Patents
Literature
412 results about "Amino acid mutation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A mutation is any random change in the DNA. The sequence of amino acids is critical to the performance of a protein; even a single amino acid in the wrong place can cause an enzyme to be non-functional or cause a disease such as Sickle Cell Anemia.
Bispecific Antibody Point Mutations for Enhancing Rate of Clearance
A mutant bispecific antibody that includes (a) a human hinge constant region from IgG having one or more amino acid mutations in the CH2 domain, (b) two scFvs; and (c) two Fvs has been constructed. This type of antibody displays enhanced clearance, which has been found to be particularly useful in the context of pre-targeting methods.
Owner:IMMUNOMEDICS INC
Anti-IL-6 Receptor Antibody
InactiveUS20110245473A1Enhanced antigen-neutralizing activity and pharmacokineticsGood treatment effectCompound screeningApoptosis detectionHigh concentrationHinge region
The present inventors succeeded in discovering specific amino acid mutations in the variable region, framework region, and constant region of TOCILIZUMAB, and this enables to reduce immunogenicity risk and the heterogeneity originated from disulfide bonds in the hinge region, as well as to improve antigen binding activity, pharmacokinetics, stability under acidic conditions, and stability in high concentration preparations.
Owner:CHUGAI PHARMA CO LTD
Anti-IL-6 Receptor Antibody
InactiveUS20130317203A1Enhanced antigen-neutralizing activity and pharmacokineticsGood treatment effectCompound screeningApoptosis detectionDisulfide bondingAntiendomysial antibodies
The present inventors succeeded in discovering specific amino acid mutations in the variable region, framework region, and constant region of TOCILIZUMAB, and this enables to reduce immunogenicity risk and the heterogeneity originated from disulfide bonds in the hinge region, as well as to improve antigen binding activity, pharmacokinetics, stability under acidic conditions, and stability in high concentration preparations.
Owner:CHUGAI PHARMA CO LTD
Heteromultimer Constructs of Immunoglobulin Heavy Chains with Mutations in the Fc Domain
InactiveUS20130336973A1Improve stabilityAnimal cellsImmunoglobulins against cell receptors/antigens/surface-determinantsImmunoglobulin heavy chainHeterologous
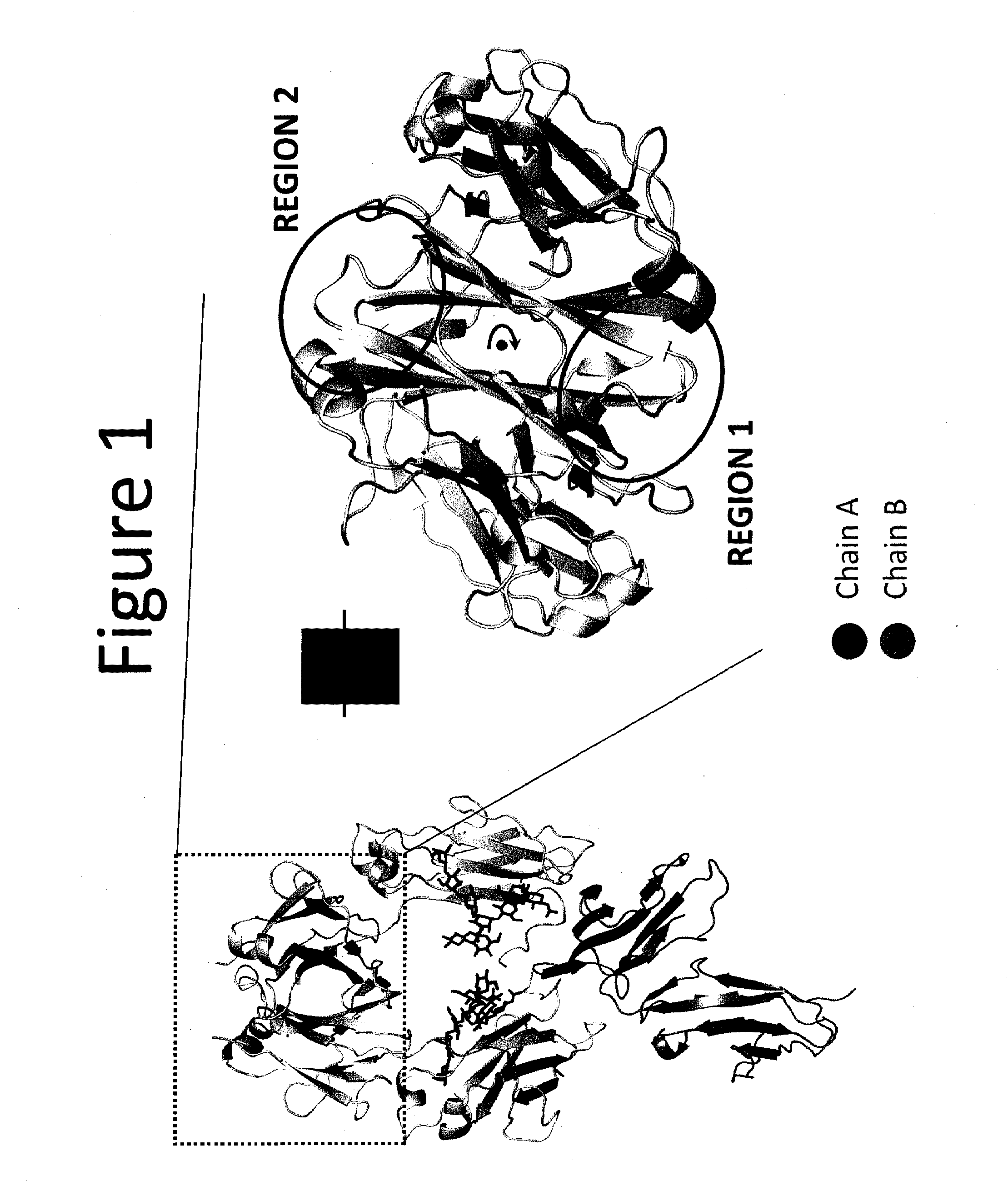
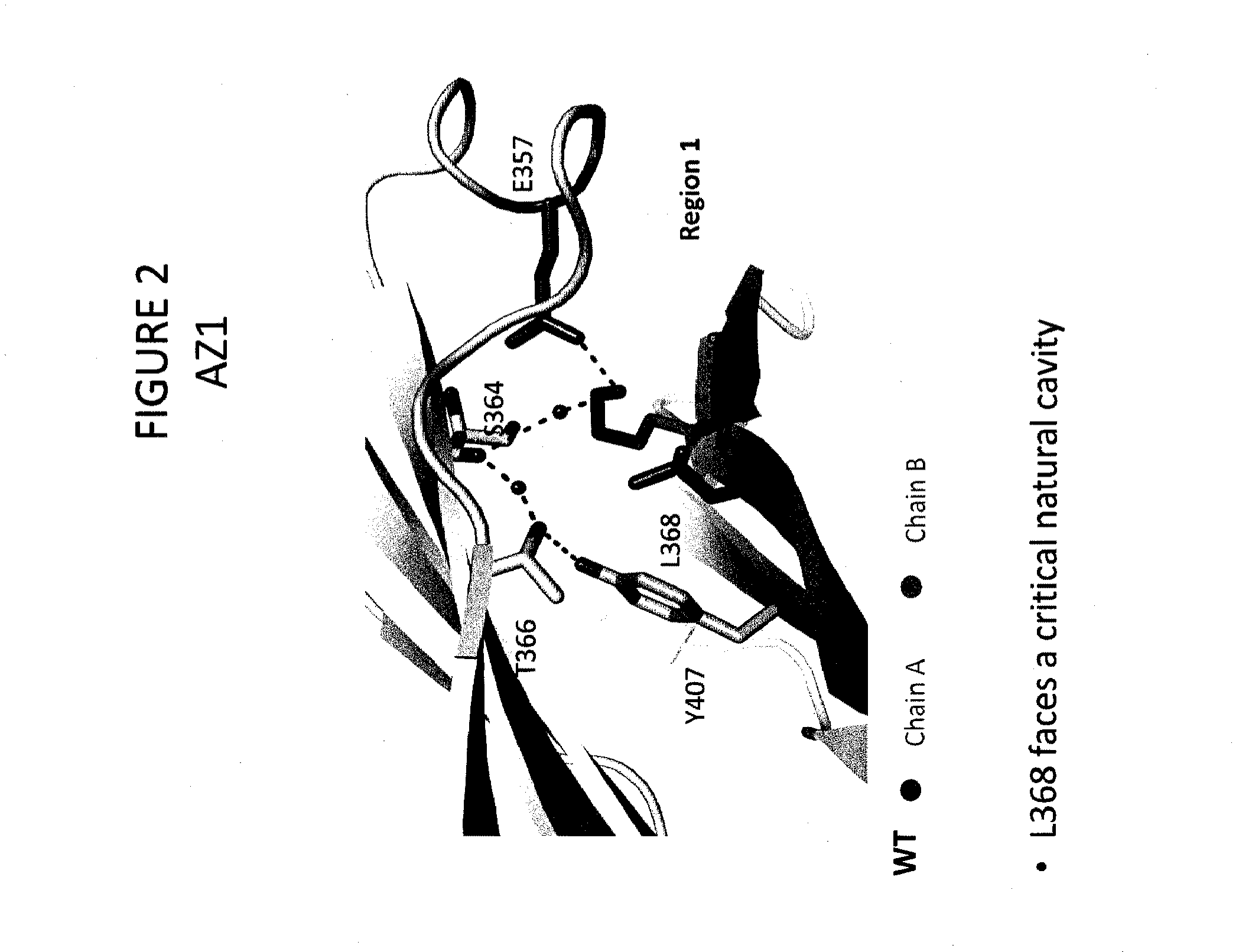
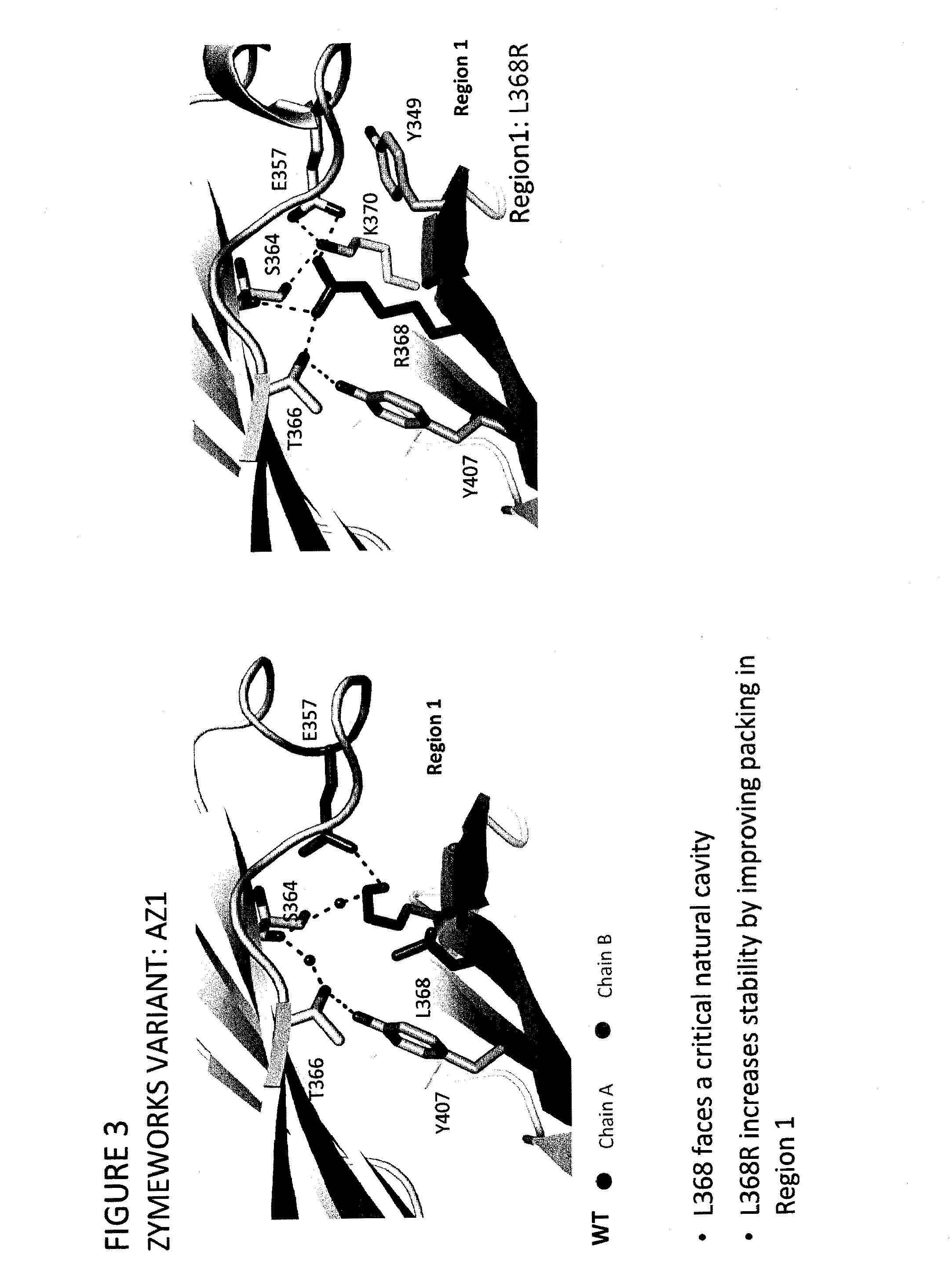
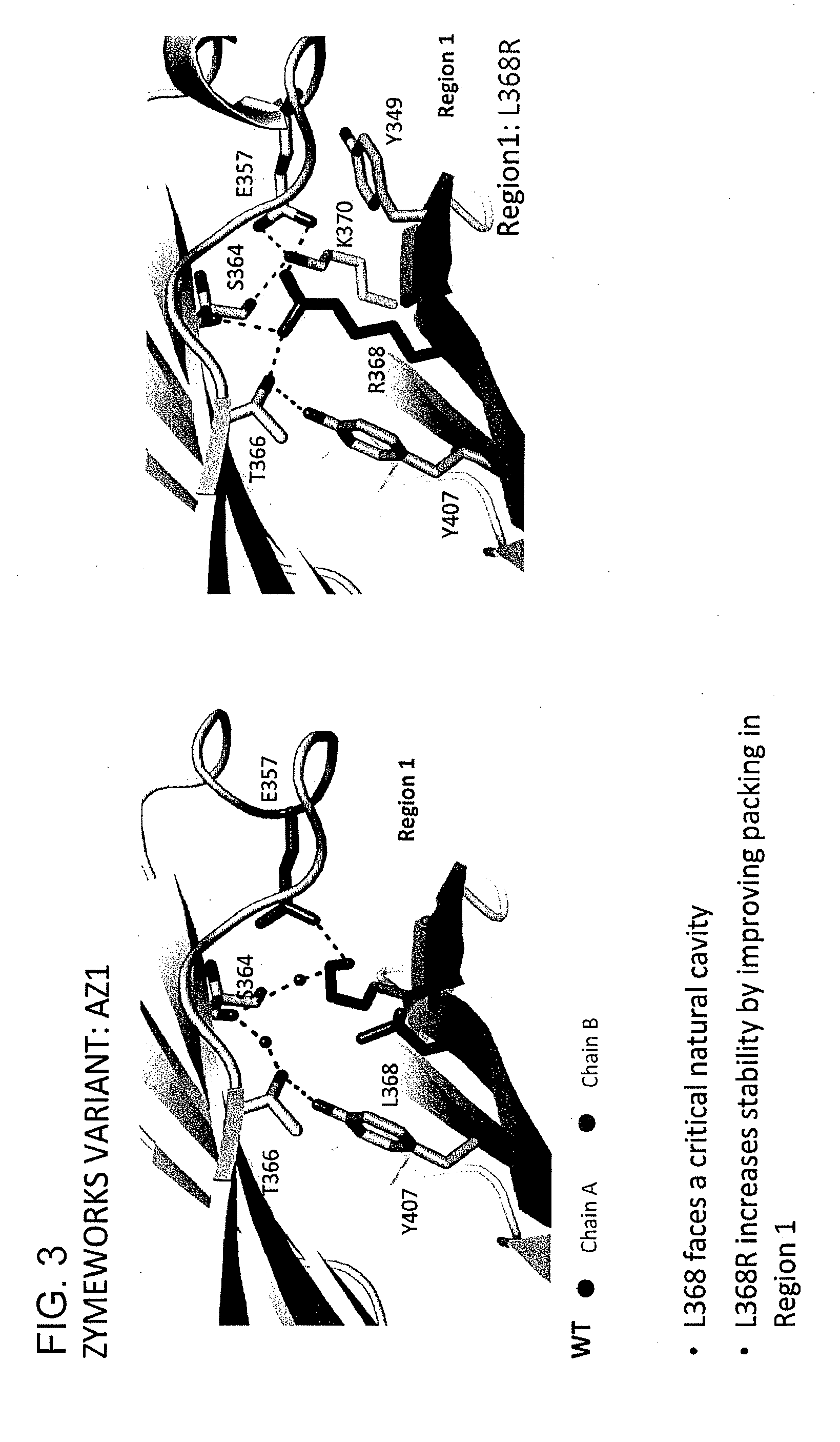
Provided herein are isolated heteromultimers comprising: at least one single domain antigen-binding construct attached to at least one monomer of a heterodimer Fc region; wherein the heterodimer Fc region comprises a variant CH3 domain comprising amino acid mutations that promote the formation of said heterodimer with stability comparable to that of a native Fc homodimer; and wherein said isolated heteromultimer is devoid of immunoglobulin light chains and optionally devoid of immunoglobulin CH1 region. These novel molecules comprise complexes of heterogeneous components designed to alter the natural way antibodies behave and that find use in therapeutics.
Owner:ZYMEWORKS INC
Form-specific antibodies for nag-1 (mic-1, gdf-15), h6d and other tgf-beta subfamily and heart disease and cancer diagnoses
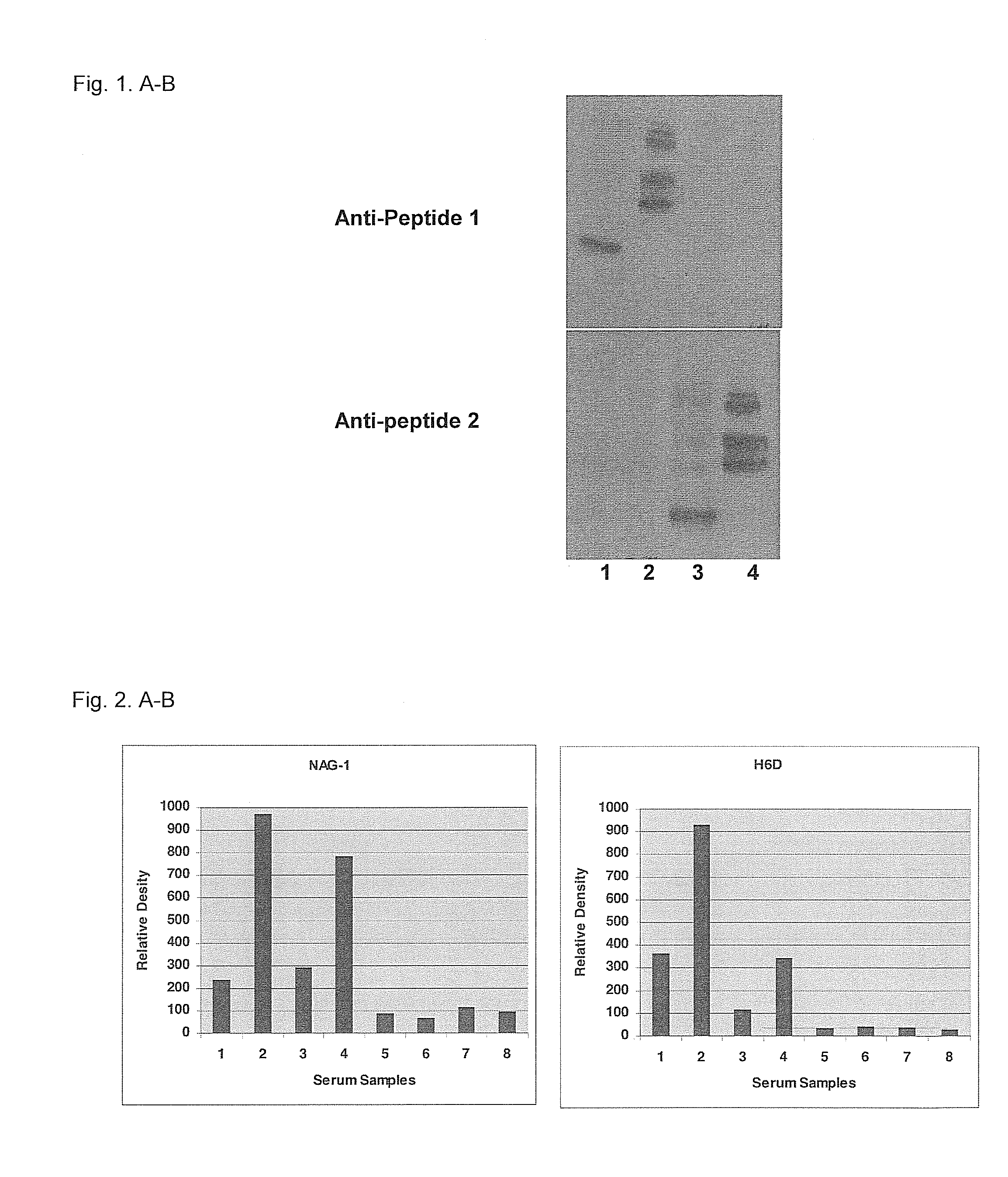
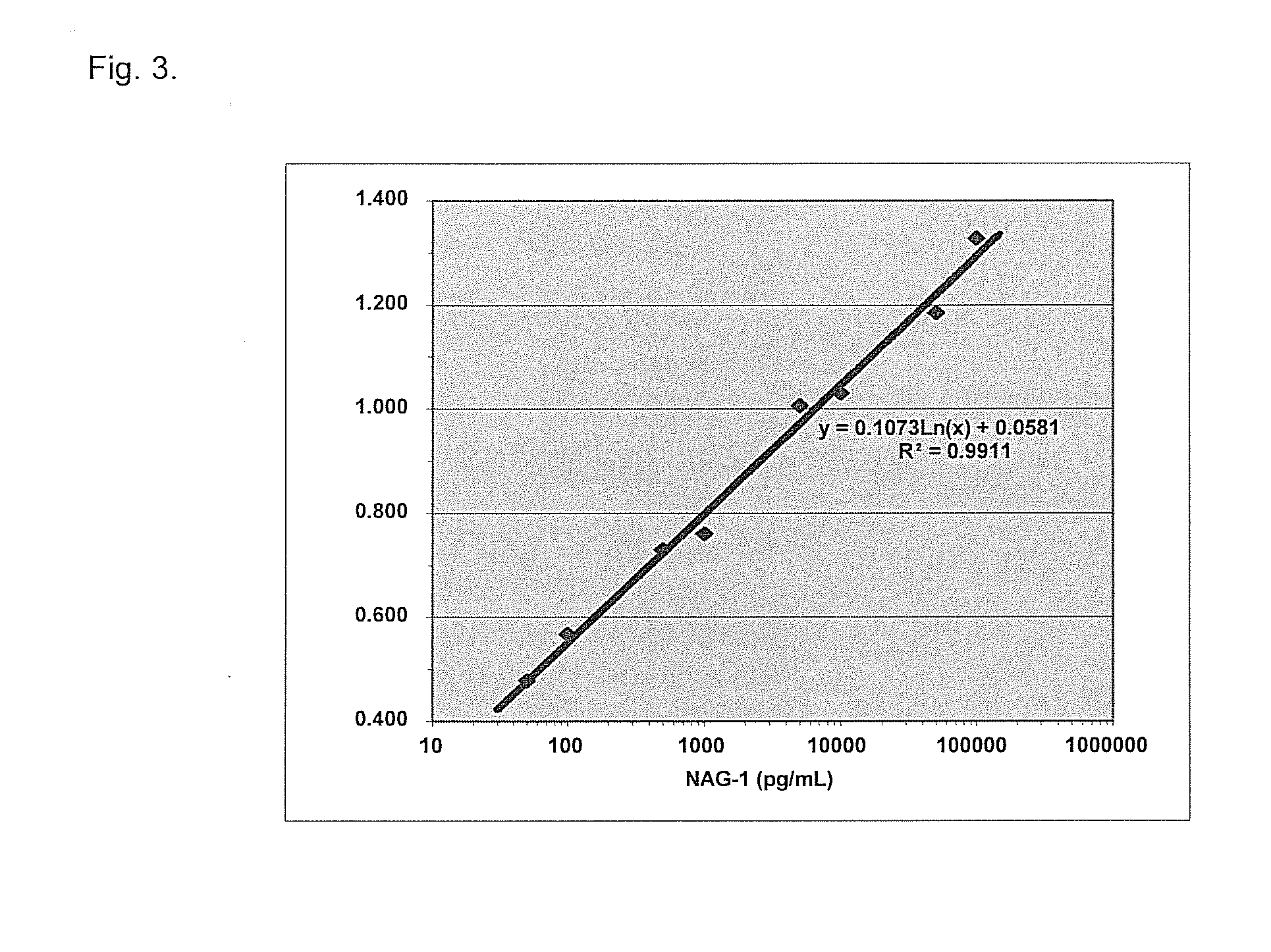
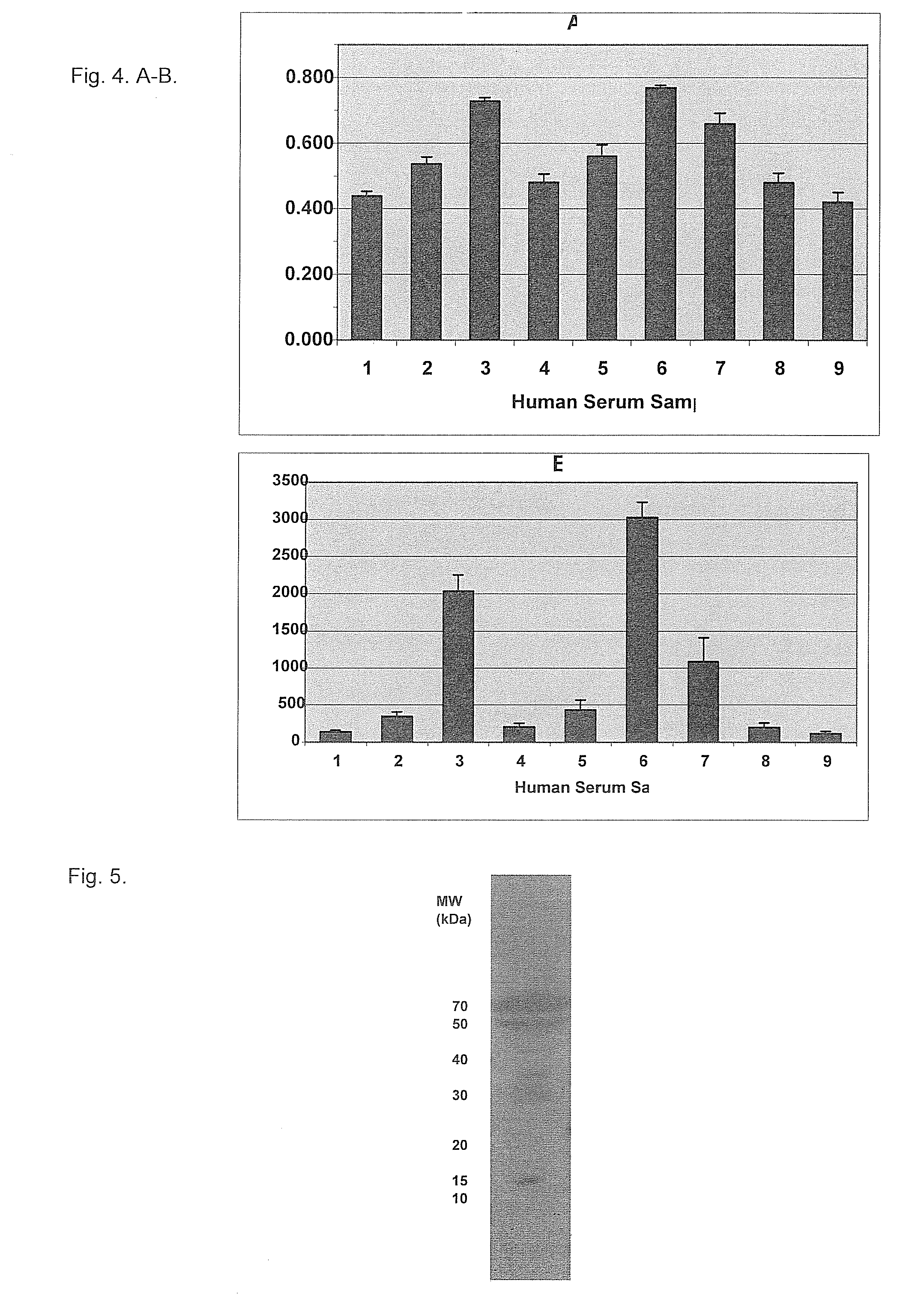
A method of producing form-specific anti-peptide antibodies for a wild type protein and its one amino acid mutated protein using a peptide antigen, by obtaining a protein sequence of the wild type protein and its one amino acid mutated protein, selecting a continuous amino acid sequence without any internal cysteine residues that includes the one amino acid mutated sequence and wild type sequence corresponding to the mutated site at the end of the sequence to obtain a synthetic mutation peptide and a synthetic wild type peptide, conjugating the synthetic peptides to a carrier protein, and immunizing an animal to produce antibodies. Methods of detecting cancer and methods of treating cancer.
