Patents
Literature
224 results about "Bone matrix" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
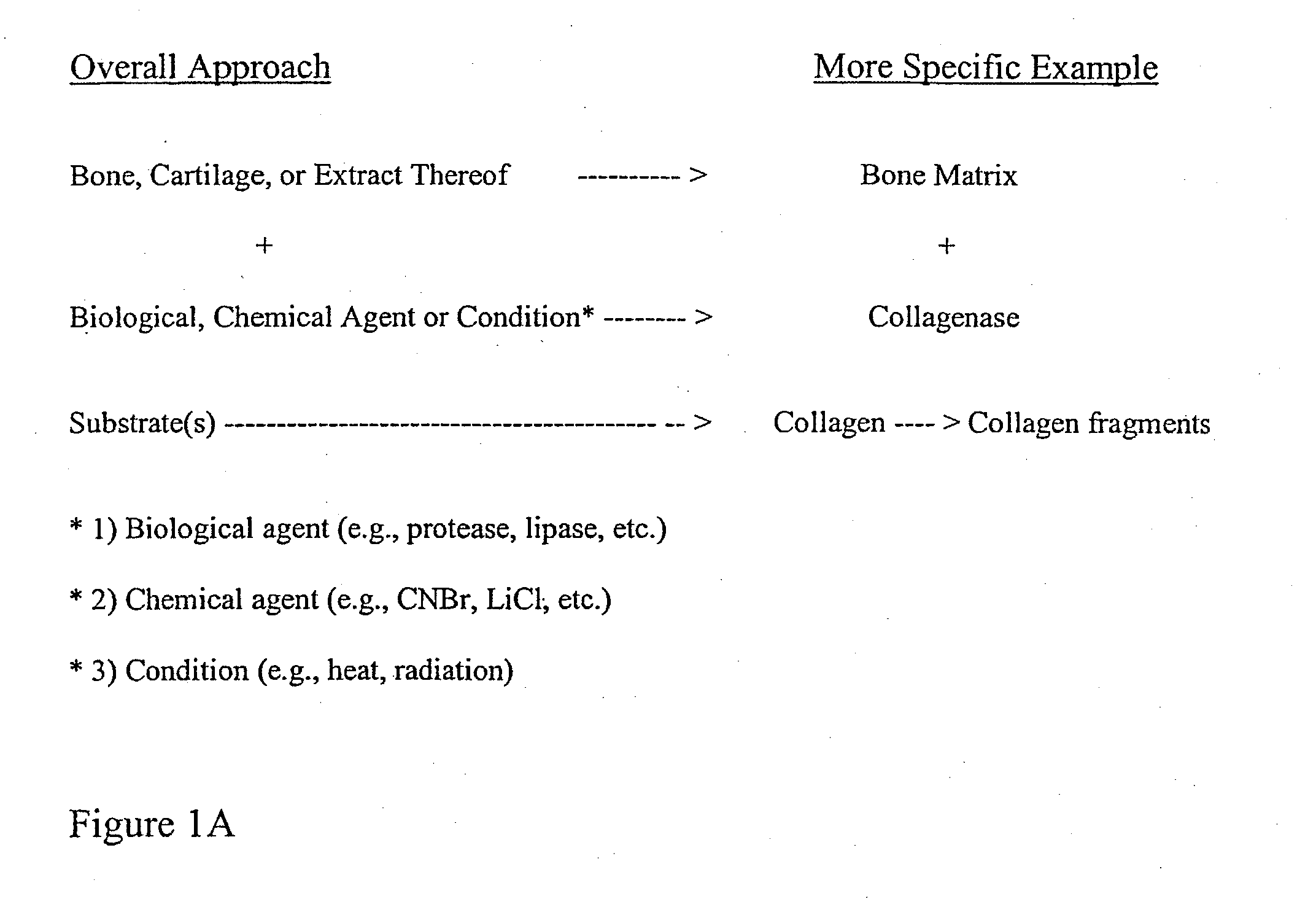
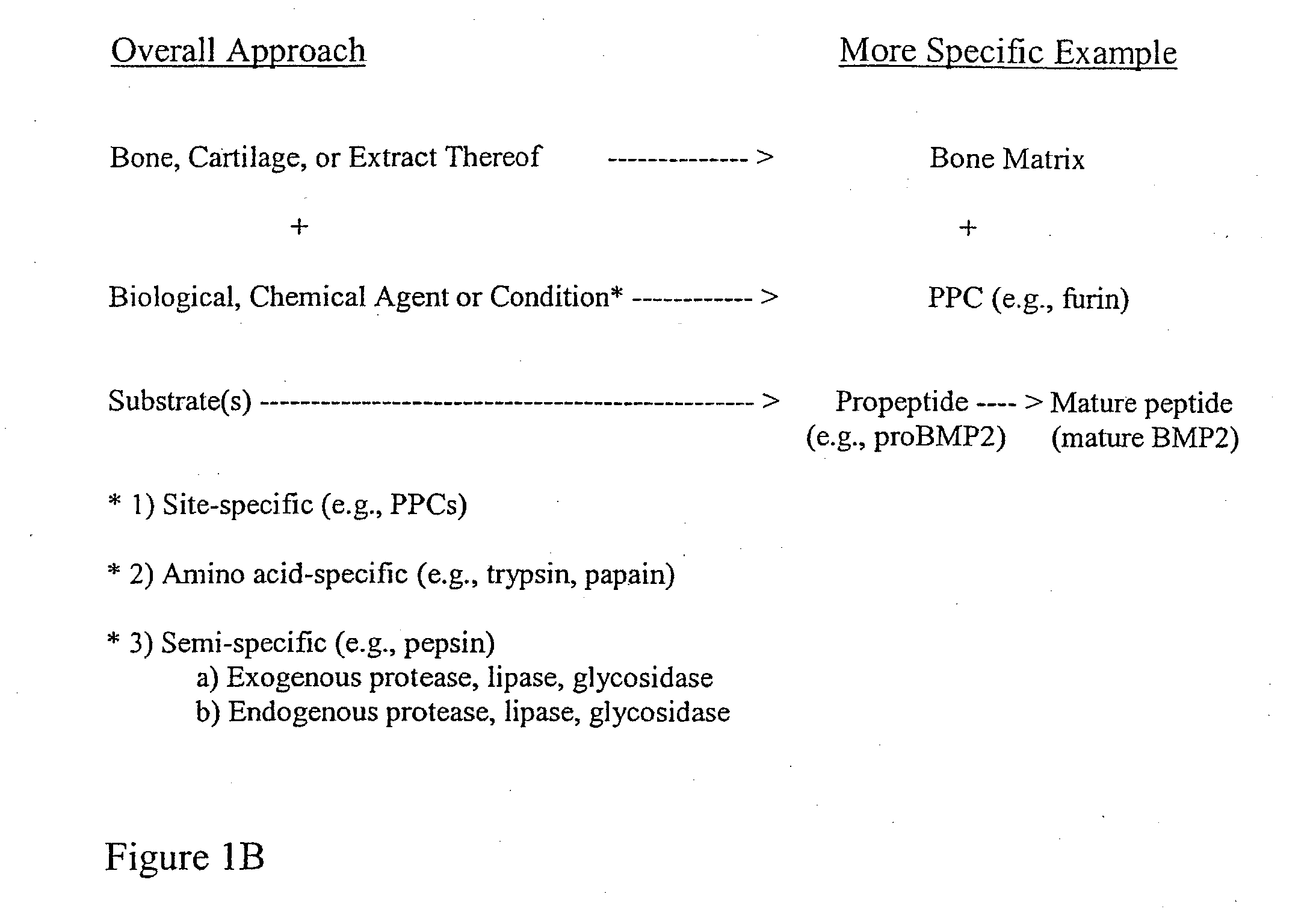
Bone matrix the intercellular substance of bone, consisting of collagenous fibers, ground substance, and inorganic salts. cartilage matrix the intercellular substance of cartilage consisting of cells and extracellular fibers embedded in an amorphous ground substance.
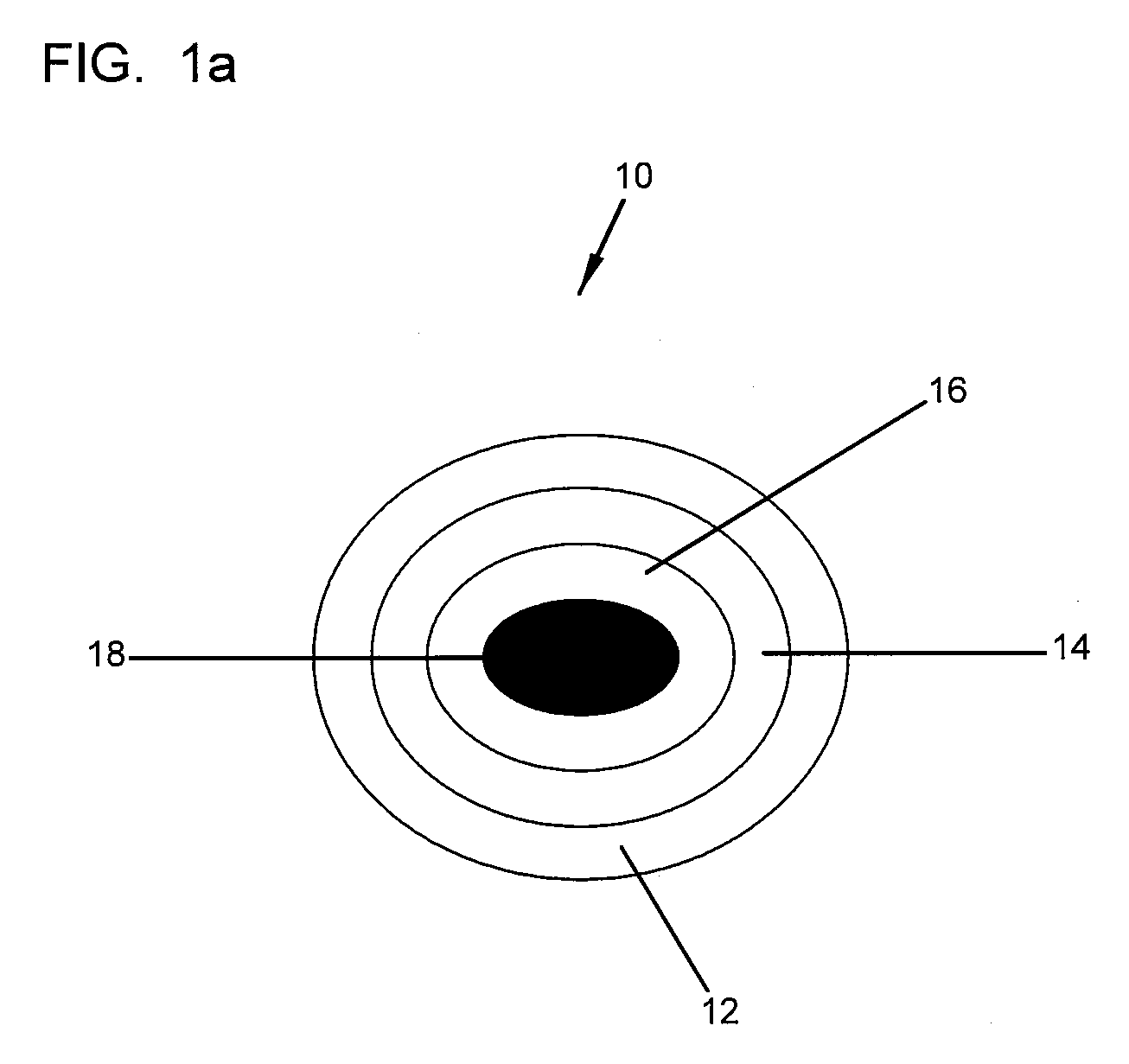
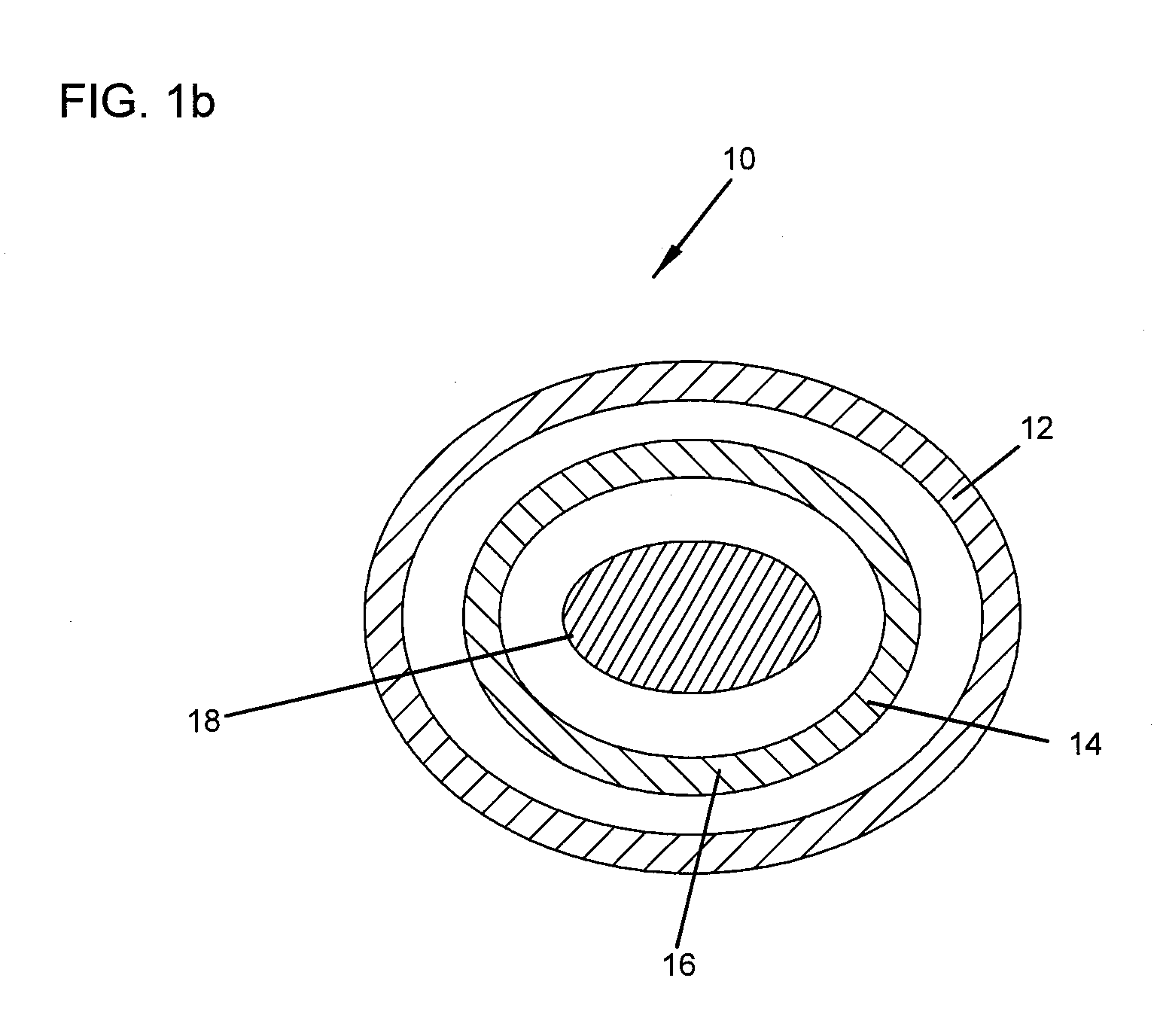
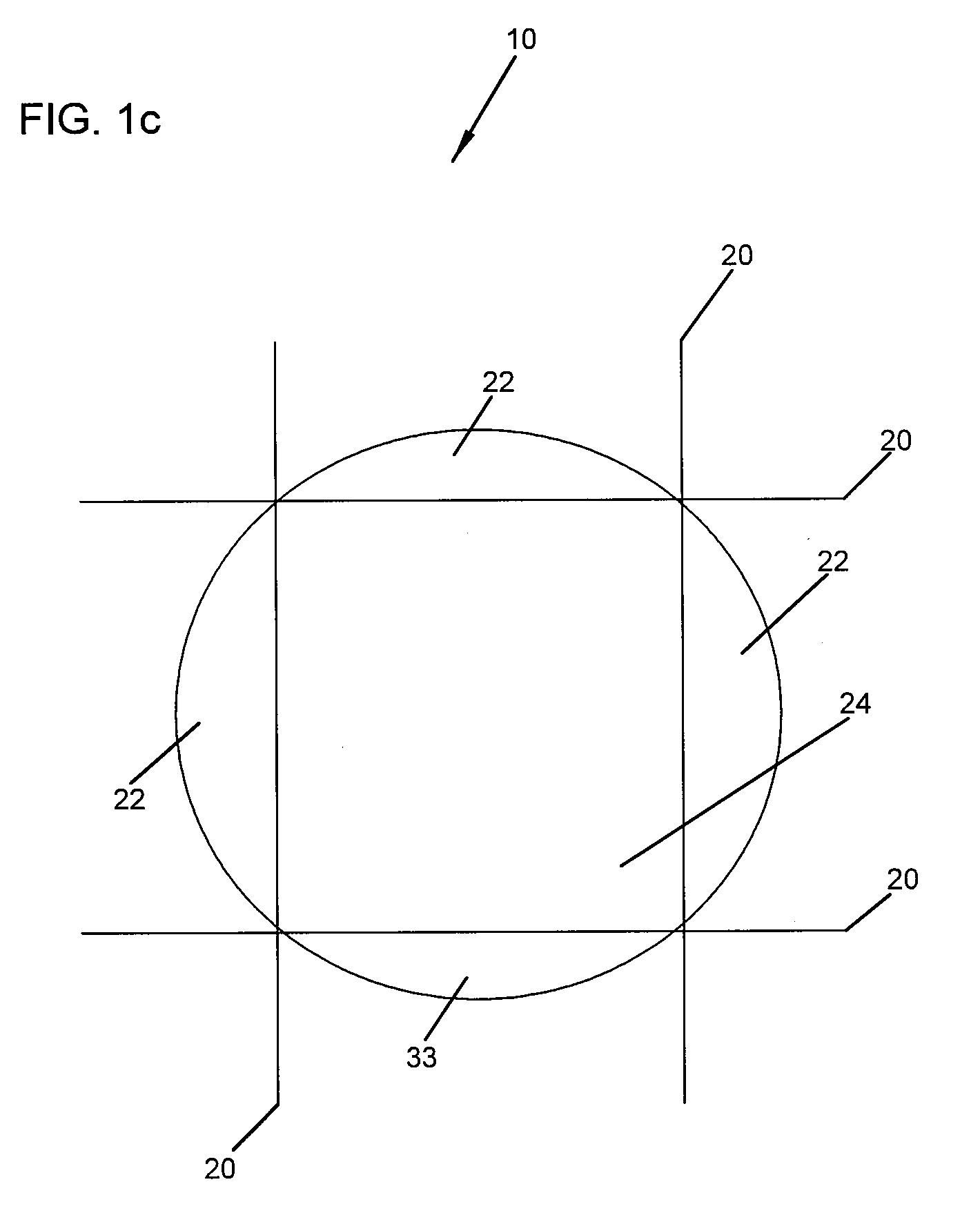
Device for lumbar surgery
InactiveUS20050171541A1Shorten recovery timeReduces the trauma to the patientInternal osteosythesisBone implantDilatorIntervertebral space
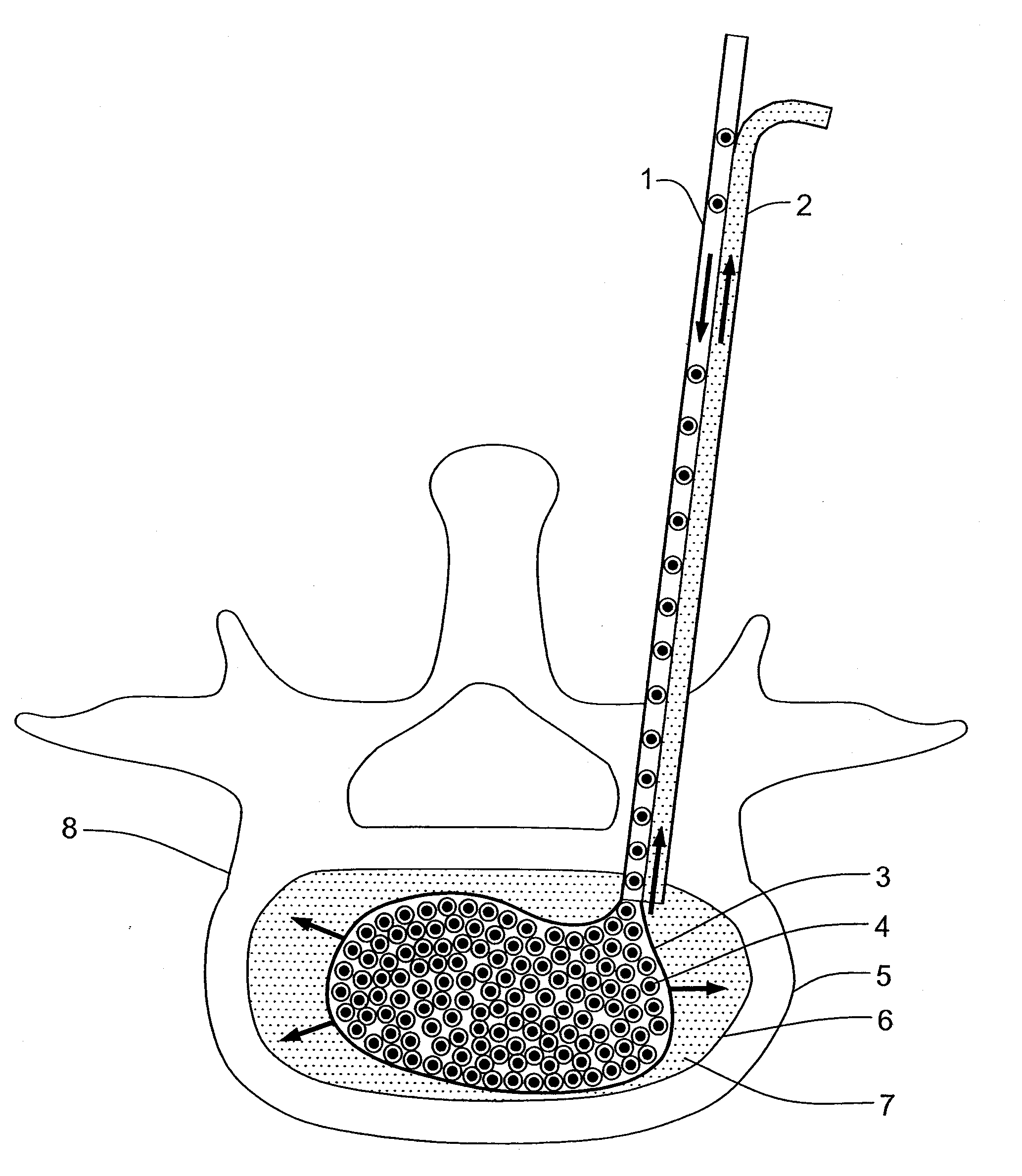
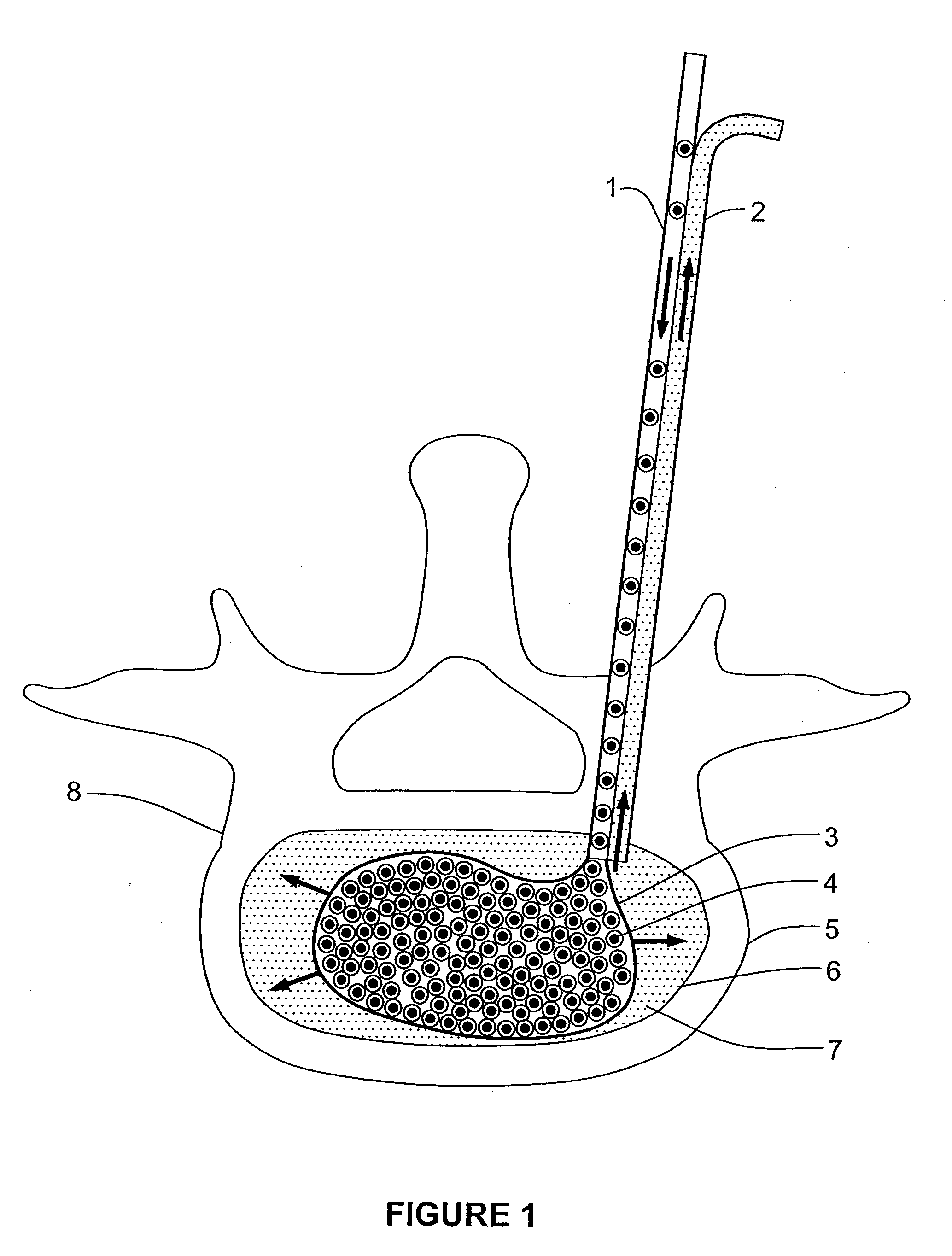
A method for performing percutaneous interbody fusion is disclosed. The method includes the steps of inserting a guide needle posteriorly to the disc space, inserting a dilator having an inner diameter slightly larger than the outer diameter of the guide needle over the guide needle to the disc space to enlarge the disc space, and successively passing a series of dilators, each having an inner diameter slightly larger than the outer diameter of the previous dilator, over the previous dilator to the disc space the gradually and incrementally increase the height of the disc space. Once the desired disc height is achieved, the guide needle and all the dilators, with the exception of the outermost dilator, are removed. An expandible intervertebral disc spacer is then passed through the remaining dilator and positioned in the disc space. The disc spacer is expanded to the required disc height, and then a bone matrix is passed through the dilator to fill the disc space. The dilator is then removed. An expandible intervertebral disc spacer is also disclosed, having a tapered bore that causes greater expansion of one end of the spacer with respect to the other. A kit for performing the percutaneous interbody fusion procedure is also disclosed.
