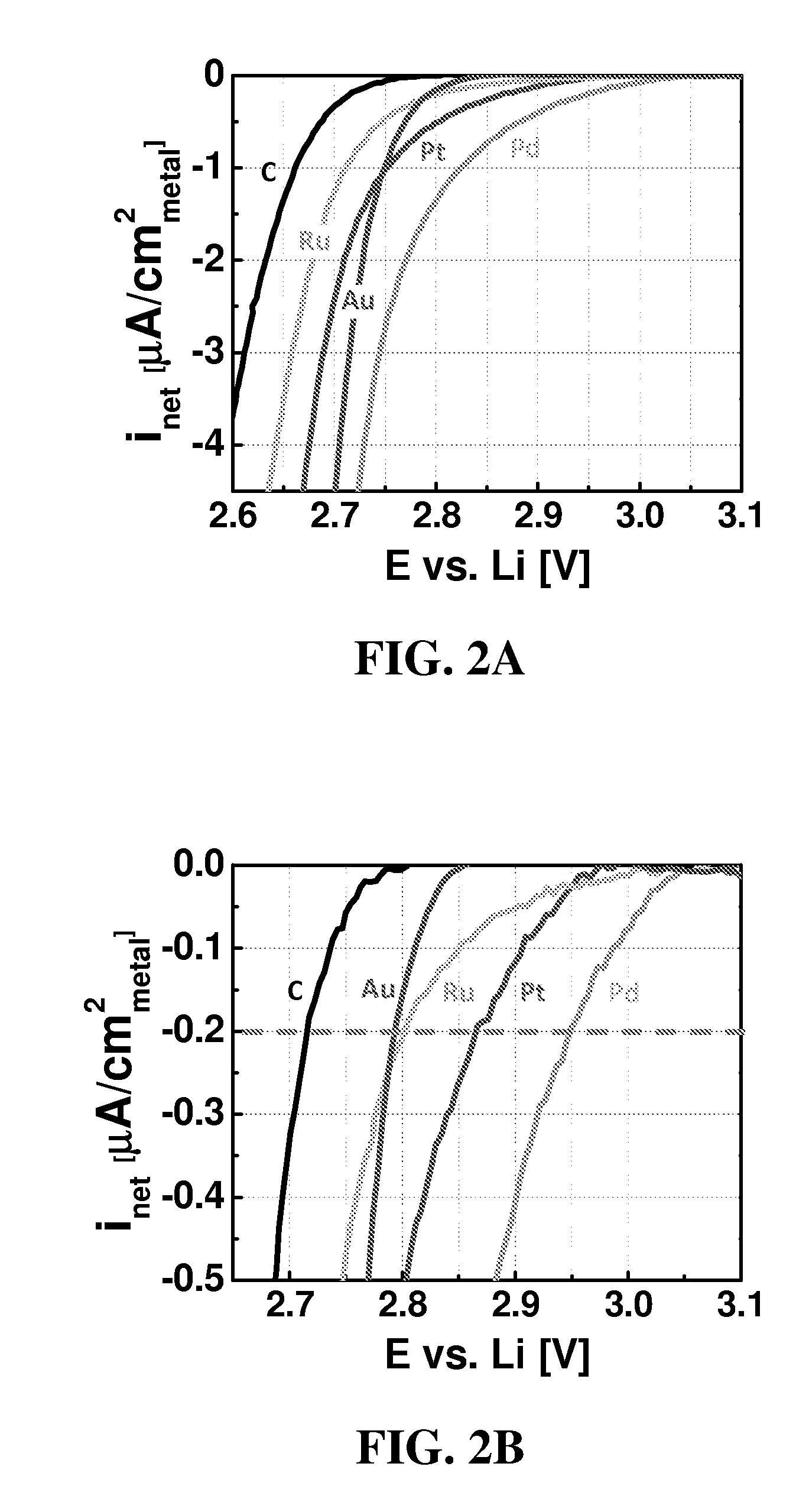
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39 results about "Metal–air electrochemical cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
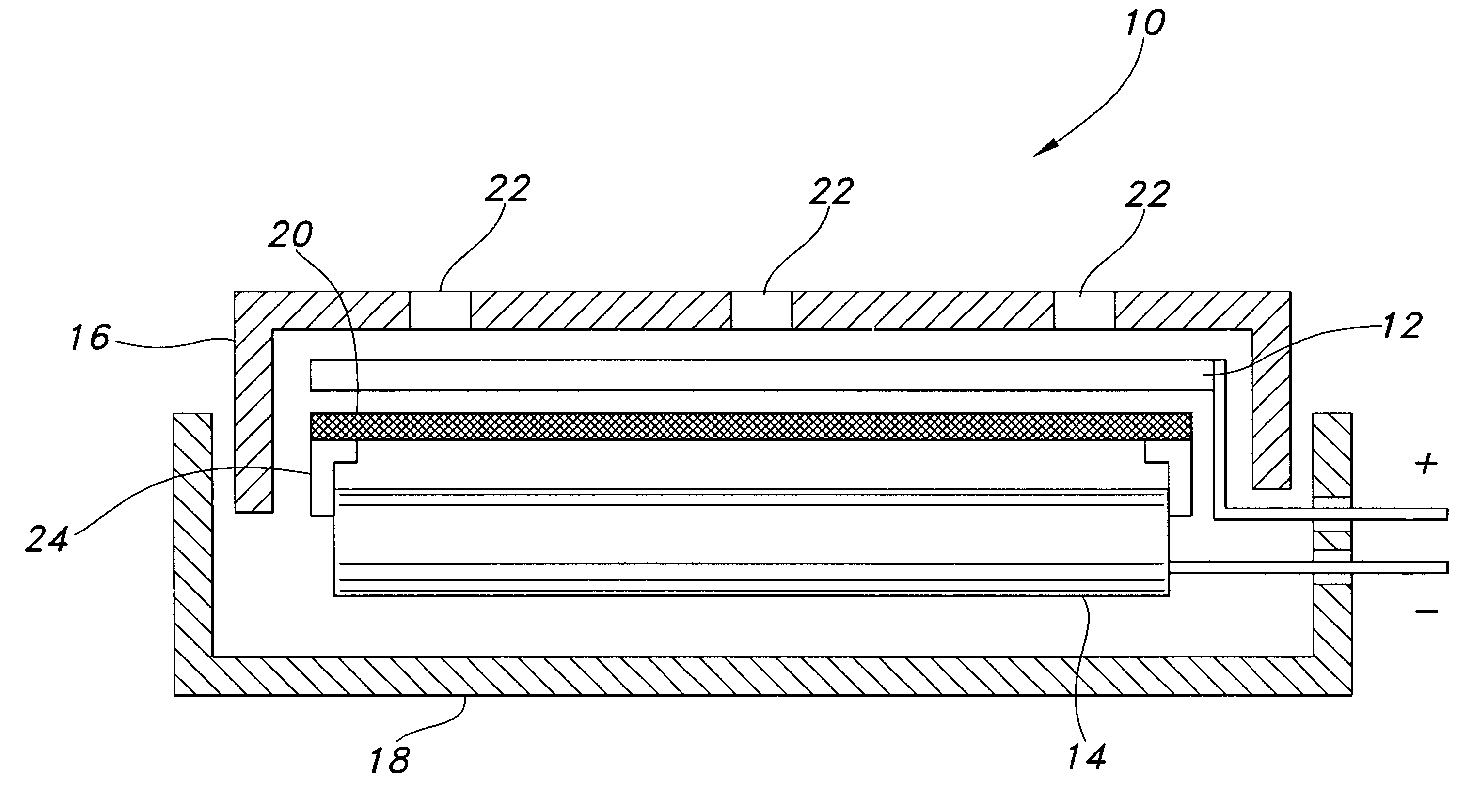
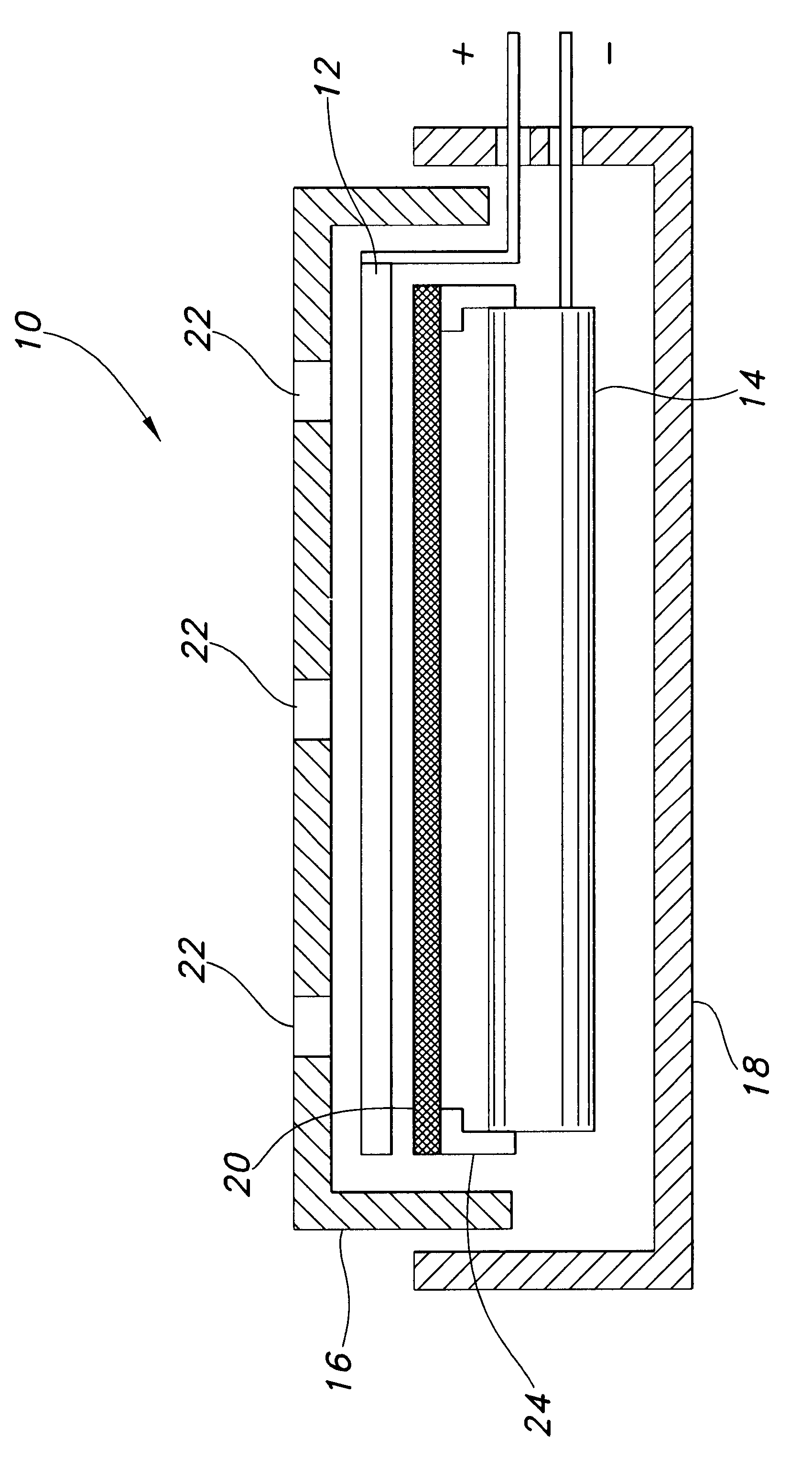
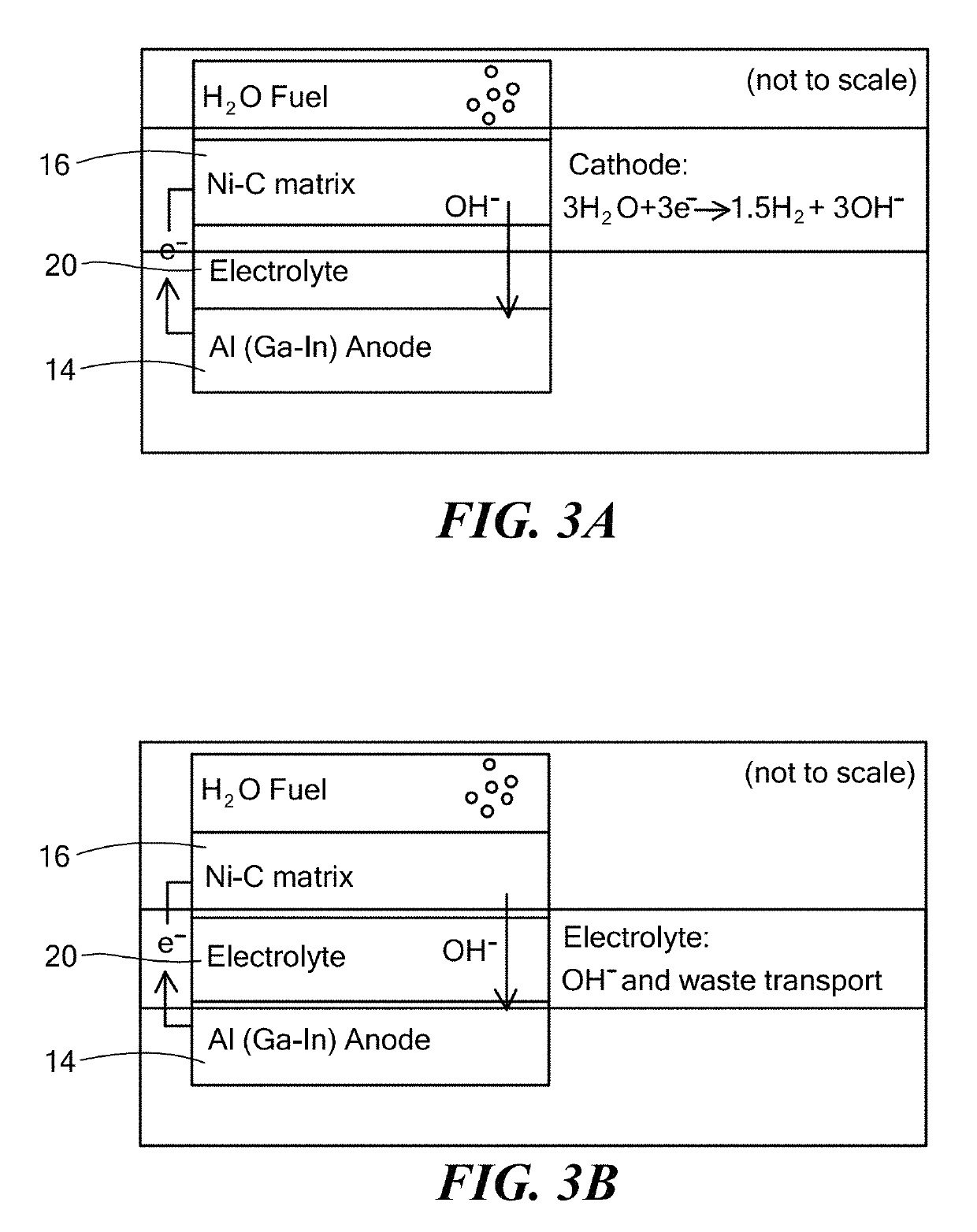
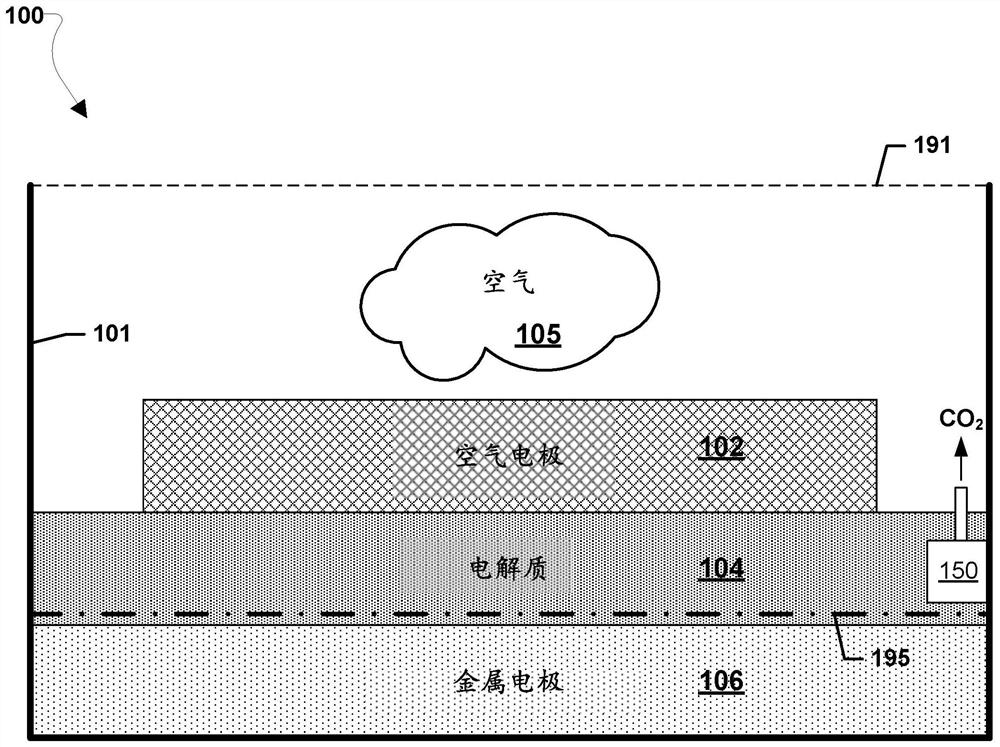
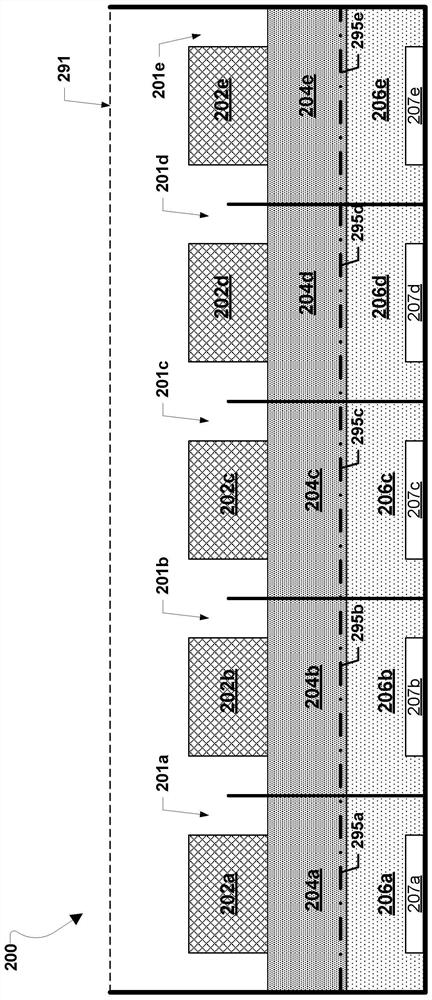
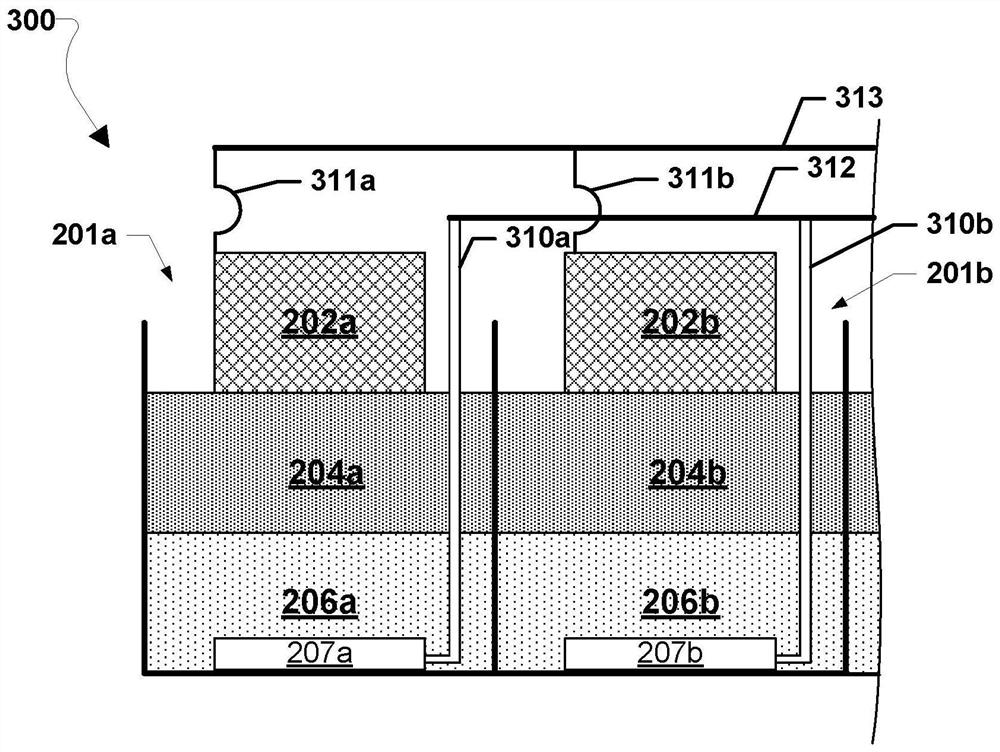
A metal–air electrochemical cell is an electrochemical cell that uses an anode made from pure metal and an external cathode of ambient air, typically with an aqueous or aprotic electrolyte. During discharging of a metal–air electrochemical cell, a reduction reaction occurs in the ambient air cathode while the metal anode is oxidized. The specific capacity and energy density of metal–air electrochemical cells is higher than that of lithium-ion batteries, making them a prime candidate for use in electric vehicles. However, complications associated with the metal anodes, catalysts, and electrolytes have hindered development and implementation of metal–air batteries.
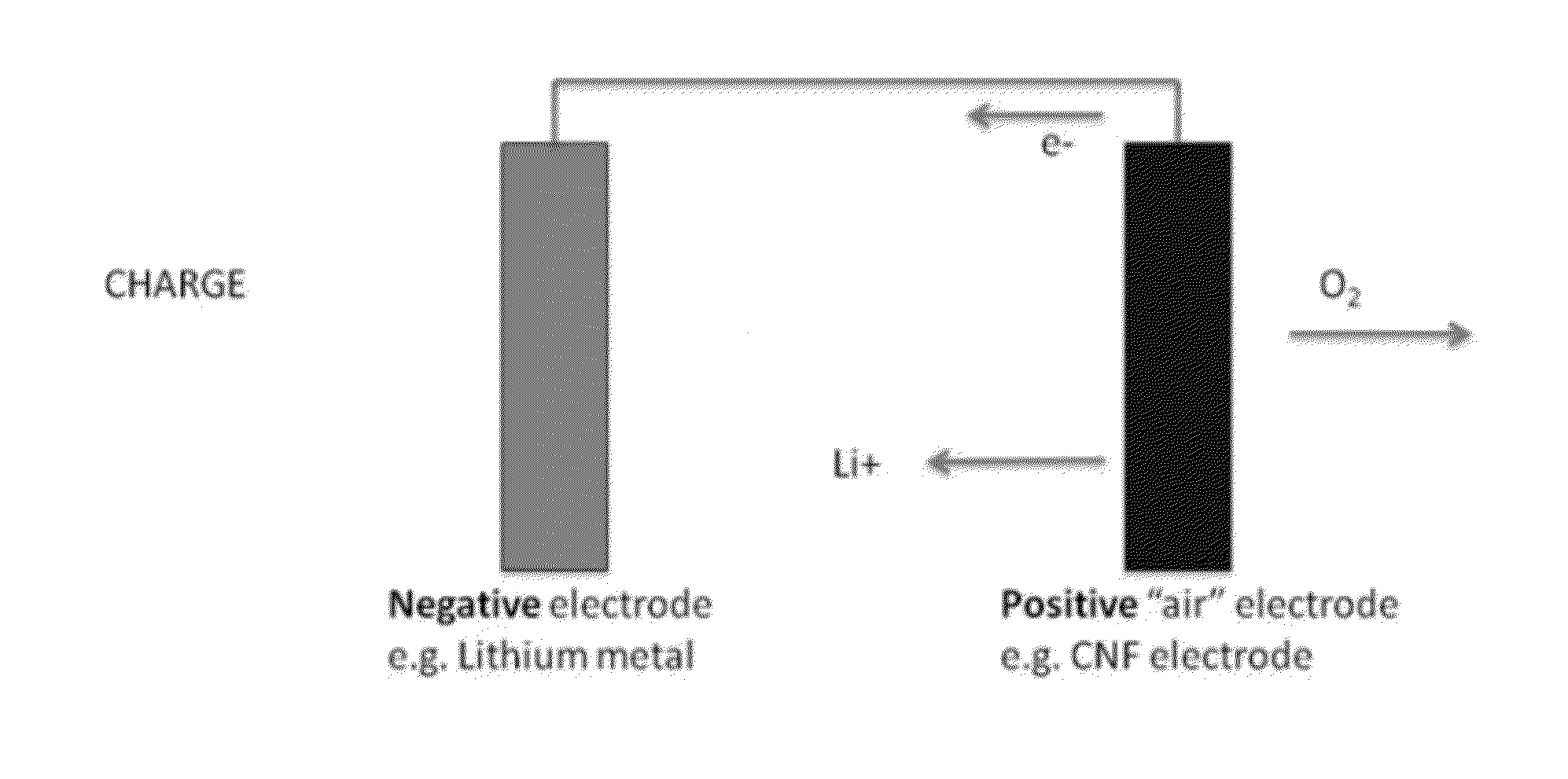
Metal-air electrochemical cell with high energy efficiency mode
ActiveUS20110250512A1Effective and efficient mannerFuel and primary cellsFuel and secondary cellsMetal–air electrochemical cellHigh energy
The present invention relates to a metal-air electrochemical cell with a high energy efficiency mode.
Owner:FORM ENERGY INC
Metal-air battery with ion-conducting inorganic glass electrolyte
InactiveUS20060063051A1Safe and reliable solid-stateIncrease energy densityFuel and primary cellsSolid electrolytesMetal–air electrochemical cellIonic conductivity
A solid-state metal-air electrochemical cell comprising: (A) a metal-containing electro-active anode; (B) an oxygen electro-active cathode; and (C) an ion-conducting glass electrolyte disposed between the metal-containing anode and the oxygen electro-active cathode. The cathode active material, which is oxygen gas, is not stored in the battery but rather fed from the environment. The oxygen cathode is preferably a composite carbon electrode which serves as the cathode current collector on which oxygen molecules are reduced during discharge of the battery to generate electric current. The glass electrolyte typically has an ion conductivity in the range of 5×10−5 to 2×10−3 S / cm. The electrolyte layer is preferably smaller than 10 μm in thickness and further preferably smaller than 1 μm. The anode metal is preferably lithium or lithium alloy, but may be selected from other elements such as sodium, magnesium, calcium, aluminum and zinc.
Owner:JANG BOR Z
Electrochemical separators with inserted conductive layers
InactiveUS20150171398A1Limiting and preventing hot spotLimiting and preventing and thermal runawayBatteries circuit arrangementsMaterial analysis by electric/magnetic meansMetal–air electrochemical cellElectrical battery
Disclosed are electrochemical cells including a composite separator capable of changing the performance of the cell by a) changing the internal electric field of the cell, b) activating lost active material, c) providing an auxiliary current collector for an electrode and / or d) limiting or preventing hot spots and / or thermal runaway upon formation of an electronic short in the system. An exemplary composite separator includes at least one electronically conducting layer and at least one electronically insulating layer. Another exemplary composite separator includes an electronically conducting layer and a solid ionic conductor. Also disclosed are methods for detecting and managing the onset of a short in an electrochemical cell and for charging an electrochemical cell.
Owner:CALIFORNIA INST OF TECH
Refuelable metal air electrochemical cell and refuelabel anode structure for electrochemical cells
