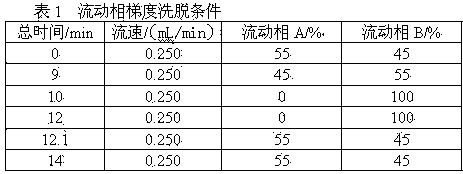
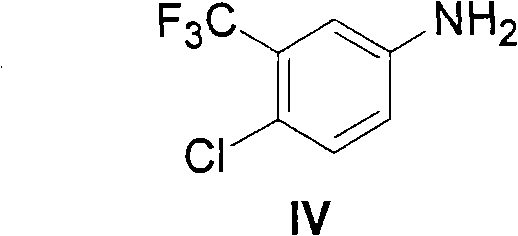
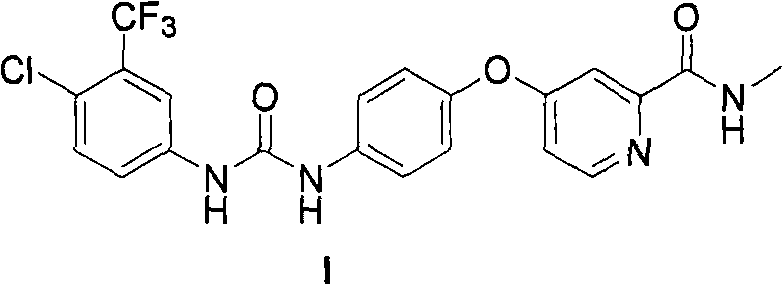
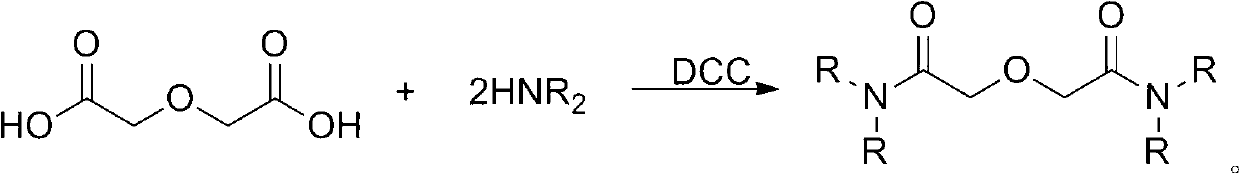Patents
Literature
394 results about "Chloroformate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
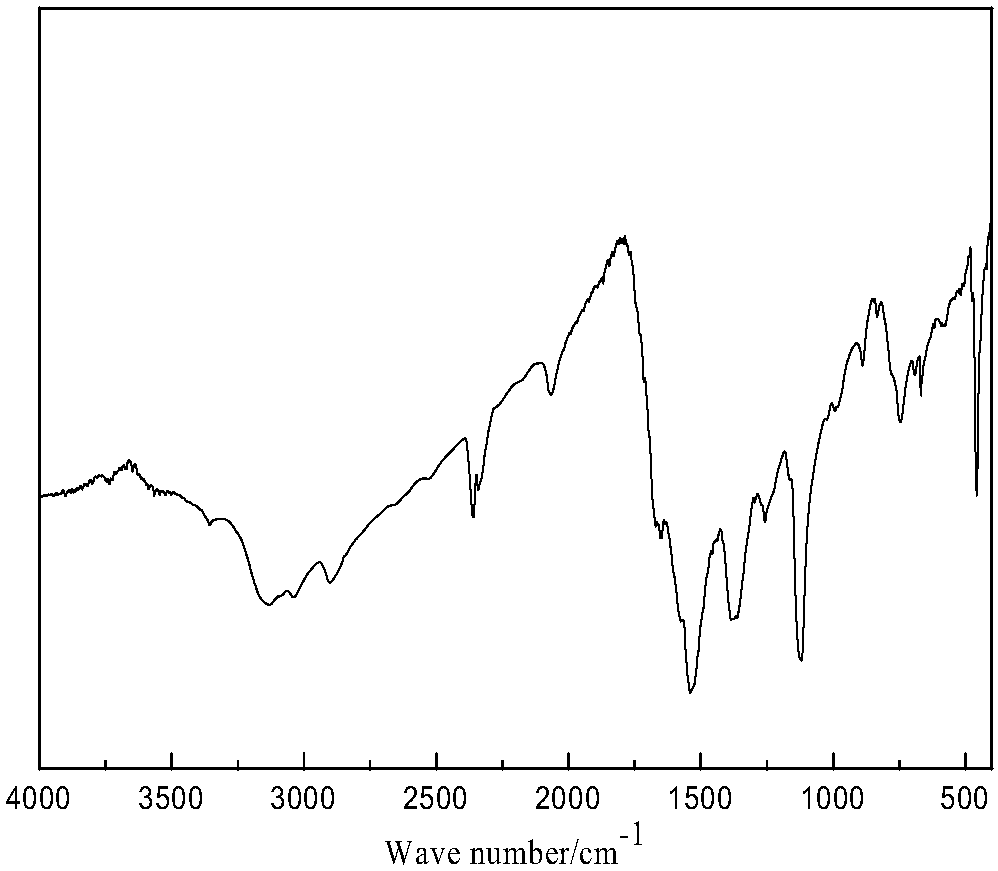
Chloroformates are a class of organic compounds with the formula ROC(O)Cl. They are formally esters of chloroformic acid. Most are colorless, volatile liquids that degrade in moist air. A simple example is methyl chloroformate, which is commercially available.
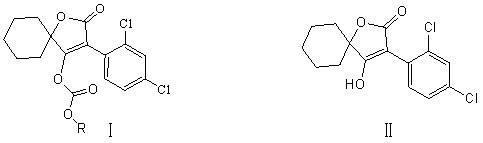
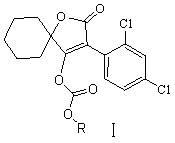
Novel spirodiclofen compound and preparation method and application thereof
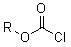
The invention relates to a novel spirodiclofen compound and a preparation method and application thereof. The structure of the novel spirodiclofen compound is shown in a general formula I. The synthesis method of the novel spirodiclofen compound comprises the steps of: in the presence of an acid binding agent, in an aprotic solvent, reacting a compound with a structure formula II with chloro-carbonic ester with a structure formula shown in the description at room temperature, and separating and purifying a product to prepare the novel spirodiclofen compound. The novel spirodiclofen compound can be used for killing pests or mites.
Owner:HANGZHOU UDRAGON CHEMICAL CO LTD
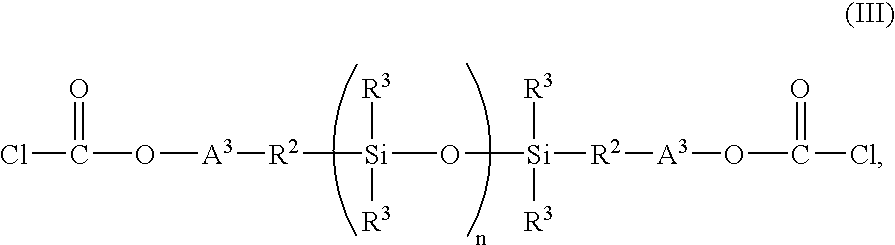
Method for preparation of copolyorganosiloxanecarbonates of high clarity
Owner:SHPP GLOBAL TECH BV
Method for preparation of copolyorganosiloxanecarbonates of high clarity
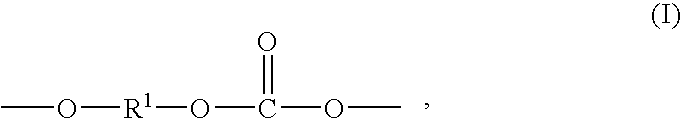
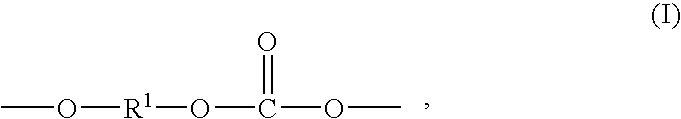
Copolyorganosiloxanecarbonates are prepared by first preparing an oligomeric aromatic polycarbonate, such as an oligomeric bisphenol A polycarbonate, in the presence of a tertiary amine as the only catalyst species; contacting the oligomeric polycarbonate mixture with a polyorganosiloxane bis(aryl)chloroformate, such as the bischloroformate of hydroxy-terminated eugenol polydimethylsiloxane; and introducing phosgene and / or chain termination agent either continuously or in stages. A feature of the process is the presence of dihydroxyaromatic compound in only one charge, at the beginning. The products have excellent physical properties, including transparency.
Owner:SHPP GLOBAL TECH BV
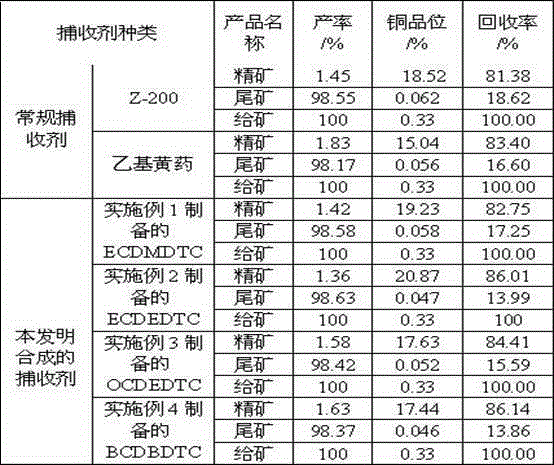
Copper sulfide flotation collector as well as preparation method and application thereof
InactiveCN104475266ASimple manufacturing methodReduce net negative chargeFlotationChloroformateCarbon chain
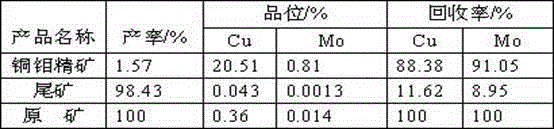
The invention discloses a copper sulfide ore flotation collector as well as a preparation method and application thereof. The collector is alkoxy carbonyl alkyl dithiocarbamate and has a structural formula as shown in the specification, wherein R1 is carbon chain 1-4 alkyl and R2 is carbon chain 2-4 alkyl. The preparation method comprises the following steps: performing nucleophilic substitution reaction on alkylamine, sodium hydroxide and carbon bisulfide to obtain sodium dithiocarbamate; reacting the sodium dithiocarbamate and alkyl chloroformate to obtain a target collector alkoxy carbonyl alkyl dithiocarbamate. The application refers to application of the copper sulfide ore flotation collector in performing flotation on copper sulfide ore and recycling valuable metal minerals in the copper sulfide ore. The collector is capable of realizing effective separation of the copper sulfide ore and pyrite under an ore pulp environment with the pH value of 6-10, reducing the lime consumption, and further effectively recycling useful metals such as gold, silver and molybdenum. Compared with the existing collector, the collector has the advantages of good selectivity and high useful metal recovery rate.
Owner:KUNMING METALLURGY INST
Detection method and application of carboxy methyl lysine ingredient in food
InactiveCN103293243AEfficient removalStable retention timeComponent separationAdditive ingredientChloroformate
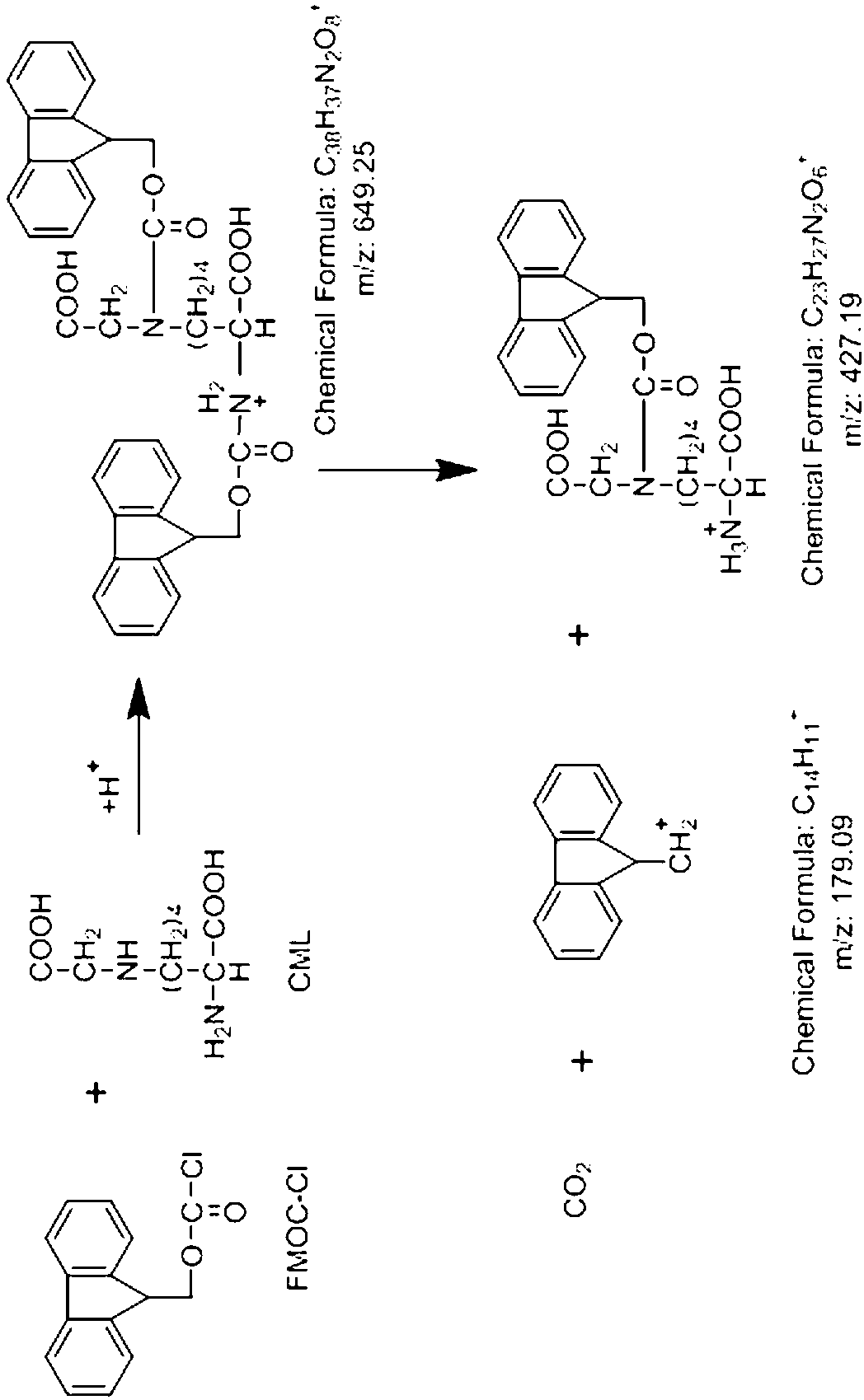
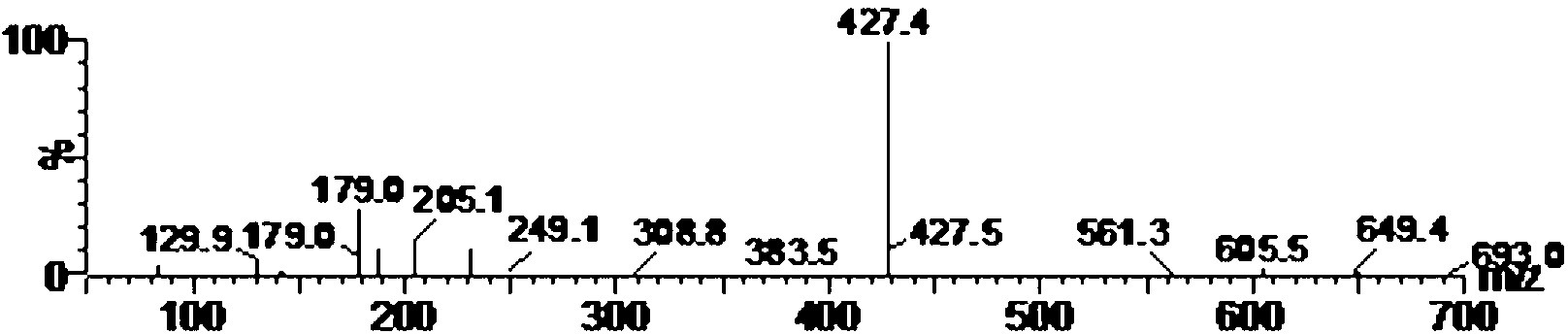
The invention relates to a detection method and application of a carboxy methyl lysine ingredient in food. The detection method comprises the following steps of: carrying out derivatization by a derivating agent after protein acid in a sample is hydrolyzed; obtaining solution to be detected from the derived solution by solid-phase extraction; carrying out qualitative and quantitative analysis on the carboxy methyl lysine ingredient in the food by adopting a tandem mass spectrometry of isotope dilution ultra-performance liquid chromatography, wherein the derivating agent is 9-fluorenylmethyl chloroformate.
