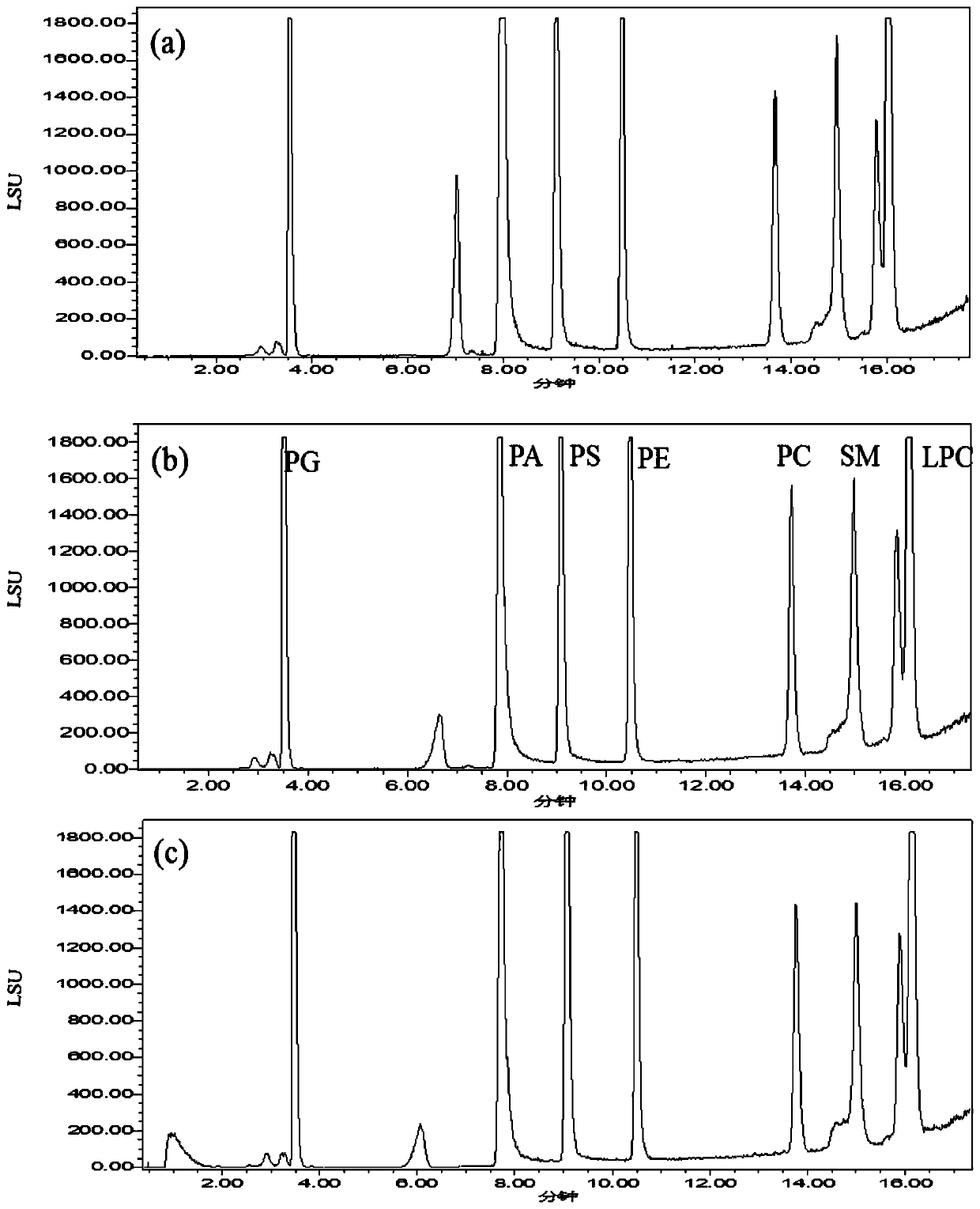
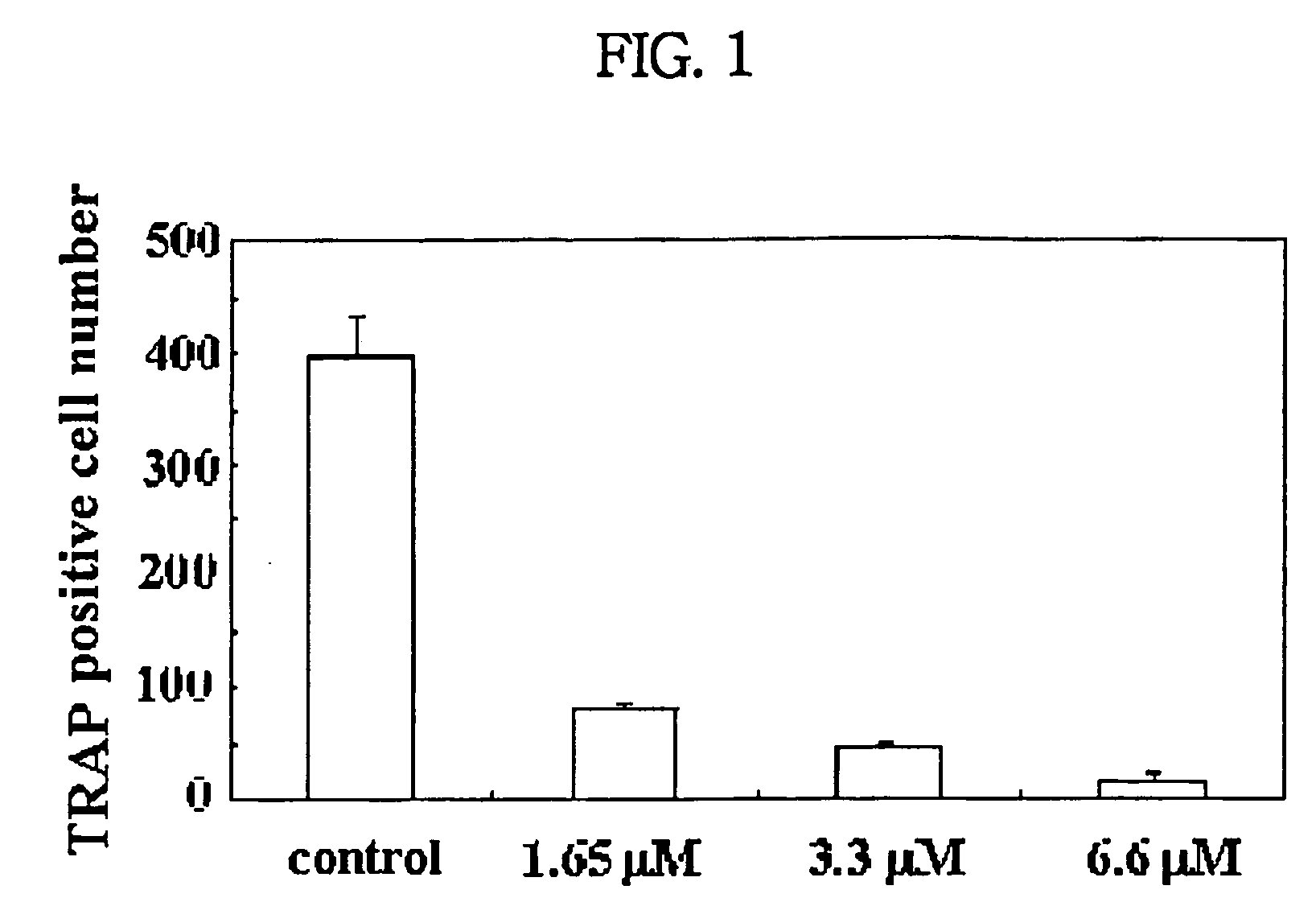
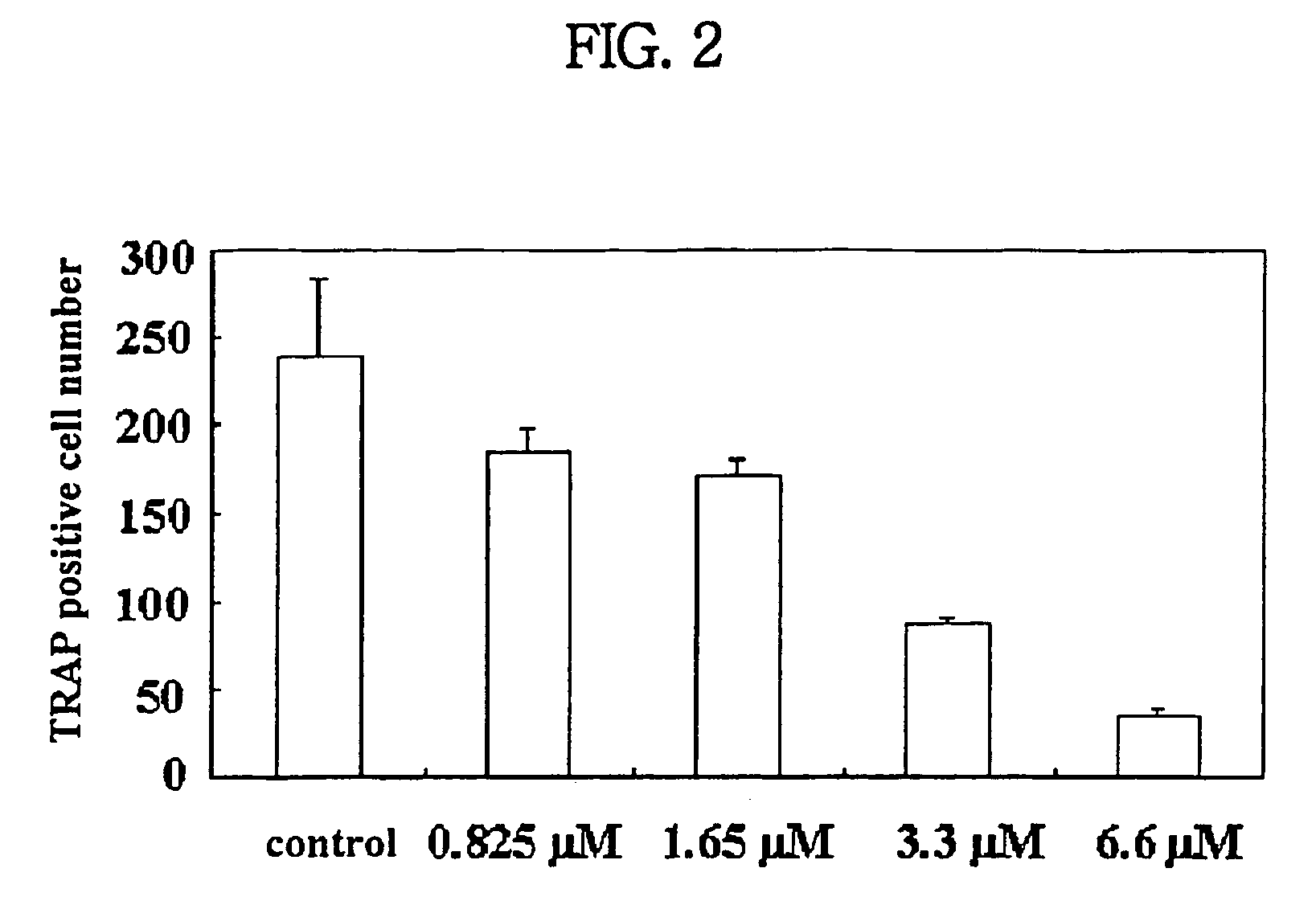
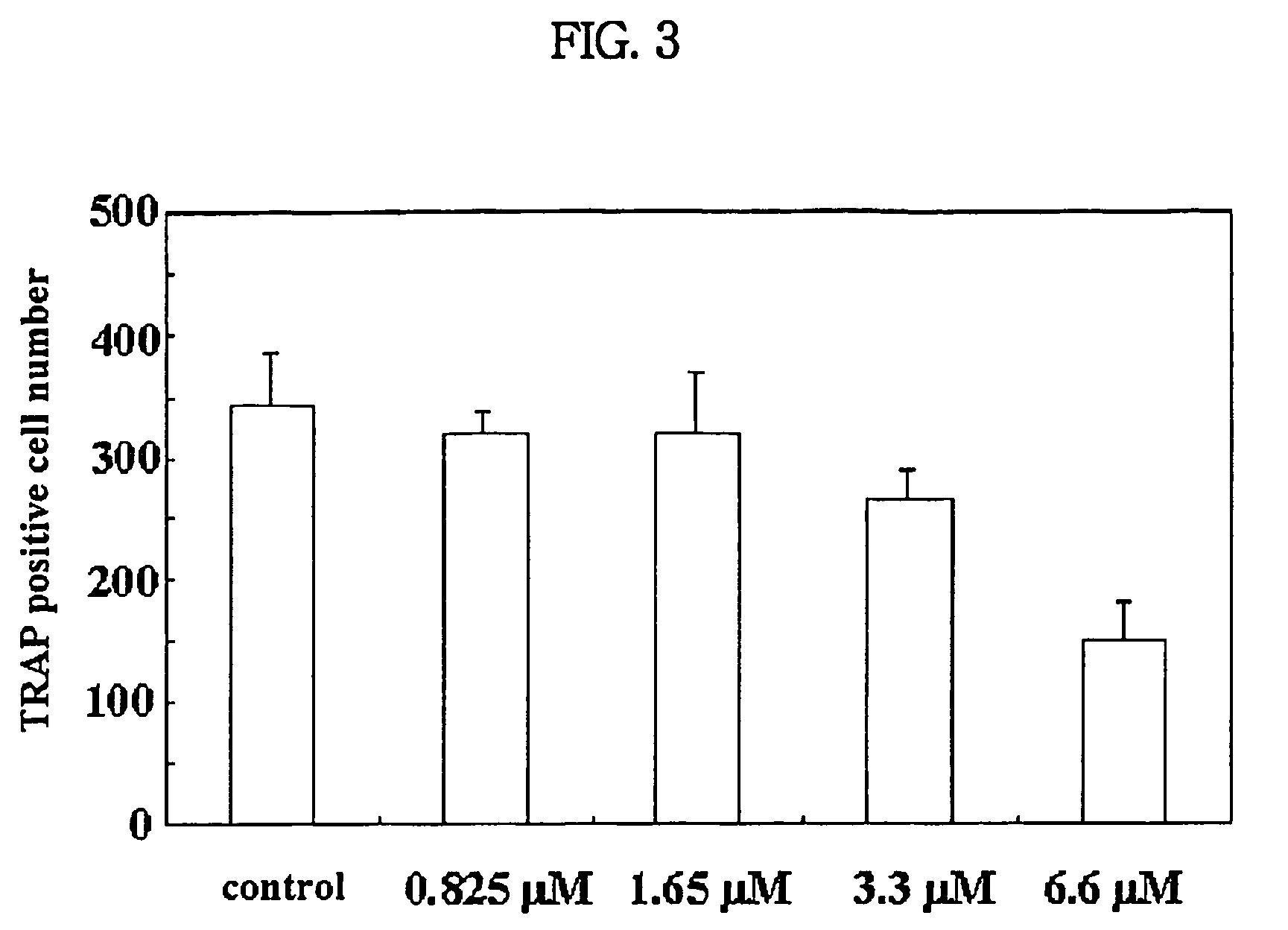
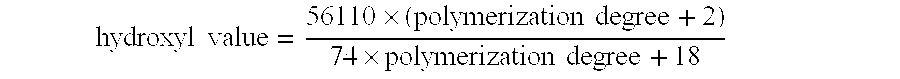Patents
Literature
135 results about "Lysophosphatidylcholine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lysophosphatidylcholines (LPC, lysoPC), also called lysolecithins, are a class of chemical compounds which are derived from phosphatidylcholines.
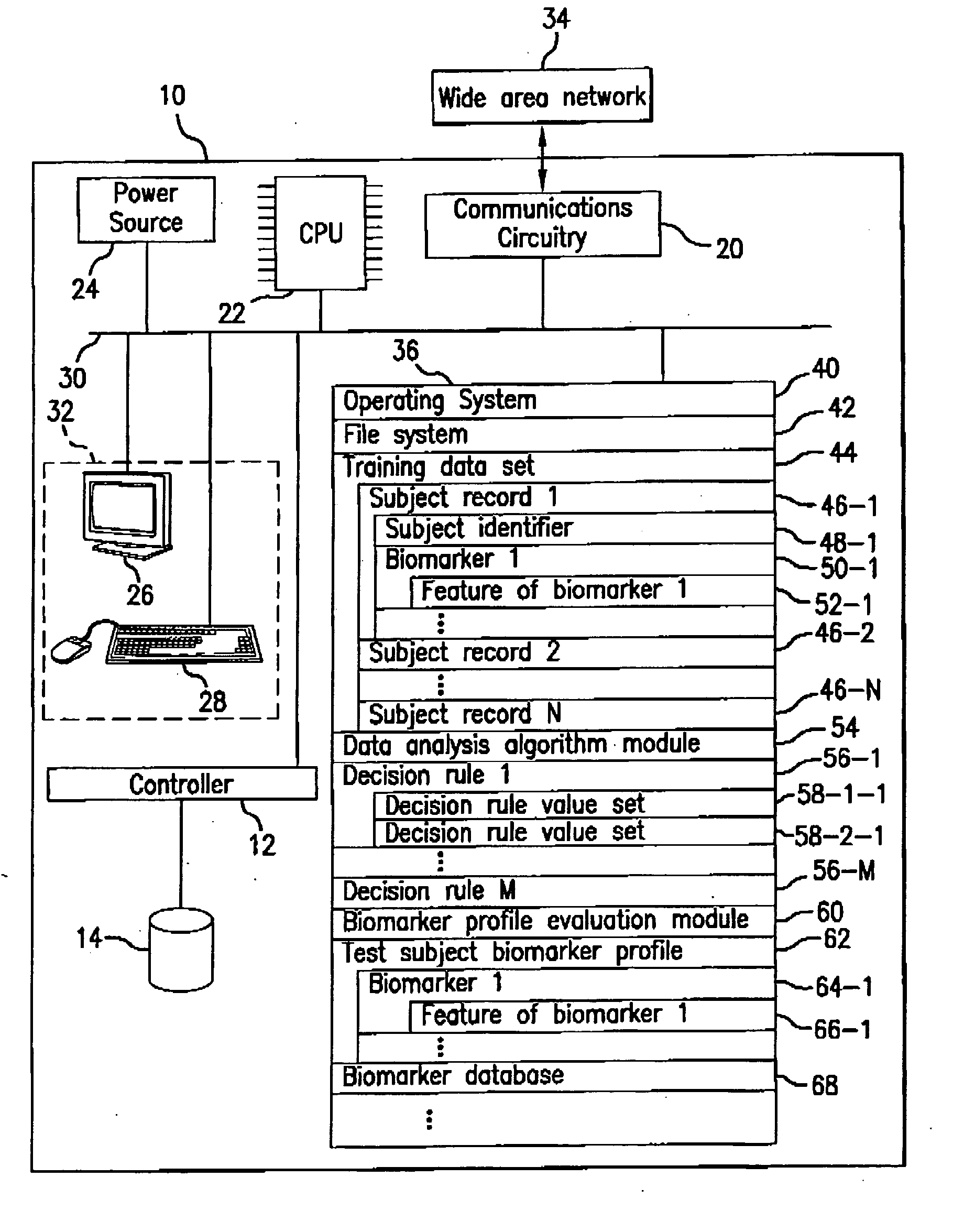
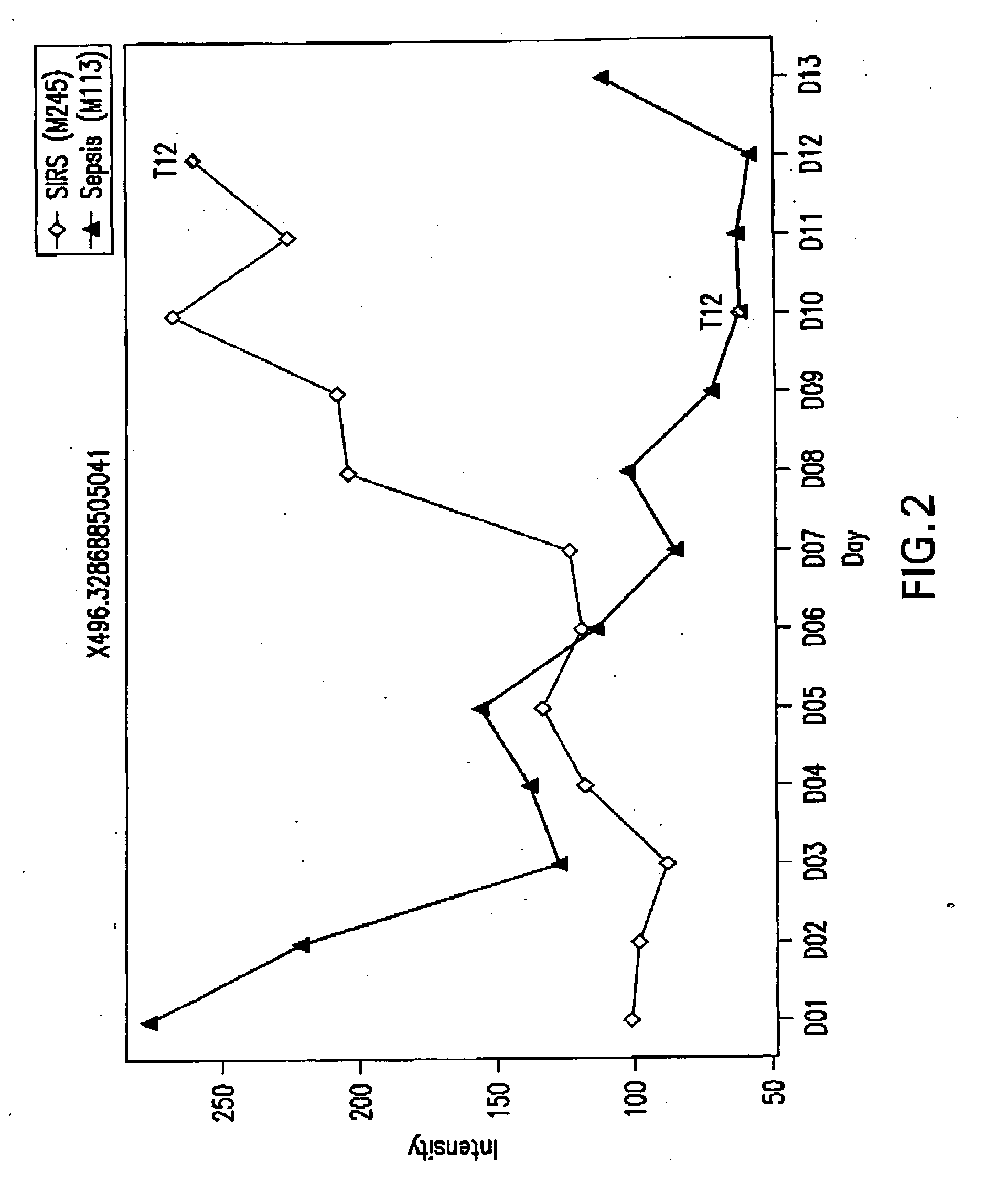
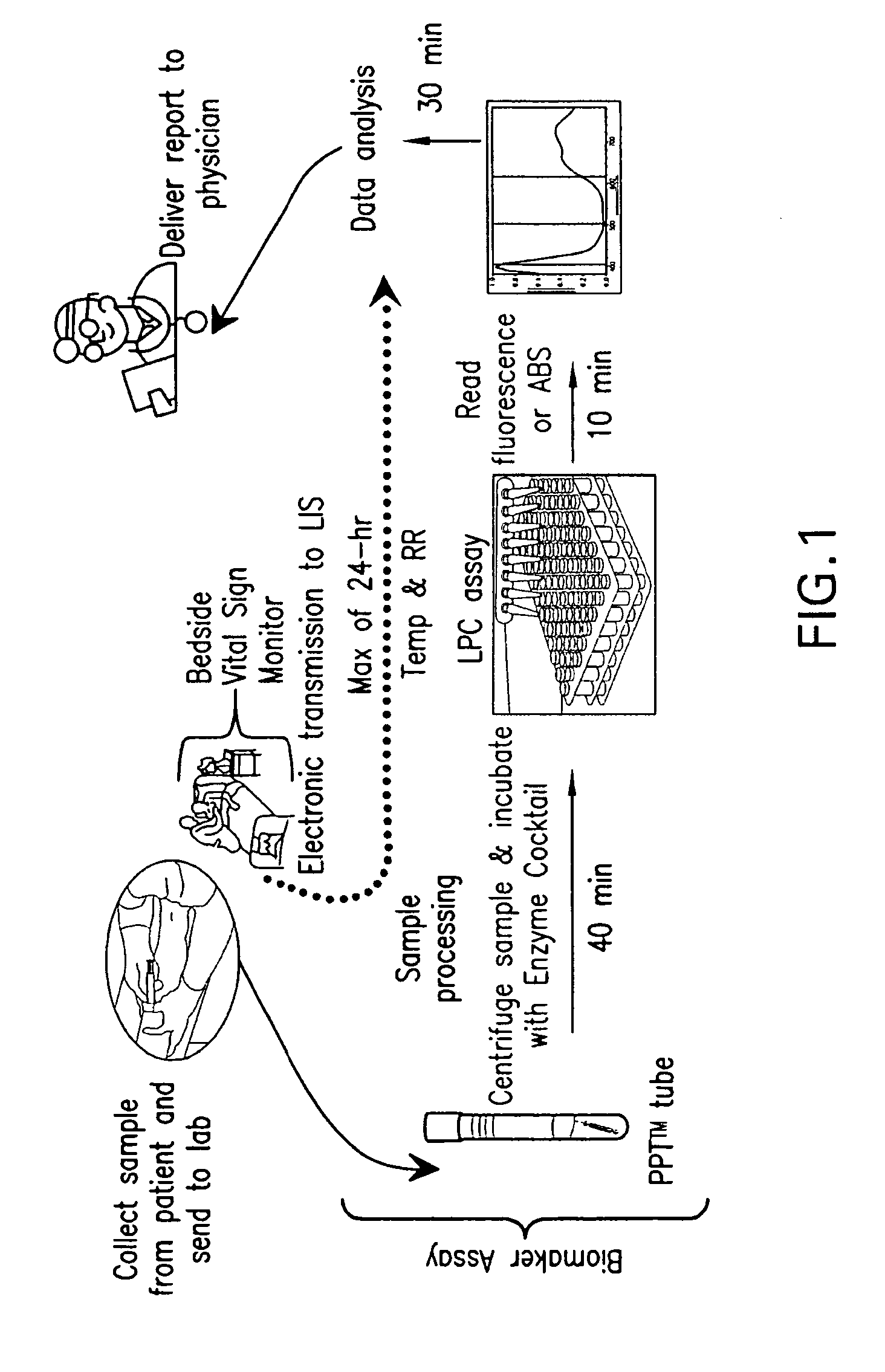
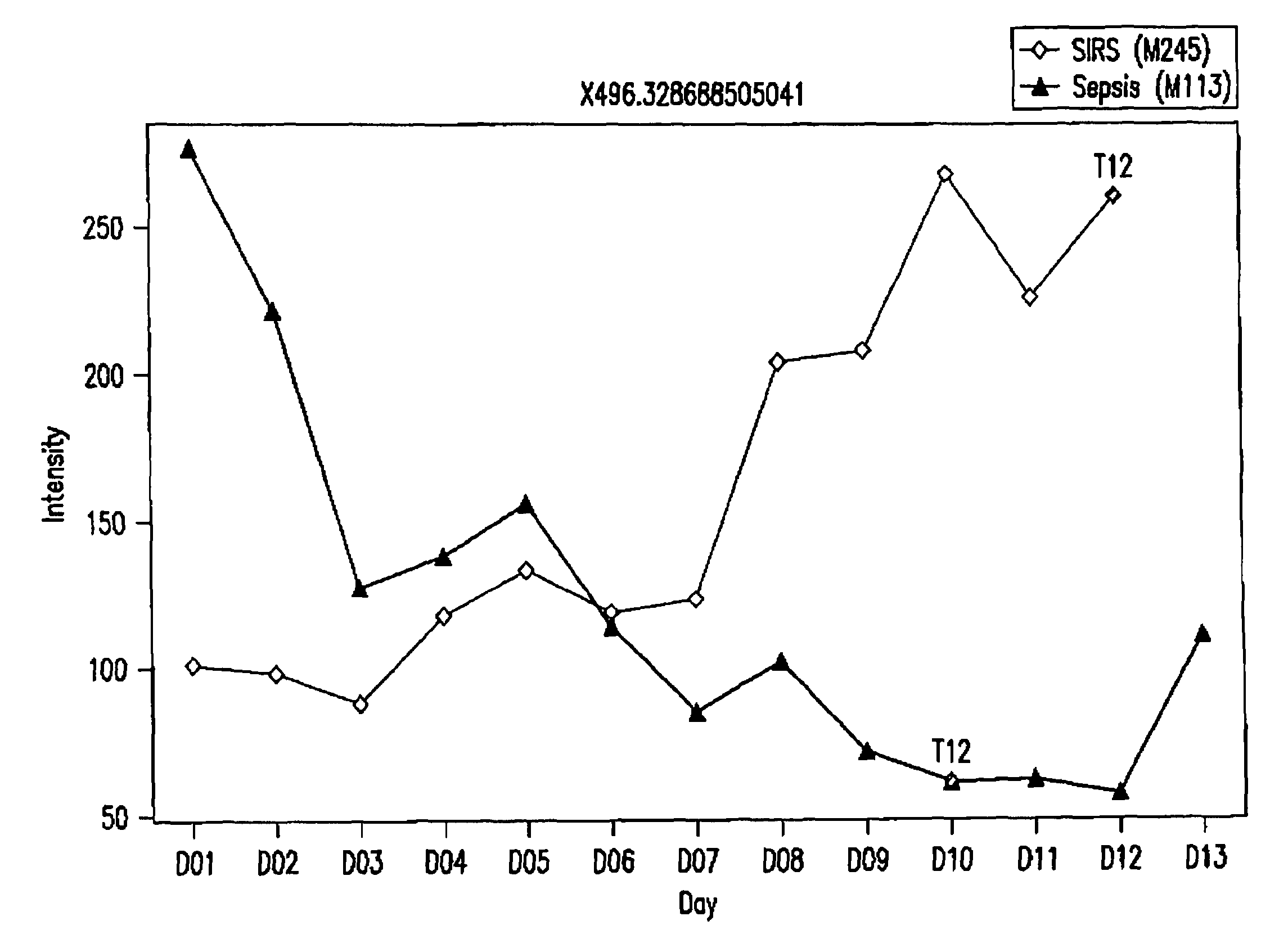
