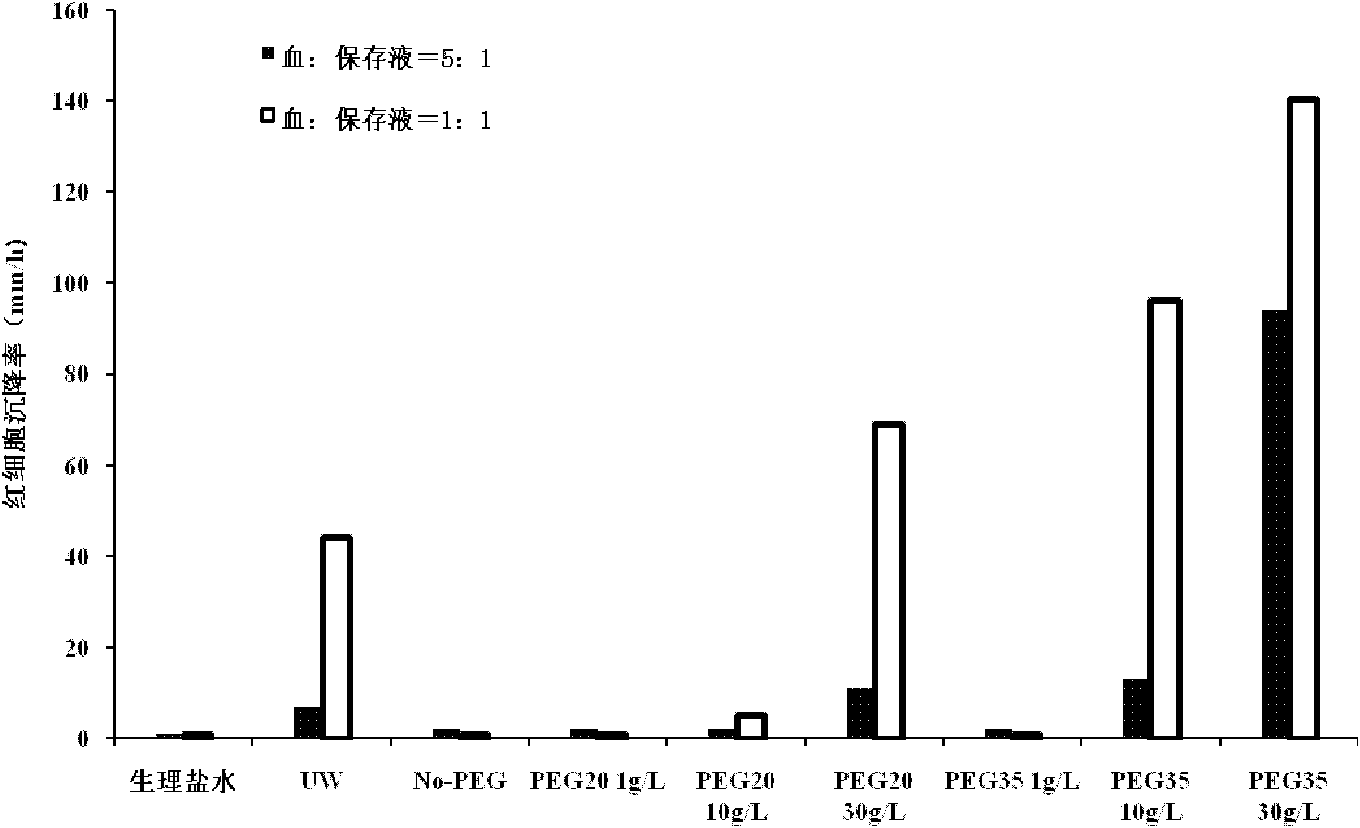
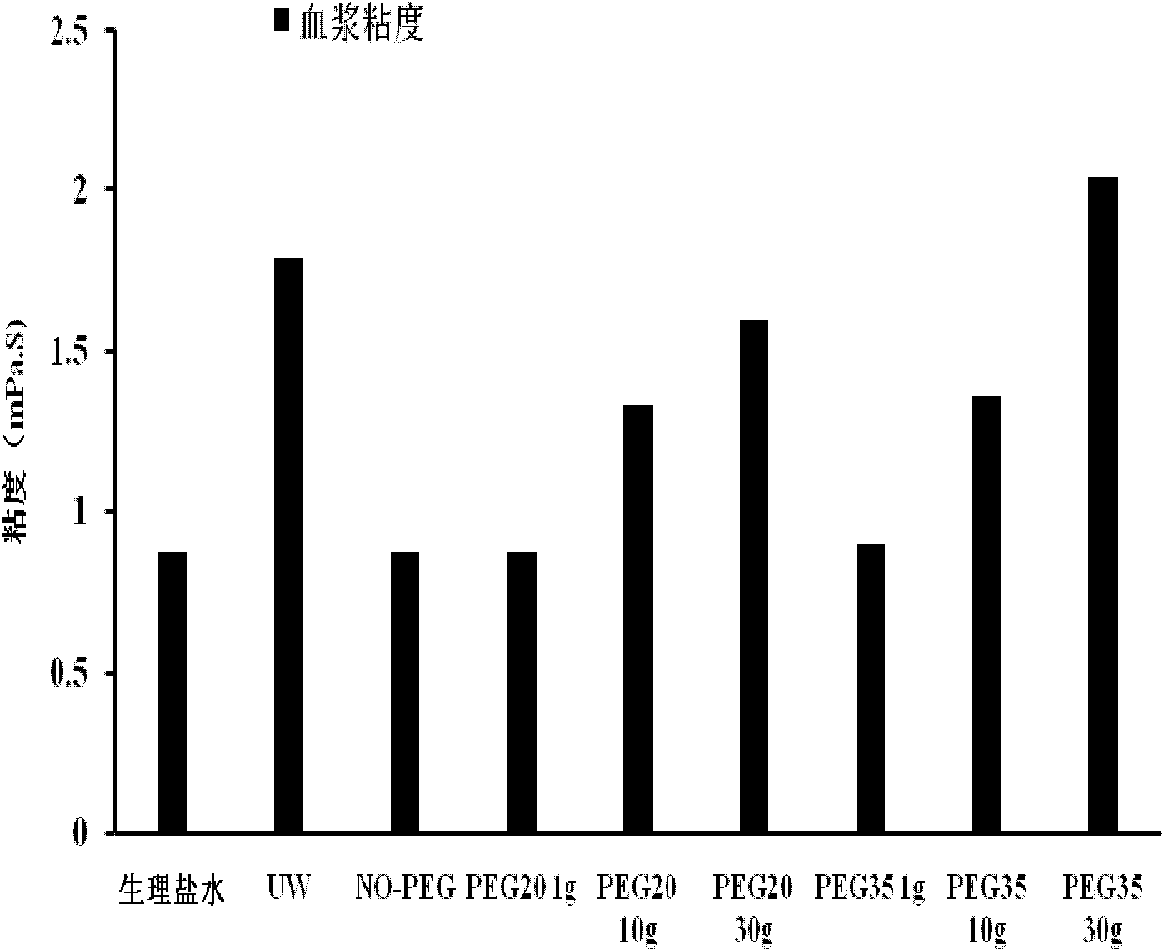
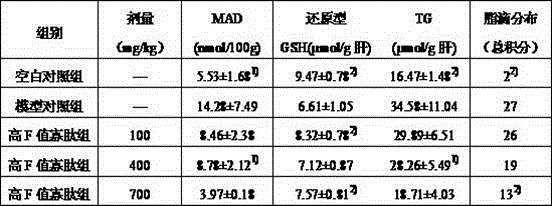
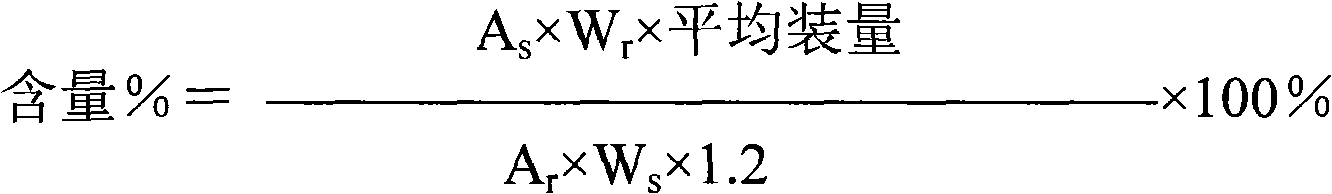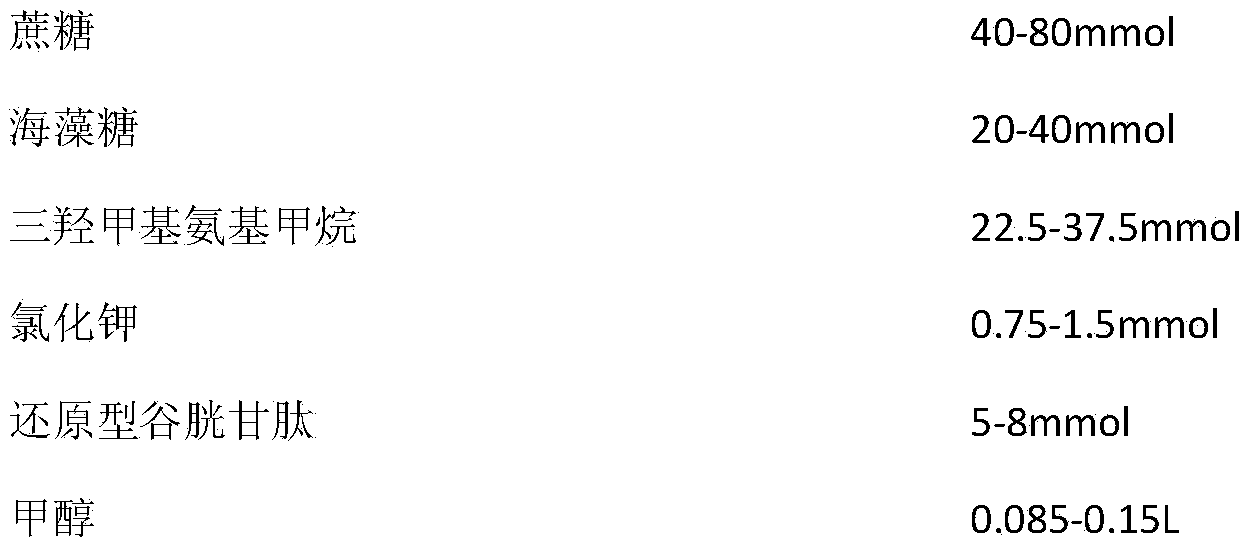Patents
Literature
320 results about "Reduce glutathione" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
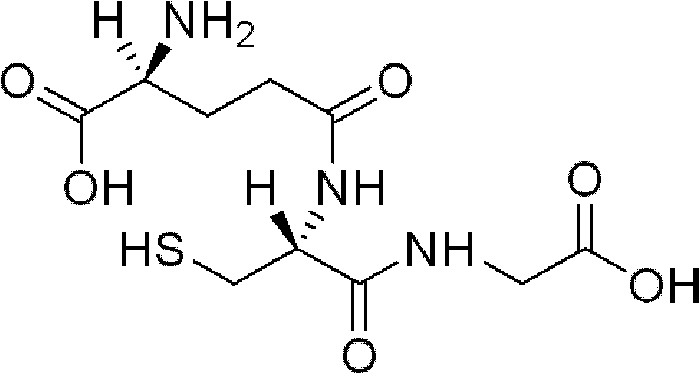
Glutathione levels in the body may be reduced by a number of factors, including poor nutrition, environmental toxins, and stress. Its levels also decline with age.