Owner:FUJIAN INSPECTION & RES INST FOR PROD QUALITY +1
Process for manufacturing N-alkoxy(or aryloxy)carbonyl isothiocyanate derivatives using N,N-dialkylarylamine as catalyst
InactiveUS6066754AHigh purityHigh yieldCarbamic acid derivatives preparationOrganic compound preparationOrganic solventChloroformate
The present invention provides a process for making N-alkoxy(or aryloxy)carbonyl isothiocyanate derivatives by reacting a chloroformate with a thiocyanate, in the presence of an organic solvent and a catalytic amount of a N,N-dialkylarylamine, to produce a N-alkoxy(or aryloxy)carbonyl isothiocyanate intermediate product, wherein the intermediate product is converted to a N-alkoxy(or aryloxy)carbonyl isothiocyanate derivative in high yield and purity.
Owner:BAYER CORP
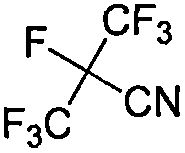
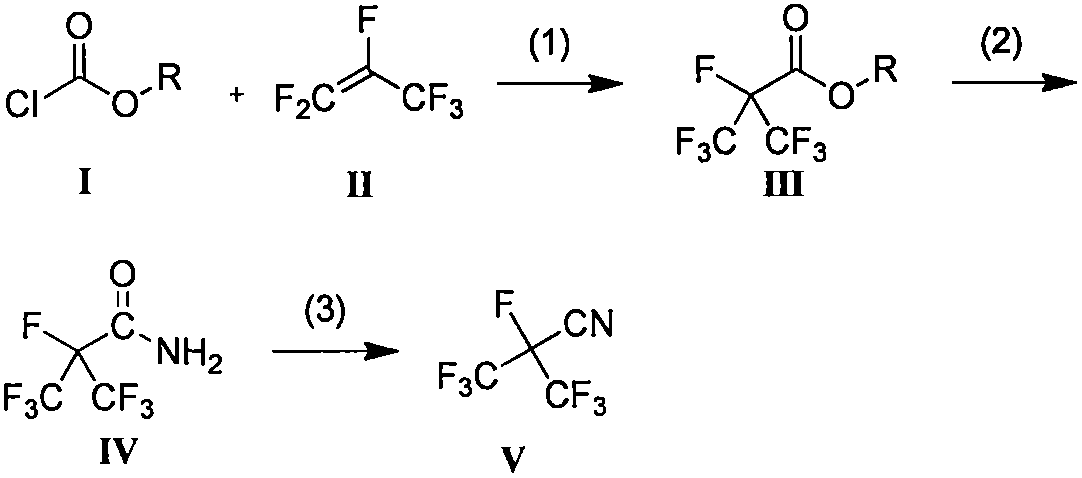
Method for synthesizing perfluoroisobutyronitrile
ActiveCN108395382ALow costHigh purityOrganic compound preparationCarboxylic acid esters preparationHexafluoropropyleneChloroformate
The invention discloses a method for synthesizing perfluoroisobutyronitrile. According to the method, industrialized products including hexafluoropropylene and chloroformic ester are used as raw materials to prepare a product; a reaction route comprises the following steps: synthesizing heptafluoroisobutyrate through the hexafluoropropylene and the chloroformic ester under the action of fluoride through a one-pot method; taking the heptafluoroisobutyrate and ammonia to react to obtain heptafluoroisobutyramide; dehydrating the heptafluoroisobutyramide through a dehydrating agent, and rectifyingand purifying to obtain the perfluoroisobutyronitrile (2,3,3,3-tetrafluoro-2-trifluoromethylpropionitrile). According to the method disclosed by the invention, the used raw materials are commerciallyavailable; the main raw materials including the chloroformic ester and the hexafluoropropylene have low cost and can be abundantly supplied; reaction conditions are moderate and the reaction conversion rate and the yield are high; the product of each step is easy to separate and the purity of the product is high; the method has the advantages of convenience and safety in operation of a technologyand easiness for realizing industrial production.
Owner:昊华气体有限公司 +1
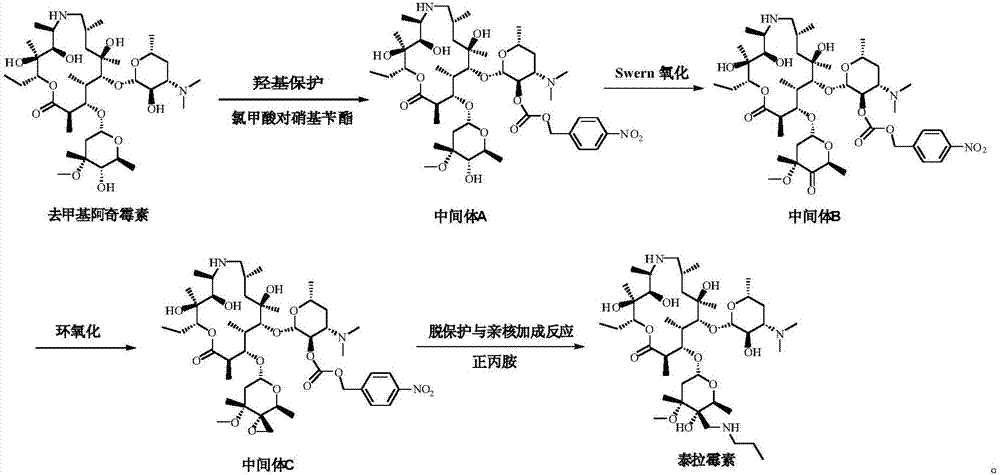
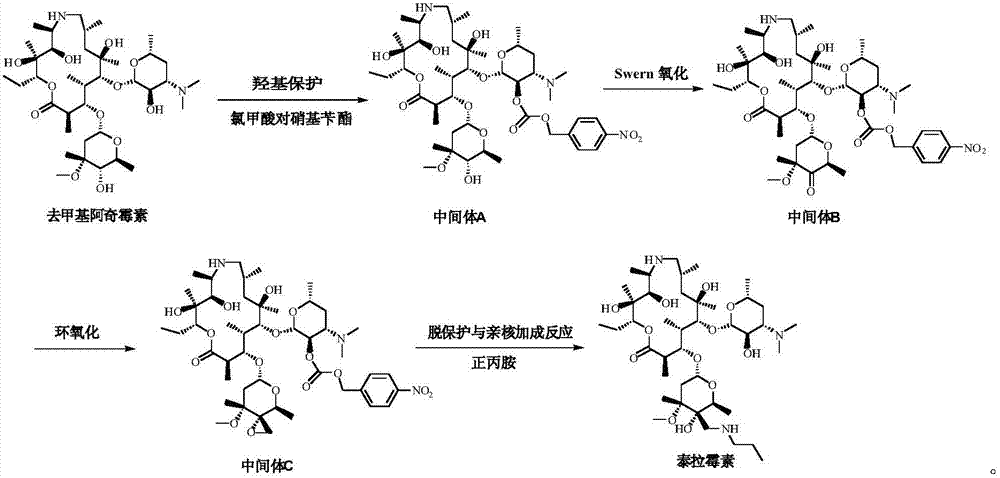
Preparation method of tulathromycin
ActiveCN106939029AImprove protectionAvoid catalytic hydrogenation methodsSugar derivativesSugar derivatives preparationAzithromycinChemical synthesis
The invention relates to the field of chemical synthesis and particularly relates to a preparation method of tulathromycin. The method comprises protecting a 2-hydroxyl group of demethyl azithromycin through 4-nitrobenzyl chloroformate, carrying out oxidation and epoxidation on a 4"-hydroxyl group, and carrying out deprotection and 4"-epoxy nucleophilic addition through n-propylamine to obtain tulathromycin. Comprised with the prior art, the preparation method has simple processes, mild conditions and a high yield, is free of palladium-carbon hydrodeprotection and is conducive to industrial production.
Owner:AMICOGEN CHINA BIOPHARM CO LTD
Pretreatment method for measuring pesticides of glyphosate and phosphinothricin in tea leaves
ActiveCN103869028AExtraction does not affectImprove derivation efficiencyComponent separationPretreatment methodPhenolic content in tea
The invention provides a pretreatment method for measuring pesticides of glyphosate and phosphinothricin in tea leaves. In general, acidic precipitation, solid phase extraction, precolumn derivatization and a liquid chromatograph / mass spectrometer are used for measuring, and a method for measuring the residual amounts of glyphosate and phosphinothricin in the tea leaves by using the liquid chromatograph / mass spectrometer is established. In the method, a large quantity of tea polyphenols in the tea leaves are precipitated, so that the deviation efficiency of solid phase extraction liquid and 9-fluorenylmethyl chloroformate is increased, the interference of impurities is reduced, and the high sensitivity is realized. The method is accurate, efficiency and stable, can meet the requirement of residue detection, and can be applied to the qualitative and quantitative analysis of the glyphosate and the phosphinothricin in the various tea leaves.
Owner:TEA RES INST CHINESE ACAD OF AGRI SCI
Process for manufacture of N-alkoxy(or aryloxy)carbonyl isothiocyanate derivatives in the presence of N,N-dialkylarylamine catalyst and aqueous solvent
InactiveUS6184412B1High purityHigh yieldCarbamic acid derivatives preparationOrganic compound preparationChloroformateSolvent
The present invention provides a process for making N-alkoxy(or aryloxy)carbonyl isothiocyanate derivatives by reacting a chloroformate with a thiocyanate, in the presence of an aqueous solvent and a catalytic amount of a N,N-dialkylarylamine, to produce a N-alkoxy(or aryloxy)carbonyl isothiocyanate intermediate product, wherein the intermediate product is converted to a N-alkoxy(or aryloxy)carbonyl isothiocyanate derivative in high yield and purity.
Owner:BAYER CORPORATION
Method for synthesizing Nexavar
InactiveCN101671299AReduce usageHigh purityOrganic chemistryAntineoplastic agentsOrganic solventHydrogen
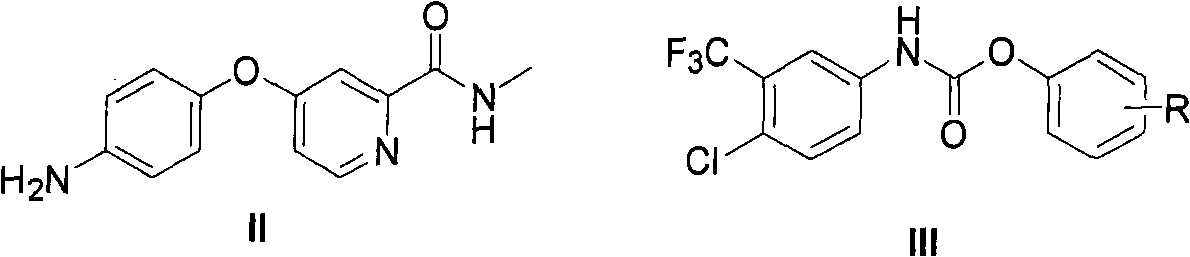
The invention provides a method for synthesizing Nexavar. The method comprises the following steps: a, dissolving a compound II and a compound III in an organic solvent inert to the compound III and adding appropriate base; b, performing reaction at a reaction temperature between 40 and 150 DEG C so as to generate a crude Nexavar product; and c, performing conventional post-treatment on the crudeproduct, wherein R is hydrogen, methyl, nitro or chlorine; the nitro is on site 4; the methyl or the chlorine is on site 2, 3 or 4; and the compound III is prepared through the reaction of a compoundIV and phenyl chloroformate or the phenyl chloroformate containing substituents on benzene rings in an organic reaction solvent inert to chloroformate at a temperature between 10 DEG C below zero and50 DEG C. The method has the advantages of applying to the preparation of Nexavar on an industrial scale, meeting standards in pharmaceutical industrial production, improving the purity and environmental compatibility of products and improving operability, safety and yield.
