Patents
Literature
2916results about "Cobalt compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
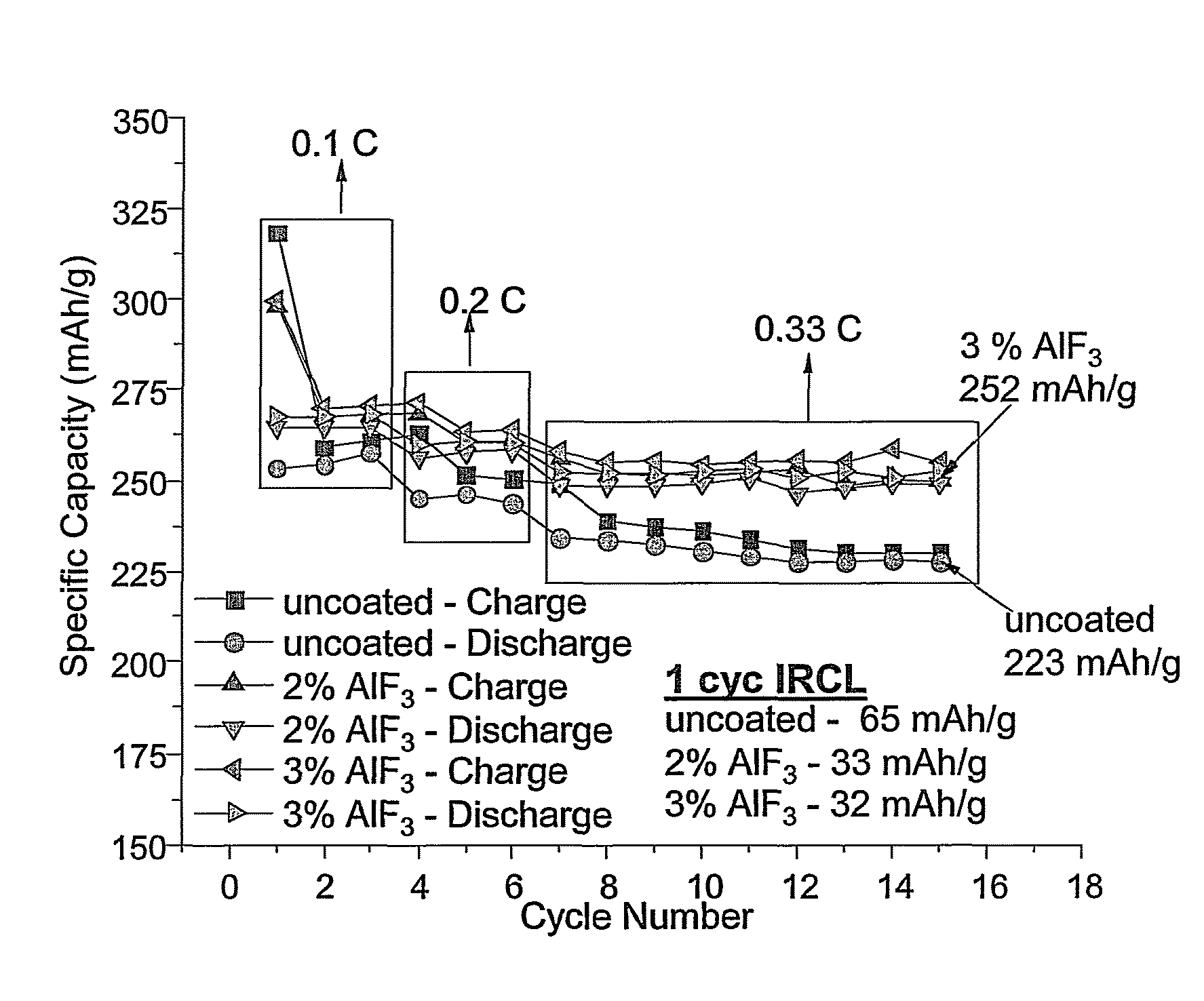
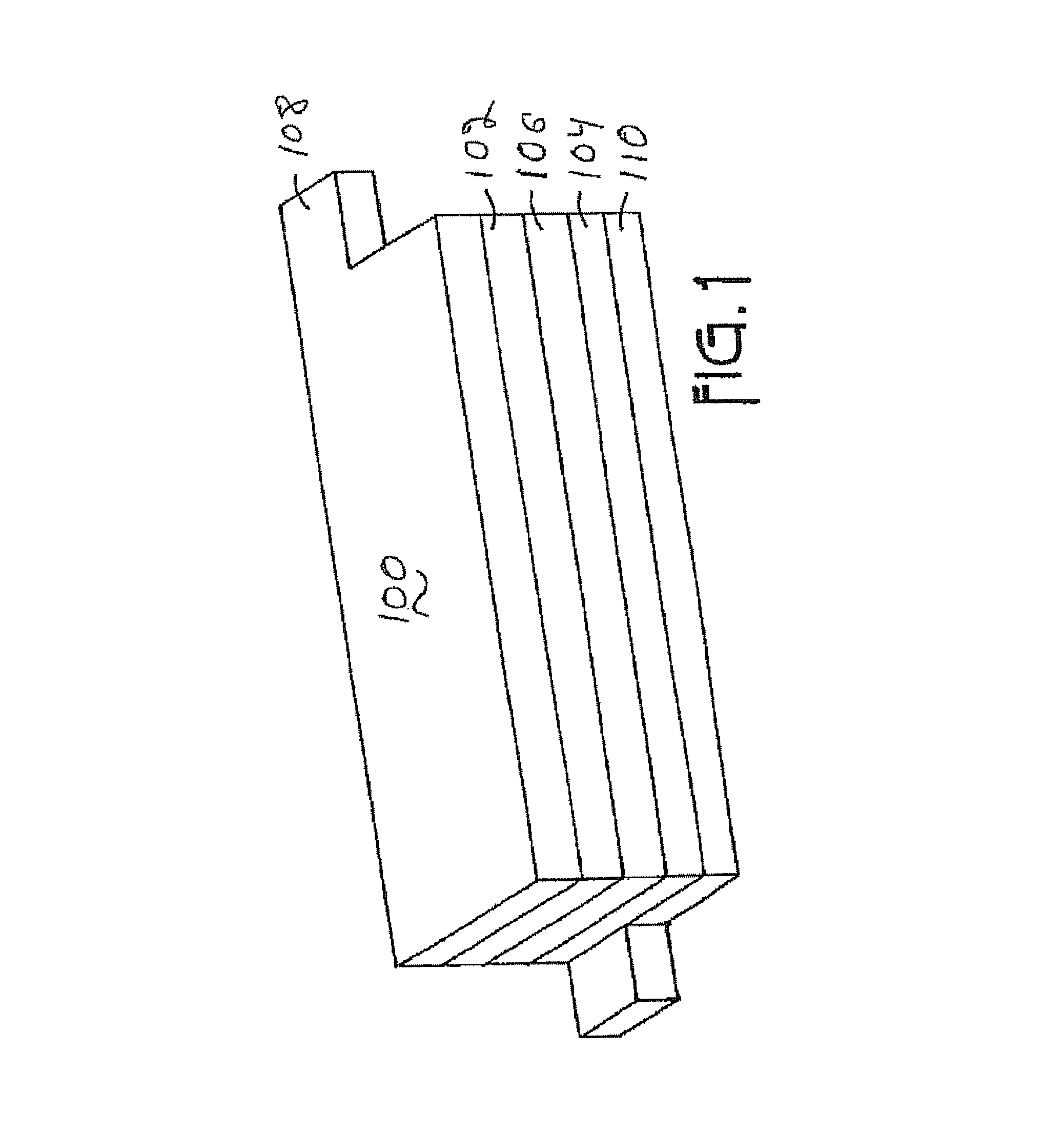
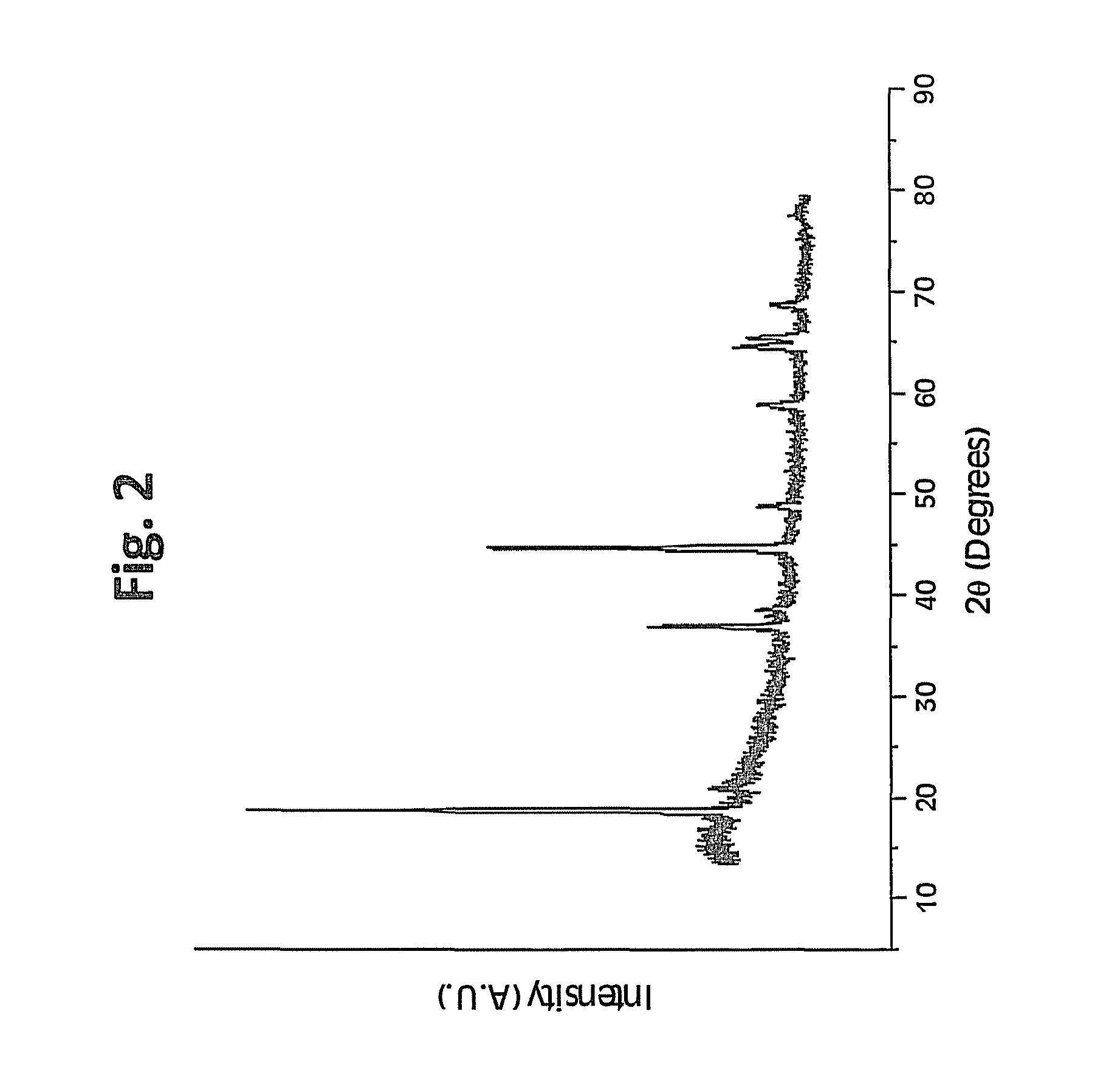
Metal oxide coated positive electrode materials for lithium-based batteries
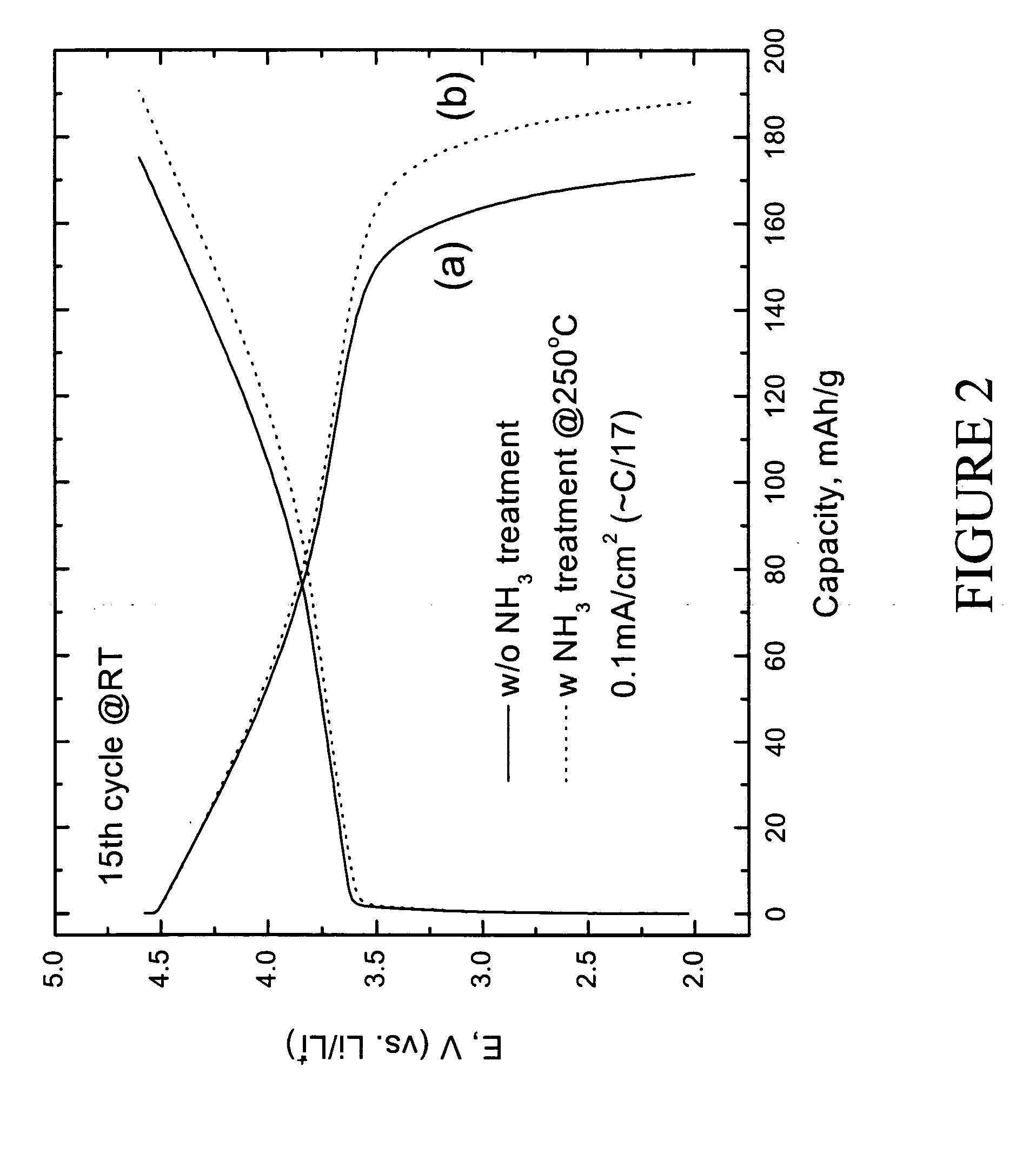
Positive electrode active materials are formed with various metal oxide coatings. Excellent results have been obtained with the coatings on lithium rich metal oxide active materials. Surprisingly improved results are obtained with metal oxide coatings with lower amounts of coating material. High specific capacity results are obtained even at higher discharge rates.
