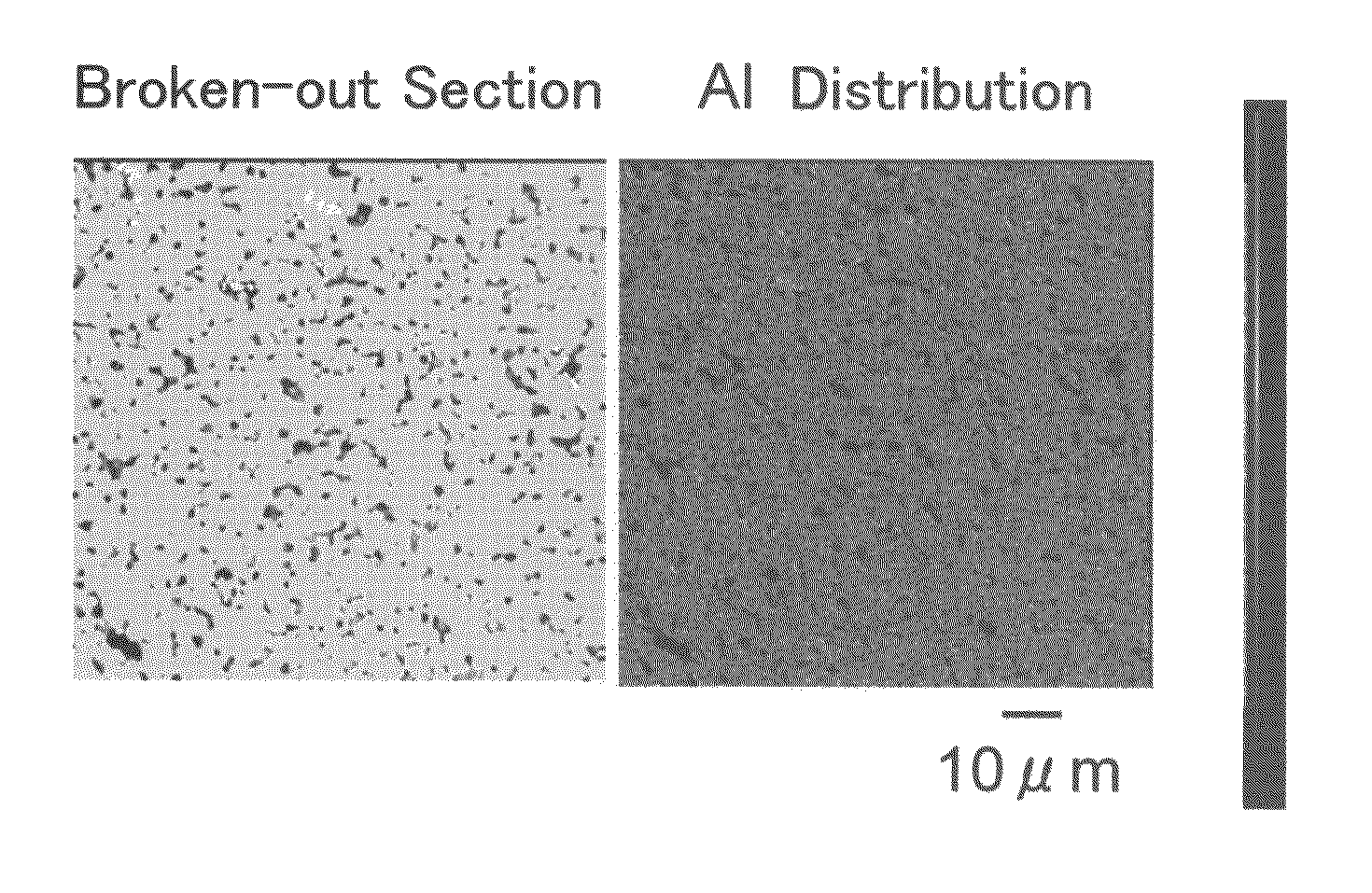
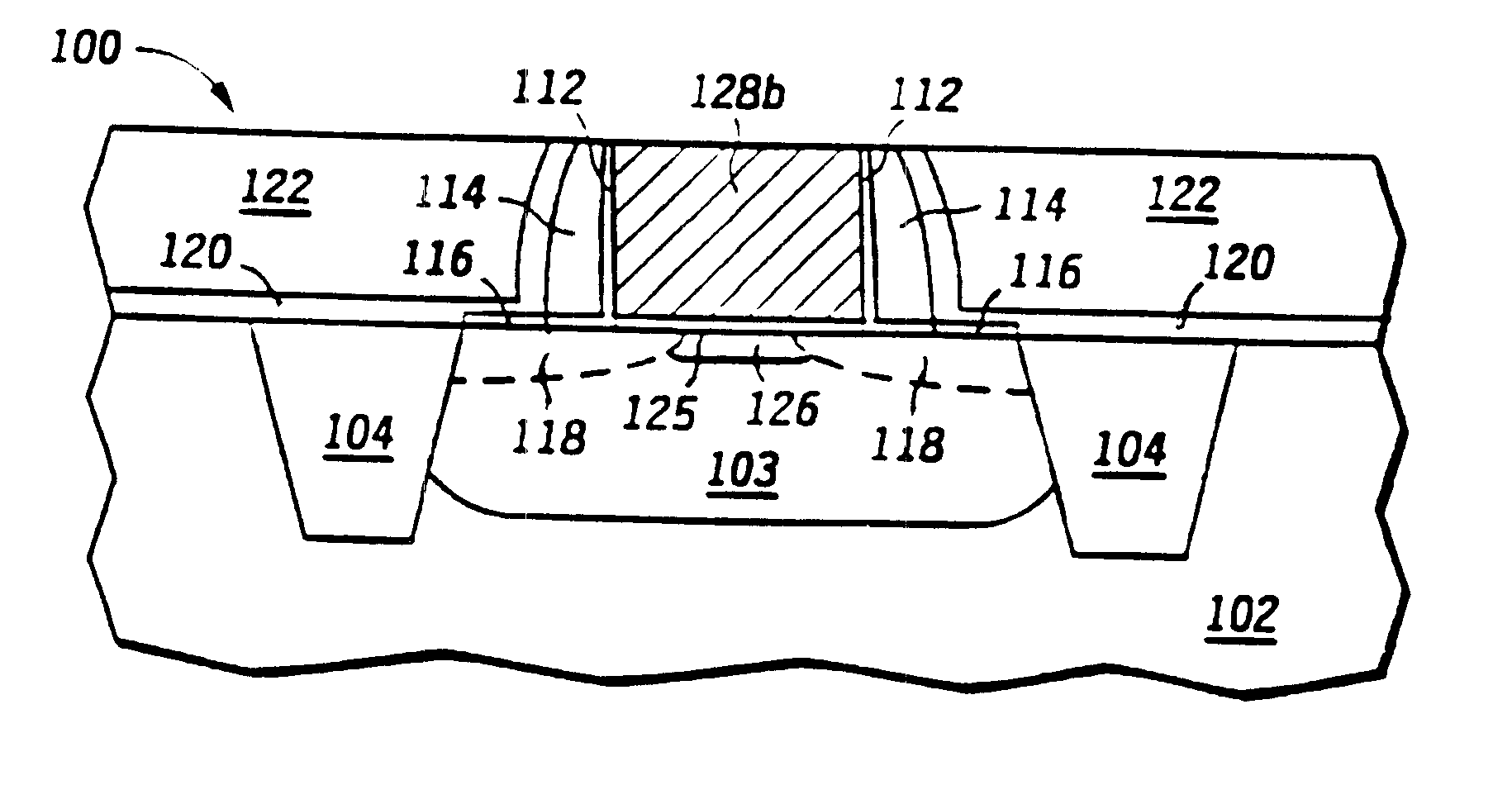
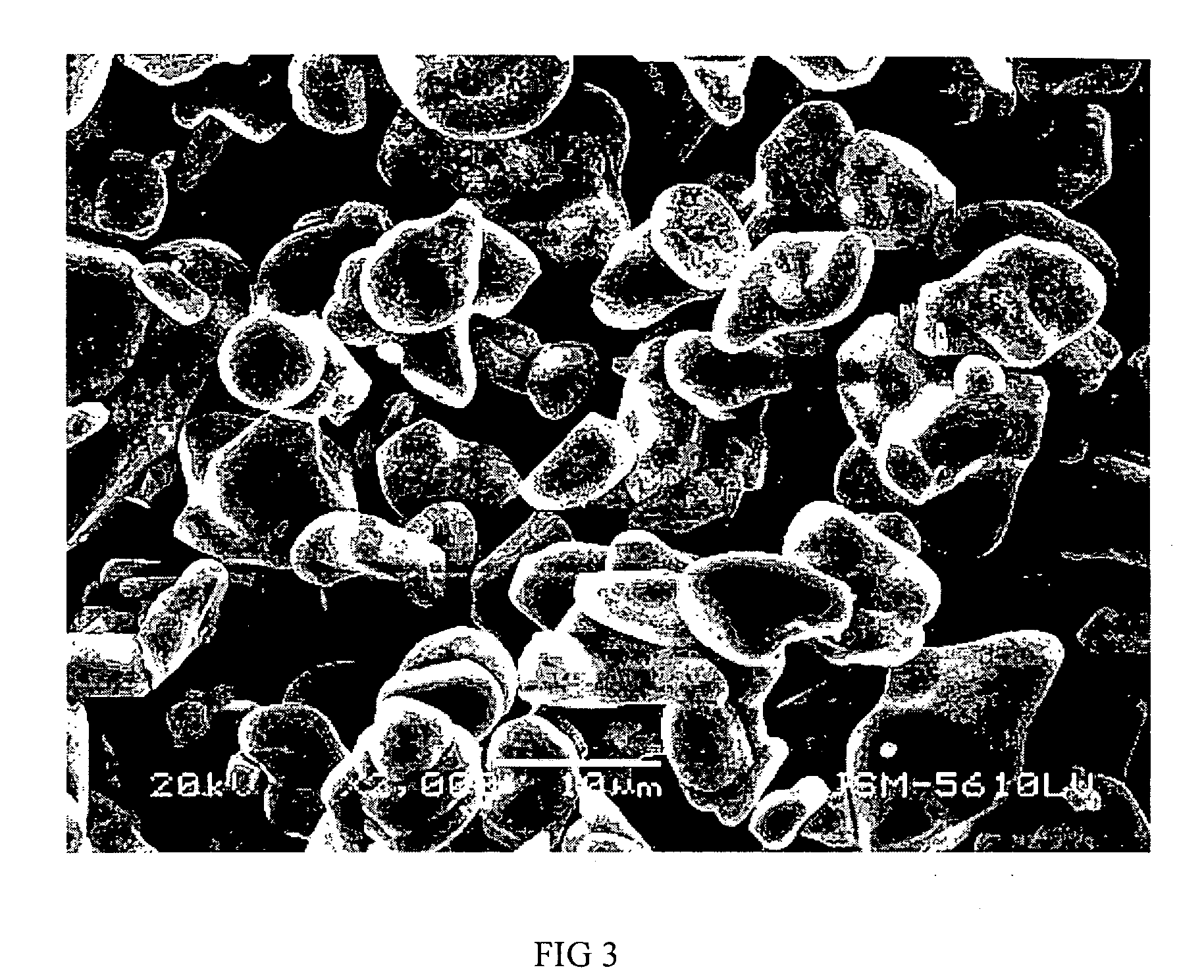
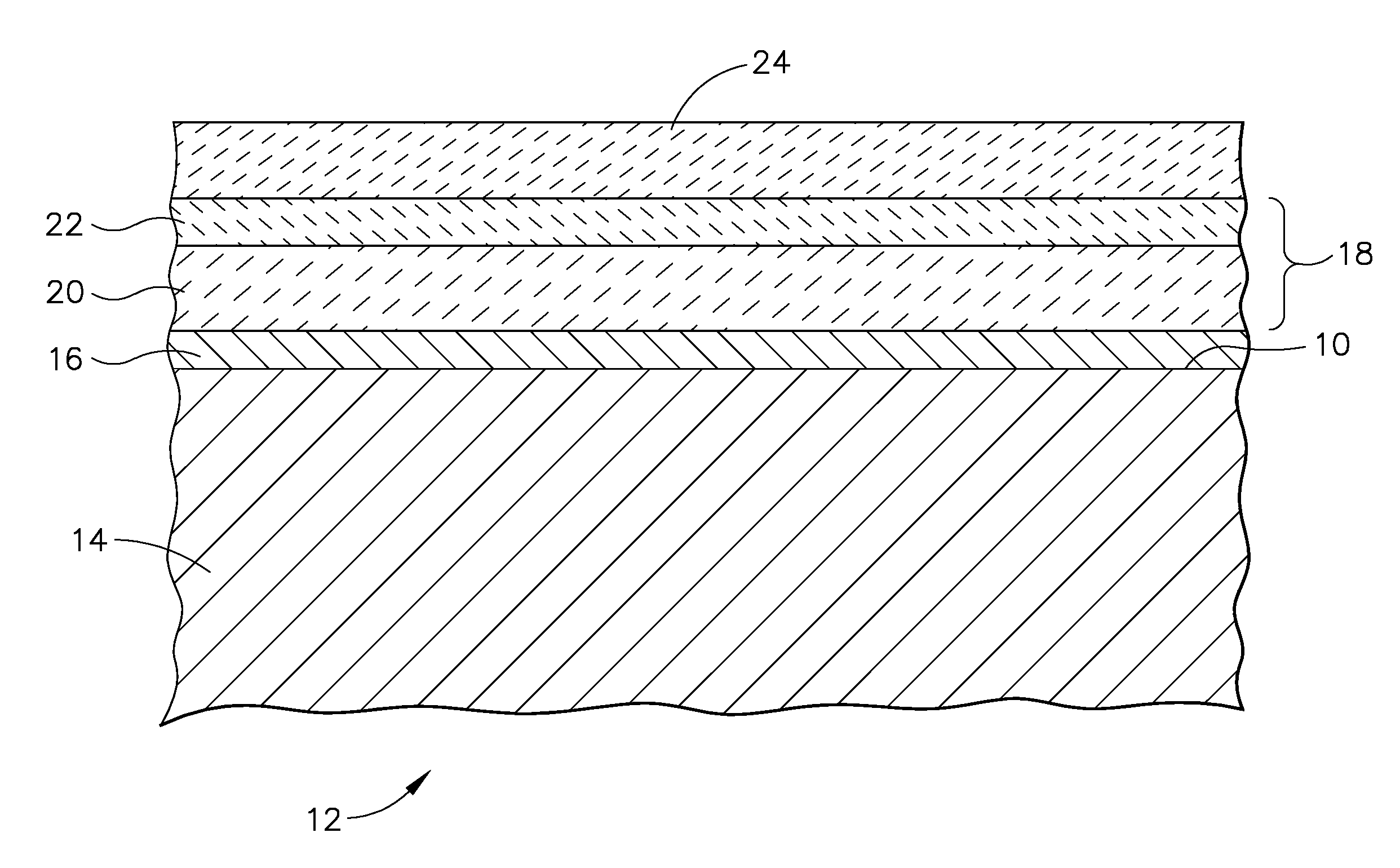
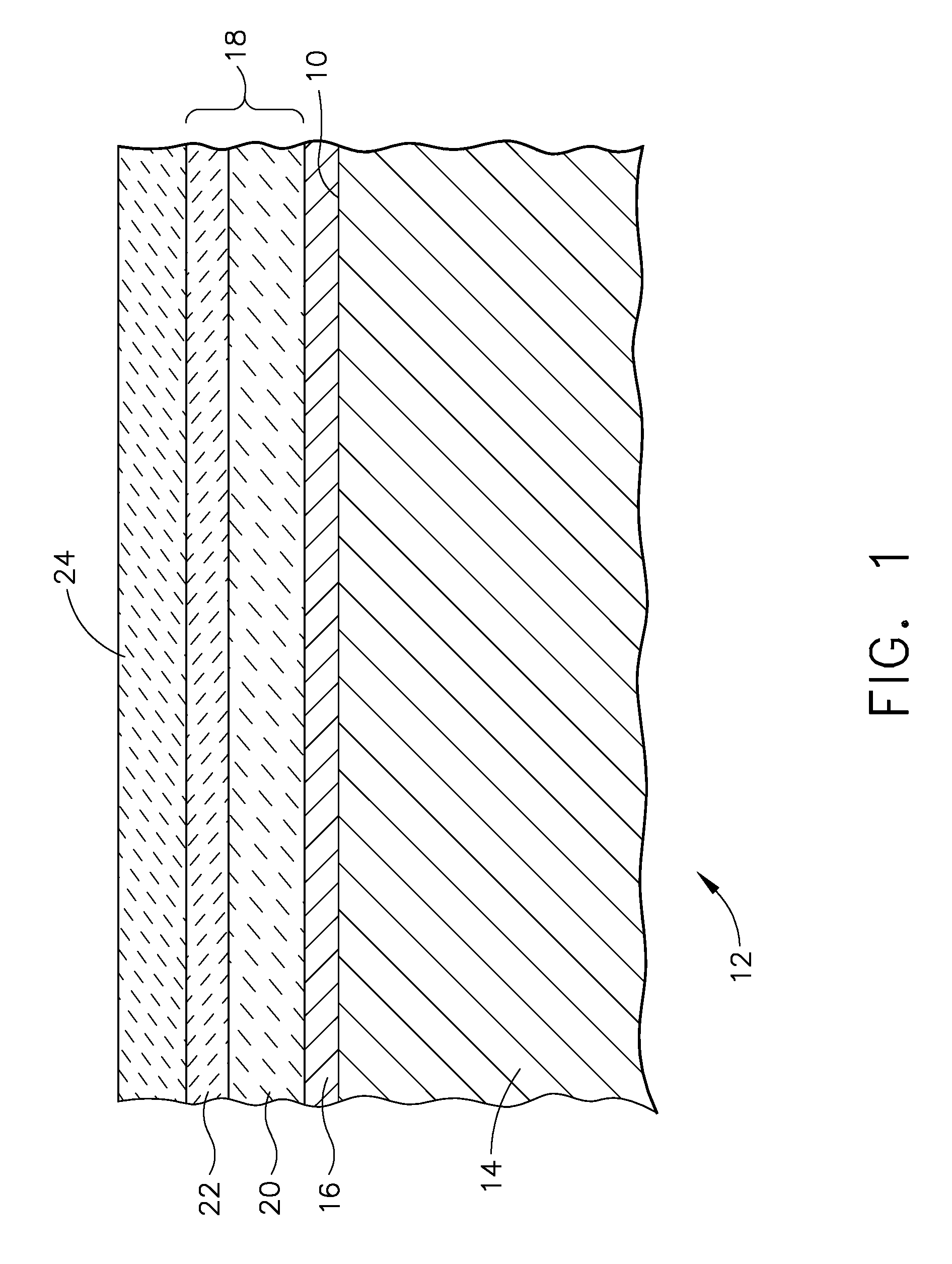
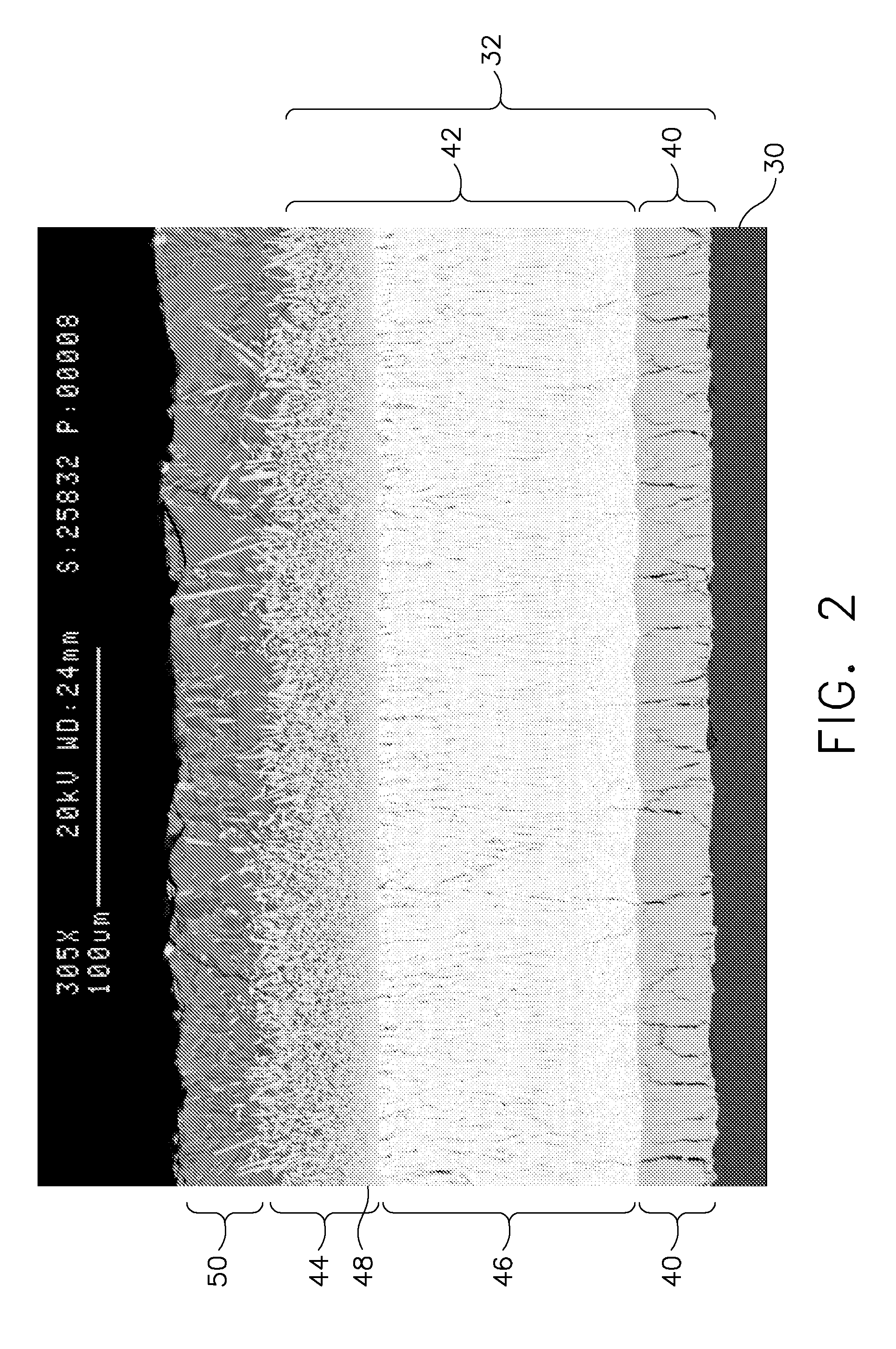
Patents
Literature
7019 results about "Lanthanum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lanthanum is a chemical element with the symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air and is soft enough to be cut with a knife. It is the eponym of the lanthanide series, a group of 15 similar elements between lanthanum and lutetium in the periodic table, of which lanthanum is the first and the prototype. It is also sometimes considered the first element of the 6th-period transition metals, which would put it in group 3, although lutetium is sometimes placed in this position instead. Lanthanum is traditionally counted among the rare earth elements. The usual oxidation state is +3. Lanthanum has no biological role in humans but is essential to some bacteria. It is not particularly toxic to humans but does show some antimicrobial activity.
