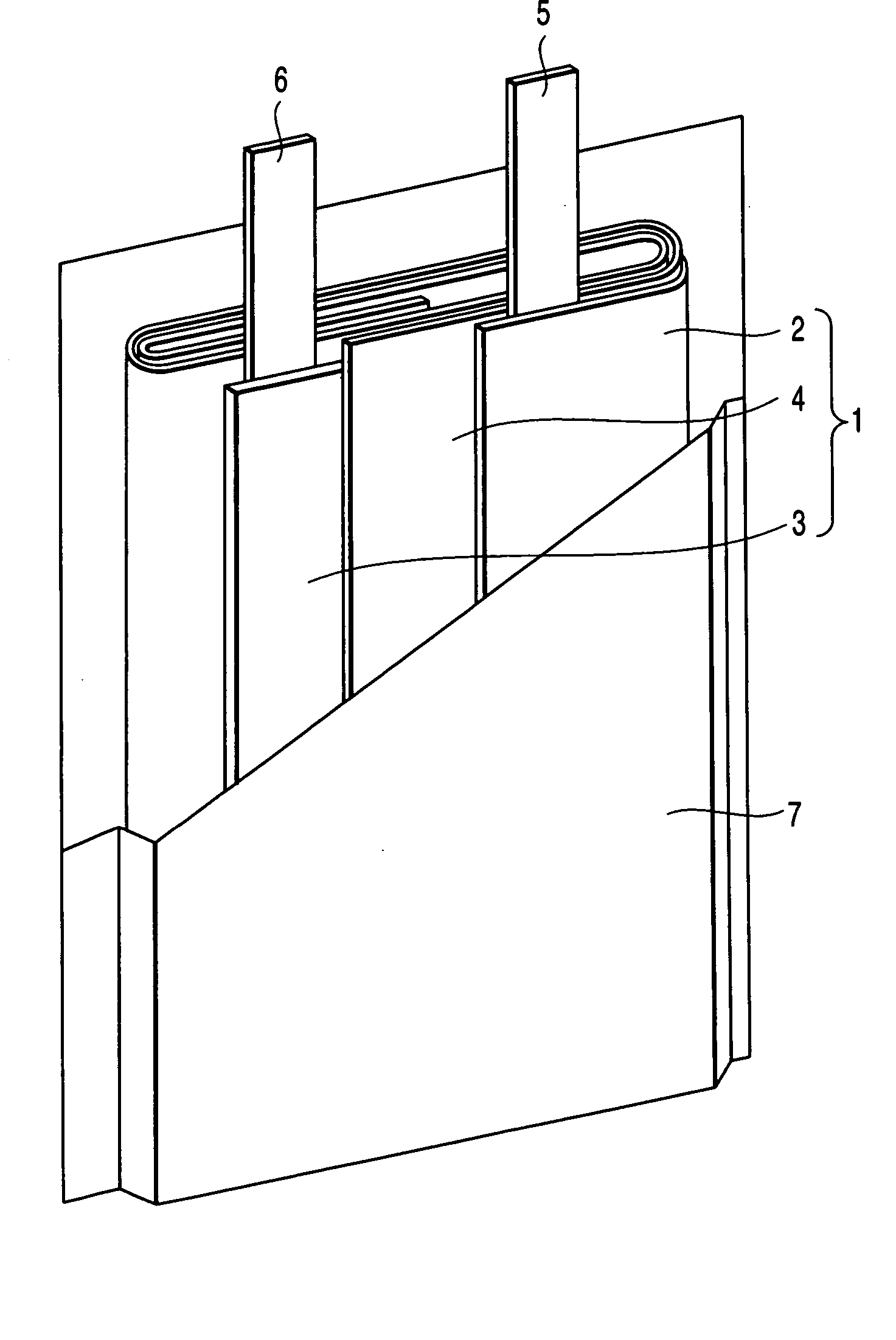
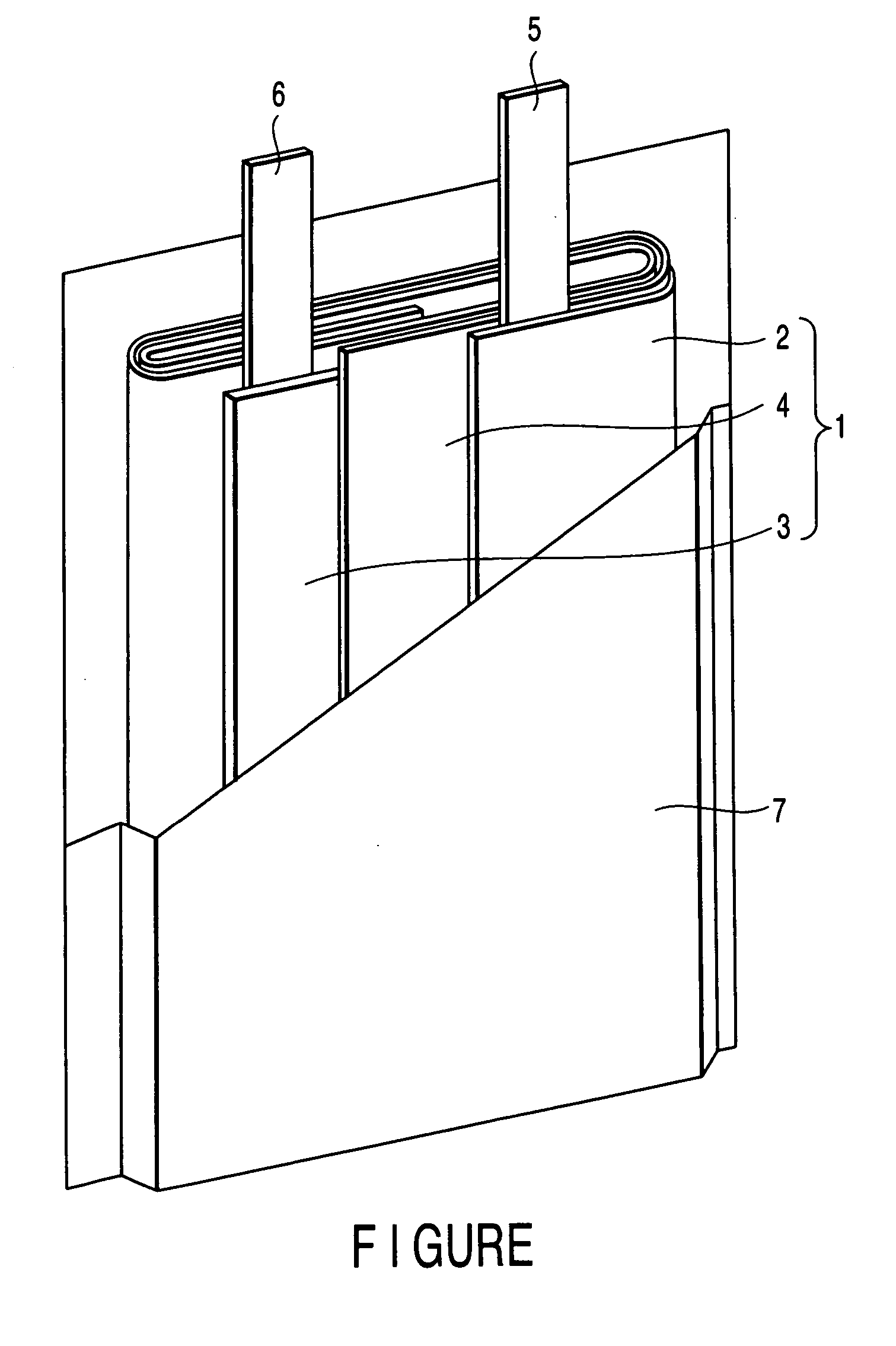
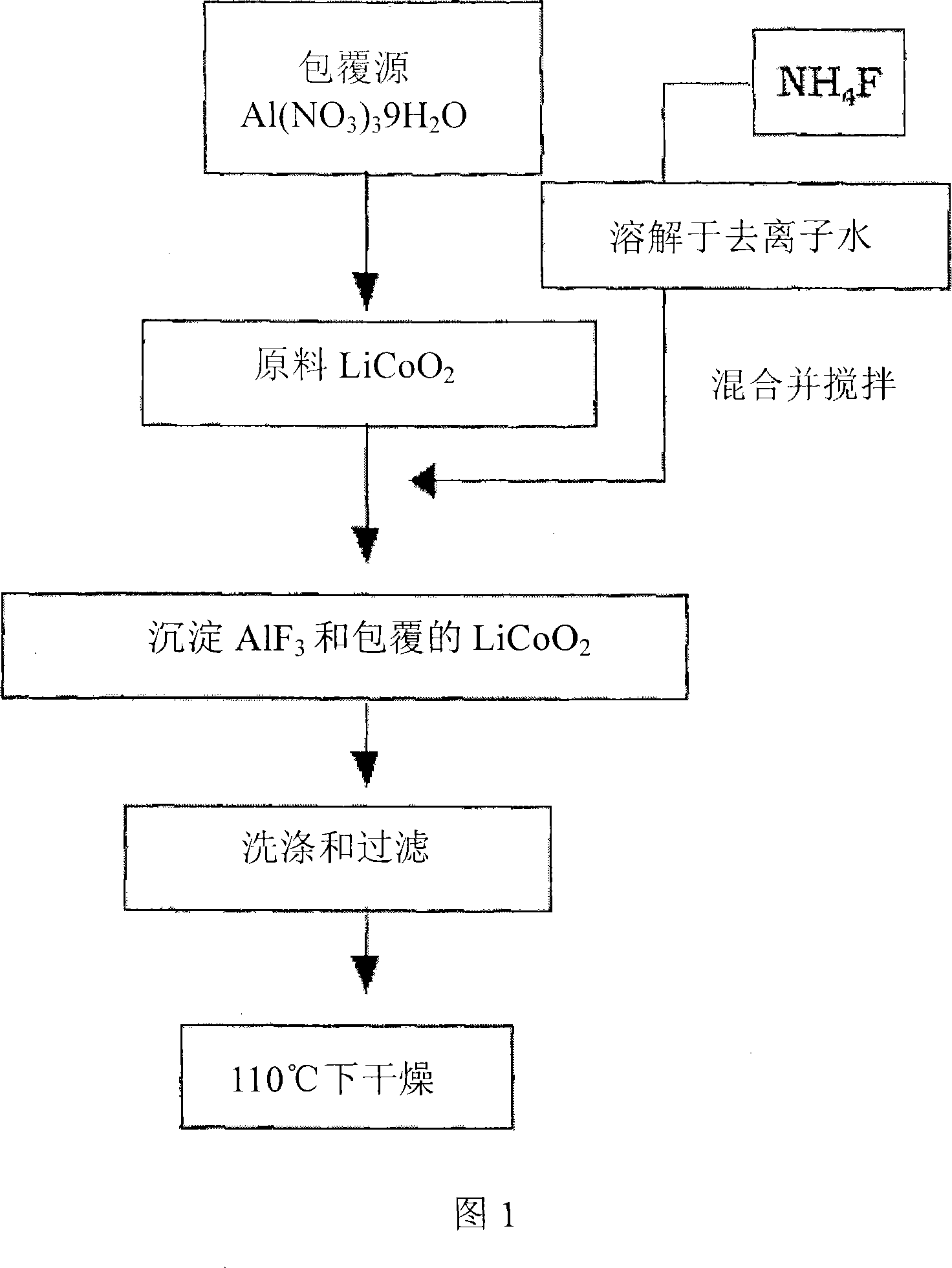
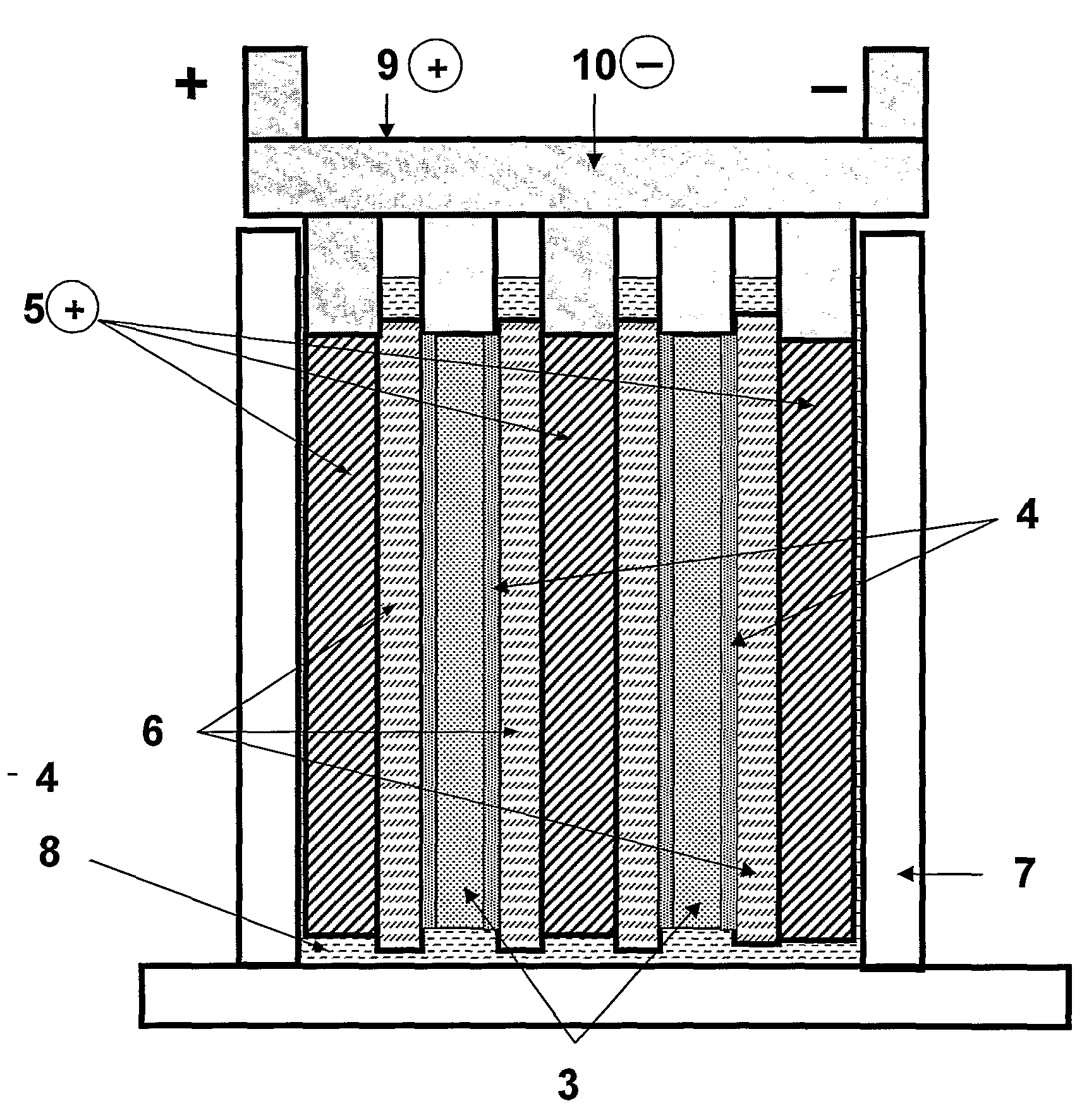
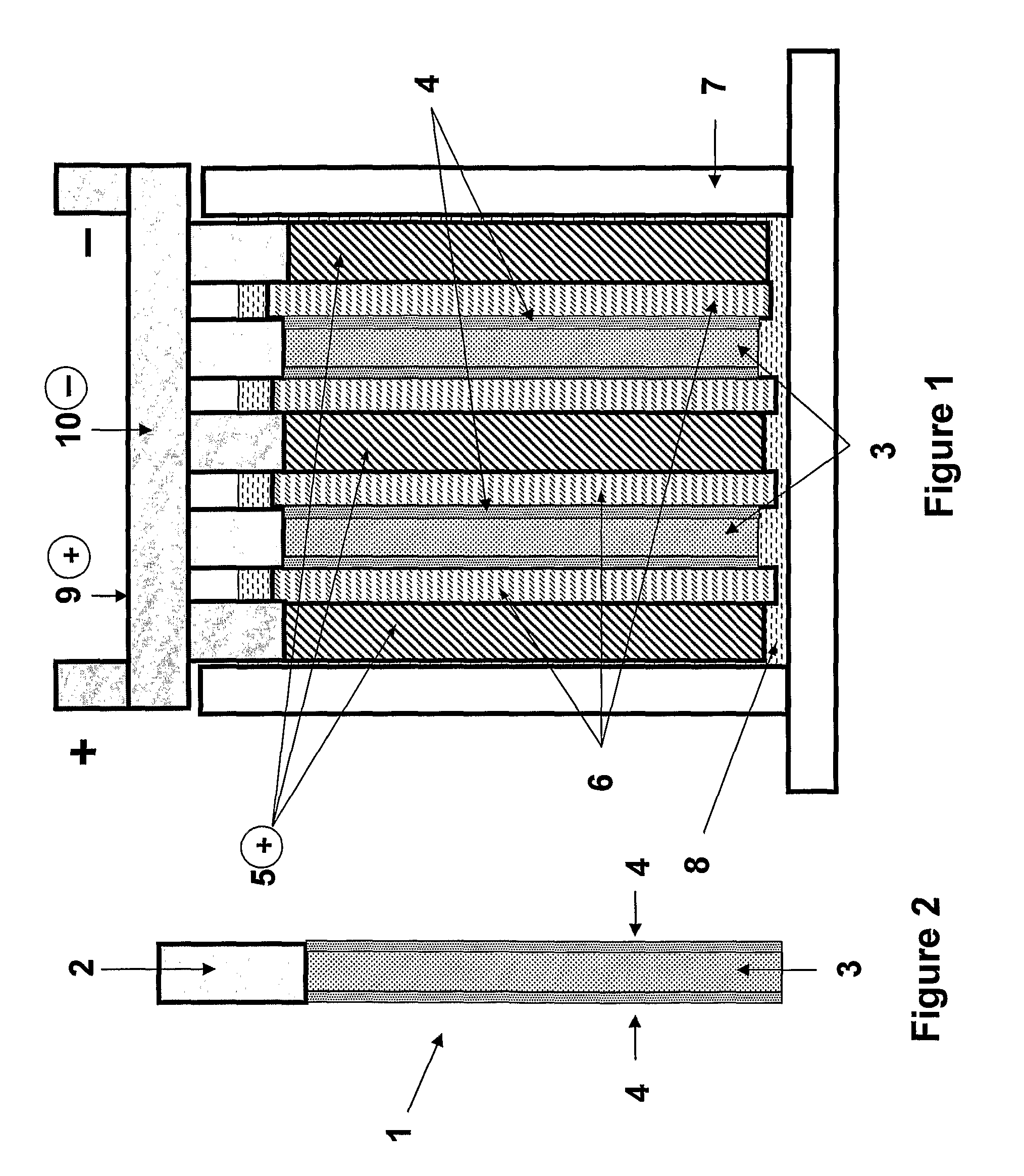
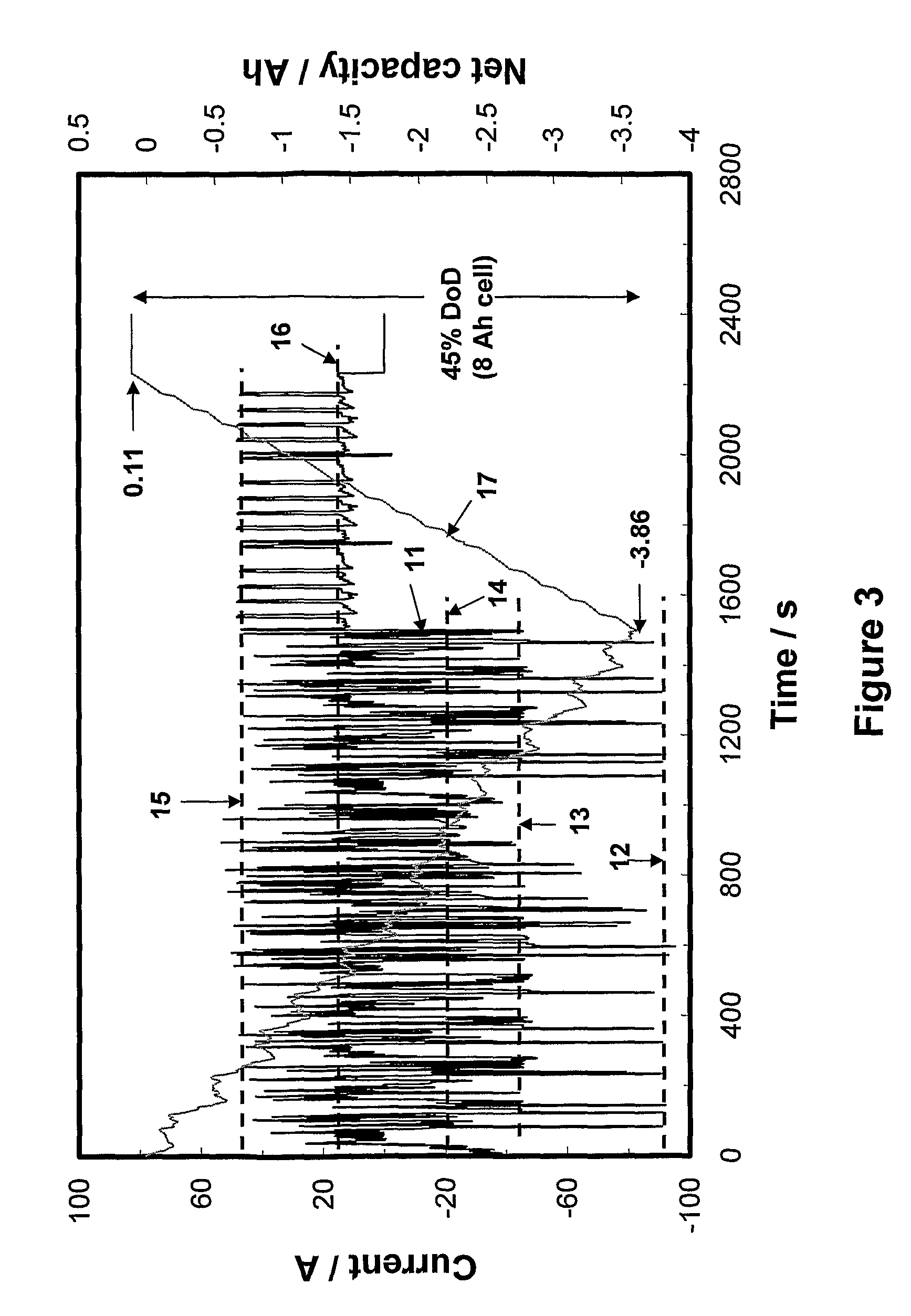
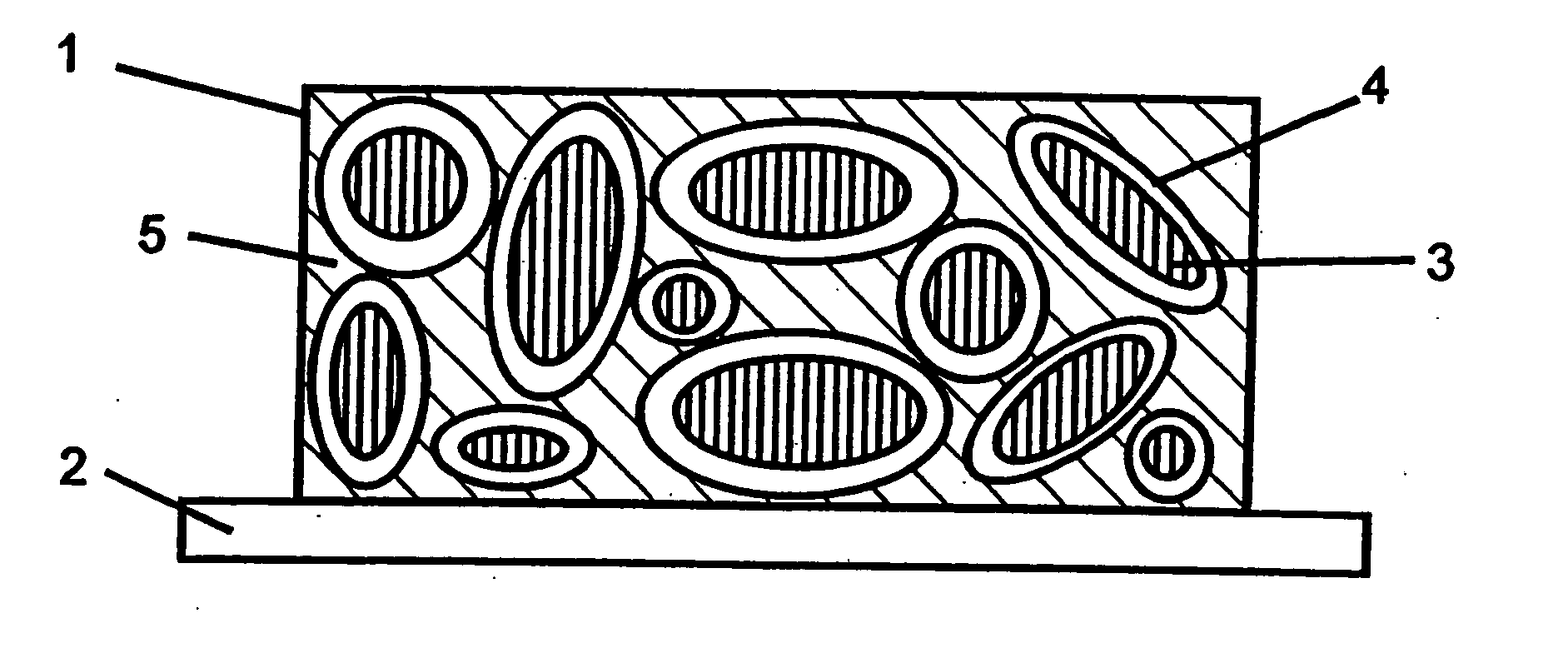
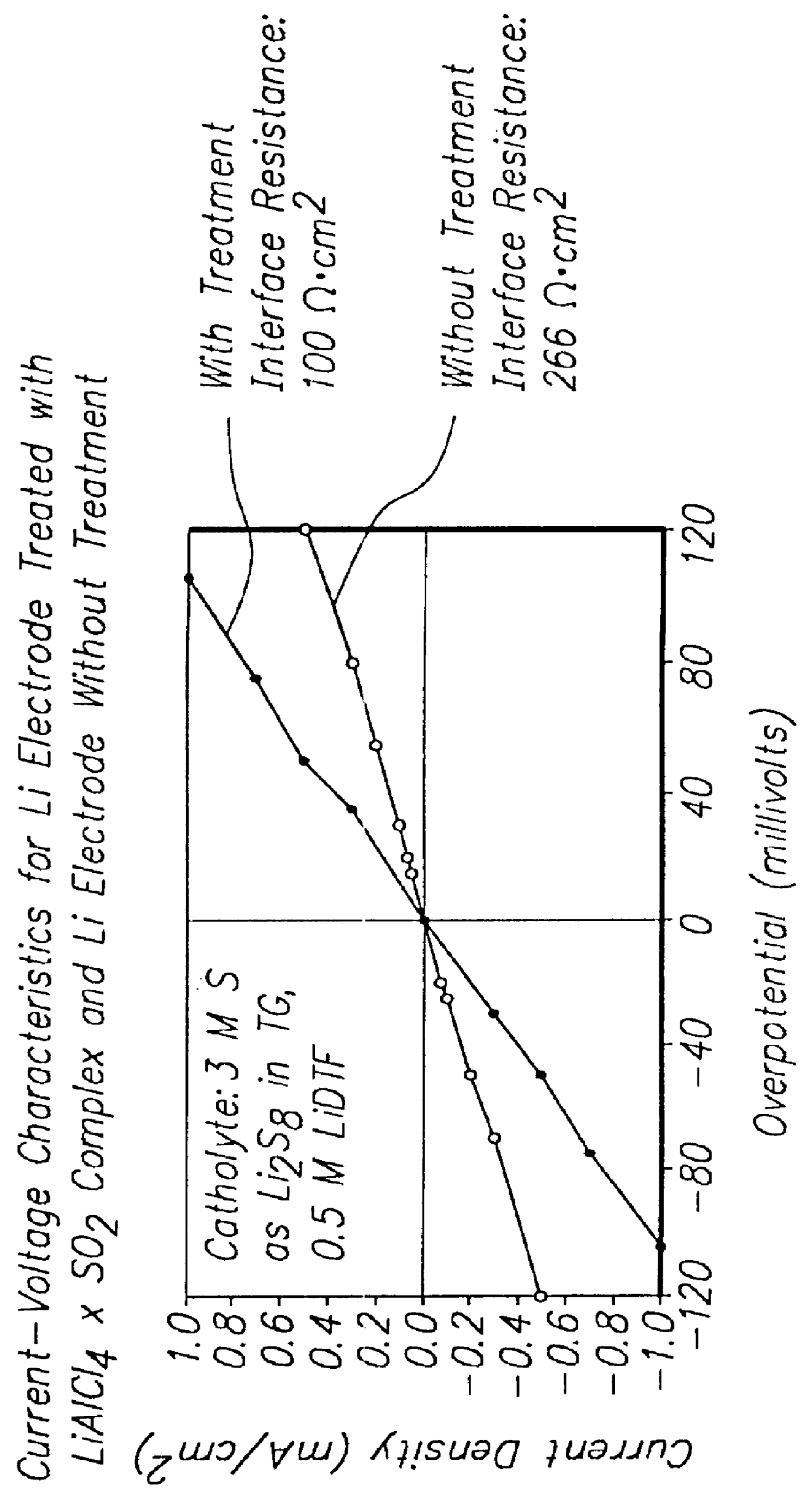
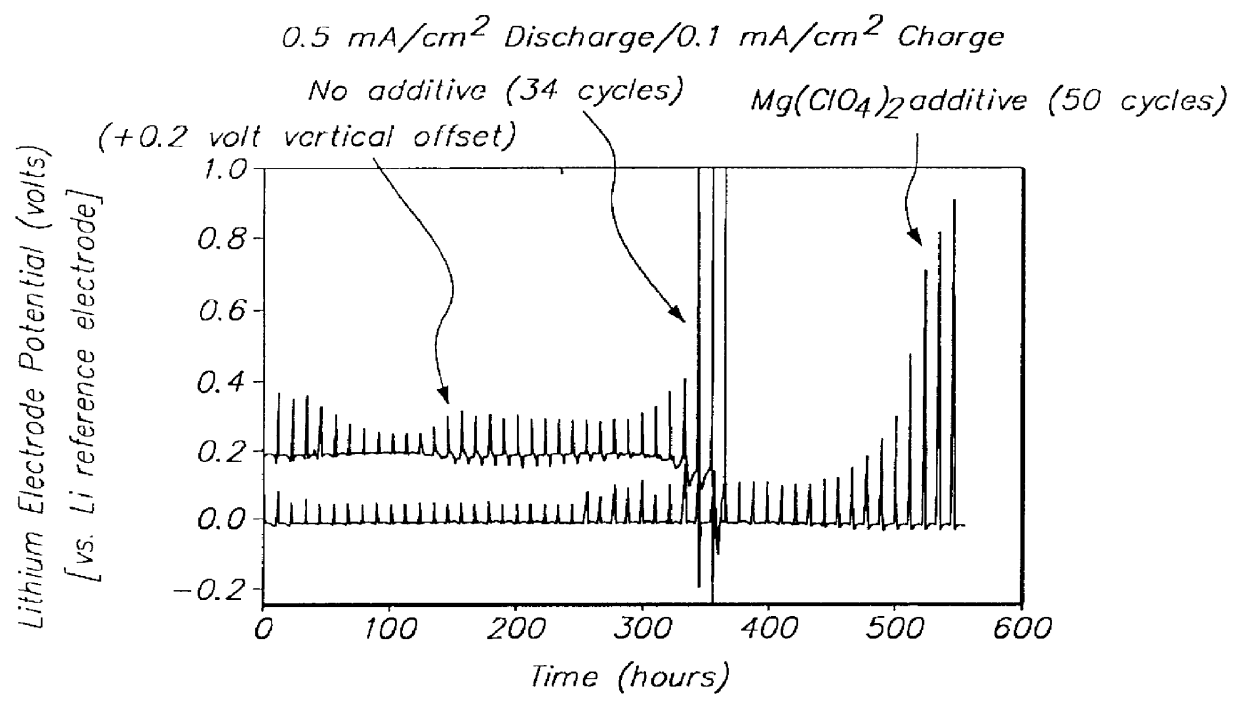
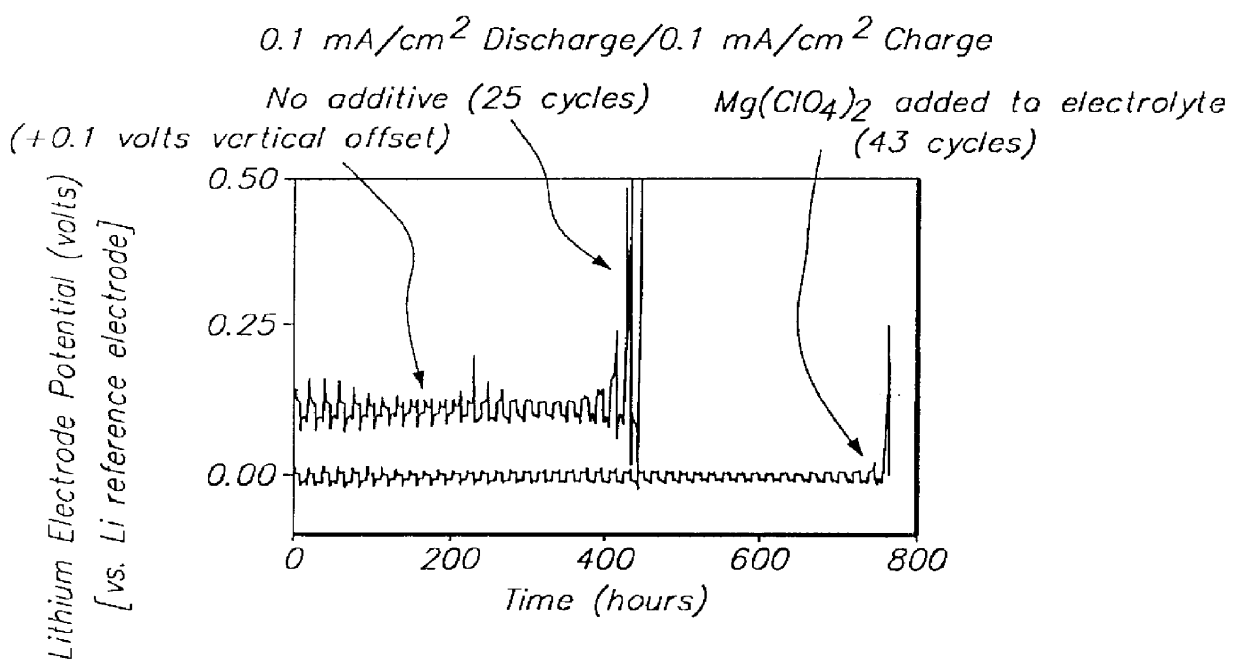
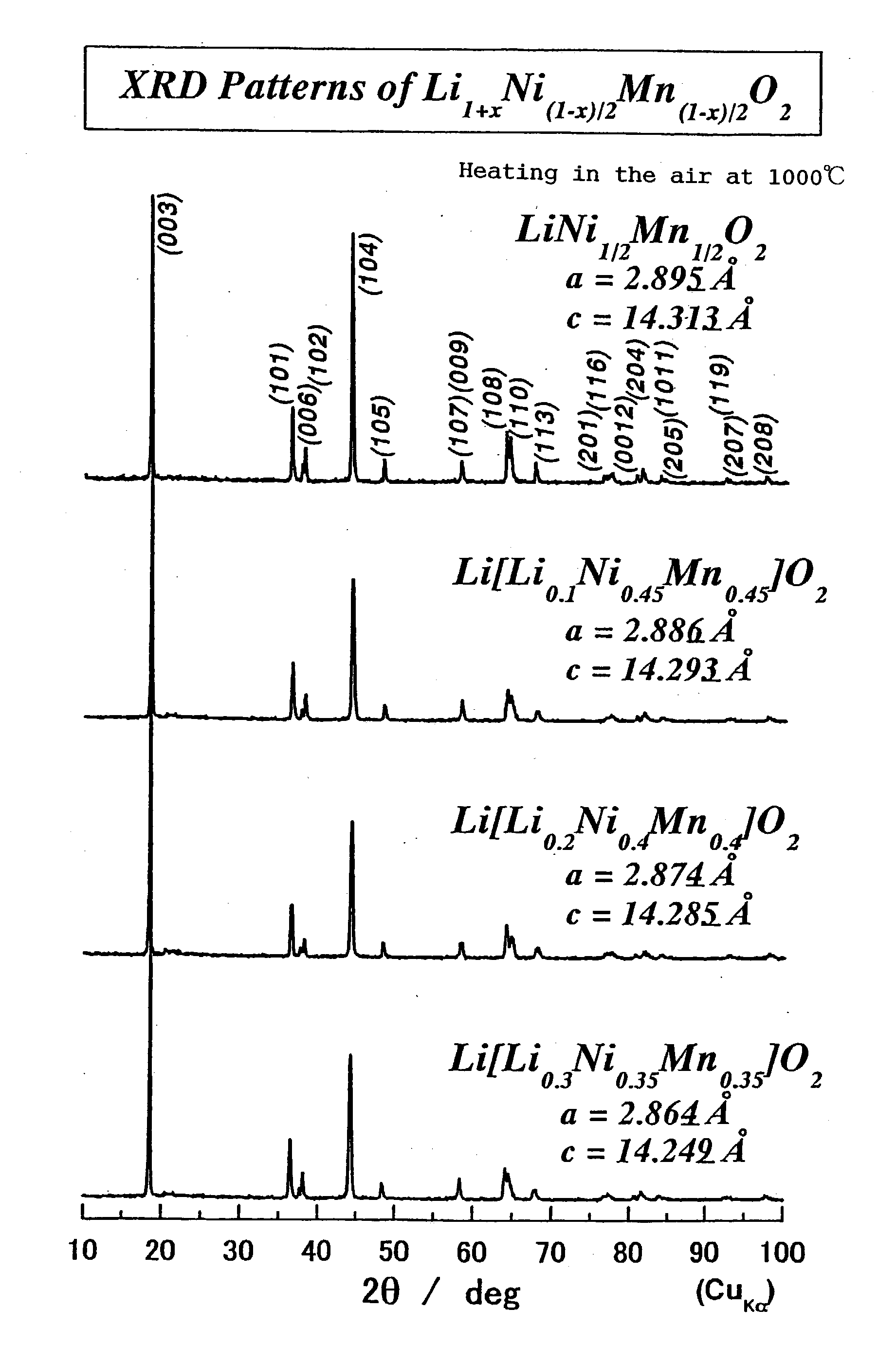
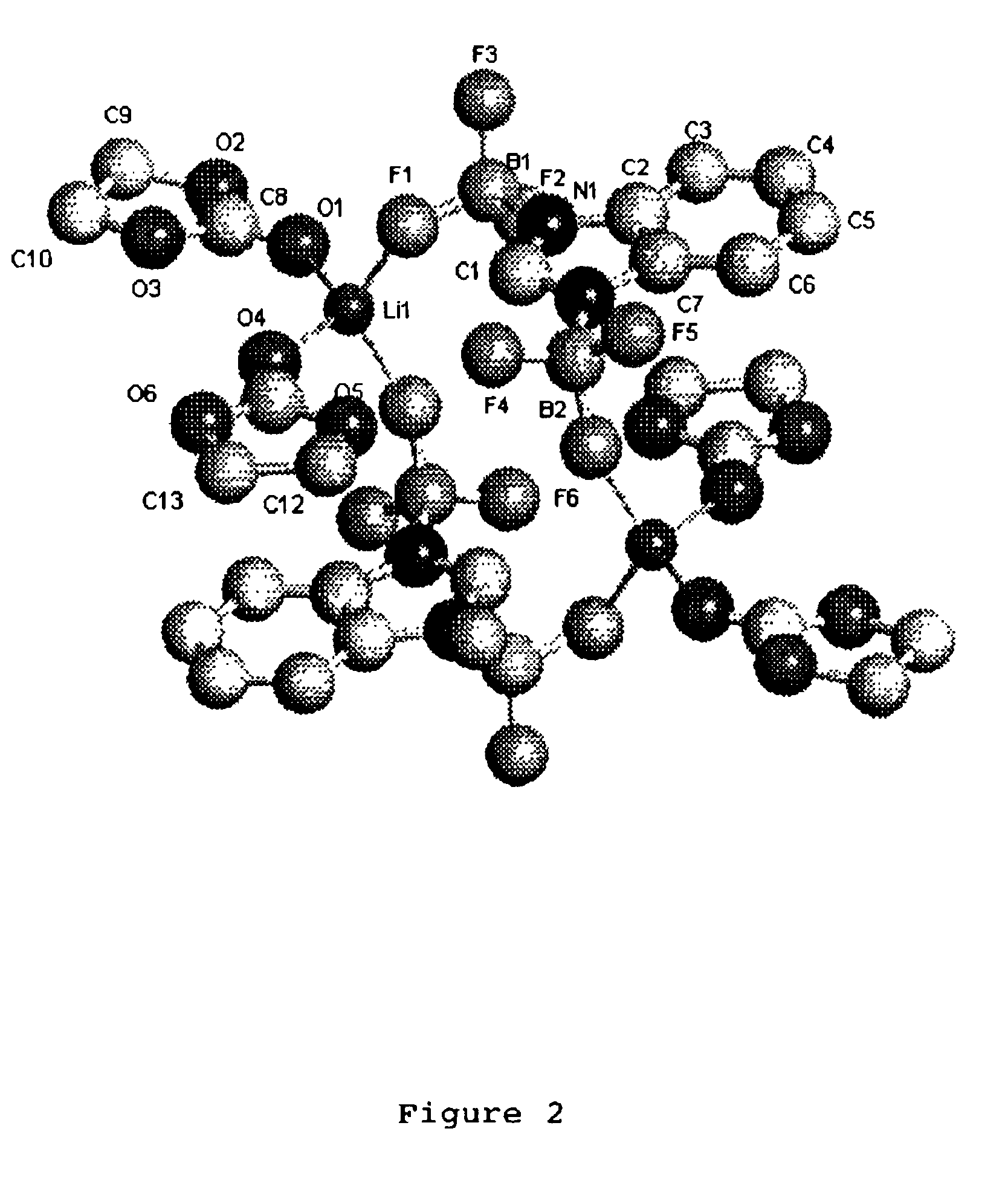
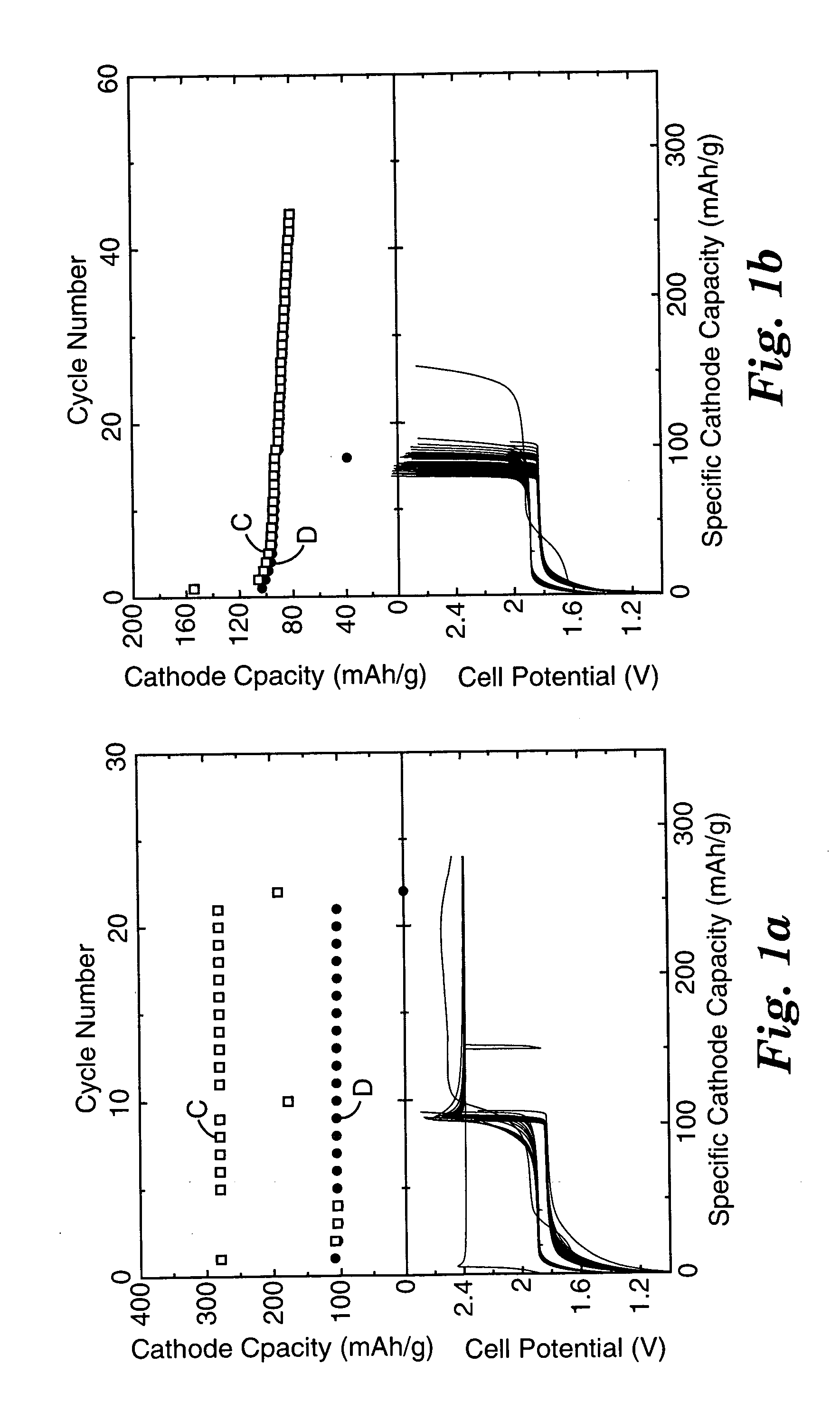
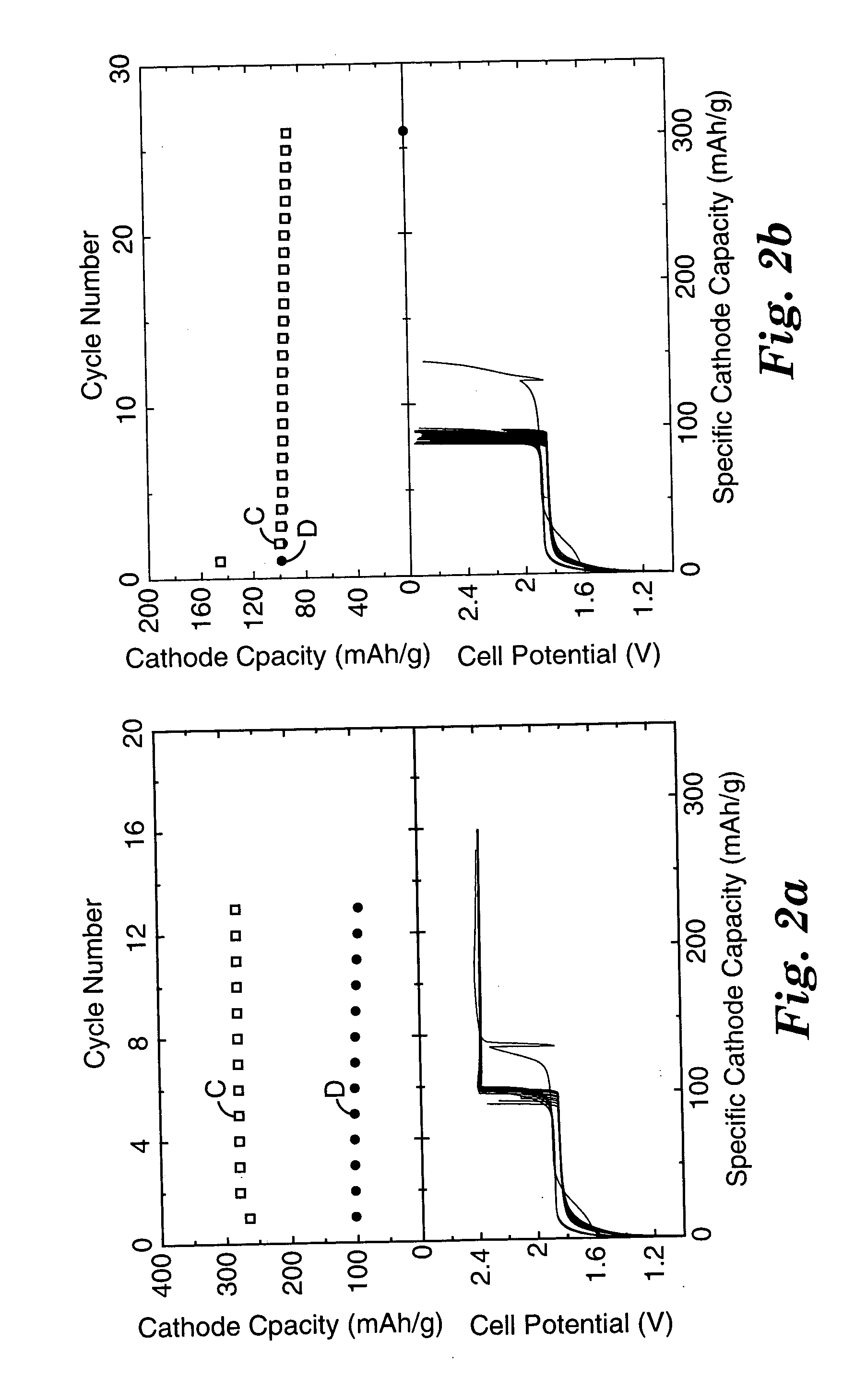
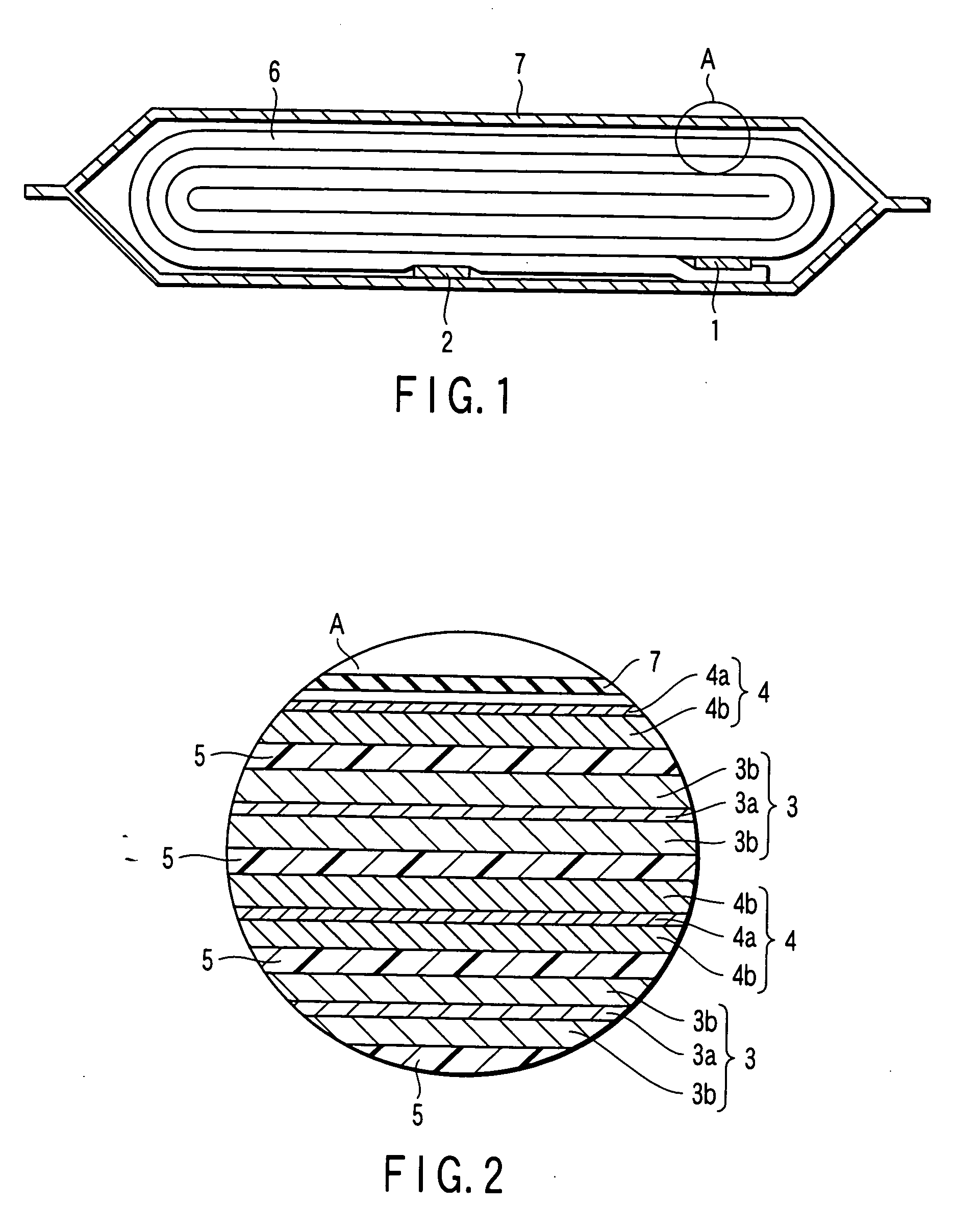
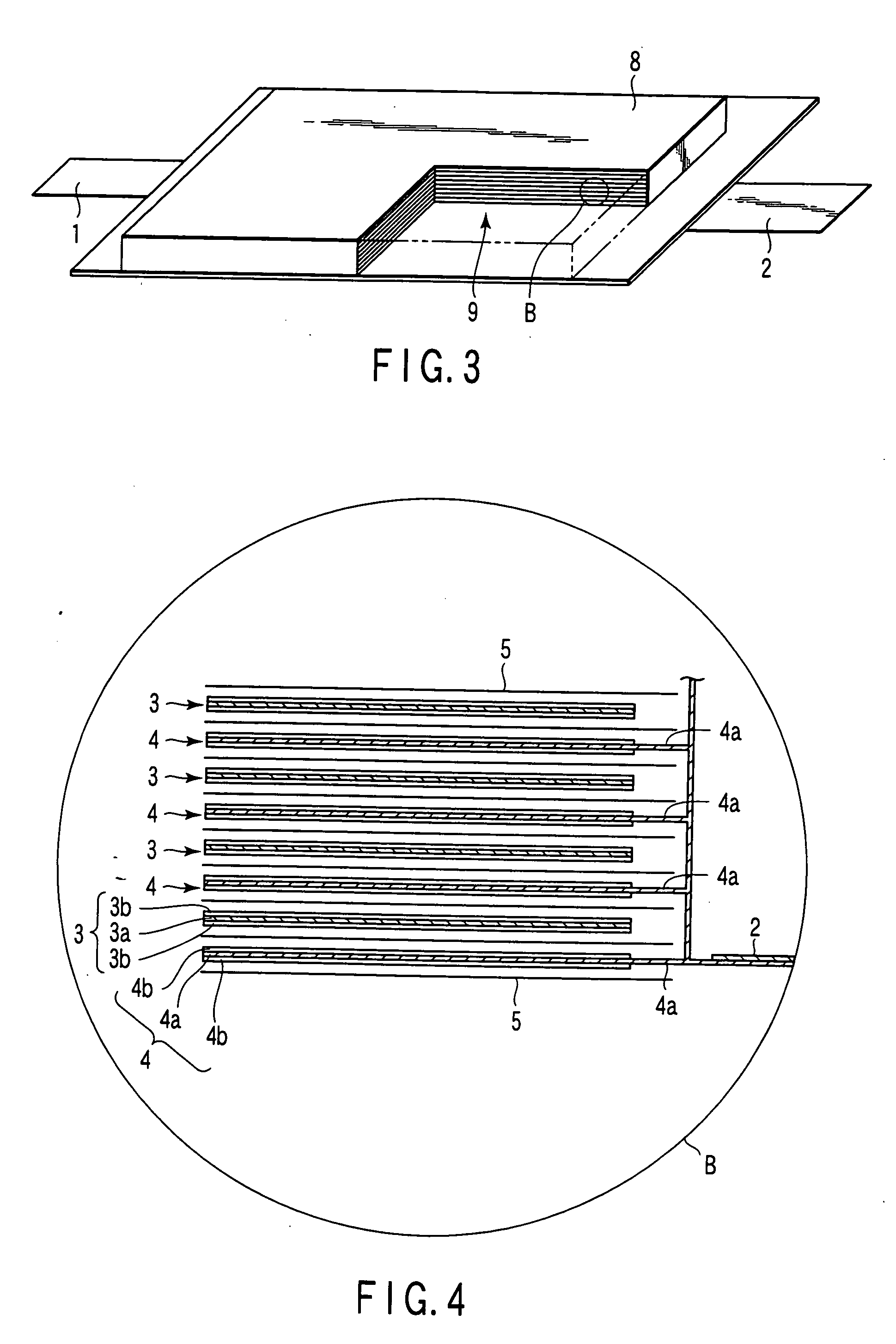
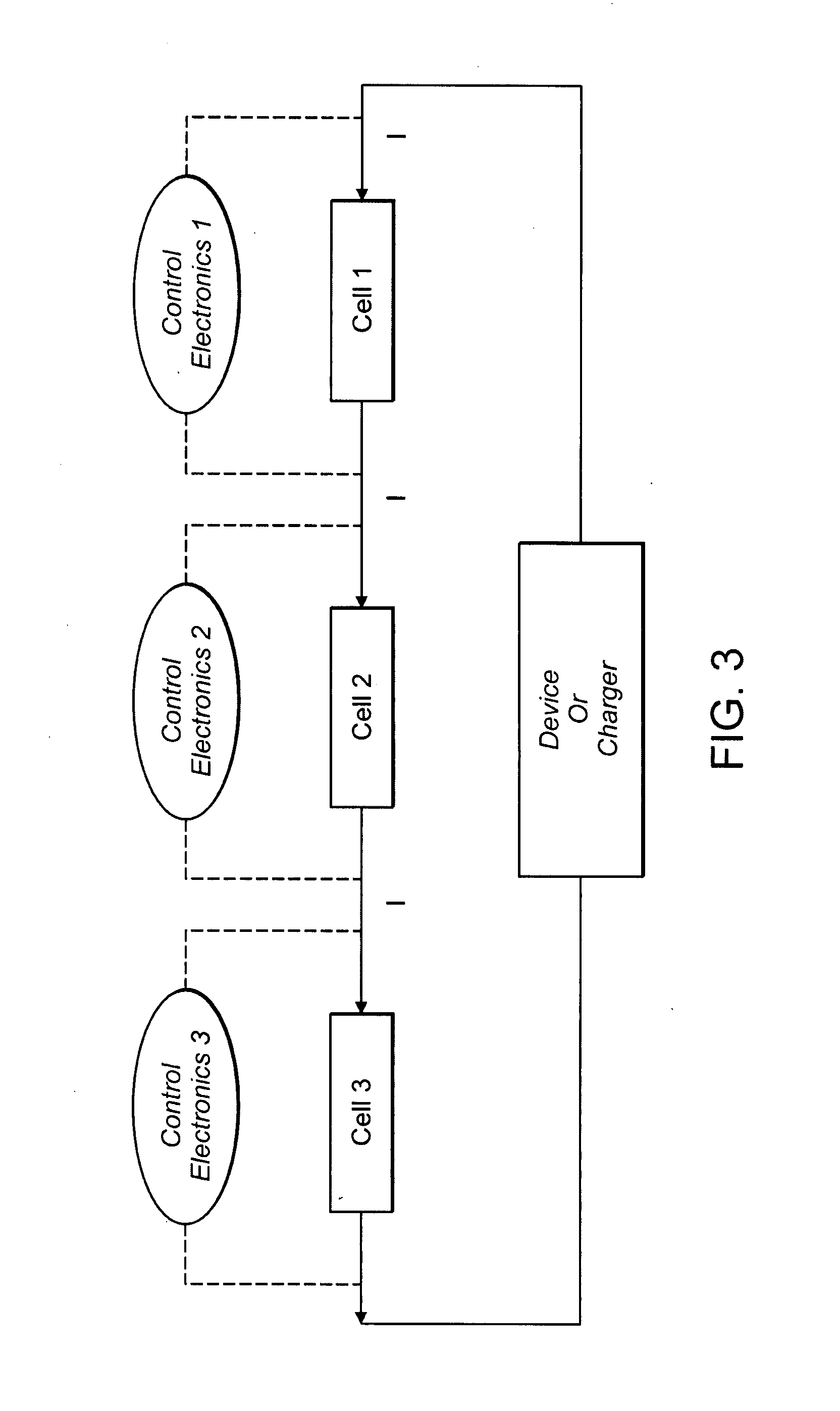
