Patents
Literature
103 results about "Electrosynthesis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Electrosynthesis in chemistry is the synthesis of chemical compounds in an electrochemical cell. The main advantage of electrosynthesis over an ordinary redox reaction is selectivity and yield which result from control of the cell potential. Electrosynthesis is actively studied as a science and also has industrial applications. Electro-oxidation has potential for wastewater treatment as well.
Nanostructured powders and related nanotechnology
InactiveUS7081267B2Low costReduce operating costsNitrogen compoundsSelenium/tellurium compundsThermal energyOxygen
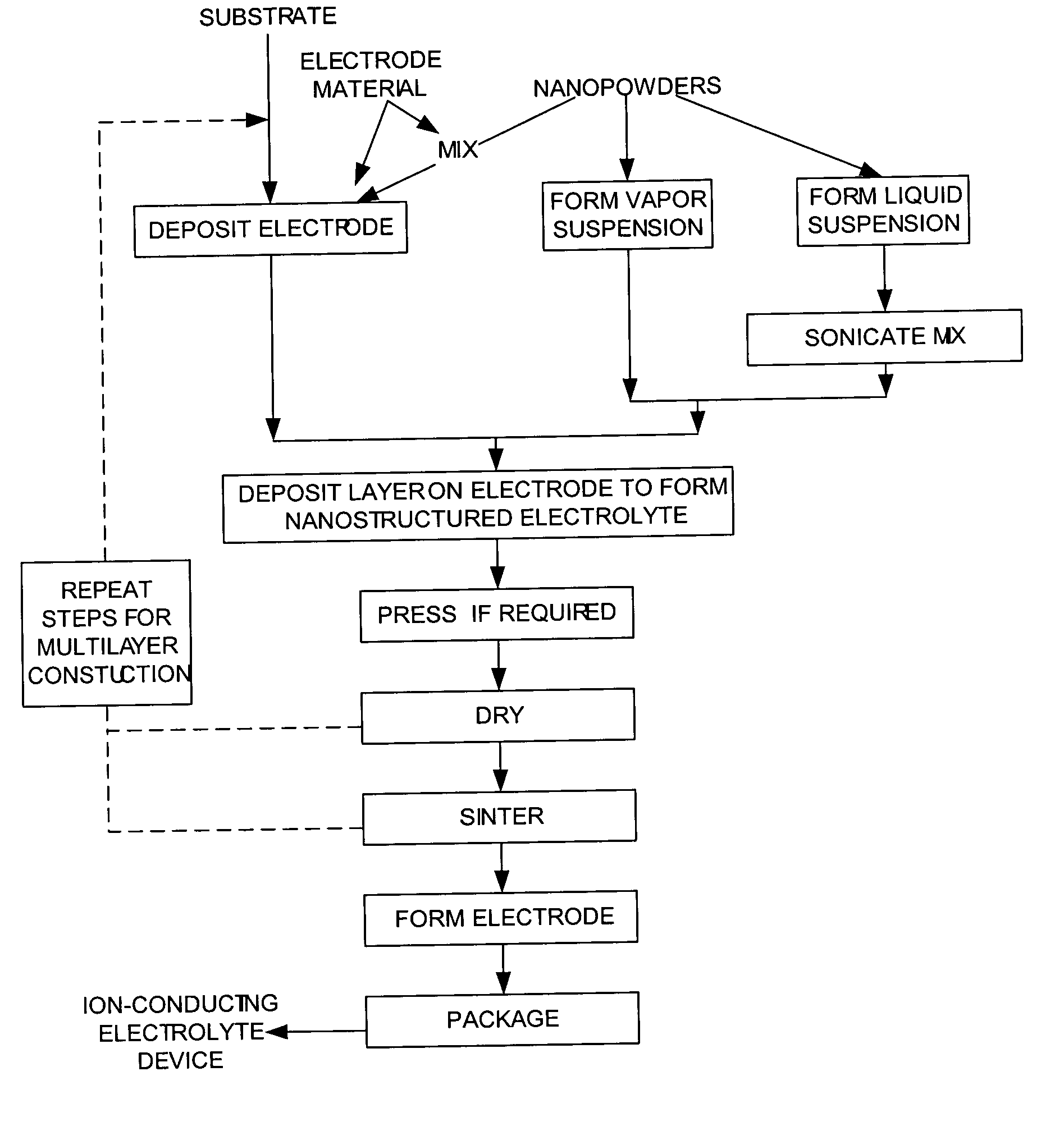
Methods to manufacture nanoscale particles comprising metals, alloys, intermetallics, ceramics are disclosed. The thermal energy is provided by plasma, internal energy, heat of reaction, microwave, electromagnetic, direct electric arc, pulsed electric arc and / or nuclear. The process is operated at some stage above 3000K and at high velocities. The invention can be utilized to prepare nanopowders for nanostructured products and devices such as ion conducting solid electrolytes for a wide range of applications, including sensors, oxygen pumps, fuel cells, batteries, electrosynthesis reactors and catalytic membranes.
Owner:PPG IND OHIO INC
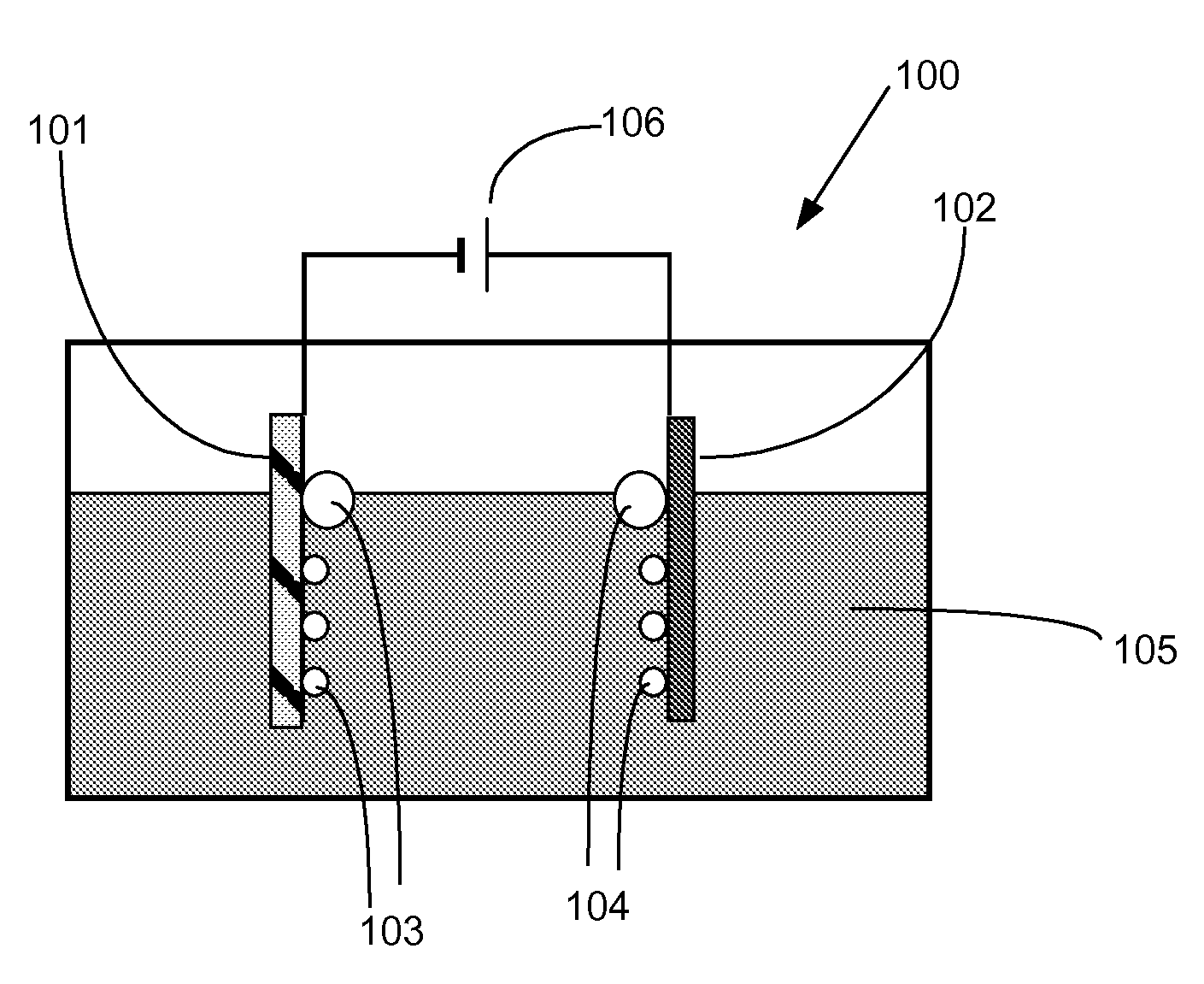
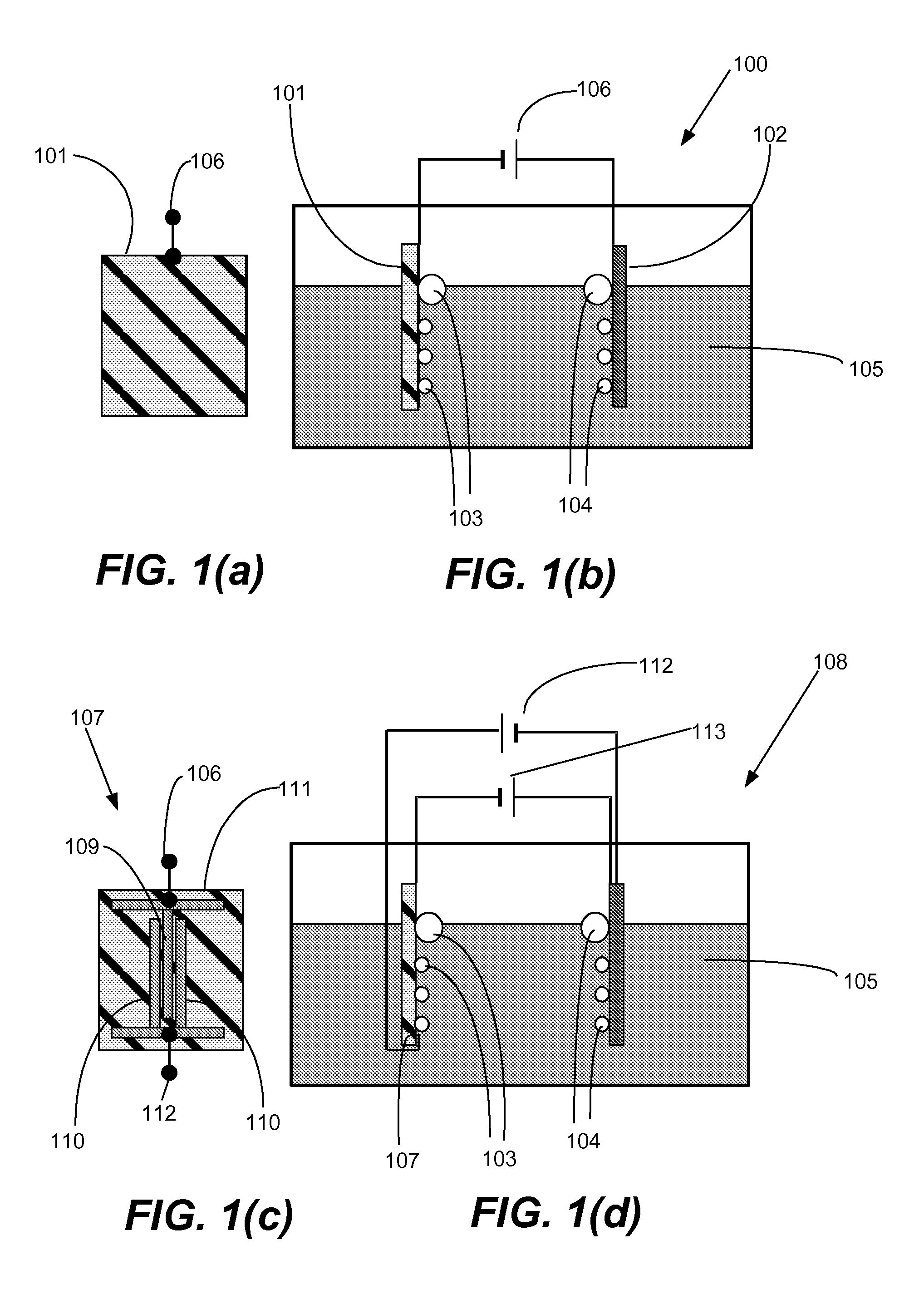
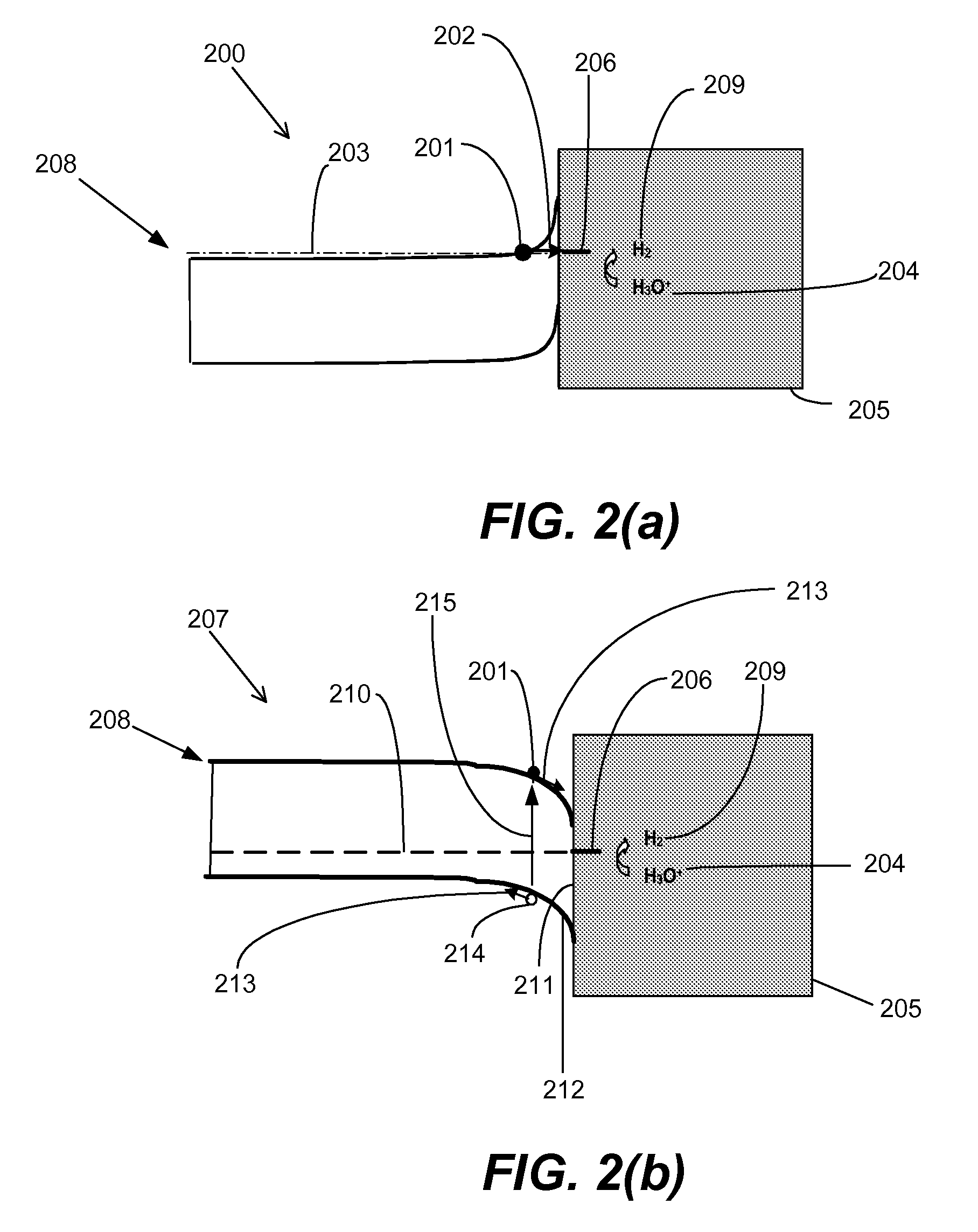
Gated electrodes for electrolysis and electrosynthesis
InactiveUS20080116080A1Good effectEnhanced charge transferCellsPhotography auxillary processesOvervoltageElectricity
A gated electrode structure for altering a potential and electric field in an electrolyte near at least one working electrode is disclosed. The gated electrode structure may comprise a gate electrode biased appropriately with respect to a working electrode. Applying an appropriate static or dynamic (time varying) gate potential relative to the working electrode modifies the electric potential and field in an interfacial region between the working electrode and the electrolyte, and increases electron emission to and from states in the electrolyte, thereby facilitating an electrochemical, electrolytic or electrosynthetic reaction and reducing electrode overvoltage / overpotential.
Owner:RGT UNIV OF CALIFORNIA
Rechargeable metal-air redox flow battery combining electrochemical preparation
InactiveCN101714680AImprove power utilization efficiencyIncrease energy densityFuel and primary cellsOxygenZinc–air battery
The invention relates to a rechargeable metal-air redox flow battery compatible with electrochemical preparation. When the battery is charged, electric energy input makes metal ions in the electrolyte flowing through a negative electrode reduced and deposited into metal to prepare for discharge, and the electric energy also makes reductive raw materials in the electrolyte flowing through a positive electrode oxidized into a product required to be synthesized. When the battery is discharged, the positive electrode is introduced with air or oxygen, the air or the oxygen is reduced on the electrode, and the positive electrode and a deposition redox flow metal negative electrode form the metal-air redox flow battery to output electric energy. In each charge and discharge cycle, the accumulation and discharge of the metal-air redox flow battery are carried out, and simultaneously electrooxidation chemical production is carried out so as to achieve the effect that one portion of electric energy is consumed and two functions are realized. The redox flow battery combines two functions of rechargeable metal-air accumulation and electrochemical preparation, realizes the combination of electrosynthesis, a deposition redox flow battery and a rechargeable metal-air battery, achieves the effects of energy conservation and high efficiency, improves the utilization rate of the electric energy and has good application prospect.
