Production method of 1,1,1,3,3-pentachloropropane
A technology of pentachloropropane and production method, applied in the field of continuous reaction synthesis, can solve problems such as difficulty in operation, and achieve the effects of accurate measurement, easy implementation and volume reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
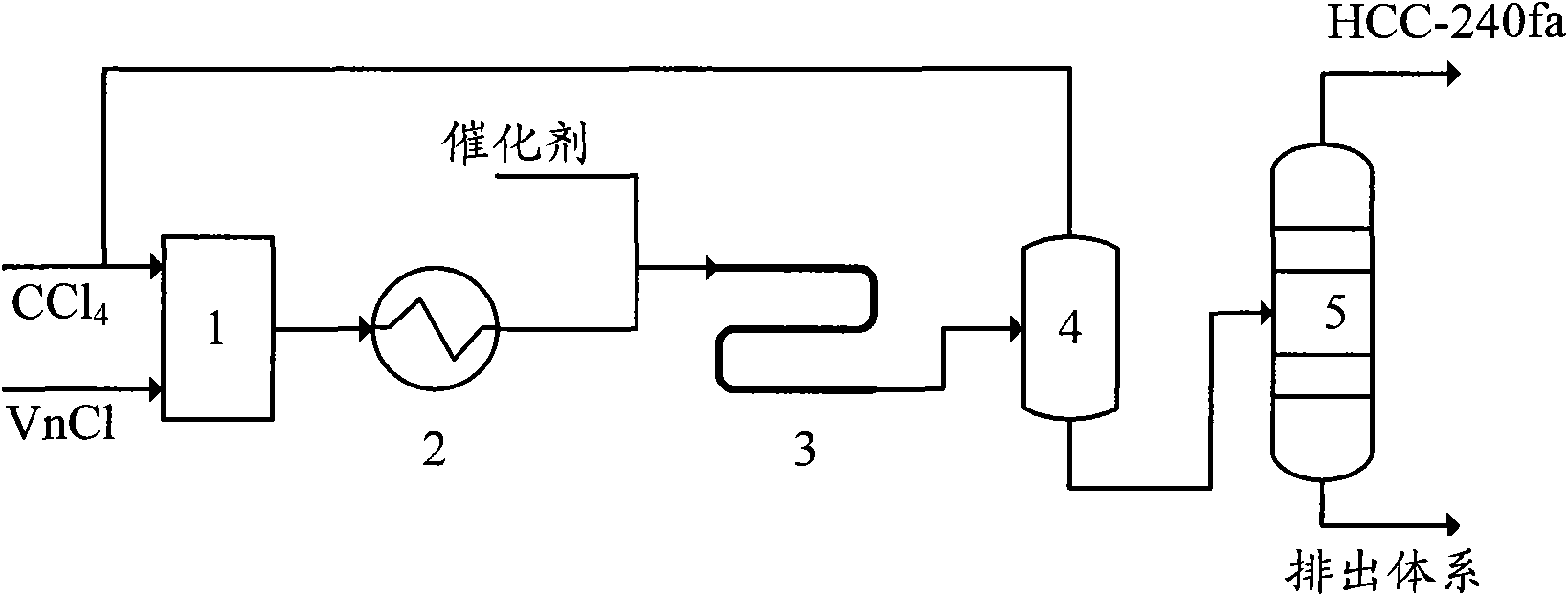
[0037] The telomerization reactor 4 in this embodiment adopts an externally heated tubular reactor with a tube diameter of ∮25×2.5mm and a length of 2 meters. The process flow is:
[0038] A. Mix carbon tetrachloride and vinyl chloride at a molar ratio of 10:1, and preheat to 150°C;
[0039] B. The preheated carbon tetrachloride and vinyl chloride enter the tubular reactor together with the catalyst. The telomerization reaction is carried out under the conditions of a reaction pressure of 1.5MPa, a reaction temperature of 140°C and a residence time of 5 minutes. The catalyst is composed of FeCl 2 , Tributyl phosphate and carbon tetrachloride, FeCl 2 , The molar ratio of tributyl phosphate and carbon tetrachloride is 1:10:20, FeCl 2 The molar ratio with vinyl chloride is 1:100;
[0040] C. The telomerization reaction product is flashed to remove unreacted carbon tetrachloride, and the unreacted carbon tetrachloride is recycled;
[0041] D. The reaction liquid after removing carbon tetra...
