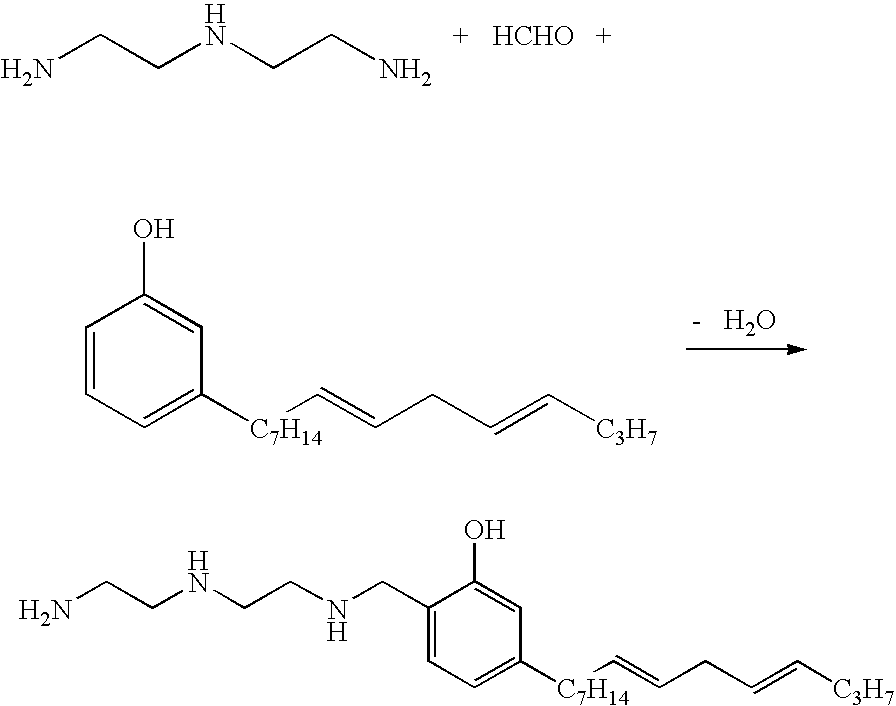
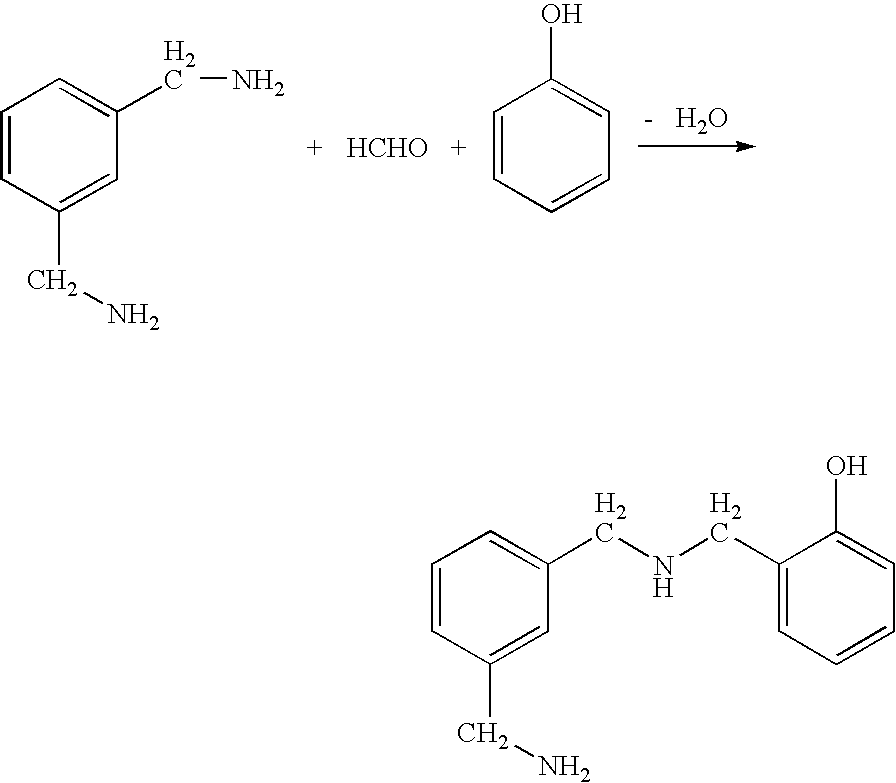
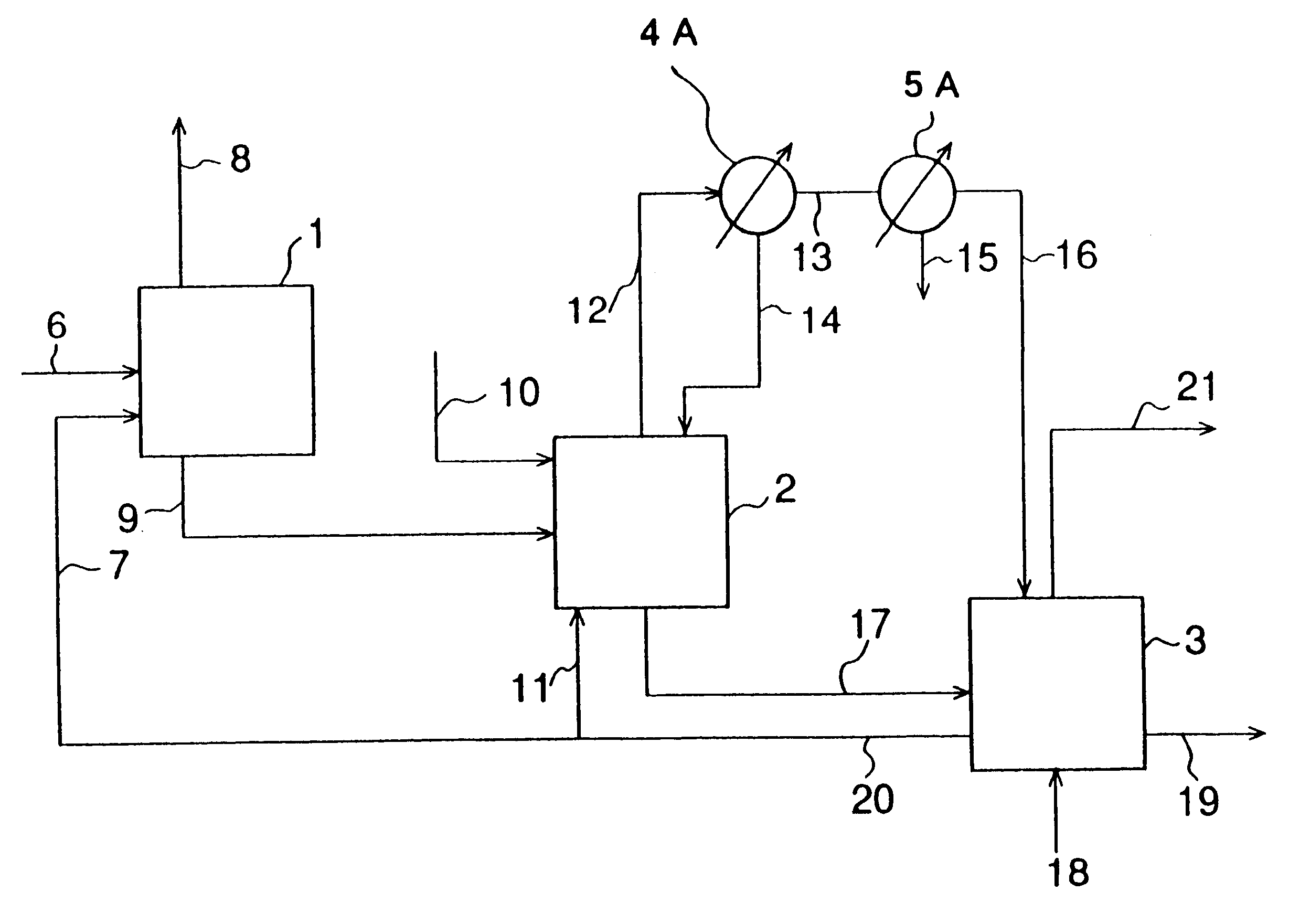
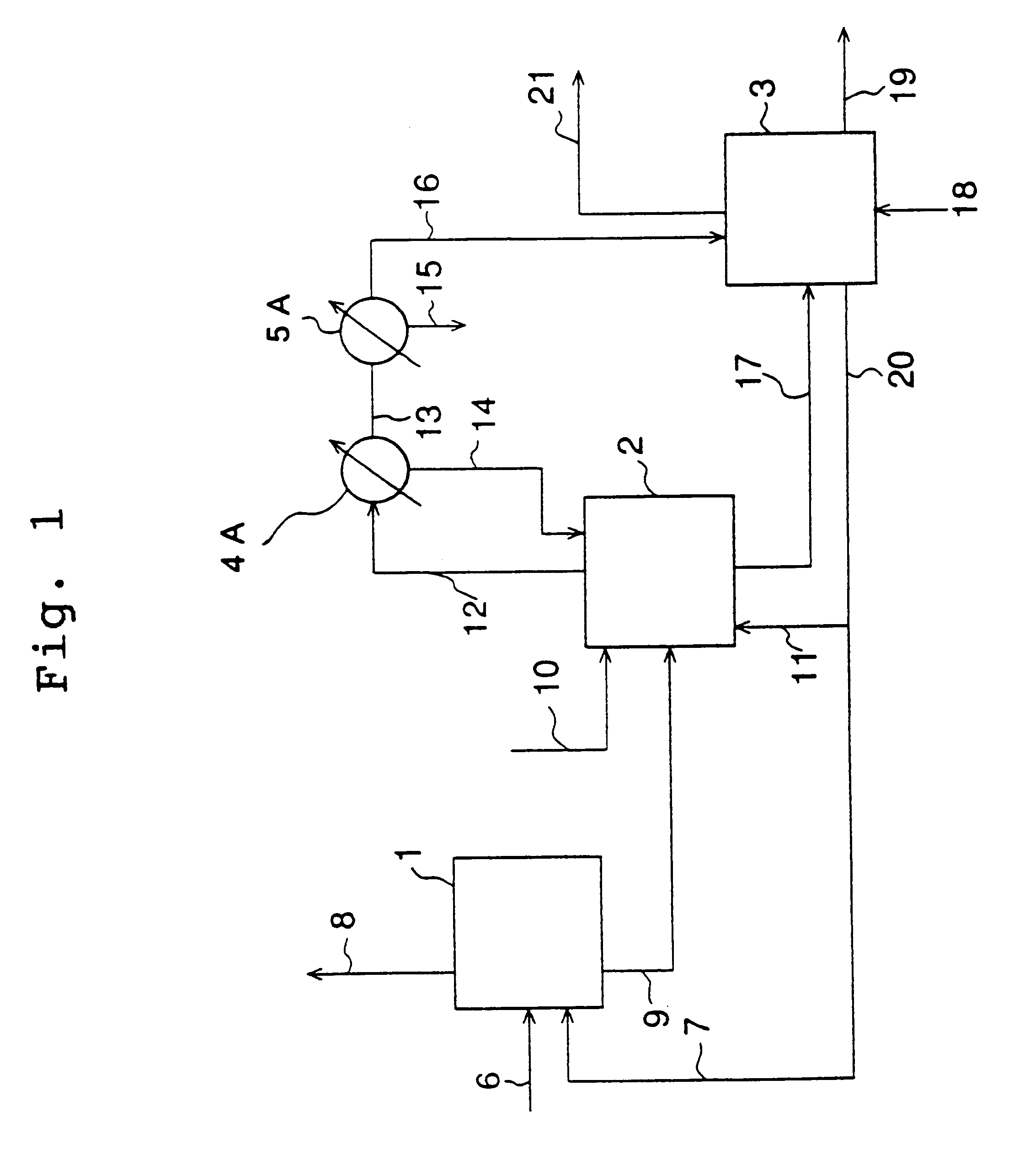
Patents
Literature
2160 results about "Vinyl chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vinyl chloride is an organochloride with the formula H₂C=CHCl that is also called vinyl chloride monomer (VCM) or chloroethene. This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC). About 13 billion kilograms are produced annually. VCM is among the top twenty largest petrochemicals (petroleum-derived chemicals) in world production. The United States currently remains the largest VCM manufacturing region because of its low-production-cost position in chlorine and ethylene raw materials. China is also a large manufacturer and one of the largest consumers of VCM. Vinyl chloride is a gas with a sweet odor. It is highly toxic, flammable, and carcinogenic. It can be formed in the environment when soil organisms break down chlorinated solvents. Vinyl chloride that is released by industries or formed by the breakdown of other chlorinated chemicals can enter the air and drinking water supplies. Vinyl chloride is a common contaminant found near landfills. In the past VCM was used as a refrigerant.
