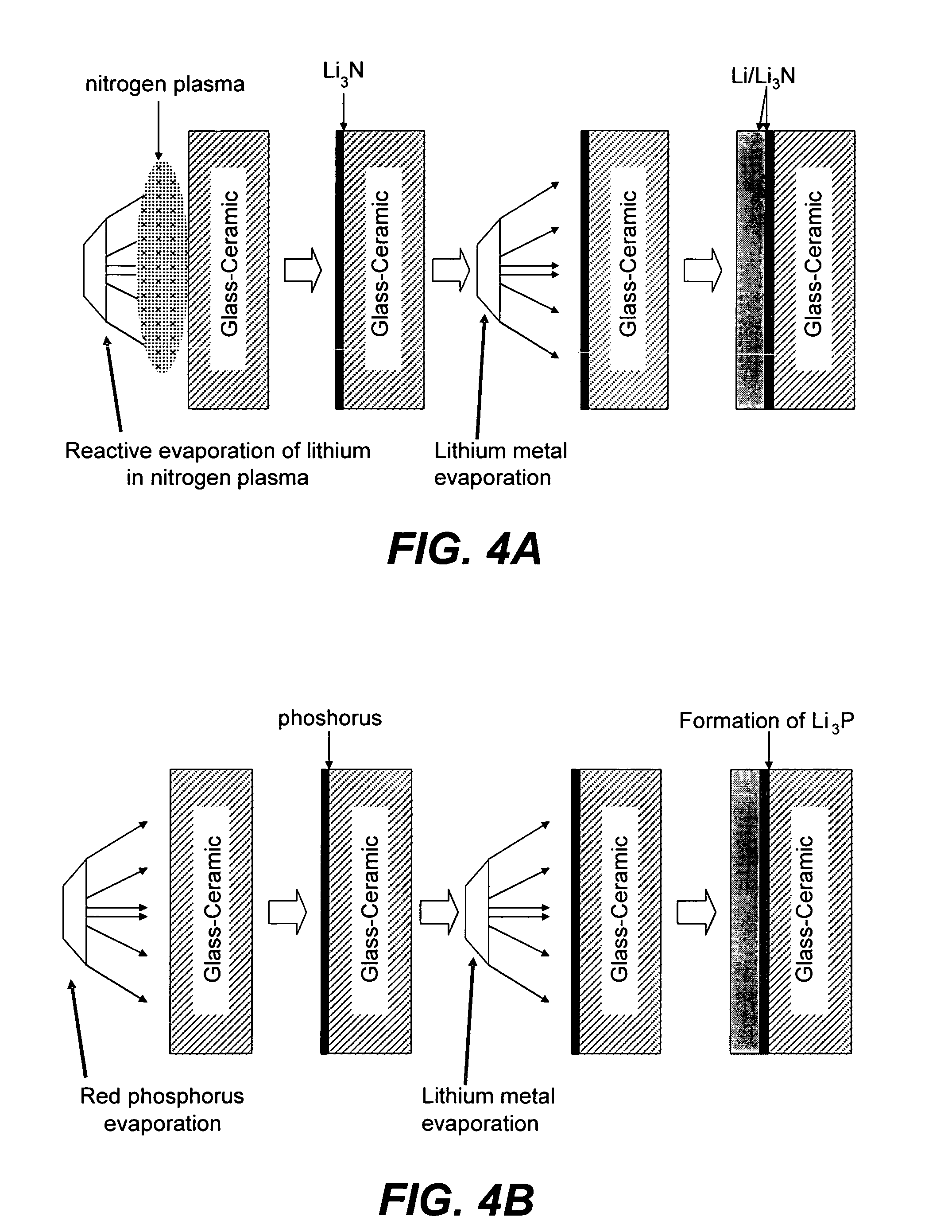
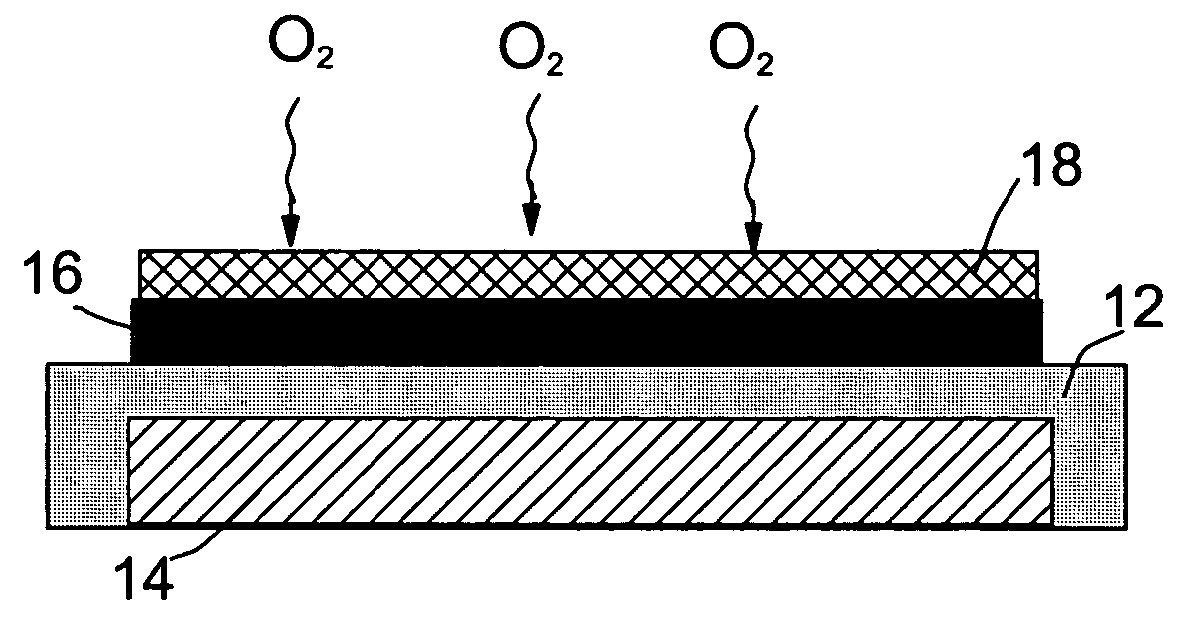
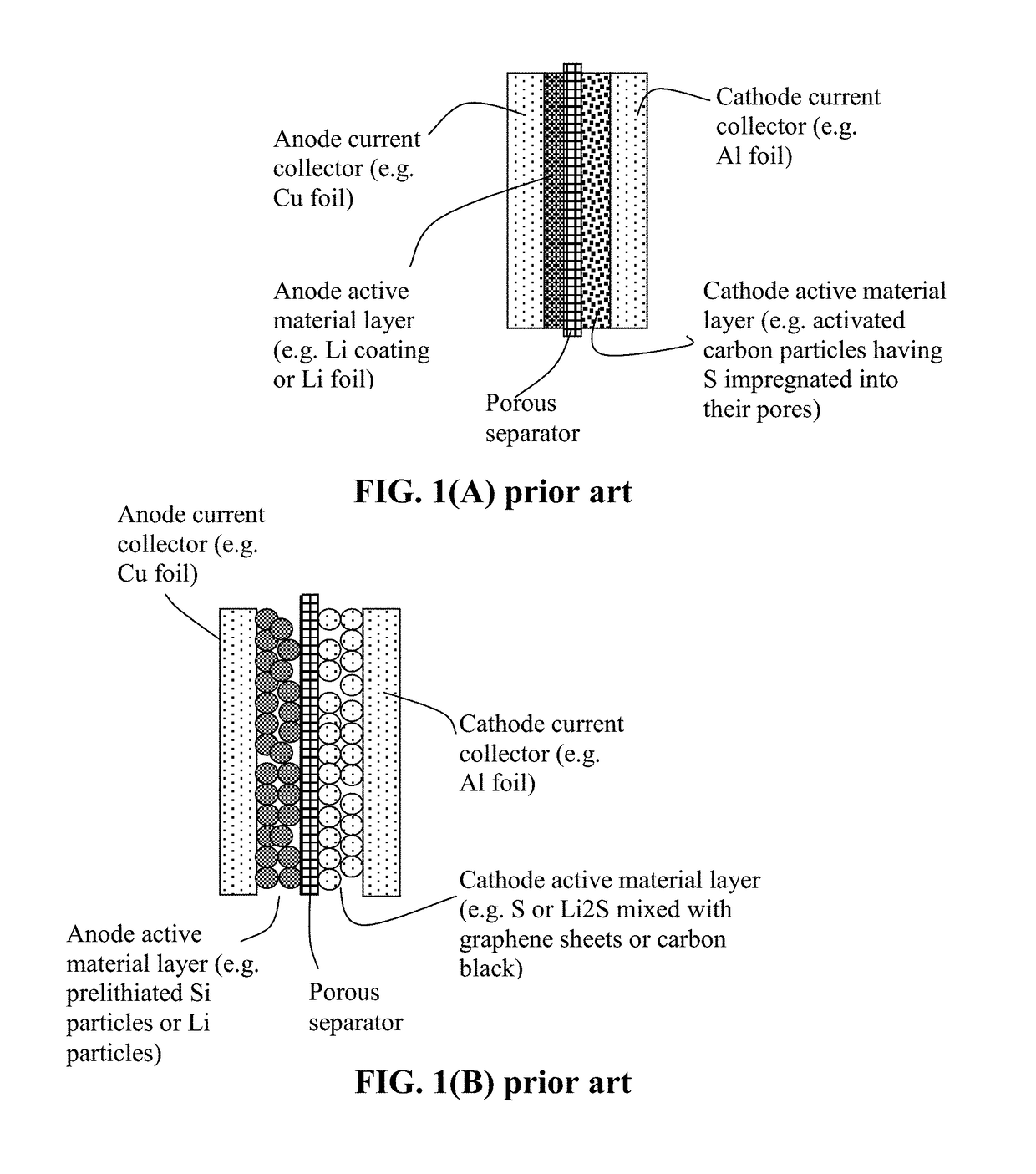
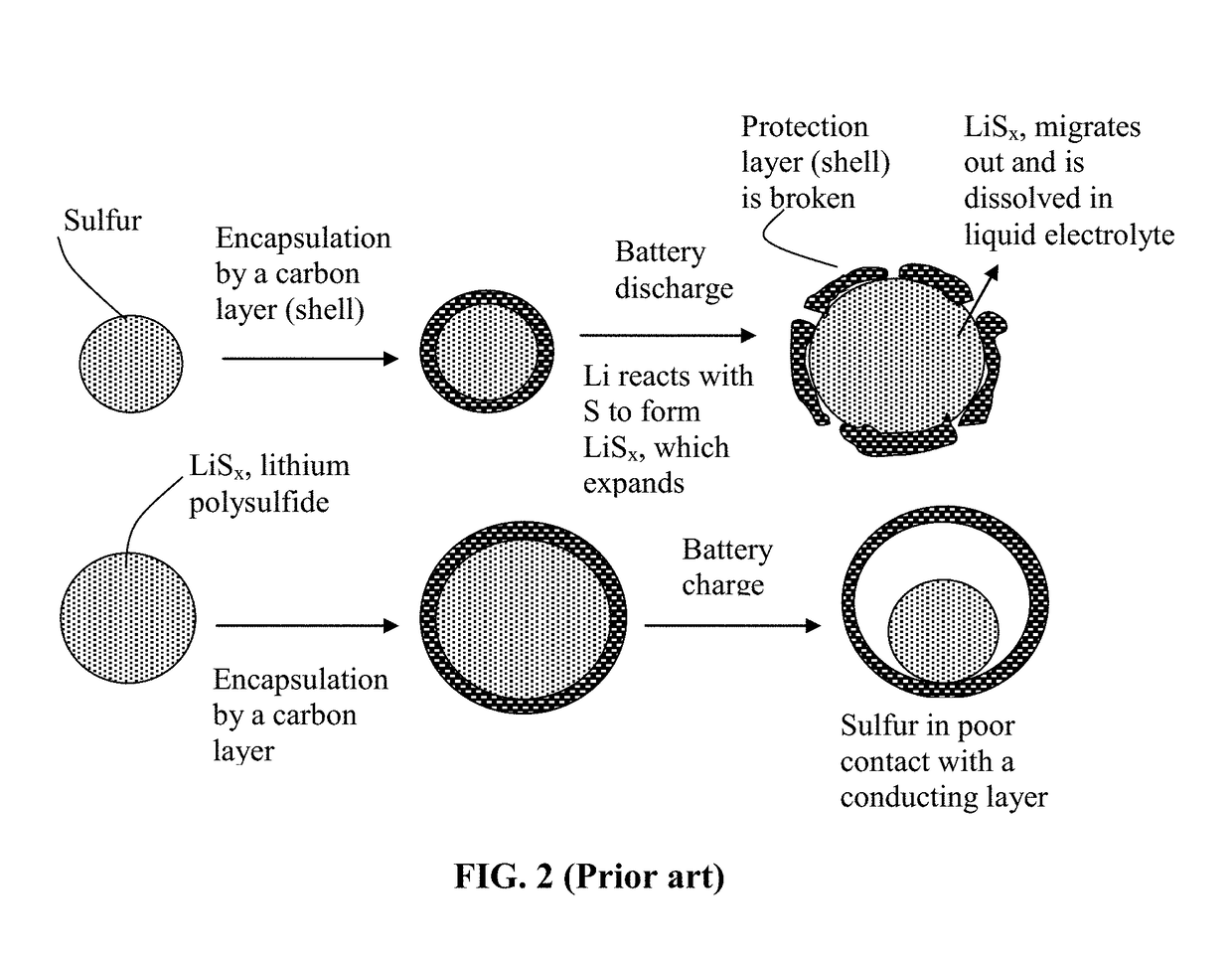
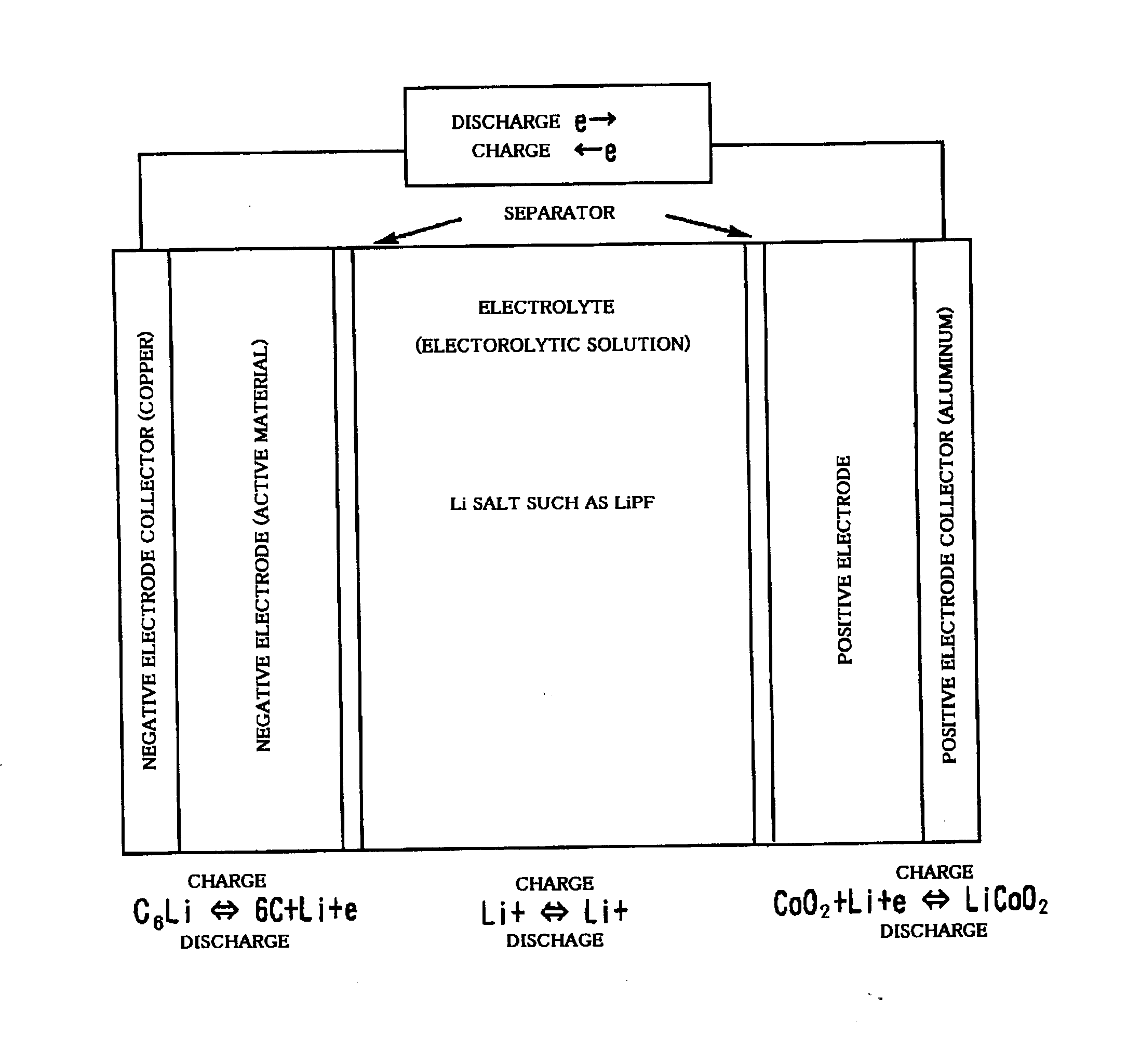
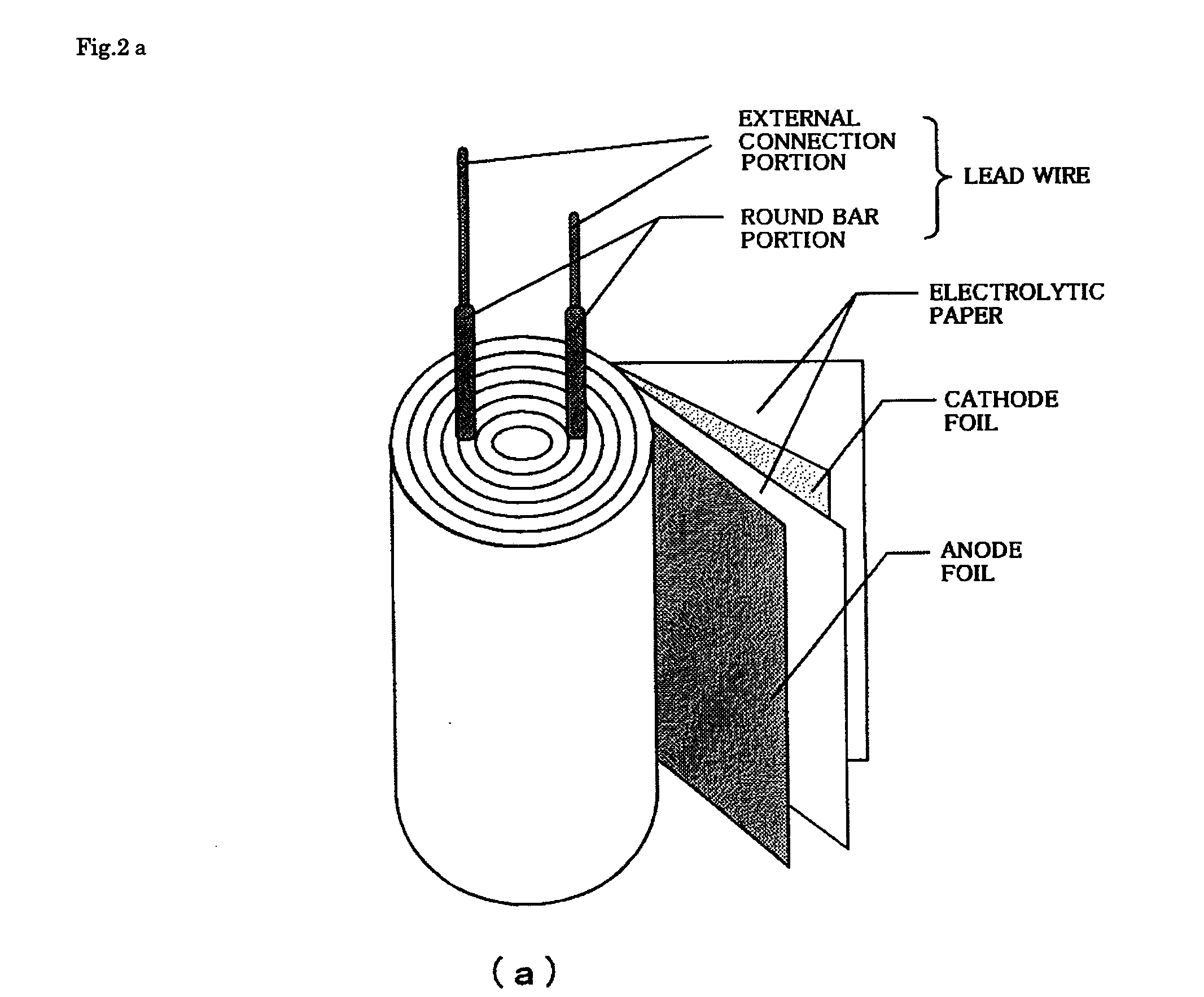
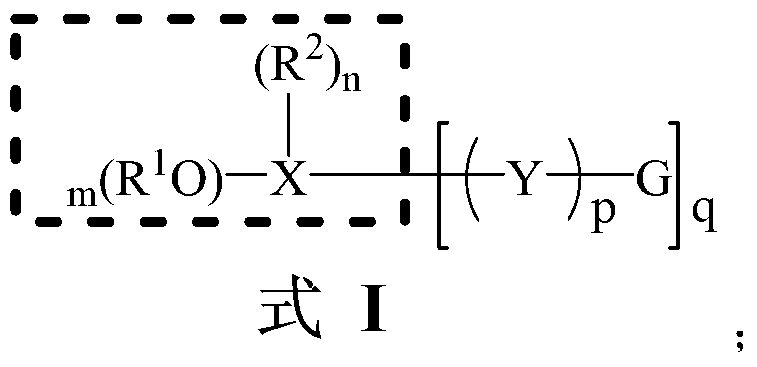Patents
Literature
2061 results about "Ionic conductivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
