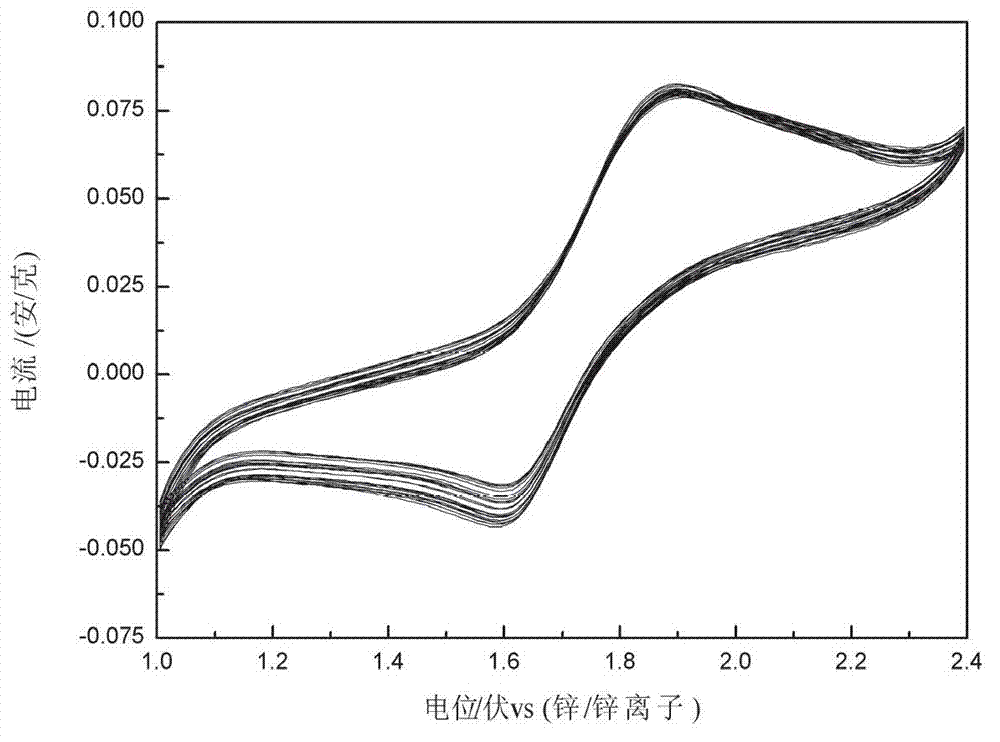
Aqueous electrolyte rechargeable zinc ion battery
A zinc ion battery and electrolyte technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of difficulty in intercalation and extraction of zinc ions, slow speed, etc., and achieve the effects of low price, simple preparation, and simple manufacturing process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Copper ferricyanide KCuFe(CN) 6 preparation of
[0022] Add a certain concentration of copper sulfate (copper chloride, zinc sulfate, zinc chloride, nickel sulfate, nickel chloride, etc.) solution and the same concentration of potassium ferricyanide solution drop by drop into continuously stirring deionized water, and wait After completion, ultrasonically shake the suspension mixed with brown precipitate for 30 minutes, then let it stand for 8 hours, then filter out the precipitate, wash it repeatedly with deionized water, and dry it in an oven to obtain ferricyanide copper powder.
[0023] Copper ferricyanide, electronic conductive graphite powder and binder polyvinylidene fluoride are evenly mixed in a mass ratio of 18:1:1, and then coated on the carbon current collector, cut into a certain size, and dried in an oven A copper ferricyanide electrode sheet was prepared. The single-electrode test uses copper ferricyanide electrode as the working electrode, a metal pla...
Embodiment 2
[0025] Mix zinc powder, electronic conductive agent graphite powder and binder polyvinylidene fluoride in a mass ratio of 18:1:1, coat it on the carbon current collector, cut it into a certain size, and dry it in an oven. Zinc electrode. The copper ferricyanide electrode prepared in Example 1 is the positive pole, the zinc electrode is the negative pole, and the electrolyte is 1mol L -1 ZnSO 4 Aqueous solutions are assembled into soft-packed rechargeable zinc-ion batteries.
