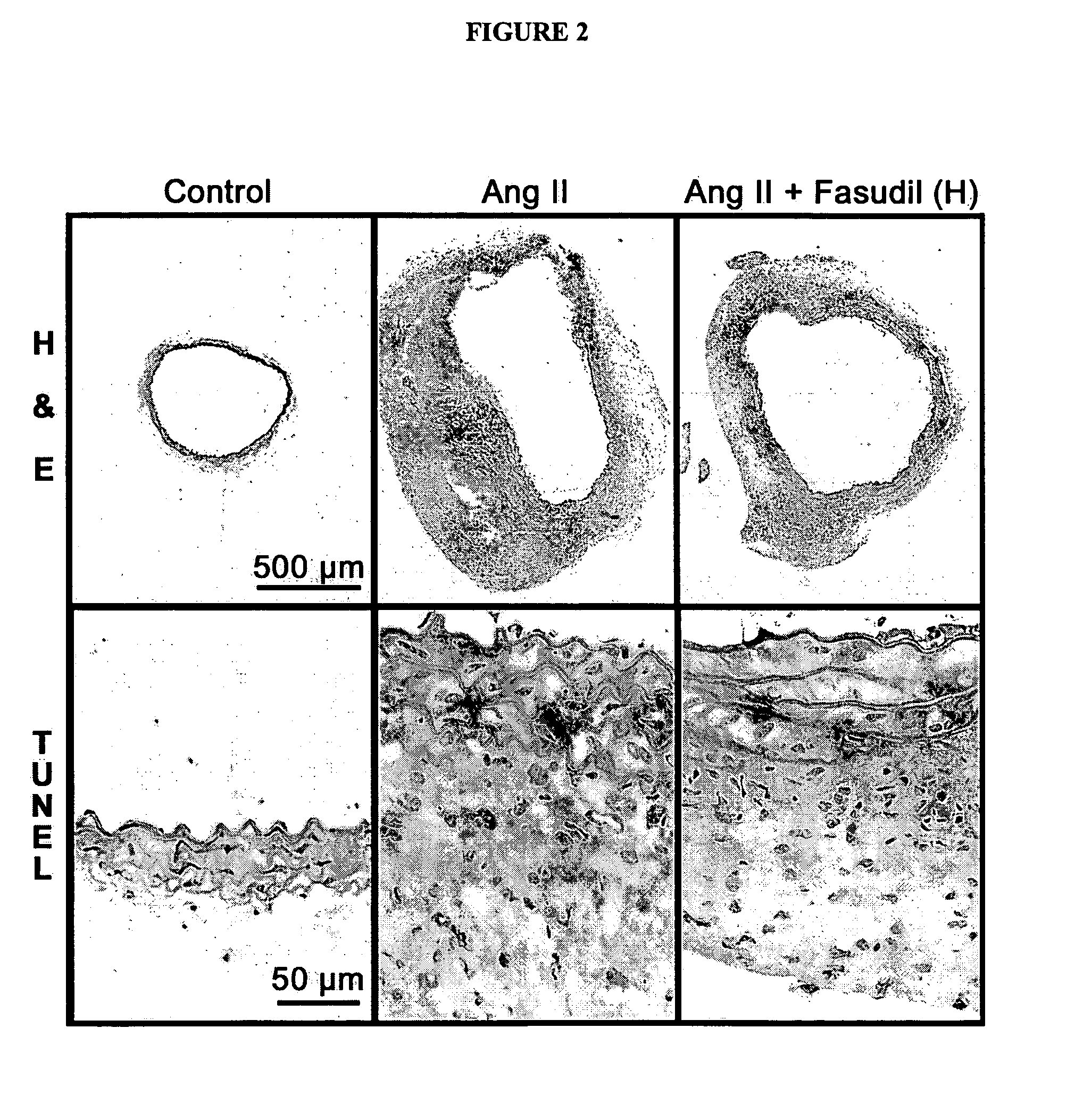
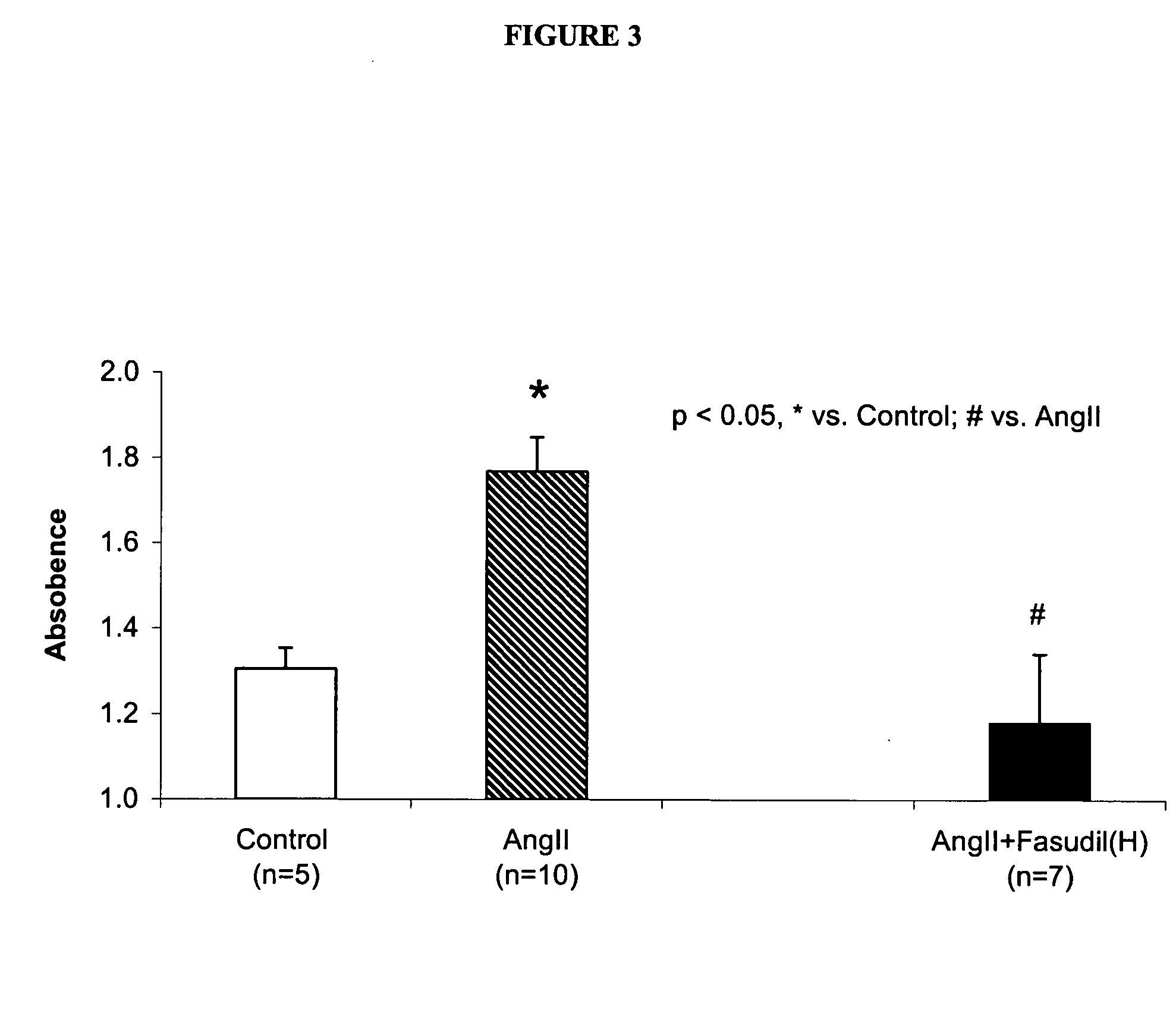
Use of Rho-kinase inhibitors in the treatment of aneurysm and cardiac hypertrophy
The present invention is directed to a method for preventing or decreasing the incidence of or treating aneurysm or cardiac hypertrophy, comprising administering an effective amount of a rho-kinase inhibitor, such as fasudil, to a subject in need thereof.
Owner:SCHERING AG
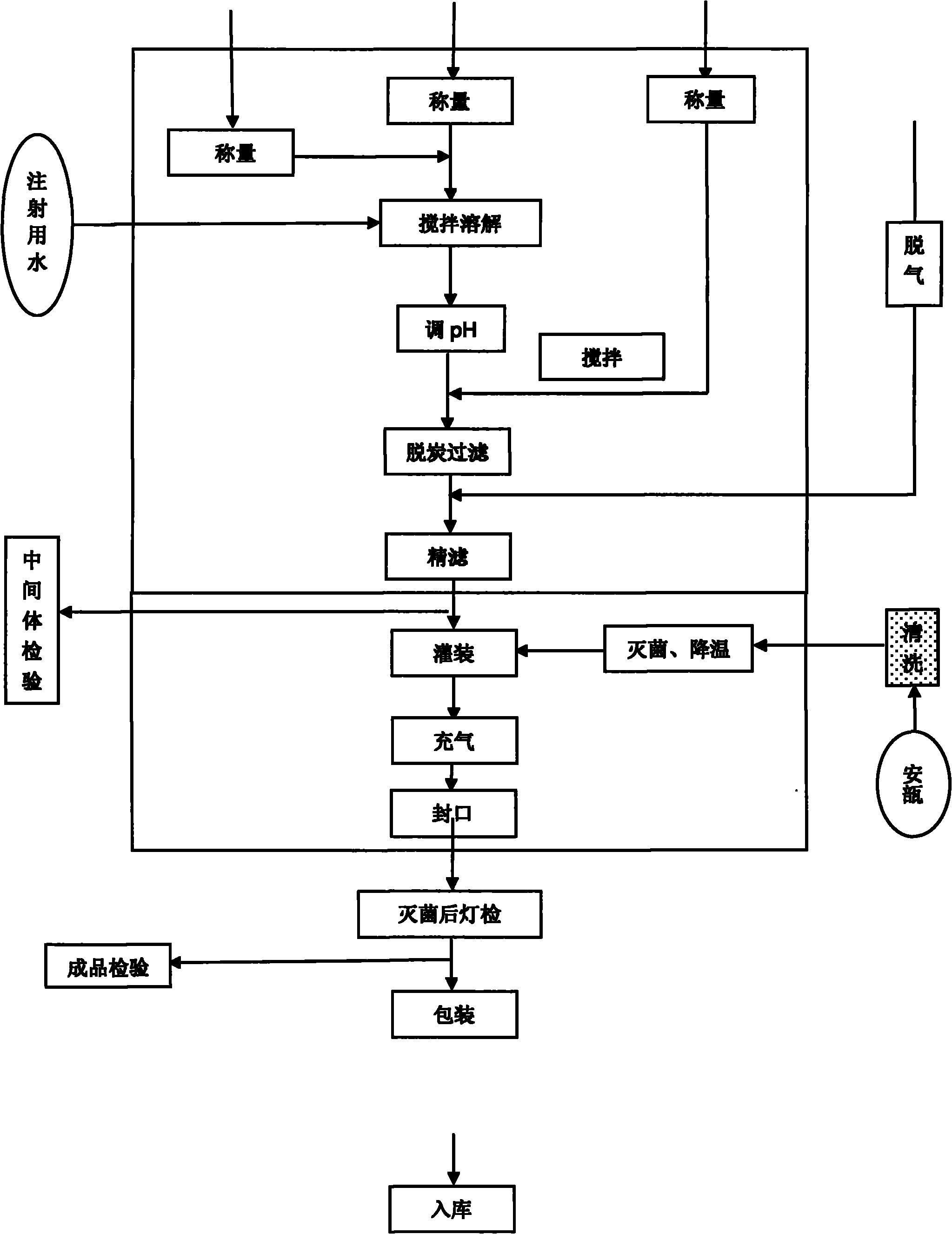
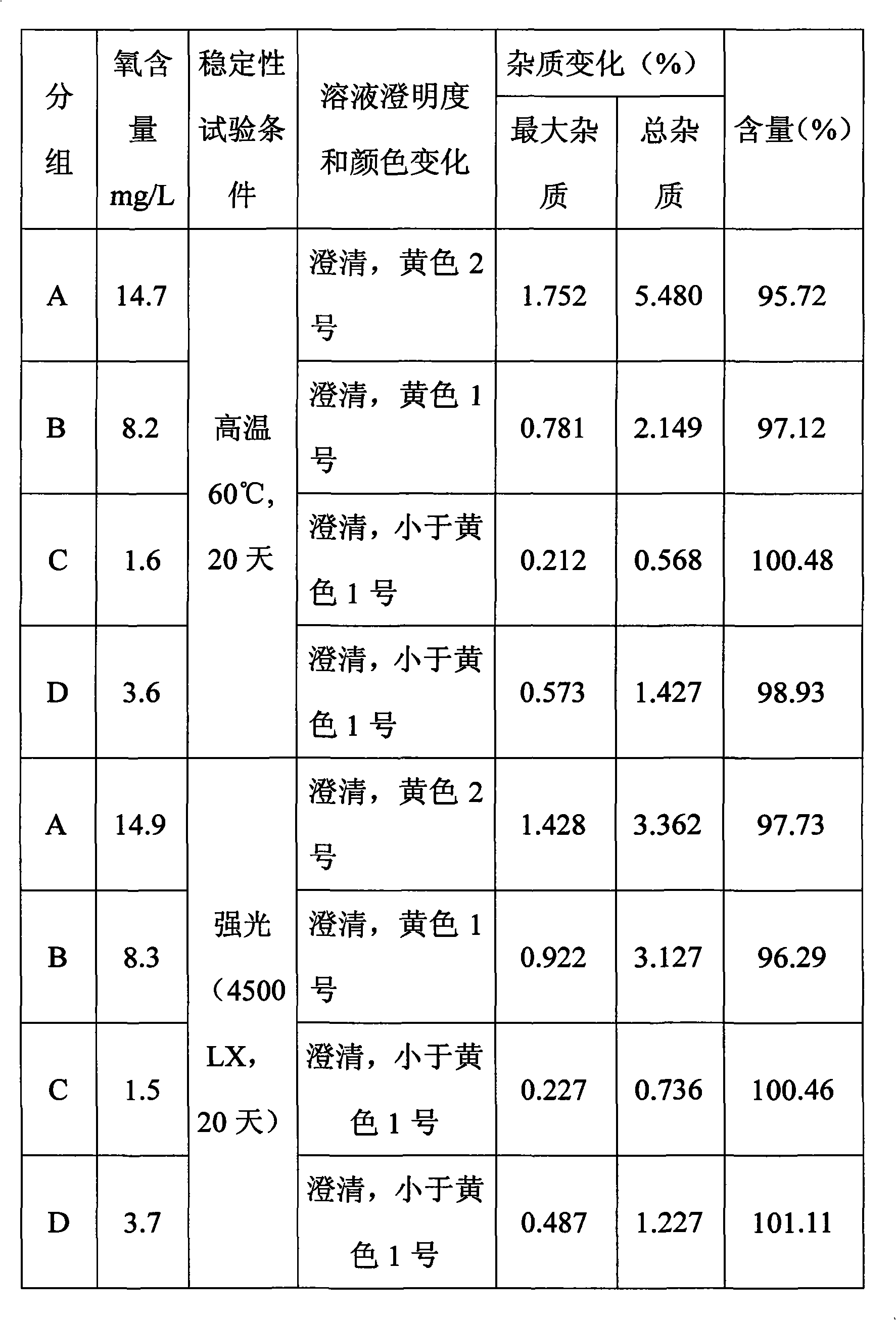
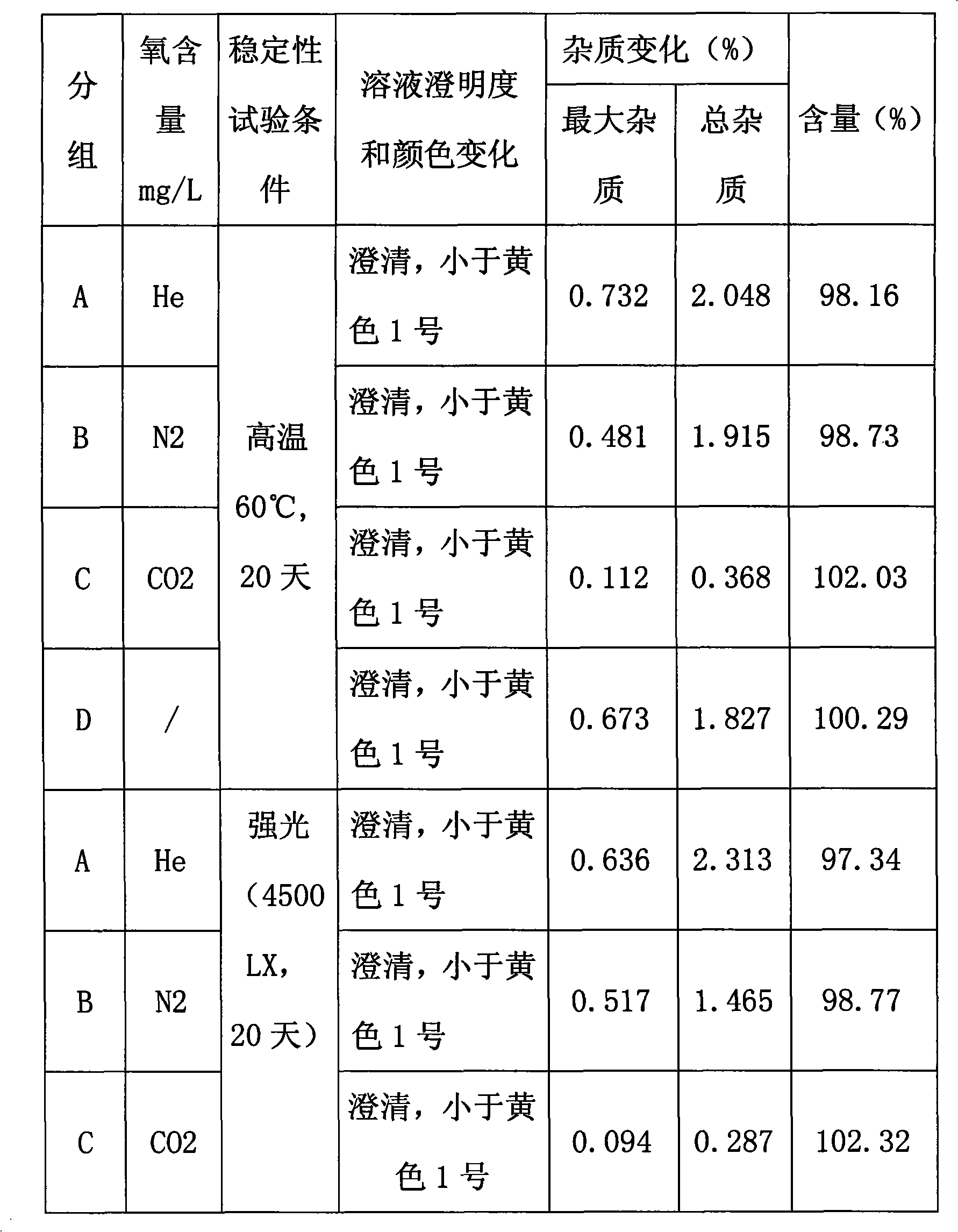
Fasudil salt injection for improving stability and preparation method thereof
InactiveCN102008433AExtended shelf lifeImprove stabilityOrganic active ingredientsInorganic non-active ingredientsInjection productOxygen content
The invention provides a fasudil salt injection for improving stability. The injection consists of the following components based on a weight ratio: the fasudil salt and medical sodium chloride are blended with water to 2,000ml according to a ratio of 100:1 to 1:100 to prepare 1,000 injections. The stability of the fasudil salt injection to light and heat is improved by reducing the oxygen content in the injection and