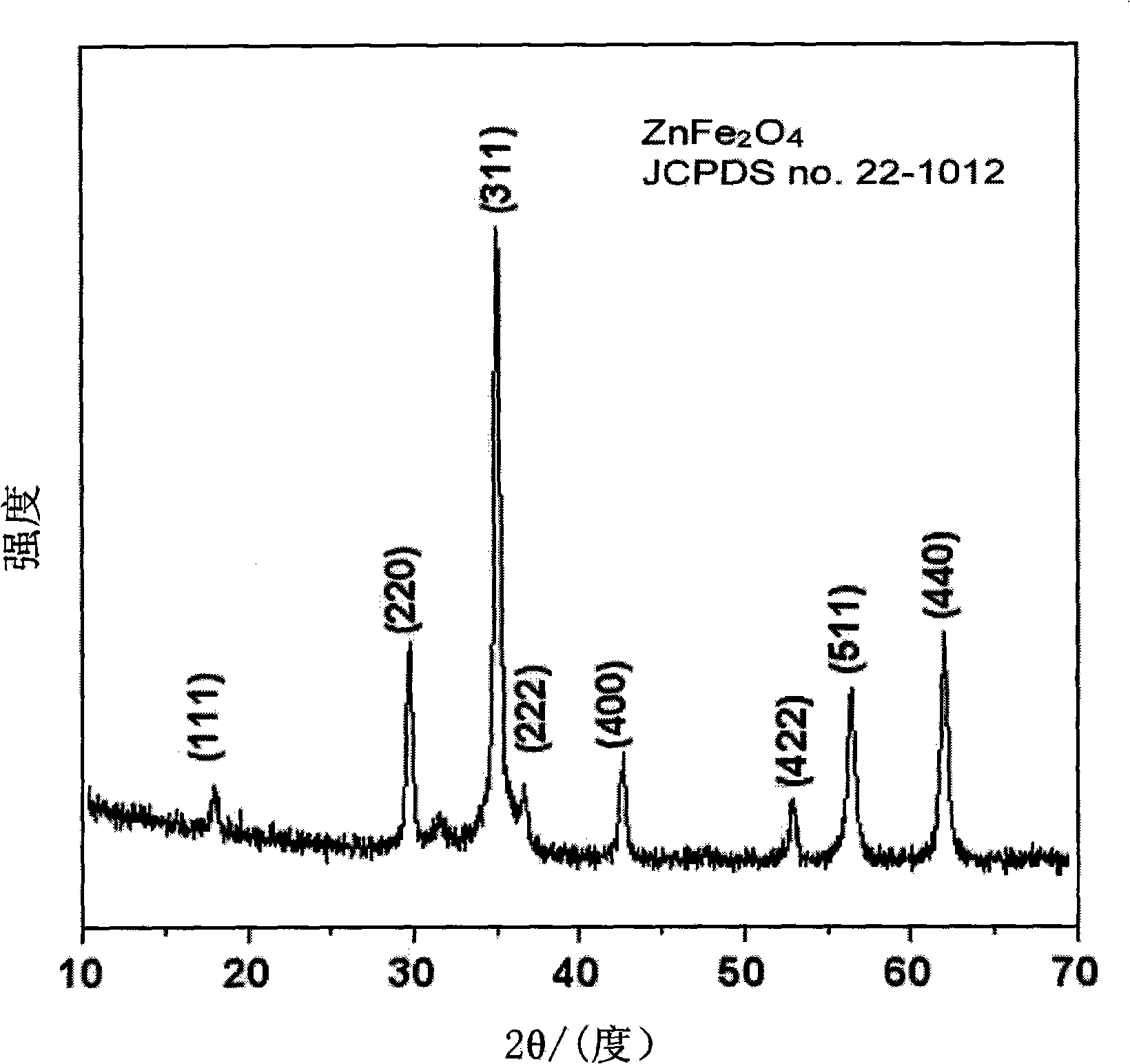
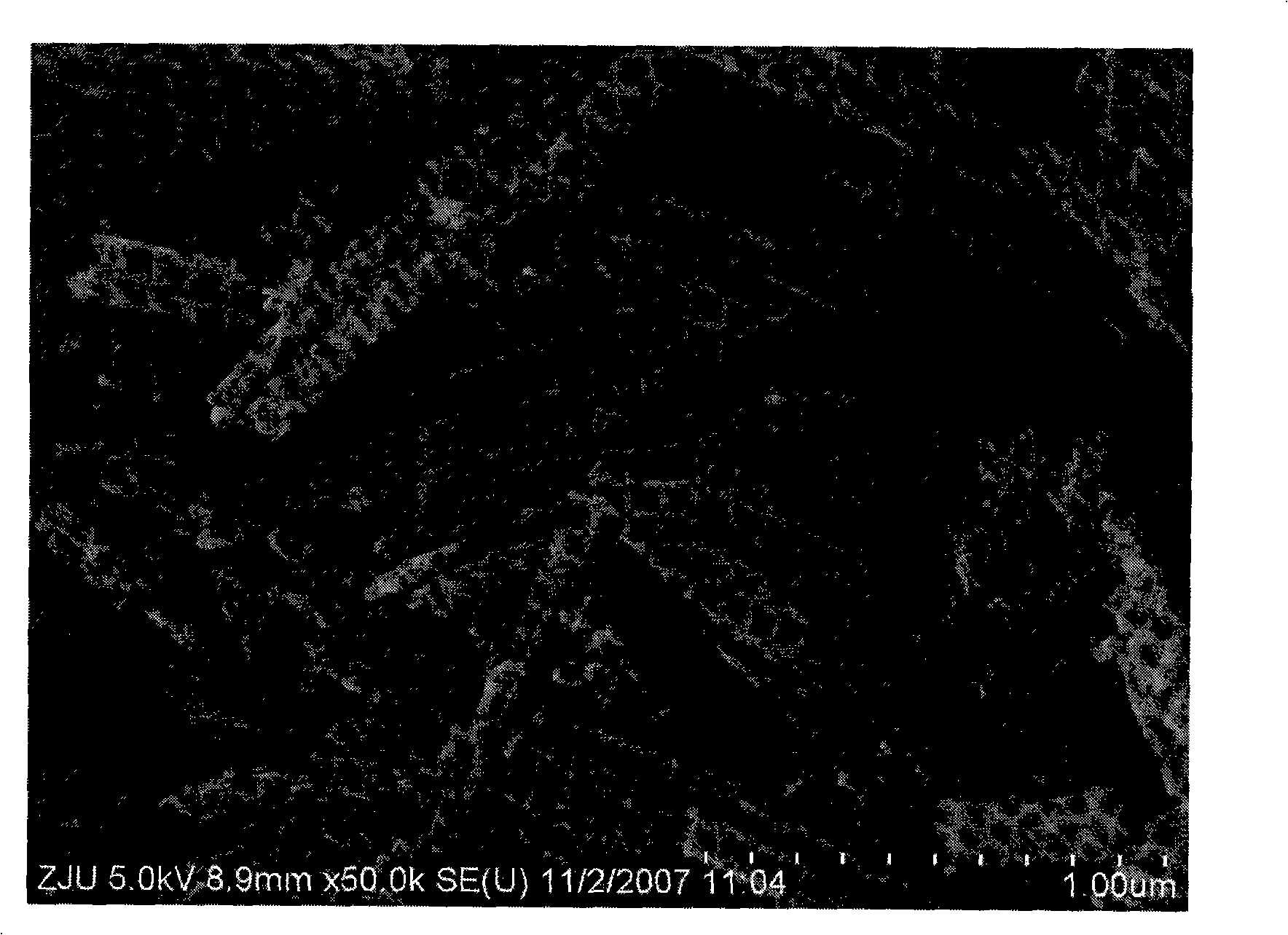
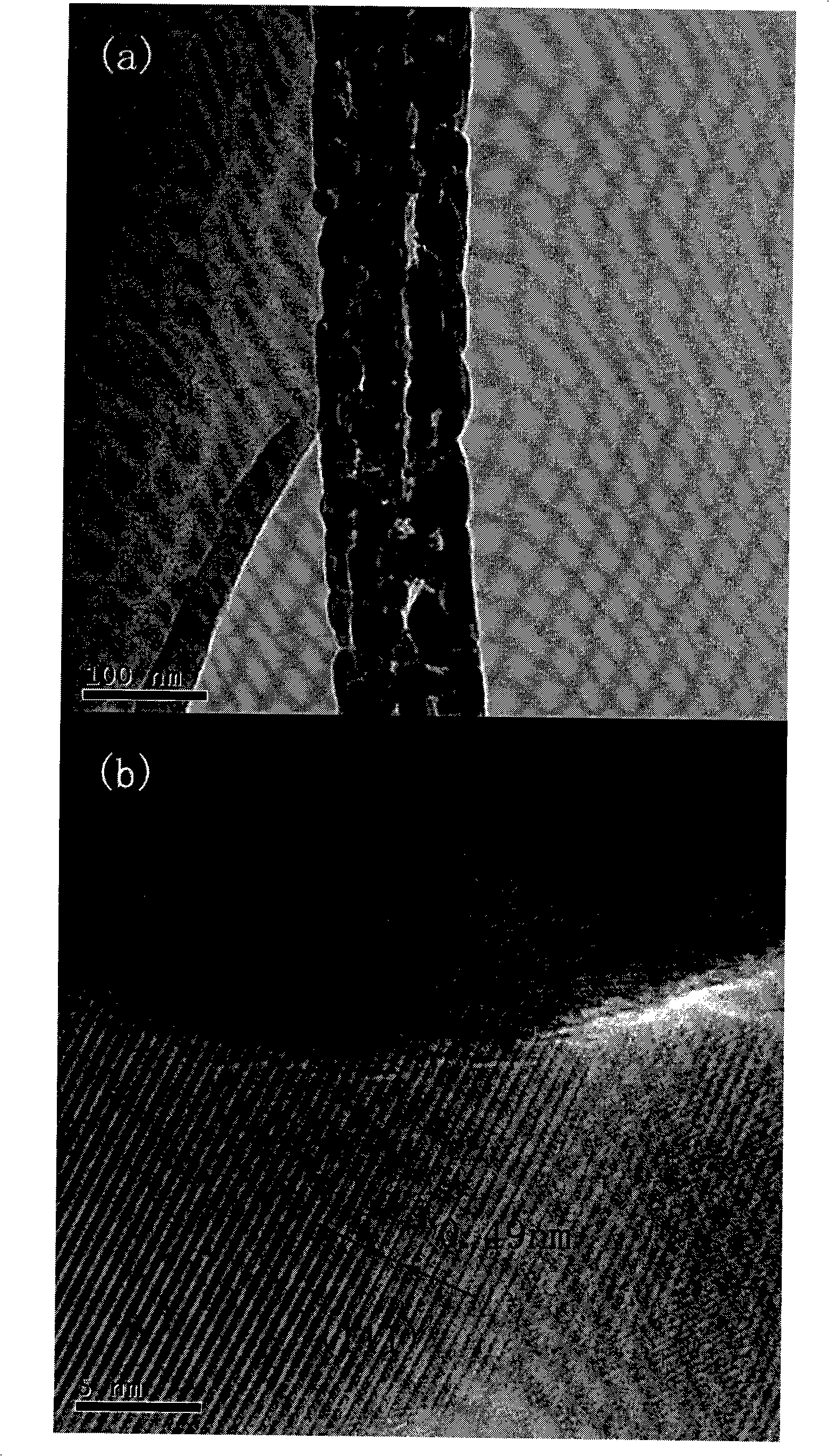
Method for preparing porous zinc ferrite nano-rods
A technology of nanorods and zinc ferrite, applied in chemical instruments and methods, zinc compounds, iron compounds, etc., can solve the problems that no one has publicly reported the preparation of one-dimensional zinc ferrite porous nanostructures, and achieve good crystallization and uniform dispersion Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] First, 0.583 g (1.6 mmol) of cetyltrimethyl bromide, 24.145 g (0.287 mole) of cyclohexane and 0.846 g (9.6 mmol) of n-pentanol were dissolved in 48 ml of deionized water, and after stirring A microemulsion system is obtained. Among them, the molar concentration of cetyl trimethyl bromide is 0.02 mol / liter, the molar ratio of deionized water to cetyl trimethyl