Reduction trigger type polypeptide modified hyaluronic acid conjugate carrier and preparation method thereof
A technology of hyaluronic acid and polypeptide modification, applied in drug combinations, microcapsules, inactive components of polymer compounds, etc., can solve the lack of targeting of small molecule chemotherapy drugs, limit the clinical use of antitumor drugs, damage organs and tissues and other problems, to achieve a good application prospect, avoid long-term accumulation, and reduce the effects of toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Synthesis of reduction-triggered T7 peptide-modified hyaluronic acid carrier (PEPHSSC)
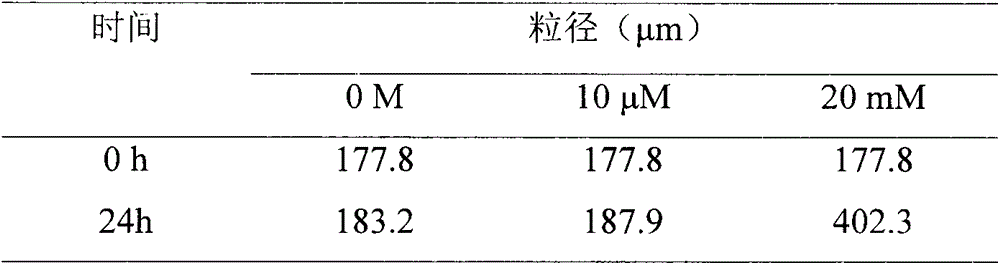
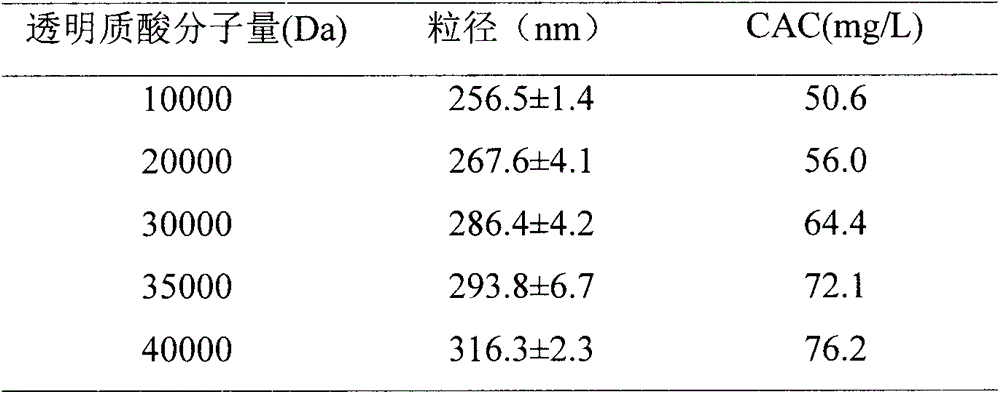
[0050] Hyaluronic acid with a molecular weight of 10000Da was selected as the hydrophilic end of the carrier. The specific preparation method is four steps:
[0051] 1) Preparation of the hydrophobic group-bonded reduction-sensitive linker: 10 mmol cystamine and 0.5 mmol pyridine were dissolved in 30 mL dichloromethane, and a dichloromethane solution of 0.5 mmol cholesterol chloroformate was slowly added dropwise. After overnight reaction at 25°C, the organic solvent was removed by rotary evaporation.
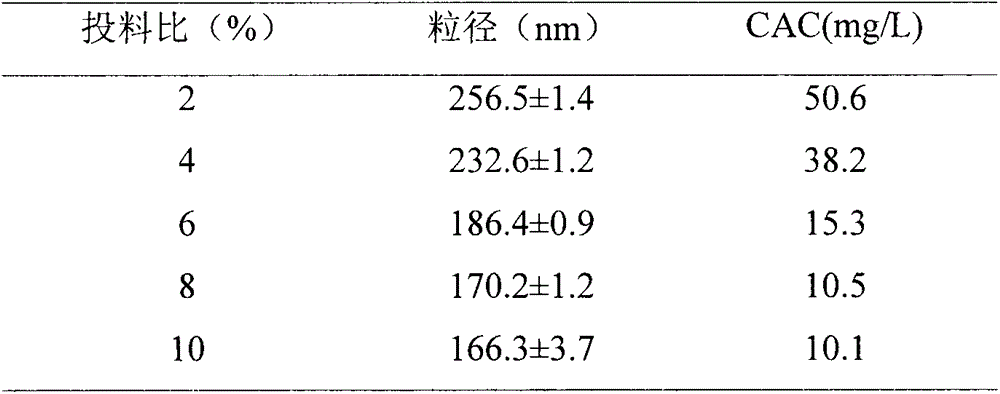
[0052] 2) Preparation of hydrophobically modified hyaluronic acid: Dissolve 0.1mmol hyaluronic acid, 0.3mmol EDC and 0.3mmol HOBT in formamide, stir for 15min, add 0.002mmol of hydrophobic groups containing reduction-sensitive linking arms to react for 24h , precipitated with excess acetone, and filtered with suction. Add water to redissolve the precipitate, dialyze with...
Embodiment 2
[0055] Example 2 Synthesis of reduction-triggered octreotide-modified hyaluronic acid carrier (PEPHSSC)
[0056] Hyaluronic acid with a molecular weight of 20000Da was selected as the hydrophilic end of the carrier. The specific preparation method is four steps:
[0057] 1) Preparation of the hydrophobic group-bonded reduction-sensitive linker: 10 mmol cystamine and 0.5 mmol triethylamine were dissolved in 30 mL dichloromethane, and a dichloromethane solution of 0.5 mmol cholesterol chloroformate was slowly added dropwise. After overnight reaction at 25°C, the organic solvent was removed by rotary evaporation.
[0058] 2) Preparation of hydrophobically modified hyaluronic acid: Dissolve 0.1mmol hyaluronic acid, 0.3mmol EDC and 0.3mmol HOBT in formamide, stir for 15min, add 0.006mmol of hydrophobic groups containing reduction-sensitive linkers to react for 24h , precipitated with excess acetone, and filtered with suction. Add water to redissolve the precipitate, dialyze with...
Embodiment 3
[0061] Example 3 Synthesis of reduction-triggered GE11 peptide-modified hyaluronic acid carrier (PEPHSSC)
[0062] Hyaluronic acid with a molecular weight of 40000Da was selected as the hydrophilic end of the carrier. The specific preparation method is four steps:
[0063] 1) Preparation of the hydrophobic group-bonded reduction-sensitive linker: 10 mmol cystamine and 0.5 mmol pyridine were dissolved in 30 mL dichloromethane, and a dichloromethane solution of 0.5 mmol cholesterol chloroformate was slowly added dropwise. After overnight reaction at 25°C, the organic solvent was removed by rotary evaporation.
[0064] 2) Preparation of hydrophobically modified hyaluronic acid: dissolve 0.1mmol hyaluronic acid, 0.3mmol EDC and 0.3mmol HOBT in formamide, stir for 15min, add 0.010mmol of hydrophobic groups containing reduction-sensitive linking arms to react for 24h , precipitated with excess acetone, and filtered with suction. Add water to redissolve the precipitate, dialyze wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com