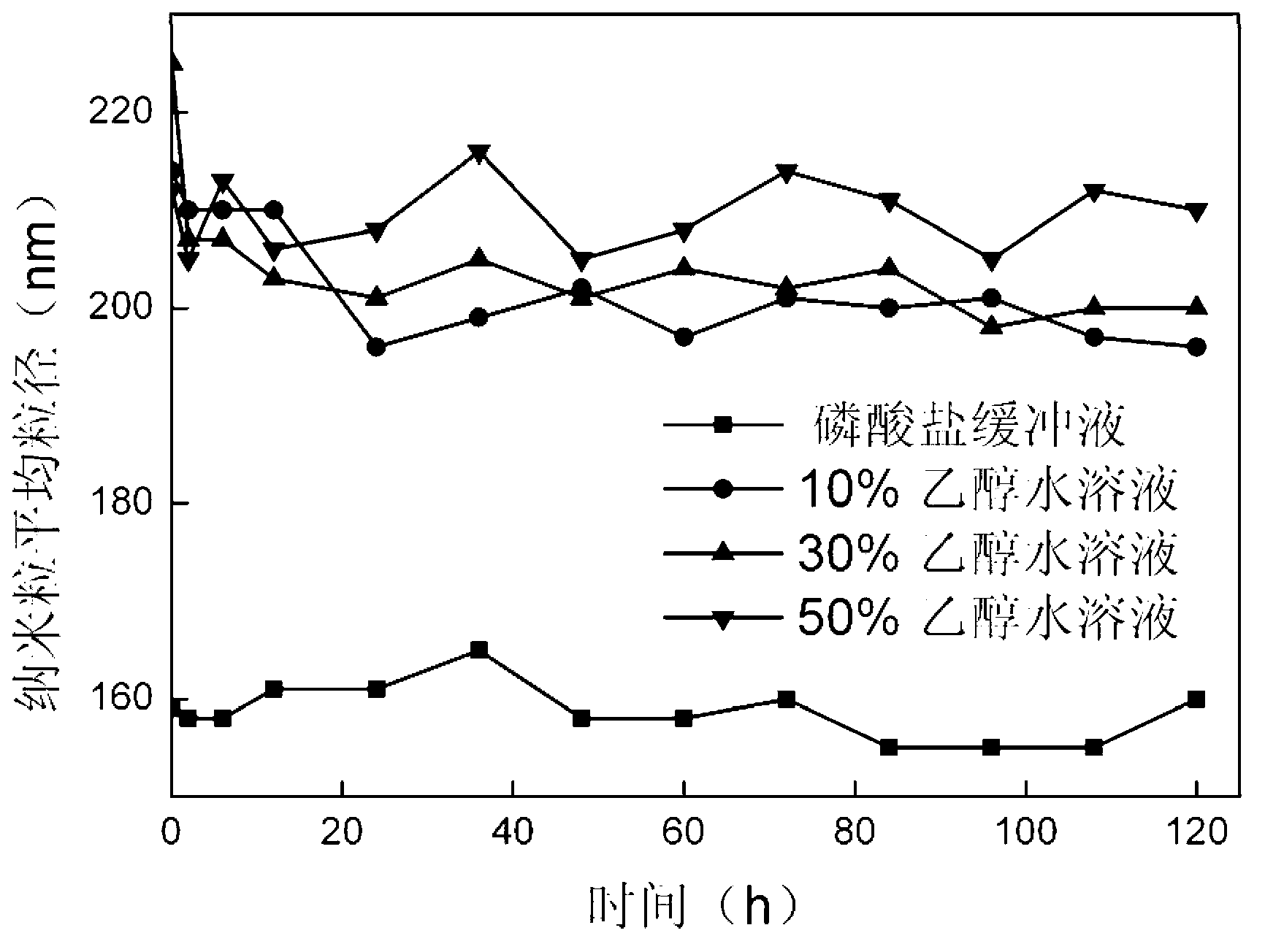
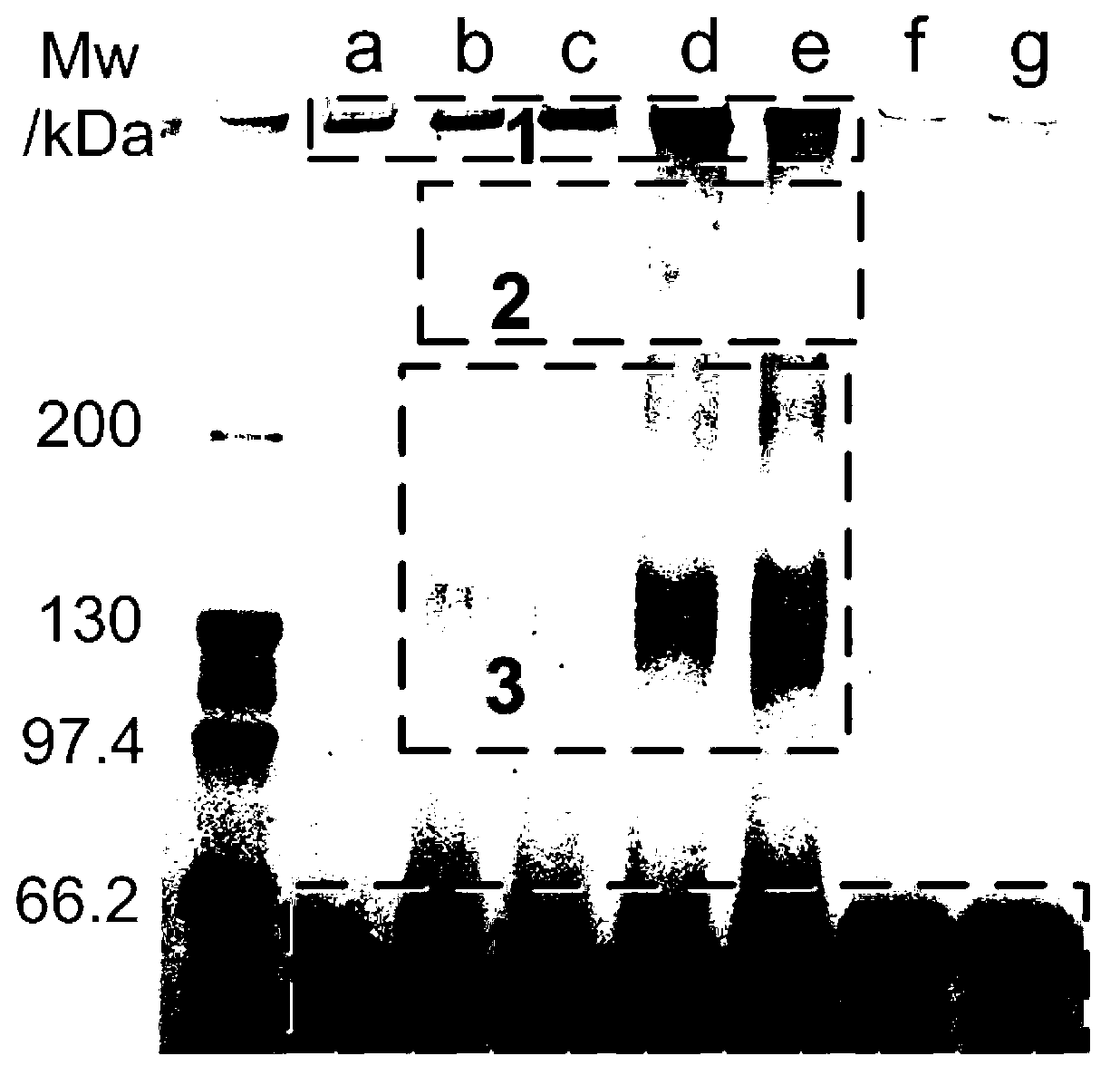
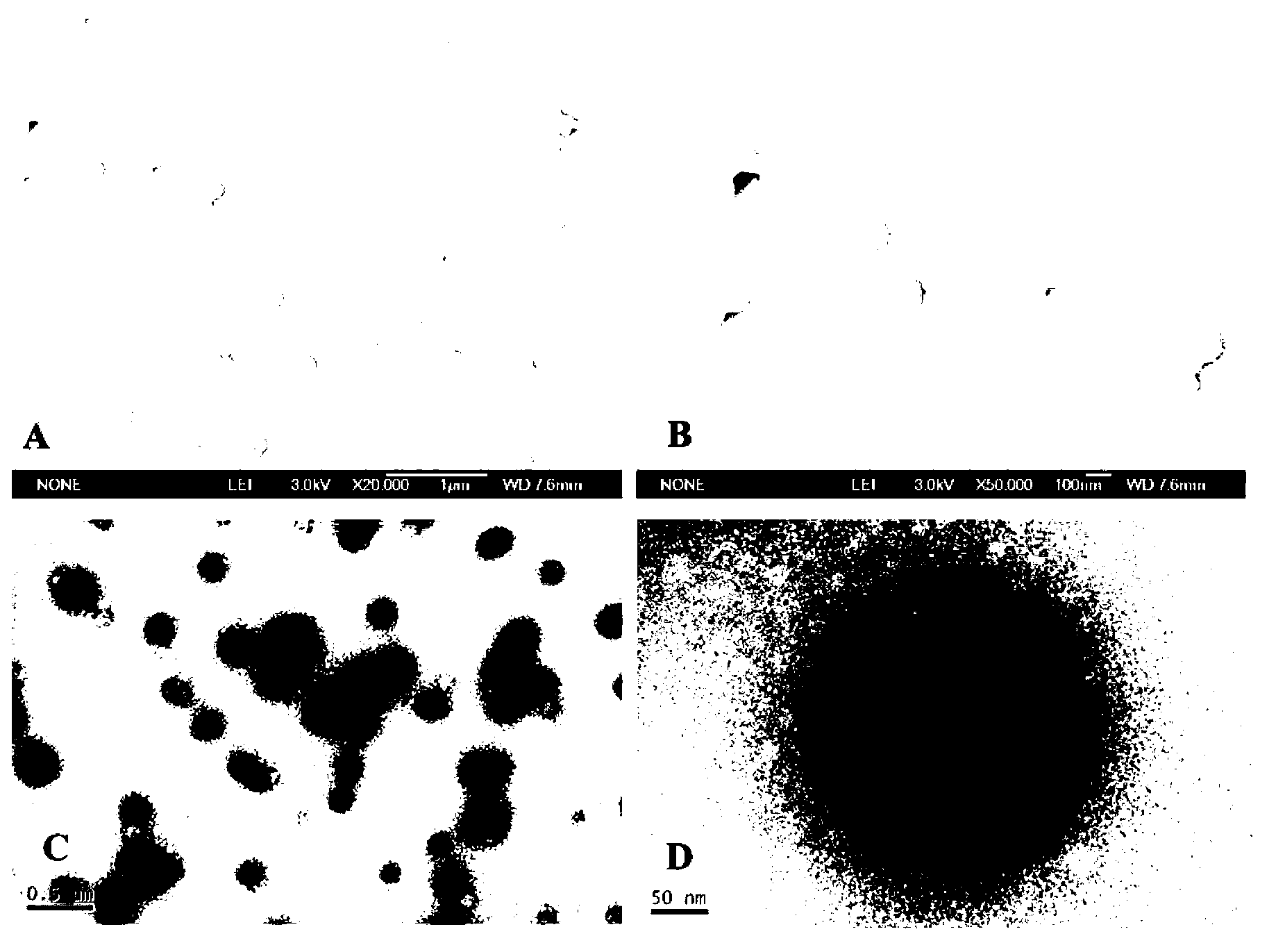
Method for preparing stable albumin nanoparticle
InactiveCN102988996AProcess stabilityPowder deliveryLyophilised deliveryRedox responsiveDisulfide bond
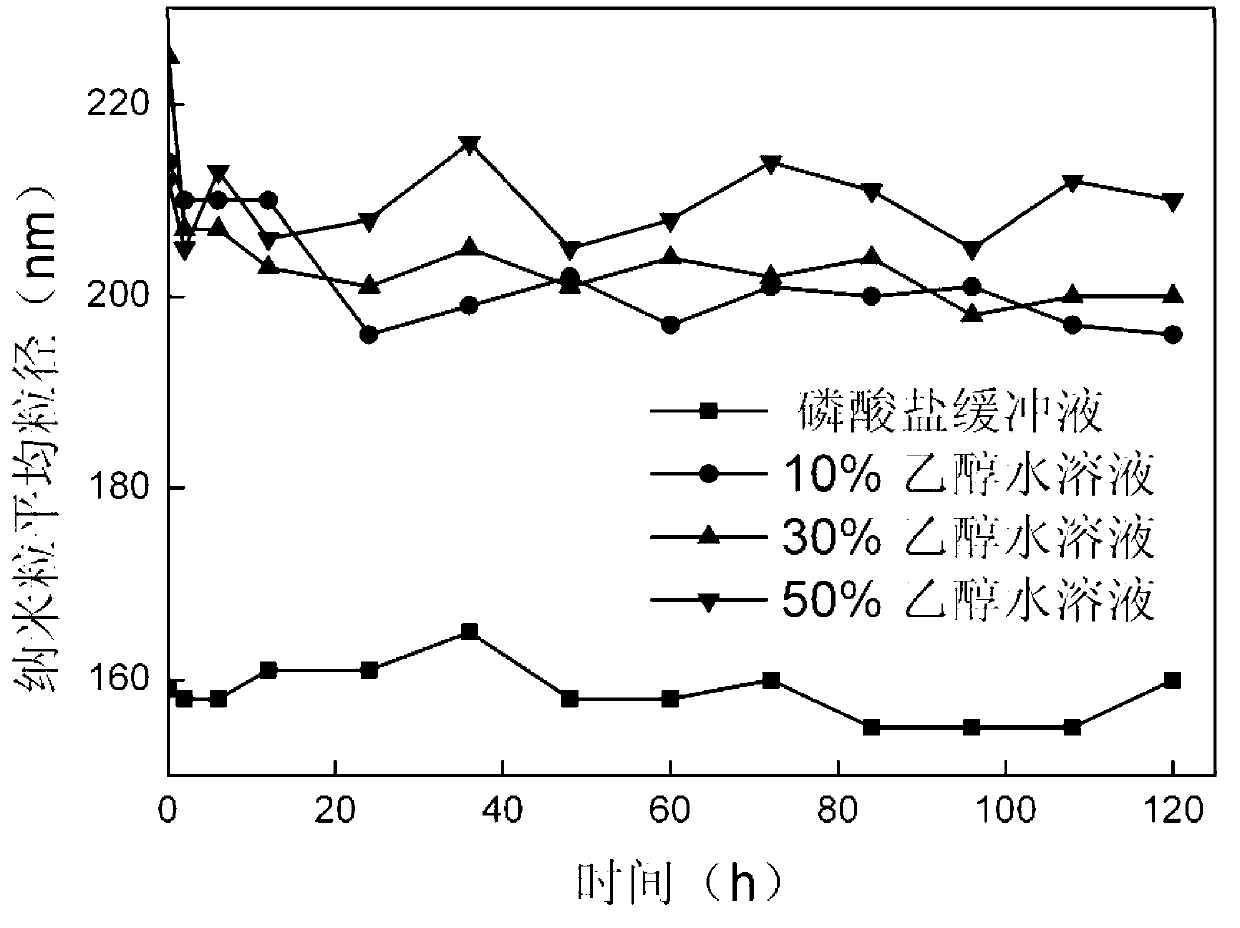
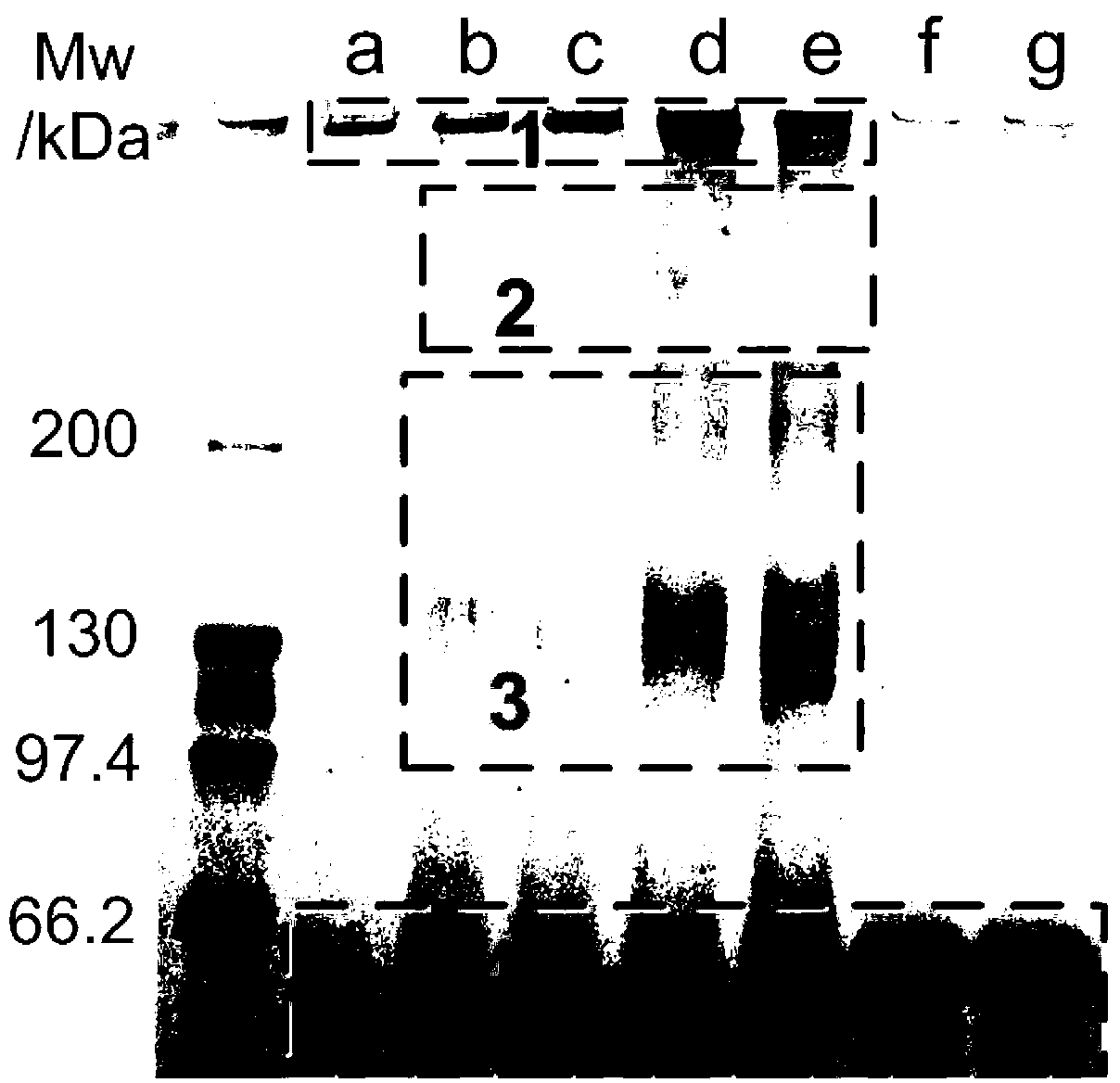
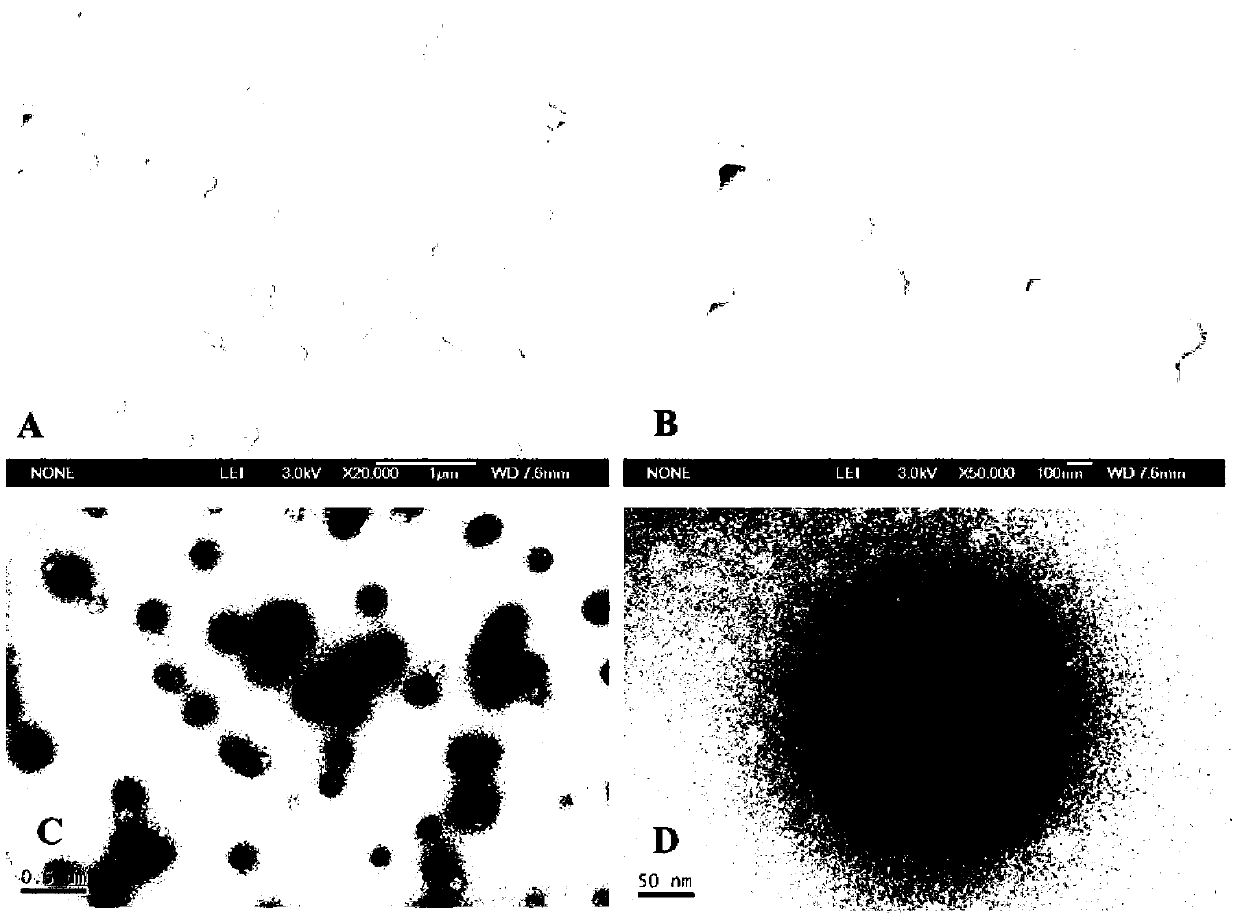
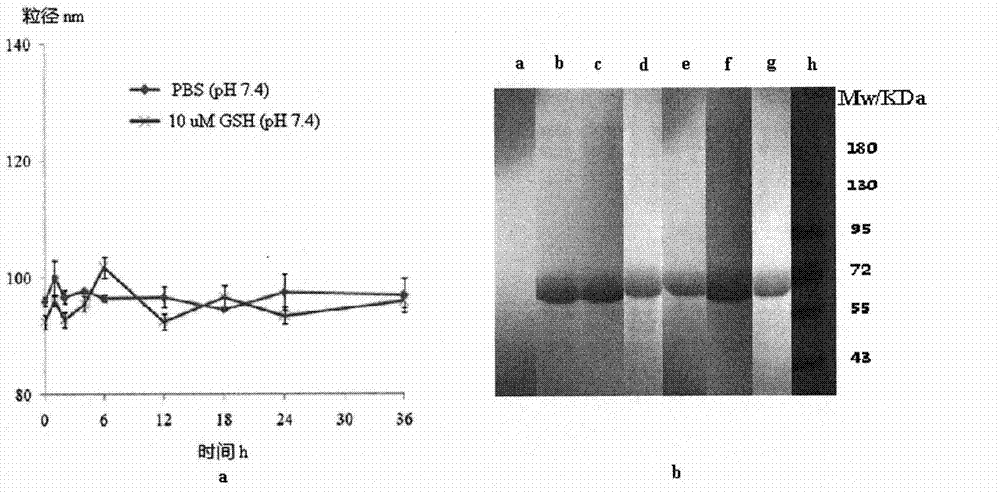
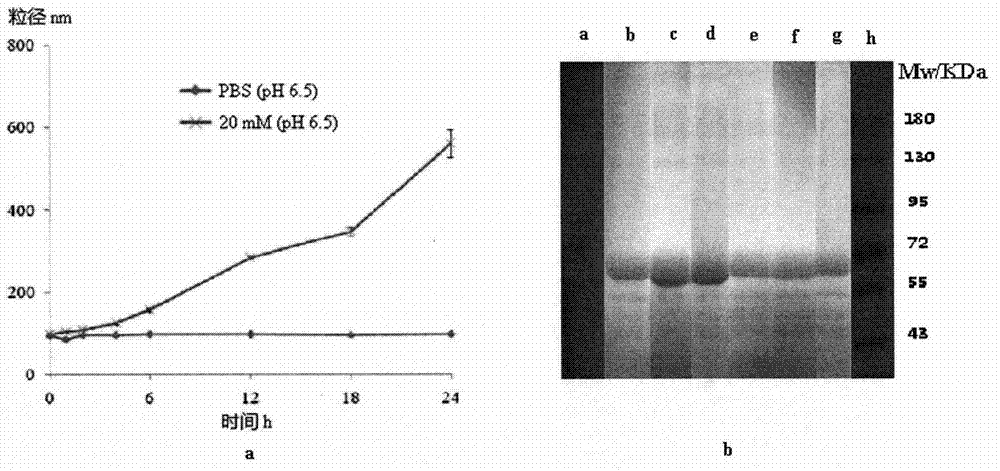
The invention relates to a method for preparing a stable albumin nanoparticle, and belongs to the technical field of preparation of biomedical materials. The method comprises the following steps: pretreating albumin by using glutathione and cysteine without biotoxicity; opening an intramolecular disulfide bond; precipitating the albumin by using anti-solvents such as alcohol and the like; and carrying out exchange reaction on a sulfydryl-disulfide bond to obtain the albumin nanoparticle containing the intramolecular disulfide bond. The prepared albumin nanoparticle can be used for the delivery of pharmacological active substances and / or diagnostic agents in an organism. The albumin nanoparticle provided by the invention has the advantages that the albumin nanoparticle has good stability under a dilution condition and gives an oxidation reduction response in a reduced environment. Based on the characteristics, the albumin nanoparticle can stably exist in a blood circulation system of the organism, and can carry out a degradation reaction in a cell under the action of reduced glutathione so as to release a wrapped medicine.
Owner:TSINGHUA UNIV
Method for preparing coating with nitric oxide (NO) catalytic activity
ActiveCN104673096AThickness is easy to controlExcellent free radical scavenging functionAntifouling/underwater paintsInorganic active ingredientsPhenolSulfur containing