Owner:IONBLOX INC
Positive electrode materials for lithium ion batteries having a high specific discharge capacity and processes for the synthesis of these materials
ActiveUS20100086853A1Electrode manufacturing processesAlkali metal oxidesDischarge rateLithium-ion battery
Owner:IONBLOX INC
Ternary oxide nanostructures and methods of making same
InactiveUS20070138459A1Suitable for preparationFrom gel stateAlkaline earth titanatesNanostructureDislocation
A single crystalline ternary nanostructure having the formula AxByOz, wherein x ranges from 0.25 to 24, and y ranges from 1.5 to 40, and wherein A and B are independently selected from the group consisting of Ag, Al, As, Au, B, Ba, Br, Ca, Cd, Ce, Cl, Cm, Co, Cr, Cs, Cu, Dy, Er, Eu, F, Fe, Ga, Gd, Ge, Hf, Ho, I, In, Ir, K, La, Li, Lu, Mg, Mn, Mo, Na, Nb, Nd, Ni, Os, P, Pb, Pd, Pr, Pt, Rb, Re, Rh, Ru, S, Sb, Sc, Se, Si, Sm, Sn, Sr, Ta, Tb, Tc, Te, Ti, Ti, Tm, U, V, W, Y, Yb, and Zn, wherein the nanostructure is at least 95% free of defects and / or dislocations.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Layer-layer lithium rich complex metal oxides with high specific capacity and excellent cycling
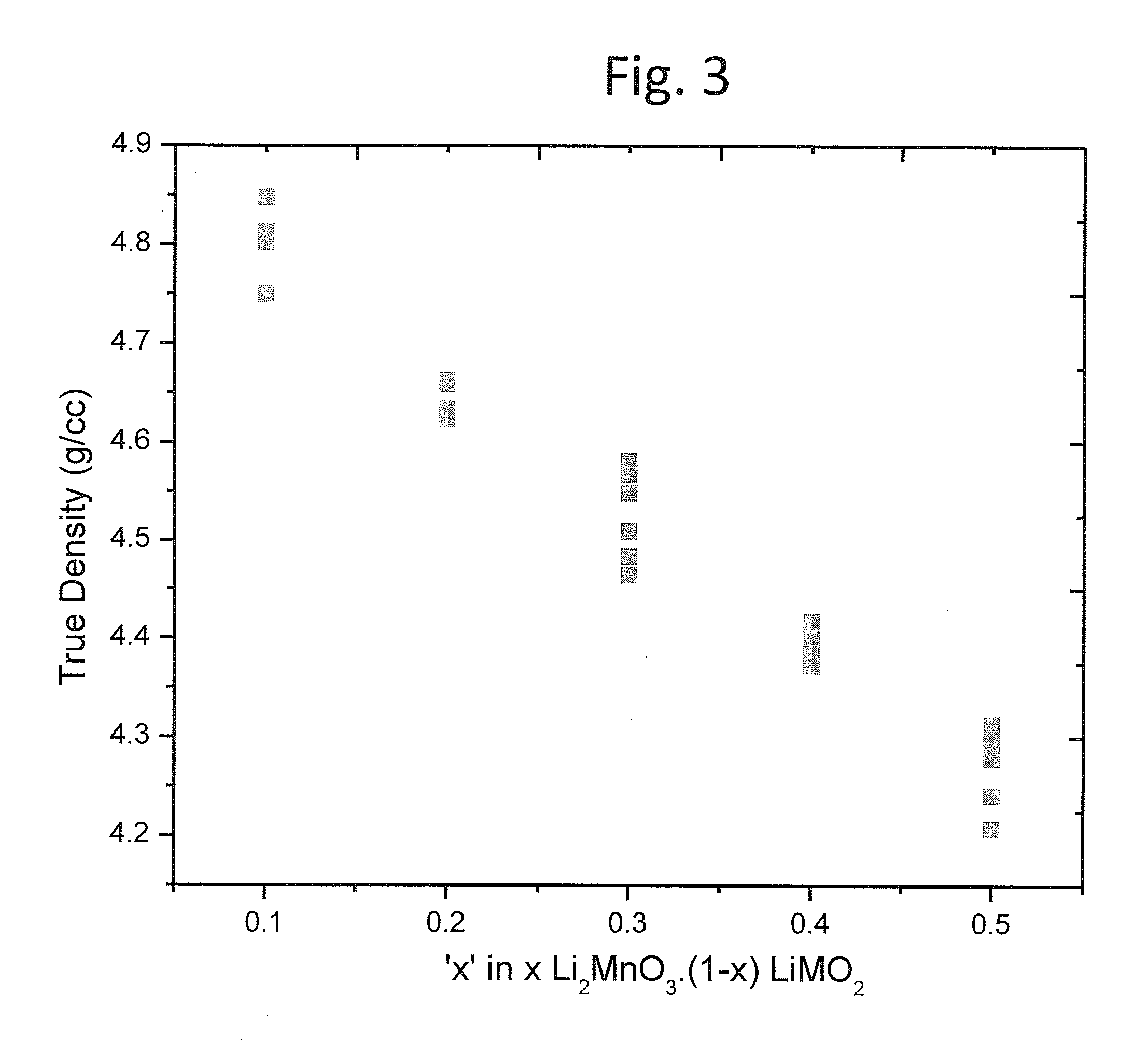
Lithium rich and manganese rich lithium metal oxides are described that provide for excellent performance in lithium-based batteries. The specific compositions can be engineered within a specified range of compositions to provide desired performance characteristics. Selected compositions can provide high values of specific capacity with a reasonably high average voltage. Compositions of particular interest can be represented by the formula, xLi2MnO3.(1−x)LiNiu+ΔMnu−ΔCowAyO2. The compositions undergo significant first cycle irreversible changes, but the compositions cycle stably after the first cycle.
Owner:IONBLOX INC
Method for preparing lithium cobaltate by directly using invalid lithium ion battery
InactiveCN102030375AReduce dispersionHigh purityCell electrodesCobalt compoundsElectrical batteryPotassium hydroxide
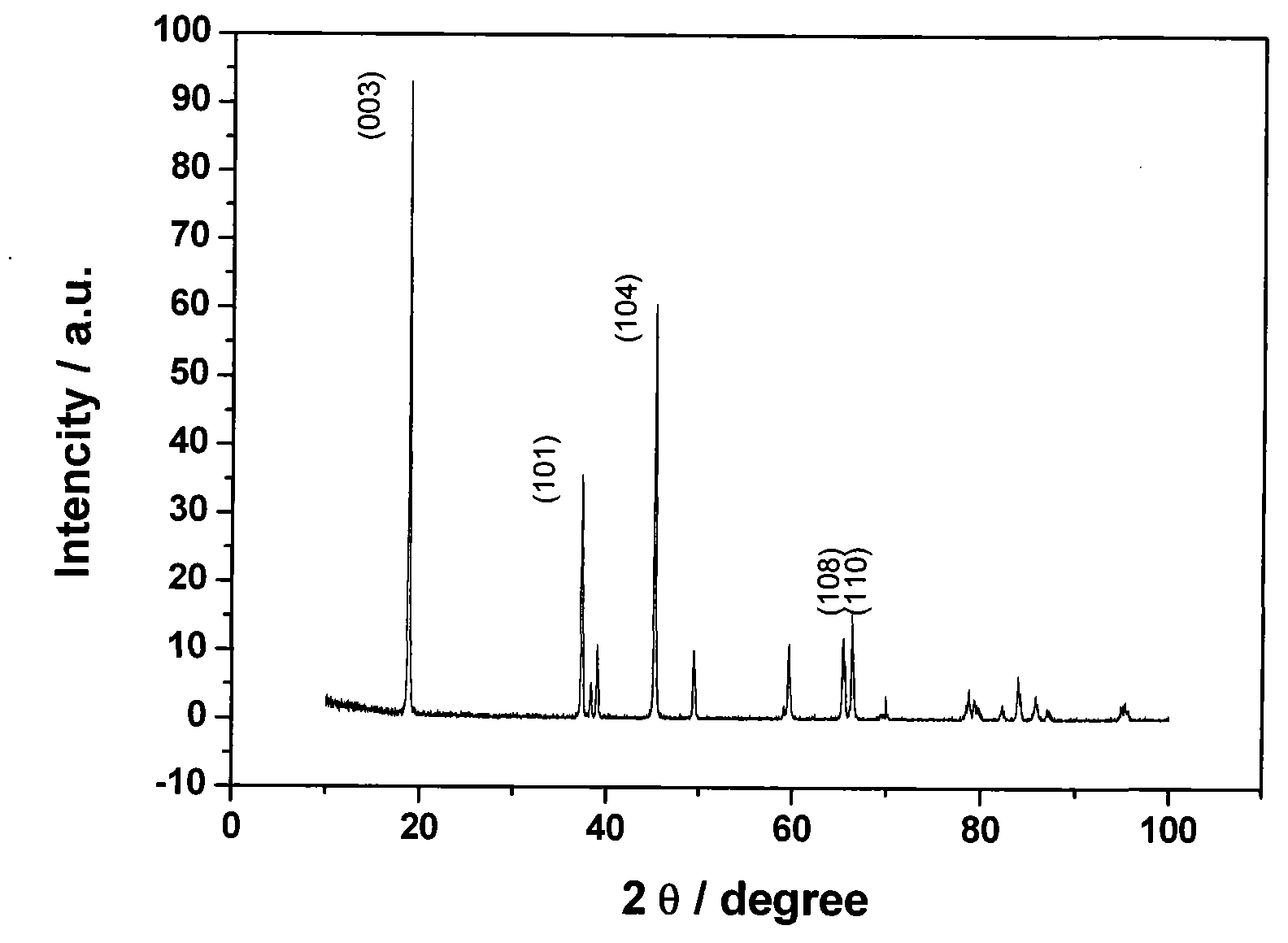
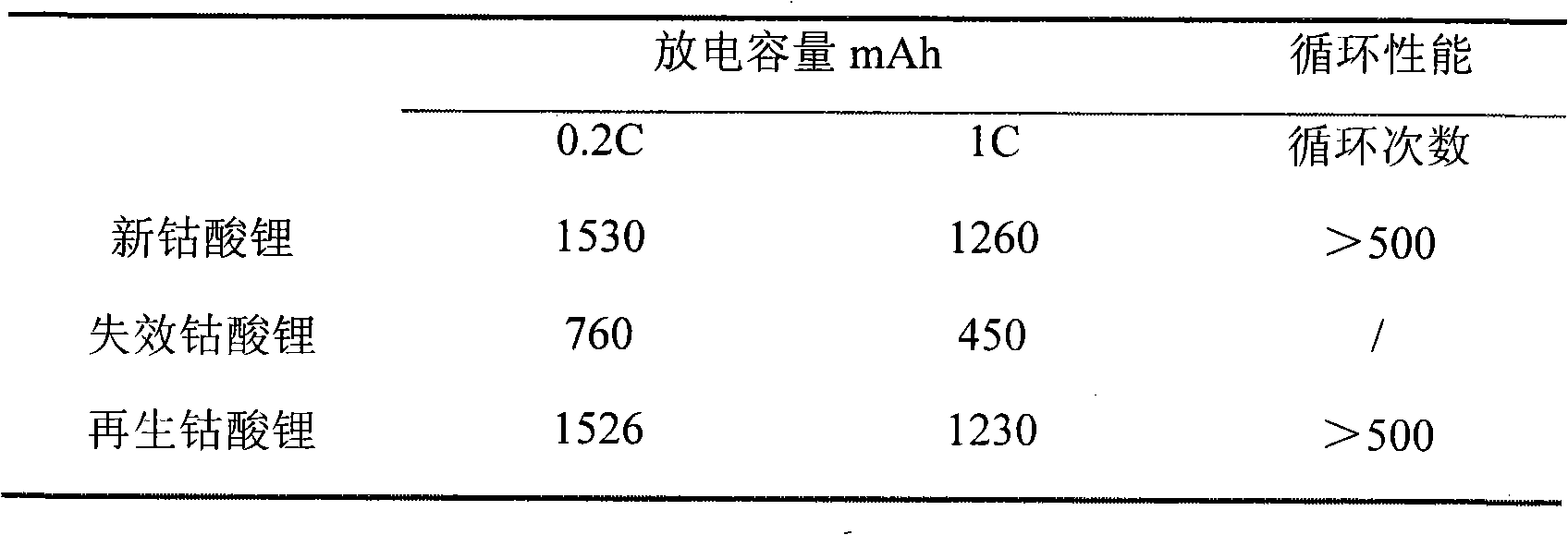
The invention provides a method for preparing lithium cobaltate by directly using an invalid lithium ion battery. The method comprises the following steps: crushing the invalid lithium ion battery or scraps generated when a lithium cobaltate battery is produced by a mechanical crusher at normal temperature; adding water and one or more of acetic acid, sulfuric acid, hydrochloric acid or nitric acid to produce mixed aqueous solution of the battery scraps and acid; filling the mixed aqueous solution into a hermetic pressure reactor, and controlling the temperature in the reactor to be between 50 and 150 DEG C; introducing or adding one leaching additive of sulfur dioxide or hydrogen, or adding hydrazine hydrate; stirring and leaching, cooling, and filtering; adding one precipitator of sodium carbonate, potassium carbonate and ammonium carbonate, or adding composite precipitator consisting of one of the sodium carbonate, the potassium carbonate and the ammonium carbonate and one of sodium hydroxide and potassium hydroxide to obtain mixture of lithium carbonate, cobalt carbonate and cobalt hydroxide; drying and calcining at high temperature to produce a lithium cobaltate product. The method is particularly suitable for the treatment scale of medium-sized and small enterprises, and is an effective method for directly materializing cobalt secondary resources.
