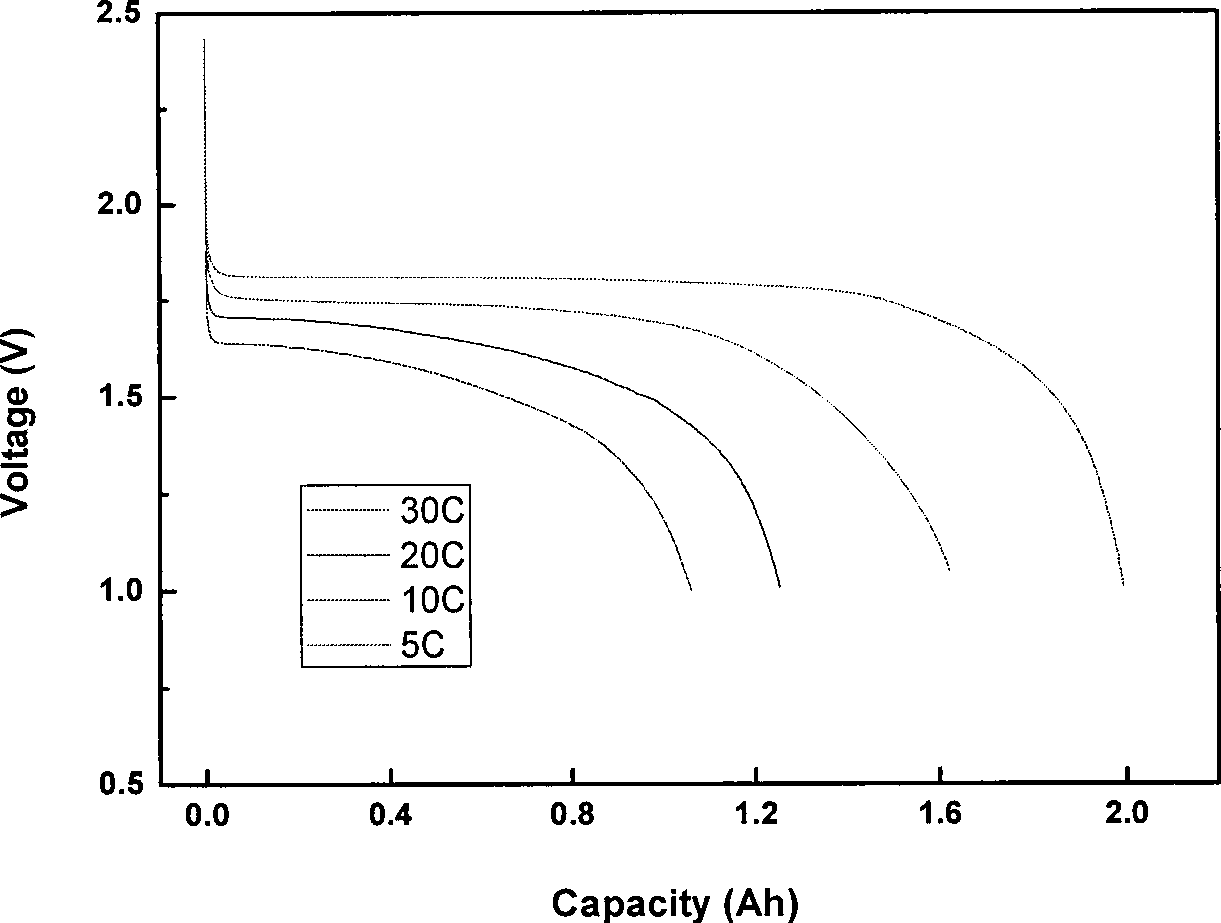
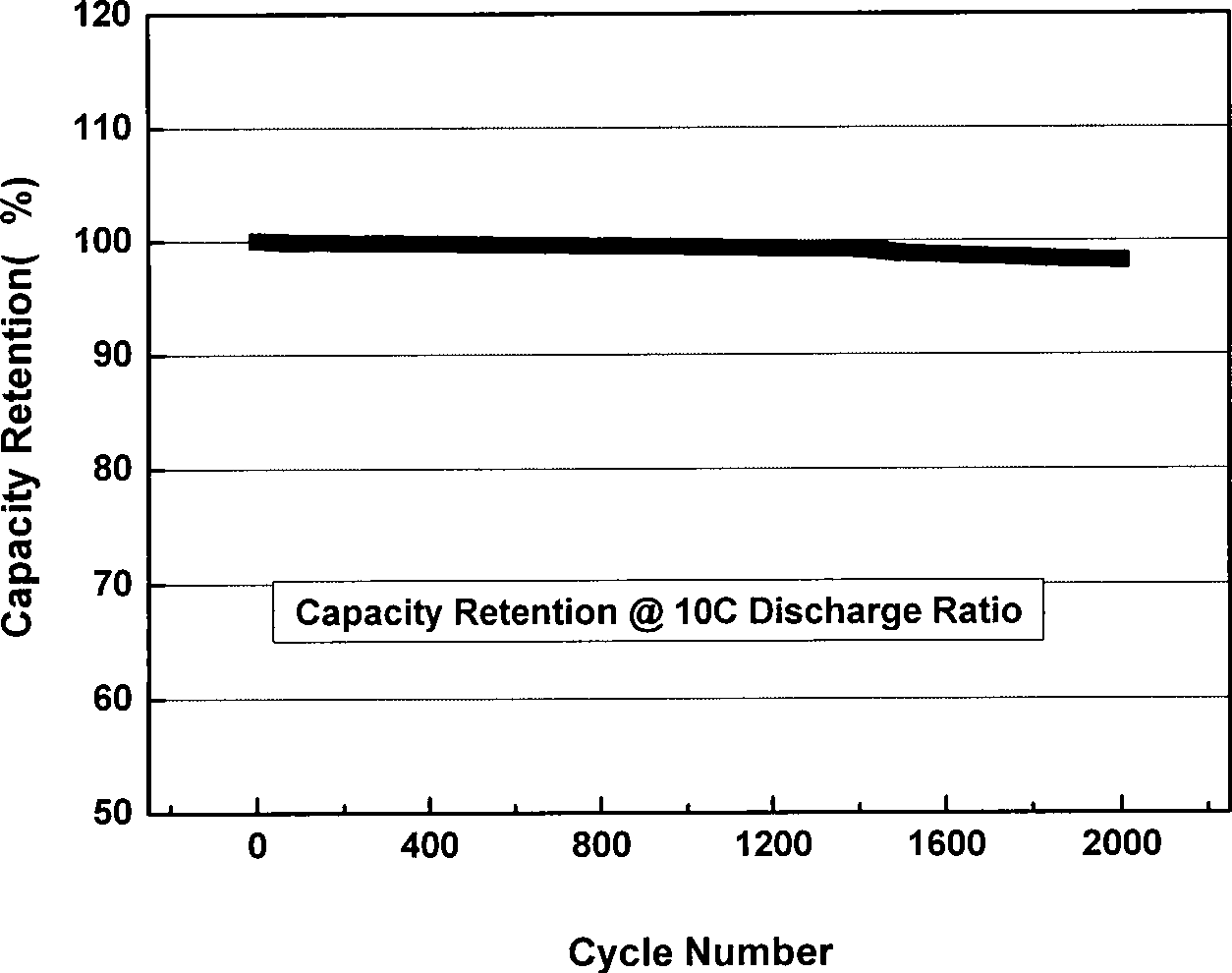
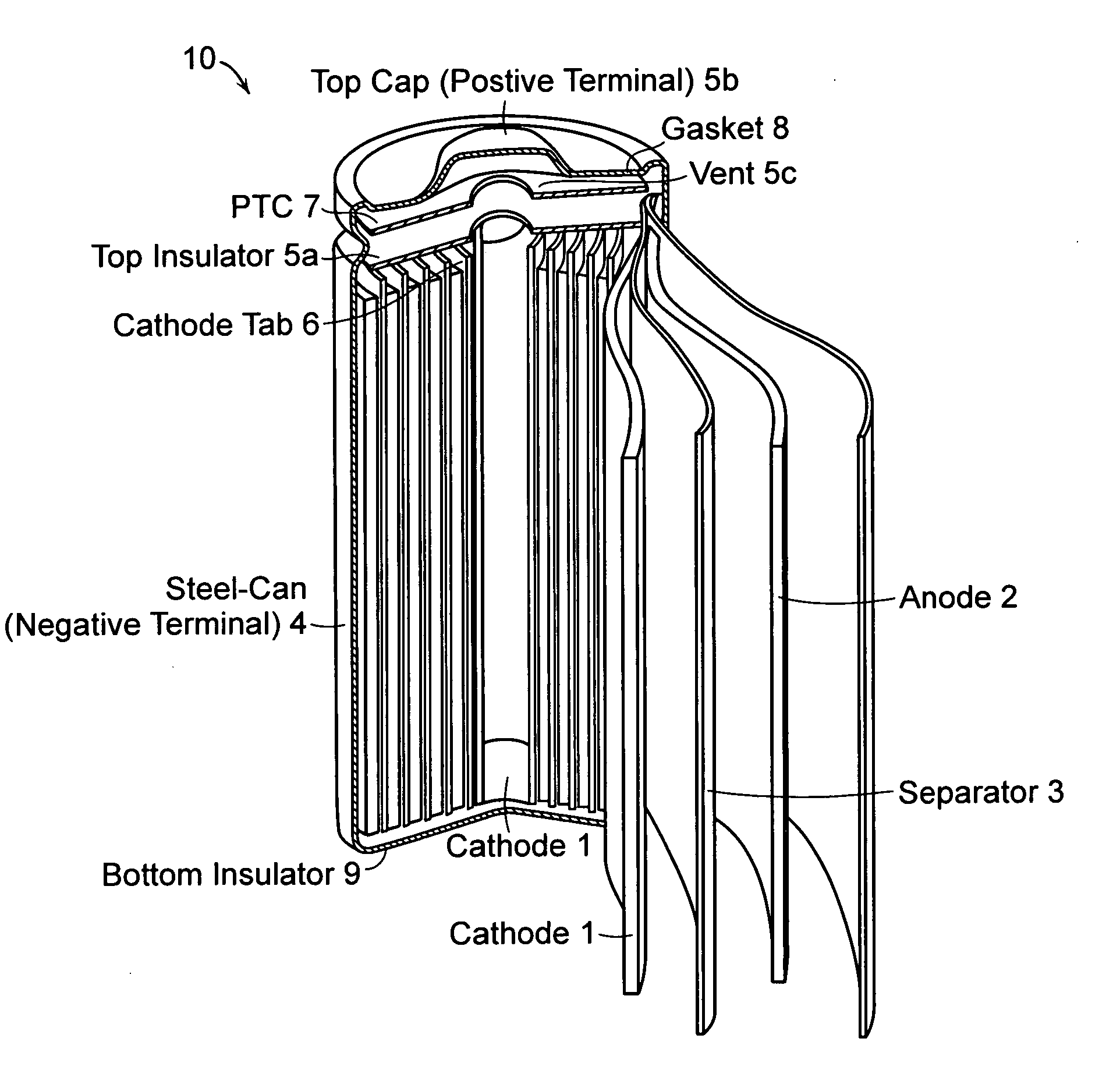
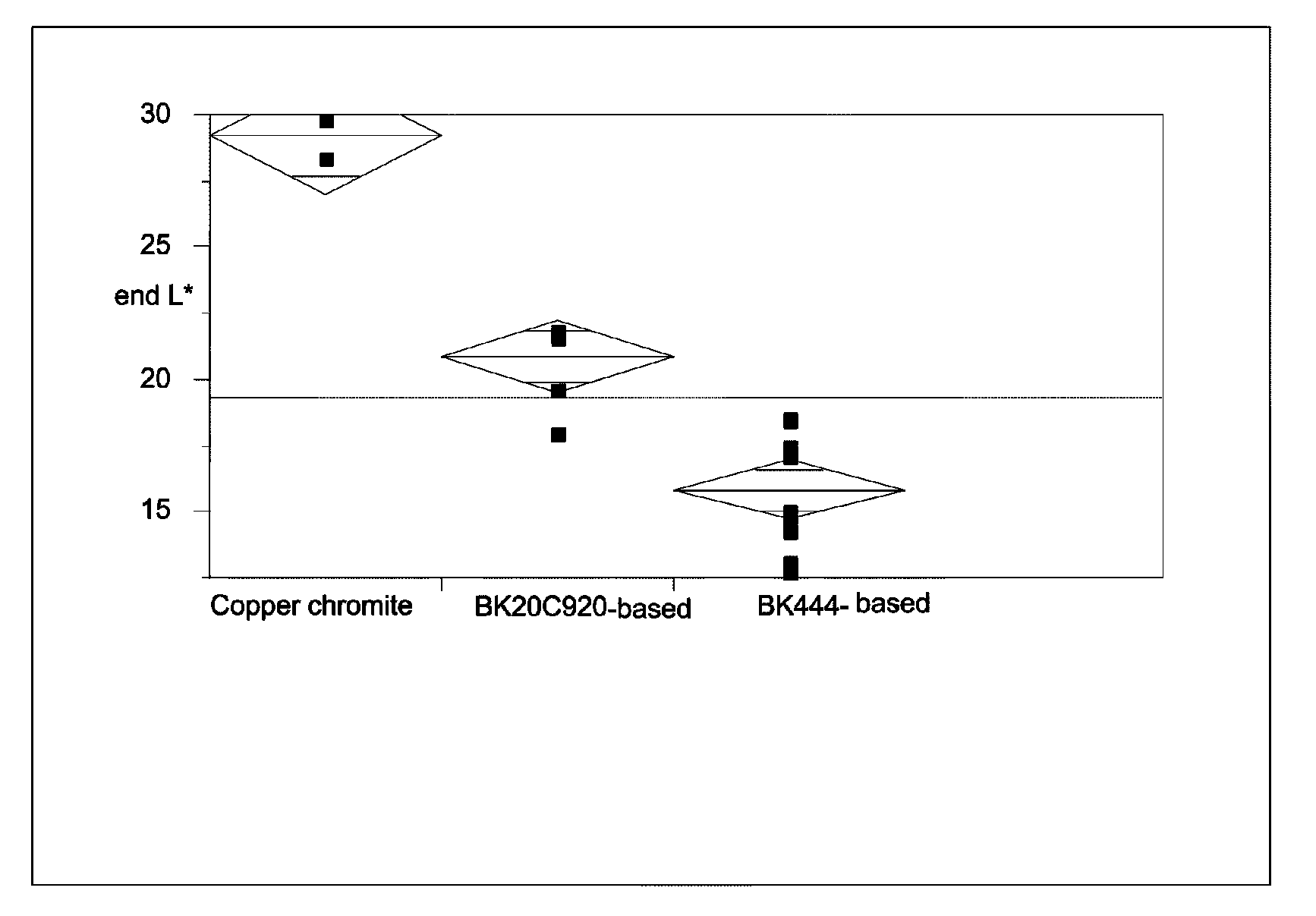
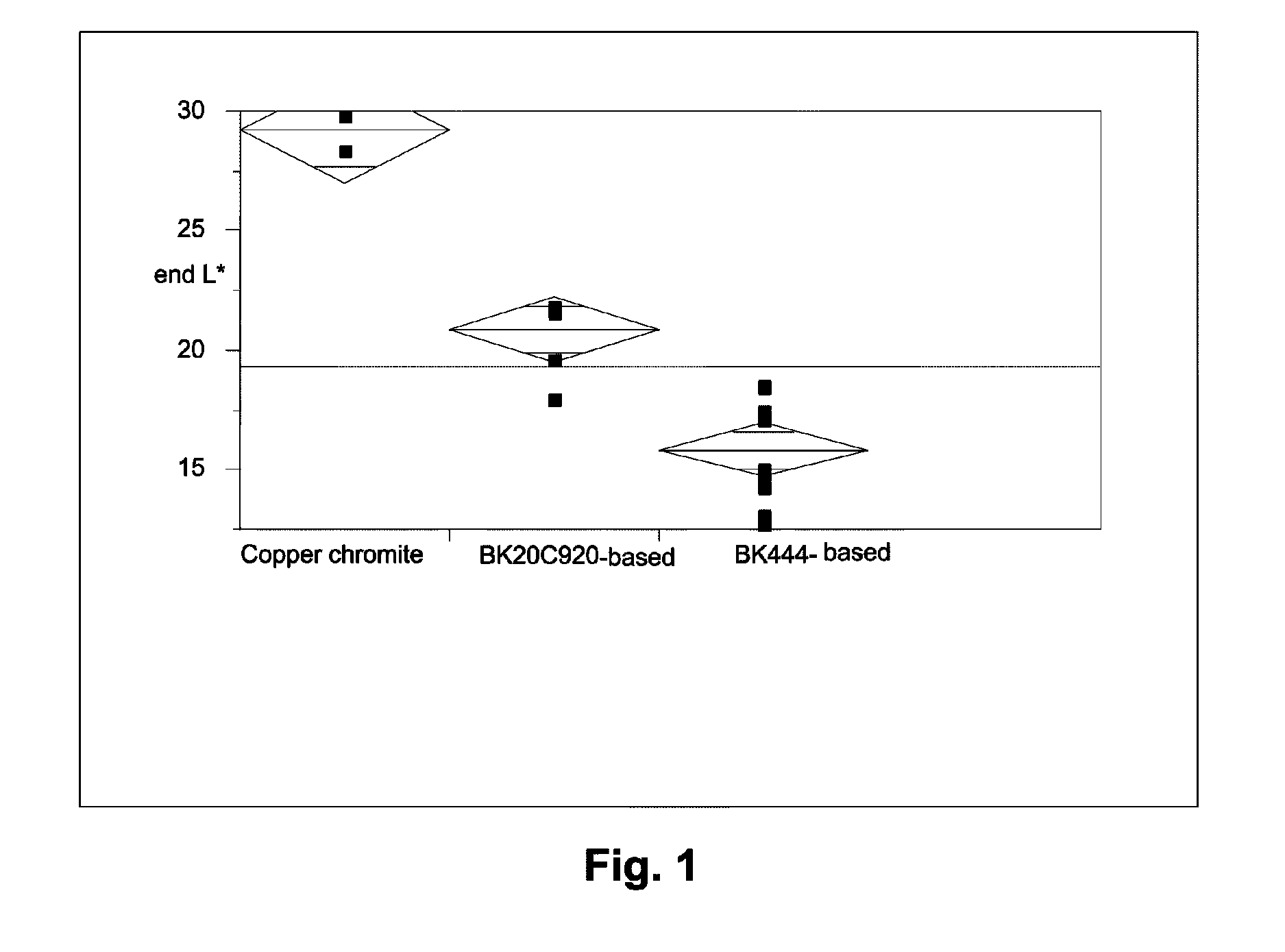
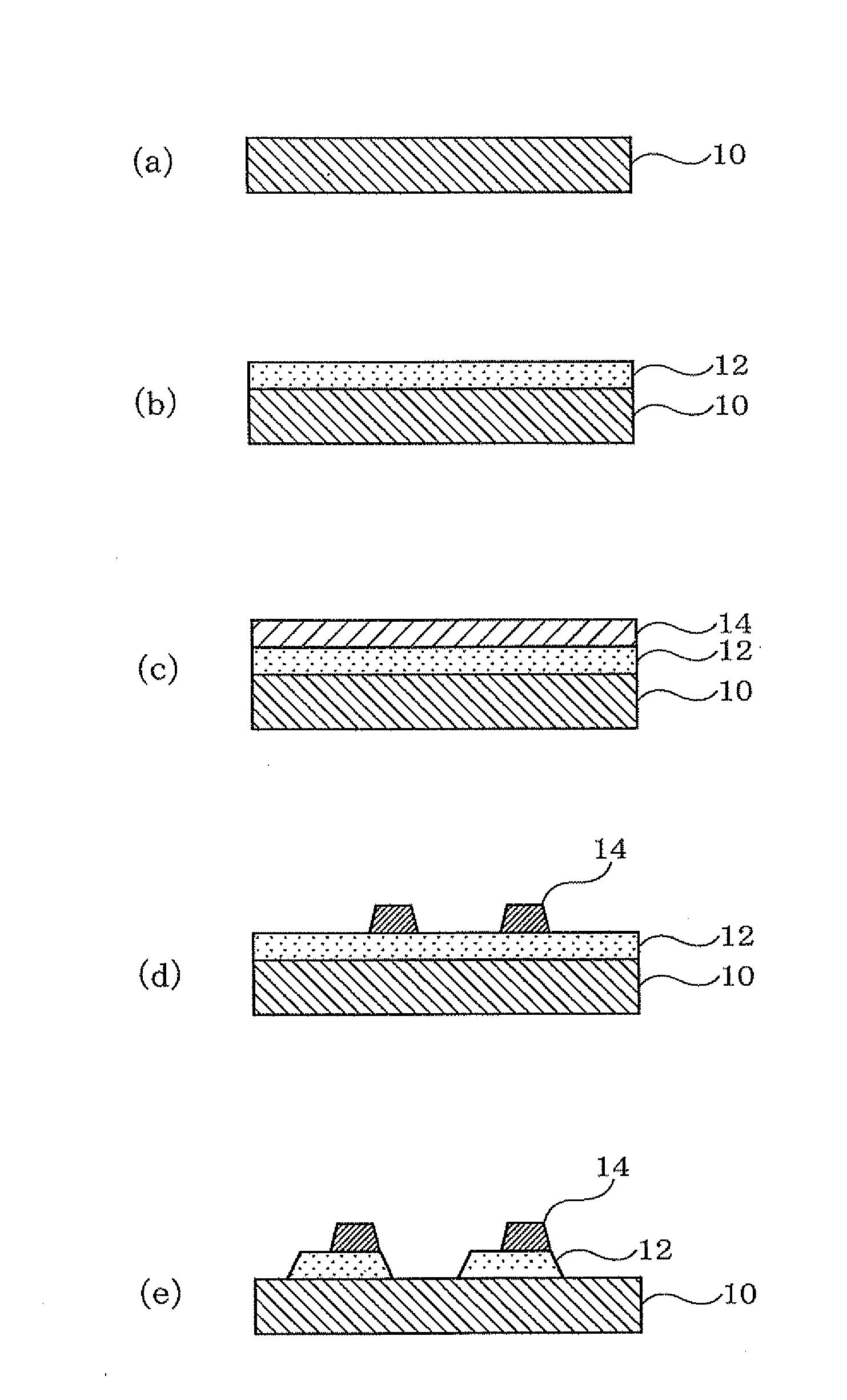
Patents
Literature
3035 results about "Spinel" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
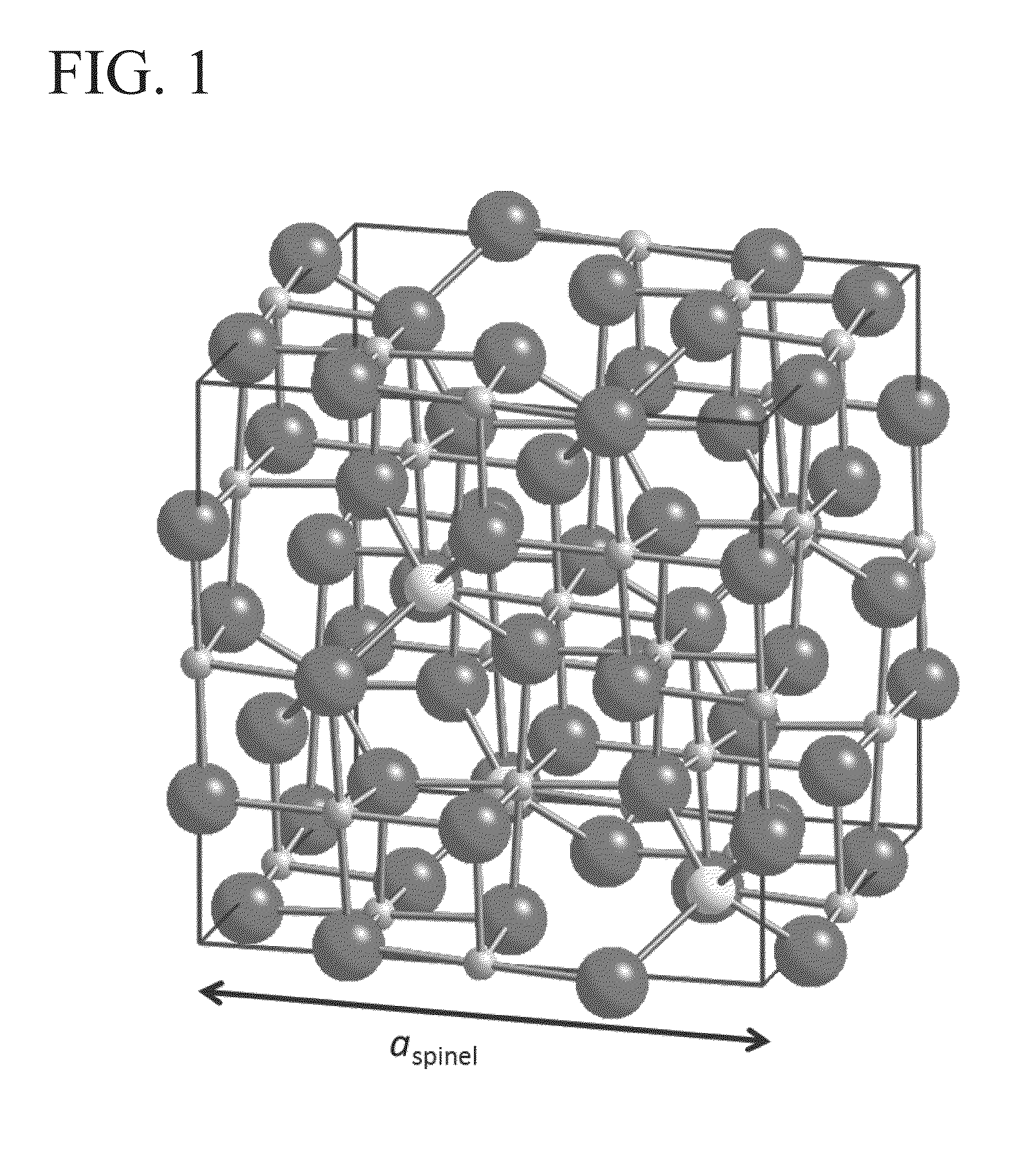
Spinel ( /spɪˈnɛl/) is the magnesium/aluminium member of the larger spinel group of minerals. It has the formula MgAl₂O₄ in the cubic crystal system. Its name comes from the Latin word "spinella", which means spine in reference to its pointed crystals.