introducing inert gas, so that the preserving period of injection products can be prolonged, and the injection has higher industrial application value. The invention provides a preparation method.
Owner:广东中润医药有限公司 +1
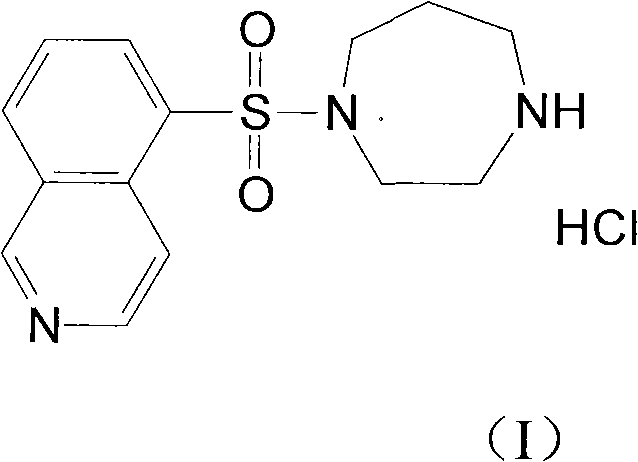
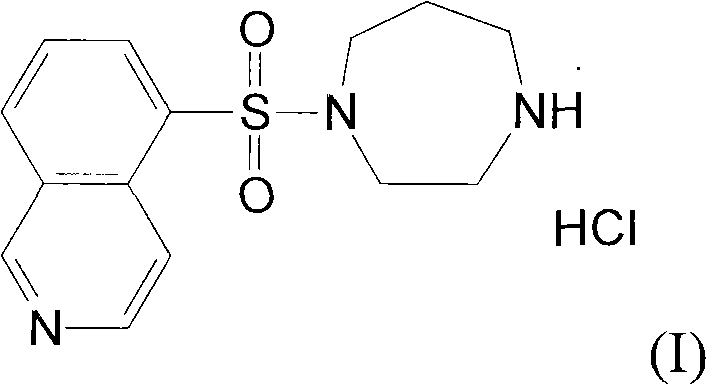
Fasudil hydrochloride compound and novel method thereof
InactiveCN101863880AImproved sulfonation reactionMild reaction conditionsOrganic chemistrySodium bicarbonateSulfonyl chloride
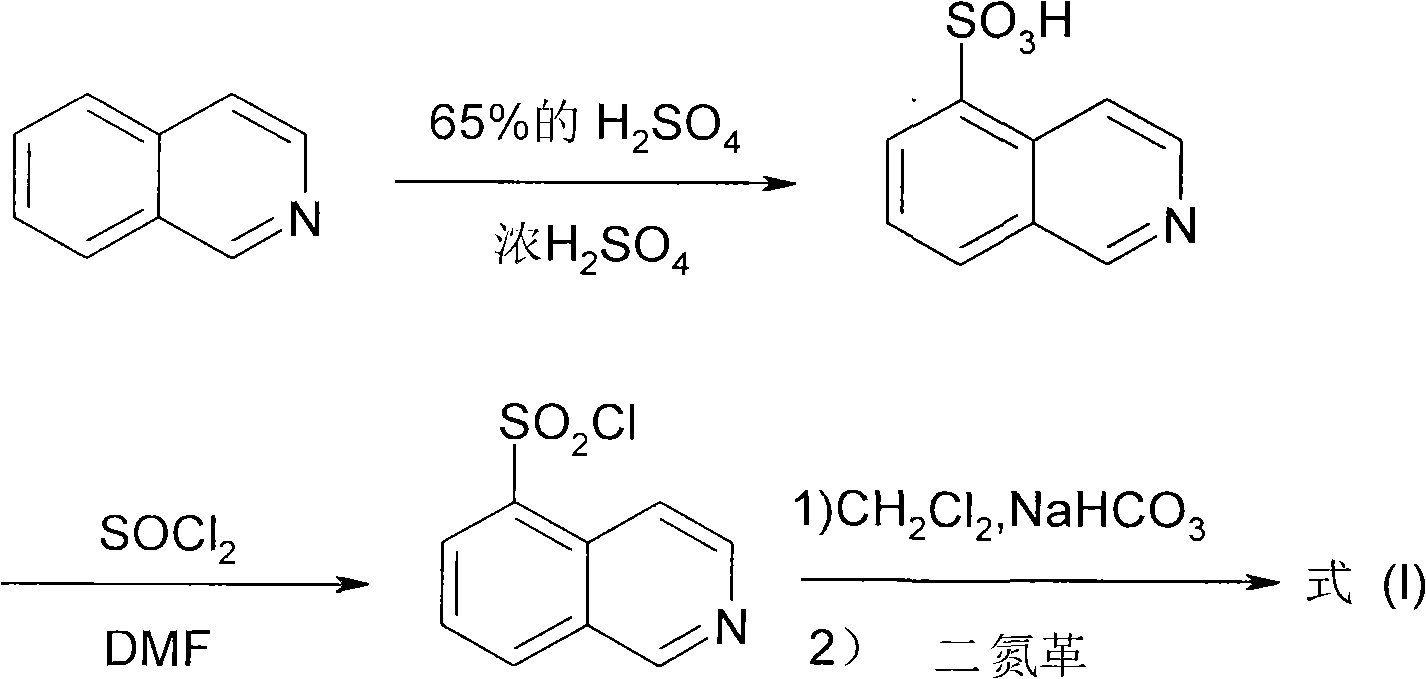
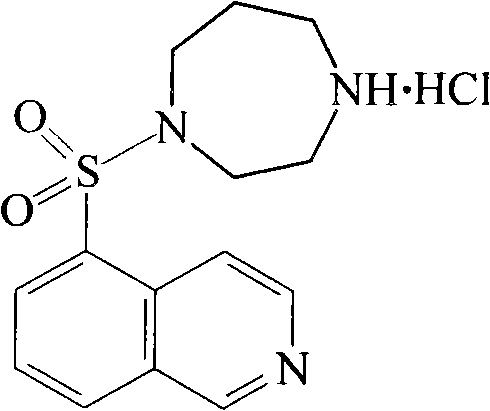
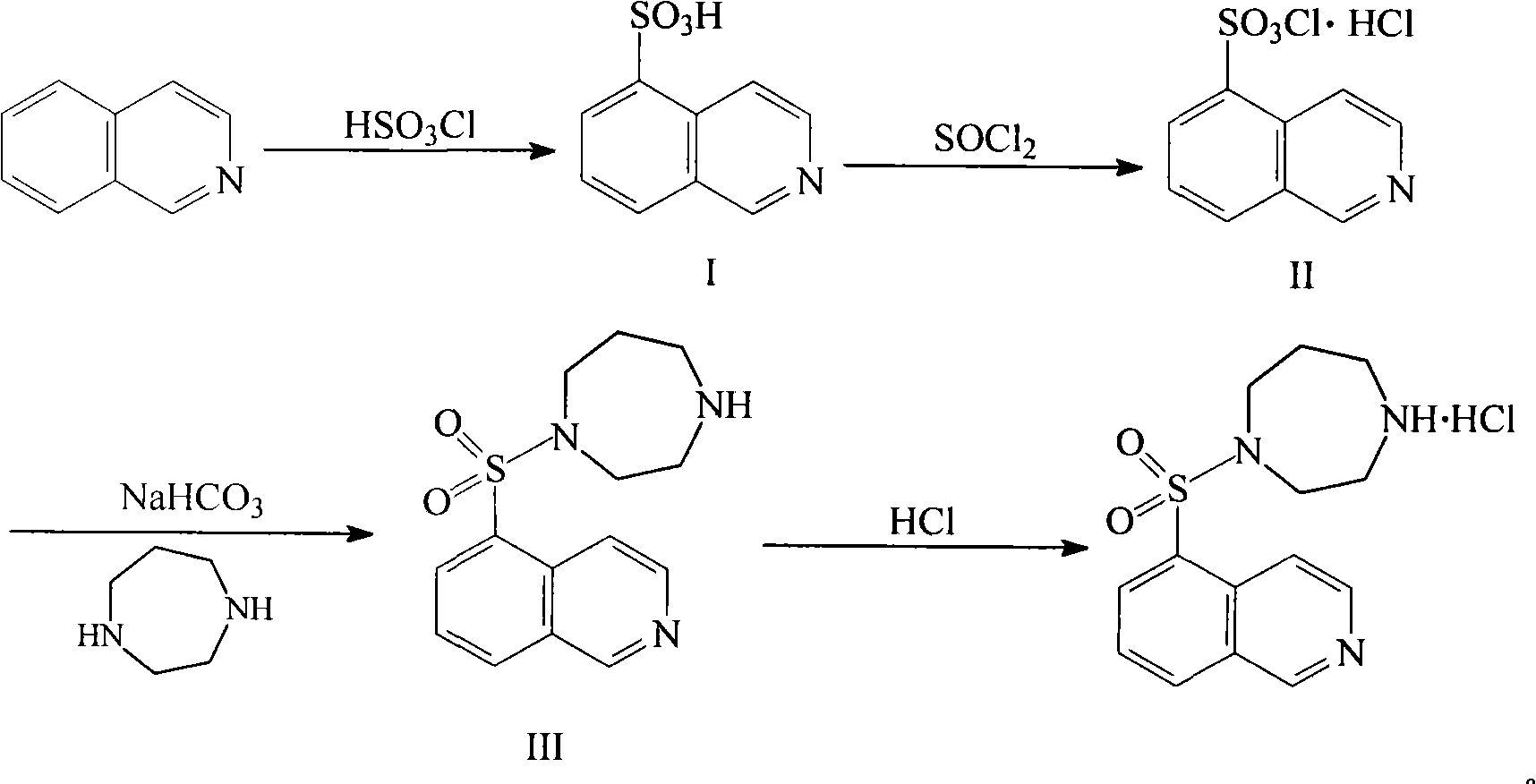
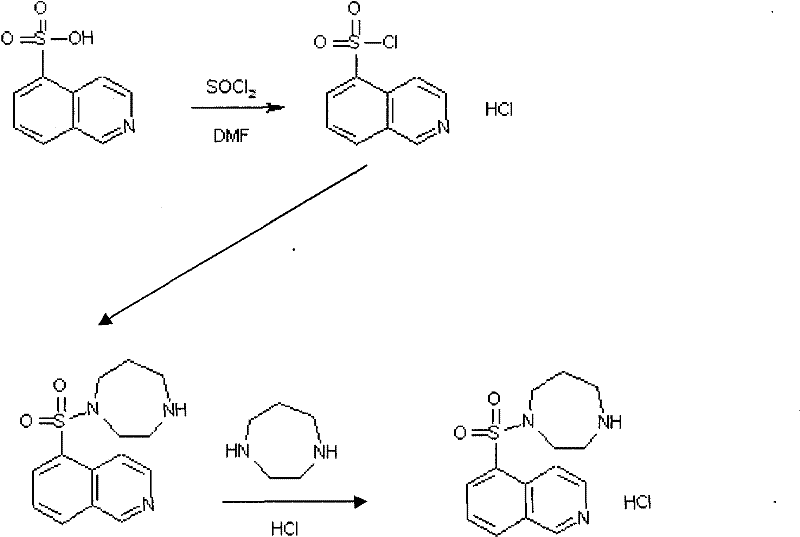
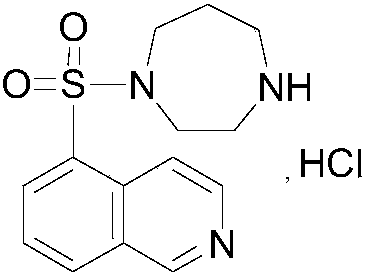
The invention relates to a fasudil hydrochloride compound and a novel method thereof. The method comprises the following steps of: in the presence of a solvent, performing sulfonation reaction on a chlorosulfonic acid serving as a sulfonating agent and isoquinoline to generate a 5-isoquinolinesulfonic acid; reacting the 5-isoquinolinesulfonic acid with thionyl chloride under a heating condition to generate isoquinoline-5-sulfonyl chloride hydrochloride; and dissolving the isoquinoline-5-sulfonyl chloride hydrochloride with