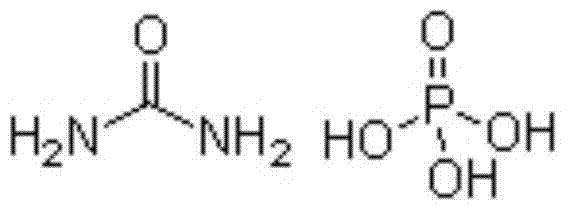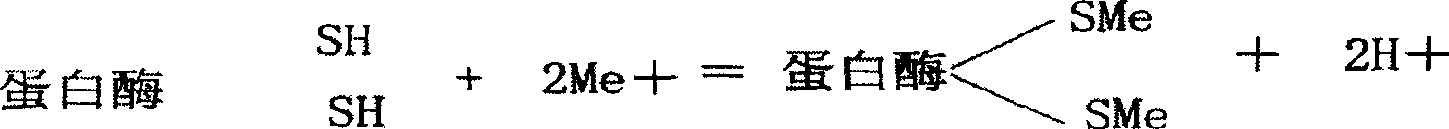Patents
Literature
41473 results about "Sodium hydroxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na⁺ and hydroxide anions OH⁻. Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burns. It is highly soluble in water, and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates NaOH·nH₂O. The monohydrate NaOH·H₂O crystallizes from water solutions between 12.3 and 61.8 °C. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, it is frequently utilized alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students.
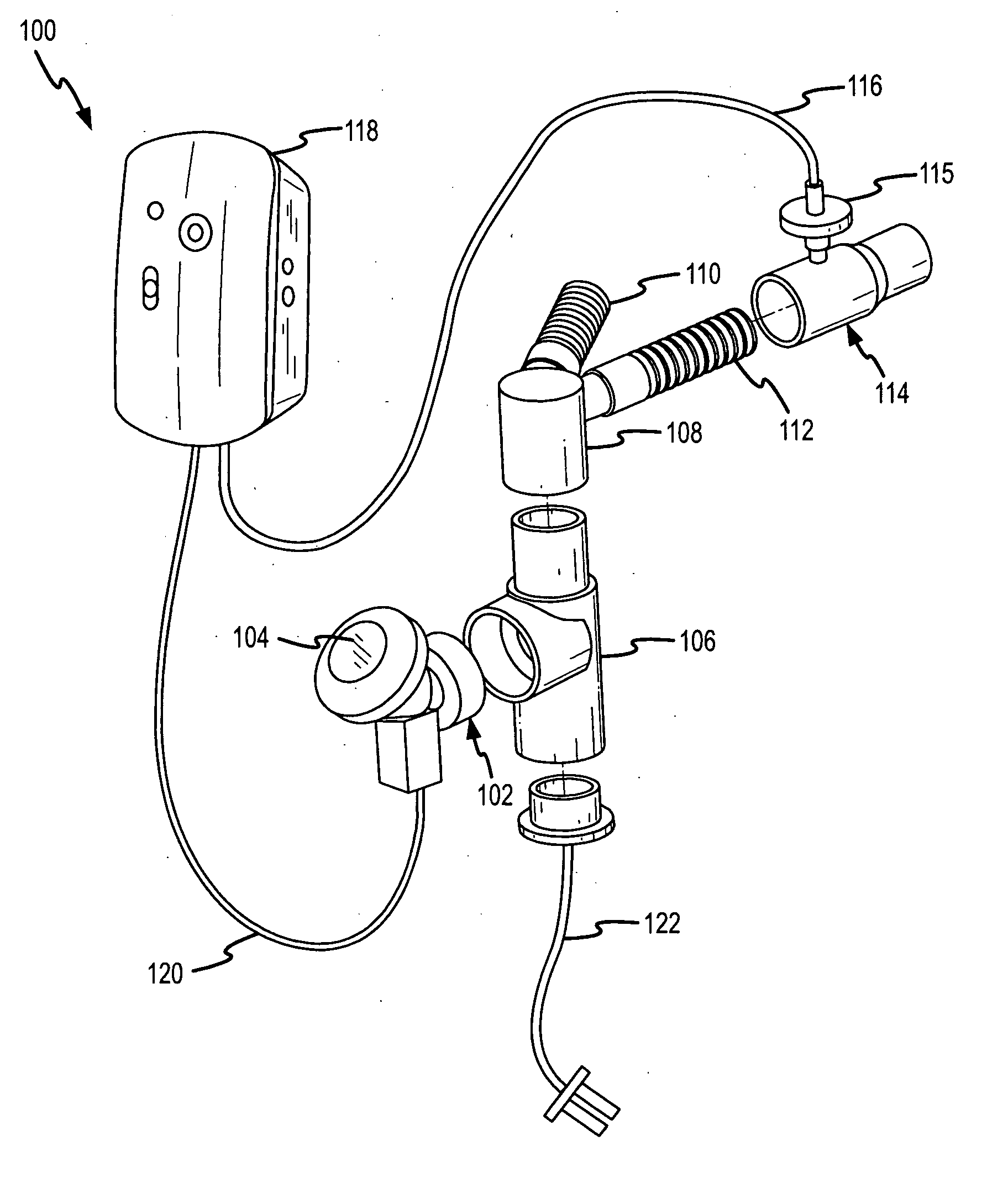
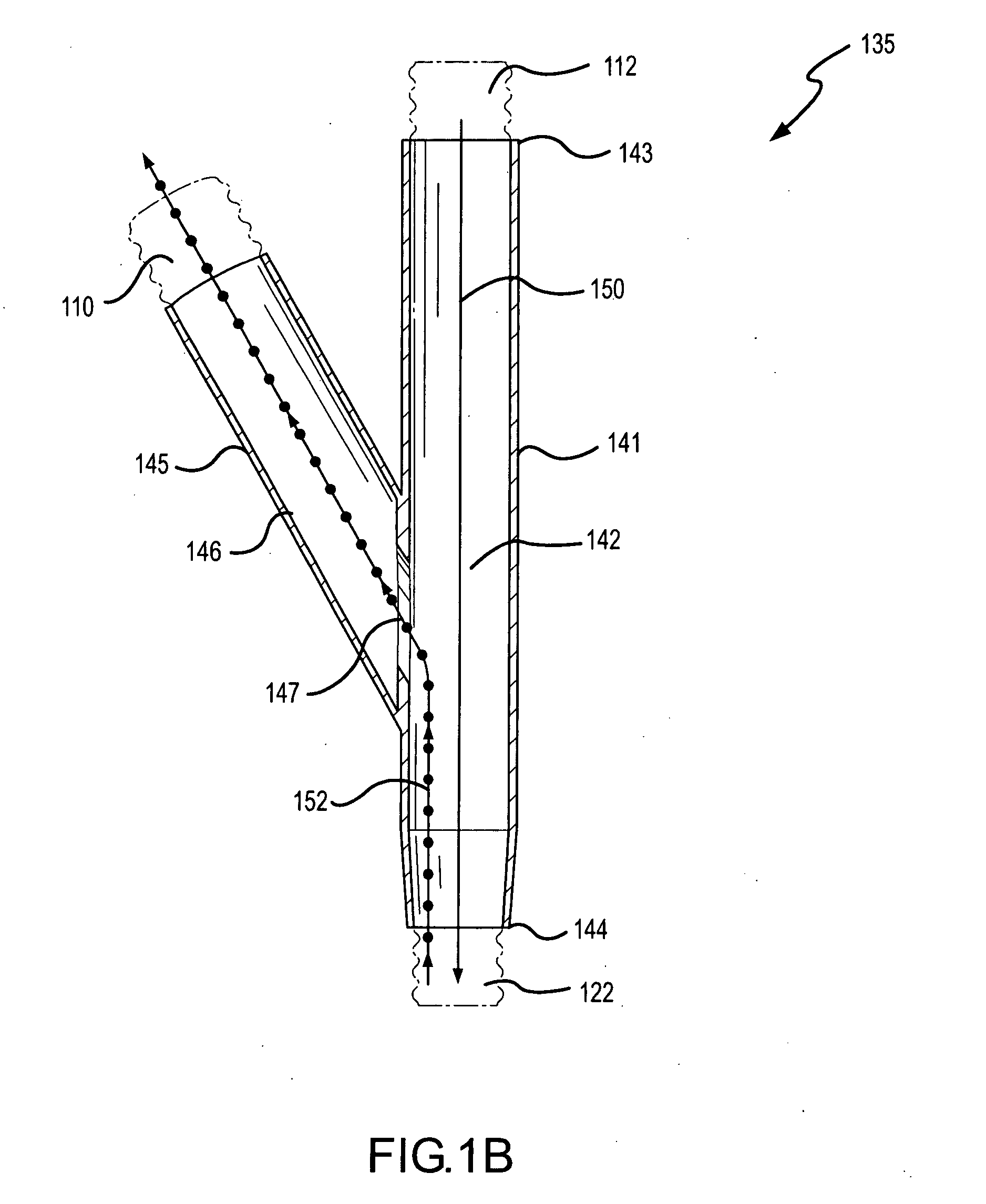
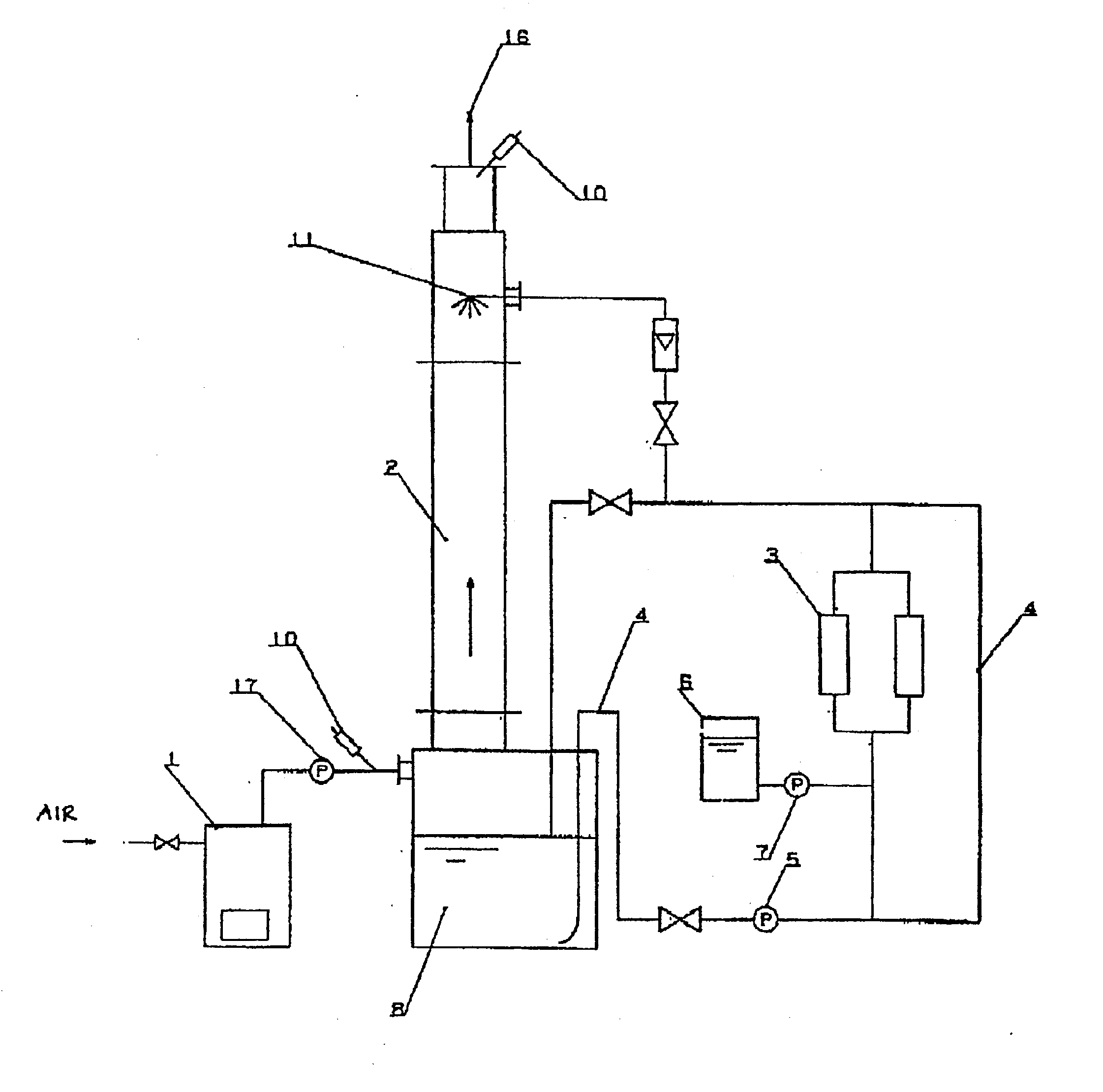
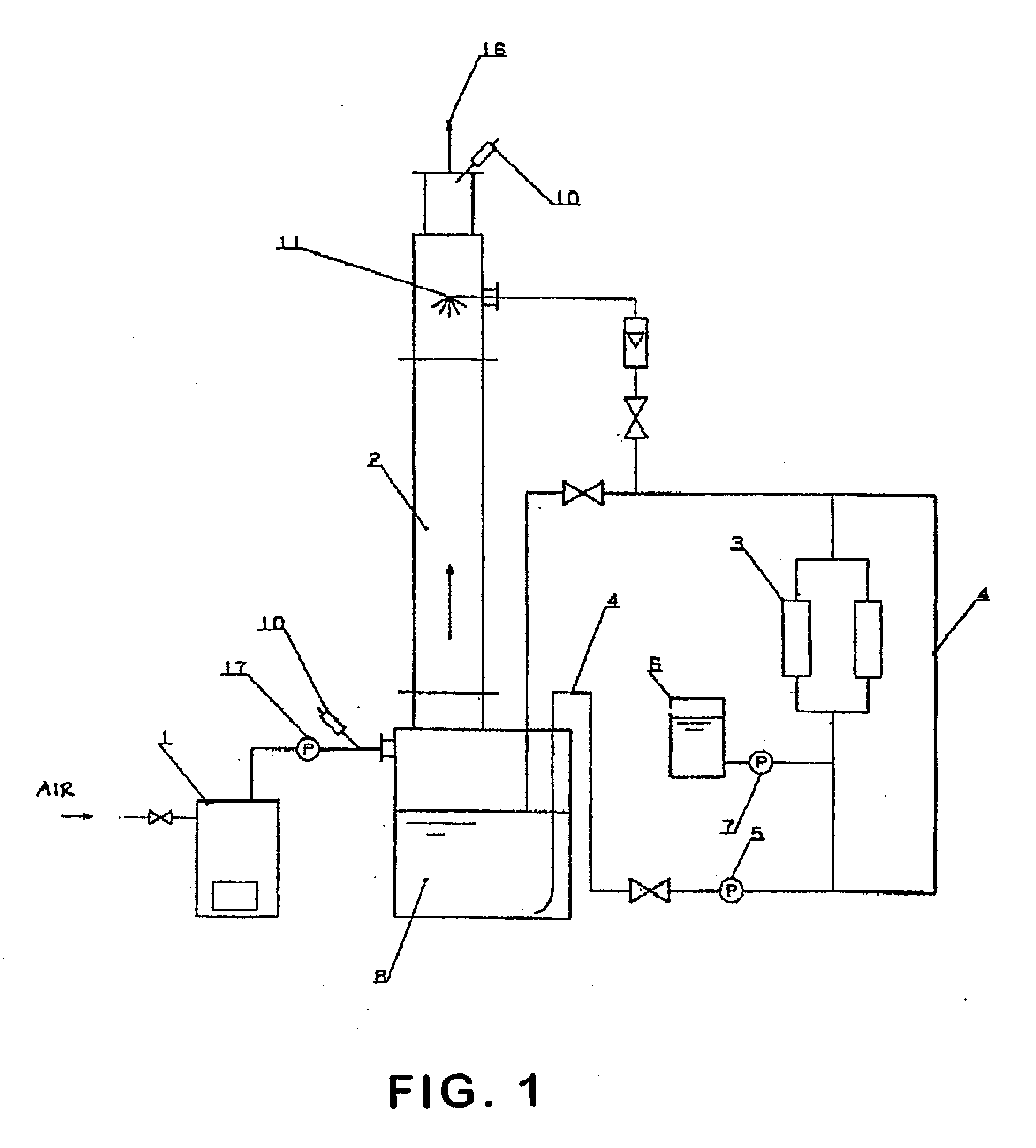
Methods and systems for operating an aerosol generator
InactiveUS20050217666A1Improve security levelImprove delivery efficiencyAntibacterial agentsOrganic active ingredientsDiseaseAmikacin
A method of treating a patient with a pulmonary disease, where the method includes delivering a dose of aerosolized medicament intermittently to a ventilator circuit coupled to the respiratory system of the patient. Also, a method of treating a patient with a pulmonary disease, where the method includes taking the patient off a ventilator, and administering to the patient, a nebulized aerosol comprising from about 100 μg to about 500 mg of a medicament. Additionally, an aerosolized medicament for the treatment of a pulmonary disease, where the medicament includes amikacin mixed with an aqueous solution having an adjusted pH from about 5.5 to about 6.3. The pH is adjusted by adding hydrochloric acid and sodium hydroxide to the aqueous solution.
Owner:NOVARTIS AG
Method for preparing lithium cobaltate by directly using invalid lithium ion battery
InactiveCN102030375AReduce dispersionHigh purityCell electrodesCobalt compoundsElectrical batteryPotassium hydroxide
The invention provides a method for preparing lithium cobaltate by directly using an invalid lithium ion battery. The method comprises the following steps: crushing the invalid lithium ion battery or scraps generated when a lithium cobaltate battery is produced by a mechanical crusher at normal temperature; adding water and one or more of acetic acid, sulfuric acid, hydrochloric acid or nitric acid to produce mixed aqueous solution of the battery scraps and acid; filling the mixed aqueous solution into a hermetic pressure reactor, and controlling the temperature in the reactor to be between 50 and 150 DEG C; introducing or adding one leaching additive of sulfur dioxide or hydrogen, or adding hydrazine hydrate; stirring and leaching, cooling, and filtering; adding one precipitator of sodium carbonate, potassium carbonate and ammonium carbonate, or adding composite precipitator consisting of one of the sodium carbonate, the potassium carbonate and the ammonium carbonate and one of sodium hydroxide and potassium hydroxide to obtain mixture of lithium carbonate, cobalt carbonate and cobalt hydroxide; drying and calcining at high temperature to produce a lithium cobaltate product. The method is particularly suitable for the treatment scale of medium-sized and small enterprises, and is an effective method for directly materializing cobalt secondary resources.