Owner:DETROIT R&D
Heteromultimer constructs of immunoglobulin heavy chains with mutations in the fc domain
InactiveUS20160257763A1Immunoglobulins against cell receptors/antigens/surface-determinantsImmunological disordersImmunoglobulin heavy chainImmunoglobulin light chain
Provided herein are isolated heteromultimers comprising: at least one single domain antigen-binding construct attached to at least one monomer of a heterodimer Fc region; wherein the heterodimer Fc region comprises a variant CH3 domain comprising amino acid mutations that promote the formation of said heterodimer with stability comparable to that of a native Fc homodimer; and wherein said isolated heteromultimer is devoid of immunoglobulin light chains and optionally devoid of immunoglobulin CH1 region. These novel molecules comprise complexes of heterogeneous components designed to alter the natural way antibodies behave and that find use in therapeutics.
Owner:ZYMEWORKS INC
Avirulent, immunogenic flavivirus chimeras
InactiveUS7094411B2Minimize and inhibit infectionStable maintenanceOrganic active ingredientsVirusesViral diseaseAmino acid mutation
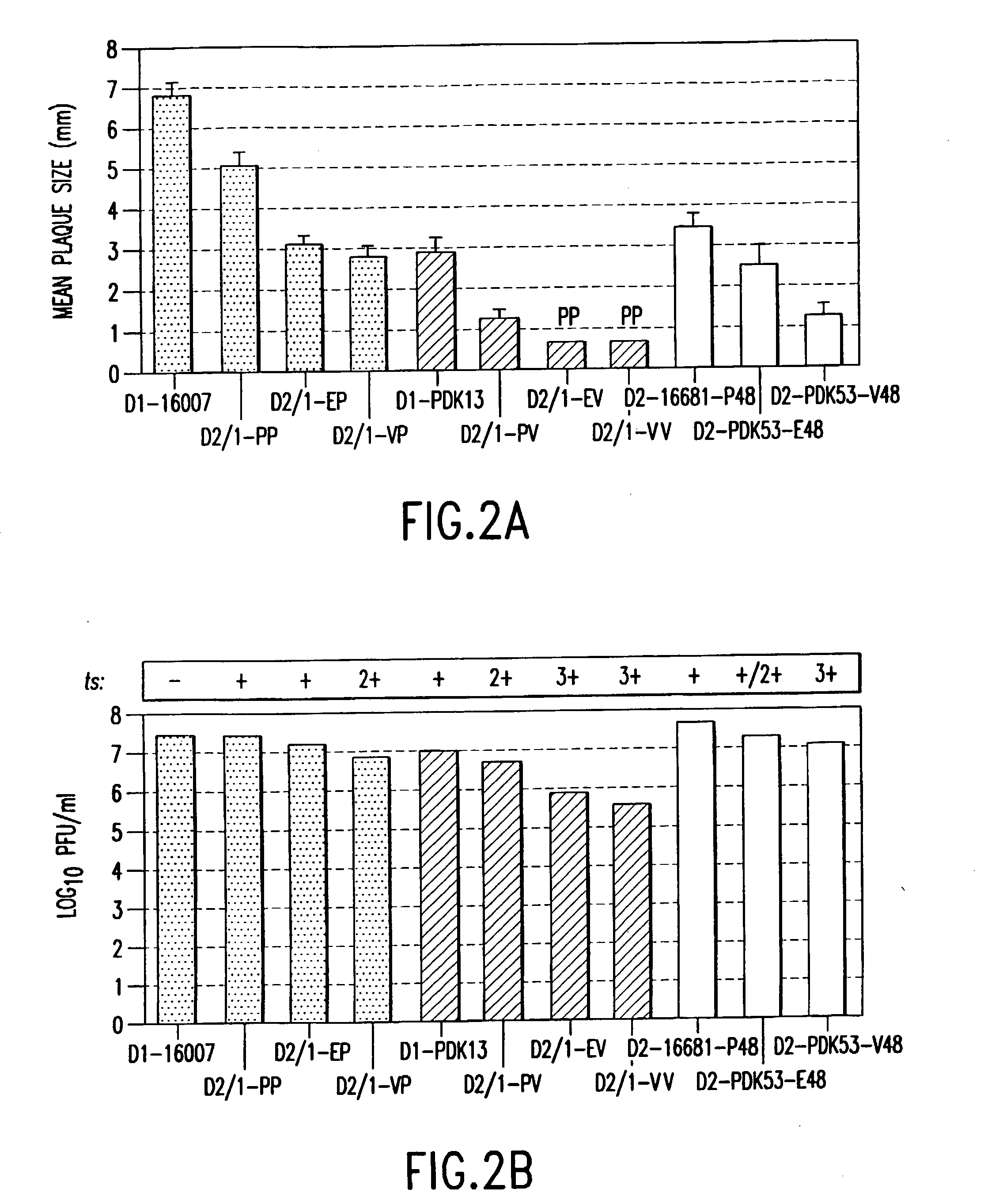
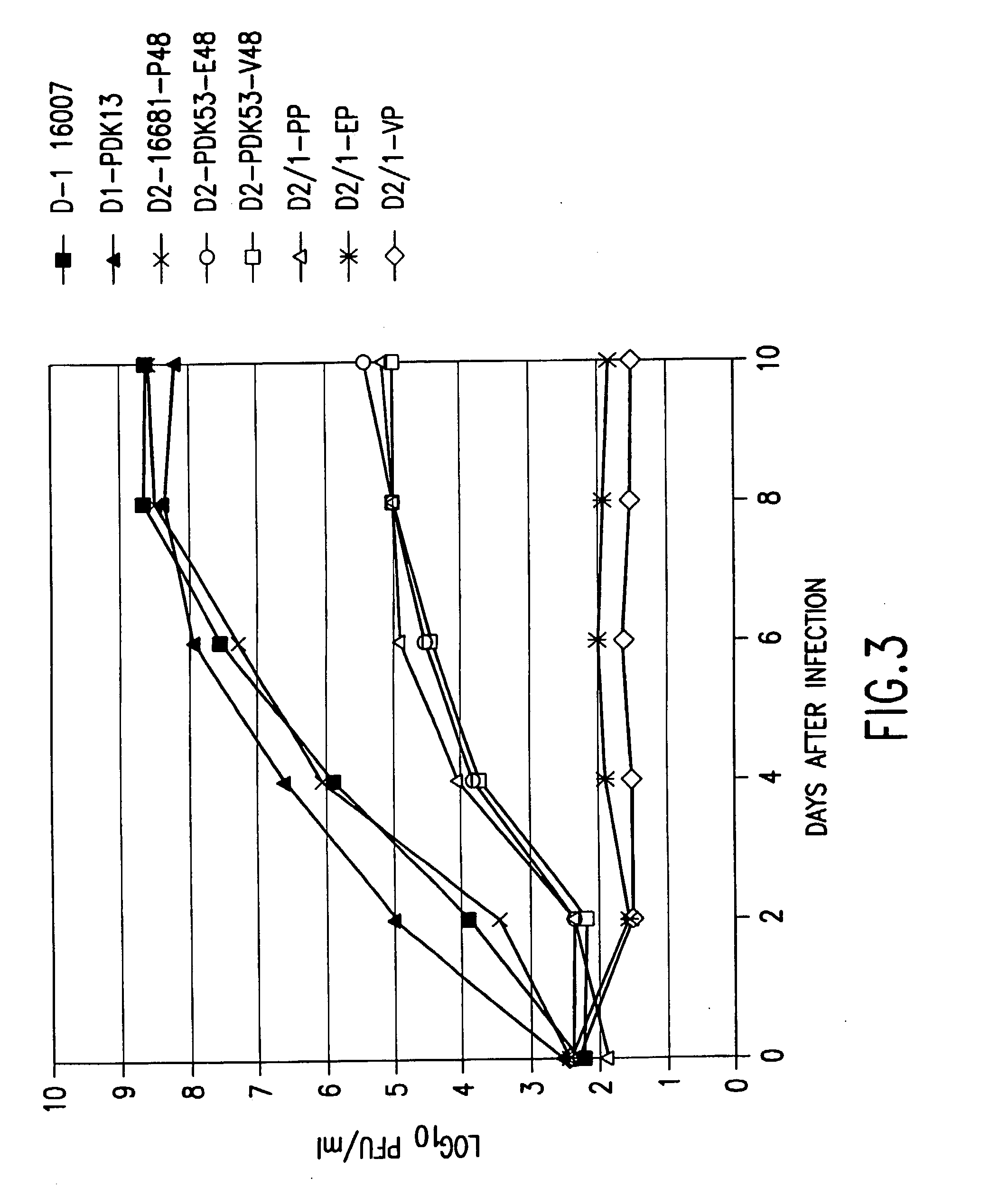
Chimeric flaviviruses that are avirulent and immunogenic are provided. The chimeric viruses are constructed to contain amino acid mutations in the nonstructural proteins of a flavivirus. Chimeric viruses containing the attenuation-mutated nonstructural genes of the virus are used as a backbone into which the structural protein genes of a second flavivirus strain are inserted. These chimeric viruses elicit pronounced immunogenicity yet lack the accompanying clinical symptoms of viral disease. The attenuated chimeric viruses are effective as immunogens or vaccines and may be combined in a pharmaceutical composition to confer simultaneous immunity against several strains of pathogenic flaviviruses.
Owner:MAHIDOL UNIV +2
Mutated anti-cd22 antibodies and immunoconjugates
ActiveUS7982011B2Immunoglobulin superfamilyAntibody mimetics/scaffoldsAntiendomysial antibodiesCancer cell
Recombinant immunotoxins are fusion proteins composed of the Fv domains of antibodies fused to bacterial or plant toxins. RFB4 (Fv)-PE38 is an immunotoxin that targets CD22 expressed on B cells and B cell malignancies. The present invention provides antibodies and antibody fragments that have improved ability to bind the CD22 antigen compared to RFB4. Immunotoxins made with the antibodies and antibody fragments of the invention have improved cytotoxicity to CD22-expressing cancer cells. Compositions that incorporate these antibodies into chimeric immunotoxin molecules that can be used in medicaments and methods for inhibiting the growth and proliferation of such cancers. Additionally, the invention provides a method of increasing the cytotoxicity of forms of Pseudomonas exotoxin A (“PE”) with the mutation of a single amino acid, as well as compositions of such mutated PEs, nucleic acids encoding them, and methods for using the mutated PEs.
Owner:UNITED STATES OF AMERICA
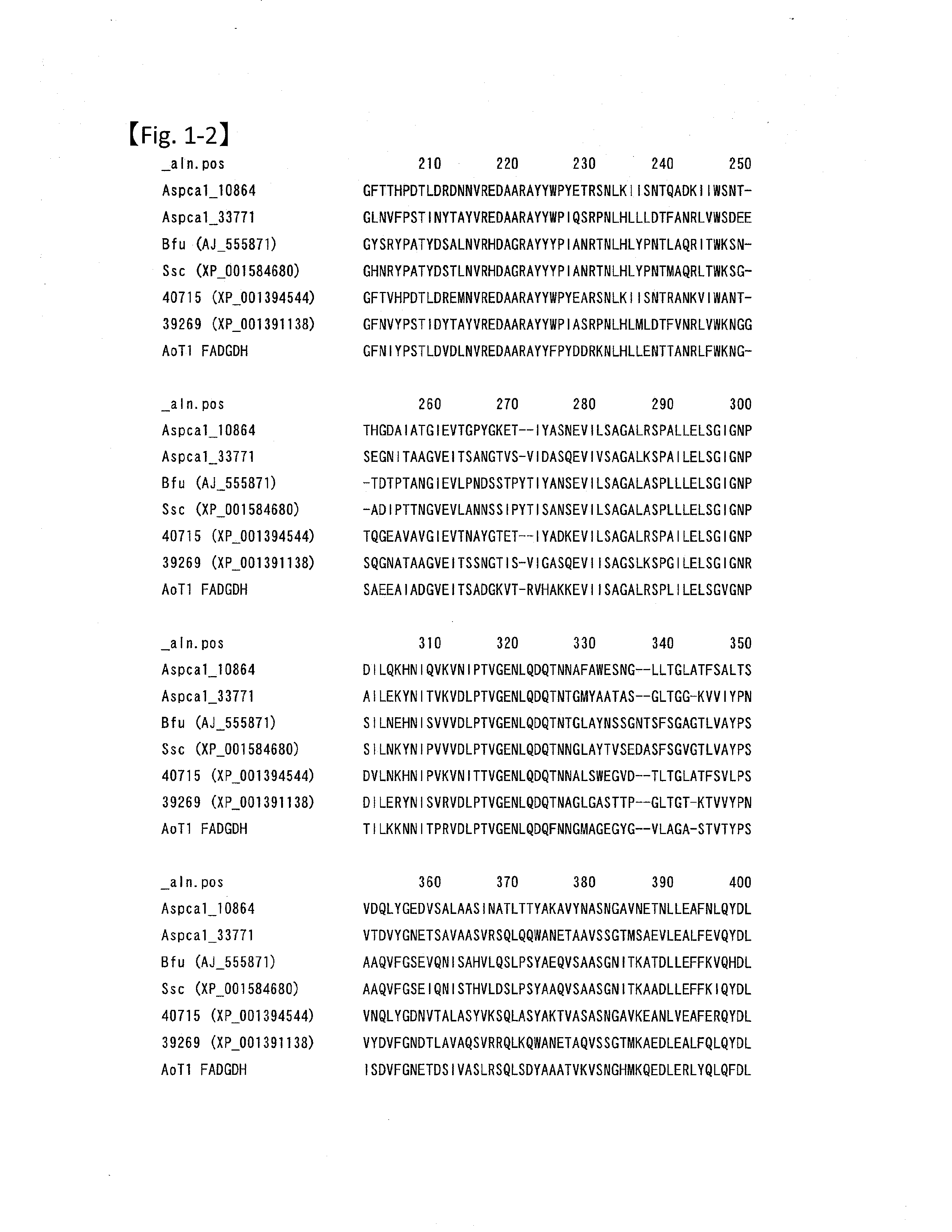
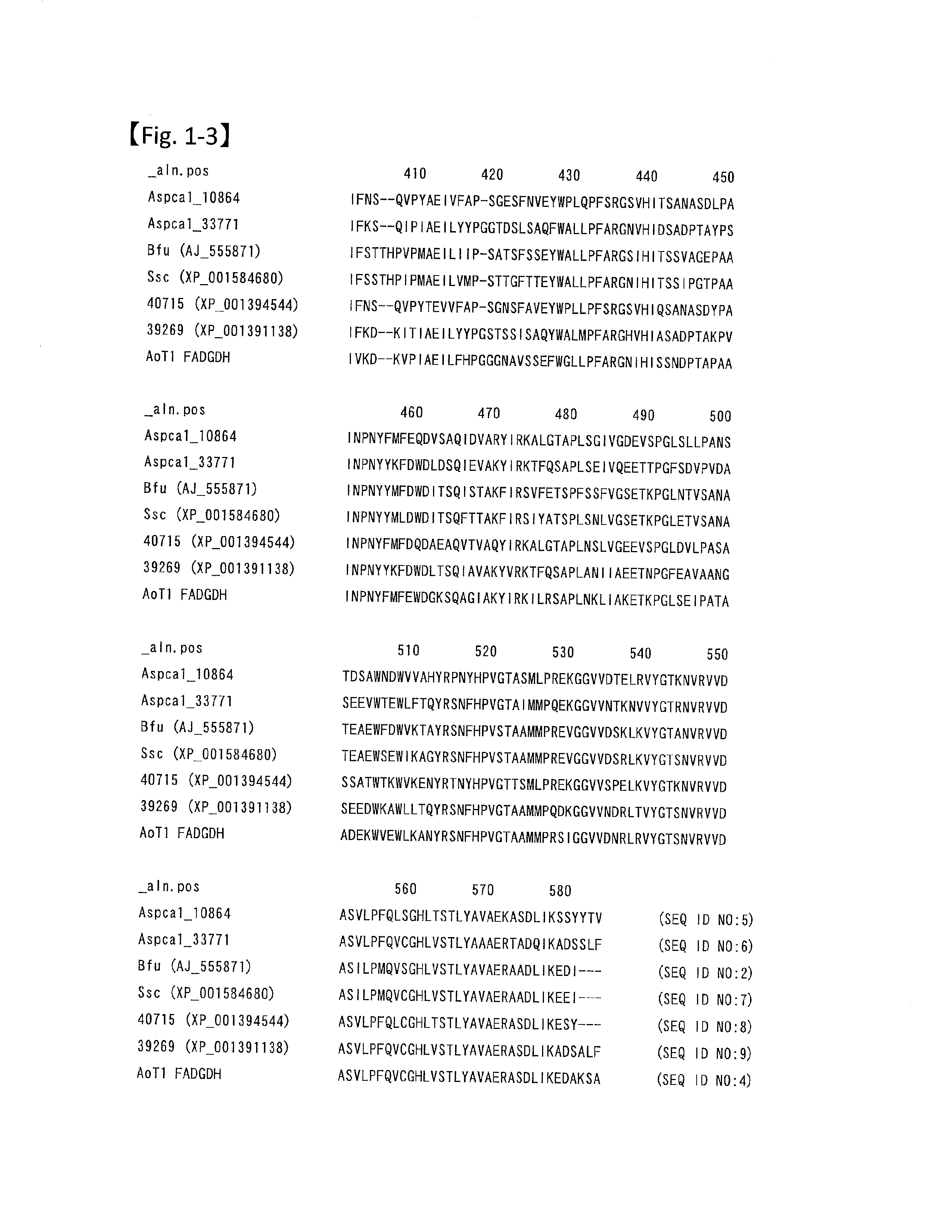
Modified pyrroloquinoline quinone (pqq) dependent glucose dehydrogenase excellent in substrate specificity
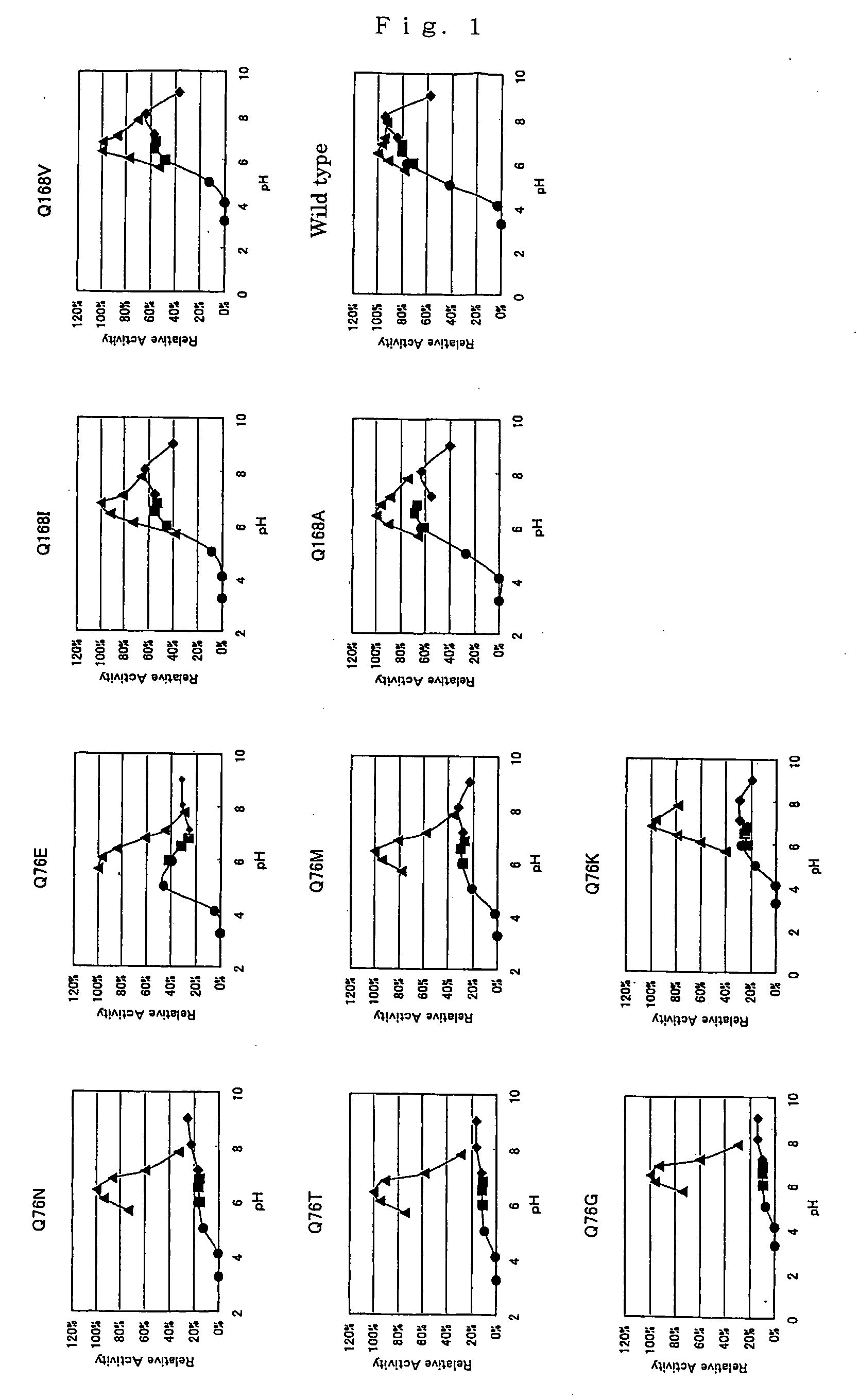
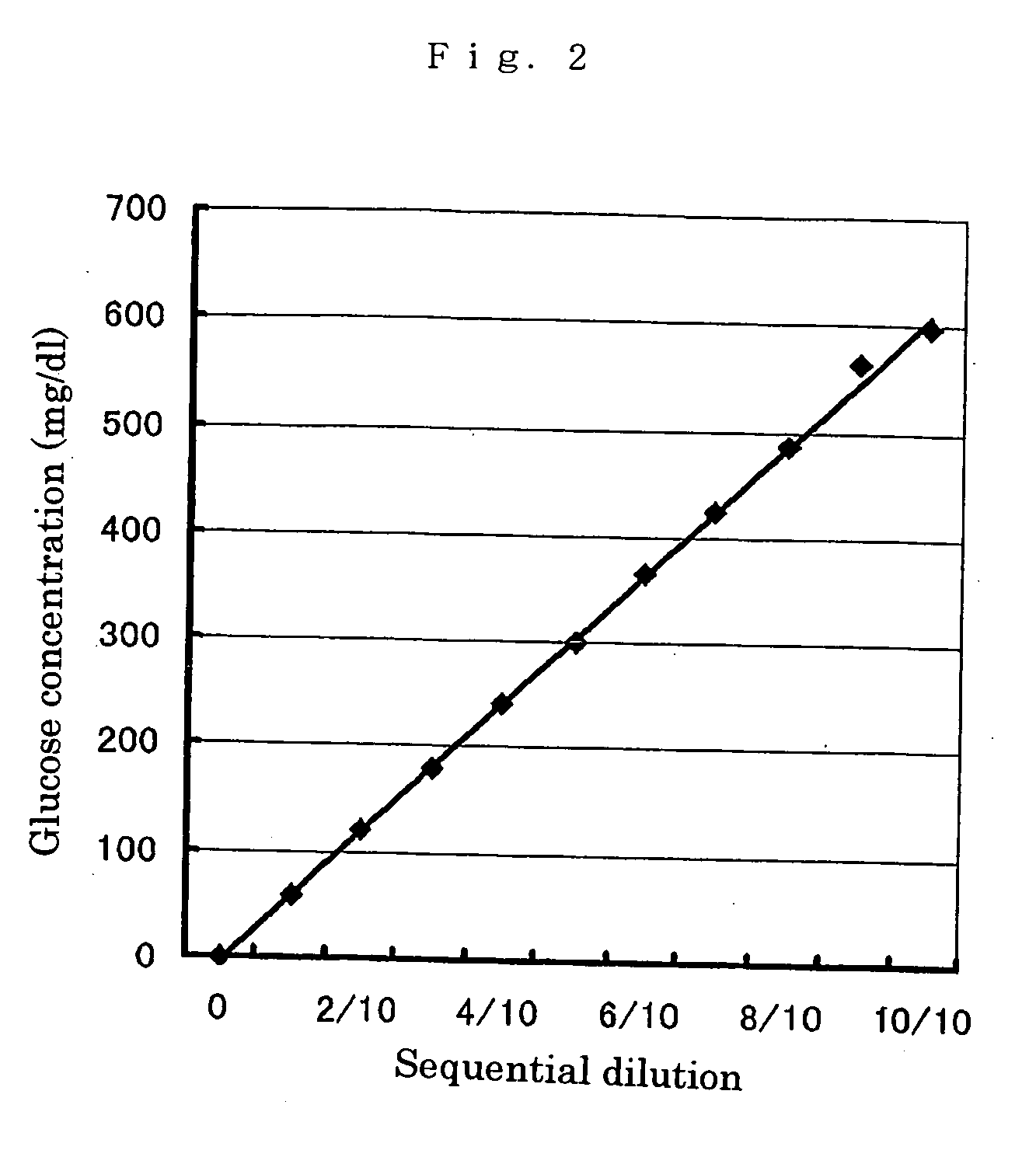
InactiveUS20070105173A1Action propertyProcess stabilityBioreactor/fermenter combinationsFungiWild typePyrroloquinoline quinone
PQQGDH having an improved substrate specificity or having an improved specific activity in an assay system using ferricyanide ion as a mediator is provided. Modified PQQGDH having the enhanced substrate specificity by introducing an amino acid mutation in a particular region of PQQGDH, and a method of enhancing the specific activity compared with a wild type in the assay system using the ferricyanide ion as the mediator by deleting, substituting, or adding one or more amino acids in an amino acid sequence of the wild type pyrroloquinoline quinone dependent glucose dehydrogenase.
Owner:TOYO TOYOBO CO LTD
Polypeptide
