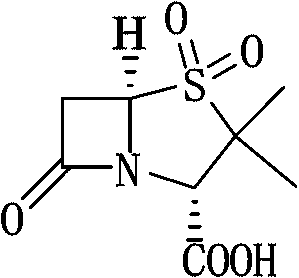
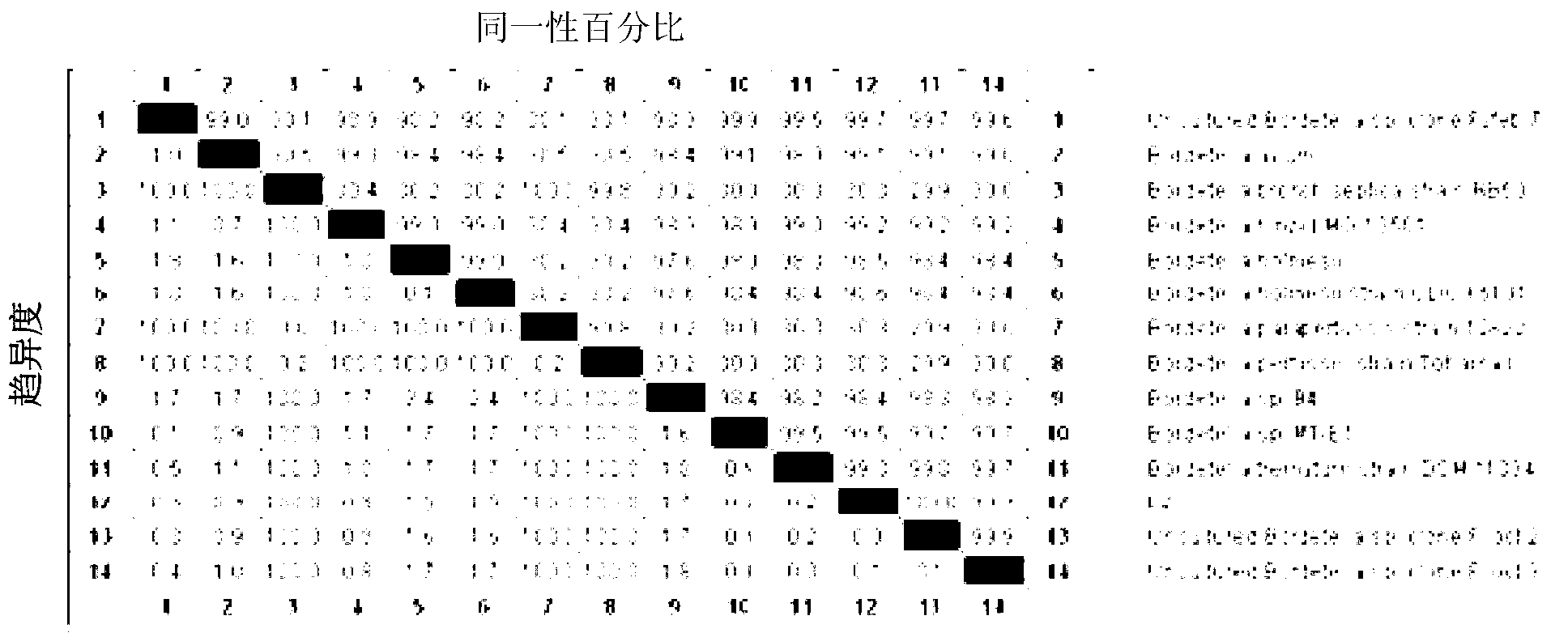
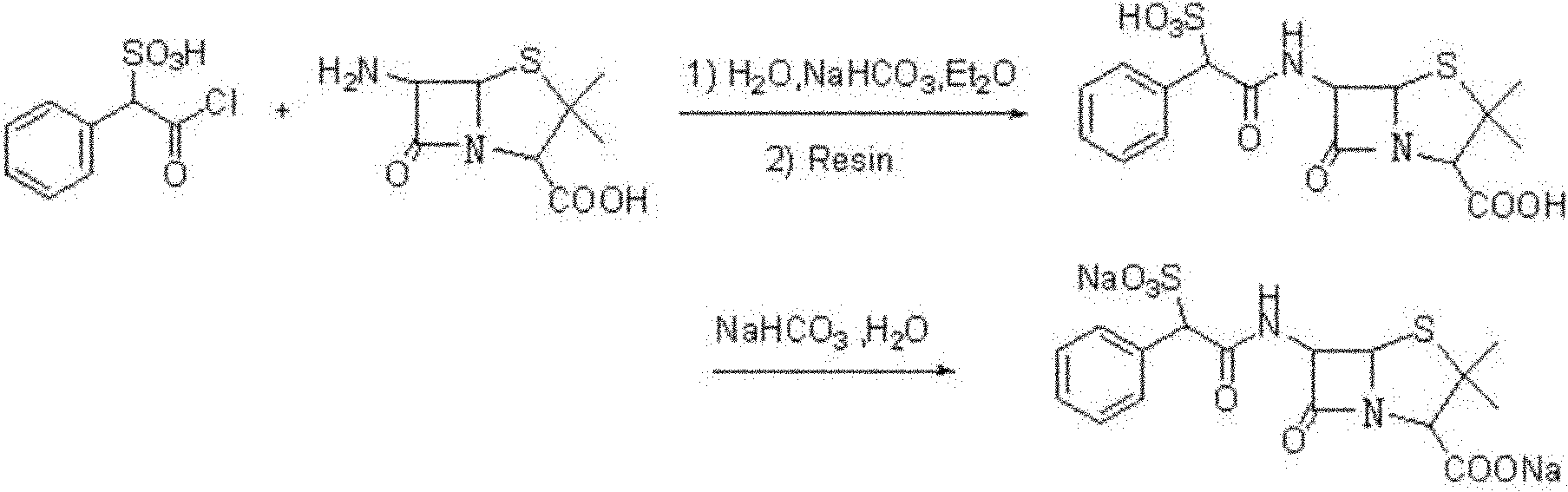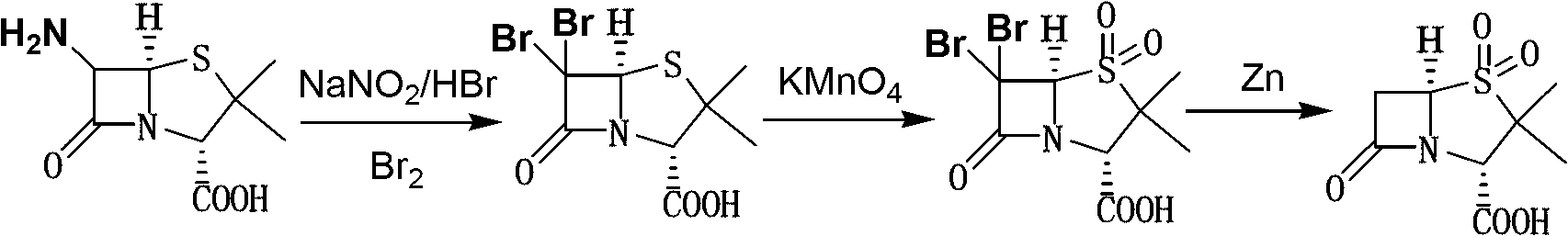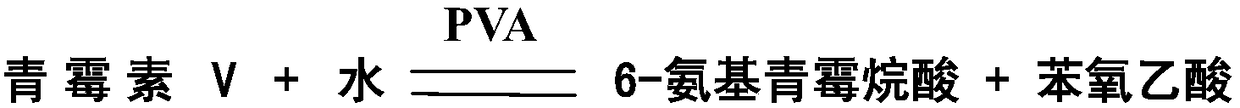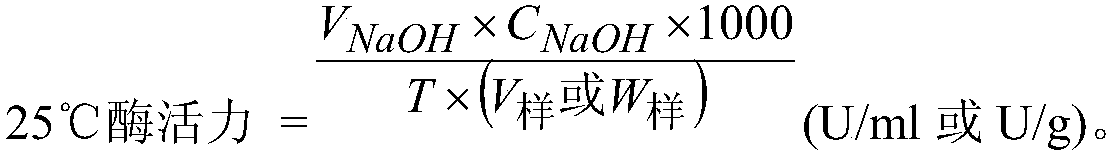Patents
Literature
76 results about "Aminopenicillanic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
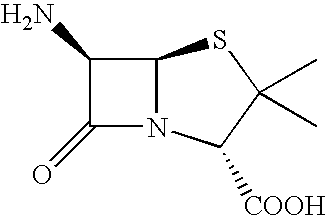
6-APA is the chemical compound (+)-6-aminopenicillanic acid. Use. 6-APA is the core of penicillins. It is obtained from the fermentation brew of the Penicillium mold and used as the main starting block for the preparation of numerous semisynthetic penicillins. History. In 1958, Beecham scientists in the UK discovered the ...
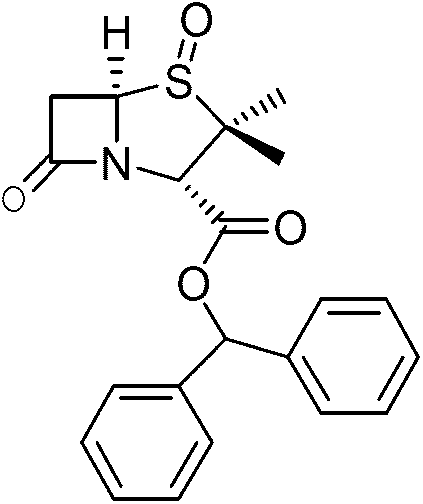
Preparation method of benzhydryl s-oxopenicillanate
ActiveCN103044447AThe synthesis steps are simpleShort reaction cycleOrganic chemistryHydrogenation reactionBromine
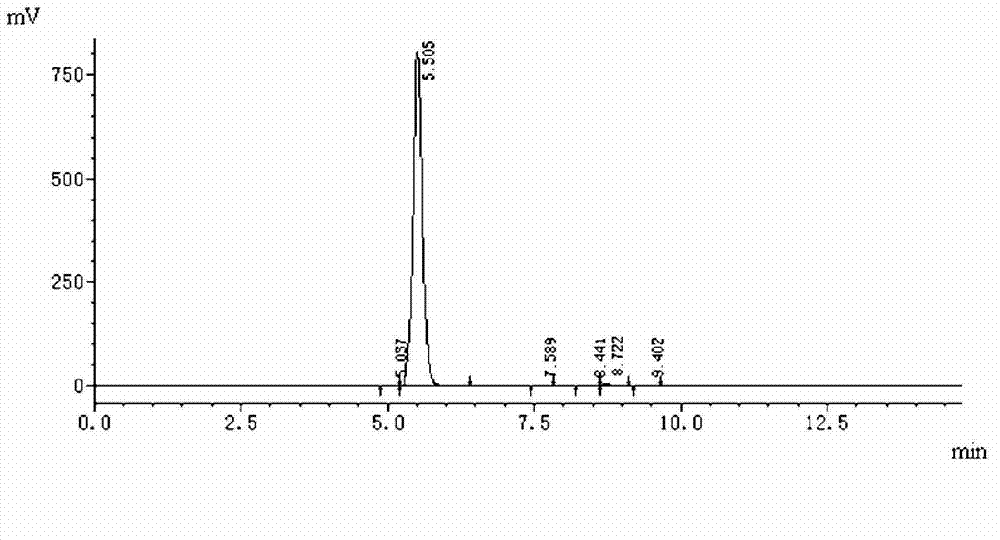
The invention provides a preparation method of benzhydryl s-oxopenicillanate, which comprises the following steps: reacting 6-aminopenicillanic acid to obtain a penicillanic acid (iii) solution; adding catalyst molybdenum acetopyruvate, and dropwisely adding oxydol while controlling the temperature; after the reaction finishes, centrifuging to obtain oxopenicillanic acid; and reacting to obtain the benzhydryl s-oxopenicillanate. The method provided by the invention has the following advantages: 1. simplified synthesis steps: the processes of bromination and reduction debromination are not adopted; 2. short reaction period: only three reaction steps are adopted, thereby greatly shortening the production period; 3. favorable reaction selectivity and high yield: the total yield of the three steps for preparing the benzhydryl s-oxopenicillanate is higher than 72%; 4. mild reaction conditions: the high-risk hydrogenation reaction is not needed; and 5. the separation of the product and the intermediates is simple to operate, the two intermediates in the benzhydryl s-oxopenicillanate synthesis route can be directly used for synthesizing the required compound without refinement, and the end product can be directly filtered and separated.