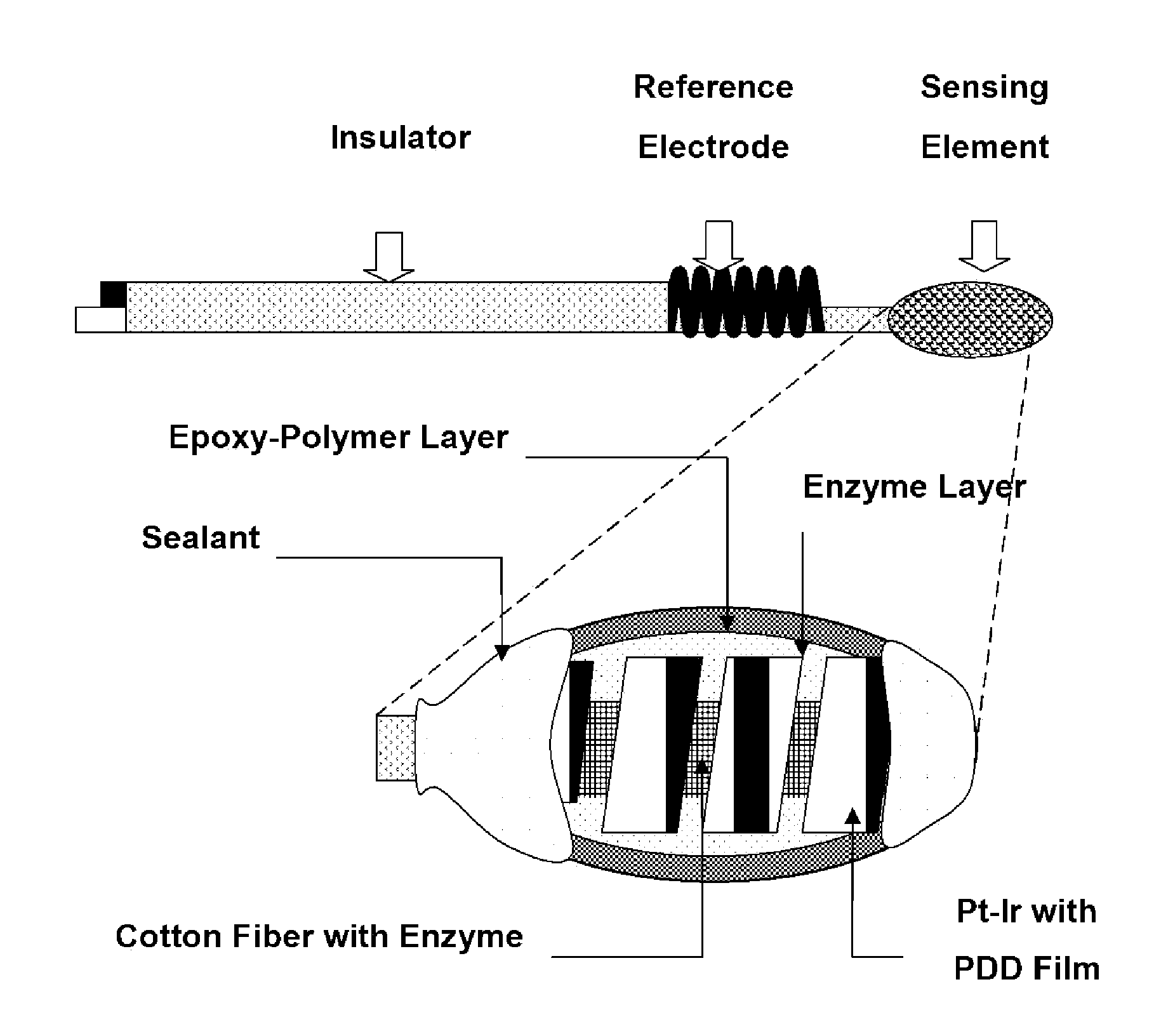
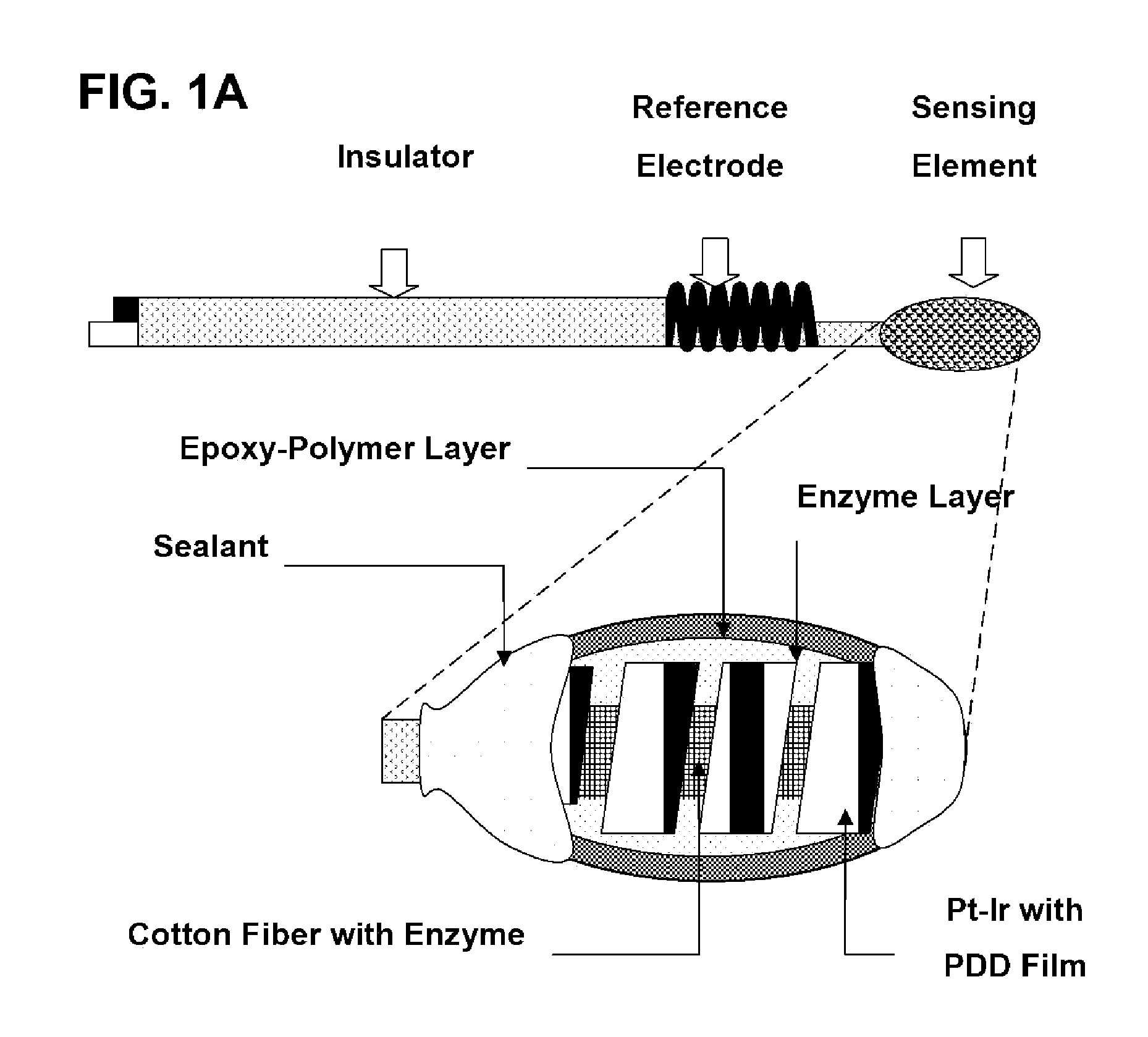
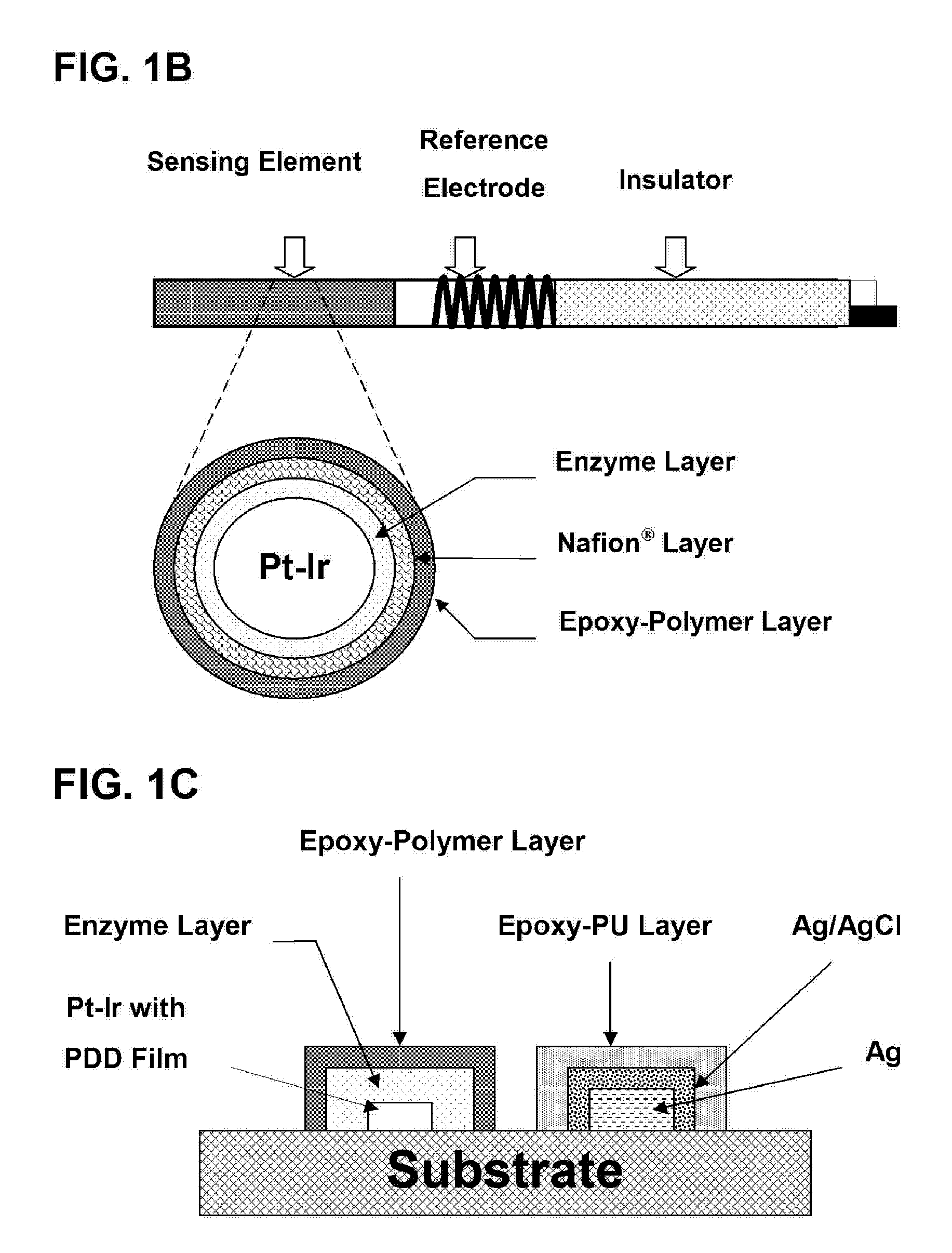
Epoxy Enhanced Polymer Membrane to Increase Durability of Biosensors
ActiveUS20060289307A1Increasing in vivo durabilityImprove long-term stabilityImmobilised enzymesBioreactor/fermenter combinationsEpoxyPolyethylene oxide
The present invention provides a polymer membrane enhanced with cured epoxy resin for use as the outer membrane of biosensors. The membrane includes approximately 30-80% epoxy resin adhesives, 10-60% polymer such as poly(vinyl chloride), polycarbonate and polyurethane and 0-30% plasticizers and 5-15% surface modifier reagent such as polyethylene oxide-containing block copolymers. Utilizing the polymer membrane of the present invention, a three-layered sensing element has been developed. This sensing element will be particularly useful for miniaturized biosensors used for in vitro blood measurements or for continuous in vivo monitoring such as implantable biosensors. This element includes an enzyme layer, an interference-eliminating layer and the novel polymer member of the present invention as the outer polymer layer. This novel sensing element shows excellent response characteristics in solutions and has an extremely long lifetime. This technology is particularly useful for improving the lifetime of implantable biosensors.
Owner:UNIV OF SOUTH FLORIDA
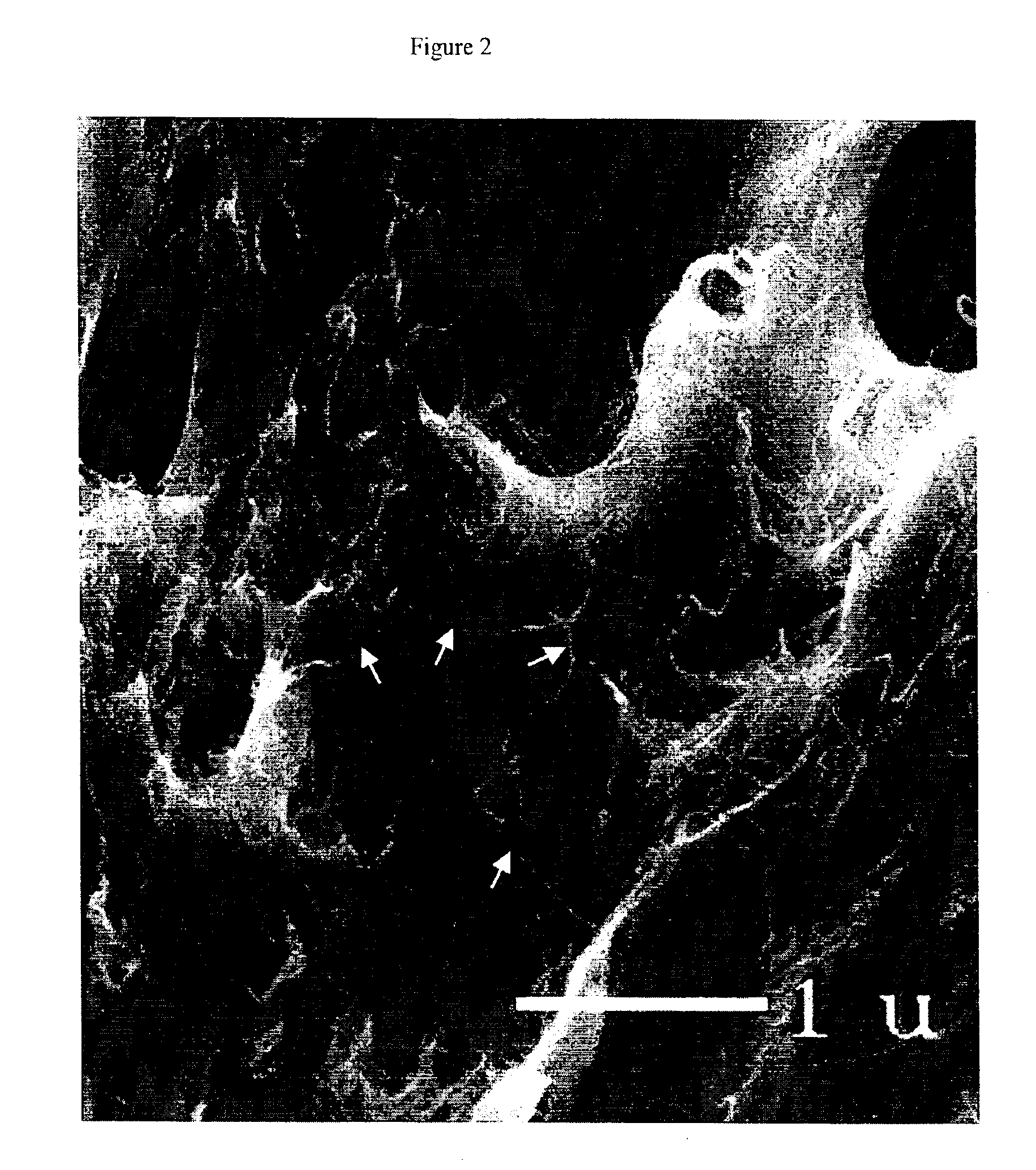
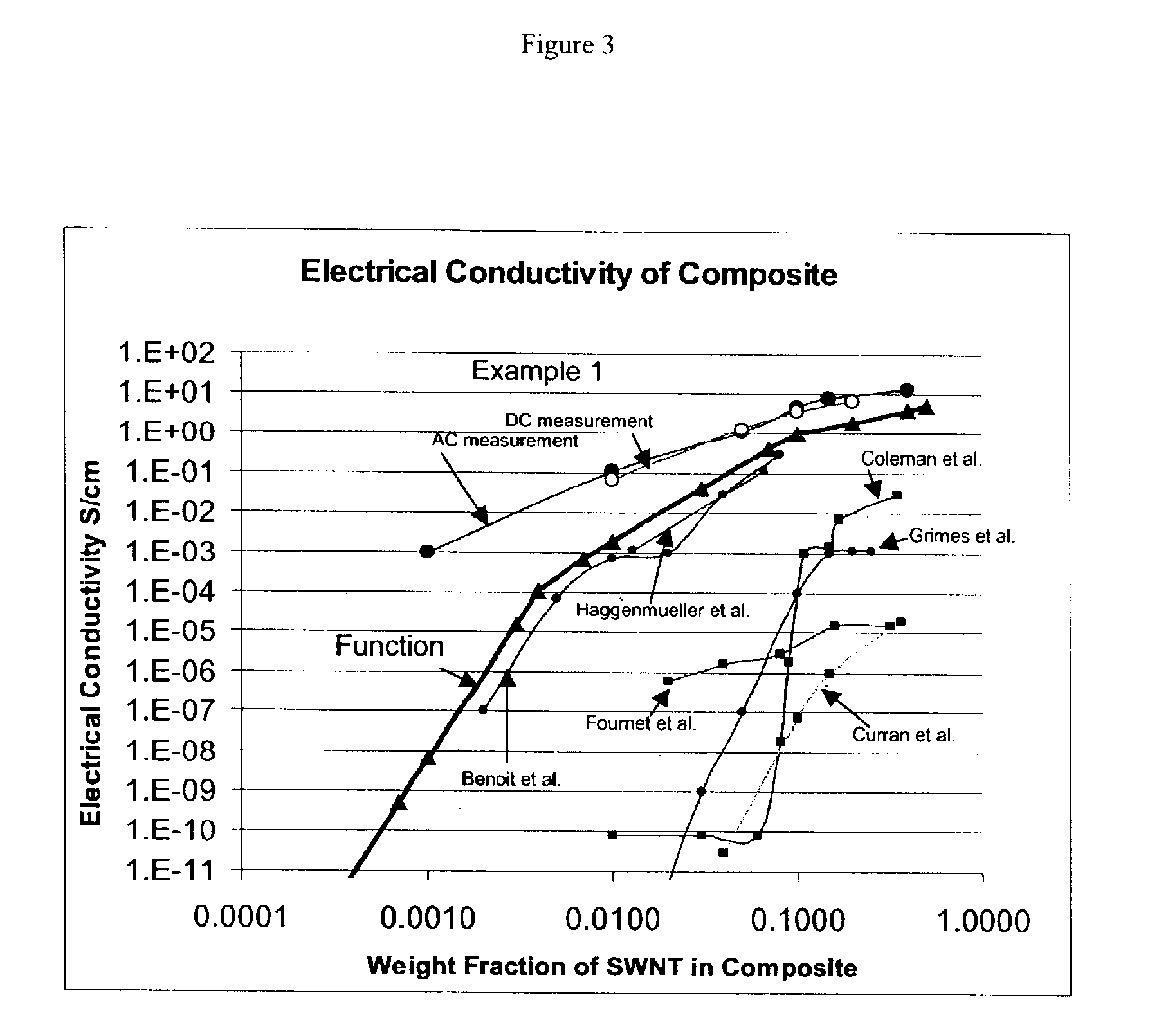
Composite materials comprising polar polymers and single-wall carbon nanotubes
InactiveUS6936653B2Improve conductivityMaterial nanotechnologyIndividual molecule manipulationPolyesterPolymer science
The invention relates to a composite comprising a weight fraction of single-wall carbon nanotubes and at least one polar polymer wherein the composite has an electrical and / or thermal conductivity enhanced over that of the polymer alone. The invention also comprises a method for making this polymer composition. The present application provides composite compositions that, over a wide range of single-wall carbon nanotube loading, have electrical conductivities exceeding those known in the art by more than one order of magnitude. The electrical conductivity enhancement depends on the weight fraction (F) of the single-wall carbon nanotubes in the composite. The electrical conductivity of the composite of this invention is at least 5 Siemens per centimeter (S / cm) at (F) of 0.5 (i.e. where single-wall carbon nanotube loading weight represents half of the total composite weight), at least 1 S / cm at a F of 0.1, at least 1×10−4 S / cm at (F) of 0.004, at least 6×10−9 S / cm at (F) of 0.001 and at least 3×10−16 S / cm (F) plus the intrinsic conductivity of the polymer matrix material at of 0.0001. The thermal conductivity enhancement is in excess of 1 Watt / m-° K. The polar polymer can be polycarbonate, poly(acrylic acid), poly(acrylic acid), poly(methacrylic acid), polyoxide, polysulfide, polysulfone, polyamides, polyester, polyurethane, polyimide, poly(vinyl acetate), poly(vinyl alcohol), poly(vinyl chloride), poly(vinyl pyridine), poly(vinyl pyrrolidone), copolymers thereof and combinations thereof. The composite can further comprise a nonpolar polymer, such as, a polyolefin polymer, polyethylene, polypropylene, polybutene, polyisobutene, polyisoprene, polystyrene, copolymers thereof and combinations thereof.
Owner:SAMSUNG ELECTRONICS CO LTD
Bisphenol a and aromatic glycidyl ether-free coatings
ActiveUS20070036903A1Good metal substrateGood inter-coat adhesionLiquid surface applicatorsSynthetic resin layered productsPolyesterMeth-
Disclosed are Bisphenol A (BPA), Bisphenol F, Bisphenol A diglycidyl ether (BADGE), and Bisphenol F diglycidyl ether (BFDGE)-free coating compositions for metal substrates including an under-coat composition containing a polyester (co)polymer, and an under-coat cross-linker; and an over-coat composition containing a poly(vinyl chloride) (co)polymer dispersed in a substantially nonaqueous carrier liquid, an over-coat cross-linker, and a functional (meth)acrylic (co)polymer. Also provided is a method of coating a metal substrate using the BPA, BPF, BADGE and BFDGE-free coating system to produce a hardened protective coating useful in fabricating metal storage containers. The coated substrate is particularly useful in fabricating multi-part foodstuffs storage containers with “easy-open” end closures.
