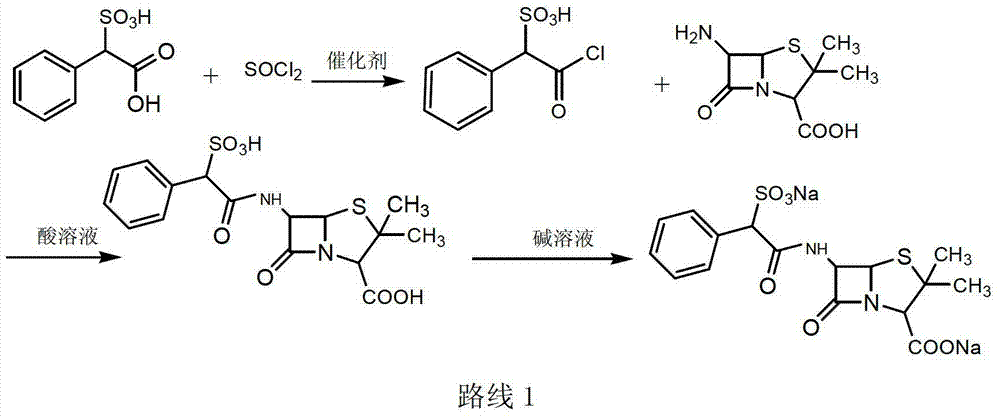
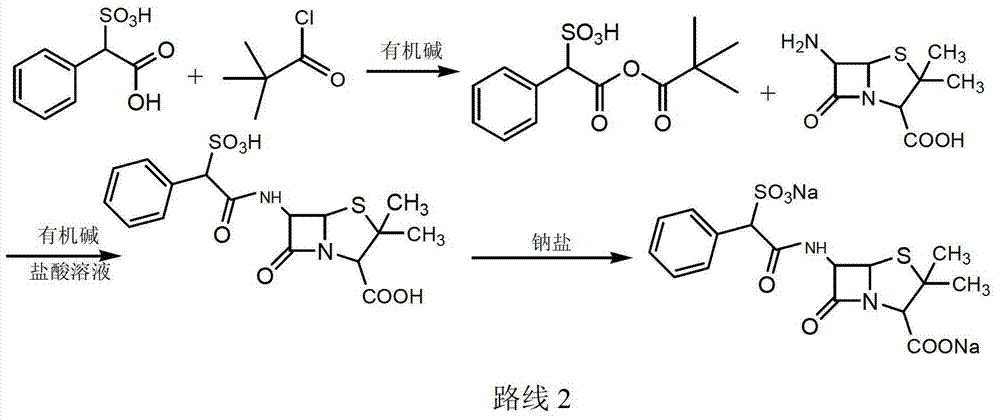
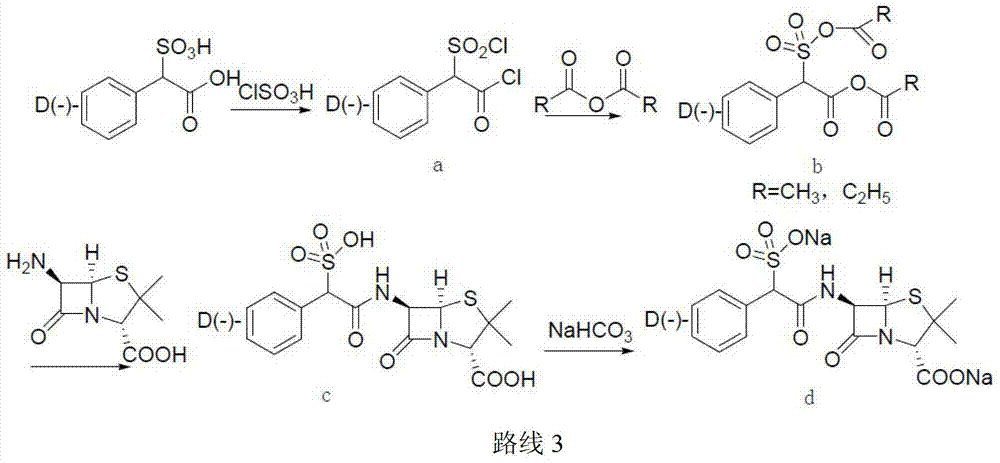
Sulbenicillin sodium preparation method
A technology of sodium sulbenicillin and sodium isooctanoate, applied in the direction of organic chemistry, can solve the problems of low conversion rate of mixed anhydrides, low yield of sulbenicillin, poor solubility, etc., and achieve shortened production cycle, easy availability and low cost low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) Add 126kg of dichloromethane to a 200L reactor, add 10kg (46.3mol) of α-sulfophenylacetic acid under rapid stirring, then add 9.37kg (92.6mol) of diisopropylamine, stir until the solution is clear, and cool down To -10°C, add 4.49kg (48.7mol) ethyl chloroformate at a uniform rate, and keep it at -10°C for 1.5h to prepare solution a, which is stored at -15°C for later use.
[0044] (2) Add 10kg (46.3mol) 6-APA (6-aminopenicillanic acid) and 101kg dichloromethane to a clean 1000L reaction kettle, stir well, cool down to 0°C, and slowly add diisopropyl Base amine 9.37kg (92.6mol), after the dropwise addition, keep the temperature at -5 ~ 5 ° C and stir to completely dissolve 6-APA, and obtain solution b.
[0045] (3) Heat preservation at -15°C and slowly add solution b to solution a. After the dropwise addition, continue to heat at -15°C for 2 hours, and the reaction ends. Add 40kg of n-butanol to the reaction solution, then slowly add cold 17% hydrochloric acid solutio...
Embodiment 2
[0049] (1) Add 126kg of dichloromethane to a 200L reactor, add 10kg (46.3mol) of α-sulfophenylacetic acid under rapid stirring, and then add 8.0kg (69.5mol) of tetramethylguanidine, and stir until the solution is clear and cooled to -10°C, add 6.65kg (48.7mol) butyl chloroformate at a constant rate, keep it at -10°C for 2 hours, and then prepare solution a, store it at -15°C for later use.
[0050] (2) Add 10kg (46.3mol) 6-APA (6-aminopenicillanic acid) and 101kg dichloromethane to a 1000L reactor, stir well, cool down to 0°C, and slowly add tetramethylguanidine dropwise 5.86kg (50.9mol), after the tetramethylguanidine is added dropwise, keep the temperature at -5~5°C and stir to dissolve the 6-APA, and store it at low temperature for later use.
[0051] (3) Keep warm at -15°C, slowly add solution b to solution a, after the dropwise addition, keep warm at -15°C for 2 hours, and the reaction ends. Add 40kg of n-butanol to the reaction solution, then slowly add cold 17% hydroch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com