Owner:JIANGXI FUSHINE PHARMA CO LTD
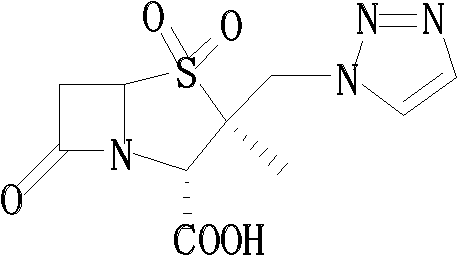
Tazobactam synthesis method
ActiveCN102643292ASteps to increase monoxidationBlocking affinityOrganic chemistryMetacresolSynthesis methods
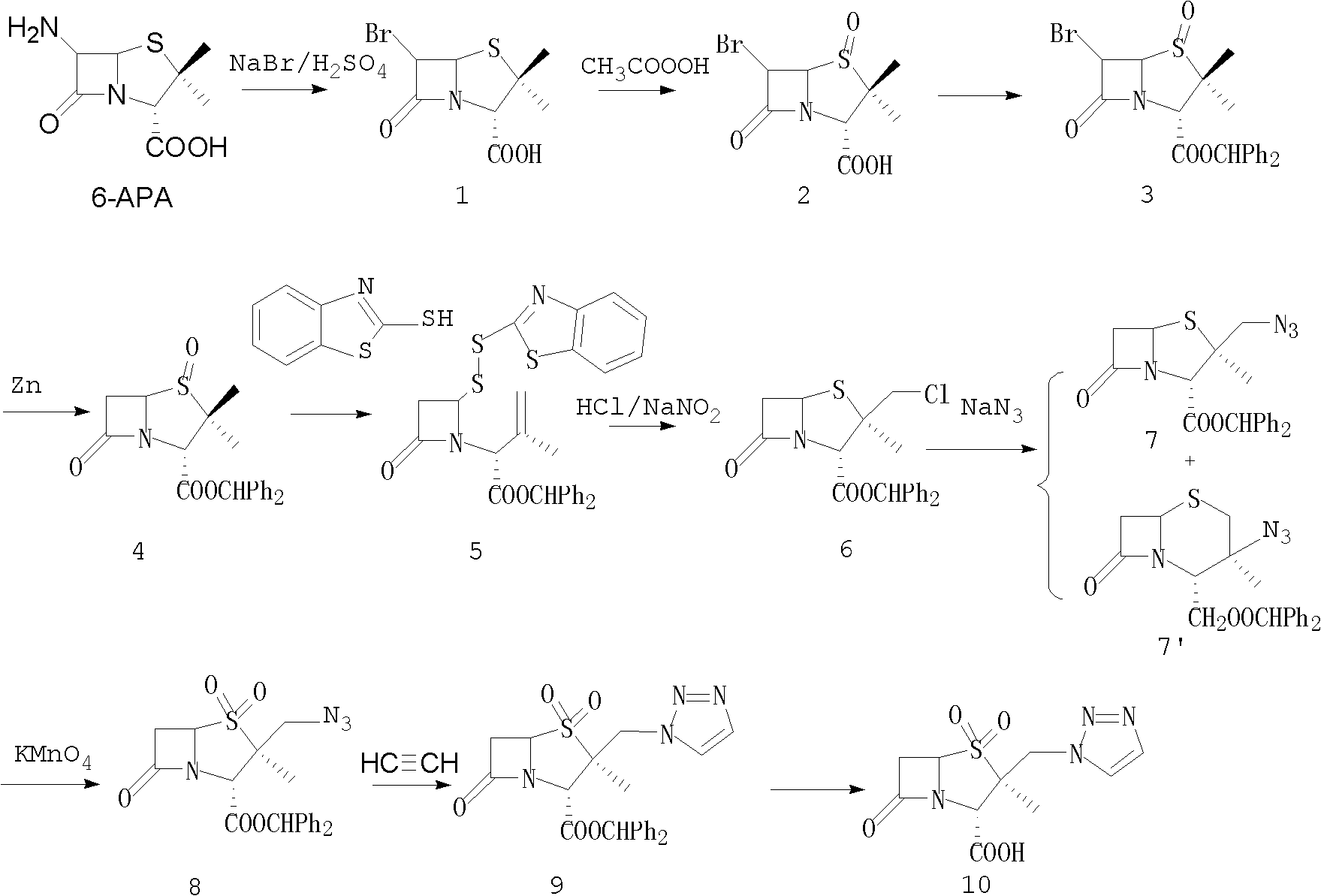
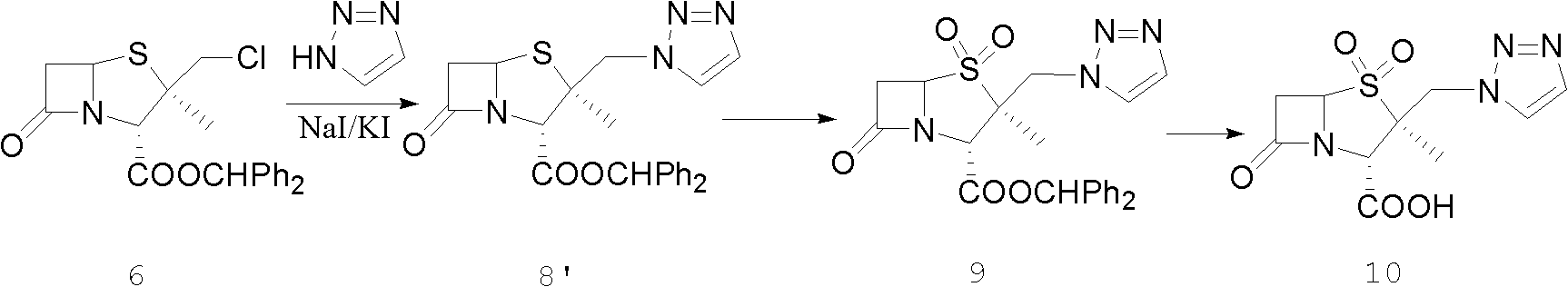
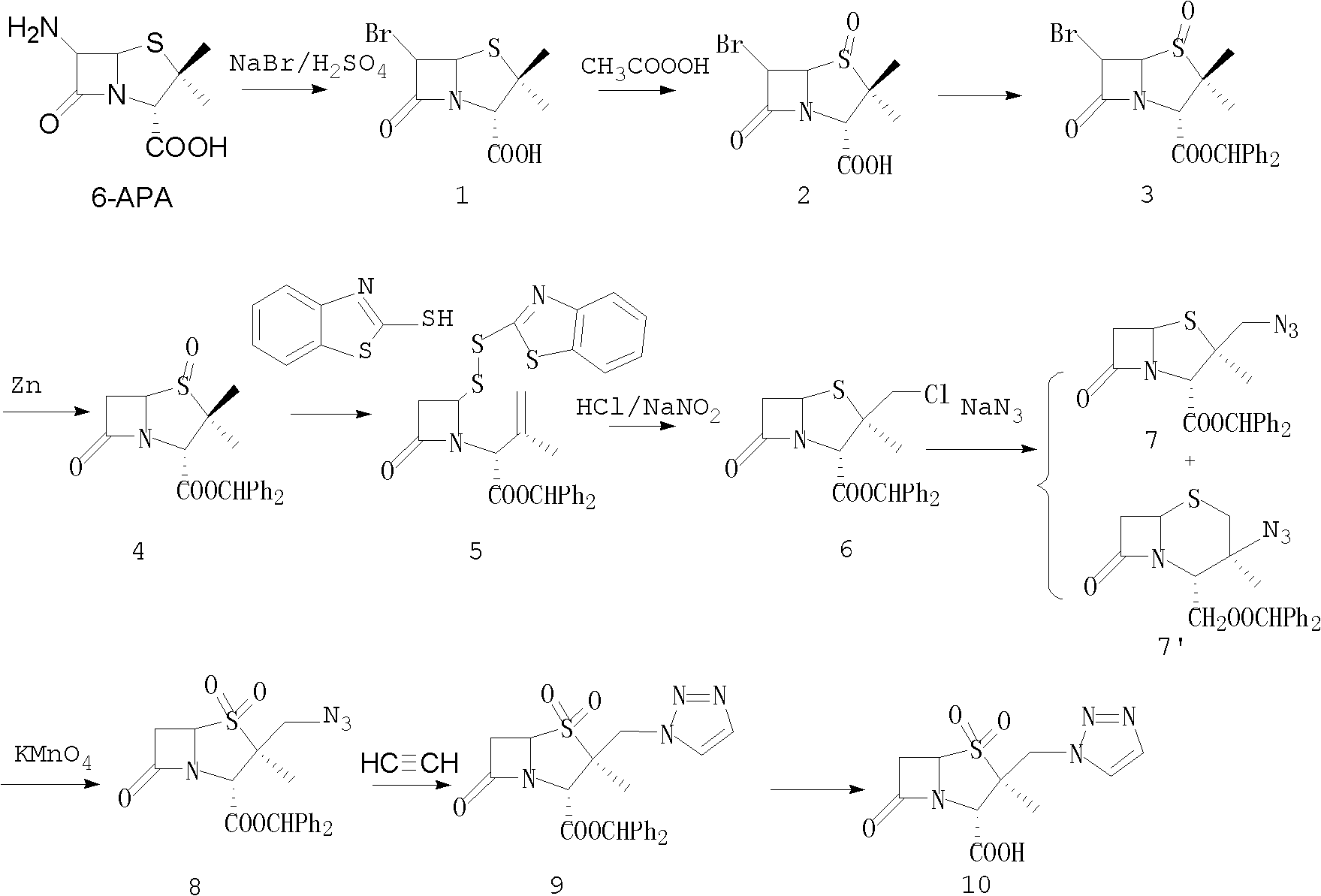
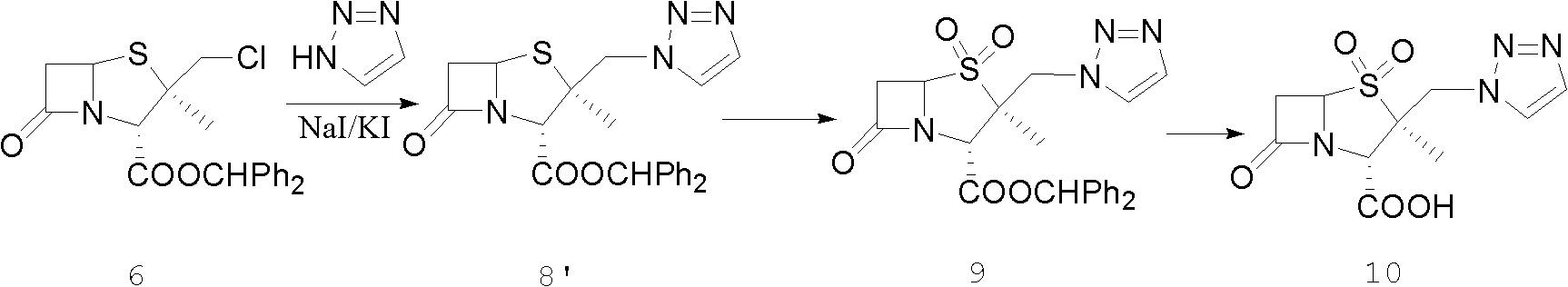
The invention discloses a tazobactam synthesis method, which belongs to the technical field of medicines, and includes the steps: firstly, enabling 6,6-dihydropenam sulfoxide acid diphenylmethyl ester serving as raw materials to undergo thermal cracking and chloromethylation reaction to obtain 2beta-chloromethyl penicillanic acid diphenylmethyl ester; secondly, adding oxidizing agent to oxidize the 2beta-chloromethyl penicillanic acid diphenylmethyl ester-1beta-oxide, enabling the oxidized 2beta-chloromethyl penicillanic acid diphenylmethyl ester-1beta-oxide to react with sodium azide to generate 2beta-hydrazoic methyl penicillanic acid diphenylmethyl ester-1beta- oxide, and then generating 2beta-hydrazoic methyl penicillanic acid diphenylmethyl ester-1,1- dioxide by means of oxidization under the action of potassium permanganate and acetic acid; and finally, preparing the tazobactam by means of deprotection under the action of acetylene cyclization and metacresol. Compared with a past 6-APA (aminopenicillanic acid) route, the tazobactam synthesis method has the advantages that the step of sulfur atom single oxidization is added, so that possibility of ring expansion due to affinity of lone pair electrons on a sulfur atom is blocked, and transformation of five-membered ring products to six-membered ring by-products during hydrazoic reaction can be effectively controlled.
Owner:山东安信制药有限公司 +1
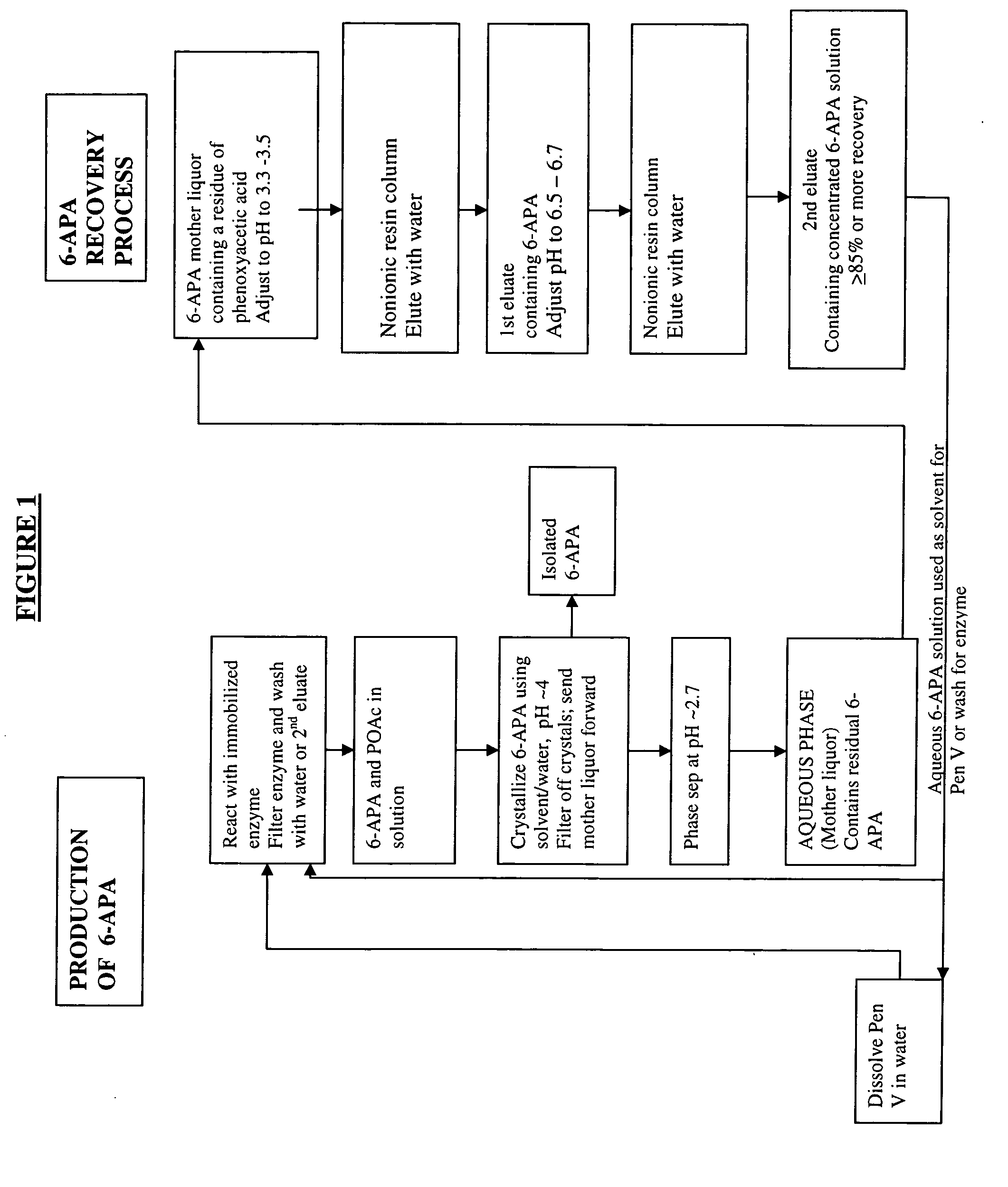
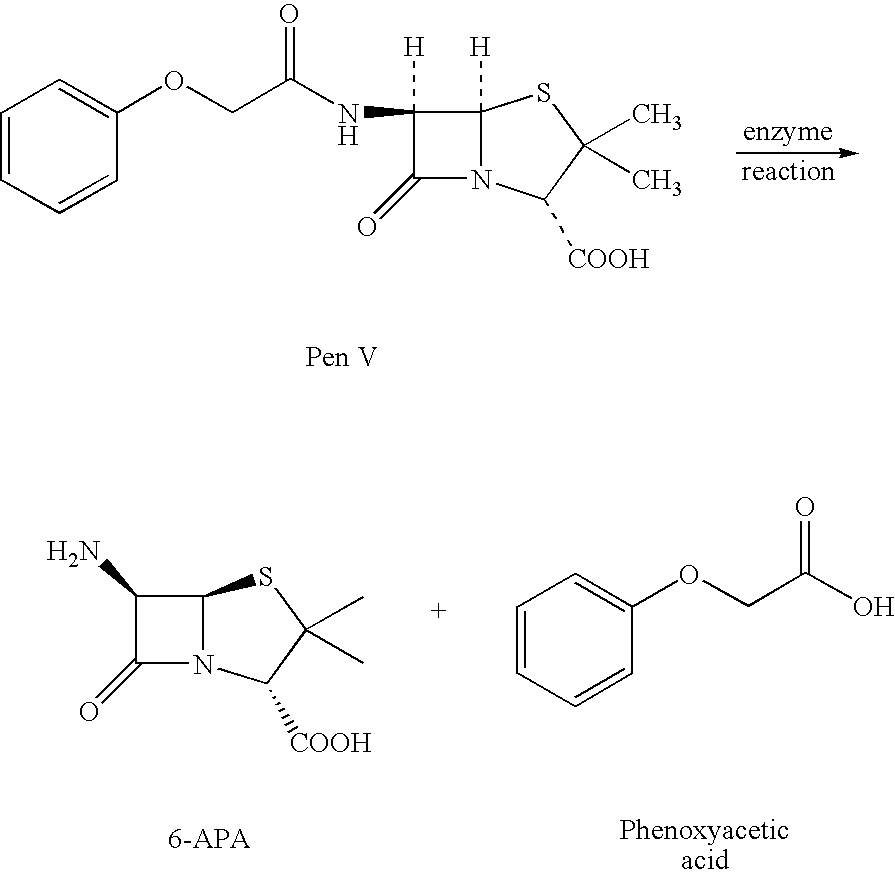
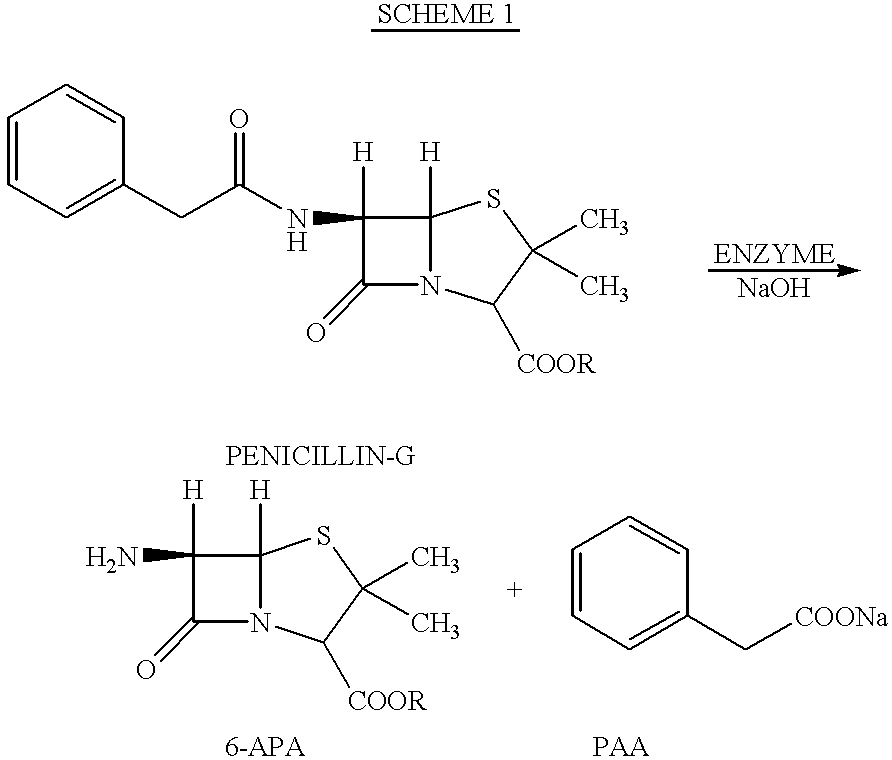
Process for producing 6-amino-penicillanic acid and phenylacetic acid
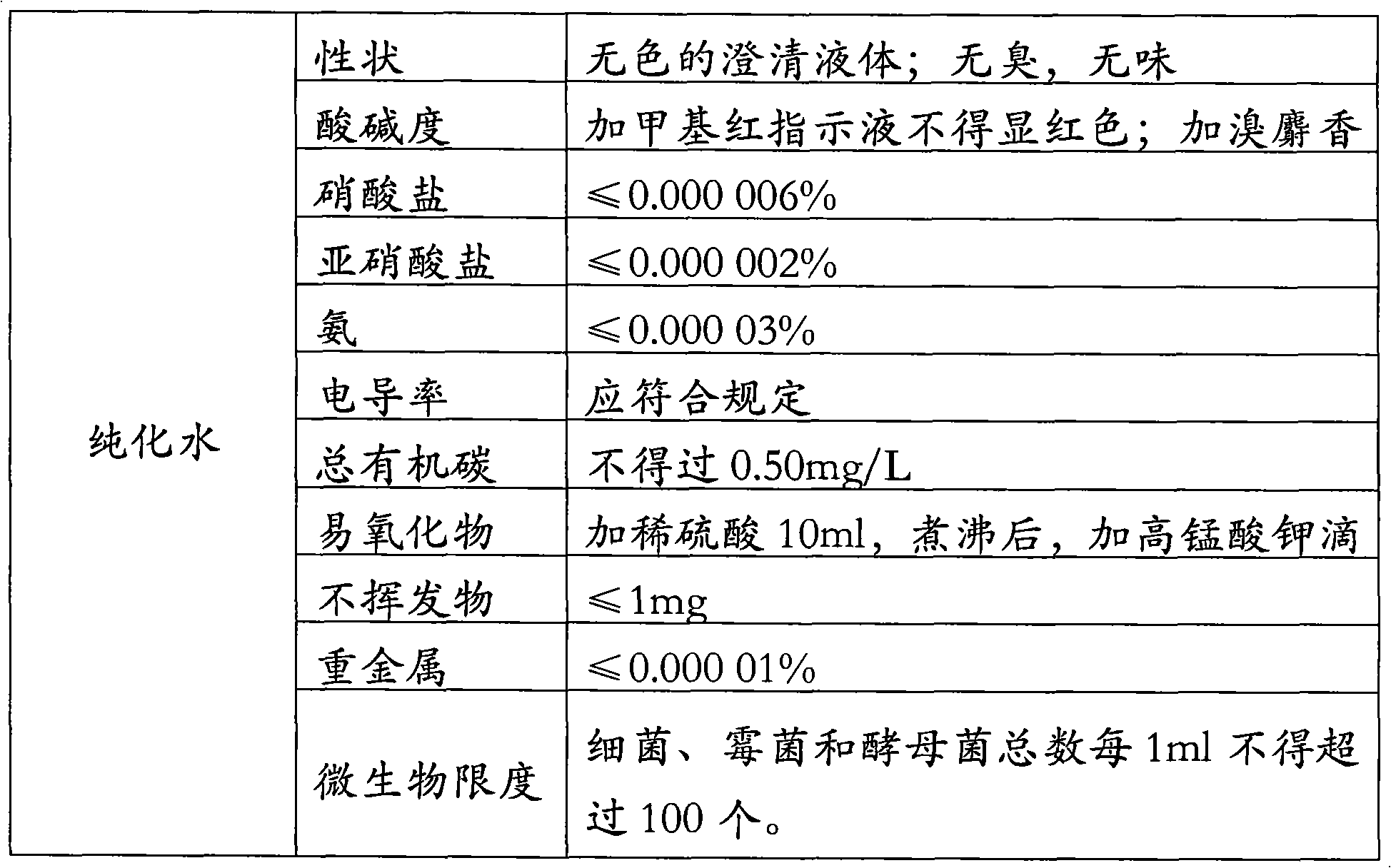
PCT No. PCT / ES97 / 00066 Sec. 371 Date Feb. 25, 1998 Sec. 102(e) Date Feb. 25, 1998 PCT Filed Mar. 14, 1997 PCT Pub. No. WO97 / 35029 PCT Pub. Date Sep. 25, 1997Alternative process for obtaining 6-aminopenicillanic acid. The process comprises replacing the stages of extraction with organic solvents and isolation and separation of the intermediate penicillin salt as a solid by a process of ultrafiltration of the culture broth in at least 2 successive stages. The first stage has a cut-off for molecular weights of 20,000 Dalton and the second, 2000 Dalton. Subsequent to the enzyme conversion stage the products from that stage are subjected to a series of anionic exchange chromatography steps.
Owner:ANTIBIOTICOS SA
Improved method for preparing amoxicillin by enzymic method
The invention relates to the field of pharmacy, and provides an improved method for preparing amoxicillin by an enzymic method, and a product obtained by the improved method for preparing amoxicillin by the enzymic method. The method comprises the following steps of: 1) dissolving 6-aminopenicillanic acid (6-APA) at the temperature of between 10 and 20 DEG C by using water or / and aqueous solution of ammonia which has the pH value of 7.0 to 8.0, and adding D-p-Hydroxyphenylglycine methyl ester hydrochlorid and penicillin G acyltransferase; 2) adjusting the pH value of a solution obtained in the step 1) to be 6.0 to 6.5, and reacting at the temperature of between 21 and 30 DEG C until the content of 6-APA is less than 5mg / ml to obtain a solution of an amoxicillin product; and 3) separating the penicillin G acyltransferase from the solution of the amoxicillin product, adjusting by using hydrochloric acid until the solution of the amoxicillin product is clarified, adding the aqueous solution of ammonia, adjusting the pH value to be 5.5 to 6.5, and crystallizing at the temperature of between 0 and 5 DEG C to obtain amoxicillin. By the improved method for preparing amoxicillin by the enzymic method, the quality of the amoxicillin product is greatly improved, and the medication safety of the amoxicillin product is further improved.