Embodiment 2
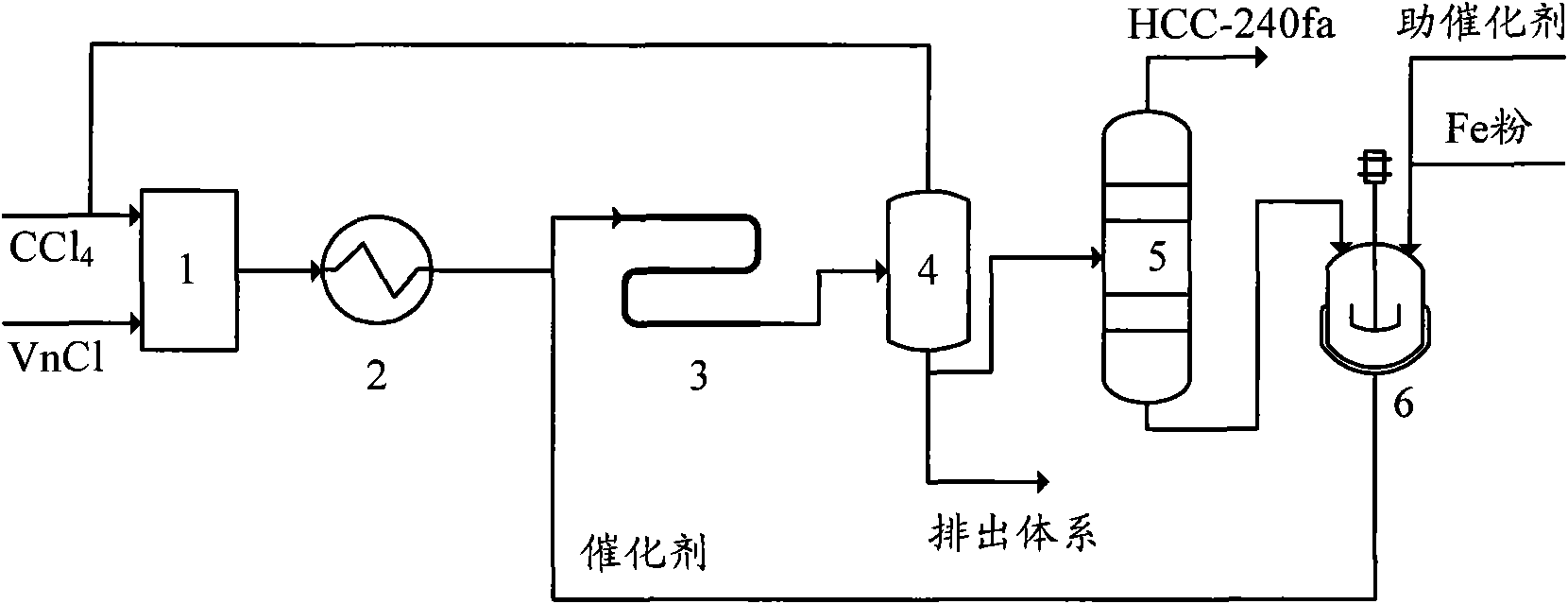
[0044] The telomerization reactor 4 in this embodiment adopts an adiabatic tubular reactor with a tube diameter of ∮25×2.5mm and a length of 2 meters. The process flow is:
[0045] A. Mix carbon tetrachloride and vinyl chloride at a molar ratio of 1:1, and preheat to 100°C;
[0046] B. The preheated carbon tetrachloride and vinyl chloride enter the tubular reactor together with the catalyst. The telomerization reaction is carried out under the conditions of a reaction pressure of 0.3MPa, a reaction temperature of 90°C, and a residence time of 60 minutes. Catalyst made of FeCl 2 , Triethyl phosphate and carbon tetrachloride, FeCl 2 , The molar ratio of triethyl phosphate and carbon tetrachloride is 1:1:50, and the catalyst is made of FeCl during circulation. 2 , Triethyl phosphate and telomerization reaction product distillation residue composition; FeCl 2 The molar ratio with vinyl chloride is 1:20;
[0047] C. The telomerization reaction product is flashed to remove unreacted carbon...
