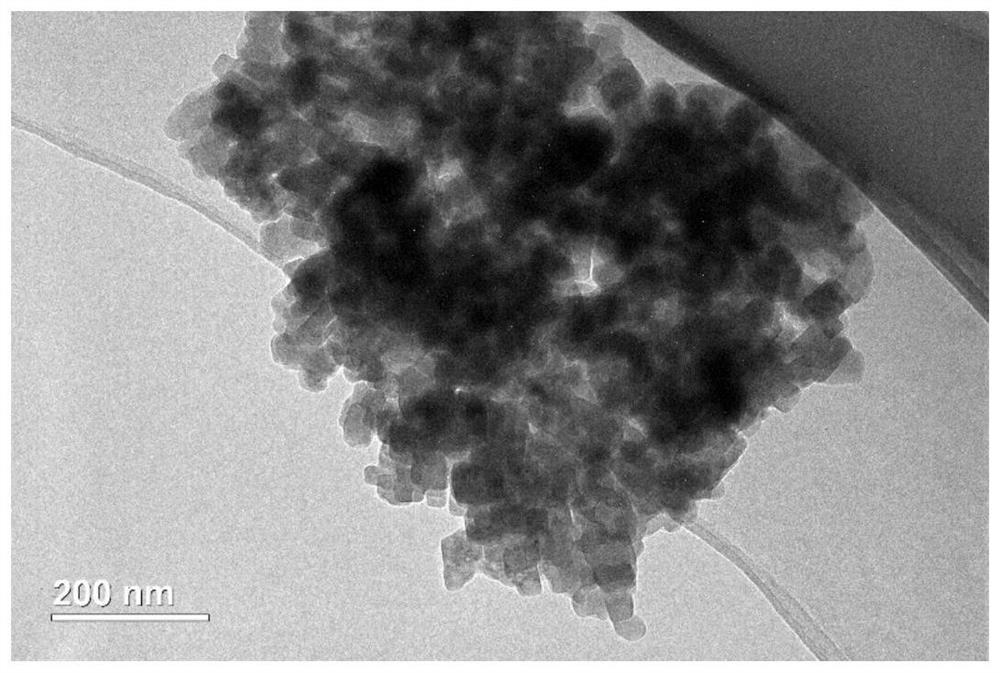
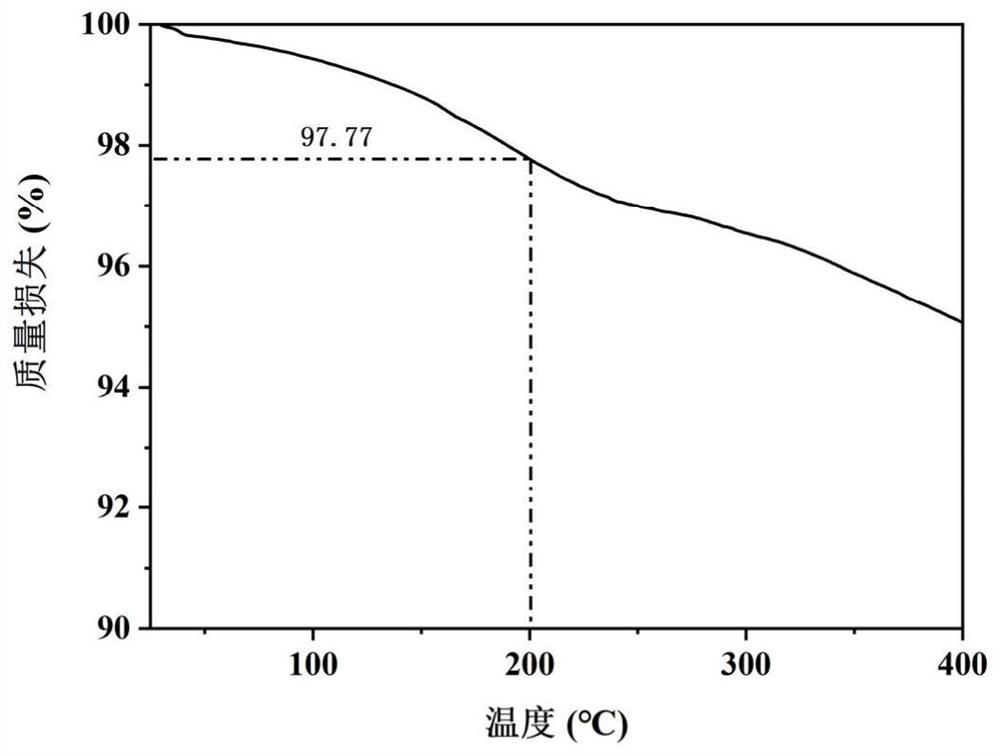
Patents
Literature
116 results about "Ferricyanide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ferricyanide is the anion [Fe(CN)₆]³⁻. It is also called hexacyanoferrate(III) and in rare, but systematic nomenclature, hexacyanidoferrate(III). The most common salt of this anion is potassium ferricyanide, a red crystalline material that is used as an oxidant in organic chemistry.
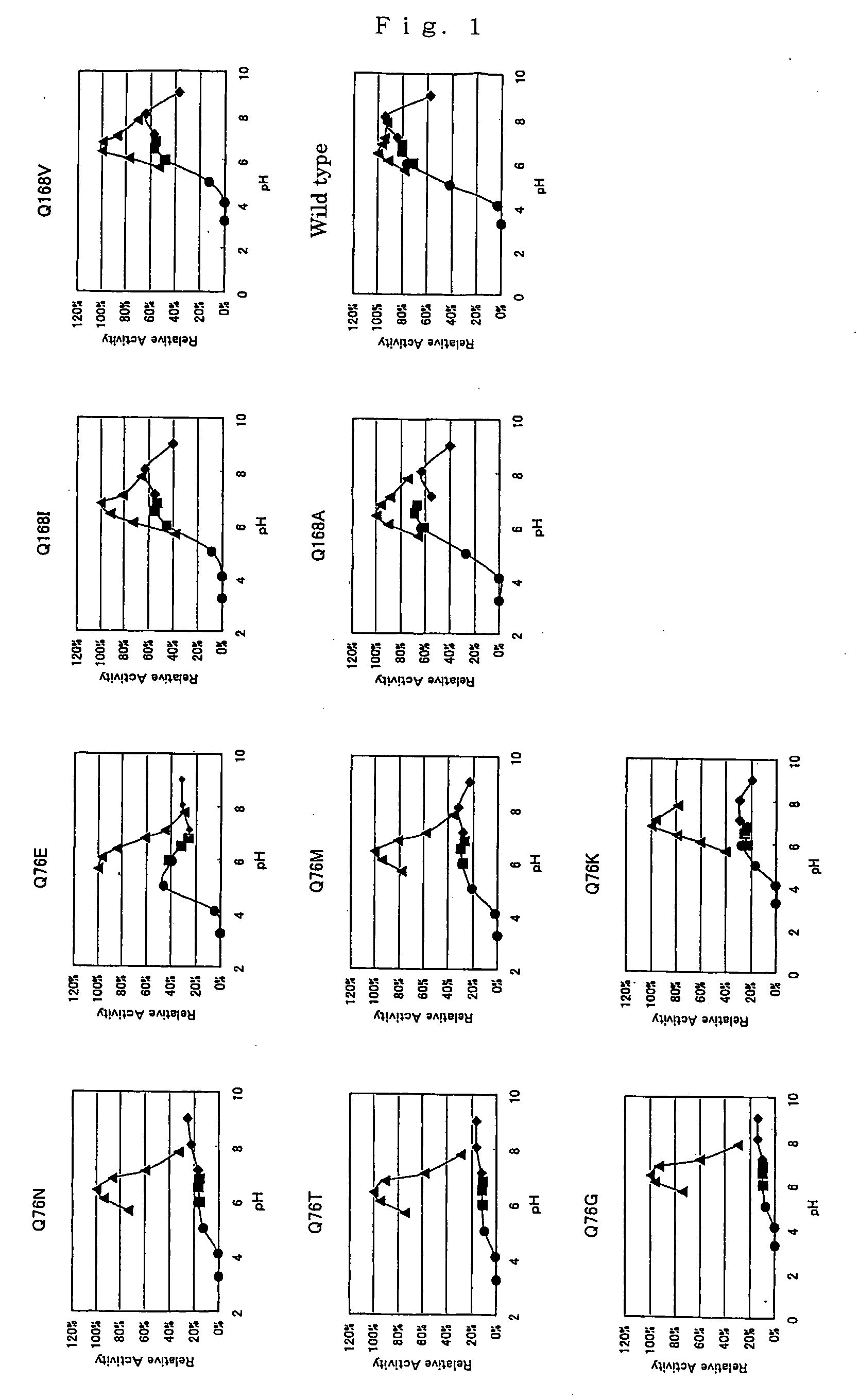
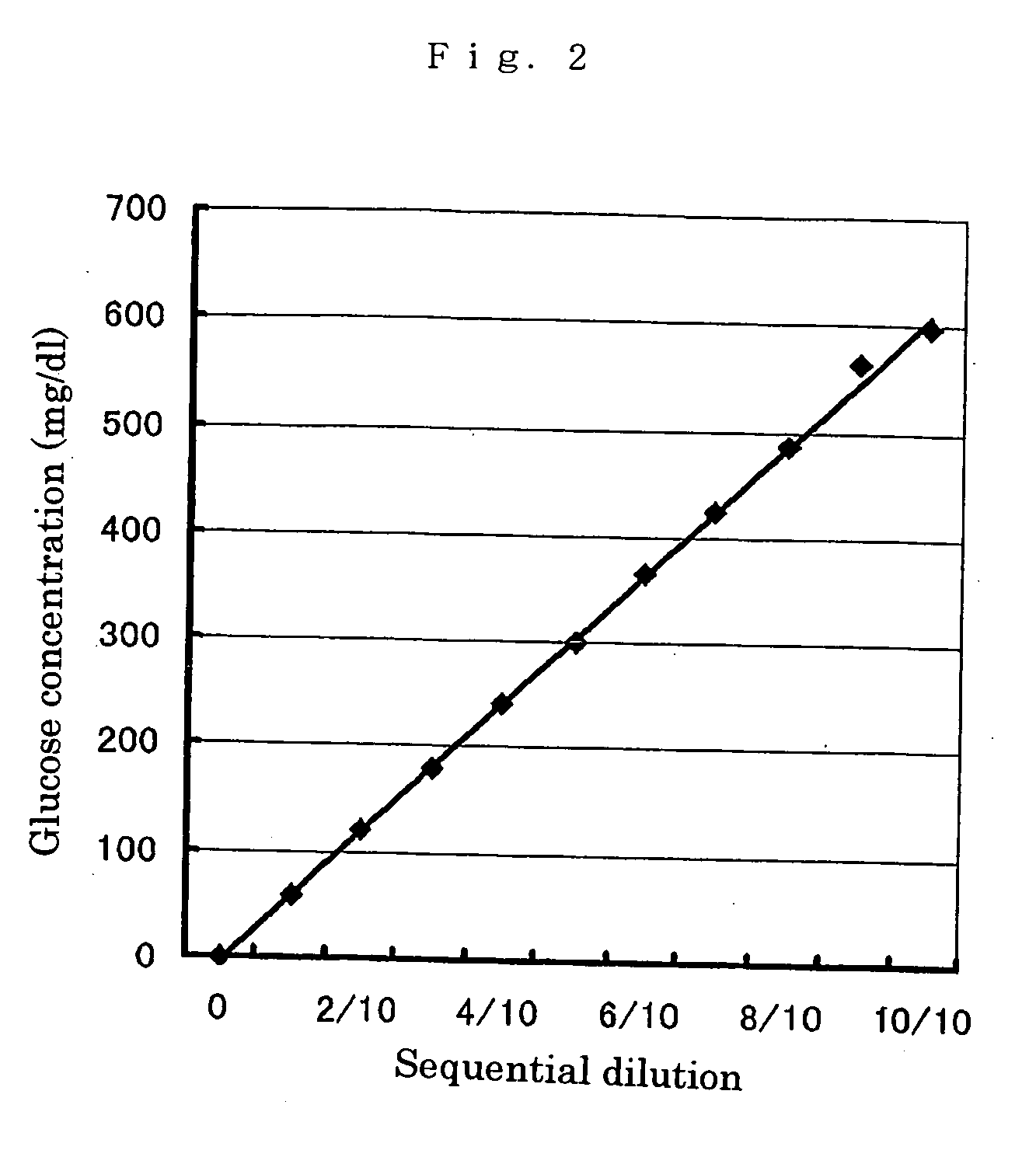
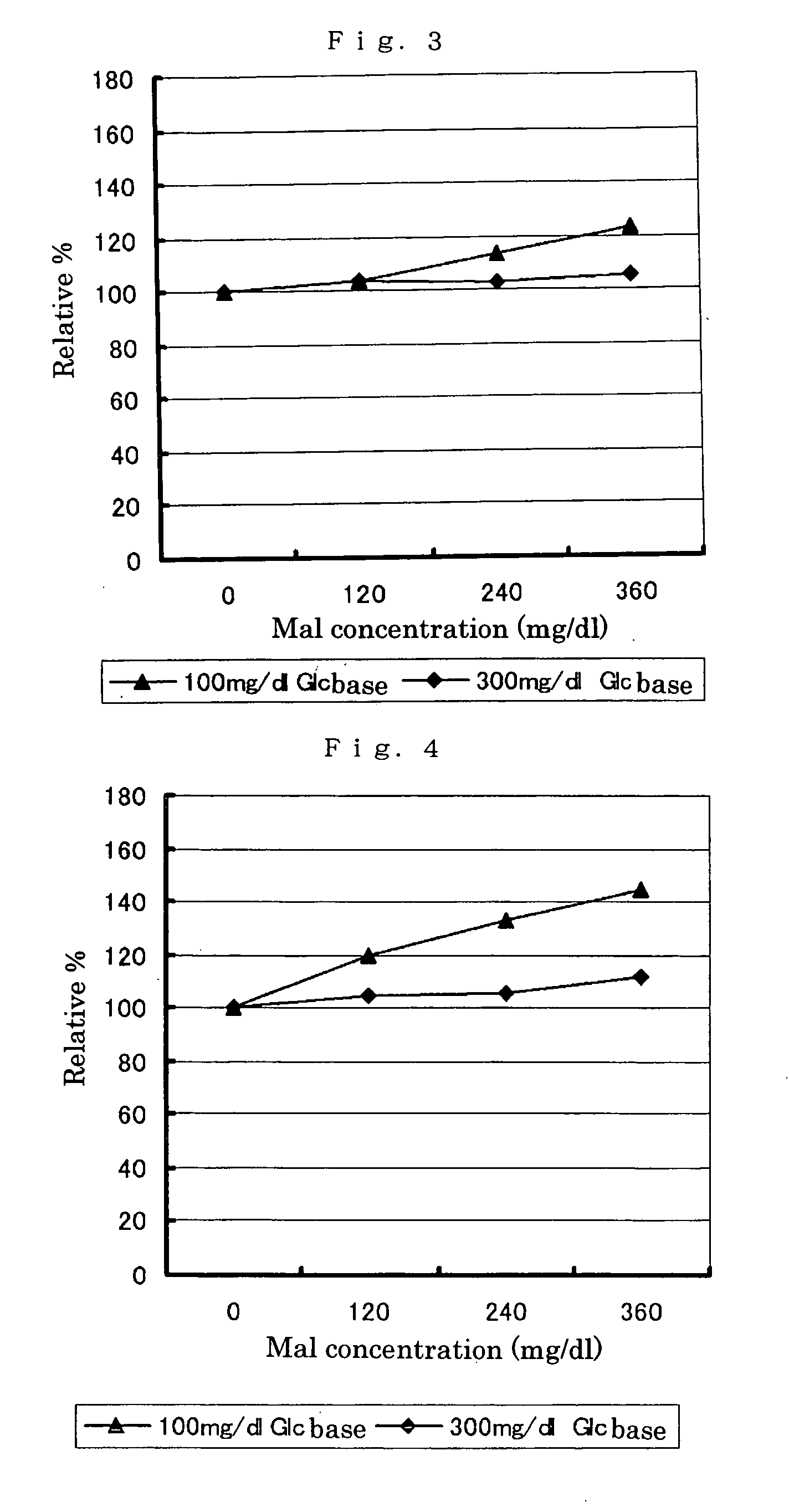
Modified pyrroloquinoline quinone (pqq) dependent glucose dehydrogenase excellent in substrate specificity
InactiveUS20070105173A1Action propertyProcess stabilityBioreactor/fermenter combinationsFungiWild typePyrroloquinoline quinone
PQQGDH having an improved substrate specificity or having an improved specific activity in an assay system using ferricyanide ion as a mediator is provided. Modified PQQGDH having the enhanced substrate specificity by introducing an amino acid mutation in a particular region of PQQGDH, and a method of enhancing the specific activity compared with a wild type in the assay system using the ferricyanide ion as the mediator by deleting, substituting, or adding one or more amino acids in an amino acid sequence of the wild type pyrroloquinoline quinone dependent glucose dehydrogenase.
Owner:TOYO TOYOBO CO LTD
Method for synthesizing high-sodium iron-based Prussian blue electrode material
InactiveCN104282908AImprove electrochemical performanceLow costCell electrodesSecondary cellsHigh sodiumSodium-ion battery
The invention provides a method for synthesizing a high-sodium iron-based Prussian blue electrode material. The high-sodium iron-based Prussian blue electrode material comprises the following raw materials in percentage by mole: 25-43 percent of iron ion-containing metal salt, 25-43 percent of ferricyanide ion-containing metal salt and 14-50 percent of a complexing agent. The method comprises the following steps: 1, adding the iron ion-containing metal salt and the complexing agent into a container according to the ratio of the raw materials, adding a solvent, mixing, thereby obtaining a solution A; 2, adding the ferricyanide ion-containing metal salt into the container according to the ratio of the raw materials, adding the solvent, mixing, thereby obtaining a solution B; 3, mixing the solution A and the solution B, and reacting for 2-24 hours; and 4, respectively washing the obtained materials for several times by using water and ethanol, removing the impurities, drying, thereby obtaining the high-sodium iron-based Prussian blue electrode material. The obtained material serves as a sodium ion battery cathode material and has excellent electrochemical performance. The method is low in cost, readily available in raw materials, simple in process and easy for industrial production.
