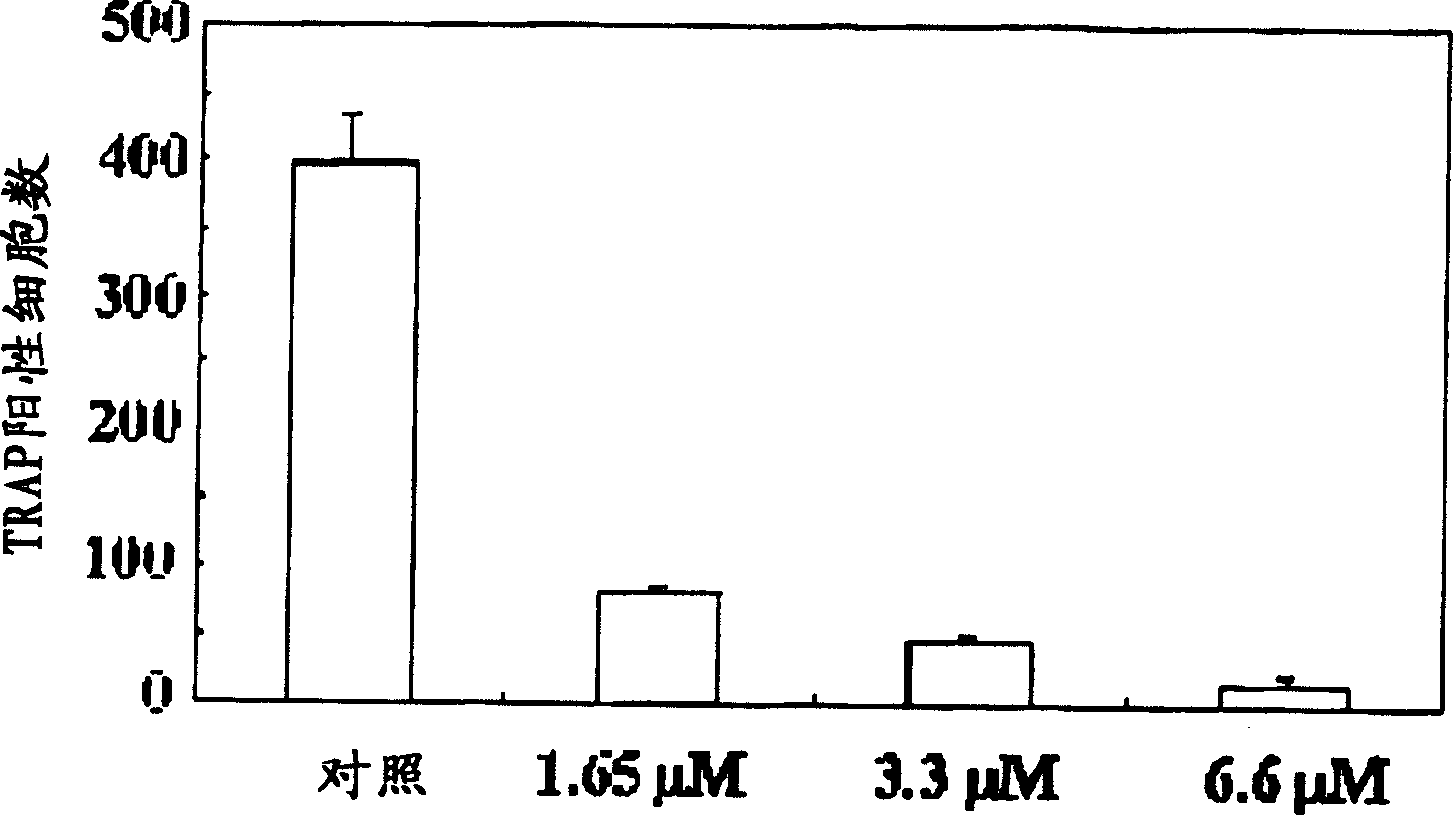
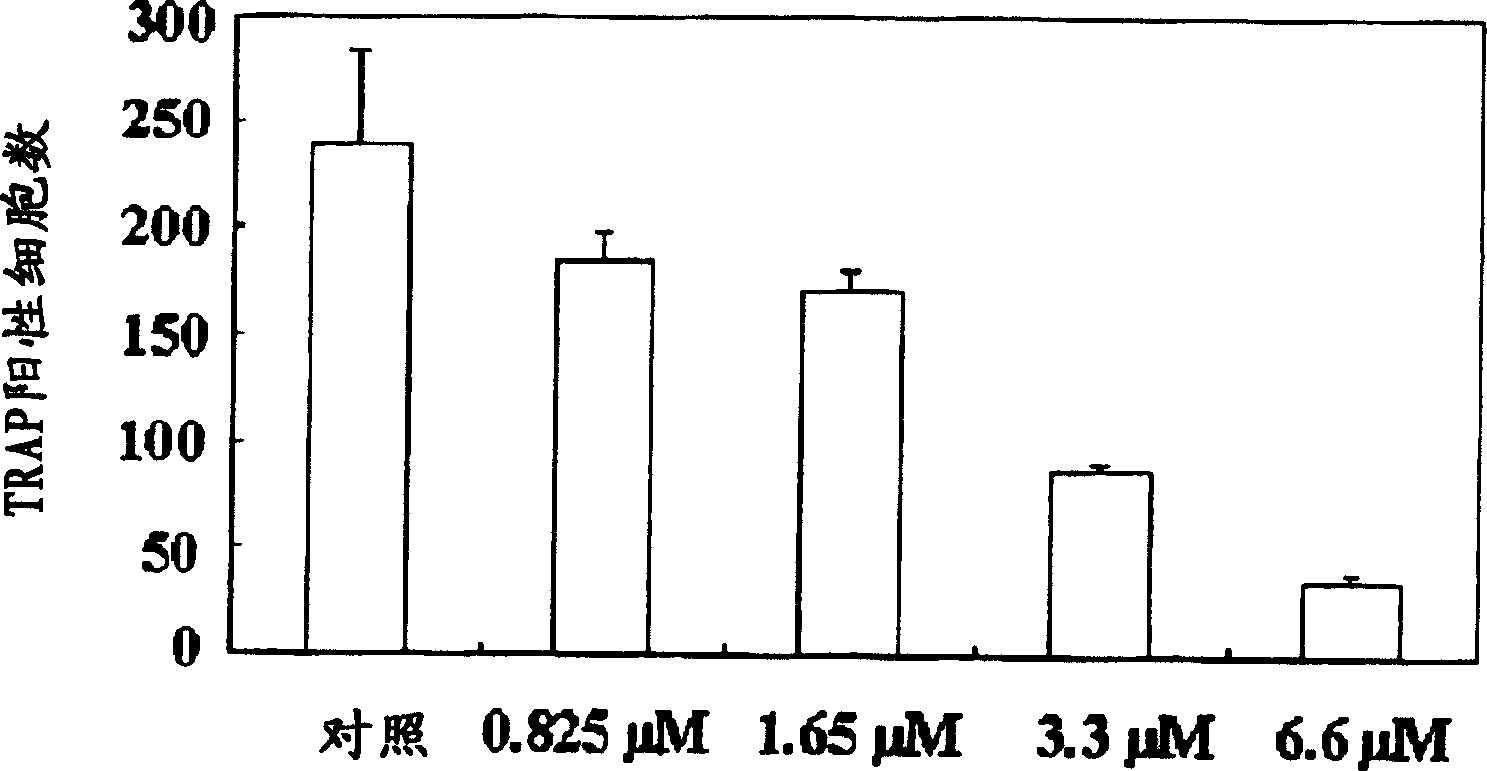
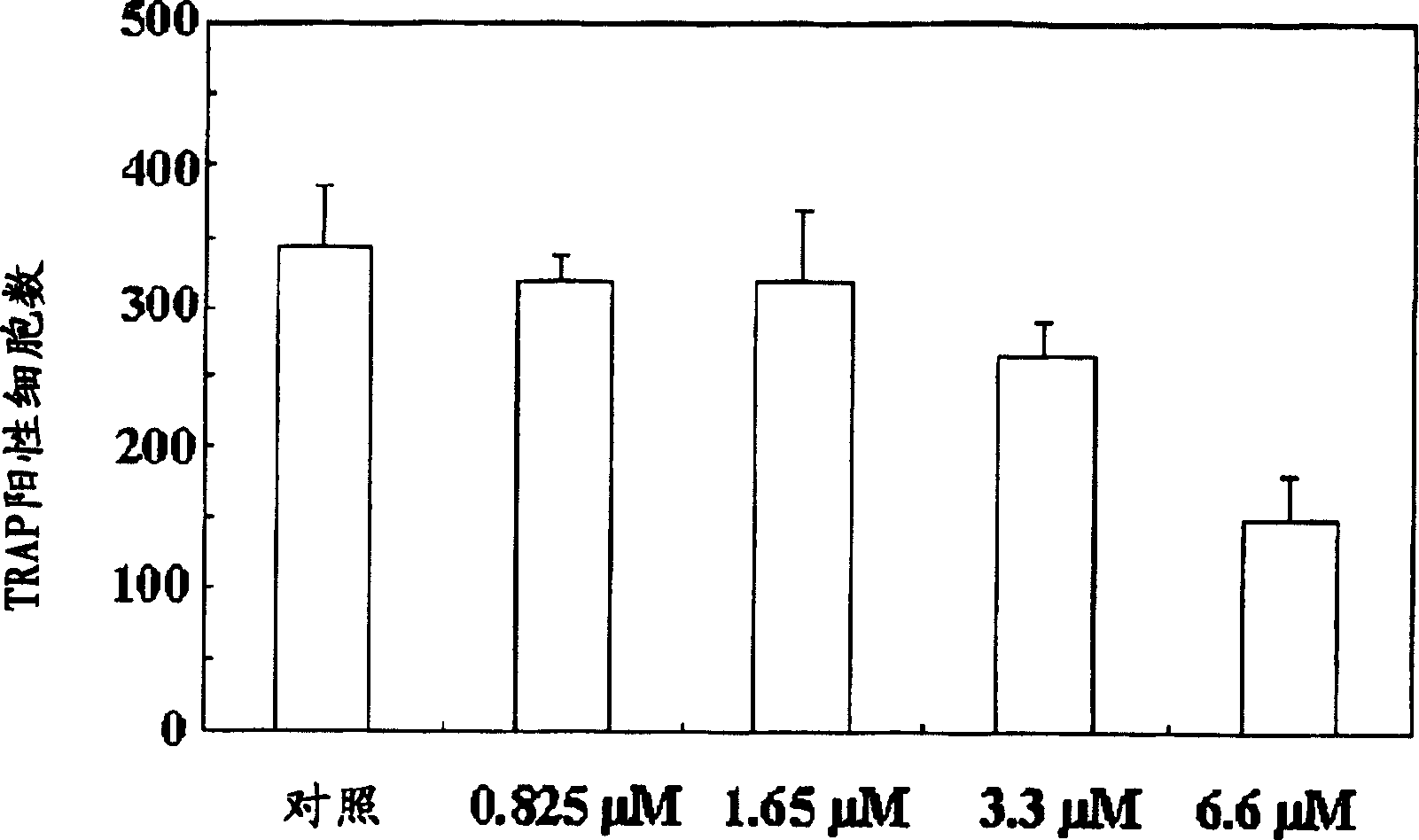
A process for preparing N-acylated lysophosphatidylcholine compounds and a pharmaceutical composition for treatment of metabolic bone disease comprising said compounds
A technology of phosphatidylcholine and composition, which is applied in the field of preparation of N-acyl lysophosphatidylcholine compound and the pharmaceutical composition containing this compound for treating metabolic bone disease, which can solve the lack of sufficient research on efficacy and side effects And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] When the production method of the present invention is applied, techniques commonly used in the art can be used for specific reaction conditions.
[0064] The typical method of generating methyl ester hydrochloride from amino acid is to react with methanol saturated with hydrochloric acid, weaken the nucleophilicity of the amino group, and selectively methylate the carboxyl group. React L-serine with methanol saturated with hydrochloric acid for 2 hours at room temperature, and recrystallize with ether / methanol to obtain serine methyl ester hydrochloride.