Owner:SWIMC LLC
Method and apparatus for reclaiming oil from waste plastic
InactiveUS6011187AIncrease productionContinuous operationPlastic recyclingIndirect and direct heating destructive distillationForeign matterBoiling point
PCT No. PCT / JP97 / 00572 Sec. 371 Date Jan. 8, 1998 Sec. 102(e) Date Jan. 8, 1998 PCT Filed Feb. 27, 1997 PCT Pub. No. WO97 / 31990 PCT Pub. Date Sep. 4, 1997This invention provides a method for reclaiming oil from waste plastic in such a way that thermosetting resins and solid foreign matter in the plastic will not pose a problem. This method greatly reduces the burden of presorting the garbage or industrial waste. To achieve this objective when oil is to be reclaimed from a waste plastic containing chlorine compounds, such as vinyl chloride, the plastic must first be stripped of chlorine. Prior to pyrolysis, while being conveyed forward in a continuous stream, the plastic is mixed with heated sand and / or an additive agent to raise its temperature to 250-350 DEG C. This creates a product which is comprised of a mixture of sand and substantially dechlorinated plastic. The product is mixed with heated sand to heat it directly to a temperature of 350-500 DEG C. It is maintained at this temperature until pyrolysis occurs. In order to obtain high-quality oil with a low boiling point, a first gas / liquid separation process separates the product obtained from the aforesaid pyrolysis into liquid high-boiling point oil, gaseous low-boiling point oil and low molecular-weight gases, and recirculates the liquid high-boiling point oil to the pyrolysis process, and a second gas / liquid separation process separates the gaseous low-boiling point oil and low molecular-weight gases into liquid low-boiling point oil and low molecular-weight gases. The first and second gas / liquid separation process are connected in sequence.
Owner:MITSUBISHI HEAVY IND LTD
Chlorinated vinyl resin/cellulosic blends: composition, processes, composites, and articles therefrom
InactiveUS7030179B2High expansion rateEasy squeezeLavatory sanitoryRadiationCellulosePolymer science
Compositions and processes for preparing extrudable powder blends containing at least one vinyl chloride resin and a cellulosic material are provided. More specifically, compositions and processes for preparing extrudable free-flowing powder blends containing PVC and wood flour (WF) are also provided for preparing foamed or nonfoamed extrudates. The processes provided herein incorporate components which may contain up to a total of 25 weight percent water. Processes for preparing foamed extrudates are also provided wherein a cooling fluid is used to increase the expansion ratio of the foam. Finally provided are composites having an extrudable thermoplastic substrate and at least one capstock layer disposed thereon containing a PVC / WF composition.
Owner:ROHM & HAAS CO
Preparation method of super-strong high temperature-resistant chlorinated polyvinyl chloride tubes
ActiveCN102134360ADoes not show brittlenessExcellent low temperature toughnessRigid pipesPolymer scienceChlorinated polyvinyl chloride
The invention discloses a preparation method of super-strong high temperature-resistant chlorinated polyvinyl chloride tubes, which is characterized in that the tubes are made by the following steps: 75 to 100 portions of chlorinated polyvinyl chloride resin, 0 to 25 portions of vinyl chloride resin, 3 to 10 portions of ABS, 1 to 8 portions of acrylic acid resin, 3 to 8 portions of bismaleimides, 1 to 5 portions of plasticizing accelerator, 1 to 5 portions of compatibilizer, 10 to 25 portions of engineering plastics, 1 to 3 portions of lubricant, 1 to 5 portions of nanometer materials, 4 to 8 portions of stabilizing agent, 2 to 15 portions of filler, 0 to 1 portion of cross-linking agent, 1 to 5 portions of processing modifier and 3 to 10 portions of impact modifier are mixed and blended according to weight proportion, and then extruded by a mould. Compared with the prior art, the super-strong high temperature-resistant chlorinated polyvinyl chloride tubes has the advantages of being high in vicat softening point, being capable of meeting the high-temperature requirement of more than 120 DEG C, and having good anti-aging performance, antiflaming and insulation performance, and compression resistance, and long service life.
Owner:SHANGHAI YUANZHOU PIPE
Oleochemical Plasticizers with Thermal and Ultraviolet Radiation Stabilizing Activity for PVC Molding Resins and Process for Obtaining Thereof
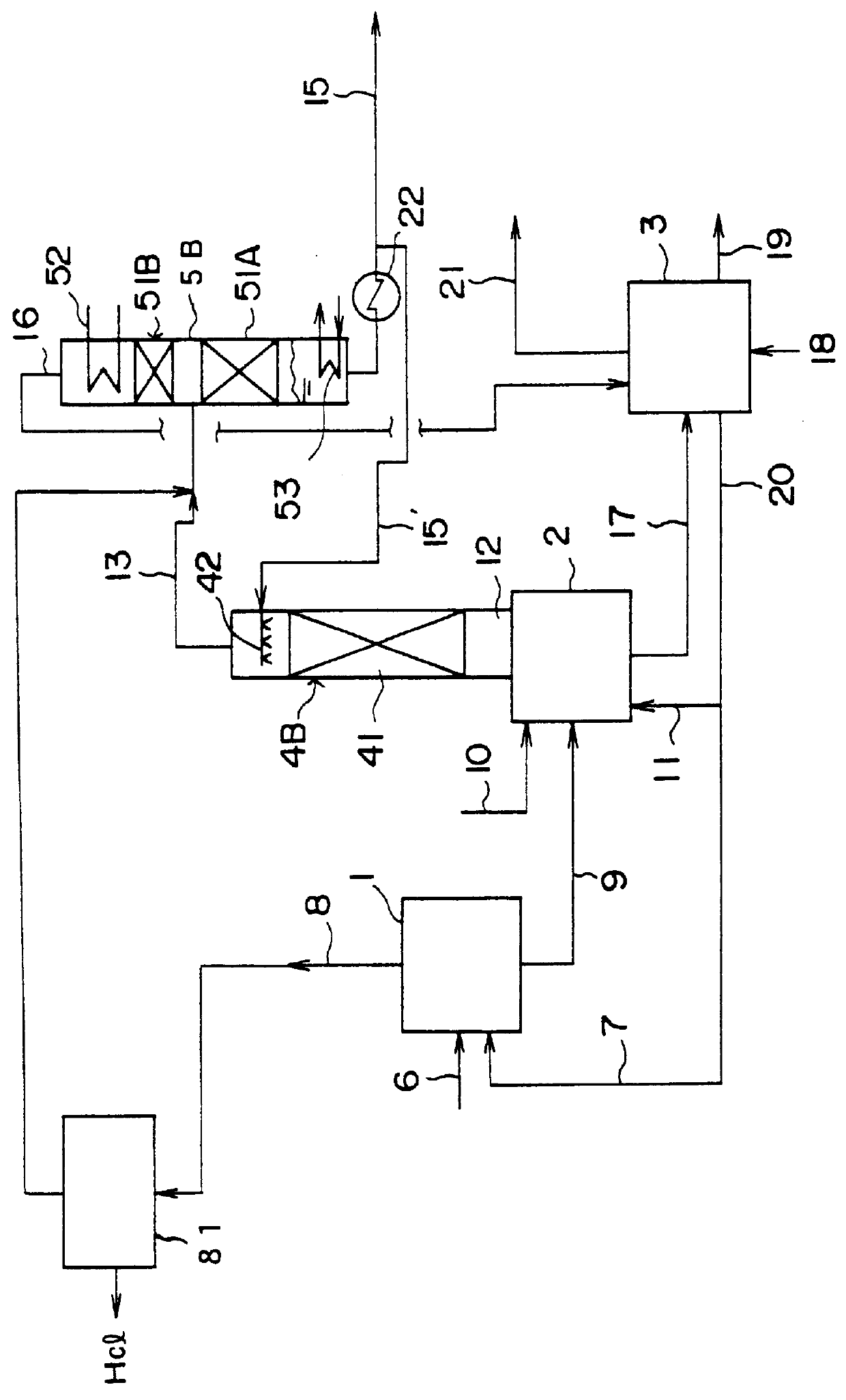
The present invention is related with bioplasticizers or primary oleochemical plasticizers and the improved process for obtaining thereof. It refers primarily to epoxydized oleochemical plasticizers produced from vegetable oils, as substitute of traditional petrochemical plasticizers. The process starts with the epoxydized product of natural oils, such as sunflower, linseed, Jatropha curcas, soybean, etc., which are transesterified with an alcohol such as ethylic or methylic, in the presence of a catalyst such as sodium methoxide or sodium hydroxide in order to produce an alkylic esters mixture of the fatty acids that were present in the oil or oil mixture used as raw material in the epoxydized oil production. When the plasticizer obtained by the process already mentioned is used for the formulation of moldable poly(vinyl chloride), PVC, resins; the resulting plastic films get adequate hardness, static and dynamic thermal stability, and plasticizer extractability by solvents, such as n-hexane, gasoline and oil. Besides, when the PVC resin is formulated with a phthalic or terephthalic plasticizers mixture and the bioplasticizer, the bioplasticizer presents a full range solubility and or compatibility with the remainder of the resin compounds. The oxyrane chemical ring of the bioplasticizer is an excellent chemical neutralizer of the HCL that might be formed from the PVC, due to the action or interference of thermal or UV radiation.
Owner:RESINAS & MATERIALES
Tea leaf-transporting apparatus
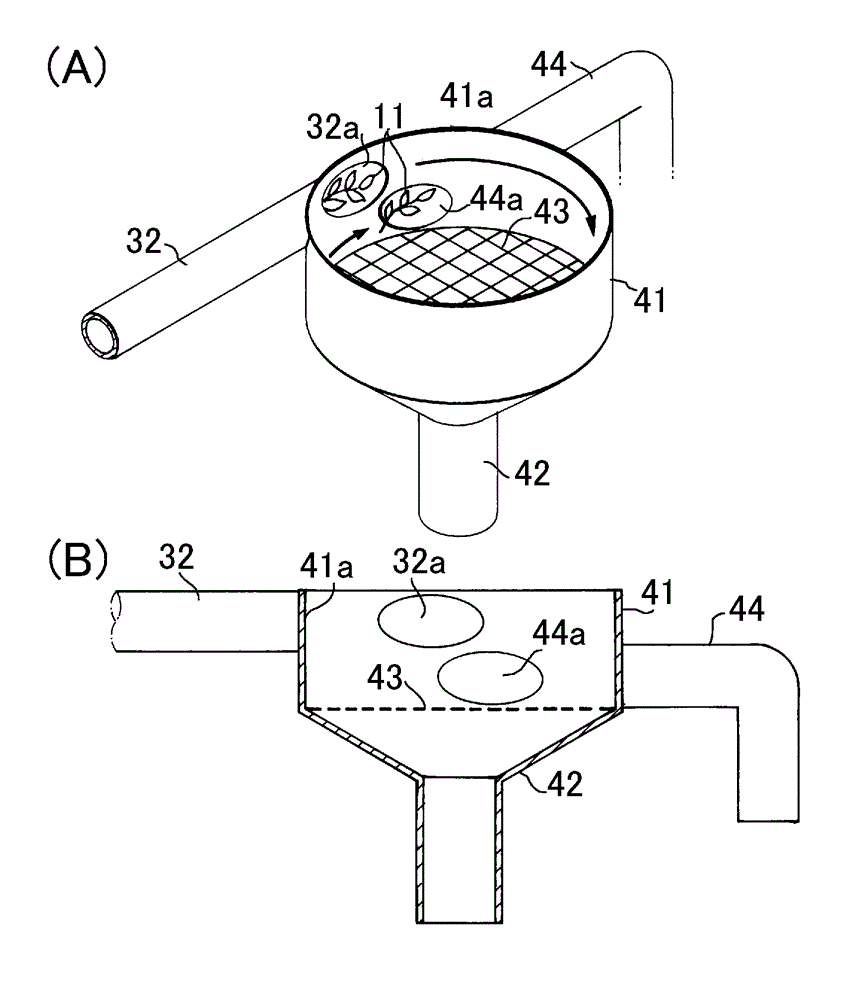
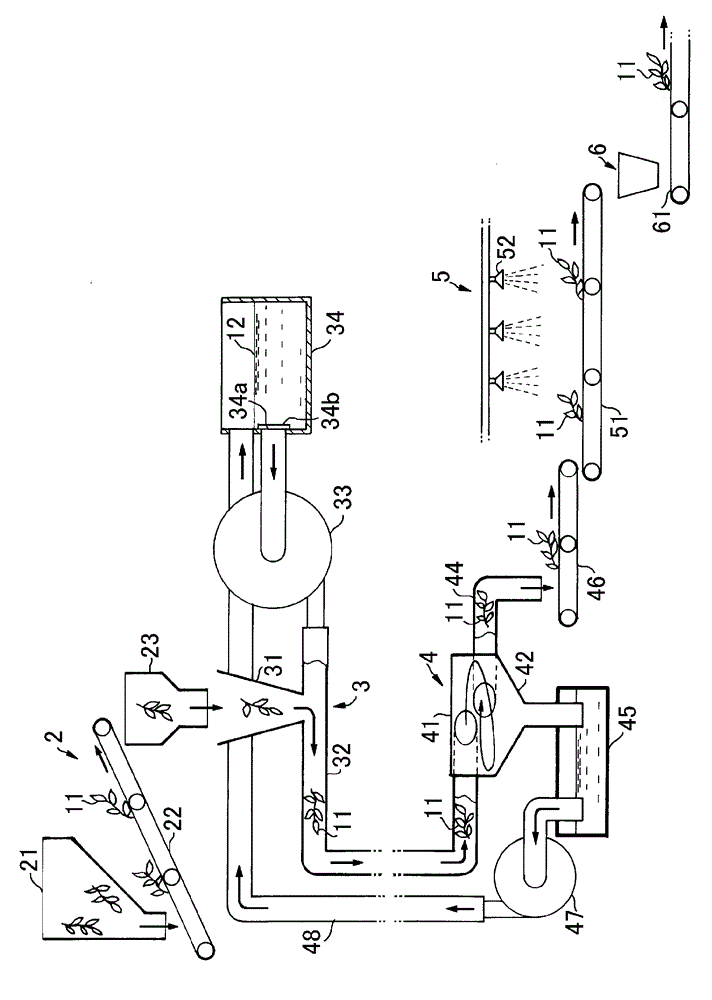
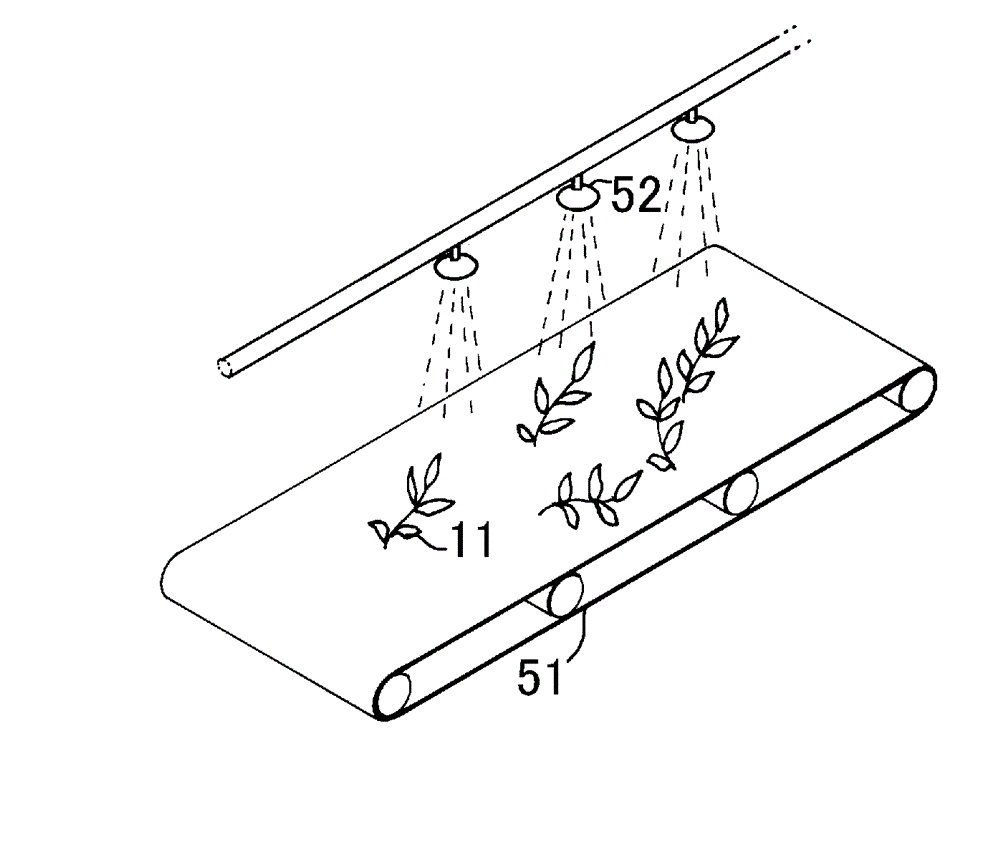
InactiveCN102753029AHandling hygieneCuticle loweringPre-extraction tea treatmentTea alkaloid content reductionEngineeringThermal water
Provided is a tea leaf-transporting apparatus whereby it is possible to transport tea leaves in a sanitary manner. The tea leaf-transporting apparatus (1) is characterized by a configuration for transporting tea leaves (11) fed into a transportation pipe (32) which is configured from a metal pipe of stainless steel or the like, or a resin pipe of vinyl chloride or the like, by means of hot water (12), and a configuration for separating the hot water (12) and the tea leaves (11) which flow out of the transportation pipe (32). Additionally, adjusting factors such as the temperature of the hot water (12) or the amount of time that the tea leaves (11) remain in the transportation pipe (32) makes it possible to sterilize the tea leaves, deactivate oxidase, reduce the cuticular layer, and reduce the amount of caffeine as said tea leaves are transported.
