Detection of lysophosphatidylcholine for prognosis or diagnosis of a systemic inflammatory condition
A systemic inflammation, phosphatidylcholine technology, applied in the direction of application, organic active ingredients, active ingredients of phosphorus compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0431] 6.1 Example 1: Analysis of serum metabolites in patients with sepsis and SIRS
[0432] Examples 1 and 2 demonstrate the use of the biomarkers of the present invention in the diagnosis and prognosis of sepsis and SIRS. Reference biomarker profiles were established for two patient volunteer populations.
[0433] Patient group
[0434] Divide the patients into two groups. The first group (SIRS group) represents patients who developed SIRS and who joined the study on "Day 1" but did not develop sepsis during hospitalization. Representatives of the second cohort ("sepsis group") also developed SIRS and entered the study on "Day 1" but generally did not develop sepsis for at least a few days after entry into the study.
[0435] Sample Collection
[0436] Blood samples were collected approximately every 24 hours from each study group. "Time 0" was when sepsis was clinically suspected in the sepsis group. In the sepsis group samples were taken at "time-12 hours", "time-36...
Embodiment 2
[0441] 6.2 Example 2: Diagnosis and prognosis of sepsis and SIRS using biomarker 496.3 or biomarker 518.3 of the present invention
[0442] This example provides a time course study of biomarkers 496.3 and 518.3 and demonstrates their use in the diagnosis, prognosis and monitoring of sepsis and SIRS. Biomarker 496.3 is 1-O-palmitoyl-2-lyso-sn-glycero-3-phosphocholine, Biomarker 518.3 is 1-O-palmitoyl-2-hemo-sn-glycero-3-phosphocholine sodium salt.
