Method and system for efficiently operating electrochemical cells
An electrochemical and battery technology, applied in the field of operating electrochemical cells and systems efficiently, can solve the problems of reducing the overall electrical efficiency of electrochemical cells, hindering the electrical efficiency of batteries, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 2
[0143] Table 1 compares the ohmic voltage drop that occurs during typical operation of a conventional alkaline electrolyzer, a PEM electrolyzer, and an electrolyzer of an embodiment of the present invention.
[0144] It should be noted that even the above-exemplified PEM electrolyzer would operate at one-twentieth of its normal operating current density (i.e., 90 mA / cm 2 , which would probably not be economically viable), it would still experience a higher voltage drop than the alkaline electrolyzer described above.
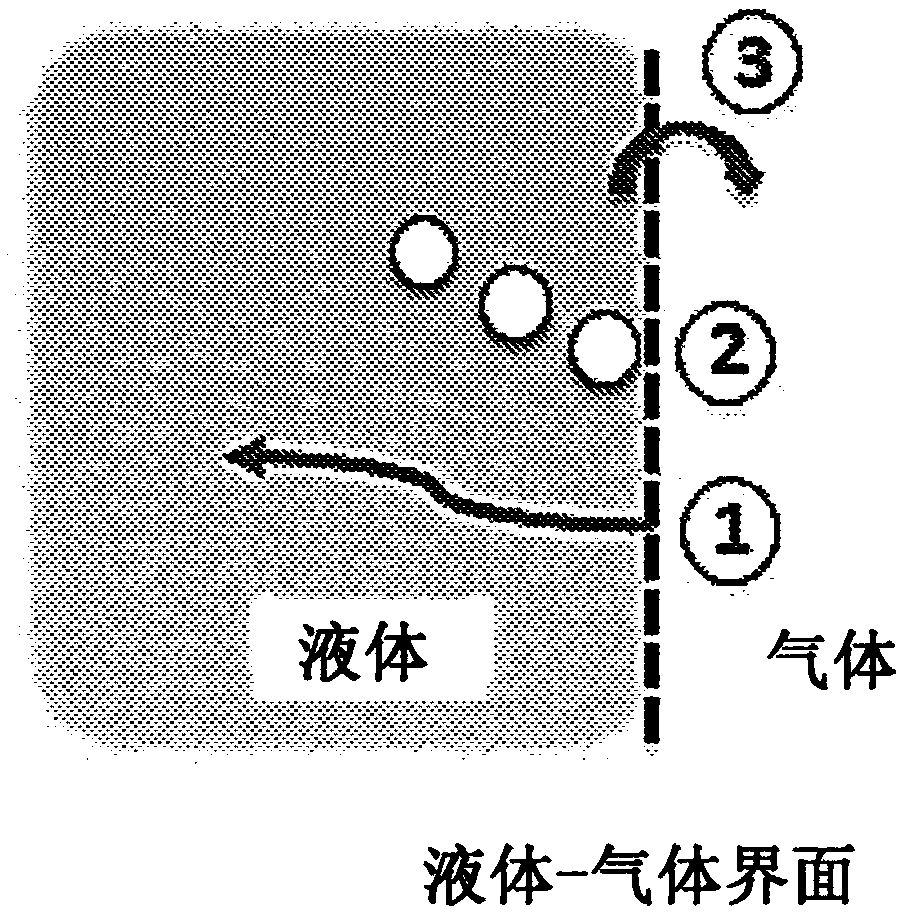
[0145]Generalizing these concepts, embodiments relate to electrochemical cells and methods of use or operation in which one or more gas generating electrodes operate in a bubble-free or substantially bubble-free manner. Electrochemical cells do not have a separator between the gas generating electrodes. Preferably, electrochemical cells utilize specific catalyst-electrolyte systems. Electrochemical cells are optimized to determine the best settings for differen...