InactiveUS20020142203A1Fuel and primary cellsFuel and secondary cellsMetal–air electrochemical cellMetal particle
A refuelable anode structure containing anode paste for a metal air electrochemical cell is provided. The anode paste comprises metal particles, a gelling agent, and a base. The spent anode structure may be removed after discharging. The anode structure may thereafter be electrically recharged to convert oxidized metal into consumable metal fuel, or mechanically emptied and refilled with fresh metal fuel paste.
Owner:EVIONYX INC
Electrolyte balance in electrochemical cells
InactiveUS20020098398A1Fuel and primary cellsElectrolyte moving arrangementsMetal–air electrochemical cellSolvent
A rechargeable metal air electrochemical cell is provided. The rechargeable metal air electrochemical cell generally includes an anode and a cathode in ionic communication via a separator and in fluid communication via one or more tubes or apertures, wherein the one or more tubes or apertures provide sufficient ionic resistance thereby preventing shorting between the electrodes while allowing liquid solvent to flow therebetween.
Owner:EVIONYX INC
Catalysts for oxygen reduction and evolution in metal-air electrochemical cells
InactiveUS20110274989A1Fuel and primary cellsBatteries circuit arrangementsPlatinumChemical reaction
Methods and devices for catalyzing reactions, e.g., in a metal-air electrochemical cell, are disclosed. In some instances, a porous positive electrode of the metal-air electrochemical cell includes a metal to catalyze a reaction at the electrode (e.g., oxidation of one or more metal-oxide species). The metal can be disposed as nanoparticles, and / or be combined with a second metal. Other aspects are directed to devices and methods that can generally promote a chemical reaction (e.g., an oxidation / reduction reaction) such as the formation of platinum containing nanoparticles that can be used to catalyze electrochemical reactions.
Owner:MASSACHUSETTS INST OF TECH
Rechargeable metal air electrochemical cell incorporating collapsible cathode assembly
InactiveUS20050019651A1Promotes Oxygen RemovalExtended service lifeFuel and primary cellsFuel and secondary cellsMetal–air electrochemical cellEngineering
The rechargeable metal air electrochemical cell generally includes a pair of air cathode portions centrally disposed and attached to each other with a collapsible mechanism. Anodes are disposed in ionic communication with each air cathode portions via a suitable electrolyte. For recharging, a pair of third charging electrodes is provided ionic communication with the anode portions.
Owner:EVIONYX INC
Methods for making oxygen reduction catalyst using micelle encapsulation and metal-air electrode including said catalyst
InactiveUS6428931B1Good dispersionInhibit transferFuel and primary cellsAlkaline accumulatorsMetal–air electrochemical cellManganese
A manganese based catalyst for use in a metal-air electrochemical cell wherein the catalyst is made by a micelle process and a method of producing the catalyst. An electrode comprising said catalyst and method of producing the electrode. The micelle encapsulation process creates catalyst particles that are submicron and easily distributed throughout the active layer of the electrode.
Owner:THE GILLETTE CO
Protective layers in lithium-ion electrochemical cells and associated electrodes and methods
ActiveCN107078277ASolid electrolytesFinal product manufactureLithium oxideMetal–air electrochemical cell
Protective layers in lithium-ion electrochemical cells, and associated electrodes and methods, are generally described. The protective layers may comprise lithium-ion-conductive inorganic ceramic materials, such as lithium oxide, lithium nitride, and / or lithium oxysulfide. The resulting lithium-ion electrochemical cells may exhibit enhanced performance, including reduced capacity fade rates and reduced self-discharge rates.