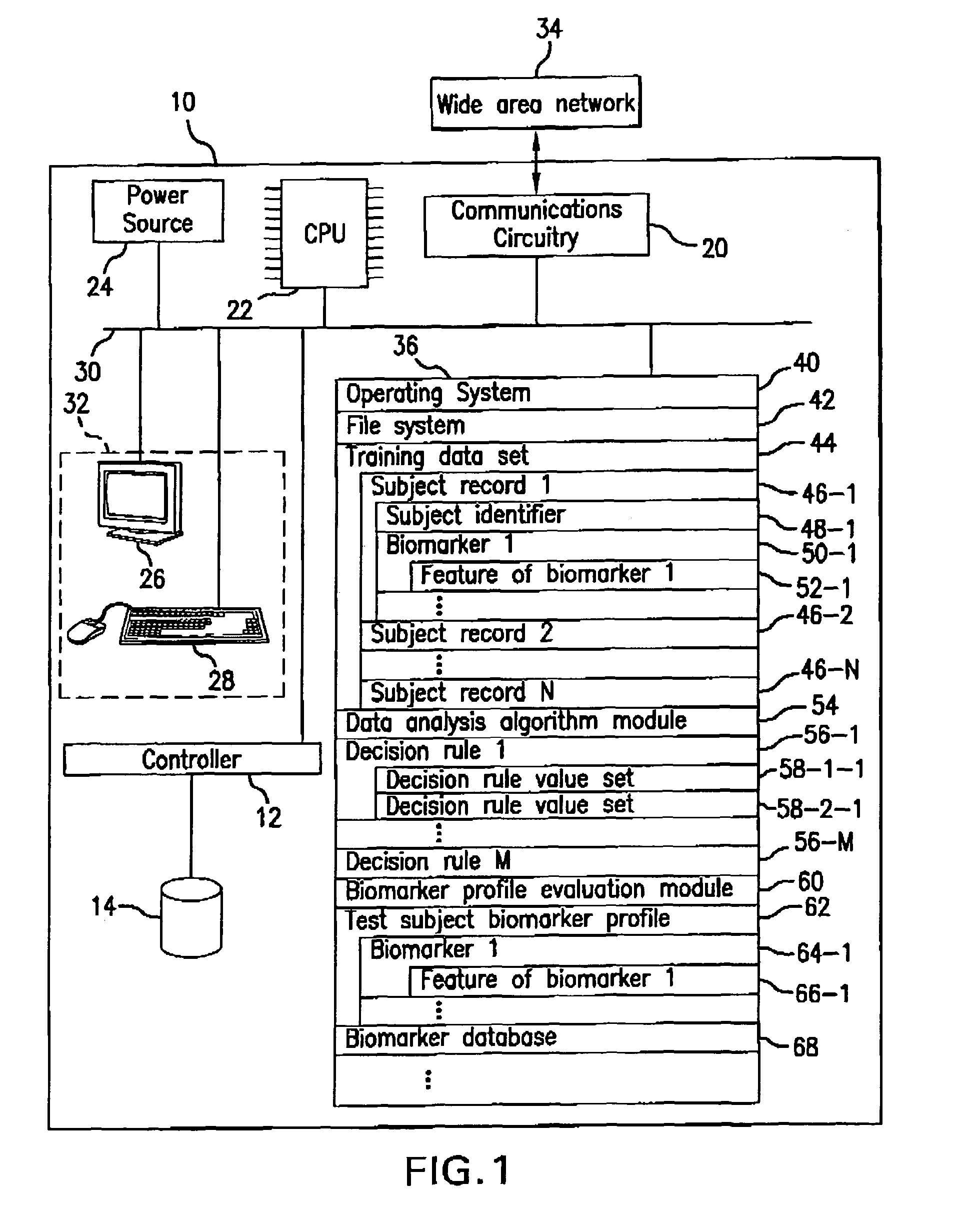
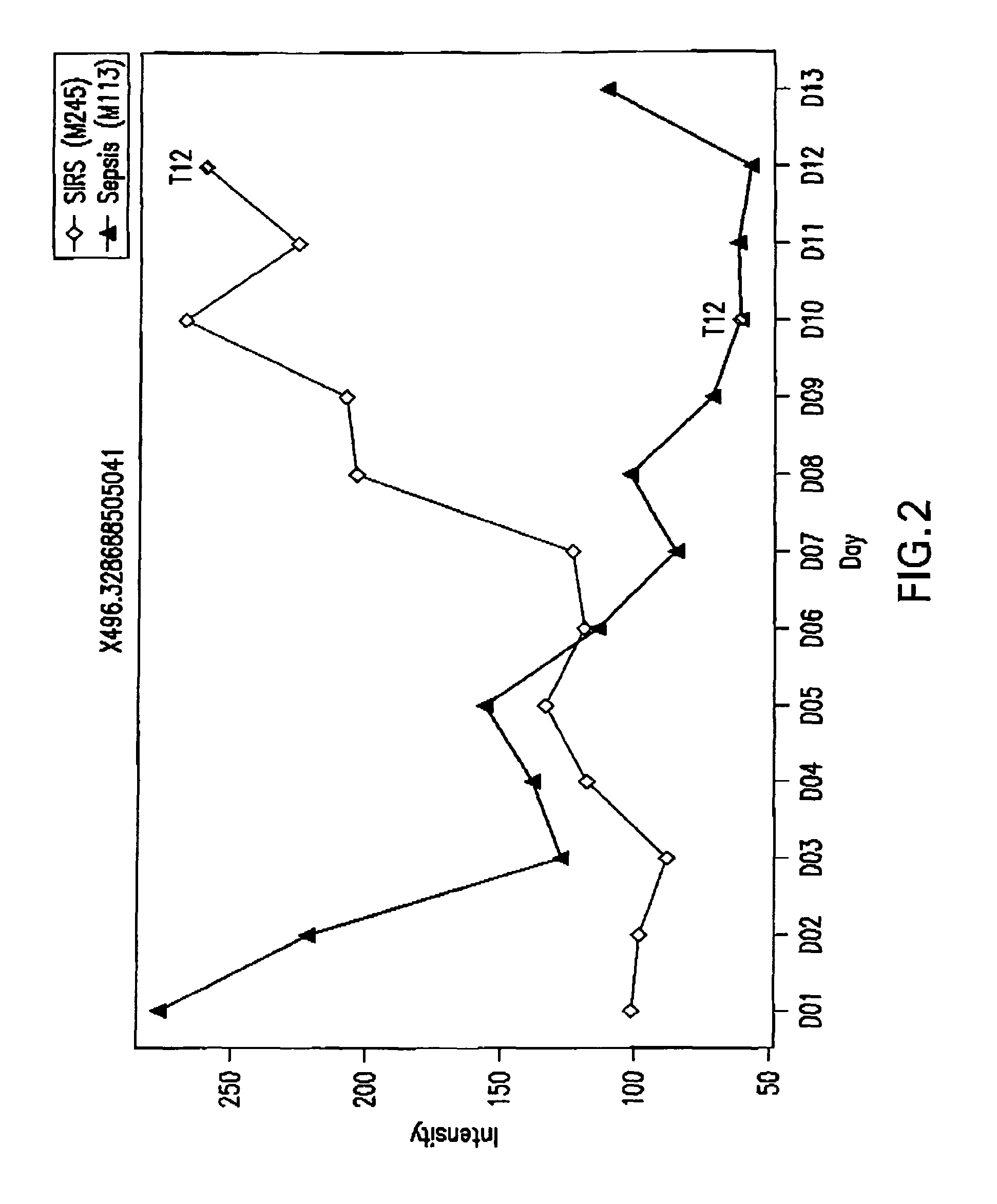
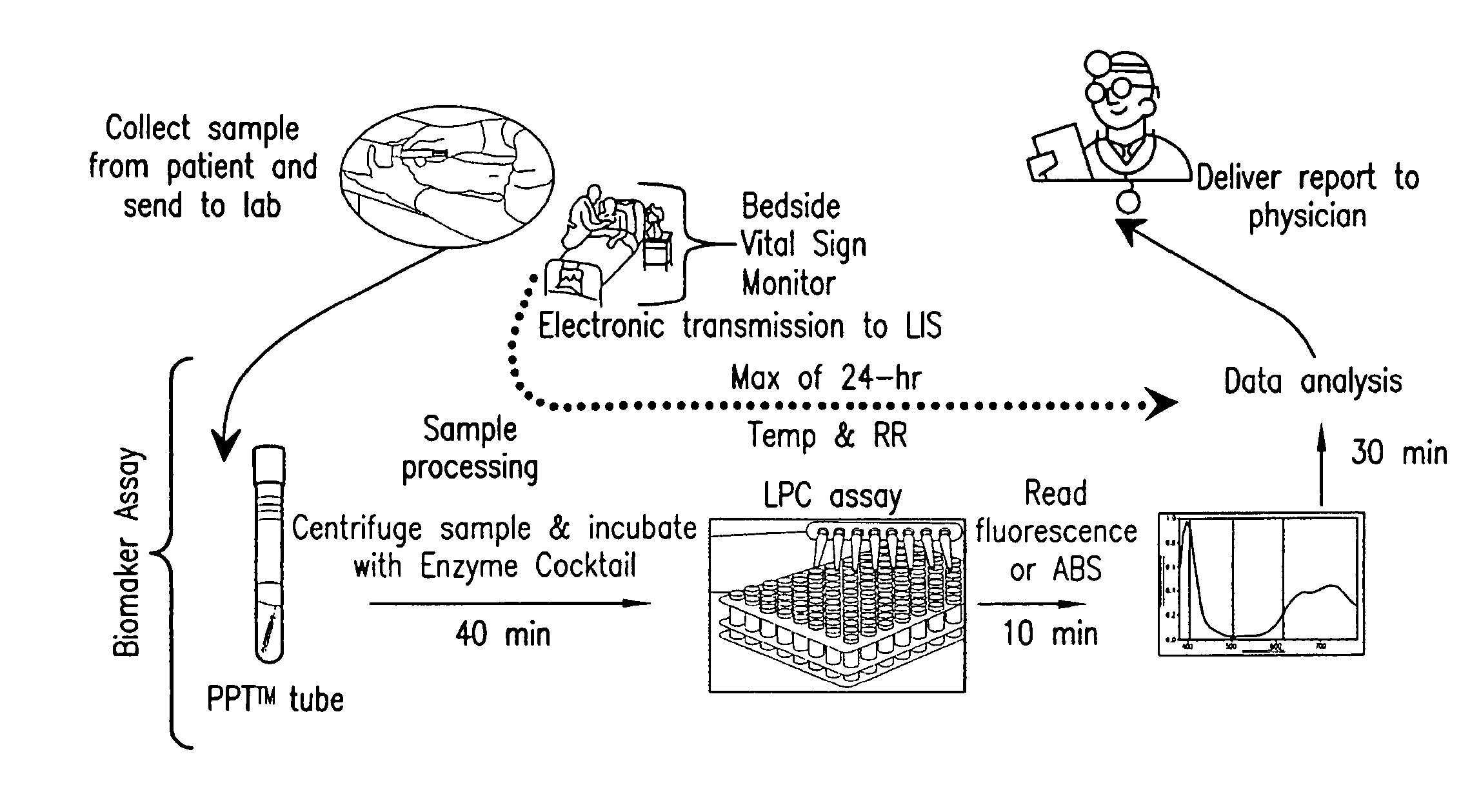
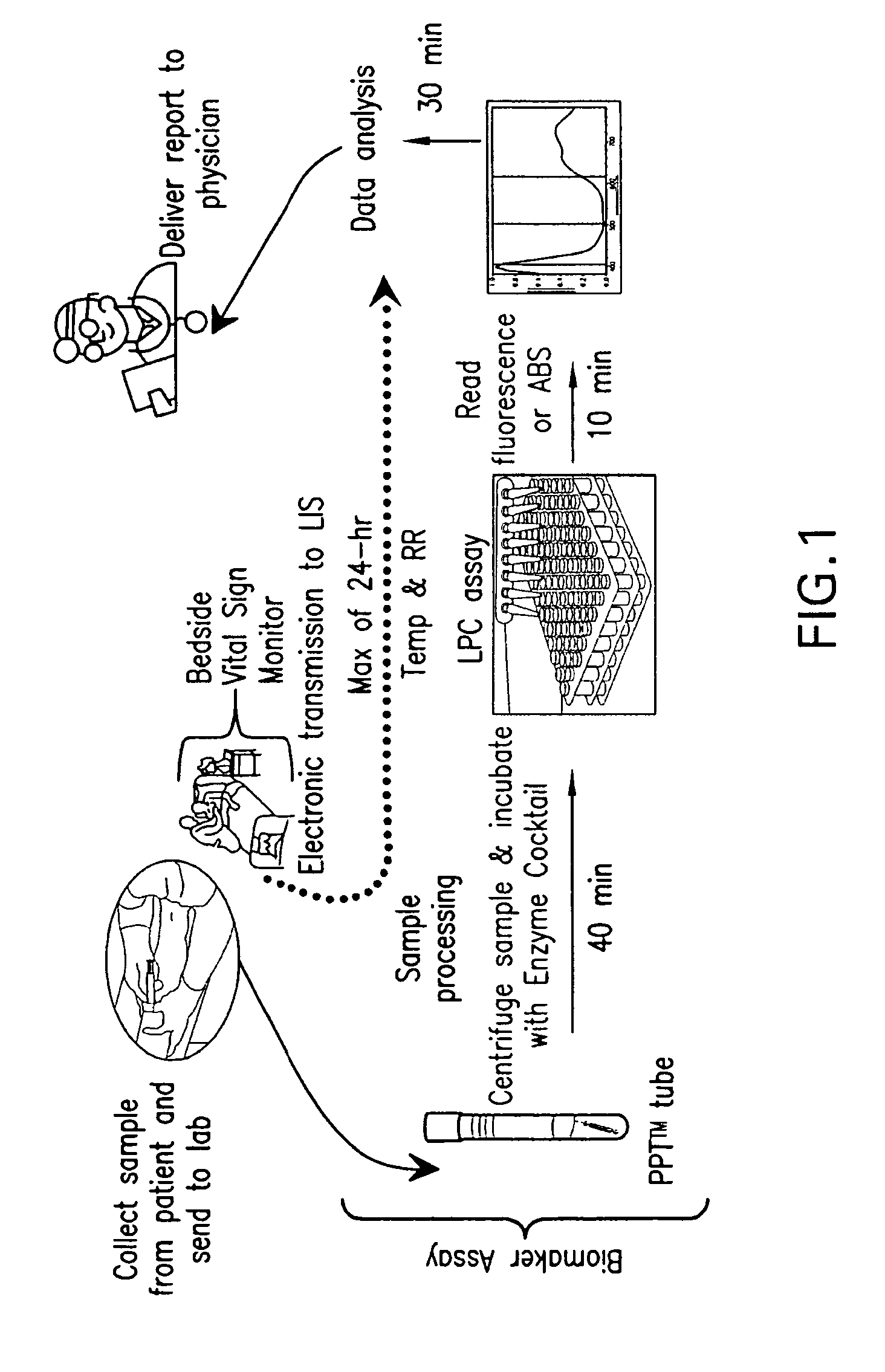
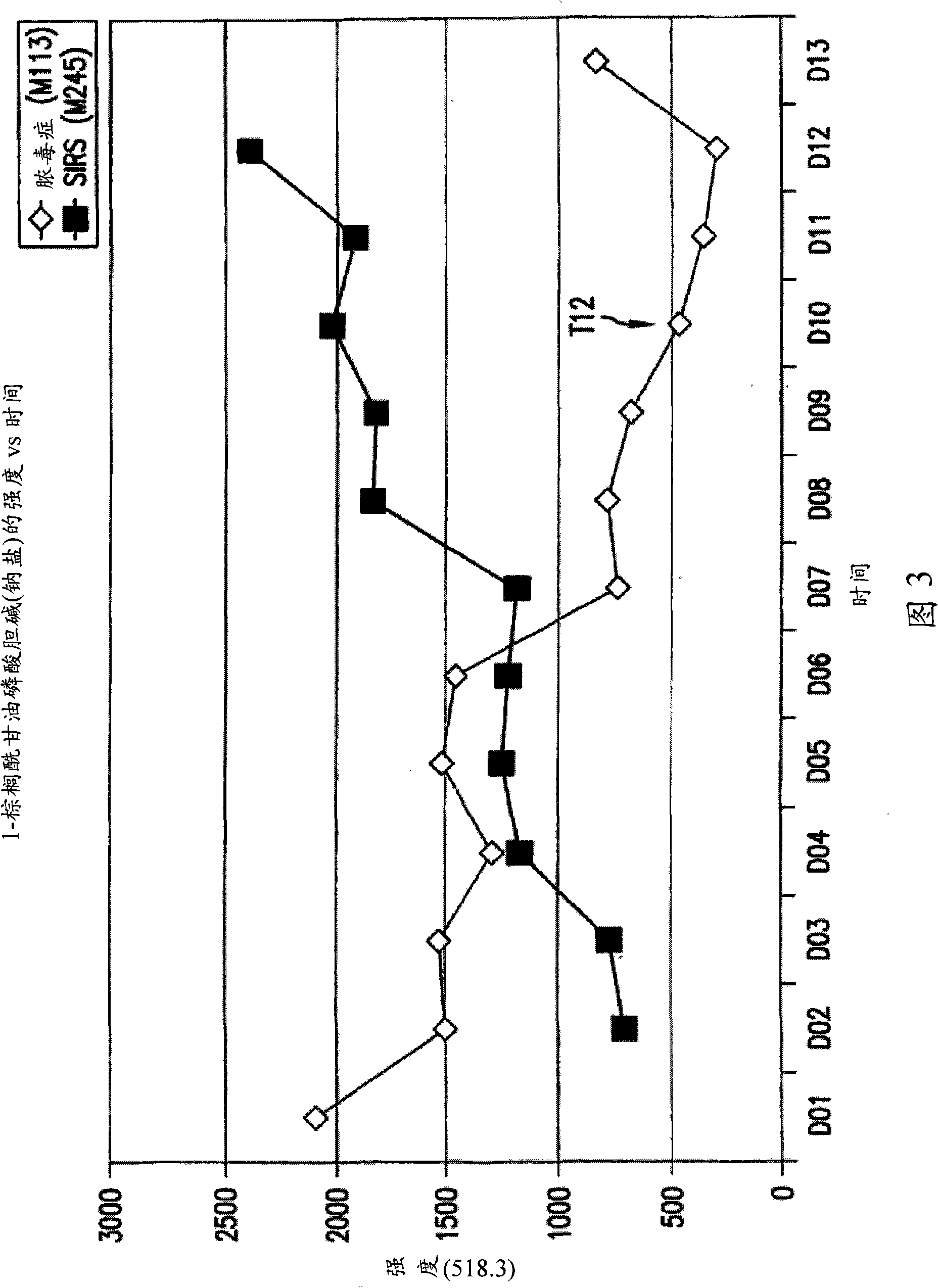
Detection of lysophosphatidylcholine for prognosis or diagnosis of a systemic inflammatory condition
ActiveUS20070111316A1Increased riskReduce riskMicrobiological testing/measurementPhosphorous compound active ingredientsPathologyLysophosphatidylcholine
The present invention provides methods and compositions useful for the diagnosis or prognosis of a systemic inflammatory condition such as sepsis.