Owner:张五星
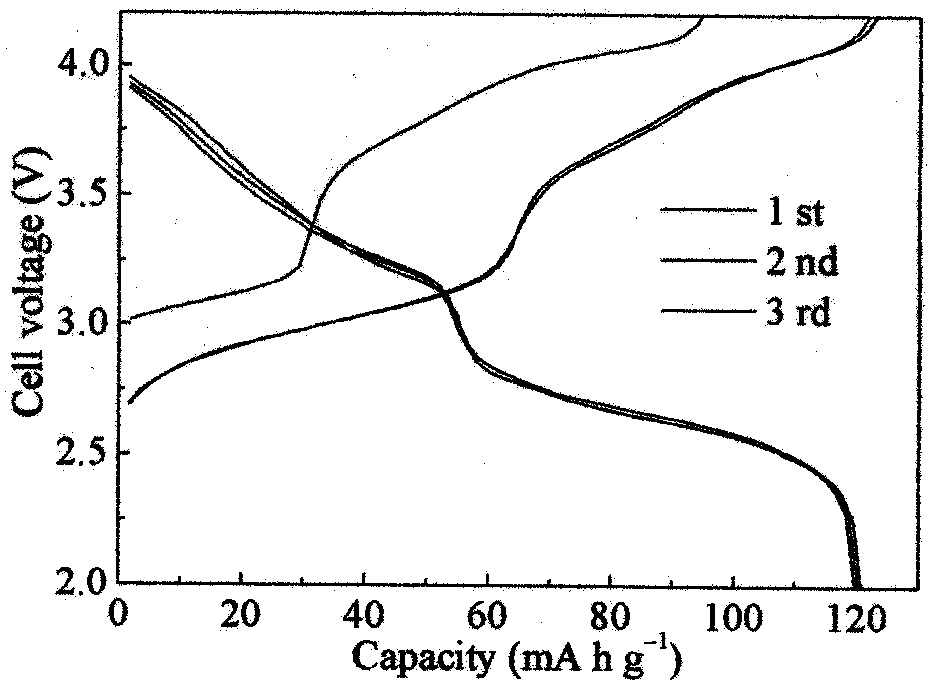
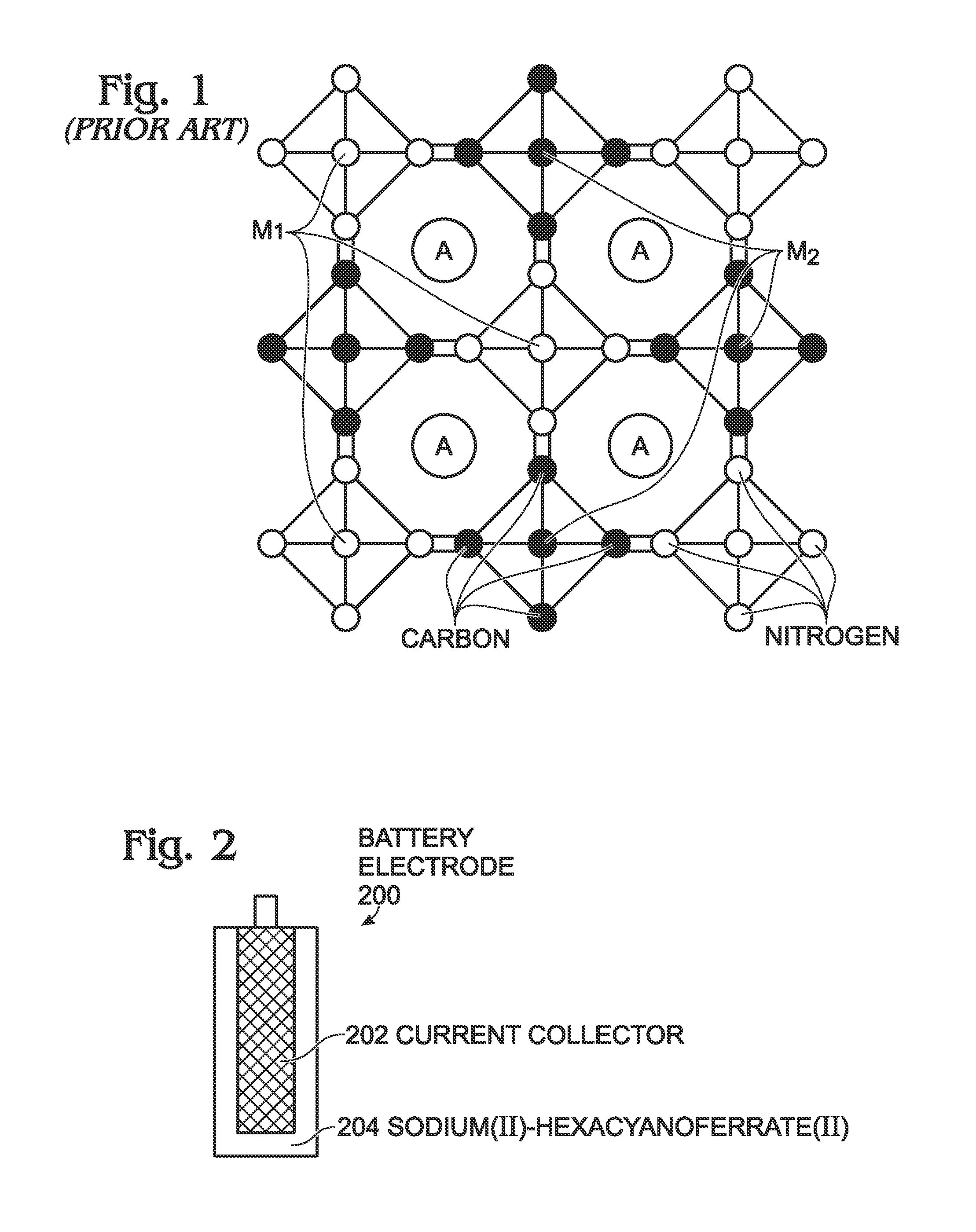
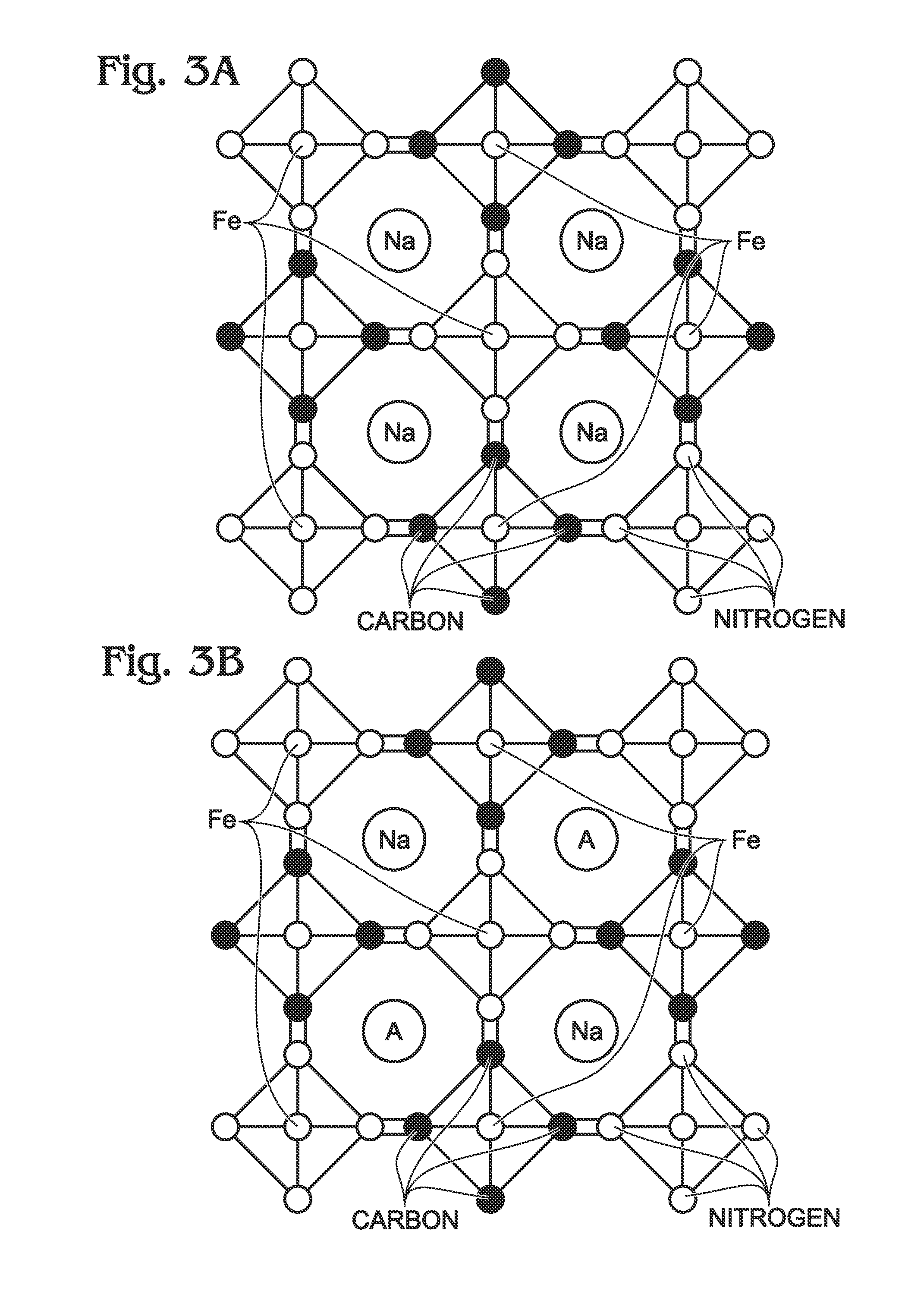
Sodium Iron(II)-Hexacyanoferrate(II) Battery Electrode and Synthesis Method
A method is provided for synthesizing sodium iron(II)-hexacyanoferrate(II). A Fe(CN)6 material is mixed with the first solution and either an anti-oxidant or a reducing agent. The Fe(CN)6 material may be either ferrocyanide ([Fe(CN)6]4−) or ferricyanide ([Fe(CN)6]3−). As a result, sodium iron(II)-hexacyanoferrate(II) (Na1+XFe[Fe(CN)6]Z.MH2O is formed, where X is less than or equal to 1, and where M is in a range between 0 and 7. In one aspect, the first solution including includes A ions, such as alkali metal ions, alkaline earth metal ions, or combinations thereof, resulting in the formation of Na1+XAYFe[Fe(CN)6]Z.MH2O, where Y is less than or equal to 1. Also provided are a Na1+XFe[Fe(CN)6]Z.MH2O battery and Na1+XFe[Fe(CN)6]Z.MH2O battery electrode.
Owner:SHARP KK
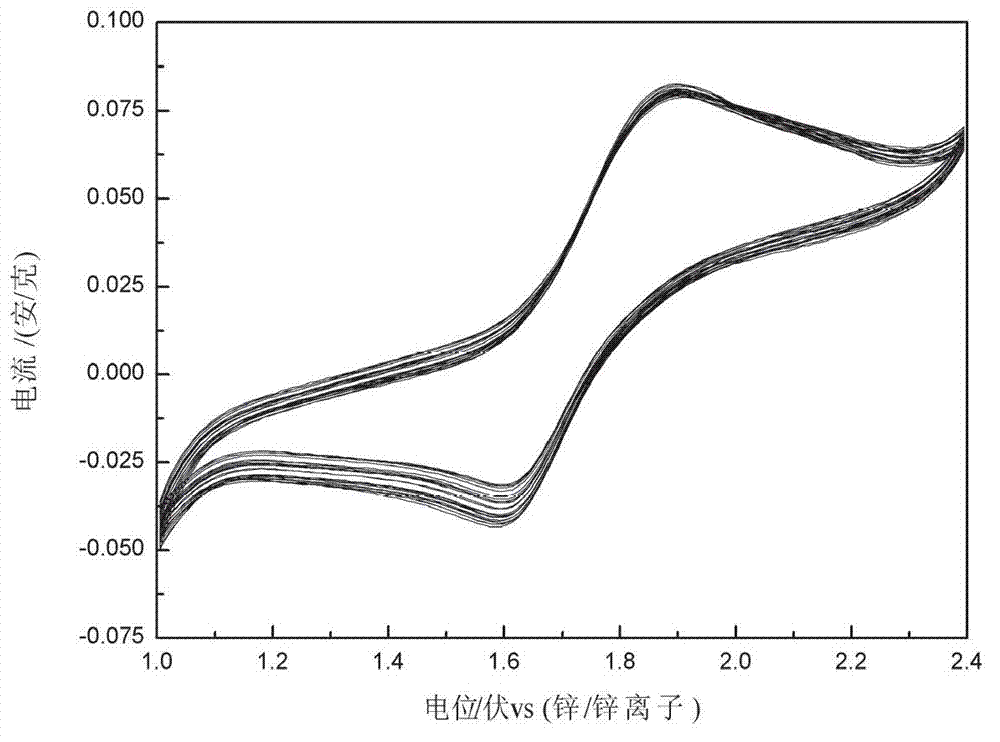
Aqueous electrolyte rechargeable zinc ion battery
InactiveCN102903917AHigh specific capacityFast chargingCell electrodesSecondary cellsZinc ionAqueous electrolyte
The invention discloses an aqueous electrolyte rechargeable zinc ion battery, belonging to the technical field of battery preparation. The battery disclosed herein is characterized in that: the cathode active material mainly comprises zinc ions (Zn<2+>)- intercalated and -removed copper (nickel) ferricyanide, the anode active material mainly comprises zinc, and the electrolyte which is liquid or gel comprises soluble salts of zinc as the solute and water as the solvent. According to the invention, the zinc ions can be intercalated in the copper (nickel) ferricyanide cathode material lattices or be removed from the copper (nickel) ferricyanide cathode material lattices, simultaneously the anode material mainly comprising zinc is subject to oxidation or reduction, thus the battery has the characteristics of high specific capacity and rapid charging, and the battery can be widely applied in the fields of portable mobile terminal, equipment, miniature electric vehicle and automobile starting battery, etc.
Owner:TSINGHUA UNIV
Preparation of Prussian blue photo-Fenton catalyst and method for degrading organic pollutant
InactiveCN102962063AReusableThe method is simpleWater/sewage treatment by irradiationMolecular sieve catalystsCatalyst degradationPotassium ferricyanide
The invention provides preparation of a Prussian blue photo-Fenton catalyst; ferric trichloride, ferric nitrate, ferric sulfate or ammonium ferrous sulfate, potassium ferricyanide, and potassium ferrocyanide are used as raw materials, and are dissolved in ultrapure water respectively to prepare a solution containing iron ions and ferricyanide ions; molecular sieve, kaolin or construction waste are used as carriers, and Prussian blue is fixed on the carriers to obtain fixed Prussian blue photo-Fenton catalyst. The invention also provides a method for degrading organic pollutants by the Prussian blue photo-Fenton catalyst, which comprises mixing of the prepared photo-Fenton catalyst with hydrogen peroxide under a light condition to degrade organic simulated pollutants. The method of the invention is simple and practical; the catalyst is suitable for recycle, can both effectively remove chroma of polluted water, and reduce COD of the polluted water.