Owner:BOEHM FR H JR +1
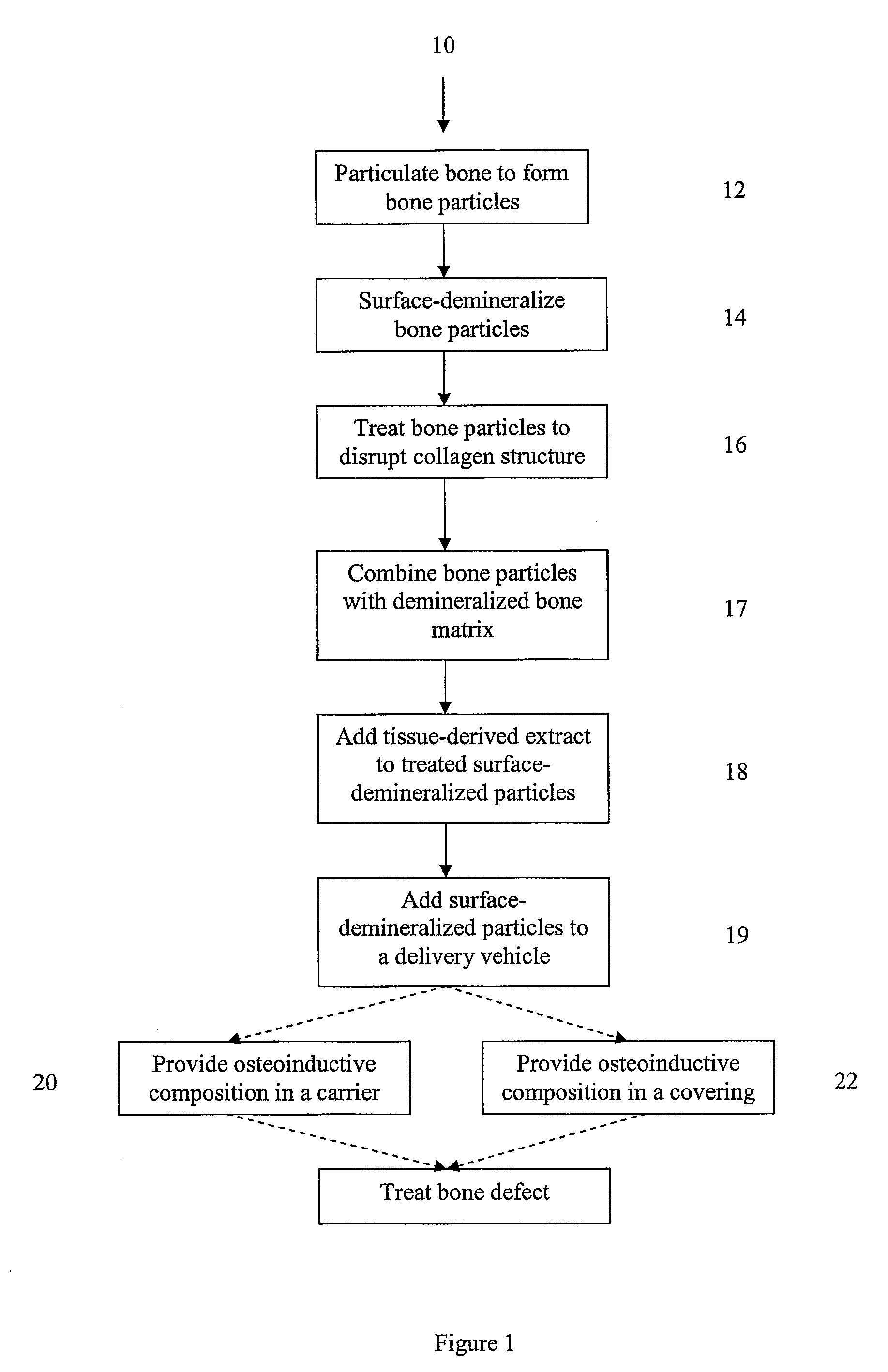
Bone matrix compositions and methods
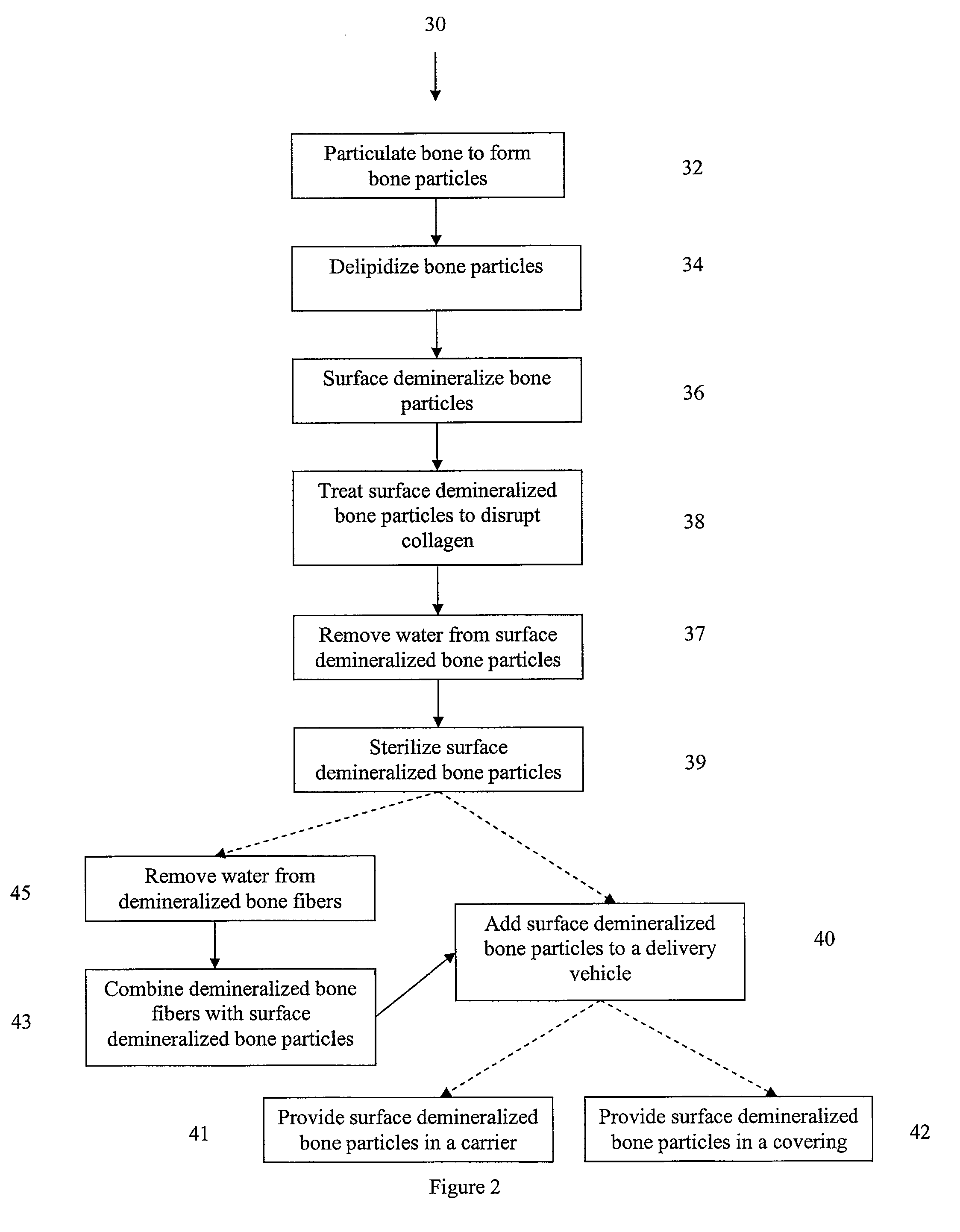
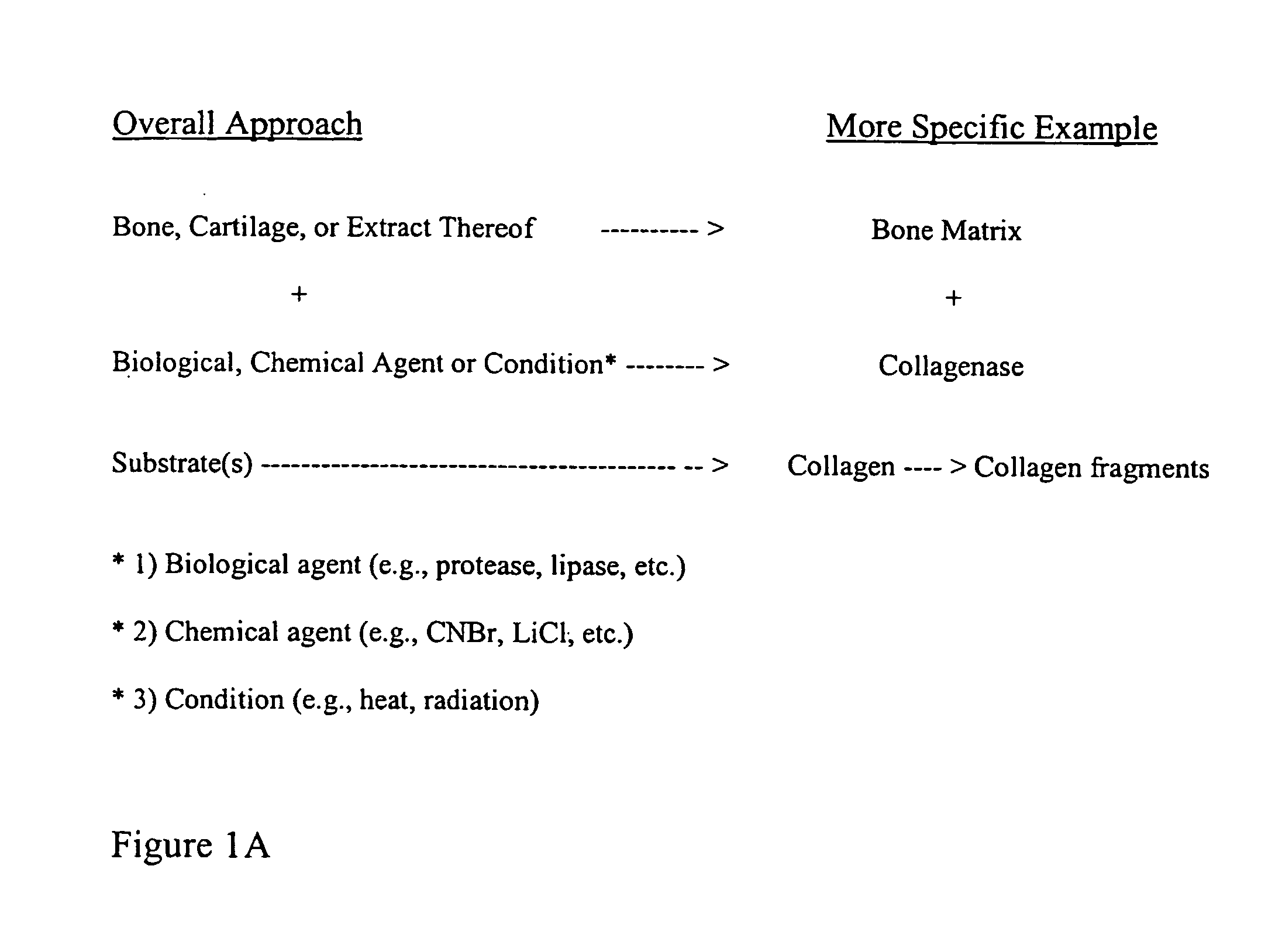
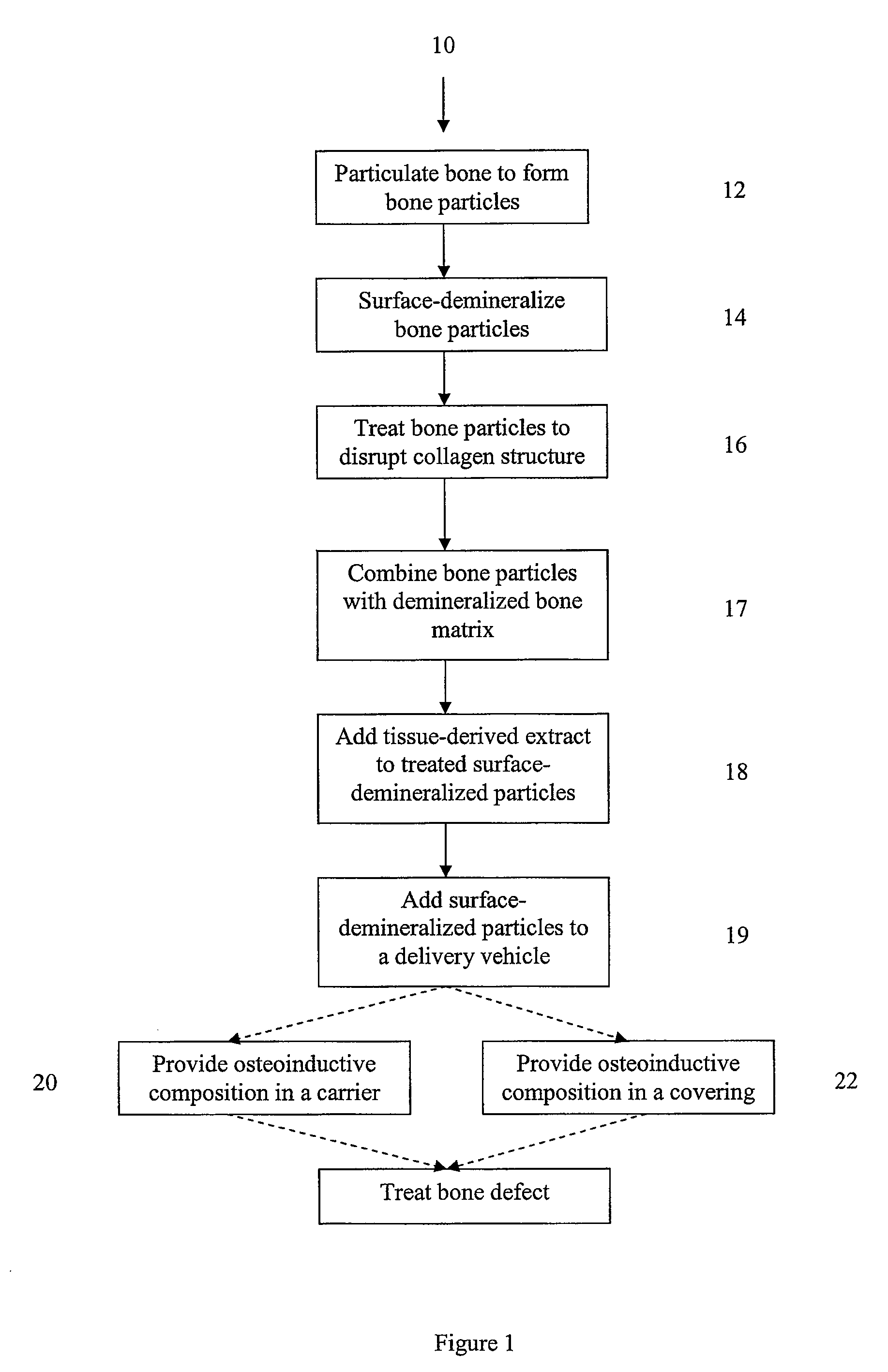
Osteoinductive compositions and implants having increased biological activities, and methods for their production, are provided. The biological activities that may be increased include, but are not limited to, bone forming; bone healing; osteoinductive activity, osteogenic activity, chondrogenic activity, wound healing activity, neurogenic activity, contraction-inducing activity, mitosisinducing activity, differentiation-inducing activity, chemotactic activity, angiogenic or vasculogenic activity, and exocytosis or endocytosis-inducing activity. In one embodiment, a method for producing an osteoinductive composition comprises providing partially demineralized bone, treating the partially demineralized bone to disrupt the collagen structure of the bone, and optionally providing a tissue-derived extract and adding the tissue-derived extract to the partially demineralized bone. In another embodiment, an implantable osteoinductive and osteoconductive composition comprises partially demineralized bone, wherein the collagen structure of the bone has been disrupted, and, optionally, a tissue-derived extract.
