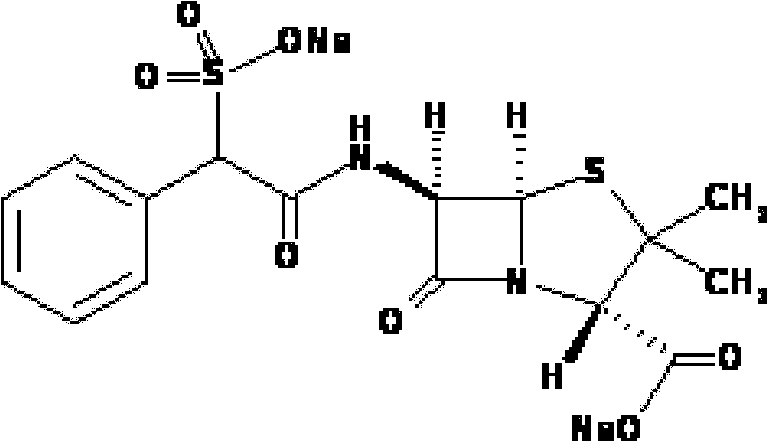
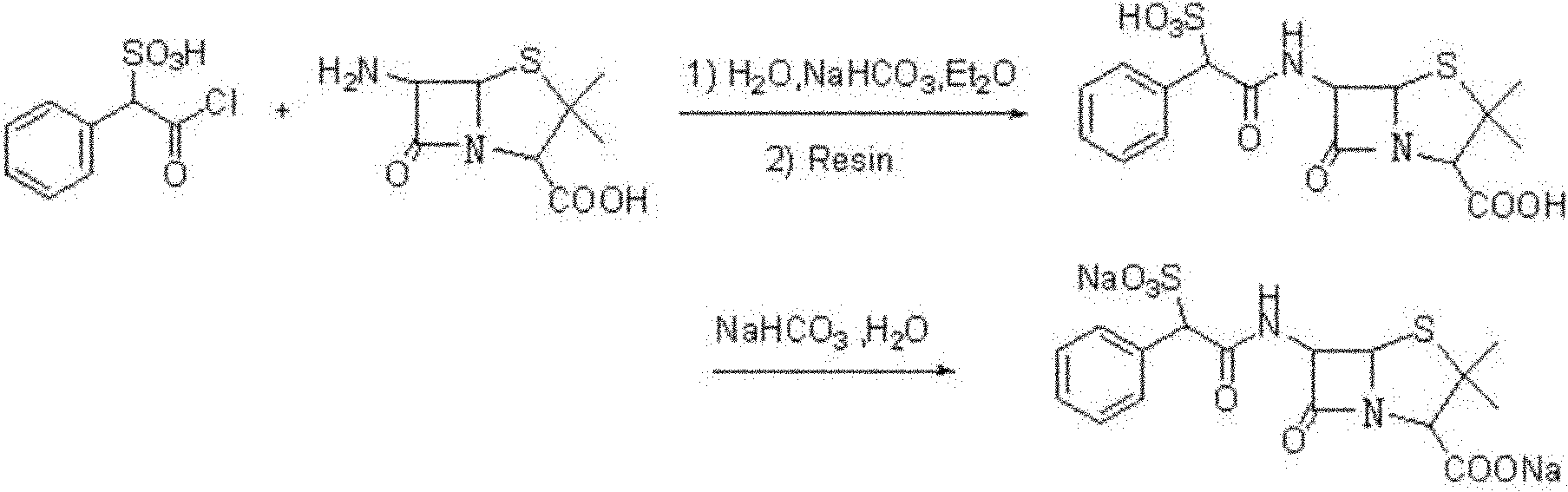Method for preparing sulbenicillin disodium
A technology of sulfobenicillin sodium and sulfophenylacetic acid, applied in directions such as organic chemistry, can solve the problems of harsh operating conditions, unsatisfactory yield, long reaction time, etc., and achieves improved reaction temperature, reduced energy consumption, and high reaction yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Stir and dissolve 52.4g of α-sulfophenylacetic acid in 100mL of ether, add 115mL of thionyl chloride dropwise at -2°C, add dropwise 2.0ml of N,N-diisopropylethylamine, and then The reaction was stirred under low pressure for 1.5 hours. After the reaction was completed, distilled to dryness under reduced pressure, and repeated 2 times to wash the residue after distillation with ether, and then distilled to dryness under reduced pressure to obtain 58.1 g of α-sulfophenylacetyl chloride with a yield of 81.0%. ;
[0038] Add 20.5g of 6-APA into a mixed solvent of 50mL of water, 29mL of ethanol and 10mL of 2-methyltetrahydrofuran, keep the temperature at 15°C, then add dropwise 10% sodium hydroxide solution to adjust the pH to 7.0, and stir at 15°C To the complete dissolution of the solid, keep the temperature and add dropwise the butyl acetate solution containing 31.2g α-sulfophenylacetyl chloride, wherein the butyl acetate solution of α-sulfophenylacetyl chloride is evenly...
Embodiment 2
[0041] Stir and dissolve 52.4g of α-sulfophenylacetic acid in 100mL of ether, add 89mL of thionyl chloride dropwise at -3°C, add dropwise 1.2ml of N,N-diisopropylethylamine, and then Stirring and reacting for 2.5 hours, after the reaction was completed, distilled to dryness under reduced pressure, repeated 2 times to wash the residue after distillation with ether, and then distilled to dryness under reduced pressure to obtain 61.2g α-sulfophenylacetyl chloride, yield 85.4 %;
[0042] Add 20.5g of 6-APA into a mixed solvent of 50mL of water, 25mL of ethanol and 12.5mL of 2-methyltetrahydrofuran, keep the temperature at 25°C, and then add dropwise a 15% sodium hydroxide solution to adjust the pH to 6.0, at 25°C Stir until the solid is completely dissolved, and then add dropwise a butyl acetate solution containing 34.0 g of α-sulfophenylacetyl chloride at the temperature. After the dropwise addition, maintain the pH of the reaction solution at 6.0, and react at room temperature f...
Embodiment 3
[0045] Stir and dissolve 52.4g of α-sulfophenylacetic acid in 100mL of ether, add 160mL of thionyl chloride dropwise at -5°C, add dropwise 1.6ml of N,N-diisopropylethylamine, and then Stirring and reacting for 2.0 hours, after the reaction was completed, distilled under reduced pressure to dryness, repeated 2 times to wash the residue after distillation with ether, and then distilled under reduced pressure to dryness to obtain 59.2g α-sulfophenylacetyl chloride, yield 82.6 %;
[0046] Add 20.5g of 6-APA into a mixed solvent of 50mL of water, 34mL of ethanol and 8.5mL of 2-methyltetrahydrofuran, keep the temperature at 20°C, and then add dropwise a 10% sodium hydroxide solution to adjust the pH to 5.6, at 20°C Stir until the solid is completely dissolved, then add dropwise a butyl acetate solution containing 30.0 g of α-sulfophenylacetyl chloride at this temperature, after the dropwise addition, maintain the pH of the reaction solution at 5.6, and react at room temperature for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com