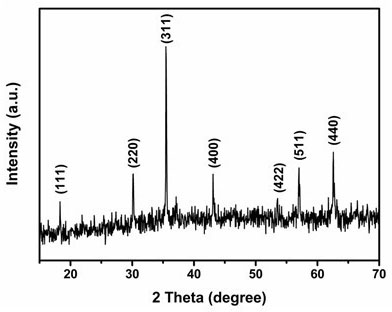
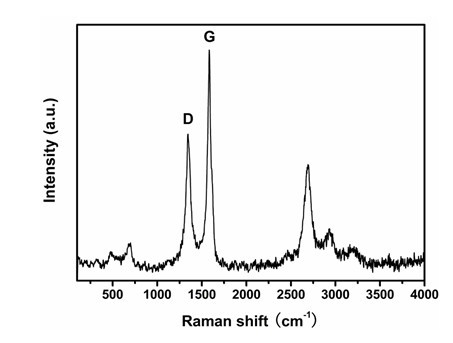
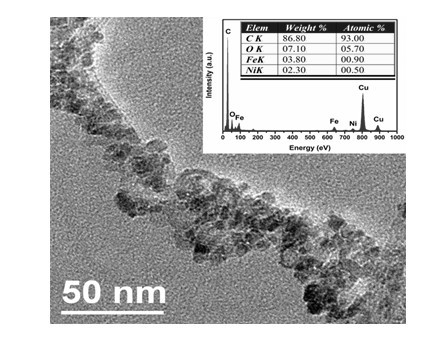
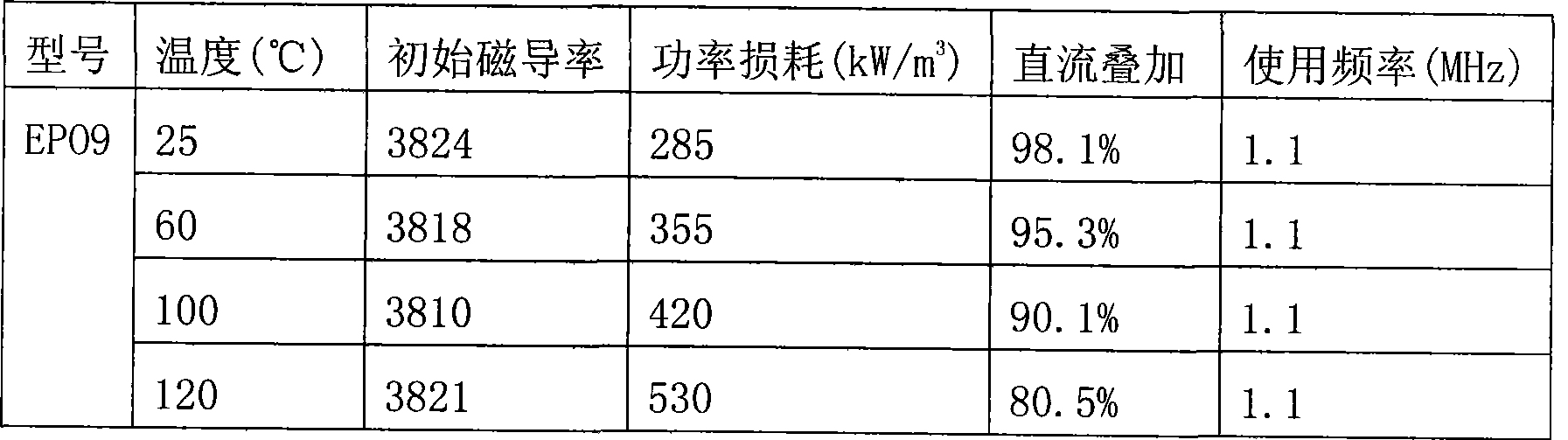
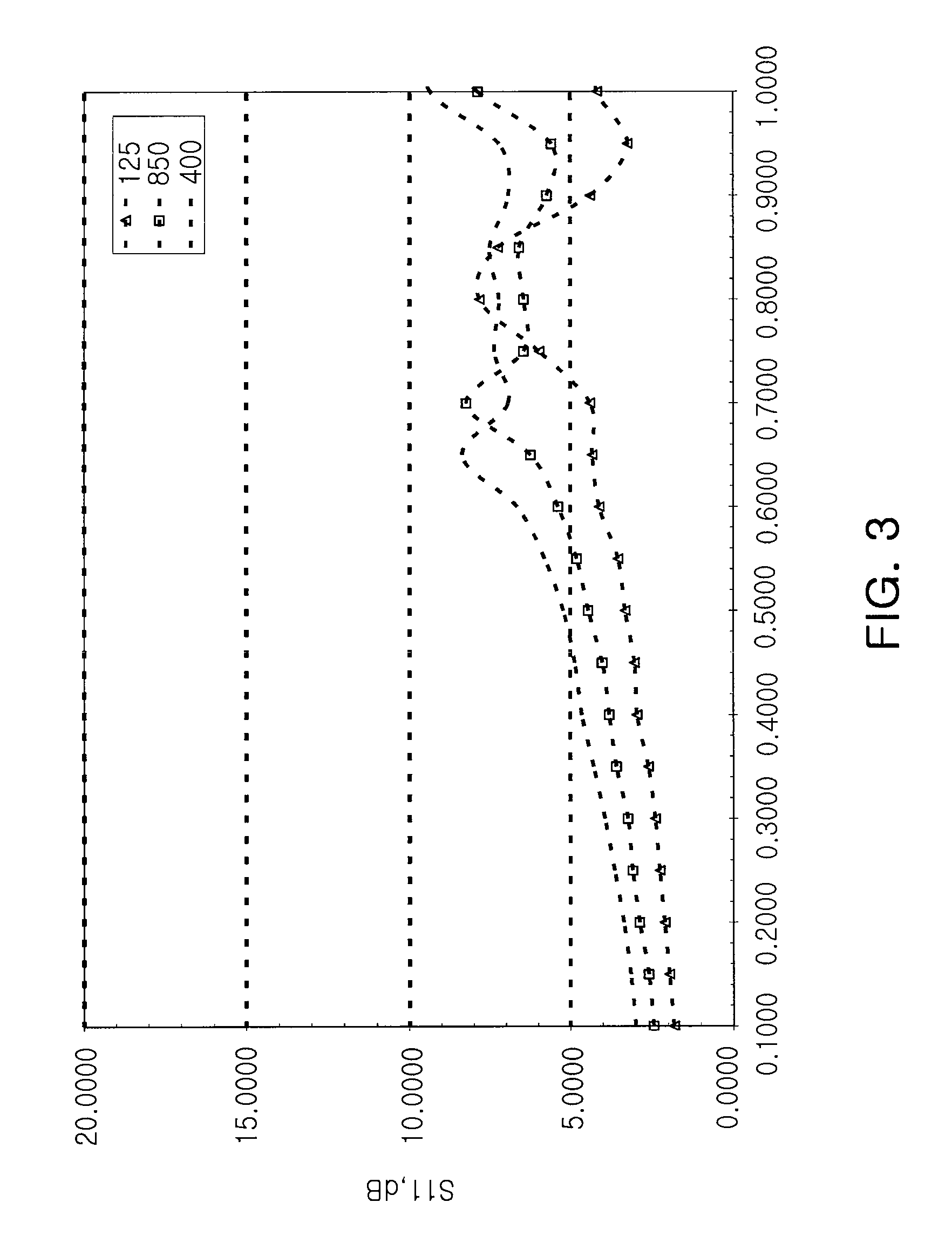
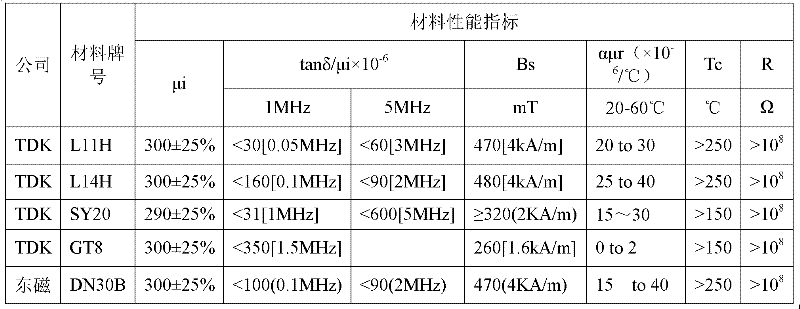
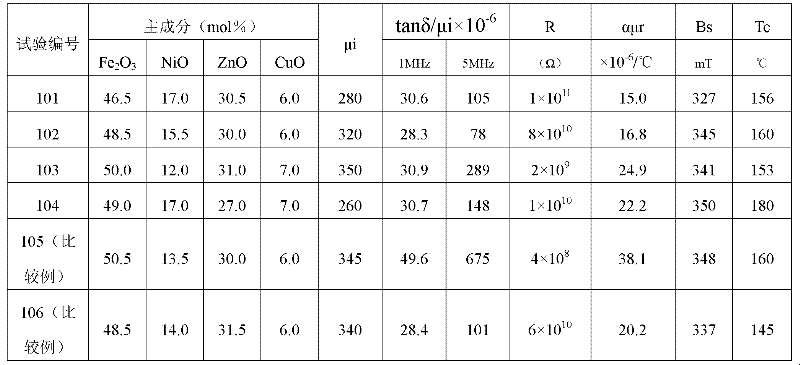
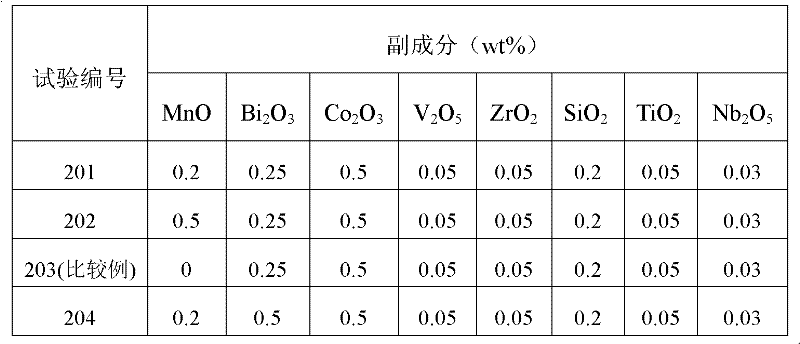
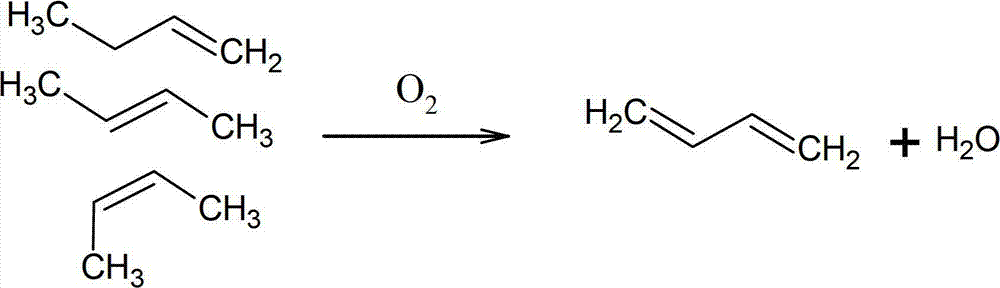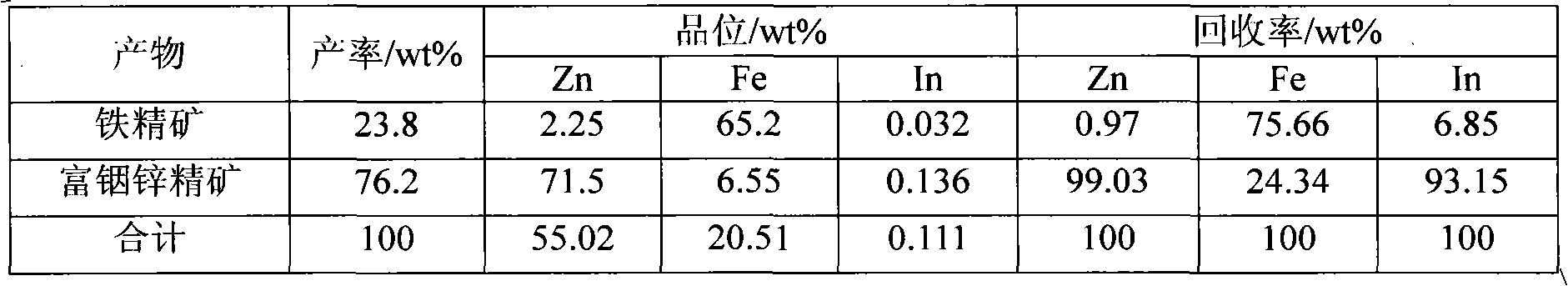Patents
Literature
770 results about "Zinc ferrite" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Zinc ferrites are a series of synthetic inorganic compounds of zinc and iron (ferrite) with the general formula of ZnₓFe₃₋ₓO₄. Zinc ferrite compounds can be prepared by aging solutions of Zn(NO₃)₂, Fe(NO₃)₃, and triethanolamine in the presence and in the absence of hydrazine, or reacting iron oxides and zinc oxide at high temperature. Spinel (Zn, Fe) Fe₂O₄ appears as a tan-colored solid that is insoluble in water, acids, or diluted alkali. Because of their high opacity, zinc ferrites can be used as pigments, especially in applications requiring heat stability. For example, zinc ferrite prepared from yellow iron oxide can be used as a substitute for applications in temperatures above 350 °F (177 °C). When added to high corrosion-resistant coatings, the corrosion protection increases with an increase in the concentration of zinc ferrite. A recent investigation shows that the zinc ferrite, which is paramagnetic in the bulk form, becomes ferrimagnetic in nanocrystalline thin film format. A large room temperature magnetization and narrow ferromagnetic resonance linewidth have been achieved by controlling thin films growth conditions.