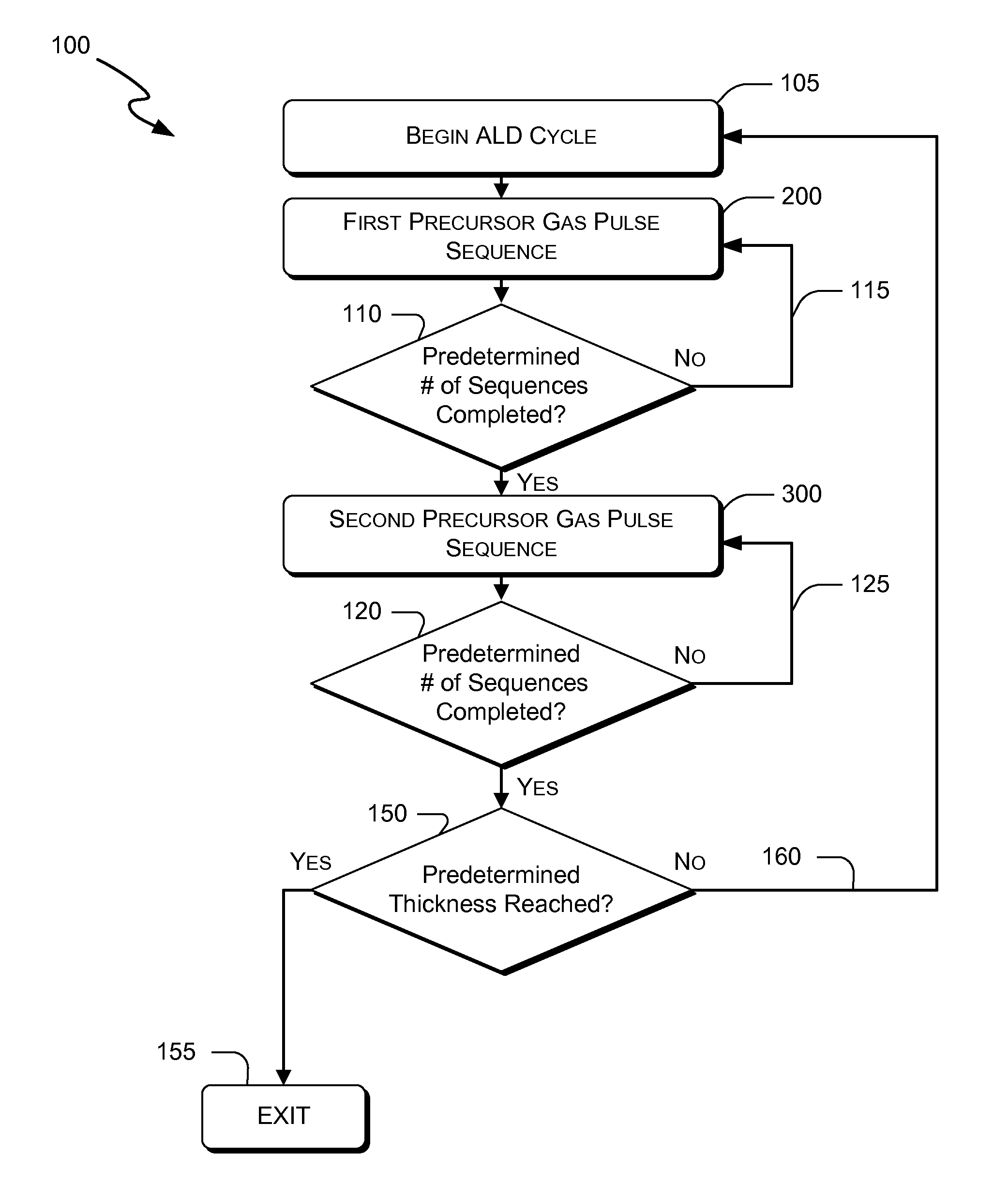
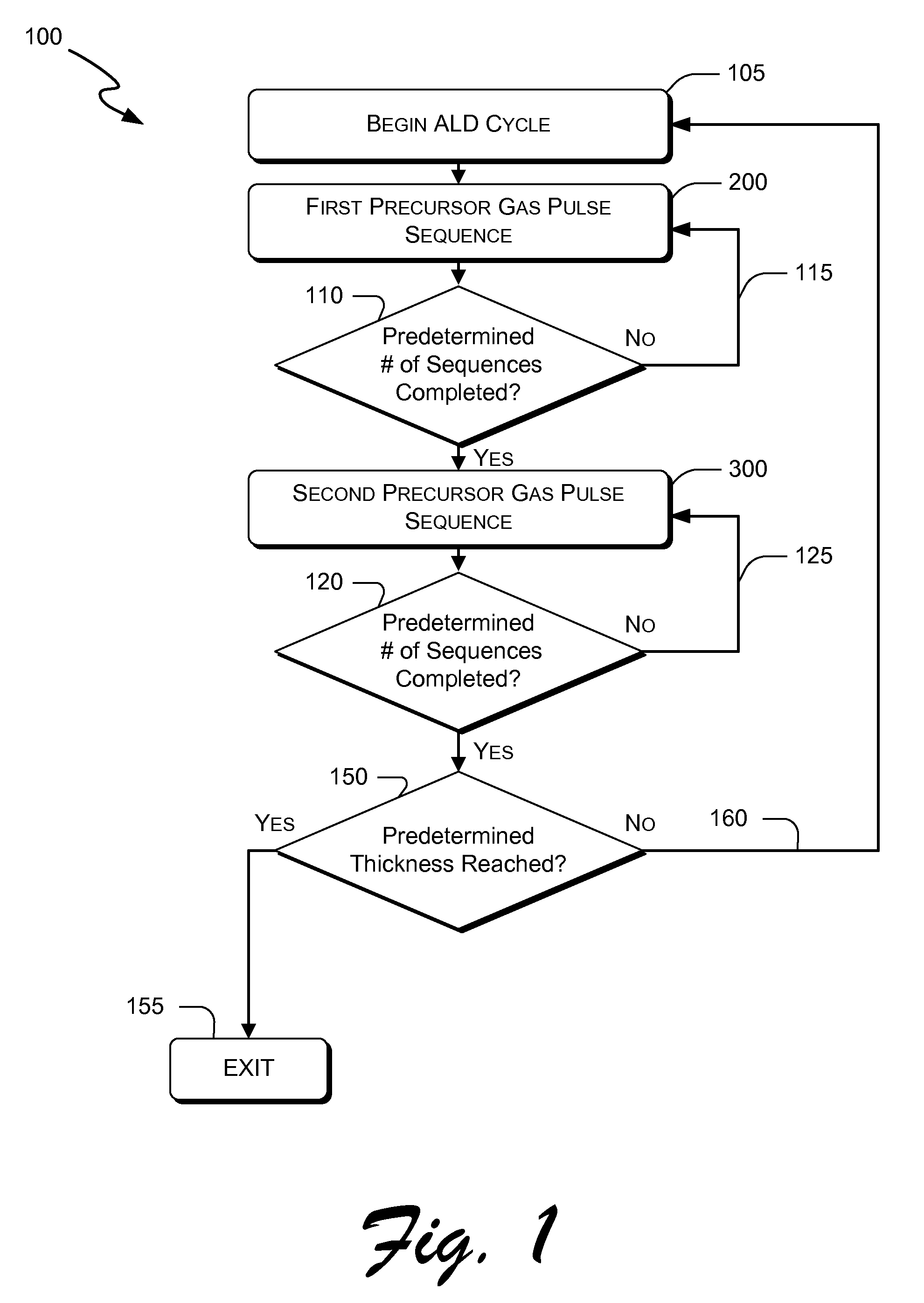
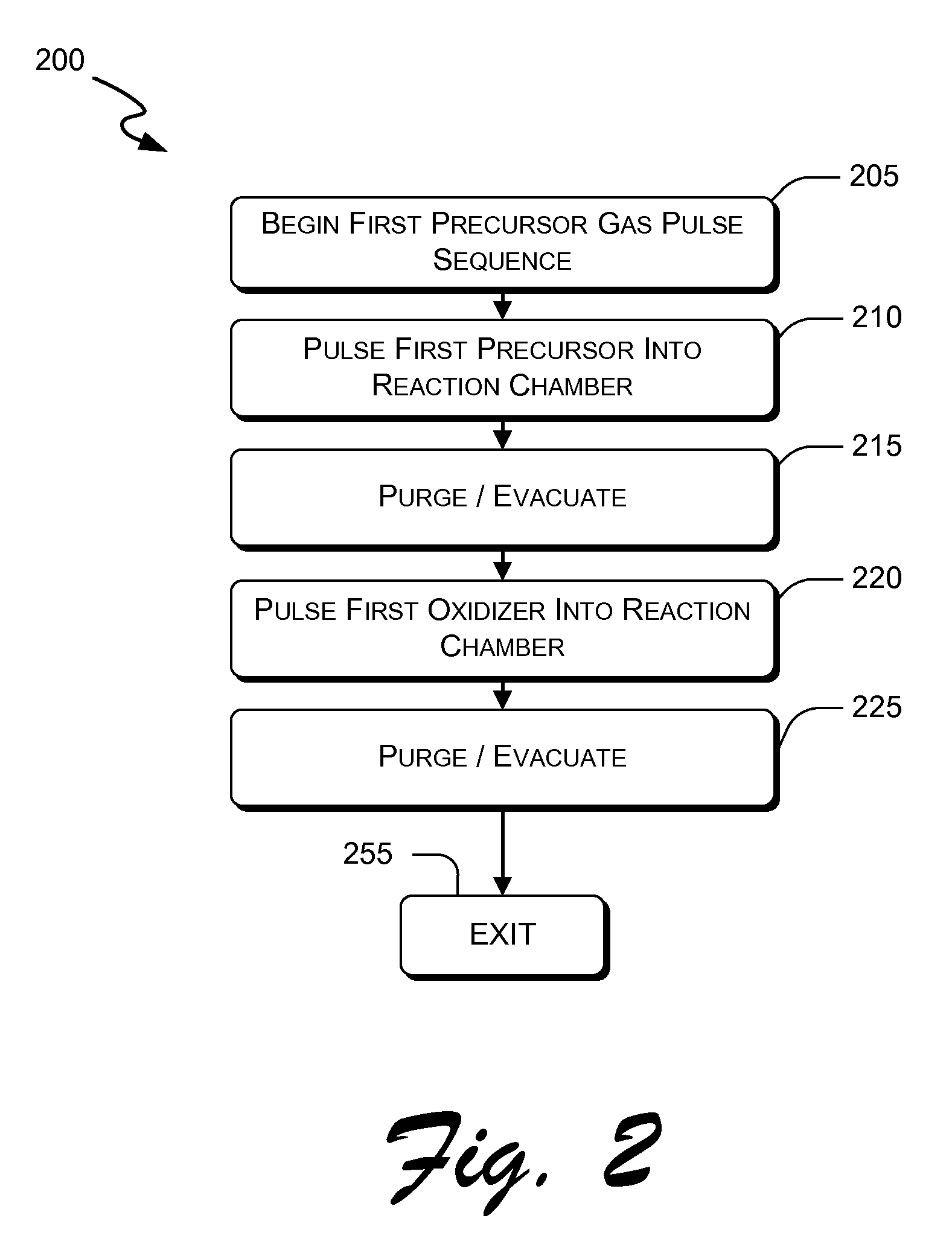
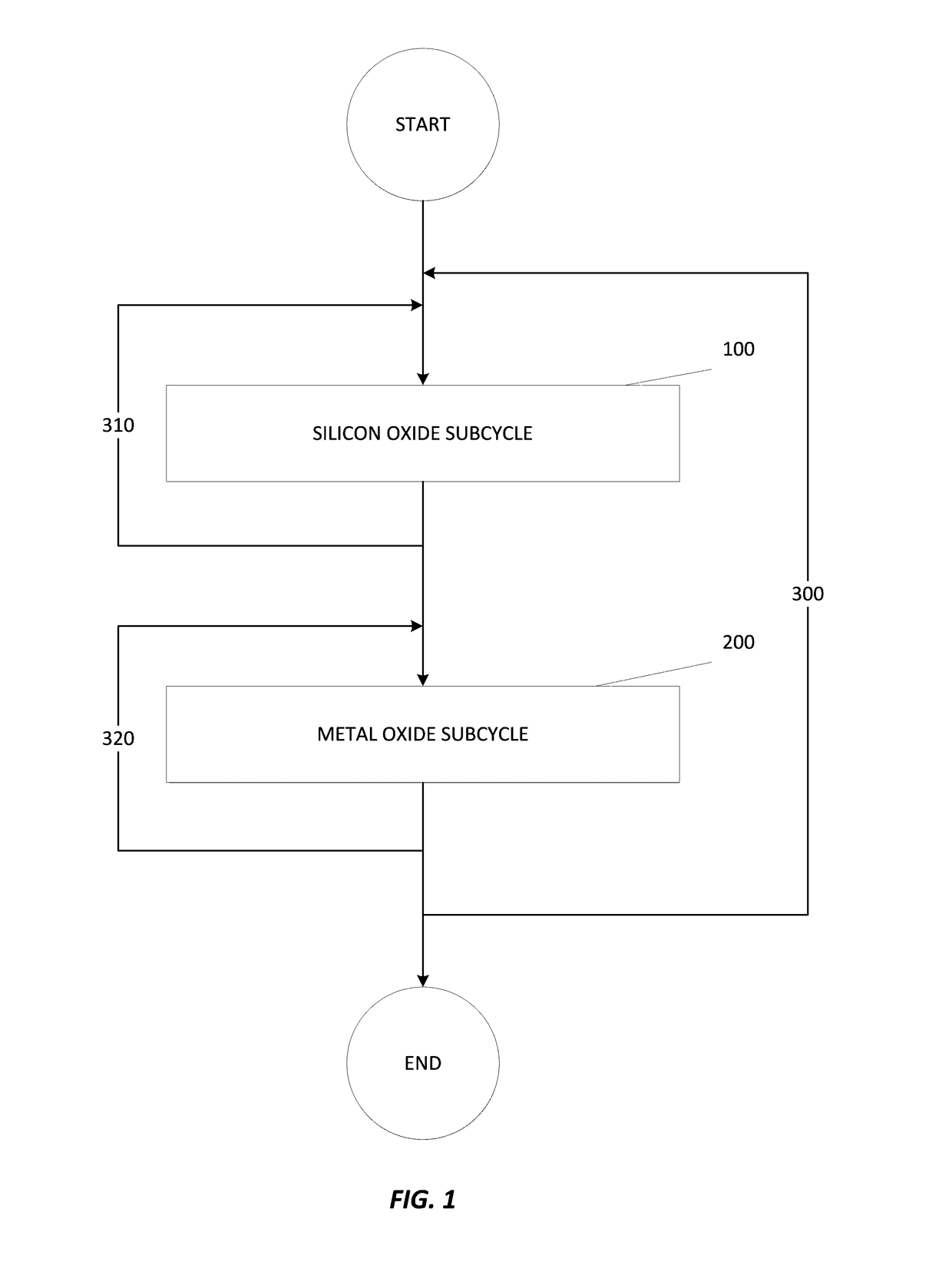
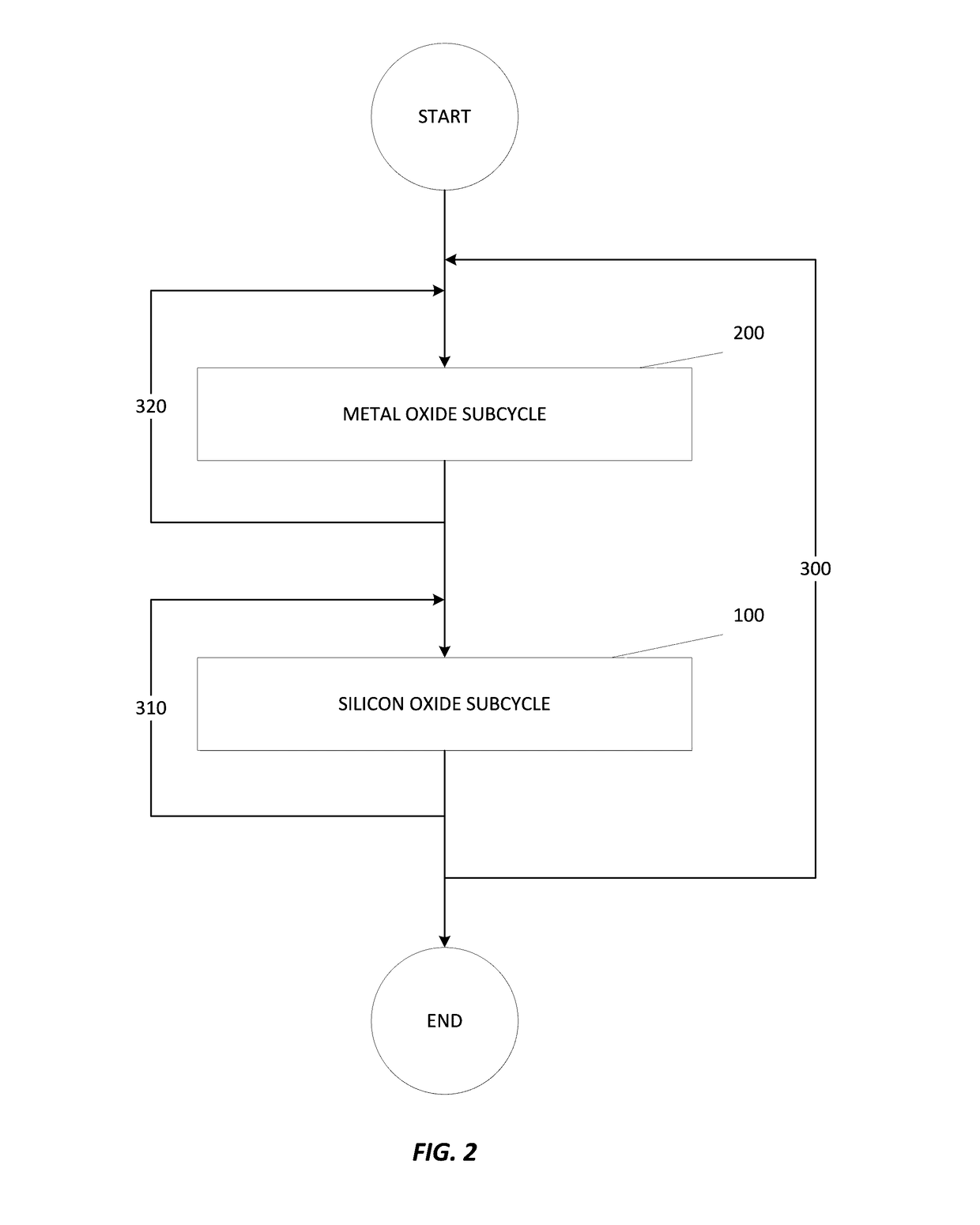
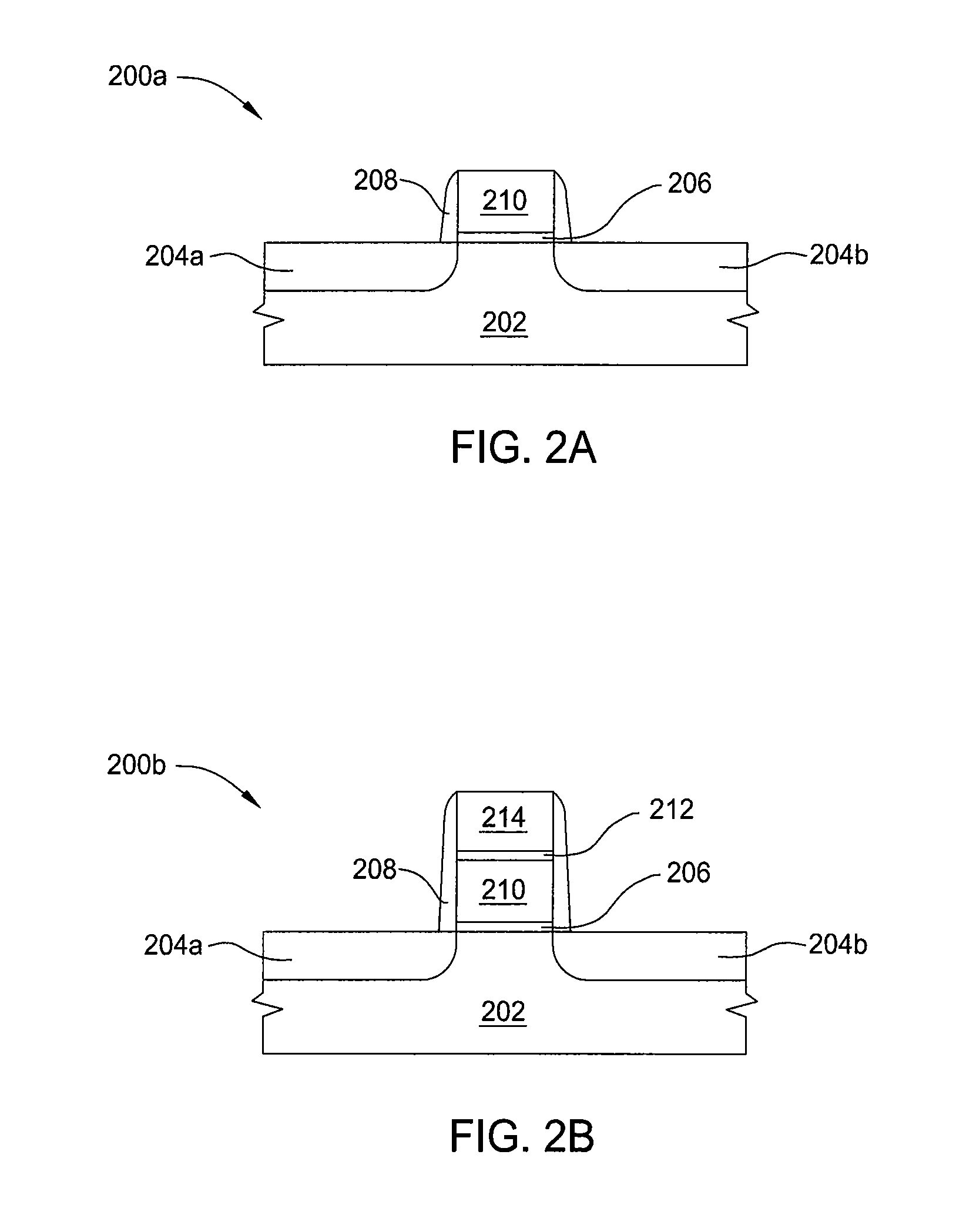
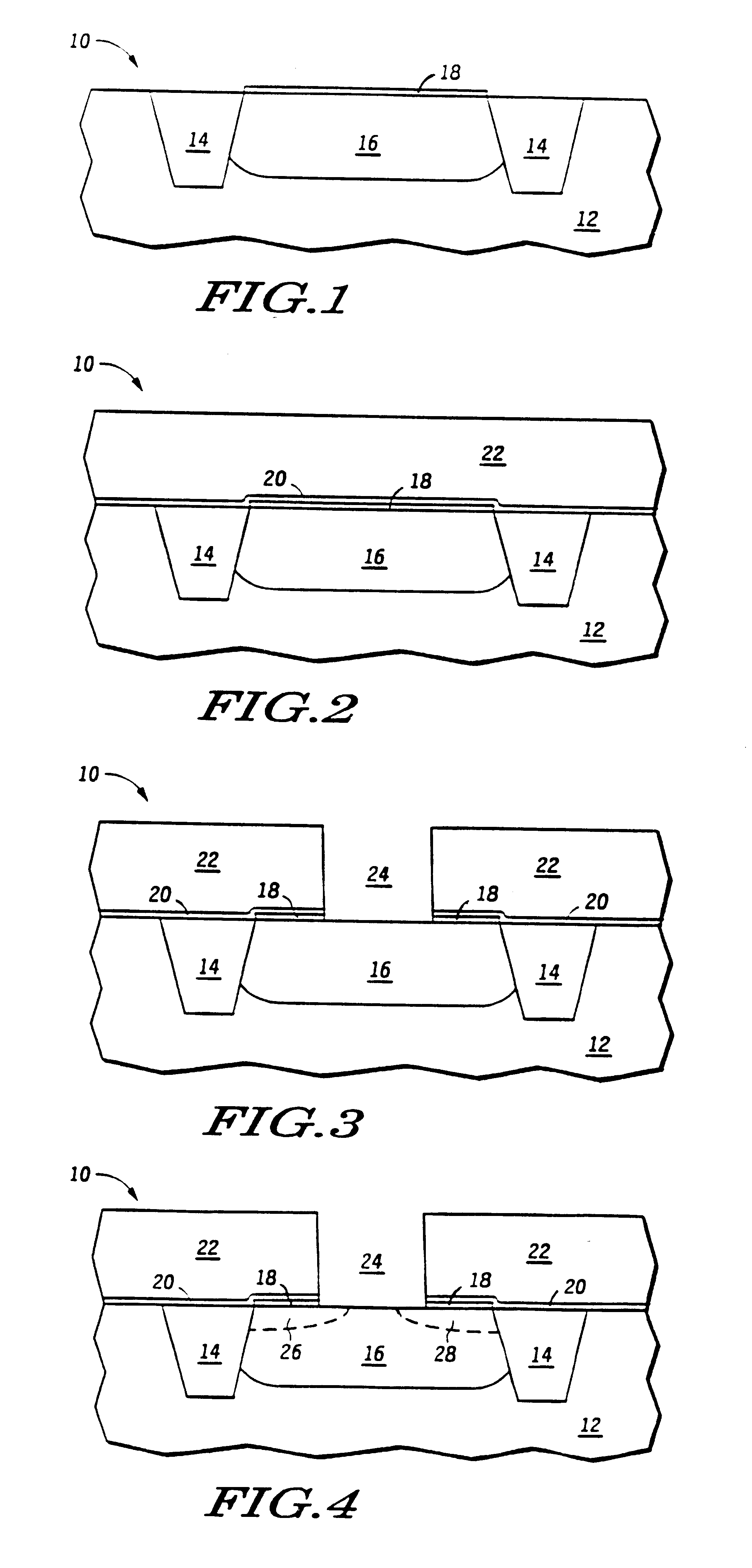
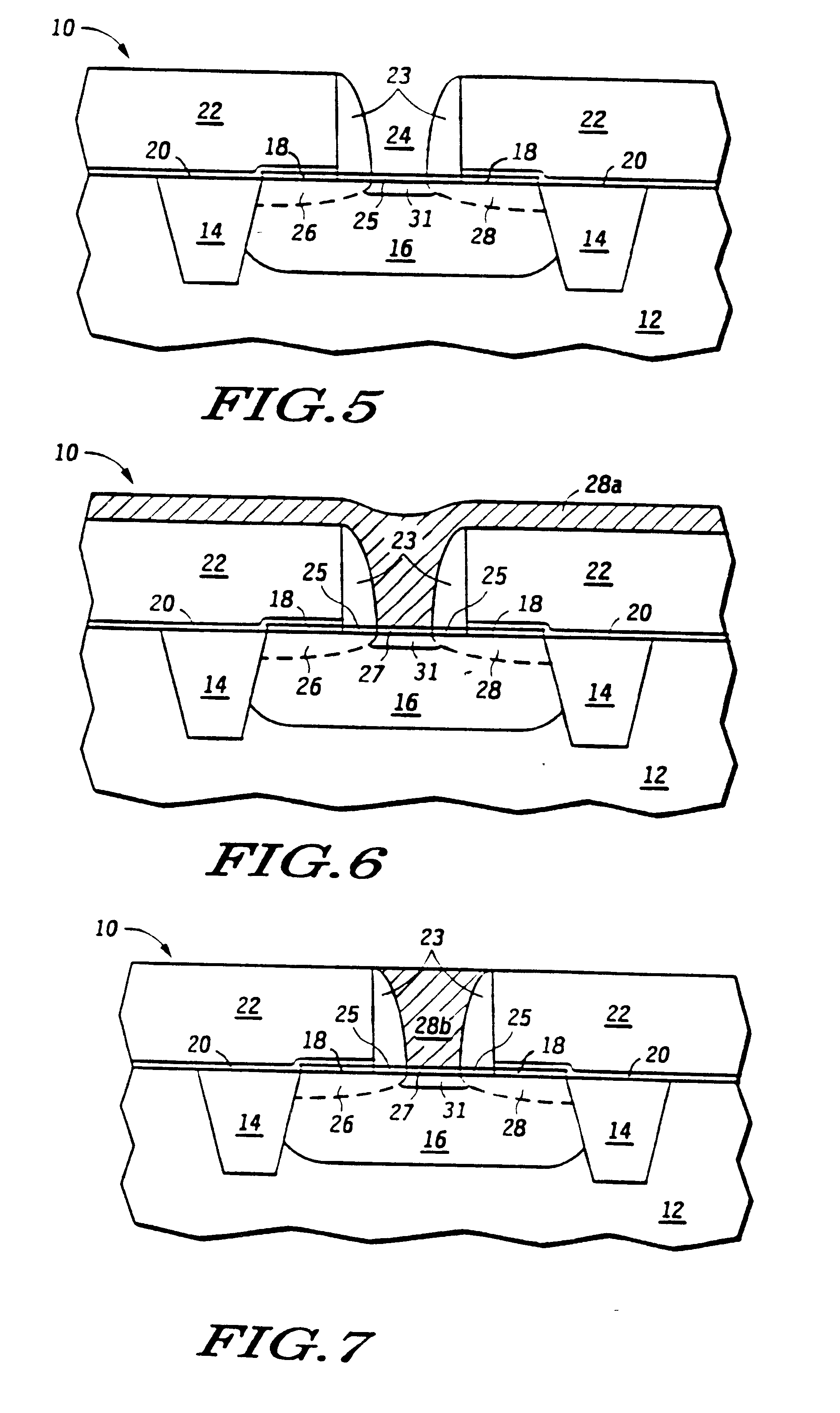
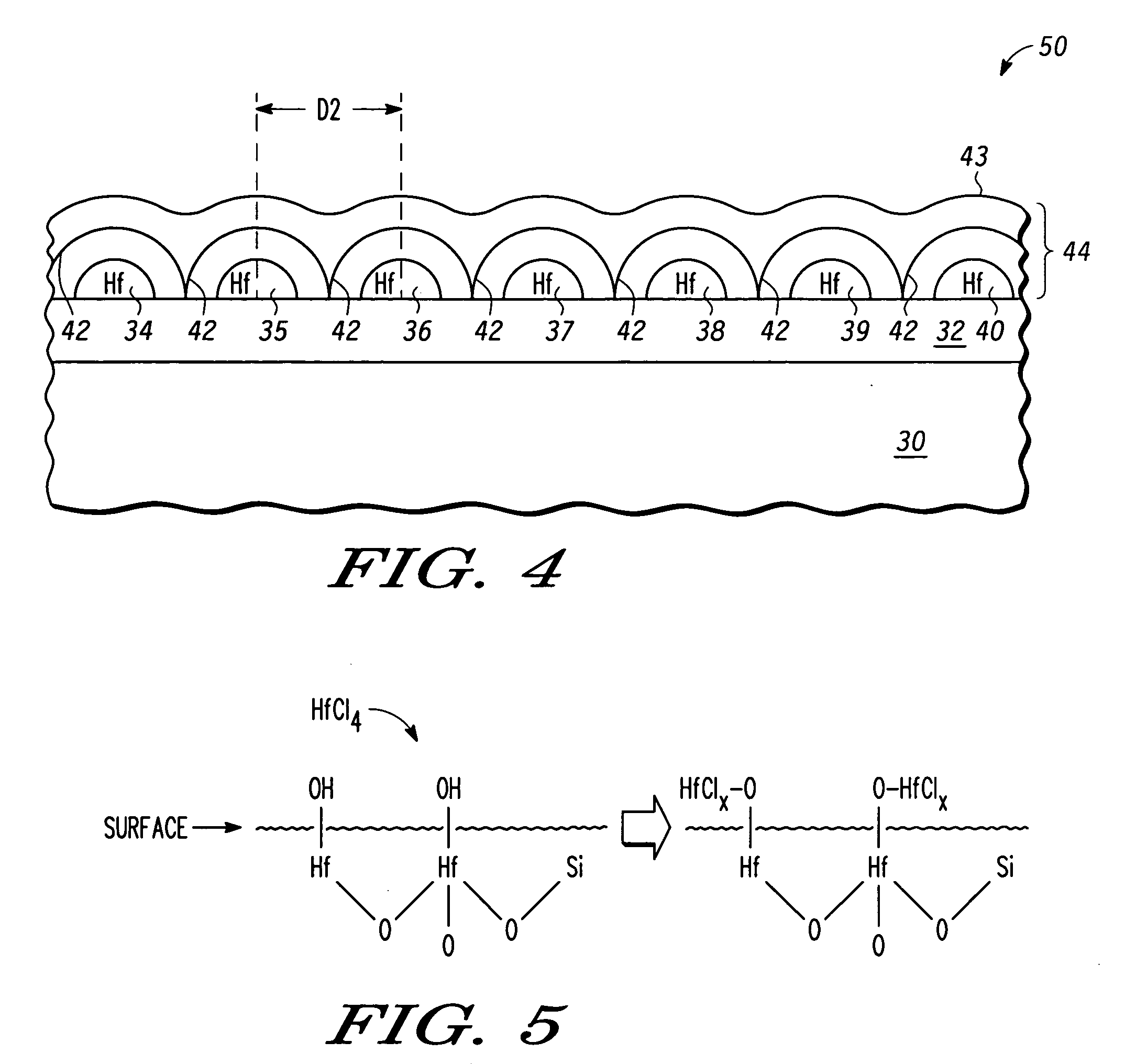
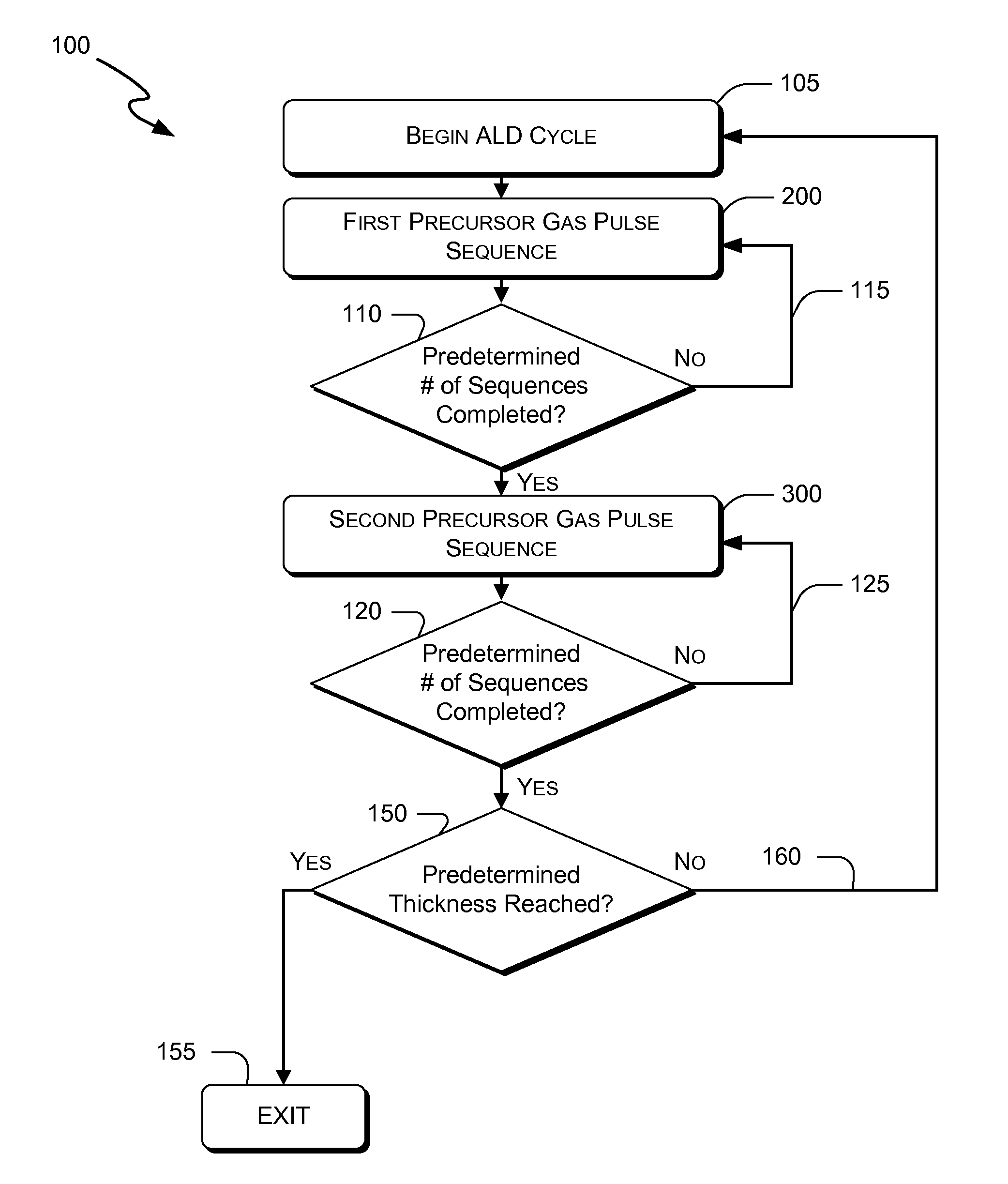
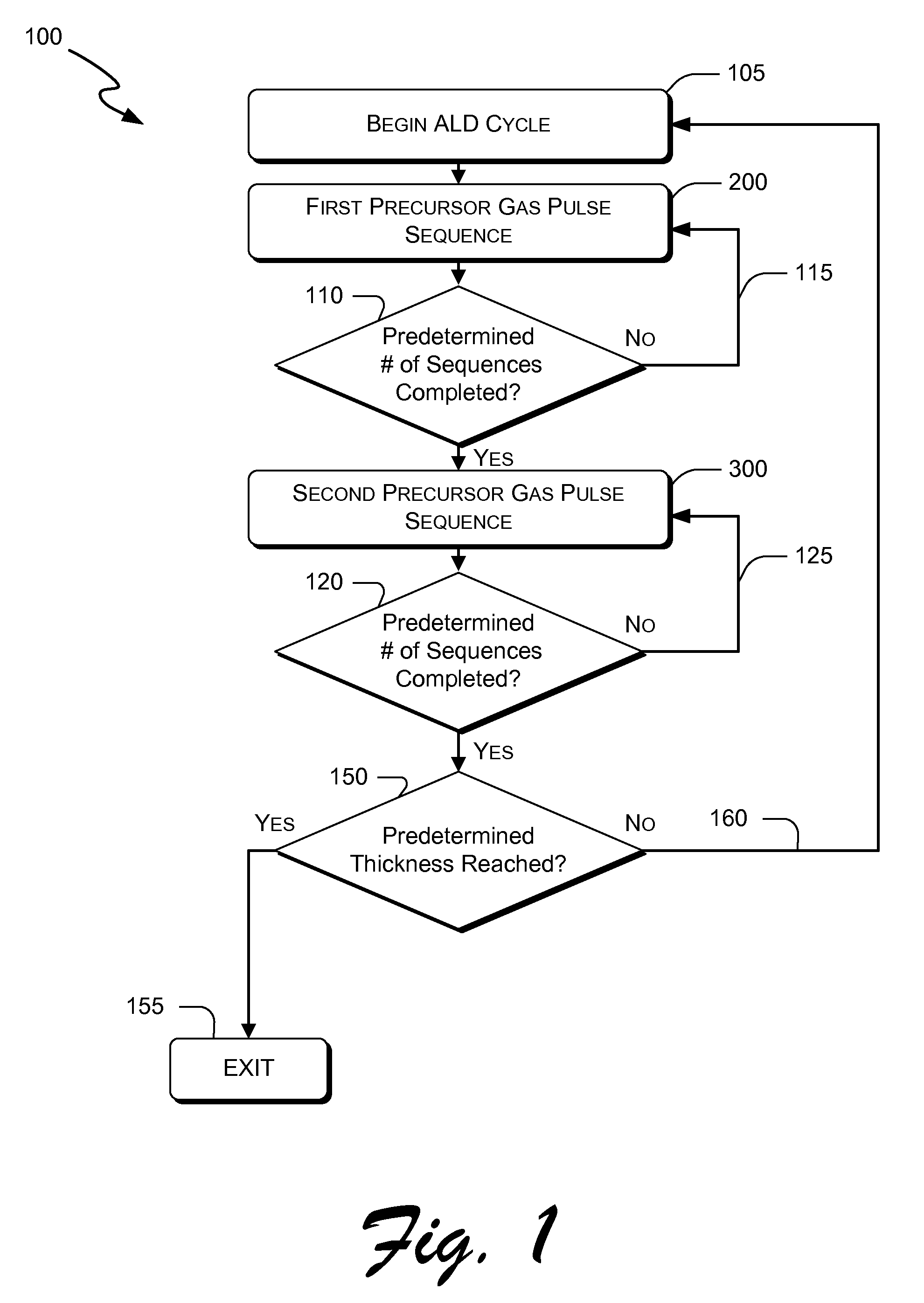
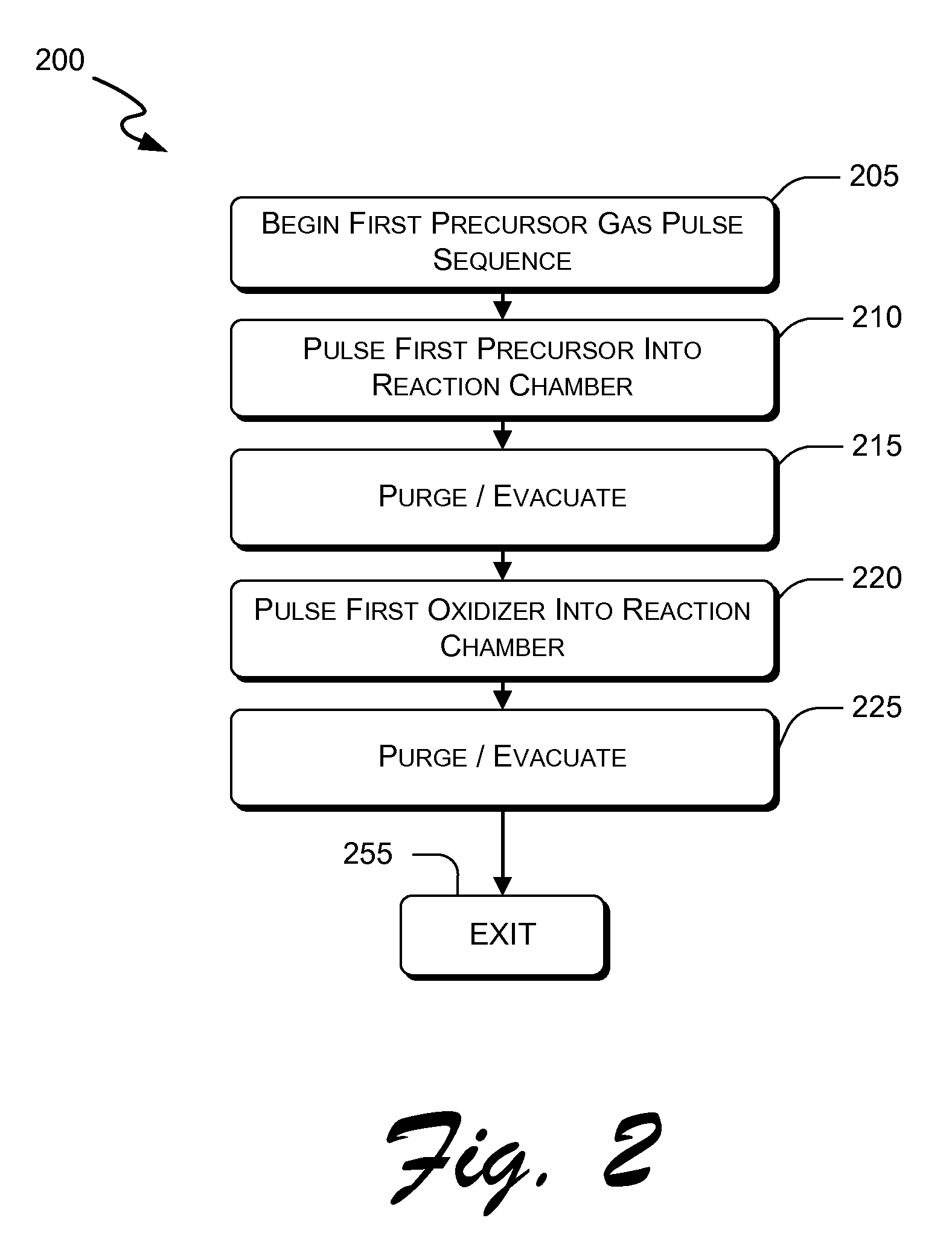
Atomic layer deposition of hafnium lanthanum oxides
There is provided an improved method for depositing thin films using precursors to deposit binary oxides by atomic layer deposition (ALD) techniques. Also disclosed is an ALD method for depositing a high-k dielectric such as hafnium lanthanum oxide (HfLaO) on a substrate. Embodiments of the present invention utilize a combination of ALD precursor elements and cycles to deposit a film with desired physical and electrical characteristics. Electronic components and systems that integrate devices fabricated with methods consistent with the present invention are also disclosed.
Owner:ASM IP HLDG BV
Implementing atomic layer deposition for gate dielectrics
PendingUS20170110313A1Semiconductor/solid-state device manufacturingChemical vapor deposition coatingGate dielectricLanthanum
A method for depositing a thin film onto a substrate is disclosed. In particular, the method forms a transitional metal silicate onto the substrate. The transitional metal silicate may comprise a lanthanum silicate or yttrium silicate, for example. The transitional metal silicate indicates reliability as well as good electrical characteristics for use in a gate dielectric material.