Owner:UNITED LAB INNER MONGOLIA CO LTD
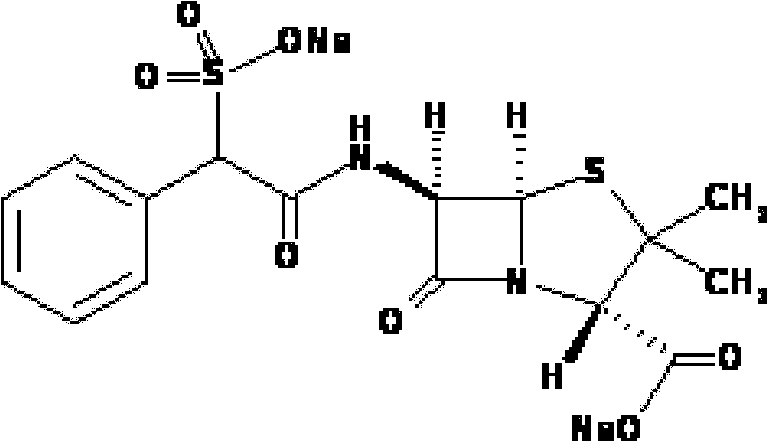
Method for preparing sulbenicillin disodium
The invention provides a method for preparing sulbenicillin disodium. The method comprises the following steps of: preparing alpha-sulfophenylacetyl chloride from alpha-sulfophenylacetic acid; reacting the alpha-sulfophenylacetyl chloride with 6-aminopenicillanic acid (APA) in the mixed solvents of water, ethanol and tetrahydro-2-methylfuran under the condition of the pH value of 5.6 to 7.0 and the temperature of 15 to 25 DEG C for 20 to 40 minutes to obtain crude sulbenicillin disodium; and obtaining the aqueous solution of the sulbenicillin disodium by post treatment and then cooling and drying the aqueous solution of the sulbenicillin disodium to obtain the final product, namely the sulbenicillin disodium. The method has the advantages of reducing energy consumption and saving production cost along with simple process, mild reaction condition and high yield.
Owner:HUNAN SANQING PHARMA +1
Process for preparing straight-through 6-aminopenicillanic acid
InactiveCN101735243AAvoid disadvantagesLarge amount of deesterificationOrganic chemistryFermentationGas phasePressure reduction
The invention discloses a process for preparing straight-through 6-aminopenicillanic acid, which comprises the following steps: a, filtering and acidizing penicillin fermentation solution, extracting the penicillin fermentation solution by using butanol, and concentrating and decoloring the extract to obtain butyl ester extracting solution of penicillin; b, back extracting the butyl ester extracting solution of the penicillin by using alkali solution to obtain brine solution of penicillin (heavy phase or RB for short); c, continuously injecting the brine solution of the penicillin into a degreasing tower in a vacuum pressure reduction state to convert the butyl ester into a gas phase from the brine solution of the penicillin, discharging the degreased brine solution of the penicillin out of a pressure reduction system from the bottom of the tower to a storage tank with a cooling device, and cooling the degreased brine solution of the penicillin for later use; and d, performing enzymatic conversion on the degreased brine solution of the penicillin, then adding 6-APA crystal seeds into the solution, growing the crystals, crystallizing the solution, and drying the crystals.
Owner:NORTH CHINA PHARMA COMPANY
Preparation method for flucloxacillin sodium
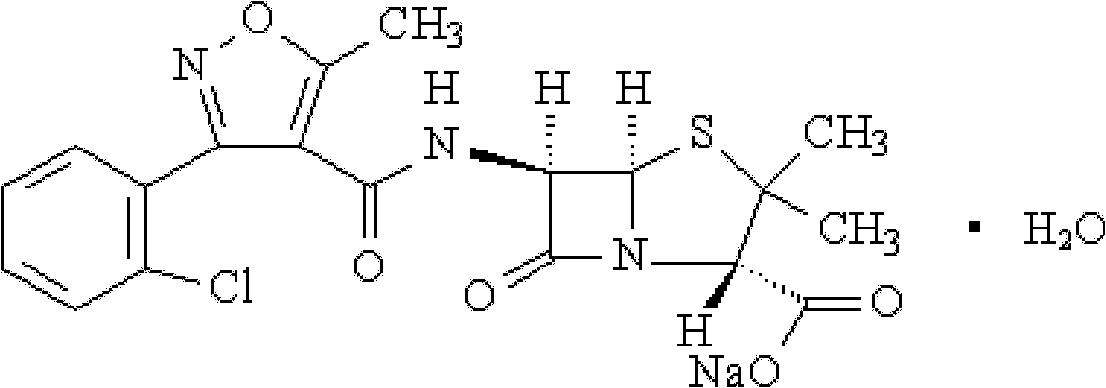
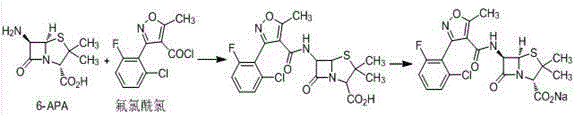
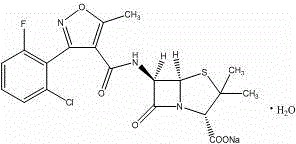
The invention relates to a preparation method for flucloxacillin sodium. The method comprises the following steps of: (1) weighing 6-aminopenicillanic acid, 3-(2-chloro-6-fluorophenyl)-5-methyl isoxazole-4-phosgene and sodium iso-octoate; (2) adding water into 6-aminopenicillanic acid, cooling in an ice bath, and adding acetone to obtain a solution A; (3) preparing 3-(2-chloro-6-fluorophenyl)-5-methyl isoxazole-4-carbonyl chloride and an organic solvent into a solution B; (4) adding the solution B into the solution A, and performing an acylation reaction; (5) after reacting, acidifying, and adding an organic solvent for extracting; (6) slowly adding a part of organic solution of sodium iso-octoate into the an extracting solution obtained in the step (5), performing a salt-forming reaction, and growing a crystal after the crystal is precipitated out; and (7) after growing the crystal, continually adding the residual sodium iso-octoate solution, preserving heat, stirring, leaching, soaking, washing and drying in vacuum. The method has the advantages of short process route, high process operability and capability of effectively increasing product quality and yield.