Owner:WARSAW ORTHOPEDIC INC
Bone Matrix Compositions and Methods
ActiveUS20070098756A1High activityEasy to addPeptide/protein ingredientsBone implantActive agentOSTEOINDUCTIVE FACTOR
An osteoinductive composition, corresponding osteoimplants, and methods for making the osteoinductive composition are disclosed. The osteoinductive composition comprises osteoinductive factors, such as may be extracted from demineralized bone, and a carrier. The osteoinductive composition is prepared by providing demineralized bone, extracting osteoinductive factors from the demineralized bone, and adding the extracted osteoinductive factors to a carrier. Further additives such as bioactive agents may be added to the osteoinductive composition. The carrier and osteoinductive factors may form an osteogenic osteoimplant. The osteoimplant, when implanted in a mammalian body, can induce at the locus of the implant the full developmental cascade of endochondral bone formation including vascularization, mineralization, and bone marrow differentiation. Also, in some embodiments, the osteoinductive composition can be used as a delivery device to administer bioactive agents.
Owner:WARSAW ORTHOPEDIC INC
Bone matrix compositions and methods
ActiveUS20070154563A1Good osteoinductivityHigh activityHydrolysed protein ingredientsBone implantOsteoblastLine of therapy
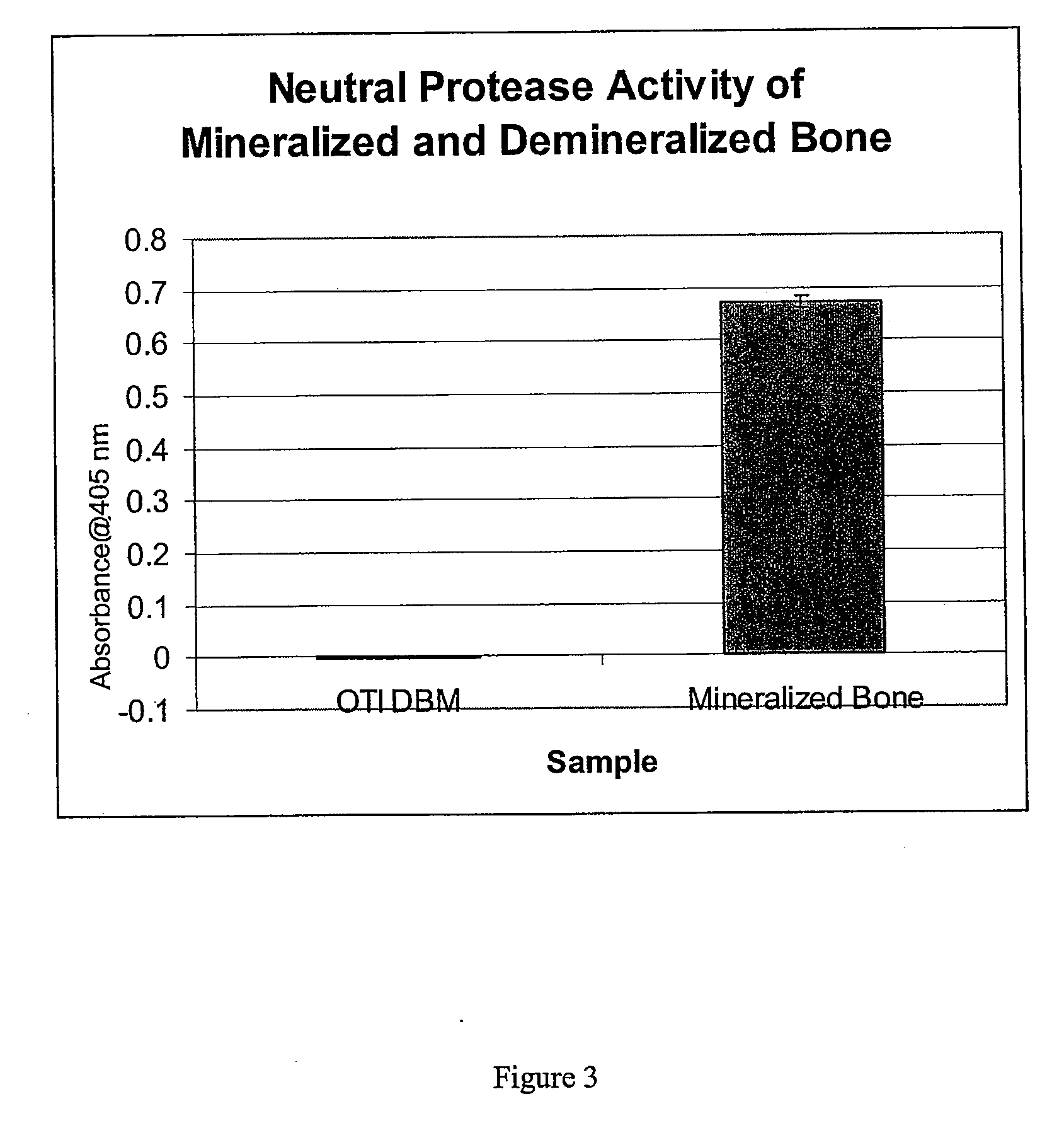
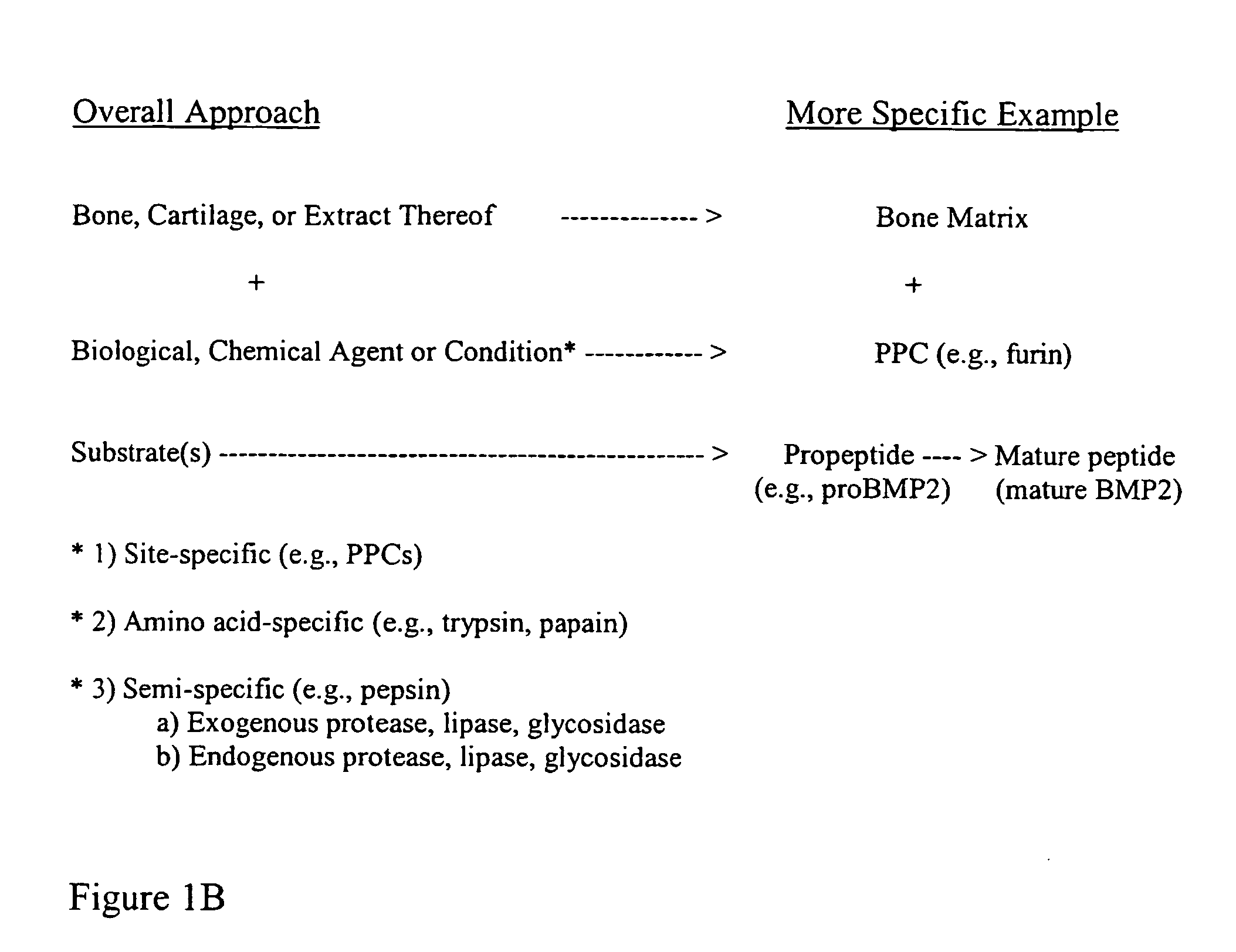
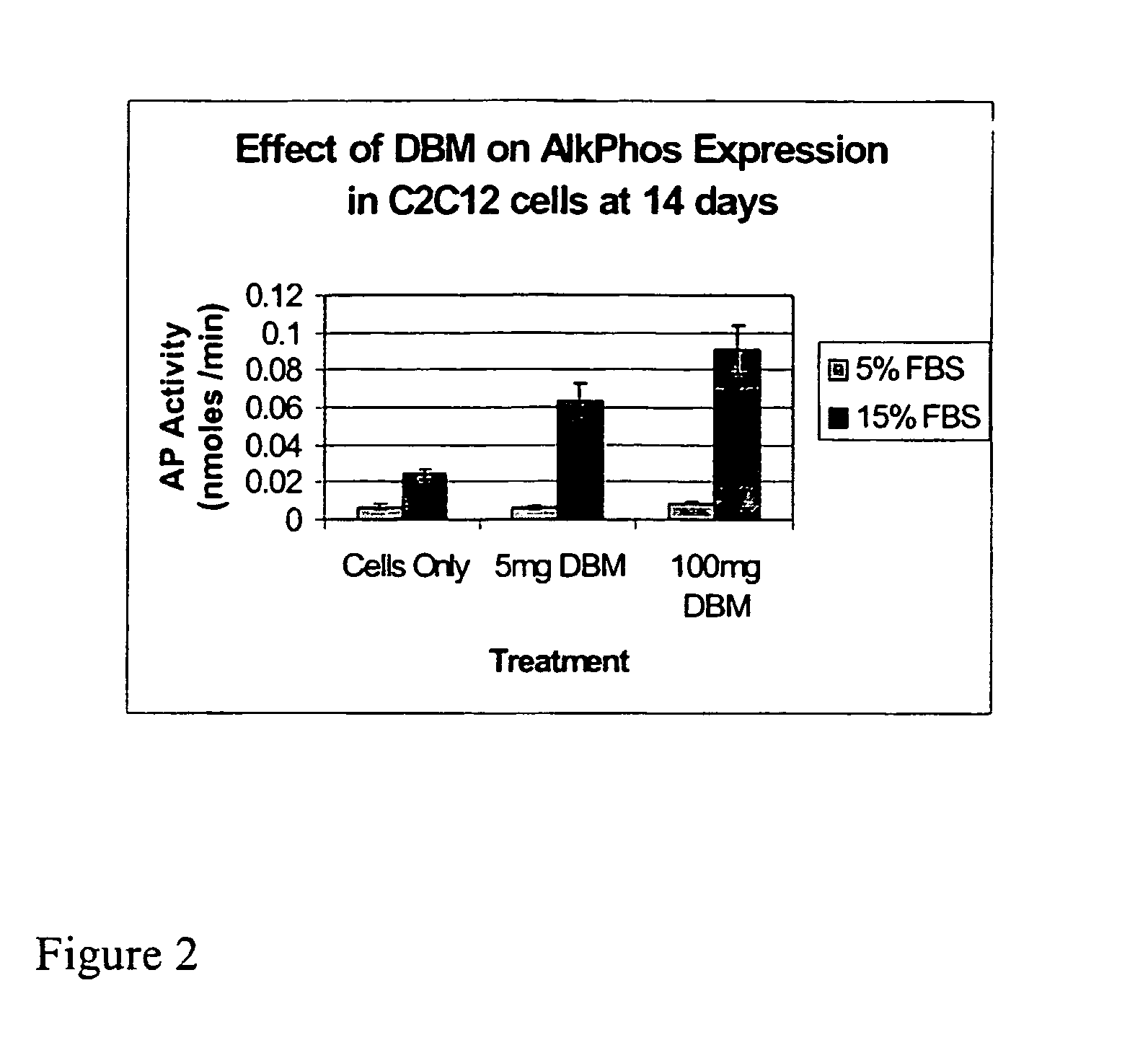
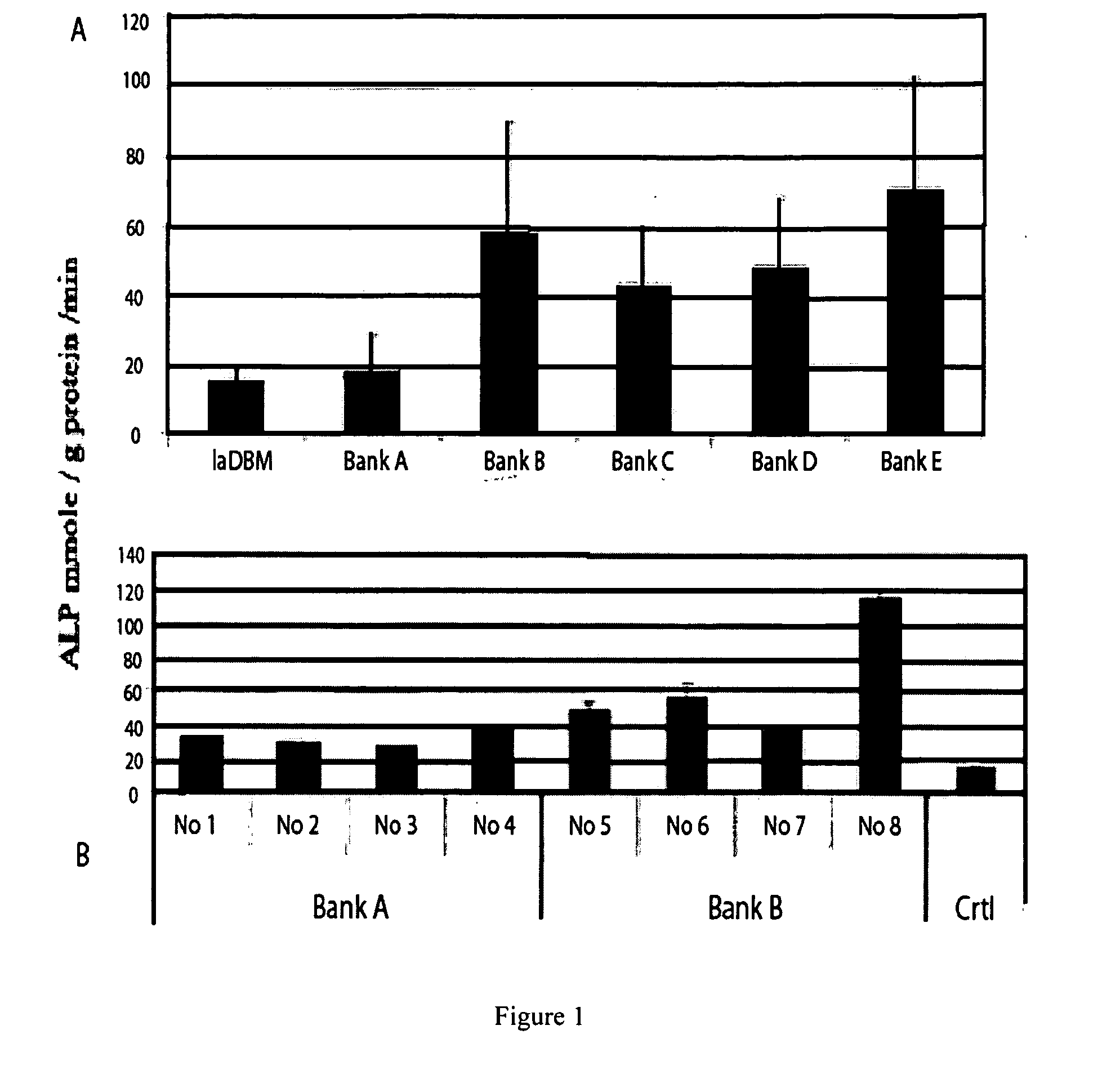
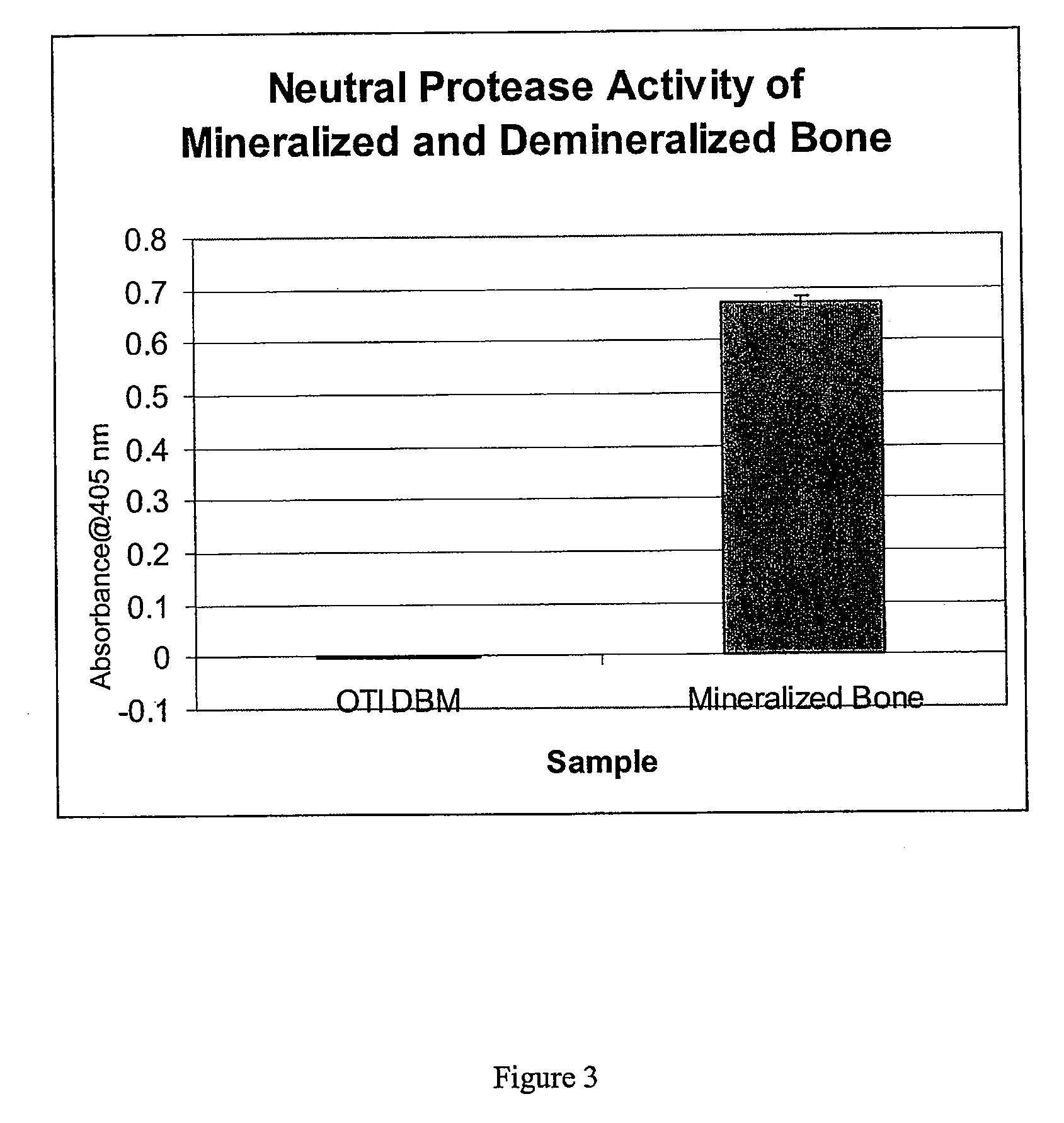
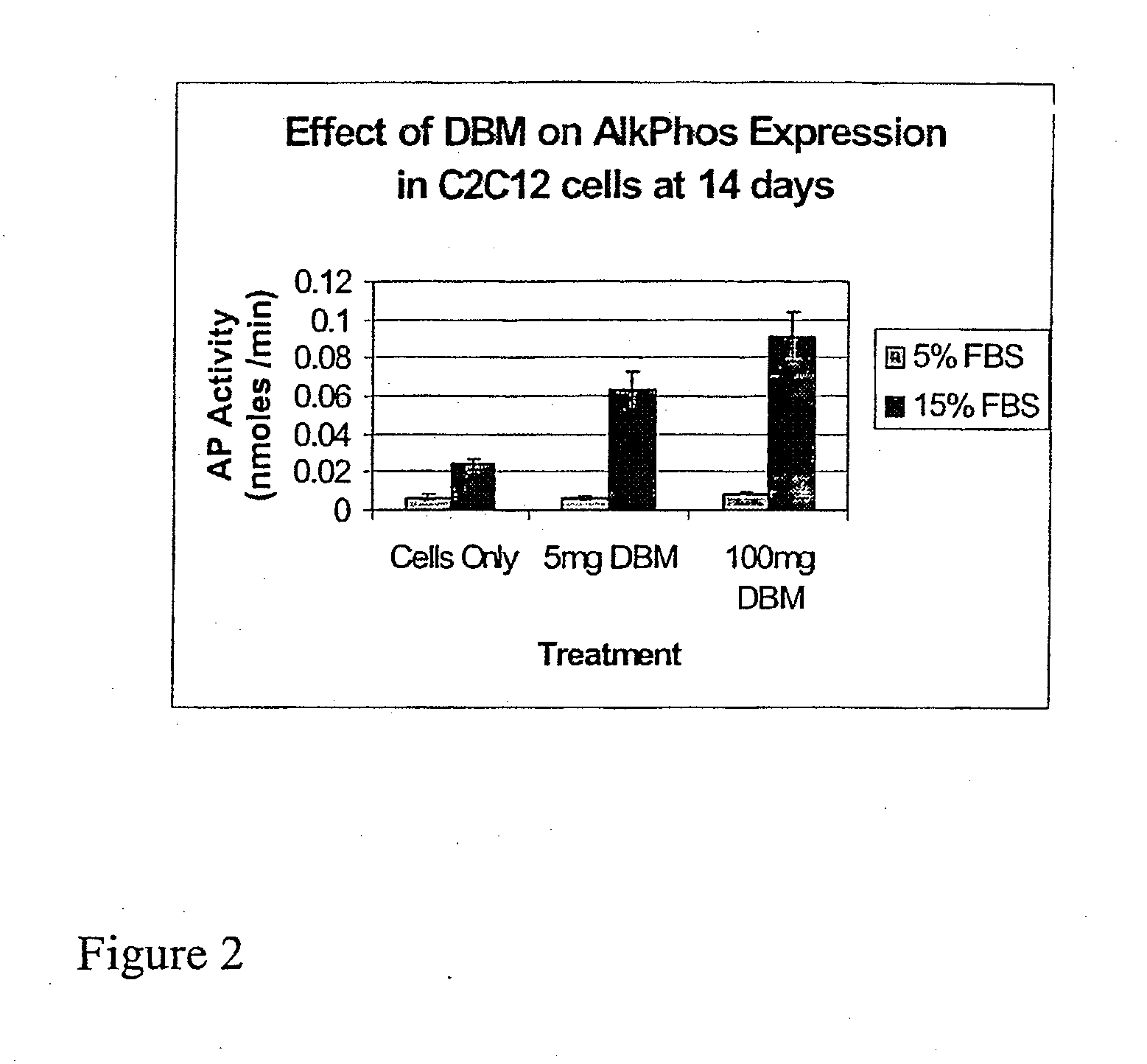
The present invention provides methods of improving the osteogenic and / or chondrogenic activity of a bone matrix, e.g., a dermineralized bone matrix (DBM), by exposing the bone matrix to one or more treatments or conditions. In preferred embodiments the bone matrix is derived from human bone. The treatment or condition may alter the structure of the bone matrix and / or cleave one or more specific proteins. Cleavage may generate peptides or protein fragments that have osteoinductive, osteogenic, or chondrogenic activity. Preferred treatments include collagenase and various other proteases. The invention further provides improved bone and cartilage matrix compositions that have been prepared according to the inventive methods and methods of treatment using the compositions. The invention further provides methods of preparing, testing, and using the improved bone matrix compositions. Ona assay comprises exposing relatively undifferentiated mesenchymal cells to a bone matrix composition and measuring expression of a marker characteristic of osteoblast or chondrocyte lineage(s). Increased expression of the marker relative to the level of the marker in cells that have been exposed to a control matrix (e.g., an inactivated or untreated matrix) indicates that the treatment or condition increased the osteogenic and / or chondrogenic activity of the bone matrix. Suitable cells include C2C12 cells. A suitable marker is alkaline phosphatase. The inventive methods increase the osteogenic and / or chondrogenic activity of human DBM when tested using this assay system.