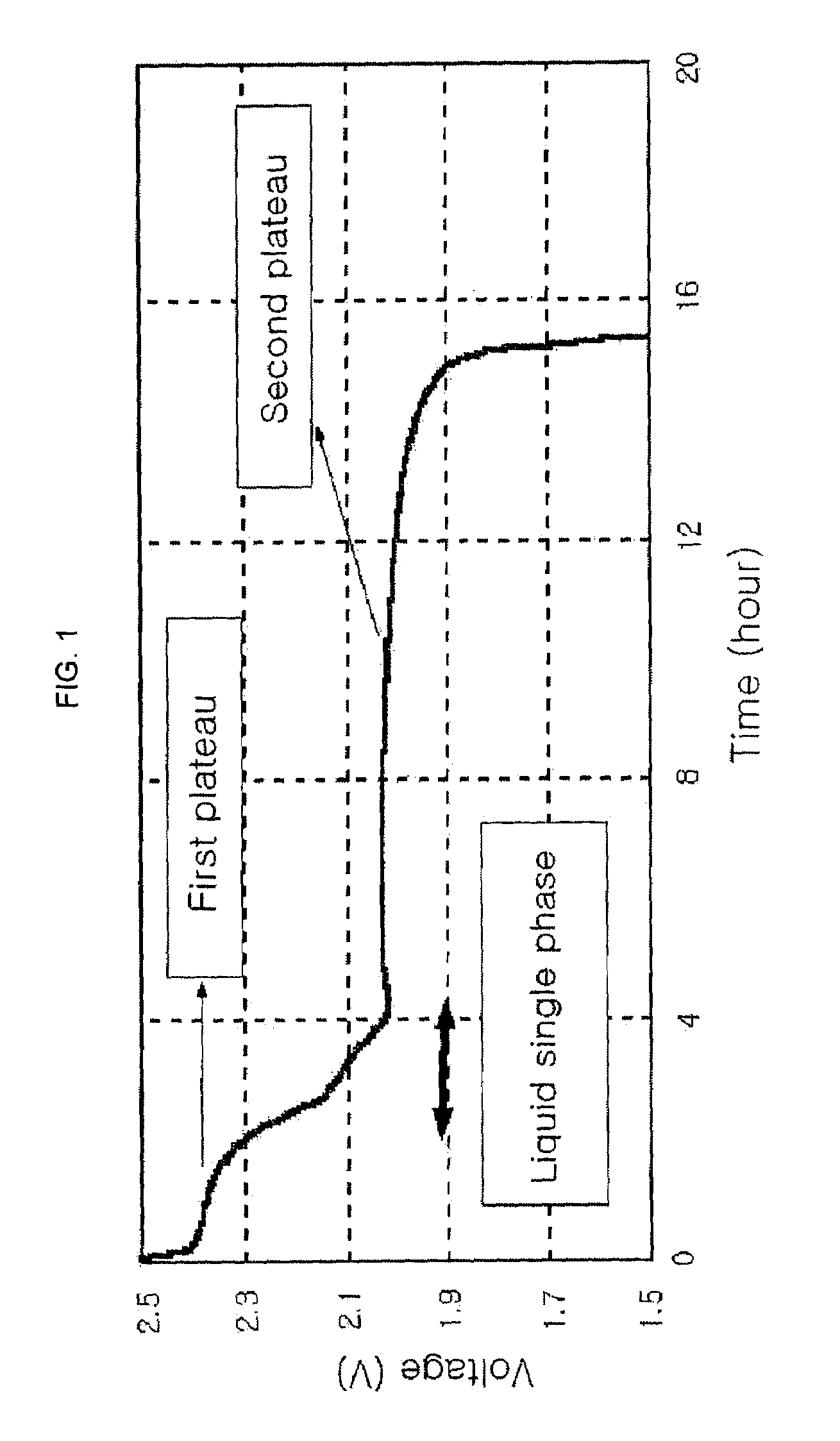
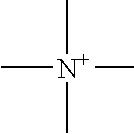Patents
Literature
11885results about "Positive electrodes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods and reagents for enhancing the cycling efficiency of lithium polymer batteries
InactiveUS6017651AImprove efficiencyElectrode rolling/calenderingElectrochemical processing of electrodesLithium metalSulfur electrode
Batteries including a lithium electrode and a sulfur counter electrode that demonstrate improved cycling efficiencies are described. In one embodiment, an electrochemical cell having a lithium electrode and a sulfur electrode including at least one of elemental sulfur, lithium sulfide, and a lithium polysulfide is provided. The lithium electrode includes a surface coating that is effective to increase the cycling efficiency of said electrochemical cell. In a more particular embodiment, the lithium electrode is in an electrolyte solution, and, more particularly, an electrolyte solution including either elemental sulfur, a sulfide, or a polysulfide. In another embodiment, the coating is formed after the lithium electrode is contacted with the electrolyte. In a more particular embodiment, the coating is formed by a reaction between the lithium metal of the lithium electrode and a chemical species present in the electrolyte.
