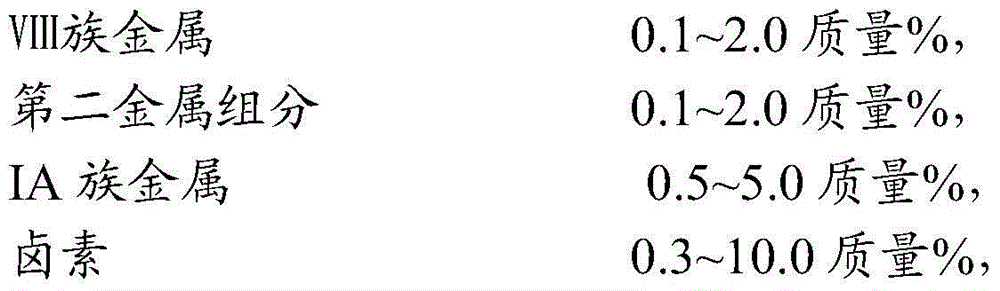Patents
Literature
606 results about "Thallium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Thallium is a chemical element with the symbol Tl and atomic number 81. It is a gray post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Chemists William Crookes and Claude-Auguste Lamy discovered thallium independently in 1861, in residues of sulfuric acid production. Both used the newly developed method of flame spectroscopy, in which thallium produces a notable green spectral line. Thallium, from Greek θαλλός, thallós, meaning "a green shoot or twig", was named by Crookes. It was isolated by both Lamy and Crookes in 1862; Lamy by electrolysis, and Crookes by precipitation and melting of the resultant powder. Crookes exhibited it as a powder precipitated by zinc at the International exhibition, which opened on 1 May that year.
Inorganic dopants, inks and related nanotechnology
InactiveUS6849109B2Facilitated DiffusionLower transition temperatureSelenium/tellurium compundsCell electrodesIndiumCerium
Ink compositions with modified properties result from using a powder size below 100 nanometers. Colored inks are illustrated. Nanoscale coated, uncoated, whisker inorganic fillers are included. The pigment nanopowders taught comprise one or more elements from the group actinium, aluminum, antimony, arsenic, barium, beryllium, bismuth, cadmuim, calcium, cerium, cesium, chalcogenide, cobalt, copper, dysprosium, erbium, europium, gadolinium, gallium, gold, hafnium, hydrogen, indium, iridium, iron, lanthanum, lithium, magnesium, manganese, mendelevium, mercury, molybdenum, neodymium, neptunium, nickel, niobium, nitrogen, oxygen, osmium, palladium, platinum, potassium, praseodymium, promethium, protactinium, rhenium, rubidium, scandium, silver, sodium, strontium, tantalum, terbium, thallium, thorium, tin, titanium, tungsten, vanadium, ytterbium, yttrium, zinc, and zirconium.
Owner:PPG IND OHIO INC
Automobile scanning system
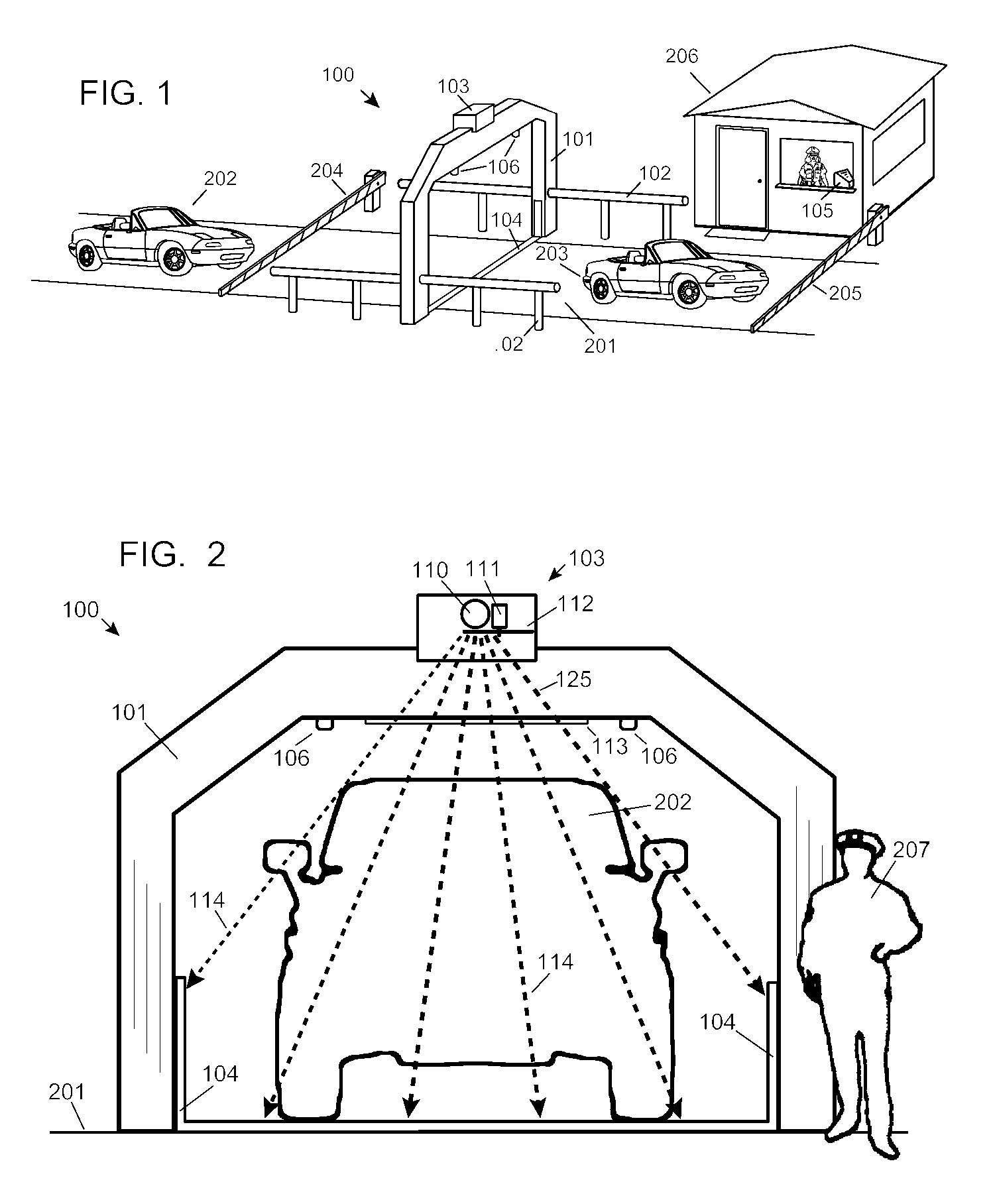
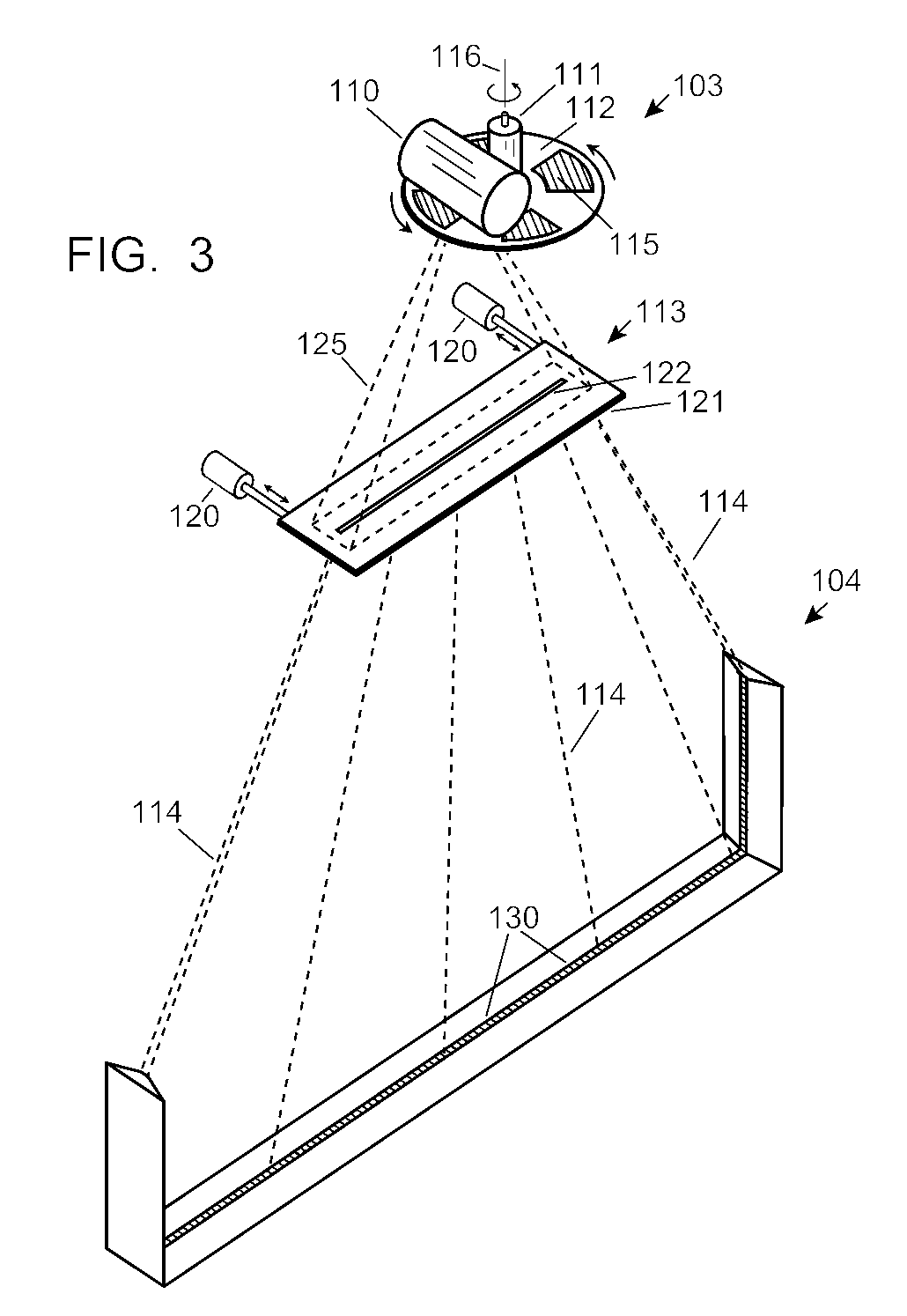
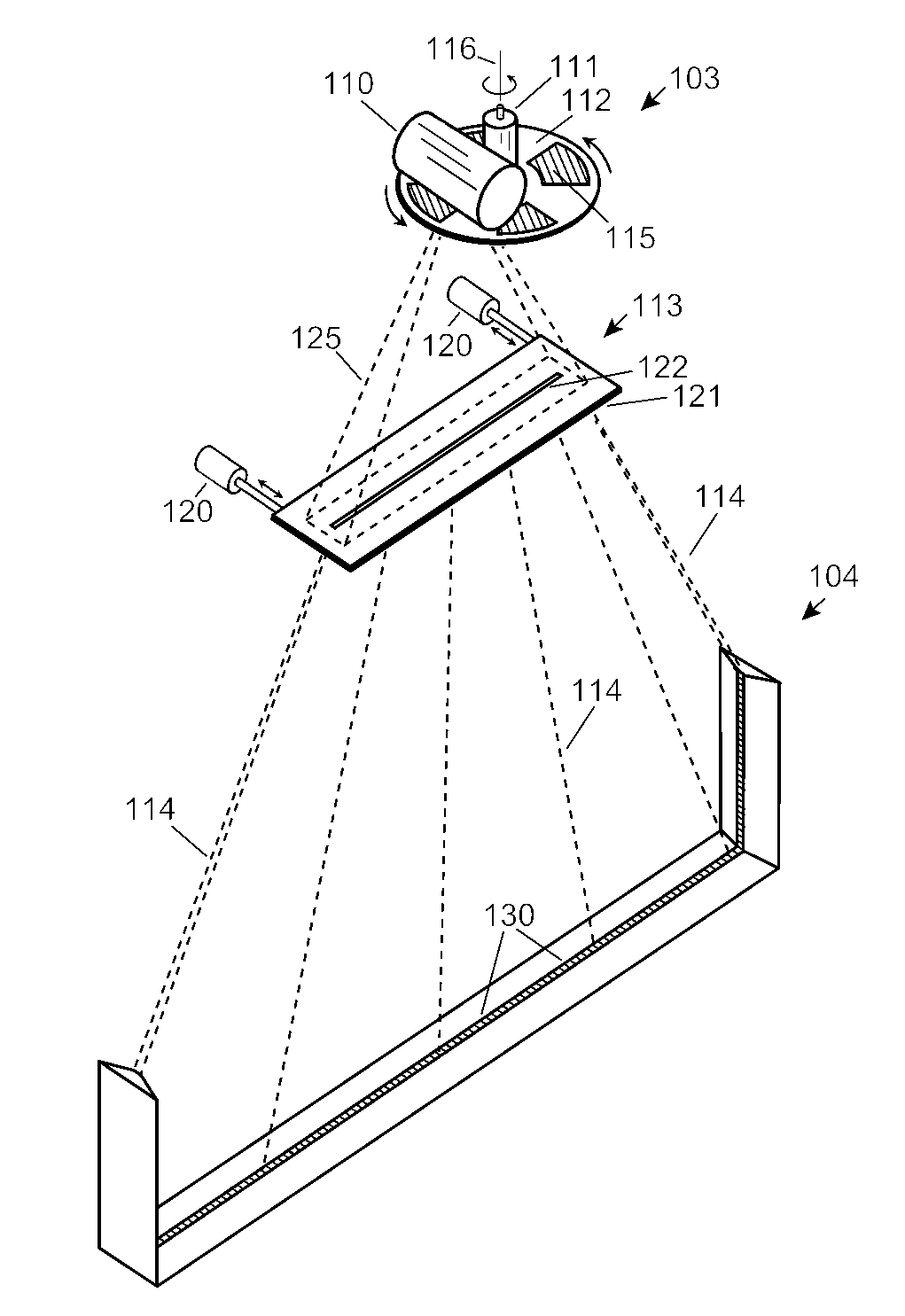
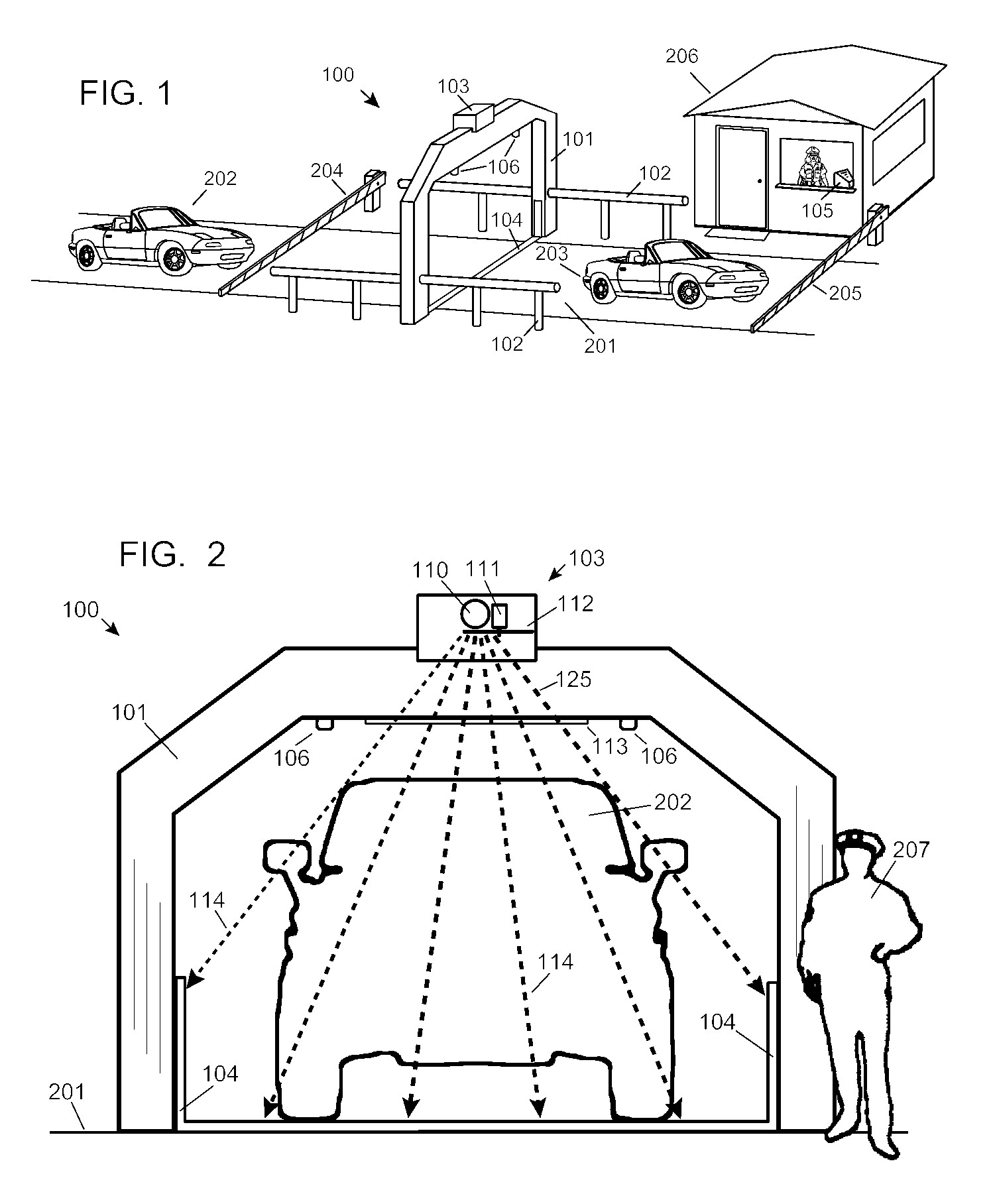
ActiveUS7742568B2Easy to checkUniform exposureX-ray apparatusMaterial analysis by transmitting radiationAtomic elementHigh energy