Owner:BECTON DICKINSON & CO
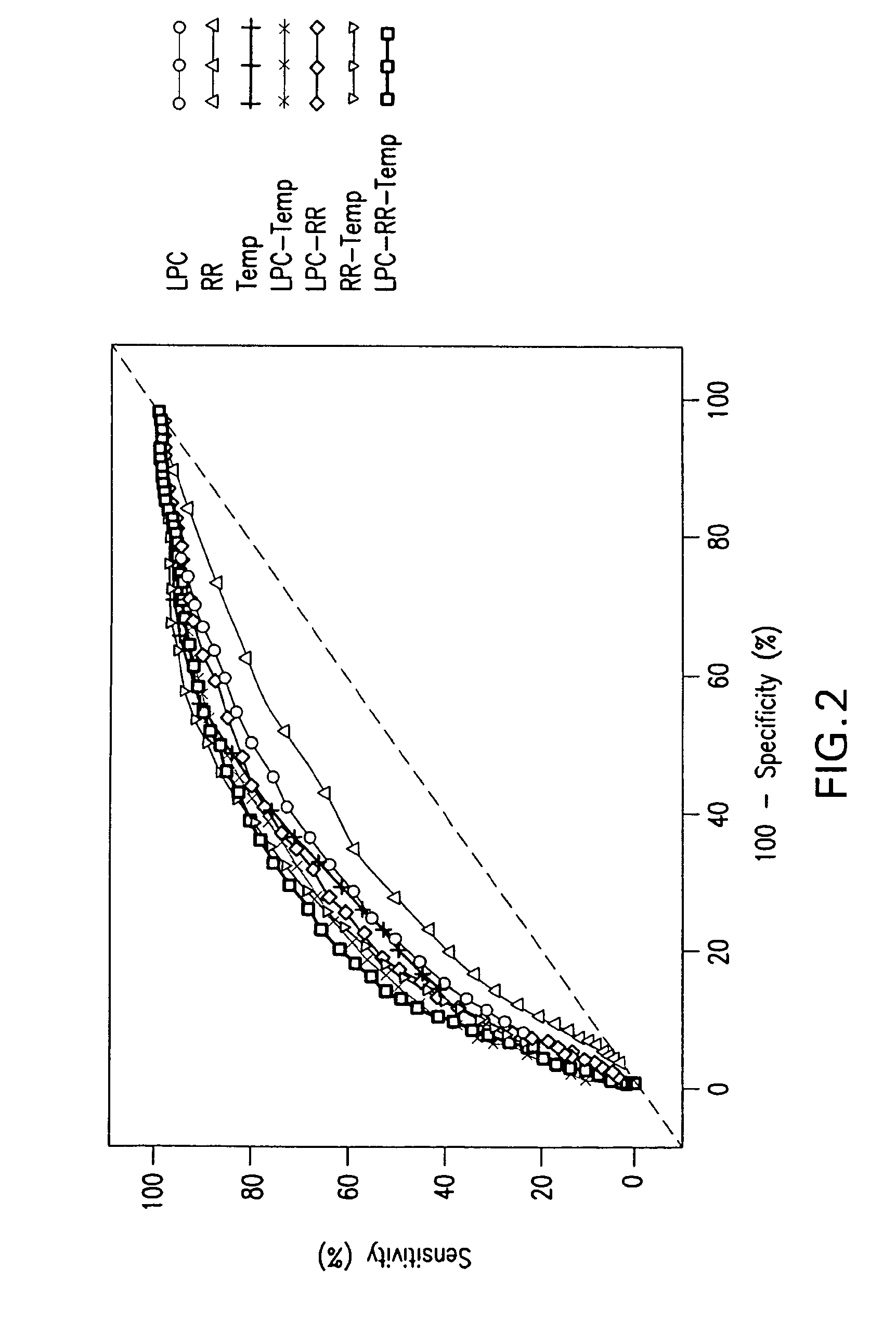
Advanced Detection of Sepsis
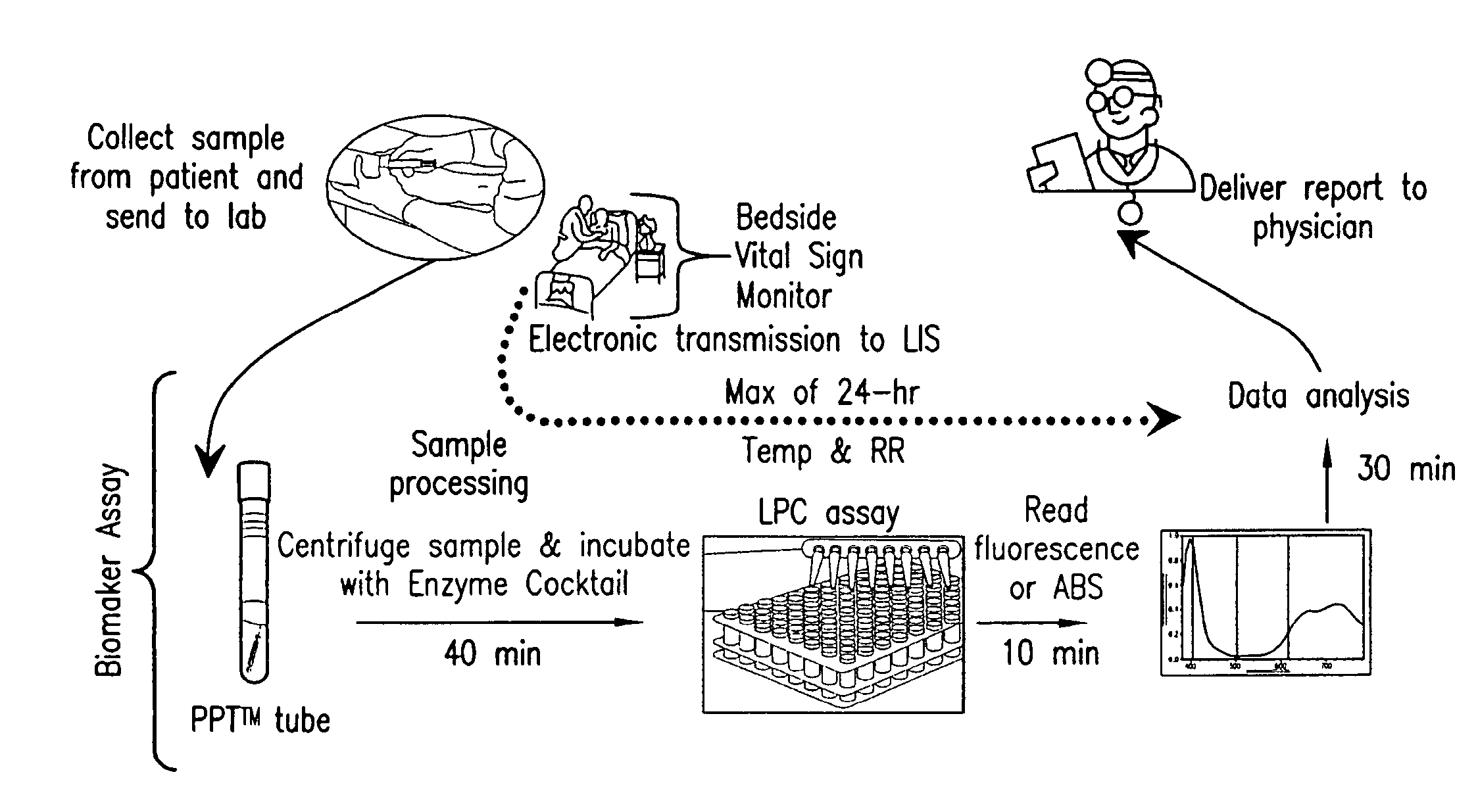
ActiveUS20110118569A1Raise the possibilityBioreactor/fermenter combinationsElectrolysis componentsPhospholipinClinical marker
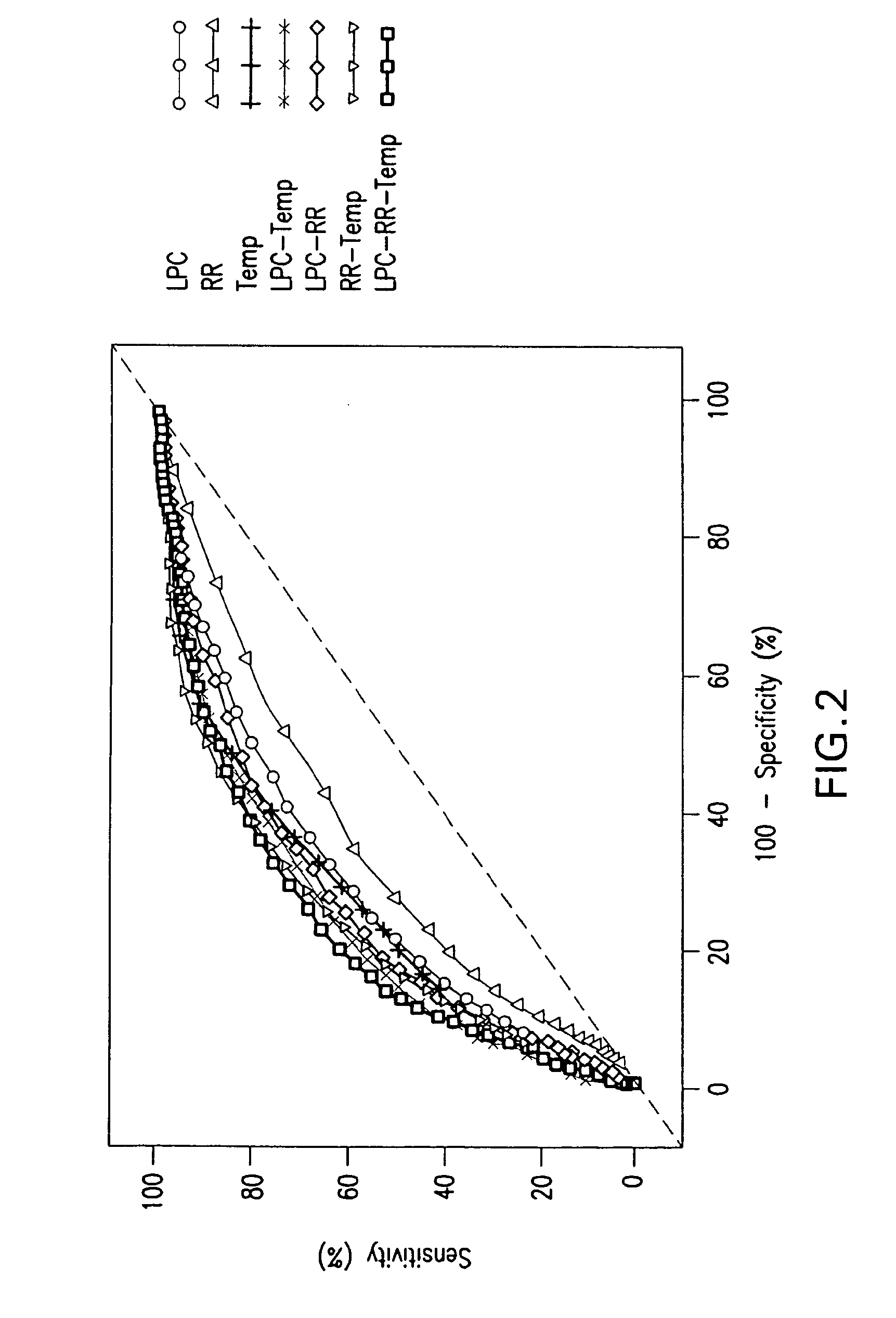
The present invention relates to methods, monitors and systems measuring lysophosphatidylcholine, its derivatives and / or procalcitonin as well as at least one clinical marker (e.g. temperature or respiration rate) and / or at least one biomarker for the early detection of sepsis in a subject.
Owner:BECTON DICKINSON & CO
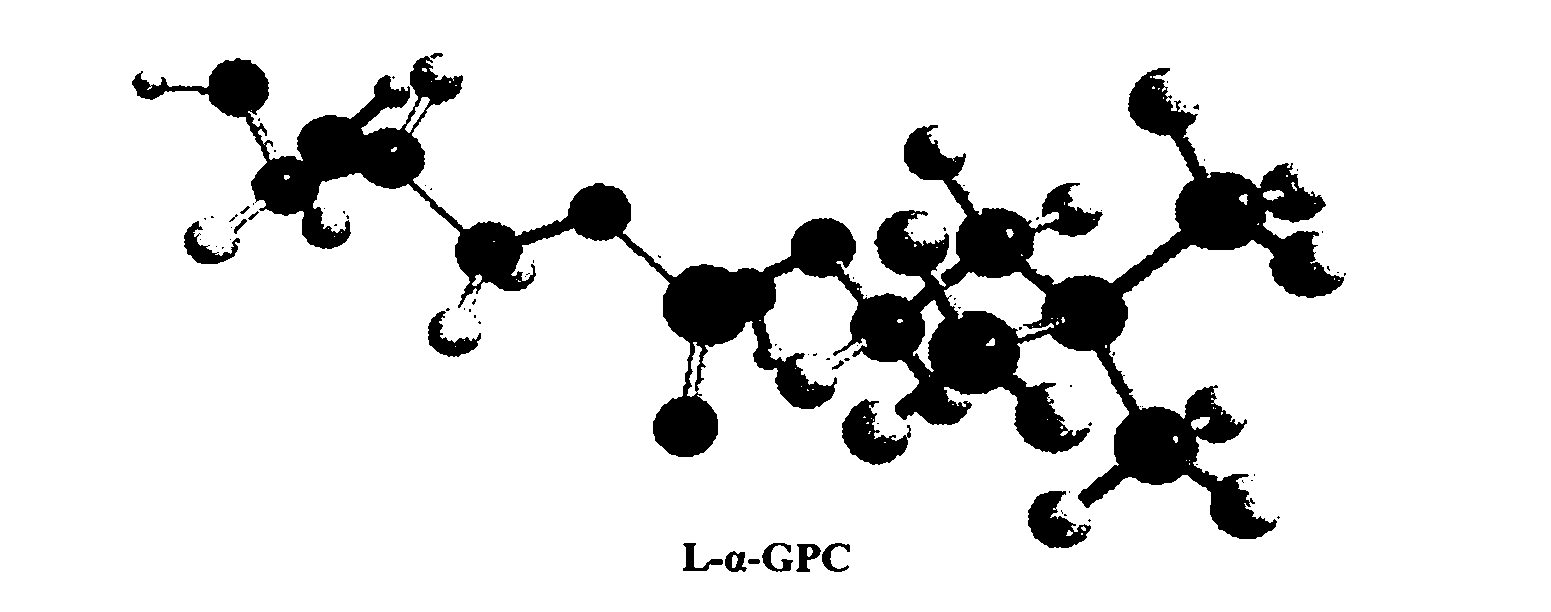
Method for separating and purifying L-alpha-glycerophosphorylcholine (L-alpha-GPC) by silica gel column chromatography
ActiveCN102093410ALow costGuaranteed optical activityFermentationPhosphorus organic compoundsChromatographic separationPurification methods
The invention discloses a method for separating and purifying L-alpha-GPC by silica gel column chromatography, which belongs to the technical field of lipid development and application and comprises the following steps: removing Ca<2+> and Cl<-> from enzymatic hydrolysis reaction solution serving as a raw material by using ion exchange resin, converting a water phase into an alcohol phase, separating L-alpha-GPC from glycerol polyglycidyl ether (GPE), lysophosphatidylcholine (LPC) and other byproducts, decolorizing by active carbon, and dewatering through vacuum concentration to obtain a colorless and transparent product; directly passing alcoholysis reaction solution serving as a raw material through a silica gel column for separation, removing Na<+> by cation exchange resin, decolorizing by active carbon, and dewatering through vacuum concentration to obtain a product. According to the test of the indexes of the product, the chemical purity is 99.6 percent, the optical purity ee is 99 percent, and the melting point (mp) is 142 to 143 DEG C (wherein C is equal to 2.6, the H2O content is 16 percent, and the pH value is 5.8). The invention provides a new way of thought and a new method for separating and purifying L-alpha-GPC, and realizes the application of the silica gel column chromatographic separation and purification method in lipid science.
Owner:JIANGNAN UNIV +1
Mixtures of and methods of use for polyunsaturated fatty acid-containing phospholipids and alkyl ether phospholipids species
Mixtures of natural phosphatidylcholine species, natural lysophosphatidylcholine species, phosphatidylserine species, phosphatidylethanolamine species, 1-hydroxy-2-acyl-phosphatidylcholine species, 1-hydroxy-2-acyl-phosphatidylserine molecular species, 1-hydroxy-2-acyl-phosphatidylethanolamine molecular species, 1-O-alkyl-2-hydroxy phosphatidylcholine species, 1-O-alkyl-2-docosaheaxnoyl phosphatidylcholine species 1-O-alkyl-2-docosahexaenoyl phosphatidylserine species, and 1-O-alkyl-2-docosahexaenoyl phosphatidylethanolamine species, Methods using the above disclosed mixtures in mammals to treat various conditions.
Owner:CHEN SU +1
Detection of lysophosphatidylcholine for prognosis or diagnosis of a systemic inflammatory condition
ActiveUS8183050B2Increased riskReduce riskSamplingMicrobiological testing/measurementPathologyLysophosphatidylcholine
The present invention provides methods and compositions useful for the diagnosis or prognosis of a systemic inflammatory condition such as sepsis.
Owner:BECTON DICKINSON & CO
Lipid nanomaterial containing lysophosphatidylcholine or derivative thereof and method for preparing same
ActiveUS20160022711A1Easy to usePromote aggregationAntibacterial agentsOrganic active ingredientsDiseasePhospholipid
The present invention relates to a lipid nanomaterial containing lysophosphatidylcholine or an ether derivative thereof, a method for preparing the same, a method for reducing erythrocyteolysis and hemagglutination using the compound, and a pharmaceutical agent containing the lipid nanomaterial. The present invention contains lysophosphatidylcholine or an ether derivative thereof as an active ingredient, and can be useful as a treatment agent for septicemia, bacterial infection diseases, and the like.