Owner:SHOKUHIN SANGYO HIGH SEP
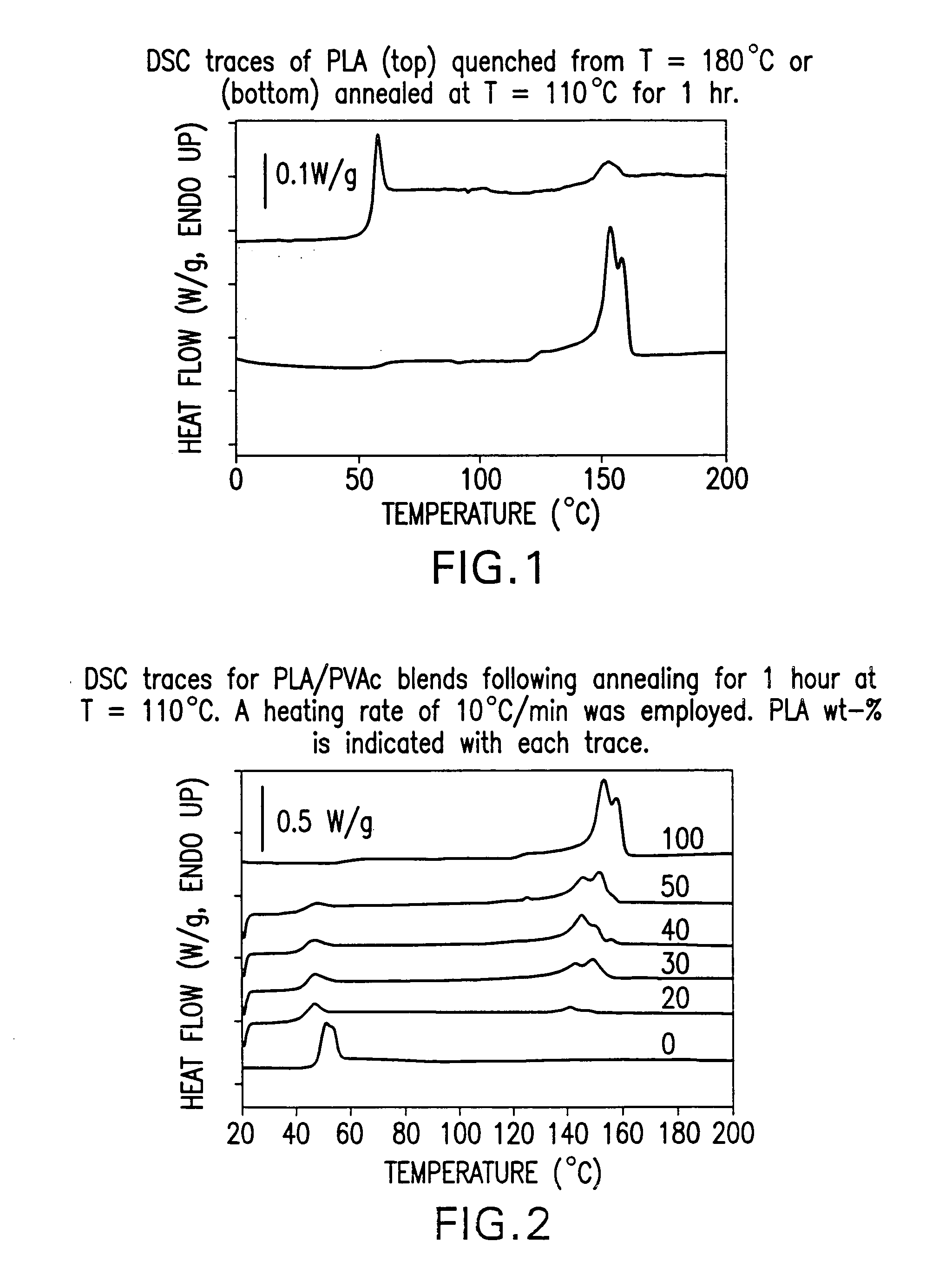
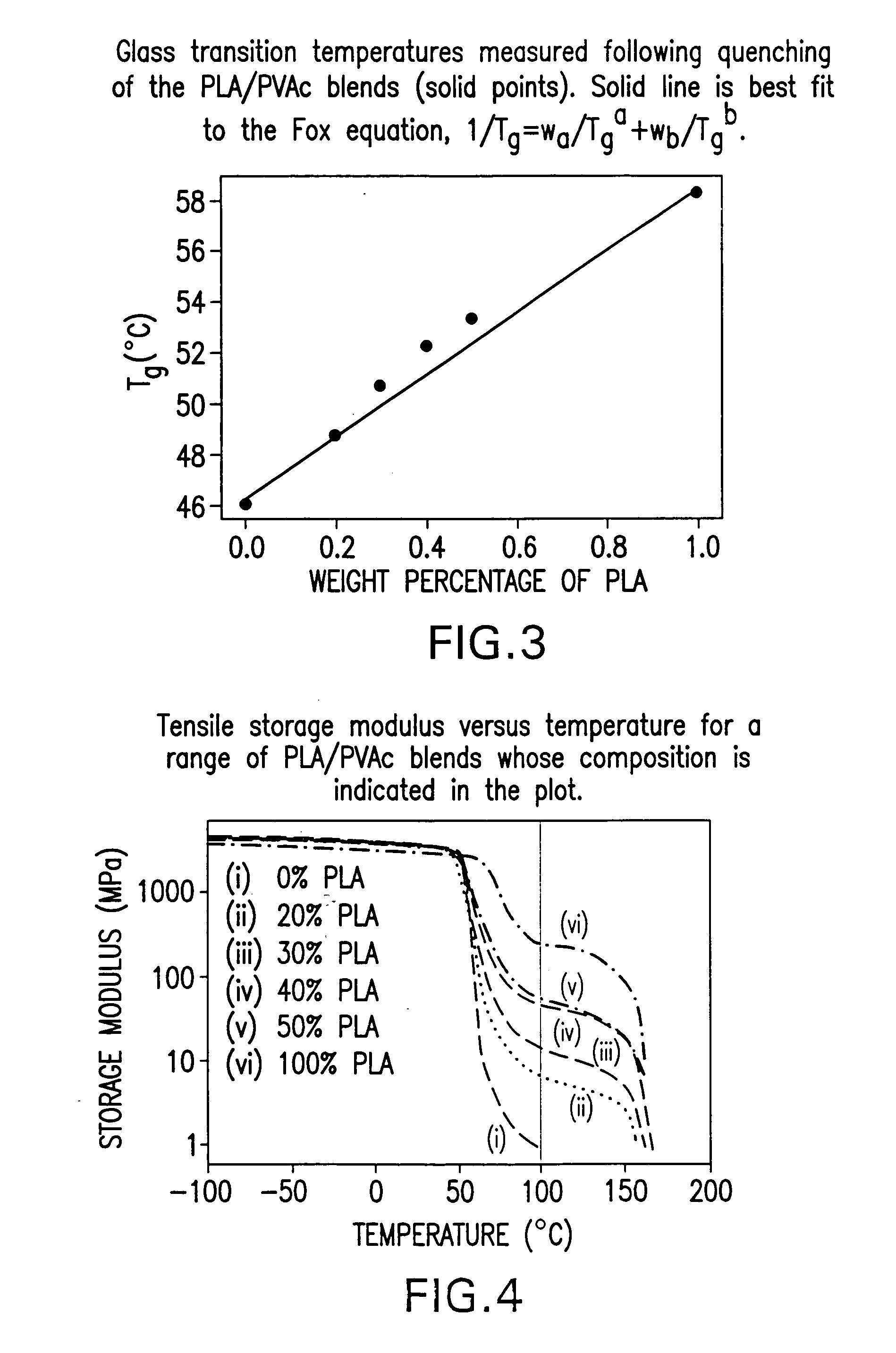
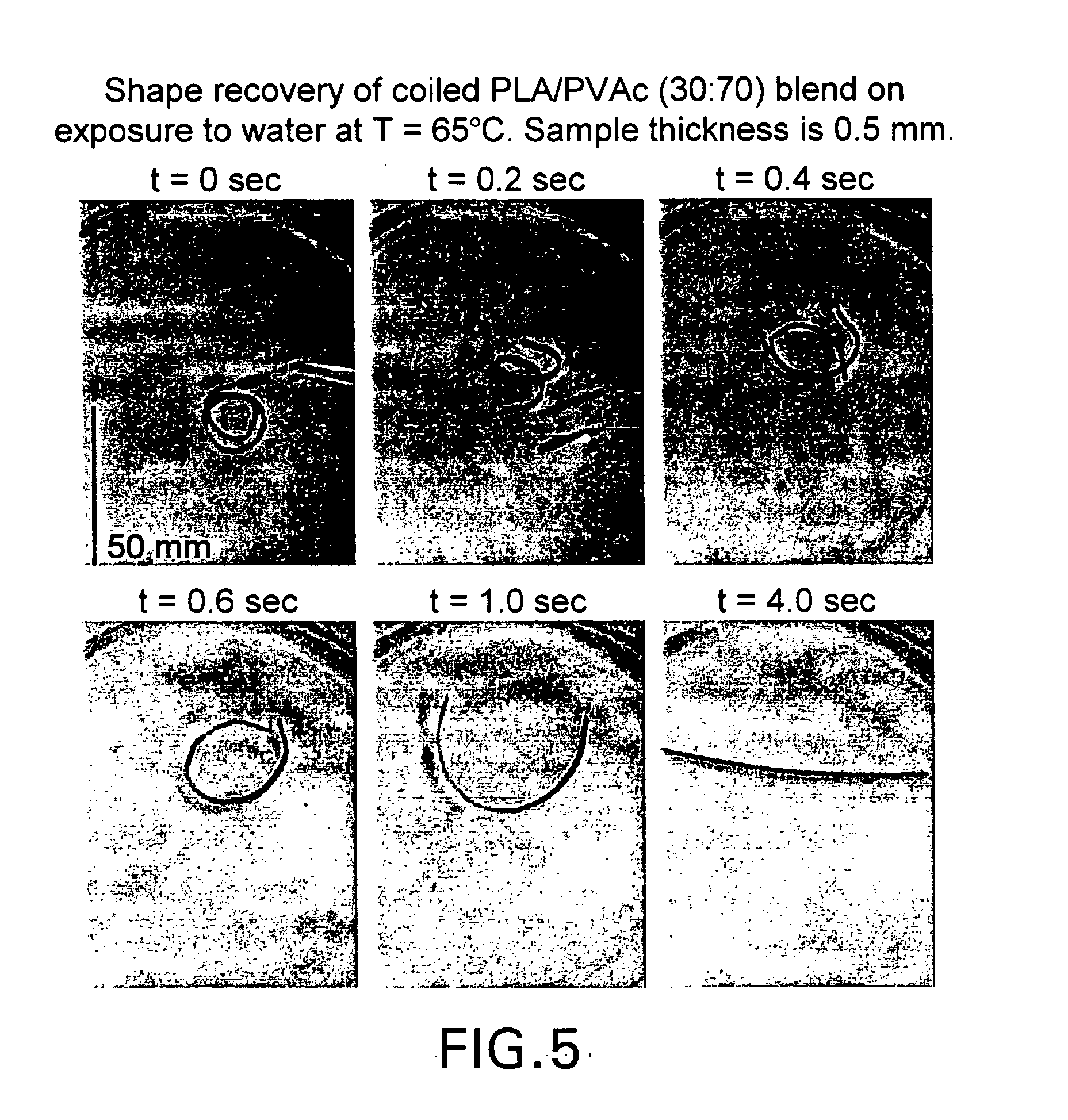
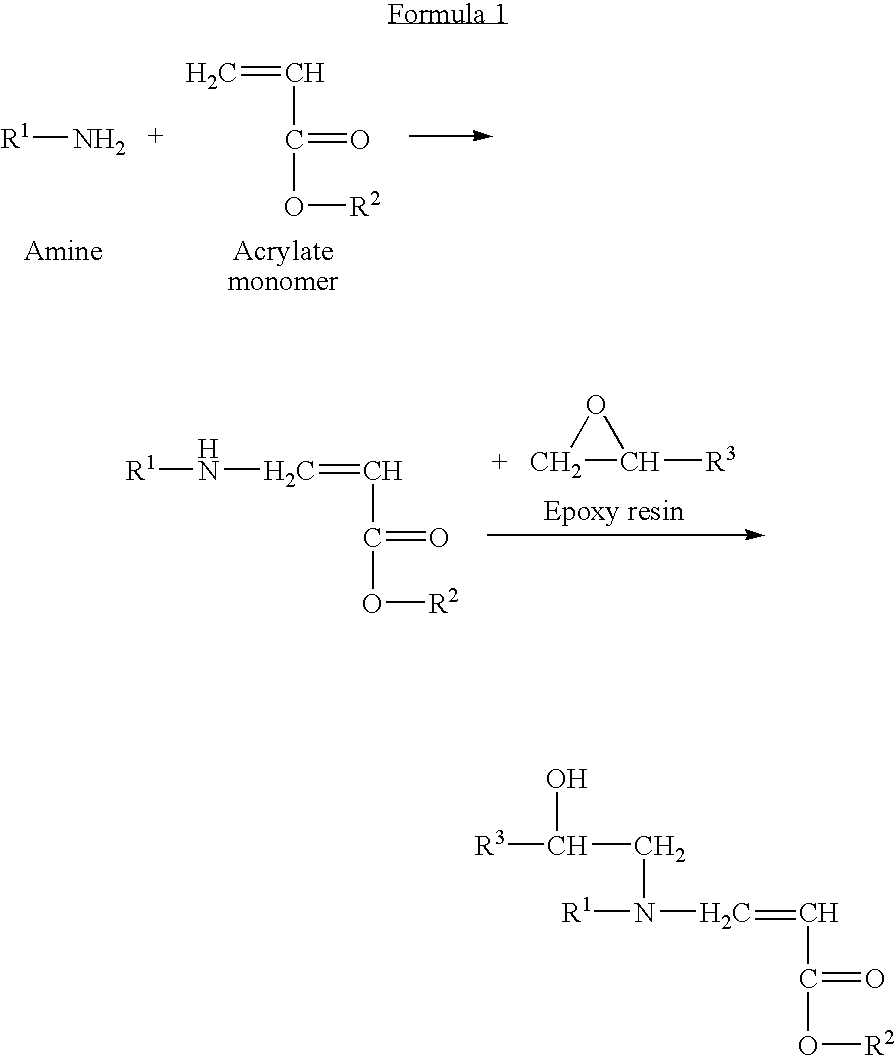
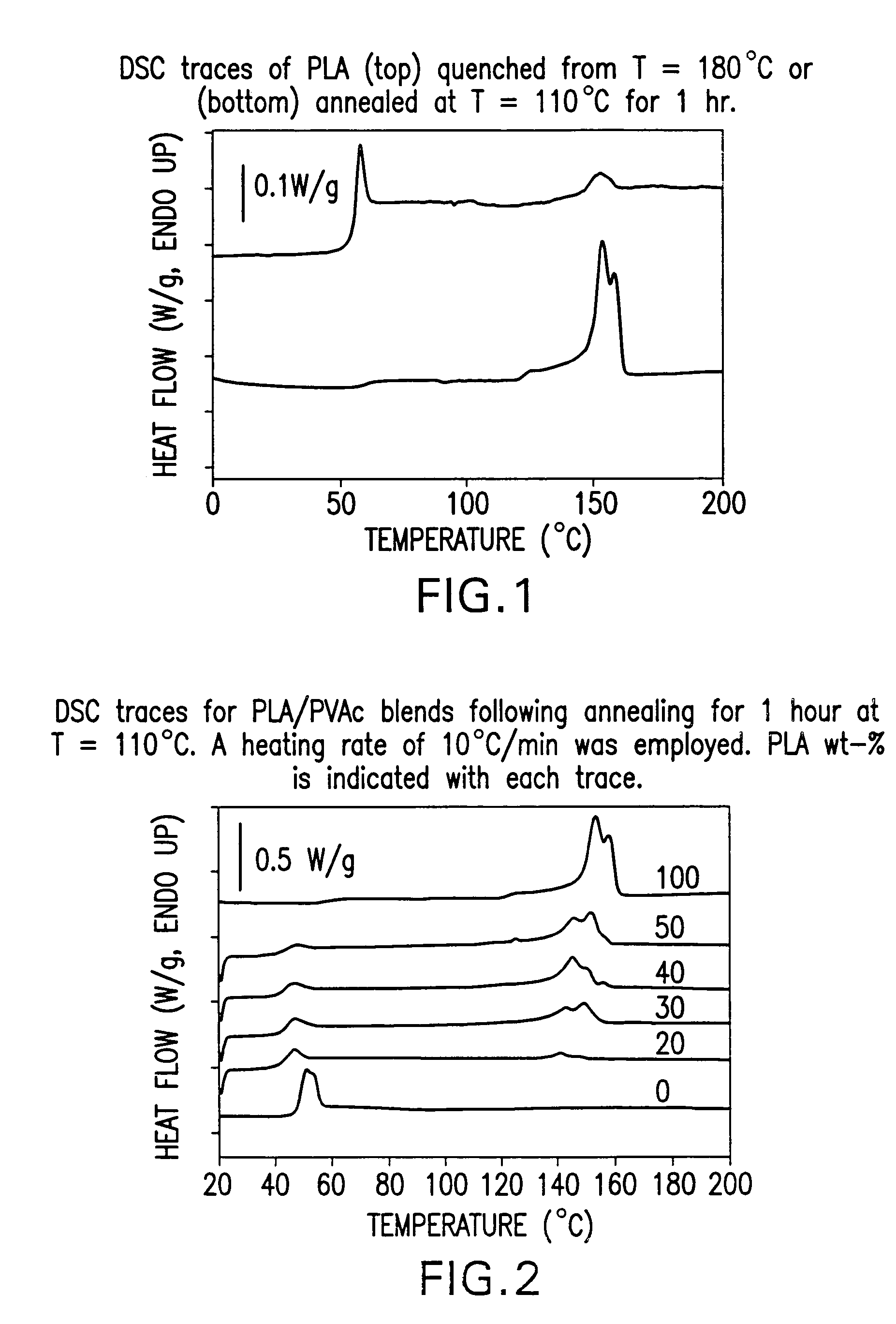
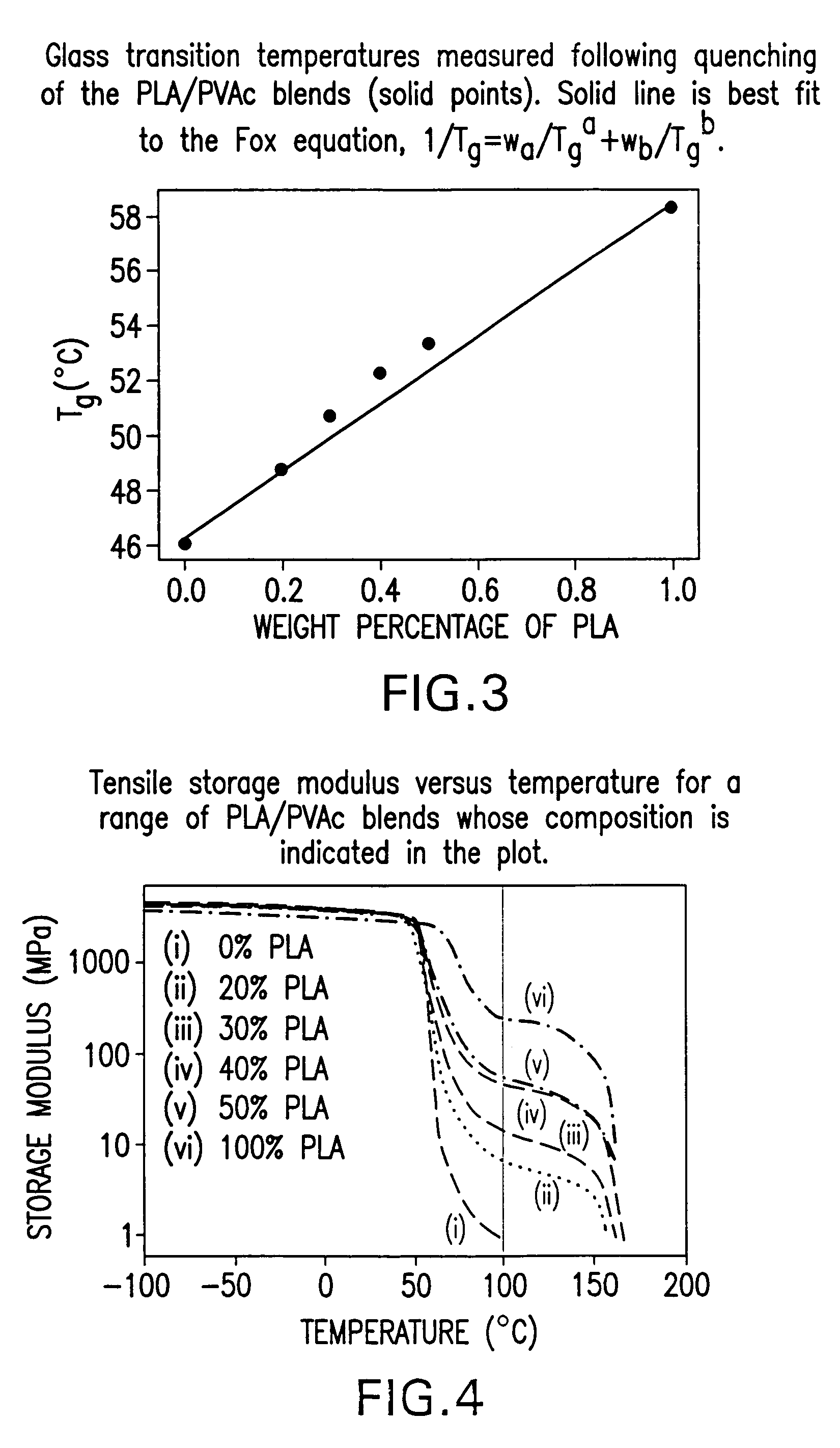
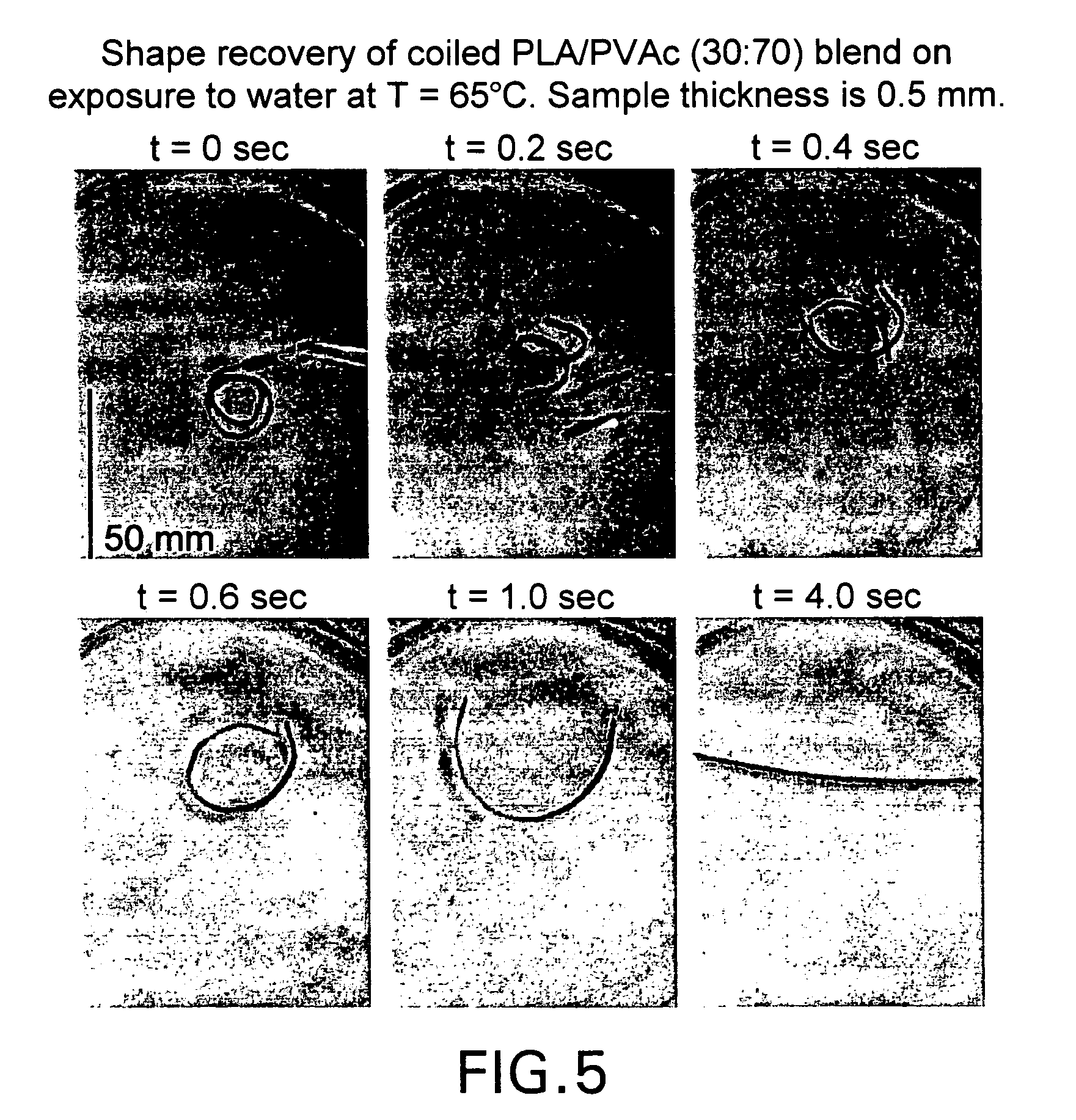
Blends of amorphous and semicrystalline polymers having shape memory properties
InactiveUS20040122174A1High modulusImprovement in critical temperatureDental impression compositionsPoly(methyl methacrylate)Ethyl acrylate
Blends of amorphous and semicrystalline polymers having shape memory properties were prepared by blending a crystalline polymer such as poly(vinylidene fluoride), polylactide, poly(hydroxxybutyrate), poly(ethylene glycol) polyethylene, polyethylene-co-vinyl acetate, poly(vinyl chloride), poly(vinylidene chloride) and copolymers of poly(vinylidene chloride) and poly(vinyle chloride) and an amorphous polymer such as poly(vinyl acetate), poly methyl acrylate, poly ethyl acrylate, atactic poly methyl methacrylate, isotactic poly methyl methacrylate, syndiotactic poly methyl methacrylate and other poly alkyl methacrylates. The method for preparing the polymeric materials and applications thereof, for example, as smart medical devices, are also disclosed.
Owner:UNIV OF CONNECTICUT
High-solid anticorrosive coating composition, high-solid rapidly-curable anticorrosive coating composition, method of coating ship or the like, high-solid anticorrosive film and rapidly cured high- anticorrosive film obtained, and coated ship and underwater structure coated with these coating films
InactiveUS20090226729A1Improve anti-corrosion performanceIncrease contentLiquid surface applicatorsAntifouling/underwater paintsOrganic solventBoiling point
A high-solids anticorrosive coating composition which comprises a main ingredient (A) comprising an epoxy resin (a1) and a hardener ingredient (B) comprising an alicyclic amine hardener (b1) and / or a Mannich type hardener (b2), the ingredient (A) and / or the ingredient (B) containing at least either of an additive (a2) selected among epoxidized reactive diluents and modified epoxy resins and a coating film modifier (ab) selected among petroleum resins, xylene resins, coumarone resins, terpene phenol resins and vinyl chloride copolymers. The high-solids anticorrosive coating composition especially of the rapidly curable type is characterized by containing a high-boiling organic solvent having a boiling point exceeding 150° C. and containing substantially no organic solvent having a boiling point of 150° C. or lower.
Owner:CHUGOKU MARINE PAINTS
Polyvinyl chloride antistatic dust-proof modified window section bar and producing method thereof
The invention discloses a kind of window PCE shaped material with antistatic and dustproof function and its manufacturing method. It raw materials are composes of PVC resin powder, antistatic agent, impact modifier, stabilizer, filler and processing aid in accordance with the weight ratio of 100:0.2-20:1-10:2-8:5-20:1-10. Then it heats and stirs the raw materials at a temperature of 100 ~ 130deg.C in the high-speed mixer, and then it slowly cools the mixture to room temperature and transfers the materials to the extruder, finally it achieves the shaped material through extrusion molding mold, vacuum cooling, traction and cutting.