Owner:SHANGHAI PUYI CHEM CO LTD
Preparation method of azilsartan intermediate and azilsartan
The invention discloses a preparation method of an intermediate 5B and azilsartan 1. The preparation method of the azilsartan 1 comprises the following steps: 1) in a solvent, mixing a compound 2B with hydroxylamine to react to obtain a compound 3B; 2) in a solvent, mixing the compound 3B prepared in the step 1) with chloroformate to react under the action of alkali to obtain a compound 4B; 3) in a solvent, carrying out cyclization reaction on the compound 4B prepared in the step 2) to obtain a compound 5B; and 4) in a solvent, carrying out esterolysis reaction on the compound 5B prepared in the step 3) under the action of alkali to obtain the azilsartan 1, wherein R is a C6-C10 aryl group or C1-C4 straight-chain or branched-chain alkyl group. The preparation method of the azilsartan intermediate 5B is described as the step 3). The preparation method has the advantages of fewer impurities, short reaction time, higher technical yield and higher product purity, and is suitable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Process for the preparation of polycarbonates and diaryl carbonate
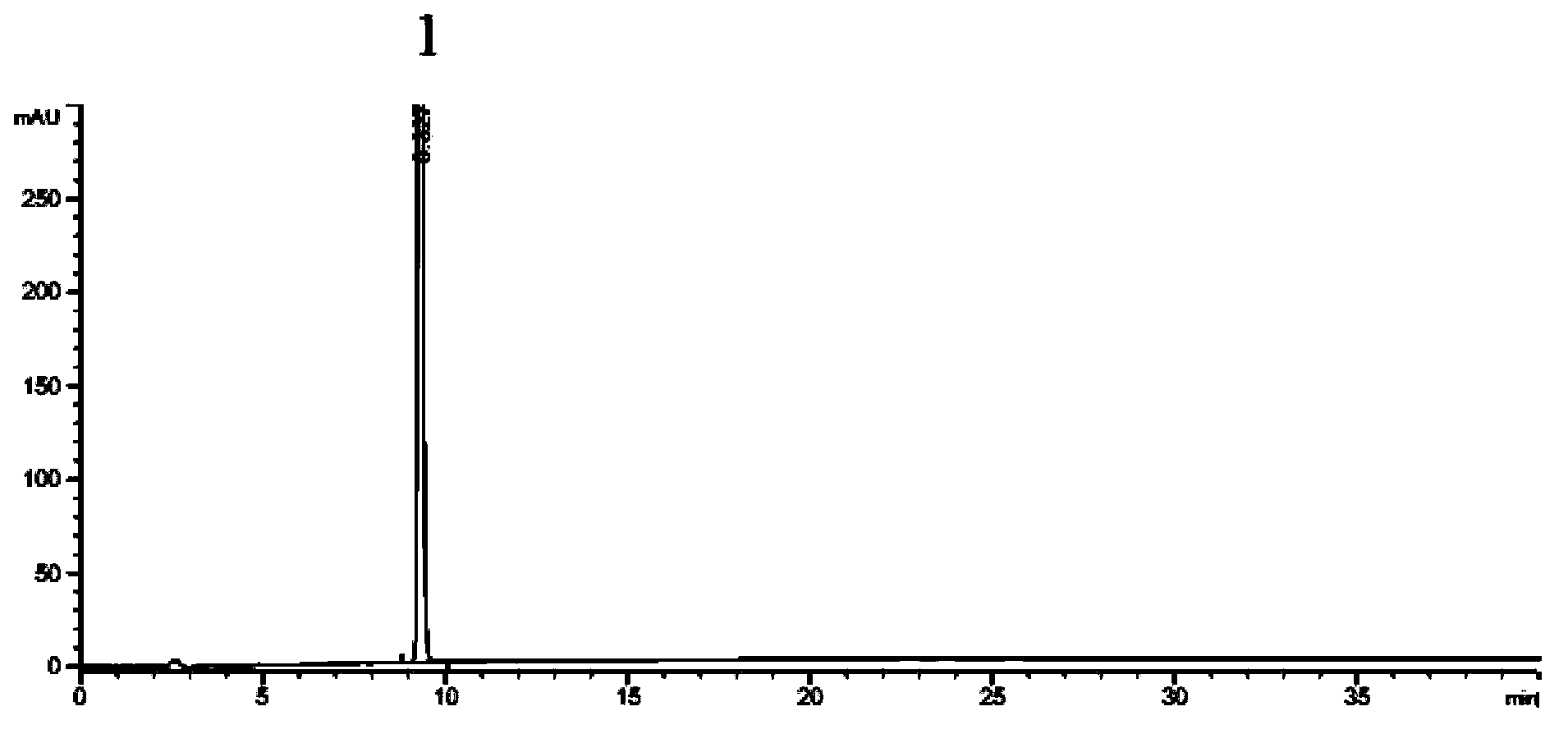
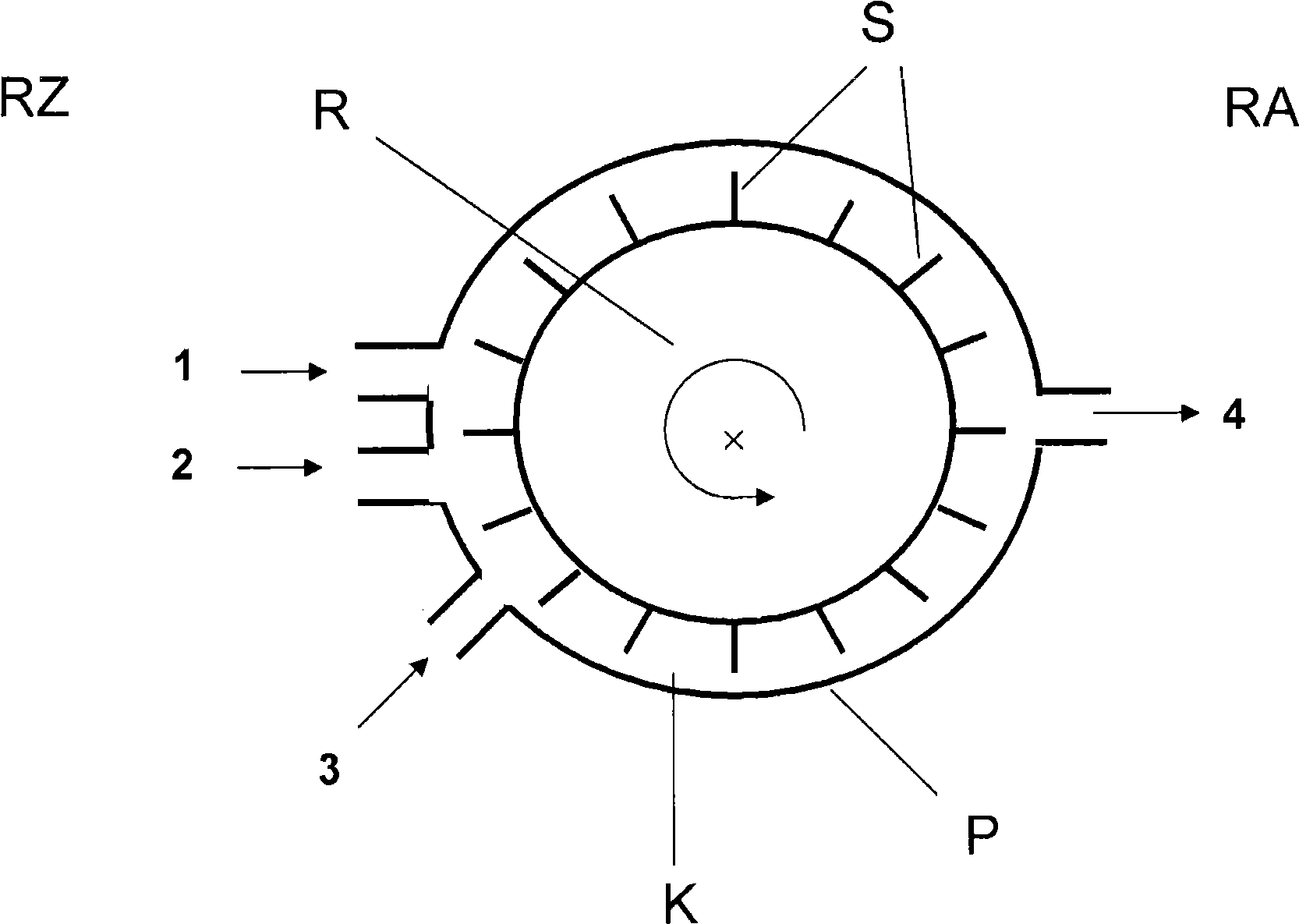
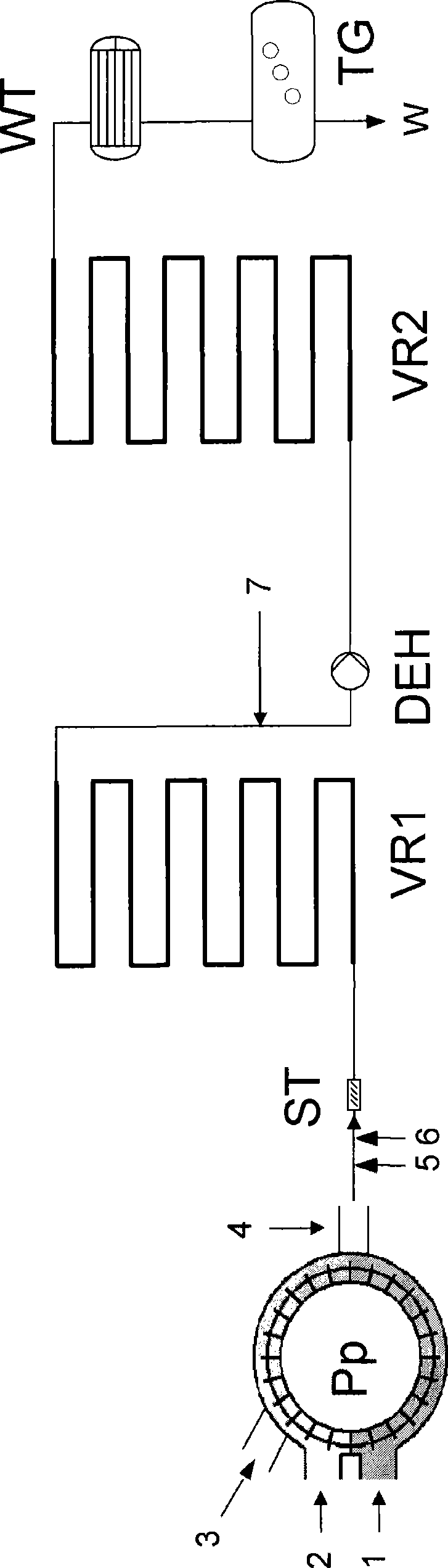
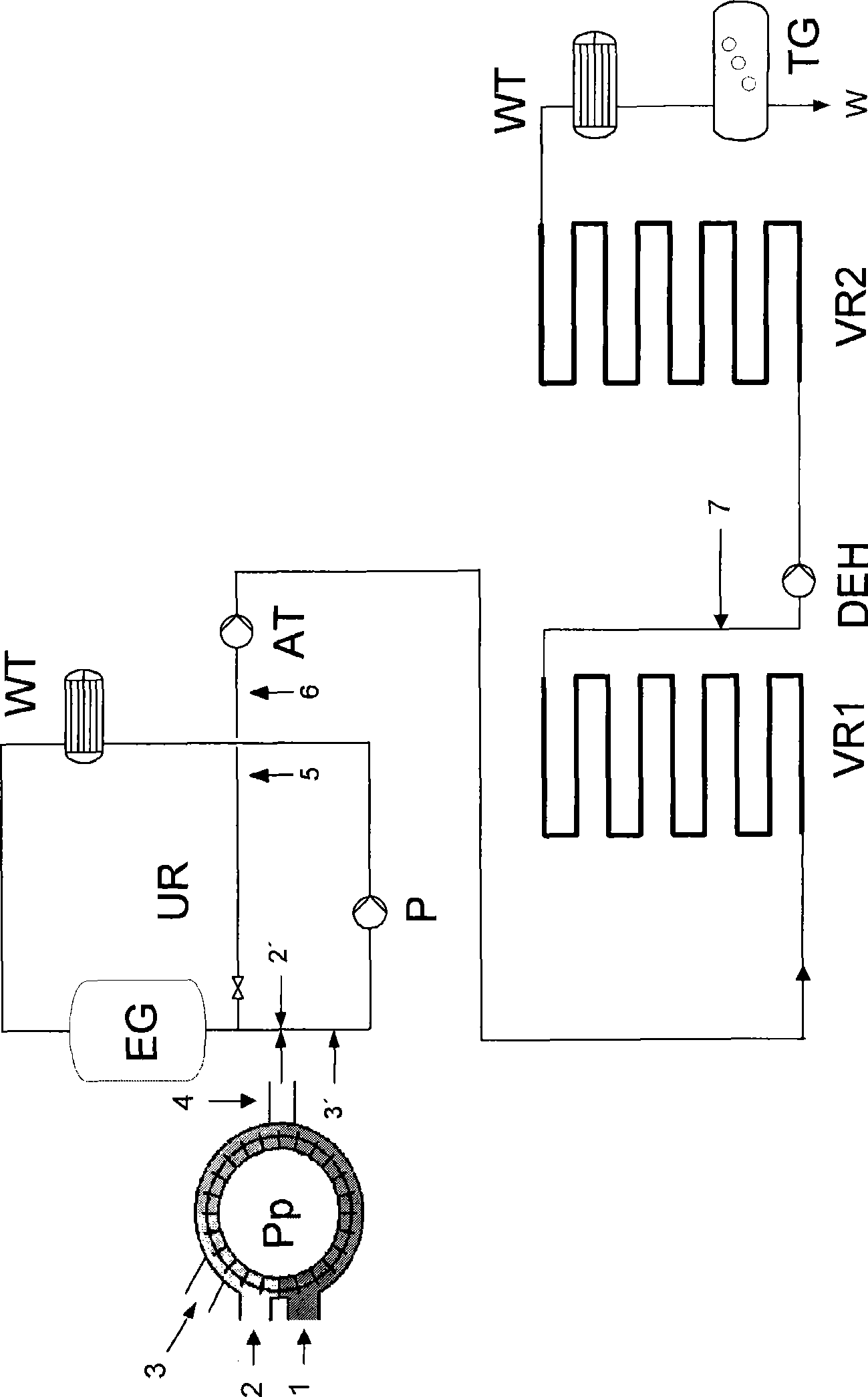
The present invention relates to a process for the continuous preparation of polycarbonates or diaryl carbonates by the method of the phase boundary process, in which both the mixing of the organic and aqueous phase and the upstream oligomerization step or aryl chloroformate and / or diaryl carbonate preparation step are effected in a special pump.