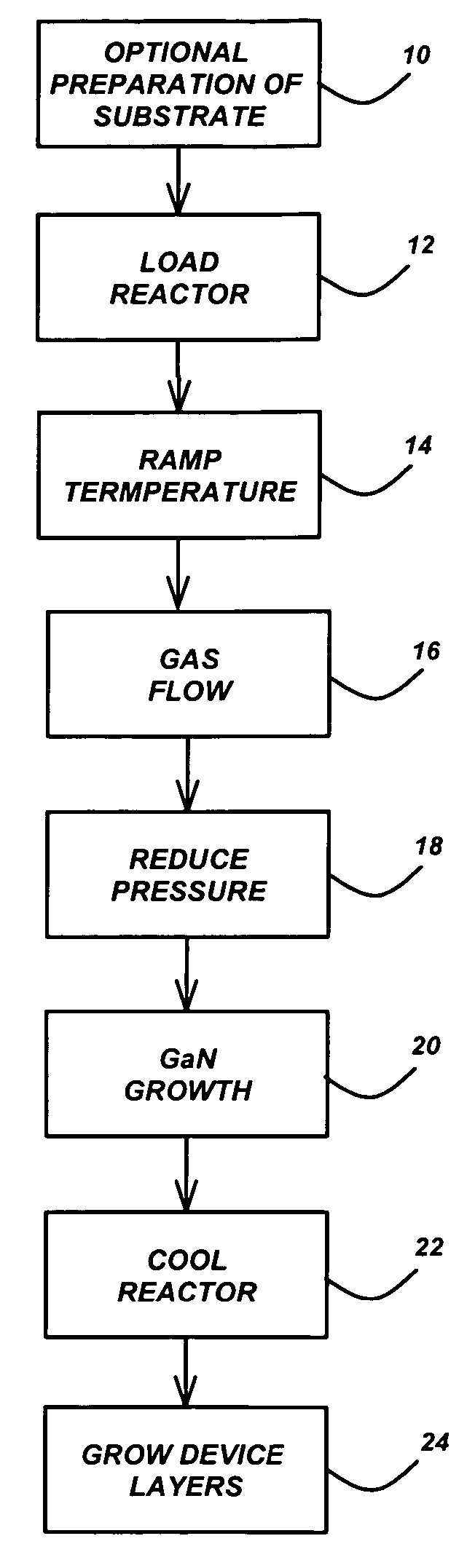
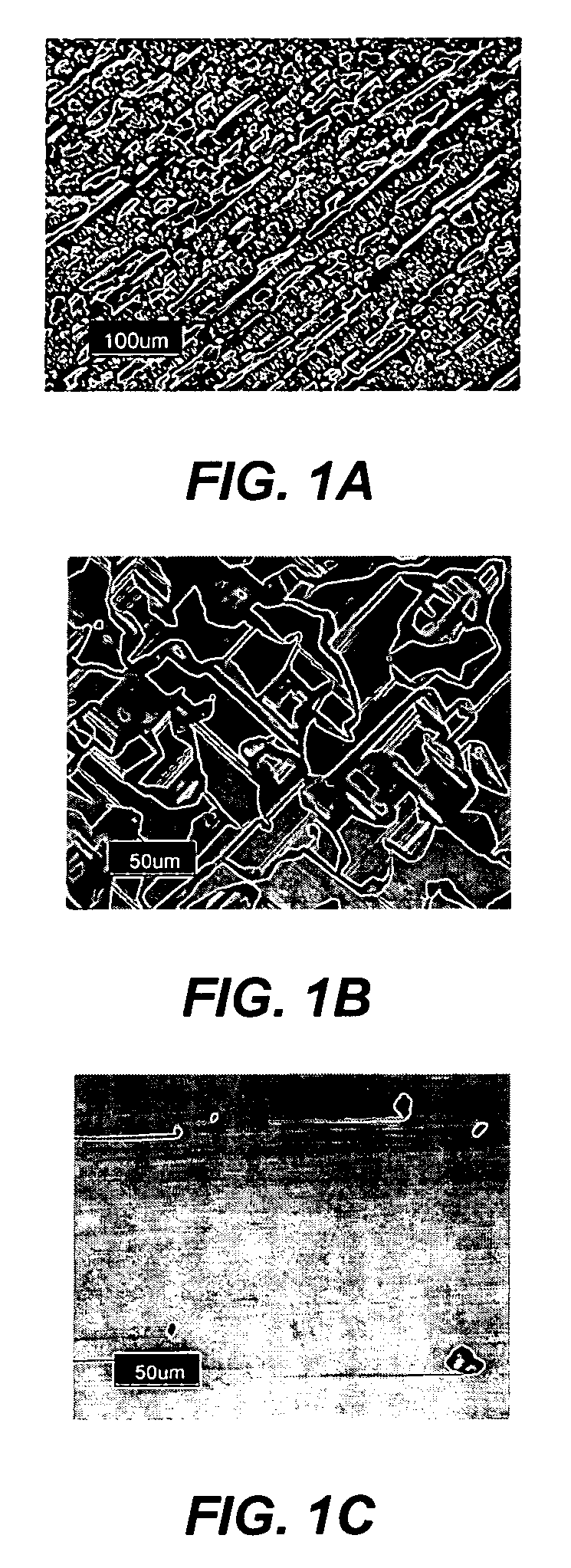
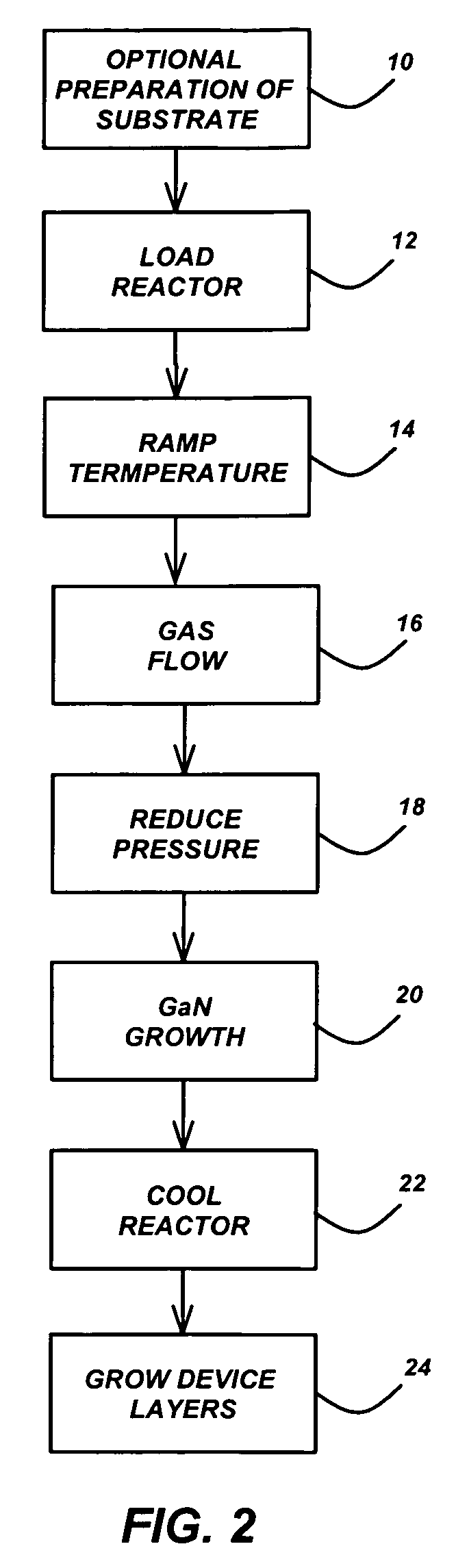
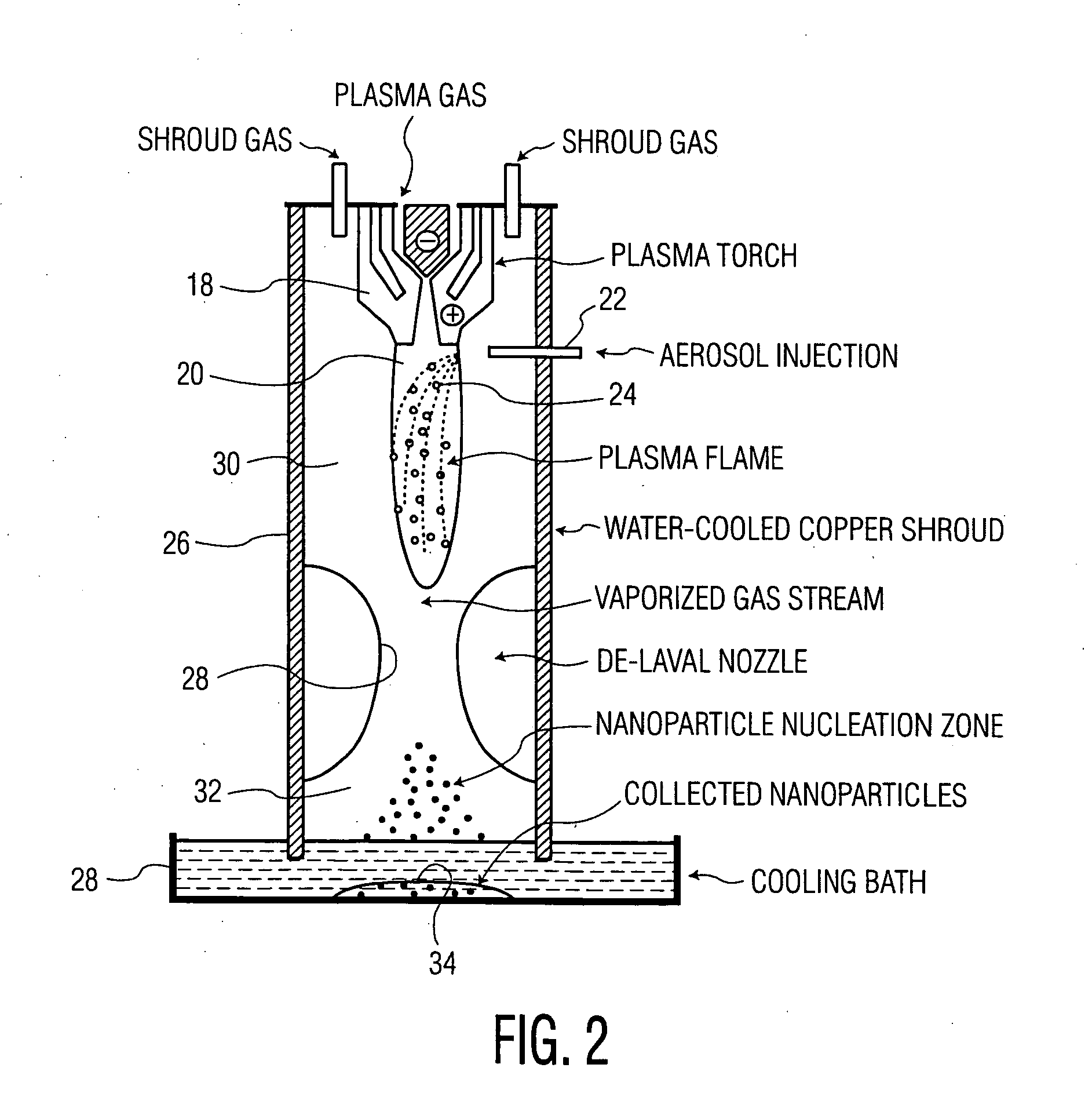
Technique for the growth of planar semi-polar gallium nitride
ActiveUS20060205199A1Reduce the impactReduce impactPolycrystalline material growthSemiconductor/solid-state device manufacturingSpinelGallium nitride
A method for growing planar, semi-polar nitride film on a miscut spinel substrate, in which a large area of the planar, semi-polar nitride film is parallel to the substrate's surface. The planar films and substrates are: (1) {10{overscore (1)}1} gallium nitride (GaN) grown on a {100} spinel substrate miscut in specific directions, (2) {10{overscore (1)}3} gallium nitride (GaN) grown on a {110} spinel substrate, (3) {11{overscore (2)}2} gallium nitride (GaN) grown on a {1{overscore (1)}00} sapphire substrate, and (4) {11{overscore (1)}3} gallium nitride (GaN) grown on a {1{overscore (1)}00} sapphire substrate
Owner:JAPAN SCI & TECH CORP
Conductor track structures and method for production thereof
InactiveUS7060421B2Simple structureProduced simply and reliablyPhotomechanical apparatusLiquid/solution decomposition chemical coatingElectrical conductorSpinel
Conductive tracks disposed on an electrically non-conductive support material by depositing a metallized layer on metal nuclei produced by using electromagnetic radiation to break up electrically non-conductive metal compounds dispersed in the support material, and a method for producing them. The electrically non-conductive metal compounds are insoluble spinel-based inorganic oxides which are thermally stable and are stable in acidic or alkaline metallization baths, and which are higher oxides with a spinel structure, and which remain unchanged in non-irradiated areas. The spinel-based inorganic oxides used are heat resistant and remain stable after being subjected to soldering temperatures. The conductor tracks are reliably and easily produced and adhere strongly to the support.
Owner:PAPST MOTOREN GMBH & CO KG
Titanium-series cathode active material and preparation method thereof, titanium-series lithium ion power battery
ActiveCN101373829AIncrease capacityHigh bulk densityElectrode manufacturing processesLi-accumulatorsHigh rateLithium titanate
The invention discloses a titanium cathode active substance, a preparation method thereof and a titanium lithium ion power battery, and aims to solve the technical problem of enhancing the rate performance of a lithium ion power battery. The formula of the titanium cathode active substance is Li4Ti5O12 / Mx, wherein Li4Ti5O12 is spinel lithium titanate, M is a dopant such as a metal simple substance, a metal compound, a nonmetallic simple substance or a nonmetallic compound; the elements or the ions contained in the dopant enter the Li4Ti5O12 crystal lattice or are compounded with the Li4Ti5O12 crystal lattice; and the preparation method comprises the following steps: the precursor mixture of compound lithium titanate is prepared, and spray drying and heat treatment are performed. The cathode of the titanium lithium ion power battery adopts Li4Ti5O12 / Mx. Compared with the prior art, the titanium cathode active substance has the advantages of high capacity, high bulk density, high volume specific capacity, good high-rate performance, good product uniformity, good battery processability, low possibility of air bulking of the battery, and low cost.
Owner:BTR NEW MATERIAL GRP CO LTD
Lithium-ion secondary battery
InactiveUS20070026315A1Safer chemistry characteristicLow cathode costPrimary cell to battery groupingFinal product manufactureManganateSpinel
A lithium-ion battery includes a cathode that includes an active cathode material. The active cathode material includes a cathode mixture that includes a lithium cobaltate and a manganate spinel a manganate spinel represented by an empirical formula of Li(1+x1)(Mn1−y1A′y2)2−x2Oz1. The lithium cobaltate and the manganate spinel are in a weight ratio of lithium cobaltate: manganate spinel between about 0.95:0.05 to about 0.55:0.45. A lithium-ion battery pack employs a cathode that includes an active cathode material as described above. A method of forming a lithium-ion battery includes the steps of forming an active cathode material as described above; forming a cathode electrode with the active cathode material; and forming an anode electrode in electrical contact with the cathode via an electrolyte.
