Method for synthesizing carbonic acid diester and sebacic acid diester in pairs
A technology of sebacic acid diester and carbonic acid diester, which is applied in the field of organic electrochemical synthesis, can solve the problems of low carbon dioxide solubility, poor atom economy, and inability to effectively use carbon dioxide, so as to improve atom economy, reduce reaction energy consumption, The effect of good industrialized values
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] Preparation of Nickel-based Nanospheres Supported Solid Alkali Electrode
[0026] (1) Use Ni 2 SO 4 :NH 3 ·H 2 O:CH 3 OH:H 2 O=5-10:5-13:10-20:70-80 The solution is heated to 70-80° C., the reaction time is 2-3 hours, and the resulting black precipitate is centrifuged to obtain nickel-based nanospheres.
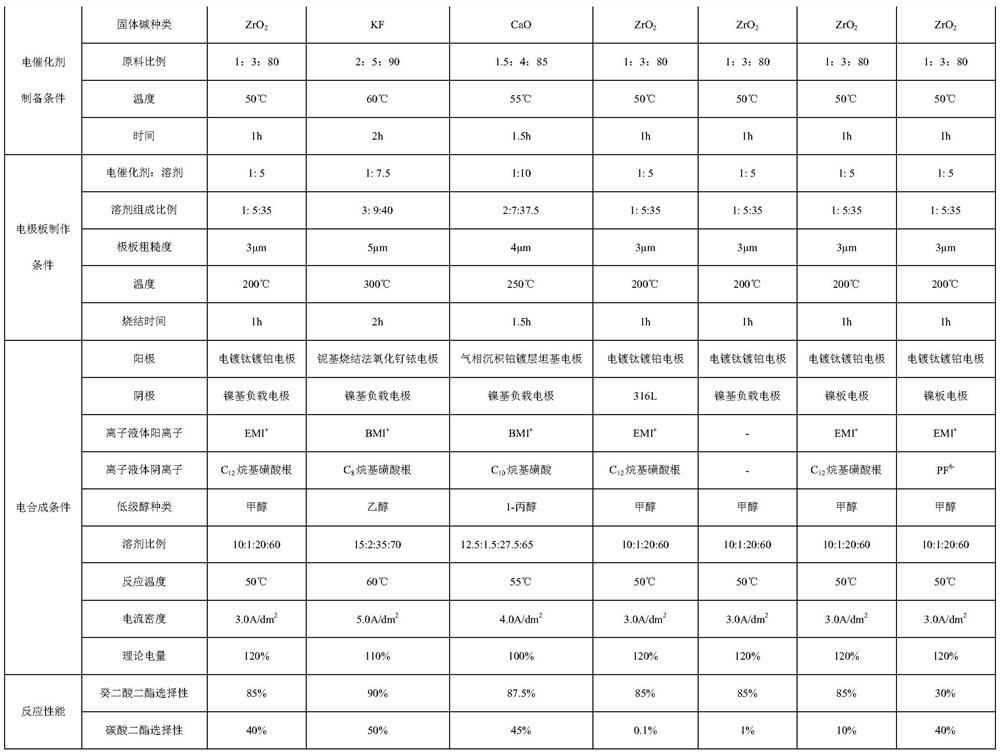
[0027] (2) Solid base catalyst can choose KF, ZrO 2 , a kind of CaO, solid base catalyst: nickel-based nanospheres: ethanol = 1-2: 3-5: 80-90, reflux heating at 50-60 ° C for 1-2 h, and then evaporate the ethanol to dryness .
[0028] (3) Put the nickel-based nanosphere-supported solid base catalyst prepared above into a mixed solvent of isopropanol: water: paraffin oil = 1-3:5-9:35-40 in a ratio of 1-2:10 dispersion. Grind the nickel plate to a roughness of 3-5μm, evenly coat the electrocatalyst on the nickel plate and sinter, the temperature is controlled between 200-300°C, the sintering time is 1-2h, and the nickel-based nanosphere-supported solid is obtain...
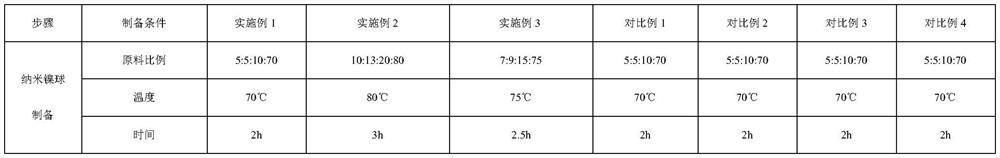
Embodiment 1
[0048] Preparation of Nickel-based Nanospheres Supported Solid Alkali Electrode
[0049] (1) Use Ni 2 SO 4 :NH 3 ·H 2 O:CH 3 OH:H 2 The O=5:5:10:70 solution was heated to 70° C., the reaction time was 2 hours, and the resulting black precipitate was centrifuged.
[0050] (2) ZrO 2 : Nickel-based nanospheres: ethanol = 1:3:80, heated under reflux at 50° C. for 1 h, and then evaporated the ethanol to dryness.
[0051] (3) Put the nickel-based nanosphere-supported solid base catalyst prepared above into a mixed solvent of isopropanol: water: paraffin oil = 1:5:35 at a ratio of 1:5 for dispersion. The nickel pole plate was polished to a roughness of 3 μm, and the electrocatalyst was evenly coated on the nickel pole plate for sintering. The temperature was controlled between 200 ° C and the sintering time was 1 h, and the solid alkali electrode supported by nickel-based nanospheres was prepared.
[0052] Pairwise electrosynthesis of carbonate diester and sebacate diester
...
Embodiment 2
[0057] Preparation of Nickel-based Nanospheres Supported Solid Alkali Electrode
[0058] (1) Use Ni 2 SO 4 :NH 3 ·H 2 O:CH 3 OH:H 2 The O=10:13:20:80 solution was heated to 80° C., the reaction time was 3 hours, and the resulting black precipitate was centrifuged.
[0059] (2) KF: nickel-based nanospheres: ethanol = 2:5:90, heated under reflux at 60° C. for 2 h, and then evaporated the ethanol to dryness.
[0060] (3) Put the nickel-based nanosphere-supported solid base catalyst prepared above into a mixed solvent of isopropanol: water: paraffin oil = 3:9:40 at a ratio of 1:7.5 for dispersion. The nickel pole plate was polished to a roughness of 5 μm, and the electrocatalyst was evenly coated on the nickel pole plate for sintering. The temperature was controlled between 300°C and the sintering time was 2h, and the solid alkali electrode supported by nickel-based nanospheres was prepared.
[0061] Pairwise electrosynthesis of carbonate diester and sebacate diester
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electric current | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com