Owner:NO 63971 TROOPS PLA
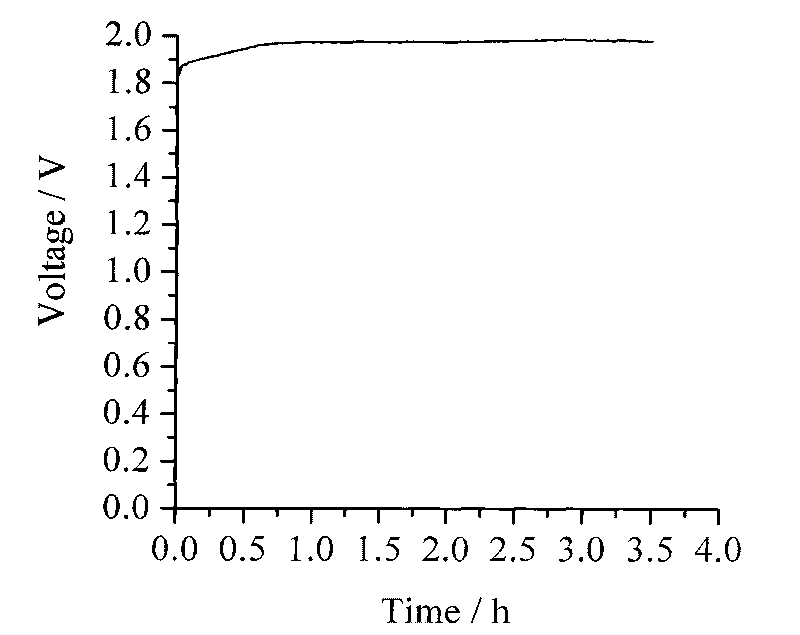
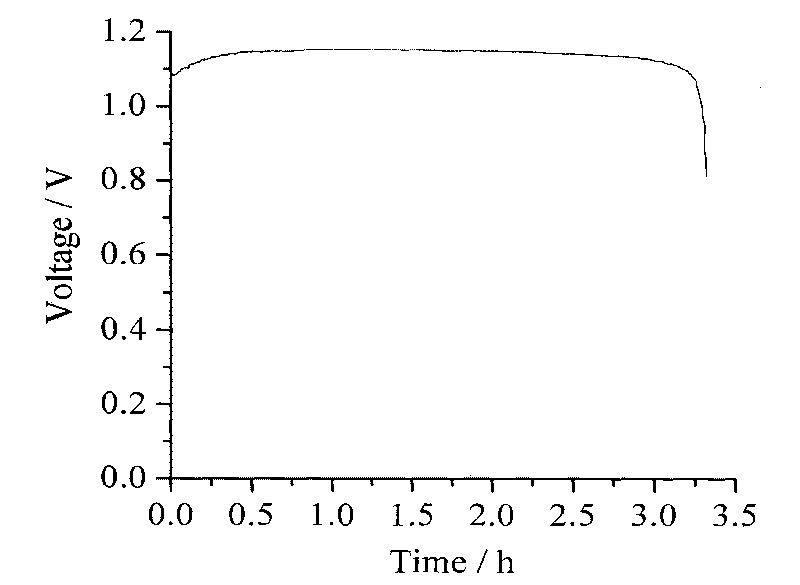
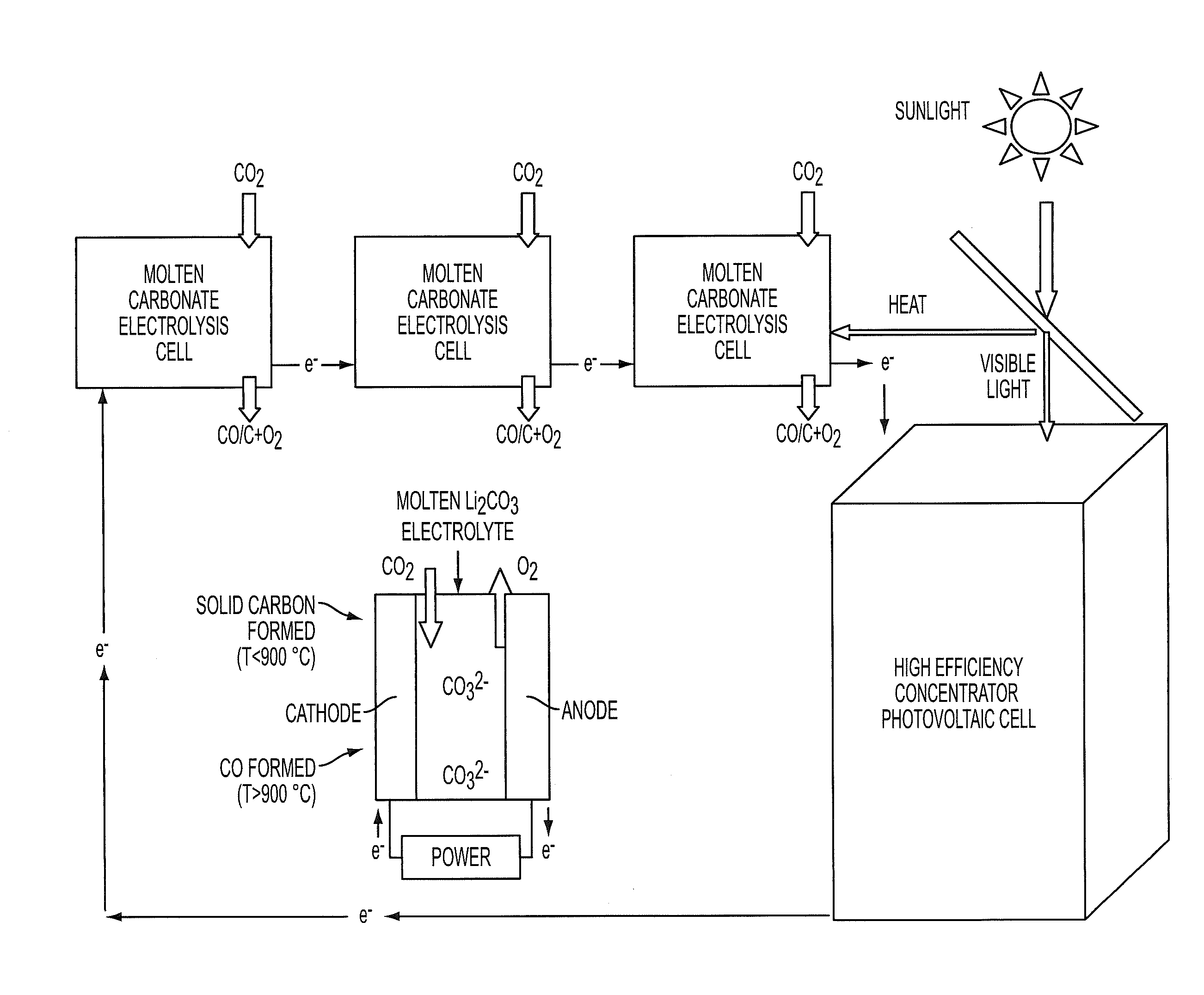
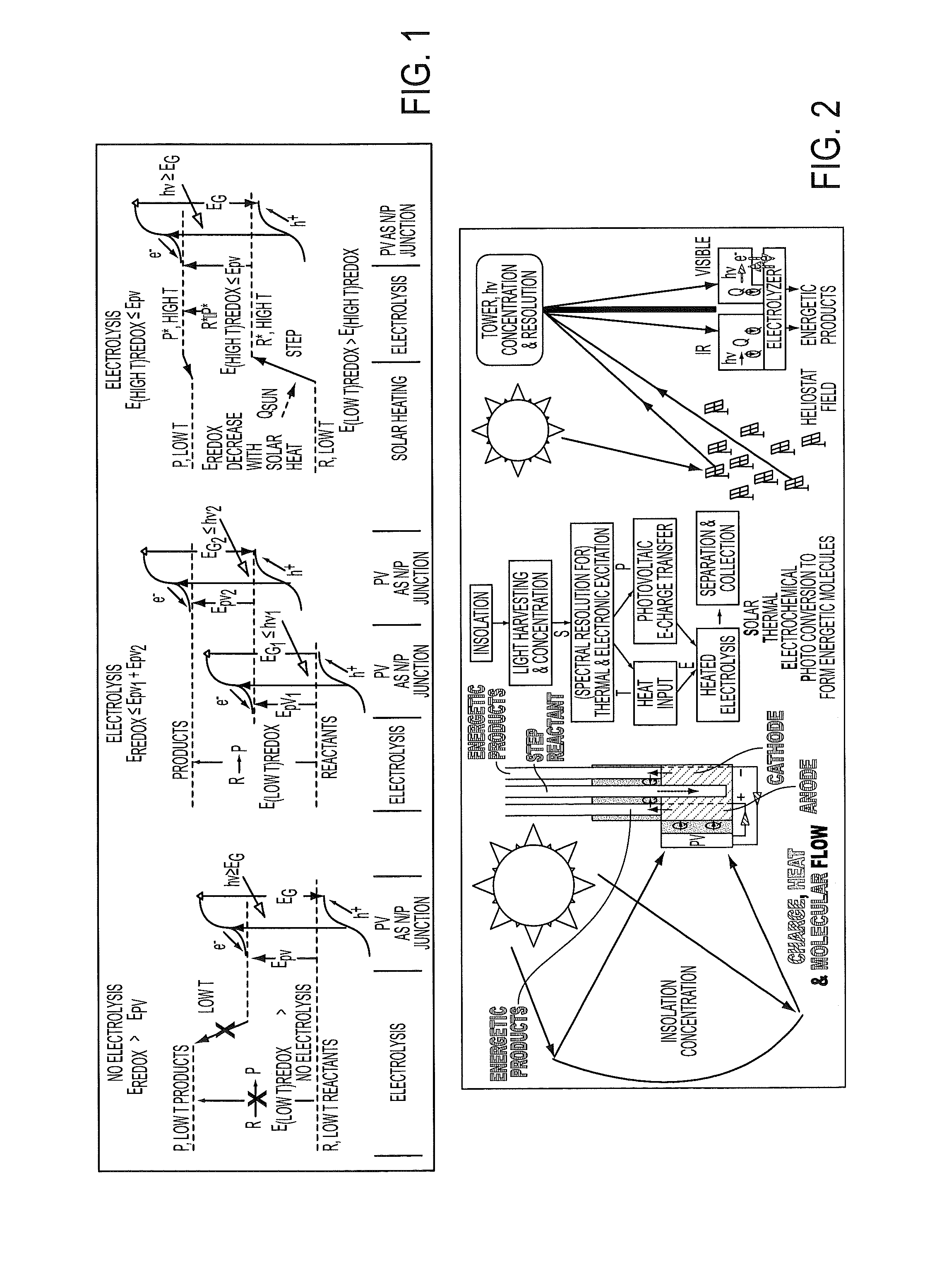
Process for electrosynthesis of energetic molecules
ActiveUS20100200418A1Energy efficiencyWeaken energyCellsPhotography auxillary processesThermal energyElectricity
A process for the production of energetically rich compounds comprising: using externally supplied thermal energy to heat an electrolyzable compound to a temperature greater than the ambient temperature; generating electricity from a solar electrical photovoltaic component; subjecting the heated electrolyzable compound to electrolysis with the solar generated electricity to generate an energetically rich electrolytic product.
Owner:C2CNT LLC
Method and apparatus for manufacturing carbon nanotube
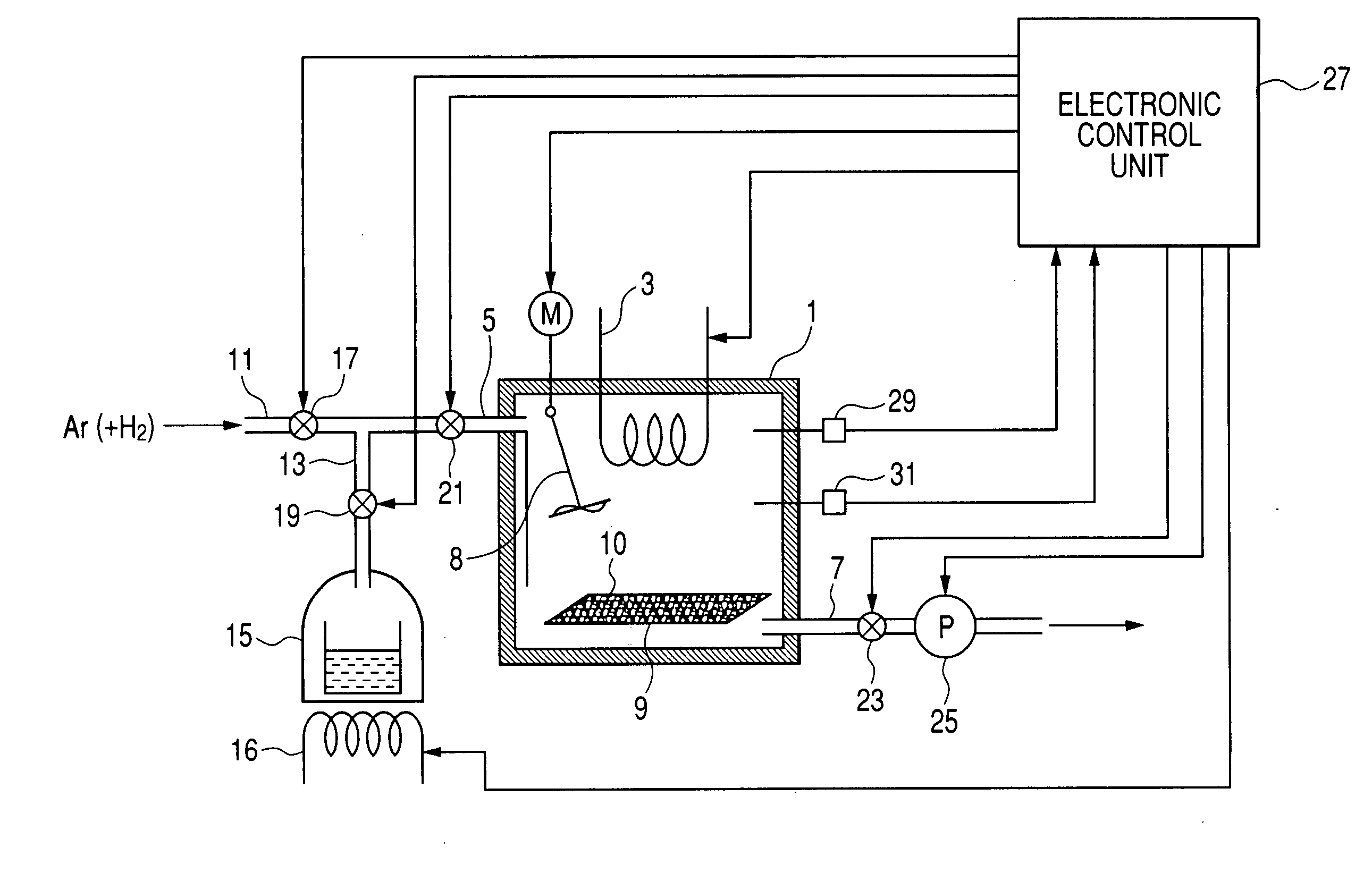
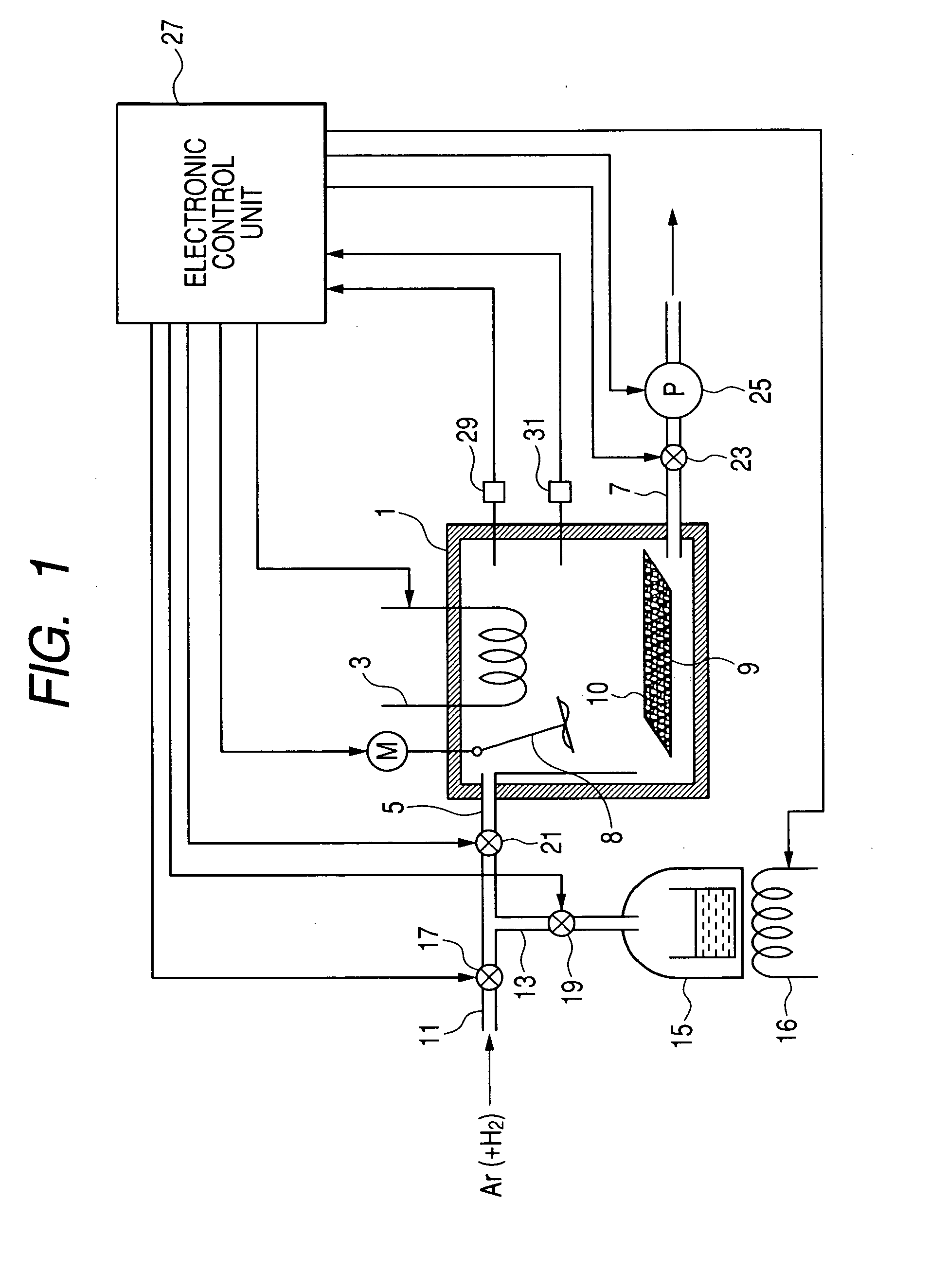
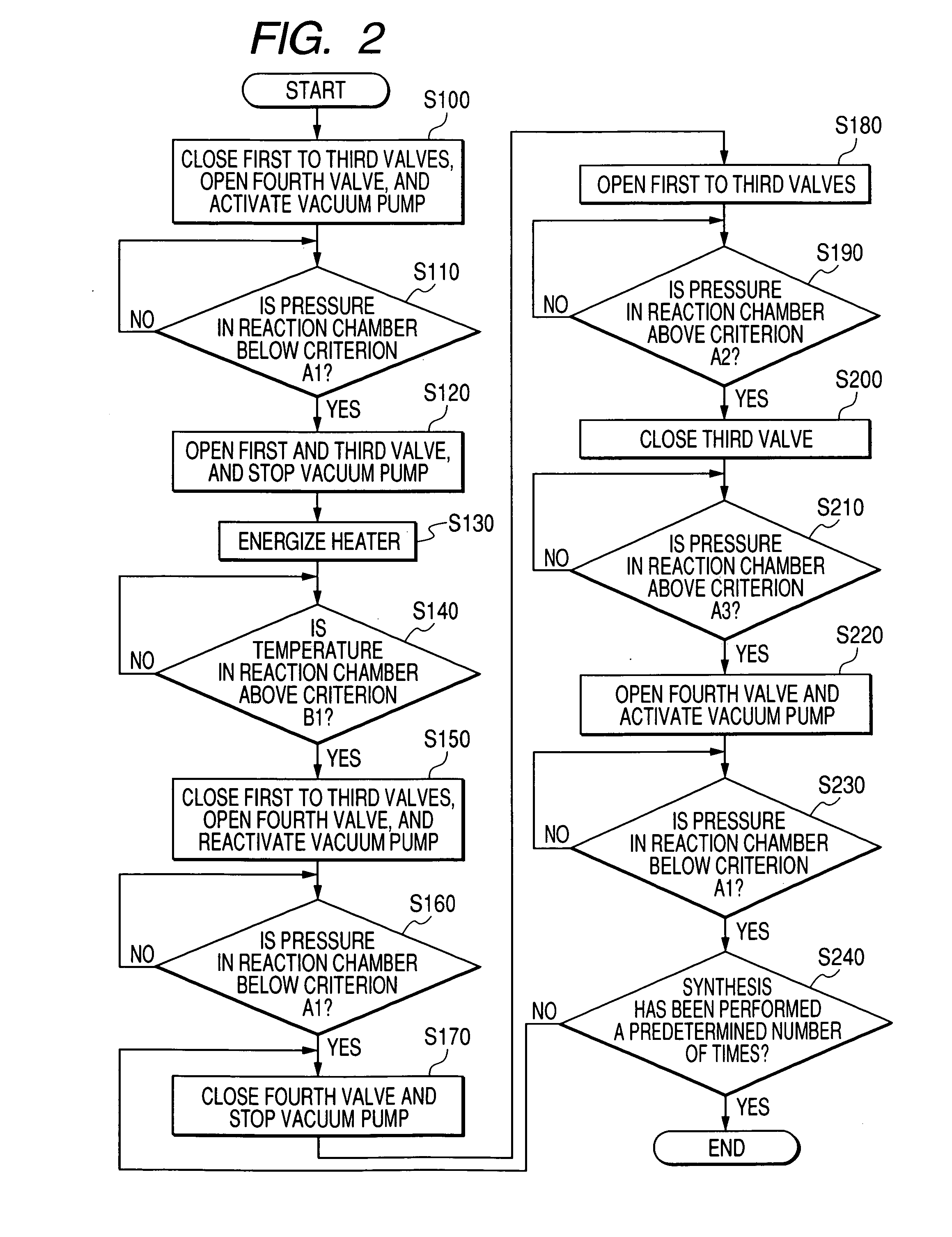
A carbon nanotube manufacturing method wherein a catalyst is heated in a reaction chamber while the reaction chamber is filled with argon gas containing hydrogen. When a predetermined temperature is reached in the reaction chamber, the reaction chamber is evacuated. Then a raw material gas as a carbon source is charged and sealed in the reaction chamber whereupon the synthesis of carbon nanotube begins. Subsequently, when a condition in which the synthesis of carbon nanotubes has proceeded to a predetermined level is detected, gases in the reaction chamber are exhausted. Then, the raw material gas is changed and sealed in the reaction tube again. Thereafter, the charging (synthesizing) operation and the exhausting operation are repeated until the carbon nanotube with a desired film thickness are synthesized. A carbon nanotube manufacturing apparatus is also disclosed
Owner:DENSO CORP
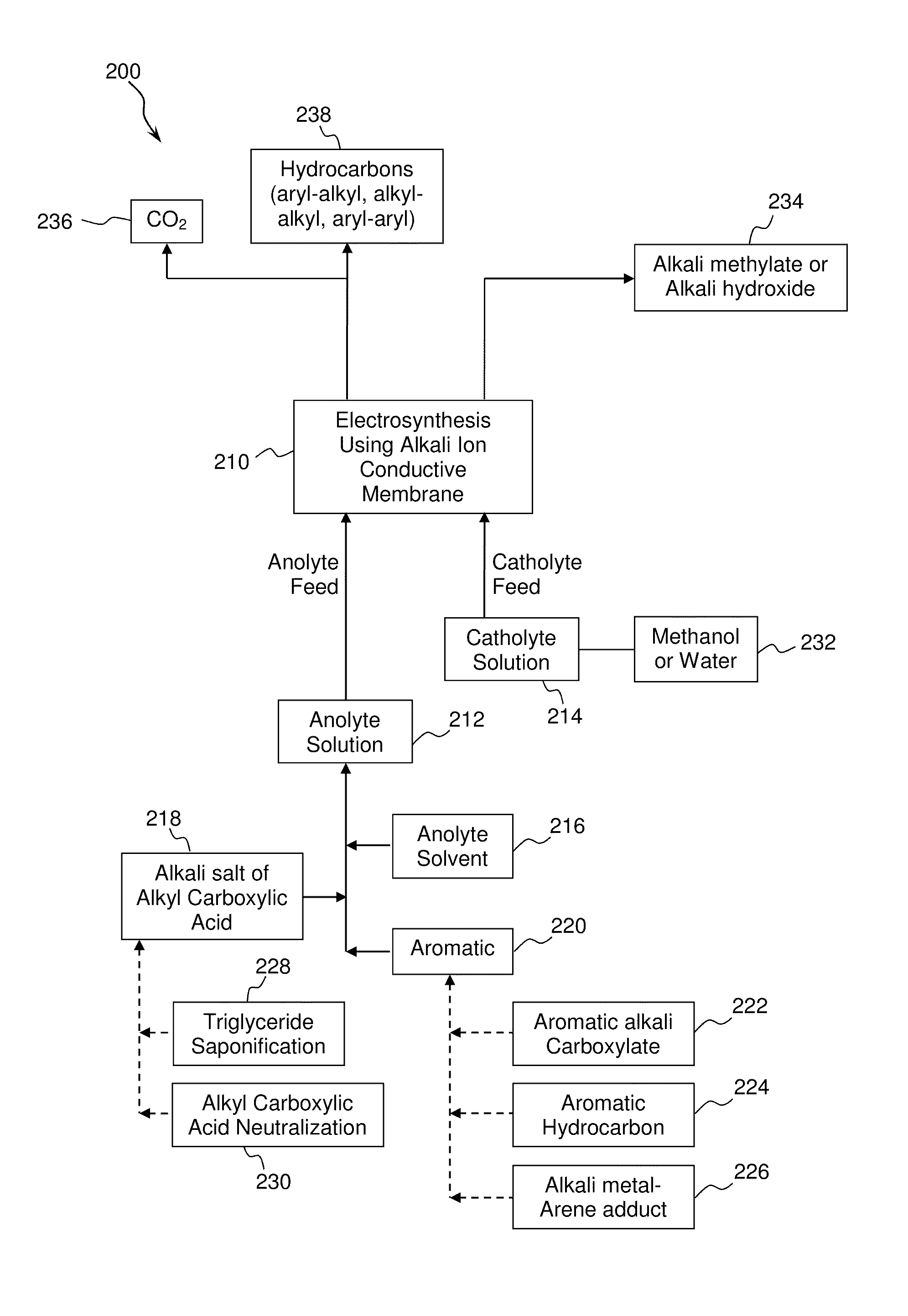
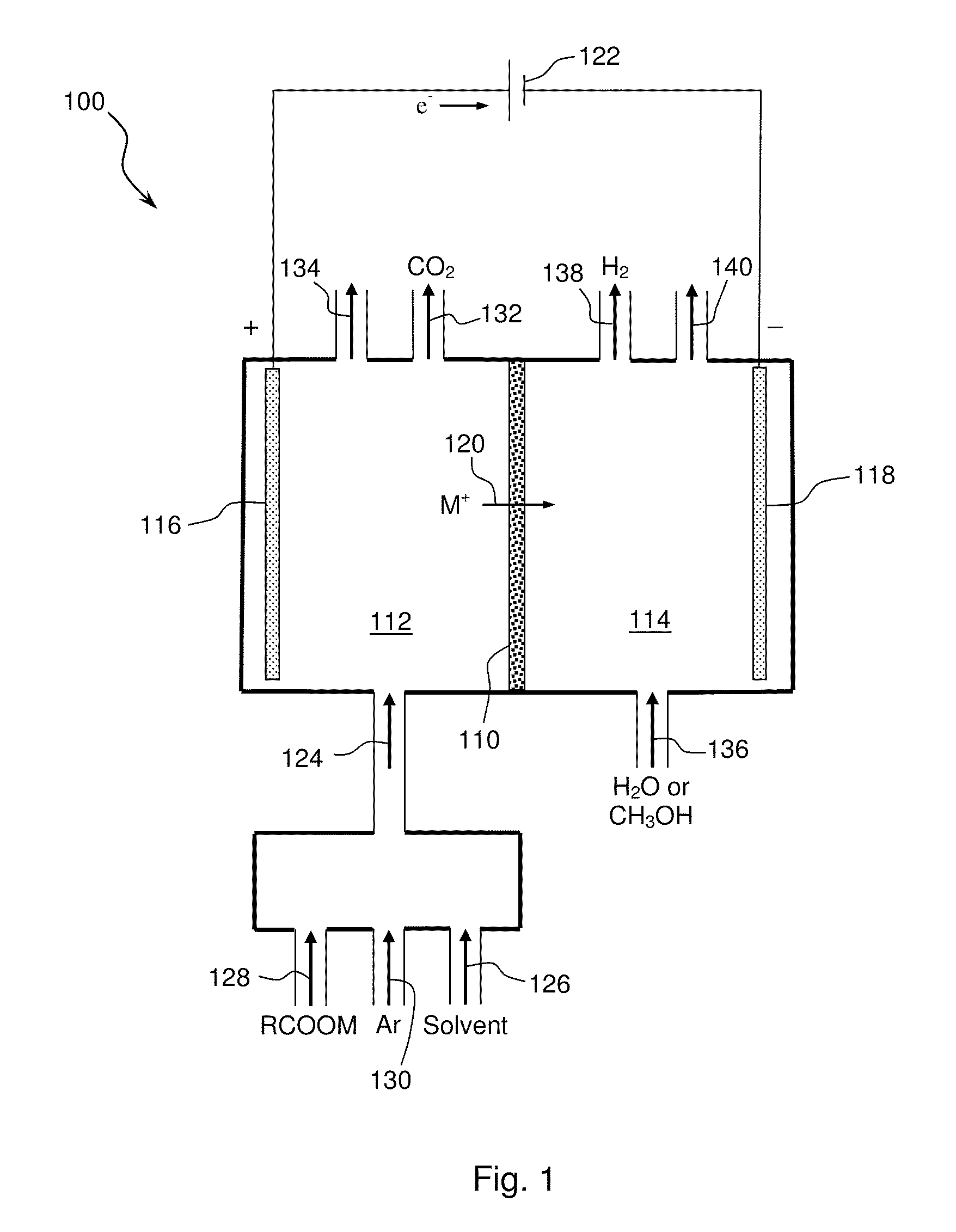
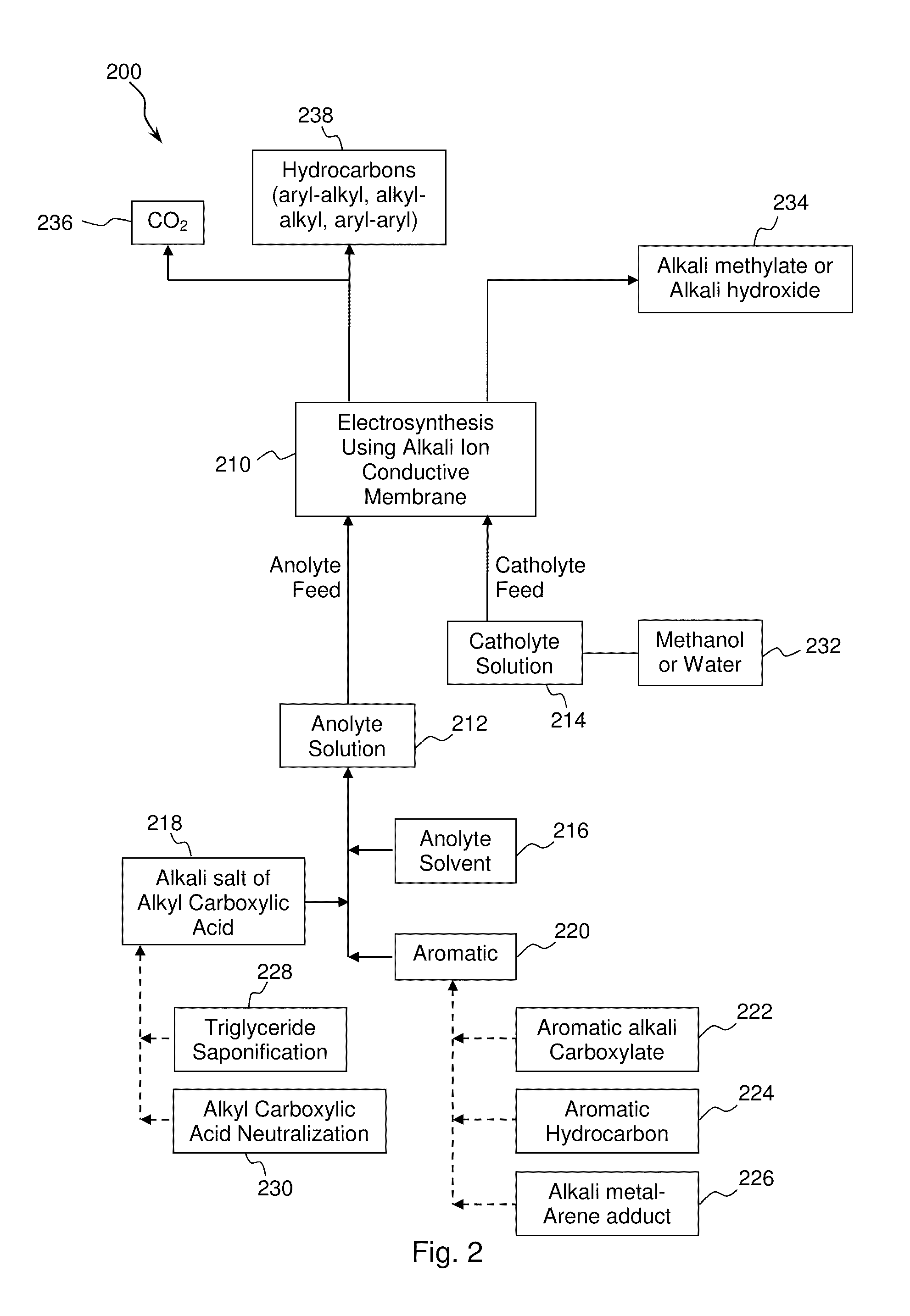
Electrochemical synthesis of aryl-alkyl surfacant precursor
An aryl-alkyl (R—Ar) hydrocarbon is prepared by an electrosynthesis process in an electrolytic cell having an alkali ion conductive membrane positioned between an anolyte compartment configured with an anode and a catholyte compartment configured with a cathode. An anolyte solution containing an alkali metal salt of an alkyl carboxylic acid and an aryl compound is introduced into the anolyte compartment. The aryl compound may include an alkali metal salt of an aryl carboxylic acid, an arene (aromatic) hydrocarbon, or an aryl alkali metal adduct (Ar−M+). The anolyte solution undergoes electrolytic decarboxylation to form an alkyl radical. The alkyl radical reacts with the aryl compound to produce the aryl-alkyl hydrocarbon.
Owner:ENLIGHTEN INNOVATIONS INC
Catalyst for electrochemically synthesizing ammonia and preparing method thereof
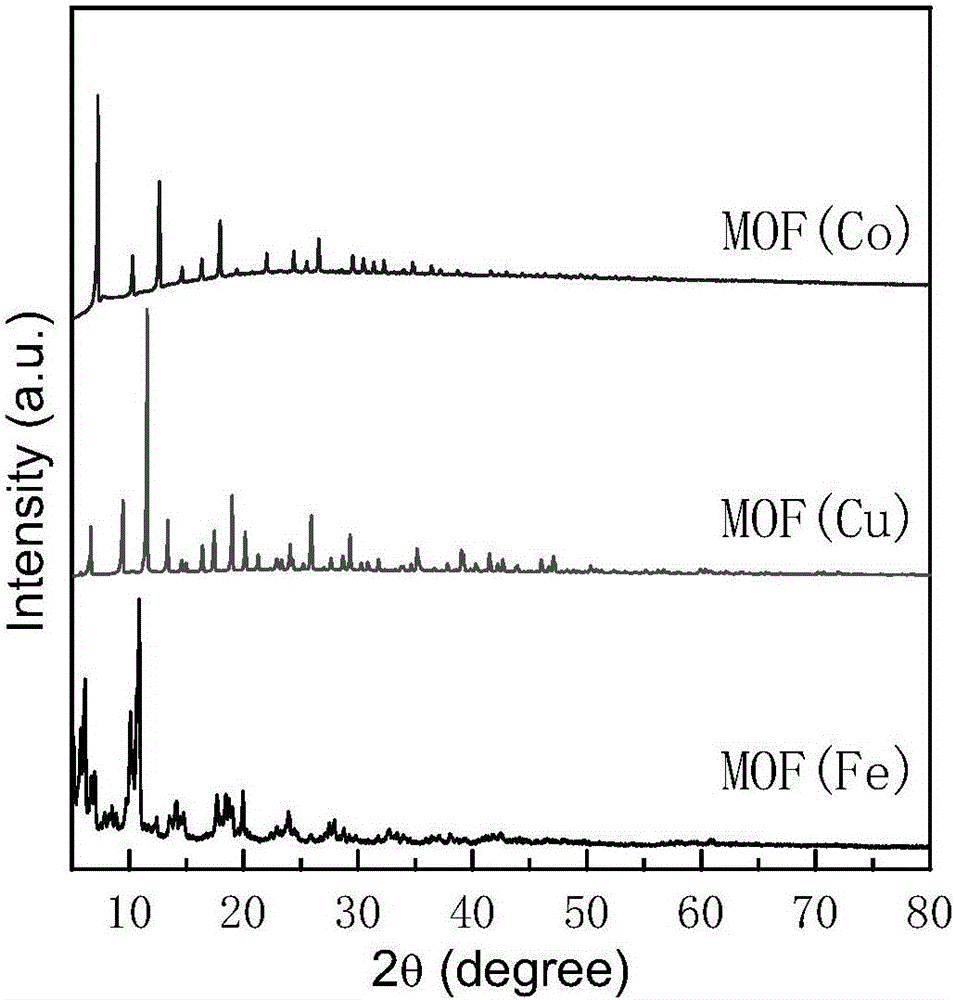
ActiveCN106111201AEasy to makeLow costOrganic-compounds/hydrides/coordination-complexes catalystsElectrodesMetal-organic frameworkElectrosynthesis
The invention belongs to the field of electrosynthesis ammonia. A catalyst for electrochemically synthesizing ammonia is formed by coating a metal organic skeleton, auxiliary carbon and a binding agent on carbon paper. The catalyst has the advantages of being easy to prepare, low in cost and high in catalysis efficiency. By using the catalyst, ammonia can be synthesized at normal pressure and low temperature, and energy consumption is greatly reduced. Air can be directly used as a raw material for synthesizing ammonia, the raw material source is enriched, raw material cost is reduced, and synthesis ammonia cost is reduced.
Owner:HANGZHOU INST OF ADVANCED MATERIAL BEIJING UNIV OF CHEM TECH
Fused salt electrosynthesis method of hydrogen storage alloy containing magnesium, lithium, sodium and potassium
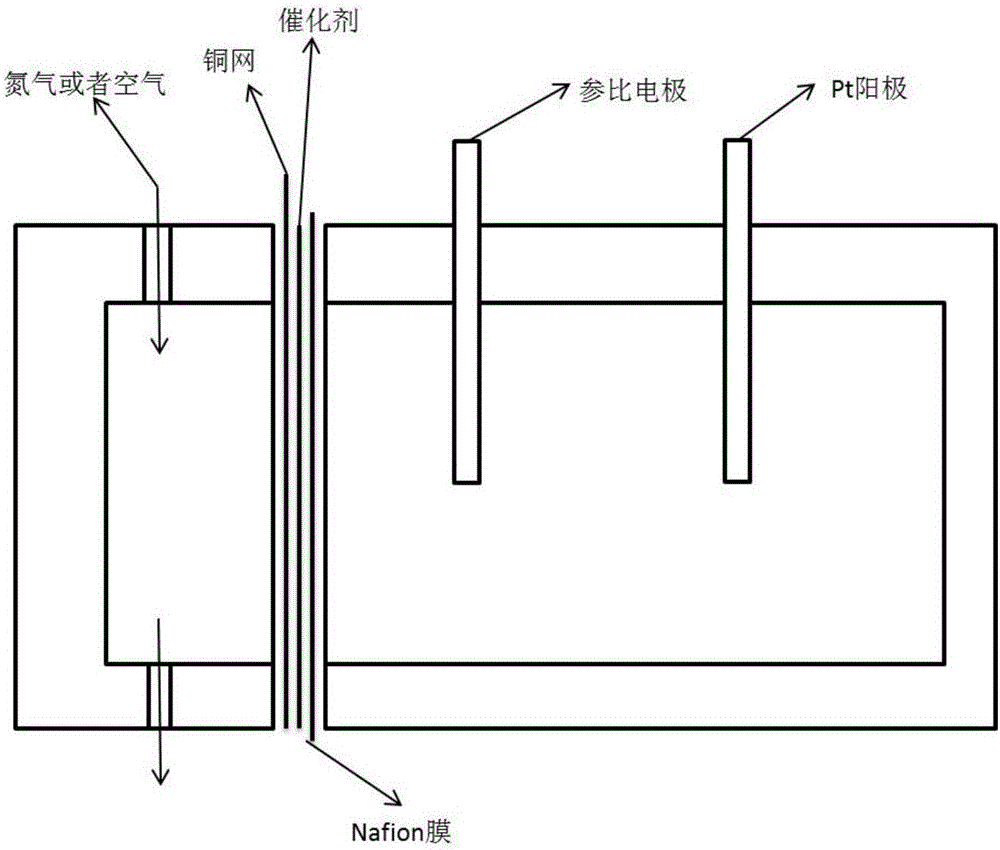
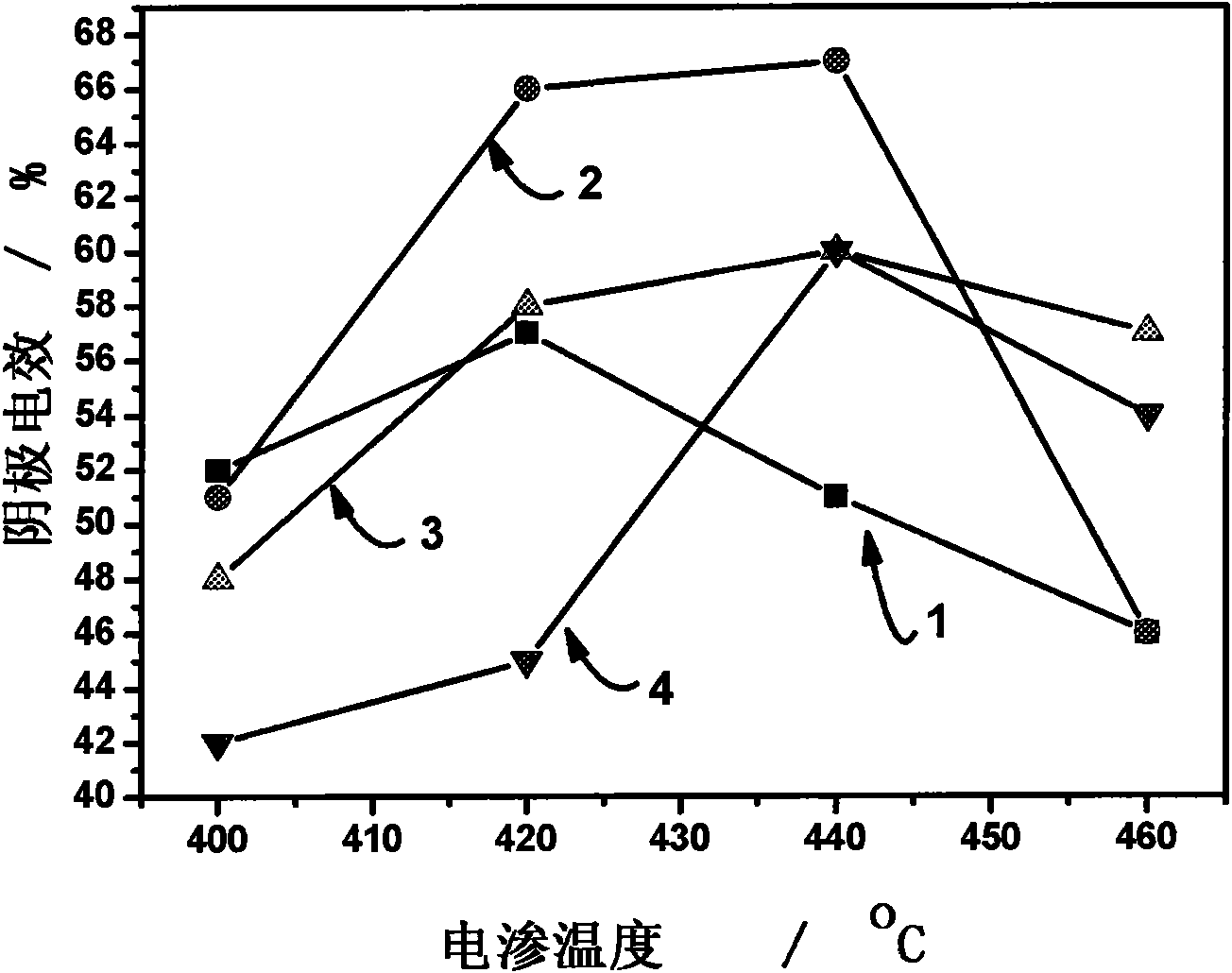
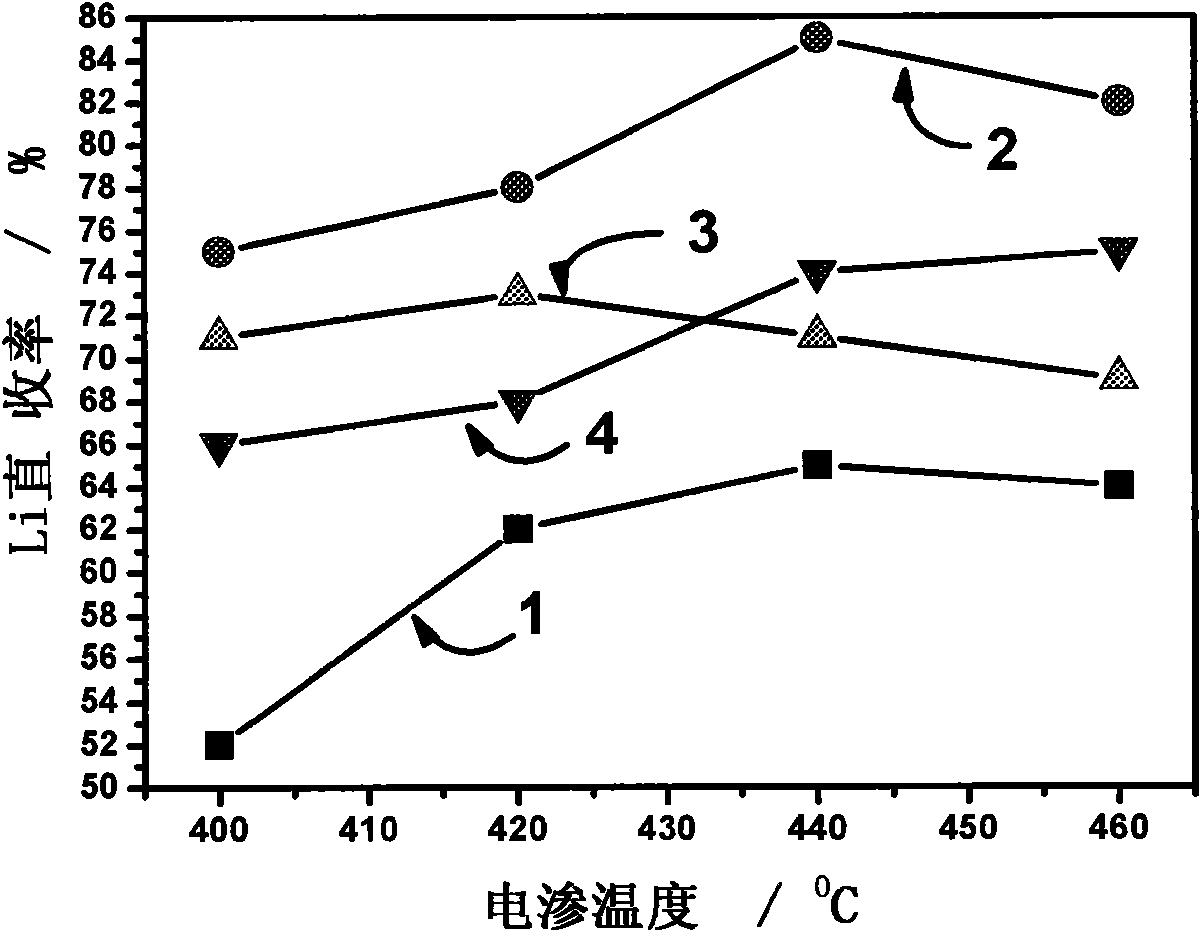
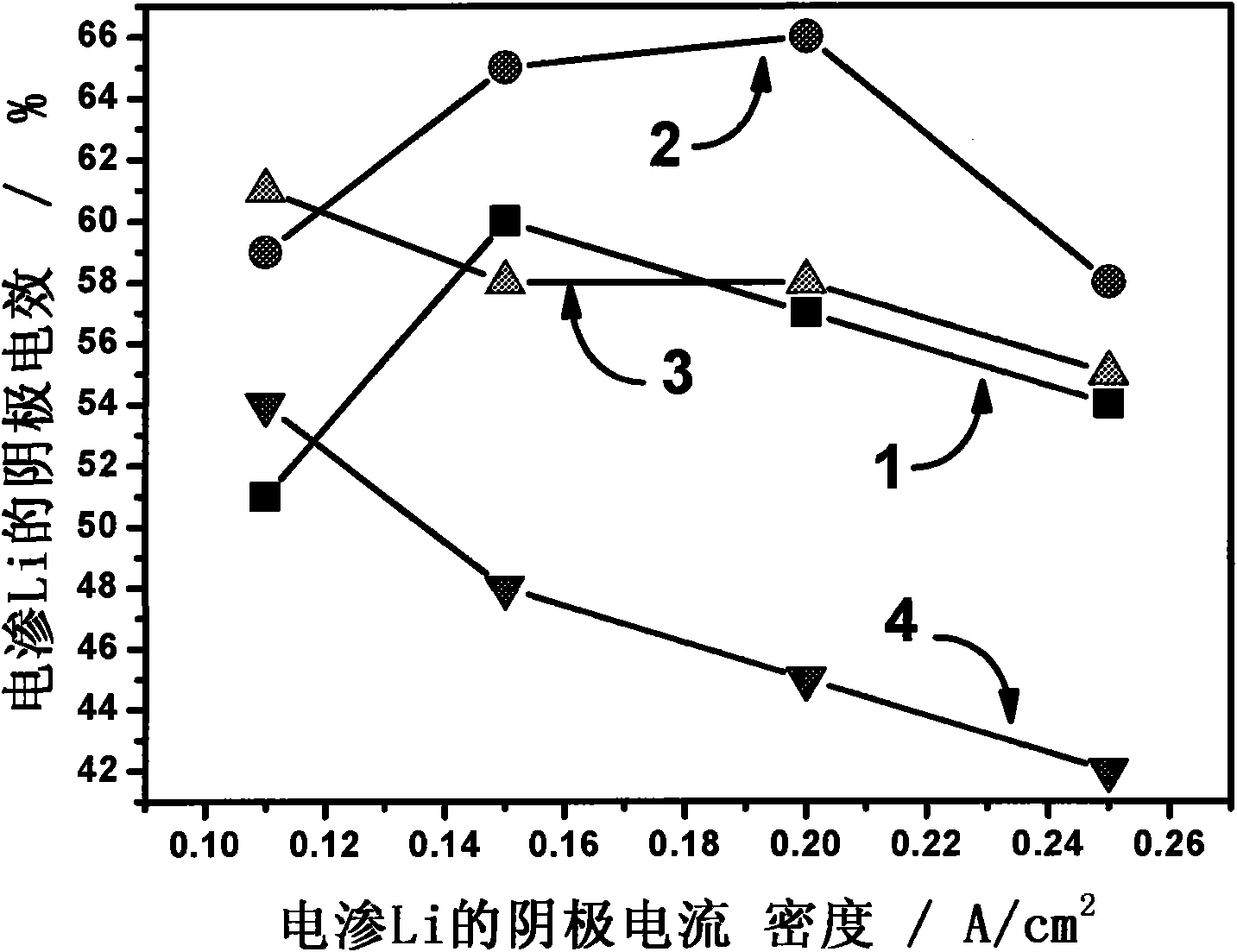