Owner:BAYER MATERIALSCIENCE AG
Fluoropyrimidine compound carbalkoxylation method
The invention relates to fluorine contained miazines compound N-4 position alkyl oxygen carbonyl acidylating method, and its use in composing antineoplastic medicine-capecitabine. The invention has the advantages of avoiding using severe toxicity reagent such as, chloro formate, or phosgene etc, stable and easily gaining raw material, easy industrialization operation etc.
Owner:ZHEJIANG HISUN PHARMA CO LTD
9-fluorenylmethyl chloroformate preparation method
InactiveCN103408427ASimple processMild conditionsOrganic compound preparationCarbonic/haloformic acid esters preparationChloroformateSolvent
The present invention relates to a 9-fluorenylmethyl chloroformate preparation method, particularly to an improved method for preparing 9-fluorenylmethyl chloroformate from 9-fluorenylmethanol. The method comprises the following steps: 1) adding chloroform, 9-fluorenylmethanol and triphosgene to a reactor, and stirring for 30 min, wherein raw materials comprise 9-fluorenylmethanol, triphosgene, chloroform and 4-dimethylaminopyridine, and a ratio of the 9-fluorenylmethanol to the triphosgene to the chloroform to the 4-dimethylaminopyridine is 20 g:46 g:200 ml:18.5 g; 2) in an ice bath, adding a chloroform solution of 4-dimethylaminopyridine in a dropwise manner, and carrying out a reaction for 2-4 h; and 3) filtering the reactant in the reactor to obtain a white solid and the filtrate, and sequentially carrying out pressure reduction solvent removing, cryogenic crystallization, organic solvent washing and drying on the filtrate to obtain the white solid 9-fluorenylmethyl chloroformate. The preparation method has characteristics of mild reaction conditions, low cost, simple process, and easy industrialization.
Owner:ZHANG JIA GANG VINSCE BIO PHARM
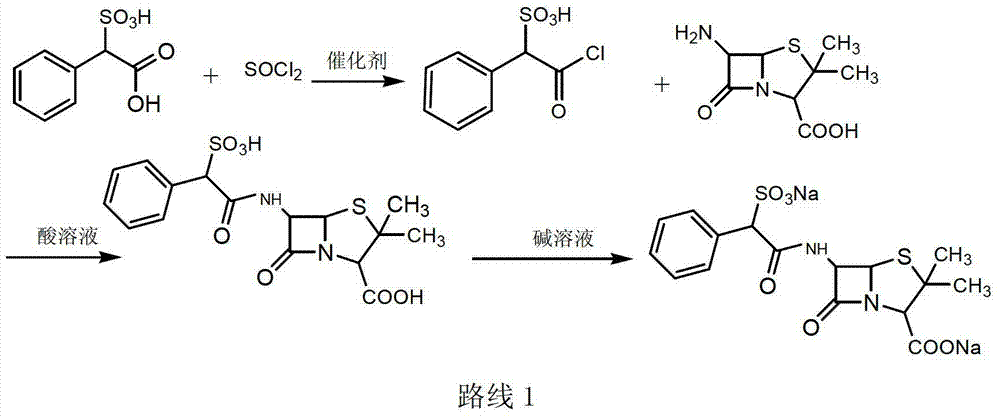
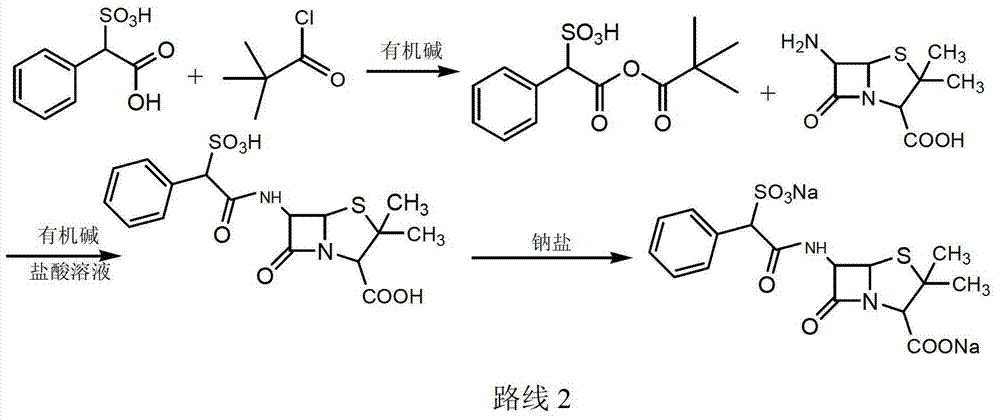
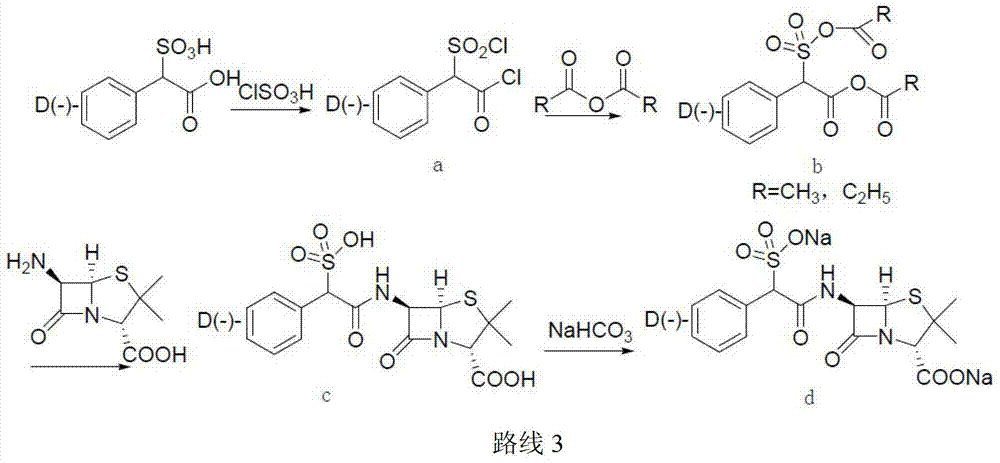
Sulbenicillin sodium preparation method
ActiveCN103319502ALow cost and readily availableEasy to removeOrganic chemistrySulbenicillinOrganic solvent
The invention discloses a sulbenicillin sodium preparation method, and belongs to the technical field of medicine. According to the preparation method, alpha-sulfophenylacetic acid reacts with chloroformate to produce a mixed anhydride, 6-APA and an organic alkali are subjected to salt formation, and then dissolved in an organic solvent, the obtained material is subjected to a condensation reaction in the mixed anhydride solution, acidification liquid separation is performed after completing the reaction, the organic phase is retained, and sodium2-ethylhexanoate is added to the organic phase to carry out salt formation to obtain the sulbenicillin sodium, wherein the sulbenicillin sodium is further subjected to sterile crystallization through a system comprising water, ethanol and acetone to obtain sulbenicillin sodium for injection. The preparation method has characteristics of low cost, good quality and easy operation, and is suitable for industrialization.
Owner:REYOUNG PHARMA
Process for the preparation of imidazo[4,5-c]-quinolin-4-amines
The present invention is a method for preparing a compound of the formula wherein R is hydrogen, C1 to C6 alkyl, C1 to C6 alkoxy or halo, R1 and R2 are independently hydrogen, C1 to C10 alkyl, C1 to C10 alkoxy, C3 to C10 cycloalkyl, C3 to C10 alkenyl, C5 to C10 cycloalkenyl, C2 to C10 alkynyl, C6 to C20 aryl or substituted C6 to C20 aryl and n is the integer 1 or 2 by reacting the corresponding N-oxide with an aqueous solution of ammonia and a C1 to C6 alkyl, C6 to C20 aryl or substituted C6 to C20 aryl chloroformate thereby forming said compound formula (1).
Owner:DZWINIEL TREVOR
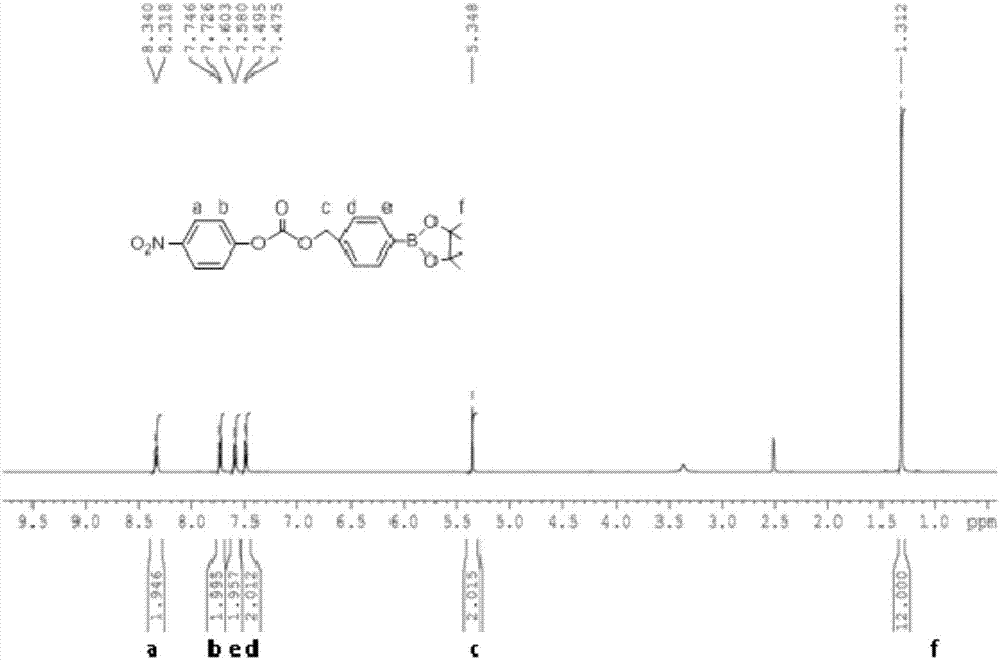
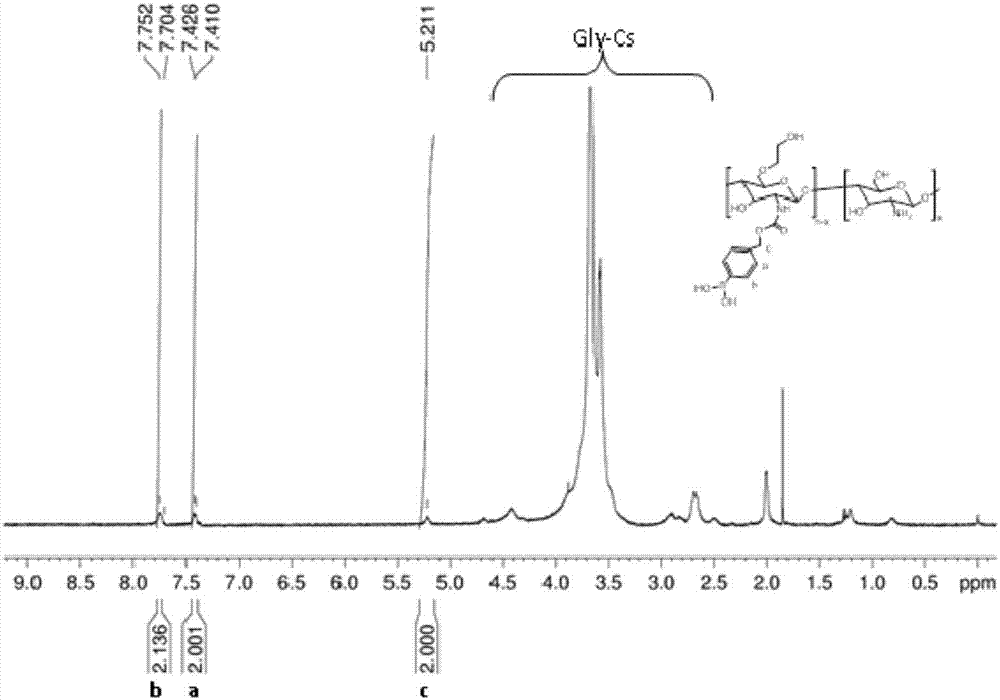
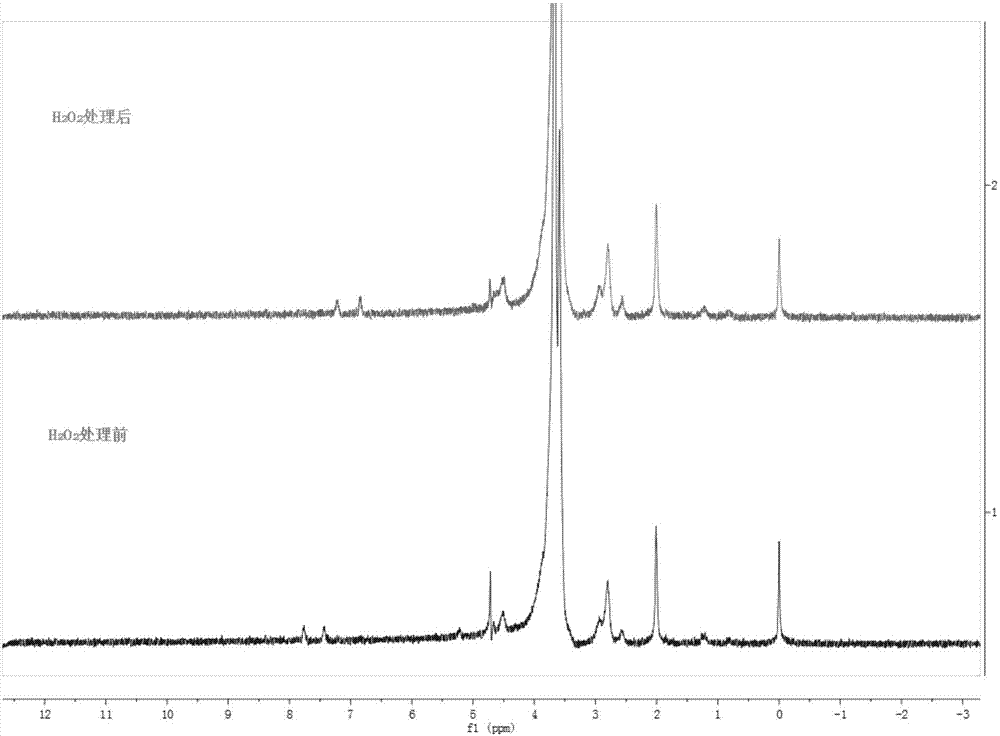
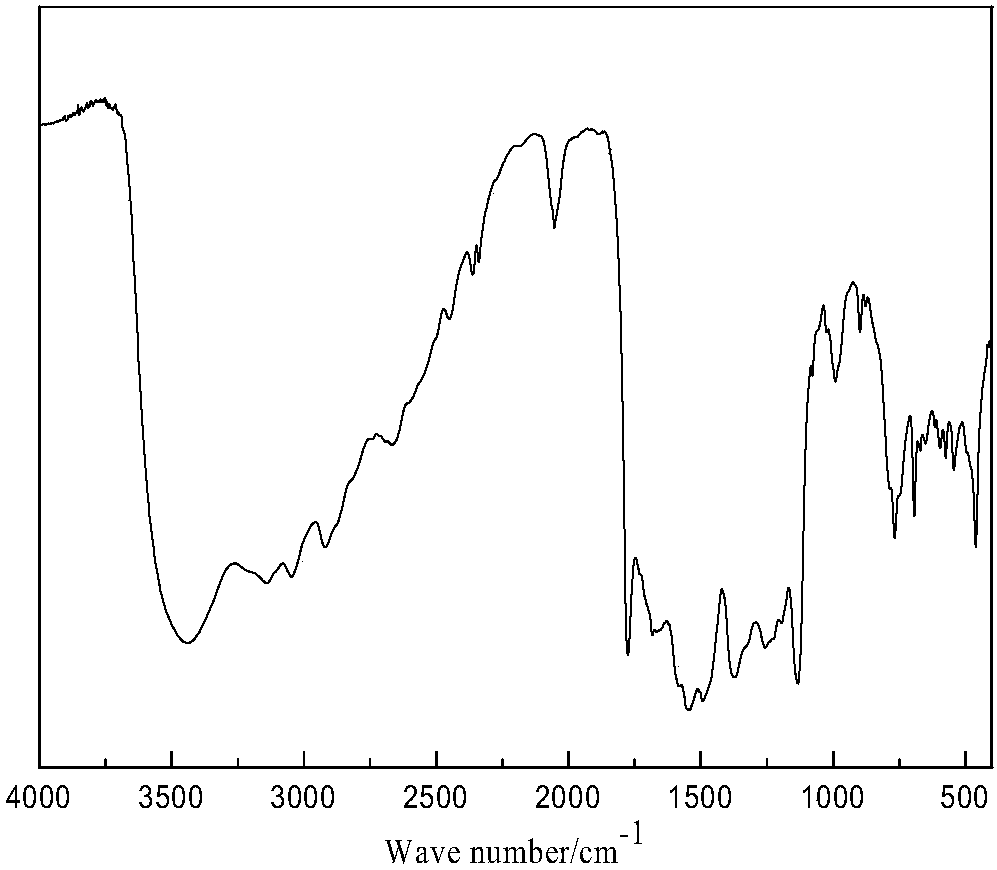
Active oxygen-responsive polymer support and preparation method thereof
ActiveCN107082828AEasy to prepareGood water solubilityOrganic active ingredientsAntipyreticSolubilityFreeze-drying
The invention discloses an active oxygen-responsive polymer support and a preparation method thereof. The preparation method comprises the following steps: mixing 4-(hydroxymethyl) phenylboronic acid pinacol ester with organic alkali and nitrophenyl chloroformate, and stirring to react to obtain NBC; dissolving a water-soluble polymer containing amino into water, adding an organic solvent, so as to form a solution A; dissolving NBC into the organic solvent, so as to obtain a solution B; adding alkali and the solution A into the solution B; stirring to react, and adjusting the pH value of the reaction system to 6.5-7.0, so as to obtain a modified polymer crude product; and purifying crude product, and carrying out freeze-drying, so as to obtain an active oxygen-responsive polymer support pure product. The preparation method of the active oxygen-responsive polymer support is simple, and the prepared arylboronic acid modified polymer support is easy to be chemically coupled with polyphenol active substances containing a catechol structure, so that the water solubility and stability of the support are improved, and the oxygen-responsive release of the support can be realized; and the support has important application prospects in the fields of anti-inflammation, anti-cancer treatment and the like.