Owner:SION POWER CORP
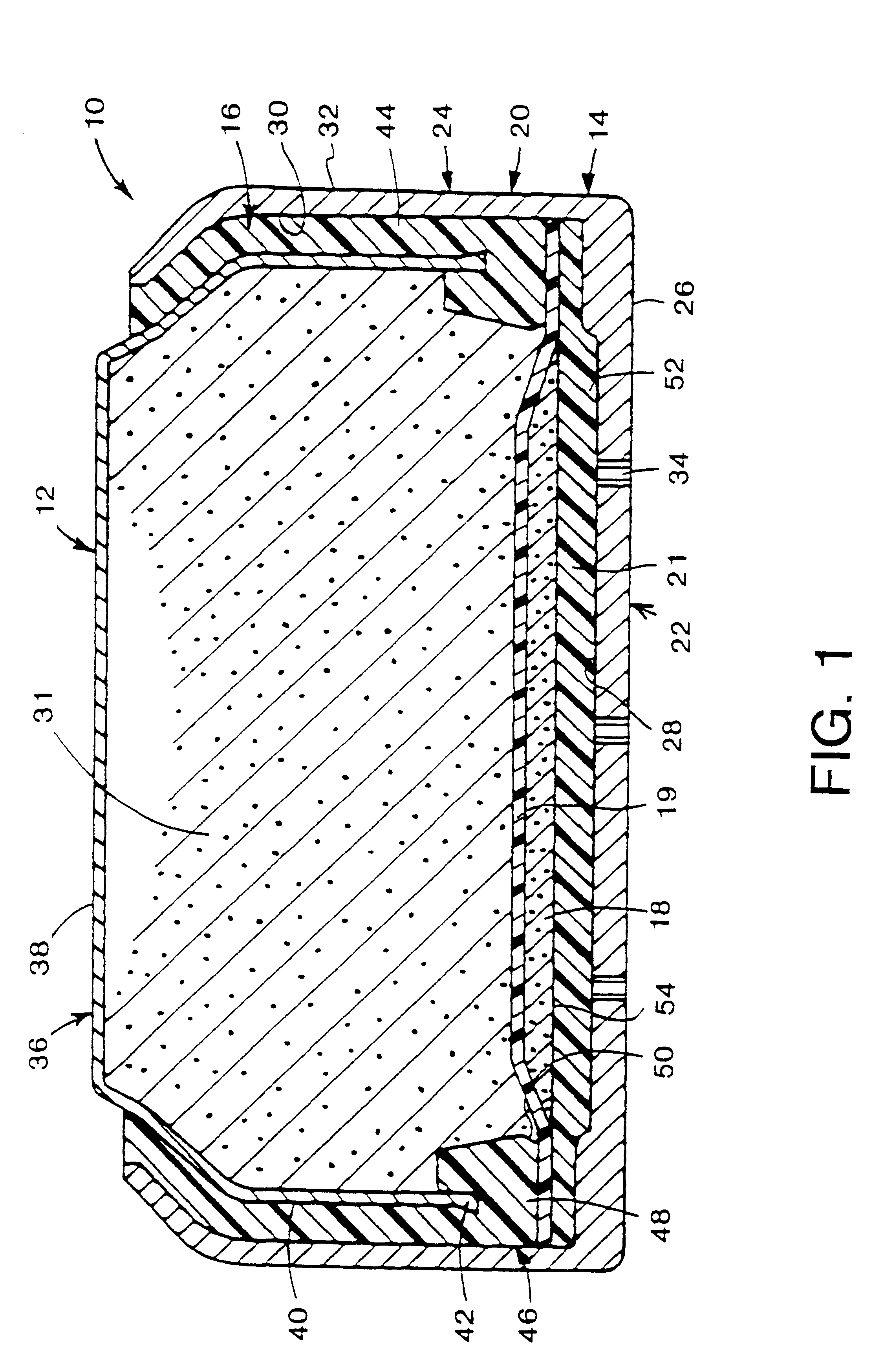
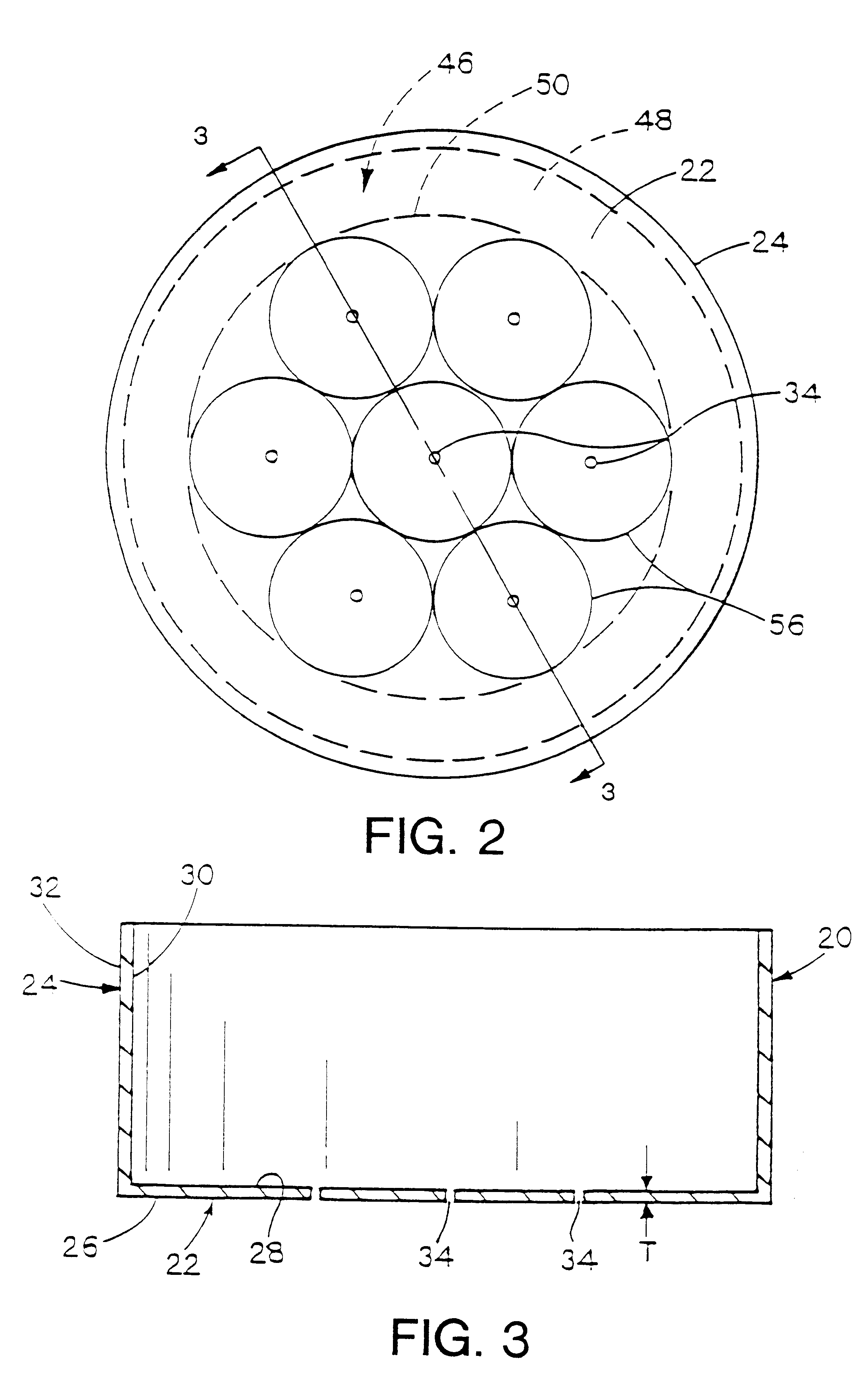
Metal-air cathode can, and electrochemical cell made therewith
InactiveUS6284400B1Small sizeIncrease in current-producing capabilityFuel and primary cellsJackets/cases materialsElectrochemical responseMetal–air electrochemical cell
This invention pertains to metal-air electrochemical cells wherein one or more air entry ports is located in the bottom of the cathode can, to provide for entry of oxygen-rich air into the cathode can, where the oxygen participates in the electrochemical reaction whereby the cell produces electrical energy. In this invention, extremely small air ports are provided, along with methods of reliably fabricating such small air ports, where a tool impression extends around the port. Generally, the use of an increased number of small air ports distributed over the bottom of the cathode can, opposite the reaction surface of the cathode assembly, wherein the overall open area of the ports is not increased, results in less moisture traversing the air ports, into or out of the cell. Accordingly, moisture loss, or gain, as a function of electrical energy produced, is thereby reduced.
Owner:ROVCAL +1
Metal-air cathode can, and electrochemical cell made therewith
InactiveUS6040074AReduce weight lossReduce the amount requiredFuel and primary cellsJackets/cases materialsElectrochemical responseMetal–air electrochemical cell
This invention pertains to metal-air electrochemical cells wherein one or more air entry ports is located in the bottom of the cathode can, to provide for entry of oxygen-rich air into the cathode can, where the oxygen participates in the electrochemical reaction whereby the cell produces electrical energy. In this invention, extremely small air ports are provided, along with methods of reliably fabricating such small air ports Generally, the use of an increased number of small air ports distributed over the bottom of the cathode can, opposite the reaction surface of the cathode assembly, wherein the overall open area of the ports is not increased, results in less moisture traversing the air ports, into or out of the cell. Accordingly, moisture loss, or gain, as a function of electrical energy produced, is thereby reduced.
Owner:THE BANK OF NEW YORK MELLON AS COLLATERAL AGENT +1
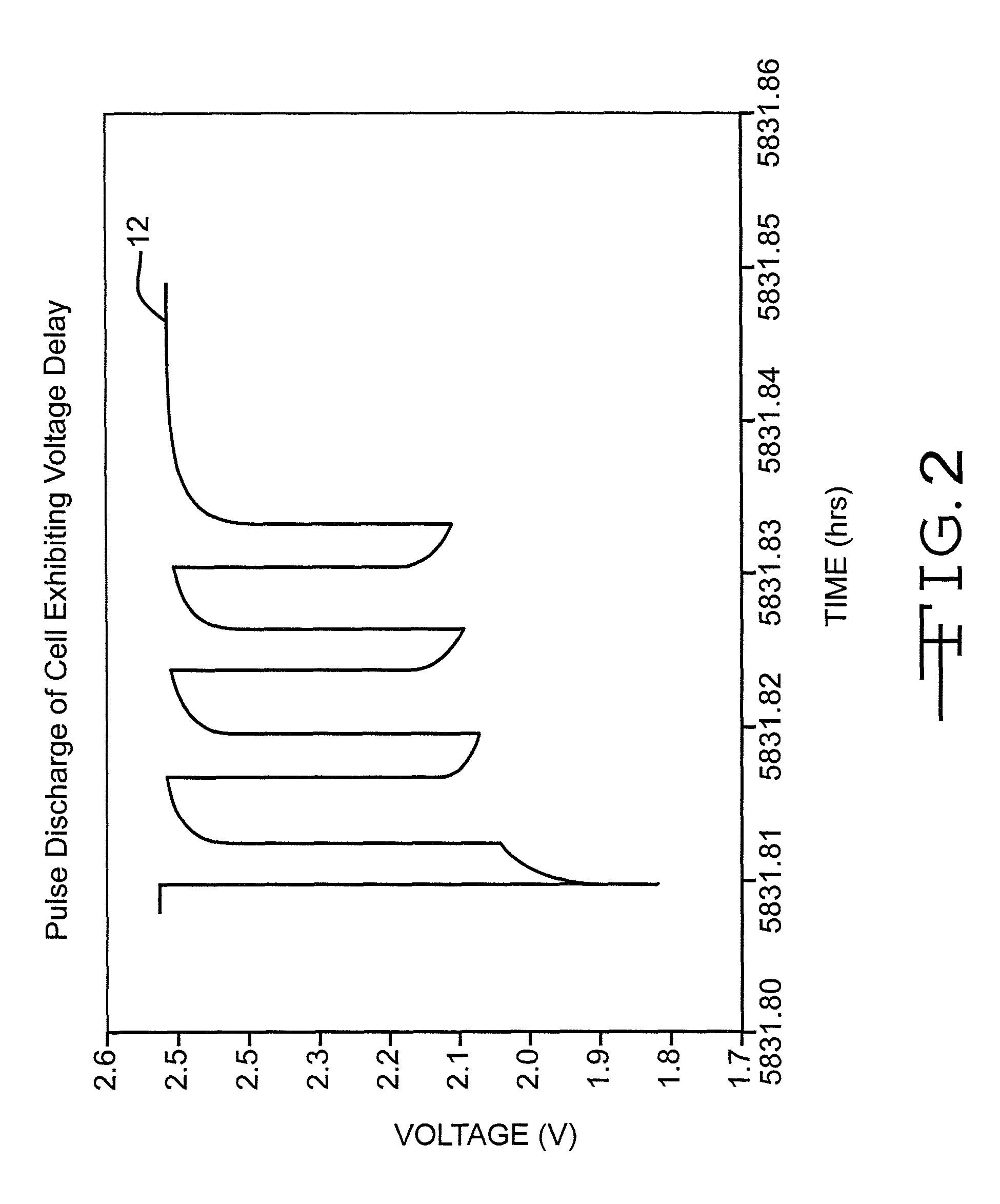
Method of controlling voltage delay and RDC growth in an electrochemical cell using low basis weight cathode material
InactiveUS7939199B1Minimal voltage delayLose weightSilver accumulatorsOrganic electrolyte cellsMetal–air electrochemical cellVanadium oxide
An electrochemical cell comprising a lithium anode, a silver vanadium oxide cathode having a relatively lower basis weight, and an electrolyte activating the anode and the cathode is described. By limiting the amount of cathode active material per unit area (i.e. basis weight) facing the anode in the Li / SVO cell, the magnitude of the passivating film growth at the solid-electrolyte interphase (SEI) and its relative impermeability to lithium ion diffusion is reduced. Therefore, by using a cathode of a relatively low basis weight active material, it is possible to eliminate or significantly reduce undesirable irreversible Rdc growth and voltage delay in the cell and to extend its useful life in an implantable medical device.
Owner:WILSON GREATBATCH LTD
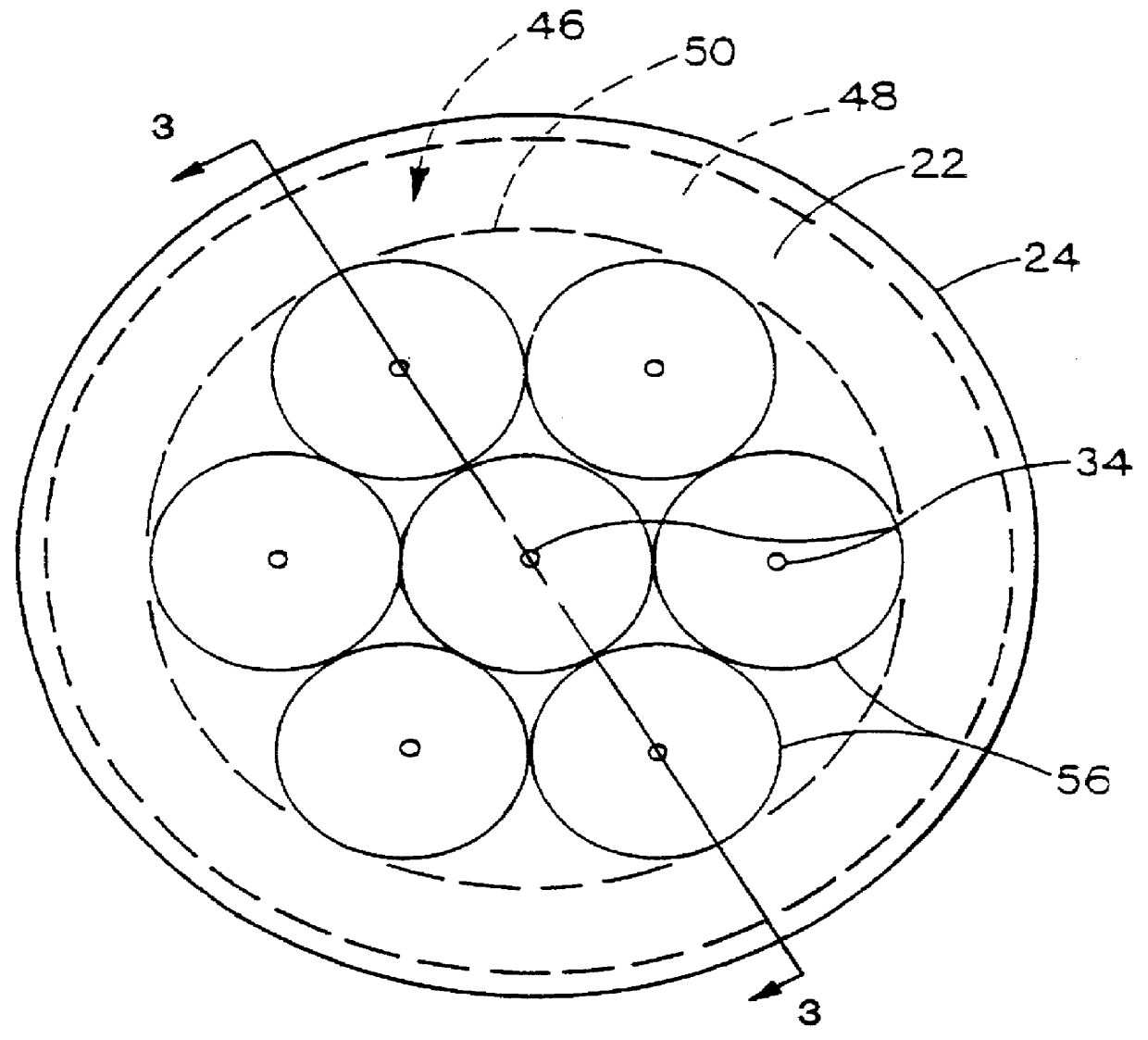
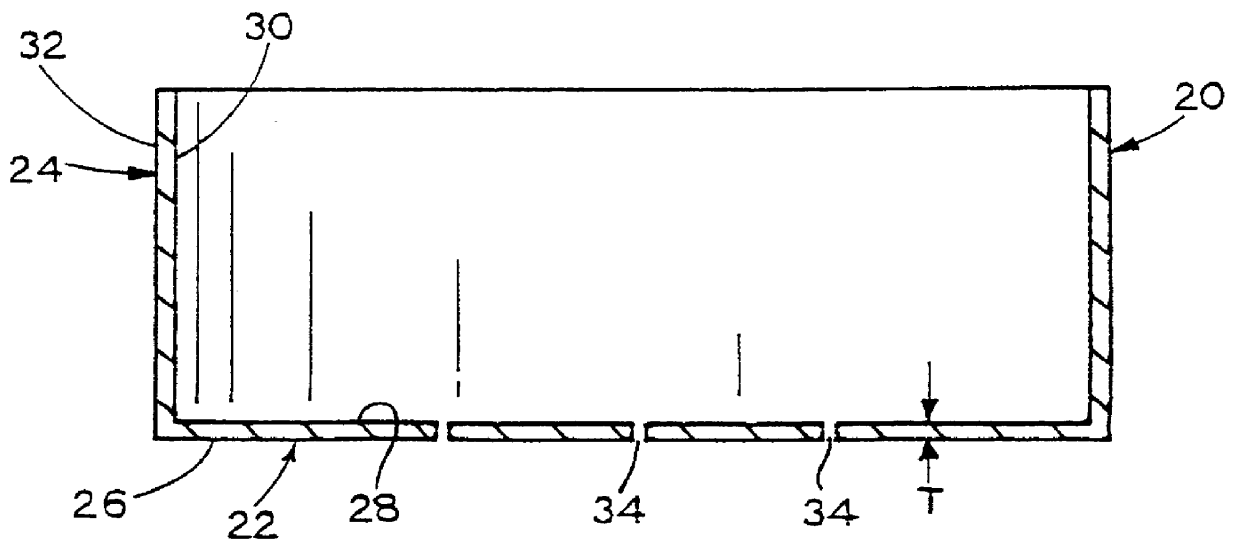
Anode structure for metal air electrochemical cells
InactiveUS6878482B2Fuel and primary cellsDeferred-action cellsMetal–air electrochemical cellOptoelectronics
An anode structure for a metal air electrochemical cell is provided. The structure includes a plurality of compartments, which are at least partially isolated from one another. A metal air cell using the anode structure includes the anode structure having one or more of the compartments partially filled with anode material, a cathode in ionic communication with the anode material, and a separator electrically isolating the cathode and the anode material. The volume of anode material included in the one or more compartments is preferably based on the expected expansion of the anode material.