The invention provides a fused salt electrosynthesis method of a hydrogen storage alloy containing magnesium, lithium, sodium and potassium, in particular to a preparation method of an electrosynthesis AB3 type hydrogen storage alloy containing magnesium, lithium, sodium and potassium, comprising a fused salt electroosmosis step and a fused salt electrolysis step which are used simultaneously. The hydrogen storage alloy containing MmNi4.2Al0.7 and the like are used as cathodes in KCl.LiCl fused salt electrolyte. The preparation method comprises the following steps of: carrying out Li electroosmosis on the cathodes, such as the hydrogen storage alloy and the like, at 420-450 DEG C; regulating the composition of the fused salt electrolyte to a quaternary mixed salt system of KCl.NaCl.LiCl.MgCl2, and also increasing the temperature; and carrying out limit electrolysis with the electrolytic cathode current density of 18-22 A / cm<2> to obtain the AB3 type hydrogen storage alloy containing magnesium, lithium, sodium and potassium. The fused salt electroosmosis step and the fused salt electrolysis step are closely linked; the Li electroosmosis is carried out at low current density, Mg is electrolyzed at high current density, and K and Na are added through compelling electrolysis so that in-situ electrodeposition is realized in a same electrolytic cell. The invention belongs to the field of a short-process touch process.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
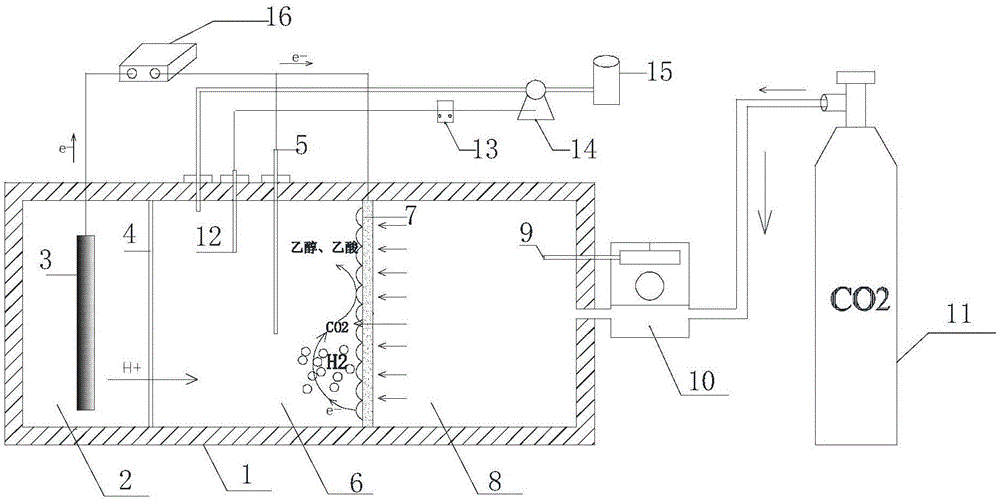
Bioelectricity synthesis system and method for synthesizing acetic acid and/or ethyl alcohol through same
ActiveCN105695319AAchieve restorationAvoid mass transfer processBioreactor/fermenter combinationsCellsPh controlEngineering
The invention discloses a bioelectricity synthesis system and a method for synthesizing acetic acid and / or ethyl alcohol through the same. The bioelectricity synthesis system comprises a reactor, an online pH control system, an air supply system and a power source. The reactor is a three-chamber reactor and comprises an air chamber, a negative electrode chamber and a positive electrode chamber, wherein the air chamber is connected with the air supply system; the negative electrode chamber is externally connected with the online pH control system, the negative electrode chamber and the air chamber are partitioned by an air negative electrode, and the negative electrode chamber is further internally provided with a reference electrode; the positive electrode chamber is internally provided with a positive electrode, the positive electrode chamber and the negative electrode chamber are partitioned by a cation exchange membrane, and the positive electrode, the air negative electrode and the reference electrode are externally connected with the power source through wires. Positive electrode electrolyte is added into the positive electrode chamber, a bioelectricity synthetic nutrient solution obtained after sterile and anaerobic treatment is added into the negative electrode chamber, and electrosynthesis bacteria are inoculated into the negative electrode chamber; carbon dioxide is sent into the air chamber through metering of the air supply system, and carbon dioxide penetrates through the air negative electrode and is converted into acetic acid and / or ethyl alcohol in the negative electrode chamber.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
Preparation method of metal organic polymer material
InactiveCN103451717AMild reaction conditionsSimple processPolycrystalline material growthFrom normal temperature solutionsElectrolytic agentMetallic electrode
The invention discloses a preparation method of a metal organic polymer material. The preparation method comprises the following steps: in an electrolytic cell, using ionic liquid as electrolyte, using a metal electrode or a titanium-based oxide electrode as an anode, using a titanium plate as a cathode, turning on a power supply, controlling the temperature and current density to carry out electric synthesis, carrying out solid-liquid separation, washing the product through a solvent, and then, washing the product through chloroform, and drying the product to obtain the metal organic polymer material; and finally, removing organic ligands, metal salt ions and solvent molecules out of pores of the synthesized metal organic polymer material and from surfaces of the pores to prepare the metal organic polymer material. The method of the invention is carried out under normal temperature and normal pressure, thereby having the advantages of mild reaction conditions, simple process, easiness for controlling the reaction, no secondary pollution, etc and is clean, sanitary and friendly to the environment.
