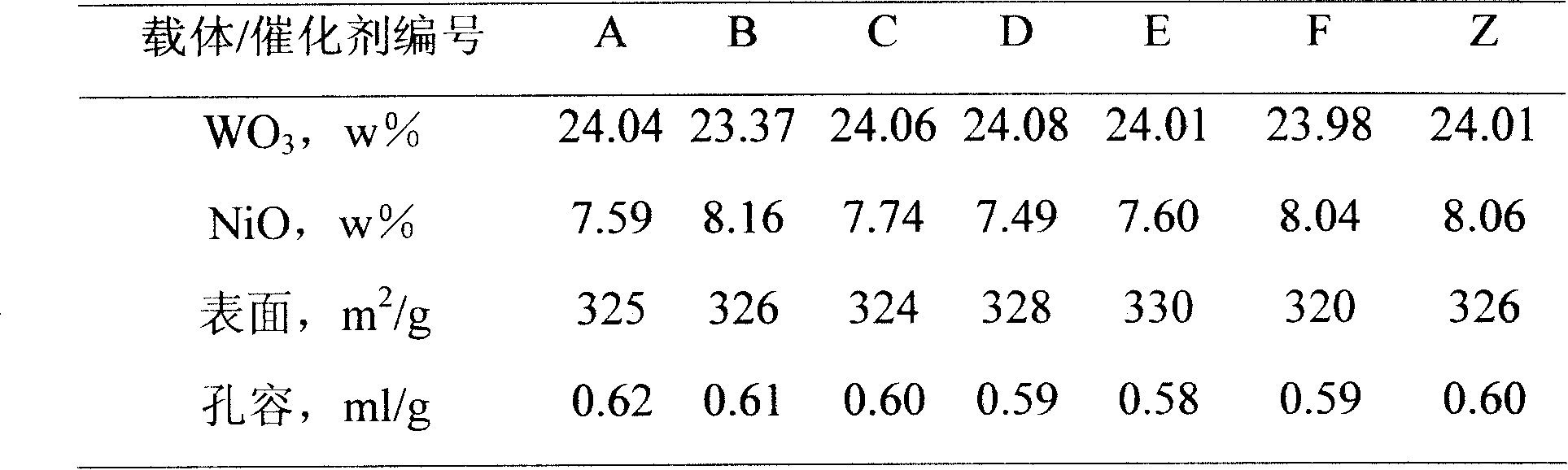
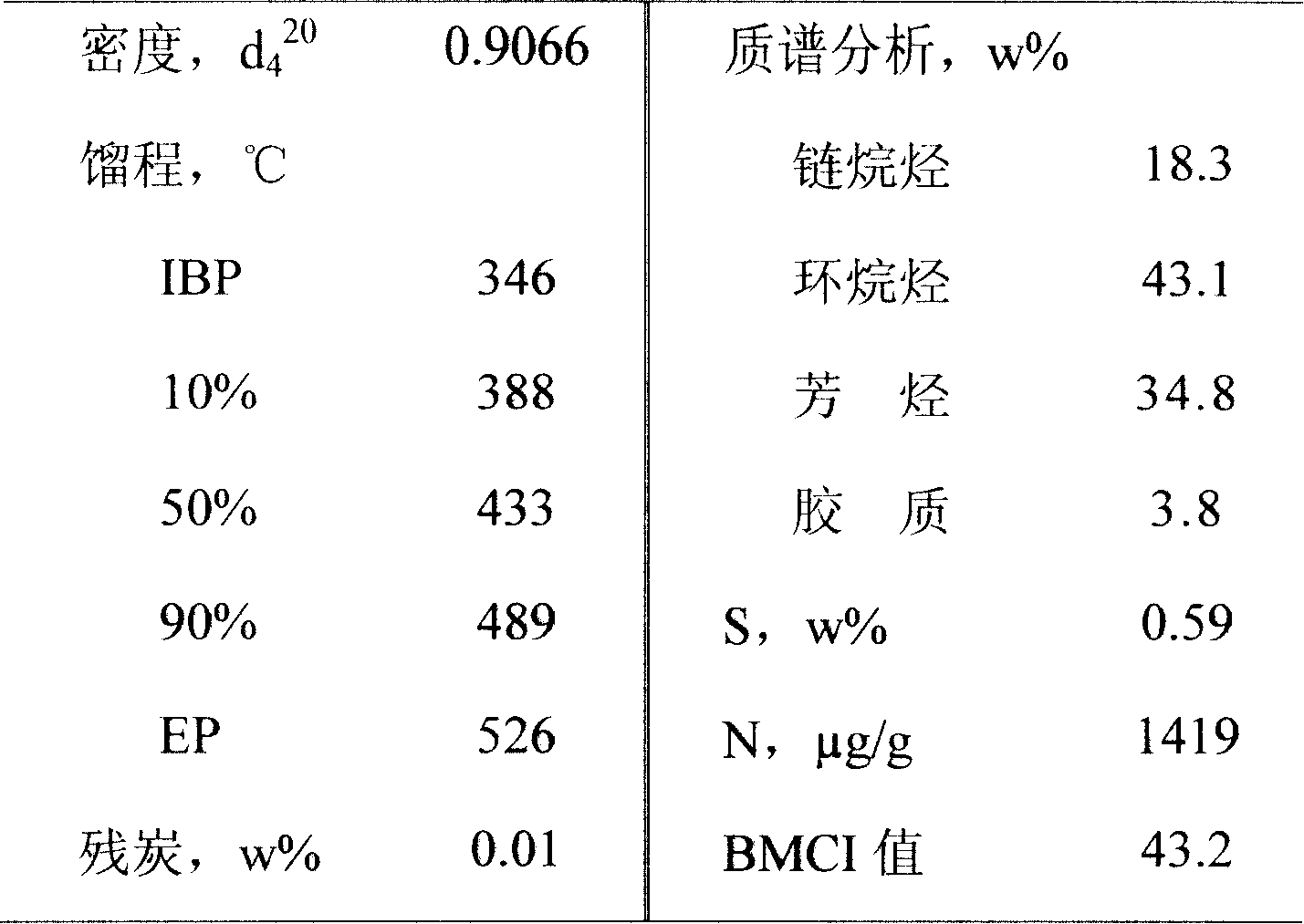
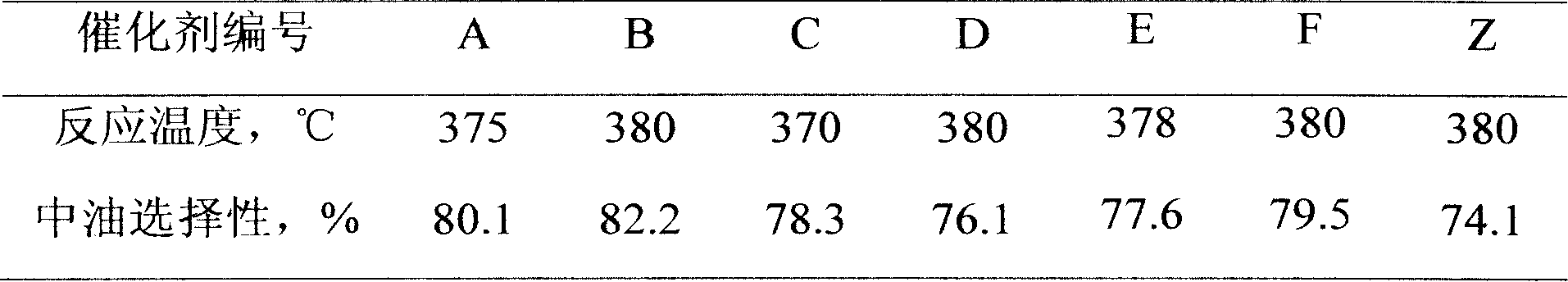
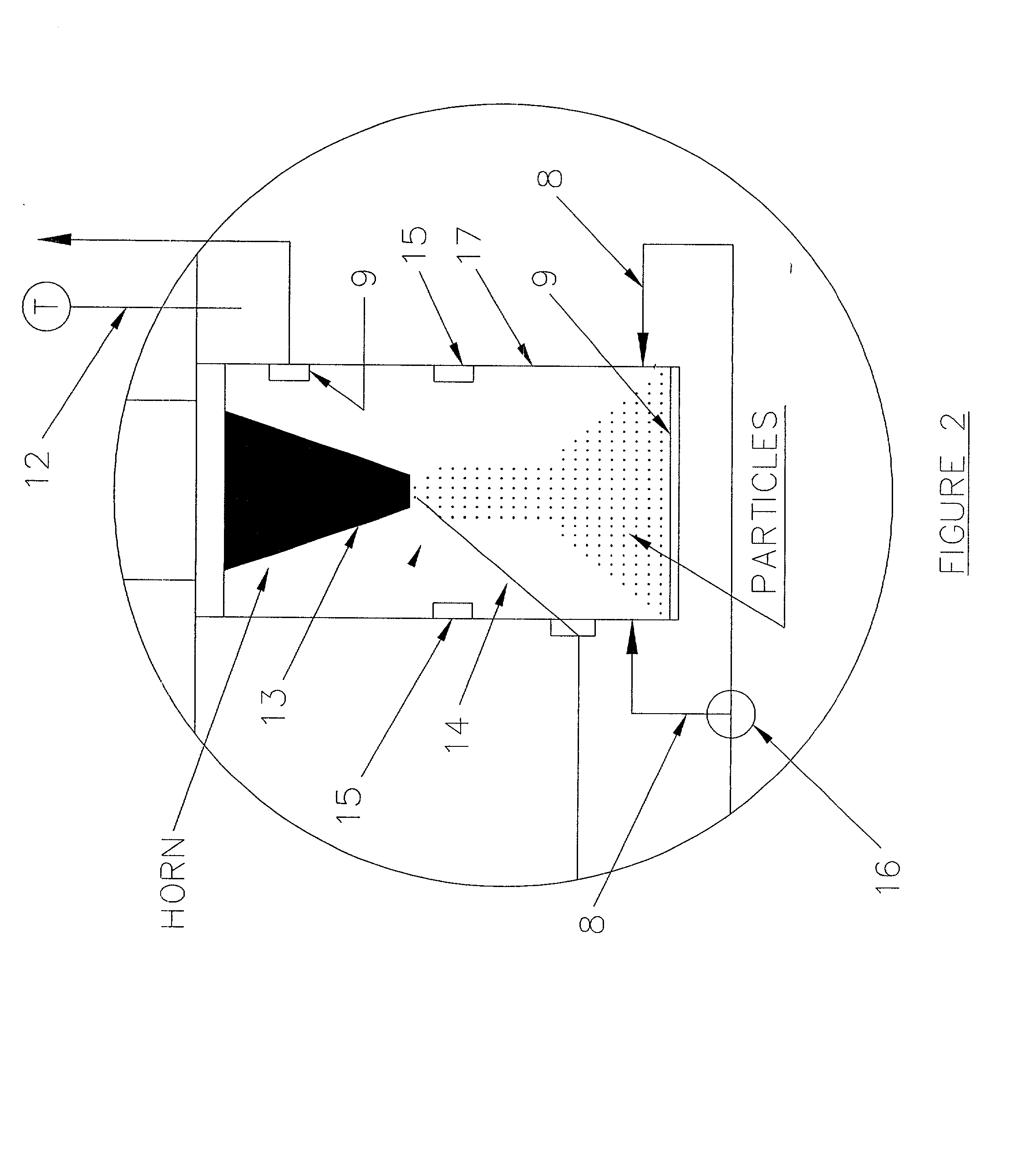
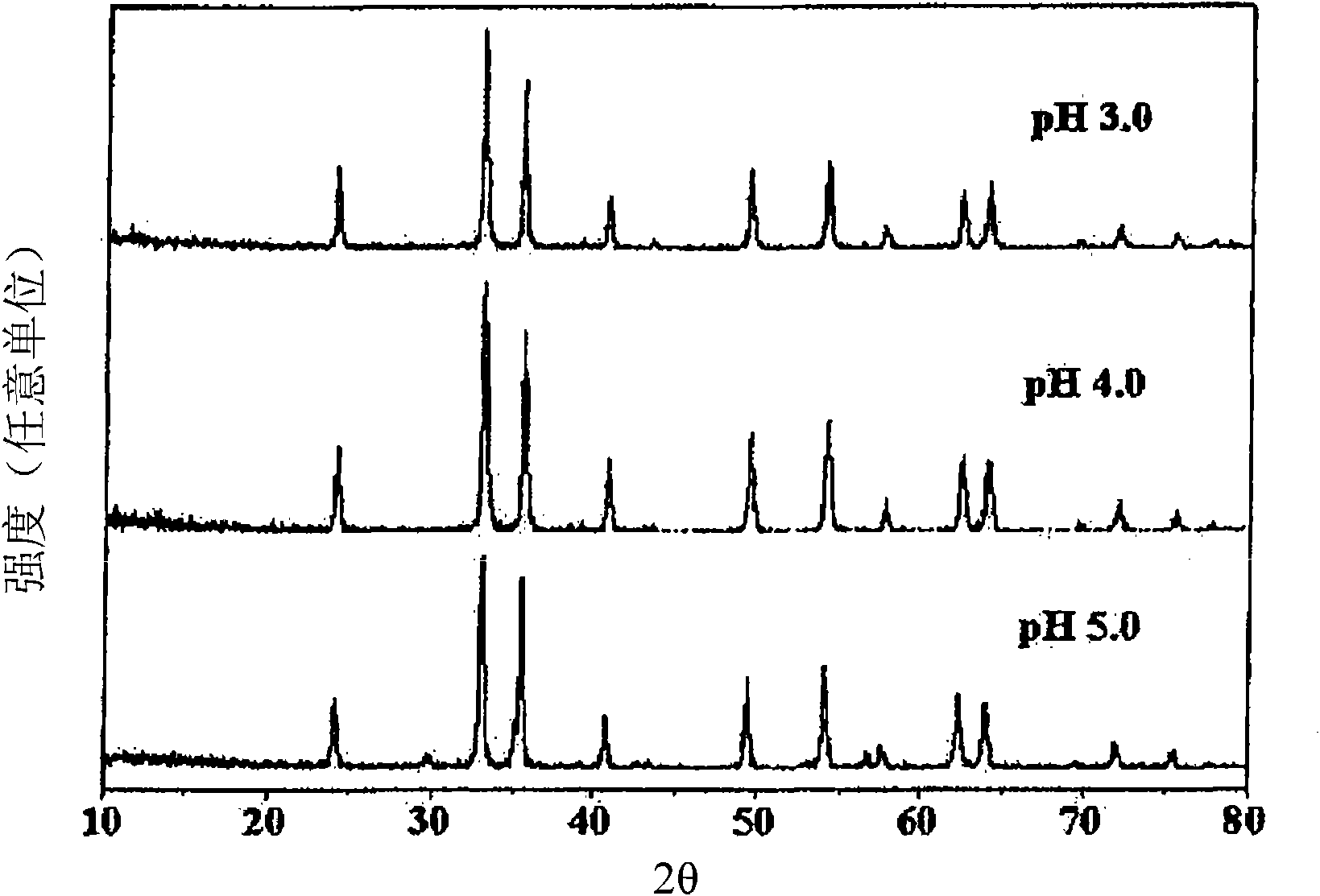
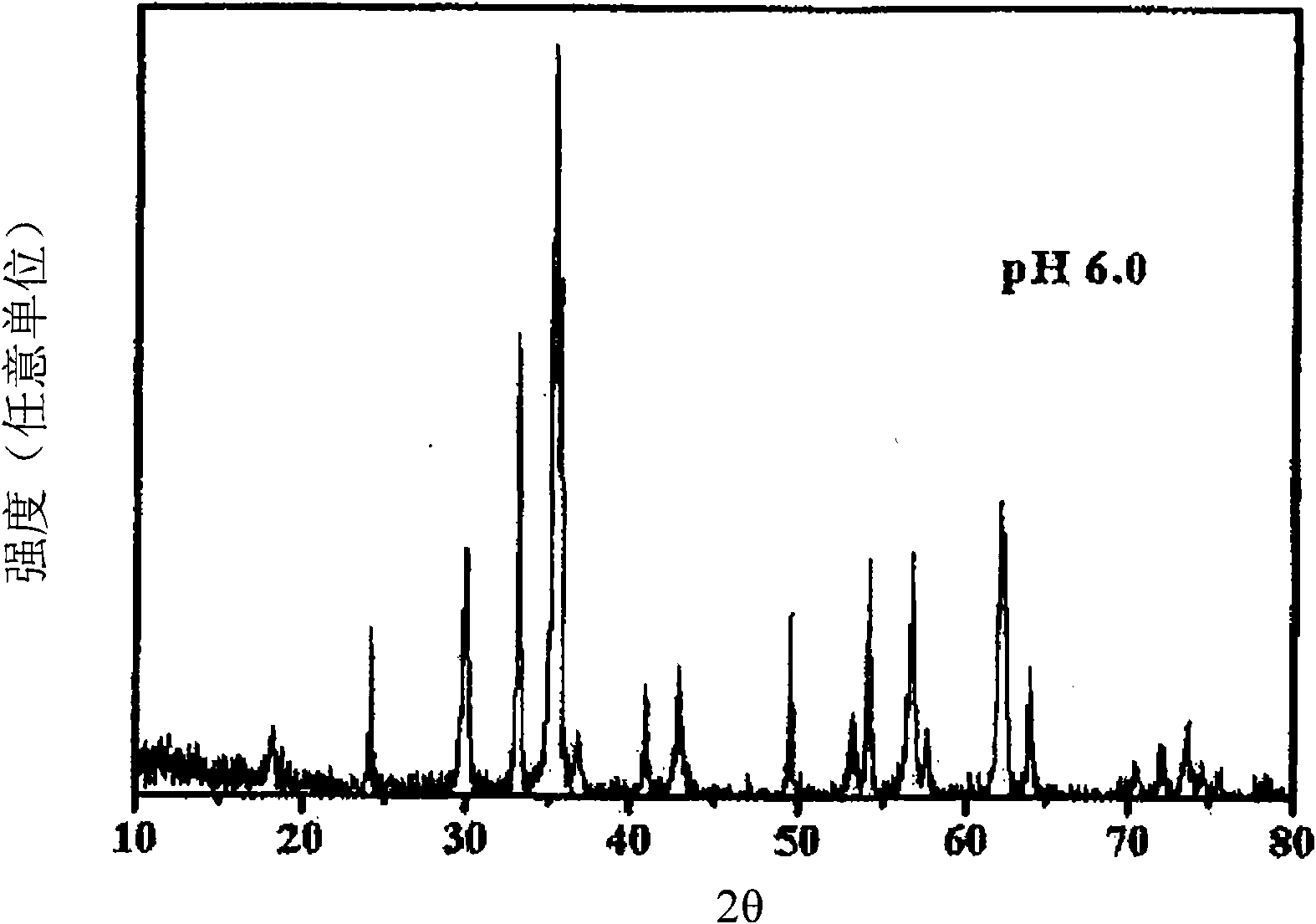
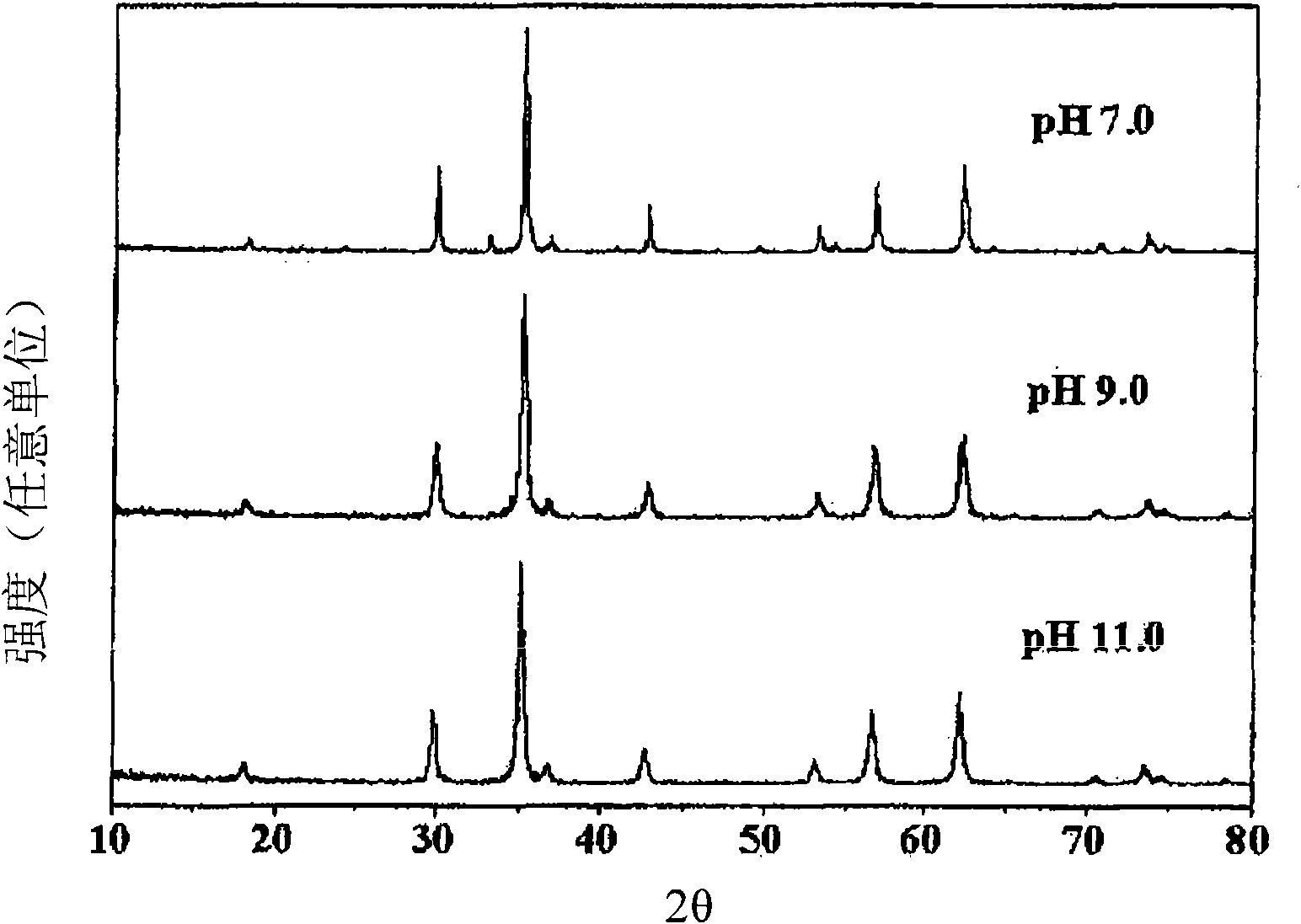
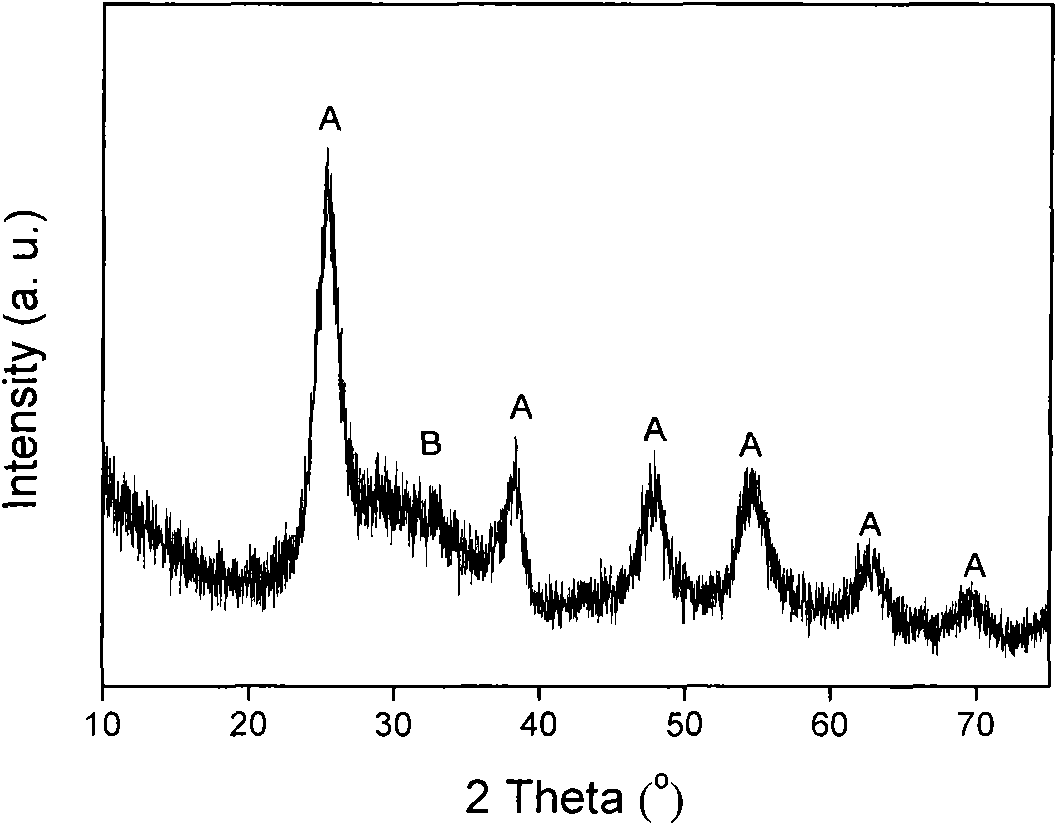
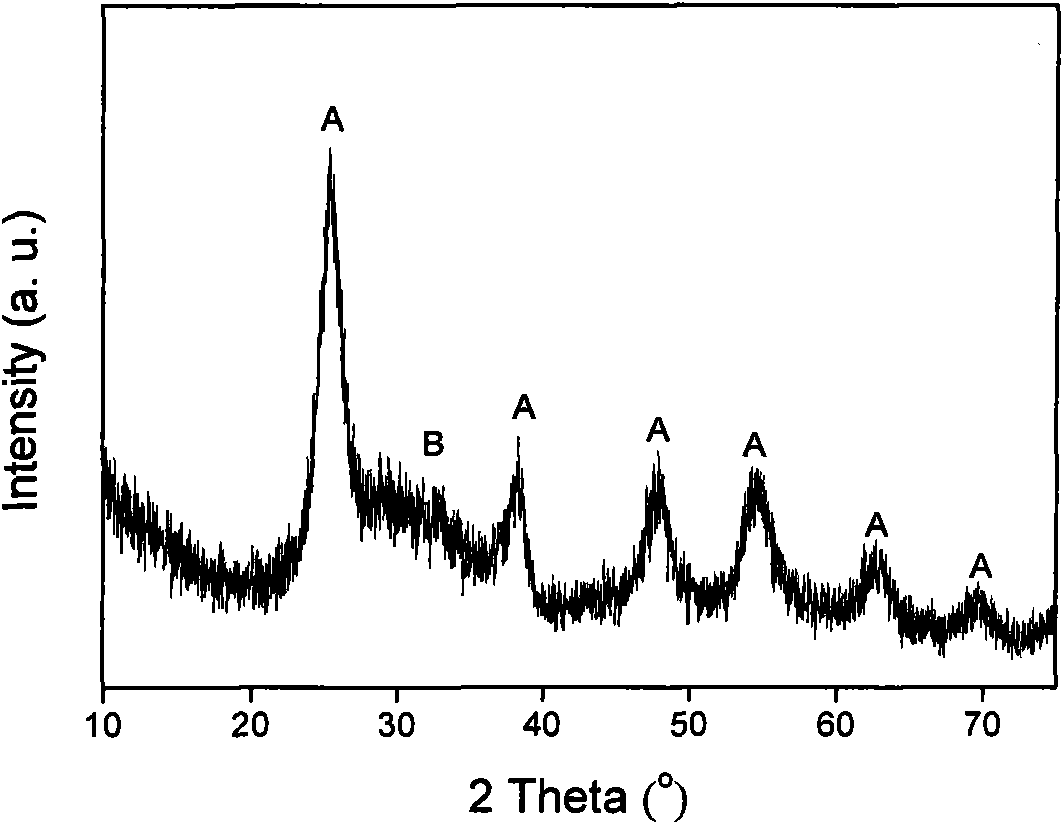
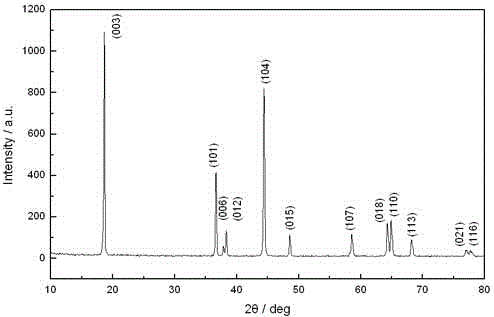
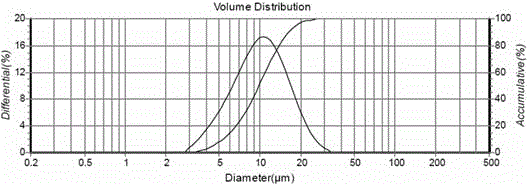
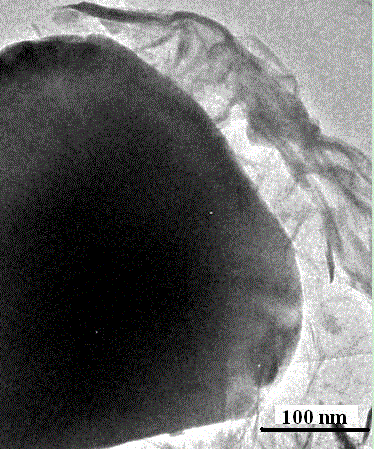
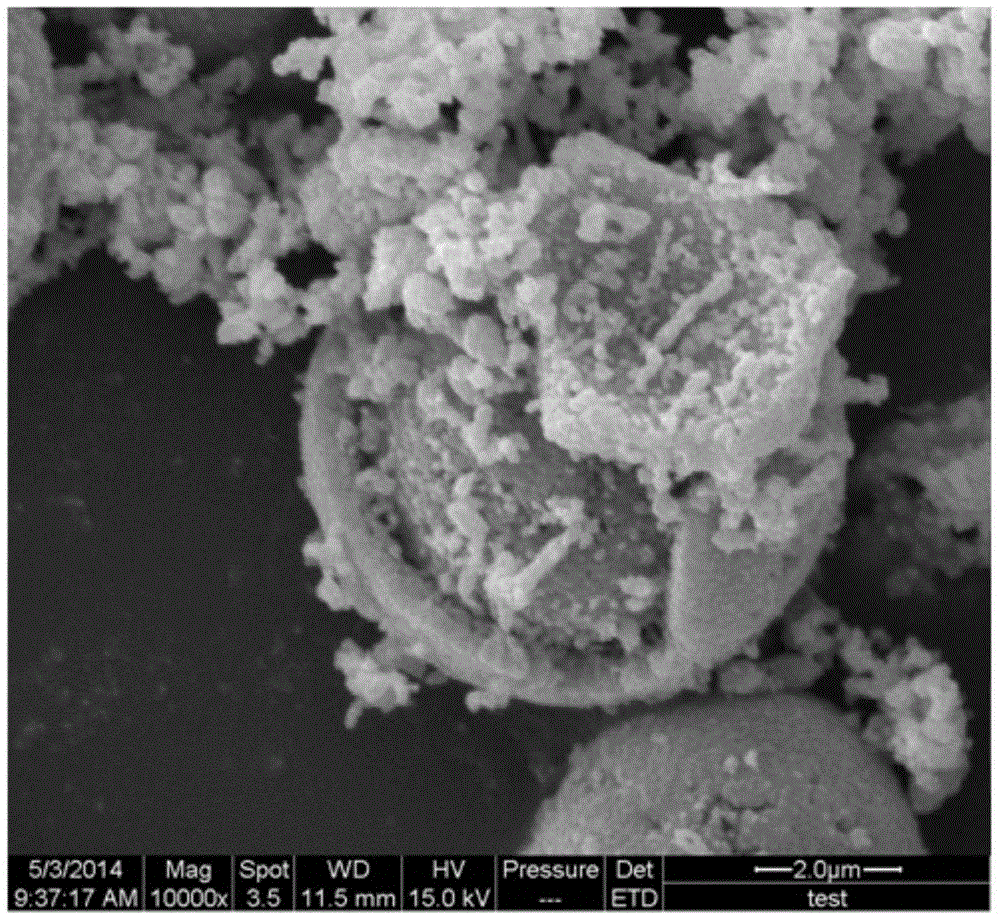
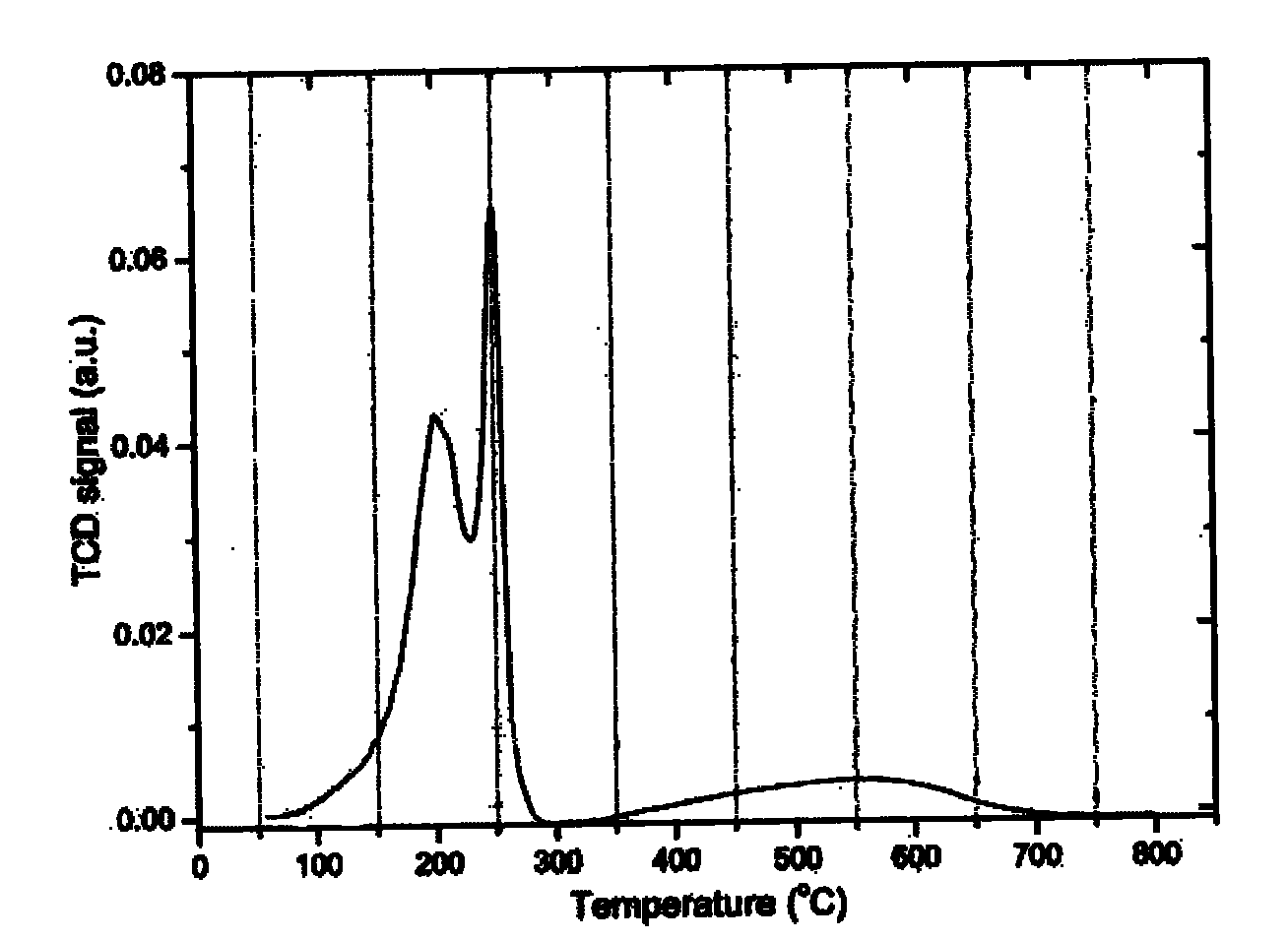
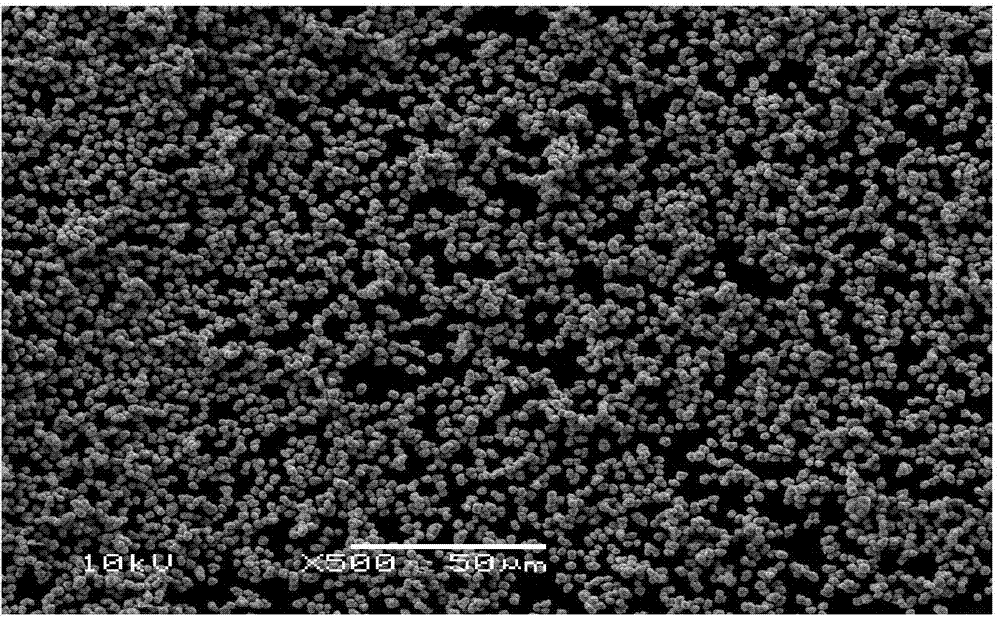
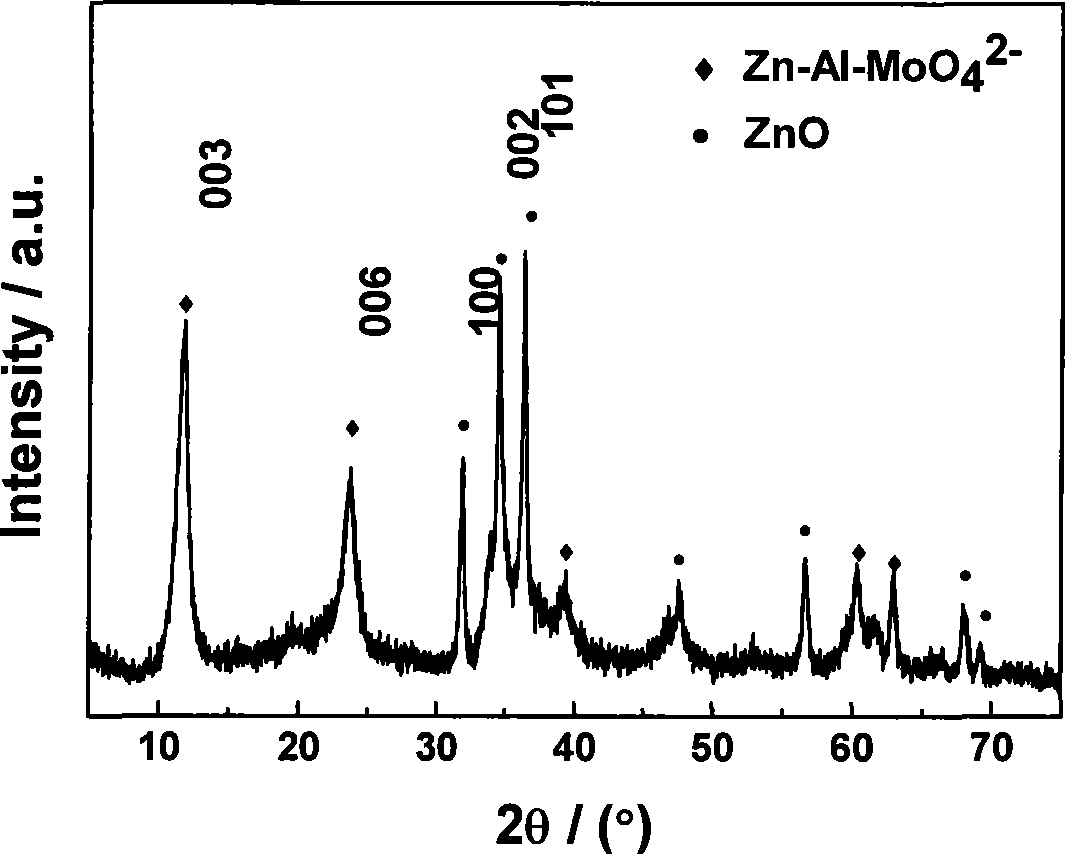
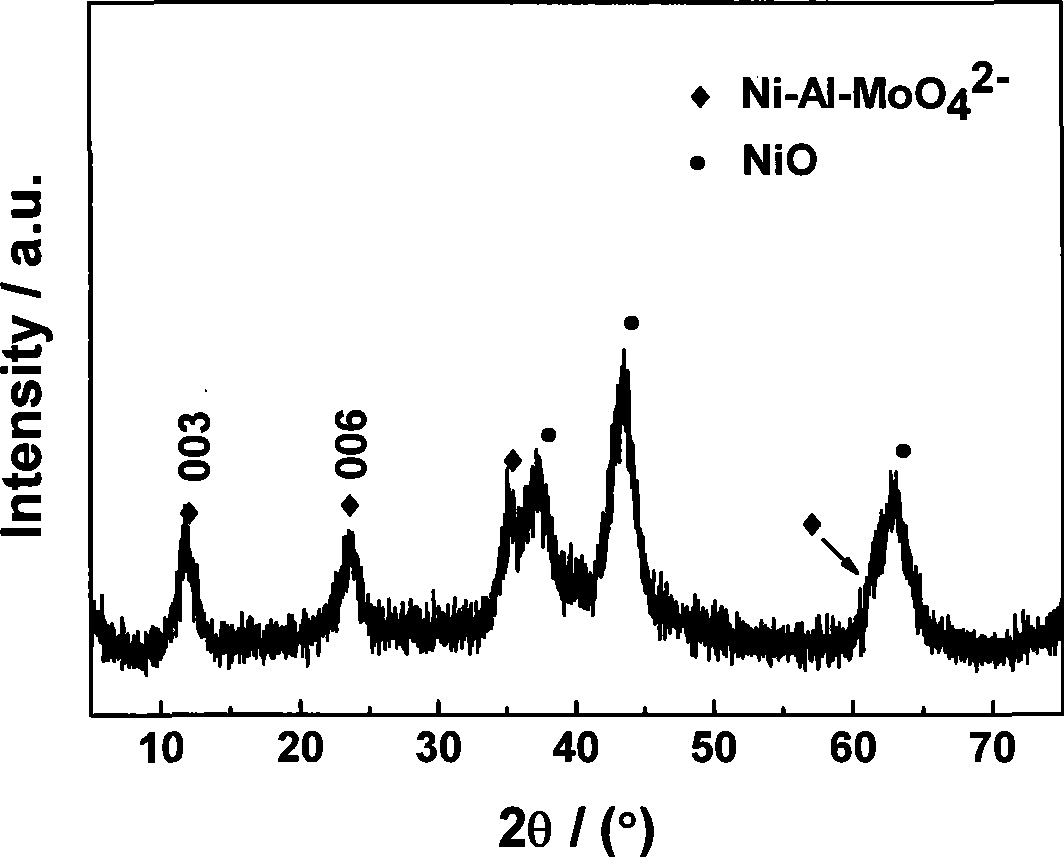
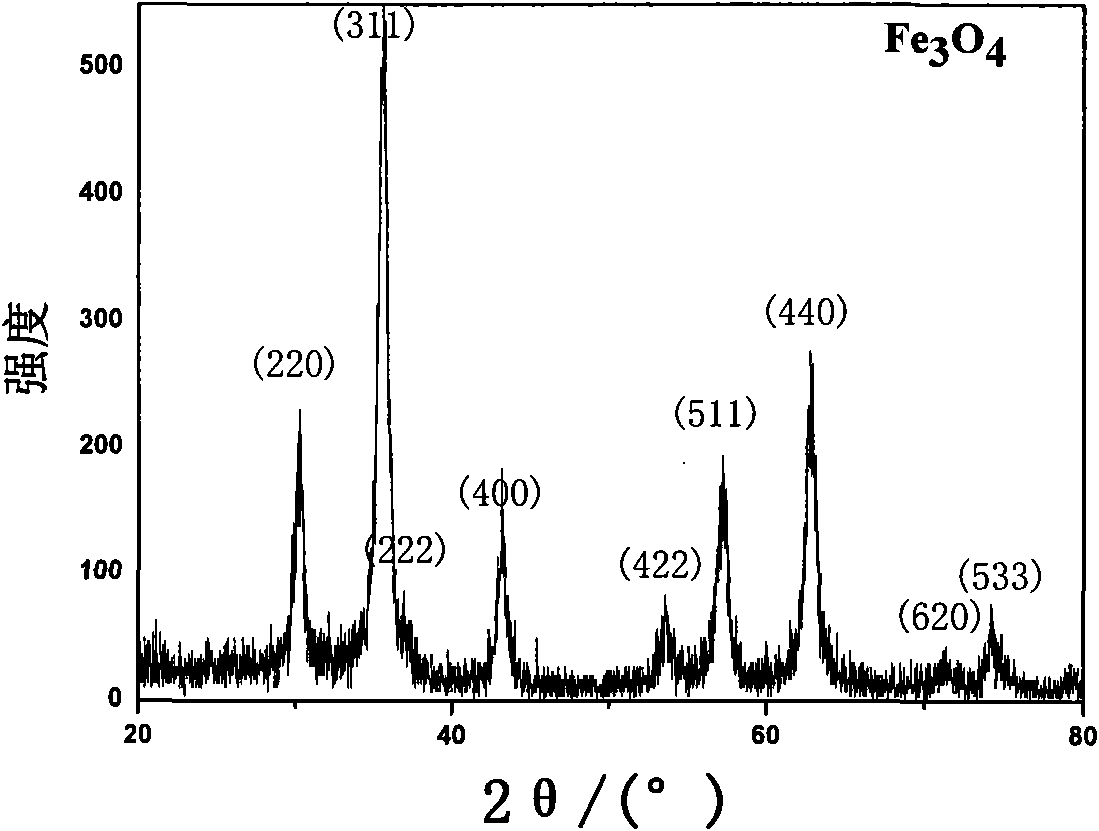
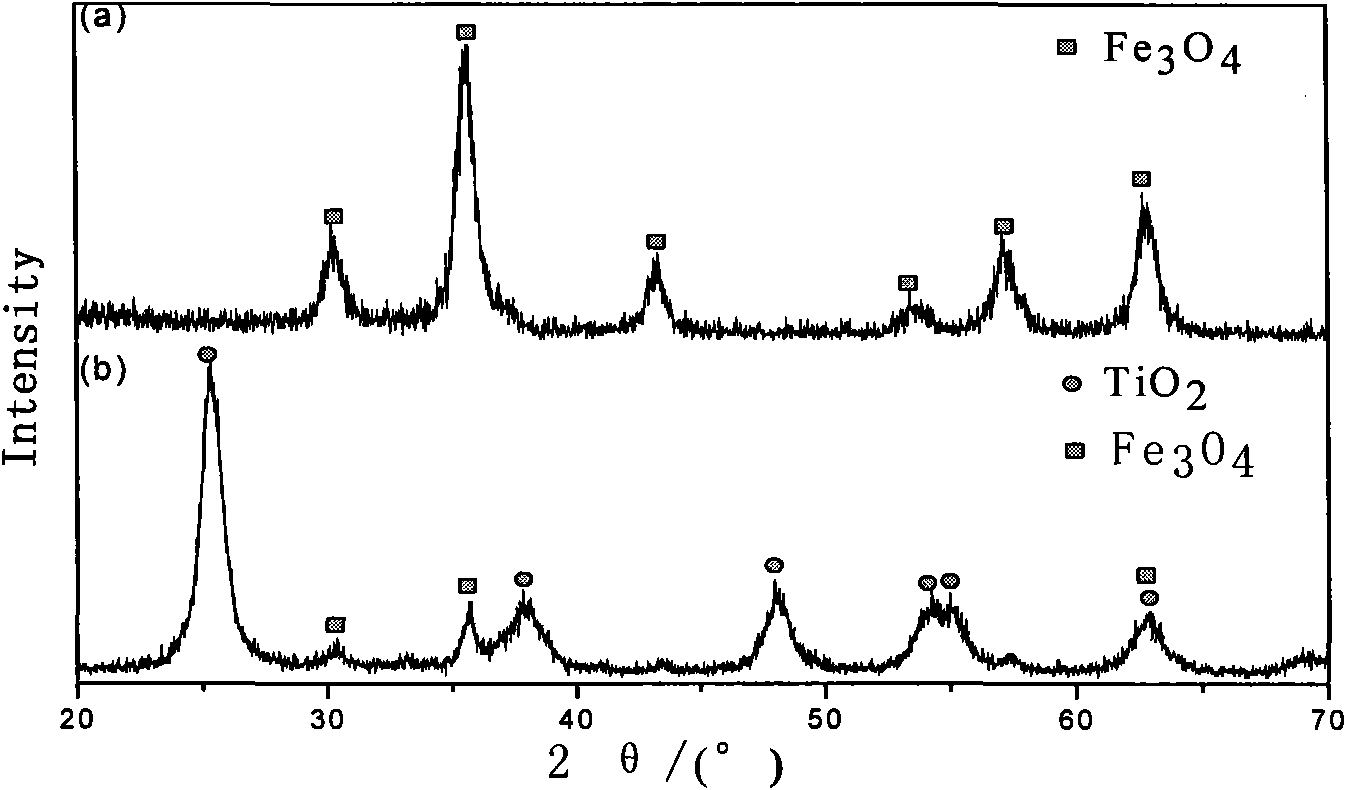
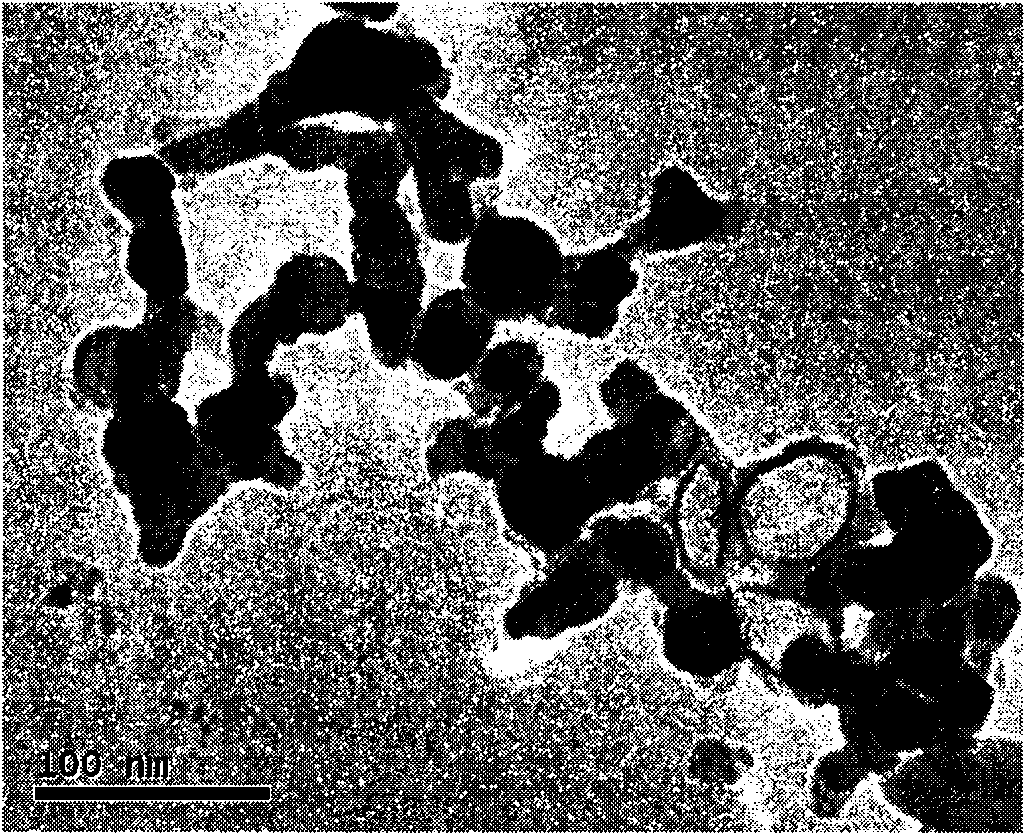
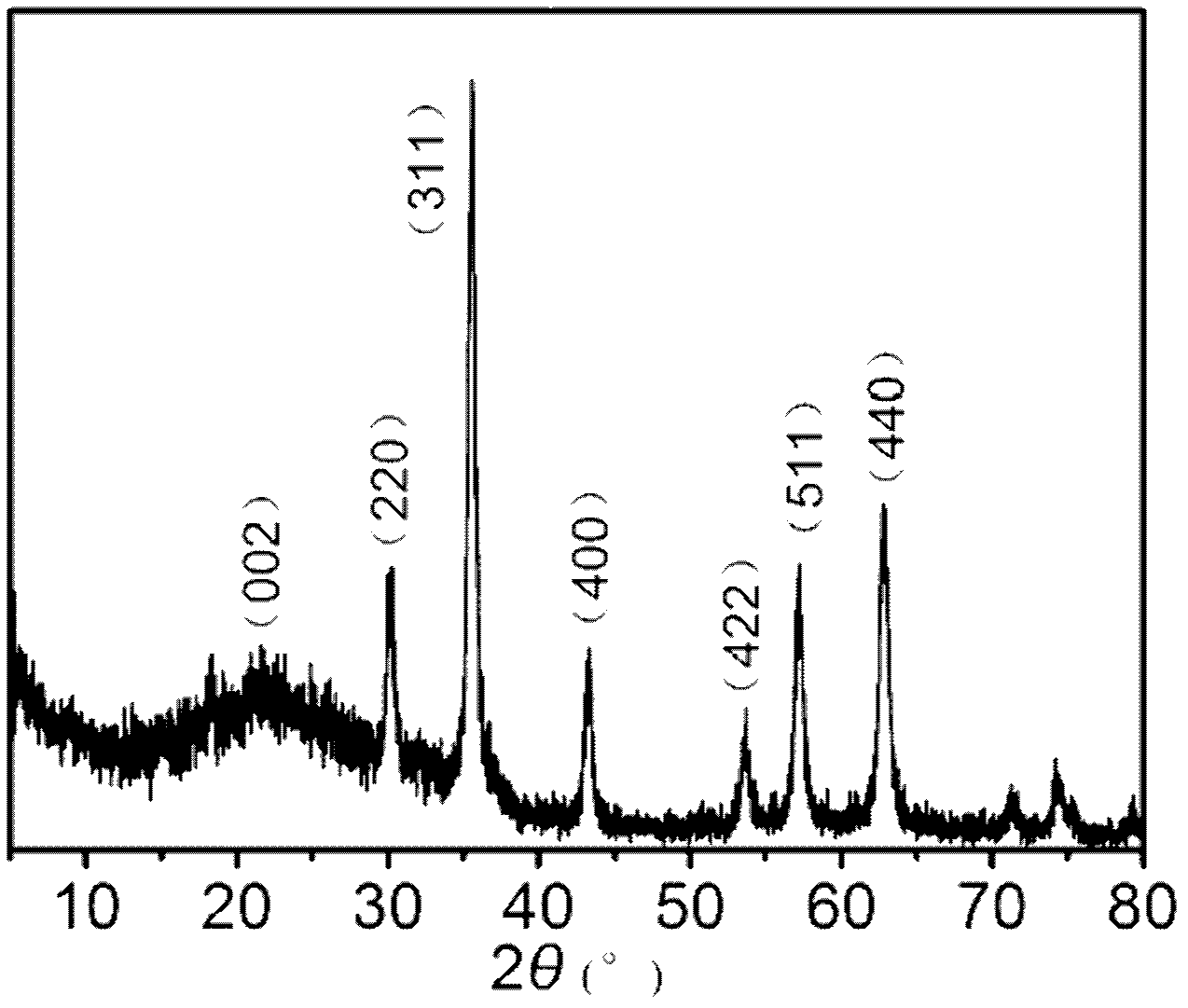
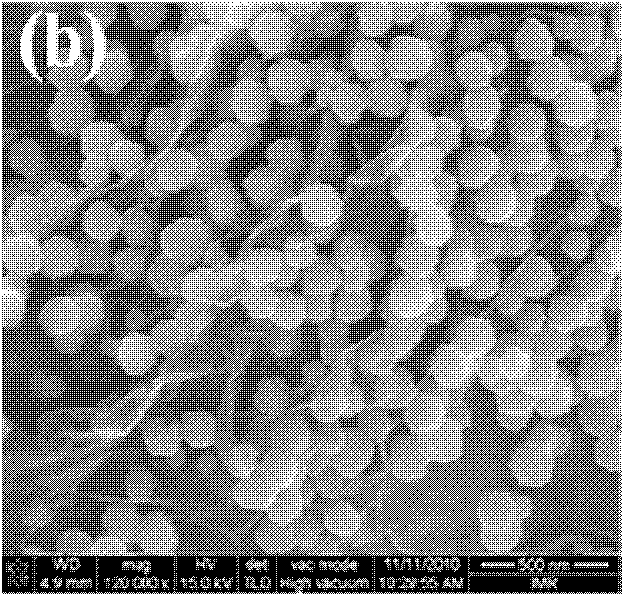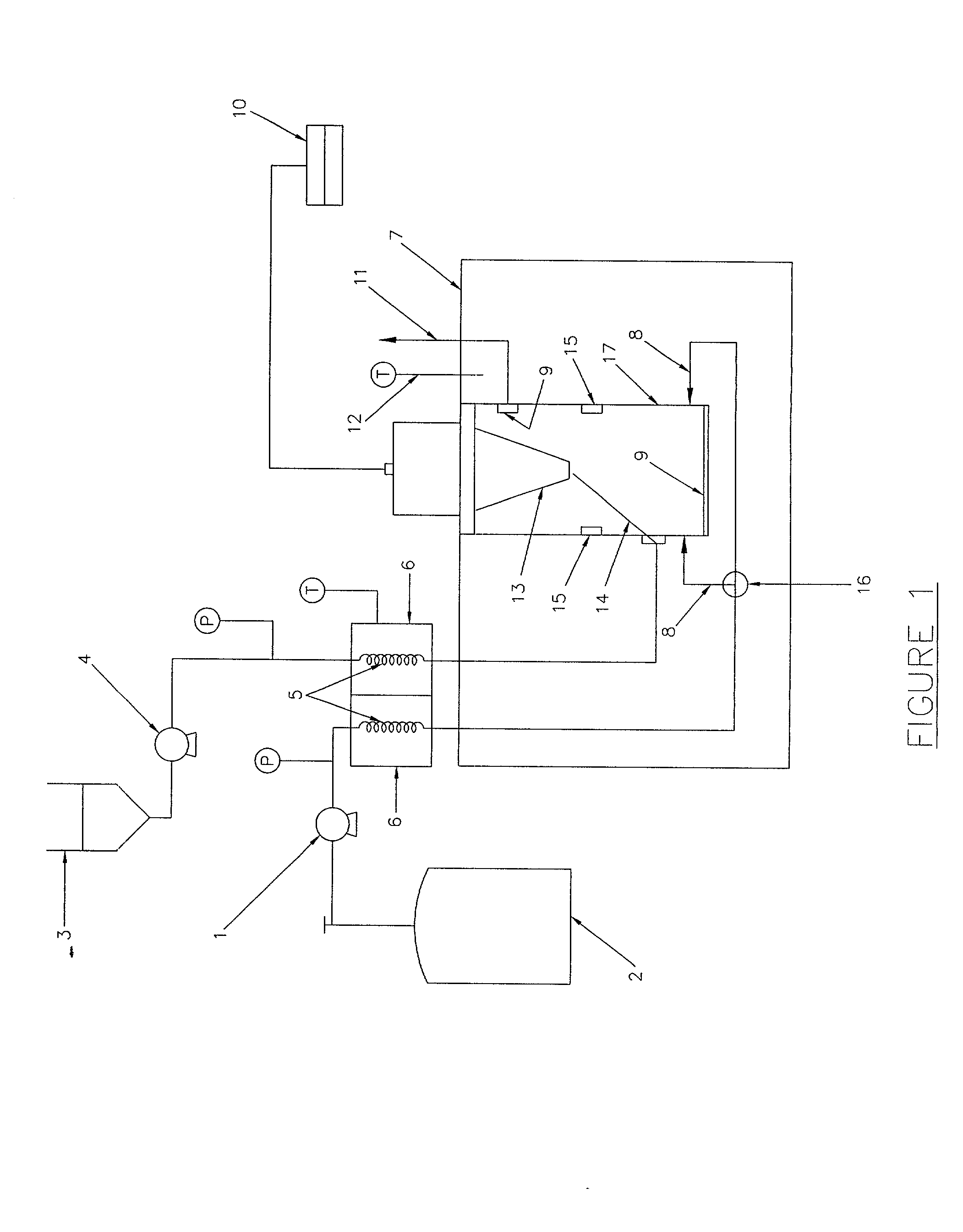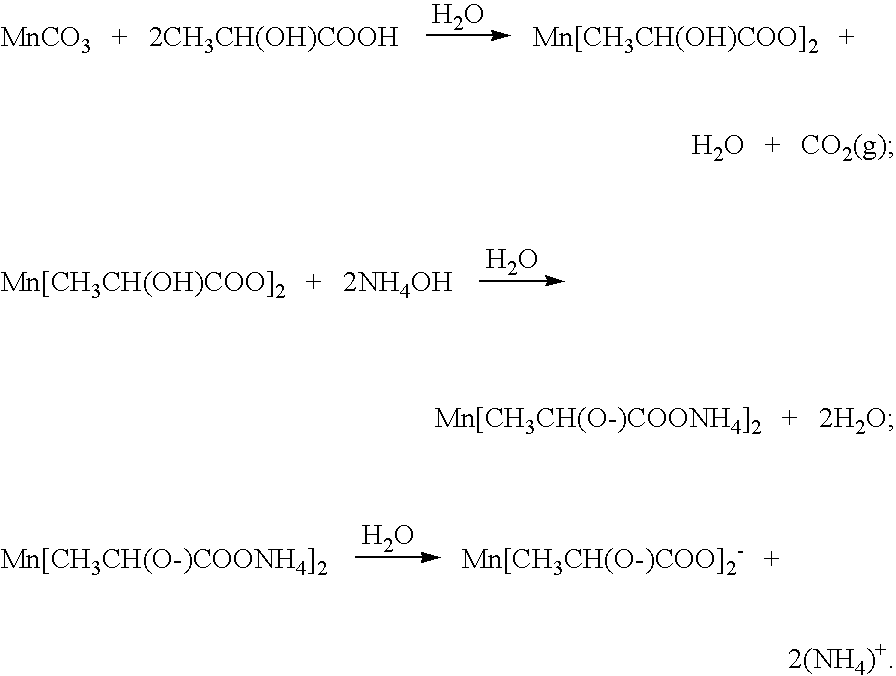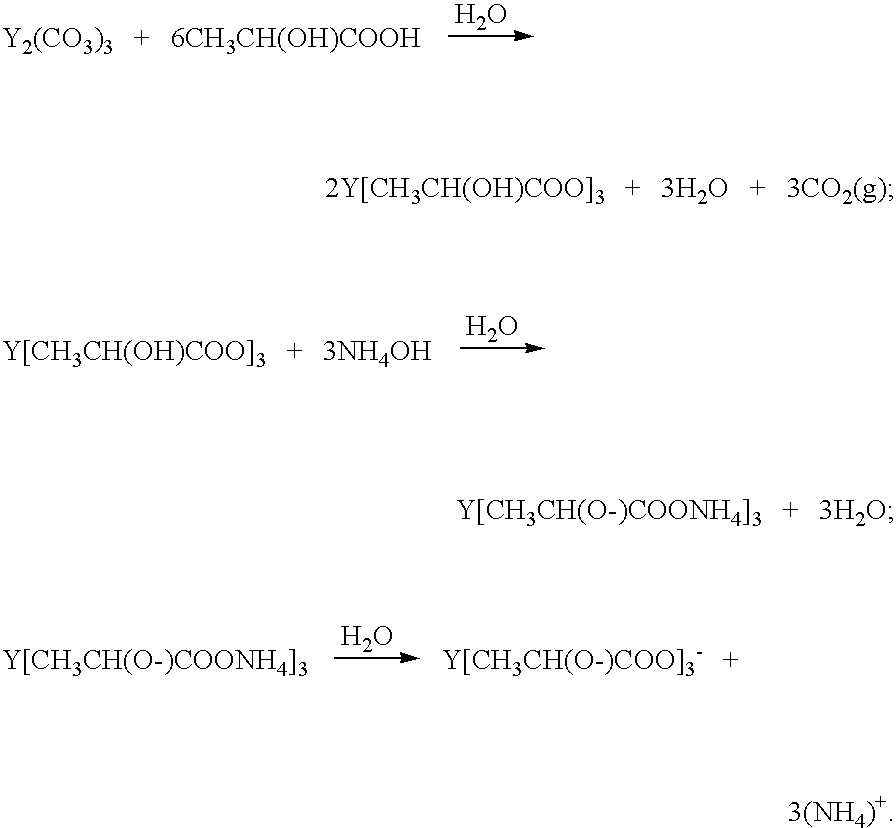Patents
Literature
3252 results about "Coprecipitation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In chemistry, coprecipitation (CPT) or co-precipitation is the carrying down by a precipitate of substances normally soluble under the conditions employed. Analogously, in medicine, coprecipitation is specifically the precipitation of an unbound "antigen along with an antigen-antibody complex".
Graphene/metal oxide composite cathode material for lithium ion battery and preparation
InactiveCN102646817APromote circulationExcellent rate performanceCell electrodesHigh energyIn situ polymerization
