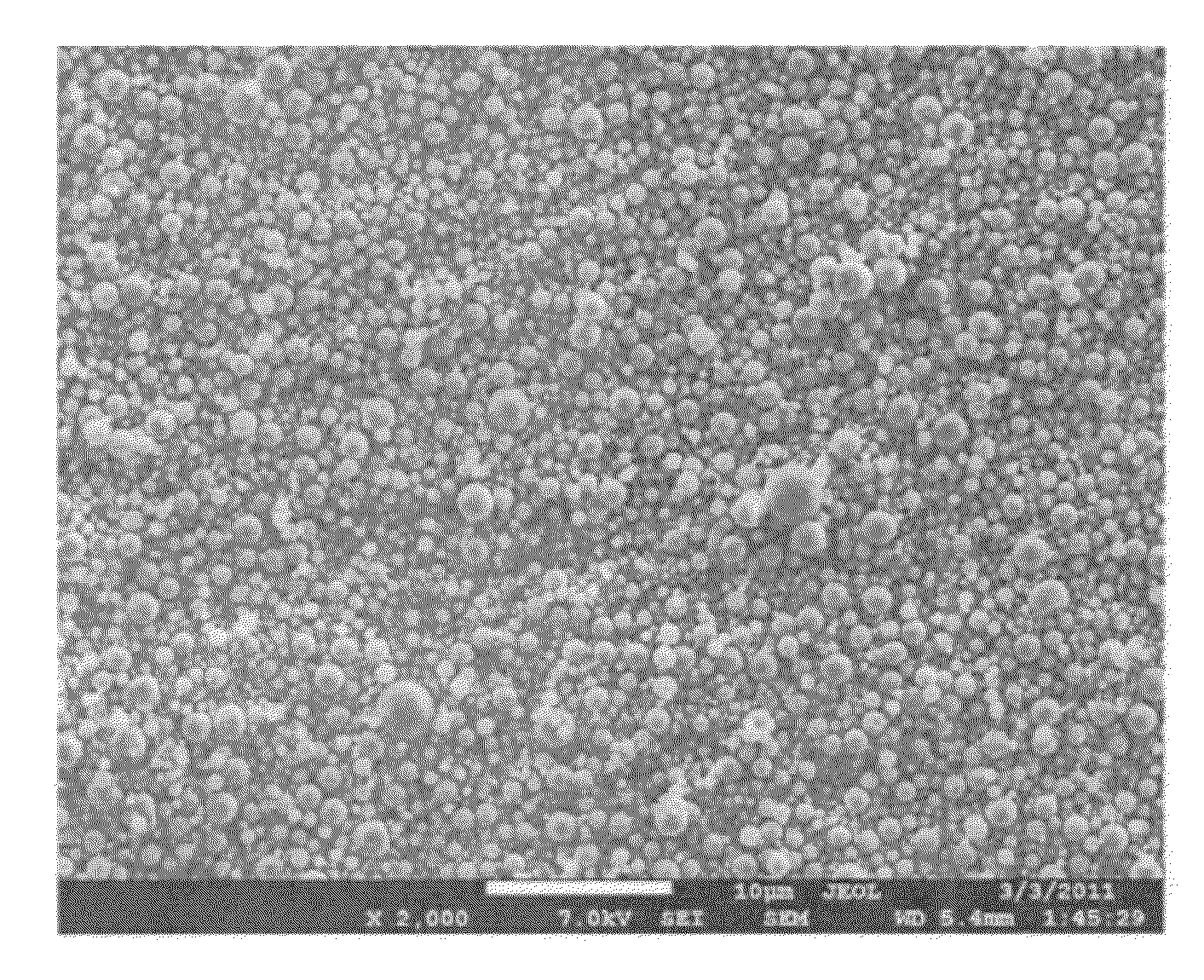Spray Pyrolysis Synthesis of Mesoporous Positive Electrode Materials for High Energy Lithium-Ion Batteries
a positive electrode material and high-energy technology, applied in the direction of lithium compounds, cell components, nickel compounds, etc., can solve the problems of pure lifepo/sub>4/sub>suffering from a low conductivity at room temperatur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
General Procedures
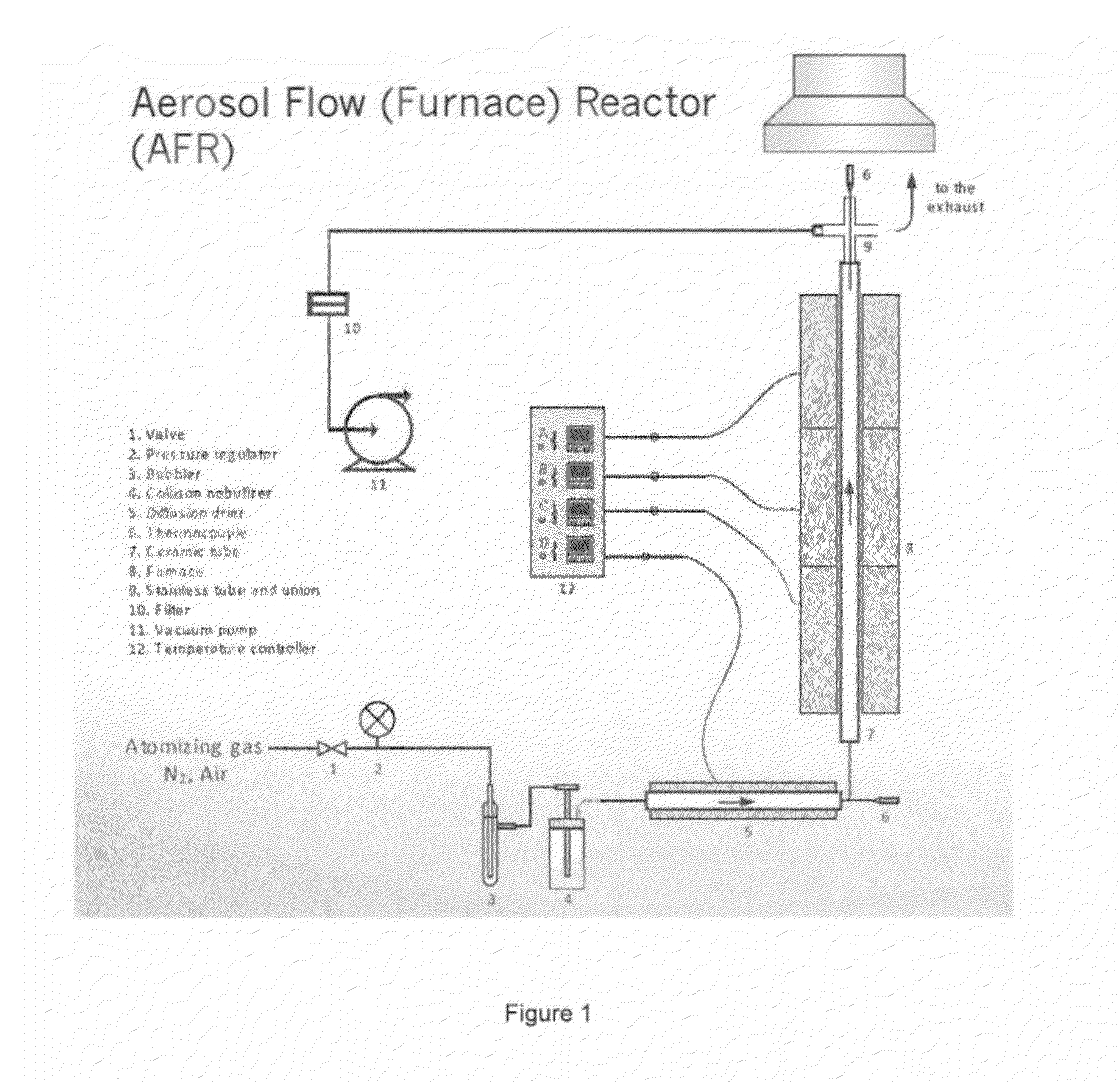
[0066]The above-described spray pyrolysis method was performed with the apparatus of FIG. 1 to produce Li-rich Li(1.2−δ)Mn0.6Ni0.2O(2−δ / 2) (0≦δ≦ 1 / 10) composite materials. The precursor solution was prepared by dissolving LiNO3, Mn(NO3)2.4H2O and Ni(NO3)2.6H2O at a ratio of (1.2−δ):0.6:0.2 in deionized water. The total molar concentrations of Mn(NO3)2.4H2O and Ni(NO3)2.6H2O were maintained at 2 M. The corresponding Li concentration was calculated based on the δ values in Li(1.2−δ)Mn0.6Ni0.2O(2−δ / 2) composites. For example, for δ=0, the composite is Li1.2Mn0.6Ni0.2O2, and the precursor solution contained 1.5 M Mn, 0.5 M Ni, and 3 M Li cations.