The invention describes a PS4 variant polypeptide derivable from a parent polypeptide having amylase activity selected from the group consisting of: (a) a polypeptide comprising an amino acid mutation at each of positions 33, 34, 121, 134, 141, 146, 157, 161, 178, 179, 223, 229, 272, 303, 307, 309 and 334; (b) a polypeptide comprising an amino acid mutation at each of positions 33, 34, 121, 134, 141, 145, 146, 157, 178, 179, 223, 229, 272, 303, 307 and 334; (c) a polypeptide comprising an amino acid mutation at each of positions 33, 34, 121, 134, 141, 146, 157, 178, 179, 223, 229, 272, 303, 307, 309 and 334; and (d) a polypeptide comprising an amino acid mutation at each of positions 3, 33, 34, 70, 121, 134, 141, 146, 157, 178, 179, 223, 229, 272, 303, 307, 309 and 334; with reference to the position numbering of a Pseudomonas saccharophilia exoamylase sequence shown as SEQ ID NO: 1, uses of such a polypeptide as a food or feed additive, and nucleic acids encoding such.
Owner:DUPONT NUTRITION BIOSCIENCES APS
Site-specific mutagenesis high temperature resistant phytase gene TP and expression vector and application thereof
ActiveCN102943083AImprove stabilityHigh expressionHydrolasesAnimal feeding stuffBiotechnologyPichia pastoris
The invention provides site-specific mutagenesis high temperature resistant phytase gene TP and an expression vector and application thereof. Amino acid in a specific area in a phytase amino acid sequence is mutated into praline and arginine so as to enable the phytase to have a special stable protein structure. Simultaneously, a gene sequence is optimized to synthesize a phytase gene according to pichia pastoris codon preference and GC content, recombinant plasmids are built and transferred to pichia pastoris, and positive converters are obtained through screening to conduct induction expression to obtain the high temperature resistant phytase. Experiments prove that the phytase obtained through expression of the pichia pastoris can resist high temperature plasmids with the temperature higher than 85 DEG C, and survival rate reaches 85%. Popularization and application of the high temperature resistant phytase have substantial economical benefit and social benefit on development of feed animal husbandry of our country, and simultaneously great ecological benefit can be obtained.
Owner:TIANJIN CHIATAI FEED TECH
Avirulent, immunogenic flavivirus chimeras
InactiveUS20060062803A1Inhibition effectPreserve immunogenicityBiocideOrganic active ingredientsViral diseaseFhit gene
Chimeric flaviviruses that are avirulent and immunogenic are provided. The chimeric viruses are constructed to contain amino acid mutations in the nonstructural proteins of a flavivirus. Chimeric viruses containing the attenuation-mutated nonstructural genes of the virus are used as a backbone into which the structural protein genes of a second flavivirus strain are inserted. These chimeric viruses elicit pronounced immunogenicity yet lack the accompanying clinical symptoms of viral disease. The attenuated chimeric viruses are effective as immunogens or vaccines and may be combined in a pharmaceutical composition to confer simultaneous immunity against several strains of pathogenic flaviviruses.
Owner:MAHIDOL UNIV +2
Lipase mutants with enhanced thermal stability
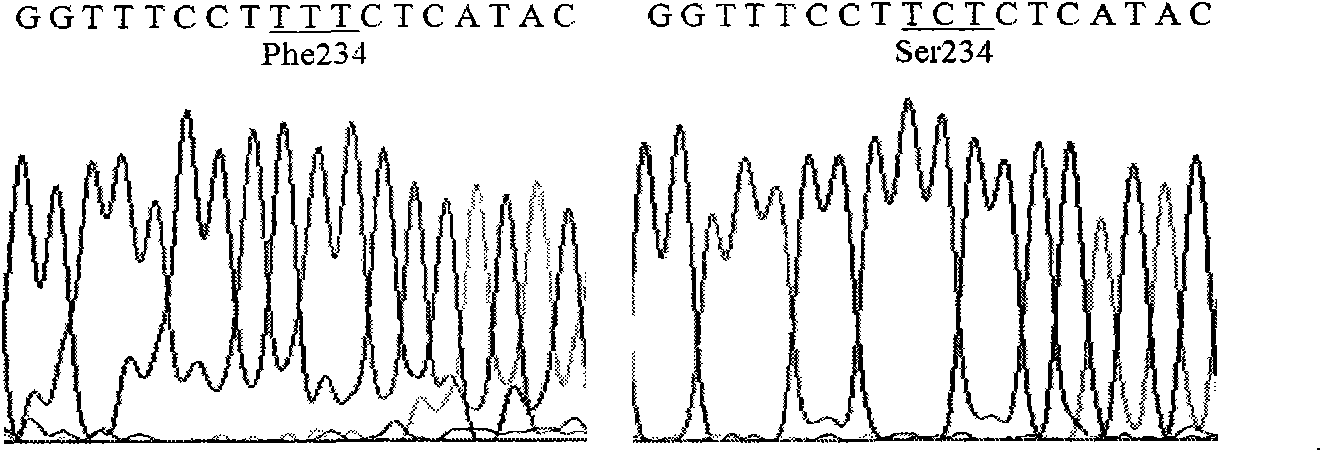
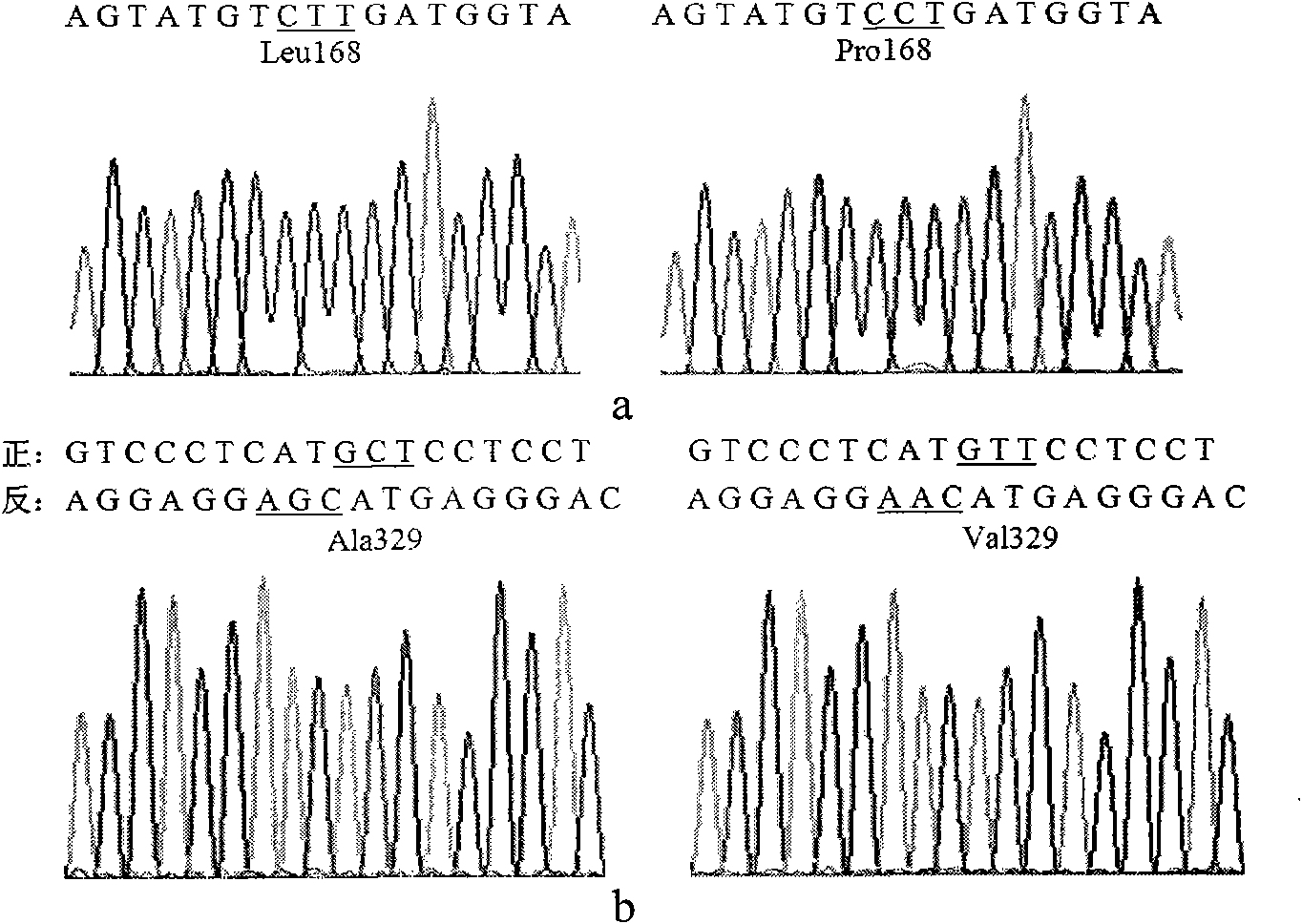
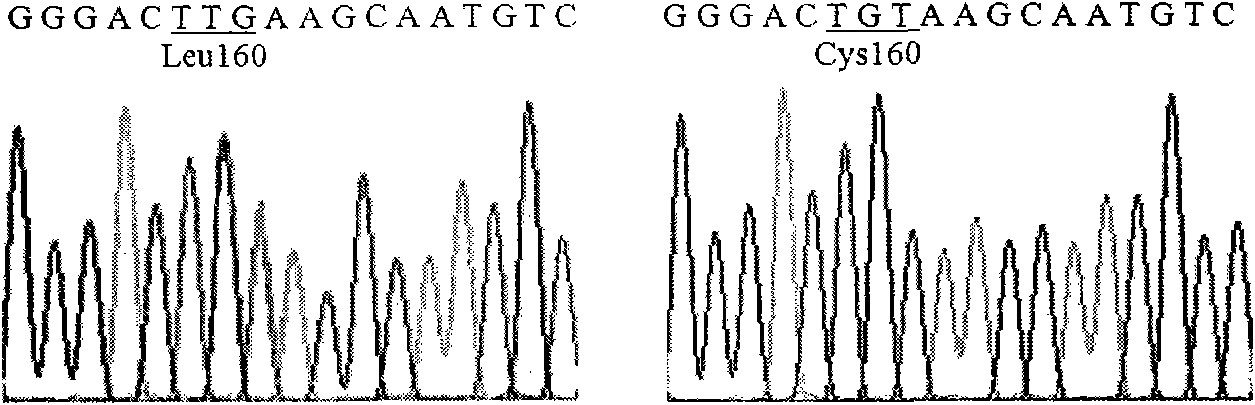
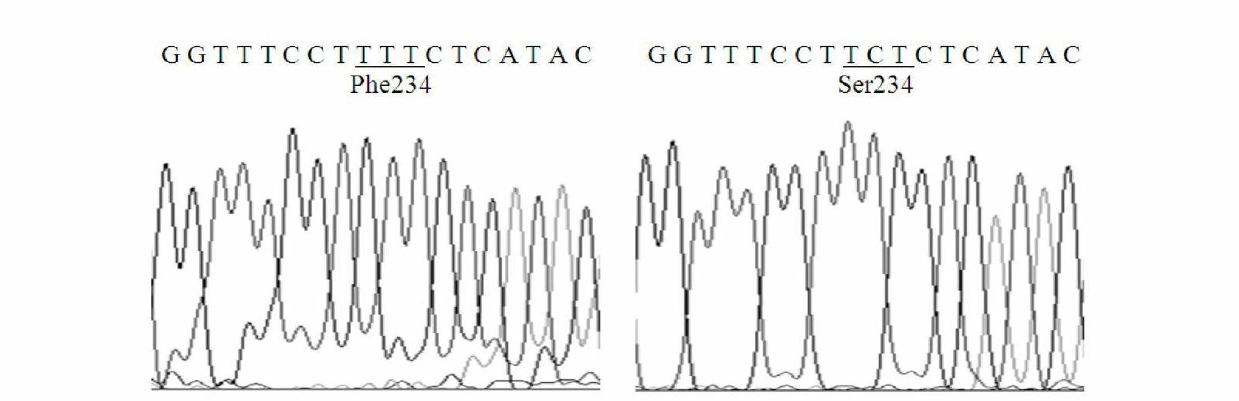
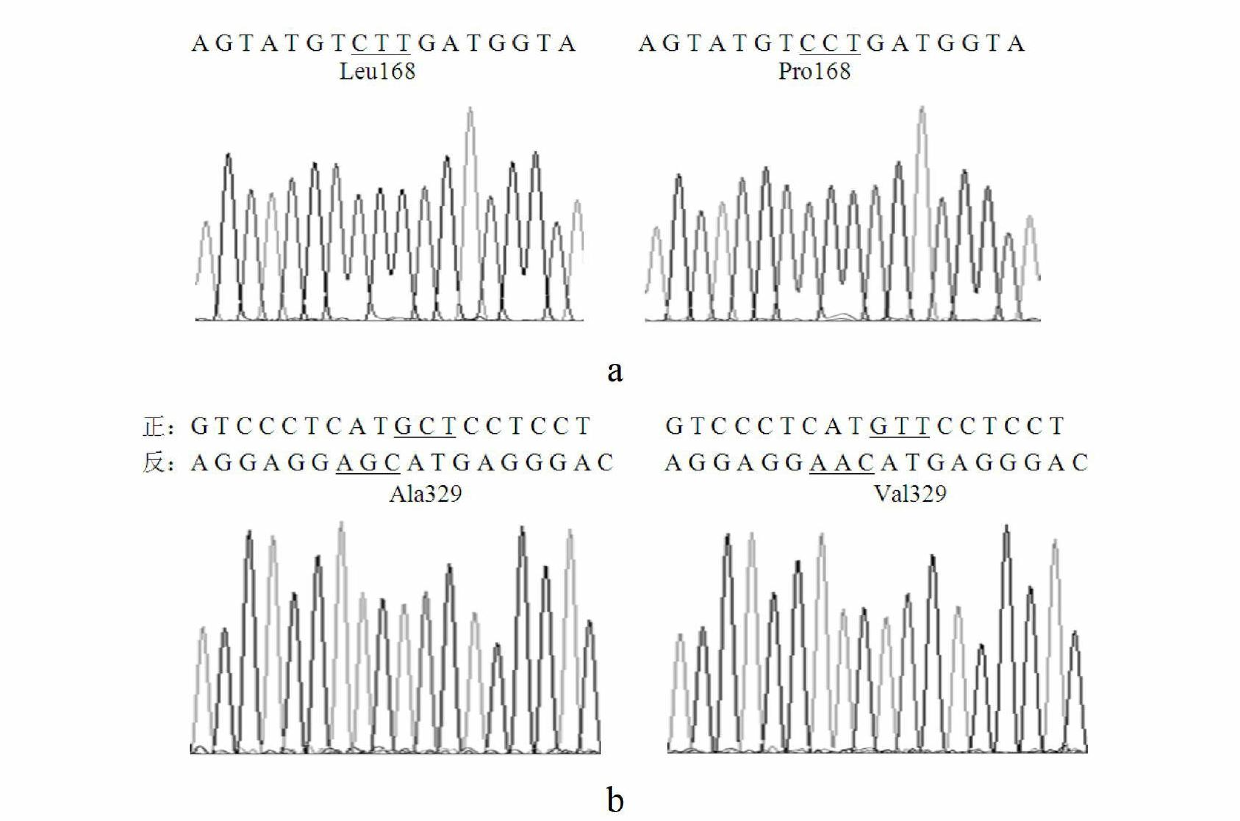
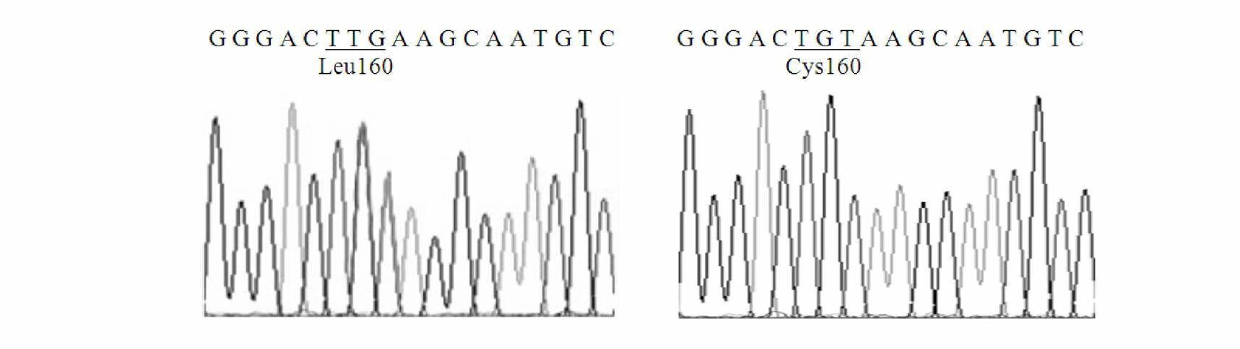
The invention relates to lipase mutants with enhanced thermal stability, which belongs to the technical field of enzyme gene engineering. Rhizopus chinensis CCTCC No.M201021 lipase used as the parent is processed by molecular biotechnology to obtain the lipase mutants with enhanced thermal stability. In the amino acid sequences of the mutants, the related amino acid mutation(s) is(are) one or a plurality of Met101Thr, Glu107Gly, Ala129Ser, Ser151Asn, Cys160Leu, Lys161Arg, Pro168Leu, Pro168His, Leu180His, Asp182Tyr, Thr183Ala, Thr218Ser, Lys219Asp, Ala230Phe, Ser234Phe, Val261Gly, His317Pro, Val329Ala, Glu363Arg, Asn366Asp and Ser373Cys. The mutants are expressed by half-life period t50 at 65 DEG C. The thermal stability of the mutants is enhanced as compared with the parent Rhizopus chinensis lipase. The invention also discloses a DNA sequence, an expression carrier and a host cell for coding the lipase mutants.