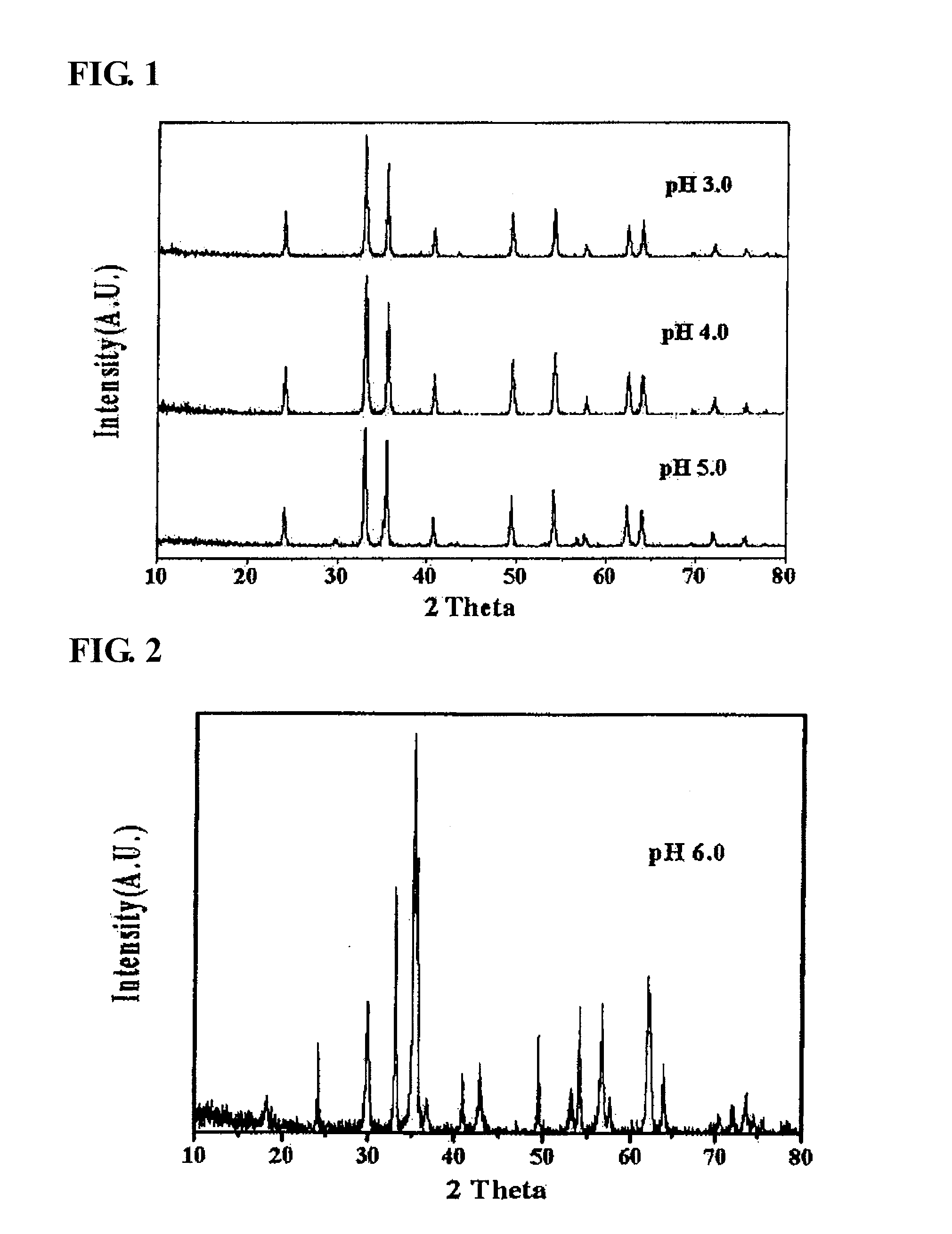
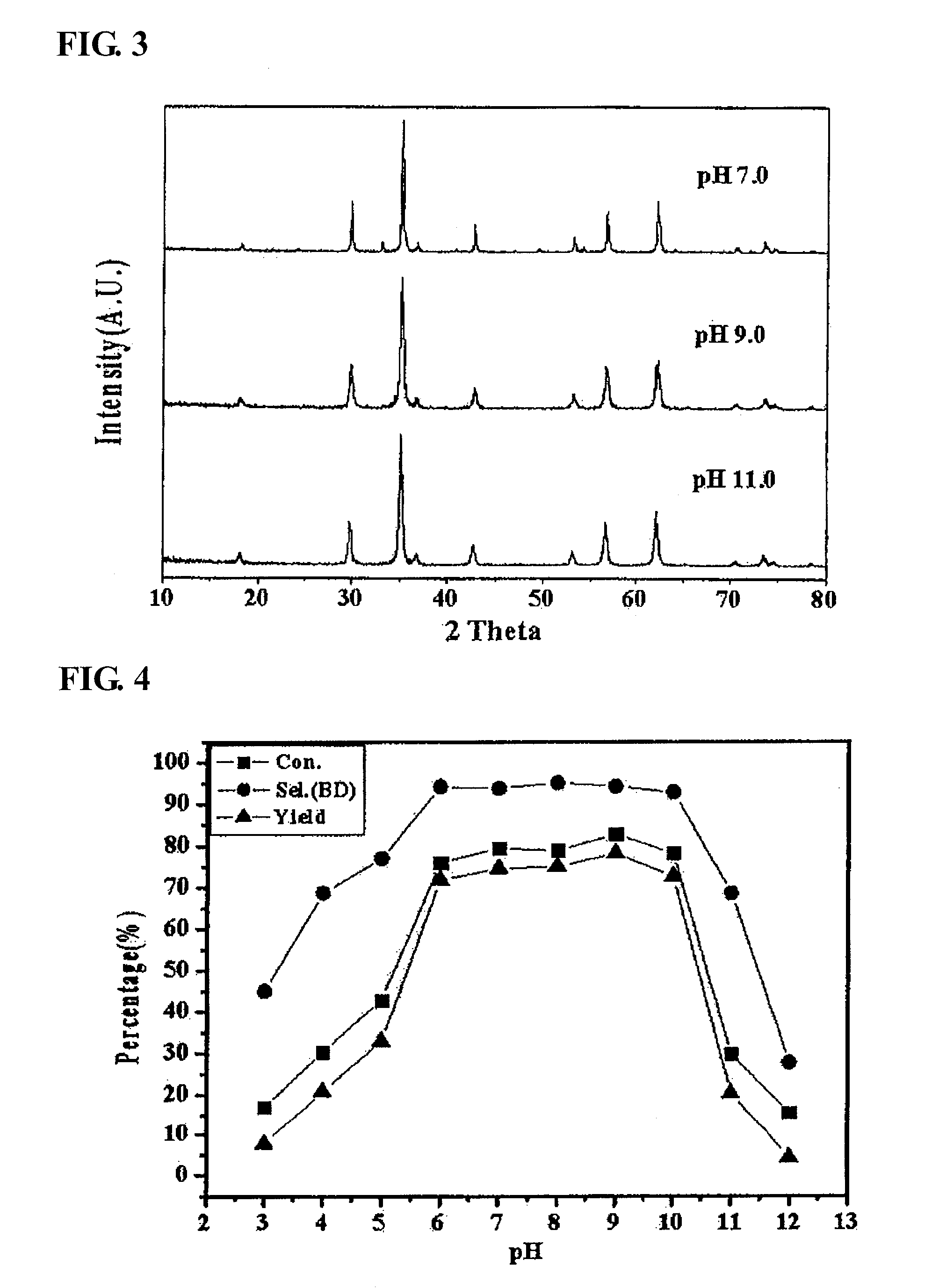
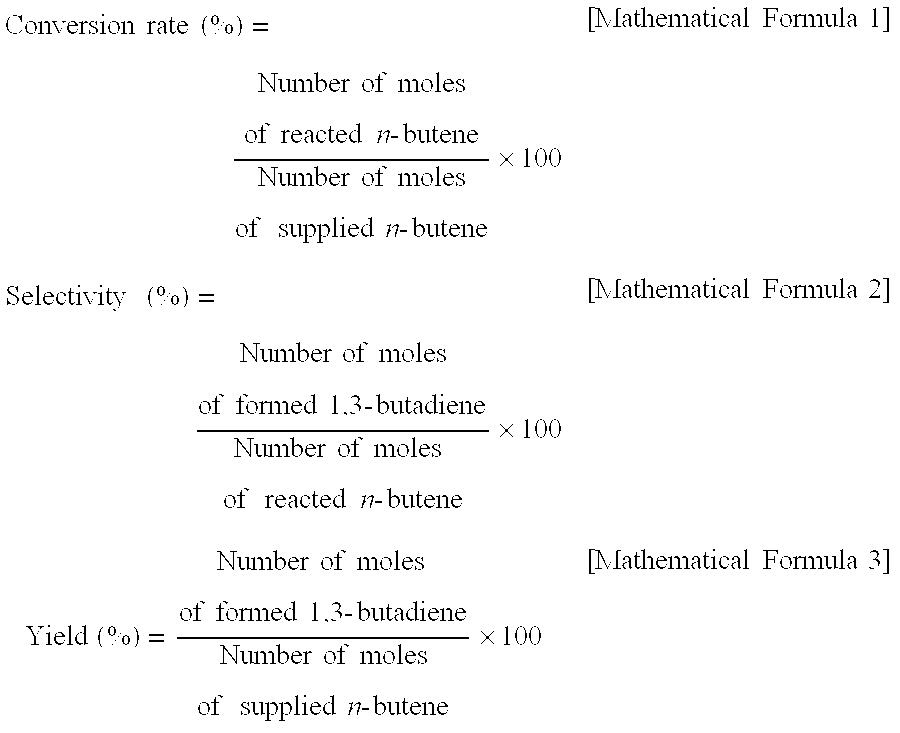
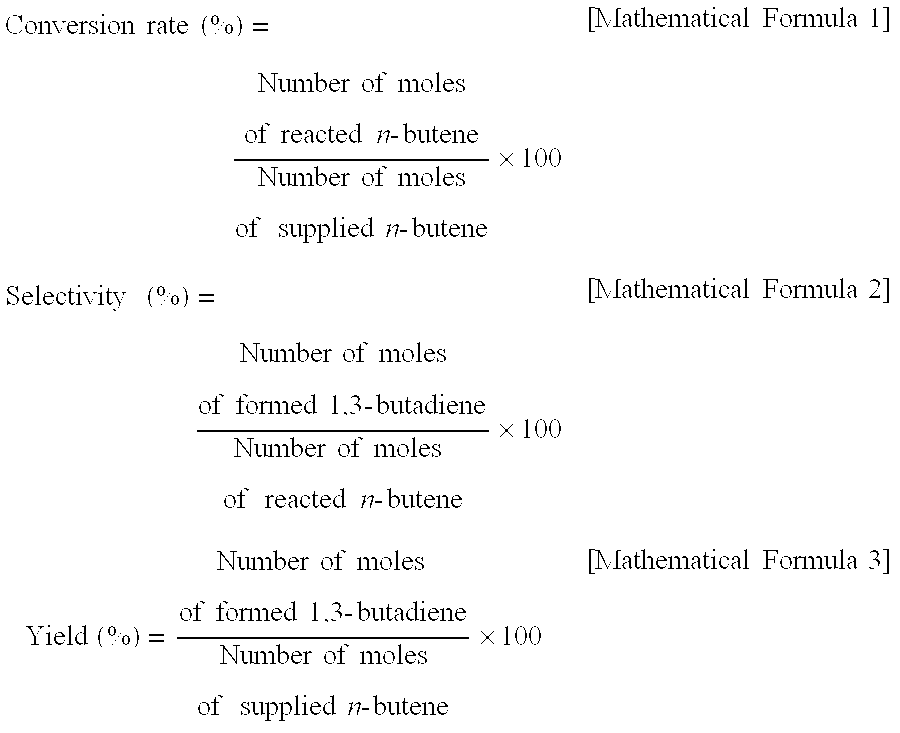
Zinc ferrite catalysts, method of preparing thereof and method of preparing 1,3-butadiene using thereof
ActiveCN101674883ASimple structureEasy to synthesizeHeterogenous catalyst chemical elementsCatalystsButeneDehydrogenation
Owner:SK INNOVATION CO LTD +1
Catalyst and method used for preparing 1,3-butadiene by oxidative dehydrogenation of n-butene
ActiveCN102824914AHigh catalytic activityImprove catalytic selectivityHydrocarbonsMetal/metal-oxides/metal-hydroxide catalystsMagnesium saltDehydrogenation
The invention discloses a catalyst and a method used for preparing 1,3-butadiene by oxidative dehydrogenation of n-butene. The catalyst is a cobalt and magnesium modified zinc ferrite catalyst which is obtained by proportioning a ferric salt, a zinc salt, a cobalt salt, a magnesium salt and a deionized water in a mole ratio, regulating pH value with ammonia water, concentrating, filtering, drying, roasting, cooling, grinding and screening. The method for preparing 1,3-butadiene by utilizing the catalyst comprises the following steps of: with C4 fraction produced by MTO (methanol to olefin) as a raw material, carrying out catalytic oxidative dehydrogenation reaction on a reaction mixture which is formed by the C4 fraction, air and vapour under the action of the cobalt and magnesium modified zinc ferrite catalyst so as to efficiently prepare1,3-butadiene, wherein the main ingredient of the C4 fraction is n-butene. The method disclosed by the invention has the advantage that the C4 fraction is not required to be refined to remove impurities such as oxygenated chemicals, thus the method disclosed by the invention is a simple and efficient method for preparing a high-additional-value product by utilizing C4 resource of the MTO.
Owner:SHAANXI COAL & CHEM TECH INST
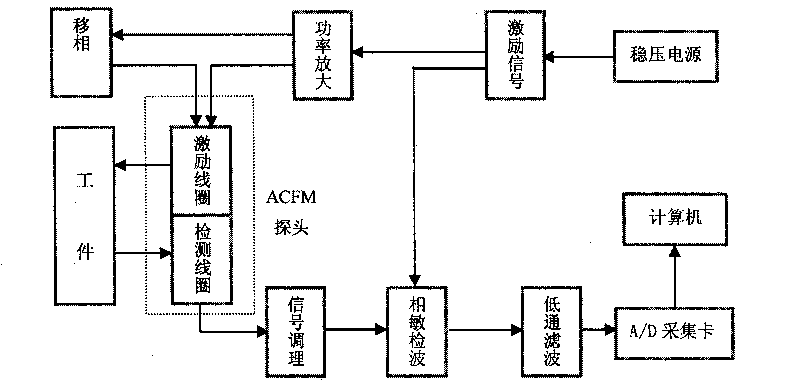
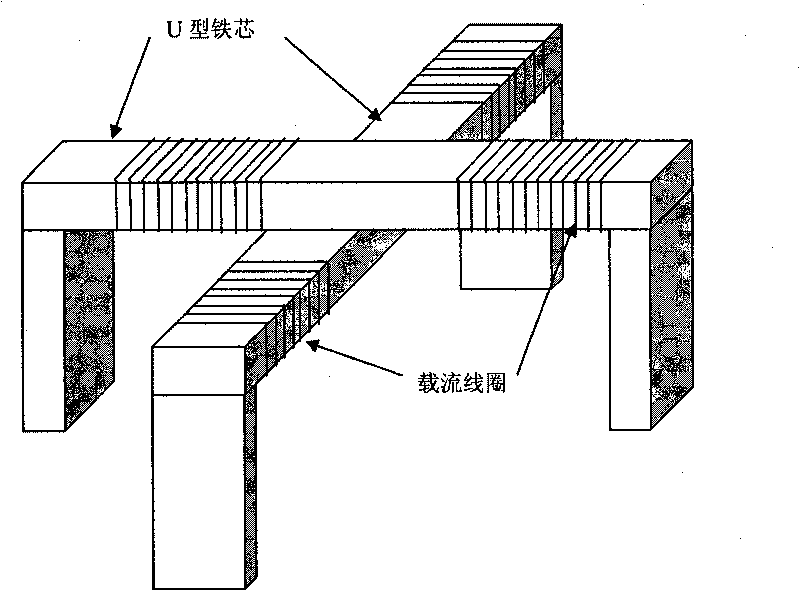
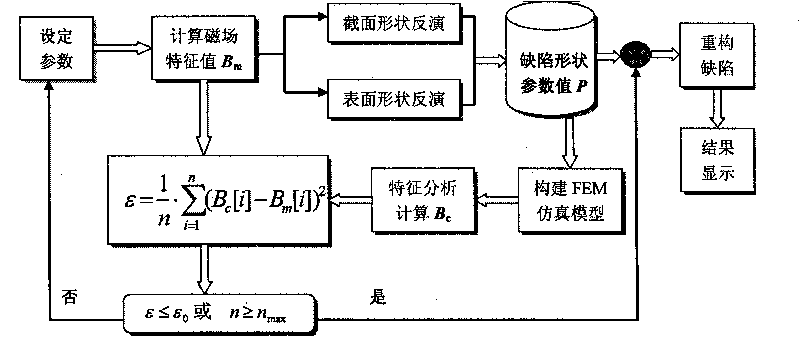
ACFM intelligent visual defect detection system
InactiveCN101701934AImprove intelligenceHigh degree of visualizationElectric/magnetic contours/curvatures measurementsUsing electrical meansThree dimensional shapeCorrelation analysis
The invention discloses an intelligent visual defect detection system based on an AC magnetic field. The system comprises a probe, an auxiliary circuit and system detection software and uses a defect quantification and shape reconstruction algorithm. An excitation part of the probe adopts a double-U-shaped structural design and consists of a mangan zinc ferrite iron core and two sets of orthogonal current-carrying coils, a detection part adopts a two-dimensional array structure, and the array probe adopts a lifting-releasing type traveling mode. The hardware processing of detection signals comprises signal conditionings such as amplification, filtering, and the like and also introduces an original excitation signal as a reference signal to carry out phase sensitive detection and low-pass filtering on the detection signals. The signals are sent into a computer through an A / D acquisition card, then the detection software is used for carrying out digital filtering and correlation analysis on the signals to draw a magnetic induction intensity curve and a butterfly diagram in real time, and size quantification and three-dimensional shape inversion are realized through the defect quantification and the shape reconstruction algorithm. The intelligent visual defect detection system gives a full play of the advantages of the computer and greatly improves the intellectualized and visual levels of AC magnetic field detection.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
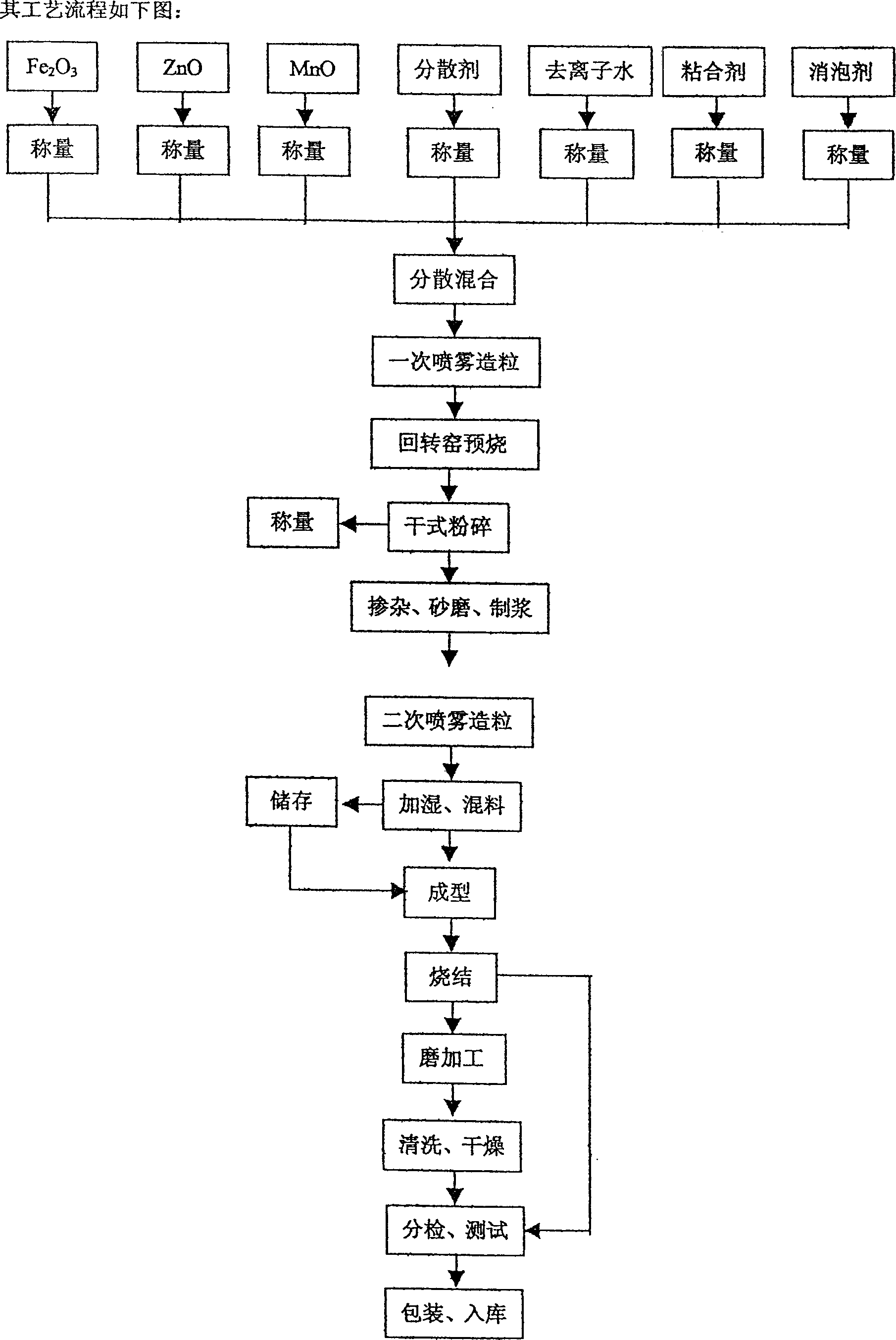
Magnanese-zinc ferrite magnetic core
InactiveCN1402266AConvenient sourceSimple preparation processInorganic material magnetismTransformers/inductances magnetic coresIron oxideSilicon dioxide
A MnZn ferrite core is prepared from Fe2O3 (52-55 mol%), ZnO (7-12 mol%), MnO (36-38 mol%), nano CaO (0.01-0.5 wt.%), and SiO2 (0.004-0.03 wt.%) through sintering at 1150-1300 deg.C for 0.5-5 hr. Its advantages are high high-frequency performance, higher saturated magnetic flux density and low magnetic loss.