Owner:河北华日药业有限公司
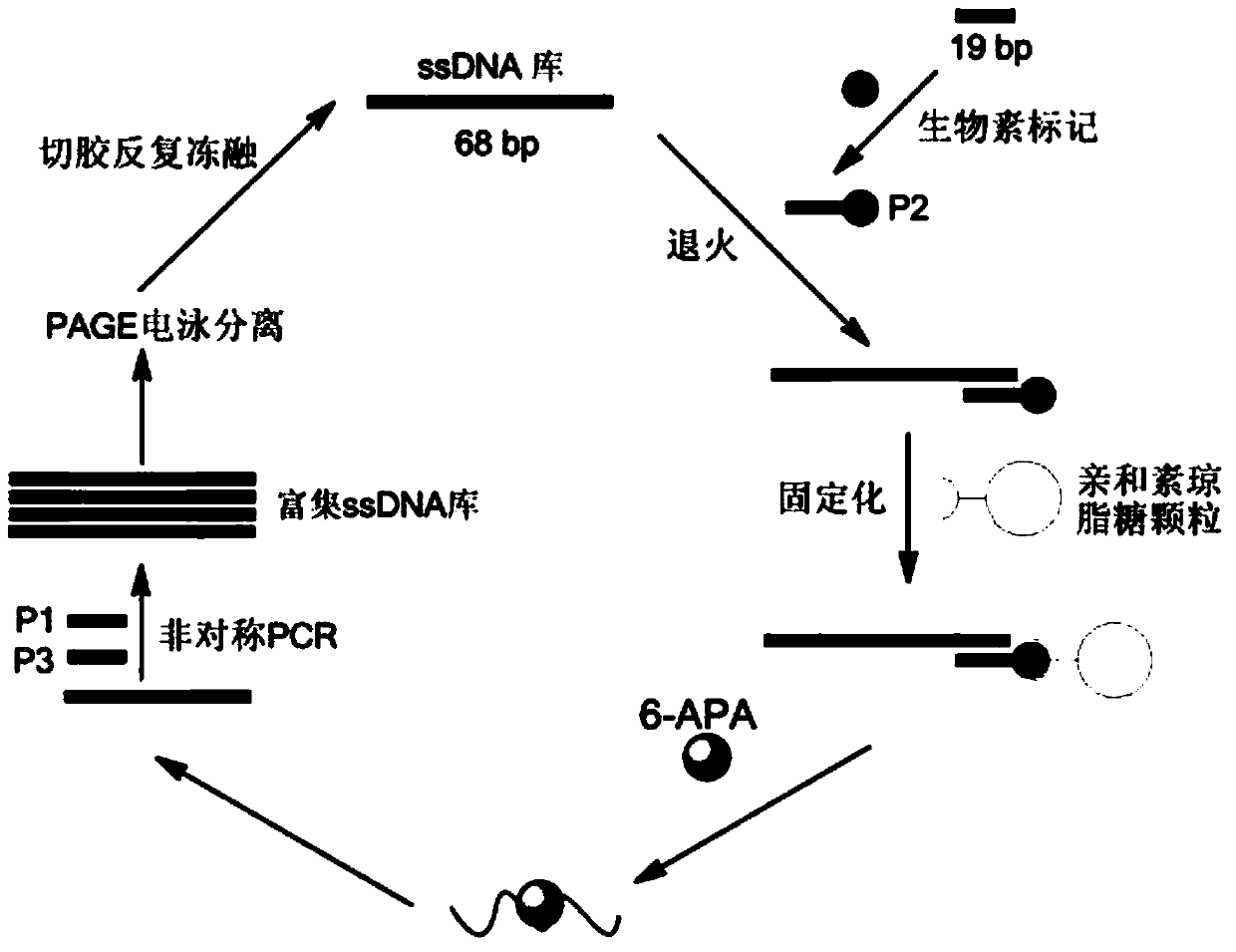
Improved penicillin antibiotic aptamer without fixed point target substance and application thereof
ActiveCN103387991AEasy to fixEasy to operateColor/spectral properties measurementsDNA/RNA fragmentationElutionMISCELLANEOUS ANTIBIOTICS
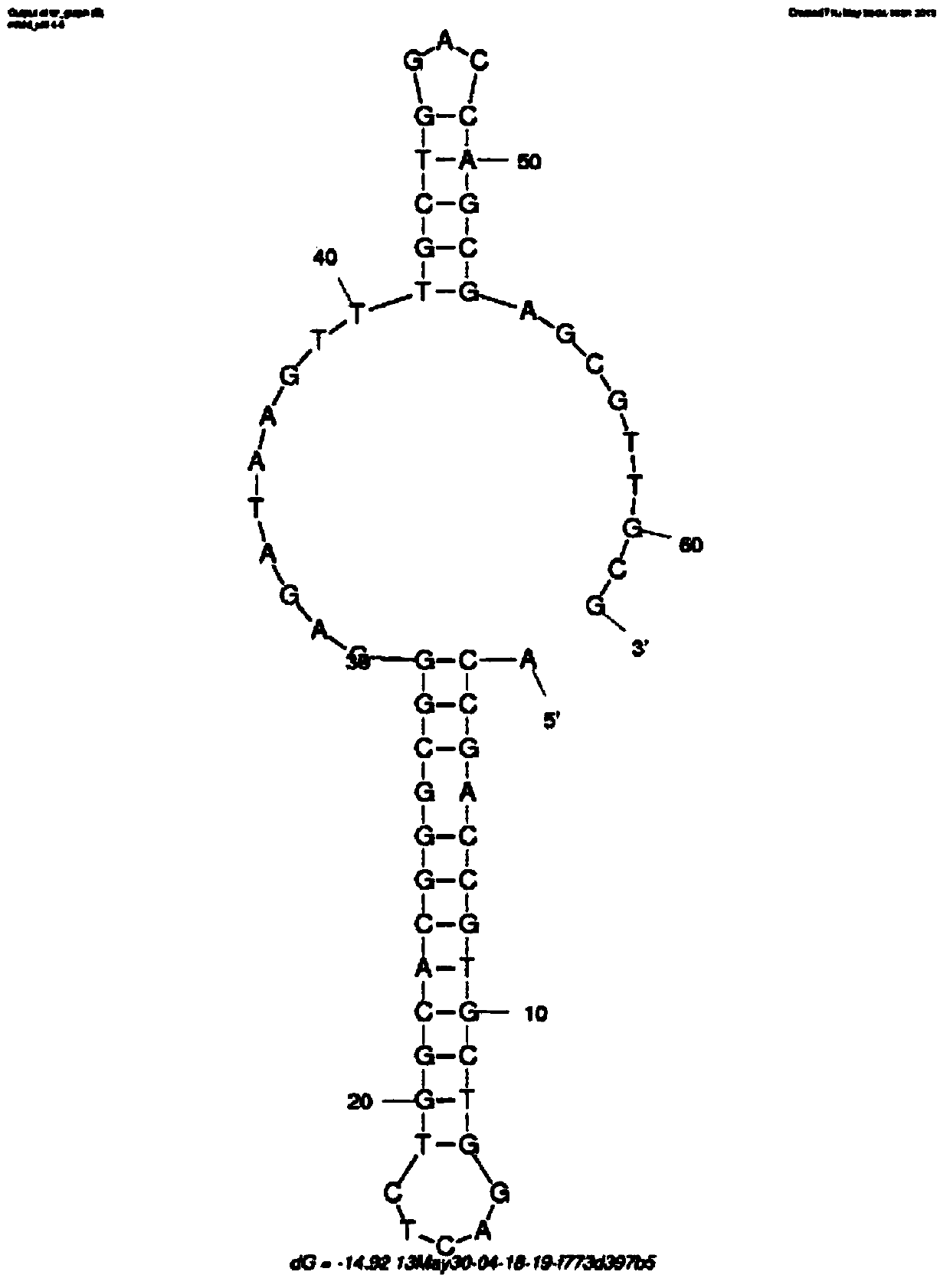
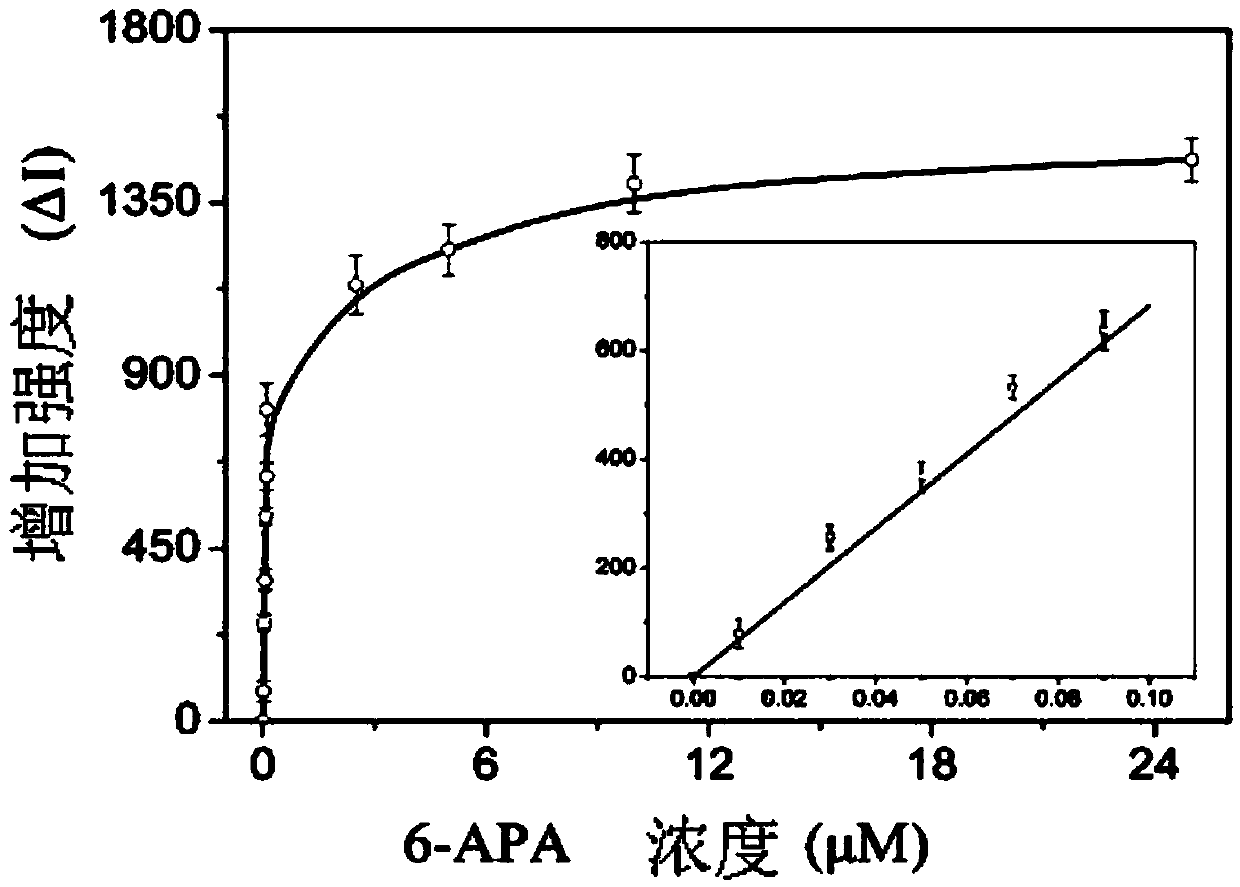
The invention discloses a penicillin antibiotic aptamer obtained by the screening technology for an improved aptamer without a fixed point target substance in the field of screening a penicillin antibiotic aptamer without a fixed point target substance, and an application thereof. The unfixed nucleic acid is eluted through a fixed oligonucleotide library; the parent nucleus 6-APA (6-aminopenicillanic acid) of the penicillin antibiotic is added in positive screening to act on the fixed nucleic acid; after eluting the nucleic acid molecules which can be combined with 6-APA, PCR (polymerase chain reaction) amplification is directly performed for next screening; and other antibiotics are added in negative screening, and the nucleic acid molecules in non-specific binding with the penicillin antibiotics are removed by elution. Through multiple rounds of positive and negative screening, 10 nucleic acid aptamers with high specificity and strong affinity with 6-APA are obtained; and the nucleic acid aptamer with a stable secondary structure is selected for developing a nucleic acid aptamer sensor for detecting 6-APA.
Owner:SHANGHAI JIAO TONG UNIV
6-aminopenicillanic acid preparation method
The invention discloses a process for preparing 6-aminopenicillanic acid, which comprises the following steps: (1) filtering the penicillin fermentation liquid, acidifying, extracting and concentrating with butanol, decoloring to obtain butyl extract of penicillin, (2) subjecting butyl extract of penicillin to back extraction with alkaline solvent, obtaining aqueous solution of penicillin salts, (3) degreasing the aqueous solution of penicillin salts, (4) loading the degreased aqueous solution of penicillin salts into penicillin acylated enzyme retort for enzyme conversion, charging 6-APA seeds, cultivating quartz, crystallizing and drying.
Owner:NORTH CHINA PHARMA GROUP CORP
Synthesizing method of sulbactam acid
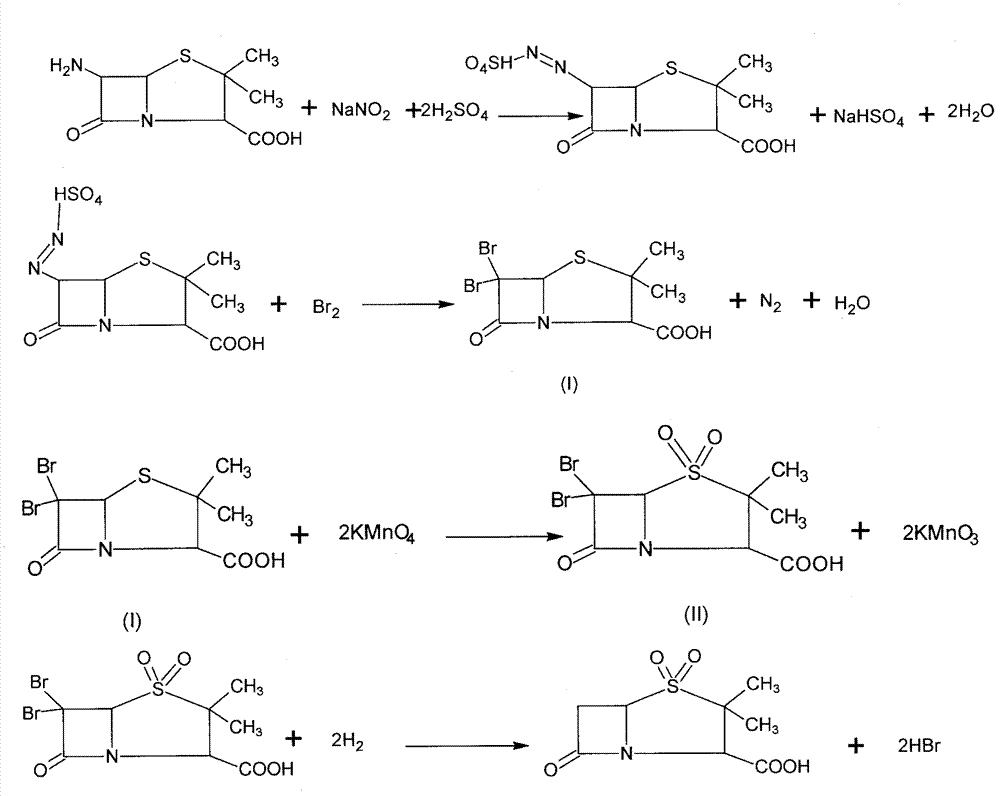
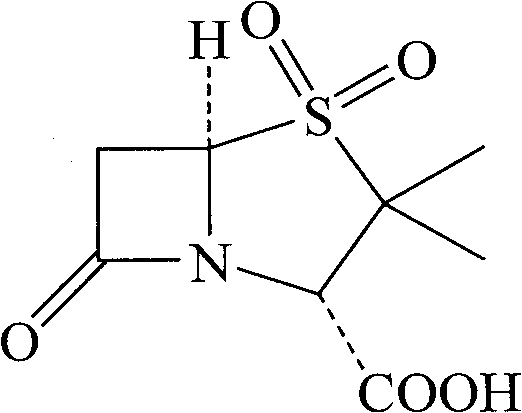
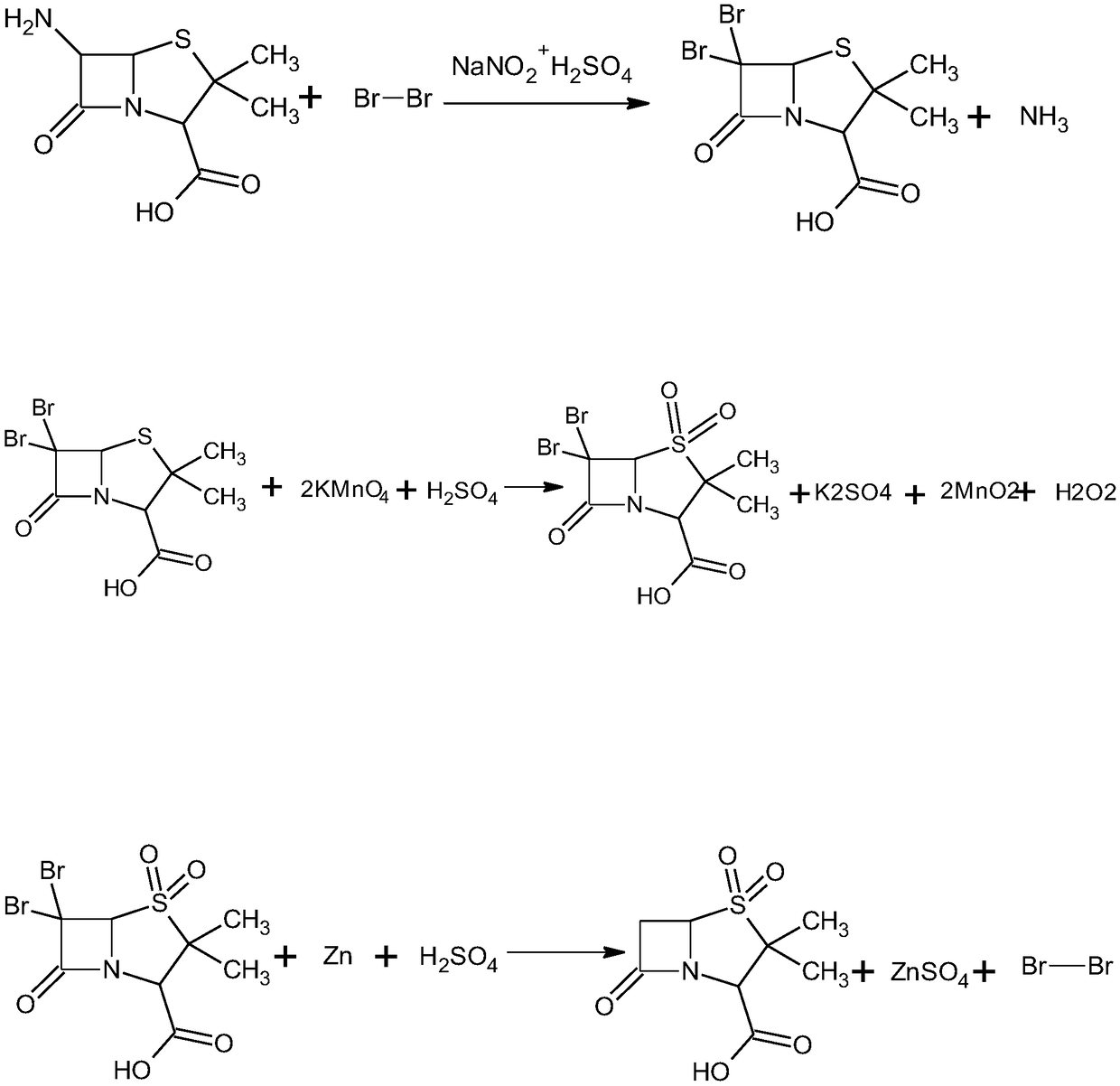
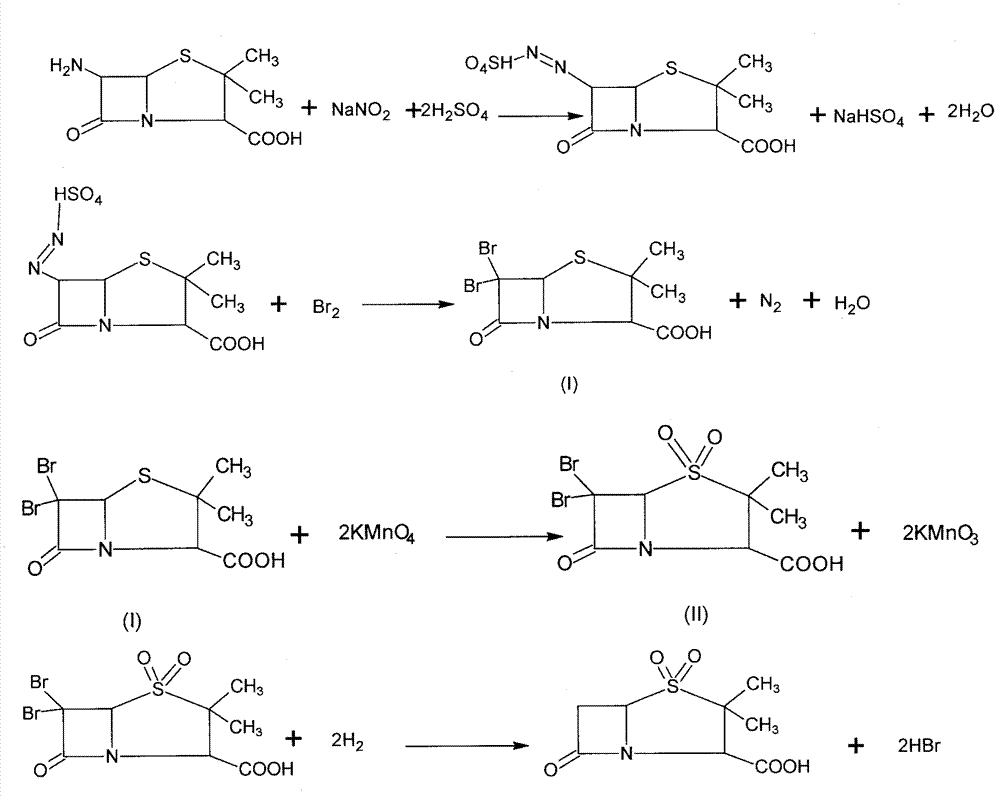
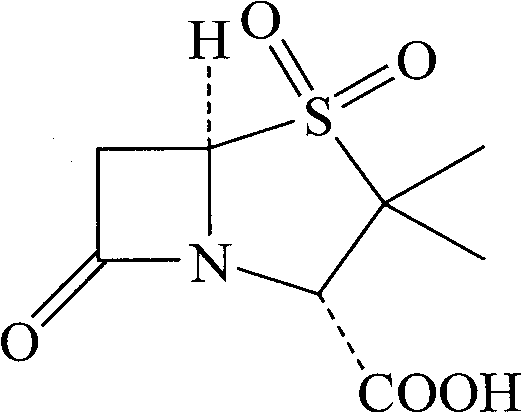
The invention discloses a synthesizing method of sulbactam acid. The method comprises the steps that: (1) under the existence of a strong acid, 6-aminopenicillanic acid, a diazotization reagent, and bromine or bromide are subjected to an insulation reaction in an organic solvent; excessive bromine is reduced; and a reaction product is extracted to a water layer; (2) an oxidant is dropped into the water-layer reaction product, and an insulation reaction is carried out; when the reaction is finished, a pH value is regulated; excessive potassium permanganate is reduced; an organic solvent is added, such that a reaction product is extracted to an organic solvent layer; (3) a catalyst is added into the organic-solvent layer product, such that hydrogenation bromine-removing is carried out; a reaction product is extracted to an organic-solvent layer; and distillation and crystallization are carried out. The organic solvent is ethyl acetate. According to the invention, ethyl acetate is adopted as the organic solvent in a four-step reaction, such that solvent consumption of the whole reaction is greatly reduced, and product total yield is effectively improved. With the method provided by the invention, by-product in the reaction can be comprehensively utilized, and advantages such as high yield, low cost, clean production, and the like are provided.