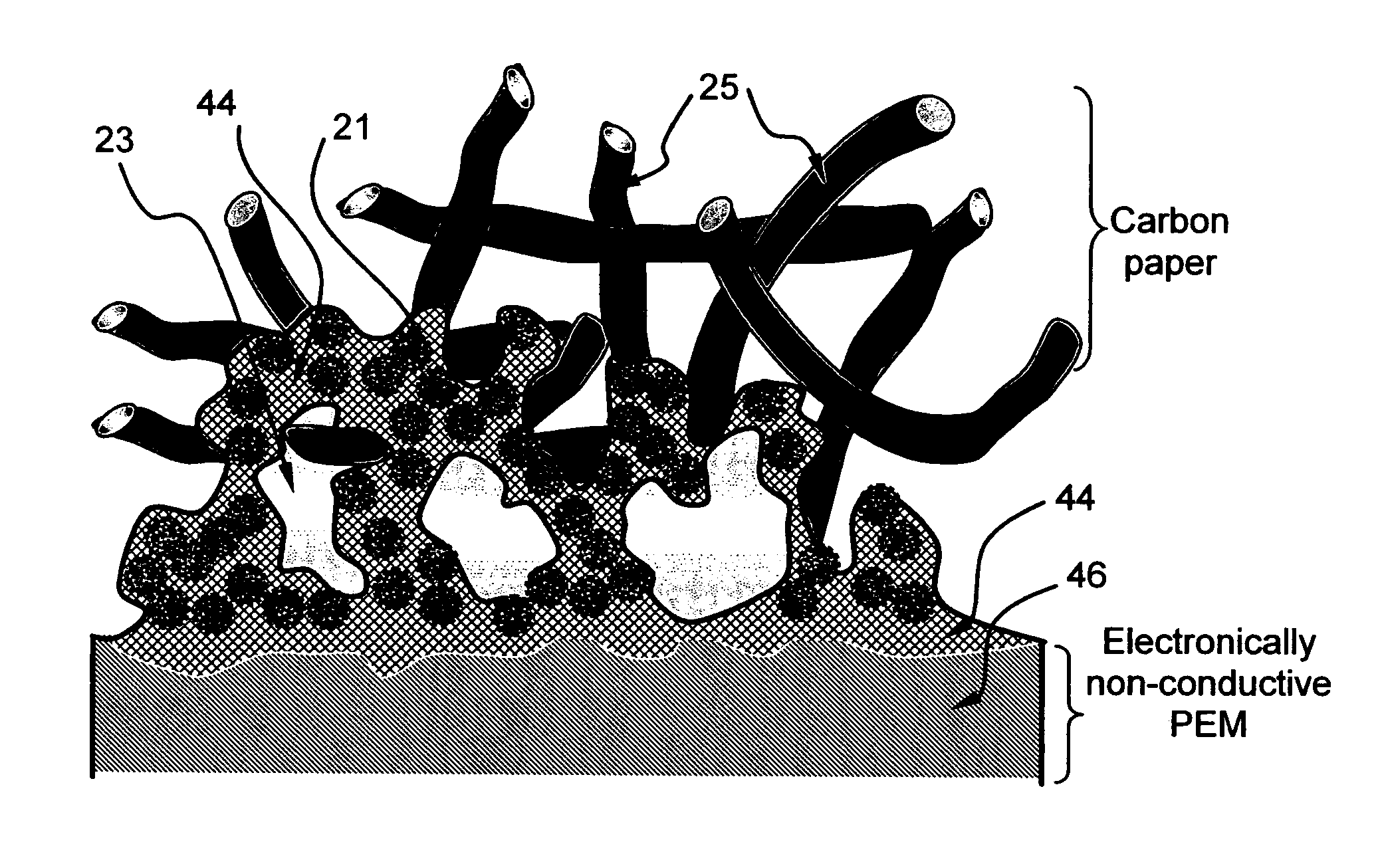
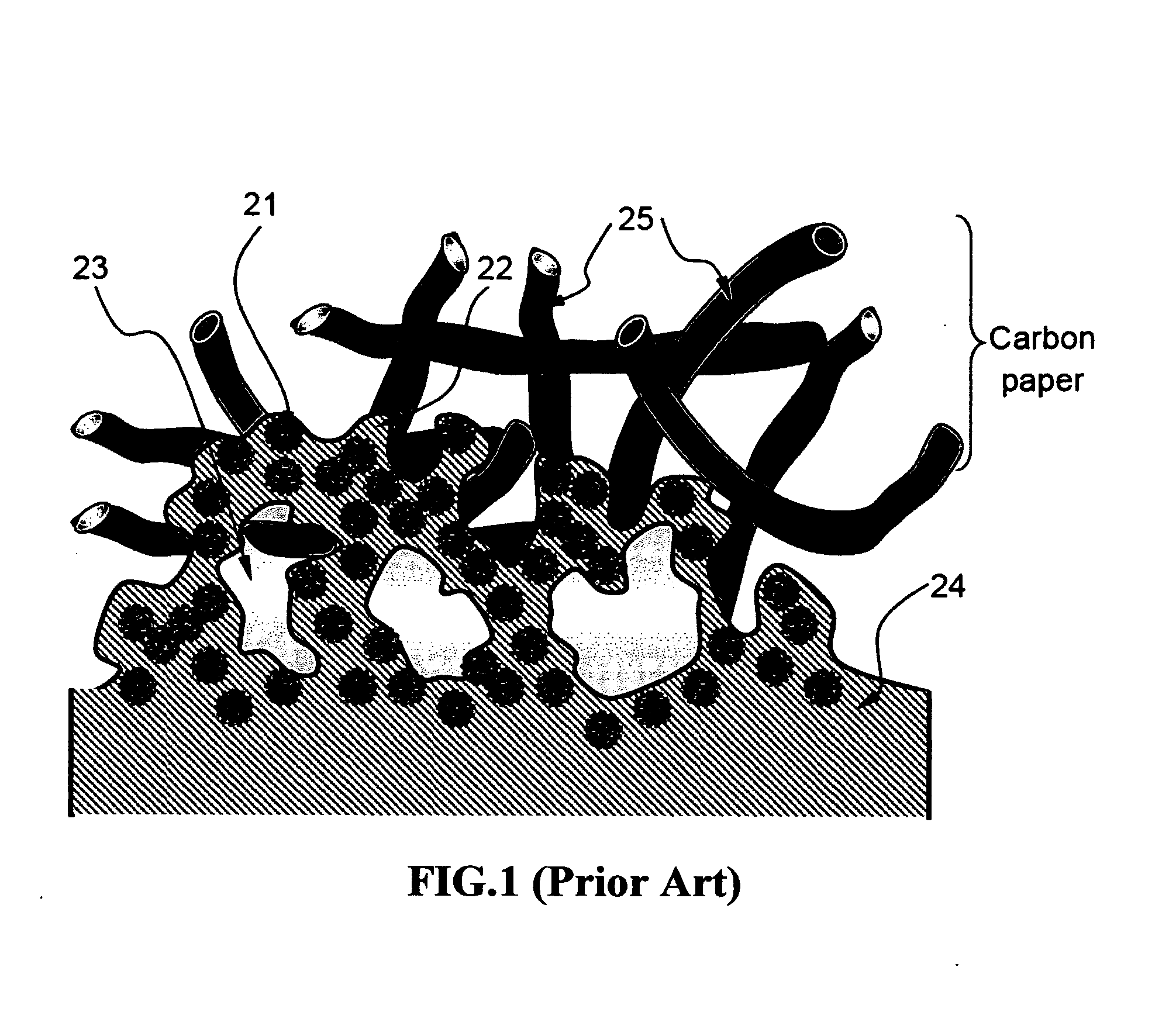
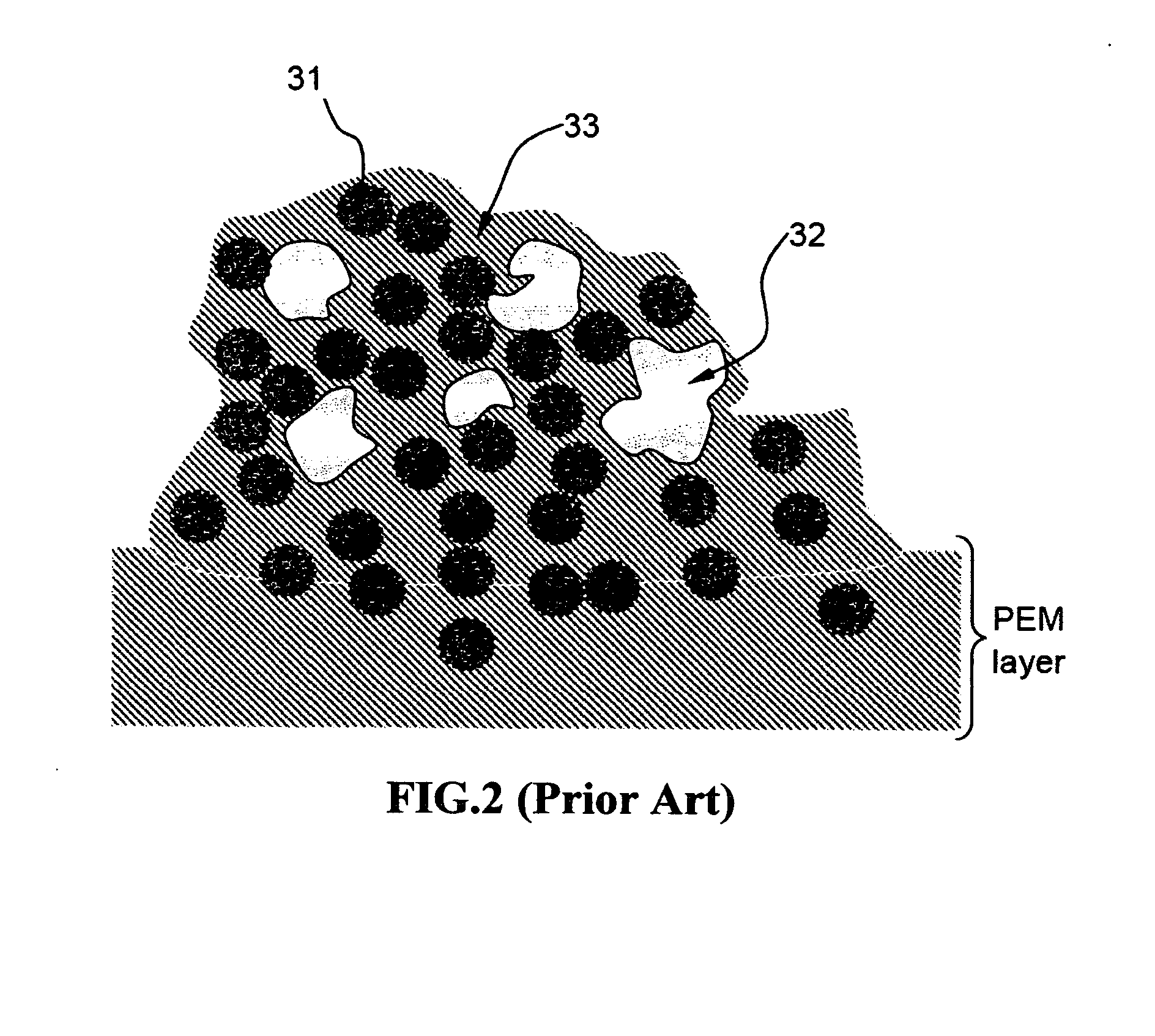
Ionic conductivity (denoted by λ) is a measure of a substance's tendency towards ionic conduction. This involves the movement of an ion from one site to another through defects in the crystal lattice of a solid or aqueous solution.