Owner:POLYPLUS BATTERY CO INC
Lithium-ion rechargeable battery based on nanostructures
A nanowire-based Li-ion rechargeable battery having superior performance with little capacity fade for use in applications including consumer electronics and medical devices is made by incorporating nanowire construction of the cathode. The nanowire-based battery system includes a nanostructured high surface area cathode structure fabricated by electrodeposition using alumina nanopore templates.
Owner:ENABLE IPC
High Capacity Anode Materials for Lithium Ion Batteries
High capacity silicon based anode active materials are described for lithium ion batteries. These materials are shown to be effective in combination with high capacity lithium rich cathode active materials. Supplemental lithium is shown to improve the cycling performance and reduce irreversible capacity loss for at least certain silicon based active materials. In particular silicon based active materials can be formed in composites with electrically conductive coatings, such as pyrolytic carbon coatings or metal coatings, and composites can also be formed with other electrically conductive carbon components, such as carbon nanofibers and carbon nanoparticles. Additional alloys with silicon are explored.
Owner:IONBLOX INC
Long cycle-life alkali metal battery
InactiveUS6203947B1Improve cycle lifeFast charging rateElectrochemical processing of electrodesElectrode carriers/collectorsHigh pressureElectrochemical cell
The present invention provides a cathode for use in a secondary electrochemical cell, such cathode being coated with a very thin, protective film, permeable to ions. The protective film of the cathode usually has a thickness of up to about 0.1 mum and it provides protection against high voltage charging and overdiscbarging. The present invention further provides a secondary electrochemical cell comprising such a cathode.
Owner:RAMOT UNIV AUTHORITY FOR APPLIED RES & INDAL DEVMENT
High discharge capacity lithium battery
InactiveUS20050233214A1Improve discharge performanceIncrease energy densityFinal product manufactureOrganic electrolyte cellsHigh rateIron disulfide
A lithium / iron disulfide electrochemical battery cell with a high discharge capacity. The cell has a lithium negative electrode, an iron disulfide positive electrode and a nonaqueous electrolyte. The iron disulfide of the positive electrode has a controlled average particle size range which allows the electrochemical cells to exhibit desired properties in both low and high rate applications. In various embodiments, the iron disulfide particles are wet milled, preferably utilizing a media mill or milled utilizing a non-mechanical mill such as a jet mill, which reduces the iron disulfide particles to a desired average particle size range for incorporation into the positive electrode.
Owner:EVEREADY BATTERY CO INC
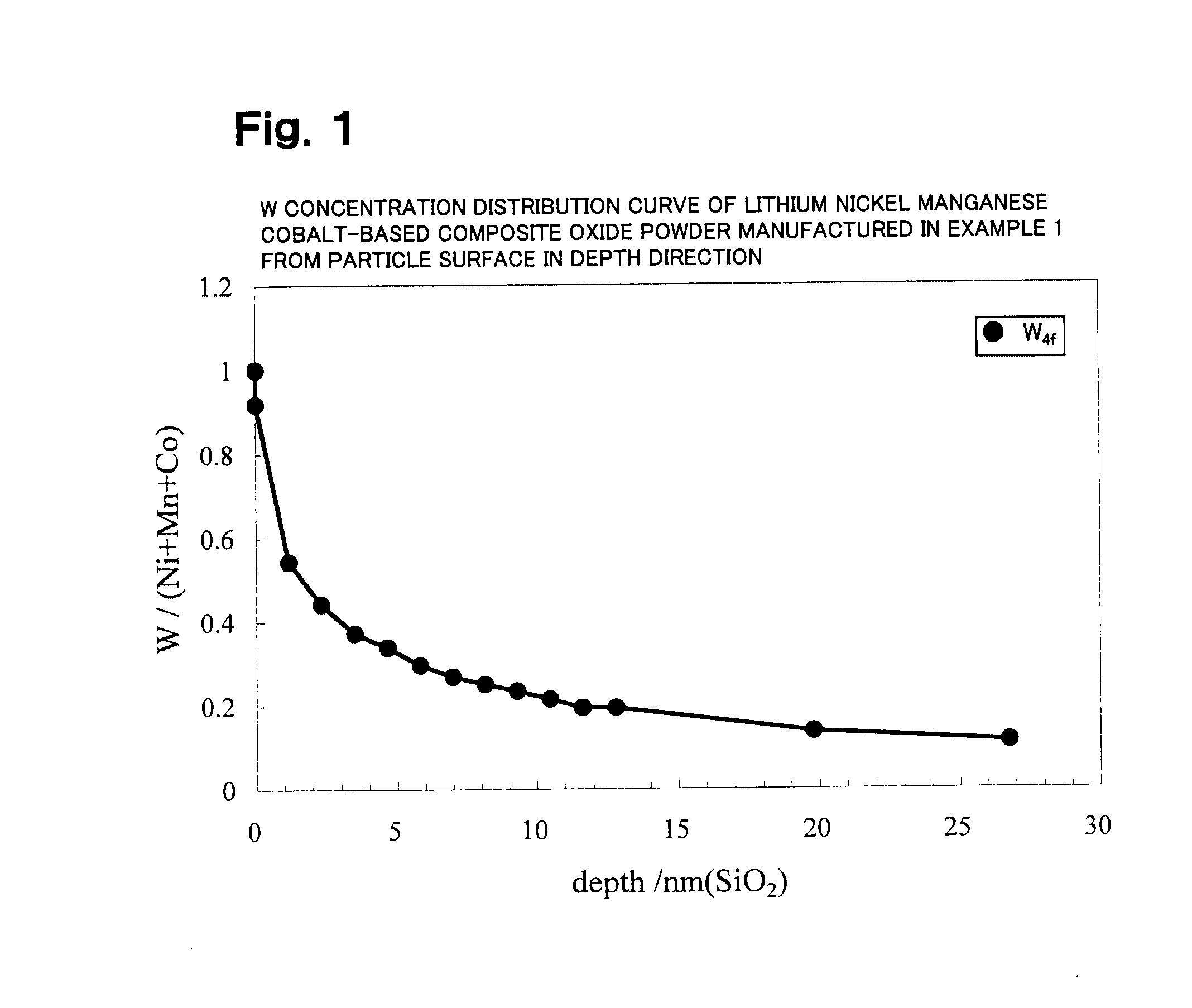
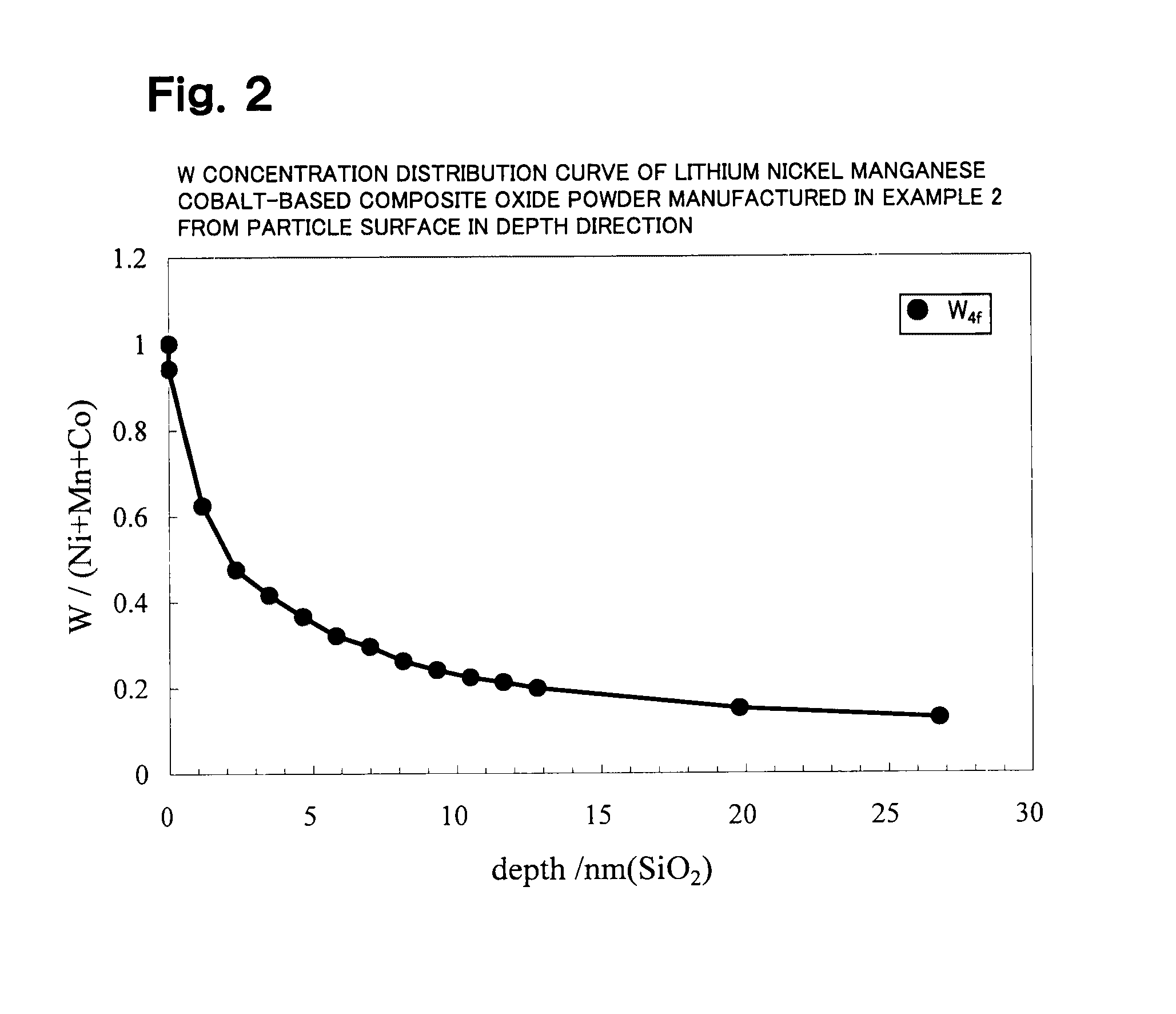
Lithium transition metal-based compound powder, method for manufacturing the same, spray-dried substance serving as firing precursor thereof, and lithium secondary battery positive electrode and lithium secondary battery using the same
ActiveUS20100209771A1Improve load characteristicsHigh densityMaterial nanotechnologyAlkaline accumulatorsLithiumHigh density
A lithium transition metal-based compound powder for a lithium secondary battery positive electrode material that can achieve both improvements of load characteristics such as rate and output characteristics and a higher density is a lithium transition metal-based compound powder containing, as a main component, a lithium transition metal-based compound that has a function of allowing elimination and insertion of lithium ions, and including a crystal structure belonging to a layer structure, wherein primary particles are aggregated to form secondary particles, the ratio A / B of a median diameter A of the secondary particles to an average diameter (average primary particle diameter B) is in the range of 8 to 100, and 0.01≦FWHM(110)≦0.5 where FWHM(110) is the half width of a (110) diffraction peak present near a diffraction angle 2θ of 64.5° in a powder X-ray diffraction analysis using a CuKα line.
Owner:MITSUBISHI CHEM CORP
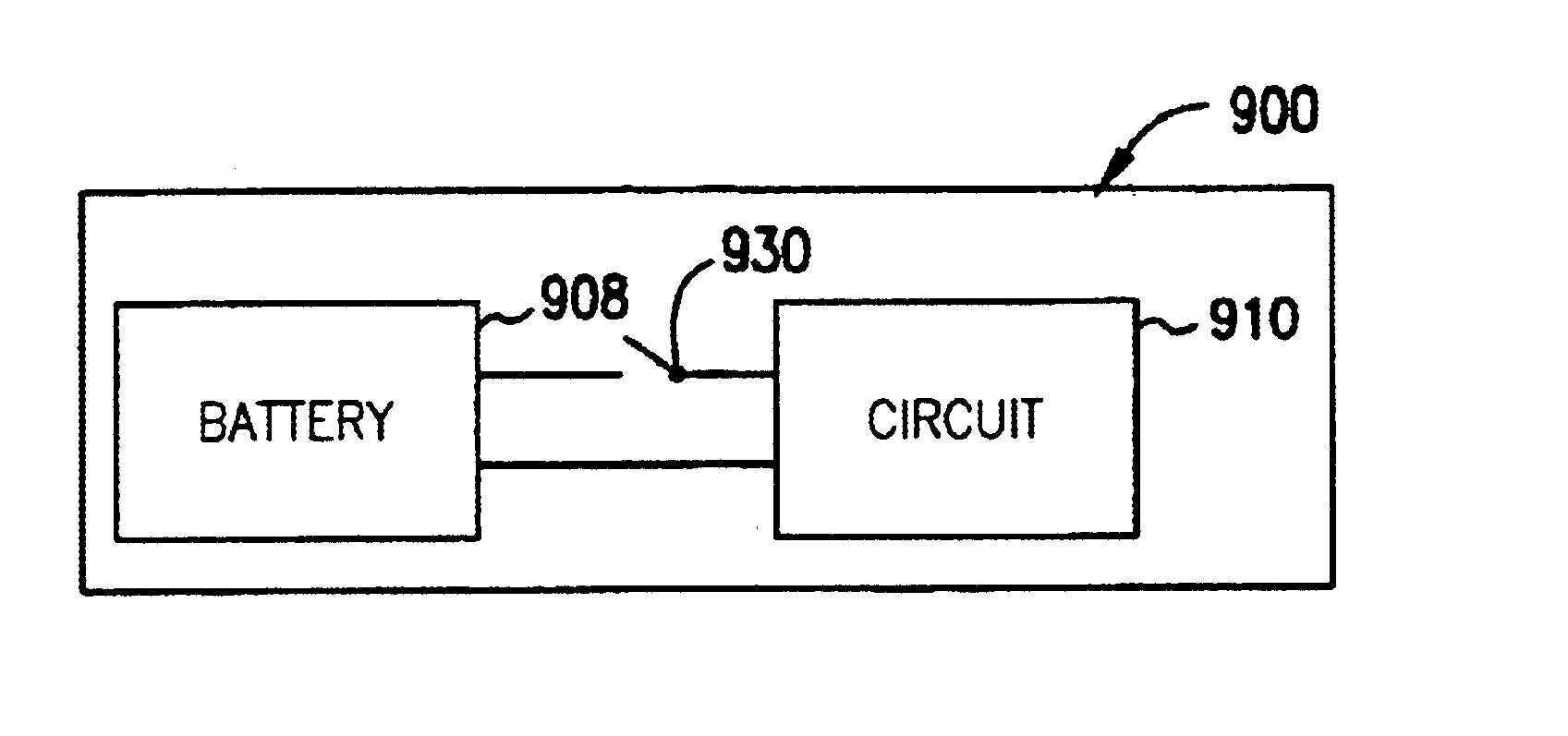
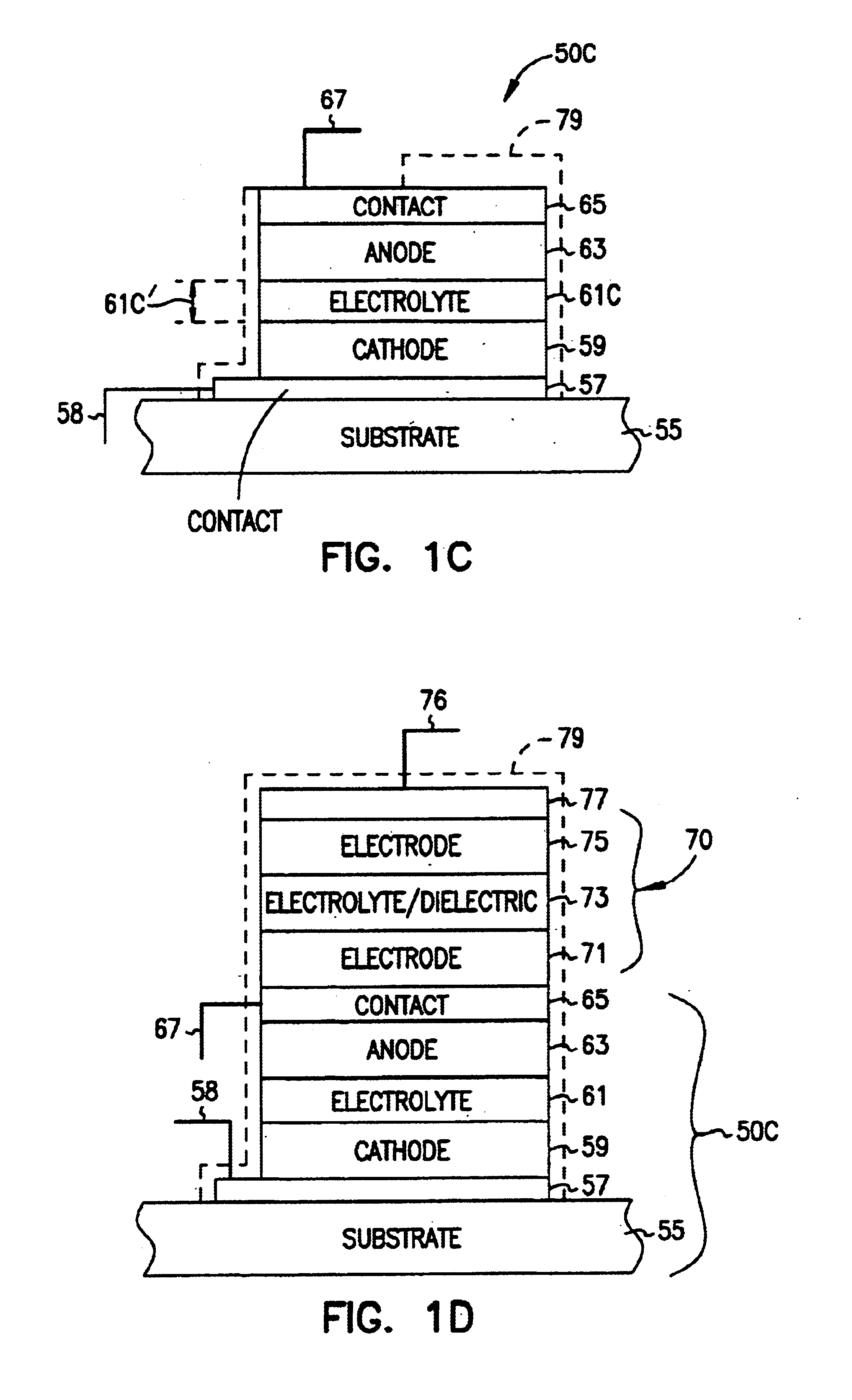
Solid state activity-activated battery device and method
InactiveUS6906436B2Sufficient energy storageBatteries circuit arrangementsFinal product manufactureEngineeringElectron
A system includes a thin-film battery and an activity-activated switch. The system is placed on a substrate with an adhesive backing. In some embodiments, the substrate is flexible. Also formed on the substrate is an electrical circuit that includes electronics. The activity-activated switch places the thin-film battery in electrical communication with the circuit and electronics. The battery and the circuit are formed on the substrate and may be comprised of one or a plurality of deposited layers.