Owner:EVIONYX INC
Anode structure for metal air electrochemical cells
InactiveUS20020182509A1Fuel and primary cellsDeferred-action cellsMetal–air electrochemical cellOptoelectronics
An anode structure for a metal air electrochemical cell is provided. The structure includes a plurality of compartments, which are at least partially isolated from one another. A metal air cell using the anode structure includes the anode structure having one or more of the compartments partially filled with anode material, a cathode in ionic communication with the anode material, and a separator electrically isolating the cathode and the anode material. The volume of anode material included in the one or more compartments is preferably based on the expected expansion of the anode material.
Owner:EVIONYX INC
Anode structure for metal air electrochemical cells and method of manufacture thereof
InactiveUS20020119368A1Fuel and primary cellsInsertable electrodesMetal–air electrochemical cellMetallurgy
The anode structure comprises a metal constituent and a base at least partially contained by a separator. Additionally, a method of manufacture of an anode structure is provided, wherein a metal constituent and a base are integrally formed into a substantially solid structure, and a separator is provided to at least partially contain the metal constituent and the base.
Owner:EVIONYX INC
Efficient treatment of wastewater using electrochemical cell
ActiveUS9440866B2Energy efficiencyLow densityWater treatment parameter controlMultiple component coatingsSupporting electrolyteMetal–air electrochemical cell
Owner:AXINE WATER TECH
Driven electrochemical cell for electrolyte state of charge balance in energy storage devices
InactiveCN105849960AElectrolyte stream managementCell electrodesElectrolysisMetal–air electrochemical cell
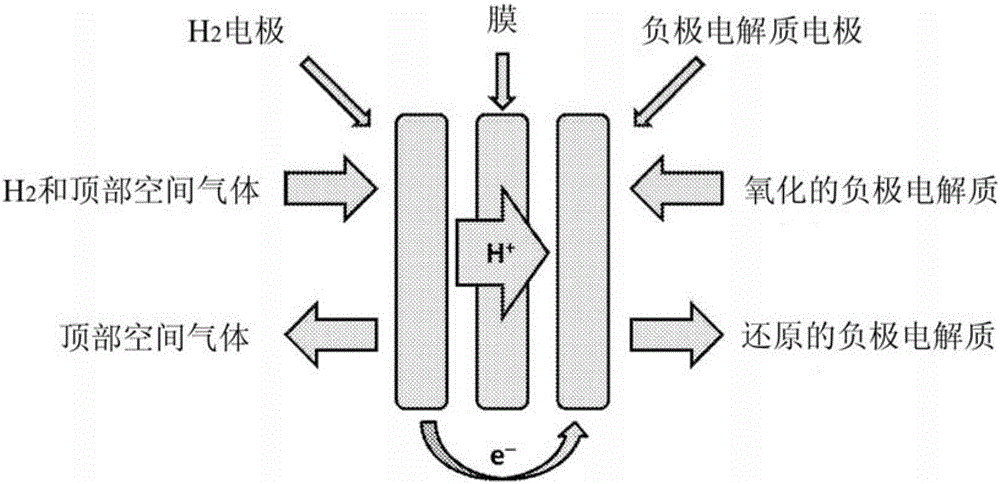
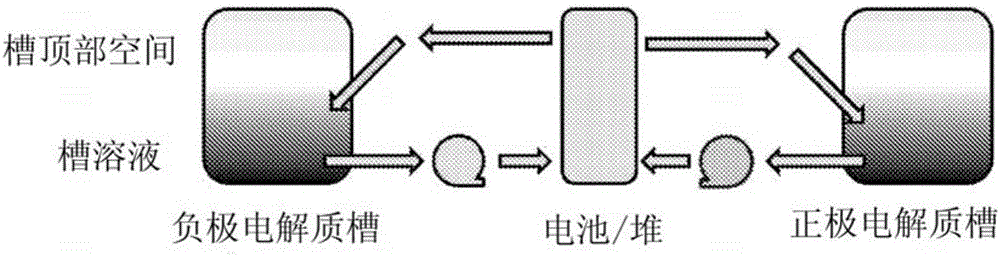
The invention concerns redox flow batteries comprising one or more electrochemical cells in fluid contact with an electrochemical balancing cell, the balancing cell comprising: (i) a first electrode comprising a gas diffusion electrode and the first electrode comprising a hydrogen oxidation catalyst, wherein the first electrode being maintained at a potential more positive than the thermodynamic potential for hydrogen evolution; (ii) a second electrode, the second electrode contacting negative electrolyte, and the second electrode being maintained at a potential sufficiently negative to reduce the negative electrolyte; (iii) a membrane disposed between the positive electrode and the negative electrode, the membrane being suitable to allow hydrogen cations to flow from the membrane to the negative electrolyte; and (iv) a means for contacting hydrogen with the first electrode.
Owner:LOCKHEED MARTIN ADVANCED ENERGY STORAGE
Electrochemical cells containing spun mercury-amalgamated zinc particles having improved physical characteristics
InactiveUS20070048576A1Good physical propertiesFuel and primary cellsAlkaline accumulatorsHydrogenMetal–air electrochemical cell
A metal-air electrochemical cell that includes spun mercury-amalgamated zinc powder particles is disclosed. The mercury-amalgamated zinc powder particles have advantageous physical properties that improve the performance characteristics of the cell including increasing the rate capability while decreasing the failure rate of the cell from buildup of hydrogen gas in the cell.
Owner:ROVCAL
Electrochemical cells containing spun mercury-amalgamated zinc particles having improved physical characteristics
InactiveUS20070048575A1Good physical propertiesFuel and primary cellsAlkaline accumulatorsHydrogenMetal–air electrochemical cell
A metal-air electrochemical cell that includes spun mercury-amalgamated zinc powder particles is disclosed. The mercury-amalgamated zinc powder particles have advantageous physical properties that improve the performance characteristics of the cell including increasing the rate capability while decreasing the failure rate of the cell from buildup of hydrogen gas in the cell.
Owner:ROVCAL
Metal-air cathode can and electrochemical cell made therewith
InactiveUS6248463B1Reduce weightReduce the amount requiredFuel and primary cellsActive material electrodesChemical reactionMetal–air electrochemical cell
This invention pertains to metal-air electrochemical cells wherein one or more air entry ports is located in the bottom of the cathode can, to provide for entry of oxygen-rich air into the cathode can, where the oxygen partcicpates in the chemical reaction whereby the cell produces electrical energy. In this invention, multiple small air entry ports are provided. Generally, the use of multiple ports distributed over the bottom of the cathode can, opposite the reaction surface of the cathode assembly, while not increasing the overall open area of the ports, results in an increase in the ratio of the cell limiting current to the rate at which moisture is lost from the cell. Accordingly, moisture loss as a function of electrical energy produced, is less.
Owner:THE BANK OF NEW YORK MELLON AS COLLATERAL AGENT
Nanofiber electrodes for energy storage devices
InactiveUS20120276458A1Fuel and primary cellsMaterial nanotechnologyPorosityMetal–air electrochemical cell
Methods and devices for enhanced energy storage in an electrochemical cell are provided. In some embodiments, an electrode for use in a metal-air electrochemical cell can include a plurality of nanofiber (NF) structures having high porosity, tunable mass, and tunable thickness. The NF structures are particularly suited for energy storage and can provide the electrode with exceptionally high gravimetric capacity and energy density when used in an electrochemical cell.
Owner:MASSACHUSETTS INST OF TECH
Anode material for electrochemical cells
InactiveUS20030162068A1Fuel and primary cellsFuel cell auxillariesPolymer electrolytesMetal–air electrochemical cell
An anode material for a metal air electrochemical cell is provided. The anode material comprises metal and / or metal oxide particles and a polymer electrolyte, particularly a polymer matrix material including electrolyte supported within the molecular structure of the polymer matrix material. Additionally, a metal air electrochemical cell is provided, using the anode material an air cathode, and a separator between the anode material and the air cathode.
Owner:WILSON JAMES D +1
Metal-air electrochemical cell with high energy efficiency mode
InactiveUS20160156082A1Effective and efficient mannerFuel and secondary cellsCells structural combinationMetal–air electrochemical cellHigh energy
The present invention relates to a metal-air electrochemical cell with a high energy efficiency mode.
Owner:FORM ENERGY INC
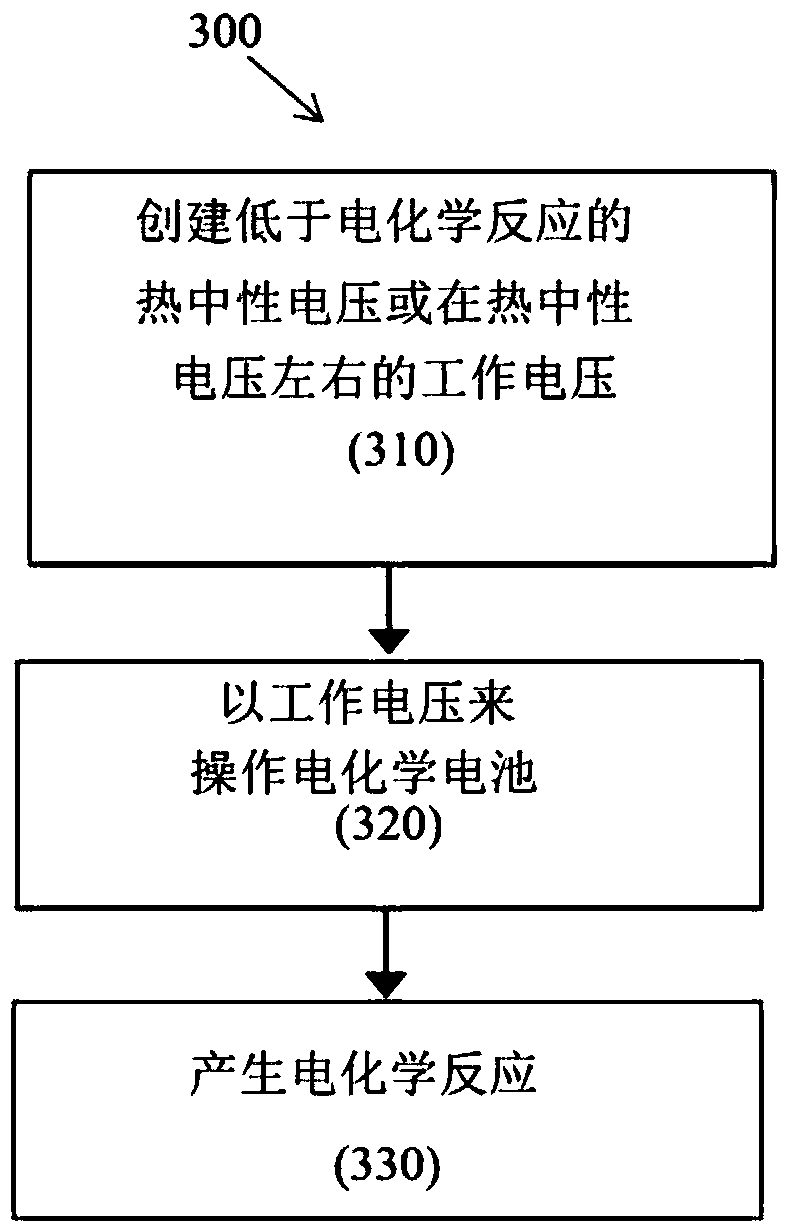
Method and system for efficiently operating electrochemical cells
Disclosed are electrochemical cells and methods of use or operation. In one aspect there is disclosed a method for management of an electrochemical cell, the method comprising operating the electrochemical cell at an operational voltage that is below or about the thermoneutral voltage for an electrochemical reaction. In another aspect there is disclosed an electrochemical cell comprising electrodes, an electrolyte between the electrodes, and a catalyst applied to at least one of the electrodes to facilitate an electrochemical reaction at an operational voltage of the electrochemical cell thatis below or about the thermoneutral voltage for the electrochemical reaction. Also disclosed are various catalysts for the electrochemical cell comprising mixtures of various catalytic materials and polytetrafluoroethylene (PTFE).