Owner:WARSAW ORTHOPEDIC INC
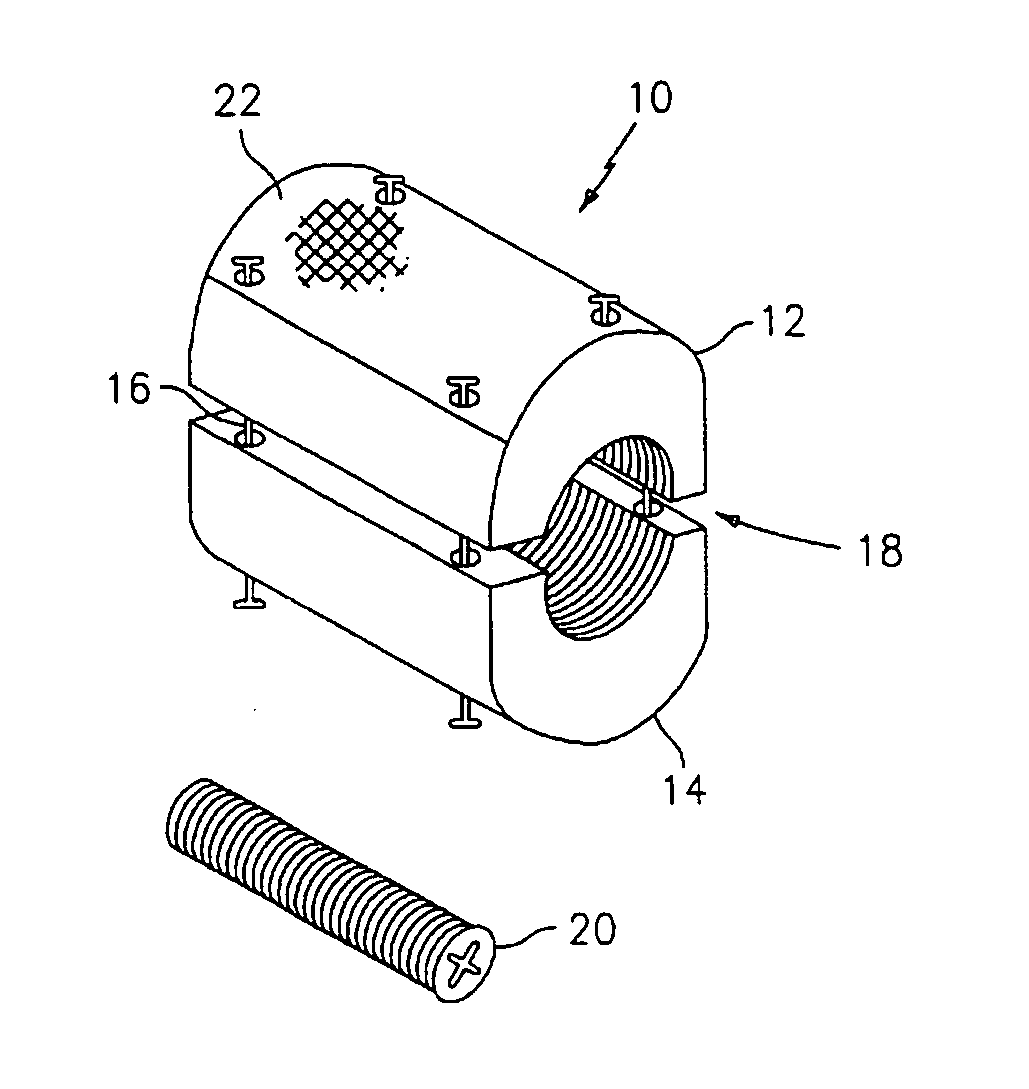
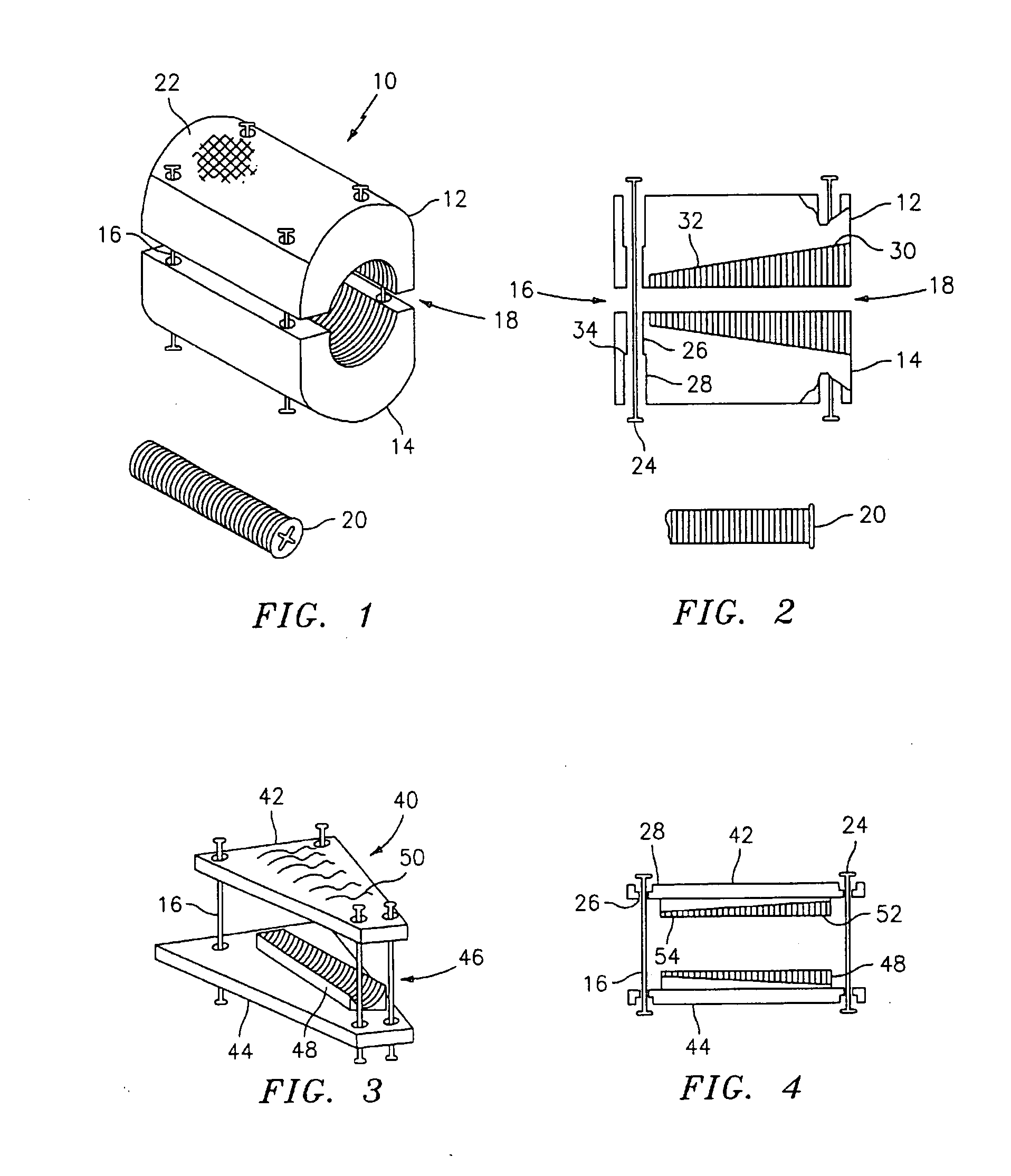
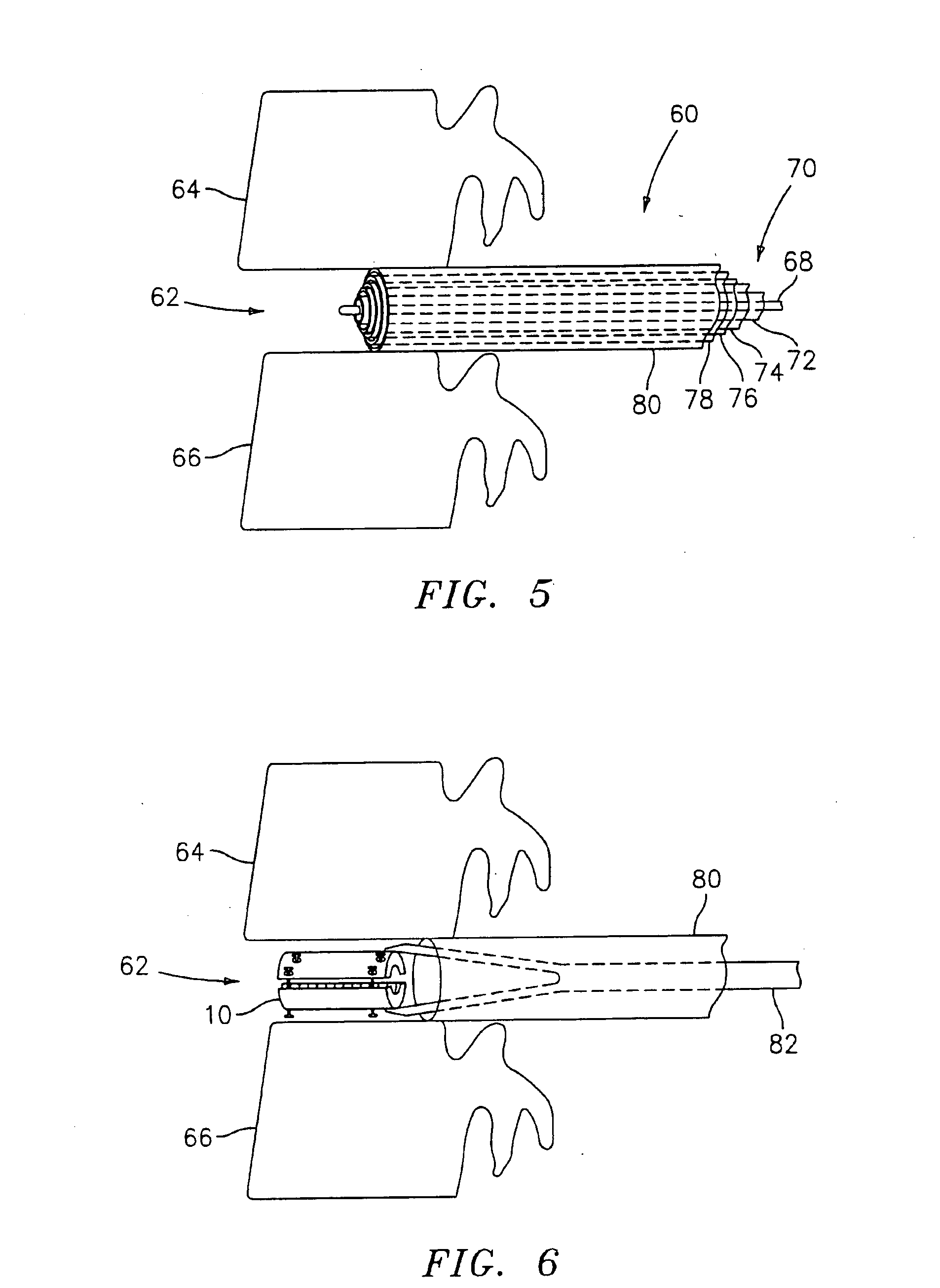
Bone screw with fluid delivery structure
A device and method to mechanically strengthen bone matrix and deliver tissue reinforcement material to weakened areas in skeletal structures. A preferred embodiment is an orthopedic screw configured to receive and directionally disperse a therapeutic fluid through the screw's channels and apertures.
Owner:MILLENNIUM MEDICAL TECH INC
Bone Matrix Compositions and Methods
ActiveUS20070110820A1High activityEasy to addPeptide/protein ingredientsImmunoglobulinsOSTEOINDUCTIVE FACTORBone implant
Owner:WARSAW ORTHOPEDIC INC
Demineralized bone matrix compositions and methods
Bone matrix compositions and, more specifically, demineralized bone matrix (DBM) having increased osteoinductive capacity and methods for its production are provided. Specifically, DBM derived from cortical bone from the periosteal layer of bone are provided. Compositions comprising a disproportionate amount of DBM prepared from bone derived from the periosteal and / or middle layer of bone are provided. Preparations of and methods of use of periosteal DBM compositions are disclosed.
Owner:WARSAW ORTHOPEDIC INC
Bone matrix compositions having nanoscale textured surfaces
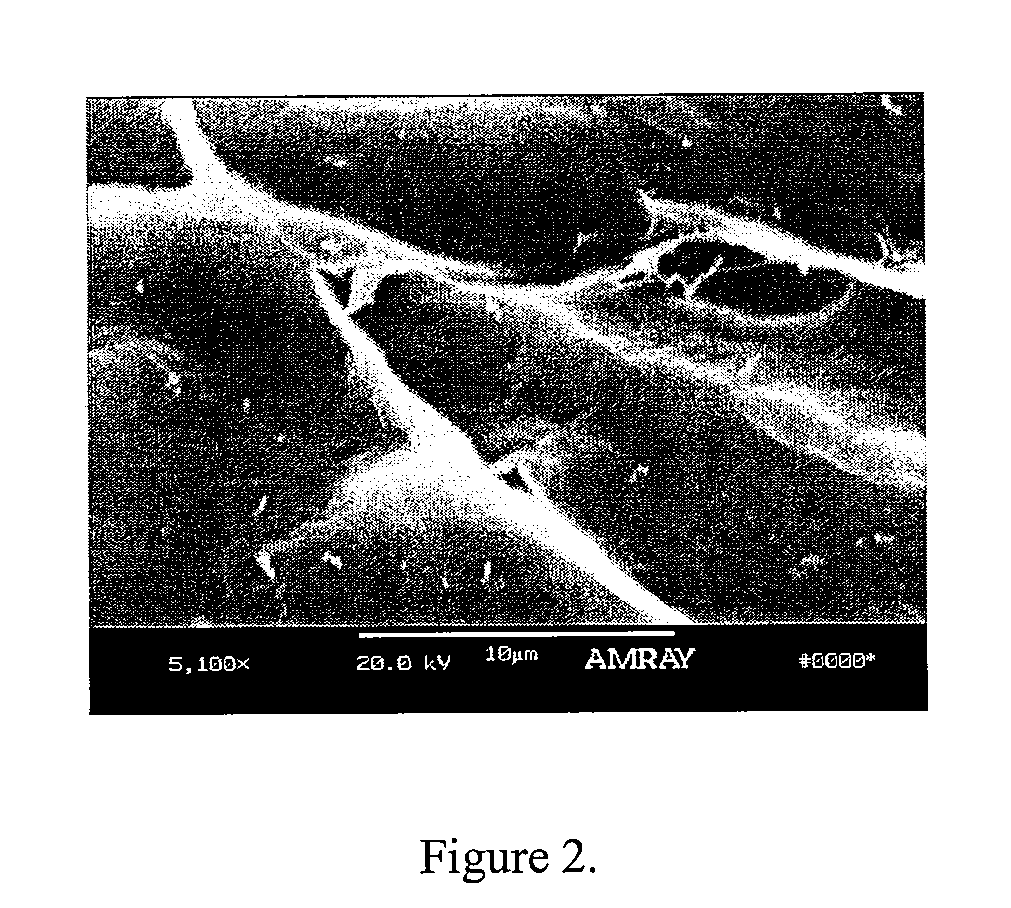
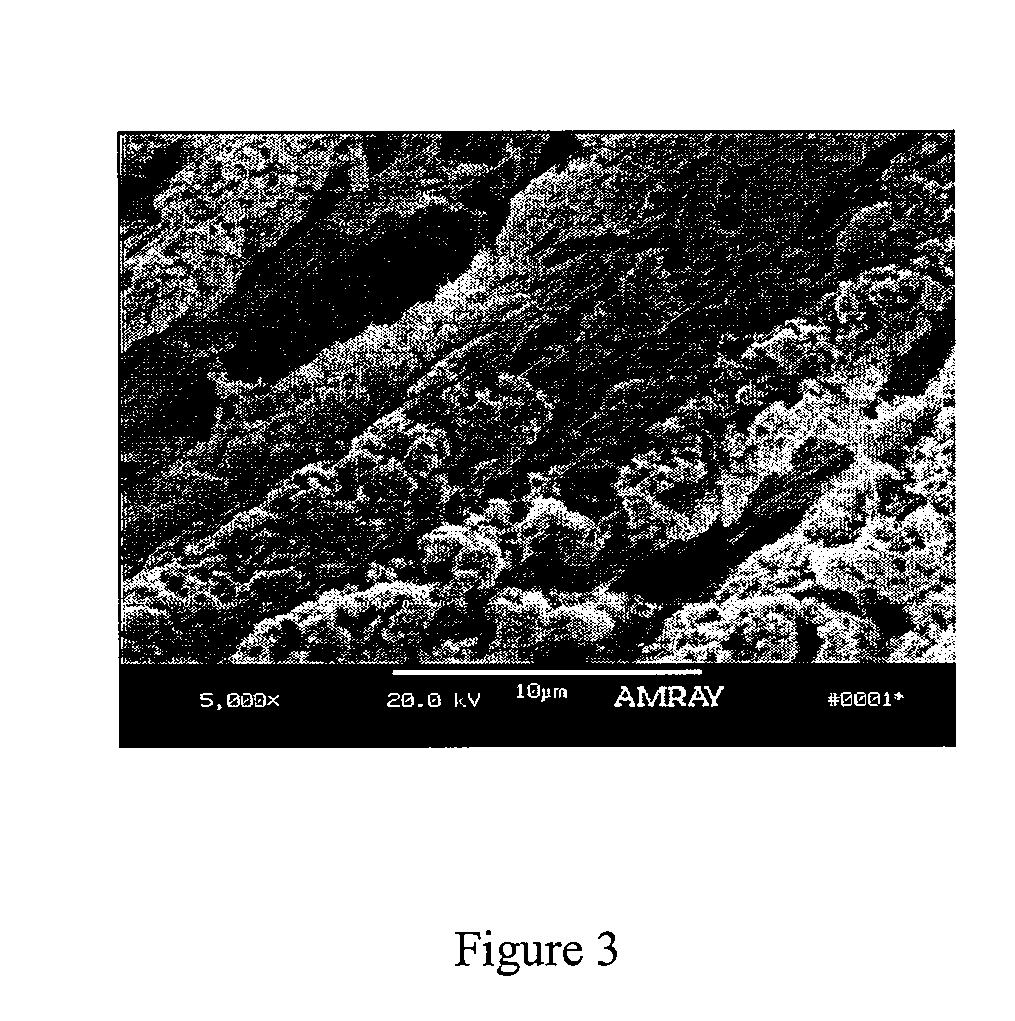
Bone matrix compositions having nanoscale textured surfaces and methods for their production are provided. In some embodiments, bone matrix is prepared for implantation and retains nanoscale textured surfaces. In other embodiments, nanostructures are imparted to bone matrix wherein collagen fibrils on the surface of the bone matrix have been compromised, thus imparting a nanoscale textured surface to the bone matrix. Generally, these methods may be applied to mineralized or demineralized bone including partially or surface demineralized bone.