Owner:BOSTON POWER INC
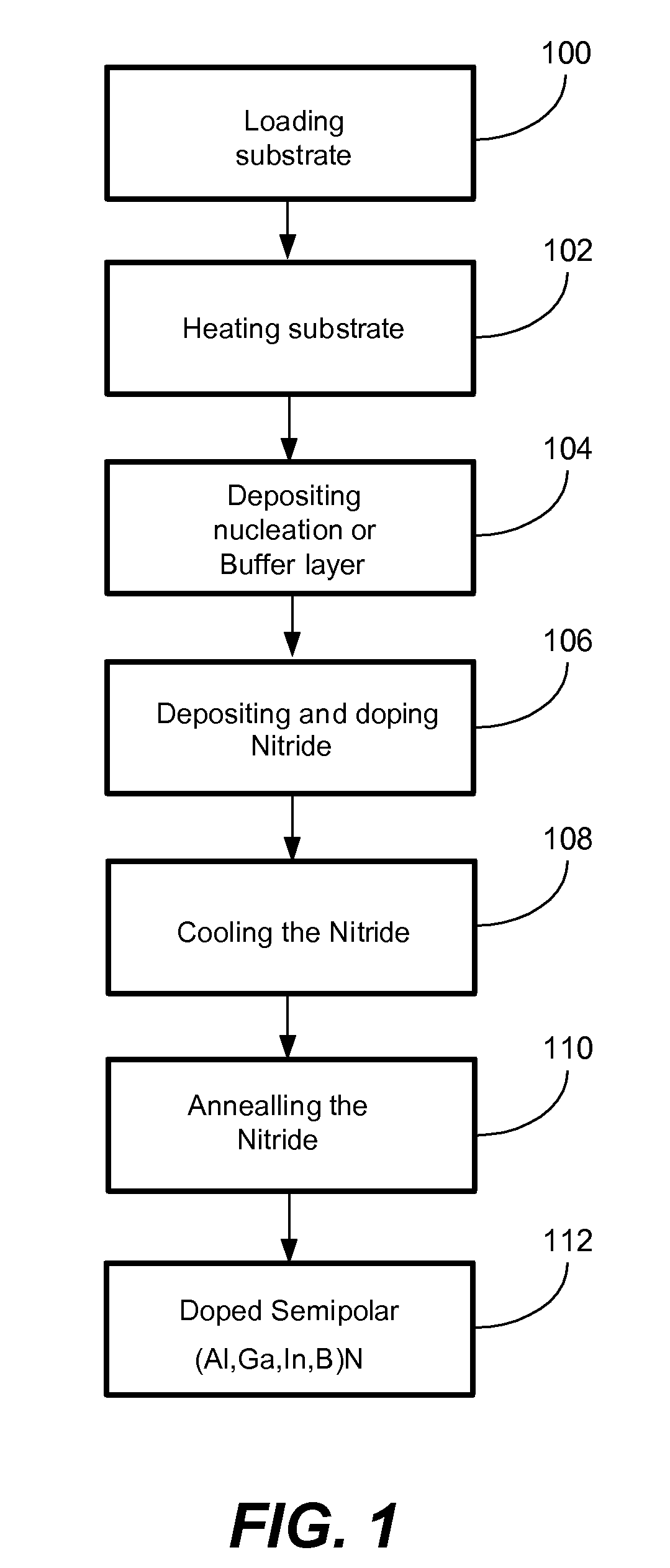
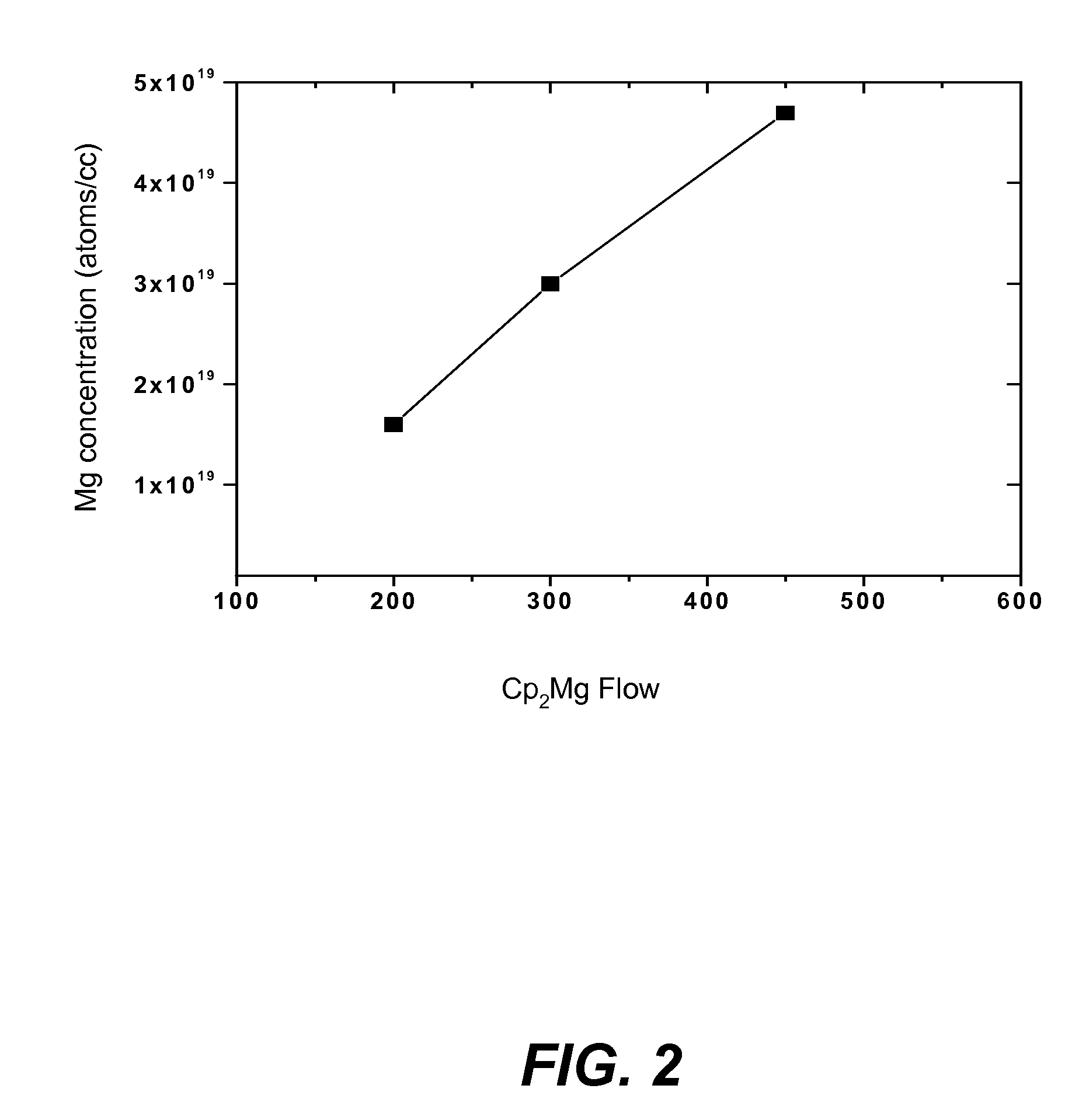
METHOD FOR CONDUCTIVITY CONTROL OF (Al,In,Ga,B)N
ActiveUS20070190758A1Improve conductivityEnhancing or tailoring conductivity propertiesSemiconductor/solid-state device manufacturingSemiconductor devicesElectronic statesSpinel
A method of controlled p-type conductivity in (Al,In,Ga,B)N semiconductor crystals. Examples include {10 11} GaN films deposited on {100} MgAl2O4 spinel substrate miscut in the <011> direction. Mg atoms may be intentionally incorporated in the growing semipolar nitride thin film to introduce available electronic states in the band structure of the semiconductor crystal, resulting in p-type conductivity. Other impurity atoms, such as Zn or C, which result in a similar introduction of suitable electronic states, may also be used.
Owner:RGT UNIV OF CALIFORNIA
Ferromagnetic Tunnel Junction Structure and Magnetoresistive Effect Device and Spintronics Device Utilizing Same
ActiveUS20130221461A1High TMR valueQuality improvementMagnetic-field-controlled resistorsSolid-state devicesCrystalline oxideSpace group
A ferromagnetic tunnel junction structure comprising a first ferromagnetic layer, a second ferromagnetic layer, and a tunnel barrier layer that is interposed between the first ferromagnetic layer and the second ferromagnetic layer, wherein the tunnel barrier layer includes a crystalline non-magnetic material having constituent elements that are similar to those of an crystalline oxide that has spinel structure as a stable phase structure; the non-magnetic material has a cubic structure having a symmetry of space group Fm-3m or F-43m in which atomic arrangement in the spinel structure is disordered; and an effective lattice constant of the cubic structure is substantially half of the lattice constant of the oxide of the spinel structure.
Owner:NAT INST FOR MATERIALS SCI
Process for activating cobalt catalysts
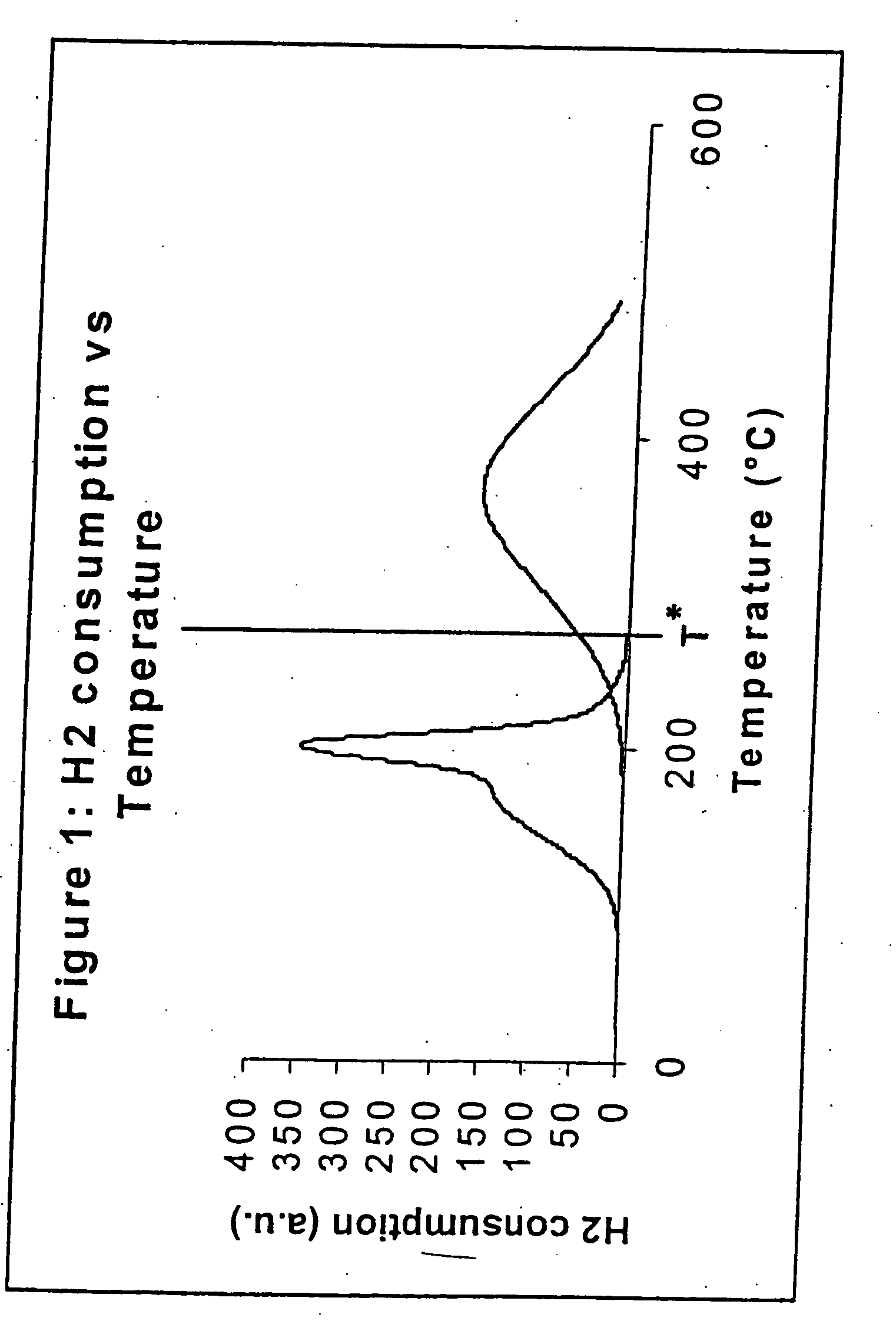
InactiveUS20050227866A1Improve reducibilityCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsParticulatesHydrogen
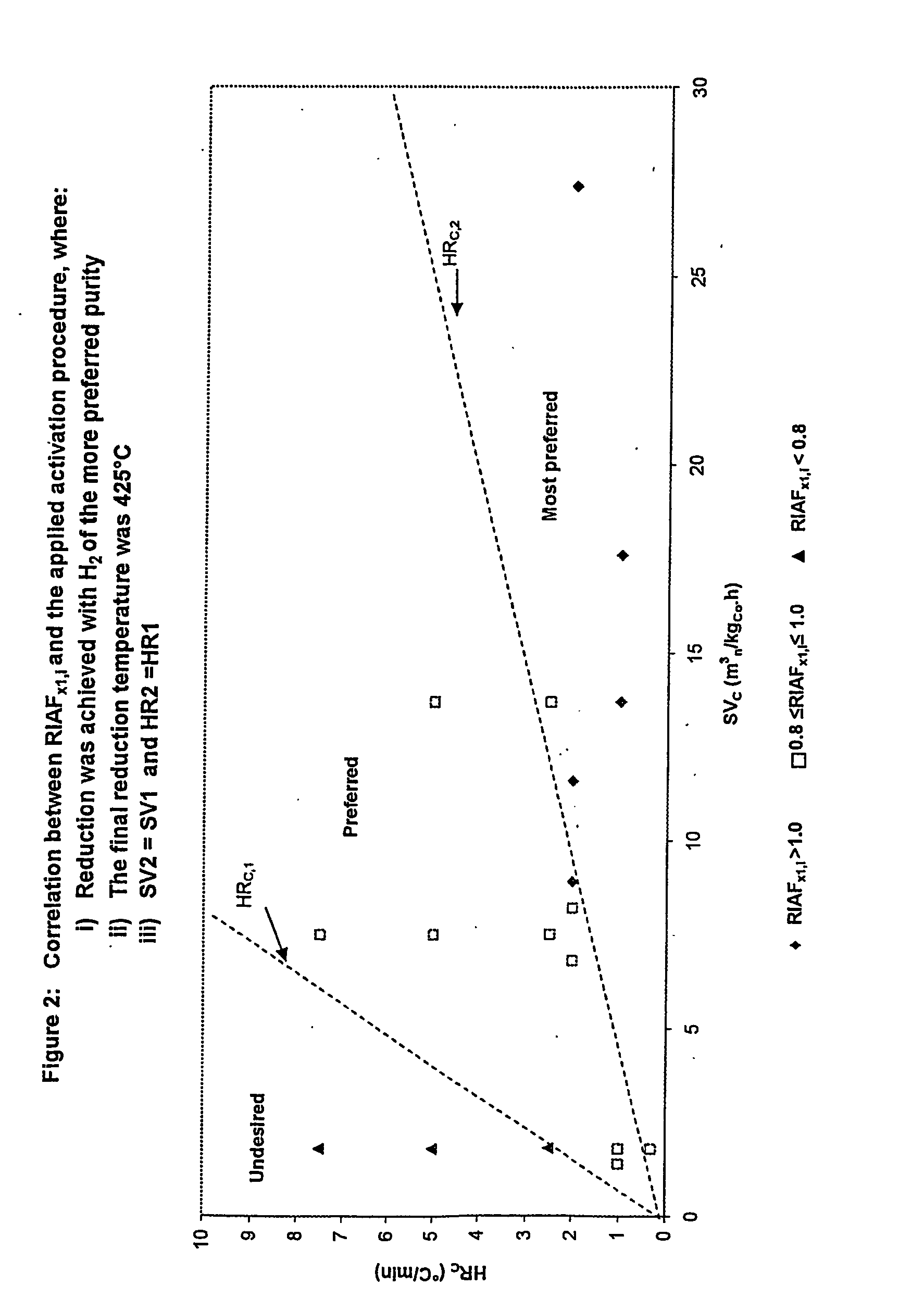
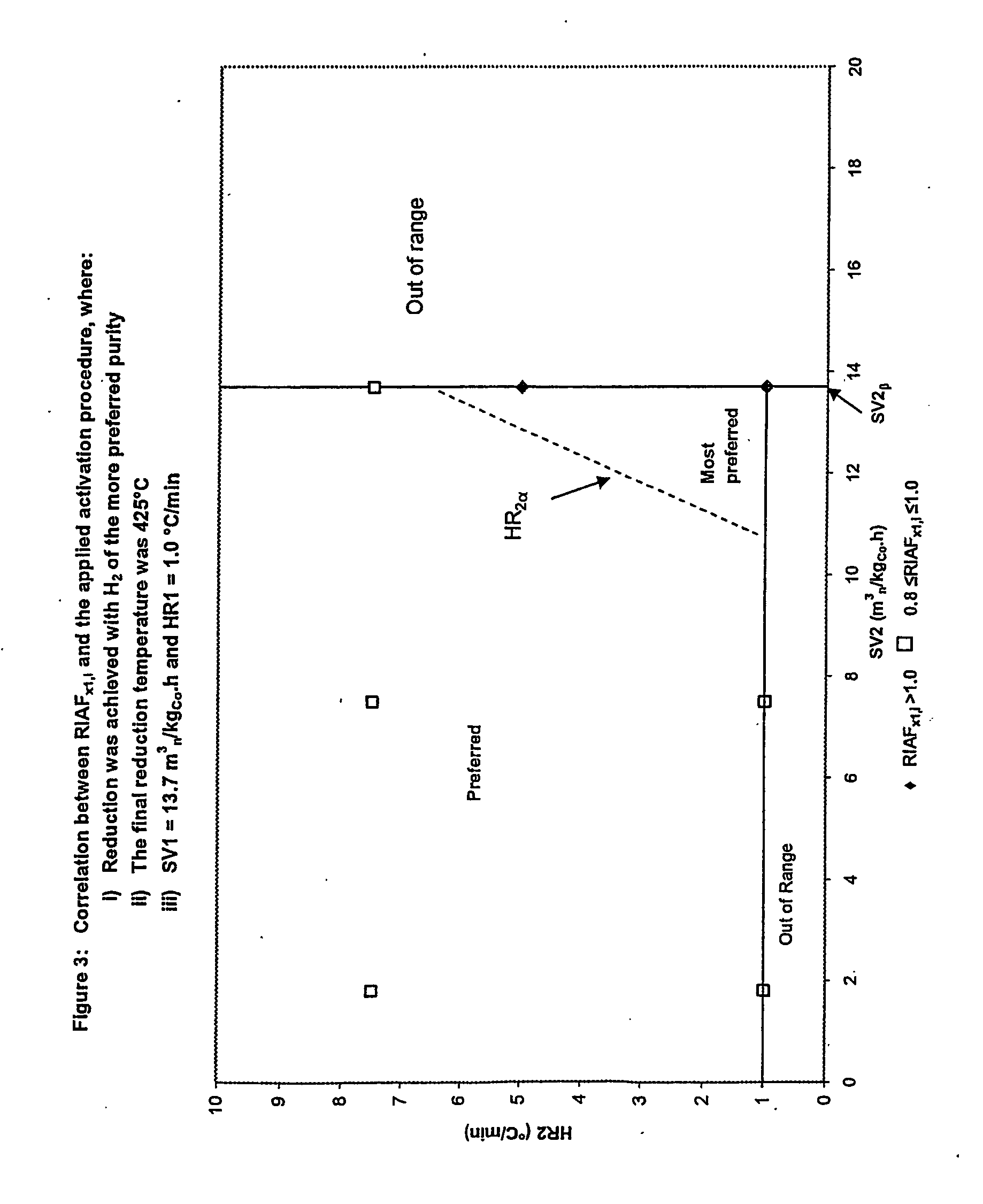
A particulate pre-reduction cobalt supported Fischer-Tropsch synthesis catalyst precursor which comprises a catalyst support impregnated with cobalt, is treated with a pure hydrogen reducing gas, at a first specific feed gas space velocity, SV1, and at a first heating rate, HR1, to obtain a partially reduced precursor. The support contains reducible cobalt oxide in a calcined state and having a formula-unit in which each mole of cobalt atoms is associated with more than 4 / 3 moles of oxygen atoms and displaying a reducible cobalt oxide specific surface area at least equal to that of Co3O4 spinel. The partially reduced precursor is then treated with a pure hydrogen reducing gas, at a second specific feed gas space velocity, SV2, and at a second heating rate, HR2, to obtain an activated supported Fischer-Tropsch catalyst, with SV2≦SV1 and / or HR2≧HR1; however, when SV2=SV1, HR2≠HR1 and when HR2=HR1, SV2≠SV1.
Owner:SASOL TEKHNOLODZHI PROPRIEHJTEHRI LTD
Electrophoretic particles, and processes for the production thereof
ActiveUS8270064B2Easy to useGood color saturationPigmenting treatmentStatic indicating devicesMagnetiteManganese oxide
An electrophoretic medium comprises at least one electrically charged particle dispersed posed in a fluid. The electrically charged particle comprises an inorganic black pigment having a surface area of at least about 7 m2 / g. Preferred pigments are magnetite and mixed metal oxides containing two or more of iron, chromium, nickel, manganese, copper and cobalt, for example copper iron manganese oxide spinel and copper chromium manganese oxide spinel. The inorganic black pigment may bear a polymer coating.