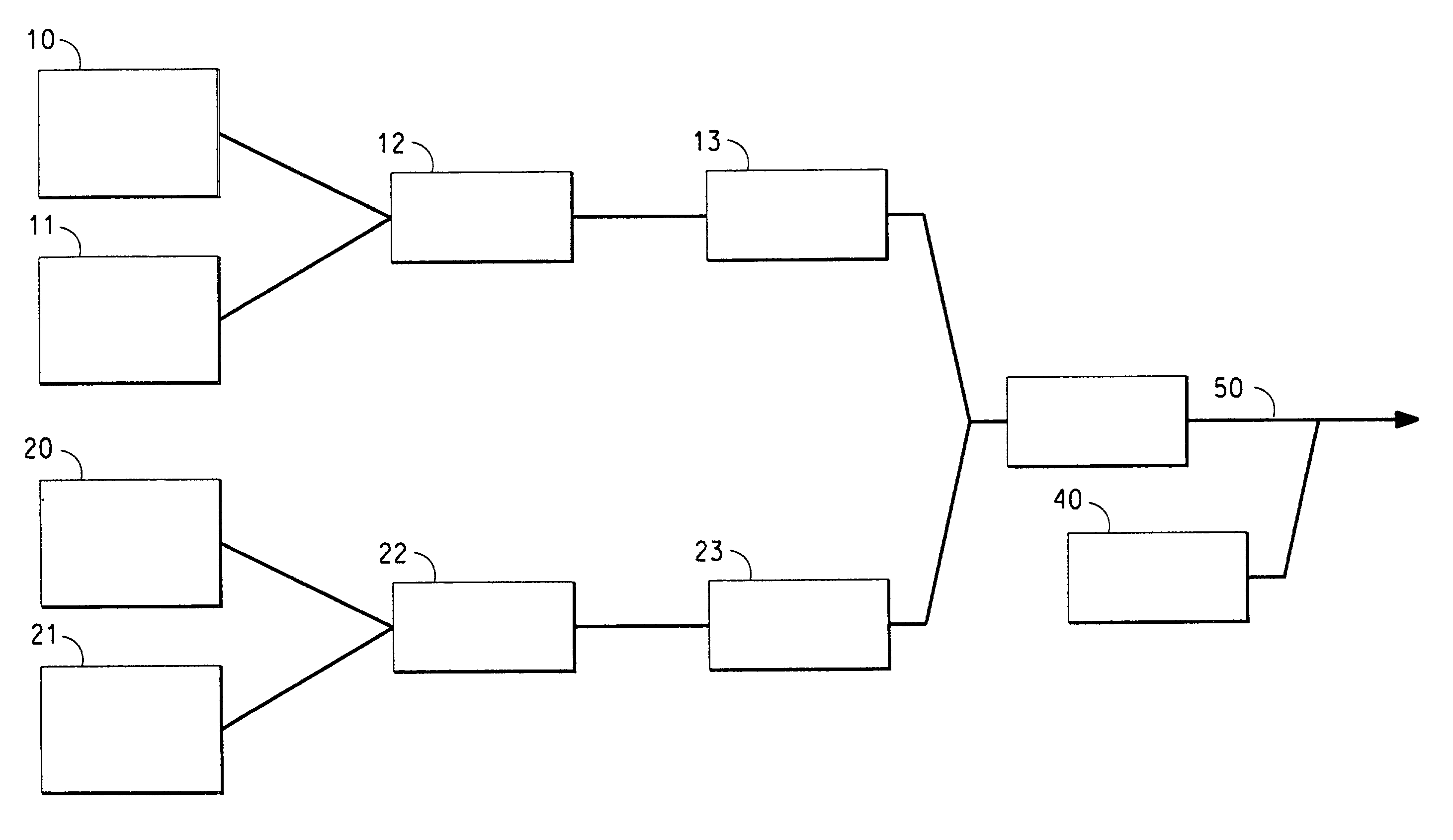
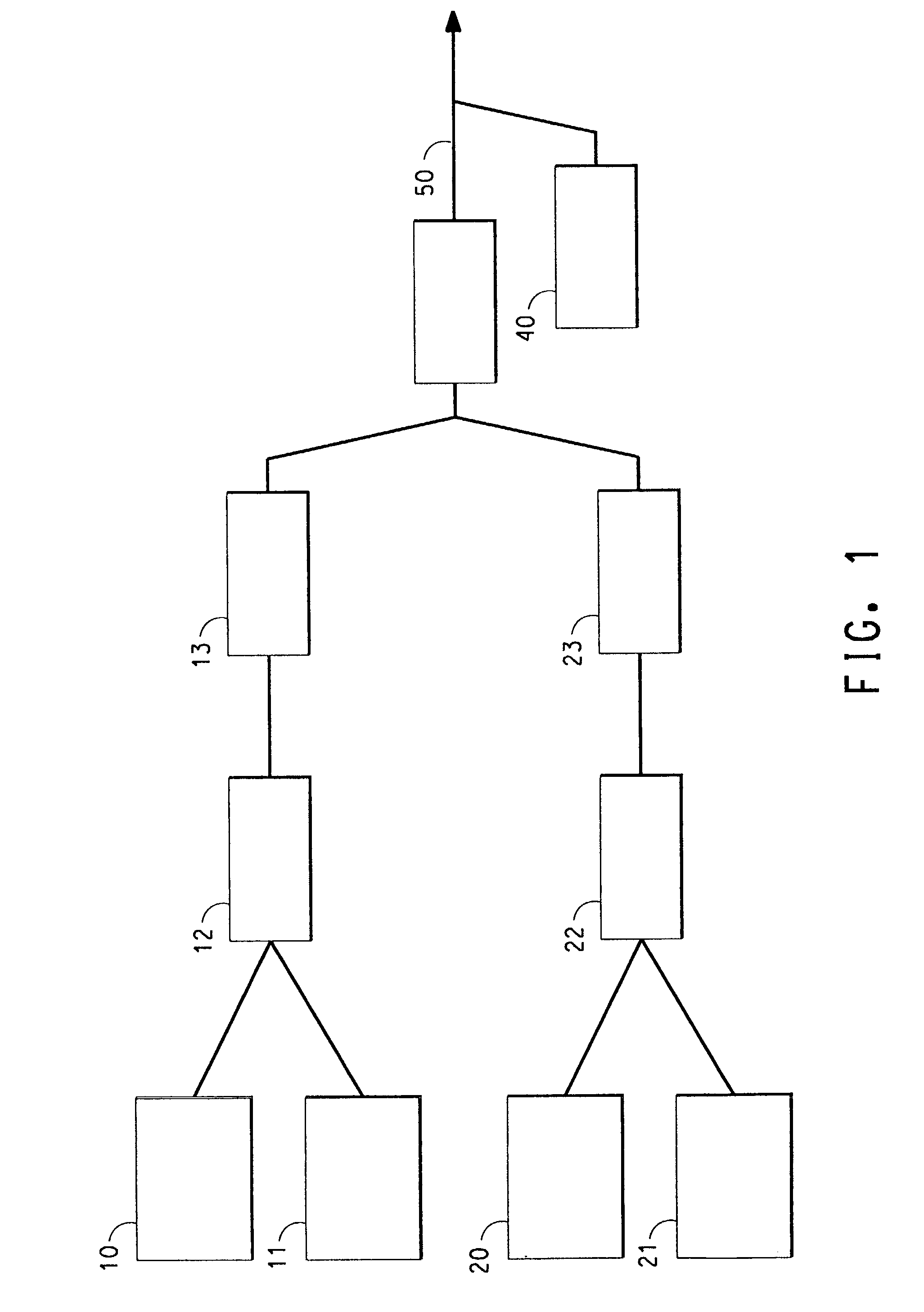
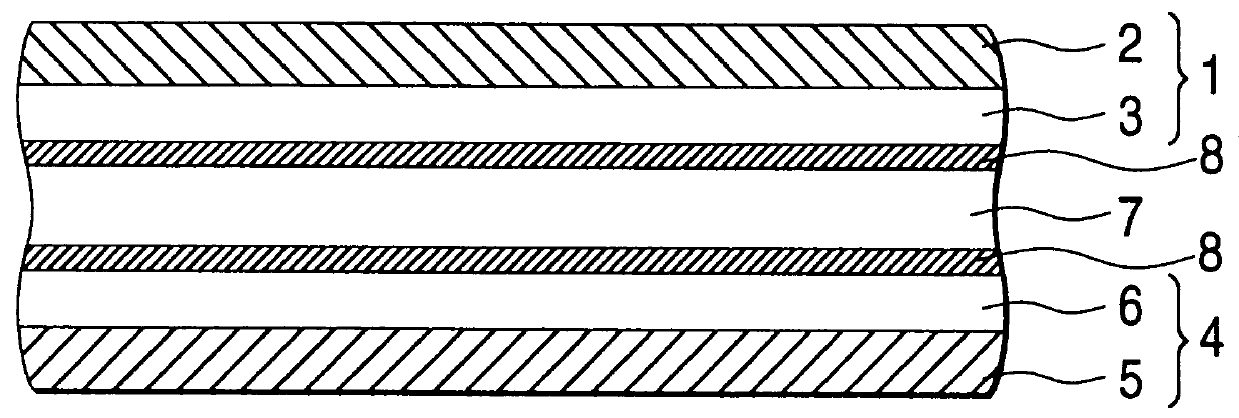
Ionically conductive composites for protection of active metal anodes
ActiveUS7282296B2Easy to manufactureImprove battery performanceFinal product manufactureElectrode carriers/collectorsChemical stabilityIonic conductivity
Disclosed are ionically conductive composites for protection of active metal anodes and methods for their fabrication. The composites may be incorporated in active metal negative electrode (anode) structures and battery cells. In accordance with the invention, the properties of different ionic conductors are combined in a composite material that has the desired properties of high overall ionic conductivity and chemical stability towards the anode, the cathode and ambient conditions encountered in battery manufacturing. The composite is capable of protecting an active metal anode from deleterious reaction with other battery components or ambient conditions while providing a high level of ionic conductivity to facilitate manufacture and / or enhance performance of a battery cell in which the composite is incorporated.
Owner:POLYPLUS BATTERY CO INC
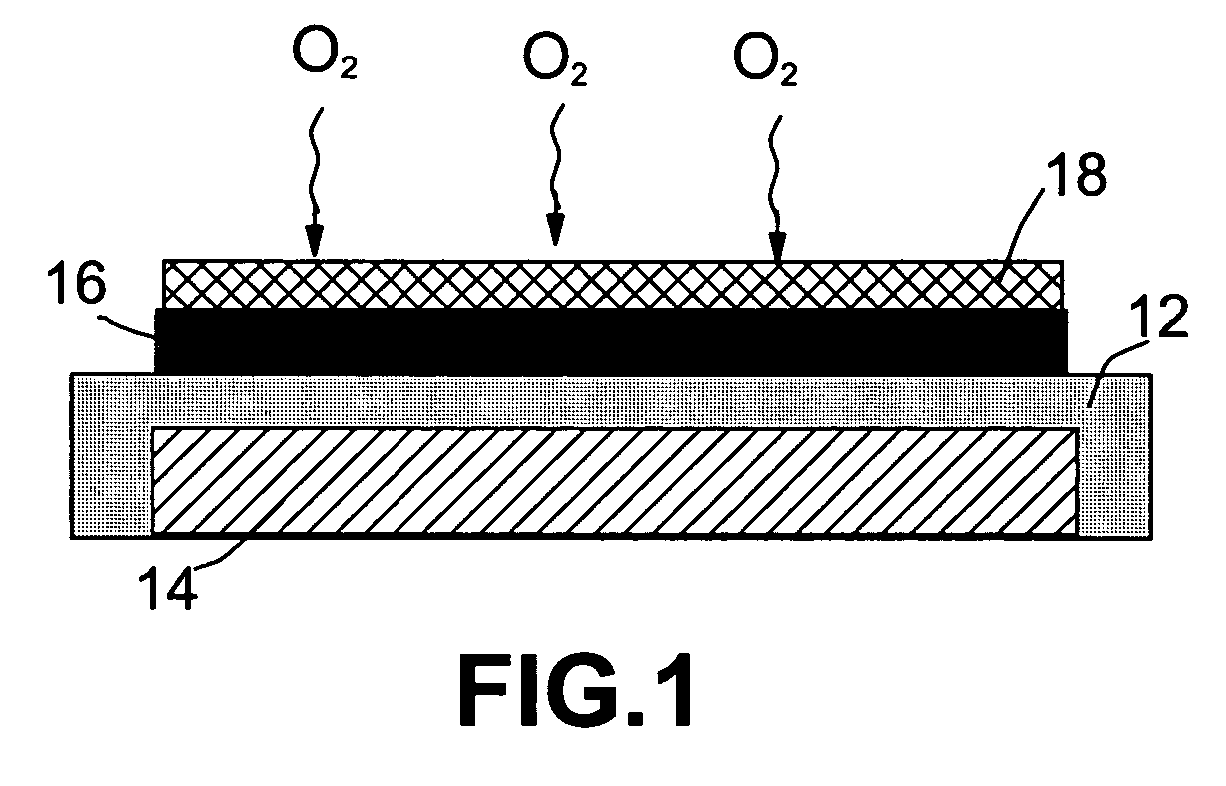
Metal-air battery with ion-conducting inorganic glass electrolyte
InactiveUS20060063051A1Safe and reliable solid-stateIncrease energy densityFuel and primary cellsSolid electrolytesMetal–air electrochemical cellIonic conductivity
A solid-state metal-air electrochemical cell comprising: (A) a metal-containing electro-active anode; (B) an oxygen electro-active cathode; and (C) an ion-conducting glass electrolyte disposed between the metal-containing anode and the oxygen electro-active cathode. The cathode active material, which is oxygen gas, is not stored in the battery but rather fed from the environment. The oxygen cathode is preferably a composite carbon electrode which serves as the cathode current collector on which oxygen molecules are reduced during discharge of the battery to generate electric current. The glass electrolyte typically has an ion conductivity in the range of 5×10−5 to 2×10−3 S / cm. The electrolyte layer is preferably smaller than 10 μm in thickness and further preferably smaller than 1 μm. The anode metal is preferably lithium or lithium alloy, but may be selected from other elements such as sodium, magnesium, calcium, aluminum and zinc.
