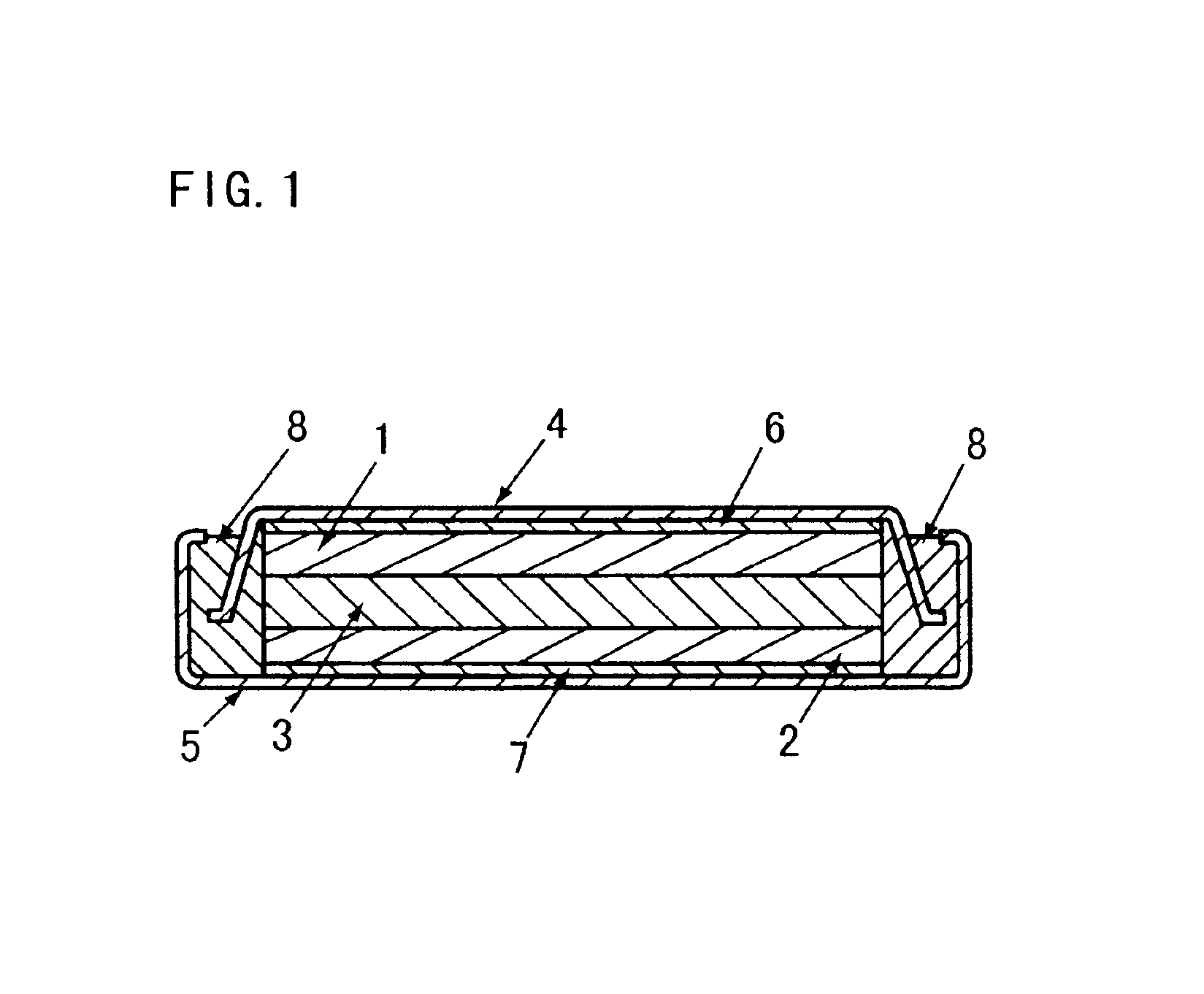
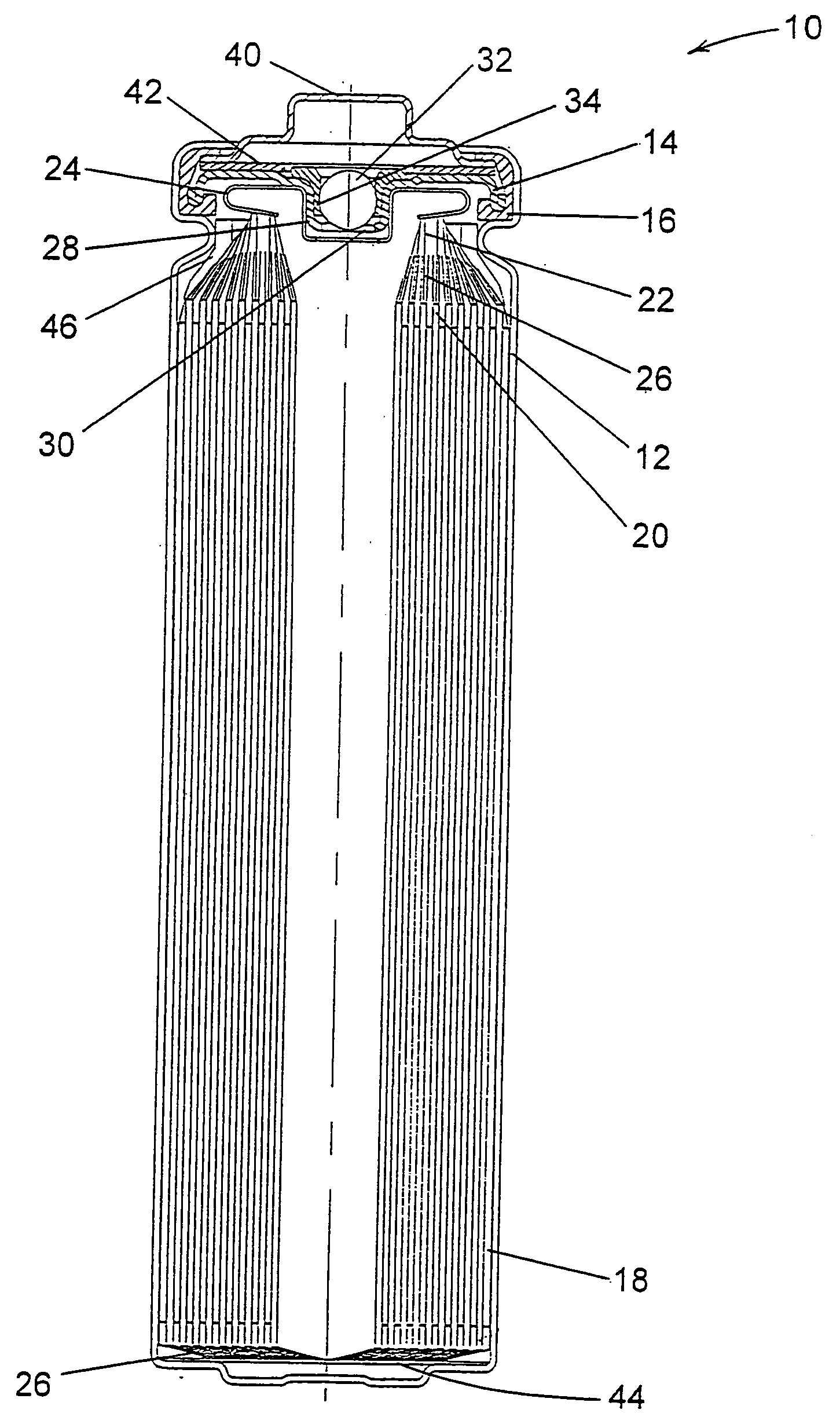
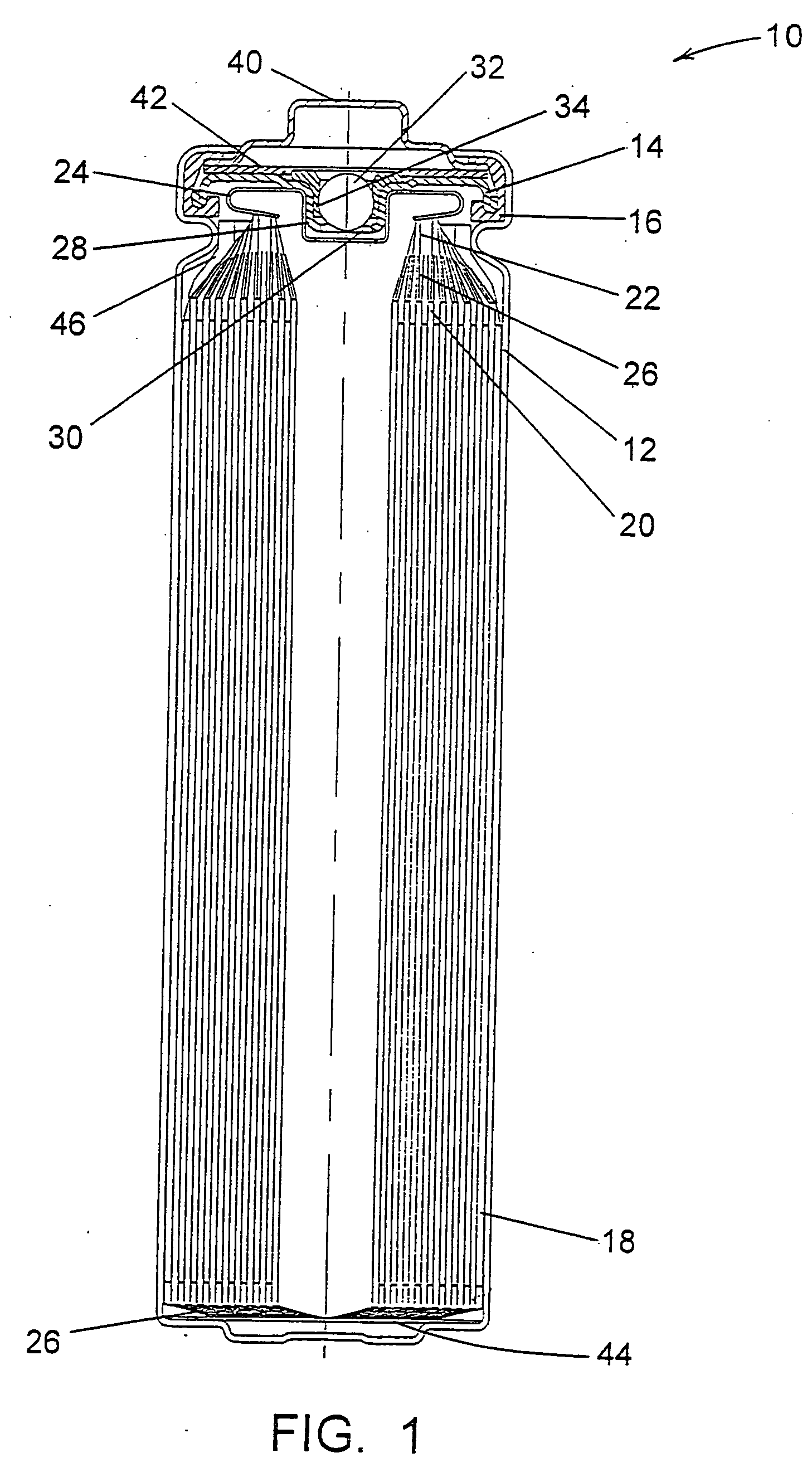
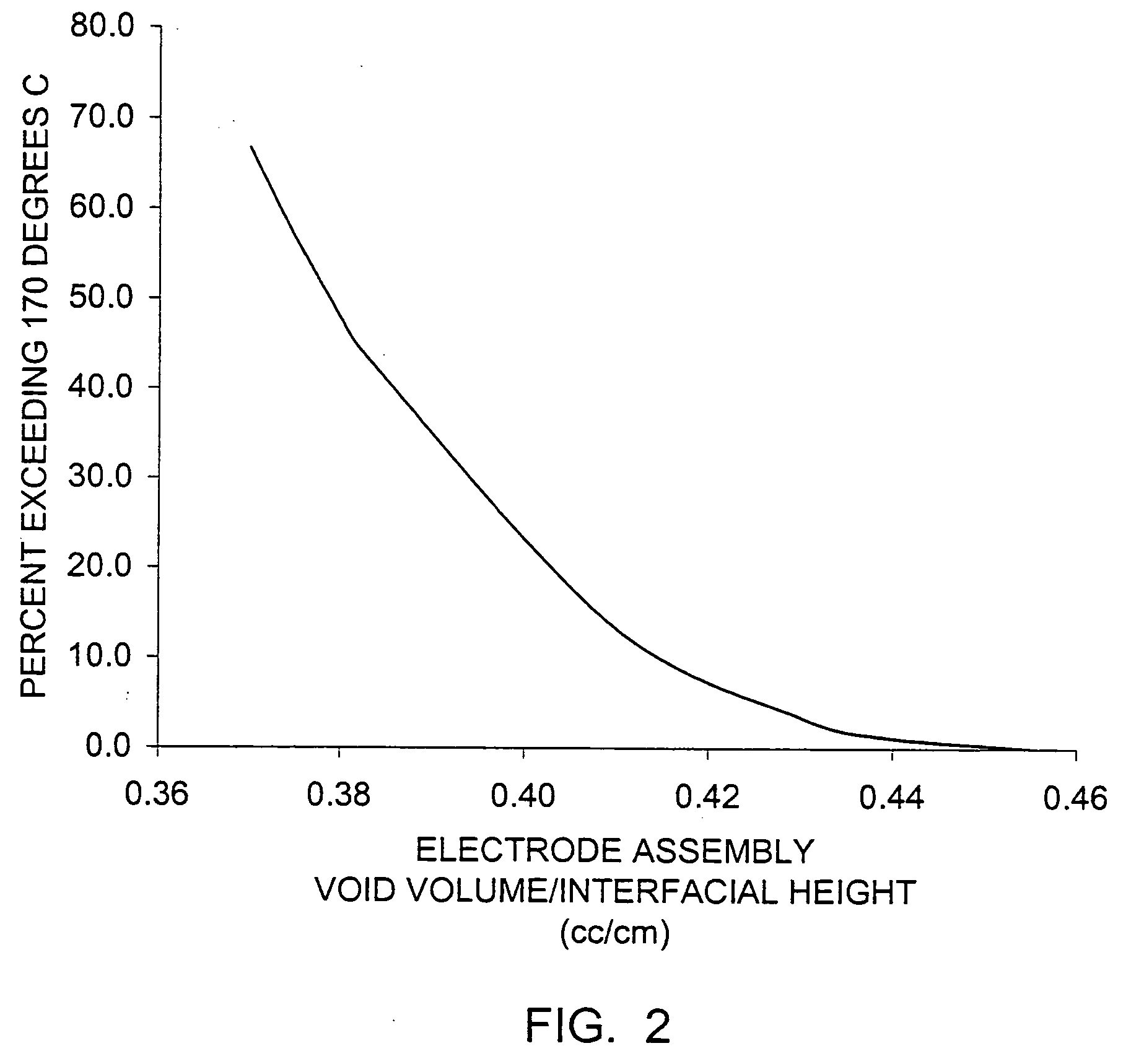
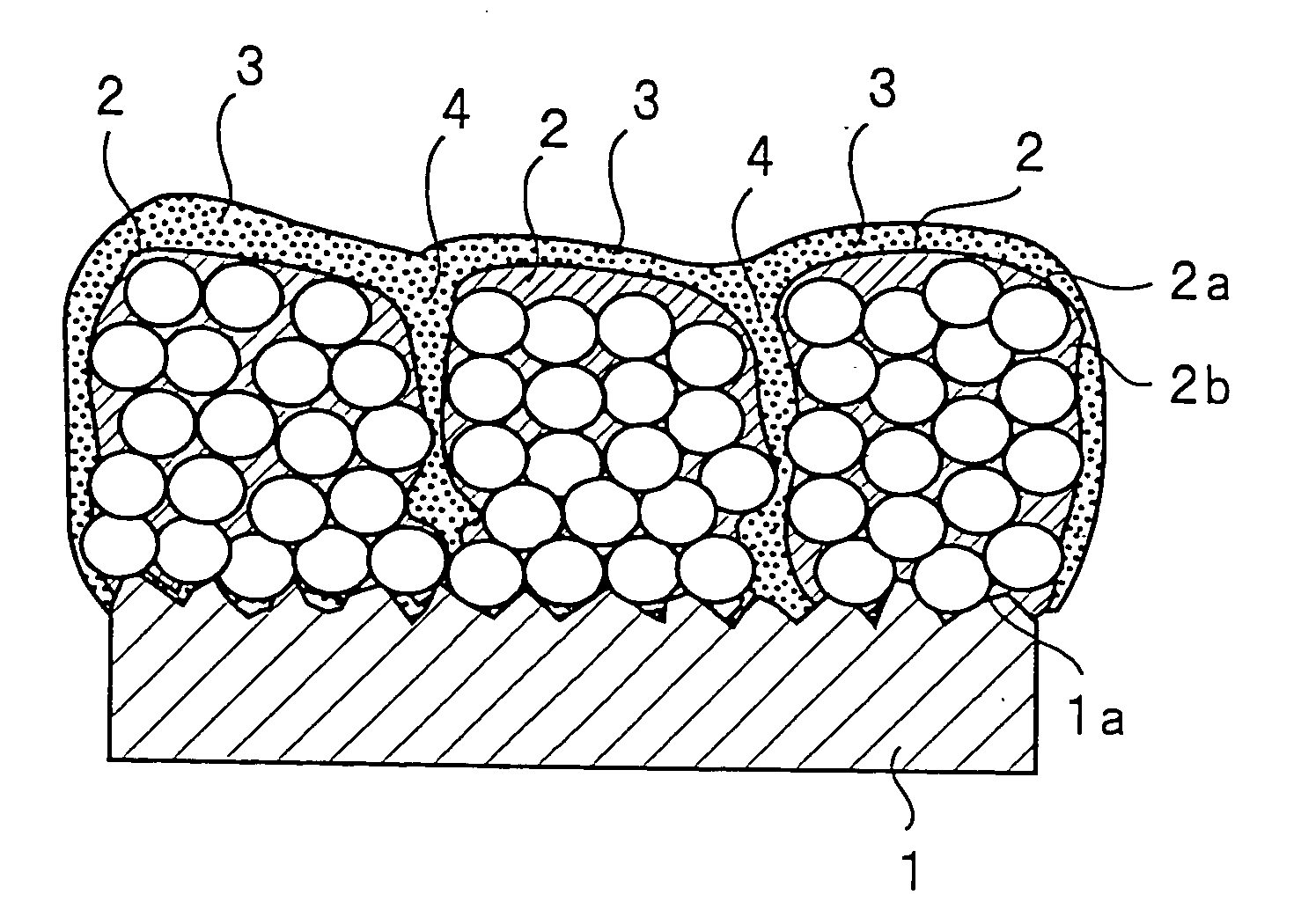
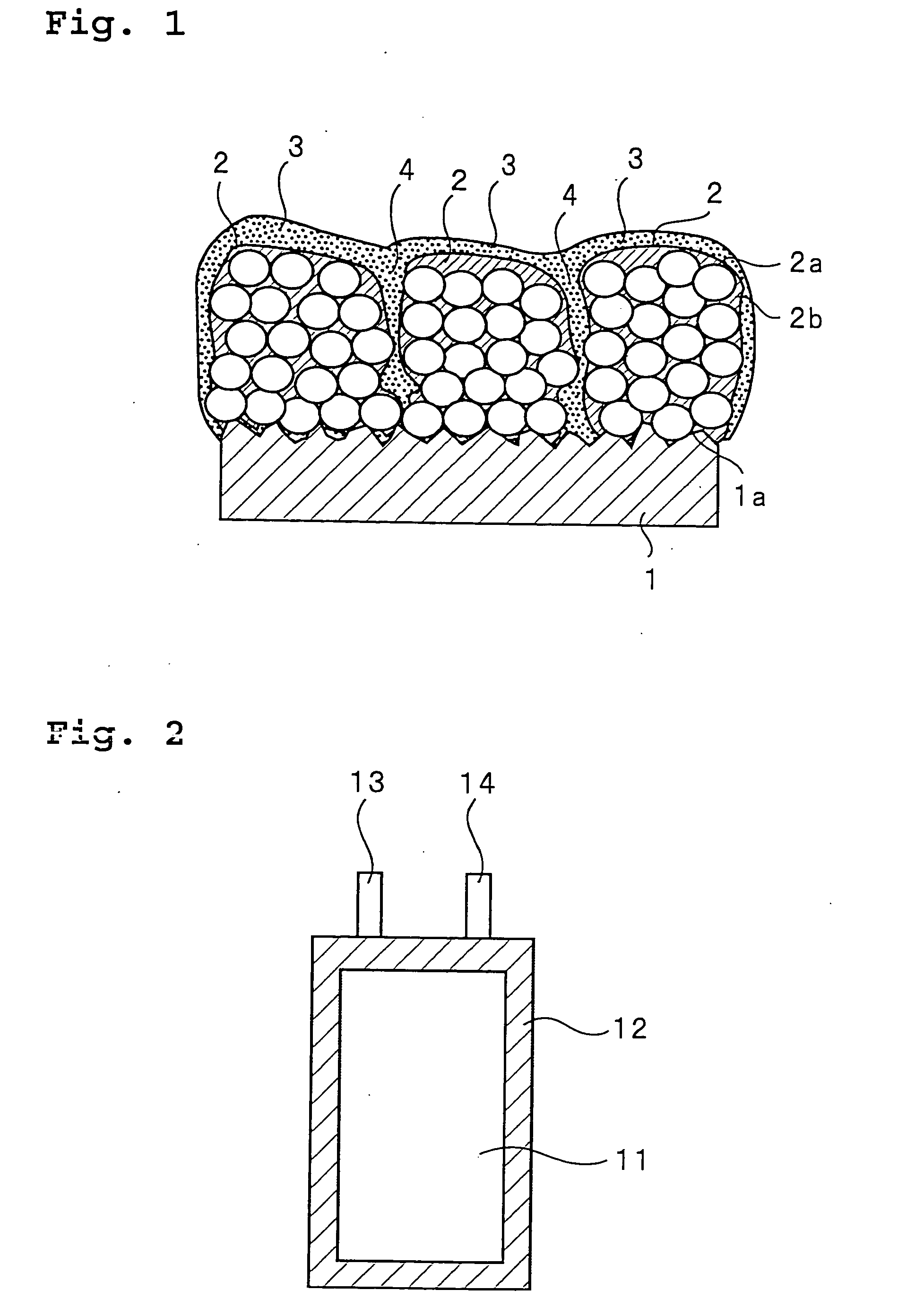
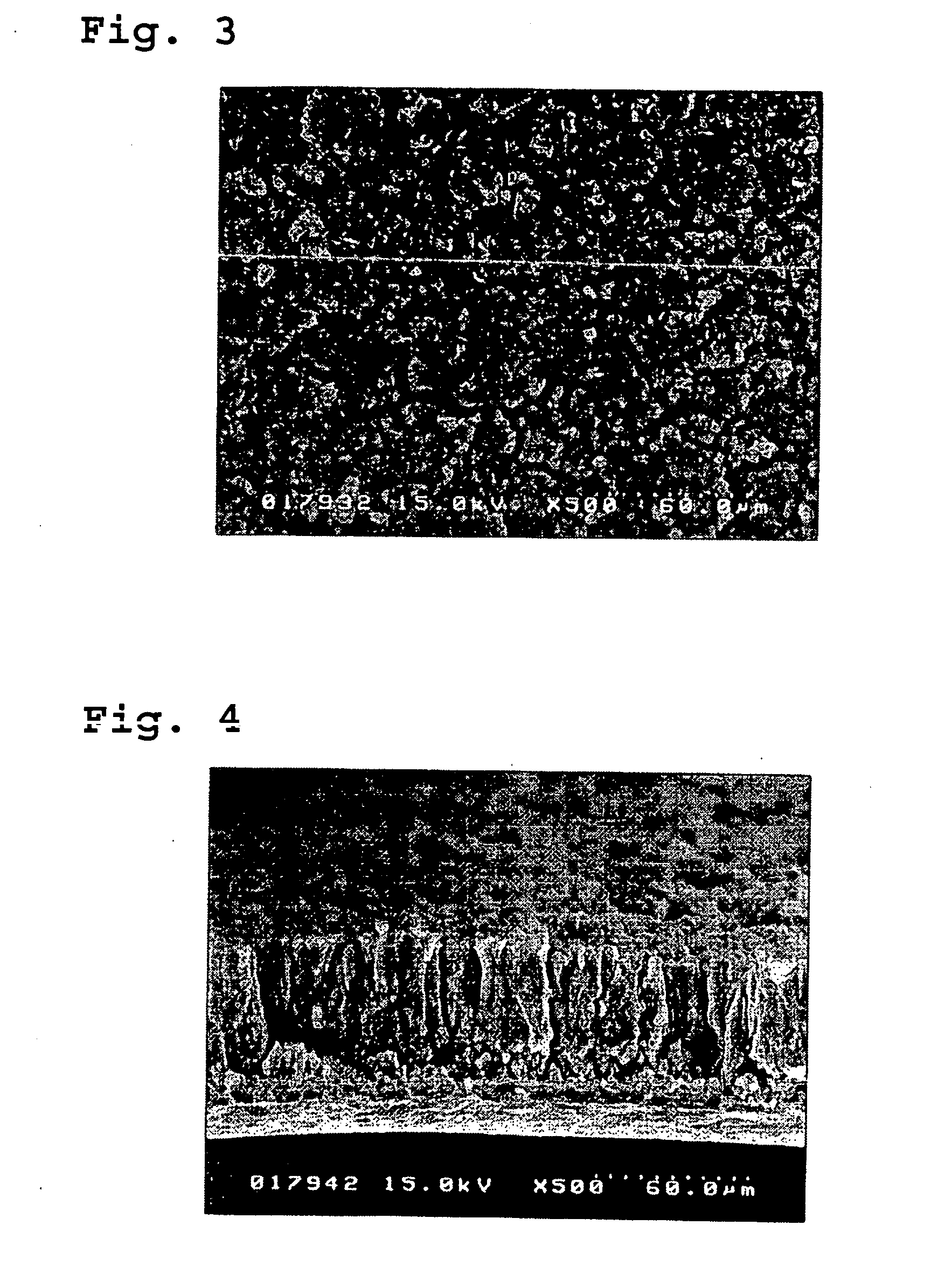
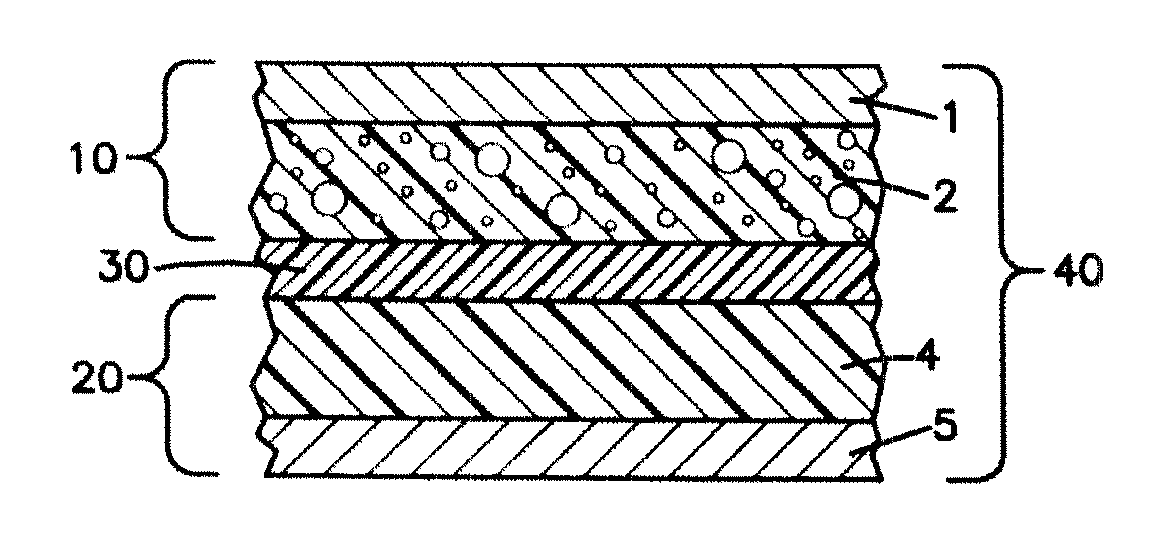
Patents
Literature
5800results about "Electrode collector coating" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
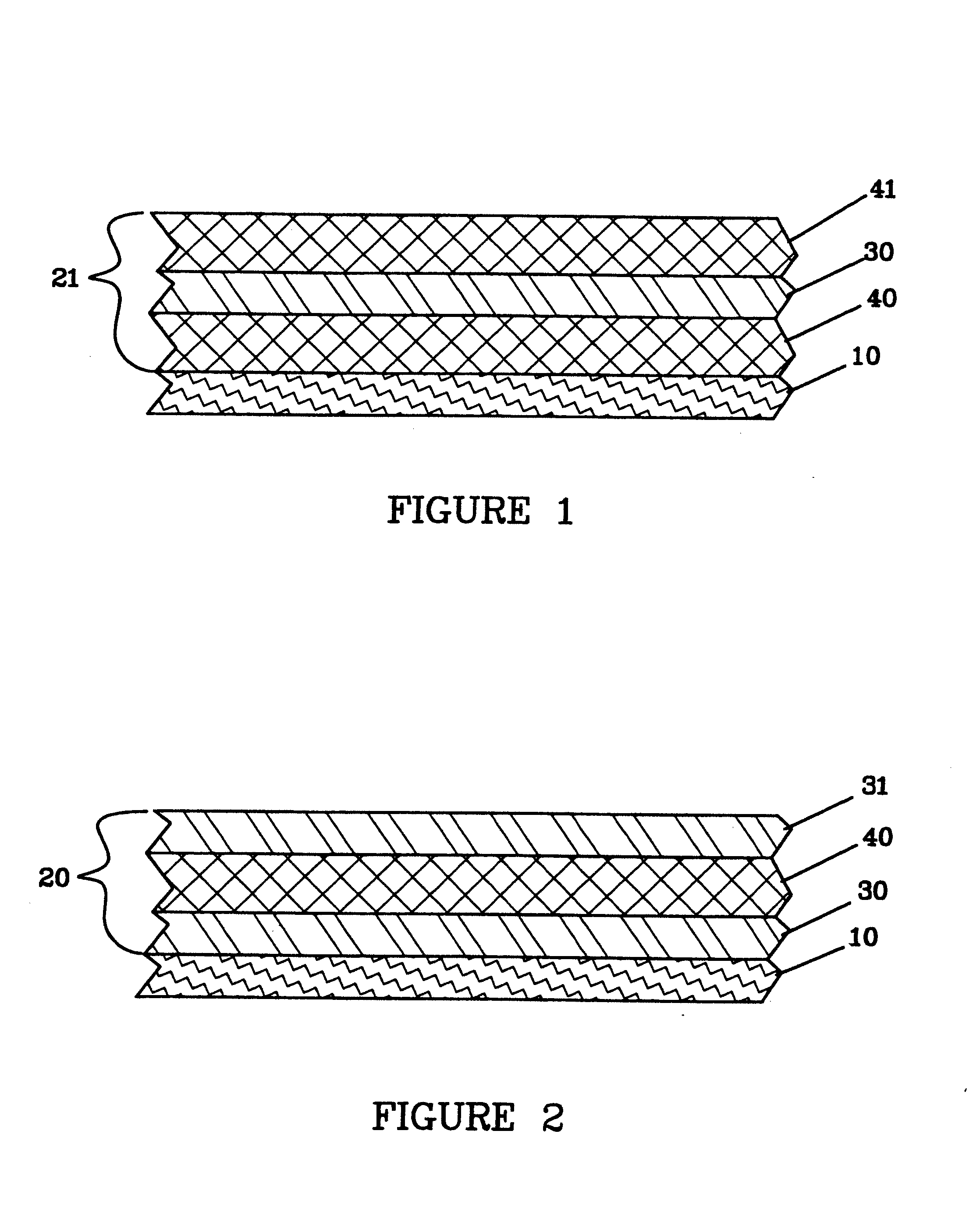
Lithium anodes for electrochemical cells
InactiveUS7247408B2Light weightFinal product manufactureElectrode carriers/collectorsLithium metalReactive gas
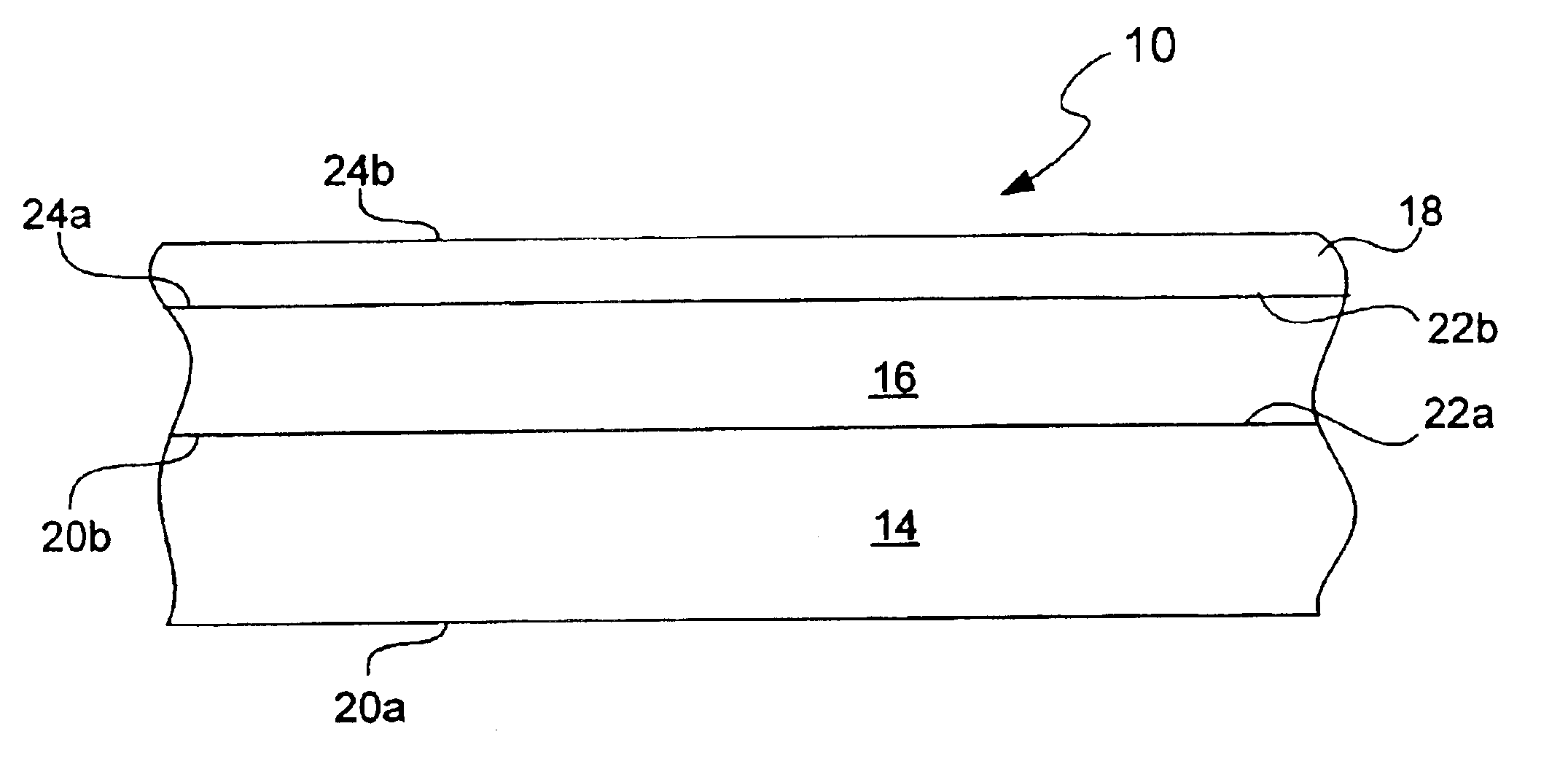
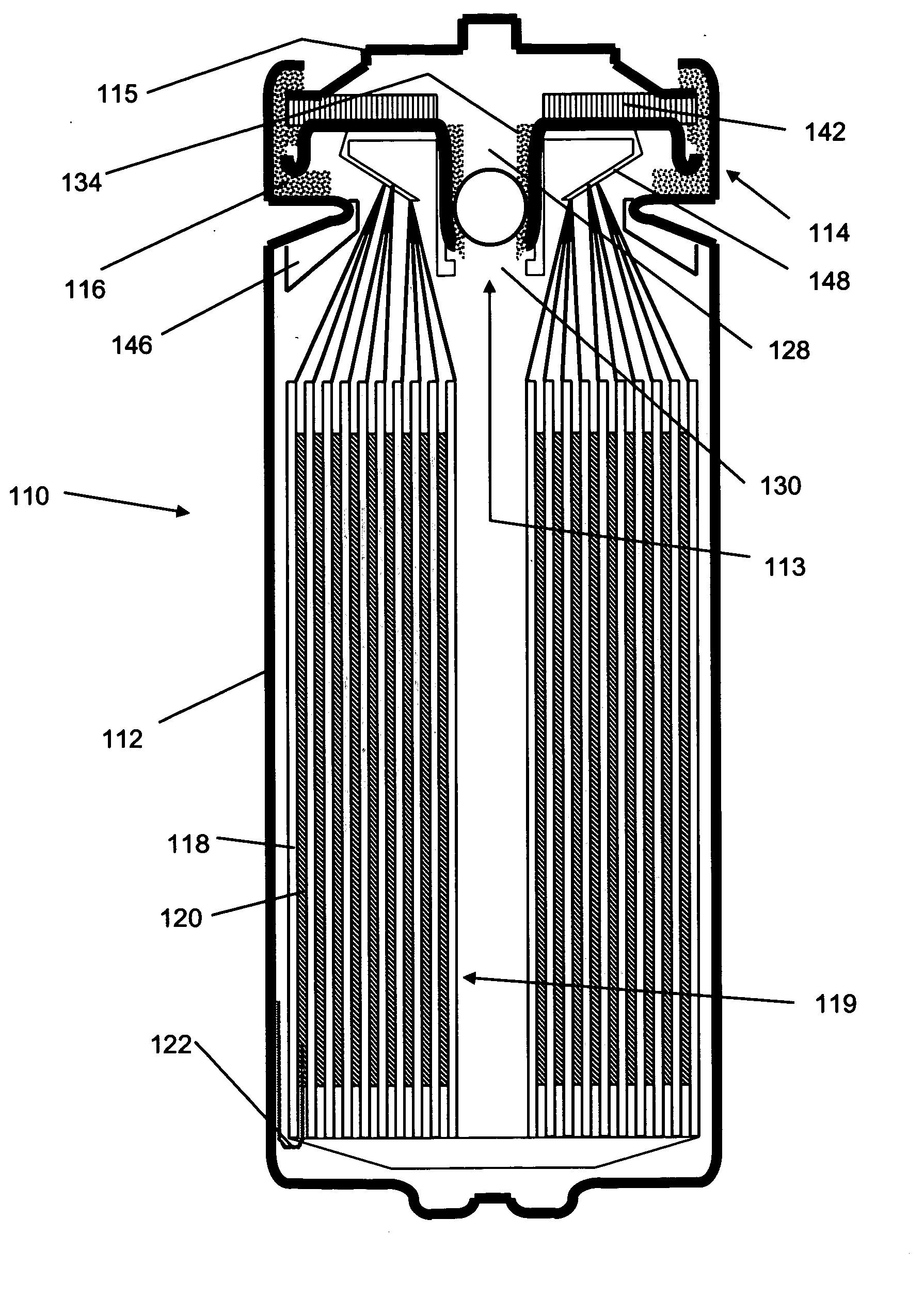