Owner:CYMBET CORP
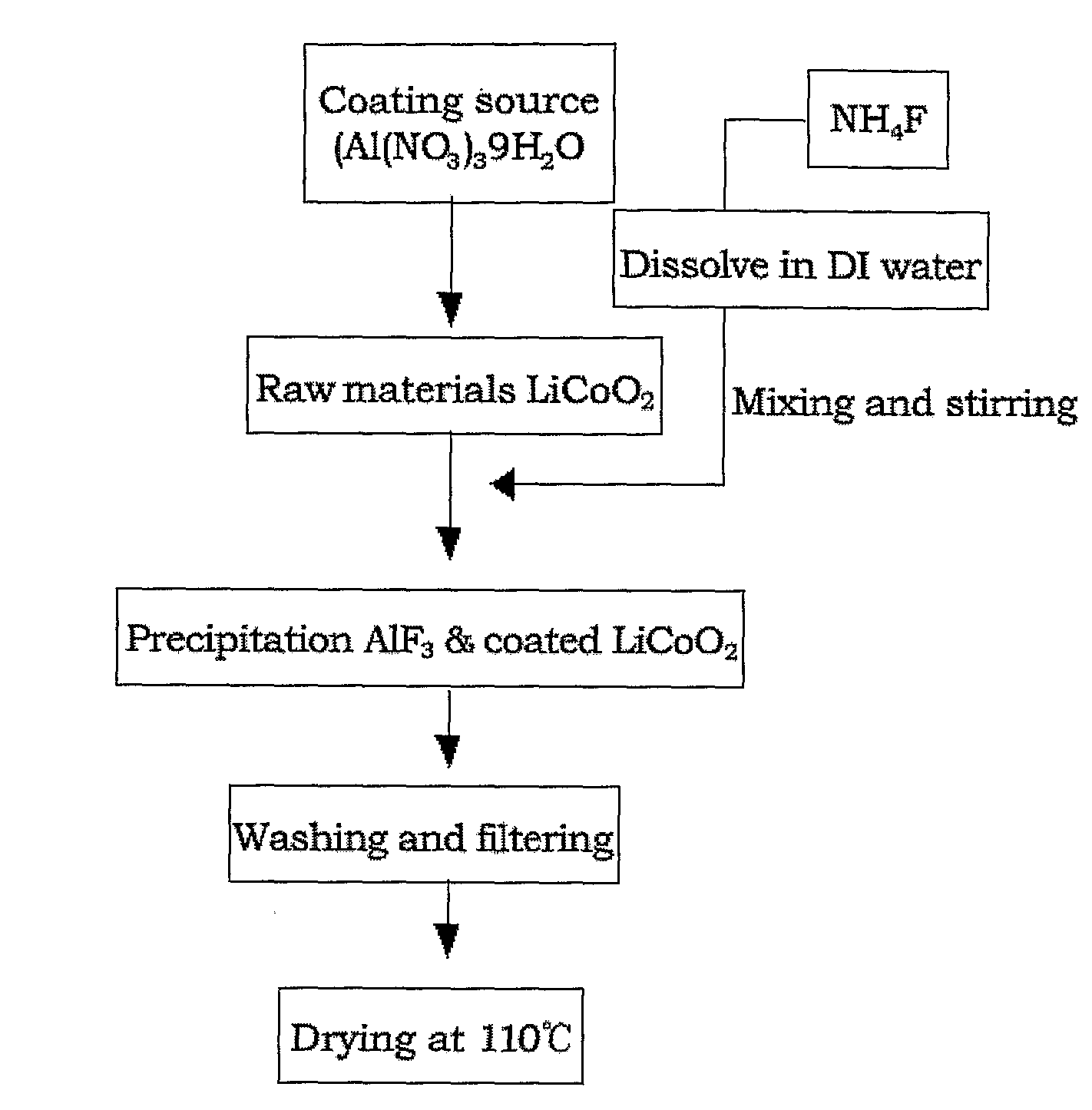
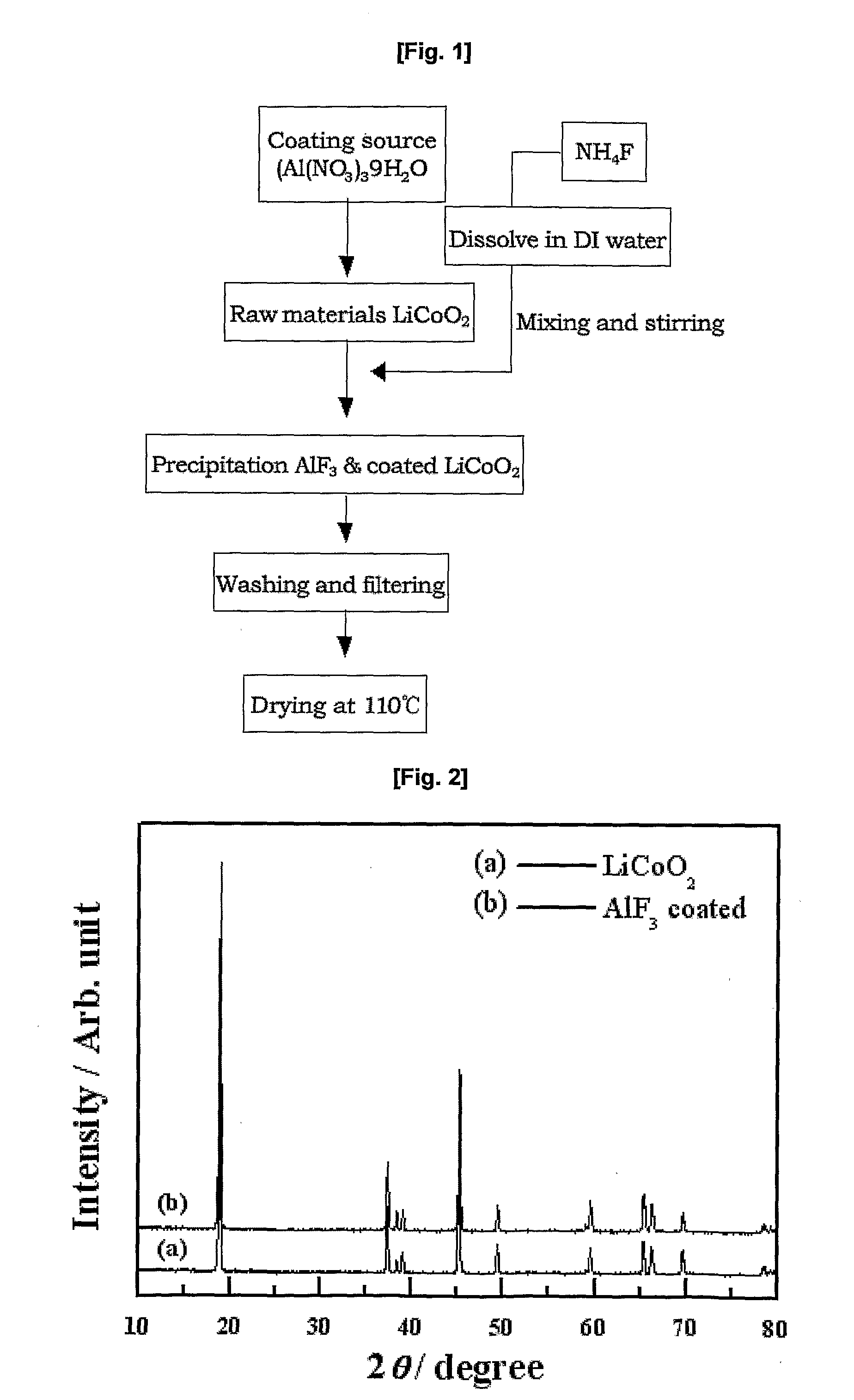
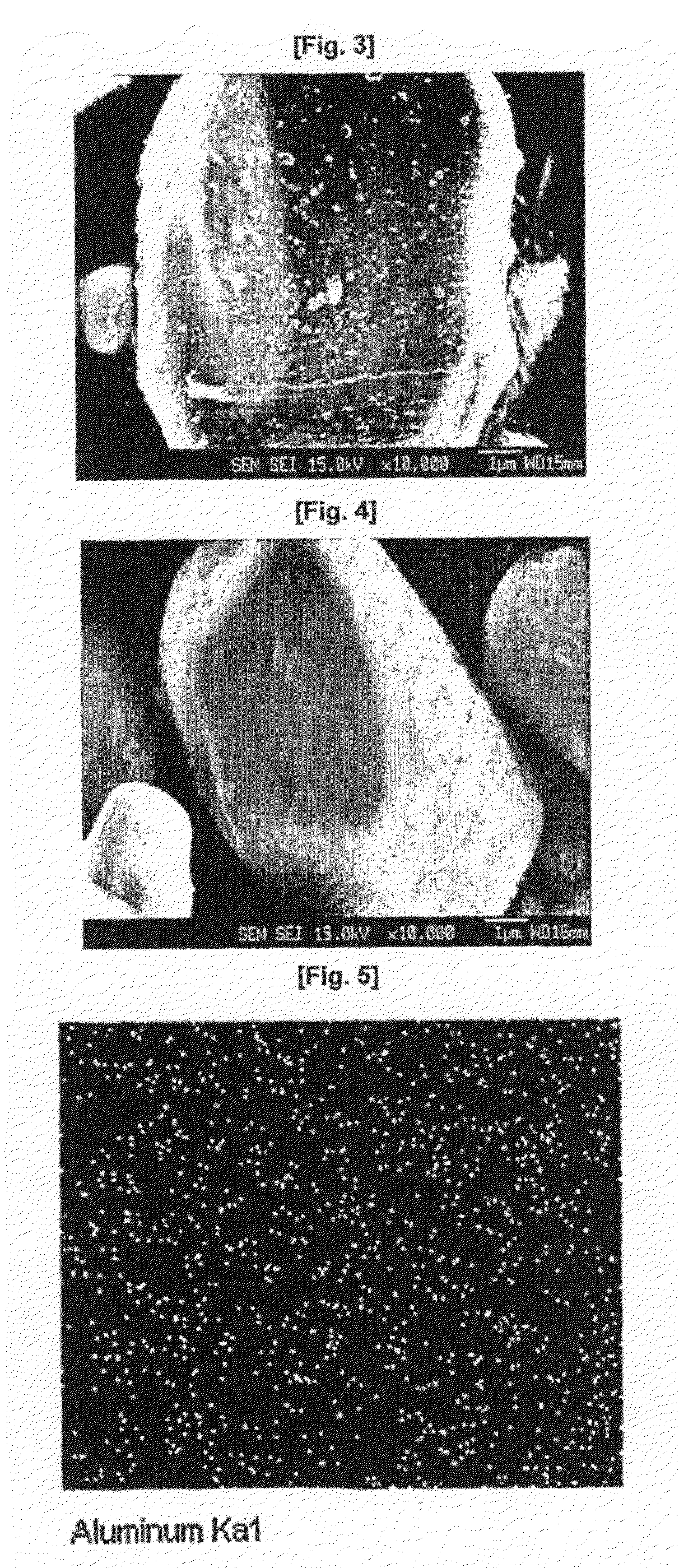
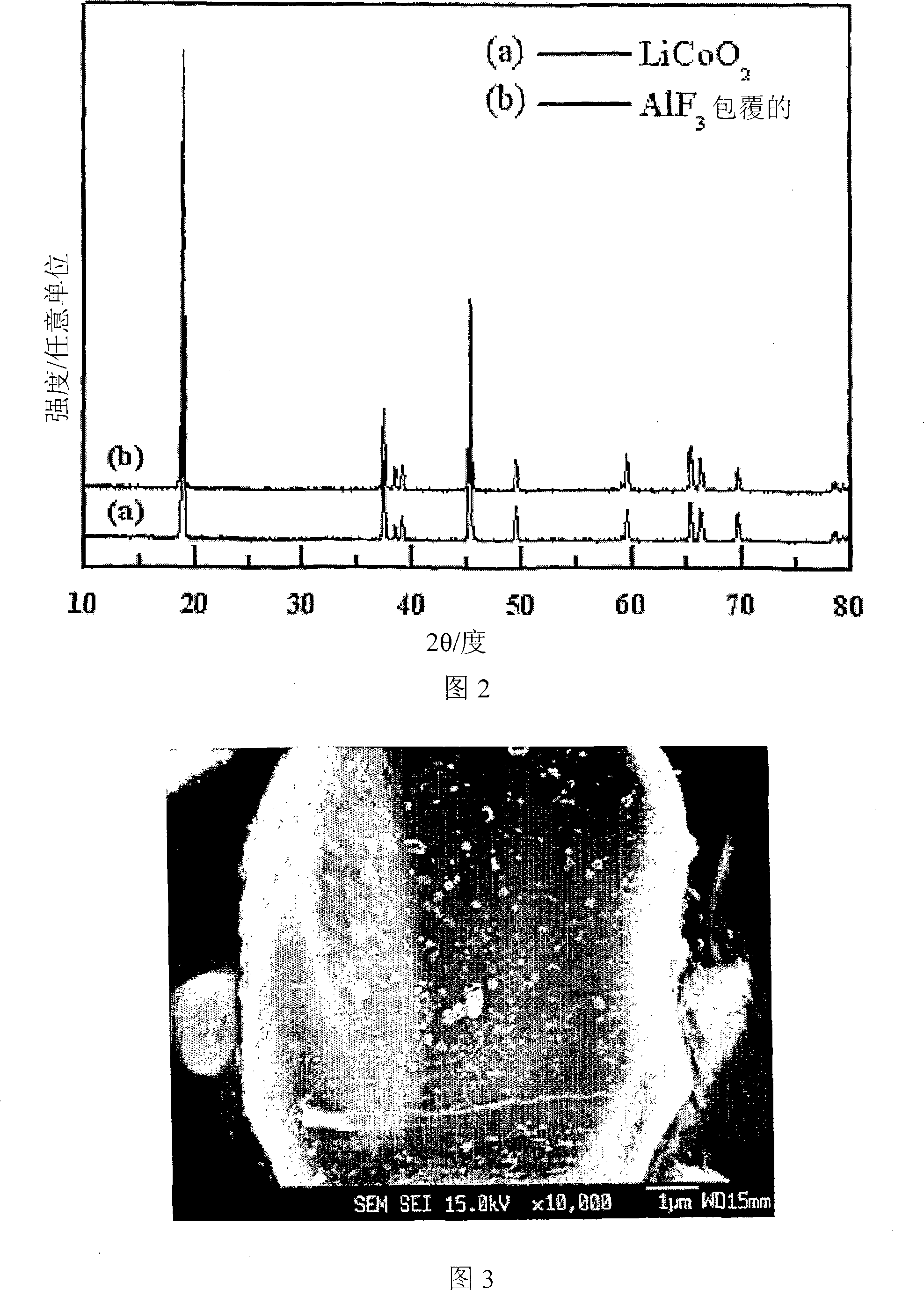
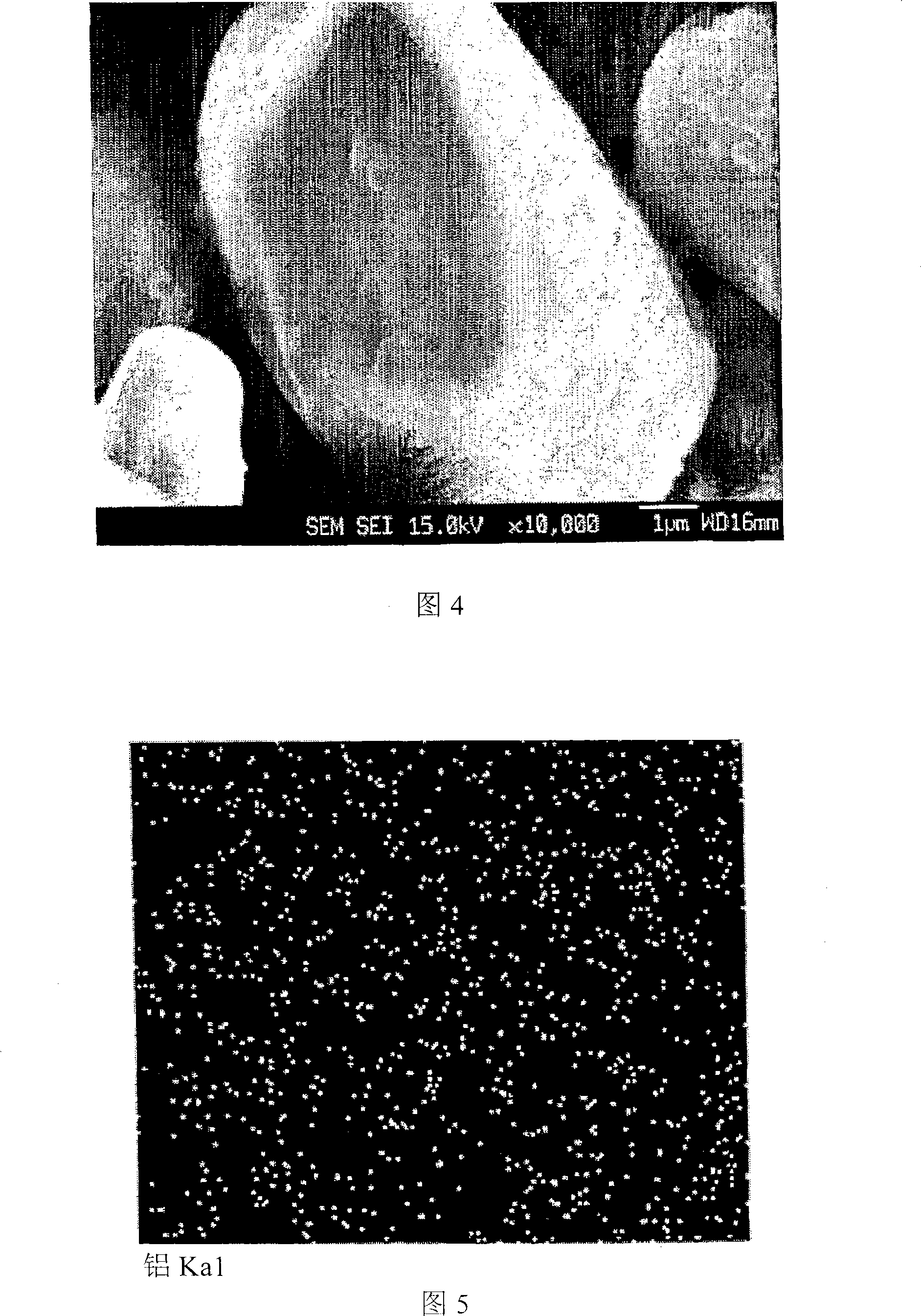
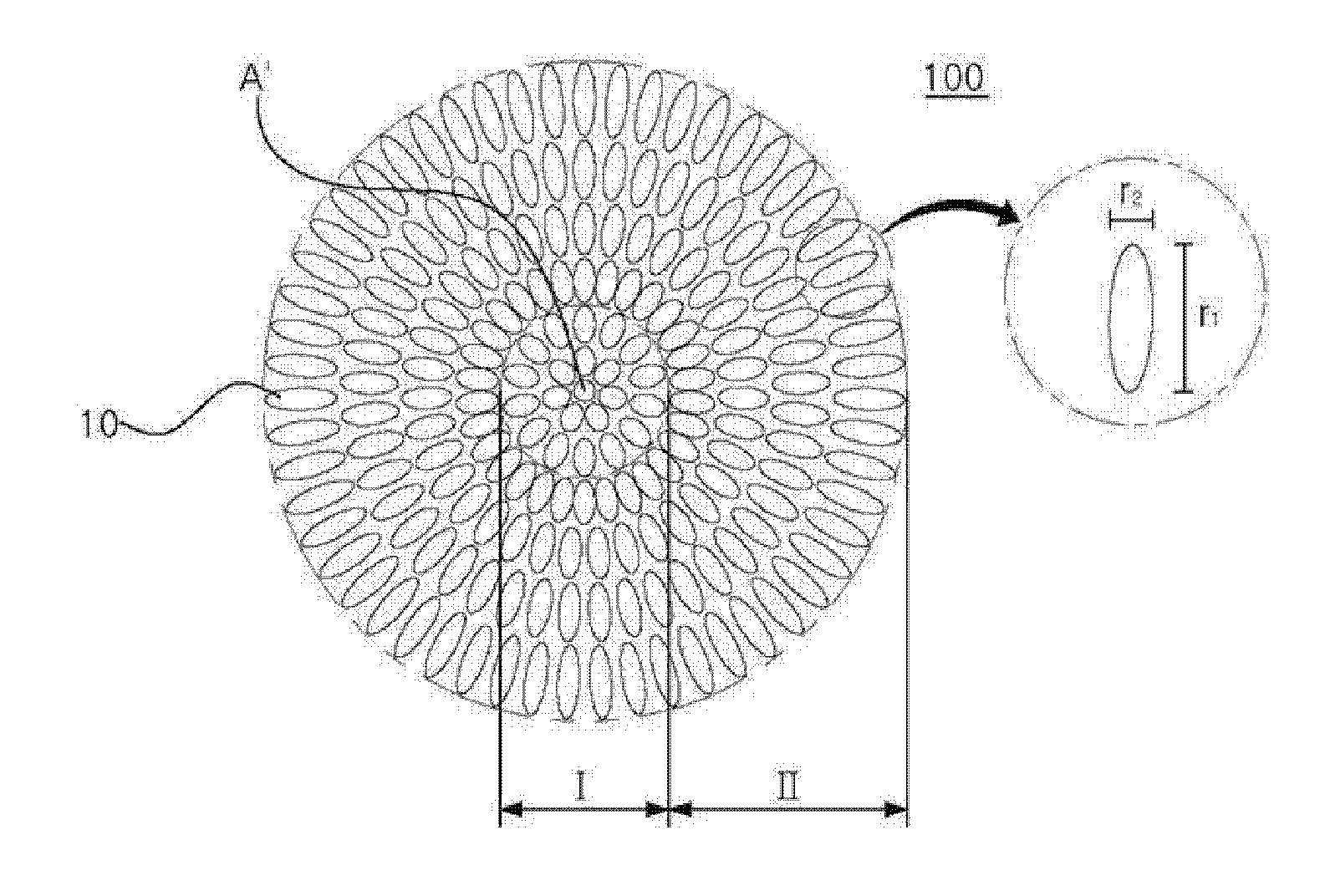
Cathode Active Material Coated With Fluorine Compound for Lithium Secondary Batteries and Method for Preparing the Same
InactiveUS20090087362A1Inhibition of performance deteriorationHigh voltageElectrode manufacturing processesLithium compoundsLithiumHigh rate
Disclosed herein is a cathode active material coated with a fluorine compound for lithium secondary batteries. The cathode active material is structurally stable, and improves the charge-discharge characteristics, cycle characteristics, high-voltage characteristics, high-rate characteristics and thermal stability of batteries.