The invention discloses a method for preparing a coating with nitric oxide catalytic activity. The method comprises the following steps: mixing a selenium-containing compound, a sulfur-containing compound and a soluble copper salt with the NO catalytic activity with a compound of an o-phenol structure, a flavone compound and a flavonol compound or a flavanone compound in a buffer solution for polymerizing. The coating with the nitric oxide catalytic activity can be applied to surface modification of matrix materials of almost all the materials, geometrical shapes and topological structures. The double selenium bonds, double sulfur bonds, copper ions and phenolic hydroxyl group in the prepared coating with the nitric oxide (NO) catalytic activity have excellent free radical scavenging functions; and the selenium bonds, sulfur bonds and chelated copper ions contained in the material have the response functions on reduced glutathione (GSH). In addition, the copper ions contained in the coating have antibacterial functions. The coating is used for catalyzing NO release and also can be applied to all the fields related to free radical scavenging and GSH response functions.
Owner:GUANGZHOU NANCHUANG EVEREST MEDICAL TECH CO LTD
Fluorescent probe and its application in reversible detection of peroxy nitrosyl
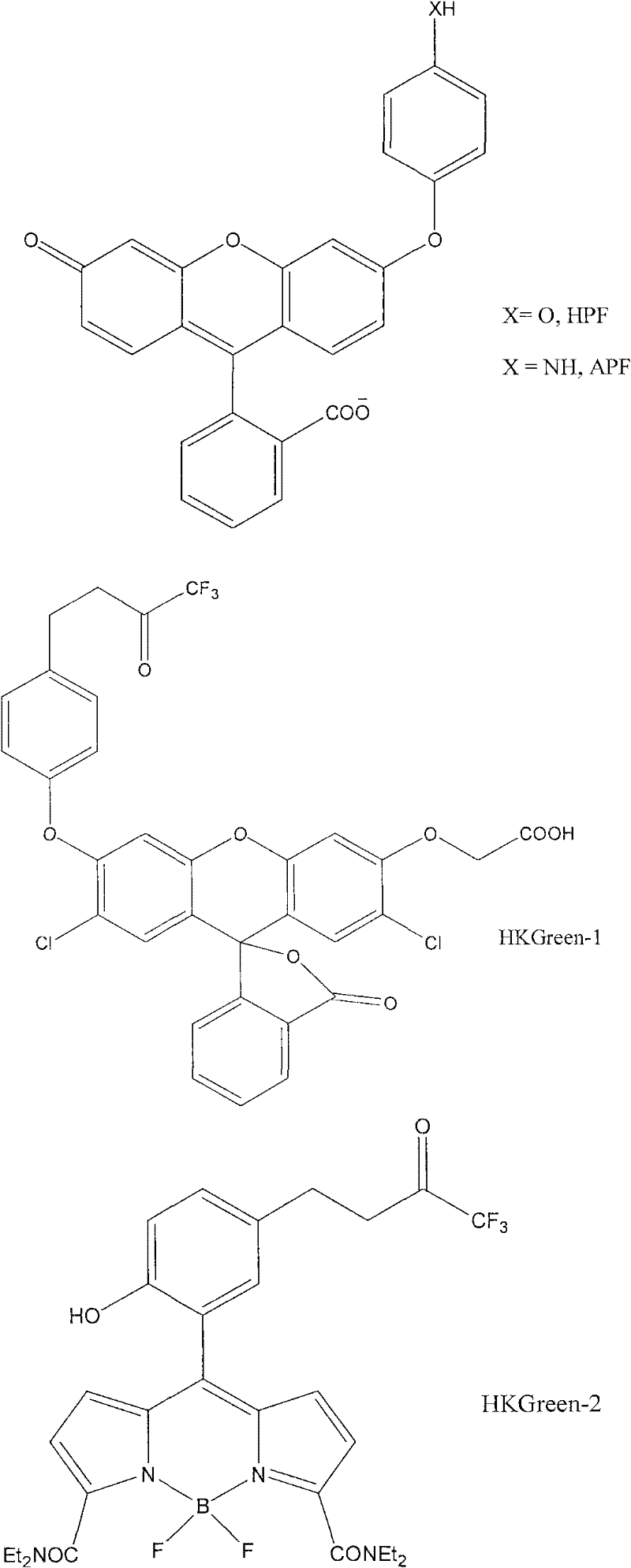
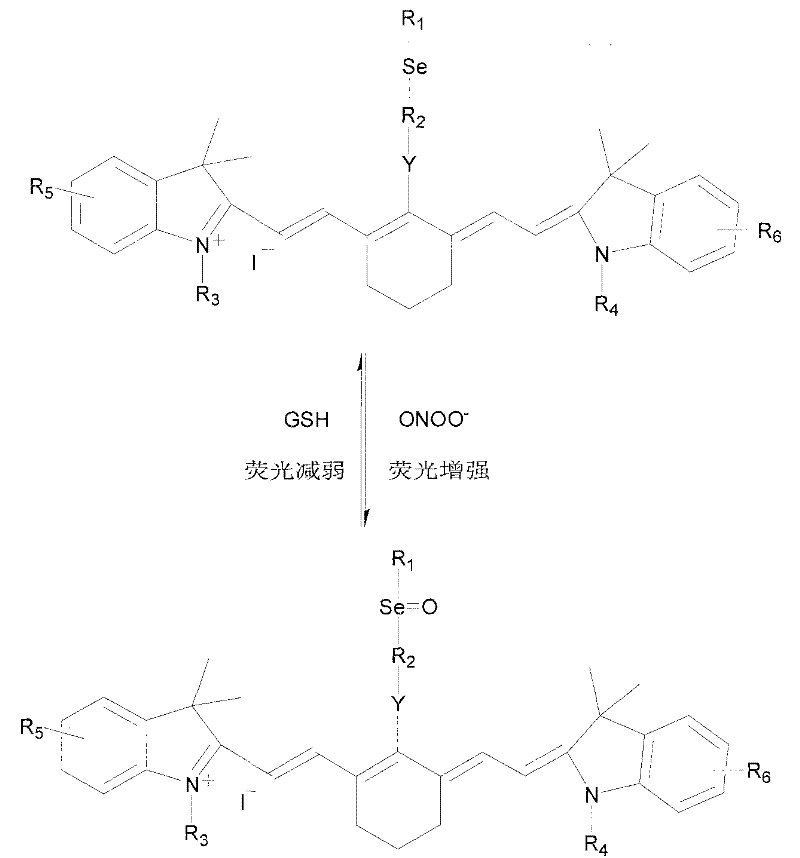
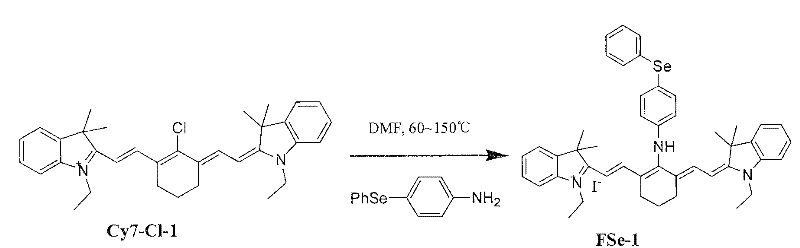
The invention relates to a fluorescent probe, wherein, the fluorescence is enhanced in the presence of ONOO-, and the fluorescence returns to original state in the presence of reducing matter. The invention provides a reversible fluorescent probe for selective detection of ONOO- in cells, characterized in that: cyanine dye is used as the fluorescent matrix, an organic selenide structure is introduced in the cyanine matrix as the active site of the reaction with ONOO- to realize the selective detection of ONOO-; the properties that organic selenium oxide formed by oxidizing organic selenide is easy to be reduced to organic selenide by reducing micromolecules in biosystem, such as cysteine, reducing glutathione, metallothionein and the like, is utilized to realize the reversibility of the probe molecules; and variations of electronic properties of organic selenide before and after the oxidation and the influence of the variations on the fluorescence property of the whole compound are utilized simultaneously to modulate the fluorescence property of the probe molecules.