Owner:BEIJING GENERAL RES INST OF MINING & METALLURGY
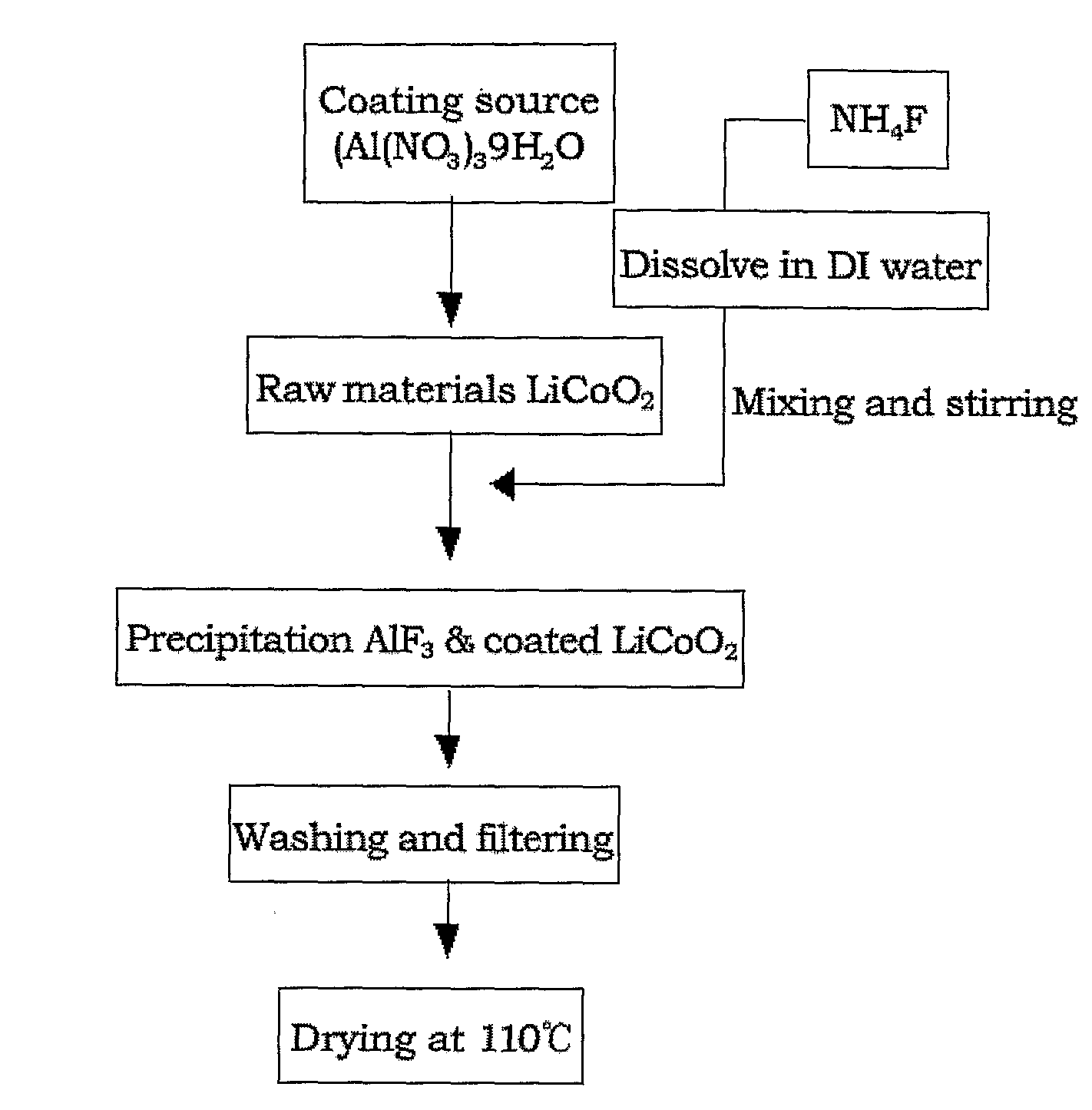
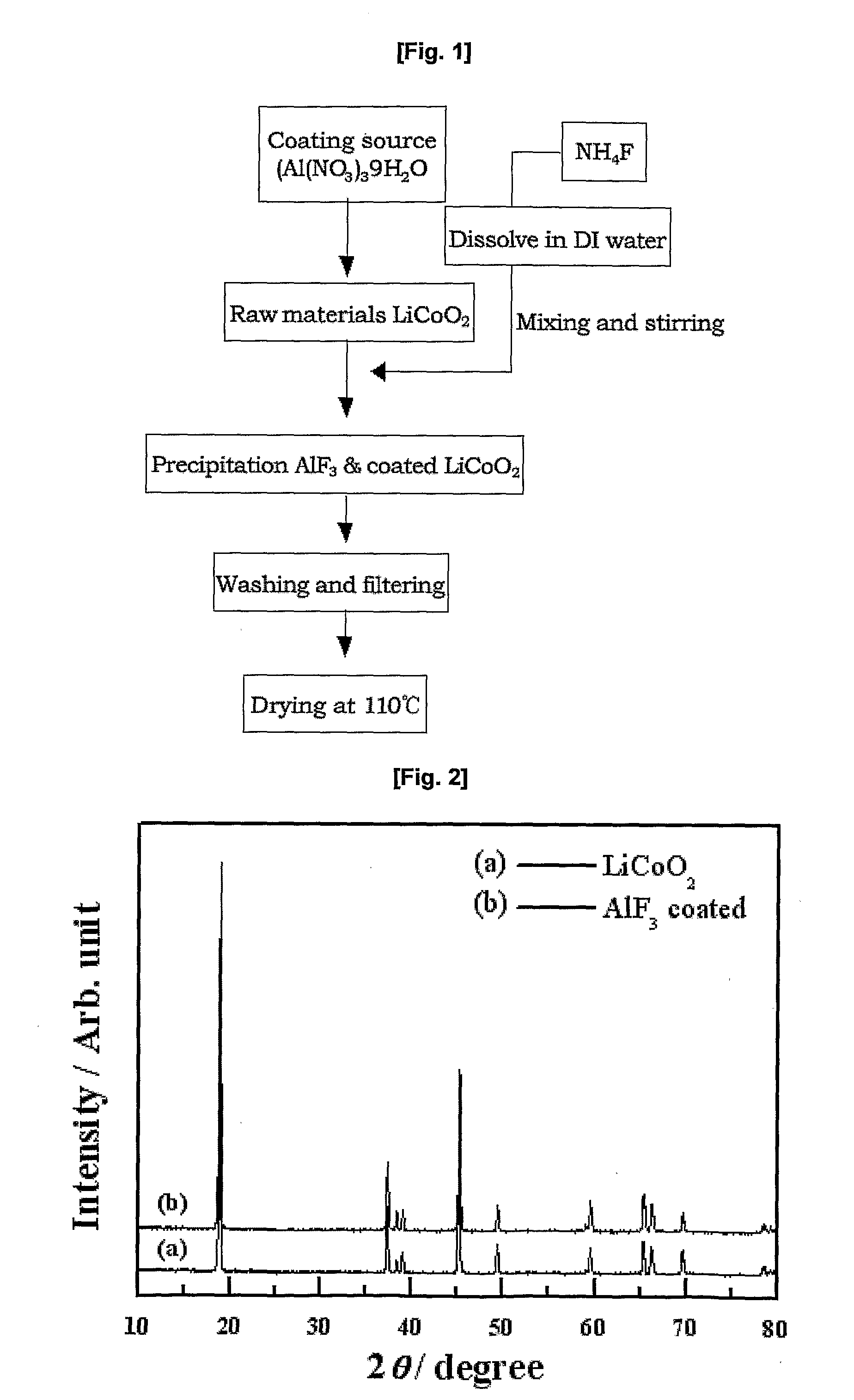
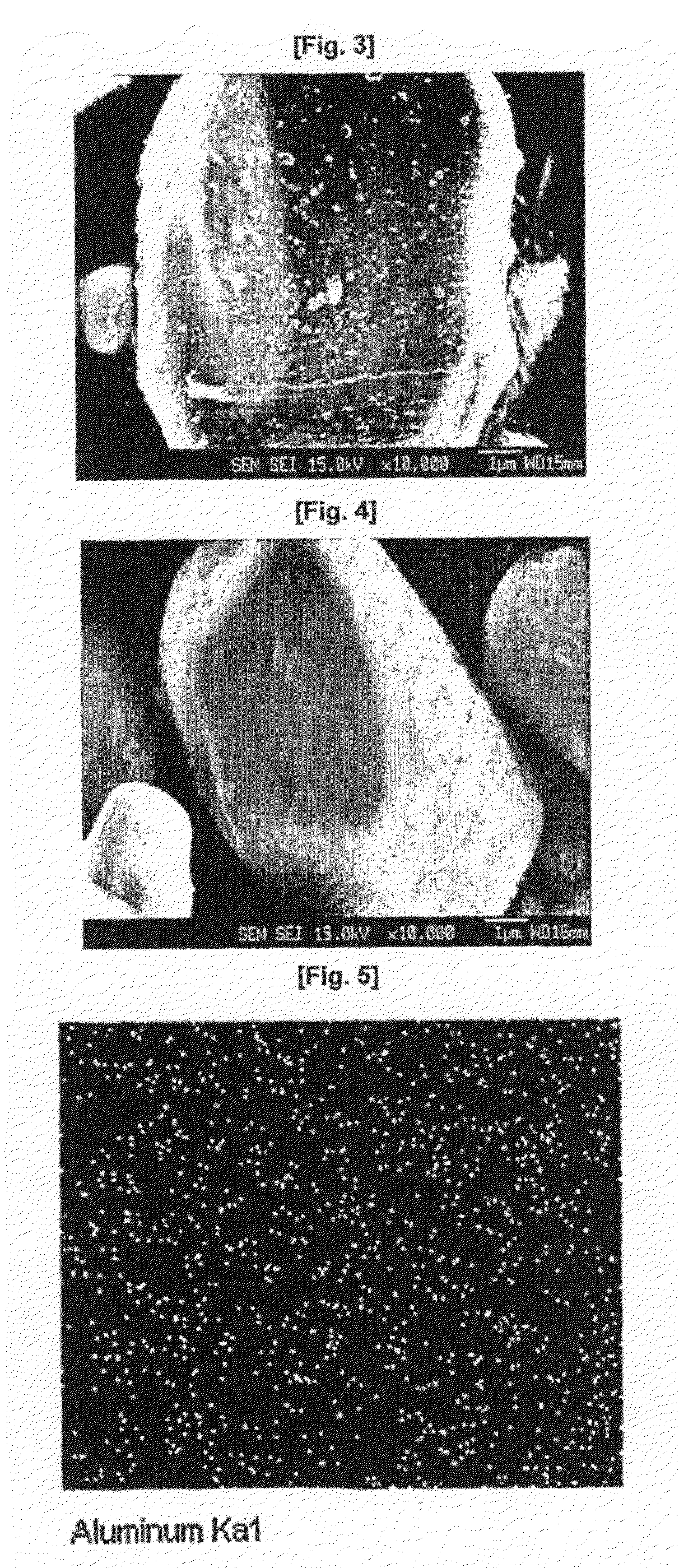
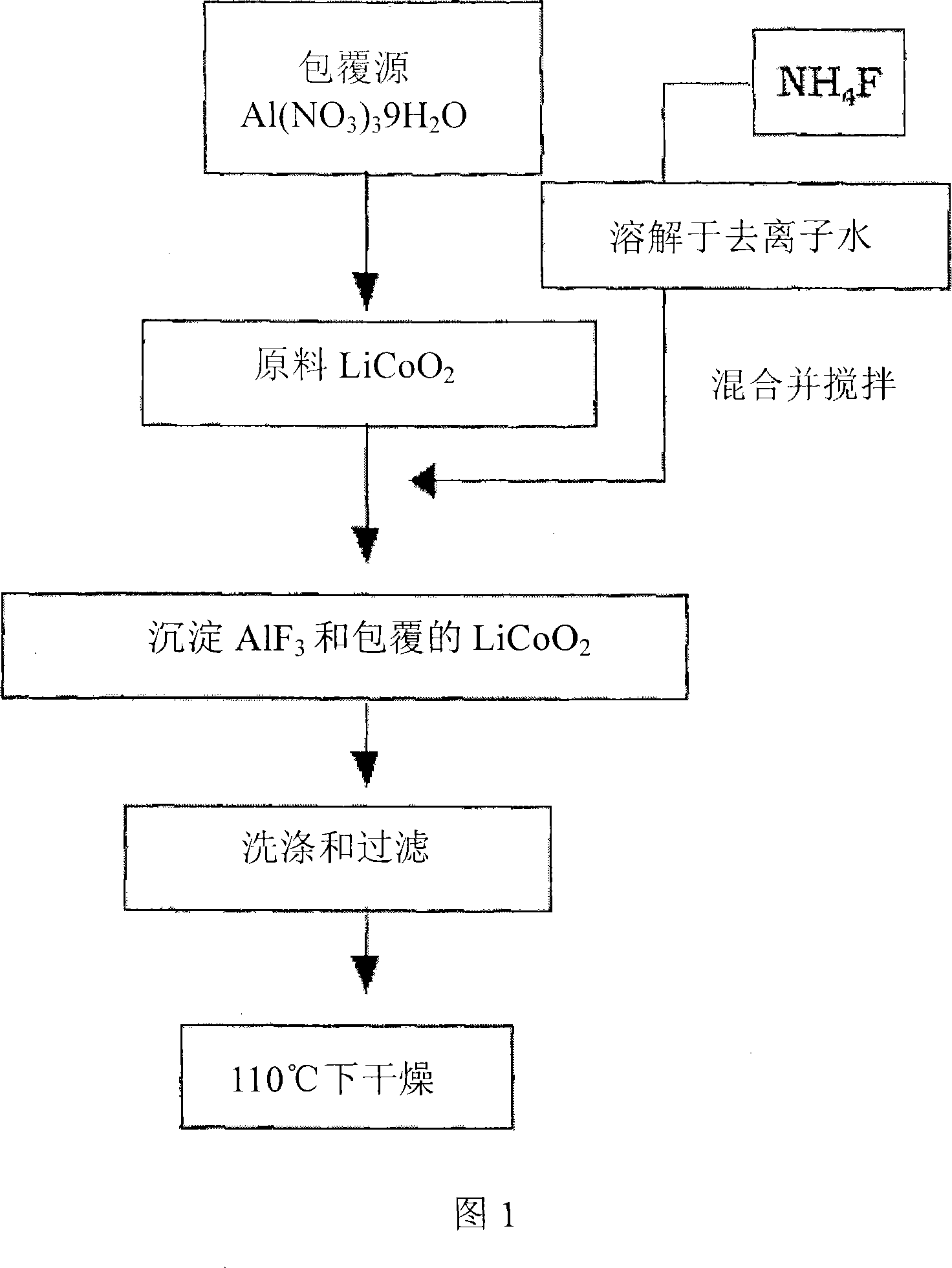
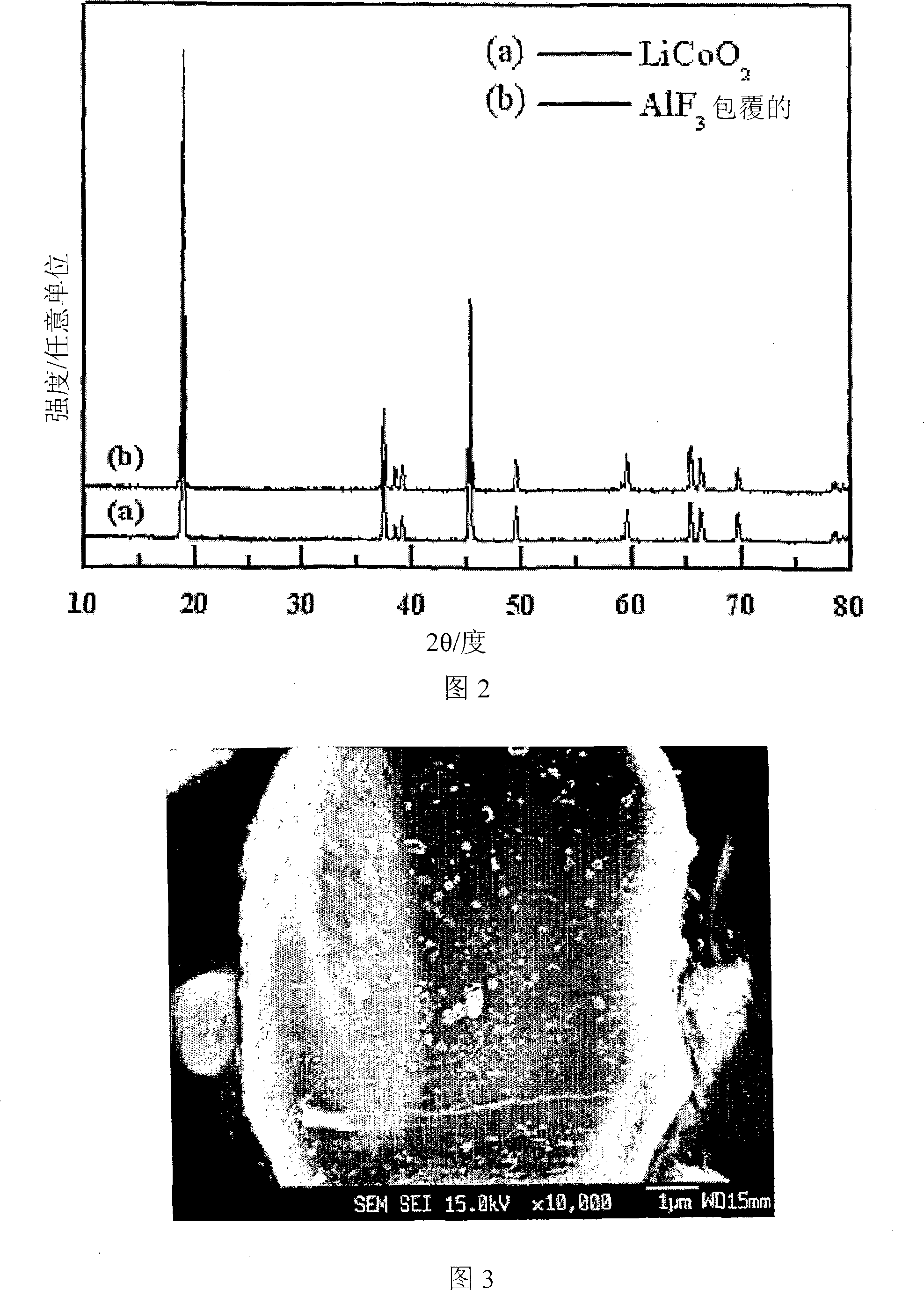
Cathode Active Material Coated With Fluorine Compound for Lithium Secondary Batteries and Method for Preparing the Same
InactiveUS20090087362A1Inhibition of performance deteriorationHigh voltageElectrode manufacturing processesLithium compoundsLithiumHigh rate
Disclosed herein is a cathode active material coated with a fluorine compound for lithium secondary batteries. The cathode active material is structurally stable, and improves the charge-discharge characteristics, cycle characteristics, high-voltage characteristics, high-rate characteristics and thermal stability of batteries.