A dual-energy x-ray imaging system searches a moving automobile for concealed objects. Dual energy operation is achieved by operating an x-ray source at a constant potential of 100 KV to 150 KV, and alternately switching between two beam filters. The first filter is an atomic element having a high k-edge energy, such as platinum, gold, mercury, thallium, lead, bismuth, and thorium, thereby providing a low-energy spectrum. The second filter provides a high-energy spectrum through beam hardening. The low and high energy beams passing through the automobile are received by an x-ray detector. These detected signals are processed by a digital computer to create a steel suppressed image through logarithmic subtraction. The intensity of the x-ray beam is adjusted as the reciprocal of the measured automobile speed, thereby achieving a consistent radiation level regardless of the automobile motion. Accordingly, this invention provides images of organic objects concealed within moving automobiles without the detritus effects of overlying steel and automobile movement.
Owner:LEIDOS
Automobile Scanning System
ActiveUS20090086907A1Easy to checkUniform radiation exposureX-ray apparatusMaterial analysis by transmitting radiationAtomic elementHigh energy
A dual-energy x-ray imaging system searches a moving automobile for concealed objects. Dual energy operation is achieved by operating an x-ray source at a constant potential of 100 KV to 150 KV, and alternately switching between two beam filters. The first filter is an atomic element having a high k-edge energy, such as platinum, gold, mercury, thallium, lead, bismuth, and thorium, thereby providing a low-energy spectrum. The second filter provides a high-energy spectrum through beam hardening. The low and high energy beams passing through the automobile are received by an x-ray detector. These detected signals are processed by a digital computer to create a steel suppressed image through logarithmic subtraction. The intensity of the x-ray beam is adjusted as the reciprocal of the measured automobile speed, thereby achieving a consistent radiation level regardless of the automobile motion. Accordingly, this invention provides images of organic objects concealed within moving automobiles without the detritus effects of overlying steel and automobile movement.
Owner:LEIDOS
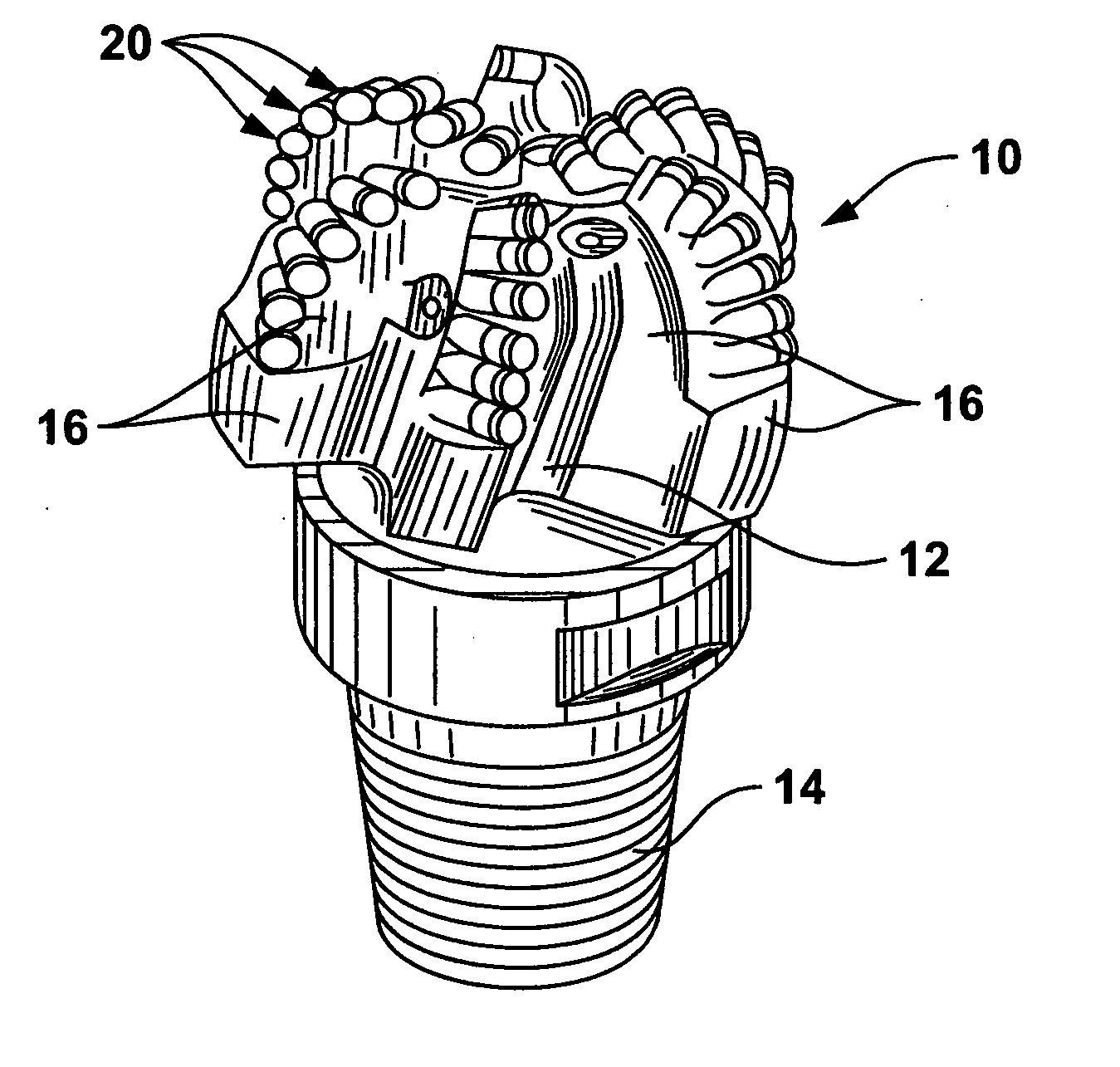
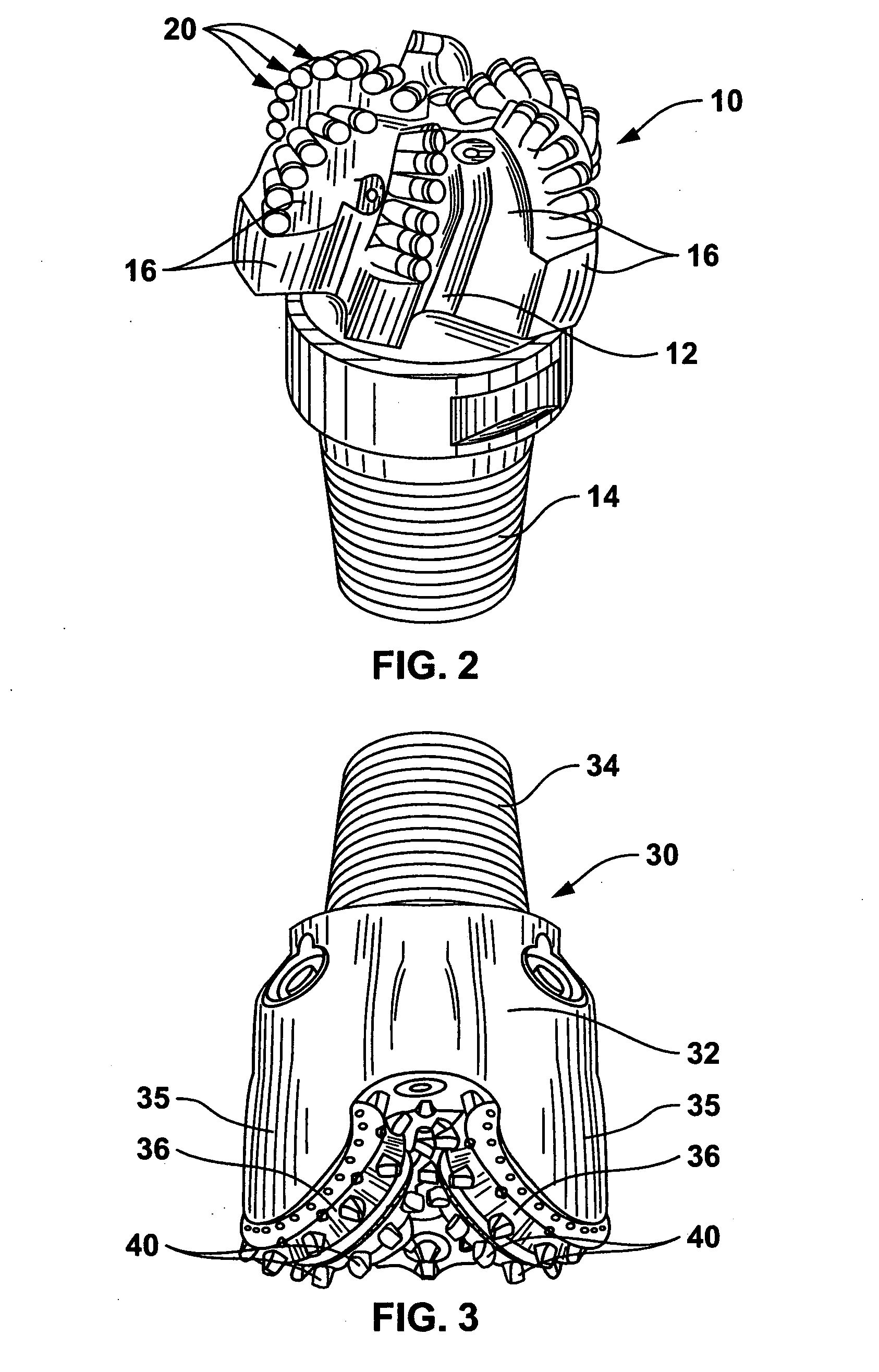
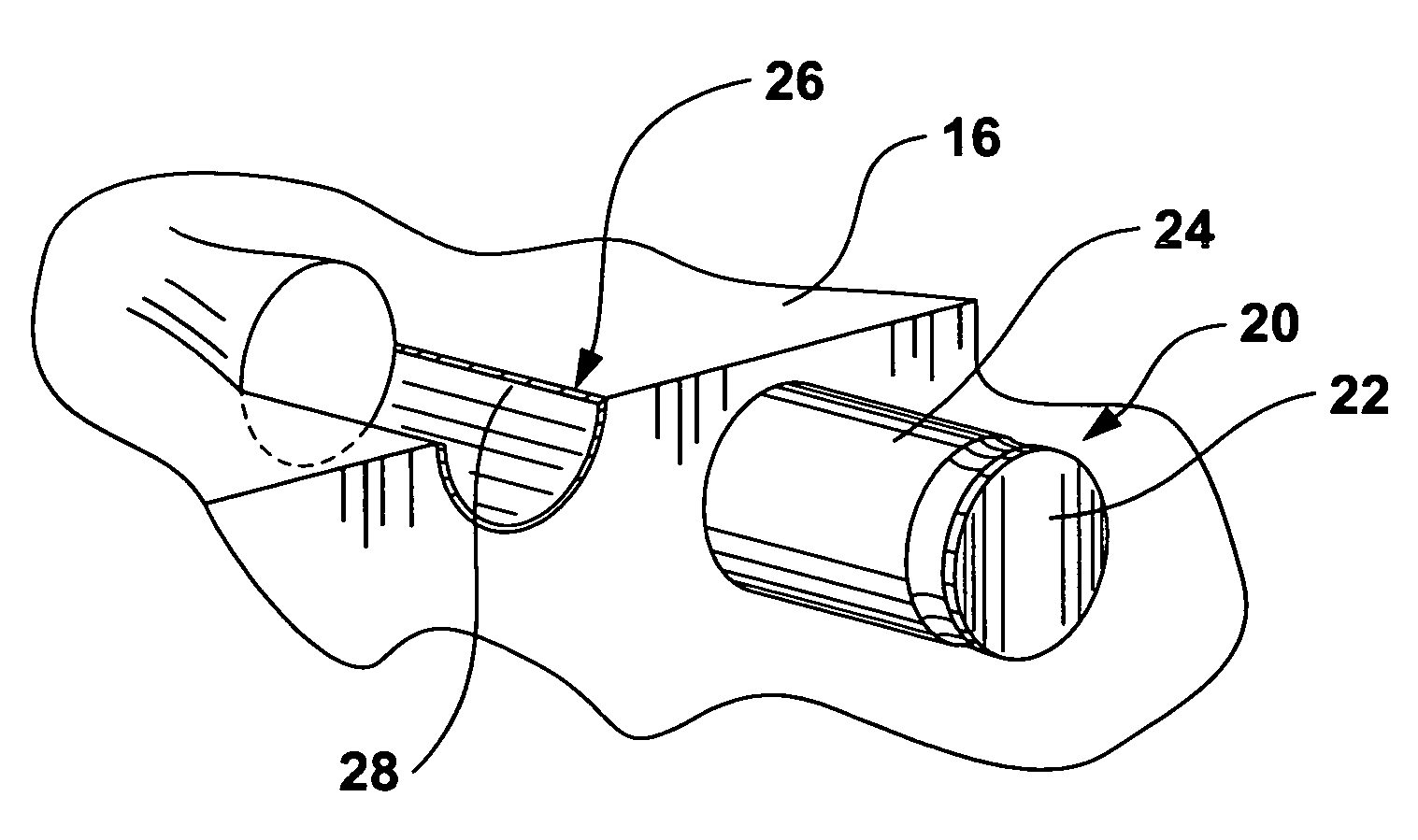
Braze alloy for drilling applications
A down hole cutting tool includes a cutting element support structure. The cutting element support structure has at least one cavity formed therein. A cutting element is disposed in the cavity. Braze alloy is also disposed in the cavity between the cutting element and the cutting element support structure. The braze alloy comprises between about 0.5% and about 10% by weight of at least one selected from the group of gallium (Ga), indium (In), thallium (Tl). Methods for building a down hole tool using the braze alloy are also disclosed.