Owner:E INK CORPORATION +1
Lithium-ion secondary battery
InactiveUS20080008933A1Safer chemistry characteristicLow cathode costPrimary cell to battery groupingElectrode carriers/collectorsManganateManganese
In one embodiment, an active cathode material comprises a mixture that includes: at least one of a lithium cobaltate and a lithium nickelate; and at least one of a manganate spinel represented by an empirical formula of Li(1+x1)(Mn1−y1A′y1)2−x1Oz1 and an olivine compound represented by an empirical formula of Li(1−x2)A″x2MPO4. In another embodiment, an active cathode material comprises a mixture that includes: a lithium nickelate selected from the group consisting of LiCoO2-coated LiNi0.8Co0.15Al0.05O2, and Li(Ni1 / 3Co1 / 3Mn1 / 3)O2; and a manganate spinel represented by an empirical formula of Li(1+x7)Mn2−y7Oz7. A lithium-ion battery and a battery pack each independently employ a cathode that includes an active cathode material as described above. A method of forming a lithium-ion battery includes the steps of forming an active cathode material as described above; forming a cathode electrode with the active cathode material; and forming an anode electrode in electrical contact with the cathode via an electrolyte. A system comprises a portable electronic device and a battery pack or lithium-ion battery as described above.
Owner:BOSTON POWER INC
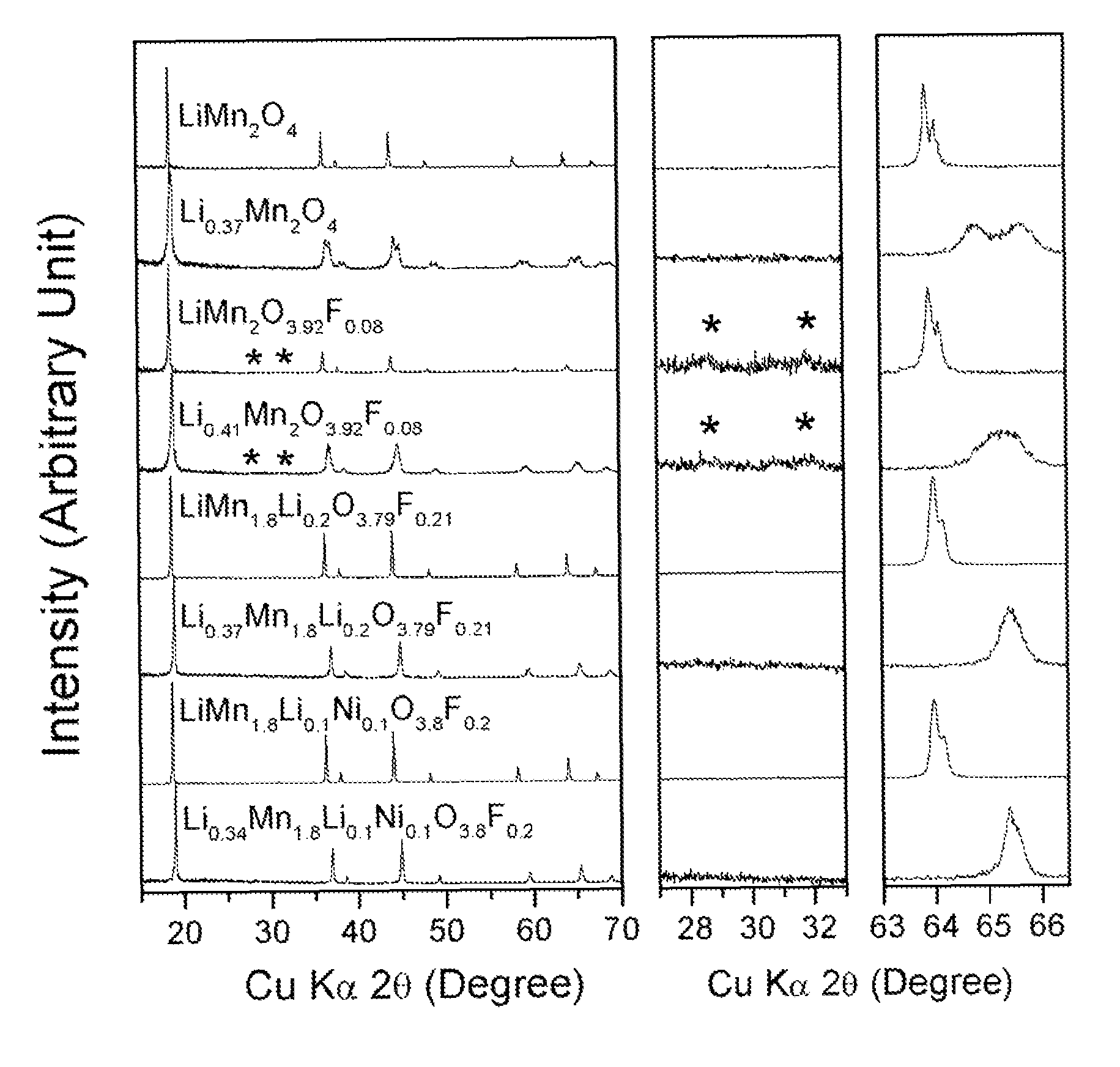
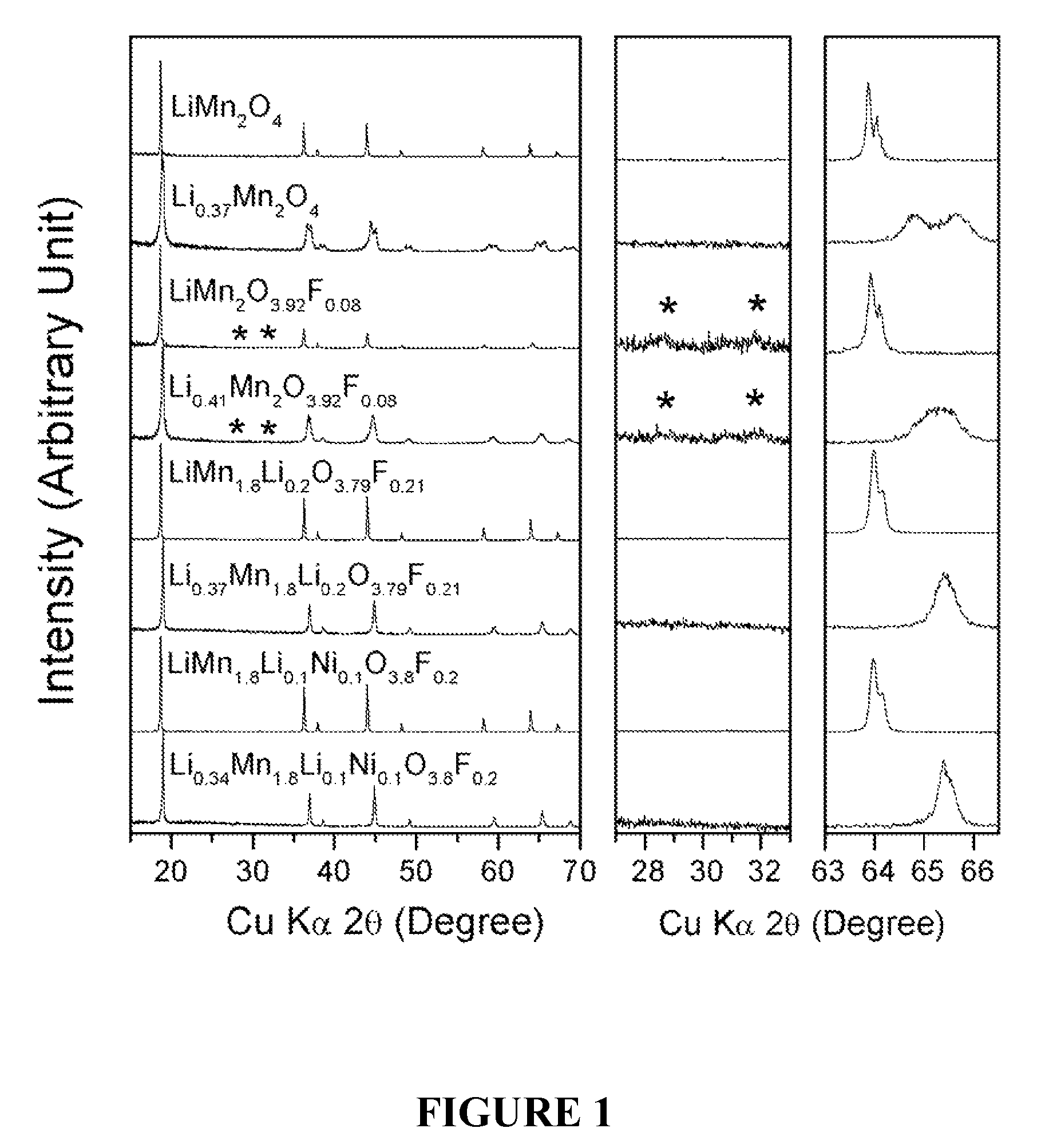
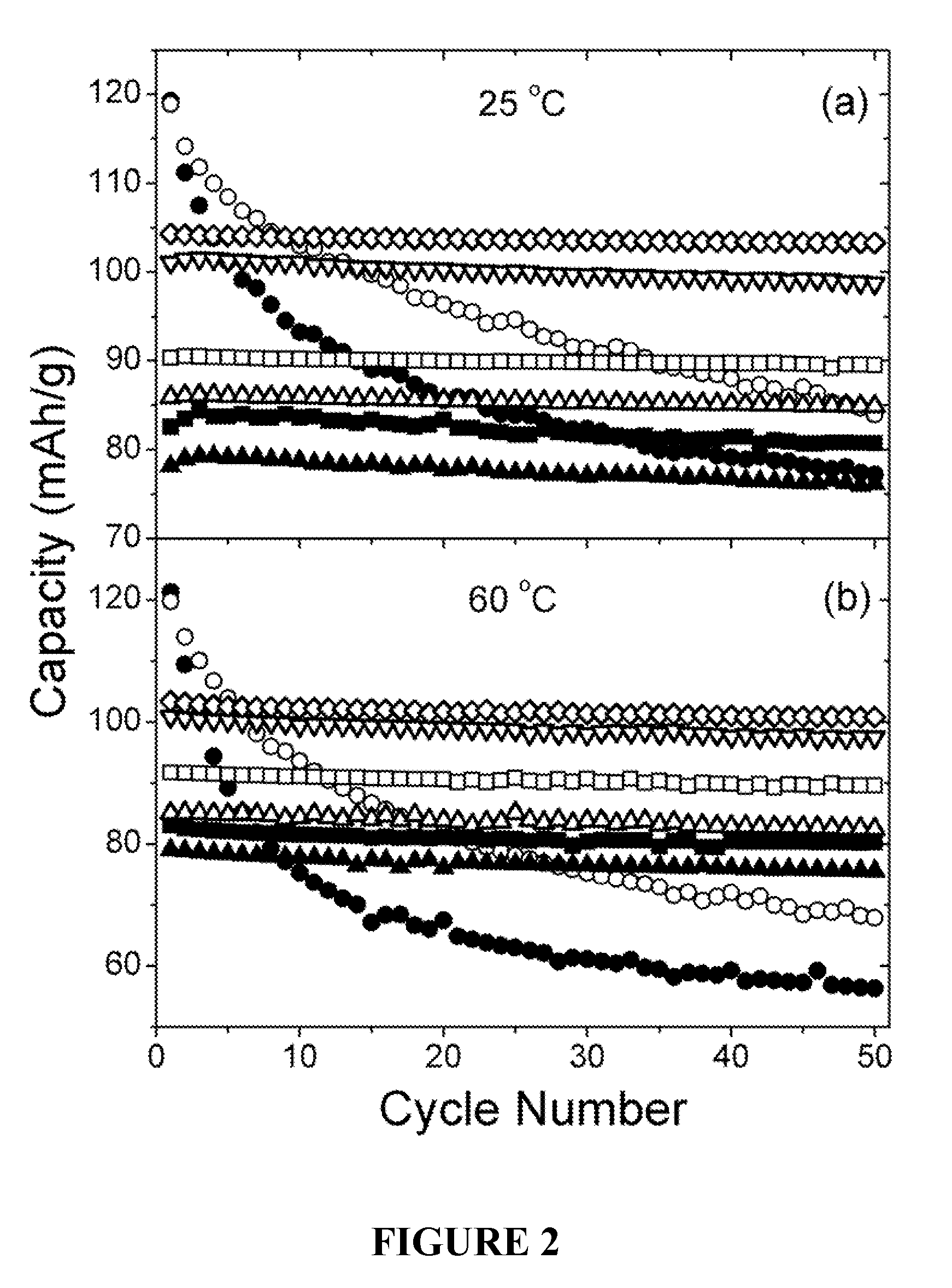
Cation-substituted spinel oxide and oxyfluoride cathodes for lithium ion batteries
InactiveUS7718319B2Cyclability at elevated temperaturesImprove rendering capabilitiesElectrode carriers/collectorsConductive materialSpinelGraphite
The present invention includes compositions and methods of making cation-substituted and fluorine-substituted spinel cathode compositions by firing a LiMn2−y−zLiyMzO4 oxide with NH4HF2 at low temperatures of between about 300 and 700° C. for 2 to 8 hours and a η of more than 0 and less than about 0.50, mixed two-phase compositions consisting of a spinel cathode and a layered oxide cathode, and coupling them with unmodified or surface modified graphite anodes in lithium ion cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Lithium ion battery and its positive material
ActiveCN102394295AImprove cycle performanceIncrease storage capacityCell electrodesSecondary cellsHigh temperature storageManganese
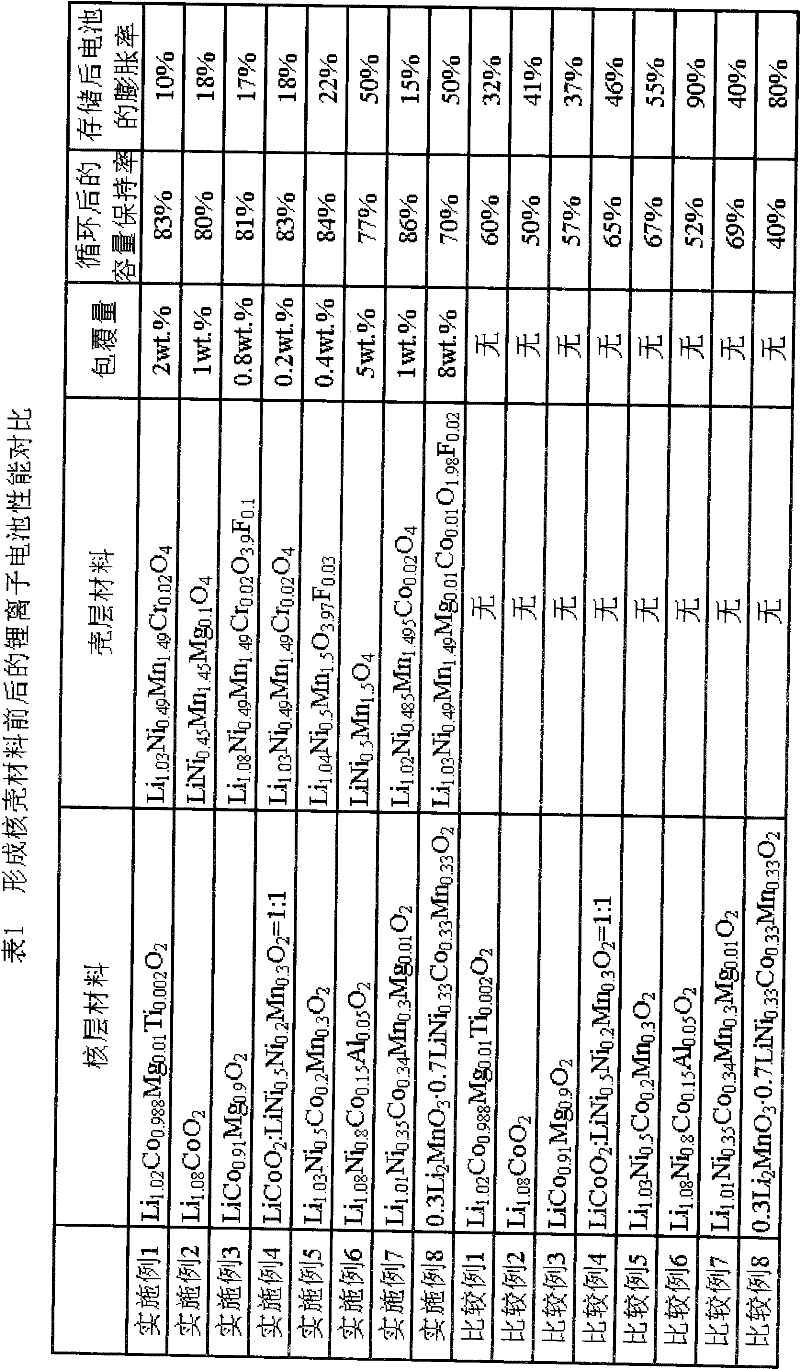
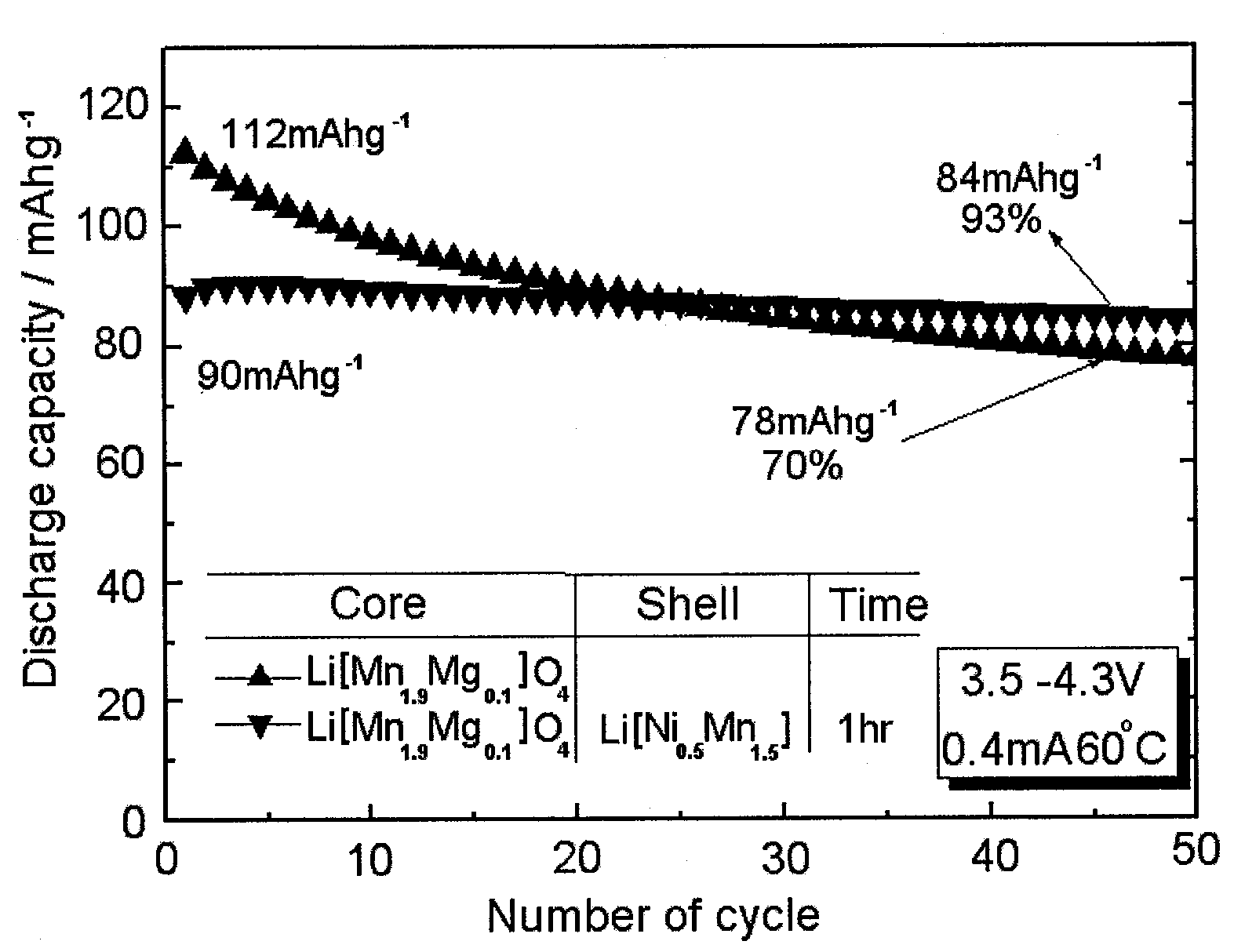
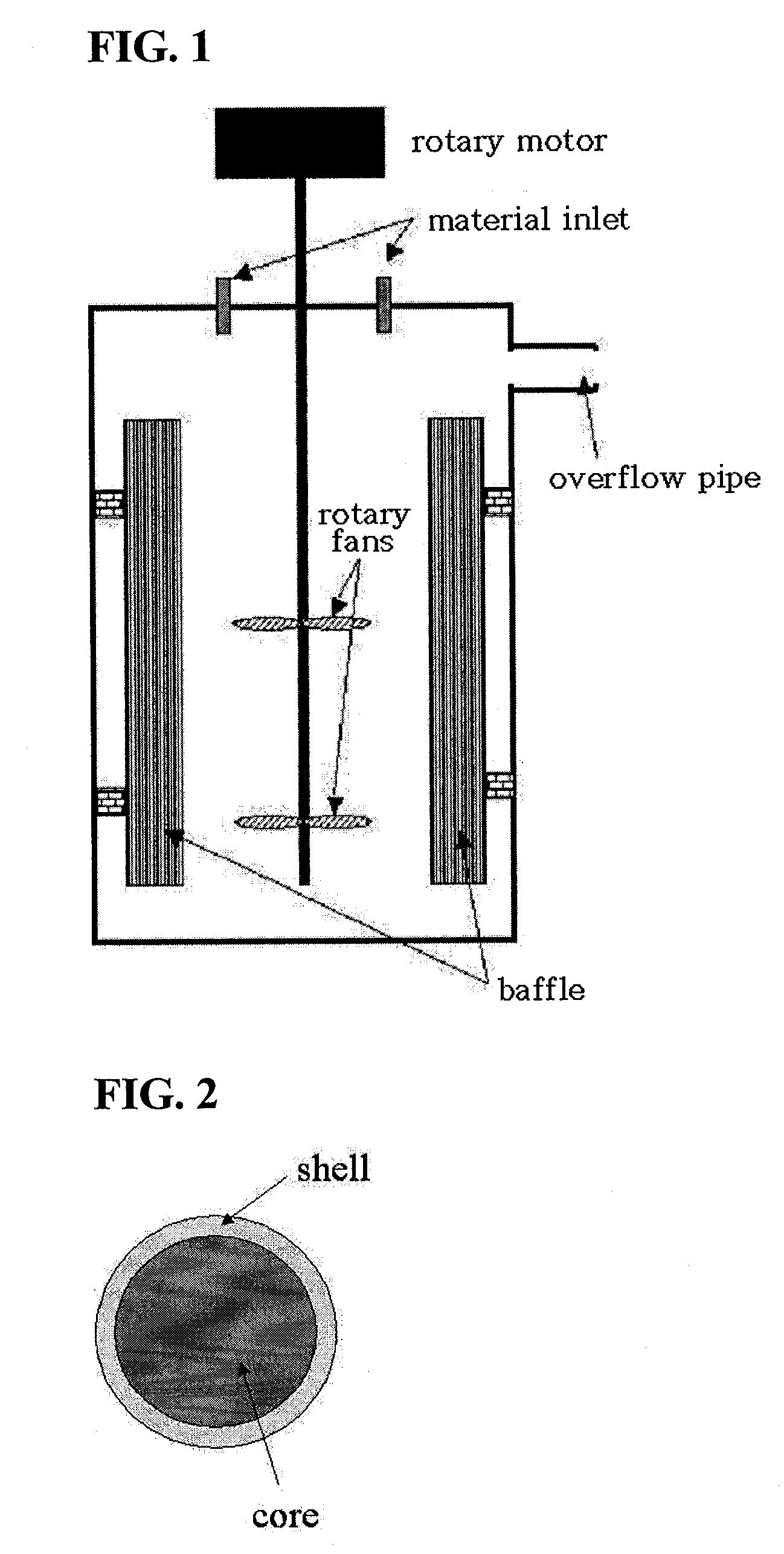
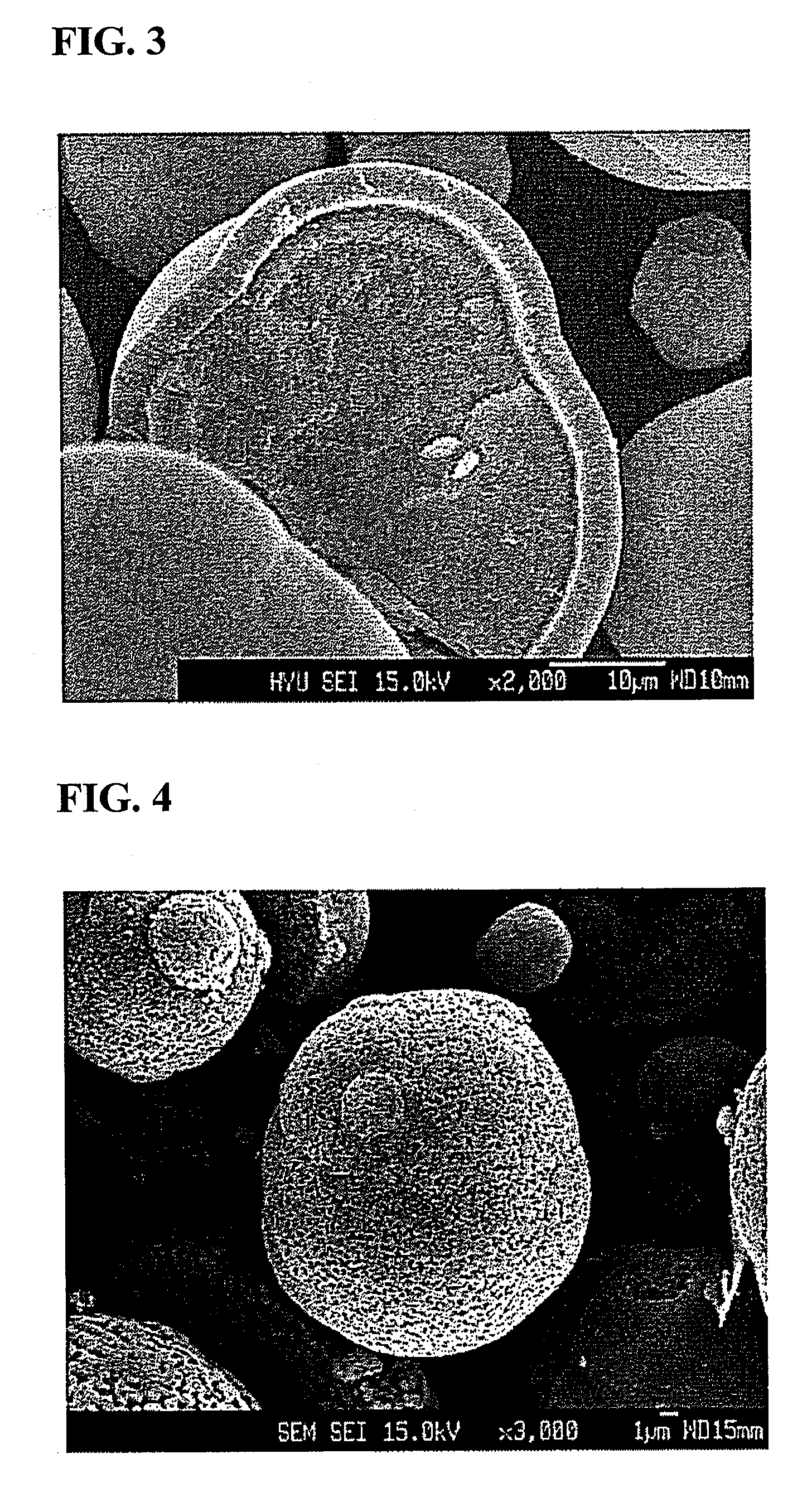
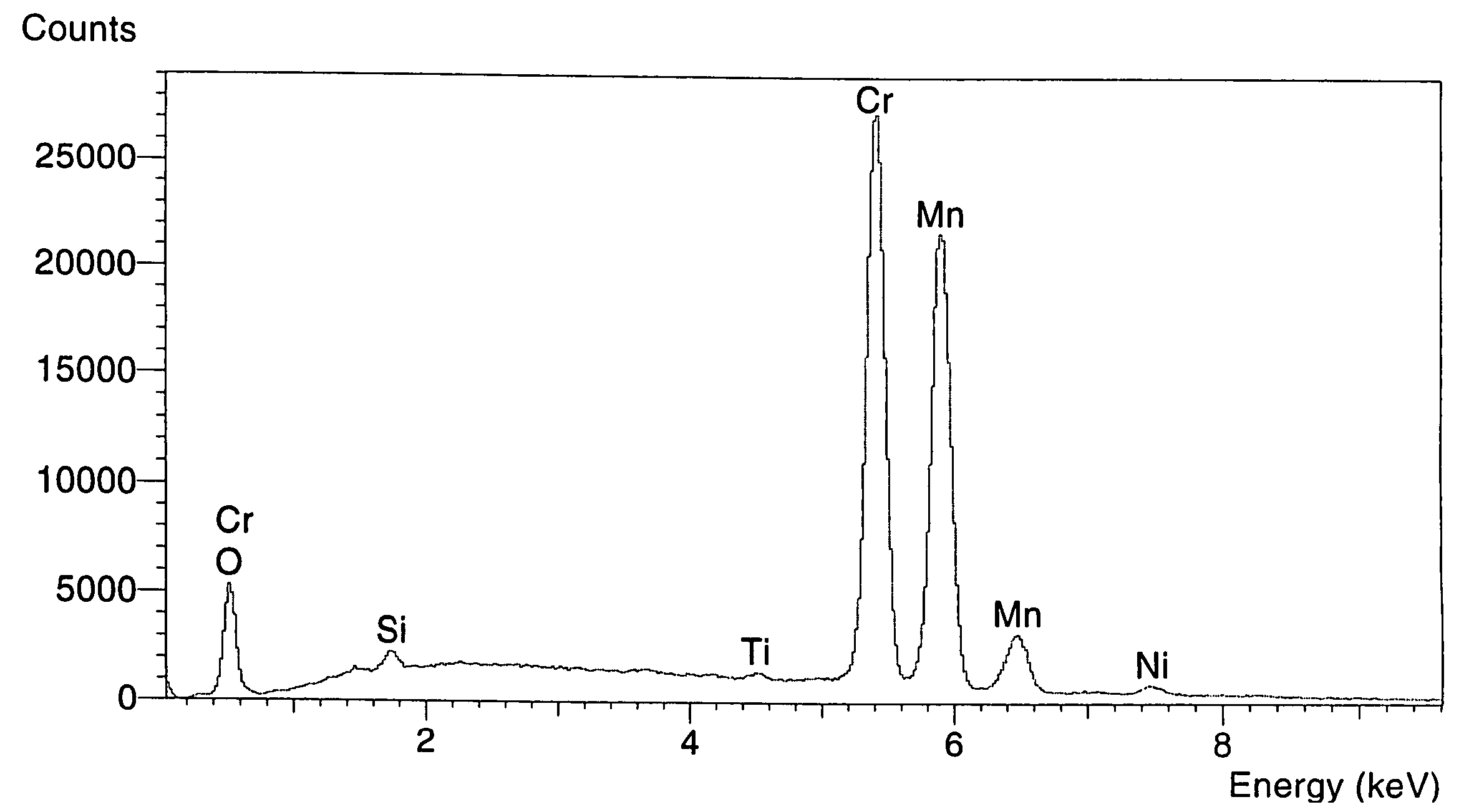