Owner:ENERCERAMIC
Cathode active material coated with fluorine compound for lithium secondary batteries and method for preparing the same
Disclosed herein is a cathode active material coated with a fluorine compound for lithium secondary batteries. The cathode active material is structurally stable, and improves the charge-discharge characteristics, cycle characteristics, high-voltage characteristics, high-rate characteristics and thermal stability of batteries.
Owner:ENERCERAMIC
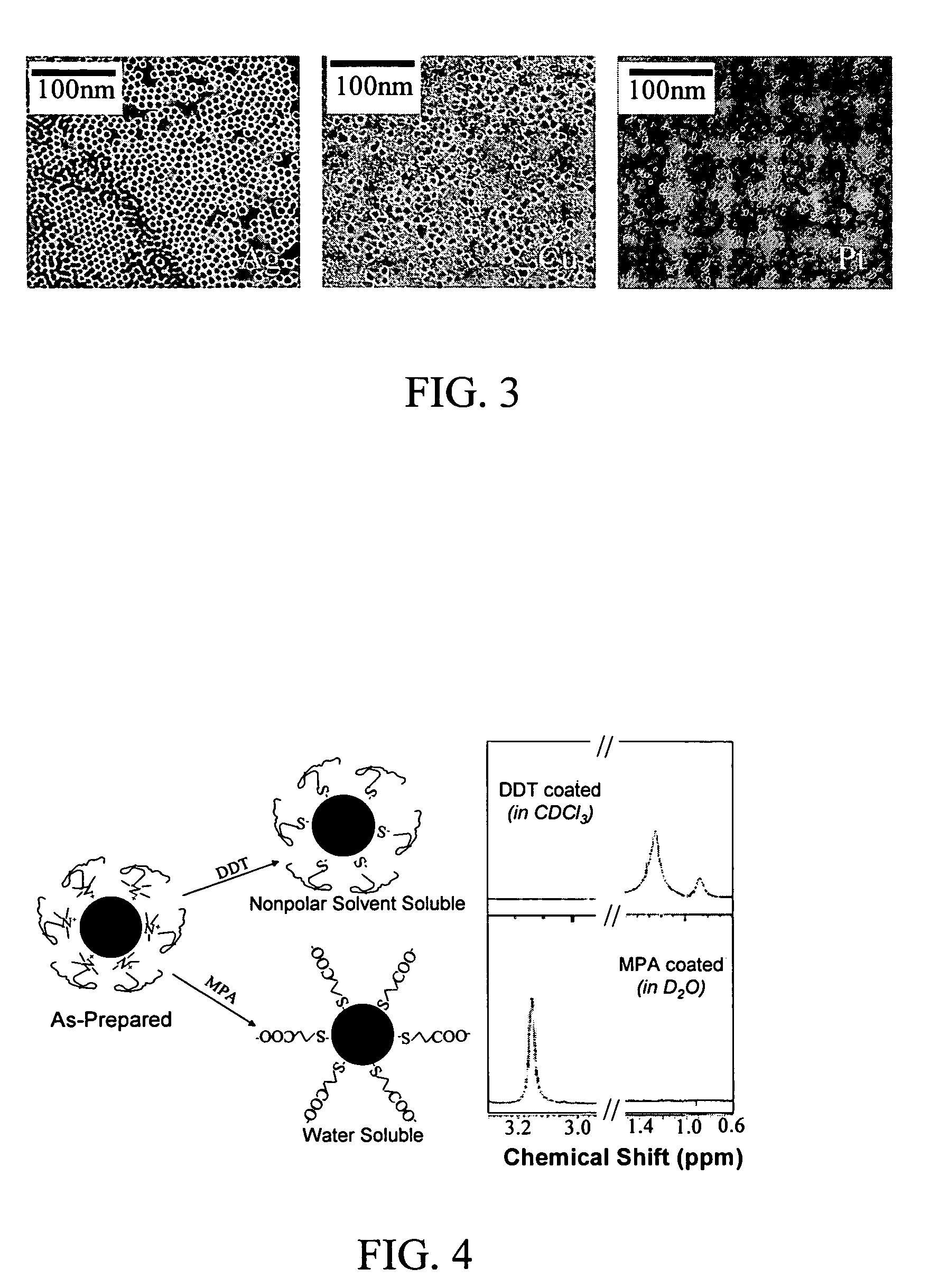
Monodisperse noble metal nanocrystals
Nanoparticle compositions of noble metals, and methods of making them, are described. The nanoparticle compositions are made by reacting a salt or complex of a noble metal, such as Au, Ag, Cu or Pt, with a weak ligand, and a reducing agent, in a single liquid phase. The noble metal is typically provided as a halide or carboxylate. The ligand is preferably a fatty acid or aliphatic amine. The reducing agent is preferably a borohydride reagent, hydrazine, or a mixture thereof. Nanocrystals in the size range of 1 nm to 20 nm are produced, and can be made in substantially monodisperse form.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
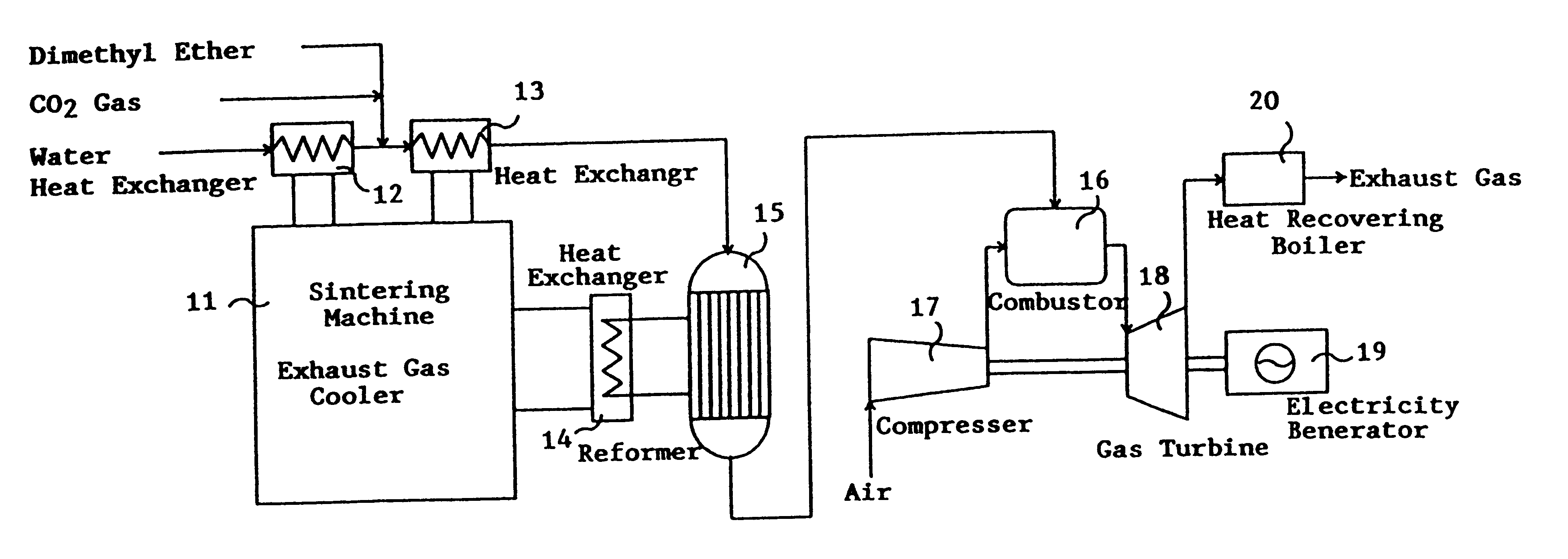
Catalyst for manufacturing hydrogen or synthesis gas and manufacturing method of hydrogen or synthesis gas
InactiveUS6361757B1Produce hydrogenEfficient productionIron compoundsCobalt compoundsIridiumForming gas
This invention provides a catalyst for producing hydrogen gas from a mixed gas comprising dimethyl ether and water vapor or carbon dioxide gas, which comprises copper, iron, cobalt, palladium, iridium, platinum, rhodium, or nickel as an active component, and a method of producing synthesis gas or hydrogen gas in a high yield at a low temperature. By using the catalyst, a fuel cell, electricity generation, reduction of iron ore and the like can be carried out.
Owner:NIPPON KOKAN KK
Ni-based lithium transition metal oxide
The present invention provides a powderous lithium transition metal oxide with the composition as represented by the below Formula and prepared by solid state reaction in air from a mixed transition metal precursor and Li2CO3, with being practically free of Li2CO3 impurity: LixMyO2 wherein M=M′l-kAk, where M′=Nil-a-b(Ni1 / 2Mn1 / 2)aCob on condition of 0.65≦a+b≦0.85 and 0.1≦b≦0.4; A is a dopant; and 0≦k≦0.05; and x+y=2 on condition of 0.95≦x≦1.05. The Ni-based lithium transition metal oxide according to the present invention has a well-layered structure, and also improved safety, cycling stability and stability against aging and low gas evolution during storage, when used as an active material for cathode of lithium secondary batteries, because it has a high sintering stability and is substantially free of soluble bases. Moreover, the lithium transition metal oxide of the present invention can be prepared by a low-cost process using a mixed transition metal precursor and Li2CO3 as raw stocks and under relatively unrestricted conditions.
Owner:LG ENERGY SOLUTION LTD
Polymer electrolyte, intercalation compounds and electrodes for batteries
Solid battery components are provided. A block copolymeric electrolyte is non-crosslinked and non-glassy through the entire range of typical battery service temperatures, that is, through the entire range of at least from about 0° C. to about 70° C. The chains of which the copolymer is made each include at least one ionically-conductive block and at least one second block immiscible with the ionically-conductive block. The chains form an amorphous association and are arranged in an ordered nanostructure including a continuous matrix of amorphous ionically-conductive domains and amorphous second domains that are immiscible with the ionically-conductive domains. A compound is provided that has a formula of LixMyNzO2. M and N are each metal atoms or a main group elements, and x, y and z are each numbers from about 0 to about 1. y and z are chosen such that a formal charge on the MyNz portion of the compound is (4-x). In certain embodiments, these compounds are used in the cathodes of rechargeable batteries. The present invention also includes methods of predicting the potential utility of metal dichalgogenide compounds for use in lithium intercalation compounds. It also provides methods for processing lithium intercalation oxides with the structure and compositional homogeneity necessary to realize the increased formation energies of said compounds. An article is made of a dimensionally-stable, interpenetrating microstructure of a first phase including a first component and a second phase, immiscible with the first phase, including a second component. The first and second phases define interphase boundaries between them, and at least one particle is positioned between a first phase and a second phase at an interphase boundary. When the first and second phases are electronically-conductive and ionically-conductive polymers, respectively, and the particles are ion host particles, the arrangement is an electrode of a battery.