[0065] The amide conjugation reaction is carried out by activating the carboxyl group with an appropriate peptide bond conjugating reagent. In particular, L-serine methyl ester hydrochloride is a primary ammonia, which is much more reactive than secondary ammonia. Therefore, using relatively cheap 1,3-dicyclohexylcarbodiimide as the peptide bond-binding reagent, the synthesis yield is also high. At this point, the ...
Embodiment 1
[0077] [Example 1] Synthesis of N-stearyl-o-phosphatidylcholine-L-serine methyl ester (CHJ-0011)
[0078] (1) Synthesis of L-serine methyl ester hydrochloride
[0079] 47.7mmol of L-serine was dissolved in 476ml of methanol, saturated with hydrochloric acid and reacted at room temperature for 2 hours. After recovering the solvent, recrystallize with methanol and ether to obtain the target compound L-serine methyl ester hydrochloride (yield: 98%, melting point: 161-162°C, [α]25D=+3.4 (c 2.0, MeOH)). The structures of the synthesized compounds were confirmed by FTIR, 1H-NMR and 13C-NMR.
[0080] FTIR (KBr, cm-1): 3349 O-H absorption peak, 2943 sp3 C-H absorption peak, 1749 ester carbonyl absorption peak
[0081] 1H NMR (CD3OD): δ4.07~4.10 (1H, t, J=3.9Hz), 3.88-3.93 (2H, m), 3.79 (3H, s) Absorption peak s of methoxyl proton: singlet , d: doublet, t: triplet, m: multiplet)