Owner:金湖县农副产品营销协会
In vivo half life increased fusion protein or peptide maintained by sustained in vivo release, and method for increasng in vivo half-life using same
ActiveUS20120094356A1Prolong half-life in vivoLoss minimizationPeptide/protein ingredientsPeptide preparation methodsPeptide drugHalf-life
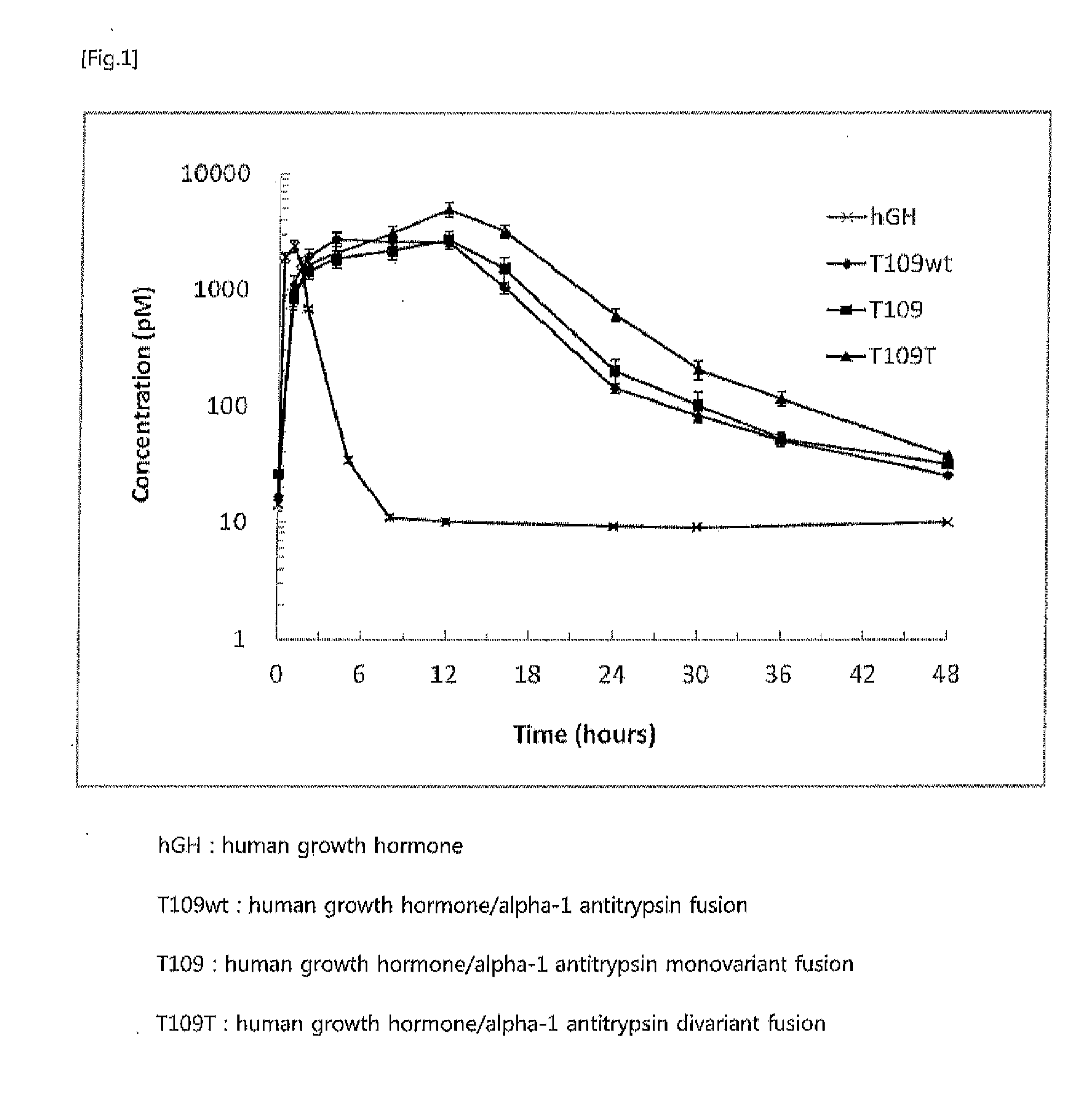
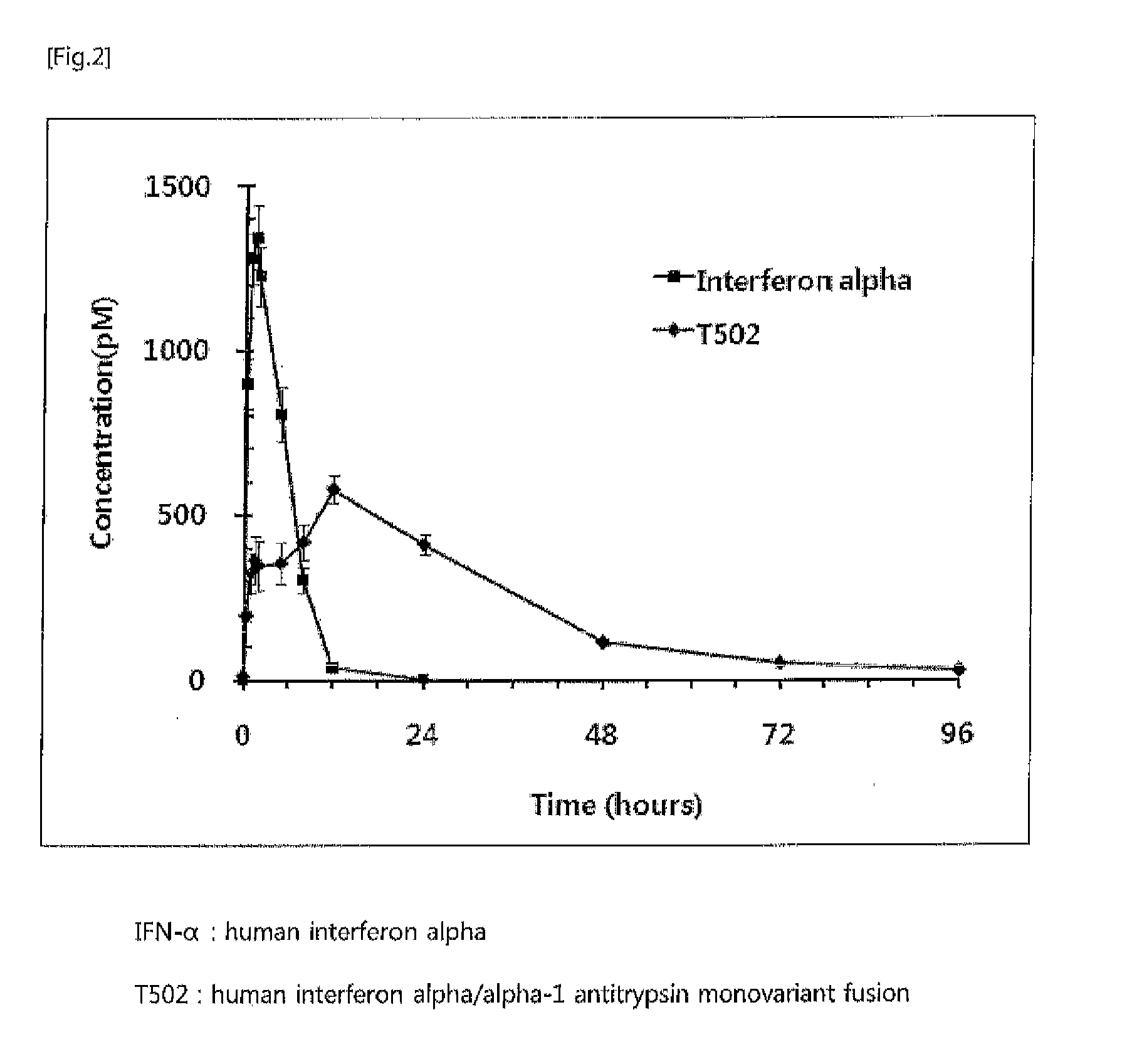
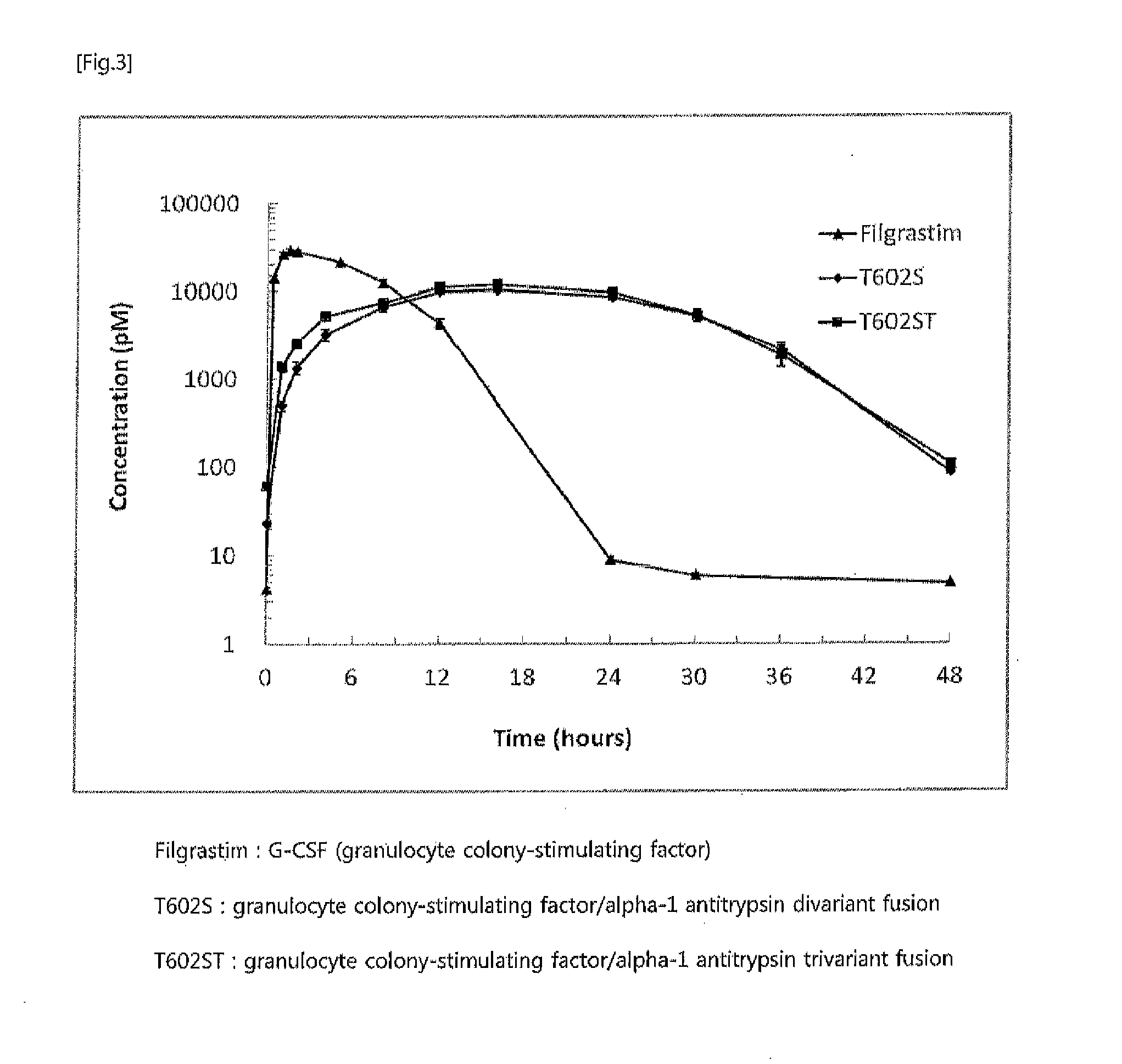
The present invention relates to a fusion protein or peptide, the in vivo half-life of which is increased by maintaining in vivo sustained release, and to a method for increasing in vivo half-life using same. A fusion protein or peptide according to the present invention has excellent in vivo stability by binding a physiologically active protein or physiologically active peptide to an alpha-1 antitrypsin or alpha-1 antitrypsin mutant with one or more amino acids mutated to maintain the in vivo sustained release and to significantly increase the half-life thereof in blood (T1 / 2) compared to an inherent physiologically active protein or physiologically active peptide. Thus, a fusion protein or peptide according to the present invention can be useful in developing a sustained-release preparation of a protein or peptide drug.
Owner:ALTEOGEN
Modified amylase from pseudomonas saccharophilia
Described is a PS4 variant polypeptide derivable from a polypeptide having amylase activity selected from: (a) a polypeptide comprising an amino acid mutation at each of positions 33, 34, 121, 134, 141, 146, 157, 161, 178, 179, 223, 229, 272, 303, 307, 309 and 334; (b) a polypeptide comprising an amino acid mutation at each of positions 33, 34, 121, 134, 141, 145, 146, 157, 178, 179, 223, 229, 272, 303, 307 and 334; (c) a polypeptide comprising an amino acid mutation at each of positions 33, 34, 121, 134, 141, 146, 157, 178, 179, 223, 229, 272, 303, 307, 309 and 334; and (d) a polypeptide comprising an amino acid mutation at each of positions 3, 33, 34, 70, 121, 134, 141, 146, 157, 178, 179, 223, 229, 272, 303, 307, 309 and 334; referring to the numbering of a Pseudomonas saccharophilia exoamylase shown as SEQ ID NO: 1.
Owner:AS DE DANSKE SUKKERFABRIKKER
Pre-fusion rsv f antigens
InactiveCN103842374ASsRNA viruses negative-senseAntibody mimetics/scaffoldsAntigenAmino acid mutation
The invention relates to pre-fusion RSV F protein and polypeptides that contain one or more amino acid mutations that stabilize the pre-fusion conformation or destabilize the post-fusion conformation. The invention also relates to methods for inducing an immune response to pre-fusion RSV F.
Owner:NOVARTIS AG
Heat-resistant reverse transcriptase mutant
Provided are: a reverse transcriptase mutant including an amino acid mutation at a position corresponding to position 55 of the amino acid sequence of wild-type reverse transcriptase derived from the Moloney murine leukemia virus, wherein the reverse transcriptase mutant is characterized in that the amino acid mutation is a substitution from threonine to another amino acid, and the other amino acid is selected from the group consisting of amino acids having a nonpolar aliphatic side chain and amino acids having a polar acidic functional group side chain; a nucleic acid that encodes the mutant; a method for producing the mutant and the nucleic acid that encodes the mutant; a method for synthesizing cDNA in which the mutant is used; and a composition and kit including the mutant.
Owner:TAKARA HOLDINGS
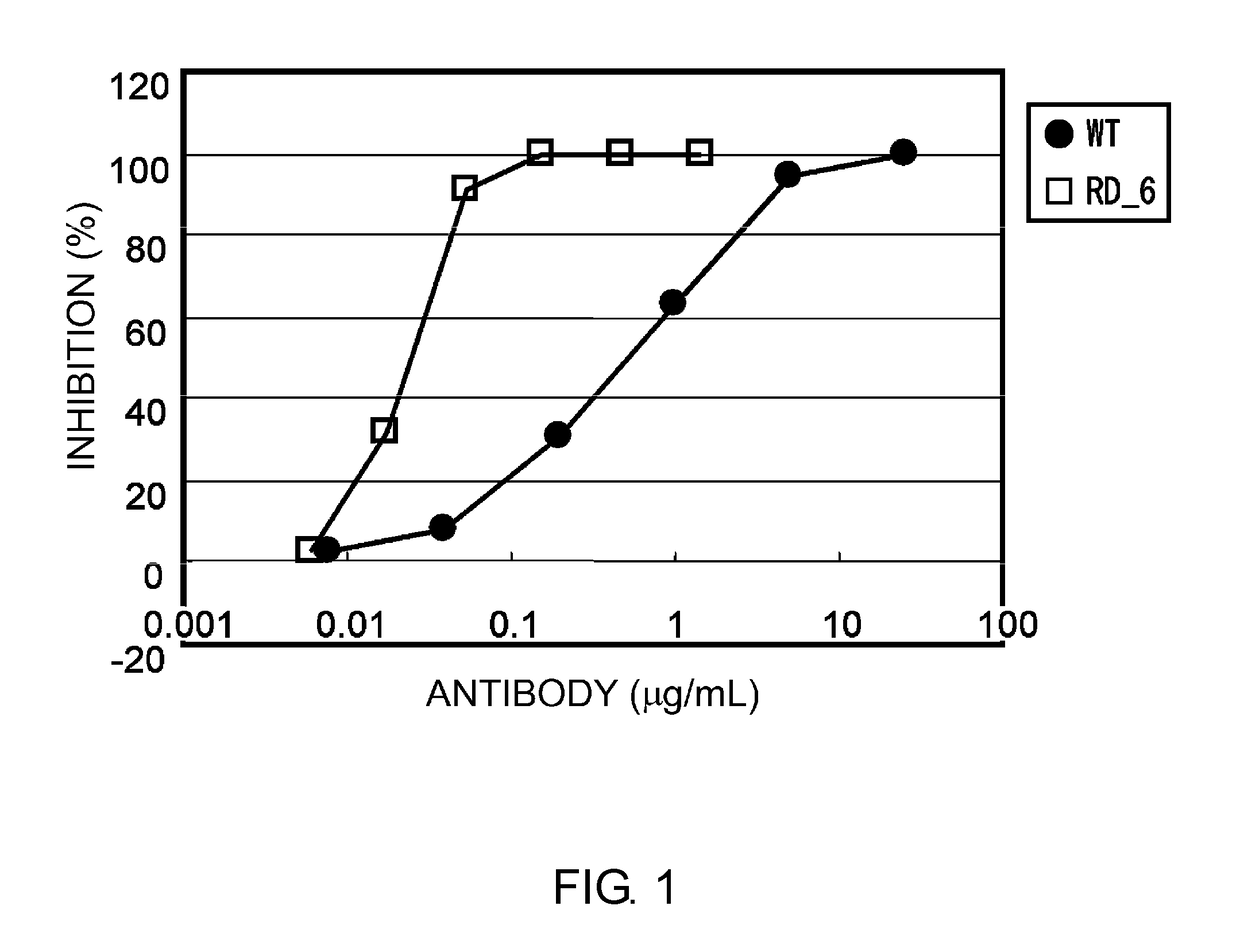
Antibody having enhanced adcc activity and method for production thereof
InactiveUS20100297103A1Enhanced ADCC activityMore cytotoxicitySugar derivativesAntibody ingredientsAmino acid mutationAntibody
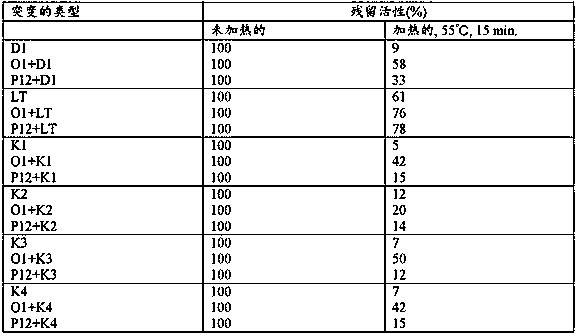
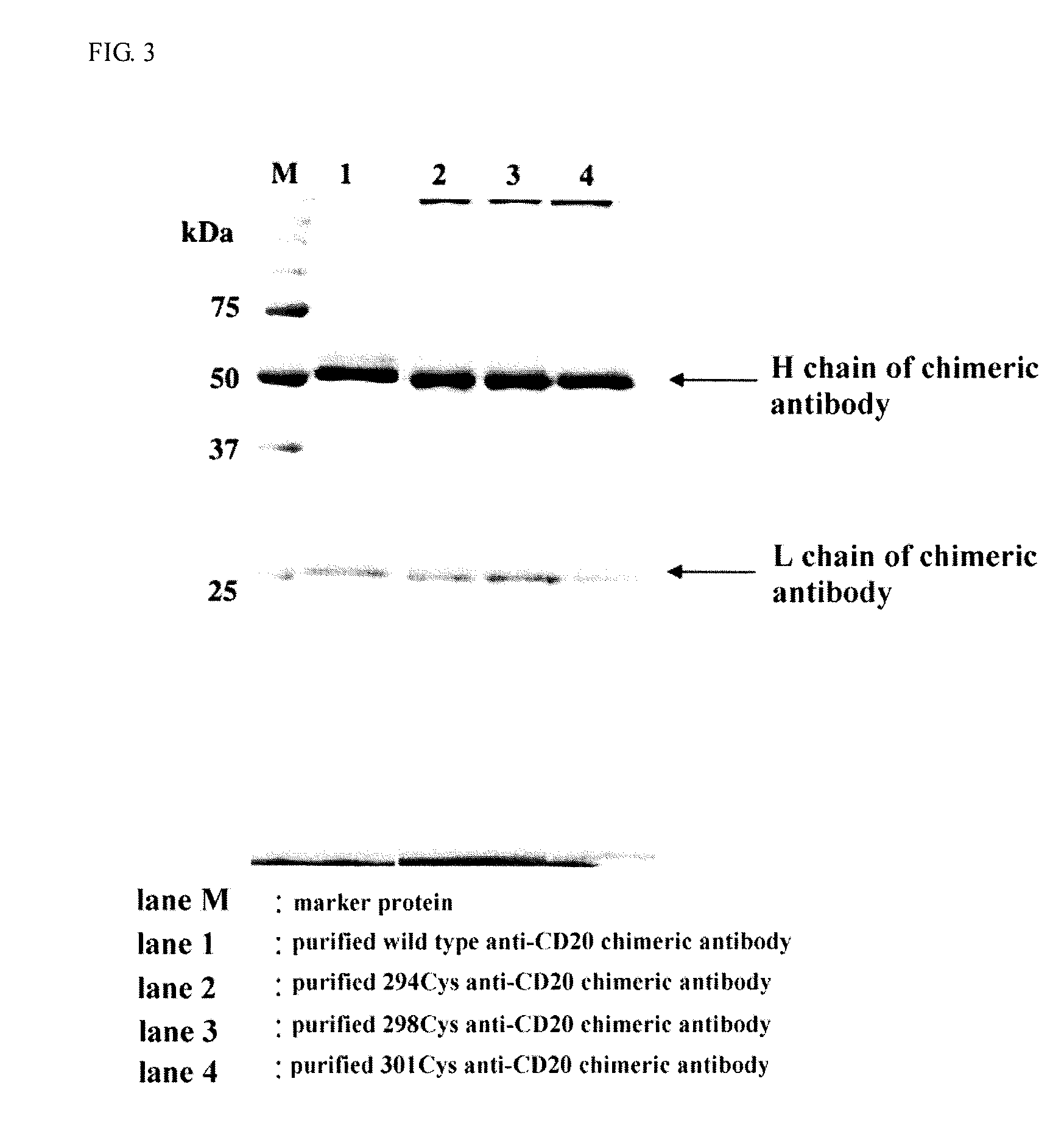
Disclosed is an antibody having an enhanced ADCC activity. Also disclosed is a method for producing the antibody. It was attempted to advance the technique of the amino acid mutation in an Fc region established by researchers of Genentech Inc. or the like, and a study was made on whether or not the ADCC activity can be enhanced by the mutation of an amino acid residue in an Fc region into cysteine (Cys) which may cause a drastic structural change that cannot be drawn by a computational search. As a consequence, a chemeric antibody is provided which has the mutation of an amino acid residue at least one position selected from the group consisting of 286th, 287th, 288th, 289th, 290th, 291st, 292nd, 294th, 298th, 301st, 302nd, 303rd, 305th, 306th, 307th, 308th and 309th positions into a Cys residue in an H-chain constant region.