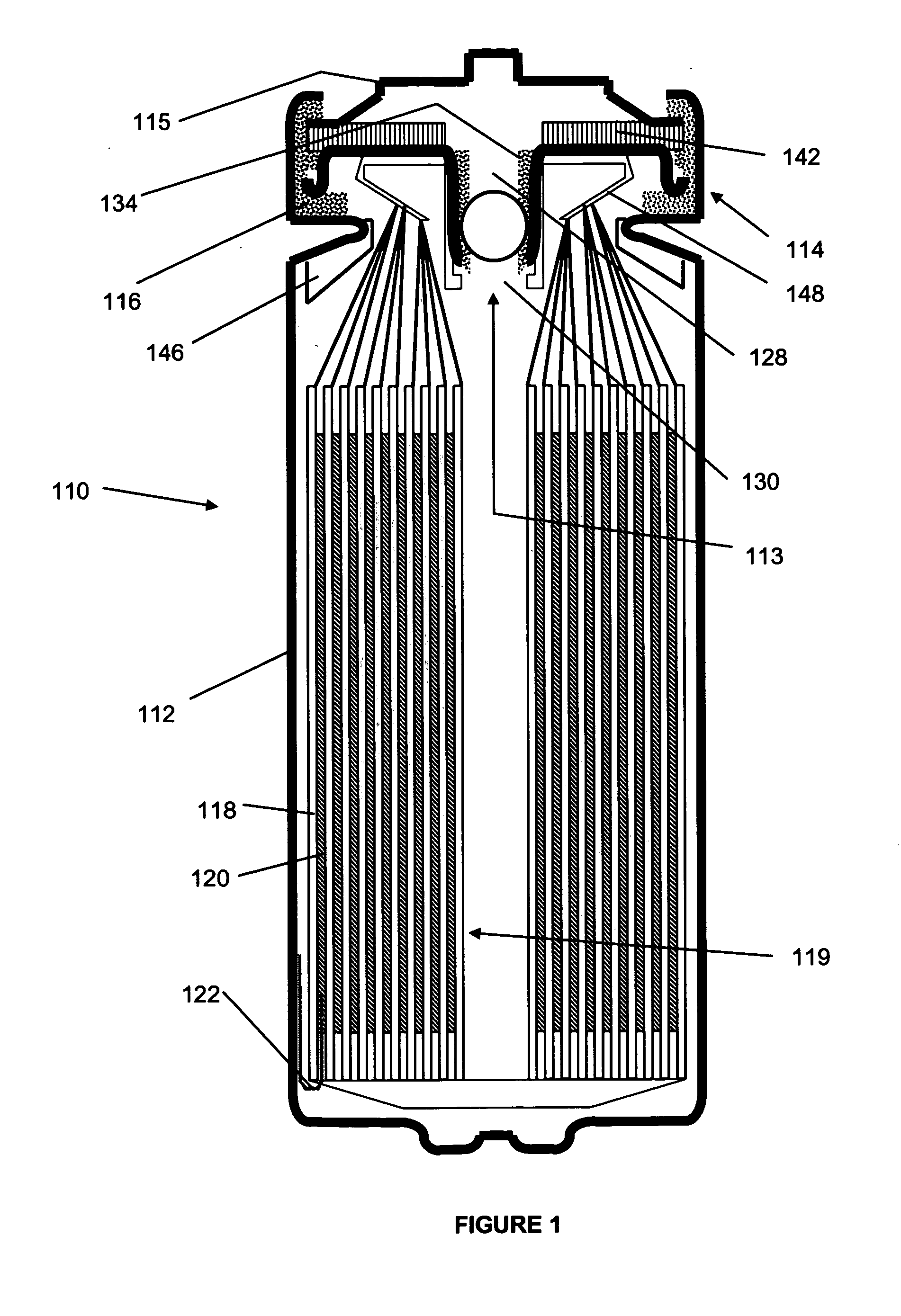
Provided is an anode for use in electrochemical cells, wherein the anode active layer has a first layer comprising lithium metal and a multi-layer structure comprising single ion conducting layers and polymer layers in contact with the first layer comprising lithium metal or in contact with an intermediate protective layer, such as a temporary protective metal layer, on the surface of the lithium-containing first layer. Another aspect of the invention provides an anode active layer formed by the in-situ deposition of lithium vapor and a reactive gas. The anodes of the current invention are particularly useful in electrochemical cells comprising sulfur-containing cathode active materials, such as elemental sulfur.