Owner:BEIJING GENERAL RES INST OF MINING & METALLURGY
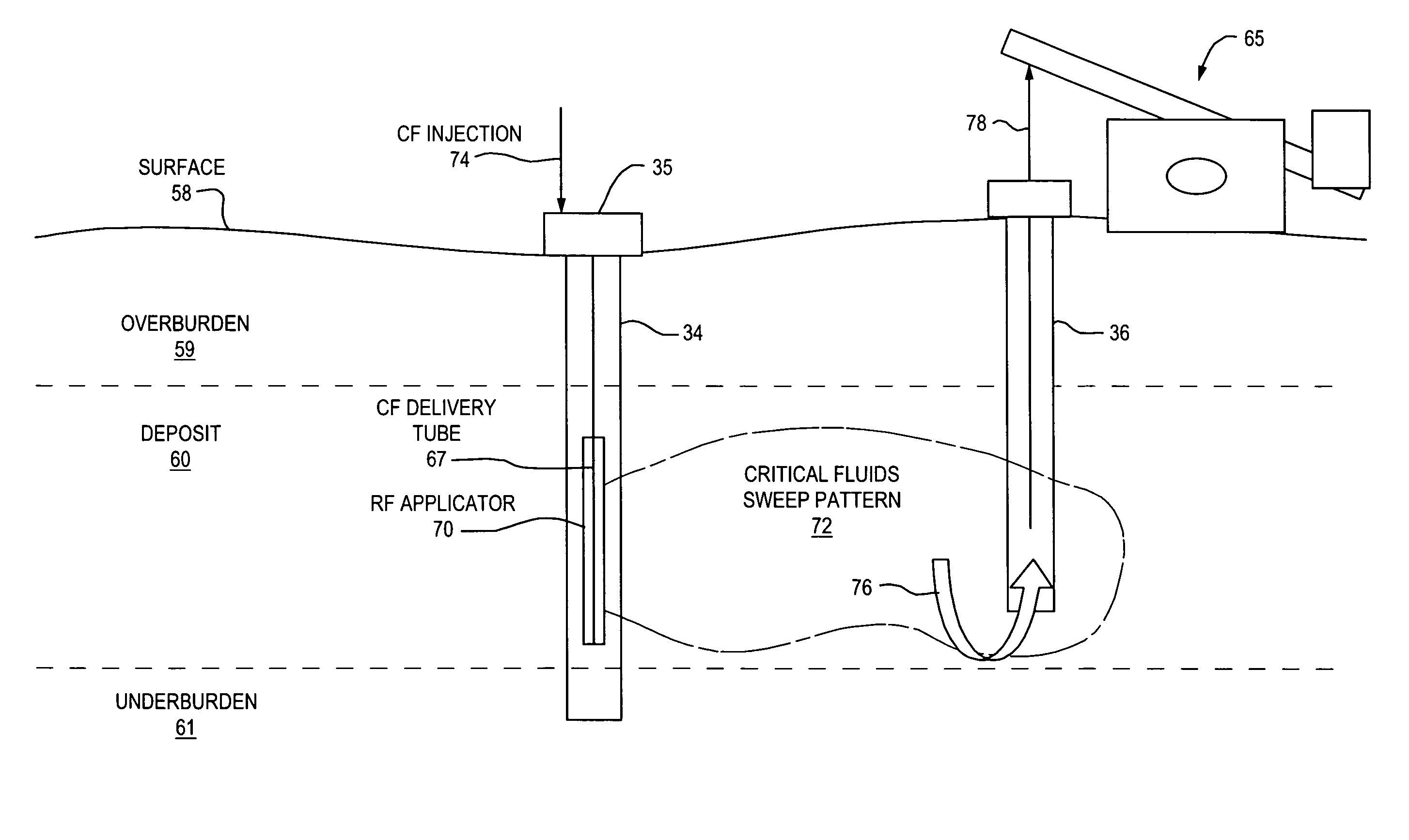
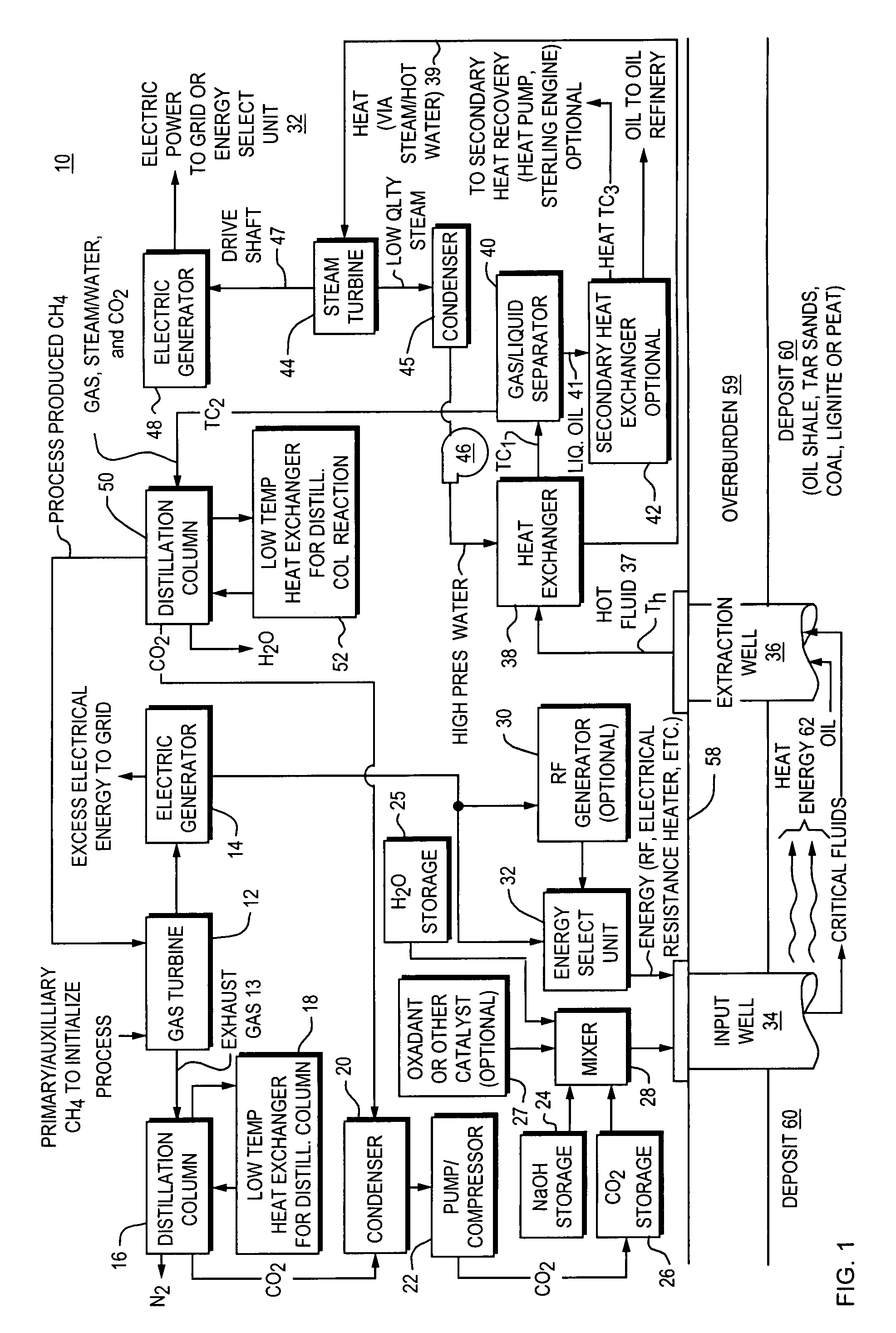
Method and apparatus for capture and sequester of carbon dioxide and extraction of energy from large land masses during and after extraction of hydrocarbon fuels or contaminants using energy and critical fluids
ActiveUS7562708B2Reduce energy consumptionMinimal pollutionSurveyOther gas emission reduction technologiesClosed loopUltra fine
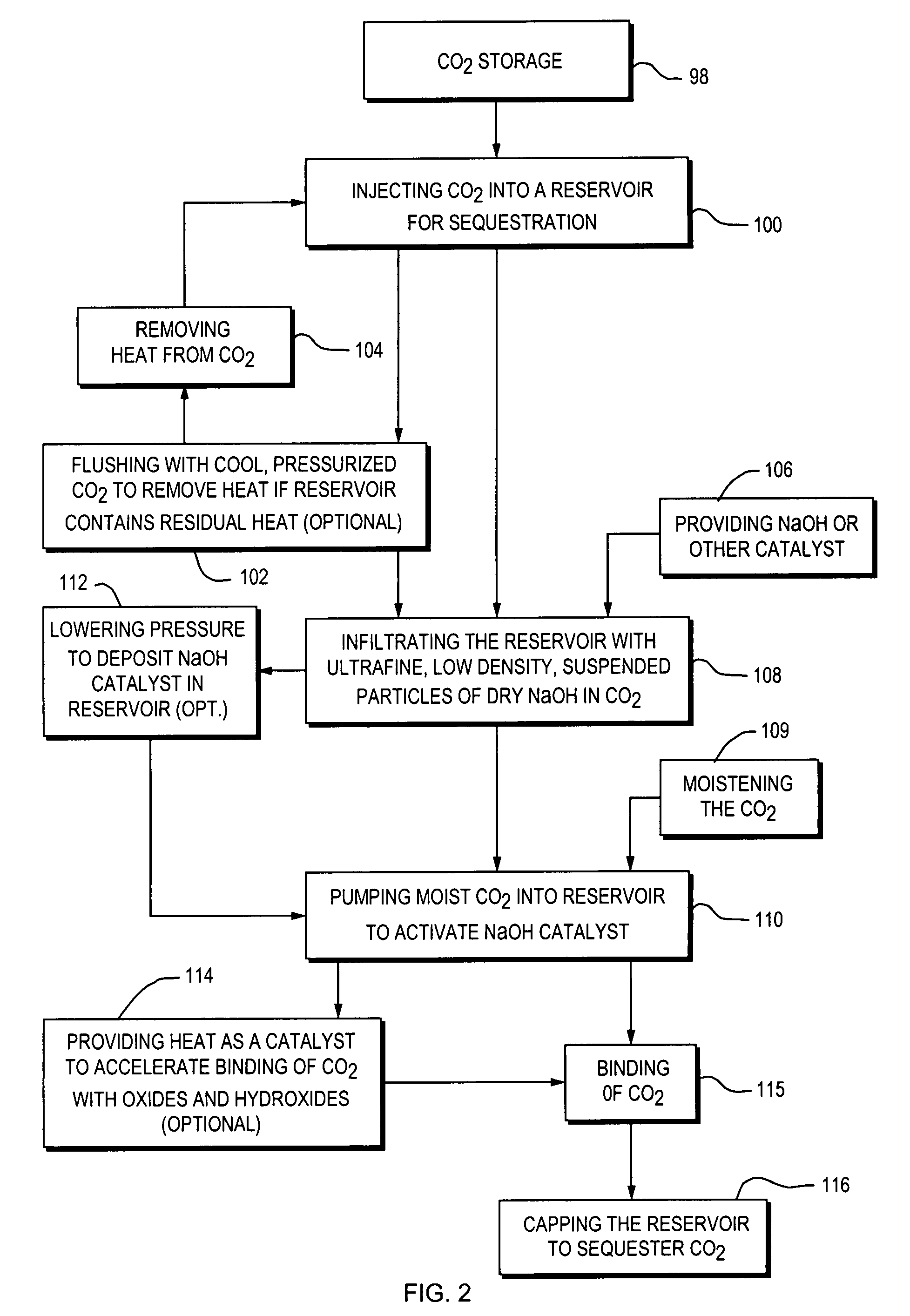
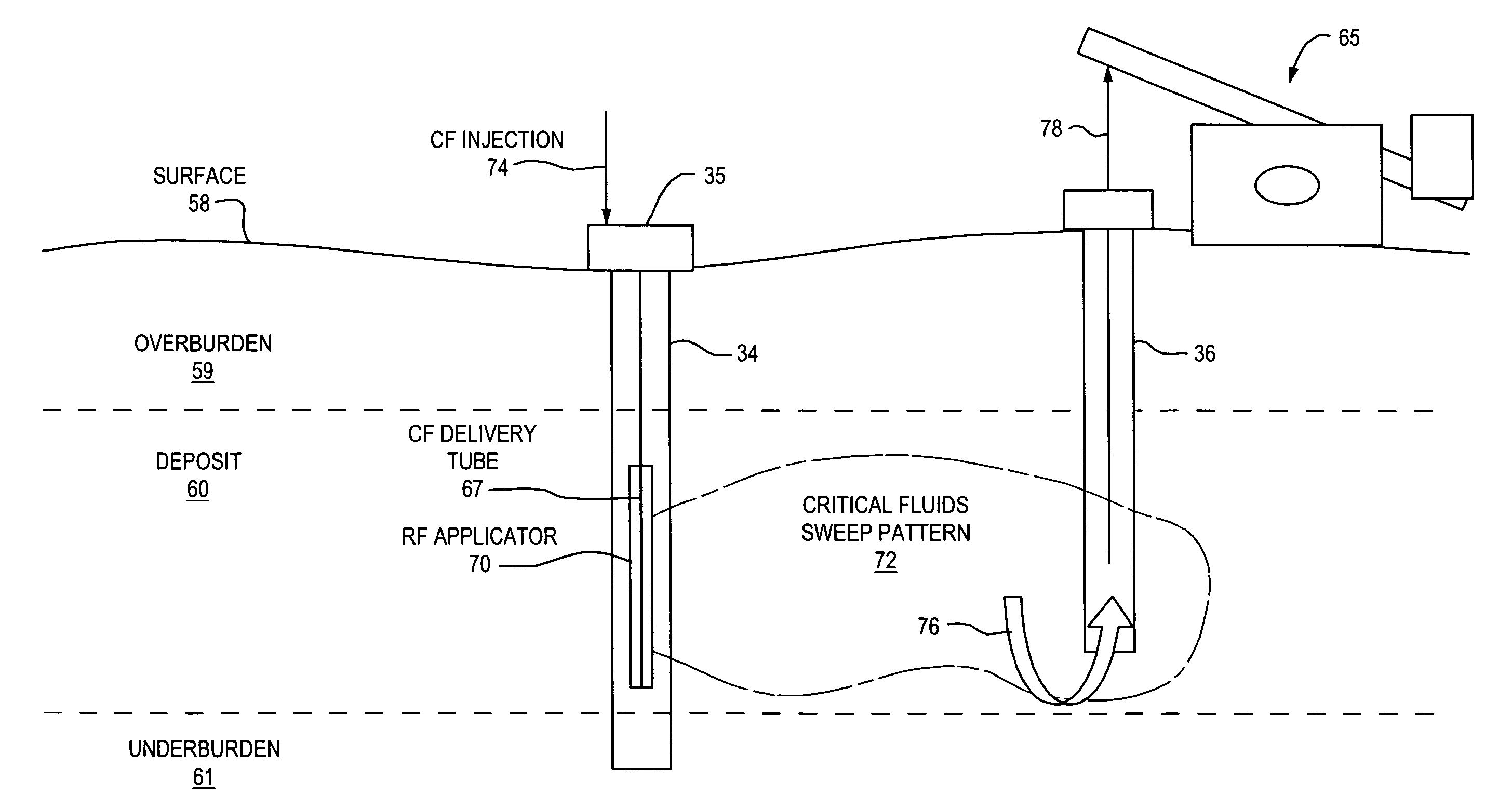
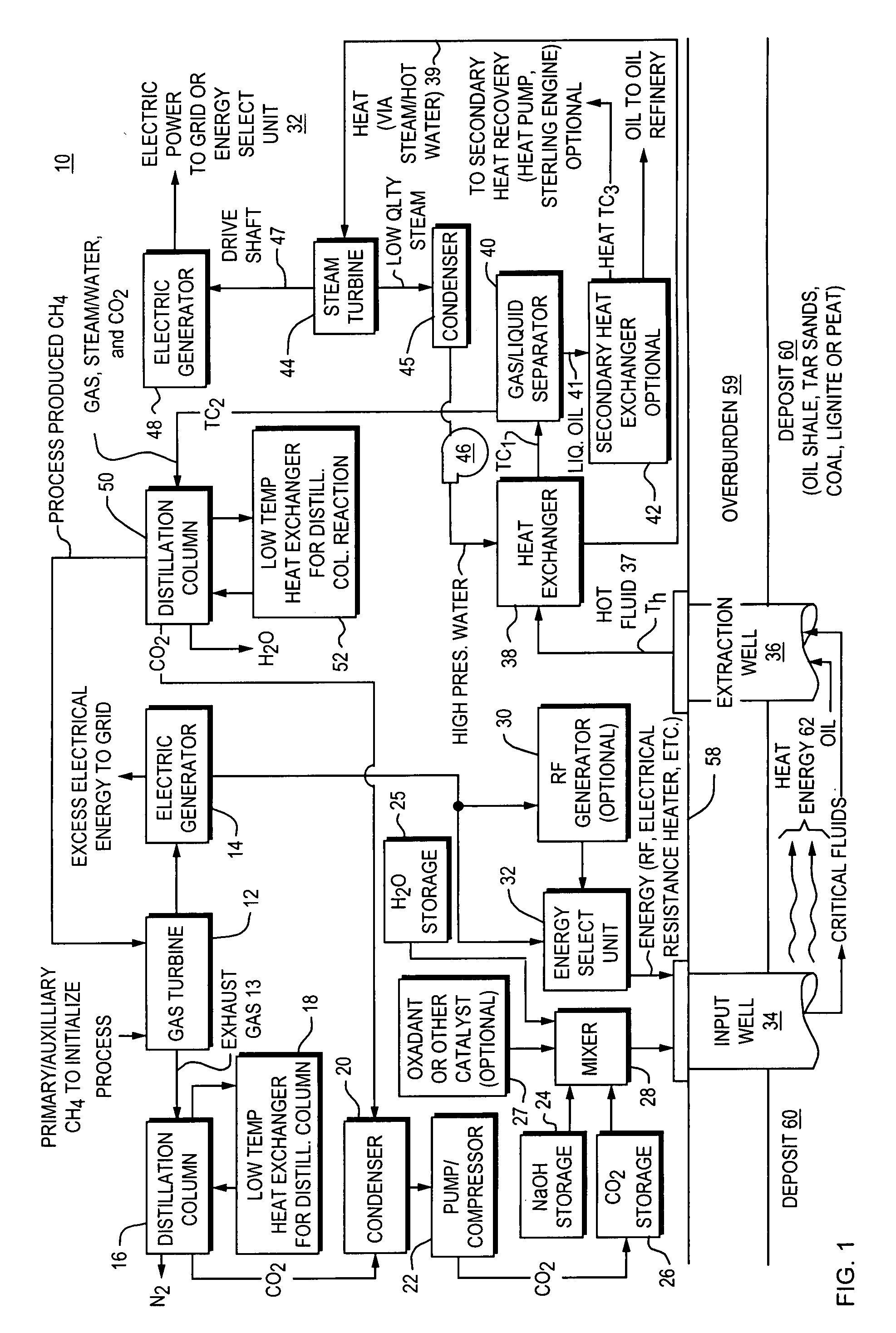
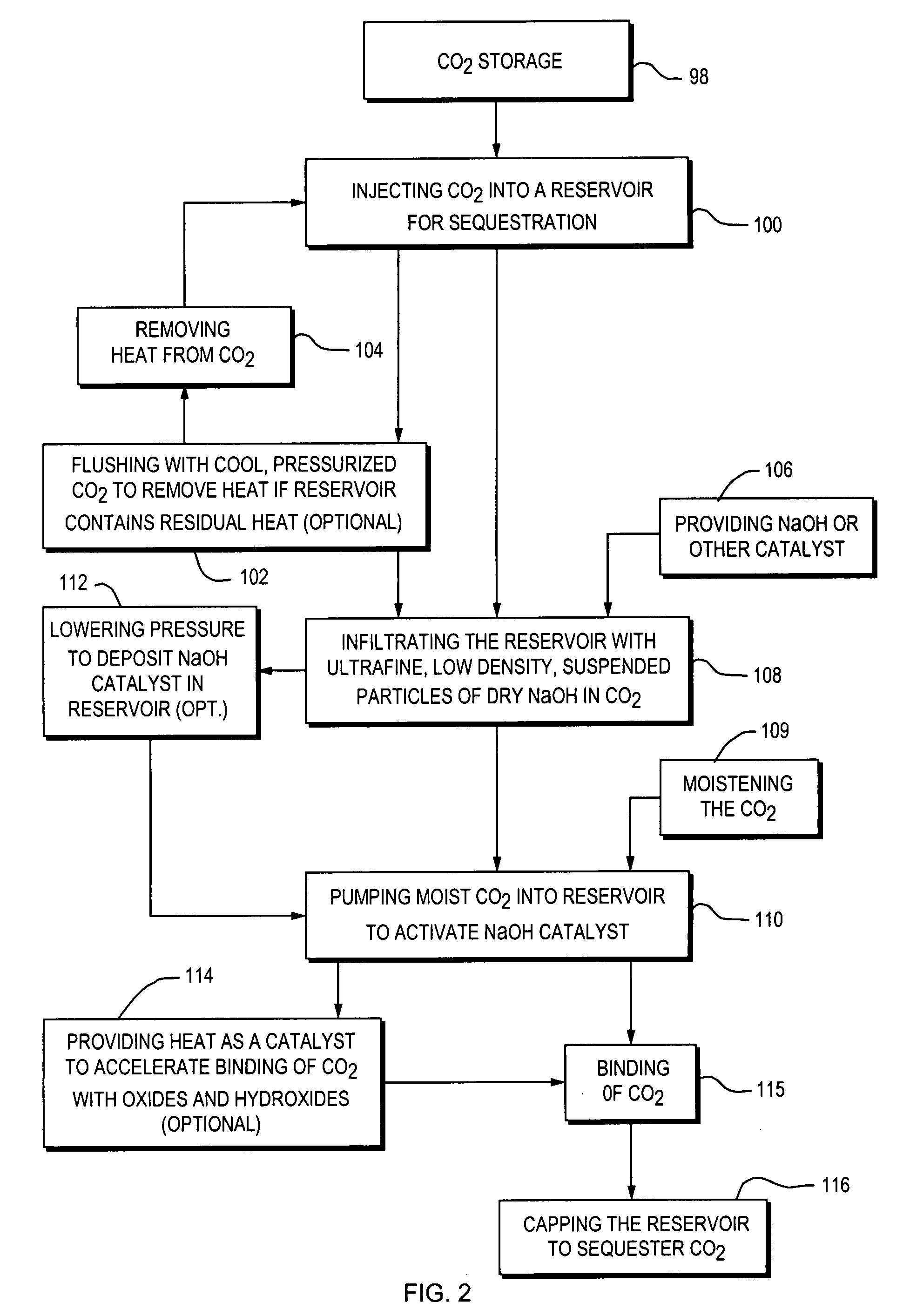
A closed loop system for increasing yield, reducing post process pollution, reducing energy consumed during and after extraction of fuels or contaminants in formations and for sequestering of carbon dioxide C02 from various sources is converted to a critical fluid for use as a flushing and cooling medium. Electrical energy heats a hydrocarbon rich formation resulting in the extraction of hot fluids which are fed to heat exchangers, gas / liquid separator, and steam turbine whereby oil, electric power, carbon dioxide and methane are produced for reuse in the system or for external use. Further, a method for sequestering of carbon dioxide in a formation comprises the steps of injecting CO2 into the reservoir, flushing with cool pressurized CO2 for heat removal, infiltrating with ultra-fine low density suspended catalyst particles of dry sodium hydroxide in CO2, pumping water moistened CO2 into the reservoir to activate the catalysts, binding the CO2 with reacting materials and capping the reservoir.