Owner:无锡晶石磁性电子器件有限公司
High saturated magnetic flux density and low loss manganese-zinc ferrite material and its preparing method
ActiveCN1749209ASimple manufacturing methodLower sintering temperatureInorganic material magnetismVolumetric Mass DensityNanometre
The present invention discloses high saturated magnetic flux density and low loss manganese manganese-zinc ferrite material and its preparation process. The manganese-zinc ferrite material contains: Fe2O3 52-54 mol%, MnO 33-40 mol%, ZnO 8-13 mol%, supplementary components CaCO3 100-600 ppm, SiO2 50-300 ppm and other metal oxides. The preparation process includes the steps of: mixing material, pre-sintering, adding supplementary components, secondary ball milling, forming and sintering. The supplementary components are common oxide grains, and this result in easy adding and low cost. The preparation process of the manganese-zinc ferrite material has relatively low pre-sintering and sintering temperature and relaxed requirement on sintering apparatus, may be completed in common vacuum sintering furnace, and easily realized in industrial production.
Owner:ZHEJIANG UNIV +1
Zinc-ferrite-based nanometer composite as well as preparation method and application thereof
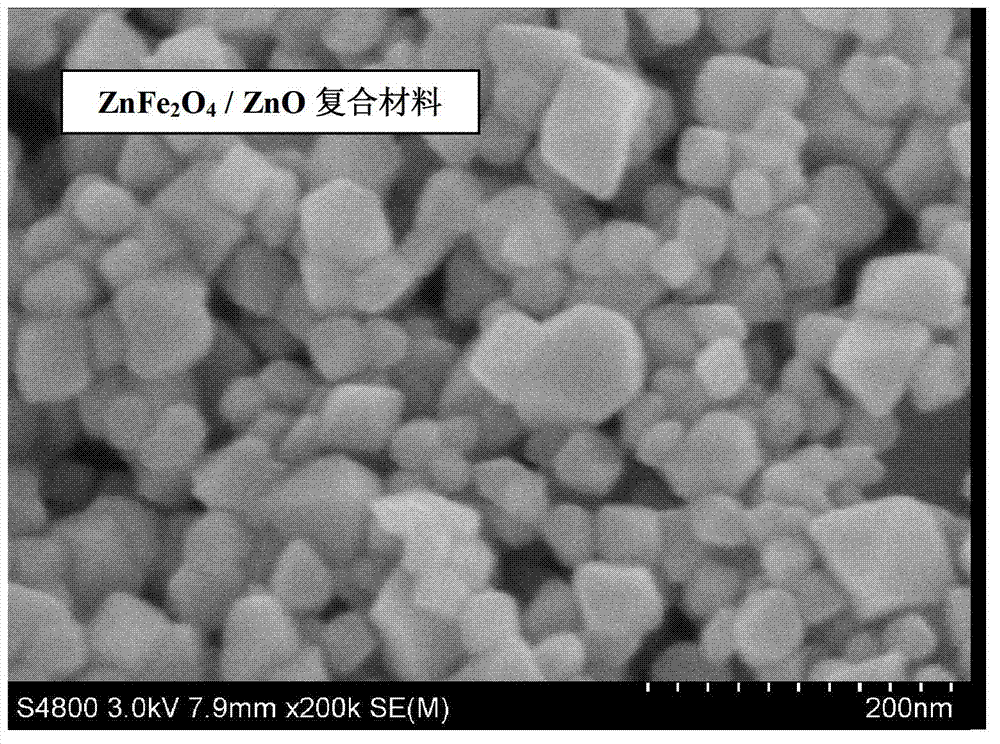
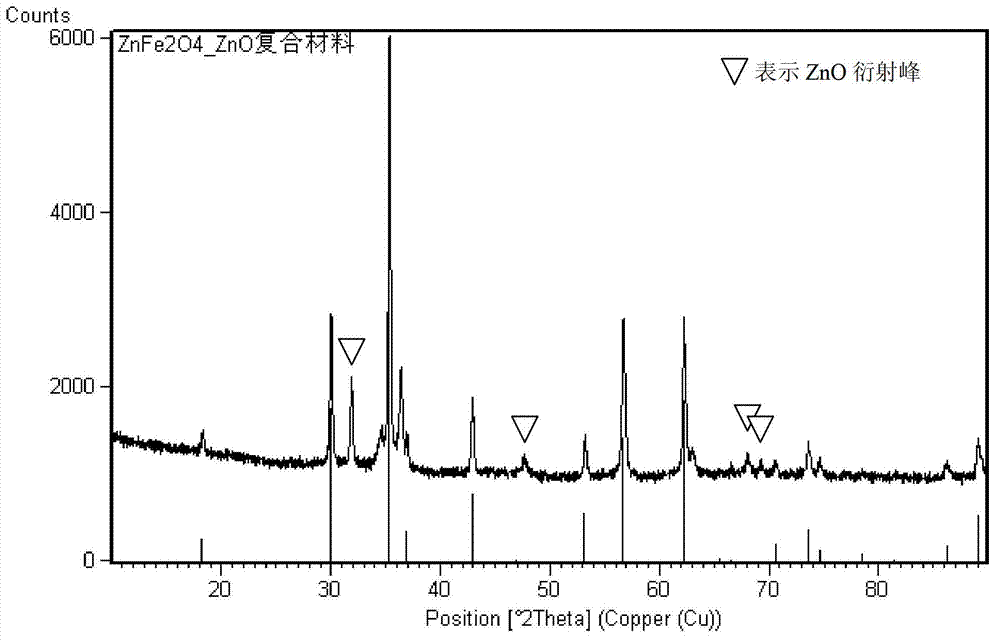
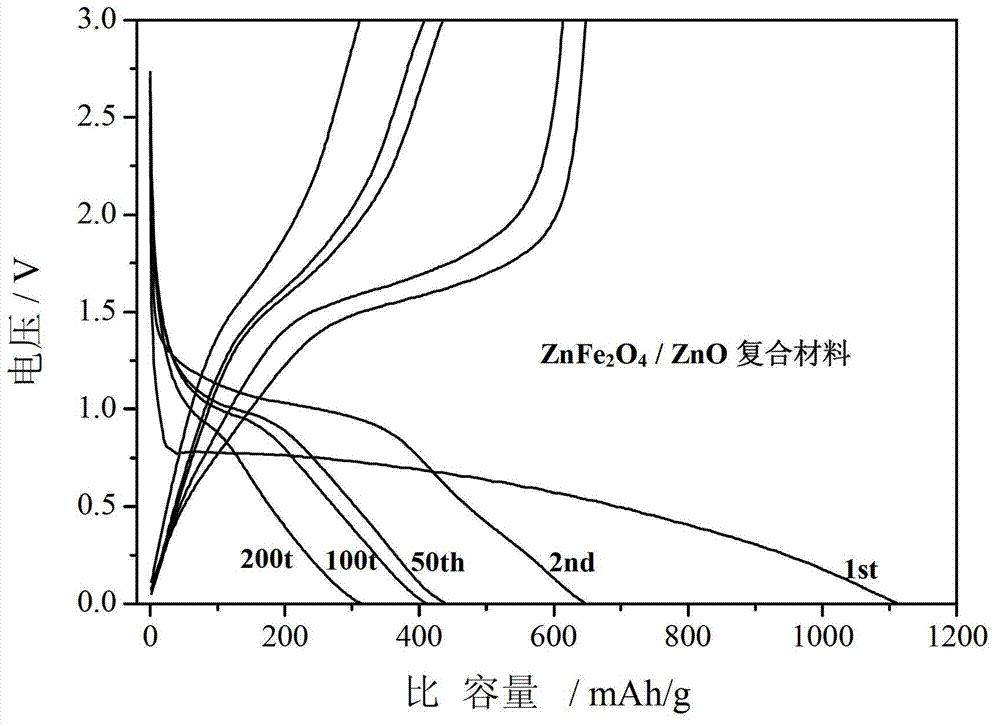
The invention relates to a ZnFe2O4-based nanometer composite. The zinc ferrite-based nanometer composite is a ZnFe2O4 / MO nanometer composite in which MO is a metal oxide. The ZnFe2O4-based nanometer composite comprises secondary particles formed by aggregating ZnFe2O4 nanoparticles with spinel structures and MO nanoparticles; and the metal oxide is ZnO, Fe2O3, CoO, NiO, CuO, MnO, TiO2, CrO3 and / or VO2. Compared with the prior art, the zinc-ferrite-based nanometer composite has the beneficial effects that the ZnFe2O4-based nanometer composite provided by the invention has high capacity and high cycle stability as a negative electrode material of a lithium ion battery, and the electrochemical performance of pure-phase ZnFe2O4 is greatly improved; the particles are uniform in size and good in dispersion; and a preparation method of the composite is simple, a production process is short without severe conditions, the cost is low, and the industrialization is easy.
Owner:BTR NEW MATERIAL GRP CO LTD
Preparation method of nano ferrate/carbon nano tube composite materials
InactiveCN102553595AUniform particle sizeImprove performanceIron compoundsMetal/metal-oxides/metal-hydroxide catalystsManganeseLithium-ion battery
The invention discloses a preparation method of nano ferrate / carbon nano tube composite materials, which utilizes a carbon nano tube as a supporting material and adopts a method of solvothermal synthesis to prepare a series of nano ferrate / carbon nano tube composite materials which are even in dispersion, have magnetic property, include nano cobalt ferrite / carbon nano tubes, ferrous acid nickel / carbon nano tubes, ferrous acid copper / carbon nano tubes, zinc ferrite / carbon nano tubes, ferrous acid manganese / carbon nano tubes and the like, and have certain universality. Compared with the prior art, the preparation method of the nano ferrate / carbon nano tube composite materials utilizes ethanol as solvent. The prepared nano materials are uniform in grain size and excellent in performance and have good application prospect and economic benefits on aspects of photocatalysis sewage treatment, lithium ion batteries and the like.
Owner:NANJING UNIV OF SCI & TECH
Ferrite magnetic core for network communication and method for producing the same
The invention relates to a manganese-zinc ferrite magnetic core for network communication and a method for preparing the same. The magnetic core comprises the following compositions in terms of mol ratio: 51 to 58 mol percent of Fe2O3, 23 to 38 mol percent of MnO, 6 to 14 mol percent of ZnO, 0.01 to 5 mol percent of NiO, and 0.01 to 3 mol percent of CuO. The method for preparing the magnetic core comprises the following steps sequentially: first time of material blending and ball milling, presintering, second time of material blending and ball milling, spraying granulation to obtain granule materials, pressing the materials into biscuits, and biscuit sintering. The sintering is performed in push board kiln in an N2 protective atmosphere, experiences different sintering temperature stages, and finally keeps the temperature for 3 to 8 hours in the N2 atmosphere until the temperature is cooled to room temperature. The magnetic core has the performances of high MU i, high superposition, broad band and low loss. The magnetic core is widely used in the fields of network communication and electronic power, in particular in an ADSL technical separator.
Owner:GUANGDONG FENGHUA ADVANCED TECH HLDG +2
High magnetic conductivity and low loss factor manganese-zinc ferrite material and manufacture process thereof
InactiveCN101905970AImprove permeabilityLow Permeability Temperature FactorLoss factorManganese-zinc ferrite
The invention discloses a high magnetic conductivity and low loss factor manganese-zinc ferrite material and a manufacture process thereof. The high magnetic conductivity and low loss factor manganese-zinc ferrite material mainly comprises the main components in percent by mole (calculated by oxide) of 51-56 mol percent of Fe2O3, 22-29 percent of MnO and 20-25 mol percent of ZnO and the auxiliarymaterials including P2O5, CaO, SiO2, Bi2O3 and the like. The manganese-zinc ferrite material with high magnetic conductivity, low magnetic conductivity temperature factor and low relevant loss factoris prepared by the steps of mixing, pre-roasting, sanding, pelleting, forming and sintering. The invention has the advantages of simple and proper formulation and advanced and reasonable manufacture process. The prepared manganese-zinc ferrite material has property exceeding CL11F-brand property and superior to the H5C2-brand material property of a TDK company in Japan.
Owner:TIANCHANG CITY ZHAOTIAN MAGNETOELECTRICITY TECH
Composition for mobile phone case and method of manufacturing mobile phone case using the same
InactiveUS20100171234A1Low absorption rateReduce lossesCeramic shaping apparatusSolid electrolyte fuel cellsPolytetramethylene terephthalateAcrylic resin
There are provided a composition for mobile phone cases and a method of manufacturing a mobile phone case using the same. The composition comprises 70 to 97% by weight of a thermoplastic resin selected from the group consisting of polycarbonate (PC), acrylonitrile-butadiene-styrene (ABS), polybutyrene terephtalate (PBT), acrylic resin and combinations thereof; and 3 to 30% by weight of ferrite selected from the group consisting of nickel-zinc ferrite, manganese-magnesium ferrite, manganese-zinc ferrite, copper-zinc ferrite, manganese-magnesium-aluminum ferrite, yttrium iron garnet (YIG) ferrite and combinations thereof, wherein the composition is a pellet resin formed by extruding the thermoplastic resin and the ferrite at a high temperature of 160 to 290° C.