Owner:MEDICAL & BIOLOGICAL LAB CO LTD
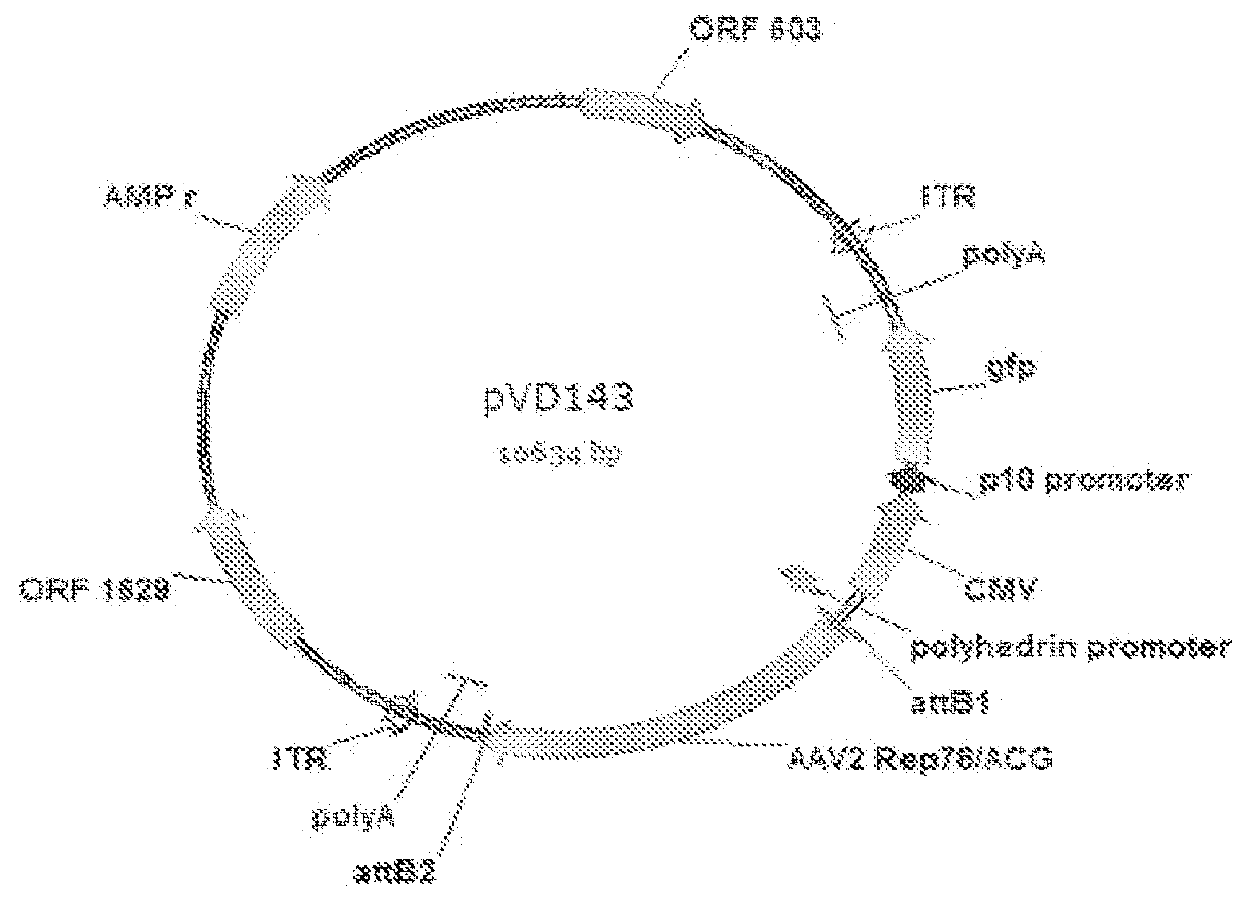
Mutated rep encoding sequences for use in AAV production
ActiveUS9228174B2Stable productionQuality improvementAnimal cellsSugar derivativesNucleotideNucleotide sequencing
Nucleic acids encoding Parvoviral Rep proteins with a mutated nuclear localization signal (NLS) are provided. Also provided is a nucleic acid comprising a nucleotide sequence encoding a Parvoviral Rep protein with a mutated zinc finger domain and a nucleic acid comprising a nucleotide sequence encoding a Parvoviral Rep protein comprising an amino acid mutation at position 43, 57, 79, 97, 120, 179, 305, 484, 493 or 571 with reference to SEQ ID NO: 2. Nucleic acid constructs and cells, such as insect cells, comprising the nucleic acids are provided as well as a method for producing a recombinant Parvoviral virion using the nucleic acids.
Owner:UNIQURE IP BV
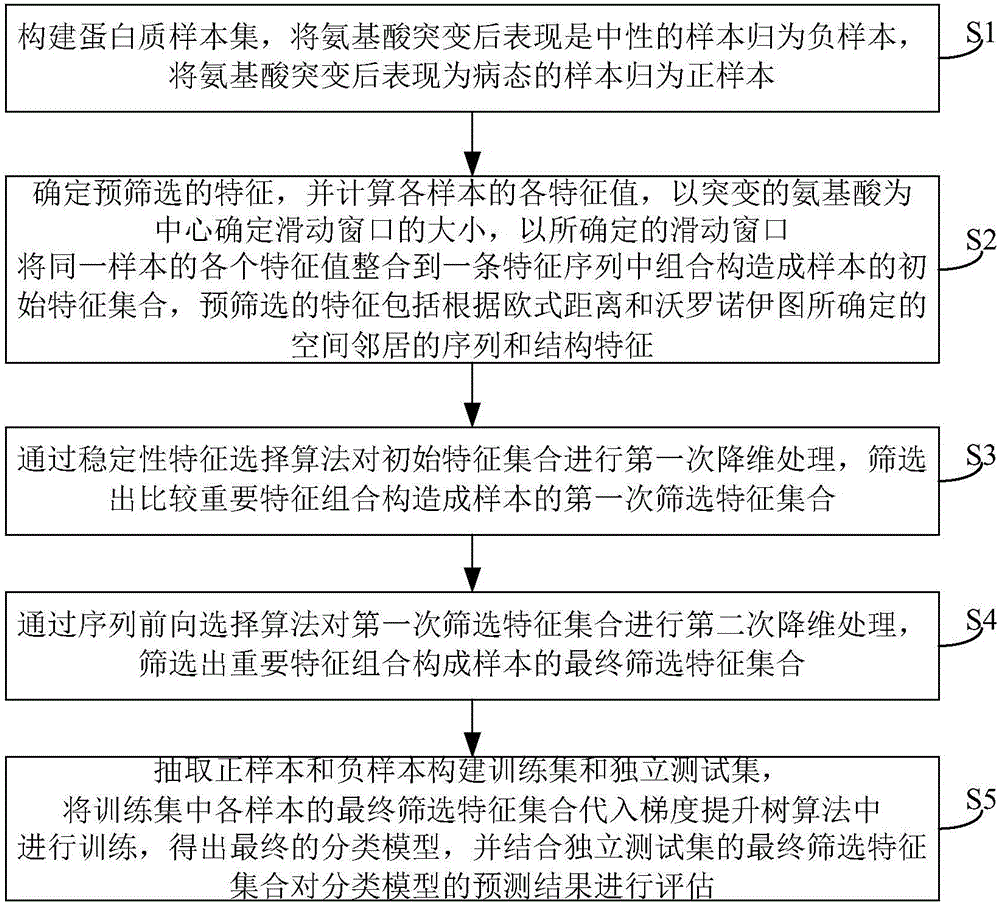
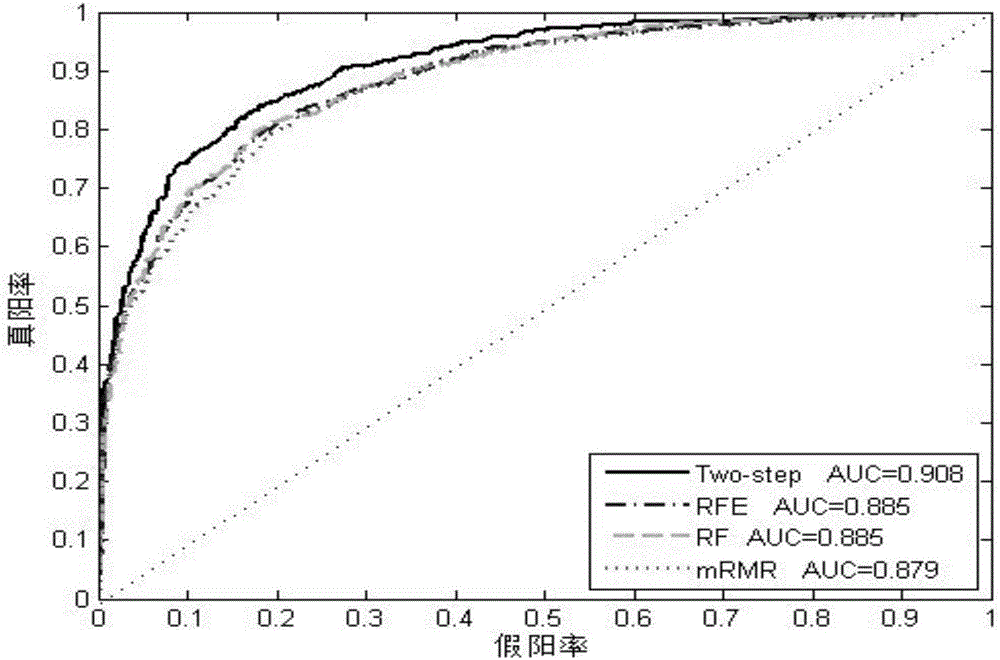
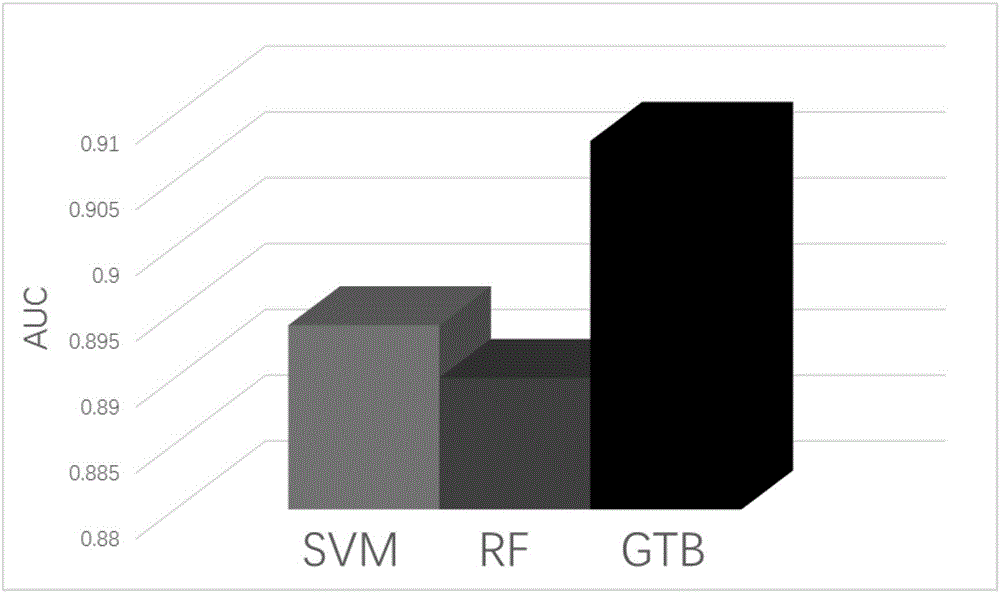
Method and system for predicting amino acid mutation
InactiveCN106650314ASolve blindnessSolve the costBiostatisticsSequence analysisPositive sampleForward selection
The invention relates to the technical field of biological information, and discloses a method and system for predicting amino acid mutation. The method and system for predicting amino acid mutation aim at improving the accuracy and the effect of prediction and effectively solving the problems that bioexperiment is blind, the cost of the bioexperiment is high and the like. The method for predicting amino acid mutation comprises the steps of establishing a protein sample set; determining characteristics of prefiltering, and integrating characteristics of the same sample into one characteristic sequence to combinedly establish an initial characteristic set of the sample; screening out relatively important characters through a stable character selection algorithm to combinedly establish a first screening out characteristic set of the sample; screening out important characters through a sequence forward selection algorithm to combinedly establish the final screening out characteristic set of the sample; selecting a positive sample and a negative sample to establish a training set and an independent test set, substituting the final screening out characteristic set of samples in the training set into a gradient promoting tree algorithm to be subjected to training so as to obtain a final disaggregated model, and conducting assessment on a prediction result of the disaggregated model by combining the final screening out characteristic set of the independent test set.
Owner:CENT SOUTH UNIV
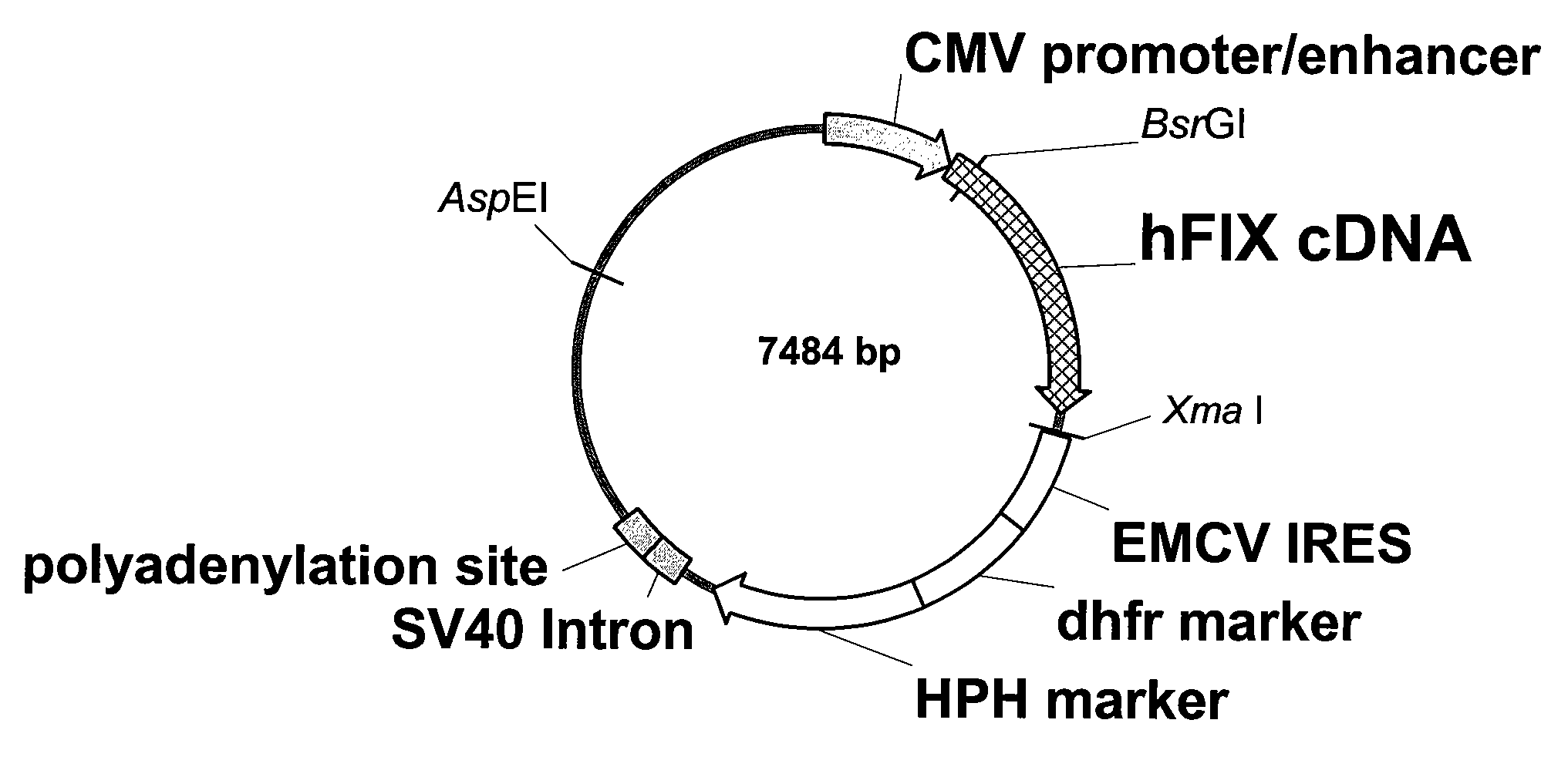
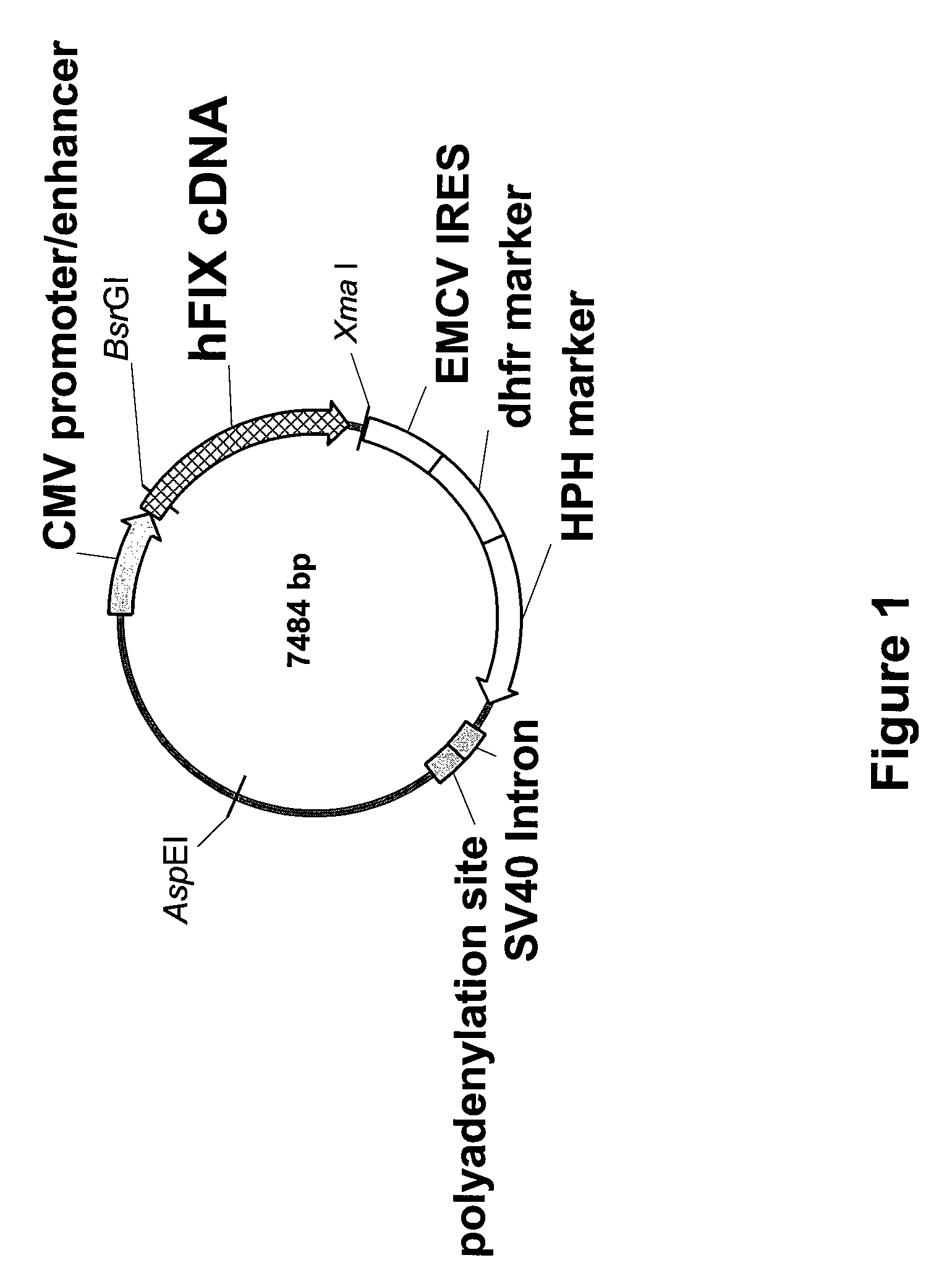
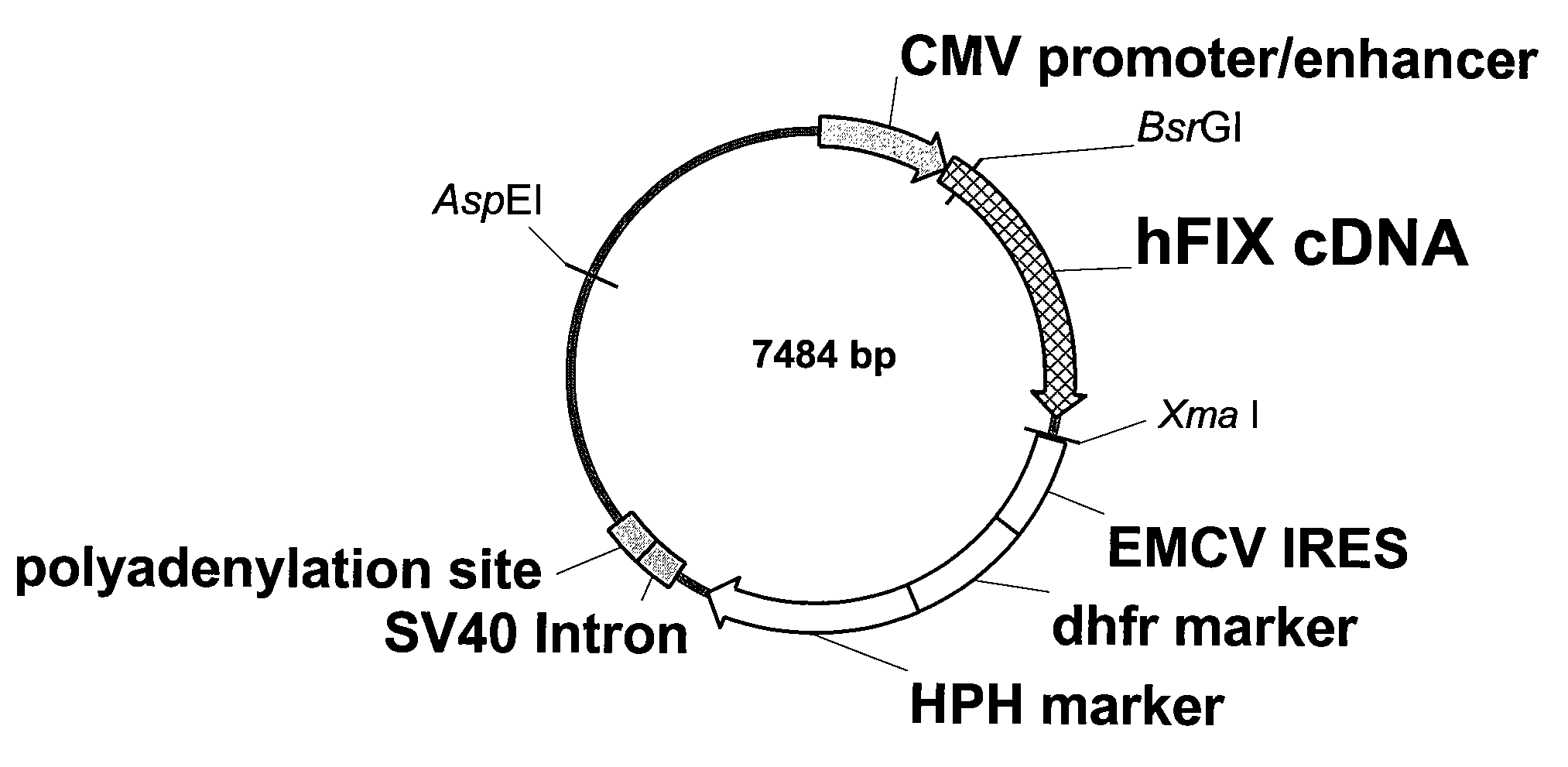
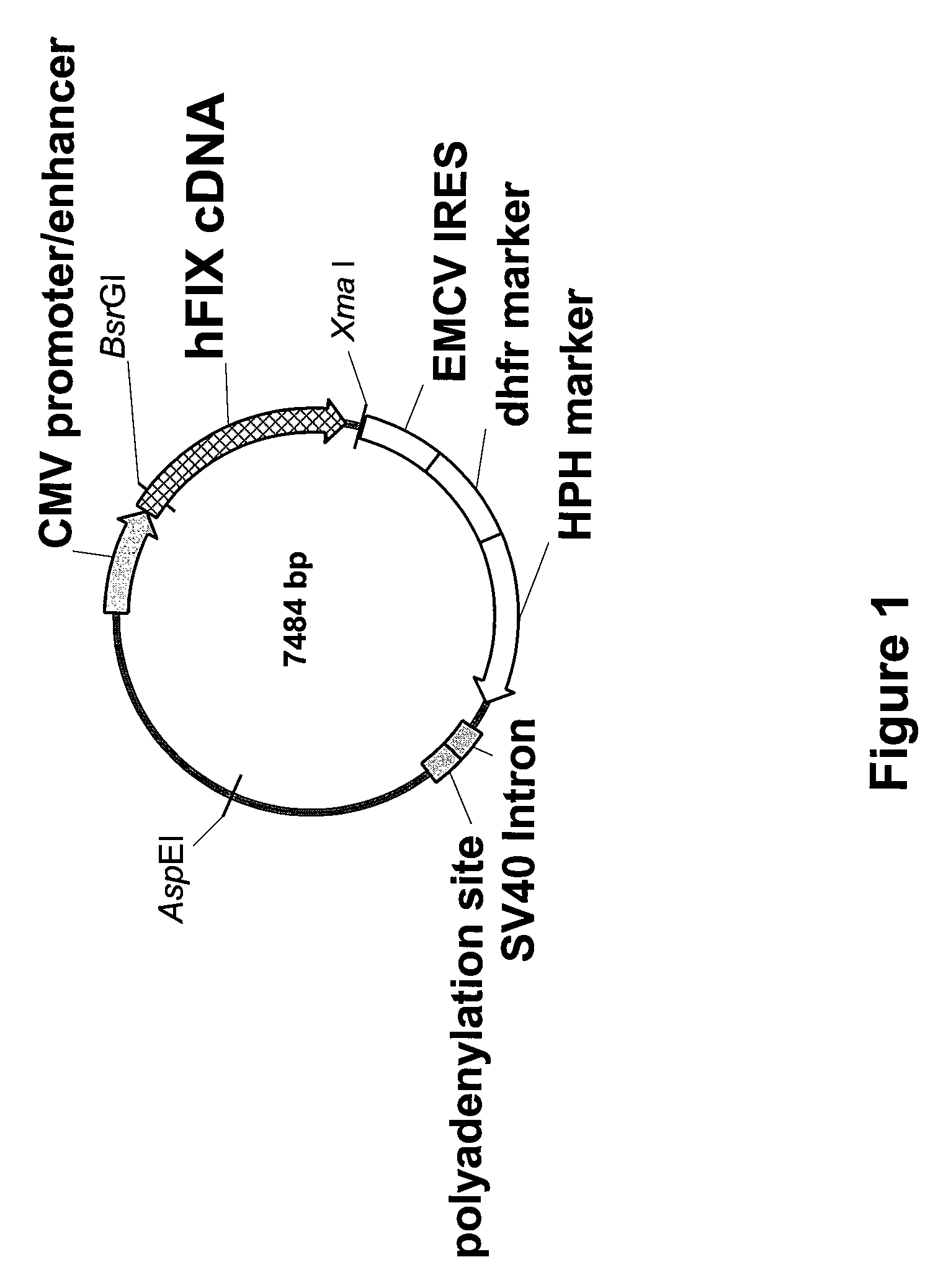
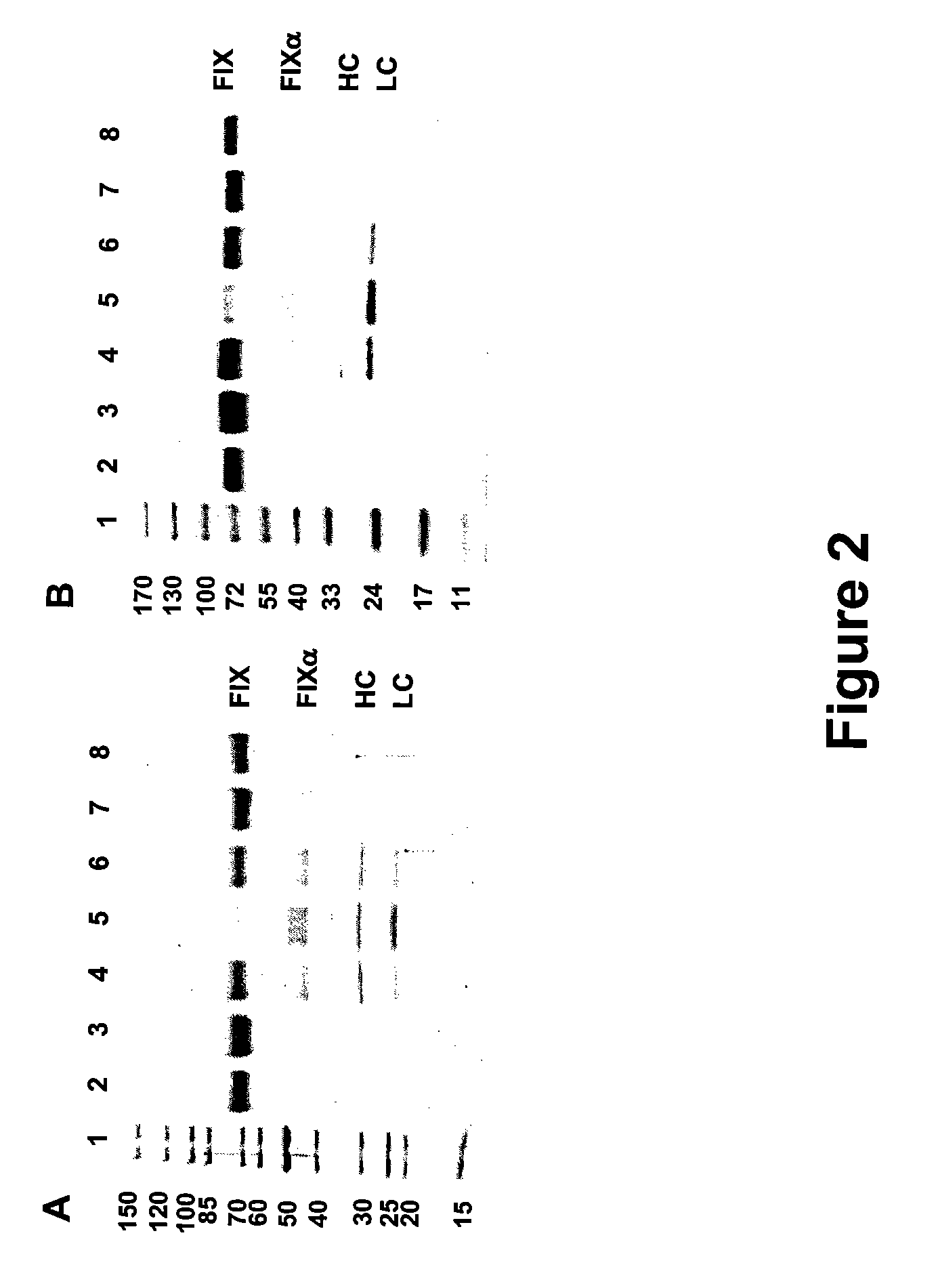
FIX-Mutant Proteins for Hemophilia B Treatment
InactiveUS20080214462A1Improved clot activityHigh activityPeptide/protein ingredientsMammal material medical ingredientsHEK 293 cellsDisease