The invention belongs to the fields of material synthesis and energy technology, and especially relates to a graphene / metal oxide composite cathode material for lithium ion batteries and a preparation method thereof. Grapheme is dispersed into various metal oxide precursor salt solutions; a graphene / metal oxide compound is obtained directly by a hydrothermal method, or an graphene / metal oxide compound is obtained by a liquid in-situ polymerization method or a coprecipitation process; and the graphene / metal oxide compound is obtained by heat treatment or hydrothermal treatment. In the invention, the novel three-dimensional composite cathode material of graphene-coated metal oxide or graphene-anchored metal oxide is prepared by carrying metal oxide particles with graphene as a carrier. The obtained composite material can be used as a lithium ion battery cathode, which has a high specific capacity, excellent cycle stability and rate capability, and is expected to be used as a lithium ion battery cathode material with a high energy density and a high power density.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
Preparation of hydrogenation catalyst
ActiveCN101172260AEasy to useEvenly dispersedMolecular sieve catalystsCatalyst activation/preparationMolecular sievePolymer science
The invention discloses a preparation method of a hydrogenation catalyst. The final catalyst is prepared by impregnation method or coprecipitation method using a carrier material containing molecular sieve and amorphous silica-alumina. The carrier material is prepared by introducing molecular sieve slurry in the process of forming amorphous silica-alumina gel, which improves the distribution of molecular sieve in the silica-alumina carrier, improves the coordinated catalytic effect of the two carrier materials, and greatly improves the performance of the catalyst. The catalyst prepared by the method of the present invention can be used in various hydrogenation processes.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method of forming nanoparticles and microparticles of controllable size using supercritical fluids with enhanced mass transfer
The current invention, Supercritical Antisolvent Precipitation with Enhanced Mass Transfer (SAS-EM) provides a significantly improved method for the production of nano and micro-particles with a narrow size distribution. The processes of the invention utilize the properties of supercritical fluids and also the principles of virbrational atomization to provide an efficient technique for the effective nanonization or micronization of particles. Like the SAS technique, SAS-EM, also uses a supercritical fluid as the antisolvent, but in the present invention the dispersion jet is deflected by a vibrating surface that atomizes the jet into fine droplets. The vibrating surface also generates a vibrational flow field within the supercritical phase that enhances mass transfer through increased mixing. Sizes of the particles obtained by this technique are easily controlled by changing the vibration intensity of the deflecting surface, which in turn is controlled by adjusting the power input to the vibration source. A major advantage of the SAS-EM technique