Owner:SAMSUNG ELECTRO MECHANICS CO LTD
Soft-magnetic nickel-copper-zinc ferrite material and preparation method thereof
The invention belongs to the field of soft magnets, and particularly provides a soft-magnetic nickel-copper-zinc ferrite material which comprises major components and minor components, wherein the major components comprise the following components in percentage by mol: 46.5-50.0 mol% of Fe2O3, 12.0-17.0 mol% of NiO, 27.0-31.0 mol% of ZnO and 4.0-8.0 mol% of CuO; and the minor components comprise manganese oxide and bismuth oxide, wherein on the basis of the total weight of the major components, the total weight percent of the minor components and standard substances thereof MnO and Bi2O3 is 0.1-1.0 wt%. Under the wide frequency of 50 KHz-10 MHz and especially high frequency of 1 MHz-10 MHz, the material provided by the invention has the characteristics of high magnetic conductivity (mu i is approximately equal to 300, and f is equal to 1 MHz), high resistivity, low specific loss, low specific temperature coefficient and the like.
Owner:HENGDIAN GRP DMEGC MAGNETICS CO LTD
Zinc ferrite catalysts, method of preparing thereof and method of preparing 1,3-butadiene using thereof
ActiveUS20100121123A1Simple structureSimple synthesis procedureHydrocarbon by hydrogenationHeterogenous catalyst chemical elementsButeneDehydrogenation
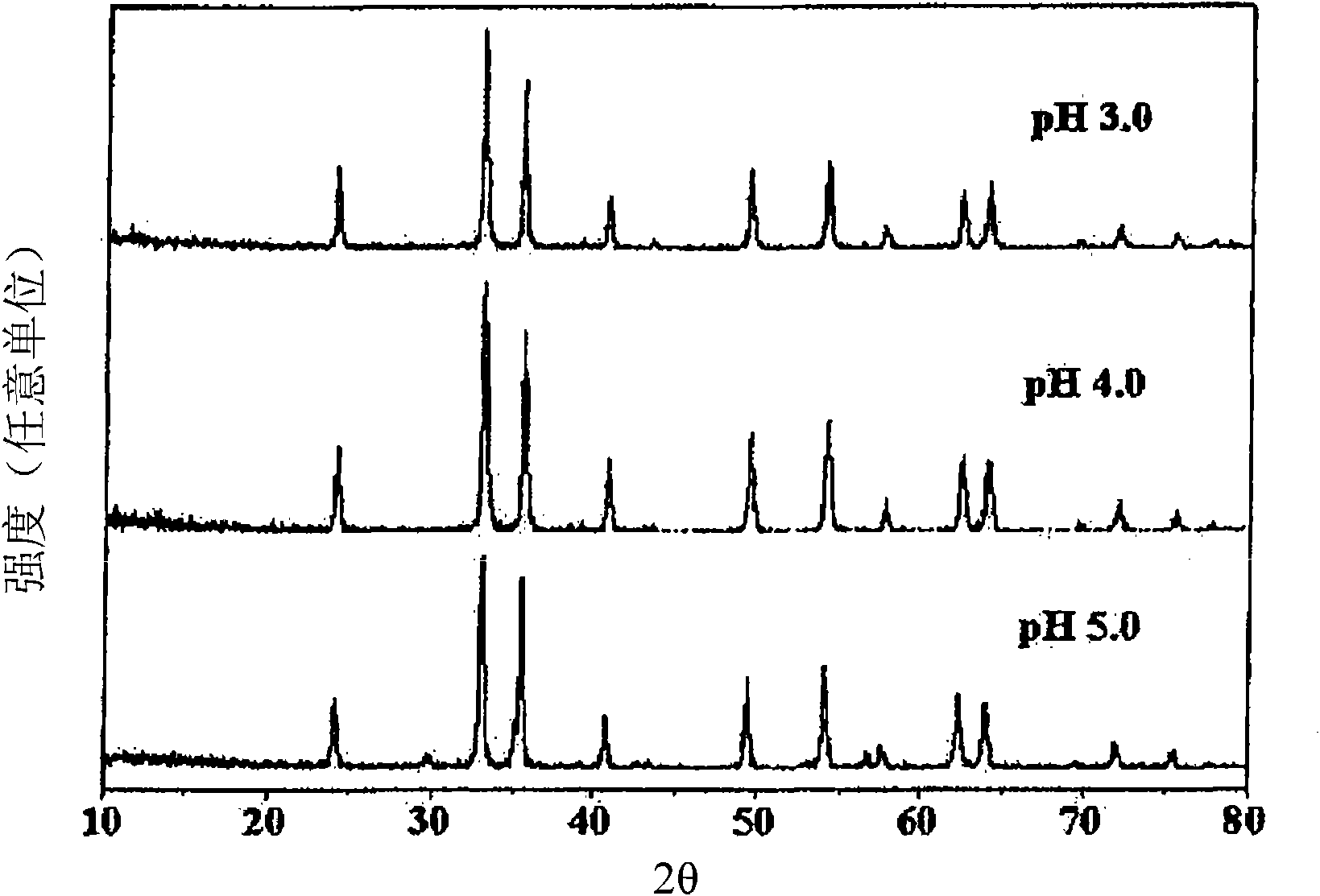
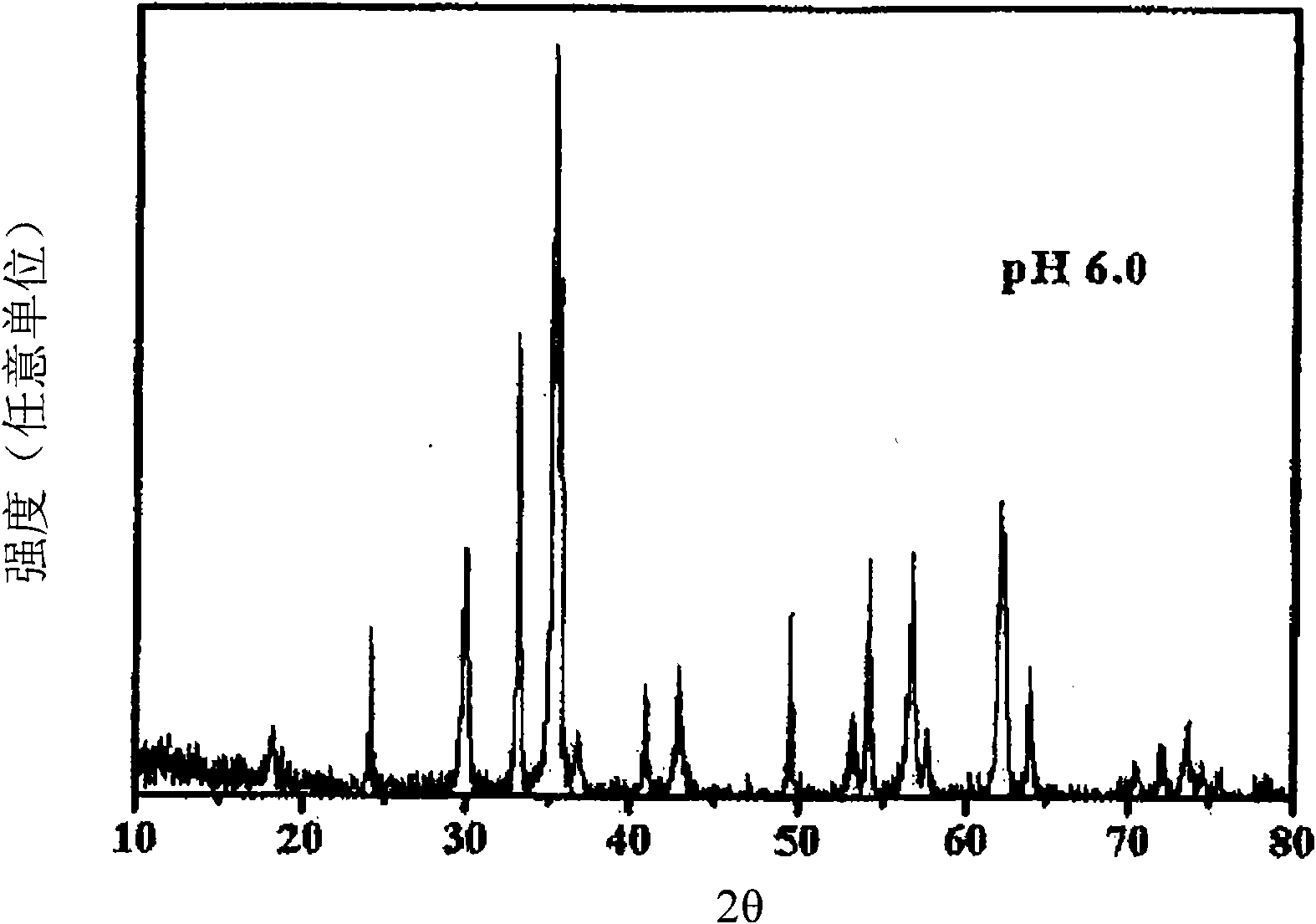
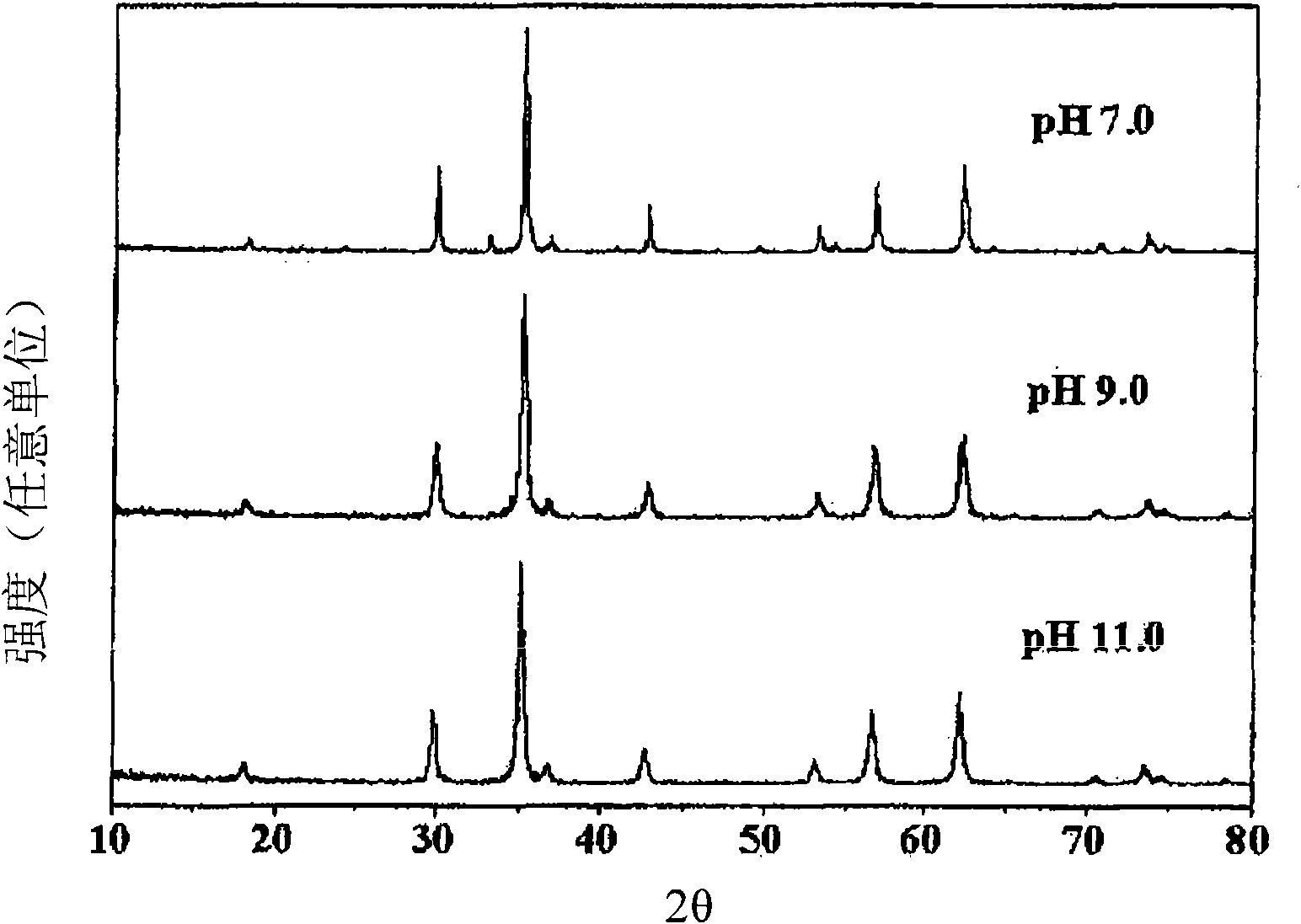
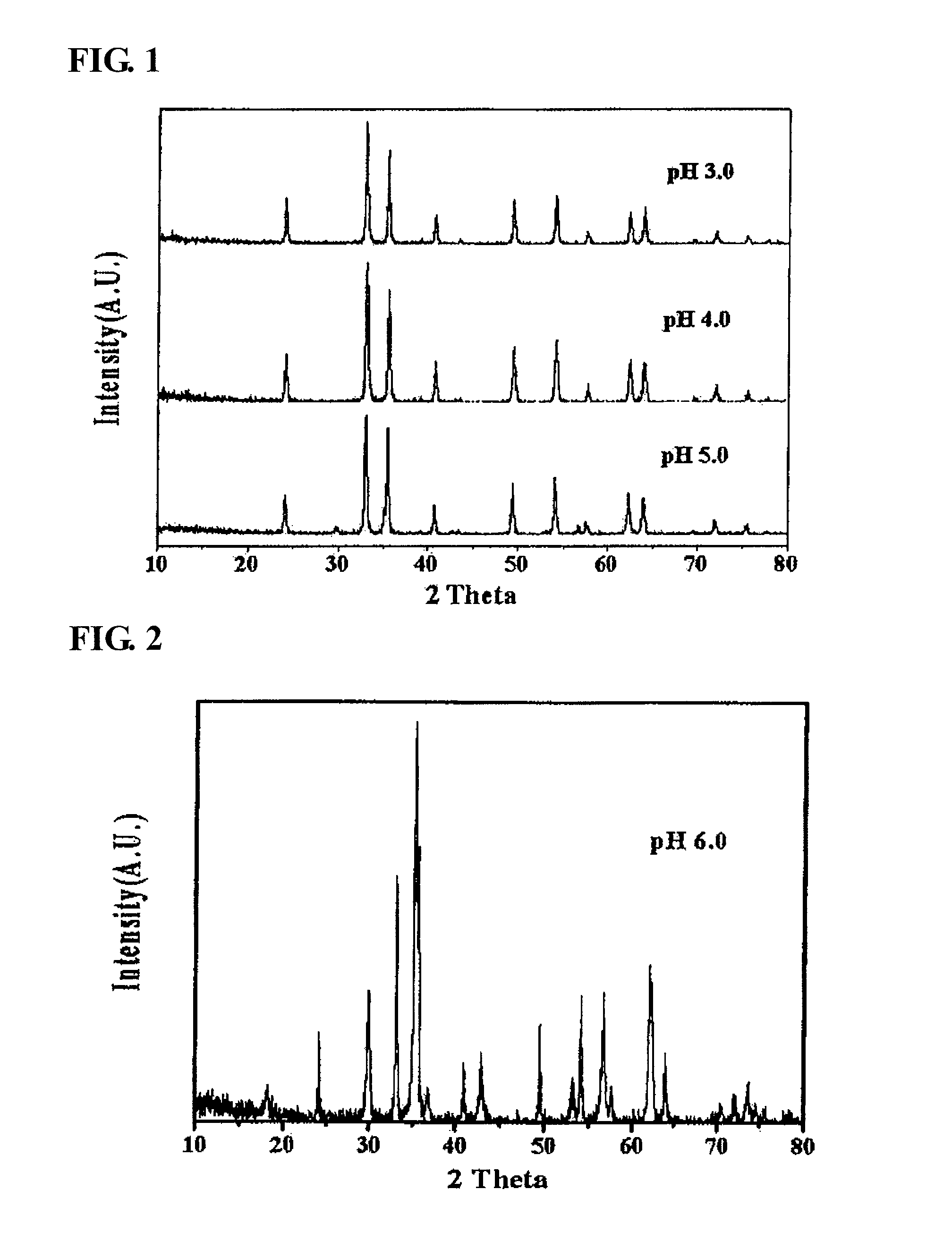
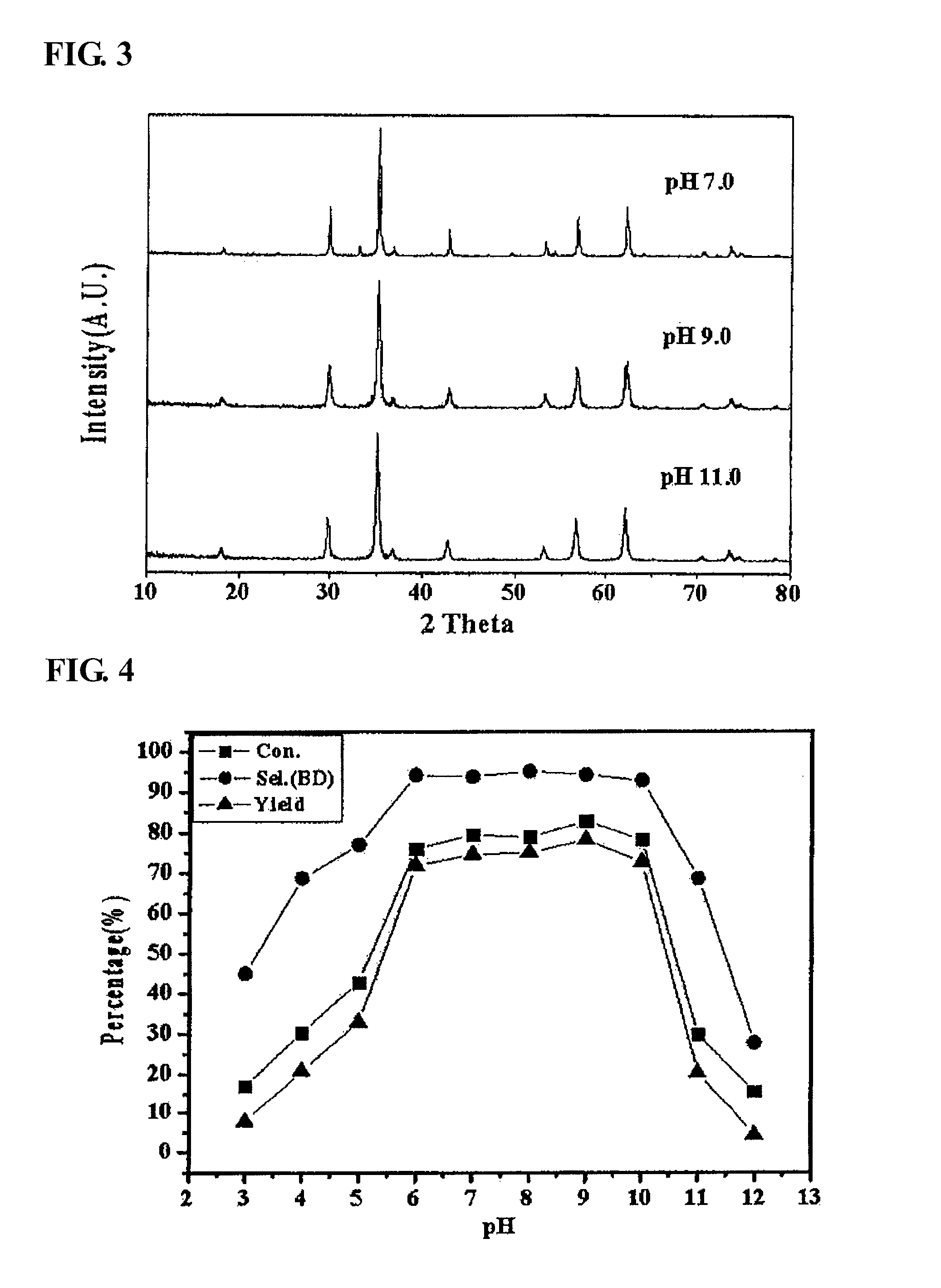
The present invention relates to a zinc ferrite catalyst, a method of producing the same, and a method of preparing 1,3-butadiene using the same. Specifically, the present invention relates to a zinc ferrite catalyst which is produced in a pH-adjusted solution using a coprecipitation method, a method of producing the same, and a method of preparing 1,3-butadiene using the same, in which the 1,3-butadiene can be prepared directly using a C4 mixture including n-butene and n-butane through an oxidative dehydrogenation reaction. The present invention is advantageous in that 1,3-butadiene can be obtained at a high yield directly using a C4 fraction without performing an additional process for separating n-butene, as a reactant, from a C4 fraction containing impurities.