ice water, adjusting the pH value of the solution with sodium bicarbonate, reacting the solution with homopiperazine, adjusting the pH value again with a hydrochloric acid, and concentrating under reduced pressure and recrystallizing to prepare the fasudil hydrochloride compound. The synthesis method of the invention has the advantages of mild reaction conditions, high yield and easy industrial production.
Owner:HAINAN LINGKANG PHARMA CO LTD
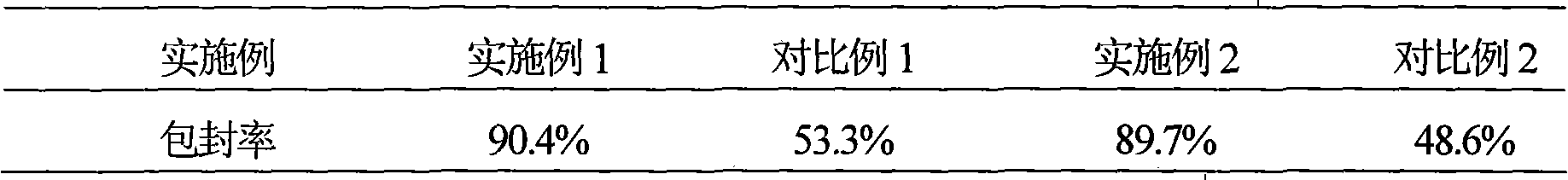
Hydrochloric acid Fasudil liposome injection and new application thereof
InactiveCN101601654AImprove stabilityHigh encapsulation efficiencyOrganic active ingredientsSkeletal disorderLeft vertebral arteryCervical spondylosis
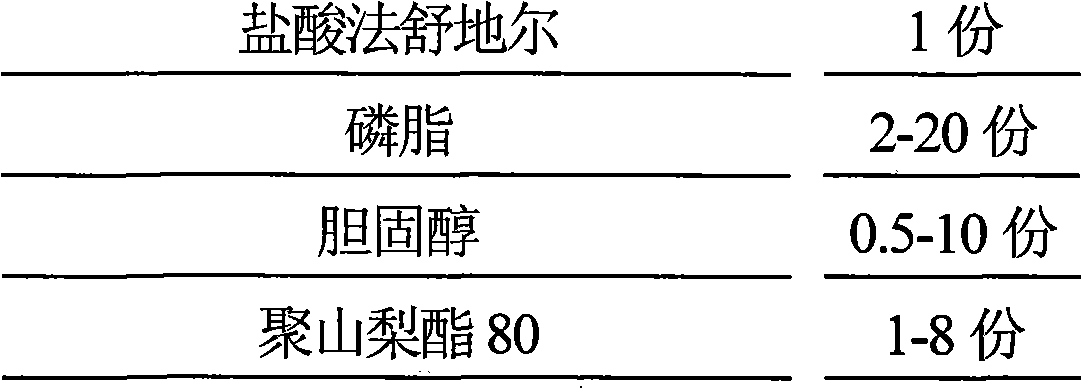
The invention discloses hydrochloric acid Fasudil liposome injection and new application thereof. The injection mainly comprises the following components according to parts by weight: 1 part of hydrochloric acid Fasudil, 2 to 20 parts of phospholipid, 0.5 to 10 parts of cholesterol and 1 to 8 parts of polysorbate 80. Simultaneously, the hydrochloric acid Fasudil liposome injection also can be used for treating vertebral artery type cervical spondylosis.