Owner:ARIBIO CO LTD
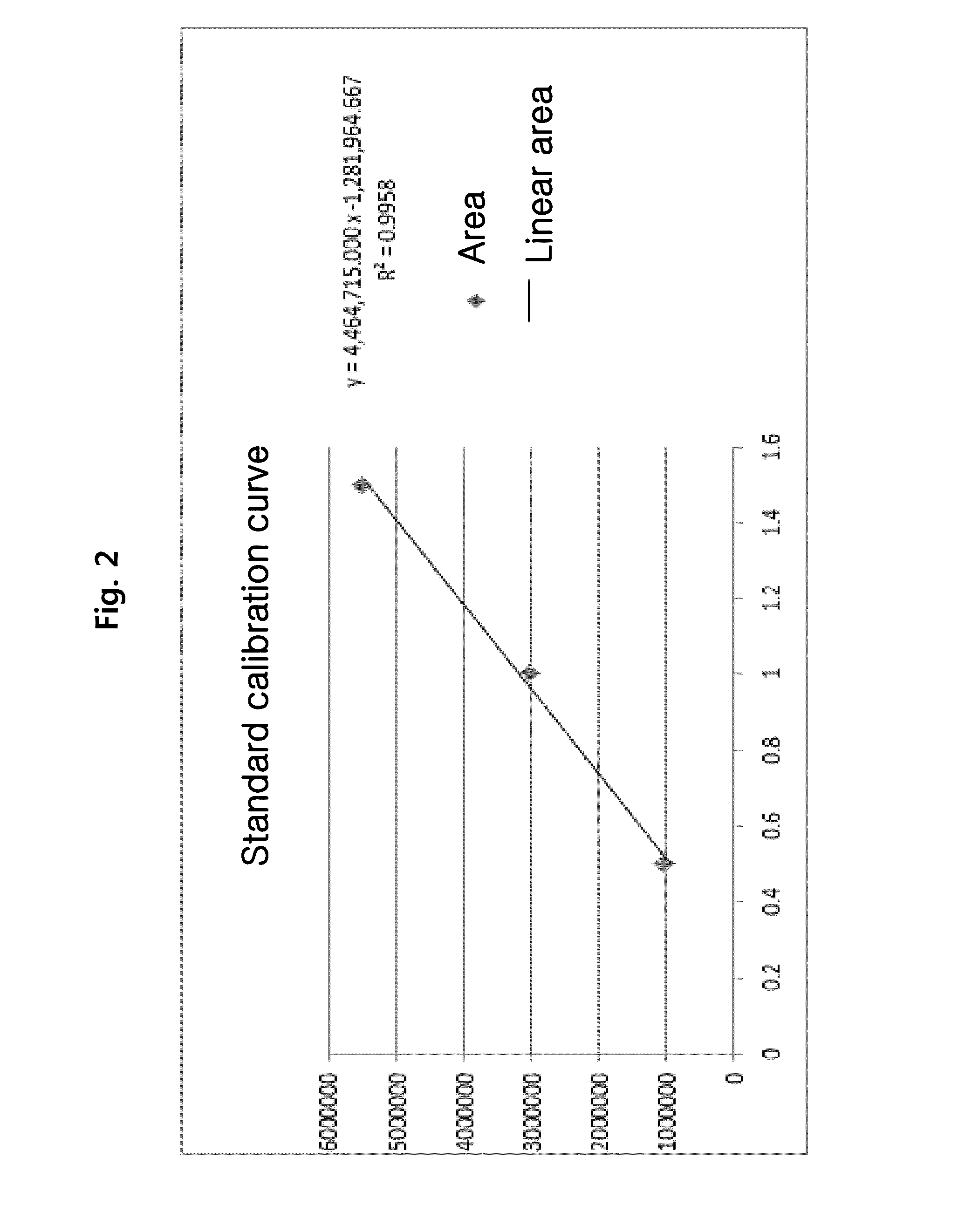
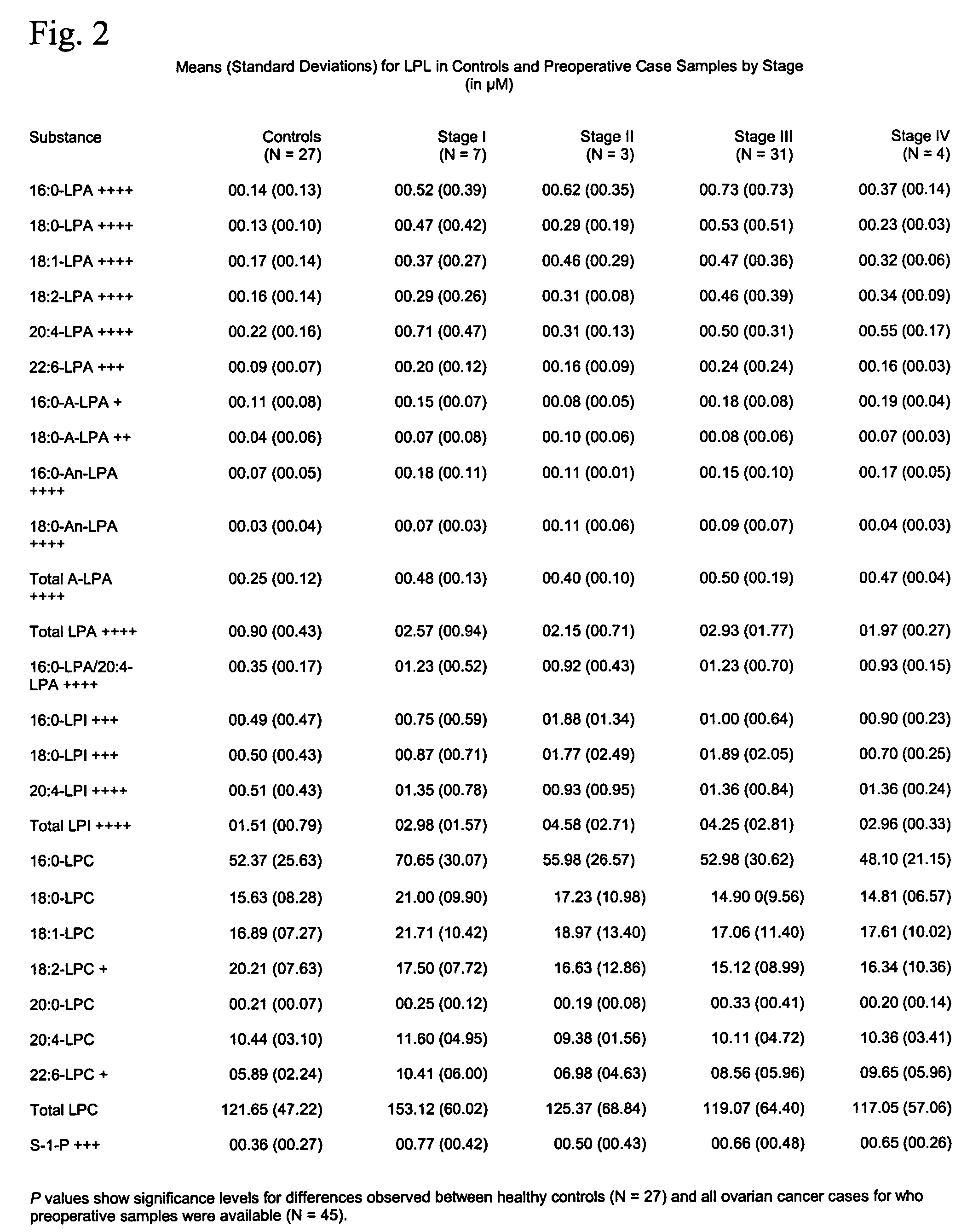
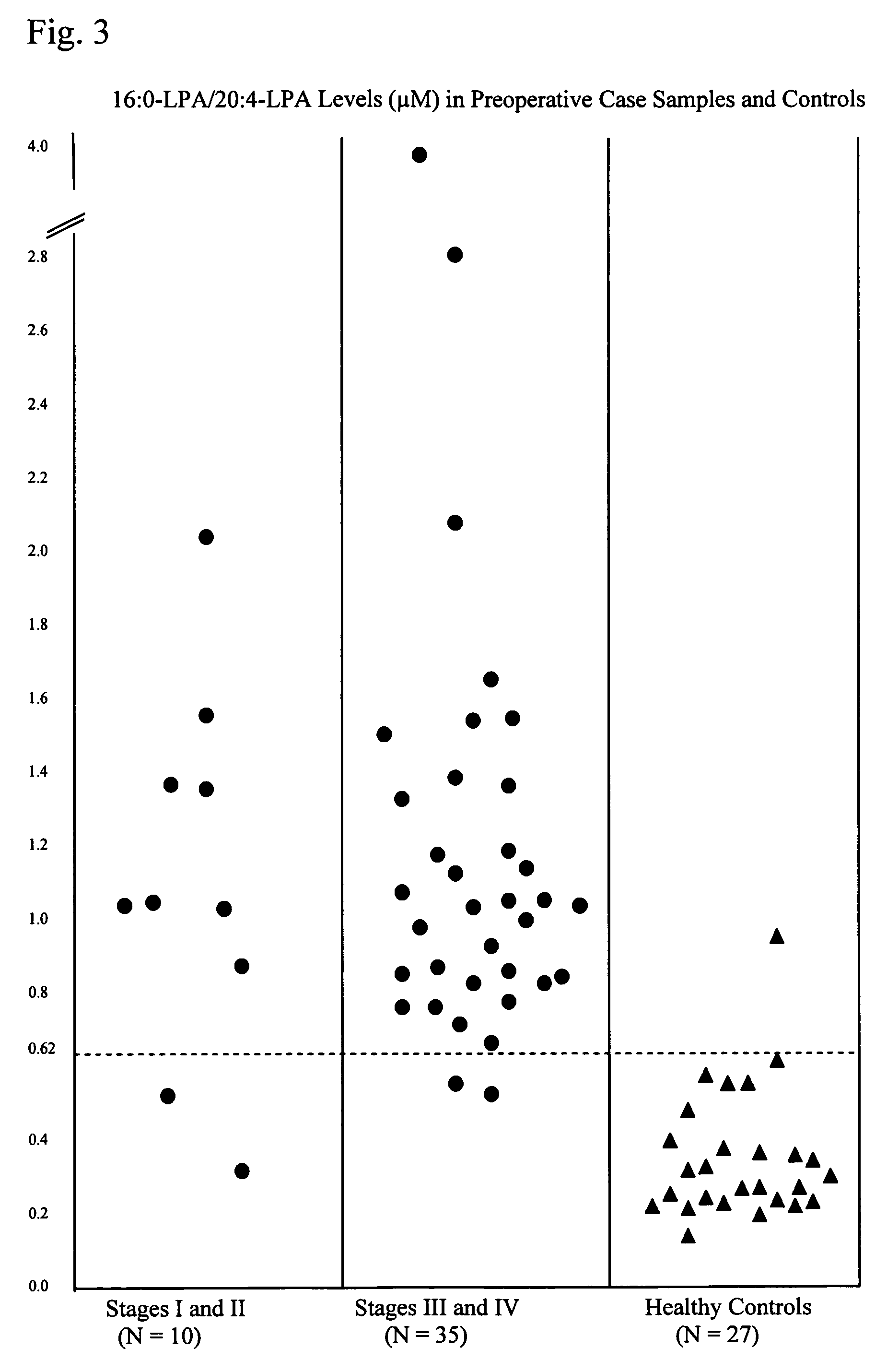
Lysophospholipids as biomarkers of ovarian cancer
InactiveUS7964408B1Improve survivalIncreased mortalityMicrobiological testing/measurementMass spectrometric analysisPhosphateSubspecies
A method of using a bioactive lysophospholipid (LL) as a biomarker for detecting the presence and recurrence of ovarian cancer. Subspecies of LL, such as lysophosphatidic acid (LPA), lysophosphatidylinositol (LPI), lysophosphatidylcholine (LPC), and lysosphingolipid sphinsosine-1-phosphate (S1P), are used alone or in conjunction to increase the specificity and sensitivity of the assay.
Owner:UNIV OF SOUTH FLORIDA
Advanced detection of sepsis
ActiveUS8669113B2Raise the possibilityBioreactor/fermenter combinationsElectrolysis componentsClinical markerBiomarker (petroleum)
The present invention relates to methods, monitors and systems measuring lysophosphatidylcholine, its derivatives and / or procalcitonin as well as at least one clinical marker (e.g. temperature or respiration rate) and / or at least one biomarker for the early detection of sepsis in a subject.
Owner:BECTON DICKINSON & CO
Method for preparing high-content phosphatidyl ethanolamine
ActiveCN104592293AAchieve decolorizationTo achieve impurity removalPhosphatide foodstuff compositionsAlkaneHplc method
The invention discloses a method for preparing high-content phosphatidyl ethanolamine. The method comprises the following steps of dissolving a cephalin crude product as a raw material with an organic solvent, eluting twice with a ternary mixed solvent comprising polyhalogenated alkanes, lower alcohol and water by column chromatography and drying to obtain the phosphatidyl ethanolamine product, wherein the obtained phosphatidyl ethanolamine product is high in content (96.0%-99.9% obtained by an HPLC method), low in content of impurities (in which the content of phosphatidylcholine is less than 0.5%, the content of lysophosphatidylcholine is less than 0.5 %, the content of free fatty acid is less than 0.2%, and the content of triglyceride is less than 0.2%), low in oxidation indexes (of which the acid number is less than 1.0, the peroxide value is less than 1.0 and the anisidine value is less than 5.0), and the product quality meets the quality requirements of drug phospholipid for injecting.
Owner:GUANGZHOU HANFANG PHARMA
Receptor for lysophosphatidylcholine in vascular endothelial cells and use thereof
InactiveUS20060067931A1Many functionsInhibit bindingImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsVascular endotheliumBlood vessel
Owner:RUSH UNIV MEDICAL CENT
A method for preparing lysophosphatidylcholine by enzymatic alcoholysis
InactiveCN102277393AImprove conversion rateImprove solubilityFermentationPhosphoric Acid EstersPhosphoric acid
The invention discloses a method for preparing lysophosphatidyl choline by enzymatic alcoholysis, which comprises: adding phosphatidylcholine into low-carbon alcohol solution, uniformly stirring and mixing at a certain temperature, adding lipase, and allowing the lipase to catalyze the alcoholysis of phosphatidylcholine with constant-temperature stirring to obtain the lysophosphatidyl choline. The method has the advantages that: (1) the solubility of phosphatidylcholine, lysophosphatidyl choline serving as the product, and aliphatic ester, glycerophosphorylcholine and very small amount of fatty acid, which serve as byproducts, in the reaction system is improved, and the conversion rate of the lysophosphatidyl choline is high; (2) the aliphatic ester, a small amount of glycerophosphate and a very small amount of fatty acid are generated in an alcoholysis reaction process, but the pH value of the system is not changed, and the influence of the solvent on the activity of the lipase is relieved and the catalytic reaction activity of the lipase is high; (3) the properties of the fatty acid, the aliphatic ester and glycerophosphorylcholine, which are products of side reactions, are very different from those of the lysophosphatidyl choline, so the lysophosphatidyl choline product can be separated and purified very easily; and (4) the alcohol, which is the product of the reaction, can be removed easily.
Owner:HENAN UNIVERSITY OF TECHNOLOGY
Method for predicating metabolizable energy level of pig diets by metabonomics technology
ActiveCN103245747AMeet nutritional needsIncrease productivityComponent separationNutritional statusAnimal science
The invention discloses a method for predicating the metabolizable energy level of pig diets by a metabonomics technology. The method is characterized in that the relative content of one to seven of eight plasma metabolic markers of a pig to be tested is plugged in equations with one to seven unknown quantities established in the invention to obtain the predicted value of metabolizable energy of the diets of the pig to be tested, wherein the plasma metabolic markers include lysophosphatidylcholine (18:3 / 0:0), myristoyl lysophosphatidylcholine (14:0 / 0:0), glycerophosphorylethanolamine (18:0 / 0:0), lysophosphatidylcholine (20:4 / 20:4), proline, phosphatidylcholine (18:2 / 0:0), arginylphenylalanylarginine and glycerophosphorylethanolamine (18:1(9Z) / 0:0), and the relative deviation between the predicted value obtained from the equation with seven unknown quantities and an actual value does not exceed + / -1 percent. The method has significant theoretical and practical significances for evaluating the nutritional status and optimizing the formula in time, meeting the nutritional requirements of pigs under physiological and pathological conditions in different environments and improving the production efficiency of pigs.
Owner:NINGBO TECH BANK
Method for preparing solubilized composition containing oil-soluble substance
ActiveUS20080070992A1Improve acid resistanceImprove heat resistanceBiocideCosmetic preparationsSucroseEthyl acetate
