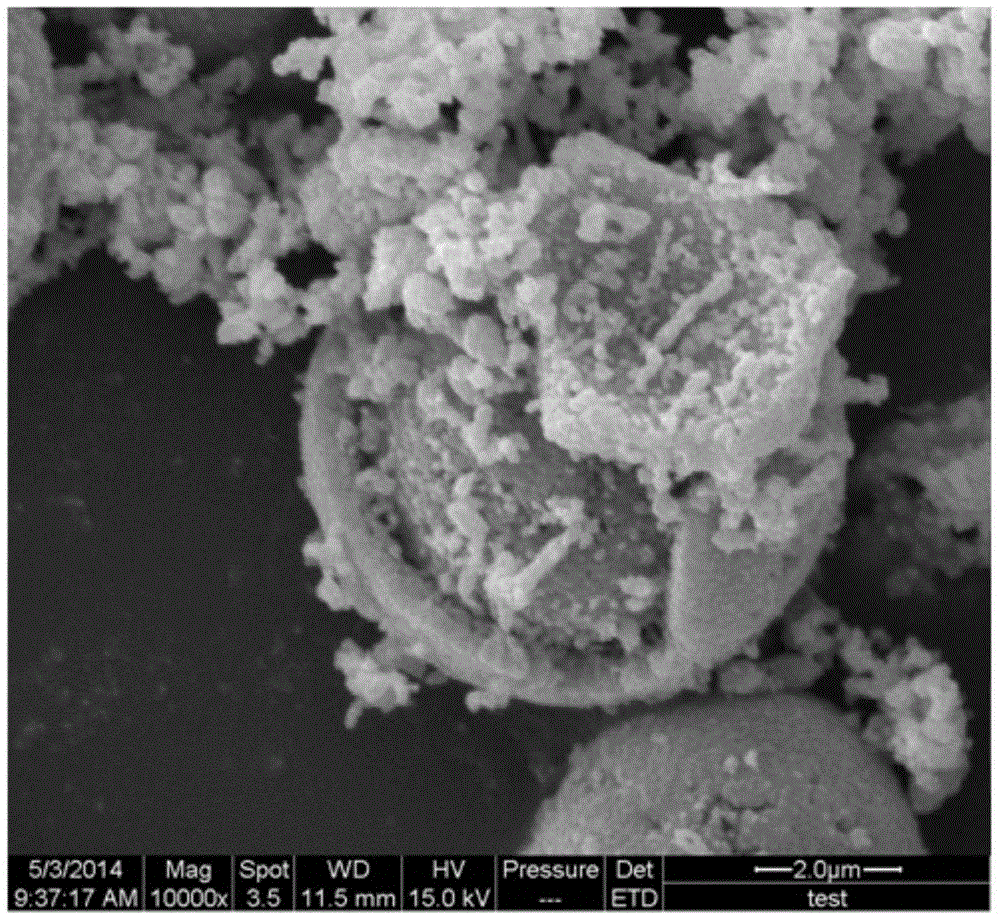
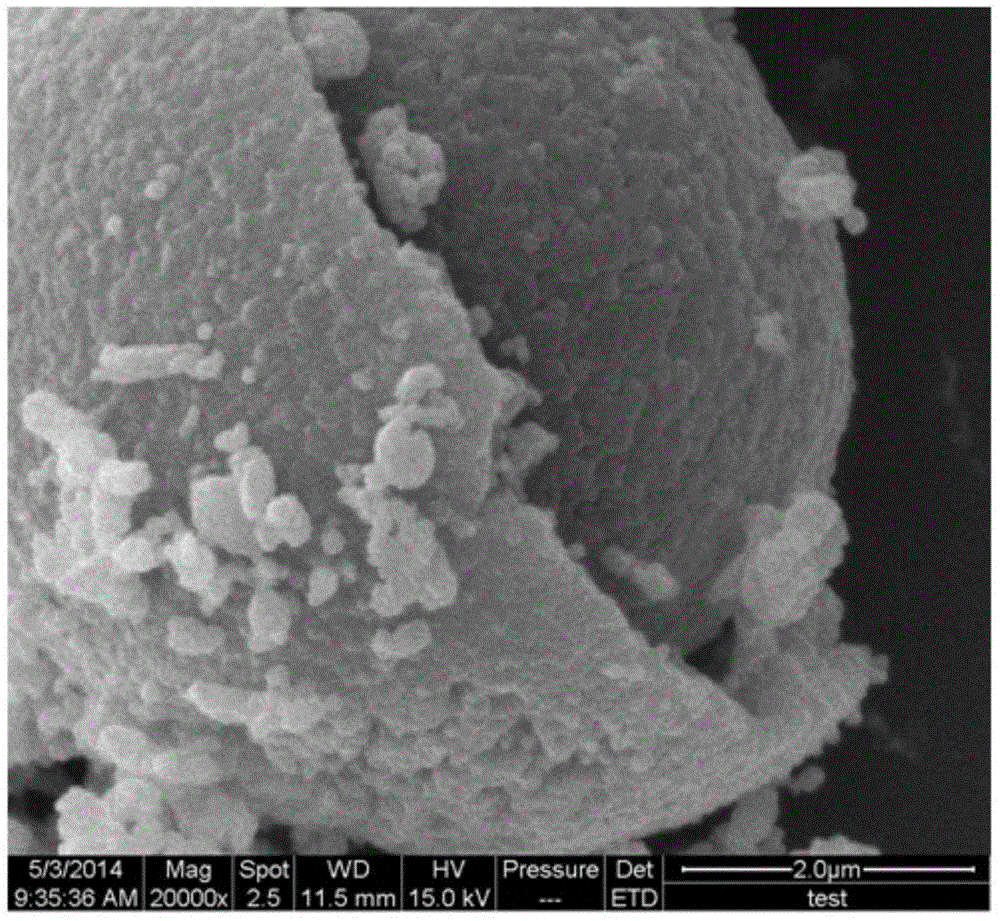
Spinel nickel manganese acid lithium and layered lithium-rich manganese-based composite cathode material with core-shell structure and preparation method thereof
A composite positive electrode material, lithium nickel manganese oxide technology, applied in the direction of structural parts, battery electrodes, electrical components, etc., can solve the problems of fast capacity decay, poor high-rate performance, and low conductivity of lithium-rich manganese-based positive electrode materials. Reliable performance improvement, improved material circulation, and improved material structure stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Weigh nickel sulfate, cobalt sulfate, and manganese sulfate according to the molar ratio Ni: Co: Mn = 0.17: 0.17: 0.66, dissolve them in deionized water and mix them evenly, and use the co-precipitation method to complex the precipitant sodium hydroxide with a certain amount Ammonia solution was added dropwise, the molar ratio of metal salt and sodium hydroxide was controlled to be 1:1, and the molar ratio of metal salt and ammonia water was 1:0.75 at the same time. Turn / minute is constantly stirred, obtains lithium-rich manganese-based material precursor (Ni 0.17 co 0.17 mn 0.66 )(OH) 2 Mother liquor;
[0034] Weigh nickel sulfate and manganese sulfate according to the molar ratio Ni:Mn=0.25:0.75, dissolve them in deionized water and mix evenly, and use the co-precipitation method to drop the precipitant sodium hydroxide and a certain amount of complexing agent ammonia into the lithium-rich Manganese-based material precursor (Ni 0.17 co 0.17 mn 0.66 )(OH) 2 In ...
Embodiment 2
