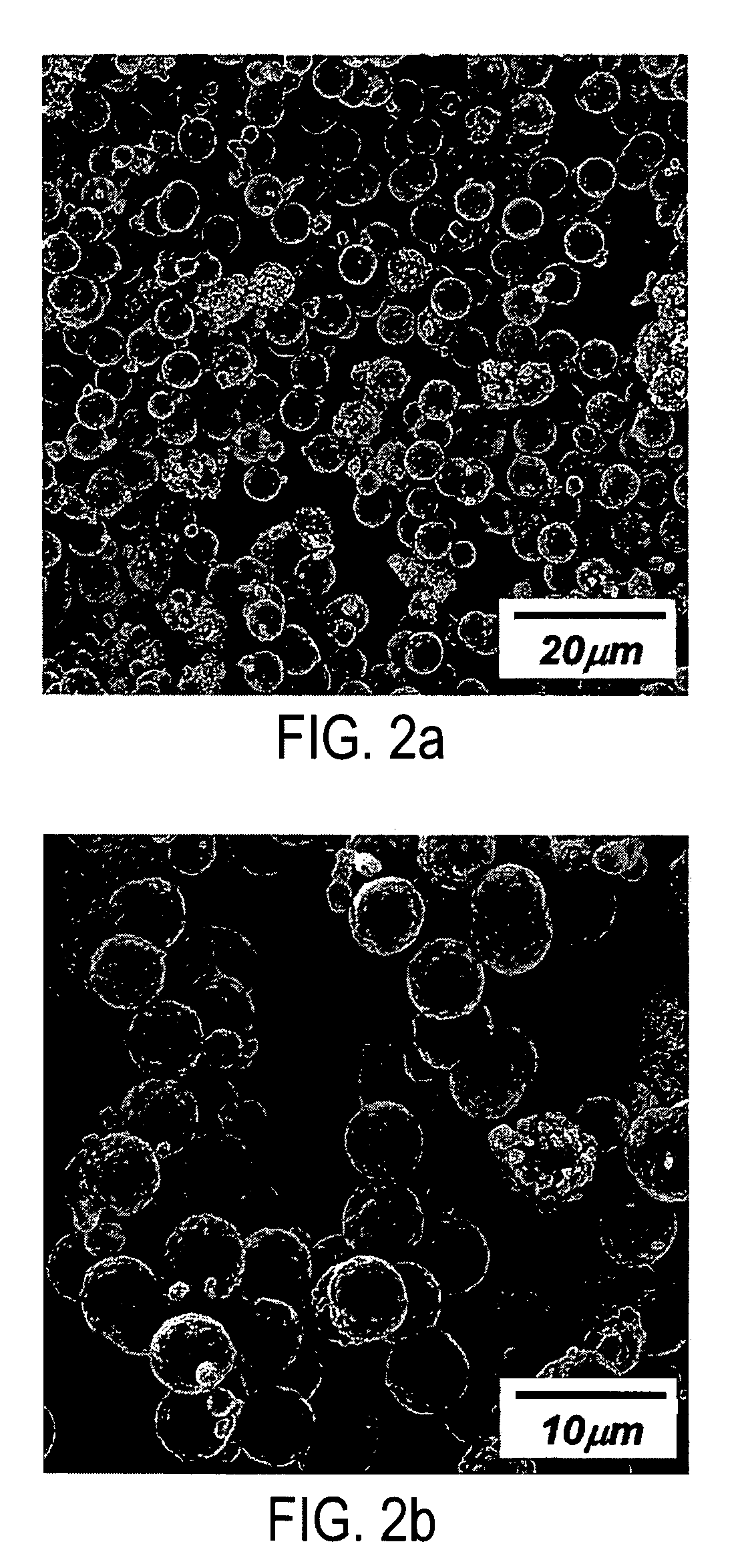
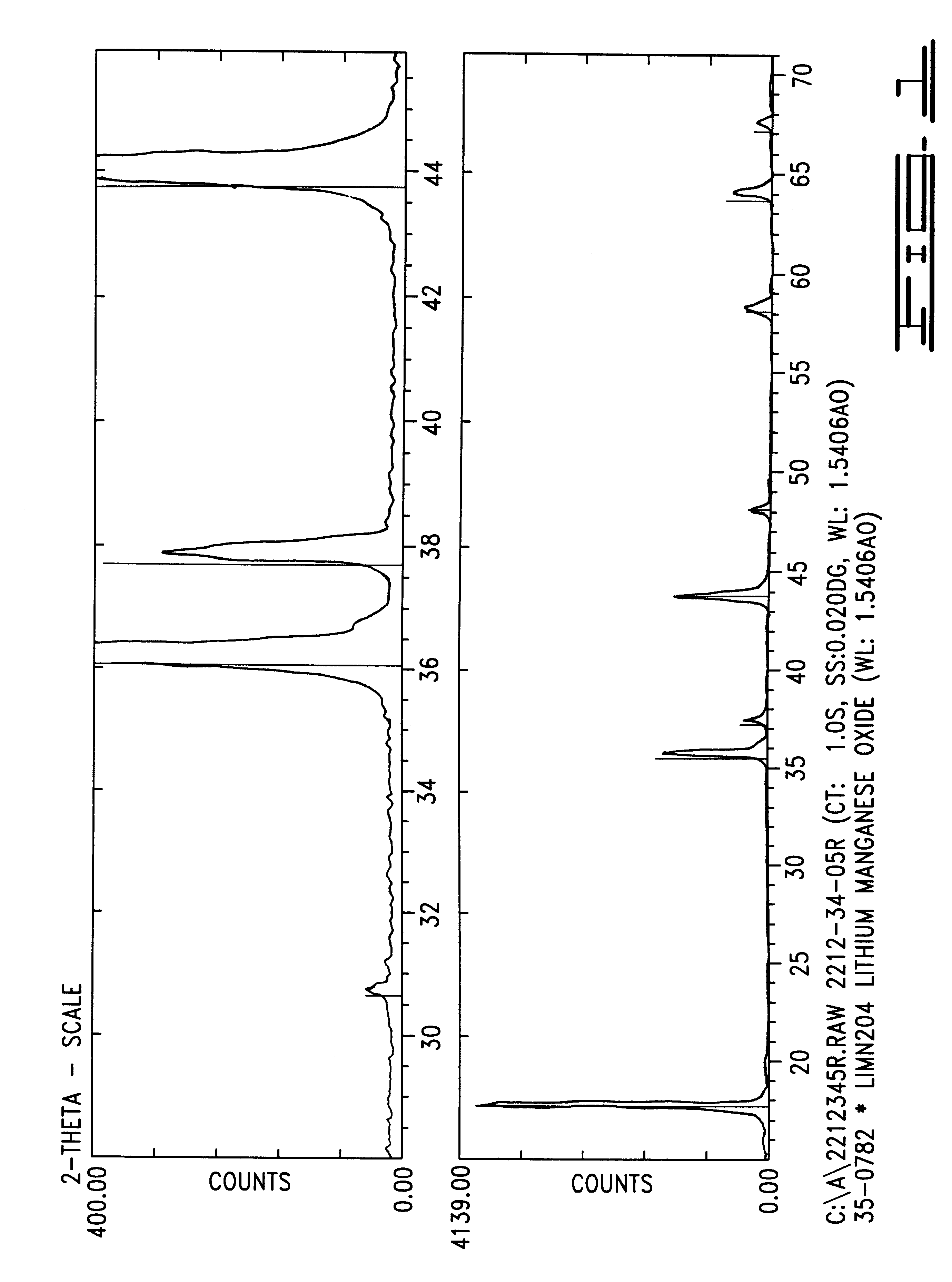
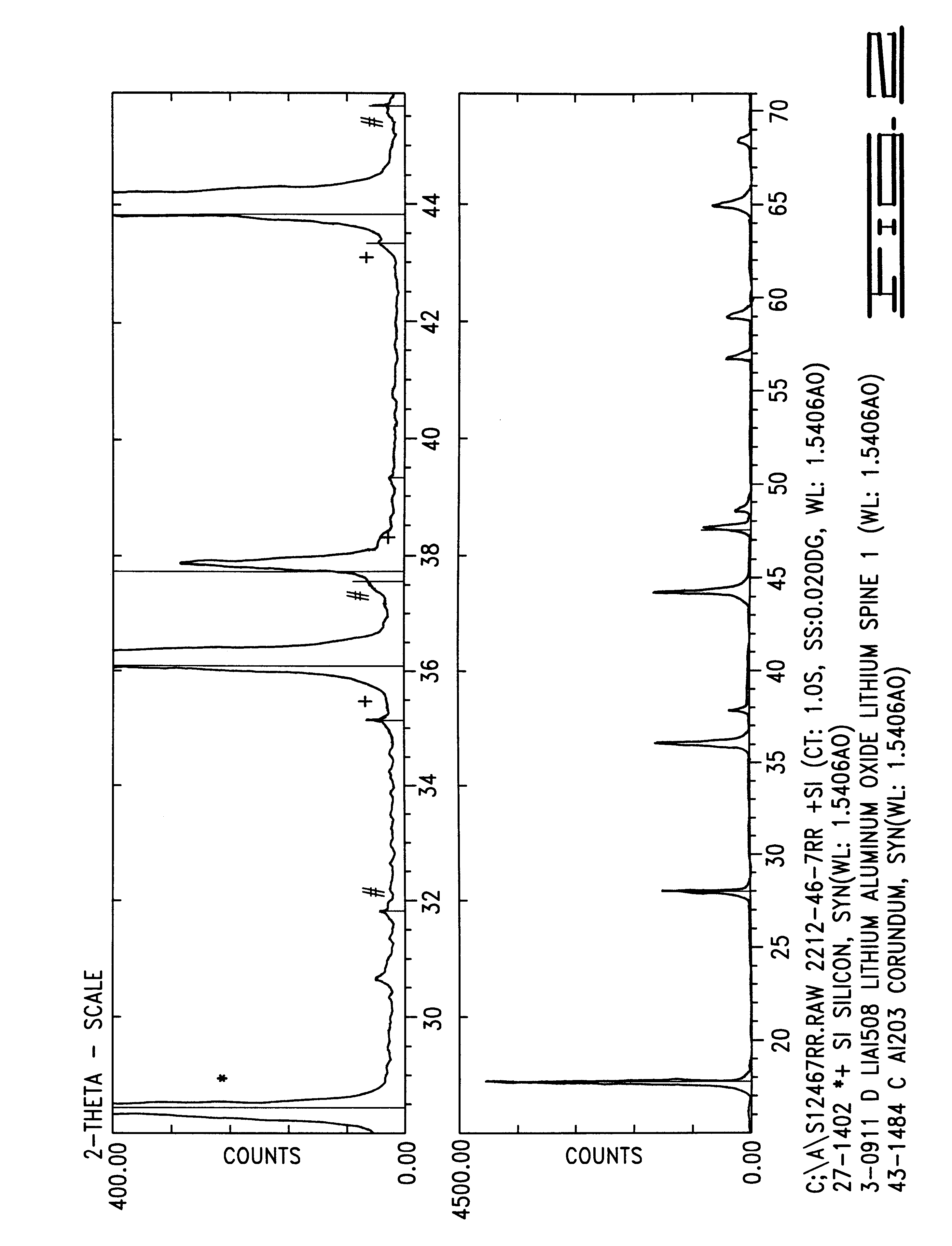
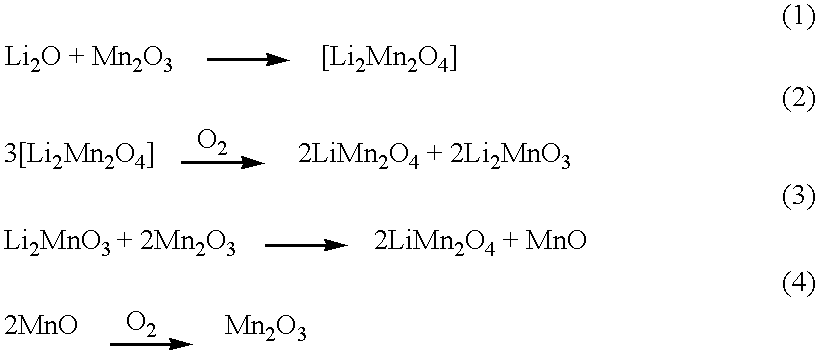Patents
Literature
1533results about "Manganese compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Metal oxide coated positive electrode materials for lithium-based batteries
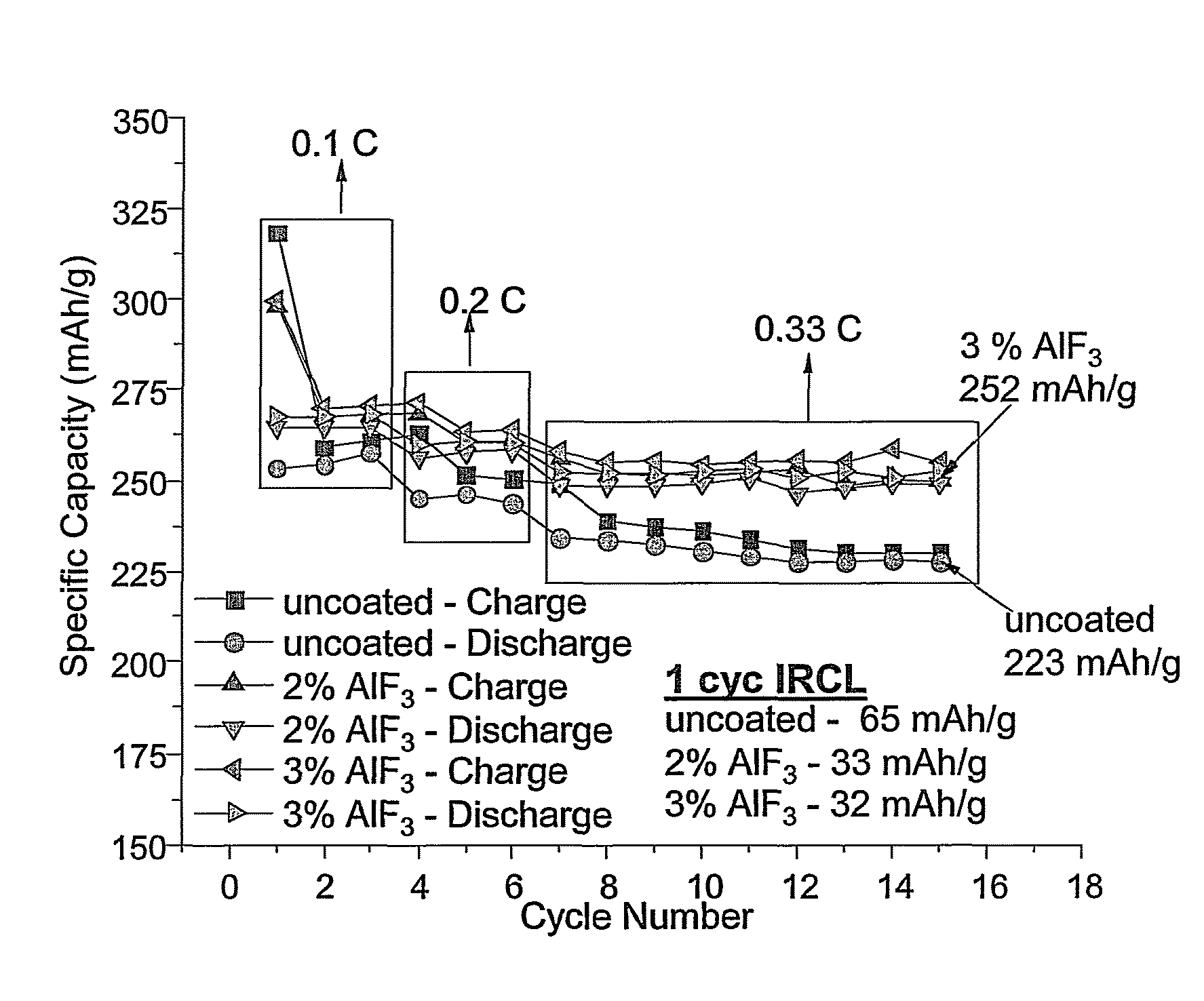
Positive electrode active materials are formed with various metal oxide coatings. Excellent results have been obtained with the coatings on lithium rich metal oxide active materials. Surprisingly improved results are obtained with metal oxide coatings with lower amounts of coating material. High specific capacity results are obtained even at higher discharge rates.