Owner:JINAN UNIVERSITY
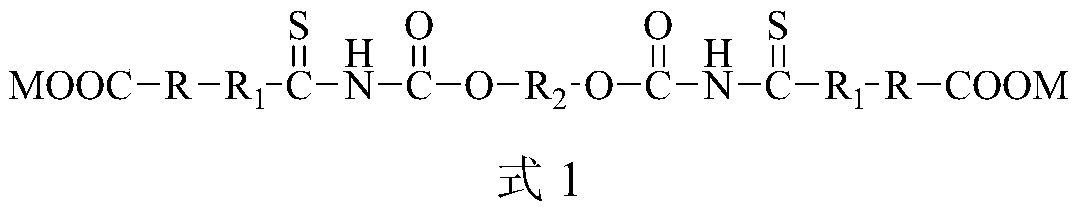
Ether-based double-sulfur amine ester derivative or ether-based bis-thiourea derivative and preparation method and application thereof
ActiveCN105801458ASpecial molecular structureHas surfactant propertiesOrganic chemistryFlotationChloroformateNickel sulfide
The invention provides an ether-based double-sulfur amine ester derivative or an ether-based bis-thiourea derivative and a preparation method and application thereof. The molecular structure of the ether-based double-sulfur amine ester derivative or the ether-based bis-thiourea derivative contains a large quantity of thioamide or thiourea or thioacid amide ether or other lipophilic groups and carboxyl hydrophilic groups. The preparation method includes the steps that bis-chloro-carbonic ester and thiocyanate are subjected to a substitution reaction, an intermediate product containing bis-acyl bis-isothiocyanate is generated, the intermediate and an alcohol compound or an amine compound or a phenol compound are subjected to an addition reaction, and then the ether-based double-sulfur amine ester derivative or the ether-based bis-thiourea derivative is obtained. The preparation method is simple, the prepared product directly serves as a non-molybdenum sulphide ore inhibitor, and floatation separation of molybdenum sulfide ore and the non-molybdenum sulphide ore can be effectively achieved. The preparation method is especially suitable for separation of molybdenite and copper sulphide ore, galena, sphalerite, pyrite, arsenopyrite, jamesonite concentrate, nickel sulfide ore, bismuth sulfide ore and the like, and the grade of molybdenum concentrate is improved.
Owner:CENT SOUTH UNIV
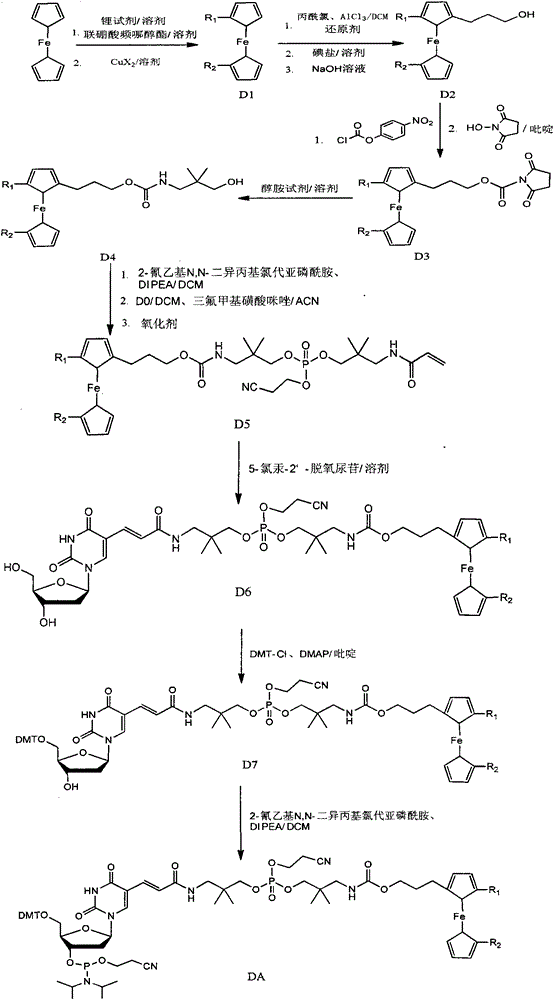
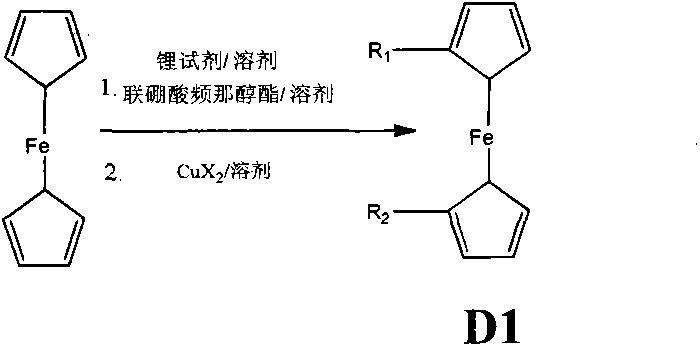
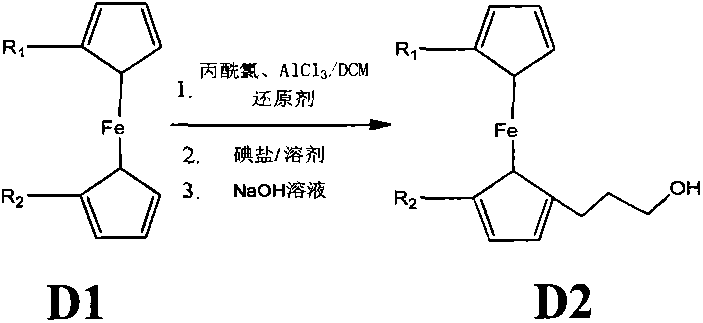
Synthetic method of ferrocene derivatives
The invention relates to a synthetic method of ferrocene derivatives. Ferrocene is used as a raw material for the ferrocene derivatives. The ferrocene derivatives which can be used for labeling special nucleotides are prepared by steps of: treating the ferrocene with a lithium reagent and bis(pinacolato)diboron to obtain a halogenated ferrocene; performing Friedel-Crafts acylation, Clemmensen reduction and hydrolysis under an alkaline condition to obtain a halogenated ferrocene alkyl alcohol; reacting with nitrophenyl chloroformate and N-hydroxy succinimide to obtain ferrocene succinimide carbonate; condensing with an alcohol amine reagent to obtain a ferrocene alkylolamide carbonate; performing substitution, oxidation, and other reactions by reacting with a phosphoramidite reagent to obtain a phosphorous ferrocene reagent; reacting with 5-chloromercuri-2'-deoxyuridine to obtain a deoxyuridine phosphorous ferrocene reagent; modifying the 5' end of the deoxyuridine through DMT-C1 to obtain a DMT-modified deoxyuridine phosphorous ferrocene reagent; and finally performing substitution by reacting with the phosphoramidite reagent. The method has operation safety, convenience, high product purity and high yield. The method has a wider application scope than other synthetic methods.
Owner:DAAN GENE CO LTD
Azilsartan intermediate and preparation method thereof
The invention discloses an azilsartan intermediate and a preparation method thereof. The preparation method comprises the following step: in a solvent, mixing a compound 3B with chloroformate to react under the action of alkali to obtain a compound 4B. The preparation method has the advantages of fewer impurities, short reaction time, higher technical yield and higher product purity, and is suitable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND +1
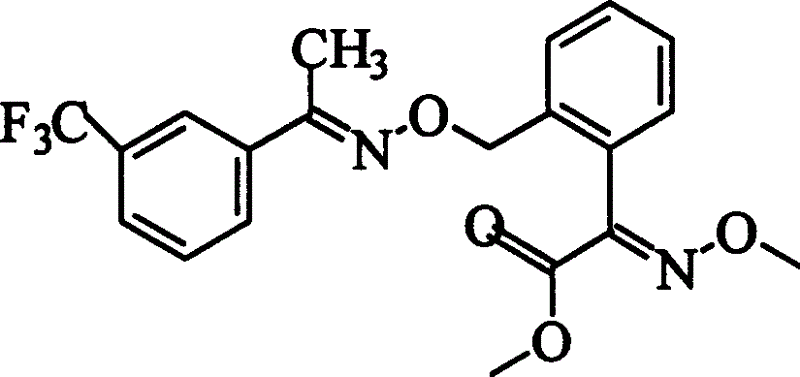
Preparation process of oxime strain ester
The invention discloses a trifloxystrobin preparing method, including the steps as follows: (a) using ortho-methyl hypnone as raw material and using potassium permanganate to oxidize so as to obtain 2-(2'-methyl-phenyl)-2-carbonyl acetic acid; (b) making the product in step (a) react with methanol to obtain 2-(2'-methyl-phenyl)-2-carbonyl methyl acetate; (c) bromizing the product in step (b) to obtain 2-(2'-bromomethyl-phenyl)-2-carbonyl methyl acetate; (d) making the product in step (c) react with methoxy amine to obtain (E)-2-(2'-bromomethyl-phenyl)-2-carbonyl methyl acetate-O-methyl ketone oxime; (e) making the product in step (d) react with meta-trifluoromethyl hypnone oxime to obtain the product trifloxystrobin. It reduces the discharge of large amount of waste water in course of oxidization reaction, avoids esterification by adopting methyl- chloroformate, most operating conditions are moderate.