Owner:WARSAW ORTHOPEDIC INC
Method and device for reducing susceptibility to fractures in vertebral bodies
InactiveUS20080051800A1Reduce sensitivityIncrease bone densityInternal osteosythesisPeptide/protein ingredientsBone growthUltimate tensile strength
The invention provides a method and a device for administering bone matrix with or without additional bone growth enhancing agents, or administering one or more bone growth enhancing agents to the interior surface of an unfractured vertebral body to enhance bone growth and strength, thus reducing susceptibility of the vertebral body to subsequent fracture.
Owner:DIAZ ROBERT +1
Transplant material and method for fabricating the same
InactiveUS6989030B1Improved bone tissue repair speedGood biocompatibilityBone implantTissue cultureCell adhesionOsteoblast
A transplant material which is capable of imparting desired mechanical properties, elevating bone tissue repair speed and improving biocompatibility. This transplant material comprises an artificial and biologically inactive material, which is to be implanted in vivo as a substitute for bone tissue, and at least one type of cells selected from among osteoblasts and precursory osteoblasts which are adhered to the surface of the artificial material so that the artificial material is coated with the bone matrix produced by the cells. The artificial material involves not only a biologically inactive material but also a biologically inactive material coated with a biologically active substrate. This transplant material is produced by culturing mesenchymal stem cells collected from a living body to differentiate into at least one type of cells selected from among osteoblasts and precursory osteoblasts and then culturing the cells together with the artificial material to thereby adhere the differentiated cells on the surface of the artificial material and coat the surface of the artificial material with the bone matrix produced by the differentiated cells.
Owner:JAPAN TISSUE ENG
Method of preparing bone material having enhanced osteoinductivity
ActiveUS20140205674A1Good osteoinductivityIncrease surface areaPeptide/protein ingredientsMammal material medical ingredientsFiberCritical point drying
Methods for increasing osteoinductivity and / or surface area of bone material are provided. The methods include providing bone material and dehydrating the bone material with a solvent at its critical point. A useful solvent for critical point dehydrating is carbon dioxide. Critical point dehydration resulting in increased osteoinductivity and / or surface area can be applied to many types of bone material including bone particles, bone chips, bone fibers, bone matrices, both demineralized and non-demineralized. An implantable composition having an enhanced osteoinductivity and / or osteoconductivity is also provided. The implantable composition contains demineralized bone matrix dried at critical point of carbon dioxide. Critical point dried fibers of a demineralized bone matrix have a BET value from about 40 m2 / gm to about 100 m2 / gm, a value that is 100 times higher than corresponding vacuum dried or lyophilized DBM fibers.
Owner:WARSAW ORTHOPEDIC INC
Device which enhances the biological activity of locally applied growth factors with particular emphasis on those used for bone repair
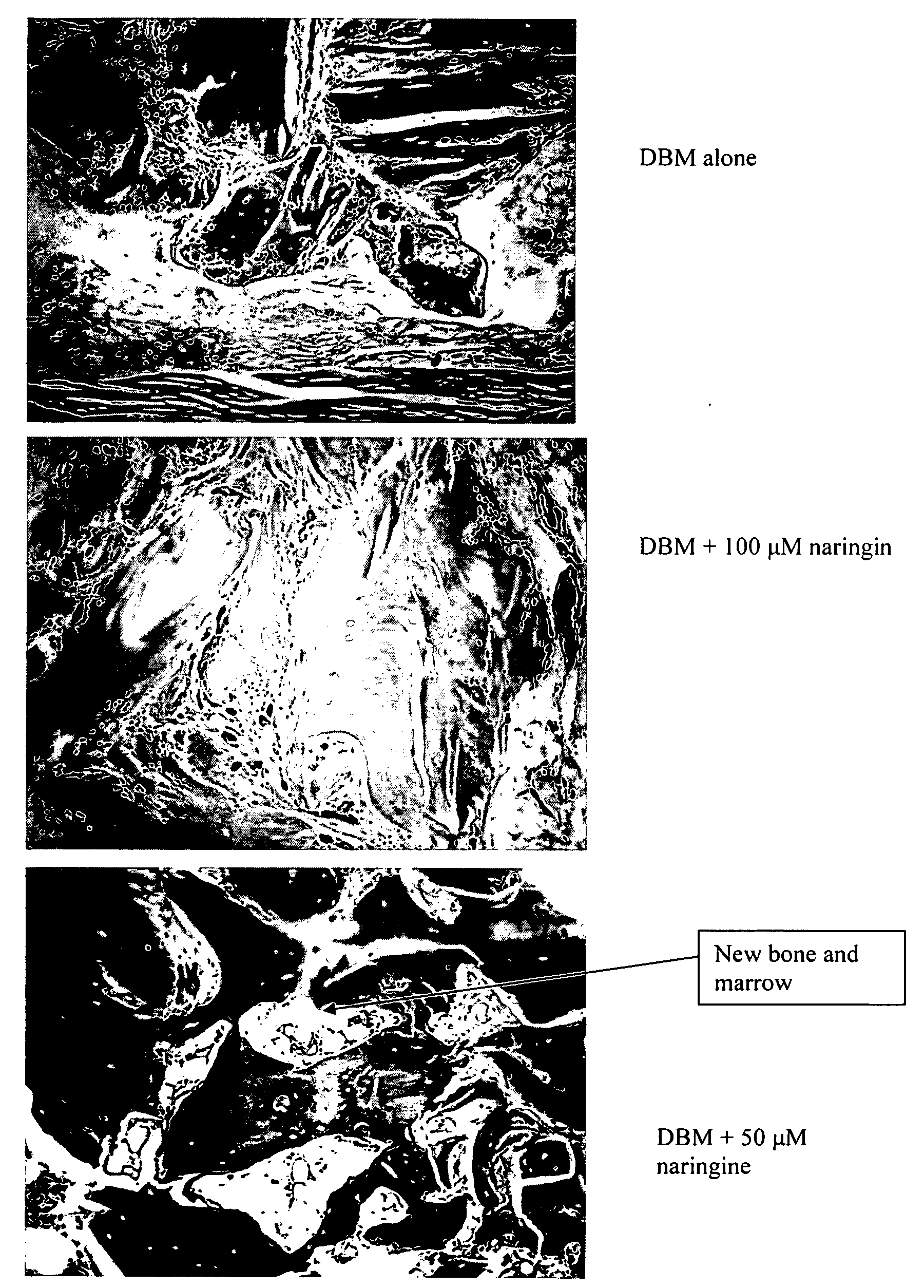
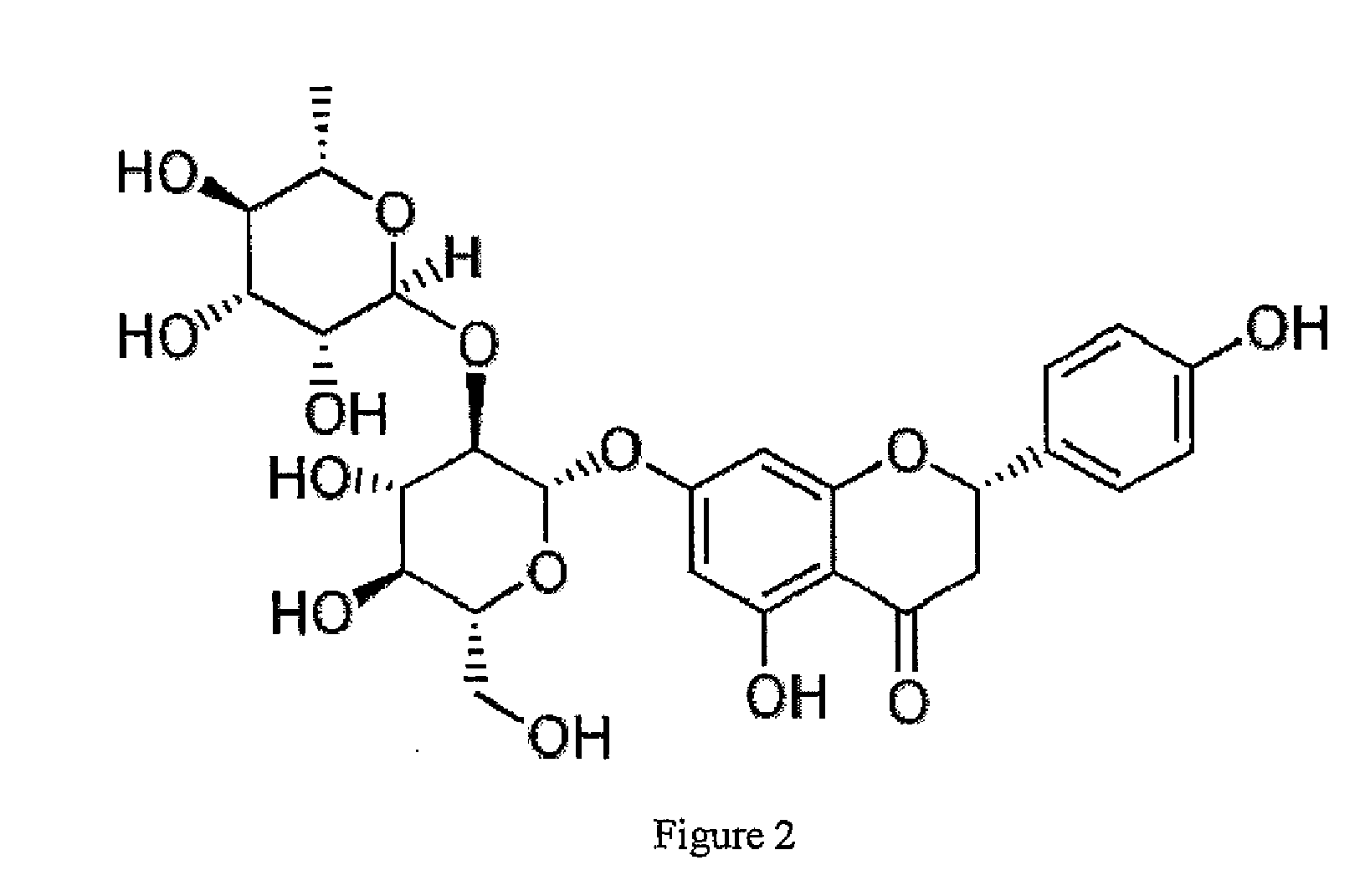
InactiveUS20080241211A1Improve biological activityReduce dose requiredBiocidePowder deliveryNaringinMedicine
This invention provides a novel medical appliance for repairing, regenerating, maintaining, and / or augmenting a bone. The medical appliance generally includes an osteoinductive agent, an osteoinductive enhancer, and a carrier matrix. Also disclosed are methods, compositions, kits, and bone matrix formulations for regenerating, maintaining, and / or augmenting a bone. Exemplary preferred osteoinductive agents include growth factors such as BMP and TGF-β. Exemplary preferred osteoinductive enhancers include phytoestrogens such as naringin.
Owner:UNIV OF SOUTHERN CALIFORNIA
Bone matrix compositions and methods
InactiveUS20110070312A1Shorten the timeMaintain biological activityUnknown materialsTissue regenerationBiomedical engineeringBone matrix
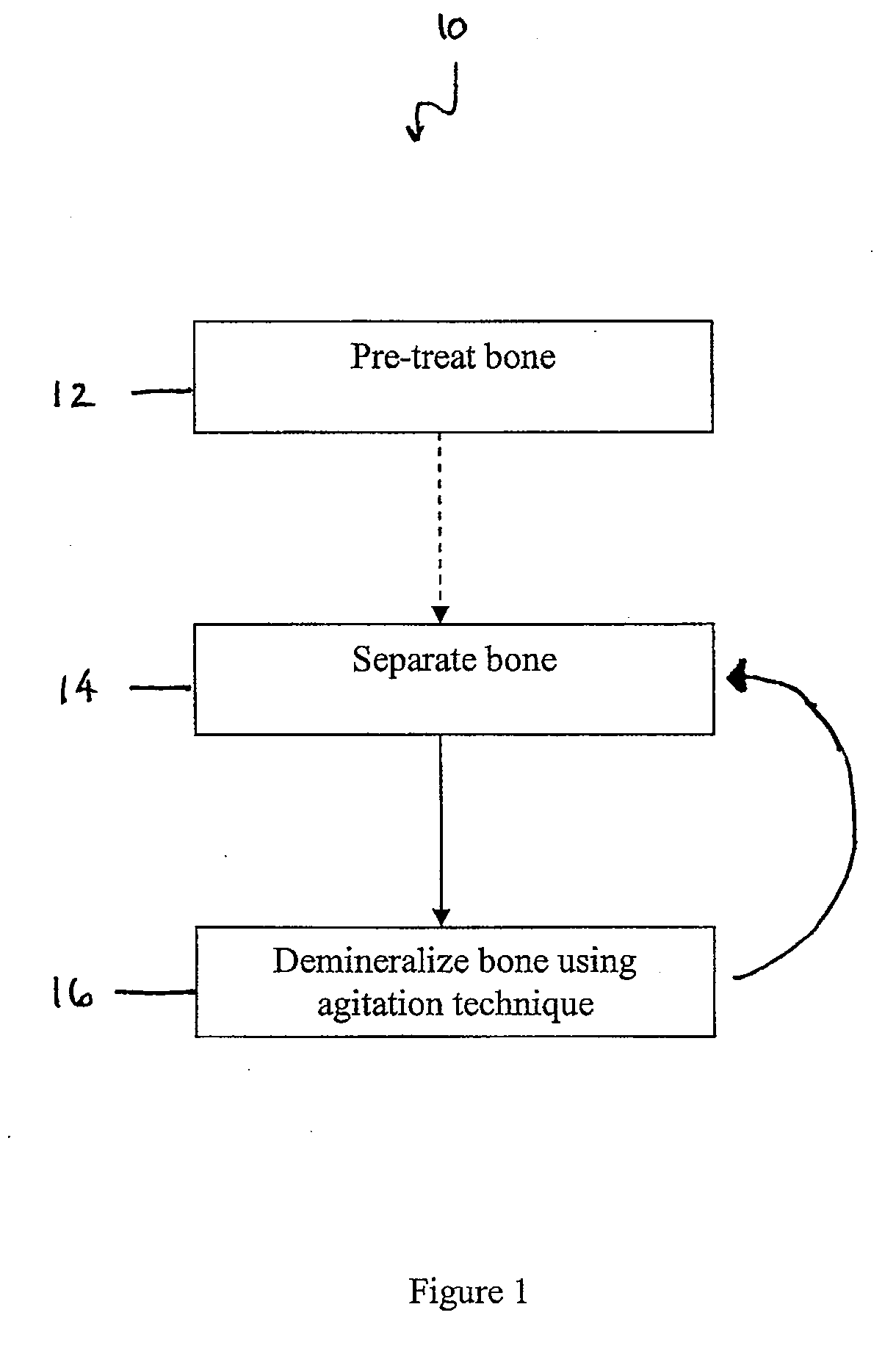
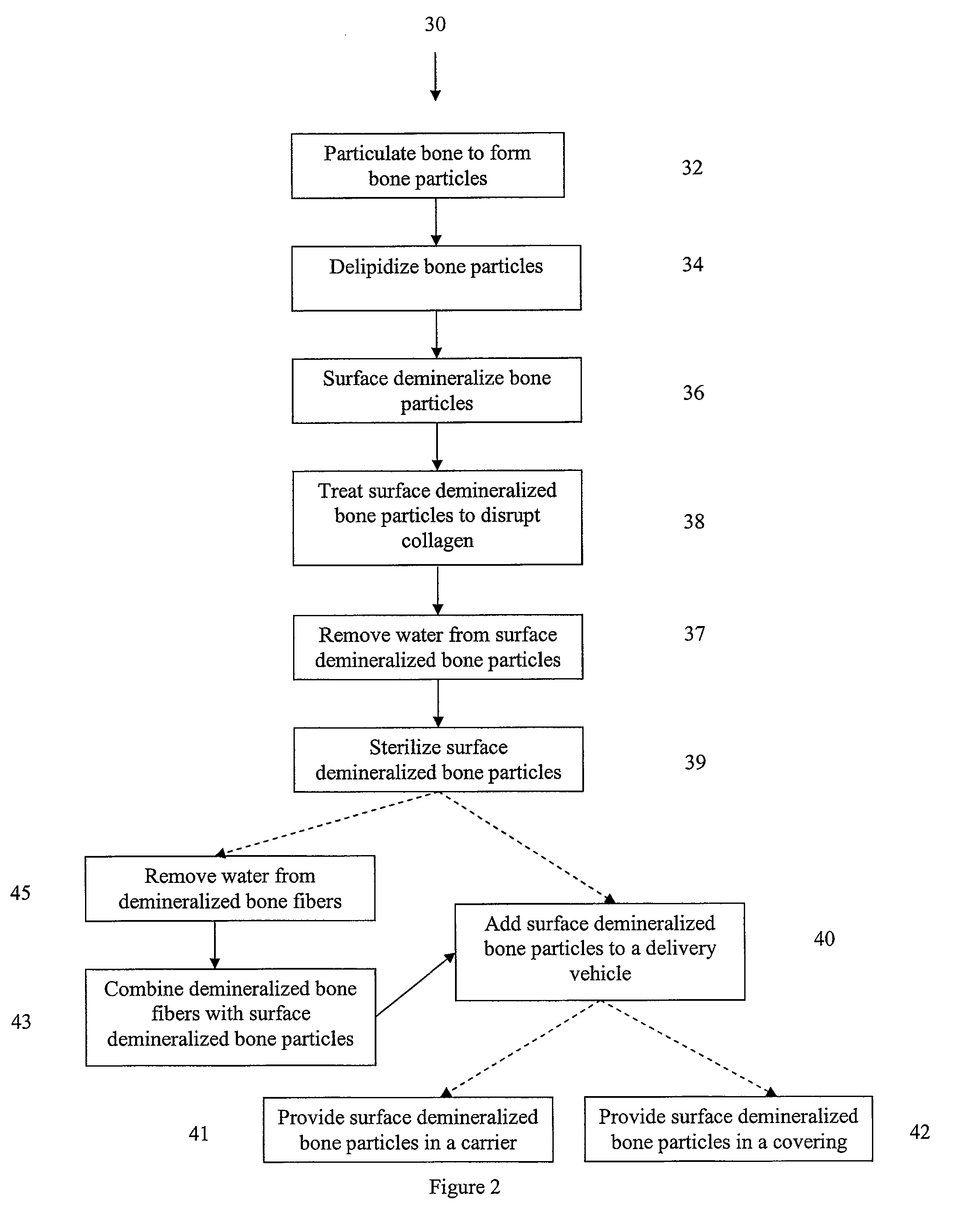
Methods for demineralizing bone are provided. In some embodiments, the methods include demineralizing the bone in a demineralization medium and separating the bone into particles during demineralization. In other embodiments, the methods include placing the bone in a demineralization medium and treating the demineralization medium while the bone is in the demineralization medium to optimize the demineralization process.
Owner:WARSAW ORTHOPEDIC INC
Demineralized bone matrix compositions and methods
ActiveUS20090226523A1Good osteoinductivityPowder deliverySkeletal disorderBone CortexDemineralized bone matrix
Bone matrix compositions and, more specifically, demineralized bone matrix (DBM) having increased osteoinductive capacity and methods for its production are provided. Specifically, DBM derived from cortical bone from the periosteal layer of bone are provided. Compositions comprising a disproportionate amount of DBM prepared from bone derived from the periosteal and / or middle layer of bone are provided. Preparations of and methods of use of periosteal DBM compositions are disclosed.
Owner:WARSAW ORTHOPEDIC INC
Bone matrix compositions and methods
Owner:WARSAW ORTHOPEDIC INC
Osteoinductive bone graft injectable cement
ActiveUS20120100225A1Sufficient load bearingFoster de novo bone growthSurgical adhesivesPeptide/protein ingredientsFiberMedicine
Osteoconductive bone graft materials are provided. These compositions contain injectable cements and demineralized bone matrix fibers. The combination of these materials enables the filling of a bone void while balancing strength and resorption.