Owner:IONBLOX INC
Extraction process for removal of impurities from an oxidizer purge stream in the synthesis of carboxylic acid
InactiveUS20050038288A1Easy to operateImprove reliabilityOrganic compound preparationOrganic chemistry methodsSingle stageMetal catalyst
Disclosed is a process that relates to the recovery of a metal catalyst from an oxidizer purge stream produced in the synthesis of carboxylic acid, typically terephthalic acid. The process involves the addition of a wash solution to a high temperature molten dispersion to recover the metal catalyst and then subjecting an aqueous mixture or purified aqueous mixture so formed to a single stage extraction to remove organic impurities to produce an extract stream and a raffinate stream comprising the metal catalyst.
Owner:GRUPO PETROTEMEX DE C V
Positive electrode materials for lithium ion batteries having a high specific discharge capacity and processes for the synthesis of these materials
ActiveUS20100086853A1Electrode manufacturing processesAlkali metal oxidesDischarge rateLithium-ion battery
Owner:IONBLOX INC
Layer-layer lithium rich complex metal oxides with high specific capacity and excellent cycling
Lithium rich and manganese rich lithium metal oxides are described that provide for excellent performance in lithium-based batteries. The specific compositions can be engineered within a specified range of compositions to provide desired performance characteristics. Selected compositions can provide high values of specific capacity with a reasonably high average voltage. Compositions of particular interest can be represented by the formula, xLi2MnO3.(1−x)LiNiu+ΔMnu−ΔCowAyO2. The compositions undergo significant first cycle irreversible changes, but the compositions cycle stably after the first cycle.
Owner:IONBLOX INC
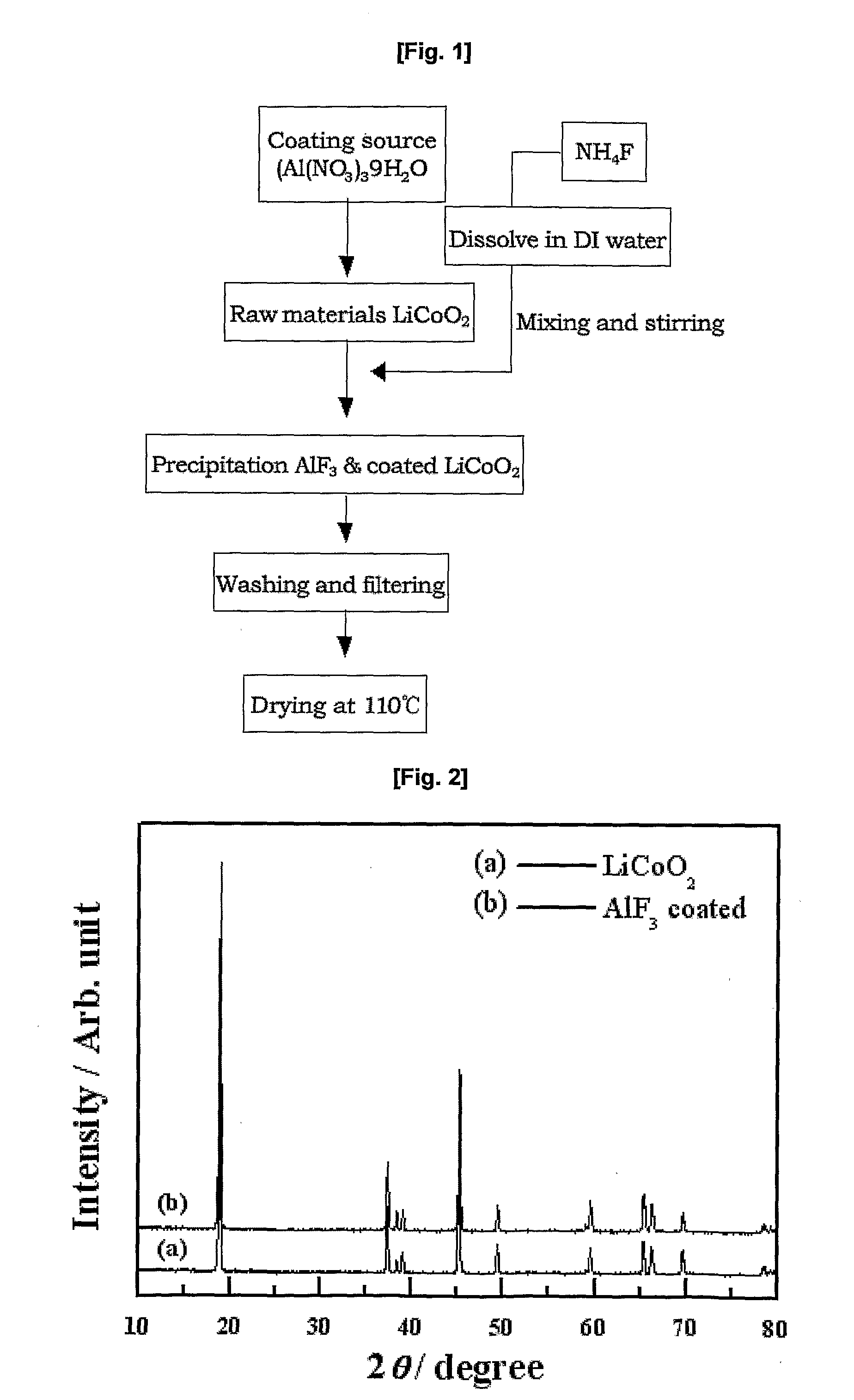
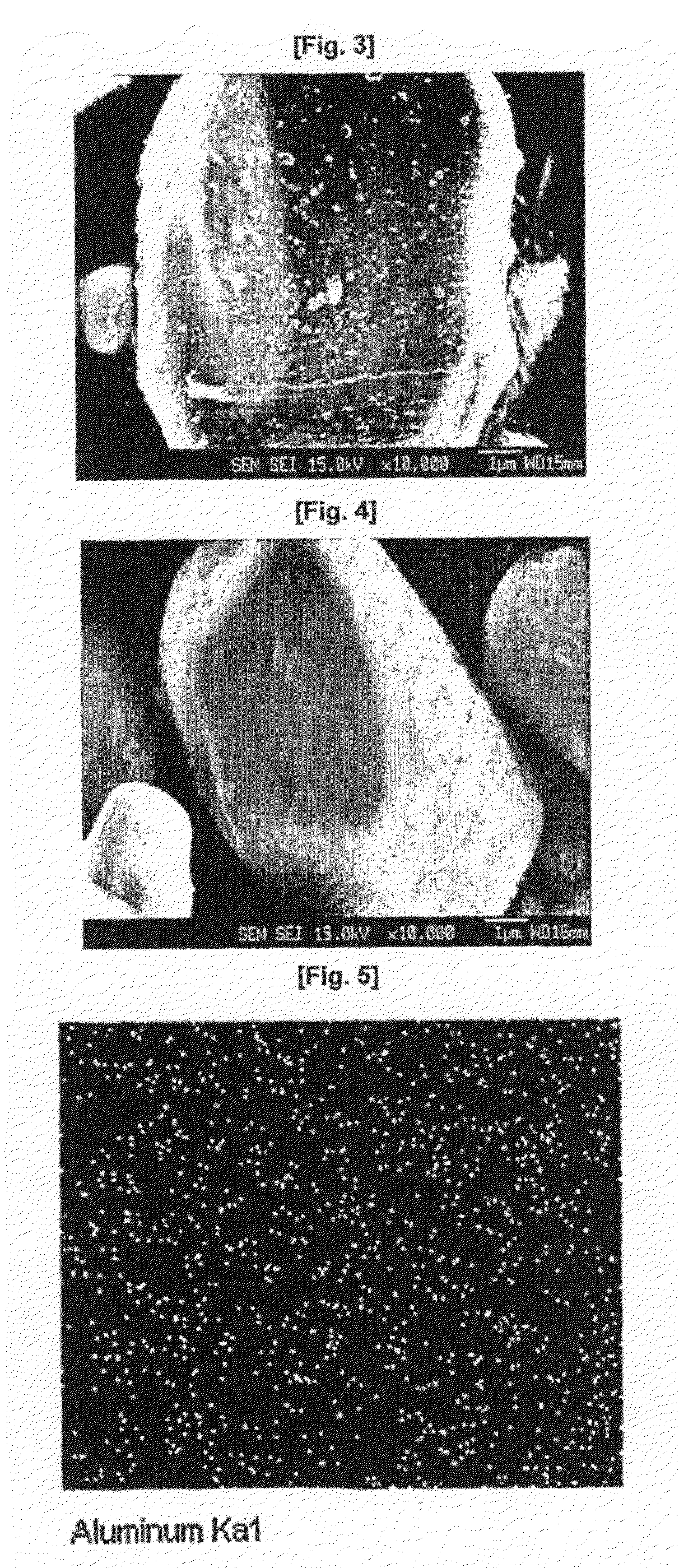
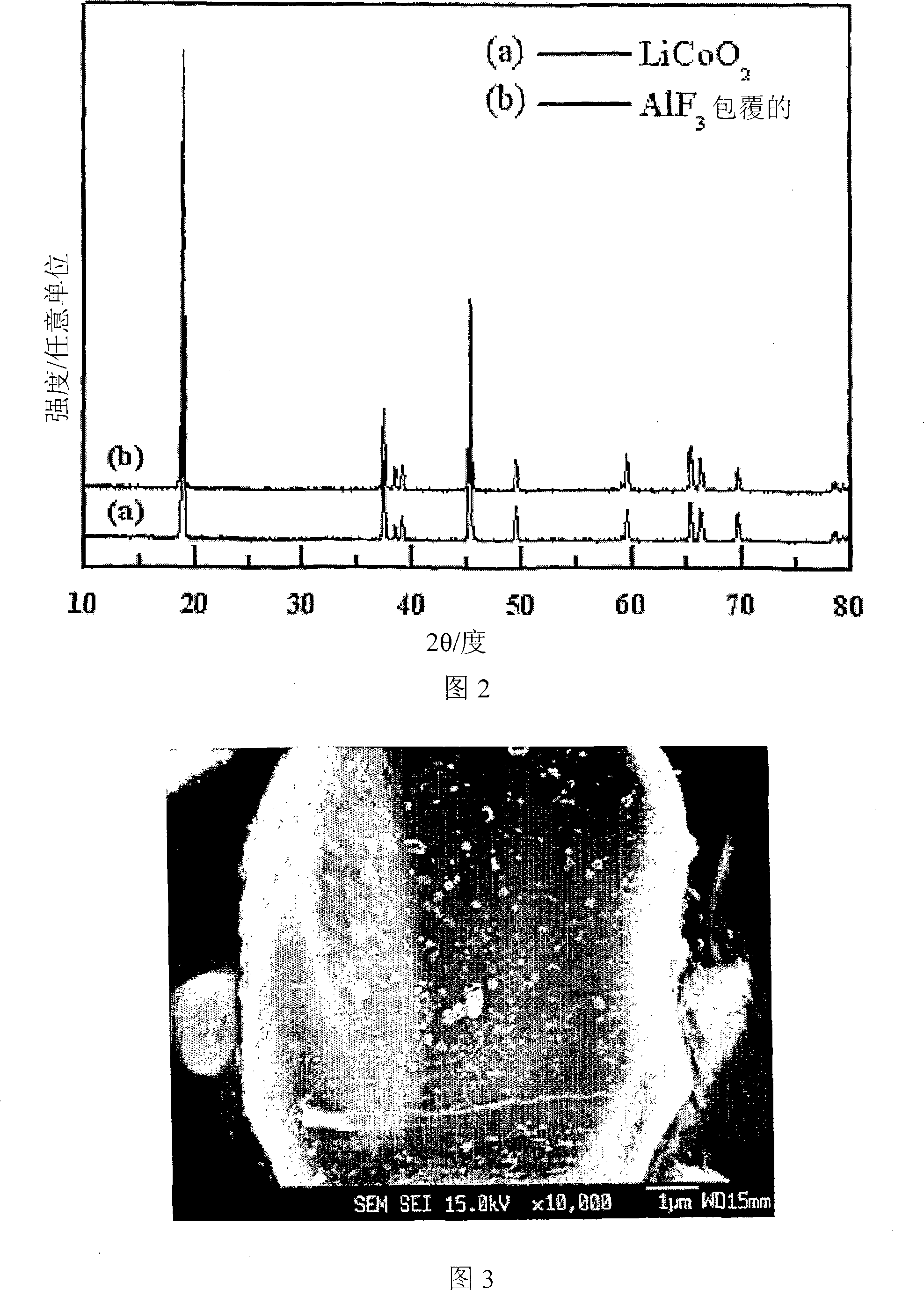
Cathode Active Material Coated With Fluorine Compound for Lithium Secondary Batteries and Method for Preparing the Same
InactiveUS20090087362A1Inhibition of performance deteriorationHigh voltageElectrode manufacturing processesLithium compoundsLithiumHigh rate
Disclosed herein is a cathode active material coated with a fluorine compound for lithium secondary batteries. The cathode active material is structurally stable, and improves the charge-discharge characteristics, cycle characteristics, high-voltage characteristics, high-rate characteristics and thermal stability of batteries.