Owner:ASM IP HLDG BV
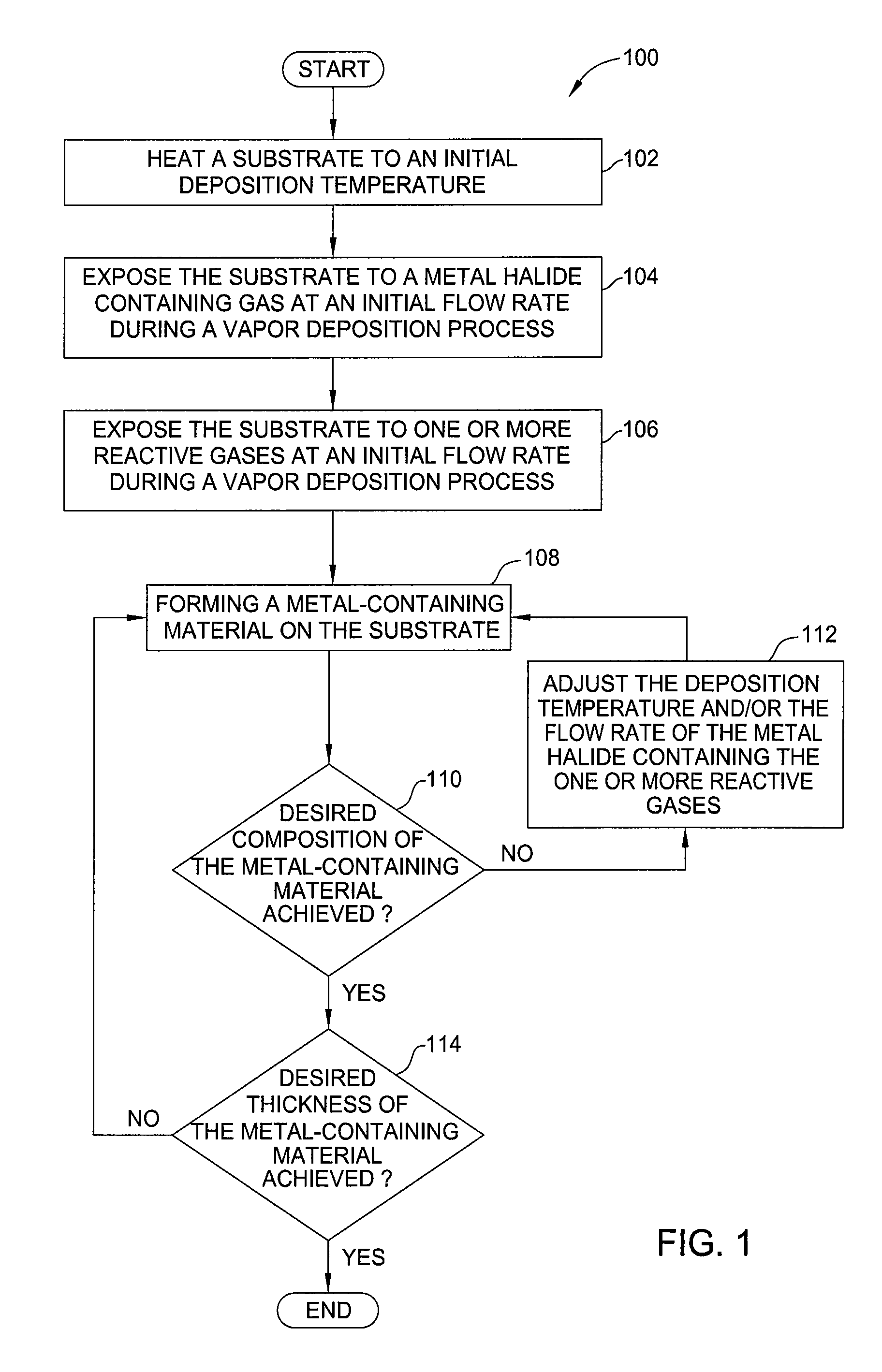
Nmos metal gate materials, manufacturing methods, and equipment using CVD and ald processes with metal based precursors
ActiveUS20110263115A1Semiconductor/solid-state device manufacturingChemical vapor deposition coatingGas phaseMetallic materials
Embodiments of the invention generally provide methods for depositing metal-containing materials and compositions thereof. The methods include deposition processes that form metal, metal carbide, metal silicide, metal nitride, and metal carbide derivatives by a vapor deposition process, including thermal decomposition, CVD, pulsed-CVD, or ALD. In one embodiment, a method for processing a substrate is provided which includes depositing a dielectric material having a dielectric constant greater than 10, forming a feature definition in the dielectric material, depositing a work function material conformally on the sidewalls and bottom of the feature definition, and depositing a metal gate fill material on the work function material to fill the feature definition, wherein the work function material is deposited by reacting at least one metal-halide precursor having the formula MXY, wherein M is tantalum, hafnium, titanium, and lanthanum, X is a halide selected from the group of fluorine, chlorine, bromine, or iodine, and y is from 3 to 5.
Owner:APPLIED MATERIALS INC
Infrared reflective color pigment
InactiveUS6454848B2Reduce heat buildupReduce energy costsInorganic pigment treatmentCoatingsIndiumCobalt
The present invention provides new solid solutions having a corundum-hematite crystalline structure which are useful as inorganic color pigments. Solid solutions according to the present invention include a host component having a corundum-hematite crystalline structure which contains as guest components one or more elements from the group consisting of aluminum, antimony, bismuth, boron, chrome, cobalt, gallium, indium, iron, lanthanum, lithium, magnesium, manganese, molybdenum, neodymium, nickel, niobium, silicon, tin, titanium, vanadium, and zinc. Solid solutions according to the present invention are formed by thoroughly mixing compounds, usually metal oxides or precursors thereof, which contain the host and guest components and then calcining the compounds to form the solid solutions having the corundum-hematite crystalline structure. Some of the new solid solutions according to the present invention exhibit relatively low Y CIE tri-stimulus values and relatively high near infrared reflectance.
Owner:FERRO CORP
Ceramic material and process for producing the same
ActiveUS20100047696A1Compactness sufficientOvercome lack of conductivitySolid electrolyte cellsCapacitor electrolytes/absorbentsLithiumCrystal structure
A ceramic material that can exhibit sufficient compactness and lithium (Li) conductivity to enable the use thereof as a solid electrolyte material for a lithium secondary battery and the like is provided. The ceramic material contains aluminum (Al) and has a garnet-type crystal structure or a garnet-like crystal structure containing lithium (Li), lanthanum (La), zirconium (Zr) and oxygen (O).
Owner:NGK INSULATORS LTD
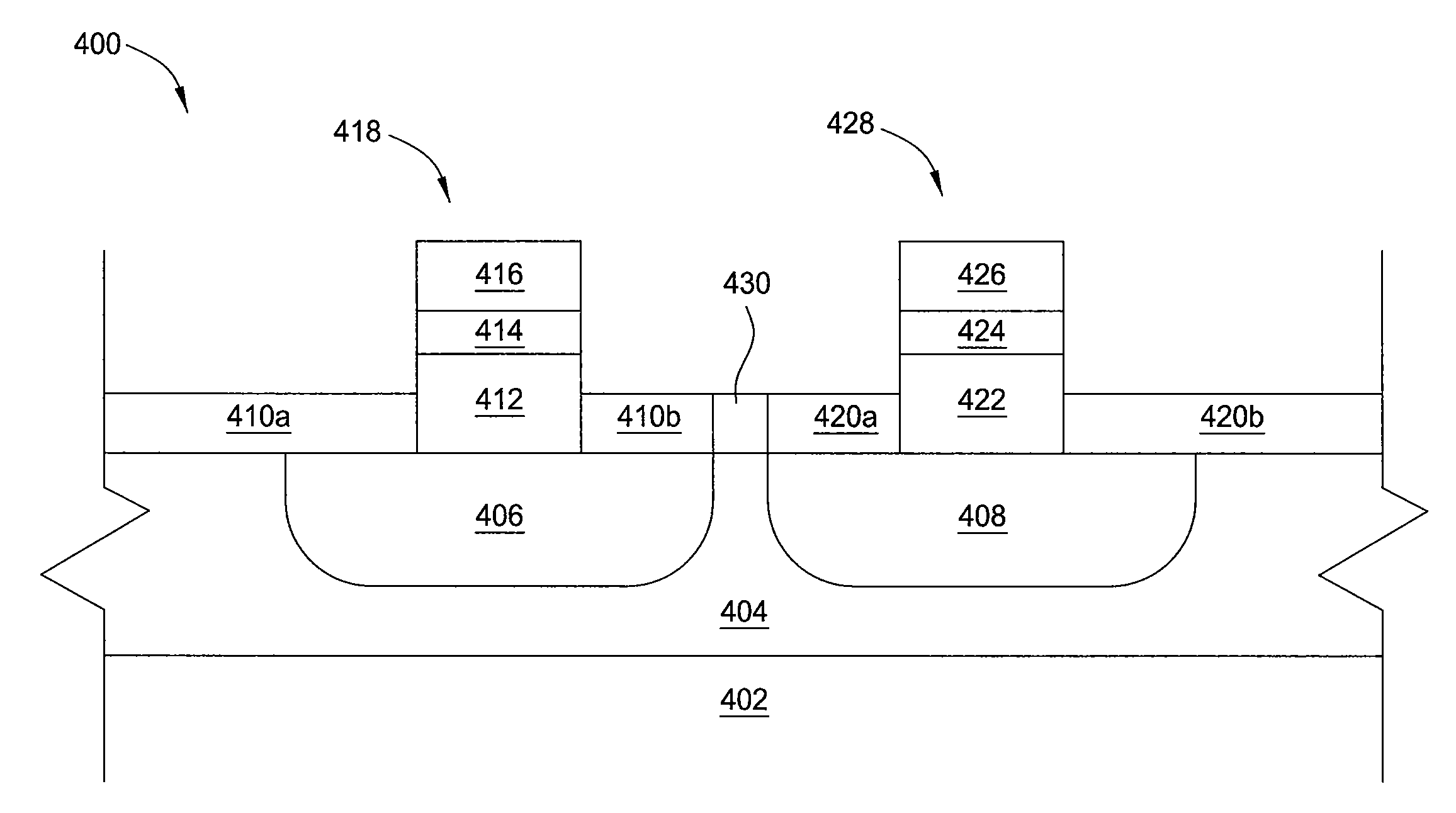
Enhanced electroless deposition of dielectric precursor materials for use in in-laid gate MOS transistors
InactiveUS6465334B1Semiconductor/solid-state device manufacturingSemiconductor devicesElectroless depositionOxygen
High quality dielectric layers, e.g., high-k dielectric layers comprised of at least one refractory or lanthanum series transition metal oxide or silicate, for use as gate insulator layers in in-laid metal gate MOS transistors and CMOS devices, are fabricated by forming an ultra-thin catalytic metal layer, e.g., a monolayer thick layer of Pd or Pd, on a Si-based semiconductor substrate, electrolessly plating on the catalytic layer comprising at least one refractory or lanthanum series transition metal or metal-based dielectric precursor layer, such as of Zr and / or Hf, and then reacting the precursor layer with oxygen or with oxygen and the semiconductor substrate to form the at least one high-k metal oxide or silicate. The inventive methodology prevents, or at least substantially reduces, oxygen access to the substrate surface during at least the initial stage(s) of formation of the gate insulator layer, thereby minimizing deleterious formation of oxygen-induced surface states at the semiconductor substrate / gate insulator interface.
Owner:GLOBALFOUNDRIES US INC
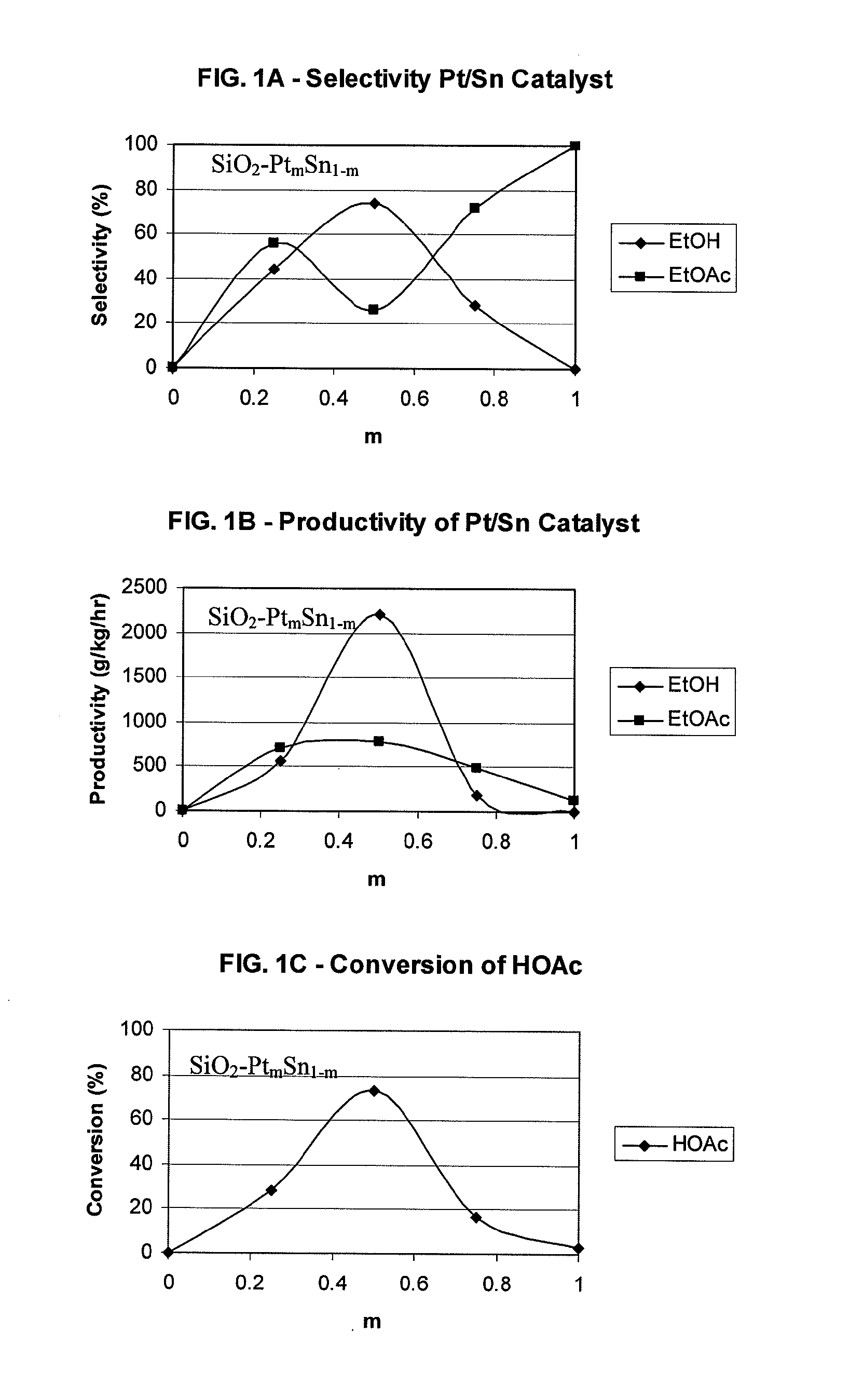
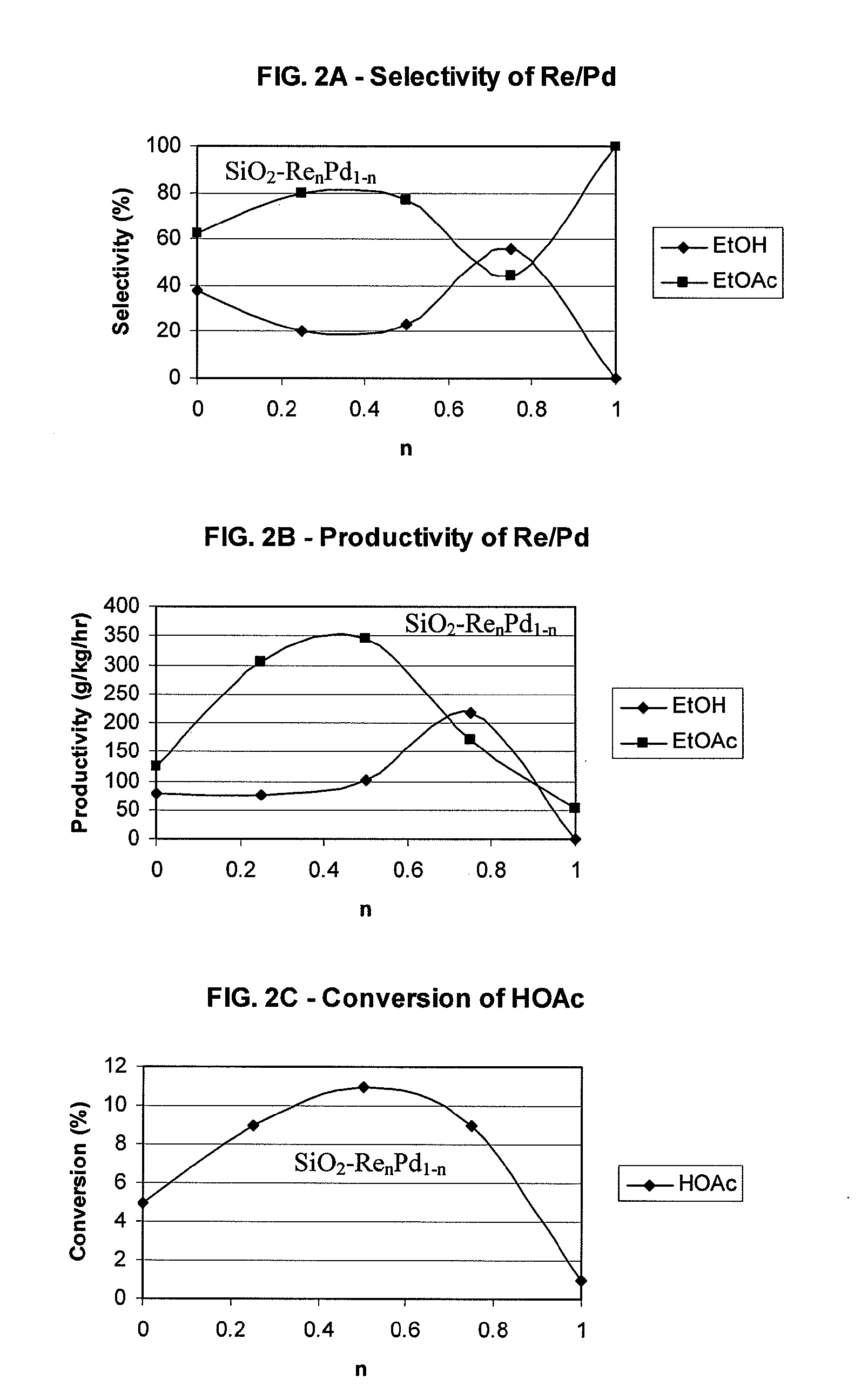
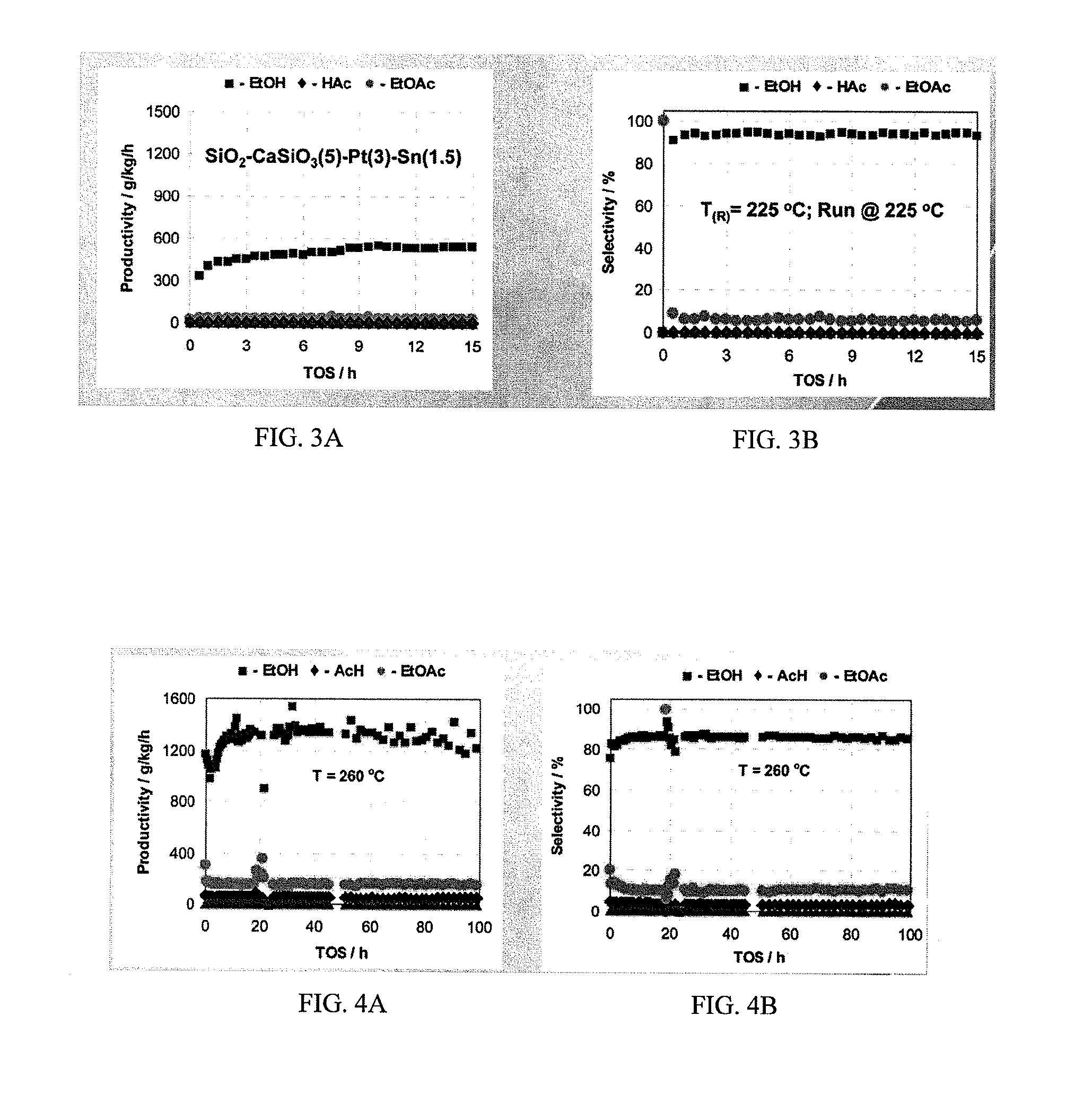
Processes for making ethanol from acetic acid
InactiveUS20100197985A1High selectivityPreparation by oxo-reaction and reductionEthylene productionCeriumCobalt
A process for selective formation of ethanol from acetic acid by hydrogenating acetic acid in the presence of first metal, a silicaceous support, and at least one support modifier. Preferably, the first metal is selected from the group consisting of copper, iron, cobalt, nickel, ruthenium, rhodium, palladium, osmium, iridium, platinum, titanium, zinc, chromium, rhenium, molybdenum, and tungsten. In addition the catalyst may comprise a second metal preferably selected from the group consisting of copper, molybdenum, tin, chromium, iron, cobalt, vanadium, tungsten, palladium, platinum, lanthanum, cerium, manganese, ruthenium, rhenium, gold, and nickel.
Owner:CELANESE INT CORP
Polymer nanocomposite implants with enhanced transparency and mechanical properties for administration within humans or animals
Polymer nanocomposite implants with nanofillers and additives are described. The nanofillers described can be any composition with the preferred composition being those composing barium, bismuth, cerium, dysprosium, europium, gadolinium, hafnium, indium, lanthanum, neodymium, niobium, praseodymium, strontium, tantalum, tin, tungsten, ytterbium, yttrium, zinc, and zirconium. The additives can be of any composition with the preferred form being inorganic nanopowders comprising aluminum, calcium, gallium, iron, lithium, magnesium, silicon, sodium, strontium, titanium. Such nanocomposites are particularly useful as materials for biological use in applications such as drug delivery, biomed devices, bone or dental implants.
