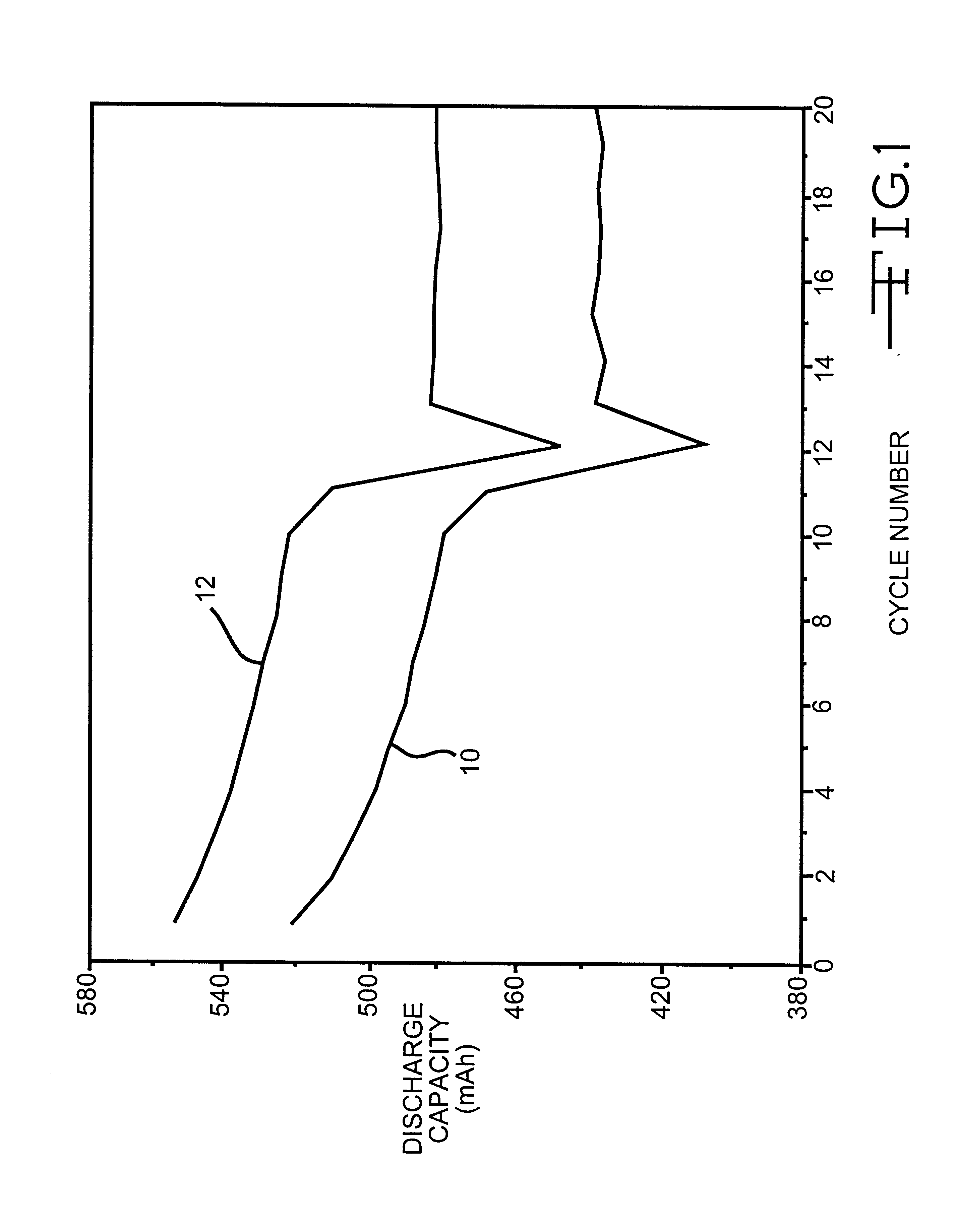
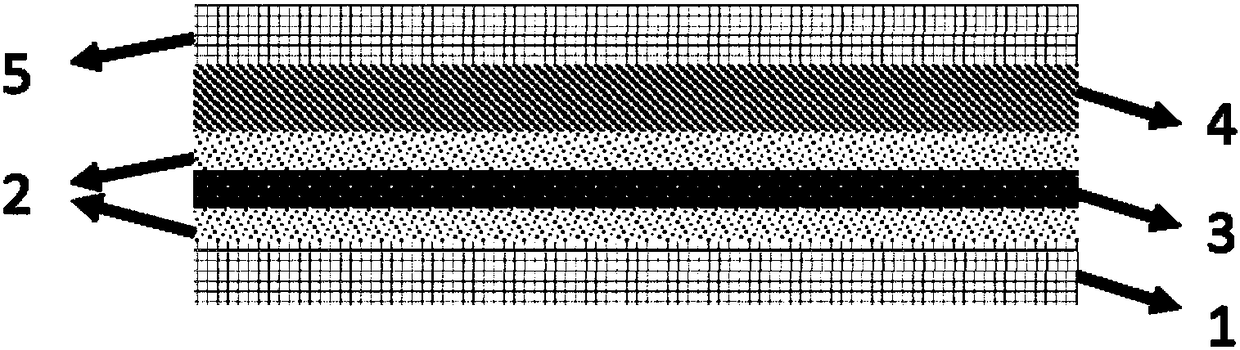
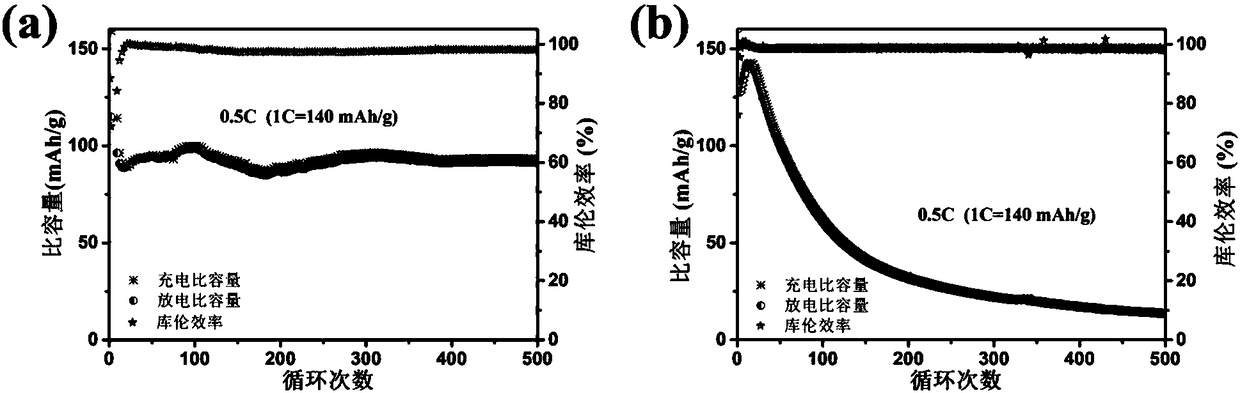
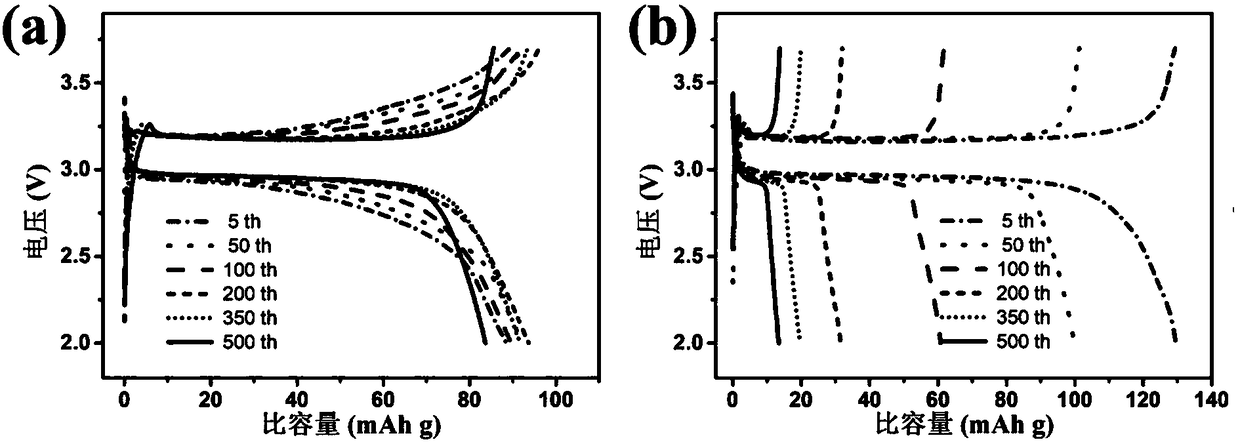
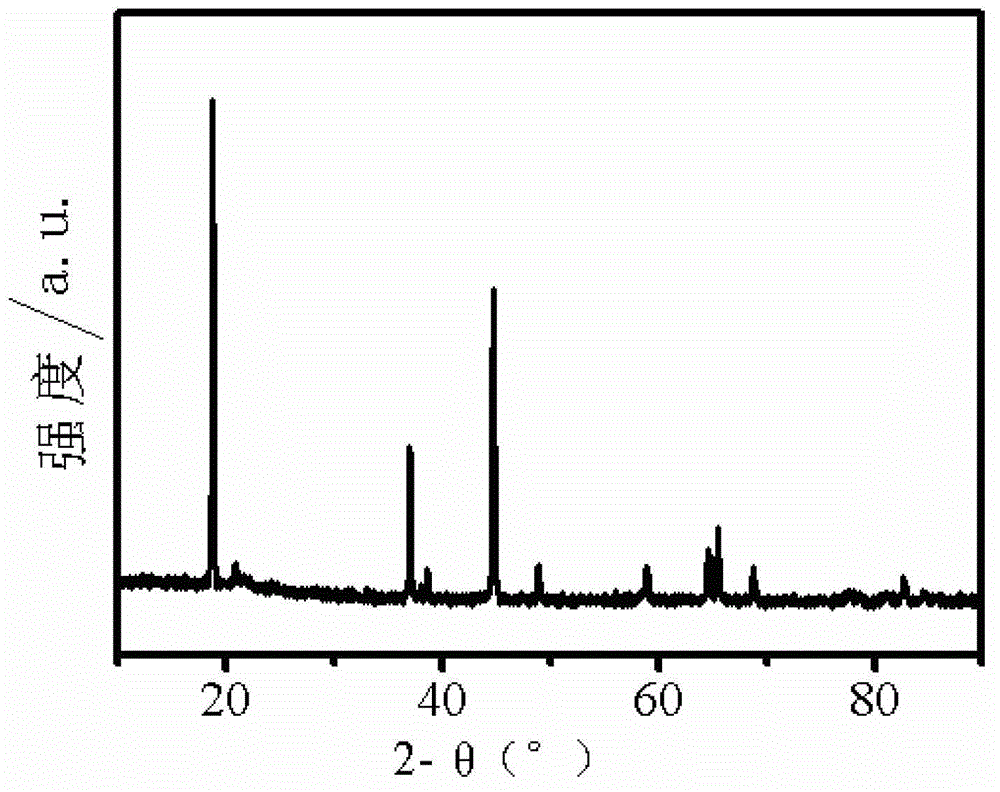
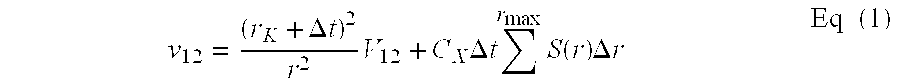Patents
Literature
347results about How to "Reduce irreversible capacity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
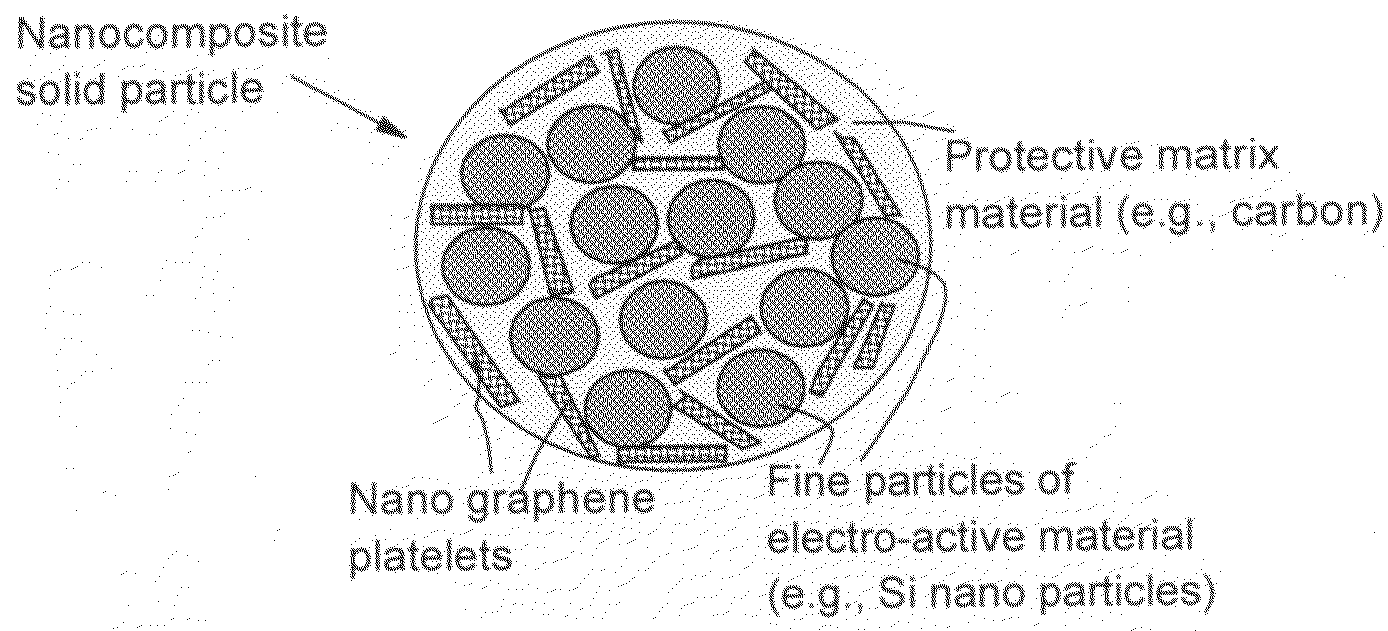
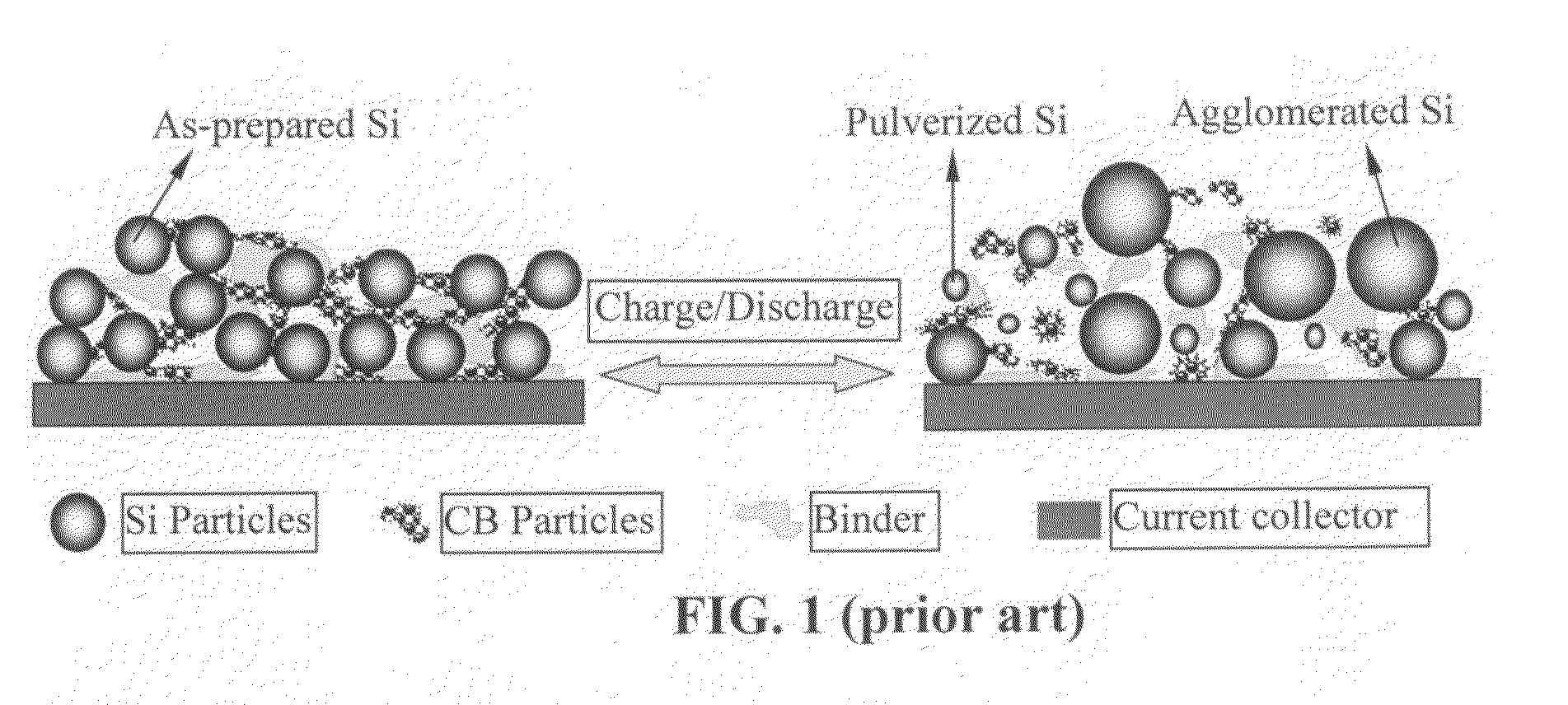
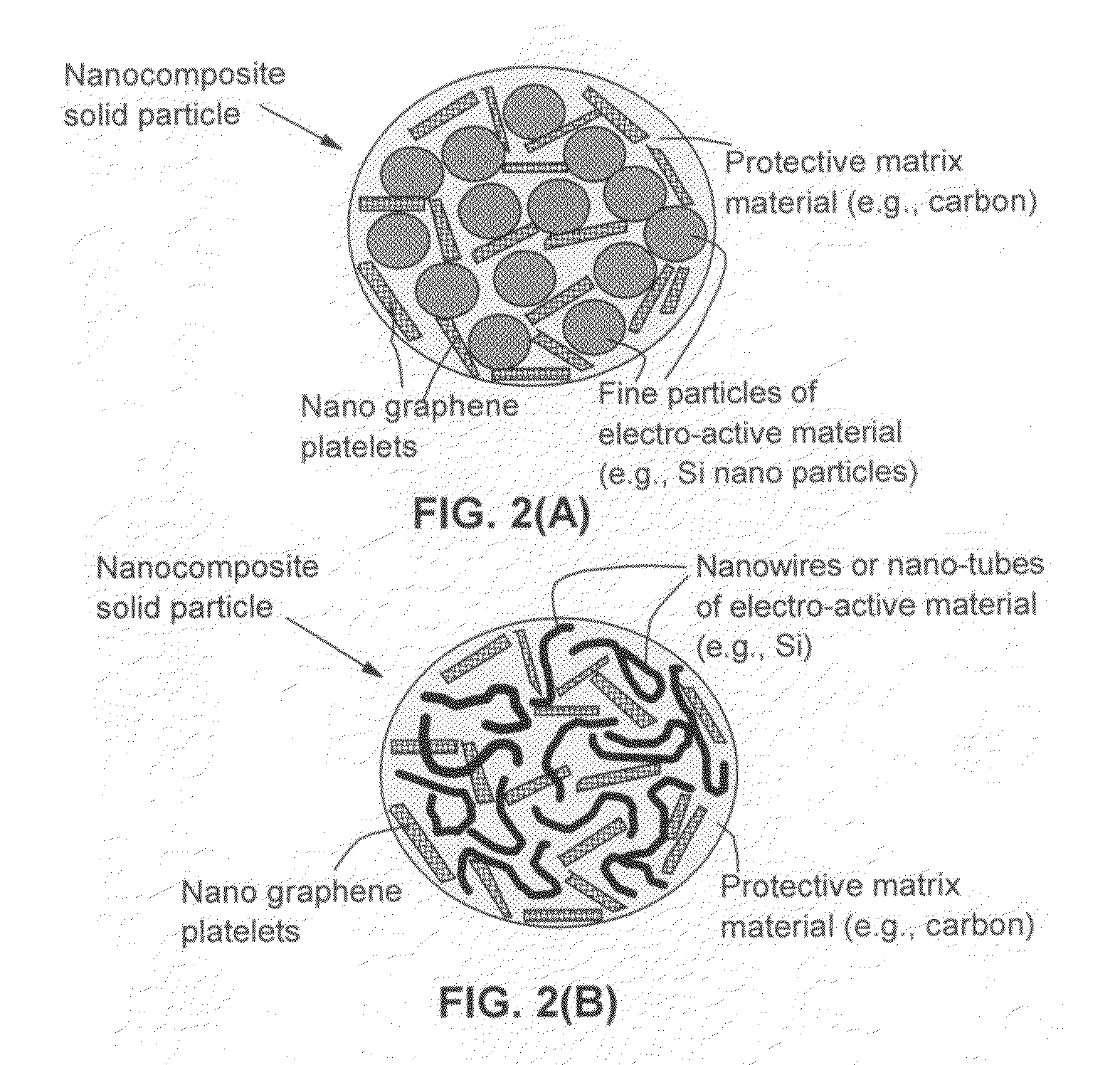
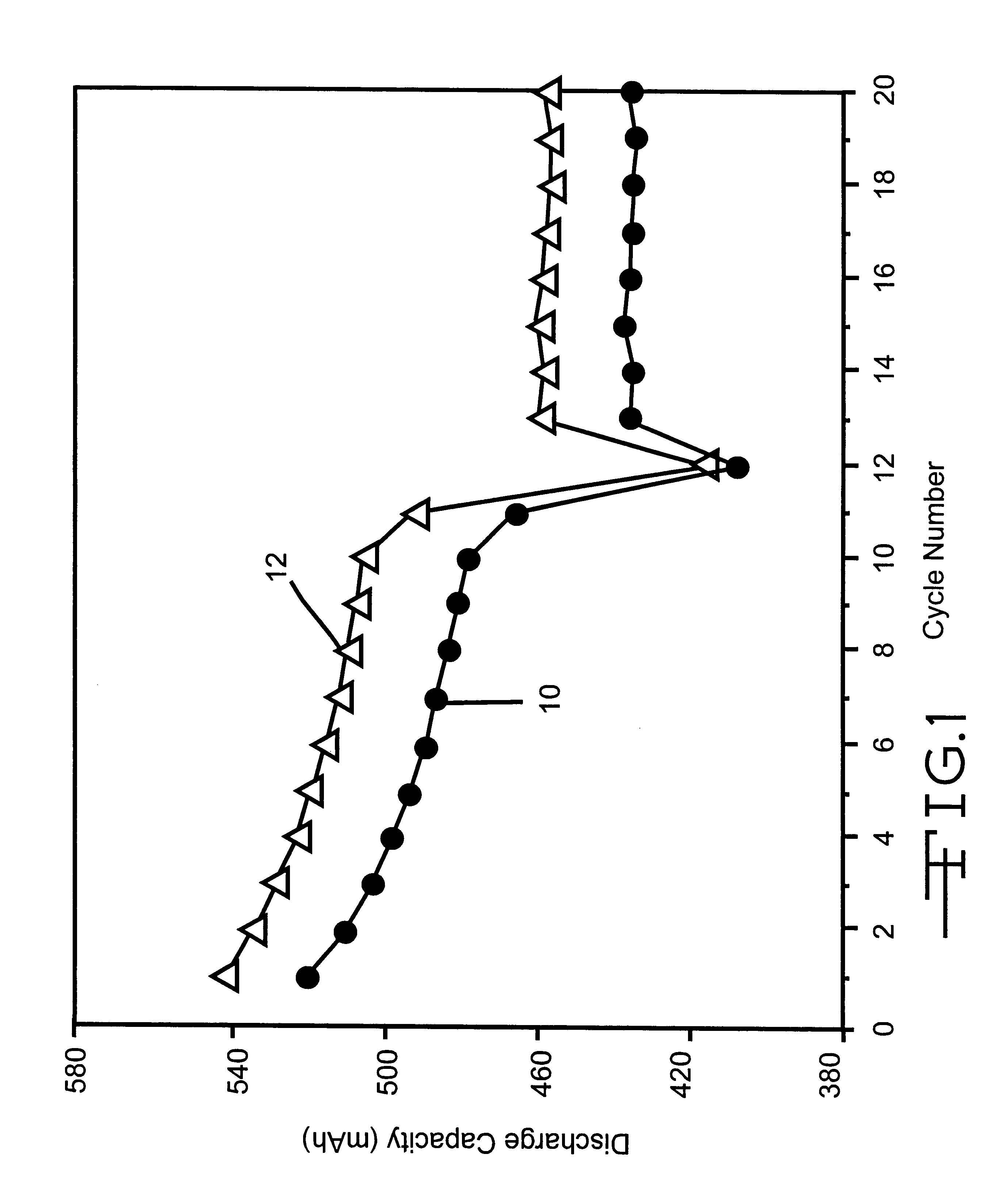
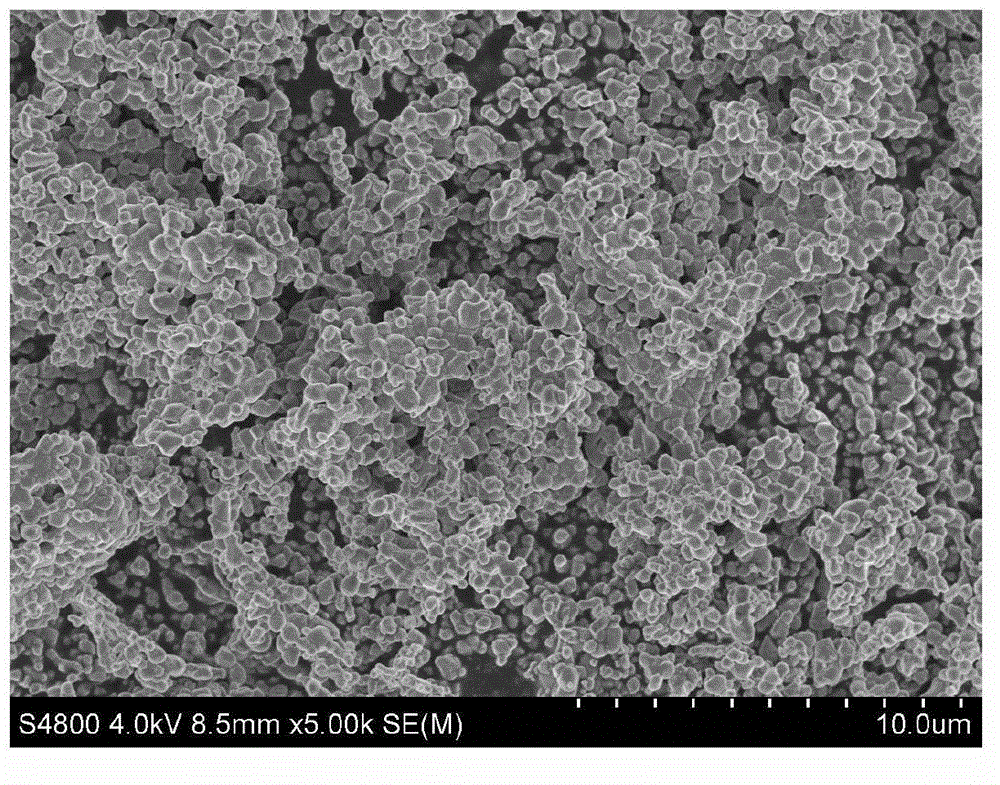
Process for producing nano graphene reinforced composite particles for lithium battery electrodes
ActiveUS20100176337A1Increase stiffnessHigh strengthCell electrodesLi-accumulatorsFiberLithium metal