Owner:RAYTHEON CO
Method and apparatus for capture and sequester of carbon dioxide and extraction of energy from large land masses during and after extraction of hydrocarbon fuels or contaminants using energy and critical fluids
ActiveUS20070261844A1Weaken energyReducing critical fluid requirementSurveyOther gas emission reduction technologiesClosed loopUltra fine
A closed loop system for increasing yield, reducing post process pollution, reducing energy consumed during and after extraction of fuels or contaminants in formations and for sequestering of carbon dioxide C02 from various sources is converted to a critical fluid for use as a flushing and cooling medium. Electrical energy heats a hydrocarbon rich formation resulting in the extraction of hot fluids which are fed to heat exchangers, gas / liquid separator, and steam turbine whereby oil, electric power, carbon dioxide and methane are produced for reuse in the system or for external use. Further, a method for sequestering of carbon dioxide in a formation comprises the steps of injecting CO2 into the reservoir, flushing with cool pressurized CO2 for heat removal, infiltrating with ultra-fine low density suspended catalyst particles of dry sodium hydroxide in CO2, pumping water moistened CO2 into the reservoir to activate the catalysts, binding the CO2 with reacting materials and capping the reservoir.
Owner:RAYTHEON CO
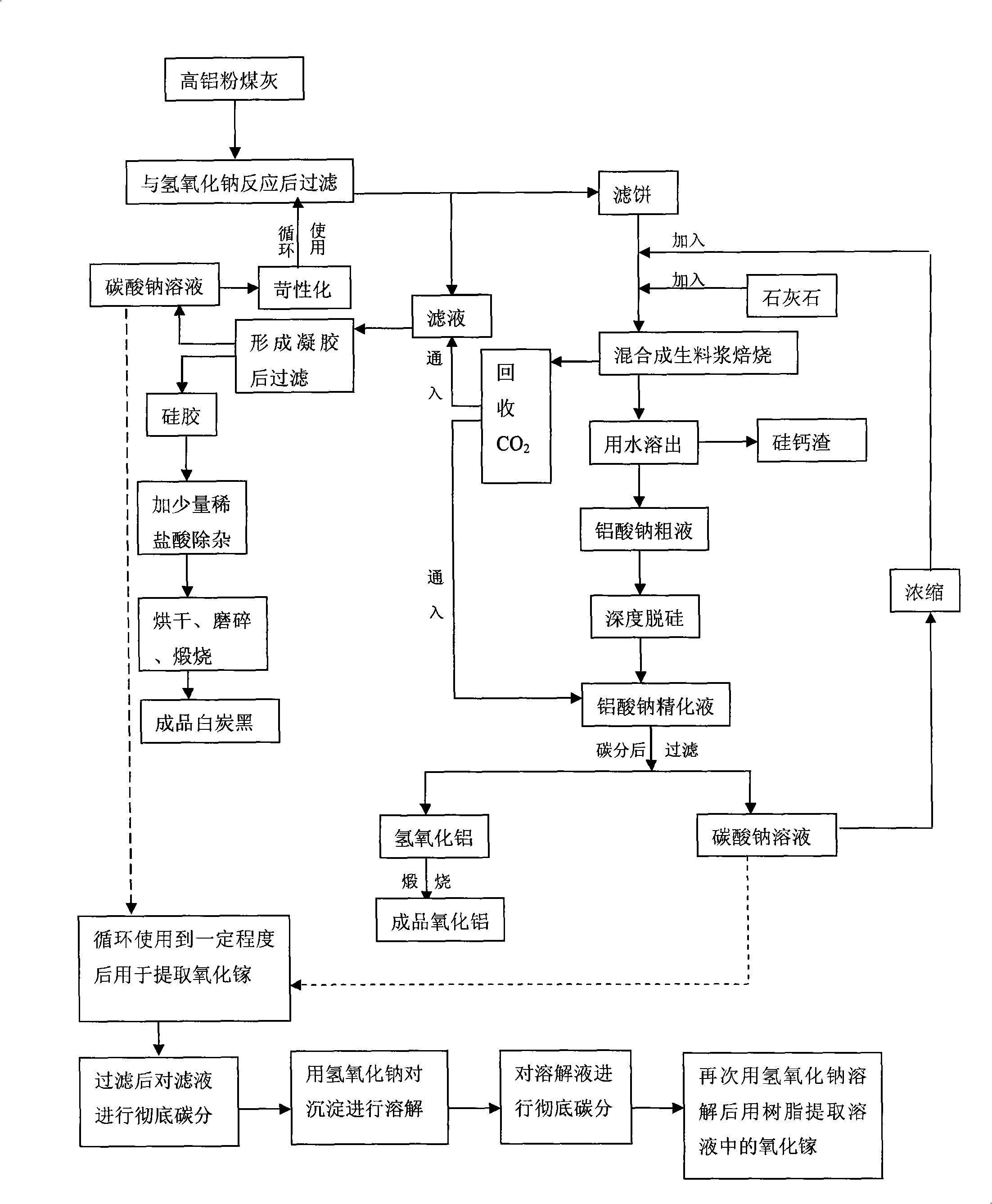
Process for abstracting earth silicon, oxide of alumina and gallium oxide from high-alumina flying ash
ActiveCN101284668AReduce the total massLow firing temperatureGallium/indium/thallium compoundsSilicon oxidesChemical industryFiltration
A method for extracting silicon dioxide, alumina and gallium oxide from high-alumina fly ash relates to the technology fields of environmental mineralogy and material, chemical industry and metallurgy. The method comprises the main steps as follows: causing the high-alumina fly ash to react with sodium hydroxide solution; filtering the solution; introducing CO2 to the filtrate for full gelation; cleaning, purifying, drying, grinding and calcining the silica gel after gel filtration to obtain finished white carbon black; adding limestone and a sodium carbonate solution into the filter mass after the reaction and filtration of the high-alumina fly ash and the sodium hydroxide solution; ball grinding the mixture into raw slurry; dissolving out the clinker obtained by baking the raw slurry; subjecting the filtrate to deep desiliconization to obtain sodium aluminate extraction liquid; filtrating the sodium aluminate extraction liquid after subjecting the sodium aluminate extraction liquid to carbon dioxide decomposition; baking the aluminum hydroxide after washing the filter mass to form the aluminum hydroxide product; and extracting the gallium oxide from the carbon dioxide decomposition mother solution and desiliconized solution. The method has the advantages of low material price, simple operating procedures, low investment, low production cost, low energy consumption and less slag.
Owner:TSINGHUA UNIV +1
Lithium ion battery positive pole material cobalt nickel oxide manganses lithium and method for making same
ActiveCN101202343AHigh specific capacityExcellent cycle characteristicsElectrode manufacturing processesLithium compoundsLithium oxideAntioxidant
The invention relates to a nickel cobalt manganese lithium oxide material used for an anode of a li-ion battery and a preparation method. The invention belongs to the li-ion battery technical field. The nickel cobalt manganese lithium oxide material used for the anode of the li-ion battery is a li-rich laminated structure with the chemical component of Li1+zM1-x-yNixCoyO2; wherein, z is less than or equal to 0.2 and more than or equal to 0.05, x is less than or equal to 0.8 and more than 0.1, and y is less than or equal to 0.5 and more than 0.1. The preparation method of the invention is that dissoluble salt of the nickel, cobalt and manganese is taken as the raw material; ammonia or ammonium salt is taken as complexing agent; sodium hydroxide is taken as precipitator; water-dissoluble dispersant and water-dissoluble antioxidant or inert gas are added for control and protection; in a cocurrent flow type the solution is added to a reaction vessel for reaction; after alkalescence disposal, aging procedure, solid-liquid separation and washing and drying, the nickel cobalt manganese oxide is uniformly mixed with the lithium raw material; the nickel cobalt manganese lithium oxide powder is obtained by sintering the mixed powder which is divided into three temperature areas. The invention has the advantages of high specific capacity, good circulation performance, ideal crystal texture, short production period, low power loss, and being suitable for industrial production, etc.
Owner:CHINA ELECTRONIC TECH GRP CORP NO 18 RES INST +1
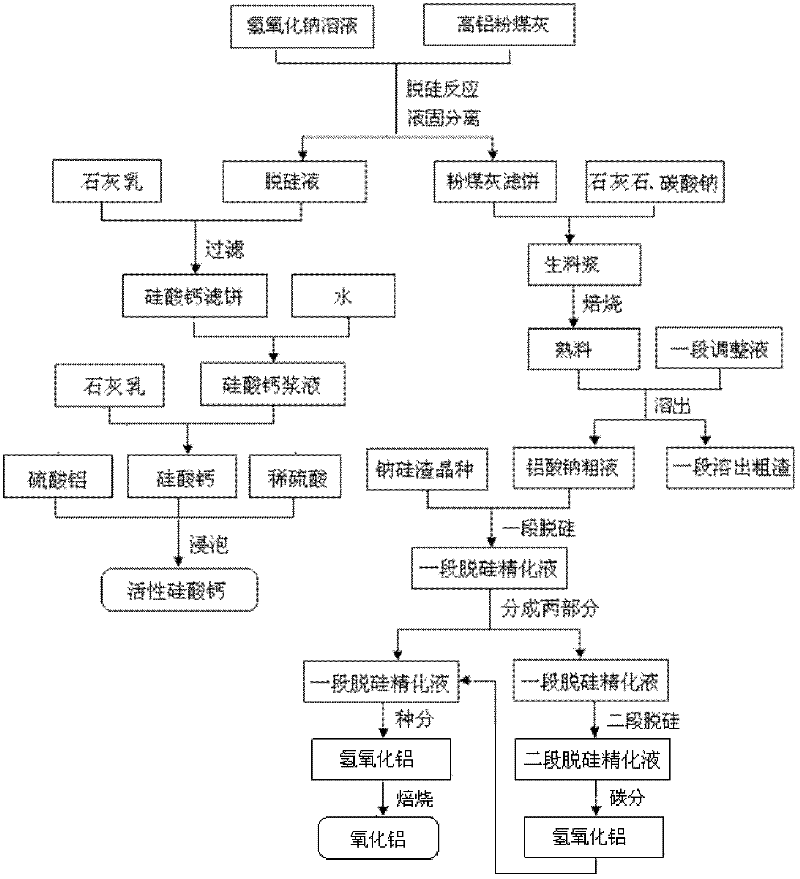
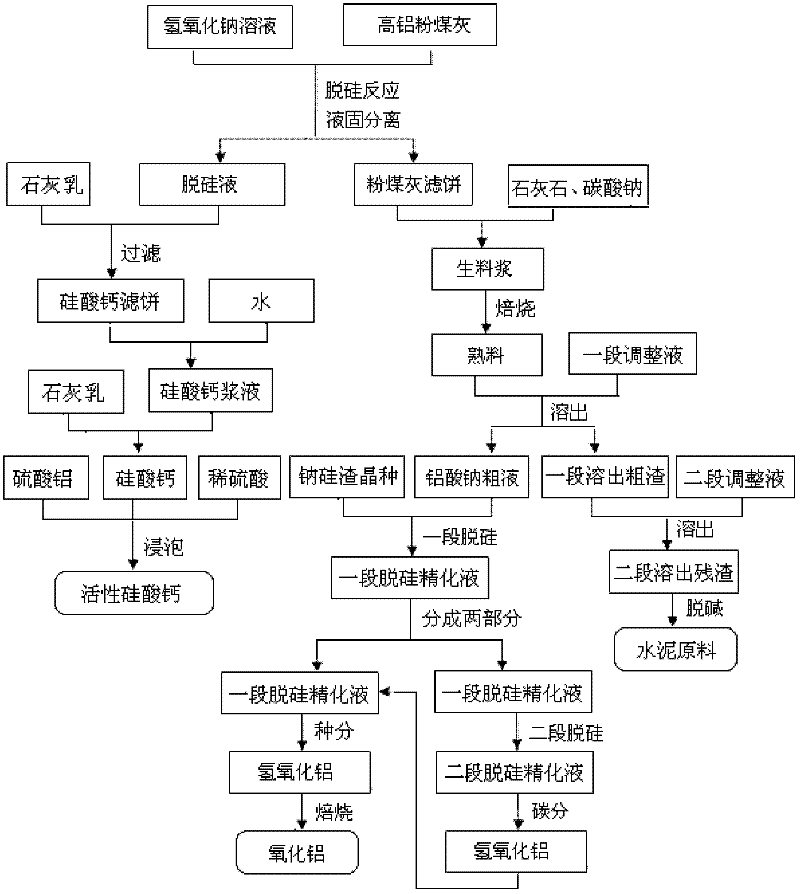
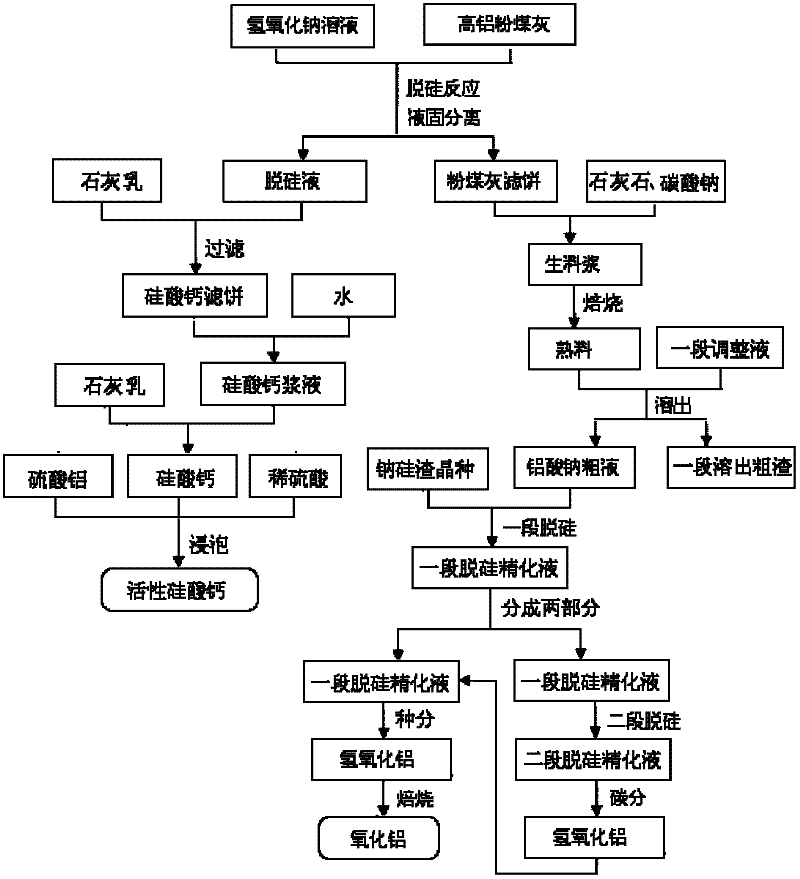
Method for producing aluminum oxide and co-producing active calcium silicate through high-alumina fly ash
ActiveCN102249253AExtraction is effective and cheapIncrease Al-Si RatioAlkaline-earth metal silicatesAluminium oxide/hydroxide preparationCalcium silicateSodium aluminate
The invention provides a method for producing aluminum oxide and co-producing active calcium silicate through high-alumina fly ash. The method comprises the following steps that: the high-alumina fly ash firstly reacts with a sodium hydroxide solution to carry out pre-desilication to obtain a liquid-phase desiliconized solution and a solid-phase desiliconized fly ash; lime cream is added to the liquid-phase desiliconized solution to carry out a causticization reaction, the resulting solid phase is active calcium silicate which is prepared through carrying out filter pressing, flash evaporation and drying to obtain the finished product; limestone and a sodium carbonate solution are added to the desiliconized fly ash to blend qualified raw slurry, then the blend qualified raw slurry is subjected to baking into the clinker, the liquid phase generated from dissolution of the clinker is a crude solution of sodium aluminate; the crude solution of the sodium aluminate is subjected to processes of first-stage deep desilication, second-stage deep desilication, carbonation, seed precipitation, baking and the like to obtain the metallurgical grade aluminum oxide meeting requirements. According to the present invention, the defects in the prior art are overcome; purposes of less material flow and small amount of slaggling are achieved; energy consumption, material consumption and production cost are relative low; extraction rate of the aluminum oxide is high; the calcium silicate with high added value is co-produced; the method provided by the present invention can be widely applicable for the field of chemical engineering.