Owner:ZHANGJIAGANG IND TECH RES INST CO LTD DALIAN INST OF CHEM PHYSICS CHINESE ACADEMY OF SCI
Liquid eye drop composition
A composition that is used as an eye treatment contains reduced glutathione, vitamin A and vitamin E, as well as one or more of zinc sulfate, boric acid and potassium as buffering agents. The composition also may contain a lubricant and a preservative. The composition is a sterile isotonic solution. The composition is used in a method of treating eyes for the alleviation of irritations and / or dryness, as well as for the prevention and treatment of cataracts.
Owner:BRASWELL A GLENN +2
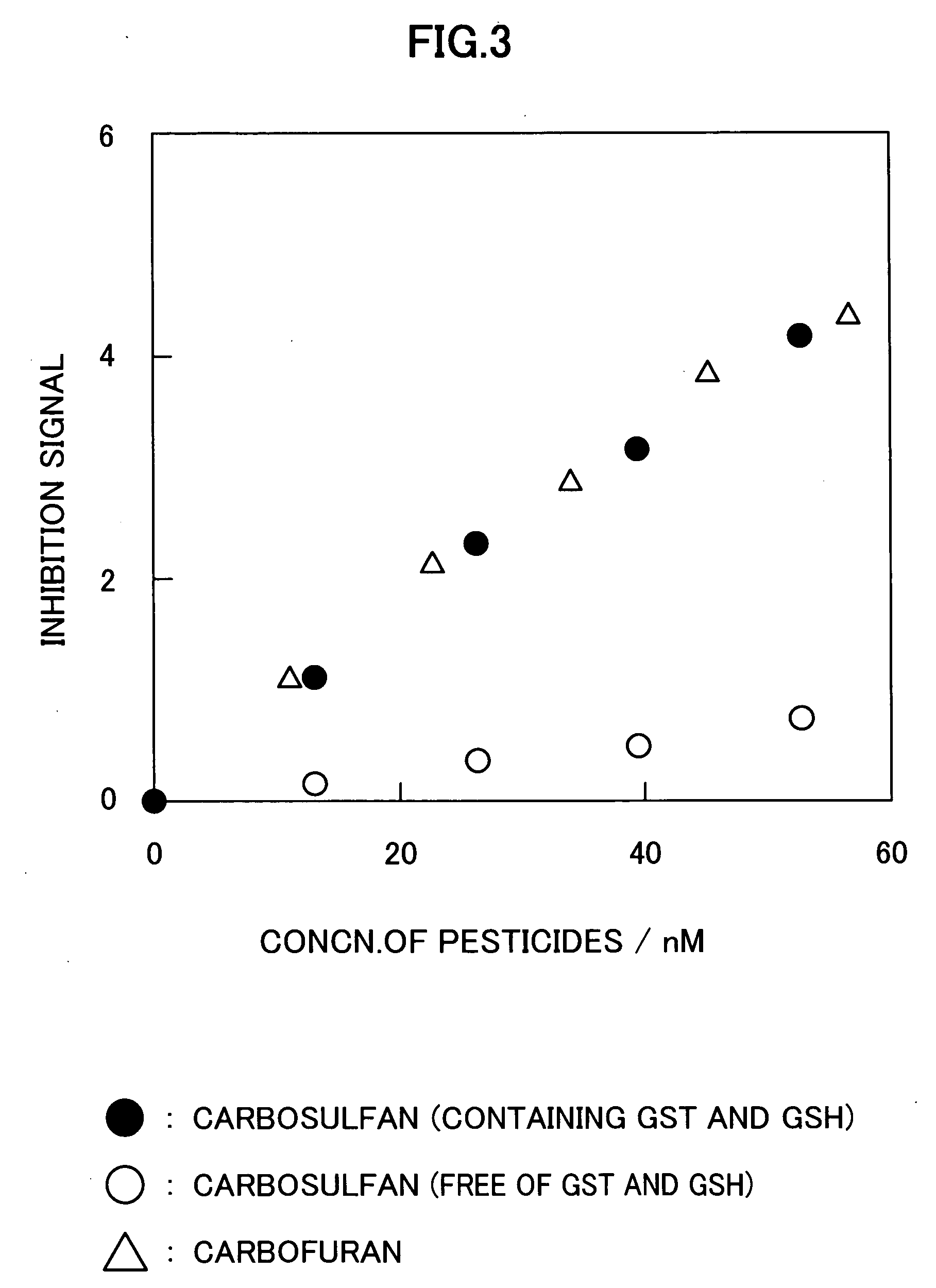
Method for analyzing residual agricultural chemical
InactiveUS20050214887A1Improve accuracyHigh detection sensitivityAnalysis using chemical indicatorsMicrobiological testing/measurementCarbofuranMethomyl
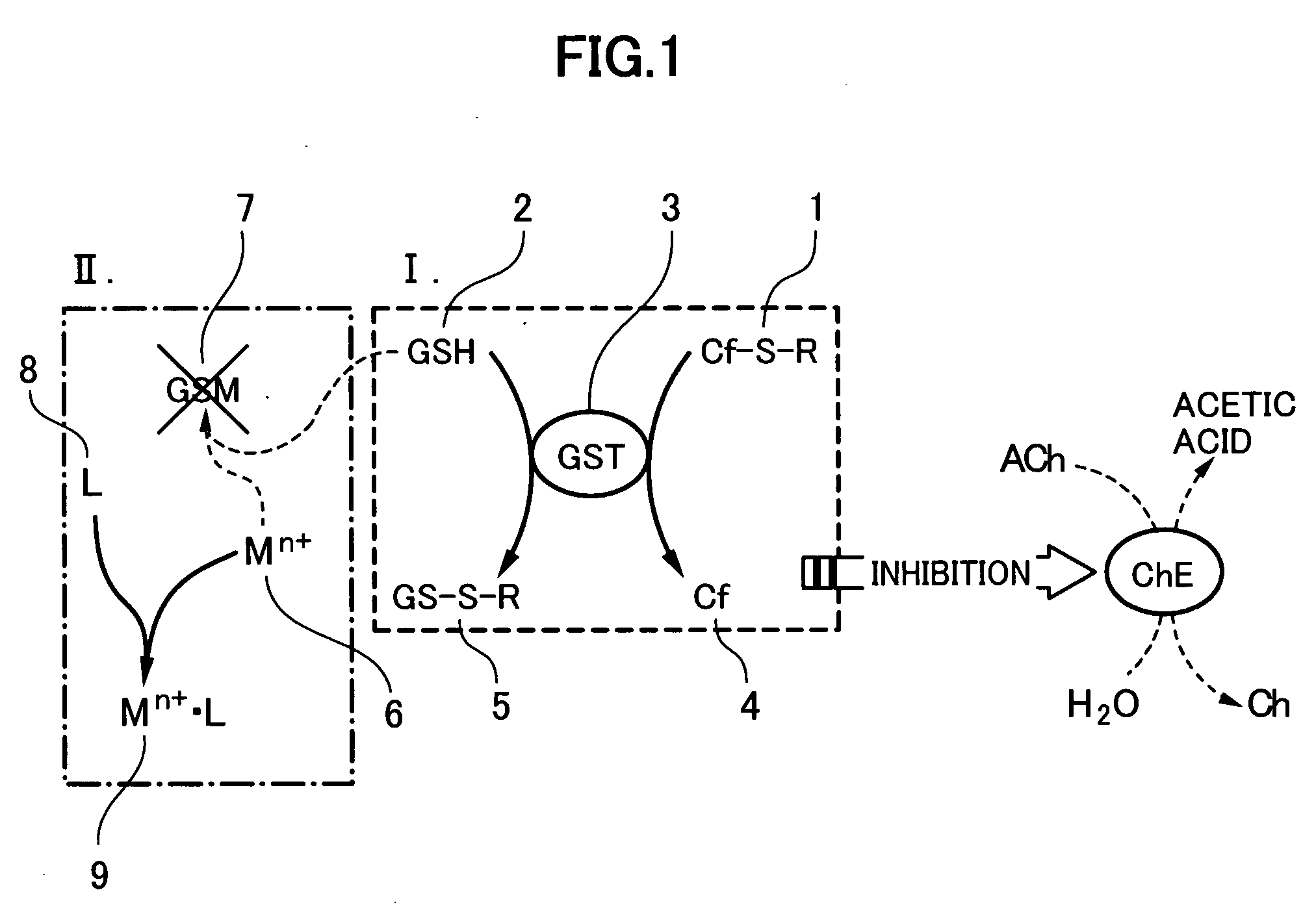
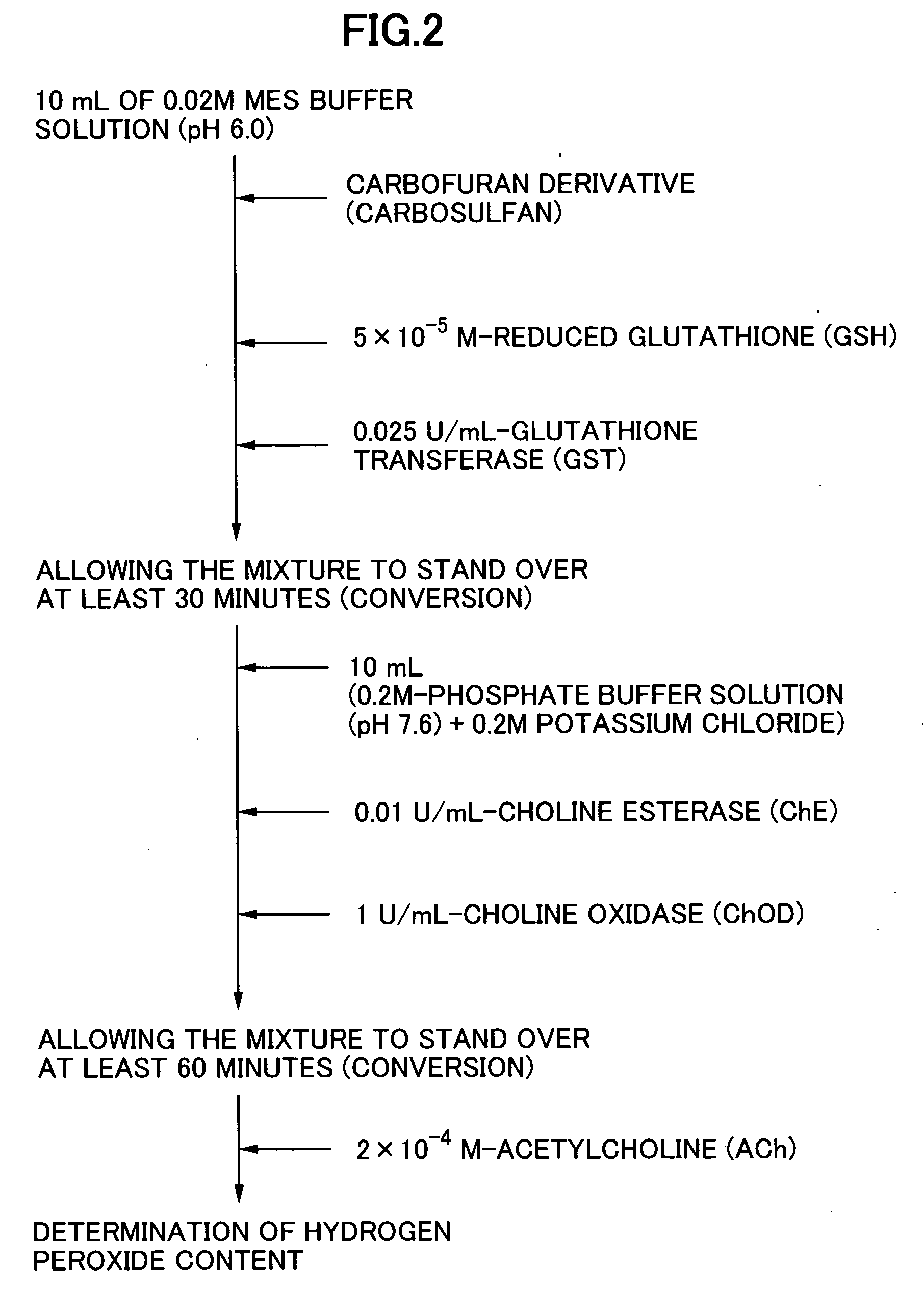
The present invention relates to a method for analyzing residual agricultural chemicals which comprises the steps of acting a reduced glutathione as a reactive substrate and a glutathione transferase serving as a catalyst for the reaction on a carbofuran derivative or a methomyl derivative as a carbamate type agricultural chemical of a new series to thus derivatize the agricultural chemical into a substance having a high choline esterase-inhibitory activity; reacting the substances formed through the derivatization reaction with a choline esterase; and then detecting the presence of the agricultural chemical as the new series of carbamate type one included in a sample to be examined on the basis of the changes in the choline esterase activity thus detected. The method of the present invention may serve as a powerful tool for the detection of the residual agricultural chemicals in grains such as rice and the detection of the content of agricultural chemicals remaining in agricultural products such as vegetables and fruits.
Owner:SATAKE CORP +1
Preserving fluid of hepatic cells for biological artificial liver and preparation method thereof
ActiveCN101919381APrevent acidificationImprove buffering effectDead animal preservationArtificial liverHydroxyethyl starch
The invention provides preserving fluid of hepatic cells for a biological artificial liver and a preparation method thereof. The preserving fluid is a solution compounded by ultrapure water. The solution contains the following components within the concentration range: 15-25mmol / L of disodium hydrogen phosphate, 1-10mmol / L of sodium hydrogen phosphate dehydrate, 4-6mmol / L of potassium citrate monohydrate, 10-30mmol / L of sodium chloride, 5-10mmol / L of magnesium chloride hexahydrate, 3-10mmol / L of disodium adenosine triphosphate, 1-5mmol / L of reducing glutathione, 0.1-0.5mmol / L of alpha-lipoic acid, 100-150mmol / L of trehalose (C6H12O5), 200 / 0.510-50g / L of hydroxyethyl starch and 2-10mg / L of matrine. The preparation method of the preserving fluid comprises the following steps of: accurately weighing all components according to the concentration requirements of the components, wherein the alpha-lipoic acid is weighed in a dark place; completely dissolving the other components except the alpha-lipoic acid by using the right amount of ultrapure water; sufficiently dissolving the alpha-lipoic acid in the dark place; and adding the ultrapure water to full dose. The preserving fluid can well protect the cell activity of the hepatic cells for the biological artificial liver and the special functions of the hepatic cells at low temperature so as to satisfy the short-term low temperature preservation of a large-scale hepatic cell bank for the biological artificial liver and / or the hepatic cell protection in the long-distance transportation process.