Owner:JANG BOR Z
Solid electrolyte, method for preparing the same, and battery using the same
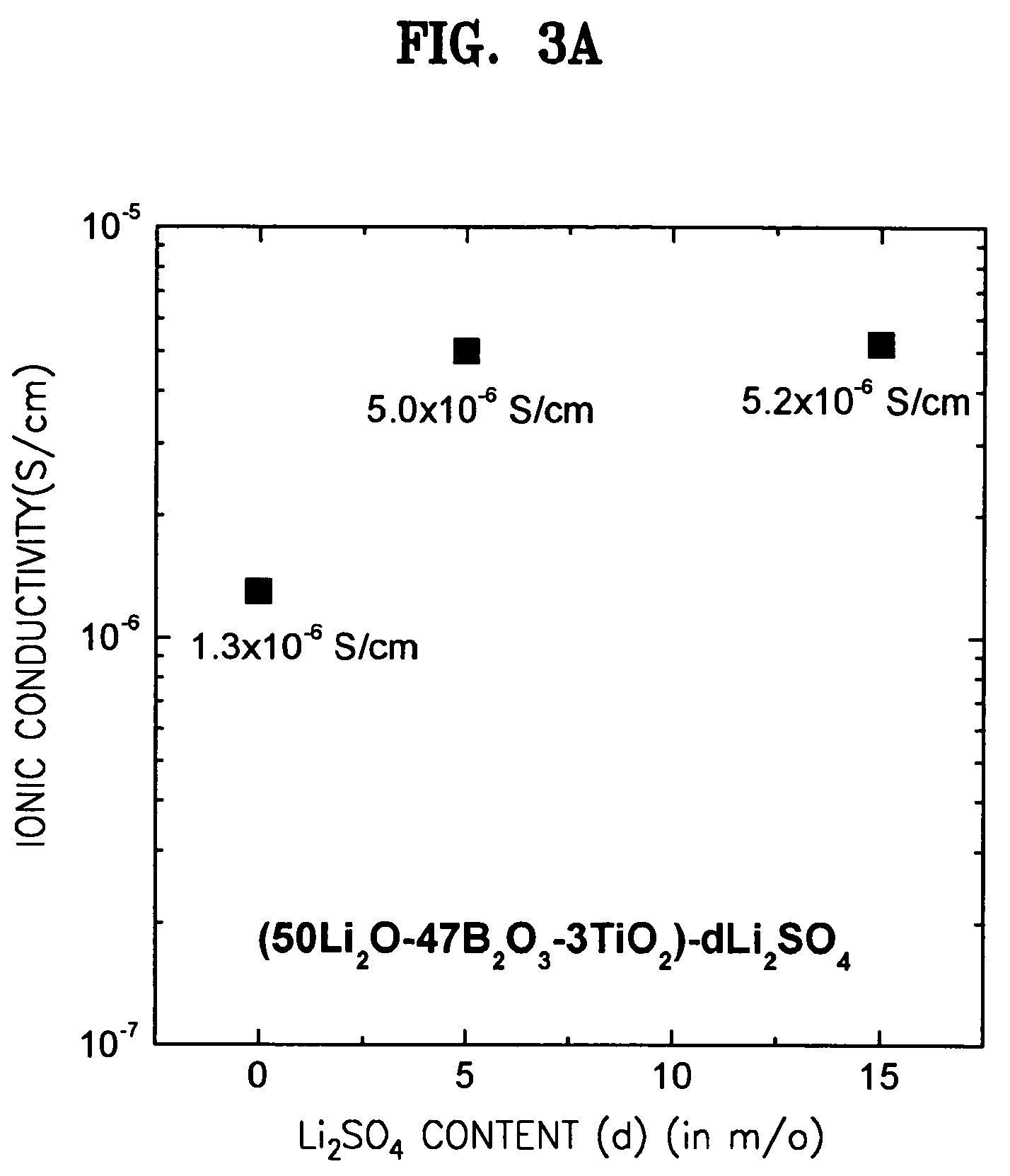
ActiveUS7273682B2Improve ion conductionImproved in charge/discharge rateNon-metal conductorsElectrolytic capacitorsLithiumPhysical chemistry
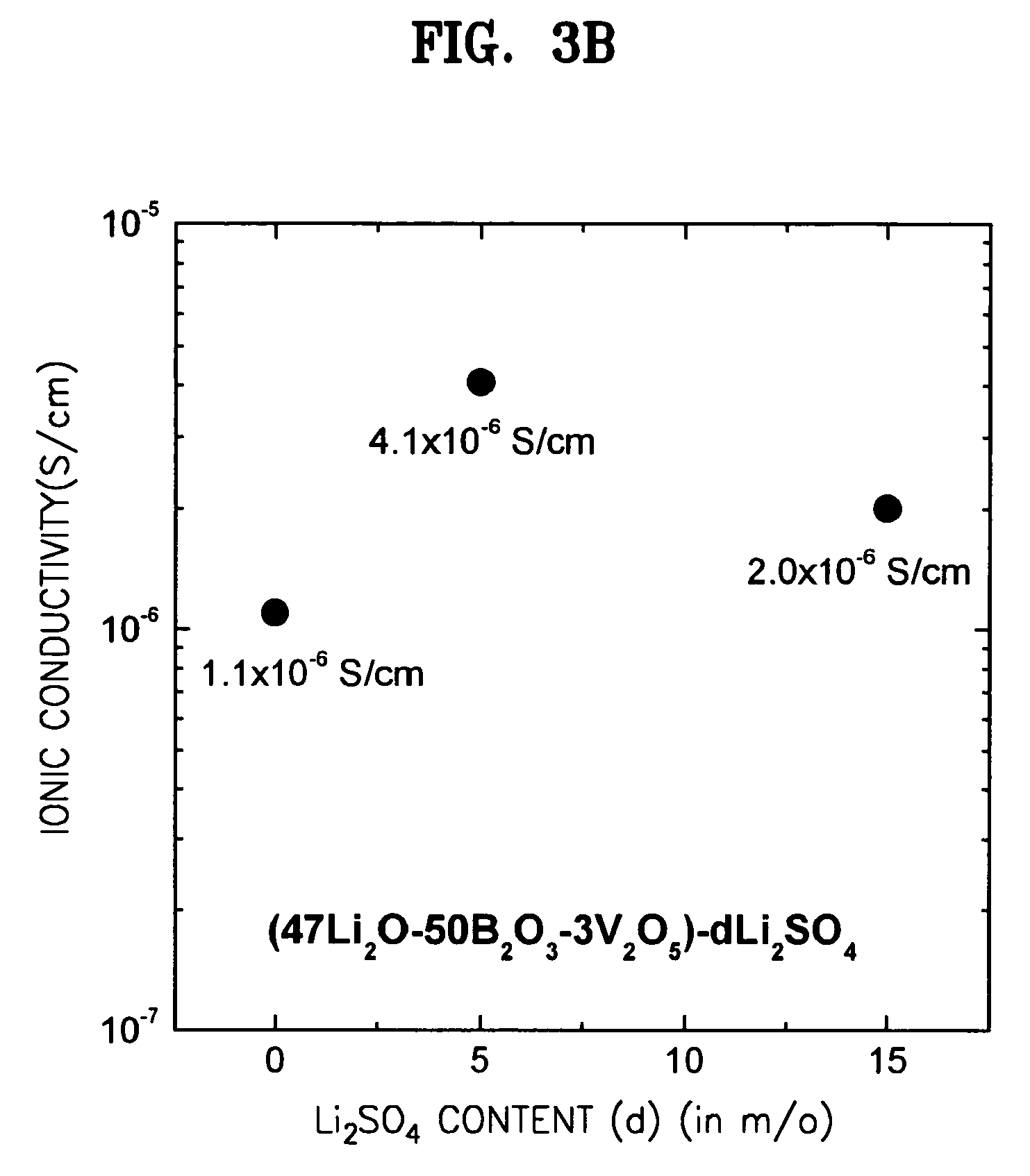
A solid electrolyte including a composition represented by Formula 1 below is provided: aLi2O-bB2O3-cM-dX (1) wherein M is at least one selected from the group consisting of TiO2, V2O5, WO3, and Ta2O5; X is at least one selected from LiCl and Li2SO4; 0.4<a<0.55; 0.4<b<0.55; 0.02<c<0.05; a+b+c=1, and 0≦d<0.2. A method for preparing the solid electrolyte and a battery using the solid electrolyte are also provided. The solid electrolyte exhibits high ionic conductivity. Lithium and thin film batteries using the solid electrolyte are improved in charge / discharge rate, power output, and cycle life.
Owner:SAMSUNG SDI CO LTD
Functionally improved battery and method of making same
InactiveUS6855441B1Extended service lifePower outputBatteries circuit arrangementsSemiconductor/solid-state device detailsElectrical batteryLiquid state
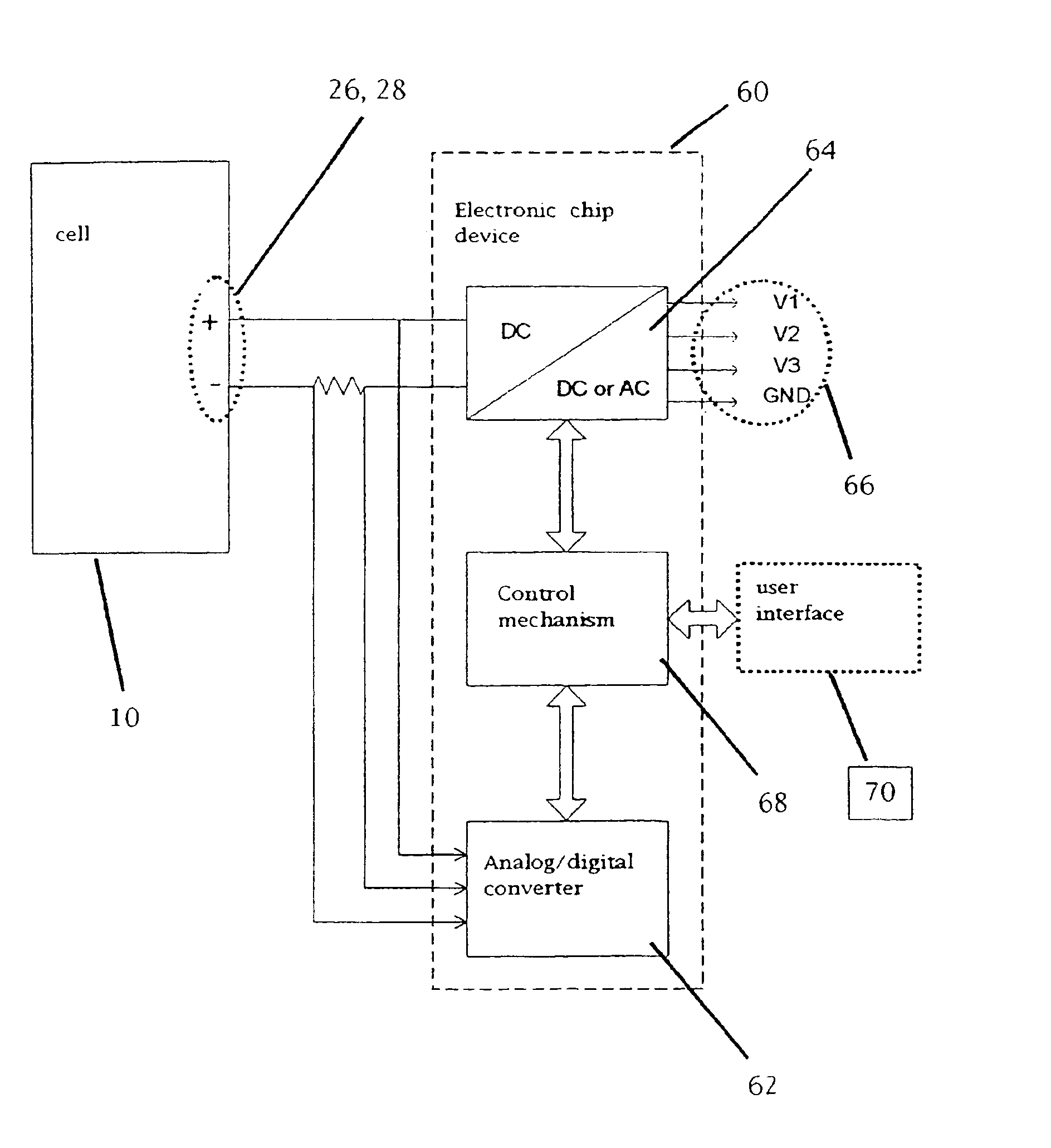
A functionally improved battery is disclosed. The battery includes a flexible thin layer open liquid state electrochemical cell (10) and an electronic chip device (60) integrally formed on or within the electrochemical cell (10). The cell (10) includes a first layer of insoluble negative pole (14), a second layer of insoluble positive pole (16) and a third layer of aqueous electrolyte (12). The third layer (12) is disposed between the first (14) and second (16) layers. The third layer (12) includes a diliquescent material for keeping the cell wet, an electroactive soluble material for ionic conductivity and a watersoluble polymer for viscosity. The viscosity adheres the first (14) and second (16) layer to the third layer (12). The chip (60) serves to improve the functionality of the battery.
Owner:POWER PAPER
Ionically conductive membranes for protection of active metal anodes and battery cells
InactiveUS20080057386A1Improve conductivityFacilitates cell fabricationSolid electrolytesCell seperators/membranes/diaphragms/spacersChemical stabilityIonic conductivity
Disclosed are ionically conductive membranes for protection of active metal anodes and methods for their fabrication. The membranes may be incorporated in active metal negative electrode (anode) structures and battery cells. In accordance with the invention, the membrane has the desired properties of high overall ionic conductivity and chemical stability towards the anode, the cathode and ambient conditions encountered in battery manufacturing. The membrane is capable of protecting an active metal anode from deleterious reaction with other battery components or ambient conditions while providing a high level of ionic conductivity to facilitate manufacture and / or enhance performance of a battery cell in which the membrane is incorporated.
Owner:POLYPLUS BATTERY CO INC
Ionically conductive composites for protection of active metal anodes
InactiveUS20080057399A1Easy to manufactureImprove battery performanceFinal product manufactureCell electrodesChemical stabilityIonic conductivity
Disclosed are ionically conductive composites for protection of active metal anodes and methods for their fabrication. The composites may be incorporated in active metal negative electrode (anode) structures and battery cells. In accordance with the invention, the properties of different ionic conductors are combined in a composite material that has the desired properties of high overall ionic conductivity and chemical stability towards the anode, the cathode and ambient conditions encountered in battery manufacturing. The composite is capable of protecting an active metal anode from deleterious reaction with other battery components or ambient conditions while providing a high level of ionic conductivity to facilitate manufacture and / or enhance performance of a battery cell in which the composite is incorporated.
Owner:POLYPLUS BATTERY CO INC
Polymer Solid Electrolyte
InactiveUS6162563AEasy to processGood moldabilityHybrid capacitor electrolytesCell electrodesLithium metalBackbone chain
PCT No. PCT / JP97 / 02854 Sec. 371 Date Mar. 11, 1999 Sec. 102(e) Date Mar. 11, 1999 PCT Filed Aug. 19, 1997 PCT Pub. No. WO98 / 07772 PCT Pub. Date Feb. 26, 1998A polymer solid electrolyte obtained by blending (1) a polyether copolymer having a main chain derived form ethylene oxide and an oligooxyethylene side chain, (2) an electrolyte salt compound, and (3) a plasticizer of an aprotic organic solvent or a derivative or metal salt of a polyalkylene glycol having a number-average molecular weight of 200 to 5,000 or a metal salt of the derivative is superior in ionic conductivity and also superior in processability, moldability and mechanical strength to a conventional solid electrolyte. A secondary battery is constructed by using the polymer solid electrolyte in combination with a lithium metal negative electrode and a lithium cobaltate positive electrode.
Owner:OSAKA SODA CO LTD
Oxide solid electrolyte material, and preparation method and application thereof
The invention discloses a lithium-lanthanum-zirconium-oxygen-base oxide solid electrolyte material and a preparation method thereof. The solid electrolyte material is composed of a base material and a doping element, wherein the base material is a lithium-lanthanum-zirconium-oxygen solid electrolyte of which the chemical formula is Li7La3Zr2O12, the doping element is selected from at least one of calcium, strontium, barium and germanium, and the weight of the doping element does not exceed 15% of that of the base material. The preparation method comprises the following steps: a lithium source compound, a lanthanum source compound, a zirconium source compound and a doping element compound are evenly mixed, calcined and sintered to obtain the lithium-lanthanum-zirconium-oxygen-base oxide solid electrolyte material. The lithium-lanthanum-zirconium-oxygen-base oxide solid electrolyte material can be prepared under the conditions of wide sources of doping element, lower sintering temperature and shorter sintering time, and the total room-temperature ionic conductivity is greater than 1*10<-4>S / cm; and thus, the material has important application value.