Owner:SAMSUNG ELECTRONICS CO LTD
Phosphonate additives for nonaqueous electrolyte in rechargeable cells
InactiveUS6200701B1Improve oxidation stabilityImprove dynamic stabilityOrganic electrolyte cellsSecondary cellsRechargeable cellPhysical chemistry
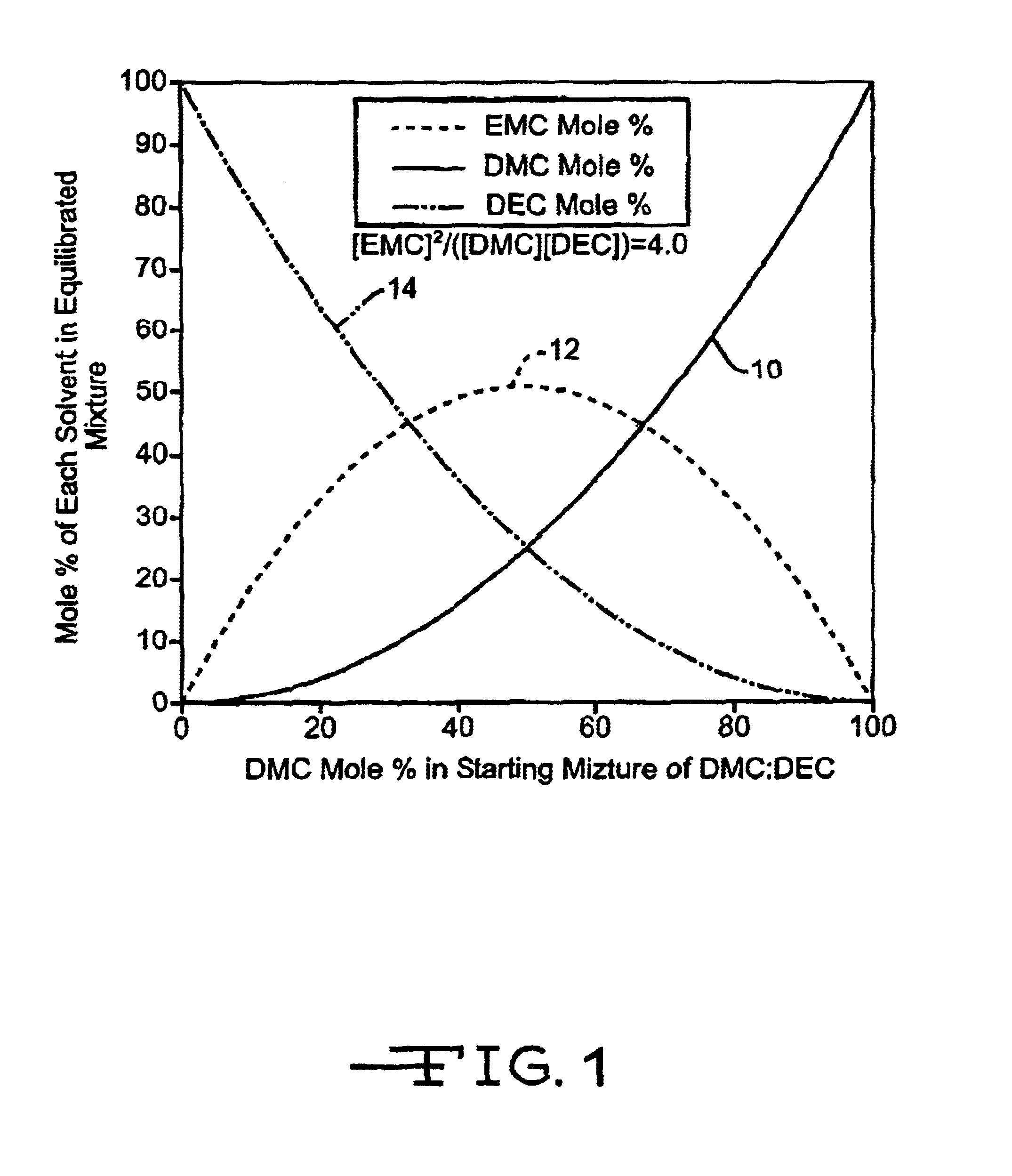
A lithium ion electrochemical cell having high charge / discharge capacity, long cycle life and exhibiting a reduced first cycle irreversible capacity, is described. The stated benefits are realized by the addition of at least one phosphonate additive to an electrolyte comprising an alkali metal salt dissolved in a solvent mixture that includes ethylene carbonate, dimethyl carbonate, ethylmethyl carbonate and diethyl carbonate. The preferred additive is an alkyl phosphonate compound.