Owner:HAINAN LINGKANG PHARMA CO LTD
Fasudil hydrochloride injection and its preparation process
InactiveCN1729985AEasy to useAvoid pollutionOrganic active ingredientsPharmaceutical delivery mechanismGlucose polymersAqueous solution
The invention provides a Fasudil hydrochloride injection, which comprises Fasudil hydrochloride, sodium chloride, glucose, amino acid, and the aqueous solution of other pharmaceutically acceptable thinning agent, wherein the content of Fasudil hydrochloride is 0.01-0.2 wt%, the content of the aqueous solution of other pharmaceutically acceptable thinning agent is 0.5-50 wt%, the content of sodium chloride is 0.9-5 wt%, the content of glucose is 5-20%, the content of amino acid is 0.5-30 wt%.
Owner:吴良信
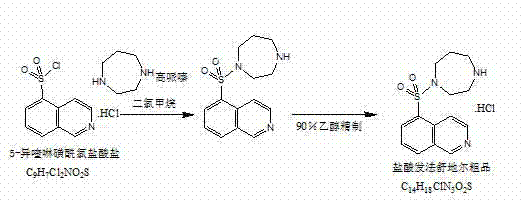
Preparation method of fasudil hydrochloride
The invention relates to a preparation method of fasudil hydrochloride. In the method, 1-(5-isoquinoline sulfonyl) homopiperazine does not undergo chromatographic column separation and purification, lots of organic solvent for elution is omitted, and multiple concentration under reduced pressure becomes unnecessary, thus the process is no longer tedious and time consuming. The crude product of fasudil hydrochloride adopts methanol as the recrystallization solvent, so that the preparation steps are reduced, the energy can be saved and the cost can be reduced. The undried 5-isoquinoline sulfonyl chloride hydrochloride that is subjected to a Karl Fischer moisture test and purified solved in water and dichloromethane, and sodium bicarbonate is added for neutralization followed by layering. The split dichloromethane solution of 5-isoquinoline sulfonyl chloride and the dichloromethane solution of homopiperazine are condensed, and water is added for washing. When the dichloromethane layer isseparated and dried, dry hydrogen chloride gas is introduced into the dichloromethane, so that the fasudil hydrochloride crude product can be obtained. The crude product is then recrystallized in methanol, thus obtaining a competitive product which is white or almost white crystalline powder. The yield of 5-isoquinoline sulfonic acid as the competitive product is calculated as 66.3%.
Owner:吉林省博大伟业制药有限公司
Preparation method of fasudil hydrochloride
InactiveCN103044403ASolve problems that are difficult to obtain through suction filtrationReduce stepsOrganic chemistryIsoquinolineKinase
The invention belongs to the technical field of medicine, and particularly relates to a preparation method of a protein kinase inhibitor fasudil hydrochloride. According to the method, as 5-isoquinoline sulfuryl chloride hydrochloride is adopted to react with homopiperzine directly, the problem that 5-5-isoquinoline sulfuryl chloride is easy to hydrolyze is avoided; and as fasudil hydrochloride is obtained by a direct water phase evaporating method, the problem that the extraction filtration of fasudil hydrochloride is difficult is solved. The preparation method is simple to operate, and higher in product yield and product purity.
Owner:成都天翼医药科技有限公司
Quality control method of fasudil hydrochloride injection
ActiveCN101008637AControl Quality CharacteristicsAccuracyComponent separationColor/spectral properties measurementsEngineeringSurgery
This invention relates to alcaine telfairic injection liquid quality control method, which comprises the following steps: property observing, content identifying; content testing and testing component volume.
Owner:TIANJIN CHASE SUN PHARM CO LTD
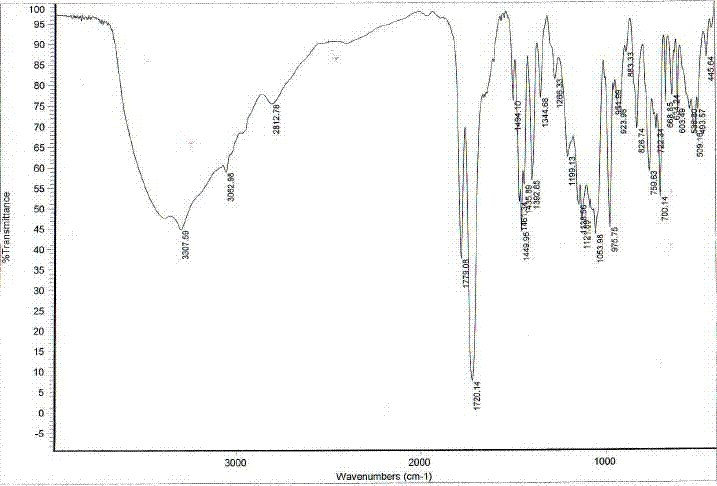
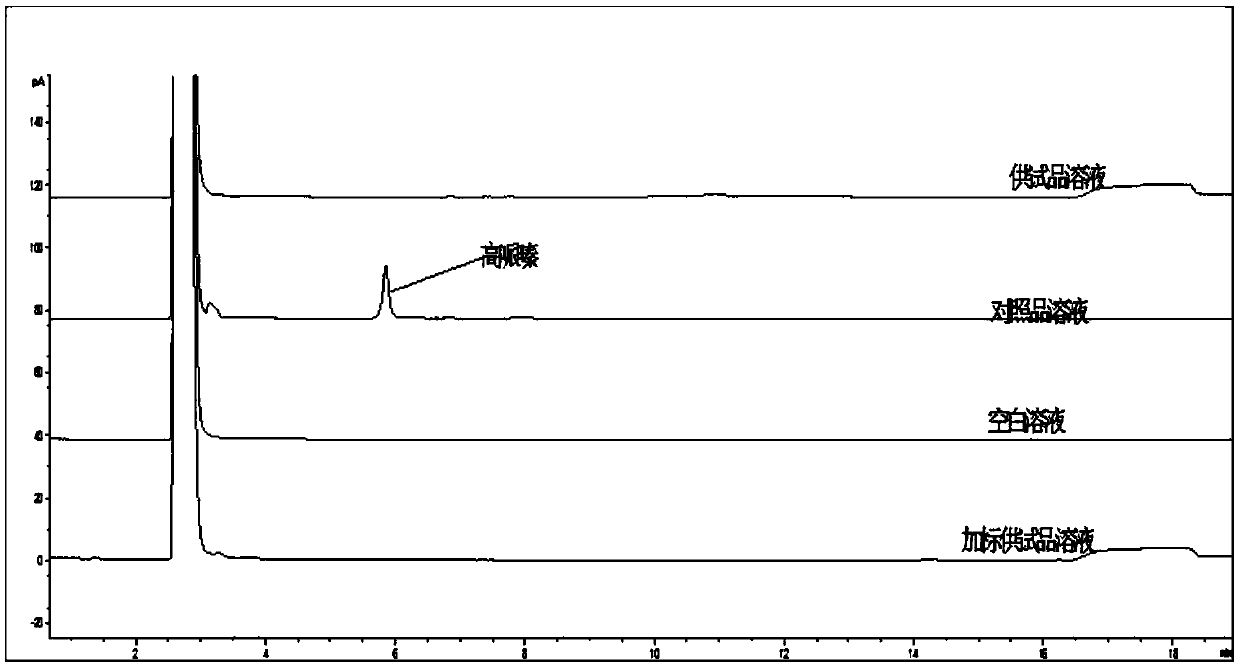
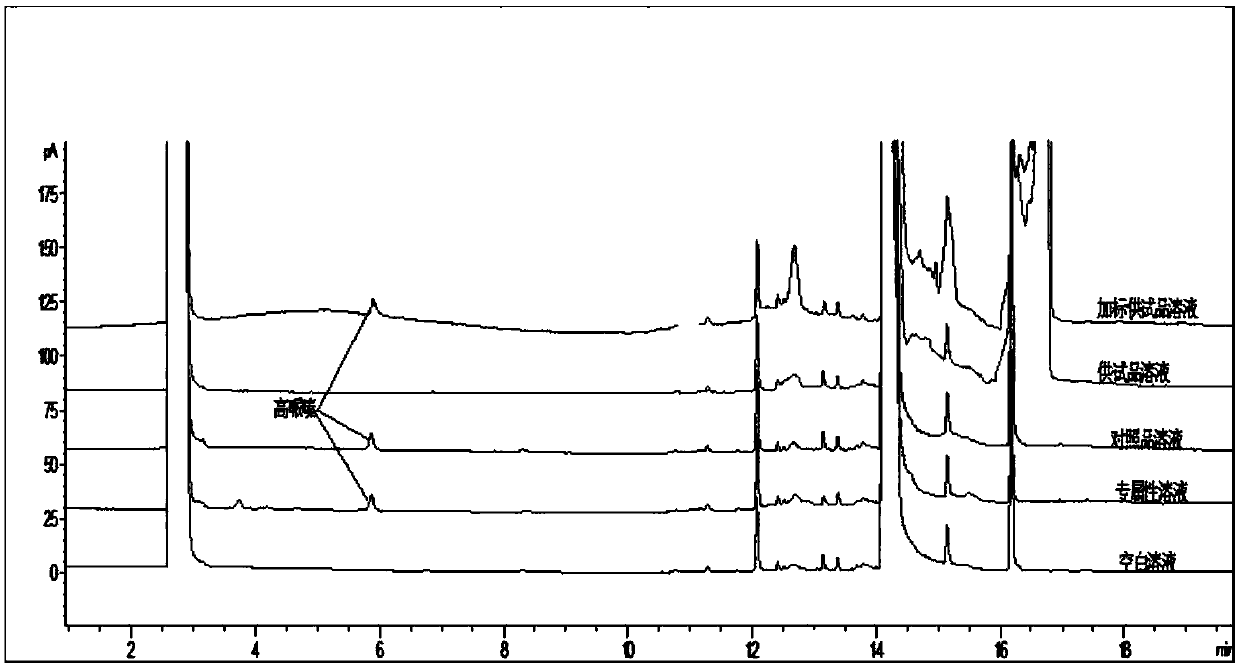
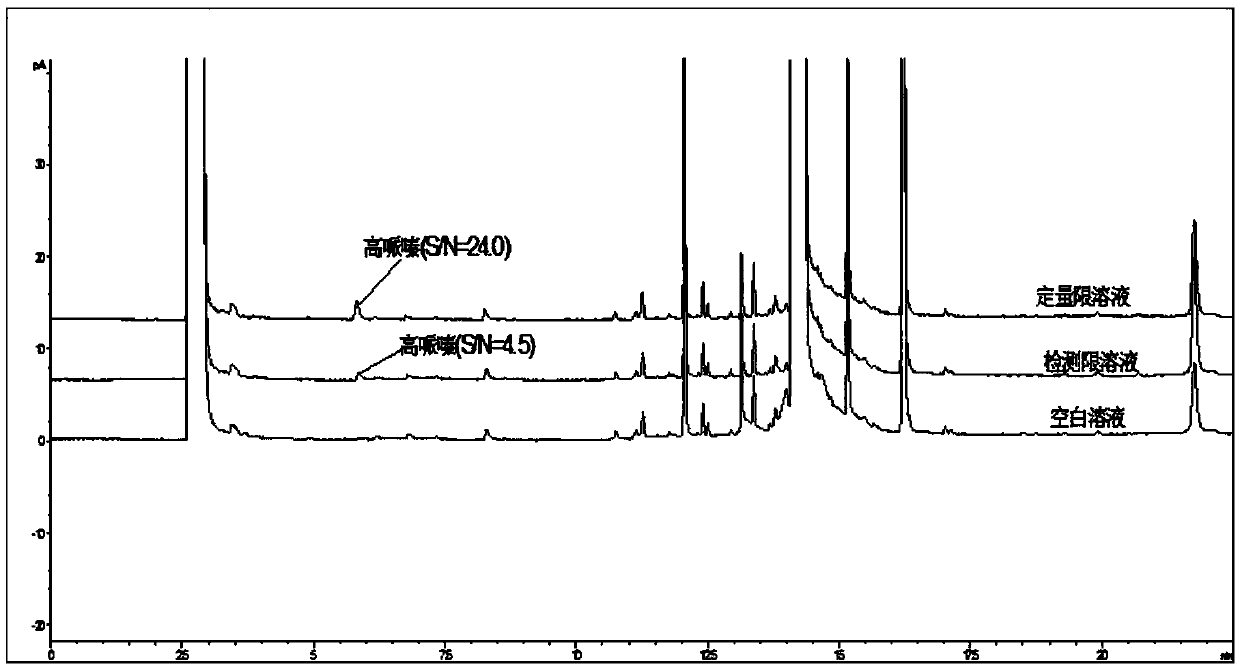
Method for determination of content of related impurity (5-methyl isoquinolinesulfonic acid) of fasudil hydrochloride
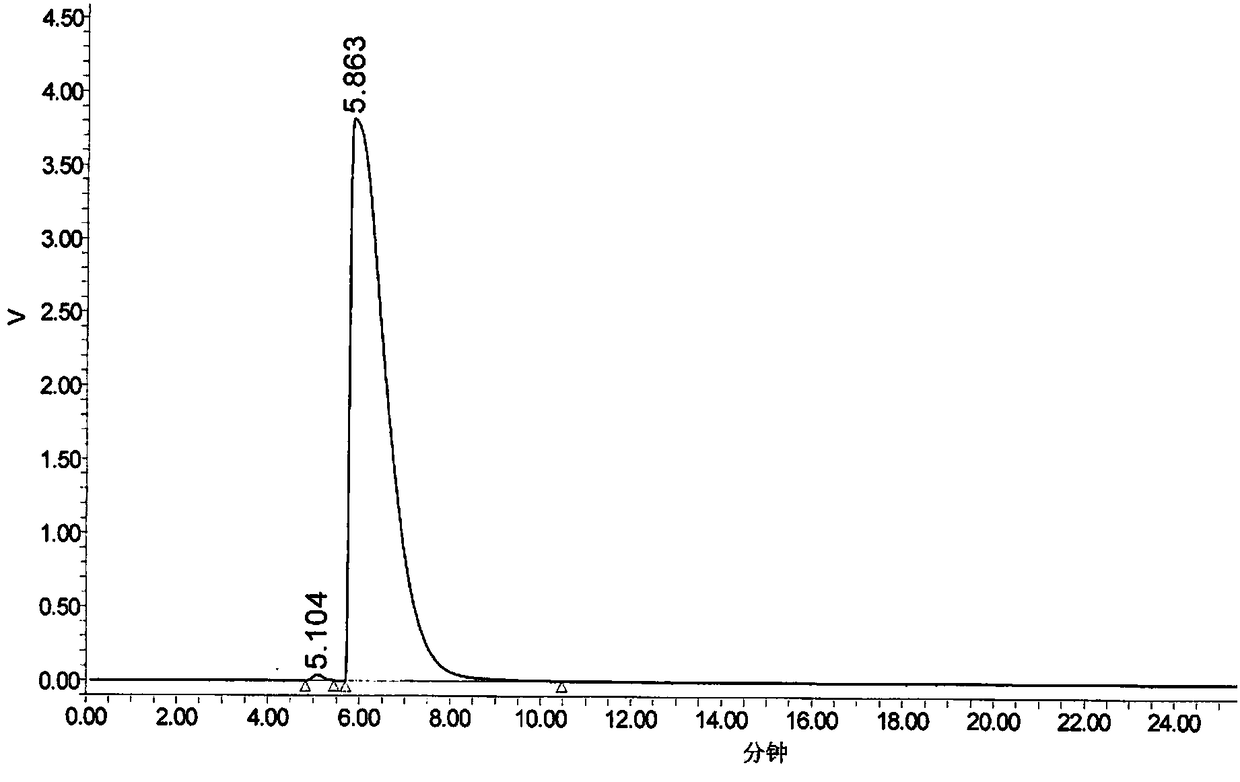
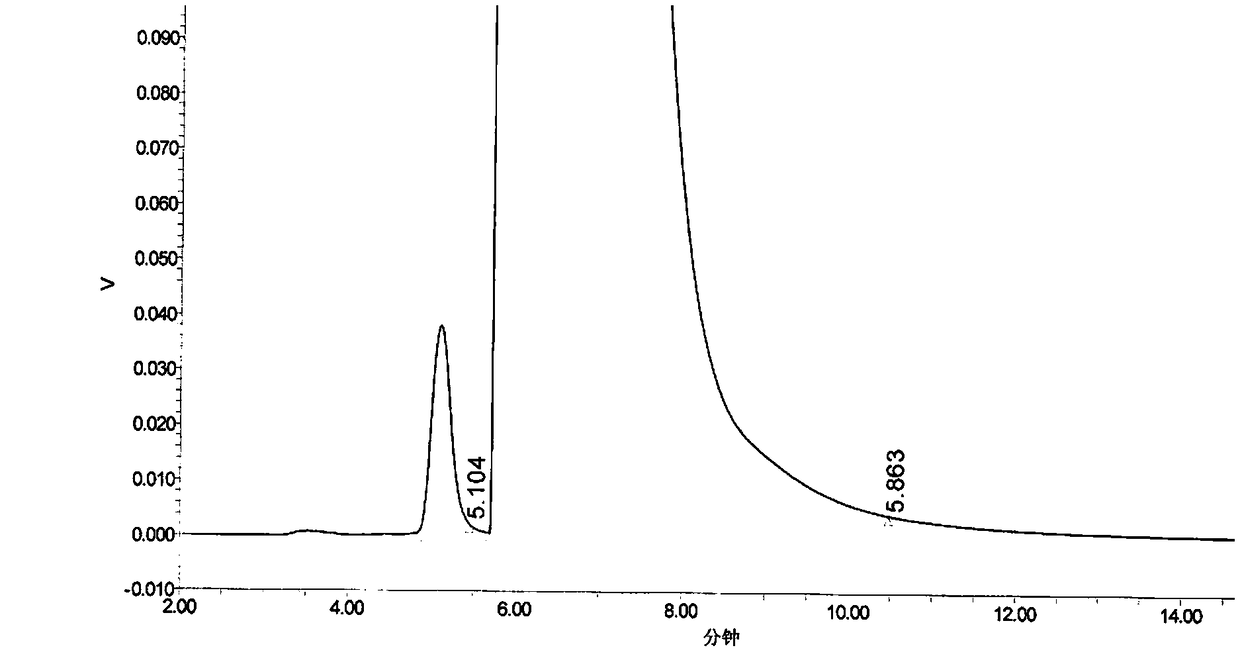