Owner:SMITH INT INC +1
Braze alloy
A braze alloy is described which includes indium and / or thallium as alloying elements. For example, between about 0.5% and about 10% by weight of indium (In) and / or thallium (Tl) can be added to a braze alloy to reduce the braze temperature without substantially effecting braze strength. Braze alloys that are alloyed to including indium (In) and / or thallium (Tl) can be use in brazing cutting elements to drilling tools. These alloying elements may also be added to braze alloys used for other applications to reduce braze temperature.
Owner:KEMBAIYAN KUMAR T
Braze alloy and method of use for drilling applications
A down hole cutting tool includes a cutting element support structure. The cutting element support structure has at least one cavity formed therein. A cutting element is disposed in the cavity. Braze alloy is also disposed in the cavity between the cutting element and the cutting element support structure. The braze alloy comprises between about 0.5% and about 10% by weight of at least one selected from the group of gallium (Ga), indium (In), thallium (Tl). Methods for building a down hole tool using the braze alloy are also disclosed.
Owner:SMITH INT INC +1
Nanocomposite scintillator, detector, and method
InactiveUS20080093557A1Material analysis by optical meansLuminescent compositionsSodium iodideTungstate
A compact includes a mixture of a solid binder and at least one nanopowder phosphor chosen from yttrium oxide, yttrium tantalate, barium fluoride, cesium fluoride, bismuth germanate, zinc gallate, calcium magnesium pyrosilicate, calcium molybdate, calcium chlorovanadate, barium titanium pyrophosphate, a metal tungstate, a cerium doped nanophosphor, a bismuth doped nanophosphor, a lead doped nanophosphor, a thallium doped sodium iodide, a doped cesium iodide, a rare earth doped pyrosilicate, or a lanthanide halide. The compact can be used in a radiation detector for detecting ionizing radiation.
Owner:RGT UNIV OF CALIFORNIA
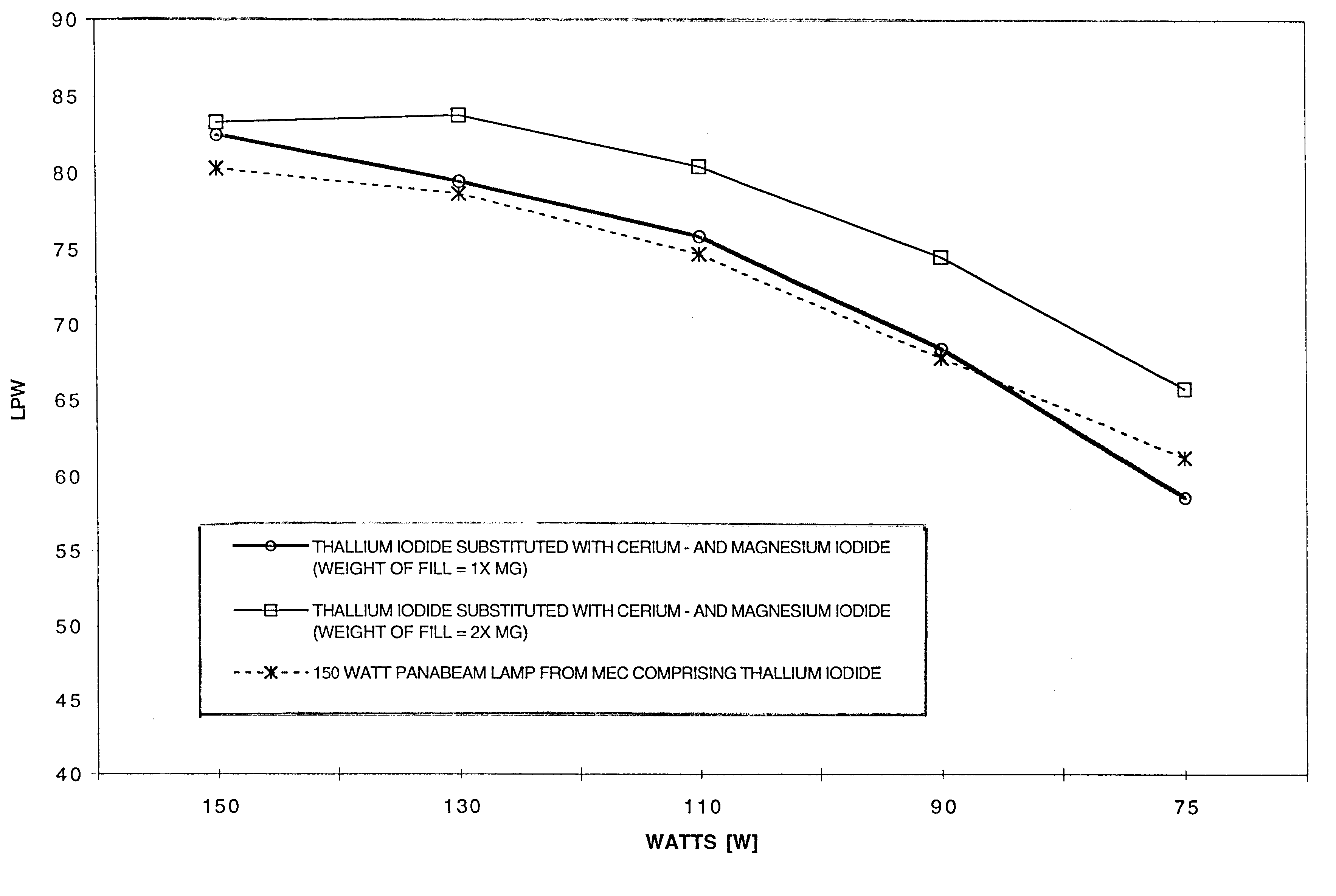
Thallium free-metal halide lamp with magnesium and cerium halide filling for improved dimming properties
InactiveUS6501220B1Increase vapor pressureReduce the temperatureSolid cathode detailsGas discharge lamp detailsMetal-halide lampCerium
A thallium-free high pressure ceramic metal halide lamp having superior dimming characteristics with a fill composition comprising MgI2 and CeI3. In addition, the fill chemistry comprises NaI and the halides of rare earth metals such as Dy, Ho and Tm.