example 1
[0465] Example 1: Empty volume and its placement
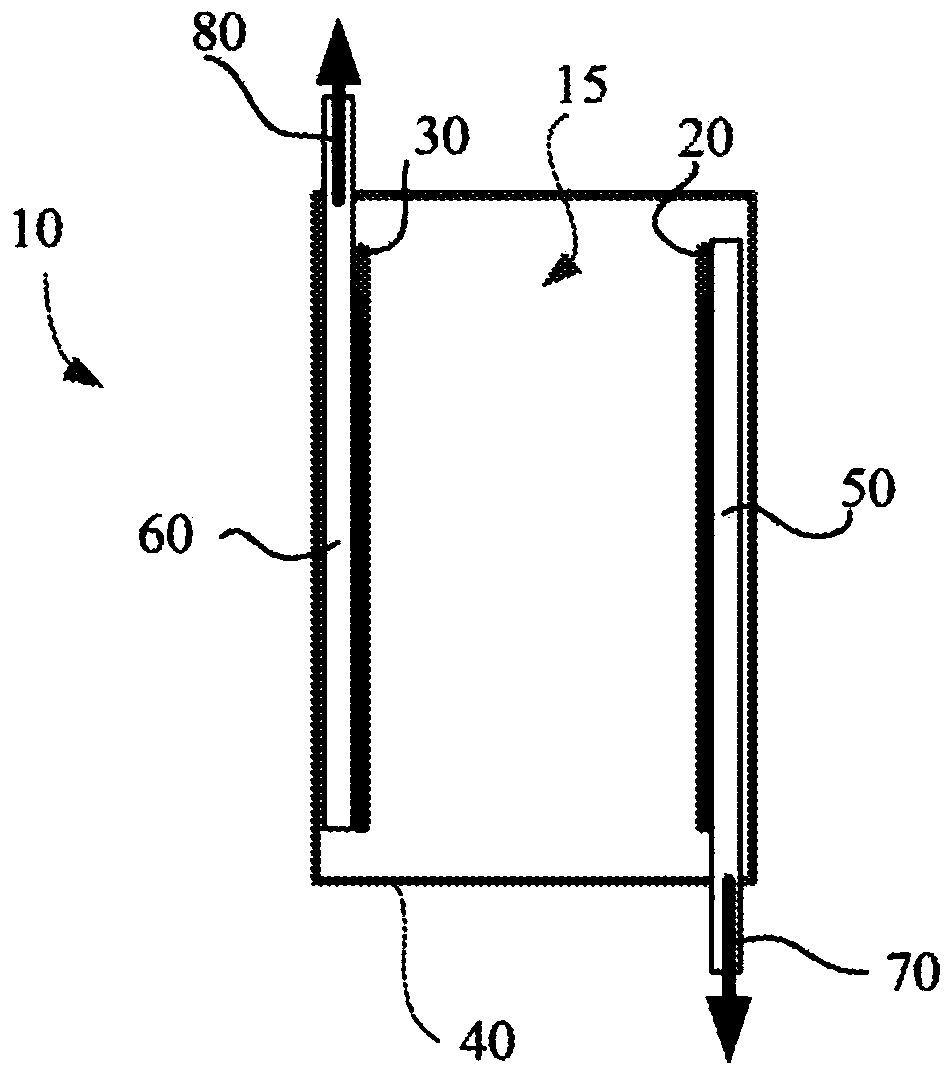
[0466] Preferably, but not exclusively, the void volume is provided by a porous structure that is impermeable to electrolyte (eg liquid electrolyte) but admits or allows the passage of gas, ie the porous structure is liquid impermeable and gas permeable. In the case of an aqueous liquid electrolyte, the void volume is preferably, but not exclusively, filled by a porous hydrophobic structure (eg, made of PTFE), such as a porous hydrophobic assembly, membrane, or hollow fiber, or a collection of such structures. Provided, it remains unfilled with liquid electrolyte during operation of the cell. In such cases, the void volume may be considered to be "artificial air bubbles" or "artificial air bubbles". Preferably, "artificial bubbles" or "artificial" bubbles are located outside the conductive pathways of the cell, or occupy only a small cross-sectional area or footprint within the conductive pathways.