Owner:TSINGHUA UNIV +1
Combined electrode of battery and preparation method thereof
InactiveCN103730630AImprove electronic conductivityImprove ionic conductivityActive material electrodesElectrolyte layer coatingSolid state electrolyteElectrical battery
The application relates to the field of energy storage materials, and discloses a combined electrode with ultrahigh electron and ionic conductivity and a preparation method thereof. The combined electrode is formed in a manner that a battery active material is uniformly tied in a three-dimensional multi-hole network formed by carbon nano tubes which are connected in a crossing manner, and meshes and the surface of the active material are filled or coated with a solid electrolyte material. According to the combined electrode, the carbon nano tubes, which are communicated with one another, can form an ultrahigh electrical transmission network, on the one hand, a solid electrolyte can provide the ultrahigh lithium-ion transmission capacity while not influencing the connection of the carbon nano tubes and the conductive capacity of the electrode; on the other hand, the three-dimensional network formed by the carbon nano tubes is also fixed by virtue of the solid electrolyte, the formation of a solid electrolyte interface is controlled, and an active material is protected under the high charge-discharge voltage. The combined electrode has the high reversible capacity and the enhanced rate capability, and can meet the requirement of a power automobile or a mixed power automobile.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL
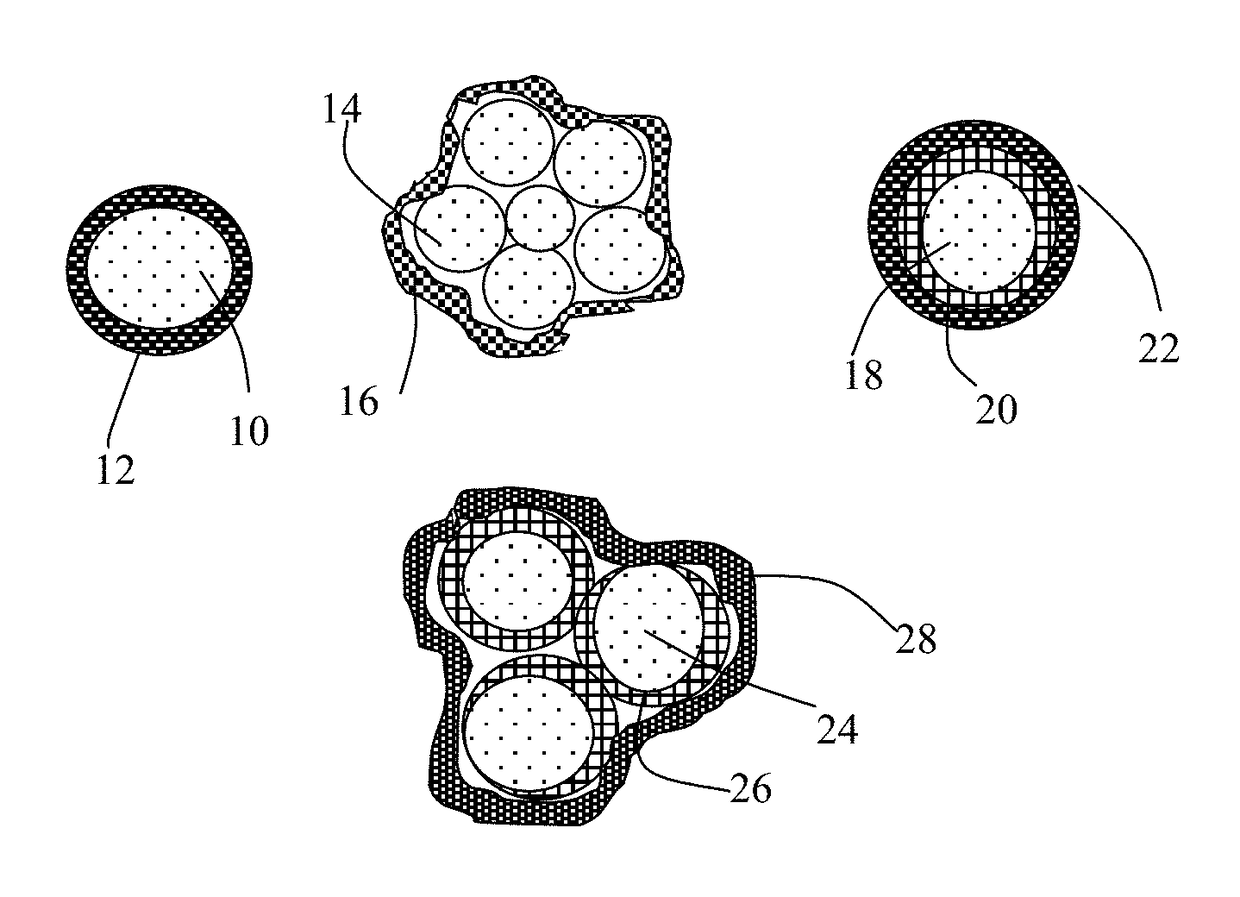
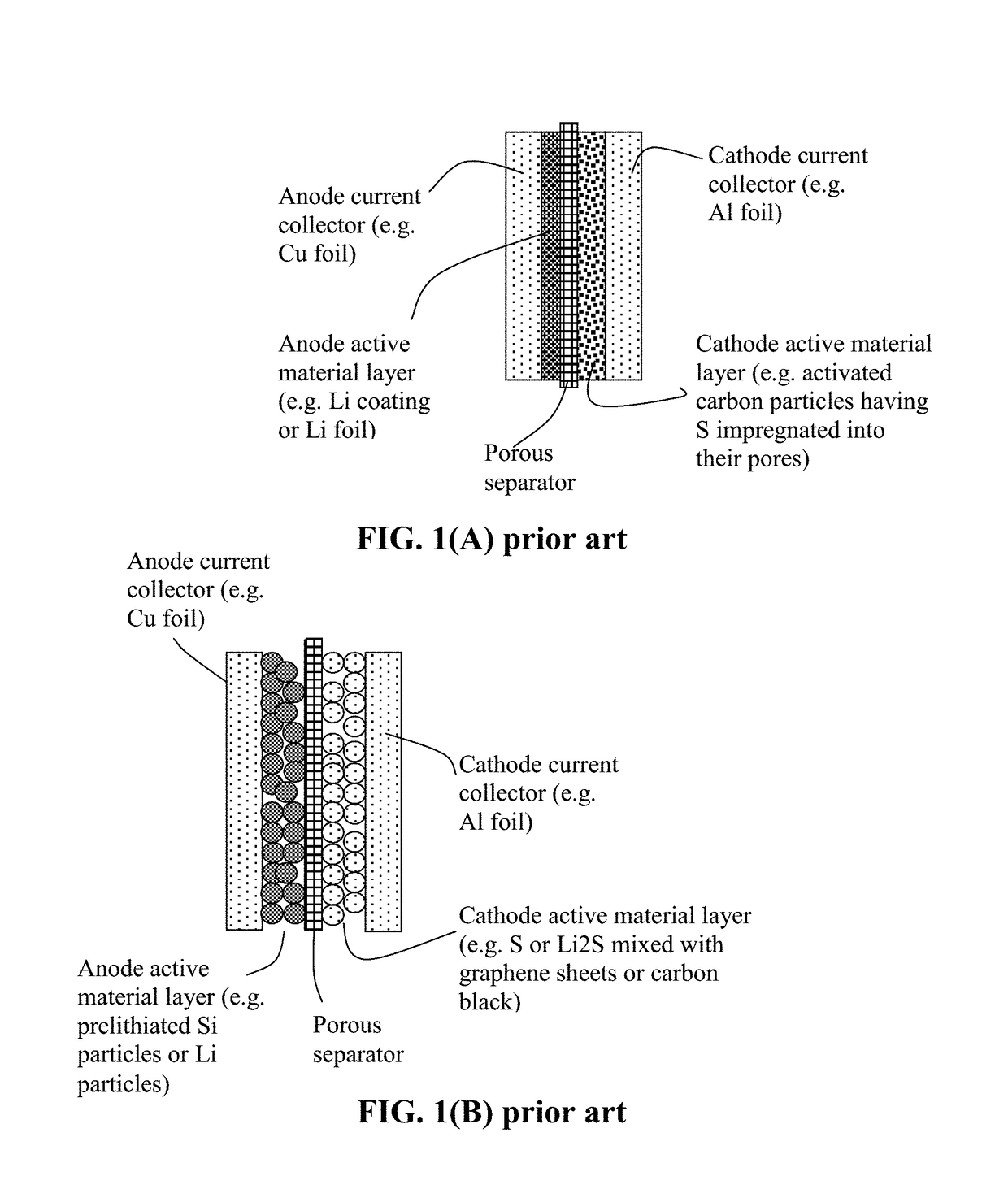
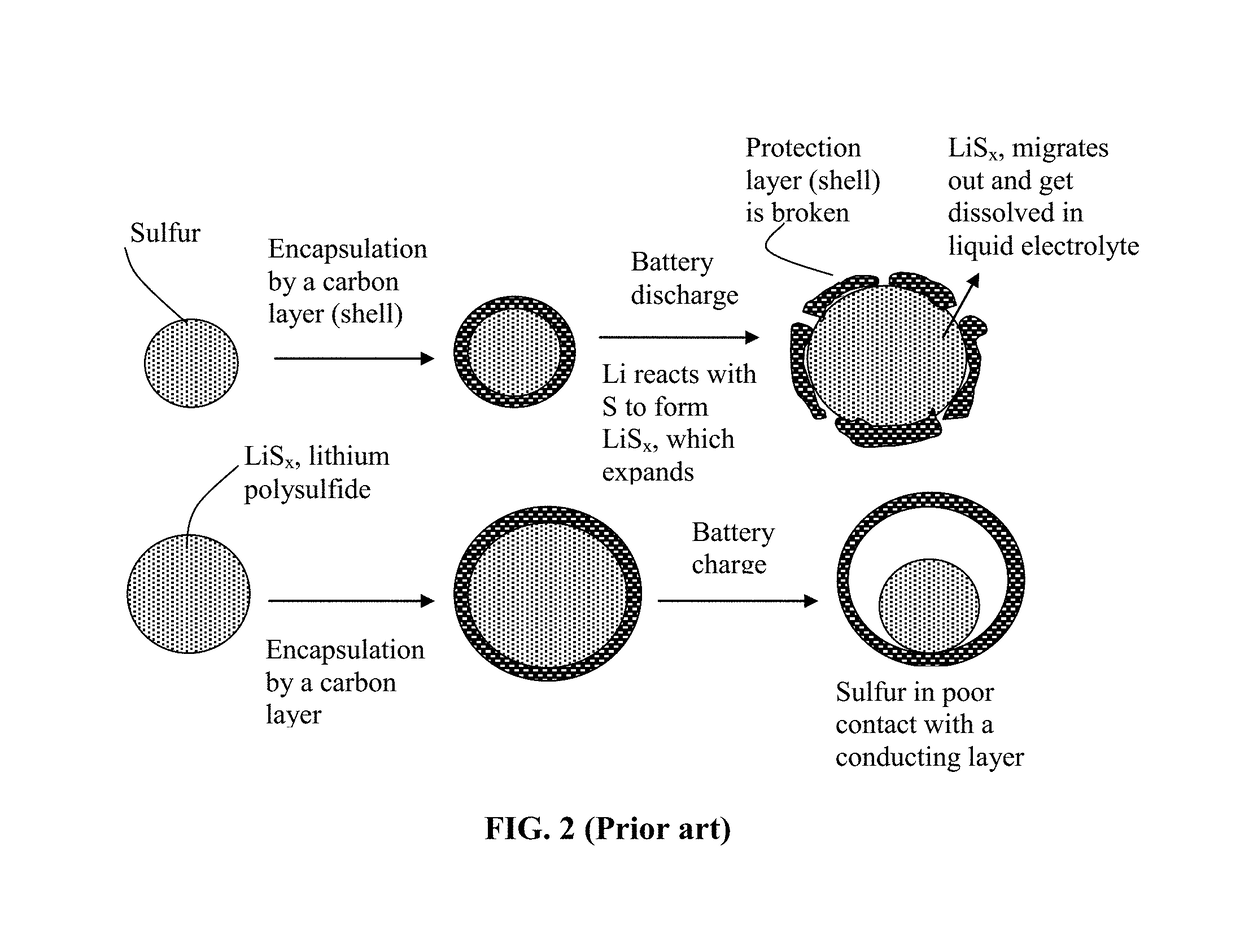
Alkali Metal-Sulfur Secondary Battery Containing a Protected Sulfur Cathode and Manufacturing Method
ActiveUS20180241031A1Inhibited DiffusionReduces and eliminates shuttling effectFinal product manufacturePositive electrodesElectrical batteryConductive polymer
Provided is a rechargeable alkali metal-sulfur cell comprising an anode active material layer, an electrolyte, and a cathode active material layer containing multiple particulates of a sulfur-containing material selected from a sulfur-carbon hybrid, sulfur-graphite hybrid, sulfur-graphene hybrid, conducting polymer-sulfur hybrid, metal sulfide, sulfur compound, or a combination thereof and wherein at least one of the particulates is composed of one or a plurality of sulfur-containing material particles being embraced or encapsulated by a thin layer of a high-elasticity polymer having a recoverable tensile strain no less than 10% when measured without an additive or reinforcement, a lithium ion conductivity no less than 10−5 S / cm at room temperature, and a thickness from 0.5 nm to 10 μm. This battery exhibits an excellent combination of high sulfur content, high sulfur utilization efficiency, high energy density, and long cycle life.
Owner:GLOBAL GRAPHENE GRP INC
Ionic compound
ActiveUS20080083626A1Improve electrochemical stabilityImprove ionic conductivityHybrid capacitor electrolytesElectrolysis componentsOrganic groupCompound (substance)
The present invention provides an ionic compound which: can exert excellent basic performance such as excellent electrochemical stability including an excellent ionic conductivity; is excellent in pH stability; and can suitably find use in a variety of applications, and an electrolyte material, an electrolytic solution, and an electrolytic capacitor each containing the ionic compound. Anionic compound of the present invention has an anion represented by a general formula (1) and a cation represented by a general formula (2): where X represents an element selected from B, C, N, O, Al, Si, P, S, As, and Se, M1 and M2 represent linking groups, Q represents a monovalent element or organic group, a represents an integer of 1 or more, and b, c, d, and e each represent an integer of 0 or more; Rs-LH⊕ (2) where L represents an element selected from C, Si, N, P, S, and O, R represents a monovalent element, functional group, or organic group, and s represents an integer of 2, 3, or 4.