The present invention provides a method for preparing a solubilized composition containing an oil-soluble substance having both acid and heat resistance, including:the step of dissolving an oil-soluble substance and two or three emulsifiers selected from(1) an emulsifier E1 comprising an ester of a fatty acid having an HLB of not less than 10 and not more than 14 carbon atoms with a polyglycerol having a polymerization degree of not less than 3,(2) an emulsifier E2 comprising an ester of a fatty acid having an HLB of not less than 10 and not more than 14 carbon atoms with sucrose, or(3) an emulsifier E3 comprising lecithin in which phosphatidylcholine accounts for not less than 50% and / or lysolecithin in which lysophosphatidylcholine accounts for not less than 50% of a phospholipid content in (a) ethanol or (b) a mixed solvent of ethanol with at least one selected from the group consisting of acetone, hexane, and ethyl acetate to prepare a transparent solution; andthe step of distilling the solvent off from the transparent solution.
Owner:TSUJI SEIYU
Method and Use of Metabolites for the Diagnosis of Inflammatory Brain Injury in Preterm Born Infants
The present invention relates to novel biomarkers for predicting the likelihood of inflammation-related brain injury in preterm born infants, using a plurality of endogenous target metabolites selected from the group consisting of acyl carnitins, diacylphosphatidylcholines, acyl-alkylphosphatidylchoines, lysophosphatidylcholines and amino acids.
Owner:INFANDX
Application of endogenous small-molecular substances in rapid detection of hepatotoxicity
The invention discloses a metabonomics-based application of endogenous small-molecular substances in rapid detection of hepatotoxicity. The endogenous small-molecular substances are 12 hepatotoxicity biomarkers, i.e., L-alanine, L-phenylalanine, L-carnitine, L-carnitine palmitoyl, tryptophan, arachidonate, cholic acid, lysophosphatidylcholine (14:0), lysophosphatidylcholine (16:1), lysophosphatidylcholine (18:2), lysophosphatidylcholine (20: 3) and hemolysis phosphatidyl ethanolamine (18:2); and the endogenous small-molecular substances also refers to 3 special hepatotoxicity biomarkers, i.e. L-carnitine, cholic acid and lysophosphatidylcholine (14:0). By adopting the hepatotoxicity biomarkers, the discovery and diagnosis of the liver injury can be earlier than those of the existing biochemical detection indexes, the hepatotoxicity biomarkers can be well used for preventing, discovering and treating the hepatotoxicity, the limitation of the existing indexes can be overcome, and accurate detection information can be provided in time.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
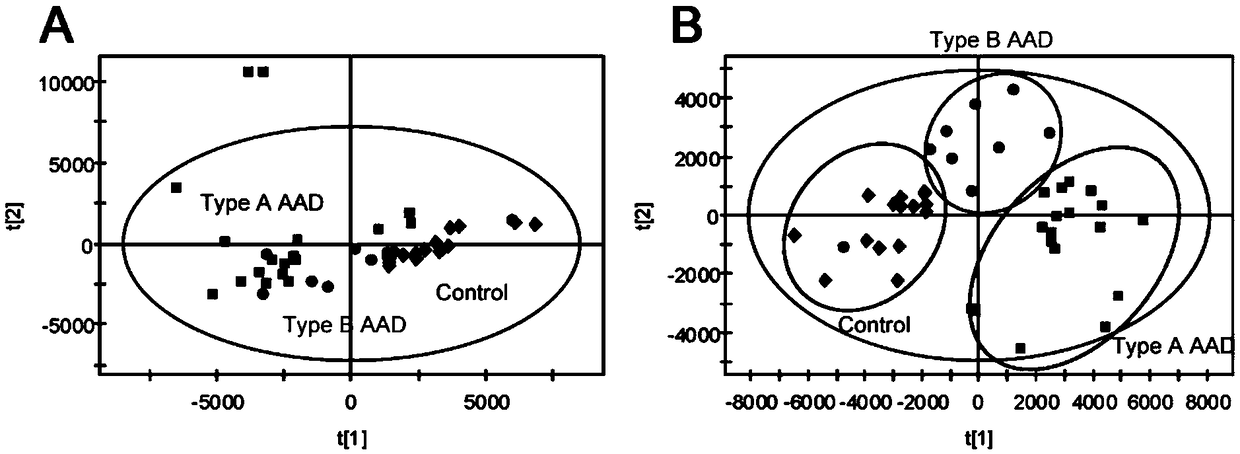
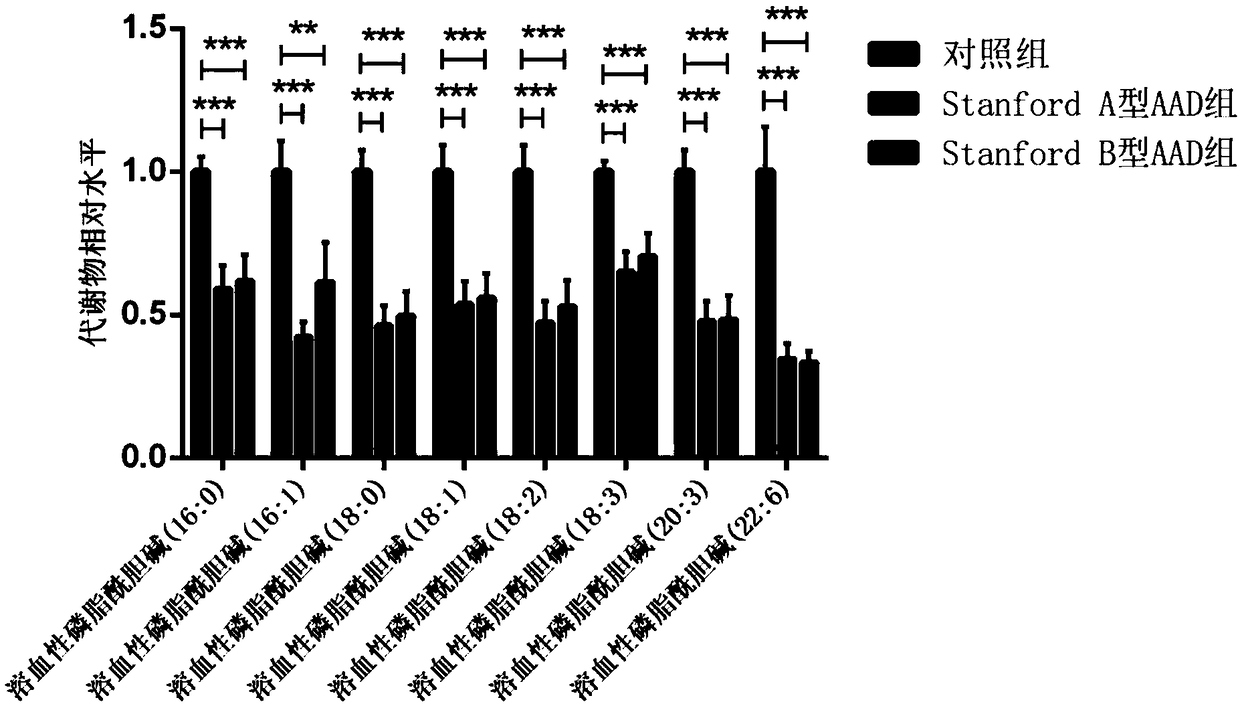
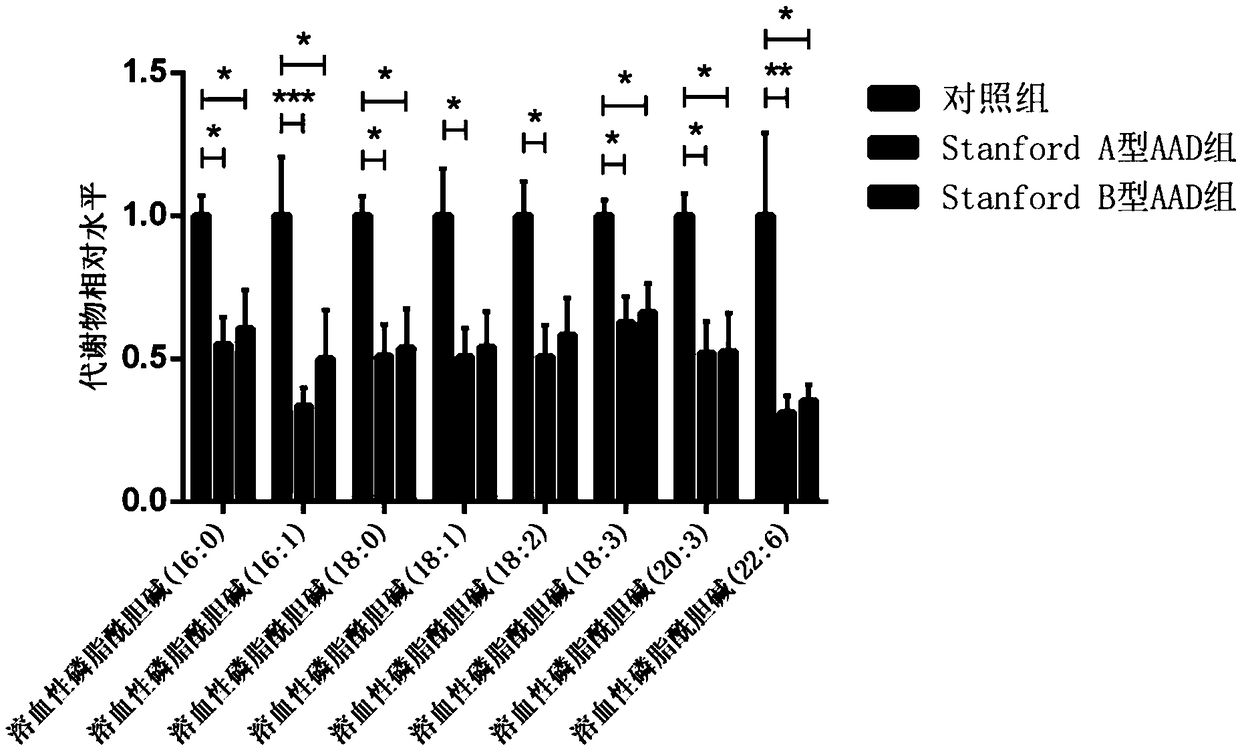
Method for establishing diagnostic criterion of acute aortic dissection
The invention discloses a method for establishing a diagnostic criterion of acute aortic dissection. The method includes the following steps: selecting metabolites such as lysophosphatidylcholine andsphingolipids as variables or elements of mathematical statistics; collecting serum samples of subjects satisfying statistical quantity requirements; separately detecting the concentration or contentof the metabolites in each of the serum samples; and performing statistics and data processing on the variables or elements, and the concentration or content by utilizing a mathematical statistics method to obtain mathematical models used for judging whether it belongs to the acute aortic dissection and whether an acute aortic dissection positive result belongs to a Stanford A type or a Stanford Btype, and used as the diagnostic criterion for thr acute aortic dissection. The invention also discloses a kit used for acute aortic dissection diagnosis.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
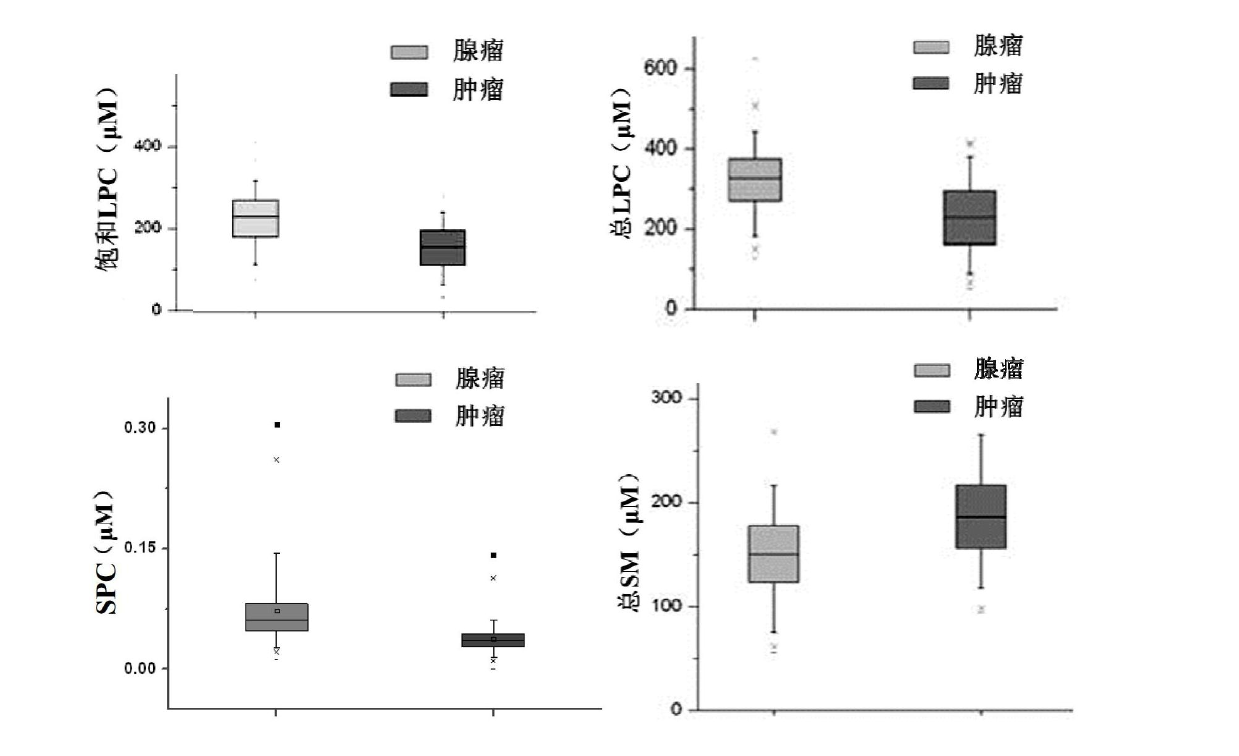
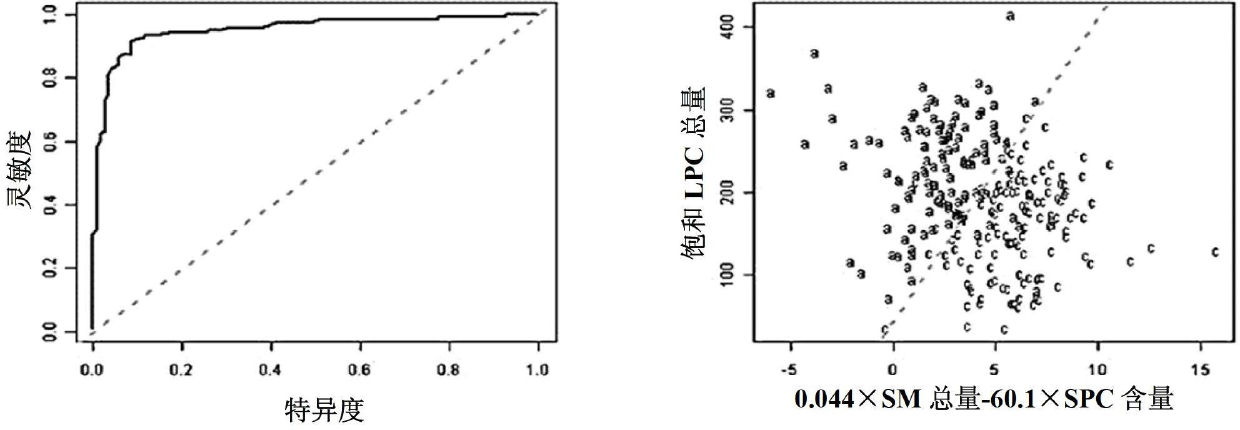
Kit for distinguishing colorectal adenomas from colorectal cancer
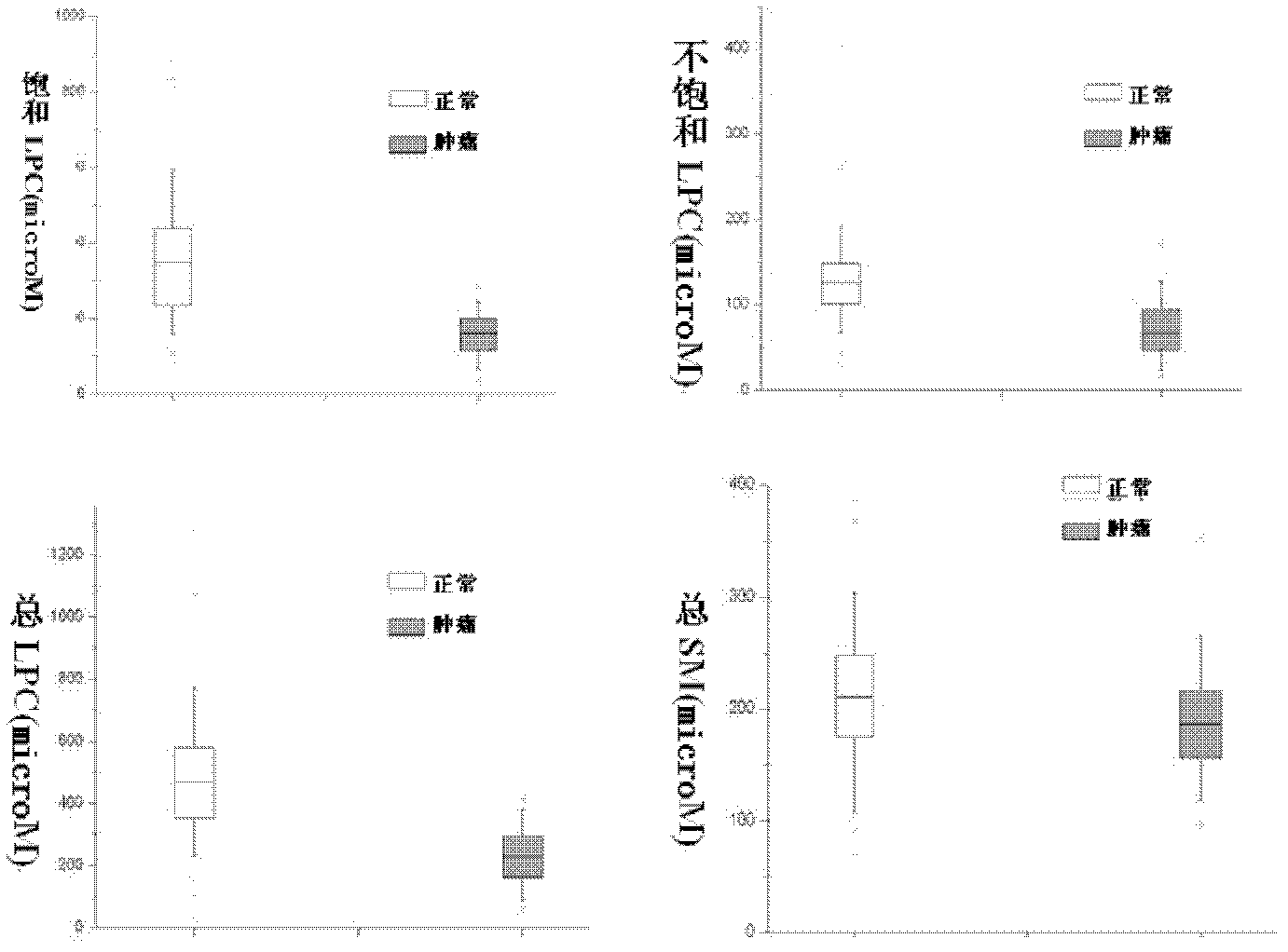
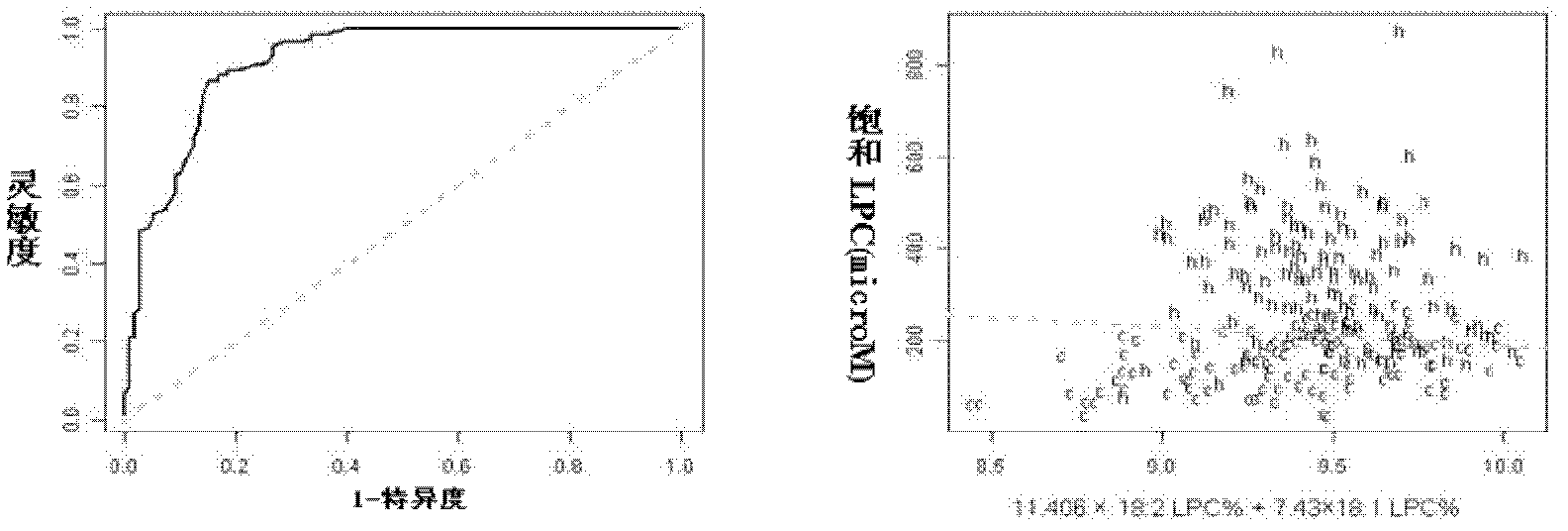
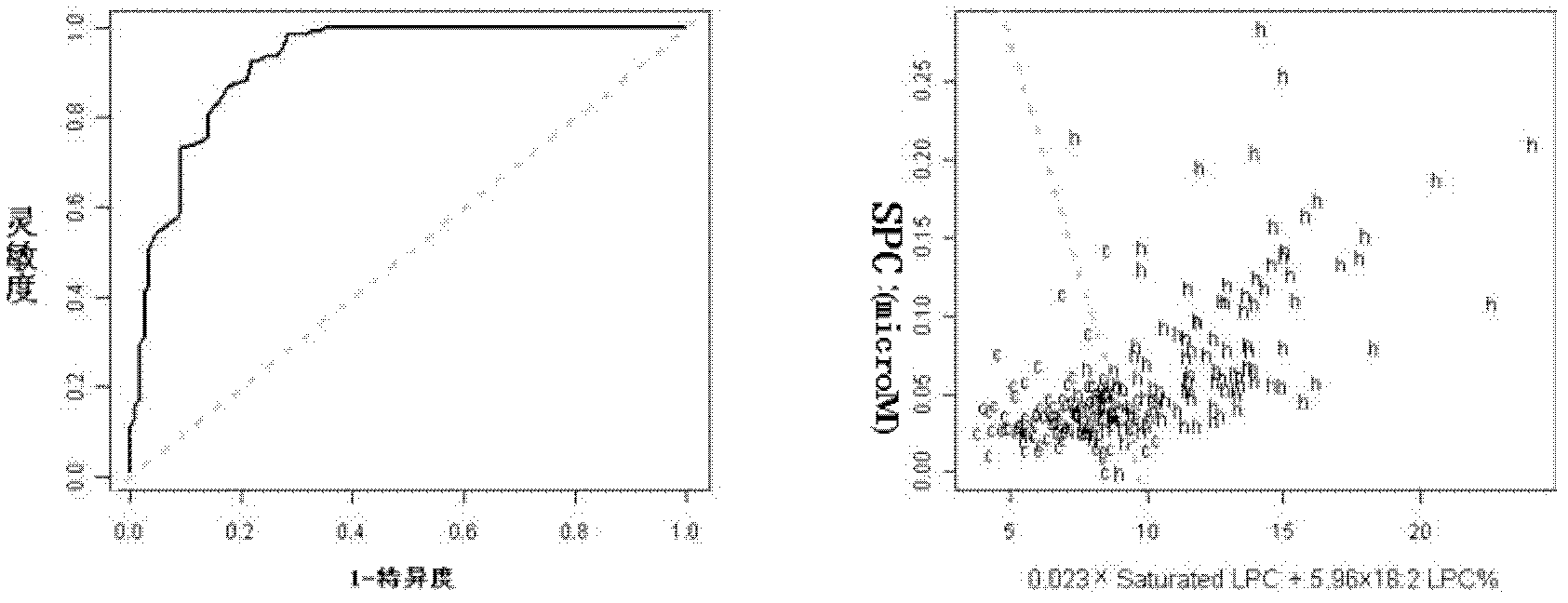
The invention discloses a kit for distinguishing colorectal adenomas from colorectal cancer. The kit for distinguishing or assisting in distinguishing the colorectal adenomas from the colorectal cancer comprises a device for detecting phospholipids content and a contrast card established by the phospholipids content, wherein phospholipids are sphingosylphosphorylcholine (SPC), 16:0 sphingomyelin (SM), 18:0SM, 14:0 lysophosphatidylcholine (LPC), 16:0LPC, 18:0LPC, 20:0LPC and 22:0LPC. Saturated LPC, SPC and SM are taken as markers, and a molecular model for distinguishing the colorectal adenomas from the colorectal cancer is established, and has colorectal cancer distinguishing sensitivity of 90 percent and colorectal cancer distinguishing specificity of 93 percent.
Owner:BEIJING NORMAL UNIVERSITY
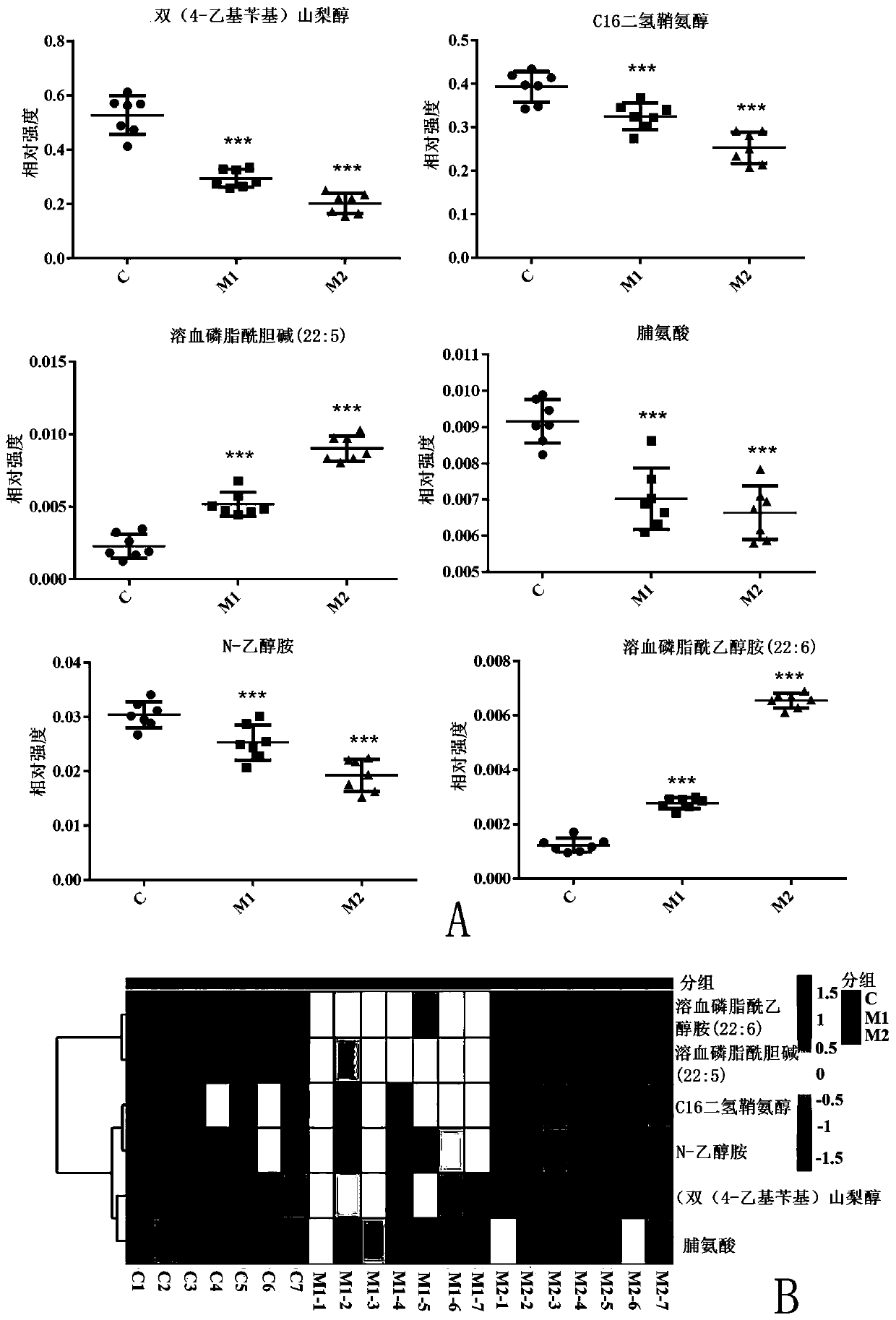
Nephrotic syndrome progression-related metabolic markers and application thereof
ActiveCN110286189AHigh sensitivityStrong specificityComponent separationDisease diagnosisCreatinine risePhosphoric acid
The present invention belongs to the technical field of screening and identification of nephrotic syndrome markers, and provides nephrotic syndrome progression-related metabolic markers and application thereof. Bis(4-ethylbenzyl)sorbitol, C16 d-sphinganine, lysophosphatidylethanolamine (LPE) (22:6), lysophosphatidylcholine (LPC) (22:5), N-ethanolamine and proline are taken as progressive markers; oleic acid amide, docosahexaenoic acid, 14S-hydroxy-docosahexaenoic acid, docosapentaenoic acid, uric acid, and LPC (20:5) and (18:1) are taken as early markers; and acylcarnitine (C18:0), linoleic acid carnitine, LPC (18:3), DL-tryptophan, indoleacrylic acid, p-cresol sulfate, 3-indol sulfate, cytosine, creatinine, sphingosine-1-phosphoric acid, L-aleuritic-dihydrosphingosine and ceramide (d18:0 / 14:0) are taken as late markers. The nephrotic syndrome progression-related metabolic markers provided by the present invention can be quantified, are fast, and have high sensitivity and strong specificity.