The invention discloses a lithium ion battery and a positive material, the positive material possesses a core-shell structure, the material of a core layer is at least one of lithium cobaltate, a ternary material and a lithium manganese material, the material of a shell layer is lithium nickel manganese spinel. The preparation method comprises the following steps: preparing the sol shell layer material, then adding the core layer material in the sol, stirring, drying and calcining to prepare the lithium ion battery positive material with the core-shell structure. In addition, the invention also discloses the lithium ion battery prepared by the positive material with the core-shell structure, the end of charge voltage is 4.3-4.7V(vs. Li), the lithium ion battery has excellent charge and discharge cycling performance and high temperature storage performance under high voltage.
Owner:DONGGUAN AMPEREX TECH +1
Coke-oven gas methanation catalyst and preparation method thereof
ActiveCN101391218AHigh activityImprove carbon resistanceHydrocarbon from carbon oxidesMetal/metal-oxides/metal-hydroxide catalystsMethanationActive component
The invention discloses a coke oven gas methanation catalyst, which takes Al2O3 as a carrier, nickel as a main active component, and MgO as an auxiliary agent; wherein: the active component nickel exists in the catalyst in the form of NiO, and the carrier Al2O3 and the auxiliary agent MgO form a carrier structure of magnesia-alumina spinel; the main components respectively include, by weight percentage: 5 percent to 20 percent of NiO, 30 percent to 80 percent of Al2O3, and 1 percent to 50 percent of MgO. The catalyst has the advantages of high strength, good activity, good thermal stability, excellent anti-coking performance and good low temperature activity, and also has the properties of transforming high hydrocarbon and good anti-oxidation. The invention also discloses a preparation method of the coke oven gas methanation catalyst.
Owner:SOUTHWEST RES & DESIGN INST OF CHEM IND
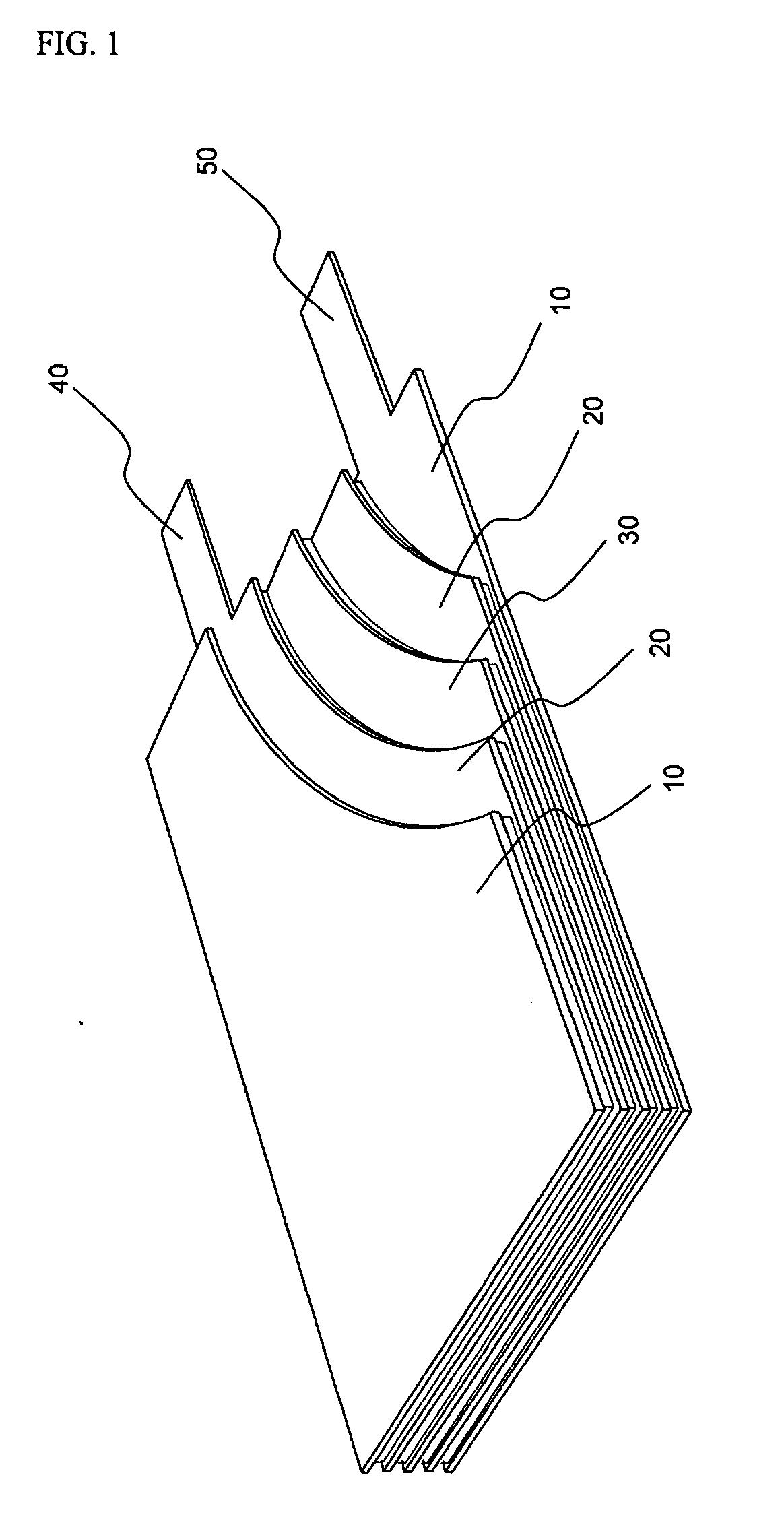
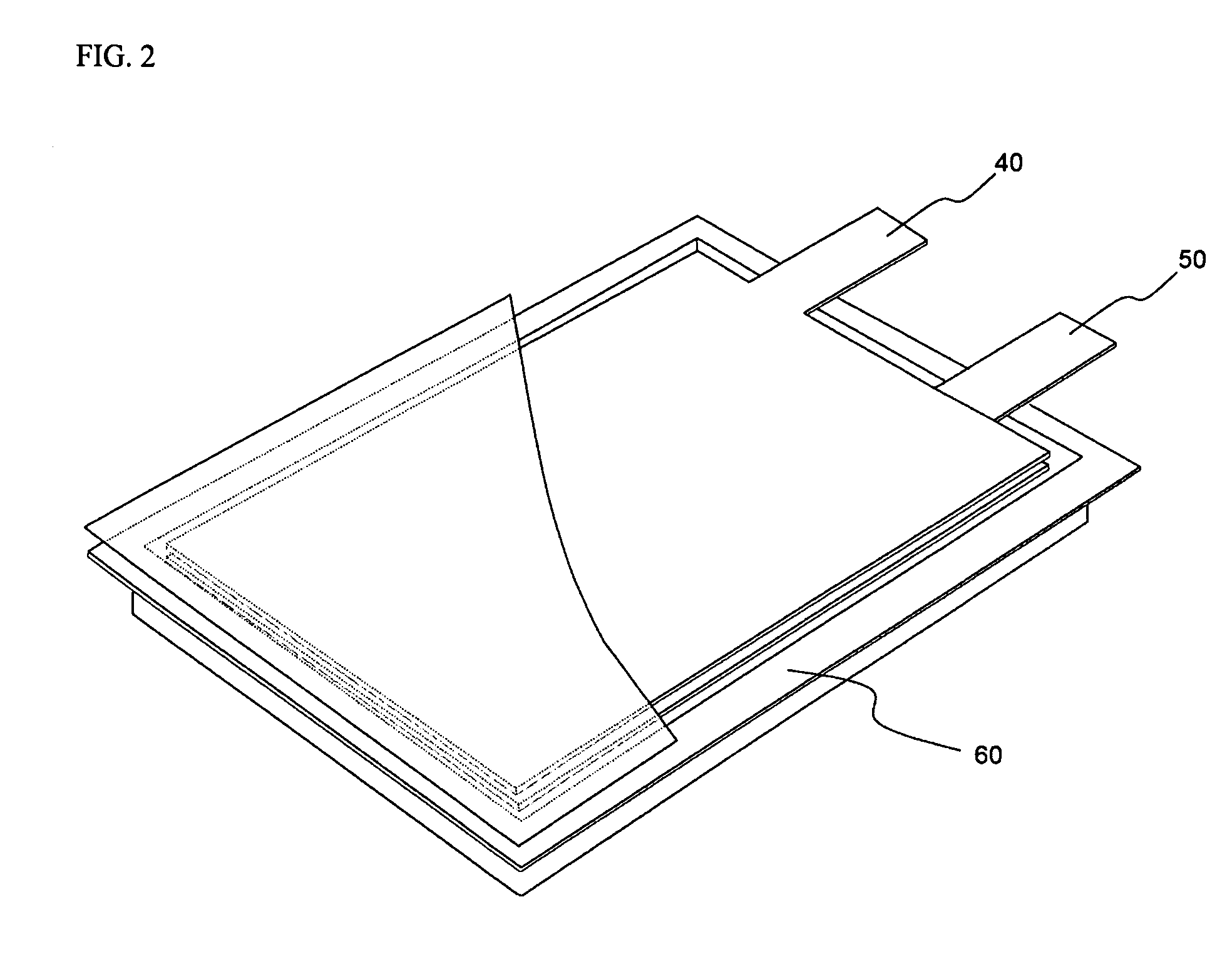
Battery pack and vehicle
ActiveUS20060216600A1Excellent in charge-discharge cycle characteristicFinal product manufactureCell lids/coversElectric capacitySpinel
A battery pack includes nonaqueous electrolyte batteries each comprising a positive electrode and a negative electrode. The positive electrode contains a lithium-transition metal oxide having a layered crystal structure. The negative electrode contains a lithium-titanium composite oxide having a spinel structure. And the positive electrodes and the negative electrodes satisfy the formula (1) given below: 1.02≦X≦2 (1) where X is a ratio of an available electric capacity of each of the negative electrodes at 25° C. to an available electric capacity of each of the positive electrodes at 25° C.