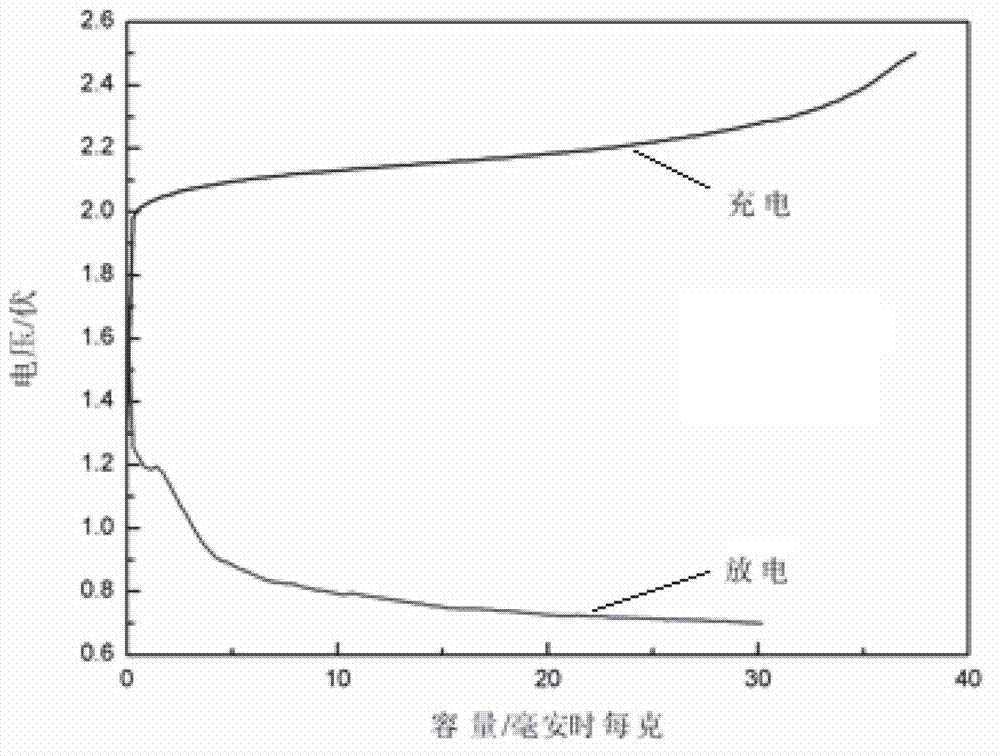
[0026] The prepared Zn-ion battery is at 200 mAg -1 For the constant current charge and discharge curve for the first time, see figure 2 (calculated based on the mass of the positive active material), as shown in the figure, a charging platform appears between the voltage range of 2.0~2.2V, and there is an obvious discharging platform at around 0.75V, with a Coulombic efficiency of 81%. The prepared Zn-ion battery is at 200 mAg -1 The constant current charge and discharge cycle curve (calculated bas...
Embodiment 3
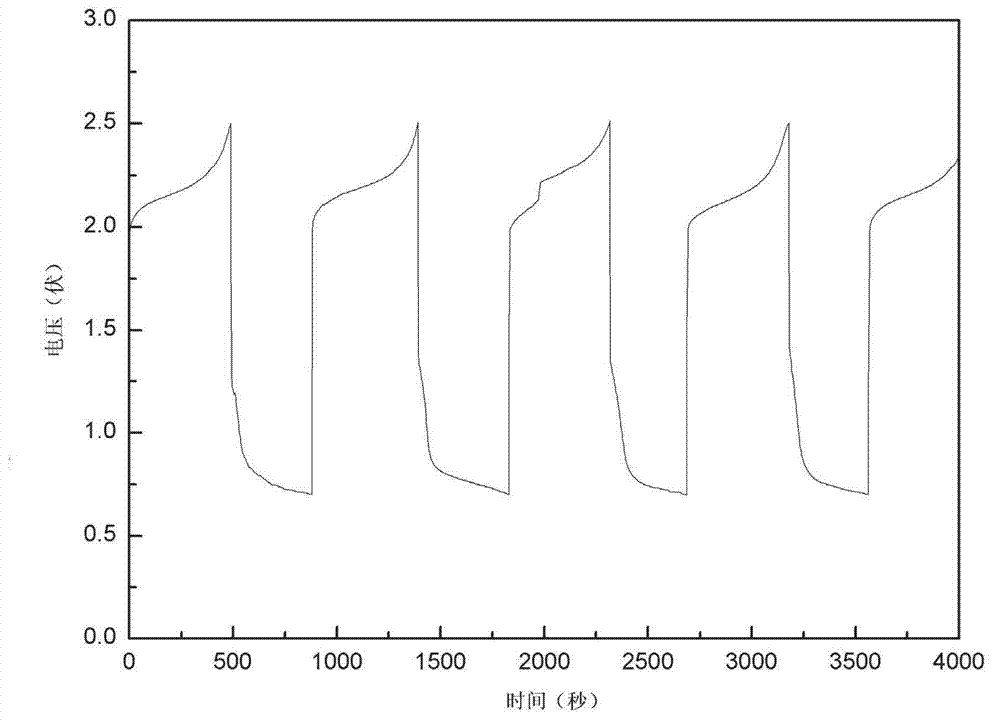
[0028] Mix zinc powder, conductive agent graphite powder, binder polyvinylidene fluoride, and corrosion inhibitor copper powder in a mass ratio of 83:8:8:1, coat them on the carbon collector, and dry them in an oven. Cut it into a certain size to make a zinc electrode. The copper ferricyanide electrode prepared in Example 1 is the positive pole, the zinc electrode is the negative pole, and the electrolyte is 1mol L -1 ZnSO 4 Aqueous solutions are assembled into soft-packed rechargeable zinc-ion batteries. The prepared Zn-ion battery is at 150 mA g -1 Constant current charge and discharge curve (calculated based on the mass of the positive electrode active material) see Figure 4 , as shown in the figure, there is a charging platform in the voltage range of 2.1~2.2V, and there is an obvious discharging platform at around 0.75V, and the Coulombic efficiency is 78%. If the structure is optimized, the battery charge and discharge characteristics and cycle stability will be sig...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com