Owner:ENERCERAMIC
Nonaqueous electrolyte
ActiveUS20050221188A1Excellent characteristicsNon-aqueous electrolyte accumulatorsElectrode carriers/collectorsLithiumMaterials science
A nonaqueous electrolyte battery includes a positive electrode containing an active material, a negative electrode, and a nonaqueous electrolyte, the negative electrode including a current collector and a negative electrode active material supported by the current collector, the negative electrode active material having a Li insertion potential not lower than 0.2V (vs. Li / Li+) and an average primary particle diameter not larger than 1 μm, and a specific surface area of the negative electrode, excluding a weight of the current collector, as determined by the BET method falls within a range of 3 to 50 m2 / g.
Owner:KK TOSHIBA
Cathode active material coated with fluorine compound for lithium secondary batteries and method for preparing the same
Disclosed herein is a cathode active material coated with a fluorine compound for lithium secondary batteries. The cathode active material is structurally stable, and improves the charge-discharge characteristics, cycle characteristics, high-voltage characteristics, high-rate characteristics and thermal stability of batteries.
Owner:ENERCERAMIC
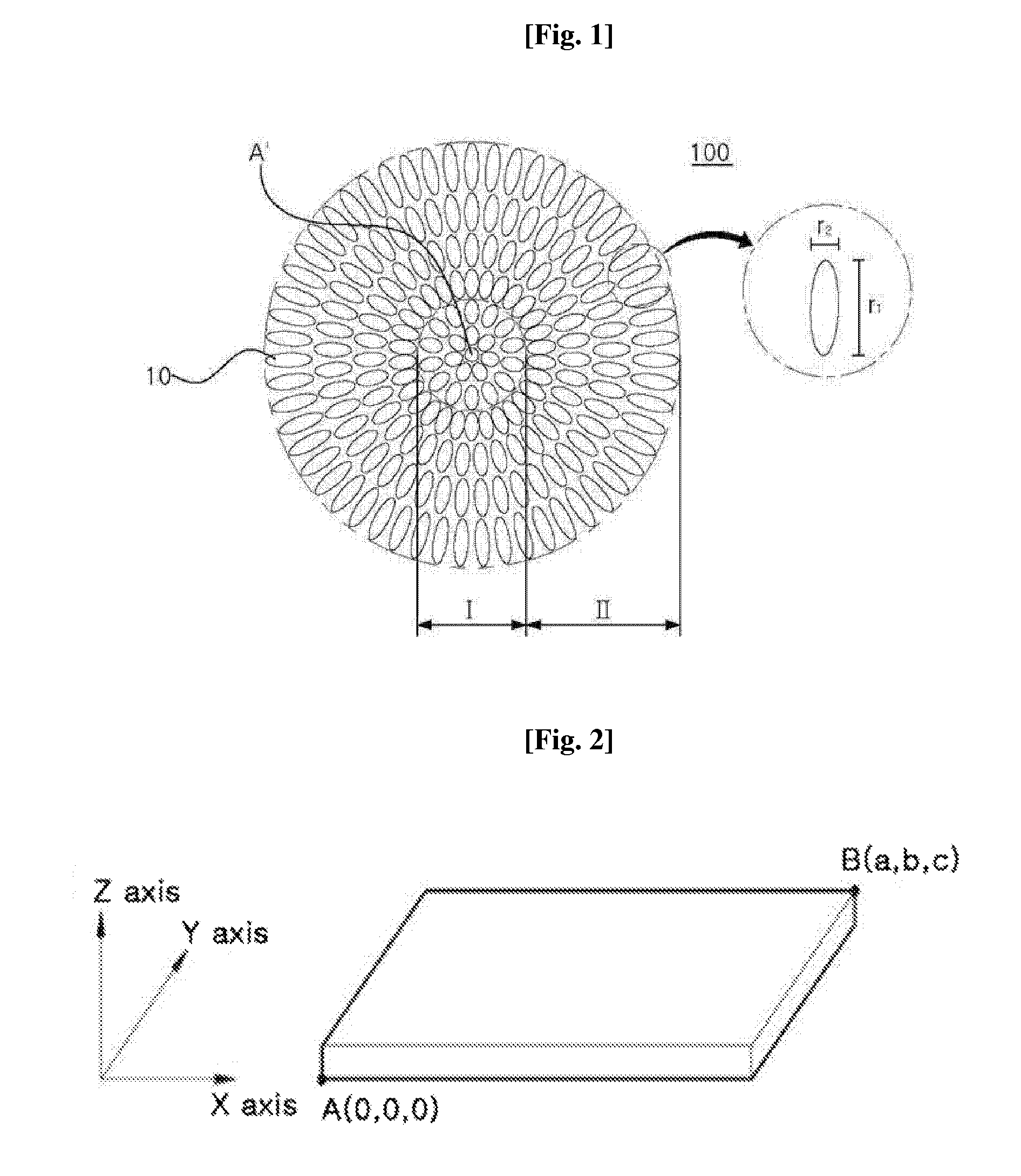
Positive electrode active material precursor for lithium secondary battery, positive electrode active material manufactured by using thereof, and lithium secondary battery including same
ActiveUS20140158932A1Large capacityEasy to insertFinal product manufactureAlkali metal oxidesLithiumEngineering
The present disclosure relates to a positive electrode active material precursor for a lithium secondary battery, a positive electrode active material manufactured by using thereof, and a lithium secondary battery comprising the same. More specifically, it relates to a positive electrode active material precursor for a lithium secondary battery as a secondary particle comprising transition metals, and formed by gathering of a plurality of primary particles having different a-axis direction length to c-axis direction length ratio, wherein the a-axis direction length to c-axis direction length ratio of the primary particle making up the secondary particle is increased from the center to the surface of the secondary particle; a positive electrode active material; and a lithium secondary battery comprising the same.
Owner:LG CHEM LTD
High Voltage and High Specific Capacity Dual Intercalating Electrode Li-Ion Batteries
ActiveUS20060269834A1High voltageHigh energyElectrode carriers/collectorsOrganic electrolyte cellsPresent methodElectrode pair
The present invention provides high capacity and high voltage Li-ion batteries that have a carbonaceous cathode and a nonaqueous electrolyte solution comprising LiF salt and an anion receptor that binds the fluoride ion. The batteries can comprise dual intercalating electrode Li ion batteries. Methods of the present invention use a cathode and electrode pair, wherein each of the electrodes reversibly intercalate ions provided by a LiF salt to make a high voltage and high specific capacity dual intercalating electrode Li-ion battery. The present methods and systems provide high-capacity batteries particularly useful in powering devices where minimizing battery mass is important.
Owner:CALIFORNIA INST OF TECH
Energy storage device
ActiveUS20100203362A1Reduce heatImprove conductivityLead-acid accumulatorsCapacitor and primary/secondary cellsTin dioxideConductive materials
An energy storage device comprising at least one negative electrode, wherein each negative electrode is individually selected from (i) an electrode comprising negative battery electrode material; (ii) an electrode comprising capacitor electrode material; (iii) a mixed electrode comprising either—a mixture of battery and capacitor electrode material or—a region of battery electrode material and a region of capacitor electrode material, or—a combination thereof, and wherein the energy storage device either comprises at least one electrode of type (iii), or comprises at least one electrode of each of types (i) and (ii),—at least one positive electrode, wherein the positive electrode comprises positive battery electrode material and a charging ability-increasing additive, such as one or a mixture of: (a) carbon nanomaterial, vapour grown carbon fibre, fullerene, or a mixture thereof, and (b) tin dioxide conductive materials.
Owner:COMMONWEALTH SCI & IND RES ORG +1
Novel composite cathodes, electrochemical cells comprising novel composite cathodes, and processes for fabricating same
The present invention pertains to composite cathodes suitable for use in an electrochemical cell, said cathodes comprising: (a) an electroactive sulfur-containing cathode material, wherein said electroactive sulfur-containing cathode material, in its oxidized state, comprises a polysulfide moiety of the formula —Sm—, wherein m is an integer equal to or greater than 3; and, (b) an electroactive transition metal chalcogenide composition, which encapsulates said electroactive sulfur-containing cathode material, and which retards the transport of anionic reduction products of said electroactive sulfur-containing cathode material, said electroactive transition metal chalcogenide composition comprising an electroactive transition metal chalcogenide having the formula MjYk(OR)l wherein: M is a transition metal; Y is the same or different at each occurrence and is oxygen, sulfur, or selenium; R is an organic group and is the same or different at each occurrence; j is an integer ranging from 1 to 12; k is a number ranging from 0 to 72; and l is a number ranging from 0 to 72; with the proviso that k and l cannot both be 0. The present invention also pertains to methods of making such composite cathodes, cells comprising such composite cathodes, and methods of making such cells.
Owner:SION POWER CORP
Lithium-ion secondary battery
InactiveUS20070026315A1Safer chemistry characteristicLow cathode costPrimary cell to battery groupingFinal product manufactureManganateSpinel
A lithium-ion battery includes a cathode that includes an active cathode material. The active cathode material includes a cathode mixture that includes a lithium cobaltate and a manganate spinel a manganate spinel represented by an empirical formula of Li(1+x1)(Mn1−y1A′y2)2−x2Oz1. The lithium cobaltate and the manganate spinel are in a weight ratio of lithium cobaltate: manganate spinel between about 0.95:0.05 to about 0.55:0.45. A lithium-ion battery pack employs a cathode that includes an active cathode material as described above. A method of forming a lithium-ion battery includes the steps of forming an active cathode material as described above; forming a cathode electrode with the active cathode material; and forming an anode electrode in electrical contact with the cathode via an electrolyte.
Owner:BOSTON POWER INC
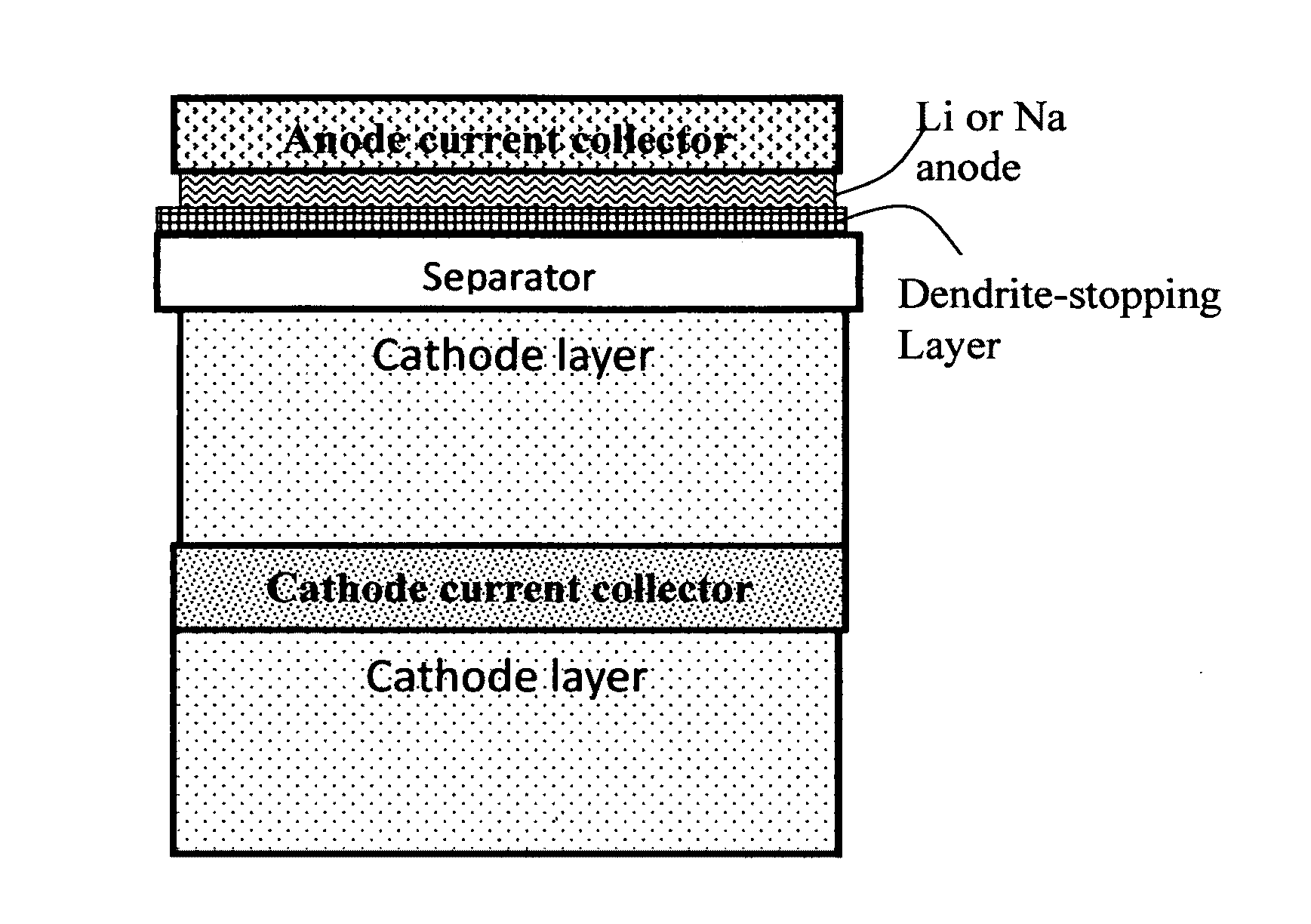
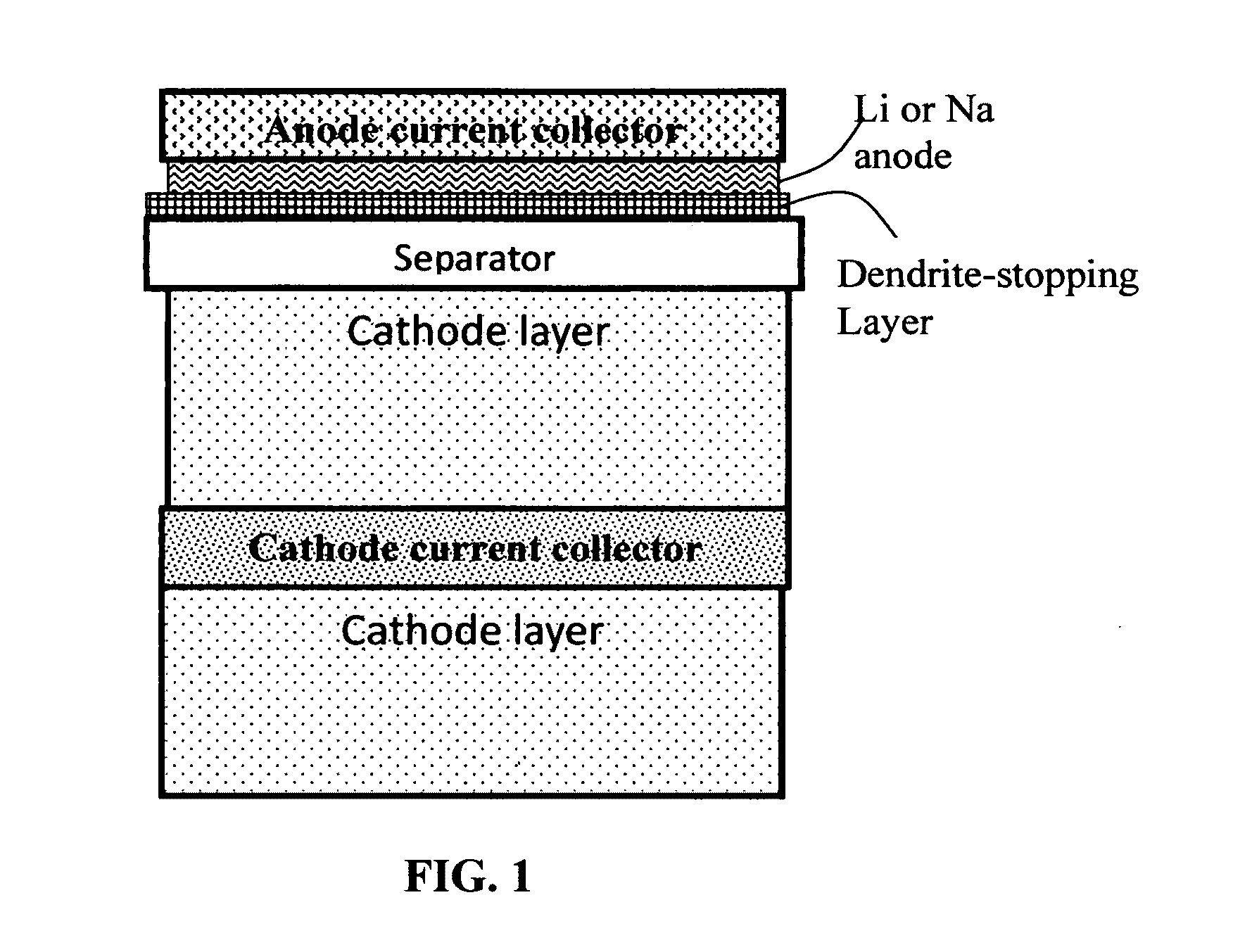
Alkali Metal Secondary Battery Containing a Carbon Matrix- or Carbon Matrix Composite-based Dendrite-Intercepting Layer
ActiveUS20160344035A1Increase energy densityReduce material costsFuel and secondary cellsPositive electrodesDendriteElectrolyte
A rechargeable alkali metal battery comprising: (a) an anode comprising an alkali metal layer and a dendrite penetration-resistant layer comprising an amorphous carbon or polymeric carbon matrix, an optional carbon or graphite reinforcement phase dispersed in this matrix, and a lithium- or sodium-containing species that are chemically bonded to the matrix and / or the optional carbon or graphite reinforcement to form an integral layer that prevents dendrite penetration, wherein the lithium- or sodium-containing species is selected from Li2CO3, Li2O, Li2C2O4, LiOH, LiX, ROCO2Li, HCOLi, ROLi, (ROCO2Li)2, (CH2OCO2Li)2, Li2S, LixSOy, Na2CO3, Na2O, Na2C2O4, NaOH, NaiX, ROCO2Na, HCONa, RONa, (ROCO2Na)2, (CH2OCO2Na)2, Na2S, NaxSOy, or a combination thereof, wherein X═F, Cl, I, or Br, R=a hydrocarbon group, x=0-1, y=1-4; (b) a cathode; and (c) a separator and electrolyte component; wherein the dendrite penetration-resistant layer is disposed between the alkali metal layer and the separator.
Owner:GLOBAL GRAPHENE GRP INC
Methods and reagents for enhancing the cycling efficiency of lithium polymer batteries
InactiveUS6165644AImprove efficiencyElectrode rolling/calenderingElectrochemical processing of electrodesLithium metalSulfur electrode
Batteries including a lithium electrode and a sulfur counter electrode that demonstrate improved cycling efficiencies are described. In one embodiment, an electrochemical cell having a lithium electrode and a sulfur electrode including at least one of elemental sulfur, lithium sulfide, and a lithium polysulfide is provided. The lithium electrode includes a surface coating that is effective to increase the cycling efficiency of said electrochemical cell. In a more particular embodiment, the lithium electrode is in an electrolyte solution, and, more particularly, an electrolyte solution including either elemental sulfur, a sulfide, or a polysulfide. In another embodiment, the coating is formed after the lithium electrode is contacted with the electrolyte. In a more particular embodiment, the coating is formed by a reaction between the lithium metal of the lithium electrode and a chemical species present in the electrolyte.
Owner:POLYPLUS BATTERY CO INC
Positive-electrode active material and nonaqueous-electrolyte secondary battery containing the same
InactiveUS20030170540A1Improve the immunityLarge ion permeabilityIron oxides/hydroxidesElectrode thermal treatmentCrystal structureOxygen
The present invention provides a high-capacity and low-cost non-aqueous electrolyte secondary battery, comprising: a negative electrode containing, as a negative electrode active material, a ssubstance capable of absorbing / desorbing lithium ions and / or metal lithium; a separator; a positive electrode; and an electrolyte, wherein the positive electrode active material contained in the positive electrode is composed of crystalline particles of an oxide containing two kinds of transition metal elements, the crystalline particles having a layered crystal structure, and oxygen atoms constituting the oxide forming a cubic closest packing structure.