Owner:SION POWER CORP
Methods and reagents for enhancing the cycling efficiency of lithium polymer batteries
InactiveUS6017651AImprove efficiencyElectrode rolling/calenderingElectrochemical processing of electrodesLithium metalSulfur electrode
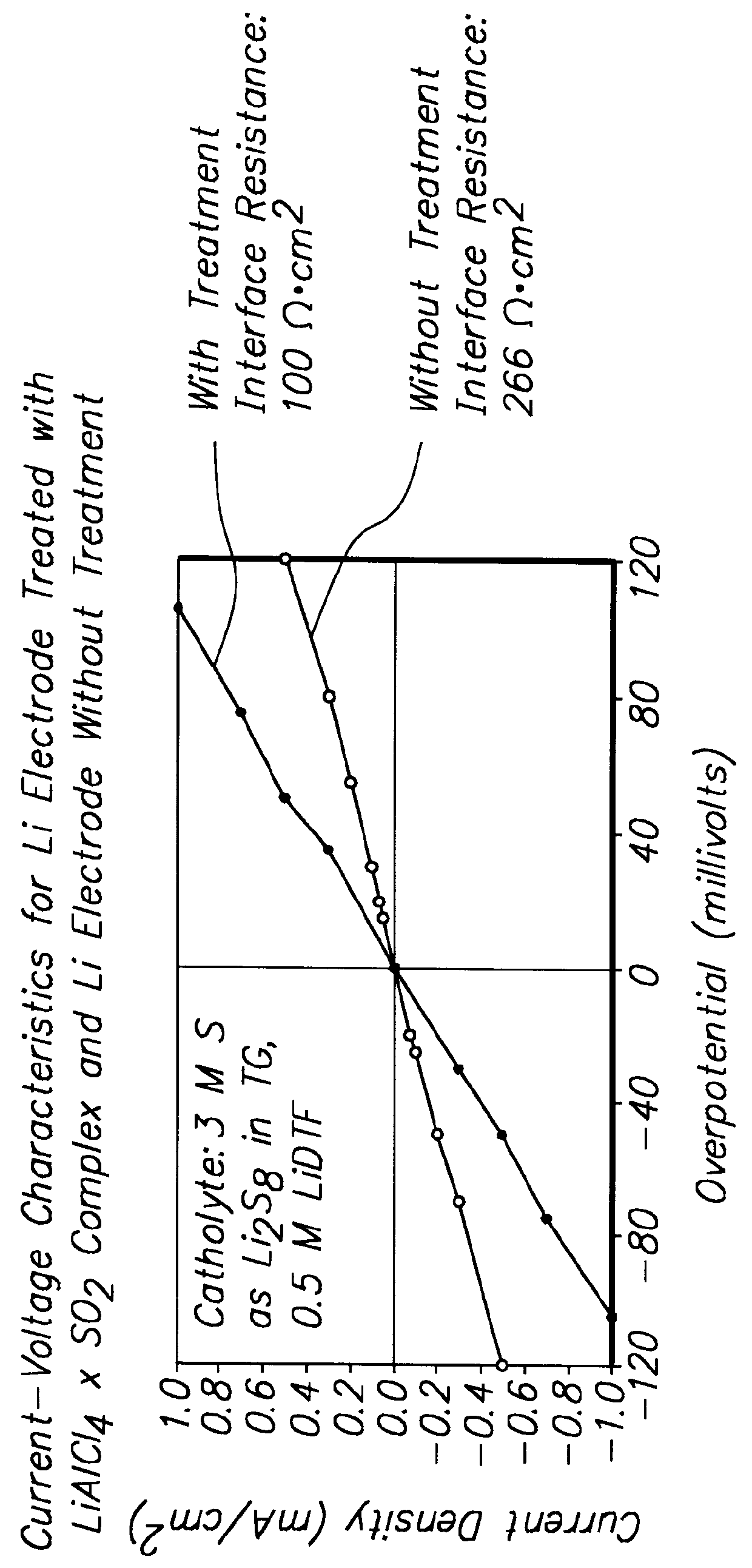
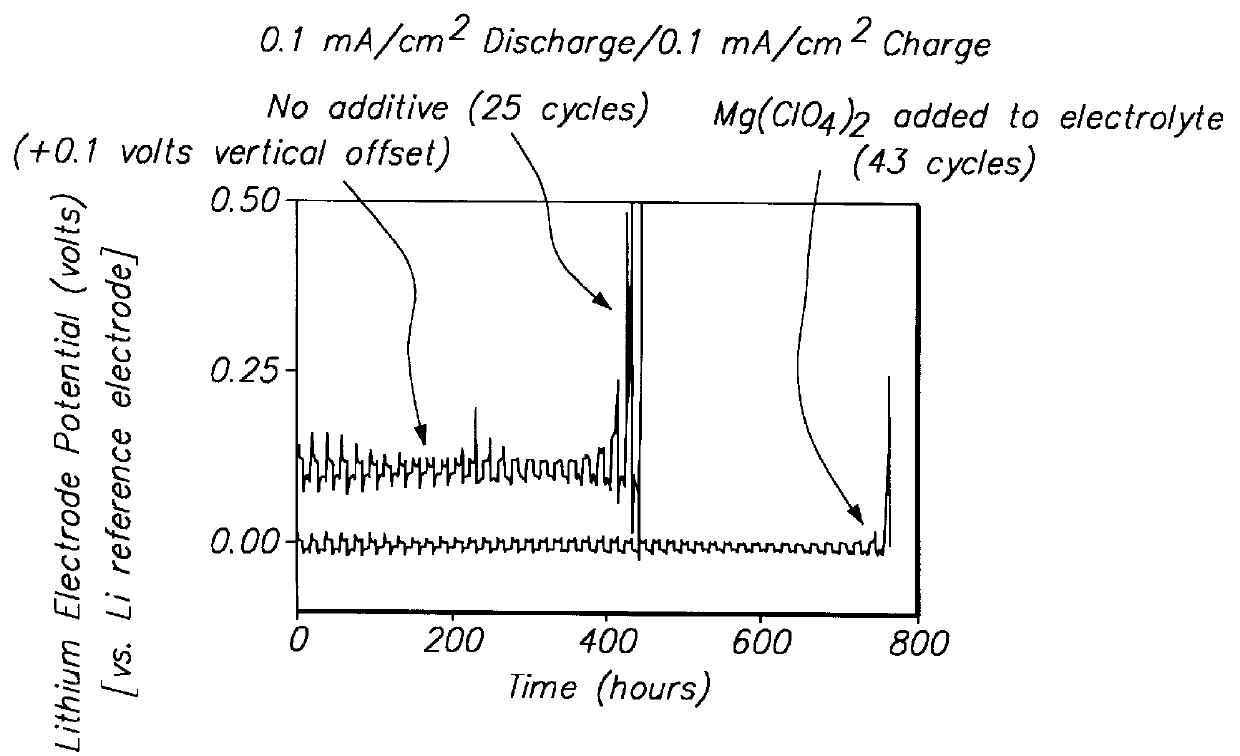
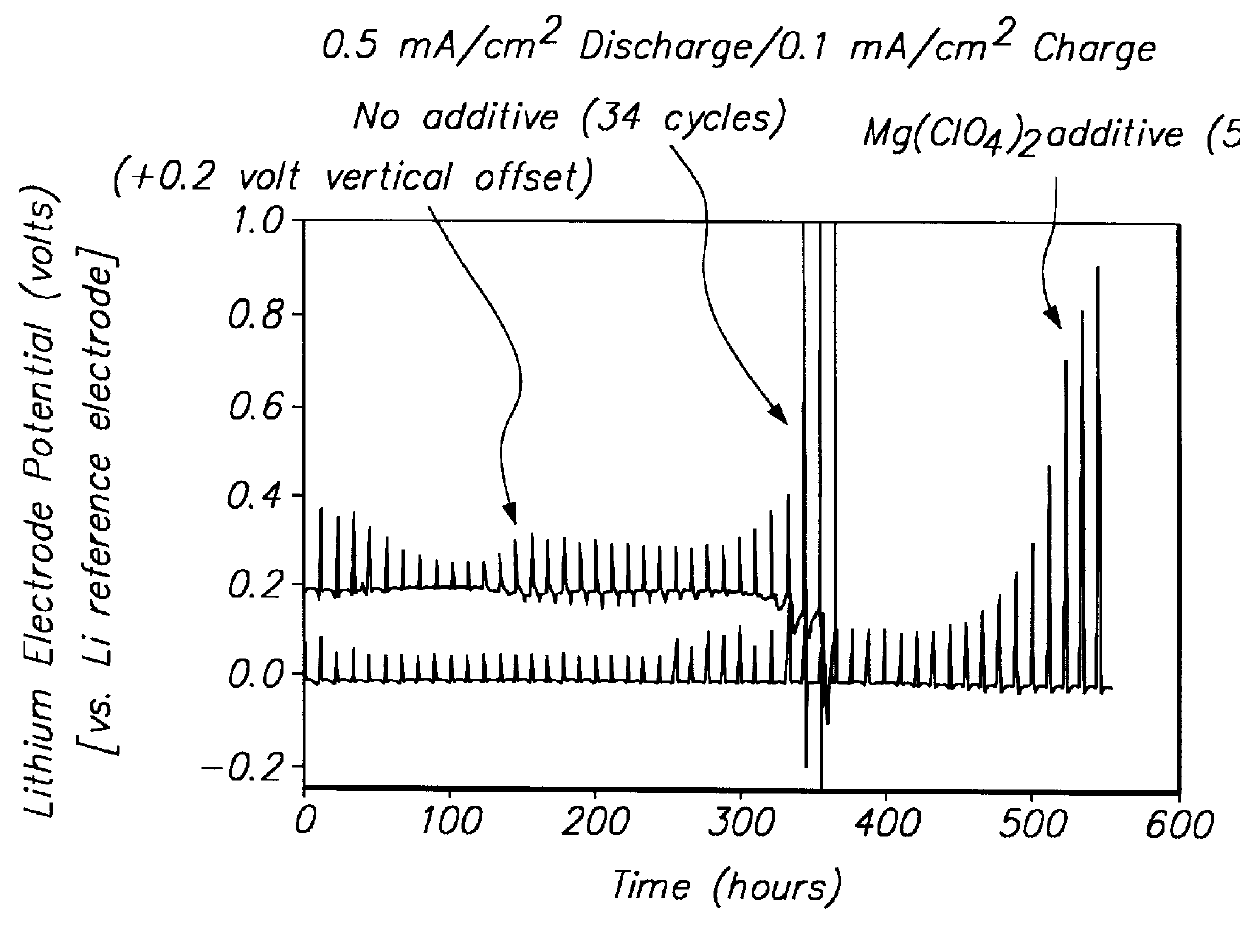
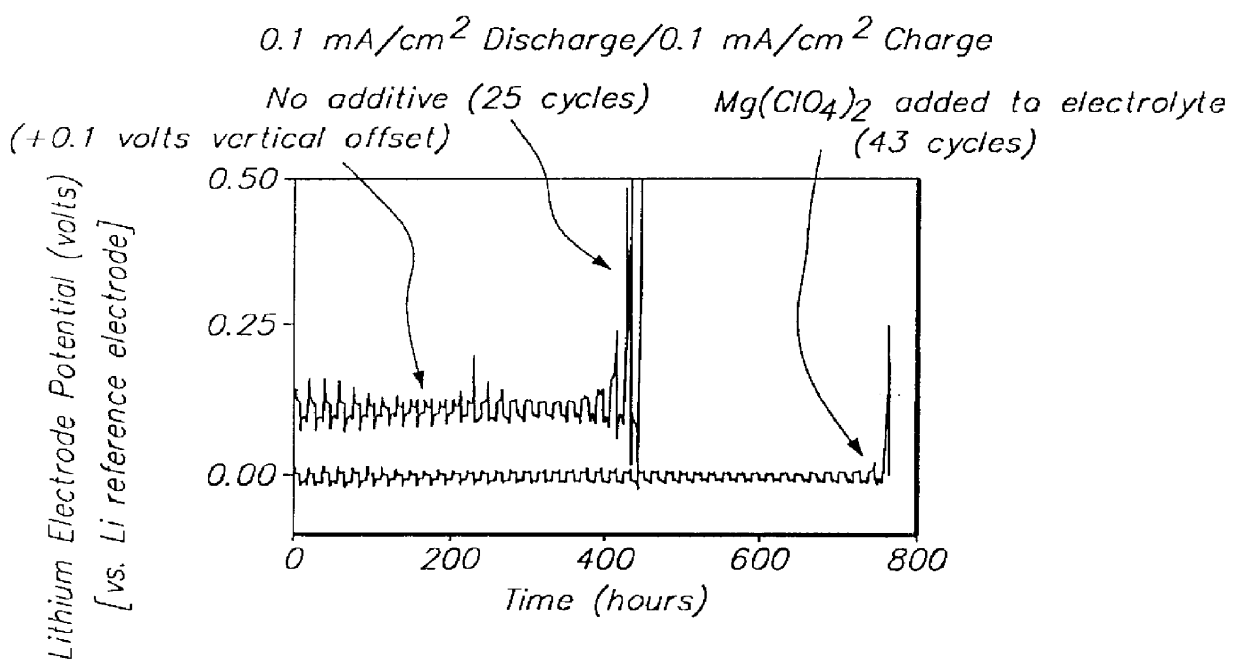
Batteries including a lithium electrode and a sulfur counter electrode that demonstrate improved cycling efficiencies are described. In one embodiment, an electrochemical cell having a lithium electrode and a sulfur electrode including at least one of elemental sulfur, lithium sulfide, and a lithium polysulfide is provided. The lithium electrode includes a surface coating that is effective to increase the cycling efficiency of said electrochemical cell. In a more particular embodiment, the lithium electrode is in an electrolyte solution, and, more particularly, an electrolyte solution including either elemental sulfur, a sulfide, or a polysulfide. In another embodiment, the coating is formed after the lithium electrode is contacted with the electrolyte. In a more particular embodiment, the coating is formed by a reaction between the lithium metal of the lithium electrode and a chemical species present in the electrolyte.
Owner:POLYPLUS BATTERY CO INC
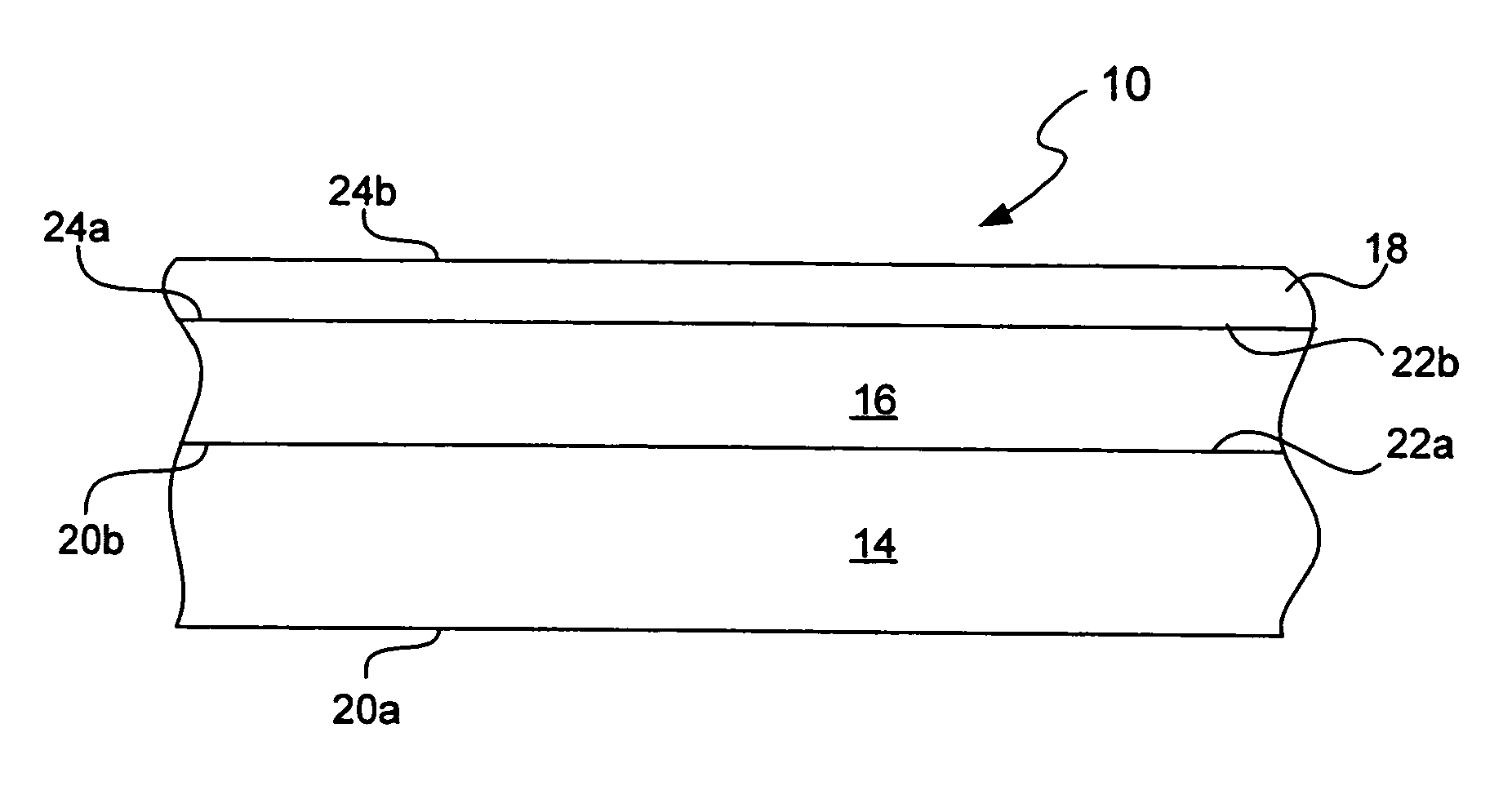
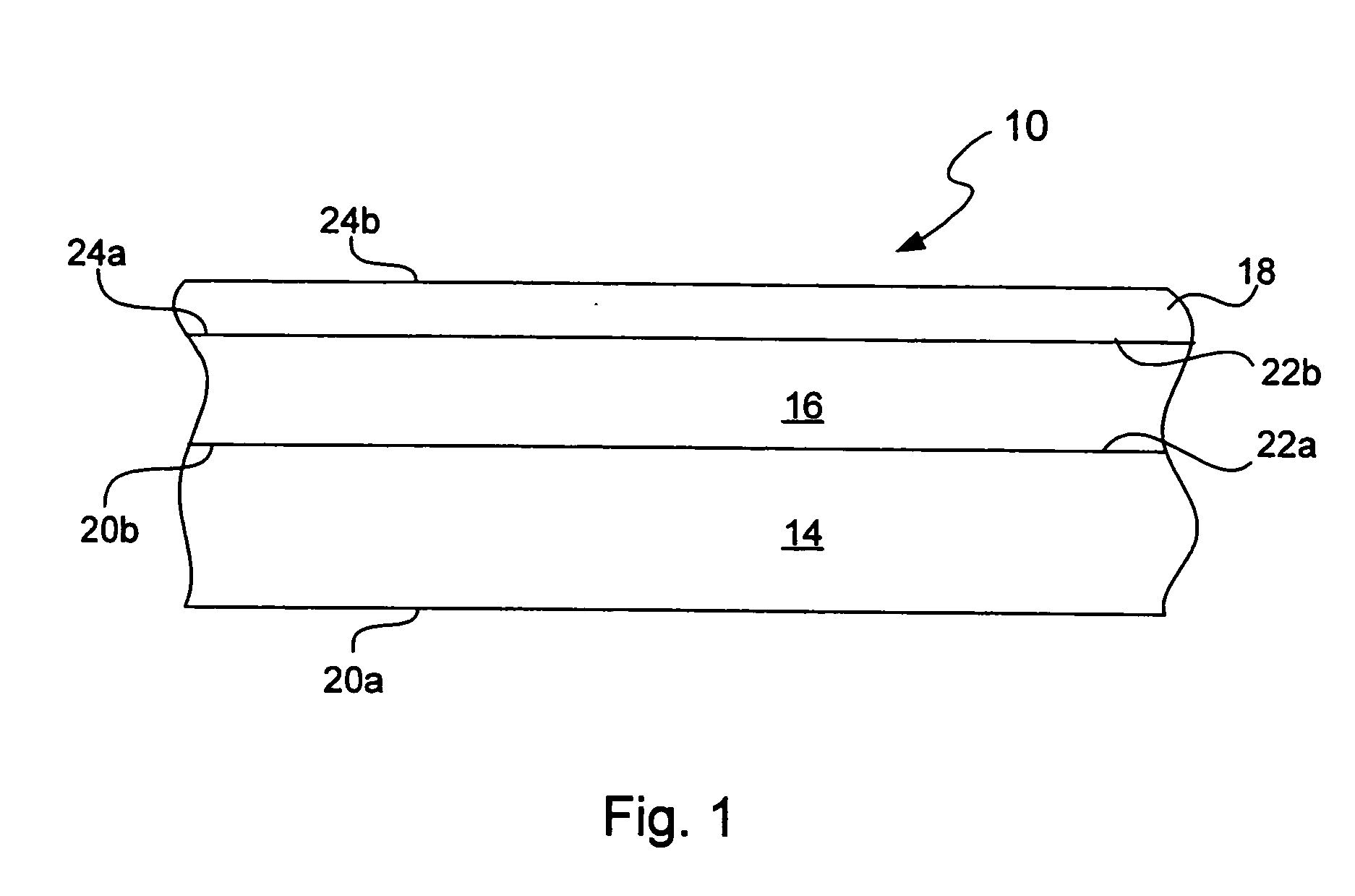
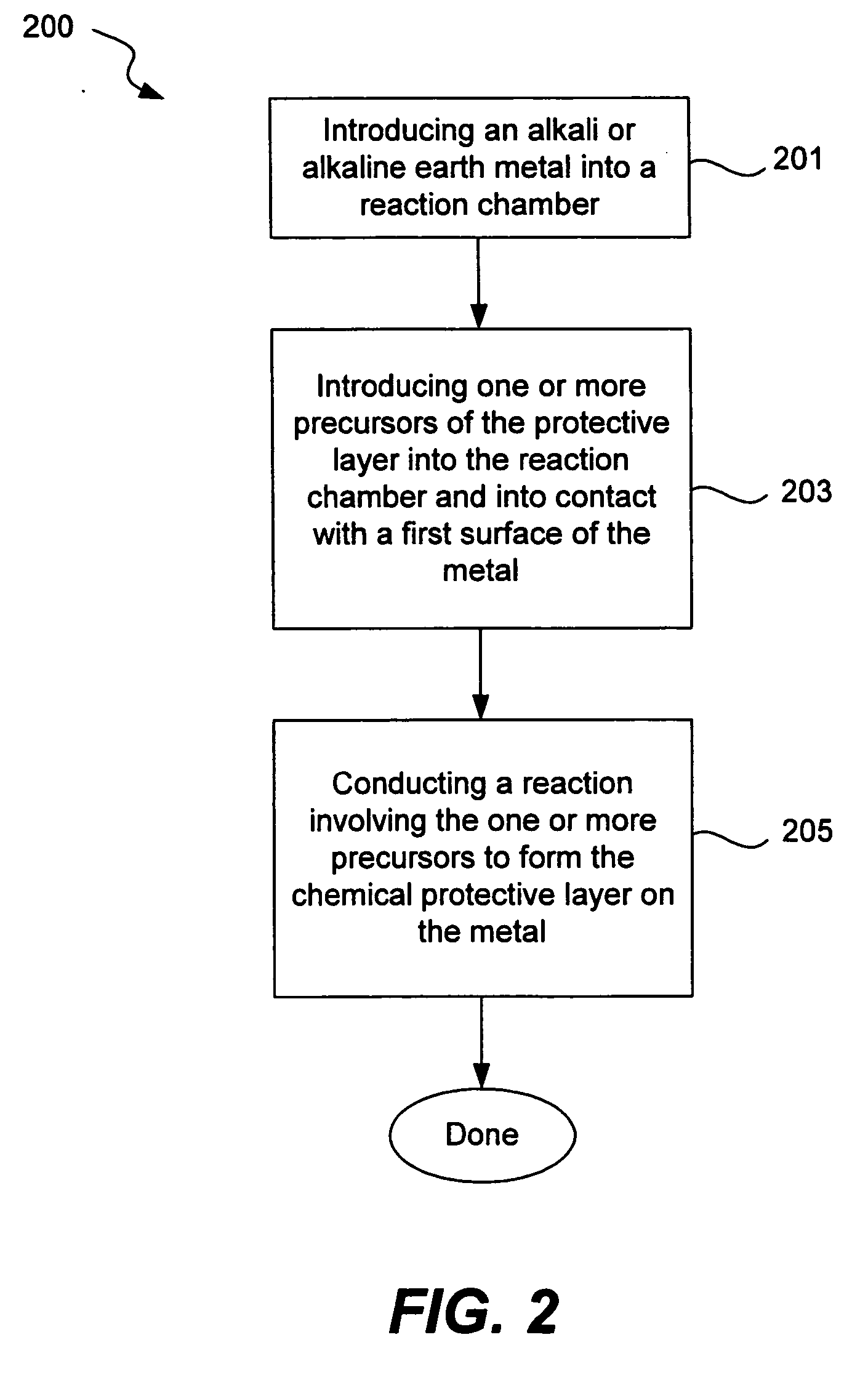
Chemical protection of a lithium surface
InactiveUS6911280B1Easy to produceSimple processElectrochemical processing of electrodesFinal product manufactureAlkaline earth metalLithium metal
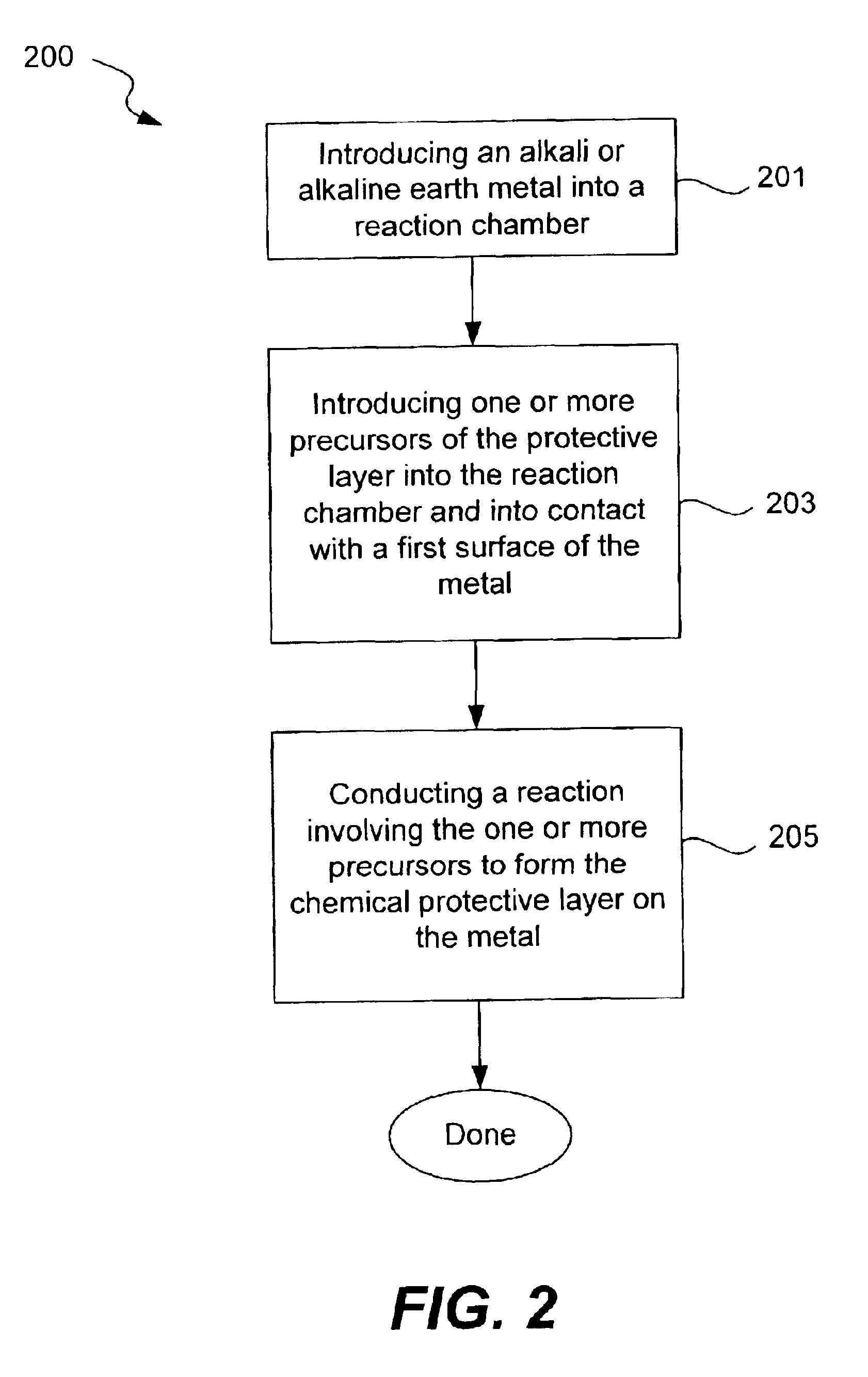
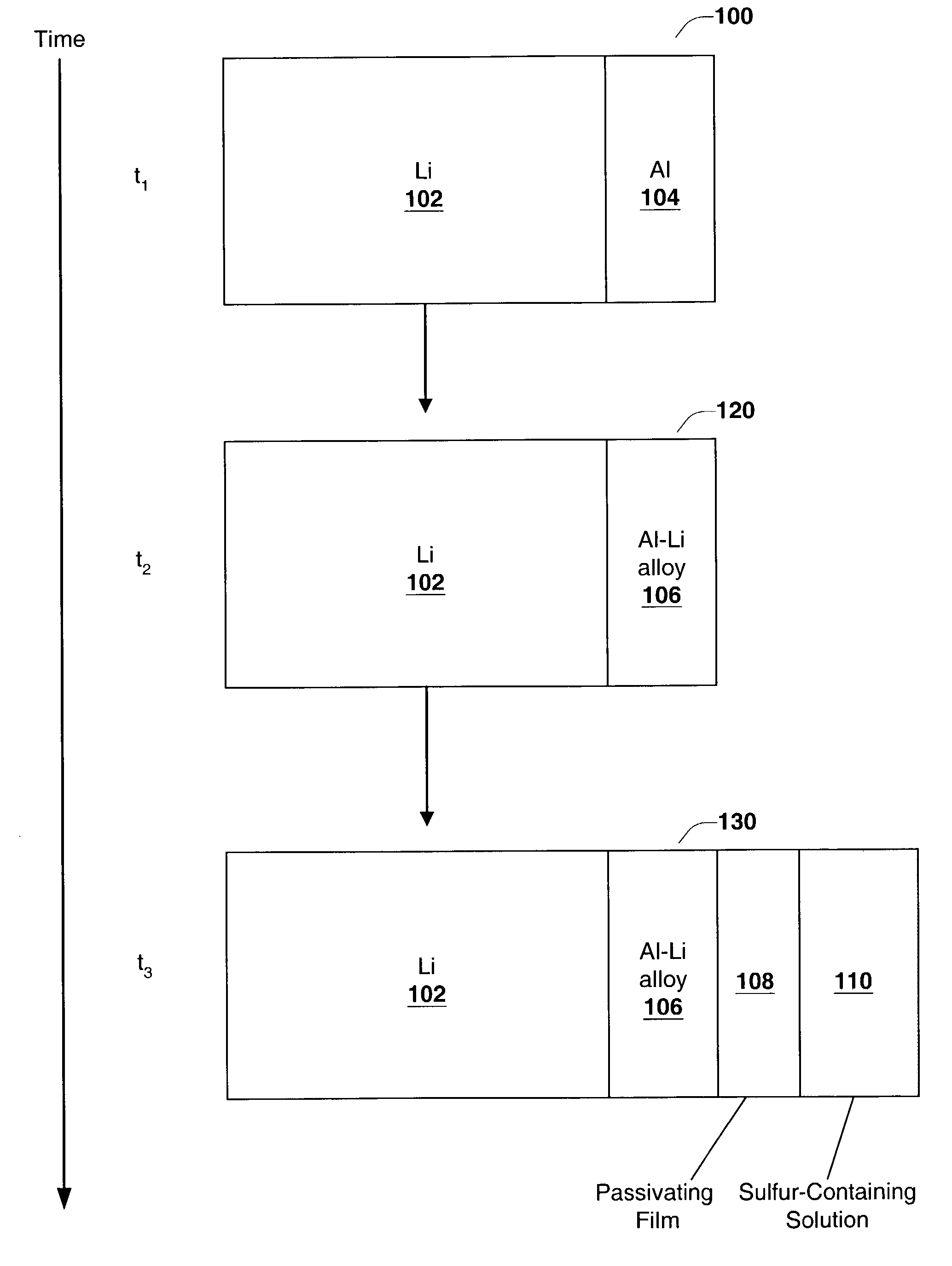
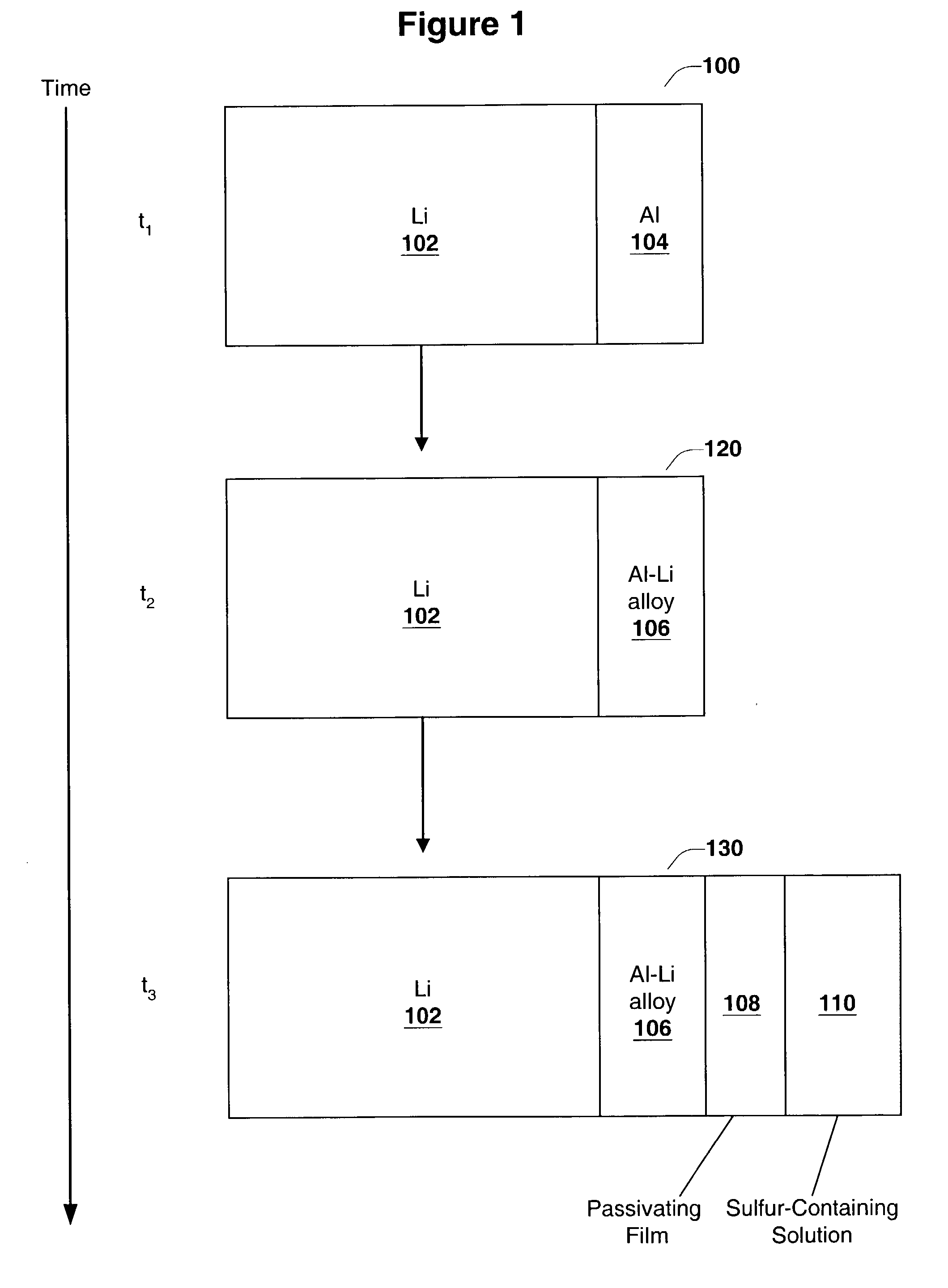
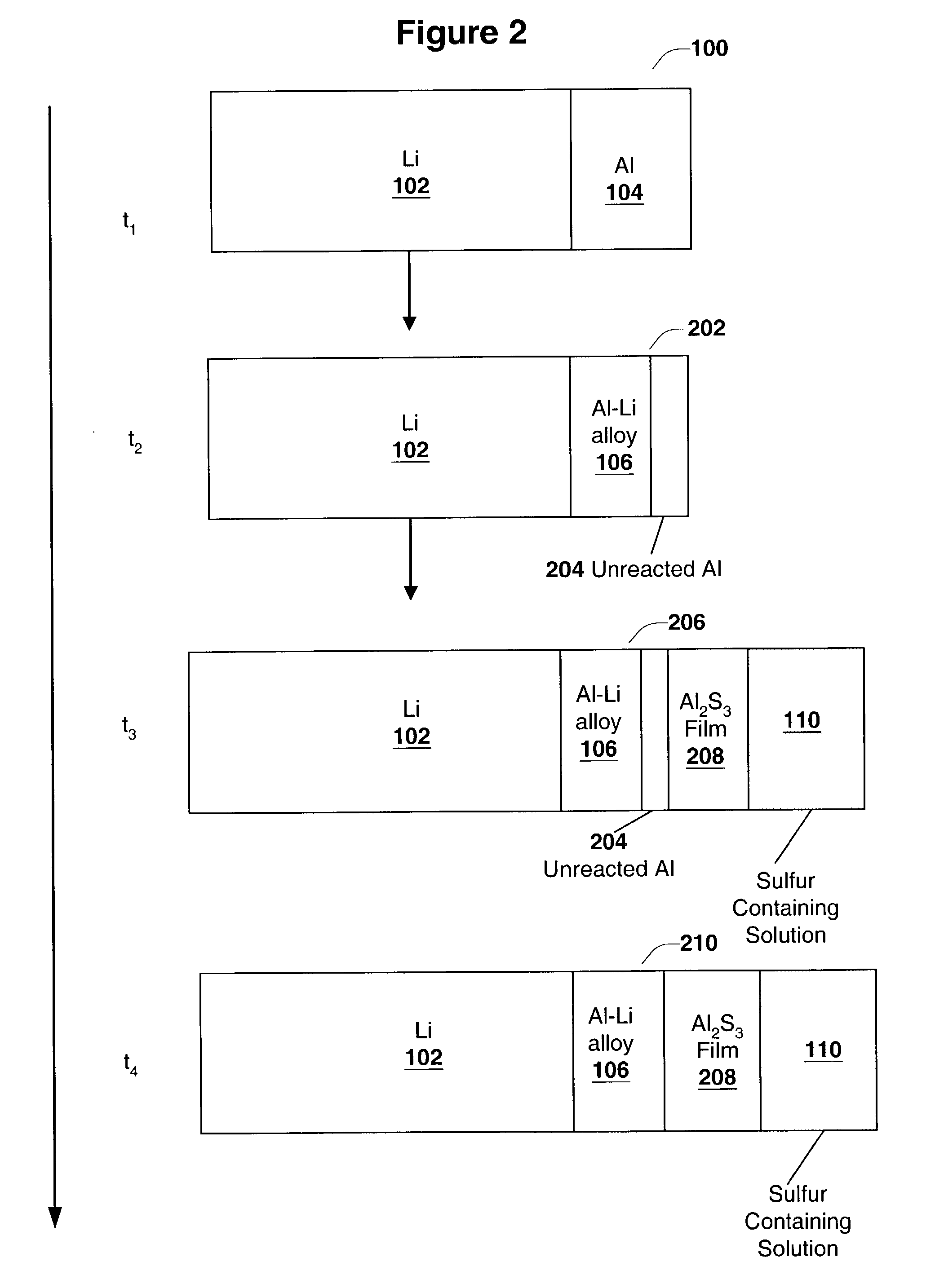
Disclosed are compositions and methods for alleviating the problem of reaction of lithium or other alkali or alkaline earth metals with incompatible processing and operating environments by creating a ionically conductive chemical protective layer on the lithium or other reactive metal surface. Such a chemically produced surface layer can protect lithium metal from reacting with oxygen, nitrogen or moisture in ambient atmosphere thereby allowing the lithium material to be handled outside of a controlled atmosphere, such as a dry room. Production processes involving lithium are thereby very considerably simplified. One example of such a process in the processing of lithium to form negative electrodes for lithium metal batteries.
Owner:POLYPLUS BATTERY CO INC
Lithium alloy/sulfur batteries
Electrochemical cells including anode compositions that may enhance charge-discharge cycling efficiency and uniformity are presented. In some embodiments, alloys are incorporated into one or more components of an electrochemical cell, which may enhance the performance of the cell. For example, an alloy may be incorporated into an electroactive component of the cell (e.g., electrodes) and may advantageously increase the efficiency of cell performance. Some electrochemical cells (e.g., rechargeable batteries) may undergo a charge / discharge cycle involving deposition of metal (e.g., lithium metal) on the surface of the anode upon charging and reaction of the metal on the anode surface, wherein the metal diffuses from the anode surface, upon discharging. In some cases, the efficiency and uniformity of such processes may affect cell performance. The use of materials such as alloys in an electroactive component of the cell have been found to increase the efficiency of such processes and to increase the cycling lifetime of the cell. For example, the use of alloys may reduce the formation of dendrites on the anode surface and / or limit surface development.