Owner:MASSACHUSETTS INST OF TECH
Anode active material, manufacturing method thereof, and non-aqueous electrolyte secondary battery
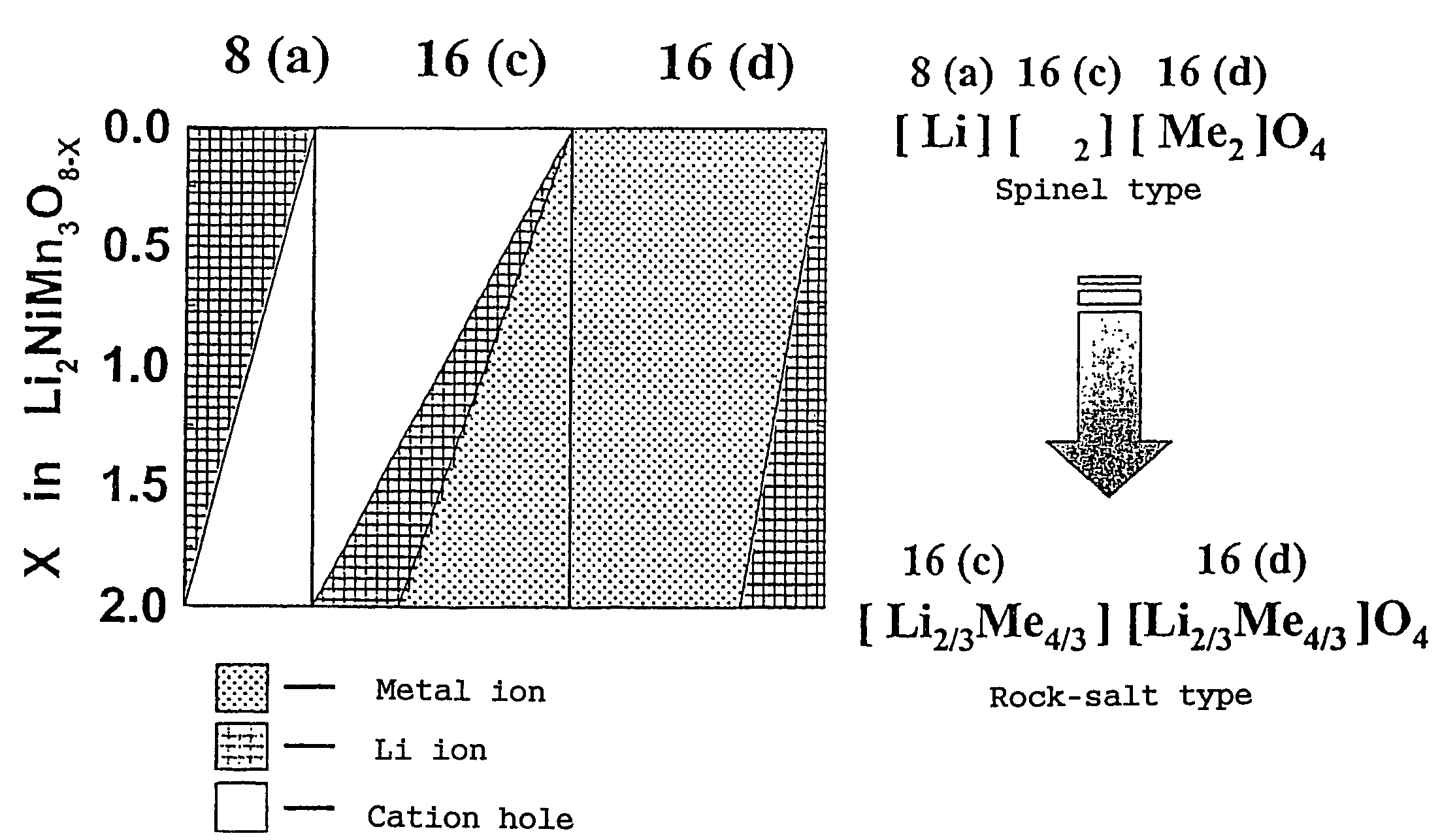
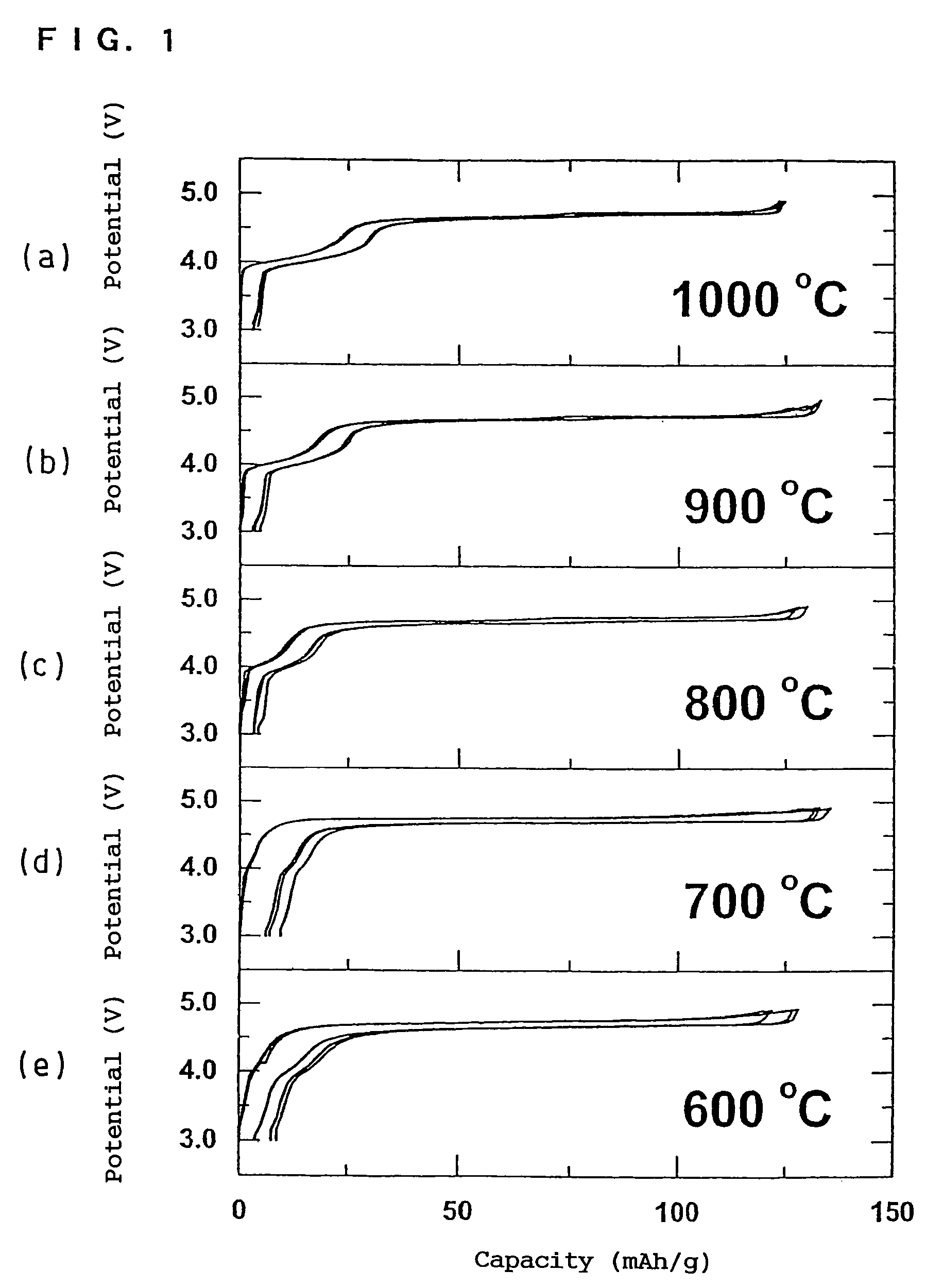
In order to provide a 3V level non-aqueous electrolyte secondary battery with a flat voltage and excellent cycle life at a high rate with low cost, the present invention provides a positive electrode represented by the formula: Li2±α[Me]4O8−x, wherein 0≦α<0.4, 0≦x<2, and Me is a transition metal containing Mn and at least one selected from the group consisting of Ni, Cr, Fe, Co and Cu, said active material exhibiting topotactic two-phase reactions during charge and discharge.
Owner:OSAKA CITY UNIV +1
Lithium ion battery positive pole material cobalt nickel oxide manganses lithium and method for making same
ActiveCN101202343AHigh specific capacityExcellent cycle characteristicsElectrode manufacturing processesLithium compoundsLithium oxideAntioxidant
The invention relates to a nickel cobalt manganese lithium oxide material used for an anode of a li-ion battery and a preparation method. The invention belongs to the li-ion battery technical field. The nickel cobalt manganese lithium oxide material used for the anode of the li-ion battery is a li-rich laminated structure with the chemical component of Li1+zM1-x-yNixCoyO2; wherein, z is less than or equal to 0.2 and more than or equal to 0.05, x is less than or equal to 0.8 and more than 0.1, and y is less than or equal to 0.5 and more than 0.1. The preparation method of the invention is that dissoluble salt of the nickel, cobalt and manganese is taken as the raw material; ammonia or ammonium salt is taken as complexing agent; sodium hydroxide is taken as precipitator; water-dissoluble dispersant and water-dissoluble antioxidant or inert gas are added for control and protection; in a cocurrent flow type the solution is added to a reaction vessel for reaction; after alkalescence disposal, aging procedure, solid-liquid separation and washing and drying, the nickel cobalt manganese oxide is uniformly mixed with the lithium raw material; the nickel cobalt manganese lithium oxide powder is obtained by sintering the mixed powder which is divided into three temperature areas. The invention has the advantages of high specific capacity, good circulation performance, ideal crystal texture, short production period, low power loss, and being suitable for industrial production, etc.
Owner:CHINA ELECTRONIC TECH GRP CORP NO 18 RES INST +1
Method and apparatus for preparation of spherical metal carbonates and lithium metal oxides for lithium rechargeable batteries
ActiveUS7435402B2Improve impedance characteristicsImproved stability of the layered oxide structureConductive materialOxide conductorsDopantLithium metal
A number of materials with the composition Li1+xNiαMnβCoγM′δO2−zFz (M′=Mg,Zn,Al,Ga,B,Zr,Ti) for use with rechargeable batteries, wherein x is between about 0 and 0.3, α is between about 0.2 and 0.6, β is between about 0.2 and 0.6, γ is between about 0 and 0.3, δ is between about 0 and 0.15, and z is between about 0 and 0.2. Adding the above metal and fluorine dopants affects capacity, impedance, and stability of the layered oxide structure during electrochemical cycling. Another aspect of the invention includes materials with the composition Li1+xNiαCoβMnγM′δOyFz (M′=Mg,Zn,Al,Ga,B,Zr,Ti), where the x is between 0 and 0.2, the α between 0 and 1, the β between 0 and 1, the γ between 0 and 2, the δ between about 0 and about 0.2, the y is between 2 and 4, and the z is between 0 and 0.5.
Owner:UCHICAGO ARGONNE LLC
Positive electrode active material for lithium secondary cell and lithium secondary cell
ActiveUS7393476B2Improve cycle performanceIncrease energy densityMaterial nanotechnologyCell temperature controlCrystallographyComposite oxide
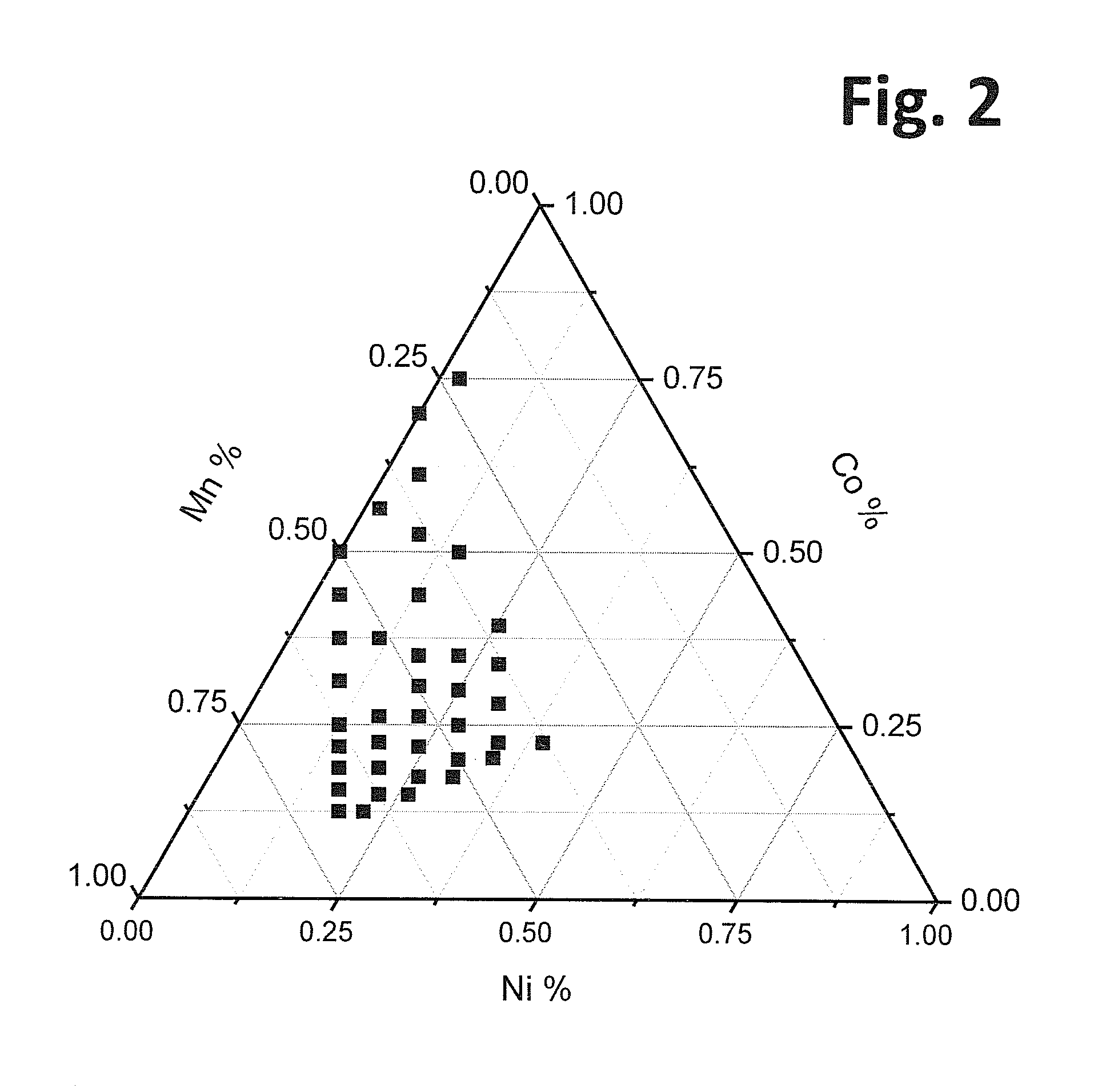
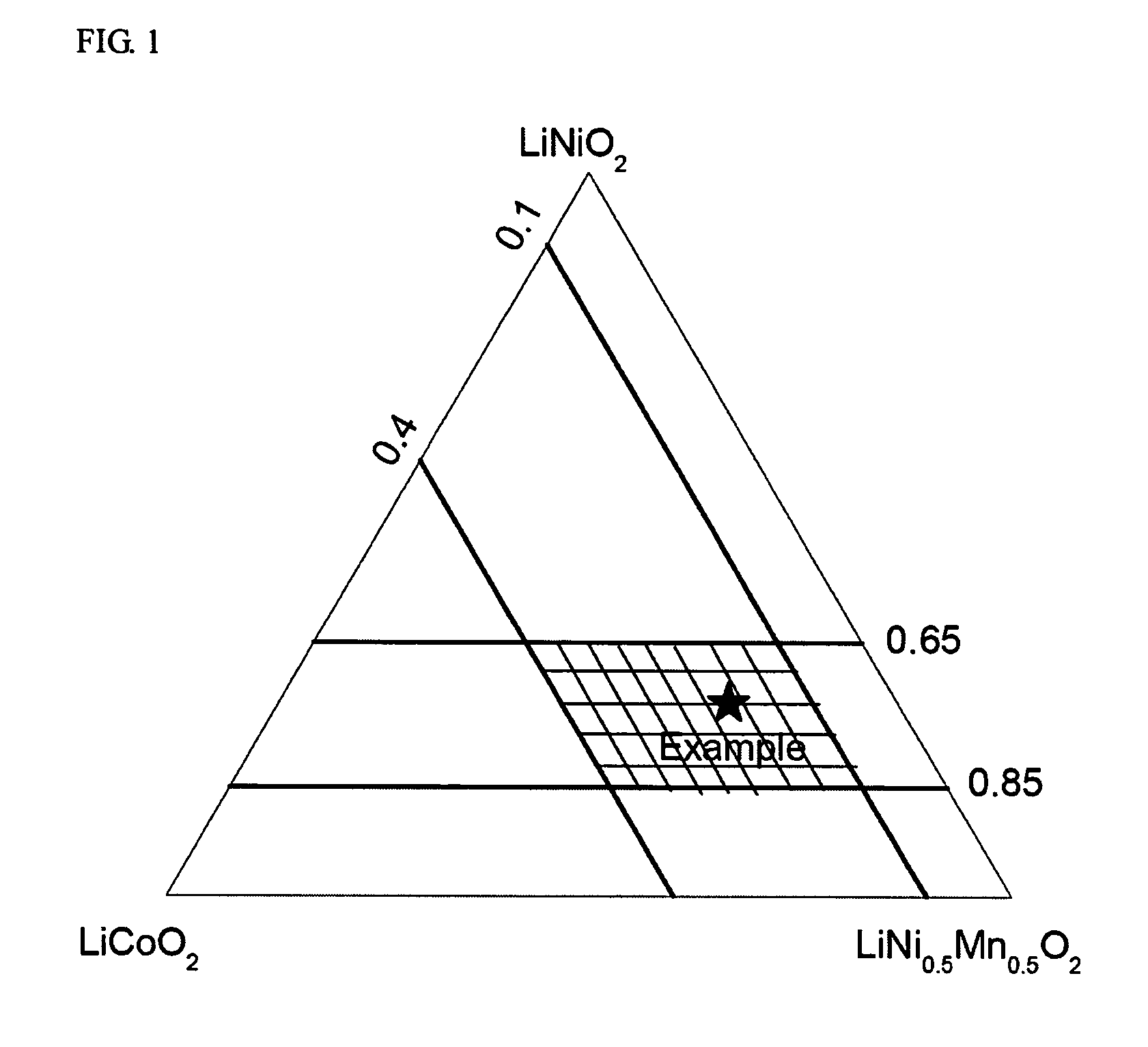
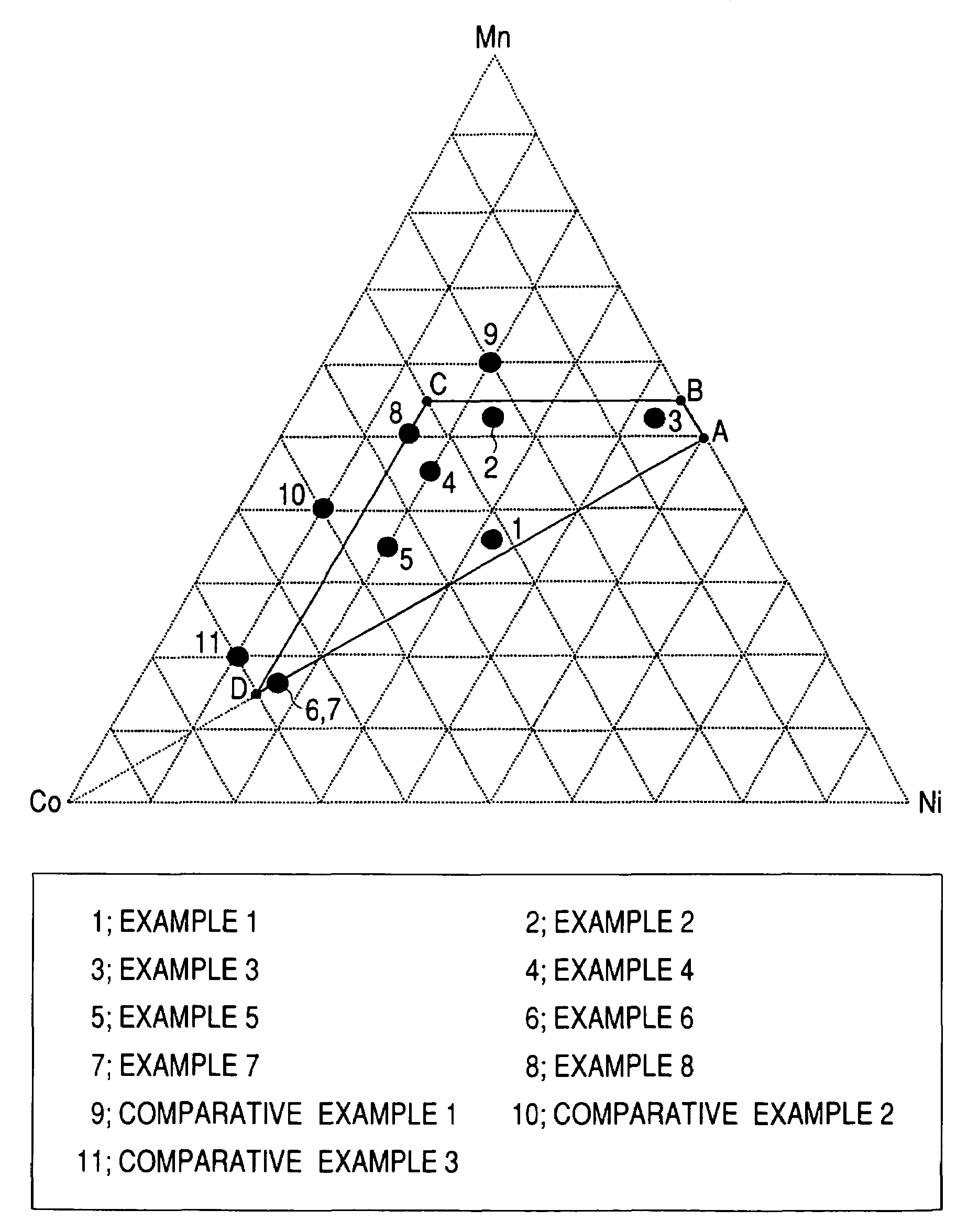
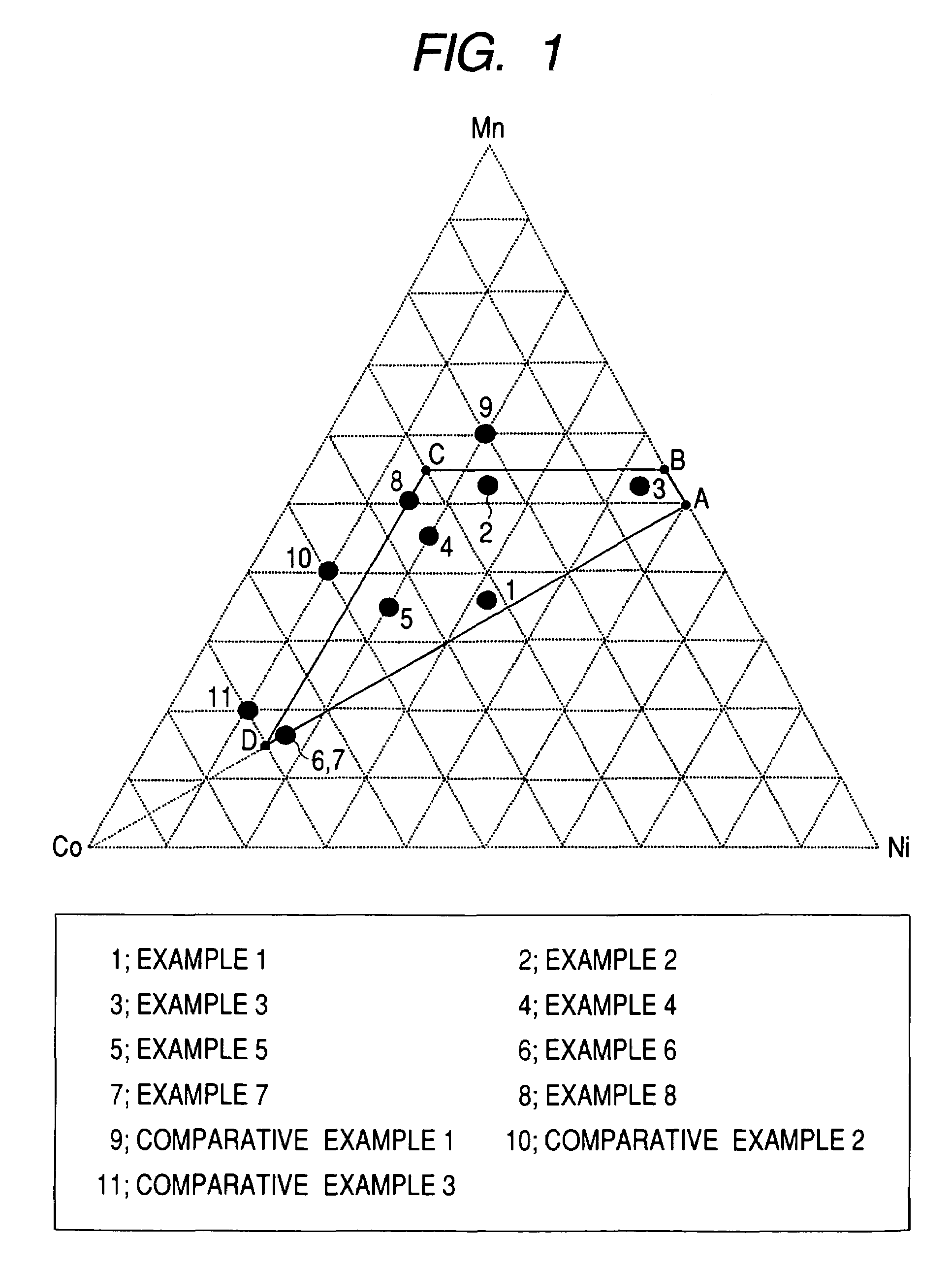
A positive active material for lithium secondary batteries, includes a composite oxide including an oxide which is represented by the composite formula LixMnaNibCocO2 and has an α-NaFeO2 structure, and an impurity phase including Li2MnO3. The values a, b, and c are within such a range that in a ternary phase diagram showing the relationship among these, (a, b, c) is present on the perimeter of or inside the quadrilateral ABCD defined by point A (0.5, 0.5, 0), point B (0.55, 0.45, 0), point C (0.55, 0.15, 0.30), and point D (0.15, 0.15, 0.7) as vertexes, and 0.95<x / (a+b+c)<1.35.
Owner:GS YUASA INT LTD
Lithium metal oxide containing multiple dopants and method of preparing same
InactiveUS6277521B1Reduce irreversible capacityHigh reversible capacityCobalt compoundsNon-aqueous electrolyte accumulator electrodesOxideLithium electrode