Owner:LIANYUNGANG HENGFEI PHARMA
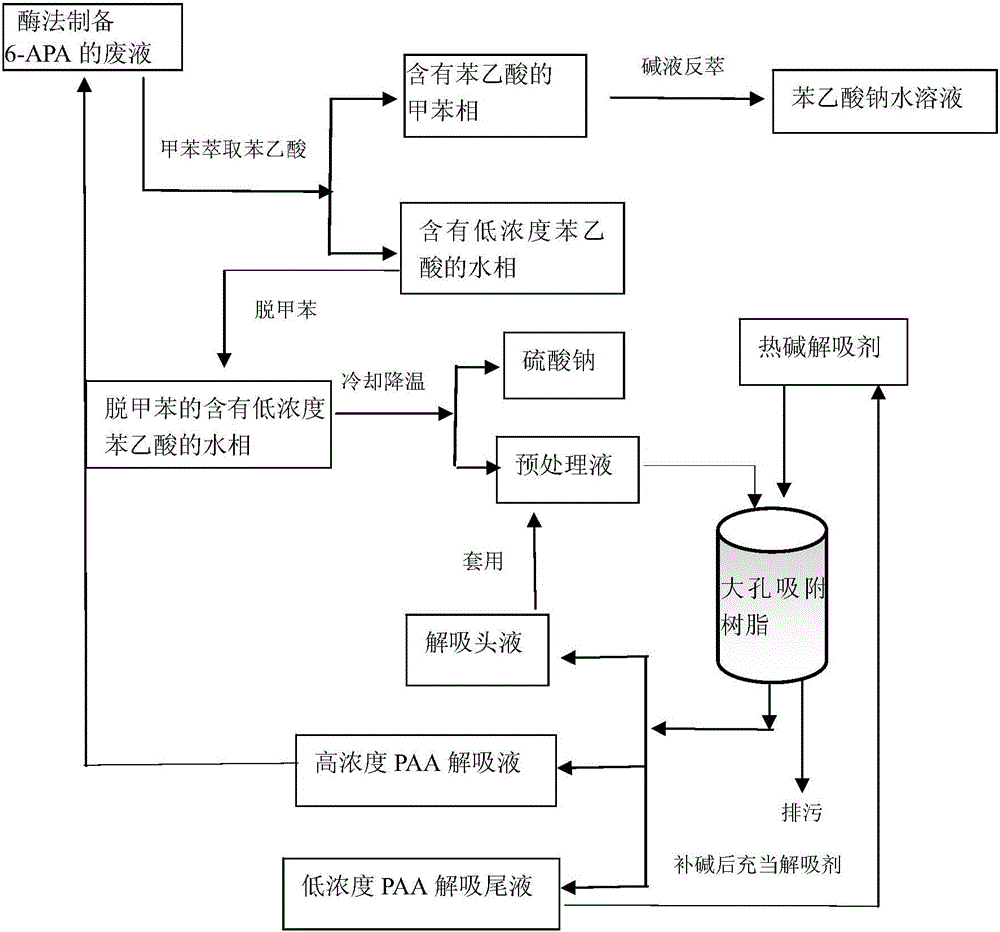
Method for recovering phenylacetic acid from waste liquid in preparation of 6-aminopenicillanic acid by enzymic method
ActiveCN106117042AQuality improvementLow incomeOrganic compound preparationAlkali metal sulfites/sulfatesPhenylacetic acidFermentation
The invention relates to a method for recovering phenylacetic acid from waste liquid in preparation of 6-aminopenicillanic acid by an enzymic method. The method comprises the following steps: 1) using toluene to extract phenylacetic acid; 2) preparing a sodium phenylacetate aqueous solution with little impurity; and 3) using macroreticular resin for recovering phenylacetic acid from a water phase containing low-concentration phenylacetic acid. According to the invention, toluene is extracted from waste liquid in preparation of 6-aminopenicillanic acid by the enzymic method, a toluene phase containing phenylacetic acid and a water phase containing low-concentration phenylacetic acid are obtained; the toluene phase containing phenylacetic acid is extracted through alkali lye to obtain the sodium phenylacetate aqueous solution with high quality, the sodium phenylacetate aqueous solution can be directly used for producing penicillin through fermentation; the water phase containing low-concentration phenylacetic acid is pretreated for recovering sodium sulfate, macroreticular resin is used for absorbing phenylacetic acid for recovery, the low concentration water after adsorption can be directly discharged, zero discharge of phenylacetic acid can be almost realized, win-win of economy and environmental protection can be achieved, the by-product sodium sulfate can be used for fermentation, and also has economic benefit.
Owner:SHANXI WEIQIDA PHARMA IND
Process for recovery of 6-aminopenicillanic acid from an aqueous discharge stream
A process for recovering residual amounts of 6-APA, on the order of 10 g / L or less, from a predominantly aqueous liquor containing phenoxyacetic acid and less than about 2% organic solvents.
Owner:BRISTOL MYERS SQUIBB CO
Method for preparing sulbactam
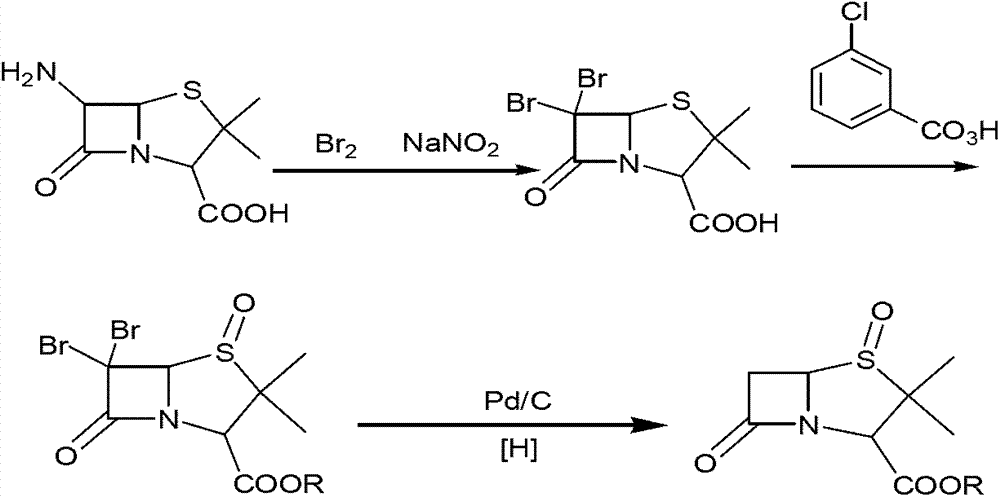
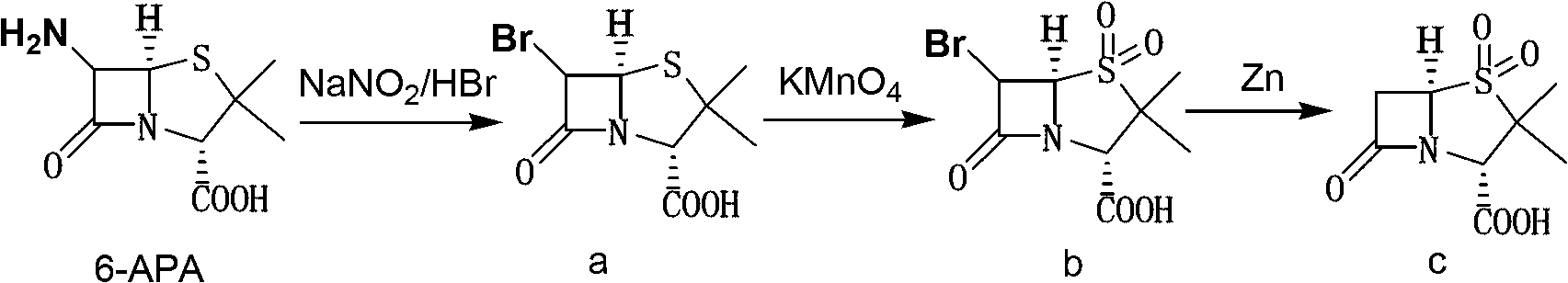
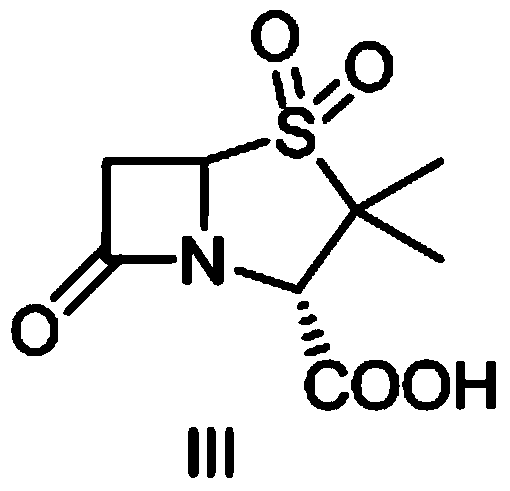
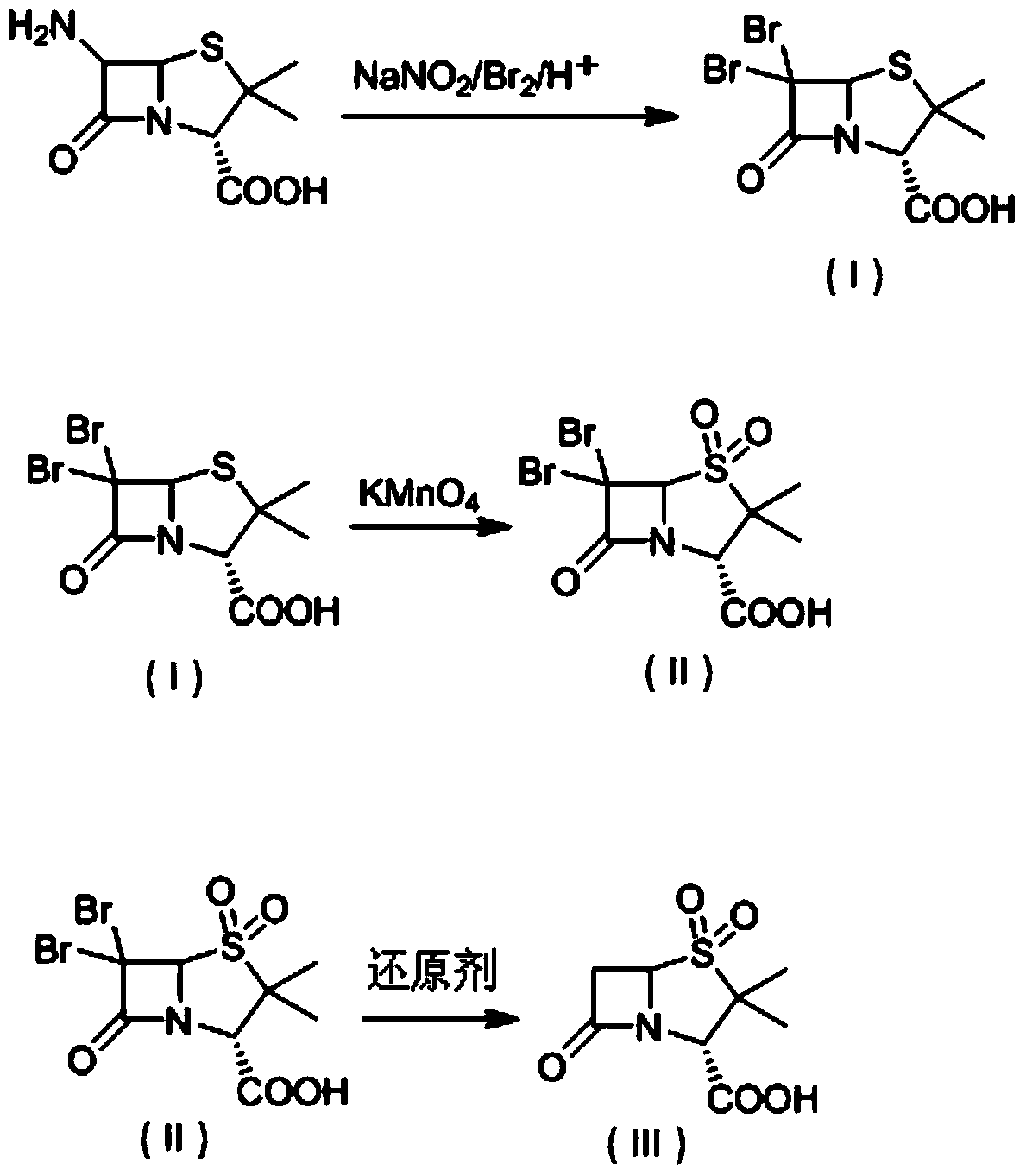
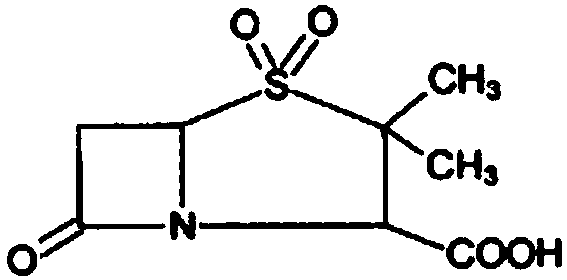
The invention discloses a method for preparing sulbactam and belongs to the technical field of pharmaceuticals. The method is characterized in that a 'one-pot' method is adopted, starting from 6-aminopenicillanic acid (6-APA), under the action of sodium nitrite and hydrobromic acid, a reaction solution which contains product 6 alpha-penicillanic acid is obtained through bromination reaction, and then, solid sulbactam is obtained through further oxidation and reduction reaction. The solid sulbactam is directly prepared during the reaction preparation process without separation, thus the method is simple to operate and easy for industrial production; the method adopts single-bromination reaction to substitute double-bromination reaction; moreover, bromine which has greater pollution is not used as a raw material, but hydrobromic acid is taken as a raw material, and the method is simple to operate and is low in cost; and the sulbactam solid which is prepared through the method is high in yield and good in quality.