is that it can be successfully used to obtain nanoparticles of materials that usually yield fibers or large crystals in SAS method. Microencapsulation via coprecipitation of two or more materials can also be achieved using the SAS-EM technique.
Owner:UNIV AUBURN
Method of forming nanoparticles and microparticles of controllable size using supercritical fluids and ultrasound
The current invention, Supercritical Antisolvent Precipitation with Enhanced Mass Transfer (SAS-EM) provides a significantly improved method for the production of nano and micro-particles with a narrow size distribution. The processes of the invention utilize the properties of supercritical fluids and also the principles of virbrational atomization to provide an efficient technique for the effective nanonization or micronization of particles. Like the SAS technique, SAS-EM, also uses a supercritical fluid as the antisolvent, but in the present invention the dispersion jet is deflected by a vibrating surface that atomizes the jet into fine droplets. The vibrating surface also generates a vibrational flow field within the supercritical phase that enhances mass transfer through increased mixing. Sizes of the particles obtained by this technique are easily controlled by changing the vibration intensity of the deflecting surface, which in turn is controlled by adjusting the power input to the vibration source. A major advantage of the SAS-EM technique is that it can be successfully used to obtain nanoparticles of materials that usually yield fibers or large crystals in SAS method. Microencapsulation via coprecipitation of two or more materials can also be achieved using the SAS-EM technique.