Owner:ENERCERAMIC
Cathode active material coated with fluorine compound for lithium secondary batteries and method for preparing the same
Disclosed herein is a cathode active material coated with a fluorine compound for lithium secondary batteries. The cathode active material is structurally stable, and improves the charge-discharge characteristics, cycle characteristics, high-voltage characteristics, high-rate characteristics and thermal stability of batteries.
Owner:ENERCERAMIC
Active material for positive electrode used in lithium secondary battery and method of manufacturing same
InactiveUS6372385B1Easy to implementEvenly dispersedElectrode thermal treatmentActive material electrodesLithium-ion batteryMetal
Disclosed is active material for a positive electrode used in lithium secondary batteries of Formula 1 below and a method manufacturing the same, a surface of the active material being coated with metal oxide. The method includes the steps of producing a crystalline powder or a semi-crystalline powder of Formula 1; coating the crystalline powder or the semi-crystalline powder with metal alkoxide sol; and heat-treating the powder coated with the metal alkoxide sol.where 0<x<=0.3, 0<=y<=0.01, andA is an element selected from the group consisting of Ni, Co and Mn; B is an element selected from the group consisting of Ni, Co, Mn, B, Mg, Ca, Sr, Ba, Ti, V, Cr, Fe, Cu and Al; and C is an element selected from the group consisting of Ni, Co, Mn, B, Mg, Ca, Sr, Ba, Ti, V, Cr, Fe, Cu and Al.
Owner:SAMSUNG ELECTRONICS DEVICES CO LTD
Polymer electrolyte, intercalation compounds and electrodes for batteries
Solid battery components are provided. A block copolymeric electrolyte is non-crosslinked and non-glassy through the entire range of typical battery service temperatures, that is, through the entire range of at least from about 0° C. to about 70° C. The chains of which the copolymer is made each include at least one ionically-conductive block and at least one second block immiscible with the ionically-conductive block. The chains form an amorphous association and are arranged in an ordered nanostructure including a continuous matrix of amorphous ionically-conductive domains and amorphous second domains that are immiscible with the ionically-conductive domains. A compound is provided that has a formula of LixMyNzO2. M and N are each metal atoms or a main group elements, and x, y and z are each numbers from about 0 to about 1. y and z are chosen such that a formal charge on the MyNz portion of the compound is (4-x). In certain embodiments, these compounds are used in the cathodes of rechargeable batteries. The present invention also includes methods of predicting the potential utility of metal dichalgogenide compounds for use in lithium intercalation compounds. It also provides methods for processing lithium intercalation oxides with the structure and compositional homogeneity necessary to realize the increased formation energies of said compounds. An article is made of a dimensionally-stable, interpenetrating microstructure of a first phase including a first component and a second phase, immiscible with the first phase, including a second component. The first and second phases define interphase boundaries between them, and at least one particle is positioned between a first phase and a second phase at an interphase boundary. When the first and second phases are electronically-conductive and ionically-conductive polymers, respectively, and the particles are ion host particles, the arrangement is an electrode of a battery.
Owner:MASSACHUSETTS INST OF TECH
Anode active material, manufacturing method thereof, and non-aqueous electrolyte secondary battery
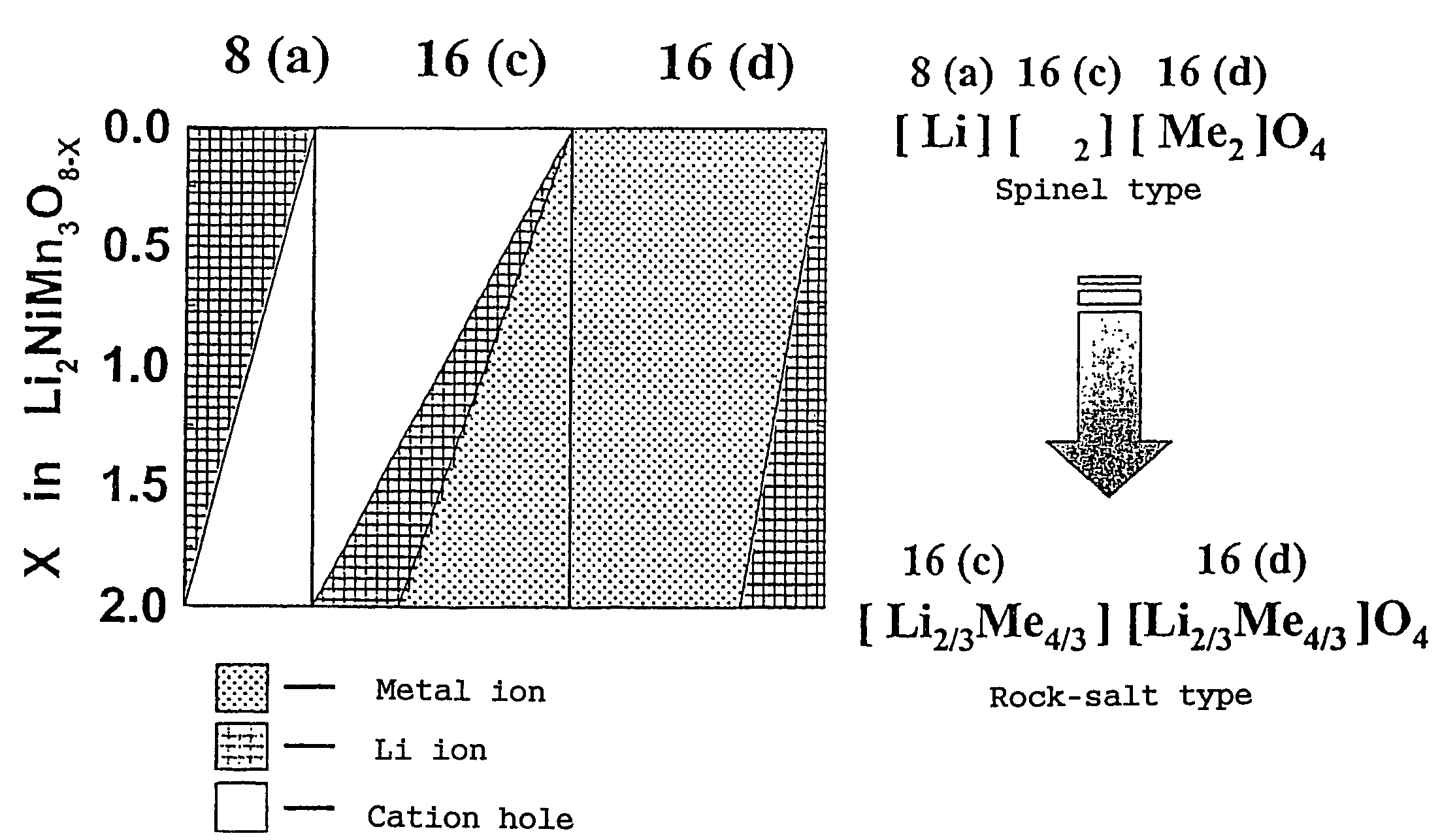
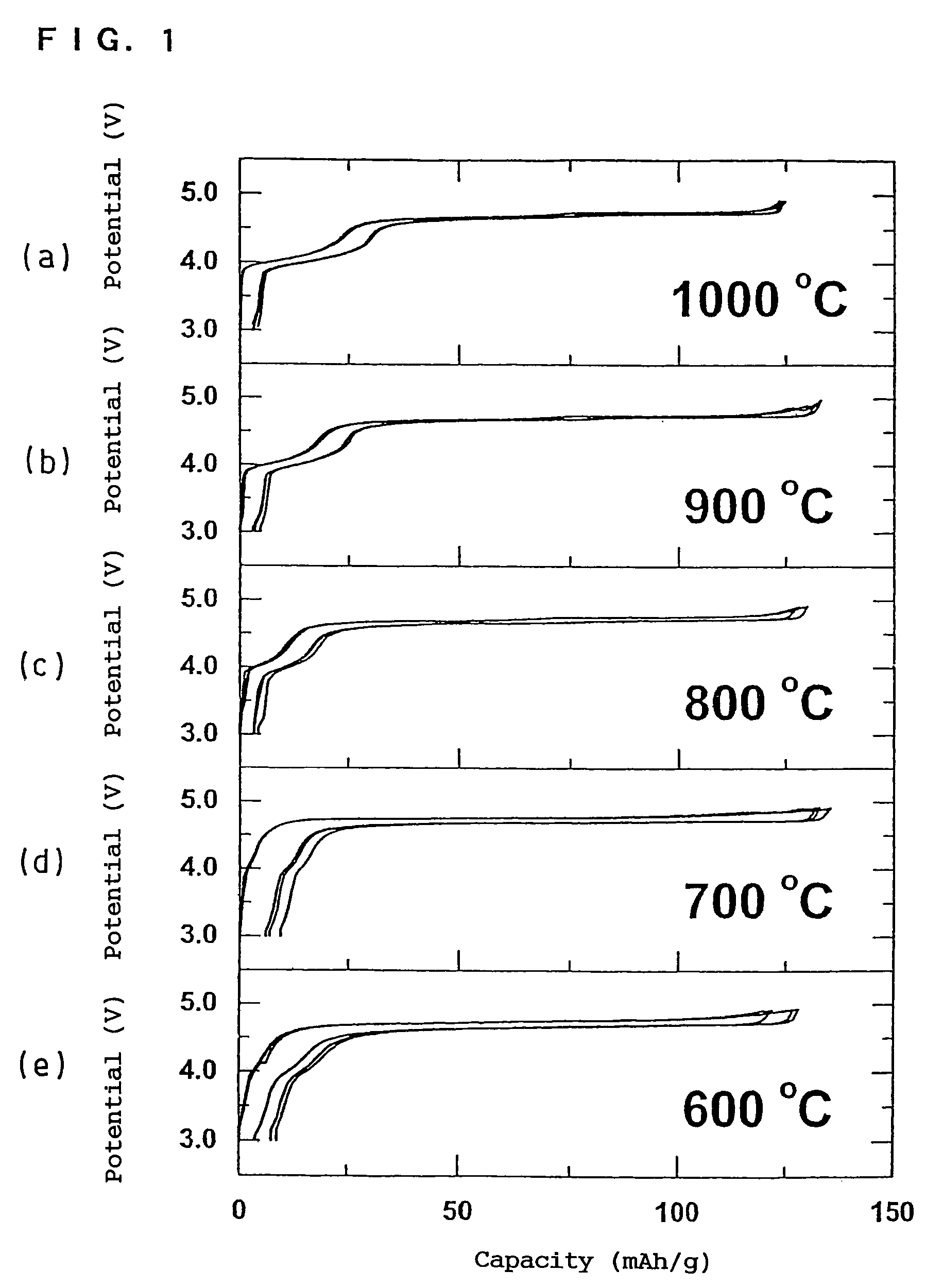
In order to provide a 3V level non-aqueous electrolyte secondary battery with a flat voltage and excellent cycle life at a high rate with low cost, the present invention provides a positive electrode represented by the formula: Li2±α[Me]4O8−x, wherein 0≦α<0.4, 0≦x<2, and Me is a transition metal containing Mn and at least one selected from the group consisting of Ni, Cr, Fe, Co and Cu, said active material exhibiting topotactic two-phase reactions during charge and discharge.