Owner:NICHICON CORP
Lithium iron phosphate having olivine structure and method for analyzing the same
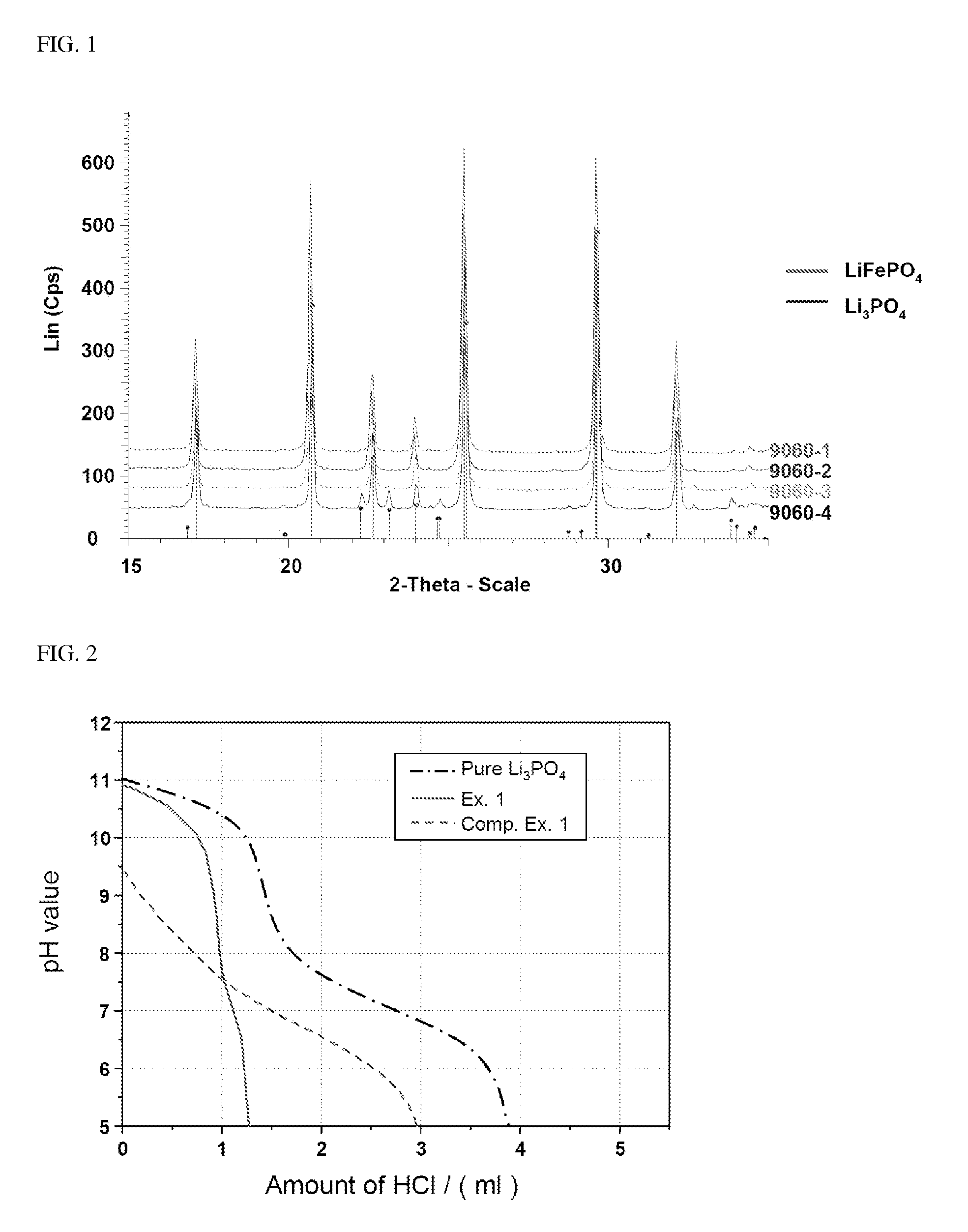
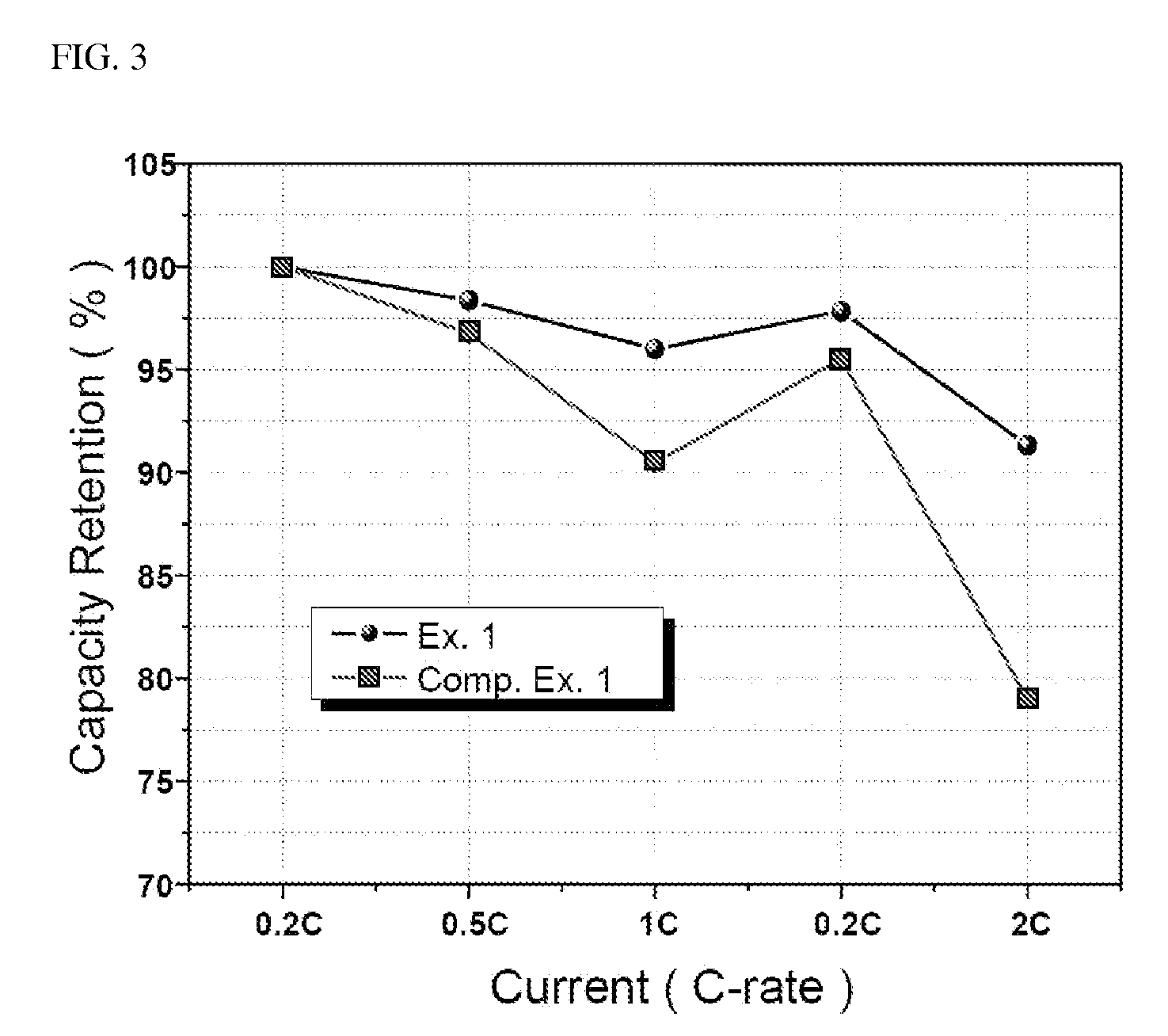
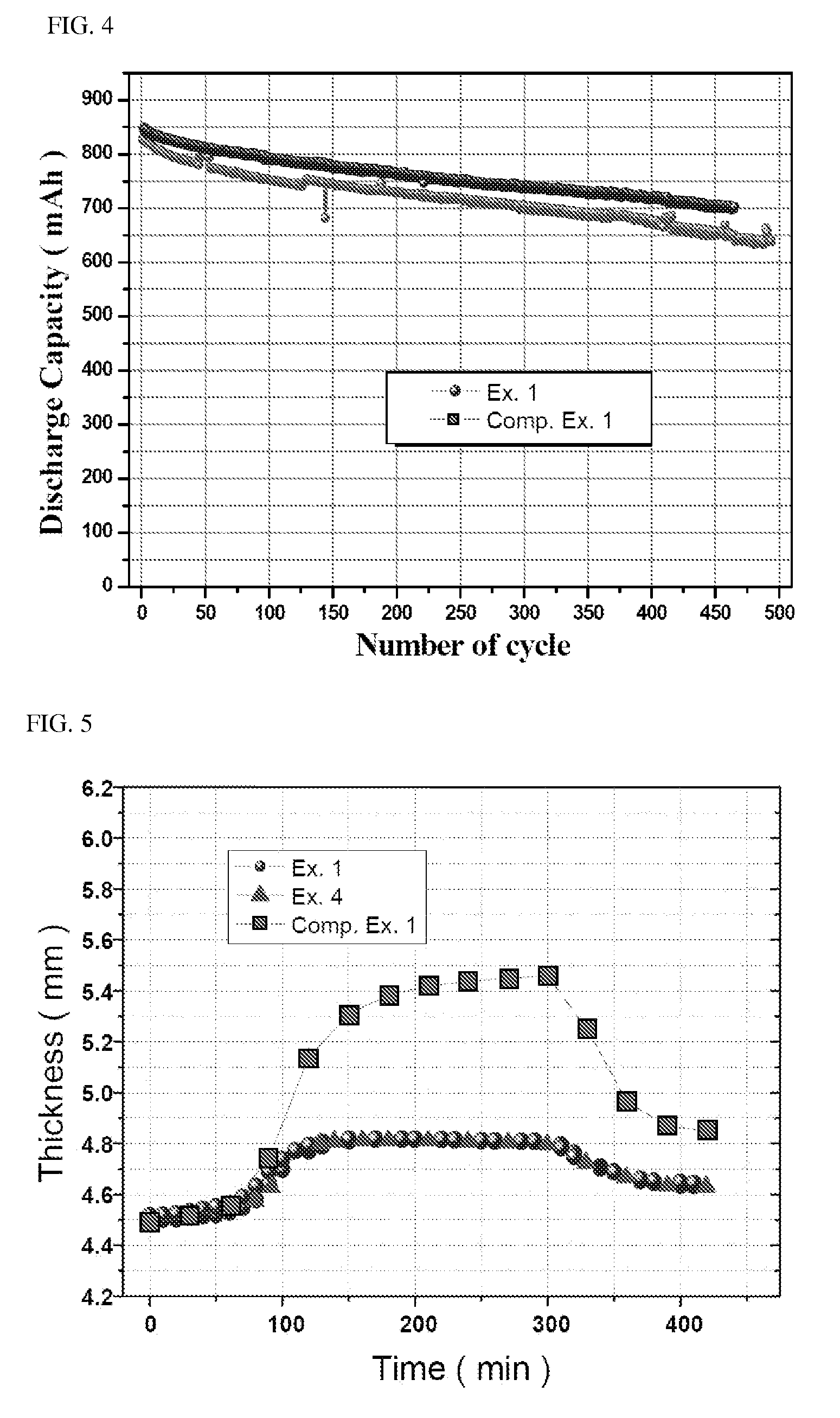
ActiveUS20100261060A1Decrease swell phenomenonHigh ionic conductivityChemical analysis using titrationPreparing sample for investigationLithium carbonateChemistry
Provided is an olivine-type lithium iron phosphate having a composition represented by Formula I, comprising 0.1 to 5% by weight of Li3PO4, and comprising no Li2CO3 or, if present, comprising Li2CO3 in an amount less than 0.25% by weight: Lii+aFe1−xMx(PO4−b)Xb (I) wherein M, X, a, x and b are as defined above.The lithium iron phosphate comprises no lithium carbonate (Li2CO3) or, if present, comprises the Li2CO3 in an extremely small amount, and comprises Li3PO4 having superior electrochemical stability, thermal stability and ionic conductivity, thus advantageously imparting high-temperature and storage stability as well as stability and rate properties to lithium secondary batteries, when used as a cathode active material for the lithium secondary batteries.
Owner:LG ENERGY SOLUTION LTD
Multilayer structure including an active electrochemical material and ionic polymer in a electrochemical cell
InactiveUS6986967B2Minimize exposureElectrode manufacturing processesFinal product manufactureEngineeringLithium-ion battery
Multilayer articles, particularly multilayer articles having electrical or ionic conductivity, are made using an improved melt extrusion process. The process includes a combination step in which a macroscopically homogeneous mass is formed prior to introduction into an extruder. The multilayer article may include an electrode layer and a separator layer that are extruded onto a metal current collector. Such structures are particularly useful in lithium-ion batteries.
Owner:EI DU PONT DE NEMOURS & CO
Alkali Metal-Sulfur Secondary Battery Containing a Polymer-Encapsulated Sulfur Cathode and Manufacturing Method
ActiveUS20180294475A1Inhibited DiffusionReduce and eliminate effectFinal product manufacturePositive electrodesConductive polymerElectrical battery
Provided is a rechargeable alkali metal-sulfur cell comprising an anode active material layer, an electrolyte, and a cathode active material layer containing multiple particulates of a sulfur-containing material selected from a sulfur-carbon hybrid, sulfur-graphite hybrid, sulfur-graphene hybrid, conducting polymer-sulfur hybrid, metal sulfide, sulfur compound, or a combination thereof and wherein at least one of the particulates is composed of one or a plurality of sulfur-containing material particles being embraced or encapsulated by a thin layer of a high-elasticity ultra-high molecular weight polymer having a recoverable tensile strain no less than 2%, a lithium ion conductivity no less than 10−6 S / cm at room temperature, and a thickness from 0.5 nm to 10 μm This battery exhibits an excellent combination of high sulfur content, high sulfur utilization efficiency, high energy density, and long cycle life.
Owner:GLOBAL GRAPHENE GRP INC
High-performance flexible composite nonwoven fabric membrane for lithium ion battery, as well as preparation method and application of membrane
InactiveCN103296240AGood flexibilityGood high current discharge performanceCell component detailsAdhesiveCharge discharge
The invention discloses a high-performance flexible composite nonwoven fabric membrane for a lithium ion battery, as well as a preparation method and an application of the membrane, which belongs to the technical field of a lithium-ion battery membrane material. The prepared composite nonwoven fabric membrane is formed by coating functional serous fluid with thermally curable or optically curable functional groups onto a substrate membrane containing an active functional group, thermally curing or optically curing the substrate membrane, removing a pore-forming agent, and hot pressing and drying the substrate. The prepared composite nonwoven fabric membrane is good in flexibility. The composite nonwoven fabric membrane has good ion electric conductivity and hot shrinkage resistance, can bear the large-current discharge, and also can improve the safety performance of the battery. Since no fluorine-containing adhesive is used in a preparation process, nano particles or nano optical fibers are connected with the substrate membrane through a chemical key, the nano particles or the nano fibers are bonded together through the chemical key and free from dropping off in the charging-discharging cycle, the stability of the coating is enhanced, and the cycling performance of the battery can be improved. The prepared flexible composite nonwoven fabric membrane is used as a membrane assembly of the lithium ion battery.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Electro-catalyst compositions for fuel cells
ActiveUS20080096093A1Simple materialOrganic-compounds/hydrides/coordination-complexes catalystsActive material electrodesElectro catalystPtru catalyst
A precursor electro-catalyst composition for producing a fuel cell electrode. The precursor composition comprises (a) a molecular metal precursor dissolved or dispersed in a liquid medium and (b) a polymer dissolved or dispersed in the liquid medium, wherein the polymer is both ion-conductive and electron-conductive with an electronic conductivity no less than 10−4 S / cm (preferably greater than 10−2 S / cm) and ionic conductivity no less than 10−5 S / cm (preferably greater than 10−3 S / cm). Also disclosed is an electro-catalyst composition derived from this precursor composition, wherein the molecular metal precursor is converted by heat and / or energy beam to form nanometer-scaled catalyst particles and the polymer forms a matrix that is in physical contact with the catalyst particles, coated on the catalyst particles, and / or surrounding the catalyst particles as a dispersing matrix with the catalyst particles dispersed therein when the liquid is removed. The fuel cell comprising such a composition in an electrode exhibits a superior power output.
Owner:NANOTEK INSTR GRP LLC
Solid electrolyte and battery employing the same
ActiveUS7220517B2Large capacityImprove cycle lifeElectrolytic capacitorsFinal product manufacturePhysical chemistryElectrochemistry
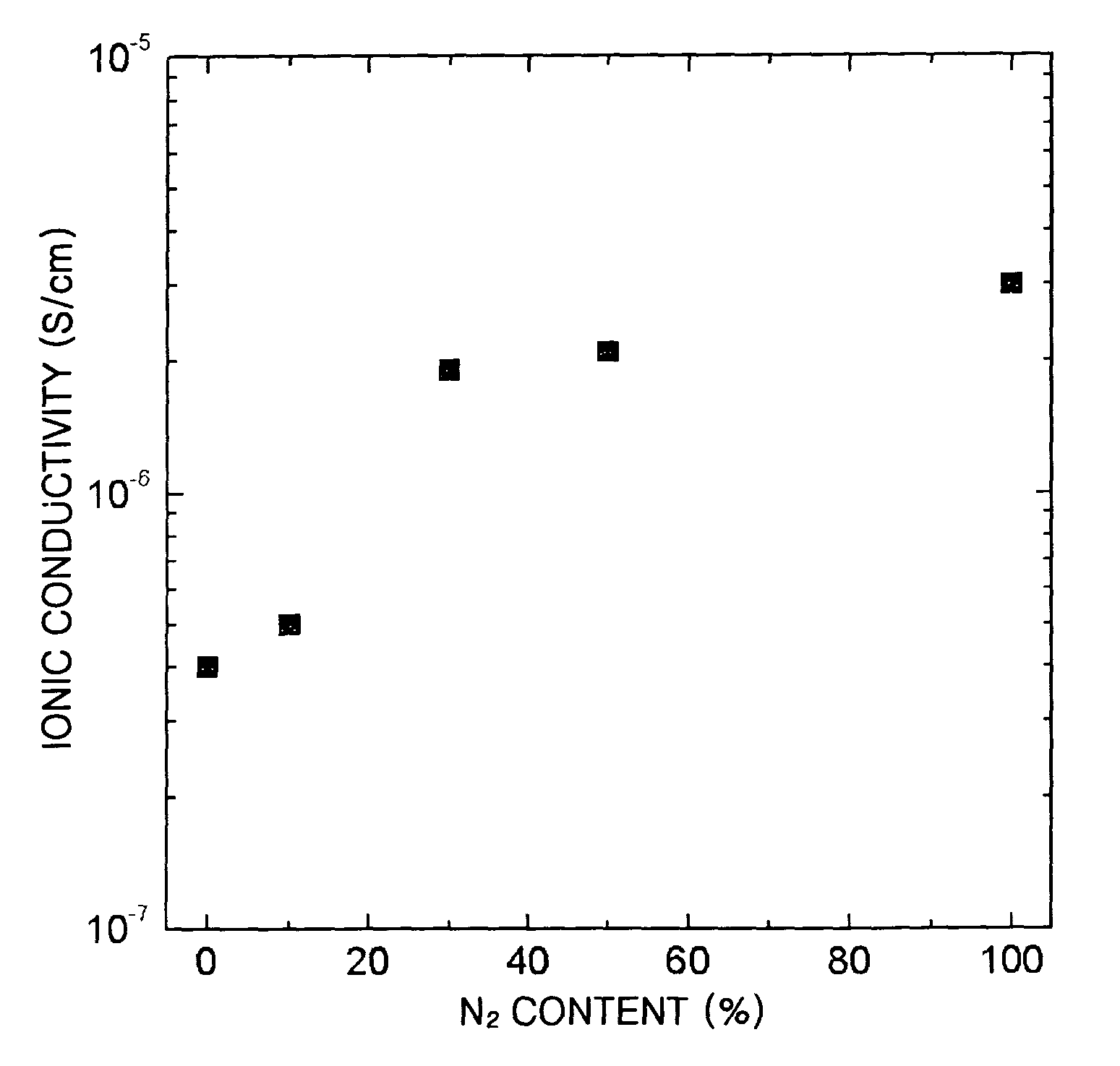
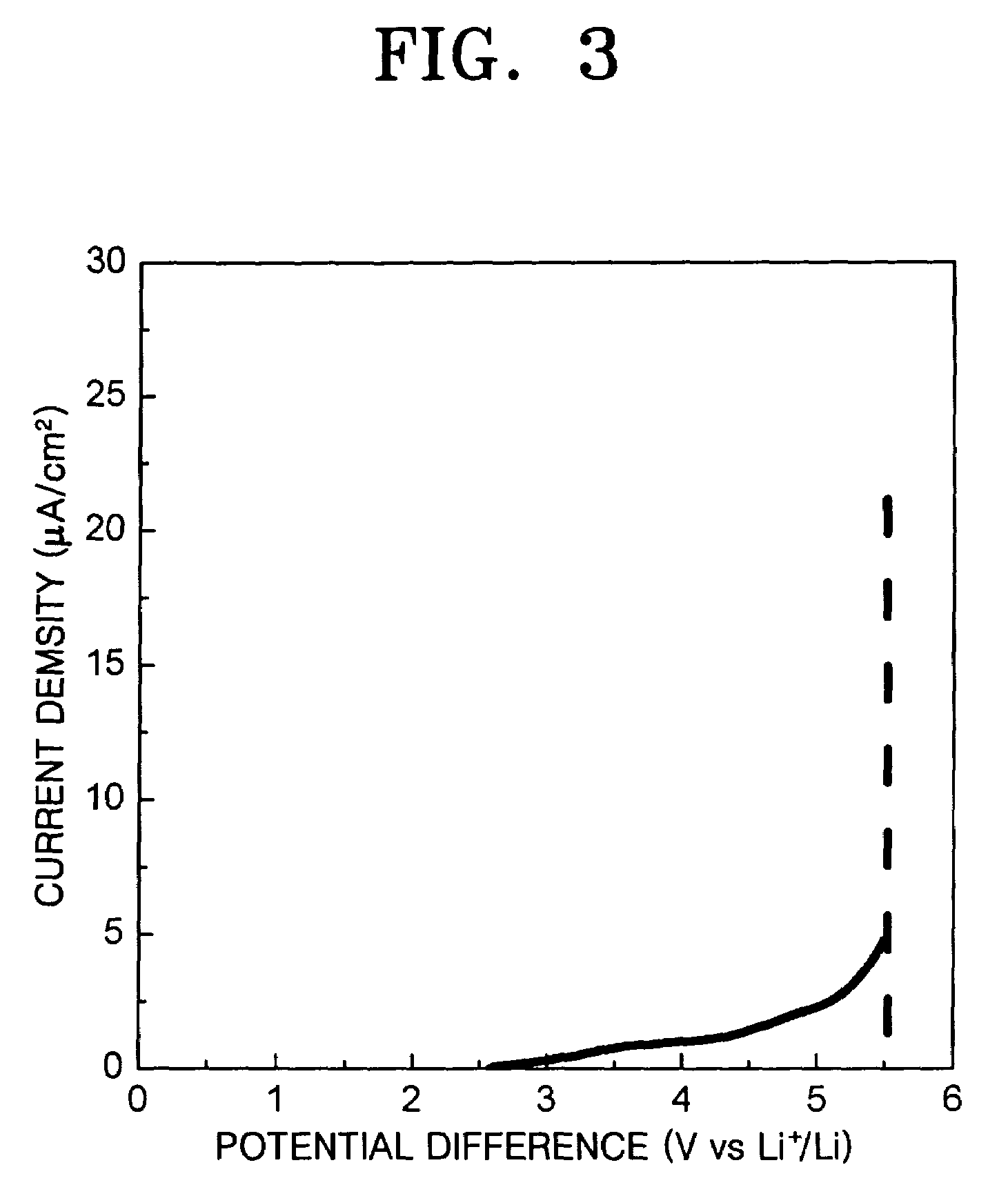
A solid electrolyte, a method of manufacturing the same, and a lithium battery and a thin-film battery that employ the solid electrolyte are provided. The solid electrolyte contains nitrogen to enhance the ionic conductivity and electrochemical stability of batteries.