Owner:SUZHOU UNIV OF SCI & TECH
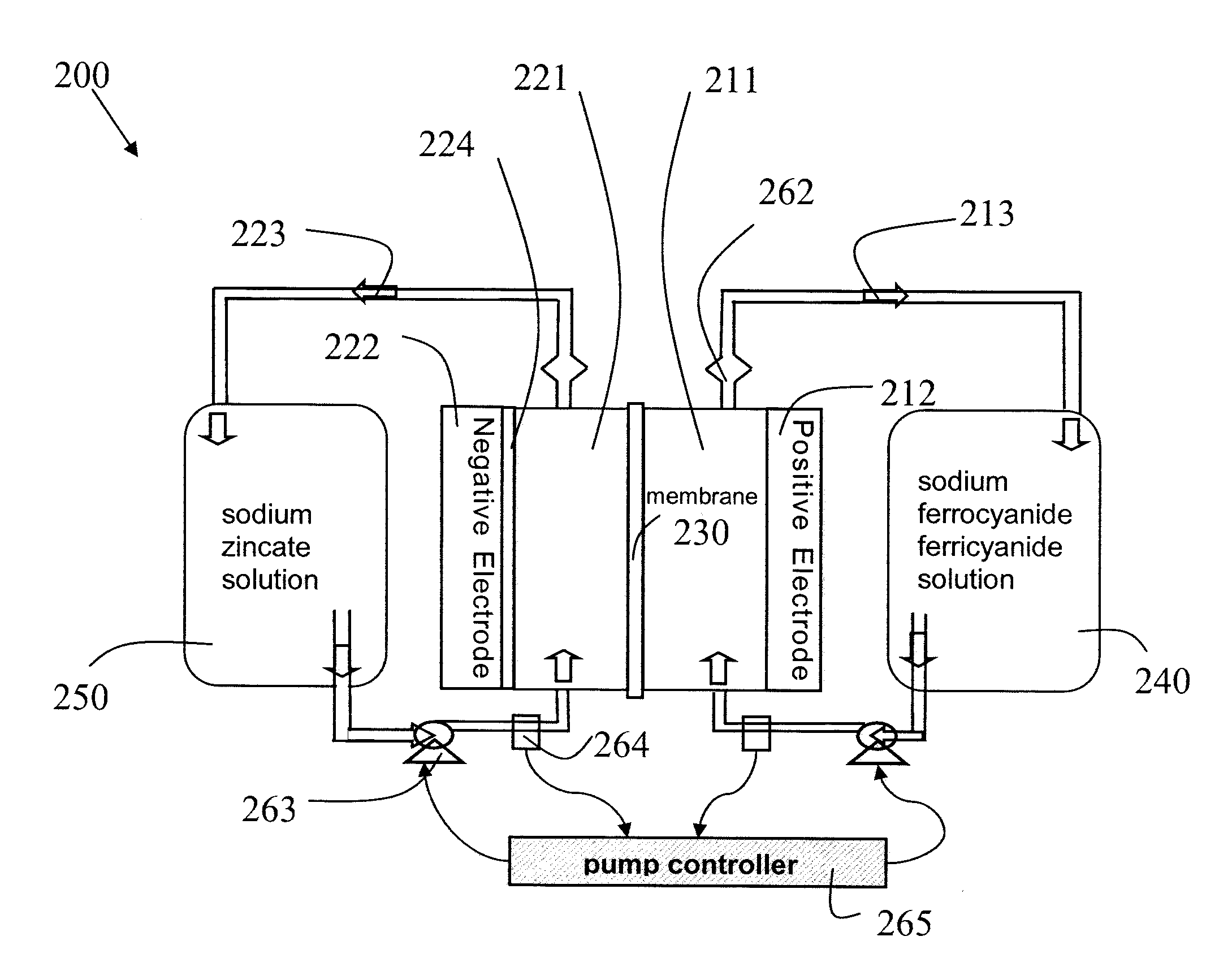
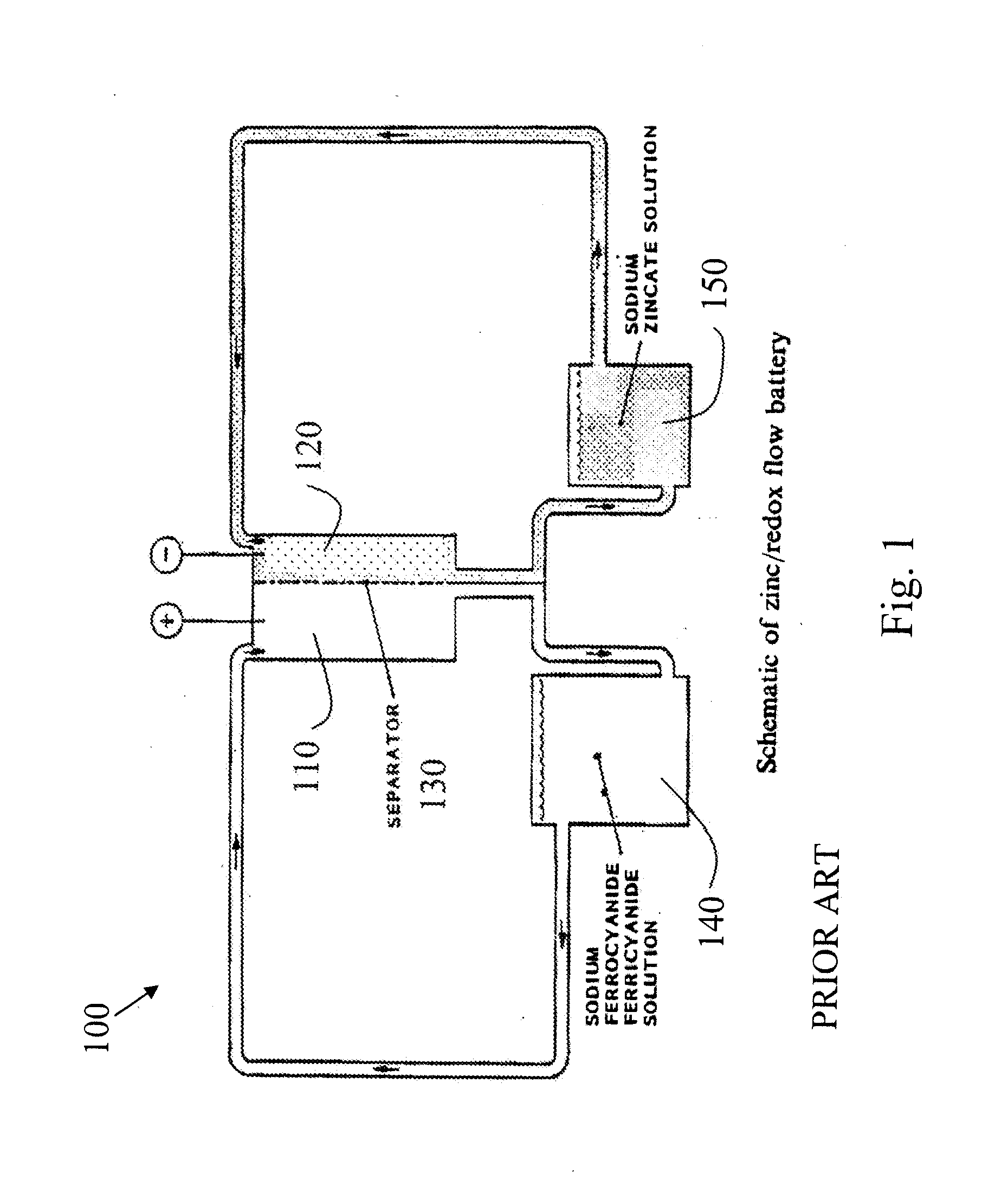
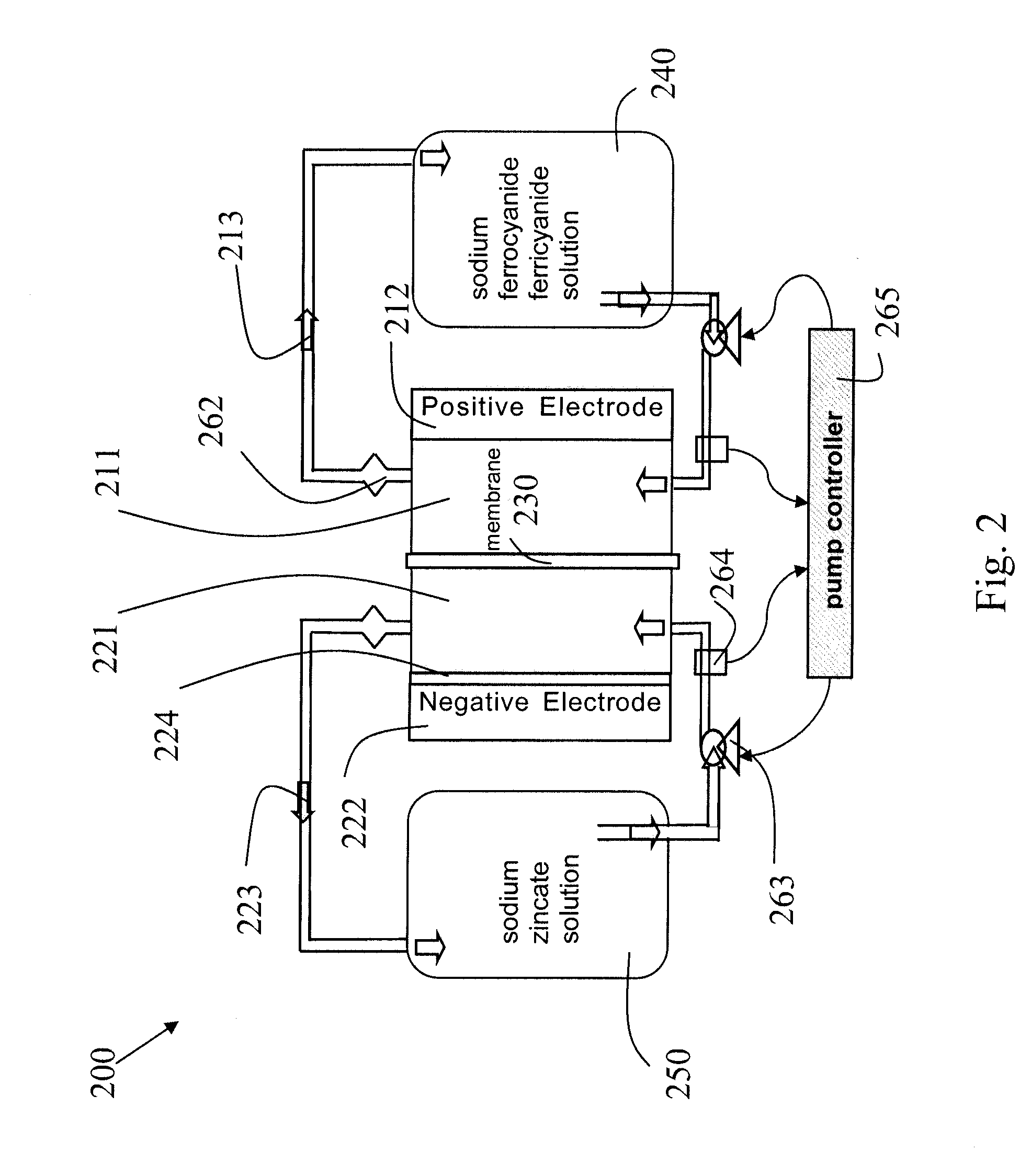
Novel flow battery and usage thereof
InactiveUS20150048777A1Promote redox processPrevent free accessBatteries circuit arrangementsReactant parameters controlCyanideMetal
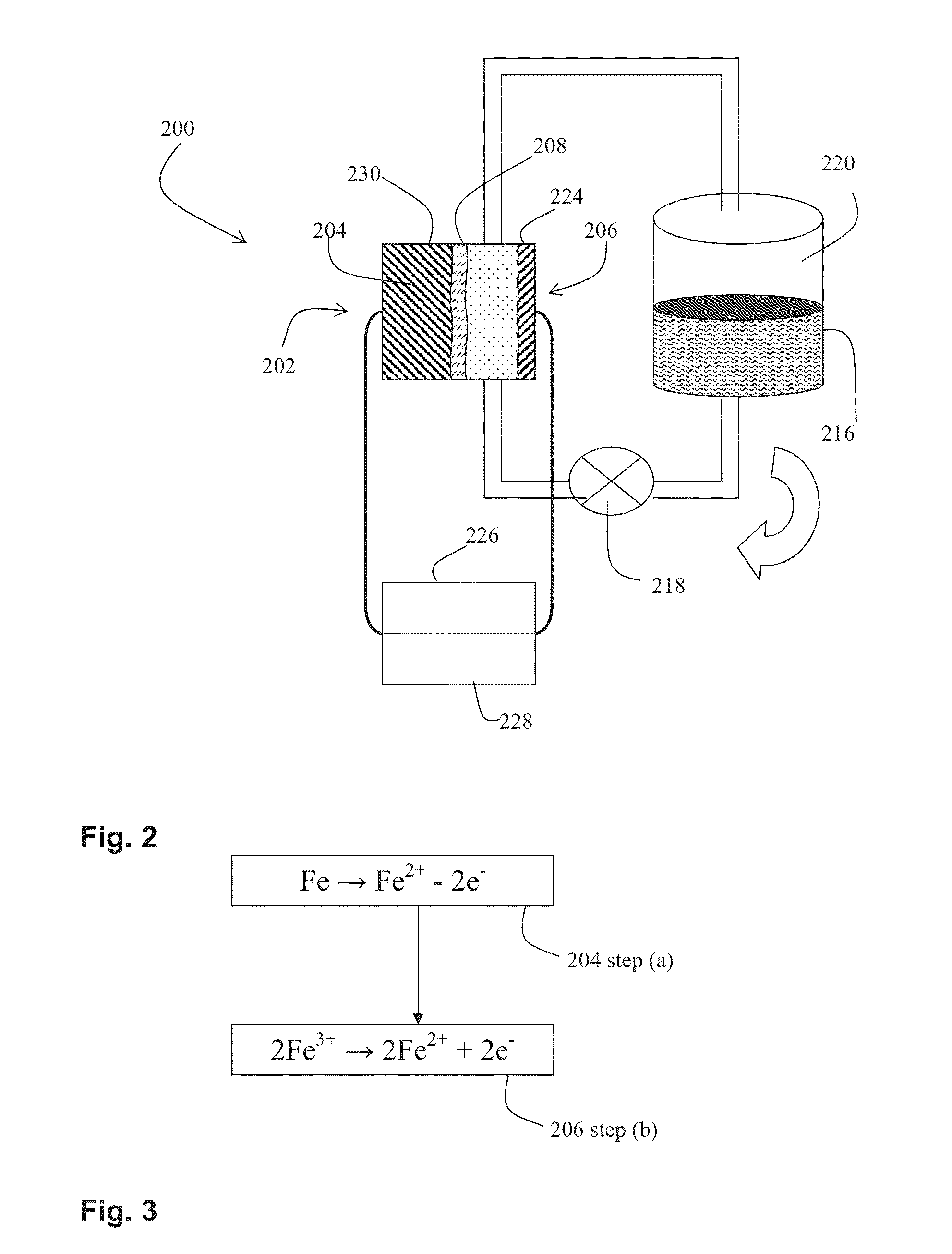
A voltaic cell comprising an iron in alkali anode where metal iron is oxidized to iron II hydroxide and a ferricyanide in alkali cathode where ferricyanide is reduced to ferrocyanide, and uses thereof.
Owner:EPSILOR ELECTRIC FUEL
Reagents for Electrochemical Test Strips
ActiveUS20130026050A1Extended shelf lifeWithout impairing qualityImmobilised enzymesBioreactor/fermenter combinationsRedox enzymesSolubility
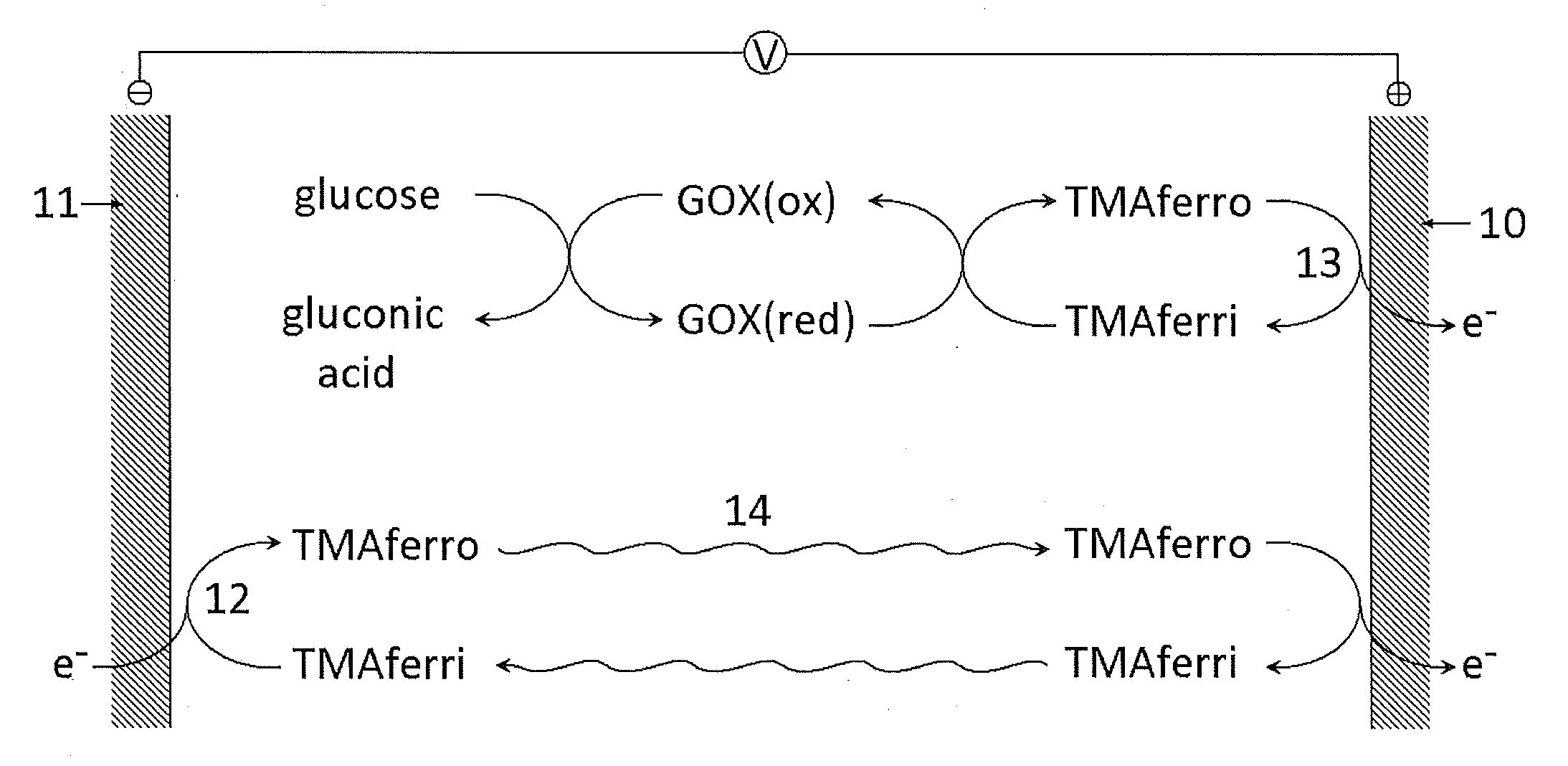
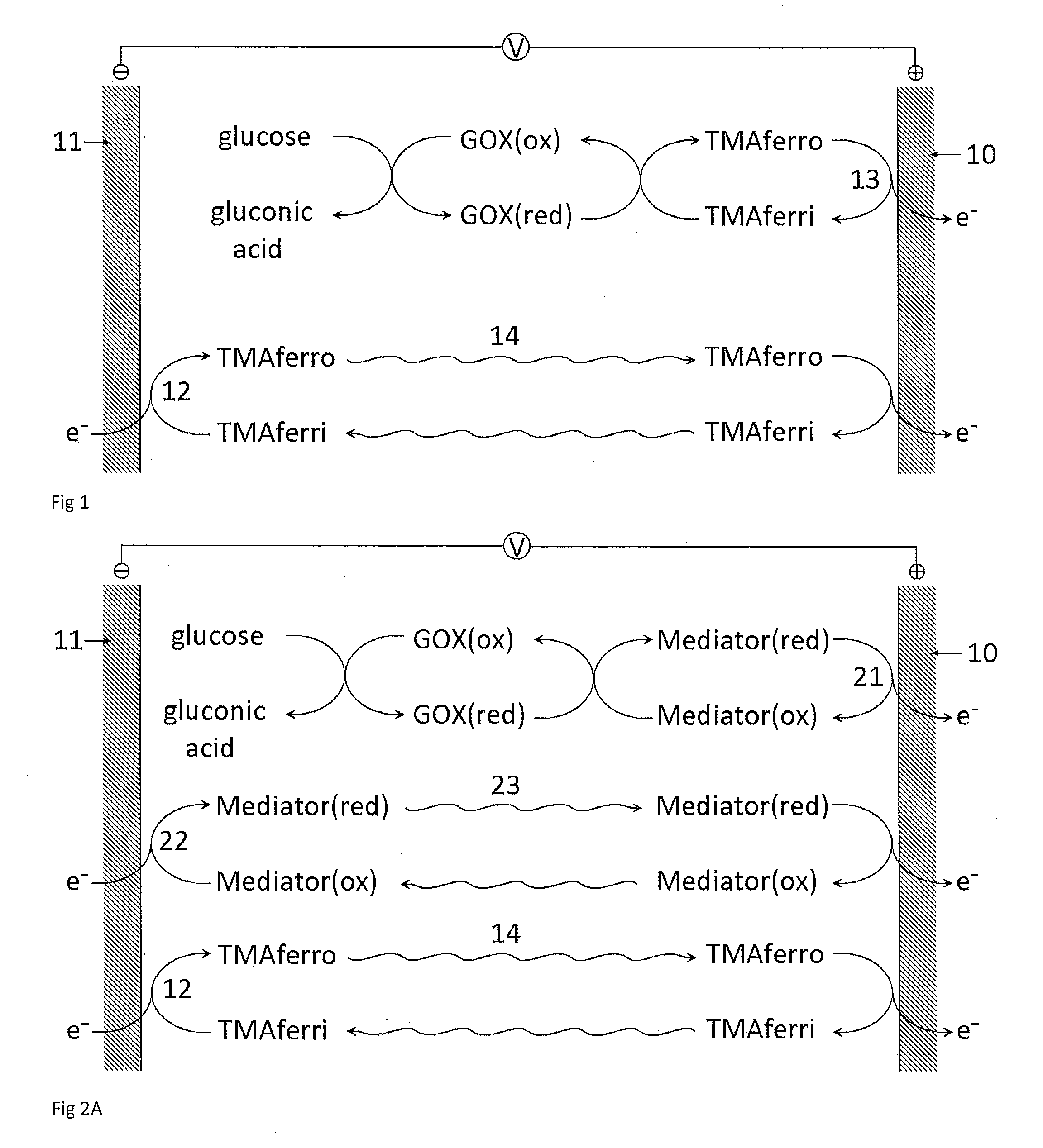
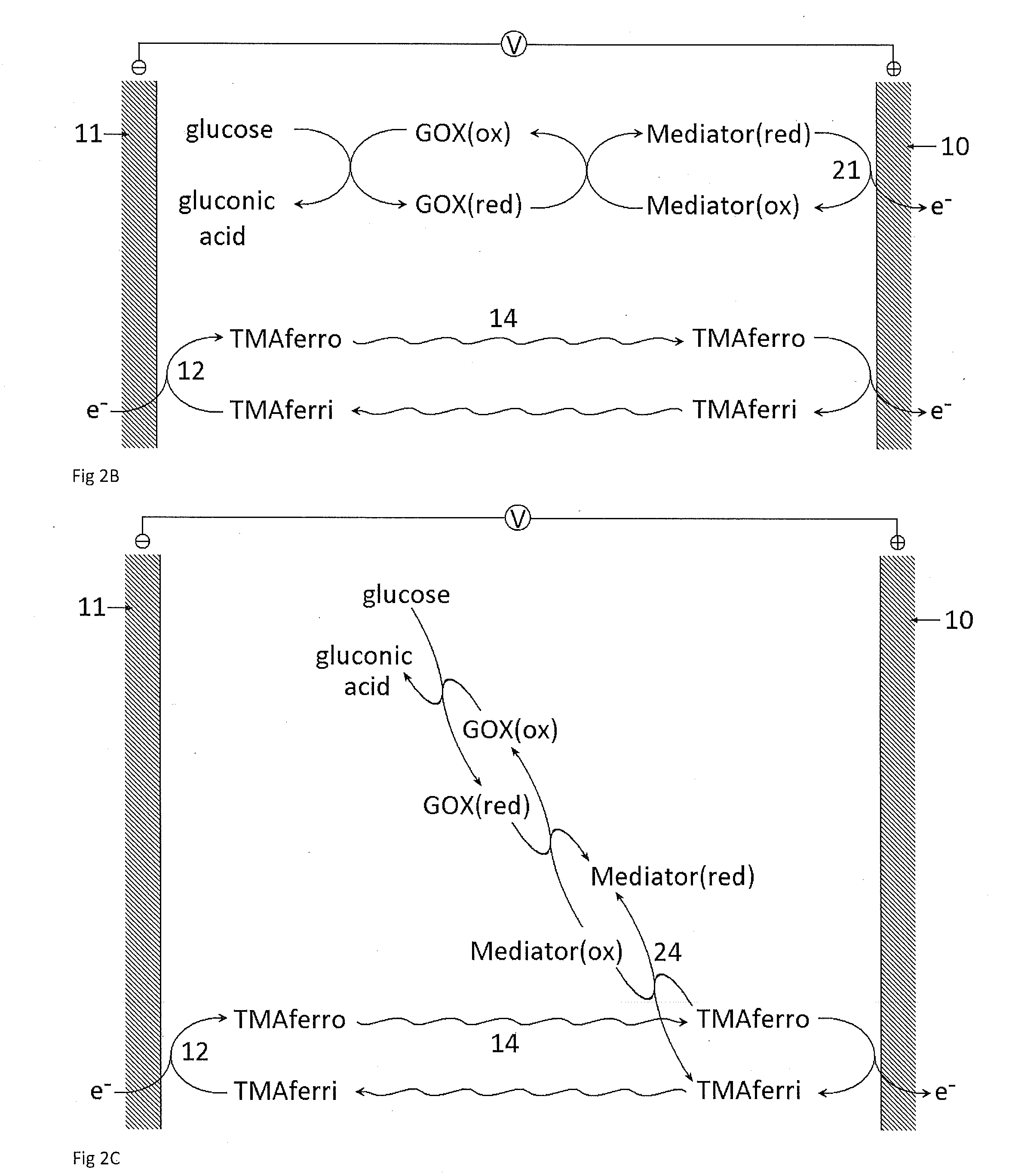
A dry reagent composition that includes an active redox enzyme that oxidizes an analyte as a specific substrate to produce an inactive reduced form of the enzyme; and a salt of ferricyanide provides improved performance in electrochemical test strips such as those used for detection of glucose. The salt of ferricyanide consists of ferricyanide and positively-charged counter ions, and the positively charged counter ions are selected such that the salt of ferricyanide is soluble in water, and such that the salt of ferricyanide or the crystalline phase of the salt of ferricyanide has a solubility in water and / or a lower E0eff at a concentration of 100 mM than potassium ferricyanide. For example, the salt of ferricyanide may be tetramethylammonium ferricyanide.
Owner:AGAMATRIX INC
Preparation method of ion exchange membrane for alkaline redox flow battery
InactiveCN110534682AHigh ion selectivityHigh proton conductivityCell electrodesRegenerative fuel cellsKetoneCarbon felt
The invention relates to the field of alkaline redox flow battery energy storage, in particular to a preparation method of an ion exchange membrane for an alkaline redox flow battery, which is mainlyused for solving the problem of the high price of a Nafion diaphragm in the alkaline redox flow battery at the present stage, so that the cost of the redox flow battery is greatly reduced. An alkalineaqueous solution of ferricyanide (such as Na3[Fe(CN)6], K3[Fe(CN)6], (NH4)3[Fe(CN)6] and the like) is used as a positive electrolyte, and a strong alkaline solution (such as KOH, NaOH and the like) is used as a negative electrolyte; graphite felt and carbon felt are selected as positive electrode materials, and a zinc plate is selected as a negative electrode material; and the ionized sulfonatedpolyether ether ketone (SPEEK) diaphragm is used as an ion exchange membrane to assemble the battery. Therefore, the alkaline redox flow battery system with low cost and high performance is obtained.The flow battery system provided by the invention has the advantages of the high open-circuit voltage, the low cost, the high efficiency, the good cycling stability, safety, reliability and the like,and has a wide application prospect.