Owner:OSAKA CITY UNIV +1
Lithium ion battery positive pole material cobalt nickel oxide manganses lithium and method for making same
ActiveCN101202343AHigh specific capacityExcellent cycle characteristicsElectrode manufacturing processesLithium compoundsLithium oxideAntioxidant
The invention relates to a nickel cobalt manganese lithium oxide material used for an anode of a li-ion battery and a preparation method. The invention belongs to the li-ion battery technical field. The nickel cobalt manganese lithium oxide material used for the anode of the li-ion battery is a li-rich laminated structure with the chemical component of Li1+zM1-x-yNixCoyO2; wherein, z is less than or equal to 0.2 and more than or equal to 0.05, x is less than or equal to 0.8 and more than 0.1, and y is less than or equal to 0.5 and more than 0.1. The preparation method of the invention is that dissoluble salt of the nickel, cobalt and manganese is taken as the raw material; ammonia or ammonium salt is taken as complexing agent; sodium hydroxide is taken as precipitator; water-dissoluble dispersant and water-dissoluble antioxidant or inert gas are added for control and protection; in a cocurrent flow type the solution is added to a reaction vessel for reaction; after alkalescence disposal, aging procedure, solid-liquid separation and washing and drying, the nickel cobalt manganese oxide is uniformly mixed with the lithium raw material; the nickel cobalt manganese lithium oxide powder is obtained by sintering the mixed powder which is divided into three temperature areas. The invention has the advantages of high specific capacity, good circulation performance, ideal crystal texture, short production period, low power loss, and being suitable for industrial production, etc.
Owner:CHINA ELECTRONIC TECH GRP CORP NO 18 RES INST +1
Lithium metal oxide electrodes for lithium batteries
ActiveUS20050026040A1Increase capacityImprove cycle stabilityCellsElectrode thermal treatmentLithium metalOxidation state
An uncycled electrode for a non-aqueous lithium electrochemical cell including a lithium metal oxide having the formula Li(2+2x) / (2+x)M′2x / (2+x)M(2-2x) / (2+x)O2-δ, in which 0≦x<1 and δ is less than 0.2, and in which M is a non-lithium metal ion with an average trivalent oxidation state selected from two or more of the first row transition metals or lighter metal elements in the periodic table, and M′ is one or more ions with an average tetravalent oxidation state selected from the first and second row transition metal elements and Sn. Methods of preconditioning the electrodes are disclosed as are electrochemical cells and batteries containing the electrodes.
Owner:UCHICAGO ARGONNE LLC
Method and apparatus for preparation of spherical metal carbonates and lithium metal oxides for lithium rechargeable batteries
ActiveUS7435402B2Improve impedance characteristicsImproved stability of the layered oxide structureConductive materialOxide conductorsDopantLithium metal
A number of materials with the composition Li1+xNiαMnβCoγM′δO2−zFz (M′=Mg,Zn,Al,Ga,B,Zr,Ti) for use with rechargeable batteries, wherein x is between about 0 and 0.3, α is between about 0.2 and 0.6, β is between about 0.2 and 0.6, γ is between about 0 and 0.3, δ is between about 0 and 0.15, and z is between about 0 and 0.2. Adding the above metal and fluorine dopants affects capacity, impedance, and stability of the layered oxide structure during electrochemical cycling. Another aspect of the invention includes materials with the composition Li1+xNiαCoβMnγM′δOyFz (M′=Mg,Zn,Al,Ga,B,Zr,Ti), where the x is between 0 and 0.2, the α between 0 and 1, the β between 0 and 1, the γ between 0 and 2, the δ between about 0 and about 0.2, the y is between 2 and 4, and the z is between 0 and 0.5.
Owner:UCHICAGO ARGONNE LLC
Cathode intercalation compositions, production methods and rechargeable lithium batteries containing the same
InactiveUS6248477B1Easy to adaptOxygen/ozone/oxide/hydroxideElectrode thermal treatmentMetalSpinel group