bromide is 30, and the molar ratio of n-amyl alcohol to cetyl trimethyl bromide is for 8. Then 8 milliliters of oxalic acid with a molar concentration of 0.2 mol / liter, 8 milliliters of a molar concentration of 0.02 mol / liter of zinc nitrate solution and 16 milliliters of a molar concentration of 0.02 mol / liter of ferrous chloride solution were poured into the above microemulsion , that is, the molar ratio of oxalic acid and zinc salt in the microemulsion is 10, and the molar ratio of zinc salt and ferrous salt is 1:2. Stir for 2 hours. ZnFe was obtained after centrifugation and drying 2 (C 2 o 4 ) 3 Nanorod...
Embodiment 2
[0022] First, 0.875 g (2.4 mmol) of cetyltrimethyl bromide, 8.138 g (96.7 mmol) of cyclohexane and 1.269 g (14.4 mmol) of n-pentanol were dissolved in 48 ml of deionized water and stirred Finally, a microemulsion system is obtained. Among them, the molar concentration of cetyltrimethyl bromide is 0.04 mol / liter, the molar ratio of deionized water to cetyltrimethyl bromide is 20, and the molar ratio of n-amyl alcohol to cetyltrimethyl bromide is for 6. Then respectively 6 milliliters of molar concentrations are 0.4 mol / liter of oxalic acid, 2 milliliters of molar concentrations of 0.04 mol / liter of zinc sulfate solution and 4 milliliters of molar concentrations of 0.04 mol / liter of ferrous sulfate solution into the above microemulsion, That is, the molar ratio of oxalic acid to zinc salt in the microemulsion is 30, and the molar ratio of zinc salt to ferrous salt is 1:2. Stir for 10 hours. ZnFe was obtained after centrifugation and drying 2 (C 2 o 4 ) 3 Nanorod precursor...
Embodiment 3
[0024] First, 1.166 g (3.2 mmol) of cetyltrimethyl bromide, 4.611 g (54.78 mmol) of cyclohexane and 1.692 g (19.2 mmol) of n-pentanol were dissolved in 32 ml of deionized water and stirred Finally, a microemulsion system is obtained. Among them, the molar concentration of cetyl trimethyl bromide is 0.08 mol / liter, the molar ratio of deionized water to cetyl trimethyl bromide is 10, and the molar ratio of n-amyl alcohol to cetyl trimethyl bromide is for 4. Then 4 milliliters of oxalic acid with a molar concentration of 0.8 mol / liter, 6.4 milliliters of a molar concentration of 0.01 mol / liter of zinc chloride solution and 12.8 milliliters of a molar concentration of 0.01 mol / liter of ferrous sulfate solution were poured into the above microemulsion , that is, the molar ratio of oxalic acid and zinc salt in the microemulsion is 50, and the molar ratio of zinc salt and ferrous salt is 1:2. Stir for 24 hours. ZnFe was obtained after centrifugation and drying 2 (C 2 o 4 ) 3 N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com