Owner:SK GEO CENTRIC CO LTD
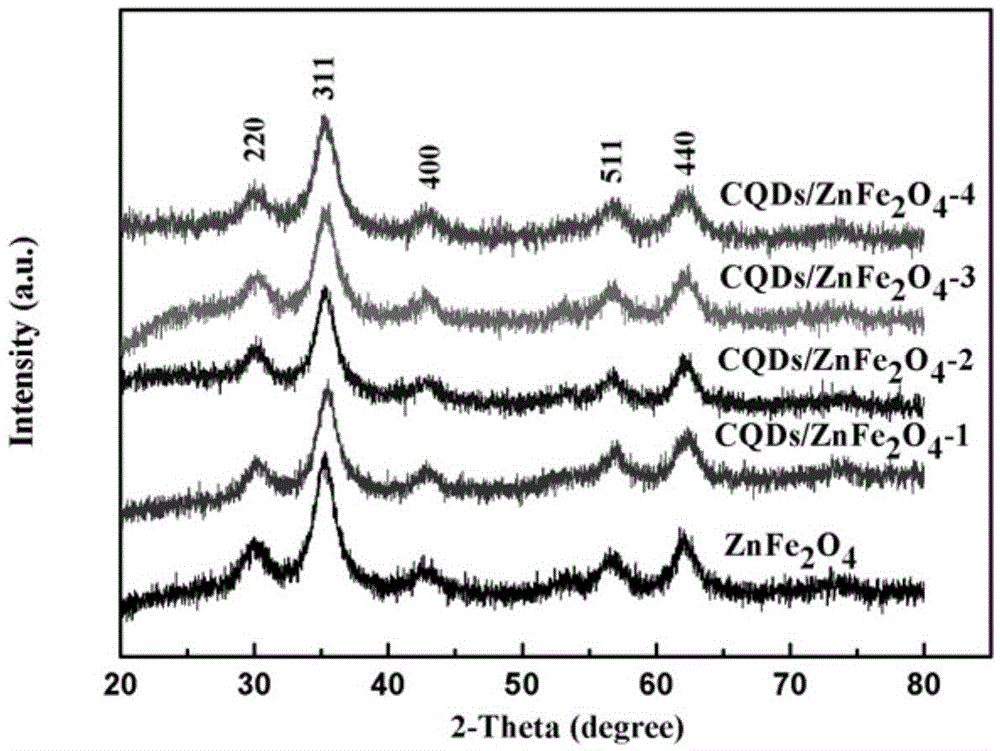
Preparation method of carbon quantum dot/zinc ferrite composite photocatalytic materials
ActiveCN105597764ALow costRaw materials are cheap and easy to getNitrous oxide captureMaterial nanotechnologyDissolutionAscorbic acid
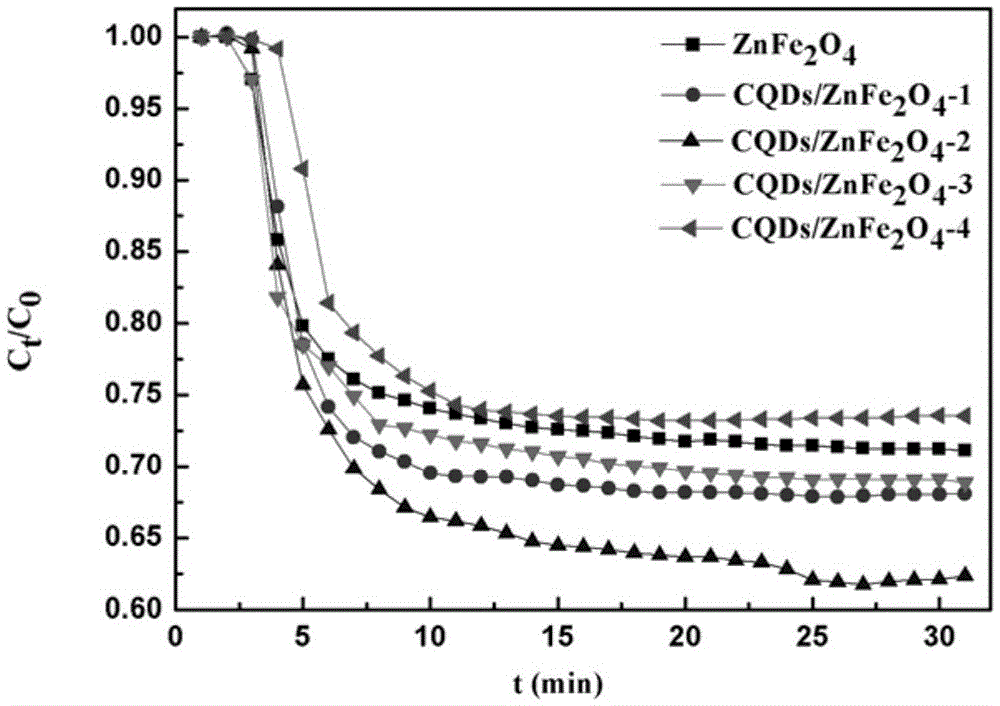
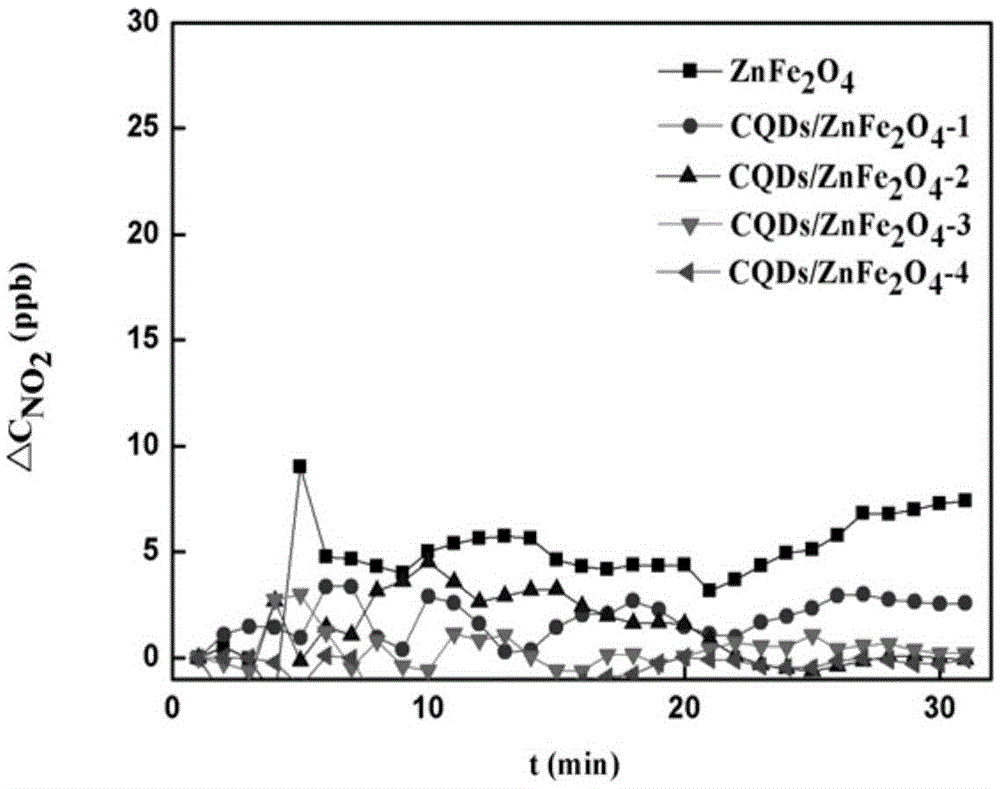
Provided is a preparation method of carbon quantum dot / zinc ferrite composite photocatalytic materials. Ascorbic acid is added into a mixed solution of ethylene glycol and deionized water and evenly stirred, reaction is performed for 1-5 h at the temperature of 100-200 DEG C, and a carbon quantum dot aqueous solution is obtained; zinc salt and Fe(NO3)3.9H2O are added into water, a pH value is adjusted to be 8-14 after dissolution, and suspension liquid is obtained; the carbon quantum dot aqueous solution is added into the suspension liquid, standing is performed after uniform stirring, hydrothermal reaction is performed for 4-10 h at the temperature of 80-180 DEG C, washing and drying are performed, and the carbon quantum dot / zinc ferrite composite photocatalytic materials are obtained. The raw materials are low in price, easy to obtain, the photocatalytic materials are low in synthesis cost, the method is easy to implement, and carbon quantum dot / zinc ferrite has higher degradation efficiency on NOx compared with pure zinc ferrite within the range of visible light.
Owner:INST OF EARTH ENVIRONMENT CHINESE ACAD OF SCI
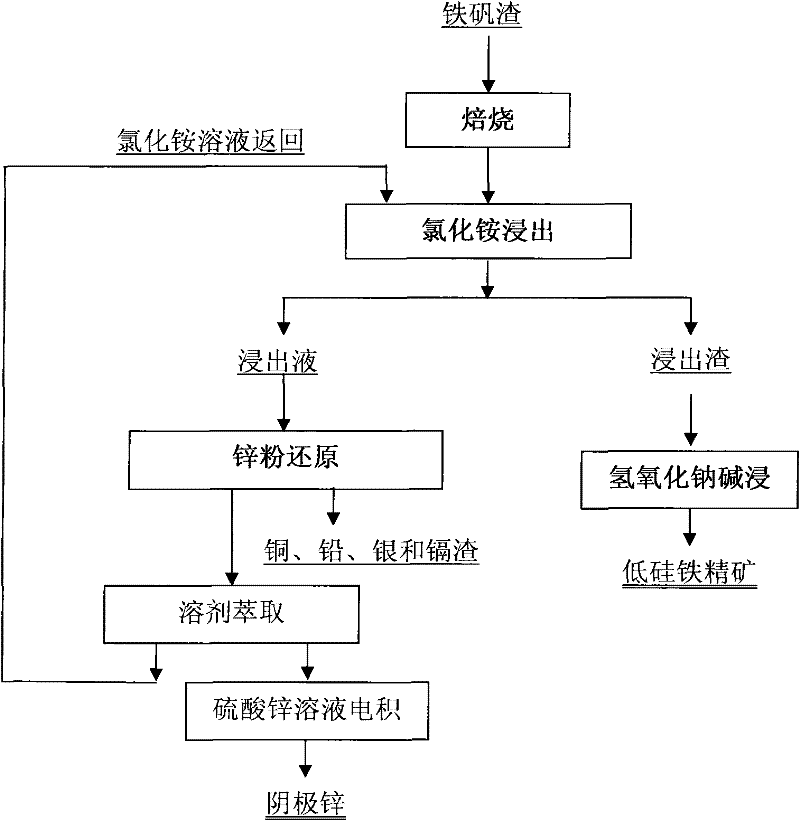
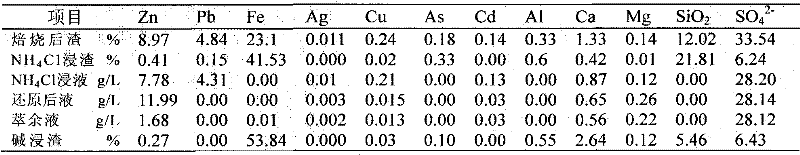
Clean metallurgic comprehensive utilization method of iron vitriol slags
InactiveCN102443701AImprove pollutionReduce consumptionProcess efficiency improvementFerrosiliconPollution
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Anti-interference magnesium-zinc ferrite and producing method
InactiveCN1587192AHigh resistivityHigh initial permeabilityInorganic material magnetismAdhesiveHigh resistivity
The anti-interference magnesium-zinc ferrite is produced with MgO, ZnO and Fe2O3 in certain proportion as main material, and through mixing, calcining at 1000-1200 deg.c for 30-90 min, adding supplementary material, crushing, mixing, adding adhesive, spray pelletizing to obtain grains of 180 micron average size, pressing to form magnetic core, sintering at 1250-1350 deg.c for 2-5 hr and cooling. The present invention has simple production process, and the produced magnesium-zinc ferrite has high initial magnetic permeability, high resistivity, excellent magnetic curve characteristic, low cost, wide application range and good use effect.