Owner:PPG IND OHIO INC
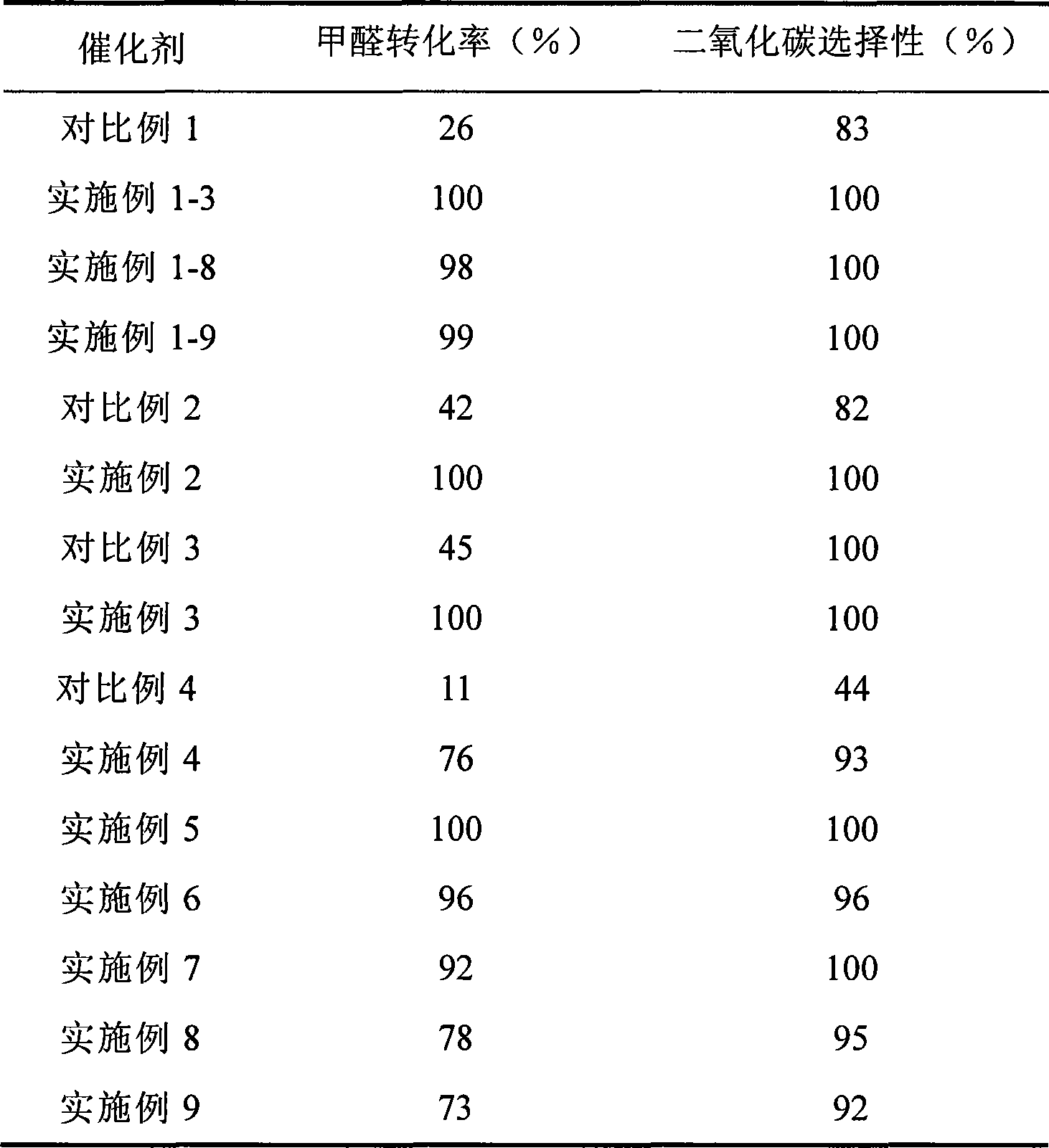
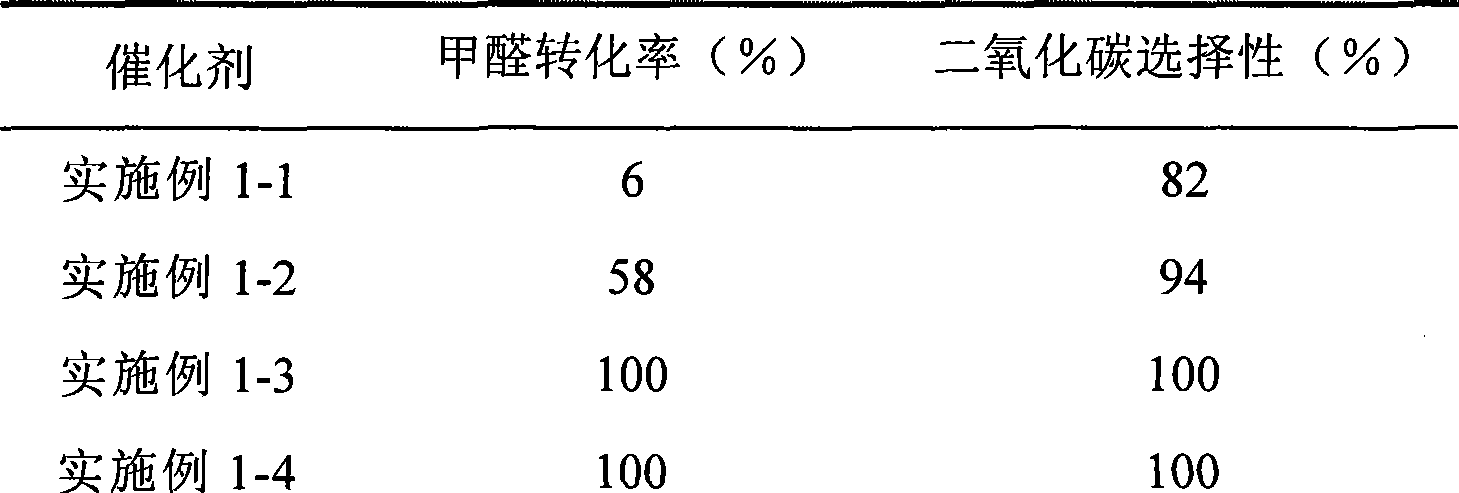
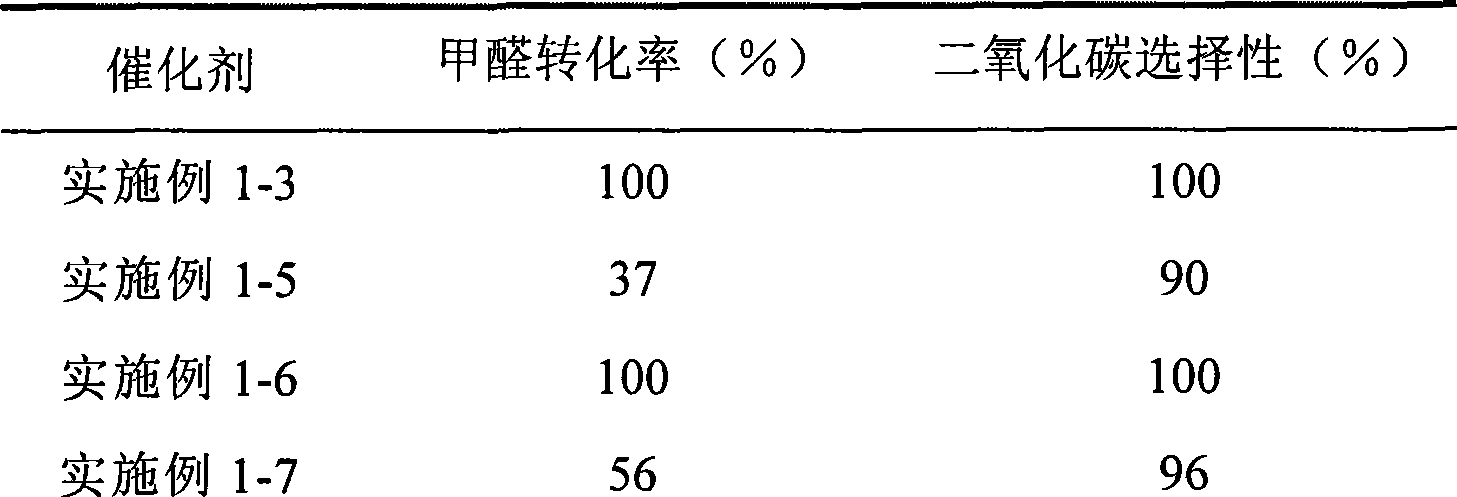
Catalyst for complete oxidation of formaldehyde at room temperature
ActiveCN101380574AEasy to makeEasy to operateDeodrantsMetal/metal-oxides/metal-hydroxide catalystsPorous carbonPt element
The invention provides a high selectivity catalyst used for catalyzing and completely oxidizing formaldehyde with low concentration at room temperature. The catalyst can catalyze formaldehyde completely so as to lead the formaldehyde to be converted into carbon dioxide and water at room temperature. In addition, the conversion rate of formaldehyde remains 100% within a long period of time, without complex auxiliary facilities such as light source, a heating oven and the like, and external conditions. The catalyst comprises three parts which are inorganic oxide carrier, noble metal component and auxiliary ingredient. Porous inorganic oxide carrier is one of cerium dioxide, zirconium dioxide, titanium dioxide, aluminium sesquioxide, tin dioxide, silicon dioxide, lanthanum sesquioxide, magnesium oxide and zinc oxide or the mixture thereof or composite oxide thereof, zeolite, sepiolite and porous carbon materials. The noble metal component of the catalyst is at least one of platinum, rhodium, palladium, gold and silver. The auxiliary ingredient is at least one of the alkali metals of lithium, sodium, kalium, rubidium and cesium. The loading of the noble metal component used in the catalyst of the invention is 0.1 to 10% according to weight converter of metal elements and the selective preference is 0.3 to 2%. The loading of the auxiliary ingredient is 0.2 to 30% according to weight converter of metal elements and the selective preference is 1 to 10%. When the loading of the auxiliary ingredient is lower than 0.2% or higher than 30%, the activity of the catalyst for catalyzing and oxidizing formaldehyde at room temperature is decreased remarkably.
Owner:广东顺德中科鸿图环境材料有限公司
Inorganic dopants, inks and related nanotechnology
InactiveUS6849109B2Facilitated DiffusionLower transition temperatureSelenium/tellurium compundsCell electrodesIndiumCerium
Ink compositions with modified properties result from using a powder size below 100 nanometers. Colored inks are illustrated. Nanoscale coated, uncoated, whisker inorganic fillers are included. The pigment nanopowders taught comprise one or more elements from the group actinium, aluminum, antimony, arsenic, barium, beryllium, bismuth, cadmuim, calcium, cerium, cesium, chalcogenide, cobalt, copper, dysprosium, erbium, europium, gadolinium, gallium, gold, hafnium, hydrogen, indium, iridium, iron, lanthanum, lithium, magnesium, manganese, mendelevium, mercury, molybdenum, neodymium, neptunium, nickel, niobium, nitrogen, oxygen, osmium, palladium, platinum, potassium, praseodymium, promethium, protactinium, rhenium, rubidium, scandium, silver, sodium, strontium, tantalum, terbium, thallium, thorium, tin, titanium, tungsten, vanadium, ytterbium, yttrium, zinc, and zirconium.
Owner:PPG IND OHIO INC
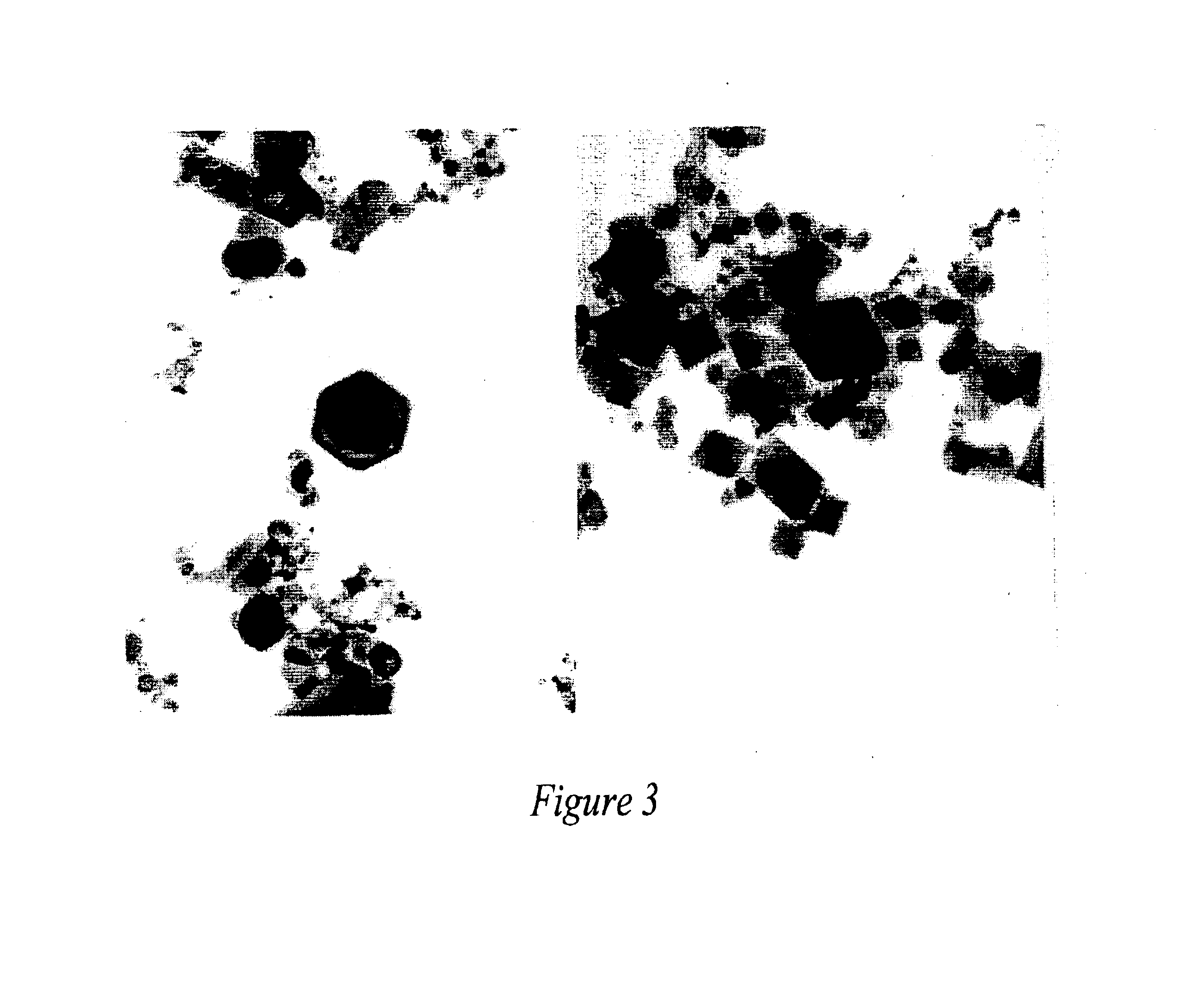
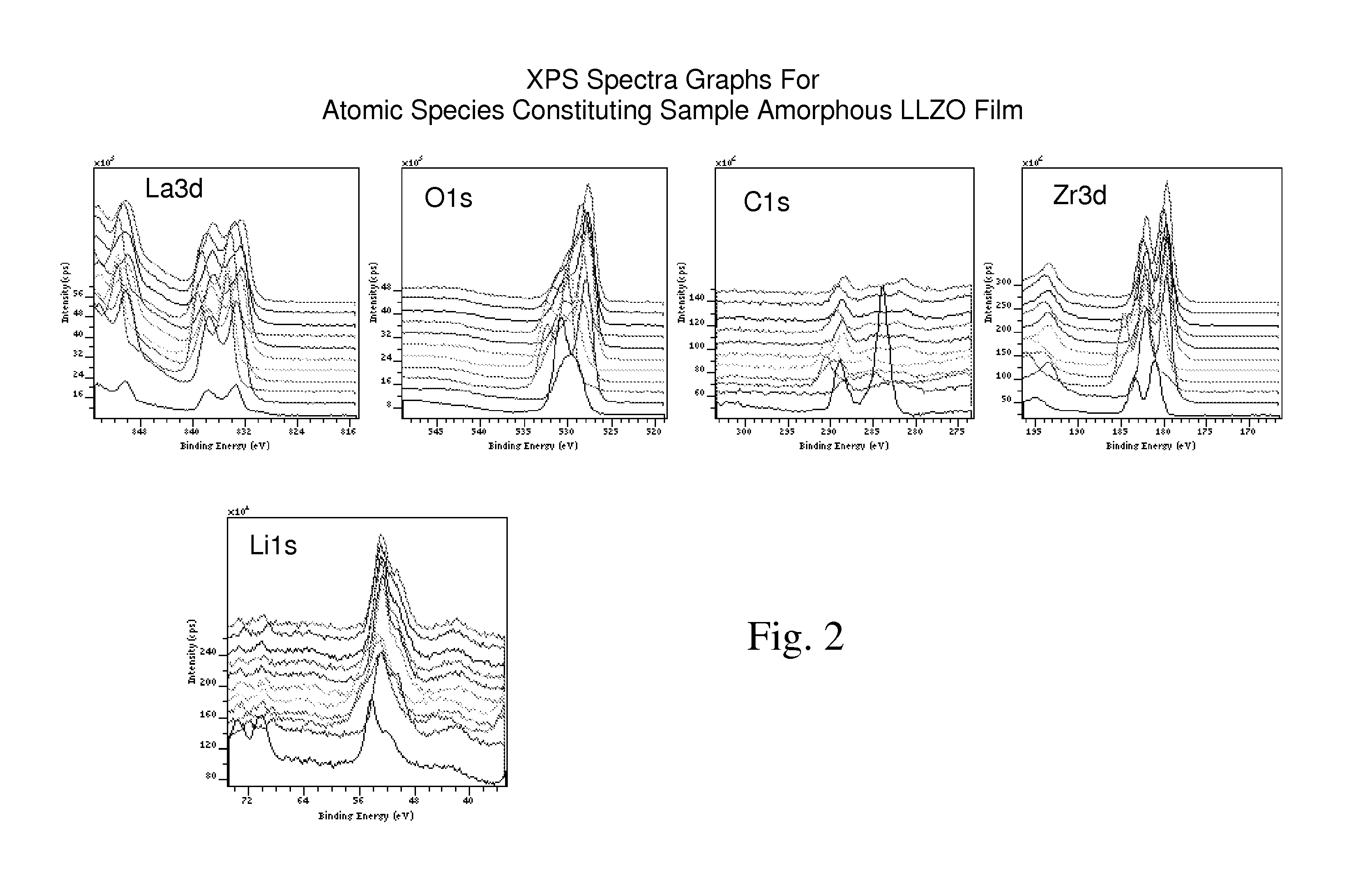
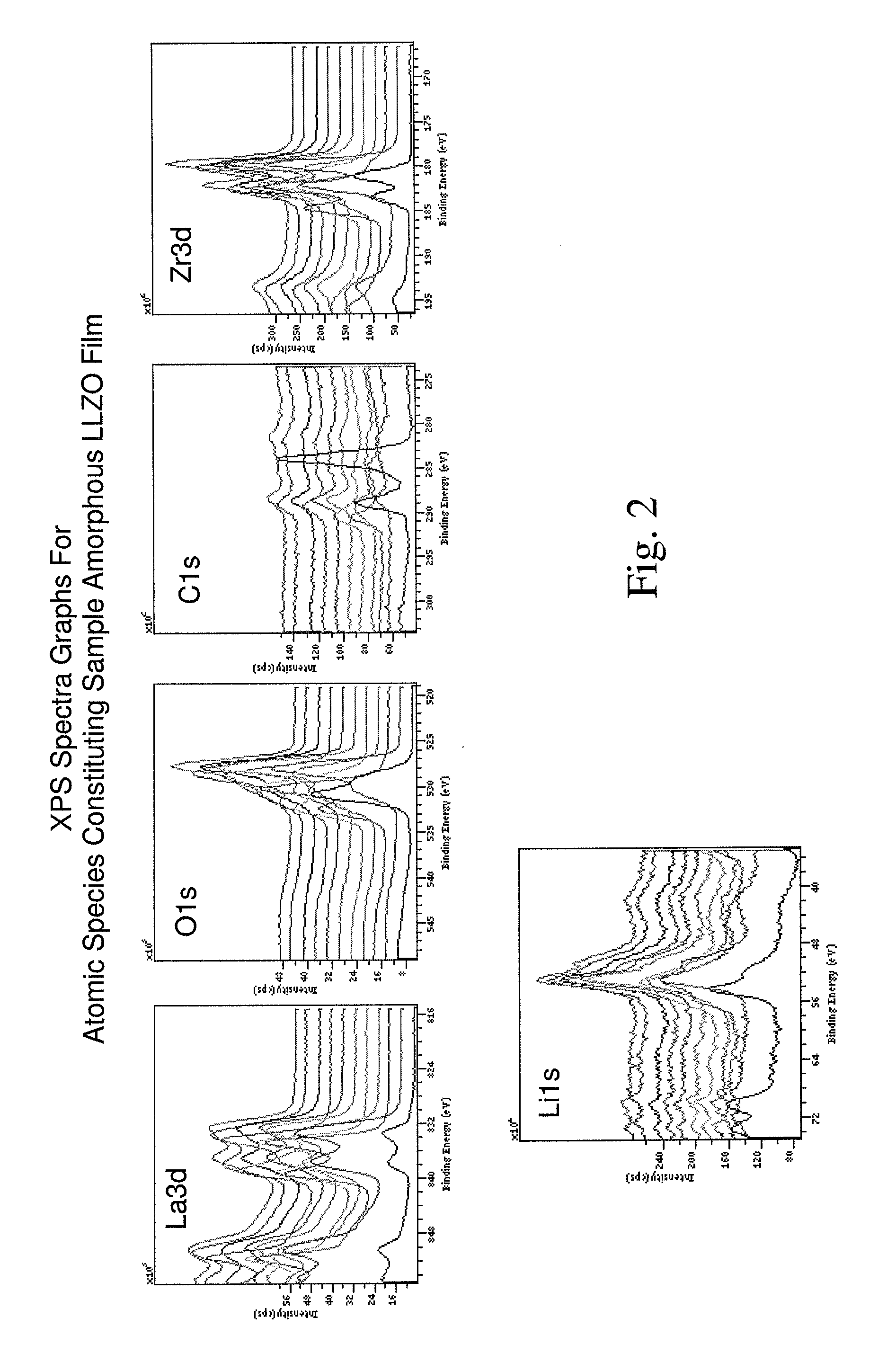
Ionically-conductive amorphous lithium lanthanum zirconium oxide
Amorphous lithium lanthanum zirconium oxide (LLZO) is formed as an ionically-conductive electrolyte medium. The LLZO comprises by percentage of total number of atoms from about 0.1% to about 50% lithium, from about 0.1% to about 25% lanthanum, from about 0.1% to about 25% zirconium, from about 30% to about 70% oxygen and from 0.0% to about 25% carbon. At least one layer of amorphous LLZO may be formed through a sol-gel process wherein quantities of lanthanum methoxyethoxide, lithium butoxide and zirconium butoxide are dissolved in an alcohol-based solvent to form a mixture which is dispensed into a substantially planar configuration, transitioned through a gel phase, dried and cured to a substantially dry phase.