Owner:AQUAHYDREX
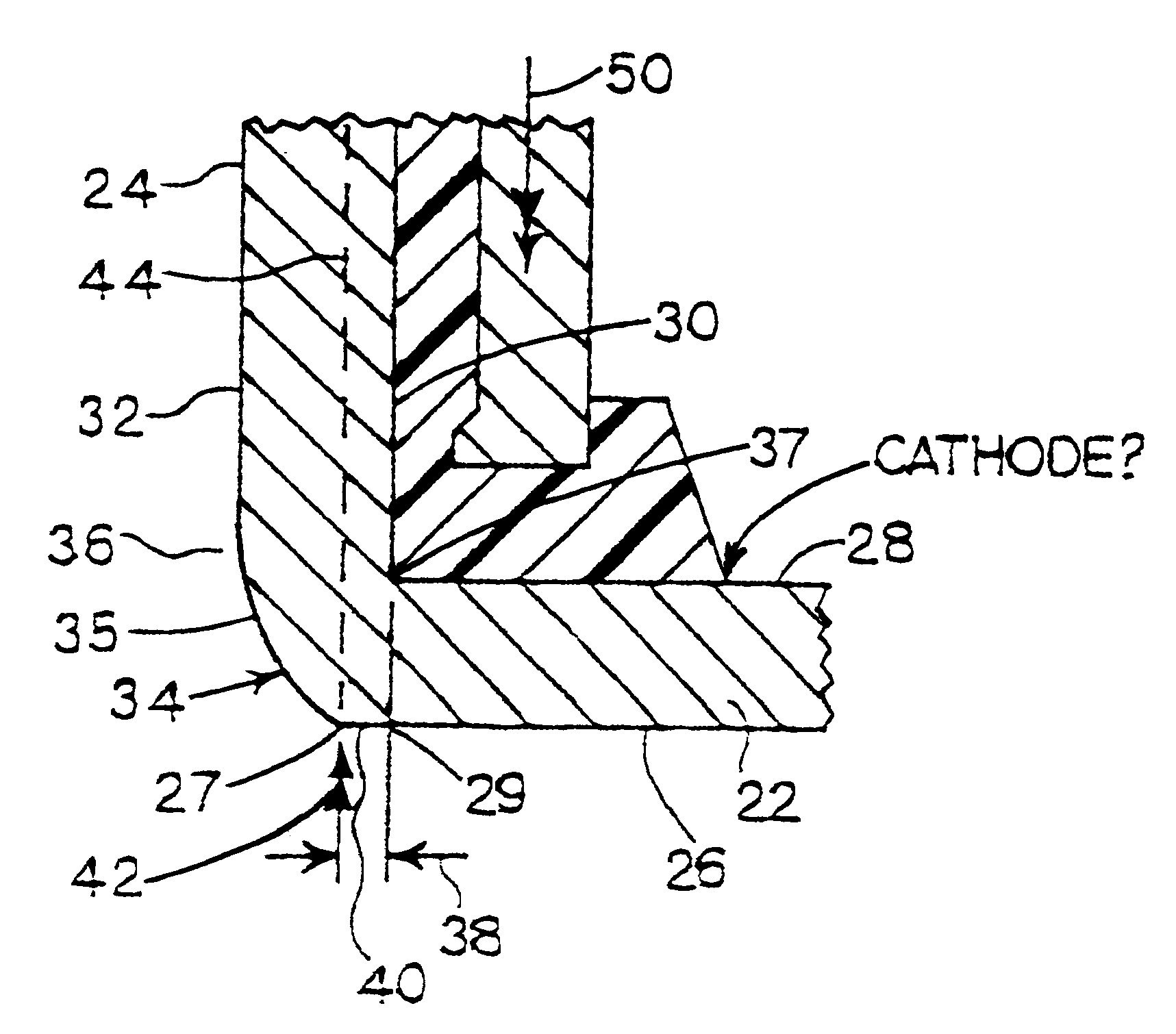
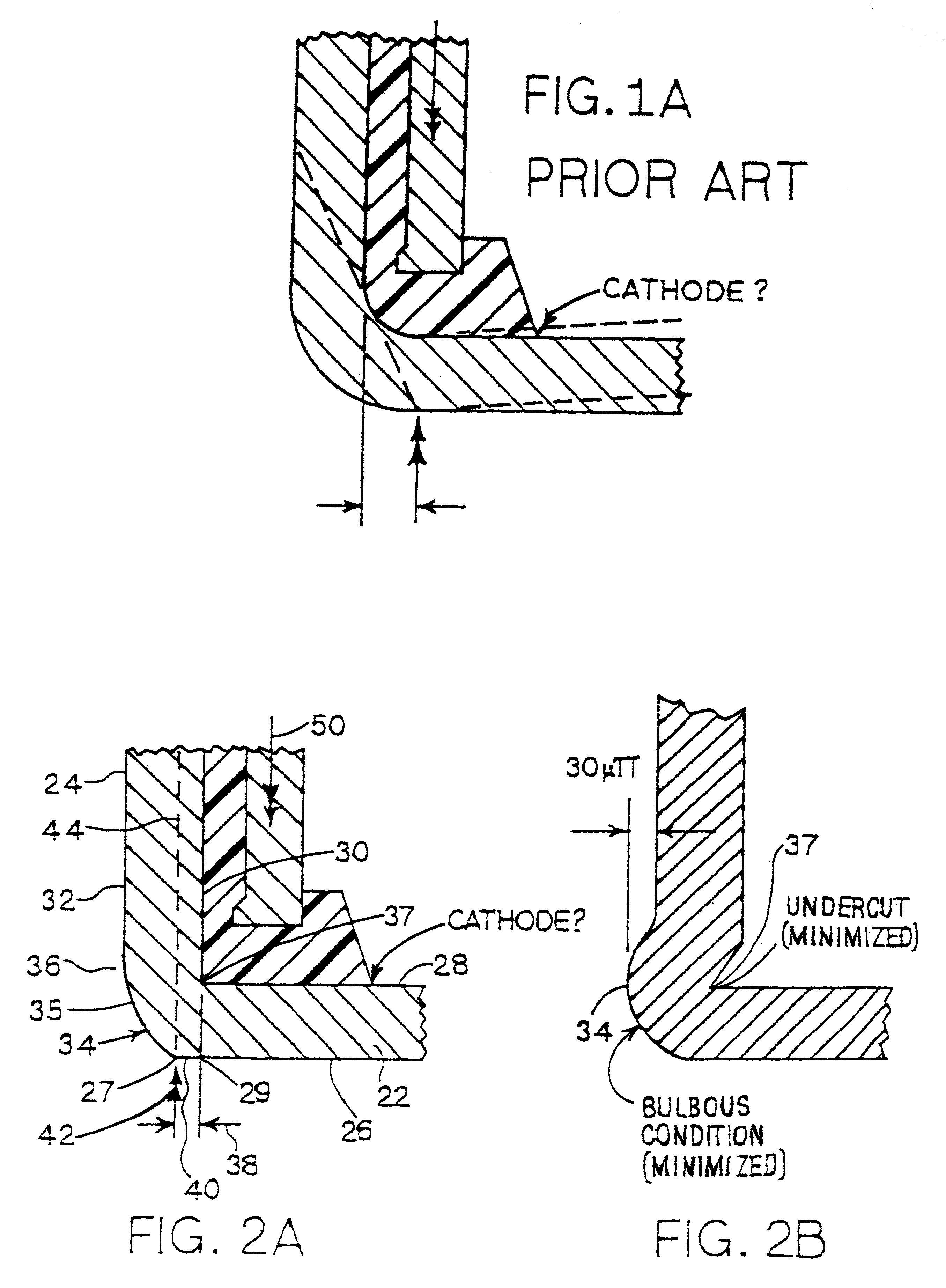
Metal-air cathode can having reduced corner and electrochemical cells made therewith
InactiveUS6280876B1Reduce volumeOvercome resistanceFuel and primary cellsFinal product manufactureMetal–air electrochemical cellButton battery
This invention pertains to electrode cans and metal air electrochemical cells made with the electrode cans. The invention provides improvide structure, and methods for making the outer edge of the closed end of the can at the joinder of the closed end of the can with an side wall extinding from the closed end. A substantially flat portion of the outer surface of the closed end of the can extends outwardly of the inner surface of the side wall. The electrochemical cells are assembled using improved assembly methods. Button-type electrochemical cells made using the invention are free of the inward dishing common to especially cathode cans in such button cells.
Owner:ROVCAL
Gas-shield-electrode and composite bifunctional air-electrode using the same for use in metal-air batteries
ActiveCN104662719AGood anode-cathode performanceFuel and secondary cellsCell electrodesMetal–air electrochemical cellElectrical battery
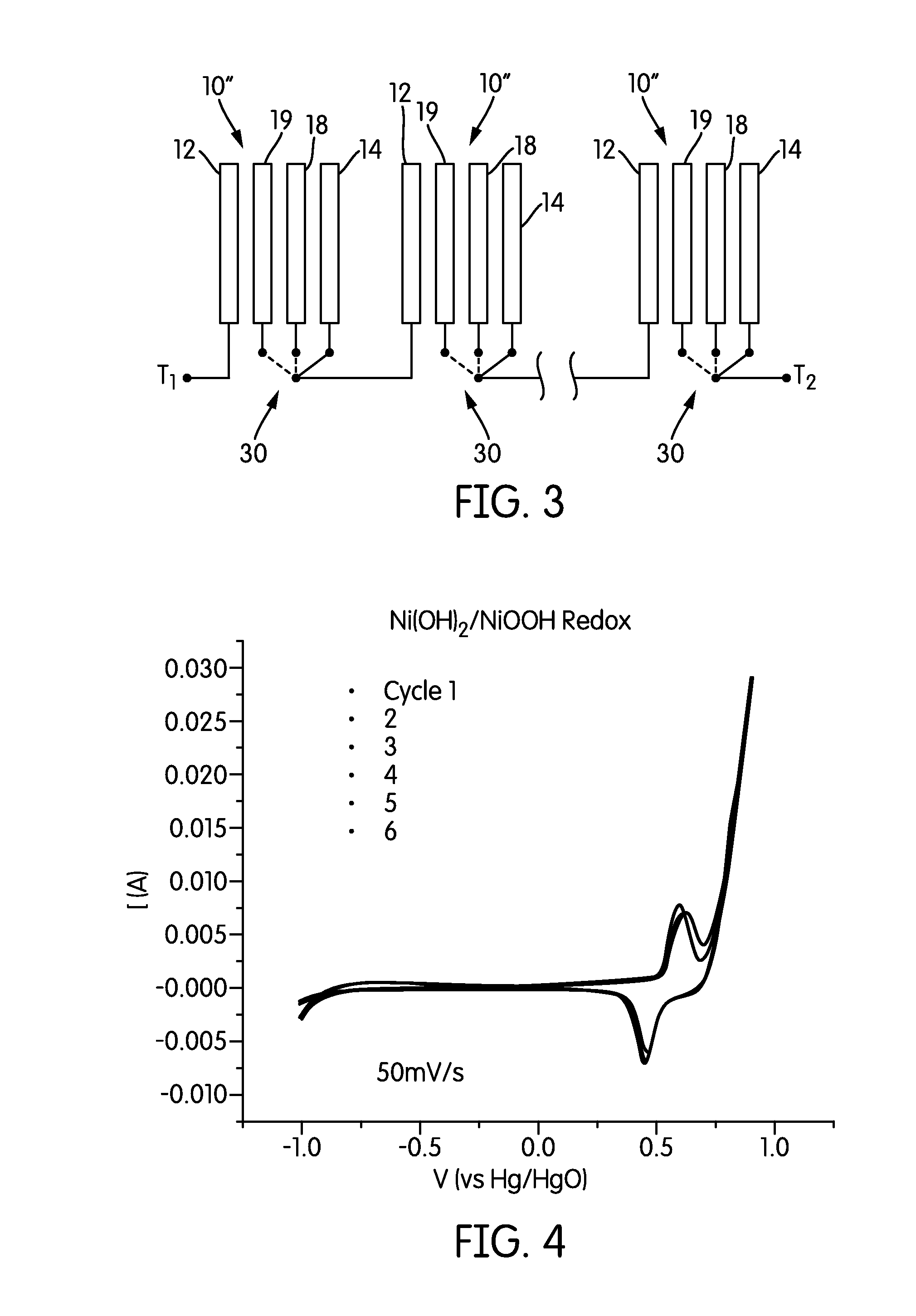
Method of operating a secondary Metal-Air electrochemical cell with a metal anode and an air cathode comprising the steps of (a) at start of a charging session, creating in less than 2 seconds an oxygen gas-shield on the electrolyte side of the air-electrode obstructing ion passage between the bulk of the electrolyte and the air-electrode; (b) charging the cell without anodic polarization of the air-electrode with the help of (i) electric conductive material placed between the electrolyte side of air-electrode and the bulk of electrolyte, and, (ii) the said oxygen gas-shield obstructing passage of ions of the electrolyte between the electrolyte side of air-electrode and the bulk of electrolyte; (c) removing the oxygen gas-shield at start of a discharging session.
Owner:AZA HLDG
Electrochemical cell having orthogonal arrangement of electrodes
ActiveUS20190348729A1Management capabilityFuel and secondary cellsLarge-sized flat cells/batteriesMetal–air electrochemical cellEngineering
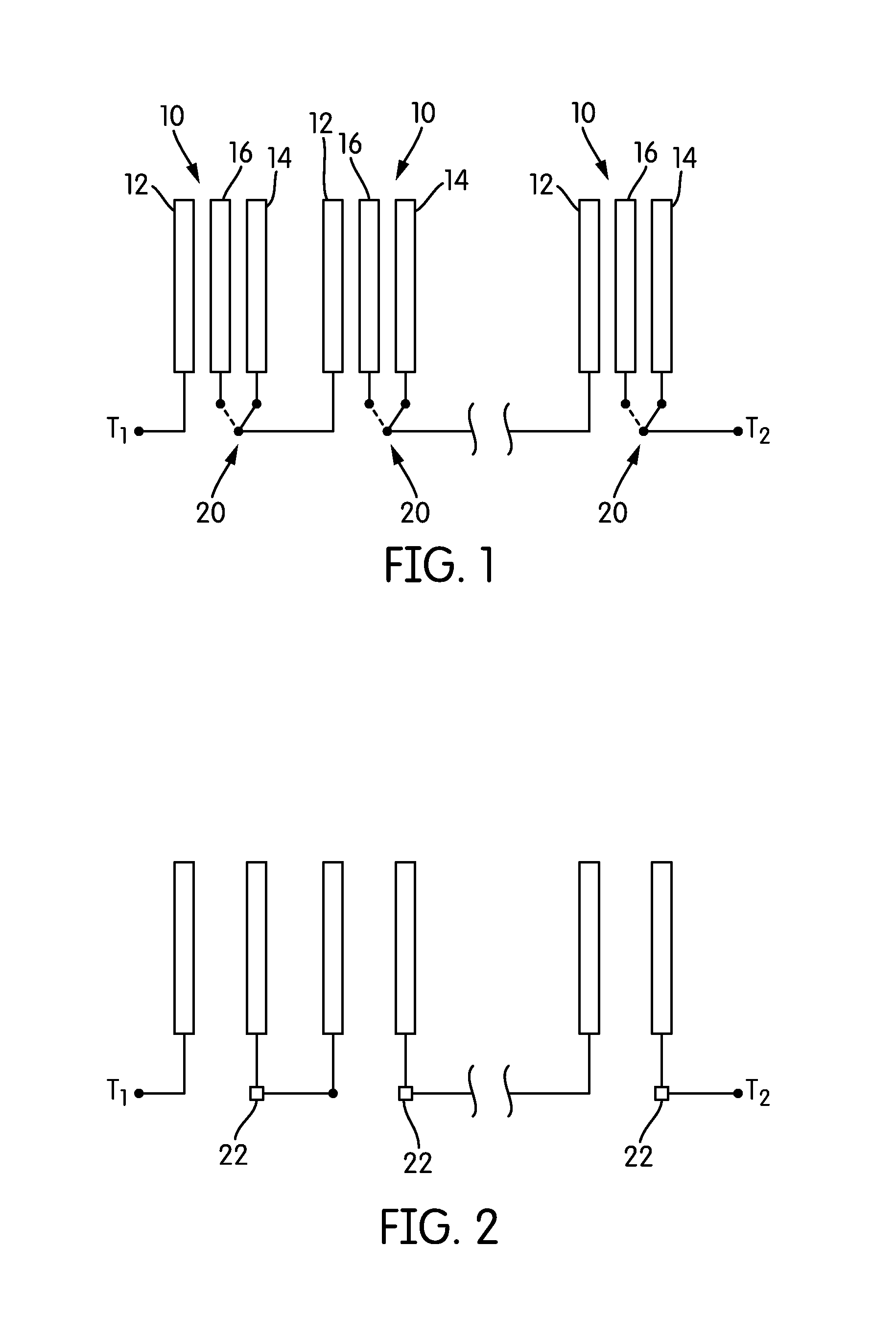
An exemplary electrochemical cell incorporates at least a first battery electrode, such as a zinc electrode, at least a second battery electrode, such as a reversible metal electrode such as nickel, and at least an oxidant reduction electrode, such as an air cathode. The oxidant reduction electrode(s) is configured in a cell housing such that it is essentially orthogonal to the first and second electrodes. The cell housing contains electrolyte therein and the electrodes are immersed, at least partially, in the electrolyte. The positioning of the oxidant reduction electrode(s) on the side of the cell and relatively perpendicular to the first and second (metal) electrodes, allows for more consistent ionic resistance across all the metal electrodes. Optionally, a fourth or oxygen evolving electrode may be provided in the cell housing, horizontal, orthogonal, and below the first and second electrodes to provide oxygen bubbles for mixing the electrolyte.