Owner:CHANGSHA UNIVERSITY OF SCIENCE AND TECHNOLOGY
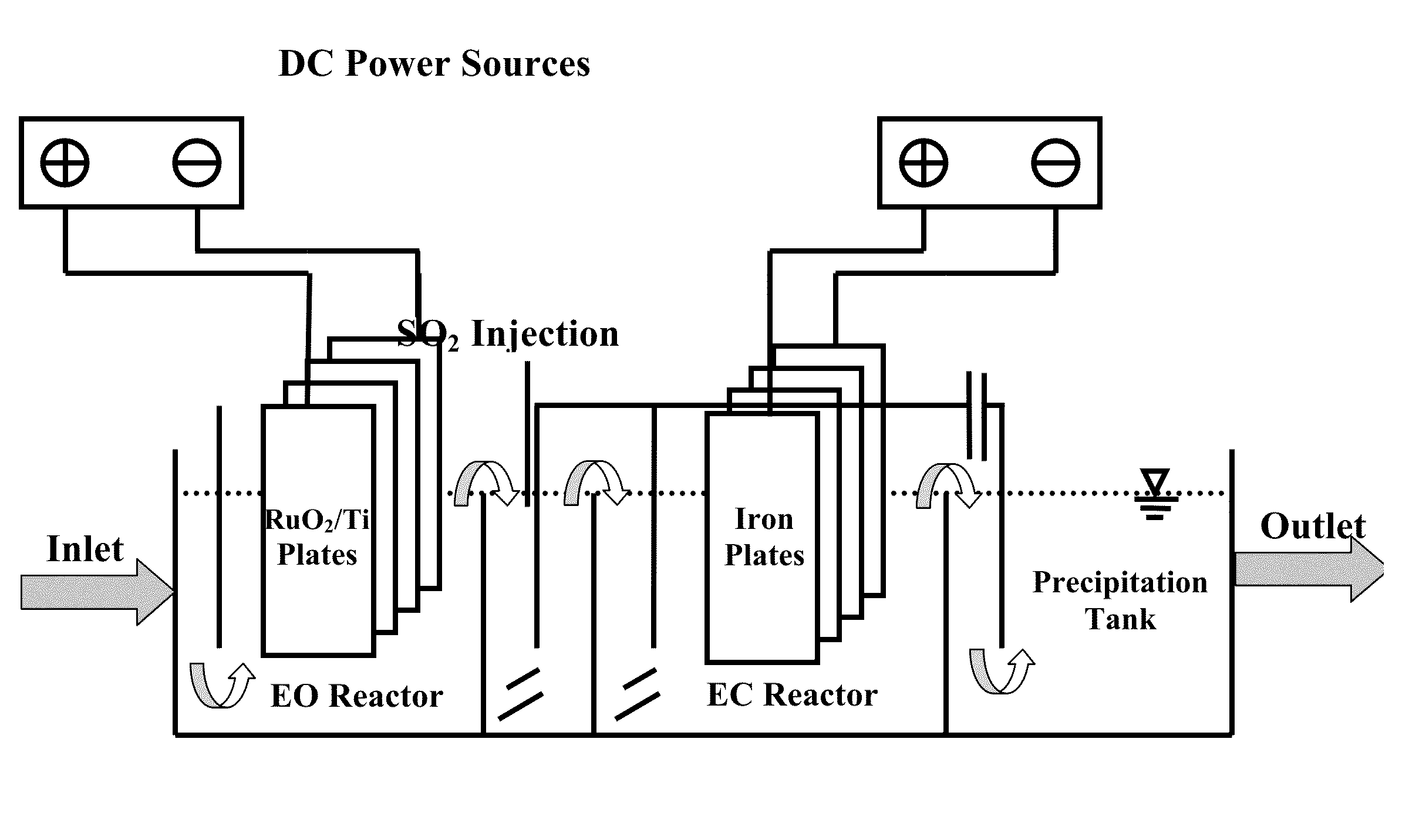
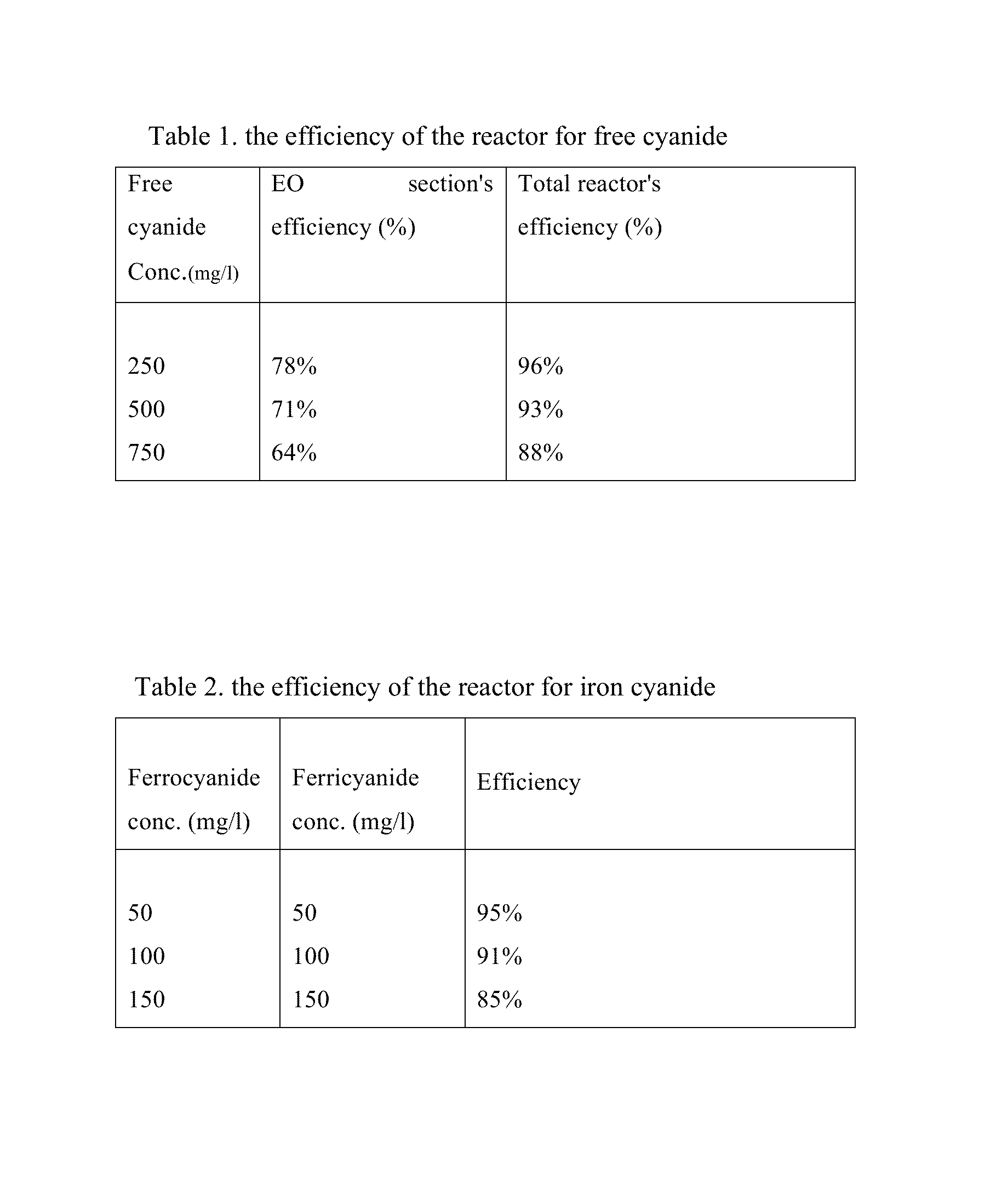
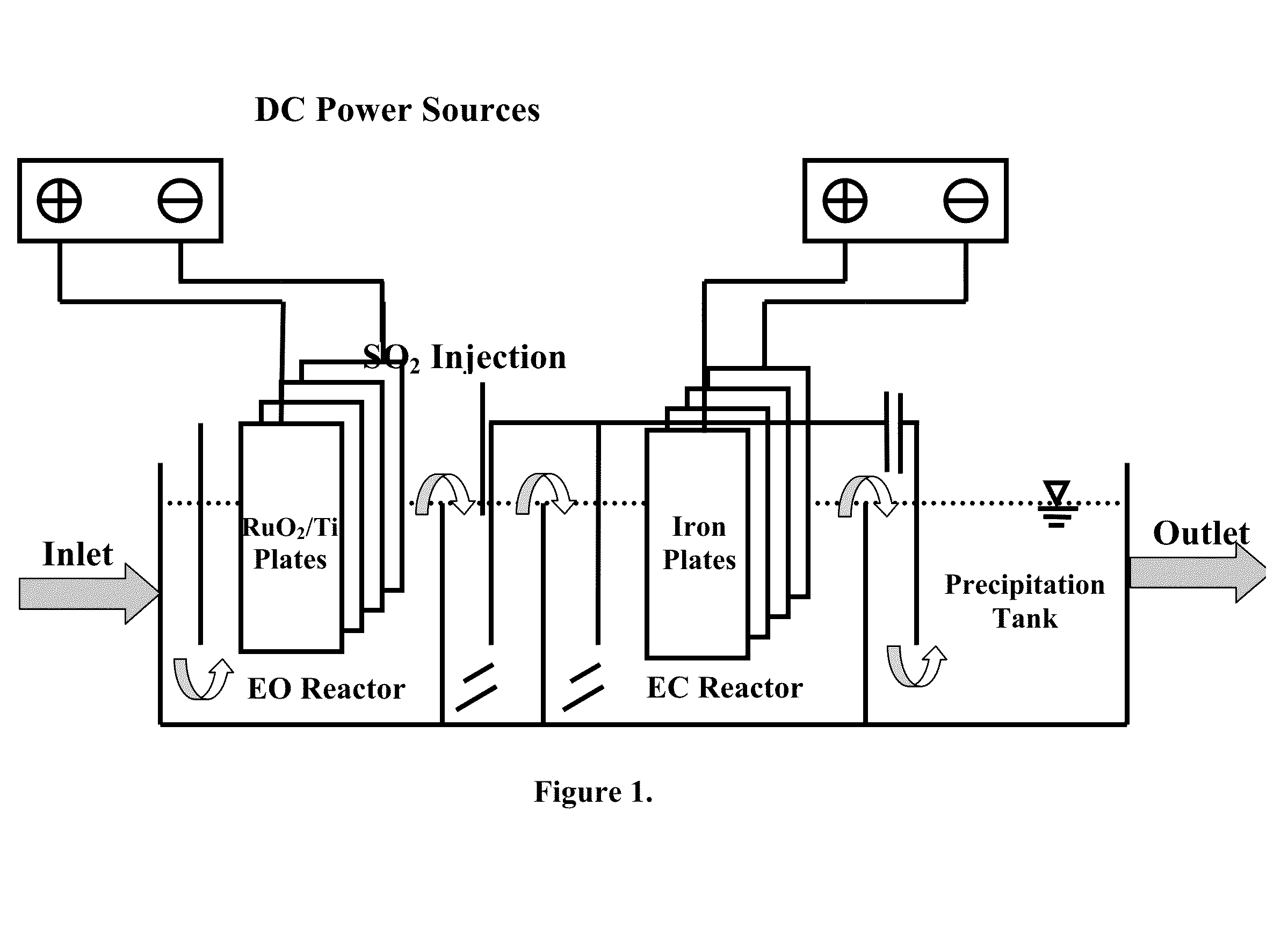
Electrochemical-based reactor for the removal of free cyanides and iron cyanide from industrial wastewater
InactiveUS20140246375A1Increase consumptionVolume of produced increaseWater contaminantsSedimentation separationElectrocoagulationIron cyanide
The present invention relates to a continuous bipolar electrochemically based reactor for the purpose of destroying free cyanide by direct and indirect oxidation and removing its strong complexes like ferrocyanide and ferricyanide by electrocoagulation. The designed reactor consists of four main sections, including electrooxidation, hydraulic mixing, electrocoagulation, and precipitation tank. The order of sections results in having a combination of reactions which separately remove cyanide and its compounds. The designed reactor shows a high flexibility in terms of handling highly variable kinds and concentrations of cyanide in the industrial wastewater effluents.
Owner:GHARIBI HAMED +1
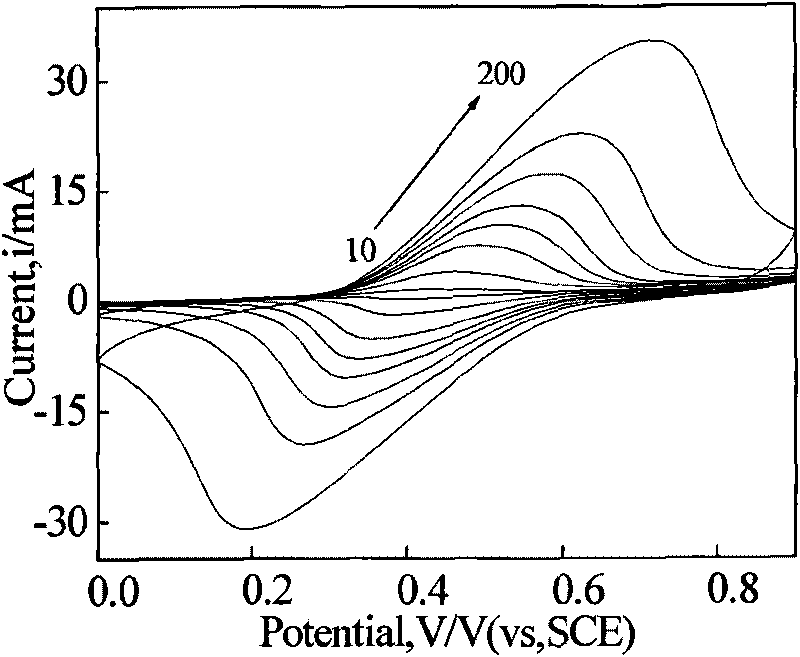
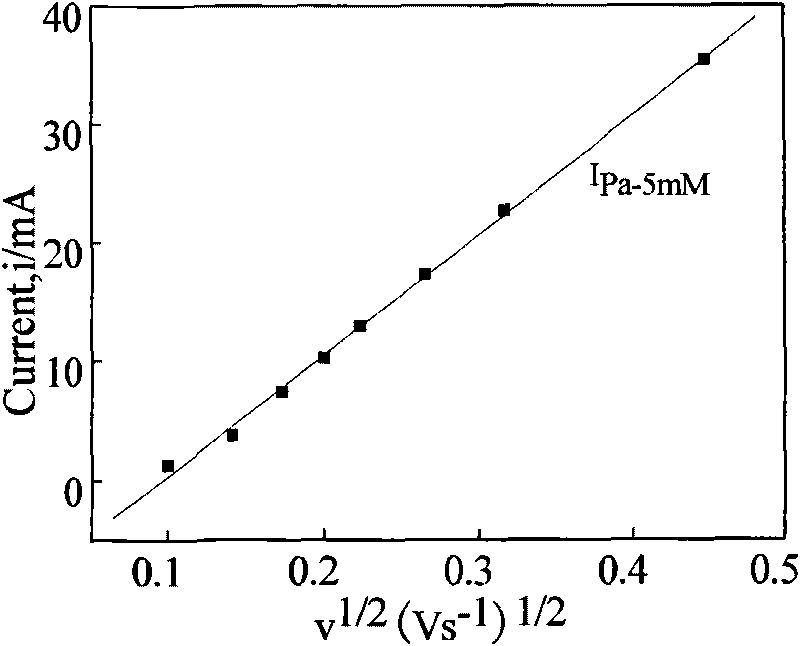
Method for measuring electroactive area of three-dimensional porous membrane electrode
InactiveCN101710058AFast electrochemical responseHigh precisionElectrical/magnetic thickness measurementsPermeability/surface area analysisPotassium cyanideInner membrane
The invention provides a method for measuring the electroactive area of a three-dimensional porous membrane electrode, which comprises the steps of: respectively measuring cyclic voltammetry curves under different scanning speeds in potassium cyanide-containing and potassium cyanide-free kali salt or sodium salt solution with a three-electrode system and a potentiostat by taking a three-dimensional porous electrode or the three-dimensional porous membrane electrode which is deposited with a transition element ferricyanide semiconductor membrane, and measuring the electroactive area and the cover degree thereof with the reversibility of the redox reaction of the membrane electrode in different solution systems; and obtaining the active volume and the average membrane depth of the inner membrane of the three-dimensional porous membrane electrode by combining with the chronocoulometry. The method is simple and reliable, and has strong practicability.