The present invention relates to recombinant blood coagulation factor IX (rFIX) mutants having improved FIX clotting activity. Three full length FIX proteins with combinations of mutations of amino acids important for functional activity of FIX and FIX wild type were cloned and expressed in HEK 293 cells. The proteins were tested by an activated partial thromboplastin time (aPTT) assays in FIX-depleted plasma. Two mutant proteins had increased specific FIX activity. Furthermore, a pre-activated FIX protein had an increased activity in FIX-depleted plasma. Therefore these FIX mutants can be used for the treatment of FIX associated bleeding disorders.
Owner:BAXTER INT INC +1
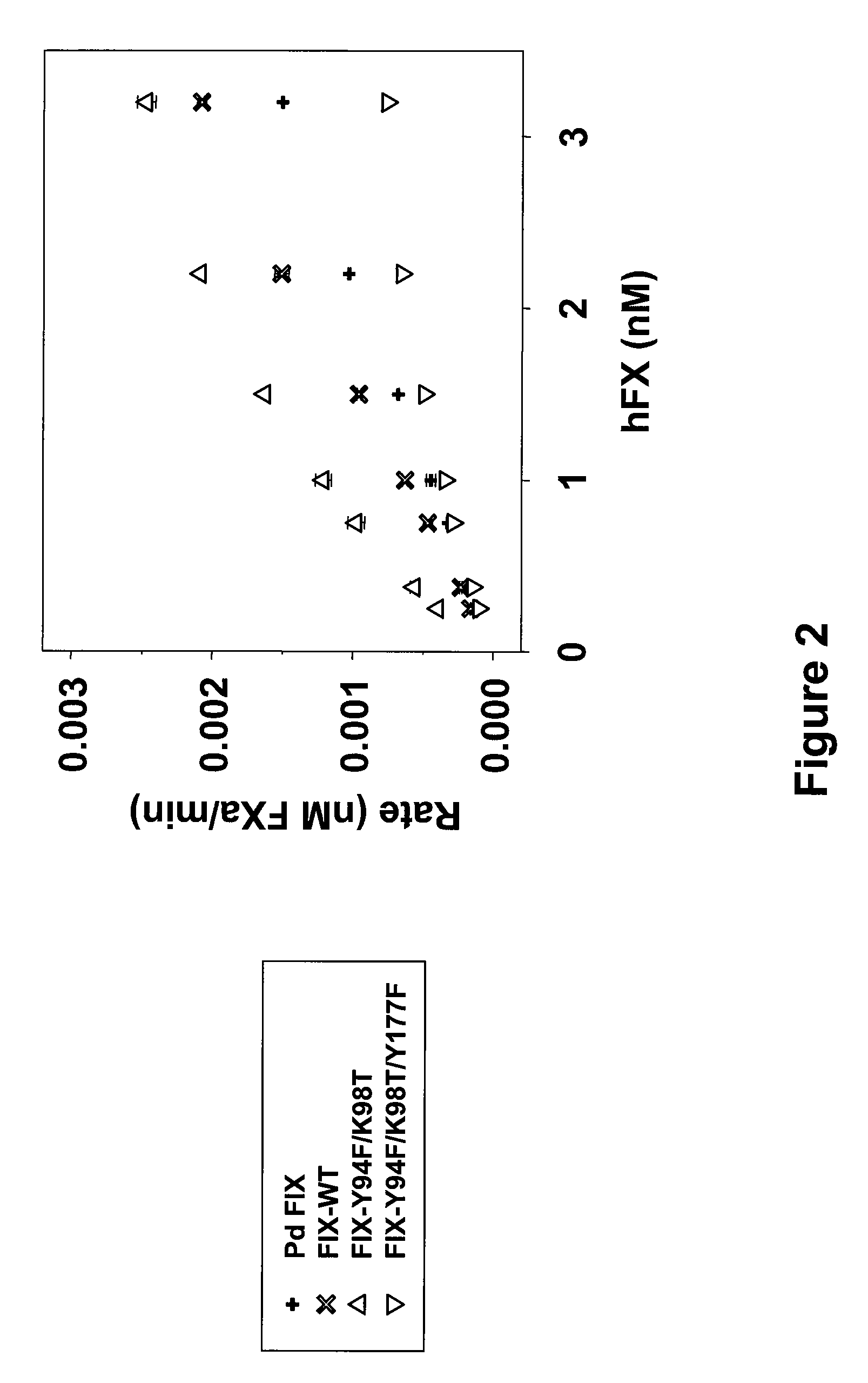
FVIII-Independent FIX-Mutant Proteins for Hemophilia A Treatment
ActiveUS20080214461A1Peptide/protein ingredientsMammal material medical ingredientsHEK 293 cellsMutated protein
The present invention relates to recombinant blood coagulation factor IX (rFIX) mutants having factor VIII (FVIII) independent factor X (FX) activation potential. Five full length FIX proteins with combinations of mutations of amino acids important for functional activity of FIX and FIX wild type were cloned and expressed in HEK 293 cells. The proteins were tested by an activated partial thromboplastin time (aPTT) assay in FVIII-depleted plasma as well as in FVIII-inhibited patient plasma. In FVIII-depleted plasma functional activity of the FIX mutants was calculated as increased FVIII equivalent activity. The mutant proteins had increased FVIII equivalent activity. In FVIII-inhibited patient plasma the FEIBA equivalent activity was calculated for analysis of FVIII independent FX activation potential. The proteins had also increased FEIBA equivalent activity. Furthermore, the pre-activated FIX proteins had an increased activity in FIX-depleted plasma containing FVIII inhibitors. Therefore these FIX mutants are alternatives as bypassing agents for treatment of FVIII inhibitor patients.
Owner:TAKEDA PHARMA CO LTD
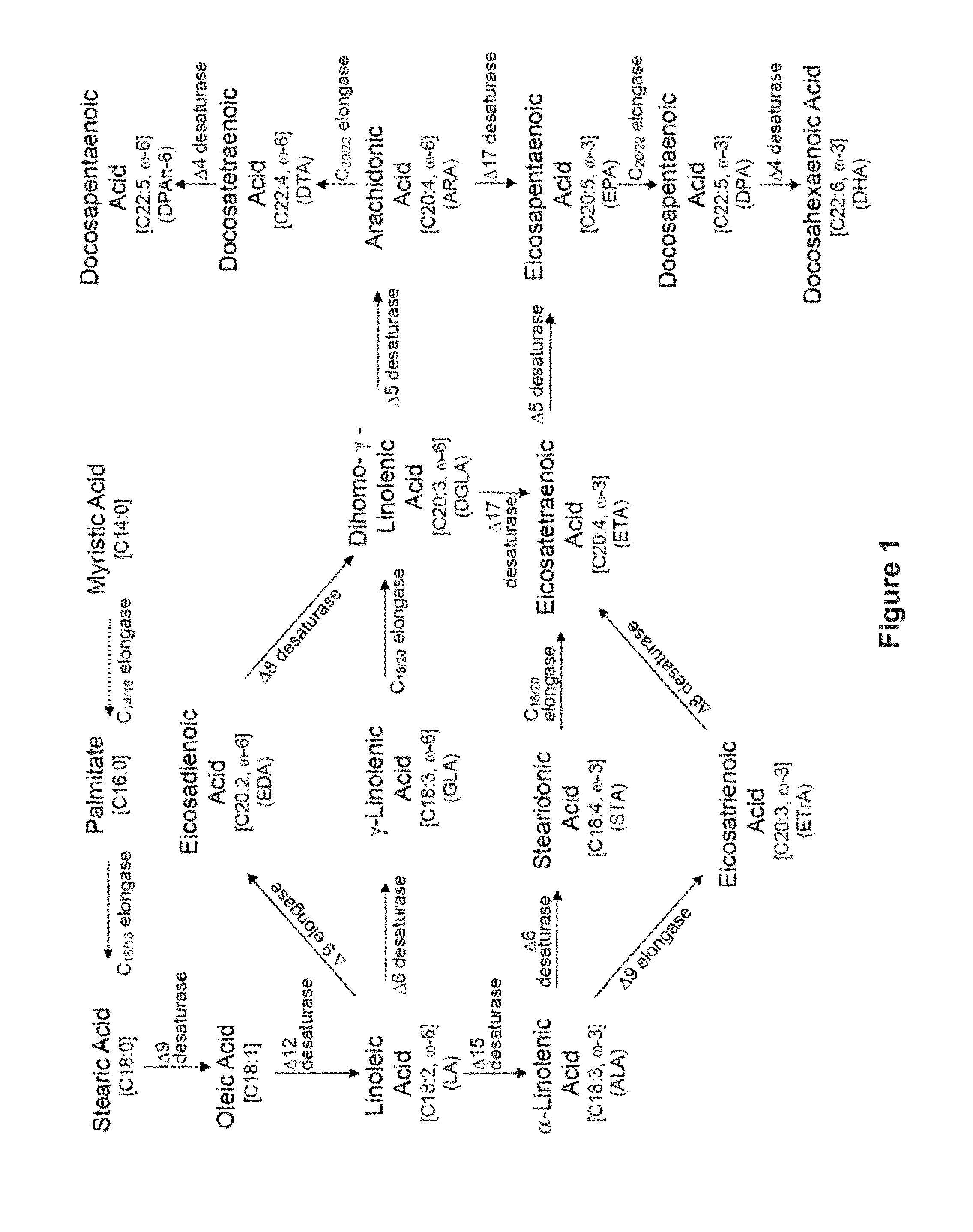
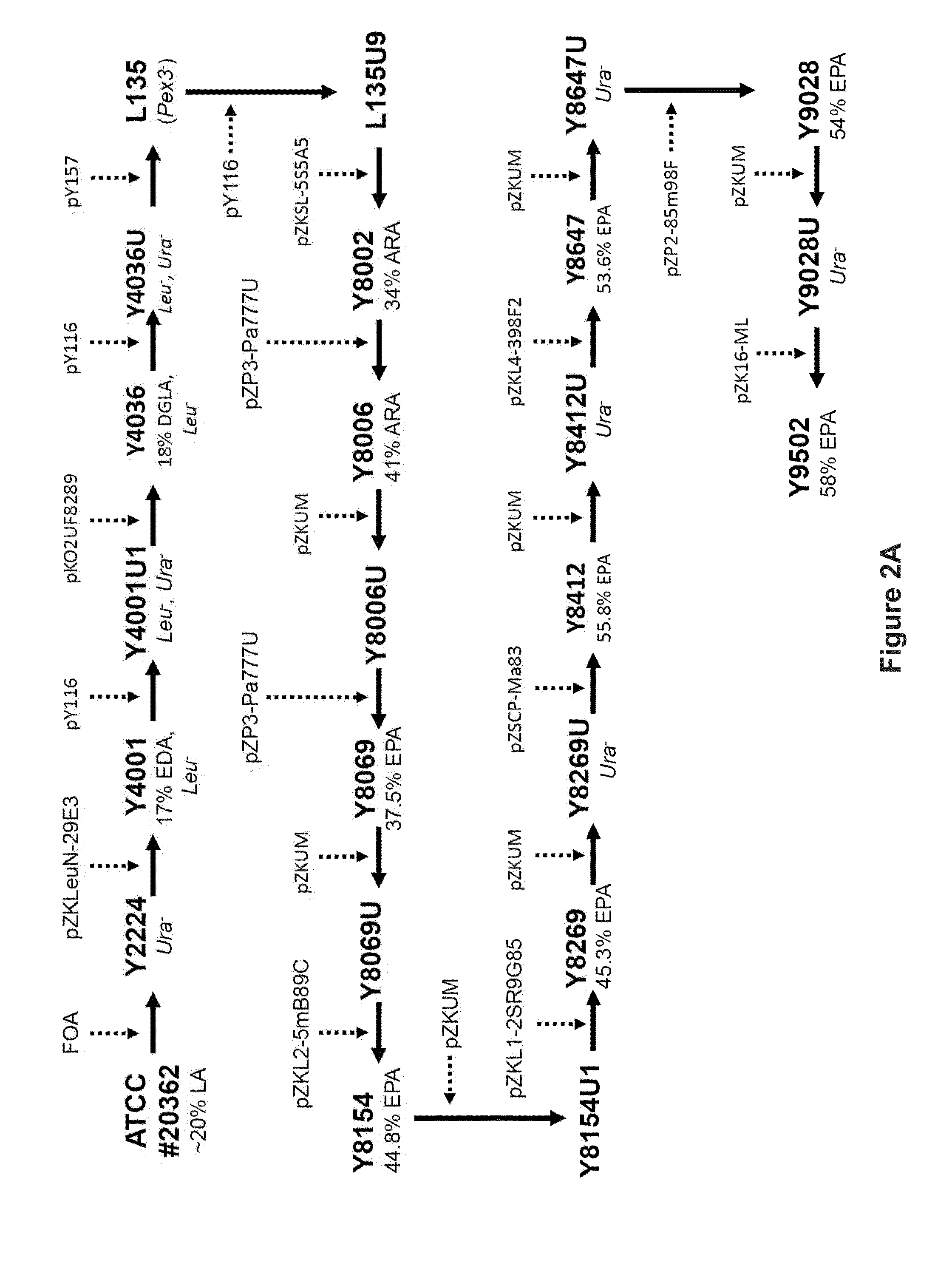
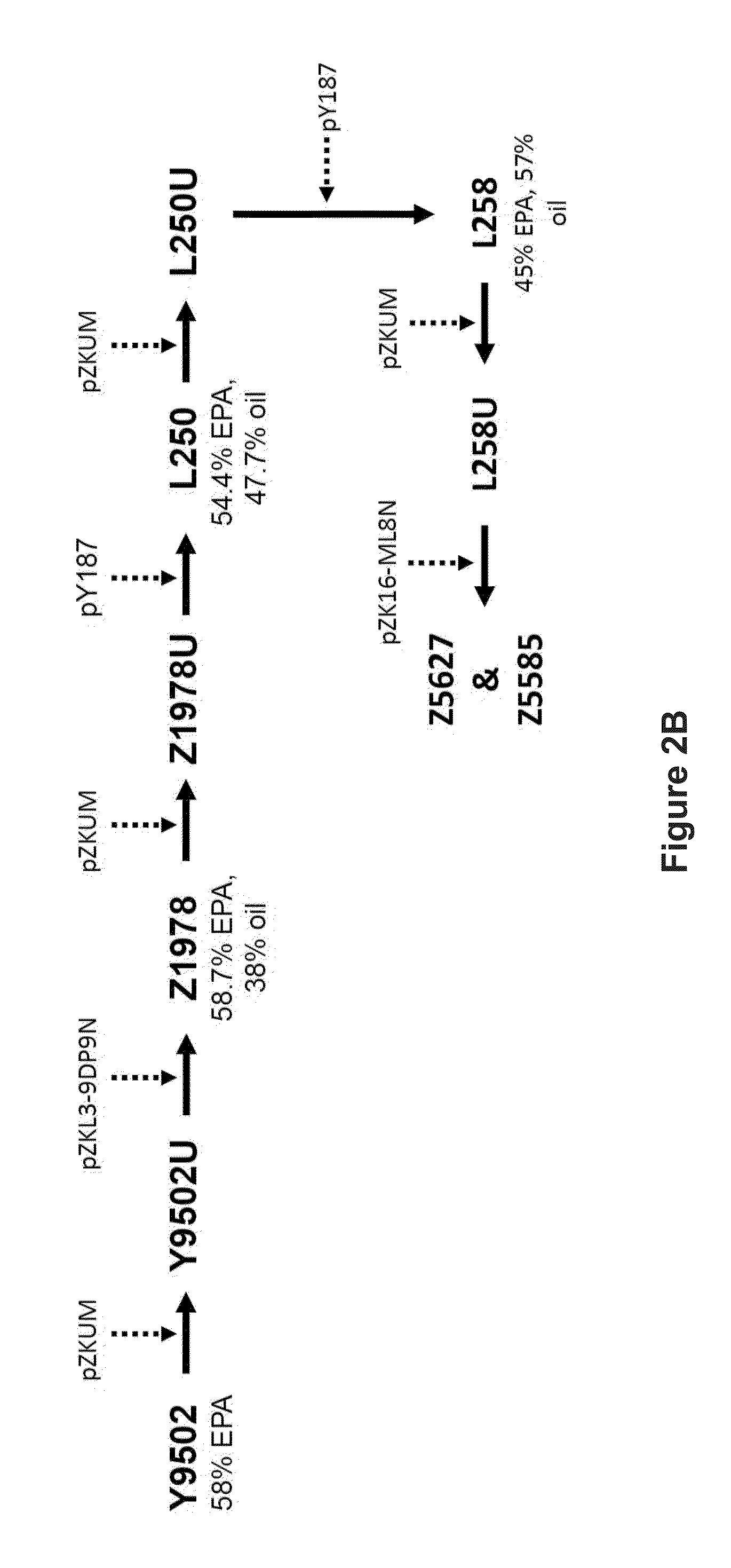
Recombinant microbial cells that produce at least 28% eicosapentaenoic acid as dry cell weight
ActiveUS20140186906A1Reduce the amount presentDecreases the total amount of sugar alcoholsFungiAcyltransferasesPhosphatidic acidPolynucleotide
Recombinant microbial cells are disclosed herein that produce an oil comprising at least 28 percent eicosapentaenoic acid (EPA) measured as a weight percent of dry cell weight. These cells may comprise a polynucleotide sequence encoding an active acyl-CoA:lysophosphatidylcholine acyltransferase (LPCAT) comprising at least one amino acid mutation in a membrane-bound O-acyltransferase motif. In addition, the cells may comprise a down-regulation of an endogenous polynucleotide sequence encoding Sou2 sorbitol utilization protein, and / or one or more polynucleotides encoding phospholipid:diacylglycerol acyltransferase (PDAT), delta-12 desaturase, a dihomo-gamma-linolenic acid (DGLA) synthase multizyme, delta-8 desaturase, malonyl-CoA synthetase (MCS), or acyl-CoA:lysophosphatidic acid acyltransferase (LPAAT). Also disclosed are methods of using the recombinant microbial cells to produce oil containing omega-3 polyunsaturated fatty acids such as EPA.