Owner:SHANXI UNIV
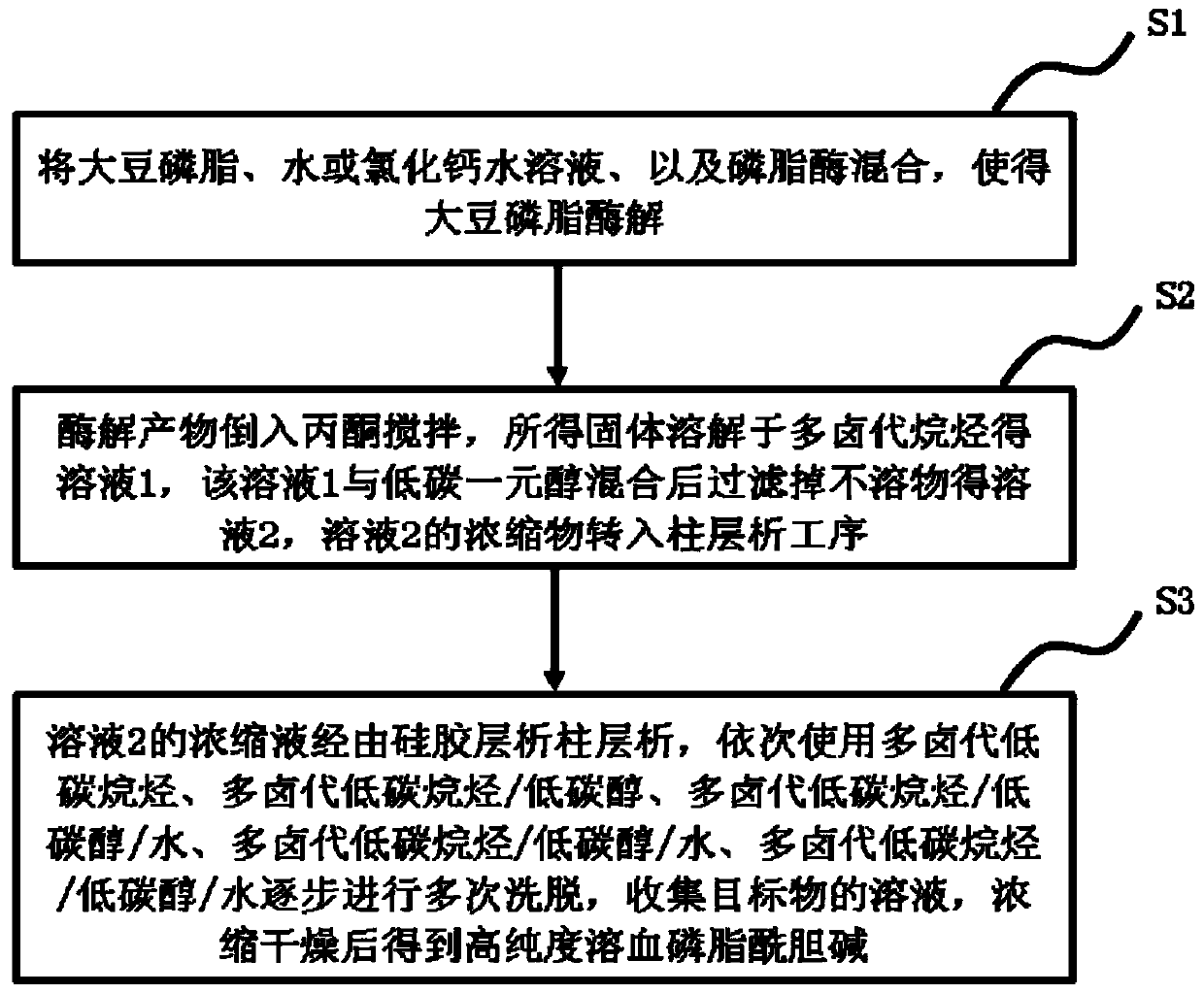
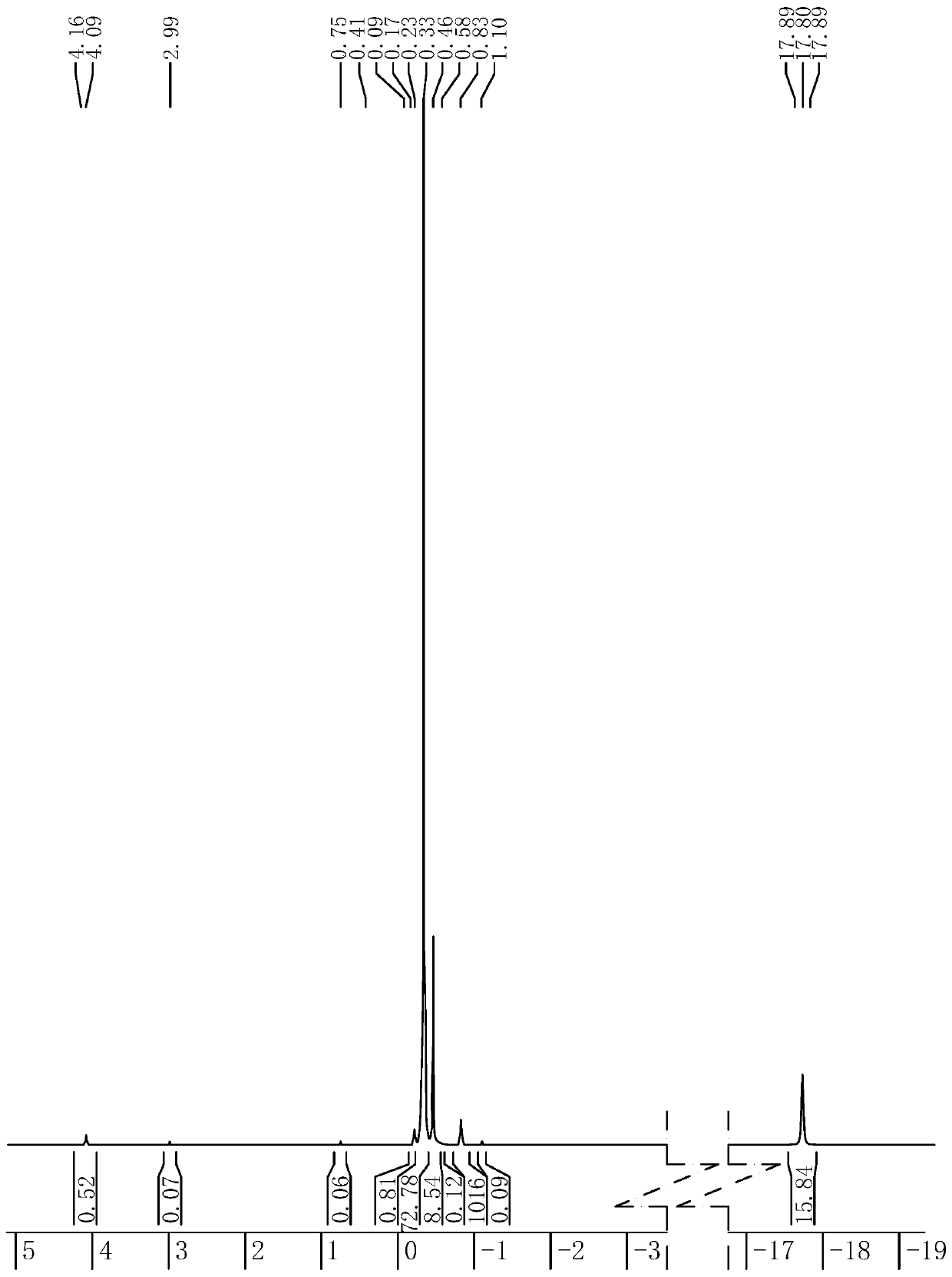
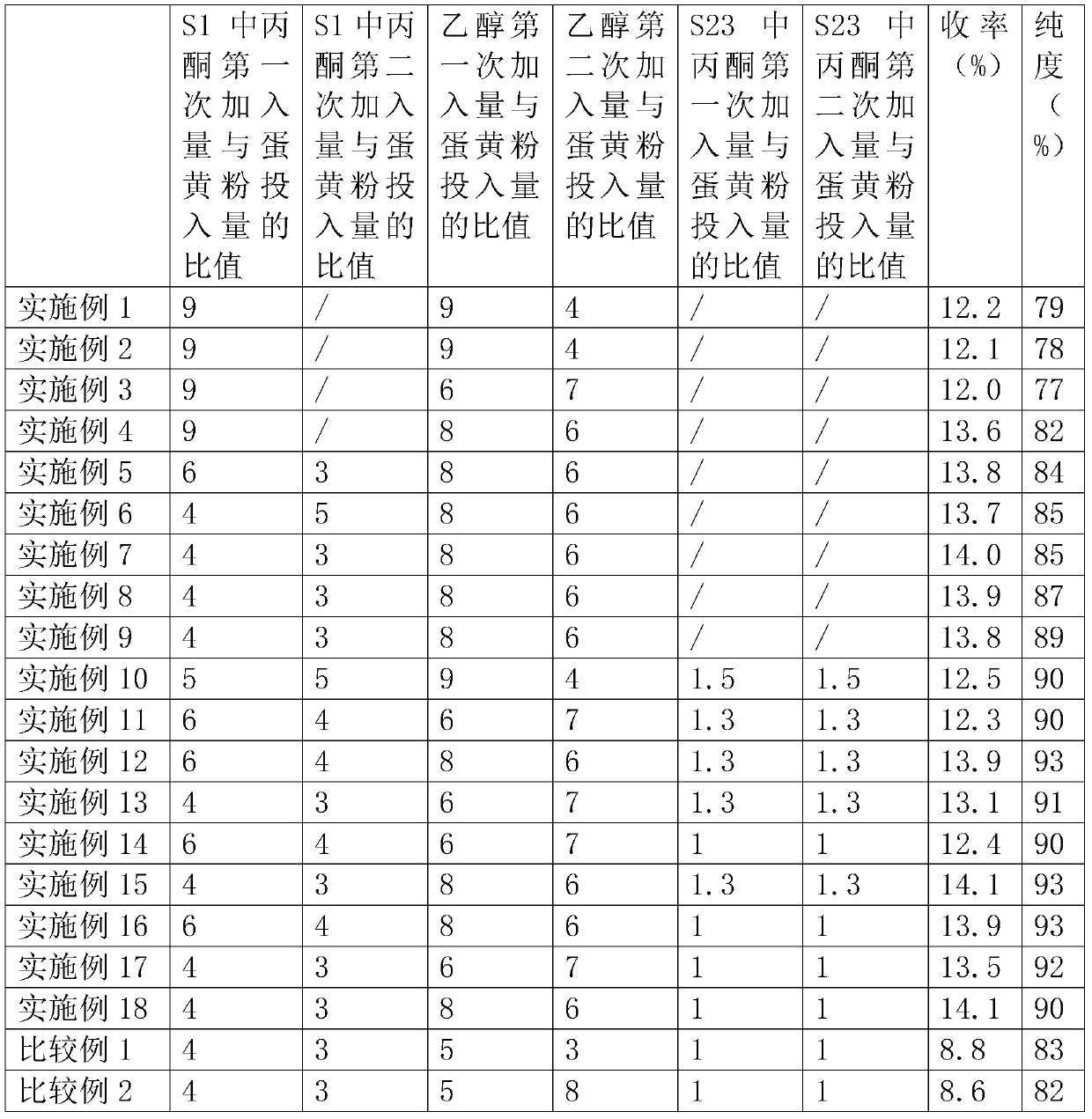
Method for preparing high-purity lysophosphatidylcholine
The invention discloses a method for preparing high-purity lysophosphatidylcholine. The method comprises the steps of enabling soybean phospholipid to dissolve in water or a calcium chloride solution,adding phospholipase, and performing an enzymolysis reaction; pretreating enzymolysis reaction products, conveying the pretreated enzymolysis reaction products into a silica gel column, performing repeated eluting by using multi-halogeno low-carbon alkane-low-carbon alcohol-water as an eluant, collecting a solution of an object, and performing concentration and drying so as to obtain high-puritylysophosphatidylcholine products. According to the method disclosed by the invention, ordinary soybean phospholipid is used as a raw material, through the enzymolysis reaction, pretreatment after enzymolysis and column chromatography and refiningtechnology, and the high-purity lysophosphatidylcholine is prepared; and the high-purity lysophosphatidylcholine has the characteristics that the raw materials are cheap and easy to obtain, the technology is simple, the maneuverability is high and product yield is high, is suitable for industrialized production, and the produced lysophosphatidylcholineis high in purity, few in by-products and stable in product quality.
Owner:杨利平
Phospholipid detection method and application
PendingCN110007032AQuality improvementEasy to separateComponent separationRetention timeAcetonitrile
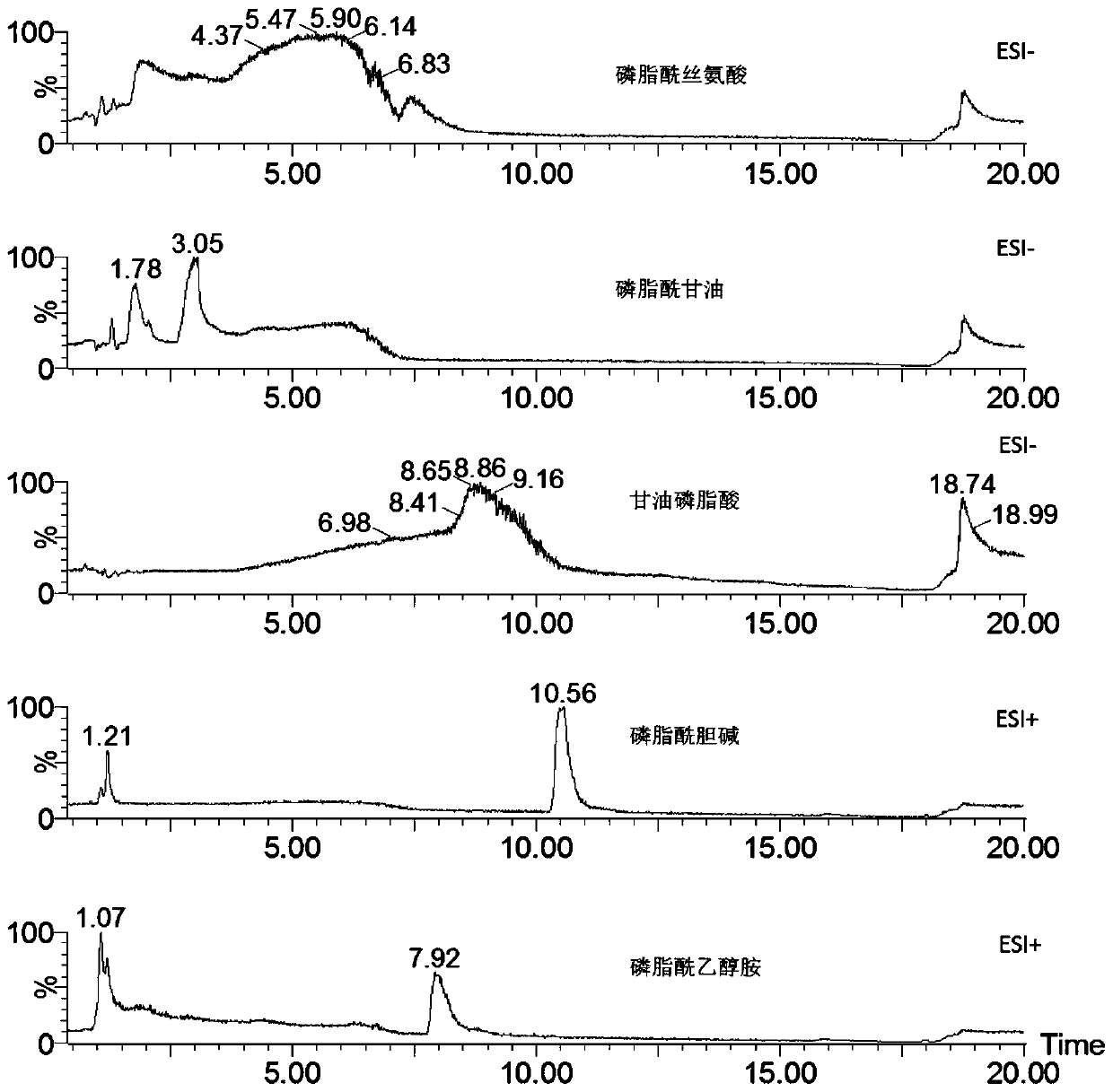
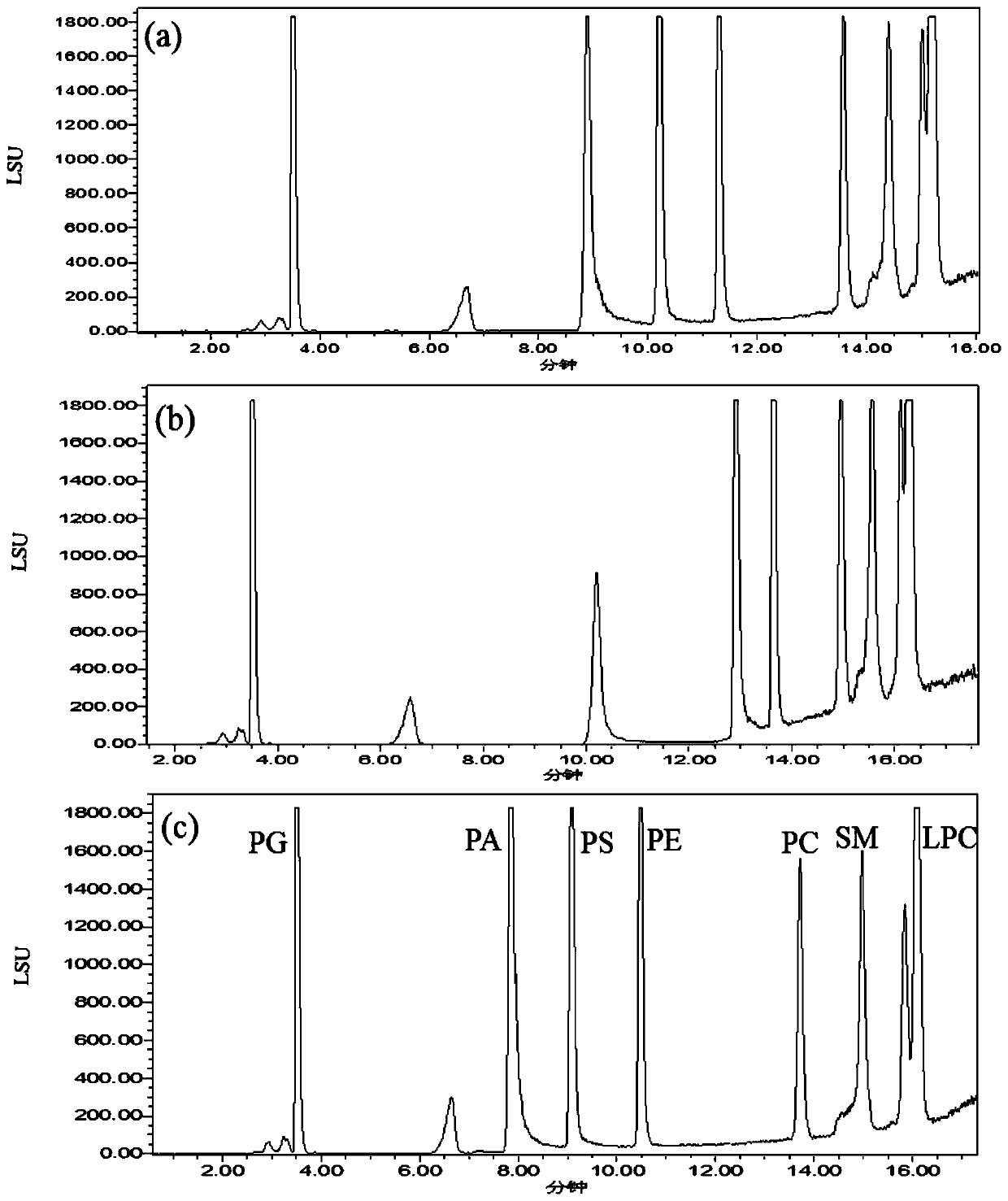
The invention discloses a phospholipid detection method and application. A phospholipid detection method is eluted with acetonitrile and ammonium acetate, and a phospholipid detection eluent comprisesacetonitrile and ammonium acetate, wherein by volume percentage, the acetonitrile accounts for 50% to 95%, and the ammonium acetate accounts for 5% to 50%. An application of the phospholipid detection method is used for detection of phosphatidylcholine, phosphatidylglycerol, phosphatidylserine, phosphatidyl ethanolamine, glycerophosphatidic acid, sphingomyelin and lysophosphatidylcholine. The analysis method provided by the invention has the advantages of simple operation, large number, good peak shape and short retention time of phospholipids analyzed, convenience and speed, reliability andpracticality.
Owner:JIANGNAN UNIV
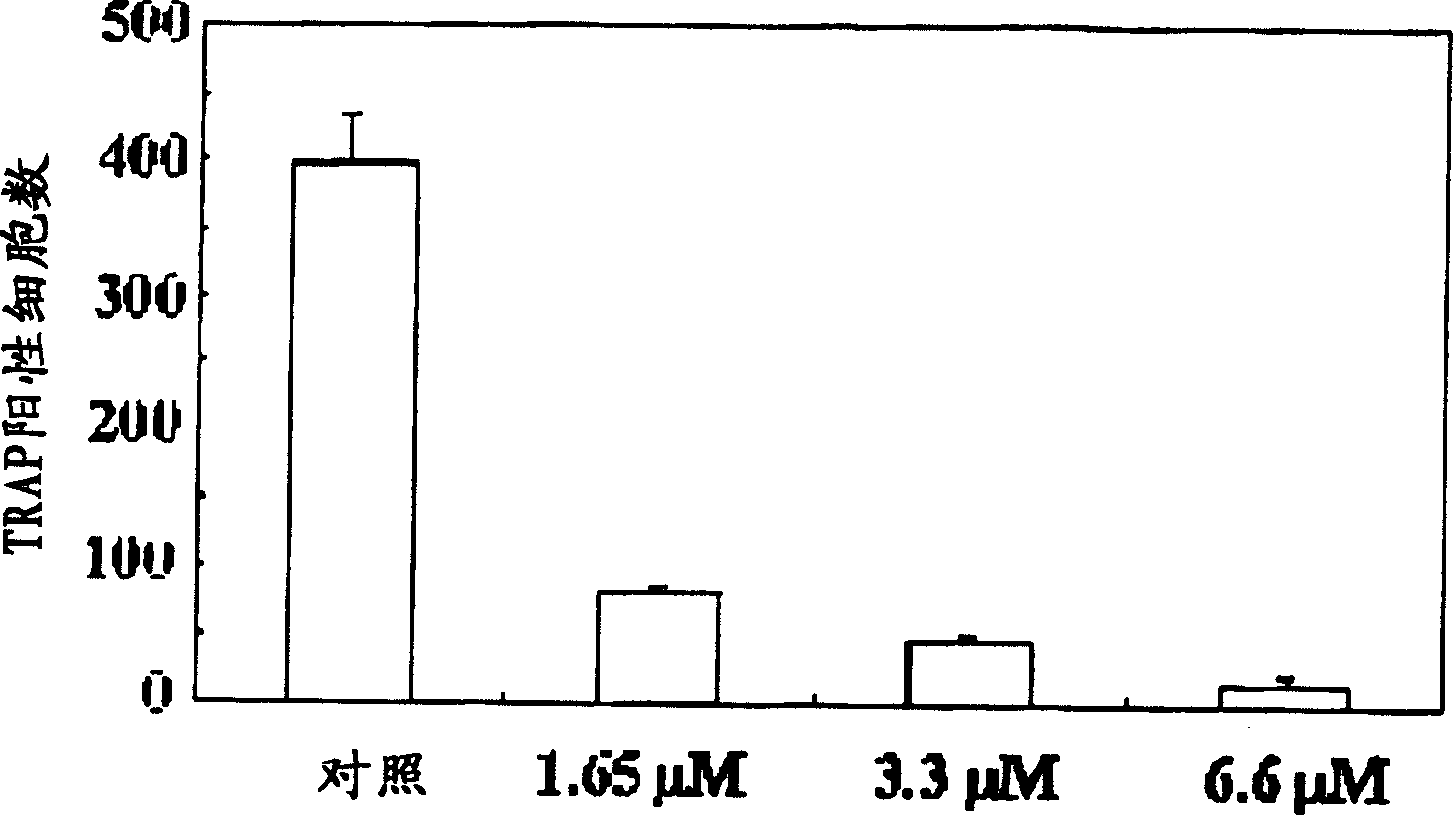
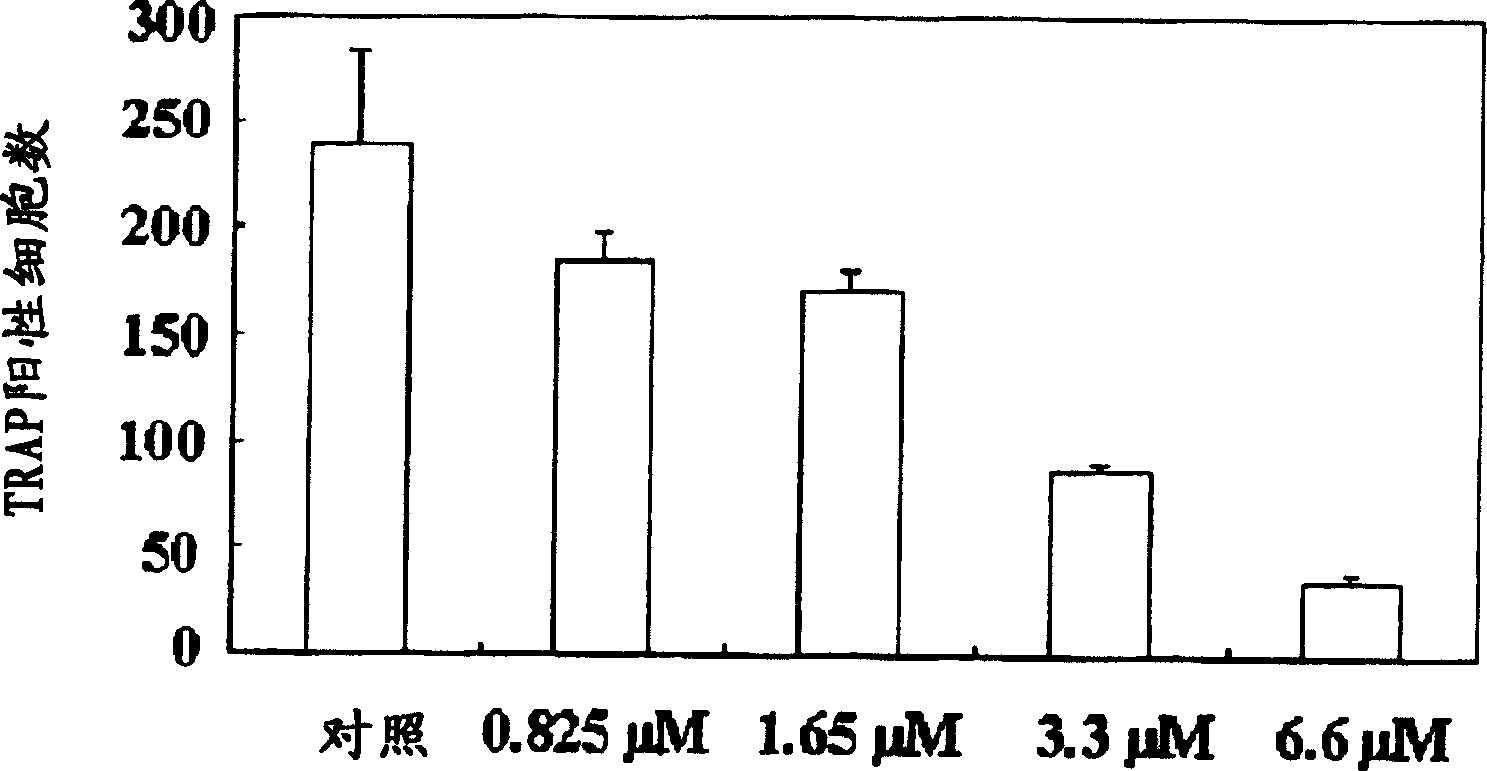
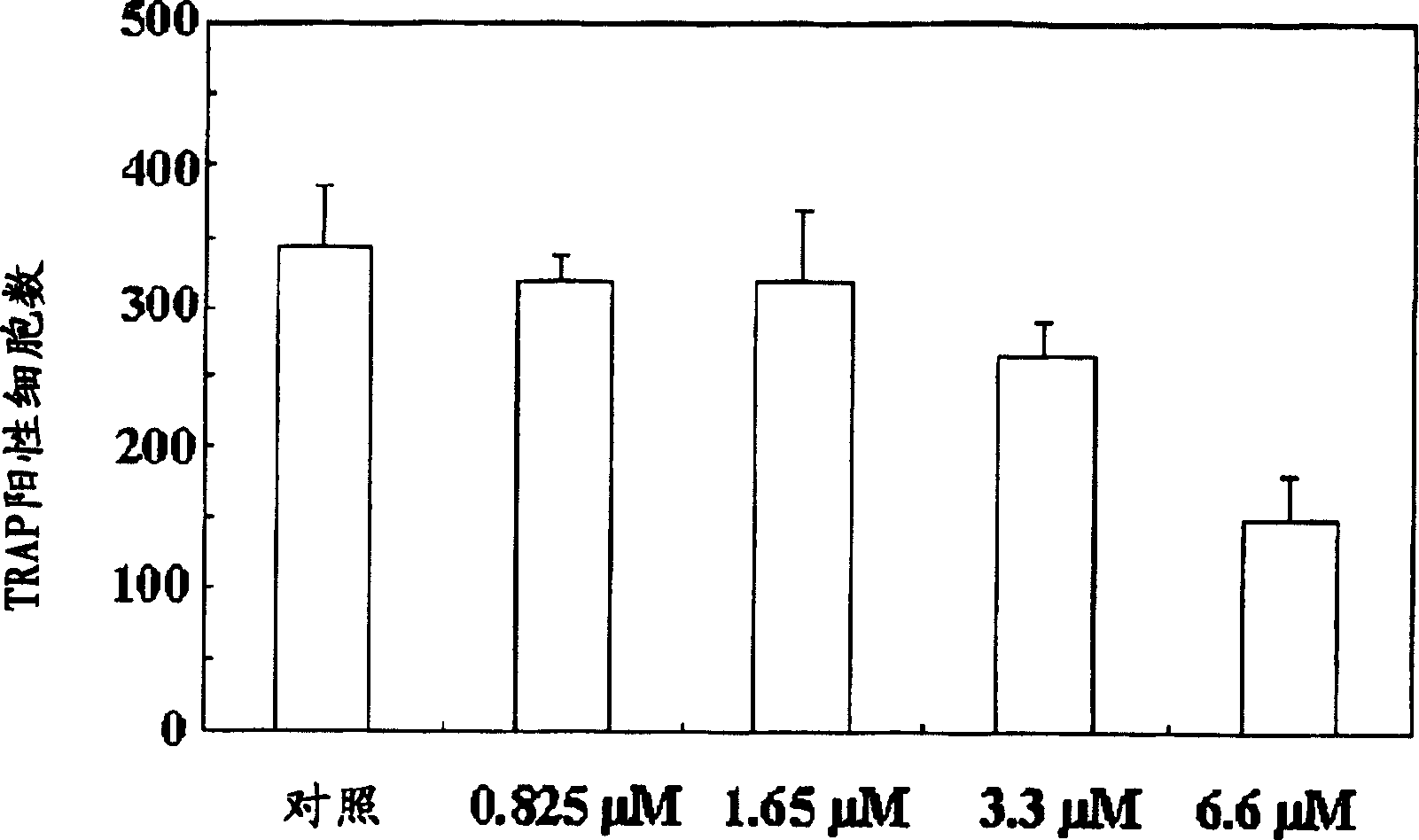
Process for preparing n-acylated lysophosphatidylcholine and pharmaceutical composition for treatment of metabolic bone disease comprising said compounds
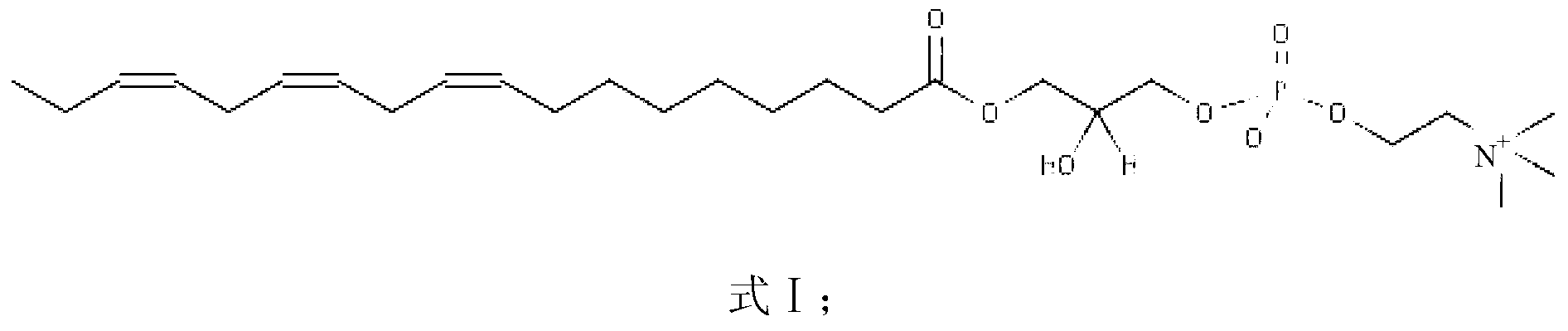
Disclosed is a pharmaceutical composition for treating and preventing metabolic bone diseases, comprising a pharmaceutically effective amount of N-acylated lysophosphatidylcholine compound represented by Formula 1 (R is a saturated or unsaturated fatty acid of C14 to C20, and R′ is methoxycarbonyl or hydroxylmethyl group), and a pharmaceutically acceptable carrier. Also, the present invention discloses a method of preparing an N-acylated lysophosphatidylcholine compound represented by Formula 1, from serine. [Formula 1]
Owner:JHON GIL JA +3
Preparation method of egg yolk lecithin
InactiveCN109912645AGood for taking offHigh yieldPhosphatide foodstuff compositionsYolkAdditive ingredient