Owner:TAIYUAN UNIV OF TECH
A complementary color photometric automatic titration device for reducing sugar concentration determination
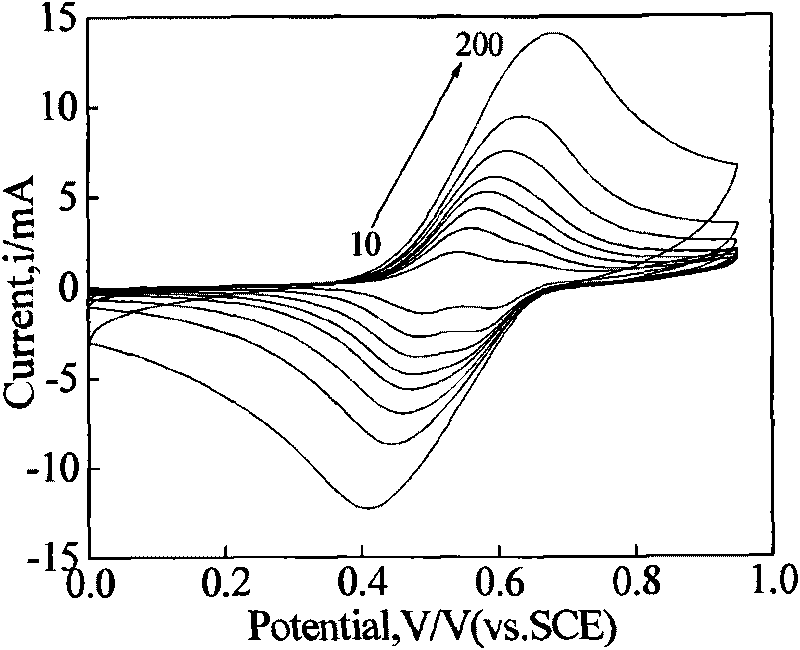
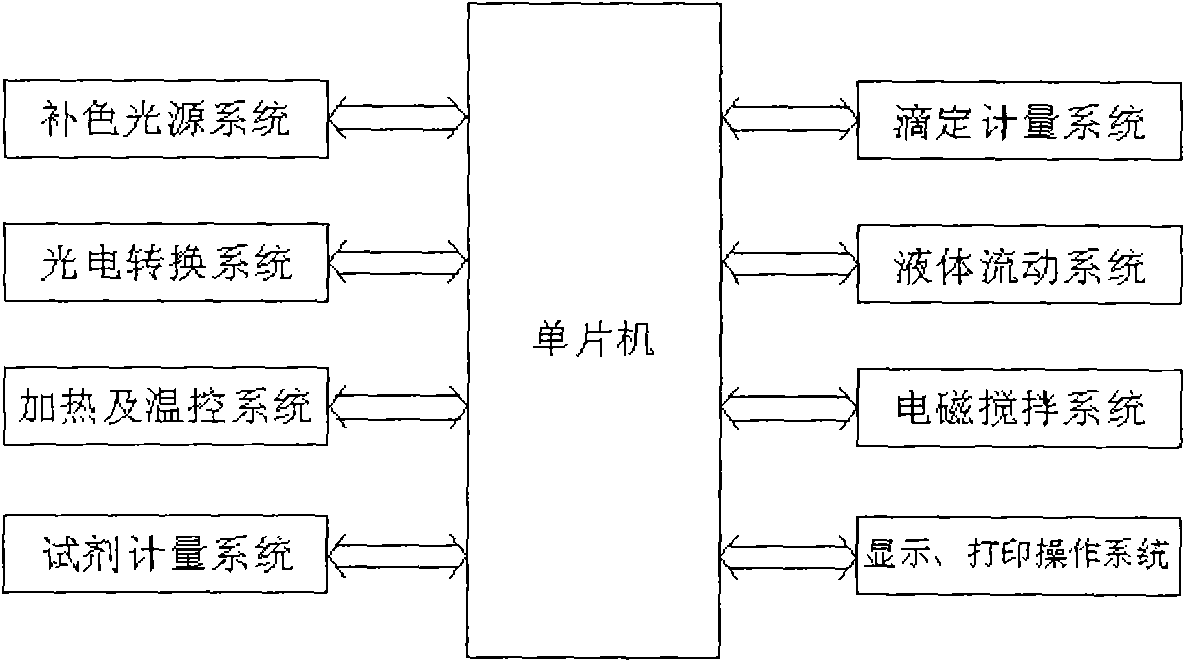
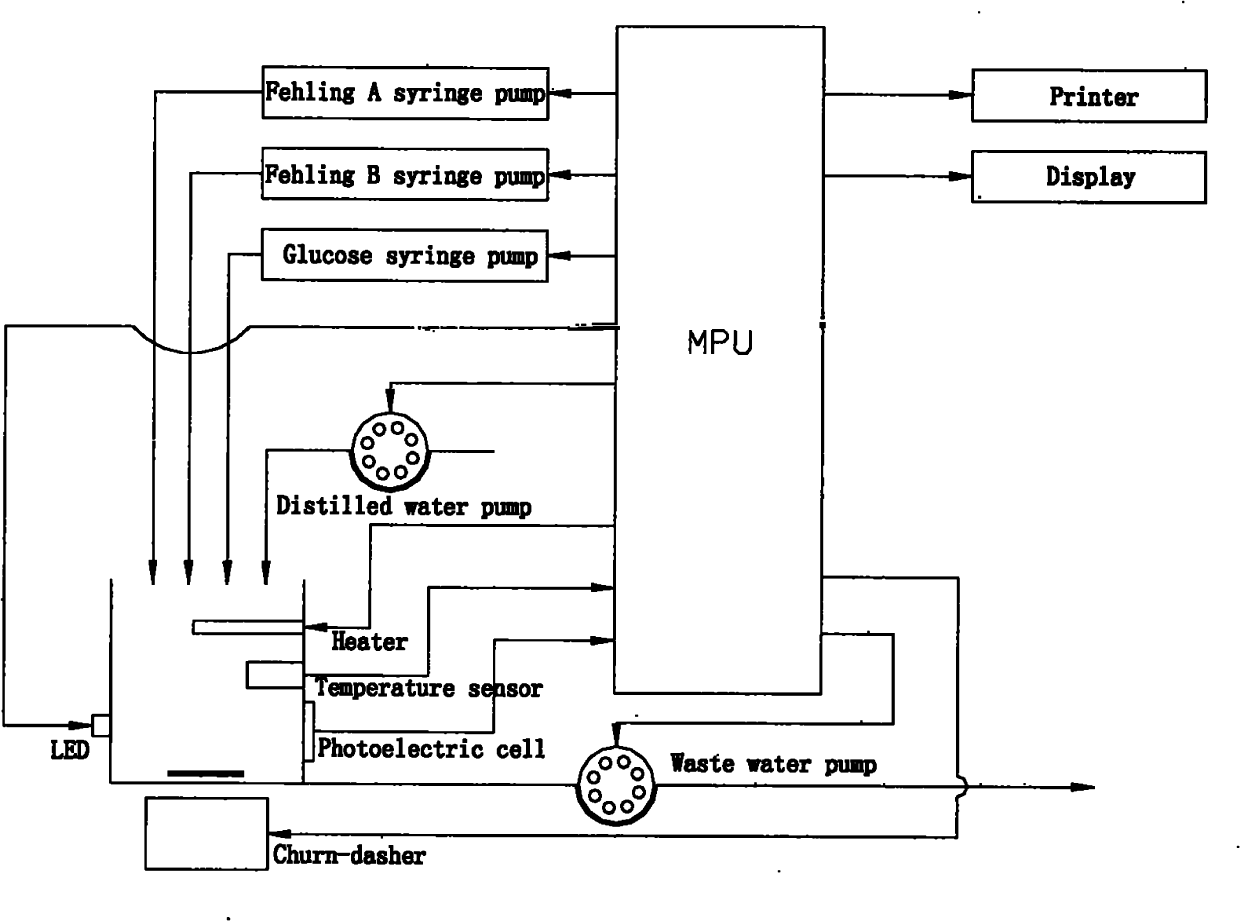
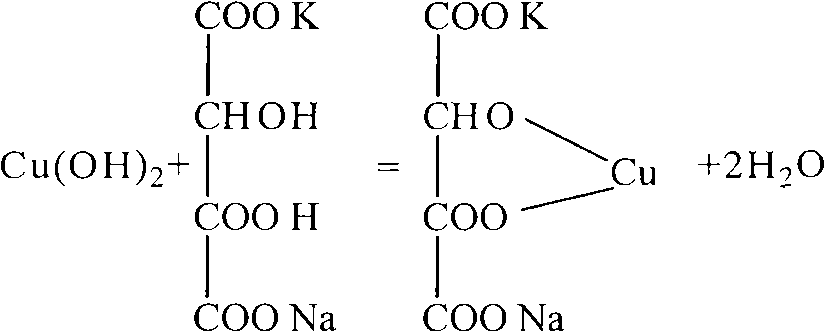
InactiveCN102262056AEasy to operateReduce titration interferenceColor/spectral properties measurementsTurbidityColor changes
The invention relates to a color-complementing photometric titration device capable of quickly and accurately measuring the concentration of reducing sugar in various samples, and belongs to the technical field of biochemical analysis instruments. The reducing sugar in the sample and Fehling’s reagent or reducing sugar and potassium ferricyanide-sodium hydroxide react in a slight boiling state. Reducing sugar will change the color of the reaction system. The reaction solution system is irradiated with a light source system with a complementary color to the reaction solution system, and a photoelectric conversion device is used to detect the transmitted light. At the end of the reaction, the received signal will change suddenly. The complementary color photometric titration established by the above method The device can be used for rapid determination of reducing sugar concentration in various samples. Complementary color photometry reduces light transmittance fluctuations, increases the intensity of mutation signals, and has almost no interference signals. Therefore, the method for determining reducing sugar concentration is not affected by sample color, turbidity, and heating bubbles.
Owner:BIOTECH CENT OF SHANDONG ACAD OF SCI
Preparation and application of ion exchange membrane of electric active nickel ferricyanide
InactiveCN1562485AReduce usageAccurate control of placementCation exchanger materialsElectrolytic agentPotassium
A process for preparing the electroactive ion-exchanging nickel ferricyanide membrane used to treat the industrial sewage containing alkali metal ions includes generating a capillary gap between electrically conductive substrate and film, filling the solution prepared from potassium ferriccyanide, 2-valence Ni salt, Na salt and water into said gap, laying aside for chemical depositing and removing film.
Owner:TAIYUAN UNIV OF TECH
Method for preparing single structure ferricyanide film using single-stage pulse electrodeposition
InactiveCN101358371ANo need to change compositionNo need to change contentElectrolytic inorganic material coatingSemiconductor/solid-state device manufacturingSupporting electrolyteSeparation technology
The invention discloses a preparation method for single-structure ferricyanide film monopolar pulse electro-deposition, which adopts a monopolar pulse electro-deposition method to prepare semi-conductive metal ferricyanide film on the surface of a conductive matrix, i.e. feeds a certain period of pulse deposition voltage and then controls the current to be zero within the interruption time to realize monopolar pulse, and prepares soluble or insoluble single-structure inorganic semi-conductive metal ferricyanide film by regulating the pulse voltage, pulse frequency, pulse make-break time (duty ratio) and other parameters in the solution containing potassium ferricyanide, transition metal salt and supporting electrolyte. The preparation method is simple and has strong operability; and the prepared single-structure metal ferricyanide film has excellent selectivity and sensitivity, and is the preferred material for the preparation of sensitive sensors and the electrical control ion separation technology.
Owner:TAIYUAN UNIV OF TECH
Method for removing heavy metal by carrying out cyanide breaking on high-concentration cyanide wastewater
ActiveCN106396205AAvoid follow-up issuesNo costWater contaminantsWaste water treatment from metallurgical processHigh concentrationEthylene diamine
The invention relates to a method for removing heavy metal by carrying out cyanide breaking on high-concentration cyanide wastewater. The method comprises the following steps: (1) feeding the high-concentration cyanide wastewater into an electrolytic bath, and carrying out electrolysis by using a direct current power supply under an alkaline condition; (2) in the electrolytic process, enabling content of cyanide to be gradually reduced, adding hydrogen peroxide to improve a reaction speed of the cyanide, and finally, carrying out electrolysis to obtain low-concentration cyanide wastewater; (3) regulating a pH value of the low-concentration cyanide wastewater obtained in the step (2) into 10 to 11, and adding EDTA (Ethylene Diamine Tetraacetic Acid) and a catalyst to perform reaction; (4) adding hydrogen peroxide into solution obtained in the step (3) to carry out deep cyanide breaking treatment; (5) for the wastewater subjected to complete cyanide breaking and obtained in the step (4), removing heavy metal in the solution by adopting a ferrite method. The method is simple to operate; not only is high-concentration cyanide removed, but also the wastewater subjected to cyanide breaking is subjected to deep treatment; particularly, ferricyanide reaches an emission standard.
Owner:盛隆资源再生(无锡)有限公司
Electrochemical method for detecting anodic oxidation aluminium formwork effective hole density
InactiveCN1987419ASimple methodImprove reliabilityPermeability/surface area analysisMaterial electrochemical variablesPlatinumEffective density
The invention especially related to electrochemical method for measuring effective density of hole in anodic aluminum oxide (AAO) template. Usual three-electrode system and potentiostat are adopted in the method. Working electrode is dish electrode of metal platinum (or gold, glassy carbon), and there is electrode of AAO template. Ferricyanide is as solution. Na2SO4 or KCl is as electrolyte. Using diffusibility of ferricyanide on surface of working electrode, the method measures effective density of hole in AAO template by using electrochemical voltmeter-ammeter method. Features are: simple method and good reliability.
Owner:FUDAN UNIV
Process to separate the vanadium contained in inorganic acid solutions
A process for recovering vanadium contained in inorganic acid solutions by precipitating the vanadium as a solid compound of vanadium and alkali metal or monovalent cation ferricyanide. Separation is carried out electrochemically by depositing the compound on to a metal immersed in the acid solution that contains vanadium, to which a ferricyanide salt of an alkali metal or a monovalent cation has been added. If the inorganic acid present in solution is different from nitric acid, the vanadium can be also separated by direct addition of a ferricyanide salt of an alkali metal or a monovalent cation to the acid solution containing vanadium. The method described allows recovery of vanadium without modifying the initial composition of the solution, except for the concentration of the vanadium dissolved.
Owner:UNIVE SIMON BOLIVAR
Preparation method of multi-purpose in-level oil-water separation material
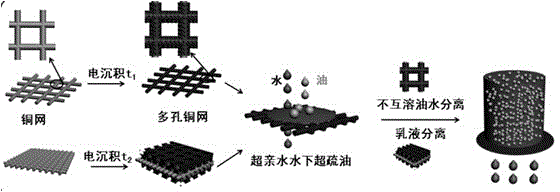
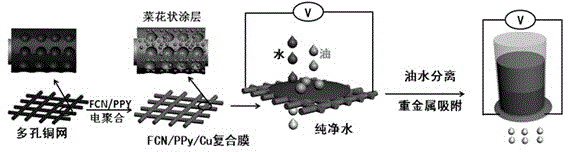
ActiveCN104941458AEasy to insertEffective specific adsorptionSemi-permeable membranesFatty/oily/floating substances removal devicesComposite filmPolypyrrole
The invention discloses a preparation method of a multi-purpose in-level oil-water separation material. The preparation method comprises the following steps: preparing a porous reticular Cu film by adopting a hydrogen bubble template; preparing an ionic imprinted polymer coating on the surface of the porous reticular Cu film, and imprinting ferricyanide(FCN) as counter ions of metal ions into polypyrrole (PPy) to prepare an FCN / PPy composite film coating. The area of a substrate of the multi-purpose in-level oil-water separation film prepared by the method provided by the invention is 3*3cm<2>, the bore diameter of the porous reticular film is 100-500 micrometers, the second-level bore diameter, namely the bore diameter of a porous structure plated on the reticular substrate is 10-120 micrometers, the ionic imprinted polypyrrole coating is cauliflower-shaped, the bore diameter of small particles is 100-800 nm, and the bore diameter of large bulges is 1-8 micrometers. The multi-purpose oil-water separation film prepared by the method provided by the invention has superoleophobic property underwater, has effective self-cleaning function, and also has excellent mechanical stability.
Owner:HARBIN INST OF TECH
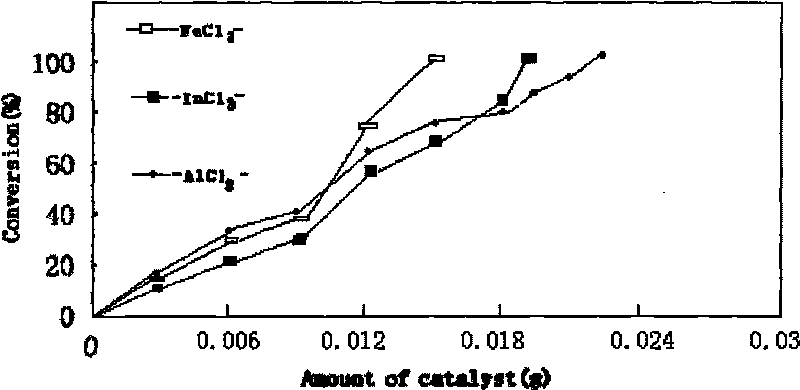
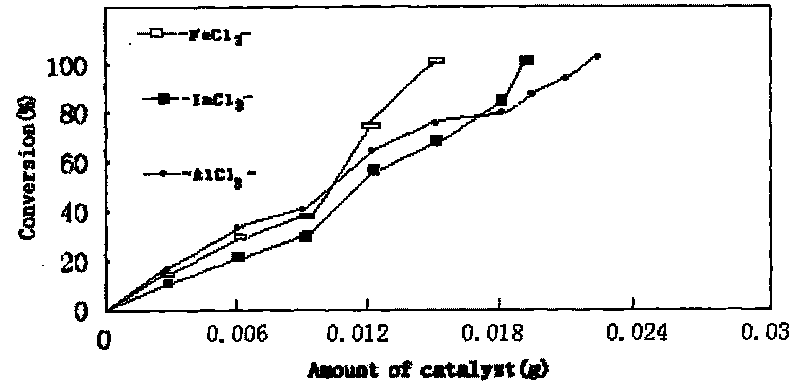
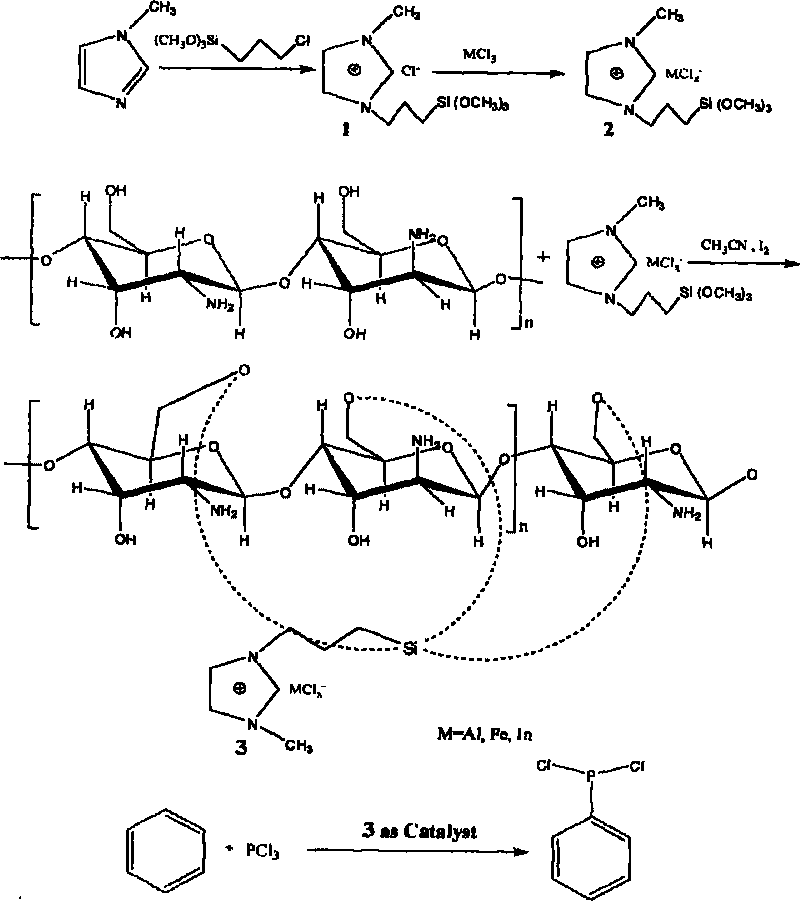
Method for preparing chitosan immobilized Lewis acidic ionic liquid and DCPP synthesized by using chitosan immobilized Lewis acidic ionic liquid in presence of high-efficient catalyst
InactiveCN101695673ACheap and easy to getMild responseGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsSilanesEvaporation
The invention relates to a method for preparing chitosan immobilized Lewis acidic ionic liquid and DCPP synthesized by using chitosan immobilized Lewis acidic ionic liquid in presence of high-efficient catalyst. The method is characterized by comprising the following steps: performing refluxing reaction on 1-methylimidazole and Y-chloropropyl trimethoxy silane in a mol ratio of 1:1 at a temperature of 70 DEG C for 70 hours; then performing reaction in a mol ratio of Lewis acid anhydrous ferric chloride to a prepared product of 2:1 at a temperature of 70 DEG C for 24 hours; dispersing the obtained ionic liquid containing ferricyanide and chitosan which is 2 times of the ionic liquid in mass in acetonitrile to reflux for 20 hours; performing rotary evaporation to remove a solvent; and performing soxhlet extraction on the obtained solid with methylene dichloride for 30 hours to obtain a chitosan immobilized acidic ionic liquid catalyst PmimCl-FeCl3-CS. Anhydrous aluminum chloride and indium chloride are used for replacing anhydrous ferric chloride and serve as a Lewis acid in turn to obtain PmimCl-AlCl3-CS and PmimCl-InCl3-CS respectively. The method has the advantages of low-price and readily available reaction raw materials, mild reaction and simple condition.
Owner:NANCHANG HANGKONG UNIVERSITY
High performance flow battery
InactiveUS20130029187A1Improve the level ofHigh rateElectrolyte moving arrangementsIndirect fuel cellsHigh current densityHigh concentration
High performance flow batteries, based on alkaline zinc / ferro-ferricyanide rechargeable (“ZnFe”) and similar flow batteries, may include one or more of the following improvements. First, the battery design has a cell stack comprising a low resistance positive electrode in at least one positive half cell and a low resistance negative electrode in at least one negative half cell, where the positive electrode and negative electrode resistances are selected for uniform high current density across a region of the cell stack. Second, a flow of electrolyte, such as zinc species in the ZnFe battery, with a high level of mixing through at least one negative half cell in a Zn deposition region proximate a deposition surface where the electrolyte close to the deposition surface has sufficiently high zinc concentration for deposition rates on the deposition surface that sustain the uniform high current density. The mixing in the flow may be induced by structures such as: conductive and non-conductive meshes; screens; ribbons; foam structures; arrays of cones, cylinders, or pyramids; and other arrangements of wires or tubes used solely or in combination with a planar electrode surface. Third, the zinc electrolyte has a high concentration and in some embodiments has a concentration greater than the equilibrium saturation concentration—the zinc electrolyte is super-saturated with Zn ions.