Owner:UNIV AUBURN
Zinc ferrite catalysts, method of preparing thereof and method of preparing 1,3-butadiene using thereof
ActiveCN101674883ASimple structureEasy to synthesizeHeterogenous catalyst chemical elementsCatalystsButeneDehydrogenation
Owner:SK INNOVATION CO LTD +1
Preparation method of low-temperature selective catalytic reduction (SCR) catalyst by removing NOx from flue gas
ActiveCN102008956AIncrease the areaLarge hole volumeNitrous oxide captureDispersed particle separationComposite oxideCoprecipitation
The invention provides a preparation method of a selective catalytic reduction (SCR) catalyst Mn-Ce-M / TiO2 by removing NOx from flue gas, wherein M represents one or more of composite oxides, namely Fe, Co, Cu, Cr, Zr and Al, the molar ratio of the elements is Ti: Mn: Ce: M =1:(0.005-1):(0.005-1):(0-1). In the invention, an improved coprecipitation method is adopted, and an intermediate compound, namely titanium nitrate Ti(NO3)4 is taken as a precursor of TiO2, thus the influence of the other impurities in the precipitation process is overcome; by changing the dispersing homogeneous degree of MnOx and CeO2 on the surface of a carrier TiO2, the activity of the catalyst is improved and the temperature of the SCR reaction is reduced; by adjusting the preparation condition of the TiO2 precursor, the sulfur resistance and water resistance of the catalyst are improved completely; and by adding the elements, such as Fe, Co, Cu, Cr, Zr and Al and the like, the activity and resistance of the catalyst are further modified.