The invention relates to the field of production of pharmaceutical ingredients and provides a preparation method of egg yolk lecithin. The preparation method of the egg yolk lecithin comprises the following steps: S1, adding acetone into egg yolk powder to deoil the egg yolk, filter and take filter residues; S2, adding the filter residues in the step S1 into ethanol of 90 to 95 percent, purifying,filtering, taking the filter residues, performing standby application on filter liquid, adding ethanol of 90 to 95 percent into the filter residues for the second time, purifying, filtering, and combining the filter liquid of two-time purification, wherein the first-time adding amount of the ethanol is 6 to 9 times the adding amount of the egg yolk powder, and the second-time adding amount of theethanol is 4 to 7 times the adding amount of the egg yolk powder; and S3, concentrating and freeze-drying the filter liquid obtained in the step S2 to obtain the egg yolk lecithin. The extraction effect of the components such as phosphatidylcholine, phosphatidyl ethanolamine, phosphatidylinositol, lysophosphatidylcholine and lysophosphatidylethanolamine is improved by controlling the ethanol concentration to be 90 to 95 percent and controlling the two-time adding amount of the ethanol, so that the yield is up to 12 to 14 percent, and the purity of the obtained products is improved.
Owner:广州隽沐生物科技股份有限公司
Detection of lysophosphatidylcholine for prognosis or diagnosis of a systemic inflammatory condition
The present invention provides methods and compositions useful for the diagnosis or prognosis of a systemic inflammatory condition such as sepsis.
Owner:BECTON DICKINSON & CO
Multi-metabolites platform for diagnosis of acute coronary syndrome
The present disclosure provides a multi-metabolite platform using one or more metabolite selected from a group consisting of tryptophan, homoserine, fatty acid (16:1), fatty acid (18:0), fatty acid (18:1), fatty acid (18:2), fatty acid (22:6), phosphatidylcholine (PC) (34:2), PC (34:3), lysophosphatidylcholine (lysoPC) (16:0), lysoPC (18:0), lysoPC (20:3), lysoPC (20:4), lysoPC (22:6), monoglyceride (18:1 / 0:0 / 0:0) and sphingomyelin (d18:2 / 16:0) as a biomarker, which can diagnose acute coronary syndrome as well as the symptoms occurring prior to the onset of the disease simply and accurately through in-vivo level analysis.
Owner:KOREA INST OF SCI & TECH
A process for preparing N-acylated lysophosphatidylcholine compounds and a pharmaceutical composition for treatment of metabolic bone disease comprising said compounds
The present invention relates to a pharmaceutical composition for preventing and / or treating metabolic bone disease. The pharmaceutical composition comprises an effective dose of an N-acyl lysophosphatidylcholine compound (chemical formula 1) and a carrier thereof that can be used in pharmaceutical preparations . Furthermore, the present invention relates to a preparation method for preparing the N-acyl lysophosphatidylcholine compound of Chemical Formula 1 from serine.
Owner:EWHA UNIV IND COLLABORATION FOUND
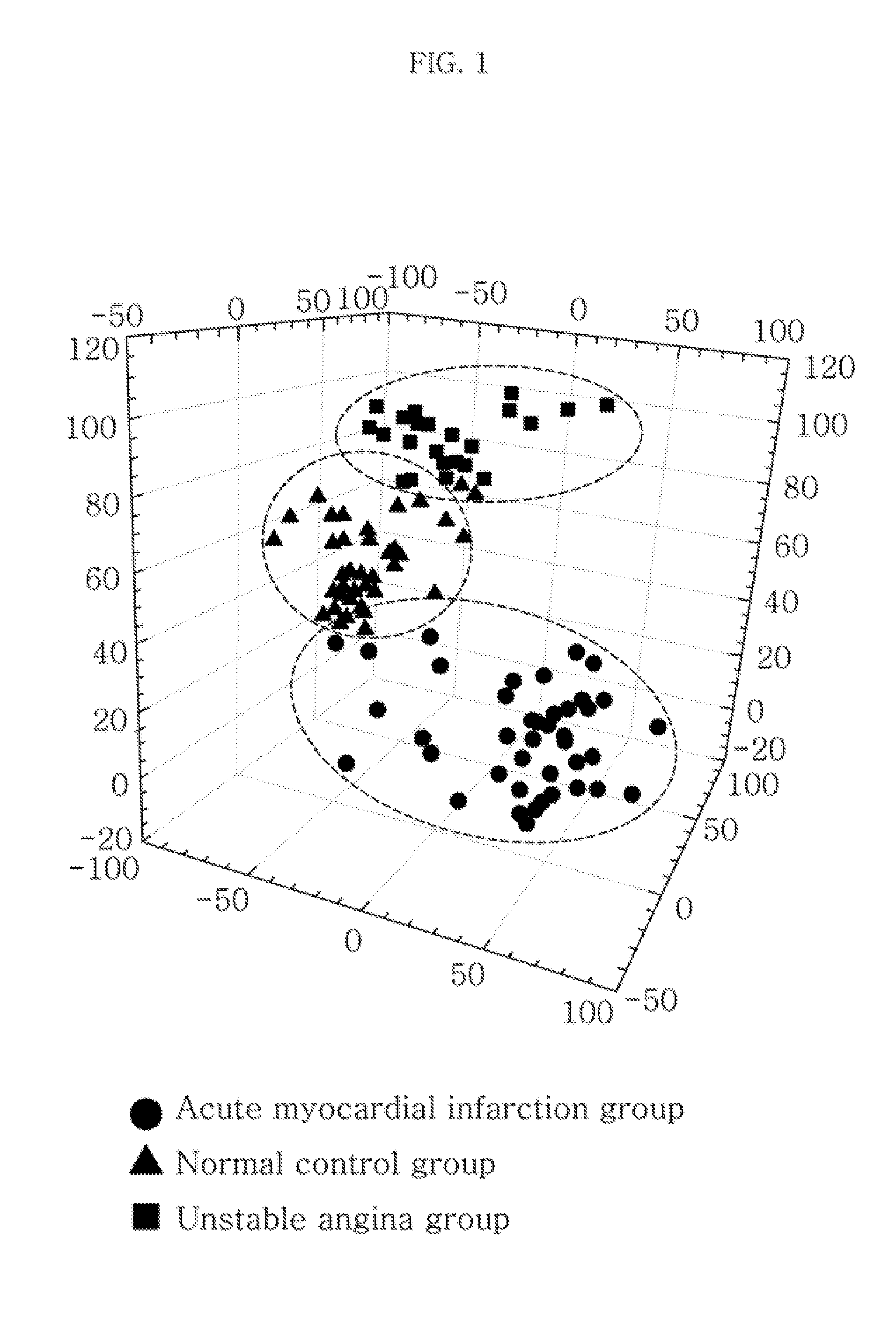
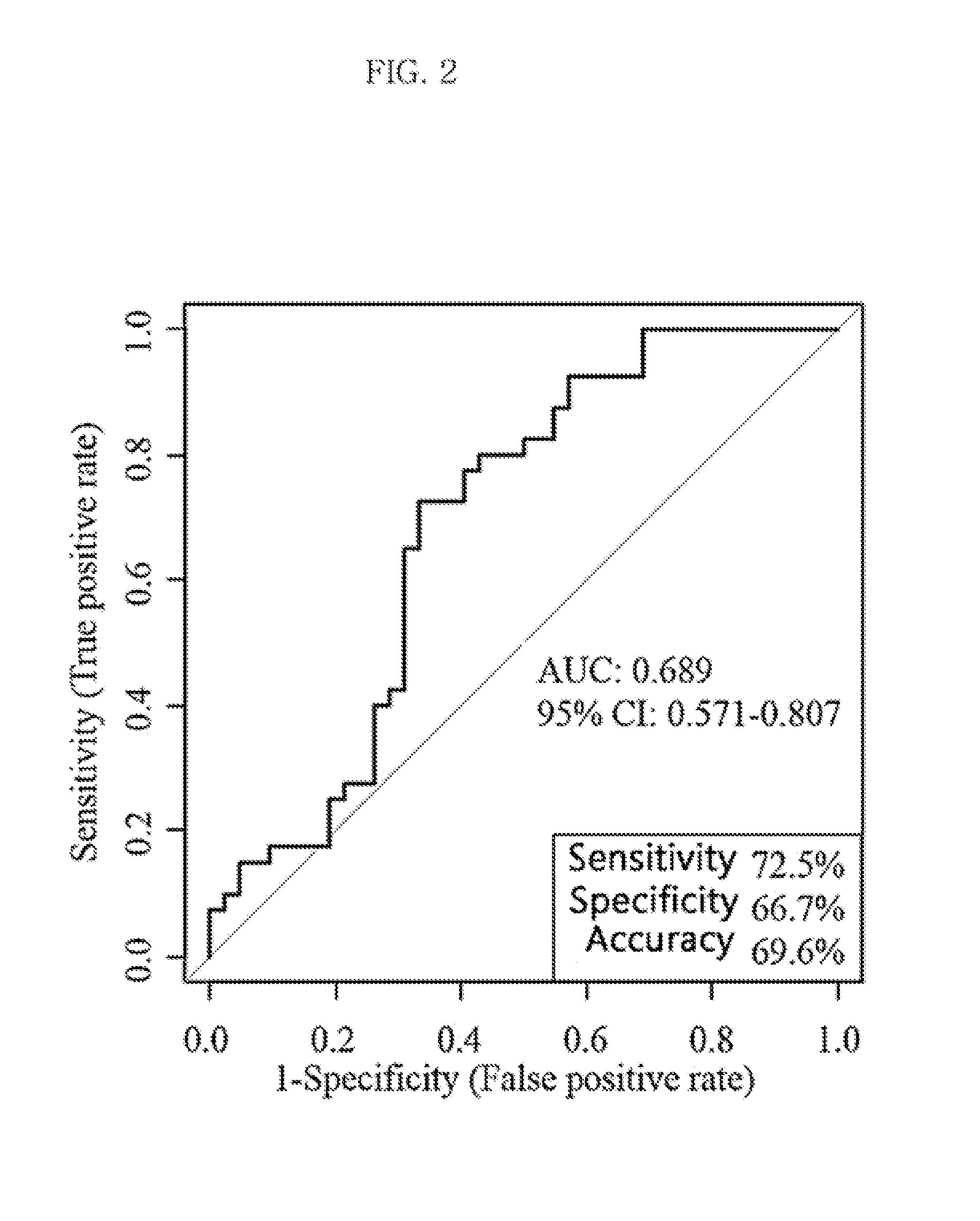
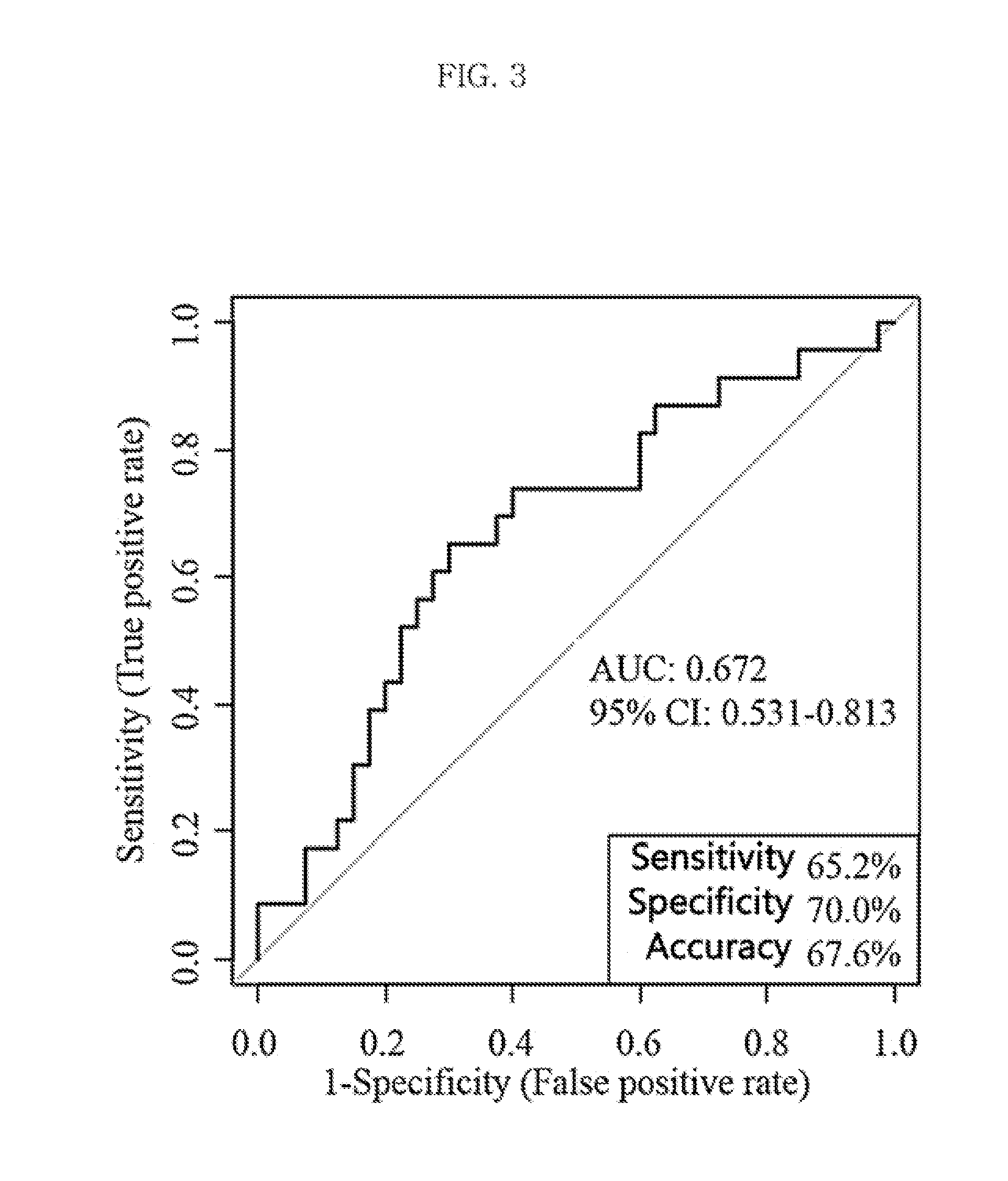
Metabolic marker for diagnosing and distinguishing unstable angina pectoris and acute myocardial infarction
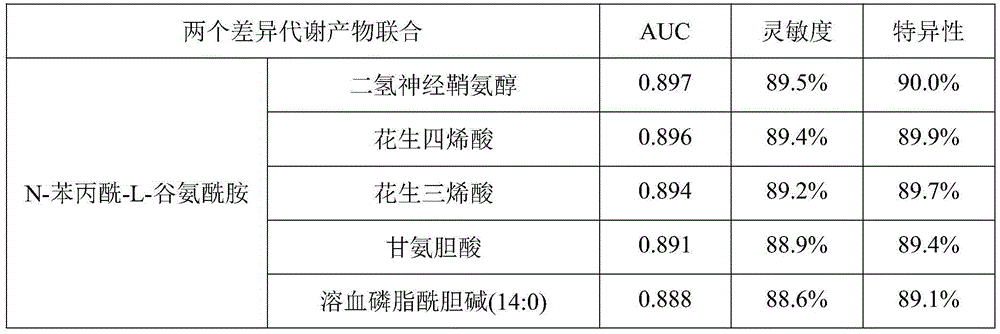
The invention discloses a metabolic marker for diagnosing and distinguishing unstable angina pectoris and acute myocardial infarction, comprising one or more of N-phenylalanyl-L-glutamine, sphinganine, arachidonic acid, eicosatrienoic acid, glycocholic acid, lysophosphatidylcholine (14:0), tryptophan-arginine-leucine tripeptide, lysophosphatidylcholine (18:2), and lysophosphatidylcholine (20:3). In the single use of diagnosing and distinguishing patients with acute myocardial infarction and patients with unstable angina pectoris, each ROC (receiver operating characteristic) AUC (area under the curve) is greater than 0.7, and clinical diagnostic significance is provided; in the joint use for diagnosis, AUC further increases as a joint quantity increases, a highest AUC up to 0.991 is obtained in a case of nine members jointed, and sensitivity and specificity are respectively 99.2% and 98.9% under an optimal cutoff value. The metabolic marker can accurately diagnose and distinguish unstable angina pectoris and acute myocardial infarction, with high accuracy and high sensitivity and specificity.