Intercalation compositions having spinel structures with crystallites of metal oxides (M2O3) dispersed throughout the structure are provided having the general formula Li1+xMyMn2-x-yO4. Methods of producing the intercalation compositions and rechargeable lithium batteries containing the compositions are also provided.
Owner:EMD ACQUISITION LLC
Positive electrode materials for lithium ion batteries having a high specific discharge capacity and processes for the synthesis of these materials
ActiveUS8389160B2Electrode manufacturing processesAlkali metal oxidesElectrical batteryDischarge rate
Positive electrode active materials are described that have a very high specific discharge capacity upon cycling at room temperature and at a moderate discharge rate. Some materials of interest have the formula Li1+xNiαMnβCOγO2, where x ranges from about 0.05 to about 0.25, α ranges from about 0.1 to about 0.4, β ranges from about 0.4 to about 0.65, and γ ranges from about 0.05 to about 0.3. The materials can be coated with a metal fluoride to improve the performance of the materials especially upon cycling. Also, the coated materials can exhibit a very significant decrease in the irreversible capacity lose upon the first charge and discharge of the cell. Methods for producing these materials include, for example, a co-precipitation approach involving metal hydroxides and sol-gel approaches.
Owner:IONBLOX INC
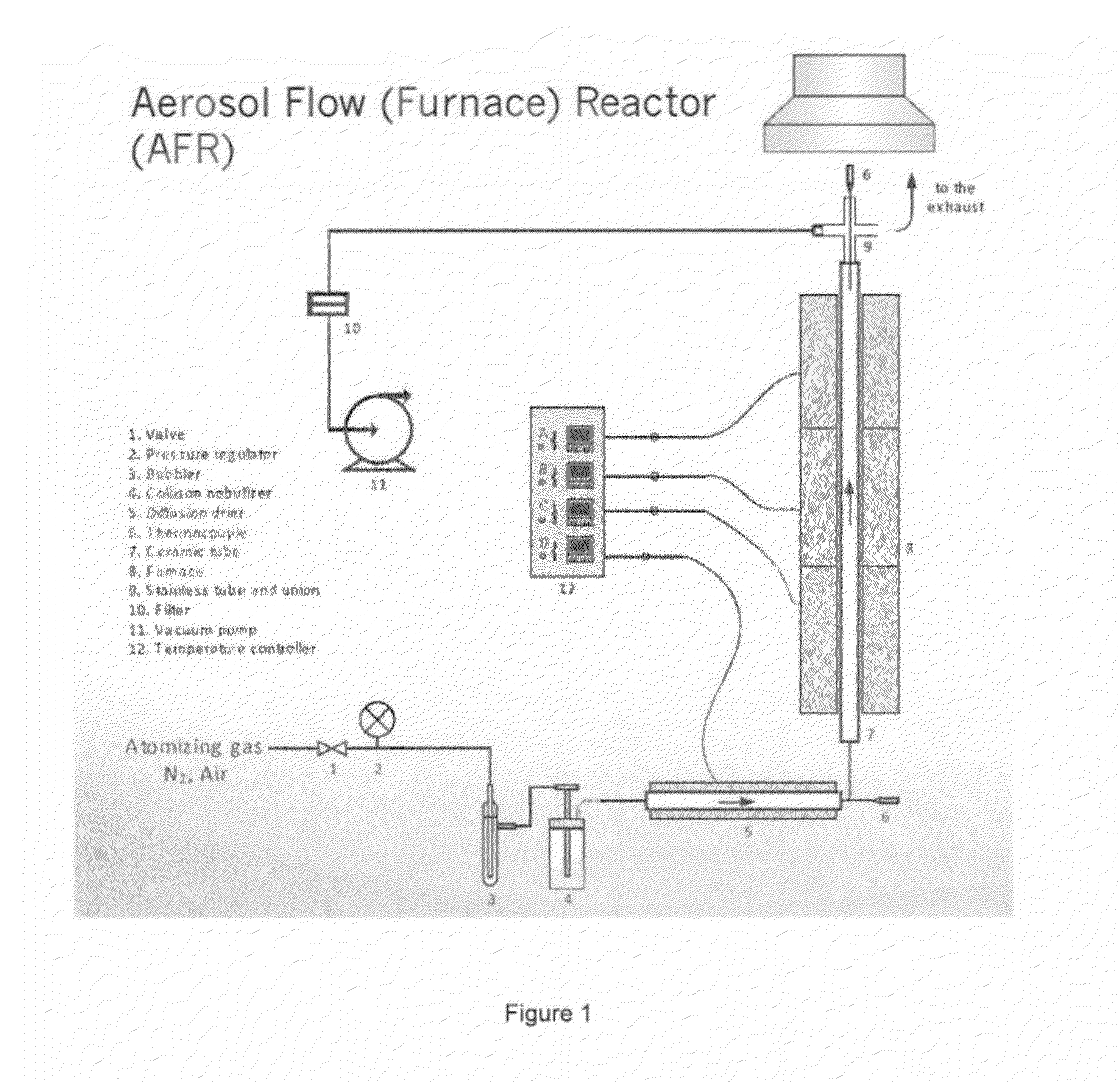
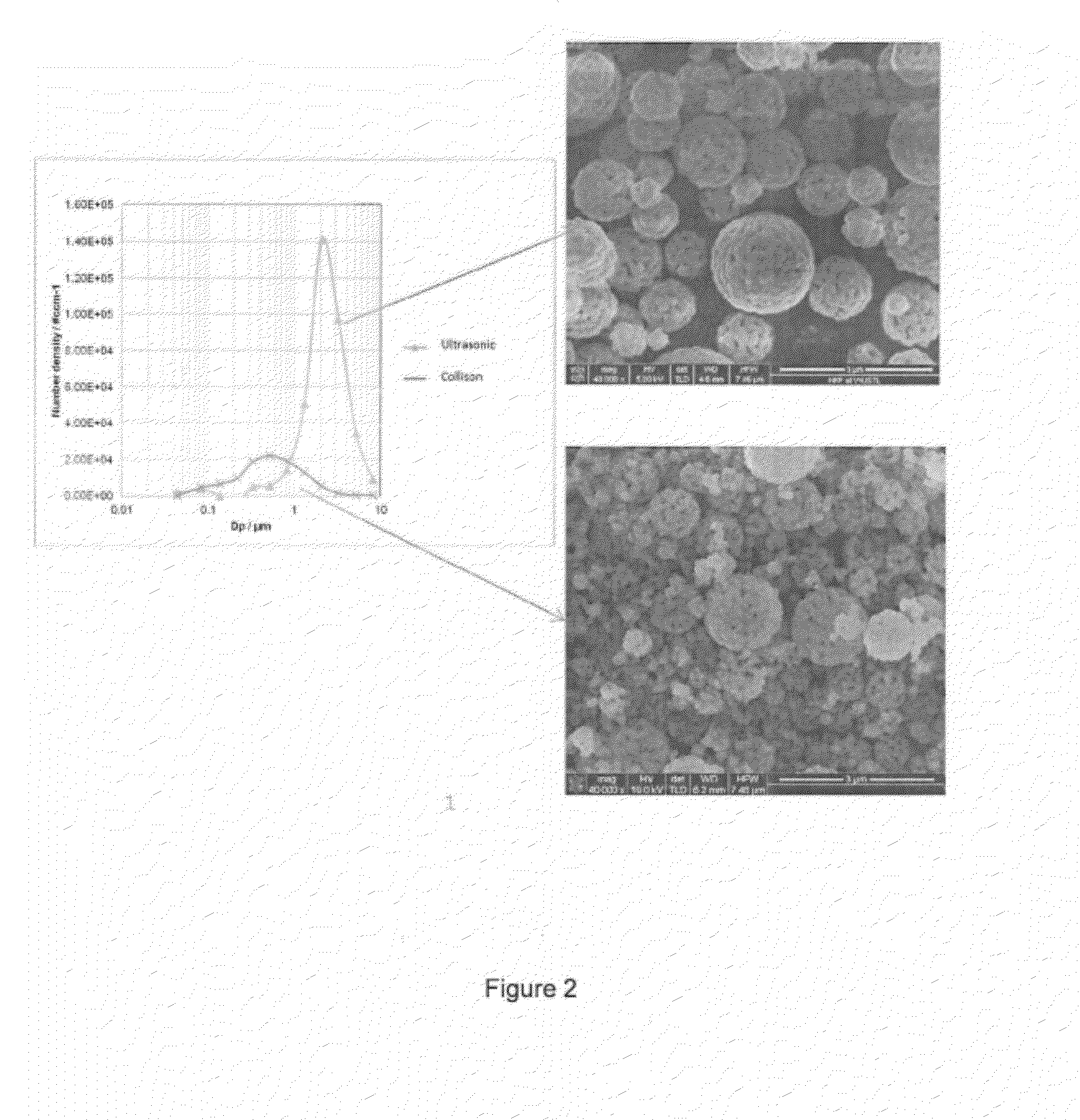
Spray Pyrolysis Synthesis of Mesoporous Positive Electrode Materials for High Energy Lithium-Ion Batteries
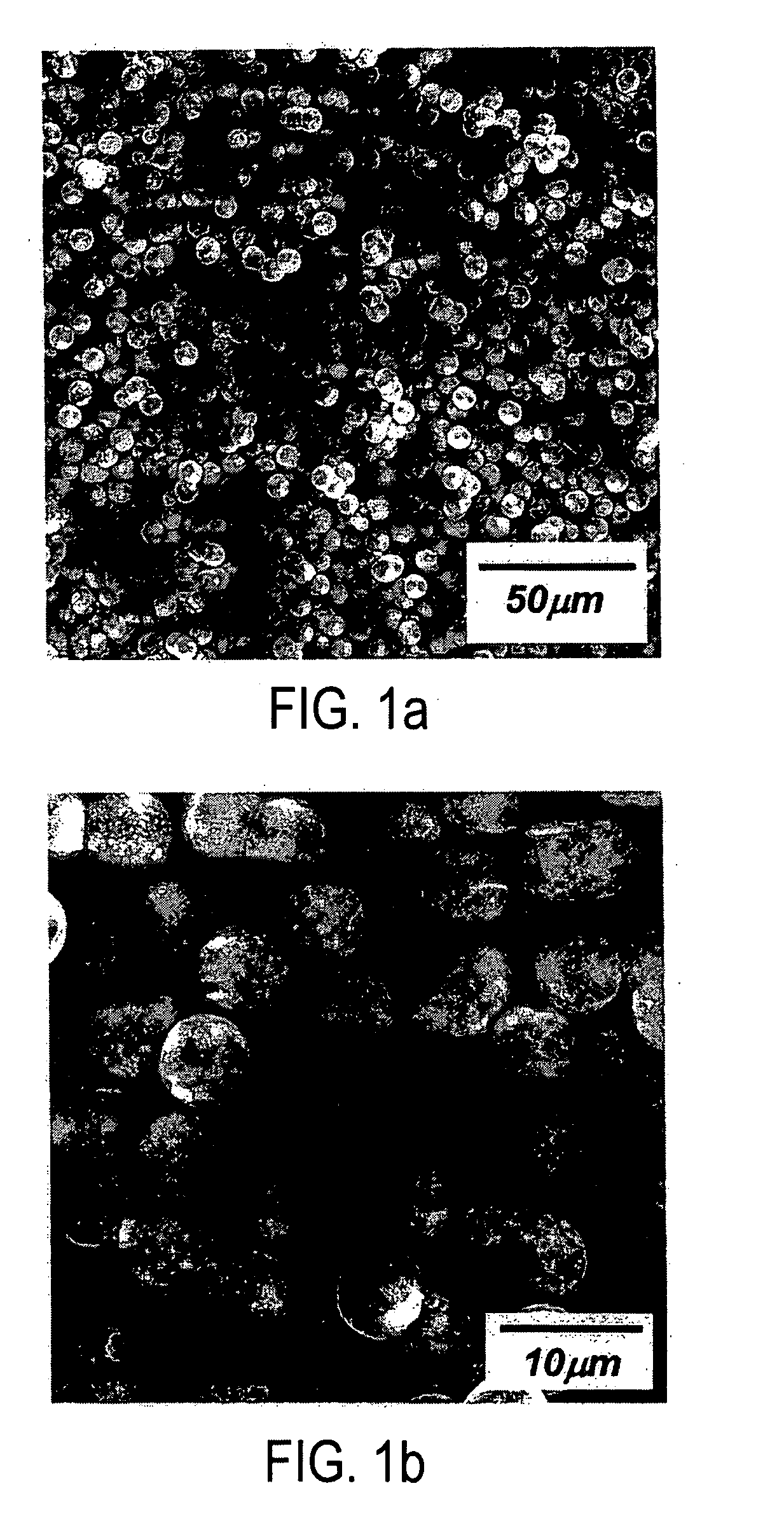
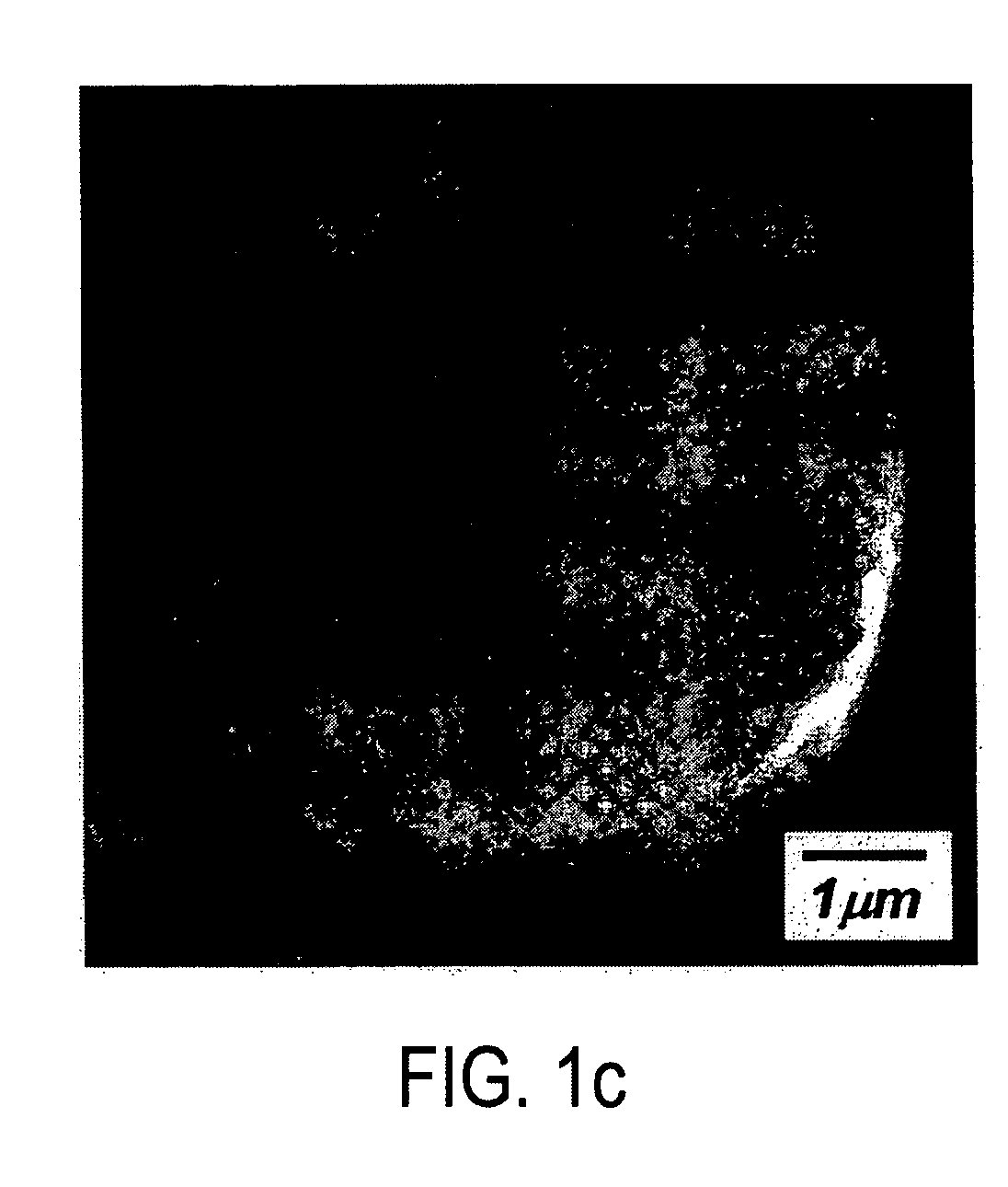
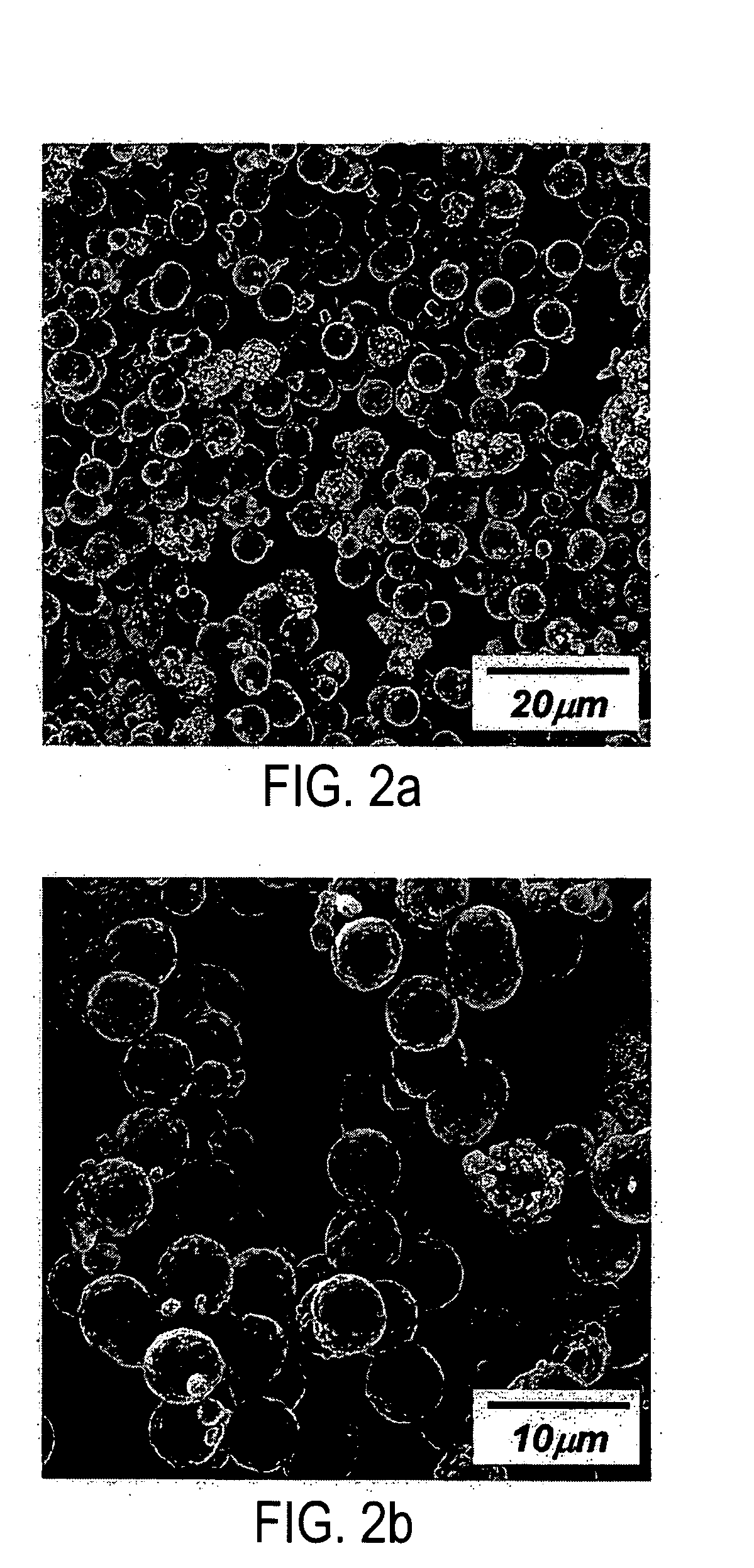
A lithium metal oxide positive electrode material useful in making lithium-ion batteries that is produced using spray pyrolysis. The material comprises a plurality of metal oxide secondary particles that comprise metal oxide primary particles, wherein the primary particles have a size that is in the range of about 1 nm to about 10 μm, and the secondary particles have a size that is in the range of about 10 nm to about 100 μm and are uniformly mesoporous.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Lithium Ion Batteries with Supplemental Lithium
Supplemental lithium can be used to stabilize lithium ion batteries with lithium rich metal oxides as the positive electrode active material. Dramatic improvements in the specific capacity at long cycling have been obtained. The supplemental lithium can be provided with the negative electrode, or alternatively as a sacrificial material that is subsequently driven into the negative electrode active material. The supplemental lithium can be provided to the negative electrode active material prior to assembly of the battery using electrochemical deposition. The positive electrode active materials can comprise a layered-layered structure comprising manganese as well as nickel and / or cobalt.
Owner:IONBLOX INC
Process for preparing high density spherical nickel-cobalt lithium manganate as anode material of lithium ion cell
InactiveCN1622371AWell mixedImprove performanceElectrode manufacturing processesLithium compoundsNickel saltManganate
The present invention relates to energy source material technology, and is preparation process of high density spherical lithium nickel-cobalt-manganate as positive electrode material for lithium ion cell. The preparation process includes the reaction of nickel salt, cobalt salt, manganese salt, ammonium hydroxide and ammonian in water solution to synthesize spherical or spheroid precursor Ni1 / 3Co1 / 3Mn1 / 3 (OTHER)2, washing, drying and mixing with lithium carbonate; and high temperature treatment in the air at 750-950 deg.c for 8-48 hr to obtain spherical lithium nickel-cobalt-manganate. The spherical lithium nickel-cobalt-manganate has great bulk density reaching 2.25-2.50 g / cu cm after vibration densifying, average grain size of 3-7 microns, and reversible specific capacity up to 172-185 mA.hr / g.