Owner:KK TOSHIBA
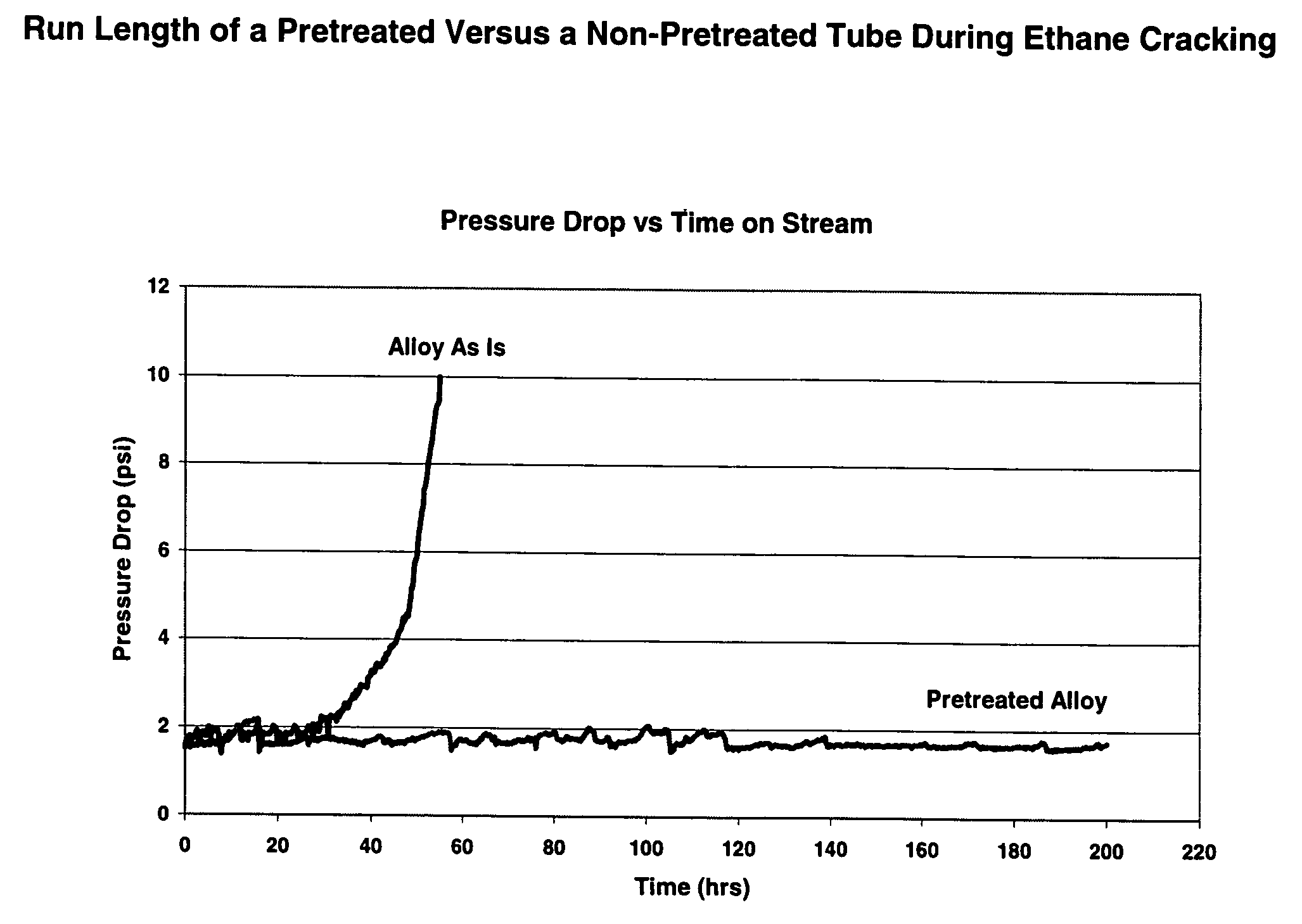
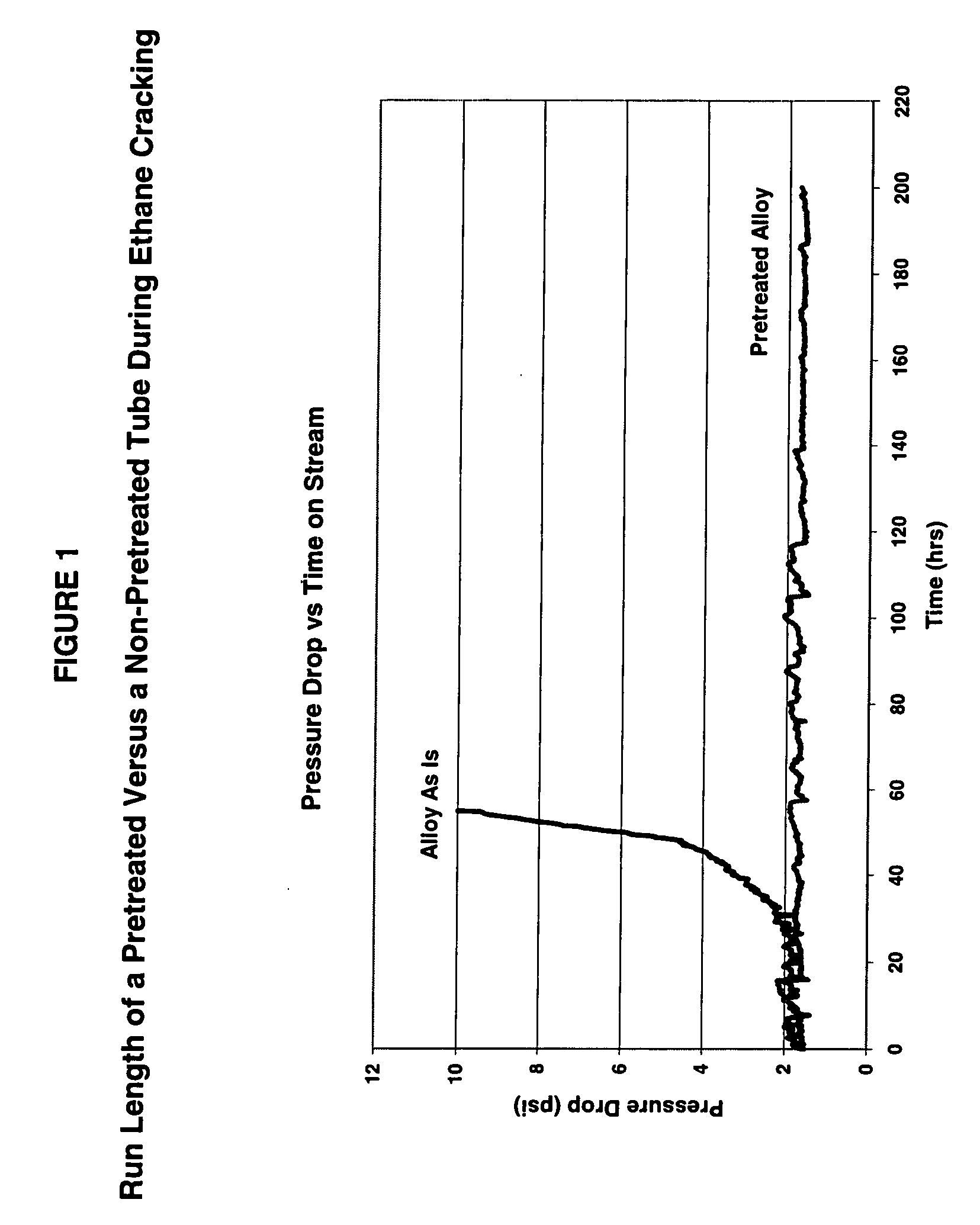
Thermal cracking process using tubes, pipes, and coils made of novel stainless steel matrix
A thermal cracking process using tubes, pipes, and coils made of.An outermost surface covering not less than 55% of stainless steel, said surface having a thickness from 0.1 to 15 microns and being a spinel of the formula MnxCr3-xO4 wherein x is from 0.5 to 2 is not prone to coking and is suitable for hydrocarbyl reactions such as furnace tubes for cracking.
Owner:NOVA CHEM (INT) SA
Copper-manganese mixed oxide cathode material for use in alkaline cells having high capacity
ActiveUS20080090138A1Cell seperators/membranes/diaphragms/spacersNon-aqueous electrolyte accumulator electrodesStructural waterMixed oxide
The present invention relates to a copper-manganese mixed oxide cathode material, which is suitable for use in a cathode of an electrochemical cell, and which has the formula MnxCuyOz,.nH2O, wherein the oxidation state of Cu is between about +1 and about +3, the oxidation state of Mn is between about +2 and about +7, x is equal to about 3−y, y is less than about 3, z is calculated or experimentally determined, using means known in the art, based on the values of x and y, as well as the oxidation states of Mn and Cu, and nH2O represents the surface and structural water present in the mixed oxide material. The present invention further relates to an electrochemical cell comprising an anode, a cathode, and a separator disposed between the anode and cathode, and an electrolyte in fluid communication with the anode, the cathode and the separator, wherein the cathode comprises the noted cathode material. The present invention still further relates to such an active cathode material, or an alternative cathode material, wherein the copper-manganese mixed oxide comprises a defect spinel-type structure, which is also suitable for use as a cathode component of an alkaline electrochemical cell.
Owner:ENERGIZER BRANDS
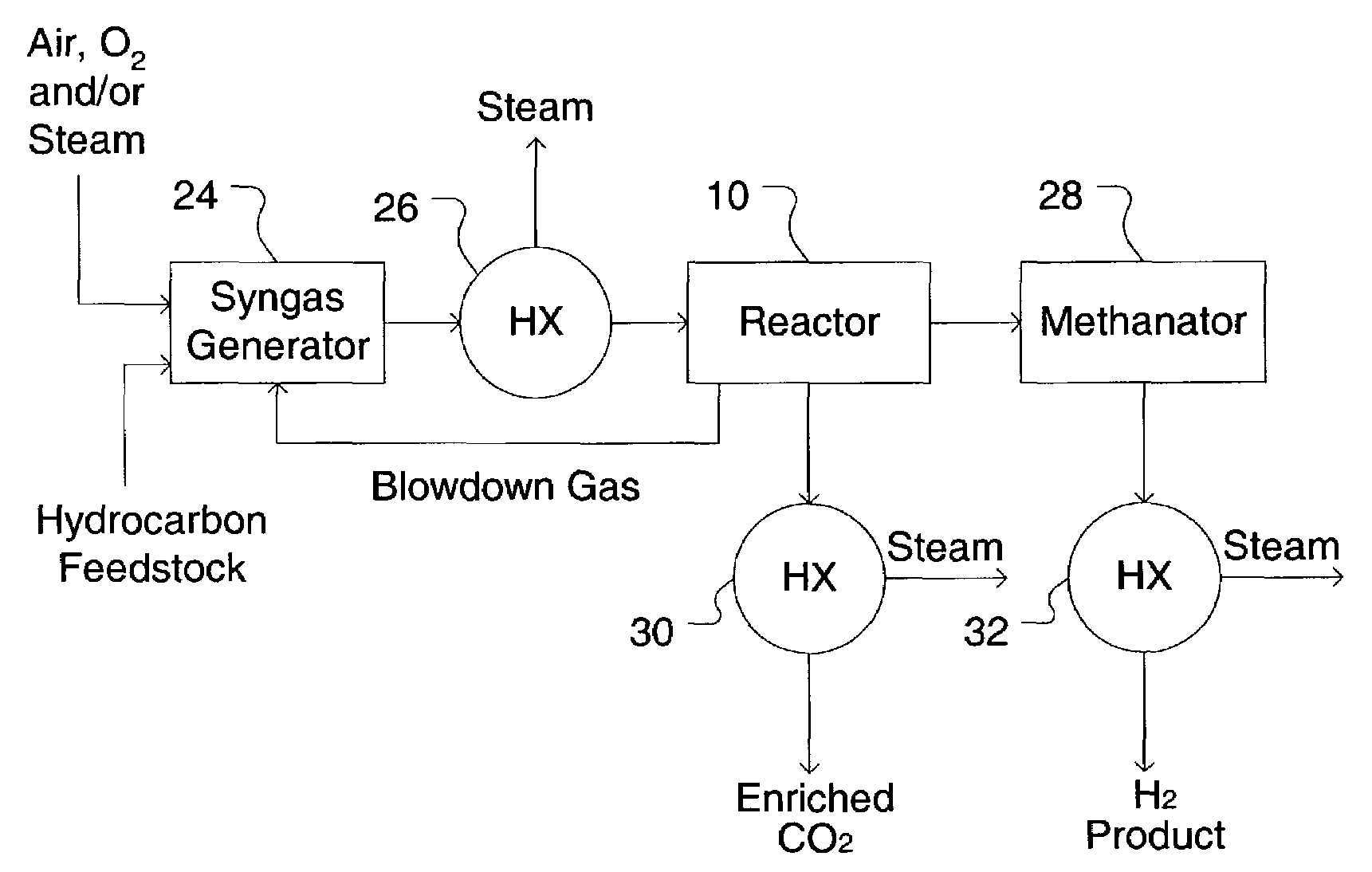
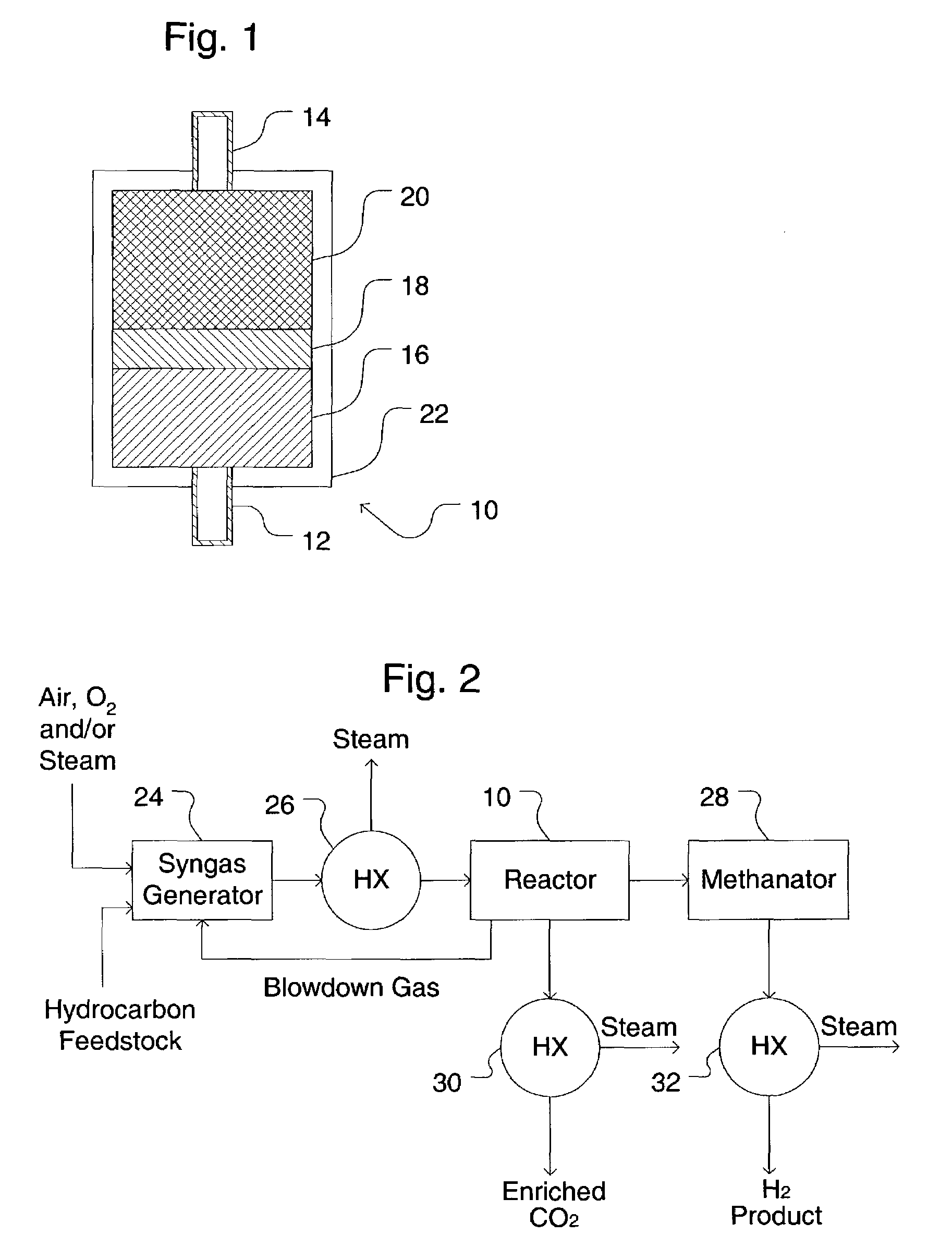
Simultaneous shift-reactive and adsorptive process to produce hydrogen
A process for producing a high temperature COx-lean product gas from a high temperature COx-containing feed gas, includes: providing a sorption enhanced reactor containing a first adsorbent, a shift catalyst and a second adsorbent; feeding into the reactor a feed gas containing H2, H2O, CO and CO2; contacting the feed gas with the first adsorbent to provide a CO2 depleted feed gas; contacting the CO2 depleted feed gas with the shift catalyst to form a product mixture comprising CO2 and H2; and contacting the product mixture with a mixture of second adsorbent and shift catalyst to produce the product gas, which contains at least 50 vol. % H2, and less than 5 combined vol. % of CO2 and CO. The adsorbent is a high temperature adsorbent for a Sorption Enhanced Reaction process, such as K2CO3 promoted hydrotalcites, modified double-layered hydroxides, spinels, modified spinels, and magnesium oxides.
Owner:AIR PROD & CHEM INC
Nanocomposite ceramic and method for producing the same
InactiveUS20070259768A1High hardnessImprove toughnessCeramic shaping apparatusClaywaresSpinelCeramic
A nanocomposite ceramic includes a uniform combination of a ceramic spinel phase and an alumina phase, wherein each phase exhibits a grain size in the range of from about 0.1 nm to 10,000 nm.
Owner:RUTGERS THE STATE UNIV
Spinel nickel manganese acid lithium and layered lithium-rich manganese-based composite cathode material with core-shell structure and preparation method thereof
ActiveCN104157831APromote circulationRaise the ratioCell electrodesSecondary cellsComposite cathodeMaterial synthesis
The invention relates to a spinel nickel manganese acid lithium and layered lithium-rich manganese-based composite cathode material with a core-shell structure and a preparation method thereof, which belongs to the technical field of material synthesis. The prepared lithium ion composite cathode material takes a layered lithium-rich manganese-based Li[Lia(NixCoyMnz)]O2 as a core material, takes spinel nickel manganese acid lithium LiNi0.5Mn1.5O4 as a shell material; a coprecipitation method is employed to obtain a core-shell precursor, the core-shell precursor and the lithium source are uniformly mixed and calcined to obtain the spinel nickel manganese acid lithium and layered lithium-rich manganese-based composite cathode material with the core-shell structure. According to the invention, the layered lithium-rich manganese-based is taken as the core material, and the spinel nickel manganese acid lithium is taken as the shell material; under the prerequisite that material gram capacity is kept, material structural stability is increased, material cycle, multiplying power and safety performances are improved, function composite and complementation of the core material and the shell layer material can be realized, and the problem that high capacity and high security can not be achieved simultaneously is solved. The composite cathode material has the advantages of simple process and obviously increased performance.
Owner:南京时拓能源科技有限公司
Positive electrodes for lithium batteries and their methods of fabrication
InactiveUS20050186474A1Low costImprove cycle performanceSecondary cellsPositive electrodesSpinelLithium manganese oxide
The present invention discloses positive electrodes and their methods of fabrication. These electrodes are low in cost. Lithium rechargeable batteries that use these positive electrodes have excellent cycling properties at high temperature. The positive electrode of the embodiments of this invention comprises of a current collector coated by two layers of active materials for positive electrodes. The active material for the first layer of coating is one or more active materials selected from the following: spinel lithium manganese oxide, and spinel lithium manganese oxide derivatives. The active material for the second layer of coating is one or more active material selected from the following: lithium cobalt oxide, lithium cobalt oxide derivatives, lithium nickel oxide, and lithium nickel oxide derivatives. To fabricate these positive electrodes, a first layer of coating comprising of the active materials stated above is applied onto a current collector and then dried before a second layer of coating is applied onto the surface of the first layer of coating. The positive electrode is obtained after the current collector with the two layers of coating is dried a second time and then pressed to form a slice.
Owner:BYD AMERICA CORP
Storage material for sulfur oxides
InactiveUS6338831B1Improve the immunityRestores original storage capacityNitrogen compoundsExhaust apparatusOxygenInternal combustion engine
A sulfur oxide storage material contains a magnesium-aluminum spinel (MgO.Al2O3) and can be used as a so-called "sulfur trap" to remove sulfur oxides from oxygen-containing exhaust gases of industrial processes. In particular, it can be used for the catalytic purification of exhaust gas from internal-combustion engines to remove the sulfur oxides from the exhaust gas in order to protect the exhaust gas catalysts from sulfur poisoning. The material displays a molar ratio of magnesium oxide to aluminum oxide in the range of over 1.1:1, and the magnesium oxide present in stoichiometric excess is homogeneously distributed in a highly disperse form in the storage material.