The present invention provides a multiple-doped lithium metal oxide and a method of preparing same for use in the positive electrodes of lithium and lithium ion batteries. The intercalation compound of the invention has the formula LiNi.sub.1-x Co.sub.y M.sub.a M'.sub.b O.sub.2, wherein M is selected from the group consisting of Ti, Zr, and combinations thereof, and M' is selected from the group consisting of Mg, Ca, Sr, Ba, and combinations thereof. The elements in the compounds are present such that x=y+a+b, x is from greater than 0 to about 0.5, y is from greater than 0 to about 0.5, a is from greater than 0 to about 0.15, and b is from greater than 0 to about 0.15.
Owner:UMICORE AG & CO KG
Lithium metal oxide electrodes for lithium batteries
ActiveUS20050026040A1Increase capacityImprove cycle stabilityCellsElectrode thermal treatmentLithium metalOxidation state
An uncycled electrode for a non-aqueous lithium electrochemical cell including a lithium metal oxide having the formula Li(2+2x) / (2+x)M′2x / (2+x)M(2-2x) / (2+x)O2-δ, in which 0≦x<1 and δ is less than 0.2, and in which M is a non-lithium metal ion with an average trivalent oxidation state selected from two or more of the first row transition metals or lighter metal elements in the periodic table, and M′ is one or more ions with an average tetravalent oxidation state selected from the first and second row transition metal elements and Sn. Methods of preconditioning the electrodes are disclosed as are electrochemical cells and batteries containing the electrodes.
Owner:UCHICAGO ARGONNE LLC
Doped positive electrode active materials and lithium ion secondary battery constructed therefrom
Positive electrode active materials comprising a dopant in an amount of 0.1 to 10 mole percent of Mg, Ca, Sr, Ba, Zn, Cd or a combination thereof are described that have high specific discharge capacity upon cycling at room temperature and at a moderate discharge rate. Some materials of interest have the formula Li1+xNiαMnβ-δCoγAδXμO2−zFz, where x ranges from about 0.01 to about 0.3, δ ranges from about 0.001 to about 0.15, and the sum x+α+β+γ+δ+μ can approximately equal 1.0. The materials can be coated with a metal fluoride to improve the performance of the materials especially upon cycling. The materials generally can have a tap density of at least 1.8 g / mL. Also, the materials can have an average discharge voltage of around 3.6 V.
Owner:IONBLOX INC
Positive electrode materials for lithium ion batteries having a high specific discharge capacity and processes for the synthesis of these materials
ActiveUS8389160B2Electrode manufacturing processesAlkali metal oxidesElectrical batteryDischarge rate
Positive electrode active materials are described that have a very high specific discharge capacity upon cycling at room temperature and at a moderate discharge rate. Some materials of interest have the formula Li1+xNiαMnβCOγO2, where x ranges from about 0.05 to about 0.25, α ranges from about 0.1 to about 0.4, β ranges from about 0.4 to about 0.65, and γ ranges from about 0.05 to about 0.3. The materials can be coated with a metal fluoride to improve the performance of the materials especially upon cycling. Also, the coated materials can exhibit a very significant decrease in the irreversible capacity lose upon the first charge and discharge of the cell. Methods for producing these materials include, for example, a co-precipitation approach involving metal hydroxides and sol-gel approaches.
Owner:IONBLOX INC
Spray Pyrolysis Synthesis of Mesoporous Positive Electrode Materials for High Energy Lithium-Ion Batteries
A lithium metal oxide positive electrode material useful in making lithium-ion batteries that is produced using spray pyrolysis. The material comprises a plurality of metal oxide secondary particles that comprise metal oxide primary particles, wherein the primary particles have a size that is in the range of about 1 nm to about 10 μm, and the secondary particles have a size that is in the range of about 10 nm to about 100 μm and are uniformly mesoporous.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Lithium Ion Batteries with Supplemental Lithium
Supplemental lithium can be used to stabilize lithium ion batteries with lithium rich metal oxides as the positive electrode active material. Dramatic improvements in the specific capacity at long cycling have been obtained. The supplemental lithium can be provided with the negative electrode, or alternatively as a sacrificial material that is subsequently driven into the negative electrode active material. The supplemental lithium can be provided to the negative electrode active material prior to assembly of the battery using electrochemical deposition. The positive electrode active materials can comprise a layered-layered structure comprising manganese as well as nickel and / or cobalt.