Owner:WARSAW ORTHOPEDIC INC
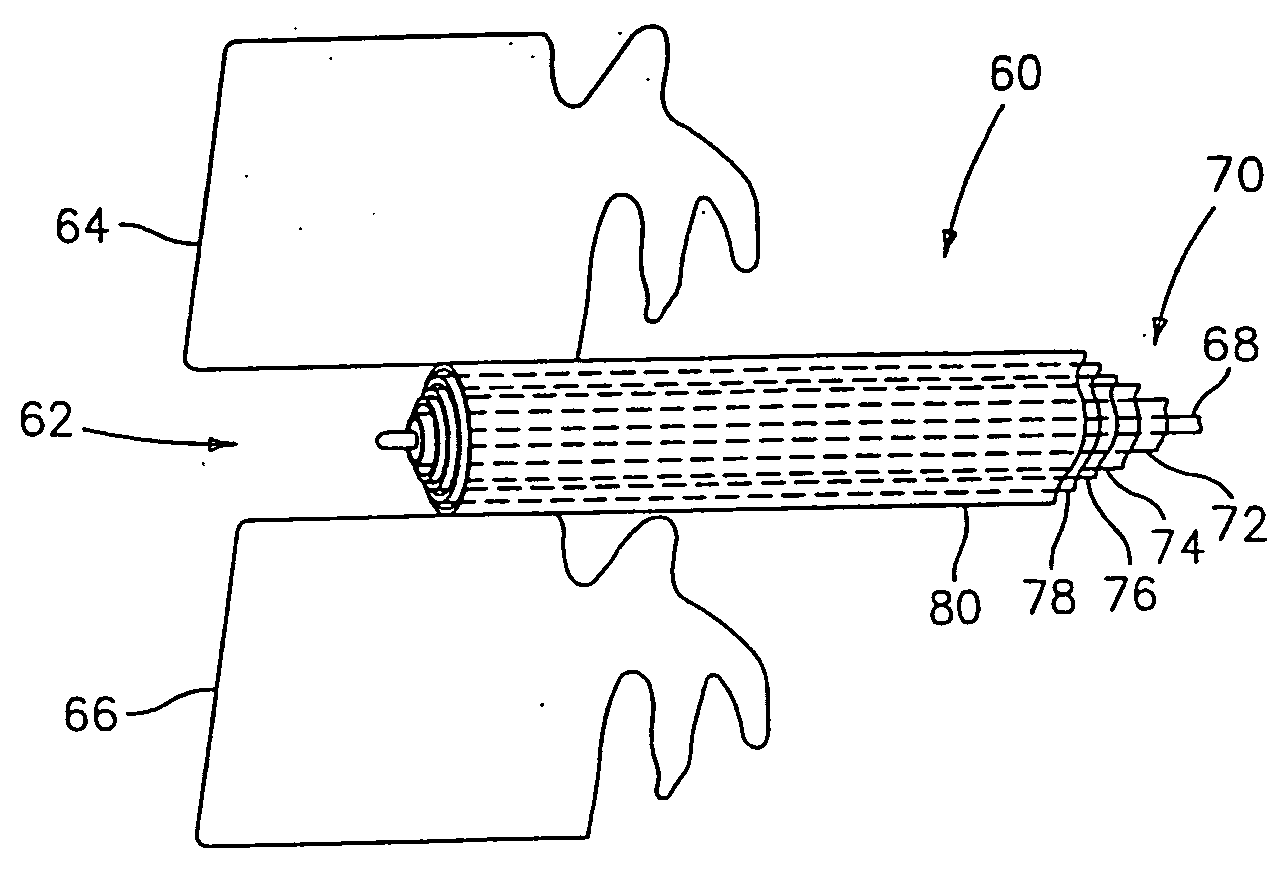
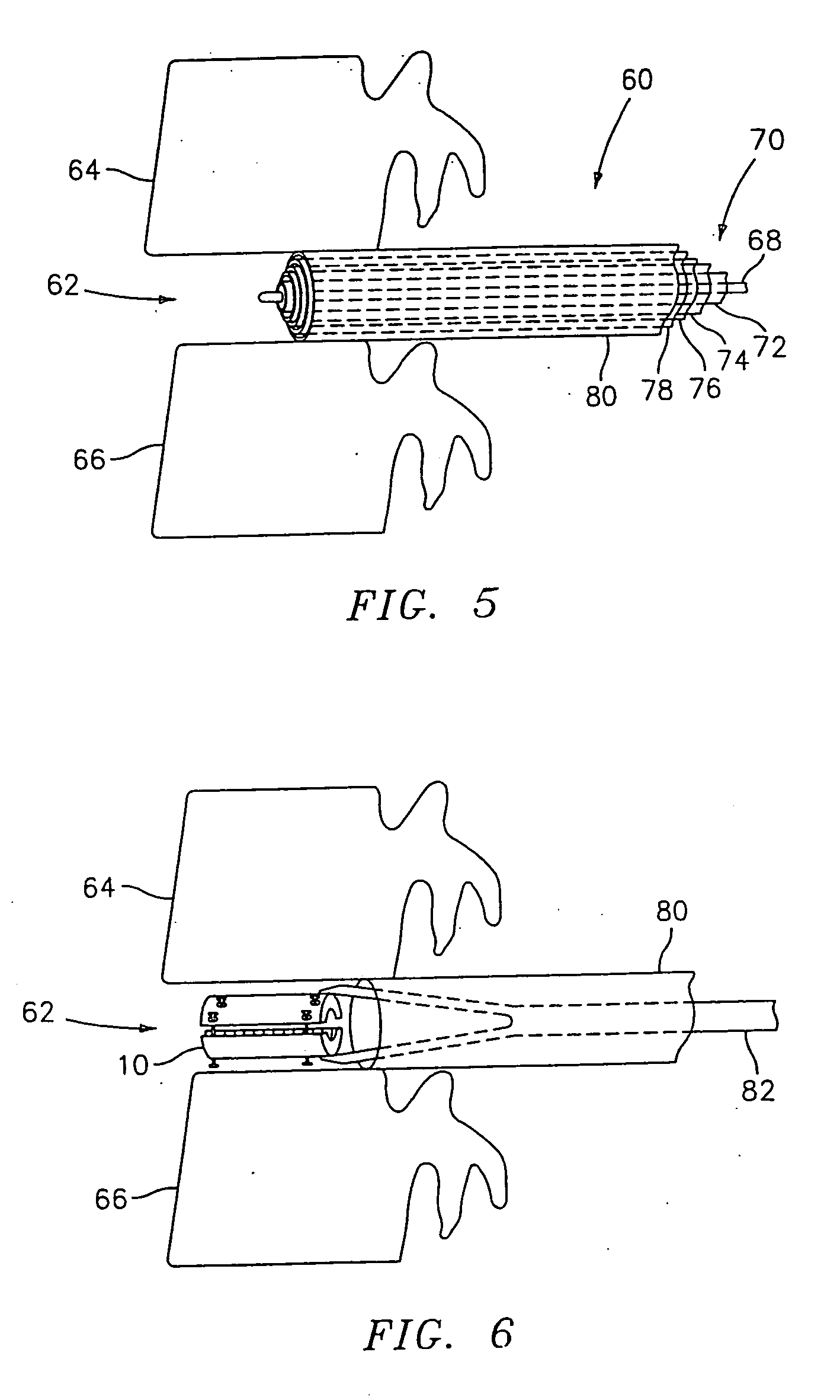
Device and method for lumbar interbody fusion
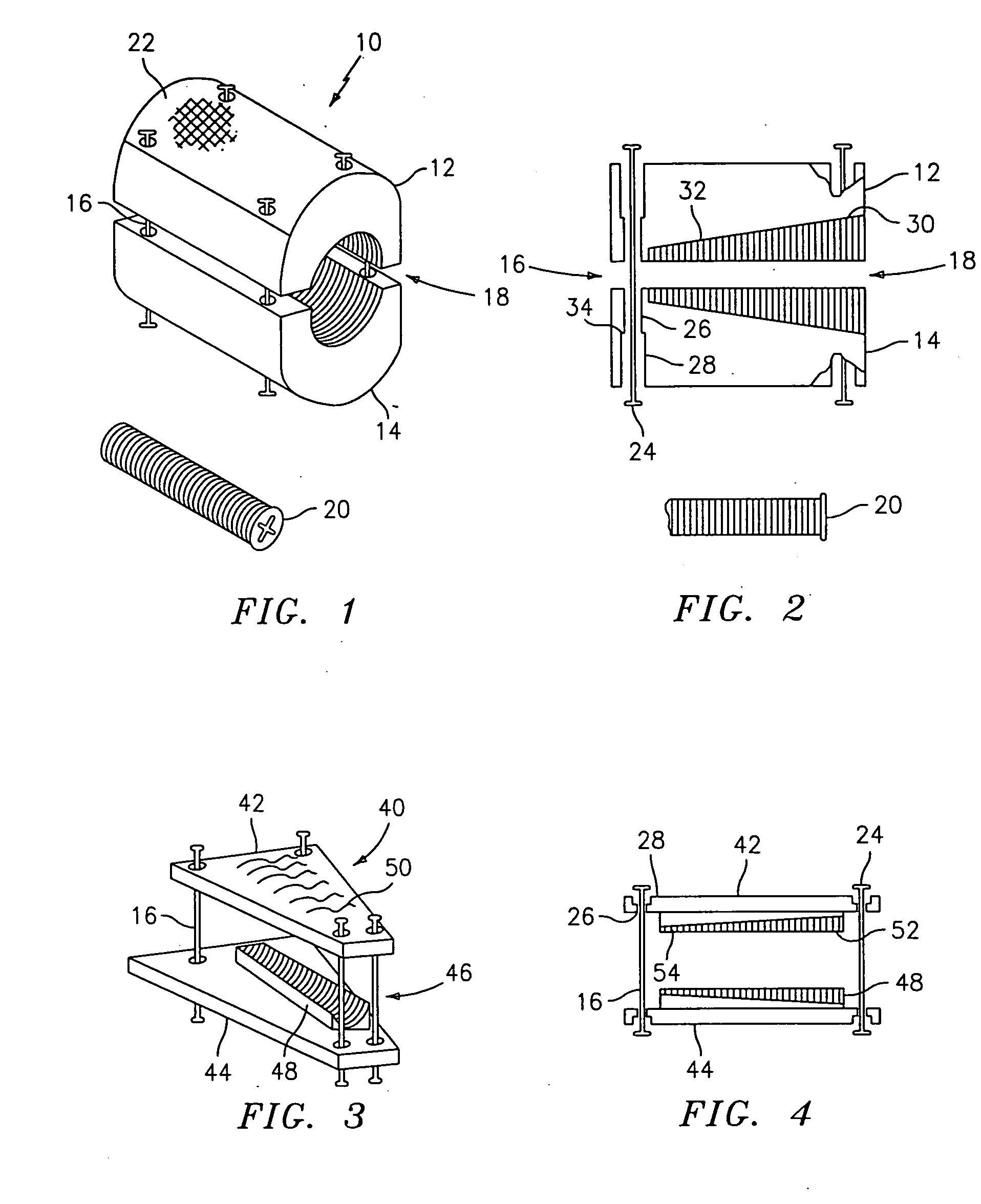
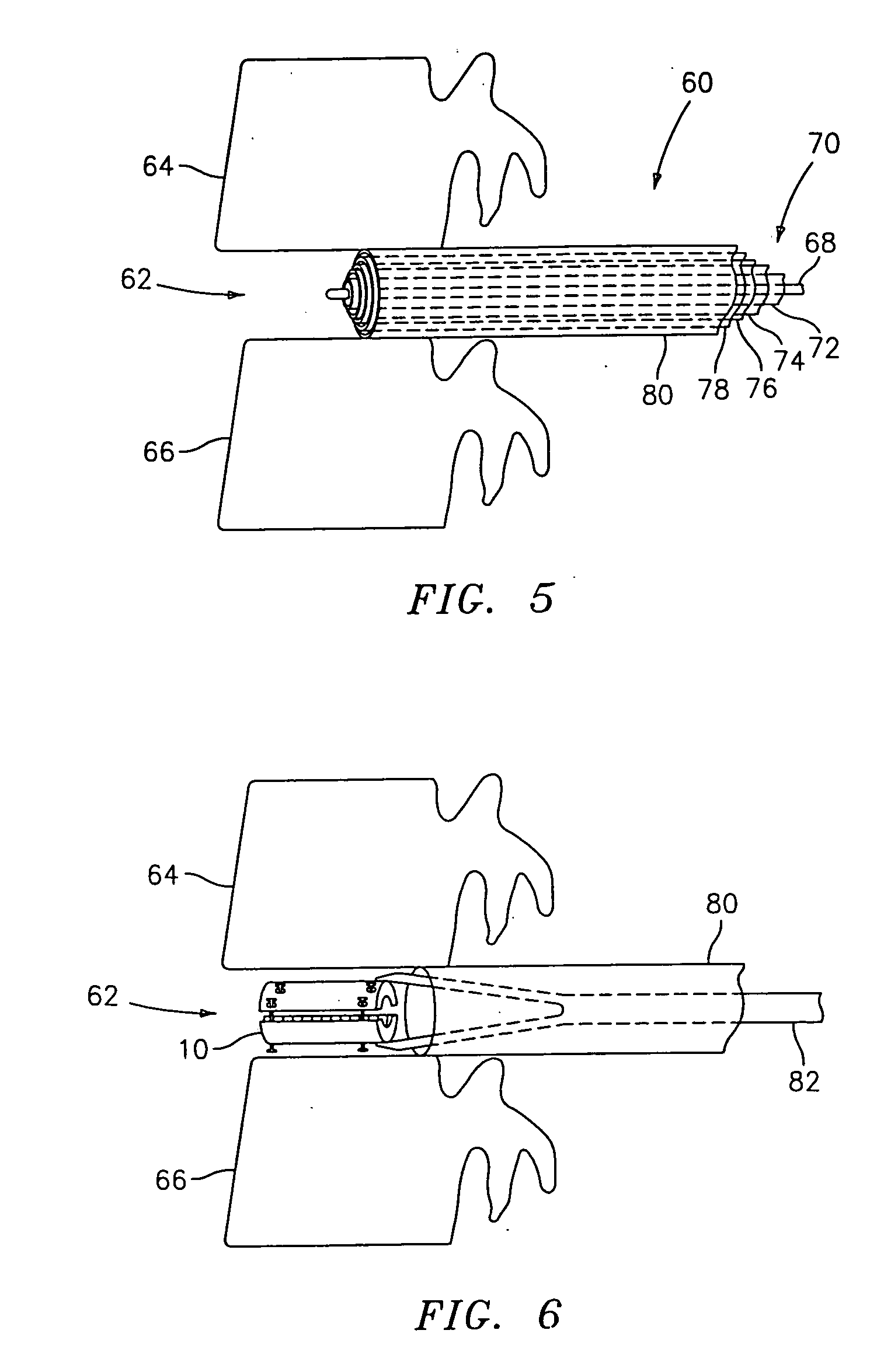
InactiveUS20050203625A1Shorten recovery timeReduces the trauma to the patientSurgical furnitureDiagnosticsMedicineDilator
A method for performing percutaneous interbody fusion is disclosed. The method includes the steps of inserting a guide needle posteriorly to the disc space, inserting a dilator having an inner diameter slightly larger than the outer diameter of the guide needle over the guide needle to the disc space to enlarge the disc space, and successively passing a series of dilators, each having an inner diameter slightly larger than the outer diameter of the previous dilator, over the previous dilator to the disc space the gradually and incrementally increase the height of the disc space. Once the desired disc height is achieved, the guide needle and all the dilators, with the exception of the outermost dilator, are removed. An expandible intervertebral disc spacer is then passed through the remaining dilator and positioned in the disc space. Th disc spacer is expanded to the required disc height, and then a bone matrix is passed through the dilator to fill the disc space. The dilator is then removed. An expandible intervertebral disc spacer is also disclosed, having a tapered bore that causes greater expansion of one end of the spacer with respect to the other. A kit for performing the percutaneous interbody fusion procedure is also disclosed.
Owner:WARSAW ORTHOPEDIC INC
Bone graft material incorporating demineralized bone matrix and lipids
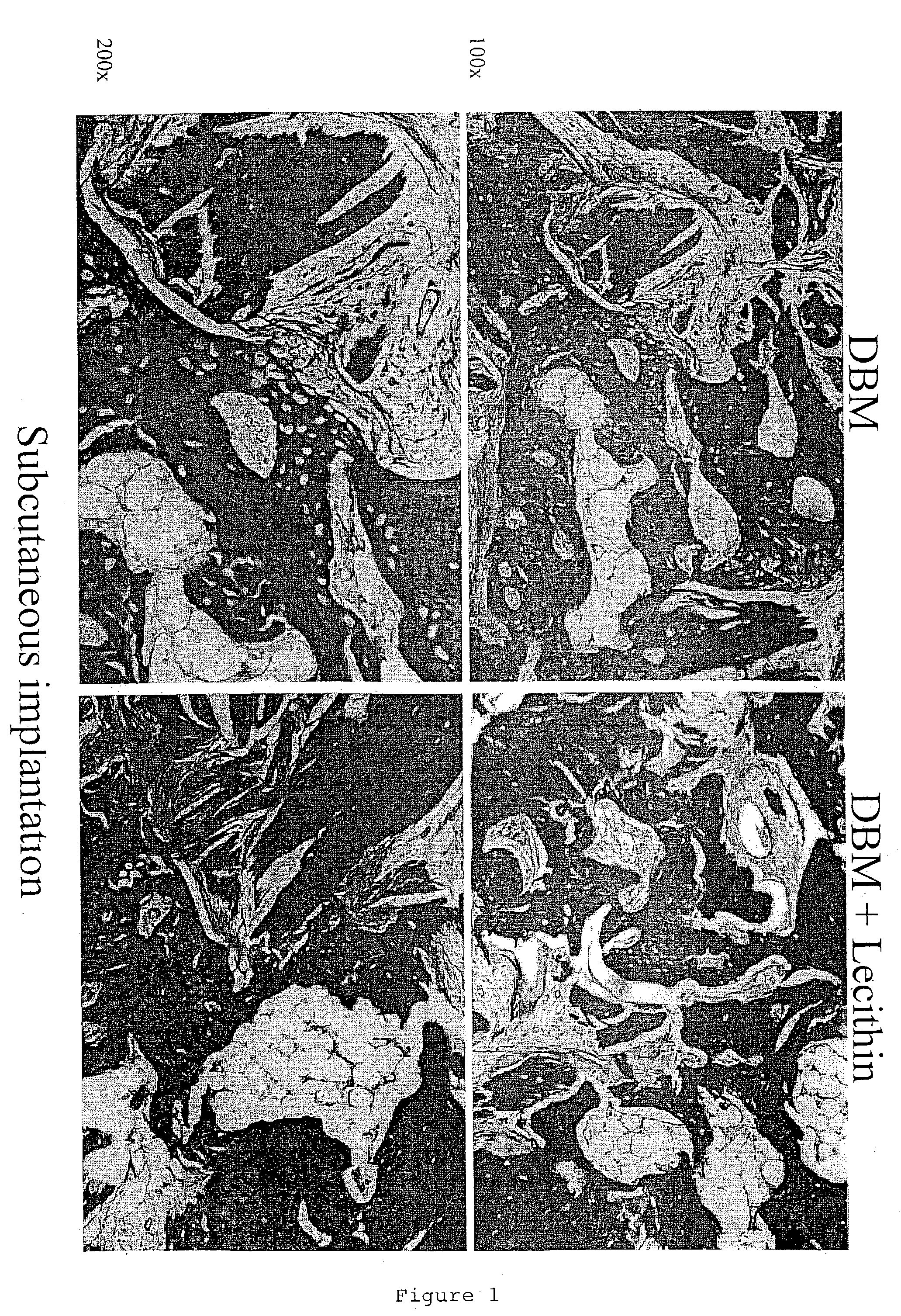
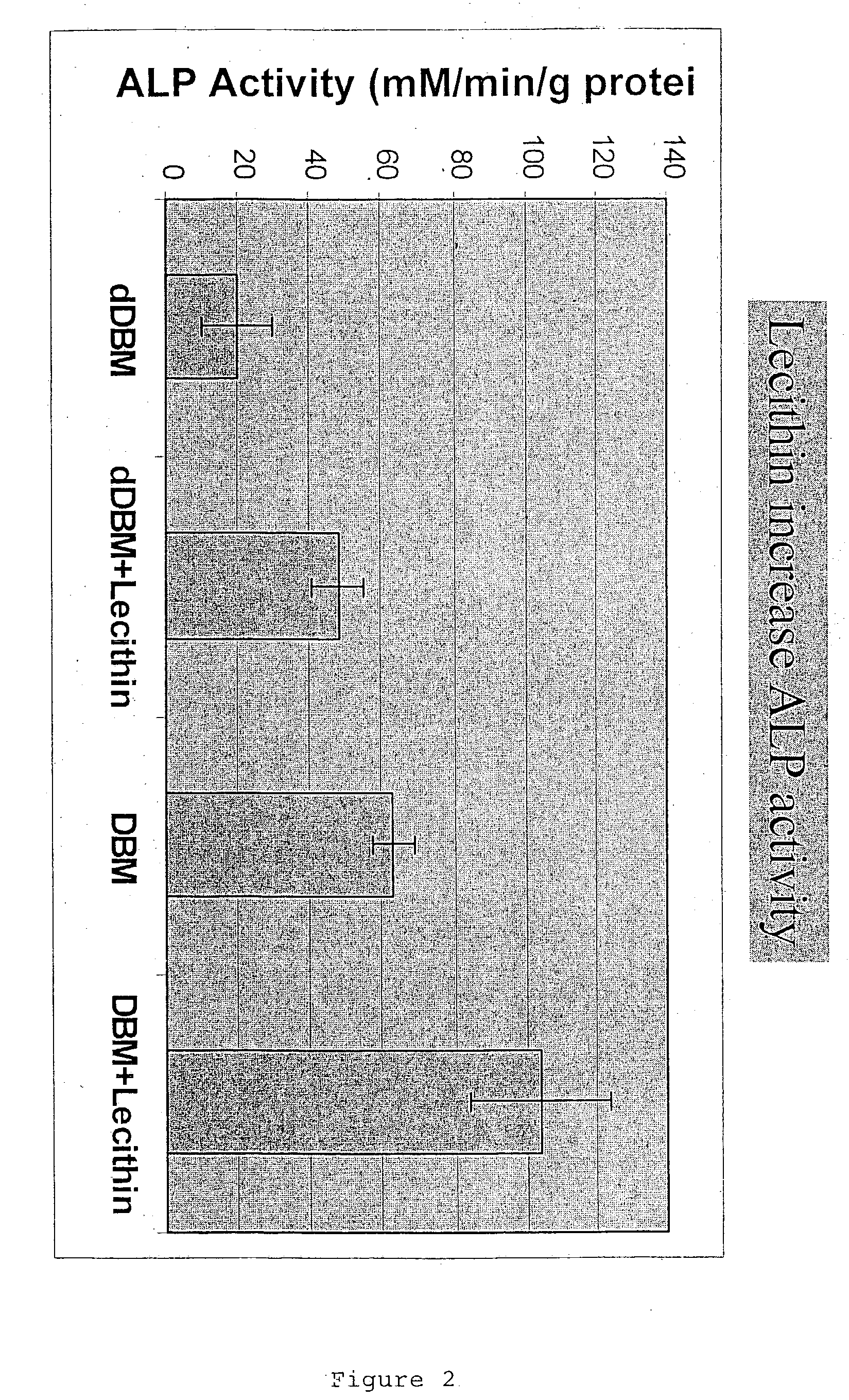
InactiveUS20040091459A1Good osteoinductivityEasy to operateBiocidePowder deliveryAntioxidantMaterials science
One embodiment of the invention is demineralized bone putty composition comprises: (1) demineralized bone matrix (DBM); and (2) a lipid fraction selected from the group consisting of lecithin and a mixture of lecithin and triglycerides containing unsaturated fatty acids. The putty composition is moldable, biocompatible, slowly resorbable, insoluble in tissue fluids, and non-extrudable. The composition delivers a biologically active product to animals and humans that will enhance bone formation at sites where bone is lost, deficient, or present in suboptimal amounts. The composition can further comprise calcium, an antioxidant such as Vitamin E or Vitamin C, or a hydrophilic polymer such as methylcellulose, a methylcellulose derivative, carboxymethyl cellulose, or hydroxypropyl methylcellulose. A second embodiment of the invention is a demineralized bone paste composition comprising: (1) about 15% to about 75% of an emulsion carrier, such as an aqueous phase; and (2) a bone-material-containing phase comprising: (a) demineralized bone matrix (DBM); and (b) an emulsifier component that is compatible with lipids. This bone paste composition is moldable, biocompatible, slowly resorbable, miscible with bone graft materials, soluble or partially soluble in tissue fluids, and extrudable.