Owner:FORM ENERGY INC
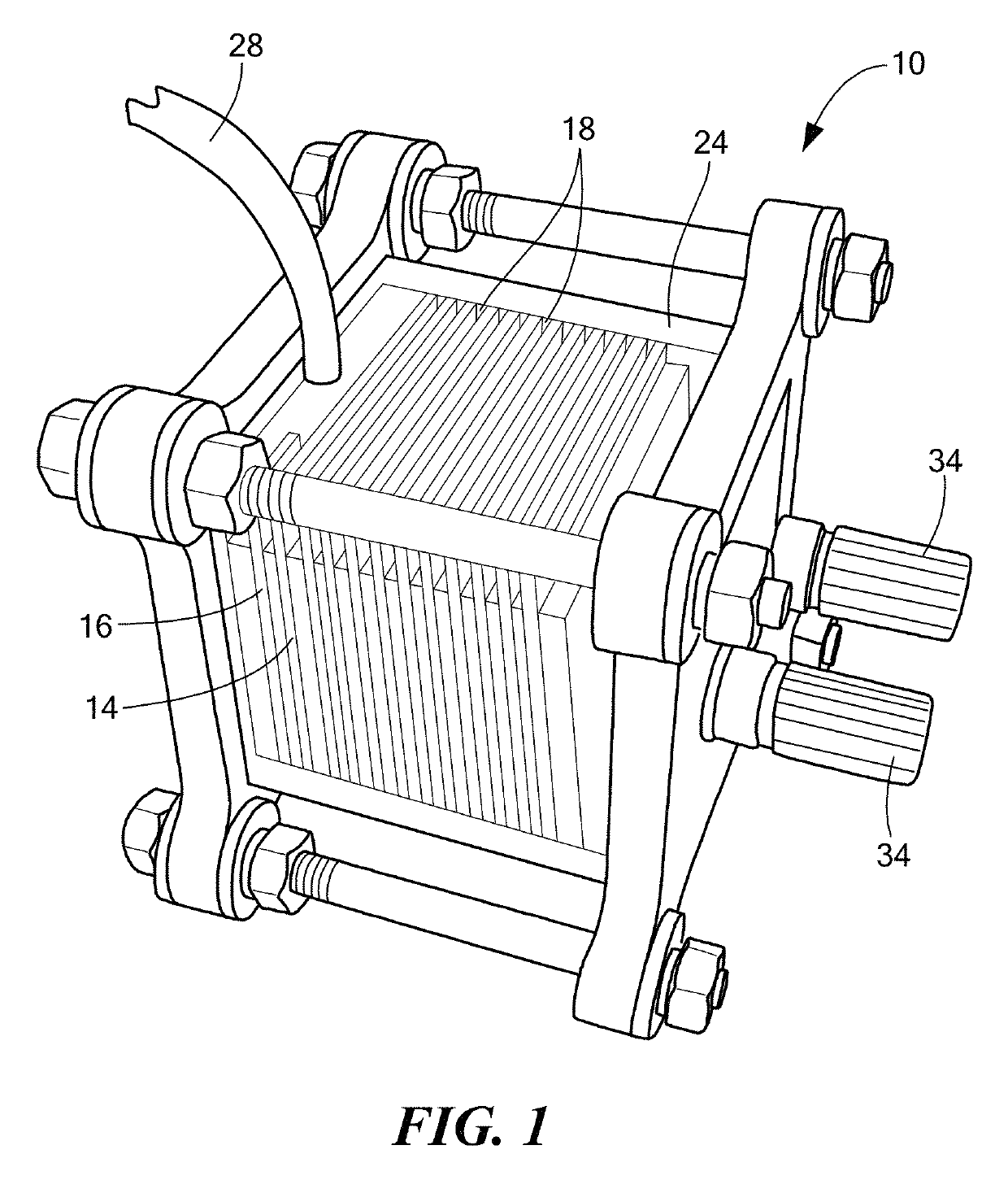
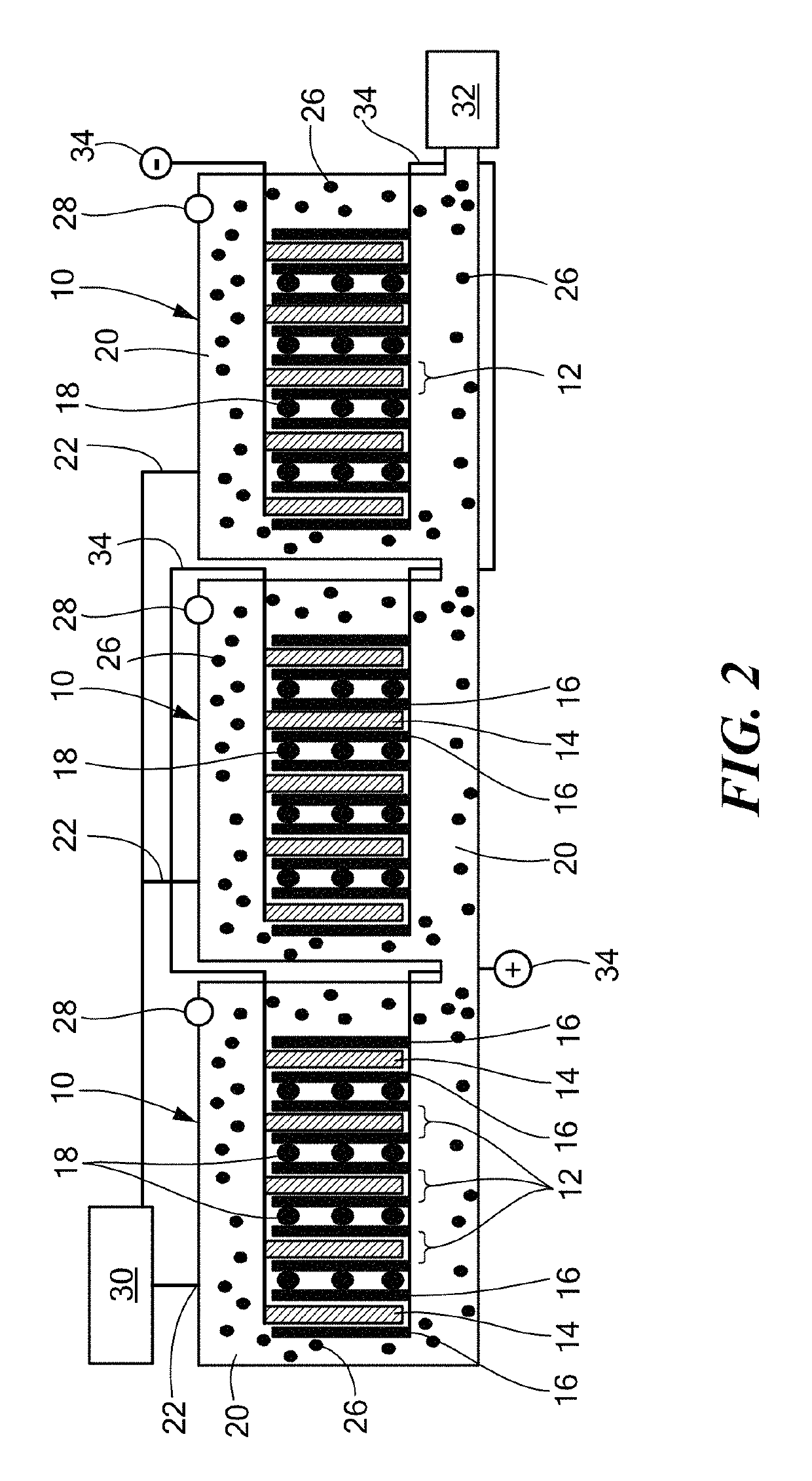
Anaerobic aluminum-water electrochemical cell
ActiveUS10516195B2Fuel and primary cellsElectrolyte moving arrangementsElectricityMetal–air electrochemical cell
An anaerobic aluminum-water electrochemical cell is provided. The electrochemical cell includes: a plurality of electrode stacks, each electrode stack featuring an aluminum or aluminum alloy anode, and at least one cathode configured to be electrically coupled to the anode; one or more physical separators between each electrode stack adjacent to the cathode; a housing configured to hold the electrode stacks, an electrolyte, and the physical separators; a water injection port, in the housing, configured to introduce water into the housing; and an amount of hydroxide base sufficient to form an electrolyte having a hydroxide base concentration of at least 0.5% to at most 13% of the saturation concentration when water is introduced between the anode and the least one cathode.
Owner:MASSACHUSETTS INST OF TECH