Owner:WILSON GREATBATCH LTD
Phosphate additives for nonaqueous electrolyte rechargeable electrochemical cells
InactiveUS6203942B1Improve oxidation stabilityImprove dynamic stabilityPrimary cell maintainance/servicingOrganic electrolyte cellsAlkylphosphateSolvent
A lithium ion electrochemical cell having high charge / discharge capacity, long cycle life and exhibiting a reduced first cycle irreversible capacity, is described. The stated benefits are realized by the addition of at least one phosphate additive to an electrolyte comprising an alkali metal salt dissolved in a solvent mixture that includes ethylene carbonate, dimethyl carbonate, ethylmethyl carbonate and diethyl carbonate. The preferred additive is an alkyl phosphate compound.
Owner:WILSON GREATBATCH LTD
Lithium metal oxide containing multiple dopants and method of preparing same
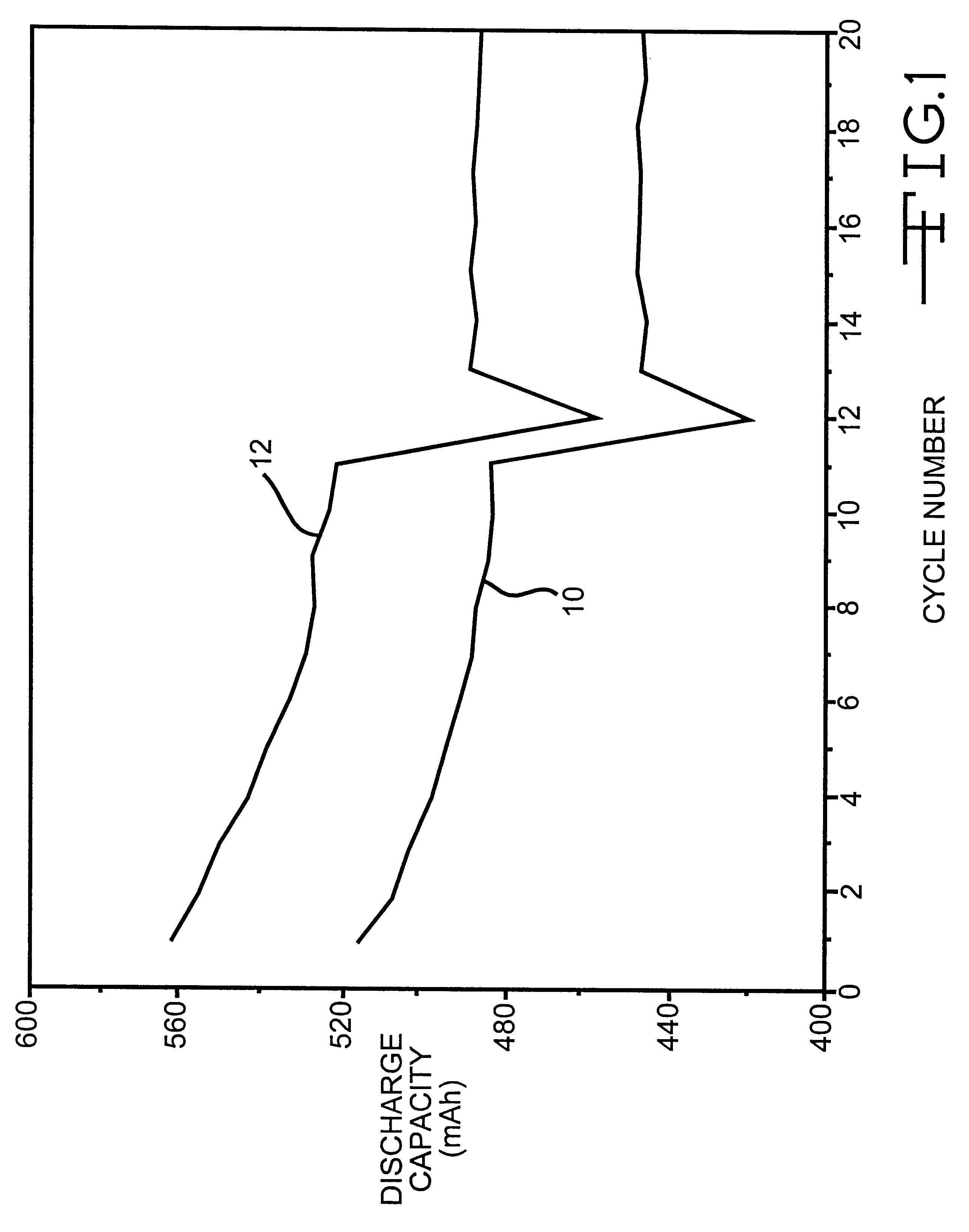
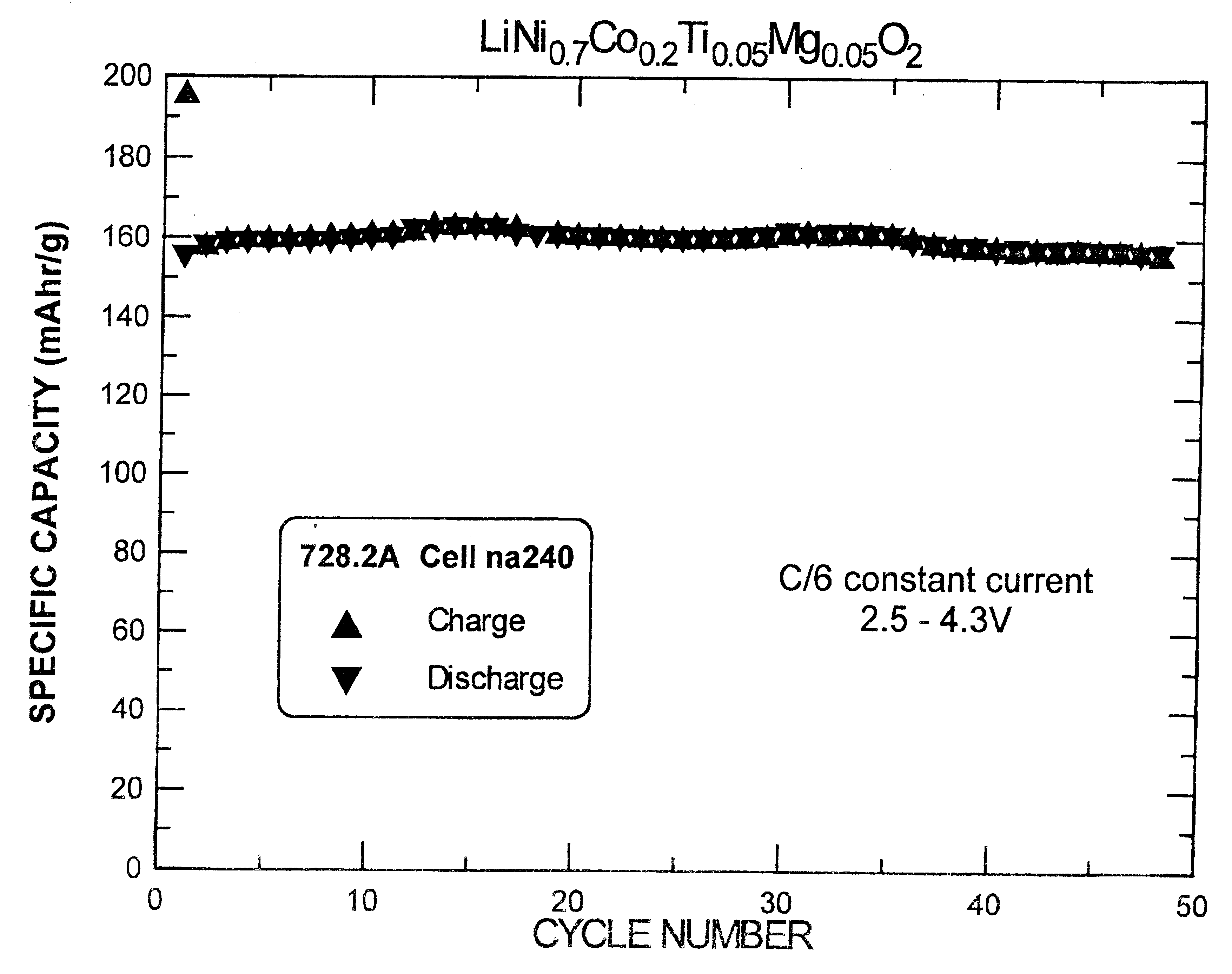
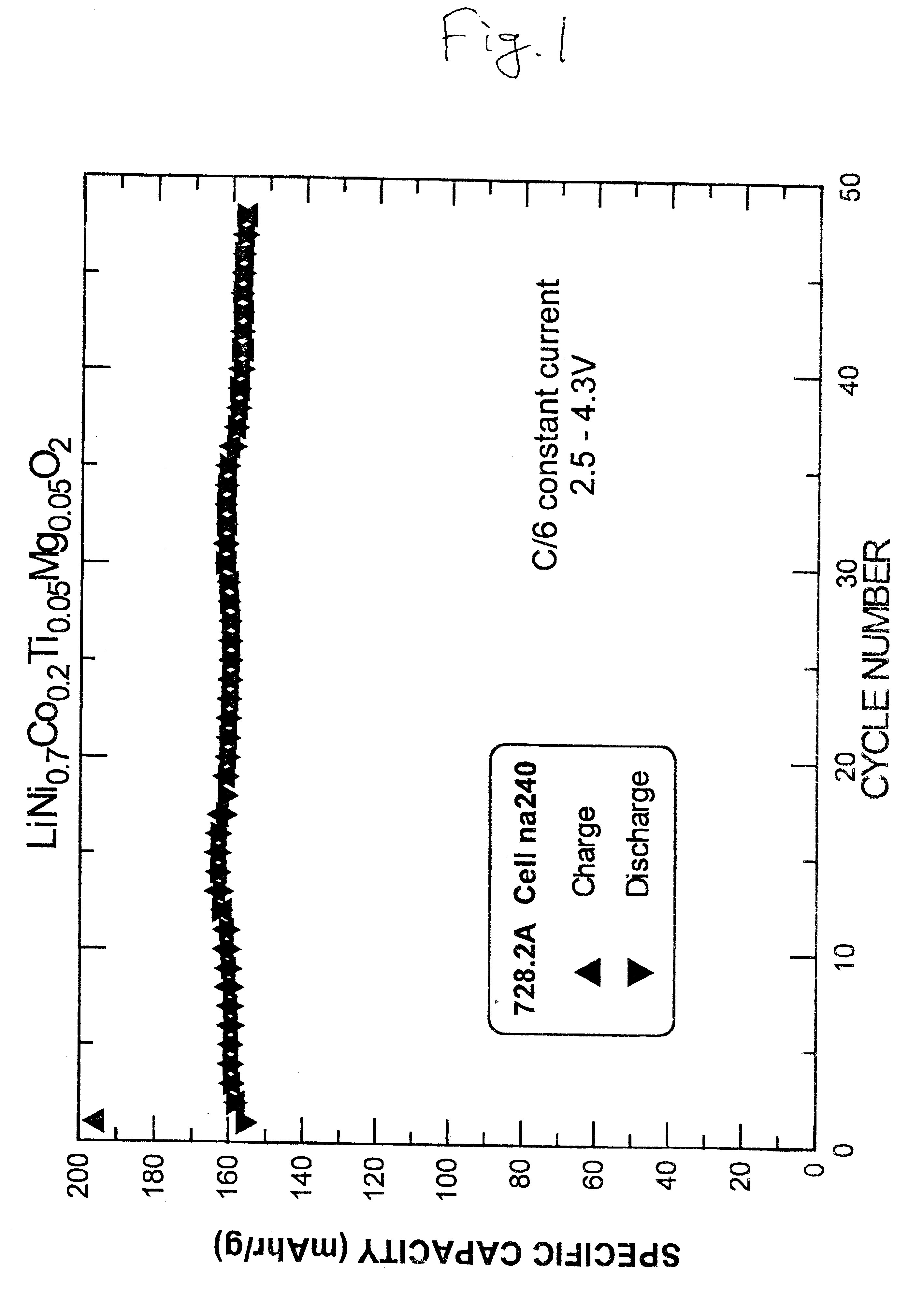
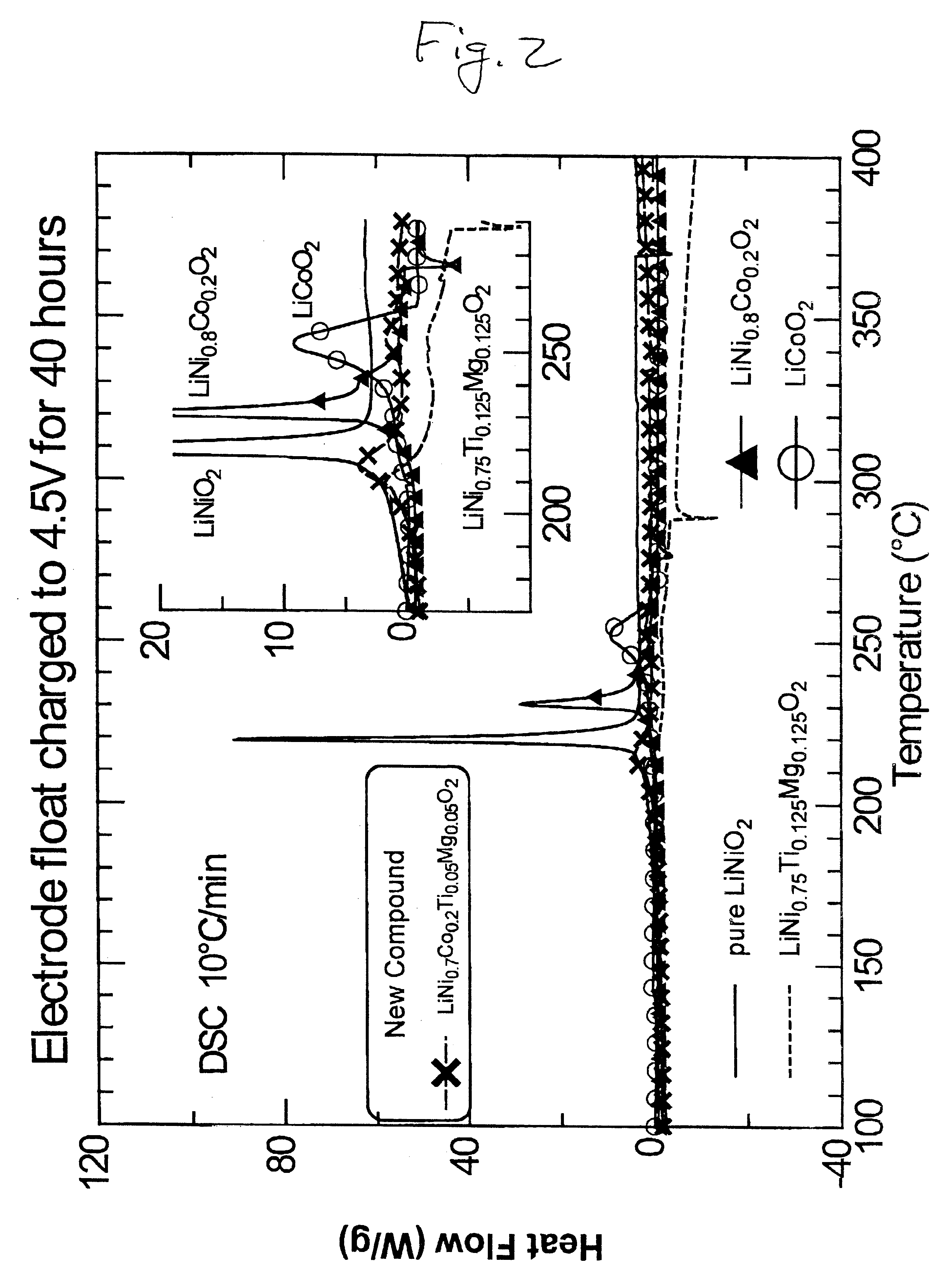
InactiveUS6277521B1Reduce irreversible capacityHigh reversible capacityCobalt compoundsNon-aqueous electrolyte accumulator electrodesOxideLithium electrode
The present invention provides a multiple-doped lithium metal oxide and a method of preparing same for use in the positive electrodes of lithium and lithium ion batteries. The intercalation compound of the invention has the formula LiNi.sub.1-x Co.sub.y M.sub.a M'.sub.b O.sub.2, wherein M is selected from the group consisting of Ti, Zr, and combinations thereof, and M' is selected from the group consisting of Mg, Ca, Sr, Ba, and combinations thereof. The elements in the compounds are present such that x=y+a+b, x is from greater than 0 to about 0.5, y is from greater than 0 to about 0.5, a is from greater than 0 to about 0.15, and b is from greater than 0 to about 0.15.