Owner:INNER MONGOLIA DATANG INT RENEWABLE RESOURCES DEV
Process for preparing high density spherical nickel-cobalt lithium manganate as anode material of lithium ion cell
InactiveCN1622371AWell mixedImprove performanceElectrode manufacturing processesLithium compoundsNickel saltManganate
The present invention relates to energy source material technology, and is preparation process of high density spherical lithium nickel-cobalt-manganate as positive electrode material for lithium ion cell. The preparation process includes the reaction of nickel salt, cobalt salt, manganese salt, ammonium hydroxide and ammonian in water solution to synthesize spherical or spheroid precursor Ni1 / 3Co1 / 3Mn1 / 3 (OTHER)2, washing, drying and mixing with lithium carbonate; and high temperature treatment in the air at 750-950 deg.c for 8-48 hr to obtain spherical lithium nickel-cobalt-manganate. The spherical lithium nickel-cobalt-manganate has great bulk density reaching 2.25-2.50 g / cu cm after vibration densifying, average grain size of 3-7 microns, and reversible specific capacity up to 172-185 mA.hr / g.
Owner:TSINGHUA UNIV
Method and device for deodorization and purification of exhaust gas or flue gas
InactiveUS20030164309A1Easy can be electrolyzedHigh densityCyanogen compoundsLighting and heating apparatusHazardous substancePotassium hydroxide
A method and device for removing, deodorizing and purifying odor, smoke and harmful substances from exhaust gas or flue gas employs a water solution containing hypohalogen acid such as hypochlorous acid soda, an alkaline electrolyte such as potassium hydroxide or sodium hydroxide and a saline electrolyte such as sodium chloride, potassium chloride, sodium bromide or potassium bromide which is electrolyzed to produce an electrolytic water solution which is fed to a deodorizing tower and brought into contact with exhaust gas or flue gas to remove odor, smoke and harmful substances in the exhaust gas or flue gas.
Owner:OMEGA CO LTD
Micropore mesopore composite molecular sieve and its preparation method
InactiveCN1597516AAdjustable silicon-aluminum ratioAdjustable aperture sizeCrystalline aluminosilicate zeolitesMordeniteSodium hydroxide
A composite millipore-mesopore molecular sieve is prepared through proportionally adding millipore Zeolite molecular sieve ZSM-5, beta-zeolite, mordenite, L-zeolite, MCM-22 and ZSM-35 to the solution of sodium hydroxide, stirring, adding the template agent of mesopore molecular sieve, crystallizing at 90-120 deg.C for 22-26 hr, regulating pH=7.5-7.9, and static crystallizing at 90-120 deg.c for 24-168 hr.
Owner:TAIYUAN UNIV OF TECH
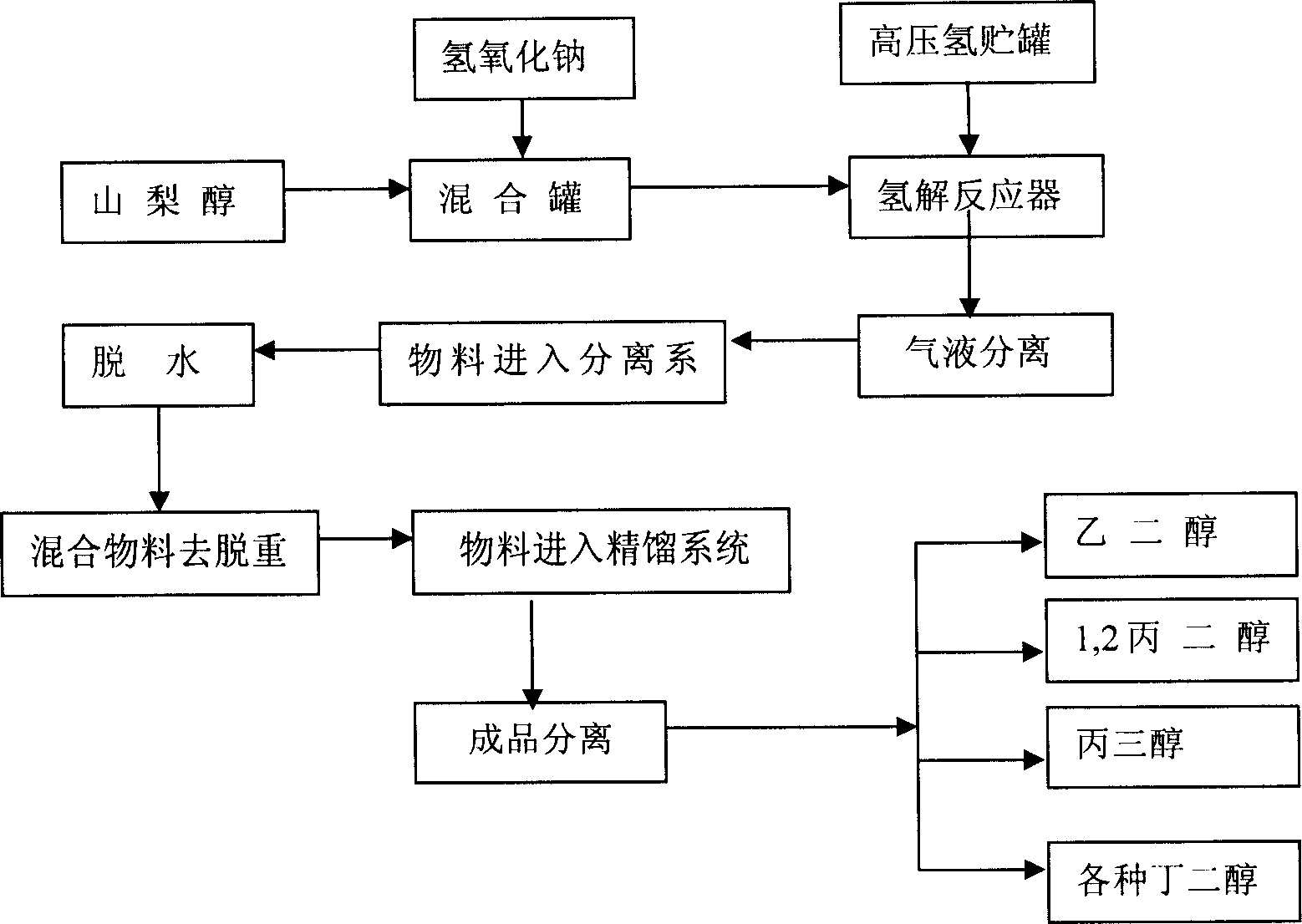
Process for producing diatomic alcohol and polyol from cracking sorbierite
InactiveCN1683293AQuality improvementIncreased production flexibilityOrganic compound preparationOrganic chemistry methodsPolyolAlcohol
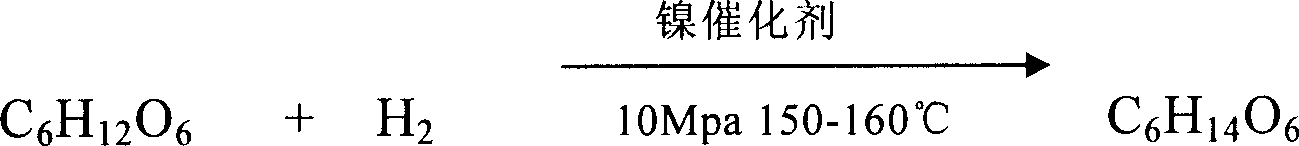
The present invention relates to sorbierite cracking process to prepare C2-C4 diatomic alcohol and polyol, and is especially the process of preparing sorbierite with corn material and hydrocracking sorbierite to prepare C2-C4 diatomic alcohol and polyol. The process includes hydrocracking sorbierite to prepare the mixture of C2-C4 diatomic alcohol and polyol at high temperature and high pressure in the presence of cracking sodium hydroxide and nickel / ruthenium catalyst, separation and refining to obtain single product. The production process of the present invention has the advantages of novel production path, unique technological condition, simultaneous production of several kinds of alcohol and high product quality. In addition, using grains, such as corn, as material to replace non-regenerable resource is significant.
Owner:王宗国
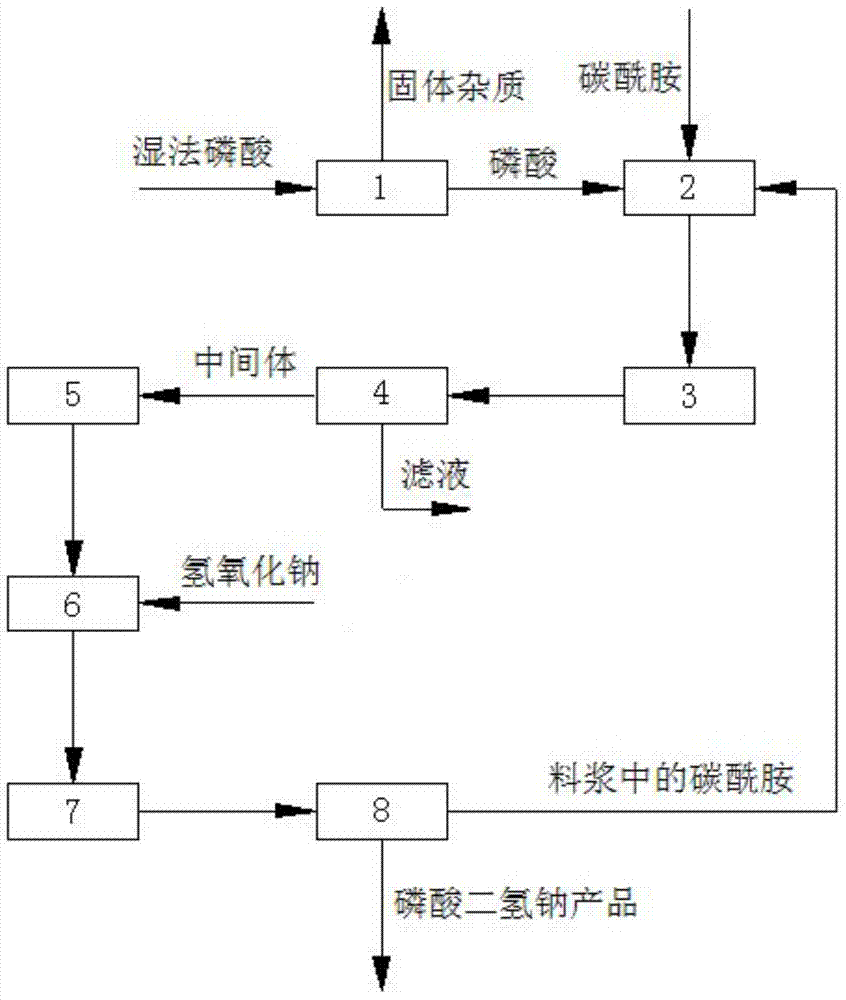
Method for preparing sodium dihydrogen phosphate by utilizing phosphoric acid by wet process
InactiveCN103787293AReduce manufacturing costShort process routePhosphorus compoundsHigh energyPhosphoric acid
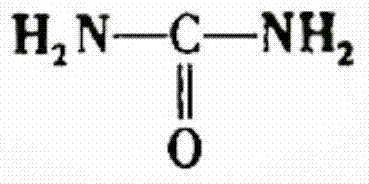
The invention discloses a method for preparing sodium dihydrogen phosphate by utilizing phosphoric acid by wet process. The method comprises the steps of carrying out reaction on carbamide and the phosphoric acid by the wet process to obtain intermediate, and then carrying out a reaction on the intermediate and sodium hydroxide to obtain the sodium dihydrogen phosphate product. The method is short in process route, low in energy consumption, stable in product quality, low in production cost, convenient to operate and safe in production; the by-product slurry can be completely recycled; the whole production process is environment-friendly, clean and free from pollution as well as emission of waste gas, waste water and waste residue; therefore, the method responses to the policy of energy conservation and emission reduction as well as clean production, and solves the problems of complicated technology, unstable product quality, high energy consumption, environmental pollution and the like in the prior art; the obtained sodium dihydrogen phosphate product has the purity of more than or equal to 98%.
Owner:GUIYANG KAILIN FERTILIZER CO LTD
Method for preparing nickel and cobalt doped lithium manganate by using waste and old lithium ionic cell as raw material
InactiveCN101450815ASimultaneous recyclingShort processManganates/permanganatesManganateManganese oxide
The invention discloses a method for preparing lithium nickel cobalt manganese oxide by taking a waste lithium ion battery as a raw material. The method is mainly characterized in that a waste lithium ion battery taking the lithium nickel cobalt manganese oxide, lithium nickel cobalt oxide and so on as a battery positive material is selected as the raw material and is pretreated through disassembly, separation, crushing, screening and so on, and then processes such as adhesive removal at high temperature and aluminum removal by sodium hydroxide are adopted to obtain an inactivated positive material containing nickel, cobalt and manganese; then a sulfuric acid and hydrogen peroxide system is adopted to leach, and P204 is adopted to remove impurities by extraction to obtain pure nickel, cobalt and manganese solution, and proper manganese sulfate, nickel sulfate or cobalt sulfate is blended to ensure that the mol ratio of nickel, cobalt and manganese elements in the solution is 1: 1: 1; and then ammonium carbonate is adopted to adjust the pH value to form a nickel cobalt manganese carbonate precursor, and then a proper amount of lithium carbonate is blended for high temperature sintering to synthesize a lithium nickel cobalt manganese oxide battery material. The first discharge capacity of the material is 150 mAh / g, the discharge capacity is still kept more than 130mAh / g after the circulation for 30 times, and the material has good electrochemical performance.
Owner:GUANGDONG BRUNP RECYCLING TECH +1
Lignin polyurethane and preparation method thereof
The invention discloses a method for preparing lignin polyurethane, which comprises the following steps of: using an organic solvent to dissolve the lignin which is extracted and separated from residues after producing ethanol from straws by sodium hydroxide; removing the residues, and depositing the mixture with water; separating the lignin; modifying the lignin with an epoxide; dissolving the lignin into a polylol; and finally compounding the lignin with raw materials of isocyanate and the like to obtain a polyurethane material. The lignin used in the method has high reactivity which can be further enhanced through modification so as to obtain a lignin polyurethane material; the polylol used in the method not only can be used as a solvent but also can take part in a synthetic reaction, has good dissolvability to the lignin, and ensures that undissolved lignin particles cannot appear in a polyurethane foam material; and the link for polyurethane synthesis uses no volatile organic solvents, so the production process causes no pollution to the environment, and simultaneously the cost of a polyurethane product is reduced.
Owner:SOUTH CHINA UNIV OF TECH
Production of biodiesel from combination of corn (maize) and other feed stocks
InactiveUS20070099278A1Increase Biodiesel production outputStable year round productionFatty oils/acids recovery from wasteOrganic compound preparationProcess systemsSodium Bentonite
A method and system to produce biodiesel from a combination of corn (maize) and other agro feedstock may be simarouba, mahua, rice, pongamia etc. Germ is separated (either by wet process or dry process) from corn, crude corn oil extracted from germ and corn starch milk / slurry is heated and cooked in jet cooker to about 105 degree Celsius, enzymes added to convert starch into fermentable sugars in liquification and saccharification process and rapidly cooled down to about 30 degree Celsius. Simarouba fruits syrup, mahua syrup is mixed with corn starch milk (after saccharification). When yeast is added the fermentation takes place for about 72 hours. Thereafter the fermented wash is distilled to produce ethanol. Water consumed in dry process is very less compared to traditional wet process system. Corn oil and mixture of other oils is fed into transesterification (reaction) vessels where ethanol with catalyst, usually sodium hydroxide is added and reaction takes place for about a period of 2-8 hours. Crude biodiesel and crude glycerin as by-products is produced. Excess ethanol removed by distillation process. Crude biodiesel washed with warm water to remove residual soaps or unused catalyst, dried and biodiesel stored for commercial use. Oil extracted from spent bleach mud (used sodium bentonite), a waste product of edible oil refineries may also be utilized for economical production of biodiesel in combination of corn oil and ethanol.
Owner:AARE PALANISWAMY RAMASWAMY
Disinfectant glass wipe
InactiveUS20050026802A1Detergent mixture composition preparationDetergent compounding agentsAmmonium compoundsPotassium hydroxide
The present invention relates to disinfecting wipes or pads that can be use to treat or clean glass or other glossy surfaces. The disinfecting wipes or pads do not depend upon quaternary ammonium compounds for disinfection, but instead depend upon relatively low solvent levels and basic pH or relatively high levels of sodium hydroxide or potassium hydroxide.