Owner:DUPONT US HLDG LLC
Glucose dehydrogenase
ActiveUS20130168263A1Improve productivityImprove thermal stabilityImmobilised enzymesBioreactor/fermenter combinationsEscherichia coliGlucose polymers
Disclosed is a modified glucose dehydrogenases that has dramatically increased productivity in Escherichia coli and dramatically increased thermal stability, which is obtained by introducing specific amino acid mutations to glucose dehydrogenase derived from Botryotinia fuckeliana. Also disclosed is a modified glucose dehydrogenases that has dramatically increased productivity in E. coli and dramatically increased thermal stability, which is obtained by replacing two amino acid residues in glucose dehydrogenase of fungal origin with cysteine residues. The novel glucose dehydrogenase has a low reactivity to xylose.
Owner:ULTIZYME INT LTD
Anti-IL-6 Receptor Antibody
InactiveUS20170121412A1Enhanced antigen-neutralizing activity and pharmacokineticsGood treatment effectCompound screeningApoptosis detectionHigh concentrationHinge region
Owner:CHUGAI PHARMA CO LTD
Firefly luciferase and gene thereof, and process for production of firefly luciferase
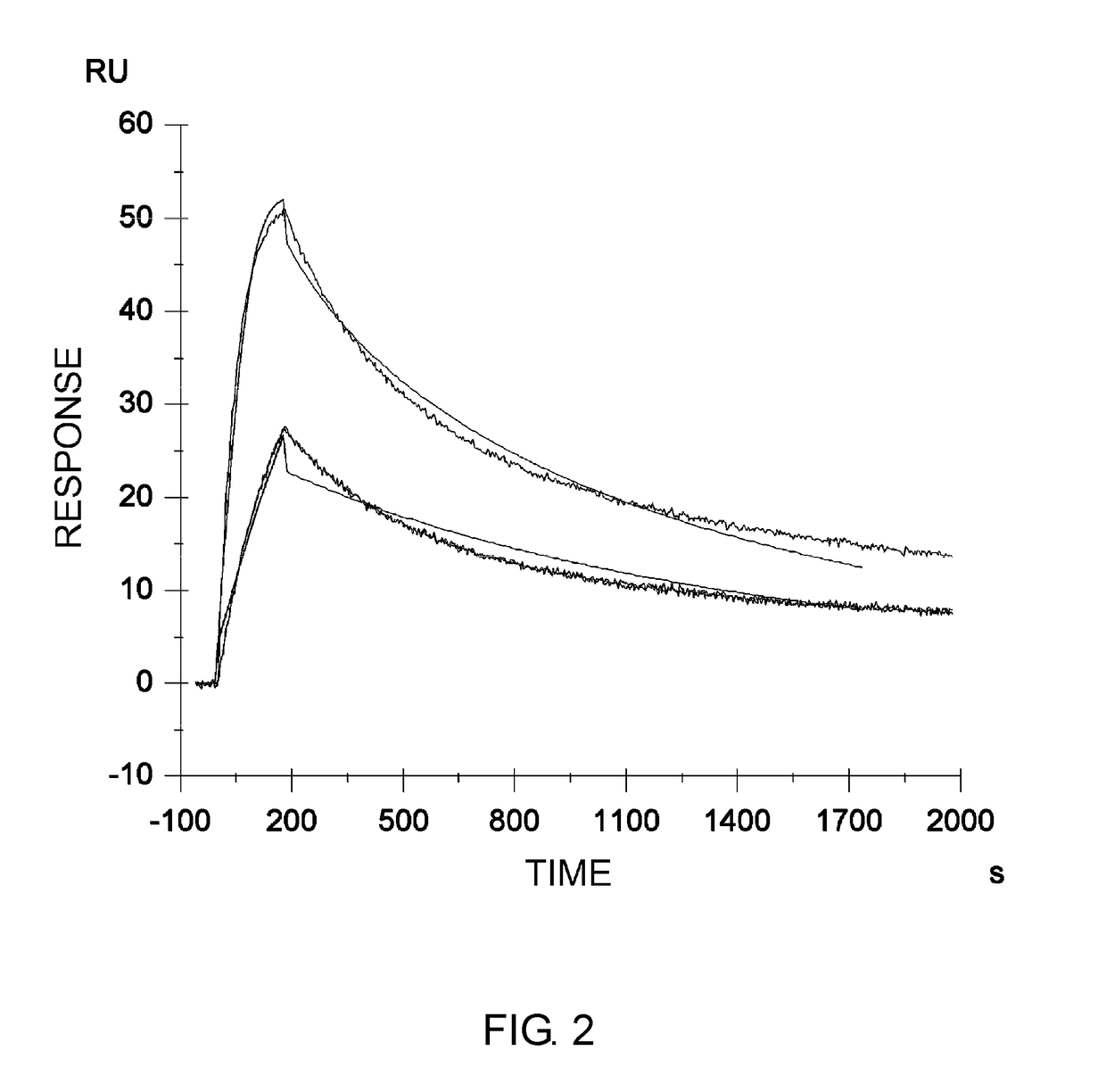
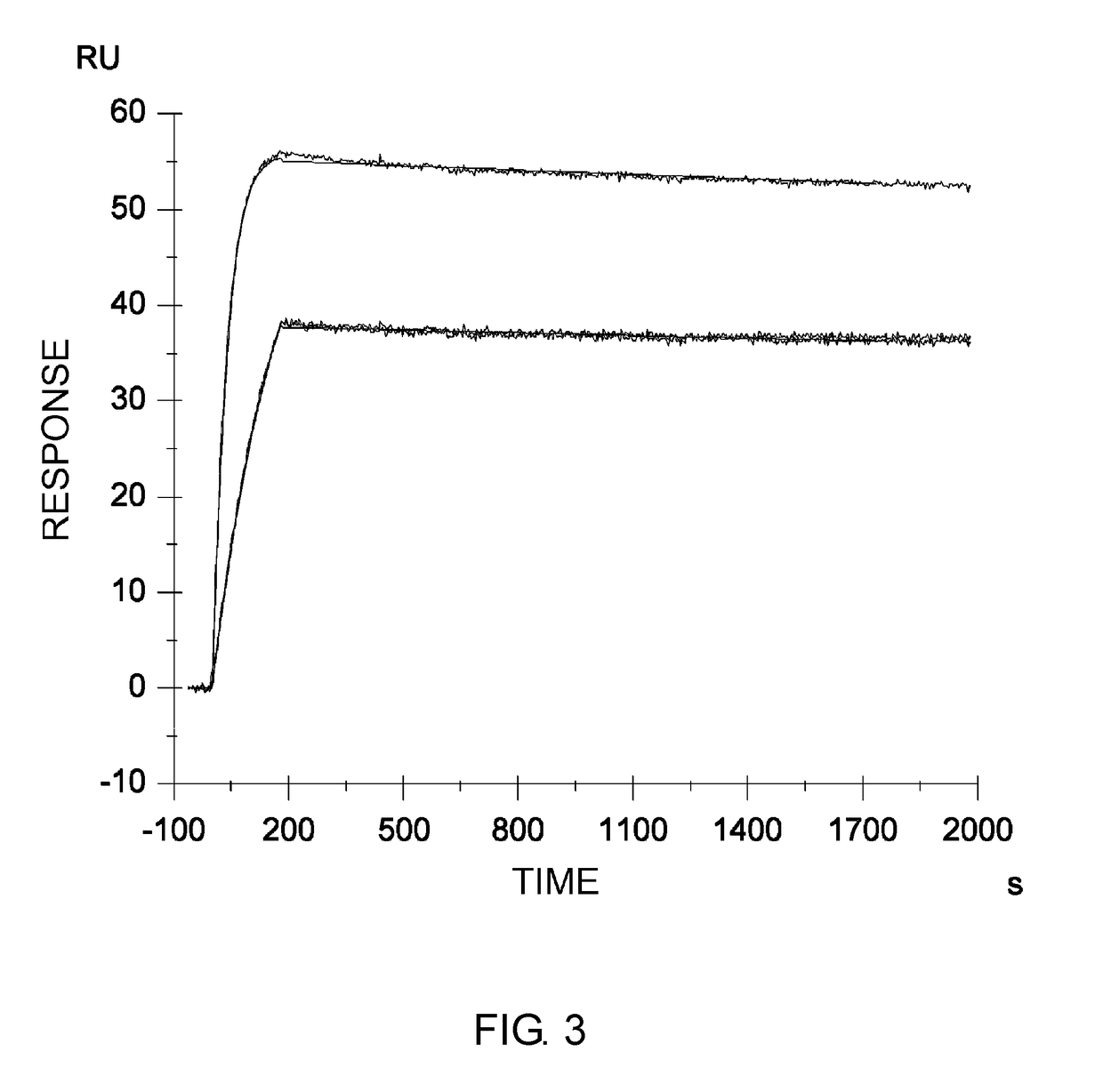
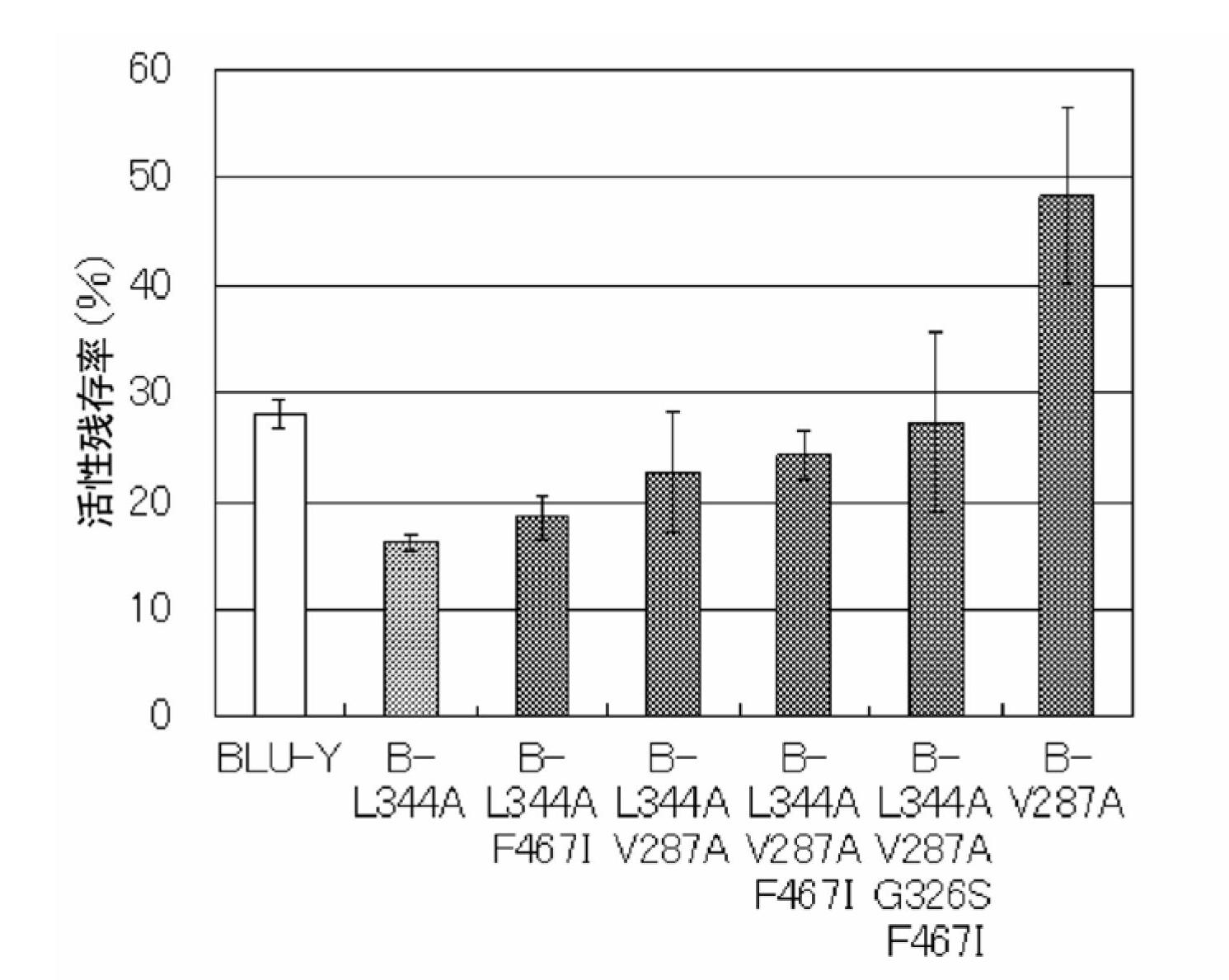
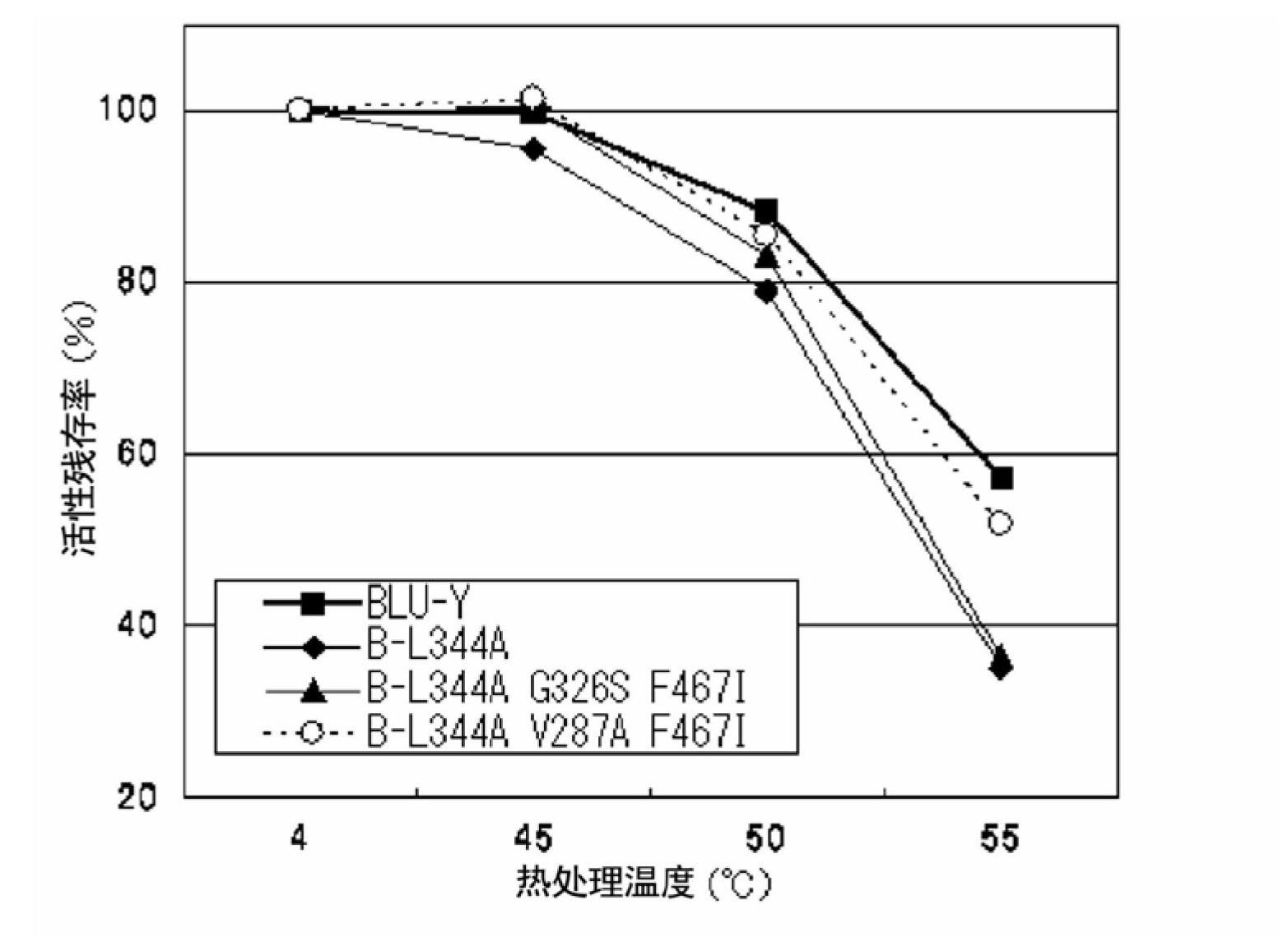
Disclosed are: a firefly luciferase having excellent thermal stability and / or storage stability; and a process for producing the firefly luciferase. Specifically disclosed are: a firefly luciferase characterized by comprising an amino acid sequence that is produced by substituting an amino acid residue located at position-287 by an alanine residue or mutating an amino acid residue located at position-392 by an isoleucine residue in the amino acid sequencer for a Japanese firefly Luciola lateralis; and a gene for the firefly luciferase. The use of the gene enables the efficient production of a firefly luciferase having improved stability. The above-mentioned mutation may be combined with a mutation produced by substituting an amino acid residue located at position-326 by a serine residue and / or a mutation produced by substituting an amino acid residue located at position-467 by an isoleucine residue in the same amino acid sequence, thereby producing a firefly luciferase having further improved stability.