Owner:ZHUJIANG HOSPITAL SOUTHERN MEDICAL UNIV
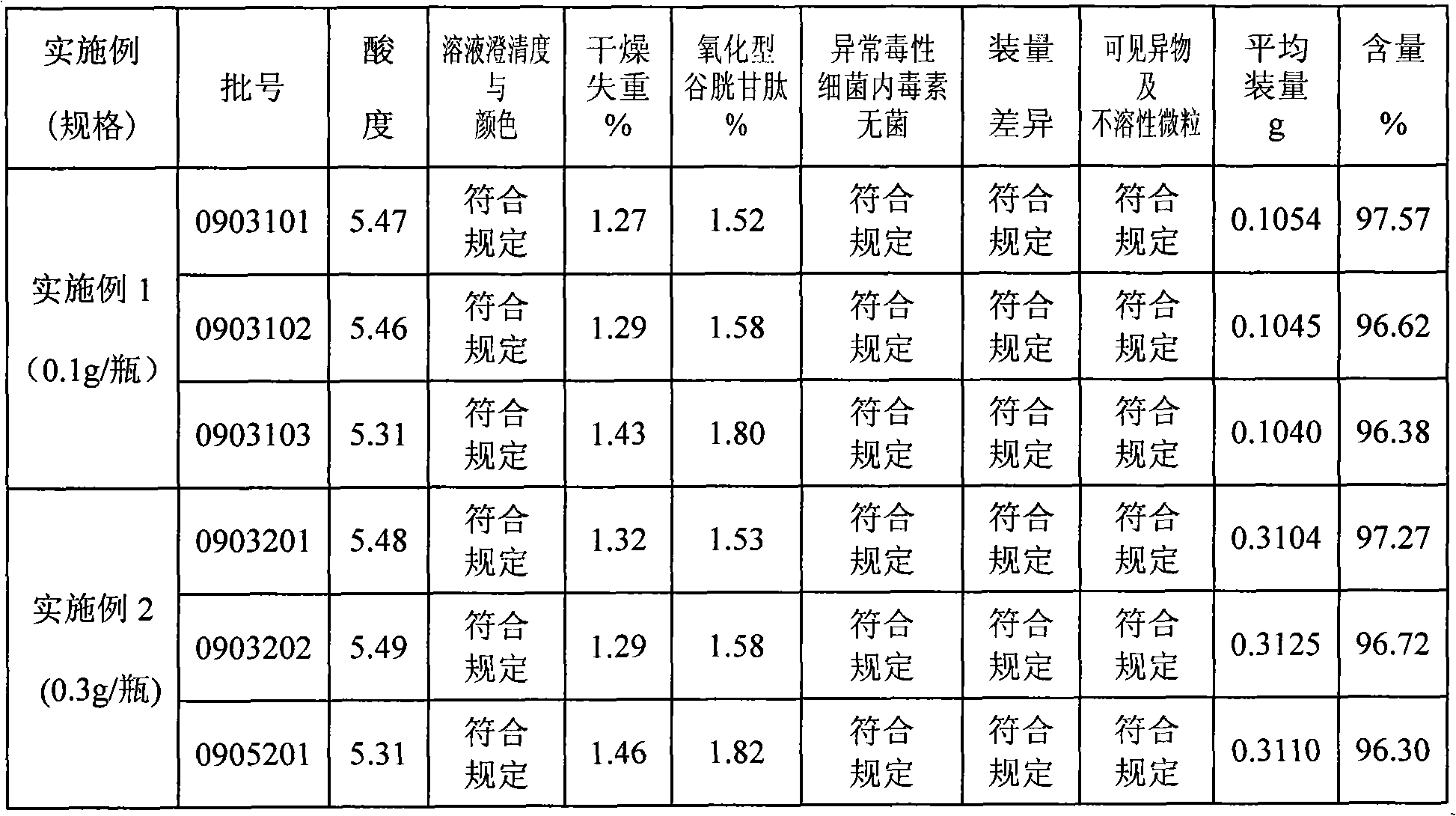
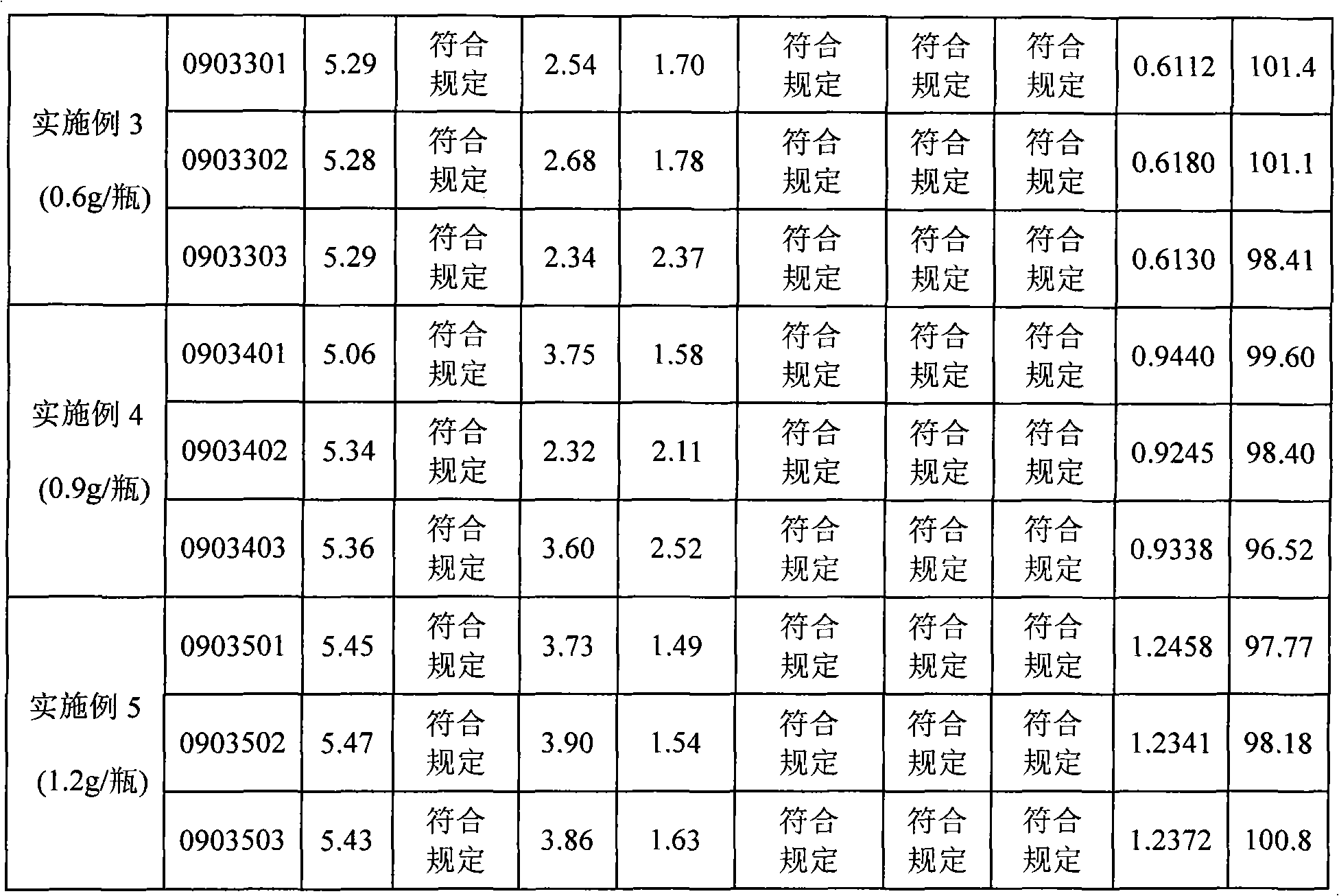
Production of injecting reductive glutathione
ActiveCN101074202AImprove product qualitySimple methodOrganic active ingredientsOrganic chemistryFreeze-dryingOrganic chemistry
Production of injection reducing glutathione is carried out by pre-freezing for reducing glutathione solution and freeze-drying. It's simple, cheap, has stable product quality and less water content and related substances and can be used for industrial production.
Owner:YAOPHARMA CO LTD
Organ preservation solution and method for preparing same
ActiveCN102726366AAvoid damageDisadvantages of Avoiding PrecipitationDead animal preservationPhosphateArginine
Owner:SHANGHAI GENEXT MEDICAL TECH
Saccharomyces cerevisiae, dry yeast rich in reduced glutathione and preparation method thereof
ActiveCN101575578APrevent oxidationAvoid degradationFungiMicroorganism based processesReduce glutathioneChemistry
The invention provides a saccharomyces cerevisiae rich in reduced glutathione, having a preserving number of CCTCC M 205130, and also provides dry yeast rich in reduced glutathione and a preparation method thereof, wherein the dry yeast rich in reduced glutathione is produced by fermenting the saccharomyces cerevisiae rich in reduced glutathione. The saccharomyces cerevisiae CCTCC M 205130 with higher content of reduced glutathione and stable property is selected through an ultraviolet mutagenesis method. The saccharomyces cerevisiae is directly used together with yeast cells without extracting and purifying glutathione so as to obtain the dry yeast rich in reduced glutathione. The invention has low production cost, simple process and less investment of permanent assets. Glutathione is not extracted and purified so that the problem of loss of glutathione does not exist, and the activity loss of glutathione is little.
Owner:ANGELYEAST CO LTD
Production method for cordyceps militaris buccal tablets
InactiveCN103652846AImprove immunityExtended shelf lifeFood shapingFood preparationCordycepsActive component
The invention provides a production method for cordyceps militaris buccal tablets with a high practical value and a good combination effect and belongs to industrial production. The technology adopted by the preparation method is characterized in that the content of active components such as reduced glutathione and a cordyceps militaris extract can be controlled, so that the aim of realizing stable quality can be fulfilled.
Owner:JIANGSU XUE FU BIOLOGICAL ENG
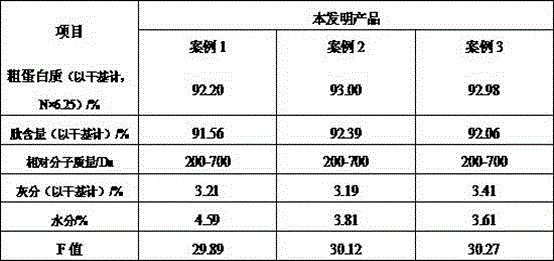
Industrial production method of high-F-value corn oligopeptide with alcohol dispelling and liver protection activities
InactiveCN105639048ARealize industrial productionSimple production processVegetable proteins working-upBiotechnologyUltrafiltration
The invention discloses an industrial production method of high-F-value corn oligopeptide with alcohol dispelling and liver protection activities. The production method comprises the following steps: carrying out high-speed shearing emulsification on corn gluten meal (CGM), carrying out enzymolysis by combining basic protein with compound flavored proteinase, carrying out enzyme deactivation and frame filtering, carrying out activated carbon adsorption to remove bitterness, colors and aromatic amino acid, carrying out separation and purification by virtue of ultrafiltration equipment, carrying out desalination by virtue of nanofiltration, and carrying out spray drying, so as to obtain the high-F-value corn oligopeptide. According to the high-F-value corn oligopeptide produced by virtue of the production method, the fatty degeneration index of liver cells of mice with alcoholic hepatic injury can be decreased, meanwhile, the contents of triglyceride (TG) and malondialdehyde (MDA) in the livers can be reduced, and the content of reduced glutathione (GSH) can be increased; and a result shows that the high-F-value corn oligopeptide produced by virtue of the production method has the effect of preventing the alcoholic hepatic injury of the mice.
Owner:乳山市华隆生物科技股份有限公司
Method for preparing stable albumin nano-particles
ActiveCN103212083AProcess stabilityKetone active ingredientsGranular deliveryIn vivoRedox responsive
The invention discloses a method for preparing stable albumin nano-particles, belonging to the technical field of biological medicine material preparation. The method comprises the following steps of: carrying out pretreatment on albumin by utilizing non-biotoxic glutathione or cysteine to open intramolecular dithio bonds, precipitating the albumin by using anti-solvents such as alcohol and the like, and carrying out sulfydryl-dithio bond exchange reaction so as to obtain albumin nano-particles with intermolecular dithio bonds. The prepared nano-particles can be used for delivering in-vivo pharmacologically active substances and / or diagnosis aids. The obtained albumin nano-particles have the advantages that the albumin nano-particles not only have good stability under dilution condition, but also have oxidation reduction response in a reduction type environment; and due to such characteristics, the particles can stably exist in in-vivo blood circulation systems of organisms and are degraded under the action of reduced glutathione so as to release the wrapped medicine.