Owner:THE GLAD PROD CO +1
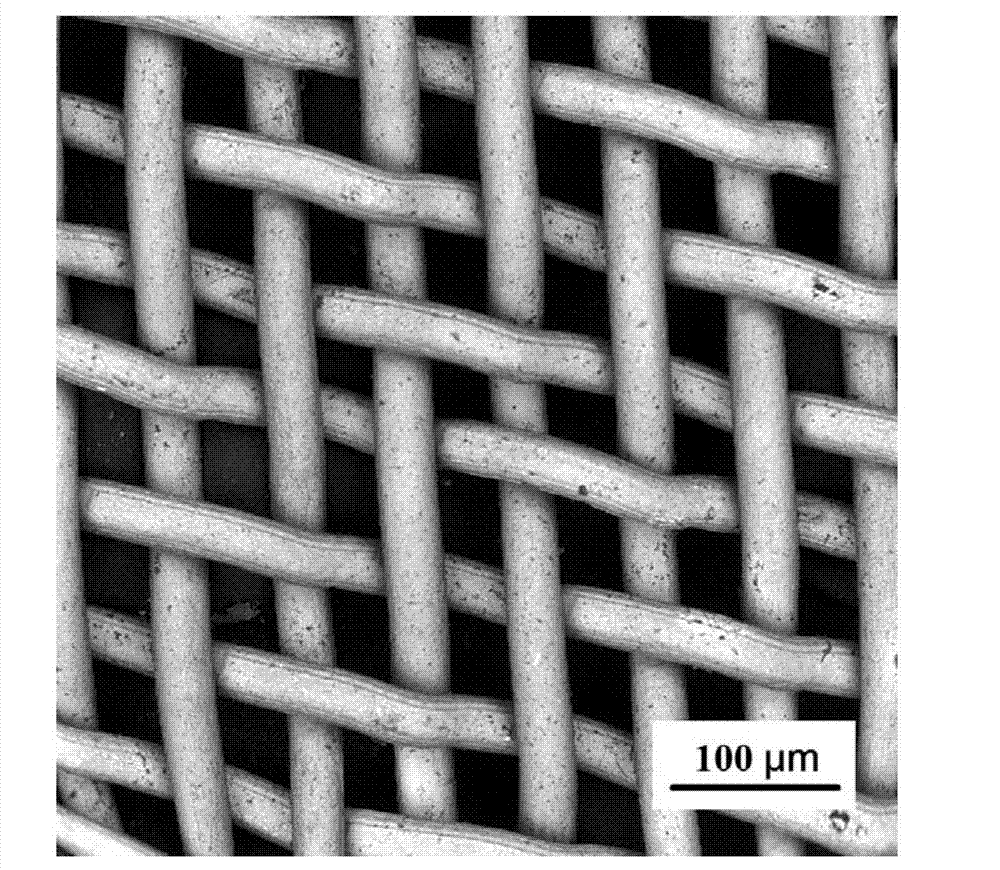
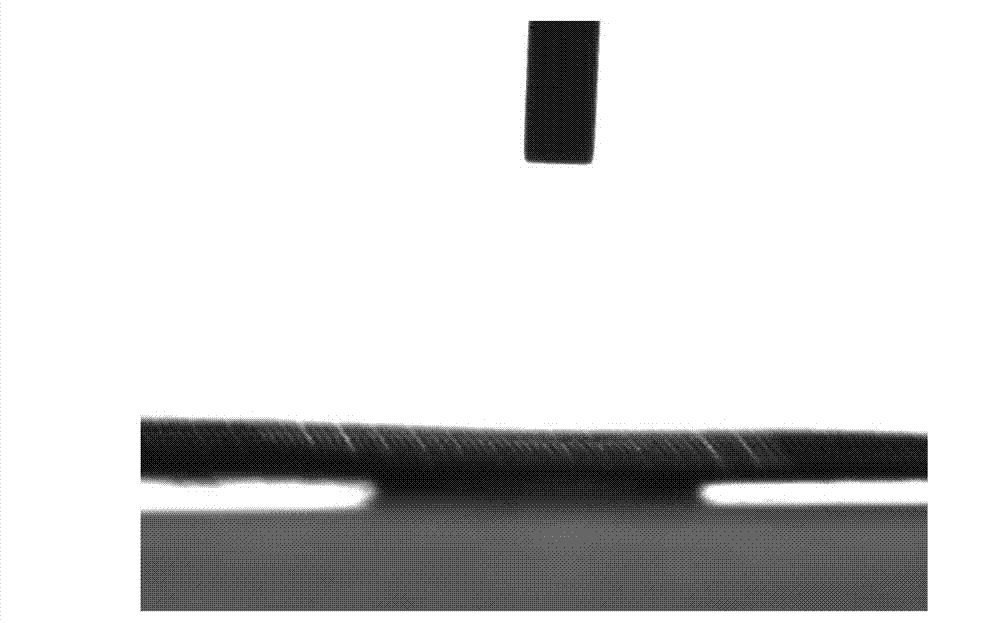
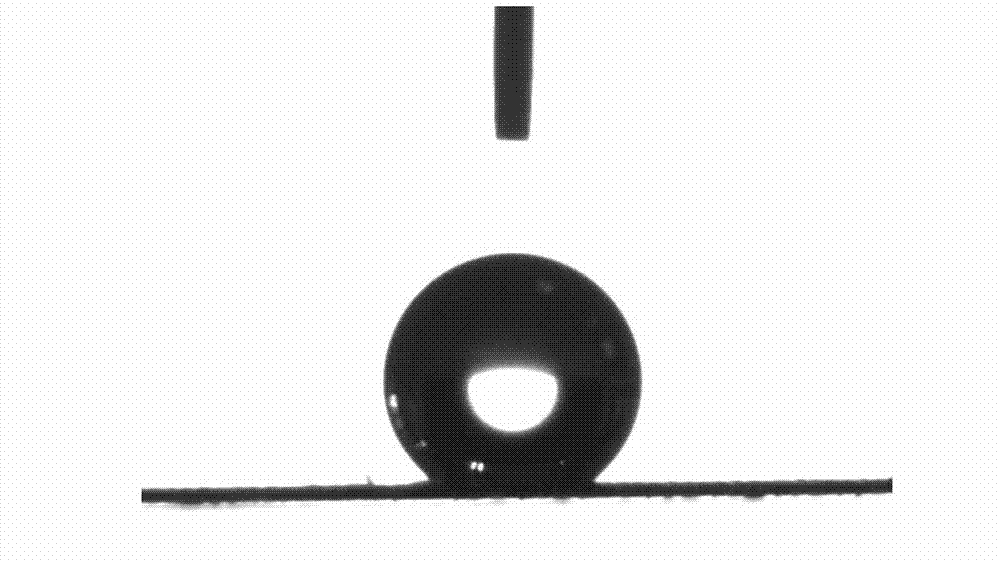
Super-hydrophobic and super-oleophylic oil-water separating mesh membrane and preparation method thereof
The invention discloses a super-hydrophobic and super-oleophylic oil-water separating mesh membrane and a preparation method thereof. The method comprises the following steps of: (1), cleaning a fabric mesh and drying the cleaned fabric mesh; (2), dissolving dopamine hydrochloride and trihytdroxy methyl-aminomethane to water to acquire a mixed solution, wherein the pH value of the mixed solution is 8.0-12.0; (3), soaking the dried fabric mesh in the mixed solution, and then getting out the soaked fabric mesh for drying; (4), dissolving mercaptan compound and sodium hydroxide in water to acquire mixed turbid liquid; (5), soaking the fabric mesh acquired in the step (3) in the mixed turbid liquid, reacting the mixture to acquire the oil-water separating mesh membrane. The oil-water separating mesh membrane disclosed by the invention has high bearing pressure to water so that oil can quickly pass, a separating effect is good, the speed is high, and the separating effect on normal hexane, petroleum ether, benzene, gasoline, diesel oil, animal and vegetable oil, crude oil and the like is good. The oil-water separating mesh membrane is non-toxic and harmless, environment-friendly, easy to clean and keep, can be reusable, and has good stability.
Owner:TSINGHUA UNIV
Anti-emulsification water-soluble metal washing agent
The invention relates to an anti-emulsification water-soluble metal washing agent. Every 100 parts of the anti-emulsification water-soluble metal washing agent include the following components according to parts by weight: 3-7 non-ionic surfactant, 3-7 bi-ion active agent, 1-5 chelator, 1-5 rust preventive, 5-10 inorganic builder and the balance water, wherein the non-ionic surfactant is any one of fatty amine polyoxypropylene ether, alkylphenol ether and fatty amine polyoxyethylene alkyl ether ammonium sulfate, the bi-ion active agent is any one of alkyl dimethylin acetic acid betaine, lauramidopropyl betaine and cocamidopropyl betaine, the chelator is any one of sodium citrate, ethylenediaminetetraacetic acid tetrasodium salt and nitrilotriacetic acid sodium salt, the rust preventive is any one of sodium borate, sodium nitrite, sodium benzoate and long carbon chain carboxylic acid amine, and the inorganic builder is any one of trisodium phosphate, sodium metasillcate, sodium carbonate, sodium bicarbonate and sodium hydroxide. The anti-emulsification water-soluble metal washing agent has the advantage of higher cleaning capacity and reutilization capacity.
Owner:NANJING KERUN LUBRICANTS
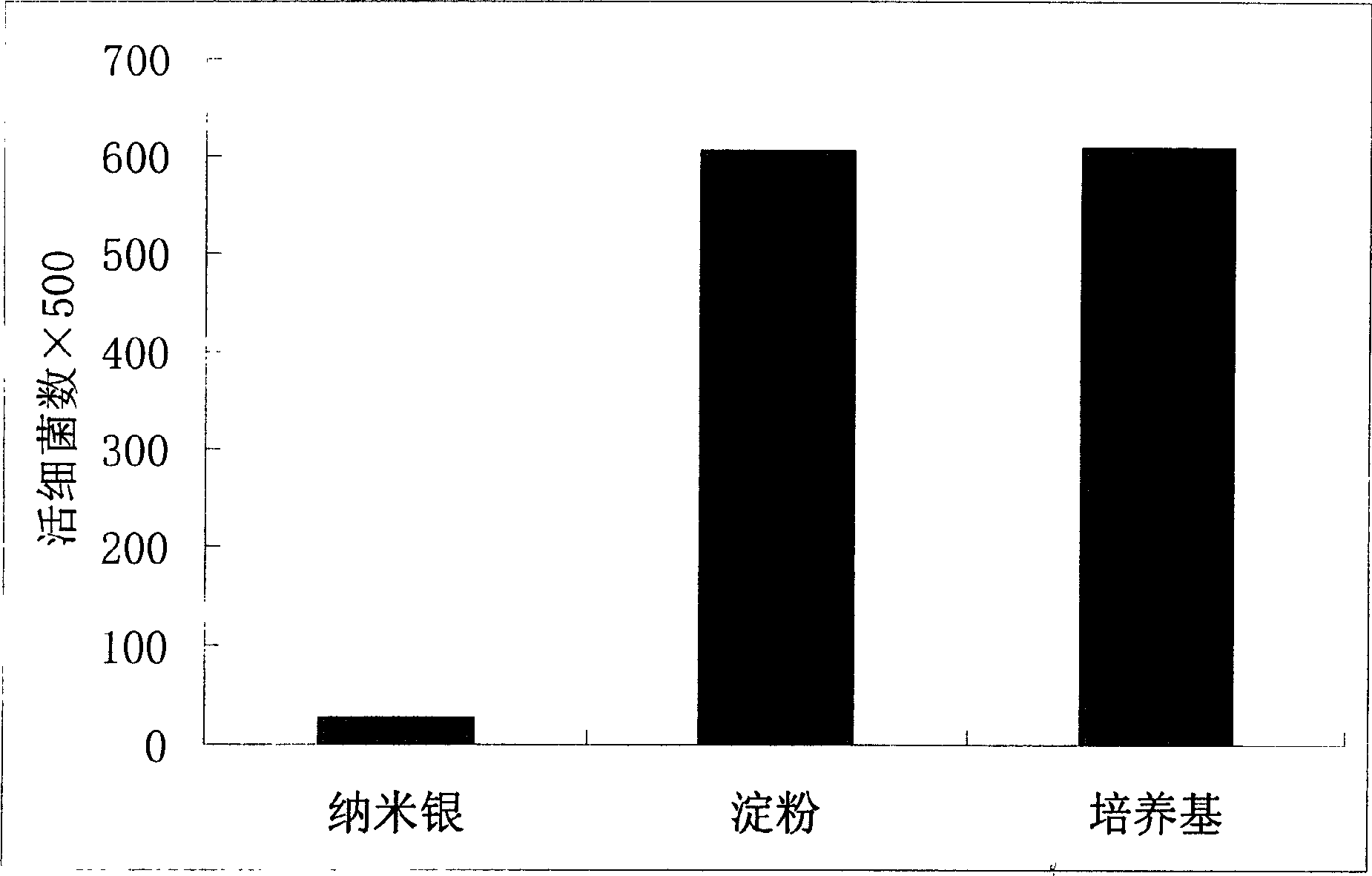
Method for preparing nanometer silver solution and nanometer silver powder by using high polymer as stabilizer
InactiveCN1653907AImprove antibacterial propertiesNo side effectsBiocideAnimal repellantsOrganic solventWater soluble
The present invention discloses the preparation process of nanometer silver solution and nanometer silver powder with polymer as stabilizer. Water soluble polymer as stabilizer in solution of 0.001-0.05 g / ml solution and silver nitrate in solution of 0.001-0.1 mol / L are compounded and reduced with water soluble reductant in solution of 0.001-0.2 mol / L to obtain nanometer silver particle of 5-100 nm size. The nanometer silver particle in solution may be further deposited and washed with sodium hydroxide solution, dried and crushed to obtain nanometer silver particle of 5-100 nm size. The prepared solution may be used in medical article, and prepared nanometer silver powder may be used widely in household appliance, plastic product, paint, etc. The present invention is environment friendly, low in cost and simple in operation, and the prepared nanometer silver particle has extremely powerful antibiotic capacity and wide antibiotic spectrum.
Owner:ZHEJIANG UNIV
Method for preparing fibrilia carboxylation cellulose nanowhiskers