Owner:UMICORE AG & CO KG
Nitrate additives for nonaqueous electrolyte rechargeable cells
InactiveUS6136477AImprove oxidation stabilityImprove dynamic stabilityOrganic electrolyte cellsSecondary cellsNitrateRechargeable cell
A lithium ion electrochemical cell having high charge / discharge capacity, long cycle life and exhibiting a reduced first cycle irreversible capacity, is described. The stated benefits are realized by the addition of at least one nitrate additive to an electrolyte comprising an alkali metal salt dissolved in a solvent mixture that includes ethylene carbonate, dimethyl carbonate, ethylmethyl carbonate and diethyl carbonate. The preferred additive is an organic alkyl nitrate compound.
Owner:WILSON GREATBATCH LTD
Nitrite additives for nonaqueous electrolyte rechargeable electrochemical cells
InactiveUS6210839B1Improve oxidation stabilityImprove dynamic stabilityOrganic electrolyte cellsSolid electrolyte cellsNitritePhysical chemistry
A lithium ion electrochemical cell having high charge / discharge capacity, long cycle life and exhibiting a reduced first cycle irreversible capacity, is described. The stated benefits are realized by the addition of at least one nitrite additive to an electrolyte comprising an alkali metal salt dissolved in a solvent mixture that includes ethylene carbonate, dimethyl carbonate, ethylmethyl carbonate and diethyl carbonate. The preferred additive is an alkyl nitrite compound.
Owner:WILSON GREATBATCH LTD
Positive electrode active material and its manufacturing method, positive electrode for lithium secondary cell using same, and lithium secondary cell
InactiveUS20060177739A1Deteriorated in battery performanceGood charge and discharge cycle performanceActive material electrodesNon-aqueous electrolyte accumulator electrodesLithiumEngineering
A positive active material is provided which can inhibit side reactions between the positive electrode and an electrolyte even at a high potential and which, when applied to a battery, can improve charge / discharge cycle performance without impairing battery performances even in storage in a charged state. Also provided are: a process for producing the active material; a positive electrode for lithium secondary batteries which employs the active material; and a lithium secondary battery which has improved charge / discharge cycle performance while retaining intact battery performances even after storage in a charged state and which can exhibit excellent charge / discharge cycle performance even when used at a high upper-limit voltage. The positive active material comprises: base particles able to dope and release lithium ions; and an element in Group 3 of the periodic table present on at least part of that part of the base particles which is able to come into contact with an electrolyte. It is produced by, e.g., a process which comprises: producing base particles containing lithium and able to dope and release lithium ions; and then imparting an element in Group 3 of the periodic table to the base particles so that the element can be present on at least part of that part of the base particles which is able to come into contact with an electrolyte.
Owner:GS YUASA INT LTD
Active material for non-aqueous electrolyte secondary battery and manufacturing method therefore
ActiveUS20080268347A1Increase capacityReduces electron conductivity and lithium diffusing abilityElectrode manufacturing processesNon-aqueous electrolyte accumulatorsDesorptionX-ray
An active material for a non-aqueous electrolyte secondary battery including a lithium-containing transition metal oxide containing nickel and manganese and having a closest-packed structure of oxygen, wherein an atomic ratio MLi / MT between the number of moles of lithium MLi and the number of moles of transition metal Mt contained in the lithium-containing transition metal oxide is greater than 1.0; the lithium-containing transition metal oxide has a crystal structure attributed to a hexagonal system, and the X-ray diffraction image of the crystal structure has a peak P003 attributed to the (003) plane and a peak P104 attributed to the (104) plane; an integrated intensity ratio I003 / I104 between the peak P003 and the peak P104 varies reversibly within a range from 0.7 to 1.5 in association with absorption and desorption of lithium by the lithium-containing transition metal oxide; and the integrated intensity ratio varies linearly and continuously.
Owner:PUBLIC UNIVERSITY CORPORATION OSAKA CITY UNIVERSITY +1
Silicon-oxygen composite negative electrode material and manufacturing method thereof
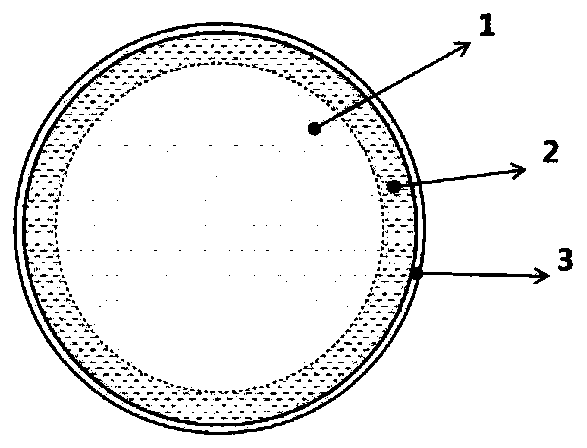
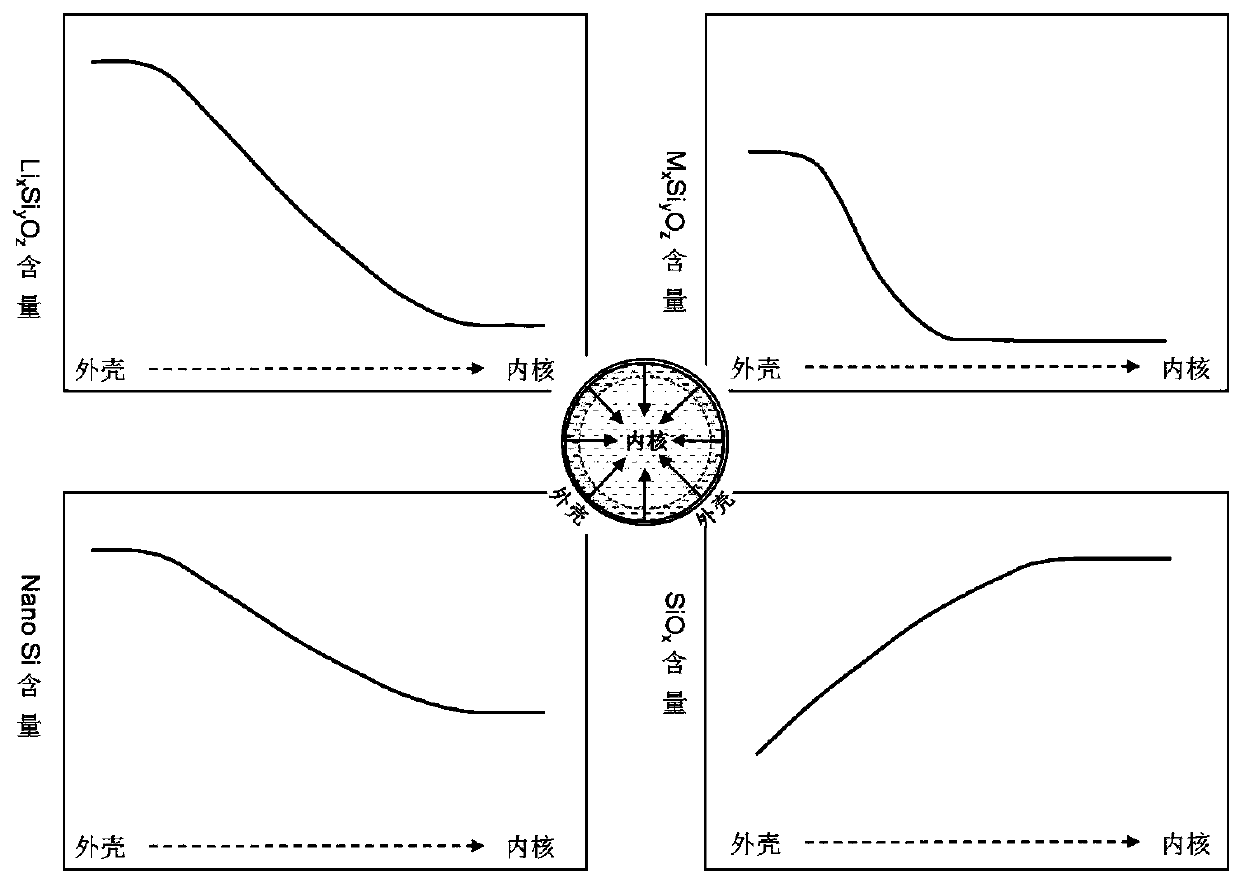
ActiveCN109755500AImprove electronic conductanceReduce lossCell electrodesLi-accumulatorsSilicon oxygenMetallurgy
The invention discloses a silicon-oxygen composite negative electrode material which is used for manufacturing a negative electrode of a lithium battery; the negative electrode material comprises an inner core, a coating layer and a middle layer, wherein the coating layer wraps the inner core, and the middle layer is positioned between the inner core and the coating layer, wherein the middle layercomprises non-lithium silicate, and the non-lithium silicate refers to non-lithium silicate, wherein the mass content of the non-lithium silicate in the middle layer is gradually decreased from the middle layer to the inner core. The decrementing comprises a gradient reduction from the middle layer to the inner core, and the gradient reduction refers to the fact that the mass-duty ratios on the circumference parts which have the same central distance from the inner core are the same, and when the distance from the center of the inner core is reduced, the mass-duty ratio is reduced step by step. The non-lithium silicate is generated in situ on the outer layer of the inner core, and has a non-water-soluble or non-alkaline or weakly alkaline compact structure, so that the dissolution of theinternal water-soluble lithium silicate can be effectively relieved; and the pH value of the ghost-eye composite negative electrode material can be lowered.
Owner:HUAWEI TECH CO LTD
Anode for nonaqueous secondary electrochemical cells
InactiveUS6730437B2Improve performanceAvoid corrosionSilver accumulatorsElectrode carriers/collectorsGraphiteMetallic sulfide
The negative electrode or anode for a secondary electrochemical cell comprising a mixture of graphite or "hairy carbon" and a lithiated metal oxide, a lithiated mixed metal oxide or a lithiated metal sulfide, and preferably a lithiated metal vanadium oxide, is described. A most preferred formulation is graphite mixed with lithiated silver vanadium oxide or lithiated copper silver vanadium oxide.
Owner:WILSON GREATBATCH LTD
Hard carbon negative electrode material of lithium ion battery, preparation method and application of hard carbon negative electrode material
InactiveCN102820455AIncrease capacityImprove the first Coulombic efficiencyCell electrodesDischarge efficiencyPhysical chemistry
The invention relates to a hard carbon negative electrode material of a lithium ion battery, and a preparation method and application of the hard carbon negative electrode material. The hard carbon negative electrode material is doped with silicon and phosphorus, wherein the mass ratio of silicon precursor, phosphorus precursor and hard carbon precursor is 1:(0.1 to 5):(5 to 30). Due to the addition of silicon / phosphorus, the hard carbon negative electrode material provided by the invention is remarkably improved in the first discharge capacity (up to 550.8mAh / g), the first charge / discharge efficiency (up to 80.9%) and the first coulombic efficiency by doping, is remarkably reduced in the irreversible capacity, and has the advantages of excellent cycle performance and rate capability, good PC (poly carbonate) consistency and high PC resistance, wherein the capacity retention ratio is above 95% after discharge cycle for 100 weeks at a high discharge rate of 10C.
Owner:天津市贝特瑞新能源科技有限公司
Compound and preparation method thereof as well as negative electrode prepared by adopting compound and lithium ion battery
ActiveCN106356508AImprove consistencyImprove dispersion uniformityCell electrodesSecondary cellsElectrical batterySilicon oxygen
The invention relates to a compound and a preparation method thereof as well as a negative electrode prepared by adopting the compound and a lithium ion battery. The compound comprises silicon, silicon oxide SiOx (x is greater than 0 and is less than or equal to 2) and silicate, wherein positive ion elements of the silicate are reductant elements, and Si, O and the reductant elements in the compound are uniformly distributed. Reductants used in the compound are heated and insulated in the environment of negative pressure, so that SiO steam reacts with reductant steam in the form of a gas phase so as to condense to obtain the compound, and then the compound is further used as a raw material so as to prepare a modified silica negative electrode material. The negative electrode material is suitable for the lithium ion battery, the prepared lithium ion battery has high charge and discharge specific capacity and excellent first coulombic efficiency, the charge capacity is 1447 mAh / g or above, the discharge capacity is 1213 mAh / g or above, and the first coulombic efficiency is 83.8% or above.
Owner:BTR NEW MATERIAL GRP CO LTD
Lithium ion battery cathode plate, lithium ion battery and preparation method of lithium ion battery
ActiveCN103441236AReduce consumptionReduce irreversible capacityFinal product manufactureNon-aqueous electrolyte accumulator electrodesLithium compoundLithium-ion battery
The invention discloses a lithium ion battery cathode plate. The lithium ion battery cathode plate comprises a cathode current collector and a cathode active material layer, wherein the cathode active material layer is distributed on the cathode current collector and contains a cathode active material, a conductive agent, an adhesive agent and a lithium compound; the lithium compound is decomposed to release lithium and gas in the formation and charging processes of a lithium ion battery. The lithium compound serves as a lithium supplementation material, a cathode potential is unchanged, the lithium compound is decomposed only in the formation and charging processes, the gas generated by decomposition can be removed in the formation process, the lithium generated by decomposition is transferred from a cathode to an anode in the charging process, a solid electrolyte inter-phase (SEI) membrane is formed on the anode, and the lithium required by forming of the SEI membrane is supplemented. Therefore, the consumption of cathode lithium ions can be reduced, the irreversible capacity of the lithium ion battery is reduced, and the cycle performance of the lithium ion battery is improved. The invention also discloses the lithium ion battery with the lithium ion battery cathode plate and a preparation method of the lithium ion battery.
Owner:DONGGUAN AMPEREX TECH +1
Method for preparing lithium-enriched manganese-based anode material of nano-oxide-coated lithium ion battery
ActiveCN103441252AReduce irreversible capacityHigh specific capacityMaterial nanotechnologyCell electrodesMass ratioManganese
The invention discloses a method for preparing a nano-oxide-coated lithium-enriched manganese-based anode material of a lithium ion battery, which comprises the following steps: (1) preparing a lithium-enriched manganese-based anode material [Li1+(1-2x) / 3Mn(2-x) / 3Mx]O2; (2) weighing nanoscale metal oxide and the prepared lithium-enriched manganese-based anode material [Li1+(1-2x) / 3Mn(2-x) / 3Mx]O2 according to the mass ratio of (0.1 to 10):(90 to 99.9) and uniformly mixing the nanoscale metal oxide and the prepared lithium-enriched manganese-based anode material; (3) after drying the mixture, heating to a temperature of 400 to 1,000 DEG C at a speed of 0.1 to 10 DEG C per minute, keeping the constant temperature for 2 to 20h, then cooling to the room temperature at a speed of 0.1 to 10 DEG C per minute and grinding to obtain the nano-oxide-coated lithium-enriched manganese-based anode material. The method reduces the first irreversible capacity of the lithium-enriched anode material, improves circulating stability and rate performance of the material, adopts a simple process and is low in cost.