Owner:LG HAUSYS TIANJIN
Method and apparatus for reclaiming oil from waste plastic
InactiveUS6172271B1Increase productionContinuous operationThermal non-catalytic crackingHydrocarbon distillationForeign matterBoiling point
Owner:MITSUBISHI HEAVY IND LTD
Use of an elastic polymer for production of a porous body in an additive manufacturing method
It is a feature of a use of an elastic polymer for production of a porous body (in an additive manufacturing method that the porous body comprises a three-dimensional network of node points joined to one another by struts, and a void volume present between the struts, where the struts have an average length of ≧200 μm to ≦50 mm and the struts (100) have an average thickness of ≧100 μm to ≦5 mm. The polymer here is an elastomer selected from the following group: thermoset polyurethane elastomers (PUR), thermoplastic copolyamides (TPA), thermoplastic copolyesters (TPC), thermoplastic olefin-based elastomers (TPO), styrene block copolymers (TPS), thermoplastic urethane-based elastomers (TPU), crosslinked thermoplastic olefin-based elastomers (TPV), thermoplastic polyvinyl chloride-based elastomers (PVC), thermoplastic silicone-based elastomers and a combination of at least two of these elastomers.
Owner:COVESTRO DEUTSCHLAND AG
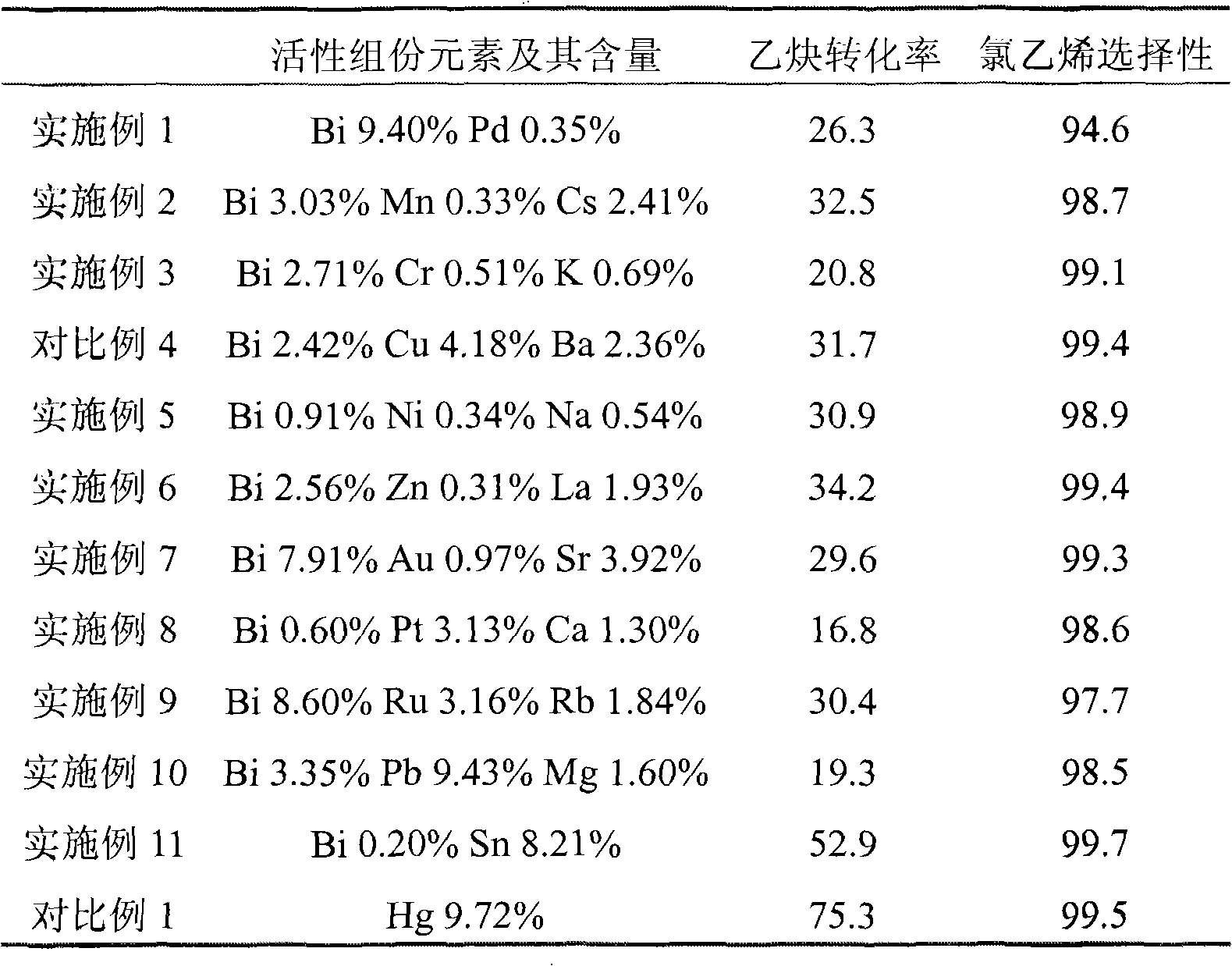
Non-mercuric catalyst used in hydrochlorination of acetylene and method for preparing vinyl chloride by using catalyst
InactiveCN102029189AGood stability in continuous useSolution to short lifePreparation by halogen halide additionOrganic-compounds/hydrides/coordination-complexes catalystsPhosphatePotassium
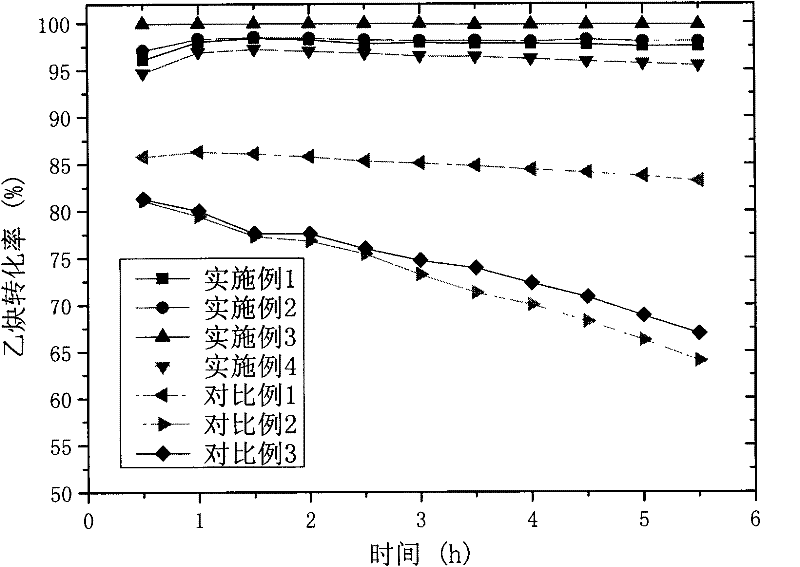
The invention relates to a non-mercuric catalyst used in hydrochlorination of acetylene and a method for preparing vinyl chloride by using the catalyst. The non-mercuric catalyst used in hydrochlorination of acetylene for preparing vinyl chloride comprises main active ingredients-gold salts, auxiliary active ingredients-non precious metal salts and a carrier, wherein the main active ingredients are gold salts and can be halides, complexes and the like of gold; gold in the gold salts accounts for 0.1-10% by weight of the catalyst; the auxiliary active ingredients are non precious metal salts and can be halides, acetates, phosphates, complexes and the like of potassium, barium, lanthanum and copper; the non precious metal salts account for 0.1-10% by weight of the catalyst; and the carrier is activated carbon, comprising coconut shell carbon, coal carbon, nutshell carbon or silica gel. The non-mercuric catalyst can be prepared by the conventional impregnation method, has simple preparation method, is environment-friendly and has the advantages of less by-products, good stability and long service life. The conversion rate of acetylene can be 90-99% and the selectivity of vinyl chloride is not lower than 99%.