The invention provides a method for preparing fibrilia carboxylation cellulose nanowhiskers, which is characterized by comprising the following steps: soaking fibrilia powder in sodium hydroxide for processing, then processing the fibrilia powder by a former treating agent and taking out the fibrilia powder to be dried in a vacuum oven to obtain preprocessed fibrilia powder; and placing the preprocessed fibrilia powder in a TEMPO oxidation system for catalytic oxidation to obtain a stable cellulose nanowhiskers suspending liquid after mechanical processing and freeze drying the suspending liquid to obtain the fibrilia carboxylation cellulose nanowhiskers having grain diameters of 3-10 nm. According to the invention, fibrilia carboxylation and nano fibrillation are realized and surfaces of prepared nanocrystalline celluloses have carboxyl functional groups, thus the surfaces generate negative charges, electrostatic repulsion among the negative charges can avoid the reunion of nanoparticles, so that the nanocrystalline celluloses can be well dispersed in water and the obtained nanocrystalline celluloses have excellent uniformity of grain sizes.
Owner:DONGHUA UNIV
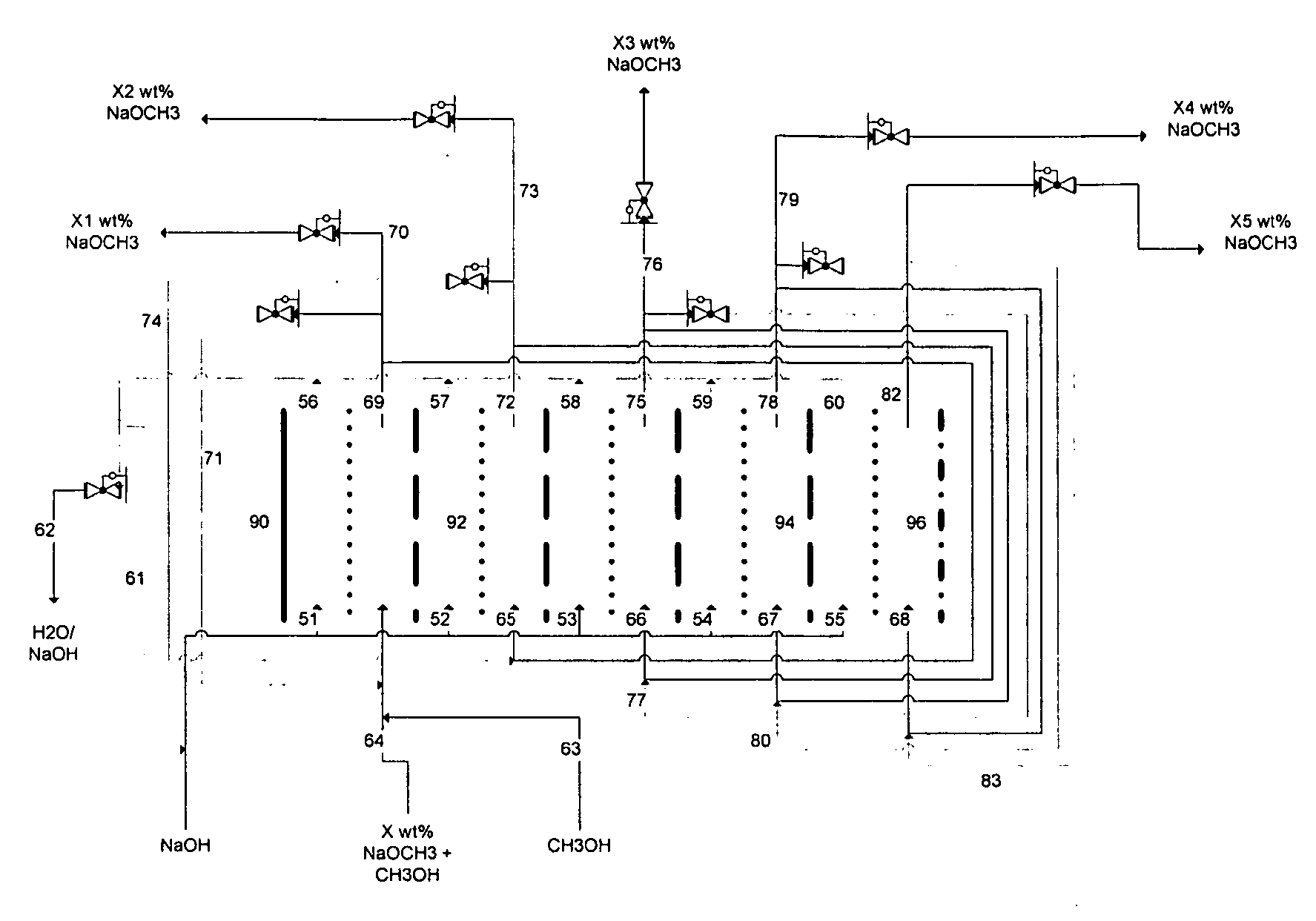
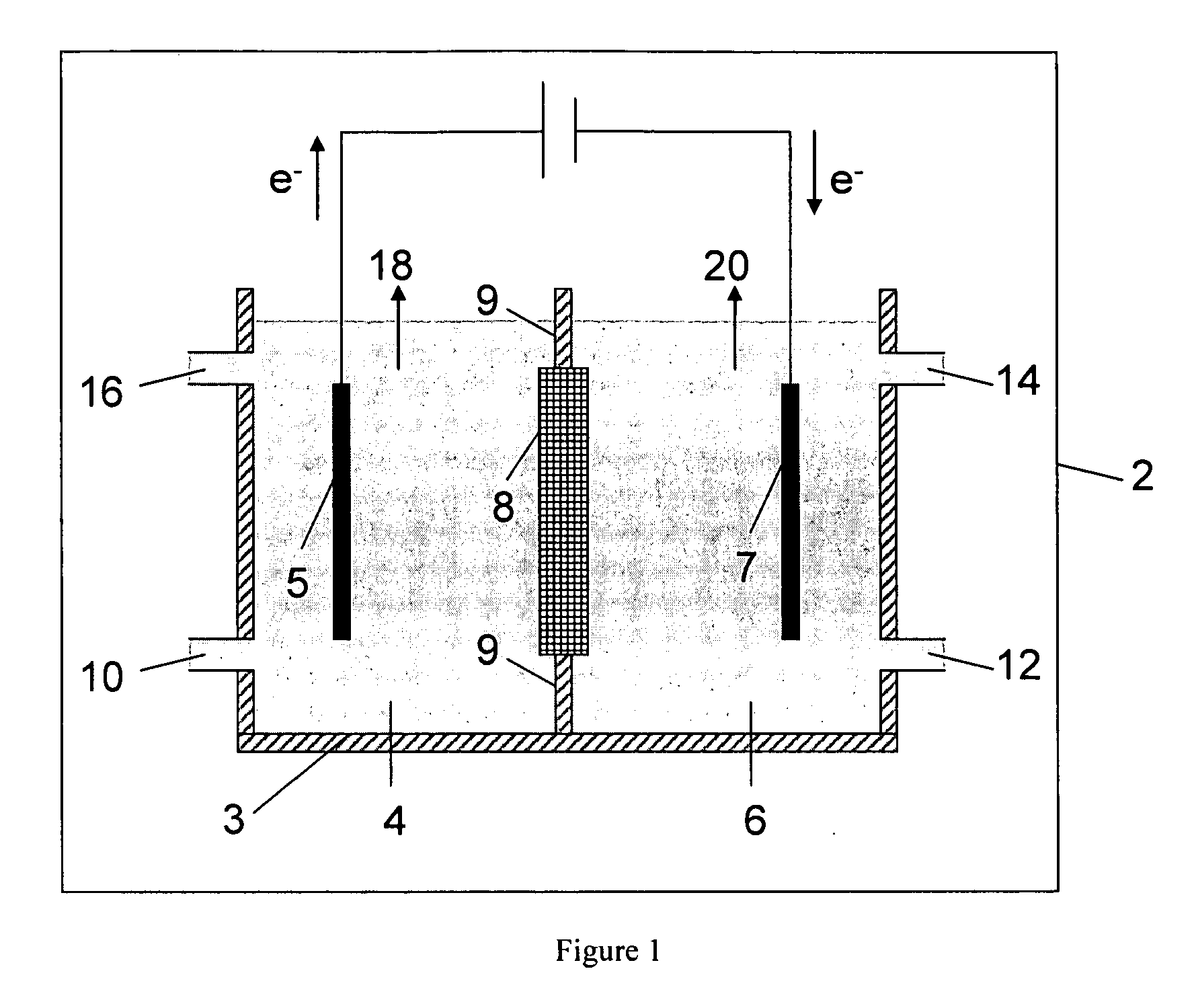
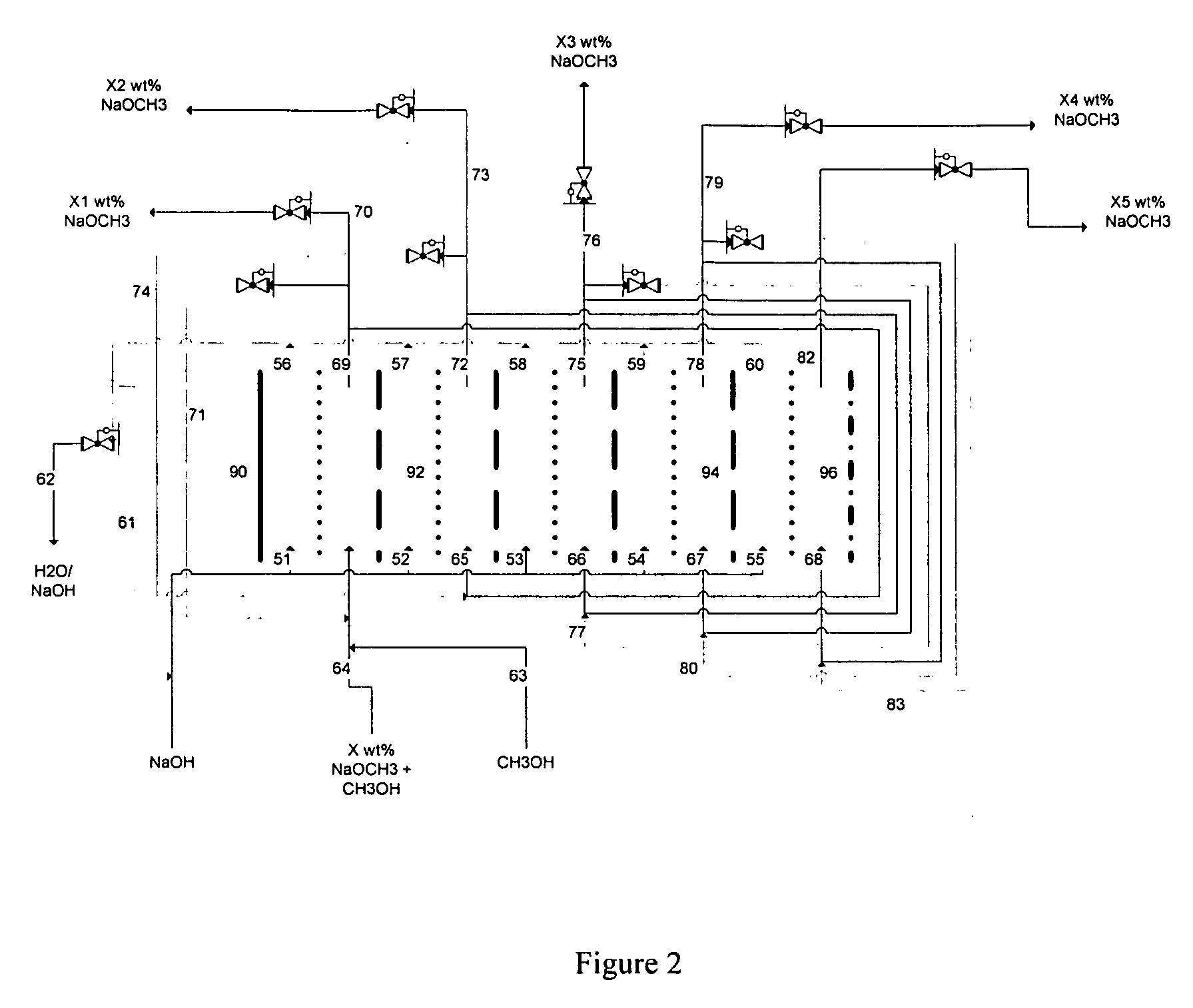
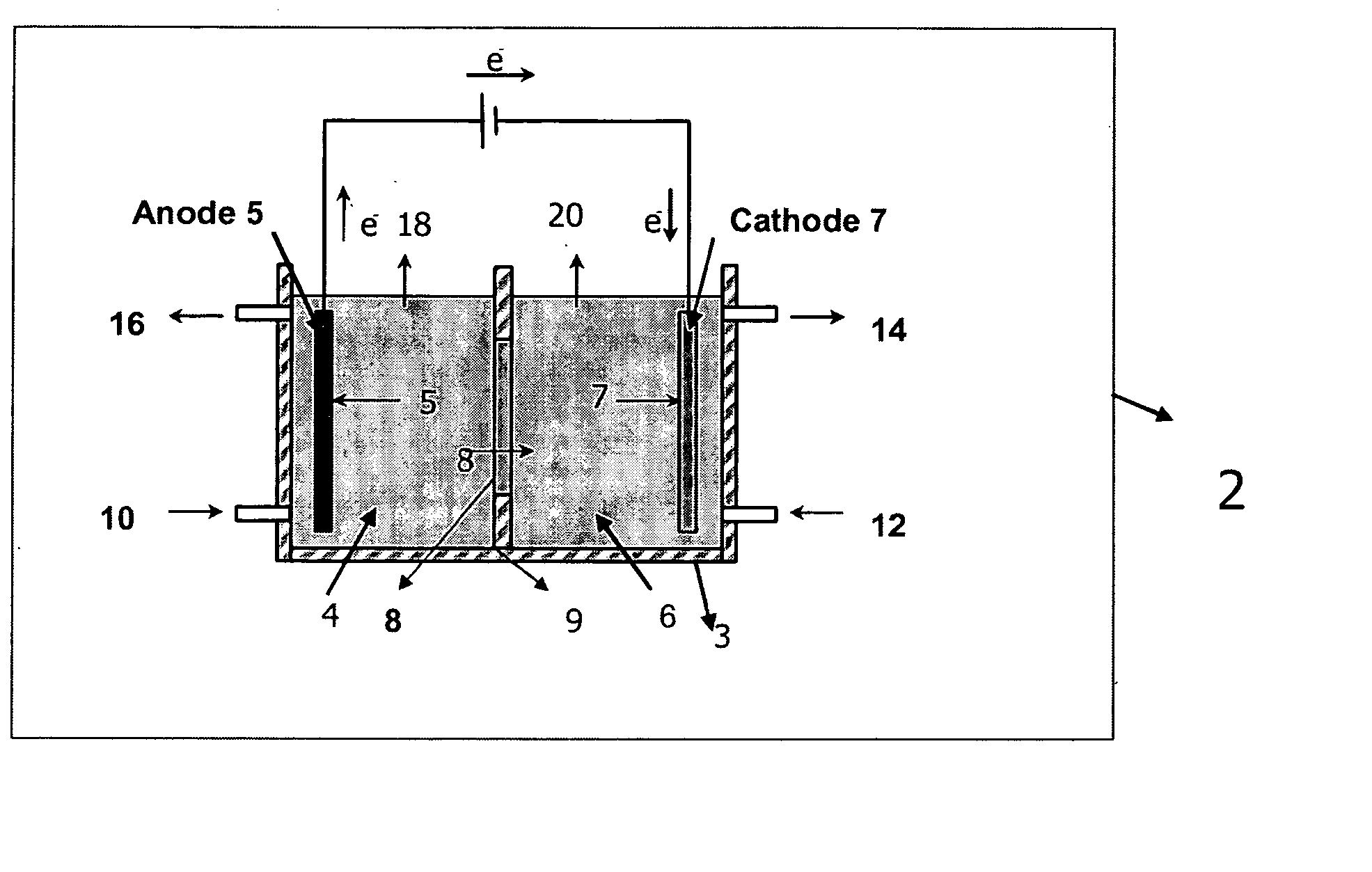
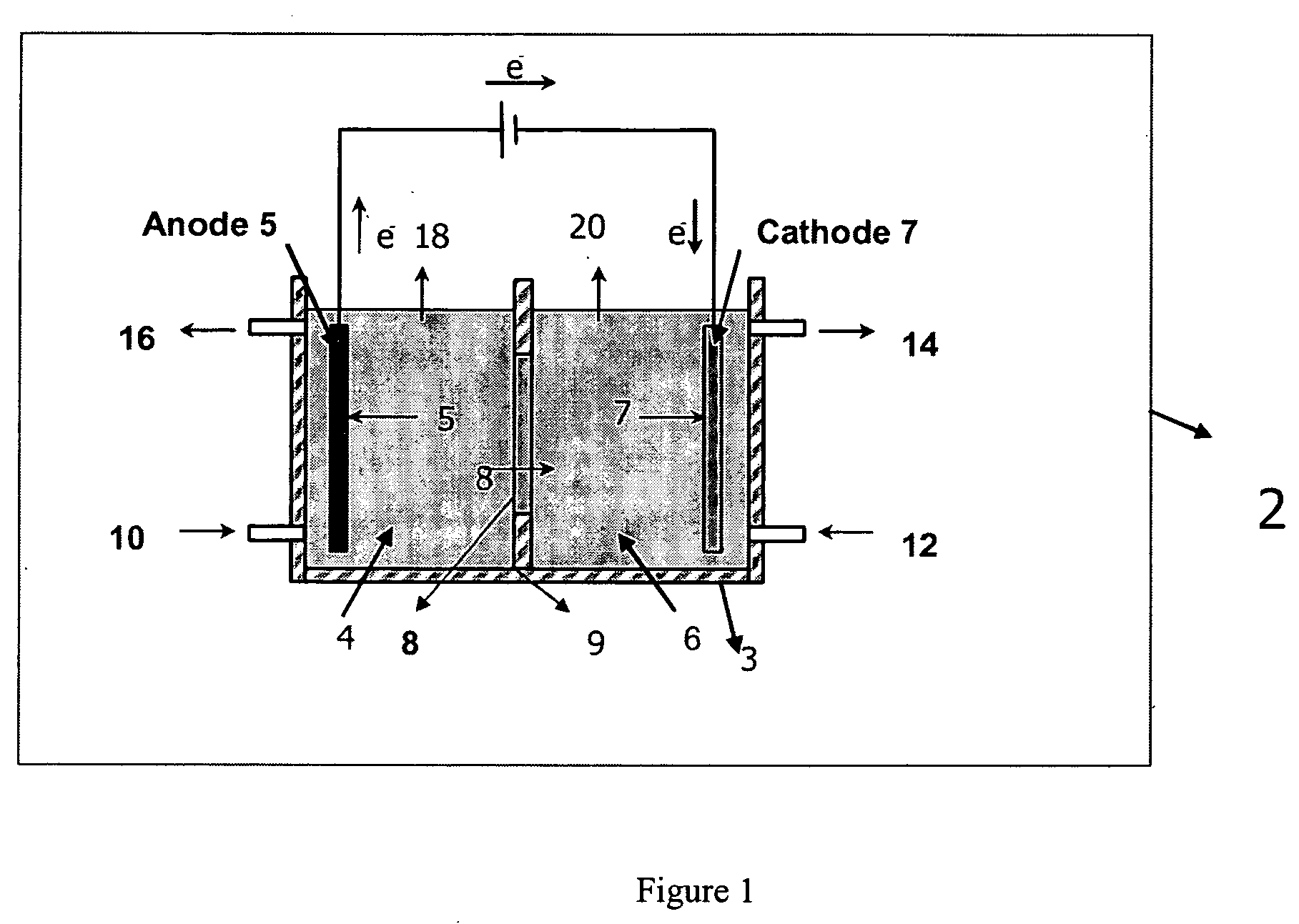
Electrolytic method to make alkali alcoholates using ceramic ion conducting solid membranes
Disclosed are processes of making solutions of alkali alkoxides in their corresponding alcohols using an electrolytic process. In one embodiment, sodium methoxide in methanol is made from methanol and aqueous sodium hydroxide solution, where the aqueous sodium hydroxide solution is present in the anolyte compartment and a solution of sodium methoxide in methanol is present in the catholyte compartment, the two compartments are separated by a ceramic membrane that selectively transports sodium ions under the influence of an electric potential, and wherein the composition of the solution of sodium methoxide in methanol in the catholyte compartment of the electrolytic cell comprises between at least about 2% by weight sodium methoxide and at most about 20% by weight sodium methoxide.
Owner:ENLIGHTEN INNOVATIONS INC
Process for producing fluoroolefins
InactiveUS20030060670A1Preparation by hydrogen halide split-offOrganic chemistry methodsPhosphonium saltPtru catalyst
A process for producing a fluoroolefin of the formula: CF.sub.3CY.dbd.CX.sub.nH.sub.p wherein Y is a hydrogen atom or a halogen atom (i.e., fluorine, chlorine, bromine or iodine); X is a hydrogen atom or a halogen atom (i.e., fluorine, chlorine, bromine or iodine); n and p are integers independently equal to 0, 1 or 2, provided that (n+p)=2; comprising contacting, in the presence of a phase transfer catalyst, a compound of the formula: CF.sub.3C(R.sup.1.sub.aR.sup.2.sub.b) C(R.sup.3.sub.cR.sup.4.sub.d), wherein R.sup.1, R.sup.2, R.sup.3, and R.sup.4 are independently a hydrogen atom or a halogen selected from the group consisting of fluorine, chlorine, bromine and iodine, provided that at least one of R.sup.1, R.sup.2, R.sup.3, and R.sup.4 is halogen and there is at least one hydrogen and one halogen on adjacent carbon atoms; a and b are independently=0, 1 or 2 and (a+b)=2; and c and d are independently=0, 1, 2 or 3 and (c+d)=3; and at least one alkali metal hydroxide. The alkali metal hydroxide can be, for example, potassium or sodium hydroxide and the phase transfer catalyst can be, for example, at least one: crown ether such as 18-crown-6 and 15-crown-5; or onium salt such as, quaternary phosphonium salt and quaternary ammonium salt. The olefin is useful, for example, as an intermediate for producing other industrial chemicals and as a monomer for producing oligomers and polymers.
Owner:HONEYWELL INT INC
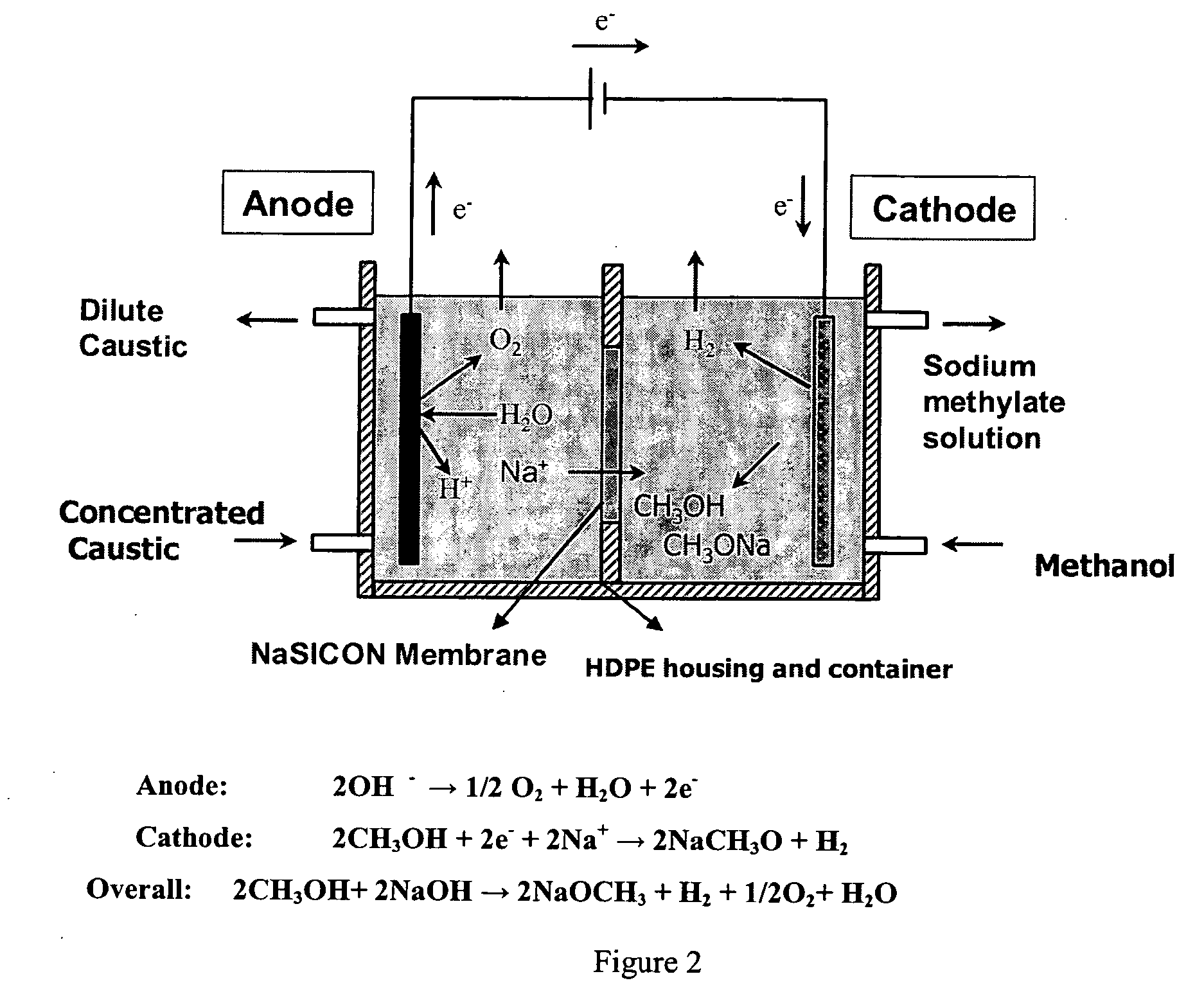
Electrolytic method to make alkali alcoholates using ceramic ion conducting solid membranes
Disclosed are processes of making solutions of metal alcoholates in their corresponding alcohols using an electrolytic process. In a preferred embodiment, sodium methylate in methanol is made from methanol and sodium hydroxide solution. The sodium hydroxide solution is placed in the anolyte compartment and the methanol is placed in the catholyte compartment, and the two compartments are separated by a ceramic membrane that selectively transports sodium under the influence of current. In preferred embodiments, the process is cost-effective and not environmentally harmful.