Owner:TIANJIN B&M SCI & TECH
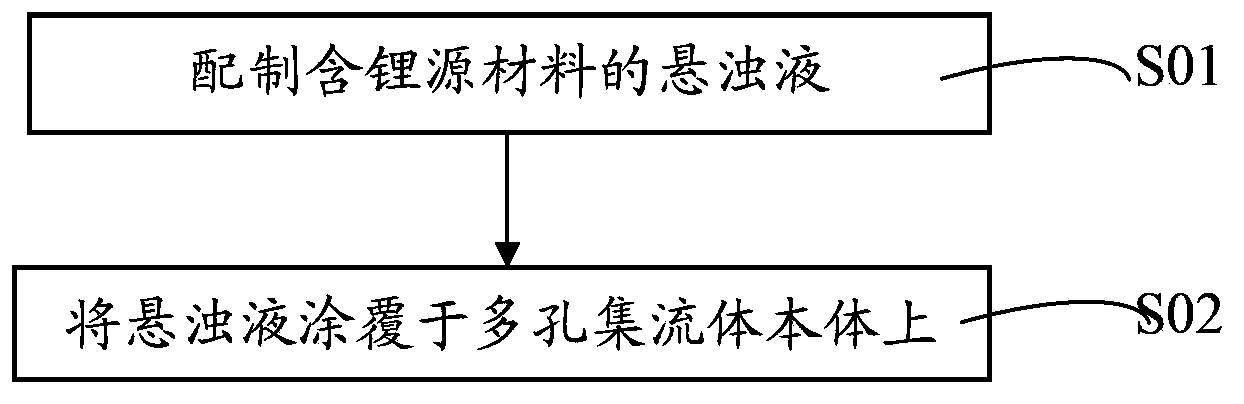
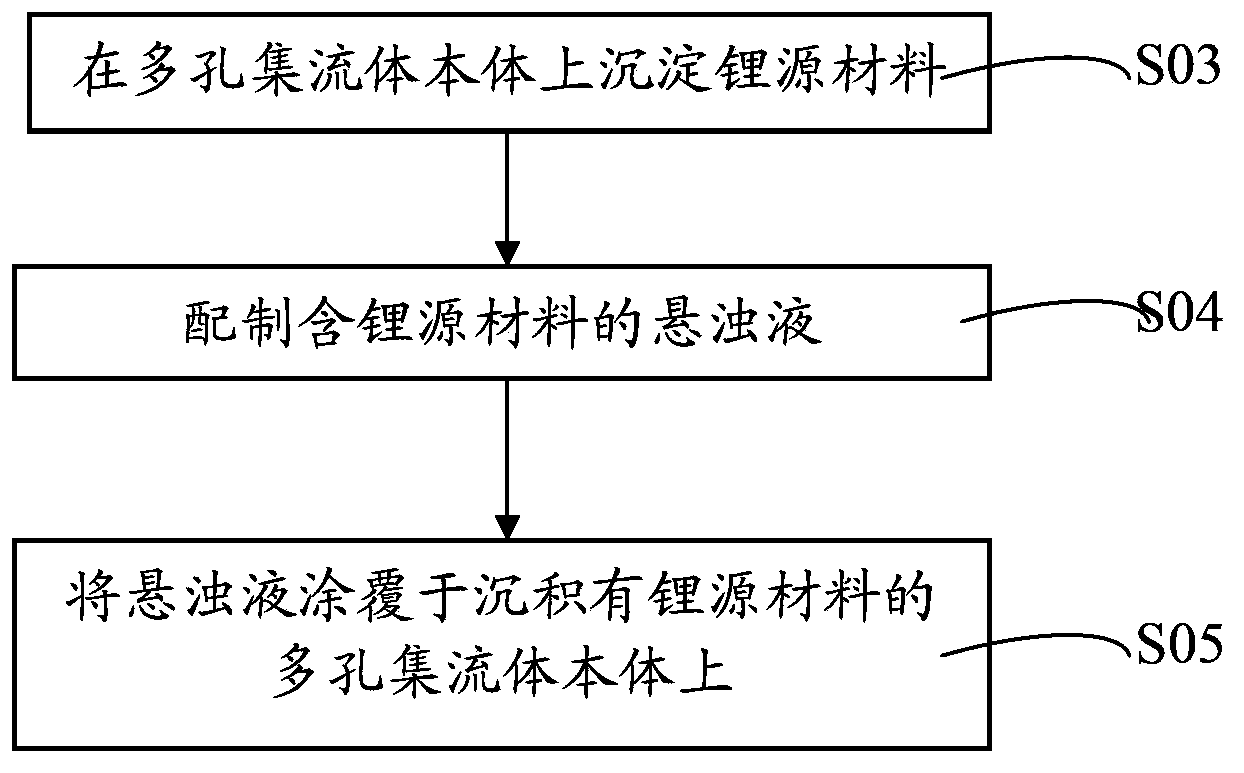
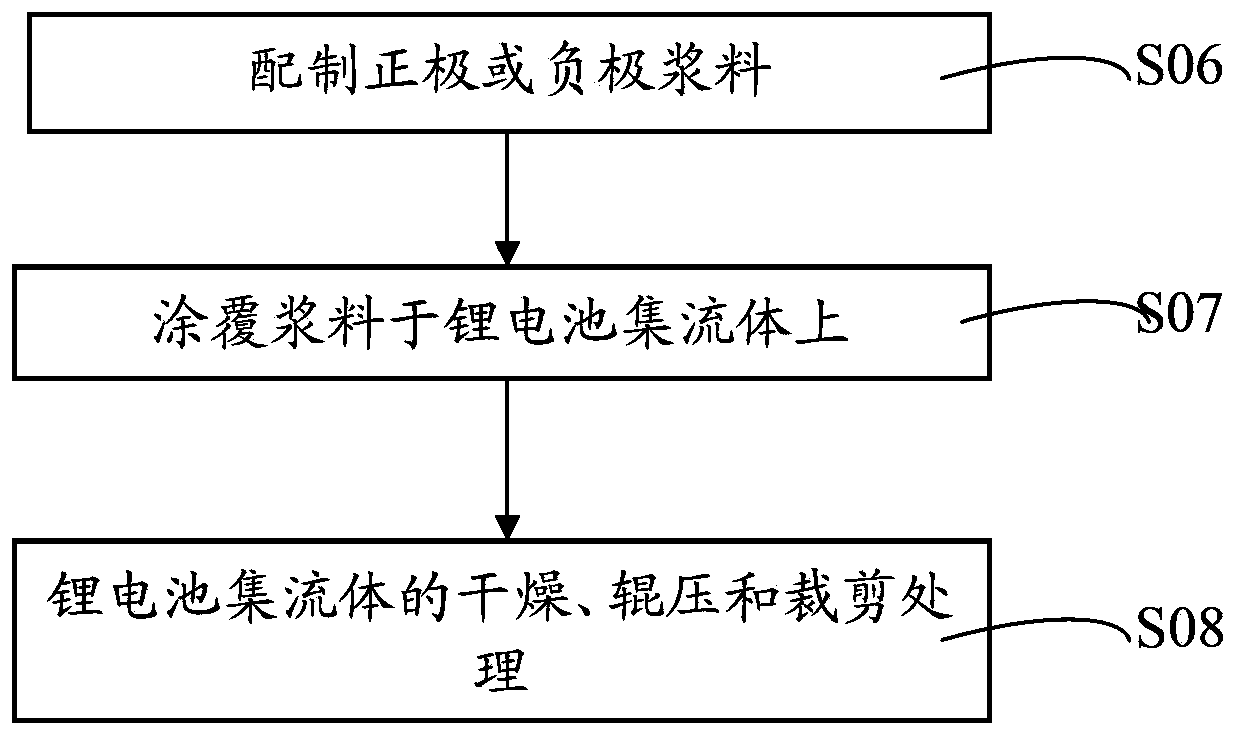
Lithium battery current collector, pole piece, lithium battery, preparation method thereof and application of lithium battery
ActiveCN103794800AEffectively fixedImprove securityFinal product manufactureElectrode carriers/collectorsLithium metalSource material
The invention discloses a lithium battery current collector and a preparation method thereof, a lithium battery pole piece and a preparation method thereof, a lithium battery and a preparation method and application thereof. The lithium battery current collector includes a porous current collector body, which is filled or / and deposited with a lithium source material. The lithium source material is a lithium metal or / and lithium-rich material. The lithium battery pole piece and the lithium battery both contain the lithium battery current collector. The lithium battery current collector provided by the invention enables the lithium source material to be effectively fixed in the current collector body. The lithium battery pole piece containing the lithium battery current collector can make the lithium in the lithium source material ionize during electrochemical activation and completely absorbed by a positive active material in an anode layer or a negative active material in a cathode layer so as to compensate the lithium ion lost in an initial charge / discharge process, thereby reducing the irreversible capacity. Therefore, the lithium battery has high initial coulombic efficiency and capacity as well as safety performance.
Owner:HUAWEI TECH CO LTD
Method for preparing artificial graphite cathode material of lithium ion battery
ActiveCN1691374AEasy to implementGood coating effectElectrode manufacturing processesNon-aqueous electrolyte accumulatorsGraphiteSodium-ion battery
The invention discloses a making method for lithium ion battery negative materials, comprising the steps of: (1) mixing the black lead, clad material and solvent, evacuating, stripping solvent and cladding the natural black lead in the clad materials; (2) polymerizing the materials and the surface of the natural black lead will get the microencapsulation cladding layer; (3) charring or charring the products of step (2) to get the carbon negative materials with the artificial black lead layer coated on the surface, this is the lithium ion battery negative materials. The detecting results of the battery negative materials are: tap density is more than 1.02, specific surface area is lese than 2.0, the first discharging capacity is more than 350mAh / g (non-reversible capacity is less than 25mAh / g), the first charging efficiency is more than 93%, after circulating 460 times, the rest first capacity is more than 90%.
Owner:NINGBO SHANSHAN NEW MATERIAL TECH
Dicarbonate additives for nonaqueous electrolyte rechargeable cells
InactiveUS6174629B1Improve oxidation stabilityImprove dynamic stabilityAlkaline accumulatorsOrganic electrolyte cellsRechargeable cellPhysical chemistry
A lithium ion electrochemical cell having high charge / discharge capacity, long cycle life and exhibiting a reduced first cycle irreversible capacity, is described. The stated benefits are realized by the addition of at least one dicarbonate additive to an electrolyte comprising an alkali metal salt dissolved in a solvent mixture that includes ethylene carbonate, dimethyl carbonate, ethylmethyl carbonate and diethyl carbonate. The preferred additive is an alkyl dicarbonate compound.
Owner:WILSON GREATBATCH LTD
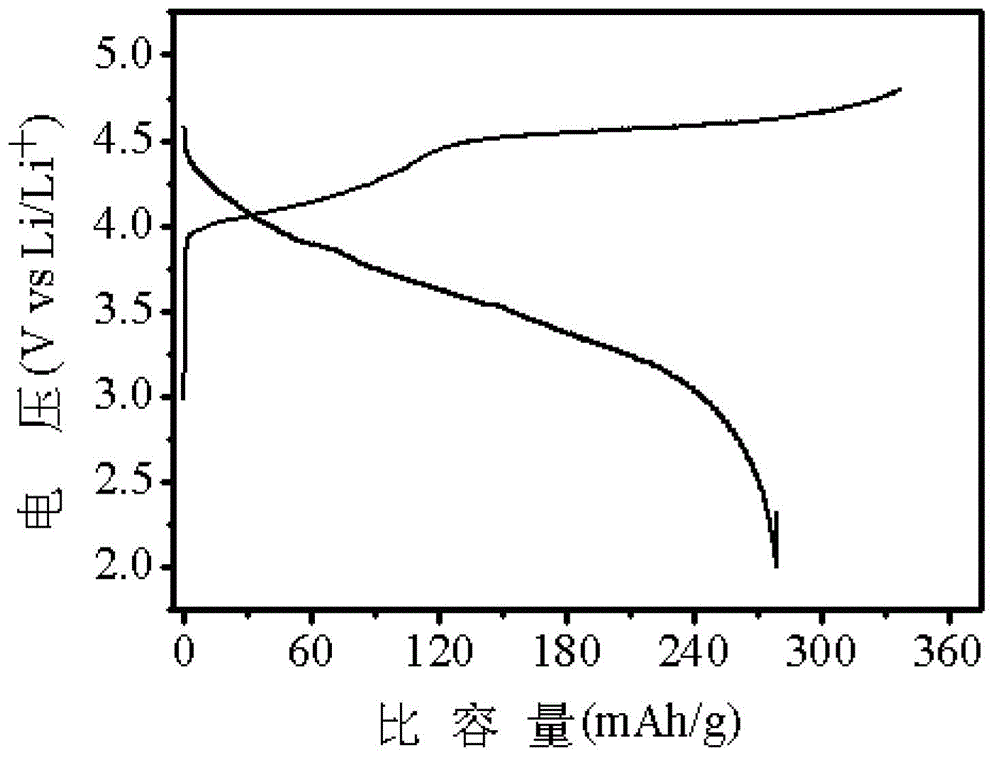
Positive active material for rechargeable lithium battery and rechargeable lithium battery including same
InactiveUS20130171524A1Improve charging effectReduce irreversible capacityNon-aqueous electrolyte accumulator electrodesMetalLithium battery
Disclosed is a positive active material for a rechargeable lithium battery and a rechargeable lithium battery including the positive active material. The positive active material includes a lithiated intercalation compound capable of reversibly intercalating and deintercalating lithium and a metal oxide represented by the following Chemical Formula 1.LixMyM′1-yO4 [Chemical Formula 1]In the above Chemical Formula M, M′, x, and y are the same as defined in the detailed description.The positive active material easily provides lithium needed for the irreversible chemical / physical reaction at a negative electrode during the initial charge reaction, and thus increases charge capacity of a battery, decreases its irreversible capacity, and resultantly improves its cycle life.
Owner:UNIST ACAD IND RES CORP
Lithium ion battery composite diaphragm, preparation method thereof and lithium ion battery
ActiveCN106129315AReduce irreversible capacityImprove first-time efficiencySecondary cellsCell component detailsPolyolefinPhysical chemistry
The invention relates to a lithium ion battery composite diaphragm, a preparation method thereof and a lithium ion battery, and belongs to the technical field of lithium ion batteries. The lithium ion battery composite diaphragm comprises a diaphragm matrix, wherein the diaphragm matrix is a polyolefin microporous membrane or a polyolefin microporous membrane whose surface is coated with a ceramic layer; a surface of the diaphragm matrix is coated with a lithium supplementing layer; and the lithium supplementing layer contains lithium powder. According to the lithium ion battery composite diaphragm, the surface of the diaphragm is provided with the lithium supplementing layer, lithium required for forming an SEI (Solid Electrolyte Interface) film is filled up, supplementationis provided for lithium ions consumed by formation of the SEI film on a surface of a negative pole piece, the irreversible capacity of the lithium ion battery is reduced, and the initial efficiency, the cycle performance and the energy density of the lithium ion battery are improved.
Owner:LUOYANG LIRONG NEW ENERGY TECH
Electrodes including novel binders and methods of making and using the same
InactiveCN101663782AReduce capacity lossReduce irreversible capacitySecondary cellsElectrode collector coatingLithiumFluoropolymer
Provided are electrode compositions for lithium-ion electrochemical cells that include novel binders. The novel binders include lithium polysalts of carboxylic and sulfonic acids, lithium salts of copolymers of acids, lithium polysulfonate fluoropolymers, a cured phenolic resin, cured glucose, and combinations thereof.