Owner:SION POWER CORP
Chemical protection of a lithium surface
InactiveUS20050186469A1Easy to produceEasy to processElectrochemical processing of electrodesFinal product manufactureAlkaline earth metalLithium metal
Disclosed are compositions and methods for alleviating the problem of reaction of lithium or other alkali or alkaline earth metals with incompatible processing and operating environments by creating a ionically conductive chemical protective layer on the lithium or other reactive metal surface. Such a chemically produced surface layer can protect lithium metal from reacting with oxygen, nitrogen or moisture in ambient atmosphere thereby allowing the lithium material to be handled outside of a controlled atmosphere, such as a dry room. Production processes involving lithium are thereby very considerably simplified. One example of such a process is the processing of lithium to form negative electrodes for lithium metal batteries.
Owner:POLYPLUS BATTERY CO INC
Method for preparing electrode material for lithium battery
InactiveUS6887511B1Improve adhesionReduce expansionElectrode carriers/collectorsVacuum evaporation coatingAmorphous siliconOptoelectronics
Owner:SANYO ELECTRIC CO LTD
High discharge capacity lithium battery
InactiveUS20050233214A1Improve discharge performanceIncrease energy densityFinal product manufactureOrganic electrolyte cellsHigh rateIron disulfide
A lithium / iron disulfide electrochemical battery cell with a high discharge capacity. The cell has a lithium negative electrode, an iron disulfide positive electrode and a nonaqueous electrolyte. The iron disulfide of the positive electrode has a controlled average particle size range which allows the electrochemical cells to exhibit desired properties in both low and high rate applications. In various embodiments, the iron disulfide particles are wet milled, preferably utilizing a media mill or milled utilizing a non-mechanical mill such as a jet mill, which reduces the iron disulfide particles to a desired average particle size range for incorporation into the positive electrode.
Owner:EVEREADY BATTERY CO INC
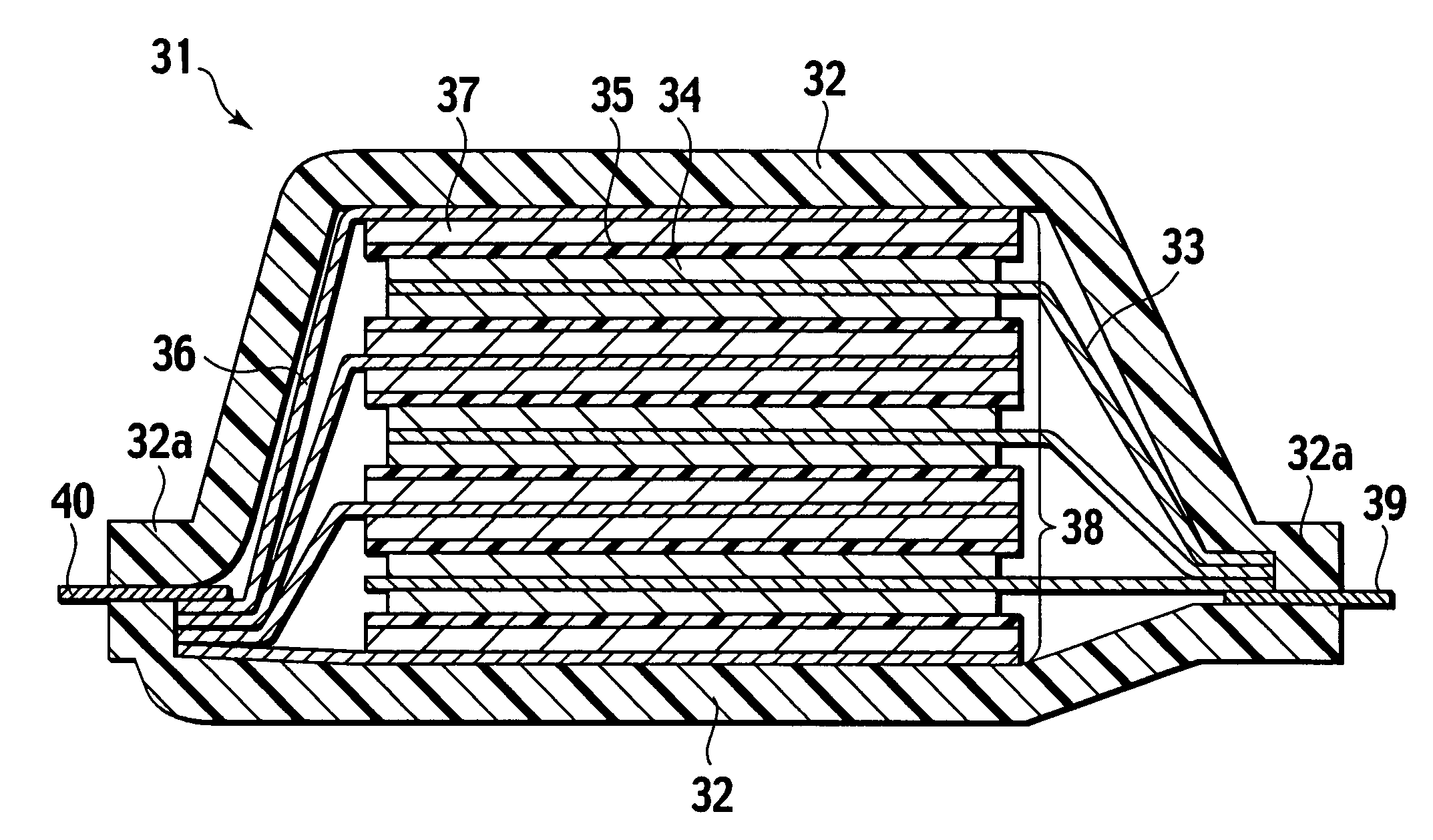
Lithium anodes for electrochemical cells
InactiveUS20060222954A1Light weightFinal product manufactureElectrode carriers/collectorsLithium metalReactive gas
Provided is an anode for use in electrochemical cells, wherein the anode active layer has a first layer comprising lithium metal and a multi-layer structure comprising single ion conducting layers and polymer layers in contact with the first layer comprising lithium metal or in contact with an intermediate protective layer, such as a temporary protective metal layer, on the surface of the lithium-containing first layer. Another aspect of the invention provides an anode active layer formed by the in-situ deposition of lithium vapor and a reactive gas. The anodes of the current invention are particularly useful in electrochemical cells comprising sulfur-containing cathode active materials, such as elemental sulfur.