Owner:TSINGHUA UNIV
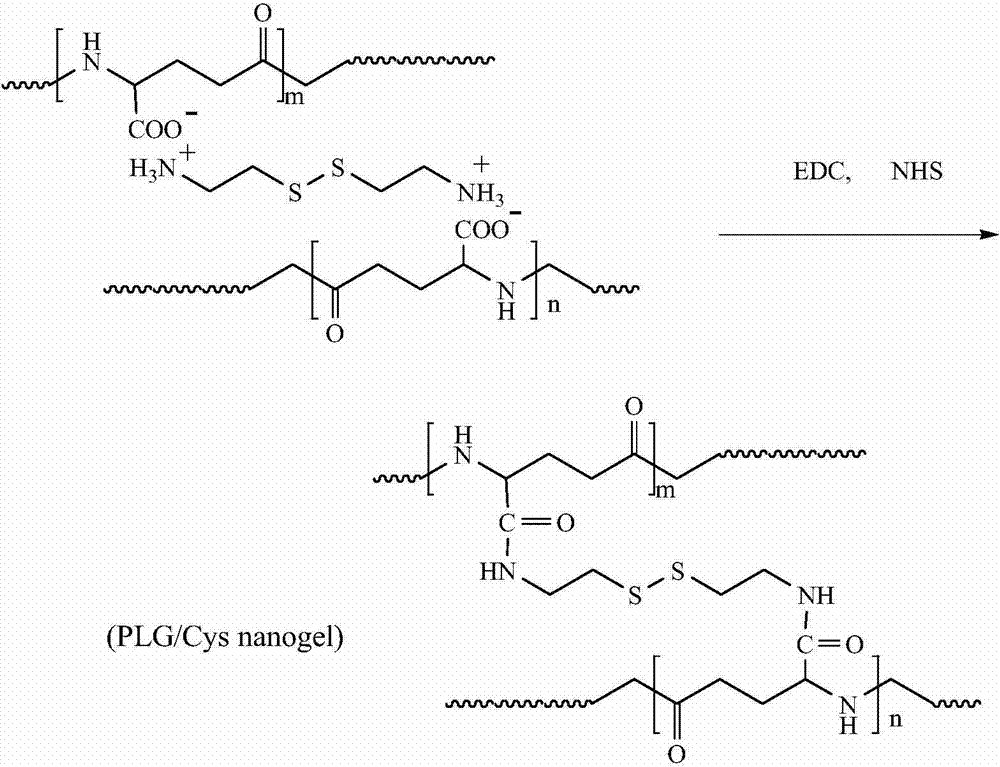
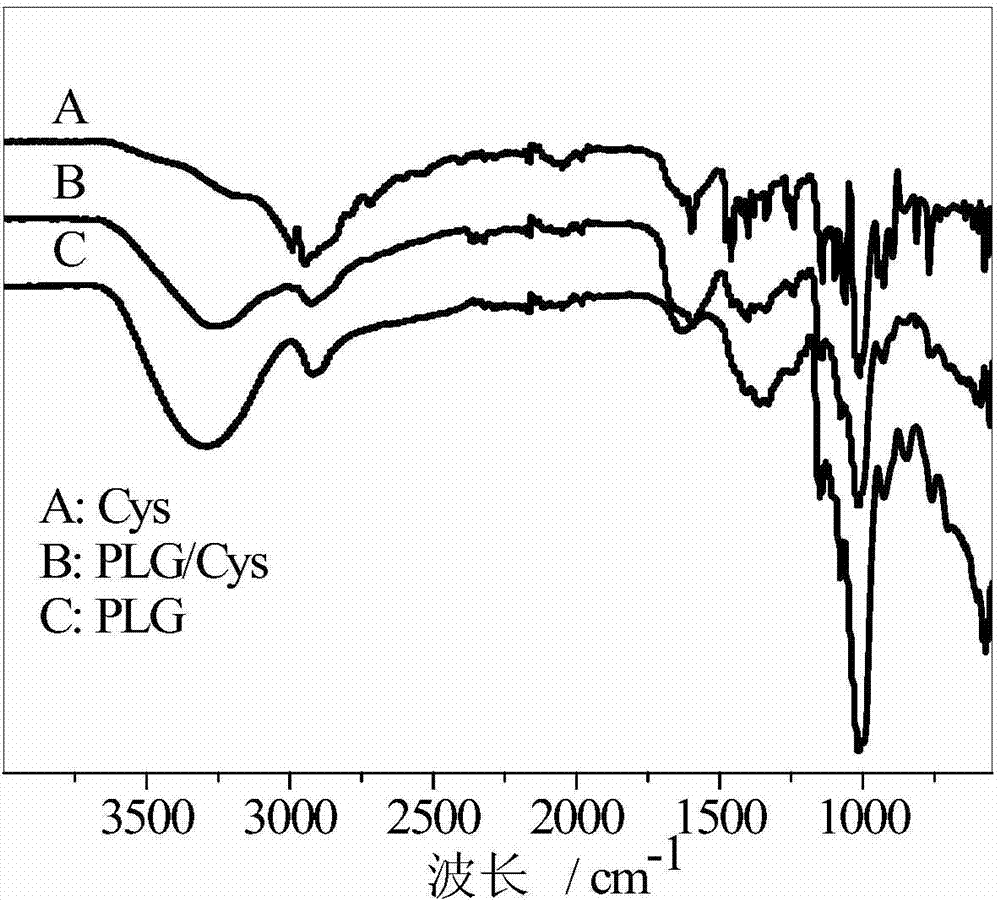
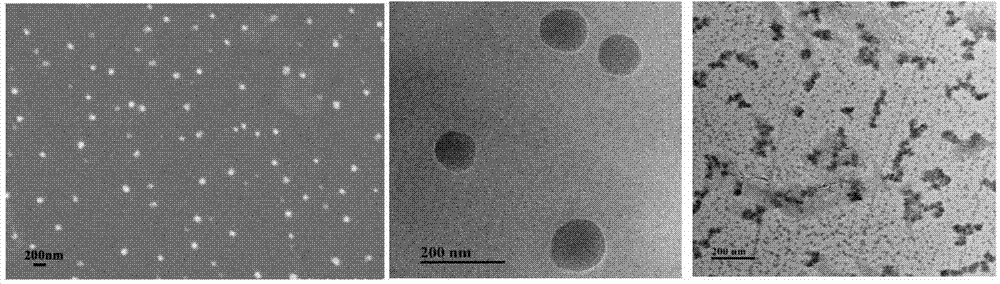
PH/reduction-sensitive nano microgel based on polyglutamic acid and cystamine
ActiveCN104491871AImprove securityIncrease drug concentrationOrganic active ingredientsAerosol deliveryHigh concentrationCancer cell
The invention provides a preparation method of pH and reduction sensitive nano microgel based on polyglutamic acid and cystamine and application of the pH and reduction sensitive nano microgel based on polyglutamic acid and cystamine in the anti-tumor aspect. The nano microgel with a particle size of 212nm is synthesized by preparing a microgel model from raw materials including rolyglutamic acid and cystamine under electrostatic interaction, and adding a catalyst to carry out crosslinking amidation reaction. Because carboxylate anions in polyglutamic acid are combined with amino cations in doxorubicin to load medicines, a high medicine lading ratio of 17.8% can be achieved. The disulfide bond in cystamine which serves as a crosslinking agent ensures that nano particles are sensitive to reduction, and the disulfide bond can break in a cancer cell containing high-concentration reduced glutathione, and thus the nano microgel is targeted at tumors and has a controlled release function. The nano microgel is directly prepared in an aqueous solution without adopting any organic solvent, and thus the safety of the medicine carrier can be guaranteed.
Owner:南通慧源塑胶有限公司
Prefreezing method in preparing injection-used reduced glutathione with freeze drying method
ActiveCN101647783ASimple process conditionsEfficient and perfect controlPowder deliveryDigestive systemFreeze-dryingNational standard
The invention discloses a prefreezing method in preparing injection-used reduced glutathione with a freeze drying method, comprising the following steps: (1) quantitatively split charging reduced glutathione solution into each tube-type bottle according to the specification of a final product, lowering the temperature below -40 DEG C in 1.5-2 hours, and keeping the temperature for 1 hour to obtaina reduced glutathione solution freezing body; (2) rising the temperature of the reduced glutathione solution freezing body obtained in step (1) to -17 DEG C in two hours, and keeping the temperaturefor one hour; (3) lowering the temperature of the reduced glutathione solution freezing body obtained in step (2) below -40 DEG C within 1-1.5 hours, and keeping the temperature for one hour. After the prefreezing method of the invention is adopted, the obtained substance is sublimated and dried to obtain a white loose block product, and surface has no appearance defect. The product obtained by the prefreezing method of the invention has small possibility of erupting in the production process, and the quality of the final product conforms to the national standard.
Owner:上海复旦复华药业有限公司 +1
Method for the treatment of infection with hhv-6 virus and the amelioration of symptoms related to virus using liposomal encapsulation for delivery of reduced glutathione
InactiveUS20090068253A1Increase intracellularIncrease extra cellular antioxidantDispersion deliveryPeptide/protein ingredientsDiseaseNucleoside Reverse Transcriptase Inhibitor
The invention is the use of a therapeutically effective amount of glutathione (reduced) in a liposome encapsulation for oral administration to improve symptoms of illnesses that are related to viruses and for the treatment and prevention of virus, particularly HHV-6 and EBV, which liposomal encapsulation of glutathione (reduced) is referred to as liposomal glutathione. The application references specifically reduced glutathione and its importance, and how to stabilize it effectively so it can be taken orally, and need not be refrigerated. New uses for tuberculosis, and asthma are discussed. The combination is proposed of reduced glutathione and Highly Active Anti-Retroviral Therapy having at least one pharmaceutical composition selected from the group of Nucleoside / tide Reverse Transcriptase Inhibitors (NRTIs), Protease Inhibitors (PIs), and Non-nucleoside Reverse Transcriptase Inhibitors (NnRTIs).
Owner:GUILFORD F TIMOTHY
Method for separating and purifying reduced glutathione (GSH) from reduced glutathione contained fermentation leaching liquid
InactiveCN103374055AMeet the requirementsSimple and fast operationPeptide preparation methodsIon-exchange resinFermentation
The invention provides a method for separating and purifying reduced glutathione (GSH) from a reduced GSH contained fermentation leaching liquid. The method comprises the following steps of: (1) treating the fermentation leaching liquid sequentially through anion exchange resin and a non-polar absorbent to obtain a reduced GSH contained solution; and (2) concentrating the reduced GSH contained solution, and then, crystallizing and drying. The reduced GSH separated and purified by using the method provided by the invention has the advantages that the purity of the reduced GSH can be up to over 98.5% (titration, drying), and relevant impurities conform to the requirement of European pharmacopoeia.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Preparation method of material with nitric oxide (NO) catalytic activity
The invention discloses a preparation method of a material with nitric oxide (NO) catalytic activity. The preparation method is implemented through the steps that a selenium-containing compound with nitric oxide catalytic activity, a sulfur-containing compound, a soluble copper salt, a compound with an o-phenol structure, a flavonoid compound, and a flavonol compound or a flavanone compound are mixed in a buffer solution and then polymerized. The material with NO catalytic activity not only can be applied to the surface modification of materials which are different in material, geometrical shape and topological structure, and also can be used as a filling material for a controlled release system. Double selenium bonds, double sulfur bonds, copper ions and phenolic hydroxyl groups, which are contained in the prepared material with nitric oxide (NO) catalytic activity, have an excellent free radical removal function; the selenium bonds, the sulfur bonds and the chelate copper ions, which are contained in the material, also have a reduced glutathione (GSH) response function; in addition, the copper ions containing in the material also have an antibacterial function; and the material, besides being used for catalyzing NO release, also can be applied to all related fields of free radical removal and GSH response function.
Owner:GUANGZHOU NANCHUANG EVEREST MEDICAL TECH CO LTD
Method for preparing stable albumin nano-particles by virtue of thermal denaturation
InactiveCN104490847AOrganic active ingredientsMacromolecular non-active ingredientsRedox responsiveDoxorubicin Hydrochloride
The invention belongs to the field of preparation of biological medical materials, and relates to a method for preparing stable albumin nano-particles by virtue of thermal denaturation. The method comprises the following steps: (1) adding vanillic aldehyde or an analogue thereof to form intermolecular disulfide bonds by virtue of inter-reaction of free sulfhydryl groups on albumin molecules under a heating condition; (2) enabling amino groups inside and among molecules to react with carboxyl so as to form amido bonds; and (3) enabling amino groups on the albumin molecules to react with aldehyde groups on vanillic aldehyde or the analogue thereof to form chemical bonds of Schiff base and the like so as to form stable nano-particles in an aqueous solution. Any organic solvent is not introduced during preparation, so that the prepared nano-particles are safe and nontoxic, and can well entrap antitumor drugs including paclitaxel, doxorubicin hydrochloride and the like. Moreover, the carrier has an oxidation reduction response in a tumor cell internal environment, and can open disulfide bonds to release drugs under the action of reducing glutathione in cells. The method provided by the invention is simple in process, convenient to operate and suitable for industrial mass production.
Owner:CHINA PHARM UNIV
Enhanced method and composition for the treatment of hiv+ tuberculosis patients with Anti-retroviral drugs and liposomal encapsulation for delivery of reduced glutathione
InactiveUS20120244212A1Increase intracellular and extra cellular antioxidantsReduced glutathioneAntibacterial agentsBiocideNucleoside Reverse Transcriptase InhibitorDisease
The invention is the use of a therapeutically effective amount of glutathione (reduced) in a liposome encapsulation for oral administration to improve symptoms of illnesses that are related to tuberculosis and HIV and more generally viruses and for the treatment and prevention of virus, particularly HHV-6 and EBV, which liposomal encapsulation of glutathione (reduced) is referred to as liposomal glutathione. The application references specifically reduced glutathione and its importance, and how to stabilize it effectively so it can be taken orally, and need not be refrigerated. New uses for tuberculosis are discussed. The combination is proposed of reduced glutathione and Highly Active Anti-Retroviral Therapy having at least one pharmaceutical composition selected from the group of Nucleoside / tide Reverse Transcriptase Inhibitors (NRTIs), Protease Inhibitors (PIs), and Non-nucleoside Reverse Transcriptase Inhibitors (NnRTIs), and further anti-tuberculosis drugs.
Owner:GUILFORD FREDERICK TIMOTHY
Method for decreasing inflammation and oxidative stress in mammals
InactiveUS20080260696A1Reduce inflammationReduce oxidative stressBiocideSenses disorderOxidized GlutathioneD-Glucose
The present invention is directed to a method for decreasing inflammation and oxidative stress in a mammal comprising; administration to a mammal a composition comprising a glucose anti-metabolite; and wherein said composition comprises amounts of the glucose anti-metabolite sufficient to decrease a level of an oxidized glutathione and / or increase the ration of reduced glutathione to oxidized glutathione in the blood of the mammal subsequent to administration of the glucose anti-metabolite.
Owner:IAMS
Method of skin care and/or treatment using glutaredoxin
InactiveUS20080050332A1Less expensiveCosmetic preparationsHydrocarbon active ingredientsVitamin CLycopene
Methods for the prevention and treatment of skin damage arising from the exposure to UV radiation, air pollution and other stressors capable of leading to the formation of free radicals, using the small-dithiol protein glutaredoxin in a dermatologically acceptable carrier that can be applied topically are disclosed. Some embodiments may include other proteins such as superoxide dismutase and / or catalase, as well as other antioxidant molecules such as reduced glutathione, vitamin E, vitamin C, lycopene, astaxanthin and / or tocotrienols.
Owner:SIVAK HANNAH NAOMI
Process for producing reduced glutathione
ActiveCN103459409AEfficient manufacturingElectrolysis componentsPeptide preparation methodsOxidized GlutathioneElectrolysis
Owner:KYOWA HAKKO BIO CO LTD
Method for preparing glutathione response shell disulfide bond crosslinking non-virogene vector
InactiveCN101265477ASimple methodDifferent transfection efficienciesVector-based foreign material introductionHigh concentrationPlasmid dna
The invention discloses a method for preparing a glutathione-response shell disulfide bond crosslinking non-virus gene carrier. The method comprises the following steps: (1) synthesizing sulfhydrylation polyethyleneimine; (2) preparing sulfhydrylation polyethyleneimine aqueous solution with concentration of 0.1-1mg / ml; (3) preparing plasmid DNA solution with concentration of 50-250Mu g / ml; and (4) adding the solution prepared in step (2) to the solution of the same volume prepared in step (3), whirling to mix, standing to obtain sulfhydrylation gene carrier solution, stirring in air, and obtaining the glutathione-response shell disulfide bond crosslinking non-virus gene carrier. Through the preparation of the shell disulfide bond bionic crosslinking non-virus gene carrier, the stability of the gene carrier in physiological salt solution can be improved. The degradation of high concentration reduced glutathione in cells on disulfide bond can release associated DNA molecule so as to realize effective gene transfection.