Owner:TAIYUAN UNIV OF TECH
Electrosynthesis of nanofibers and nano-composite films
InactiveUS20060021879A1Material nanotechnologySurface reaction electrolytic coatingComposite filmNanofiber
A method for producing an array of oriented nanofibers that involves forming a solution that includes at least one electroactive species. An electrode substrate is brought into contact with the solution. A current density is applied to the electrode substrate that includes at least a first step of applying a first substantially constant current density for a first time period and a second step of applying a second substantially constant current density for a second time period. The first and second time periods are of sufficient duration to electrically deposit on the electrode substrate an array of oriented nanofibers produced from the electroactive species. Also disclosed are films that include arrays or networks of oriented nanofibers and a method for amperometrically detecting or measuring at least one analyte in a sample.
Owner:BATTELLE MEMORIAL INST
Organic electrosynthesis method of phenothiazine/phenoxazine compound
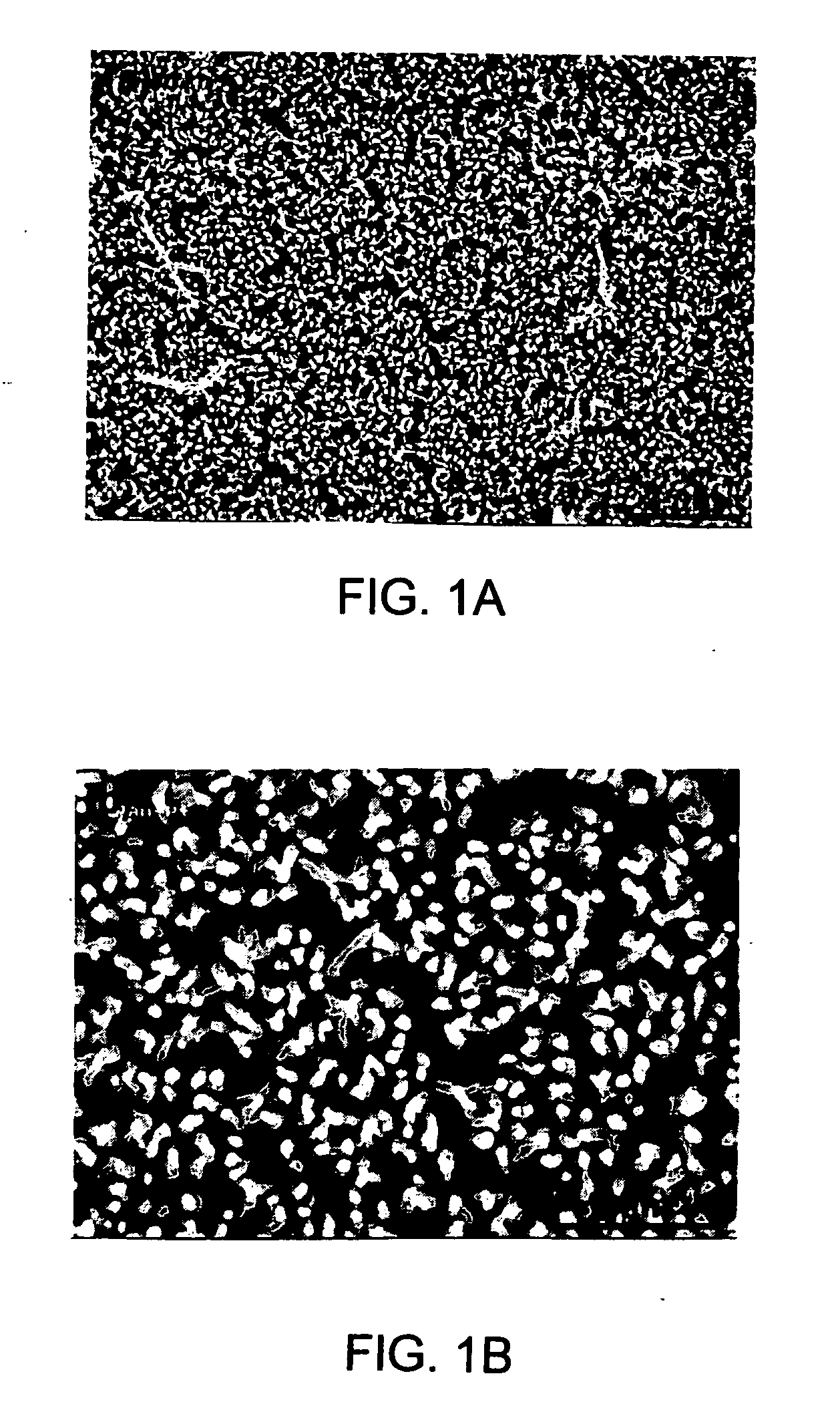
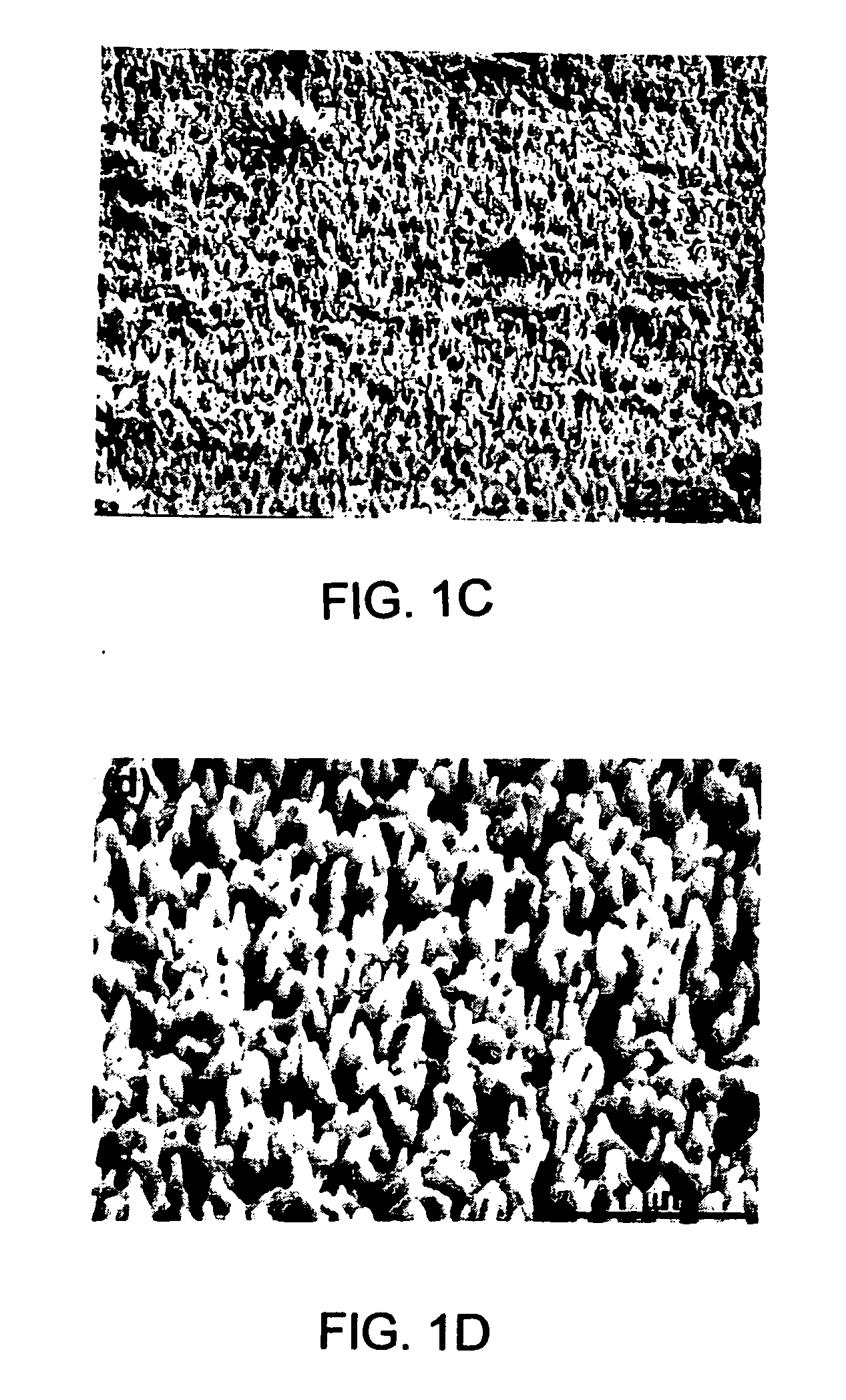
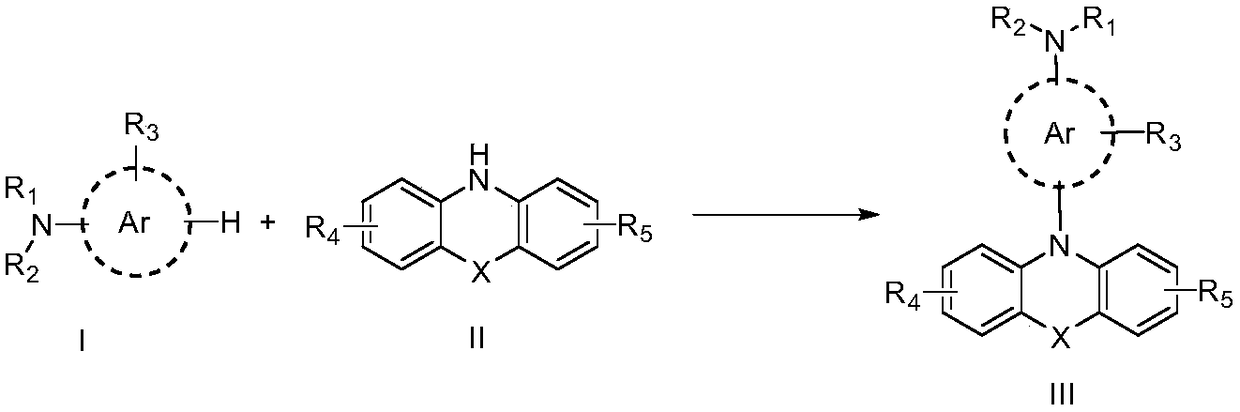
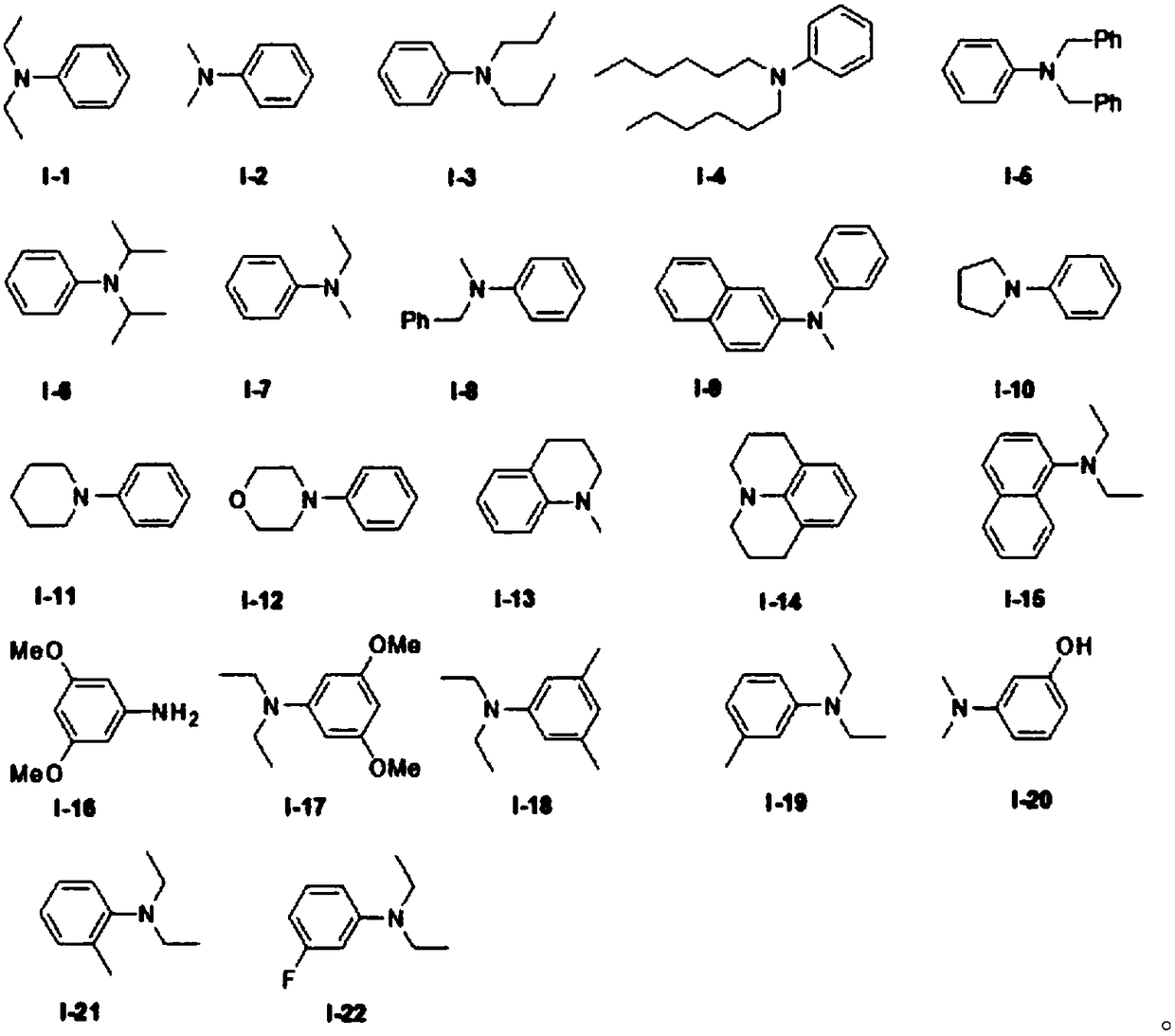
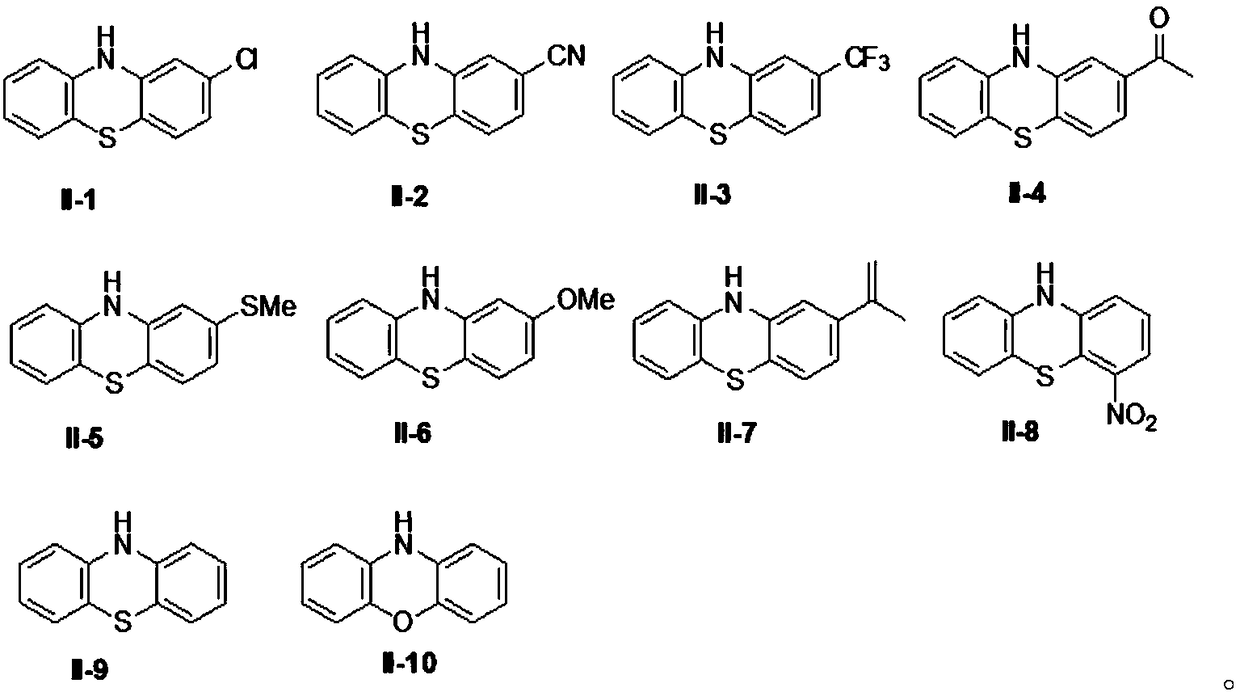
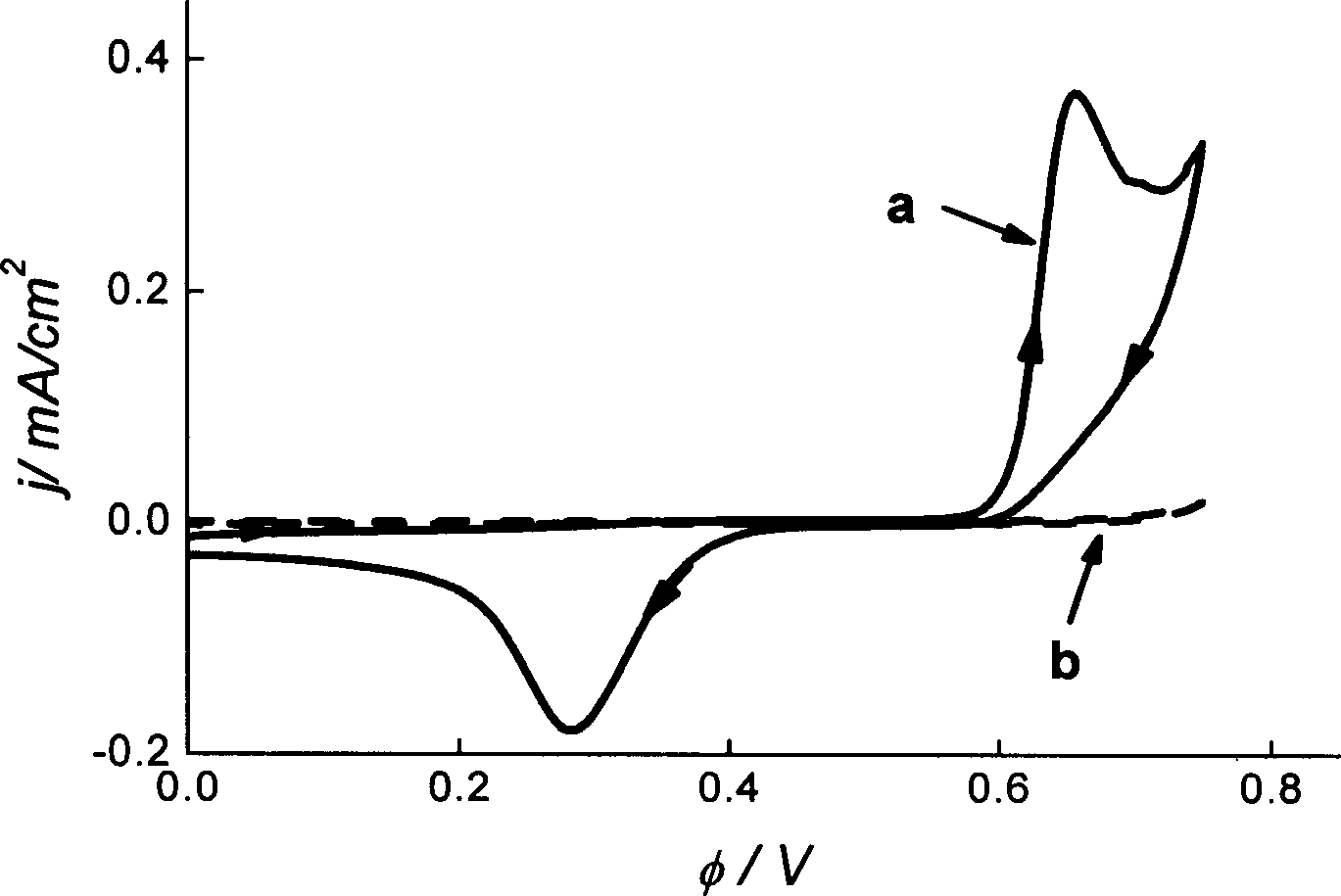
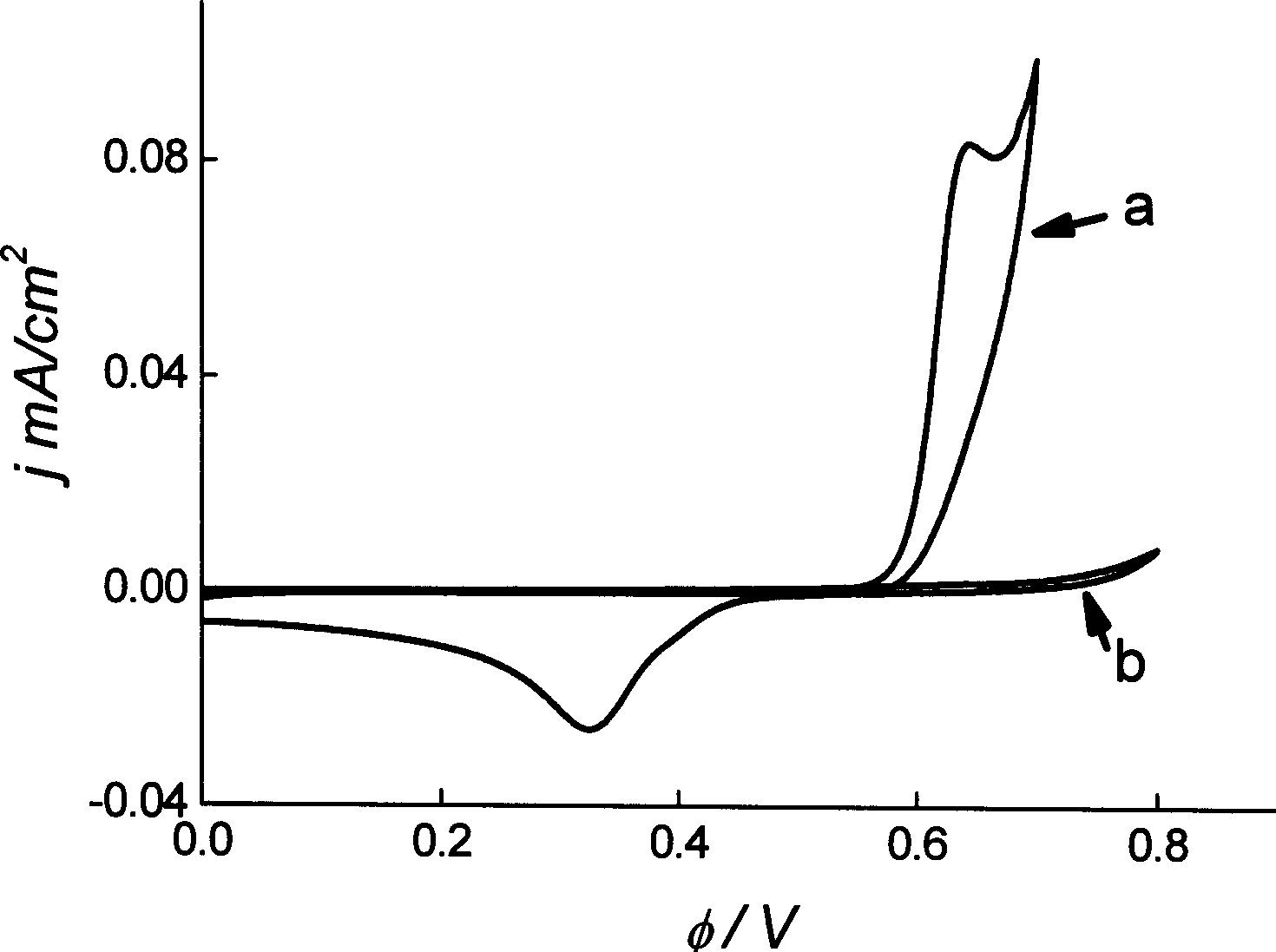
ActiveCN108863982AReduce processEasy to operateElectrolysis componentsOrganic chemistryPhenoxazineElectrosynthesis
The invention discloses an organic electrosynthesis method of a phenothiazine / phenoxazine compound. The organic electrosynthesis method adopts an organic electrosynthesis means for a compound shown ina formula I and a compound shown in a formula II to prepare the phenothiazine / phenoxazine compound. The organic electrosynthesis method disclosed by the invention has the beneficial effects that a target product shown in a formula III can be obtained by directly activating C-H without need of expensive halogenated raw materials and additional reduction and substitution processes, so that the process flow is obviously shortened, the operation is simplified, the preparation efficiency is improved, and the production cost is reduced; the reaction condition is simple, mild and environmentally friendly, the atom economy is high, the adaptation range of a reaction substrate is wide, and the yield of a target product is high.
Owner:NANCHANG HANGKONG UNIVERSITY
Bivalent and trivalent iron inorganic compound suitable fo sgnthesizing perferrate
InactiveCN1786282ALow priceSelect the electrochemical catalytic performance is goodElectrolysis componentsElectricityIron powder
The invention relates to manufacturing new type ferrous and ferric iron inorganic compound applied in electrosynthesis ferrate. They are used as anode material to form ferrous at alkali condition, restrains electrochemistry bleeding oxygen reaction. The compound has good reversibility. Thus it can use as green quadric chemical electric power source anode material. The material manufacturing is easy to do industrialization production. The raw material can utilize blue dust with low price. Its manufacturing process is simple. It is good for environment, and can fully reclaim blue dust.
Owner:国家高技术绿色材料发展中心
Method for synthesizing 3-methyl-4-nitrobenzoic acid by using stepped heating method and indirect electrosynthesis method