Owner:SION POWER CORP
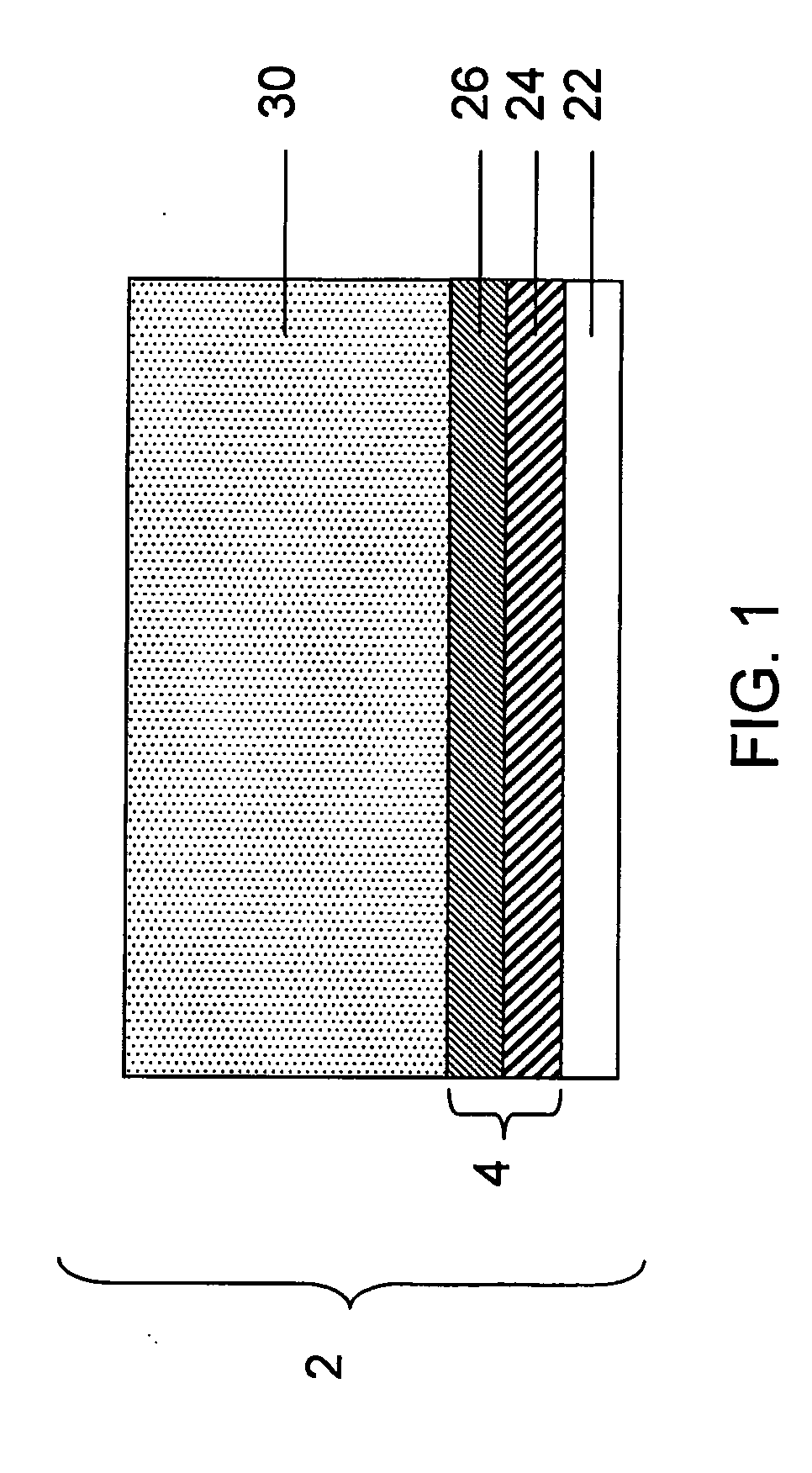
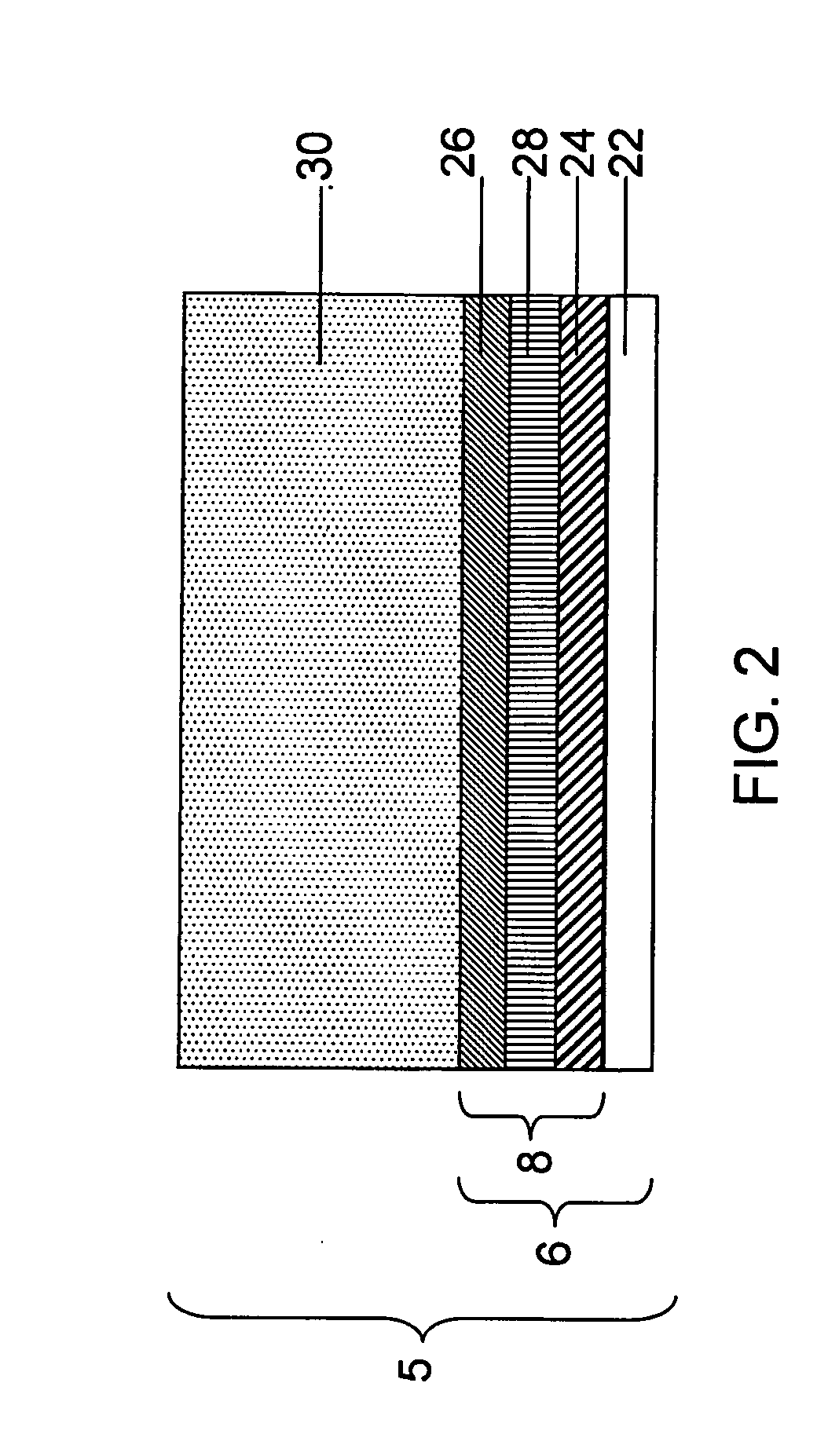
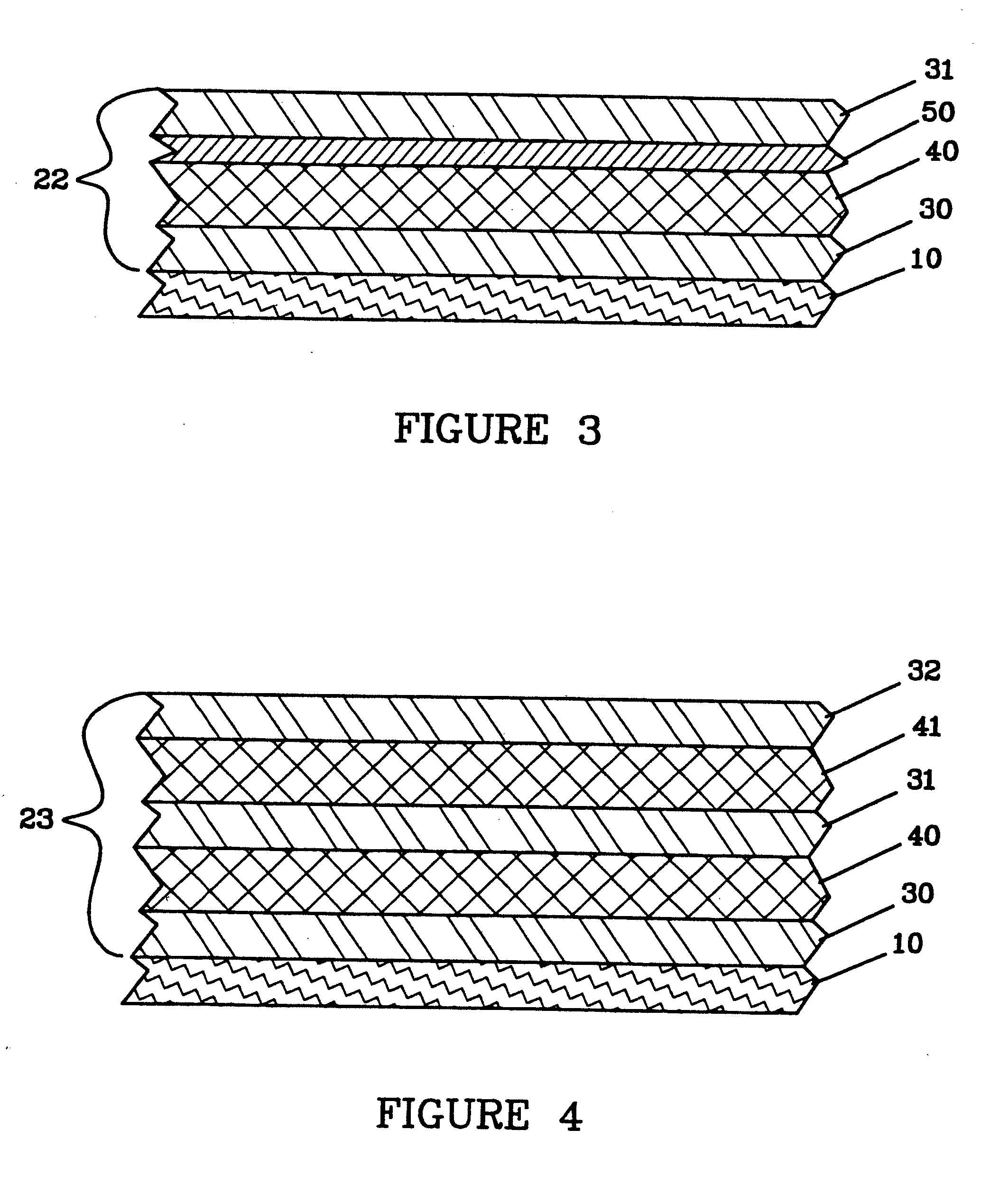
Release system for electrochemical cells
InactiveUS20110068001A1Active material electrodesElectrical-based machining electrodesEngineeringElectrochemical cell
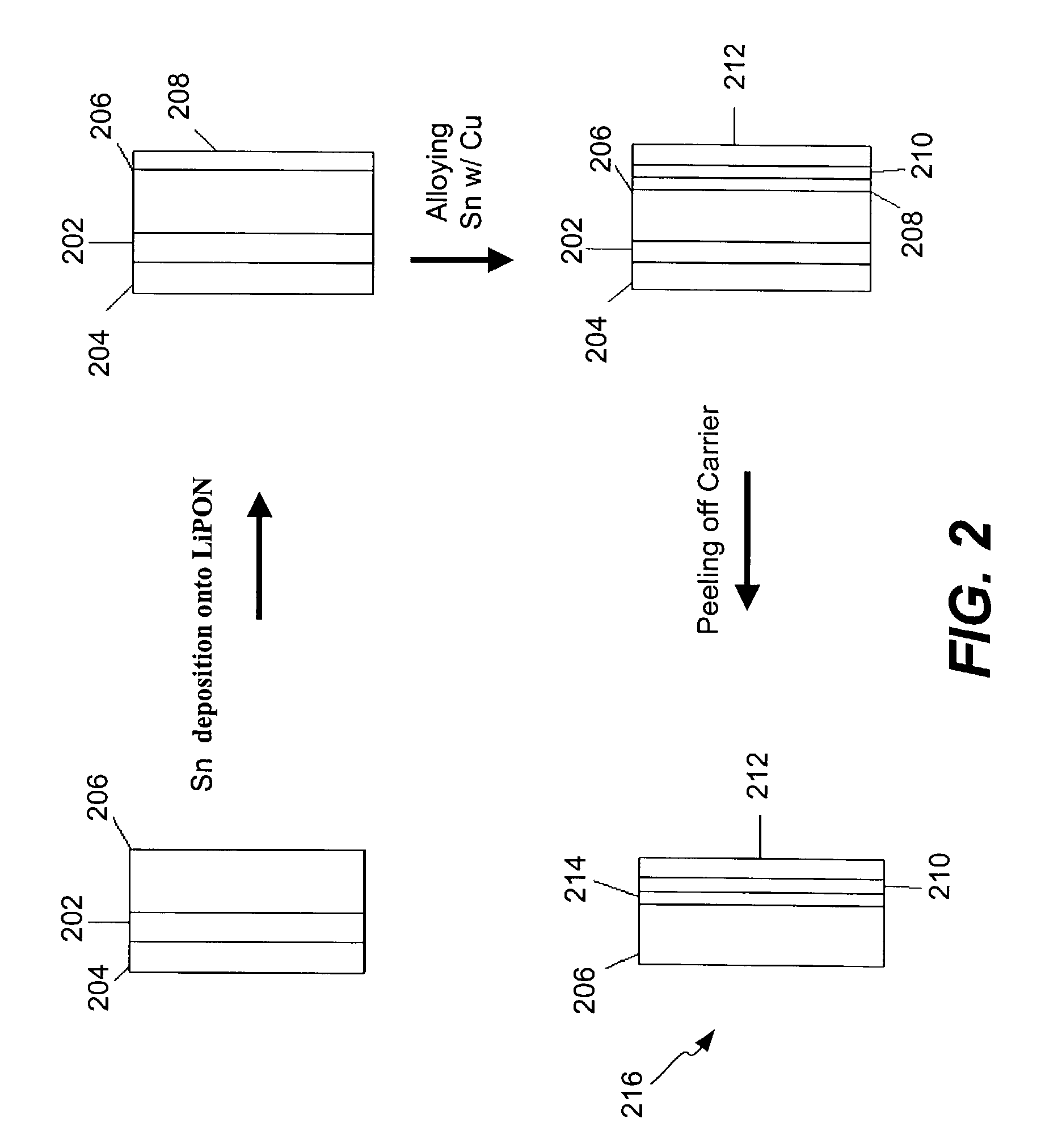
Electrochemical cells, and more specifically, release systems for the fabrication of electrochemical cells are described. In particular, release layer arrangements, assemblies, methods and compositions that facilitate the fabrication of electrochemical cell components, such as electrodes, are presented. In some embodiments, methods of fabricating an electrode involve the use of a release layer to separate portions of the electrode from a carrier substrate on which the electrode was fabricated. For example, an intermediate electrode assembly may include, in sequence, an electroactive material layer, a current collector layer, a release layer, and a carrier substrate. The carrier substrate can facilitate handling of the electrode during fabrication and / or assembly, but may be released from the electrode prior to commercial use.
Owner:SION POWER CORP
Non-sintered type thin electrode for battery, battery using same and process for same
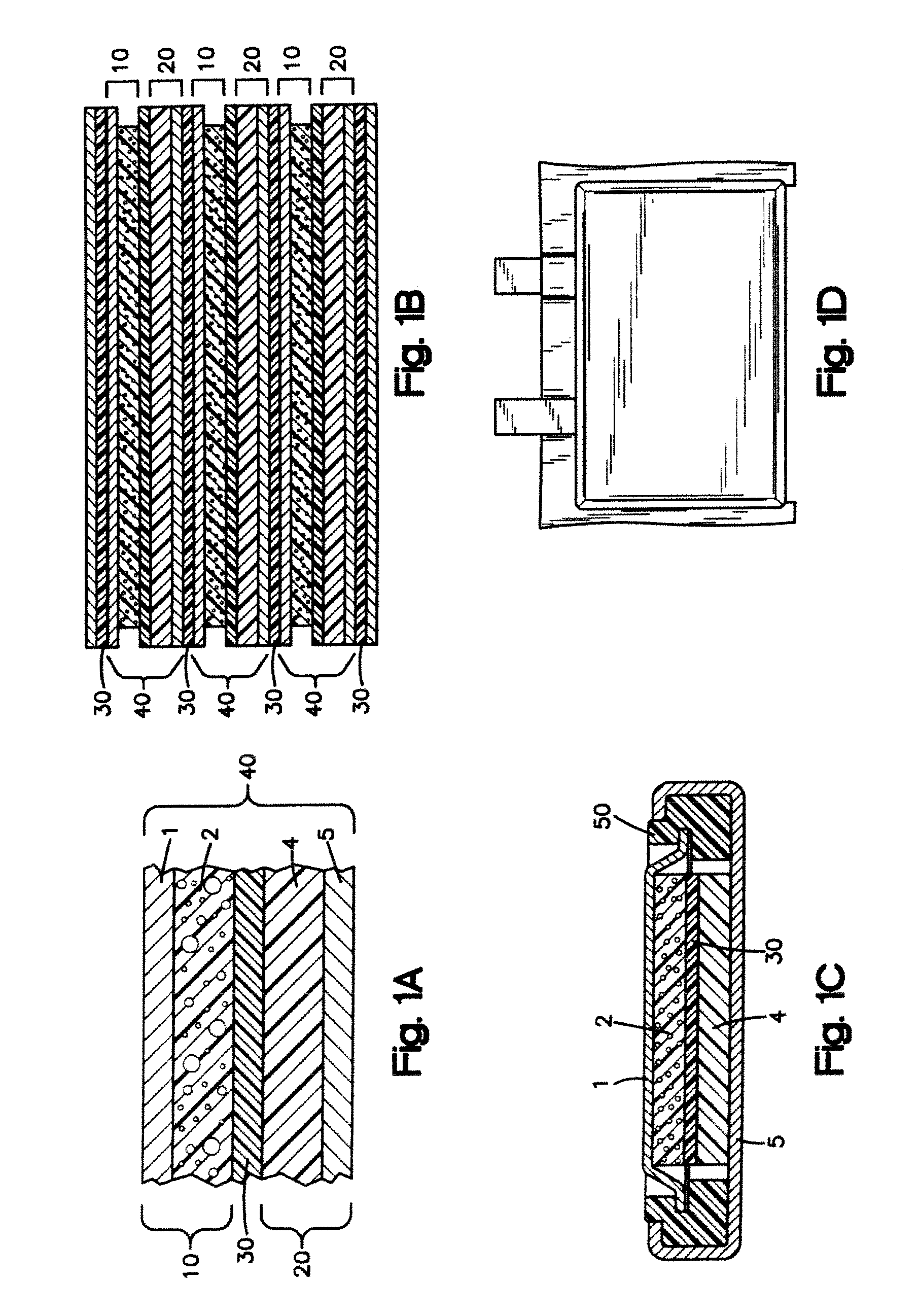
InactiveUS20050019664A1Prevent peelingPrevent materialElectrode carriers/collectorsActive material electrodesElectrical batteryEngineering
An electrode substrate is formed by mechanically processing a nickel foil so as to be made three dimensional through the creation of concave and convex parts, and then, this substrate is filled with active material or the like so that an electrode is manufactured, wherein the above described concave and convex parts are rolling pressed so as to incline in one direction. Furthermore, an electrode for secondary battery is formed by using the above described method.