Owner:GUODIAN SCI & TECH RES INST +1
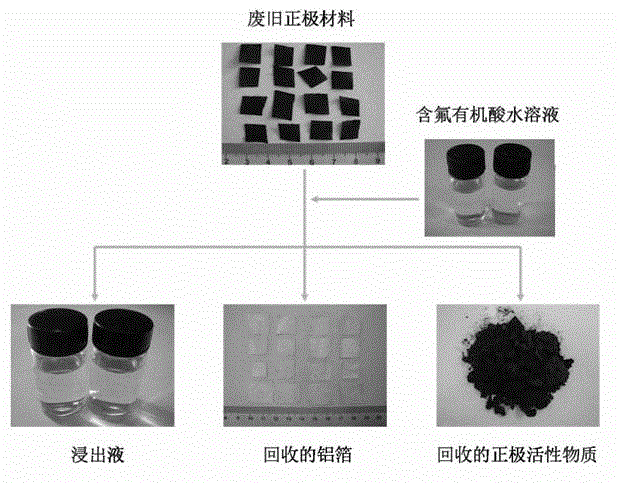
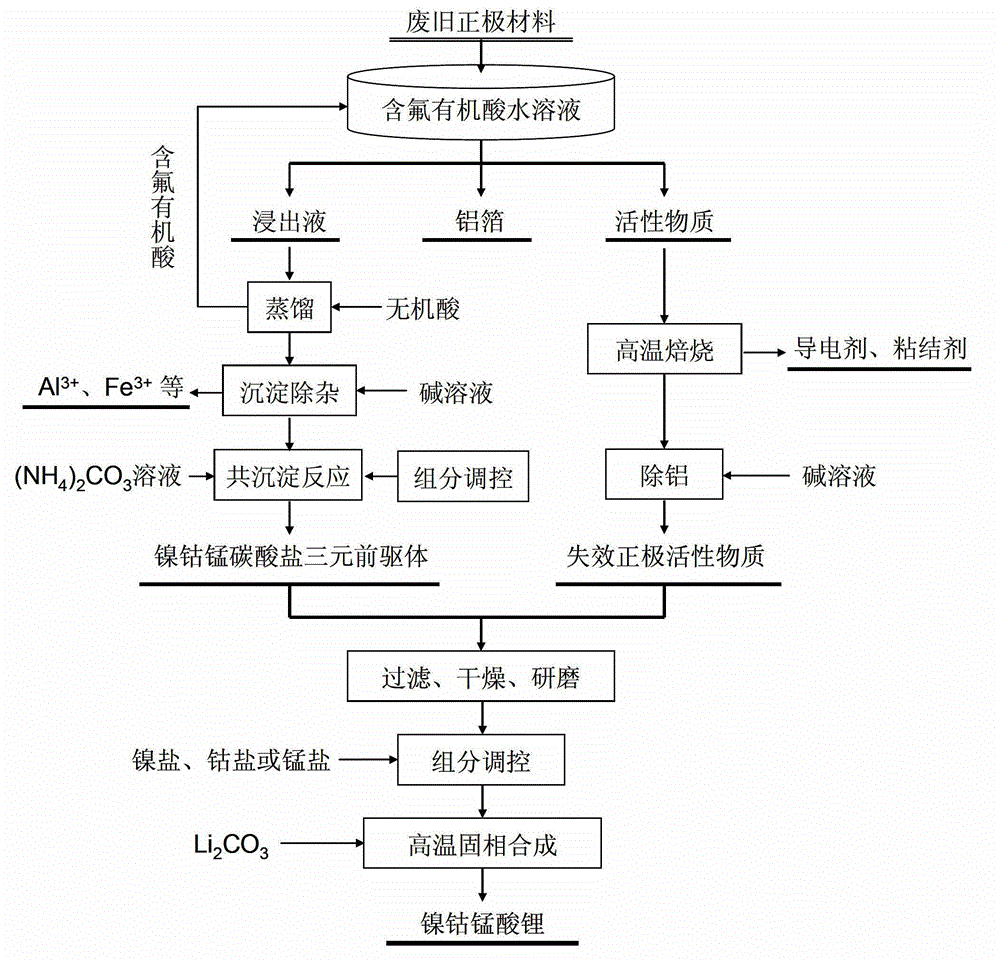
Full-component resource reclamation method for waste positive electrode materials of lithium ion batteries
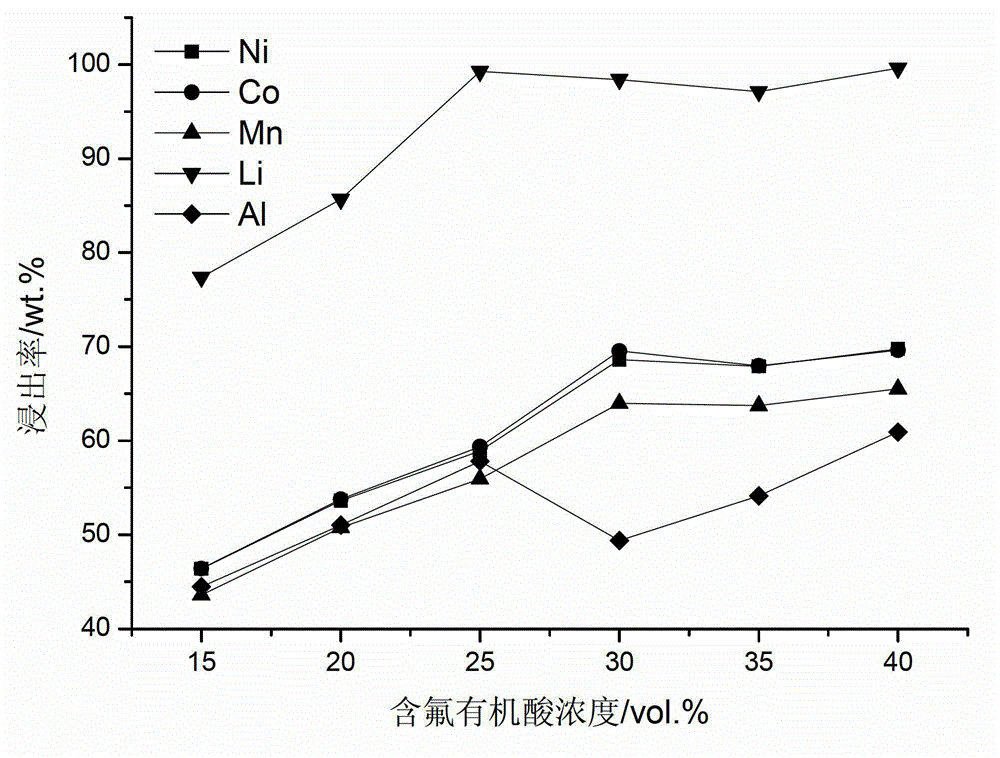
ActiveCN102751549AAvoid secondary pollutionImprove separation efficiencyWaste accumulators reclaimingBattery recyclingRecovery methodDistillation
The invention provides a full-component resource reclamation method for waste positive electrode materials of lithium ion batteries. The method comprises the following steps: 1) separating active substances and aluminum foils in waste positive electrode materials of lithium ion batteries by using an aqueous solution of fluorine-containing organic acid and carrying out liquid-solid-solid separation so as to obtain leachate, the lithium-containing active substances and the aluminum foils; 2) respectively carrying out high temperature roasting and impurity removal with alkali liquor on the lithium-containing active substances; 3) respectively carrying out recovery of the fluorine-containing organic acid through addition of acid and distillation, deposition of impurity ions through addition of alkali and ammonium carbonate coprecipitation on the leachate so as to prepare nickel-cobalt-manganese carbonate ternary precursor; and 4) carrying out component regulation on a mixture of the treated active substances and the nickel-cobalt-manganese carbonate ternary precursor, adding lithium carbonate in a certain proportion and carrying out high temperature solid phase sintering so as to prepare a lithium nickel cobalt manganese oxide ternary positive electrode material. The method provided in the invention has the following advantages: the application scope of the method is wide; separation efficiency of the lithium-containing active substances and the aluminum foils is high; short-flow direct re-preparation of positive electrode materials in waste lithium ion batteries is realized; and the method is applicable to large-scale resource reclamation of waste lithium ion batteries.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Preparation method of hydrogenation catalyst composition
ActiveCN102451703AImprove adhesionEasy to shapeCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsAluminateHigh activity
The invention discloses a preparation method of a hydrogenation catalyst composition. According to the method, sodium meta-aluminate is adopted as an aluminum source in the preparation of a mixture of an NixWyOz composite oxide precursor and an Al2O3 precursor through a coprecipitation process, and a proper amount of a CO2 gas is accessed in the colloid forming process, so a difficult shaping problem of a bulk phase catalyst is solved, physicochemical properties of the catalyst are adjusted, and the composition which has the characteristics of large specific surface area and uniform aperture distribution and makes many metal active sites be exposed on the catalyst surface allows the utilization rate of active metals to be improved. The catalyst composition of the invention, which has increased apertures and pore volumes, allows the Ni-W high activity sites to be fully utilized and complex macrostructure molecules to easily contact with the active sites, so the catalyst composition is especially suitable for ultra-deep desulfurization reactions for the production of ultraclean diesel oil.
Owner:CHINA PETROLEUM & CHEM CORP +1
Nickel system hydrogenation catalyst, preparation method and application
ActiveCN101037613AImprove hydrogenation activityImprove stabilityHydrocarbon by hydrogenationRefining to eliminate hetero atomsResistAdjuvant
The invention realtes to a nickel based hydrogenation catalyst, preparation and application thereof. Using alumina or and monox silica as carrier, the catalyst prepared by means of coprecipitation, main containing active component Ni, is characterized in catalyst composed by active component Ni, La, adjuvant X1 and carrier X2O. Calculated by weight of component, the catalyst including: NiO 40-70%, La2O3 2-5%, X2O 20-50%, wherein X1 is selected from one or more of Cu, Mg, Zr, X2 is selected from Al or and Si; surface area to volume ratio thereof is 80-200 m<2> / g, pore volume ratio is 0.4-0.8 ml / g. The catalyst is suit for mono olefin hydrogenation, especially for hydrogenation of pyrolysis C9 distillation, which has higher hydrogenation activity, definited sulfur poison resist and carboid resist to fit for higher require of high colloid raw material hydrogenation which of catalyst has high hydrogenation activity and good stability.
Owner:PETROCHINA CO LTD
Preparation method for ternary positive electrode material of graphene composite lithium ion battery
ActiveCN104157854ASolving Dispersion ProblemsLower polarization internal resistanceCell electrodesSecondary cellsManganeseCoprecipitation
The invention particularly relates to a preparation method for a ternary positive electrode material of a graphene composite lithium ion battery. The preparation method comprises the following steps of firstly preparing a ternary positive electrode material precursor by a crystallization control-coprecipitation method; performing multi-step sintering to prepare the ternary positive electrode material, wherein the mole ratio of nickel to manganese to cobalt (namely x to y to z) is equal to (0.30-0.90) to (0.50-0.80) to (0.05-0.50), and x+y+z=1; and finally preparing the ternary positive electrode material of the graphene composite lithium ion battery. According to the preparation method disclosed by the invention, the problem that graphene is difficult to disperse in the ternary positive electrode material is solved, the internal polarization resistance is greatly reduced, and high-current rate discharge is realized; furthermore, high discharge capacity and long cycle life are kept. The technology is simple, and the preparation method is low in energy consumption and favorable for large-scale production.
Owner:SHANDONG YUHUANG NEW ENERGY TECH
Coprecipitation method for preparing ultra fine zinc oxide powder possessing high electric conductivity
ActiveCN1590302AImprove conductivityVolume resistivity is stableZinc oxides/hydroxidesIndiumGas phase
The invention relates to a preparation method for preparing nano-scale oxidized zinc powder with high conductivity. The method simultaneously drip mixed salt solution of zincic soluble salt and doping elements such as aluminum, gallium, indium, Yt, scandium, tin, germanium, silicon, as well as precipitating agent into water, to generate coprecipitation to generate doped zinc bloom precursor basic zinc carbonate in condition of controlling temperature and PH value of entire reaction system, and at last, calcining the product in mixed gas atmospheres of hydrogen gas and argon gas, doped superfine zinc bloom conductive powder material can be obtained. The powder material prepared by the invention has small particle-size, uniform grain fineness distribution, and which mean particle diameter is about 10 to 80 nanometer. The electric volume resistivity of the powder can reach 2.5*10^-3 omega.cm, thus its electro conductivity is better than the sample prepared by plasma method and gas-phase method in current market. The preparation method further enhances whiteness degree and conductivity of the oxidized zinc powder, and further reduces the cost.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Spinel nickel manganese acid lithium and layered lithium-rich manganese-based composite cathode material with core-shell structure and preparation method thereof
ActiveCN104157831APromote circulationRaise the ratioCell electrodesSecondary cellsComposite cathodeMaterial synthesis
The invention relates to a spinel nickel manganese acid lithium and layered lithium-rich manganese-based composite cathode material with a core-shell structure and a preparation method thereof, which belongs to the technical field of material synthesis. The prepared lithium ion composite cathode material takes a layered lithium-rich manganese-based Li[Lia(NixCoyMnz)]O2 as a core material, takes spinel nickel manganese acid lithium LiNi0.5Mn1.5O4 as a shell material; a coprecipitation method is employed to obtain a core-shell precursor, the core-shell precursor and the lithium source are uniformly mixed and calcined to obtain the spinel nickel manganese acid lithium and layered lithium-rich manganese-based composite cathode material with the core-shell structure. According to the invention, the layered lithium-rich manganese-based is taken as the core material, and the spinel nickel manganese acid lithium is taken as the shell material; under the prerequisite that material gram capacity is kept, material structural stability is increased, material cycle, multiplying power and safety performances are improved, function composite and complementation of the core material and the shell layer material can be realized, and the problem that high capacity and high security can not be achieved simultaneously is solved. The composite cathode material has the advantages of simple process and obviously increased performance.
Owner:南京时拓能源科技有限公司
Lithium ion battery gradient core shell cathode material and synthetic method thereof
ActiveCN103236537AGuaranteed cycle performanceGuaranteed rate performanceCell electrodesNickel compoundsElectrical batteryPhysical chemistry
The invention provides a lithium ion battery gradient core shell cathode material and synthetic method thereof, and relates to a lithium ion battery cathode material and synthetic method thereof. The lithium ion battery gradient core shell cathode material provided by the present invention may have two kinds of core shell structures as follows: a two-layer structure: a ternary material is used as a core material, and a binary material or a unitary material is casing material, and the ternary material external layer is covered by the binary material or the unitary material; three-layer structure: the ternary material is used as a core material, and the binary material and the unitary material are casing materials, and the ternary material external layer is covered with the binary material, and the binary material is covered with the unitary material. The synthetic method includes: employing a coprecipitation method for obtaining a precursor, and then adding lithium source, calcining and coating to obtain the ternary gradient core shell material. Under the prerequisite that the structure stability of the material is kept, the cost is reduced, and the gram capacity of the material is improved, and the material circulating performance and rate capability of the material are improved, and the safety performance and low temperature performance of the ternary cathode material are increased, and the preparation technology is optimized and improved.
Owner:HARBIN INST OF TECH
Composite catalyst flue gas denitrating under low-temperature condition and method of preparing the same
InactiveCN101254464ALarge specific surface areaImprove thermal stabilityDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsManganeseCerium
The invention relates to a composite catalyst used for denitrification of flue gases under low-temperature conditions. The catalyst contains a Ti / Zr composite oxide as carrier, an oxide of manganese as catalytically-active component, and at least one oxide of vanadium, chromium, iron, copper, nickel and cerium as auxiliary agent. The Ti / Zr composite oxide is prepared by coprecipitation method and loads the catalytically-active component and the auxiliary agent by immersion method, wherein the mole ratio of Ti / Zr in the composite oxide is controlled in a range from 0.25 to 4, the oxide of manganese accounts for 1-15% of the weight of the Ti / Zr composite oxide, and the auxiliary agent accounts for 1-5% of the weight of the Ti / Zr composite oxide. The catalyst has good catalytic activity at low temperature and high NO conversion rate up to 100% at 100-300 DEG C, and has good prospect in industrial application.
Owner:NANJING UNIV OF TECH
Nano Au catalyst for eliminating formaldehyde at room temperature and preparation method thereof
InactiveCN101612578AGood resistance to water vapor poisoningImprove stabilityDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsWater vaporRoom temperature
The invention relates to a loading type nano Au catalyst for eliminating formaldehyde in a room, a car and a microenvironment and a preparation method thereof. The corresponding nano Au catalyst is prepared mainly by adopting a coprecipitation method, a depositing precipitation method, an improved soaking method, and the like, taking metallic oxide, composite carrier oxide MOx / Al2O3, composite carrier oxide MOx / CeO2, glass, honeycomb ceramics and a metal mesh as matrixes and taking metallic oxide MOx as a second carrier. Compared with the prior art, the invention has the advantages that (1) the effect of eliminating the formaldehyde by 100 percent at room temperature can be achieved; (2) the nano Au catalyst has good steam poisoning resistance; (3) the CO is catalyzed to be completely converted into CO2; (4) the nano Au catalyst has the favorable active stability and the service life; and (5) the nano Au catalyst can be made into a molded catalyst to be applied in various occasions.