Owner:TSINGHUA UNIV
Metal oxide coated positive electrode materials for lithium-based batteries
Positive electrode active materials are formed with various metal oxide coatings. Excellent results have been obtained with the coatings on lithium rich metal oxide active materials. Surprisingly improved results are obtained with metal oxide coatings with lower amounts of coating material. High specific capacity results are obtained even at higher discharge rates.
Owner:IONBLOX INC
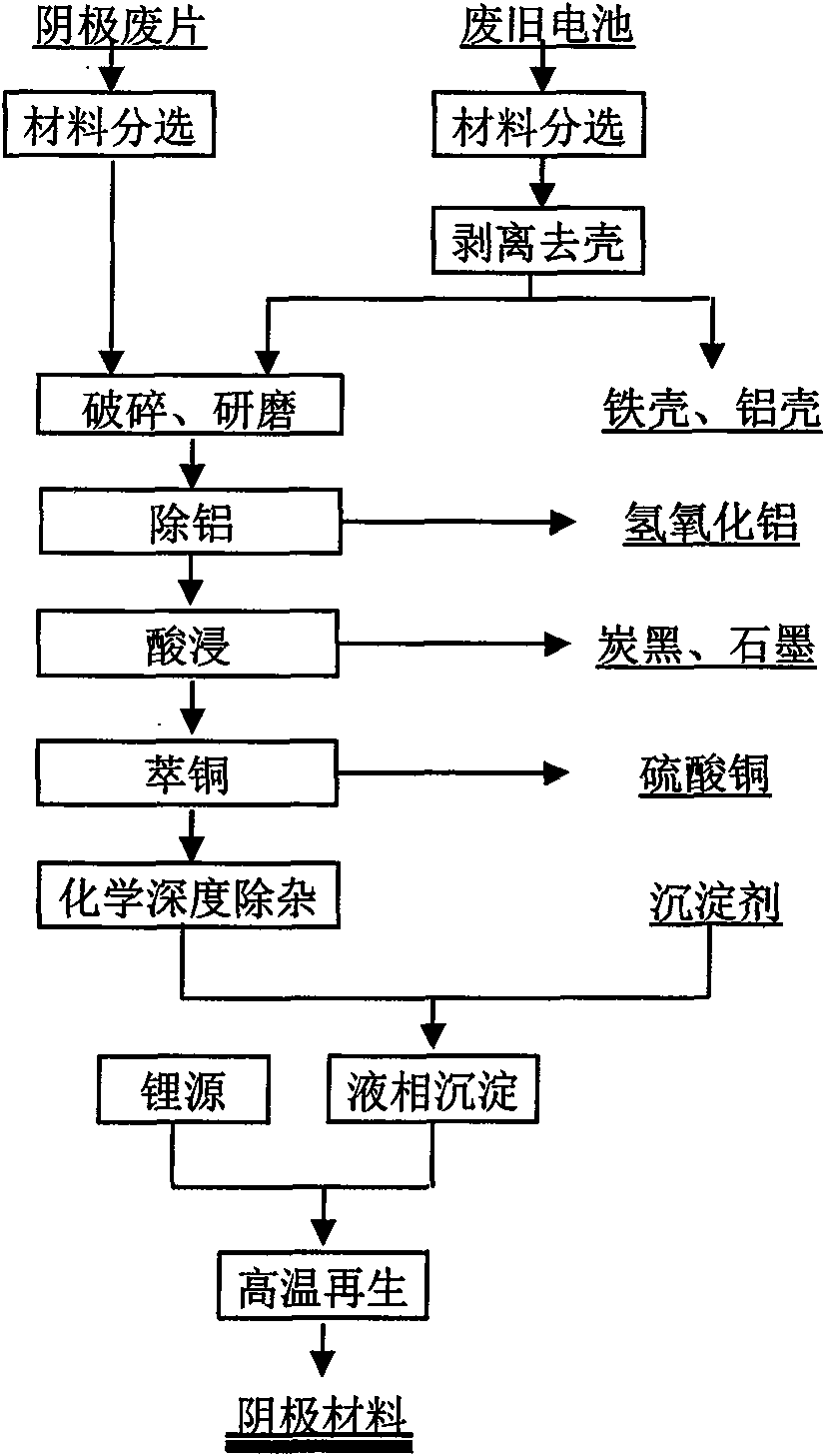
Method for recovering and recycling waste lithium ion battery cathode material
InactiveCN101555030AAchieve recyclingAchieve regenerationCobalt compoundsNickel compoundsEngineeringLithium-ion battery
The invention discloses a method for recovering and recycling waste lithium ion battery cathode material, belonging to the fields of lithium ion battery material preparation and recycling use of waste resources. The method has the technical proposal with the key points as follows: the recovered waste batteries are sorted, cathode waste material (after being sorted) which is stripped to remove the shell and generated in the production process of the lithium ion battery is directly crushed and ground, and then processed by the procedures such as aluminum removing, acid dipping, copper extracting, chemical purification and the like under different conditions, so that byproduct materials such as aluminum hydroxide, carbon black, graphite, copper sulphate and the like are generated; reaction products are added with precipitator with certain concentration for liquid-phase precipitation and then added with lithium source, so that cathode material can be generated in the high temperature environment. The technical proposal successfully realizes the valuable constituent recovery and cathode material regeneration of the waste lithium ion battery cathode material.
Owner:GUANGDONG BRUNP RECYCLING TECH
Active material for non-aqueous electrolyte secondary battery and manufacturing method therefore
ActiveUS20080268347A1Increase capacityReduces electron conductivity and lithium diffusing abilityElectrode manufacturing processesNon-aqueous electrolyte accumulatorsDesorptionX-ray
An active material for a non-aqueous electrolyte secondary battery including a lithium-containing transition metal oxide containing nickel and manganese and having a closest-packed structure of oxygen, wherein an atomic ratio MLi / MT between the number of moles of lithium MLi and the number of moles of transition metal Mt contained in the lithium-containing transition metal oxide is greater than 1.0; the lithium-containing transition metal oxide has a crystal structure attributed to a hexagonal system, and the X-ray diffraction image of the crystal structure has a peak P003 attributed to the (003) plane and a peak P104 attributed to the (104) plane; an integrated intensity ratio I003 / I104 between the peak P003 and the peak P104 varies reversibly within a range from 0.7 to 1.5 in association with absorption and desorption of lithium by the lithium-containing transition metal oxide; and the integrated intensity ratio varies linearly and continuously.
Owner:PUBLIC UNIVERSITY CORPORATION OSAKA CITY UNIVERSITY +1
Core-shell composite anode material for lithium ion battery and preparation method thereof
ActiveCN101740752AImprove conductivityHigh specific capacitySecondary cellsIron compoundsLithium iron phosphateElectrical battery
The invention discloses a core-shell composite anode material for a lithium ion battery. The composite anode material has a core-shell structure; the core-shell structure consists of a core-layer active material and a shell-layer active material; the core-layer active material is LiFePO4 or lithium manganate; the shell-layer active material is carbon-containing LiFePO4; the LiFePO4 has an Li1-XMXFePO4 or LiFe1-yMyPO4 structure; the lithium manganate has a LiMnO2 or LiMn2O4 structure; the carbon is selected from one or more of carbon nano tubes, superfine conductive black and an agraphitic carbon material; and the composite anode material comprises 65 to 99 mass percent of core-layer active material and 1 to 35 mass percent of shell-layer active material. The composite anode material of the invention has stable performance and excellent electrochemical properties; and the lithium ion battery manufactured by using the material has relatively high charge-discharge capacity and excellent cycle performance, can perform quick charging and large multiplying-factor discharging, can adapt to an ultra-low temperature working environment and is safe and stable.