[0443] Blood was collected daily from patients enrolled in the study as described in Example 1 for up to 13 days from the day of admission to the ICU. After extraction with cold methanol, the relative concentrations of the metabolite biomarkers disclosed in this patent were determined by ESI-MS, also described in Example 1.
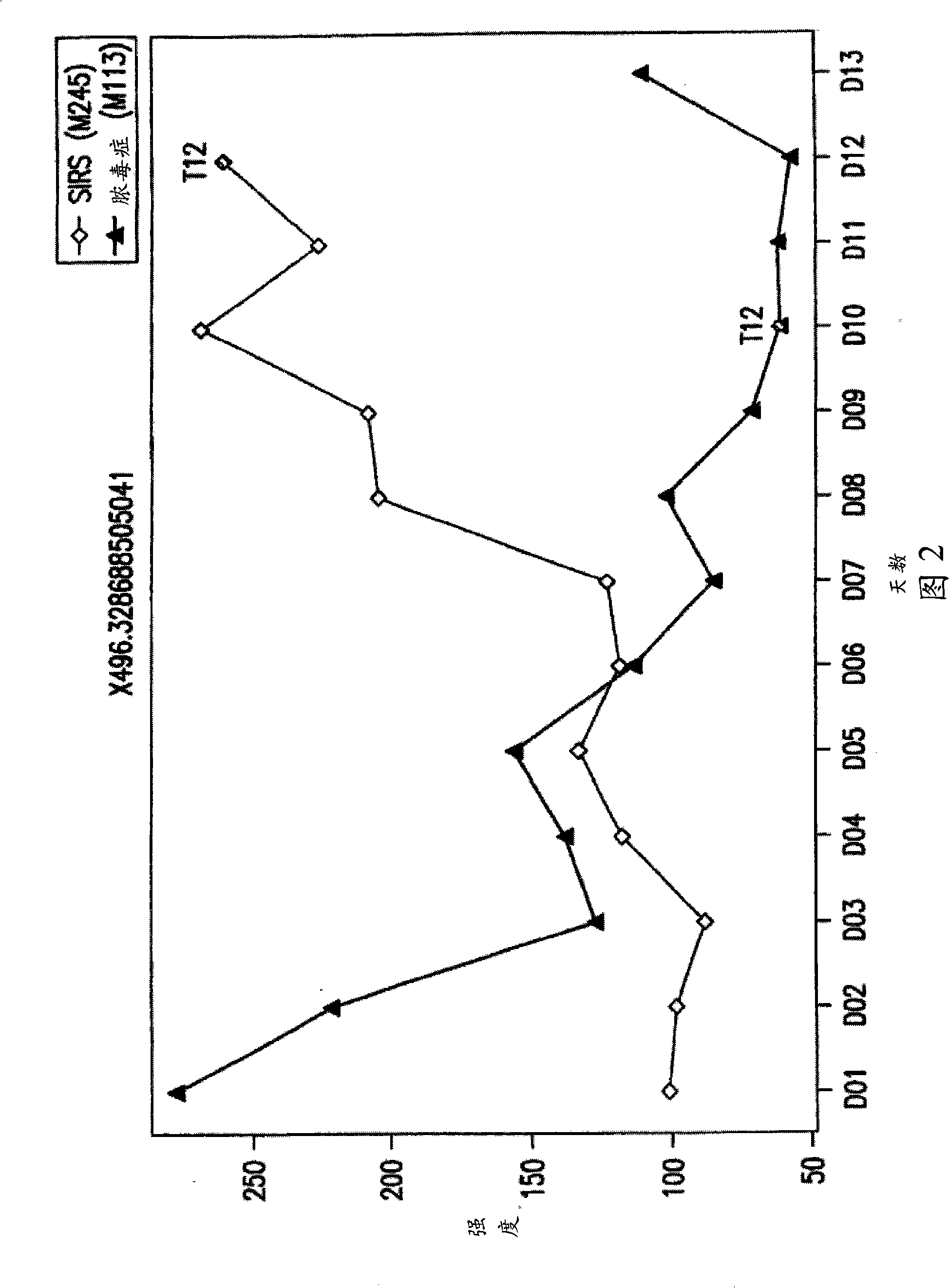
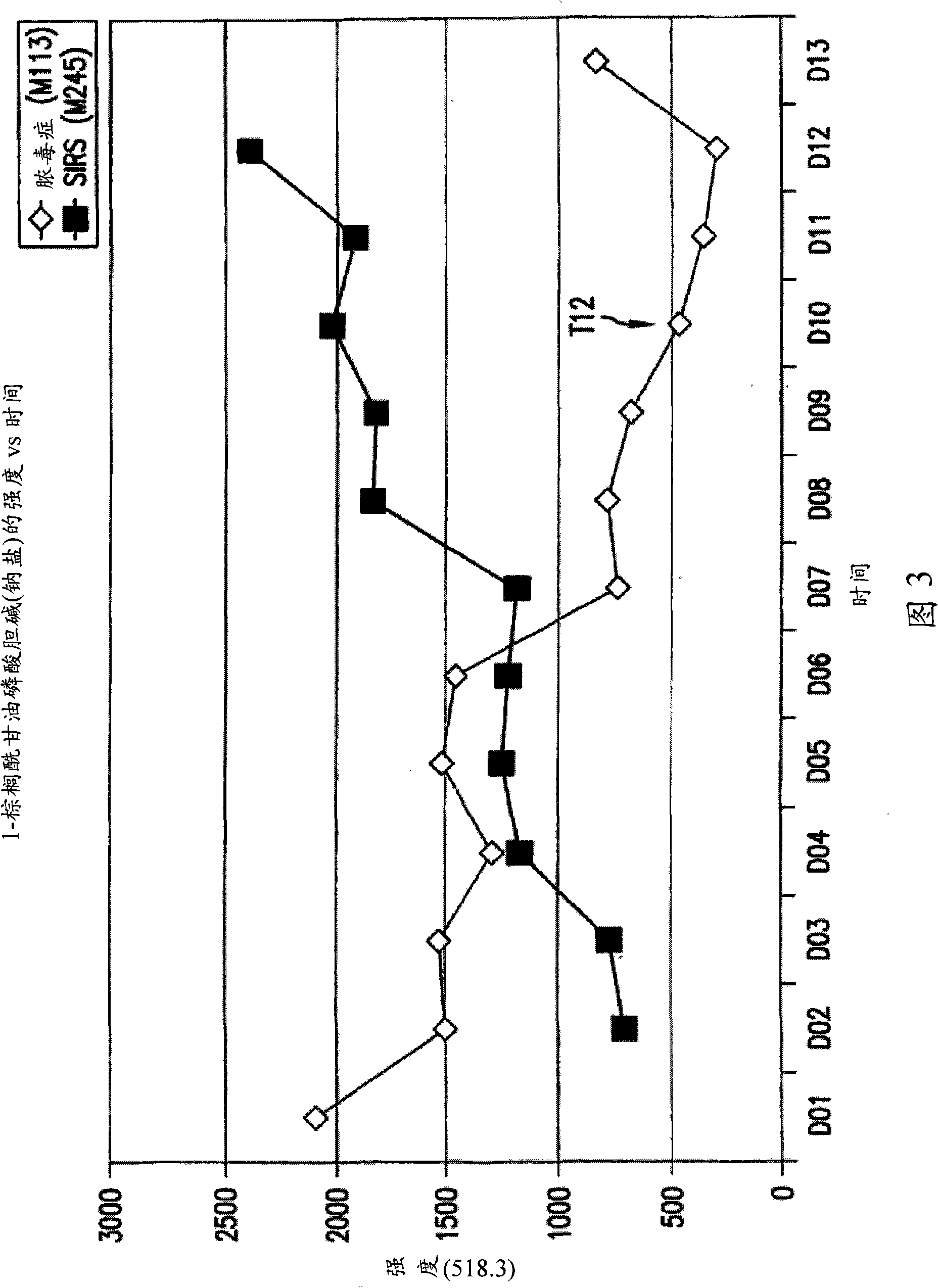
[0444] Figures 2 and 3 provide the relative concentration changes of biomarkers 496.3 and 518.3 over 13 days for sepsis patient M113 and SIRS patient M245.
[0445] As seen in Figure 2, the biomarker 496.3 ...
Embodiment 3
[0452] 6.3 Example 3: Identification of biomarkers by MS / MS
[0453] Biomarkers are typically identified by performing tandem MS on MS peak signals from plasma samples. The MS / MS peak signals were then assigned to the corresponding fragments of the proposed biomarkers. Biomarkers were finally confirmed by MS / MS spectra of commercially purchased compounds.
[0454] Table 1. MS / MS for 496.3 (1-O-palmitoyl-2-hemolys-sn-glycero-3-phosphocholine)
[0455] Ion Peak (Daltons)
[0456] MS / MS of Table 2.524.3 (1-O-stearyl-2-hemolys-sn-glycero-3-phosphocholine)
[0457] Ion Peak (Daltons)
[0458] 6.4 Example 4: Least squares results of 1-O-palmitoyl-2-hemolysate-sn-glycero-3-phosphocholine as a diagnosis of sepsis by least squares analysis
[0459] This example demonstrates how well the diagnosis of sepsis is based on the marker 1-O-palmitoyl-2-hemolys-sn-glycero-3-phosphocholine. In the analysis of these examples, least squares curve fitting was used to model t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com