[0067]The precursor solutions were aerosolized with air-assisted nebulizers (or atomizer or sprayer). Specifically, a one-jet collision nebulizer from BGI Inc. was used to aerosolize the precursor solution to form fine precursor droplets in the micron-size range. The atomizing gas was air flowing at 3.3 liters per minute with t...
example 2
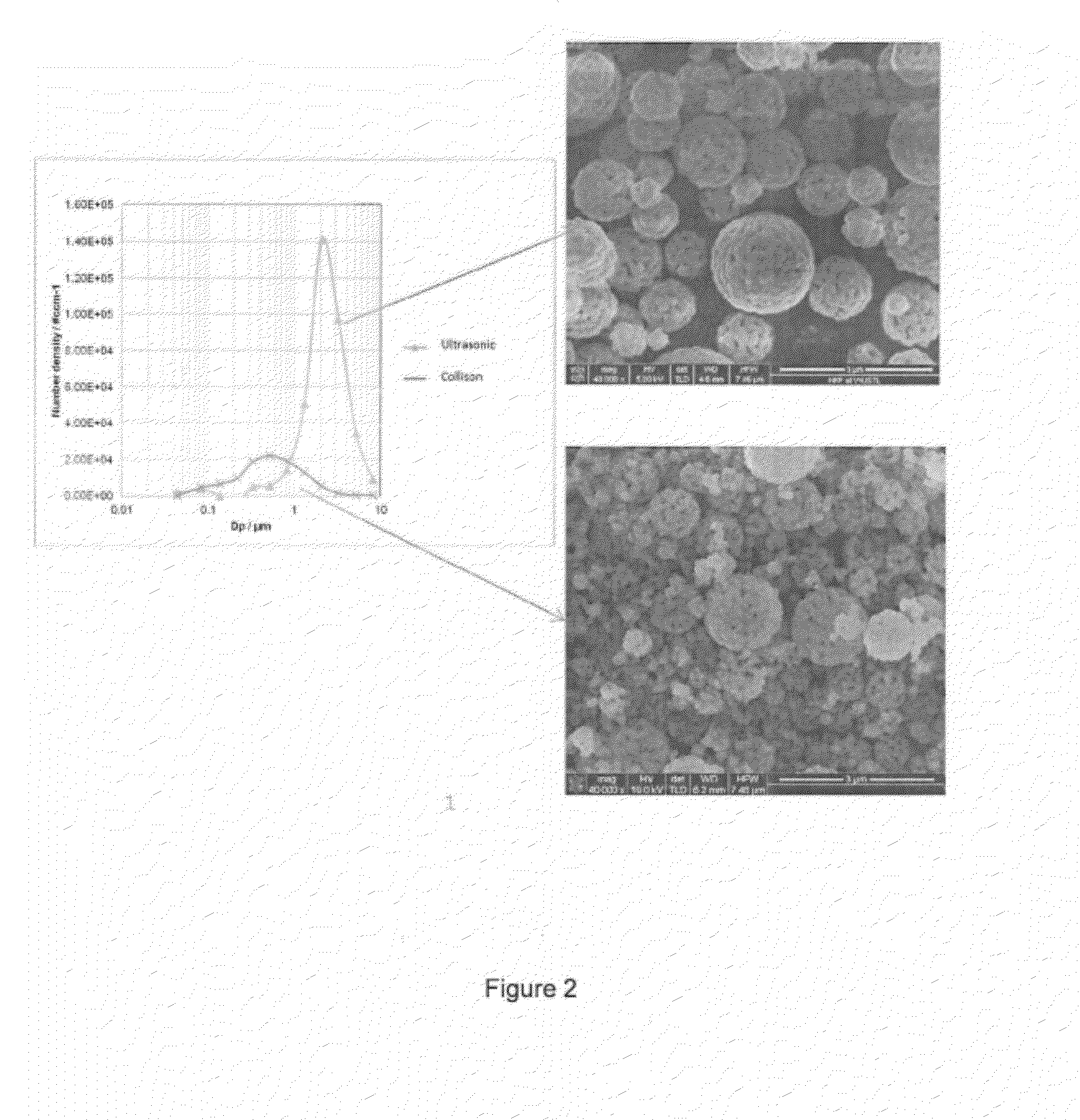
[0092]The above-described spray pyrolysis method was performed with a precursor solution was comprising metal nitrates dissolved in deionized water where the composition of metals nitrates in the precursor solution was prepared to yield Li1.2Mn0.53Ni0.13Co0.13O2and 2.5 M. Upon being subjecting this solution to spray pyrolysis, a layered-layered composite with the alternative formula 0.5Li2MnO3.0.5Li(Ni1 / 3Mn1 / 3Co1 / 3)O2 was to have been formed. In this example, the precursor solution was aerosolized with a SONAER ultrasonic nebulizer, which has the larger size distribution shown in FIG. 2. The preheater (dryer) temperature was 200° C. and the tube furnace wall temperature was 550° C. The air flow rate through the ultrasonic nebulizer was 6 liters per minute. The powder collected was subjected to a heat treatment of 900° C. for 2 hours.
[0093]To prepare the positive electrode, the active material (i.e., Li1.2Mn0.53Ni0.13Co0.13O2or, alternatively, 0.5Li2MnO3.0.5Li(Ni1 / 3Mn1 / 3Co1 / 3)O2), 12...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com