The invention provides a method for determination of the content of a related impurity (5-methyl isoquinolinesulfonic acid) of fasudil hydrochloride. The method is characterized in that a reversed-phase high-performance liquid chromatographic peak area normalization method is adopted for quantitative analysis; for the chromatographic conditions, the chromatographic column is an ACCHROM XAmide column; the flow speed is 0.9-1.1ml / min; the detection wavelength is 238nm; the column temperature is 28-32 DEG C; the sample injection amount is 20mu l; a mobile phase A is 0.05mol / l ammonium dihydrogenphosphate aqueous solution; a mobile phase B is methanol; A:B is equal to (91-94): (6-9) (V:V). The method provided by the invention is convenient to control the product quality in the processes of production and quality control, and has the advantages of low cost, simpleness, easy implementation, high accuracy and precision, good stability and reproducibility and high sensitivity.
Owner:SHANDONG XINHUA PHARMA CO LTD
Freeze dry formulation of fasudil hydrochloride and its preparation process
InactiveCN1729984AGood light stabilityEasy to transportOrganic active ingredientsPowder deliveryGlycineGlucose polymers
The invention relates to a freeze dried powder injection of Fasudil hydrochloride and its preparation, which comprises (by weight percent) Fasudil hydrochloride 1-5%, excipient 4-40%. The excipient can be mannitol, lactose, sodium chloride, glucose, glycine, and / or other pharmaceutically acceptable excipient.
Owner:吴良信
Method for refining fasudil
ActiveCN102002036ALow toxicityLow costOrganic chemistryCardiovascular disorderAcetic acidEthyl ester
The invention discloses a method for refining fasudil. In the method, recrystallization by ethyl acetate and normal hexane can be used for removing some polar small impurities from coarse fasudil, and the obtained product contains less than 0.05 percent of small polar impurities and can meet requirements of standards completely. Compared with the conventional column chromatography, the method hasthe characteristics of simple operation, low production cost and high finished product purity, and can be used in the industrial production of coarse fasudil.
Owner:JIANGSU CAREFREE PHARM CO LTD
Application of Fasudil in preparing medicaments for treating glaucoma through ophthalmic drug delivery
ActiveCN102028694ASignificant miosis and lowering of intraocular pressureOptically activeOrganic active ingredientsSenses disorderHydrobromideCITRATE ESTER
The invention relates to the application of Fasudil in preparing medicaments for treating glaucoma through ophthalmic drug delivery, which belongs to the technical field of medicines. The Fasudil comprises pharmaceutically acceptable saline forms such as hydrochloride, nitrate, sulfate, phosphate, hydrobromide, mesylate, citrate and the like as well as pharmaceutically acceptable forms such as crystallization form, hydrate form and the like. The medicament is a pharmaceutic preparation composition prepared by using the Fasudil as an active pharmaceutical ingredient.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Fasudil hydrochloride injection composition and preparation method thereof
ActiveCN104840418AImprove stabilitySolve the problem of insoluble particulatesOrganic active ingredientsPharmaceutical delivery mechanismHydrochlorideReduce glutathione
The invention provides a fasudil hydrochloride injection composition and a preparation method thereof. According to the fasudil hydrochloride injection composition, fasudil hydrochloride, reduced glutathione, poloxamer 188, sodium chloride and water for injection are mixed, and the pH of the mixture is adjusted to 4.5-5.5 by using a pH adjusting agent. The fasudil hydrochloride injection composition is high in product percent of pass and steady in the presence of light, cannot generate insoluble particles and has good long-term stability; and therefore, the safety of drug application is improved, and the fasudil hydrochloride injection composition is suitable for industrial production.
Owner:SHANDONG NEWTIME PHARMA
Rho kinase inhibitors for use in treating amyotrophic lateral sclerosis
ActiveUS20150031683A1Extended service lifeImprove survivalBiocideNervous disorderDimethylfasudilAmyotrophic lateral sclerosis
The invention relates to a new use of a known Rho kinase inhibitor, fasudil or a fasudil derivative selected from hydroy-fasudil or dimethylfasudil, in the treatment of amyotrophic lateral sclerosis (ALS).
