Zinc ferrite catalysts, method of preparing thereof and method of preparing 1,3-butadiene using thereof
一种丁二烯、催化剂的技术,应用在制备1,制备该铁酸锌催化剂领域,能够解决催化剂难商业化、改变催化剂活性、产率降低等问题,达到简单结构和合成过程、提高经济效益、高度可重现性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
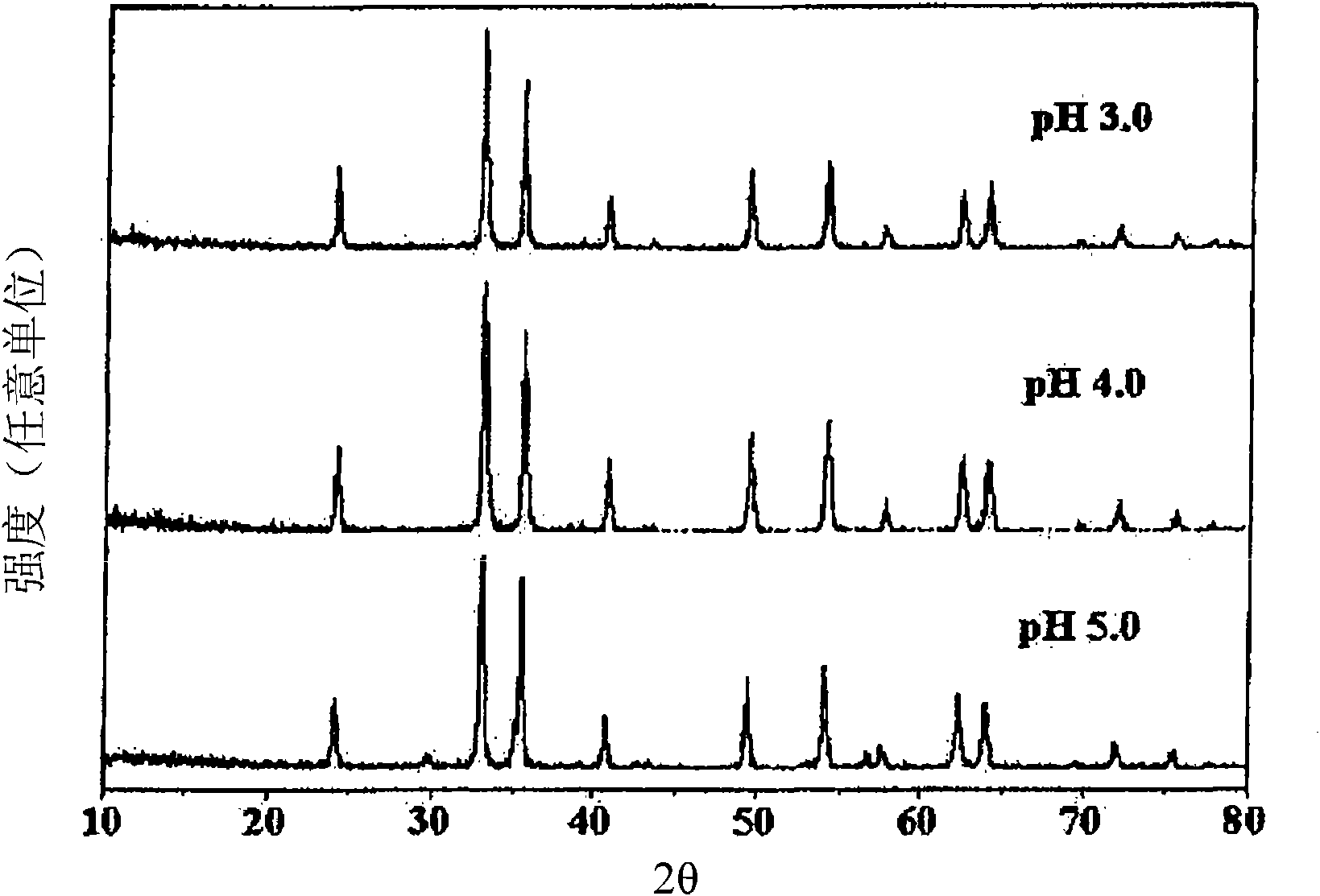
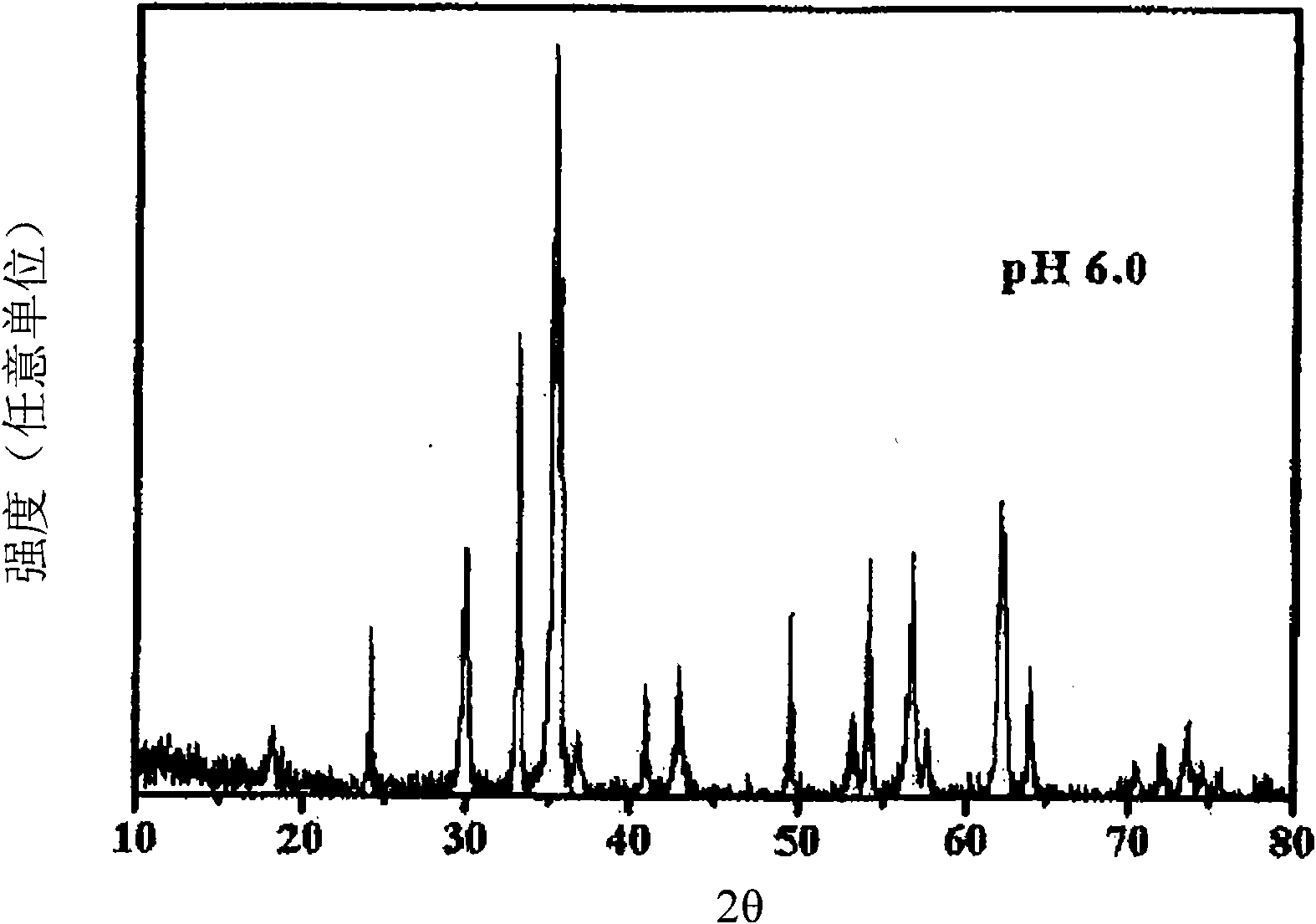
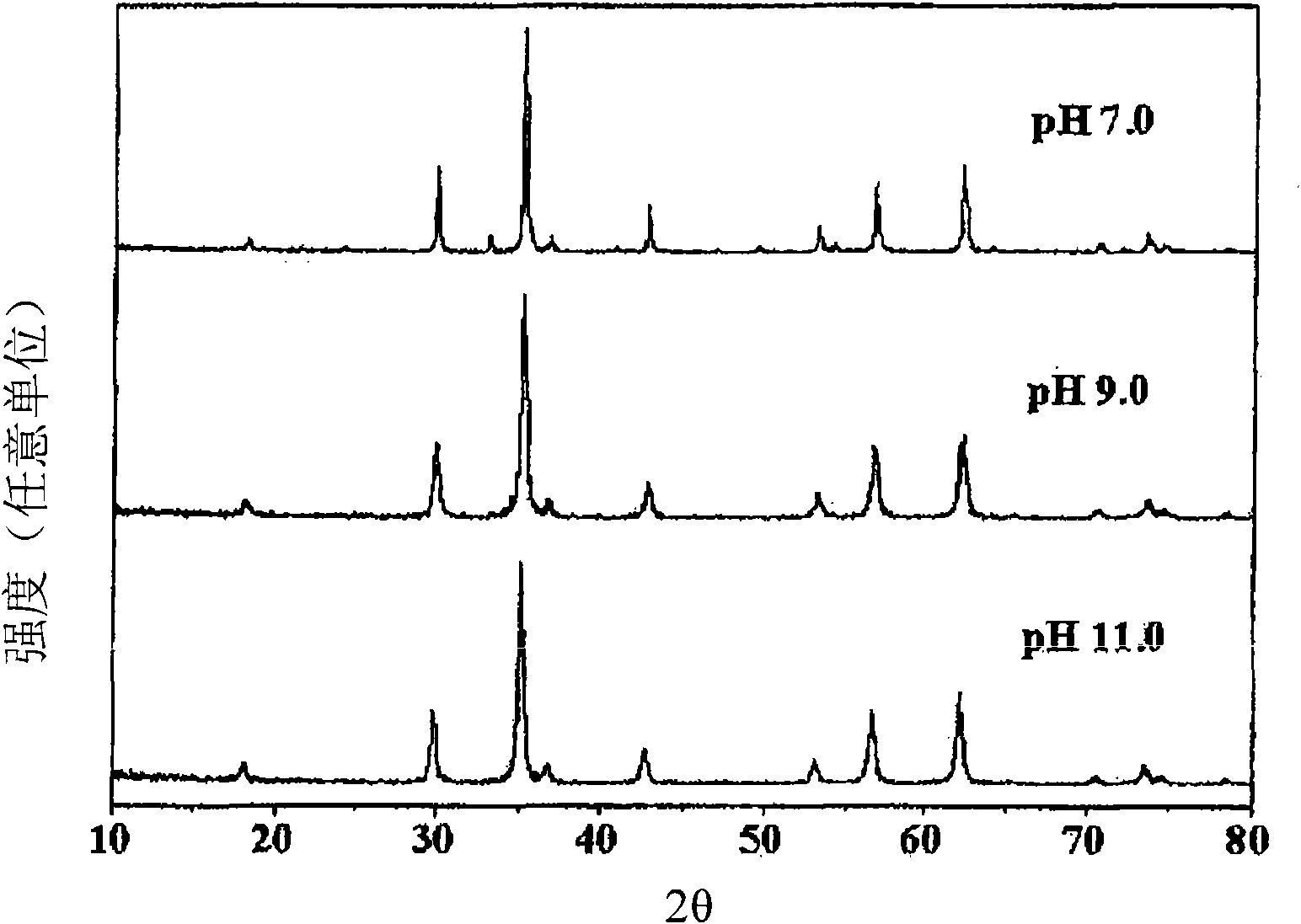
[0034] According to Preparation Example 1 of the present invention, the phase characteristics of the co-precipitated catalysts at different pHs were compared by X-ray diffraction analysis, and it was found that the characteristics of the catalyst lattice changed with the pH of the co-precipitated solution. Specifically, it was found that the catalysts coprecipitated at pH 3–5, mainly formed α-iron(III) oxide (α-Fe 2 o 3 )(III), instead of zinc ferrite, this α-iron(III) oxide is known to have low selectivity to 1,3-butadiene due to complete oxidation in the oxidative dehydrogenation of n-butene, so This catalyst is not suitable as a catalyst for the present invention. In contrast, it was found that the catalyst coprecipitated at pH 6, mainly formed zinc ferrite, and the catalyst coprecipitated at pH 7-12, formed single-phase zinc ferrite (see Figure 1 to Figure 3 ).