Owner:NO 63971 TROOPS PLA
Active material for lithium secondary battery, lithium secondary battery, and method for producing the same
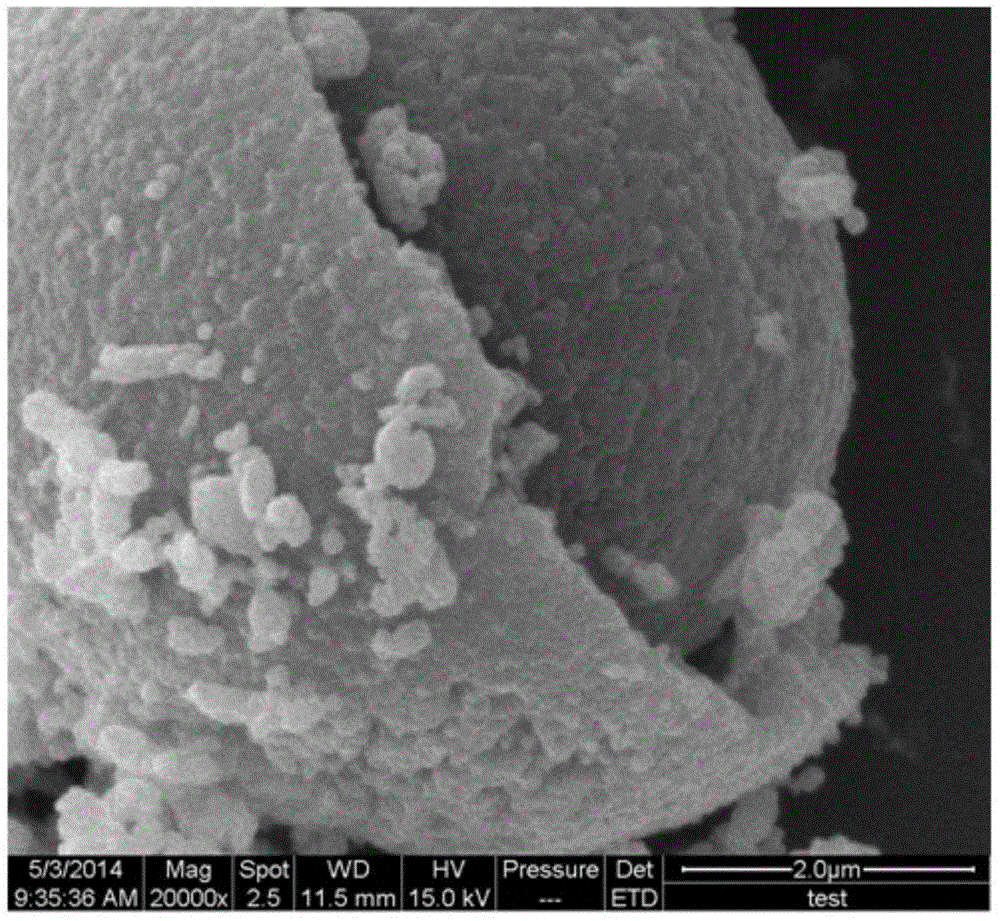
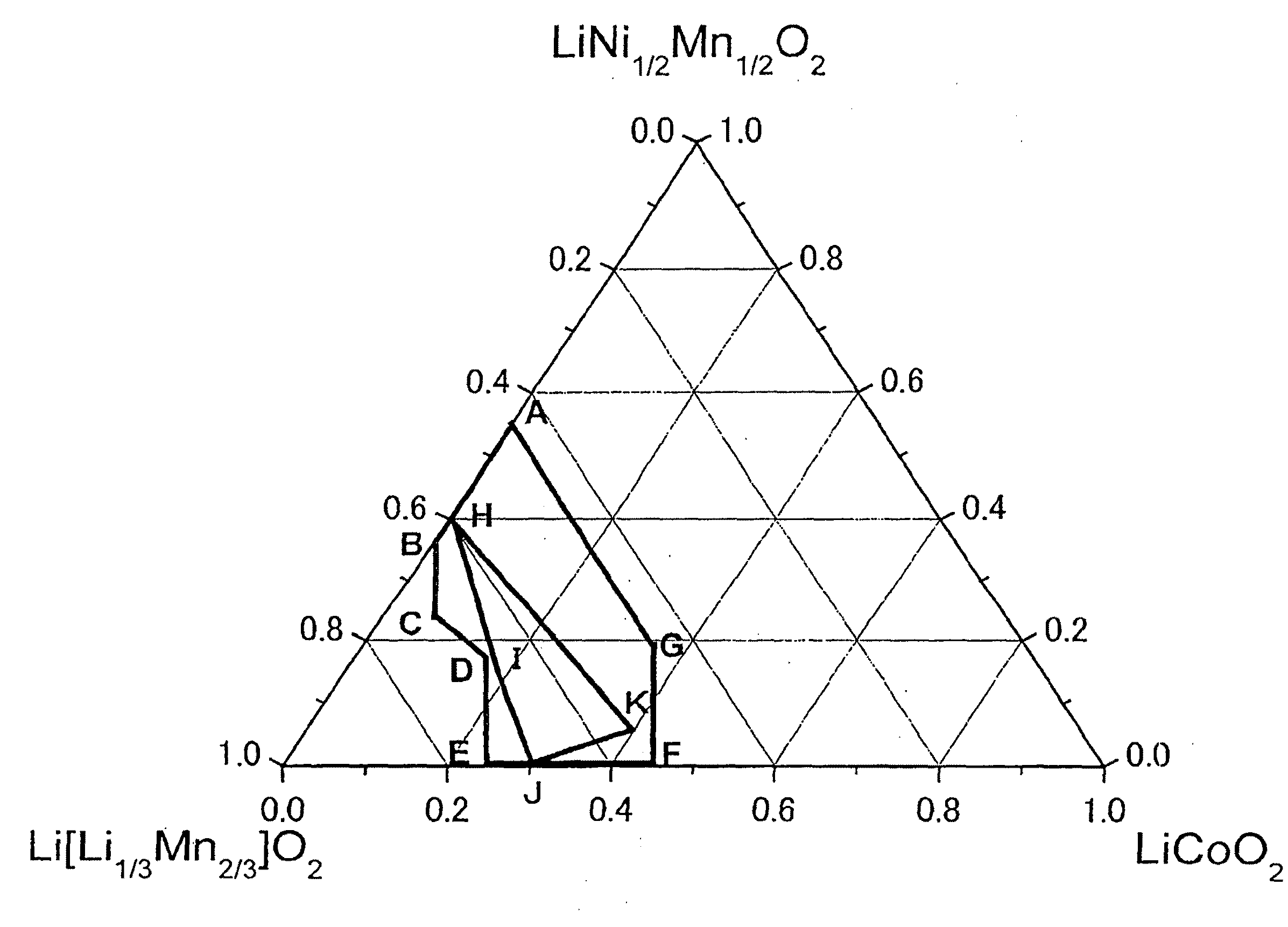
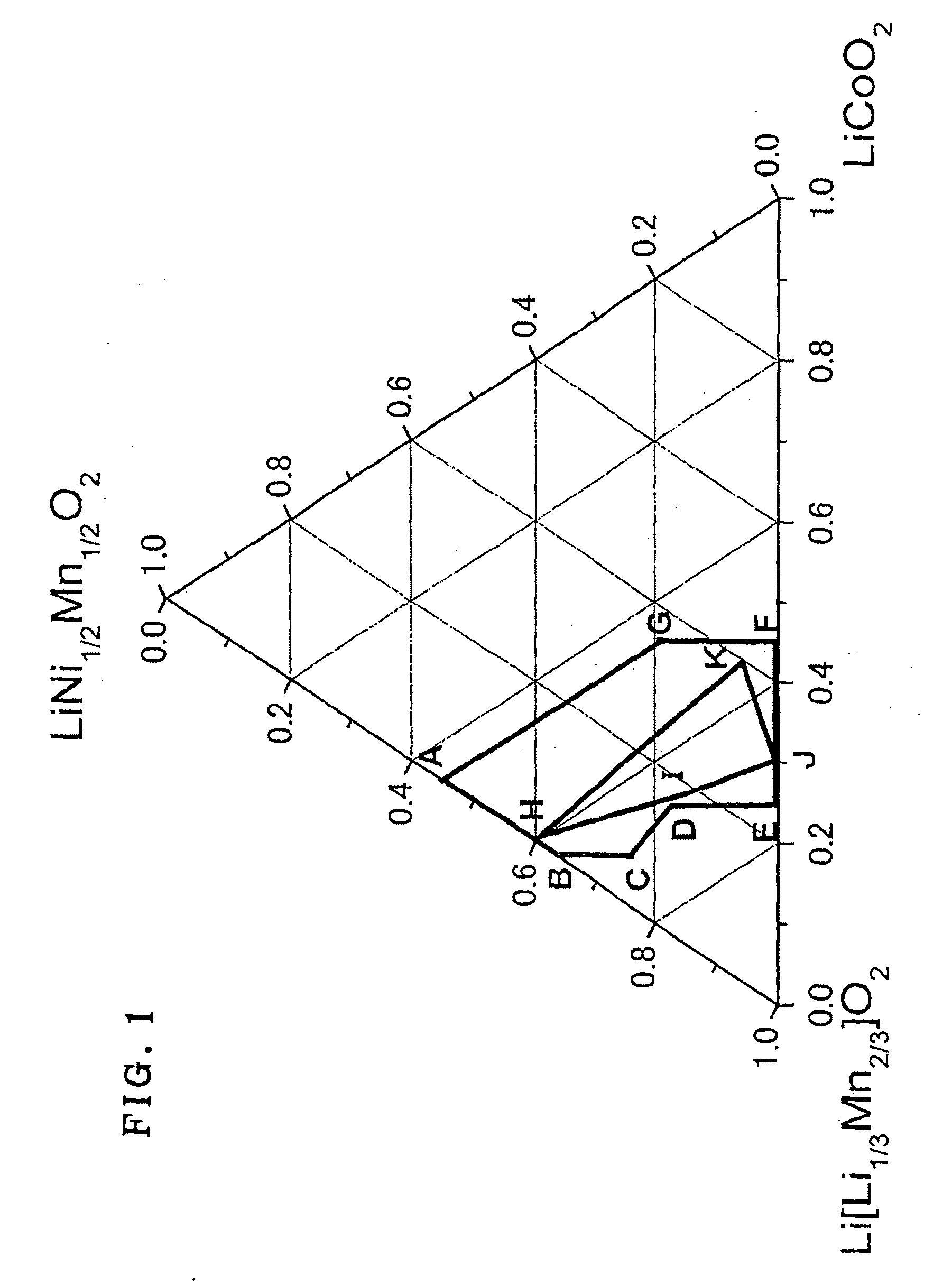
ActiveUS20100233542A1Decrease in battery performanceSufficient discharge capacityBatteries circuit arrangementsElectrode manufacturing processesX-rayCrystal structure
The present invention provides an active material for a lithium secondary battery with a high discharge capacity, particularly for a lithium secondary battery that can increase the discharge capacity in a potential region of 4.3 V or lower, a method for producing the same, a lithium secondary battery having a high discharge capacity, and a method for producing the same. The active material for a lithium secondary battery includes a solid solution of a lithium transition metal composite oxide having an α-NaFeO2 type crystal structure, in which the composition ratio of Li, Co, Ni, and Mn contained in the solid solution satisfies Li1+(1 / 3)xCo1−x−yNi(1 / 2)yMn(2 / 3)x+(1 / 2)y (x+y≦1, 0≦y and 1−x−y=z); in an Li[Li1 / 3Mn2 / 3]O2(x)-LiNi1 / 2Mn1 / 2O2(y)-LiCoO2(z) type ternary phase diagram, (x, y, z) is represented by values in a range present on or within a line of a heptagon (ABCDEFG) defined by the vertexes; point A(0.45, 0.55, 0), point B(0.63, 0.37, 0), point C(0.7, 0.25, 0.05), point D(0.67, 0.18, 0.15), point E(0.75, 0, 0.25), point F(0.55, 0, 0.45), and point G(0.45, 0.2, 0.35); and the intensity ratio between the diffraction peaks on (003) plane and (104) plane measured by X-ray diffractometry before charge-discharge is I(003) / I(104)≧1.56 and at the end of discharge is I(003) / I(104)>1. The invention provides a method for producing the active material for a lithium secondary battery using a coprecipitation method, a lithium secondary battery including a positive electrode containing the active material and a method for producing the lithium secondary battery.
Owner:GS YUASA INT LTD
Catalyst for preparing ethylene diamine through amination of ethylene glycol and preparation method thereof
InactiveCN102233272AImprove performanceOrganic compound preparationCatalyst activation/preparationEthylenediamineManganese
The invention provides a solid catalyst for preparing ethylene diamine through catalytic amination of ethylene glycol. The catalyst takes copper and nickel as main components and zirconium as an auxiliary component; and other metal components can be one or more than one of zinc, aluminum, titanium, manganese and cerium. The invention further provides a preparation method of the solid catalyst. The preparation method is characterized in that the catalyst is prepared through the processes of roasting, reducing and re-roasting by utilizing a coprecipitation method.
Owner:ZHANGJIAGANG HUIER CHEM TECH
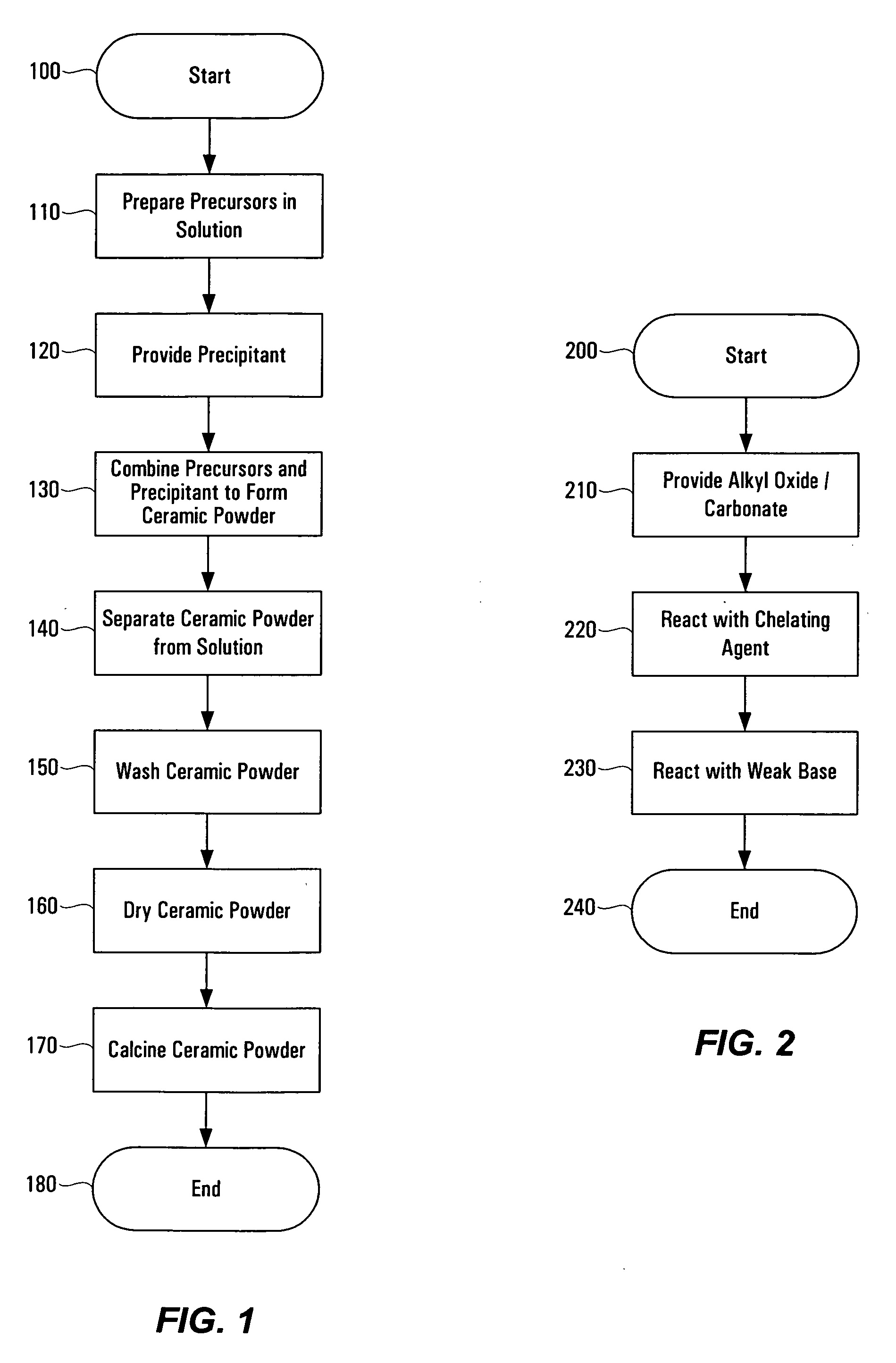
Method of preparing ceramic powders using chelate precursors
InactiveUS20070148065A1Oxide/hydroxide preparationStacked capacitorsBarium titanateAmmonium hydroxide
Wet-chemical methods involving the use of water-soluble hydrolytically stable metal-ion chelate precursors and the use of a nonmetal-ion-containing strong base can be used in a coprecipitation procedure for the preparation of ceramic powders. Examples of the precipitants used include tetraalkylammonium hydroxides. A composition-modified barium titanate is one of the ceramic powders that can be produced. Certain metal-ion chelates can be prepared from 2-hydroxypropanoic acid and ammonium hydroxide.