Owner:TONGJI UNIV
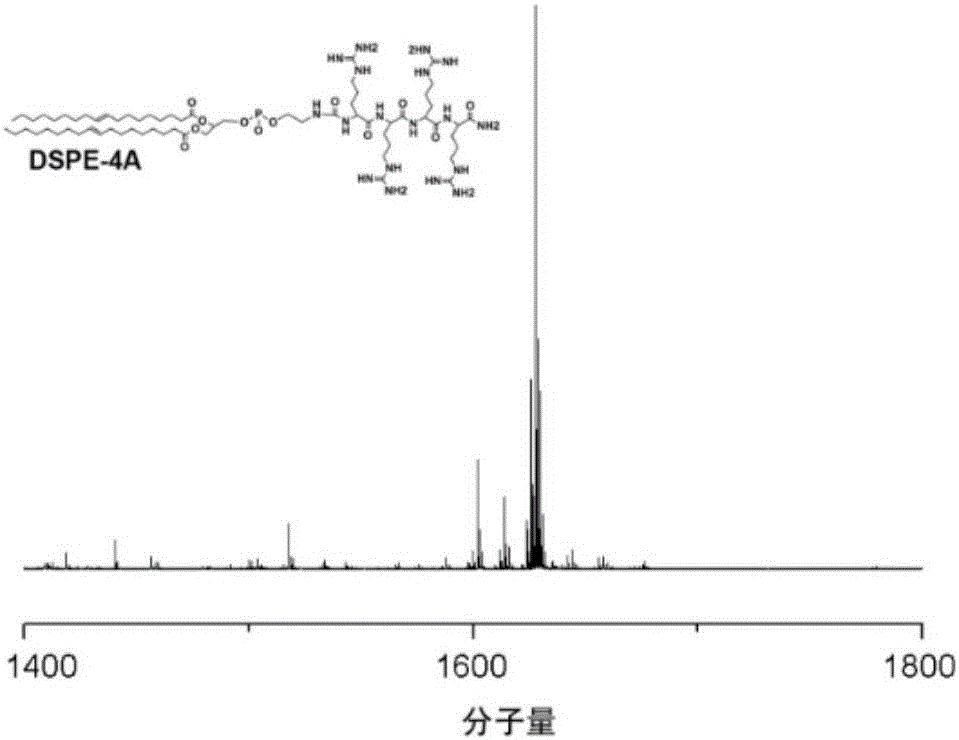
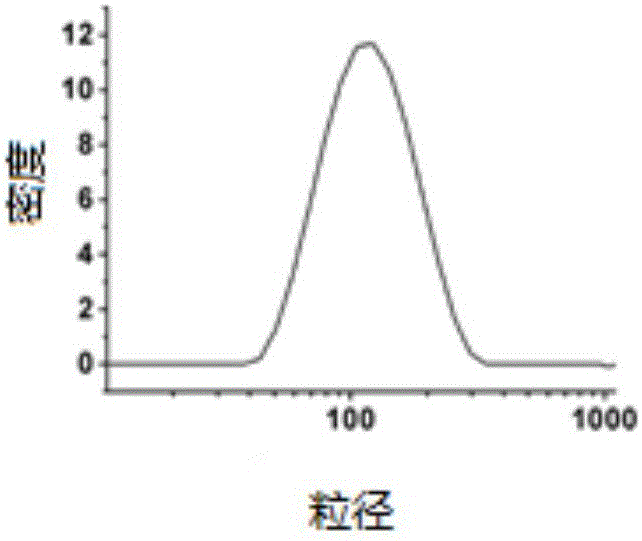
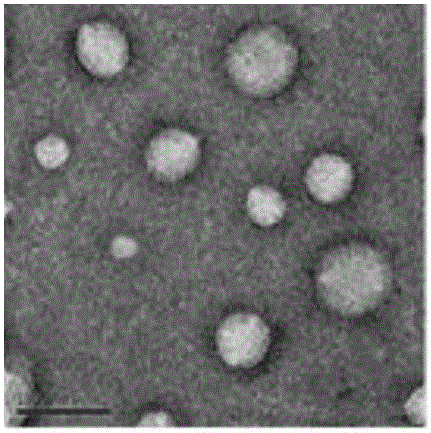
Oligoarginine modified phospholipid, nanoparticles assembled by oligoarginine modified phospholipid, preparation method of oligoarginine modified phospholipid and application of nanoparticles
ActiveCN105801668ASpeed up entryImprove delivery efficiencyPeptide preparation methodsNanotechnologyChloroformateIn vivo
The invention relates to oligoarginine modified phospholipid, nanoparticles assembled by the oligoarginine modified phospholipid, a preparation method of the oligoarginine modified phospholipid and an application of the nanoparticles. After oligoarginine peptide chains are activated by DCC (dicyclohexylcarbodiimide) / NHS (N-hydroxysuccinimide), the oligoarginine peptide chains react with phosphatidyl ethanolamine, purification is performed, and oligoarginine modified phosphatidyl ethanolamine is obtained; or the oligoarginine peptide chains react with DCC / NHS activated phosphatidic acid, and oligoarginine modified phosphatidic acid is obtained through purification; or the oligoarginine peptide chains react with nitrophenyl chloroformate activated phosphatidylcholine, and oligoarginine modified phosphatidylcholine is obtained through purification. The nanoparticles assembled by the oligoarginine modified phospholipid is used for supporting genes, small-interfering RNA, polypeptides or proteinic drugs, antibodies and chemical drugs, and the formed aqueous dispersion of the drug-carrying nanoparticles is used for delivering drugs for in-vitro cells or in-vivo local intravenous injection. The nanoparticles are effectively promoted to enter the cells, and the intracellular drug delivery efficiency is improved.
Owner:天津渤化讯创科技有限公司
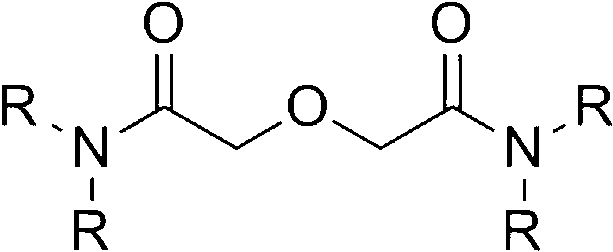
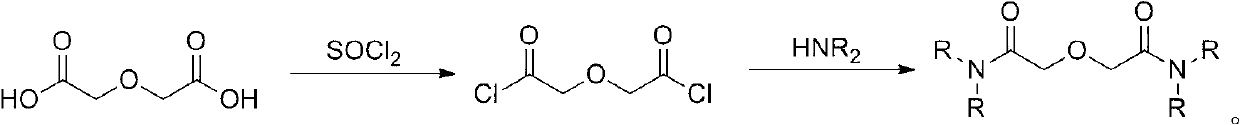
Method for synthesizing diamide podand extraction agent
ActiveCN102993042AEasy to purifyMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationLiquid wasteFuel reprocessing
The invention discloses a method for synthesizing diamide podand extraction agent, wherein chloro-carbonic ester and diglycolic anhydride react to generate mixed anhydride under the action of tertiary amine, and then the reaction is performed with amine to generate the diamide podand extraction agent. The method has mild reaction condition, can be performed at a low temperature, and has high reaction speed and short consumed time; the product purification operation is simple and easy; the obtained diamide podand extraction agent can meet the extraction purity requirement, and is beneficial to establishing flow that the diamide podand is used for treating high-level liquid waste in spent fuel reprocessing plant; the used chloro-carbonic ester is easy to prepare and has a low price, therefore, the cost for preparation of a great amount of extraction agent is greatly reduced; and besides, the yield of the extraction agent is high, so that the method is very suitable for industrial production and application.
Owner:SICHUAN UNIV
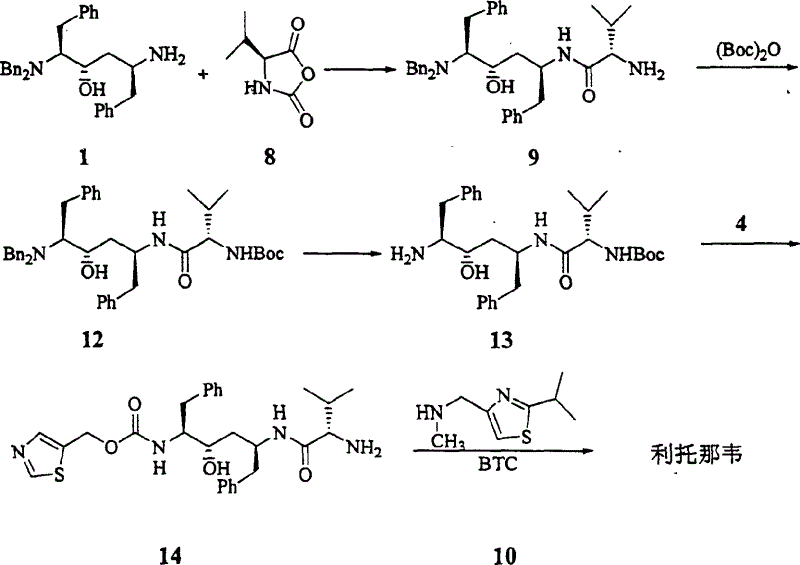
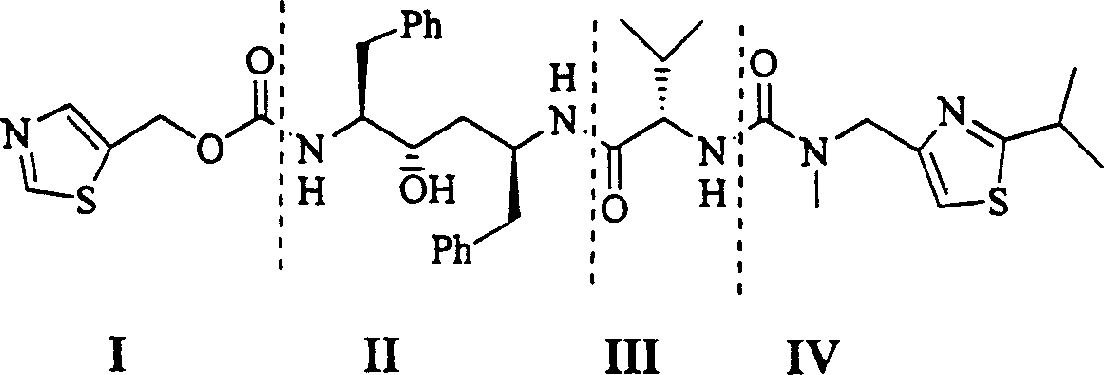
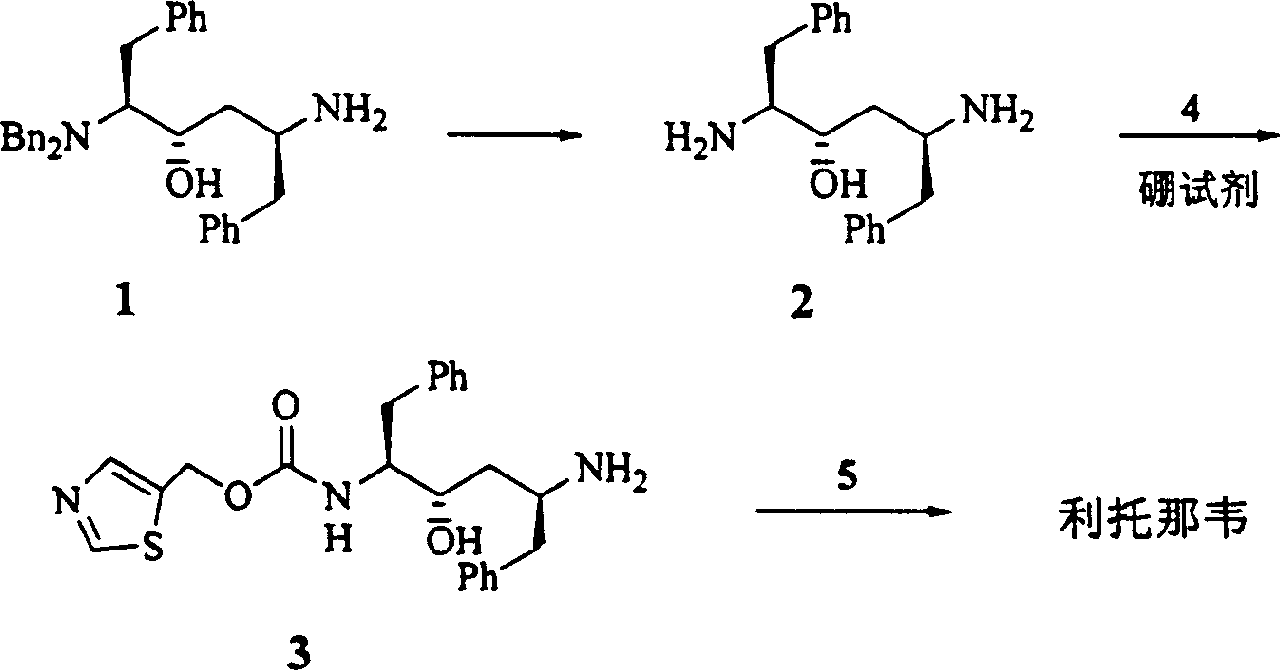
Process for synthesizing ritonavir
InactiveCN1554647AStrong response specificityLow costOrganic chemistryAntiviralsChloroformateHydrolysis
The present invention relates to the synthesis process of Ritonavir as one proteinase inhibitor for resisting AIDS. The synthesis process includes the condensation between benzylamino alcohol and valine NCA to obtain valyl benzylamino alcohol, the reaction between valyl benzylamino alcohol and ditert-butyl dicarbonate to obtain tert-butoxy acyl valyl benzylamino alcohol, the hydrogenolysis and debenzylation of tert-butoxy acyl valyl benzylamino alcohol in ammonium formate and Pd-C to obtain tert-butoxy acyl valyl amino alcohol, the active esterification reaction between tert-butoxy acyl valyl amino alcohol and 5-methylol thiazole, subsequent hydrolysis to obtain thiazolyl-5-methoxycarbonyl valyl amino alcohol, and the reaction of thiazolyl-5-methoxycarbonyl valyl amino alcohol and isopropyl thiazolyl methylamine under the action of BTC to obtain final product Ritonavir. The present invention has lowered cost and raised atomic utilization.