Owner:MATSUSHITA ELECTRIC WORKS LTD
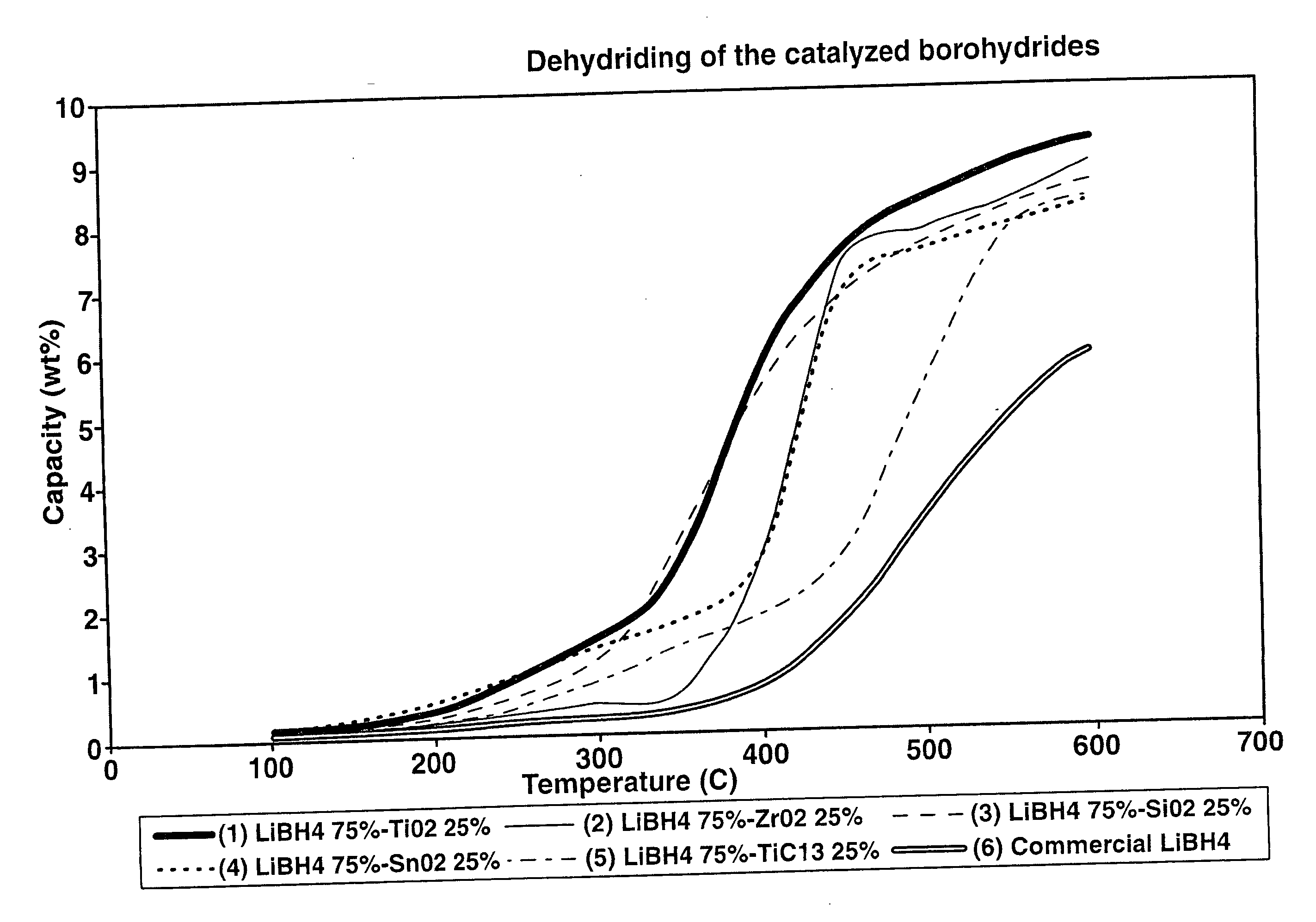
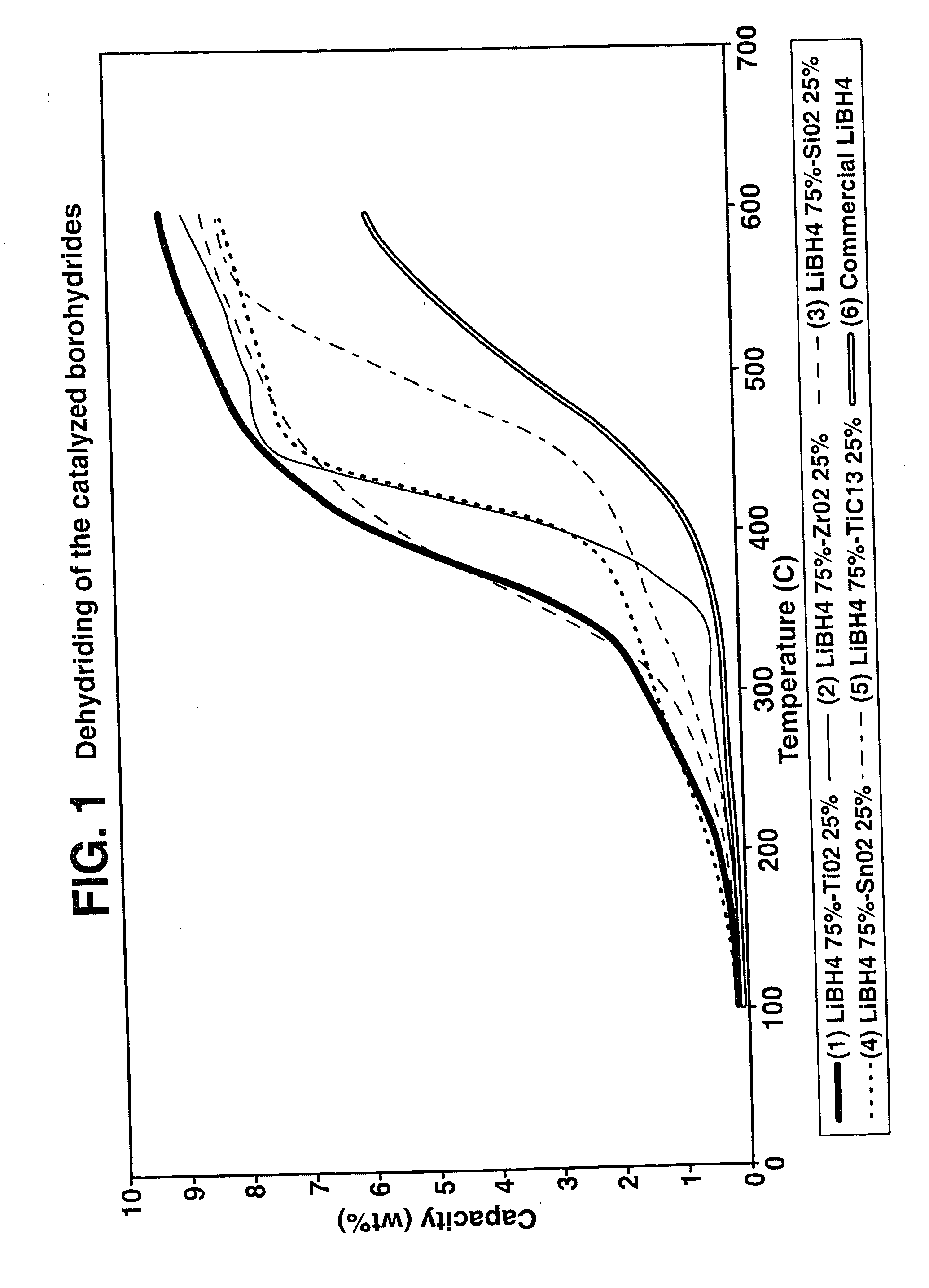
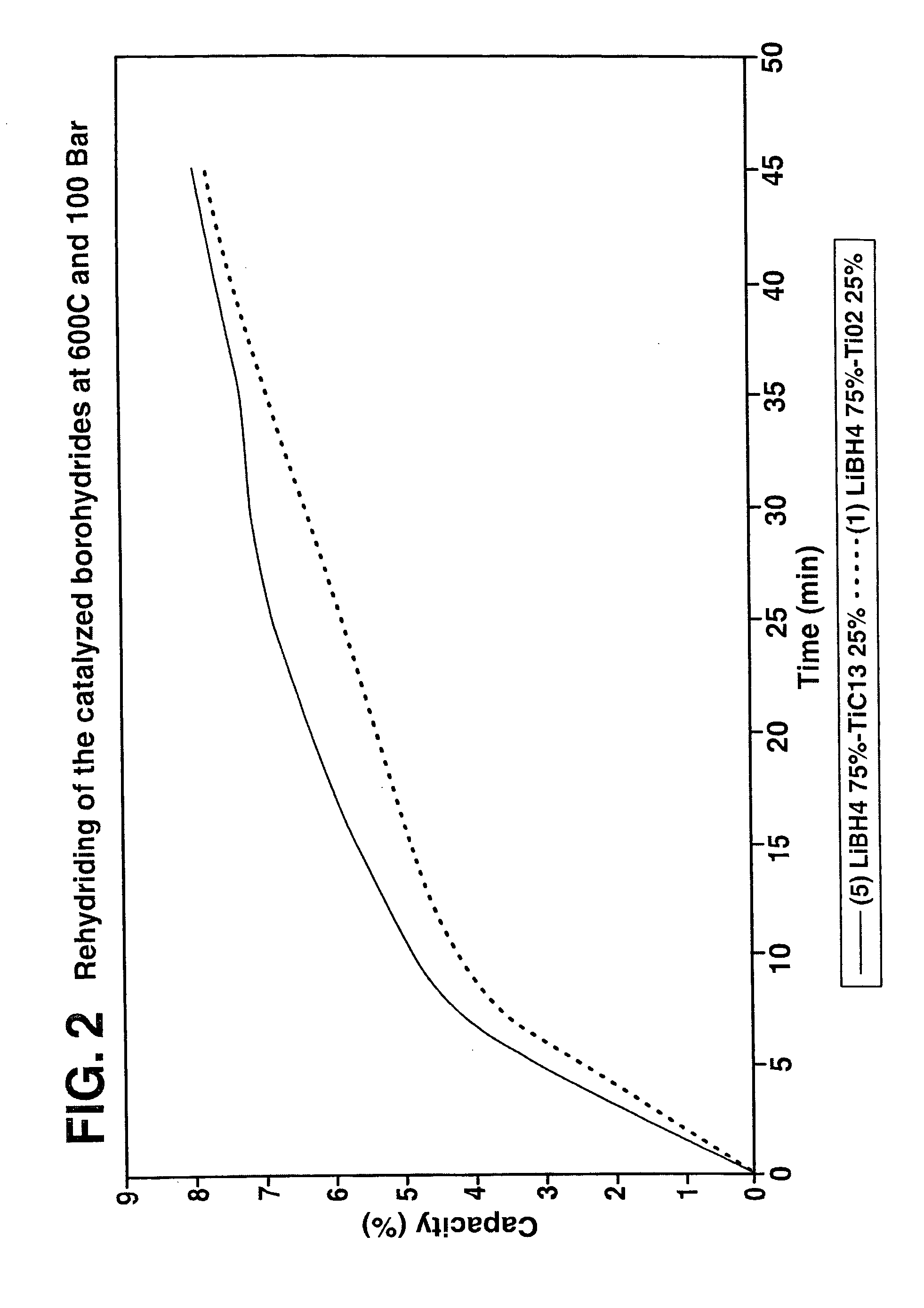
Destabilized and catalyzed borohydrided for reversible hydrogen storage
InactiveUS20060194695A1Reduce the temperatureImproved hydrogen binding/release kineticsHydrogenPhysical/chemical process catalystsIndiumCobalt
A hydrogen storage material and process is provided in which catalyzed alkali borohydride materials and partially substituted borohydride materials are created and which may contain effective amounts of catalyst(s) which include transition metal oxides, halides, and chlorides of titanium, zirconium, tin, vanadium, iron, cobalt and combinations of the various catalysts and the destabilization agents which include metals, metal hydrides, metal chlorides and complex hydrides of magnesium, calcium, strontium, barium, aluminum, gallium, indium, thallium and combinations of the various destabilization agents. When the catalysts and destabilization agents are added to an alkali borodydride such as a lithium borohydride, the initial hydrogen release point of the resulting mixture is substantially lowered. Additionally, the hydrogen storage material may be rehydrided with weight percent values of hydrogen of at least about nine percent.