Owner:ZINC AIR
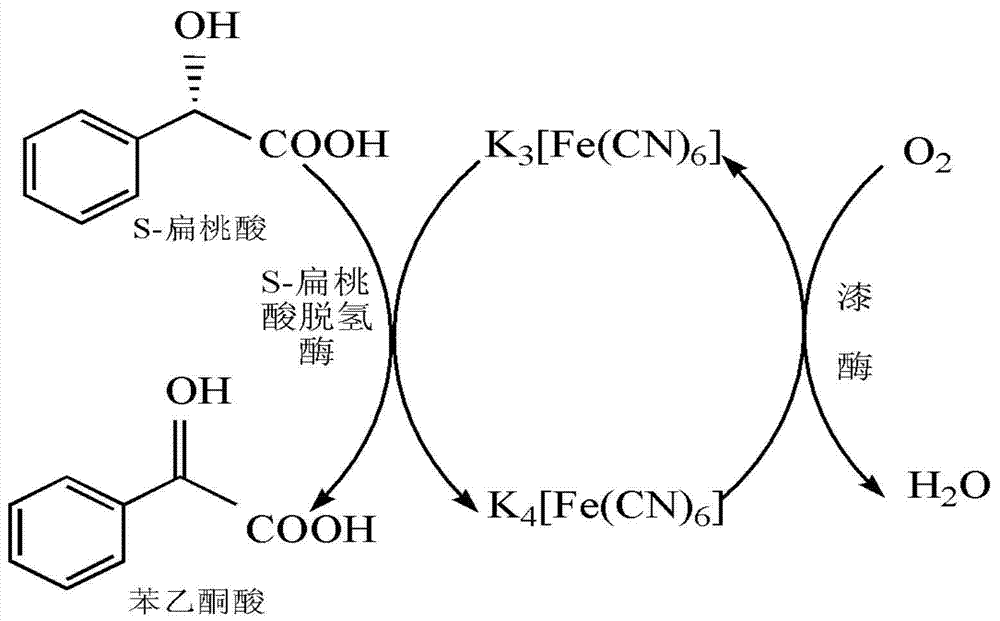
Method for preparing benzoyl formic acid and R-mandelic acid by coupling reaction of S- mandelic acid dehydrogenase and laccase
The invention provides a method for catalyzing S-mandelic acid to be converted into benzoyl formic acid and R-mandelic acid by coupling reaction of S-mandelic acid dehydrogenase and laccase. The method is characterized by continuously regenerating reaction media by laccase to continuously regenerate coenzyme FMN (Flavin Mononucleotide) of S-mandelic acid dehydrogenase, so that the S-mandelic acid dehydrogenase and the laccase are coupled to carry out catalytic reaction. Therefore, the conversion efficiency of independently using the S-mandelic acid dehydrogenase is improved by sufficiently utilizing the high efficiency of enzyme catalysis and continuously catalyzing and oxidizing the S- mandelic acid through less reaction media; and the reaction cost is reduced. According to the method disclosed by the invention, the S-mandelic acid dehydrogenase and laccase are coupled to catalyze the S-mandelic acid to be converted into benzoyl formic acid; and the enantiomer excess of the product R-mandelic acid can reach 99.8%. Moreover, only a small quantity of ferrocyanide or ferricyanide is needed as the reaction medium; the process is simple; the cost is low; and the environment pressure is small; and besides, the method is convenient for industrial production and of a great application value to chiral resolution of racemic mandelic acid.
Owner:NANJING UNIV OF SCI & TECH
Method for extracting gold from auriferous ores through potassium ferricyanide
The invention relates to a method for extracting gold through potassium ferricyanide. The method is characterized in that the gold is extracted from the auriferous ores under an alkaline environment at normal temperature and pressure with nontoxic ferricyanide serving as a gold leaching agent and calcium peroxide serving as a leaching adding agent; and the method is a completely novel cyanide-free gold leaching method. According to the method, some auriferous ores which are difficultly treated are preprocessed and then leached for 2 to 20 hours at a normal-temperature alkaline environment and under the conditions that the concentration of potassium ferricyanide is 2 to 15%, the dosage of CaO2 is 1 to 10g / L and the liquid-solid ratio is 2: 1 to 10: 1; and the leaching rate of the gold can reach more than 94%. The method is simple and reliable in process and high in gold leaching rate and nearly not pollutes the environment; the reaction is carried out at the alkaline environment, so that equipment is nearly not corroded, and the requirement and cost are low, and the method has an important industrial application prospect.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
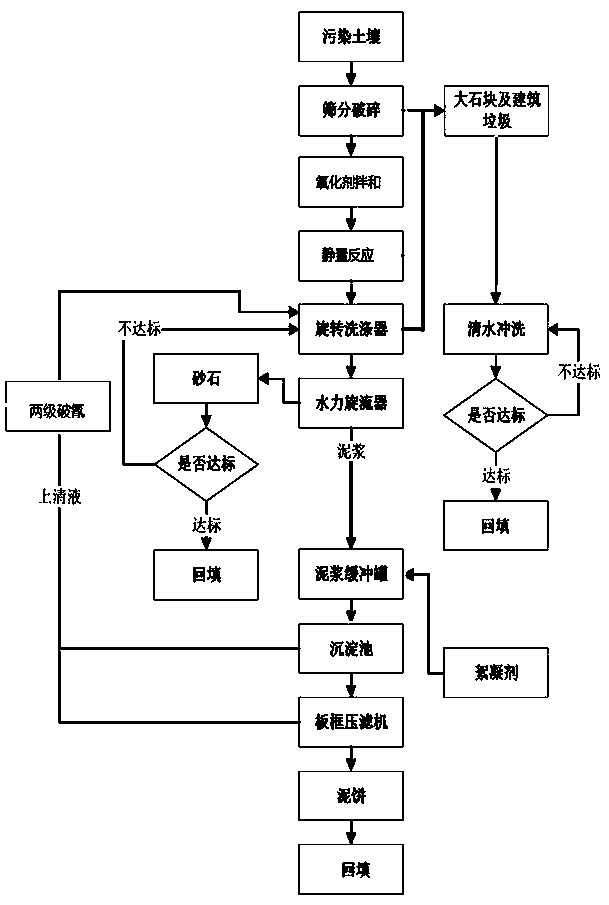
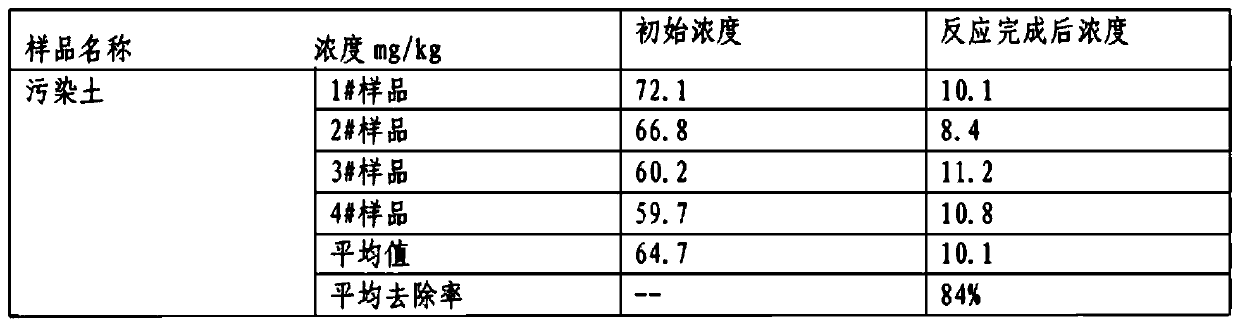
Engineering remediation method for cyanide contaminated soil
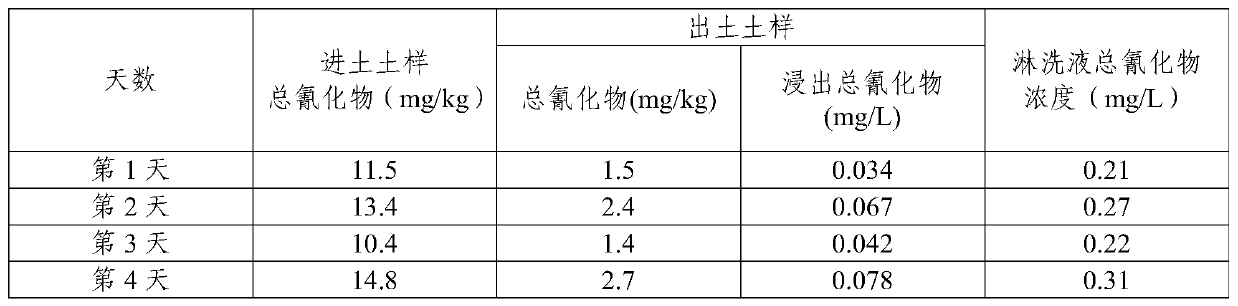
InactiveCN111408612AReduce processing costsAvoid it happening againContaminated soil reclamationCyanide compoundSoil science
The invention provides a cyanide contaminated soil remediation technology, which is characterized in that a soil oxidation technology and a soil leaching technology are combined to treat moderate andsevere cyanide contaminated soil. An efficient oxidizing agent is selected, free radicals with high oxidation reduction potential are matched with a proper activation technology, a binding form of cyanide is destroyed, and a stable ferricyanide complexing state is converted into an easy-to-release state; then the cyanide in the easily released state is leached from the soil by utilizing a leachingtechnology to reach a remediation target value. The method can be applied to an engineering implementation process and has a good effect; in the remediation process, wastewater and waste residues aretreated, secondary pollution is prevented, and green remediation in the whole remediation process can be achieved; automation is achieved, and a large amount of manpower investment is not needed in the whole process.
Owner:TIANJIN ECOLOGY CITY ENVIRONMENTAL PROTECTION
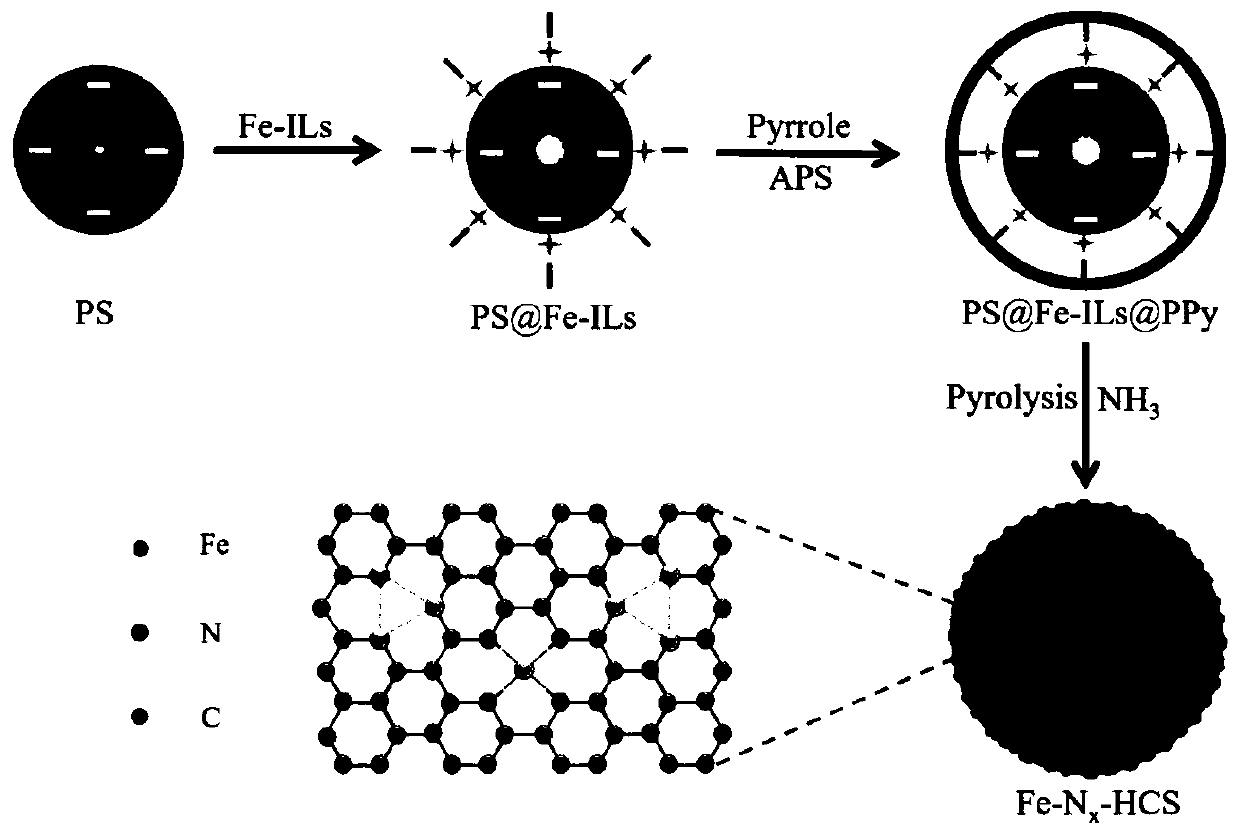
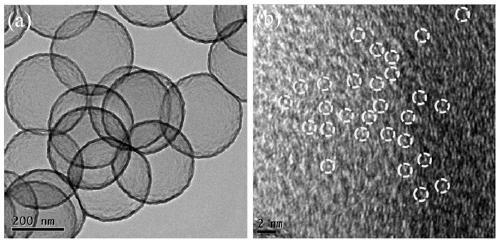
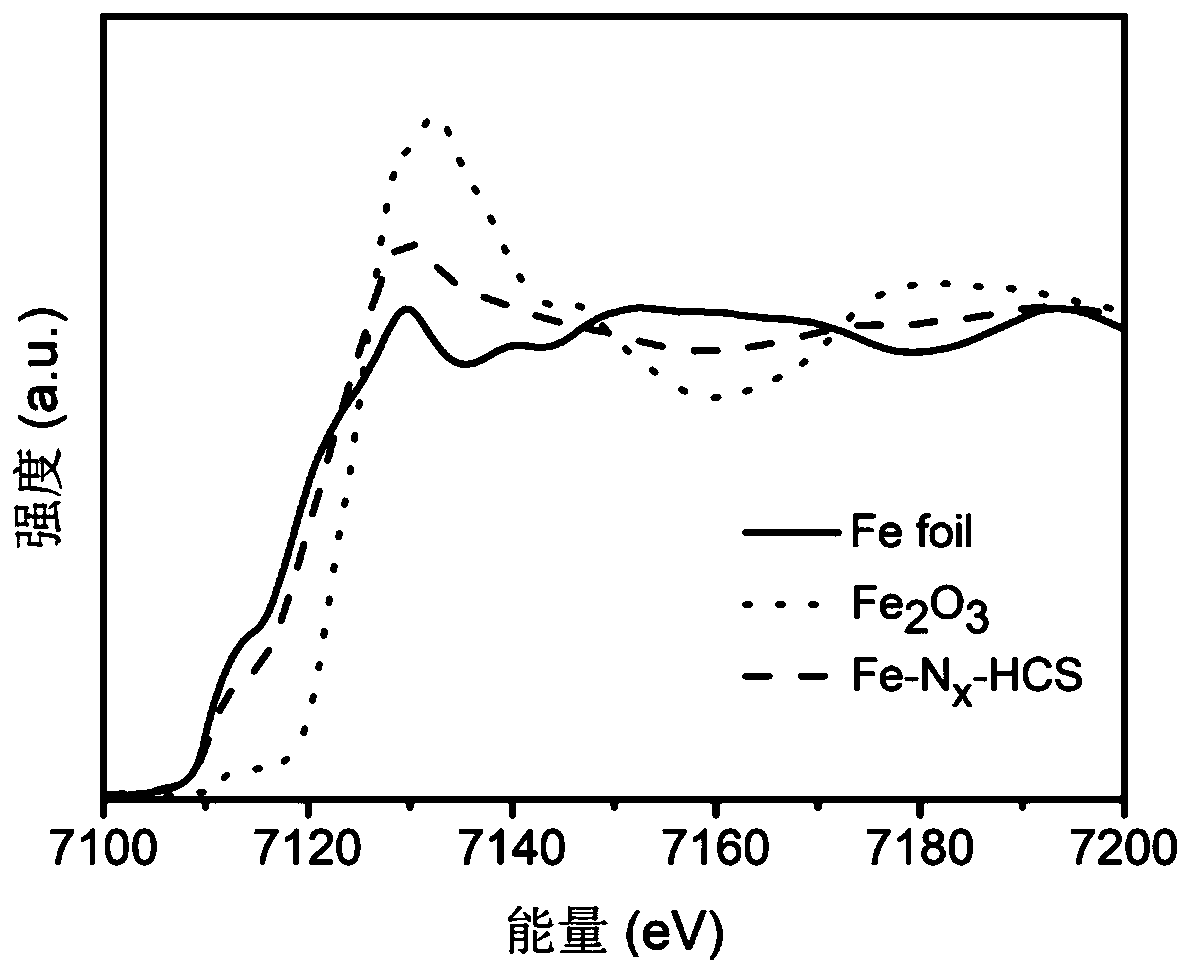
Atomic-scale iron active site catalyst as well as preparation method and application thereof
ActiveCN111584891AImprove catalytic performanceIncrease mass transfer rateCell electrodesPtru catalystAir cell
The invention belongs to the field of nano materials, and discloses an atomic-scale iron active site catalyst as well as a preparation method and application thereof. The preparation method provided by the invention comprises the following steps: 1) preparing ferricyanide dimethylimidazole ionic liquid; 2) constructing a core-shell structure precursor with uniformly dispersed iron ions; and 3) preparing the atomic-scale iron active site catalyst. The atomic-scale iron active site catalyst obtained by the preparation method disclosed by the invention is microporous nanospheres loaded with atomic-scale iron active sites comprising Fe-N4 ligands and Fe3 clusters. The catalyst is low in density, high in stability and resistant to acid and alkali corrosion; porous properties are also exhibited;the reaction activation energy can be greatly reduced, the electron / ion transfer and the mass transfer rate of an intermediate product are accelerated, and thus, the catalytic activity and the cycling stability of the catalyst are improved, and the maximum atom utilization rate (100%) is realized; and the catalyst is suitable for being used as a catalyst material for electrocatalytic oxygen reduction (ORR) and oxygen evolution (OER) reactions in fuel cells and metal-air cells.