The invention discloses a method for synthesizing 3-methyl-4-nitrobenzoic acid by using a stepped heating method and an indirect electrosynthesis method. The method is implemented by taking 2,4-dimethylnitrobenzene and chromium sulfate as main raw materials through the following steps of: firstly, carrying out electrolytic oxidation on chromium sulfate by using an electrolytic process so as to obtain chromium trioxide; then, selectively oxidizing 2,4-dimethylnitrobenzene into a 3-methyl-4-nitrobenzoic acid by using chromium trioxide through using the stepped heating method. According to the invention, through adopting the stepped heating method, the oxidation reaction rate of 2,4-dimethylnitrobenzene is controlled, and the selectivity and conversion rate of reaction are remarkably improved. The conversion rate of chromium trioxide obtained by using an electrolytic oxidation method is 85-95%, and the conversion rate of 3-methyl-4-nitrobenzoic acid obtained by using the stepped heating method can reach 65-86%. According to the invention, the problem that in the process of reaction, the reaction rate declines due to the consumption of acids is solved; through carrying out electrolytic oxidation on chromium sulfate produced after reaction, chromium trioxide is circularly produced, thereby not only avoiding the environment pollution, but also greatly saving the cost.
Owner:HUNAN UNIV OF SCI & TECH
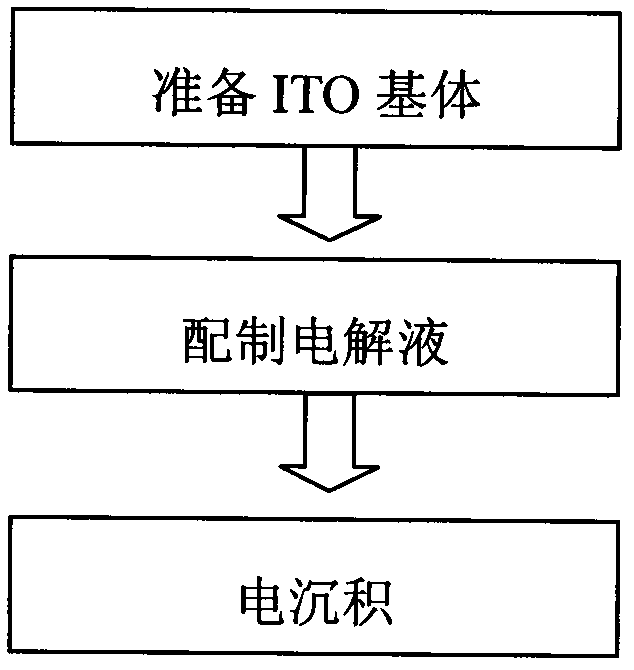
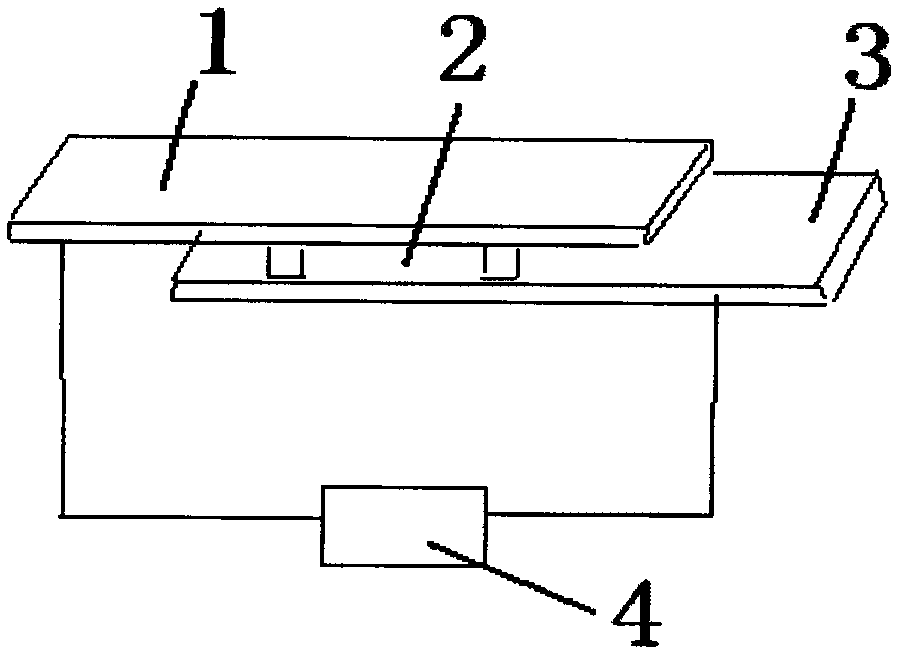
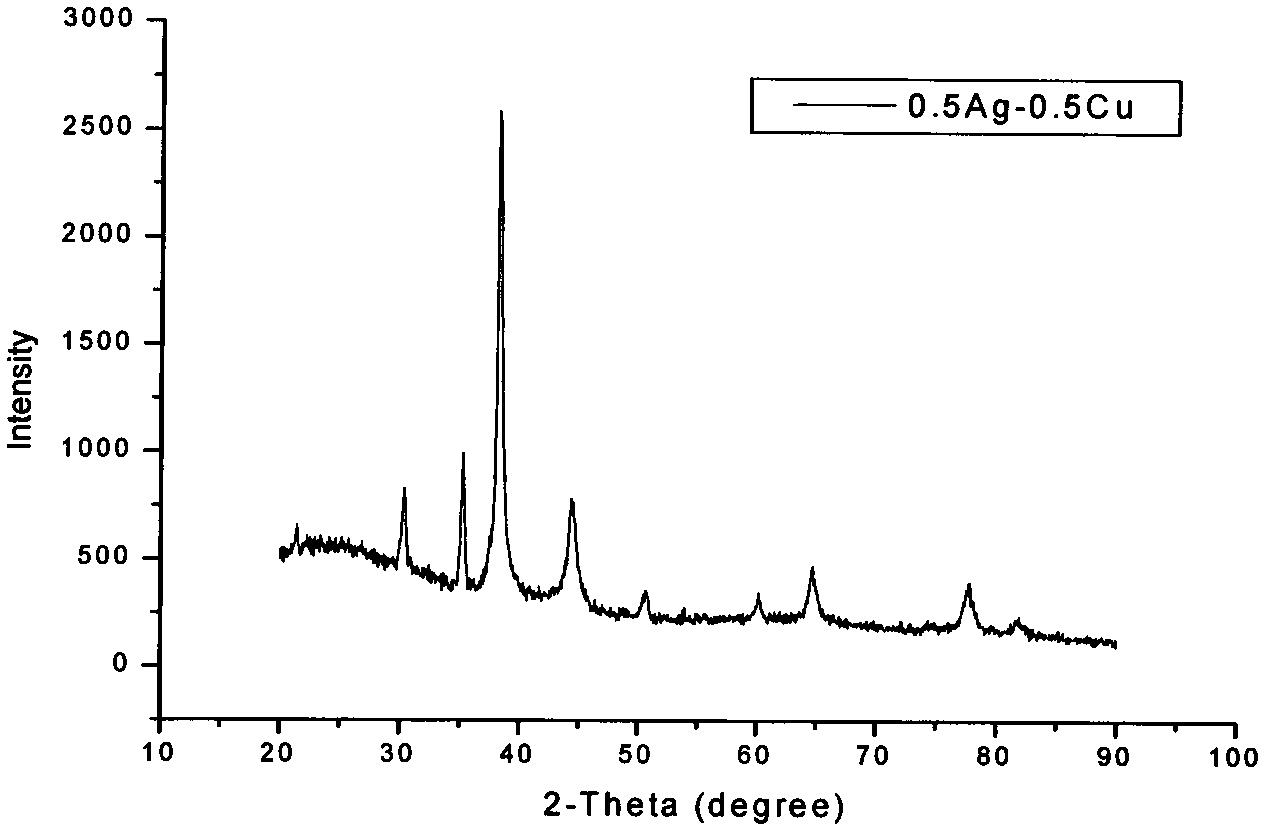
Silver-copper nano alloy and electrosynthesis method thereof
The invention discloses a silver-copper nano alloy and an electrosynthesis method thereof. The silver-copper nano alloy comprises Ag and Cu; the molar components of Ag and Cu are shown as a formula of xAg-(1-x)Cu, wherein x=0.5-0.9; the phase of the alloy is a face-centered cubic structure silver single phase solid solution; the (111) diffraction peak of the alloy is consistent with the (111) diffraction peak of pure metal Ag; and the alloy has a surface plasma resonance peak and has a dendritic shape, the secondary dendrite arm spacing is 100 to 300nm, and the tertiary dendrite arm length is 100 to 500nm. The silver-copper nano alloy with the dendritic structure is grown under constant potential. Electrolyte for preparation does not contain any surfactant molecules. The surface plasma resonance frequency of the alloy is modulated by controlling components of the alloy, and along with the increase of the silver content in the silver-copper alloy, the plasma absorption peak of the alloy generates red shift. The formed silver-copper nano dendrite has a rough and clean surface and a large specific surface area, and is better applied to the aspects such as surface enhanced spectrums, surface plasma optical catalysis, surface plasma enhanced solar absorption and the like.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Method for electrosynthesis of adiponitrile by using dimensionally stable anode
InactiveCN102061482ASolve the disadvantages of being easily corrodedElectrolytic organic productionElectrodesSupporting electrolyteIridium
The invention relates to a method for electrosynthesis of adiponitrile by using a dimensionally stable anode. The method comprises the following steps of: carrying out electrosynthesis of the adiponitrile in a non-diaphragm type electrolytic bath by utilizing titanium as a substrate, utilizing the dimensionally stable anode coated with oxides, such as ruthenium, iridium, tantalum and the like as an anode and utilizing plumbum or cadmium as a cathode; introducing a direct current of 500 A.m-2-3,000A.m-2 at the temperature of 20-60 DEG C for electrolysis; and maintaining the flow rate of interelectrode electrolytes to be more than 1m.s<-1>, wherein the electrolytes contain 0.5-7% by mass of acrylonitrile, 5-20% by mass of potassium phosphate, sodium phosphate, acid type potassium phosphate or acid type sodium phosphate as supporting electrolytes, 0.1-5.0% by mass of buffer, 0.1-10.0% by mass of guiding ion source quaternary ammonium salt and the balance of water, the pH value of the electrolyte is 6-10. An organic phase containing a product of adiponitrile, a by-product of propionitrile and a raw material AN (Acrylic Nitrile) which does not participate in the reaction is generated from electrolysis. The adiponitrile and the propionitrile are obtained from the organic phase through rectification. The method overcomes the defect of easy corrosion of the anode in the electrolysis, the yield of the adiponitrile reaches more than 80%, and the current efficiency is more than 75%.