Owner:THE UNIV OF QUEENSLAND
Method of manufacturing solid electrolyte battery
InactiveUS20050132562A1High trafficAvoid excessive power outputNon-aqueous electrolyte accumulatorsFinal product manufacturePolymer electrolytesBattery cell
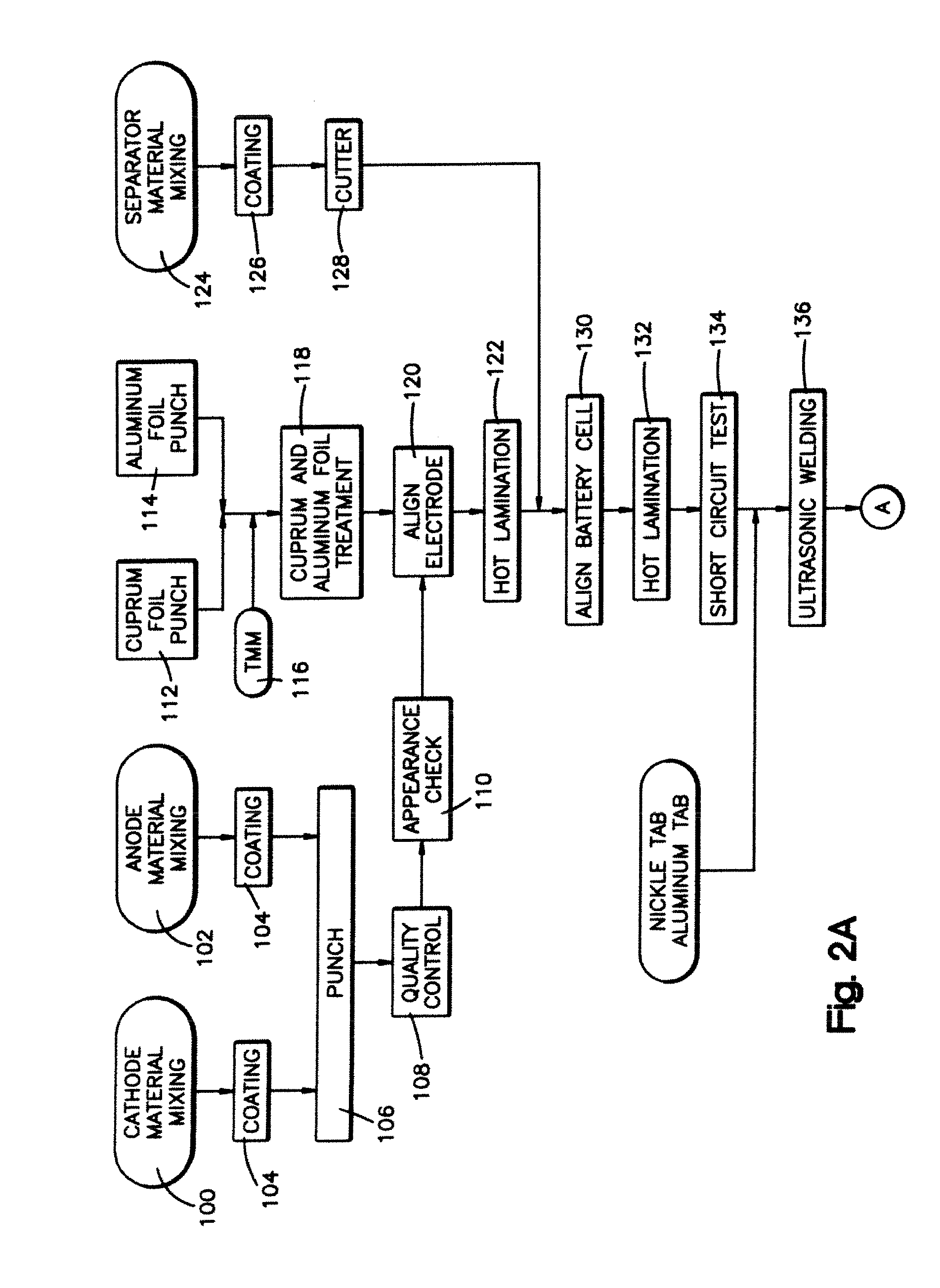
A method of manufacturing a solid electrolyte battery includes a step of thermally pressing a composite layer including a positive electrode ink layer, an electrolyte ink layer and a negative electrode ink layer that are formed by coating a positive electrode ink, an electrolyte ink and a negative electrode ink. Further, the positive electrode ink, the electrolyte ink and the negative electrode ink contain a polymer electrolyte. By this method, it is possible to improve the flow of ions across respective interlayers of a positive electrode active material layer, a solid electrolyte layer and a negative electrode active material layer.
Owner:NISSAN MOTOR CO LTD
Primer for battery electrode
ActiveUS20100291442A1Electrode carriers/collectorsActive material electrodesPolyvinyl alcoholCrosslinked polymers
Primer arrangements that facilitate electrical conduction and adhesive connection between an electroactive material and a current collector are presented. In some embodiments, primer arrangements described herein include first and second primer layers. The first primer layer may be designed to provide good adhesion to a conductive support. In one particular embodiment, the first primer layer comprises a substantially uncrosslinked polymer having hydroxyl functional groups, e.g., polyvinyl alcohol. The materials used to form the second primer layer may be chosen such that the second primer layer adheres well to both the first primer layer and an electroactive layer. In certain embodiments including combinations of first and second primer layers, one or both of the first and second primer layers comprises less than 30% by weight of a crosslinked polymeric material. A primer including only a single layer of polymeric material is also provided.
Owner:SION POWER CORP
Aqueous polyvinylidene fluoride composition
ActiveUS20100304270A1Dry fastUseful electrodeLiquid electrolytic capacitorsConductive materialInterconnectivityPolyvinylidene difluoride
The invention relates to an aqueous fluoropolymer, and preferably polyvinylidene fluoride (PVDF), composition for manufacturing electrodes for use in non-aqueous-type electrochemical devices, such as batteries and electric double layer capacitors. The composition contains aqueous PVDF binder, and one or more powdery electrode-forming materials. In one embodiment, the composition is free of fluorinated surfactant In another embodiment, one or more fugitive adhesion promoters are added. The electrode formed from the composition of the invention exhibits interconnectivity and irreversibility that is achieved from the use of aqueous PVDF binder.
Owner:ARKEMA INC
Novel composite cathodes, electrochemical cells comprising novel composite cathodes, and processes for fabricating same
The present invention pertains to composite cathodes suitable for use in an electrochemical cell, said cathodes comprising: (a) an electroactive sulfur-containing cathode material, wherein said electroactive sulfur-containing cathode material, in its oxidized state, comprises a polysulfide moiety of the formula —Sm—, wherein m is an integer equal to or greater than 3; and, (b) an electroactive transition metal chalcogenide composition, which encapsulates said electroactive sulfur-containing cathode material, and which retards the transport of anionic reduction products of said electroactive sulfur-containing cathode material, said electroactive transition metal chalcogenide composition comprising an electroactive transition metal chalcogenide having the formula MjYk(OR)l wherein: M is a transition metal; Y is the same or different at each occurrence and is oxygen, sulfur, or selenium; R is an organic group and is the same or different at each occurrence; j is an integer ranging from 1 to 12; k is a number ranging from 0 to 72; and l is a number ranging from 0 to 72; with the proviso that k and l cannot both be 0. The present invention also pertains to methods of making such composite cathodes, cells comprising such composite cathodes, and methods of making such cells.
Owner:SION POWER CORP
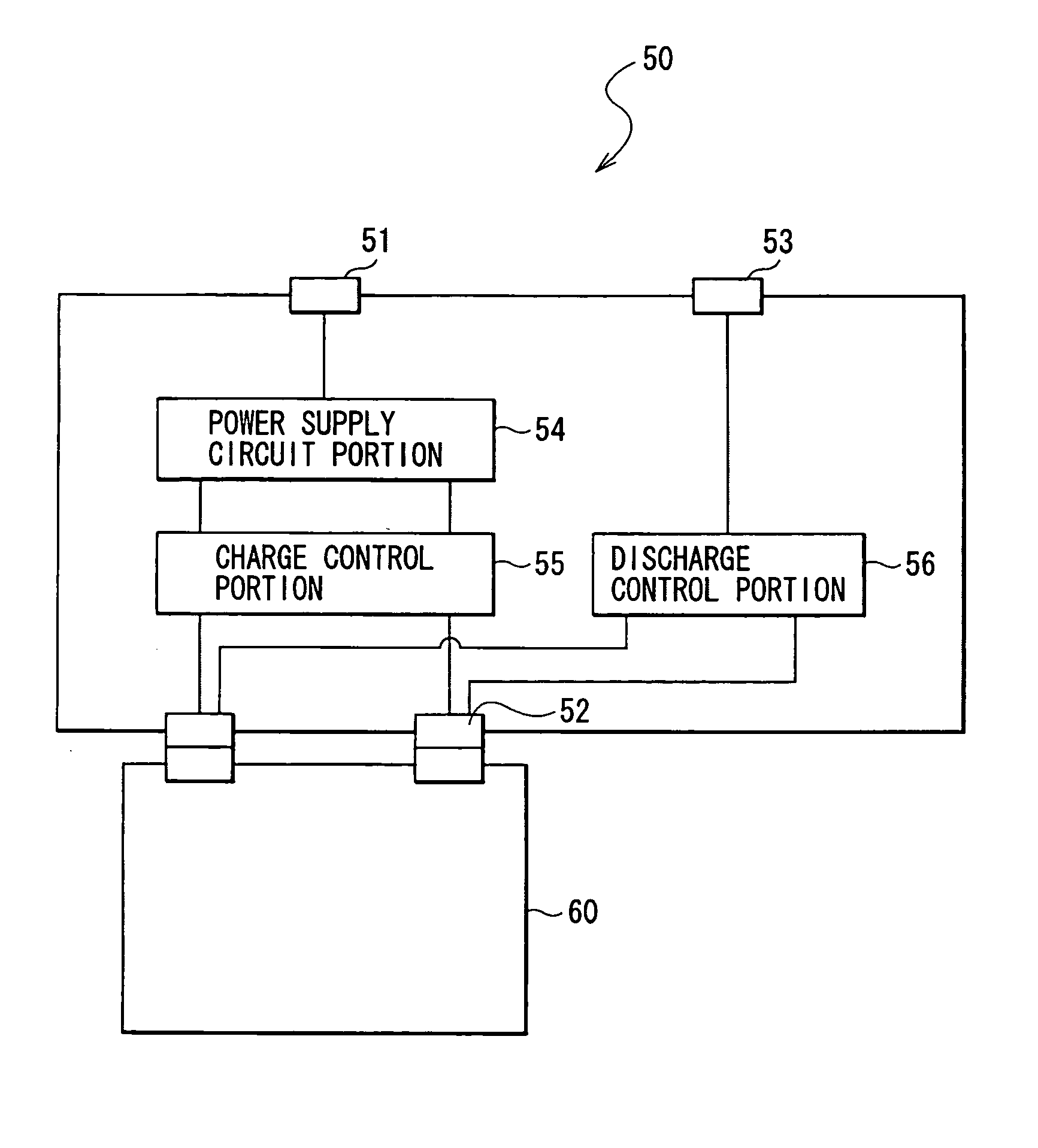
Battery, method of charging and discharging the battery and charge-discharge control device for the battery
InactiveUS20050214646A1Excellent cycle characteristicsSimple structureElectrode thermal treatmentElectrode carriers/collectorsOptoelectronicsAlloy
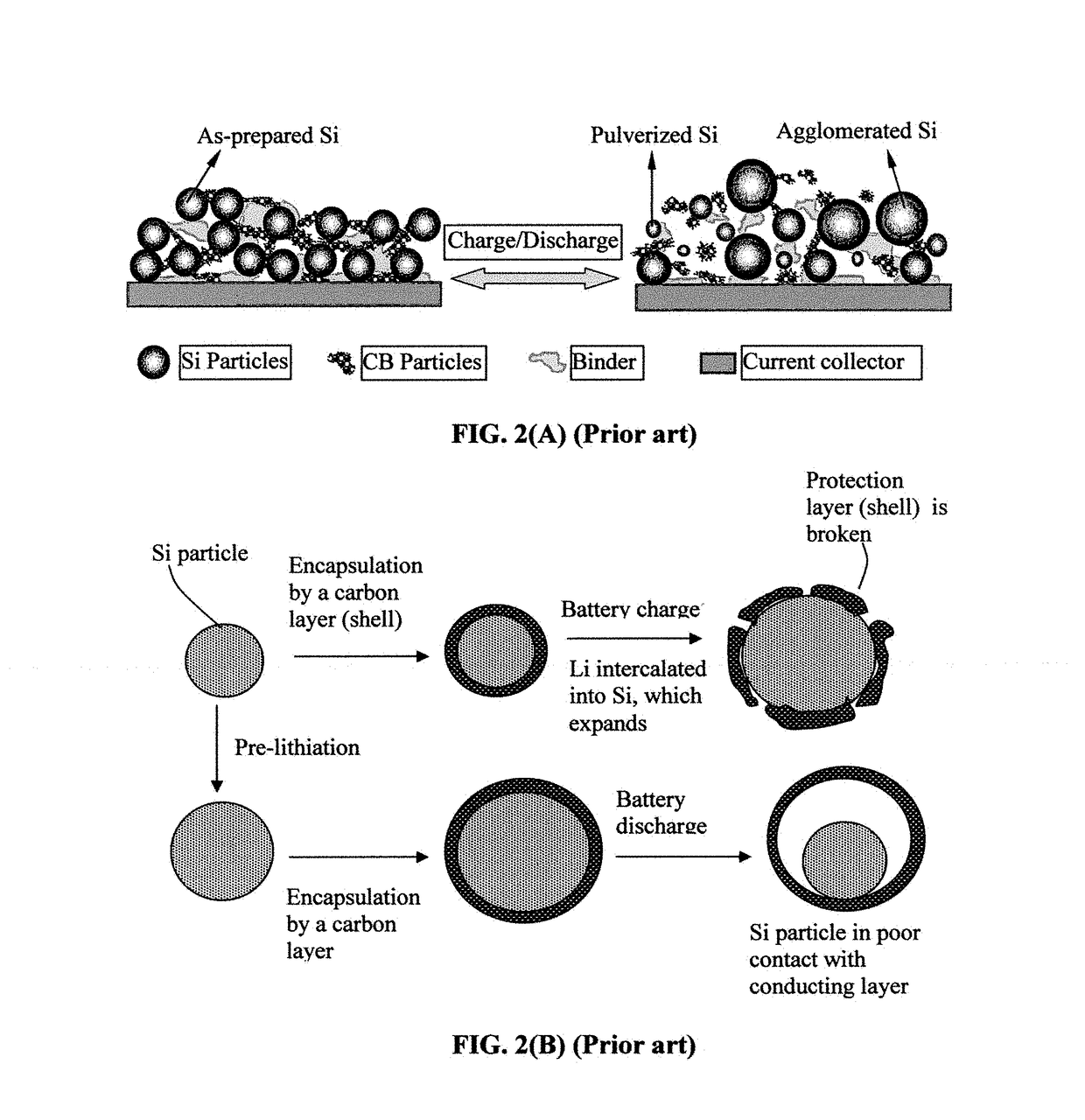
Provided is a battery capable of improving cycle characteristics through reducing a structural fracture according to charge and discharge of an anode and a reaction of the with an electrolyte. An anode active material layer includes at least one kind selected from the group consisting of a simple substance and alloys of Si capable of forming an alloy with Li. A cathode and an anode are formed so that the molar ratio Li / Si in the anode at the time of charge is 4.0 or the potential of the anode vs. Li is 0.04 V or more through adjusting, for example, a ratio between a cathode active material and an anode active material. Moreover, the cathode and the anode are formed so that the molar ratio Li / Si in the anode at the time of discharge is 0.4 or more, or the potential of the anode vs. Li is 1.4 V or less.
Owner:SONY CORP
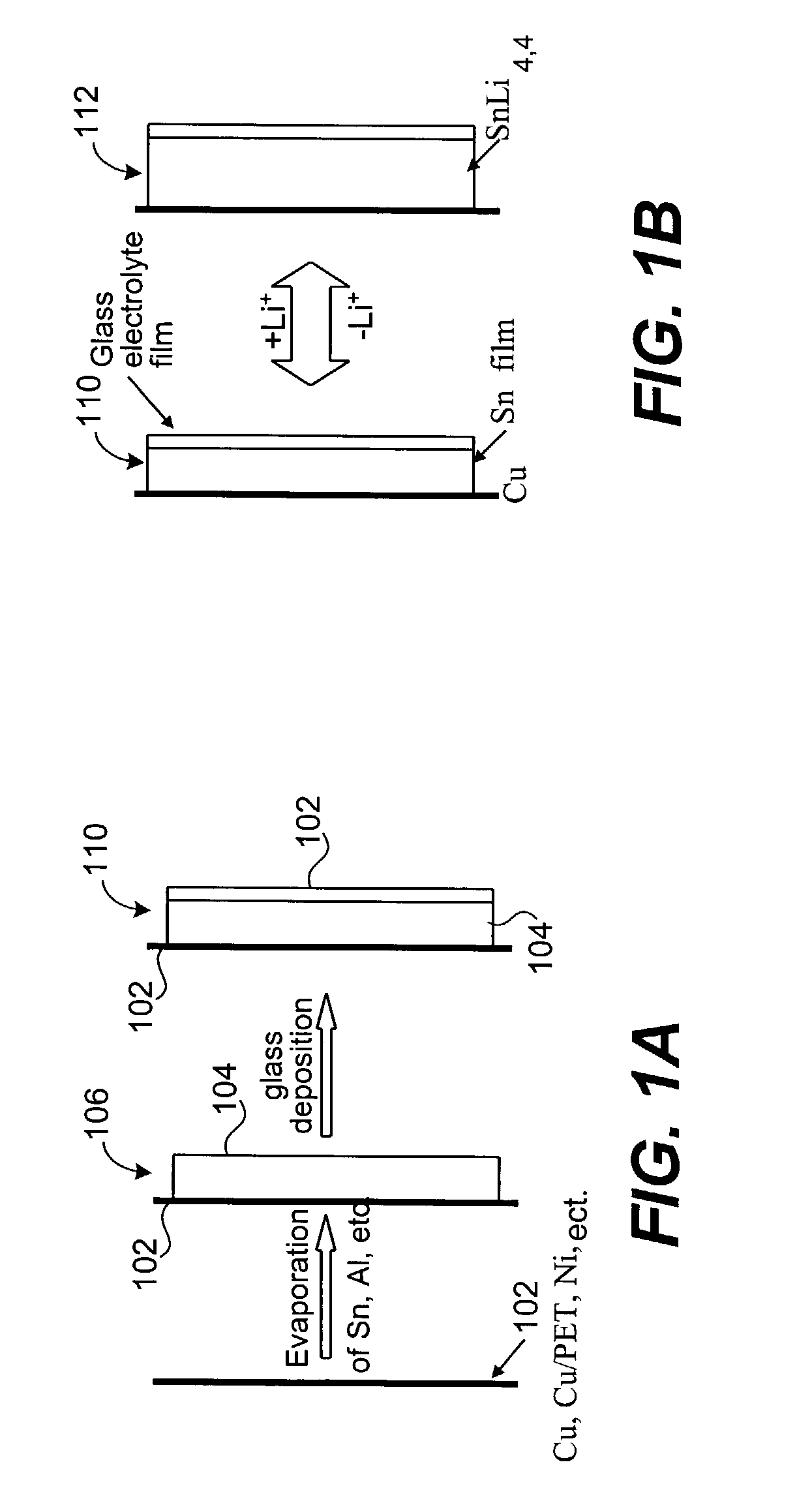
Method of making an ion-switching device without a separate lithiation step
InactiveUS20100007937A1Inhibition formationEfficient processingFinal product manufactureVacuum evaporation coatingChemical physicsElectrical conductor
In any manufacturing sequence for making a lithium ion-switching device, lithium has to be introduced at some stage into the device. An electrode inside the device is filled and depleted with lithium through an ion conductor at every use cycle of the device. Prior-art methods to introduce the lithium are: direct sputtering of lithium on the electrode, or electrochemically loading the electrode in an electrochemical cell, or indirectly loading the electrode after an ion conductor has been deposited on top of the electrode and still other methods. The inventive method disclosed makes such a separate lithiation step obsolete. The lithium is introduced at the same time as the ion-conductor is put on the electrode. This can be achieved by using an oxygen super-stoichiometric compound for the electrode.
Owner:SAGE ELECTROCHROMICS
Methods and reagents for enhancing the cycling efficiency of lithium polymer batteries
InactiveUS6165644AImprove efficiencyElectrode rolling/calenderingElectrochemical processing of electrodesLithium metalSulfur electrode
Batteries including a lithium electrode and a sulfur counter electrode that demonstrate improved cycling efficiencies are described. In one embodiment, an electrochemical cell having a lithium electrode and a sulfur electrode including at least one of elemental sulfur, lithium sulfide, and a lithium polysulfide is provided. The lithium electrode includes a surface coating that is effective to increase the cycling efficiency of said electrochemical cell. In a more particular embodiment, the lithium electrode is in an electrolyte solution, and, more particularly, an electrolyte solution including either elemental sulfur, a sulfide, or a polysulfide. In another embodiment, the coating is formed after the lithium electrode is contacted with the electrolyte. In a more particular embodiment, the coating is formed by a reaction between the lithium metal of the lithium electrode and a chemical species present in the electrolyte.