Owner:EAST CHINA UNIV OF SCI & TECH
Method of treatment of a textile or non-woven substrate to render same water and oil repellent
The present invention relates to a method of treating a non-woven substrate or textile, comprising the step of applying to said non-woven substrate or textile a fluorochemical composition comprising a fluoropolymer that comprises:(a) between 10 and 97 mole % of units that can be derived from fluorinated monomer selected from the group consisting of monomers according to the general formula: Rf-X—OC(O)—C(R)═CH2 wherein Rf represents a perfluorinated aliphatic group having 3 or 4 carbon atoms, X is an organic divalent linking group and R represent hydrogen or a lower alkyl group having 1 to 4 carbon atoms; (b) between 3 and 75 mole % of units derived from a chlorine containing comonomer selected from the group consisting of vinylidene chloride, vinyl chloride and mixtures thereof; and (c) optionally further units derived from monomers other than a fluorinated monomer and said chlorine containing comonomers; wherein the amount of units (a), (b) and (c) adding up to 100%, whereby said fluorochemical composition is applied in such amount that the weight of fluoropolymer on said non-woven substrate or textile is not more than 3% by weight based on the weight of said non-woven substrate or textile.
Owner:3M INNOVATIVE PROPERTIES CO
Cold-resistant PVC cable material and preparation method thereof
ActiveCN101824193ADoes not change manufacturabilityReduce manufacturing costPlastic/resin/waxes insulatorsVinyl chlorideBrittleness
The invention relates to a cold-resistant PVC cable material which can resist the cold of minus 50DEG C, and a preparation method thereof; the material comprises PVC cable material; and after being added with the multipolymer of vinyl chloride and vinyl acetate, the cold resistant temperature of the cable material reaches minus 50DEG C. The invention has the advantages that: 1. the low-temperature impact brittleness temperature of the prepared low temperature resistant cable material can reaches minus 50DEG C; and 2. not only the process performance of PVC is not changed but also the manufacturing cost is low.
Owner:HANGZHOU GAOXIN RUBBER & PLASTIC MATERIALS CO LTD
Process for the start-up of an epoxidation process, a process for the production of ethylene oxide, a 1,2-diol, a 1,2-diol ether, a 1,2-carbonate, or an alkanolamine
InactiveUS20090281339A1Improve catalytic selectivityHigh selectivityProductsOrganic compound preparationOrganic chloride compoundOxygen
A process is provided for the start-up of an ethylene epoxidation process comprising: (a) contacting a catalyst bed comprising a high selectivity epoxidation catalyst with a feed comprising ethylene, oxygen and an organic chloride for a period of time until an increase of at least 1×10−5 mole-% of vinyl chloride (calculated as the moles of vinyl chloride relative to the total gas mixture), preferably 2×10−5 mole-% of vinyl chloride is detected in a reactor outlet gas or a recycle gas loop; and (b) subsequently adjusting the quantity of organic chloride in the feed to a value sufficient to produce ethylene oxide at a substantially optimum selectivity.
Owner:SHELL OIL CO
Blends of amorphous and semicrystalline polymers having shape memory properties
InactiveUS7208550B2High modulusDental impression compositionsPoly(methyl methacrylate)Glycol synthesis
Blends of amorphous and semicrystalline polymers having shape memory properties were prepared by blending a crystalline polymer such as poly(vinylidene fluoride), polylactide, poly(hydroxxybutyrate), poly(ethylene glycol) polyethylene, polyethylene-co-vinyl acetate, poly(vinyl chloride), poly(vinylidene chloride) and copolymers of poly(vinylidene chloride) and poly(vinyle chloride) and an amorphous polymer such as poly(vinyl acetate), poly methyl acrylate, poly ethyl acrylate, atactic poly methyl methacrylate, isotactic poly methyl methacrylate, syndiotactic poly methyl methacrylate and other poly alkyl methacrylates. The method for preparing the polymeric materials and applications thereof, for example, as smart medical devices, are also disclosed.
Owner:UNIV OF CONNECTICUT
Hot stamping foil applied to overlapping hot stamping on gold stamping layer and preparation method of hot stamping foil
ActiveCN103465668AImprove trimming effectTransfer completelyDuplicating/marking methodsPolyurea/polyurethane coatingsHot stampingCellulose
The invention discloses a hot stamping foil applied to overlapping hot stamping on a gold stamping layer and a preparation method of the hot stamping foil. When overlapping hot stamping is carried out on an existing hot stamping foil, the defects that the transferring is incomplete, the hot stamping is not carried out on some portions due to omission, scumming is caused, powder falls off, and the edge of a hot stamping layer is not clear can happen. The hot stamping foil is composed of a base membrane layer, a release layer, a color coating layer, a vacuum aluminum coated layer and a gumming layer. The gumming layer is prepared by raw materials which comprise, by weight, 1-6 parts of chloroethylene-vinyl acetate resin, 1-10 parts of polyvinyl alcohol, 1-10 parts of acrylic ester, 1-5 parts of nitrocellulose, 1-5 parts of fumed silica, 20-70 parts of ethyl acetate, 10-50 parts of ethyl alcohol and 1-5 parts of propyl acetate. According to the hot stamping foil applied to the overlapping hot stamping on the gold stamping layer, the hot stamping foil has the good stamping performance and the good trimming performance, the overlapping hot stamping layer is complete in transferring, the edge is smooth and free of burrs, the adhesion is firm, the phenomena of dusting, powder falling and the like do not exist, the hot stamping effect is strong in third dimension, and the anti-fake performance is more outstanding.
Owner:云南玉溪东魅包装材料有限公司
Wood flooring composed of wpl, base and soundproof layer
InactiveUS20060172118A1Improve surface strengthEnhance natural textureCovering/liningsLighting and heating apparatusWood veneerHigh density
Owner:LG CHEM LTD
Low noble metal mercury-free catalyst for acetylene hydrochlorination reaction, preparation method and application thereof
ActiveCN103894208AHigh catalytic activityImprove stabilityPreparation by halogen halide additionCatalyst activation/preparationActive componentMass content
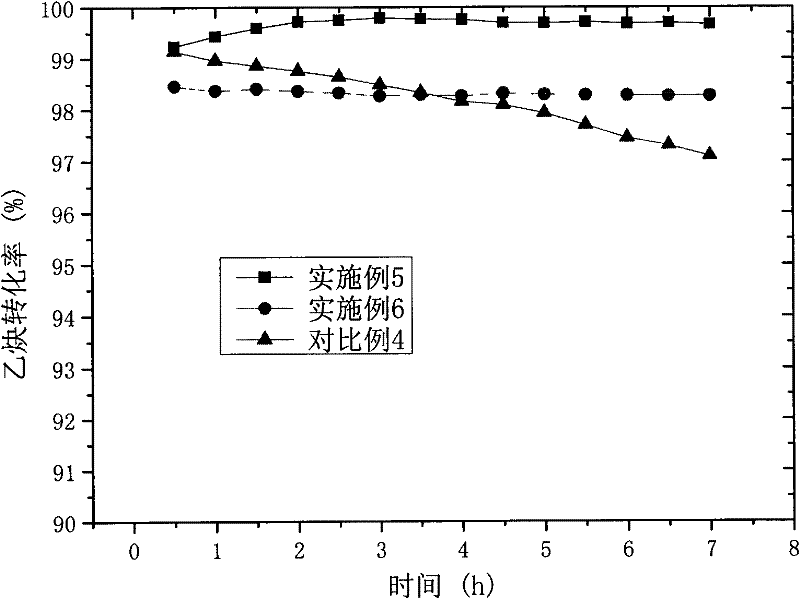
This invention relates to a low noble metal mercury-free catalyst for a vinyl chloride synthesis reaction through an acetylene hydrochlorination reaction, a preparation method and an application thereof. The catalyst comprises an active carbon carrier and a mixed active component comprising one or a plurality of noble metal compounds and one or a plurality of general metal compounds according to a certain ratio, wherein the mass content of the noble metal is 0.01-0.04%, and the mass content of the general metal is 0.1-1%. The low noble metal mercury-free catalyst has characteristics of low noble metal content, low catalyst cost, high catalyst activity and long service life.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI +1
Anticorrosive pigment composition and coating compositions containing the same
A nontoxic anticorrosive pigment composition free from toxic heavy metals comprises a condensed phosphoric acid aluminum salt, typically aluminum dihydrogen tripolyphosphate, and magnesium silicate, typically magnesium hexasilicate. The anticorrosive pigment composition enhances the thermal resistance of paint films when formulated in a coating composition containing a chlorine-containing vinyl polymer such as vinyl chloride-vinylidene chloride copolymer emulsion paints.
Owner:TAYCA CORP
A kind of preparation method of mercury-free catalyst for the synthesis of vinyl chloride by acetylene method
InactiveCN102259007AHigh catalytic activityImprove catalytic stabilityPreparation by halogen halide additionCatalyst activation/preparationPolyvinyl chlorideBULK ACTIVE INGREDIENT
The invention discloses a method for preparing a mercury-free catalyst for the synthesis of vinyl chloride by acetylene method. In the method, the active components of the catalyst are highly dispersed and bonded to the surface of the carrier by carrying out surface modification treatment on the catalyst carrier and applying ultrasonic loading technology. On the group, a firm load is formed in the internal pores of the carrier, so that while improving its catalytic performance and stability, it can greatly reduce the loading of the required active components and reduce the cost of the catalyst. The method is especially suitable for preparing supported noble metal catalysts. The supported noble metal catalysts prepared by the method of the present invention can reach and exceed the catalytic activity of the currently used mercuric chloride catalysts in the case of a very small amount of loading. And stability, has industrial application value, and is of great significance in environmental protection and promoting the sustainable development of my country's polyvinyl chloride industry.
Owner:李伟
Polymeric extenders for surface effects
A polymer extender composition comprising monomers copolymerized in the following percentages by weight: (a) from about 5% to about 90% of a monomer of the formula I: R1—OC(O)—C(R)═CH2 (I) (b) from about 5% to about 85% of vinylidene chloride, vinyl chloride, vinyl acetate, or a mixture thereof, (c) from about 0.5% to about 3% of a monomer of the formula II: HO—CH2—NH—C(O)—C(R)═CH2 (II) (d) from about 0.5% to about 3% of a monomer of the formula III HO—CH2CH2—OC(O)—C(R)═CH2 (III) and (e) from about 1% to about 5% of a monomer of the formula IV: H—(OCH2CH2)m—O—C(O)—C(R)═CH2 (IV) (f) from 0% to about 25% of methyl methacrylate, vinylbenzyl chloride, styrene or a mixture thereof, wherein each R is independently H or CH3; R1 is a linear or branched or cyclic alkyl chain having from about 4 to about 18 carbon atoms, and m is 2 to about 10.
Owner:EI DU PONT DE NEMOURS & CO
Aqueous rust-conversion rust-inhibiting primer
The invention relates to a water-based rust conversion antirust primer which is characterized in that: the primer comprises the following components with the corresponding percentage by weight : main conversion agent 5-35, auxiliary conversion agents 2-15, film forming material 40-55, penetrant 0.2-1.0, humectant 1-10, anti-forming agent 0.1-0.5, corrosion inhibitor 0.2-0.5 and solvent 0.5-8; the process is as follows: adding deionized water to a charging basket and heating the water to 60-80 DEG C with aliphatic alcohol polyethenoxy ether added, adding propylene glycol and starting a high-speed dispersion machine for agitation, later on adding tributyl phosphate, potassium sorbate, tannic acid and one of the auxiliary conversion agents and cooling down to 20-33 DEG C after agitating for 30-55 minutes, and adding vinyl chloride-modified acrylic resin latex and discharging material after agitating for 10-20 minutes. The invention has the advantages of direct application to steel surfaces with rust, thorough conversion of rust, excellent adhesive force of a conversion film, good flexibility, adaptation to various forms, and good compatibility with various top coatings.
Owner:安泰能(上海)高分子材料有限公司
Method for synthesizing and regenerating mercury-free catalyst for hydrochlorination of acetylene and application thereof
InactiveCN101670293AHigh activityGood choicePreparation by halogen halide additionCatalyst regeneration/reactivationSpray GranulationHydrogen
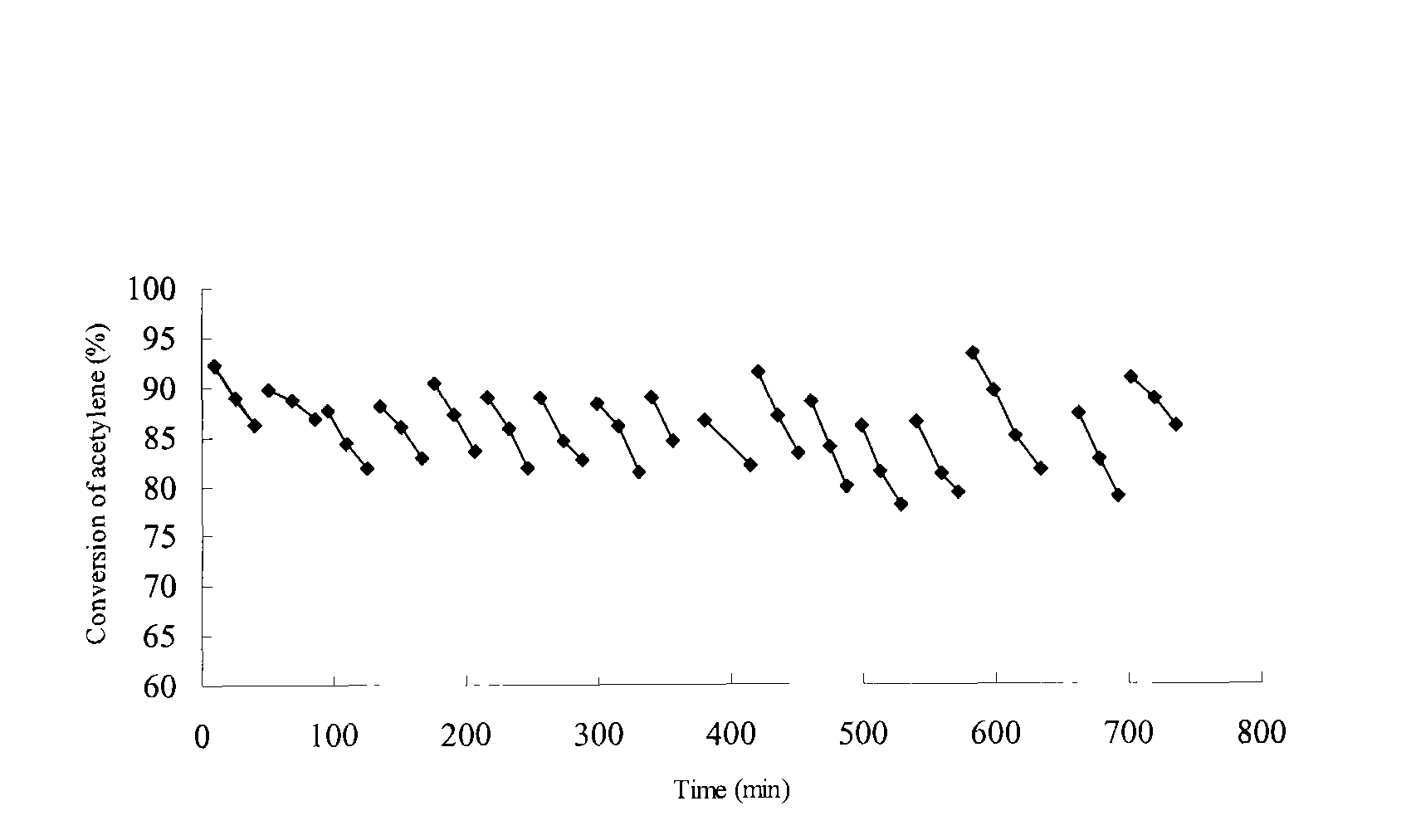
The invention relates to a method for synthesizing and regenerating a mercury-free catalyst for hydrochlorination of acetylene and an application thereof, which belongs to the technical field of preparation of the mercury-free catalysts. The catalyst contains bismuth, phosphorous, a component for promoting catalysis and a carrier. The content of bismuth element accounts for 0.1-68.8wt% of the weight of the catalyst, the content of phosphorous element accounts for 0.1-40.8wt% of the weight of the catalyst, the content of the component for promoting the catalysis accounts for 0.1-63.6wt%, and the content of the carrier accounts for 50-99.5%. The coke-burning regeneration is carried out on the catalyst in hydrogen, water vapor, oxygen, air or other oxygen-containing oxidative atmosphere at the temperature of 300-600 DEG C after the inactivation. The catalyst is prepared by the impregnation method, the co-precipitation method or the spray granulation method. The catalyst provided by the invention has the advantages of good activity, high selectivity, high strength and regeneration, and can be used for producing vinyl chloride by the hydrochlorination of the acetylene.