Owner:山东润兴化工科技有限公司
Method for paired electrolysis during synthesis of benzaldehyde, sorbitol and mannitol
InactiveCN103628086AImprove conversion rateImprove current efficiencyElectrolysis componentsElectrolytic organic productionSupporting electrolyteBenzaldehyde
A method for aired electrolysis during synthesis of benzaldehyde, sorbitol and mannitol comprises the followings: a cationic membrane is processed firstly, then electrode preparation and pretreatment are performed, and finally paired electrosynthesis is carried out, wherein paired electrosynthesis refers to that an H-type diaphragm electrolytic cell is provided with a cationic exchange membrane, both a cathode electrolytic bath and an anode electrolytic bath use a sodium sulfate solution as supporting electrolyte, sodium hydroxide is used for adjusting the alkalinity of the solution, then benzyl alcohol is added into an anode pool, glucose is added into a cathode pool, after stirring, the reaction temperature is adjusted through thermostatic waterbath, constant-current electrolysis is performed through setting electrolytic current, the benzyl alcohol is selectively oxidized to be benzaldehyde at the anode, and the glucose is reduced to be the sorbitol and the mannitol at the cathode. According to the method, in the same electrolytic process, two compounds of the benzyl alcohol and the glucose are both taken as raw materials, so that the benzaldehyde, the sorbitol and the mannitol are obtained simultaneously, the current efficiency and the time and space efficiency are improved greatly, the manufacturing cost and the energy consumption are reduced, the cathode product conversion rate is increased by more than 10 percent, and the economic benefit is very obvious.
Owner:TAIYUAN UNIV OF TECH
Gated electrodes for electrolysis and electrosynthesis
InactiveUS8147659B2Good effectEnhanced charge transferCellsPhotography auxillary processesElectricityOvervoltage
A gated electrode structure for altering a potential and electric field in an electrolyte near at least one working electrode is disclosed. The gated electrode structure may comprise a gate electrode biased appropriately with respect to a working electrode. Applying an appropriate static or dynamic (time varying) gate potential relative to the working electrode modifies the electric potential and field in an interfacial region between the working electrode and the electrolyte, and increases electron emission to and from states in the electrolyte, thereby facilitating an electrochemical, electrolytic or electrosynthetic reaction and reducing electrode overvoltage / overpotential.
Owner:RGT UNIV OF CALIFORNIA
Preparation method and application of composite cathode integrating efficient in-situ electrosynthesis of hydrogen peroxide and catalytic performance
ActiveCN112479317AEfficient degradationGood effectWater treatment compoundsWater contaminantsFerrocobaltNitrogen gas
The invention discloses a preparation method and application of a composite cathode integrating efficient in-situ electrosynthesis of hydrogen peroxide and catalytic performance, and relates to the technical field of preparation of electrode materials. The method comprises steps: soaking carbon nanotubes in a cobalt chloride solution and then complexing with potassium ferricyanide to form ferrocobalt prussian blue analogue loaded carbon nanotubes; preparing ferrocobalt alloy carbon nano tubes through nitrogen calcination, loading the ferrocobalt alloy carbon nano tubes on a foamed nickel metalelectrode of the carbon nano tube by adopting a dispensing process, preparing the composite cathode integrating in-situ electrosynthesis of hydrogen peroxide and catalytic performance, and successfully applying the composite cathode to a heterogeneous electro-Fenton system to degrade organic pollutants.
Owner:BEIJING UNIV OF TECH
Low-Ag-content Pb-RE-Ag alloy electrode
A low-Ag-content Pb-RE-Ag alloy electrode comprises (by weight percentage) rare earth (RE) 0.001-2.0, silver (Ag) 0.001-0.6 and lead in balance. RE is selected from at least one of La, Ce, Nd, Sm, Eu, Tb, Dy, Ho, Er, Dm, Yb, and Lu, and the total RE content is 0.001wt.%-2.0wt.%. With the invention, alloy has low cost, high mechanical strength, good corrosion resistance, and good electrochemical property, can find wide application in fields including metal electrodeposition, electroplating, wastewater treatment and organic electrosynthesis, and is suitable for industrial application.
Owner:CENT SOUTH UNIV
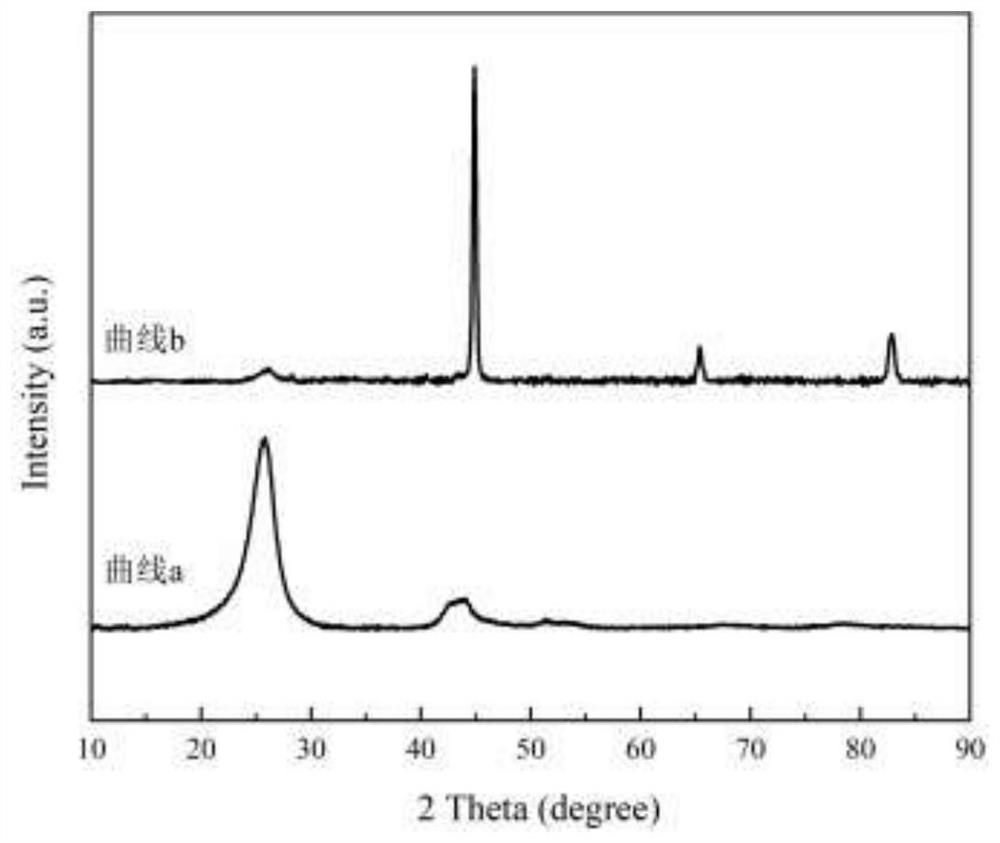
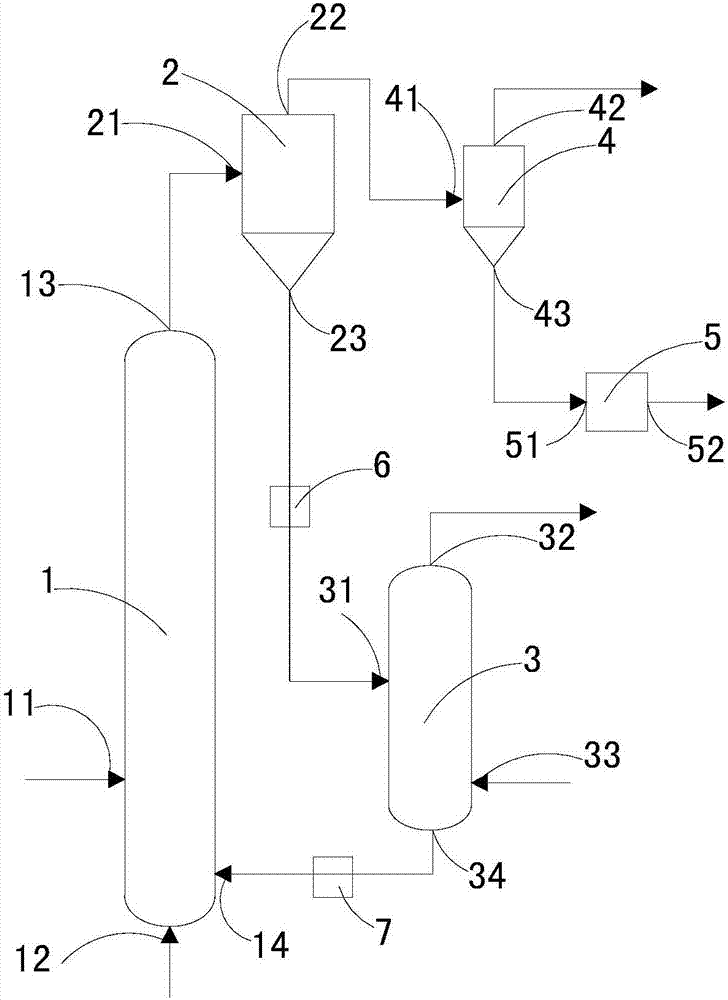
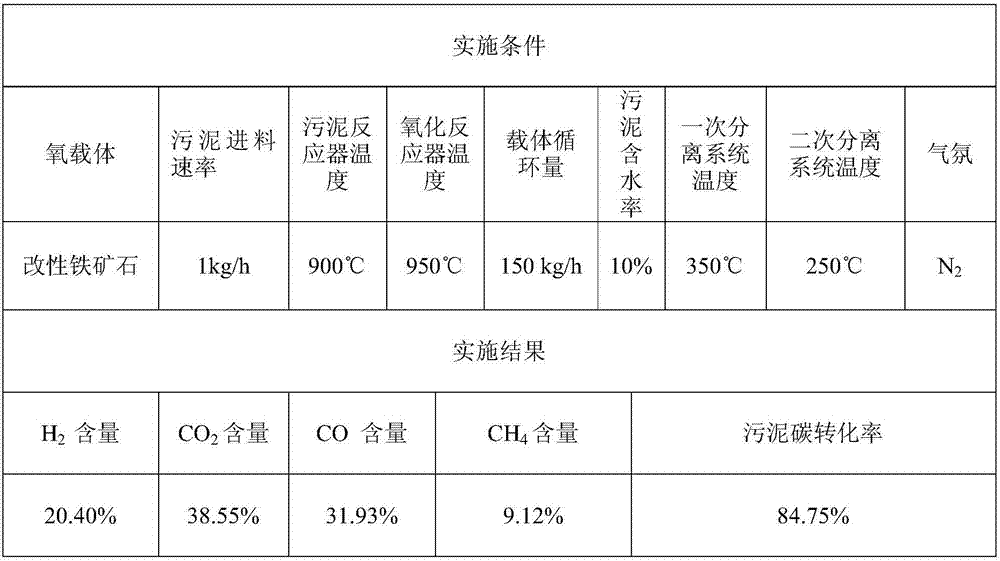
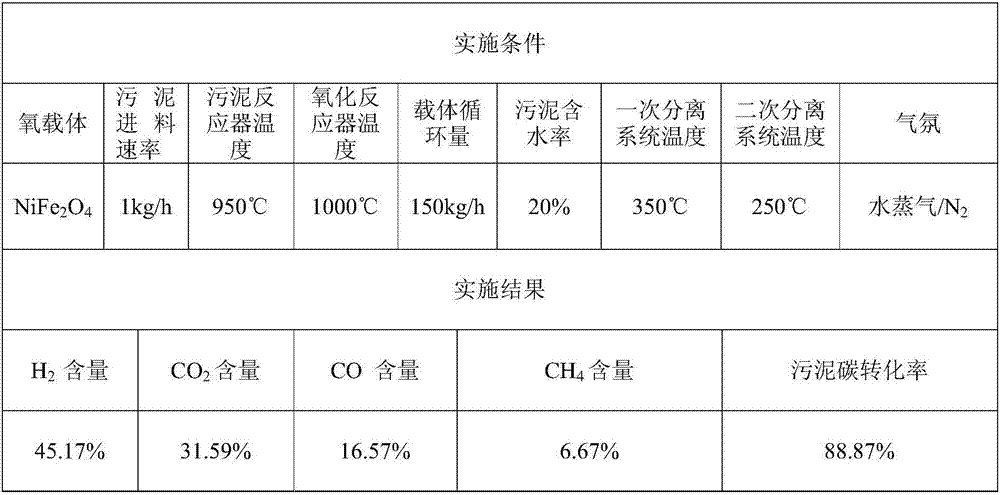
Method for directionally producing syngas by chemical chain gasification of sludge
InactiveCN107142125AEasy to operateEase of mass productionGasification processes detailsCombustible gas productionSyngasCatalytic pyrolysis
The invention discloses a method for directionally producing syngas by chemical chain gasification of sludge. The method comprise the steps of 1, inputting an oxygen carrier, carrier gas and sludge into a sludge reactor to perform a chemical chain gasification reaction, wherein reaction temperature is 850-950 DEG C, and an oxygen carrier reaction time is 4-15s to acquire the syngas, sludge ash and the reduction state oxygen carrier; 2, inputting the acquired syngas, sludge ash and reduction oxygen carrier into a primary separation system to separate the reduction state oxygen carrier to acquire a mixture of the syngas and the sludge ash, wherein separation temperature is 350-500 DEG C, and cutting diameters are 2-4mm. According to the method for directionally producing syngas by chemical chain gasification of sludge provided by the invention, chemical chain gasification is used for directionally converting organics in the sludge into the high-quality syngas, gas proportion in the syngas can be directionally adjusted via the different reactions, and the oxygen carrier has a catalytic pyrolysis effect on tar generated by the reactions; the syngas is relatively low in tar content, and applicable to gas power generation, FT synthesis or fuel cells.