Owner:齐炼文
Method for determining liver fat amount and method for diagnosing nafld
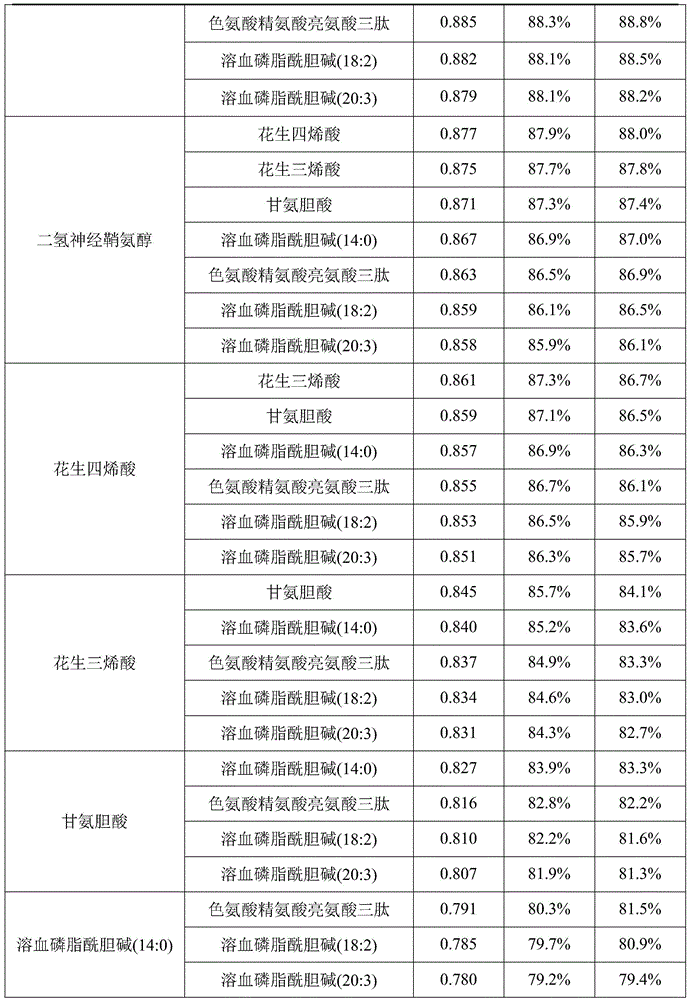
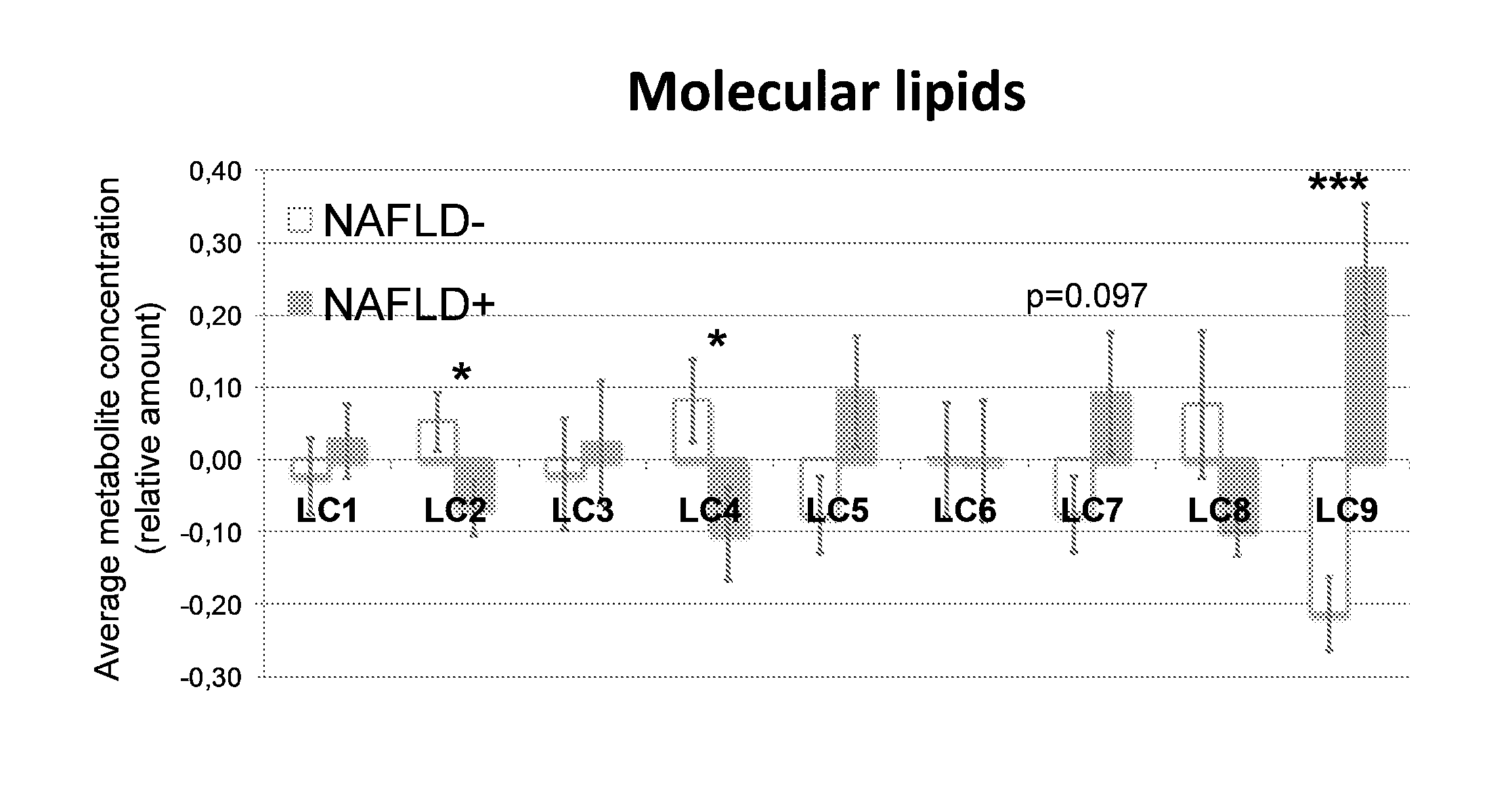
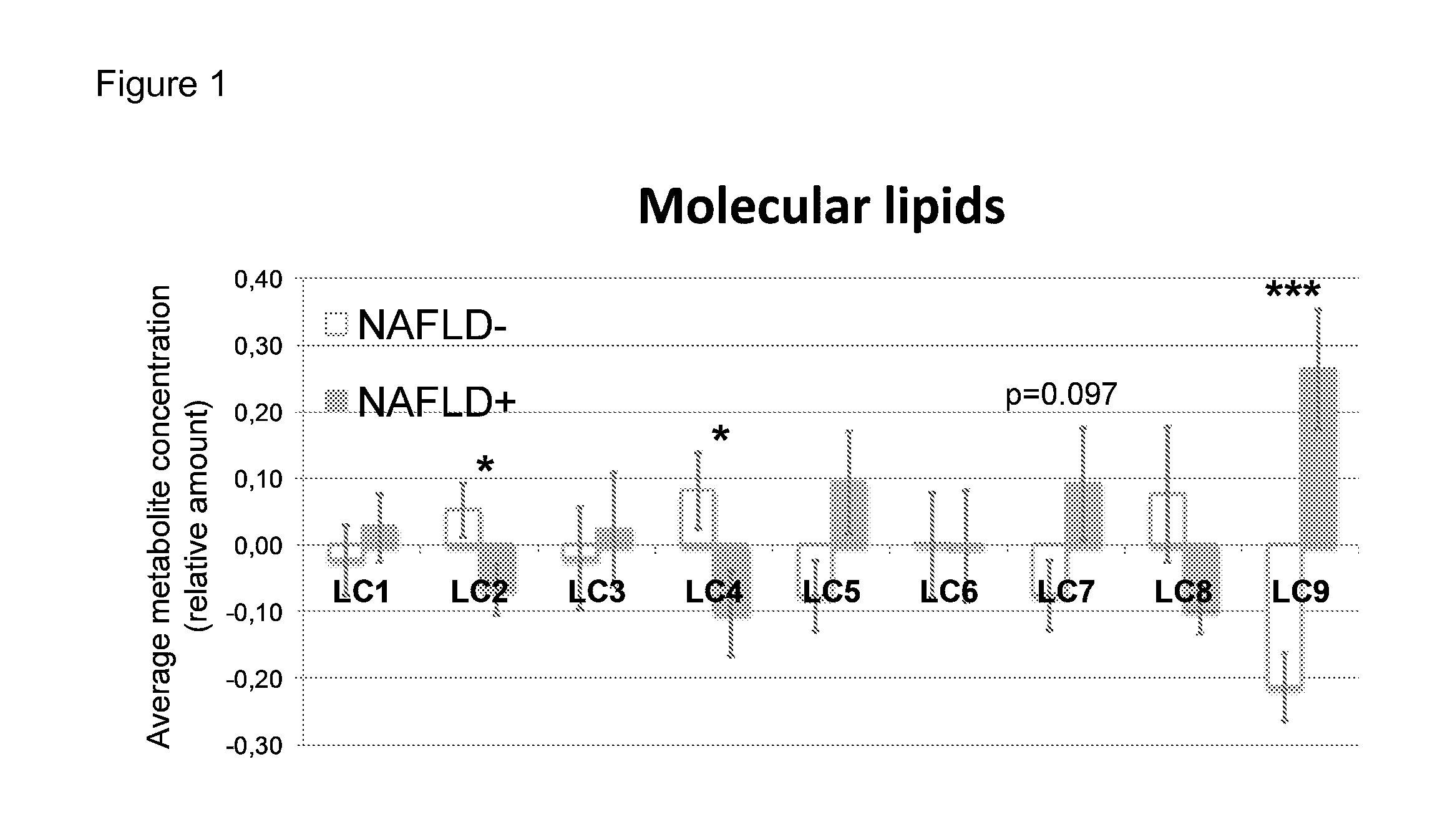
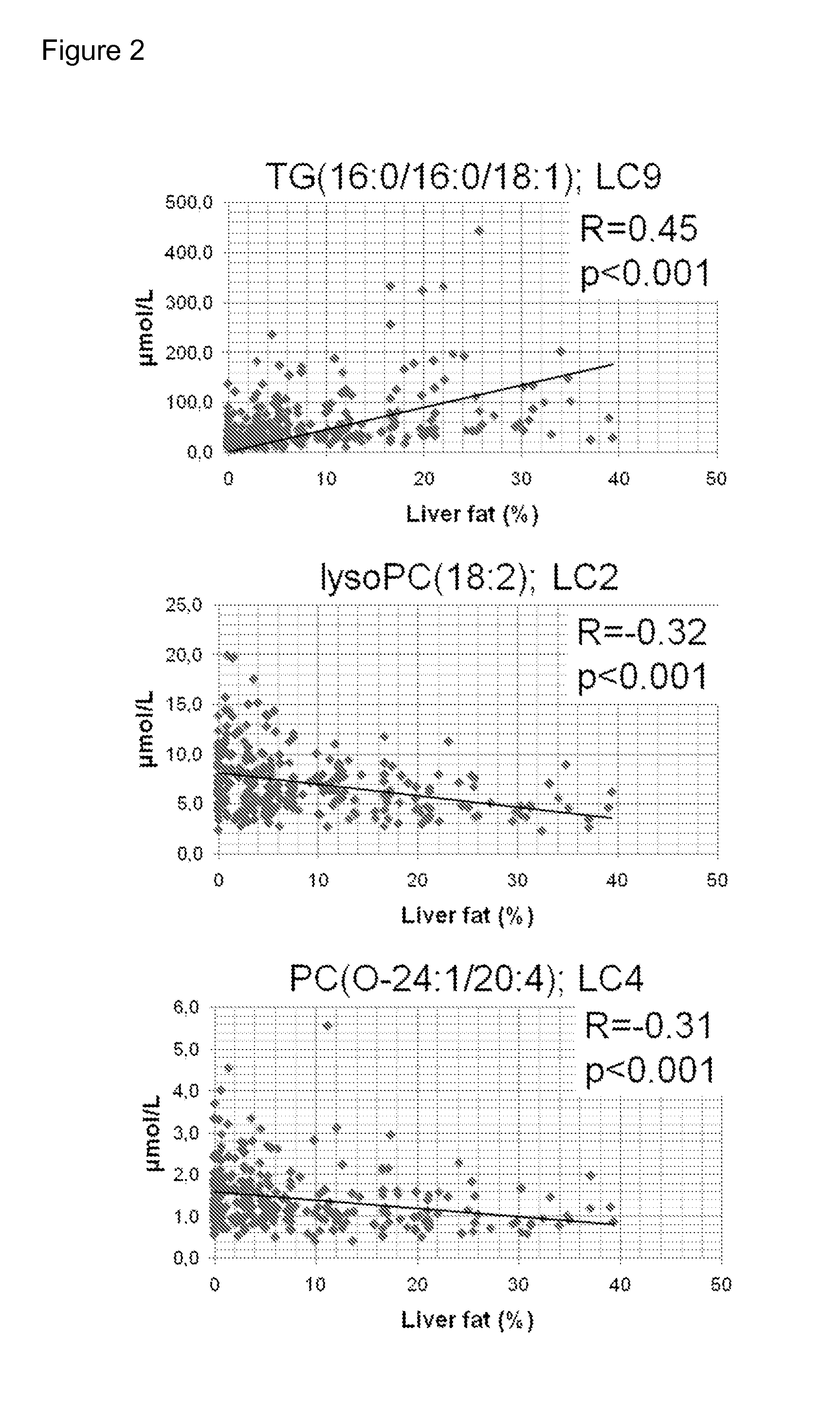
InactiveUS20150011424A1Simple and sensitive and non-invasive toolDiagnosing NAFLDParticle separator tubesComponent separationBlood plasmaBiology
The present invention is based on the idea of determining certain molecular lipids from a subject's blood sample, for example from serum or plasma sample, and based on the amounts of the determined lipids determining the amount of liver fat and / or diagnosing NAFLD in the subject. More specifically the subjects with elevated liver fat amount and NAFLD are characterized by elevated triglycerides with low carbon number and double bond content in the blood sample. Lysophosphatidylcholines, ether phospholipids, sphingomyelins and PUFA-containing phospholipids are diminished in the blood samples of subjects with an elevated liver fat amount and NAFLD. The method of the present invention can be further used for monitoring the subject's response to the treatment of NAFLD or to the treatment of lowering of the liver fat amount in the subject.
Owner:TEKNOLOGIAN TUTKIMUSKESKUS VTT
Kit for screening colorectal cancer
The invention discloses a kit for screening colorectal cancer, which comprises a device and a comparison card, wherein the device is used for detecting the content of phosphatide; the comparison card is established by utilizing the content of phosphatide; and the phosphatide is SPC (sphingosylphosphorylcholine), 14:0 LPC (lysophosphatidylcholine), 16:0 LPC, 18:2 LPC, 18:1 LPC, 18:0 LPC, 20:4 LPC, 20:0 LPC, 22:6 LPC and 22:0 LPC. A test of the kit proves that an effective early diagnosis molecule mark for the colorectal cancer is developed, a molecule detection model is established, the detection sensitivity and specificity can reach 88% and 80% according to the model, and a distinguishing effect for comparing the colorectal cancer with the normal state obtained by utilizing the lipid molecules is better than the one obtained by using the marks for researching the early diagnosis of the colorectal cancer in recent years.
Owner:BEIJING NORMAL UNIVERSITY
Quantitative analysis method for phosphatidylcholine in serum through capillary electrophoresis-mass spectrum and application
ActiveCN108020592AHigh PC contentHigh recovery rateMaterial analysis by electric/magnetic meansLinear regressionNitrogen gas
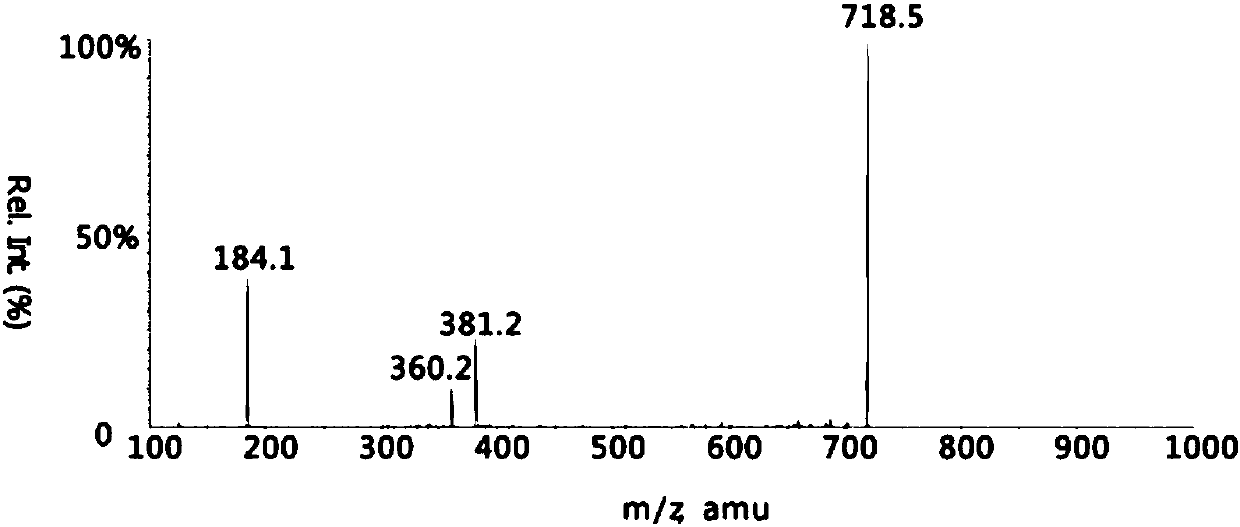
The invention discloses a quantitative analysis method for phosphatidylcholine in serum through capillary electrophoresis-mass spectrum and application. The quantitative analysis method includes the steps of S1, serum sample treating, wherein a serum sample is taken and added into a phosphatidylcholine internal standard substance, methyl alcohol is directly added, incubation on ice is carried out,centrifugation is carried out, supernatant is transferred, the product is dried with nitrogen and stored, and is dissolved with the methyl alcohol before CE-MS quantitative analysis, supernatant is removed through centrifugation, and capillary electrophoresis sample feeding is prepared, wherein the phosphatidylcholine internal standard substance is phosphatidylcholine 17:0 / 14:1, MW 717.996; S2, capillary electrophoresis condition conducting; S3, mass spectrum condition conducting; S4, quantitative analysis, wherein according to the concentration and the peak area of a standard solution, linear regression is carried out, a linear equation is obtained, and then according to the liner equation and the peak area obtained through detection, the concentration of the phosphatidylcholine and theconcentration of lysophosphatidylcholine in the serum sample are calculated. The application of the method is the application to detecting alzheimer disease. Relative quantitative analysis is carriedout on the PC / PLC in the serum in a multistage reaction monitoring mode (MRM), a large quantity of interfering ions are excluded, the detection limit is 0.0001 ng / mu L, the reproducibility is good, and the recycling rate and the detection sensitivity are high.
Owner:WUHAN INST OF PHYSICS & MATHEMATICS CHINESE ACADEMY OF SCI
Method for separating and measuring lysophosphatidylcholine in drug preparations
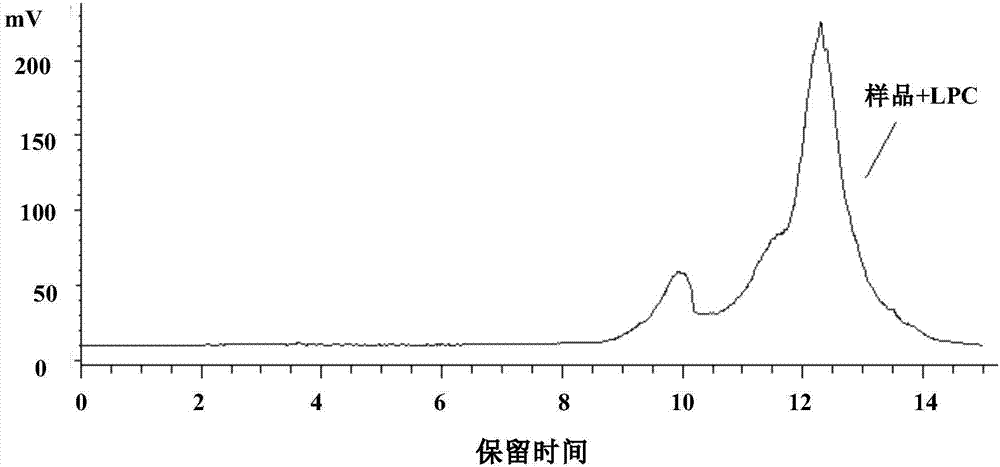
ActiveCN107957467AAvoid interferenceReduced service lifeComponent separationChromatography columnHplc mass spectrometry
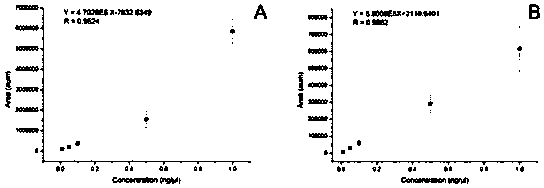
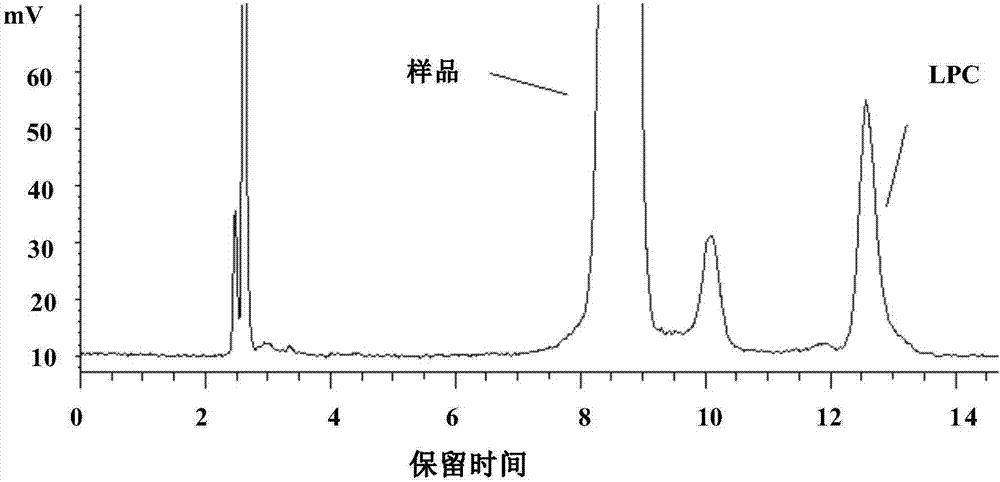
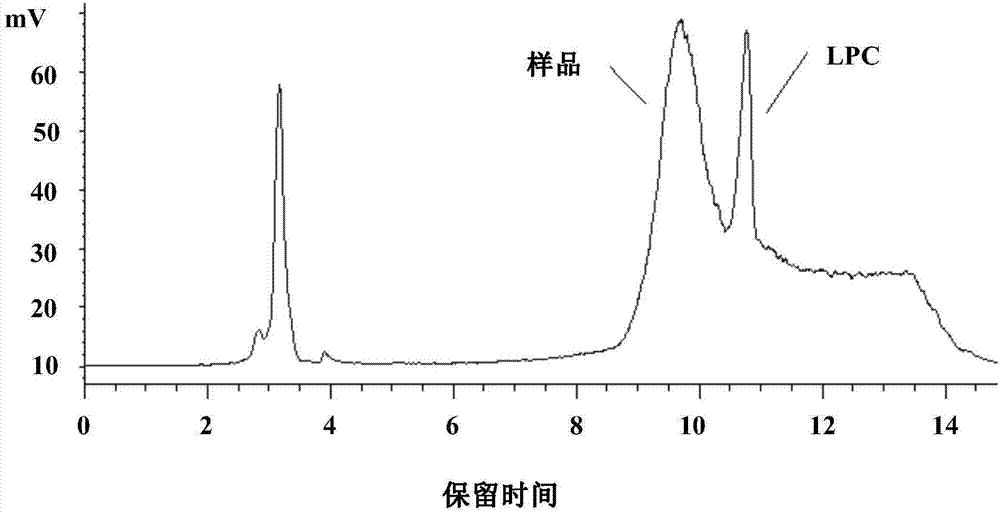
The invention provides a method for separating and measuring lysophosphatidylcholine in drug preparations. According to the method, a high performance liquid chromatograph is combined with an evaporative light scattering detector to separate and detect drug preparation samples to be detected. When the drug preparation samples to be detected are separated and measured by the high performance liquidchromatograph, a flowing phase is absolute methanol-absolute ethyl alcohol-glacial acetic acid, the volume ratio of the absolute methanol, the absolute ethyl alcohol to the glacial acetic acid is (300-800):(300-800):(15-25), and a pH (potential of hydrogen) value is adjusted to reach 5.6-6.4. The method is simple, efficient, high in accuracy and good in repeatability, good separation can be achieved, separation degree is larger than 1.5, performance of a chromatographic column of the HPLC (high performance liquid chromatograph) cannot be affected, the service life of the chromatographic column cannot be obviously shortened, and the content of lysophosphatidylcholine in drug preparations to be detected can be effectively monitored and controlled, so that drug quality can be effectively guaranteed.
Owner:SHANGHAI JINGFENG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com