Owner:JOHNSON IP HLDG LLC
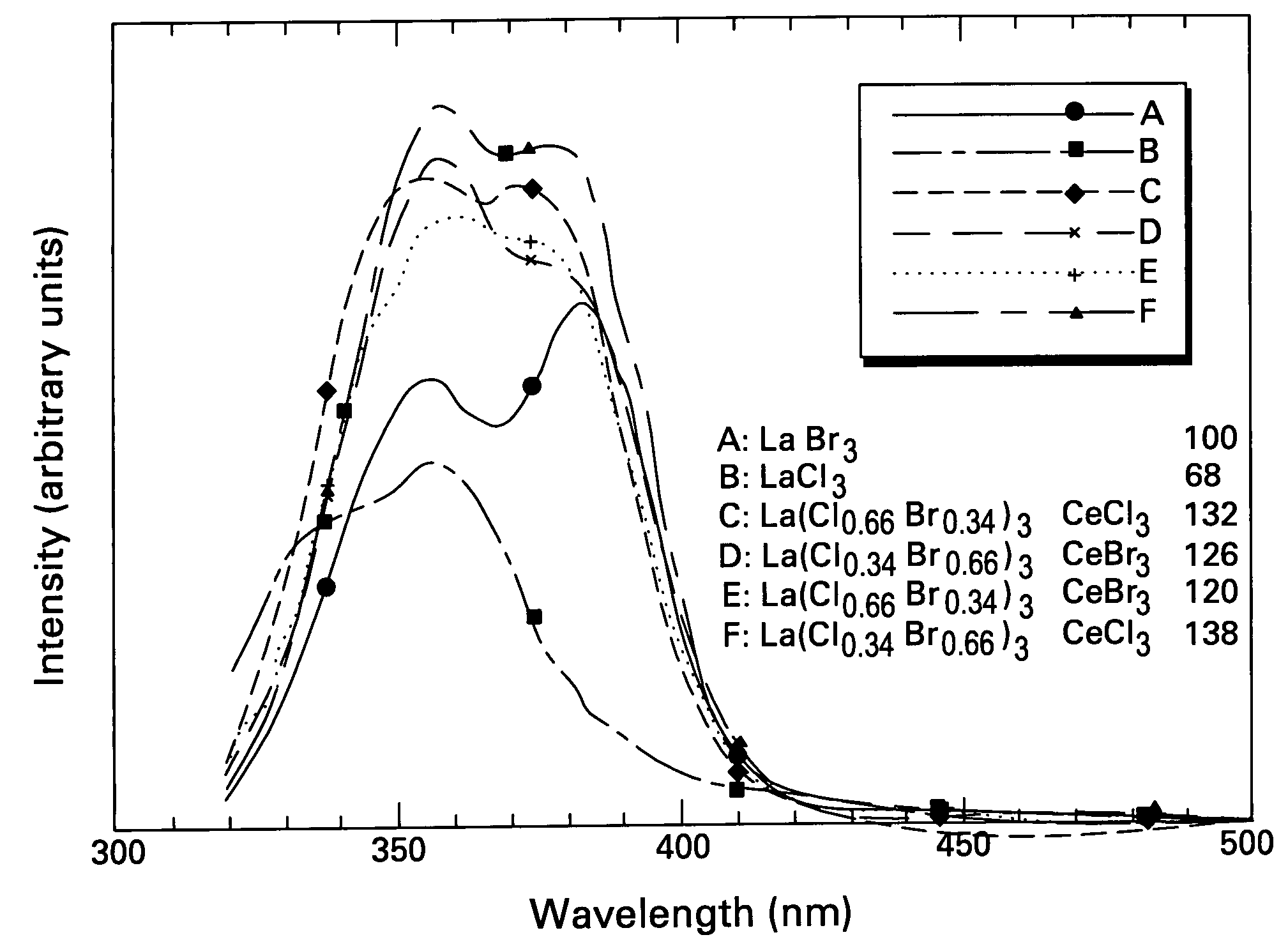
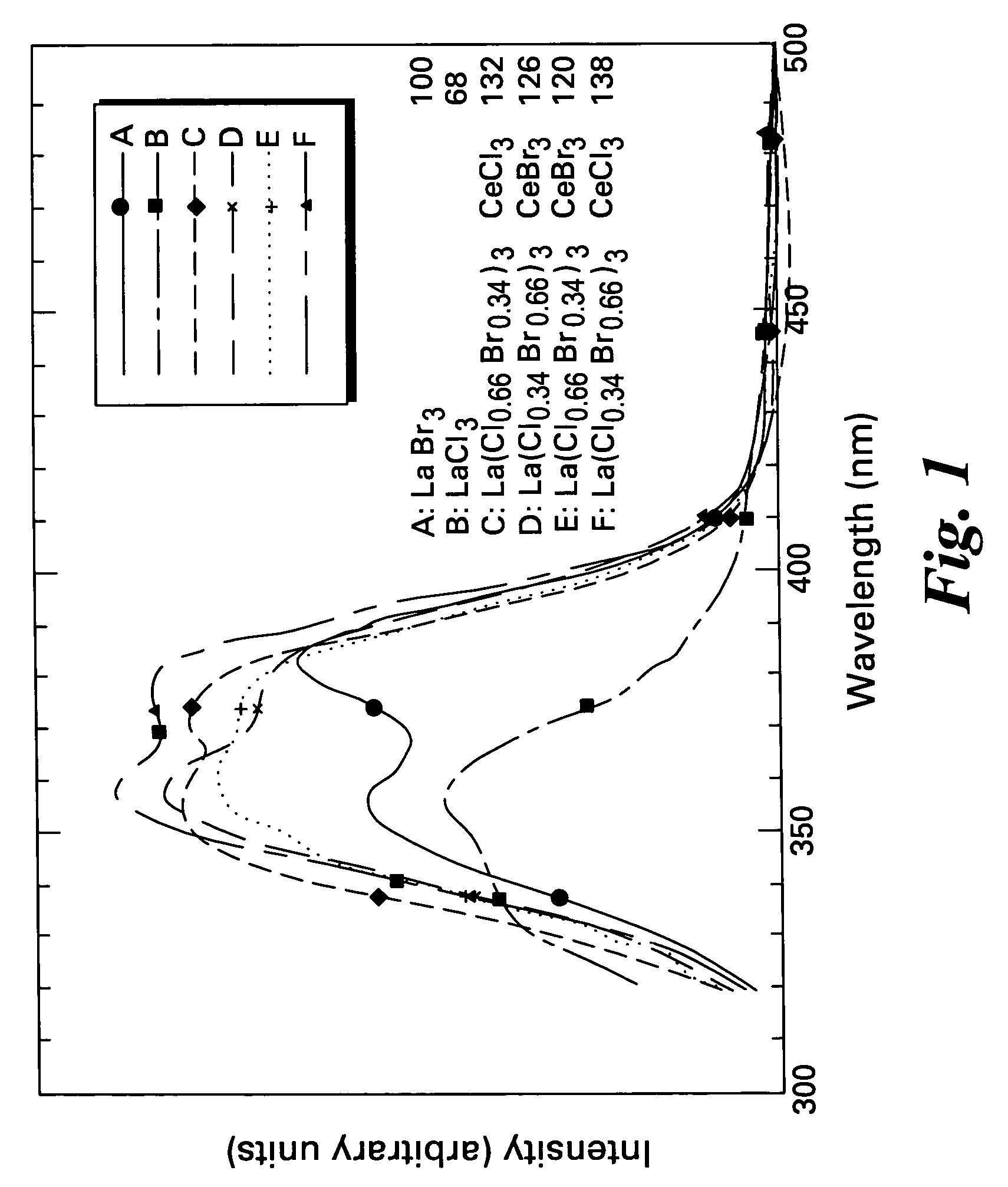
Scintillator compositions, and related processes and articles of manufacture
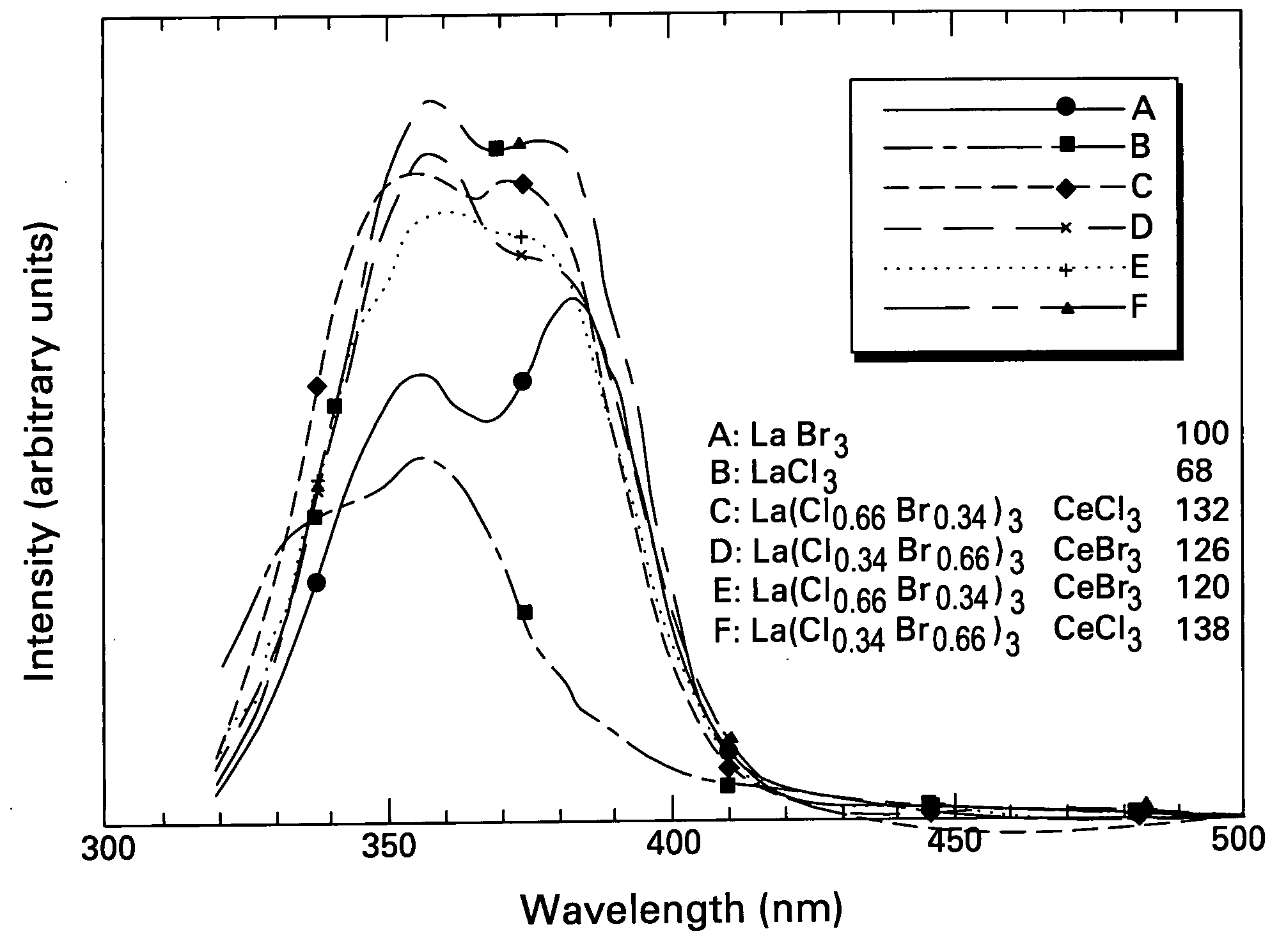
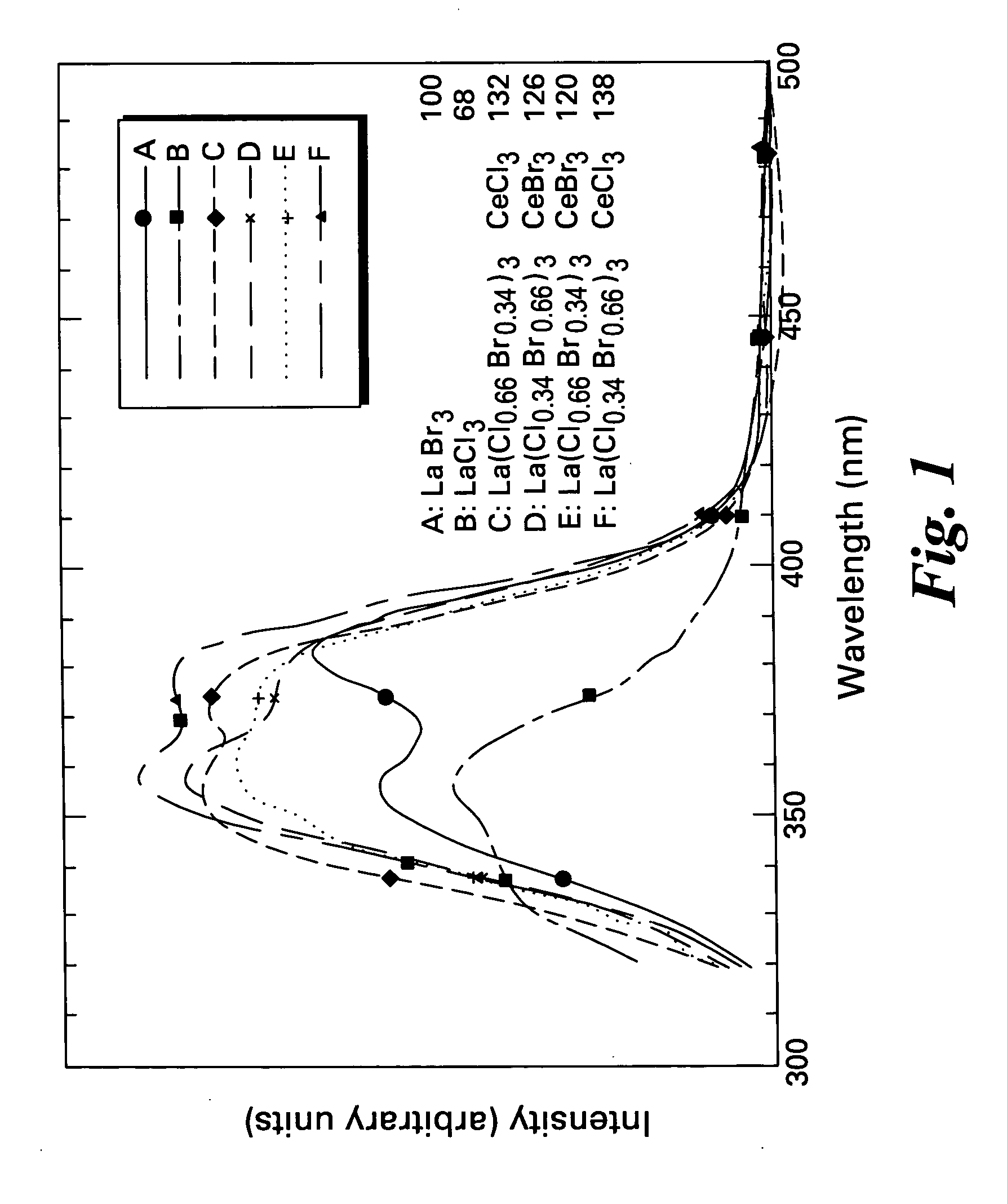
ActiveUS20050082484A1Improve performanceExcellent and reproducible scintillation responseMaterial analysis by optical meansLuminescent compositionsIodideHigh energy
Scintillator materials based on certain types of halide-lanthanide matrix materials are described. In one embodiment, the matrix material contains a mixture of lanthanide halides, i.e., a solid solution of at least two of the halides, such as lanthanum chloride and lanthanum bromide. In another embodiment, the matrix material is based on lanthanum iodide alone, which must be substantially free of lanthanum oxyiodide. The scintillator materials, which can be in monocrystalline or polycrystalline form, also include an activator for the matrix material, e.g., cerium. Radiation detectors that use the scintillators are also described, as are related methods for detecting high-energy radiation.
Owner:BAKER HUGHES OILFIELD OPERATIONS LLC
Method of depositing rare earth oxide thin films
InactiveUS6858546B2Increase probabilityIncrease ratingsPolycrystalline material growthSemiconductor/solid-state device manufacturingDeposition temperatureGadolinium
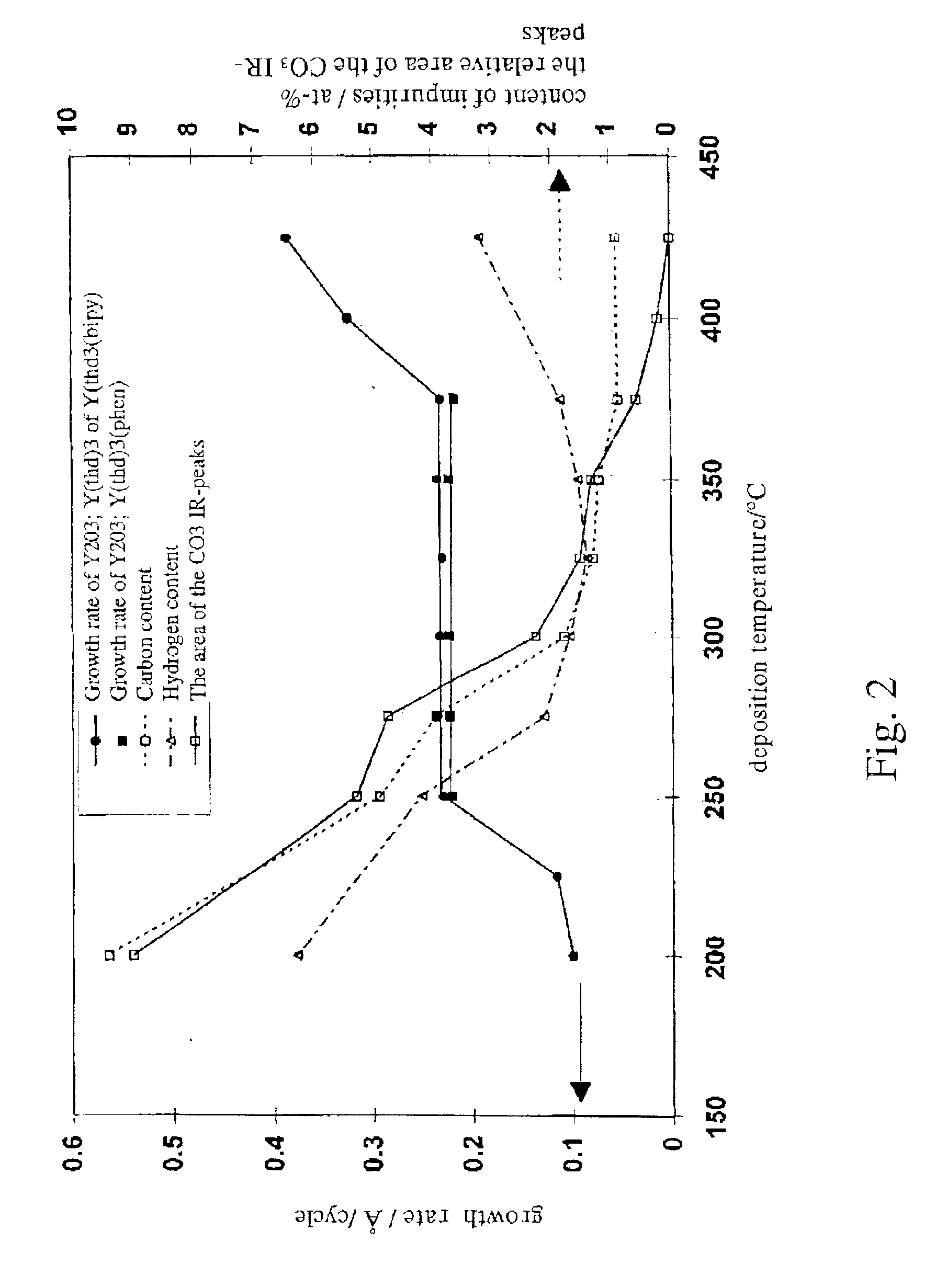
The present invention concerns a process for depositing rare earth oxide thin films, especially yttrium, lanthanum and gadolinium oxide thin films by an ALD process, according to which invention the source chemicals are cyclopentadienyl compounds of rare earth metals, especially those of yttrium, lanthanum and gadolinium. Suitable deposition temperatures for yttrium oxide are between 200 and 400° C. when the deposition pressure is between 1 and 50 mbar. Most suitable deposition temperatures for lanthanum oxide are between 160 and 165° C. when the deposition pressure is between 1 and 50 mbar.
Owner:ASM INTERNATIONAL
Method of forming copper interconnections with enhanced electromigration resistance and reduced defect sensitivity
A method of providing sub-half-micron copper interconnections with improved electromigration and corrosion resistance. The method includes double damascene using electroplated copper, where the seed layer is converted to an intermetallic layer. A layer of copper intermetallics with halfnium, lanthanum, zirconium or tin, is provided to improve the electromigration resistance and to reduce defect sensitivity. A method is also provided to form a cap atop copper lines, to improve corrosion resistance, which fully covers the surface. Structure and methods are also described to improve the electromigration and corrosion resistance by incorporating carbon atoms in copper intersititial positions.
Owner:GLOBALFOUNDRIES INC
Electrolytic deposition of dielectric precursor materials for use in in-laid gate MOS transistors
InactiveUS6300203B1Semiconductor/solid-state device manufacturingSemiconductor devicesElectrolysisOxygen
High quality dielectric layers, e.g., high-k dielectric layers comprised of at least one refractory or lanthanum series transition metal oxide or silicate, for use as gate insulator layers in in-laid metal gate MOS transistors and CMOS devices, are formed by electrolytically plating a metal or metal-based dielectric precursor layer comprising at least one refractory or lanthanum series transition metal, on a semiconductor substrate, typically a silicon-based substrate, and then reacting the precursor layer with oxygen or with oxygen and the semiconductor substrate to form the at least one refractory or lanthanum series transition metal oxide or silicate. The inventive methodology prevents, or at least substantially reduces, oxygen access to the substrate surface during at least the initial stage(s) of formation of the gate insulator layer, thereby minimizing deleterious formation of oxygen-induced surface states at the semiconductor substrate / gate insulator interface.
