Method for simultaneous removal of cadmium and thallium in raw water
A technology of raw water and water intake, applied in the direction of chemical instruments and methods, water pollutants, water/sewage multi-stage treatment, etc., to achieve the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
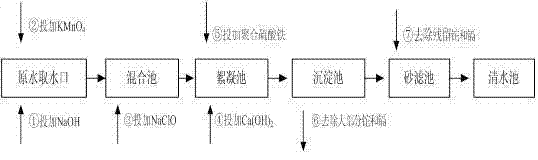
[0043] 1) Take 1L of raw water, add sodium hydroxide to adjust the pH value to 8.5;
[0044] 2) Add 0.6mg / L potassium permanganate and react for 5 minutes;
[0045] 3) Add sodium hypochlorite solution, add effective chlorine concentration of 2.0mg / L, and react for 3 minutes:
[0046] 4) Add lime water 25mg / L and react for 1 minute;
[0047] 5) Add 5.0 mg / L polyferric sulfate, react for 20 minutes, precipitate for 20 minutes, and filter.
[0048] When the cadmium content in the raw water before treatment was 25 μg / L and the thallium content was 0.25 μg / L, the cadmium content in the water after precipitation was 2.7 μg / L and the thallium content was 0.05 μg / L, and the removal rate of cadmium during the precipitation process was 89.2% respectively. , 80%; the cadmium content in the filtered water is 0.08μg / L, the thallium content is 0.02μg / L, and the total removal rates are 99.7% and 92% respectively.
Embodiment example 2
[0050] 1) Take 1L of raw water, add sodium hydroxide to adjust the pH to 8.0;
[0051] 2) Add 0.8 mg / L potassium permanganate and react for 5 minutes;
[0052]3) Add sodium hypochlorite solution, the concentration of available chlorine added is 1.5mg / L, and react for 3 minutes;
[0053] 4) Add lime water 30mg / L and react for 1 minute;
[0054] 5) Add 5.0 mg / L polyferric sulfate, react for 20 minutes, precipitate for 20 minutes, and filter.
[0055] When the cadmium content in the raw water before treatment was 20 μg / L and the thallium content was 0.18 μg / L, the cadmium content in the water after precipitation was 1.8 μg / L and the thallium content was 0.04 μg / L, and the removal rates were 91% and 77.8% respectively; The cadmium content in the back water was 0.05 μg / L, the thallium content was 0.02 μg / L, and the total removal rates were 99.8% and 88.9% respectively.
Embodiment 3
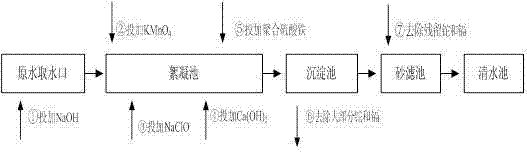
[0057] Such as figure 1 Show: and They are the dosing points of sodium hydroxide and potassium permanganate, which are generally set at the water intake or water pumping station. If neither of them has the dosing conditions, they can be set in the mixing tank or flocculation tank of the water plant; The sodium hypochlorite dosing point is located in the mixing tank; if the mixing tank needs to add other chemicals, the dosing point can be moved to the front of the flocculation tank; The lime water dosing point is located in the front section of the flocculation tank; The dosing point of polymerized ferric sulfate is located in the front section of the flocculation tank, after the lime water dosing point. The residence time of the advection sedimentation tank should not be less than 1h, and the residence time of the inclined tube sedimentation tank should not be less than 0.5h. The sand filter uses a quartz sand filter l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com