Owner:秦会斌 +1
Power type nickel-zinc ferrite material and preparation method thereof
ActiveCN106587977AThe preparation method is simple and easyEasy to operateInorganic material magnetismNickel-zinc ferriteZinc
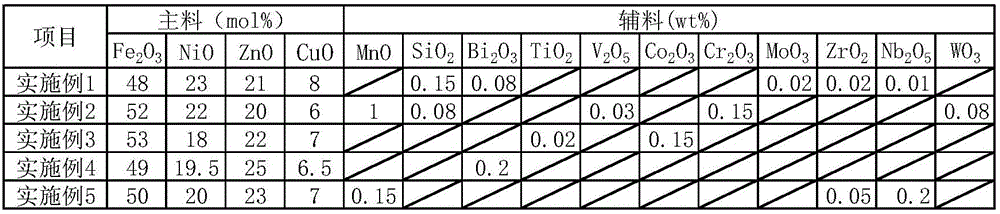
The invention discloses a power type nickel-zinc ferrite material. The power type nickel-zinc ferrite material is prepared from a main material and an auxiliary material. The main material is prepared from, by molar percentage, 48-53 mol% of Fe2O3, 18-23 mol% of NiO, 20-25 mol% of ZnO and 6-8 mol% of CuO. The auxiliary material is selected from at least one of MnO, SiO2, Bi2O3, TiO2, V2O5, Co2O3, Cr2O3, MoO3, ZrO2, Nb2O5 and WO3. The power type nickel-zinc ferrite material has high magnetic conductivity, ultrahigh saturation flux density, ultralow power loss and high high-temperature-resisting soldering tin temperature, and the application range of the nickel-zinc ferrite material can be widened. The invention further provides a preparation method of the power type nickel-zinc ferrite material. The preparation method is simple in step, high in operability and suitable for industrial production.
Owner:HENGDIAN GRP DMEGC MAGNETICS CO LTD
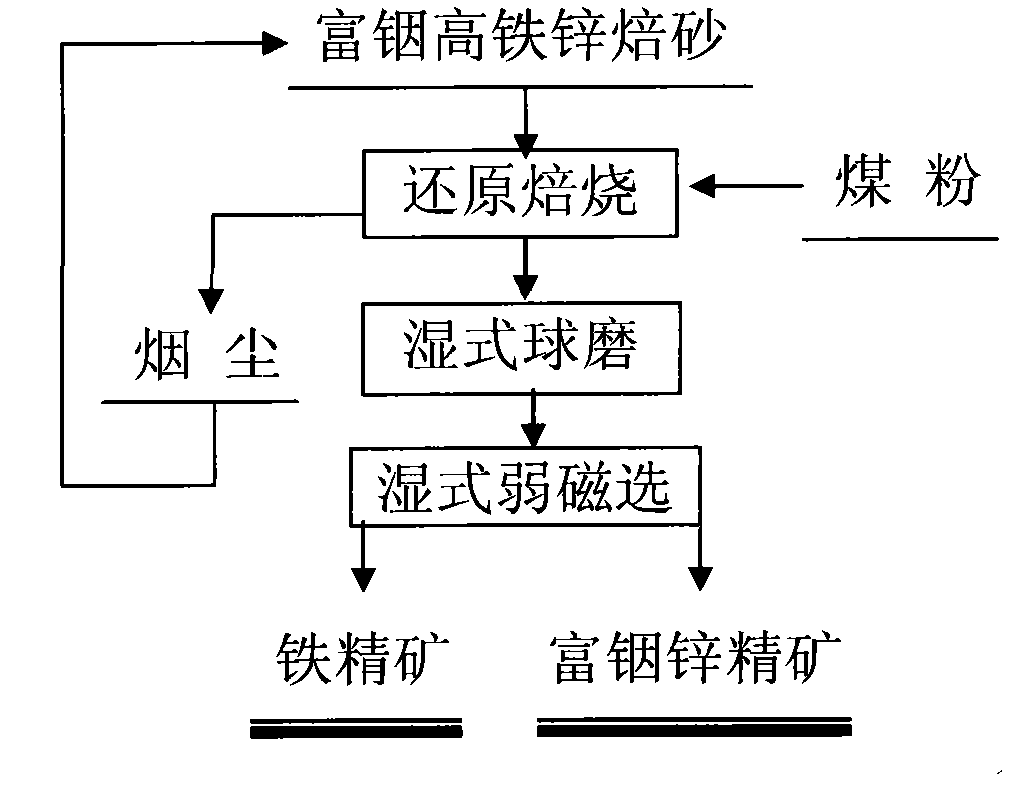
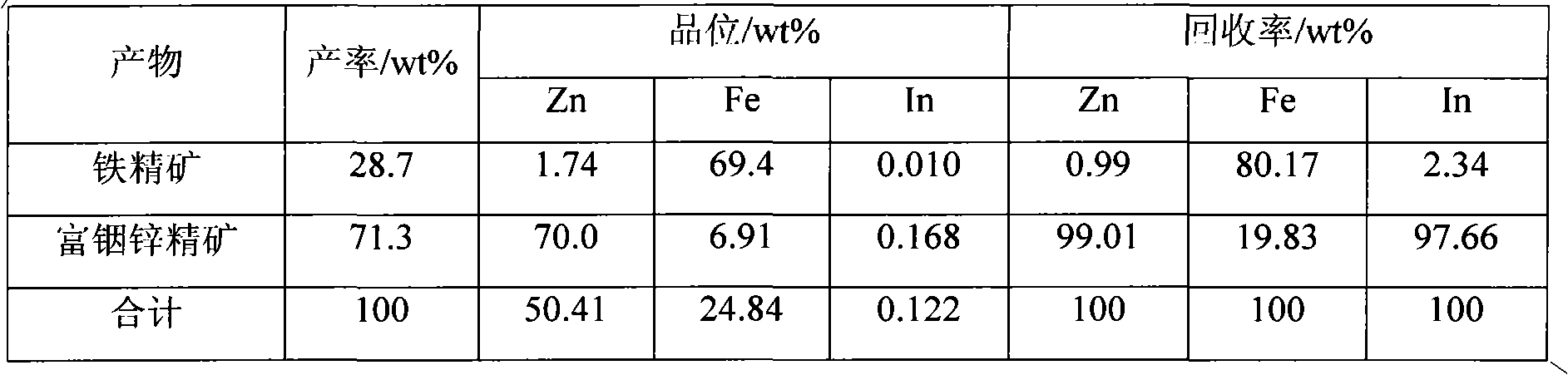
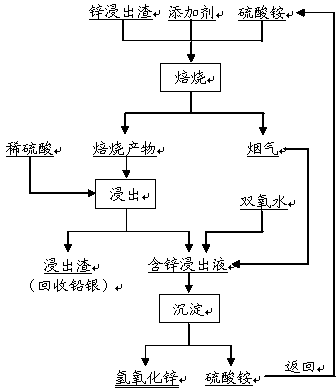
Method for separating zinc and indium and iron from indium-enriched high-iron high-zinc calcine through reduction-magnetic separation
InactiveCN102134655AReduce dosageReduce energy consumptionMagnetic separationReduction treatmentIndium
The invention relates to a method for separating zinc and indium and iron from indium-enriched high-iron high-zinc calcine through reduction-magnetic separation, belonging to the technical field of mineral processing. The invention is characterized in that the method adopts the technical means that mineral dressing is combined with smelting and performs smelting firstly and mineral dressing secondly; and the method is as follows: the waste heat of the indium-enriched high-iron zinc calcine obtained through fluidized roasting is utilized, the indium-enriched high-iron zinc calcine is introduced to perform low-temperature weak reduction treatment at below 570 DEG C and ensure that zinc ferrite is decomposed and reduced to ZnO, Fe3O4 and iron, the reduced calcine is levigated to prepare slurry, and zinc and indium and iron is separated through wet-type magnetic separation to obtain iron ore concentrates and indium-enriched zinc- enriched ore concentrates. The method has low energy consumption and low dosage of a reducing agent, is simple in operation, easy in control and high in metal recovery rate. Therefore, the indium embedded and distributed in zinc ferrite can be released, the loss caused by the high temperature volatilization of indium can be avoided, and the zinc and indium and iron of the indium-enriched high-iron high-zinc calcine can be separated in an ore dressing manner before leaching.
Owner:KUNMING UNIV OF SCI & TECH
Zinc ferrite-loaded carbon nano tube catalyst prepared by microwave-hydrothermal method and application of catalyst in degrading organic pollutants in water
ActiveCN104383930AImprove absorbing performanceIncrease temperatureWater/sewage treatment by irradiationMetal/metal-oxides/metal-hydroxide catalystsCarbon nanotubeCarbon nanotube supported catalyst
The invention relates to a zinc ferrite-loaded carbon nano tube catalyst prepared by a microwave-hydrothermal method and an application of the catalyst in degrading organic pollutants in water. A preparation method of the catalyst comprises the steps of dissolving FeCl3 and ZnCl2 into deionized water, adding a pretreated carbon nano tube into a solution, performing ultrasonic treatment for 1.0-3.0min, adjusting the pH to 7.5-11.5, stirring and transferring into a polytetrafluoroethylene reaction tank, putting the reaction tank into a microwave digester, performing hydrothermal reaction under the pressure of 0.3-1.5MPa for 10-40min, washing a product obtained by the reaction with the deionized water until the product is neutral, filtering the product, drying the product under the constant temperature of 70 DEG C, grinding the product, and screening the product with a screen of 100 meshes to obtain the zinc ferrite-loaded carbon nano tube catalyst. The catalyst provided by the invention is combined with microwaves to degrade the organic pollutants in water; the catalyst preparation speed is high, the degrading efficiency is high, the rate is high, and the cost is low; no intermediate product or secondary pollution is generated.
Owner:LIAONING UNIVERSITY
Zinc smelting technology
ActiveCN103540765AImprove leaching rateHigh recovery rateProcess efficiency improvementMagnetic separationNon magneticCalcination
The invention discloses a zinc smelting technology. The zinc smelting technology comprises the following step that high-iron sphalerite concentrate is subjected to calcination, neutral leaching and low-acid leaching; zinc ferrite is separated from the low-grade leaching residues by a magnetic separator and the non-magnetic leaching residues are further treated by high-acid leaching; and the zinc ferrite is decomposed into ferroferric oxide and zinc oxide by reduction roasting, and the ferroferric oxide and the zinc oxide are used respectively as a magnetic seed and a neutralizer used in a leachate magnetofluid iron-removal technology. Through combination of a wet method and a fire method, a zinc leaching rate and a lead and silver recovery rate are improved, and the calcinations of the zinc ferrite are used in the magnetofluid iron-removal technology so that an iron-removal technology cost is effectively reduced and iron residues are pure, have high iron content and are conducive to iron residue comprehensive utilization. The zinc smelting technology can efficiently prepare high-quality zinc leachate, utilize agents having wide sources and a low cost, prepare a very-low iron-content zinc leachate, greatly improve the efficiency of the zinc wet method smelting technology, basically prevent a valuable metal loss and promote resource comprehensive utilization.