Owner:BIOMET MFG CORP
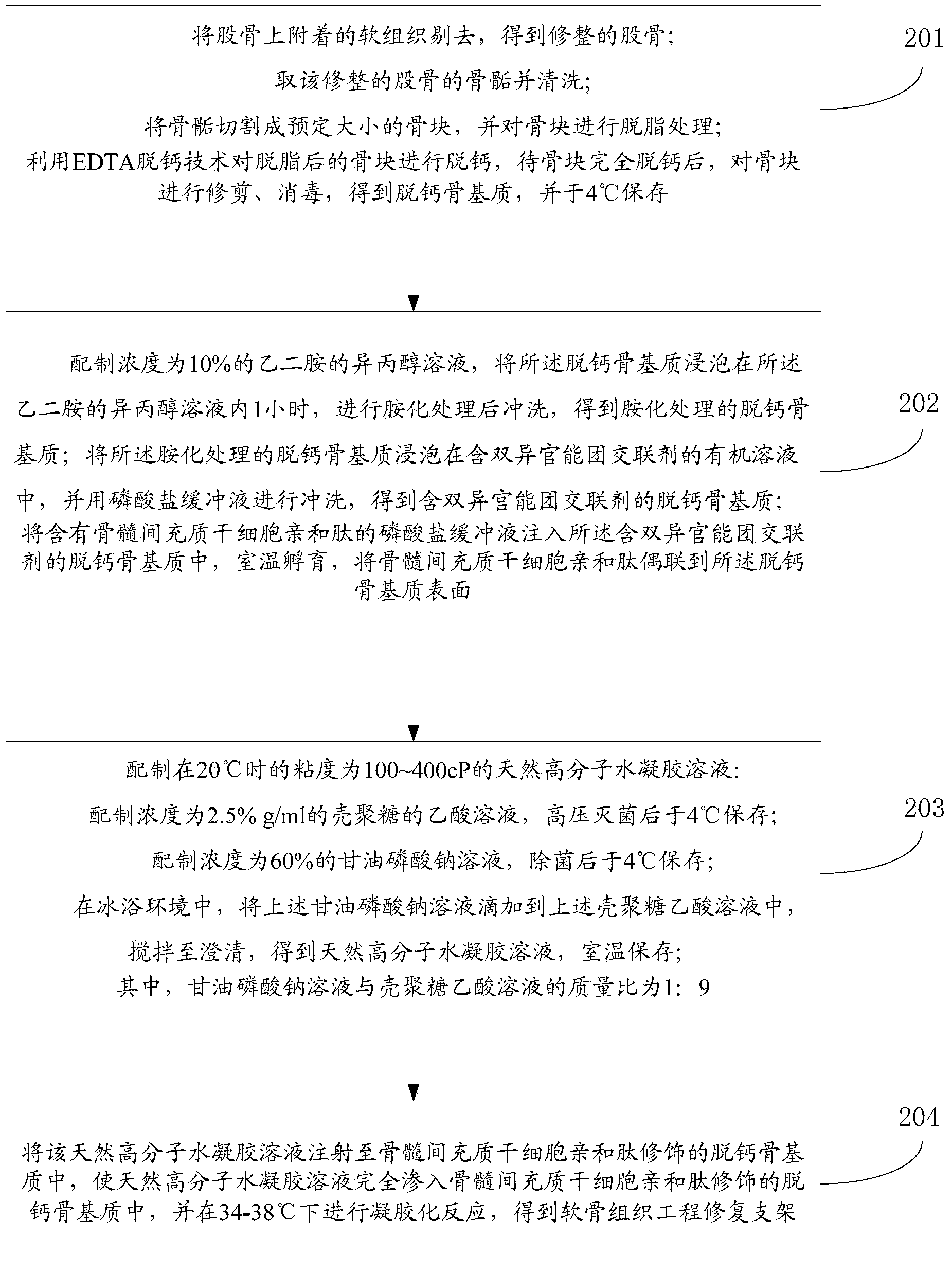
Method for preparing tissue-engineered bone/cartilage integrated scaffold
The invention discloses a method for preparing a tissue-engineered bone / cartilage integrated scaffold. An integrated scaffold structure of which internal pores are aligned and pore diameters are changed in a gradient way along a thickness direction and which has good mechanical properties is obtained by simulating the composition and spatial structure characteristics of natural articular cartilage and using extracellular matrixes and bone matrix gelatin of the cartilage as main raw materials by methods such as a different concentration superposition method, a directional crystallization method, a freeze-drying method, a cross-linking reaction method and the like. The high biocompatibility of the scaffold is favorable for the adhesion and proliferation of cells; a special pore structure of the scaffold ensures that the content of matrixes secreted by the cells is also changed in a gradient way; and meanwhile, a bone matrix gelatin substrate provides good mechanical support for the instant fixation of the scaffold and an injured part.
Owner:BEIJING SHENYOU BIO TECH
Cartilage tissue engineering repair bracket and preparation method thereof
ActiveCN103877616AHigh mechanical strengthImprove induction abilityCoatingsProsthesisBone Marrow Stromal CellCartilage repair
The invention discloses a cartilage tissue engineering repair bracket and a preparation method thereof, and belongs to the cartilage tissue repair field. The bracket comprises natural high polymer water-gel and decalcified bone matrix which is coupled with affinitive peptides of mesenchymal stem cells on surface; the affinitive peptides of the mesenchymal stem cells are coupled on the surface of the decalcified bone matrix to obtain the cartilage tissue engineering repair bracket with special mesenchymal stem cell affinity. By being placed in a cartilage defective region, the cartilage tissue engineering repair bracket can collect a plurality of BMSC (bone marrow stromal cells); and in the bracket, under mating effect of the natural high polymer water-gel and the decalcified bone matrix, the BMSC can be retained in the cartilage defective region for a long time, so that the cartilage repair has a longer curative effect period. The invention further discloses a preparation method of the bracket, wherein the natural high polymer water-gel aqueous liquor is injected in the decalcified bone matrix which is coupled with affinitive peptides of mesenchymal stem cells on surface to carry out gelation reaction to obtain the bracket with higher mechanical strength and inducibility. The method is simple and high in practicability.
Owner:PEKING UNIV THIRD HOSPITAL
Method for preparing similar bone bioactivity coatings medical material by galvano-chemistry method
InactiveCN101156963AThe preparation process conditions are simpleEfficient preparation process conditionsElectrolytic inorganic material coatingElectrolysisApatite
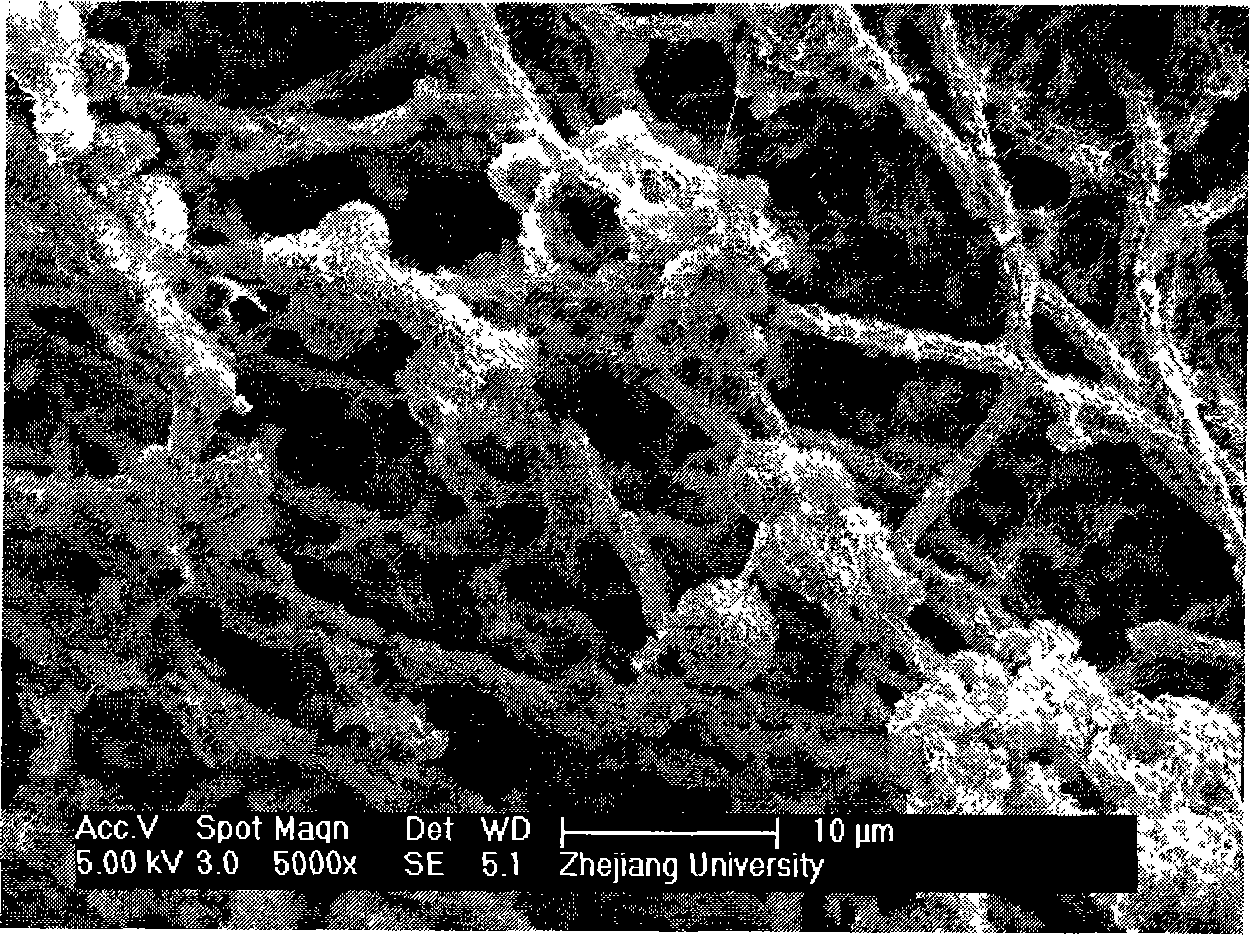
The invention discloses bone-like bioactive coating medical material which is prepared by adopting an electrochemical method. The invention adopts an electrolytic deposition method and imitates the forming process of a natural bone. Electrolyte solution contains calcium, a phosphorous compound and bone matrix collagen, a medical metal transplant body is taken as a working electrode, platinum is taken as a reference electrode, the electrode reaction causes partial pH value of the electrolyte solution around the medical metal transplant body to ascend, thereby leading collagen to be gelatinized, in cooperation with the deposition of calcium phosphate mineral, the bone-like bioactive coating is formed on the medical metal surface. Through the adoption of the invention method, the bioactive coating acquired on the surface of the metal transplant body is similar to the natural bone in the component and the structure, and has favorable biological activity, thus the disadvantages that the biological activity of the business use hydroxyapatite coating is limited, the required curing time is long, etc. are solved. The preparation process of the invention is simple, highly effective and easy to be industrialized.
Owner:ZHEJIANG UNIV
Collagen base bionic bone matrix
Owner:TIANJIN SANNIE BIOENG TECH
Device and method for lumbar interbody fusion
InactiveUS20080294171A1Reduces the trauma to the patientShorten recovery timeSurgical furnitureBone implantDilatorLumbar vertebrae
A method for performing percutaneous interbody fusion is disclosed. The method includes the steps of inserting a guide needle posteriorly to the disc space, inserting a dilator having an inner diameter slightly larger than the outer diameter of the guide needle over the guide needle to the disc space to enlarge the disc space, and successively passing a series of dilators, each having an inner diameter slightly larger than the outer diameter of the previous dilator, over the previous dilator to the disc space the gradually and incrementally increase the height of the disc space. Once the desired disc height is achieved, the guide needle and all the dilators, with the exception of the outermost dilator, are removed. An expandable intervertebral disc spacer is then passed through the remaining dilator and positioned in the disc space. The disc spacer is expanded to the required disc height, and then a bone matrix is passed through the dilator to fill the disc space. The dilator is then removed. An expandable intervertebral disc spacer is also disclosed, having a tapered bore that causes greater expansion of one end of the spacer with respect to the other. A kit for performing the percutaneous interbody fusion procedure is also disclosed.
Owner:WARSAW ORTHOPEDIC INC
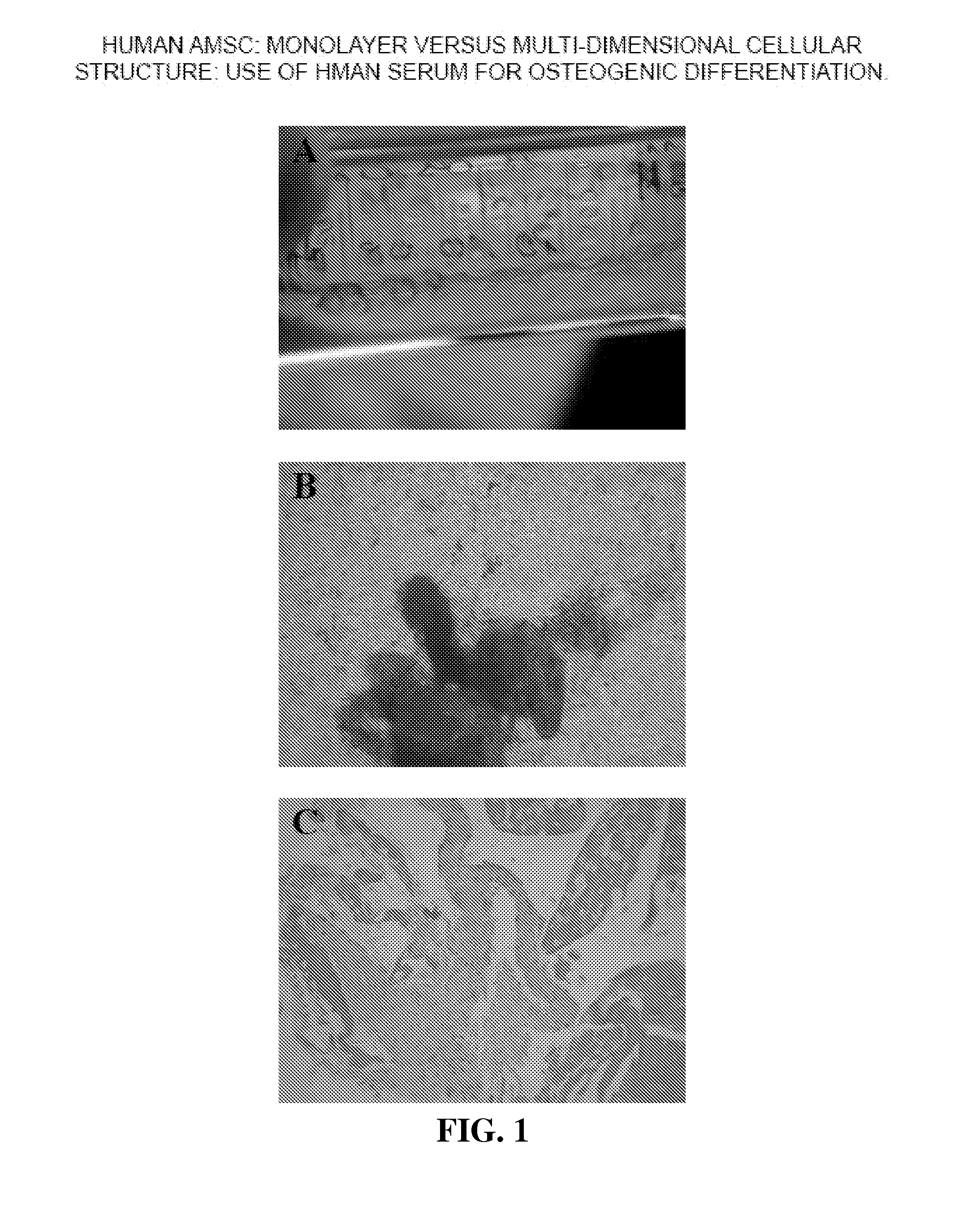
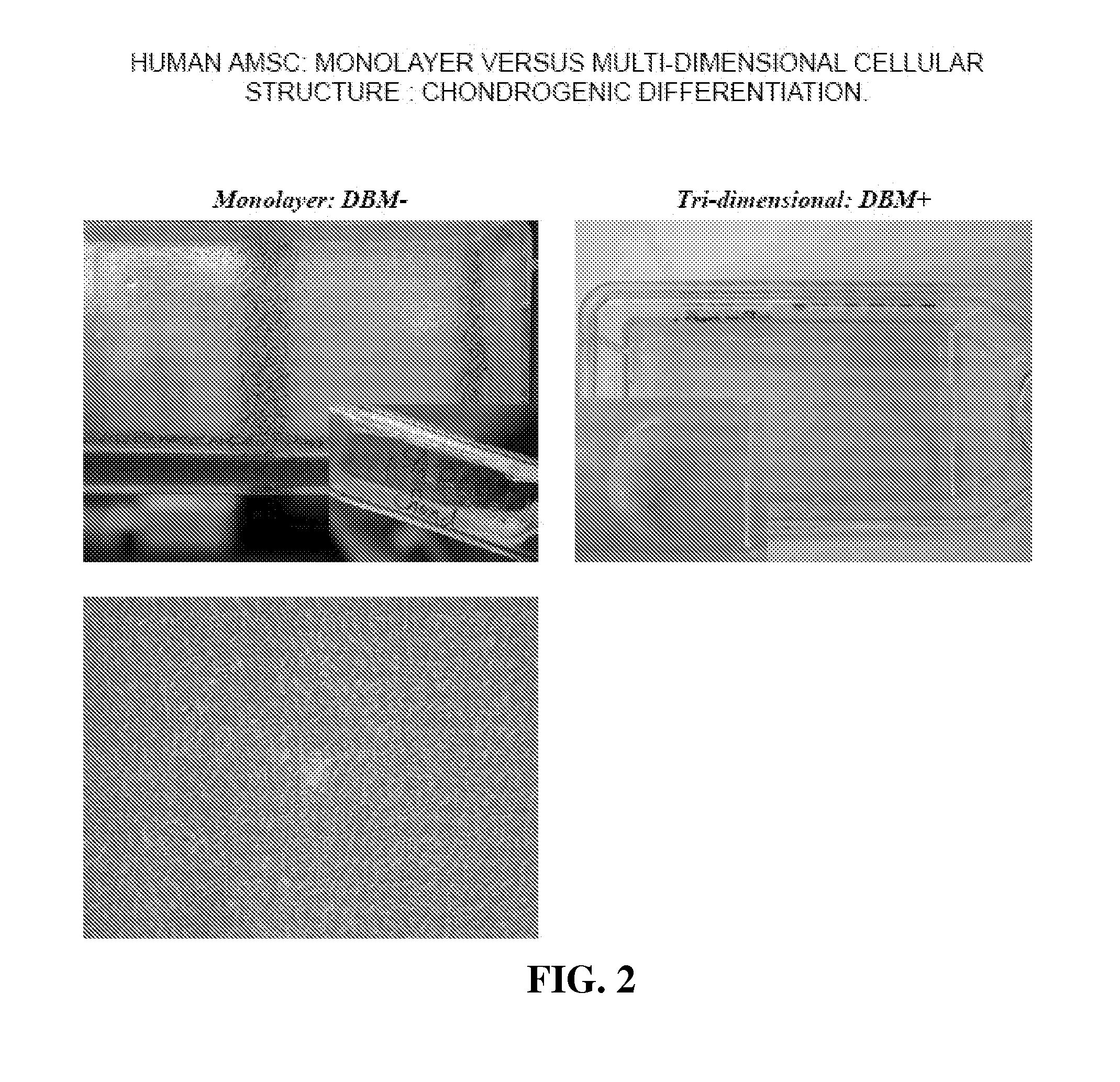
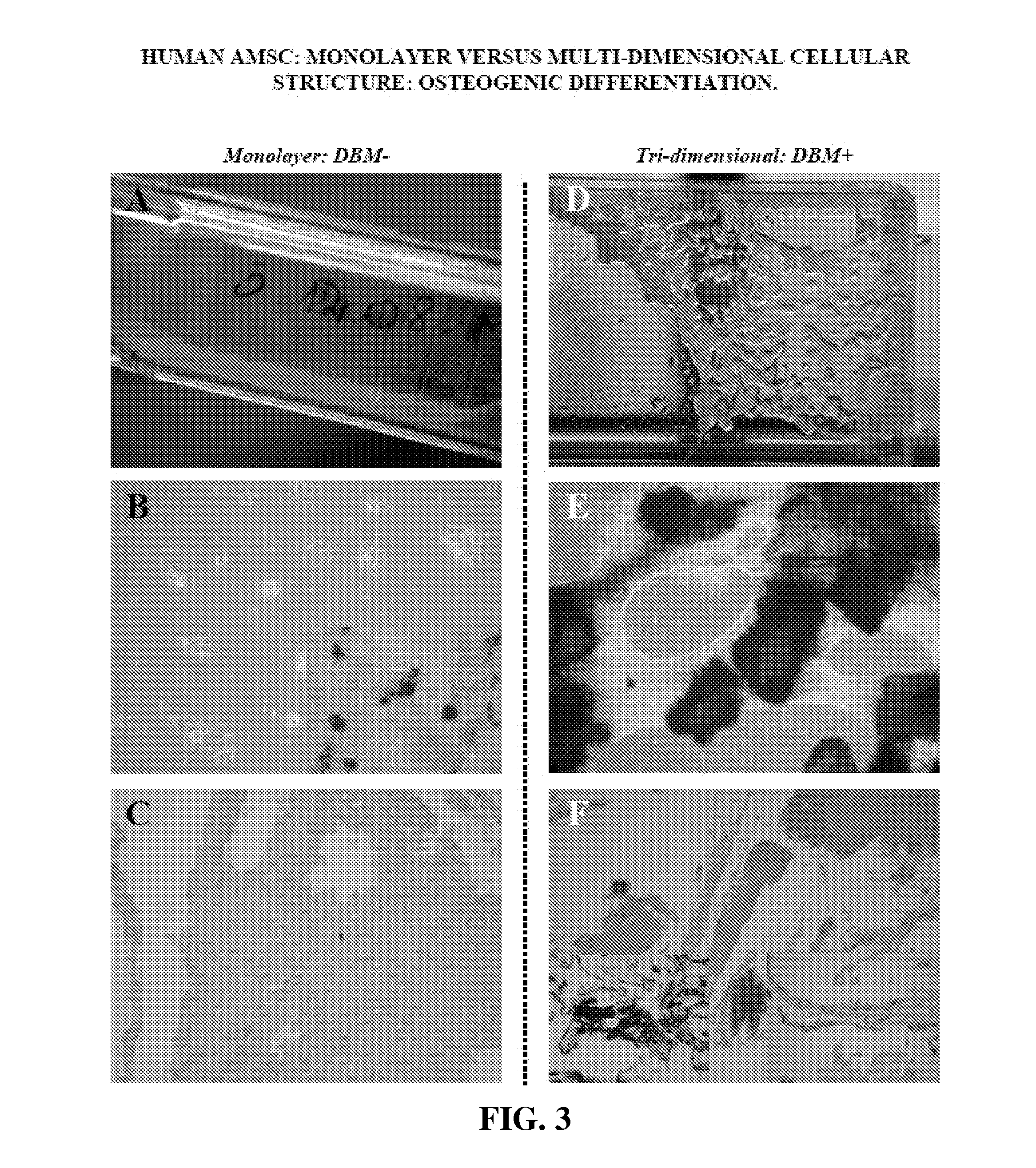
Multi-Dimensional Biomaterial and Method for Producing the Same
ActiveUS20120087958A1Reduce frequencySufficient amountBiocidePowder deliveryDemineralized bone matrixBiological materials
Biomaterial having a multi-dimensional structure and comprising differentiated MSCs tissue and demineralised bone matrix, wherein said demineralised bone matrix is dispersed within the differentiated MSCs tissue, method for producing the same and use thereof.