[0035] Therefore, preferably, the catalyst for preparing 1,3-butadiene of the present invention is a single...
preparation Embodiment 1
[0045] Identification of precursors and solvents for the production of zinc ferrite catalysts
[0046] Zinc chloride (ZnCl 2 ) as the zinc precursor, ferric chloride hexahydrate (FeCl 3 ·6H 2 O) as an iron precursor. Both the zinc precursor and the iron precursor are easily soluble in distilled water, the zinc precursor and the iron precursor are respectively dissolved in distilled water, and then mixed with each other to form a precursor aqueous solution. A predetermined amount of distilled water is provided as a co-precipitation medium, and then the precursor aqueous solution is added to the distilled water, and at the same time, a predetermined amount of sodium hydroxide aqueous solution is added to adjust the pH, thereby forming a mixed solution. At this time, in order to precisely adjust the pH, the aqueous precursor solution and the aqueous sodium hydroxide solution were slowly added dropwise to the distilled water. In order to obtain a sample with a uniform compos...
Embodiment 1
[0051] Oxidative Dehydrogenation of C4-Raffinate-3 Mixture or C4 Mixture over Zinc Ferrite Catalyst
[0052] Using the zinc ferrite catalyst produced in Preparation Example 1, the oxidative dehydrogenation reaction of n-butene was carried out under the following experimental conditions.
[0053] In the present invention, a C4 mixture whose composition is shown in Table 1 was used as a reactant in the oxidative dehydrogenation reaction of n-butene. The C4 mixture as a reactant was introduced into a reactor in the form of a mixed gas together with air and steam, and a straight-type Pyrex glass fixed-bed reactor was used as the reactor. The composition ratio of the reactants was set based on the amount of n-butene in the C4 mixture, and the ratio was set so that the mixing ratio of n-butene:air:steam was 1:3.75:15. The steam formed by the vaporization of liquid phase water is mixed with other reactants (for example: C4 mixture and air) and then introduced into the reactor. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com