Owner:3M INNOVATIVE PROPERTIES CO
Carbon nano tube/graphene composite negative pole material, preparation method thereof and lithium battery
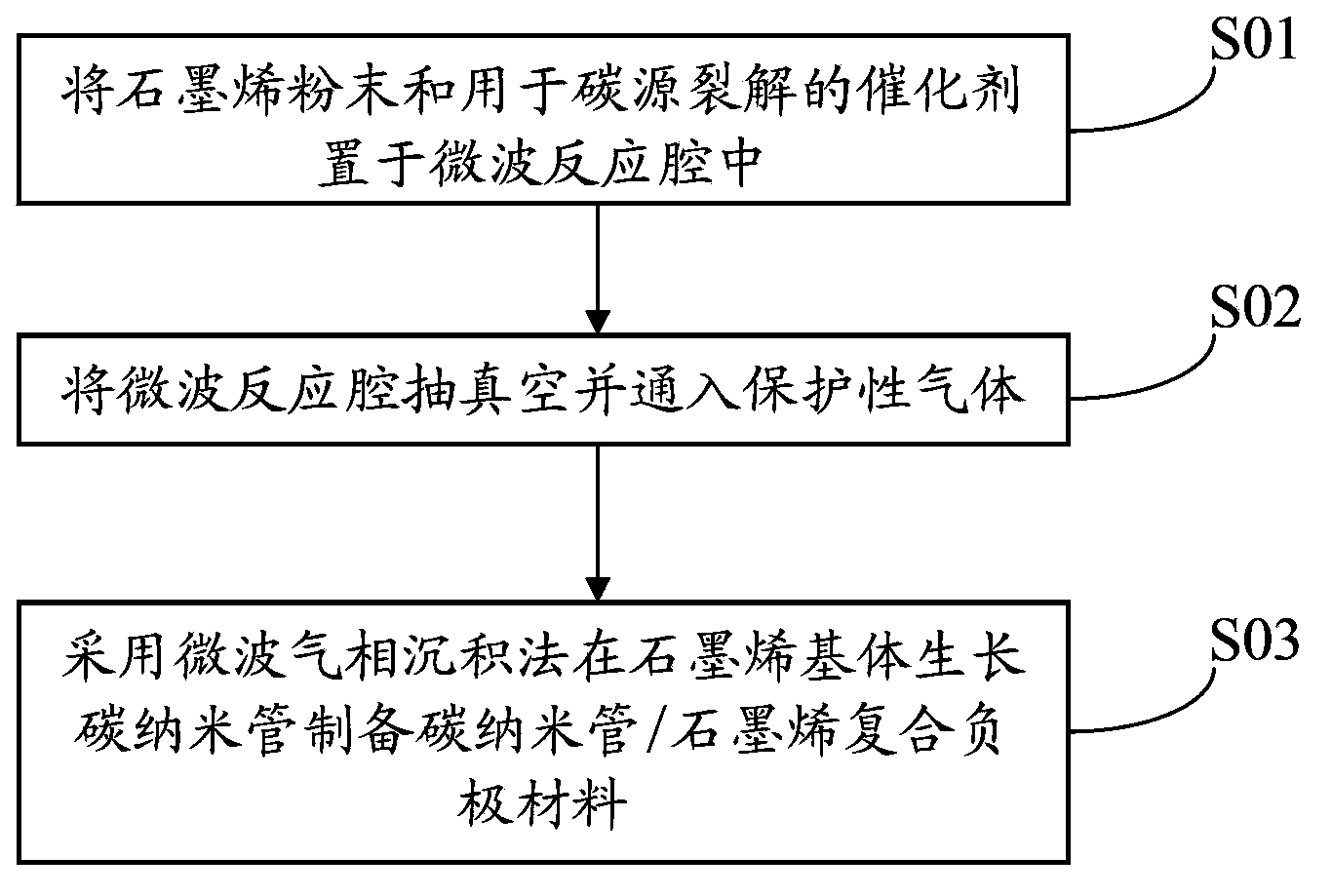
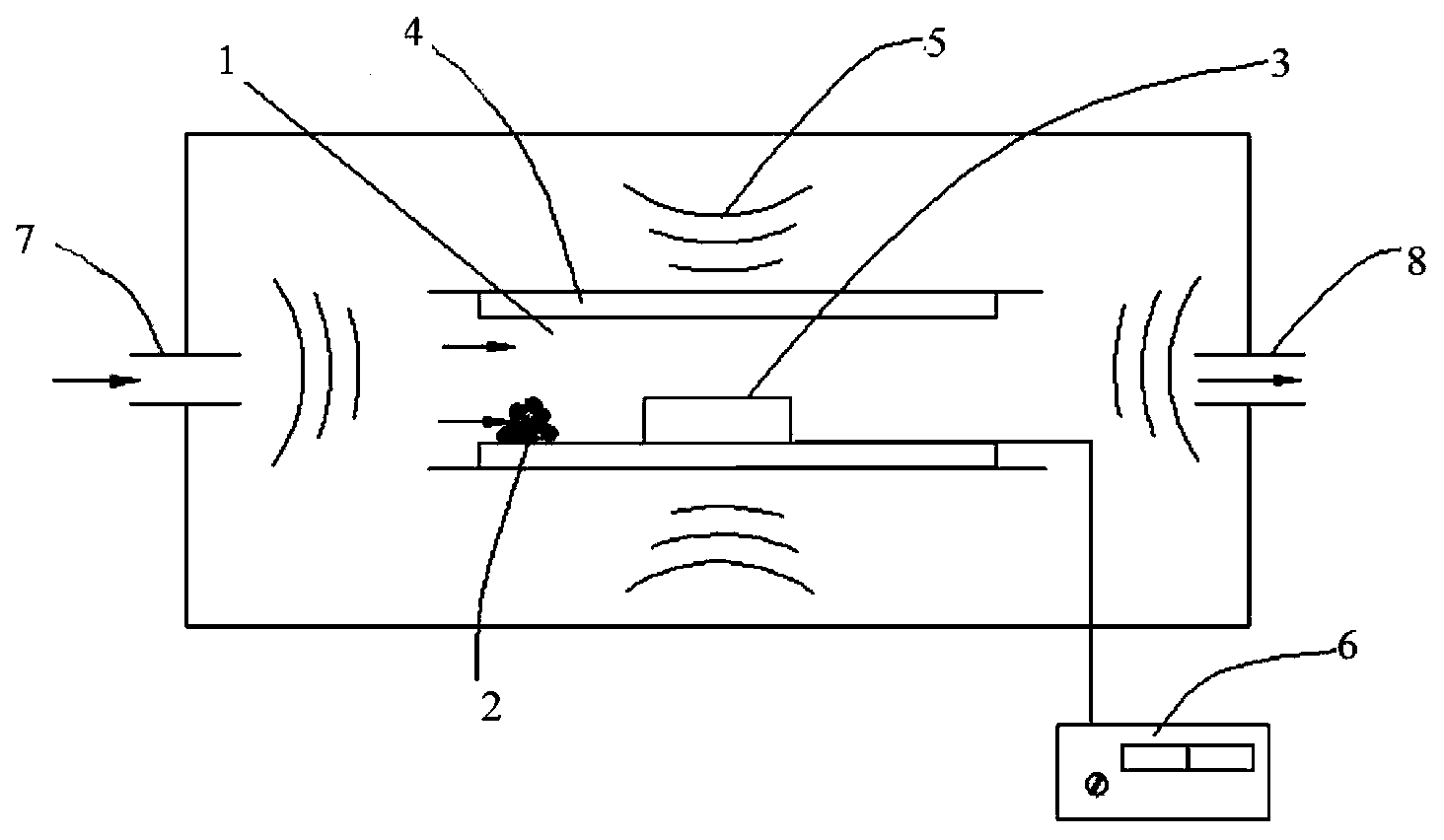
The invention discloses a carbon nano tube / graphene composite negative pole material, a preparation method thereof and a lithium battery. The preparation method of the carbon nano tube / graphene composite negative pole material comprises the steps of placing graphene powder and a catalyst for carbon source splitting decomposition in a microwave reaction cavity, vacuumizing the microwave reaction cavity and leading protective gas into the microwave reaction cavity and using a microwave vapor deposition method to prepare the carbon nano tube / graphene composite negative pole material on a graphene base body growing carbon nano tube. A negative pole of the lithium battery contains the carbon nano tube / graphene composite negative pole material. The preparation method of the carbon nano tube / graphene composite negative pole material adopts the microwave vapor deposition method to perform in-situ preparation of the carbon nano tube / graphene composite negative pole material, does not needs a pre-synthesis process, reduces the production cost, adopts microwave heating and is efficient, low in energy consumption and short in production period. Due to the fact that the lithium battery contains the carbon nano tube / graphene composite negative pole material, embedding and taking-out of lithium are facilitated, the inreversible capacity of first-time charging and discharging is reduced, and the lithium battery is good in safety and high in power.
Owner:RESEARCH INSTITUTE OF TSINGHUA UNIVERSITY IN SHENZHEN
Material for electrode of power storage device, power storage device, and electrical appliance
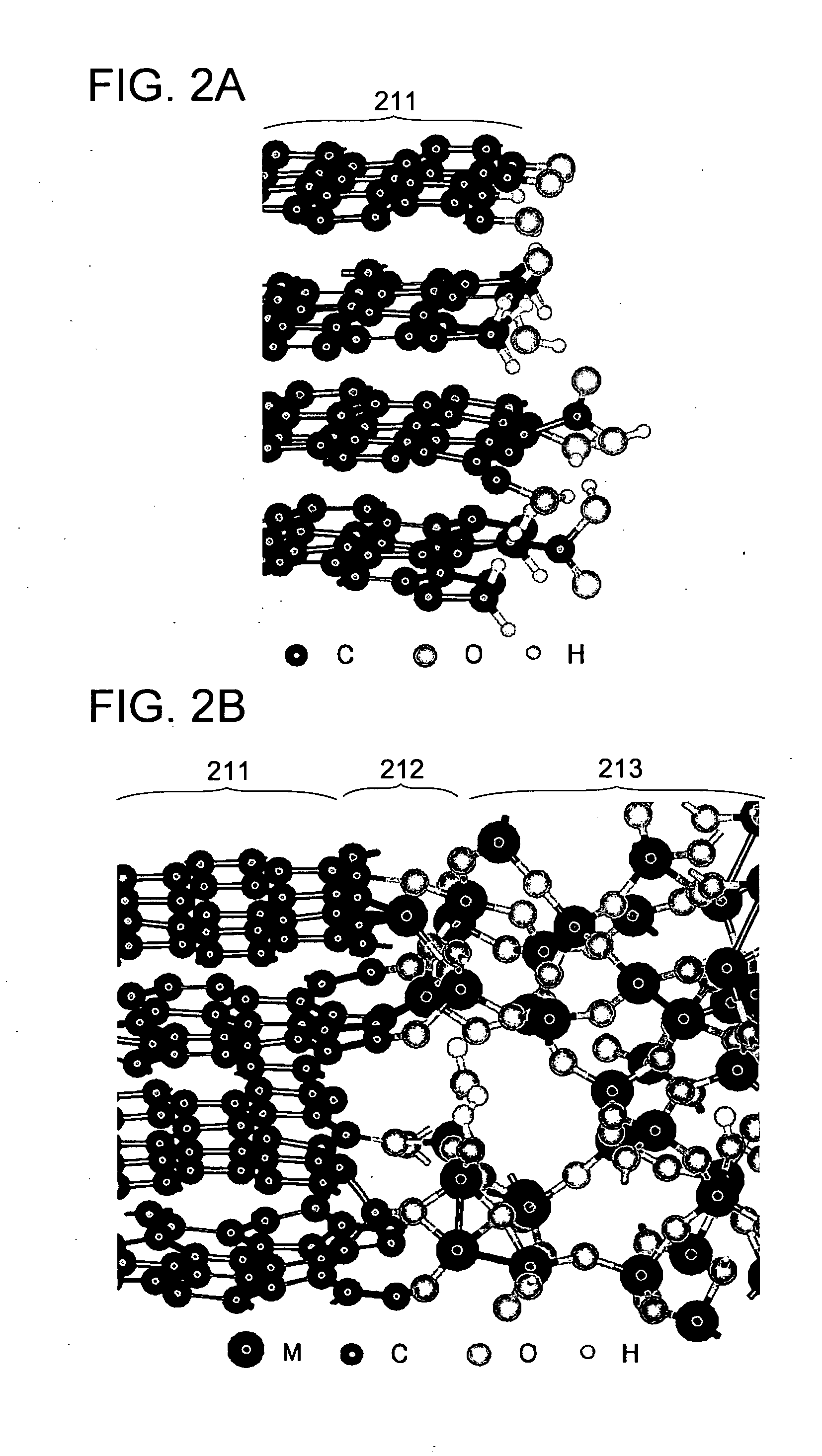
ActiveUS20140099554A1Reduce irreversible capacityReduce in quantityAluminium silicatesLiquid electrolytic capacitorsReductive decompositionSilicon
To improve the reliability of a power storage device. A granular active material including carbon is used, and a net-like structure is formed on part of a surface of the granular active material. In the net-like structure, a carbon atom included in the granular active material is bonded to a silicon atom or a metal atom through an oxygen atom. Formation of the net-like structure suppresses reductive decomposition of an electrolyte solution, leading to a reduction in irreversible capacity. A power storage device using the above active material has high cycle performance and high reliability.