Owner:IONBLOX INC
Electromechanical actuators
InactiveUS7090785B2Increased doping of BaTiOImprove propertiesAlkaline earth titanatesLighting support devicesElectroactive materialsMaterials science
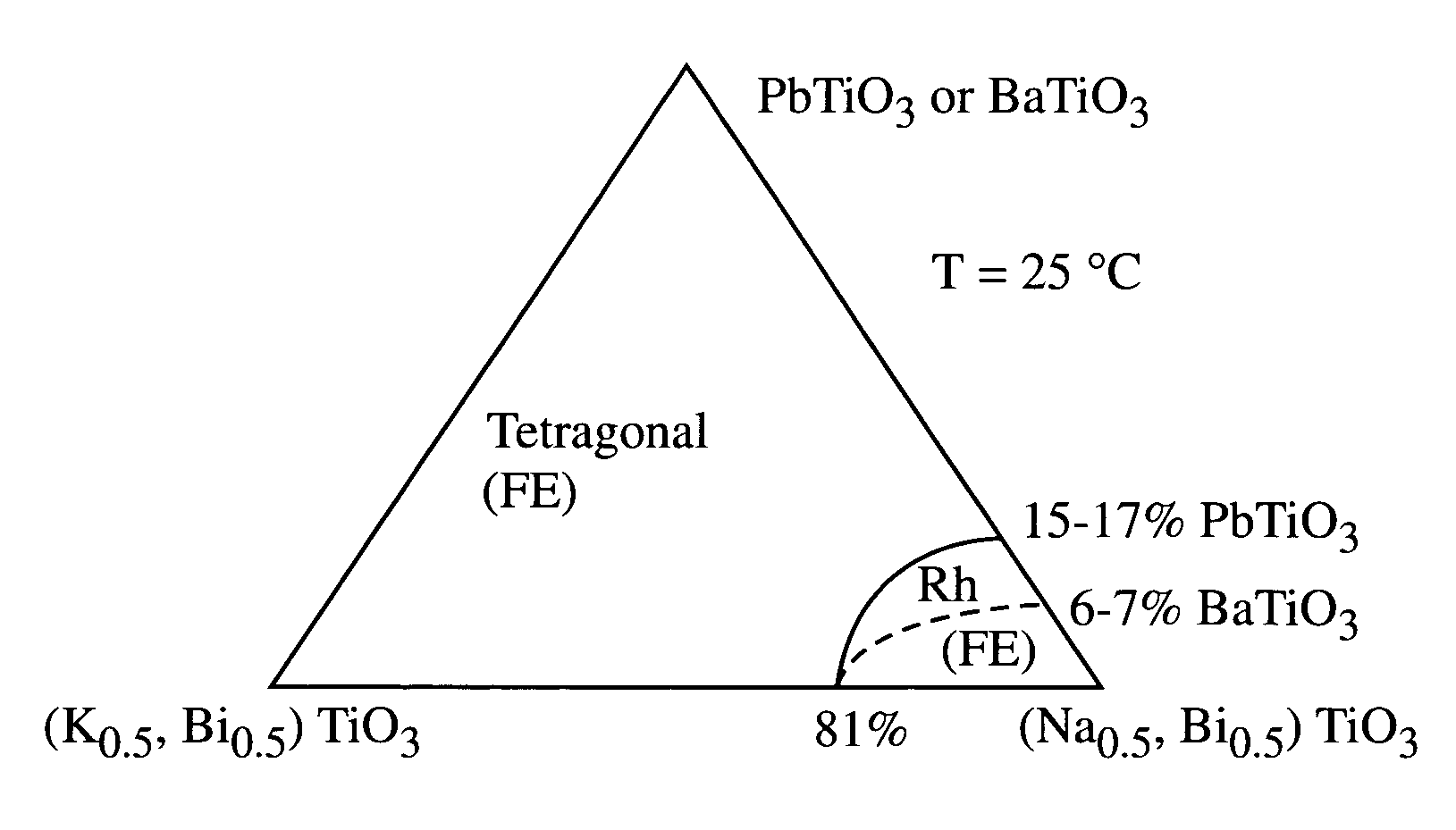
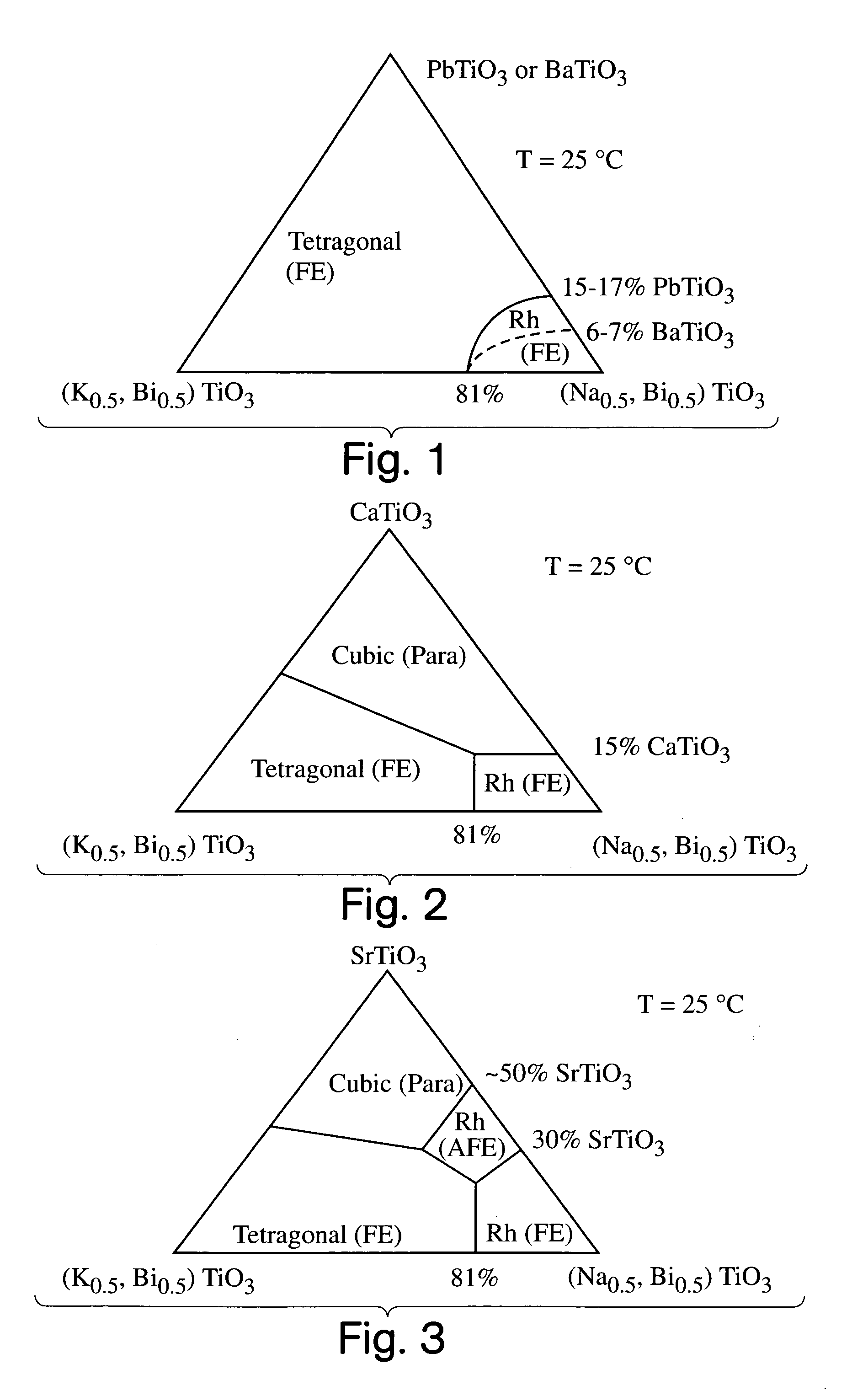
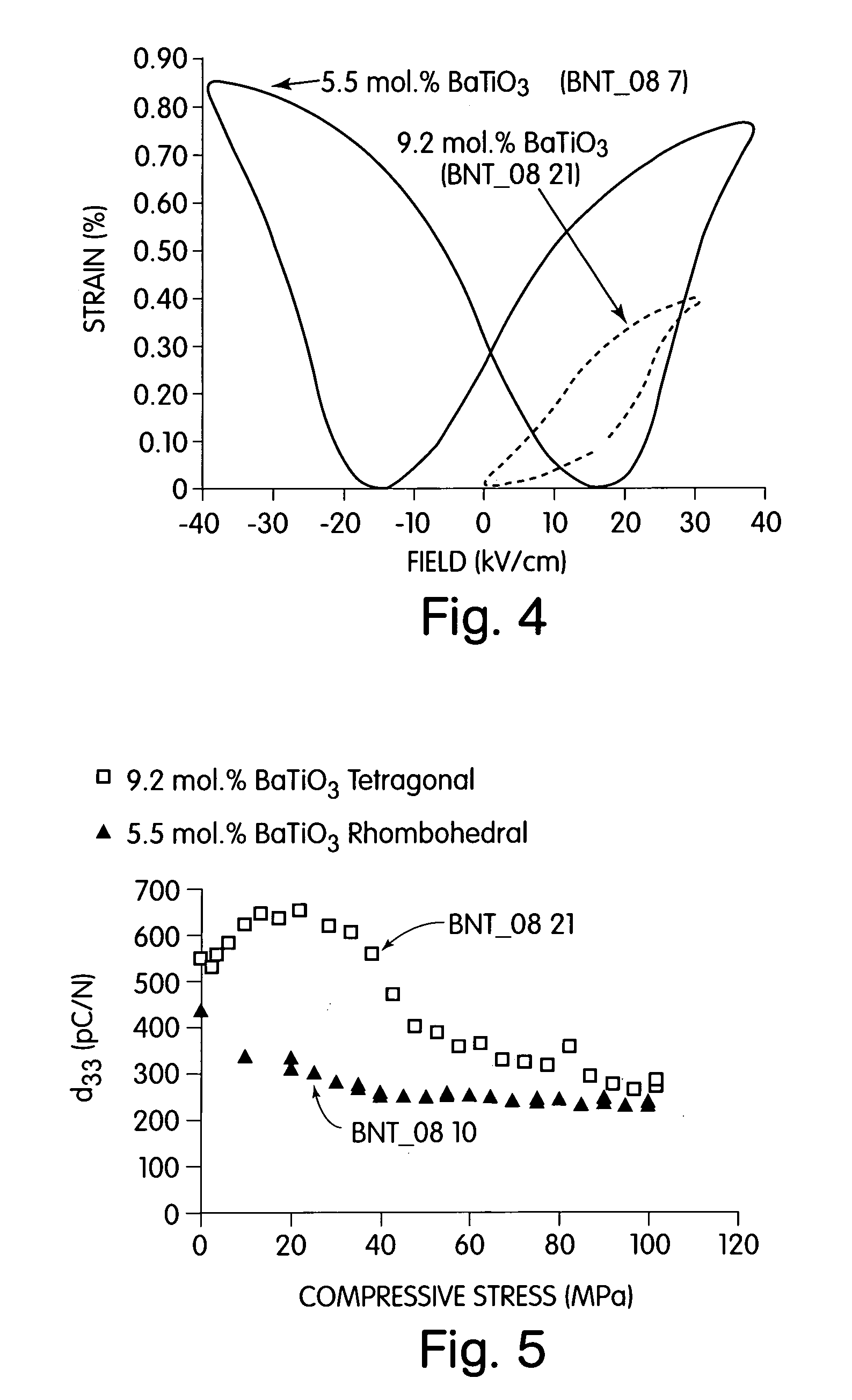
A perovskite compound of the formula, (Na1 / 2Bi1 / 2)1-xMx(Ti1-yM′y)O3±z, where M is one or more of Ca, Sr, Ba, Pb, Y, La, Pr, Nd, Sm, Eu, Gd, Th, Dy, Ho, Er, Tm, Yb and Lu; and M′ is one or more of Zr, Hf, Sn, Ge, Mg, Zn, Al, Sc, Ga, Nb, Mo, Sb, Ta, W, Cr, Mn, Fe, Co and Ni, and 0.01<x<0.3, and 0.01<y<0.3, and z<0.1 functions as an electromechanically active material. The material may possess electrostrictive or piezoelectric characteristics.
Owner:MASSACHUSETTS INST OF TECH
Lithium metal oxide electrodes for lithium batteries
InactiveUS7314682B2Electrode thermal treatmentActive material electrodesLithium metalOxidation state
An uncycled electrode for a non-aqueous lithium electrochemical cell including a lithium metal oxide having the formula Li(2+2x) / (2+x)M′2x / (2+x)M(2−2x) / (2+x)O2−δ, in which 0≦x<1 and δ is less than 0.2, and in which M is a non-lithium metal ion with an average trivalent oxidation state selected from two or more of the first row transition metals or lighter metal elements in the periodic table, and M′ is one or more ions with an average tetravalent oxidation state selected from the first and second row transition metal elements and Sn. Methods of preconditioning the electrodes are disclosed as are electrochemical cells and batteries containing the electrodes.