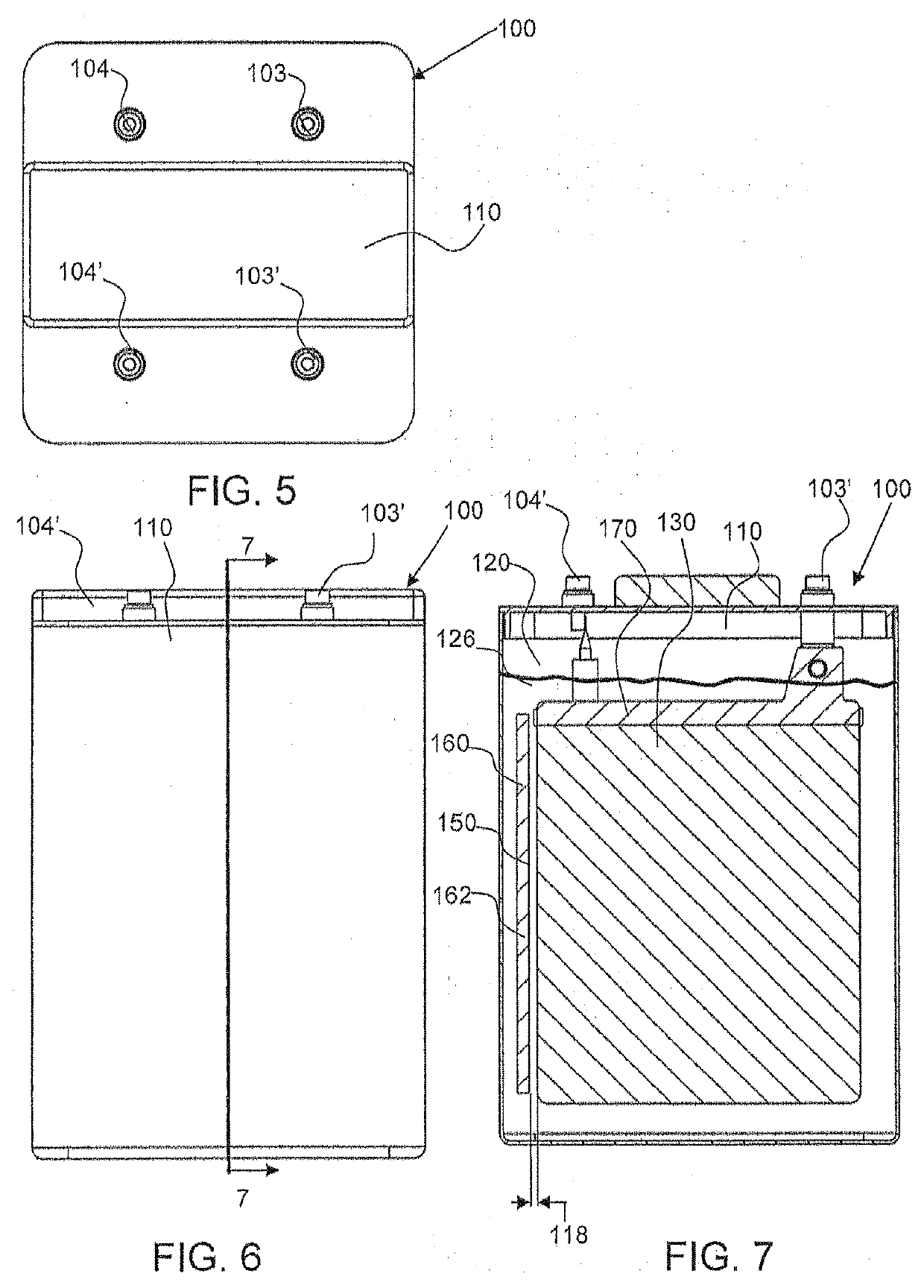
Metal air electrochemical cell architecture
PendingCN112805868AFuel and secondary cellsDeferred-action cellsElectrochemical responseMetal–air electrochemical cell
Systems and methods of the various embodiments may provide metal air electrochemical cell architectures. Various embodiments may provide a battery, such as an unsealed battery or sealed battery, with an open cell arrangement configured such that a liquid electrolyte layer separates a metal electrode from an air electrode. In various embodiments, the electrolyte may be disposed within one or more vessel of the battery such that electrolyte serves as a barrier between a metal electrode and gaseous oxygen. Systems and methods of the various embodiments may provide for removing a metal electrode from electrolyte to prevent self-discharge of the metal electrode. Systems and methods of the various embodiments may provide a three electrode battery configured to operate each in a discharge mode, but with two distinct electrochemical reactions occurring at each electrode.