Owner:PANASONIC CORP +1
Non-aqueous electrolytes for lithium electrochemical cells
InactiveUS6852446B2Improve conductivityImprove stabilityAlkaline accumulatorsOrganic electrolyte cellsHydrogen atomHydrogen
A non-aqueous electric current producing electrochemical cell is provided comprising an anode and a cathode, an ionically permeable separator interposed between the anode and the cathode, and a non-aqueous electrolyte, the electrolyte comprising an ionically conducting salt in a non-aqueous medium, the ionically conducting salt corresponding to the formula:M+(Z*(J*)j(X*)x)−, wherein:M is a lithium atom,Z* is an anion group containing two or more Lewis basic sites and comprising less than 50 atoms not including hydrogen atoms,J* independently each occurance is a Lewis acid coordinated to at least one Lewis basic site of Z*, and optionally two or more such J* groups may be joined together in a moiety having multiple Lewis acidic functionality,X* independently each occurrence is selected from the group consisting of H, C1-C4 alkyl, alkoxide, halide and mixtures thereof,j is an integer from 2 to 12, andx is an integer from 0 to 4.
Owner:EAGLE PICHER TECH LLC
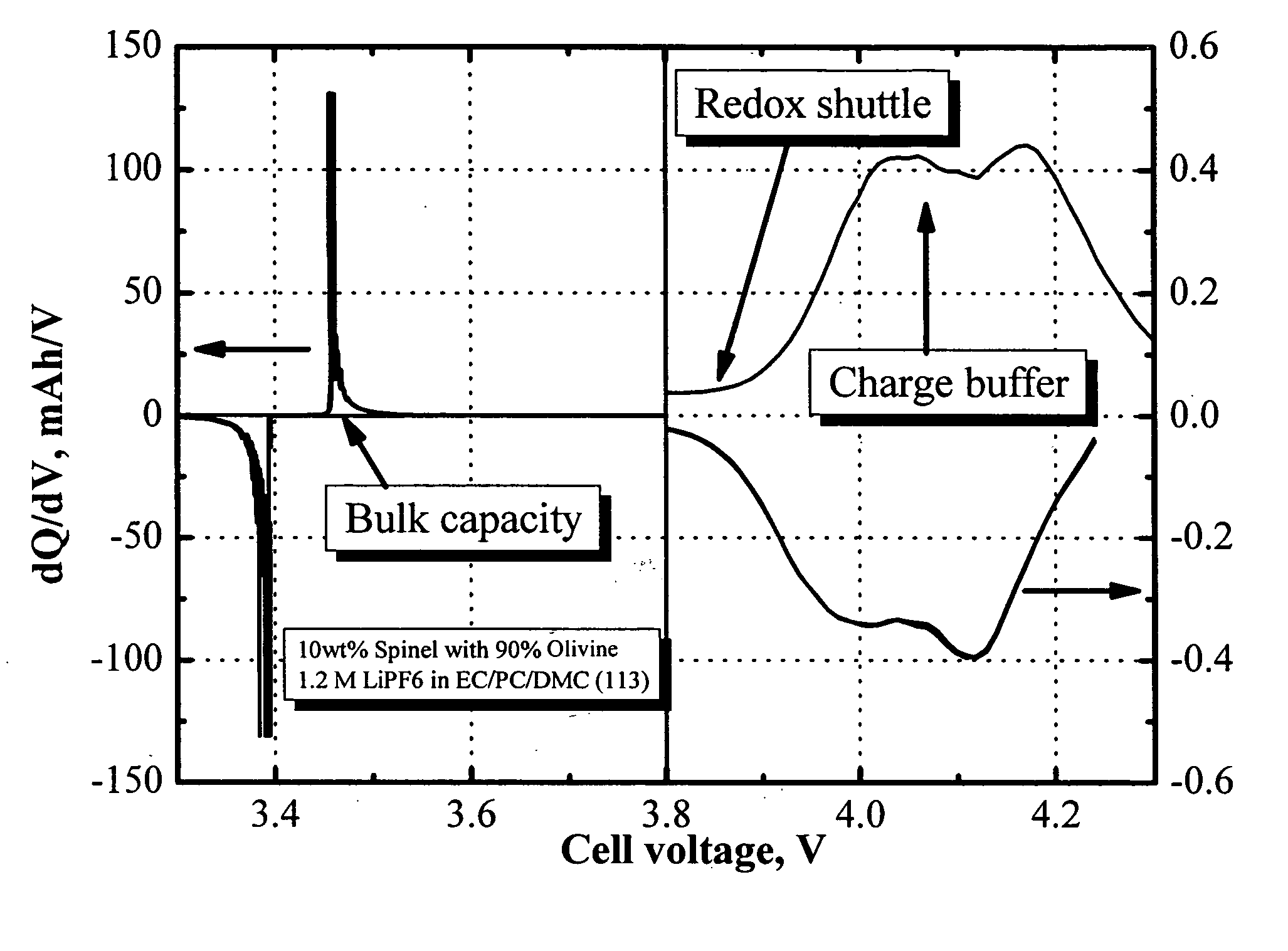
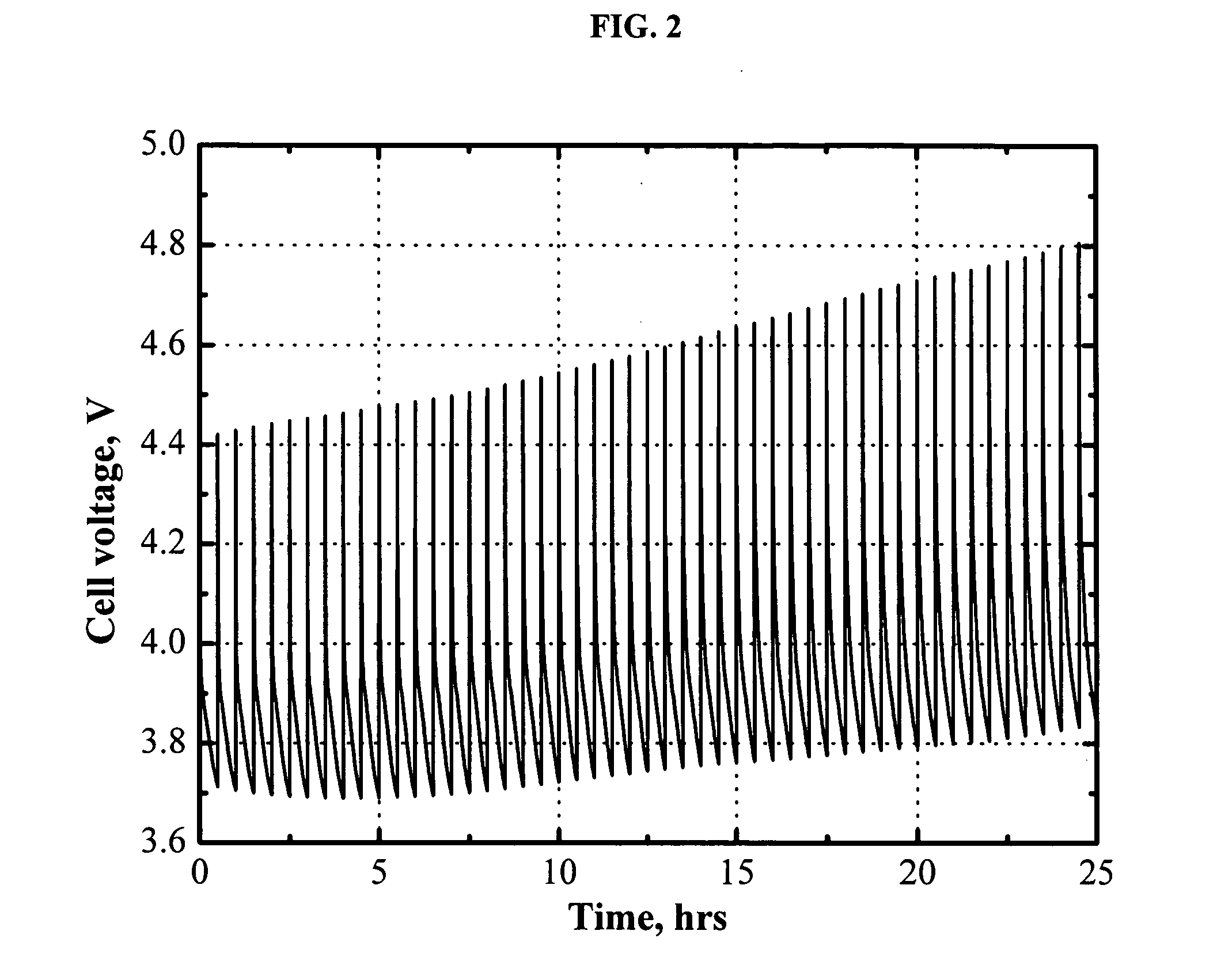
Redox shuttle for rechargeable lithium-ion cell
InactiveUS20050221196A1Excellent repeated overcharge stabilityNon-aqueous electrolyte accumulatorsOrganic electrolyte cellsLithiumAlkoxy group
A redox chemical shuttle comprising an aromatic compound substituted with at least one tertiary carbon organic group and at least one alkoxy group (for example, 2,5-di-tert-butyl-1,4-dimethoxybenzene) provides repeated overcharge protection in rechargeable lithium-ion cells.
Owner:3M INNOVATIVE PROPERTIES CO
Lithium-ion batteries with intrinsic pulse overcharge protection
The present invention relates in general to the field of lithium rechargeable batteries, and more particularly relates to the positive electrode design of lithium-ion batteries with improved high-rate pulse overcharge protection. Thus the present invention provides electrochemical devices containing a cathode comprising at least one primary positive material and at least one secondary positive material; an anode; and a non-aqueous electrolyte comprising a redox shuttle additive; wherein the redox potential of the redox shuttle additive is greater than the redox potential of the primary positive material; the redox potential of the redox shuttle additive is lower than the redox potential of the secondary positive material; and the redox shuttle additive is stable at least up to the redox potential of the secondary positive material.
Owner:UCHICAGO ARGONNE LLC
Nonaqueous electrolyte battery, battery pack and positive electrode active material
InactiveUS20060134520A1Improve storage characteristicsOrganic electrolyte cellsPositive electrodesLithium oxideLithium hydroxide
A nonaqueous electrolyte battery includes a case, a positive electrode housed in the case and including a positive electrode active material containing a lithium-nickel composite oxide and at least one of lithium hydroxide and lithium oxide, the sum of lithium hydroxide and lithium oxide falling within not less than 0.1% to not more than 0.5% by weight based on the total amount of the positive electrode active material, a negative electrode housed in the case and capable of lithium intercalation-deintercalation, and a separator sandwiched between the positive electrode and the negative electrode and impregnated with a nonaqueous electrolyte containing γ-butyrolactone.
Owner:KK TOSHIBA
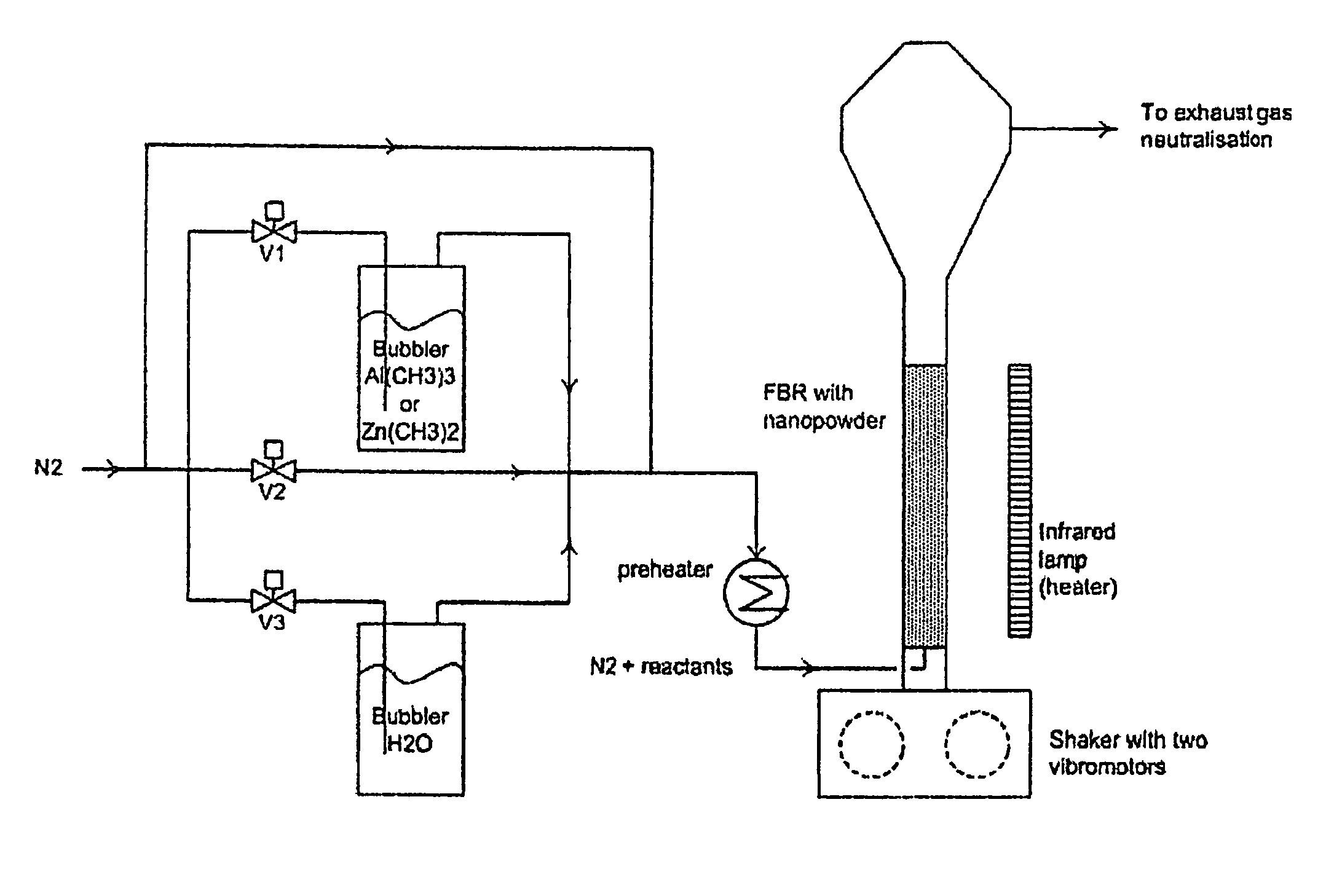
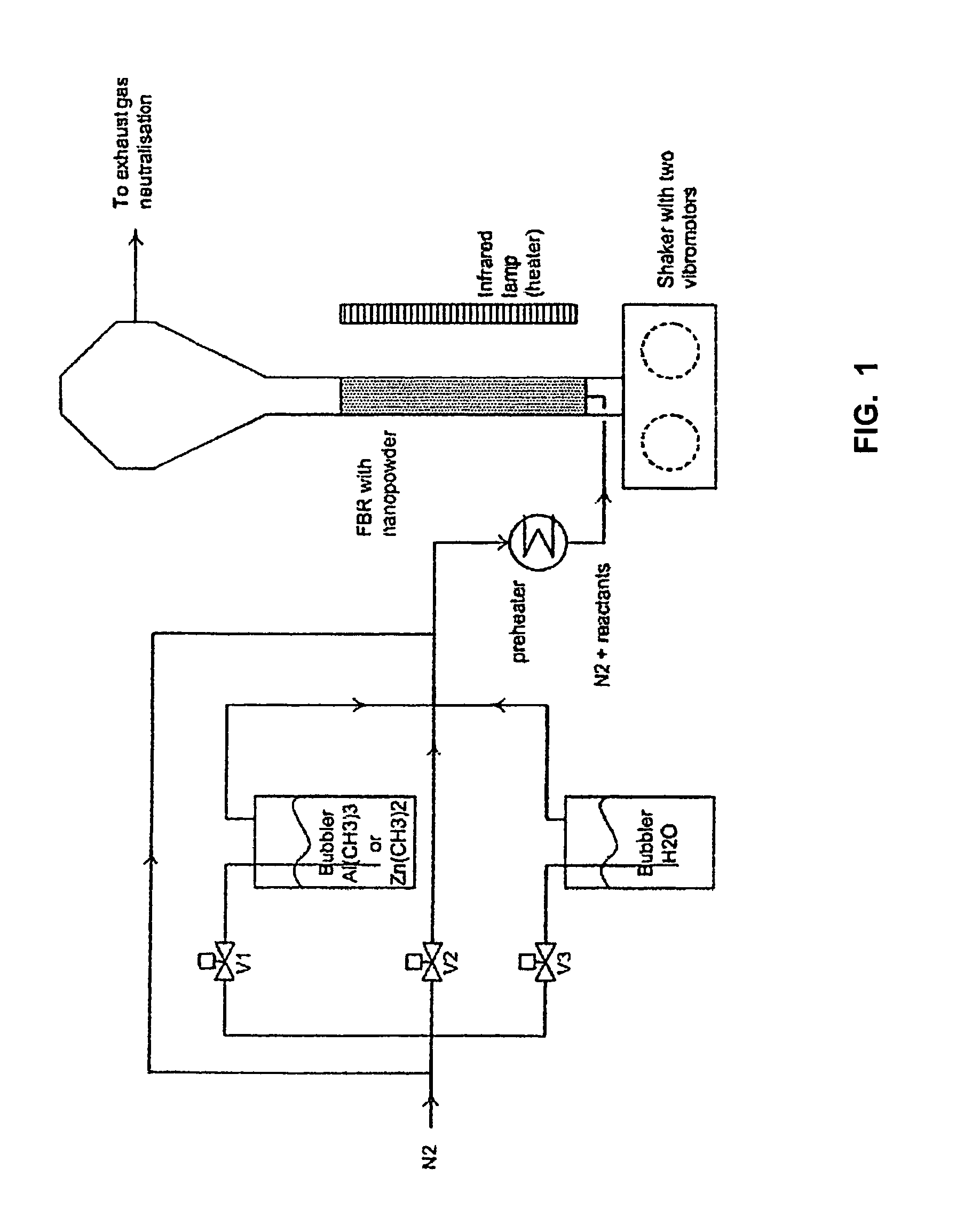
Method for covering particles, especially a battery electrode material particles, and particles obtained with such method and a battery comprising such particle
A method for covering particles having a diameter of maximally 60 μm by means of atomic layer deposition, whereby said method comprises the step of fluidizing said particles in a fluidized bed reactor using a first reactant gas comprising a first reactant for substantially completely covering said particles with a monolayer of said first reactant.
Owner:PNEUMATICOAT TECH LLC
Battery cathode
InactiveUS6858349B1Improve conductivityOvercome lack of conductivityPositive electrodesPrimary cell electrodesFiberCarbon fibers
A primary alkaline battery includes a cathode having a cathode active material and carbon fibers, an anode, a separator and an alkaline electrolyte. The carbon fibers have diameters less than about 250 nanometers.
Owner:DURACELL U S OPERATIONS
Sodium ion batteries
InactiveUS6872492B2High operation potentialHigh specific capacityOrganic electrolyte cellsSecondary cellsPhosphateSodium-ion battery
Sodium ion batteries are based on sodium based active materials selected among compounds of the general formula:AaMb(XY4)cZd,wherein A comprises sodium, M comprises one or more metals, comprising at least one metal which is capable of undergoing oxidation to a higher valence state, Z is OH or halogen, and XY4 represents phosphate or a similar group. The anode of the battery includes a carbon material that is capable of inserting sodium ions. The carbon anode cycles reversibly at a specific capacity greater than 100 mAh / g.
Owner:VALENCE TECH INC
Lithium-ion secondary battery
InactiveUS20080008933A1Safer chemistry characteristicLow cathode costPrimary cell to battery groupingElectrode carriers/collectorsManganateManganese
In one embodiment, an active cathode material comprises a mixture that includes: at least one of a lithium cobaltate and a lithium nickelate; and at least one of a manganate spinel represented by an empirical formula of Li(1+x1)(Mn1−y1A′y1)2−x1Oz1 and an olivine compound represented by an empirical formula of Li(1−x2)A″x2MPO4. In another embodiment, an active cathode material comprises a mixture that includes: a lithium nickelate selected from the group consisting of LiCoO2-coated LiNi0.8Co0.15Al0.05O2, and Li(Ni1 / 3Co1 / 3Mn1 / 3)O2; and a manganate spinel represented by an empirical formula of Li(1+x7)Mn2−y7Oz7. A lithium-ion battery and a battery pack each independently employ a cathode that includes an active cathode material as described above. A method of forming a lithium-ion battery includes the steps of forming an active cathode material as described above; forming a cathode electrode with the active cathode material; and forming an anode electrode in electrical contact with the cathode via an electrolyte. A system comprises a portable electronic device and a battery pack or lithium-ion battery as described above.