Owner:QILU TIANHE PHARMA
Synthetic method of salbactam acid
ActiveCN104262359APrevent decomposition reactionAvoid self-decomposition reactionsOrganic chemistryAqueous solutionImpurity
The invention discloses a synthetic method of salbactam acid. The synthetic method comprises the following steps: carrying out diazotized bromination reaction on 6-aminopenicillanic acid to form bromopenicillanic acid; and then, carrying out oxidation reaction and reduction reaction to obtain salbactam acid, wherein in the diazotized bromination reaction, 6-aminopenicillanic acid is continuously added in form of an acidic solution, the acidic solution of 6-aminopenicillanic acid is 5-8% sulfuric acid aqueous solution, 13-15% hydrobromic acid aqueous solution or 5-8% hydrochloric acid aqueous solution. 6-aminopenicillanic acid is dropwise added in form of the acidic solution, so that dust pollution is avoided and the work environment of field personnel is improved. Meanwhile, 6-aminopenicillanic acid exists in form of stable salt, decomposition reaction is avoided, and the reaction quality is improved. The three-step yield of the method is over 70%, the HPCL purity is over 99.7%, the individual impurity content is less than 0.1% and the total impurity content is less than 0.5%.
Owner:JIANGXI FUSHINE PHARMA CO LTD
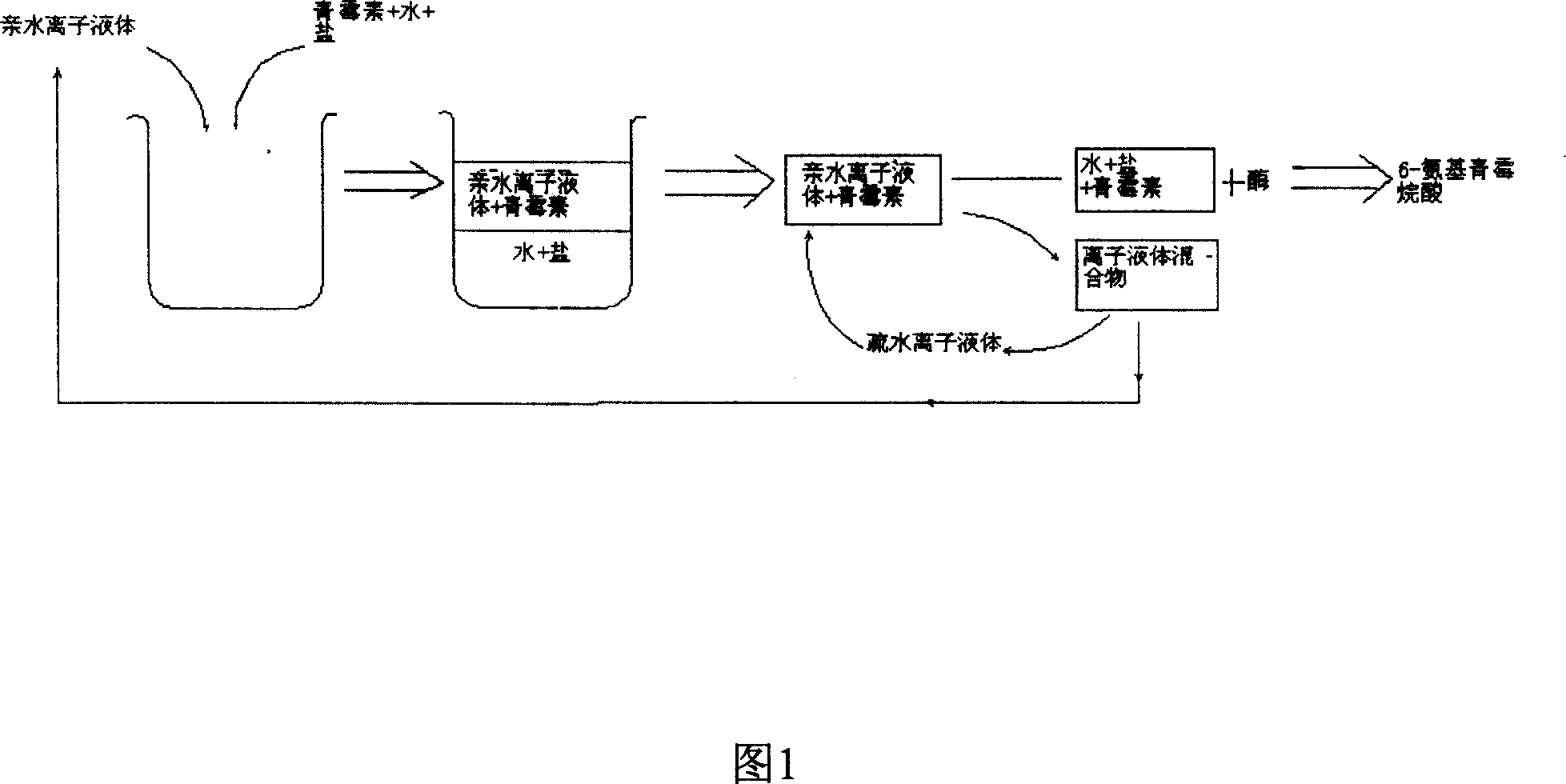
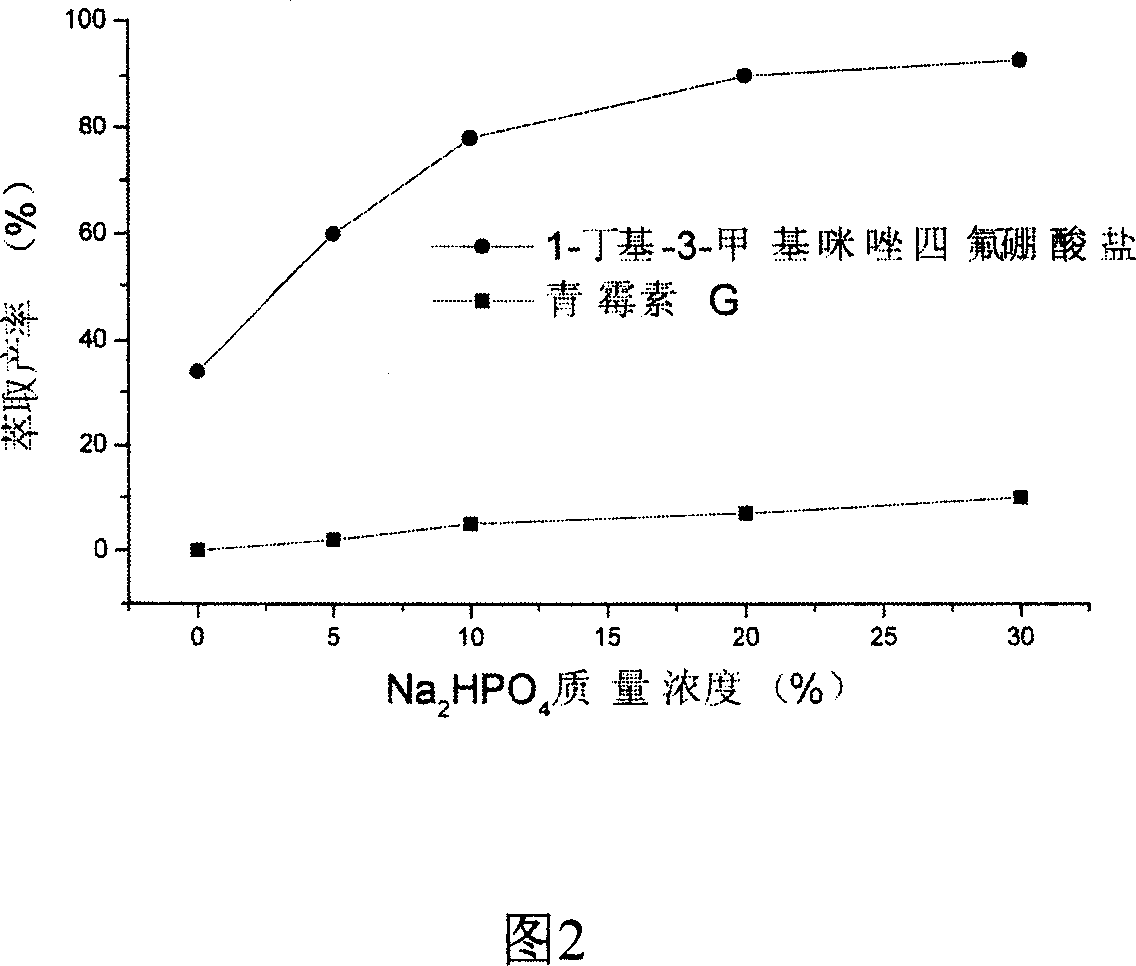
Method of preparing semisynthetic antibiotic 6-amino penicillanic acid by ion liquid extraction penicillin and enzymic catalytic reaction coupling
InactiveCN1948316AAchieve separationReduced hydrophilic ionic liquid contentOrganic chemistryFermentationOrganic solventHydrophile
The present invention relates to a technological process for preparing semi-synthetic antibiotic 6-aminopenicillanic acid by utilizing integrative process composed of penicillin fermentation liquor extraction and enzymatic catalysis reaction. It is characterized by that it utilizes the aqueous two-phase formed by hydrophilic ionic liquor to make penicillin be extracted into the upper phase containing ionic liquor, then utilizes hydrophobic ionic liquor to make secondary extraction so as to make the hydrophilic ionic liquor be extracted into hydrophobic phase, and the penicillin aqueous solution of raffinate phase can be directly undergone the process of enzymatic catalysis reaction, the enzyme activity can be up to above 80%.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Synthesis of austrastaph
ActiveCN1613859ASimple processSimple and fast operationOrganic chemistryPenicillin synthesisOrganic solvent
Synthesis of cloxacillin benxethine is carried out by: dissolving 6-aminopenicillanic acid in aqueous organic solvent to form a sodium salt, condensing with o-chlorbenzylisoxazoleacyl cyanide to obtain cloxacillin benxethine solution directly, and having metathesis with N,N-dibenzyl quadol diacetate. It is simple and costs low.
Owner:石药集团中诺药业(石家庄)有限公司
Technology for preparing ampicillin by adopting enzymic method
The invention discloses a technology for preparing ampicillin by adopting an enzymic method. The technology comprises the following steps of (a) mixing 6-aminopenicillanic acid, a phenylglycine derivative and penicillin G acylase into water to obtain mixed liquid; (b) adjusting and controlling the pH value of the mixed liquid to be 5.5-6.1 through acid or alkaline, and reacting under the temperature of 0-40 DEG C; (c) after the reaction is finished, performing separation, cleaning reaction liquid containing a coarse ampicillin product through the acid, crystallizing the reaction liquid through the alkaline, and performing grain cultivation, washing and drying to obtain an ampicillin product. The preparation technology provided by the invention is simple, convenient to operate, low in energy consumption, high in safety and high in stability; furthermore, the pH value required by a reaction system is easy to control, the reaction time is short, the conversion rate is up to over 99 percent, the product purity is high, and a solvent does not need to be recycled; and therefore, the low-cost and high-efficiency production technology can be popularized and applied in the industrial production.
Owner:NORTH CHINA PHARM GRP SEMISYNTECH CO LTD
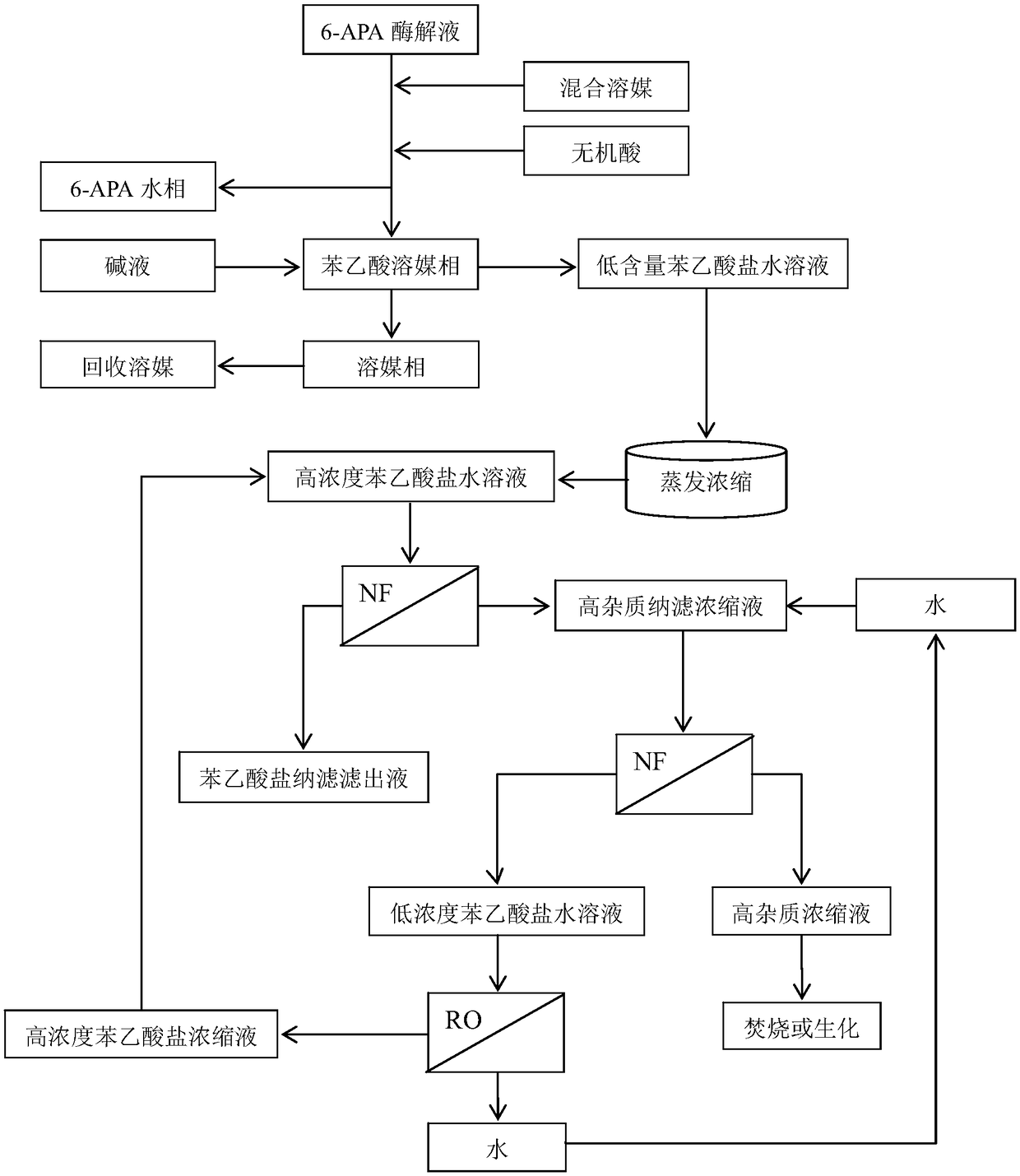
Method for recovering phenylacetic acid in enzymatic aqueous solution of 6-aminopenicillanic acid
ActiveCN108658757ALess dischargeReduce usageCarboxylic compound separation/purificationPhenylacetic acidEvaporation
The invention relates to a method for recovering phenylacetic acid (PAA) in an enzymatic aqueous solution of 6-aminopenicillanic acid (6-APA). The method comprises the following steps: firstly, usinga mixed solvent to extract PAA from the enzymatic aqueous solution of 6-APA to a solvent phase, so as to enable 6-APA to be separated from PAA; then, performing lye stripping and evaporation concentration on the solvent phase to obtain an aqueous solution containing phenylacetate; and finally, recovering PAA in the aqueous solution in a manner of combining secondary nanofiltration with reverse osmosis. The method provided by the invention can directly separate and obtain high-quality aqueous solution of phenylacetate from the enzymatic aqueous solution of 6-APA in a manner of combining extraction, stripping, concentration and nanofiltration, can be directly used for fermentation to produce penicillin, further treats high-impurity nanofiltration concentrate in a manner of combining water dilution, nanofiltration and reverse osmosis, realize zero discharge of PAA, and minimizes discharged wastes by recycling of a solvent and water in the process.