Owner:GLOBALFOUNDRIES US INC
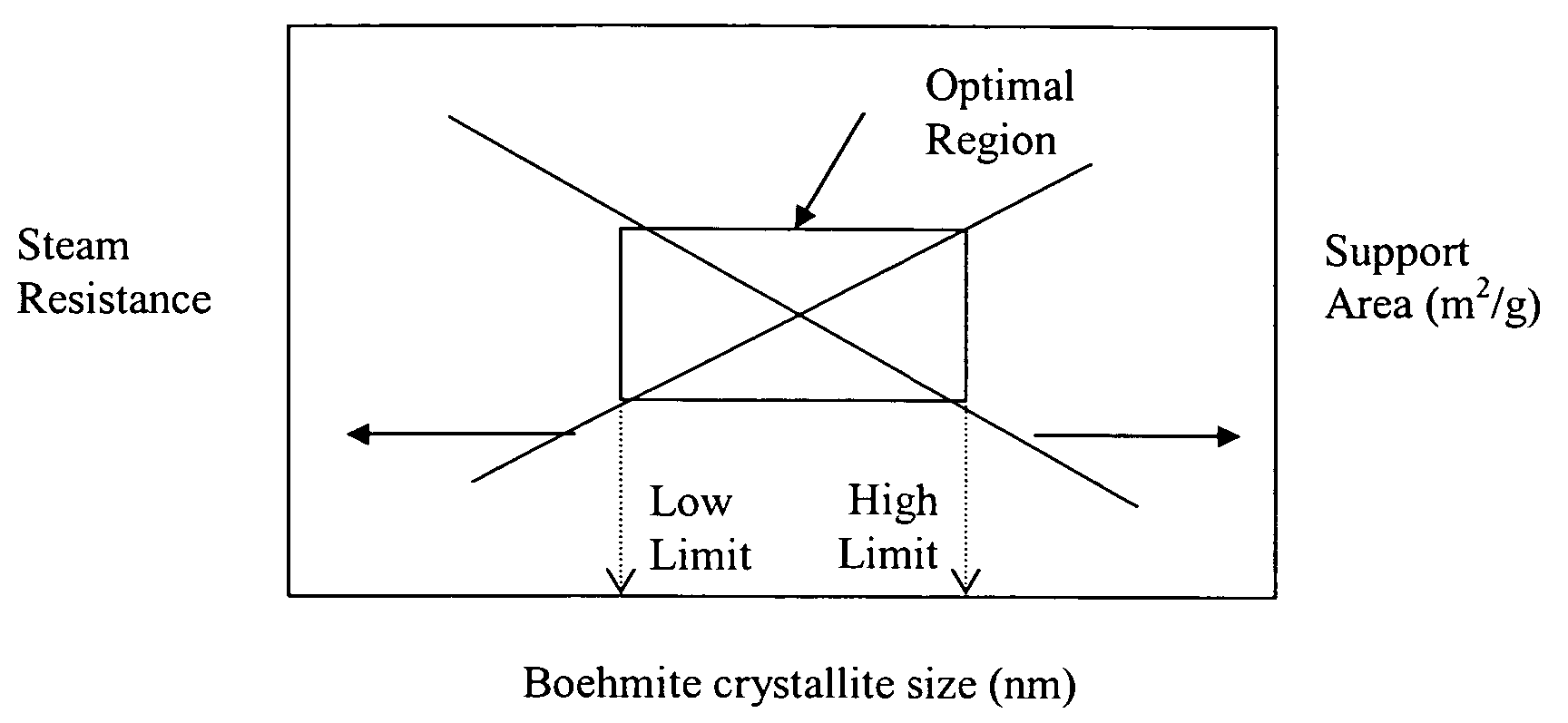
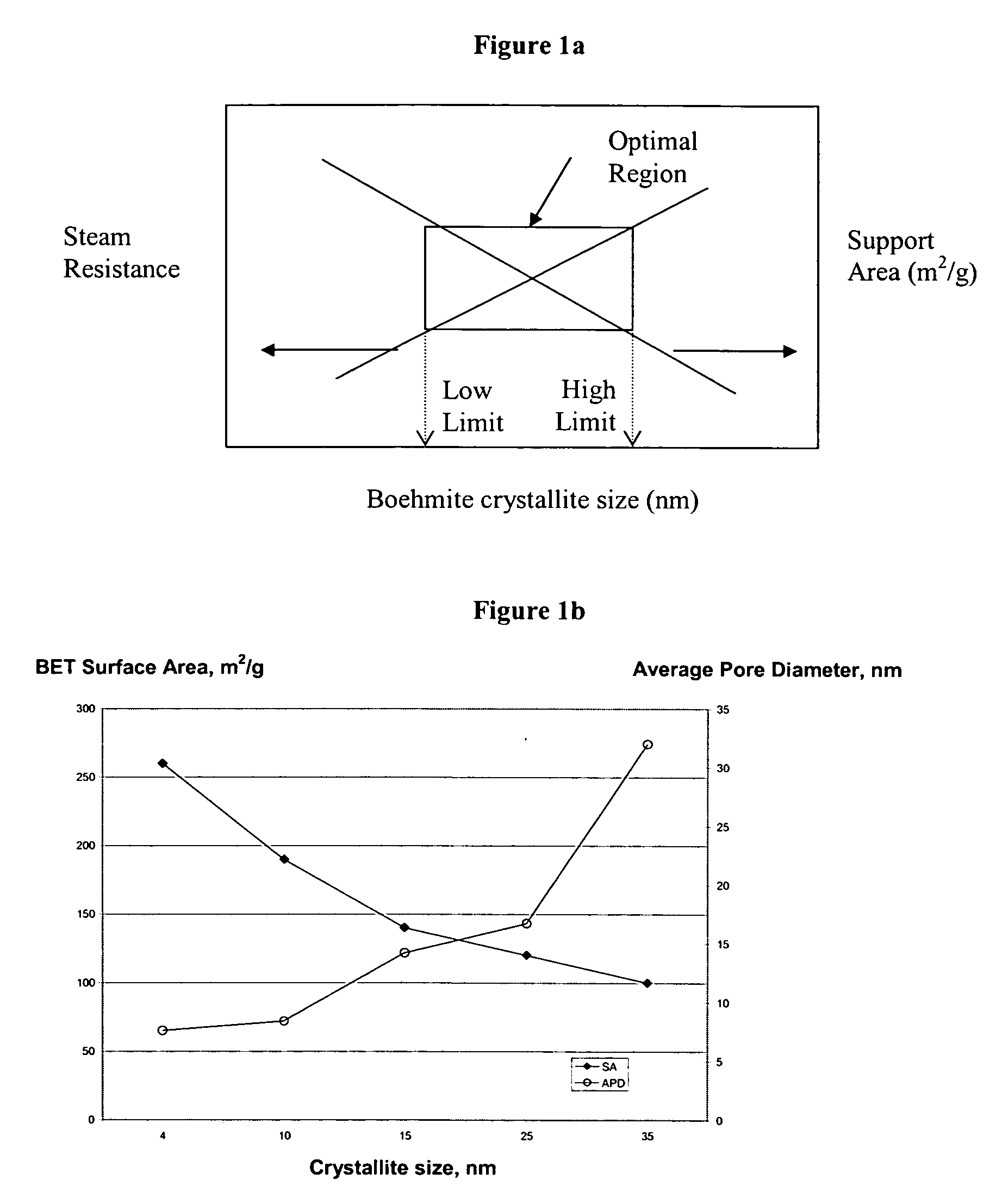
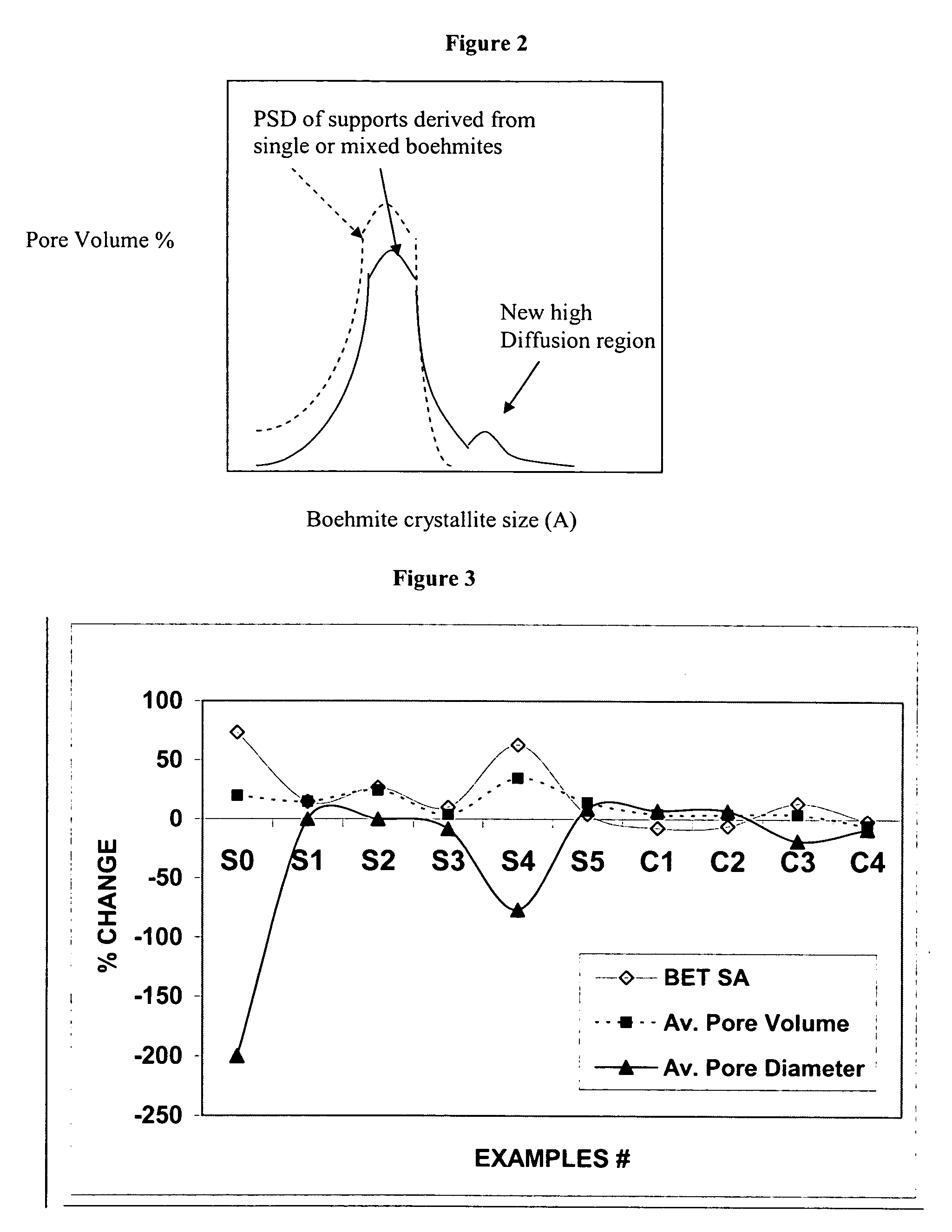
Stabilized boehmite-derived catalyst supports, catalysts, methods of making and using
ActiveUS20050234137A1Good hydrothermal stabilityImprove stabilityMaterial nanotechnologyCatalyst carriersPorosityAluminium hydroxide
A stabilized catalyst support having improved hydrothermal stability, catalyst made therefrom, and method for producing hydrocarbons from synthesis gas using said catalyst. The stabilized support is made by a method comprising treating a crystalline hydrous alumina precursor in contact with at least one structural stabilizer or compound thereof. The crystalline hydrous alumina precursor preferably includes an average crystallite size selected from an optimum range delimited by desired hydrothermal resistance and desired porosity. The crystalline hydrous alumina precursor preferably includes an alumina hydroxide, such as crystalline boehmite, crystalline bayerite, or a plurality thereof differing in average crystallite sizes by at least about 1 nm. The crystalline hydrous alumina precursor may be shaped before or after contact with the structural stabilizer or compound thereof. The treating includes calcining at 450° C. or more. Preferred structural stabilizers can include cobalt, magnesium, manganese, manganese, zirconium, boron, aluminum, barium, silicon, lanthanum, oxides thereof, or combinations thereof.
Owner:CLARIANT INT LTD
Materials for positive electrodes of lithium ion batteries and their methods of fabrication
InactiveUS20050130042A1Cycle wellEasy dischargeElectrode thermal treatmentSecondary cellsCeriumSolvent
This invention discloses materials for positive electrodes of secondary batteries and their methods of fabrication. Said materials comprise of granules of an active material for positive electrodes coated with an oxide layer. The active material is one or more of the following: oxides of lithium cobalt, oxides of lithium nickel cobalt, oxides of lithium nickel cobalt manganese, oxides of lithium manganese, LiCoO2, LiNi1-xCoxO2, LiNi1 / 3Co1 / 3Mn1 / 3O2, and LiMn2O4. The non-oxygen component in the oxide layer is one or more of the following: aluminum, magnesium, zinc, calcium, barium, strontium, lanthanum, cerium, vanadium, titanium, tin, silicon, boron, Al, Mg, Zn, Ca, Ba, Sr, La, Ce, V, Ti, Sn, Si, and B. Said non-oxygen component of the granules is between 0.01 wt. % to 10 wt. % of said granules of active material. The methods of fabrication for said materials includes the steps of mixing an additive and an active material for positive electrodes uniformly in water or solvent, evaporating said solvent or water, and heat treating the remaining mixture at 300° C. to 900° C. for between 1 hour to 20 hours. The additive is a compound of one or more of the following elements: aluminum, magnesium, zinc, calcium, barium, strontium, lanthanum, cerium, vanadium, titanium, tin, silicon, boron, Al, Mg, Zn, Ca, Ba, Sr, La, Ce, V, Ti, Sn, Si, and B where the element is between 0.01 wt. % to 10 wt. % of said active material. Using the materials of positive electrodes disclosed above or materials for positive electrodes fabricated in the methods disclosed above in batteries produces batteries with excellent cycling and high temperature properties.
Owner:BYD AMERICA CORP
Layered thermal barrier coatings containing lanthanide series oxides for improved resistance to CMAS degradation
InactiveUS20070160859A1Avoid damageElimination of expensiveBlade accessoriesEfficient propulsion technologiesReaction layerCerium
A coating applied as a two layer system. The outer layer is an oxide of a group IV metal selected from the group consisting of zirconium oxide, hafnium oxide and combinations thereof, which are doped with an effective amount of a lanthanum series oxide. These metal oxides doped with a lanthanum series addition comprises a high weight percentage of the outer coating. As used herein, lanthanum series means an element selected from the group consisting of lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), lutetium (Lu) and combinations thereof, and lanthanum series oxides are oxides of these elements. When the zirconium oxide is doped with an effective amount of a lanthanum series oxide, a dense reaction layer is formed at the interface of the outer layer of TBC and the CMAS. This dense reaction layer prevents CMAS infiltration below it. The second layer, or inner layer underlying the outer layer, comprises a layer of partially stabilized zirconium oxide.
Owner:GENERAL ELECTRIC CO
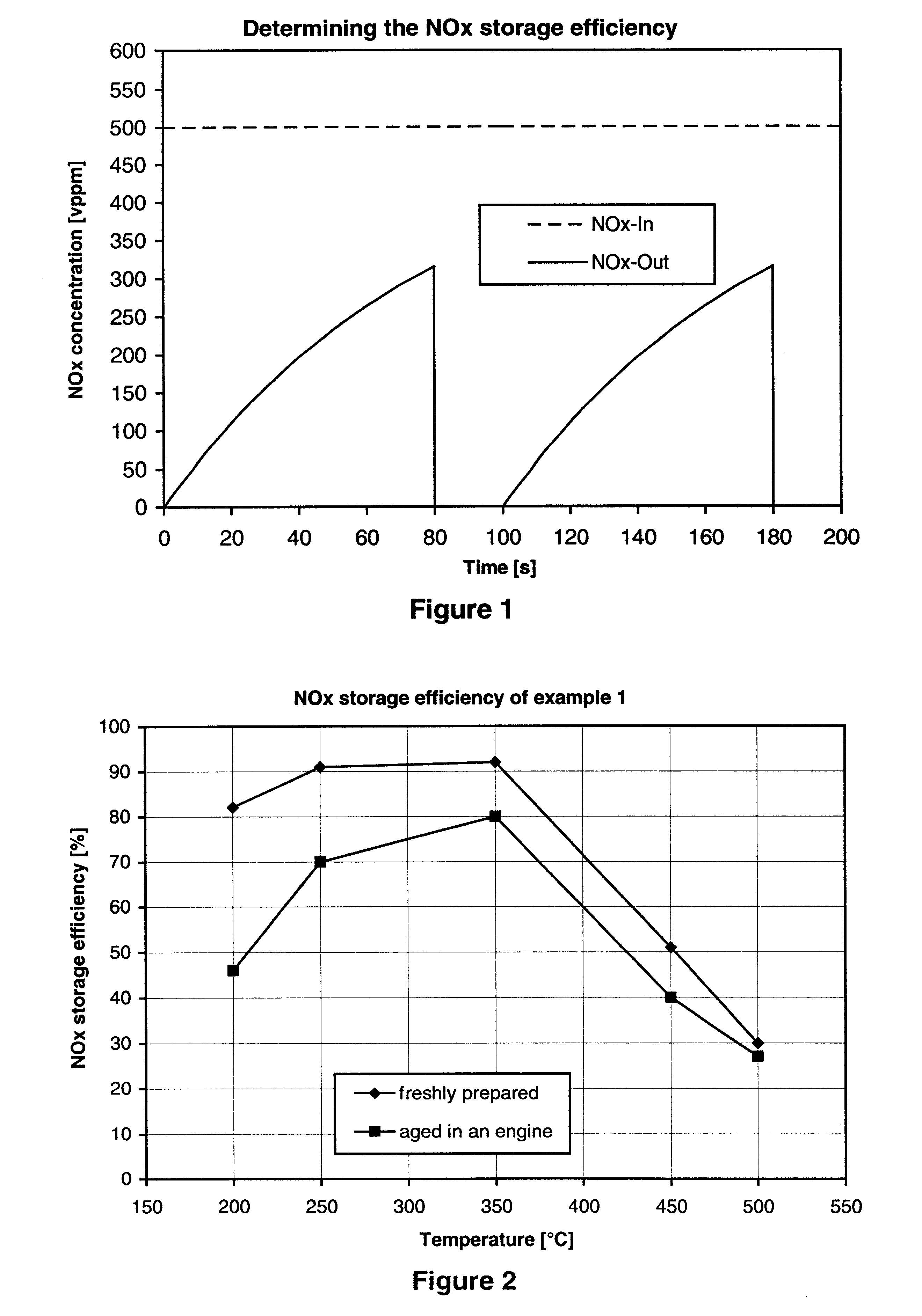
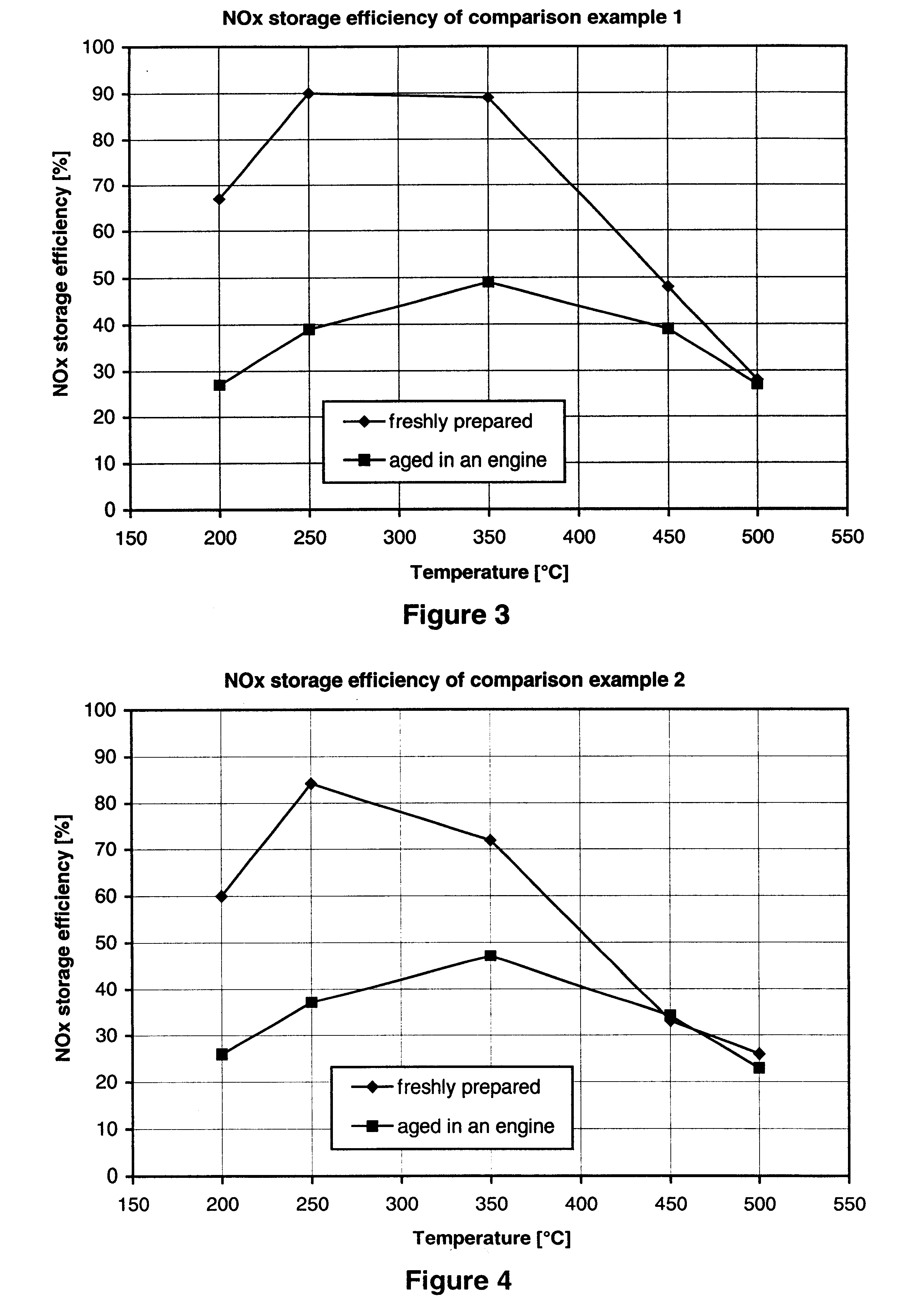
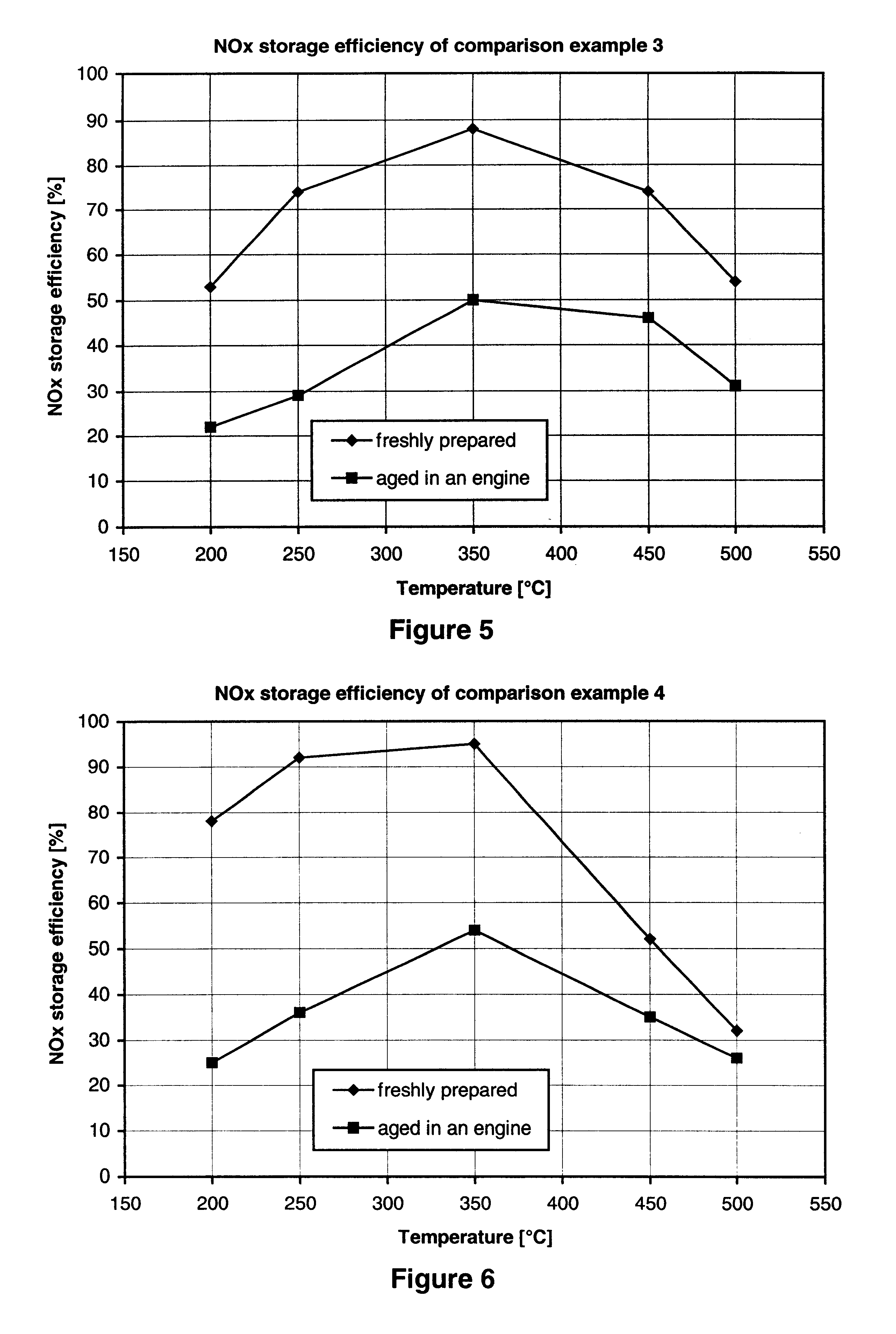
Nitrogen oxide storage material and nitrogen oxide storing catalyst prepared therefrom
InactiveUS6350421B1Determine efficiencyNitrogen compoundsExhaust apparatusAlkaline earth metalCuprate
A nitrogen oxide storage material is disclosed which contains at least one storage component for nitrogen oxides in the form of an oxide, mixed oxide, carbonate or hydroxide of the alkaline earth metals magnesium, calcium, strontium and barium and the alkali metals potassium and caesium on a high surface area support material. The support material can be doped cerium oxide, cerium / zirconium mixed oxide, calcium titanate, strontium titanate, barium titanate, barium stannate, barium zirconate, magnesium oxide, lanthanum oxide, praseodymium oxide, samarium oxide, neodymium oxide, yttrium oxide, zirconium silicate, yttrium barium cuprate, lead titanate, tin titanate, bismuth titanate, lanthanum cobaltate, lanthanum manganate and barium cuprate or mixtures thereof.