Owner:EESTOR
Cerium copper titanium composite oxide catalyst for flue gas denitration, preparation method and using method
ActiveCN101954281AAdapt to a wide temperature windowActiveDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsFlue gasCerium
The invention discloses a cerium copper titanium composite oxide catalyst for flue gas denitration and a preparation method. The catalyst comprises the following components: CeO2, CuO and TiO2. The catalyst is prepared by a coprecipitation method and can promote the generation of an ideal denitration effect. The catalyst has the advantages that: 1) the catalyst has the high activity for the catalytic reduction of nitric oxides in flue gas; 2) the adaptive temperature window of the catalyst is wide, namely the catalyst has certain activity within the integral temperature range from 150 to 450 DEG C; 3) the operating performance of the catalyst under the condition of SO2 and H2O can be improved effectively, and the catalyst has the strong adaptivity to severe working conditions in the presence of sulfur dioxide, water and the like and has the wide application range; 4) raw materials for preparation are easy to obtain, a preparation process is simple and the cost of the catalyst is low.
Owner:ZHEJIANG UNIV
High-density small-particle-size nickel-cobalt-manganese hydroxide and preparing method thereof
The invention relates to a high-density small-particle-size nickel-cobalt-manganese hydroxide and a preparing method thereof. The nickel-cobalt-manganese hydroxide and the preparing method thereof are characterized in that: the general chemical formula of the nickel-cobalt-manganese hydroxide is NixCoyMnz(OH)2, wherein the sum of the x, the y and the z is 1, the x is not more than 0.8 and not less than 0.3, the y is not more than 0.4 and not less than 0.1, and the z is not more than 0.4 and not less than 0.1. The nickel-cobalt-manganese hydroxide is provided, so that problems of small-particle-size nickel-cobalt-manganese hydroxides prepared by methods at present, namely nonuniform element distribution, poor particle appearance, loose surfaces, difficult particle size control, nonuniformity, low tap density, and the like are overcome. A complexing control crystallization coprecipitation method is adopted. By a special technical process of producing nucleuses, growing, and subjecting particles to continuous frictional collision under a continuously increased solid liquid ratio, an aqueous solution of a nickel-cobalt-manganese soluble salt and an aqueous sodium hydroxide solution are subjected to coprecipitation under complexing of ammonia to obtain the small-particle-size nickel-cobalt-manganese hydroxide having characteristics of uniform element distribution, good degree of sphericity, uniform particle size distribution and high tap density.
Owner:宁夏中色金辉新能源有限公司
Method for preparing corrosion inhibition anion intercalated layered double hydroxides/oxide composite material and application
InactiveCN101418154AFacilitated releaseImprove anti-corrosion performanceAnti-corrosive paintsEpoxy resin coatingsOxide compositeReaction temperature
The invention provides a corrosion-inhibiting anionic intercalated hydrotalcite / nano oxide composite material, a preparation method thereof and application of the corrosion-inhibiting anionic intercalated hydrotalcite / nano oxide composite material. The preparation method is as follows: corrosion-inhibiting anions are directly inserted between hydrotalcite layers by the one-step coprecipitation method and the roasting restoring method; the nano oxide is generated by means of in situ synchronization during the process of generation of hydrotalcite crystals by controlling the mixture ratio of divalent metal ions to trivalent metal ions, the pH value of a reaction solution and the reaction temperature; and the corrosion-inhibiting anionic intercalated hydrotalcite / nano oxide composite material with the anti-corrosive function is prepared. The invention is mainly characterized in that the in situ preparation method for the hydrotalcite / oxide composite material provided with a nano lamellar structure improves the release amount of a corrosion inhibitor and reduces the water absorption of a coating by leading into the corrosion inhibitor with the anti-corrosive function and the nano oxide which is generated in situ during the reaction process. The composite material can be used to be pigment of an anti-corrosive metallic coating system, and particularly has potential application value in improving the anti-corrosive performance of a magnesium alloy anti-corrosive coating.
Owner:HARBIN ENG UNIV
Supported noble metal catalyst and preparation and application thereof
ActiveCN104923225AGood dispersionEnhanced strong interactionHydrocarbon from carbon oxidesDispersed particle separationMethanationHigh activity
The invention relates to a preparation method and a catalytic application of a supported noble metal catalyst. Active components are noble metals Ru, Rh and Pd, and a carrier is MxOy or perovskite MAlO3 or spinel MAl2O4. The composite oxide carrier is prepared by an impregnation method or a coprecipitation method and is roasted under a medium-high temperature condition of 650-1200 DEG C ultimately, so that while the formed MxOy, MAlO3 or MAl2O4 or even a mixture thereof has a closer effect with an Al2O3 carrier, the number of defects and holes on the surface of Al2O3 is greatly increased, and thus prepared noble metal nanoparticles have high dispersion degree and strong stability. The catalyst preparation method has the advantages of simple operation, convenience for production and application, good product repeatability, high activity in a carbon dioxide methanation reaction and a carbon monoxide oxidation reaction, strong stability in a storage state and reaction, and quite good application prospects.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Superparamagnetism mesoporous silicon dioxide composite ball and preparing method thereof
InactiveCN101256864ALarge specific surface areaHigh content of magnetic substancesSilicaInorganic material magnetismParticulatesMesoporous silica
A superparamagnetism mesoporous silicon dioxide composite ball of the present invention and manufacture method thereof belong to nucleocapsid type technology field of magnetic nano particle. The shape of the composite ball is global; inner core being made of magnetic ferrite nano particle cluster, shell coating layer being made of mesoporous silicon dioxide; quality ratio of magnetic ferrite nano particle in particulate being 40-80%. Method of manufacturing has that producing magnetic ferrite nano particle is by using coprecipitation method; executing surface modification by adding oleic acid stirring; ultrasonic forming oil-in-water emulsion in the mix solution of ethyl orthosilicate and cyclohexane, cetyl trimethyl ammonium bromide as surfactant; ethyl orthosilicate hydrolytic condensation forming mesoporous silicon dioxide coating layer by adding ammonia spirit; finally taking off molding plate and getting products. Product of the invention has bigger specific surface area and stronger magnetic separation capacity, and has good dispersancy in water, and is further functionalization after being decorated at surface. The method of the present invention has simple process, and lower equipment requirement.
Owner:JILIN UNIV
Acid-resistant magnetic chitosan microspheres as well as preparation method and application thereof
InactiveCN104437395AImprove acid resistanceHigh amino contentOther chemical processesWater contaminantsMicrosphereHydrolysis
The invention relates to an acid-resistant magnetic chitosan microsphere adsorbent as well as a preparation method and the application thereof, and in particular relates to acid-resistant magnetic chitosan microspheres which have the excellent absorption performance and recycling performance for acid waste water containing hexavalent chromium, a preparation method of the acid-resistant magnetic chitosan microspheres and the application of the acid-resistant magnetic chitosan microspheres. The preparation method of the acid-resistant magnetic chitosan microspheres comprises the following steps: (1) preparing Fe3O4 nano-particles modified by citric acid by using a coprecipitation method; (2) preparing single-coated magnetic SiO2 nano-particles by using a sodium silicate hydrolysis method or a sol-gel method; and (3) preparing the chitosan microspheres coated with magnetic SiO2 by taking chitosan powder as a raw material by adopting an emulsion crosslinking method. Furthermore, in order to further improve the adsorbing capacity of the microspheres, in the method, dendritic polyethylene imine is taken as a functional group for modifying the adsorbent, so that the modified chitosan microspheres can be obtained. The magnetic chitosan microspheres prepared by adopting the method not only have the excellent acid resistance, but also have the good absorption performance on Cr (VI) ions.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Catalyst for synthesizing low carbon mellow with synthesis gas and preparation method thereof
ActiveCN101185895AHigh conversion rate of COHigh selectivityOrganic compound preparationHydroxy compound preparationIsobutanolPropanol
The invention discloses a catalyst for synthesizing low carbon mixed alcohol with synthetic gas and a preparation method. The main components of the catalyst are copper oxide, zinc oxide, chromium oxide, alumina, and an appropriate amount of other assistants, which are expressed by the general formula Cu(Zn)a(Cr)b(Al)c(M)d(A)e(O)x; the catalyst is prepared by coprecipitation, and the assistant is added by impregnation. Under appropriate conditions, the catalyst can lead CO and hydrogen to react to produce a mixture of oxidation compounds which comprises methanol, ethanol, propanol, isobutanol, pentanol, and a small amount of C5.
Owner:SINOPEC NANJING RES INST OF CHEM IND CO LTD
Method for manufacturing magnetic loading type nanometer catalyst TiO2/Fe2O4
InactiveCN101816937ALow costImprove photocatalytic activityWater/sewage treatment by irradiationMetal/metal-oxides/metal-hydroxide catalystsSulfateWastewater
The invention relates to a method for manufacturing a magnetic loading type nanometer catalyst TiO2 / Fe2O4. The method comprises the following steps of: using ferrous sulfate, ferric sulfate and ammonia as raw materials to prepare black nanometer Fe3O4 particles with good dispersivity through a coprecipitation process; coating one layer of TiO2 with optical catalyst on the Fe3O4 surface by a sol-gel process, and forming a TiO2 / Fe3O4 composite material with a core-shell structure; carrying out heat treatment and recycling to obtain a magnetic loading type nanometer catalyst TiO2 / Fe2O4. The catalyst not only has good optical catalytic activity, but also can utilize the outer magnetic field to realize convenient and quick recovering and reutilization, thereby decreasing the cost of waste water treatment and realizing the practical application of nanometer TiO2 in wastewater treatment.
Owner:LANZHOU UNIVERSITY OF TECHNOLOGY
Method for preparing catalyst for acrolein and acrylic acid
InactiveCN101690900AOrganic compound preparationCatalyst activation/preparationTrace elementActive component
The invention discloses a method for preparing a catalyst for acrolein and acrylic acid, which is characterized in that: the catalyst consists of an active component supporter and an inert alumina carrier; the main composition elements of the active component are selected from Mo, Bi, Co and / or Ni and Fe, and the active component also contains trace elements such as K, Na, Rb, Cs, Mg, Ca, Zn, B, P and W; the active component supported on the carrier accounts for 5 to 70 percent of the total weight of the catalyst; and the active component supporter is not prepared by coprecipitation, instead, initial raw materials of the elements of the above expression formula containing the active component are precipitated in aqua ammonia of ammonium molybdate step by step to prepare an active component precursor with core-shell distribution, and the active component precursor is dried and baked to prepare the active component.
Owner:CHINA NAT OFFSHORE OIL CORP +1
Method for preparing magnetic nanometer ferroferric oxide-graphene composite catalyst
ActiveCN102553593AReduce usageNo pollution in the processWater contaminantsMetal/metal-oxides/metal-hydroxide catalystsMagnetic separationGraphite oxide
The invention relates to a method for preparing a magnetic nanometer ferroferric oxide-graphene composite catalyst, which includes steps of: 1) dispersing graphite oxide in ethanol and water system; 2) respectively mixing soluble trivalent ferric salt and divalent ferric salt in ethanol; 3) mixing and stirring systems obtained in step 1) and step 2); 4) heating reaction system of 3) at temperature of 50 DEG C to 90 DEG C, and adjusting pH to be 9-11 for reaction by adding ammonia water; and 5) performing magnetic separation on products of step 4), washing through deionized water, and obtaining magnetic nanometer ferroferric oxide-graphene composite catalyst. The method provides a simple and practical coprecipitation method for preparing the nanometer ferroferric oxide-graphene composite catalyst which is uniform in particular sizes, even in dispersing and good in magnetism. The nanometer ferroferric oxide-graphene composite catalyst prepared by the method has excellent catalytic activity and is easy in preparing process and environment-friendly.
Owner:HUAWEI TEHCHNOLOGIES CO LTD
Method for preparing nickel-cobalt lithium aluminate as anode material of lithium ion battery
InactiveCN103094546AImprove electrical performanceImprove cycle performanceCell electrodesLithium aluminateOxygen
The invention discloses a method for preparing nickel-cobalt lithium aluminate as an anode material of a lithium ion battery. The method comprises the following steps: (1) mixing a nickel-cobalt metal salt water solution, a sodium metaaluminate solution, a complexing agent and a precipitant, regulating the pH value of a reaction system to be 9 to 12, and then maintaining a stirring state to carrying out a reaction at the temperature of 30 to 80 DEG C for 20 to 200 hours, thereby obtaining nickel-cobalt aluminum hydroxide precipitates; (2) washing the nickel-cobalt aluminum hydroxide precipitates by using pure water of 50 to 100 DEG C, drying, screening the part of precipitates capable of passing through a sieve being 300 meshes, adding a lithium source to the precipitates, mixing evenly, and sintering at the temperature of 600 to 1000 DEG C, wherein oxygen is filled during the sintering process; and finally sintering for 5 to 50 hours, thereby obtaining the nickel-cobalt lithium aluminate. According to the method, the sodium metaaluminate is adopted as the lithium source, so that the nickel-cobalt aluminum elements can evenly form a coprecipitation, so that the aluminum is evenly distributed in the nickel-cobalt lithium aluminate material. As a result, the electrical performance of the material is improved, and especially the cycling performance of the material is improved.
Owner:HUNAN BRUNP RECYCLING TECH +1
Method for preparing hydrogenation catalyst composite
ActiveCN101722007AOvercome preparation difficultiesOvercome stabilityMetal/metal-oxides/metal-hydroxide catalystsRefining to eliminate hetero atomsCoprecipitationHigh activity
The invention discloses a method for preparing a hydrogenation catalyst composite, comprising the following steps of: (1) preparing a NixWyOz composite oxide precursor by using a coprecipitation method; (2) pulping, mixing and filtering the NixWyOz composite oxide precursor and MoO3; and (3) forming and activating the NixWyOz composite oxide precursor and the MoO3 to form a final catalyst, wherein the process of manufacturing the NixWyOz composite oxide precursor comprises the steps of: preparing acid solution A containing an active metal Ni component according to a component content proportion of the catalyst; preparing solution B containing an active metal W component according to the component content proportion of the catalyst; merging the solution A and the solution B, and adding the mixture of the solution A and the solution B into a reaction tank to gelatinize; and generating a deposited mixture to obtain the precursor containing the composite oxide NixWyOz. The catalyst prepared by the method has the advantages of uniform dispersion of metals, high use performance, and particularly higher activity for deep impurity removal process of hydrocarbon. The method is simple and convenient, and has low metal loss. The method is mainly used for preparing a bulk phase catalyst with higher metal content.
Owner:CHINA PETROLEUM & CHEM CORP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com