Owner:SEMICON ENERGY LAB CO LTD
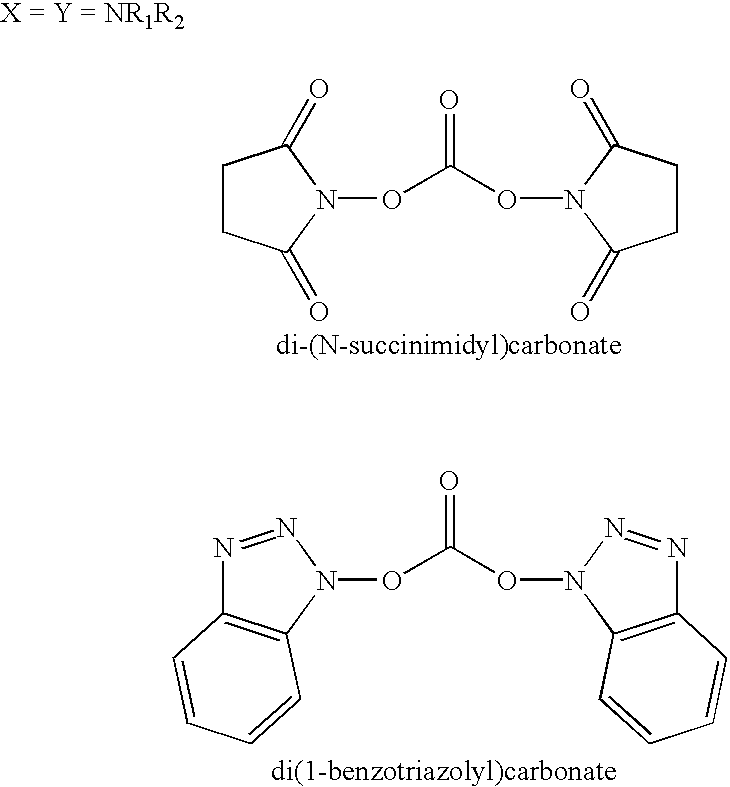
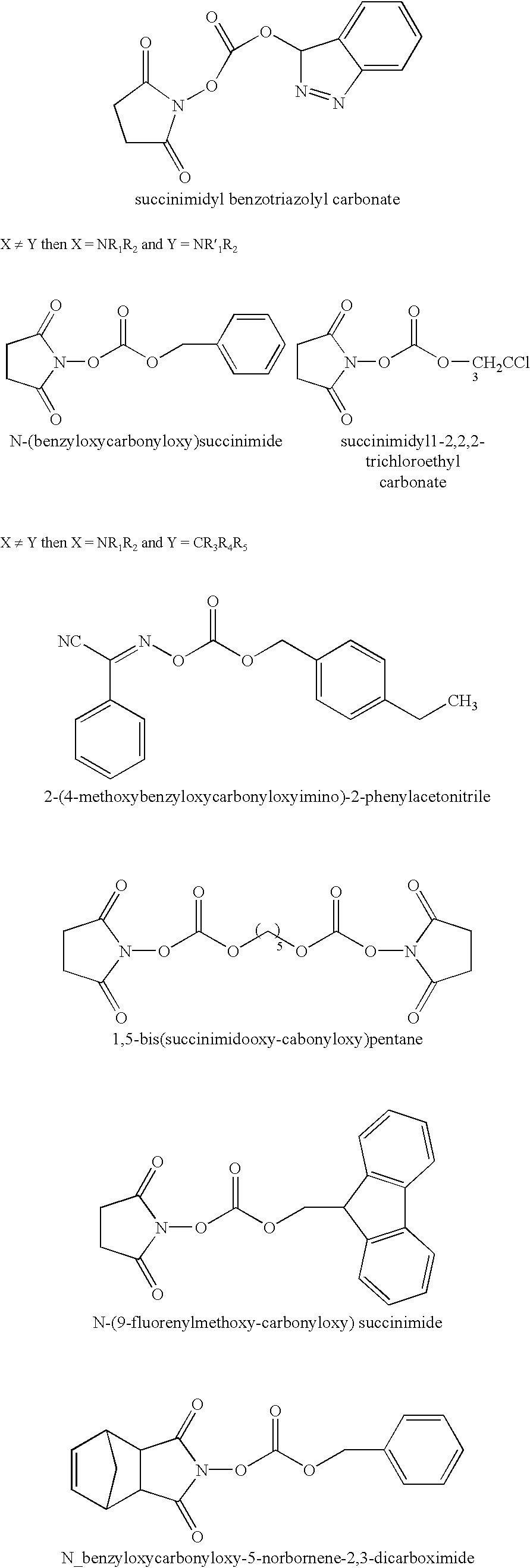
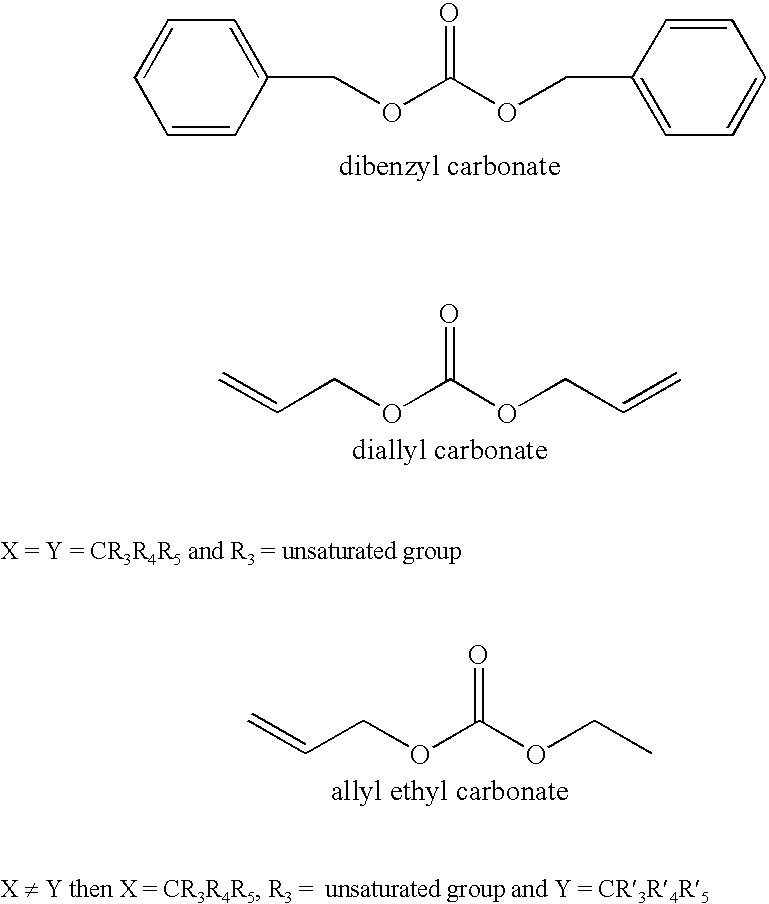
Organic carbonate additives for nonaqueous electrolyte rechargeable electrochemical cells
InactiveUS20030129500A1Improve oxidation stabilityImprove dynamic stabilityElectrode carriers/collectorsOrganic electrolyte cellsMethyl carbonateSolvent
A lithium ion electrochemical cell having high charge / discharge capacity, long cycle life and exhibiting a reduced first cycle irreversible capacity, is described. The stated benefits are realized by the addition of at least one carbonate additive to an electrolyte comprising an alkali metal salt dissolved in a solvent mixture including ethylene carbonate, dimethyl carbonate, ethyl methyl carbonate and diethyl carbonate. The preferred additive is either a linear or cyclic carbonate containing covalent O-X and O-Y bonds on opposite sides of a carbonyl group wherein at least one of the O-X and the O-Y bonds has a dissociation energy less than about 80 kcal / mole.
Owner:WILSON GREATBATCH LTD
Application of high-molecular coating in aluminium negative electrode, aluminium negative electrode, preparation method thereof and secondary battery
ActiveCN108155363AAvoid erosionAvoid reactionCell electrodesSecondary cellsElectrochemistryElectrochemical energy storage
The invention discloses application of a high-molecular coating in an aluminium negative electrode, the aluminium negative electrode, a preparation method thereof and a secondary battery and relates to the field of electrochemical energy storage devices. For the application of the high-molecular coating in the aluminium negative electrode, the aluminium negative electrode is used as a negative current collector and a negative active material simultaneously. The aluminium negative electrode is used as the negative current collector and the negative active material simultaneously and is coated with the high-molecular coating; the secondary battery comprises the aluminium negative electrode. The application disclosed by the invention has the beneficial effects that the problems that the volume of the aluminium negative electrode used as the negative current collector and the negative active material is expanded and the capacity is attenuated due to an unstable solid electrolyte membrane are relieved; after the high-molecular coating is applied on the aluminium negative electrode, electrolytic solution and the aluminium negative electrode can be effectively isolated, the aluminium negative electrode can be prevented from being eroded and reacted, the coulombic efficiency is effectively improved, the irreversible capacity can be reduced, the cyclic stability of the battery can be improved, simultaneously certain role is played in inhibiting powdering of the aluminium negative electrode in the process of volume expansion, and the integrity of the aluminium negative electrode structure can be ensured.
Owner:SHENZHEN INST OF ADVANCED TECH
Lithium-rich manganese-based anode material and method for manufacturing same
ActiveCN102916169AImprove featuresUniform structureCell electrodesSecondary cellsPtru catalystActive agent
The invention discloses a lithium-rich manganese-based anode material and a method for manufacturing the same. The method includes steps of (a), providing mixed solution containing lithium compounds, nickel compounds and manganese compounds, optional titanium compounds, optional iron compounds, optional cobalt compounds or an optional combination of the titanium compounds, the ion compounds and the cobalt compounds; (b), adding complexing agents, catalysts and surfactants into the mixed solution to form pre-coagulated substances; and (c), calcining the pre-coagulated substances to obtain the lithium-rich manganese-based anode material Li[LixNiaMnbM1-a-b-x]O2 or a combination of lithium-rich manganese-based anode materials. The complexing agents, the catalysts and the surfactants are used for forming the pre-coagulated substances, the complexing agents contain resorcinol and formaldehyde, in the molecular formula of the lithium-rich manganese-based anode material, the M represents Ti, Fe, Co or a combination of the Ti, the Fe and the Co, the x is larger than 0 and is smaller than or equal to 0.4, the a is larger than 0 and is smaller than or equal to 0.5, the b is larger than or equal to 0.33 and smaller than or equal to 0.6, and a result of 1-a-b-x is larger than or equal to 0. The lithium-rich manganese-based anode material is of a multi-channel porous structure, is small in grain size, uniform in grain distribution, advanced in porosity and stable in electrochemical performance.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Anode for nonaqueous secondary electrochemical cells