Owner:DMC2 DEGUSSA METALS +1
High performance lithium ion battery anode material lithium manganate and preparation method thereof
The invention provides a high performance lithium ion battery anode material lithium manganate and a preparation method of the material. The lithium manganate is a doped lithium manganate LiMn2-yXy04 which is doped with one kind or a plurality of other metal elements X, wherein X element is at least one kind selected form the group of aluminium, lithium, fluorine, silver, copper, chromium, zinc, titanium, bismuth, germanium, gallium, zirconium, stannum, silicon, cobalt, nickel, vanadium, magnesium, calcium, strontium, barium and rare earth elements lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium and lutetium, and y is larger than 0 but less than or equal to 0.11. The lithium ion battery anode material lithium manganate provided in the invention has extraordinary charge and discharge cycle performance both in the environments of normal temperature and high temperature. According to the invention, the preparation method of the material is a solid phase method, the operation is simple and controllable and the cost is low so that it is easy to realize large-scale productions.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
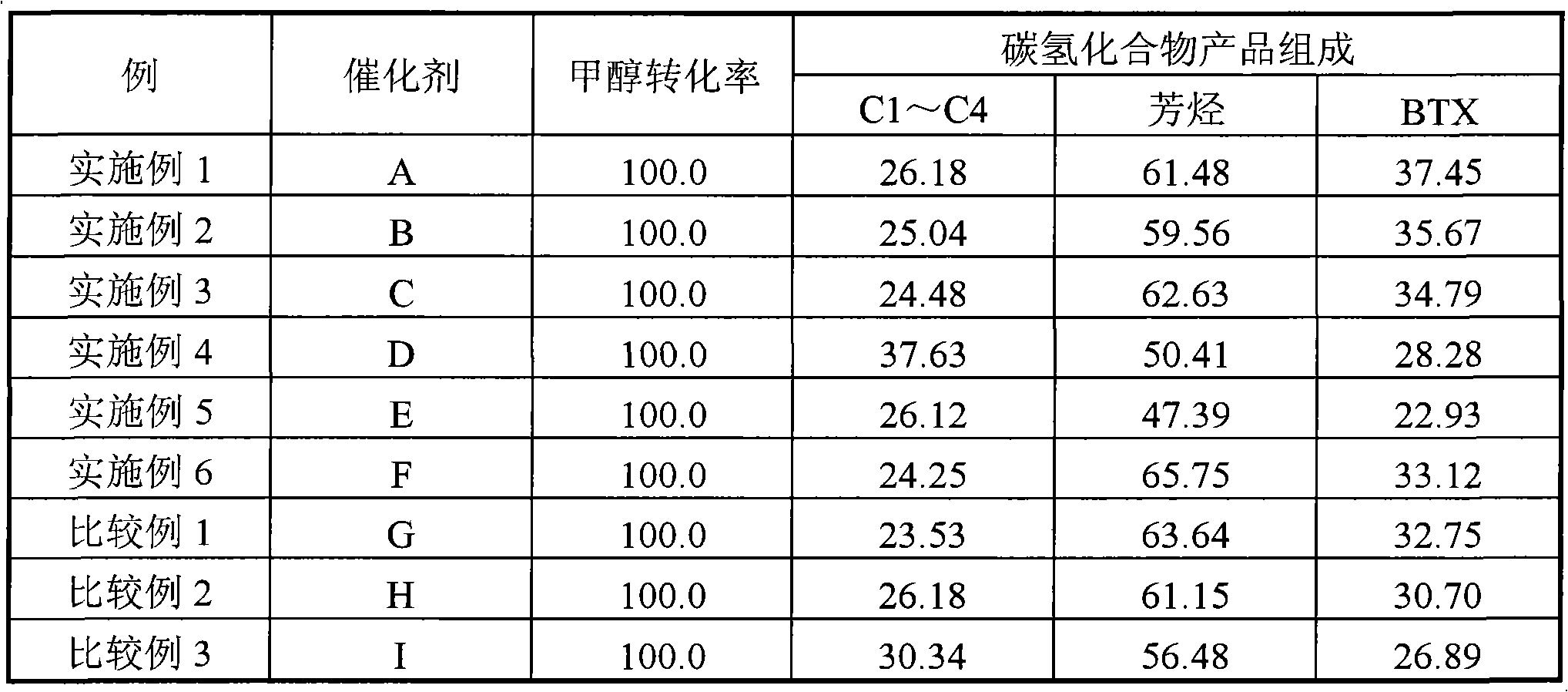
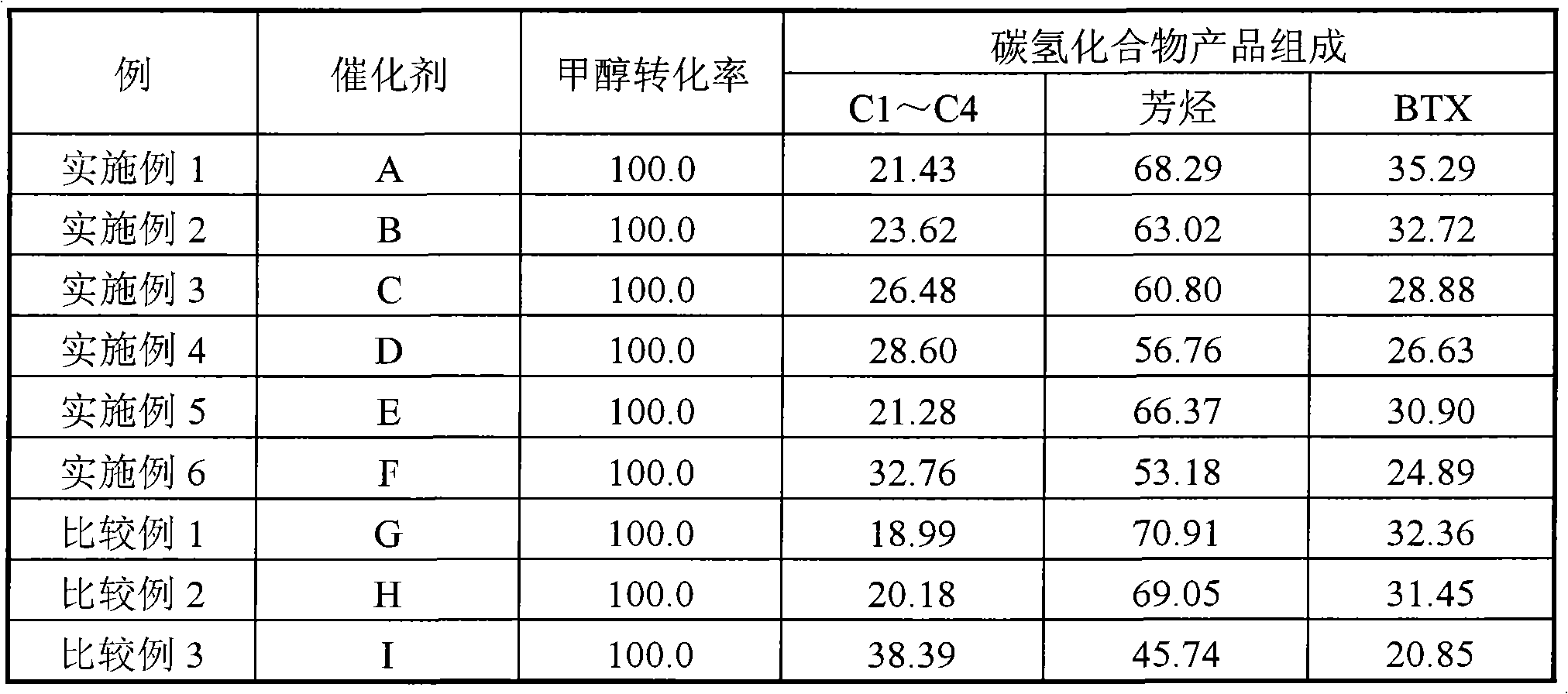
Catalyst for preparing aromatic hydrocarbons by methanol conversion and preparation method thereof
ActiveCN102371177AMolecular sieve catalystsHydrocarbon from oxygen organic compoundsMolecular sieveMass ratio
The invention relates to a catalyst for preparing aromatic hydrocarbons by methanol conversion and a preparation method thereof, and mainly solves problems of complex technical route flow and low object product selectivity of a prior art. The catalyst comprises a) 20-80 parts of molecular sieve carrier, b) 0.1-20 parts of gallium element or oxide thereof carried by the molecular sieve carrier, c) 0.1-12 parts of lanthanum or phosphor element or oxide thereof, d) 20-80 parts of binder; and the molecular sieve comprises HZSM-5 and HMCM-22 in a mass ratio of 0.1-10:1. The invention also provides a preparation method of the catalyst. The above technical scheme well solves the problems and can be applied to industrial production of aromatic hydrocarbons by methanol conversion.
Owner:CHINA PETROLEUM & CHEM CORP +1
Catalyst for preparing aromatic hydrocarbon through methanol conversion and preparation method thereof
ActiveCN102371176AHigh selectivityHigh activityMolecular sieve catalystsHydrocarbon by hydrocarbon and non-hydrocarbon condensationMolecular sievePhosphor
The invention relates to a catalyst for preparing aromatic hydrocarbon through methanol conversion and a preparation method thereof, and mainly solves the problems of complex technological process and low selectivity of the target product in the prior art. According to the invention, the catalyst comprises the following components of: by weight, a) 20-80 parts of a molecular sieve carrier; b) 0.5-12 parts of lanthanum element or its oxide which is carried on a); c) 0.5-10 parts of phosphor element or its oxide; d) 20-80 parts of a binder, wherein the molecular sieve comprises a mixture of HZSM-5 and at least one component selected from HZSM-11 or Hbeta molecular sieves; and the weight ratio of HZSM-5 to the at least one component selected from HZSM-11 or Hbeta is 0.1-10: 1. The technical scheme provided by the invention greatly solves the problems and can be used in the industrial production of aromatic hydrocarbon through methanol conversion.
Owner:CHINA PETROLEUM & CHEM CORP +1
Scintillator compositions, and related processes and articles of manufacture
ActiveUS7084403B2Excellent and reproducible scintillation responseImprove performanceMaterial analysis by optical meansLuminescent compositionsHigh energyIodide
Scintillator materials based on certain types of halide-lanthanide matrix materials are described. In one embodiment, the matrix material contains a mixture of lanthanide halides, i.e., a solid solution of at least two of the halides, such as lanthanum chloride and lanthanum bromide. In another embodiment, the matrix material is based on lanthanum iodide alone, which must be substantially free of lanthanum oxyiodide. The scintillator materials, which can be in monocrystalline or polycrystalline form, also include an activator for the matrix material, e.g., cerium. Radiation detectors that use the scintillators are also described, as are related methods for detecting high-energy radiation.
Owner:BAKER HUGHES OILFIELD OPERATIONS LLC
Aluminum titanate ceramic articles and methods of making same
ActiveUS7259120B2Reduced strengthLower firing temperatureInternal combustion piston enginesSilencing apparatusAlkaline earth metalRare earth
An aluminum titanate ceramic article having a predominant crystal phase of aluminum titanate and a material composition including aluminum, titanium, silica, an alkaline earth metal (e.g., at least one selected from the group of strontium, calcium, barium, or combinations), and a rare earth metal (e.g., at least one selected from the group consisting of yttrium, lanthanum, and combinations) and methods of making such aluminum titanate bodies are described. An oxide of yttrium metal or lanthanide metals is preferably used as a sintering aid in combination with the other compositional components to enable firing of the resulting green body at a lower heating temperature of less than 1500° C., and more preferably between 1400°-1450° C., with a preferable hold time of less than 8 hours, more preferably of 6 to 8 hours.
Owner:CORNING INC
Amorphous ionically conductive metal oxides and sol gel method of preparation
Amorphous lithium lanthanum zirconium oxide (LLZO) is formed as an ionically-conductive electrolyte medium. The LLZO comprises by percentage of total number of atoms from about 0.1% to about 50% lithium, from about 0.1% to about 25% lanthanum, from about 0.1% to about 25% zirconium, from about 30% to about 70% oxygen and from 0.0% to about 25% carbon. At least one layer of amorphous LLZO may be formed through a sol-gel process wherein quantities of lanthanum methoxyethoxide, lithium butoxide and zirconium butoxide are dissolved in an alcohol-based solvent to form a mixture which is dispensed into a substantially planar configuration, transitioned through a gel phase, dried and cured to a substantially dry phase.
Owner:JOHNSON IP HLDG LLC
Hydroisomerization catalyst, preparation method and application thereof, and hydroisomerization method for hydrocracked tail oil
ActiveCN105582992AHigh catalytic activityHigh isomerization reaction selectivityMolecular sieve catalystsHydrocarbon oils refiningMolecular sieveHalogen
The invention discloses a hydroisomerization catalyst, a preparation method and an application thereof. The preparation method comprises the following steps: a) providing a catalyst precursor containing a carrier, and a compound containing noble metals in the VIII group and a compound containing a second metal which are both supported on the carrier, wherein the two compounds hereinabove are both non-oxides, the second metal is one or more than two selected from the metal in the VB group, the metal in the VIB group, non-noble metal in the VIII group and lanthanum-series metals, the carrier containing mesoporous molecular sieve; and b) roasting the catalyst precursor in an atmosphere formed by a gas containing an oxidizing gas and a halogen-containing compound. The invention also discloses a hydroisomerization method for hydrocracked tail oil with the hydroisomerization catalyst. The hydroisomerization catalyst, when being used in the hydroisomerization for the hydrocracked tail oil, can be used for producing an isomerized product having high viscosity index and low pour point, which is suitable for being used as lubricant basic oil.
Owner:CHINA PETROLEUM & CHEM CORP +1
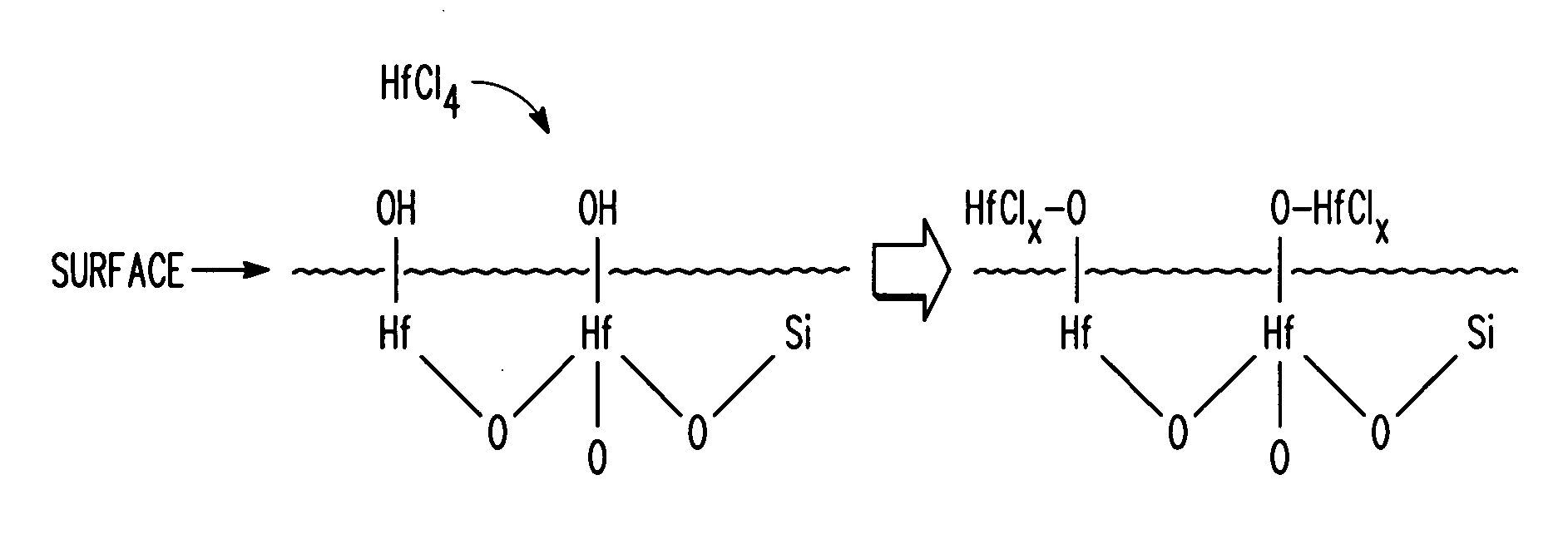
Method for treating a semiconductor surface to form a metal-containing layer
ActiveUS20050277294A1Semiconductor/solid-state device manufacturingChemical vapor deposition coatingRare earthHafnium
A method for treating a semiconductor surface to form a metal-containing layer includes providing a semiconductor substrate having an exposed surface. The exposed surface of the semiconductor substrate is treated by forming one or more metals overlying the semiconductor substrate but not completely covering the exposed surface of the semiconductor substrate. The one or more metals enhance nucleation for subsequent material growth. A metal-containing layer is formed on the exposed surface of the semiconductor substrate that has been treated. The treatment of the exposed surface of the semiconductor substrate assists the metal-containing layer to coalesce. In one embodiment, treatment of the exposed surface to enhance nucleation may be performed by spin-coating, atomic layer deposition (ALD), physical layer deposition (PVD), electroplating, or electroless plating. The one or more metals used to treat the exposed surface may include any rare earth or transition metal, such as, for example, hafnium, lanthanum, etc.
Owner:NXP USA INC +1
Method of forming copper interconnections with enhanced electromigration resistance and reduced defect sensitivity
A method of providing sub-half-micron copper interconnections with improved electromigration and corrosion resistance. The method includes double damascene using electroplated copper, where the seed layer is converted to an intermetallic layer. A layer of copper intermetallics with hafnium, lanthanum, zirconium or tin, is provided to improve the electromigration resistance and to reduce defect sensitivity. A method is also provided to form a cap atop copper lines, to improve corrosion resistance, which fully covers the surface. Structure and methods are also described to improve the electromigration and corrosion resistance by incorporating carbon atoms in copper interstitial positions.
Owner:GLOBALFOUNDRIES INC
Atomic layer deposition of hafnium lanthanum oxides
ActiveUS20100270626A1Desired characteristicSemiconductor/solid-state device detailsSolid-state devicesDielectricHafnium
There is provided an improved method for depositing thin films using precursors to deposit binary oxides by atomic layer deposition (ALD) techniques. Also disclosed is an ALD method for depositing a high-k dielectric such as hafnium lanthanum oxide (HfLaO) on a substrate. Embodiments of the present invention utilize a combination of ALD precursor elements and cycles to deposit a film with desired physical and electrical characteristics. Electronic components and systems that integrate devices fabricated with methods consistent with the present invention are also disclosed.
Owner:ASM IP HLDG BV
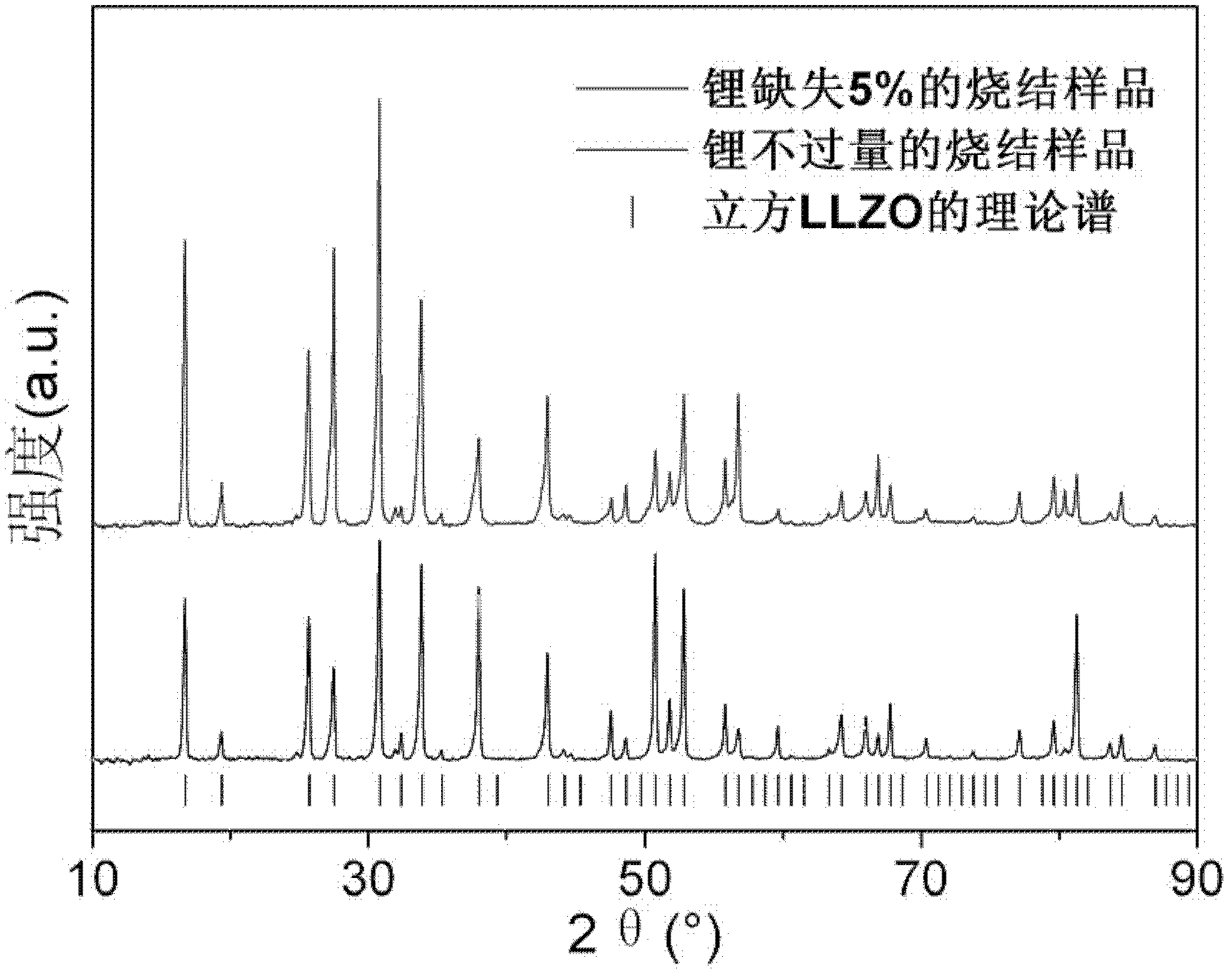
Lithium lanthanum zirconium oxide solid electrolyte material and its preparation method and application
The invention discloses a lithium lanthanum zirconium oxygen solid electrolyte material. The molecular formula of the material is shown as Li7+xLa3Zr2O12+0.5x, wherein -0.35≤x≤+0.35. The structure of the lithium lanthanum zirconium oxygen solid electrolyte material is a cubic garnet structure; the total ion conductivity of the lithium lanthanum zirconium oxygen solid electrolyte material at room temperature is greater than 1×10-4 S / cm. The method comprises the steps of: mixing the lithium source compound, the lanthanum source compound and the zirconium source compound according to the molar ratio of Li, La and Zr (7+x):3:2, and then calcining and sintering to obtain the lithium Lanthanum zirconium oxide solid electrolyte material; wherein, -0.35≤x≤+0.35. This material can prepare lithium lanthanum zirconium oxide solid electrolyte material at lower lithium content and lower sintering temperature, and the total room temperature ion conductivity can be greater than 1×10-4S / cm, which has important applications value.
Owner:TSINGHUA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com