Owner:CHANGSHA HASKY ENVIRONMENTAL PROTECTION TECH DEV CO LTD
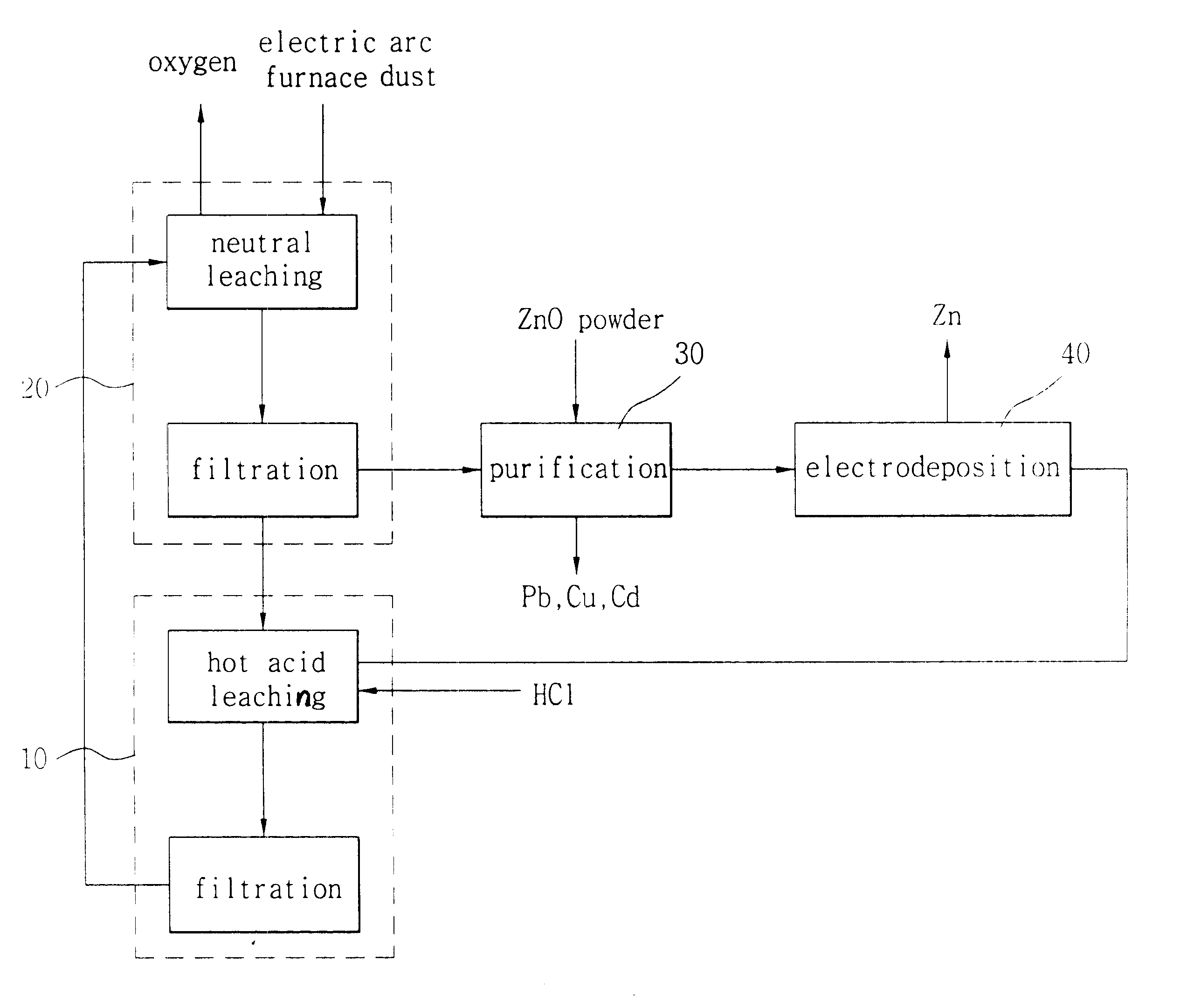
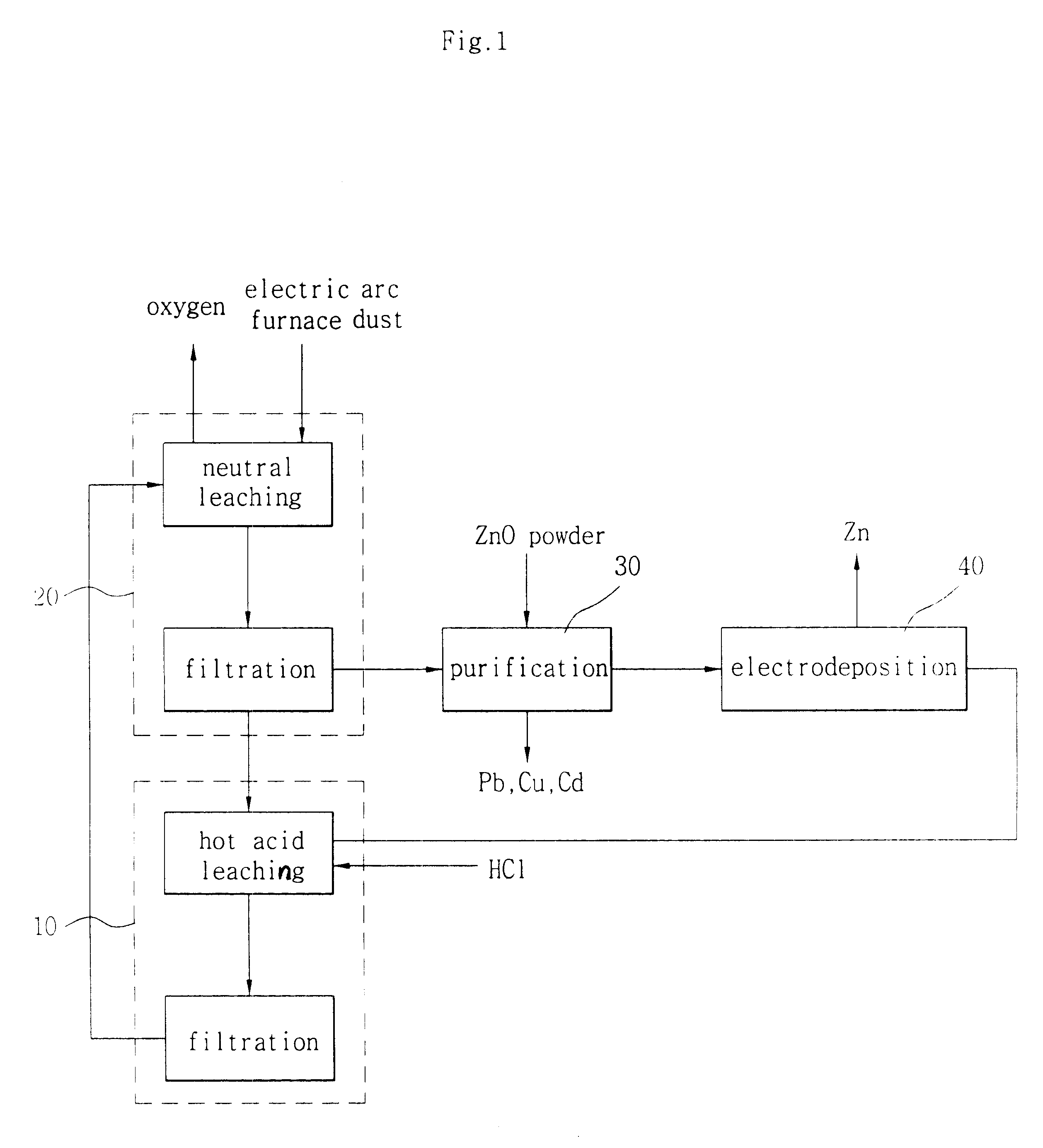
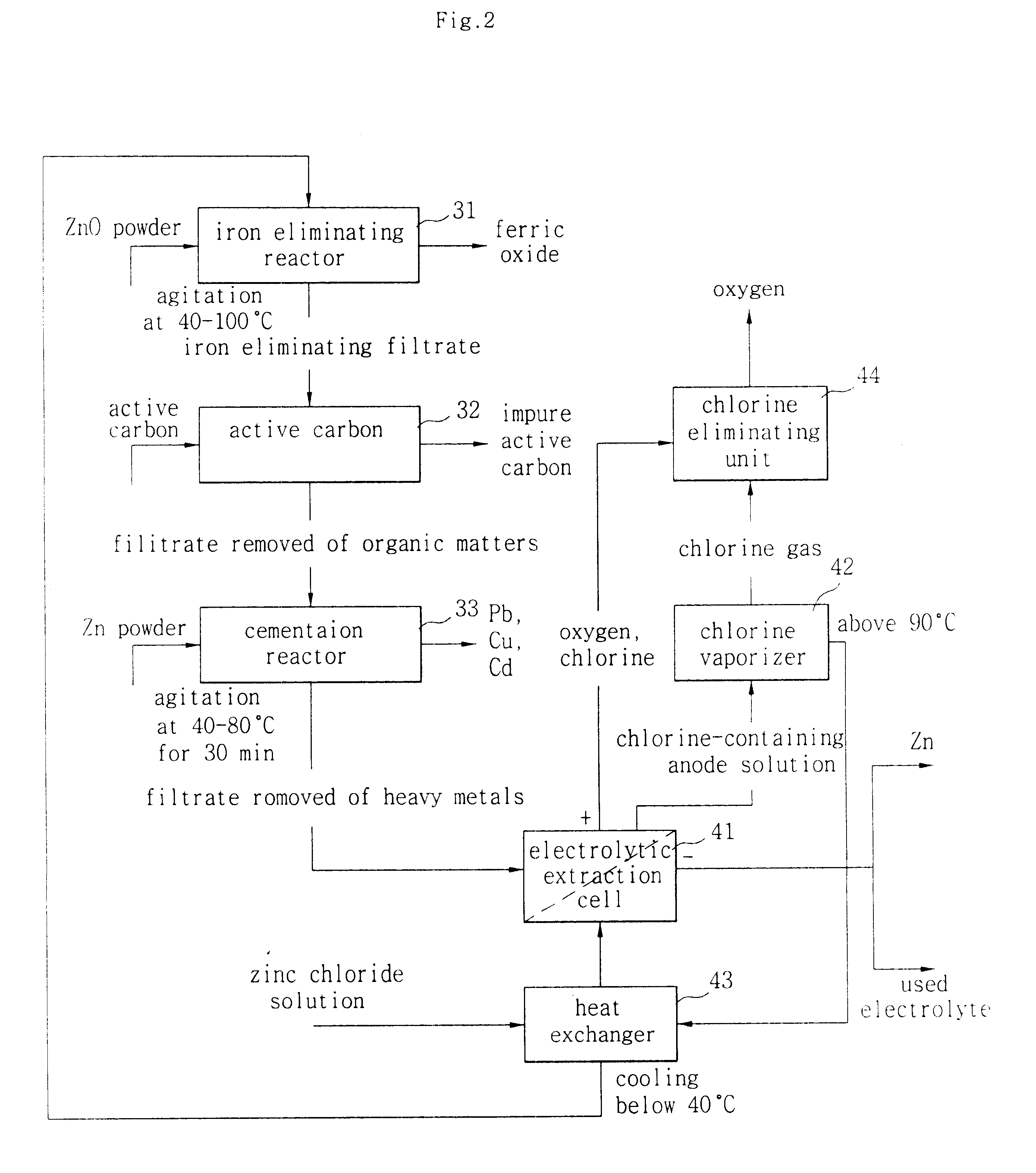
Hydrometallurgical method for recovery of zinc from electric arc furnace dust
InactiveUS6338748B1Current efficiency is deterioratedLow costPhotography auxillary processesSolvent extractionMembrane currentElectric arc furnace
The process uses hydrochloric acid solutions to extract zinc from electric arc furnace dusts containing zinc oxide and zinc ferrite. To selectively leach zinc and minimize iron dissolution by precipitating as FeO.OH and Fe2O3, hot acid leaching with the aqueous solution containing 37 g / l-74 g / l HCl and 104 g / l-270 g / l ZnCl2 is used. New dust is introduced to remove iron from the filtrate of the hot acid leaching. The zinc chloride solutions purified by activated carbon and metallic zinc powder is electrolysed in electrowinning cells which had cation exchange membrane to produce high purity zinc metal and to regenerate hydrochloric acid. Electrolysing an aqueous solution of zinc chloride with Zn concentration of 50-130 g / litre below 40° C. in a cell divided by cation exchange membrane, whereby coherent zinc is yielded at the cathode with high current efficiency of exceeding 90%. HCl is directly regenerated with a very small loss below 2% and low energy consumption below 5.0 kWh / kg-Zn during Zn-electrodeposition at a cathodic current density in the range of 300-2000 A / m2, and a membrane current density in the range of 750-2000 A / m2. The spent electrolyte with 1-2N HCl is used to leach the residue in the hot acid leaching vessel.
Owner:SANGWON ENC +1
A nickel-copper-zinc ferrite and its making method
InactiveCN101236819AUniform grain sizeUniform sizeInorganic material magnetismCurie temperatureVanadium oxide
The invention relates to a nickel-copper-zinc ferrite and a manufacturing method thereof. The ferrite of the invention comprises principal constituents and auxiliary constituents, wherein, the principal constituents are respectively iron oxides, nickel protoxide, zinc oxides and cupric oxides; the contents of the principal constituents calculated by respective reference substances are that: 48 mol percent to 50 mol percent Fe2O3, 13 mol percent to 16 mol percent NiO, 29 mol percent to 31.5 mol percent ZnO and 4.5 mol percent to 6.5 mol percent CuO; the auxiliary constituents comprise vanadium oxides, molybdenum oxides and titanium oxides; compared with the gross of the principal constituents, the total content of the auxiliary constituents calculated by respective reference substances V2O5, MoO3 and TiO2 is 0.01 weight percent to 0.08 weight percent. The nickel-copper-zinc ferrite has higher Curie temperature and high initial permeability, high saturation magnetic flux density and low loss under the condition of wide-temperature range, thereby comprehensive performance requirements of development of electric equipment towards directions of miniaturization, mobilization, integration and multifunction can be better met.
Owner:RUYUAN DONGYANGGUANG MAGNETIC MATERIAL
Wide-temperature low-distortion mangan zinc ferrite and preparation method thereof
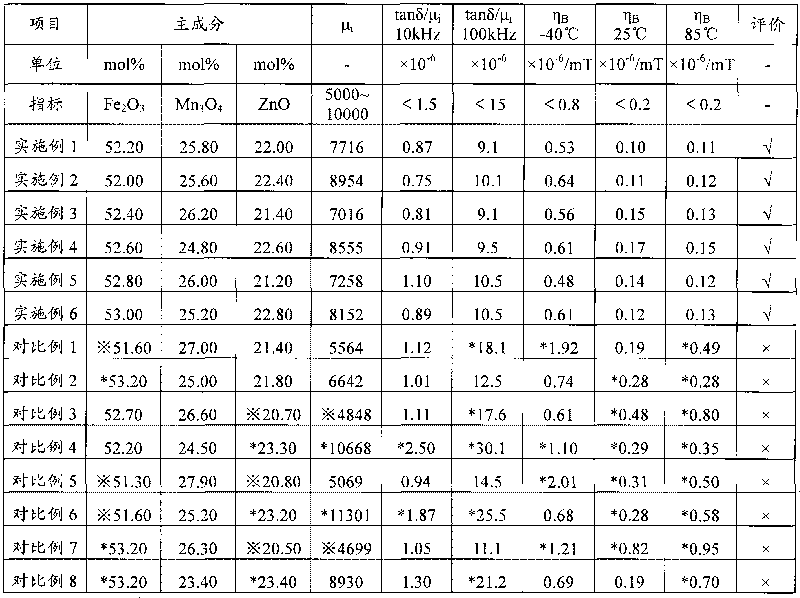
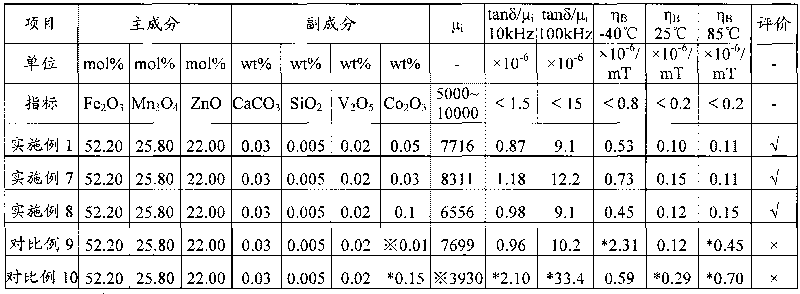
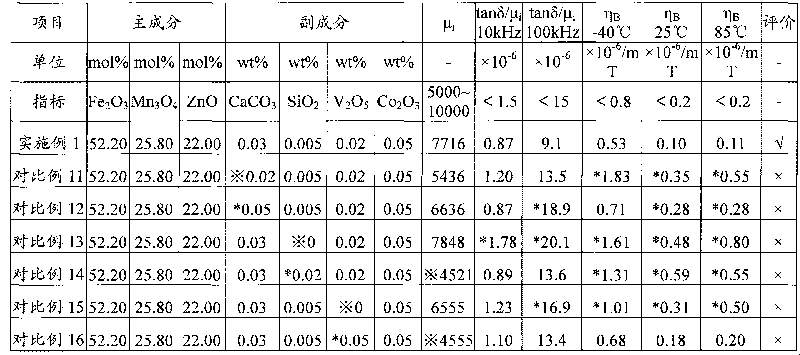
ActiveCN101728048AReduce production energy consumptionBurn-in lowInorganic material magnetismLow distortionTransformer
The invention relates to a wide-temperature low-distortion mangan zinc ferrite applicable to a broadband network transformer and a preparation method thereof. The mangan zinc ferrite mainly comprises the following components: 52.0 to 53.0 mol percent of iron oxide computed by Fe2O3, 21.0 to 23.0 mol percent of zinc oxide computed by ZnO, and the balance of trimanganese tetroxide, and also comprises the following auxiliary components in percentage by weight (wt %) computed by respective standard substance CaCO3, SiO2, V2O5 and Co2O3: 0.03 to 0.04 of CaCO3, 0.005 to 0.01 of SiO2, 0.01 to 0.03 of V2O5 and 0.03 to 0.1 of Co2O3. The mangan zinc ferrite is prepared by an oxide method and sintered under the condition of bell-type furnace densification. A product has higher initial magnetic permeability mu i, low relative loss factor tan delta / mu i, and wide-temperature low-magnetic hysteresis coefficient eta B (-40 to 85 DEG C), is capable of reducing waveform distortion, reducing transmission errors and prolonging transmission distance in the process of transmitting signals, and meets the requirement of the application in outdoor severe environment.
Owner:TDG HLDG CO LTD
Zinc ferrite catalysts, method of preparing thereof and method of preparing 1,3-butadiene using thereof
ActiveUS8513479B2Simple procedureSimple structureHydrocarbon by hydrogenationHeterogenous catalyst chemical elementsButeneDehydrogenation
The present invention relates to a zinc ferrite catalyst, a method of producing the same, and a method of preparing 1,3-butadiene using the same. Specifically, the present invention relates to a zinc ferrite catalyst which is produced in a pH-adjusted solution using a coprecipitation method, a method of producing the same, and a method of preparing 1,3-butadiene using the same, in which the 1,3-butadiene can be prepared directly using a C4 mixture including n-butene and n-butane through an oxidative dehydrogenation reaction. The present invention is advantageous in that 1,3-butadiene can be obtained at a high yield directly using a C4 fraction without performing an additional process for separating n-butene, as a reactant, from a C4 fraction containing impurities.