Owner:DMC2 DEGUSSA METALS +1
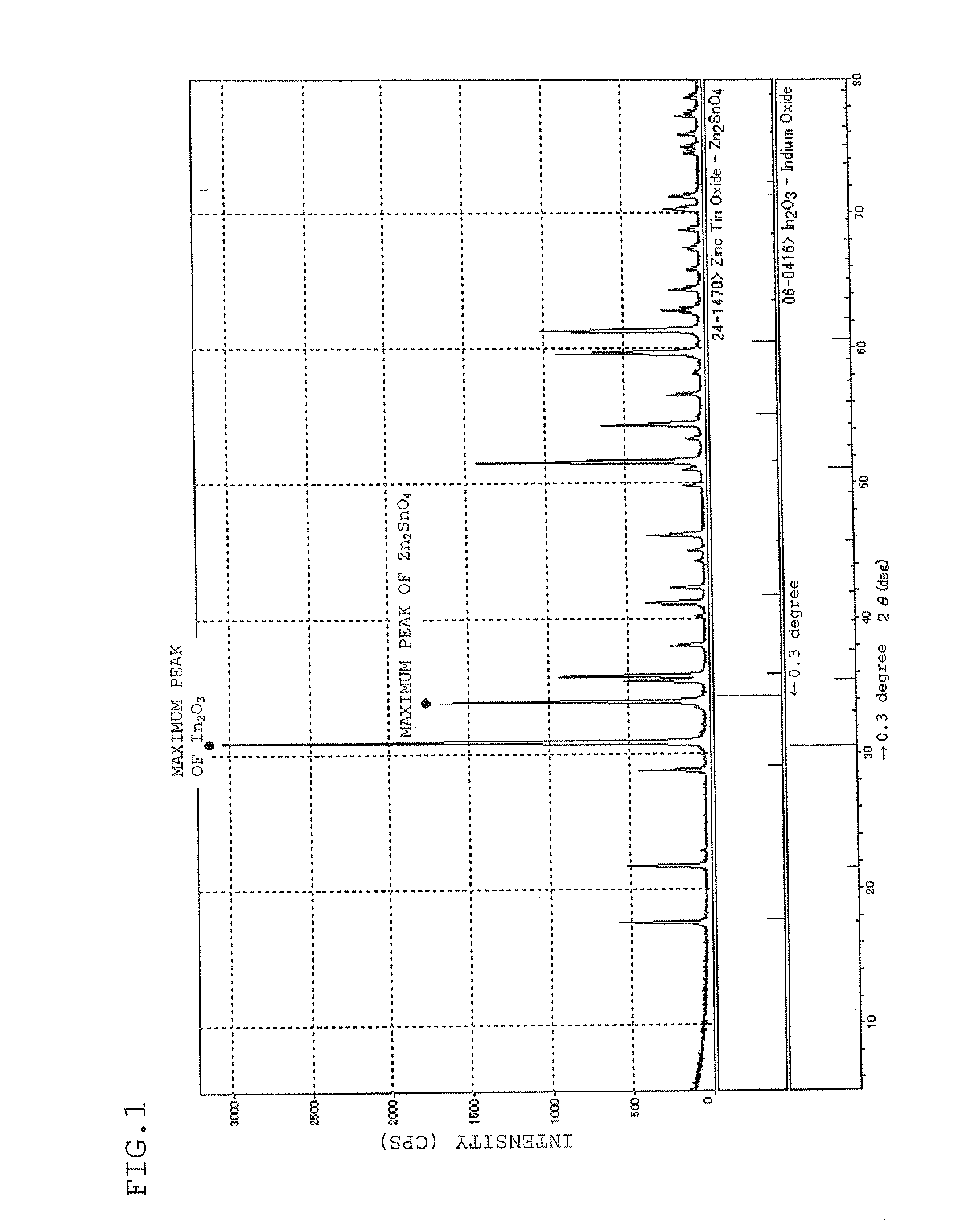
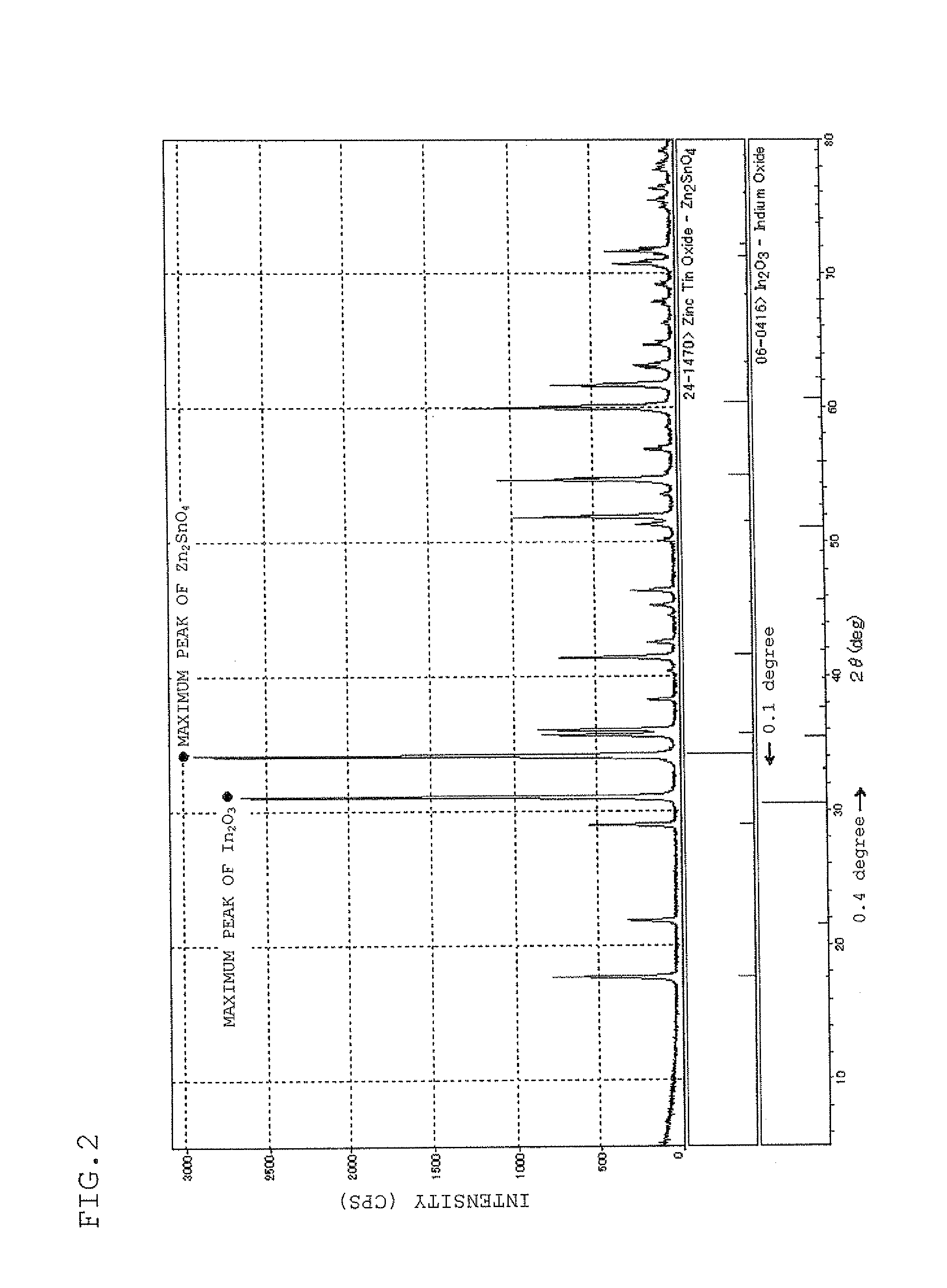
Sputtering target, transparent conductive film and transparent electrode
ActiveUS20100170696A1Lower resistanceHigh densityNon-insulated conductorsConductive materialIndiumX-ray
A sputtering target which is composed of a sintered body of an oxide which contains at least indium, tin, and zinc and includes a spinel structure compound of Zn2SnO4 and a bixbyite structure compound of In2O3. A sputtering target includes indium, tin, zinc, and oxygen with only a peak ascribed to a bixbyite structure compound being substantially observed by X-ray diffraction (XRD).
Owner:IDEMITSU KOSAN CO LTD
Nonaqueous secondary cell
InactiveUS20070134558A1Inhibit deteriorationImprove cycle performanceNon-aqueous electrolyte accumulatorsOrganic electrolyte cellsSpinelManganese oxide
Using a positive electrode active material including spinel type manganese oxide as the main constituent, a novel low cost and high output power flat type nonaqueous secondary cell for HEVs that has increased safety at overcharge, and superior storage properties and cycle life is provided. A flat type nonaqueous secondary cell that has increased safety and is superior in storage and cycle properties even though the cell is a laminate type cell which does not have a blocking mechanism can be obtained by blending the spinel type lithium manganese oxide of the positive electrode and 5 wt % to 40 wt % of layered type lithium manganese oxide, to suppress storage deterioration at a high temperature and to simultaneously achieve safety when overcharged, and further, by adding a Li compound having a structure as shown in Formula (1) structure, to suppress deterioration of a mixed positive electrode active material during a high temperature cycle.
Owner:HITACHI LTD +1
Positive electrode active material, method of manufacturing the positive electrode active material, and non-aqueous electrolyte secondary battery using the positive electrode active material
ActiveUS20110076564A1Improve cycle performanceDeterioration of cycle performanceAlkali metal oxidesLi-accumulatorsCrystal structureSpinel
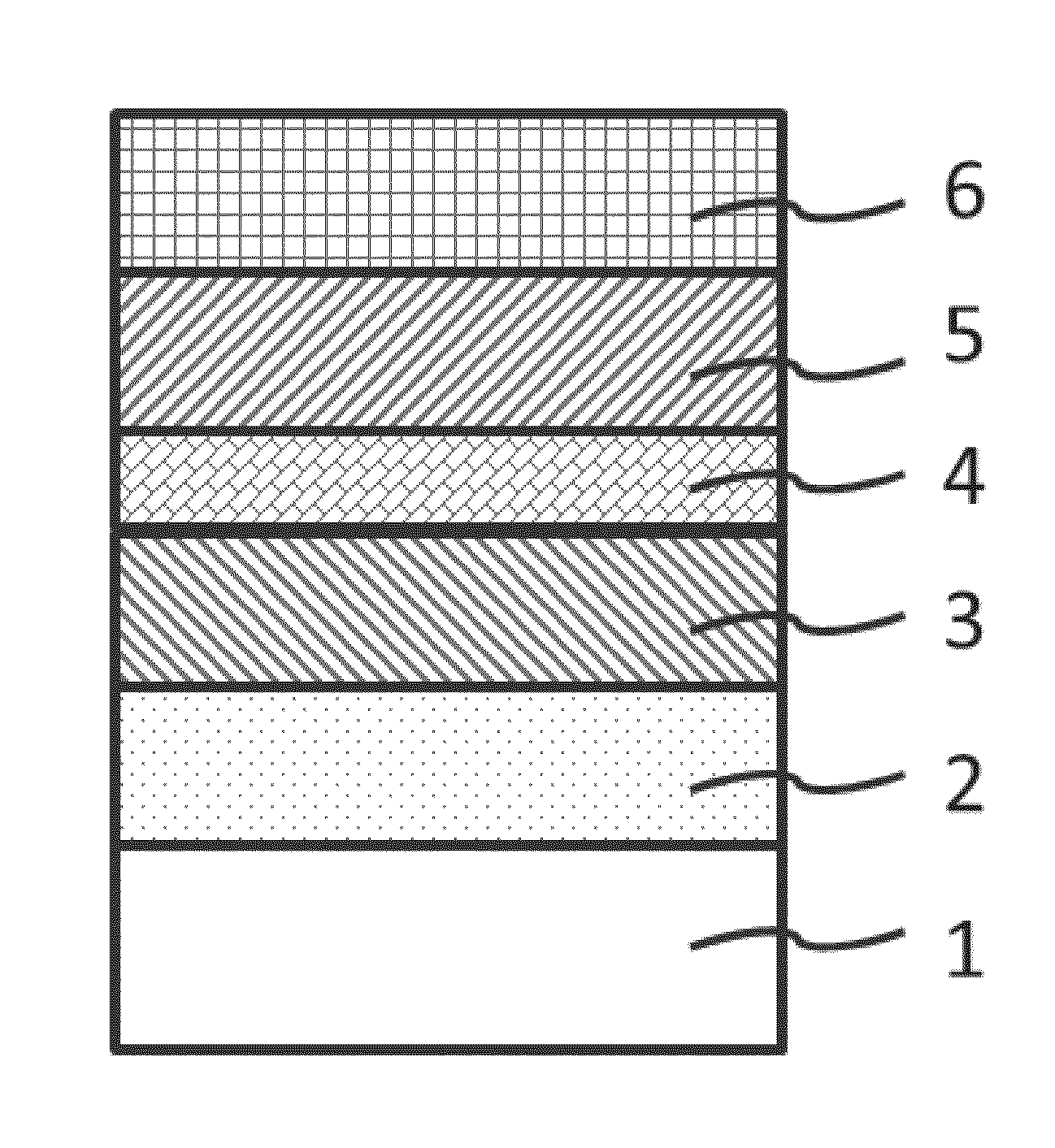
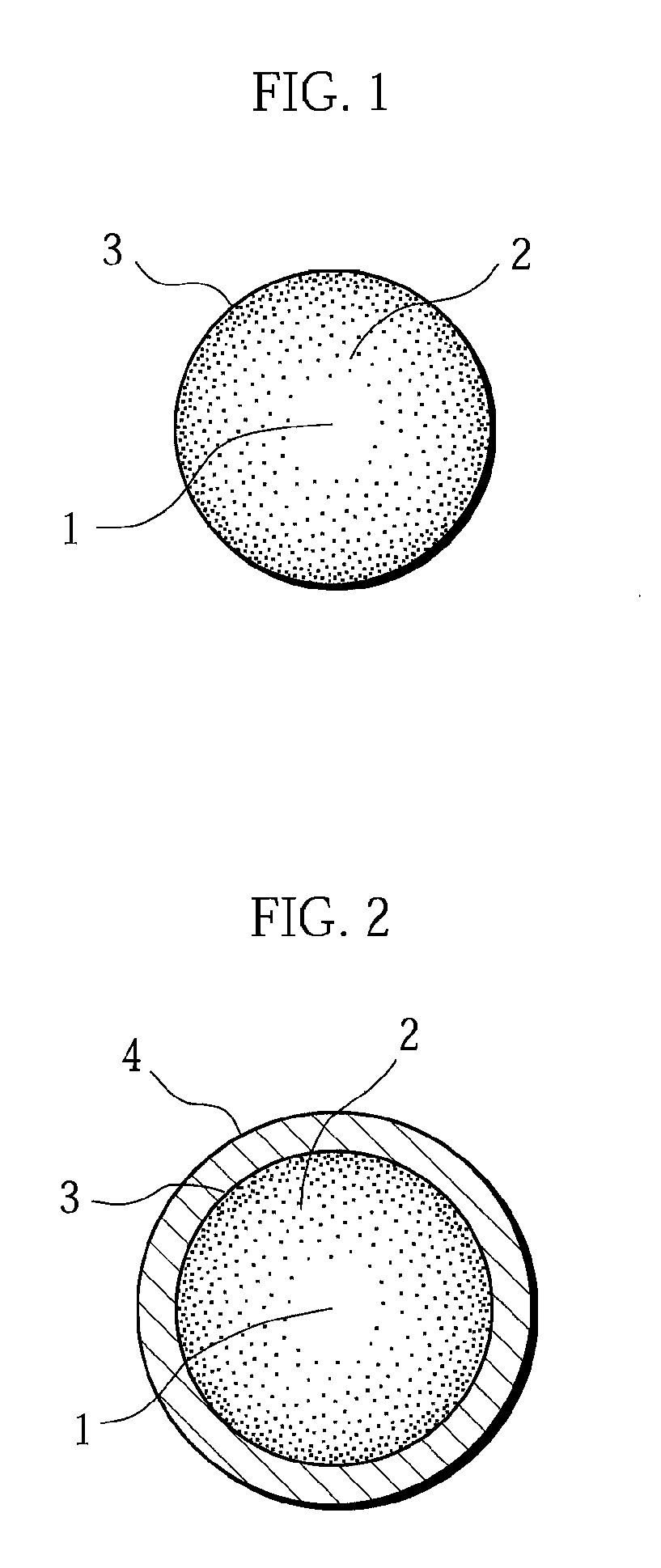
A positive electrode active material having a lithium-excess lithium-transition metal composite oxide particle represented by the chemical formula Li1.2Mn0.54Ni0.13Co0.13O2. The lithium-excess lithium-transition metal composite oxide particle has an inner portion (1) having a layered structure and a surface adjacent portion (2) having a crystal structure gradually changing from a layered structure to a spinel structure from the inner portion (1) toward the outermost surface portion (3). The layered structure and the spinel structure have an identical ratio of the amount of Mn and the total amount of Ni and Co.
Owner:PANASONIC ENERGY CO LTD
Self-discharge screening method for lithium ion phosphate battery
ActiveCN102117937ASolving the Difficult Problem of Self-Discharge ScreeningImprove consistencyFinal product manufactureElectrolyte accumulators manufacturePhosphateScreening method
The invention discloses a self-discharge screening method for a lithium ion phosphate battery, belongs to the technical field of lithium ion batteries, and aims to provide a method for effectively screening the lithium ion phosphate battery with high self-discharge rate by using shelving in a charging state. According to the technical key points, the method comprises the following steps of: adding laminar lithium nickel cobalt manganese oxide or spinel lithium nickel manganese oxide which comprises 0.5 to 5 weight percent of lithium ion phosphate and has high voltage platform into a lithium ion phosphate-containing compound positive electrode; assembling the lithium ion phosphate battery by taking graphite as a negative electrode; fully charging the battery and then shelving the battery at an ambient temperature of between 20 and 45 DEG C; recording voltages before and after the shelf and shelf time; calculating the voltage difference before and after the shelf or a voltage variation value in unit time; and determining a critical value of the voltage difference of the batteries with the high self-discharge rate which are shelved in the same period or voltage variation in the unit time, and determining that the self-discharge rate of the battery of which the voltage difference or the voltage variation value in the unit time is greater than the critical value is high.
Owner:HEFEI GUOXUAN HIGH TECH POWER ENERGY
Layered Core-Shell Cathode Active Materials For Lithium Secondary Batteries, Method For Preparing Thereof And Lithium Secondary Batteries Using The Same
InactiveUS20080193841A1Large capacityElectrode manufacturing processesSecondary cellsManganeseSpinel
Disclosed herein is a layered core-shell cathode active material for secondary lithium batteries, in which the core layer has a structural formula of Li1+a[MxMn1-x]2O4 (M is selected from a group consisting of Ni, Co, Mg, Zn, Ca, Sr, Cu, Zr, P, Fe, Al, Ga, In, Cr, Ge, Sn and combinations thereof, 0.01≦x≦0.25, 0≦a≦0.1) and the shell layer has a structural formula of Li1+a[MyMn1−y]2O4 (M′ is selected from a group consisting of Ni, Mg, Cu, Zn and combinations thereof, 0.01≦y≦0.5, 0≦a≦0.1). In the layered cathode active material, the core layer, corresponding to a 4V spinel-type manganese cathode, functions to increase the capacity of the active material while the shell layer, corresponding to a 5 V spinel-type transition metal mix-based cathode, is electrochemically stable enough to prevent the reaction of the components with electrolytes and the dissolution of transition metals in electrolytes, thereby improving thermal and lifetime characteristics of the active material.
Owner:SK ENERGY CO LTD (KR)
Surface on a stainless steel matrix
InactiveUS7488392B2Not to damage surfaceSolid state diffusion coatingQuenching agentsChemical reactionSpinel
A stainless steel comprising at least 20 weight % of chromium and at least 1.0 weight % of manganese is adapted to support an overcoating having a thickness from 1 to 10 microns of a spinel of the formula MnxCr3−xO4 wherein x is from 0.5 to 2. Preferably the overcoating is on chromia and has stability against chemical reaction at temperatures at least 25° C. higher than the uncoated chromia.