Owner:ZHEJIANG UNIV
Acipenser dabryanus sperm preserving fluid liquid and preparing method and application
InactiveCN104054697AAchieve cryopreservationRealize comprehensive applicationDead animal preservationMotilitySimple component
The invention discloses acipenser dabryanus sperm preserving fluid liquid and a preparing method and application. The acipenser dabryanus sperm preserving fluid liquid comprises saccharose, mycose, trihydroxy aminomethane, potassium chloride, reduced glutathione and carbinol. The acipenser dabryanus sperm preserving fluid liquid has simple components and is low in cryopreservation cost. The added reduced glutathione plays a good role in antioxygenation, well protects sperm plasma membranes and reduces damage to DNA molecules. The added mycose has the effects of preventing protein denaturation and effectively protecting sperms. By means of the acipenser dabryanus sperm preserving fluid liquid, motility of unfreezed sperms is higher than 70%, and the fertility rate reaches 45%.
Owner:YANGTZE RIVER FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
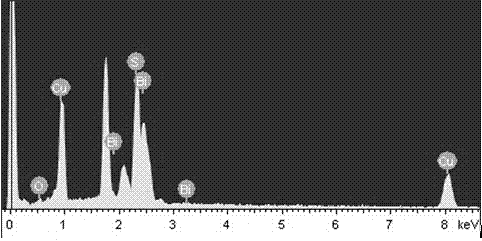
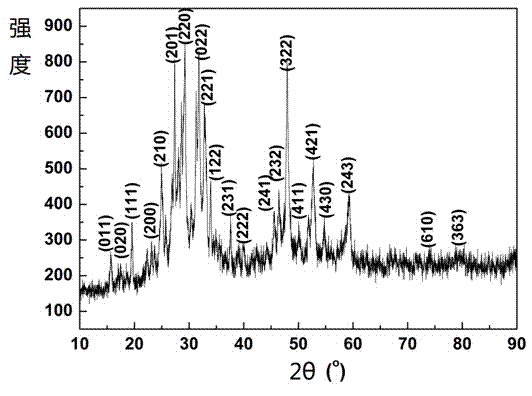
Preparation method for Cu3BiS3 micro/nanosheet
InactiveCN104709941ASimple processLow costMaterial nanotechnologyBismuth compoundsThioureaCopper nitrate
The invention discloses a preparation method for a Cu3BiS3 micro / nanosheet. The preparation method comprises the following steps: 1, dissolving a copper-containing precursor, a bismuth-containing precursor, a sulfur-containing precursor and a chelating agent in water to prepare a solution, wherein the copper-containing precursor is copper nitrate, copper sulfate or copper acetate, the bismuth-containing precursor is bismuth nitrate or bismuth acetate, the sulfur-containing precursor is thiourea, thioacetamide, sublimed sulfur or sodium sulfate, and the chelating agent is reduced glutathione; 2, heating the solution prepared in the step 1, producing a reaction completely, cooling to a room temperature, performing centrifugal treatment, washing sediments obtained by centrifuging with water and ethanol, drying, and preparing the Cu3BiS3 micro / nanosheet. The preparation method is simple in process, adopts low-price copper source, bismuth source and sulfur source as the precursors, uses solid-state nontoxic reduced glutathione as the chelating agent and uses the water as a solvent, so that the preparation method is low in cost and favorable for popularization and application.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
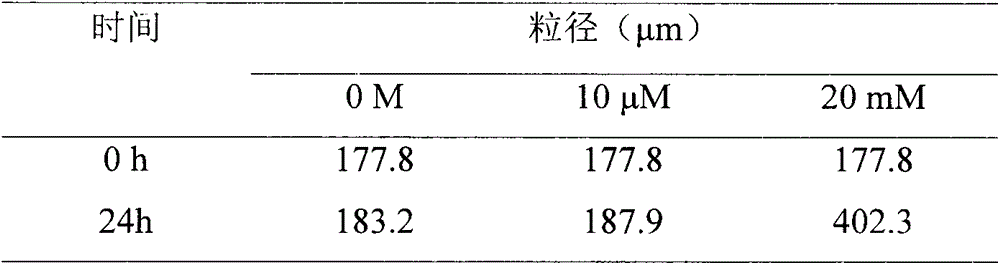
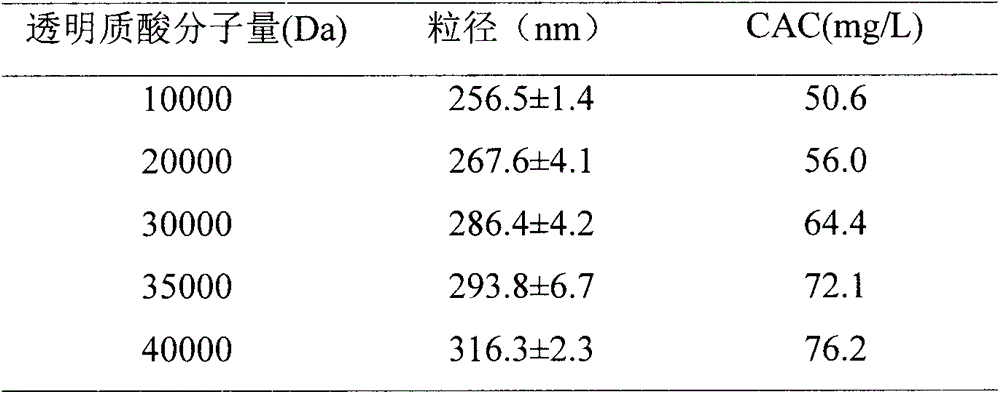
Reduction trigger type polypeptide modified hyaluronic acid conjugate carrier and preparation method thereof
InactiveCN104586816AImprove utilization efficiencyGood carrier stabilityOrganic active ingredientsMacromolecular non-active ingredientsSolubilityTumor target
The invention discloses a reduction trigger type polypeptide modified hyaluronic acid conjugate carrier and a preparation method thereof. The carrier is a nanoparticle mainly consisting of hyaluronic acid, hydrophobic groups, tumor targeted hydrophilic groups and a medicament, wherein the hydrophobic groups are bonded with hyaluronic acid through reduction sensitive bonds. The conjugate carrier can be self-assembled into the nanoparticle in an aqueous medium and is capable of supporting anti-tumor active medicine molecules. The reduction trigger type polypeptide modified hyaluronic acid conjugate carrier has the main advantages that (1) the carrier has a dual targeting capacity, so that the transfer efficiency of drugs in tumors is improved, and the untoward effect is reduced; (2) a disulfide bond linking arm is intruded into the conjugate and can be specifically degraded by high-concentration reduced glutathione in the tumor cells, so that the drugs are rapidly released, and the bioavailability of the drugs is improved; and (3) the anti-tumor drugs are loaded by virtue of a physical embedding action, so that the water solubility of the anti-tumor drugs is improved. The preparation method is simple, a process is mature, and the carrier has a good application prospect.
Owner:CHINA PHARM UNIV
Autologous-repair nutrient injection and application method thereof
PendingCN109528642ASolve potential safety hazardsImprove timelinessOrganic active ingredientsHydrolysed protein ingredientsVitamin CMedicine
The invention discloses autologous-repair nutrient injection. The injection comprises the following main components: collagen and physiological saline. By further improvement, the injection can further comprise lidocaine, reduced glutathione, vitamin C, beta thymosin, and hyaluronic acid with low molecular weight. The invention solves the problems that absorbable injectants have no lasting effectand need repeated injection, and also solves the problem that the non-absorbable injectants have potential safety hazards. The injection completely performs autologous repair on the growing skin, causes no rejection and allergy, and has long timeliness and natural effect. The injection is most suitable for people who pursue safe and natural beautifying, people who seek to recover within a short period without influencing daily work and life, and people who seek to maintain their own characteristics with exquisite beautifying.
Owner:上海欧邦医疗管理有限公司
Mixed cultivation technology of recombinant escherichia coli for producing glutathione and application of mixed cultivation technology
ActiveCN110387379AAvoidance of placementRaw materials are cheap and easy to getBacteriaMicroorganism based processesEscherichia coliPhosphorylation
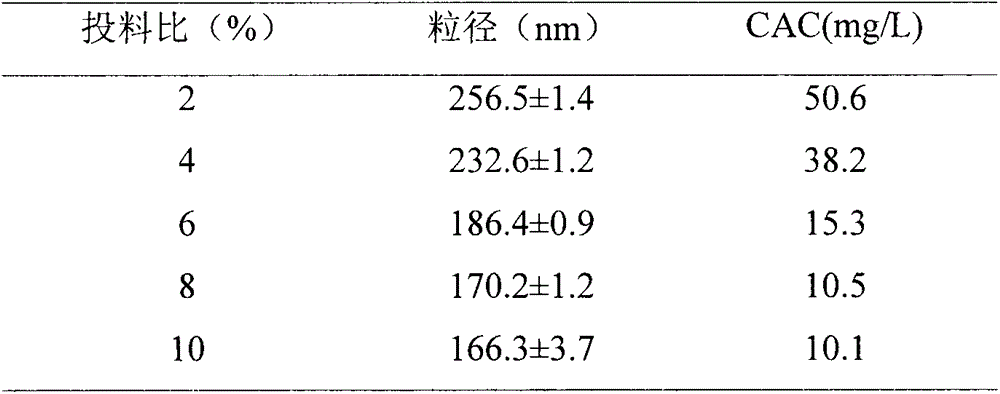
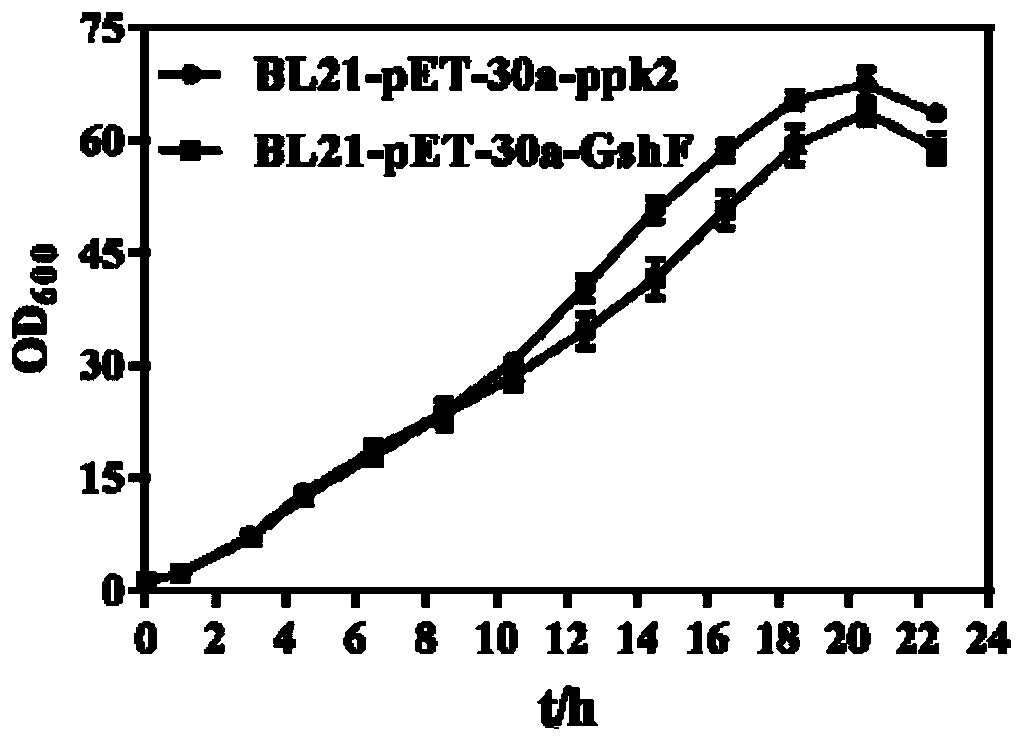
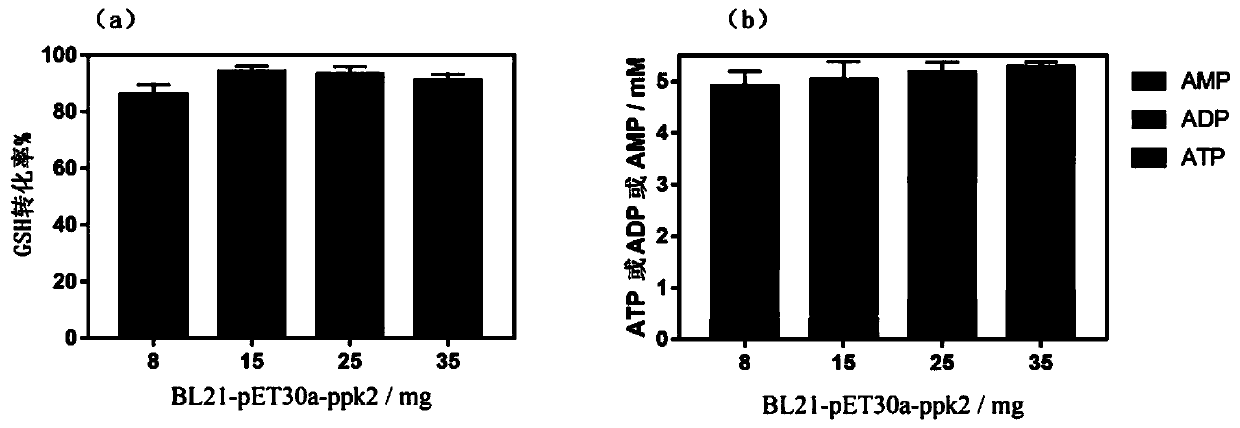
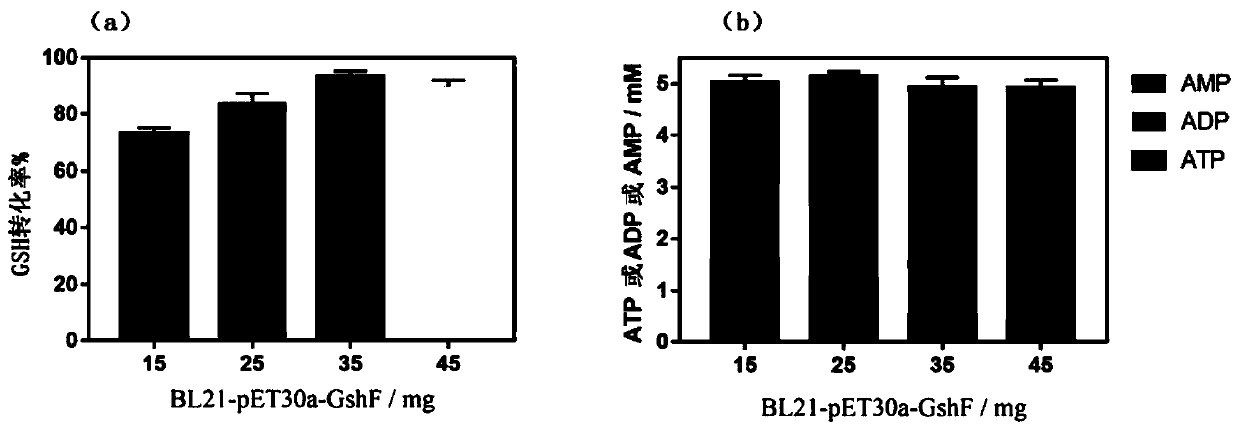
The invention discloses a mixed cultivation technology of recombinant escherichia coli for producing glutathione and application of the mixed cultivation technology. According to the mixed cultivationtechnology, a DshF gene and a ppk2 gene are correspondingly integrated on a plasmid vector, escherichia coli containing the GshF gene and escherichia coli containing the ppk2 gene are obtained, and mixedly cultivated in the proportion of 5 to 4-5 to 2 to obtain a mixed bacteria cell. Under the condition of the mixed bacteria cell at37-45 DEG C with the pH of 6-7.5, reduced glutathione is catalyzed and synthesized through inducible expression. In the presence of low-cost sodium hexametaphosphate, the phosphorylation of ADP and AMP can be catalyzed, and high-energy phosphorylation bond breakingof a catalysate provides energy for the synthesis of the glutathione. The cost is saved, the problem of placement of remaining cells due to the high reaction efficiency is avoided, operation is easy,convenient and easy to control, and good application prospects are achieved.