Owner:ENLIGHTEN INNOVATIONS INC
Oleochemical Plasticizers with Thermal and Ultraviolet Radiation Stabilizing Activity for PVC Molding Resins and Process for Obtaining Thereof
The present invention is related with bioplasticizers or primary oleochemical plasticizers and the improved process for obtaining thereof. It refers primarily to epoxydized oleochemical plasticizers produced from vegetable oils, as substitute of traditional petrochemical plasticizers. The process starts with the epoxydized product of natural oils, such as sunflower, linseed, Jatropha curcas, soybean, etc., which are transesterified with an alcohol such as ethylic or methylic, in the presence of a catalyst such as sodium methoxide or sodium hydroxide in order to produce an alkylic esters mixture of the fatty acids that were present in the oil or oil mixture used as raw material in the epoxydized oil production. When the plasticizer obtained by the process already mentioned is used for the formulation of moldable poly(vinyl chloride), PVC, resins; the resulting plastic films get adequate hardness, static and dynamic thermal stability, and plasticizer extractability by solvents, such as n-hexane, gasoline and oil. Besides, when the PVC resin is formulated with a phthalic or terephthalic plasticizers mixture and the bioplasticizer, the bioplasticizer presents a full range solubility and or compatibility with the remainder of the resin compounds. The oxyrane chemical ring of the bioplasticizer is an excellent chemical neutralizer of the HCL that might be formed from the PVC, due to the action or interference of thermal or UV radiation.
Owner:RESINAS & MATERIALES
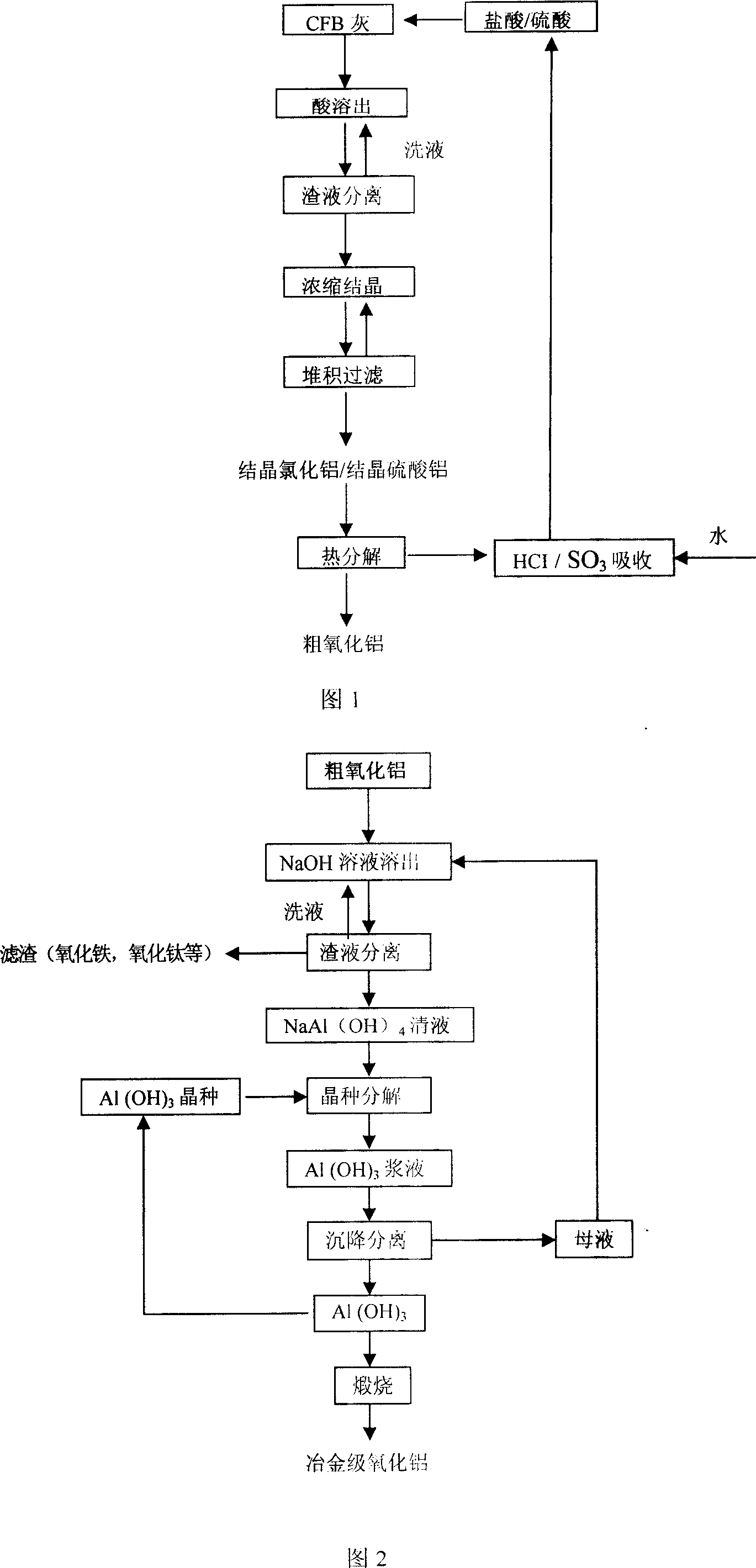
Preparation method of alumina
ActiveCN1927716AReduce pollutionReduce consumptionAluminium oxide/hydroxide preparationAluminium oxides/hydroxidesAluminium hydroxideDecomposition
The process of preparing alumina includes: the reaction of flyash from circular fluidizing bed and acid to obtain aluminum chloride solution, eliminating silicon impurity, concentrating to crystallize and heating to decompose and to obtain crude alumina product; reaction of crude alumina product and hot alkali solution to obtain sodium aluminate solution; eliminating iron, titanium and other impurity, adding aluminum hydroxide crystal seed into sodium aluminate solution for seed separating decomposition to obtain aluminum hydroxide precipitate; and final calcining aluminum hydroxide to obtain metallurgical alumina. The normal pressure process has no any cosolvent added, and the alumina product has Al2O3 content up to 98 %. The process has small sodium hydroxide consumption, reuse of most of sodium hydroxide, simple operation, low cost, low power consumption, capacity of reducing flyash pollution and other advantages.
Owner:SHENHUA ZHUNGER ENERGY
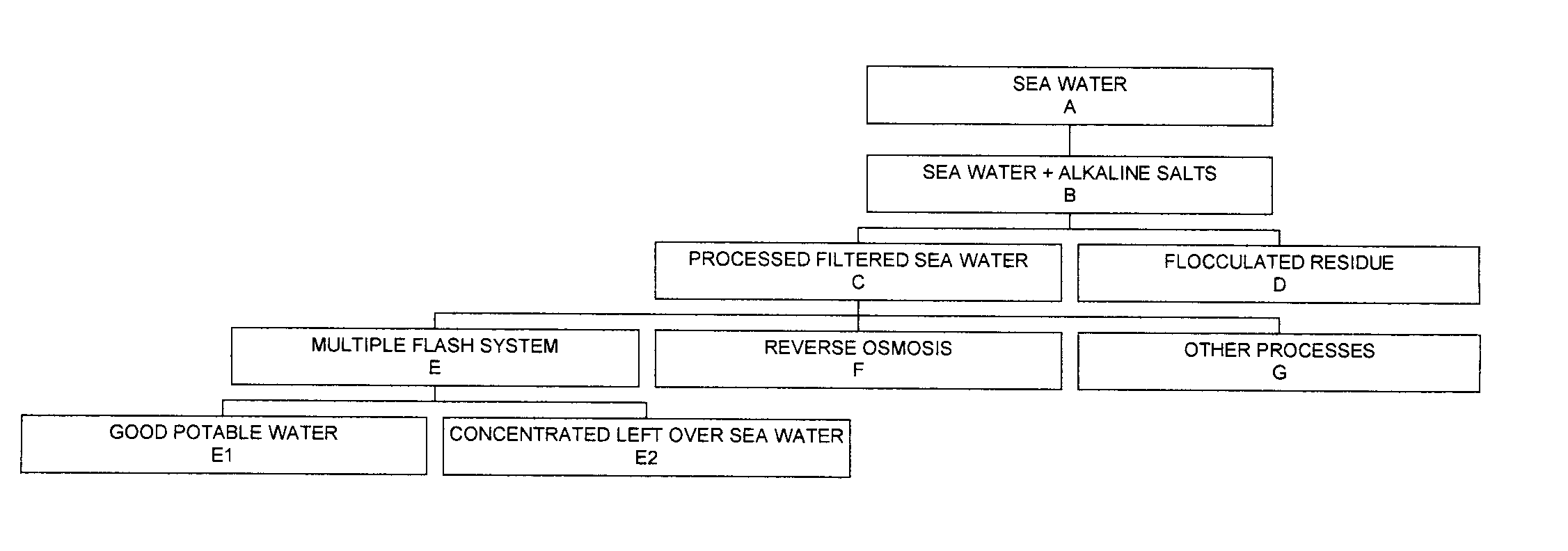
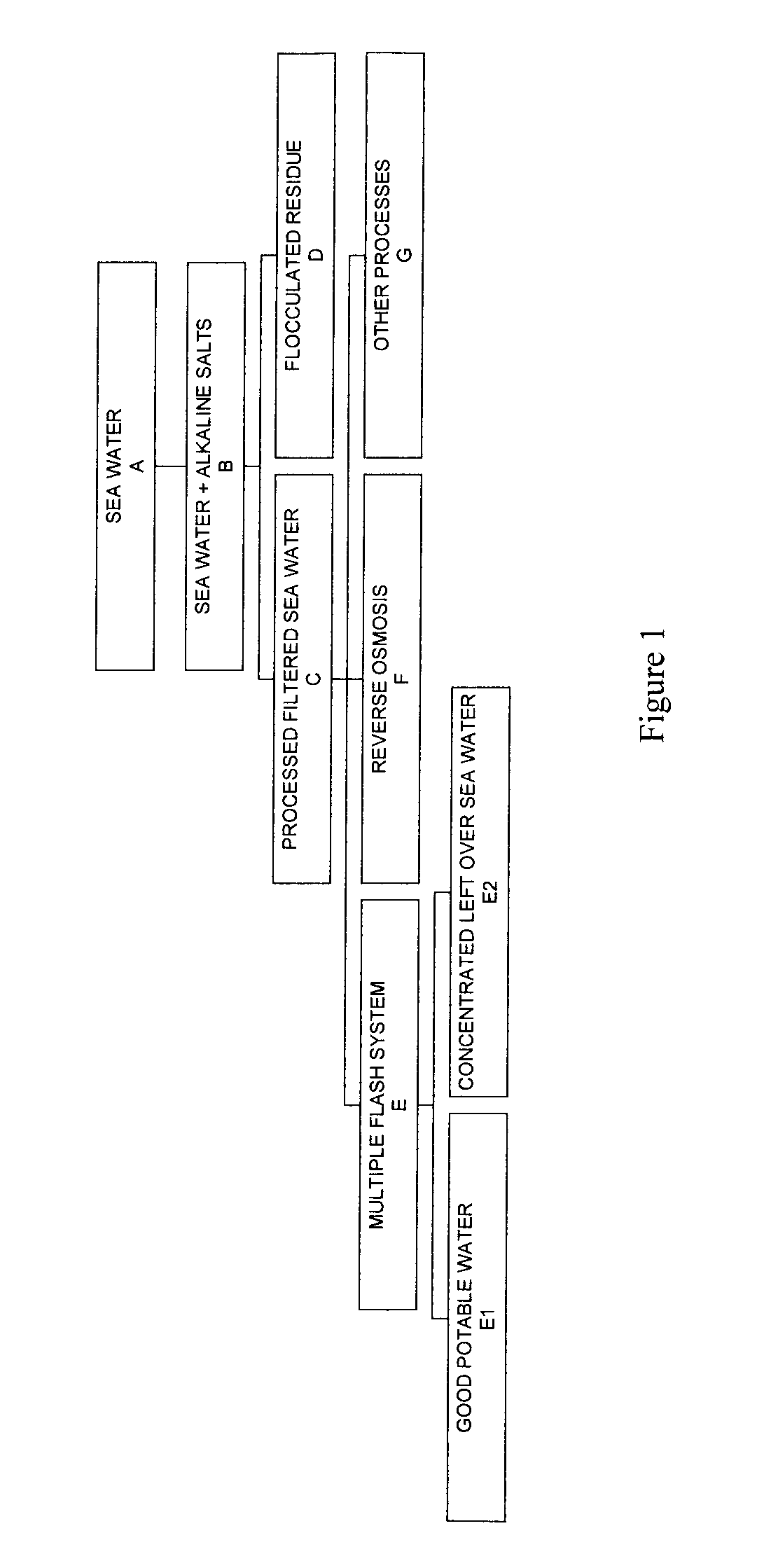
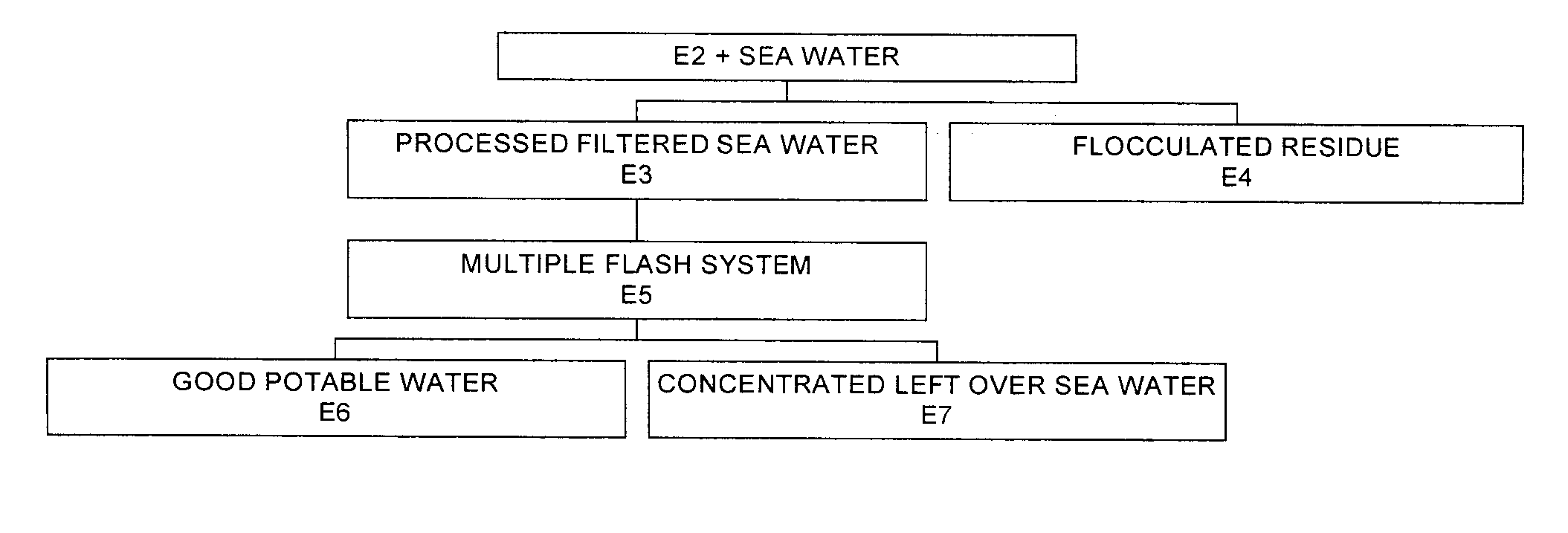
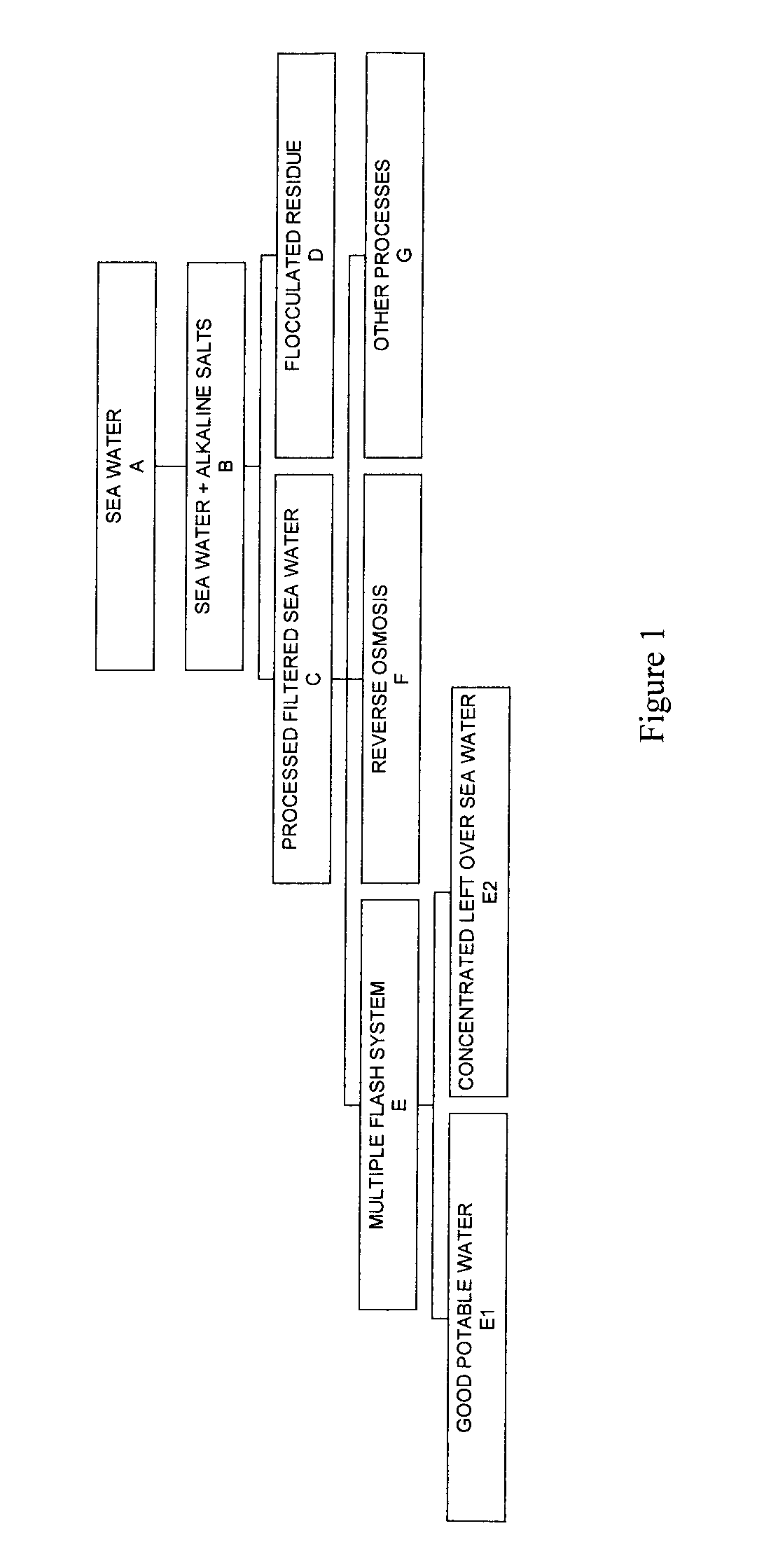
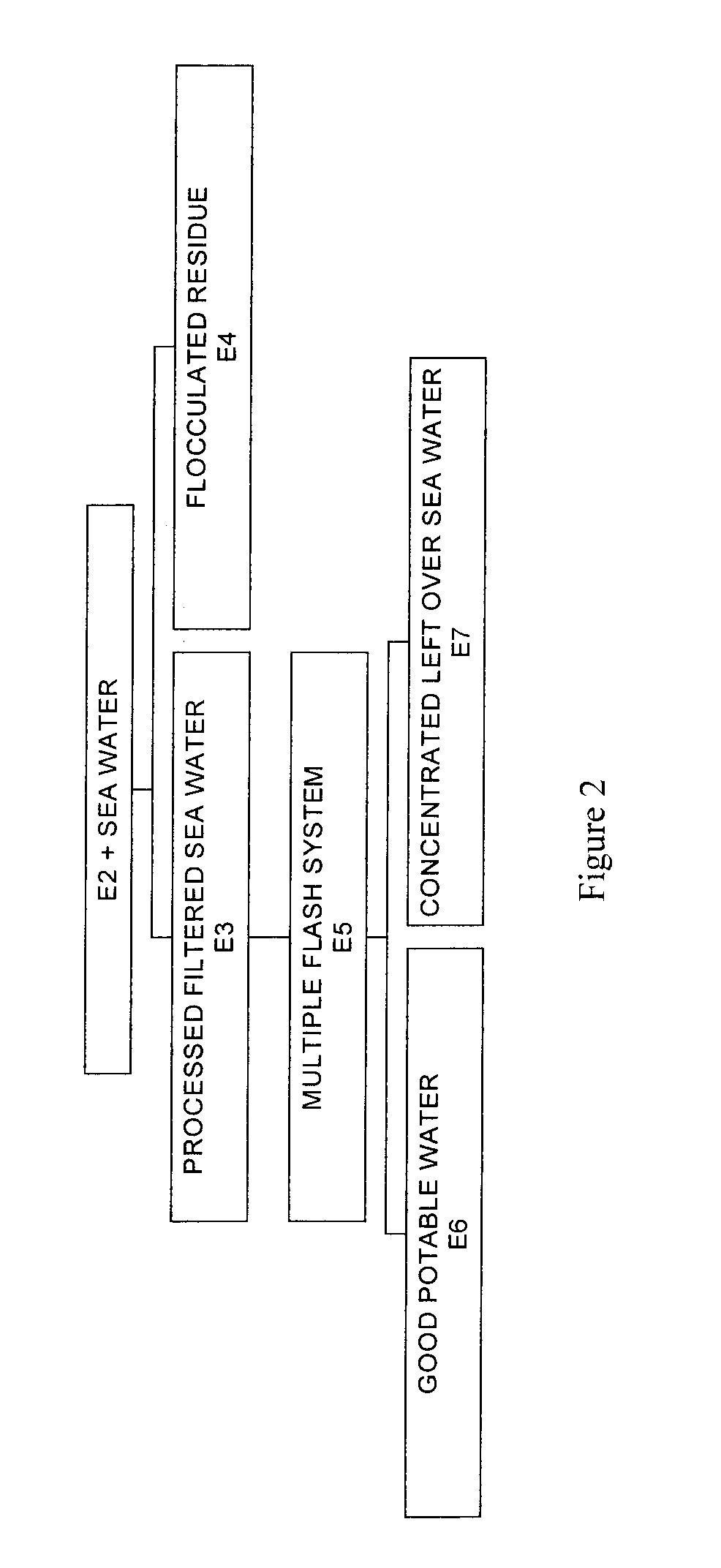
Process for pre-treating and desalinating sea water
ActiveUS20050098499A1Reduce maintenanceExtend equipment lifeGeneral water supply conservationSeawater treatmentCalcium bicarbonatePotassium hydroxide
Water containing dissolved salts, such as calcium sulfate, calcium chloride, magnesium sulfate, magnesium chloride, sodium carbonate, sodium chloride, sodium sulfate, calcium bicarbonate, and mixtures thereof, is treated to reduce the concentration of those salts. About 0.1 to about 60 g / L of sodium hydroxide, sodium carbonate, potassium hydroxide, potassium carbonate, calcium hydroxide, calcium carbonate, aluminum hydroxide, aluminum sulfate, aluminum potassium sulfate, and mixtures thereof is added to the water, whereby a precipitate forms in the water. The precipitate is separated from said water and the water is desalinated using reverse osmosis, flash evaporation, or another method. The process is preferably performed by first adding calcium oxide or calcium hydroxide, separating the precipitate that forms, then adding sodium hydroxide and sodium carbonate to form a second precipitate.