Owner:NANTONG UNIVERSITY
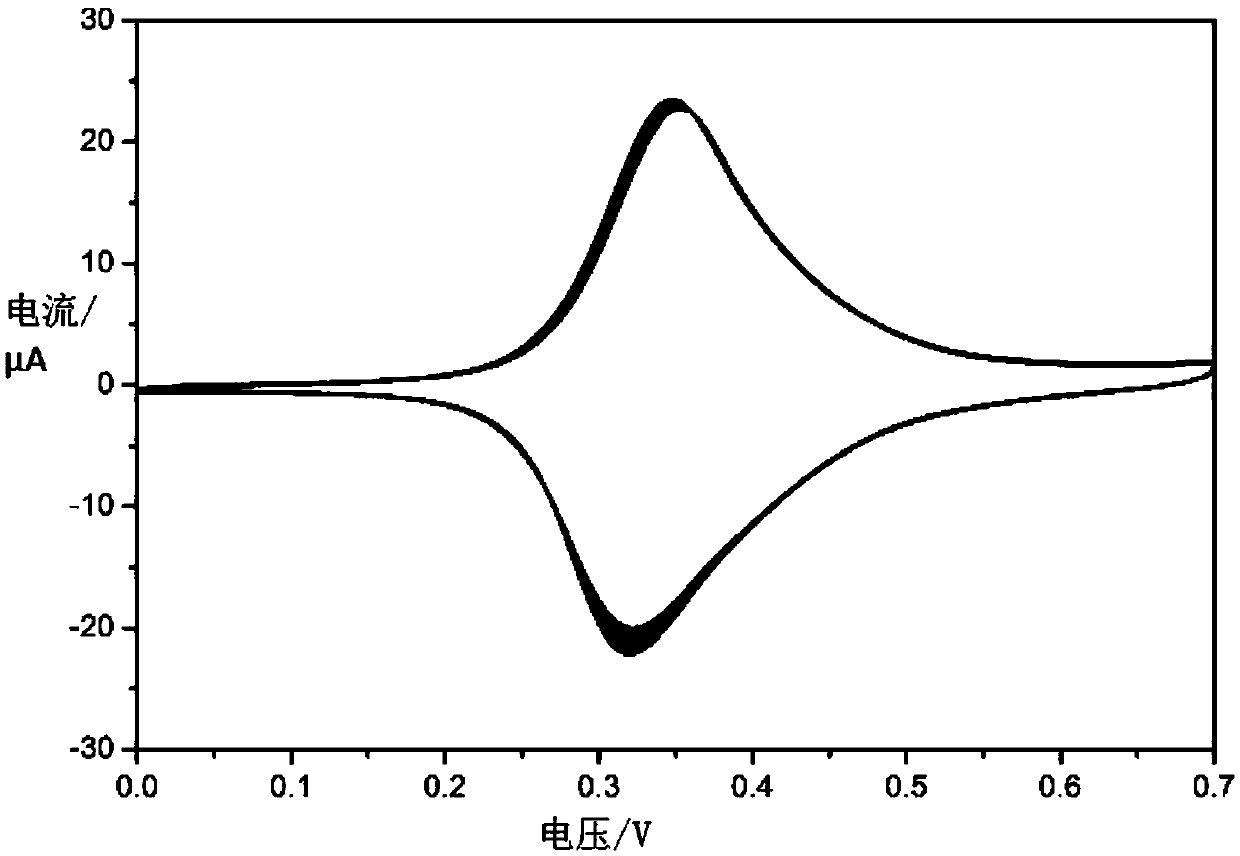
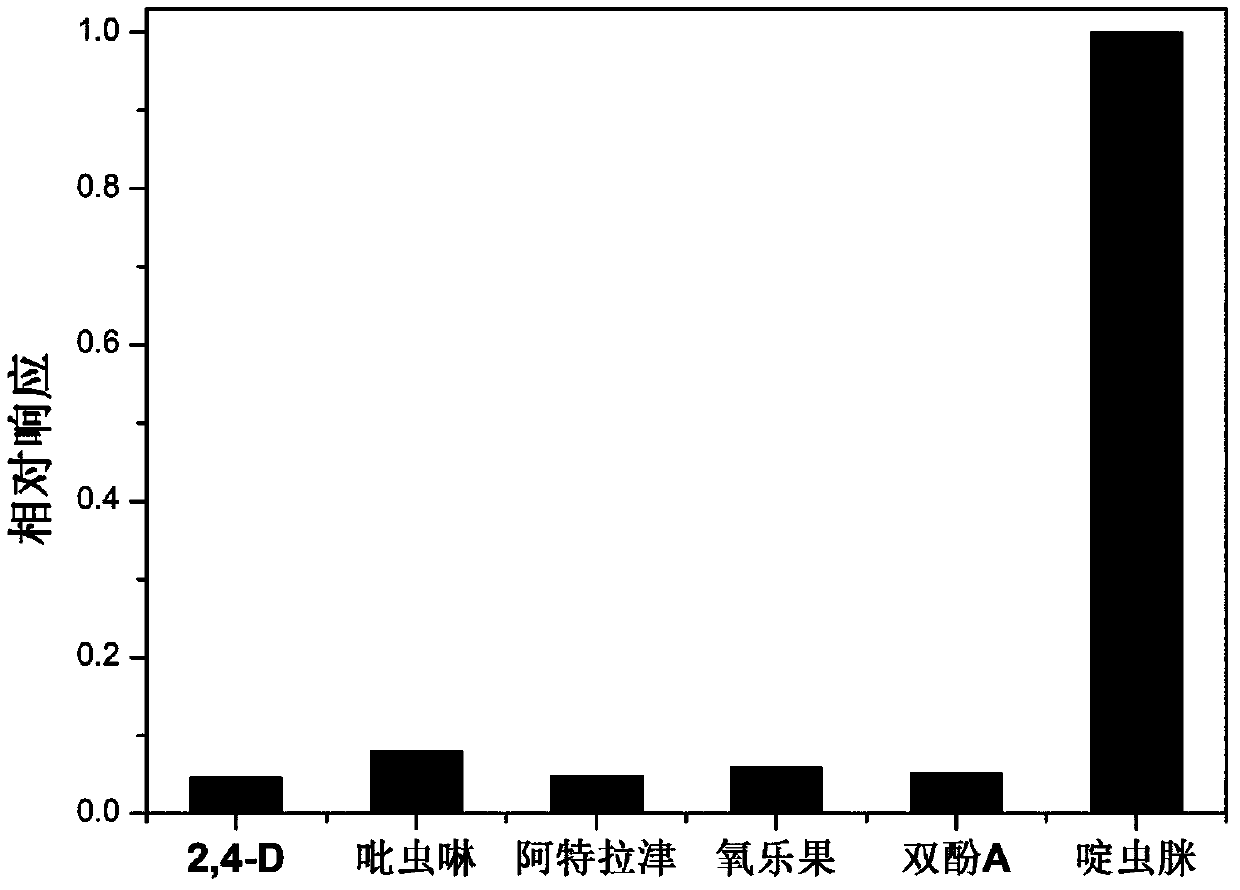
Method for detecting acetamiprid based on nickel-ferricyanide complex nanoparticles as indication probe
ActiveCN107655958AHigh selectivityReduce analysis costsMaterial electrochemical variablesNanoparticleElectrochemistry
The invention relates to a method for detecting acetamiprid based on nickel-ferricyanide complex nanoparticles as an indication probe. The problems of high cost and poor selectivity of existing acetamiprid detection methods and the problems of tedious operation, high price and long time caused by electroactive group labeling of aptamer or addition of an electroactive substance into a test system in the construction process of previous electrochemical aptamer sensors for acetamiprid are solved. The nickel-ferricyanide complex nanoparticles used as the indication probe are deposited on the surface of an electrode in situ, and a highly-sensitive electrochemical analysis technology is effectively combined with a high-affinity nuclear aptamer to construct an electrochemical aptamer sensor for acetapamidine. The method realizes highly sensitive analysis of the acetamiprid and obtaining of high selectivity in a complex environment medium, has the advantages of simple device, low analysis costand fast response, and provides a new detection method for assessing the pesticide acetamiprid residues in foods and the environment.
Owner:SHANXI UNIV
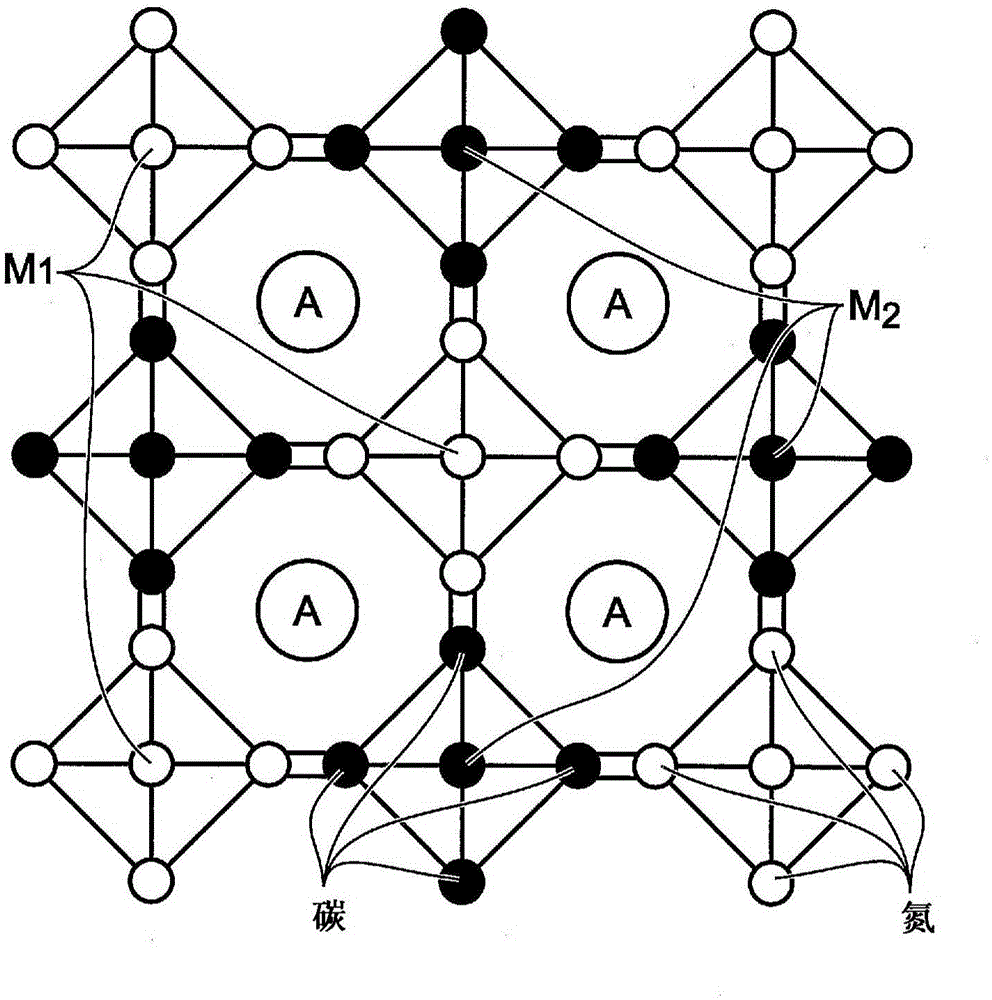
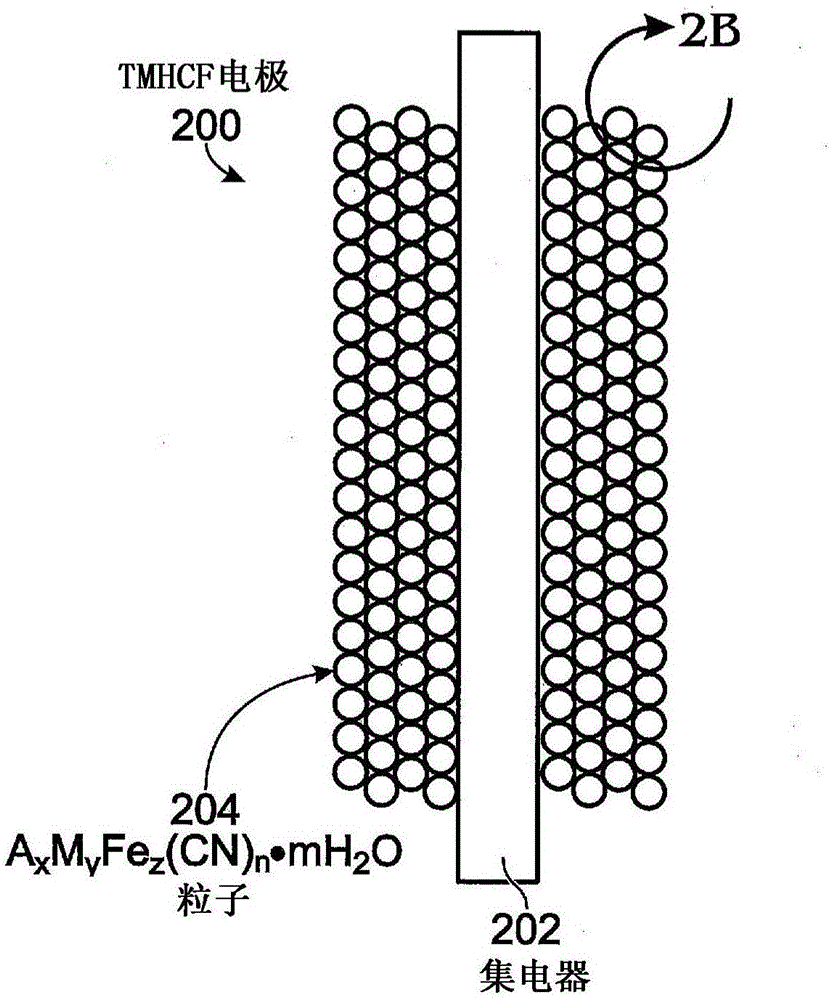
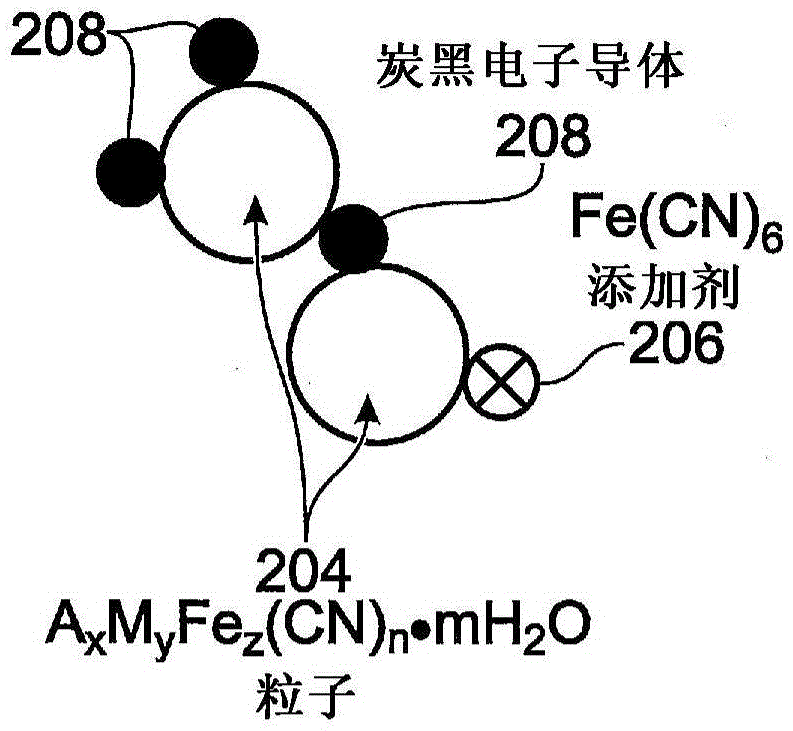
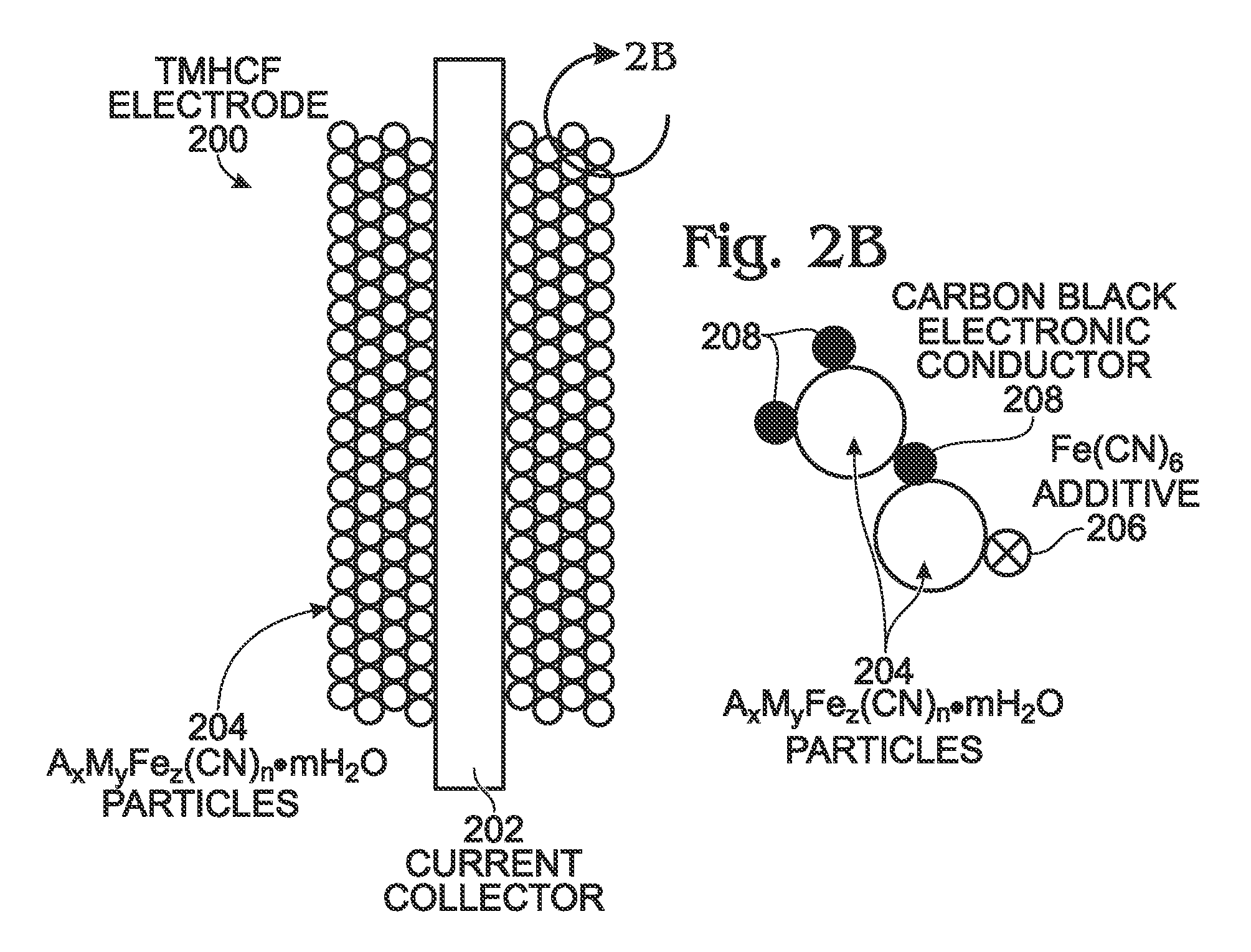
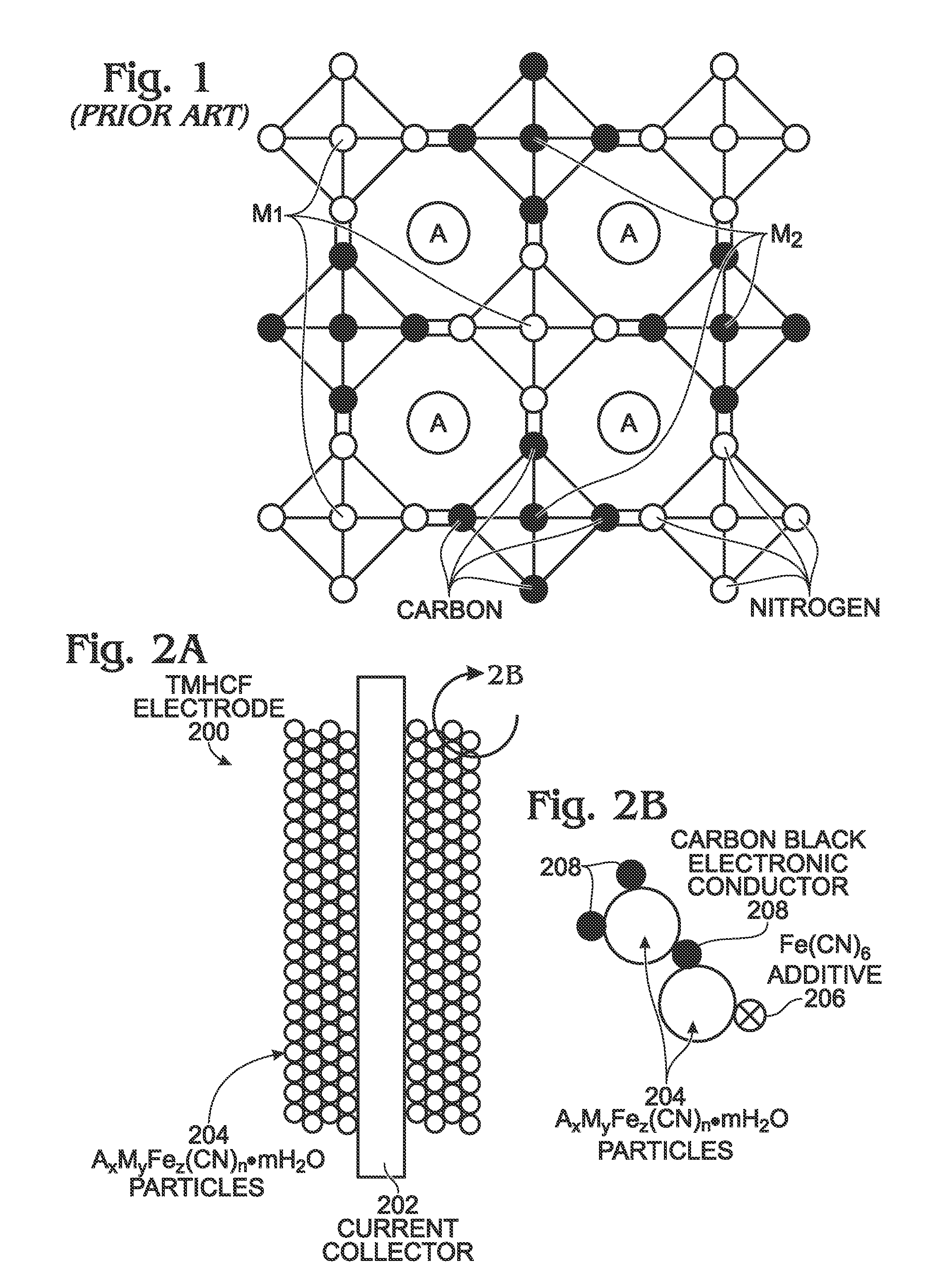
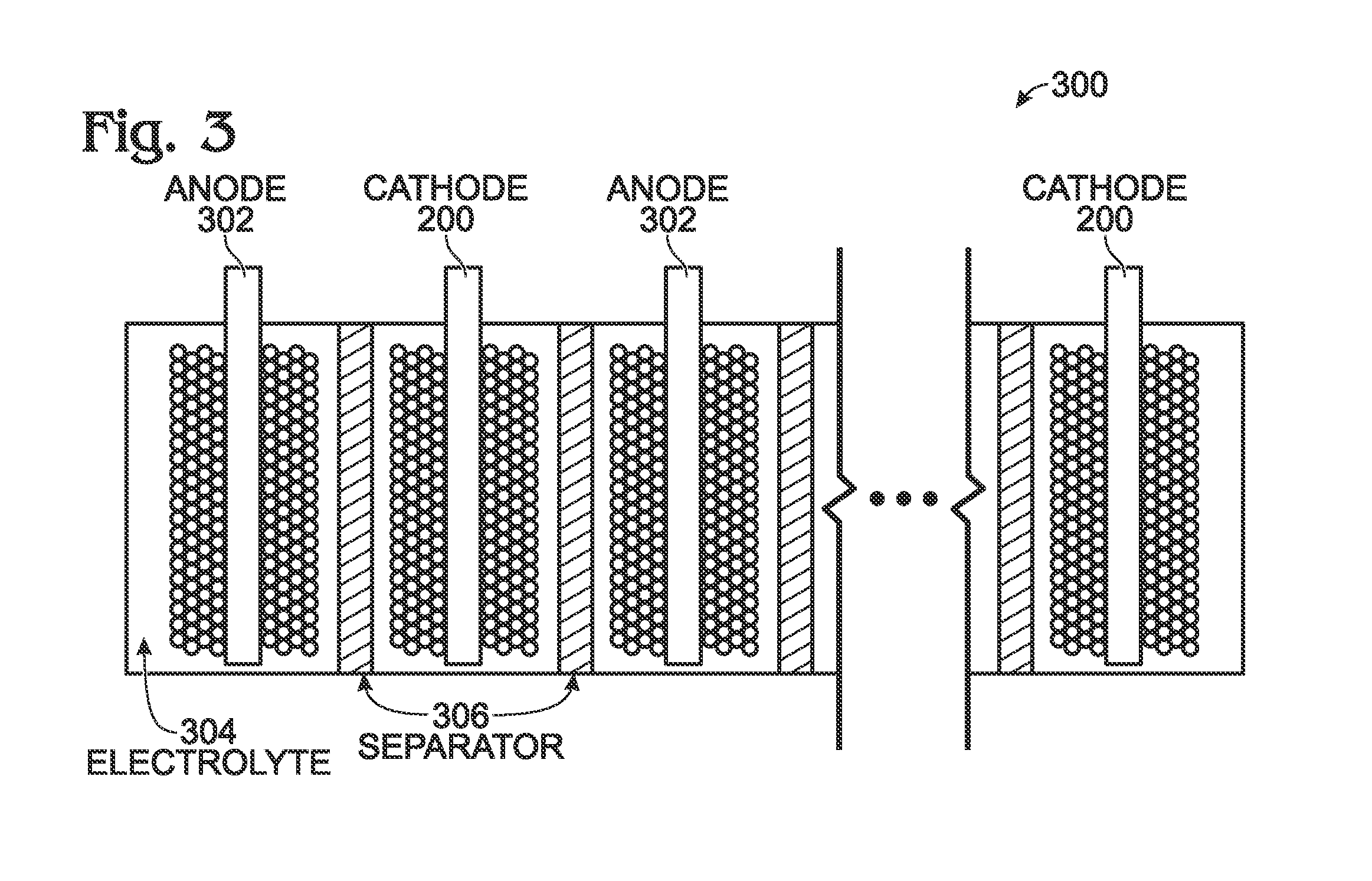
Hexacyanoferrate battery electrode modified with ferrocyanides or ferricyanides
A transition metal hexacyanoferrate (TMHCF) battery electrode is provided with a Fe(CN)6 additive. The electrode is made from AxMyFez(CN)n.mH2O particles overlying a current collector, where the A cations are either alkali and alkaline-earth cations such as sodium (Na), potassium (K), calcium (Ca), or magnesium (Mg), and M is a transition metal. A Fe(CN)6 additive modifies the AxMyFez(CN)n.mH2O particles. The Fe(CN)6 additive may be ferrocyanide ([Fe(CN)6]4-) or ferricyanide ([Fe(CN)6]3-). Also provided are a related TMHCF battery with Fe(CN)6 additive, TMHCF fabrication process, and TMHCF battery fabrication process.
