Electrodes including novel binders and methods of making and using the same
A technology of binder and electrode composition, which is applied in the direction of electrode manufacturing, battery electrodes, electrode collector coating, etc., can solve the problem of irreversible loss of battery capacity, and achieve the reduction of irreversible capacity and attenuation, and the loss of irreversible capacity in the first cycle Effect of reducing and improving cycle life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
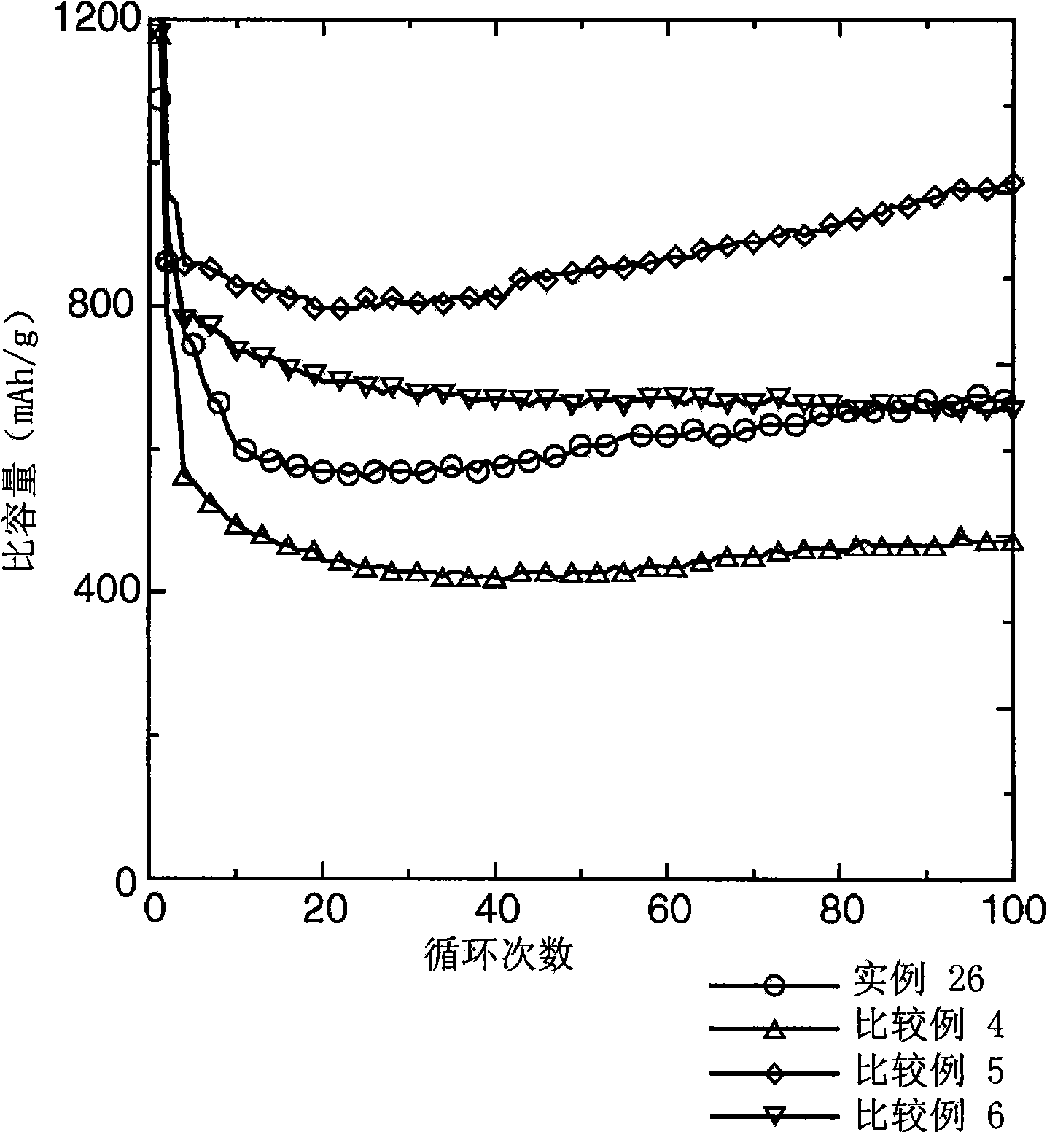
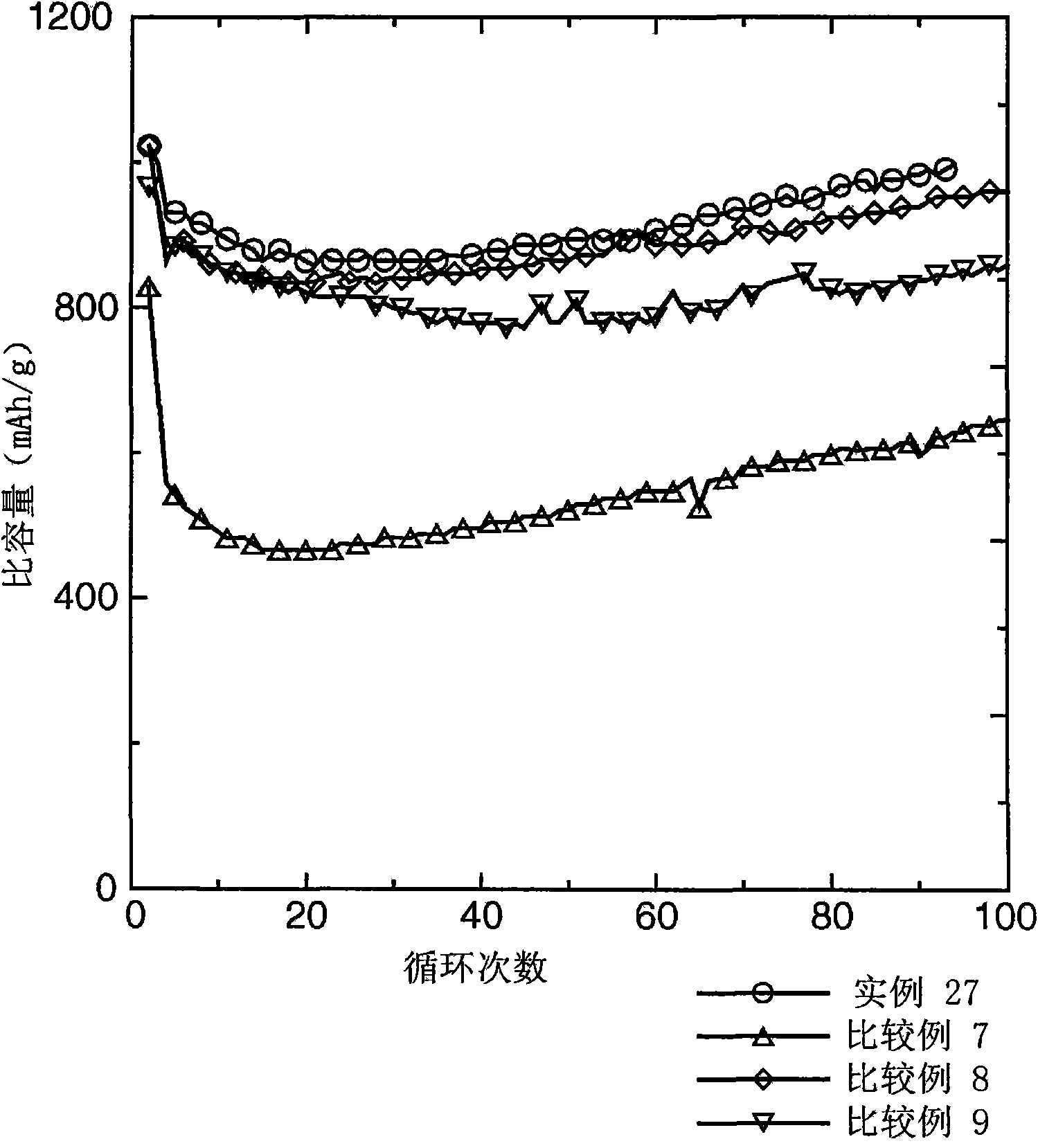
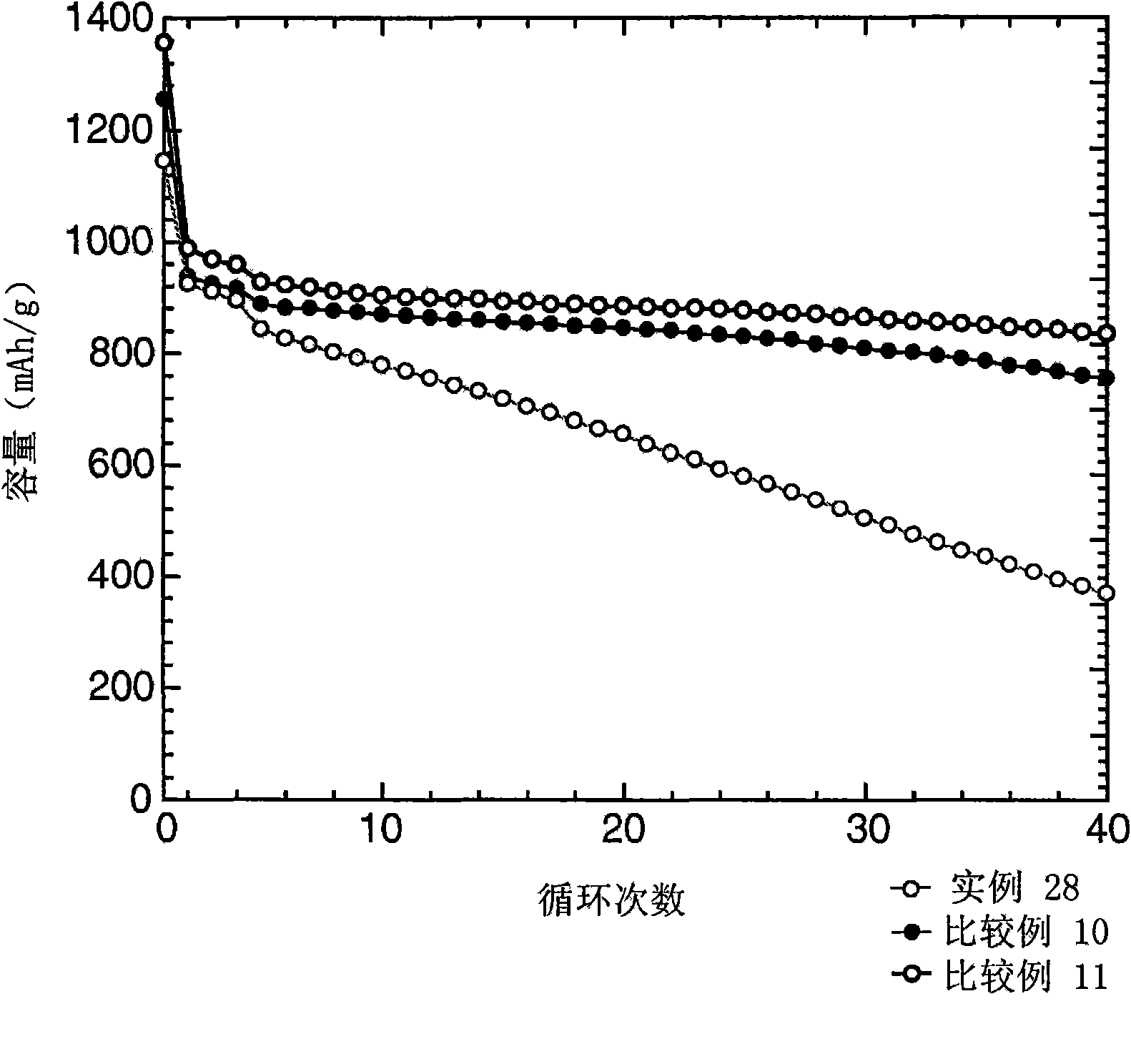
Examples
preparation example 1
[0072] Preparation Example 1-Preparation of Si 70 Fe 10 Ti 10 C 10 alloy
[0073] Si 70 Fe 10 Ti 10 The preparation was as follows: silicon block (65.461 g) (Alfa Aesar / 99.999%, Ward Hill, MS), iron sheet (18.596 g) (Alfa Aesar / 99.97%) and titanium sponge (15.943 g) (Alfa Aesar / 99.97%) were melted in an electric arc furnace. / 99.7%). Si 70 Fe 10 Ti 10 The alloy ingot was broken into small pieces and processed in a hammer mill to prepare alloy powder particles having an average particle size of 150 μm.