Owner:XIAMEN UNIV
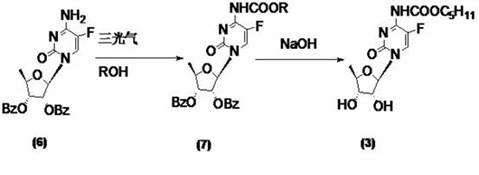
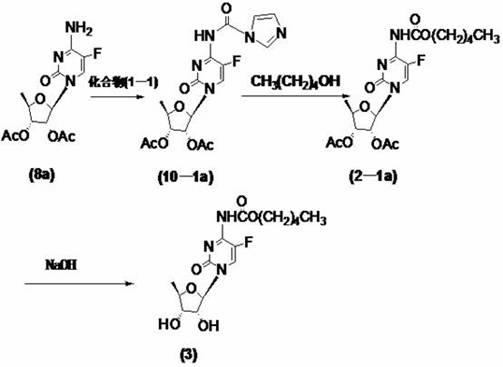
Preparation method of 2'3'-di-O-acetyl-5'-desoxy-5-fluoro-N4-(pentyloxycarbonyl)cytidine
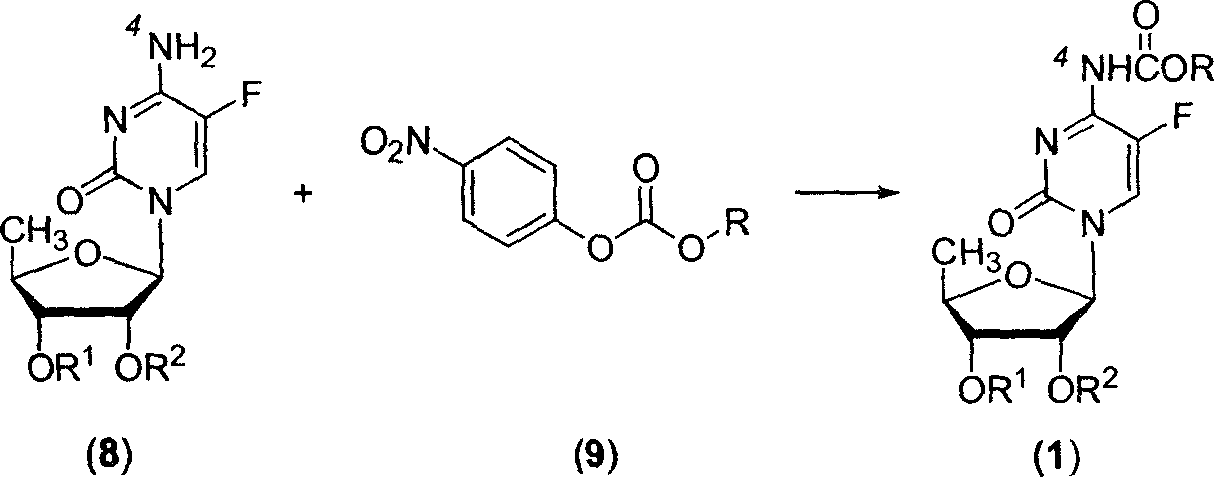
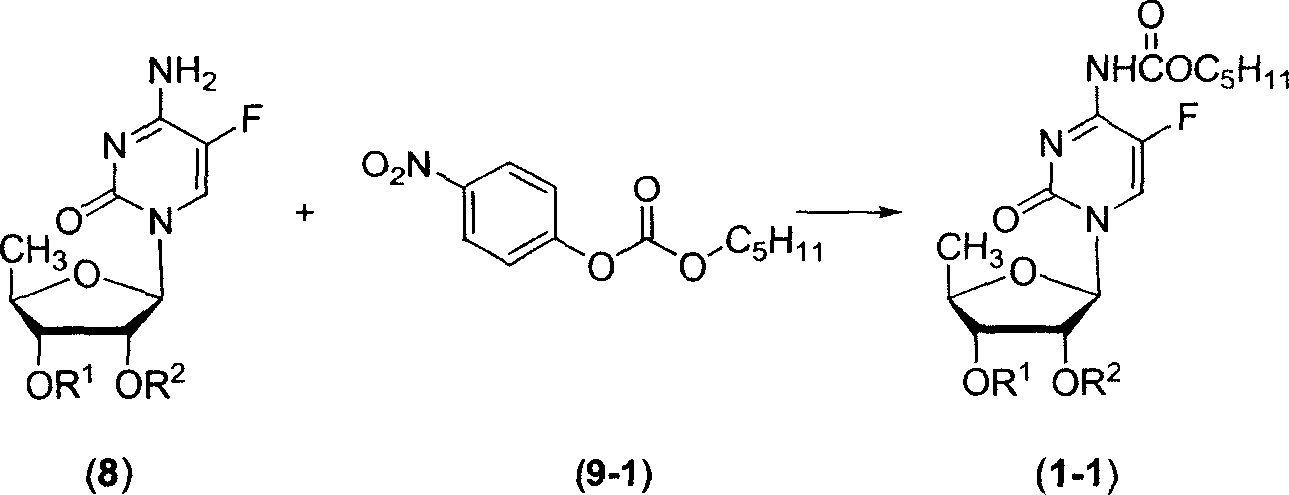
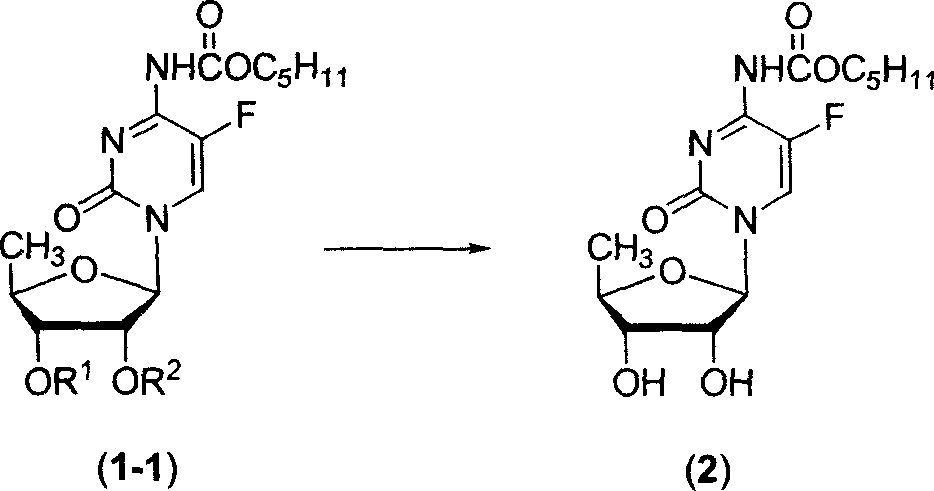
InactiveCN102977169AHarm reductionHigh yieldSugar derivativesSugar derivatives preparation5-fluorocytidineChloroformate
The invention relates to a preparation method of 2'3'-di-O-acetyl-5'-desoxy-5-fluoro-N4-(pentyloxycarbonyl)cytidine, belonging to the technical field of medicine. The compound is an important intermediate of Capecitabine. The preparation method comprises the following step: by using anhydrous sodium carbonate or anhydrous potassium carbonate as alkali, quaternary ammonium salt as a phase-transfer catalyst and 4-substituted-pyridine as a catalyst, carrying out amidation reaction on 2'3'-di-O-acetyl-5'-desoxy-5-fluorocytidine and amyl chloroformate to obtain the 2'3'-di-O-acetyl-5'-desoxy-5-fluoro-N4-(pentyloxycarbonyl)cytidine. The invention adopts the anhydrous sodium carbonate or anhydrous potassium carbonate instead of the high-toxicity organic alkali pyridine, and obtains ideal yield and product purity. The method has the advantages of accessible raw materials, low cost, small environmental hazard, high safety and reliability, short reaction time and good product quality.
Owner:QILU TIANHE PHARMA
Preparation process of ammonia-ester-bond cross-linked poly(ethylene imine) polycation carrier
InactiveCN102443169ALow toxicityEfficientOther foreign material introduction processesVector-based foreign material introductionCross-linkFreeze-drying
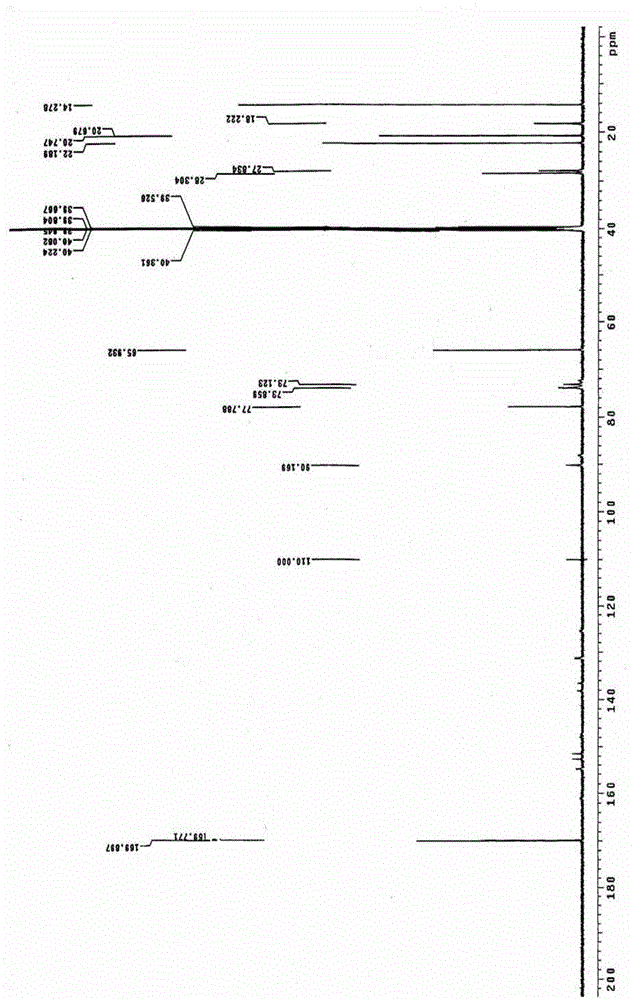
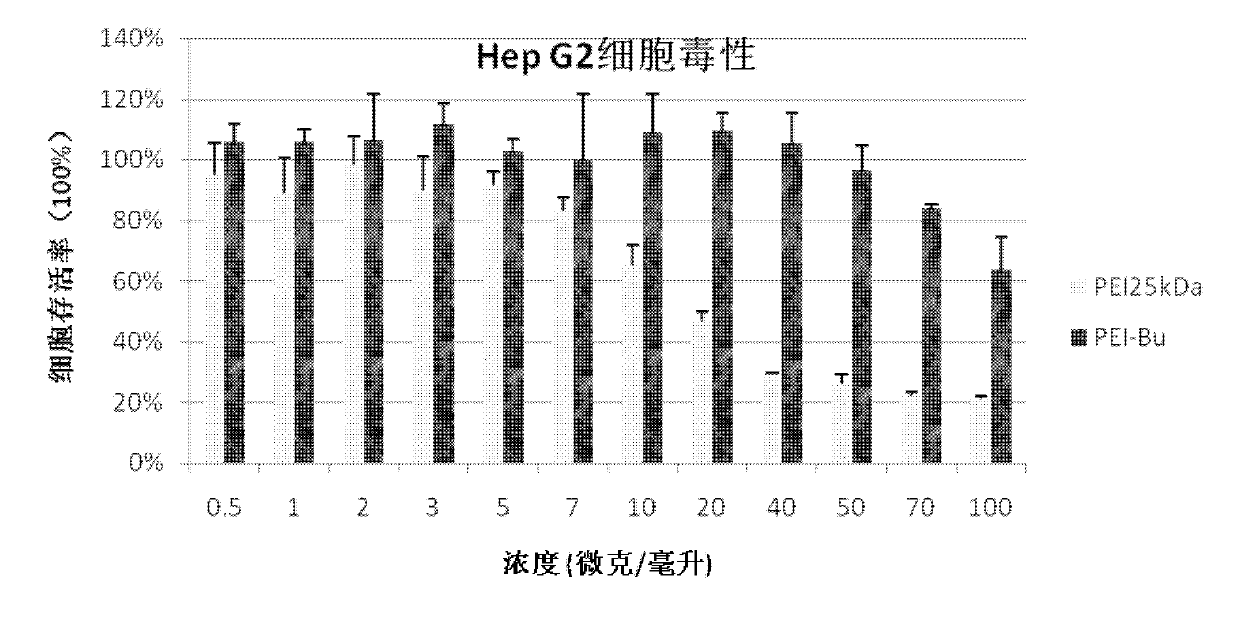
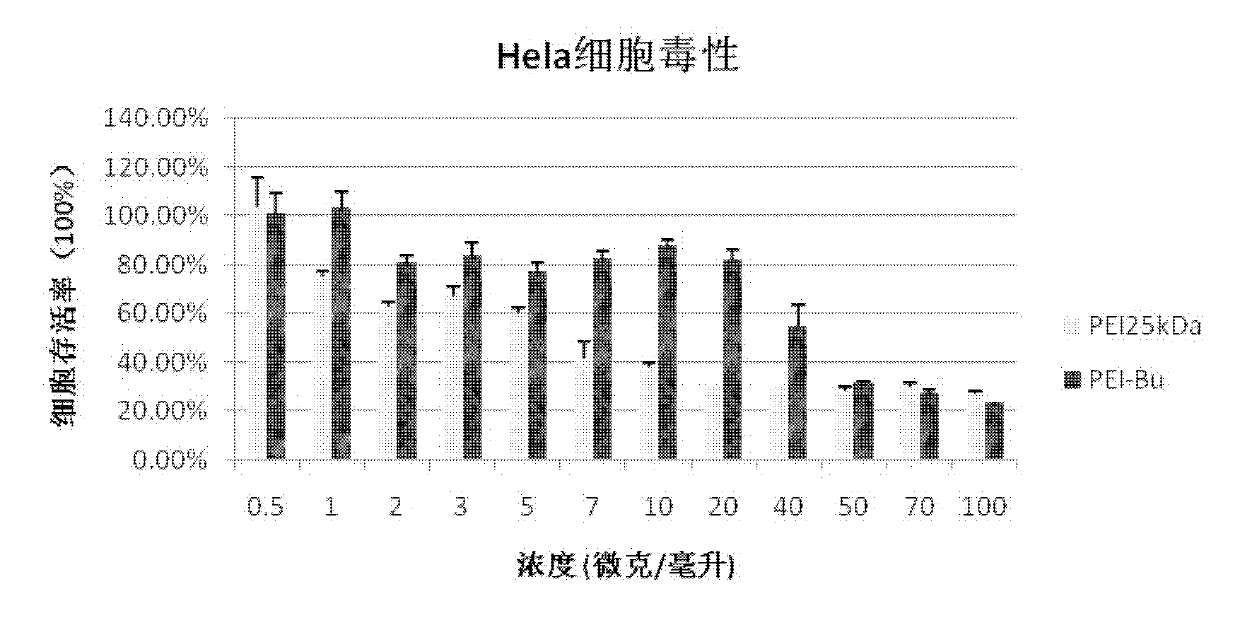
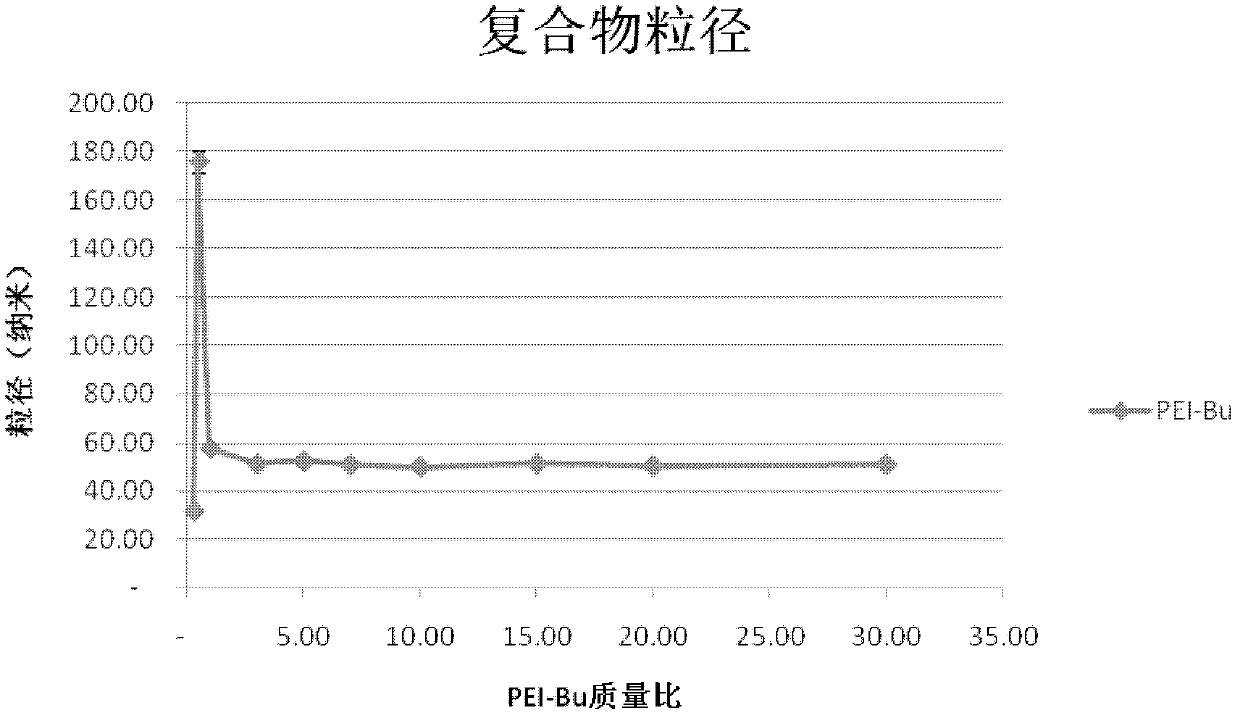
The invention relates to a preparation process of an ammonia-ester-bond cross-linked poly(ethylene imine) polycation carrier. The preparation process comprises the steps of: adding small-molecular-weight poly(ethylene imine) (PEI), 1,4-butanediol bischloroformate and triethylamine to a reaction solvent, stirring, shaking or vibrating the reaction system to carry out condensation reaction, then dialyzing with a 3500Da reactivated dialysis bag for 48 hours, finally filtering with a 0.22 mu m microporous filtering membrane, and freeze-drying to obtain the product. As compared with the prior art, the preparation process provided by the invention has the advantages that: a purified ammonia-ester poly(ethylene imine) derivative PEI-Bu is prepared; and as compared with poly(ethylene imine) biscarbamate conjugate (PEIC), PEI-Bu displays the highest transfection activity and lower cytotoxicity at a lower mass ratio of PEI-Bu to DNA (deoxyribonucleic acid), is an efficient, low-toxicity gene substance carrier, and can be used for conveying gene substances.
Owner:SHANGHAI JIAO TONG UNIV
Method for carrying out carbalkoxylation acylation on fluorouracil compound with active coupling agent
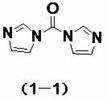
InactiveCN102219817AQuality improvementRaw materials are easy to getSugar derivativesSugar derivatives preparationAlcoholChloroformate
The invention discloses a method for carrying out carbalkoxylation acylation on fluorouracil compound with an active coupling agent, and applications thereof in synthesizing capecitabine. The method comprises the steps of carrying out N-4 site acylation reaction of coupling agent shown as the formula (1) and fluorouracil complex shown as the formula (8), introducing active carbonyl at N-4 site to obtain acylate, directly reacting the acylate with alcohol or phenol without separation, and carrying out N-4 site carbalkoxylation acylation reaction, thus obtaining carbalkoxylation acylate of fluorouracil compound. The carbalkoxylation acylation method can be used for preparing capecitabine. The method conducts acylation and carbalkoxylation acylation on cytidine by adopting coupling agent and alcohol or phenol, thus avoiding use of chlorocarbonic ester or phosgene acylating agent, being used for preparing capecitabine, and being stable and easy to obtain raw materials, high in product purity, safe and environment-friendly for operation environment, and easy for industrial operation.