Owner:GUANGZHOU INST OF ENERGY CONVERSION - CHINESE ACAD OF SCI
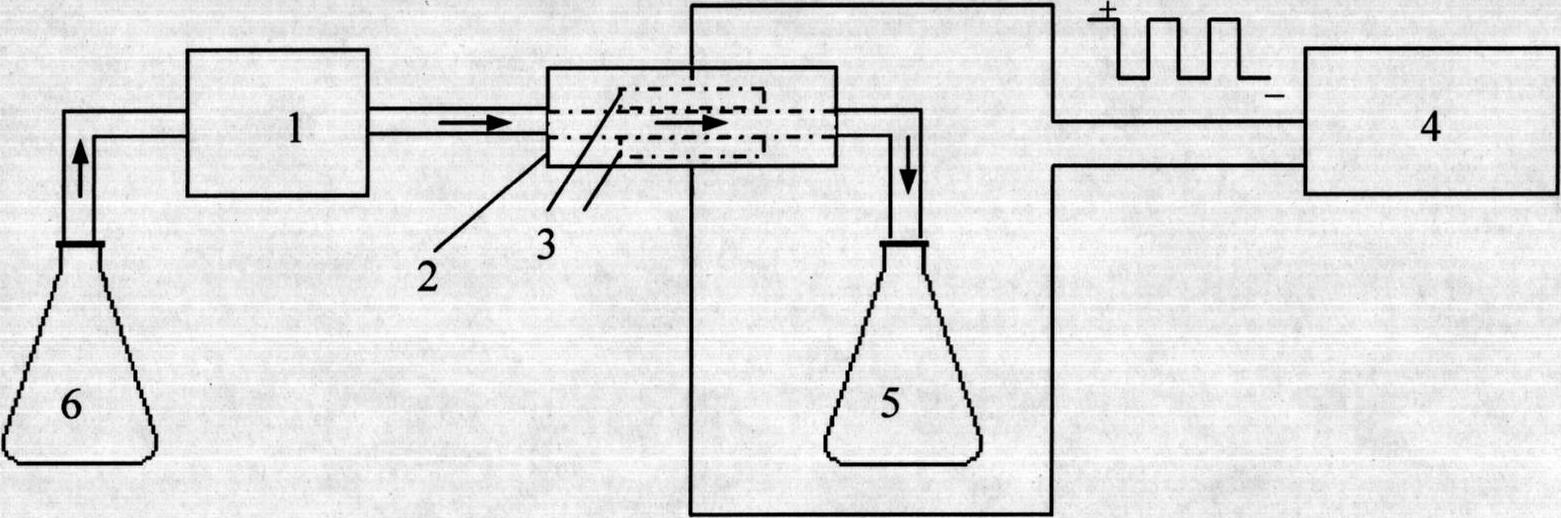
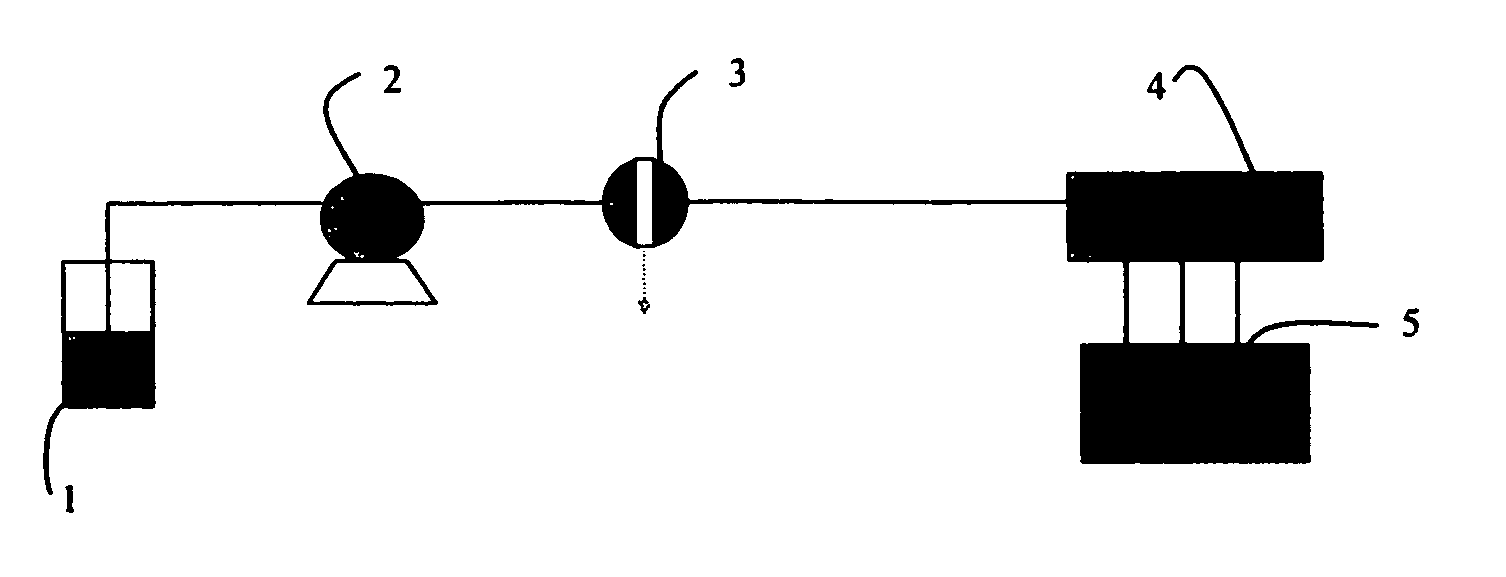
Method for continuously and electrically synthesizing spiro[4.5]trienone by using micro-reaction device
ActiveCN111235598AImprove reaction efficiencySimple and fast operationElectrolysis componentsElectrolytic organic productionElectrolysisDiselenide
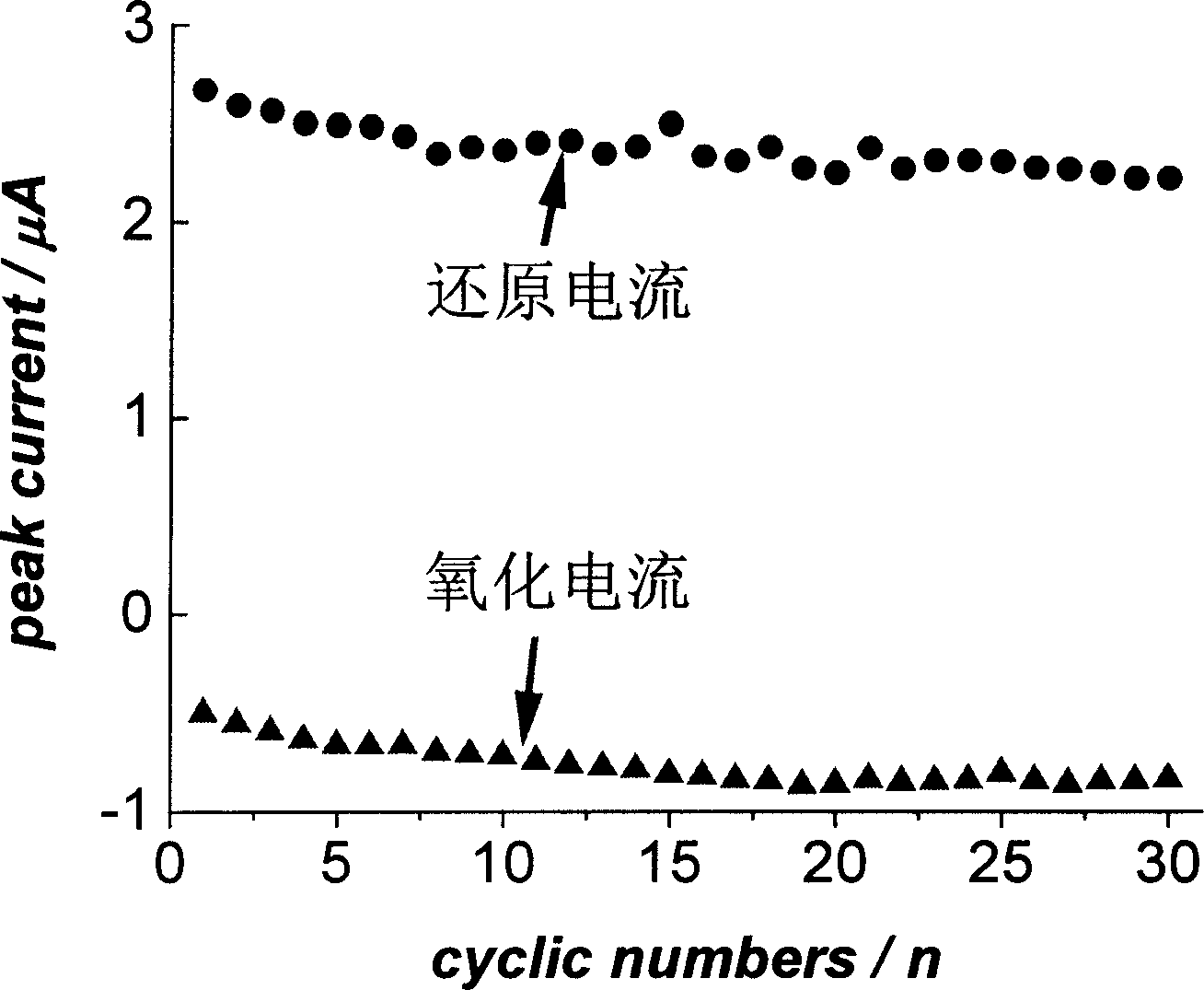
The invention discloses a method for continuously and electrically synthesizing spiro[4.5]trienone by using a micro-reaction device. According to the method, an alkynyl compound and diselenide are taken as reaction raw materials, a graphite plate is taken as an anode and a platinum sheet is taken as a cathode, a continuous electrolytic reaction is carried out in a micro-channel reaction device inthe presence of an electrolyte and a solvent, and the spiro[4.5]trienone is obtained through a free radical cascade reaction and dearomatization. The invention provides the novel preparation method ofthe spiro[4.5]trienone, and the preparation method is green, safe and efficient in technological operation and mild in reaction condition.
Owner:CHINA PHARM UNIV
Method for synthesizing monascus yellow pigment through direct electroreduction of monascus red pigment
ActiveCN103074640AStable in natureReduce manufacturing costElectrolysis componentsArtificial dyesBiotechnologyAnimal science
The invention relates to a monascus yellow pigment preparation method, in particular to a method for synthesizing a monascus yellow pigment through direct electroreduction of a monascus red pigment. In the method, the monascus red pigment is taken as a raw material, an organic electrosynthesis method is used according to the following steps: dissolving the monascus red pigment in a small amount of ethanol, then adding distilled water and electrolyte to the monascus red pigment solution; preparing an electrolyte solution as an anode liquor; by taking an diaphragm electrolytic cell as an electrolytic bath and using a constant current method, evaporating anode electrolysate in a rotating way so as to remove the ethanol; separating out monascus yellow pigment precipitates; filtering the monascus yellow pigment precipitates so as to remove the electrolytes; and drying the filtered monascus yellow pigment precipitates so as to obtain the monascus yellow pigment. According to the invention, by using an electrochemical method, the monascus yellow pigment is directly modified to obtain the monascus yellow pigment; toxic substances are not used in the modifying process, the modifying process is safe and environment friendly, and the property of the obtained yellow pigment is stable; and moreover, the method for synthesizing the monascus yellow pigment through direct electroreduction of the monascus red pigment has the advantages of low production cost, simplicity and convenience in operation, obvious economic benefits and obvious application prospects.
Owner:厦门金色田园生物科技有限公司
A method for preparing concave cubic platinum-rare earth alloy nanocrystals based on a eutectic solvent
InactiveCN108767267APrecise particle size controlEnhance poisoning abilityMaterial nanotechnologyCell electrodesRare earthAlloy
A method for preparing concave cubic platinum-rare earth alloy nanocrystals based on a eutectic solvent is disclosed, and belongs to the technical field of fuel cell catalyst preparation. The eutecticsolvent which is novel, green and non-aqueous is adopted as a medium to prepare the concave cubic platinum-rare earth alloy nanocrystals with a surface of a {hk0} high-index crystal face structure byelectrochemical cyclic voltammetry without the need of any surfactant. The high-index crystal face has an open type surface structure so that a high number of active sites can be provided and catalyst performance can be significantly improved through a double-function mechanism of an alloyed surface electron structure, and therefore, the nanocrystals have excellent electrocatalytic performance for fuel molecules such as formic acid, methanol and ethanol, and have broad application prospects in the field of fuel cells, the field of electrosynthesis, and the like.
Owner:XUZHOU NORMAL UNIVERSITY
Electrolytic apparatus, methods purification of aqueous solutions and synthesis of chemicals
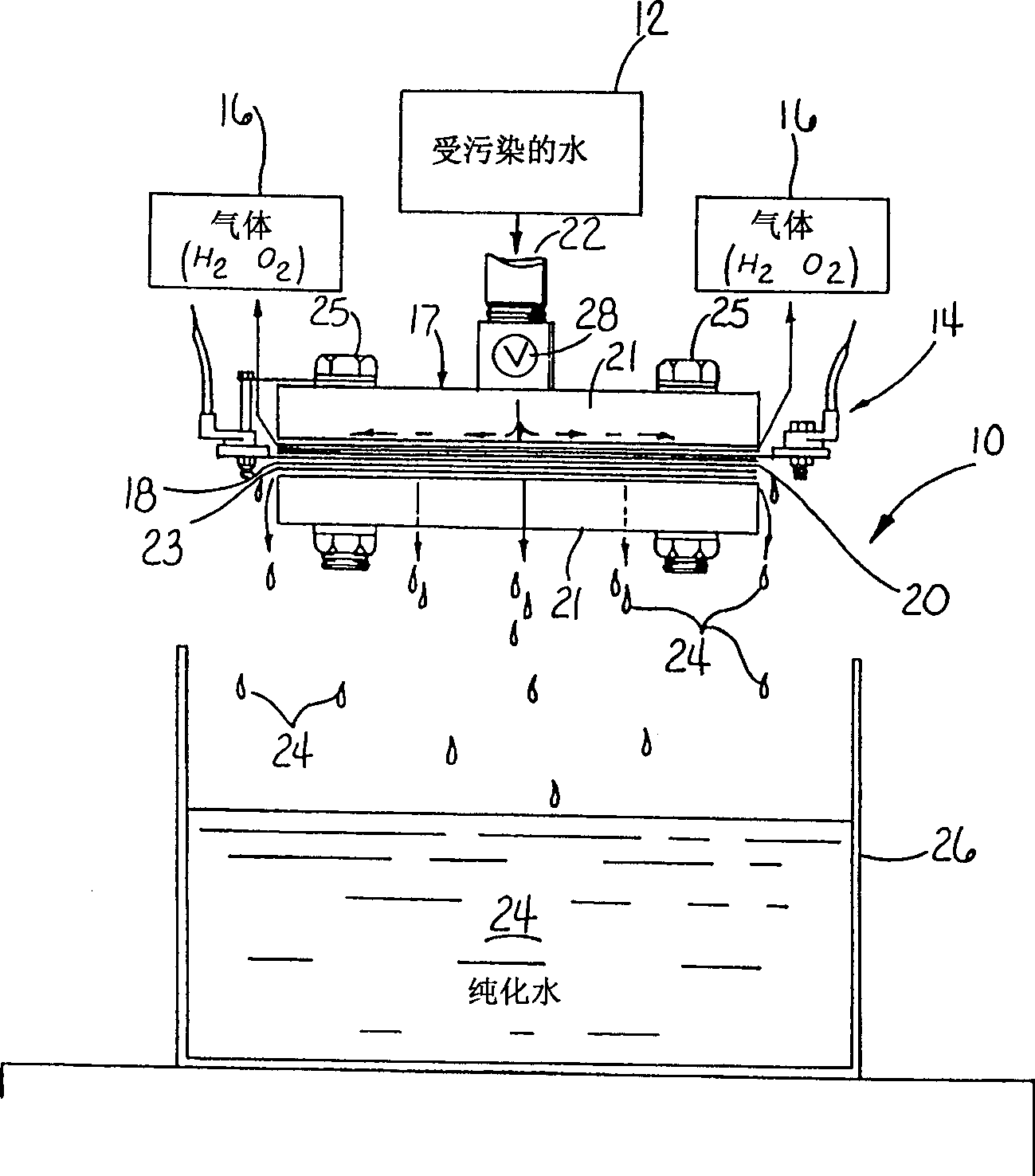
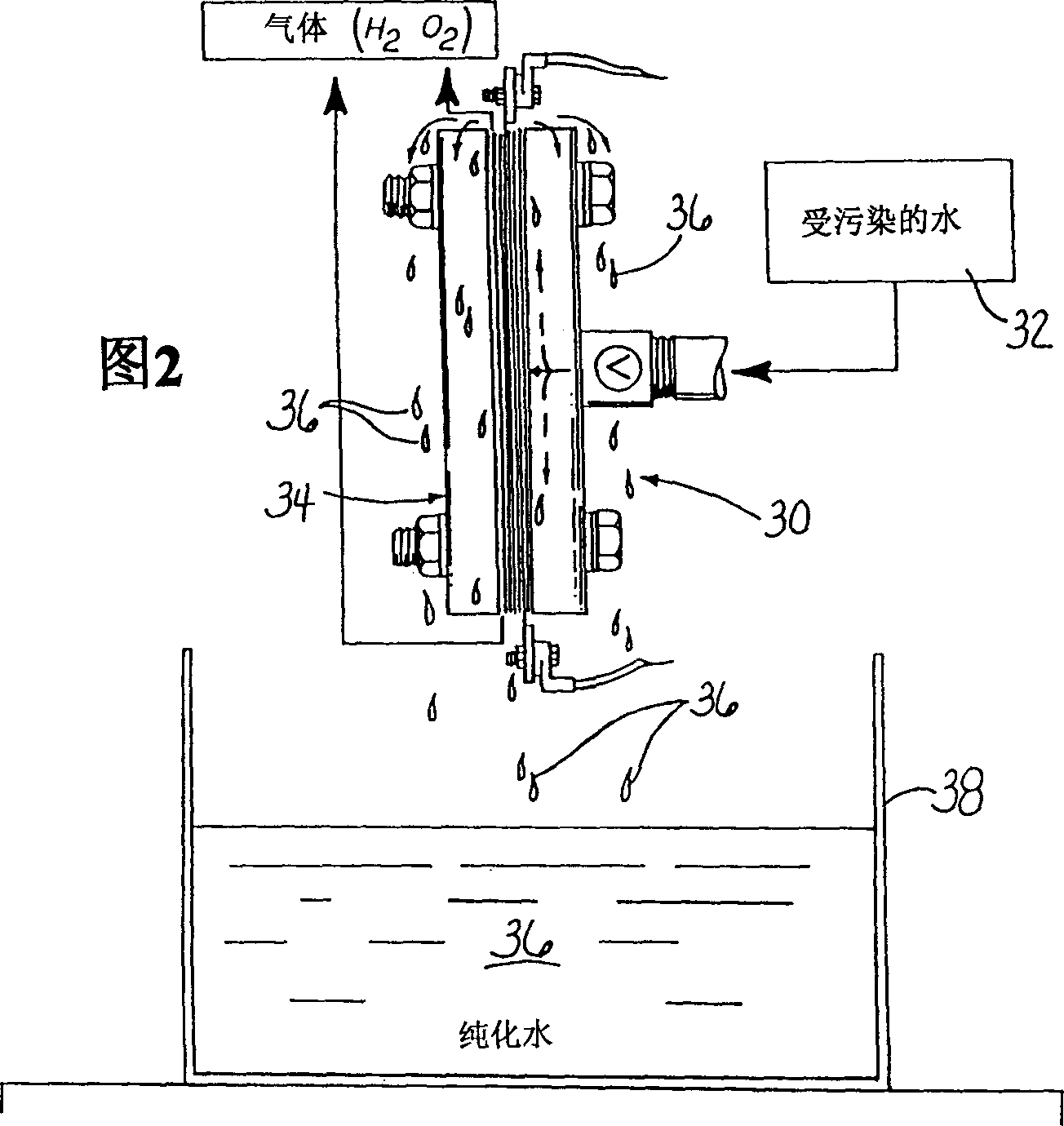
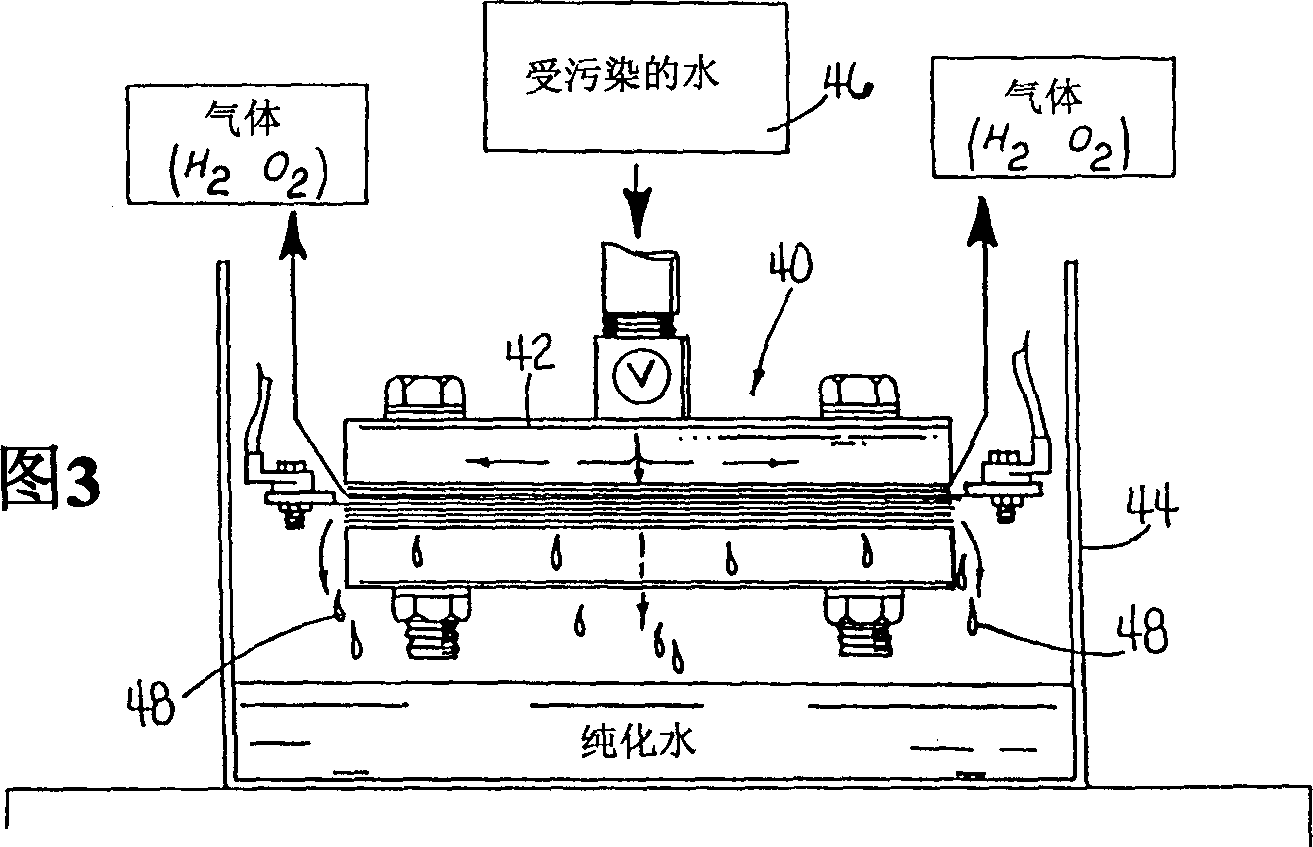
InactiveCN1329576AEconomically competitiveImprove economyCellsWater treatment compoundsHypochloriteElectrolysis
Electropurification of contaminated aqueous media, such as ground water and wastewater from industrial manufacturing facilities like paper mills, food processing plants and textile mills, is readily purified, decolorized and sterilized by improved, more economic open configuration electrolysis cell designs with electrodes comprising a plurality of conductive porous elements in electrical contact with one another. The cells may be divided or undivided, and connected in monopolar or bipolar configuration. When coupled with very narrow capillary gap electrodes more economic operation, particularly when treating solutions of relatively low conductivity is assured. The novel cell design is also useful in the electrosynthesis of chemicals, both organic and inorganic types, such as hypochlorite bleaches and other oxygenated species.