Owner:GEORG AUGUST UNIV GOTTINGEN STIFTUNG OFFENTLICHEN RECHTS UNIVSMEDIZIN
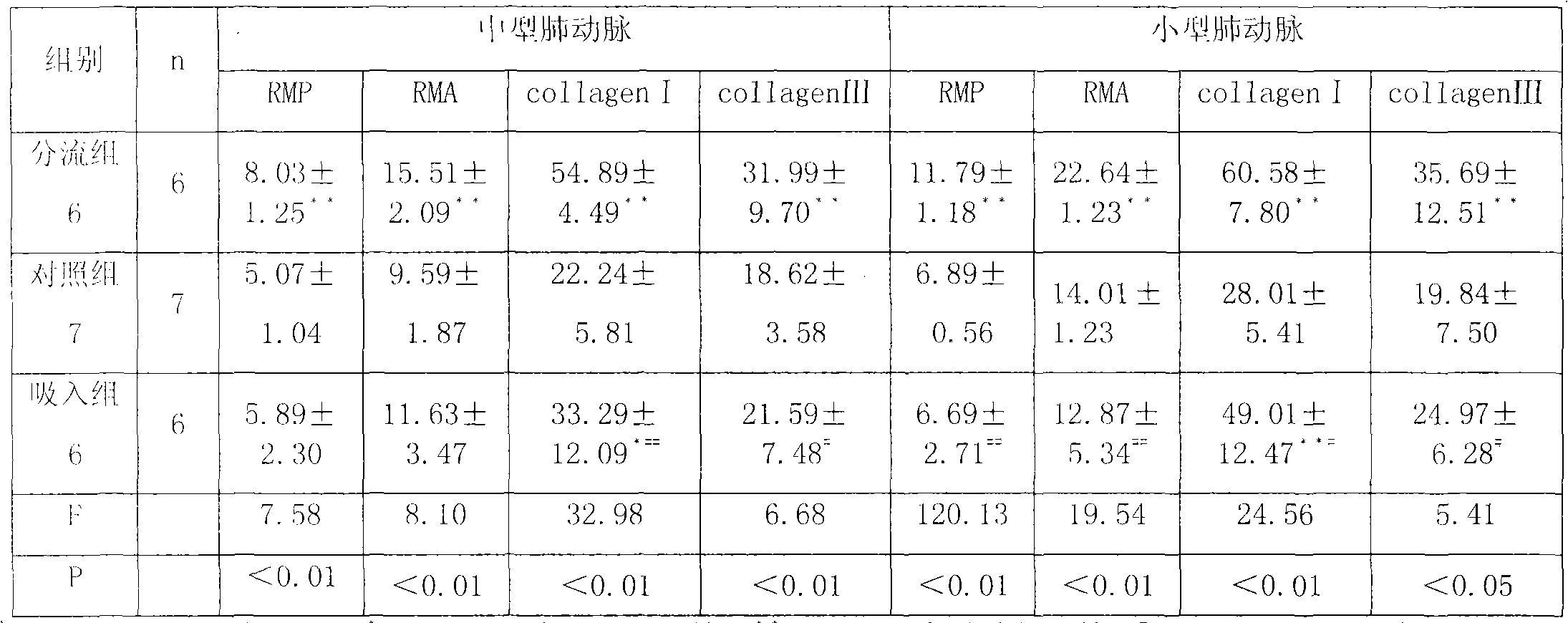
Application of Fasudil in preparing drug for treating pulmonary hypertension by atomized inhalation
The invention belongs to the technical field of medicine, particularly relating to an application of Fasudil in preparing a drug for treating pulmonary hypertension by atomized inhalation. The Fasudil comprises the modes of pharmaceutically acceptable salts, such as hydrochloride, nitrate, sulphate, phosphate, hydrobromide, mesylate, citrate and the like of the Fasudil, and comprises the pharmaceutically acceptable modes, such as a crystallization mode, a hydrate mode and the like of the Fasudil. The drug is a drug preparation composition prepared by taking the Fasudil as the drug active ingredient.
Owner:TIANJIN CHASE SUN PHARM CO LTD
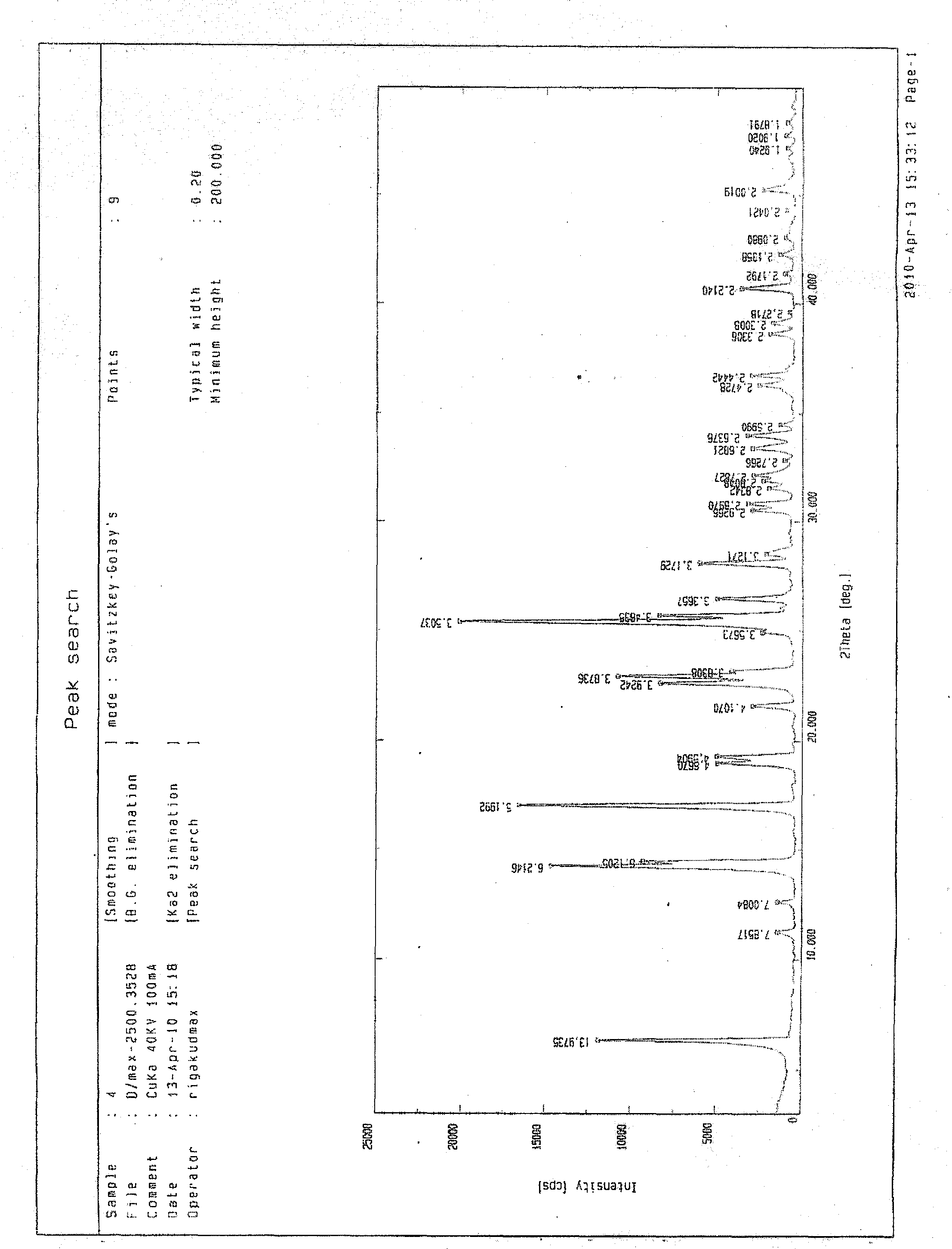
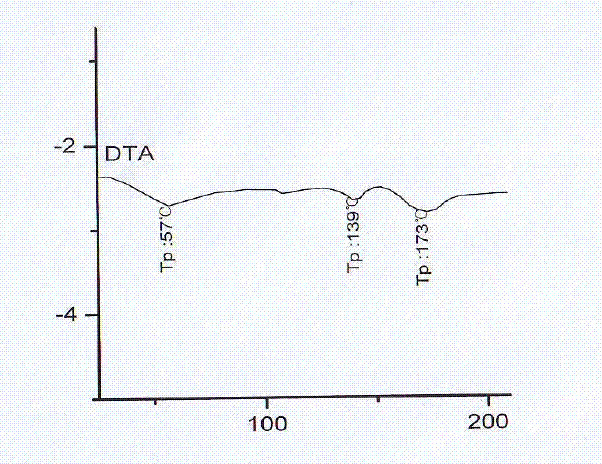
Fasudil crystal formation IV as well as preparation method and application thereof
The invention particularly relates to a Fasudil crystal formation IV as well as a preparation method and application thereof, belonging to the technical field of medicines. For the Fasudil hydrochloride V crystal formation, Cu-Ka radiation is adopted, and X-ray powder represented by an angle of 2 theta is diffracted by angles of 6.320, 14.240, 14.460, 17.040, 22.640, 22.940, 25.400, 25.700 and 28.100 degrees, wherein the angle of 2 theta has a characteristic peak. The Fasudil hydrochloride crystal formation prepared with the preparation method provided by the invention has the advantages of high physical stability, high purity and the like when being stored and used at normal temperature and is further suitable for industrial production. The invention further discloses the application of the Fasudil hydrochloride in improving and preventing of ischemic cranial vascular diseases caused by various reasons.
Owner:TIANJIN CHASE SUN PHARM CO LTD
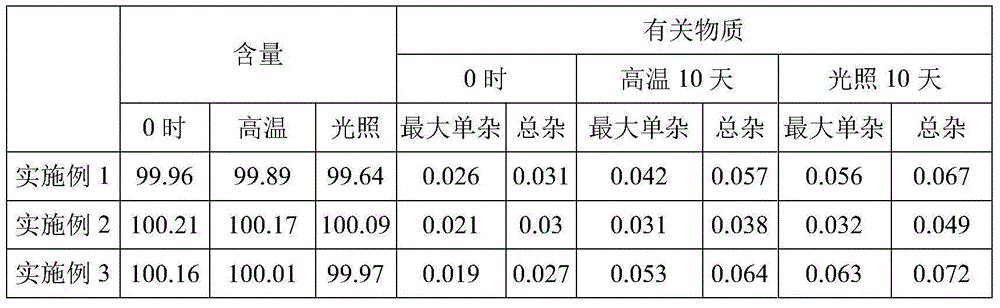
Quality control method for fasudil hydrochloride
ActiveCN105866263AQuality improvementHigh precisionComponent separationMaterial analysis by optical meansSide reactionImpurity
The invention discloses a quality control method for fasudil hydrochloride. The quality control method comprises the following steps: observation of properties; identification of contents; inspection of the contents; and determination of the contents of components included in the contents; wherein inspection of the contents comprises determination of the contents of related substances and determination of the content of homopiperazine. In the process of a synthesis reaction, fasudil hydrochloride is prone to generation of degradation impurities and to a side reaction for production of a dimer impurity and a starting reaction material homopiperazine is hard to thoroughly remove, so purity and security of fasudil hydrochloride are influenced; thus, the contents of impurities in fasudil hydrochloride should be determined in time in the production process so as to control product quality in the production process. The method provided by the invention has additional steps of determination of dimer content and determination of homopiperazine content on the basis of conventional control method, so the quality control method further improves the quality of fasudil hydrochloride, perfects current industrial standards, has the advantages of simplicity, easy practicability and high precision and accuracy and is of great significance to improvement of product quality.