[0082] 13C NMR (CD3OD): δ52.69, 55.10, 59.67, 168.37 carbonyl absorption peaks
[0083] (2) Synthesis...
Embodiment 2
[0091] [Example 2] Synthesis of N-stearyl-o-phosphatidylcholine-L-serine hydroxymethyl (CHJ-0013)
[0092] (1) Synthesis of L-serine methyl ester hydrochloride
[0093] 47.7mmol of L-serine was dissolved in 476ml of methanol, saturated with hydrochloric acid and reacted at room temperature for 2 hours. After recovering the solvent, recrystallize with methanol and ether to obtain the target compound L-serine methyl ester hydrochloride (yield: 98%, melting point: 161-162°C, [α]25D=+3.4 (c 0.2, MeOH)). The structures of the synthesized compounds were confirmed by FTIR, 1H-NMR and 13C-NMR.
[0094] FTIR (KBr, cm-1): 3349 O-H absorption peak, 2943 sp3 C-H absorption peak, 1749 ester carbonyl absorption peak
[0095] 1H NMR (CD3OD): δ4.07~4.10 (1H, t, J=3.9Hz), 3.88~3.93 (2H, m), 3.79 (3H, s) the absorption peak of the methoxy proton (s: singlet , d: doublet, t: triplet, m: multiplet)
[0096] 13C NMR (CD3OD): δ2.69, 55.10, 59.67, 168.37 carbonyl absorption peaks
[0097] (2) S...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com