Owner:SHANXI WEIQIDA PHARMA IND
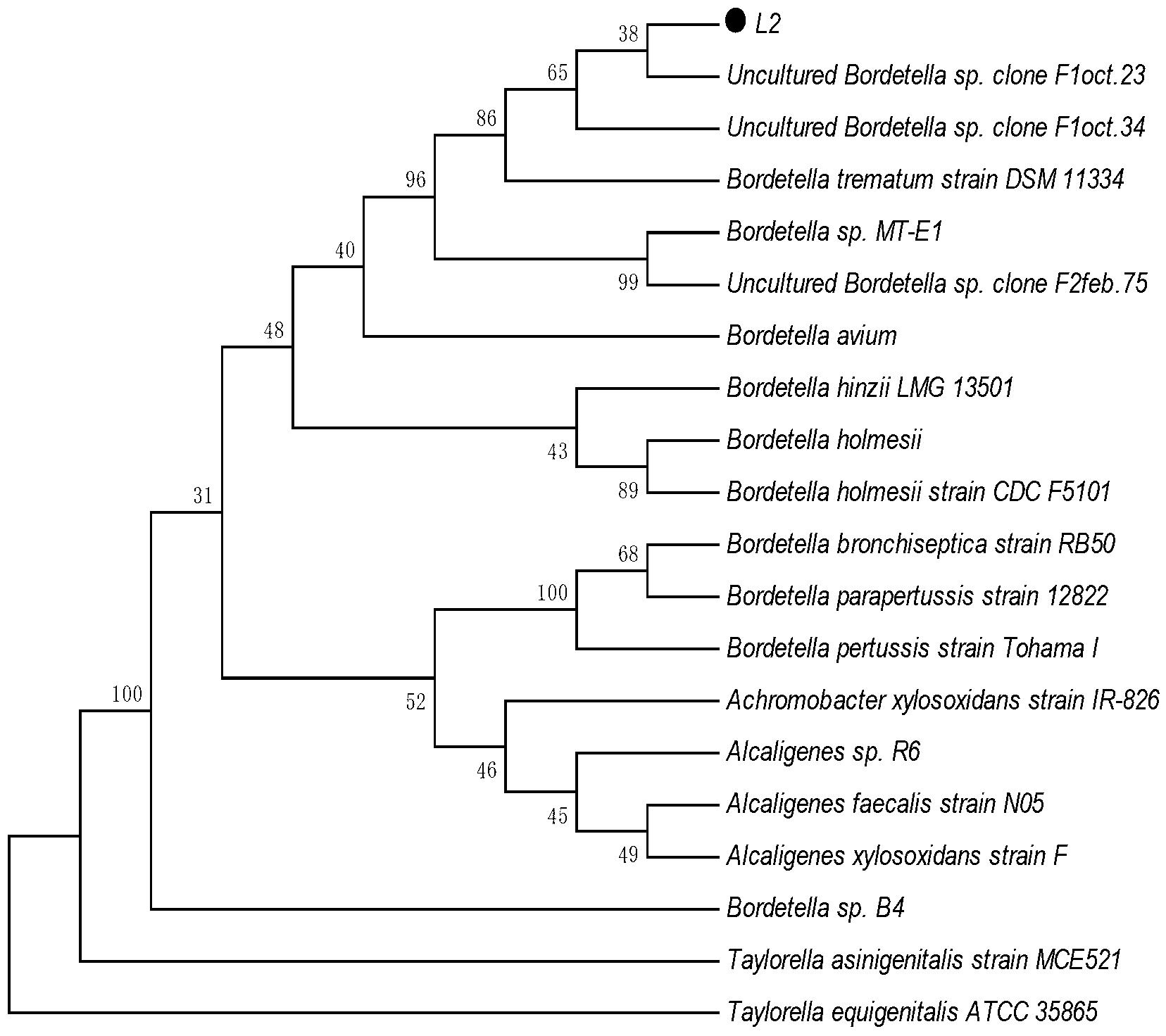
6-aminopenicillanic acid degrading bacterium and screening method thereof
ActiveCN103184177AImprove the ability to hydrolyze 6-aminopenicillanic acidBacteriaWater contaminantsMicroorganismScreening method
The invention relates to a 6-aminopenicillanic acid (6-APA) degrading bacterium and a screening method thereof. Particularly, the invention relates to a strain of 6-APA degrading bacterium Bordetella sp.L2, which is preserved in China General Microbiological Culture Collection Center (CGMCC) on October 18, 2012, with the preservation number of CGMCC No. 6691; and the GenBank registration number of the 16S rRNA gene sequence of the 6-APA degrading bacterium Bordetella sp.L2 is HQ840720. The 6-APA degrading bacterium Bordetella sp.L2 is an aerobic bacillus brevis; and when the pH value is 8.0, the 6-APA degradation rate of the 6-APA degrading bacterium Bordetella sp.L2 is 28%. The strain achieves higher 6-APA degradation capability and has a practical significance and an important engineering application value for treatment of waste water containing 6-APA cephalosporin.
Owner:CHINA THREE GORGES CORPORATION
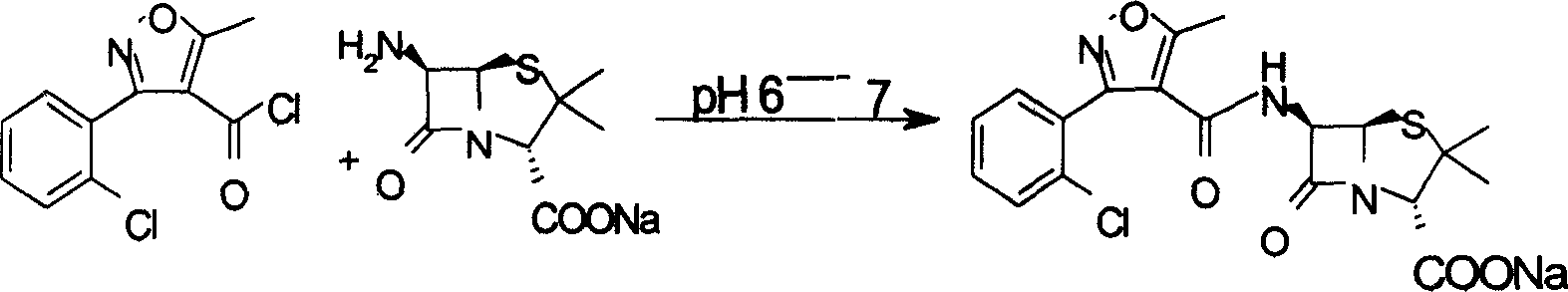
Method for crystallizing cloxacillin sodium
InactiveCN102070653AOvercome the defects of fine and difficult to filterSolve problems such as non-conformityOrganic chemistryFiltrationDistillation
The invention provides a method for crystallizing cloxacillin sodium, which comprises the following steps: weighing 6-aminopenicillanic acid and 3-chloro-O-phenyl-5-methyl-4-isoxazole acyl chloride according to a mass ratio of 1.0 / 0.5 to 2.5; adding water and acetone into the 6-aminopenicillanic acid to prepare a solution A; adding the 3-chloro-O-phenyl-5-methyl-4-isoxazole acyl chloride into the acetone to prepare a solution B; adding the solution B into the solution A to be uniformly stirred for carrying out acidylation reaction for 0.5 to 4h; adding acid for acidification after the reaction is completed, and adding mixed liquid of lower alcohol and butyl acetate for extraction; adding sodium iso-octoate organic solution for carrying out salt forming reaction, and carrying out reduced pressure distillation and crystal growing; and carrying out suction filtration, washing and drying. In the method provided by the invention, the product quality and the yield of the cloxacillin sodium can be effectively improved, the production period is shortened, and the production cost is reduced.
Owner:河北华日药业有限公司
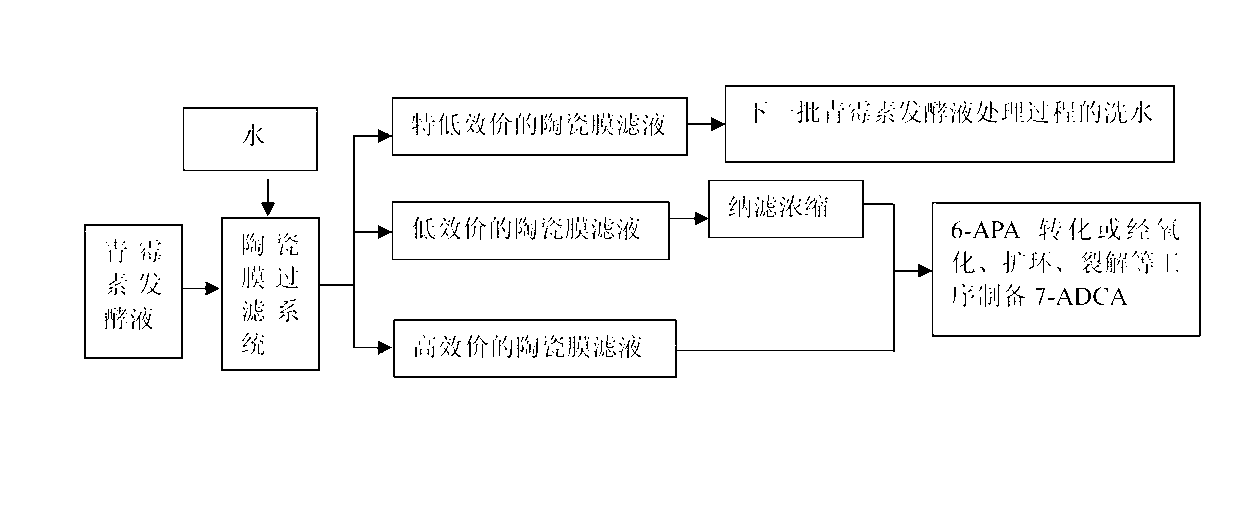
Penicillin fermentation broth treating technology
ActiveCN103214498ADetermining the concentrationDetermine the quantitySemi-permeable membranesOrganic chemistryCross-flow filtrationCephalosporanic Acids
The invention discloses a penicillin fermentation broth treating technology, which comprises the following steps of: cooling an original penicillin fermentation broth, filtering the cooled penicillin fermentation broth by a closed ceramic-membrane cross-flow filtration system, and collecting high-titer ceramic-membrane filtrate; during filtration, when the wet solid content in the penicillin fermentation broth is enhanced to 1.8-2 times that of the original fermentation broth, adding water with weight accounting for 2 times that of the original fermentation broth for dialyzing to obtain and collect low-titer ceramic-membrane filtrate, and then nano-filtering, concentrating and dewatering the low-titer ceramic-membrane filtrate for later use; continuing to add water with weight accounting for 2 times that of the original fermentation broth for dialyzing to obtain and collect ultra-low-titer ceramic-membrane filtrate; stopping filtration until the titer of penicillin in the penicillin fermentation broth is low to 500-800U; collecting bacterium dregs intercepted by the ceramic-membrane filtration system; putting the high-titer ceramic-membrane filtrate in 6APA (6-aminopenicillanic acid) for conversion or oxidization, ring enlargement and cracking, thus preparing 7-ADCA (7-aminodeacetoxy cephalosporanic acid); and collecting the obtained bacterium dregs and adding engineering bacteria for decomposing the bacterium dregs.
Owner:河北美邦工程科技股份有限公司 +1
Enzymatic conversion in a solvent mixture containing water and flourinated, non-chlorinated solvent
InactiveUS6383772B1Reduce capacityOrganic compound preparationOn/in organic carrierPhenylacetic acidFiltration
A first compound is converted to a second compound enzymatically in a solvent mixture containing water and a fluorinated, non-chlorinated alkane, alkene, or alkyne having up to 4 carbon atoms. Lactams, for example 6-aminopenicillanic acid (6-APA), may be prepared by enzymatic conversion of a first compound, for example penicillin-G, in a solvent mixture comprising water and a non-aqueous organic solvent, for example 1,1,1, 2-tetrafluoroethane. The 6-APA can be caused to precipitate, isolated by filtration and optionally derivatized to produce a desired compound. A by-product of the enzymatic conversion, for example phenylacetic acid, can be isolated by solvent extraction, suitably using a solvent which also comprises 1, 1, 1, 2-tetrafluoroethane.
Owner:ADVANCED PHYTONICS OSSET
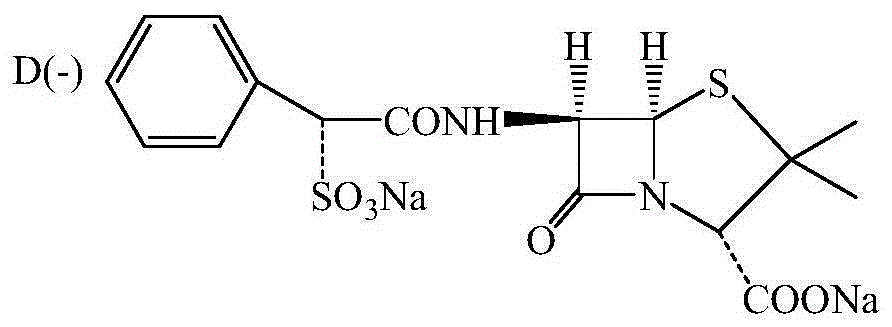
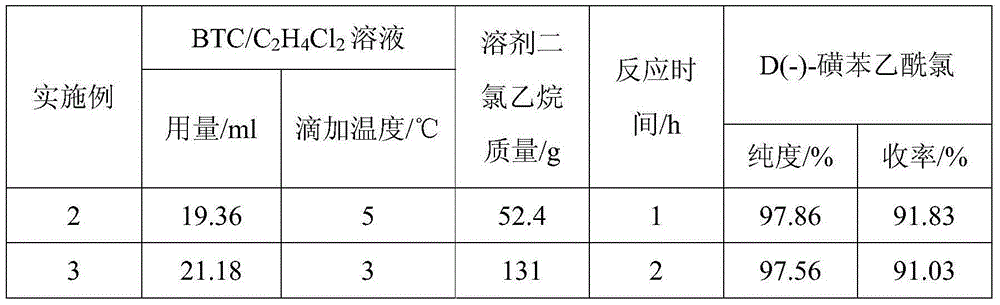
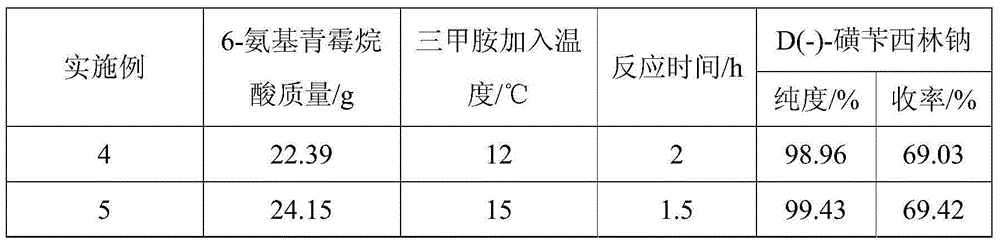
Preparing method for D(-)-sulbenicillin disodium
InactiveCN105218562AImprove reaction efficiencyLess side effectsOrganic chemistryEthane DichlorideSide reaction
The invention provides a preparing method for D(-)-sulbenicillin disodium. The preparing method includes the concrete operation steps that D(-)-sulfophenylacetic acid, dichloroethane and a catalyst dimethylformamide are added into a reaction container, a BTC / C2H4Cl2 solution serves as an acylating chlorination agent, and a dichloroethane solution of D(-)-sulfophenylacetyl chloride is obtained through preparation; D(-)-sulfophenylacetyl chloride and 6-aminopenicillanic acid are prepared into D(-)-sulbenicillin disodium through condensation and refining. The BTC / C2H4Cl2 solution is adopted as the acylating chlorination agent, reaction efficiency is high, the number of side reactions is small, sulfur dioxide pollution is avoided, and the preparing method is of significance in environment-friendly production; acetone and 75% ethyl alcohol are adopted as solvent in the condensation reaction, the pH value is controlled to be seven through trimethylamine, the condensation reaction is carried out at about 10 DEG C, reaction conditions are moderate, the process is simple and easy to operate, the purity and the yield of the obtained D(-)-sulbenicillin disodium are high, and the preparing method is suitable for industrial production.