Owner:SAMSUNG ELECTRONICS CO LTD
Water system high-voltage mixed ion secondary battery based on zinc-lithium ferric manganese phosphate
InactiveCN105826520AIncrease capacityExcellent rate performanceCell electrodesSecondary cellsElectrolytic agentElectrical battery
The invention relates to a water system high-voltage mixed ion secondary battery. A positive pole material of the battery is a high-voltage battery positive pole material, namely zinc-lithium ferric manganese phosphate (LiFe1-xMnxPO4), the element zinc serves as the majority of a negative pole material, and electrolyte is a liquid-state or gel-state material which is formed by lithium bis(trifluoromethane sulfonimide) (LiTFSI) and soluble zinc salt as solute and water as solvent and has ionic conductivity. The battery is based on the energy storage mechanisms of a dissolution-out / deposition reaction of zinc ions (Zn2+) on a negative pole and a reversible embedding / ejection reaction of the zinc ions (Zn2+) on a positive pole, meanwhile, through the water-in-salt electrolyte formed by high-concentration LiTFSI, the electrochemical water decomposition process is inhibited, a potential window of the water system electrolyte is remarkably broadened, the zinc-lithium mixed ion secondary battery has the advantages of being high in capacity, long in cycling life, safe, environmentally friendly, low in cost and the like, and the battery can be applied to the fields such as consumer electronic equipment, electromobiles and large-scale energy storage.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Solid electrolyte material system for all solid state lithium battery and preparation method
ActiveCN101013761AImprove conductivityImprove ionic conductivitySolid electrolyte cellsChemical/physical/physico-chemical processesCrystal systemAll solid state
The invention relates to one solid electrolyte materials and its process method for fix lithium battery, which is characterized by the following: Li2S and other sulfur compound B / S and A / I according to certain mole proportion for compounds to form non-crystal system to provide space for lithium ion transmission to get higher ion conductive rate, wherein, the materials have wider heat stable range to provide one ideal electrolyte prepare materials for solid lithium ion battery.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI
Non-aqueous electrolytes having an extended temperature range for battery applications
InactiveUS20050123835A1Improve ionic conductivityLow pour pointFinal product manufactureOrganic electrolyte cellsPhysical chemistryAqueous electrolyte
The present invention discloses non-aqueous electrolytes having an extended temperature range for battery applications. The electrolyte comprises an electrolyte salt, e.g. LiPF6, a first non-aqueous solvent, and a second non-aqueous solvent having a structure of formula I or II. The electrolyte of the present invention has higher ionic conductivity, lower freezing point, and lower vapor pressure at high temperature than commercial electrolytes. These non-aqueous electrolytes can be used, for example, in lithium-ion batteries. Methods of making lithium-ion batteries are also described.
Owner:POLICELL TECH
Garnet-type solid electrolyte and method for preparing the same
ActiveUS20160190639A1High densityImprove ionic conductivityFinal product manufactureSolid electrolyte cellsCrystal structureIonic conductivity
Disclosed are a garnet-type solid electrolyte and a method for preparing the same. The garnet-based solid electrolyte of the present invention is prepared by adding Al2O3 to a precursor containing hydroxide, thereby enhancing sintered density and ionic conductivity while having a pure cubic phase crystal structure without including impurities.
Owner:HYUNDAI MOTOR CO LTD
Lithium-sulfur system solid electrolyte material for all solid state lithium battery and preparation method
ActiveCN101013753ARich structure typeImprove ionic conductivitySolid electrolyte cellsSulfide conductorsCrystal systemAll solid state
The invention relates to one solid electrolyte materials and its process method for fix lithium battery, which is characterized by the following:four types of different sulfur compounds as Li2S:A / S:P2S5=6:0.1-4.0:1.5 for compound to form non-crystal system to provide more effective paths for lithium ion transmission to get higher ion conductive rate; the said A for Ag, Zn, Al or Zr with sample of Li2S-ZrS2-P2S5 with room ion battery conductive rate as 9.60X10-6S / cm and lower electron conductive rate under 150 degrees for 3.30X10-4S / cm.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI
Lithium cationic single-ion conducting inorganic filler-containing composite polymer electrolyte for lithium secondary battery and method of manufacturing the same
ActiveUS20050196677A1High mechanical strengthPrevent increase of inner resistanceNon-metal conductorsSolid electrolytesLithiumPolymer science
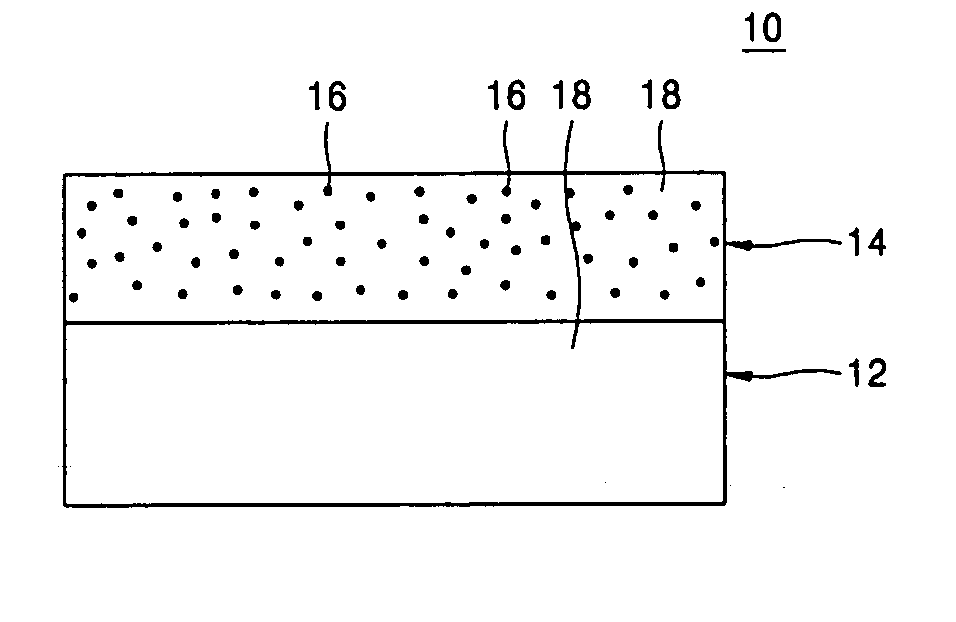
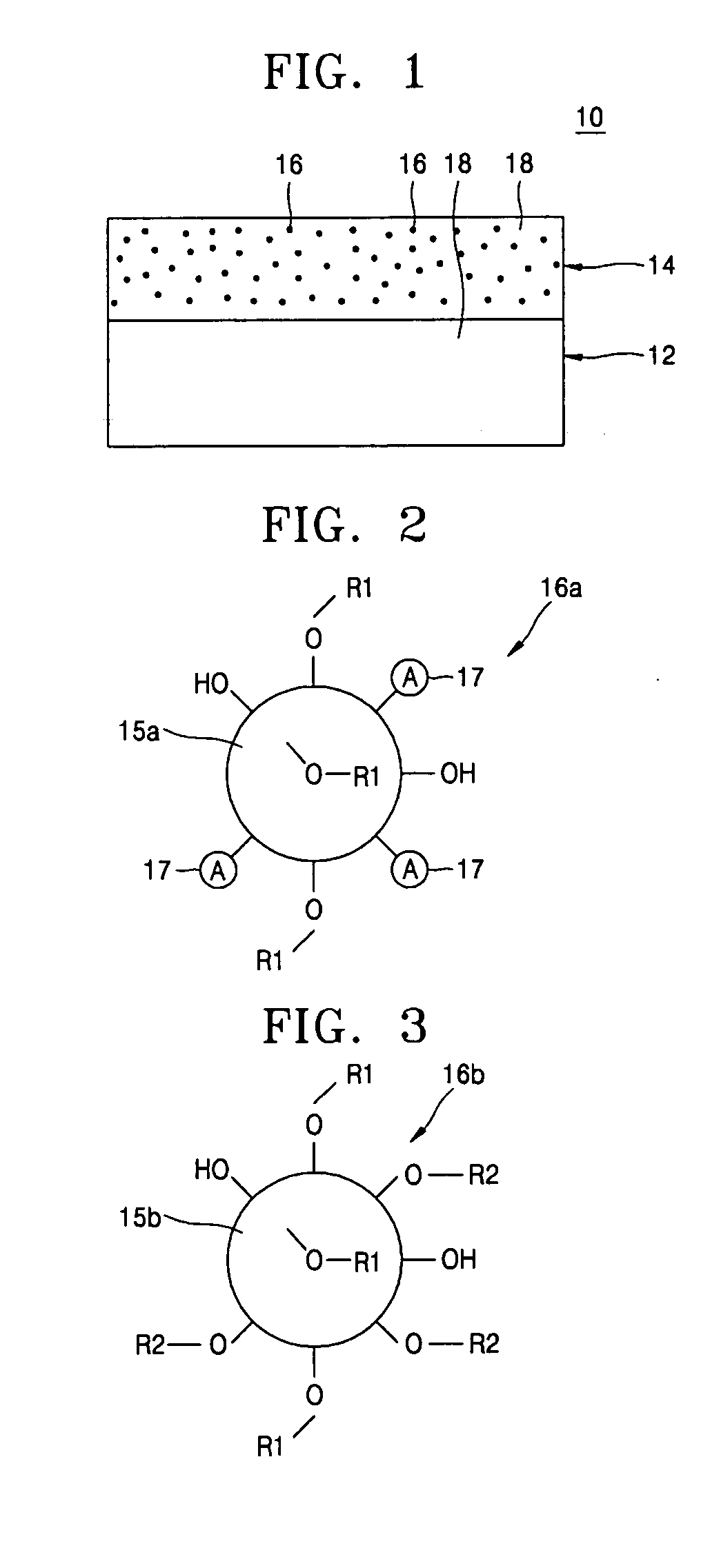
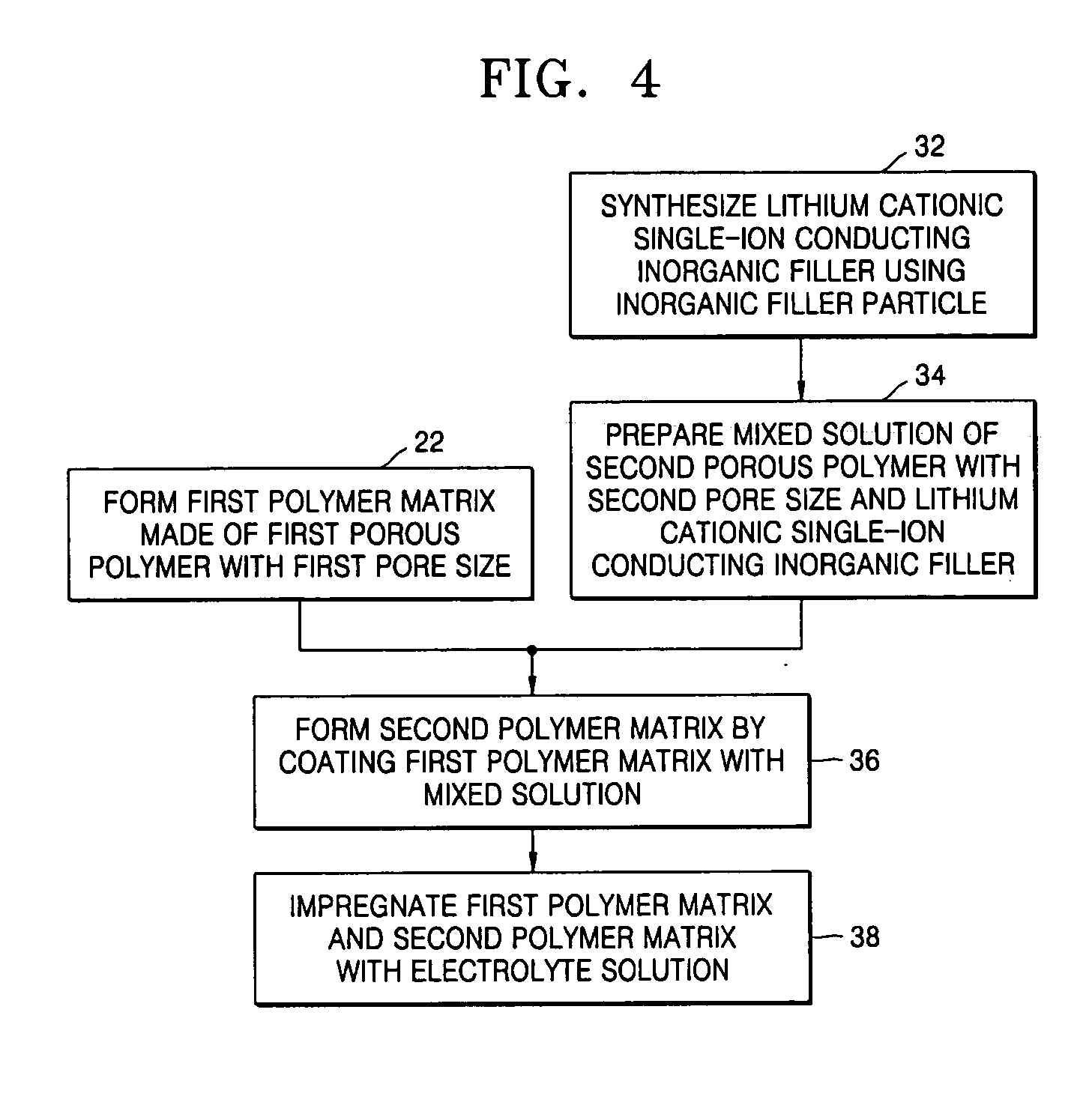
Provided are a composite polymer electrolyte for a lithium secondary battery in which a composite polymer matrix multi-layer structure composed of a plurality of polymer matrices with different pore sizes is impregnated with an electrolyte solution, and a method of manufacturing the same. Among the polymer matrices, a microporous polymer matrix with a smaller pore size contains a lithium cationic single-ion conducting inorganic filler, thereby enhancing ionic conductivity, the distribution uniformity of the impregnated electrolyte solution, and maintenance characteristics. The microporous polymer matrix containing the lithium cationic single-ion conducting inorganic filler is coated on a surface of a porous polymer matrix to form the composite polymer matrix multi-layer structure, which is then impregnated with the electrolyte solution, to manufacture the composite polymer electrolyte. The composite polymer electrolyte is used in a unit battery. The composite polymer matrix structure can increase mechanical properties. The introduction of the lithium cationic single-ion conducting inorganic filler can provide excellent ionic conductivity and high rate discharge characteristics.