Owner:SAVANNAH RIVER NUCLEAR SOLUTIONS
Remediation of metal contaminants with hydrocarbon-utilizing bacteria
Methods and apparatus are disclosed for remediating metal contaminants using hydrocarbons which stimulate the growth of hydrocarbon-utilizing bacteria. The metal contaminants may include heavy metals such as arsenic, antimony, beryllium, cadmium, chromium, copper, lead, mercury, iron, manganese, magnesium, radium, nickel, selenium, silver, thallium and zinc. The hydrocarbon may include alkanes, alkenes, alkynes, poly(alkene)s, poly(alkyne)s, aromatic hydrocarbons, aromatic hydrocarbon polymers and aliphatic hydrocarbons. Butane is a particularly suitable hydrocarbon which stimulates the growth of butane-utilizing bacteria. Remediation may occur in-situ or ex-situ, and may occur under aerobic, anaerobic or dual aerobic / anaerobic conditions. Examples of applications include the remediation of heavy metals, the remediation of arsenic impacted surface water, groundwater and / or soil, the remediation of acid mine drainage, and the treatment of spent metal plating solutions.
Owner:GLOBAL BIOSCI
Deep purification process for thallium-containing waste water
The invention relates to a deep purification process for thallium-containing waste water, which belongs to the technical field of sewage treatment. The deep purification process for the thallium-containing waste water comprises the following steps: (1) adding excess oxidizing agent into the thallium-containing waste water so as to oxidize Tl<+> into Tl<3+>; (2) adjusting the pH value of the thallium-containing waste water to 7 to 13 so as to produce thallium hydroxide precipitate; (3) carrying out solid-liquid separation to remove the thallium hydroxide precipitate in the thallium-containing waste water so as to obtain preliminarily purified water; (4) adding a reducing agent into the preliminarily purified water so as to allow redox potential of the preliminarily purified water to be less than 200 mV; and (5) introducing the preliminarily purified water treated in the step (4) into an ion exchange column equipped with an adsorption resin for purification so as to obtain deeply purified water, wherein the adsorption resin is a macroporous chelate resin containing a mercapto group. The deep purification process enables the thallium-containing waste water to be deeply purified; and the obtained deeply-purified water has total thallium content of less than 0.1 [mu]g / L.
Owner:HUNAN YONKER ENVIRONMENTAL PROTECTION RES INST
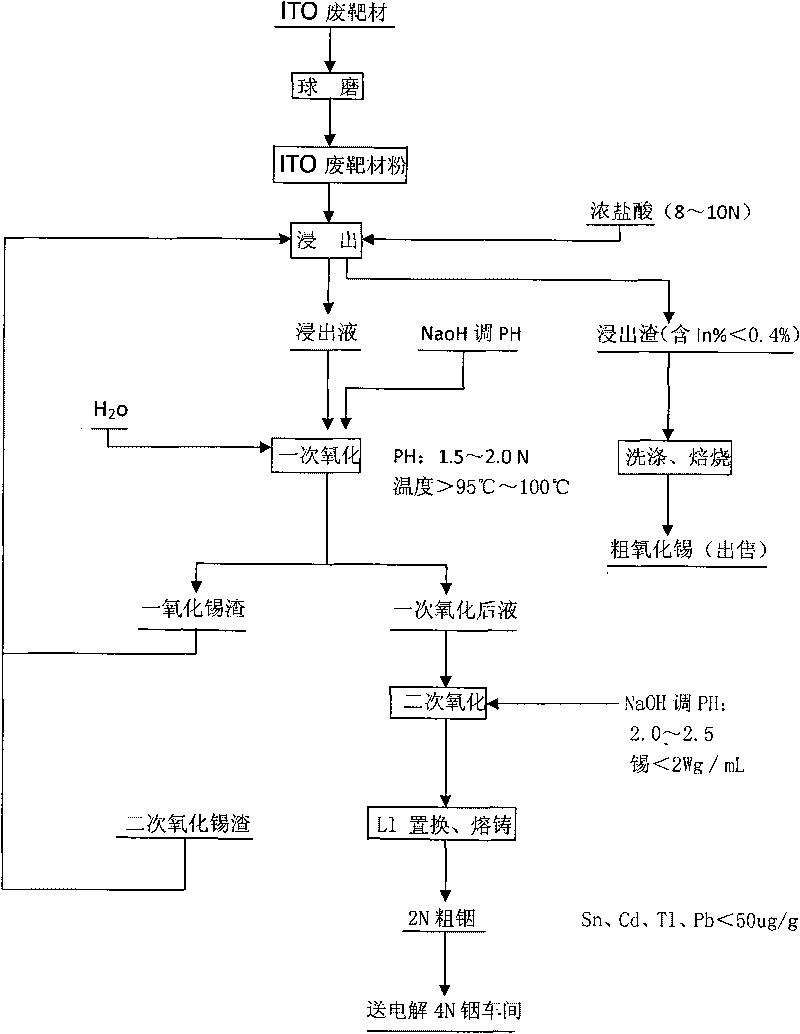
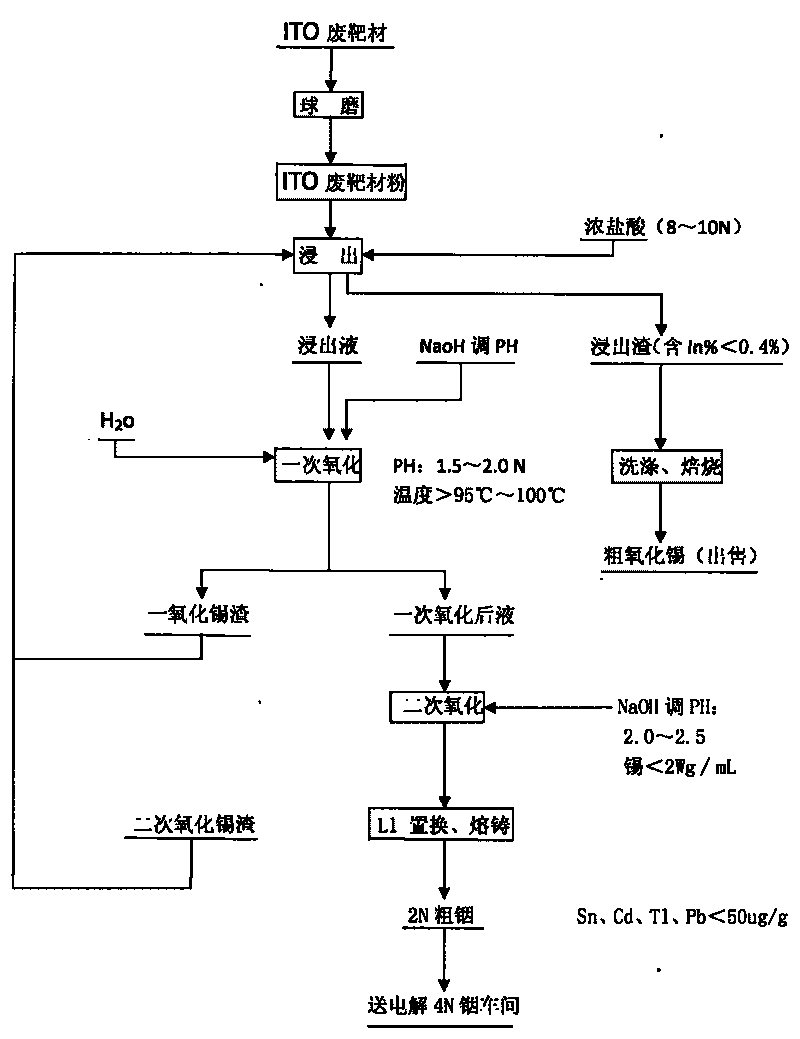
Method for recovering indium and tin from ITO waste targets by utilizing oxidation method
InactiveCN101701292AStable recoveryPhotography auxillary processesProcess efficiency improvementVulcanizationIndium
The invention utilizes the characteristics that tin has two states of bivalence and tetravalence in the solution, and the difference of the pH values is great when the tetravalent tin and trivalent indium ions precipitate in the solution, so the tetravalent tin and trivalent indium ions can be controlled under a certain pH value; oxidant is utilized to oxidize tin ions into tetravalence to generate hydrolysis precipitation, while indium still remains in the water solution, thus achieving the complete separation of indium from tin. At the moment, the tin contained in the indium solution is lowered to 2ppm, aluminium cutter replacement can be directly carried out after vulcanization to ensure that the content of tin, cadmium, thallium and lead is less than 100 PPM, and the content of indium is greater than 99%; while the oxidized tin dregs return and leach, and finally coarse tin oxide can be obtained for sale after washing and roasting the leached dregs, and the grade of indium content is lower than 0.2%.
Owner:南京中锗科技有限责任公司
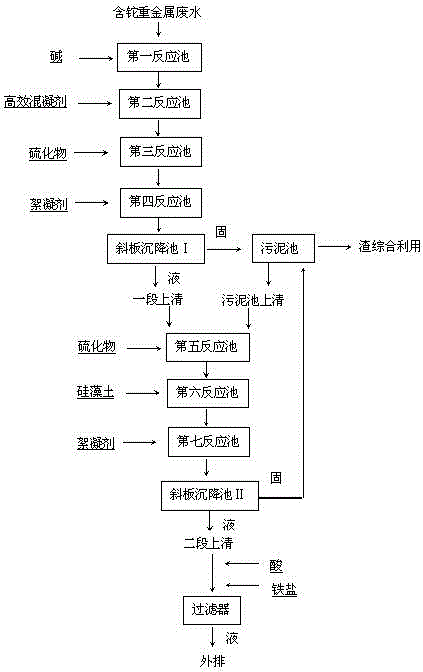
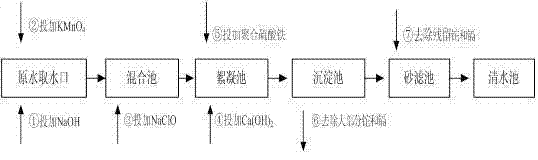
Process for removing thallium from thallium-containing heavy metal wastewater through neutralization and flocculation
InactiveCN104445732AGuaranteed deep removalRealize deep purificationWater contaminantsMultistage water/sewage treatmentFlocculationIron salts
The invention discloses a process for removing thallium from thallium-containing heavy metal wastewater through neutralization and flocculation. The process comprises the following steps: performing primary treatment, namely adjusting the pH value of thallium-containing heavy metal wastewater to be more than 7through acid and alkali, adding an efficient coagulant, sulfide and a flocculant, and performing solid-liquid separation through a slanting board sedimentation pool; performing secondary treatment, namely adding sulfide, diatomite and the flocculant into supernatant purified water after primary treatment, and performing solid-liquid separation through the slanting board sedimentation pool; adjusting the purified water subjected to secondary treatment to be neutral, adding iron salt, and finally filtering through a filter and directly discharging to the outside. Due to the adoption of the technical scheme, the removal rate of thallium and other heavy metal ions in the heavy metal wastewater can be effectively increased, the environmental pollution can be avoided, and the medicament cost can be reduced.
Owner:ZHUZHOU SMELTER GRP
Dehydrogenation catalyst composition
Owner:UOP LLC
Scintillator materials which are useful for detecting radiation, and related methods and articles
A scintillator composition is described, including a matrix material and an activator. The matrix material includes at least one alkali metal or thallium; at least one alkaline earth metal or lead; and at least one halide compound. The activator is usually cerium, praseodymium, or mixtures thereof. Radiation detectors which include the scintillator composition are also described. Methods for detecting high-energy radiation also form part of this disclosure.