Owner:POLYPLUS BATTERY CO INC
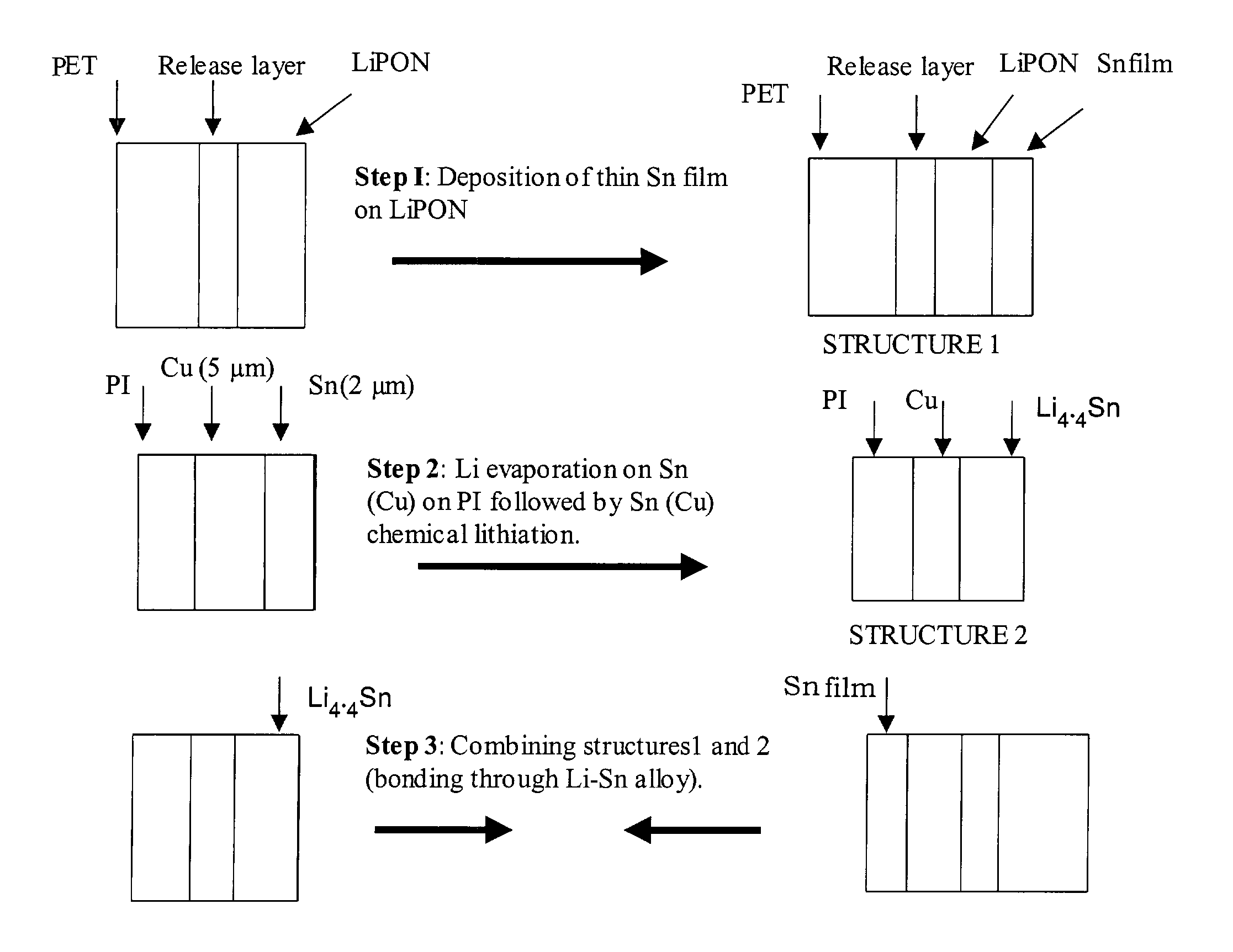
Coated lithium electrodes
InactiveUS6955866B2Improve cycle lifeIncrease storageabilityCellsOrganic electrolyte cellsSulfur electrodePolysulfide
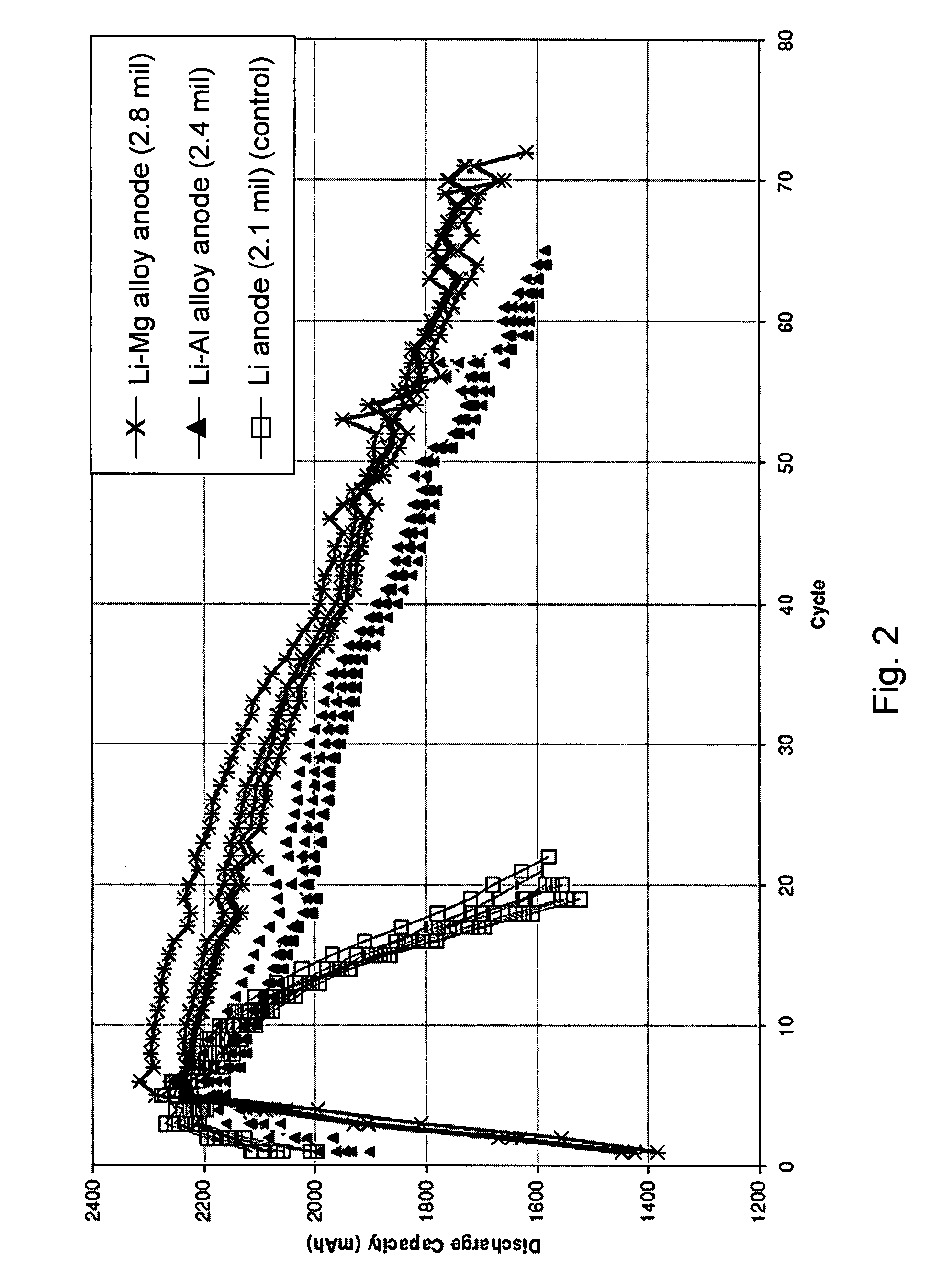
Batteries including a lithium anode stabilized with a metal-lithium alloy and battery cells comprising such anodes are provided. In one embodiment, an electrochemical cell having an anode and a sulfur electrode including at least one of elemental sulfur, lithium sulfide, and a lithium polysulfide is provided. The anode includes a lithium core and a ternary alloy layer over the lithium core where the ternary alloy comprises lithium and two other metals. The ternary alloy layer is effective to increase cycle life and storageability of the electrochemical cell. In a more particular embodiment, the ternary alloy layer is comprised of lithium, copper and tin.
Owner:POLYPLUS BATTERY CO INC
Lithium secondary battery and method for manufacturing thereof
InactiveUS20040072067A1Increase currentExtended discharge cycleFinal product manufactureElectrode carriers/collectorsLithiumElectrochemistry
A lithium secondary battery comprising an electrode in which an active material layer which includes an active material that electrochemically occludes and releases lithium is formed on a current collector, wherein cracks are formed in the active material layer by occlusion and release of lithium ions and thereafter a solid electrolyte is formed in the cracks in the active material layer.
Owner:SANYO ELECTRIC CO LTD
Nano-lithium-ion batteries and methos for manufacturing nano-lithium-ion batteries
The disclosure describes nano-lithium-ion batteries having a cathode, an anode including lithium titanium oxide nanoparticles, a separator including silicon dioxide nanoparticles and an electrolyte. In a preferred embodiment, the cathode is composed of 70-95 wt % lithium cobalt oxide, 1-6 wt % of a conductive carbon, and a synthetic resin including at least one thermoplastic; the anode is composed of 75-90 wt % lithium titanium oxide nanoparticles, 1-5 wt % of a conductive carbon, and a synthetic resin including at least one thermoplastic; and the separator is composed of silicon dioxide nanoparticles and a synthetic resin comprising at least one thermoplastic. The disclosure also describes methods of manufacturing the nano-lithium-ion batteries.
Owner:FU ZHIGUO +2
Lithium secondary battery and method of manufacturing the same
ActiveUS20080124631A1Avoid fracturesImprove cycle performanceElectrode carriers/collectorsNegative electrodesSilicon alloyMaterials science
A lithium secondary battery includes an electrode assembly having a positive electrode (1), a negative electrode (2) having a negative electrode current collector and a negative electrode active material layer formed on a surface of the negative electrode current collector and composed of a binder and negative electrode active material particles containing silicon and / or a silicon alloy, and a separator (3) interposed between the electrodes. The electrode assembly is impregnated with a non-aqueous electrolyte. The binder contains a polyimide resin represented by the following chemical formula (1):where R contains at least a benzene ring, and n is within the range of from 10 to 100,000, and the negative electrode active material particles have an average particle size of 5 μm or greater.
Owner:SANYO ELECTRIC CO LTD
Functional polymer film-coated electrode and electrochemical device using the same
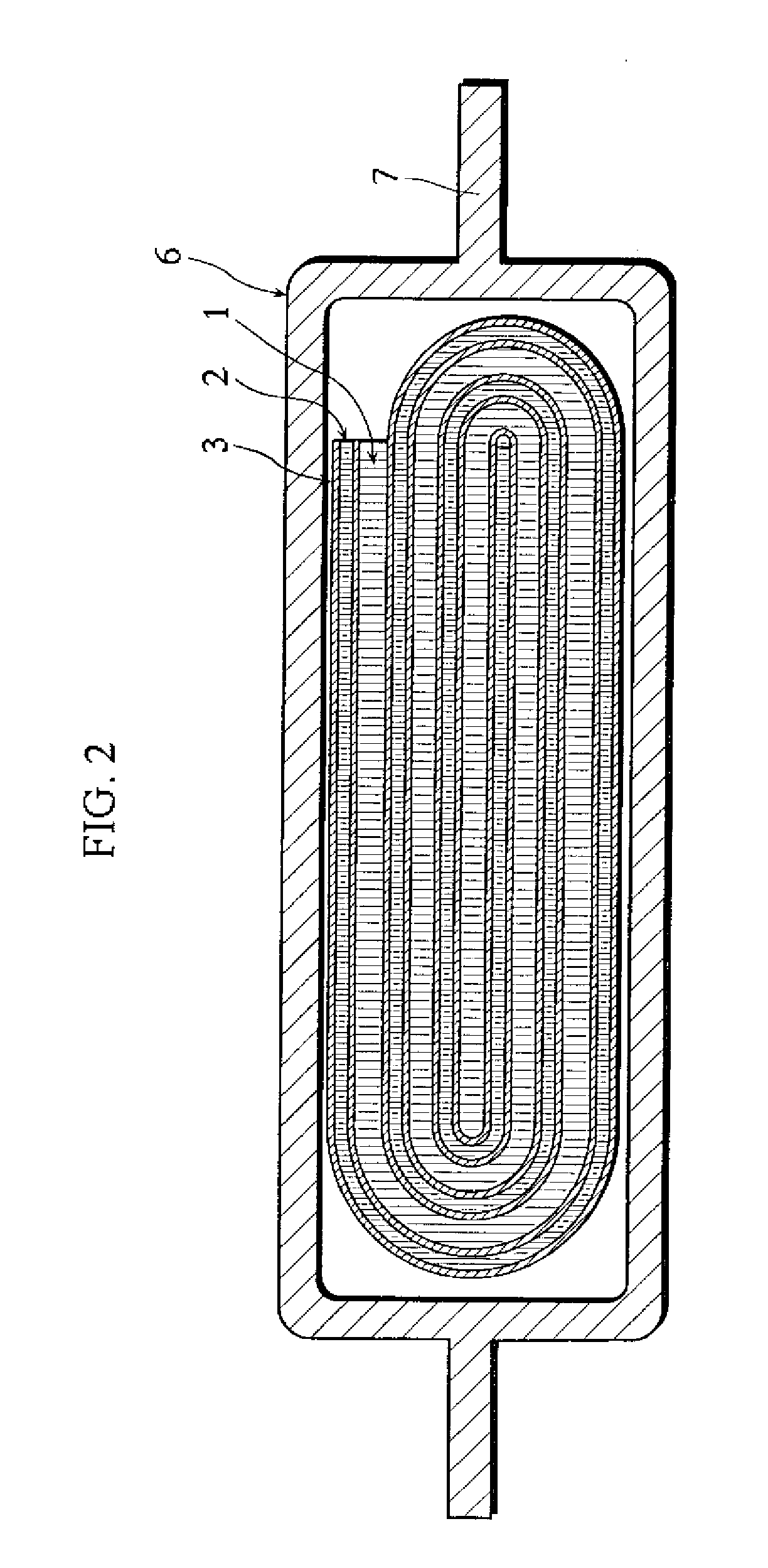
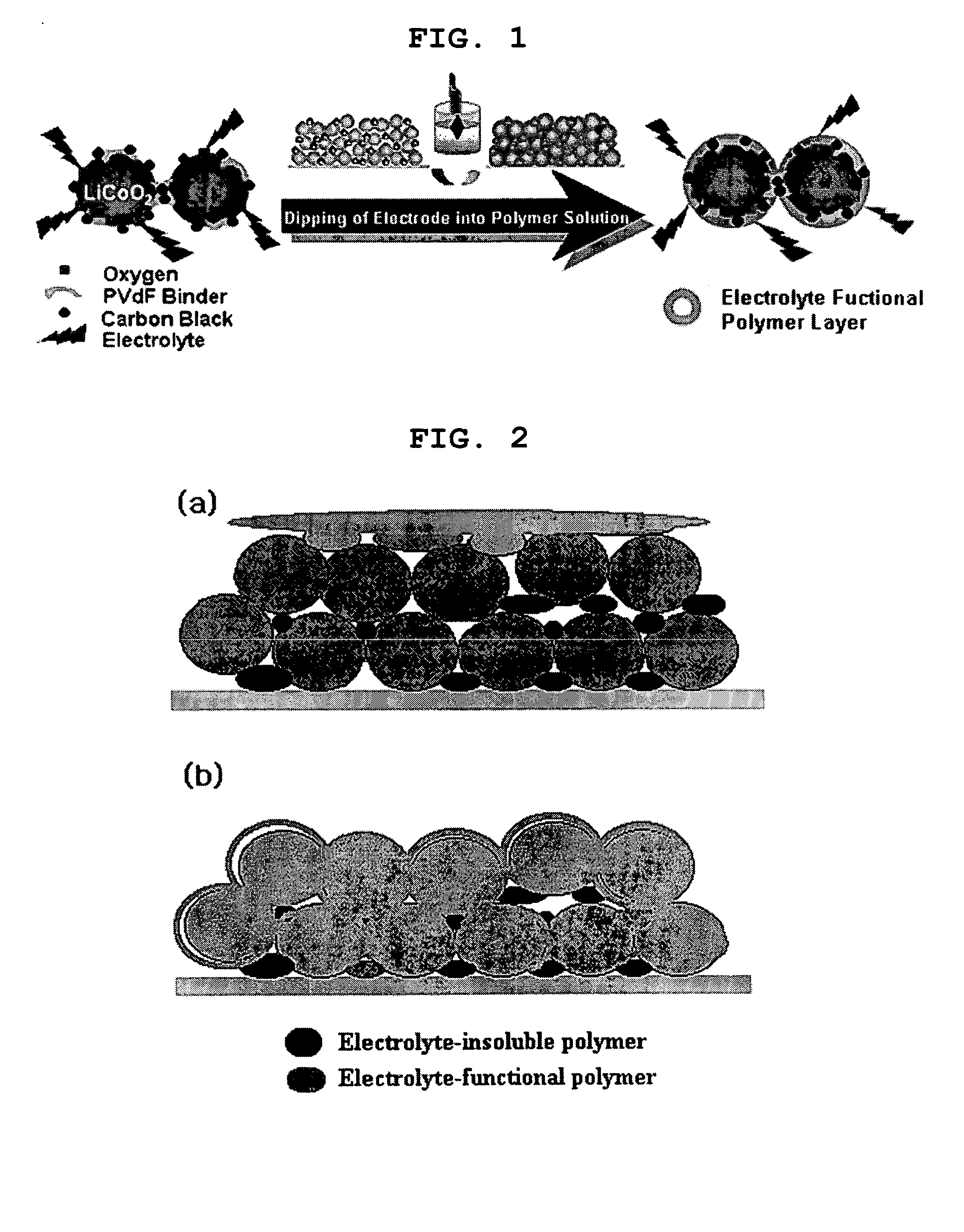
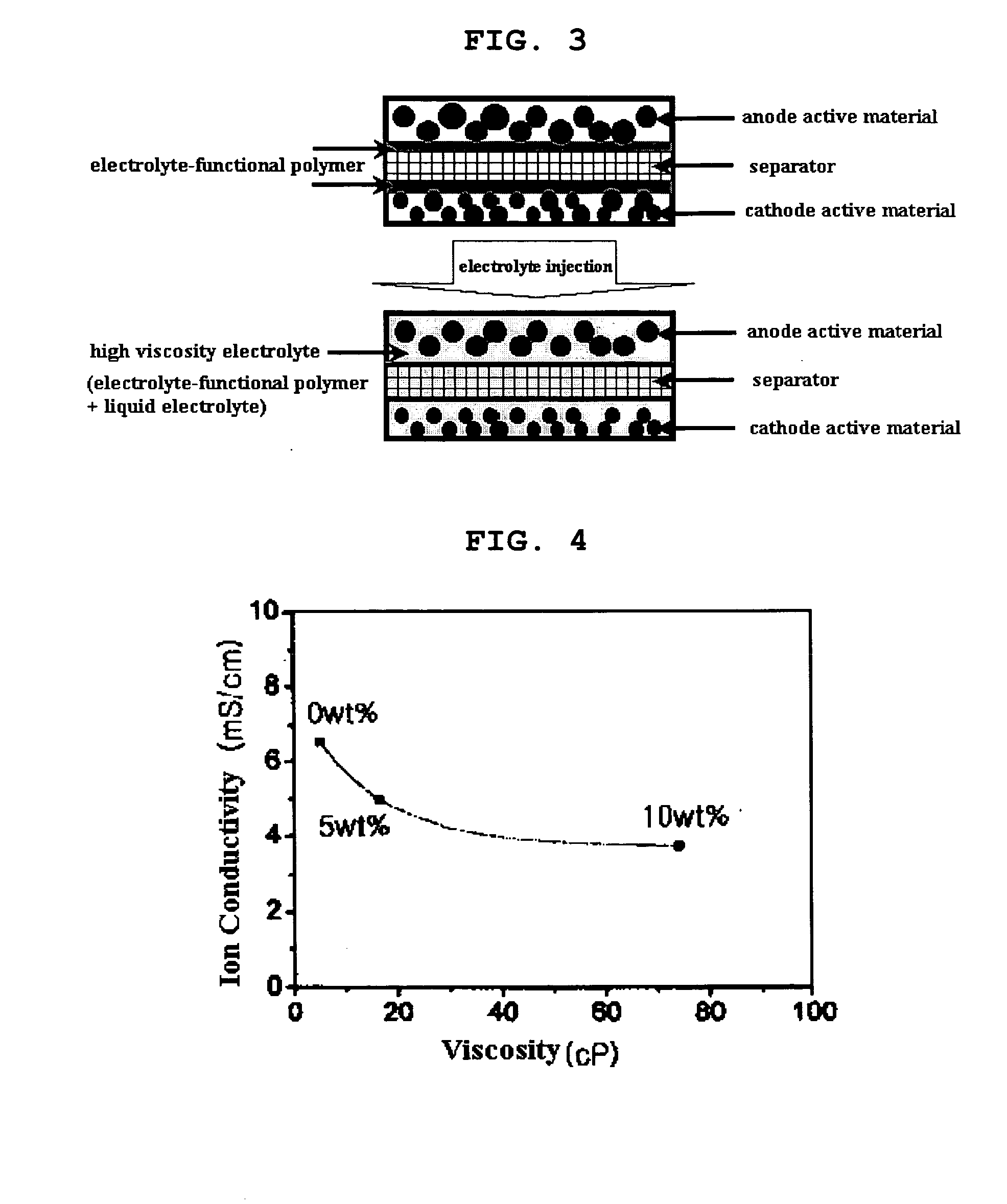
ActiveUS20050118508A1Lower performance requirementsImprove battery safetyGel electrodesElectrode carriers/collectorsSlurryPolymer thin films
The present invention provides an electrode in which an electrode active material particles as being interconnected are applied on current collector, wherein the interconnected surface of electrode active material particles is coated with a polymer, the polymer being present as an independent phase, while maintaining a pore structure formed among the interconnected electrode active material particles as well as an electrochemical device including the electrode. Also, the present invention provides a method for manufacturing an electrode coated with a polymer present on an interconnected surface of electrode active material as an independent phase, while maintaining a pore structure formed among the electrode active material particles, which comprises the steps of: (a) coating slurry for an electrode including an electrode active material on a current collector and drying it to form an electrode; and (b) dipping the electrode obtained from step (a) into a solution containing the polymer dissolved therein and a method for manufacturing an electrochemical device comprising the electrode obtained by the above method. The electrode coated with a polymer as an independent phase provides an electrochemical device with improved safety and prevents degradation of performance of an electrochemical device.