Embodiment 3
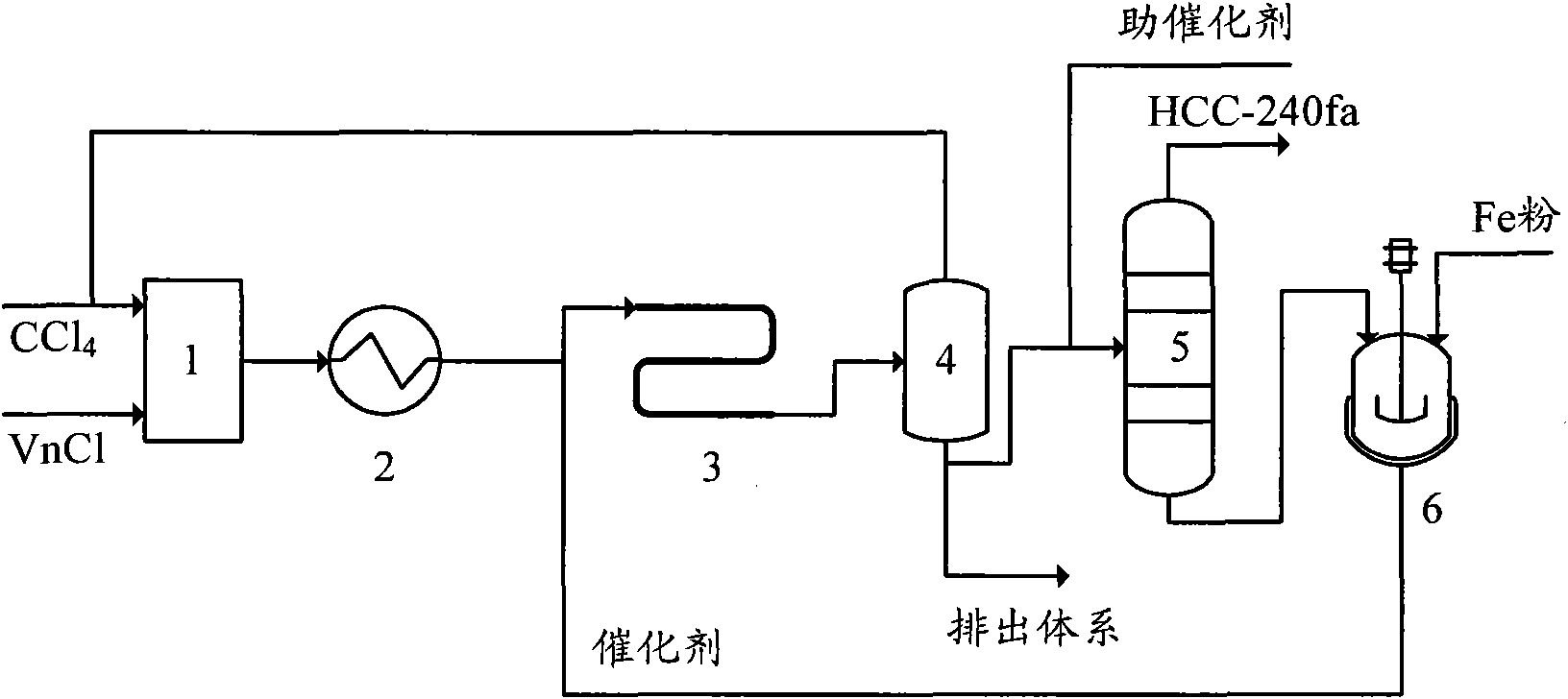
[0053] The telomerization reactor 4 in this embodiment adopts an adiabatic tubular reactor lined with tetrafluoroethylene with a diameter of ∮38×2.5mm and a length of 2 meters. The process flow is:
[0054] A. Mix carbon tetrachloride and vinyl chloride at a molar ratio of 1.5:1, and preheat to 130°C;
[0055] B. The preheated carbon tetrachloride and vinyl chloride enter the tubular reactor together with the catalyst. The telomerization reaction is carried out under the conditions of a reaction pressure of 0.6MPa, a reaction temperature of 120°C, and a residence time of 30 minutes. By FeCl 2 , Triethyl phosphite and carbon tetrachloride, FeCl 2 , The molar ratio of triethyl phosphate and carbon tetrachloride is 1:3:20, and the catalyst is composed of FeCl during circulation. 2 , Triethyl phosphite and telomerization reaction product distillation residue composition; FeCl 2 The molar ratio with vinyl chloride is 1:50;
[0056] C. The telomerization reaction product is flashed to remo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com