Owner:TSINGHUA UNIV
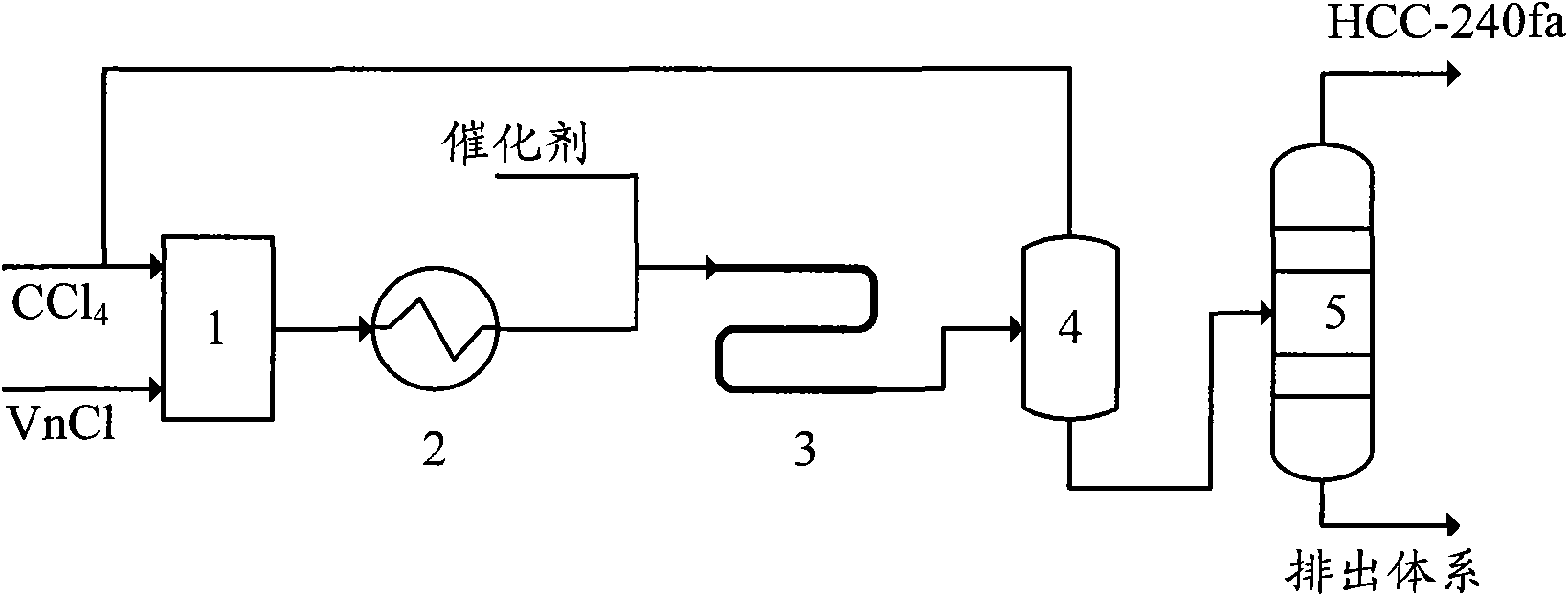
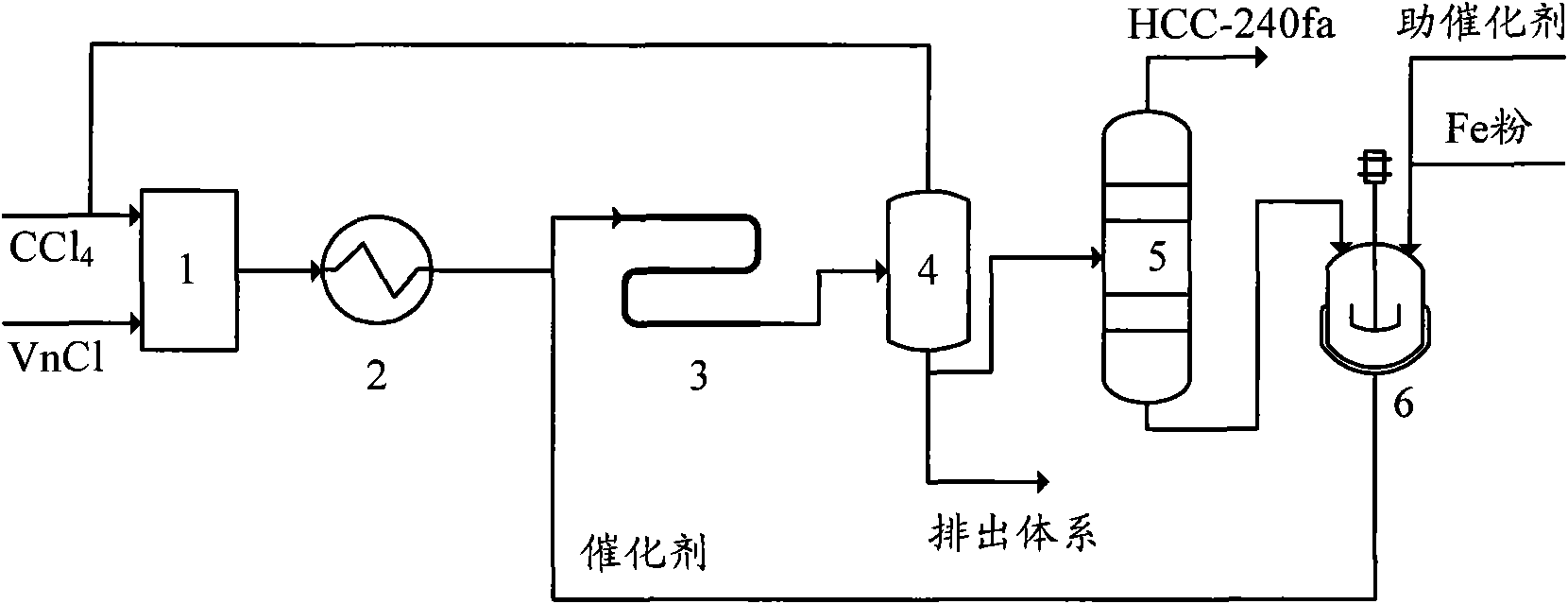
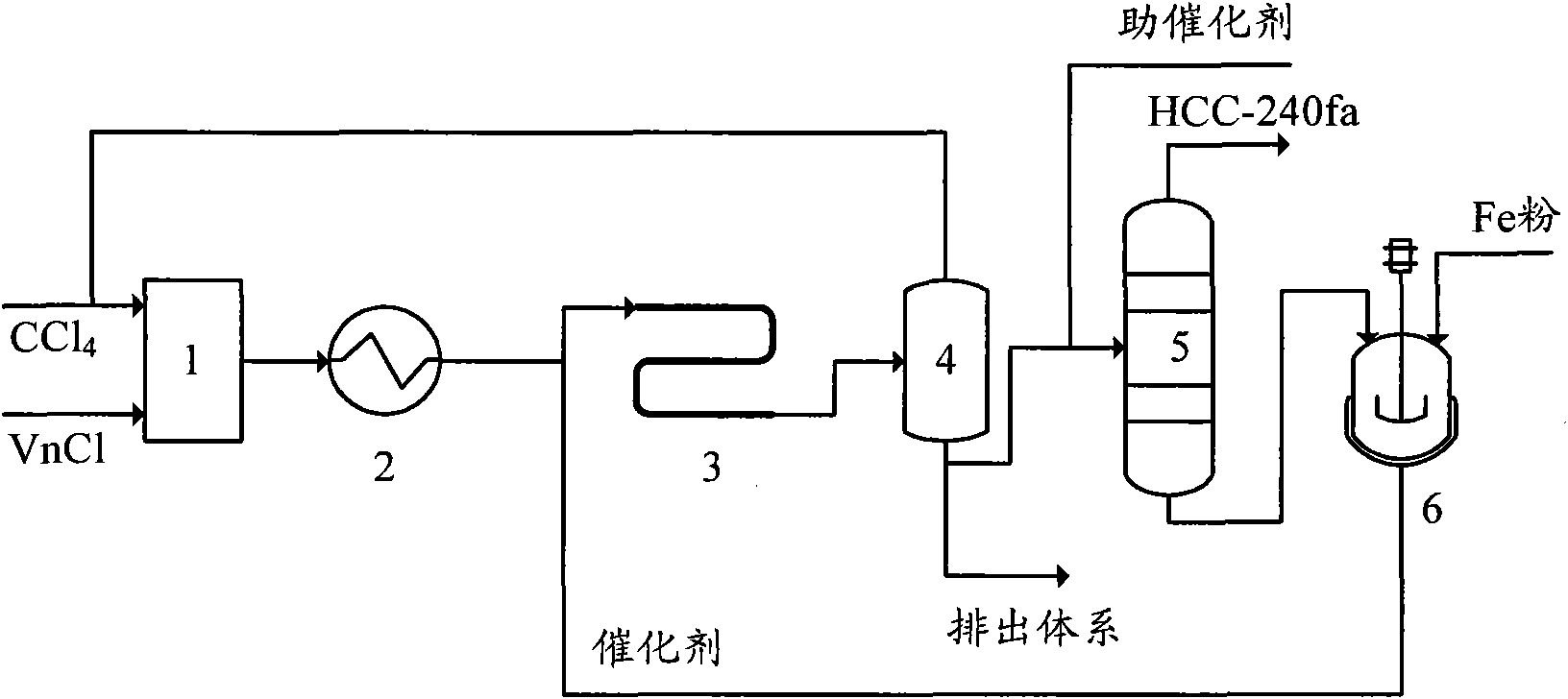
Production method of 1,1,1,3,3-pentachloropropane
InactiveCN101913980AEasy to transportAccurate measurementOrganic-compounds/hydrides/coordination-complexes catalystsChemical recyclingReaction temperatureSilicon tetrachloride
The invention provides a production method of 1,1,1,3,3-pentachloropropane (HCC-240fa). The 1,1,1,3,3-pentachloropropane is synthesized by continuously reacting the materials of carbon tetrachloride and chloroethylene in a tubular reactor. The method comprises the steps of firstly mixing the carbon tetrachloride with the chloroethylene and preheating the mixture to a reaction temperature, then introducing the mixture together with a catalyst into the tubular reactor for telomeric reaction, removing the unreacted carbon tetrachloride and chloroethylene through flash evaporation, and then further distilling to obtain the HCC-240fa, wherein the unreacted carbon tetrachloride and chloroethylene are recycled and the catalyst is recycled after being subjected to the activating treatment. The invention adopts the liquid as the activated system and uses the tubular reactor for reaction, thereby ensuring simple and convenient operation as well as continuous production.
Owner:XIAN MODERN CHEM RES INST
Gold complex catalyst for hydrochlorinating acetylene
ActiveCN102631947AImprove stabilityHigh activityPreparation by halogen halide additionOrganic-compounds/hydrides/coordination-complexes catalystsGold contentPotassium
The invention discloses a gold complex catalyst, and particularly relates to a non-mercury catalyst which is applicable to synthesizing vinyl chloride by acetylene hydrochlorination, and a preparation method of the non-mercury catalyst. The non-mercury catalyst comprises potassium aurate tetra-thiocyanate taken as a main active component, one or a combination of more than two of potassium chloride, copper chloride, cobalt chloride and zinc chloride taken as auxiliary active components, and carrier activated carbon, wherein gold complex accounts for 0.1-2.5% of the weight of the catalyst, and the auxiliary active components account for 0.05-15% of the total weight of the catalyst. According to the gold complex catalyst prepared by the invention, the gold content is lower, so that the cost of noble metal catalyst can be remarkably reduced; and the novel non-mercury catalyst is good in activity, high in stability and strong in selectivity. The gold complex catalyst has the advantages of being simple in production technology, short in production cycle and environment-friendly.
Owner:XINJIANG CORPS MODERN GREEN CHLOR ALKALI CHEM ENG RES CENT LTD +1
Composite metal salt catalyst for hydrochlorination reaction of acetylene
ActiveCN102631942AGuaranteed stabilityImprove stabilityPhysical/chemical process catalystsPreparation by halogen halide additionPotassiumCarbon nanotube
The invention discloses a composite metal salt catalyst for a hydrochlorination reaction of acetylene, belonging to the technical field of catalysts. The composite metal salt catalyst takes gold as active metal, and the reduction deactivation of the composite metal salt catalyst is reduced by reducing reduction potential of metal through a complexing action of a thiocyanate radical or a cyanate radical. Carbon deposition in a reaction process is restrained by introducing one or more of potassium, cerium and lanthanum elements. The composite metal salt catalyst is loaded on active carbon or carbon nanometer pipe with the specific surface area of not less than 100m<2>*g<-1>, wherein the mass fraction of a gold load is 0.05-0.50%, the mass fraction of a copper load is 0.1-5.0%, and the mass fraction of the potassium / cerium / lanthanum load is 0.1-5.0%. In a reaction for preparing vinyl chloride, composite salt of gold and copper has excellent activity, selectivity and stability; furthermore, one or two of cerium and lanthanum are added to a catalyst so as to be capable of effectively restraining the carbon deposition and catalyzing a hydrochlorination process of the acetylene in low cost and high efficiency.