[0038] According to the molar ratio Ni: Co: Mn=0.17: 0.17: 0.66, the molar ratio of nickel sulfate and nickel oxalate is 1: 1 mixture, the molar ratio of cobalt sulfate and cobalt oxalate is 1: 1 mixture, and the molar ratio of manganese sulfate and manganese oxalate is 1:1 mixture, and dissolved in deionized water and mixed evenly, the precipitant sodium carbonate and a certain amount of complexing agent ammonia were added dropwise by the method of co-precipitation, and the molar ratio of metal salt and sodium carbonate was controlled to be 1:1, and at the same time The molar ratio of metal salt to ammonia water is 1:0.5, the pH value of the reaction is 7.5, and the reaction is carried out at 60° C. for 10 h, and the speed is 700 revolutions per minute under continuous stirring to obtain the lithium-rich manganese-based material precursor (Ni 0.17 co 0.17 mn 0.66 )CO 3 Mother liquor;
[0039] According to the molar ratio Ni:Mn=0.25:0.75, weigh the mixture of nickel sulfate...
Embodiment 3
[0043] Weigh nickel oxalate and manganese oxalate according to the molar ratio Ni:Mn=0.25:0.75, dissolve them in deionized water and mix them evenly, and add the precipitating agent sodium hydroxide and a certain amount of complexing agent ammonia water dropwise by co-precipitation method, The molar ratio of control and sodium hydroxide is 1:1, while the molar ratio of metal salt metal salt to ammonia water is 1:0.75, the pH value of the reaction is 10.5, react at 60°C for 12h, and continuously stir at a speed of 800 rpm to obtain Lithium-rich manganese-based material precursor core-(Ni 0.25 mn 0.75 )(OH) 2 Mother liquor;
[0044] Weigh nickel oxalate and manganese oxalate according to the molar ratio Ni:Mn=0.25:0.75, dissolve them in deionized water and mix evenly, and use the co-precipitation method to drop the precipitating agent sodium hydroxide and a certain amount of complexing agent ammonia water into the lithium-rich Manganese-based material precursor (Ni 0.25 mn ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com