Owner:SHENZHEN DYNANONIC
Lithium doped cathode material
Lithium dopant is introduced into lithium rich high capacity positive electrode active materials as a substitution for manganese within the complex metal oxides. In some embodiments, the lithium doped compositions can be written in a two component notation as x.Li2MnO3.(1−x)LiNiu+ΔMnu−Δ−dLidCowO2, where d ranges from about 0.004 to about 0.25 and 2u+w is approximately equal to 1. The materials are believed to form a layer-layer composite crystal structure that has very good cycling properties at high voltages, although the materials exhibit significant first cycle irreversible capacity loss.
Owner:IONBLOX INC
Lithium ion batteries with supplemental lithium
ActiveUS20120105007A1Batteries circuit arrangementsElectrode carriers/collectorsManganeseElectrochemistry
Supplemental lithium can be used to stabilize lithium ion batteries with lithium rich metal oxides as the positive electrode active material. Dramatic improvements in the specific capacity at long cycling have been obtained. The supplemental lithium can be provided with the negative electrode, or alternatively as a sacrificial material that is subsequently driven into the negative electrode active material. The supplemental lithium can be provided to the negative electrode active material prior to assembly of the battery using electrochemical deposition. The positive electrode active materials can comprise a layered-layered structure comprising manganese as well as nickel and / or cobalt.
Owner:ZENLABS ENERGY INC
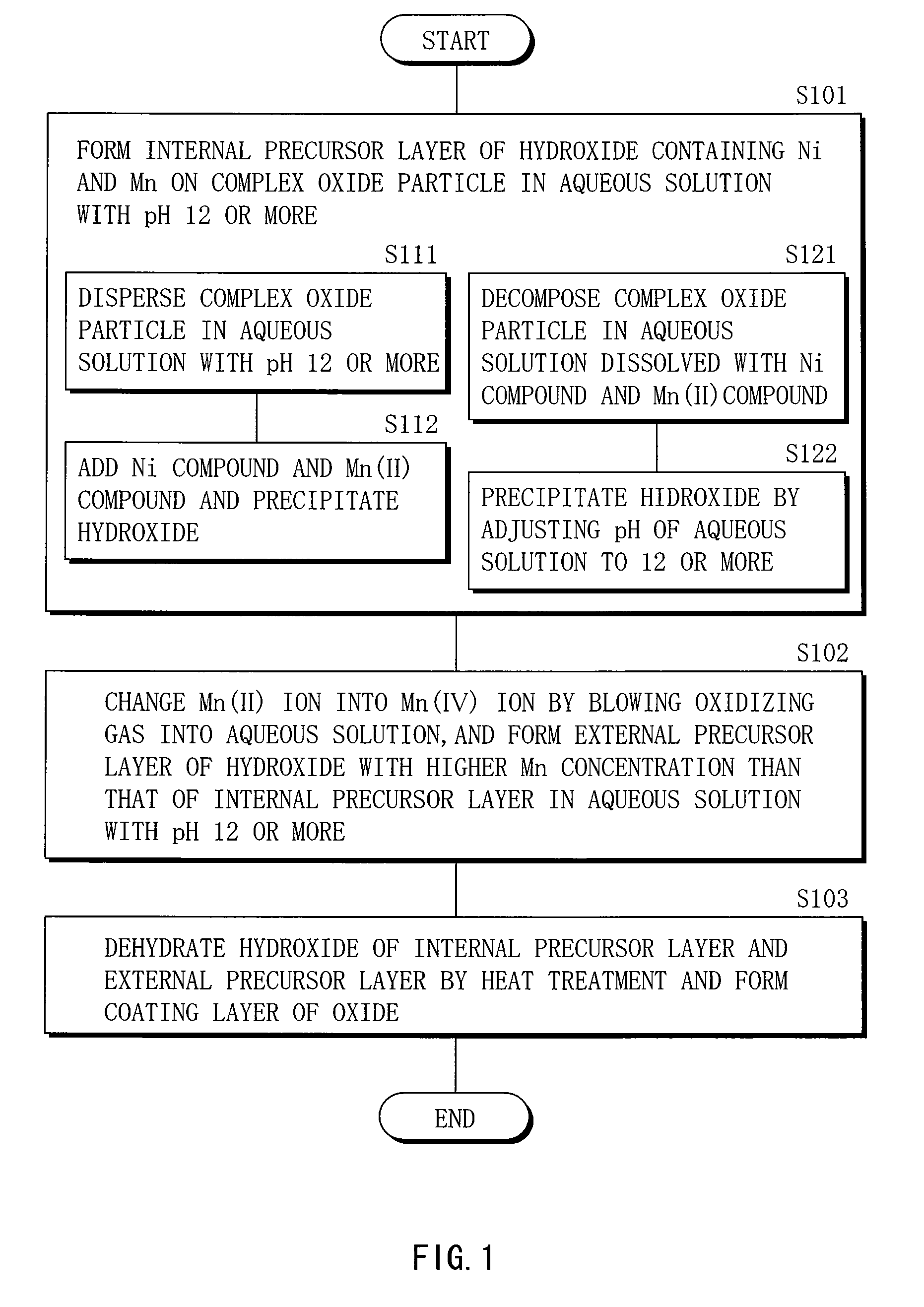
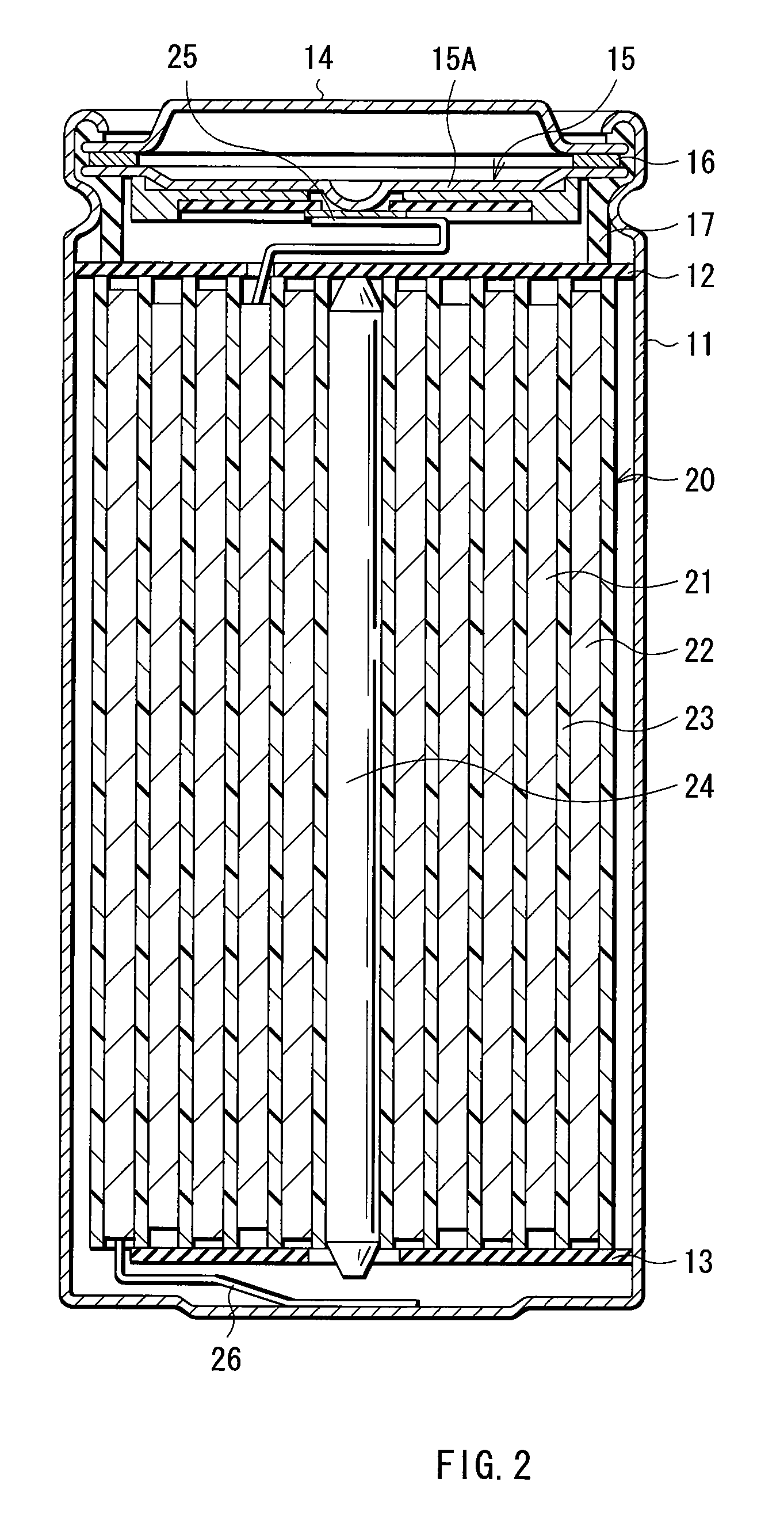
Cathode active material, method of manufacturing it, cathode, and battery
ActiveUS20060275667A1Large capacityGood chemical stabilityElectrode manufacturing processesNon-aqueous electrolyte accumulatorsLithiumManganese
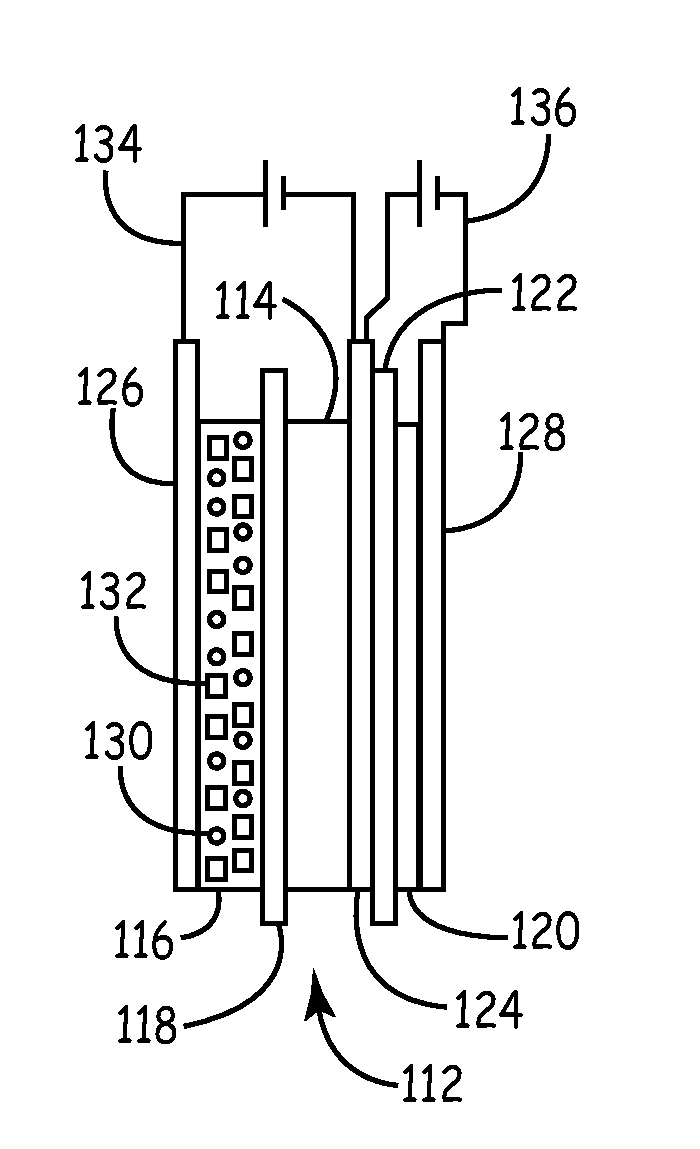
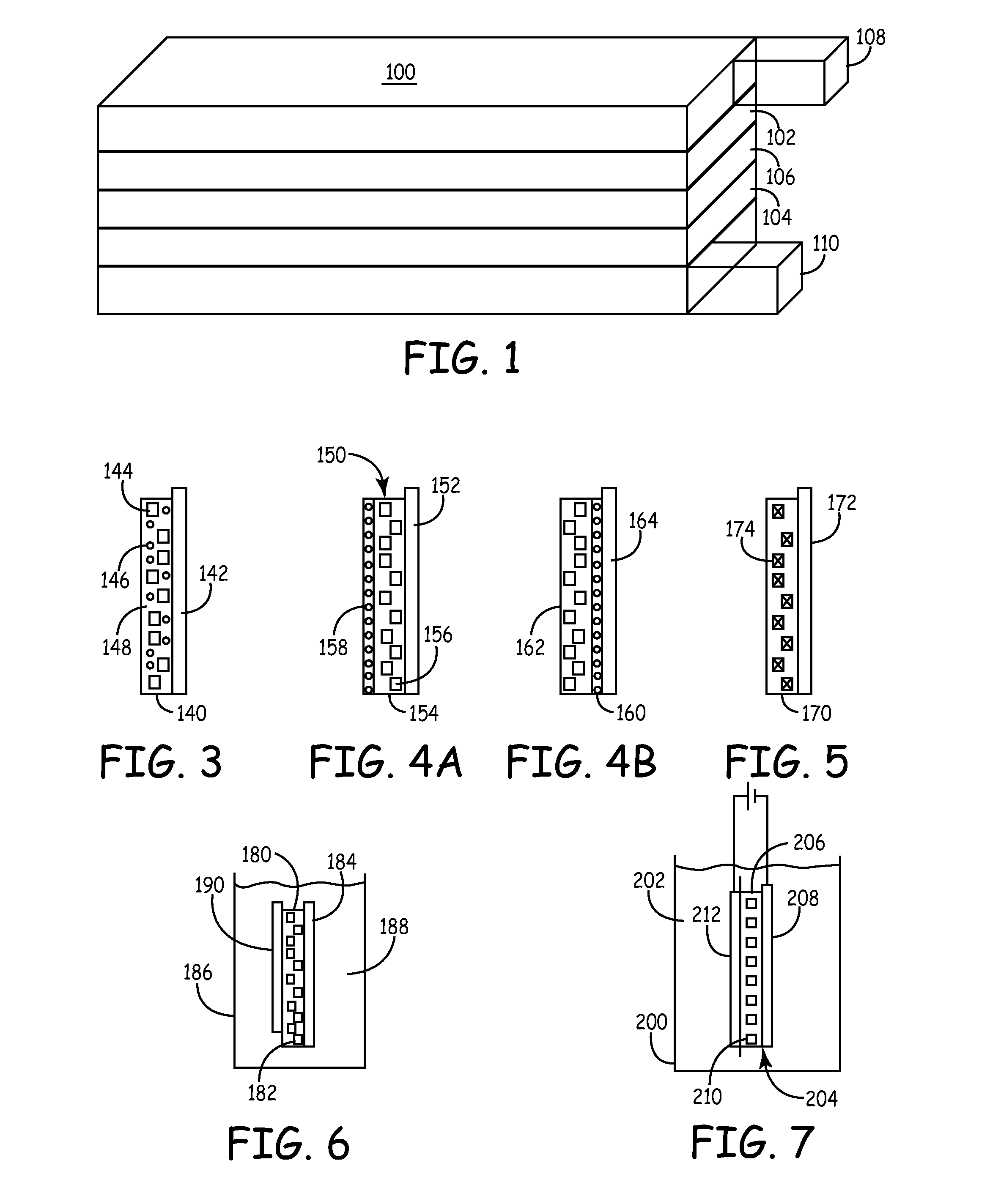
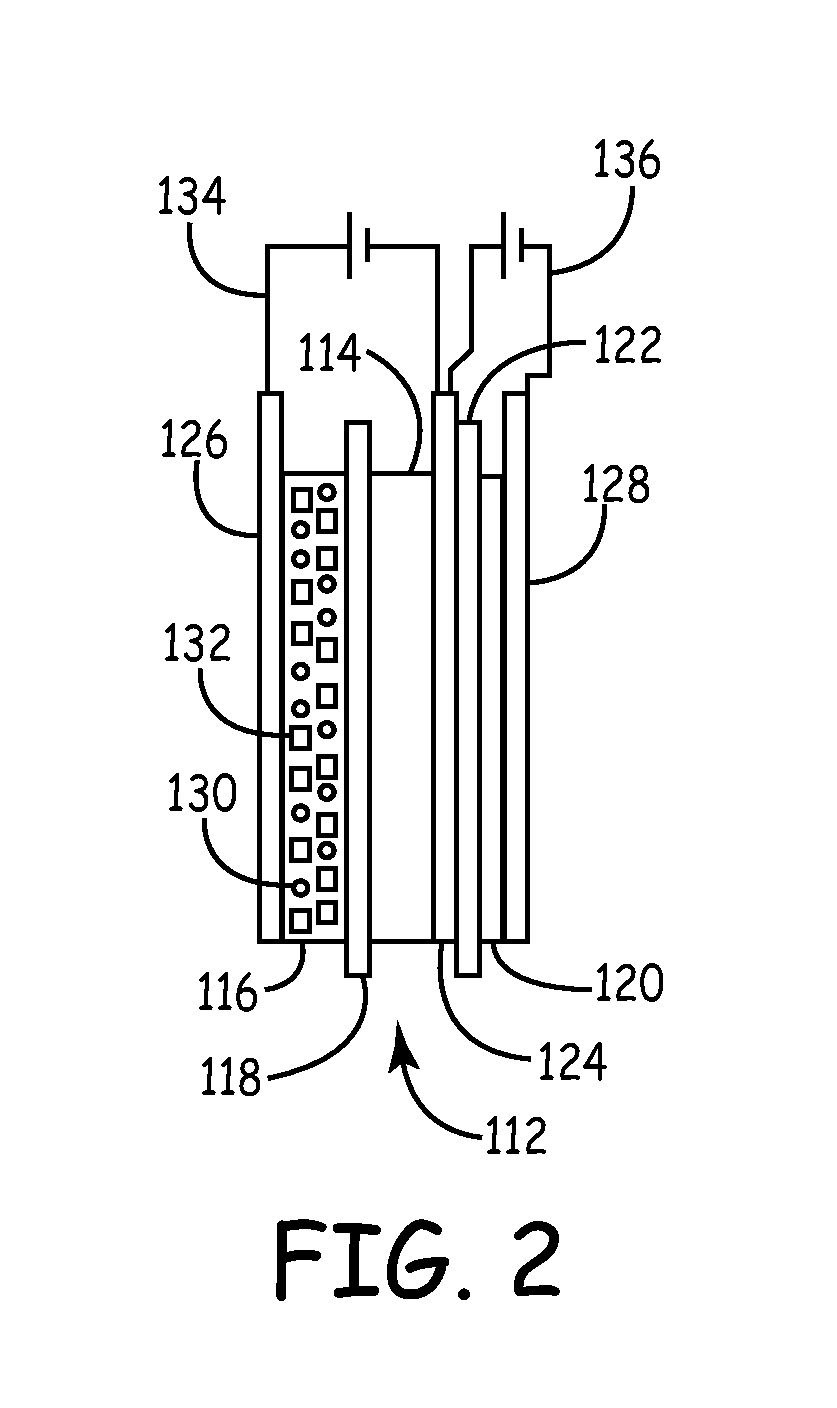
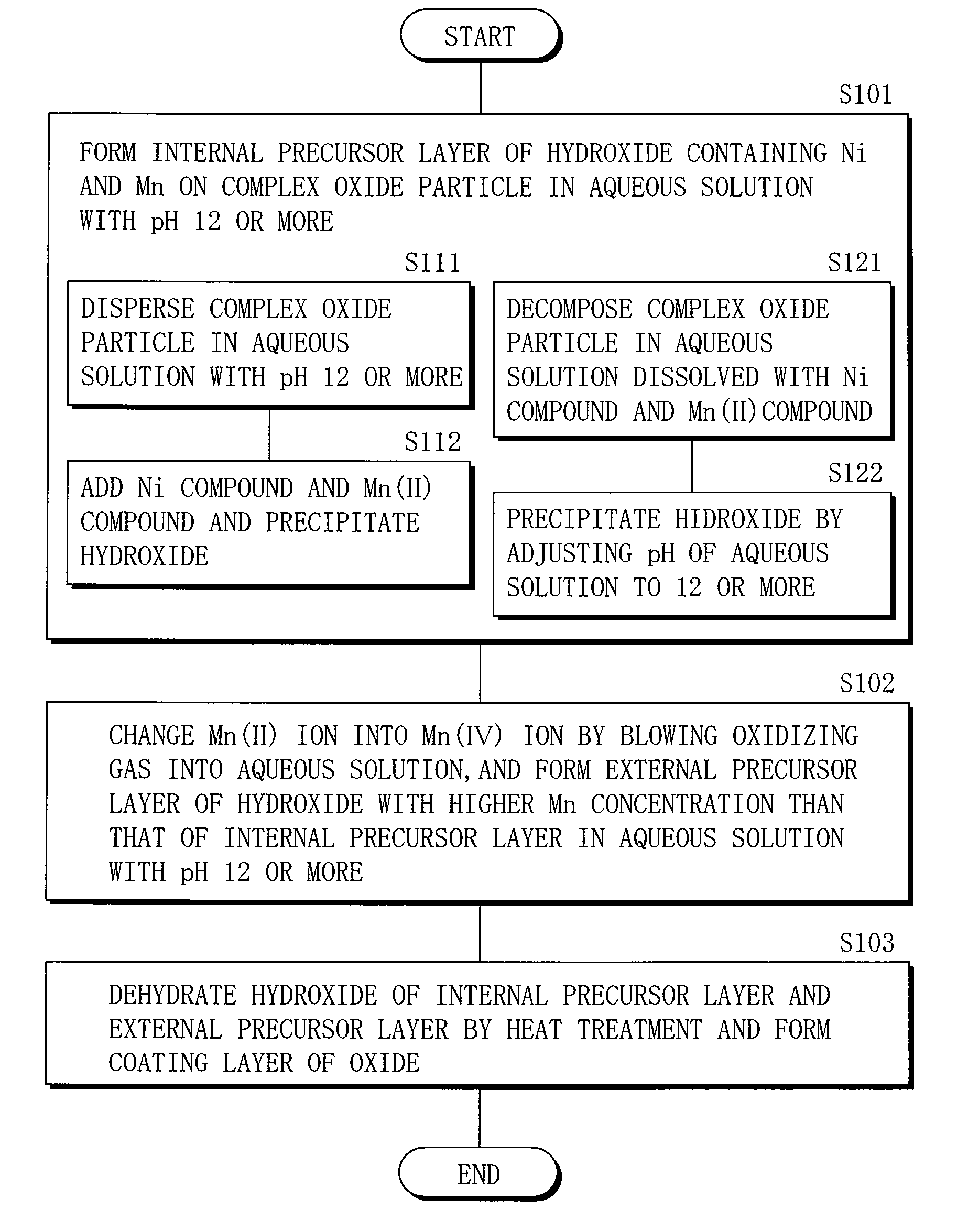
A cathode active material capable of increasing a capacity and improving high temperature characteristics or cycle characteristics, a method of manufacturing it, a cathode using the cathode active material, and a battery using the cathode active material are provided. In a cathode active material contained in a cathode, a coating layer is provided on at least part of a complex oxide particle containing at least lithium (Li) and cobalt (Co). The coating layer is an oxide which contains lithium (Li) and at least one of nickel (Ni) and manganese (Mn).
Owner:MURATA MFG CO LTD
Cathode material for lithium secondary battery and method of producing same
InactiveUS20060121350A1Stable supplyImprove sinterabilityOxygen/ozone/oxide/hydroxideElectrode manufacturing processesElectrical batteryPhysical chemistry
Stable supply of a cathode material for a lithium secondary battery that exels in sinterbility and composition stability and can exhibit satisfactory battery performance is accomplished by reducing to 100 ppm or less both the contents of Na and S being impurity elements in multiple oxides as materials for a cathode material for a lithium secondary battery and carbonic salts as precursor materials for the production of a cathode material for a lithium secondary battery.
Owner:NIKKO MATERIALS CO LTD
Desulfurization process
InactiveUS6869522B2Extended service lifeReduced valenceOther chemical processesZirconium compoundsSorbentOrganosulfur compounds
In a desulfurization process for the removal of organosulfur compounds from a hydrocarbon fluid stream such as cracked-gasoline or diesel fuel wherein a bifunctional sorbent system is employed, surface treatment of the bifunctional sorbent during the use of same for desulfurization results in an extension of the useful life of the bifunctional sorbent prior to the regeneration and reactivation of same for further use in the desulfurization of the hydrocarbon fluid stream.
Owner:CHINA PETROCHEMICAL CORP
Layer-layer lithium rich complex metal oxides with high specific capacity and excellent cycling