Owner:GENERAL ELECTRIC CO
Catalyst for preparing olefin from low-carbon alkane by dehydrogenation and preparation method of catalyst
ActiveCN105214657AImprove carbon storage capacityImprove stabilityHydrocarbonsMetal/metal-oxides/metal-hydroxide catalystsAlkaneIndium
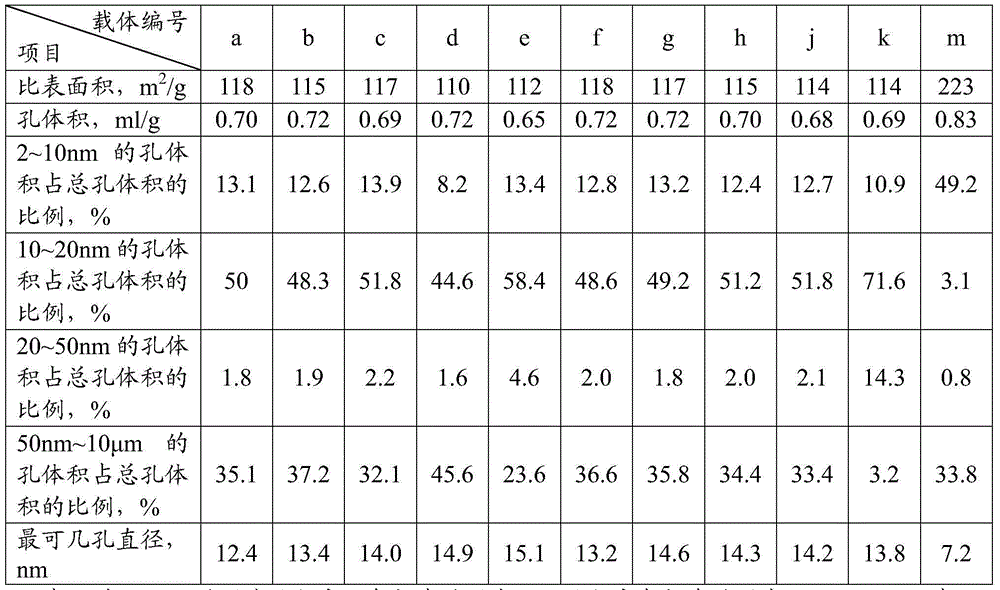
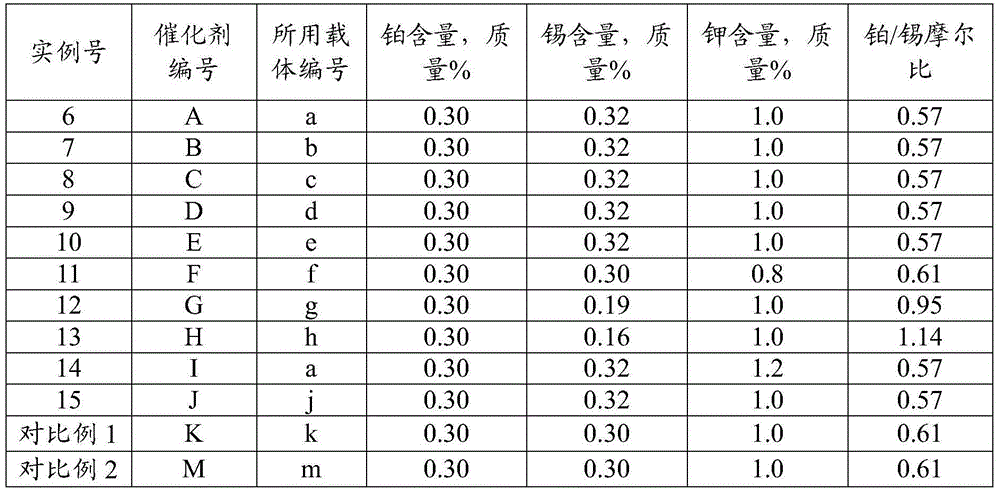
The invention provides a catalyst for preparing olefin from low-carbon alkane by dehydrogenation. The catalyst comprises an aluminum oxide carrier and the following active components with the carrier as datum by mass: 0.1 to 2.0% of a group-VIII metal, 0.1 to 2.0% of a second metal component, 0.5 to 5.0% of a group-IA metal and 0.3 to 10.0% of halogen, wherein the aluminum oxide carrier has a pore volume of pores with a diameter of 2 to 10 nanometers accounting for 4 to 15% of a total pore volume, a pore volume of pores with a diameter of 10 to 20 nanometers accounting for 40 to 60% of the total pore volume, a pore volume of pores with a diameter of 20 to 50 nanometers accounting for 1.0 to 5.0% of the total pore volume, and a pore volume of pores with a diameter of more than 50 nanometers and less than 10 microns accounting for 20 to 50% of the total pore volume; and the second metal component is selected from the group consisting of tin, germanium, lead, indium, gallium or thallium. The catalyst is applied to preparation of propylene from propane by dehydrogenation and has high activity and selectivity and low coke deposition rate.
Owner:CHINA PETROLEUM & CHEM CORP +1
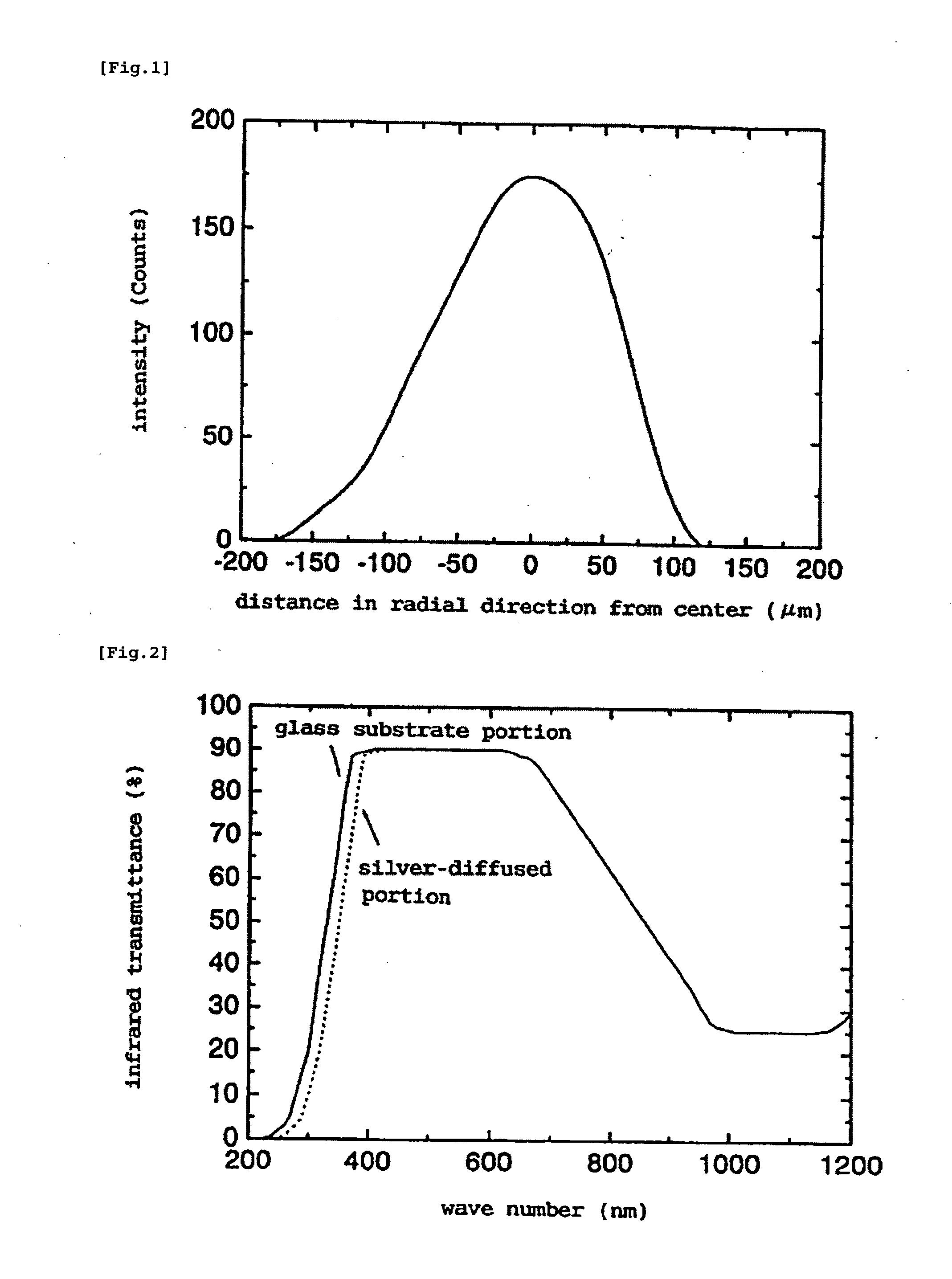
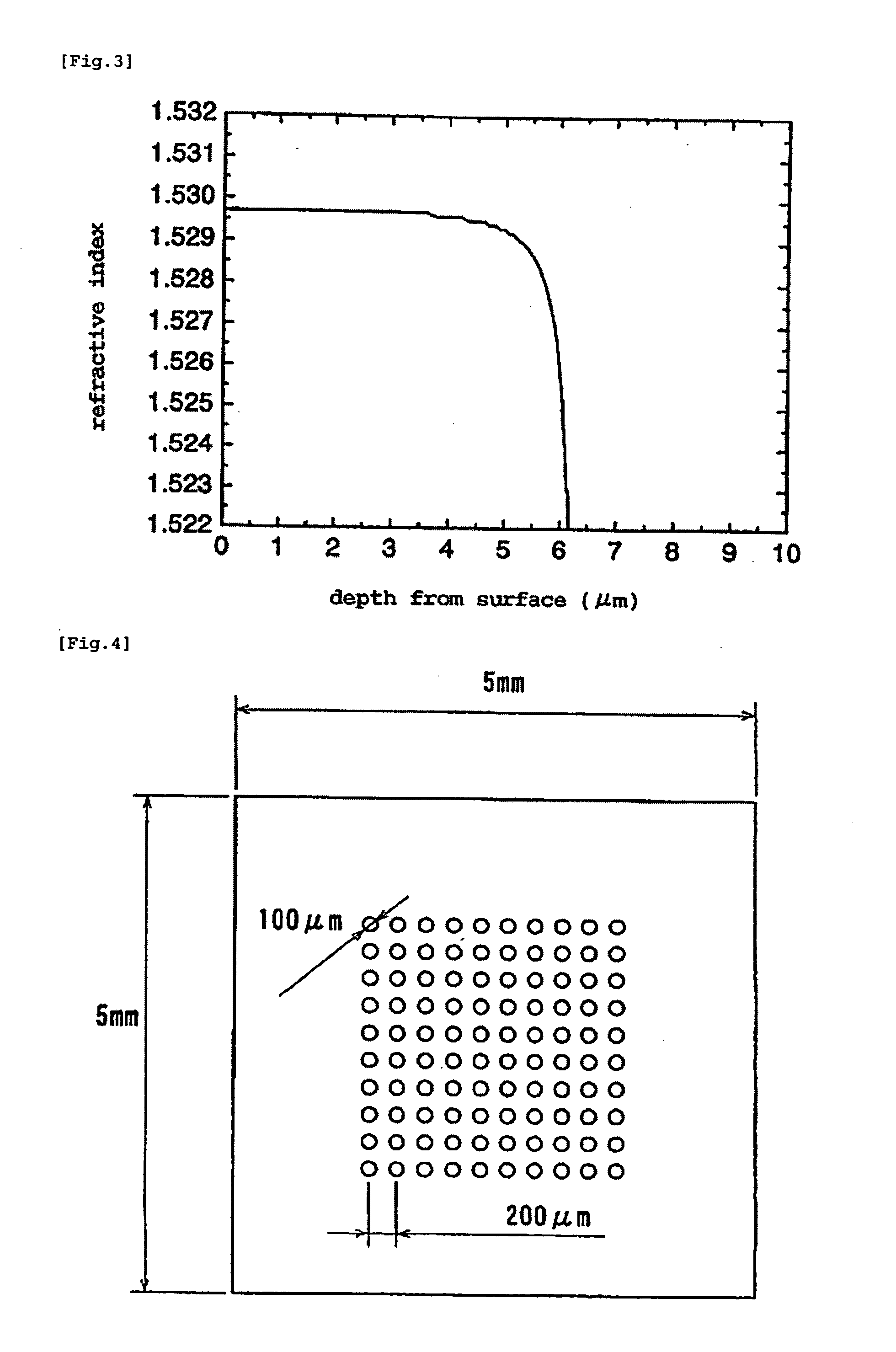
Method for Manufacturing Gradient-Index Optical Element Having Infrared Absorbing Ability
InactiveUS20100165454A1Low costProductionElectrostatic spraying apparatusCoatingsRubidium compoundGradient-index optics
A method of readily producing a gradient optical element having infrared absorbing ability by easily forming a refractive index distribution in a desired portion of a glass substrate having infrared absorbing ability without requiring a specific treatment atmosphere nor using a molten salt.More specifically, the present invention provides a method for producing a gradient-index optical element having infrared absorbing ability, the method comprising applying a paste containing an organic resin, an organic solvent, and at least one compound selected from the group consisting of lithium compounds, potassium compounds, rubidium compounds, cesium compounds, silver compounds, copper compounds, and thallium compounds onto a glass substrate containing an alkali metal component, at least one member selected from the group consisting of iron, copper, cobalt and vanadium, and over 3 wt. % of iron, when contained singly among iron, copper, cobalt and vanadium, on an Fe2O3 basis, taking the total weight of the glass as 100 wt. %, and heating the glass substrate at a temperature below the softening temperature of the glass substrate.