Owner:FORM ENERGY INC
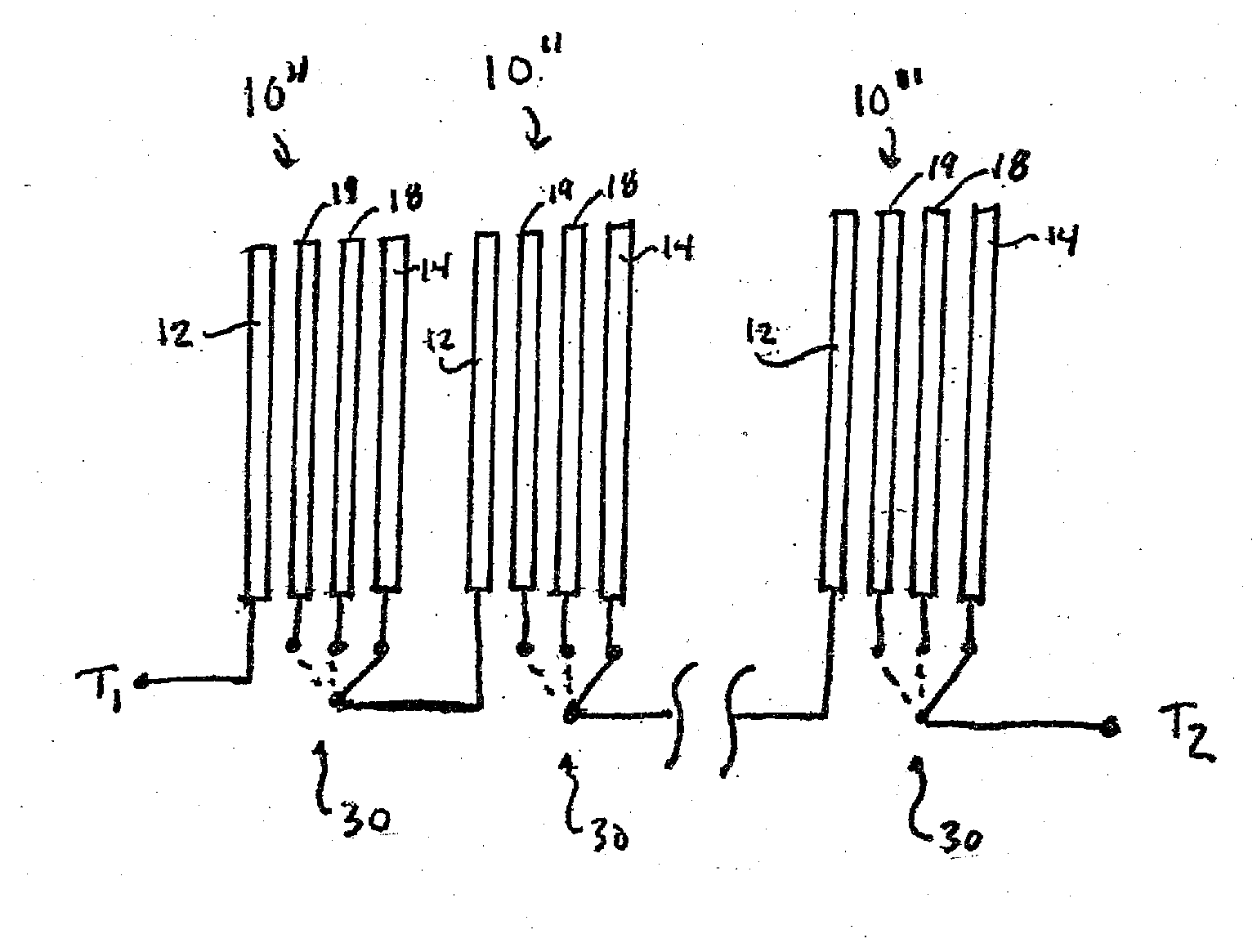
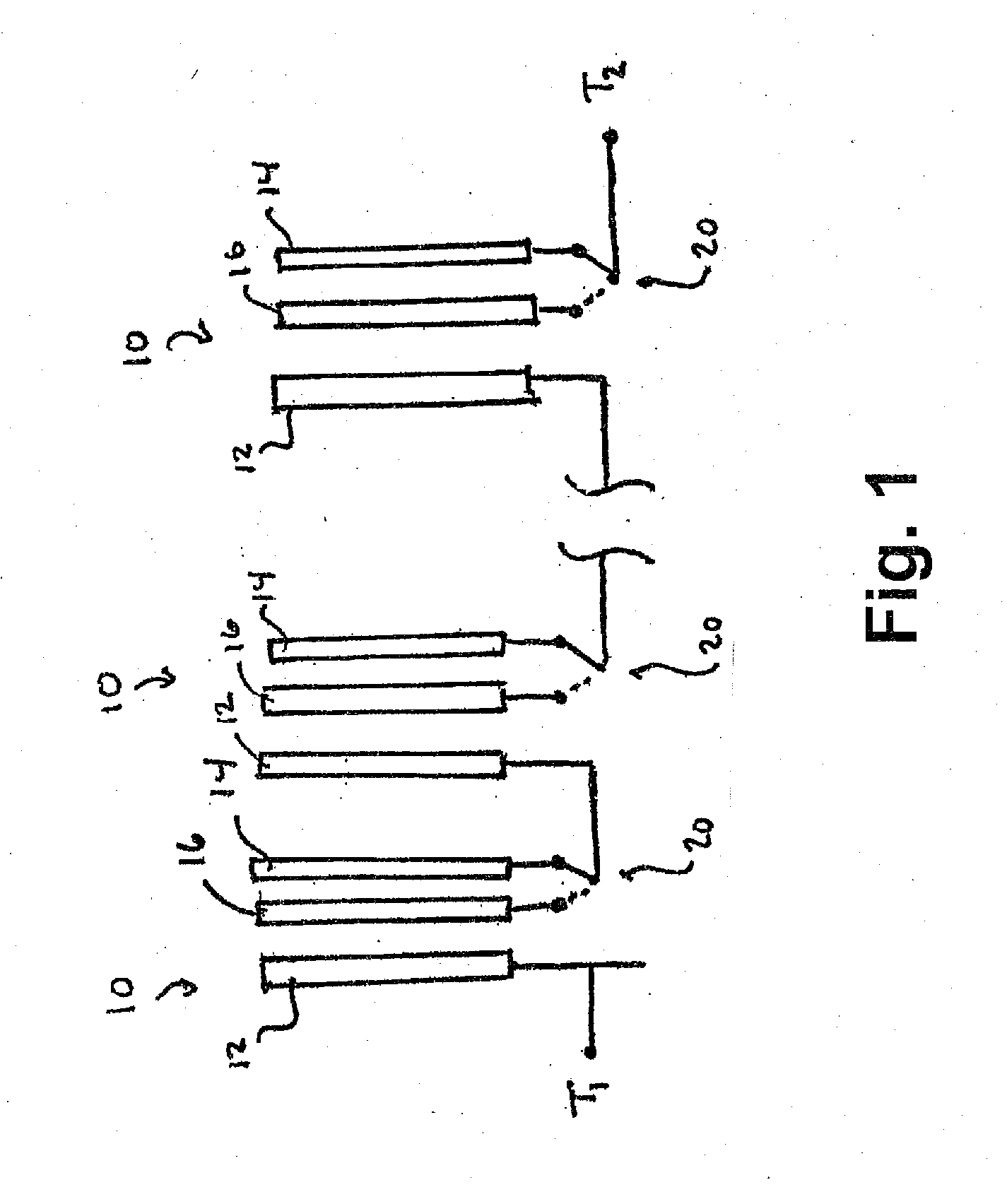
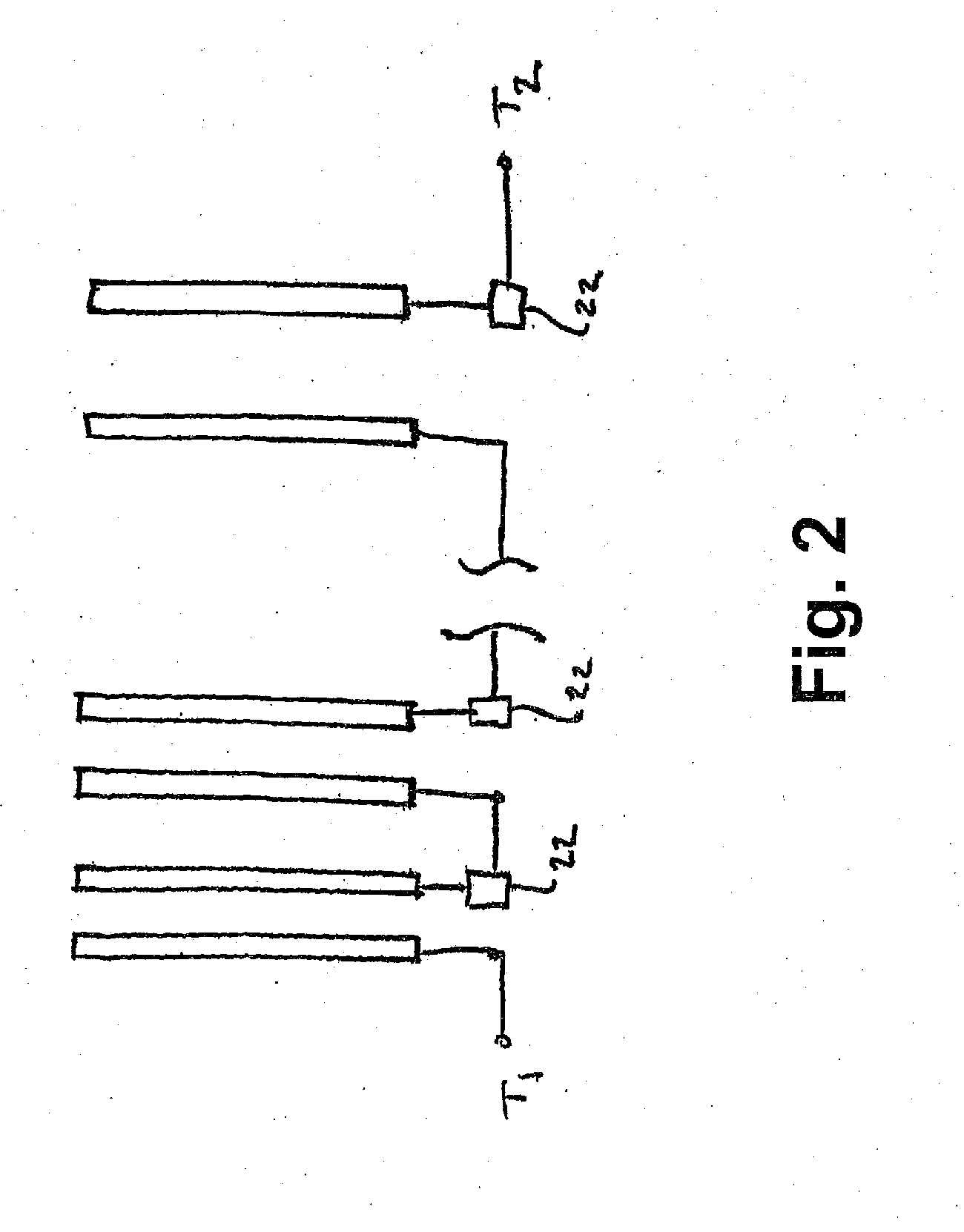
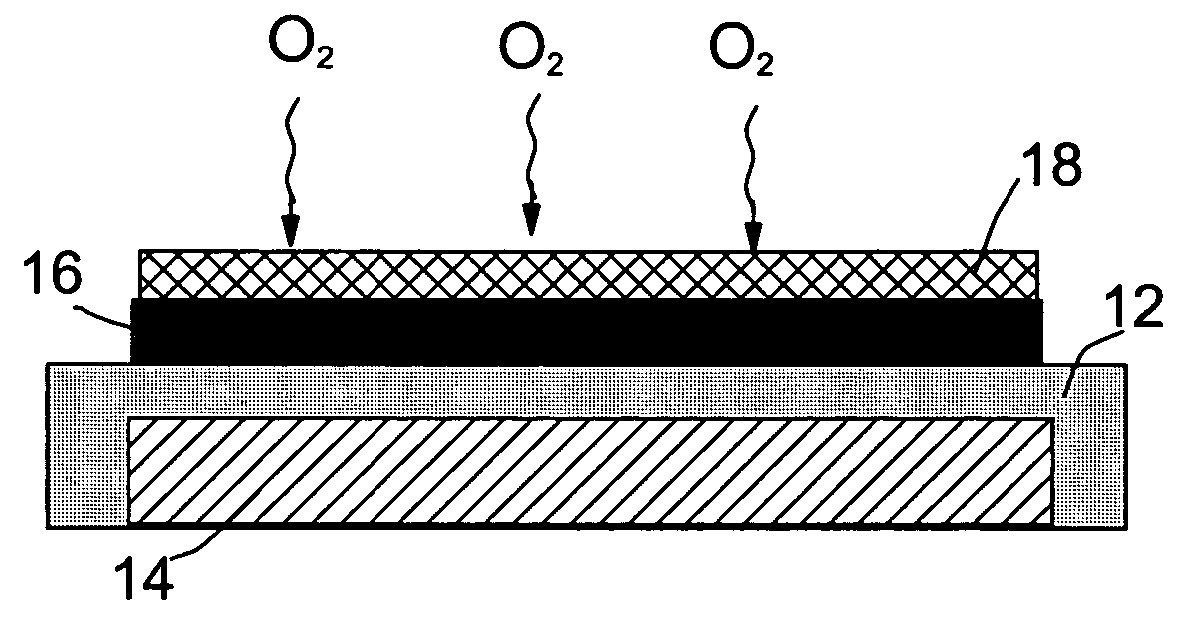
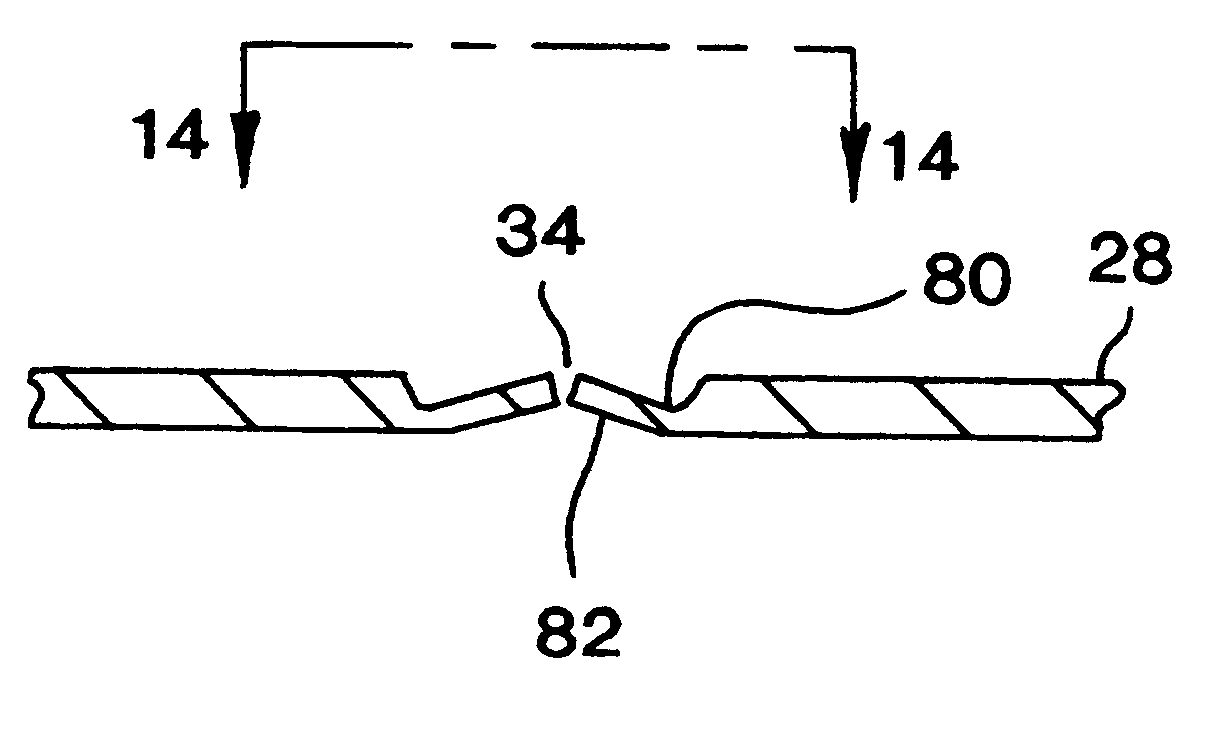
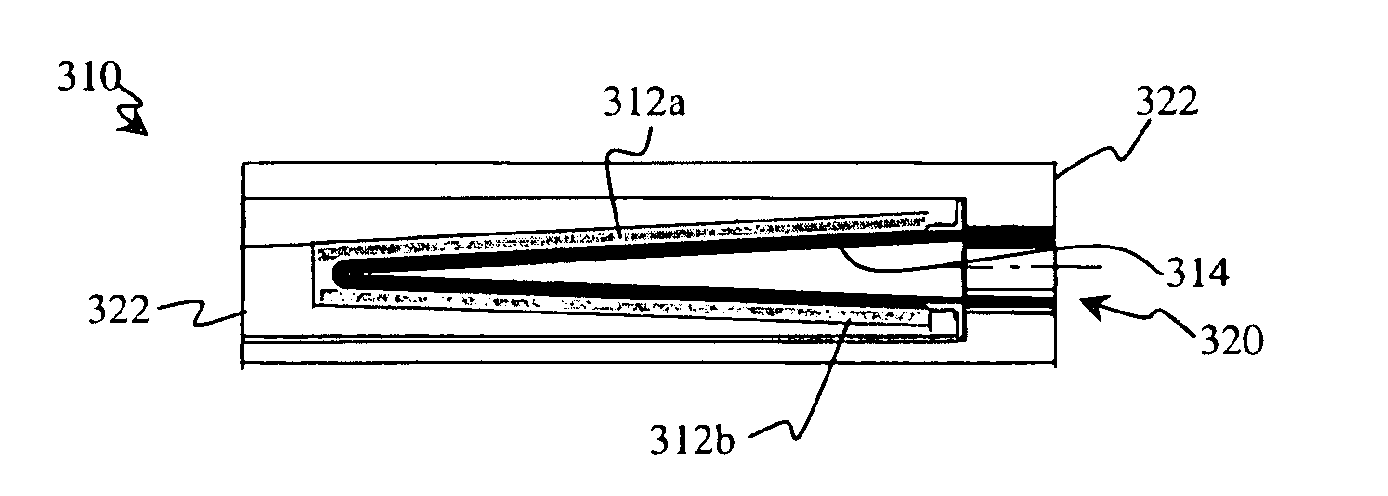
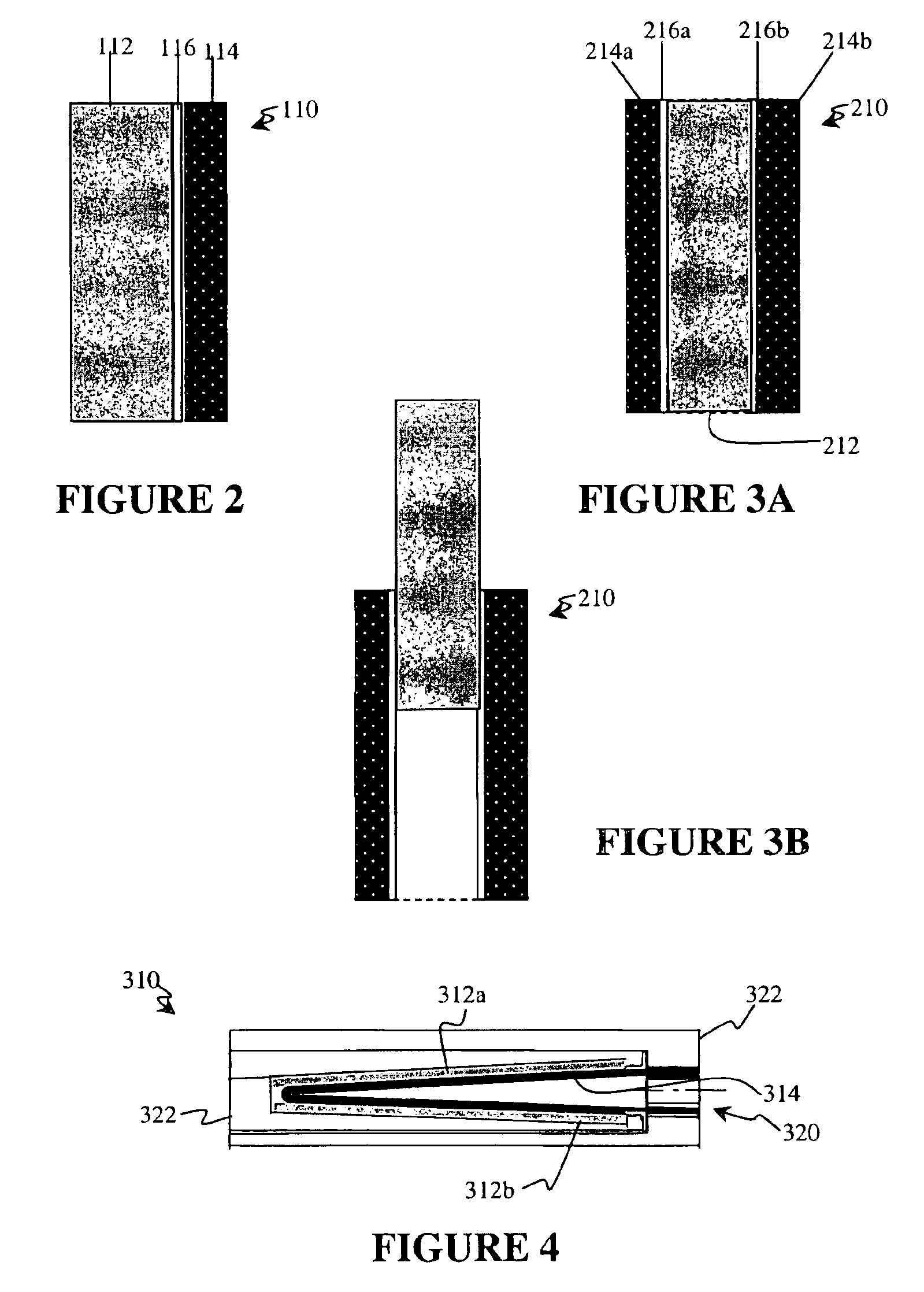
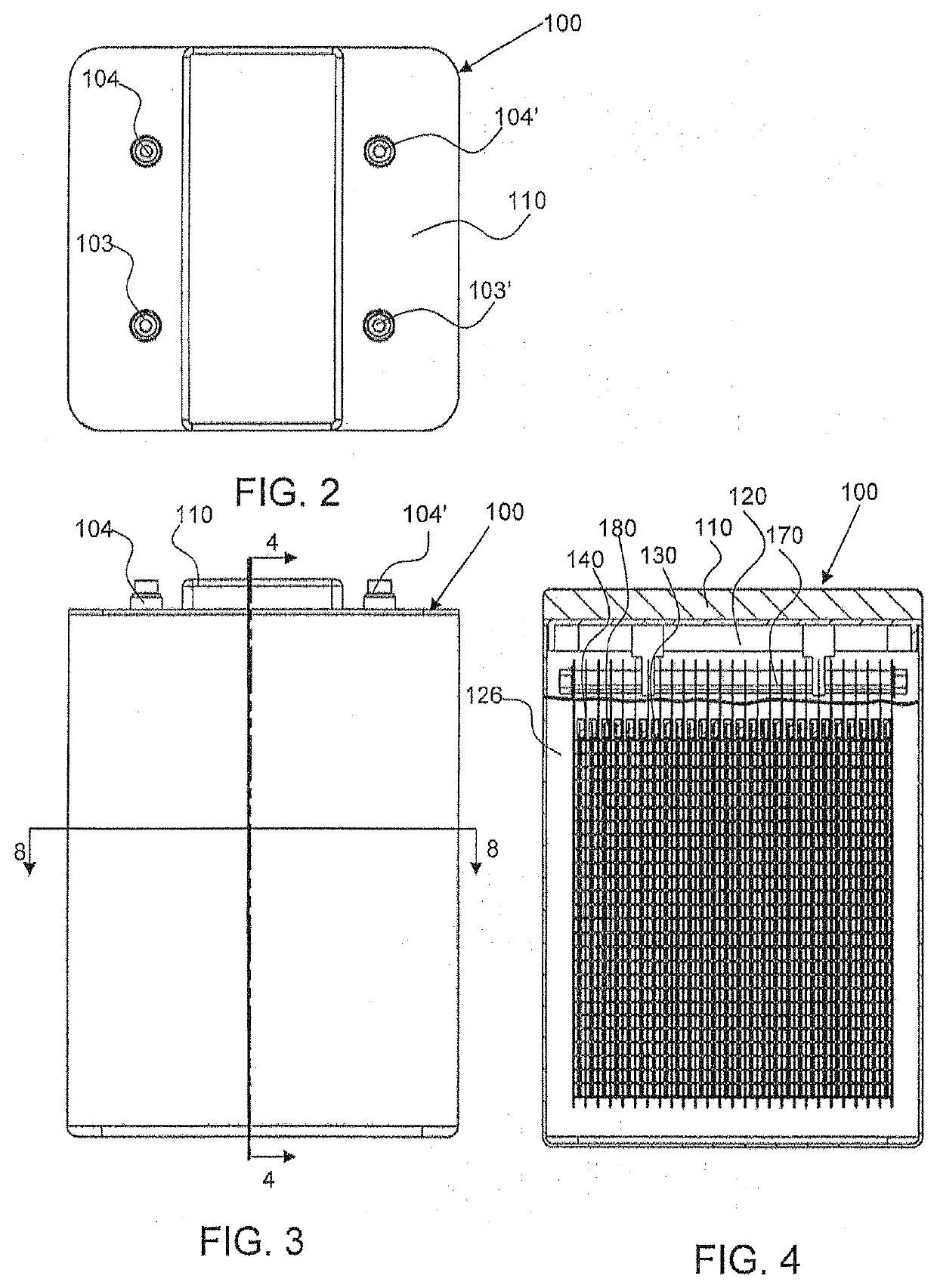
Tab system for a metal-air electrochemical cell
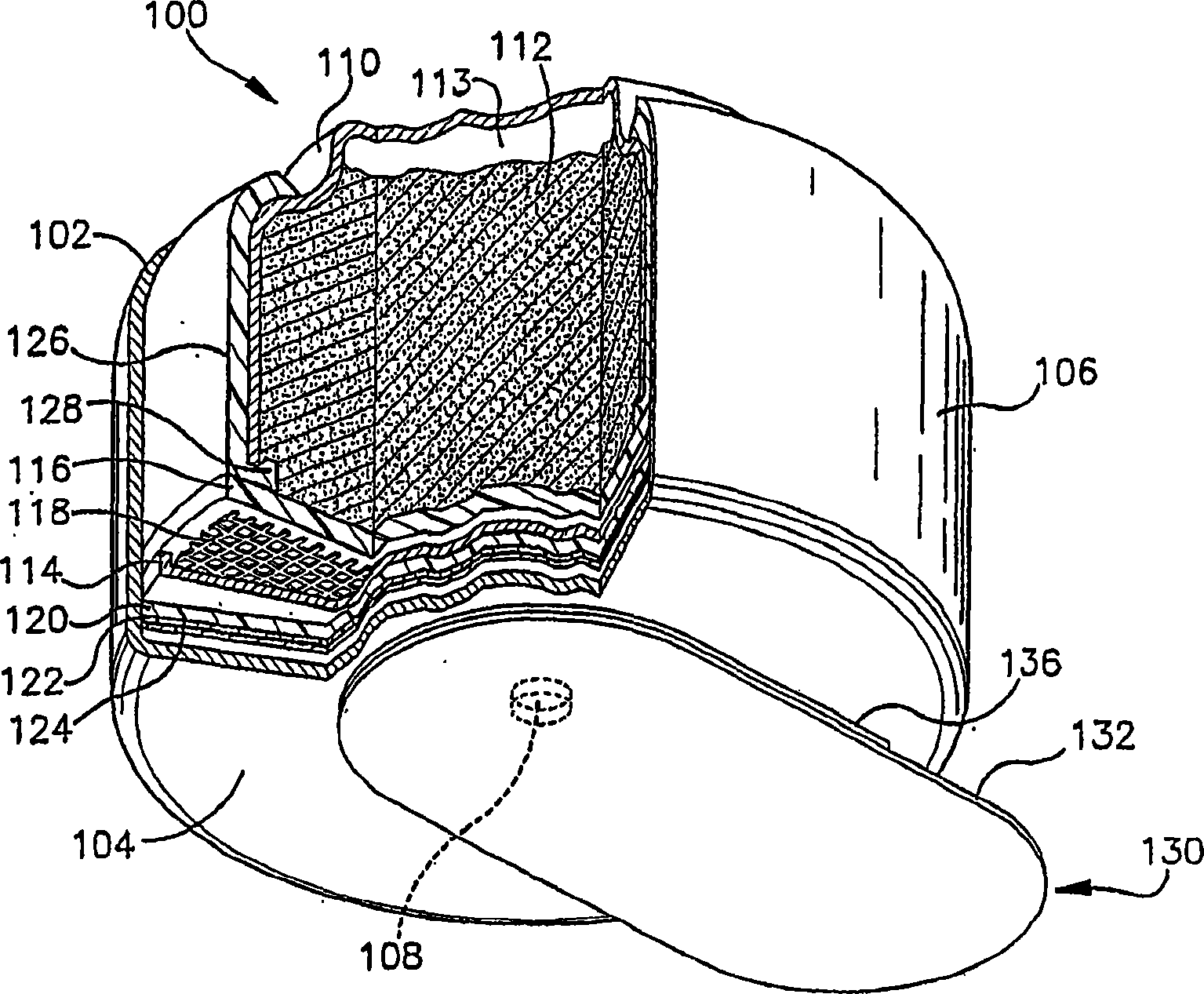
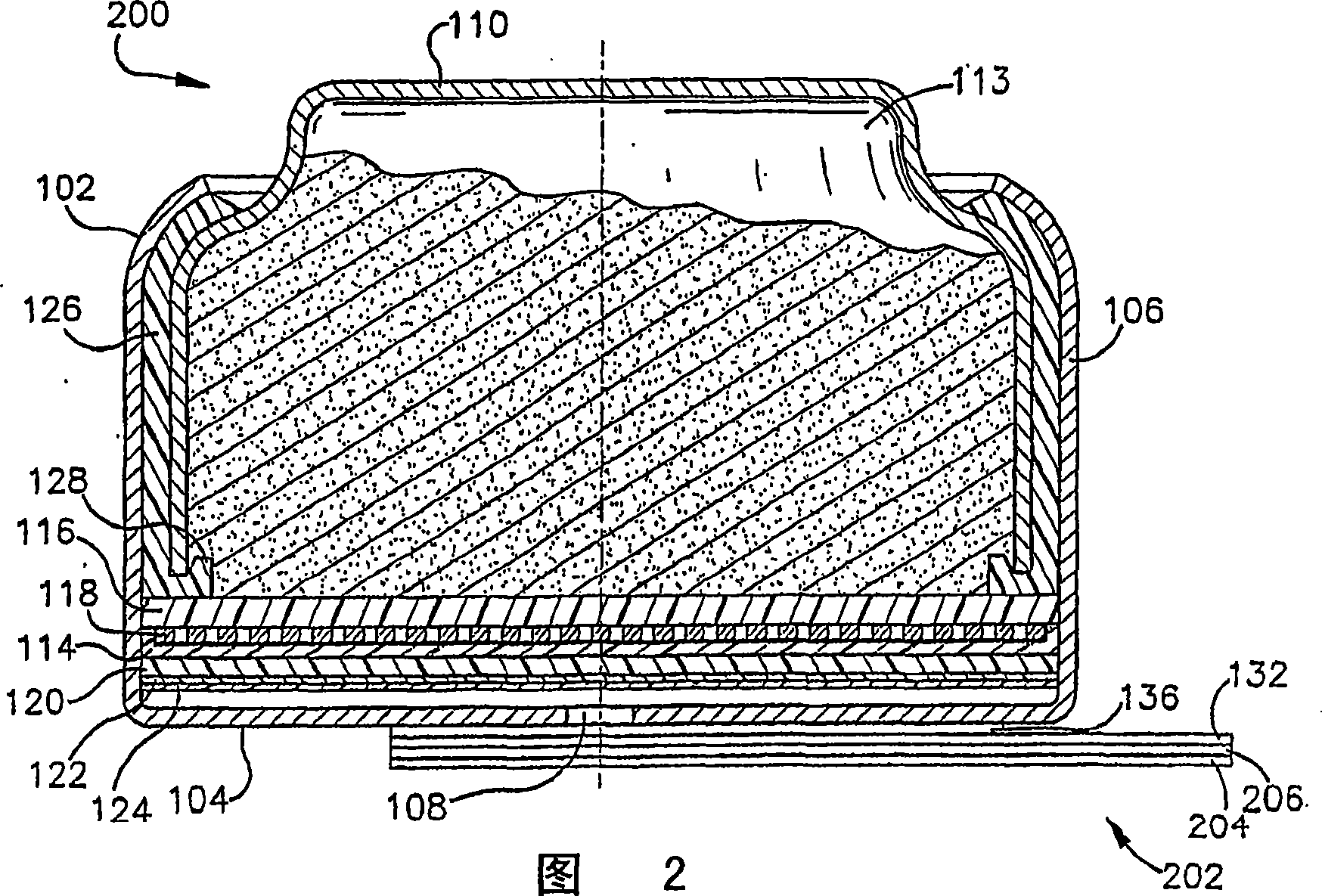
ActiveCN1918742AFuel and primary cellsDeferred-action cellsMetal–air electrochemical cellEngineering
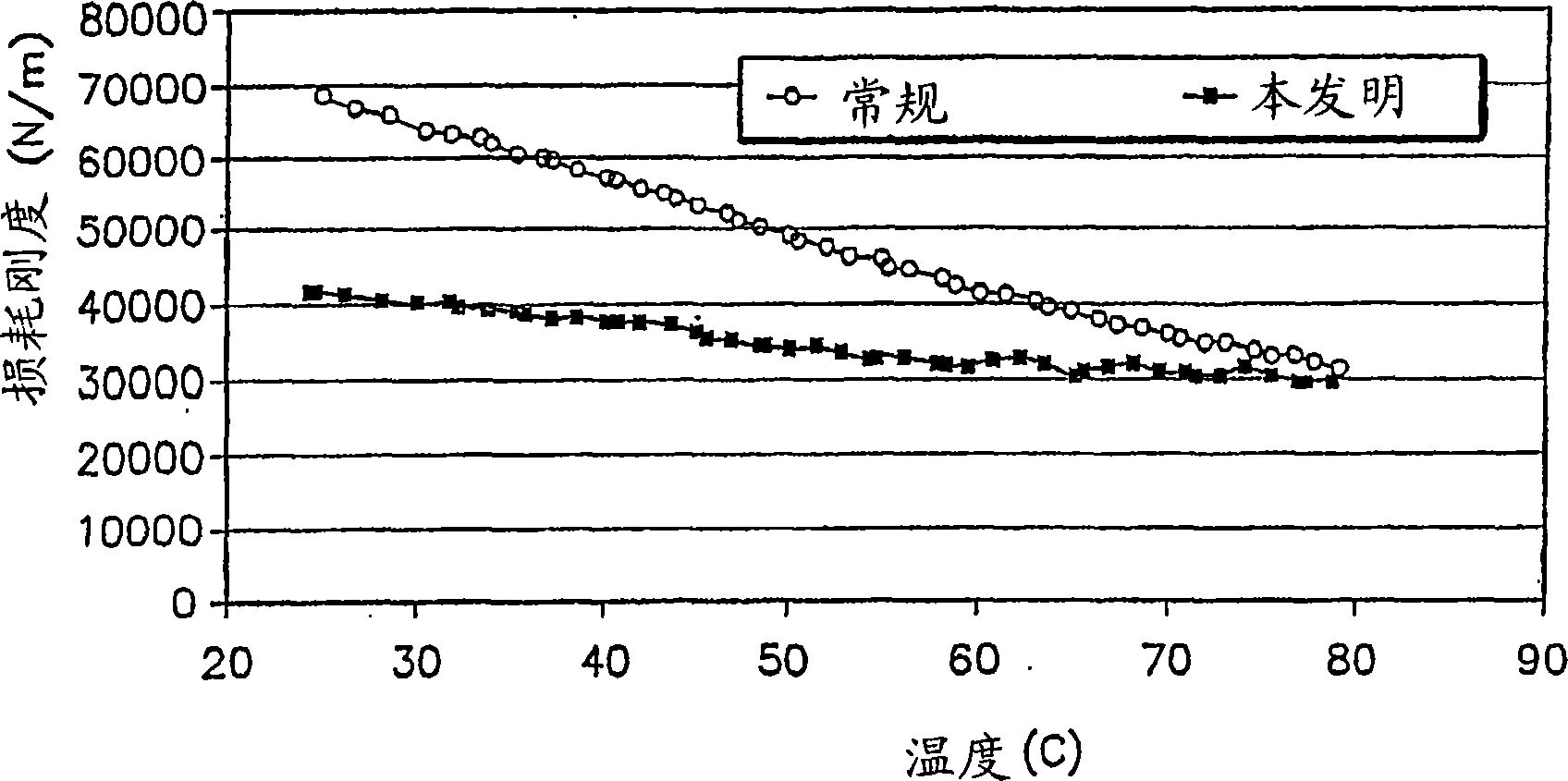
Various embodiments of a metal-air cell having a tab system that covers an air entry port of the metal-air cell are provided. In one representative embodiment the tab system includes polymer layer and an adhesive layer between the metal-air cell and the polymer layer. The tab system has a loss stiffness of less than 55,000 N / m at 20° C. to 25° C.
Owner:ENERGIZER BRANDS
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com