Owner:BOSTON POWER INC
Positive active material of a lithium-sulfur battery and method of fabricating same
InactiveUS7029796B2Increase capacityImprove cycle lifeMaterial nanotechnologyPositive electrodesLithium–sulfur batteryMaterials science
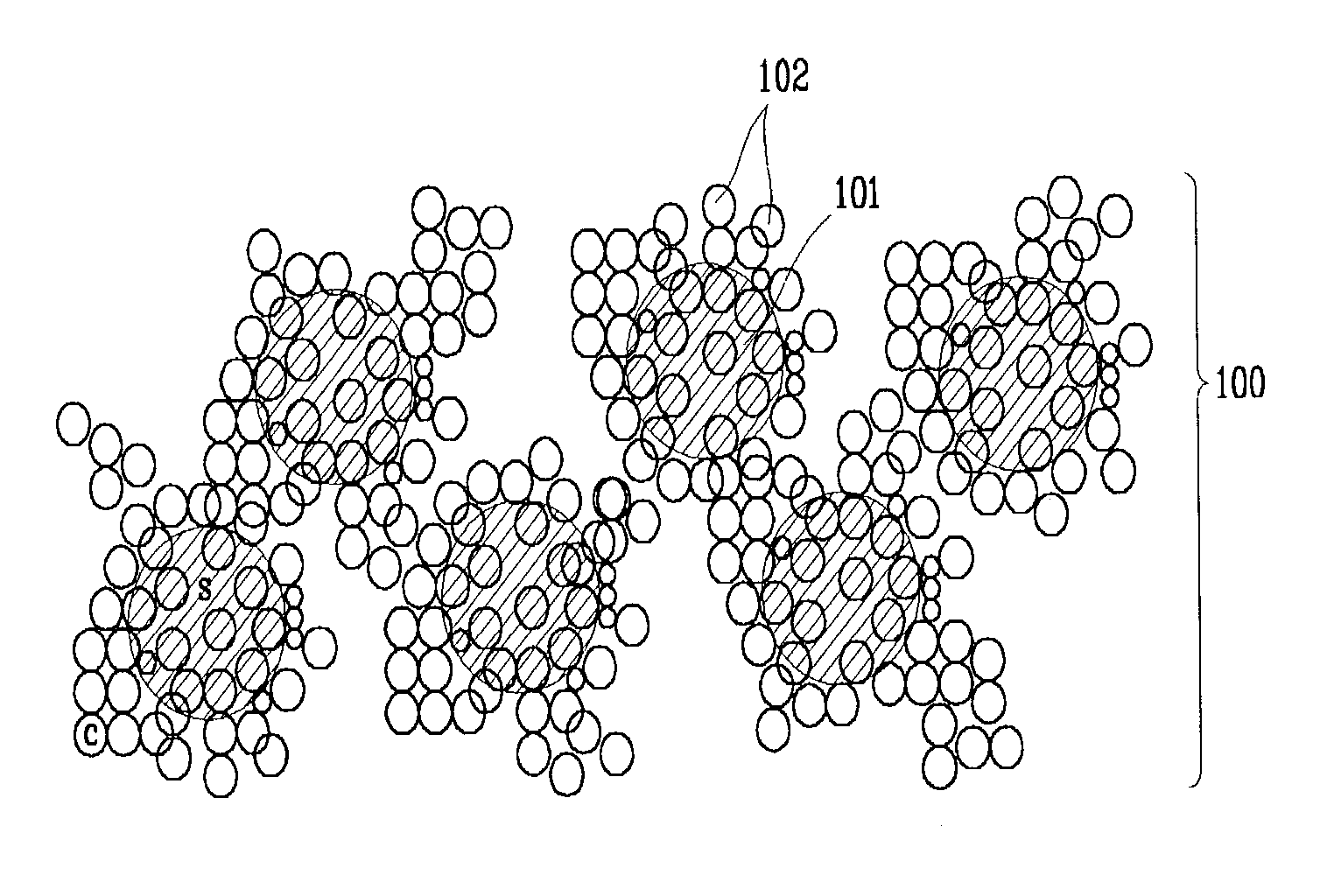
A positive active material of a lithium-sulfur battery includes a sulfur-conductive agent-agglomerated complex in which a conductive agent particle is attached onto a surface of a sulfur particle having an average particle size less than or equal to 7 μm. The sulfur-conductive agent-agglomerated complex is manufactured by mixing a sulfur powder and a conductive agent powder to form a mixture, and milling the mixture.
Owner:SAMSUNG SDI CO LTD
Doped positive electrode active materials and lithium ion secondary battery constructed therefrom
Positive electrode active materials comprising a dopant in an amount of 0.1 to 10 mole percent of Mg, Ca, Sr, Ba, Zn, Cd or a combination thereof are described that have high specific discharge capacity upon cycling at room temperature and at a moderate discharge rate. Some materials of interest have the formula Li1+xNiαMnβ-δCoγAδXμO2−zFz, where x ranges from about 0.01 to about 0.3, δ ranges from about 0.001 to about 0.15, and the sum x+α+β+γ+δ+μ can approximately equal 1.0. The materials can be coated with a metal fluoride to improve the performance of the materials especially upon cycling. The materials generally can have a tap density of at least 1.8 g / mL. Also, the materials can have an average discharge voltage of around 3.6 V.
Owner:IONBLOX INC
Cathode active material, method of preparing the same, and cathode and lithium battery containing the material
ActiveUS20060257745A1Improve high voltage stabilityImprove thermal stabilityWalking sticksElectrode rolling/calenderingComposite cathodeHigh rate
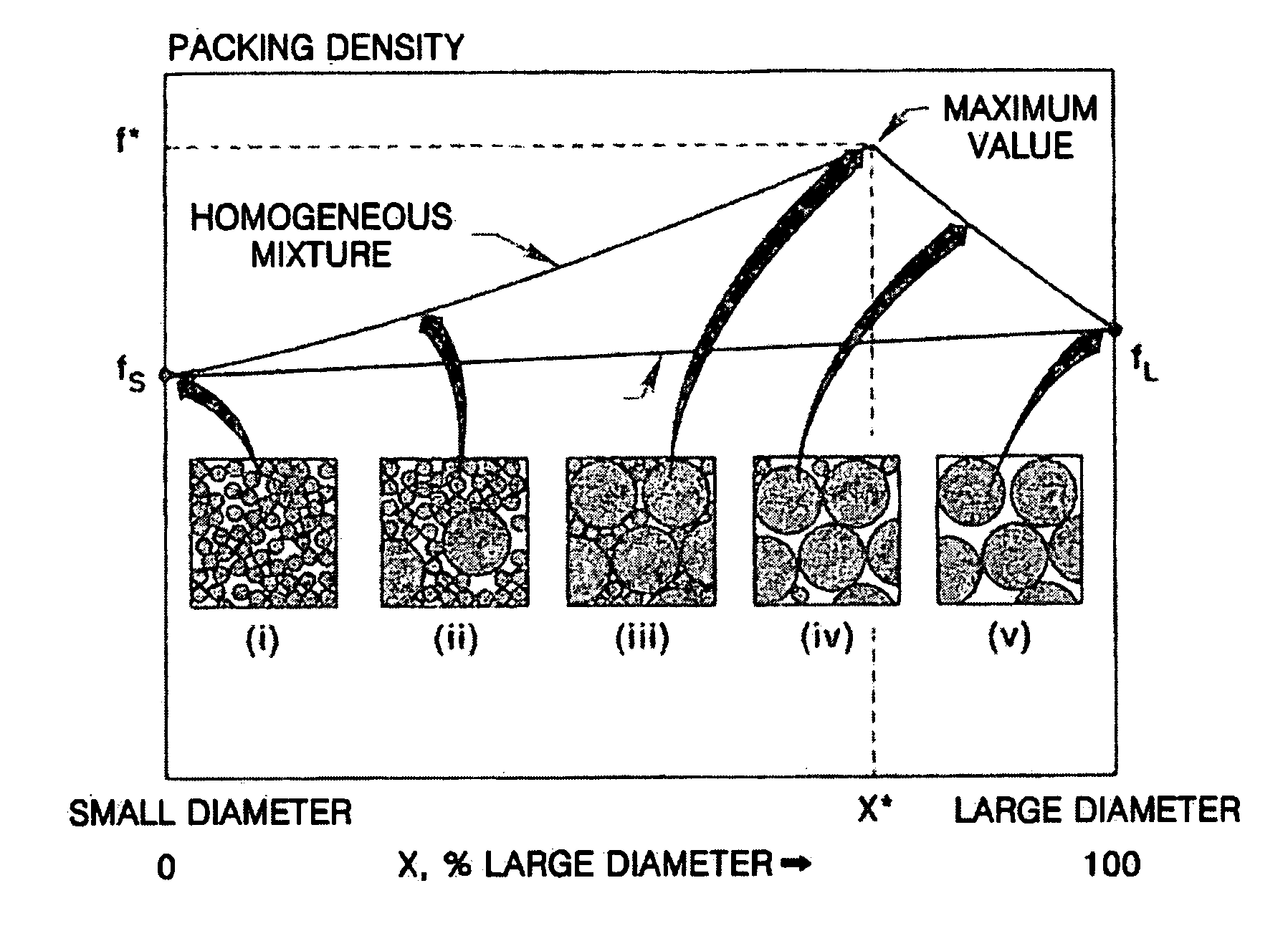
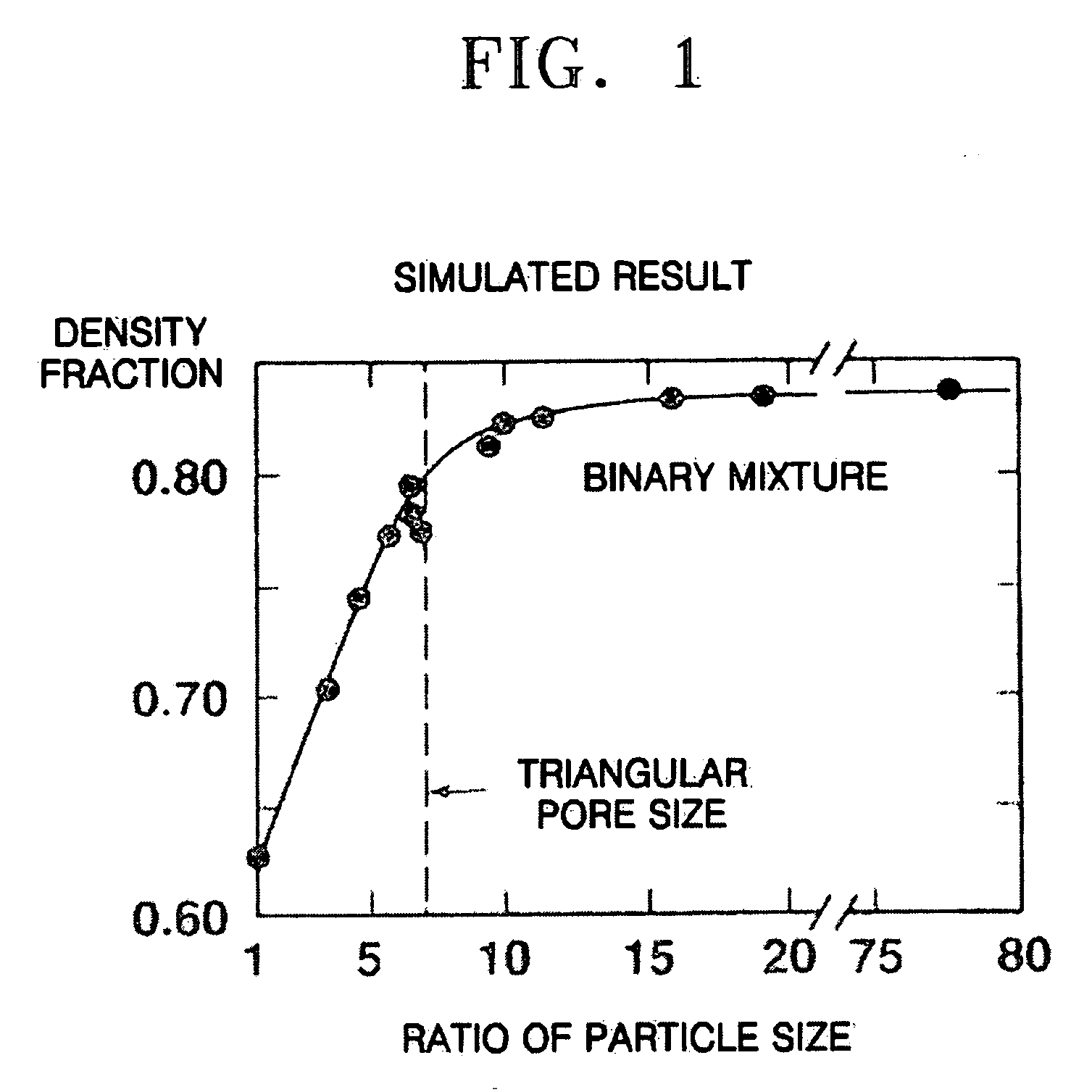
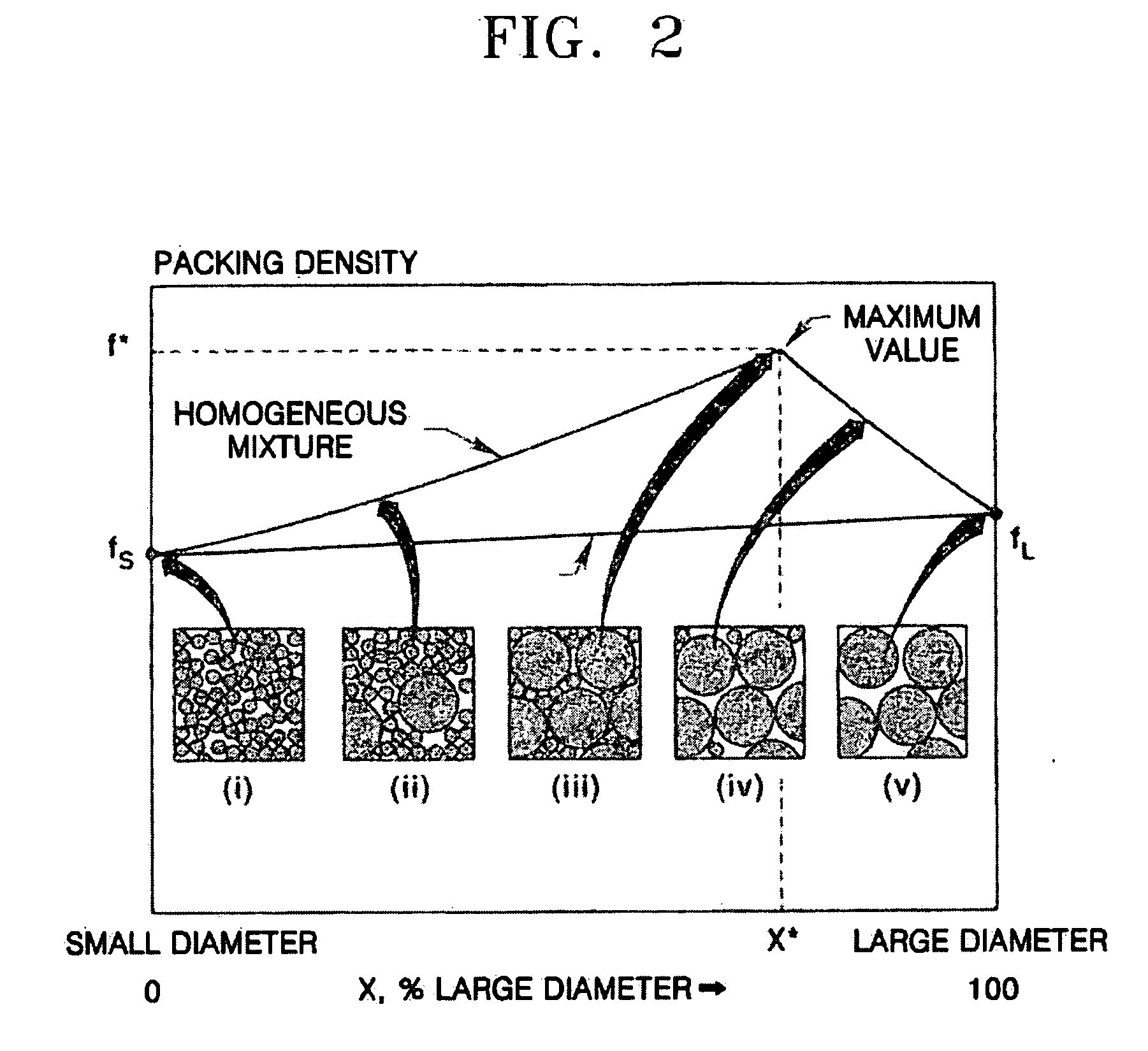
Composite cathode active materials having a large diameter active material and a small diameter active material are provided. The ratio of the average particle diameter of the large diameter active material to the average particle diameter of the small diameter active material ranges from about 6:1 to about 100:1. Mixing the large and small diameter active materials in a proper weight ratio improves packing density Additionally, including highly stable materials and highly conductive materials in the composite cathode active materials improves volume density, discharge capacity and high rate discharge capacity.
Owner:SAMSUNG SDI CO LTD
Cathode electrode, method for manufacturing the same and lithium battery containing the same
InactiveUS20030113624A1Continuous fillingNon-aqueous electrolyte accumulatorsFinal product manufactureSimple Organic CompoundsConductive materials
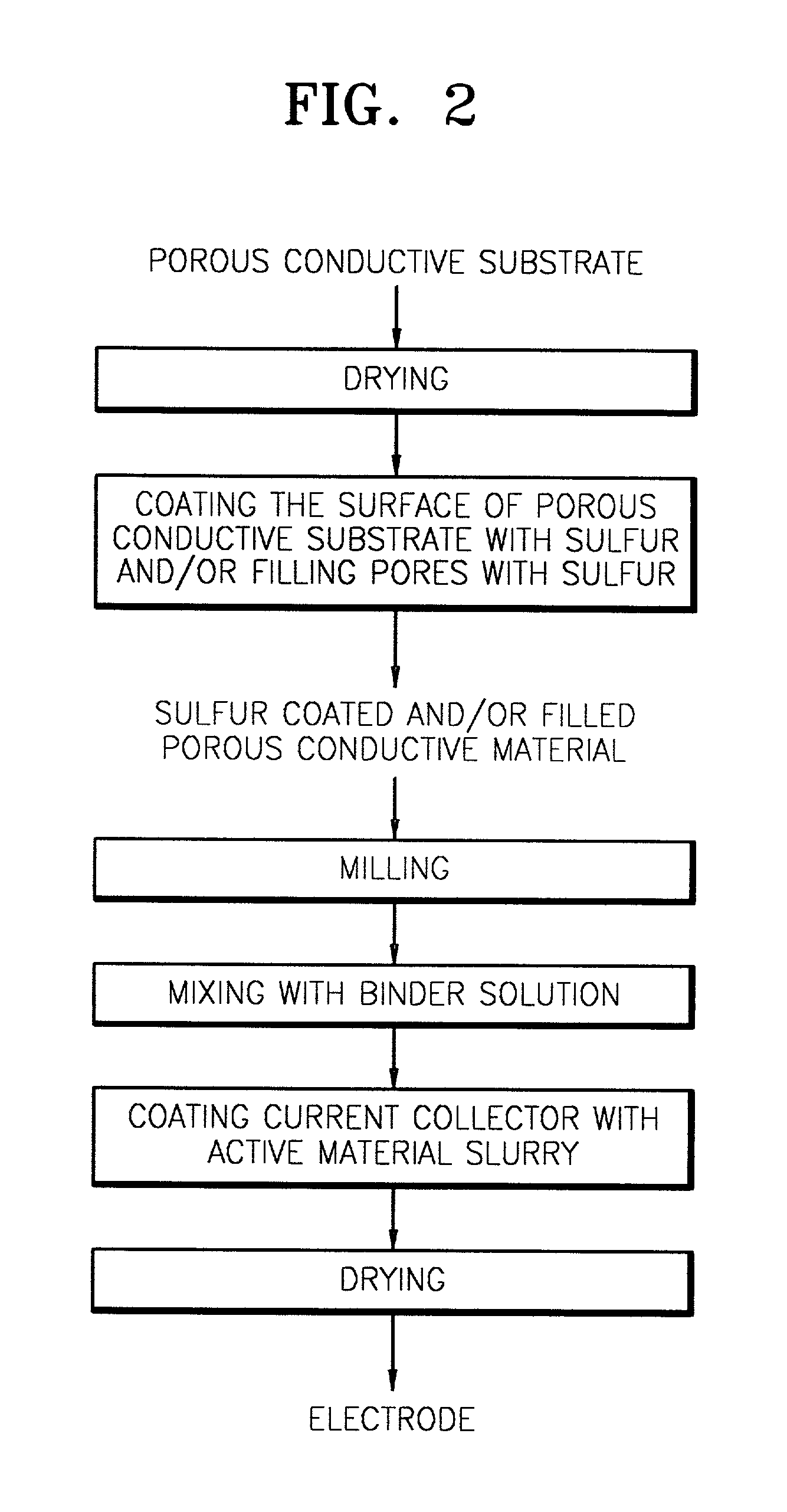
Disclosed is a cathode electrode having a cathode active material layer stacked on a current collector. The cathode active material layer includes a porous conductive material having a surface coated with sulfur and / or a sulfur-containing organic compound and / or pores filled with sulfur and / or a sulfur-containing organic compound. A lithium secondary battery employing the cathode electrode also is disclosed. The cathode electrode is structurally stable during charging and discharging since the structure of the cathode active material layer can be maintained even at the phase transition of sulfur during charging and discharging.
Owner:SAMSUNG SDI CO LTD
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com