[0467] In an alternative...
example 3
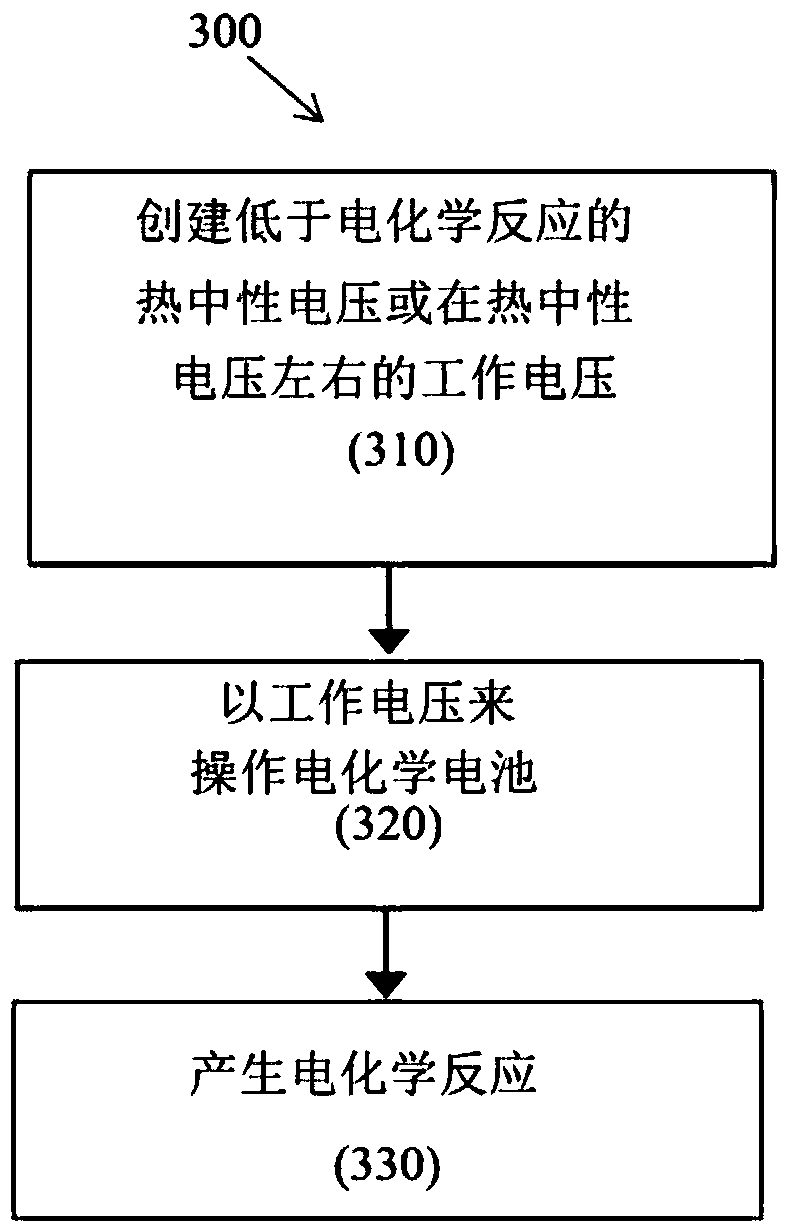
[0505] Example 3: Efficient Operation of Example Embodiment Electrochemical Cells Using Catalysts Operating Below Thermal Neutral Voltage to Promote Endothermic Electrochemical Reactions. Catalysts and catalytic materials are preferred.
[0506] In a preferred embodiment, void volumes may be associated with electrodes. That is, the void volume may form the gas side of the gas diffusion electrode, where the gas side of the electrode is outside or substantially outside the conductive path of the cell between the electrodes, and where the gas side of the gas diffusion electrode facilitates the movement of gas into or out of the cell. Gas-diffusion electrodes can be used to transport gases generated rapidly and suddenly at the electrodes out of the cell. Examples of such batteries include electrosynthesis or electrical energy batteries.
[0507]Example electric batteries of the type described herein and including but not limited to in WO2013 / 185170, WO2015 / 013764, WO2015 / 013765,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com