Owner:LG ENERGY SOLUTION LTD
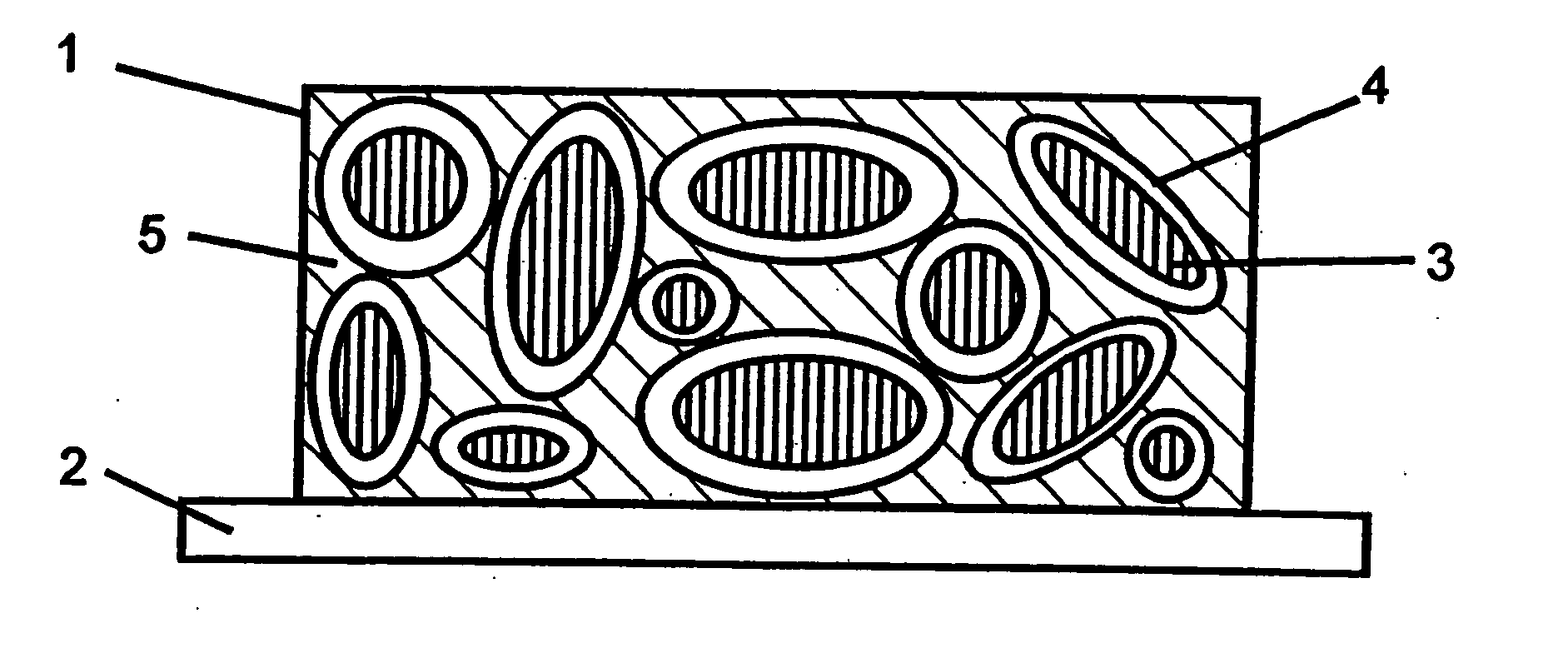
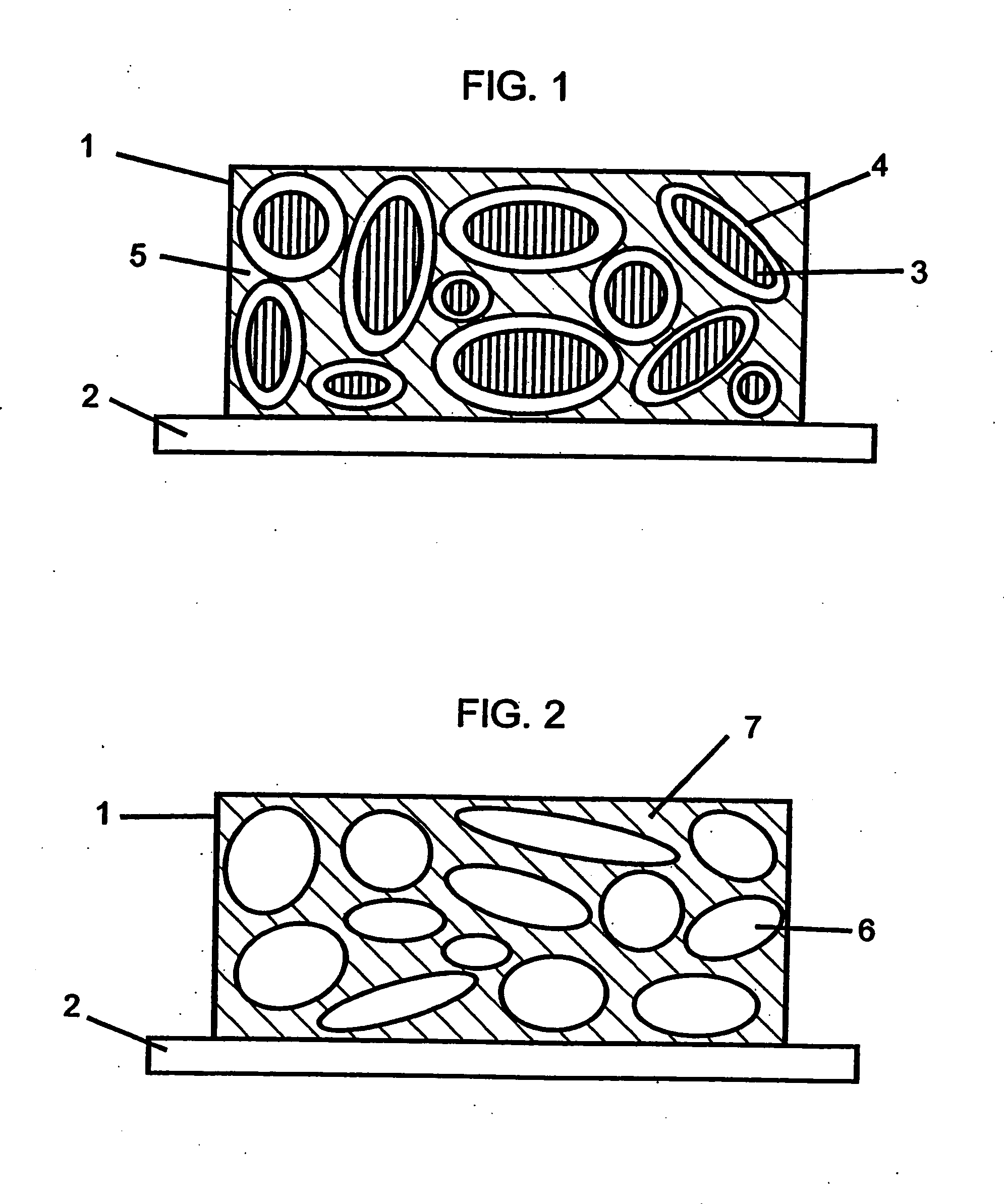
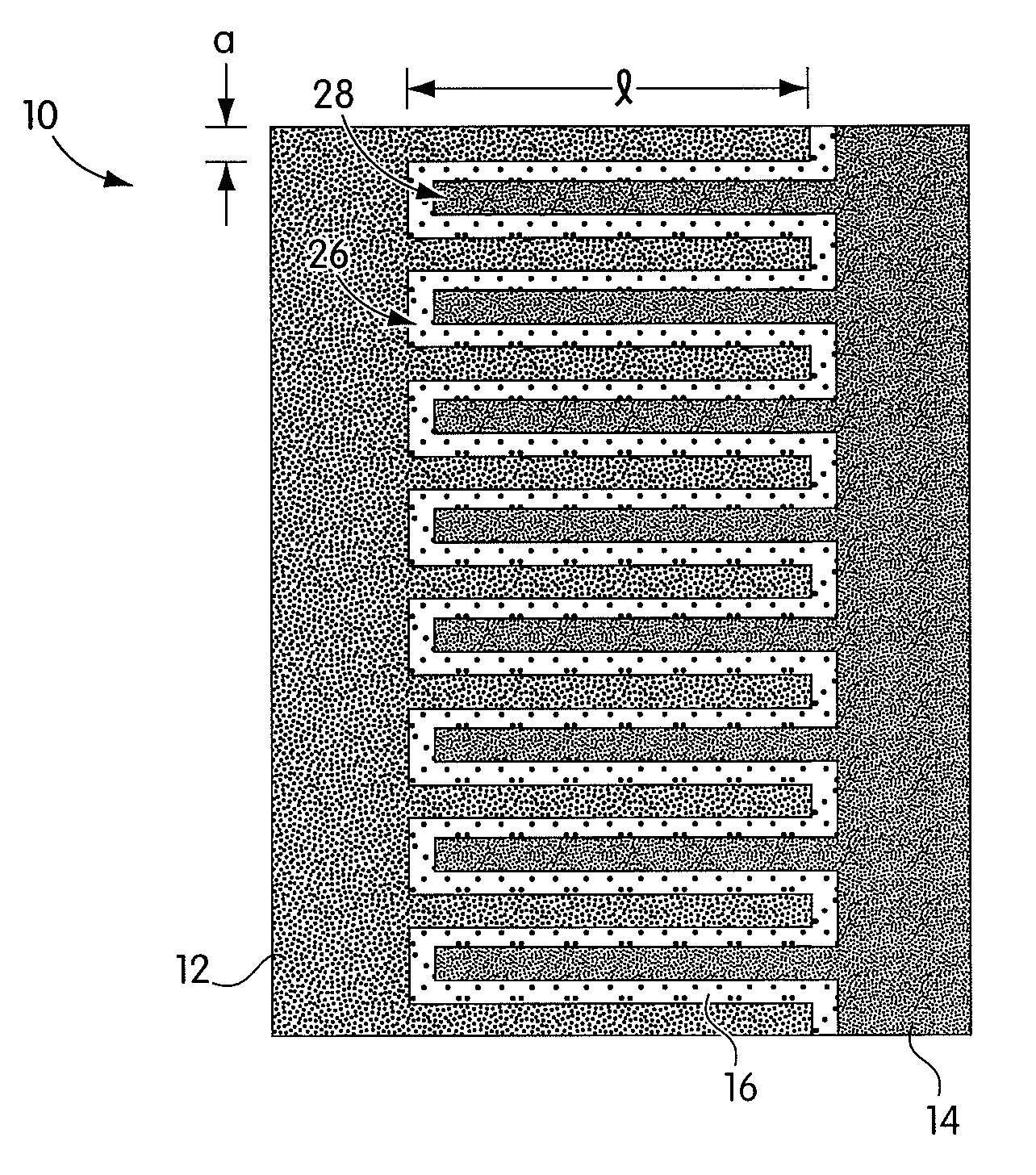
Reticulated and controlled porosity battery structures
InactiveUS7553584B2Boost energyHigh porosityElectrode thermal treatmentElectrolytic capacitorsEngineeringTortuous retinal vessels
The effective ionic conductivity in a composite structure is believed to decrease rapidly with volume fraction. A system, such as a bipolar device or energy storage device, has structures or components in which the diffusion length or path that electrodes or ions must traverse is minimized and the interfacial area exposed to the ions or electrons is maximized. The device includes components that can be reticulated or has a reticulated interface so that an interface area can be increased. The increased interfacial perimeter increases the available sites for reaction of ionic species. Many different reticulation patterns can be used. The aspect ratio of the reticulated features can be varied. Such bipolar devices can be fabricated by a variety of methods or procedures. A bipolar device having structures of reticulated interface can be tailored for the purposes of controlling and optimizing charge and discharge kinetics. A bipolar device having graded porosity structures can have improved transport properties because the diffusion controlling reaction kinetics can be modified. Graded porosity electrodes can be linearly or nonlinearly graded. A bipolar device having perforated structures also provides improved transport properties by removing tortuosity and reducing diffusion distance.
Owner:MASSACHUSETTS INST OF TECH
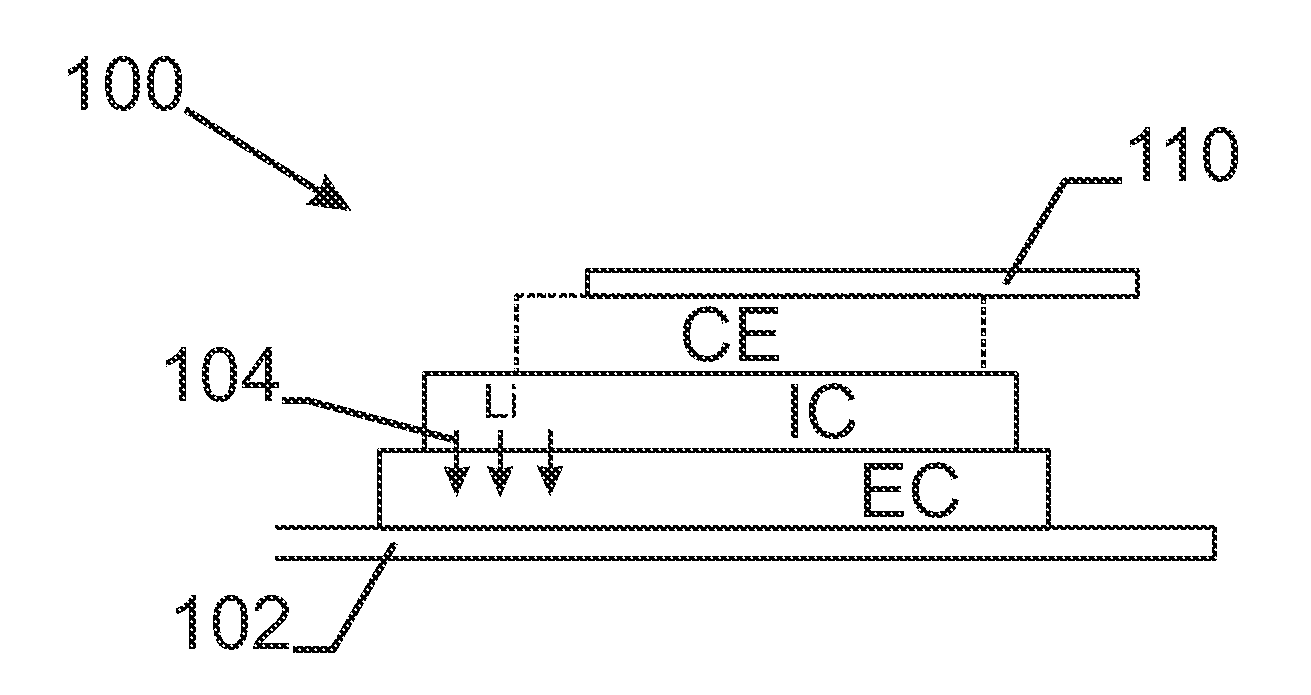
Elastomer-Encapsulated particles of high-capacity anode active materials for lithium batteries
ActiveUS20170288211A1Improve lithium ion conductivityFuel and secondary cellsElectrode thermal treatmentParticulatesElastomer
Provided is an anode active material layer for a lithium battery. This layer comprises multiple particulates of an anode active material, wherein at least a particulate is composed of one or a plurality of particles of a high-capacity anode active material being encapsulated by a thin layer of elastomeric material that has a lithium ion conductivity no less than 10−7 S / cm (preferably no less than 10−5 S / cm) at room temperature and an encapsulating shell thickness from 1 nm to 10 μm, and wherein the high-capacity anode active material (e.g. Si, Ge, Sn, SnO2, Co3O4, etc.) has a specific capacity of lithium storage greater than 372 mAh / g (the theoretical lithium storage limit of graphite).
Owner:GLOBAL GRAPHENE GRP INC
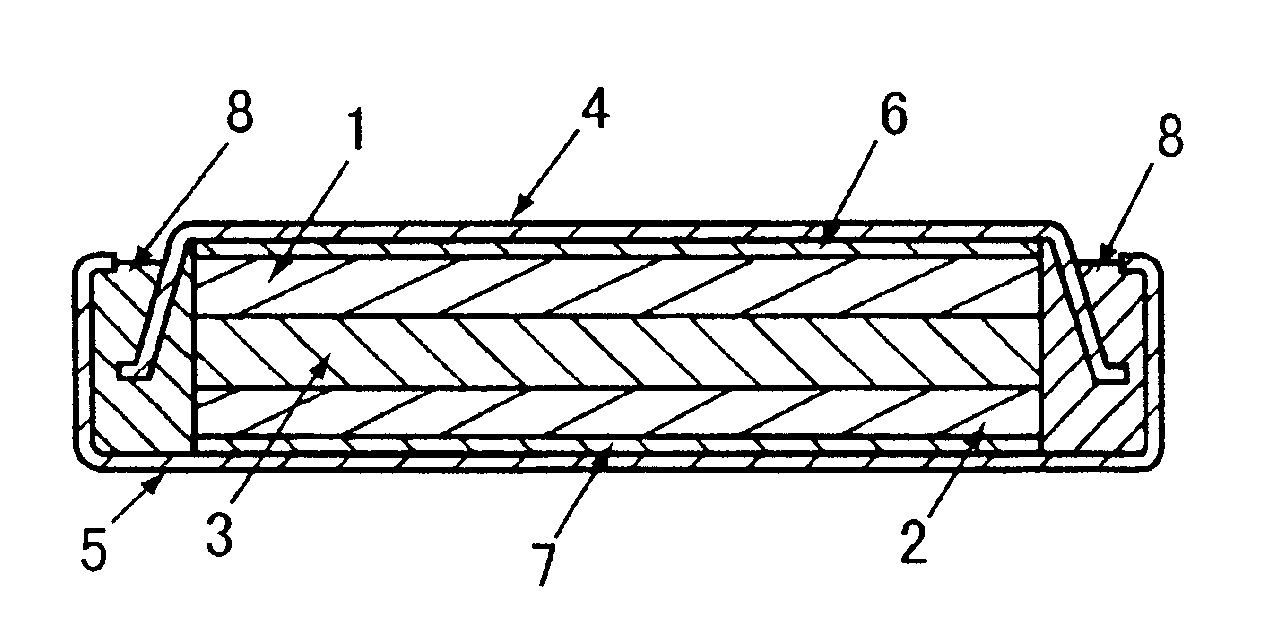
Lithium-iron disulfide cylindrical cell with modified positive electrode
InactiveUS20080026293A1Improve battery performanceMaterial Utilization OptimizationFinal product manufactureElectrode carriers/collectorsEngineeringAlloy
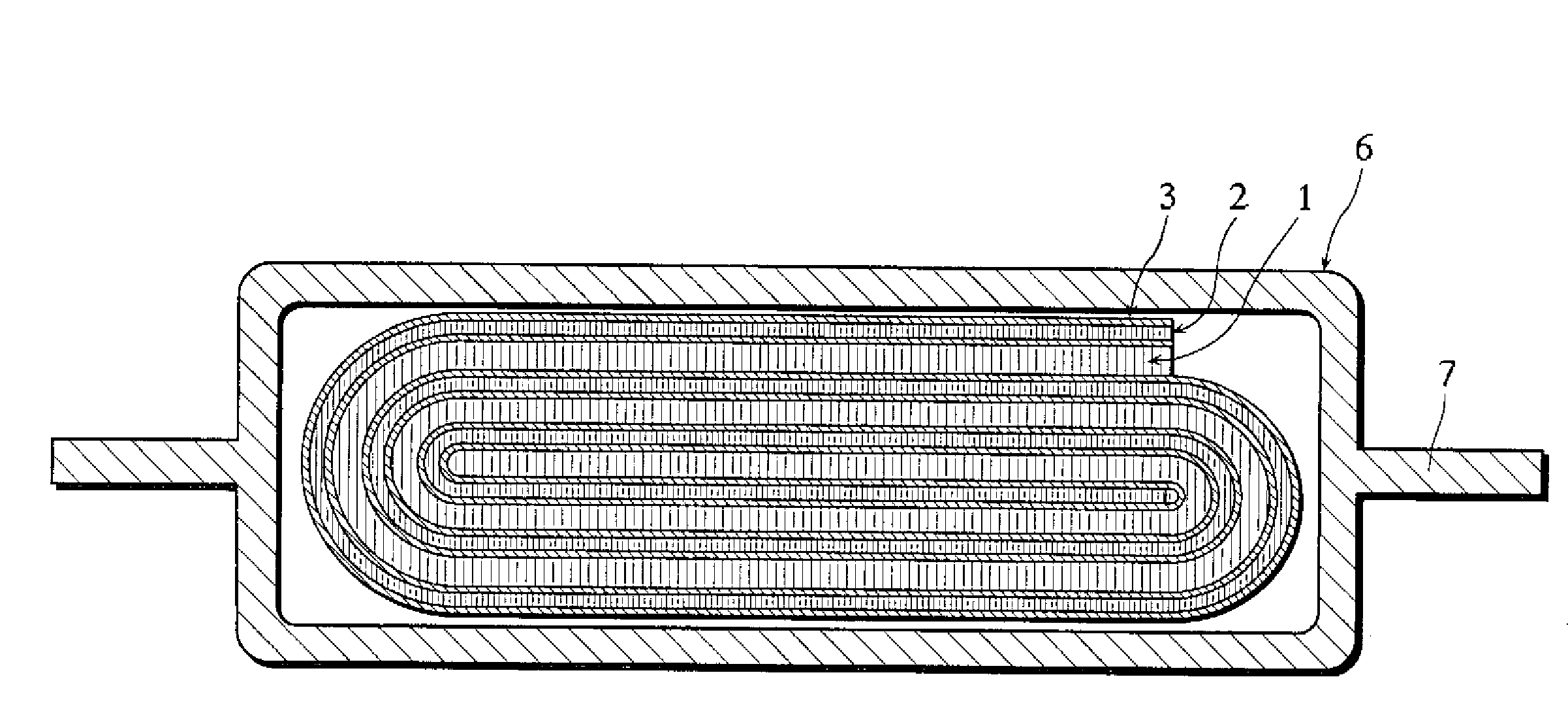
A primary electrochemical cell, and a method for making the same, relies upon a jellyroll electrode with a positive electrode material deposited on a conductive carrier having partially uncoated portion wherein electrochemically active material is coated on only one side of the carrier in order to achieve superior performance in comparison to a cell having no such uncoated portion. The partially uncoated portion is oriented along a longitudinal axis of the jellyroll. The positive electrode material is preferably iron disulfide, whereas the negative electrode comprises lithium or a lithium alloy.
Owner:EVEREADY BATTERY CO INC
Electrode, electrochemical device, method for manufacturing electrode, and method for manufacturing electrochemical device
InactiveUS20050064289A1Distinguish clearlyReliably obtainedFixed capacitor electrodesActive material electrodesInternal resistanceElectrochemistry
The electrode of the present invention is provided with an active material-containing layer comprising as the structural material composite particles composed of an electrode active material, a conductive additive and a binder, and a current collector in electrical contact with the layer. The composite particles are formed by integrating the conductive additive and binder with the electrode active material particles. The active material-containing layer is formed by subjecting powder comprising at least the composite particles to pressurization treatment to form a sheet, and placing the sheet at the location of the current collector at which the active material-containing layer is to be formed. The electrode active material and conductive additive in the active material-containing layer are non-isolated and electrically linked. This construction allows an electrode with excellent electrical characteristics to be realized, which exhibits adequately reduced internal resistance and easily permits increased energy density to be achieved for electrochemical devices.
Owner:TDK CORPARATION
Lithium anodes for electrochemical cells
InactiveUS20080014501A1Light weightFinal product manufactureElectrode carriers/collectorsLithium metalReactive gas
Provided is an anode for use in electrochemical cells, wherein the anode active layer has a first layer comprising lithium metal and a multi-layer structure comprising single ion conducting layers and polymer layers in contact with the first layer comprising lithium metal or in contact with an intermediate protective layer, such as a temporary protective metal layer, on the surface of the lithium-containing first layer. Another aspect of the invention provides an anode active layer formed by the in-situ deposition of lithium vapor and a reactive gas. The anodes of the current invention are particularly useful in electrochemical cells comprising sulfur-containing cathode active materials, such as elemental sulfur.
Owner:SION POWER CORP
Lithium secondary battery
InactiveUS20050008939A1Improve featuresElectrochemical processing of electrodesFinal product manufactureLithiumPhosphate
A lithium secondary battery comprising a positive electrode, a negative electrode, and a nonaqueous electrolyte, wherein the positive electrode or the negative electrode is an electrode obtained by depositing a thin film of active material capable of lithium storage and release on a current collector, the thin film is divided into columns by gaps formed therein in a manner to extend in its thickness direction and the columnar portions are adhered at their bottoms to the current collector, and the nonaqueous electrolyte contains at least one selected from phosphate ester, phosphite ester, borate ester and carboxylic ester having a fluoroalkyl group.
Owner:SANYO ELECTRIC CO LTD
Electrode material for anode of rechargeable lithium battery, electrode structural body using said electrode material, rechargeable lithium battery using said electrode structural body, process for producing said electrode structural body, and process for producing said rechargeable lithium battery
InactiveUS6949312B1Improve featuresLarge capacitySilver accumulatorsFinal product manufactureElectrochemical responseElectrode material
An electrode material for an anode of a rechargeable lithium battery, containing a particulate comprising an amorphous Sn.A.X alloy with a substantially non-stoichiometric ratio composition. For said formula Sn.A.X, A indicates at least one kind of an element selected from a group consisting of transition metal elements, X indicates at least one kind of an element selected from a group consisting of O, F, N, Mg, Ba, Sr, Ca, La, Ce, Si, Ge, C, P, B, Pb, Bi, Sb, Al, Ga, In, Tl, Zn, Be, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, As, Se, Te, Li and S, where the element X is not always necessary to be contained. The content of the constituent element Sn of the amorphous Sn.A.X alloy is Sn / (Sn+A+X)=20 to 80 atomic %. An electrode structural body for a rechargeable lithium battery, comprising said electrode material for an anode and a collector comprising a material incapable of being alloyed with lithium in electrochemical reaction, and a rechargeable lithium battery having an anode comprising said electrode structural body.
Owner:CANON KK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com