Owner:ISUZU GLASS +1
Glass structure and method for producing the same
InactiveUS7217448B2Easy to processGood shape controllabilityLayered productsGlass reforming apparatusAbsorbed energyCerium
A limited portion of a surface of a glass substrate is removed by application of a laser beam on the limited portion of the glass substrate to thereby produce a glass structure according to the invention. The glass substrate contains at least one element such as titanium, iron, vanadium, bismuth, lead, thallium, tin, cerium, rhodium or cobalt capable of absorbing energy of the laser beam and has a threshold of not larger than 1.0 J / cm2 per laser beam pulse in terms of machining energy of the laser beam. When such a glass substrate 21 is used, a glass structure having a through-hole 61 or cavity optional in sectional shape can be formed by irradiation with the laser beam 10.
Owner:NIPPON SHEET GLASS CO LTD
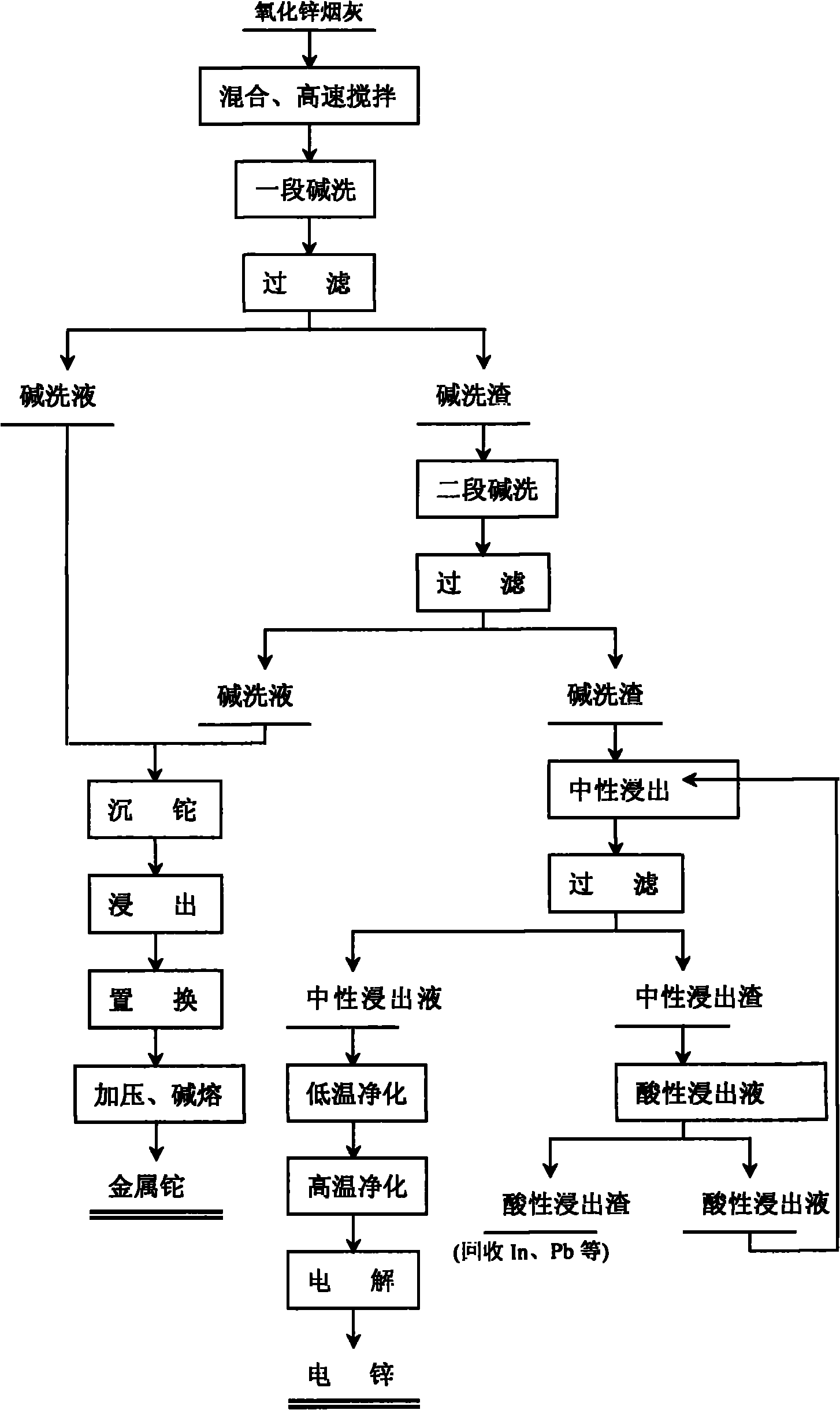
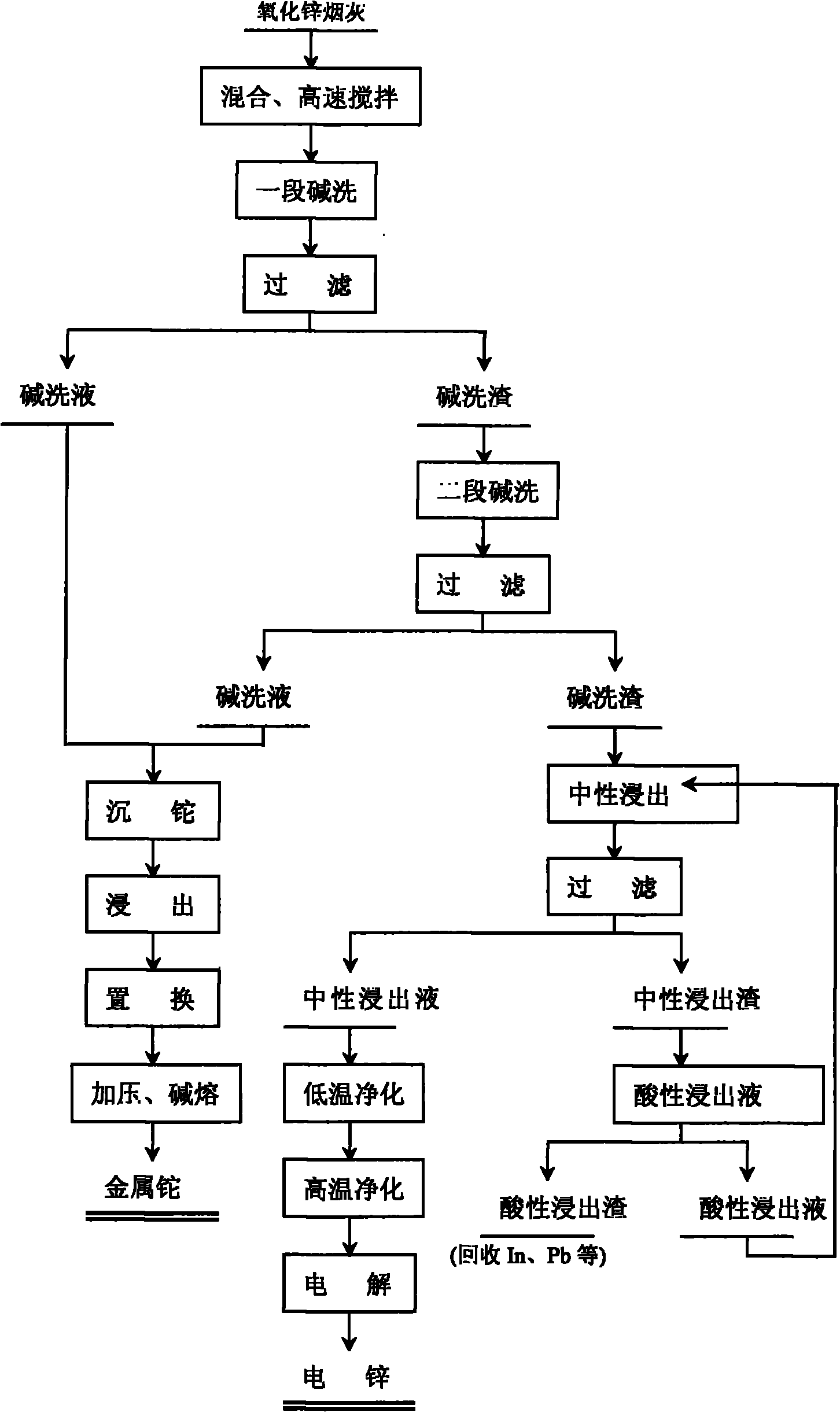
Comprehensive recovery method of zinc oxide fume dust
The invention relates to a comprehensive recovery method of zinc oxide fume dust. The method comprises the following steps: 1. performing alkali-washing on zinc oxide fume dust with Na2CO3 and NaOH in different stages: in the first stage of alkali wash, evenly mixing the zinc oxide fume dust and solid Na2CO3, adding water, stirring at high speed to dissolve the solid into solution with slag, and filtering to obtain first-stage alkali wash liquid and alkali wash slag; in the second stage of alkali wash, adding solid Na2CO3 and NaOH into the first-stage alkali wash slag, adding water, stirring to dissolve the solid, regulating the pH value, and filtering to obtain second-stage alkali wash liquid and alkali wash slag; and carrying out neutral leaching on the second-stage alkali wash slag, purifying and electrodepositing to obtain electric zinc; 2. leaching, displacing and the like to process the low temperature purified slag to obtain sponge cadmium; and 3. processing acid leached slag to obtain coarse indium, using high-temperature high-acidity leached slag as the raw material for recovering lead, and using alkali wash liquid for recovering thallium. In the invention, metals in ZnCl2, ZnF2 and PbCl2 form carbonate solid slag, chlorine and fluorine are dissolved in solution in the form of sodium salts, the slag and the liquids are respectively processed subsequently, and the products in every stage are refined processed to comprehensively recover zinc, cadmium, lead, thallium and indium.
Owner:ZHUZHOU SMELTER GRP
Organic light emitting diode display
ActiveUS20160380235A1Improve lighting efficiencyHigh refractive indexSolid-state devicesSemiconductor/solid-state device manufacturingThalliumRefractive index
An organic light emitting diode display including: a substrate; an organic light emitting diode on the substrate; a capping layer on the organic light emitting diode and including a high refractive layer including an inorganic material having a refractive index that is equal to or greater than about 1.7 and equal to or less than about 6.0; and a thin film encapsulation layer covering the capping layer and the organic light emitting diode, the inorganic material including at least one selected from the group consisting of CuI, thallium iodide (TlI), BaS, Cu2O, CuO, BiI, WO3, TiO2, AgI, CdI2, HgI2, SnI2, PbI2, BiI3, ZnI2, MoO3, Ag2O, CdO, CoO, Pr2O3, SnS, PbS, CdS, CaS, ZnS, ZnTe, PbTe, CdTe, SnSe, PbSe, CdSe, AlAs, GaAs, InAs, GaP, InP, AlP, AlSb, GaSb, and InSb.