Owner:UCHICAGO ARGONNE LLC
Method for recovering and preparing lithium cobalt oxide by using disused lithium battery
ActiveCN101673859ASolve the hazardSolve resource problemsLithium compoundsWaste accumulators reclaimingWater bathsOrganic acid
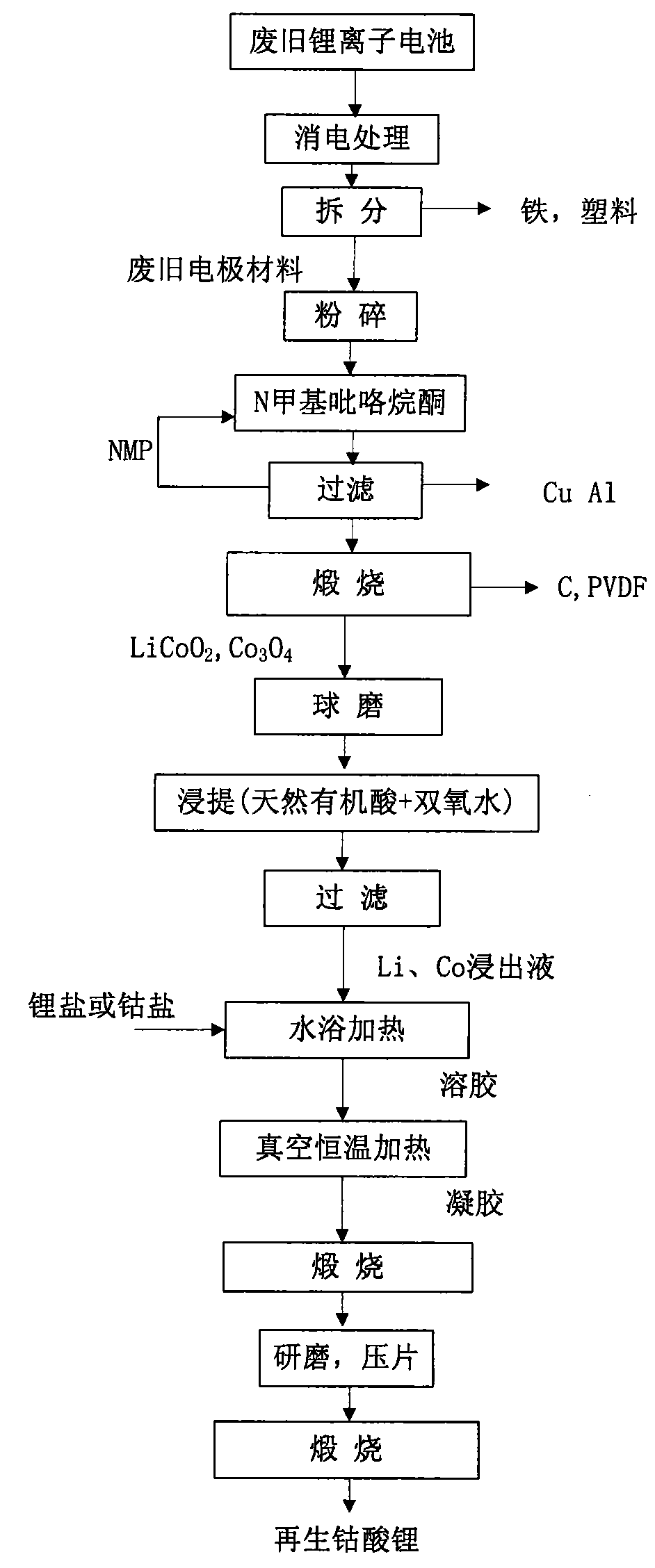
The invention relates to a method for recovering and preparing lithium cobalt oxide by using a disused lithium battery, belonging to the technical field of recovery and recycle of electrode materials.The method comprises the steps of: discharging, disassembling, smashing, NMP processing and burning a disused lithium battery sequentially, to obtain a disused LiCoO2 material; ball-milling the disused LiCoO2 material and adding natural organic acid and hydrogen peroxide to obtain a solution of Li<+> and Co<2+>; adding lithium salt or cobalt salt after filtering, and then heating by water bath; dropwise adding ammonia water in the solution to prepare a xerogel; and performing secondary burning to obtain an electrode material of lithium cobalt oxide. The method has the advantages that the electrochemical properties of the electrode material of the disused lithium battery can be recycled with obvious effect as well as simple and easy operation; the natural organic acid used in the process of acid dipping has small damage to apparatus; and the method is environment friendly and efficient, and has low cost, simple technique, high recovery rate and industrialized promotion.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Process for preparing high density spherical nickel-cobalt lithium manganate as anode material of lithium ion cell
InactiveCN1622371AWell mixedImprove performanceElectrode manufacturing processesLithium compoundsNickel saltManganate
The present invention relates to energy source material technology, and is preparation process of high density spherical lithium nickel-cobalt-manganate as positive electrode material for lithium ion cell. The preparation process includes the reaction of nickel salt, cobalt salt, manganese salt, ammonium hydroxide and ammonian in water solution to synthesize spherical or spheroid precursor Ni1 / 3Co1 / 3Mn1 / 3 (OTHER)2, washing, drying and mixing with lithium carbonate; and high temperature treatment in the air at 750-950 deg.c for 8-48 hr to obtain spherical lithium nickel-cobalt-manganate. The spherical lithium nickel-cobalt-manganate has great bulk density reaching 2.25-2.50 g / cu cm after vibration densifying, average grain size of 3-7 microns, and reversible specific capacity up to 172-185 mA.hr / g.
Owner:TSINGHUA UNIV
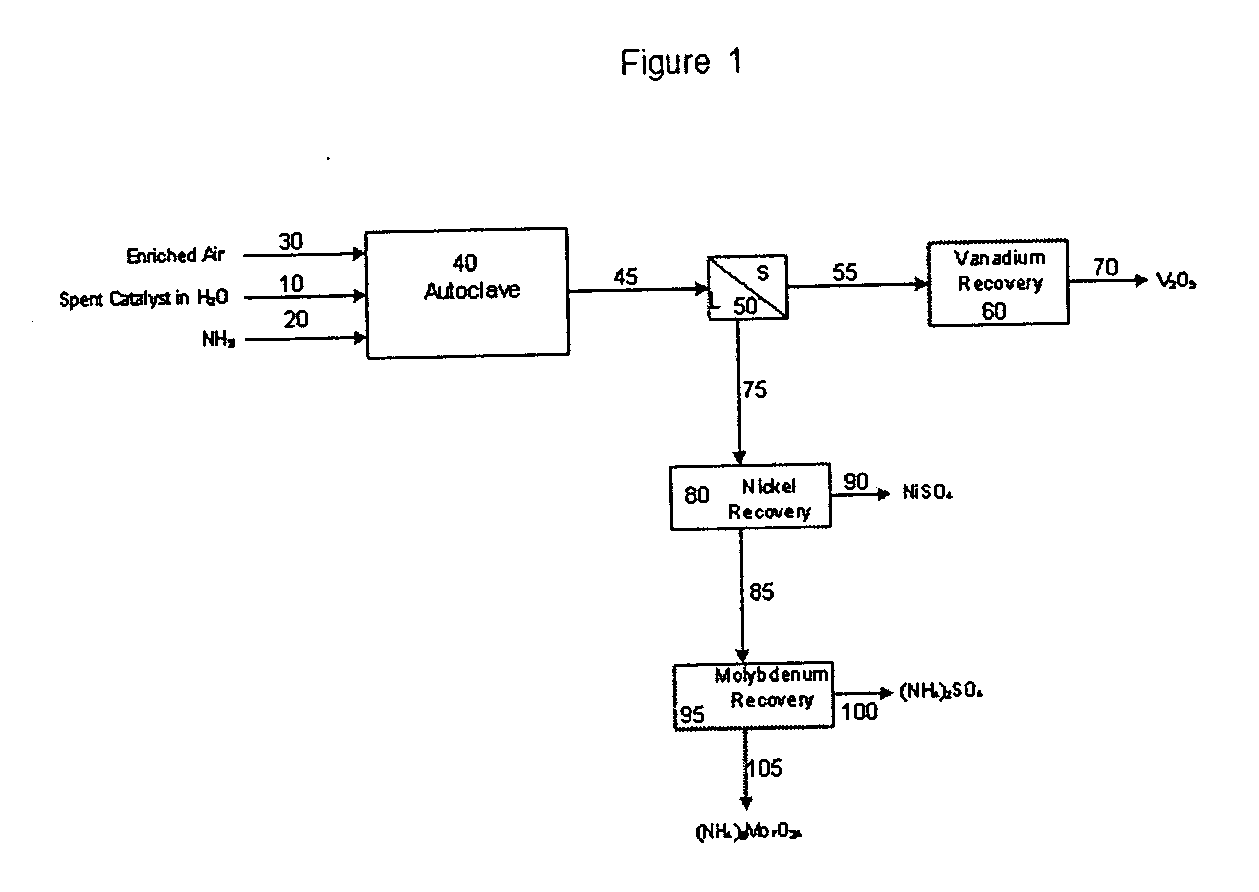
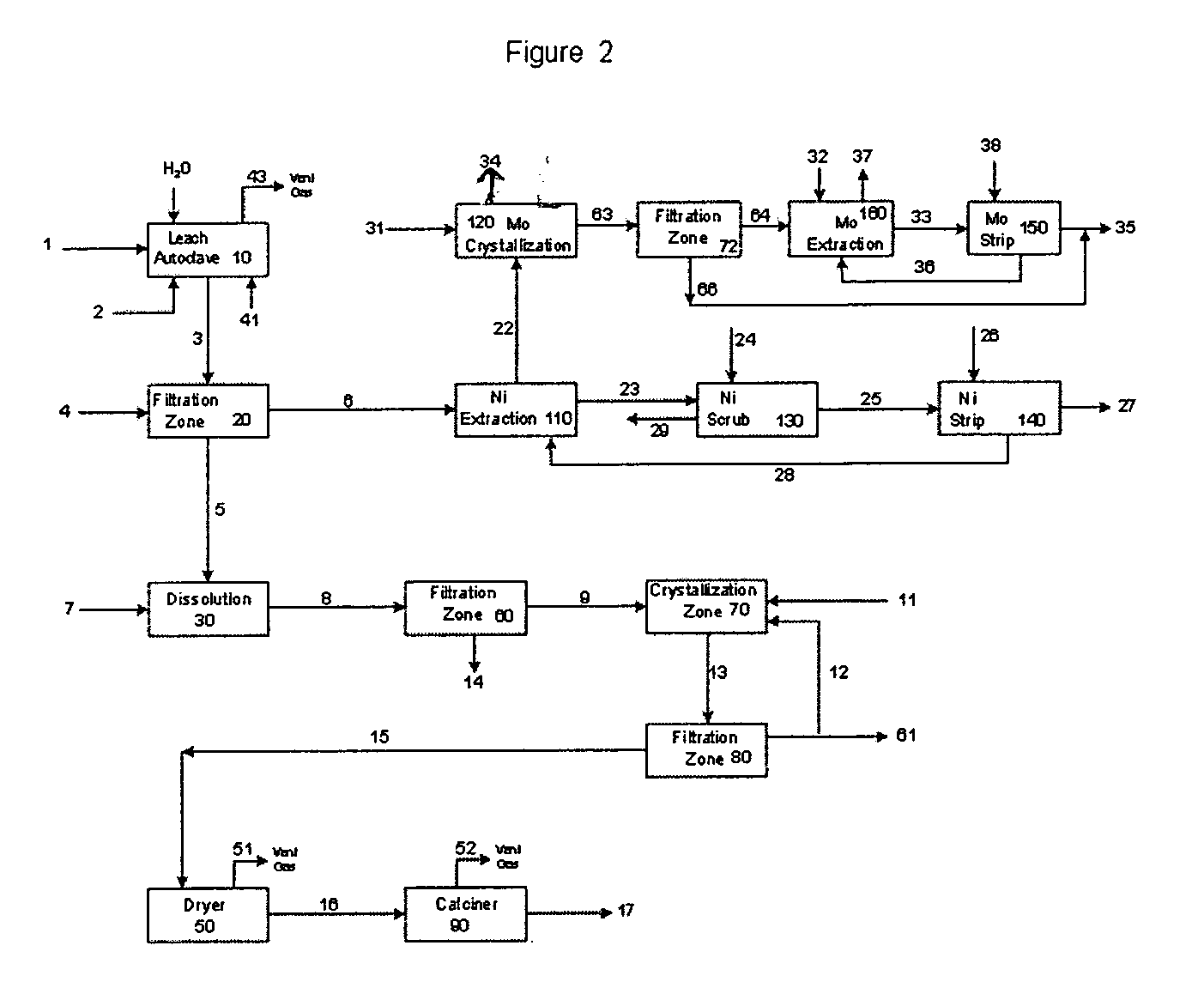
Process for metals recovery from spent catalyst
The process of this invention is directed to the removal of metals from an unsupported spent catalyst. The catalyst is subjected to leaching reactions. Vanadium is removed as a precipitate, while a solution comprising molybdenum and nickel is subjected to further extraction steps for the removal of these metals. Molybdenum may alternately be removed through precipitation.
Owner:CHEVROU USA INC
Metal oxide coated positive electrode materials for lithium-based batteries
Positive electrode active materials are formed with various metal oxide coatings. Excellent results have been obtained with the coatings on lithium rich metal oxide active materials. Surprisingly improved results are obtained with metal oxide coatings with lower amounts of coating material. High specific capacity results are obtained even at higher discharge rates.
Owner:IONBLOX INC
Nano-sized particles, processes of making, compositions and uses thereof
InactiveUS20070140951A1Economical and efficientQuality improvementMaterial nanotechnologyToilet preparationsSolventPharmaceutical formulation
The present invention describes methods for preparing high quality nanoparticles, i.e., metal oxide based nanoparticles of uniform size and monodispersity. The nanoparticles advantageously comprise organic alkyl chain capping groups and are stable in air and in nonpolar solvents. The methods of the invention provide a simple and reproducible procedure for forming transition metal oxide nanocrystals, with yields over 80%. The highly crystalline and monodisperse nanocrystals are obtained directly without further size selection; particle size can be easily and fractionally increased by the methods. The resulting nanoparticles can exhibit magnetic and / or optical properties. These properties result from the methods used to prepare them. Also advantageously, the nanoparticles of this invention are well suited for use in a variety of industrial applications, including cosmetic and pharmaceutical formulations and compositions.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Lithium ion secondary battery, cathode active material therefor and production thereof
A substituted lithium cobalt composite oxide for use as a cathode active material in a lithium ion secondary battery, which has the general formula:in which M represents at least one metallic element selected from the group consisting of Ti, Mo and; x is a numeral in the range of 0.8 to 1.2; and y is a numeral in the range of 0.001 to 0.10.
Owner:HONJIYOU CHEM
Positive active material for rechargeable lithium battery and method of preparing same
InactiveUS20020055042A1Improve thermal stabilityElectrode manufacturing processesZirconium compoundsPhysical chemistryLithium battery
Disclosed is a positive active material for a rechargeable lithium battery. The positive active material includes at least one compound represented by formulas 1 to 4 andl a metal oxide or composite metal oxide layer formed on the compound. <table-cwu id="TABLE-US-00001"> <number>1< / number> <tgroup align="left" colsep="0" rowsep="0" cols="3"> <colspec colname="OFFSET" colwidth="42PT" align="left" / > <colspec colname="1" colwidth="77PT" align="left" / > <colspec colname="2" colwidth="98PT" align="center" / > <row> <entry>< / entry> <entry>< / entry> < / row> <row> <entry>< / entry> <entry namest="OFFSET" nameend="2" align="center" rowsep="1">< / entry> < / row> <row> <entry>< / entry> <entry>LixNi1-yMnyF2< / entry> <entry>(1)< / entry> < / row> <row> <entry>< / entry> <entry>LixNi1-yMnyS2< / entry> <entry>(2)< / entry> < / row> <row> <entry>< / entry> <entry>LixNi1-y-zMnyMzO2-aFa< / entry> <entry>(3)< / entry> < / row> <row> <entry>< / entry> <entry>LixNi1-y-zMnyMzO2-aSa< / entry> <entry>(4)< / entry> < / row> <row> <entry>< / entry> <entry namest="OFFSET" nameend="2" align="center" rowsep="1">< / entry> < / row> < / tgroup> < / table-cwu> (where M is selected from the group consisting of Co, Mg, Fe, Sr, Ti, B, Si, Ga, Al, Sc, Y, La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Ac, Th, Pa, U, Np, IPu, Am, Cm, Bk, Cf, Es, Fm, Md, No and Lr, 0.95<=x<=1.1, 0<=y<=0.99, 0<=,z<=0.5, and 0<=a<=0.5)
Owner:SAMSUNG SDI CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com