Owner:TSINGHUA UNIV +1
Material containing metal ion ligand complex producing nitric oxide in contact with blood
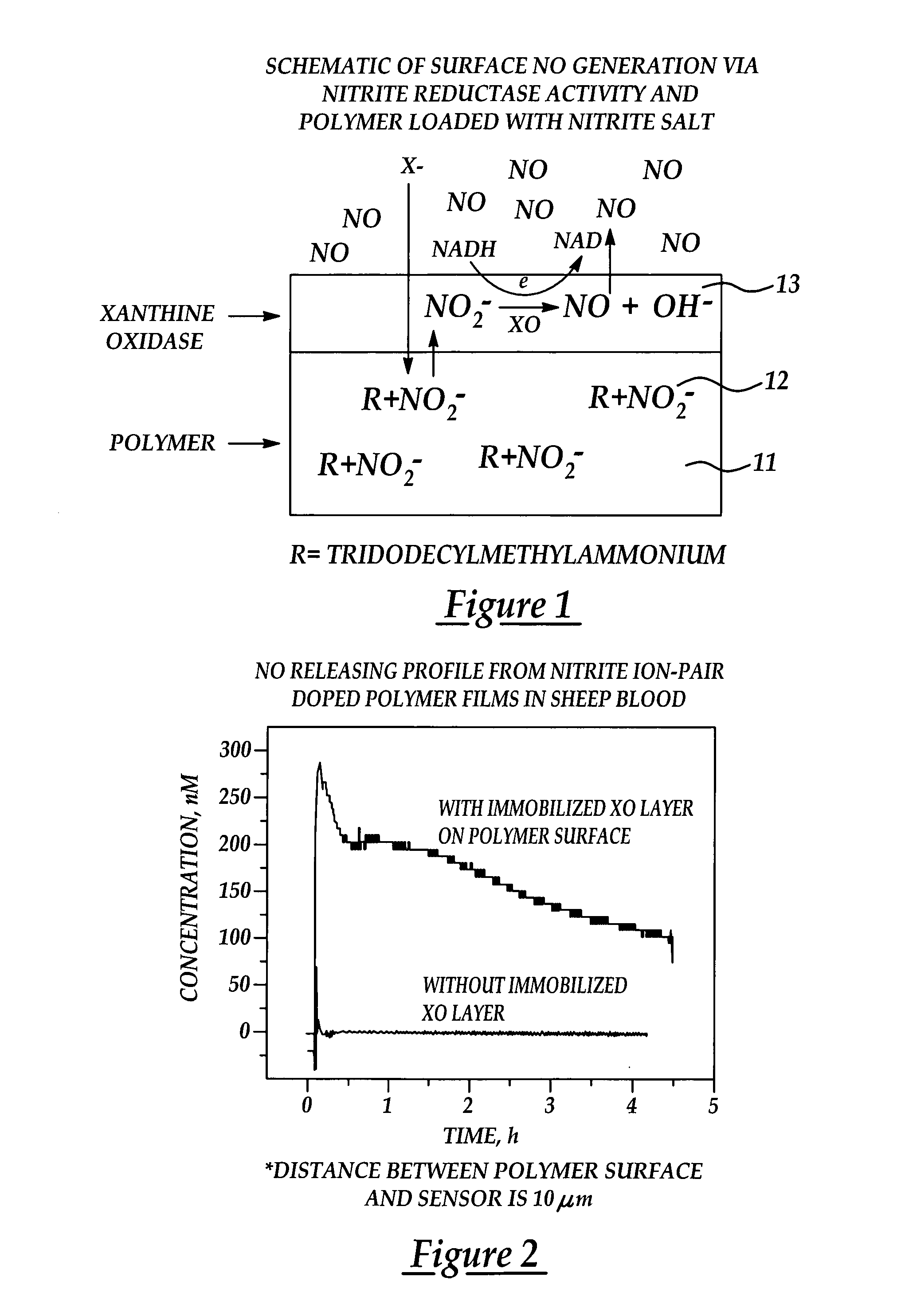
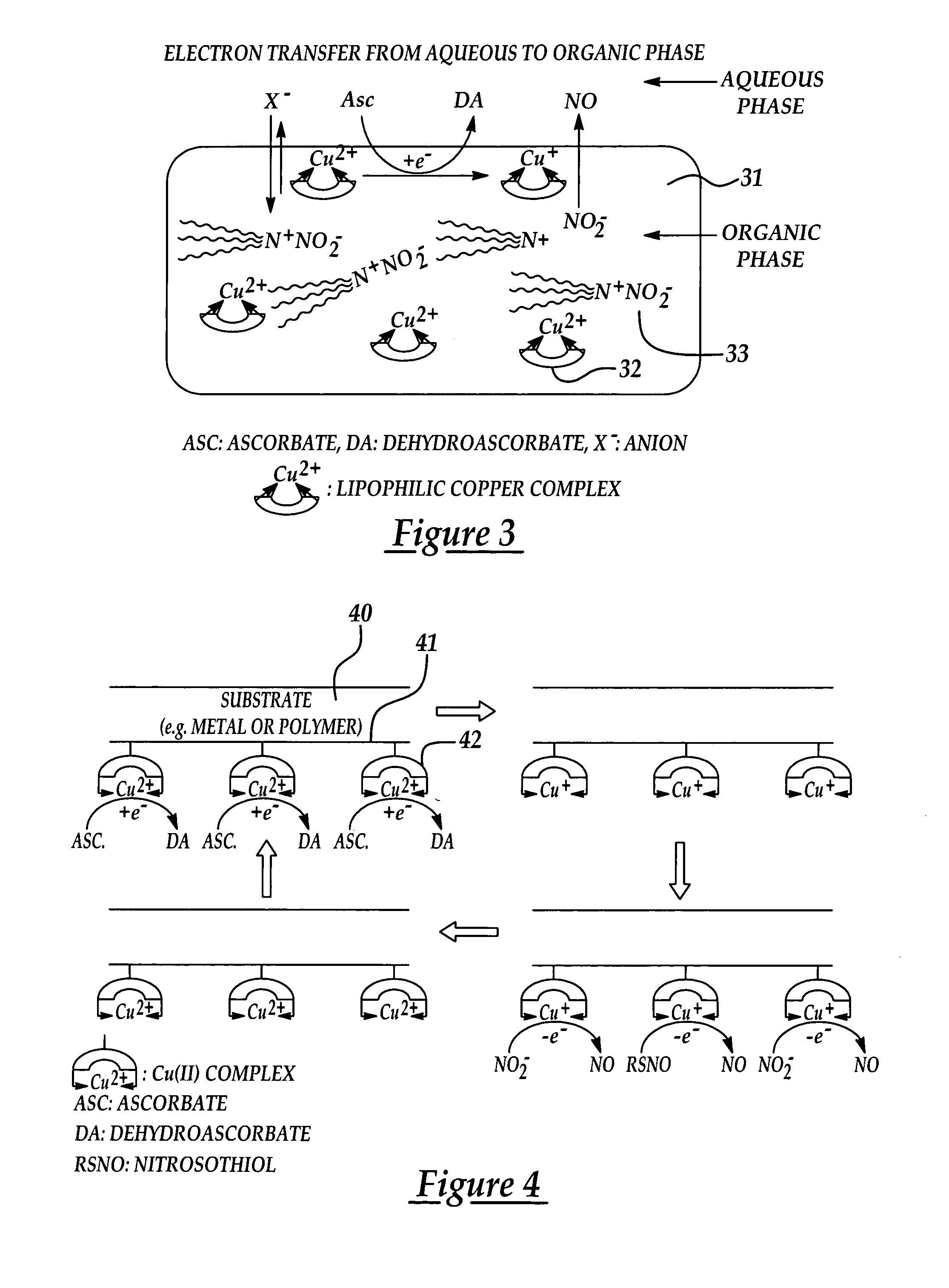
InactiveUS7128904B2Good biocompatibilityPeptide/protein ingredientsOther blood circulation devicesPolymer thin filmsNitric oxide
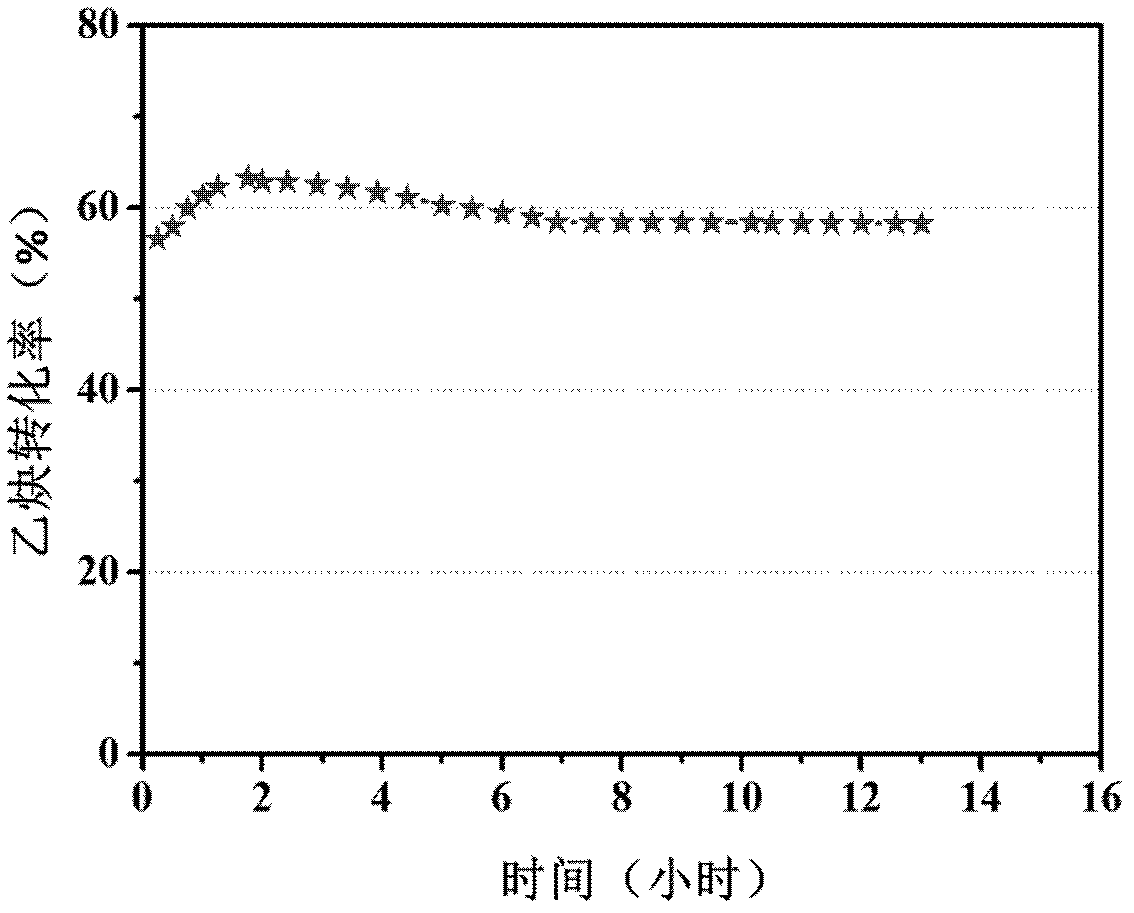
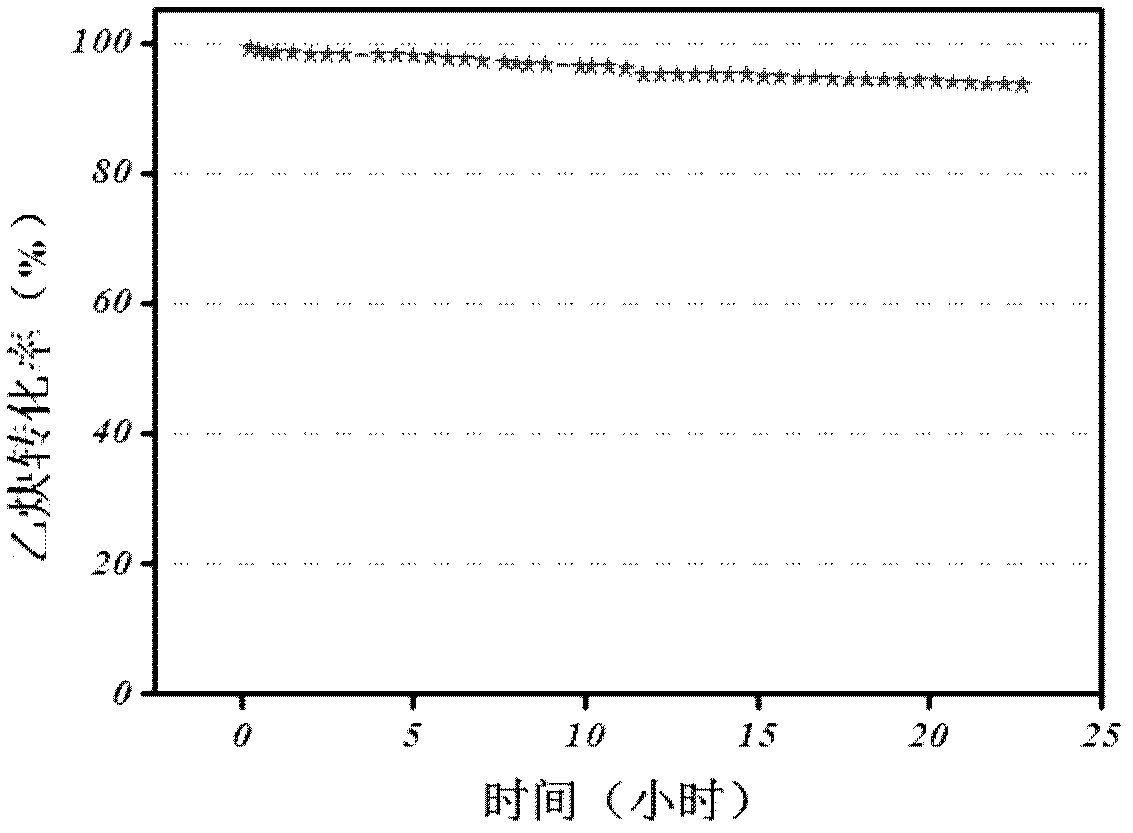
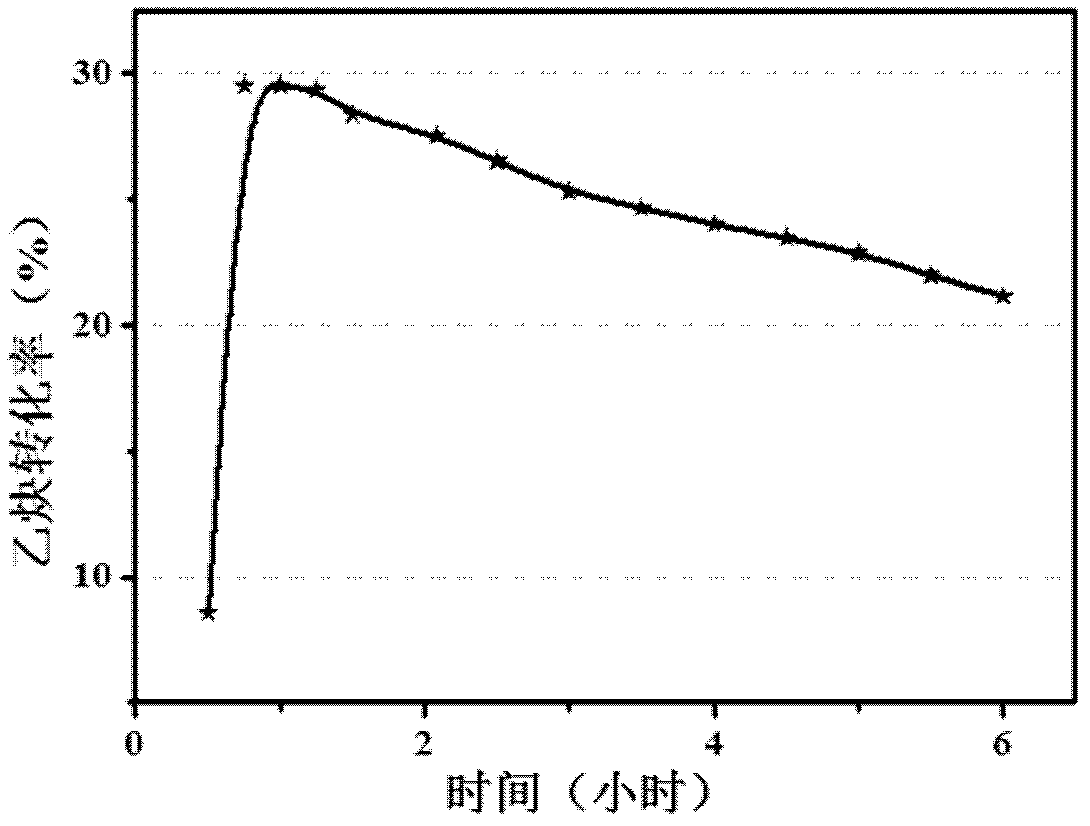
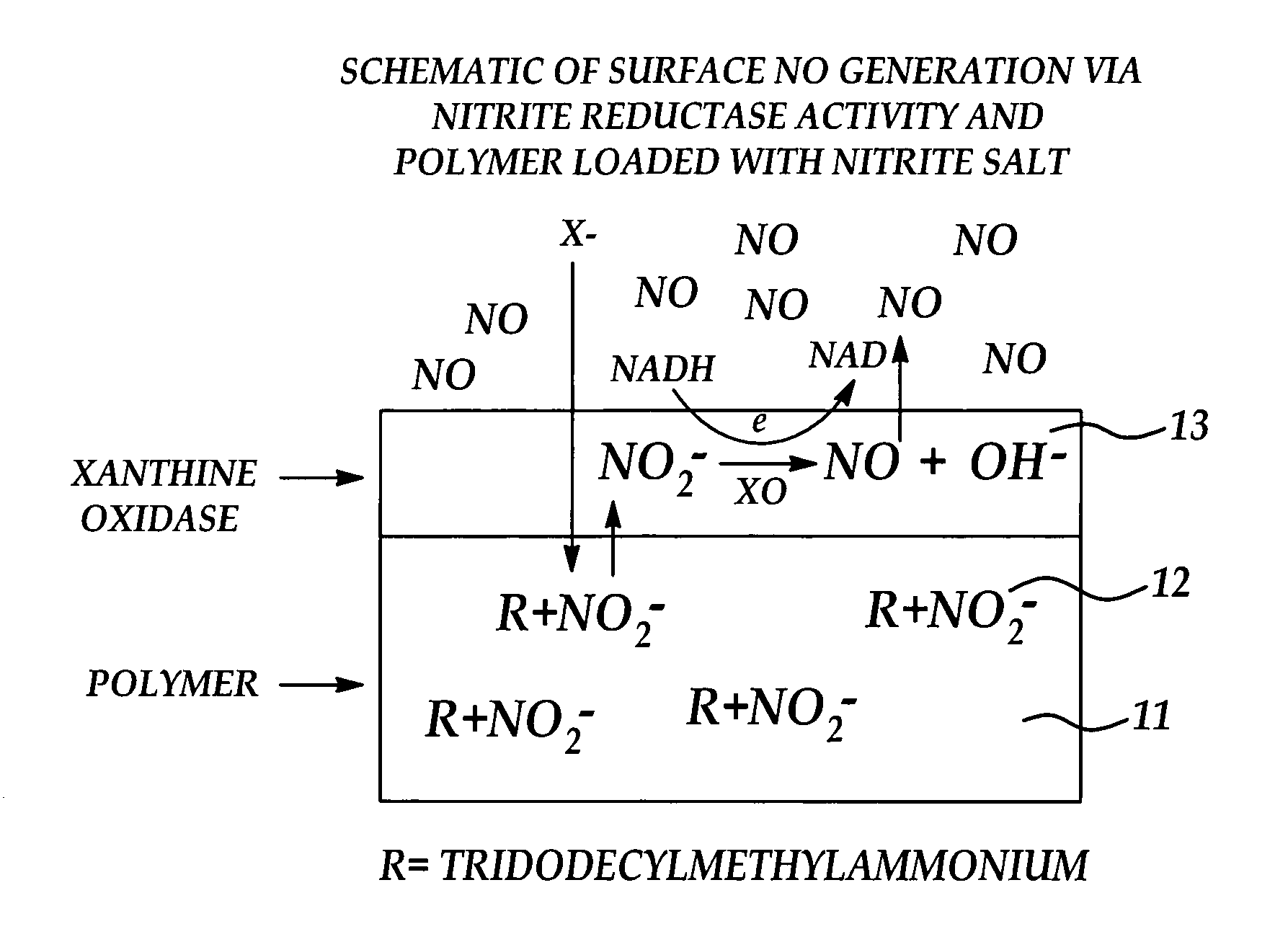
Biocompatible materials that have the ability to release nitric oxide (NO) in situ at the surface-blood interface when in contact with blood. The materials which may be polymers (e.g., polyurethane, poly(vinyl chloride), silicone rubbers), metals, such as stainless steel, carbon, and the like are provided with biocatalysts or biomimetic catalysts on their surface that have nitrite, nitrate, and / or nitrosothiol-reducing capability. Illustratively, the catalysts are adsorbed or immobilized at the surface of the material. The catalysts can act on endogenous nitrite, nitrate, or nitrosothiols within the blood creating a local increase in the NO levels at the surface of the material. An illustrative enzymatic biocatalyst is mammalian xanthine oxidase. In another illustrative embodiment, a biomimetic catalyst is a copper (Cu(II)-ligand complex, e.g. dibenzo[e,k]-2,3,8,9-tetraphenyl-1,4,7,10-tetraaza-cyclododeca-1,3,7,9-tetraene. In some cases, lipophilic salts of nitrite / nitrate (e.g., tridodecylmethylammonium nitrite (TDMA+NO2− / NO3−)) or certain salts of nitrosothiols can be doped within a polymer material, or an underlying polymeric film, to create a reservoir of nitrite or nitrosothiol that continuously leaks into the immobilized catalytic layer. Adequate levels of endogenous reducing equivalents are present within blood to provide catalytically-generated surface levels of NO that are above the threshold reportedly required to prevent platelet adhesion or activation.
Owner:RGT UNIV OF MICHIGAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com