Owner:SAMSUNG DISPLAY CO LTD
Water treatment method for removing Tl<+> and/or Cd2<+> by producing nanometer iron and manganese oxides in situ
ActiveCN102145947AHigh electronegativityLarge specific surface areaWater contaminantsMultistage water/sewage treatmentFerric hydroxideWater source
The invention discloses a water treatment method for removing Tl<+> and / or Cd2<+> by producing nanometer iron and manganese oxides in situ, relating to a water treatment method of thallium and / or cadmium-containing source water and solving the problems of complex process, high running cost and low removing efficiency of thallium and / or cadmium existing in the conventional water treatment technology specific to thallium and / or cadmium-polluted source water. The method comprises the following steps of: adding permanganate and ferrous salt into Tl<+> and / or Cd2<+>-containing water; stirring to obtain a mixed solution; adding a coagulant; and performing conventional water treatment. A nanometer ferric hydroxide-manganese dioxide oxide composite adsorbent which has a large specific surface area and high electronegativity and is easy for precipitation separation is produced in situ by making permanganate react with the ferrous salt, so that Tl<+> and / or Cd2<+> can be removed effectively andspecifications in the national Sanitary Standard for Drinking Water are met. The method has the advantages of high removing efficiency, simple process, flexibility and convenience for operation, no change of the original treatment process of a water plant, low running cost and the like, and can be applied to emergency treatment of a water pollution event.
Owner:HARBIN INST OF TECH
Catalyst for the oxidation of a mixed aldehyde feedstock to methacrylic acid and methods for making and using same
InactiveUS20070021296A1Improve distributionOrganic compound preparationHeterogenous catalyst chemical elementsIndiumCerium
A heteropolyacid catalyst for oxidation of isobutyraldehyde, methacrolein or mixtures or combinations thereof to methacrylic acid is disclosed where the heteropolyacid catalyst includes at least molybdenum (Mo), phosphorus (P), vanadium (V), and a first component including bismuth (Bi) and / or boron (B). The heteropolyacid catalyst can also optionally include a second component including potassium (K), rubidium (Rb), cesium (Cs), and / or thallium (Tl) and optionally a third component including antimony (Sb), cerium (Ce), niobium (Nb), indium (In), iron (Fe), chromium (Cr), cobalt (Co), nickel (Ni), manganese (Mn), arsenic (As), silver (Ag), zinc (Zn), germanium (Ge), gallium (Ga), zirconium (Zr), magnesium (Mg), barium (Ba), lead (Pb), tin (Sn), titanium (Ti), aluminum (Al), silicon (Si), tantalum (Ta), tungsten (W), and / or lanthanum (La). The heteropolyacid catalyst can also include an ammonium-containing compound designed to increase a value of medium pores in the final heteropolyacid catalyst. A method for oxidizing isobutanal to methacrylic acid using the heteropolyacid catalyst is also disclosed.
Owner:SAUDI BASIC IND CORP SA
Method for simultaneous removal of cadmium and thallium in raw water
ActiveCN103693774ASimple processEasy to implementWater contaminantsMultistage water/sewage treatmentCadmium CationTreated water
The invention provides a method for simultaneous removal of cadmium and thallium in raw water. The method comprises the following steps: step A, adding sodium hydroxide into raw water, adjusting a pH value to alkalescence and then adding potassium permanganate with a concentration of 0.3 to 0.8 mg / L; step B, adding sodium hypochlorite or liquid chlorine, wherein the concentration of added sodium hypochlorite or liquid chlorine is 0.5 to 2.5 mg / L in term of effective chlorine; step C, adding limewash into raw water having undergone a full oxidation reaction and adjusting a pH value to 8.5 to 9.0; step D, adding a flocculating agent, carrying out a flocculation reaction for 10 to 20 min and then carrying out deposition for at least 0.5 h so as to remove cadmium, thallium and colloids of manganese hydroxide and iron hydroxide through coprecipitation, wherein the pH value of water after precipitation drops to 7.0 to 8.5; and step E, filtering raw water obtained after precipitation by using quartz sand. The invention has the following beneficial effects: cadmium concentration of treated water is as small as the limit of a detection method, i.e., 0.02 mu g / L, or less than 0.02 mu g / L; thallium concentration of treated water is as small as the limit of a detection method, i.e., 0.01 mu g / L, or less than 0.01 mu g / L; and the pH value, manganese ions and iron ions of the treated water all meet requirements prescribed in drinking water quality standards.
Owner:SHENZHEN WATER GRP CO LTD
Catalyst for methacrolein oxidation and method for making and using same
InactiveUS7851397B2Reduce the amount of solutionIncrease the amount addedOrganic compound preparationHeterogenous catalyst chemical elementsOrganic acidRubidium
Owner:SAUDI BASIC IND CORP SA
Remediation of metal contaminants with hydrocarbon-utilizing bacteria
Methods and apparatus are disclosed for remediating metal contaminants using hydrocarbons which stimulate the growth of hydrocarbon-utilizing bacteria. The metal contaminants may include heavy metals such as arsenic, antimony, beryllium, cadmium, chromium, copper, lead, mercury, iron, manganese, magnesium, radium, nickel, selenium, silver, thallium and zinc. The hydrocarbon may include alkanes, alkenes, Aalkynes, poly(alkene)s, poly(alkyne)s, aromatic hydrocarbons, aromatic hydrocarbon polymers and aliphatic hydrocarbons. Butane is a particularly suitable hydrocarbon which stimulates the growth of butane-utilizing bacteria. Remediation may occur in-situ or ex-situ, and may occur under aerobic, anaerobic or dual aerobic / anaerobic conditions. Examples of applications include the remediation of heavy metals, the remediation of arsenic impacted surface water, groundwater and / or soil, the remediation of acid mine drainage, and the treatment of spent metal plating solutions.
Owner:GLOBAL BIOSCI
2XXX series aluminum alloy
An A-rated, aluminum alloy suitable for machining, said alloy consisting essentially of: about 4-5.75 wt. % copper, about 0.2-0.9 wt. % bismuth, about 0.12-1.0 wt. % tin, the ratio of bismuth to tin ranging from about 0.8:1 to 5:1, up to about 0.7 wt. % iron, up to about 0.4 wt. % silicon, up to about 0.3 wt. % zinc, the balance aluminum, incidental elements and impurities. On a preferred basis, this alloy contains about 4.4-5.0 wt. % copper, about 0.4-0.75 wt. % bismuth, about 0.2-0.5 wt. % tin, the ratio of bismuth to tin ranging from about 1:1 to 3:1, about 0.2 wt. % or less iron and about 0.2 wt. % or less silicon. The alloy is substantially lead-free, cadmium-free and thallium-free. There is further disclosed an improved method for making screw machine stock or wire, rod and bar product from this alloy by casting, preheating, extruding, solution heat treating, cold finishing and aging the same.
Owner:ALUMINUM CO OF AMERICA
Novel thallium doped sodium, cesium or lithium iodide scintillators
InactiveUS20170355905A1Solve the low detection efficiencyShort decay timeX/gamma/cosmic radiation measurmentLuminescent compositionsSodium iodideThallium
The present invention provides for a composition comprising a crystal composition or inorganic scintillator comprising a thallium doped sodium iodide, cesium iodide, or lithium iodide scintillator useful for detecting nuclear material.
Owner:RGT UNIV OF CALIFORNIA
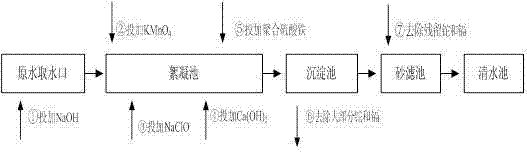
Method adopting combined technology of pre-oxidation and coagulating sedimentation to process wastewater containing thallium and ammonia-nitrogen
InactiveCN105293775AShort operating timeLess investmentWater contaminantsMultistage water/sewage treatmentNitrogen gasHydrolysis
The invention discloses a method adopting a combined technology of pre-oxidation and coagulating sedimentation to process wastewater containing thallium and ammonia-nitrogen. According to the method, a sodium hypochlorite oxidizing agent is added into a wastewater collecting tank so as to oxidize metal ions in wastewater, the monovalent thallium is fully oxidized into trivalent thallium, monovalent thallium is converted into complex under the effect of strong oxidant, and at the same time, the nitrogen in ammonia-nitrogen is degraded and removed in the form of nitrogen gas. After pre-oxidation, the wastewater is lifted to an integral processing facility through a self-sucking pump; ferrous sulfate and poly aluminum chloride (PAC) are added to form alumen ustum flocculus in a precipitation unit, the precipitate is wrapped, the thallium complex is adsorbed, then quicklime is added to adjust the solution to an alkaline environment; in the alkaline environment, Fe<3+>, Al<3+>, and prepolymer products thereof carry out hydrolysis quickly to form Fe(OH)3 flocculus and Al(OH)3 flocculus; before the flocculus becomes big, the adsorption sites on the surface of flocculus form covalent bonds with Ti<3+>, the flocculus becomes bigger and bigger very quickly and goes on absorbing Ti<3+> in water; at the same time, Ti<3+>, Fe<3+>, Al<3+>, Zn<2+>, lead, and cadmium carry out co-precipitation reactions, and thus the heavy metal ions in water are removed.
Owner:HUNAN LIHONG NEW MATERIAL TECH CO LTD
Thallium-containing heavy metal wastewater advanced treatment method
ActiveCN103693819AEliminate secondary pollutionLow costMultistage water/sewage treatmentPretreatment methodFiltration
The invention belongs to a thallium-containing heavy metal wastewater advanced treatment method. The method comprises the following steps: (1) pretreating thallium-containing heavy metal wastewater: adjusting the pH value of wastewater to 9.5-11.5 with alkaline, and adding a chemical thallium removing agent according to the mass ratio of the chemical thallium removing agent to thallium of (0.5-1.5):1; (2) medicating according to the mass ratio of the chemical thallium removing agent to wastewater after the reaction in step (1) for 15-30 minutes, and carrying out mixed reaction for 15-30 minutes; (3) adding liquid alkaline into the solution obtained from the step (2), adjusting the pH value to 11.0-11.5, reacting for 10-20 minutes, adding 5-50g / m<3> of flocculating agents, reacting for 10-20 minutes, carrying out solid-liquid separation by oblique plate deposition or plate frame press filtration, standing for 1-2 hours, and discharging or recycling supernate. The thallium contained heavy metal wastewater treatment method is simple in process, has no secondary pollution, is high in treatment efficiency, runs stably and low in cost, and has very high practicability.
Owner:CENT SOUTH UNIV +1
Method for removing trace of thallium in sewage
InactiveCN104773878AStrong oxidizingImprove adsorption capacityWater contaminantsMultistage water/sewage treatmentFenton reagentSulfate
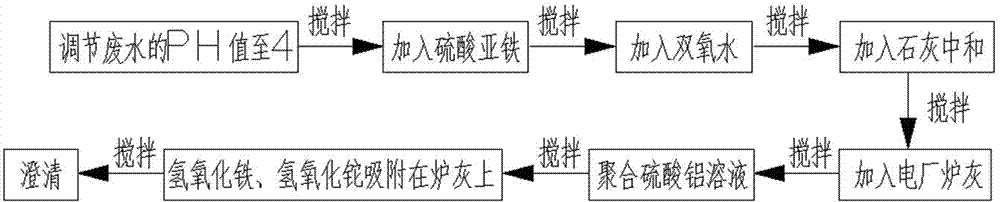
The invention relates to a method for removing a trace of thallium in sewage. The method includes the process steps of regulating the PH value of the thallium-contained sewage to 4, adding a ferrous sulfate solution to the sewage, evenly mixing the sewage with the ferrous sulfate solution, adding hydrogen peroxide to the sewage again, conducting stirring, oxidizing fluorosis univalence thallium (T1+) in the sewage into tervalence thallium (T13+), mixing ferrous iron and hydrogen peroxide into a fenton reagent, adding lime to the sewage and evenly conducting stirring so that the PH value of the sewage can be neutralized to be 7 to 9, hydrolyzing tervalence iron (Fe3+) in the sewage at the same time to form flocculent ferric hydroxide, adding an appropriate amount of power plant stove ash (electric duct collector stove ash) to the sewage, evenly conducting stirring, and adding a polyaluminium sulfate solution so that ferric hydroxide and thallium hydroxide can be adsorbed to the stove ash. Flocculent sediment generated through hydrolysis has the great surface area and quite strong adsorption capacity; the power plant stove ash can adsorb the flocculent sediment and colloid particles in the sewage and sink.
Owner:广东云测环境科技有限公司
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com