Owner:HUSSAIN MOHAMMED AZAM
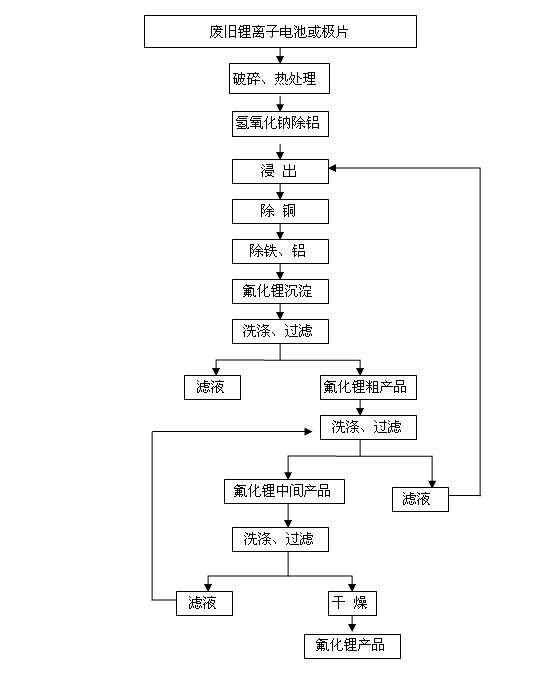
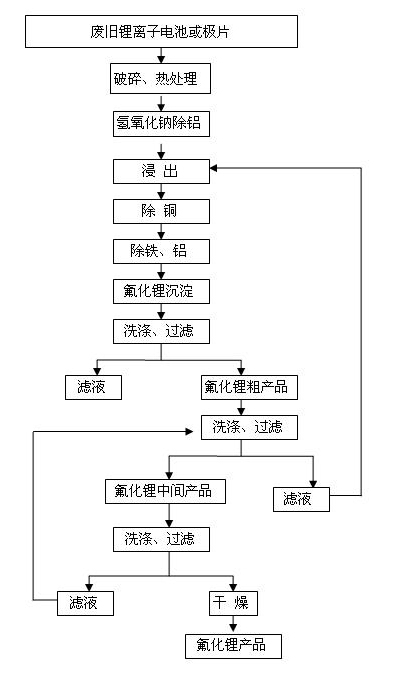
Method for recovering lithium from waste lithium ion battery and waste pole piece
ActiveCN101942569AHigh recovery rateEffectively realize comprehensive recyclingProcess efficiency improvementPhysical chemistryLithium-ion battery
The invention discloses a method for recovering lithium from a waste lithium ion battery and a waste pole piece. The method comprises the following steps of: (1) crushing the waste lithium ion battery or the waste pole piece by using a crusher, placing the crushed material in a high-temperature furnace and removing an adhesive from the crushed material by thermal processing to obtain powder; (2) removing aluminum from the powder by dissolving the aluminum in sodium hydroxide solution and filtering the solution to obtain low-aluminum filter mud; (3) leaching the low-aluminum filter mud with acid and a reducing agent to obtain lixivium; (4) removing impurities such as iron, copper, aluminum and the like from the lixivium by a chemical method; (5) precipitating lithium in the lixivium with fluorine salt to obtain a lithium fluoride rough product; (6) washing the lithium fluoride rough product, filtering and drying to obtain a lithium fluoride product; and (7) returning filtrate obtained after the lithium fluoride rough product is washed to the step (3) for processing. By the method of the invention, the lithium fluoride product has purity of over 98.0 percent and the primary recoveryrate of lithium is between 75 and 92 percent; and the method of the invention has the advantages of simple process, low cost, easy industrial production and high economic benefit.
Owner:HUNAN BRUNP RECYCLING TECH
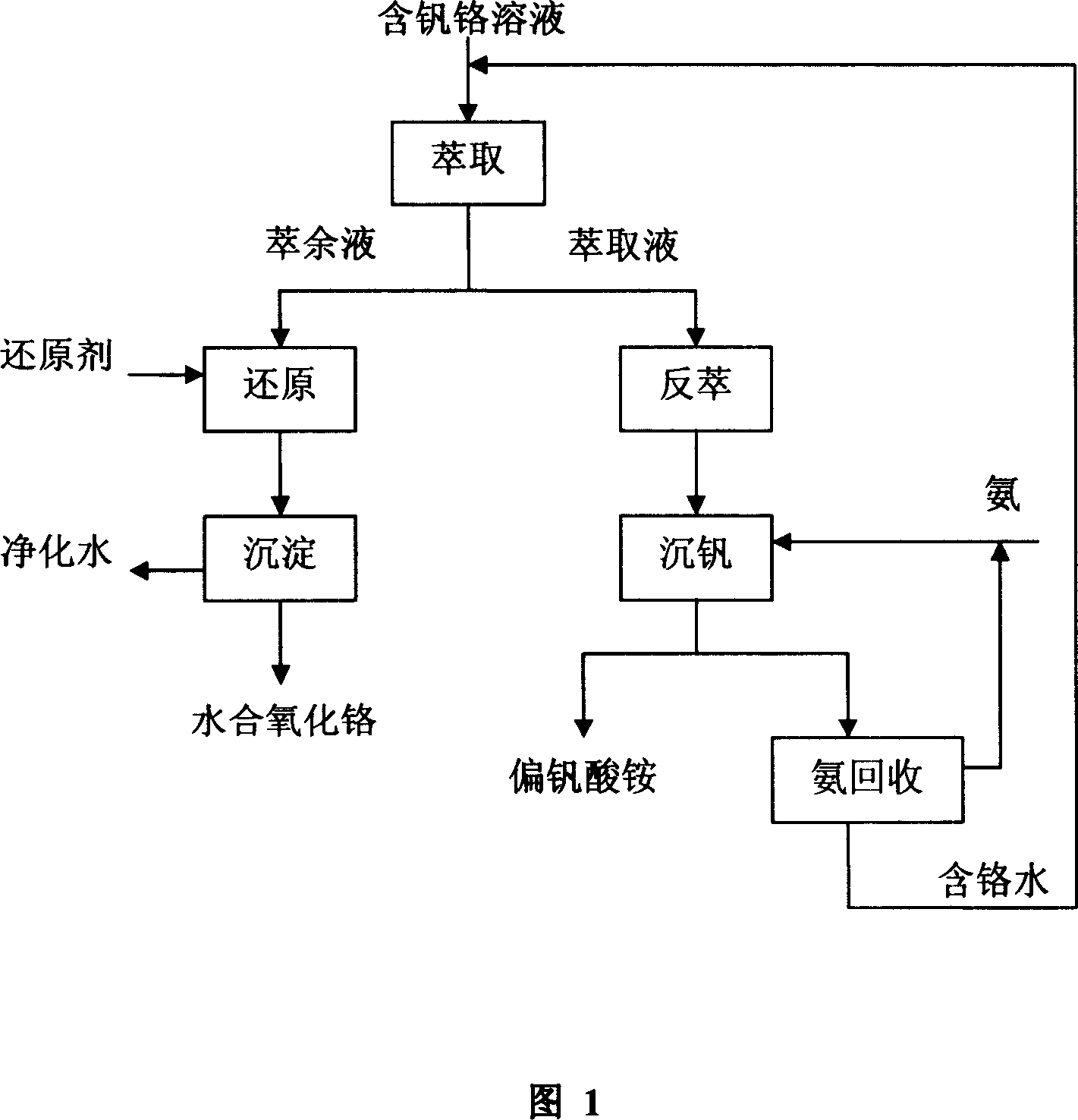
Method for separating and reclaiming vanadium and chromium from solution containing vanadium and chromium
ActiveCN101121962ALow costSimple processProcess efficiency improvementHydration reactionDistillation
This invention relates to an entirely new technology of completely recovering chromium and vanadium from vanadium-chromium miscible liquid. The main procedures include: first a primary-secondary compound amine extracting agent contacts the vanadium-chromium miscible liquid by means of countercurrent contact and extract, so as to extract most of vanadium and a small amount of chromium into a organic phase while most of chromium stays into a aqueous phase; and a reduction reaction is conducted with pH of acid adjustable faffinate (aqueous phase) and a certain amount of a reducing agent; the sodium hydroxide is used for adjusting pH value of the solution and filter, and finally the product is hydrous chromium oxide; at that time, the lye is used as a stripping agent; the vanadium is stripped from the vanadium-rich organic phase into water in the manner of countercurrent contact; and the vanadium is separated from the solution witthe method of ammonium precipitation and in the form of ammonium metavanadate; and finally the supernatant clear solution of the one is processed with deposited vanadium with a high-efficient distillation technology, and the strong aqua ammonia is left in the tower top and deamidization solution is left in the tower bottom until the extraction process is reached. The invention uses the primary-secondary compound amine as the extracting agent, extracts and separates vanadium and chromium selectively at a low temperature. The invention not only has a simple process flow, but also is low-cost, quite applicable in large-scale industrial production. In addition, the invention also provides high-purity ammonium metavanadate and 16 percentage strong aqua ammonia, and makes sure the vanadium and chromium can be completely recovered through re-use of the solution.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Apparatus and method of removing water soluble support material from a rapid prototype part
ActiveUS20050103360A1Low costEasy to useAdditive manufacturing apparatusHollow article cleaningSupport removalPotassium hydroxide
The support removal apparatus comprising in combination a retention tank having a manifold assembly comprising a plurality of nozzle heads in hydraulic communication with the discharge side of a pump, collectively configured for agitating an aqueous cleaning solution comprised of sodium or potassium hydroxide, sodium or potassium carbonate, and water; a heating element mounted within the retention tank for heating the aqueous cleaning solution to a predetermined temperature set point; a basket strainer mounted within the retention tank in hydraulic communication with the intake side of the pump to mitigate passage of small rapid prototype parts and residual support material therethrough and into the pump and manifold assembly; a work surface mounted atop the retention tank and having a movable lid fitted with a basket for containing small rapid prototype parts; a thermocouple for maintaining the temperature within a tolerable range for optimum removal of support material; a level indicator to ensure adequate solution level in the retention tank for operability of the pump and heating element; a cabinet having interface controller mounted on an exterior panel thereof for setting timer and heat functions; and a microprocessor having capabilities for making minute adjustments to the heating element via feedback from the thermocouple and controlling operation of the pump and heating element for a pre-set time interval.
Owner:TAFOYA DAVID JONATHAN
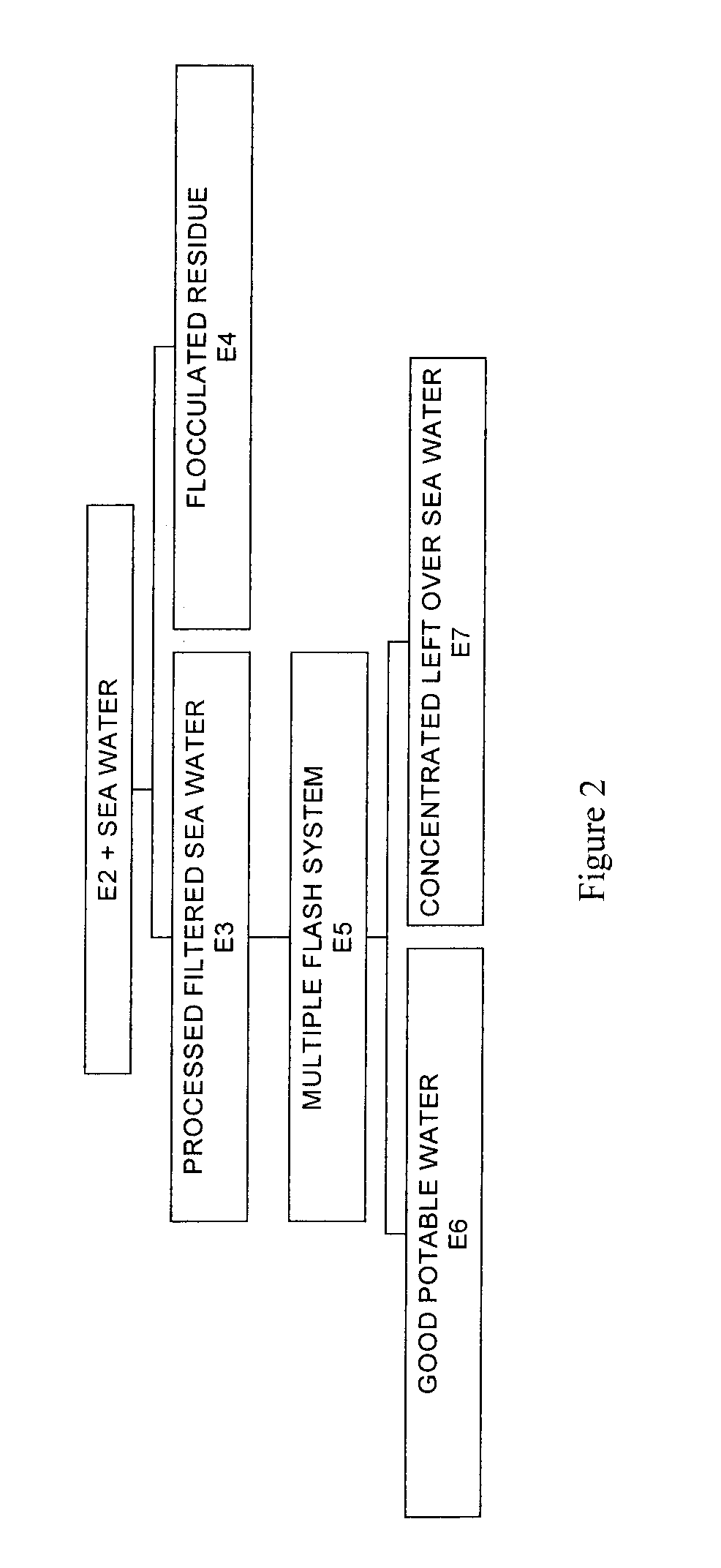
Process for pre-treating and desalinating sea water
ActiveUS7198722B2Extend equipment lifeReduce maintenanceGeneral water supply conservationSeawater treatmentCalcium bicarbonateReverse osmosis
Owner:HUSSAIN MOHAMMED AZAM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com