Owner:连云港杰瑞药业有限公司
Method for preparing amine-terminated polyether by leaving group method
ActiveCN103087308AGood reaction selectivityIncrease contentCarbamic acid derivatives preparationOrganic compound preparationCarbamatePtru catalyst
The invention relates to a method for for preparing amine-terminated polyether by a leaving group method, including esterification reaction and amination reaction. The method comprises the following steps of: dropping the mixed solution of polyols and a catalyst into the mixed solution of chloro-carbonic ester and solvent, ventilating nitrogen, controlling the temperature of a reaction system not bigger than 20 degrees centigrade, heating the reaction liquid to 20-150 degrees centigrade after dropping, carrying out vacuum filtration after adequate reaction to obtain the solvent-containing carbonate seal end polyether; adding the solvent-containing carbonate seal end polyether into polyamine, stirring the same for 10-120 minutes under normal temperature and pressure, removing excess polyamine, solvent and by-product of mother solution by a molecular still at distillation temperature of 110-170 degrees centigrade and under pressure of 1*10<-2>Pa-200Pa, thereby obtaining the mine-terminated polyether containing carbamate group. The method for preparing amine-terminated polyether by the leaving group method disclosed by the invention has the advantages of mild reaction conditions, low operation cost, no high temperature and pressure and few by-product during the whole process, and is simple in process and by-product is easy remove.
Owner:山西省建筑科学研究院集团有限公司
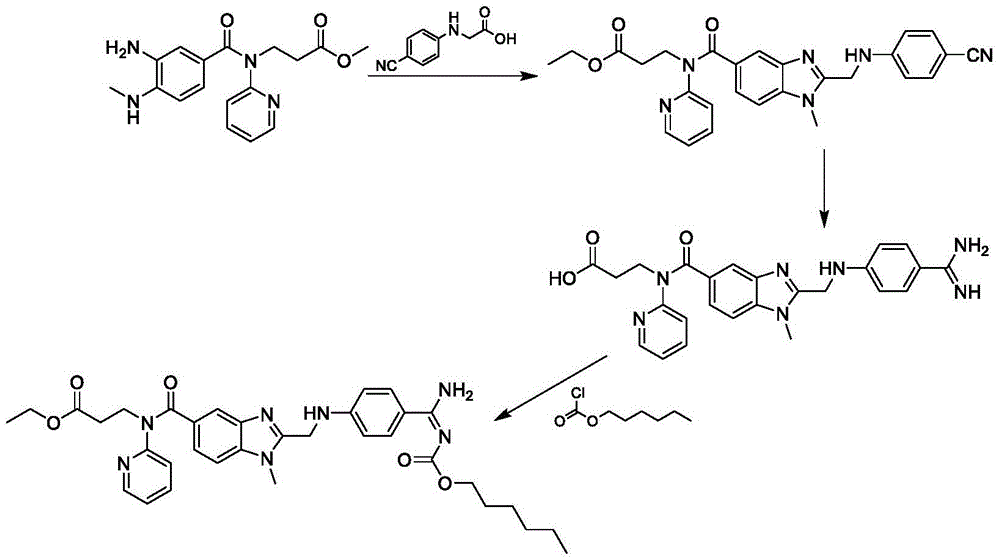
Preparation method of dabigatran etexilate mesylate
InactiveCN105566297ASimple and fast operationHigh selectivityOrganic chemistryPropanoic acidChloroformate
The invention discloses a preparation method of dabigatran etexilate mesylate, and belongs to the technical field of medicine. The preparation method comprises the following steps: taking 3-[(3-amino-4-methylaminobenzoyl)pyridine-2-ylamino]ethyl propanoate and N-(4-cyanphenyl)amino acetic acid as the raw materials to synthesize an intermediate (S3); making the intermediate (S3) carry out ring-closure reactions to generate an intermediate (S4); subjecting the intermediate (S4) to acid splitting in the presence of a hydrogen chloride-ethanol solution at first, then carrying out ammonification in the presence of ammonia water to generate an intermediate (S5); carrying out reactions between the intermediate (S5) and n-hexyl chloroformate under an alkaline condition to generate an intermediate (S6); dissolving the intermediate (S6), and finally carrying out reactions between the intermediate (S6) and methylsulfonic acid to obtain dabigatran etexilate mesylate. The preparation method has the advantages of simpleness, controllable and mild conditions, high yield, high product purity, stable product property, and suitability for industrial production.
Owner:HARBIN PHARMA GROUP TECH CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
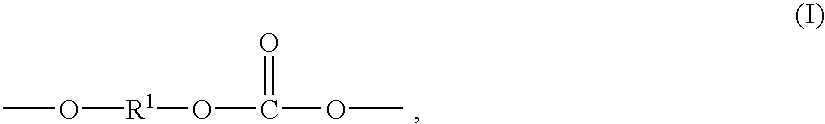
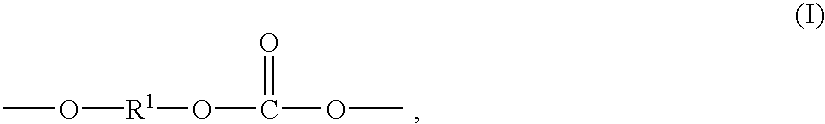
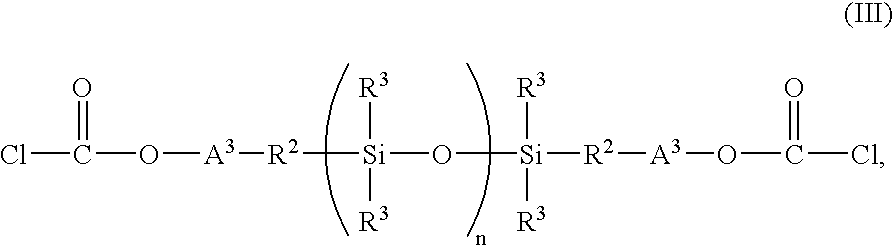
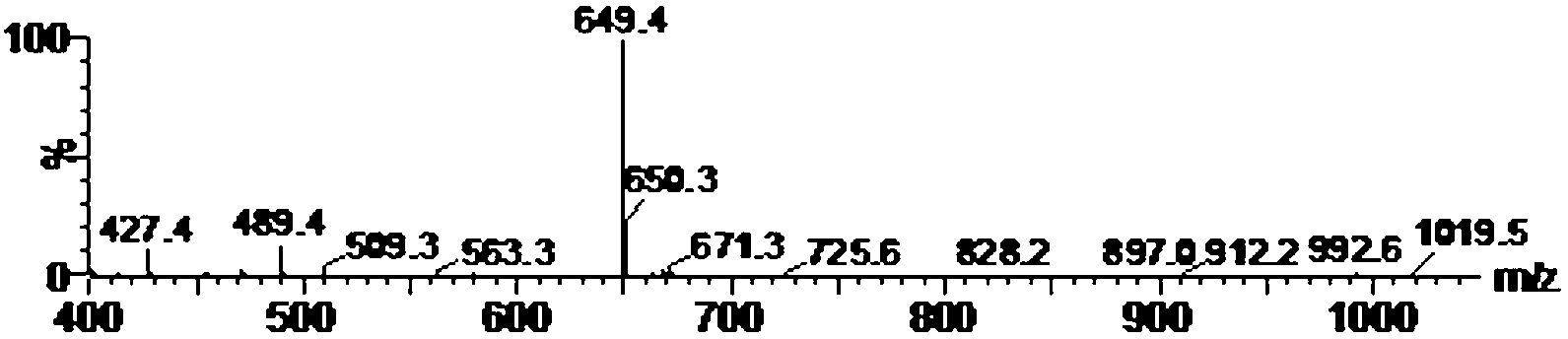



![Process for the preparation of imidazo[4,5-c]-quinolin-4-amines Process for the preparation of imidazo[4,5-c]-quinolin-4-amines](https://images-eureka.patsnap.com/patent_img/168728c9-081a-4897-b871-a887a3a87657/US20070100146A1-20070503-C00001.png)
![Process for the preparation of imidazo[4,5-c]-quinolin-4-amines Process for the preparation of imidazo[4,5-c]-quinolin-4-amines](https://images-eureka.patsnap.com/patent_img/168728c9-081a-4897-b871-a887a3a87657/US20070100146A1-20070503-C00002.png)
![Process for the preparation of imidazo[4,5-c]-quinolin-4-amines Process for the preparation of imidazo[4,5-c]-quinolin-4-amines](https://images-eureka.patsnap.com/patent_img/168728c9-081a-4897-b871-a887a3a87657/US20070100146A1-20070503-C00003.png)