InactiveUS20030003362A1Improve performanceAvoid corrosionSilver accumulatorsElectrode carriers/collectorsGraphiteMetallic sulfide
The negative electrode or anode for a secondary electrochemical cell comprising a mixture of graphite or "hairy carbon" and a lithiated metal oxide, a lithiated mixed metal oxide or a lithiated metal sulfide, and preferably a lithiated metal vanadium oxide, is described. A most preferred formulation is graphite mixed with lithiated silver vanadium oxide or lithiated copper silver vanadium oxide.
Owner:WILSON GREATBATCH LTD
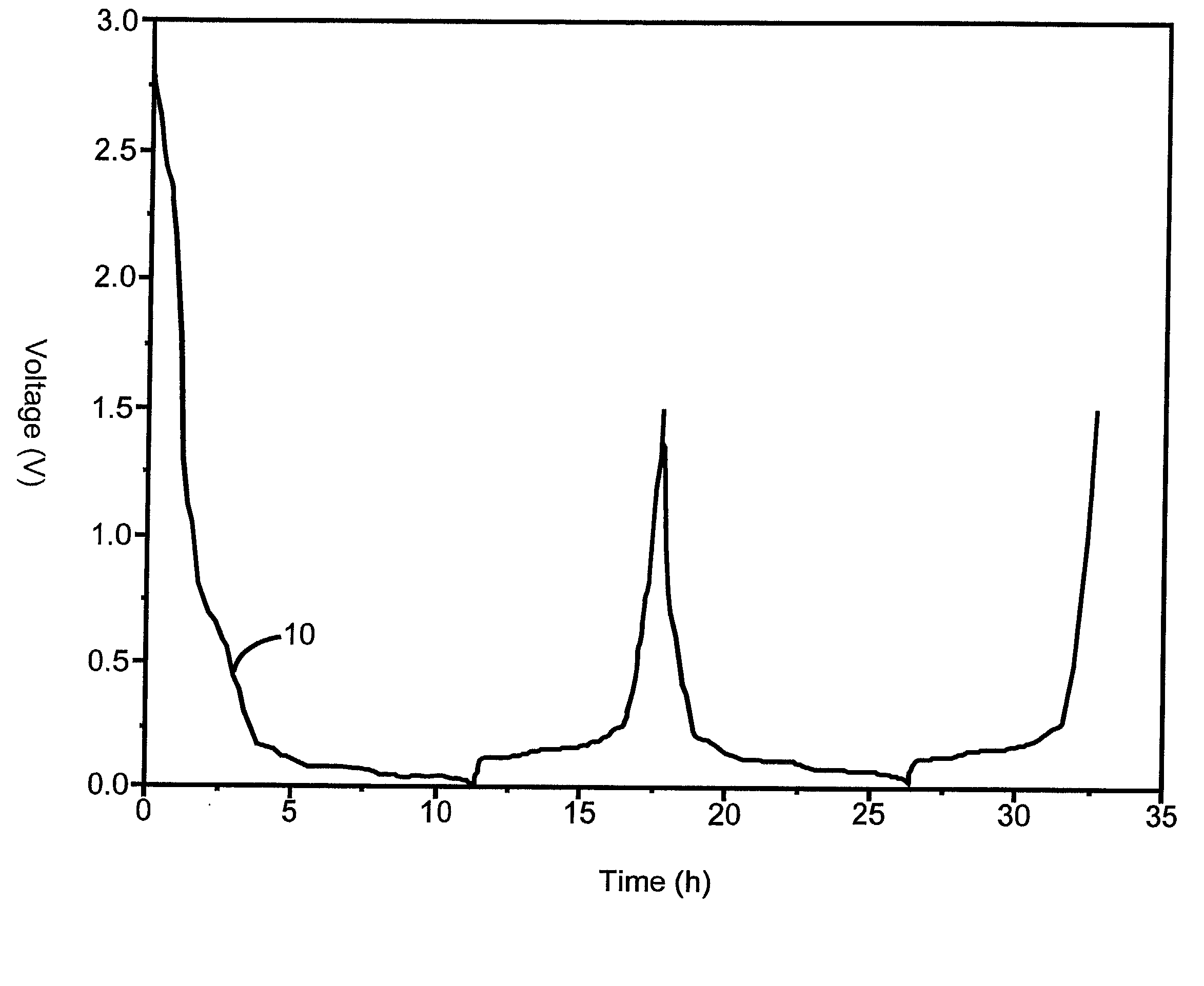
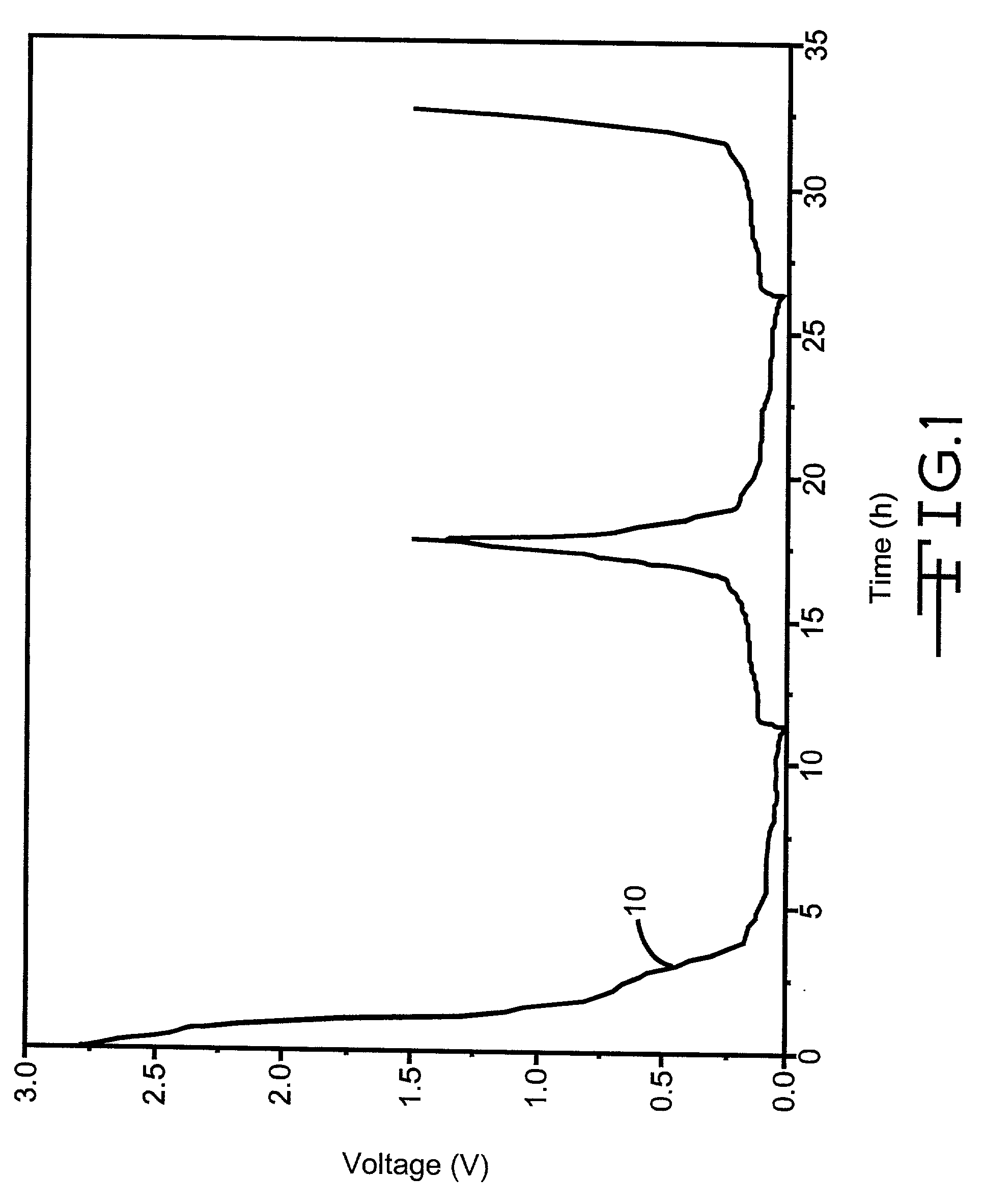
Negative electrode material for lithium ion secondary battery
InactiveUS20050158550A1Good effectTrimming is irreversibleSynthetic resin layered productsCellulosic plastic layered productsLaser scatteringLithium
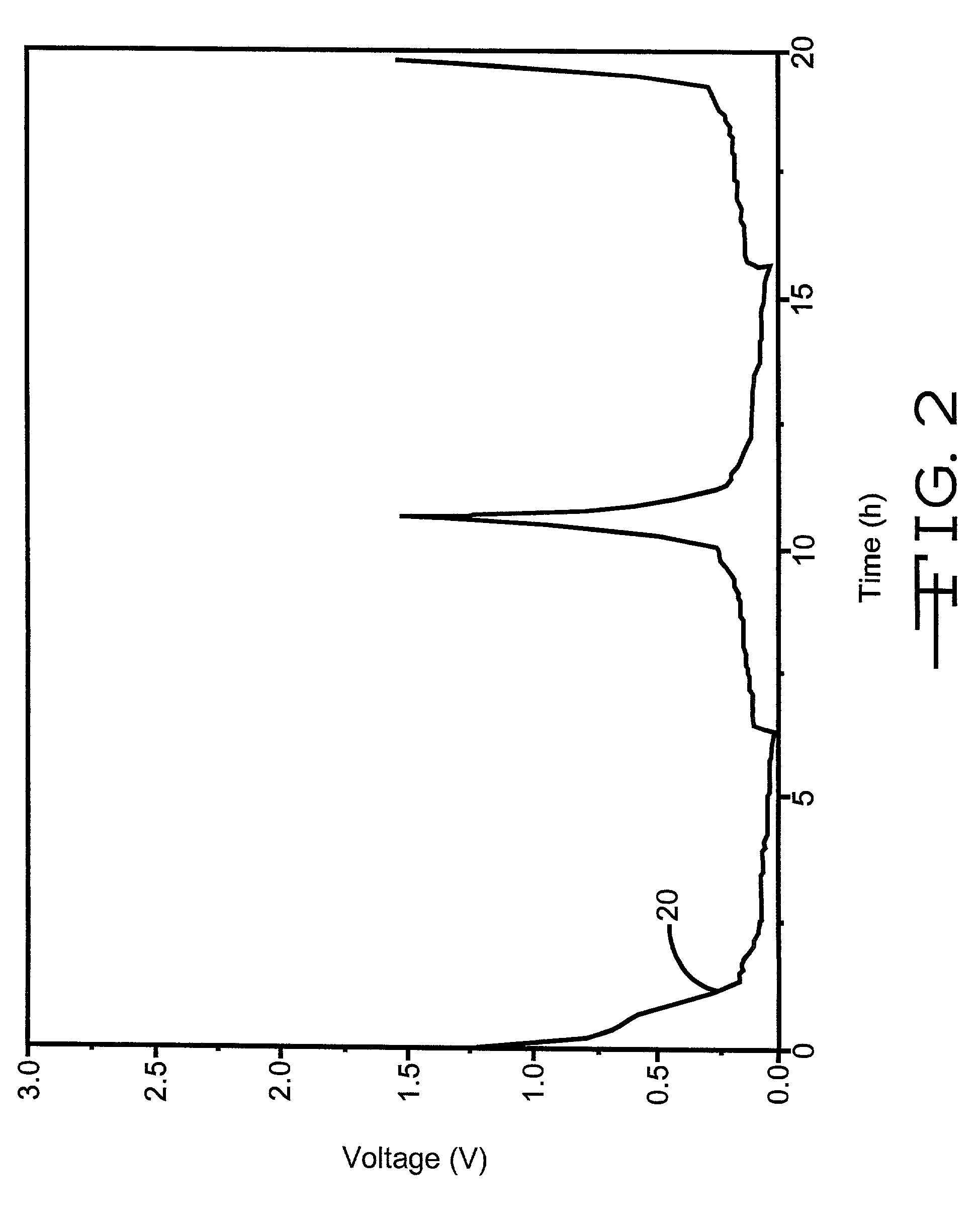
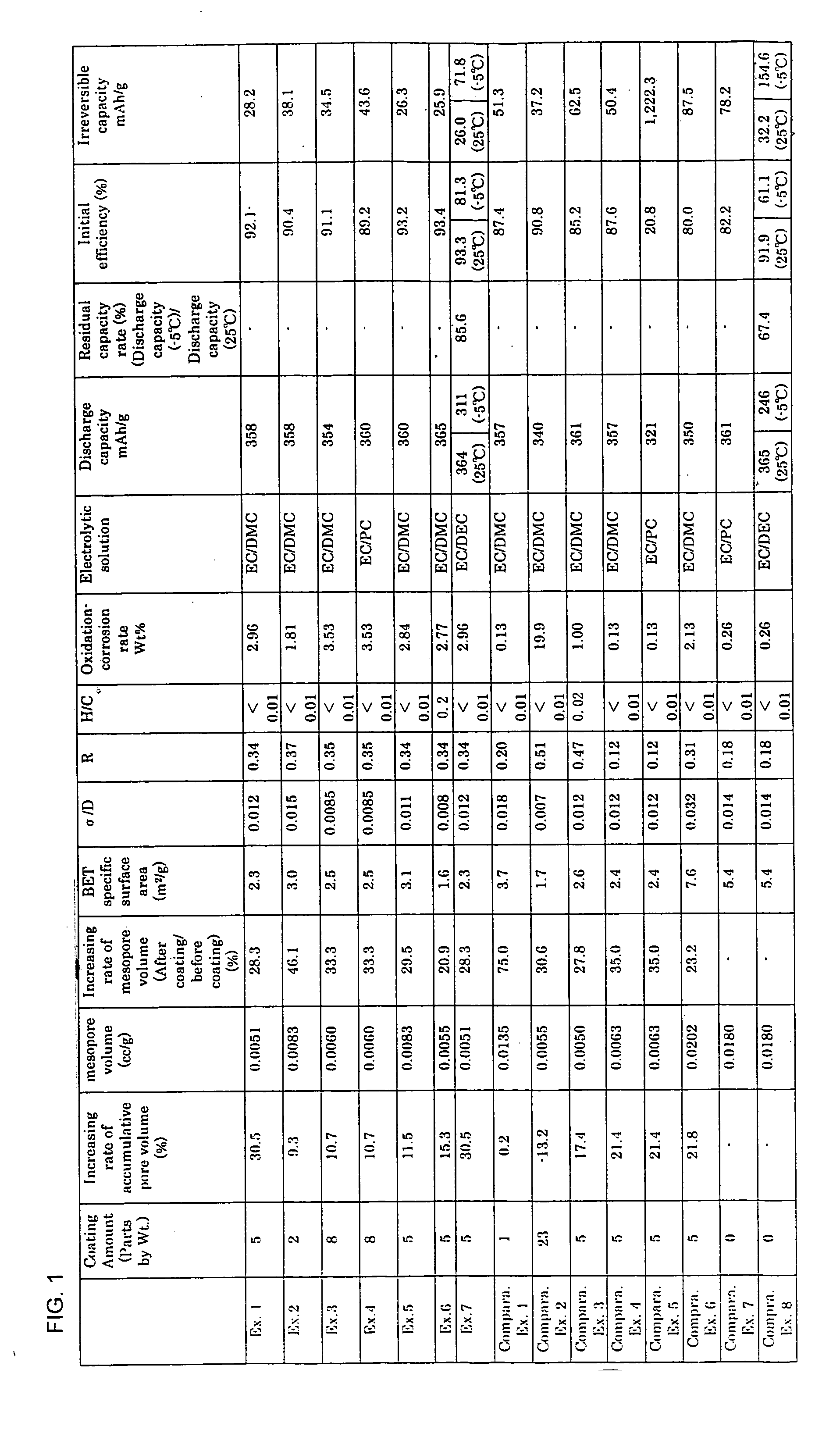
The invention provides an anode material for lithium ion secondary battery using a coated graphite powder as a raw material. The coated graphite powder is coated with carbonized material of thermoplastic resin of a carbonization yield of not more than 20 wt % in a proportion of not more than 10 parts by weight the carbonized material per 100 parts by weight graphite powder. The graphite powder as coated with thermoplastic resin increases 5% or more in accumulative pore volume of the graphite powder having a pore size of 0.012 μm to 40 μm as measured by a mercury porosimeter method, as compared with the graphite powder before coated with the thermoplastic resin. The coated graphite powder has a mesopore volume defined by IUPAC of 0.01 cc / g or less as calculated with the BJH method as viewed from desorption isotherm, which is also equal to 60% or less of the pore volume of the graphite powder before coated with the thermoplastic resin, an average particle size ranging from 10 μm to 50 μm, as measured by a laser-scattering-particle-size-distribution measuring device, and a ratio of standard deviation to the average particle size (σ / D) of 0.02 or less.
Owner:TOYO TANSO KK
Negative electrode material for non-aqueous electrolyte secondary battery, negative electrode for non-aqueous electrolyte secondary battery and method of producing the same, and non-aqueous electrolyte secondary battery
ActiveUS20160351947A1Excellent resistance to an organic solvent and a water solventReduce irreversible capacityElectrode thermal treatmentFinal product manufactureLithium compoundBattery capacity
The present invention is a negative electrode material for a non-aqueous electrolyte secondary battery, including negative electrode active material particles containing a silicon compound expressed by SiOx, where 0.5≦x≦1.6, the silicon compound containing in its interior a lithium compound and one or more ions selected from Group 1 metal ions, Group 2 metal ions, and substitutable ammonium ions. This negative electrode material for a non-aqueous electrolyte secondary battery can increase the battery capacity and improve the cycle performance and the battery initial efficiency.
Owner:SHIN ETSU CHEM IND CO LTD
Lithium ion battery
InactiveUS20100092869A1Good rate characteristicsReduce resistanceCell electrodesOrganic electrolyte cellsDecompositionPhysical chemistry
To provide high energy density, good cycle properties and rate characteristics and long-term safety of a lithium ion battery containing at least an ionic liquid and a lithium salt. The above problems are solved by suppressing reduction and decomposition of the ionic liquid on an anode by using a graphite coated with an amorphous carbon or onto which an amorphous carbon is deposited as an anode active material.
Owner:NEC ENERGY DEVICES LTD
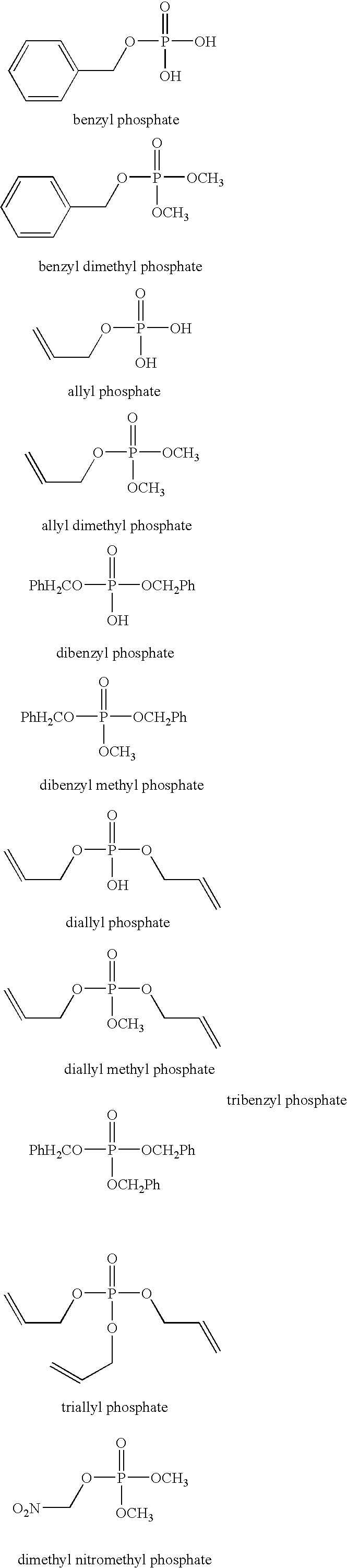
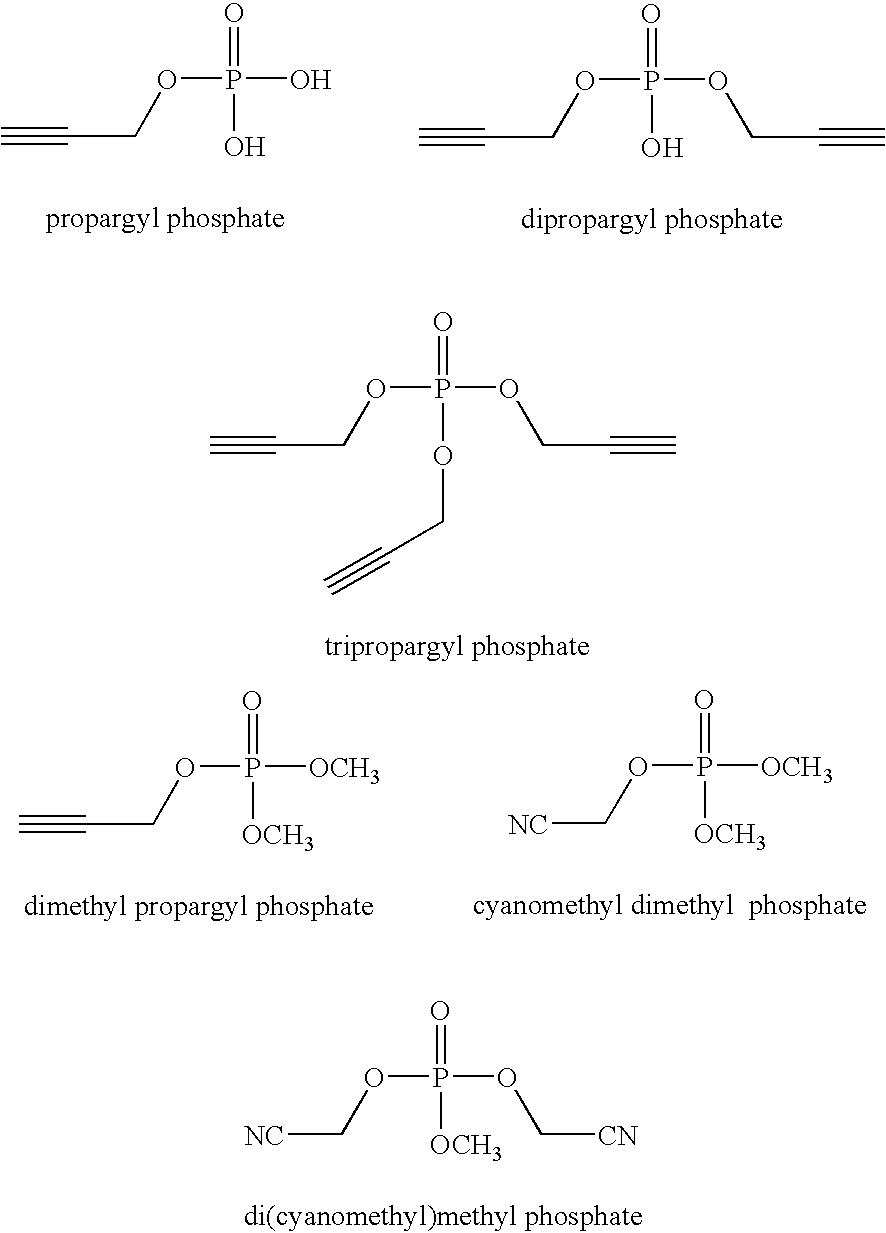
Phosphate additives for nonaqueous electrolyte rechargeable electrochemical cells
InactiveUS6919141B2Acceptable cycling efficienciesReduce the temperatureOrganic electrolyte cellsSecondary cellsLithiumHydrogen
A lithium ion electrochemical cell having high charge / discharge capacity, long cycle life and exhibiting a reduced first cycle irreversible capacity, is described. The stated benefits are realized by the addition of at least one phosphate additive having the formula: (R1O)P(═O) (OR2) (OR3) and wherein R1, R2 and R3 are the same or different, wherein at least one, but not all three, of the R groups is hydrogen, or at least one of the R groups has at least 3 carbon atoms and contains an sp or sp2 hybridized carbon atom bonded to an sp3 hybridized carbon atom bonded to the oxygen atom bonded to the phosphorous atom.
Owner:CAPCHEM TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com