Owner:INNOBIO CORP LTD
Separation and cultivation method of human adipose stem cells
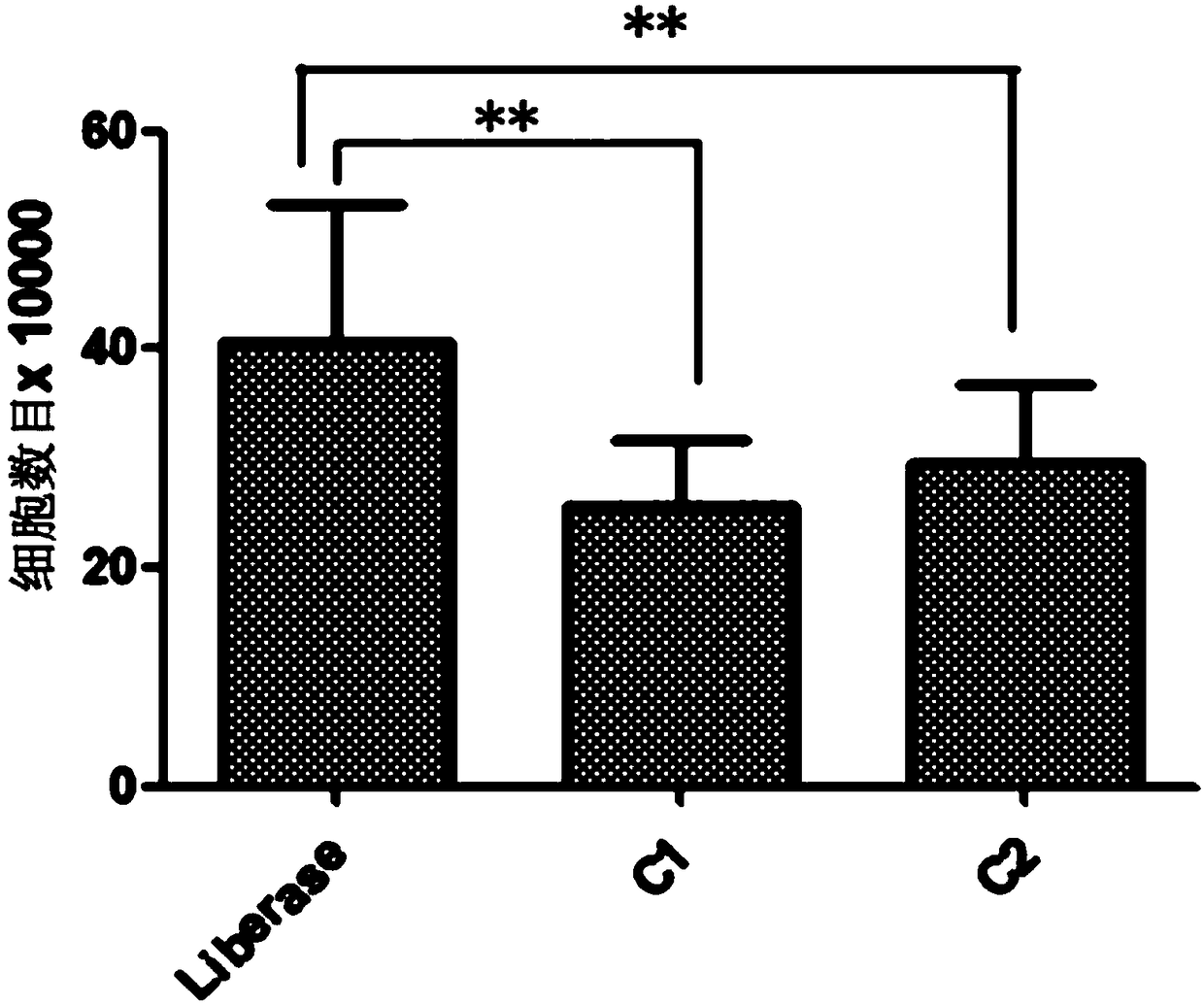
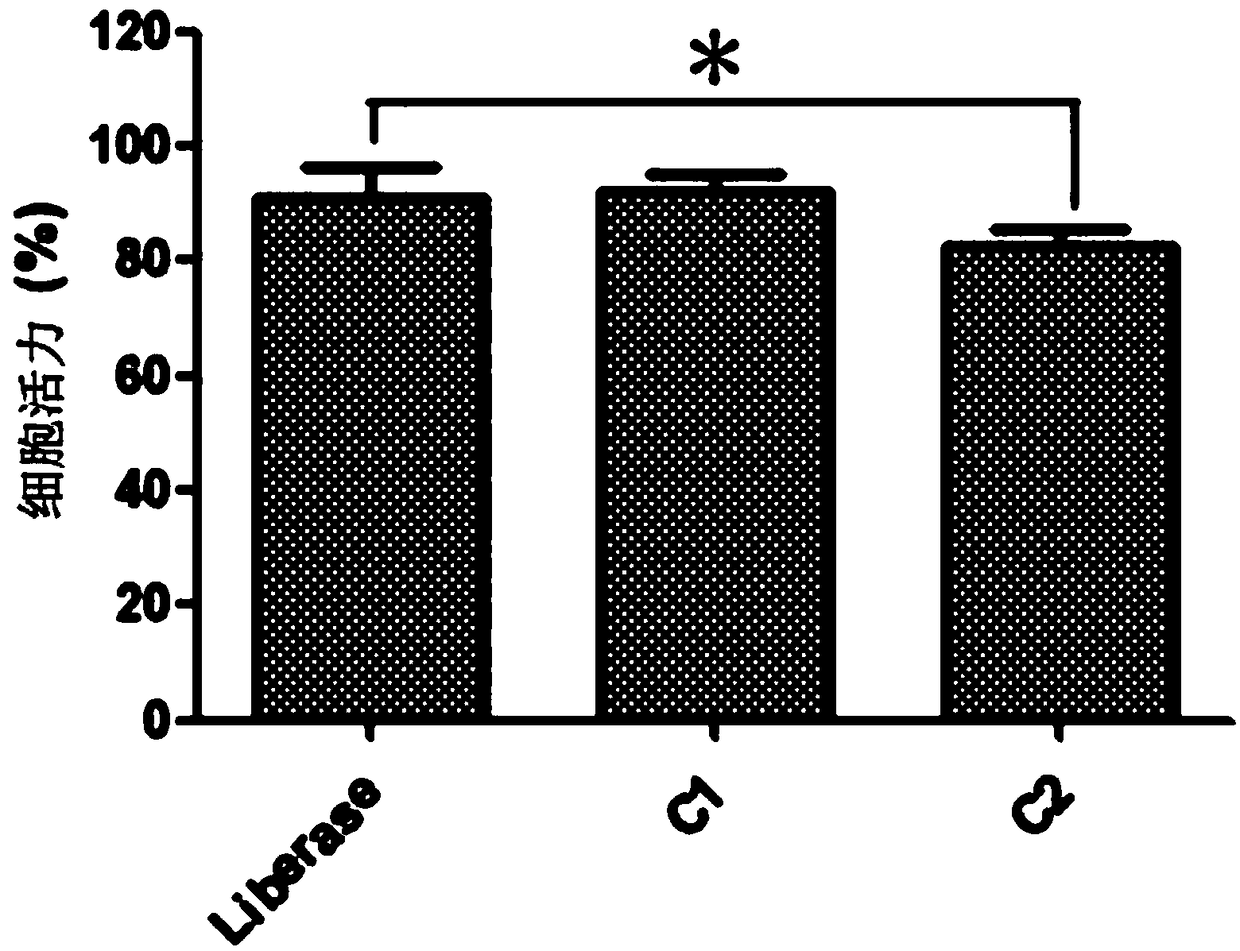
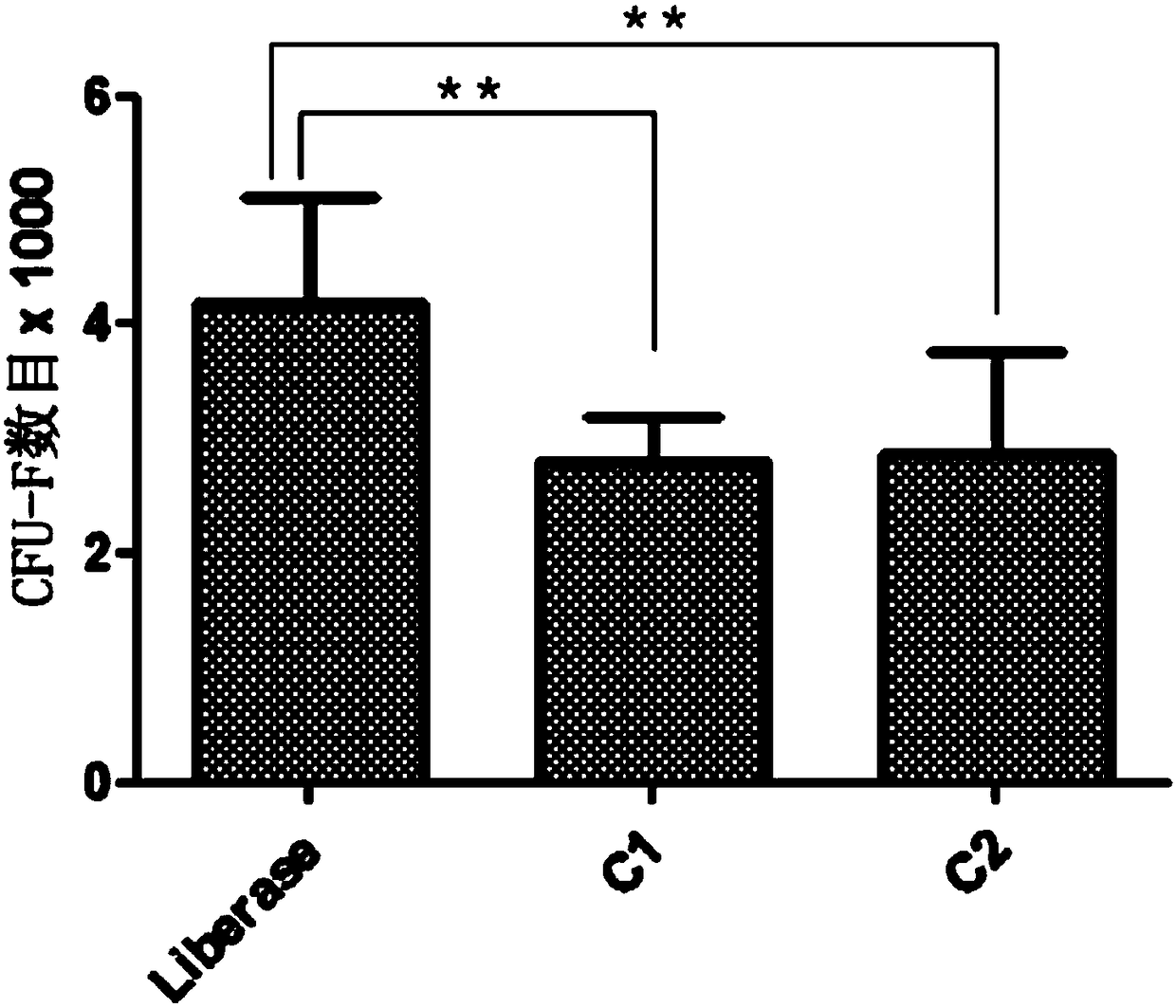
ActiveCN108220230ANormal growthNormal metabolismCell dissociation methodsCulture processSerum free mediaParenchyma
The invention discloses a separation and cultivation method of human adipose stem cells. The method comprises the following steps: first, digesting a fat suction matter by using a Liberase digestive enzyme solution, neutralizing digestive enzyme after digestion is ended, centrifuging, filtering, and further removing parenchyma cells by using a lymphocyte separation solution so as to obtain the human adipose stem cells; second, cultivating the human adipose stem cells by using a serum-free medium, wherein following components are added in the serum-free medium: a recombinant human epidermal growth factor, a recombinant human basic fibroblast growth factor, a recombinant human transforming growth factor-beta, a recombinant human platelet-derived growth factor-BB, a recombinant human stem cell factor, reduced glutathione, coenzyme A, biotin, MEM vitamin solution, MEM amino acid solution, MEM non-essential amino acid solution, a GlutaMAX additive, insulin-transferrin-selenium solution andgentamicin. The method can remarkably increase the yield of adipose stem cells and improve the activity of the adipose stem cells, so as to obtain more adipose stem cells with proliferation ability.
Owner:SHANGHAI LIFE SCI & TECH CO LTD
Universal procedure for refolding recombinant proteins
A universal folding method that has been demonstrated to be effective in refolding a variety of very different proteins expressed in bacteria as inclusion bodies has been developed. Representative proteins that can be dissolved and refolded in biologically active form, with the native structure, are shown in Table I. The method has two key steps to unfold and then refold the proteins expressed in the inclusion bodies. The first step is to raise the pH of the protein solution in the presence of denaturing agents to pH greater than 9, preferably 10. The protein solution may be maintained at the elevated pH for a period of up to about 24 hours, or the pH immediately decreased slowly, in increments of about 0.2 pH units / 24 hours, until the solution reaches a pH of about 8.0, or both steps used. In the preferred embodiment, purified inclusion bodies are dissolved in 8 M urea, 0.1 M Tris, 1 mM glycine, 1 mM EDTA, 10 mM beta-mercaptoethanol, 10 mM dithiothreitol (DTT), 1 mM reduced glutathione (GSH), 0.1 mM oxidized glutathione (GSSG), pH 10. The absorbance at 280 nm (OD280) of the protein solution is 5.0. This solution is rapidly diluted into 20 volumes of 20 mM Tris base. The resulting solution is adjusted to pH 9.0 with 1 M HCl and is kept at 4° C. for 24 hr. The pH is adjusted to pH 8.8 and the solution is kept at 4° C. for another 24 hrs. This process is repeated until the pH is adjusted to 8.0. After 24 hr at pH 8.0, the refolded proteins can be concentrated by ultrafiltration and applied to a gel filtration column for purification.
Owner:OKLAHOMA MEDICAL RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com