Owner:SICHUAN SUNNYHOPE PHARM CO LTD
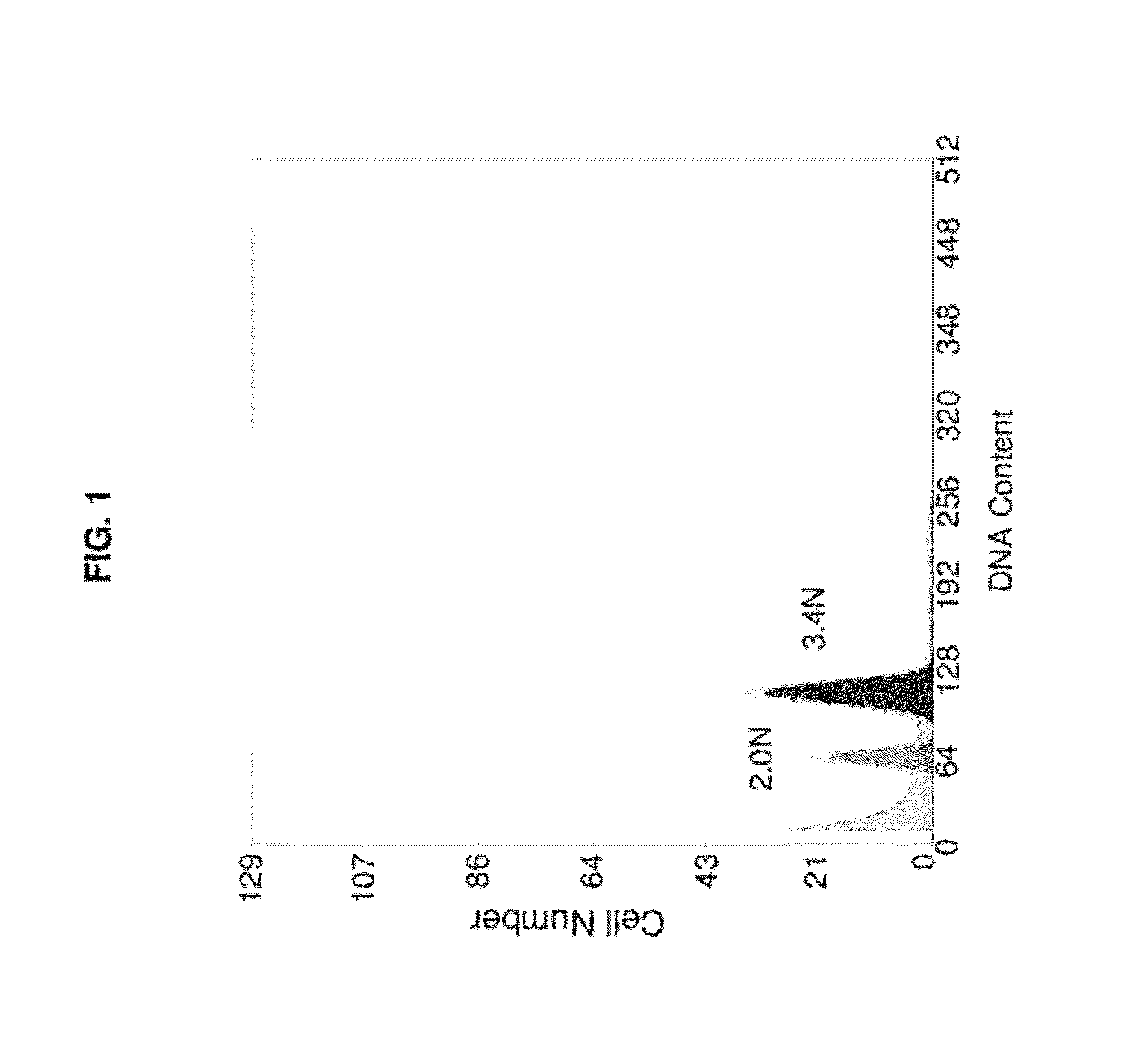
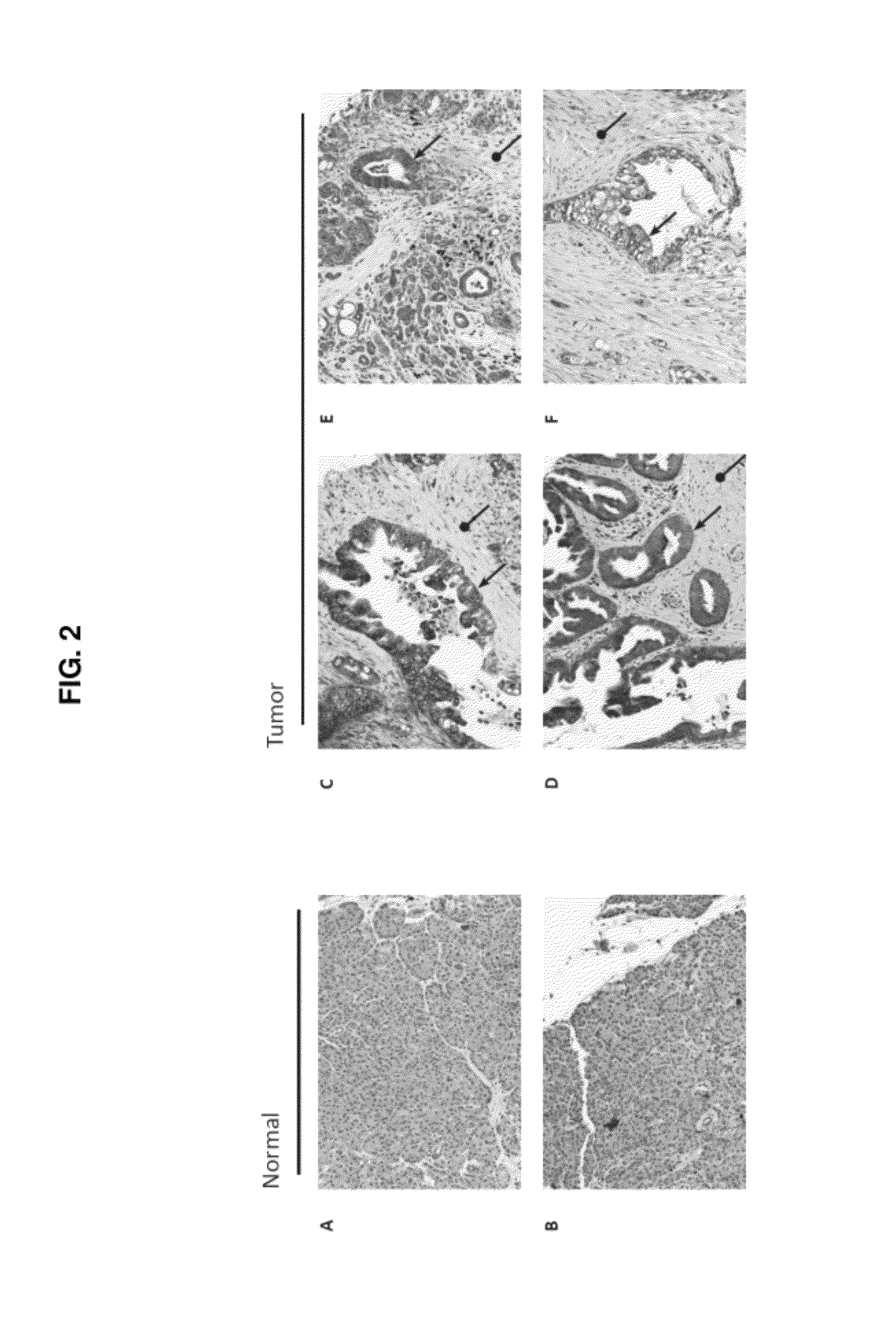
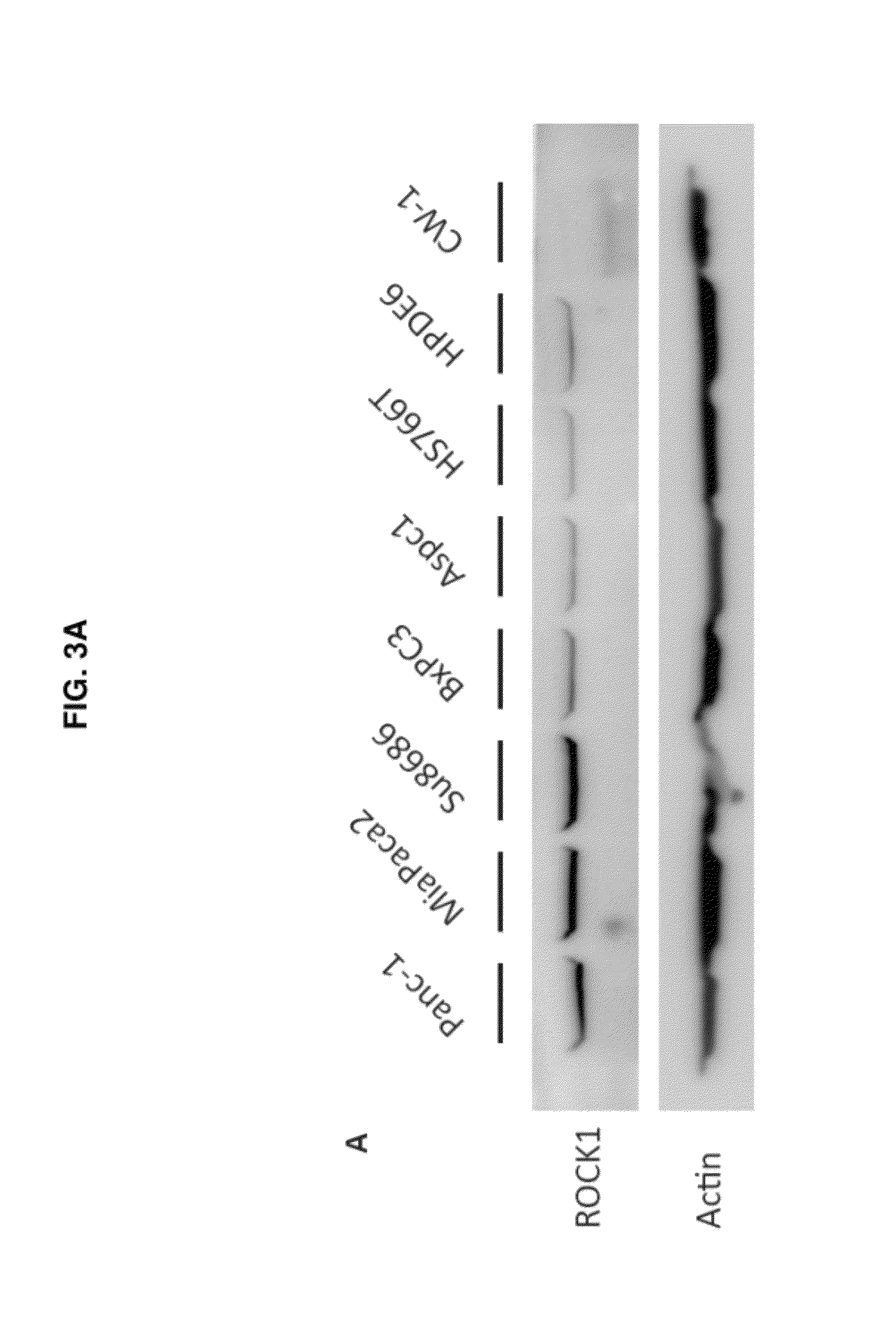
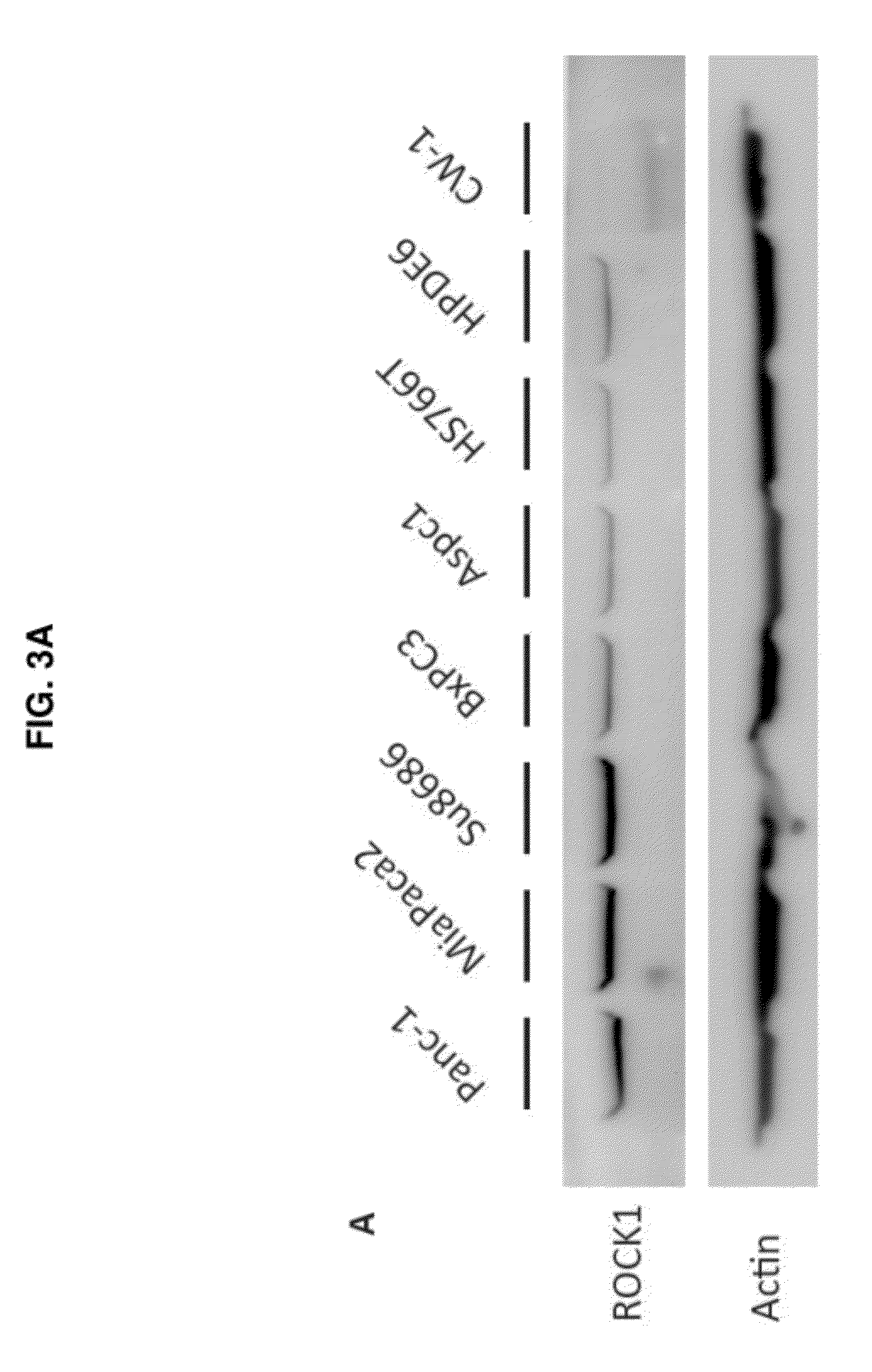
Therapeutic target for pancreatic cancer cells
ActiveUS8703736B2Reducing stromal contributionInhibit migrationBiocideMicrobiological testing/measurementPancreas CancersPancreatic cancer cell
This invention provides a therapeutic target for pancreatic cancer. The invention further provides methods of screening of new therapeutic agents using the target. The invention also provides a pharmaceutical composition comprising fasudil or derivatives thereof for pancreatic cancer treatment, and a kit comprising such a pharmaceutical composition.