Owner:KIKKOMAN CORP
Modified carbohydrate processing enzyme
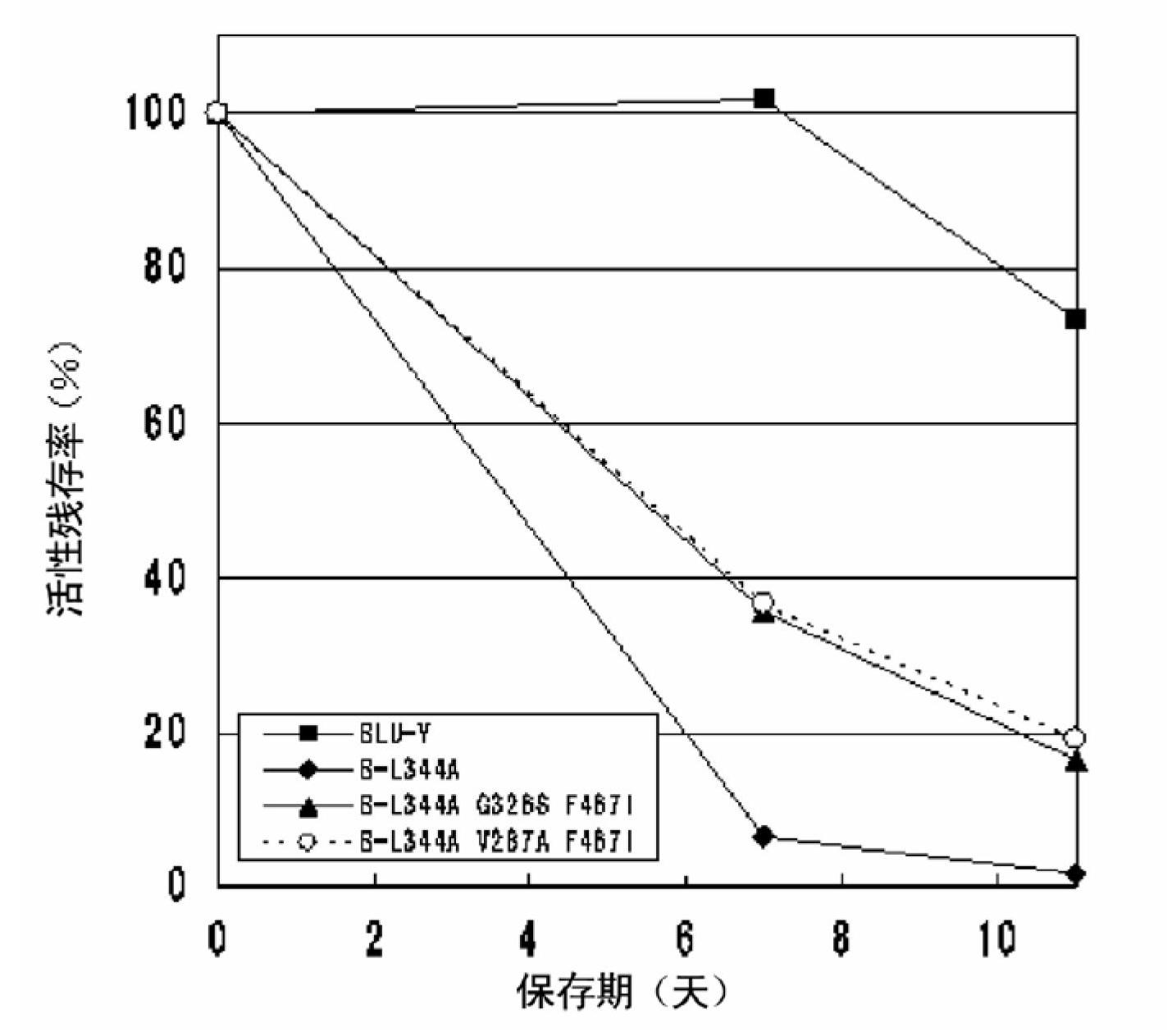
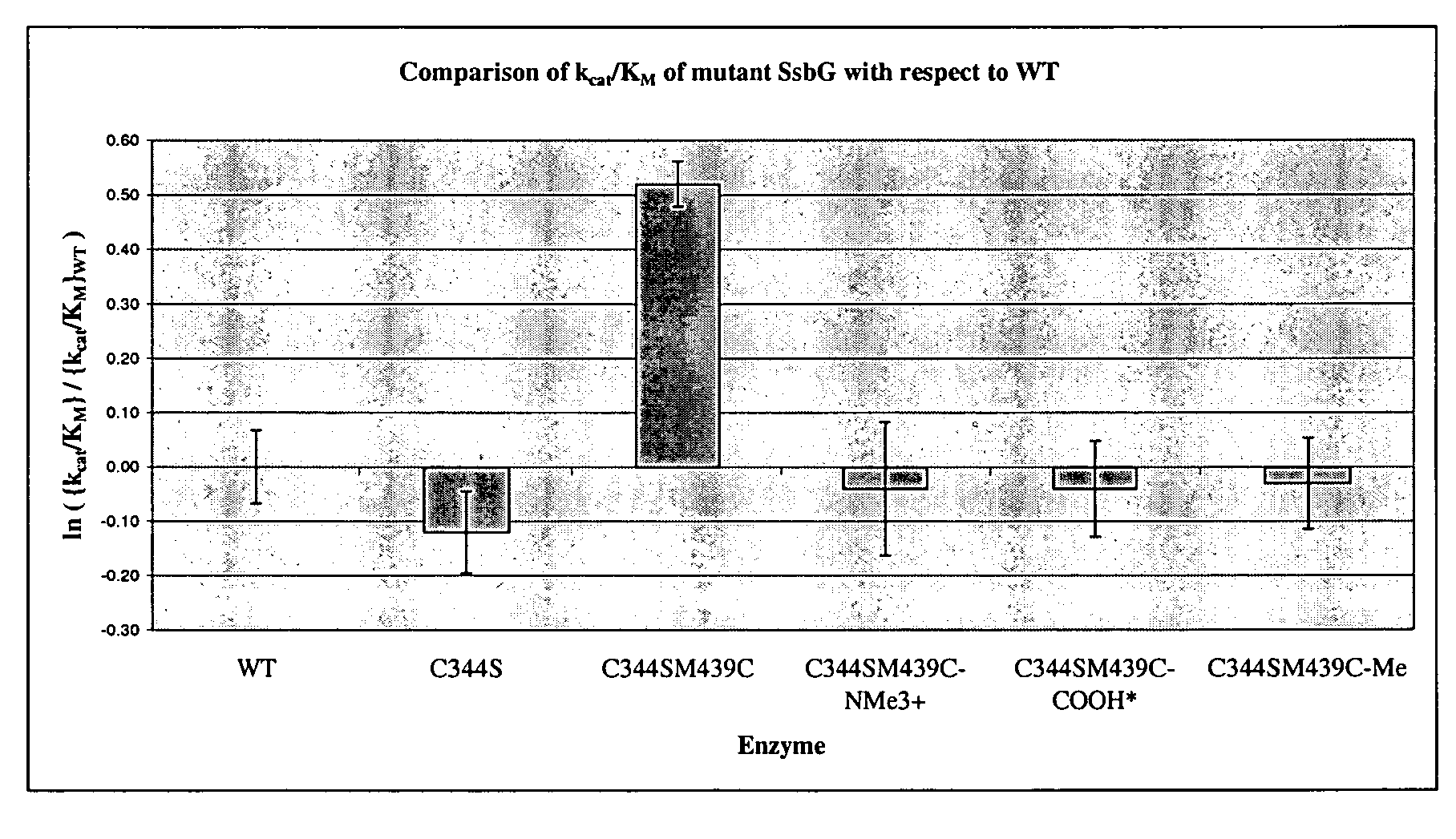
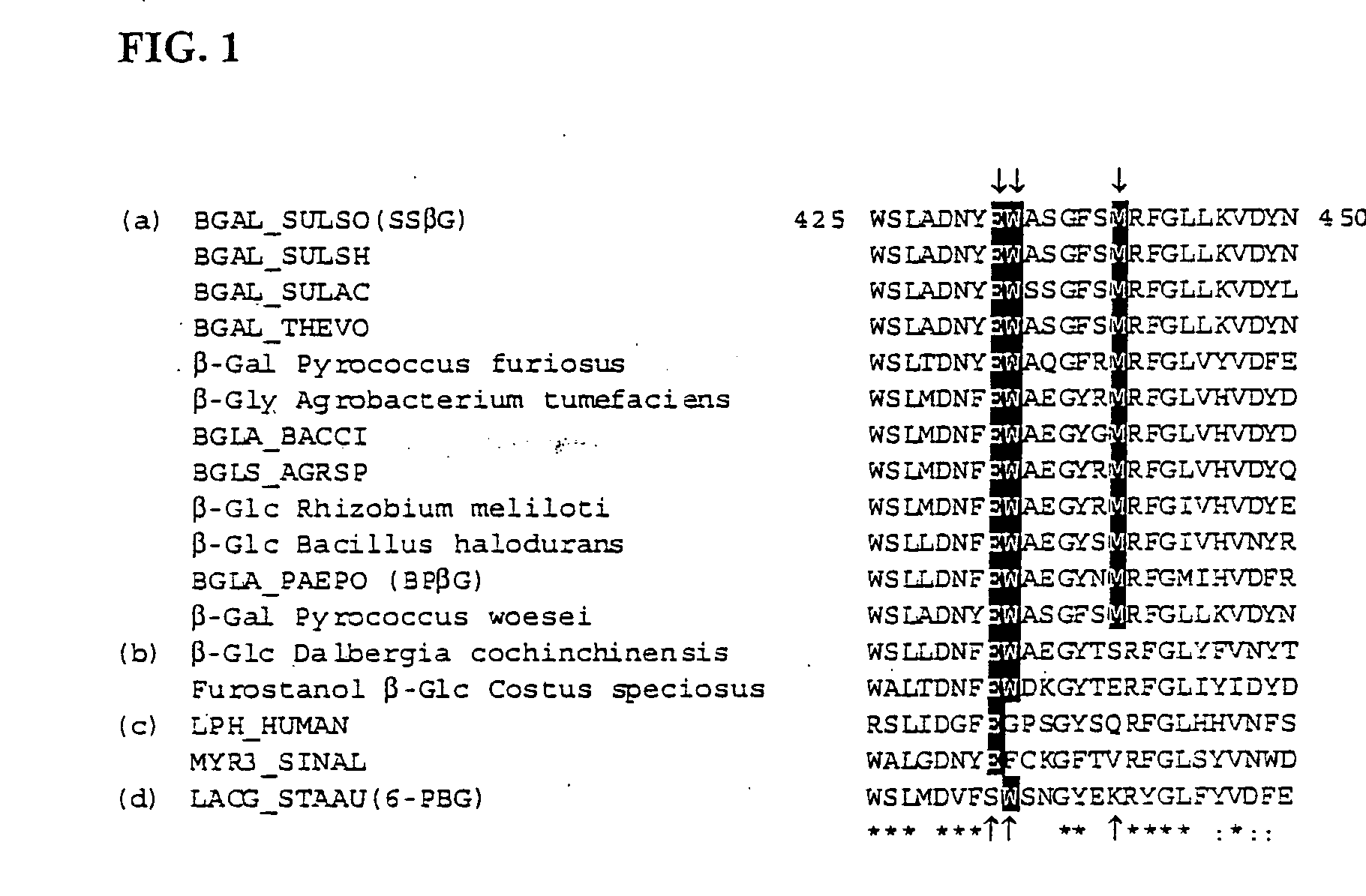
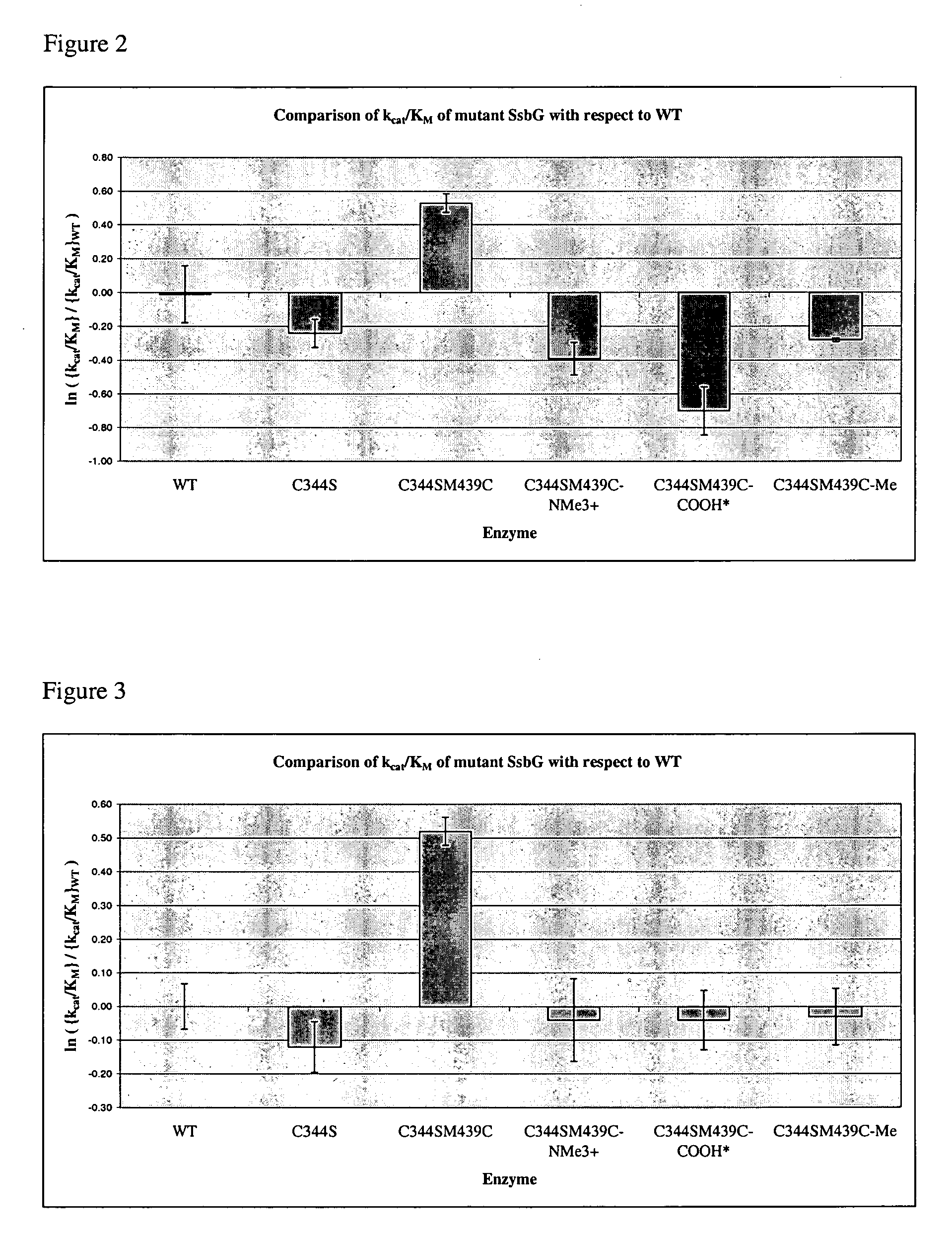
A modified polypeptide having carbohydrate processing enzymatic activity is provided, said polypeptide comprising an amino acid sequence selected from: (a) the amino acid sequence of SEQ ID NO:2 comprising a mutation in at least one of W433, E432 and M439; (b) the amino acid sequence of an enzyme of glycosyl hydrolase family 1, comprising at least one mutation at an amino acid residue equivalent to W433, E432 or M439 of SEQ ID NO:2; and (c) a variant of (a) or (b) having carbohydrate processing enzymatic activity and comprising at least one amino acid mutation at a position equivalent to W433, E432 or M439 of SEQ ID NO:2.
Owner:ISIS INNOVATION LTD
Thermal stability improved lipase mutant constructed through orthogenesis
The invention relates to a thermal stability improved lipase mutant constructed through orthogenesis, belonging to the technical field of enzymatic genetic engineering. The invention discloses a thermal stability improved lipase mutant obtained through a molecular biological technology by using a Rhizopuschinensis CCTCC M201021 lipase as a parent. In amino acid sequences of each mutant, the related amino acid mutant is one or more of Met101Thr, Glu107Gly, Ala129Ser, Ser151Asn, Cys160Leu, Lys161Arg, Pro168Leu, Pro168His, Leu180His, Asp182Tyr, Thr183Ala, Thr218Ser, Lys219Asp, Ala230Phe, Ser234Phe, Val261Gly, His317Pro, Val329Ala, Glu363Arg, Asn366Asp and Ser373Cys. The mutants are represented by a half-life period of t50 at 65 DEG C, and the thermal stability of the mutants are improved compared with the parent of Rhizopuschinensis lipase. The invention also discloses a DNA (Deoxyribonucleic Acid) sequence, an expression vector and a host cell for encoding the lipase mutant.
Owner:金湖县农副产品营销协会
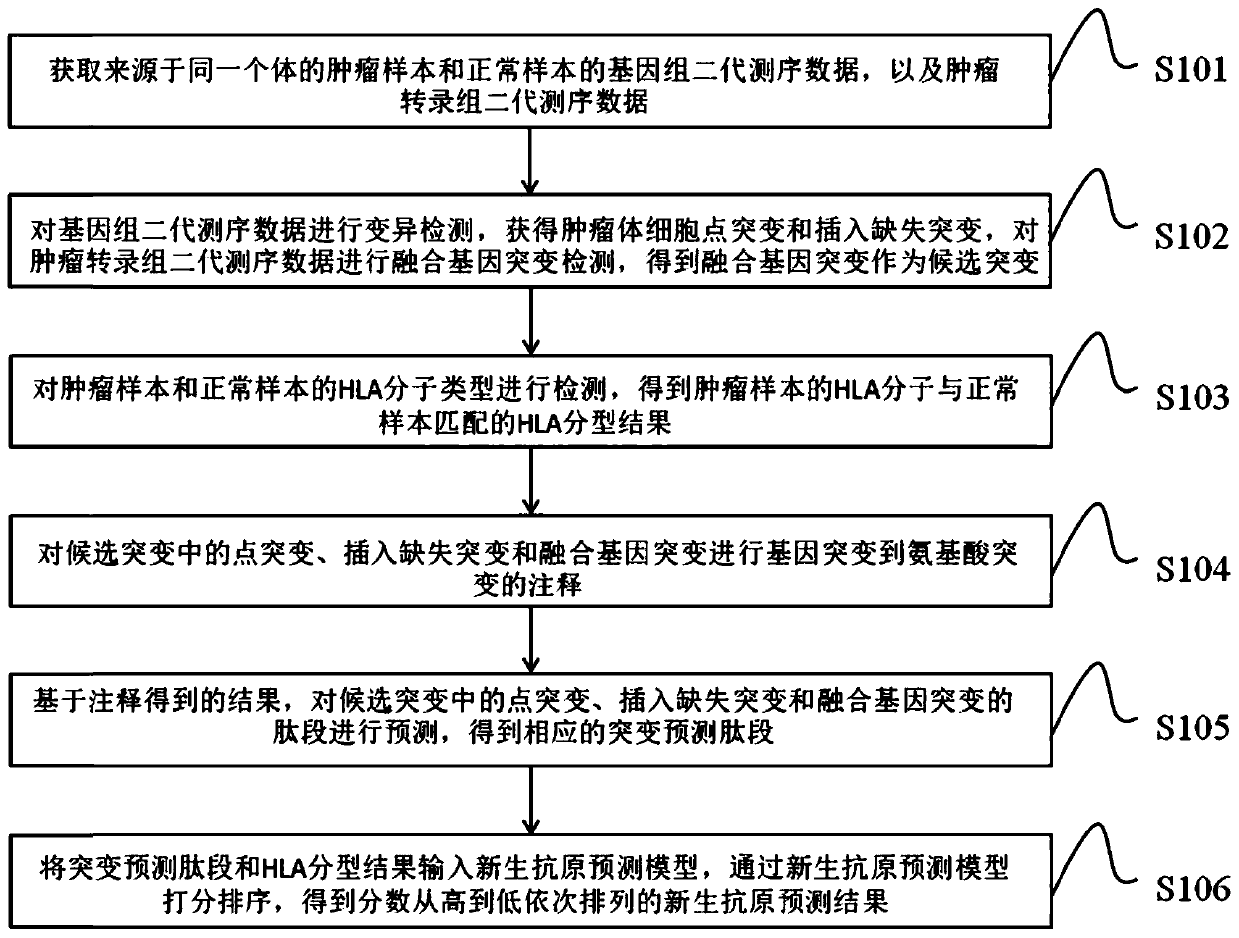
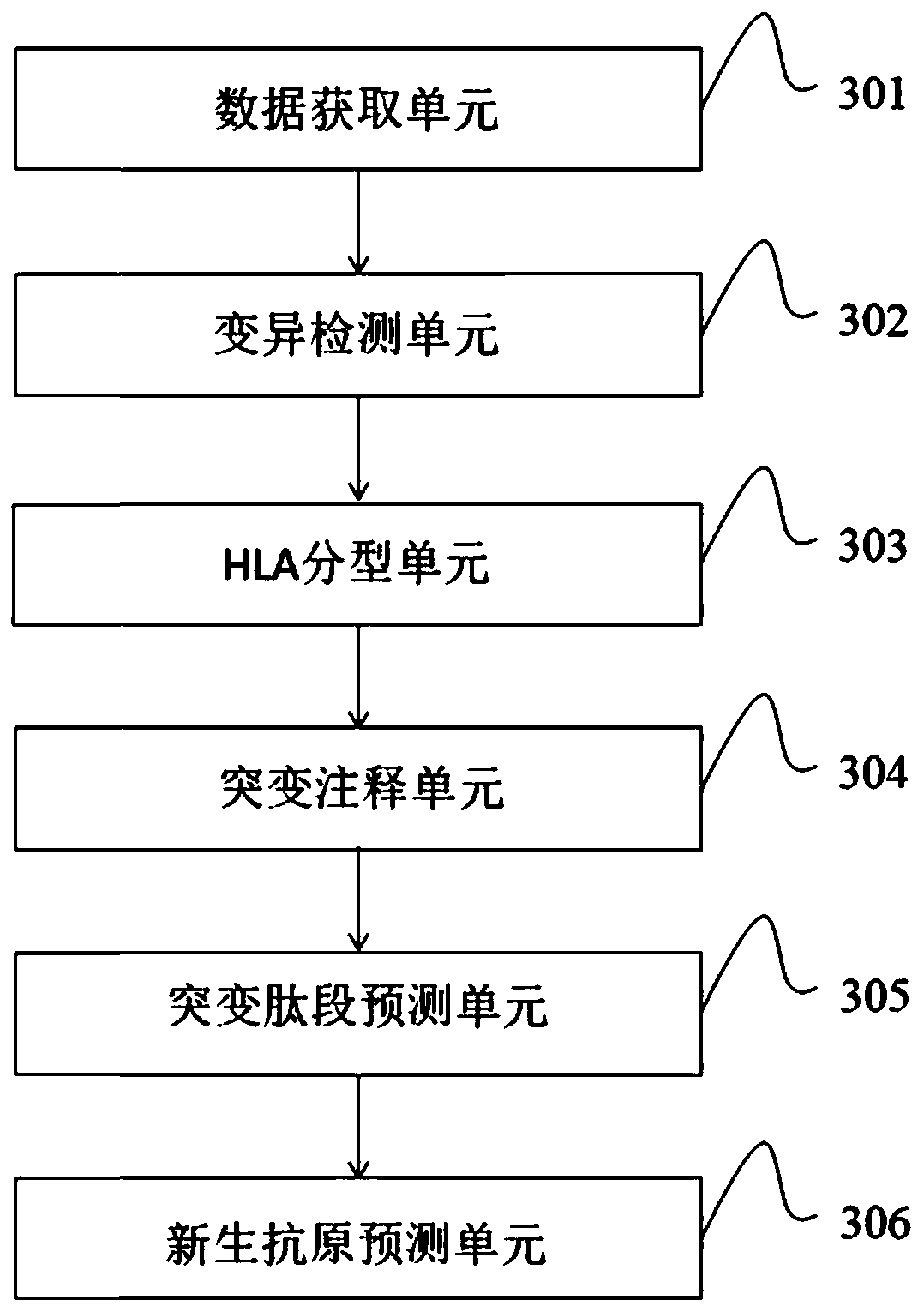
Neoantigen prediction method and device based on next-generation sequencing and storage medium
PendingCN110752041AQuality improvementConvenient treatmentProteomicsDrug referencesGenomic sequencingGenes mutation
Owner:深圳裕策生物科技有限公司
Adenine base editing tool and application thereof
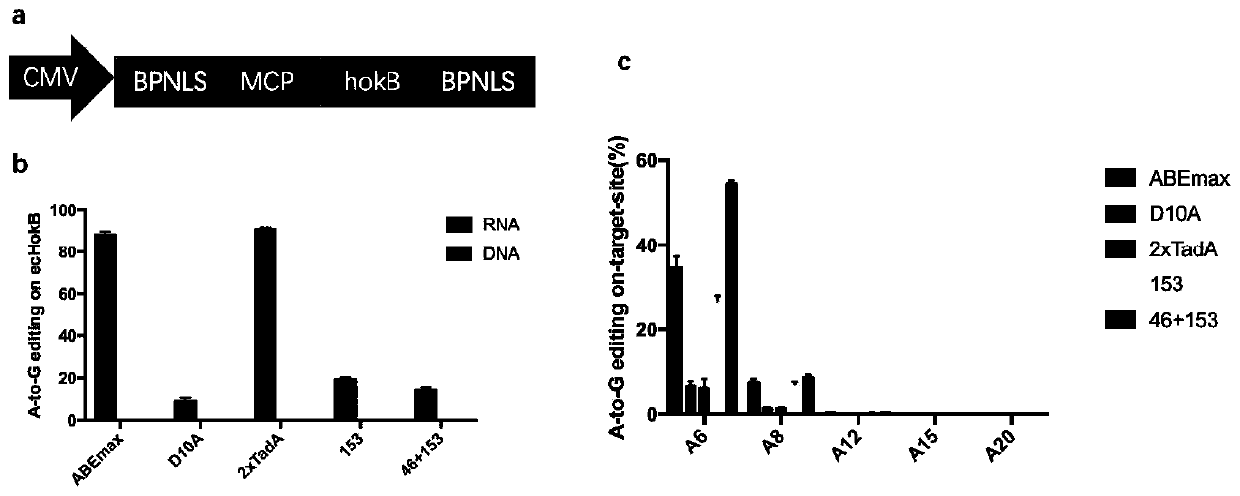
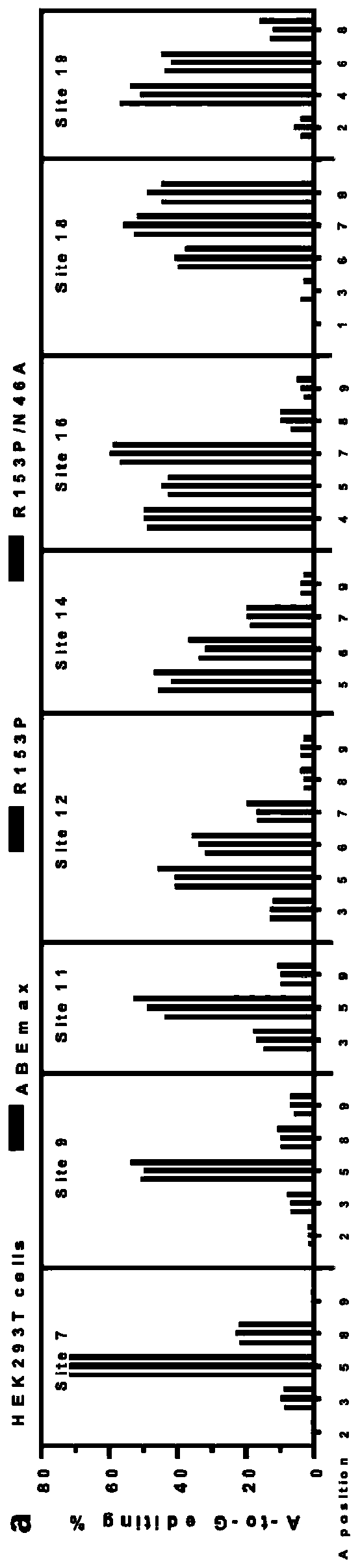
The invention relates to the field of biotechnology, in particular to an adenine base editing tool and application thereof. The invention provides a fusion protein. The fusion protein includes an ecTadA-ecTadA* dimer fragment and an SpCas9-D10A nickase fragment, and the ecTadA-ecTadA* dimer fragment includes an ecTad fragment and an ecTadA* fragment. According to the provided adenine base editingtool, in the ecTadA-ecTadA* dimer fragment, compared with wild-type existing R153P and N46A amino acid mutations, binding of ecTadA and RNA can be restricted, and during base editing of A at the 4-7 position of sgRNA 5' end mutating to G, the RNA off-target effect of the adenine base editing tool can be greatly lowered or even eliminated.
Owner:SHANGHAI TECH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com