[0074] by (above) Si 70 Fe 10 Ti 10 Alloy powder and graphite (TIMREX SFG44, TimCal GmbH, Baudio, Switzerland), prepared Si by reactive ball milling in a high dynamic ball mill (SIMOLOYER, CM20-201m, Zoz GmbH, Wenden, Germany) 70 Fe 10 Ti 10 C 10 alloy. Si 70 Fe 10 Ti 10 1.4423kg of alloy powder samples, 0.0577kg of graphite and 25kg of chromium steel balls with a diameter of 4.76mm are loaded into the mill. The grinder was run for 180 cyc...
preparation example 2-S
[0075] Preparation 2-Si 66.4 Fe 11.2 Ti 11.2 C 11.2 Alloy powder
[0076] Si 74.8 Fe 12.6 Ti 12.6 The alloy composition was prepared as follows: silicon block (123.31 g) (Alfa Aesar / 99.999%, Ward Hill, MS), iron sheet (41.29 g) (Alfa Aesar / 99.97%) and titanium sponge (35.40 g) were melted in an electric arc furnace. ) (Alfa Aesar / 99.7%). The alloy ingot was broken into small pieces and processed in a hammer mill to produce alloy powder particles of approximately 150 μm.
[0077] by Si 74.8 Fe 12.6 Ti 12.6 Alloy powder (2.872 g) and graphite (0.128 g) (TIMREXSFG44, TimCal Ltd., Bodio, Switzerland) were processed in a Spex mill with sixteen tungsten carbide balls (3.2 mm diameter) (USA, NJ) under an argon atmosphere. Si was prepared by reactive ball milling in Tuchen's SpexCERTIPREP Group for one hour 66.4 Fe 11.2 Ti 11.2 C 11.2 .
preparation example 3
[0078] Preparation example 3-preparation of lithium polyacrylate
[0079] Lithium polyacrylate was prepared by adding an aqueous lithium hydroxide solution to an aqueous poly(acrylic acid) solution. Different molar ratios were used for lithium hydroxide to carboxylic acid groups. Typically, a 20% by weight lithium hydroxide aqueous solution and a 34% by weight poly(acrylic acid) aqueous solution are used. Deionized water was added to bring the final lithium polyacrylate solution to 10% solids by weight. Poly(acrylic acid) at 100,000 (Mw) and 250,000 (Mw) was obtained as an aqueous solution from Aldrich Chemical Company, Milwaukee, Wisconsin. A 65% by weight lithium polyacrylate sample neutralized by LiOH was prepared by adding 185.56 g of deionized water, 60.41 g of a 20% by weight lithium hydroxide solution, and 100 g of a poly(acrylic acid) (PAA) solution (34% by weight in water) to give 100,000Mw and 250,000Mw. The result was a 64% neutralized lithium polyacrylate sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com