Owner:ZHEJIANG ESIGMA BIOTECH CO LTD
Tazobactam synthesis method
ActiveCN102643292BSteps to increase monoxidationBlocking affinityOrganic chemistryMetacresolSynthesis methods
The invention discloses a tazobactam synthesis method, which belongs to the technical field of medicines, and includes the steps: firstly, enabling 6,6-dihydropenam sulfoxide acid diphenylmethyl ester serving as raw materials to undergo thermal cracking and chloromethylation reaction to obtain 2beta-chloromethyl penicillanic acid diphenylmethyl ester; secondly, adding oxidizing agent to oxidize the 2beta-chloromethyl penicillanic acid diphenylmethyl ester-1beta-oxide, enabling the oxidized 2beta-chloromethyl penicillanic acid diphenylmethyl ester-1beta-oxide to react with sodium azide to generate 2beta-hydrazoic methyl penicillanic acid diphenylmethyl ester-1beta- oxide, and then generating 2beta-hydrazoic methyl penicillanic acid diphenylmethyl ester-1,1- dioxide by means of oxidization under the action of potassium permanganate and acetic acid; and finally, preparing the tazobactam by means of deprotection under the action of acetylene cyclization and metacresol. Compared with a past 6-APA (aminopenicillanic acid) route, the tazobactam synthesis method has the advantages that the step of sulfur atom single oxidization is added, so that possibility of ring expansion due to affinity of lone pair electrons on a sulfur atom is blocked, and transformation of five-membered ring products to six-membered ring by-products during hydrazoic reaction can be effectively controlled.
Owner:山东安信制药有限公司 +1
Synthesis process of o-chloro-benzathine penicillin
InactiveCN105566349AOvercome serious pollution problemsIncrease conversion rate per passOrganic chemistryEthylenediamineDecomposition
The invention discloses a synthesis process of o-chloro-benzathine penicillin. 6-aminopenicillanic acid is firstly dissolved in a dilute alkali solution to be prepared into sodium salt in an aqueous solution of an organic solvent, then subjected to condensation with isoxazolyl o-chlorobenzoyl chloride to directly obtain a cloxacillin sodium solution, and the cloxacillin sodium solution is subjected to double decomposition reaction with N, N-dibenzyl ethylenediamine diacetate to obtain the o-chloro-benzathine penicillin. The synthesis process of the o-chloro-benzathine penicillin disclosed by the invention has benefits of simple process, simple and convenient operation, low cost and easy industrial production.
Owner:QINGDAO SHOUTAI AGRI SCI & TECH CO LTD
Preparation method of 6-aminopenicillanic acid
InactiveCN105567778AHigh catalytic efficiencyIncrease productivityOrganic chemistryFermentationMedicinal chemistryPenicillin Acylase
The invention discloses a method for preparing 6-aminopenicillanic acid, a penicillin acylase activator and application thereof in preparing the 6-aminopenicillanic acid. The penicillin acylase activator is a compound with a novel structure separated from a dry mature fruit of citrus chirocarpus, the compound is first reported, has an effect for improving the transforming activity of the penicillin acylase and can be used for preparing the 6-aminopenicillanic acid. A test result shows that after the penicillin acylase activator is added, not only can the yield of the 6-aminopenicillanic acid (6-APA) be increased, but also the pyrolysis time can be remarkably shortened, and the production efficiency is three times of that when the penicillin acylase activator is not added; therefore, the penicillin acylase activator can remarkably improve the transforming activity of the penicillin acylase and has prominent substantive characteristics and remarkable progress compared to the prior art.
Owner:QINGDAO MUNICIPAL HOSPITAL
Synthesis method of sulbactam acid
The invention relates to the field of medicine synthesis and provides a synthesis method of sulbactam acid, aiming at the problem of low yield of a traditional synthesis manner. The synthesis method comprises the following steps: S1, carrying out diazotization and bromination reaction: adding bromine, a dilute sulfuric acid solution and a sodium nitrite solid into 6-aminopenicillanic acid, whereinthe concentration of the dilute sulfuric acid solution is 20 to 25 percent; reacting to obtain a first intermediate; S2, carrying out oxidization reaction: dropwise adding potassium permanganate andthe dilute sulfuric acid solution into the first intermediate, wherein the concentration of the dilute sulfuric acid solution is 20 to 25 percent; S3, carrying out hydrogenation reaction: dropwise adding zinc powder and the dilute sulfuric acid solution into a second intermediate and reacting to obtain the sulbactam acid. The dilute sulfuric acid solution with the concentration of 20 to 25 percentis used for reacting, which facilitates the improvement of the activity of the diazotization and bromination reaction and the oxidization reaction, and reactants are enabled to react more completely,so that the improvement of the yield of the diazotization and bromination reaction and the oxidization reaction is facilitated, and the total yield of the reaction is further improved.
Owner:常州红太阳药业有限公司
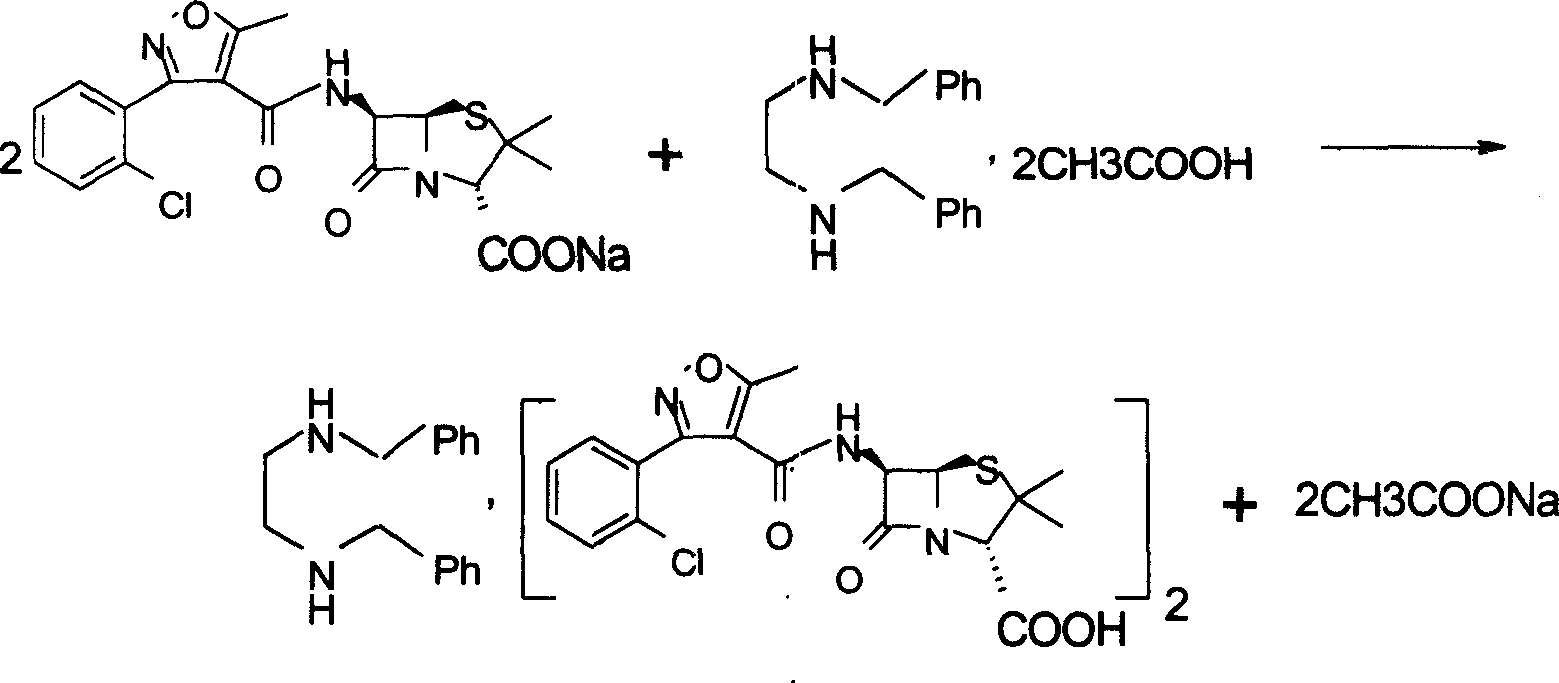
Method for compounding flucloxacillin sodium-hydrate
ActiveCN104910183AReduce pollutionReduce the use effectOrganic chemistryAcylationInorganic chemistry
The invention discloses a method for compounding flucloxacillin sodium-hydrate, which belongs to the technical field of drug synthesis, and comprises the steps: 6-aminopenicillanic acid (6-APA) is salified, then 3-(2-chloro-6-fluorophenyl)-5- methyl isoxazole-4-formyl chloride or equivalents thereof are added to do an acylation reaction, and then acids are added drop by drop to adjust a potential of hydrogen (pH) value to obtain flucloxacillin acid aqueous solutions. Organic solution is used to extract, and organic phases are washed, dried and filtered to obtain flucloxacillin acid solutions through saturated salt water, then white solids are dissolved out in the flucloxacillin acid solutions which are added with sodium iso-octoate solutions, and products are obtained by controlling temperature and crystallizing. The method for compounding the flucloxacillin sodium-hydrate does not separate intermediate flucloxacillin acids, obtained flucloxacillin acids are directly salified with sodium iso-octoate after being extracted trough the organic solution, reduces separation steps and operation process, also reduces usage amount and times of organic solution simultaneously, greatly reduces discharge amount of organic solution relative to patent documentation CN 102964356A, reduces production cost above 20%, and obviously improves economic and environmental values.
Owner:CHENGDU LIKAI CHIRAL TECH
Penicillin V acylase mutant, encoding array, recombinant expression vector, genetically engineered bacterium and application
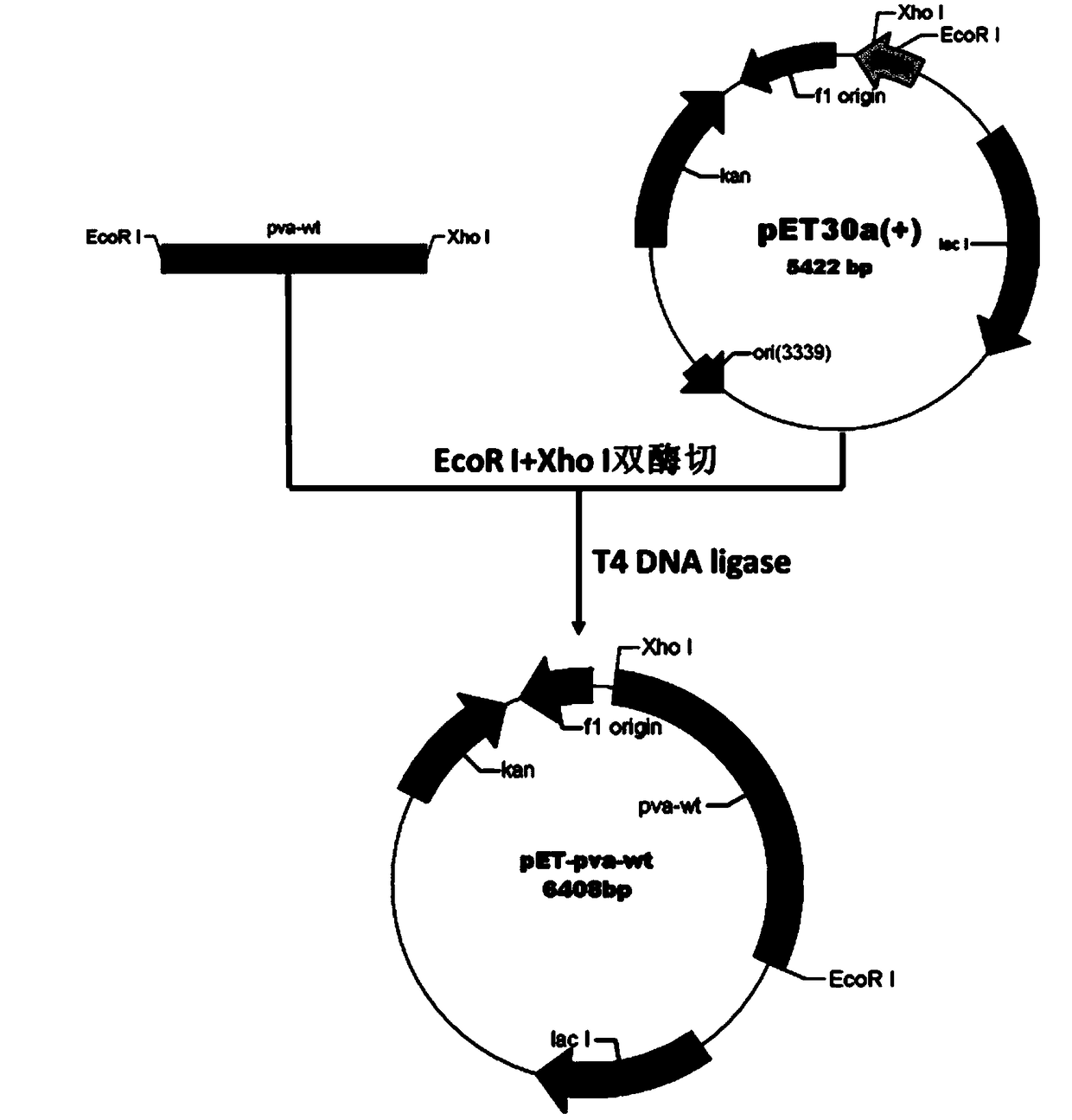
ActiveCN109161540AHigh catalytic activityRapid catalytic hydrolysisHydrolasesOn/in organic carrierContinuous useMutant
The invention provides a penicillin V acylase mutant, an encoding array, a recombinant expression vector, a genetically engineered bacterium and application. Penicillin V acylase derived from Bacillussphaericus is mutated, the specific activity of the obtained mutant PVA-3 is improved by nine times, the immobilization activity is improved to 820U / g from 92U / g, and after being incubated for one hour under a condition of 30% penicillin V sylvite, the mutant has activity residue of 89.1%; the reaction time that the immobilized mutant PVA-3 is adopted to prepare 6-APA (6-Aminopenicillanic Acid) by splitting 25% penicillin V under a condition that the pH value is 6.0 at 25 DEG C is shortened to 60 minutes from 180 minutes, a substrate conversion rate is increased to 99.5% or greater, the activity is not greatly lost after continuous use for 600 times or greater, and good operation stability can be achieved. By adopting the mutated PVA-3, the production cost can be greatly reduced, the production efficiency can be improved, and the mutant is applicable to industrial application.
Owner:HUNAN FLAG BIOTECHNOLOGY CO LTD
Synthesizing method of sulbactam acid
The invention discloses a synthesizing method of sulbactam acid. The method comprises the steps that: (1) under the existence of a strong acid, 6-aminopenicillanic acid, a diazotization reagent, and bromine or bromide are subjected to an insulation reaction in an organic solvent; excessive bromine is reduced; and a reaction product is extracted to a water layer; (2) an oxidant is dropped into the water-layer reaction product, and an insulation reaction is carried out; when the reaction is finished, a pH value is regulated; excessive potassium permanganate is reduced; an organic solvent is added, such that a reaction product is extracted to an organic solvent layer; (3) a catalyst is added into the organic-solvent layer product, such that hydrogenation bromine-removing is carried out; a reaction product is extracted to an organic-solvent layer; and distillation and crystallization are carried out. The organic solvent is ethyl acetate. According to the invention, ethyl acetate is adopted as the organic solvent in a four-step reaction, such that solvent consumption of the whole reaction is greatly reduced, and product total yield is effectively improved. With the method provided by the invention, by-product in the reaction can be comprehensively utilized, and advantages such as high yield, low cost, clean production, and the like are provided.
Owner:LIANYUNGANG HENGFEI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com