Owner:SK GEO CENTRIC CO LTD
Wide-temperature-range low-power-consumption high-Curie-temperature manganese/zinc ferrite material and preparation method thereof
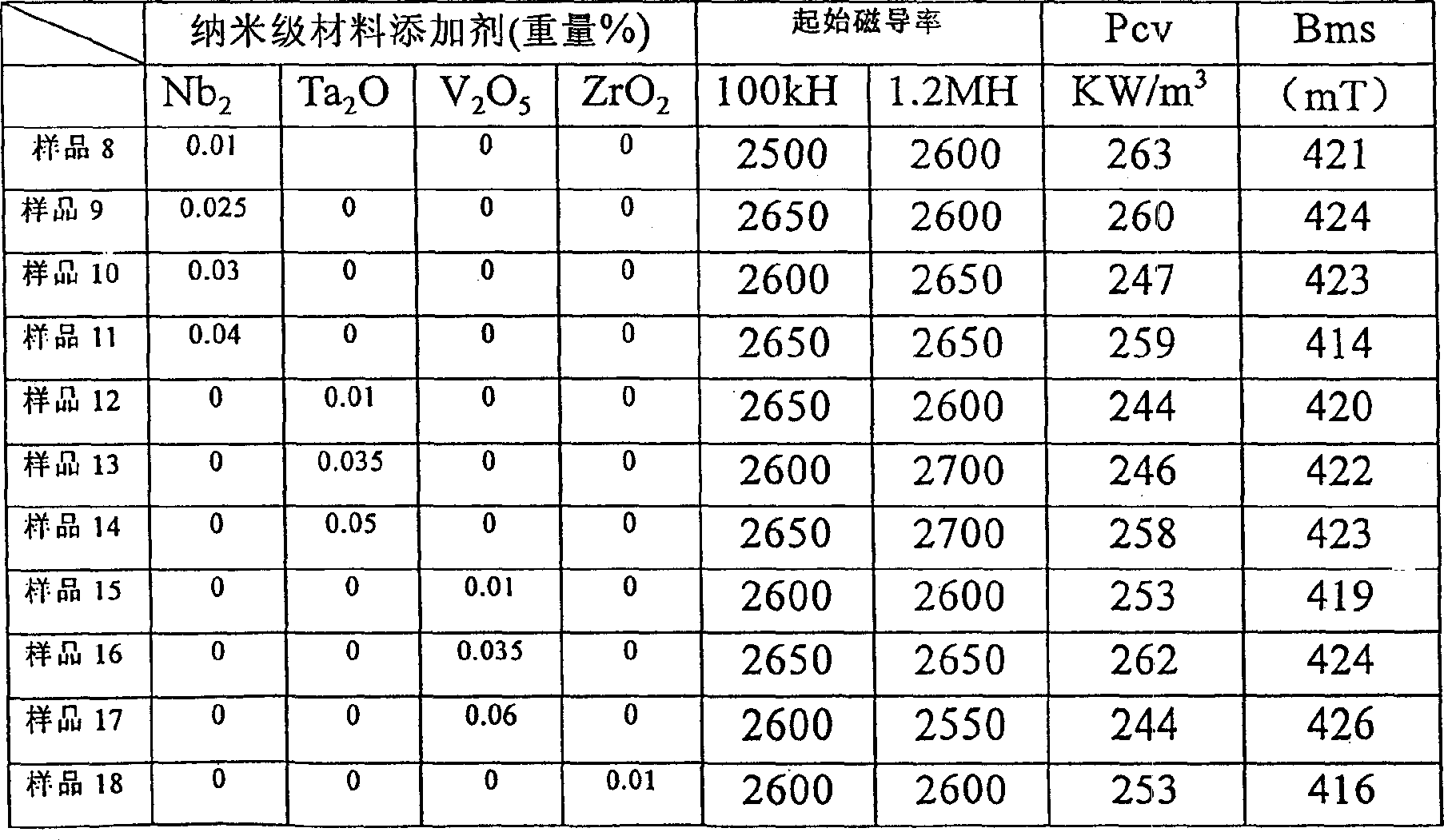
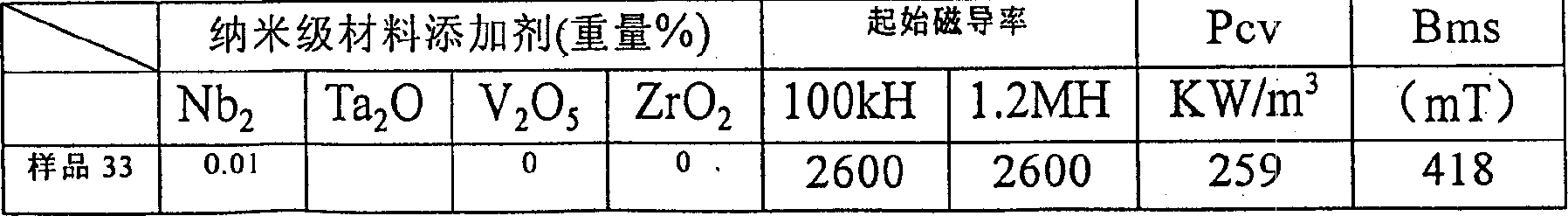
The invention relates to a wide-temperature-range superposed low-power-consumption high-Curie-temperature manganese / zinc ferrite material and a preparation method thereof, belonging to the technical field of soft magnetic ferrite materials. The manganese / zinc ferrite material comprises main components and additives, wherein the main components comprises the following materials in percentage by mass measured by oxide: 69.0-72.5% of Fe2O3, 6-10% of ZnO and the balance of Mn3O4, Fe2O3, with the sum of the Mn3O4 and ZnO being 100%; the additives comprises the following materials by weight percent measured by oxide: 0.02-0.07% of CaO, 0.02-0.08% of Nb2O5, 0.02-0.08% of V2O5, 0.05-0.25% of TiO2, 0.08-0.6% of CO2O3, 0.003-0.10% of ZrO2, 0.04-0.4% of NiO, 0.02-0.10% of MgO, and the like. The manganese / zinc ferrite material disclosed by the invention is mainly used in the fields of home electronics, communications, optoelectronics, automotive electronics and like, and is especially applicable for new fields of electrodeless lamp electromagnetic coupler core markets as well as automotive electronics, strong current, inverter air conditioners, etc. The manganese / zinc ferrite material has the beneficial effects of low power consumption, wide temperature range, high Curie temperature and the like.
Owner:NANTONG GUANYOUDA MAGNET
High-frequency nickel-copper-zinc ferrite and preparation thereof
The invention relates to a high frequency nickel-copper-zinc ferrite and a method for preparing the same, which belong to the technical field of nickel series soft magnetism ferrite. The main components of the ferrite are ferric oxide, nickel oxide, zinc oxide, and copper oxide, the accessory components comprise cobalt oxide, bismuth oxide, and silicon oxide, and the ferrite is prepared by an oxide method. The invention adopts an iron deficiency formulation, a low calcination temperature, and a low sintering temperature so that the prepared high frequency nickel-copper-zinc ferrite has good performances of low initial permeability, low relative dissipation factor between 50MHz and 200MHz, and high Curie temperature, and can better satisfy the use requirement of communication electronic parts and components; besides, the preparation process is stable, and the production process has low energy consumption.
Owner:TDG HLDG CO LTD
Method for preparing porous zinc ferrite nano-rods
InactiveCN101289228AEvenly dispersedPromote crystallizationIron compoundsZinc compoundsCalcinationNanorod
The invention discloses a preparation method of a nano-rod of porous zinc ferrite. The preparation method adopts a four-component micro emulsion system comprising hexadecyl trimethyl bromide / water / cyclohexane / n-amyl alcohol, dissoluble zinc salt, dissoluble ferrite and oxalic acid as reactants to firstly synthesize the ZnFe2(C2O4)3 precursor of nano-rod, and then is calcined at the temperature of 500 DEG C to 900 DEG C, the nano-rod of the porous zinc ferrite, the chemical formula of which is ZnFe2O4, is obtained owing to the separation of oxalic acid radical from the precursor in the process of calcination. The nano-rod of the porous zinc ferrite is provided with a plurality of nano-pores, the products obtained by the preparation method is uniformly dispersed with good crystallization, diameter being 30nm to100nm and length being 1mum to 10mum.
Owner:ZHEJIANG SCI-TECH UNIV
Nickel-zinc ferrite material for wireless signal sensing, sheet core and preparation method thereof
ActiveCN102807361AImprove frequency characteristicsHigh resistivityInorganic material magnetismTransformers/inductances detailsSurface mountingNano sio2
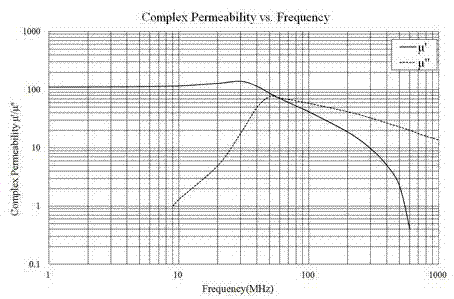
The invention relates to a nickel-zinc ferrite material for wireless signal sensing, a sheet core and a preparation method thereof. The nickel-zinc ferrite material comprises main components which are calculated by the following oxides in mole percent: 48.0-62.5mol% of Fe2O3, 15.3-25.5mol% of NiO, 18.5-23.5mol% of ZnO and 3-10mol% of CuO; and the nickel-zinc ferrite material additionally comprises auxiliary components which are calculated by the following standard substances in weight percent relative to the total weight of the main components: 0.05-0.10wt% of nano CaCO3, 0.30-0.85wt% of nano SiO2, 1.50-2.50wt% of Mn3O4, 0.05-0.35wt% of Co2O3 and 1.00-1.50wt% of Bi2O3. The nickel-zinc ferrite material is prepared by adopting an oxidation method. The sheet core is a reticular sheet, the length is 45-250mm, the width is 45-250mm, the thickness is 0.05-0.3mm, the sheet core is formed by small sheets in a connecting way, the gaps among the small sheets are less than 50mum, and the sheet core is directly molded and then is sintered, or a magnetic bar is molded and then is sliced into small sheets which form the sheet core through an SMT (surface mount device) technology. At frequency of 13.56MHz, the material has the electromagnetic performance that mu' is equal to 125 plus or minus 20% and the mu'' is less than or equal to 4. Therefore, the material can satisfy the requirement of high-frequency low consumption on the ferrite material for wireless signal sensing.
Owner:TDG HLDG CO LTD
Method for separating zinc and iron from zinc leaching residues
InactiveCN104178642AAchieve regenerationIncrease incidenceProcess efficiency improvementZinc hydroxideSulfate zinc
The invention discloses a method for separating zinc and iron from zinc leaching residues. The method comprises the following steps: firstly, mixing the zinc leaching residues with ammonium sulfate and an additive, then performing roasting to ensure that zinc ferrite and the like in the zinc leaching residues are changed into soluble zinc sulfate and insoluble ferric oxide; secondly, directly leaching out a roasted product by using a dilute sulfuric acid solution; and finally, introducing fume which is produced during roasting and mainly contains ammonia gas into a zinc leaching solution to perform deposition so as to produce zinc hydroxide and an ammonium sulfate solution, and concentrating and crystallizing the ammonium sulfate solution to prepare ammonium sulfate which is returned to the ammonium sulfate roasting process. The ammonium sulfate roasting process in the method can ensure that a zinc ferrite phase in the zinc leaching residues is changed into soluble zinc sulfate, the leaching rate of zinc is more than 97%, and the leaching rate of iron is lower than 2%, so that the separation of zinc and iron is effectively realized; the ammonia gas produced in the ammonium sulfate roasting process is directly used for depositing zinc from the leaching solution, and the regeneration of ammonium sulfate can be realized while a zinc hydroxide product is generated; the comprehensive recovery rate of zinc is more than 96%; the method can realize closed-loop circulation and is relatively environment-friendly.
Owner:CENT SOUTH UNIV
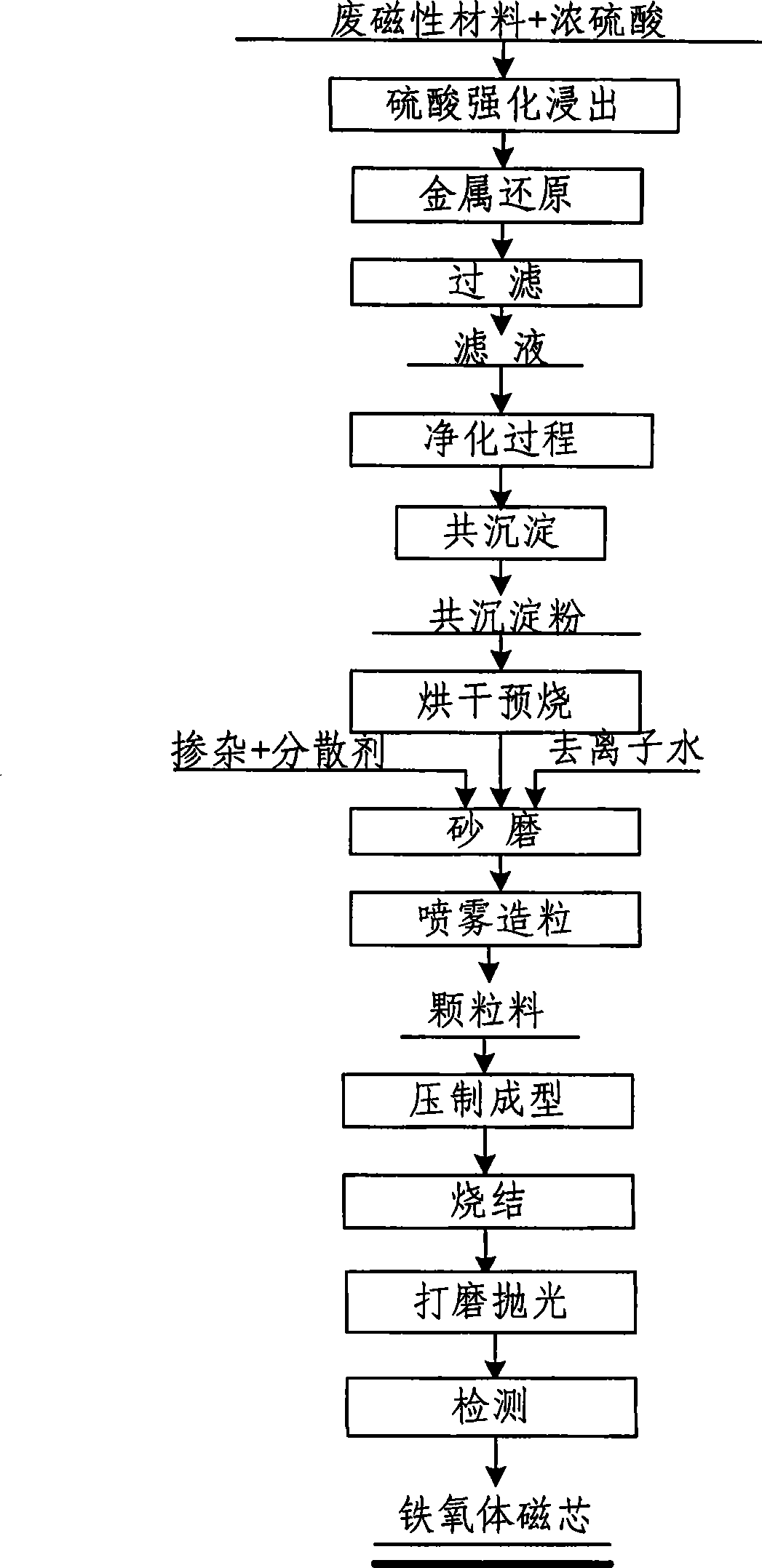
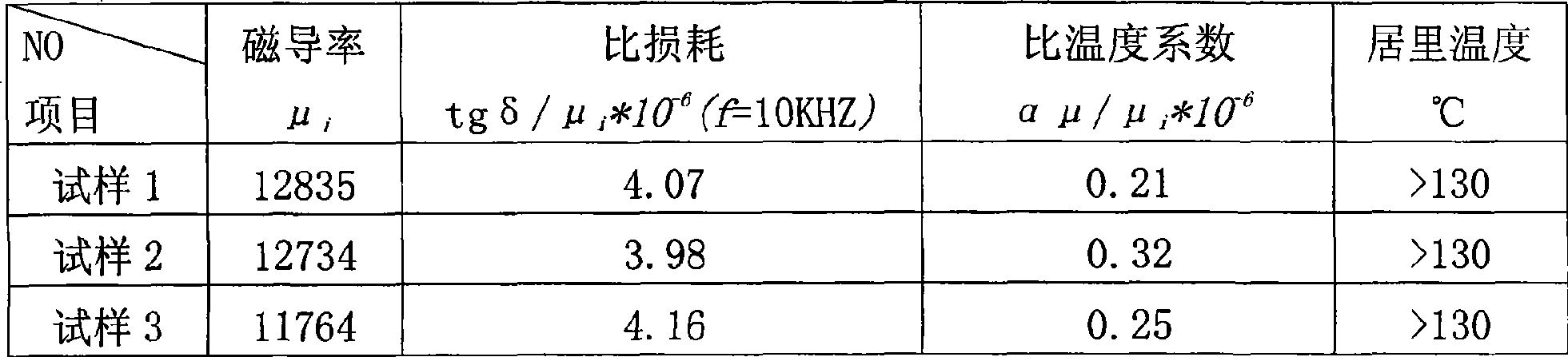
Fabrication method of broad-band high-conductive manganese-zinc ferrite magnetic core
InactiveCN101521071AReduce manufacturing costEliminate pollutionInorganic material magnetismTransformers/inductances magnetic coresSpray GranulationManganese
The invention provides a fabrication method of a broad-band high-conductive manganese-zinc ferrite magnetic core, which utilizes manganese-zinc soft magnetic waste as a raw material and obtains compound powder with low impurity content by reinforced lixiviation, reduction and purification and coprecipitation. The compound powder is dried and pre-burned and then refined; then the powder is ground and mixed in a ball mill; an additive is added for spray granulation; and finally the magnetic core is formed by burning after pressing and shaping. The method comprises the steps of first fabricating the pre-burned powder with high purity by wet processing, then adding the additive for improving the frequency characteristic and loss characteristic of materials and adjusting the burning process, thus being capable of stably fabricating the manganese-zinc ferrite magnetic core material of which the magnetic permeability reaches 12K, Curie temperature reaches 130 DEG C and the usage frequency amounts to 300 KHz; and simultaneously, the fabrication method sharply reduces the fabrication cost of the manganese-zinc ferrite magnetic core material and enhances the market competitiveness of products.
Owner:CENT SOUTH UNIV
Sol of nano titania and preparation method
The prepn. steps are: creating amorphous neutral titanium oxide pulp material by chemical method, washing chlorion to less than 50 ppm by deionized water, proceeding ultrasonic dispersion to the pulp after adding lanthanum nitrate or zinc ferrite then senting the pulp into reaction boiler, increasing temp. to 80-200 deg.C, taking it out after being crystallized 8-72 hours, the nanometre titanium dioxide sol with photochemical function is produced. The sol has good water-solubility, 99.9 percent bactericidal ratio with 0.8 percent density and on condition of natural lighting.
Owner:辛华鹏
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com