Lithium rich and manganese rich lithium metal oxides are described that provide for excellent performance in lithium-based batteries. The specific compositions can be engineered within a specified range of compositions to provide desired performance characteristics. Selected compositions can provide high values of specific capacity with a reasonably high average voltage. Compositions of particular interest can be represented by the formula, xLi2MnO3.(1−x)LiNiu+ΔMnu−ΔCowAyO2. The compositions undergo significant first cycle irreversible changes, but the compositions cycle stably after the first cycle.
Owner:IONBLOX INC
Method and apparatus for preparation of spherical metal carbonates and lithium metal oxides for lithium rechargeable batteries
ActiveUS20050058588A1Improve impedance characteristicsImprove stabilityConductive materialOxide conductorsDopantLithium metal
A number of materials with the composition Li1+xNiαMnβCoγM′δO2−zFz (M′=Mg,Zn,Al,Ga,B,Zr,Ti) for use with rechargeable batteries, wherein x is between about 0 and 0.3, α is between about 0.2 and 0.6, β is between about 0.2 and 0.6, γ is between about 0 and 0.3, δ is between about 0 and 0.15, and z is between about 0 and 0.2. Adding the above metal and fluorine dopants affects capacity, impedance, and stability of the layered oxide structure during electrochemical cycling. Another aspect of the invention includes materials with the composition Li1+xNiαCoβMnγM′δOyFz (M′=Mg,Zn,Al,Ga,B,Zr,Ti), where the x is between 0 and 0.2, the α between 0 and 1, the β between 0 and 1, the γ between 0 and 2, the δ between about 0 and about 0.2, the y is between 2 and 4, and the z is between 0 and 0.5.
Owner:UCHICAGO ARGONNE LLC
Lithium ion battery gradient core shell cathode material and synthetic method thereof
ActiveCN103236537AGuaranteed cycle performanceGuaranteed rate performanceCell electrodesNickel compoundsElectrical batteryPhysical chemistry
The invention provides a lithium ion battery gradient core shell cathode material and synthetic method thereof, and relates to a lithium ion battery cathode material and synthetic method thereof. The lithium ion battery gradient core shell cathode material provided by the present invention may have two kinds of core shell structures as follows: a two-layer structure: a ternary material is used as a core material, and a binary material or a unitary material is casing material, and the ternary material external layer is covered by the binary material or the unitary material; three-layer structure: the ternary material is used as a core material, and the binary material and the unitary material are casing materials, and the ternary material external layer is covered with the binary material, and the binary material is covered with the unitary material. The synthetic method includes: employing a coprecipitation method for obtaining a precursor, and then adding lithium source, calcining and coating to obtain the ternary gradient core shell material. Under the prerequisite that the structure stability of the material is kept, the cost is reduced, and the gram capacity of the material is improved, and the material circulating performance and rate capability of the material are improved, and the safety performance and low temperature performance of the ternary cathode material are increased, and the preparation technology is optimized and improved.
Owner:HARBIN INST OF TECH
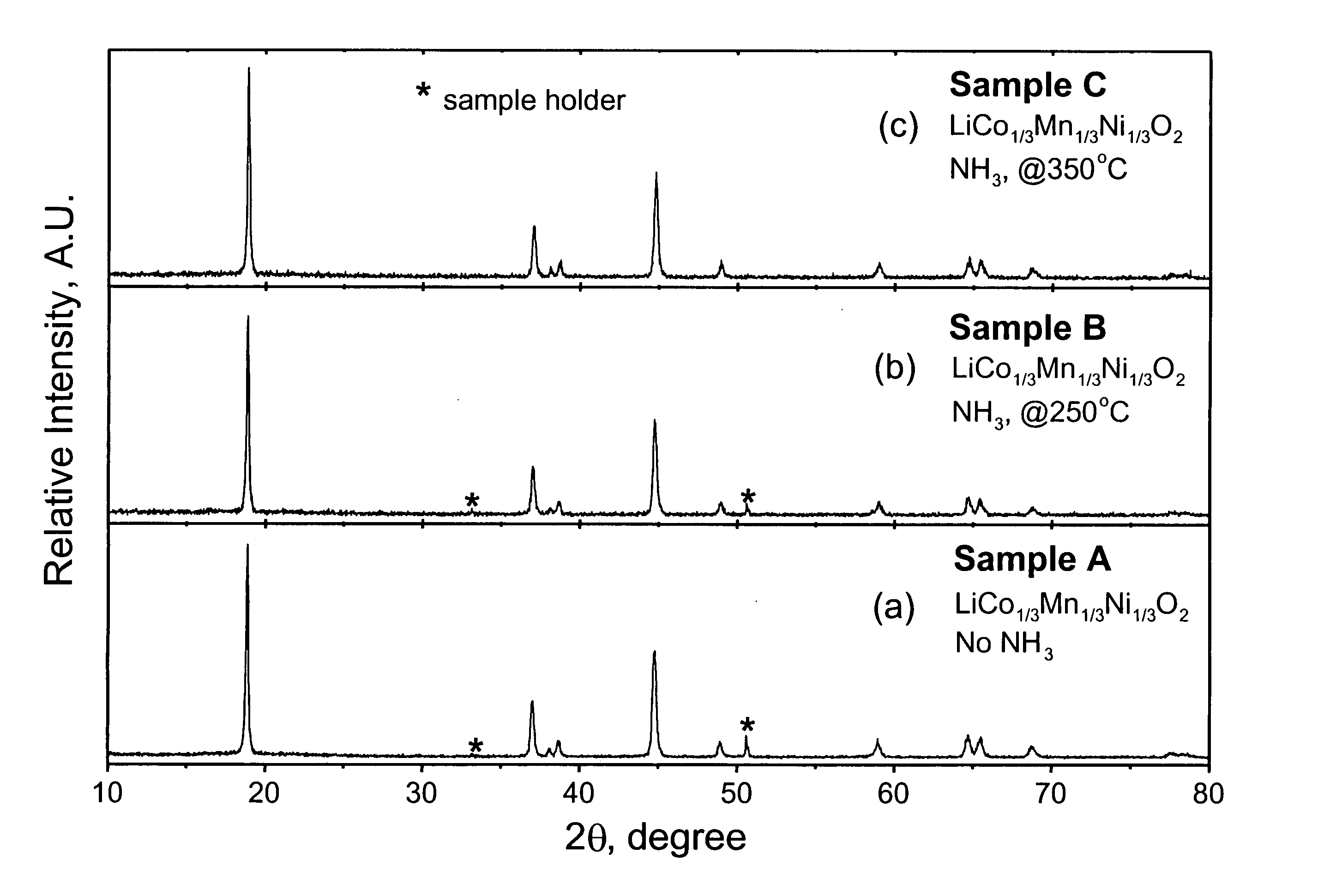
Lithium transition metal complex oxide for lithium ion secondary battery cathode active material and method for producing the same, lithium ion secondary battery cathode active material, and lithium ion secondary battery
InactiveUS20080241693A1Improve featuresAvoid security issuesIron compoundsCobalt compoundsLithium compoundSilicon
A lithium transition metal complex oxide for a lithium ion secondary battery cathode active material contains 100 to 1000 ppm of silicon and 300 to 900 ppm of fluorine. A method for producing the lithium transition metal complex oxide includes the step of mixing a lithium compound, a transition metal compound, a fluorine compound, and a silicon compound to prepare a raw material mixture, and the step of firing the raw material mixture to produce the lithium transition metal complex oxide.
Owner:NIPPON CHECMICAL IND CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com