Owner:UNIVERSITE CATHOLIQUE DE LOUVAIN +1
Tissue engineering cartilage construction method using bone matrix gelatin
A tissue engineering cartilage construction method using bone matrix gelatin which comprises, using cartilage cell for isolated culture or base material stem cell for isolated culture to evoke cartilage cell i.e. seed cell, fetching New Zealand rabbit or human embryon os longum and metaphysic trabecular bone to construct bone matrix gel BMG, inoculating the seed cells harvested through isolated culture onto cortex bone or cancellous bone BMG for extracorporal culture.
Owner:XI AN JIAOTONG UNIV
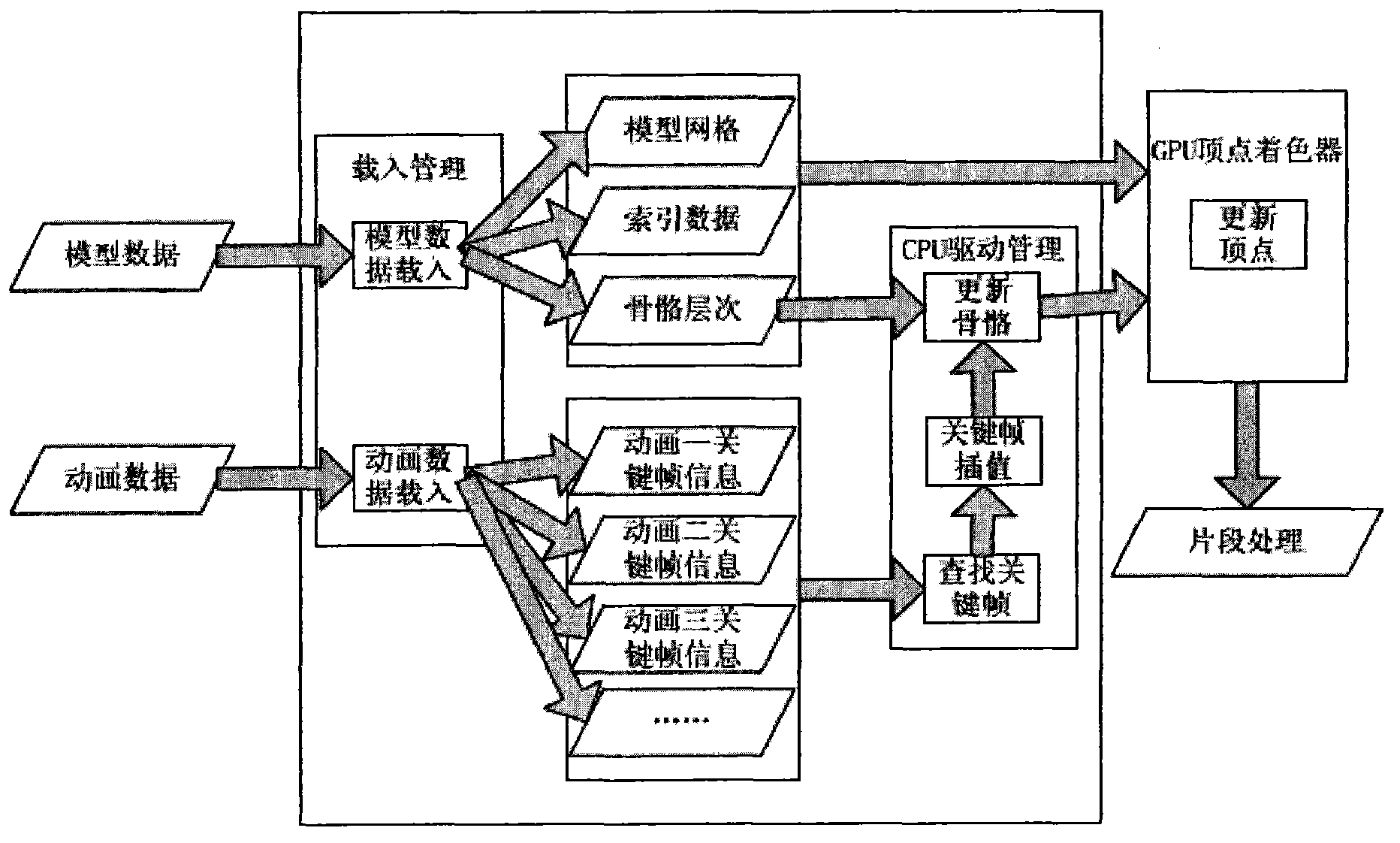
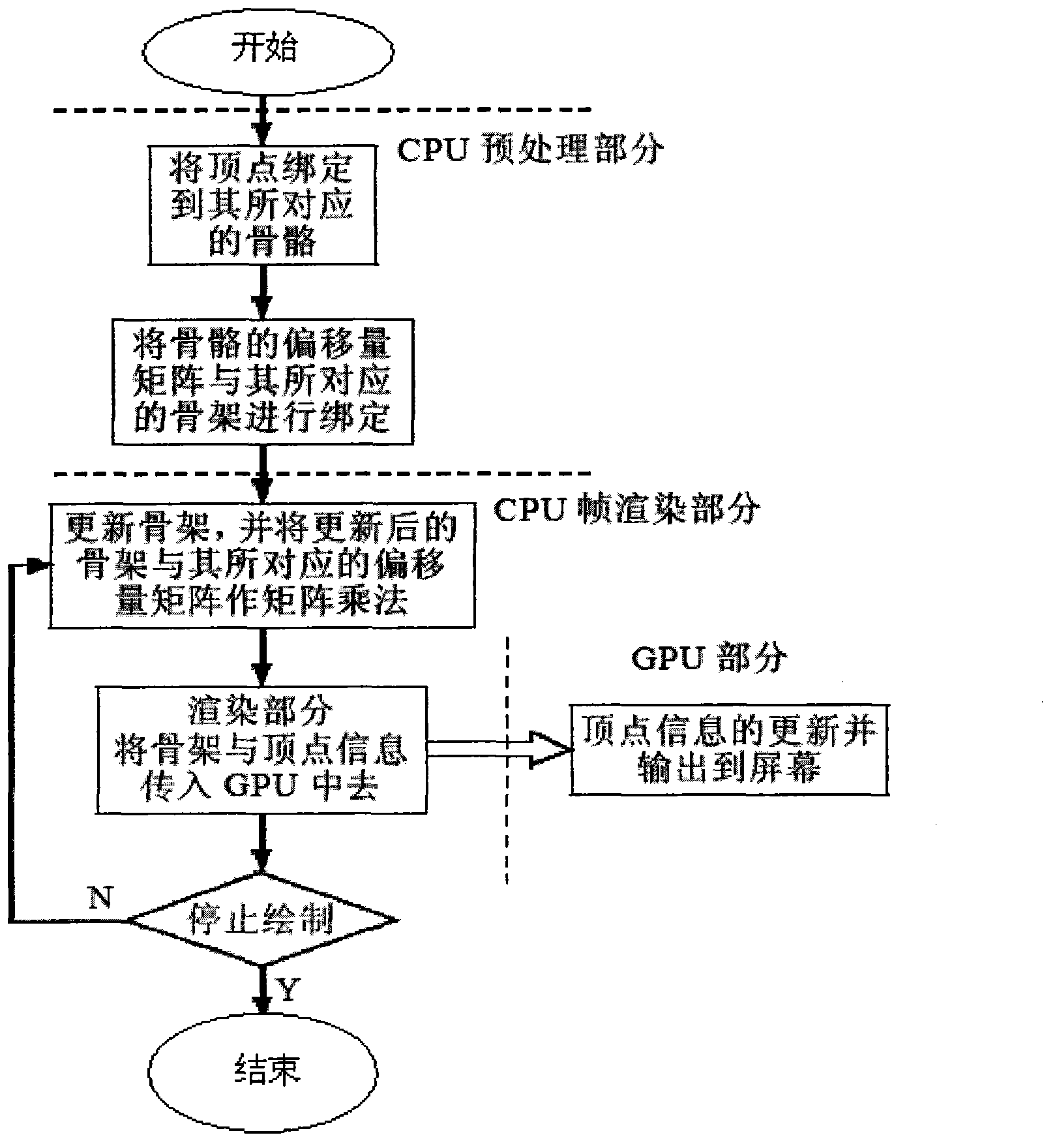
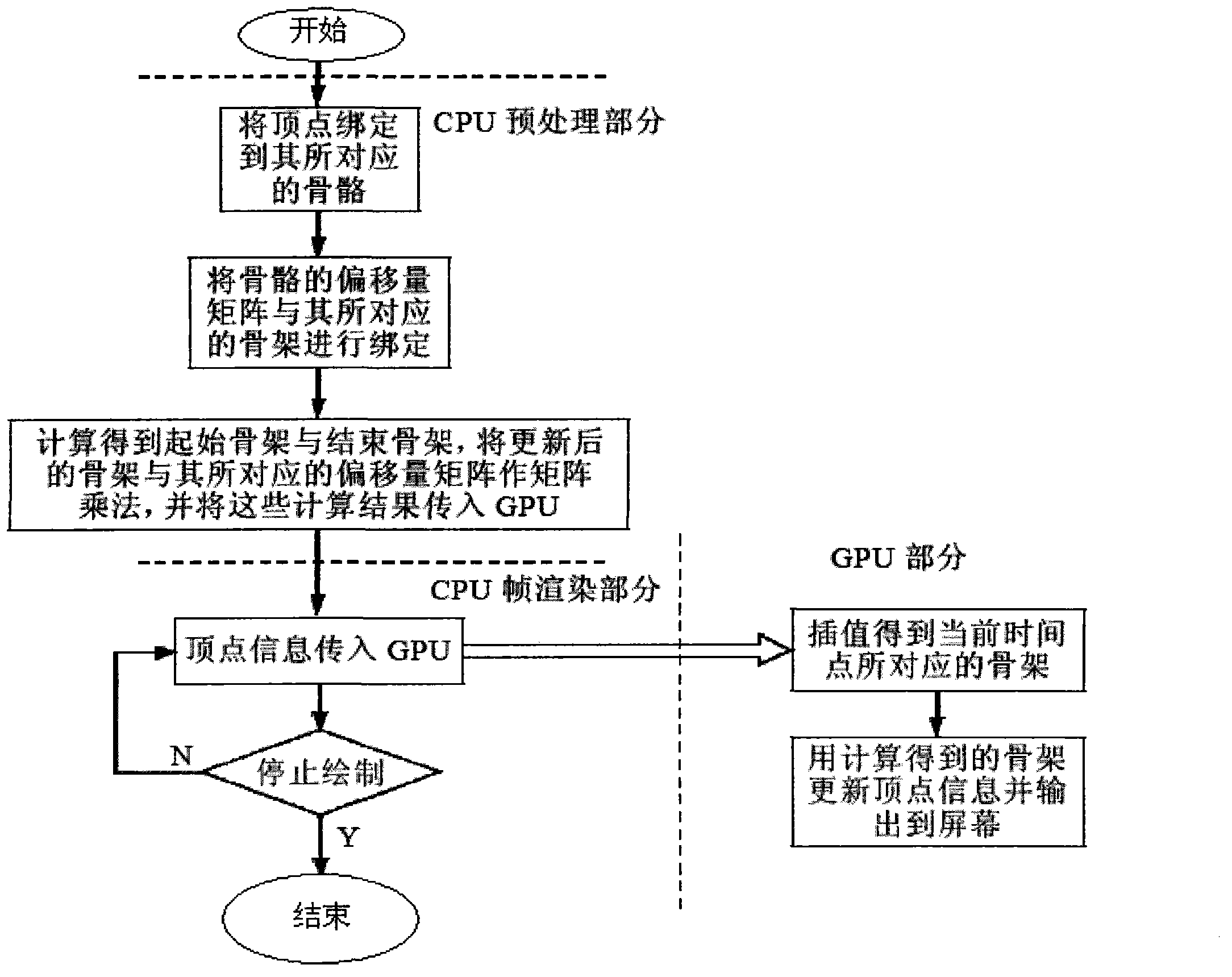
Bone animation processing method based on programmable graphics processing unit (GPU)
The invention aims at providing a bone animation processing method based on a programmable graphics processing unit (GPU). Calculation tasks of different parts of bone animation are placed into the GPU to enable parts of or all of calculation amounts to be transferred from the GPU to the GPU, and accordingly the GPU is liberated from burdensome vertex transformation and bone update. The bone animation processing method based on the GPU comprises: step one, binding vertexes in the GPU to corresponding bones; step two, binding offset matrixes of the bones and corresponding skeletons in the GPU; step three, updating skeletons in the GPU and performing matrix multiplication to updated skeletons and corresponding offset matrixes and transmitting updated bone matrixes and vertex information to the GPU; and step four, utilizing transmitted bone matrixes to update the vertex information in the GPU.
Owner:HUNAN NORMAL UNIVERSITY
Cartilidge and bone induction by artificially perforated organic bone matrix augmented by undifferentiated cells suspended in bone gel
InactiveUS20050074476A1Process can be usedBone implantJoint implantsBiomedical engineeringUndifferentiated cell
A novel method for cartilage and bone induction is introduced whereby an artificially perforated bone organic bone matrix augmented by live human undifferentiated cells suspended in a gel is used as a surgical implant.
Owner:GENDLER FAMILY PARTNERSHIP
Bone matrix compositions and methods
ActiveUS20110195052A1Improve biological activityFacilitated releaseBiocideHydrolysed protein ingredientsOsteoblastSpecific protein
The present invention provides methods of improving the osteogenic and / or chondrogenic activity of a bone matrix, e.g., a dermineralized bone matrix (DBM), by exposing the bone matrix to one or more treatments or conditions. In preferred embodiments the bone matrix is derived from human bone. The treatment or condition may alter the structure of the bone matrix and / or cleave one or more specific proteins. Cleavage may generate peptides or protein fragments that have osteoinductive, osteogenic, or chondrogenic activity. Preferred treatments include collagenase and various other proteases. The invention further provides improved bone and cartilage matrix compositions that have been prepared according to the inventive methods and methods of treatment using the compositions. The invention further provides methods of preparing, testing, and using the improved bone matrix compositions. On a assay comprises exposing relatively undifferentiated mesenchymal cells to a bone matrix composition and measuring expression of a marker characteristic of osteoblast or chondrocyte lineage(s). Increased expression of the marker relative to the level of the marker in cells that have been exposed to a control matrix (e.g., an inactivated or untreated matrix) indicates that the treatment or condition increased the osteogenic and / or chondrogenic activity of the bone matrix. Suitable cells include C2C12 cells. A suitable marker is alkaline phosphatase. The inventive methods increase the osteogenic and / or chondrogenic activity of human DBM when tested using this assay system.
Owner:WARSAW ORTHOPEDIC INC
Bone matrix material containing various proteins secreted by umbilical cord mesenchymal stem cells and preparation method thereof
ActiveCN103480040AStrong osteoinductive activityGood osteoinductive functionSkeletal/connective tissue cellsProsthesisBone tissueTissue engineered bone
The invention relates to a bone matrix material containing various proteins secreted by umbilical cord mesenchymal stem cells and a preparation method thereof. The material is an animal bone matrix material with the umbilical cord mesenchymal stem cells inoculated on the surface of a decalcified bone matrix and used for secreting osteogenic induction function proteins to promote osteogenesis. The bone matrix material has natural structures and characteristics of bone tissues and a good osteogenic induction effect, has a better osteogenesis promoting effect in a bone defect repair process, and is helpful for large-scale preparation and clinical application of tissue-engineered bones.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Abnormal decelled bone based material and its preparation
A cell-removed heterogeneous bone matrix is prepared from the rib and extremity bone of pig through physical and chemical processing, and removing cells and the heterogeneous protein from tissue by use of proteolytic enzyme and Triton-X100. Its advantages are natural components and biologic chemical strength, low antigenicity, high compatibility and bone conductivity, and low cost.
Owner:INST OF FIELD OPERATION SURGERY NO 3 MILITARY MEDICL UNIV PLA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com