Owner:NOVA CHEM (INT) SA
Non-aqueous electrolyte battery
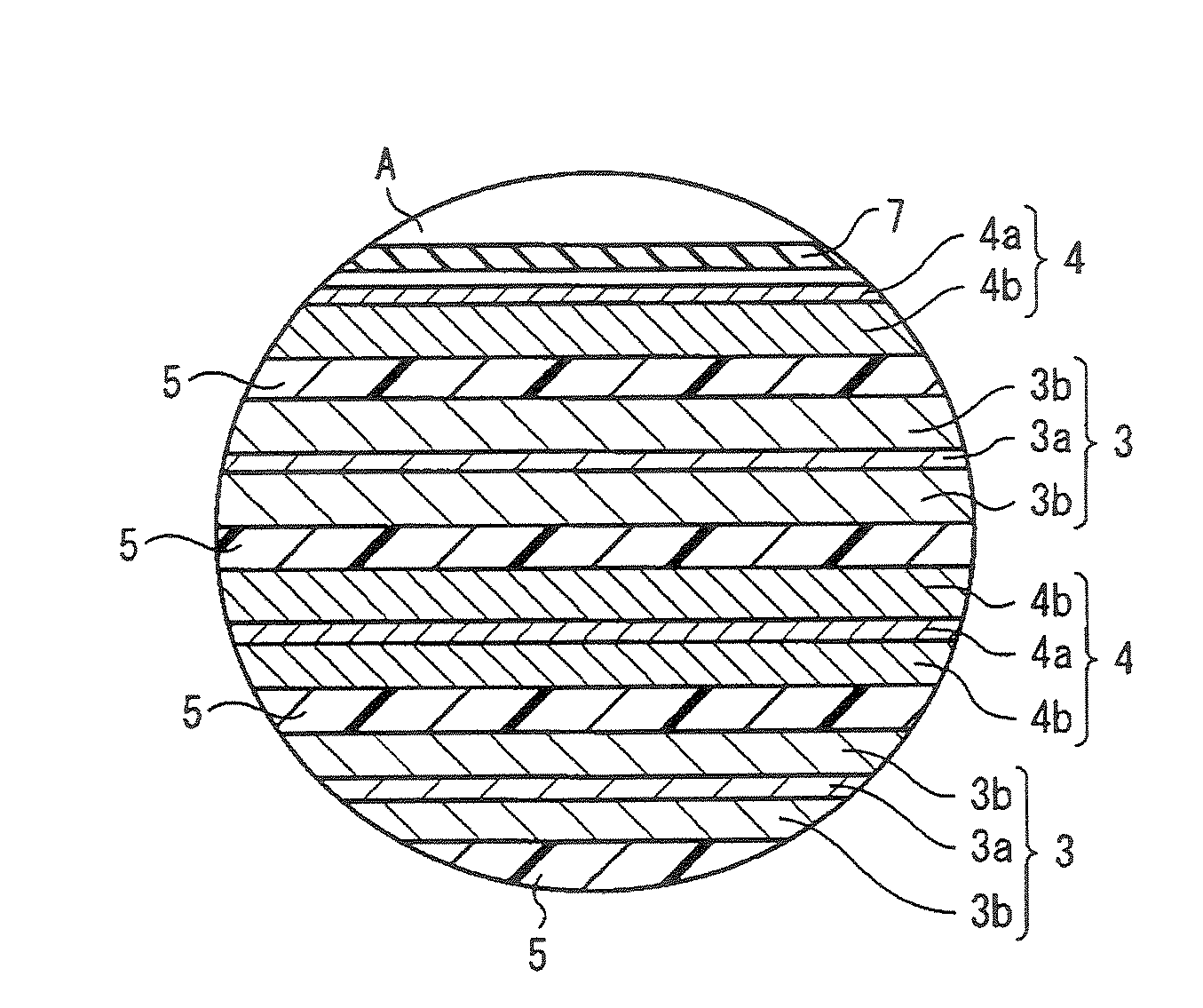
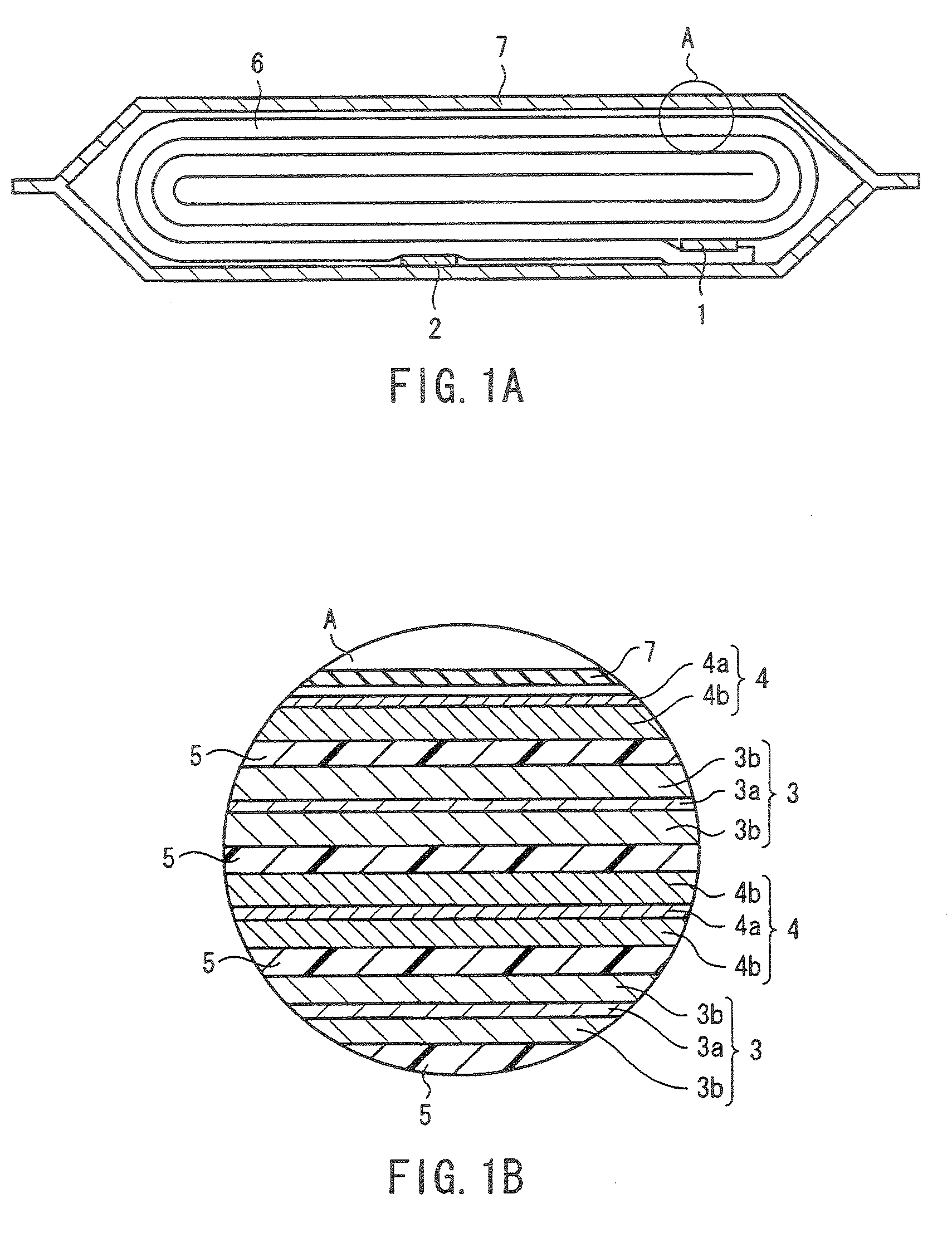
InactiveUS20060019151A1Avoid depositionIncrease probabilityFinal product manufactureCell temperature controlSpinelEngineering
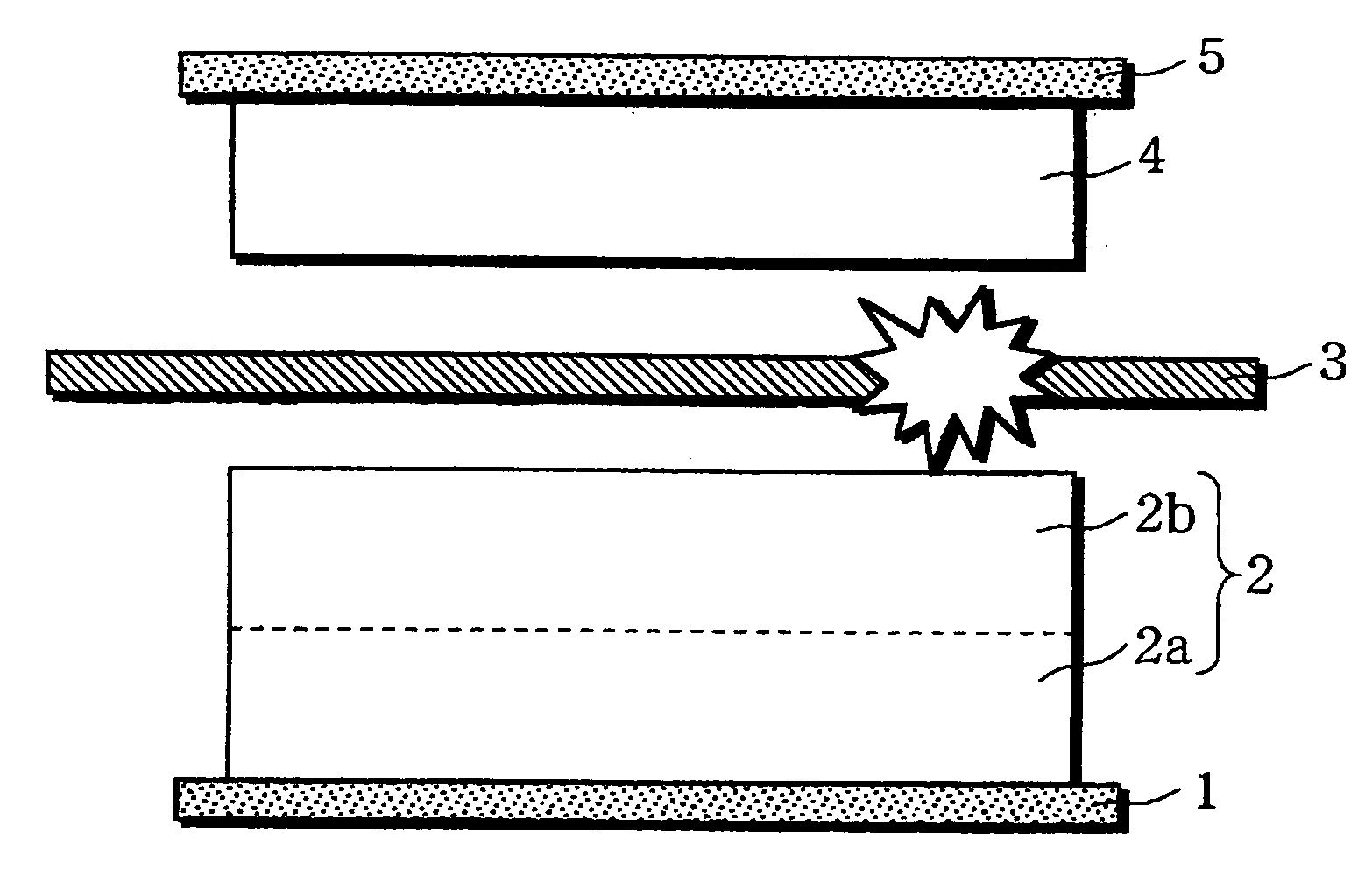
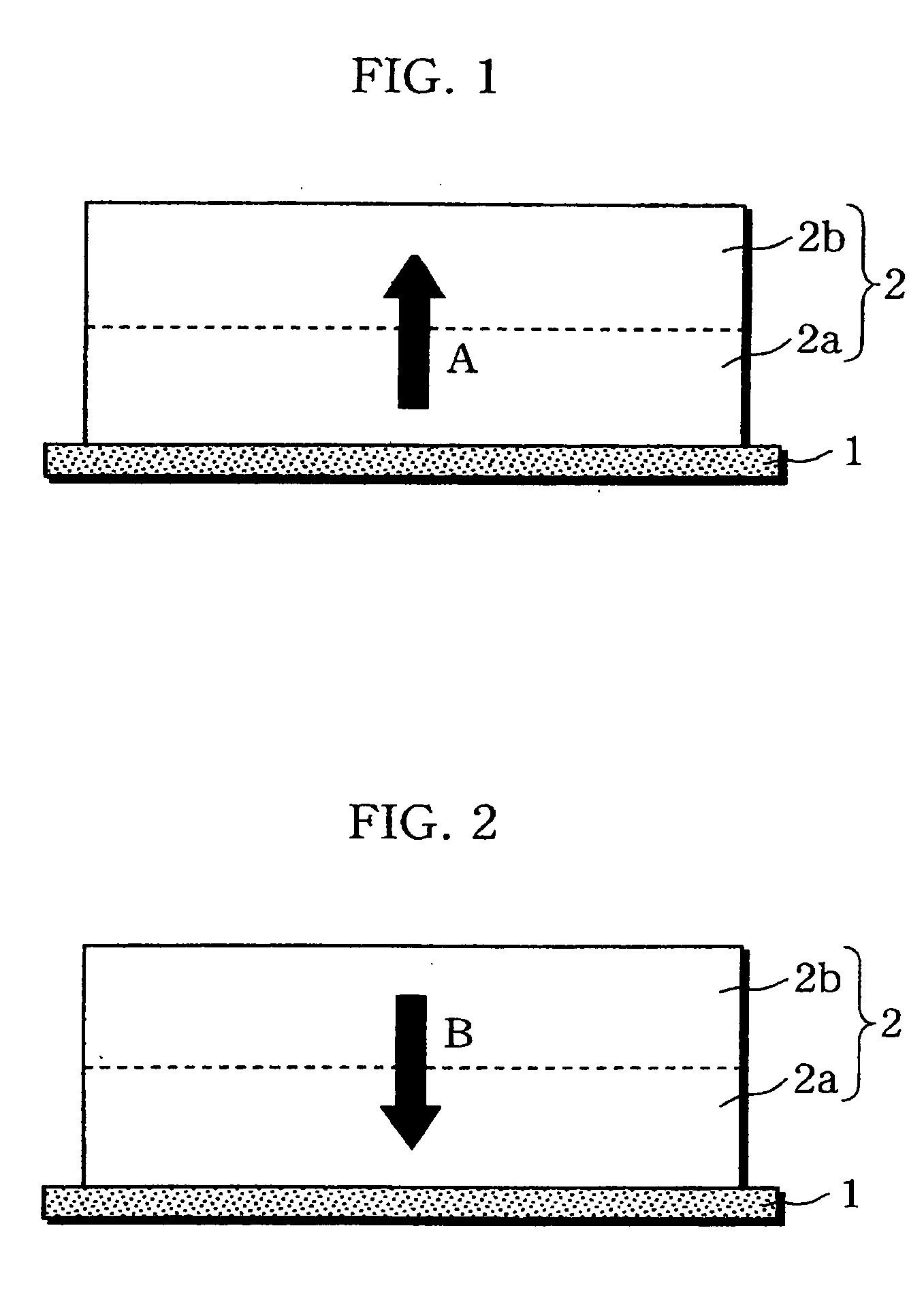
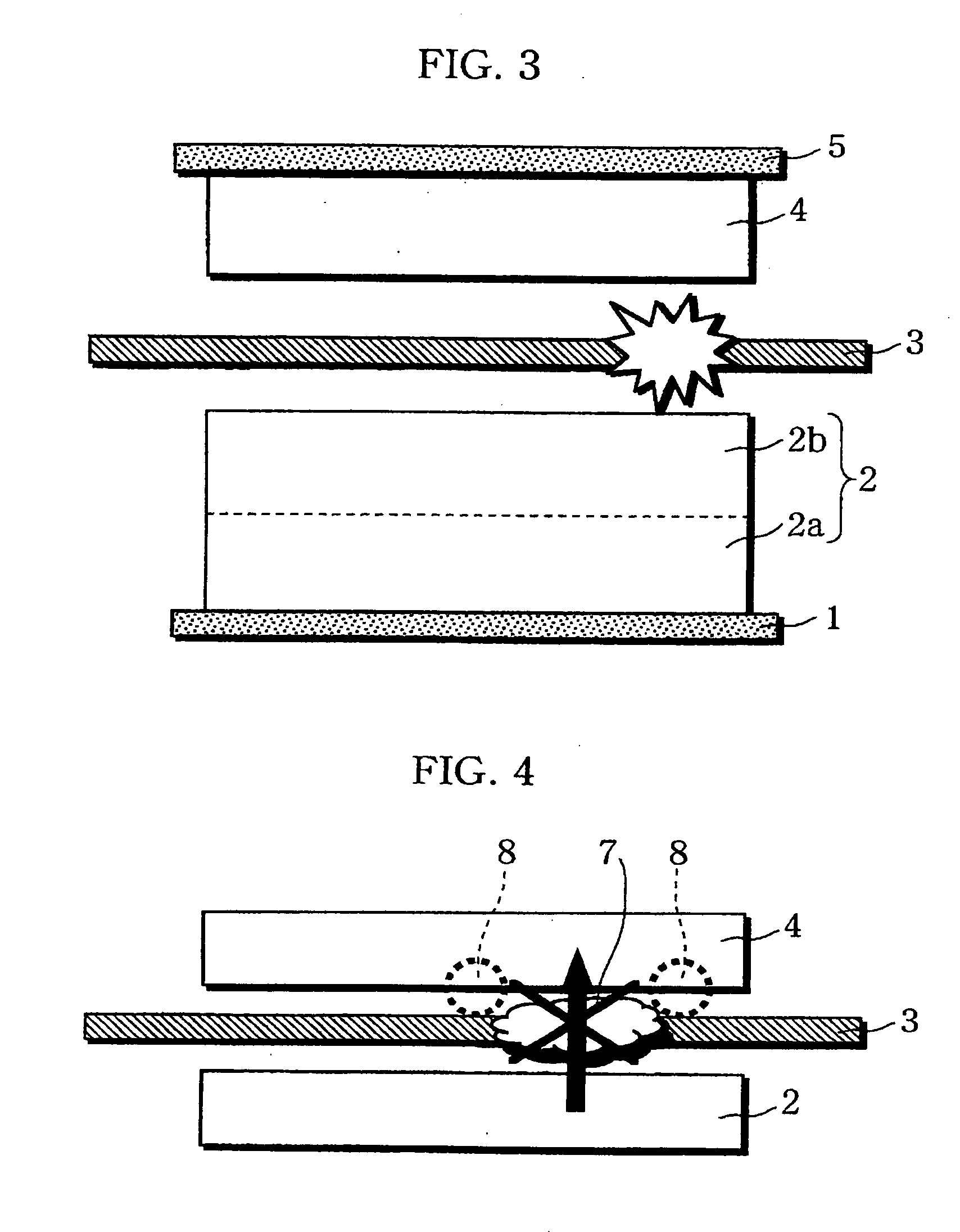
A non-aqueous electrolyte battery is provided that is capable of improving safety, particularly the tolerance of the battery to overcharging, without compromising conventional battery constructions considerably. A non-aqueous electrolyte battery is furnished with a positive electrode including a positive electrode active material-layer (2) containing a plurality of positive electrode active materials and being formed on a surface of a positive electrode current collector (1), a negative electrode including a negative electrode active material layer (4), and a separator (3) interposed between the electrodes. The positive electrode active material-layer (2) is composed of two layers (2a) and (2b) having different positive electrode active materials, and of the two layers (2a) and (2b), the layer (2b) that is nearer the positive electrode current collector contains, as its main active material, a spinel-type lithium manganese oxide or an olivine-type lithium phosphate compound.
Owner:SANYO ELECTRIC CO LTD
Lithium secondary battery with high power
ActiveUS20050271943A1Improves high-temperature cycle characteristicReduce concentrationAlkali metal oxidesIron compoundsElectrical batteryManganese
The present invention provides a non-aqueous electrolyte-based high power lithium secondary battery having a long-term service life and superior safety at both room temperature and high temperature, even after repeated high-current charging and discharging, wherein the battery comprises a mixture of a particular lithium manganese-metal composite oxide (A) having a spinel structure and a particular lithium nickel-manganese-cobalt composite oxide (B) having a layered structure, as a cathode active material.
Owner:LG ENERGY SOLUTION LTD
Supported noble metal catalyst and preparation and application thereof
ActiveCN104923225AGood dispersionEnhanced strong interactionHydrocarbon from carbon oxidesDispersed particle separationMethanationHigh activity
The invention relates to a preparation method and a catalytic application of a supported noble metal catalyst. Active components are noble metals Ru, Rh and Pd, and a carrier is MxOy or perovskite MAlO3 or spinel MAl2O4. The composite oxide carrier is prepared by an impregnation method or a coprecipitation method and is roasted under a medium-high temperature condition of 650-1200 DEG C ultimately, so that while the formed MxOy, MAlO3 or MAl2O4 or even a mixture thereof has a closer effect with an Al2O3 carrier, the number of defects and holes on the surface of Al2O3 is greatly increased, and thus prepared noble metal nanoparticles have high dispersion degree and strong stability. The catalyst preparation method has the advantages of simple operation, convenience for production and application, good product repeatability, high activity in a carbon dioxide methanation reaction and a carbon monoxide oxidation reaction, strong stability in a storage state and reaction, and quite good application prospects.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Nonaqueous electrolyte battery, battery pack and vehicle
ActiveUS20070231693A1High voltageNon-aqueous electrolyte accumulatorsFinal product manufactureLithiumCapacitance
A nonaqueous electrolyte battery includes a positive electrode containing a lithium-transition metal oxide having a layered crystal structure; a negative electrode containing a lithium-titanium composite oxide having a spinel structure; and a nonaqueous electrolyte. The positive electrode and the negative electrode satisfy the formula (1) given below:1.25≦X , (1)where X is a ratio of an available electric capacity, represented by “(B / A)”, A is an available electric capacity (mAh) at 25° C. per cm2 of the positive electrode, and B is an available electric capacity (mAh) at 25° C. per cm2 of the negative electrode.
Owner:KK TOSHIBA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com