Owner:SHARP KK
Hexacyanoferrate Battery Electrode Modified with Ferrocyanides or Ferricyanides
InactiveUS20130266860A1Improve electrode performanceHigh capacity retentionElectrode manufacturing processesPrimary cellsAlkaline earth metalCyanide
A transition metal hexacyanoferrate (TMHCF) battery electrode is provided with a Fe(CN)6 additive. The electrode is made from AxMyFez(CN)n.mH2O particles overlying a current collector, where the A cations are either alkali and alkaline-earth cations such as sodium (Na), potassium (K), calcium (Ca), or magnesium (Mg), and M is a transition metal. A Fe(CN)6 additive modifies the AxMyFez(CN)n.mH2O particles. The Fe(CN)6 additive may be ferrocyanide ([Fe(CN)6]4−) or ferricyanide ([Fe(CN)6]3−). Also provided are a related TMHCF battery with Fe(CN)6 additive, TMHCF fabrication process, and TMHCF battery fabrication process.
Owner:SHARP KK
MnO2/Fe2O3@ amorphous carbon composite material, aptamer sensor and preparation method and application thereof
ActiveCN109813787AHigh detection sensitivityGood choiceMaterial electrochemical variablesDNA/RNA fragmentationAptamerPolypyrrole
The invention relates to a MnO2 / Fe2O3@ amorphous carbon composite material, an aptamer sensor and a preparation method and application thereof. The preparation method of the composite material comprises the following steps of: 1) conducting a reaction of soluble bivalent manganese salt, soluble ferricyanide and citrate in a solvent to prepare a ferromanganese Prussian blue analog; 2) conducting anoxidative polymerization reaction of the ferromanganese Prussian blue analog, pyrrole and an oxidant in the solvent to obtain a polypyrrole-coated ferromanganese Prussian blue analog; 3) calcining the polypyrrole-coated ferromanganese Prussian blue analog in a protective atmosphere to obtain the composite material. The bimetallic oxide particles and the amorphous carbon in the composite materialprepared by the method have a synergistic effect, so that the electrochemical activity of a substrate material is improved, stronger binding force is applied between the bimetallic oxide particles andan aptamer chain, the stability of G-quadruplexes formed between the aptamer and PTK7 is improved through the biometric identification ability.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
Secondary iron-based composite-copper ferricyanide battery and manufacturing method thereof
ActiveCN107516735AImprove stabilityAvoid spontaneous dischargeCell electrodesSecondary cellsRedoxCopper
The invention discloses a manufacturing method of a secondary iron-based composite-copper ferricyanide battery, the manufacturing method comprises the steps of: (1) preparing an iron-based composite; (2) preparing a battery negative electrode sheet; (3) preparing a battery positive electrode sheet; (4) assembling a iron-based composite-copper ferricyanide battery. In the invention, the iron-based composite is used as a negative electrode, the copper ferricyanide is used as a positive electrode and an electrolyte is an acidic solution. The negative electrode of the new type battery is a composite with iron as the main component, and has relatively good stability in the acidic solution to avoid spontaneous discharge of the battery; the positive electrode of the battery is the copper ferricyanide, which is stable in the acidic solution and has excellent redox properties. When the battery is discharging, the negative electrode is a process of forming metal ions by the iron-based composite and the positive electrode is a process of forming copper ferrocyanide by the copper ferricyanide. When the battery is charging, the metal ions are deposited on the negative electrode and the copper ferrocyanide in the positive electrode is reduced to be the copper ferricyanide.
Owner:HUNAN UNIV OF SCI & TECH
Prussian blue as well as preparation method and application thereof
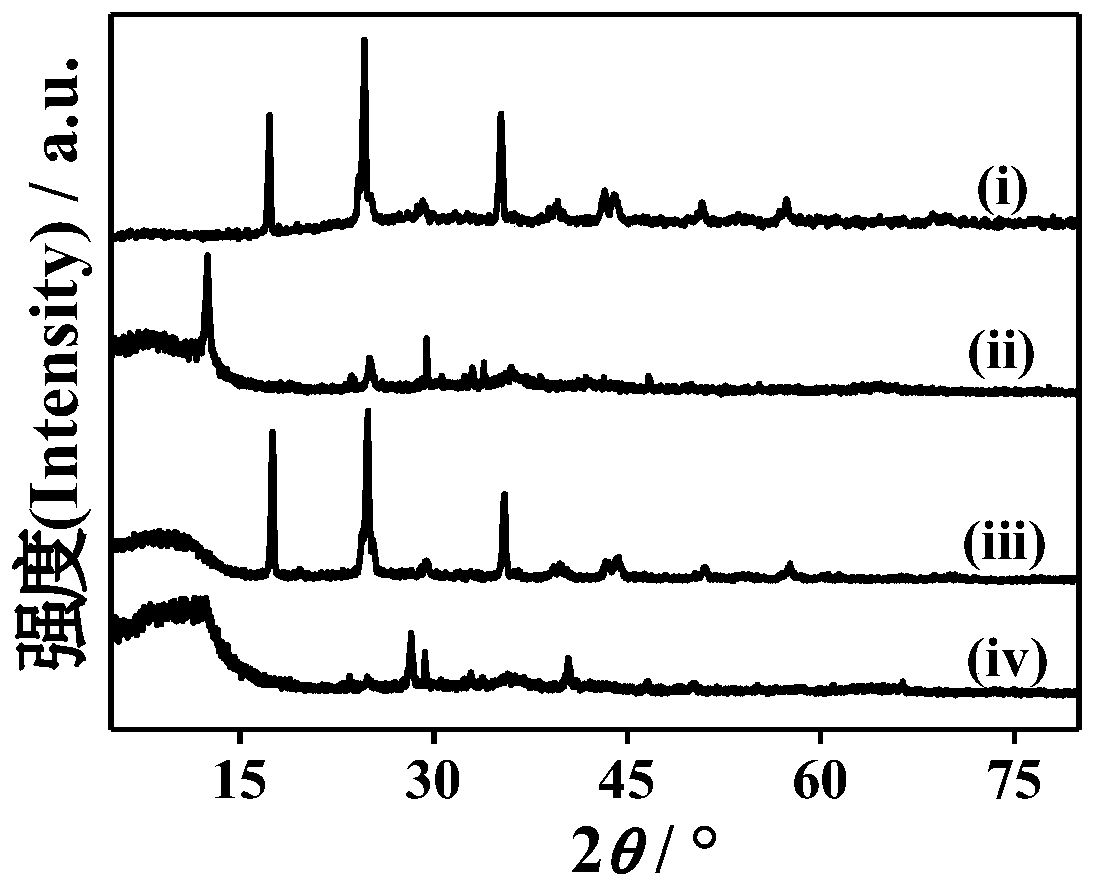
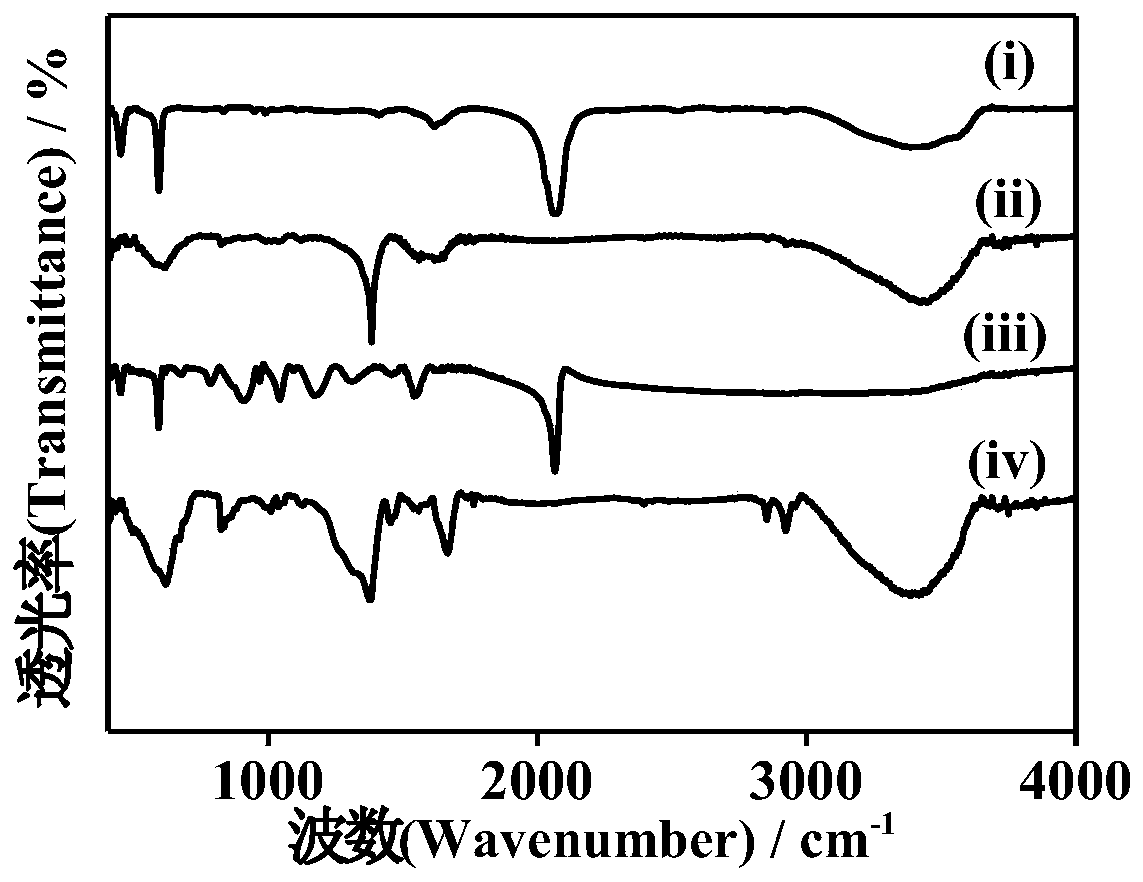
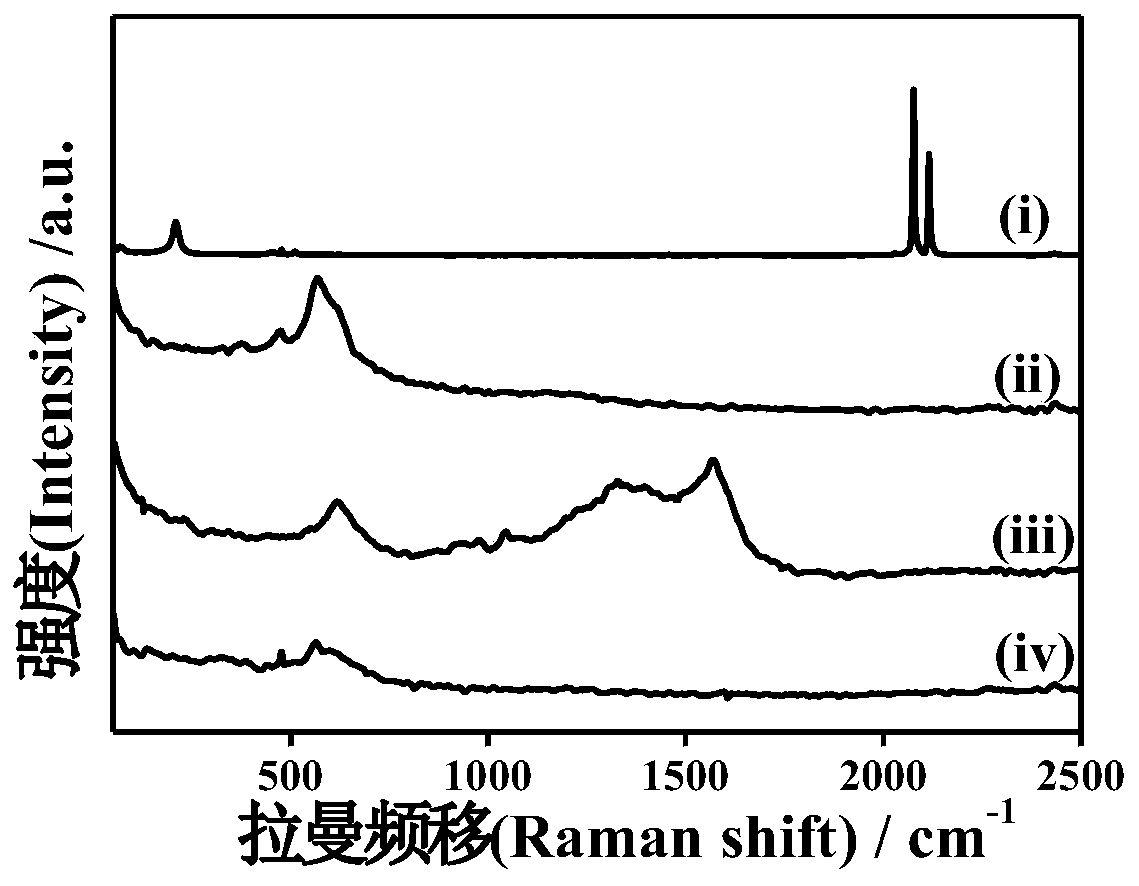
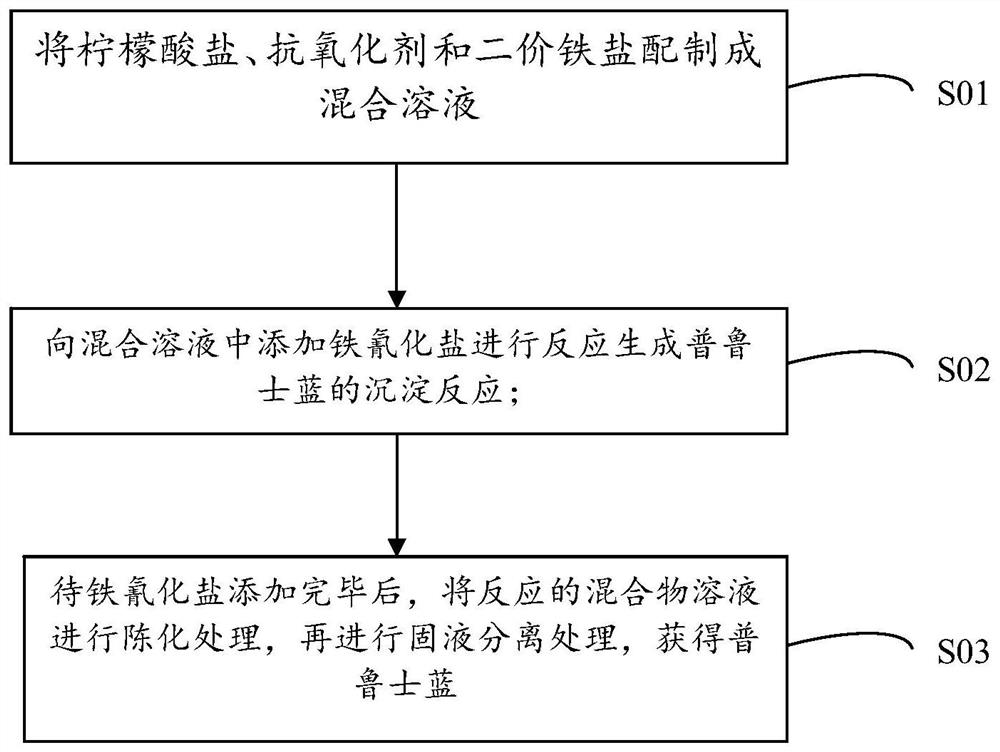
The invention discloses Prussian blue as well as a preparation method and application thereof. The prussian blue is prepared by taking ferricyanide and ferrous salt as iron sources and carrying out complex reaction in the presence of citrate and an antioxidant. The Prussian blue crystal form is complete, vacancy and structural water are less or the Prussian blue does not contain vacancy and structural water, the crystal structure is stable in the ion intercalation and deintercalation process, high reversible gram volume is given to the Prussian blue crystal form, ion diffusion is facilitated, and the Prussian blue crystal form has a good capacity retention rate characteristic under large current. The preparation method of the Prussian blue can ensure that the prepared Prussian blue is stable in structure and electrochemical performance, is high in efficiency, saves the production cost, does not generate toxic and harmful components, and is high in safety. Therefore, the Prussian blue can be applied as a positive electrode material.
Owner:EVERGRANDE NEW ENERGY TECH SHENZHEN CO LTD
Preparation method of metal ferricyanide adsorbent particles for liquid rubidium and cesium resource extraction
ActiveCN113509910AImprove water absorptionIncrease loadOther chemical processesAlkali metal oxides/hydroxidesRubidiumSorbent
The invention discloses a preparation method of metal organic framework compound adsorbent particles for extracting rubidium and cesium resources from salt lake brine, seawater and underground water. The adsorbent particles are prepared by taking a water-absorbing polymer as a carrier, adding transition metal ferrous ferrocyanide, potassium cobalt cyanide, potassium nickel ferrocyanide, potassium titanium ferrocyanide, potassium copper ferrocyanide, potassium cadmium ferrocyanide or other metal organic framework compound adsorbents in a high loading amount, and carrying out secondary cross-linking. The preparation process is simple and is suitable for industrial production. The prepared adsorbent particles have the characteristics of high elasticity, porosity, high water absorption, good permeability and the like. A resin matrix is resistant to strong acid, strong alkali and high-salt environments, the polyhydroxy structure on the surface of the matrix can effectively adsorb adsorbent particles, the solution loss rate is effectively reduced, the method can be applied to extraction of rubidium and cesium elements in salt lake raw brine, old brine, seawater and underground water resources, and meanwhile the high-strength corrosion-resistant matrix is suitable for an industrial adsorption column process.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com