Owner:TRANSLATIONAL GENOMICS RESEARCH INSTITUTE
Fasudil hydrochloride injection and preparing method thereof
ActiveCN105168224ASimple preparation processLow costOrganic active ingredientsPharmaceutical delivery mechanismEthylenediamineAcetic acid
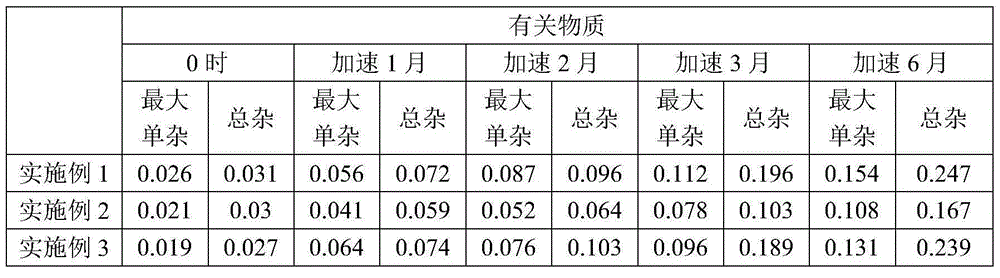
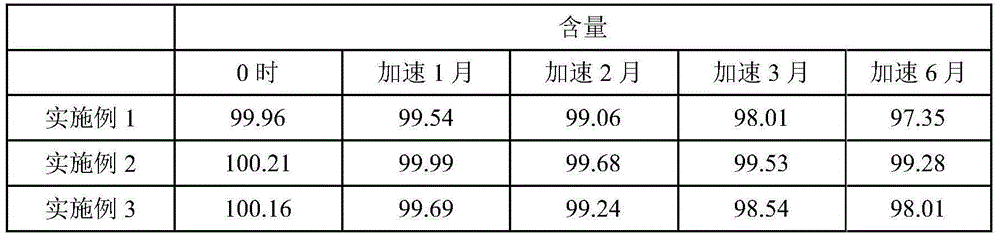
The invention belongs to the technical field of drug preparation, and particularly relates to fasudil hydrochloride injection and a preparing method thereof. The fasudil hydrochloride injection is prepared from, by mass, 30 parts of fasudil hydrochloride, 5-15 parts of sodium citrate, 0.1-0.4 part of ethylenediamine tetraacetic acid disodium, and 1500-2500 parts of injection water. Compared with the prior art, the fasudil hydrochloride injection has the advantages that the ph value, content and related material results of every embodiment are still qualified after the fasudil hydrochloride injection is exposed to high temperature, illumination and acceleration for 6 months and 24 months, and therefore the quality of the whole product is stable; furthermore, the preparing process is simple, cost is quite low, and industrialized production is facilitated.
Owner:CISEN PHARMA
Therapeutic target for pancreatic cancer cells
ActiveUS20120252753A1Inhibiting pancreatic tumor cell proliferationInhibit migrationBiocideMicrobiological testing/measurementMedicinePancreatic cancer cell
This invention provides a therapeutic target for pancreatic cancer. The invention further provides methods of screening of new therapeutic agents using the target. The invention also provides a pharmaceutical composition comprising fasudil or derivatives thereof for pancreatic cancer treatment, and a kit comprising such a pharmaceutical composition.
Owner:TRANSLATIONAL GENOMICS RESEARCH INSTITUTE
Pharmaceutical composition containing fasudil and sildenafil, and preparation method and application thereof
The invention relates to a pharmaceutical composition containing fasudil and sildenafil for treating pulmonary hypertension, and a preparation method thereof, belonging to the field of pharmacy. The composition provided by the invention uses fasudil and sildenafil as the pharmaceutical active components, and the composition also comprises other pharmaceutically acceptable carriers, such as a disintegrant, an adhesive, an excipient, a thinner, a lubricant, a skeletal material for controlling the release rate of the active components in a sustained or controlled release preparation, and the like, and the suitable carriers are selected for different dosage forms.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Stable fasudil hydrochloride injection and preparation method thereof
ActiveCN105919931AStable lightingHigh clarityOrganic active ingredientsNervous disorderMass ratioFiltration
The invention belongs to the field of pharmaceutical preparation, and particularly relates to stable fasudil hydrochloride injection and a preparation method thereof. The fasudil hydrochloride injection comprises fasudil hydrochloride, stabilizers, anhydrous citric acid, sodium citrate dihydrate, sodium chloride and water for injection, wherein the stabilizers comprise N-carboxymethyl chitosan and reduced glutathione according to the mass ratio of 1:(0.05-0.35). The preparation method includes the steps: dissolving components in the formula by the water for injection after pyrogen removal; adjusting pH (potential of hydrogen); adding medicinal carbon for stirring adsorption to further remove pyrogen and perform discoloration; performing filtration and decarburization; performing filtration, sterilization, sub-package, potting and sterilization to obtain the fasudil hydrochloride injection. The fasudil hydrochloride injection has fine light stability and simple in formula, insoluble particles are effectively suppressed, the preparation process is easily operated, and industrialization is facilitated.
Owner:中润药业有限公司
Compound of stable Fasudil hydrochloride hydrate
The invention which belongs to the technical field of medicines concretely relates to a Fasudil hydrochloride hydrate and a preparation method thereof. Fasudil hydrochloride prepared in the invention which has three and a half crystal waters has the advantages of high purity, good stability, unapparent weight gain of moisture absorption in a high humidity condition and good formability. The invention also relates to an application of the hydrate in preparing drugs for improving and preventing ischemic cerebrovascular diseases caused by a plurality of reasons, such as cerebral infarction, vertebrobasilar artery insufficiency, delayed cerebrovascular disease caused by subarachnoid hemorrhage, cerebral vasospasm caused by brain surgery and interventional therapies, and transient ischemic attack.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Method for detecting homopiperazine in fasudil hydrochloride
The invention discloses a method for detecting homopiperazine in fasudil hydrochloride, which uses gas chromatography to qualitatively or quantitatively detect the homopiperazine in the fasudil hydrochloride. The diluent used in the sampling detection solution in the gas chromatography comprises DBU and organic solvent. According to the method for detecting the homopiperazine in the fasudil hydrochloride, the homopiperazine in the fasudil hydrochloride can be detected effectively and veritably, and the quality and the safety of fasudil hydrochloride are guaranteed.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
Stable large-capacity fasudil hydrochloride injection
ActiveCN102138894ALower pHRisk of causing secondary pollutionOrganic active ingredientsPharmaceutical delivery mechanismHydrochlorideChloride sodium
The invention discloses a stable large-capacity fasudil hydrochloride injection, which comprises 30-120mg of fasudil hydrochloride and 5%-20% of glucose, or comprises 30-120mg of fasudil hydrochloride, 5%-10% of glucose and 0.9% of sodium chloride, wherein the pH (potential of hydrogen) value of the solution is 3.5-4.0, and the pH value is regulated through hydrochloric acid or NaOH.
Owner:GUANGDONG QIFANG MEDICINES CO LTD
Oral sustained-release preparation of fasudil hydrochloride
InactiveUS20060280793A1Reduce the burden onHigh activityOrganic active ingredientsPill deliveryDissolutionBULK ACTIVE INGREDIENT
Disclosed is an oral sustained-release preparation which contains at least one active-ingredient selected from the group consisting of fasudil hydrochloride and a hydrate thereof, the preparation comprising at least one sustained-release coated particle comprising a core having a surface and a coating formed on the surface of the core, wherein the core contains the active ingredient and the coating comprises a coating base material and a specific insoluble auxiliary material, and wherein the preparation exhibits, with respect to the active ingredient, a specific dissolution rate, as measured by the dissolution test. By using the oral sustained-release preparation of the present invention, it becomes possible to surely control the release of fasudil hydrochloride from the preparation, so that the effect of the active ingredient is maintained for a long period of time. Therefore, the burden of the patient who has to take the preparation can be decreased and the compliance with respect to the administration of the preparation can be improved. Also disclosed is a method for evaluating an oral sustained-release preparation containing the active ingredient, wherein the evaluation is conducted with respect to the sustained-release ability of the active ingredient.
Owner:ASAHI KASEI PHARMA
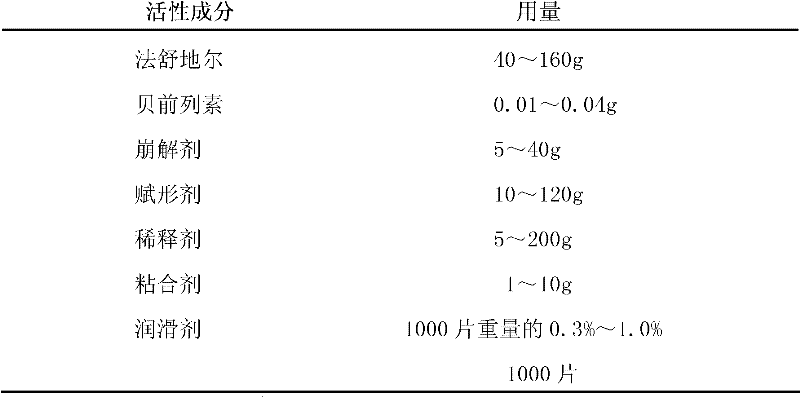
Compound preparation of Fasudil and Beraprost as well as preparation method and application thereof
The invention belongs to the pharmaceutical field and relates to a compound preparation of Fasudil and Beraprost as well as a preparation method and application thereof to treating pulmonary hypertension. The compound preparation in the invention comprises Fasudil, Beraprost and auxiliary materials chosen from auxiliary materials acceptable in pharmacy, such as a disintegrating agent, an adhesive, an excipient, diluents, a lubricant, framework materials used for controlling the release of active ingredients in slow and controlled release agents, and the like. Proper carriers are chosen according to different types of drugs.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com