Owner:ZAPPI WATER PURIFICATION SYST
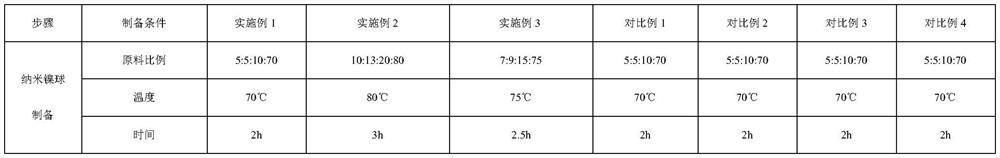
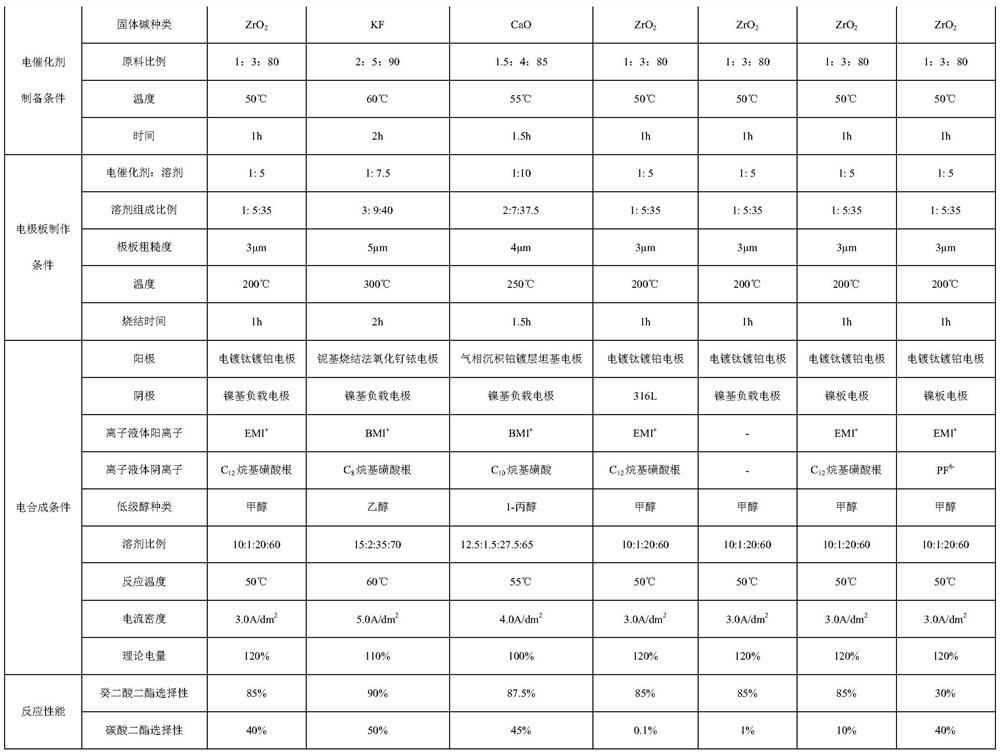
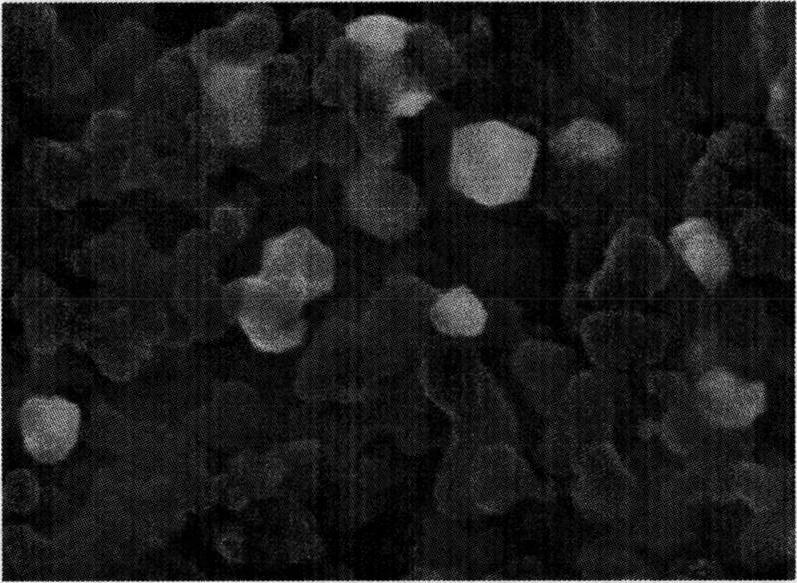
Method for synthesizing carbonic acid diester and sebacic acid diester in pairs
PendingCN112609201APromote enrichmentSolve the problem of fragmentationElectrolysis componentsElectrolytic organic productionElectrolytic agentCombinatorial chemistry
The invention relates to a method for synthesizing carbonic acid diester and sebacic acid diester in pairs. The method realizes electrolytic synthesis of sebacic acid diester and carbonic acid diester in the same electrochemical synthesis system by designing an electrolyte and electrodes. Compared with a traditional electric synthesis process, the atom economy of a reaction is greatly improved, and energy consumption is reduced; and the method has good industrial prospects.
Owner:WANHUA CHEM (SICHUAN) CO LTD +1
Method for preparing Pt/C nanometer catalyst and device thereof
ActiveCN101554597BIncrease output powerHigh selectivityElectrolysis componentsCell electrodesPtru catalystFuel cells
Owner:XIAMEN UNIV
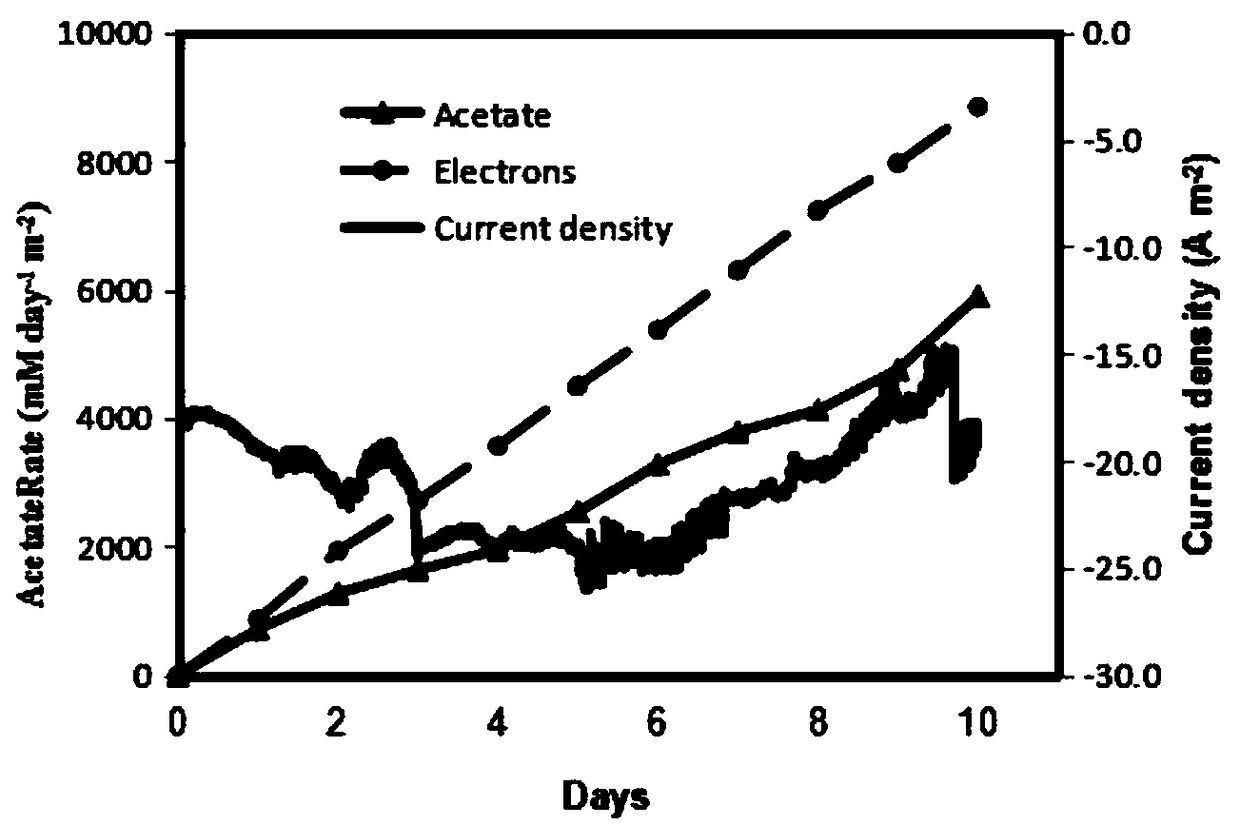
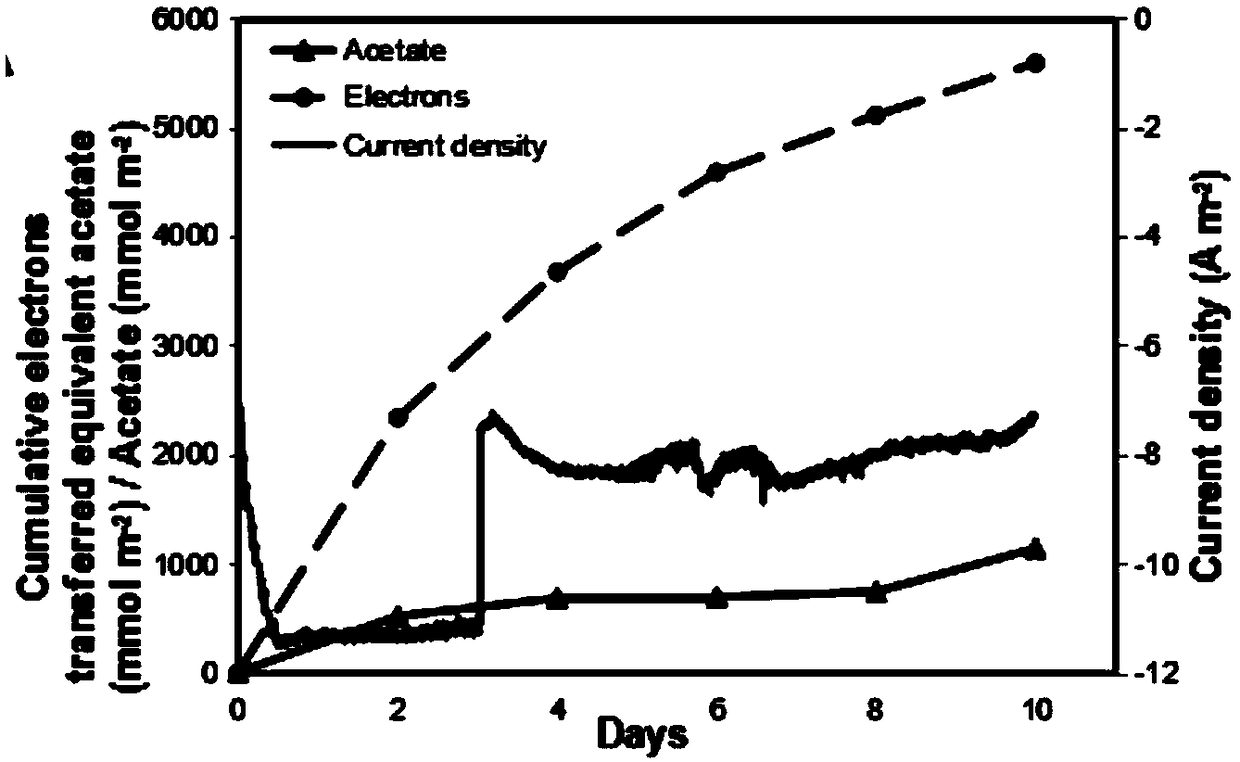
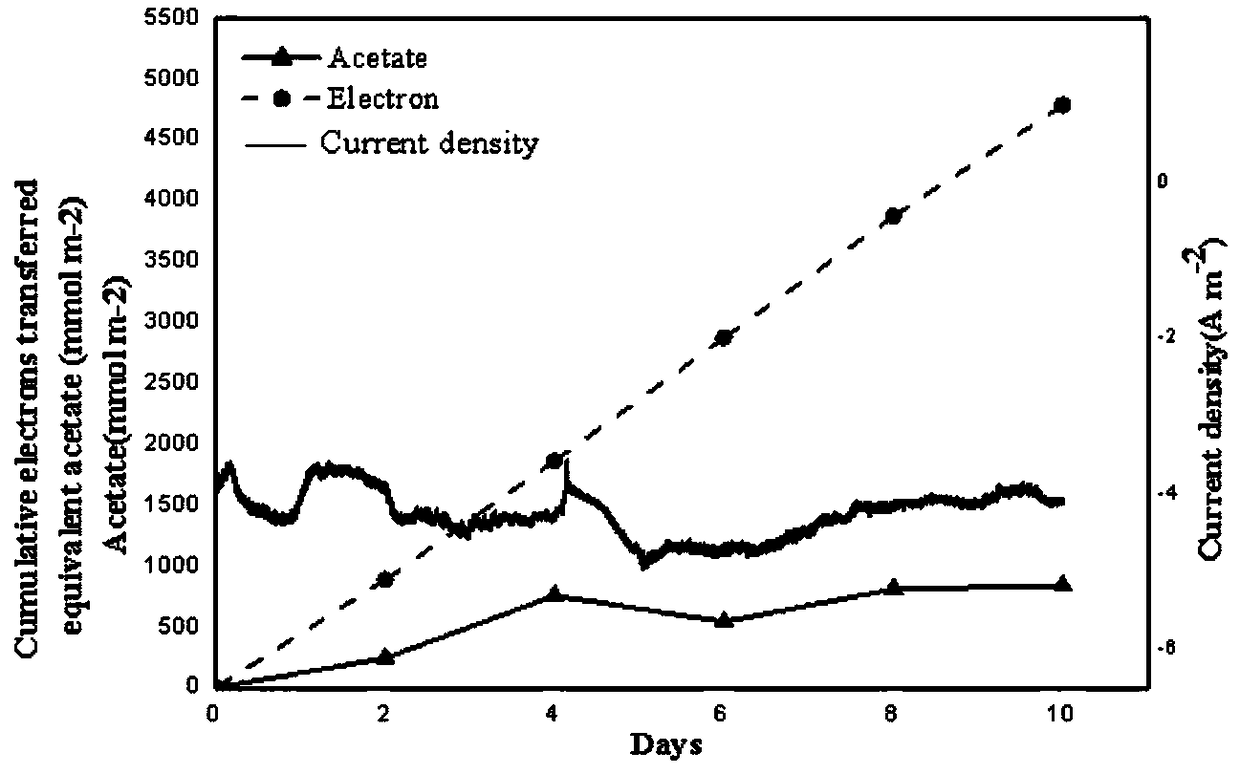
Method for improving biological reduction of CO2 for electrosynthesis of acetic acid through graphene-foam copper composite cathode
ActiveCN109371418AExcellent Physical ChemistryGood biocompatibilityMicroorganism based processesElectrolytic organic productionMaterials scienceBioelectrochemistry
The invention belongs to the field of bioelectrochemistry, and particularly relates to a method for improving biological reduction of CO2 for electrosynthesis of acetic acid through a graphene-foam copper composite cathode. The method comprises the steps that firstly, microorganism culture is conducted; secondly, a double-chamber H-shaped electrolytic tank is constructed, the graphene-foam coppercomposite cathode is adopted as a cathode, microorganism fungi are inoculated into a cathode chamber of the double-chamber H-shaped electrolytic tank to be cultured, the electric potential of the electrode is -990 mV vs SHE, an anode chamber is continuously inflated with N2-CO2(80:20) gas, a cathode chamber is continuously inflated with N2-CO2-H2(83:10:7) gas at the beginning, after running is conducted for 5-6 days, continuous inflation of N2-CO2(80:20) gas is conducted, and running is continuously conducted for 10 days; and a fungus solution is collected, and the content of the acetic acid is measured. A method for acetic acid synthesis through an MES with graphene-foam copper composite materials as the cathode is provided, and the method has the high acetic acid production rate.
Owner:WUHAN UNIV OF TECH
Aeration-free electro-Fenton water treatment method and aeration-free electro-Fenton water treatment device
PendingCN110937667AIncrease productionLow running costWater treatment compoundsWater/sewage treatment by oxidationElectrochemical responseSingle electrode
The invention belongs to the technical field of sewage treatment, and particularly provides an aeration-free electro-Fenton water treatment method and an aeration-free electro-Fenton water treatment device based on an air active diffusion cathode, wherein the method and the device have the advantages of high pollutant removal rate, low energy consumption and high stability. According to the invention, by using an air active diffusion electrode as a cathode of an electrochemical reactor, a large amount of H2O2 can be generated in situ through an oxygen reduction reaction and react with Fe<2+> to generate .OH with high oxidation capacity, so that organic pollutants in wastewater are efficiently degraded; with the method and the device, the defect that the conventional electrochemical water treatment device needs an aeration device to provide oxygen for in-situ generation of H2O2 is overcome, the investment and the operation energy consumption of aeration equipment are reduced, the yieldof H2O2 is improved, and the removal efficiency of pollutants is improved; and the novel water treatment device is simple in structure, low in electrode material cost and mild in operation condition,and can efficiently and electrically synthesize H2O2 in a wide current range so as to be used for electro-Fenton reaction treatment of wastewater.
Owner:NANKAI UNIV
Tin-antimony oxide coating titanium anode material for electrosynthesis of ferrate
The present invention provides a preparation method of Sn-Sb oxide coated anode material, which is used in direct electrochemical synthesis of super ferrate. This anode material has very high over-voltage of oxygen evolution and excellent stability and therefore electrochemical oxidation reaction can happen to trivalent iron compound, forming super ferrate without oxygen evolution. Besides, electrochemical redox reaction between super ferrate and trivalent iron compound on this anode material has good reversibility. It is easy to realize mass production. The anode material is cheap and does not pollute environment, therefore, the method is suitable for the preparation of high specific energy second batteries. It also has application value and potential in the treatment of alkaline waste water.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

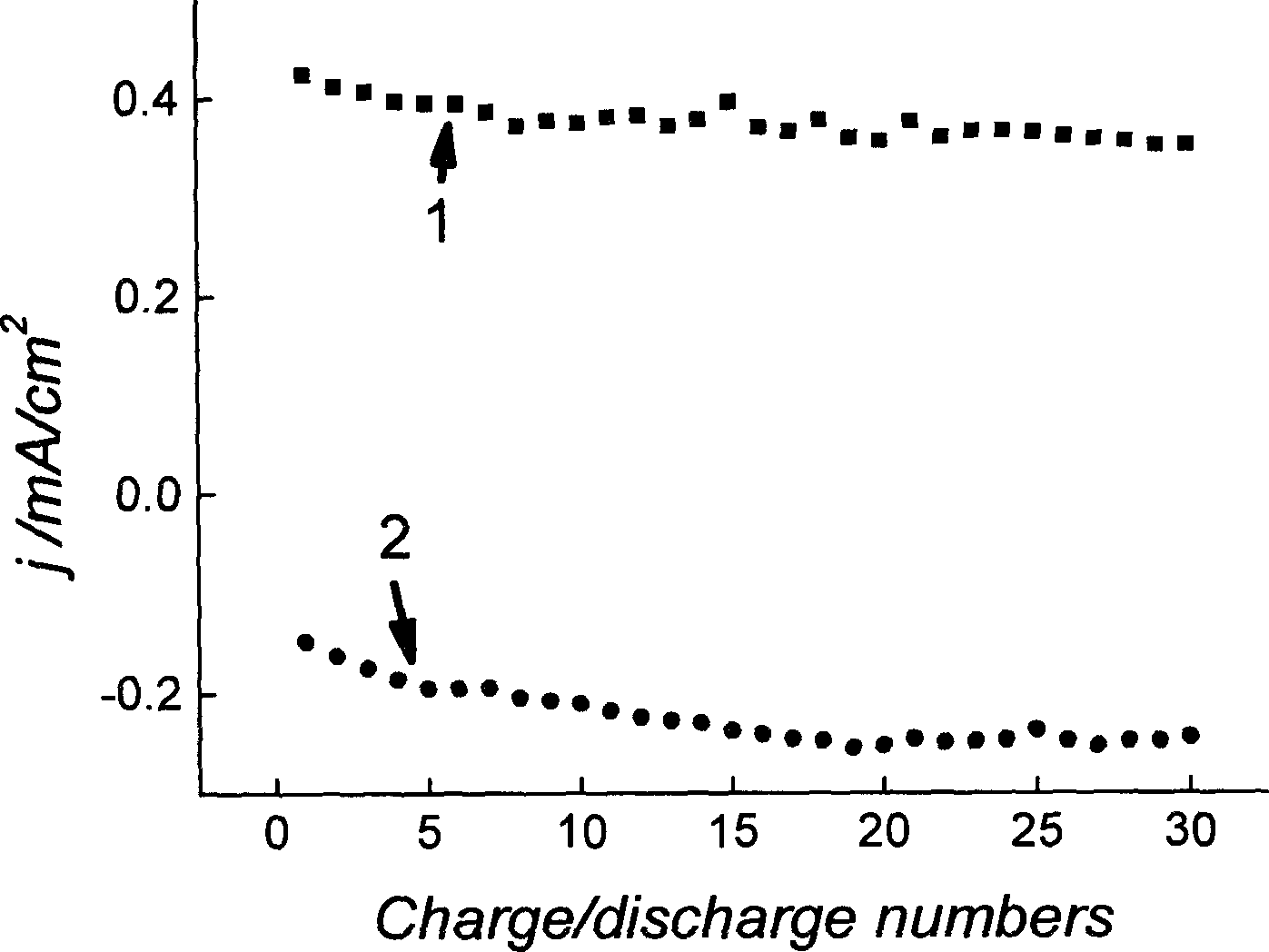
![Method for continuously and electrically synthesizing spiro[4.5]trienone by using micro-reaction device Method for continuously and electrically synthesizing spiro[4.5]trienone by using micro-reaction device](https://images-eureka.patsnap.com/patent_img/437cf3cf-fb84-4fb4-a35f-de8fc3c0f4af/HDA0002369971260000011.png)
![Method for continuously and electrically synthesizing spiro[4.5]trienone by using micro-reaction device Method for continuously and electrically synthesizing spiro[4.5]trienone by using micro-reaction device](https://images-eureka.patsnap.com/patent_img/437cf3cf-fb84-4fb4-a35f-de8fc3c0f4af/FDA0002369971240000011.png)
![Method for continuously and electrically synthesizing spiro[4.5]trienone by using micro-reaction device Method for continuously and electrically synthesizing spiro[4.5]trienone by using micro-reaction device](https://images-eureka.patsnap.com/patent_img/437cf3cf-fb84-4fb4-a35f-de8fc3c0f4af/FDA0002369971240000012.png)