Owner:ELECTRONICS & TELECOMM RES INST
Lithium ion secondary battery and method of fabricating thereof
InactiveUS6051342AFinal product manufactureElectrode carriers/collectorsElectrolytic agentAdhesion strength
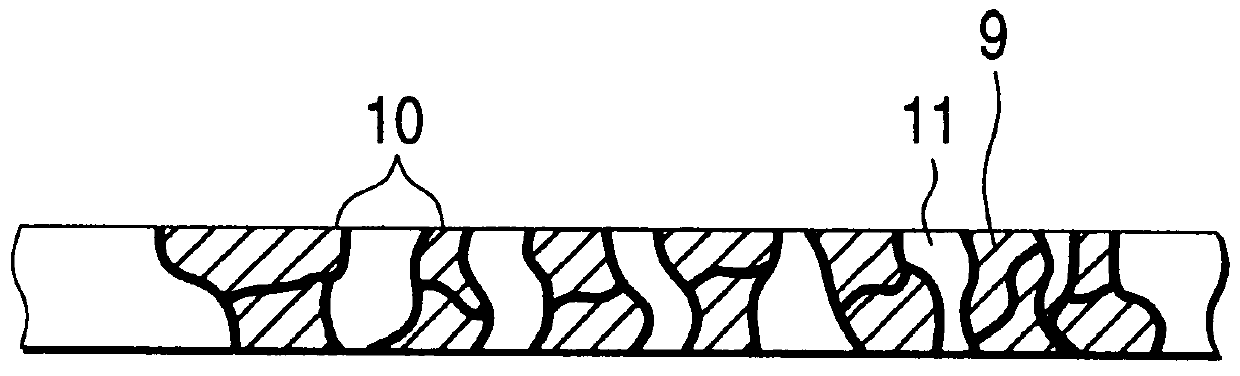
In a lithium ion secondary battery which is composed of a positive electrode 1, a negative electrode 4 and a separator 7 which contains a Li ion-containing non-aqueous electrolytic solution, both of the ionic conductivity and adhesion strength were ensured by making an adhesive resin layer 8 which bonds the positive electrode 1 to the separator 7 and the negative electrode 4 to the separator 7 into a mixture phase consisting of an electrolytic solution phase 9, an electrolytic solution-containing a polymer gel phase 10 and a polymer solid phase 11.
Owner:MITSUBISHI ELECTRIC CORP
Electrochemical device
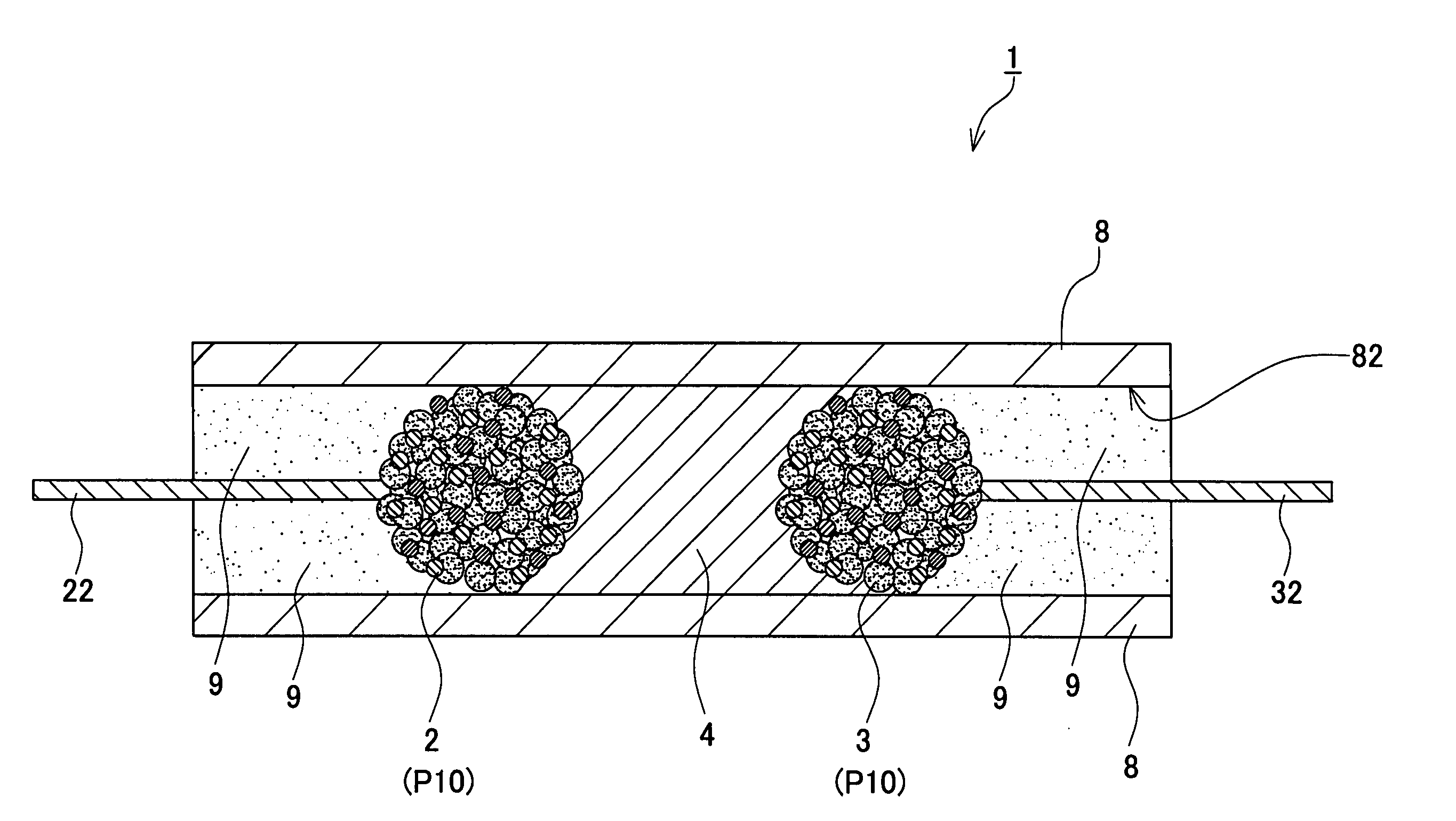
InactiveUS20050034299A1Convenient to accommodateSmall structureFinal product manufactureElectrode carriers/collectorsIonic conductivityElectronic conductivity
An electrochemical device comprises, at least, a first electrode, a second electrode, and an electrolyte layer having an ionic conductivity. The first and second electrodes oppose each other by way of the electrolyte layer. The first and second electrodes comprise a composite particle containing an electrode active material, a conductive auxiliary agent having an electronic conductivity, and a binder adapted to bind the electrode active material and conductive auxiliary agent to each other. In the composite particle, the electrode active material and conductive auxiliary agent are electrically coupled to each other without being isolated.
Owner:TDK CORPARATION
Sulfide solid electrolytes, preparation method thereof and all-solid lithium secondary battery
ActiveCN103531841AImprove air stabilityImprove ionic conductivitySolid electrolytesCell electrodesOxideElectrolyte
The invention provides sulfide solid electrolytes shown by a formula (I) and sulfide solid electrolytes shown by a formula (II). The invention further provides a design thought and a preparation method of the sulfide solid electrolytes. A certain amount of oxide is doped and added to or compounded to the sulfide solid electrolytes, the air stability of the sulfide solid electrolytes is improved, and the ionic conductivity of the sulfide solid electrolytes is kept.
Owner:ZHEJIANG FUNLITHIUM NEW ENERGY TECH CO LTD
Polyvinylidene-fluoride-based composite fibrous membrane, preparation method and application thereof
InactiveCN103147224AHigh porosityIncrease membrane fluxCell component detailsNon-woven fabricsFiberLithium-ion battery
The invention provides a polyvinylidene-fluoride-based composite fibrous membrane, which comprises a polyvinylidene fluoride fibrous layer with the thickness ranging from 5 mu m to 120 mu m, wherein the diameters of the fibers range from 80 nm to 1500 nm; the polyvinylidene fluoride fibrous layer comprises the components in percentage by mass as follows: 15%-95% of polyvinylidene fluoride and 5%-85% of a modifier; and the modifier comprises a polymer and / or inorganic oxide. The preparation method is simple and feasible; the used raw materials and equipment are cheap; the prepared composite fibrous membrane has the advantages of high porosity and membrane flux, good mechanical strength and thermal stability, uniform thickness, controllability and the like; and when applied to a lithium ion battery, the composite fibrous membrane can improve the ionic conductivity and the charge-discharge performance of the high current of the battery, and shows good electrochemical performance and circulative performance.
Owner:HARBIN INST OF TECH SHENZHEN GRADUATE SCHOOL
Oxide solid electrolytes based on lithium halide-doping and low-temperature sintering method thereof
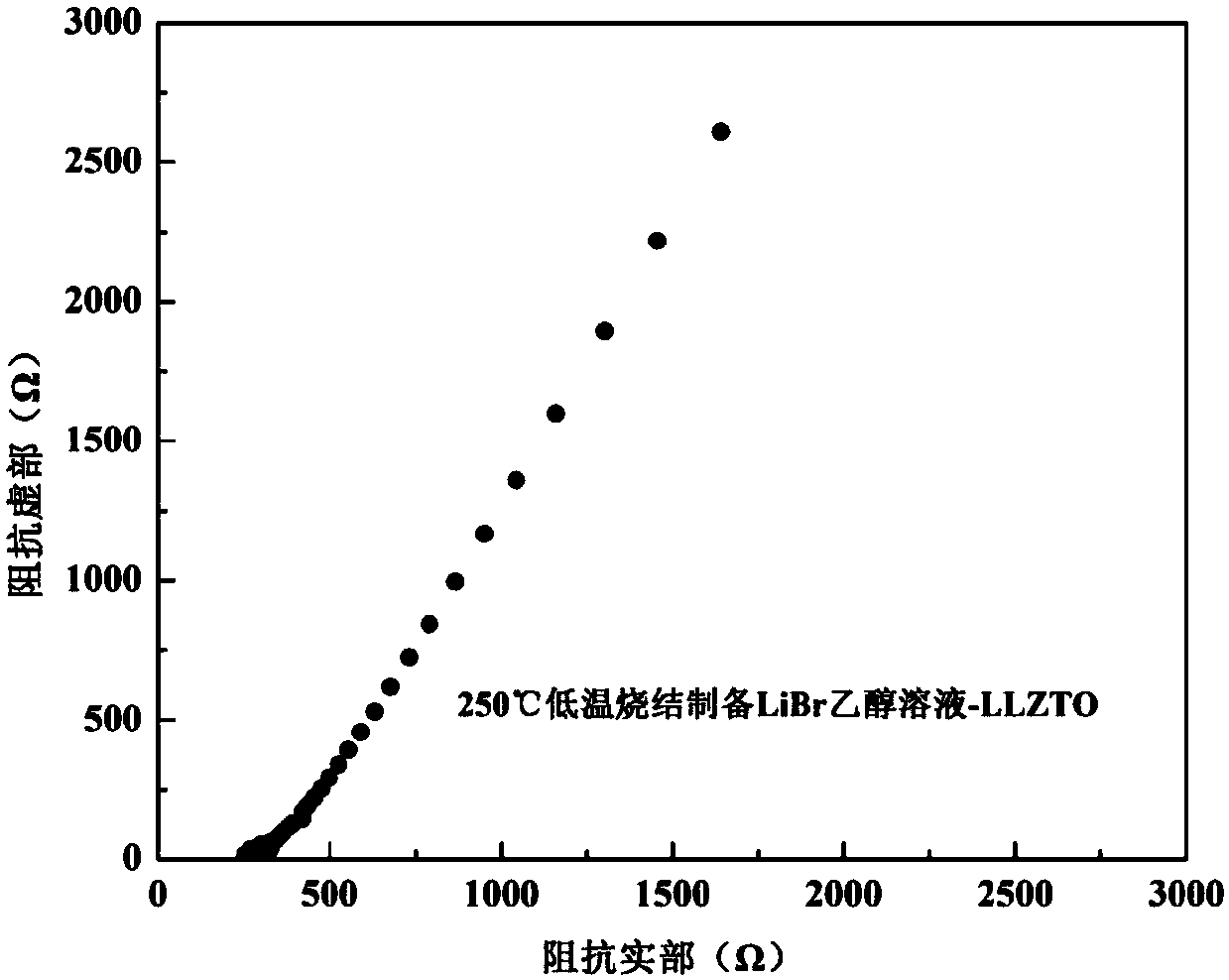
InactiveCN107732295AImprove ionic conductivityImprove performanceSecondary cellsElectrolytesSolid state electrolyteLanthanum
Provided is an oxide solid electrolyte based on lithium halide-doping. The oxide solid electrolyte is a lithium solid electrolyte, wherein electrolytes in a perovskite type, an NASICON type and a garnet type are taken as substrates, and a lithium halide solution and the oxide solid electrolytes are compounded and sintered at a low temperature. A preparing method comprises the steps that LATP, LLTOand LLZO solid electrolytes are subjected to ball-milling, or self-produced cubic-phase lithium-lanthanum-zirconium-oxygen solid electrolyte powder is subjected to ball-milling and sintered to prepare cubic-phase LLZO solid electrolyte powder, an LIX solution is added to the solid electrolyte powder, and the mixture is pressed into a slice or painted into a membrane, then placed in a muffle furnace at a low temperature of 100-250 DEG C and sintered for 1-10 hours. The oxide solid electrolyte is simple in technology and low in cost, and the prepared lithium solid electrolyte has high ionic conductivity and high repeatability; the oxide solid electrolyte can be on a par with electrolytes prepared through a traditional high-temperature technology, and meanwhile low-temperature sintering canavoid high-temperature diffusion reaction with cathode materials.
Owner:YANSHAN UNIV
Garnet-Type Oxide Sintered Body and Method for Producing Same
ActiveUS20180175446A1High densityImprove ionic conductivityZirconium compoundsSecondary cellsLithiumHigh density
A garnet-type oxide sintered body according to the present invention includes crystal grains composed of a garnet-type oxide containing Li, La and Zr and a grain boundary composition containing boron and silicon and filling gaps between the crystal grains. The oxide sintered body has the characteristics of high density and high ion conductivity. A production method of the sintered body includes a step of providing a precursor material by mixing a garnet-type oxide powder containing Li, La and Zr with a sintering aid; a step of forming the precursor material into a formed body; and a sintering step of sintering the formed body. The sintering aid contains oxygen, boron, silicon and lithium. The oxygen and boron, or the oxygen and silicon, contained in the sintered aid form a compound.
Owner:CENT GLASS CO LTD
Electrolytic-solution-supporting polymer film and secondary battery
InactiveUS6291106B1Improve puncture strengthAvoid it happening againCell seperators/membranes/diaphragms/spacersFinal product manufactureLithiumPolymer science
A high-strength, heat-resistant and high-safety electrolytic-solution-supporting polymer film which can be applied to secondary batteries typified by lithium and lithium ion secondary batteries and which has an ionic conductivity of at least 5x10-4 S / cm at 25° C., a puncture strength of at least 300 g and a mechanical heat resistance of at least 300° C.
Owner:TEIJIN LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com